Abstract
Rationale
Recent recognition of the need to improve pain management in cats has led to the investigation of the pharmacokinetics and efficacy of opioid analgesic drugs in this species. The results of these studies may be difficult to interpret because the effect of these drugs varies with dose, route of administration and the method used to assess them. As equipotency of different opioids is not known, it is hard to compare their effects. Animals do not verbalise the pain they feel and, in cats, it may be more difficult to recognise signs of pain in comparison with other species such as dogs.
Aim
This article reviews the use of opioid analgesics in cats. It must be remembered that not all drugs are licensed for use in cats, and that marketing authorisations vary between different countries.
Introduction
In recent years research into pain assessment and management in cats has increased. This interest and focus may have been in response to reports that cats have been undertreated for pain in comparison with other species.1 –4 Advances in knowledge have led to internationally recognised veterinary bodies issuing guidelines on pain management in cats, including those very recently from the American Animal Hospital Association and the American Association of Feline Practitioners. 5 In 2014, the World Small Animal Veterinary Association also published guidelines on the recognition, assessment and treatment of pain. 6
Pain assessment in cats
Pain assessment and quantification in animals is challenging because pain is a subjective sensory and emotional experience and animals are unable to verbalise their suffering. The most reliable way to assess pain in cats is thought to be through a combination of behavioural observations and interaction with the animal. 7 Various scales have been created to try to quantify pain in cats. For example, the Colorado State University Veterinary Medical Center has designed a feline acute pain scale, 8 whereby psychological and behavioural components are evaluated in conjunction with the cat’s response to palpation of the site of surgery and its body tension. In many studies the visual analogue scale (VAS) is used: an assessor marks a point on a 100 mm line that best correlates with the cat’s pain intensity; VAS is based on visual observation and ‘0’ is considered no pain, while ‘100’ is considered the worst pain imaginable. The so-called dynamic interactive visual analogue scale (DIVAS) also includes interaction with the cat, such as palpation of the wound. Recently, a multidimensional composite scale for assessing postoperative pain in cats has been validated. 9 This tool combines evaluations of postoperative psychomotor changes, reaction to palpation of the wound area and vocal expression of pain, together with appetite and arterial blood pressure. It is important to remember that increased arterial blood pressure can be a sign of stress, fear and anxiety, and not necessarily pain. Another method with the potential to assess pain in cats is the evaluation of facial expressions. 10
Limitations of pain assessment using the aforementioned methods include the subjective nature of the evaluation and the difficulties in recognising behavioural clues that may be indicative of pain, particularly in a hospital environment. However, use of a pain scale has the advantages that it focuses the attention of members of staff on pain and also means that an individual cat’s response to analgesic administration can be assessed and monitored.
Nociceptive threshold testing has been used to assess the antinociceptive effects of analgesic drugs in a more objective way. In cats, mechanical, thermal and electrical threshold testing has been employed in experimental settings to evaluate the effects of opioids.11,12 For each of these techniques a baseline nociceptive threshold is measured, an analgesic is then administered and the thresholds are measured again at specific time points. An increase in the threshold is usually accepted as evidence of a drug’s anti-nociceptive effect. Nociceptive threshold testing does have a number of limitations, 13 including the lack of evaluation of the emotional components of pain. Despite these limitations it is a useful tool for identifying drugs and dosages for further evaluation in clinical cases. In a clinical setting, mechanical nociceptive testing has also been used, in conjunction with subjective assessments of pain, in cats undergoing surgery. 14
There are numerous publications investigating the effects of drugs on the minimum alveolar concentration (MAC) of inhalant anaesthetics in cats. However, this is not an effective way to evaluate the analgesic effects of opioids 15 since it only indicates the immobilising potency of the inhalation anaesthetic at the spinal cord level, while pain also involves supraspinal pathways. Several opioids, including morphine, buprenorphine, methadone, butorphanol, hydromorphone, fentanil, alfentanil, remifentanil and tramadol, have been proven to decrease, to different degrees, the MAC of inhalant anaesthetics, but the effects were not always clinically relevant and were not as profound as have been reported in other species, including dogs.16 –23 Thus, a significant reduction in volatile anaesthetic concentration after opioid administration should not be expected in the clinical setting.
When discussing opioids, opioid receptors and comparisons between different opioid drugs, some concepts should be made clear. Affinity refers to how avidly a drug binds to a receptor, efficacy indicates the magnitude of the effect produced by the drug–receptor interaction, and potency indicates the quantity of drug needed to produce a maximal effect. 24
Opioids and cats
Opioids play an important role in the clinical management of pain in cats. Ideally, analgesics should be administered before noxious stimulation (preventive analgesia) with the aim of preventing sensitisation of the central nervous system, which could lead to the development of hyperalgesia. A multimodal approach is also recommended; this involves the concurrent administration of different classes of analgesics, such as opioids, non-steroidal anti-inflammatory drugs (NSAIDs), local anaesthetics and α2-adrenoreceptor agonists, with the aim of decreasing the doses and thus the side effects of individual agents, while improving the control of pain in the animal. 6
In cases of acute pain, opioids are effective and versatile analgesics, with wide therapeutic margins and relatively minor side effects in cats. 6 Their effects can also be antagonised if required. Historically there was a reluctance to administer opioids to cats due to concerns over the potential excitatory effects reported by Wikler in 1944. 25 However, ‘morphine mania’ was elicited at doses 100-fold higher (15 mg/kg) than those used in the clinical setting. 26 Sufentanil and alfentanil administration in cats is associated with an increase in sympathetic outflow and central stimulatory effects,27,28 but a study investigating morphine administered to cats at clinical (0.3 mg/kg) and supraclinical (0.6–2.4 mg/kg) doses reported no excitement. 29 At clinically used doses the behavioural effects of opioids include euphoria, manifesting as purring, rubbing, rolling and kneading with the forepaws.30 –35
Opioids cause mydriasis in cats, 36 which outlasts their analgesic effects and may affect vision. In dogs, the incidence of vomiting and salivation following morphine, hydromorphone and oxymorphone administration was reduced by prior administration of acepromazine. 37 This has not been studied in cats but it is possible that a similar effect may be seen. Decreased intestinal motility is a potential adverse effect of opioids but the clinical relevance of this in healthy cats is questionable, and in a study where buprenorphine was administered with acetylpromazine the orocaecal transit time, assessed by the breath hydrogen method, was not affected. 38
Postanaesthetic hyperthermia, defined as a rectal temperature higher than 39.2°C, has been associated with opioid administration in cats. Moreover, it was shown in a prospective clinical study of 40 healthy adult cats that body temperature at extubation was inversely related to the degree of postanaesthetic hyperthermia; that is, the colder the cat was at the end of anaesthesia, the higher the temperature was reported to be during recovery. 39 In a prospective randomised crossover study, buprenorphine (0.02 mg/kg IM), butorphanol (0.2 mg/kg IM), morphine (0.5 mg/kg IM) and hydromorphone (0.05–0.2 mg/kg IM) caused mild/moderate and self-limiting increases in body temperature (<40.1°C). 40 Alfentanil infusion increased body temperature in isoflurane-anaesthetised cats, 20 but not in propofol-anaesthetised cats. 41 An increase in body temperature has been detected with transdermal fentanyl patches in cats undergoing onychectomy, with mean rectal temperatures ranging from 38.8–39.4°C in the postoperative period. 30 Morphine and pethidine may cause hyperthermia at doses much higher than those recommended.42,43 In the light of these findings, it is recommended that a cat’s body temperature is closely monitored during anaesthesia and in the perioperative period.
Other side effects of opioid therapy in people are opioid-induced hyperalgesia and tolerance, 44 but these have not been reported in cats to date. This may be due to the relatively short duration of opioid administration in animals compared with people (where they may be used for long periods, particularly in the palliative care setting), meaning that opportunities for detecting these phenomena are limited. Challenges associated with determining whether changes in response to opioids are due to hyperalgesia/tolerance or disease progression, or due to different metabolisation of drugs in cats compared with people, might also be a factor. 15
As in people, great variability in the response of individual cats to opioids has been observed;7,45,46 a phenomenon known as ‘pharmacogenomics’. 47 Hence it is important to tailor the analgesic protocol to fit the individual patient’s needs in order to maximise pain relief while minimising side effects. Although adverse events may occur, opioids are effective analgesic drugs to be used in cases of moderate/severe pain and the risk of severe side effects is low in comparison with other classes of analgesics, such as NSAIDs. 48
In this review of the use of opioids in cats, opioids licensed for use in cats are discussed before those that are not licensed. Licensing of drugs is country-specific and readers need to be aware of the prescribing laws pertaining to their own country.
Pethidine (meperidine)
Pethidine is a synthetic full µ receptor agonist 49 that has a marketing authorisation at a dose of 3.3 mg/kg by intramuscular (IM) injection in cats in some European countries, including the UK. It should not be administered intravenously (IV) because it causes histamine release. 50 Vomiting is a rare side effect. 43
Clinical and experimental studies have evaluated various doses of pethidine, ranging from 2–10 mg/kg, alone and in combination with other drugs (Table 1). The onset of analgesic effect is rapid (30 mins) and the duration of action is dose-dependent, ranging from 1–2 h.11,14,55 These characteristics mean that pethidine may be a good option in cats that require short-term pain relief and/or frequent re-evaluation of the neurological system. However, other opioids may be more suitable where a longer duration of analgesia is required, such as after surgery.
Table 1.
Studies evaluating pethidine in cats
| Type of study | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Experimental | P 2.5mg/kg + DEXM 10 µg/kg IM |
Subjective evaluation of nociceptive response to digital pad clamp and tail clamp | Decrease in pain score (multifactorial scale) 30 mins after P administration; the protocol allowed completion of several clinical manipulations | 51 |
| Experimental | P 5 mg/kg IM | TNT MNT ENT |
TNT increased at 15 and 60 mins after treatment. MNT increased at 30 and 45 mins after treatment. No change in ENT detected | 12 |
| Experimental | P 5 mg/kg IM | TNT | TNT increased at 30, 45 and 60 mins after treatment | 11 |
| Experimental | P 5 mg/kg IM | PK | T½ el = 216 mins Clp = 20.8 ml/kg/min |
52 |
| Clinical: castration | P 5 mg/kg IM | MNT VAS |
Lower VAS pain scores at 0.5 and 1 h postsurgery in cats being administered P in comparison with cats not receiving P. In control cats significant hyperalgesia was recorded at all postoperative time points | 14 |
| Clinical: OHE | B 6 μg/kg IM, P 5 mg/kg IM, KETO 2 mg/kg SC |
VAS | Cats in KETO group had lower pain scores than cats in other groups from 1 h postoperatively | 53 |
| Clinical: neutering | P 3.3 mg/kg IM, CAR 4 mg/kg SC |
VAS | P provided less analgesia than CAR from 4 h after OHE and at 18–24 h after castration | 54 |
| Clinical: OHE | P 5 mg/kg IM, P 10 mg/kg IM, CAR 1–2–4 mg/kg SC |
VAS DIVAS |
P 10 mg/kg provided better analgesia than CAR up to 2 h post-extubation. From 2–20 h post-extubation CAR 4 mg/kg provided better analgesia than P | 55 |
‘+’ identifies where drugs were given in combination; where individual drugs were compared in different groups of animals, these are separated by commas
B = buprenorphine; CAR = carprofen; Clp = plasma clearance; DEXM = dexmedetomidine; DIVAS = dynamic interactive visual analogue scale; ENT = electrical nociceptive threshold; KETO = ketoprofen; MNT = mechanical nociceptive threshold; OHE = ovariohysterectomy; P = pethidine; PK = pharmacokinetics; T½ el = elimination half-life; TNT = thermal nociceptive threshold; VAS = visual analogue scale
Methadone
Methadone has recently received marketing authorisation for use in cats at a dose of 0.3–0.6 mg/kg in the United Kingdom, Italy and some other European countries. The results of various studies on methadone in cats are summarised in Table 2. Methadone is a synthetic µ opioid receptor agonist drug, consisting of a racemic mixture of D and L enantiomers. In addition to its interaction with the µ opioid receptor, the D isomer exerts an antagonistic action at the N-methyl-D-aspartate (NMDA) receptor. 60 Moreover, methadone plays a role in the descending pain pathways by inhibiting the reuptake of serotonin and noradrenaline, and by blocking the nicotinic cholinergic receptors.61,62 These receptor interactions could explain the good analgesic and possible anti-hyperalgesic effects that have been demonstrated using mechanical nociceptive testing in cats receiving methadone as part of pre-anaesthetic medication before ovariohysterectomy. 31
Table 2.
Studies evaluating methadone in cats
| Type of study | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Clinical: neutering | MET 0.5 mg/kg IM, B 0.02 mg/kg IM, BUT 0.4 mg/kg IM |
IVAS MNT |
IVAS pain scores were not different between groups at any time point up to 6 h after premedication. Postoperatively in female cats there was no significant variation in MNT over time in the MET group; in the B and BUT groups MNT decreased over time, becoming lower than baseline |
31 |
| Experimental | MET 0.3 mg/kg IV, MET 0.6 mg/kg OTM |
MNT | IV group: increased MNT values from 10 mins to 4 h postadministration. OTM group: increased MNT values from 10 mins to 6 h postadministration. MNT values higher in the IV group in comparison with the OTM group at 10 mins and 1 h postadministration |
33 |
| Experimental | MET 0.3 mg/kg IV | Tail clamp technique | Sevoflurane MAC after MET administration decreased by 25%, 15% and 7% at 26, 76 and 122 mins, respectively | 17 |
| Experimental | MET 0.2 mg/kg SC | TNT MNT |
TNT increased at 1–3 h and MNT increased at 45–60 mins after MET administration | 32 |
| Clinical: orthopaedic surgery | Levomethadone 0.3 mg/kg SC q8h for 5 days | NRT VAS MNT |
Analgesic effect of levomethadone was similar to B 0.01 mg/kg administered SC q8h. Higher MNT values were observed in both groups from 30 mins post-extubation until the end of day 4 |
56 |
| Clinical: OHE | MET 0.6 mg/kg IM | Wound palpation | Analgesia up to 4 h postoperatively, except in one cat | 57 |
| Clinical: OHE | MET 0.5 mg/kg IM | Behavioural observation Wound palpation |
Analgesia for 1.5–6.5 h | 58 |
| Experimental | MET 0.3 mg/kg OTM, MOR 0.2 mg/kg OTM, HYDRO 0.1 mg/kg OTM, OXY 0.25 mg/kg OTM |
Bioavailability/pharmacokinetic study | Mean ± SE bioavailability was 44.2 ± 7.9%, 36.6 ± 5.2%, 22.4 ± 6.9% and 18.8 ± 2.0% for buccal administration of MET, MOR, HYDRO and OXY, respectively | 59 |
See footnote to Table 1
B = buprenorphine; BUT = butorphanol; HYDRO = hydromorphone; IVAS = interactive visual analogue scale; MAC = minimum alveolar concentration; MET = methadone; MNT = mechanical nociceptive threshold; MOR = morphine; NRT = numerical rating scale; OHE = ovariohysterectomy; OTM = oral transmucosal; OXY = oxymorphone; TNT = thermal nociceptive threshold; VAS = visual analogue scale
In the experimental setting, methadone administration (0.2–0.6 mg/kg) resulted in antinociception to thermal and mechanical stimuli.32,33 The duration of antinociception to the thermal stimulus was longer than that to the mechanical stimulus, and also depended on the dose and route of administration; the duration of antinociception to the mechanical stimulus after 0.2 mg/kg SC was only 45–60 mins, whereas it was up to 4 h after 0.3 mg/kg IV. This suggests that the IV route is preferable where a longer duration of action is required.
In clinical studies, methadone, at doses of 0.3–0.5 mg/kg IM or SC, provided a dose-dependent period of analgesia lasting from 1.5–6.5 h.31,56 –58
In some countries the levorotatory enantiomer is available; when levomethadone (0.3 mg/kg IM) was compared with racemic methadone (0.6 mg/kg IM) it produced satisfactory postoperative analgesia after ovariohysterectomy. 57 Mechanical nociceptive threshold testing has been used to compare oral transmucosal (OTM) (0.6 mg/kg) with IV (0.3 mg/kg) administration of methadone. 33 A similar duration of antinociception to a mechanical stimulus was reported, although a less profound response for up to 1 h after administration was evident, suggesting a slower onset of full effect after OTM administration. It is worth noting that the OTM dose was double that administered IV, but these data suggest that methadone is absorbed by this route and recently a mean bioavailability of 44.2% was reported after buccal administration of methadone in cats. 59
The OTM route does look promising for administering methadone to difficult-to-inject cats, but further studies are required to determine the efficacy in clinical cases. Assessing the depth of anaesthesia and titrating the amount of anaesthetic agent to obtain a suitable depth of anaesthesia is important in all anaesthetised animals. Methadone (0.3 mg/kg IV) has been reported to decrease the MAC of sevoflurane in cats by 7–25%, so the vaporiser setting may need to be reduced. 17 As already mentioned, MAC reduction cannot be considered as a surrogate for analgesia.
