Abstract
Objectives
Anatomical and methodological detail is lacking regarding local anesthetic peripheral nerve block techniques for distal pelvic limb surgery in cats. The aim of this study was to develop, describe and test nerve block methods based on cadaveric dissections and dye injections.
Methods
Ten pairs of feline pelvic limbs (n = 20) were dissected and the tibial nerve (T n.), common fibular (peroneal) nerve (CF n., and its two branches, the superficial fibular [peroneal] nerve [SpF n.] and the deep fibular [peroneal] nerve [DpF n.]) and the saphenous nerve (Sa n.) were identified. Based on these dissections, a ‘distal crus block’ (selective blockade of the CF n., T n. and Sa n.) and a ‘distal pes block’ (selective blockade of the SpF n., DpF n., T n. and Sa n.) were developed for surgical procedures in two different regions of the distal pelvic limb. Techniques were tested using new methylene blue (NMB) dye injections in feline pelvic limbs (n = 12). Using a 25 G × 5/8 inch needle and 1 ml syringe, 0.1 ml/kg of NMB dye solution was injected at the site of the CF n., and 0.05 ml/kg was injected at the sites of the SpF n., DpF n., Sa n. and T n. The length and circumference (fully or partially stained) of each stained nerve were measured.
Results
Positive staining of nerves was observed in 12/12 limbs. The lengths stained for the CF n., DpF n., SpF n., Sa n. and T n. were 27.19 ± 7.13, 20.39 ± 5.57, 22.82 ± 7.13, 30.89 ± 6.99 and 25.16 ± 8.09 mm, respectively. The nerves were fully stained in 12, 12, 10, 11 and 11 out of 12 limbs, respectively.
Conclusions and relevance
These two, three-point injection methods may be an effective perioperative analgesia technique for feline distal pelvic limb procedures.
Introduction
Local and regional nerve blocks can be included in a multimodal anesthesia/analgesia plan to enhance and maximize perioperative pain control in animals undergoing painful procedures.1–3 Nerve blocks are commonly utilized preoperatively in an attempt to block nociceptive input, avoid central sensitization, allow a decrease in administration of general anesthesia agents and provide continued analgesia during the postanesthesia and early postoperative recovery.1,4,5 A successfully performed nerve block is one of the most effective means to manage pain;4,6 however, one of the limitations of local anesthetics is their relatively short duration of action compared with the postoperative analgesic period desired. 2 Non-steroidal anti-inflammatory drugs (NSAIDs), transdermal fentanyl patches and transmucosal buprenorphine are used for providing postoperative analgesia in the early at-home period in cats.2,7 There are concerns related to the side effects of NSAIDs, the potential for abuse and safety of fentanyl patches in the home environment, and the difficulty for owners to administer transmucosal buprenorphine effectively; therefore, all these pain management options have some limitations.2,8 Recently, an extended-release formulation of the local anesthetic bupivacaine (EXPAREL; Pacira Pharmaceuticals) became available for humans,9,10 and its counterpart (NOCITA; Aratana Therapeutics) was recently approved for use in dogs.11,12 This drug, if accurately injected over pertinent nerves, may be of significant use in bridging the pain management gap in cats between in-hospital and at-home recovery periods. However, thus far, to our knowledge, this liposome-encapsulated bupivacaine has not been clinically evaluated for nerve blocks in dogs or cats.
Trauma, neoplasia and infected nail beds are reasons for surgical intervention in the distal pelvic limb of cats. The surgical procedures that can be considered include digit amputation, biopsy and fracture repair. For mid-to-distal pelvic limb surgery, femoral and sciatic nerve blocks using ultrasound or nerve-stimulation guidance, has been recommended in cats.13–16 Performing these nerve blocks requires access to certain equipment and a level of familiarity with using the equipment, which may not be routine in primary care practices. Additionally, complications of proximal limb nerve blocks, such as infection, hematoma and nerve injury may be considered more problematic than similar complications in a more peripheral location. 17 Specifically, local anesthesia of the distal pelvic limb can be achieved by selective blockade of the tibial nerve (T n.), common fibular (peroneal) nerve (CF n.) (or its two branches, the superficial fibular [peroneal] nerve [SpF n.] and the deep fibular [peroneal] nerve [DpF n.]) and the saphenous nerve (Sa n.).17,18 Selective blockade of these nerves are commonly used local anesthetic techniques for foot and ankle surgery in humans, and they have been shown to be safe and effective for these surgeries.19,20 Comparable approaches for these nerves in cats have also been previously described, but there is a lack of anatomical detail and specific direction for performing the blocks as accurately as possible.17,18 Similar to our previous study of the feline distal thoracic limb nerve blocks, 21 the aims of this study were to review the anatomical location of the relevant pelvic limb nerves and identify clearly definable injection sites that when utilized may provide a comprehensive blockade of the feline distal pelvic limb. An additional aim of this study was to test the defined sites using volumetrically relevant injections of dye in cadaver limbs.