Buprenorphine
Buprenorphine is a highly lipophilic semi-synthetic partial µ agonist opioid, 63 with a marketing authorisation for use in cats in the USA and several European countries. A wide variation in the duration of analgesic and antinociceptive effects has been reported for buprenorphine (Table 3). This variation may be attributed to different doses, routes of administration and methods of assessment, and individual variation between cats.
Table 3.
Studies evaluating buprenorphine in cats
| Type of study | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Experimental | B 0.02 mg/kg IV, B 0.02 mg/kg IM, B 0.02 mg/kg SC |
TNT | B 0.02 mg/kg IV: increased TNT values from 15–480 mins. B 0.02 mg/kg IM: increased TNT values at 30 and 60 mins. TNT values were higher in IV than IM or SC groups at 15, 60, 120 and 180 mins. SC group showed erratic absorption and disposition |
64 |
| Clinical: neutering | B 0.02 mg/kg IM, MET 0.5 mg/kg IM, BUT 0.4 mg/kg IM |
VAS MNT test |
B provided as effective analgesia as M during the 6 h test period | 31 |
| Clinical: OHE | B + CAR, B + MEL, BUT + CAR, BUT + MEL; B 180 μg/m2 IM, BUT 6 μg/m2 IM, CAR 4 mg/kg SC, MEL 0.3 mg/kg SC |
VAS DIVAS TNT |
All protocols tested provided low pain scores with no differences between groups | 65 |
| Clinical: OHE | SRB 0.11 mg/kg SC, B 0.02 mg/kg OTM |
VAS CSUCPSVFF |
SRB provided analgesia for up to 72 h postsurgery. No rescue analgesia required. SRB as efficacious as OTM B administered every 12 h |
66 |
| Clinical: OHE | B 0.01 mg/kg IV, B 0.01 mg/kg IM, B 0.01 mg/kg OTM |
DIVAS SDS |
No differences between groups detected with SDS. DIVAS pain scores higher in the OTM than IV or IM group at 1, 3, 4, 8 and 12 h. DIVAS pain scores after SC administration significantly higher than IV and IM administration at 2, 3, 4, 8, 12 and 24 h. OTM and SC groups required more rescue analgesia than IV and IM groups |
67 |
| Clinical | B 0.02 mg/kg + DEXM 0.02 mg/kg IM, B 0.02 mg/kg + DEXM 0.02 mg/kg OTM |
NRS to evaluate sedation | OTM treatment produced less sedation than IM treatment for IV catheterisation | 68 |
| Clinical: various surgeries | B 0.01–0.02 mg/kg IM, BUT 0.4 mg/kg IM |
SDS | Overall cats in B group had lower pain scores than BUT group. At 2 and 24 h time points B pain scores were lower. B provided better and longer lasting postoperative analgesia than BUT |
69 |
| Experimental | B 0.01 mg/kg IV (B1), B 0.02 mg/kg IV (B2), B 0.04 mg/kg IV (B4) |
TNT MNT |
Increased TNT values for 4, 2 and 8 h for B1, B2 and B4 groups, respectively. Increased MNT values at 15 and 45 mins for B2, and 30 and 45 mins, and 1 and 2 h for B4. B2 and B4 produced more mechanical antinociception and a longer duration of action than B1, respectively. No dose response effect to thermal stimulation detected |
70 |
| Experimental | B 12.5 μg/kg EPI, MOR 100 μg/kg EPI, SAL EPI |
TNT | TNT increased from 1–10 h in B group and from 1–16 h in MOR group in comparison with SAL. The maximum cut-off temperature of 55°C was reached 0, 74 and 11 times in SAL, MOR and B groups, respectively | 71 |
| Experimental | B 35 μg/kg TD | TNT | No significant changes in TNT during the 16 h test period | 72 |
| Experimental | B 0.02 mg/kg IM, BUT 0.2 mg/kg IM, SAL IM |
TNT | Compared with baseline, B increased TNT from 35 mins to 5 h post-treatment. Similar antinociception between B and BUT. Large inter-cat variation in magnitude and duration of response |
73 |
| Experimental | B 12.5 μg/kg EPI, MOR 100 μg/kg EPI |
MAC determination by tail clamp technique | No significant MAC-sparing effect in either B or MOR group | 74 |
| Experimental | B 0.02 mg/kg SC, MET 0.2 mg/kg SC, MOR 0.2 mg/kg SC |
TNT MNT |
TNT significantly increased at 45 mins after B administration; MNT increased at 30–45 mins after B administration | 32 |
| Clinical: OHE | B 0.01 mg/kg PO, B 0.01 mg/kg SC, MEL 0.3 mg/kg SC, MEL 0.3 mg/kg PO |
IVAS | IVAS scores were higher for B groups than MEL groups | 75 |
| Experimental | B 0.02 mg/kg IV, B 0.02 mg/kg OTM |
PK TNT |
TNT increased between 30 and 360 mins in both IV and OTM groups. OTM treatment was as effective as IV treatment |
46 |
| Experimental | B 0.01 mg/kg IV, B 0.01 mg/kg OTM |
PK | PK by the OTM and IV routes were similar | 76 |
| Experimental | B 0.01 mg/kg IM | TNT | TNT increased between 4 and 12 h post-B administration. Euphoria was recorded for up to 24 h in some cats. Mild sedation noted |
34 |
| Experimental | B 0.005 mg/kg IV, B 0.05 mg/kg IV |
MAC determination by tail clamp technique | Maximal MAC reductions were 17 ± 7% and 11 ± 6% with the lowest and highest doses of B, respectively, and were considered not clinically relevant | 16 |
| Clinical: various surgeries or invasive diagnostic investigations | B 0.01 mg/kg IM, MOR 0.1 mg/kg IM |
VAS | B provided better postoperative analgesia than MOR at 60, 120 and 180 mins postanaesthesia. Rescue analgesia was necessary in 5/14 and 3/18 cats in MOR and B groups, respectively |
77 |
| Clinical: onychectomy± neutering | B 0.01 mg/kg IV, OXY 0.05 mg/kg IM, KETO 2 mg/kg IM |
Cumulative pain scores VAS |
B cumulative pain scores were lower than OXY and KETO at 12 h post-extubation and lower than OXY at 4 h | 78 |
| Experimental | B 0.01 mg/kg IV and IM | PK | IV: mean ± SD T½ el = 416 ± 176.8 mins Clp = 16.7 ± 6.2 ml/kg/min Vdss = 7.1 ± 3.2 l/kg IM: mean ± SD T½ el = 380.2 ± 131 mins Clp = 23.7 ± 12.6 ml/kg/min Vdss = 8.9 ± 5.9 l/kg |
52 |
| Clinical: OHE | B 6 μg/kg IM, P 5 mg/kg IM, KETO 2 mg/kg SC |
VAS | Cats in KETO group had lower pain scores than cats in other groups from 1 h postoperatively | 53 |
| Experimental | B 20 μg/kg EPI, MEDET 10 μg/kg EPI, B 10 μg/kg + MEDET 5 μg/kg EPI |
TNT MNT |
TNT increased from 30 mins to 1 h after B and at 45 mins after MEDET. MNT increased from 45 mins to 2 h after B, from 15 mins to 1 h after MEDET and at 30, 45 mins and 2 h after B + MEDET. TNTs were above the upper 95% CI from 15 mins to 24 h after B, from 15 mins to 4 h after MEDET and from 15 mins to 8 h after B + MEDET. MNTs were above the upper 95% CI from 15 mins to 5 h, and at 8, 12 and 24 h after B, from 15 mins to 6 h after MEDET and from 15 mins to 6 h and at 12 and 24 h after B + MEDET | 79 |
| Experimental | B 0.24 mg/kg/day SC for 9 days, B 0.72 mg/kg/day SC for 9 days, B 1.20 mg/kg/day SC for 9 days, SAL |
Safety study | Young cats tolerated the different doses well. Adverse events related to B administration were noted in two cats being administered the 0.24 and 0.72 mg/kg/day dose, and consisted of mydriasis and behavioural changes such as hyperactivity, difficult handling, agitation and disorientation | 80 |
See footnote to Table 1
B = buprenorphine; BUT = butorphanol; CAR = carprofen; CI = confidence interval; Clp = plasma clearance; CSUCPS = Colorado State University Cat Pain Scale; DIVAS = dynamic interactive visual analogue scale; DEXM = dexmedetomidine; EPI = epidural; IVAS = interactive visual analogue scale; KETO = ketoprofen; MAC = minimum alveolar concentration; MEDET = medetomidine; MEL = meloxicam; MET = methadone; MNT = mechanical nociceptive threshold; MOR = morphine; NRS = numerical rating scale; OHE = ovariohysterectomy; OTM = oral transmucosal; OXY = oxymorphone; P = pethidine; PK = pharmacokinetics; SAL = saline; SDS = simple descriptive scale; SRB = sustained release buprenorphine; T½ el = elimination half-life; TD = transdermal; TNT = thermal nociceptive threshold; VAS = visual analogue scale; Vdss = volume of distribution at steady state; VFF = von Frey filaments
In experimental studies buprenorphine 0.01–0.02 mg/kg given IV or IM had a thermal antinociceptive effect lasting from 30 mins to 12 h.34,46,73 The dose-related antinociceptive effects of intravenous buprenorphine have been investigated; buprenorphine 0.02 mg/kg and 0.04 mg/kg produced a greater degree of mechanical antinociception than the 0.01 mg/kg dose, but no dose-related response was found with a thermal threshold model. 70
Clinical studies have indicated that buprenorphine appears to be an effective analgesic in cats undergoing various procedures including ovariohysterectomy, onychectomy and orthopaedic surgery.53,65,69,77,78
The pharmacokinetics of buprenorphine administered by the IV, IM, SC, transdermal and OTM routes have been described.46,52,64,72 Buprenorphine has good bioavailability when administered by the OTM route and experimental studies suggested that it was both effective and well tolerated by cats.46,76,81 Clinical studies have reported conflicting results regarding the analgesic efficacy of OTM buprenorphine.67,68,75 This is possibly due to the timing of drug administration, the concomitant use of α2-adrenoreceptor agonists (which could cause vasoconstriction and potentially reduce the uptake of buprenorphine across the oral mucous membranes), and the volume and dilution of buprenorphine. Experimental data suggested that SC and transdermal administration of buprenorphine resulted in erratic absorption and disposition, and a limited intensity of antinociception.32,64,72
Two studies evaluated the thermal antinociceptive effects of epidurally administered buprenorphine, which lasted from 1–10 h and from 15 mins to 24 h, at doses of 0.0125 mg/kg and 0.02 mg/kg, respectively.71,79 In another study, epidural administration of buprenorphine did not reduce the MAC of isoflurane. 74
Vomiting and dysphoria are rarely associated with buprenorphine administration and its efficacy and long duration of action make it a good analgesic for cats in the perioperative period. The reported variability in intensity and duration of analgesia reflects the different doses and routes of administration used in different studies. These factors, coupled with the differences in response between cats, emphasise why it is important to monitor the response to treatment and titrate analgesic therapy to suit the individual’s needs.
A sustained-release preparation of buprenorphine that may produce analgesia for up to 72 h after SC injection has been produced; this shows promise for providing analgesia in cats following ovariohysterectomy, as it was as effective as the standard formulation of buprenorphine administered by the OTM route q12h. 66 Very recently, the safety of long-acting buprenorphine administered SC has been tested in young cats. 80 Cats were administered buprenorphine for 9 consecutive days at a dose of 0.24, 0.72 or 1.20 mg/kg/day. These doses represent 1 x, 3 x and 5 x the licensed dose and they were reported to be well tolerated by cats.
A review of studies describing the clinical application of buprenorphine in cats is now available. 82
Butorphanol
Butorphanol is a synthetic opioid analgesic with agonist/antagonist activity. 83 Its pharmacology is complex and it has species-specific affinity for the μ-, δ- and κ-opioid receptor subtypes. 84 Butorphanol has a marketing authorisation for use in cats in several European countries and in North America, where it is widely used. In general, butorphanol is administered at doses from 0.1–0.4 mg/kg via the IV, IM or SC route. The OTM route has been investigated but it was not efficacious due to the limited systemic absorption in comparison with IM administration. 85
Many experimental studies have evaluated the analgesic effects of butorphanol. Some of the results are summarised in Table 4. The effects and duration of action vary according to the dose administered, the route of administration, the type of pain studied (visceral, somatic) and the type of pain model (electrical, mechanical, thermal threshold, colonic balloon, surgery).34,45,73,89,90,94,95,98,99 Experimental studies suggest that butorphanol provides short-lasting antinociception lasting from 5–165 mins,34,45,89,90,94,95 with the exception of one study where thermal nociceptive threshold values were increased for up to 8 h after 0.2 mg/kg butorphanol IM. 73 Early clinical studies showed that butorphanol decreased the stress response to surgery,100,101 and provided more analgesia than saline in cats undergoing onychectomy. 102 Subsequent studies reported that butorphanol administered to cats undergoing onychectomy or onychectomy plus neutering provided short-lasting analgesia for up to 2 h.30,96,97
Table 4.