Materials and methods
Cadaver dissections
Ten pairs of pelvic limbs were obtained from feral domestic shorthair cats (three intact males and seven intact females), that had been euthanized with an intravenous overdose of pentobarbital (Fatal Plus solution; Vortech Pharmaceuticals) for reasons unrelated to this study. Mean ± SD body weight for the 10 cats was 3.1 ± 0.2 kg. The relative maturity of euthanized animals was estimated by evaluating the appearance of the degree of closure of the distal femoral physis on a lateral radiograph and all cats were considered to be of mature age. The pelvic limbs and pelvis were disarticulated as a unit at the level of the L5–L6 intervertebral space and stored immediately at −18°C, then thawed at room temperature overnight prior to use. Anatomical dissection of the CF n., SpF n., DpF n., T n. and Sa n. from the level of the mid-thigh distally was performed to determine palpable landmarks, anatomical variations, fascial planes and associated structures along the nerve pathways. During dissection, potential injection points and landmarks that would guide injection were recorded and a similarly sized mature feline skeleton was frequently referred to for corroboration of palpable bony prominences.
Certain bone structures and the musculotendinous junction of the common calcanean tendon were established as reference landmarks because they are relatively fixed and easily palpable in the live animal. The location of each putative injection point was recorded using measurements taken relative to the selected anatomical landmarks in the transverse and sagittal planes by the same person (ME) (see ‘Measurements’ and Figure 1), and simultaneously the descriptive relationship to anatomical landmarks was recorded. Based on these findings, subcutaneous perineural injection techniques were developed for the CF n., SpF n., DpF n., T n. and Sa n.
Figure 1.
Description of the measured variables used to evaluate the proposed injection sites for distal limb nerves. Lateral view (A): the distance from the fibular head (Fh) to the common fibular nerve (CF n.); (B) the distance from Fh to the lateral malleolus (Lm). Cranial/dorsal view (D): the distance from the central tarsal bone (Ctb) to the deep fibular nerve (DpF n.); (E) the width of the tarsus at the level of Ctb; (F) the length from the medial malleolus of the tibia (Mm) to the first metatarsal bone (Mt 1); (G) the length from the Ctb to the Mt 1; (J): the distance from the Ctb to the superficial fibular nerve (SpF n.). Medial view (L): the distance from the musculotendinous junction (mtj) to the tibial nerve (T n.); (M): the distance from the medial surface of the tuber calcanei (TC) to mtj; (N): the distance from Mm to the medial condyle of the tibia (Tmc); (P): the distance from Mm to where the saphenous nerve (Sa n.) passed across the center point of the shaft of the tibia. MSa v. crbr = medial saphenous vein cranial branch
Dye studies
Based on the results of the cadaver dissection study, two independent perineural, three-point injection techniques were developed for ‘blockade’ of the CF n., SpF n., DpF n., T n. and Sa n. Method one (named the ‘distal crus block’) was developed with the intention of providing analgesia of the limb from approximately the distal third of the crus and distally by selective blockade of the CF n., T n. and Sa n. Method two (named the ‘distal pes block’) was developed with the intention of providing analgesia of the limb distal to the tarsus by selective blockade of the SpF n., DpF n., T n. and Sa n. Twelve additional pelvic limbs from six feral cats (one intact male and five intact females) that were euthanized for reasons unrelated to this study were used. The relative maturity of euthanized animals was estimated using the same radiographic survey method as already described, and all cats were considered to be of mature age. Mean ± SD body weight was 3.20 ± 0.59 kg, and individual body weights were used to calculate the volume of 0.5% bupivacaine that would be used in a dose of 1 mg/kg per limb. Using a 25 G × 5/8 inch needle and a 1 ml syringe, an equivalent volume (0.2 ml/kg per limb) of new methylene blue (NMB [New Methylene Blue Stain; Jorgensen]) was distributed at the nerve block sites in the following volumes: 0.1 ml/kg for the CF n., 0.05 ml/kg for the T n. and 0.05 ml/kg for the Sa n. For the distal pes block the 0.1 ml/kg allocated to the CF n. was divided into 0.05 ml/kg for the SpF n. and 0.05 ml/kg for the DpF n. Approximately 10 mins after the injections, dissection was performed to expose each nerve. The length of the stained portion of each nerve was measured as previously described.21,22 The degree of circumferential staining of the nerve (fully or partially stained) was visually assessed. The proximity of dye to unstained or poorly stained nerves and any adjacent structures stained were noted.
Measurements
All measurements were made using Dial Calipers (Dial caliper 505-633-50; Mitutoyo). To minimize error due to limb positioning, all measurements were made with the tarsus extended at 145° for the CF n., SpF n. and DpF n., and so that the long digital extensor tendon could be easily visualized and palpated. Measurements of the T n. and Sa n. were made with the limb lying on its lateral surface and the tarsus positioned at 90° flexion, and this allowed ready palpation of the musculotendinous junction of the common calcanean tendon. The measurements made are detailed in Figure 1. The location where the CF n. passes over the fibula was established as the site of injection of the CF n., in order to block this nerve before it divided into its branches. The distance from the fibular head to the CF n. (Figure 1, A – lateral view) and the distance from the fibular head to the lateral malleolus (Figure 1, B – lateral view) were measured. The ratio of A/B (Table 1 , C) was calculated. The preferred injection site for the SpF n. and the DpF n. was determined from dissection to be slightly distal to the tarsocrural joint on the dorsal aspect because at this location the nerves are not covered with a retinaculum and run relatively superficial. The distance from the central tarsal bone to the DpF n. (Figure 1, D – dorsal view), the width of the tarsus at the level of the central tarsal bone (Figure 1, E – dorsal view), the length from the medial malleolus of the tibia to the first metatarsal bone (Figure 1, F – dorsal view) and the length from the central tarsal bone to the first metatarsal bone (Figure 1, G – dorsal view) were measured. The ratios of D/E (Table 1, H) and G/F (Table 1, I) were calculated. The distance from the central tarsal bone to the SpF n. (Figure 1, J – dorsal view) was measured and the ratio of J/E (Table 1, K) was calculated. In the distal-third of the medial crus the T n. and Sa n. pass relatively close together in a similar sagittal plane, and this location was determined to be an optimal injection site. During the dissection, the musculotendinous junction of the common calcanean tendon was established as a palpable anatomical landmark for guiding the blockade of the T n. and Sa n. The distance from the musculotendinous junction to the T n. (Figure 1, L – medial view), the distance from the medial surface of the tuber calcanei to the musculotendinous junction (Figure 1, M – medial view) and the distance from the medial malleolus to the medial condyle of the tibia (Figure 1, N – medial view) were measured. The ratio of M/N (Table 1, O) was calculated. The distance from the medial malleolus to where the Sa n. passed across the center point of the shaft of the tibia (Figure 1, P – medial view) was measured and the ratio of P/N (Table 1, Q) was calculated. Measurements of nerve location were made using the center of the nerve.
Table 1.