Studies evaluating butorphanol in cats
| Type of study | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Clinical: neutering | B 0.02 mg/kg IM, MET 0.5 mg/kg IM, BUT 0.4 mg/kg IM |
VAS MNT test |
BUT and B produced similar sedation during the 6 h test period | 31 |
| Experimental | MID 0.4 mg/kg + BUT 0.4 mg/kg IM, MID 0.4 mg/kg + BUT 0.4 mg/kg + KETA 3 mg/kg IM, MID 0.4 mg/kg + BUT 0.4 mg/kg + DEXM 5 µg/kg IM, KETA 3 mg/kg + DEXM 5 µg/kg IM |
Sedation score in response to tactile and auditory stimulation. NRS to assess recovery |
MID + BUT was associated with the lowest sedation score and the poorest quality of recovery. KETA + DEXM was associated with the highest sedation score and best quality recovery. Stroke volume decreased by 24%, 21%, 24% and 36%, and cardiac output by 23%, 34%, 54% and 53% in MID + BUT, MID + BUT + KETA, MID + BUT + DEXM and KETA + DEXM treatment, respectively |
86 |
| Experimental | ACE 0.1 mg/kg + BUT 0.25 mg/kg IM, ACE 0.1 mg/kg + BUT 0.25 mg/kg IM + KETA 1.5 mg/kg IV |
Echocardiography | In ACE + BUT + KETA group heart rate increased significantly; in ACE + BUT group systolic blood pressure decreased significantly. The two sedation protocols did not alter echocardiography variables significantly, with the exception of a mild decrease in left ventricular end-diastolic dimensions and a mild increase in left ventricular end-diastolic wall thickness | 87 |
| Experimental | DEXM 20 µg/kg IM, DEXM 10 µg/kg + PETH 2.5 mg/kg IM, DEXM 10 µg/kg + BUT 0.4 mg/kg IM |
Multifactorial sedation scale. Subjective pain score to digital pad clamp and tail clamp. Subjective assessment of muscle tone |
No statistically significant differences between groups regarding sedation, analgesia and muscle relaxation | 51 |
| Clinical: OHE | B + CAR, B + MEL, BUT + CAR, BUT + MEL; B 180 μg/m2 IM, BUT 6 μg/m2 IM, CAR 4 mg/kg SC, MEL 0.3 mg/kg SC |
VAS DIVAS TNT |
All protocols tested provided low pain scores, with no differences between groups | 65 |
| Clinical | KETA 5 mg/kg + MID 0.2 mg/kg + BUT 0.3 mg/kg, SEVO |
Monitoring of physiological parameters | Both groups achieved adequate restraint for blood collection. SEVO was associated with a faster recovery. Hypotension (SAP <70 mmHg) requiring intervention was reported in 42% and 84% of cats in the KETA + MID + BUT and SEVO groups, respectively | 88 |
| Clinical: various surgeries | BUT 0.4 mg/kg IM, B 0.01–0.02 mg/kg IM |
SDS | Overall, cats in the B group had lower pain scores than those in the BUT group. At 2 and 24 h time points, B pain scores were lower. B provided better and longer lasting postoperative analgesia than BUT | 69 |
| Experimental | BUT 0.2 mg/kg IM | MNT | At 30 mins after BUT administration MNT was higher than baseline | 89 |
| Experimental | BUT 0.4 mg/kg IM, BUT 0.4 mg/kg OTM |
PK | IM: mean ± SD T ½ el = 6.28 ± 2.77 h Cl/F = 775.01 ± 302.17 ml/h/kg Vd/F = 7603.2 ± 6276.89 ml/kg Cmax = 0.35 h OTM: mean ± SD T½ el = 5.23 ± 1.72 h Cl/F = 2120.27 ± 392.87 ml/h/kg Vd/F = 15,633.54 ± 4697.48 ml/kg Cmax = 1.1 h OTM BUT absorption was 37.16% |
85 |
| Experimental | SAL, T + BUT, T + HYDRO ;T 8.6 mg/kg PO, T 11.6 mg/kg PO, BUT 0.4 mg/kg IV, HYDRO 0.1 mg/kg IV |
Tail clamp | Mean ± SEM MAC for sevoflurane after SAL was 2.45 ± 0.22%. MAC decreased to 1.48 ± 0.20%, 1.20 ± 0.16%, 1.76 ± 0.15%, 1.48 ± 0.20% and 1.85 ± 0.20% with T, BUT, HYDRO, T + BUT and T + HYDRO, respectively. Naloxone reversed the reductions in MAC | 18 |
| Experimental | BUT 0.4 mg/kg SC | MNT | MNT values increased from baseline for 45 mins after BUT administration. Maximum increase was recorded at 10 mins | 90 |
| Experimental | BUT 0.2 mg/kg IM, B 0.02 mg/kg IM, SAL IM |
TNT | Compared with baseline, BUT increased TNT from 50 mins to 8 h post-treatment. Similar antinociception between B and BUT. TNT of BUT was different from SAL at 50 mins. Large inter-cat variation in magnitude and duration of response reported |
73 |
| Clinical: OHE | BUT 0.44 mg/kg IM before surgery, CAR 2.2 mg/kg PO before surgery, KETO 2.2 mg/kg SC before surgery, BUPI 1.1 mg/kg local infiltration |
VAS IVAS |
VAS and IVAS pain scores were not different in BUT, CAR or KETO groups at any time point. In BUPI group, VAS and IVAS pain scores were higher than BUT group at 1 and 2 h after surgery |
91 |
| Clinical: onychectomy ± neutering | BUT 0.4 mg/kg SC prior to GA, MEL 0.3 mg/kg SC prior to GA |
VAS Composite pain score Gait lameness score |
In comparison with BUT, MEL group showed a lower VAS pain score, composite pain score and gait lameness score from 1–24 h following surgery. Rescue analgesia was required more often in BUT than MEL group | 92 |
| Clinical: OHE | CAR 4 mg/kg SC at induction, BUT 0.4 mg/kg SC at the end of surgery, SAL SC |
Composite pain scale until 24 h postoperatively | There were no differences between CAR and BUT in pain scores at any time points. Pain scores were increased from baseline in BUT and CAR groups for 12 h postsurgery | 93 |
| Experimental | HYDRO 0.1 mg/kg IM, BUT 0.4 mg/kg IM, SAL IM |
TNT | TNT values were higher compared with SAL in BUT group from 15–165 mins, and in HYDRO group from 15–345 mins | 94 |
| Experimental | BUT 0.1 mg/kg IV | TNT | Mean TNT values increased from baseline from 15–450 mins after BUT administration. Nausea was reported in 4/6 cats | 95 |
| Experimental | BUT 0.2 mg/kg IM | TNT | Significant increase in TNT values only 5 mins after BUT administration. Hyperalgesia was present 2 h after BUT administration. Euphoria was recorded for less than 30 mins after drug administration. Mild sedation reported | 34 |
| Clinical: onychectomy | TFP 25 µg/h, BUT 0.2 mg/kg administered at the time of sedation, at extubation, and every 4 h thereafter for 12 h. |
Plasma cortisol concentrations Subjective pain scores |
Lower pain scores in the TFP group than BUT group only at 8 h after surgery. Plasma cortisol concentrations not different between groups | 30 |
| Clinical: OHE | BUT 0.1 mg/kg IM, MEDET 15 µg/kg IM, SAL IM |
Subjective sedation and pain score | BUT provided better analgesia than MEDET and SAL during the 120 mins test period | 96 |
| Experimental | BUT 0.08 mg/kg IV, B 0.8 mg/kg IV |
MAC determination by tail clamp technique | Maximal MAC reductions were 19 ± 3% and 18 ± 4% with the lowest and highest doses of B, respectively, and were considered clinically relevant | 16 |
| Clinical: onychectomy, onychectomy + OHE, onychectomy + castration | TFP 25 µg/h, BUT 0.5 mg/kg IM and repeated at extubation at 0.2 mg/kg IM |
Subjective pain score Lameness assessed with pressure-sensitive mat |
Pain scores for TFP group were significantly lower than scores for BUT group at all time points from 30 mins after extubation to the end of the study. Lameness score was significantly lower for TFP group than BUT group the day after surgery and 2 days after surgery. Mean ratios of digital pad-to-metacarpal pad force were not significantly different between groups at any time point |
97 |
See footnote to Table 1
ACE = acepromazine; B = buprenorphine; BUPI = bupivacaine; BUT = butorphanol; CAR = carprofen; Cl = apparent clearance; Cmax = maximum plasma concentration; DEXM = dexmedetomidine; DIVAS = dynamic interactive visual analogue scale; F = relative bioavailability; GA = general anaesthesia; HYDRO = hydromorphone; KETA = ketamine; IVAS = interactive visual analogue scale; MAC = minimum alveolar concentration; MEL = meloxicam; MET = methadone; MID = midazolam; MEDET = medetomidine; MNT = mechanical nociceptive threshold; NRS = numerical rating scale; OHE = ovariohysterectomy; OTM = oral transmucosal; PETH = pethidine; PK = pharmacokinetics; SAL = saline; SAP = systolic arterial pressure; SDS = simple descriptive scale; SEVO = sevoflurane; T1/2 el = elimination half-life; T = tramadol; TFP = transdermal fentanyl patch; TNT = thermal nociceptive threshold; VAS = visual analogue scale; Vd = apparent volume of distribution
In a multicentre study, butorphanol (0.4 mg/kg) provided poorer analgesia, and for a shorter time duration, than buprenorphine (0.01–0.02 mg/kg) after a variety of surgeries. 69 In contrast to these findings, another study, of cats undergoing ovariohysterectomy, 65 reported no differences in analgesia between cats receiving butorphanol vs buprenorphine; these results may reflect the fact that an NSAID was administered in combination with the opioid prior to surgery. 65 NSAIDs have been reported to be more efficacious analgesics in the postoperative period than butorphanol in cats undergoing ovariohysterectomy and onychectomy,92,93 but another study showed similar pain-associated behaviour after ovariohysterectomy. 91
Butorphanol has isoflurane and sevoflurane MAC-sparing effects;16,18 moreover, butorphanol is a versatile agent that can be used in combination with other drugs to provide the sedation required to perform clinical and diagnostic procedures when only mild pain is anticipated.51,86,87 Most clinical studies report a few hours’ analgesic effect for butorphanol;93,100,101 those reporting a longer duration of action included repeat dosing.30,97,102 Frequent re-dosing in order to provide analgesia would be impractical when pain is expected to last for a long period postoperatively. 7
Morphine
Morphine is a full agonist at the µ, δ and 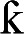 opioid receptors.
103
Although morphine is not licensed for use in veterinary species, it is generally considered the ‘gold standard’ opioid.
104
Several of the experimental and clinical studies that have evaluated the use of morphine in cats are summarised in Table 5.
opioid receptors.
103
Although morphine is not licensed for use in veterinary species, it is generally considered the ‘gold standard’ opioid.
104
Several of the experimental and clinical studies that have evaluated the use of morphine in cats are summarised in Table 5.
Table 5.
Studies evaluating morphine in cats
| Type of study | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Case report | MOR 0.06 mg/kg + BUPI 0.33 mg/kg in 0.13 ml/kg SAL intrathecal | Clinical | Within minutes of injection, a decrease in heart rate (from 150 to 110 bpm) and hypotension (MAP 60 mmHg) were recorded and resolved with the administration of 0.01 mg/kg glycopyrrolate and 10 ml/kg Ringer’s solution | 105 |
| Case report | MOR 0.43 mg in 0.86 ml SAL EPI | Clinical | Cat showed chronic urinary retention, constipation and decreased perineal reflex | 106 |
| Experimental | MOR 0.5 mg/kg IM, HYDRO 0.05–0.1– 0.2 mg/kg IM, B 0.02 mg/kg IM, BUT 0.2 mg/kg IM |
Body temperature measured with a thermistor | All treatments caused an increase in body temperature in comparison with baseline values | 40 |
| Experimental | MOR 0.1 mg/kg EPI, T 1 mg/kg EPI, SAL 0.22 ml/kg EPI |
SDS VAS Tail clamp test |
T group had a higher SDS and VAS score when compared with MOR at 8, 10 and 12 h postepidural. SAL group had a higher SDS and VAS score at all time points when compared with T and MOR groups. Euphoria was observed in five cats from MOR group and four from T group, and persisted up to 12 h postepidural | 107 |
| Experimental | MOR 100 μg/kg EPI, B 12.5 μg/kg EPI, SAL EPI |
TNT | TNT increased from 1–16 h in MOR group and from 1–10 h in B group in comparison with SAL | 71 |
| Experimental | MOR 0.2 mg/kg SC | TNT MNT |
TNT significantly increased from 45–60 mins, while MNT increased at 45–60 mins and 3–5 h after MOR administration | 32 |
| Experimental | MOR 0.2 mg/kg IM | TNT | TNT increased from 4 and 6 h, and euphoria was observed for 2–3 h after MOR administration. Mild sedation | 34 |
| Clinical | MOR EPI, MOR + BUPI EPI |
Mean dose ± SEM of MOR and MOR + BUPI was 0.16 ± 0.02 mg/kg and 1.16 ± 0.14 mg/kg, respectively. Two cats had urine retention | 108 | |
| Experimental | MOR 0.1 mg/kg IV, MOR 1 mg/kg IV |
MAC determination by tail clamp technique | Maximal MAC reductions were 28 ± 9% and 12 ± 4% with the lowest and highest doses of MOR, respectively. MOR 1 mg/kg IV provided clinically relevant isoflurane MAC reduction | 16 |
| Clinical: various surgeries or invasive diagnostic investigations | B 0.01 mg/kg IM, MOR 0.1 mg/kg IM |
VAS | B provided better postoperative analgesia than MOR at 60, 120 and 180 mins postanaesthesia. Rescue analgesia was necessary in 5/14 and 3/18 cats in MOR and B groups, respectively |
77 |
| Experimental | MOR 0.2 mg/kg IV and IM | PK | IV: mean ± SD T½ el = 76.3 ± 17.6 mins Clp = 24.1 ± 10.3 ml/kg/min Vdss = 2.6 ± 1.3 l/kg IM: mean ± SD T½ el = 93.6 ± 7.5 mins Clp = 13.9 ± 4 ml/kg/min Vdss= 1.7 ± 0.8 l/kg |
52 |
See footnote to Table 1
B = buprenorphine; bpm = beats per minute; BUPI = bupivacaine; BUT = butorphanol; Clp = plasma clearance; EPI = epidural; HYDRO = hydromorphone; MAC = minimum alveolar concentration; MAP = mean arterial pressure; MNT = mechanical nociceptive threshold; MOR = morphine; PK = pharmacokinetics; SAL = saline; SDS = simple descriptive scale; T = tramadol; T½ el = elimination half-life; TNT = thermal nociceptive threshold; VAS = visual analogue scale; Vdss = volume of distribution at steady state
Pharmacokinetic data for morphine (0.2 mg/kg IV and IM) have been reported. 52 In comparison with other species, the production of the metabolite morphine-6-glucuronide is limited in cats. Morphine-6-glucuronide is responsible for some of morphine’s analgesic effects in people, 109 and the lack of production of this metabolite in cats may be the reason why morphine (0.1 mg/kg) appears to be less effective than buprenorphine (0.01 mg/kg) in cats undergoing various surgeries or invasive diagnostic procedures. 77
Thermal nociceptive threshold testing has been used to evaluate morphine (0.2 mg/kg IM); the thermal threshold was increased from 4–6 h after injection. 34 When the same dose was administered SC, an increase in thermal threshold was measured 45 mins and 1 h after injection and pressure thresholds were increased compared with baseline at 45–60 mins and 3–6 h after injection. 32 Adverse effects after IV injection are vomiting and histamine release. 49 Morphine’s relative hydrophilicity means that its administration by the epidural or subarachnoid route provides long lasting analgesia.49,71,103,107 Morphine can also be combined with bupivacaine and administered by the epidural route. 108 Hypotension is a side effect of epidural anaesthesia with local anaesthetic agents, while very rarely reported side effects of morphine also include urinary retention, pruritus, and chronic urinary and bowel dysfunction.105,106,108
Morphine can also exert a significant isoflurane MAC-sparing effect when administered at a dose of 1 mg/kg IV, but this dose is considerably higher than that usually used in clinical settings and physiological and behavioural effects were not reported. 16 Until the behavioural and physiological effects of such a high dose are established, it would be prudent to continue to use more conventional doses (0.1–0.2 mg/kg) when administering morphine by the IV route.
Hydromorphone
Hydromorphone is a semi-synthetic full µ-agonist analgesic that is widely used in the United States. It does not have a marketing authorisation for administration to animals in Europe. It has higher potency than morphine. 110 The analgesic effects of hydromorphone are similar to those of oxymorphone, but it is cheaper.111,112
Adverse effects in cats include hypersalivation, nausea, vomiting, respiratory depression and postanaesthetic hyperthermia.111 –113 In patients admitted to an intensive care unit for painful procedures, hydromorphone (0.05 mg/kg) appeared to provide adequate analgesia with a similar efficacy to oxymorphone. 112 IV administration of 0.1 mg/kg hydromorphone was more efficacious than a 0.025 or 0.05 mg/kg dose in a thermal antinociception model. 114 The epidural administration of 0.05 mg/kg hydromorphone caused thermal and some mechanical antinociception without hyperthermia. 115
SC administration of hydromorphone provides a slower onset of peak effect, shorter duration of antinociception and more undesirable side effects (emesis and salivation) than IV or IM administration, so this route of administration is not recommended. 116 Hydromorphone increases skin temperature in cats; patients should be monitored closely for postanaesthetic hyperthermia.39,113 It also has sevoflurane MAC-sparing effects. 18
Studies evaluating hydromorphone are summarised in Table 6.
Table 6.
Studies evaluating hydromorphone in cats
| Type of study | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Experimental | HYDRO 0.05 mg/kg EPI, SAL EPI |
TNT MNT |
In comparison with baseline, MNT and TNT values were significantly higher at 15, 120 and 180 mins in HYDRO group. In comparison with SAL, TNT and MNT values were higher in HYDRO group at 30 mins, 15 mins and from 200–300 mins. No hyperthermia detected | 115 |
| Experimental | HYDRO 0.1 mg/kg SC | TNT | Significant increase in TNT values at 15, 60 and 210 mins. Time to peak TNT values was 105 mins. 5/6 cats vomited; 2/6 showed marked dysphoria |
116 |
| Clinical: intensive care setting | OXY 0.05 mg/kg IV, HYDRO 0.05 mg/kg IV |
Cumulative pain score scale | OXY and HYDRO showed similar potency and efficacy (total number of doses administered, time between first and second dosing, rescue analgesia). Four cats in the HYDRO group and one in the OXY group experienced nausea |
112 |
| Experimental | T + BUT, T + HYDRO, SAL; T 8.6 mg/kg PO, T 11.6 mg/kg PO, BUT 0.4 mg/kg IV, HYDRO 0.1 mg/kg IV |
Tail clamp test | Mean ± SEM MAC for sevoflurane after SAL was 2.45 ± 0.22%. MAC decreased to 1.48 ± 0.20%, 1.20 ± 0.16%, 1.76 ± 0.15%, 1.48 ± 0.20% and 1.85 ± 0.20% with T, BUT, HYDRO, T + BUT and T + HYDRO, respectively. Naloxone reversed the reductions in MACs | 18 |
| Experimental | HYDRO 0.025 mg/kg IV, HYDRO 0.05 mg/kg IV, HYDRO 0.1 mg/kg IV |
TNT | Dose-related antinociceptive effects of HYDRO. HYDRO 0.05 mg/kg IV increased TNT values from 5–80 mins and from 35–80 mins in comparison with baseline values and lower dose, respectively. HYDRO 0.1 mg/kg IV increased TNT values from 5–200 mins in comparison with baseline values and lower doses. A 1–2°C increase in skin temperature was reported |
114 |
| Clinical: OHE, castration, onychectomy | HDK, HP, MDK, MP; HDK = HYDRO 0.1 mg/kg SC + DIAZEPAM 0.1 mg/kg IV + KETA 5 mg/kg IV, HP = HYDRO 0.1 mg/kg SC + PROPOFOL 6 mg/kg IV, MDK = MEDETOMIDINE 7.5 µg/kg SC + DIAZEPAM 0.1 mg/kg IV + KETA 5 mg/kg IV, MP = MEDETOMIDINE 7.5 µg/kg SC + PROPOFOL 6 mg/kg IV |
Postanaesthetic body temperature | Postanaesthetic body temperatures higher than basal temperatures were reported for 86%, 80%, 25% and 34% of observations in groups HDK, HP, MDK and MP, respectively | 39 |
| Retrospective | HYDRO 0.05–0.2 mg/kg IM or SC, B 0.01–0.02 mg/kg IM, SC or OTM |
Postanaesthetic body temperature Hyperthermia = >40°C |
There is an association between HYDRO administration and postanaesthetic hyperthermia | 113 |
| Experimental | HYDRO 0.1 mg/kg IV | PK TNT |
Median ± SEM T1/2β = 98.9 ± 10.87 mins Vc = 1272 ± 132.24 ml/kg Vdss = 2957 ± 293.4 ml/kg Cl = 24.6 ± 2.35 ml/min/kg. TNT increased from baseline from 15–450 mins |
117 |
| Experimental | HYDRO 0.1 mg/kg IM | TNT | TNT increased from baseline from 15–345 mins. A statistically significant increase in skin temperature was reported | 90 |
See footnote to Table 1
B = buprenorphine; BUT = butorphanol; Cl = clearance; EPI = epidural; HYDRO = hydromorphone; KETA = ketamine; MAC = minimum alveolar concentration; MNT = mechanical nociceptive threshold; OHE = ovariohysterectomy; OTM = oral transmucosal; OXY = oxymorphone; PK = pharmacokinetics; SAL = saline; T = tramadol; TNT = thermal nociceptive threshold; T1/2β = terminal half-life; Vc = apparent volume of distribution of the central compartment; Vdss = apparent volume of distribution at steady state
Oxymorphone
Oxymorphone is a semi-synthetic derivative of morphine, characterised by higher potency (lower dose required) and a faster onset of action than morphine. 118 Like hydromorphone, it is used in the USA but it does not have a marketing authorisation for administration to animals in Europe.
Pharmacokinetic data after IV administration of 0.1 mg/kg oxymorphone suggest a moderate volume of distribution and a short terminal half-life. 119 Oxymorphone administration does not seem to be associated with vomiting, hyperthermia or adverse behavioural changes and the clinical efficacy of oxymorphone is comparable with hydromorphone, the latter being cheaper.111,112 When compared with buprenorphine in cats undergoing onychectomy or onychectomy and neutering, oxymorphone seemed to be a less effective analgesic; however, as commented by the authors of the study, the results might have been influenced by the methodology of measuring pain, and it would have been appropriate to include other more sensitive evaluations. 78
Studies evaluating oxymorphone are summarised in Table 7.
Table 7.
Studies evaluating oxymorphone in cats
| Type of study | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Experimental | OXY 0.1 mg/kg IV | PK | Median ± SEM values for Vc and Vss were 1.1 ± 0.2 and 2.5 ± 0.4 l/kg, respectively. Harmonic mean ± jackknife pseudo-SD values for Cl and T1/2β were 26 ± 7 ml/kg/min and 96 ± 49 min, respectively | 119 |
| Clinical: intensive care setting | OXY 0.05 mg/kg IV, HYDRO 0.05 mg/kg IV |
Cumulative pain score scale | OXY and HYDRO showed similar potency and efficacy (total number of doses administered, time between first and second dosing, rescue analgesia). Four cats in the HYDRO group and one in the OXY group experienced nausea |
112 |
| Clinical: onychectomy ± neutering | B 0.01 mg/kg IM, OXY 0.05 mg/kg IM, KETO 2 mg/kg IM |
Cumulative pain scores VAS |
B cumulative pain scores were lower than OXY and KETO at 12 h post-extubation and lower than OXY at 4 h | 78 |
See footnote to Table 1
B = buprenorphine; Cl = clearance; HYDRO = hydromorphone; OXY = oxymorphone; PK = pharmacokinetics; KETO = ketoprofen; T1/2β = terminal half-life; VAS = visual analogue scale; Vc = apparent volume of distribution of the central compartment; Vss = apparent volume of distribution at steady state
Fentanyl
Fentanyl is a very potent short-acting, lipid soluble, synthetic µ agonist. 49 Studies evaluating fentanyl in cats are summarised in Table 8.
Table 8.
Studies evaluating fentanyl in cats
| Type of study | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Experimental | FEN LD 5 µg/kg IV, FEN 5 µg/kg/h IV for 2 h, SAL IV |
TNT MNT |
SAL administration did not significantly change MNT or TNT over time. In FEN group, TNT was higher than baseline from 0.25–0.75 h; MNT was higher than baseline from 0.25–1 h. Plasma FEN concentrations <1.33 ± 0.30 ng/ml were not associated with antinociception. Mild sedation recorded | 120 |
| Clinical: orthopaedic surgery in traumatised cats | FEN + ISO (end-tidal 1%), FEN + PPF; FEN 0.02 mg/kg/h IV, PPF 12 mg/kg/h IV |
Monitoring of physiological parameters | Mean end-expiratory isoflurane concentration was 1.19 ± 0.19%, while PPF infusion rate was 11.4 ± 0.8 mg/kg/h. Heart rate was not different between groups. Arterial blood pressure was better maintained in FEN + PPF; MAP was significantly lower in FEN + ISO in comparison with FEN + PPF group during skin incision, during surgery without intense surgical stimulation and during surgery with intense surgical stimulation. 1/11 cats and 9/11 cats required IPPV in FEN + ISO and FEN + PPF groups, respectively. Oxygen saturation was not different between groups |
121 |
| Experimental | FEN 10 µg/kg IV, SAL IV |
PK TNT |
Median maximum plasma concentration was 6.6 ng/ml at 2 mins after FEN administration. Plasma FEN concentration was <0.2 ng/ml at 95 mins after administration. TNT increased above baseline from 5–110 mins; TNT was different from SAL from 5–110 mins and at 125 and 155 mins. Mild euphoria recorded |
122 |
| Clinical: unilateral onychectomy | TFP 25 µg/h, BUT IM, BUPI topical |
Gait analysis | Limb function was better in TFP and BUT groups than BUPI group. Limb function still reduced 12 days after surgery |
123 |
| Experimental | TFP 25 µg/h, TFP 50 µg/h |
MAC evaluation (tail clamp method and standard technique) | ISO MAC was reduced by 17.8 ± 7.4% and 18.1 ± 10.3 % in TFP 25 µg/h and TFP 50 µg/h groups, respectively. MAC reductions between groups were not statistically significant | 19 |
| Clinical: OHE | Full or partial (half) exposure to TFP 25 µg/h | Plasma FEN concentration Subjective pain scores |
Steady-state plasma FEN concentrations significantly lower in half than in full TFP exposure groups (1.14 ± 0.86 vs 1.78 ± 0.92 ng/ml; mean ± SD). Pain scores were not different between groups | 124 |
| Experimental | PPF + SAL, PPF + FEN, PPF + SUF, PPF + ALF; PPF 7 mg/kg + 0.2 mg/kg/min IV, FEN 0.1 µg/kg/min IV, SUF 0.01 µg/kg/min IV, ALF 0.5 µg/kg/min IV |
Interdigital skin clamp to detect MIR | In comparison with baseline values, PPF + FEN group showed a decrease in heart rate from 30–90 mins of infusion; the decrease in heart rate was significantly greater 30 mins after anaesthetic induction in the PPF + ALF group than in other groups. In the PPF + FEN group, systolic blood pressure was significantly lower than baseline values from 15–90 mins of infusion, while mean blood pressure and diastolic blood pressure were not different from baseline. Respiratory rate decreased and ETCO2 increased from baseline from 15–90 mins of infusion |
41 |
| Experimental | TFP + 4 h GA + hypothermia TFP + 4 h GA, TFP 25 µg/h |
FEN serum concentrations and temperature monitoring | In the hypothermia group, FEN serum concentrations were significantly lower than baseline from 1–4 h of GA. Independently from body temperature, serum FEN concentrations returned to baseline values within 1 h of the end of GA |
125 |
| Experimental, preclinical: OHE |
TFP, TFP + GA, TFP + GA + OHE; TFP 25 µg/h |
PK | Mean plasma FEN concentrations at different time points and other PK parameters were not different between groups. Halothane anaesthesia and GA + OHE did not significantly alter plasma FEN concentrations. High individual variability reported in parameters measured within and between groups | 126 |
| Clinical: onychectomy | TFP 25 µg/h, BUT 0.2 mg/kg administered at the time of sedation, at extubation, and every 4 h thereafter for 12 h |
Plasma cortisol concentrations Subjective pain scores |
Lower pain scores in the TFP group than BUT group only at 8 h after surgery. Plasma cortisol concentrations not different between groups | 30 |
| Preclinical: OHE | TFP 25 µg/h, No TFP |
SDS for pain Cortisol and glucose plasma concentrations |
In TFP cats cortisol concentration was lower than in No TFP cats during surgical procedure (3.3 vs 4.7 µg/dl) and early postsurgical period (14–25 h post-TFP application) (3.7 vs 5.5 µg/dl). Glucose concentration was lower in TFP cats than in No TFP cats during surgical and early postsurgical period (75 vs 93 mg/dl). Pain scores not significantly different between groups |
127 |
| Clinical: onychectomy, onychectomy + OHE, onychectomy + castration | TFP 25 µg/h, BUT 0.5 mg/kg IM, and repeated at extubation at 0.2 mg/kg |
Subjective pain score Lameness assessed with pressure-sensitive mat |
Pain scores for TFP group cats were significantly lower than for BUT group cats at all evaluation times from 30 mins post-extubation to the end of the study. Lameness score was significantly lower in TFP than BUT group cats the day after surgery and 2 days after surgery. Mean ratios of digital pad-to-metacarpal pad force were not significantly different between groups at any time point |
97 |
| Experimental | FEN 7.19 ± 1.17 µg /kg IV, FEN 25 µg/h TD |
PK | IV group: mean ± SEM T½ el = 2.35 ± 0.57 h Clp = 1.19 ± 0.16 l/kg/h Vdβ = 3.43 ± 0.58 l/kg TD group: mean ± SEM Rab = 8.48 ± 1.7 µg/h Css = 1.88 ± 0.14 ng/ml |
128 |
| Experimental | FEN 4 µg/kg EPI + MED 10 µg/kg EPI | Electrical cutaneous stimulation | Compared with baseline values, MED and FEN increased electrical threshold values in the hindlimb from 20–245 mins and at 20 mins, respectively. Compared with baseline values, MED increased electrical threshold values in the forelimb from 15–120 mins, while FEN did not have any effect |
129 |
| Experimental | FEN 4 µg/kg EPI, MED 10 µg/kg EPI, SAL EPI |
Monitoring of physiological parameters | In the MED group, MAP was significantly increased from 5–20 mins and decreased from 30–120 mins post-injection. In the FEN group, MAP was decreased from 5–120 mins post-injection compared with baseline. In the MED and FEN groups, heart rate and respiratory rate were lower from 5–120 mins compared with baseline |
130 |
See footnote to Table 1
ALF = alfentanil; BUPI = bupivacaine; BUT = butorphanol; Css = concentration at steady state; Clp = plasma clearance; EPI = epidural; FEN = fentanyl; GA = general anaesthesia; IPPV = intermittent positive pressure ventilation; ISO = isoflurane; LD = loading dose; MAC = minimum alveolar concentration; MAP = mean arterial pressure; MED = medetomidine; MIR = minimum infusion rate; MNT = mechanical nociceptive threshold; OHE = ovariohysterectomy; PK = pharmacokinetics; PPF = propofol; Rab = rate of absorption; SAL = saline; SDS = simple descriptive scale; SUF = sufentanil; T½ el = elimination half-life; TD = transdermal; TFP = transdermal fentanyl patch; TNT = thermal nociceptive threshold; Vdβ = apparent volume of distribution
A pharmacokinetic study of IV administered fentanyl in cats reported rapid distribution and elimination. 128 A more recent study showed that, following a single dose of fentanyl (10 µg/kg IV), the onset of action was rapid and thermal antinociception could be detected from 5–110 mins; antinociception was detected at plasma values higher than 1.07 ng/ml. 122 In conscious cats, the pharmacokinetics and pharmacodynamics of a 5 µg/kg/h fentanyl infusion, following a 5 µg/kg loading dose, and its effect on mechanical and thermal threshold have recently been studied. 120 Side effects consisted of mild sedation and salivation following the loading dose in 1/7 cats; antinociception could be detected at fentanyl plasma concentrations higher than 1.3 ng/ml. In an experimental setting an infusion of fentanyl at 6 µg/kg/h combined with a continuous rate infusion of propofol resulted in satisfactory anaesthesia in cats. 41 One clinical study in injured cats undergoing anaesthesia reported that a fentanyl infusion (20 µg/kg/h) combined with a propofol infusion (12 mg/kg/h) maintained the haemodynamic variables better than fentanyl and isoflurane anaesthesia, although respiratory depression was more marked and intermittent positive pressure ventilation was required. 121
The analgesic and cardiovascular effects of epidural fentanyl (4 µg/kg) have been evaluated in isoflurane-anaesthetised cats. Electrical threshold was increased 20 mins post-injection and no side effects were reported in one study. 129 In a separate study by the same investigators cardiopulmonary effects included a decrease in mean arterial pressure, heart rate and respiratory rate from 5–120 mins post-injection, and an increase in arterial partial pressure of carbon dioxide from 15–120 mins post-injection. 130
The need for a ‘hands off’ approach to longer-term analgesia in cats led to an interest in transdermally administered fentanyl and many studies have been carried out over the past 15 years.19,30,97,123 –128 In a pharmacokinetic study, steady state plasma concentrations were reached 12–24 h after the application of a fentanyl patch (25 µg/kg). Sustained plasma fentanyl concentrations were detected throughout a 5 day period, with a mean concentration at steady state of 1.58 ng/ml. The mean calculated delivery rate of fentanyl was 8.48 µg/h, with high variability among cats. 128 A few clinical studies have suggested that fentanyl patches can be considered effective analgesics in cats undergoing onychectomy or ovariohysterectomy;30,97,123,127 however, if a fentanyl patch is chosen to provide perioperative analgesia, the patch has to be applied 12–24 h before the surgical procedure.7,19,124,128
It is important to note that the presence of a fentanyl patch does not negate the need for pain assessment. In fact, this becomes imperative since there is considerable variability in the absorption of fentanyl, and thus analgesic efficacy. Also, a cat with a painful condition will require administration of additional analgesics, particularly in the time before the fentanyl patch becomes effective. The primary advantages of administering fentanyl by the transdermal route are the avoidance of repeated injections and a decrease (approximately 18%) in the MAC of isoflurane, which may promote more stable haemodynamics during anaesthesia.19,30 Moreover this method of fentanyl delivery can be used in cats weighing less than 4 kg by decreasing the amount of patch-exposed surface area. 124
However, there are also some disadvantages associated with the use of fentanyl patches. As mentioned, there is great individual variability in drug absorption by this method.19,128 Although anaesthesia and/or surgery do not appear to alter plasma fentanyl concentrations, hypothermia during anaesthesia can cause a reduction in serum fentanyl concentration.125,126 Skin permeability, altered skin perfusion and hypovolaemia are other factors that may affect plasma levels of fentanyl. 7 For all of these reasons, cats with transdermal fentanyl patches should be carefully monitored (mental status, behaviour, physiological variables) to assess efficacy and any potential adverse effects.126,128
There are also important safety considerations associated with the use of fentanyl patches, particularly if cats are discharged into their owners’ care after application of the patch. Fentanyl is an addictive drug that can be abused by people, and the risk of ingestion by the treated animal, other animals or humans has to be considered. There are reports of fatalities caused by ingestion of fentanyl patches by children and drug addicts, among others, as well as by monkeys.131 –133 There may also be legal implications associated with dispensing fentanyl patches; in the UK, fentanyl is a Schedule 2 controlled drug.
MAC reduction reported 24 h after placement of a 25 µg/h fentanyl patch (corresponding to a possible delivery dose of 5.8 µg/kg/h) and antinociception after fentanyl infusion of 5 µg/kg/h were achieved with different fentanyl plasma levels, of 0.54 ± 0.41 and >1.3 ng/ml, respectively.19,120
Fentanyl analogues
Alfentanil, remifentanil and sufentanil are potent µ agonists of the anilidopiperidine family and are characterised by a more rapid onset of action and shorter context-sensitive half-life after prolonged infusion in comparison with the structural analogue fentanyl. 134 They are used mainly in the intraoperative period. 49 These are Schedule 2 controlled drugs in the UK and do not have marketing authorisations for administration to animals. In the UK they can only be prescribed under the ‘cascade’ when their use can be justified in an individual animal and with informed owner consent. Further information on the use of the cascade and unlicensed drugs in the UK can be found on the Veterinary Medicines Directorate website (www.gov.uk/government/organisations/veterinary-medicines-directorate).