Measured and calculated variables for the location of proposed injection sites for distal limb nerves (n = 20 limbs)
| Mean | Maximum | Minimum | Median | SD | CC | |
|---|---|---|---|---|---|---|
| Common fibular nerve | ||||||
| (A) Distance from the fibular head (mm) | 9.27 | 12.30 | 5.30 | 8.65 | 1.94 | 0.42* |
| (B) Distance from the fibular head to the lateral malleolus (mm) | 93.30 | 97.50 | 88.90 | 93.85 | 2.92 | 0.43 |
| (C) Length % (the ratio of A/B expressed as a %) | 9.93 | 13.23 | 5.68 | 9.15 | 2.05 | 0.41 |
| Deep fibular nerve | ||||||
| (D) Distance from the central tarsal bone (mm) | 7.40 | 8.90 | 6.00 | 7.30 | 0.66 | 0.58* |
| (E) Width of the tarsus at the level of the central tarsal bone (mm) | 16.89 | 18.30 | 15.70 | 16.80 | 0.84 | 0.25 |
| (F) Length from the medial malleolus to the first metatarsal bone (mm) | 24.28 | 26.60 | 21.50 | 24.35 | 1.63 | −0.06 |
| (G) Length from the central tarsal bone to the first metatarsal bone (mm) | 12.57 | 13.65 | 11.50 | 12.60 | 0.69 | −0.37 |
| (H) Width % (the ratio of D/E expressed as a %) | 43.86 | 51.25 | 37.74 | 43.81 | 3.82 | 0.44* |
| (I) Width % (the ratio of G/F expressed as a %) | 51.84 | 55.41 | 47.06 | 51.86 | 2.42 | −0.35 |
| Superficial fibular nerve | ||||||
| (J) Distance from the central tarsal bone (mm) | 8.78 | 10.30 | 7.70 | 8.75 | 0.54 | −0.25 |
| (K) Width % (the ratio of J/E expressed as a %) | 52.09 | 63.98 | 46.93 | 51.62 | 4.03 | −0.37 |
| Tibial nerve | ||||||
| (L) Distance from the musculotendinous junction (mm) | 6.74 | 8.30 | 4.60 | 7.00 | 0.98 | 0.04 |
| (M) Distance from the tuber calcanei to the musculotendinous junction (mm) | 28.93 | 32.20 | 26.60 | 28.30 | 1.91 | 0.24 |
| (N) Distance from the medial malleolus to the medial condyle of the tibia (mm) | 104.52 | 111.40 | 98.40 | 104.80 | 3.42 | 0.16 |
| (O) Length % (the ratio of M/N expressed as a %) | 27.69 | 31.02 | 25.48 | 27.02 | 1.83 | 0.16 |
| Saphenous nerve | ||||||
| (P) Distance from medial malleolus (mm) | 34.20 | 41.30 | 24.30 | 35.25 | 4.57 | 0.10 |
| (Q) Length % (the ratio of P/N expressed as a %) | 32.75 | 39.84 | 22.56 | 32.83 | 4.53 | 0.08 |
Significant correlation to body weight (P <0.05)
CC = correlation coefficient
Injection technique
Each limb was clipped from just proximal to the stifle, distally to the level of the proximal metatarsus. The blocks were performed as described below and illustrated in Figure 2 (a–c). To perform the ‘distal crus block’ the CF n., T n. and Sa n. should be blocked. To perform the ‘distal pes block’ the SpF n., DpF n., T n. and Sa n. should be blocked.
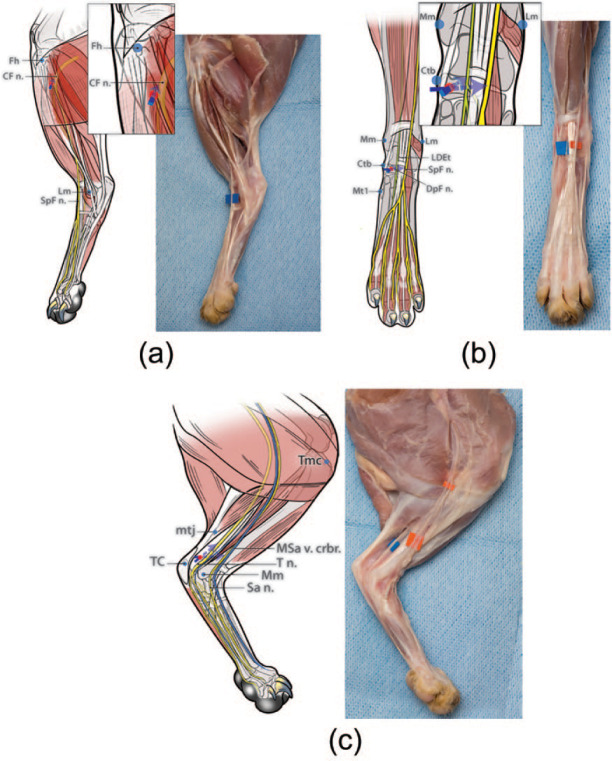
Figure 2.
Diagrams illustrating the developed and tested injection technique for each nerve. To perform the ‘distal crus block’ the common fibular nerve (CF n.), tibial nerve (T n.) and saphenous nerve (Sa n.) should be blocked. To perform the ‘distal pes block’ the superficial fibular nerve (SpF n.), deep fibular nerve (DpF n.), T n. and Sa n. should be blocked. (a) CF n. (for distal crus block): the fibular head (Fh) is readily identified by palpating the fibular shaft from a distal to proximal direction. The injection site is identified on the shaft of the fibula approximately 10 mm distal to the Fh (ie, about 10% of the distance from the Fh to the lateral malleolus [Lm]). A 25 G × 5/8 inch needle is gently inserted at this location, perpendicular to the lateral aspect of the limb until the tip of the needle contacted the shaft of the fibula, only a few mm deep to the skin. The needle is then withdrawn slightly and the injection performed (0.1 ml/kg). (b) SpF n. and DpF n. (for distal pes block): the injection target for the SpF n. and DpF n. is identified on a transverse plane level with the palpable medial surface of the central tarsal bone (Ctb) (ie, approximately at the midpoint between the medial malleolus of the tibia [Mm] and the base of the first metatarsal bone [Mt 1]) and approximately 9 mm from the medial surface of the Ctb towards the limb axis (ie, immediately adjacent to the palpable long digital extensor tendon [LDEt]). A 25 G × 5/8 inch needle is passed subcutaneously, medially to laterally, inserted at the medial surface of the Ctb, and with the bevel facing upwards. The needle is advanced until its tip is located superficial to the dorsal surface of the LDEt and then the injection is performed (0.05 ml/kg). Then, the needle is withdrawn a few mm and redirected deeply in the same transverse plane towards the medial edge of the LDEt (ie, from a dorsomedial direction) to block the DpF n. The needle is advanced a few mm until its tip is located deep to the medial edge of LDEt and then the injection is performed (0.05 ml/kg). (c) T n. and Sa n. (for distal crus block and distal pes block): the injection site for the T n. is identified at approximately 30 mm proximal to the medial surface of the tuber calcanei (TC) (ie, at the musculotendinous junction [mtj] of the common calcanean tendon) and 7 mm cranial to the mtj (ie, in the fossa bound cranially by the distal caudal surface of the tibia and caudally by the mtj of the common calcanean tendon). A 25 G × 5/8 inch needle, with the bevel facing up, is inserted medially, subcutaneously from a distal to proximal direction in the fossa until the tip of the needle is at the same level as the mtj of the common calcanean tendon. Then the injection is performed (0.05 ml/kg). To block the Sa n. using the same needle insertion point, the needle is redirected cranially towards the tibia (ie, towards the medial saphenous vein cranial branch [MSa v. crbr.] passing over the medial shaft of the tibia). The needle is advanced until its tip is located over the center of the tibial shaft and then the injection is performed (0.05 ml/kg). Red dots indicate the site of needle insertion. The blue arrows represent the direction of needle insertion to reach the desired injection site
CF n. (for distal crus block)
The limb was positioned with the tarsus in 145° extension and the lateral surface facing upwards. The fibular head was readily identified by palpating the fibular shaft from a distal to proximal direction. The injection site was identified on the shaft of the fibula approximately 10 mm distal to the fibular head (ie, about 10% of the distance from the fibular head to the lateral malleolus). A 25 G × 5/8 inch needle was gently inserted at this location, perpendicular to the lateral aspect of the limb until the tip of the needle contacted the shaft of the fibula, only a few mm deep to the skin. The needle was then withdrawn slightly and 0.1 ml/kg NMB injected.