Alfentanil
Studies evaluating alfentanil in cats are summarised in Table 9. Alfentanil administered IV to conscious cats at a dose of 50 µg/kg produced analgesic effects for approximately 21 mins, as assessed by applying a clamp to the base of the tail, and the alfentanil was rapidly metabolised. 137 Recently the disposition of alfentanil in isoflurane-anaesthetised cats was studied. 135 The results were broadly similar to those published by Pascoe et al, 137 but the volume of the central compartment and volume of distribution at steady state were greater. 135 The same research group showed that plasma levels of alfentanil of 500 ng/ml had MAC-sparing effects on isoflurane, which, compared with isoflurane alone, increased heart rate, mean arterial pressure, stroke index, cardiac index, haemoglobin and oxygen delivery index, and blunted haemodynamic responses to a noxious stimulant. 28 Another study showed that a plasma alfentanil concentration of 500 ng/ml produced a maximal isoflurane MAC reduction of 35%; mild metabolic acidosis and decreased arterial partial pressure of oxygen were reported as adverse effects. 20
Table 9.
Studies evaluating alfentanil in cats
| Type of study | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Experimental | ALF 100 µg/kg | PK | Volume of the central compartment was 0.10 ± 0.01 (0.07–0.14) l/kg (mean ± SEM [range]); volume of distribution at steady state was 0.89 ± 0.16 (0.68–1.83) l/kg (mean ± SEM [range]); clearance was 11.6 ± 2.6 (9.2–15.8) ml/min/kg (harmonic mean ± pseudo-SD [range]); and terminal half-life was 144 (118–501) mins (median [range]) | 135 |
| Clinical: OHE | PPF + ALF 10 µg/kg, 0.8 µg/kg/min IV, PPF + REMI 2.5 µg/kg, 0.2 µg/kg/min IV; PPF 0.3 mg/kg/h IV |
Monitoring of cardiovascular parameters | In both groups, RT, RR, ETCO2 and SpO2 values were not significantly different when compared with baseline, with the exception that RT was lower at skin closure compared with baseline. In the ALF group, but not in the REMI group, HR was higher at some time points during the surgical procedure when compared with baseline. During ligation of the ovary, SAP was higher in the ALF group; from ligation of the ovary to skin closure, SAP was higher in both groups when compared with baseline. There were no significant differences between groups for RR, ETCO2, HR and SpO2. From coeliotomy until ligation of the ovaries, SAP was lower in ALF compared with the REMI group. Overall, the infusion rates of REMI and ALF were 0.24 ± 0.05 mg/kg/min and 0.97 ± 0.22 mg/kg/min, respectively | 136 |
| Experimental | PPF + SAL, PPF + SUF, PPF + FEN, PPF + ALF; PPF 7 mg/kg + 0.2 mg/kg/min IV, SUF 0.01 µg/kg/min IV, FEN 0.1 µg/kg/min IV, ALF 0.5 µg/kg/min IV |
Interdigital skin clamp to detect MIR | In comparison with baseline values, the ALF group showed a decrease in HR from 30–90 mins of infusion; the decrease in HR was significantly greater 30 mins after anaesthetic induction in the ALF group than in other groups. In the ALF group diastolic blood pressure and mean blood pressure were significantly lower than baseline values from 30–90 and from 15–90 mins after induction, respectively. RR decreased from baseline from 30–90 mins of infusion; ETCO2 increased from 15–90 mins of infusion |
41 |
| Experimental | ALF administered IV in order to achieve estimated plasma concentration of 500 ng/ml |
MAC determination | ALF reduced isoflurane MAC and caused a significant increase in body temperature, heart rate, mean arterial pressure, mean pulmonary arterial pressure, stroke index, cardiac index, haemoglobin, oxygen delivery index, dopamine, epinephrine, norepinephrine and cortisol values, and a significant decrease in arterial and venous pH | 28 |
| Experimental | ALF administered IV in order to achieve plasma concentrations of 50, 100, 250, 500, 750 and 1000 ng/ml | Isoflurane MAC reduction was estimated to be maximal (35.0 ± 6.6%) at a plasma ALF concentration of 500 ng/ml | 20 | |
| Experimental | ALF 50 μg/kg IV | Tail clamp test | Harmonic mean for the half-life of the rapid distribution was 4.12 mins; slow distribution was 18.8 mins; and elimination phase was 119.2 mins. Analgesia lasted for 21.7 ± 14 mins. ALF caused a transient increase in blood pressure, and respiratory and metabolic acidosis | 137 |
See footnote to Table 1
ALF = alfentanil; ETCO2 = end-tidal carbon dioxide; FEN = fentanyl; HR = heart rate; MAC = minimum alveolar concentration; MIR = minimum infusion rate; OHE = ovariohysterectomy; PK = pharmacokinetics; PPF = propofol; REMI = remifentanil; RR = respiratory rate; RT = rectal temperature; SAP = systolic arterial pressure; SAL = saline; SpO2 = oxygen saturation of haemoglobin; SUF = sufentanil
A clinical study evaluated total IV anaesthesia with propofol (0.2 mg/kg/min) alone or in combination with fentanyl (0.1 µg/kg/min), alfentanil (0.5 µg/kg/min) or sufentanil (0.01 µg/kg/min) infusions. In cats treated with alfentanil, diastolic blood pressure and mean blood pressure were significantly decreased compared with baseline from 30–90 and from 15–90 mins after induction, respectively. 41 More recently, a clinical study evaluated the combination of alfentanyl and propofol in cats undergoing ovariohysterectomy: propofol was infused at 0.3 mg/kg/h while the overall infusion rate of alfentanil was 0.97 ± 0.22 µg/kg/min. 136 In this study intermittent assisted ventilation was provided during anaesthesia in order to maintain normocapnia.
Remifentanil
Remifentanil is metabolised by non-specific plasma and tissue esterases. 138 In people and dogs it is characterised by a short context-sensitive half-time (time required for the plasma concentration to decrease by 50% after termination of an infusion) that does not depend on the duration of the infusion; thus there are no cumulative effects after prolonged infusions.139,140 Moreover, the extrahepatic metabolism of remifentanil is potentially advantageous in cats that lack some hepatic metabolic pathways,52,141 and especially in cats with liver and kidney disease.
Studies evaluating remifentanil in cats are summarised in Table 10. Pharmacokinetics data have been published. In conscious and isoflurane-anaesthetised cats an IV infusion of remifentanil at 1 µg/kg/min over 5 mins resulted in a rapid distribution to peripheral compartments, and a high clearance and a relatively short terminal half-life (17.4 and 15.7 mins in awake and anaesthetised cats, respectively). 142 Anaesthesia decreased the volume of the central compartment. 142 However, another study in cats showed that the MAC of isoflurane decreased significantly after a 30 min remifentanil infusion; 21 according to the authors this might have been caused by a ‘cumulative effect of repeated infusions of remifentanil or by a modified response to the repeated electrical stimulation’ (electrical stimulation was the method used to determine MAC), or by the use of ‘high doses which might have facilitated the cumulative effects’. 21 In the same study, three remifentanil constant rate infusions (CRIs) were examined, 0.25, 0.5 and 1 µg/kg/min, and the MAC reduction from baseline ranged from 23–30% with no statistical difference between groups. This may mean that there is a ceiling to the isoflurane-sparing effect. The study also reported that the remifentanil CRI was associated with an increase in heart rate of 26% and an increase in systolic arterial pressure of 23%. 21
Table 10.
Studies evaluating remifentanil in cats
| Type of study | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Clinical: OHE | PPF + REMI LD 2.5 µg/kg + 0.2 µg/kg/min IV, PPF + ALF LD 10 µg/kg + 0.8 µg/kg/min IV; PPF 0.3 mg/kg/h IV |
Monitoring of cardiovascular parameters | In both groups, RT, RR, ETCO2 and SpO2 values were not significantly different when compared with baseline, with the exception that RT was lower at skin closure compared with baseline. During ligation of the ovary, SAP was higher in the ALF group; from ligation of the ovary to skin closure, SAP was higher in both groups when compared with baseline. There were no significant differences between groups for RR, ETCO2, HR and SpO2. From coeliotomy until ligation of the ovaries, SAP was lower in the ALF group compared with the REMI group. Overall, the infusion rates of REMI and ALF were 0.24 ± 0.05 mg/kg/min and 0.97 ± 0.22 mg/kg/min, respectively. Mean (range) extubation time was 9.5 (6–10) and 12 (8–27) mins for REMI and ALF groups, respectively, and this was statistically significant | 136 |
| Experimental | REMI 0.0625, 0.125, 0.25, 0.5, 1, 2, 4, 8 and 16 µg/kg/min IV | Isoflurane MAC determination by tail clamp technique TNT in awake cats |
REMI infusions did not significantly change isoflurane MAC. Mean ± SEM median effective concentration for REMI and its active metabolite, GR90291, for TNT test was 1 ± 0.35 ng/ml and 307 ± 28 ng/ml of blood, respectively. There was no relationship between REMI immobilising potency (MAC determination) and its analgesic potency (TNT). Euphoria was detected in awake cats during REMI infusions at 8 and 16 µg/kg/min |
15 |
| Experimental | REMI 0.25 µg/kg/min IV, REMI 0.5 µg/kg/min IV, REMI 1 µg/kg/min IV |
Isoflurane MAC determination by electrical stimulation | Compared with MACBASAL, MACREMI 0.25, MACREMI 0.5 and MACREMI 1 were significantly decreased by 23.4 ± 7.9%, 29.8 ± 8.3% and 26 ± 9.4%, respectively. MAC did not significantly differ between groups. Heart rate and SBP increased by 26% and 23%, respectively, during REMI infusion | 21 |
| Experimental | REMI 1 µg/kg/min IV, REMI 1 µg/kg/min IV + ISO 1.63% |
PK in conscious and anaesthetised cats | Median (range) T1/2β = 17.4 (5.6–920.3) and 15.7 (3.8–410.3) mins; Vc = 1.596 (1.164–2.111) and 567 (278–641) ml/kg; Vss = 7.632 (2.284–76.039) and 1.651 (446–29,229) ml/kg; Cl = 766 (408–1473) and 371 (197–472) ml/min/kg in conscious and anaesthetised cats, respectively. Large variability in disposition of REMI was observed between cats | 142 |
| Experimental, Preclinical: OHE |
REMI 0.2 + PPF, REMI 0.3 + PPF, REMI + PPF + OHE; REMI 0.2 µg/kg/min, REMI 0.3 µg/kg/min, PPF 0.3 mg/kg/min |
Electrical stimulation Surgery |
No significant differences in arterial blood pressure between the two infusions in PPF-anaesthetised cats. REMI 0.3 group did not respond to noxious stimulation from 30–90 mins of infusion. During OHE the highest REMI dosage (mean ± SEM) that prevented cardiovascular response was 0.23 ± 0.01 µg/kg/min. Recovery time from REMI + PPF anaesthesia ranged from 115–140 mins |
143 |
See footnote to Table 1
ALF = alfentanil; Cl = clearance; ETCO2 = end-tidal carbon dioxide; HR = heart rate; ISO = isoflurane; LD = loading dose; MACREMI 0.25 = isoflurane minimum alveolar concentration determined during infusion of 0.25 µg remifentanil/kg/min; MAC = minimum alveolar concentration; MACBASAL = basal isoflurane minimum alveolar concentration; MACREMI 0.5 = isoflurane minimum alveolar concentration determined during infusion of 0.5 µg remifentanil/kg/min; MACREMI 1 = isoflurane minimum alveolar concentration determined during infusion of 1 µg remifentanil/kg/min; mmHg = millimetres of mercury; OHE = ovariohysterectomy; PK = pharmacokinetics; PPF = propofol; REMI = remifentanil; RR = respiratory rate; RT = rectal temperature; SAP = systolic arterial pressure; SBP = systolic blood pressure; SpO2 = oxygen saturation of haemoglobin; TNT = thermal nociceptive threshold; T1/2β = terminal half-life; Vc = apparent volume of distribution of the central compartment; Vss = apparent volume of distribution at steady state
IV infusion of remifentanil was shown to result in a dose-dependent increase in thermal threshold in conscious cats. 15 Behaviours suggestive of euphoria were apparent in conscious cats when infusion rates were equal to or higher than 1 µg/kg/min. In the same study a relationship between the immobilising potency of remifentanil, assessed as a MAC-sparing effect, and analgesic potency was not detected; nor was hyperalgesia detected on termination of the infusion. 15 Another study evaluated remifentanil infusions in association with propofol anaesthesia (0.3 mg/kg/min). 143 Remifentanil infusion rates ranging from 0.2–0.27 µg/kg/min were necessary in cats undergoing ovariohysterectomy to prevent cardiovascular responses, while a remifentanil CRI of 0.3 µg/kg/min was necessary to prevent motor responses to electrical stimulation. Bradycardia (lowest heart rate recorded = 68 beat per minutes) and hypotension (lowest mean arterial pressure = 49 mmHg) were noted in some cats. 143 Similarly, in an investigation involving propofol-anaesthetised cats (0.3 mg/kg/min) undergoing ovariohysterectomy, the time to extubation of the trachea was faster in cats that received a remifentanil infusion at 0.24 ± 0.05 µg/kg/min than in those that received an alfentanil infusion at 0.97 ± 0.22 µg/kg/min. 136
It must be emphasised that in the aforementioned clinical studies intermittent positive pressure ventilation was required as remifentanil produces a significant degree of respiratory depression. 140
Sufentanil
Sufentanil has a more rapid onset and shorter duration of action than fentanyl in people. 144 Sufentanil administration can elicit centrally mediated sympathetic stimulation, resulting in effects such as an increase in blood pressure and heart rate. 27
Studies evaluating the use of sufentanil in cats are summarised in Table 11. To the authors’ knowledge, there are currently no published studies evaluating the analgesic or antihyperalgesic effects of sufentanil in cats. Very recently an experimental study evaluated the pharmacokinetics of sufentanil in isoflurane-anaesthetised cats and demonstrated that the drug has a rapid disposition due to a small volume of distribution and moderate clearance. 135 An experimental study evaluated the cardiorespiratory effects of propofol (induction dose of 7 mg/kg, followed by an infusion at 0.2 mg/kg/min IV) alone or in combination with sufentanil (loading dose of 0.1 µg/kg, followed by an infusion at 0.01 µg/kg/min IV). 41 The cats breathed spontaneously during the 90 min study period, although an increase in expired carbon dioxide level, up to 69 mmHg, was noted, as well as a decrease in respiratory rate and heart rate in comparison with baseline values. Mean blood pressure and oxygen saturation did not change from baseline. 41
Table 11.
Studies evaluating sufentanil in cats
| Type of study | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Experimental | SUF 1 μg/kg | PK | Volume of the central compartment was 0.06 ± 0.01 (0.04–0.10) l/kg (mean ± SEM [range]); volume of distribution at steady state was 0.77 ± 0.07 (0.63–0.99) l/kg (mean ± SEM [range]); clearance was 17.6 ± 4.3 (13.9–24.3) ml/min/kg (harmonic mean ± pseudo-SD [range]); and terminal half-life was 54 (46–76) mins (median [range]) | 135 |
| Experimental | PPF + SAL, PPF + SUF, PPF + FEN, PPF + ALF; PPF 7 mg/kg + 0.2 mg/kg/min IV, SUF 0.01 µg/kg/min IV, FEN 0.1 µg/kg/min IV, ALF 0.5 µg/kg/min IV |
Interdigital skin clamp to detect MIR | In comparison with baseline values, SUF + PPF group showed a decrease in HR from 30–90 mins of infusion; RR decreased from baseline from 15–90 mins of infusion; ETCO2 increased (from 34–56 mmHg) from 15–90 mins of infusion. From 70–90 mins, mean infusion rate of PPF was significantly lower when cats were treated with SUF than when they were treated with FEN | 41 |
See footnote to Table 1
ALF = alfentanil; ETCO2 = end-tidal carbon dioxide; FEN = fentanyl; HR = heart rate; MIR = minimum infusion rate; PK = pharmacokinetics; PPF = propofol; RR = respiratory rate; SAL = saline; SUF = sufentanil
Tramadol
Tramadol is a centrally acting analgesic, consisting of two enantiomers, which exerts its analgesic effect by binding to the opioid receptors (mainly µ) and by interfering with the neuronal release and reuptake of serotonin and noradrenaline in the descending inhibitory pathways. 145 The (+) enantiomer and its metabolite O-desmethyltramadol (M1) bind the opioid receptors and appear to contribute significantly to the analgesic effect of tramadol. The opioid effect of tramadol is believed to be related, at least in part, to its metabolite O-desmethyltramadol. In cats O-desmethyltramadol rapidly appears in plasma following tramadol administration and has a moderate half-life. 146
Tramadol does not have a marketing authorisation for use in cats, but is licensed for use in dogs in some European countries. There is a great deal of interest in using tramadol for analgesia in companion animals. It is available in tablet form, facilitating administration by owners at home, and could be an alternative to NSAIDs in cats in which these drugs are poorly tolerated and/or contraindicated, or used in addition to NSAIDs in animals with more severe pain.
Tramadol has recently been classified as a Schedule 3 controlled drug in the UK, but exempted from the safe custody requirements, and is a Schedule IV drug under the Federal Controlled Substances Act in the United States. The potential for human abuse should be carefully considered, as well as the potential for toxicity in cats. Recently, symptoms related to serotonin syndrome secondary to tramadol overdose (80 mg/kg administered PO twice) have been reported for the first time in a cat. 147 The serotonin syndrome is induced by pharmacological treatment with serotonergic agents that increase serotonin activity, including selective serotonin reuptake inhibitors, tricyclic antidepressants (amitriptyline), monoamine oxidase inhibitors, lithium, carbamazepine, amphetamine and derivatives, dextromethorphan, tramadol and meperidine; and also by St John’s wort (Hypericum perforatum). In people symptoms include cognitive behavioural changes, neuromuscular excitability and autonomic instability. 148
Studies evaluating the use of tramadol in cats are summarised in Table 12. Experimental studies have investigated the antihyperalgesic and MAC-sparing effects of various doses of tramadol administered by the SC, epidural or PO route. Steagall and colleagues reported that tramadol (1 mg/kg) administered by the SC route had no effect on mechanical nociceptive thresholds and only a limited effect on thermal thresholds, which increased above the 95% confidence interval only at 45 mins, 3 and 6 h. 35 Moreover, large variability in the antinociceptive response to tramadol was detected between cats. In the same study tramadol combined with acepromazine increased the pressure threshold values from 30 mins to 3 h after administration. This finding was unexpected since no antinociceptive effects were detected when tramadol was administered alone, and acepromazine is generally considered not to be analgesic. 154 It is possible that the acepromazine enhanced the tramadol-induced analgesia.
Table 12.
Studies evaluating tramadol in cats
| Type of study | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Clinical: neutering | T 2 mg/kg IV | Subjective pain scoring | Pain scores were low and no rescue analgesia was necessary during the 6 h study period | 149 |
| Experimental | T 0.5 mg/kg PO, T 1 mg/kg PO, T 2 mg/kg PO, T 3 mg/kg PO, T 4 mg/kg PO |
TNT | Thermal threshold was significantly higher than the baseline value at 80 and 120 mins for the 0.5 mg/kg dose; at 80 and from 120–360 mins for the 2 mg/kg dose; from 40–360 mins for the 3 mg/kg dose; and from 60–360 mins for the 4 mg/kg dose | 150 |
| Clinical: OHE | T for 3 days, VEDA for 3 days, T + VEDA for 3 days; SAL for 3 days; T 2 mg/kg SC q8h, VEDA 0.5 mg/kg PO q24h |
IVAS CPS VFF |
Pain scores higher than pre-surgical values were seen in T + VEDA and T cats up to 4 h, in VEDA cats up to 32 h, and in SAL cats up to 32 and 56 h postoperatively by CPS and IVAS evaluation, respectively. Pain scores in SAL were higher than T 1 h postsurgery and than T + VEDA from 1–72 h postsurgery. Pain scores in VEDA were higher than T 1 h postsurgery, and higher than T + VEDA from 1–56 h postsurgery. MNT was significantly reduced 1 h postsurgery in SAL and 1, 4 and 32 h postsurgery in VEDA cats; thresholds were not reduced in T + VEDA and T cats. SAL presented a lower mechanical nociceptive threshold than T + VEDA and T up to 4 h postsurgery. VEDA presented a lower mechanical nociceptive threshold than T up to 4 h postsurgery, and than T + VEDA up to 32 h postsurgery. Rescue analgesia was administered to all SAL and VEDA cats, and 50% of T cats. No T + VEDA cats needed rescue analgesia | 151 |
| Clinical: OHE | T 2 mg/kg SC q8h, VEDA 0.5 mg/kg PO q24h |
There were no differences for platelet aggregation, blood platelets and bleeding time either between the groups or over time within each group. Perioperative use of VEDA, T or their combination did not modify primary homeostasis and renal, liver or gastrointestinal function |
152 | |
| Experimental | T 1 mg/kg EPI, MOR 0.1 mg/kg EPI, SAL 0.22 ml/kg EPI |
SDS VAS Tail clamp test |
T group had a higher SDS and VAS score when compared with the MOR group at 8, 10 and 12 h postepidural. SAL group had higher SDS and VAS score at all time points when compared with T and MOR groups. Euphoria, observed in five cats in MOR group and four in T group, persisted for up to 12 h postepidural | 107 |
| Experimental | SAL, T + BUT, T + HYDRO;T 8.6 mg/kg PO, T 11.6 mg/kg PO, BUT 0.4 mg/kg IV, HYDRO 0.1 mg/kg IV |
Tail clamp | Mean ± SEM MAC for sevoflurane after SAL was 2.45 ± 0.22%; MAC decreased to 1.48 ± 0.20%, 1.20 ± 0.16%, 1.76 ± 0.15%, 1.48 ± 0.20% and 1.85 ± 0.20% with T, BUT, HYDRO, T + BUT and T + HYDRO, respectively. Naloxone reversed the reductions in MAC | 18 |
| Experimental | T 1 mg/kg SC, ACP 0.1 mg/kg SC, T + ACP SC, SAL 0.3 ml SC |
TNT MNT |
After T administration, thermal threshold was above the 95% confidence interval at 0.75, 3 and 6 h while pressure threshold did not vary from baseline. After T + ACP pressure threshold increased above baseline from 0.5–3 h. Pressure thresholds increased above baseline from 0.25–2 h after ACP |
35 |
| Experimental | T 1 mg/kg IV, T 2 mg/kg IV, T 4 mg/kg IV |
Assessment of ventilation outputs | T 2mg/kg and T 4mg/kg reduced sensitivity of peripheral and central chemoceptors to carbon dioxide and increased the apnoeic threshold. Total carbon dioxide sensitivity was reduced by 31%, 59% and 68% by 1, 2 and 4 mg/kg T, respectively |
153 |
| Experimental | T 2 mg/kg IV, T 5 mg/kg PO |
PK | After IV administration, the apparent volume of distribution of the central compartment, apparent volume of distribution at steady state, clearance and terminal half-life (mean ± SEM) were 1553 ± 118 ml/kg, 3103 ± 132 ml/kg, 20.8 ± 3.2 ml/min/kg, and 134 ± 18 mins, respectively. Systemic availability and terminal half-life after oral administration were 93 ± 7% and 204 ± 8 mins, respectively. O-desmethyltramadol rapidly appeared in plasma following T administration and had a terminal half-life of 261 ± 28 and 289 ± 19 mins after IV and PO T administration, respectively | 146 |
| Case report | T overdose (80 mg/kg PO administered twice) |
Clinical evaluation | Signs of T toxicity: agitation, altered mentation, hypersalivation, jerky head movement, hypertension and tachycardia. Stabilisation and therapy included fluid therapy, cyproheptadine and buprenorphine. The cat recovered |
147 |
See footnote to Table 1
ACP = acepromazine; BUT = butorphanol; CPS = composite pain score; EPI = epidural; HYDRO = hydromorphone; IVAS = interactive visual analogue scale; MAC = minimum alveolar concentration; MNT = mechanical nociceptive threshold; MOR = morphine; OHE = ovariohysterectomy; PK = pharmacokinetics; SAL = saline; SDS = simple descriptive scale; T = tramadol; TNT = thermal nociceptive threshold; VFF = von Frey filaments; VAS = visual analogue scale; VEDA = vedaprofen
In another study the thermal antinociceptive effects of 0.5–4 mg/kg tramadol administered PO were investigated. 150 A dose-dependent antinociceptive effect was detected and significant analgesic effects that lasted up to 6 h were reported with doses of 2 and 4 mg/kg. In the same study a pharmacokinetic simulation was performed and it was predicted that a dose of 4 mg/kg q6h would maintain adequate analgesia in cats.
Orally administered tramadol (8.6–11.6 mg/kg) produces a significant reduction in the MAC of sevoflurane from 2.45 ± 0.2% to 1.48 ± 0.2%, and so it could be used in a multimodal anaesthetic protocol. 18 Another experimental study compared the effect of 1 mg/kg tramadol or 0.1 mg/kg morphine administered by the epidural route in cats with the use of a tail clamp test. 107 Tramadol provided analgesia comparable to morphine for up to 6 h, while morphine provided superior analgesia from 6–12 h after administration. The use of preservative-containing preparations of tramadol for epidural injection should be avoided as the toxicity of the preservatives on neuronal tissue in cats has not been established. Preservative-free preparations of tramadol are available in some countries.
In a clinical study, cats undergoing elective ovariohysterectomy received tramadol (2 mg/kg SC q8h for 3 days) or vedaprofen (0.5 mg/kg PO q24h for 3 days) alone or in combination. Cats receiving the two drugs combined had lower pain scores than cats that received one or other of the drugs on its own. 151 Haemostatic, biochemical and gastrointestinal function was not affected by the perioperative use of vedaprofen and/or tramadol in cats. 152 More recently, tramadol (2 mg/kg IV) provided adequate analgesia after neutering for up to 6 h. 149 In the aforementioned studies respiratory depression was not noted; however, in an experimental study in cats, tramadol at 2 and 4 mg/kg administered IV in α-chloralose-urethane anaesthetised cats exerted a depressant effect on ventilation by reducing the sensitivity of peripheral and central chemoceptors to carbon dioxide and increasing the apnoeic threshold. 153
Naloxone
Naloxone is a pure opioid antagonist, with no intrinsic effect, used to antagonise the effects of opioids. It antagonises the analgesic effects as well as the adverse effects such as excessive sedation, bradycardia and respiratory depression.49,155 Naloxone has a rapid onset of action (1–2 mins) and a duration of effect of approximately 30–60 mins. 155 It should be administered slowly IV (0.002–0.04 mg/kg). Renarcotisation can occur when the duration of action of the opioid agonist is longer than the antagonist. 155 The appropriate dosage in cats has not been evaluated. The authors would suggest preparing a syringe with 0.002 mg/kg of naloxone diluted with saline and titrating administration to effect; ie, until the adverse effects of opioids have disappeared. Some clinicians have suggested that if naloxone is not available, butorphanol can be used for antagonism of respiratory depression, while maintaining a certain degree of analgesia, but there are no studies evaluating this in cats. Nevertheless, butorphanol was used to reverse the effects of fentanyl and sufentanil in rats and rabbits.156 –158 By contrast, in dogs, butorphanol was not proven to reverse oxymorphone-induced postoperative respiratory depression. 159
The effects of a combination of buprenorphine and naloxone have recently been investigated. In people and rats naloxone can enhance the analgesic/antihyperalgesic effects of buprenorphine. 160 However, this did not appear to be the case in cats and the study showed that naloxone antagonised the thermal antinociceptive effects of clinically analgesic doses of buprenorphine in cats (Table 13). 161
Table 13.
Studies evaluating naloxone in cats
| Type of route | Dose and route* | Assessment | Results | Reference |
|---|---|---|---|---|
| Experimental | B 0.01 mg/kg IM, N 0.67 μg/kg IM, B + N (15:1) IM |
MNT TNT |
B and N combination did not enhance B antinociception | 161 |
See footnote to Table 1
B = buprenorphine; N = naloxone; MNT = mechanical nociceptive threshold; TNT = thermal nociceptive threshold
Conclusions
In summary, opioids can be used in cats, especially in cases of moderate to severe pain. Their effects should be closely monitored so that pain treatment is tailored to best suit the individual animal’s needs.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
Accepted: 5 January 2015
JFMS Premier Reviews are invited state-of-the-art review papers on key issues in feline medicine and surgery. Written by expert international authors, these reviews are made freely available to maximise their impact.
References
- 1. Dohoo SE, Dohoo IR. Factors influencing the postoperative use of analgesics in dogs and cats by Canadian veterinarians. Can Vet J 1996; 37: 552–556. [PMC free article] [PubMed] [Google Scholar]
- 2. Watson AD, Nicholson A, Church DB, et al. Use of anti-inflammatory and analgesic drugs in dogs and cats. Aust Vet J 1996; 74: 203–210. [DOI] [PubMed] [Google Scholar]
- 3. Lascelles BD, Capner CA, Waterman-Pearson AE. Current British veterinary attitudes to perioperative analgesia for dogs. Vet Rec 1999; 145: 95–99. [DOI] [PubMed] [Google Scholar]
- 4. Joubert KE. The use of analgesic drugs by South African veterinarians. J S Afr Vet Assoc 2001; 72: 57–60. [DOI] [PubMed] [Google Scholar]
- 5. Epstein ME, Rodan I, Griffenhagen G, et al. 2015 AAHA/AAFP pain management guidelines for dogs and cats. J Feline Med Surg 2015; 17: 251–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mathews K, Kronen PW, Lascelles D, et al. Guidelines for recognition, assessment and treatment of pain. J Small Anim Pract 2014; 55: E10–68. [DOI] [PubMed] [Google Scholar]
- 7. Robertson SA. Managing pain in feline patients. Vet Clin North Am Small Anim Pract 2008; 38: 1267–1290. [DOI] [PubMed] [Google Scholar]
- 8. Hellyer PW, Uhrig SR, Robinson NG. Feline acute pain scale. Colorado State University Veterinary Medical Center, Fort Collins, CO. http://www.vasg.org/pdfs/CSU_Acute_Pain_Scale_Kitten.pdf (2006, accessed January 10, 2014). [Google Scholar]
- 9. Brondani JT, Mama KR, Luna SP, et al. Validation of the English version of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in cats. BMC Vet Res 2013; 9: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holden E, Calvo G, Collins M, et al. Evaluation of facial expression in acute pain in cats. J Small Anim Pract 2014; 55: 615–621. [DOI] [PubMed] [Google Scholar]
- 11. Dixon MJ, Robertson SA, Taylor PM. A thermal threshold testing device for evaluation of analgesics in cats. Res Vet Sci 2002; 72: 205–210. [DOI] [PubMed] [Google Scholar]
- 12. Millette VM, Steagall PV, Duke-Novakovski T, et al. Effects of meperidine or saline on thermal, mechanical and electrical nociceptive thresholds in cats. Vet Anaesth Analg 2008; 35: 543–547. [DOI] [PubMed] [Google Scholar]
- 13. Love EJ, Murrell J, Whay HR. Thermal and mechanical nociceptive threshold testing in horses: a review. Vet Anaesth Analg 2011; 38: 3–14. [DOI] [PubMed] [Google Scholar]
- 14. Slingsby LS, Jones A, Waterman-Pearson AE. Use of a new finger-mounted device to compare mechanical nociceptive thresholds in cats given pethidine or no medication after castration. Res Vet Sci 2001; 70: 243–246. [DOI] [PubMed] [Google Scholar]
- 15. Brosnan RJ, Pypendop BH, Siao KT, et al. Effects of remifentanil on measures of anesthetic immobility and analgesia in cats. Am J Vet Res 2009; 70: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 16. Ilkiw JE, Pascoe PJ, Tripp LD. Effects of morphine, butorphanol, buprenorphine, and U50488H on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res 2002; 63: 1198–1202. [DOI] [PubMed] [Google Scholar]
- 17. Ferreira TH, Steffey EP, Mama KR, et al. Determination of the sevoflurane sparing effect of methadone in cats. Vet Anaesth Analg 2011; 38: 310–319. [DOI] [PubMed] [Google Scholar]
- 18. Ko JC, Abbo LA, Weil AB, et al. Effect of orally administered tramadol alone or with an intravenously administered opioid on minimum alveolar concentration of sevoflurane in cats. J Am Vet Med Assoc 2008; 232: 1834–1840. [DOI] [PubMed] [Google Scholar]
- 19. Yackey M, Ilkiw JE, Pascoe PJ, et al. Effect of transdermally administered fentanyl on the minimum alveolar concentration of isoflurane in cats. Anaesth Analg 2004; 31: 183–189. [DOI] [PubMed] [Google Scholar]
- 20. Ilkiw JE, Pascoe PJ, Fisher LD. Effect of alfentanil on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res 1997; 58: 1274–1279. [PubMed] [Google Scholar]
- 21. Ferreira T, Aguiar AJA, Valverde A. Effects of remifentanil hydrochloride administered via constant rate infusion on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res 2009; 70: 581–588. [DOI] [PubMed] [Google Scholar]
- 22. Credie RG, Teixeira Neto FJ, Ferreira TH, et al. Effects of methadone on the minimum alveolar concentration of isoflurane in dogs. Vet Anaesth Analg 2010; 37: 240–249. [DOI] [PubMed] [Google Scholar]
- 23. Monteiro ER, Teixeira Neto FJ, Campagnol D, et al. Effects of remifentanil on the minimum alveolar concentration of isoflurane in dogs. Am J Vet Res 2010; 71: 150–156. [DOI] [PubMed] [Google Scholar]
- 24. Cross M, Plunkett E. Affinity, efficacy and potency. In: Physics, pharmacology and physiology for anaesthetists. 1st ed. Cambridge: Cambridge University Press, 2008, pp 93–96. [Google Scholar]
- 25. Wikler A. Studies on the action of morphine on the central nervous system of cat. J Pharm Pharmacol 1944; 80: 176–178. [Google Scholar]
- 26. Fertziger AP, Stein EA, Lynch JJ. Suppression of morphine-induced mania in cats [letter]. Psychopharmacologia 1974; 36: 185–187. [DOI] [PubMed] [Google Scholar]
- 27. Gaumann DM, Yaksh TL, Tyce GM, et al. Sympathetic stimulating effects of sufentanil in the cat are mediated centrally. Neurosci Lett 1988; 91: 30–35. [DOI] [PubMed] [Google Scholar]
- 28. Pascoe PJ, Ilkiw JE, Fisher LD. Cardiovascular effects of equipotent isoflurane and alfentanil/isoflurane minimum alveolar concentration multiple in cats. Am J Vet Res 1997; 58: 1267–1273. [PubMed] [Google Scholar]
- 29. Kamata M, Nagahama S, Kakishima K, et al. Comparison of behavioral effects of morphine and fentanyl in dogs and cats. J Vet Med Sci 2012; 74: 231–234. [DOI] [PubMed] [Google Scholar]
- 30. Gellasch KL, Kruse-Elliott KT, Osmond CS, et al. Comparison of transdermal administration of fentanyl versus intramuscular administration of butorphanol for analgesia after onychectomy in cats. J Am Vet Med Assoc 2002; 220: 1020–1024. [DOI] [PubMed] [Google Scholar]
- 31. Bortolami E, Murrell JC, Slingsby LS. Methadone in combination with acepromazine as premedication prior to neutering in the cat. Vet Anaesth Analg 2013; 40: 181–193. [DOI] [PubMed] [Google Scholar]
- 32. Steagall PV, Carnicelli P, Taylor PM, et al. Effects of subcutaneous methadone, morphine, buprenorphine or saline on thermal and pressure thresholds in cats. J Vet Pharmacol Ther 2006; 29: 531–537. [DOI] [PubMed] [Google Scholar]
- 33. Ferreira TH, Rezende ML, Mama KR, et al. Plasma concentrations and behavioral, antinociceptive, and physiologic effects of methadone after intravenous and oral transmucosal administration in cats. Am J Vet Res 2011; 72: 764–771. [DOI] [PubMed] [Google Scholar]
- 34. Robertson SA, Taylor PM, Lascelles BD, et al. Changes in thermal threshold response in eight cats after administration of buprenorphine, butorphanol and morphine. Vet Rec 2003; 153: 462–465. [DOI] [PubMed] [Google Scholar]
- 35. Steagall PV, Taylor PM, Brondani JT, et al. Antinociceptive effects of tramadol and acepromazine in cats. J Feline Med Surg 2008; 10: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wallenstein MC, Wang SC. Mechanism of morphine-induced mydriasis in the cat. Am J Physiol 1979; 236: R292–296. [DOI] [PubMed] [Google Scholar]
- 37. Valverde A, Cantwell S, Hernández J, et al. Effects of acepromazine on the incidence of vomiting associated with opioid administration in dogs. Vet Anaesth Analg 2004; 31: 40–45. [DOI] [PubMed] [Google Scholar]
- 38. Sparkes AH, Papasouliotis K, Viner J, et al. Assessment of orocaecal transit time in cats by the breath hydrogen method: the effects of sedation and a comparison of definitions. Res Vet Sci 1996; 60: 243–246. [DOI] [PubMed] [Google Scholar]
- 39. Posner LP, Gleed RD, Erb HN, et al. Post-anesthetic hyperthermia in cats. Vet Anaesth Analg 2007; 34: 40–47. [DOI] [PubMed] [Google Scholar]
- 40. Posner LP, Pavuk AA, Rokshar JL, et al. Effects of opioids and anesthetic drugs on body temperature in cats. Vet Anaesth Analg 2010; 37: 35–43. [DOI] [PubMed] [Google Scholar]
- 41. Mendes GM, Selmi AL. Use of a combination of propofol and fentanyl, alfentanil, or sufentanil for total intravenous anaesthesia in cats. J Am Vet Med Assoc 2003; 223: 1608–1613. [DOI] [PubMed] [Google Scholar]
- 42. Clark WG, Cumby HR. Hyperthermic responses to central and peripheral injections of morphine sulphate in the cat. Br J Pharmacol 1978; 63: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Booth N, Rankin AL. Evaluation of meperidine hydrochloride in the cat. Vet Med 1954; 49: 249–252. [Google Scholar]
- 44. Lee M, Silverman SM, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011; 14: 145–161. [PubMed] [Google Scholar]
- 45. Lascelles BD, Robertson SA. Use of thermal threshold response to evaluate the antinociceptive effects of butorphanol in cats. Am J Vet Res 2004; 65: 1085–1089. [DOI] [PubMed] [Google Scholar]
- 46. Robertson SA, Lascelles BD, Taylor PM, et al. PK-PD modeling of buprenorphine in cats: intravenous and oral transmucosal administration. J Vet Pharmacol Ther 2005; 28: 453–460. [DOI] [PubMed] [Google Scholar]
- 47. Sadhasivam S, Chidambaran V. Pharmacogenomics of opioids and perioperative pain management. Pharmacogenomics 2012; 13: 1719–1740. [DOI] [PubMed] [Google Scholar]
- 48. Papich MG. Pharmacologic considerations for opiate analgesic and nonsteroidal anti-inflammatory drugs. Vet Clin North Am Small Anim Pract 2000; 30: 815–837. [DOI] [PubMed] [Google Scholar]
- 49. Lamont LA, Mathews KA. Opioids, nonsteroidal anti-inflammatories, and analgesic adjuvants. In: Tranquilli WJ, Thurmon JC, Grimm KA. (eds). Lumb & Jones’ veterinay anesthesia and analgesia. 4th ed. Ames: Blackwell Publishing, 2007, pp 241–271. [Google Scholar]
- 50. Kaye AD, Hoover JM, Baber SR, et al. The effects of meperidine in the pulmonary vascular bed of the cat. J Cardiothorac Vasc Anesth 2006; 20: 691–695. [DOI] [PubMed] [Google Scholar]
- 51. Nagore L, Soler C, Gil L, et al. Sedative effects of dexmedetomidine, dexmedetomidine-pethidine and dexmedetomidine-butorphanol in cats. J Vet Pharmacol Ther 2012; 36: 222–228. [DOI] [PubMed] [Google Scholar]
- 52. Taylor PM, Robertson SA, Dixon MJ, et al. Morphine, pethidine and buprenorphine disposition in the cat. J Vet Pharmacol Ther 2001; 24: 391–398. [DOI] [PubMed] [Google Scholar]
- 53. Slingsby LS, Waterman-Pearson AE. Comparison of pethidine, buprenorphine and ketoprofen for postoperative analgesia after ovariohysterectomy in the cat. Vet Rec 1998; 143: 185–189. [DOI] [PubMed] [Google Scholar]
- 54. Balmer TV, Irvine D, Jones RS, et al. Comparison of carprofen and pethidine as postoperative analgesics in the cat. J Small Anim Pract 1998; 39: 158–164 [DOI] [PubMed] [Google Scholar]
- 55. Lascelles BD, Cripps P, Mirchandani S, et al. Carprofen as an analgesic for postoperative pain in cats: dose titration and assessment of efficacy in comparison to pethidine hydrochloride. J Small Anim Pract 1995; 36: 535–541. [DOI] [PubMed] [Google Scholar]
- 56. Mollenhoff A, Nolte I, Kramer S. Anti-nociceptive efficacy of carprofen, levomethadone and buprenorphine for pain relief in cats following major orthopaedic surgery. J Vet Med A Physiol Pathol Clin Med 2005; 52: 186–198. [DOI] [PubMed] [Google Scholar]
- 57. Bley CR, Neiger-Aeschbacher G, Busato A, et al. Comparison of perioperative racemic methadone, levo-methadone and dextromoramide in cats using indicators of post-operative pain. Vet Anaesth Analg 2004; 31: 175–182. [DOI] [PubMed] [Google Scholar]
- 58. Dobromylskyj P. Assessment of methadone as an anaesthetic premedicant in cats. J Small Anim Pract 1993; 34: 604–608. [Google Scholar]
- 59. Pypendop BH, Ilkiw JE, Shilo-Benjamini Y. Bioavailability of morphine, methadone, hydromorphone, and oxymorphone following buccal administration in cats. J Vet Pharmacol Ther 2014; 37: 295–300. [DOI] [PubMed] [Google Scholar]
- 60. Inturrisi CE. Pharmacology of methadone and its isomers. Minerva Anestesiol 2005; 71: 435–437. [PubMed] [Google Scholar]
- 61. Codd EE, Shank RP, Schupsky JJ, et al. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception. J Pharmacol Exp Ther 1995; 274: 1263–1270. [PubMed] [Google Scholar]
- 62. Xiao Y, Smith RD, Caruso FS, et al. Blockade of rat alpha3beta4 nicotinic receptor function by methadone, its metabolites, and structural analogs. J Pharmacol Exp Ther 2001; 299: 366–371. [PubMed] [Google Scholar]
- 63. Cowan A, Lewis JW, Macfarlane IR. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol 1977; 4: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Steagall PV, Pelligand L, Giordano T, et al. Pharmacokinetic and pharmacodynamic modeling of intravenous, intramuscular and subcutaneous buprenorphine in conscious cats. Vet Anaesth Analg 2013; 40: 83–89. [DOI] [PubMed] [Google Scholar]
- 65. Polson S, Taylor PM, Yates D. Analgesia after feline ovariohysterectomy under midazolam-medetomidine-ketamine anaesthesia with buprenorphine or butorphanol, and carprofen or meloxicam: a prospective, randomised clinical trial. J Feline Med Surg 2012; 14: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Catbagan DL, Quimby JM, Mama KR, et al. Comparison of the efficacy and adverse effects of sustained-release buprenorphine hydrochloride following subcutaneous administration and buprenorphine hydrochloride following oral transmucosal administration in cats undergoing ovariohysterectomy. Am J Vet Res 2011; 72: 461–466. [DOI] [PubMed] [Google Scholar]
- 67. Giordano T, Steagall PV, Ferreira TH, et al. Postoperative analgesic effects of intravenous, intramuscular, subcutaneous or oral transmucosal buprenorphine administered to cats undergoing ovariohysterectomy. Vet Anaesth Analg 2010; 37: 357–366. [DOI] [PubMed] [Google Scholar]
- 68. Santos LC, Ludders JW, Erb HN, et al. Sedative and cardiorespiratory effects of dexmedetomidine and buprenorphine administered to cats via oral transmucosal or intramuscular routes. Vet Anaesth Analg 2010; 5: 417–424. [DOI] [PubMed] [Google Scholar]
- 69. Taylor PM, Kirby JJ, Robinson C, et al. A prospective multi-centre clinical trial to compare buprenorphine and butorphanol for postoperative analgesia in cats. J Feline Med Surg 2010; 12: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Steagall PV, Mantovani FB, Taylor PM, et al. Dose-related antinociceptive effects of intravenous buprenorphine in cats. Vet J 2009; 182: 203–209. [DOI] [PubMed] [Google Scholar]
- 71. Pypendop BH, Siao KT, Pascoe PJ, et al. Effects of epidurally administered morphine or buprenorphine on the thermal threshold in cats. Am J Vet Res 2008; 69: 983–987. [DOI] [PubMed] [Google Scholar]
- 72. Murrell JC, Robertson SA, Taylor PM, et al. Use of a transdermal matrix patch of buprenorphine in cats: preliminary pharmacokinetic and pharmacodynamic data. Vet Rec 2007; 17: 578–583. [DOI] [PubMed] [Google Scholar]
- 73. Johnson JA, Robertson SA, Pypendop BH. Antinociceptive effects of butorphanol, buprenorphine, or both, administered intramuscularly in cats. Am J Vet Res 2007; 68: 699–703. [DOI] [PubMed] [Google Scholar]
- 74. Pypendop BH, Pascoe PJ, Ilkiw JE. Effects of epidural administration of morphine and buprenorphine on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res 2006; 67: 1471–1475. [DOI] [PubMed] [Google Scholar]
- 75. Gassel AD, Tobias KM, Egger CM, et al. Comparison of oral and subcutaneous administration of buprenorphine and meloxicam for preemptive analgesia in cats undergoing ovariohysterectomy. J Am Vet Med Assoc 2005; 12: 1937–1944. [DOI] [PubMed] [Google Scholar]
- 76. Robertson SA, Taylor PM, Sear JW. Systemic uptake of buprenorphine by cats after oral mucosal administration. Vet Rec 2003; 152: 675–678. [DOI] [PubMed] [Google Scholar]
- 77. Stanway GW, Taylor PM, Brodbelt DC. A preliminary investigation comparing pre-operative morphine and buprenorphine for postoperative analgesia and sedation in cats. Vet Anaesth Analg 2002; 29: 29–35. [DOI] [PubMed] [Google Scholar]
- 78. Dobbins S, Brown NO, Shafer FS. Comparison of the effects of buprenorphine, oxymorphone hydrochloride, and ketoprofen for post operative analgesia after onychectomy or onychectomy and sterilization in cats. J Am Anim Hosp Assoc 2002; 38: 507–514. [DOI] [PubMed] [Google Scholar]
- 79. Steagall PV, Millette V, Mantovani FB, et al. Antinociceptive effects of epidural buprenorphine or medetomidine, or the combination, in conscious cats. J Vet Pharmacol Ther 2009; 32: 477–484. [DOI] [PubMed] [Google Scholar]
- 80. Sramek MK, Haas MC, Coleman GD, et al. The safety of high-dose buprenorphine administered subcutaneously in cats. J Vet Pharmacol Ther. Epub ahead of print 27 January 2015. DOI: 10.1111/jvp.12203. [DOI] [PubMed] [Google Scholar]
- 81. Bortolami E, Slingsby L, Love EJ. Comparison of two formulations of buprenorphine in cats administered by the oral transmucosal route. J Feline Med Surg 2012; 14: 534–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Steagall PV, Monteiro-Steagall BP, Taylor PM. A review of the studies using buprenorphine in cats. J Vet Intern Med 2014; 28: 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pircio AW, Gylys JA, Cavanagh RL, et al. The pharmacology of butorphanol, a 3,14-dihydroxymorphinan narcotic antagonist analgesic. Arch Int Pharmacodyn Ther 1976; 220: 231–257. [PubMed] [Google Scholar]
- 84. Horan P, Ho IK. Comparative pharmacological and biochemical studies between butorphanol and morphine. Pharmacol Biochem Behav 1989; 34: 847–854. [DOI] [PubMed] [Google Scholar]
- 85. Wells SM, Glerum LE, Papich MG. Pharmacokinetics of butorphanol in cats after intramuscular and buccal transmucosal administration. Am J Vet Res 2008; 69: 1548–1554. [DOI] [PubMed] [Google Scholar]
- 86. Biermann K, Hungerbühler S, Mischke R, et al. Sedative, cardiovascular, haematologic and biochemical effects of four different drug combinations administered intramuscularly in cats. Vet Anaesth Analg 2012; 39: 137–150. [DOI] [PubMed] [Google Scholar]
- 87. Ward JL, Schober KE, Fuentes VL, et al. Effects of sedation on echocardiographic variables of left atrial and left ventricular function in healthy cats. J Feline Med Surg 2012; 14: 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Killos MB, Graham LF, Lee J. Comparison of two anesthetic protocols for feline blood donation. Vet Anaesth Analg 2010; 37: 230–239. [DOI] [PubMed] [Google Scholar]
- 89. Dixon MJ, Taylor PM, Slingsby L, et al. A small, silent, low friction, linear actuator for mechanical nociceptive testing in veterinary research. Lab Anim 2010; 44: 247–253. [DOI] [PubMed] [Google Scholar]
- 90. Dixon MJ, Taylor PM, Steagall PV, et al. Development of a pressure nociceptive threshold testing device for evaluation of analgesics in cats. Res Vet Sci 2007; 82: 85–92. [DOI] [PubMed] [Google Scholar]
- 91. Tobias KM, Harvey RC, Byarlay JM. A comparison of four methods of analgesia in cats following ovariohysterectomy. Vet Anaesth Analg 2006; 33: 390–398. [DOI] [PubMed] [Google Scholar]
- 92. Carroll GL, Howe LB, Peterson KD. Analgesic efficacy of preoperative administration of meloxicam or butorphanol in onychectomized cats. J Am Vet Med Assoc 2005; 226: 913–919. [DOI] [PubMed] [Google Scholar]
- 93. Al-Gizawiy MM, Rudé EP. Comparison of preoperative carprofen and postoperative butorphanol as postsurgical analgesics in cats undergoing ovariohysterectomy. Vet Anaesth Analg 2004; 31: 164–174. [DOI] [PubMed] [Google Scholar]
- 94. Lascelles BD, Robertson SA. Antinociceptive effects of hydromorphone, butorphanol, or the combination in cats. J Vet Intern Med 2004; 18: 190–195. [DOI] [PubMed] [Google Scholar]
- 95. Robertson SA, Lascelles BD, Taylor P. Effect of 0.1, 0.2, 0.4 and 0.8 mg/kg of IV butorphanol on thermal antinociception in cats. Vet Anaesth Analg 2003; 30: 107. [DOI] [PubMed] [Google Scholar]
- 96. Ansah OB, Vainio O, Hellsten C, et al. Postoperative pain control in cats: clinical trials with medetomidine and butorphanol. Vet Surg 2002; 31: 99–103. [DOI] [PubMed] [Google Scholar]
- 97. Franks JN, Boothe HW, Taylor L, et al. Evaluation of transdermal fentanyl patches for analgesia in cats undergoing onychectomy. J Am Vet Med Assoc 2000; 217: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 98. Sawyer DC, Rech RH. Analgesia and behavioral effects of butorphanol, nalbuphine, and pentazocine in the cat. J Am Anim Hosp Assoc 1987; 23: 438–446. [Google Scholar]
- 99. Mandsager PE, Raffe MR. The efficacy of butorphanol tartrate as an analgesic agent in the cat using somatic pain model [abstract]. Proceedings of the Annual Meeting of the American College of Veterinary Anesthesia, 1988, p 251. [Google Scholar]
- 100. Lin HC, Benson GJ, Thurmon JC, et al. Influence of anesthetic regimens on the perioperative catecholamine response associated with onychectomy in cats. Am J Vet Res 1993; 54: 1721–1724. [PubMed] [Google Scholar]
- 101. Smith JD, Allen SW, Quandt JE, et al. Indicators of postoperative pain in cats and correlation with clinical criteria. Am J Vet Res 1996; 57: 1674–1678. [PubMed] [Google Scholar]
- 102. Carroll GL, Howe LB, Slater MR, et al. Evaluation of analgesia provided by postoperative administration of butorphanol to cats undergoing onychectomy. J Am Vet Med Assoc 1998; 213: 246–250. [PubMed] [Google Scholar]
- 103. Gutstein HB, Akil H. Opioid analgesics. In: Harman JG, Limbird LE, Goodman Gilman A. (eds). Goodman and Gilman’s the pharmacological bases of therapeutics. 10th ed. New York: McGraw-Hill, 2001, pp 569–619. [Google Scholar]
- 104. Hall LW, Clarke KW. Principles of sedation, analgesia and premedication. In: Veterinary anaesthesia. 9th ed. London: WB Saunders, 1991, pp 51–79. [Google Scholar]
- 105. Bauquier SH. Hypotension and pruritus induced by neuraxial anaesthesia in a cat. Aust Vet J 2012; 90: 402–403. [DOI] [PubMed] [Google Scholar]
- 106. Song RB, Cross JR, Golder FJ, et al. Suspected epidural morphine analgesia induced chronic urinary and bowel dysfunction in a cat. J Feline Med Surg 2011; 13: 602–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Castro DS, Silva MF, Shih AC, et al. Comparison between the analgesic effects of morphine and tramadol delivered epidurally in cats receiving a standardized noxious stimulation. J Feline Med Surg 2009; 11: 948–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Troncy E, Junot S, Keroack S, et al. Results of preemptive epidural administration of morphine with or without bupivacaine in dogs and cats undergoing surgery: 265 cases (1997–1999). J Am Vet Med Assoc 2002; 221: 666–672. [DOI] [PubMed] [Google Scholar]
- 109. Murthy BR, Pollack GM, Brouwer KL. Contribution of morphine-6-glucuronide to antinociception following intravenous administration of morphine to healthy volunteers. J Clin Pharm 2002; 42: 569–576. [DOI] [PubMed] [Google Scholar]
- 110. Wright A, Mather L, Smith M. Hydromorphone-3-glucuronide. A more potent neuro-excitant than its structural analogue, morphine-3-glucuronide. Life Sci 2001; 69: 409–420. [DOI] [PubMed] [Google Scholar]
- 111. Pettifer G, Dyson D. Hydromorphone: a cost-effective alternative to the use of oxymorphone. Can Vet J 2000; 41: 135–137. [PMC free article] [PubMed] [Google Scholar]
- 112. Bateman SW, Haldane S, Stephens JA. Comparison of the analgesic efficacy of hydromorphone and oxymorphone in dogs and cats: a randomized blinded study. Vet Anaesth Analg 2008; 35: 341–347. [DOI] [PubMed] [Google Scholar]
- 113. Niedfeldt RL, Robertson SA. Postanesthetic hyperthermia in cats: a retrospective comparison between hydromorphone and buprenorphine. Vet Anaesth Analg 2006; 33: 381–389. [DOI] [PubMed] [Google Scholar]
- 114. Wegner K, Robertson SA. Dose-related thermal antinociceptive effects of intravenous hydromorphone in cats. Vet Anaesth Analg 2007; 34: 132–138. [DOI] [PubMed] [Google Scholar]
- 115. Ambros B, Steagall PV, Mantovani F, et al. Antinociceptive effects of epidural administration of hydromorphone in conscious cats. Am J Vet Res 2009; 70: 1187–1192. [DOI] [PubMed] [Google Scholar]
- 116. Robertson SA, Wegner K, Lascelles BD. Antinociceptive and side-effects of hydromorphone after subcutaneous administration in cats. J Feline Med Surg 2009; 11: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wegner K, Robertson SA, Kollias-Baker C, et al. Pharmacokinetic and pharmacodynamic evaluation of intravenous hydromorphone in cats. J Vet Pharmacol Therap 2004; 27: 329–336. [DOI] [PubMed] [Google Scholar]
- 118. Torske K, Dyson D, Conlon P. Cardiovascular effects of epidurally administered oxymorphone and an oxymorphone-bupivicaine combination in halothane-anesthetized dogs. Am J Vet Res 1999; 60: 194–200. [PubMed] [Google Scholar]
- 119. Siao KT, Pypendop BH, Stanley SD, et al. Pharmacokinetics of oxymorphone in cats. J Vet Pharmacol Ther 2011; 34: 594–598. [DOI] [PubMed] [Google Scholar]
- 120. Ambros B, Alcorn J, Duke-Novakovski T, et al. Pharmacokinetics and pharmacodynamics of a constant rate infusion of fentanyl (5 μg/kg/h) in awake cats. Am J Vet Res 2014; 75: 716–721. [DOI] [PubMed] [Google Scholar]
- 121. Liehmann L, Mosing M, Auer U. A comparison of cardiorespiratory variables during isoflurane-fentanyl and propofol-fentanyl anaesthesia for surgery in injured cats. Vet Anaesth Analg 2006; 33: 158–168. [DOI] [PubMed] [Google Scholar]
- 122. Robertson SA, Taylor PM, Sear JW, et al. Relationship between plasma concentrations and analgesia after intravenous fentanyl and disposition after other routes of administration in cats. J Vet Pharmacol Ther 2005; 28: 1–7. [DOI] [PubMed] [Google Scholar]
- 123. Romans CW, Gordon WJ, Robinson DA, et al. Effect of postoperative analgesic protocol on limb function following onychectomy in cats. J Am Vet Med Assoc 2005; 227: 89–93. [DOI] [PubMed] [Google Scholar]
- 124. Davidson CD, Pettifer GR, Henry JD., Jr. Plasma fentanyl concentrations and analgesic effects during full or partial exposure to transdermal fentanyl patches in cats. J Am Vet Med Assoc 2004; 224: 700–705. [DOI] [PubMed] [Google Scholar]
- 125. Pettifer GR, Hosgood G. The effect of rectal temperature on perianesthetic serum concentrations of transdermally administered fentanyl in cats anesthetized with isoflurane. Am J Vet Res 2003; 64: 1557–1561. [DOI] [PubMed] [Google Scholar]
- 126. Egger CM, Glerum LE, Allen SW, et al. Plasma fentanyl concentrations in awake cats and cats undergoing anesthesia and ovariohysterectomy using transdermal administration. Vet Anaesth Analg 2003; 30: 229–236. [DOI] [PubMed] [Google Scholar]
- 127. Glerum LE, Egger CM, Allen SW, et al. Analgesic effect of the transdermal fentanyl patch during and after feline ovariohysterectomy. Vet Surg 2001; 30: 351–358. [DOI] [PubMed] [Google Scholar]
- 128. Lee DD, Papich MG, Hardie EM. Comparison of pharmacokinetics of fentanyl after intravenous and transdermal administration in cats. Am J Vet Res 2000; 61: 672–677. [DOI] [PubMed] [Google Scholar]
- 129. Duke T, Cox AK, Remedios AM, et al. The analgesic effects of administering fentanyl or medetomidine in the lumbosacral epidural space of cats. Vet Surg 1994; 23: 143–148. [DOI] [PubMed] [Google Scholar]
- 130. Duke T, Cox AK, Remedios AM, et al. The cardiopulmonary effects of placing fentanyl or medetomidine in the lumbosacral epidural space of isoflurane anesthetized cats. Vet Surg 1994; 23: 143–148. [DOI] [PubMed] [Google Scholar]
- 131. Deschamps JY, Gaulier JM, Podevin G, et al. Fatal overdose after ingestion of a transdermal fentanyl patch in two non-human primates. Vet Anaesth Analg 2012; 39: 653–656. [DOI] [PubMed] [Google Scholar]
- 132. Mrvos R, Feuchter AC, Katz KD, et al. Whole fentanyl patch ingestion: a multi-center case series. J Emerg Med 2012; 42: 549–552. [DOI] [PubMed] [Google Scholar]
- 133. Teske J, Weller JP, Larsch K, et al. Fatal outcome in a child after ingestion of a transdermal fentanyl patch. Int J Legal Med 2007; 121: 147–151. [DOI] [PubMed] [Google Scholar]
- 134. Stoelting RK. Opioid agonists and antagonists. In: Stoelting RK. (ed). Pharmacology and physiology in anesthetic practice. Philadelphia: Lippincott Williams & Wilkins, 1999, pp 77–112. [Google Scholar]
- 135. Pypendop BH, Brosnan RJ, Majewski-Tiedeken CR, et al. Pharmacokinetics of fentanyl, alfentanyl, and sufentanil in isoflurane-anesthetized cats. J Vet Pharmacol Ther 2014; 37: 13–17. [DOI] [PubMed] [Google Scholar]
- 136. Padilha ST, Steagall PV, Monteiro BP, et al. A clinical comparison of remifentanil or alfentanil in propofol-anesthetized cats undergoing ovariohysterectomy. J Feline Med Surg 2011; 13: 738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Pascoe PJ, Ilkiw JE, Black WD, et al. The pharmacokinetics of alfentanil in healthy cats. Vet Anaesth Analg 1993; 20: 9–13. [Google Scholar]
- 138. Feldman PL, James MK, Brackeen MF. Design, synthesis, and pharmacological evaluation of ultrashort- to long-acting opioid analgesics. J Med Chem 1991; 34: 2202–2208. [DOI] [PubMed] [Google Scholar]
- 139. Michelsen LG, Salmenperä M, Hug CC, Jr, et al. Anesthetic potency of remifentanil in dogs. Anesthesiology 1996; 84: 865–872. [DOI] [PubMed] [Google Scholar]
- 140. Glass PS, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg 1999; 89 Suppl 4: S7–S14. [DOI] [PubMed] [Google Scholar]
- 141. Court MH, Greenblatt DJ. Molecular genetic basis for deficient acetaminophen glucuronidation by cats: UGT1A6 is a pseudogene, and evidence for reduced diversity of expressed hepatic UGT1A isoforms. Pharmacogenetics 2000; 10: 355–369. [DOI] [PubMed] [Google Scholar]
- 142. Pypendop BH, Brosnan RJ, Siao KT, et al. Pharmacokinetics of remifentanil in conscious cats and cats anesthetized with isoflurane. Am J Vet Res 2008; 69: 531–536. [DOI] [PubMed] [Google Scholar]
- 143. Correa Mdo A, Aguiar AJ, Neto FJ, et al. Effects of remifentanil infusion regimens on cardiovascular function and responses to noxious stimulation in propofol-anesthetized cats. Am J Vet Res 2007; 68: 932–940. [DOI] [PubMed] [Google Scholar]
- 144. Rosow CE. Sufentanil citrate: a new opioid analgesic for use in anesthesia. Pharmacotherapy 1984; 4: 11–19. [DOI] [PubMed] [Google Scholar]
- 145. Raffa RB, Friderichs E, Reimann W, et al. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther 1992; 260: 275–285. [PubMed] [Google Scholar]
- 146. Pypendop BH, Ilkiw JE. Pharmacokinetics of tramadol, and its metabolite O-desmethyl-tramadol, in cats. J Vet Pharmacol Ther 2008; 31: 52–59. [DOI] [PubMed] [Google Scholar]
- 147. Indrawirawan Y, McAlees T. Tramadol toxicity in a cat: case report and literature review of serotonin syndrome. J Feline Med Surg 2014; 16: 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ener RA, Meglathery SB, Van Decker WA, et al. Serotonin syndrome and other serotonergic disorders. Pain Med 2003; 4: 63–74. [DOI] [PubMed] [Google Scholar]
- 149. Cagnardi P, Villa R, Zonca A, et al. Pharmacokinetics, intraoperative effect and postoperative analgesia of tramadol in cats. Res Vet Sci 2011; 90: 503–509. [DOI] [PubMed] [Google Scholar]
- 150. Pypendop BH, Siao KT, Ilkiw JE. Effects of tramadol hydrochloride on the thermal threshold in cats. Am J Vet Res 2009; 70: 1465–1470. [DOI] [PubMed] [Google Scholar]
- 151. Brondani JT, Luna SP, Beier SL, et al. Analgesic efficacy of perioperative use of vedaprofen, tramadol or their combination in cats undergoing ovariohysterectomy. J Feline Med Surg 2009; 11: 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Brondani JT, Luna SP, Marcello GC, et al. Perioperative administration of vedaprofen, tramadol or their combination does not interfere with platelet aggregation, bleeding time and biochemical variables in cats. J Feline Med Surg 2009; 11: 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Teppema LJ, Nieuwenhuijs D, Olievier CN, et al. Respiratory depression by tramadol in the cat: involvement of opioid receptors. Anesthesiology 2003; 98: 420–427. [DOI] [PubMed] [Google Scholar]
- 154. Lemke KA. Anticholinergics and sedatives. In: Lumb and Jones’ veterinary anaesthesia and analgesia. Tranquilli WJ, Turmon JC, Grimm KA. (eds). 4th ed. Ames, IA: Blackwell Publishing, pp; 203–239. [Google Scholar]
- 155. Kerr C. Pain management: systemic analgesics. In: Seymour C, Duke-Novakovski T. (eds). Manual of small animal anaesthesia and analgesia. 2nd ed. Gloucester: BSAVA, 2007, pp 89–103. [Google Scholar]
- 156. Flecknell PA, Liles JH, Wootton R. Reversal of fentanyl/fluanisone neuroleptanalgesia in the rabbit using mixed agonist/antagonist opioids. Lab Anim 1989; 23: 147–155. [DOI] [PubMed] [Google Scholar]
- 157. Hu C, Flecknell PA, Liles JH. Fentanyl and medetomidine anaesthesia in the rat and its reversal using atipamazole and either nalbuphine or butorphanol. Lab Anim 1992; 26: 15–22. [DOI] [PubMed] [Google Scholar]
- 158. Hedenqvist P, Roughan JV, Flecknell PA. Sufentanil and medetomidine anaesthesia in the rat and its reversal with atipamezole and butorphanol. Lab Anim 2000; 34: 244–251. [DOI] [PubMed] [Google Scholar]
- 159. McCrackin MA, Harvey RC, Sackman JE, et al. Butorphanol tartrate for partial reversal of oxymorphone-induced postoperative respiratory depression in the dog. Vet Surg 1994; 23: 67–74. [DOI] [PubMed] [Google Scholar]
- 160. La Vincente SF, White JM, Somogyi AA, et al. Enhanced buprenorphine analgesia with the addition of ultra-low-dose naloxone in healthy subjects. Clin Pharmacol Ther 2008; 83: 144–152. [DOI] [PubMed] [Google Scholar]
- 161. Slingsby LS, Murrell JC, Taylor PM. Buprenorphine in combination with naloxone at a ratio of 15:1 does not enhance antinociception from buprenorphine in healthy cats. Vet J 2012; 192: 523–524. [DOI] [PubMed] [Google Scholar]