SpF n. and DpF n. (for distal pes block)
The limb was positioned with the dorsal surface facing upwards and the tarsus at 145° extension. An injection target for the SpF n. and DpF n. was identified on a transverse plane level with the palpable medial surface of the central tarsal bone (ie, approximately at the midpoint between the medial malleolus of the tibia and the base of the first metatarsal bone) and approximately 9 mm from the medial surface of the central tarsal bone towards the limb axis (ie, immediately adjacent to the palpable long digital extensor tendon). A 25 G × 5/8 inch needle was passed subcutaneously, medially to laterally, inserted at the medial surface of the central tarsal bone, and with the bevel facing upwards. The needle was advanced until its tip was located superficial to the dorsal surface of the long digital extensor tendon and 0.05 ml/kg of NMB was injected. Then, the needle was withdrawn a few mm and redirected deeply in the same transverse plane towards the medial edge of the long digital extensor tendon (ie, from a dorsomedial direction) to block the DpF n. The needle was advanced a few mm until its tip was located deep to the medial edge of the long digital extensor tendon and 0.05 ml/kg NMB injected.
T n. and Sa n. (for distal crus block and distal pes block)
The limb was positioned with the medial surface facing upwards (ie, lying on its lateral surface) and the tarsus in 90° of flexion. The injection site for the T n. was identified at approximately 30 mm proximal to the medial surface of the tuber calcanei (ie, at the musculotendinous junction of the common calcanean tendon) and 7 mm cranial to the musculotendinous junction (ie, in the fossa bound cranially by the distal caudal surface of the tibia and caudally by the musculotendinous junction of the common calcanean tendon). A 25 G × 5/8 inch needle, with bevel facing up, was inserted and advanced subcutaneously from a distal to proximal direction in the fossa so that the tip of the needle was located at the same level as the musculotendinous junction of the common calcanean tendon. NMB, 0.05 ml/kg, was subcutaneously injected. To block the Sa n. using the same needle insertion point, with the limb maintained in position, the needle was redirected towards the tibia in a cranial direction (ie, towards the cranial branch of the medial saphenous vein passing over the medial shaft of the tibia). The needle was advanced until its tip was located over the center of the tibial shaft and then 0.05 ml/kg of NMB was subcutaneously injected.
Statistics
Statistical analyses were performed using JMP software (JMP Pro 12; SAS). Wilcoxon’s signed rank tests were used to evaluate pairwise differences in measurements A through Q between left and right limbs and to compare body weights between cadaver dissection and dye injection parts of the study. Correlations between body weight and measurements A through Q were determined using Spearman’s rank correlation coefficient. Differences were considered significant at P <0.05.
Results
Cadaver dissections
There were no significant differences between the right limb and left limb for any measurements (A–Q). The mean, SD, median, minimum and maximum of the measured and calculated variables for the 20 limbs are shown in Table 1. The correlation coefficient of the measured or calculated variable with body weight is given for each variable. There were significant positive correlations between body weight and A, D and H. There was no significant relationship between body weight and the measurements B, C, E, F, G, I, J, K, L, M, N, O, P and Q.
Dye test
There was no significant difference in body weight between the cats in the dissection study and the cats in the dye study.
The length stained and the degree of circumferential staining of each nerve are recorded in Table 2. Positive dye staining of all nerves was observed in 12/12 limbs. When targeting the CF n., the dye was deposited deep to the biceps femoris muscle, and stained the nerve well in all cases. When targeting the DpF n., the dye was located deep to the long digital extensor tendon, and stained the nerve well in all cases. The SpF n. was partially stained in two limbs. In these cases, the dye appeared to spread mainly into the connective tissue between the SpF n. and the long digital extensor tendon, or deep to the long digital extensor tendon, owing to the injection being made too deeply. This resulted in only the plantar aspect of the nerve being stained well. When targeting the T n., the dye accumulated in the connective tissue lateral to the T n. in 1/12 limbs, because the tip of the needle was inserted too deeply. This resulted in patchy staining of the nerve. When targeting the Sa n., in 1/12 limbs, the tip of the needle was located too caudally and deeply, which resulted in patchy staining of the Sa n. Excluding the two incidences of patchy staining, overall it was determined that nerves targeted were stained well and consistently with these proposed distal crus and distal pes injection methods (Figure 2a–c).
Table 2.
Degree of new methylene blue distribution on the surface of each nerve (n = 12)
| Nerves | Length of nerve stained (mm) |
Circumference fully stained (number of nerves) | ||||
|---|---|---|---|---|---|---|
| Mean | Maximum | Minimum | Median | SD | ||
| Common fibular nerve | 27.19 | 37.20 | 12.60 | 27.75 | 7.13 | 12 |
| Deep fibular nerve | 20.39 | 29.20 | 11.30 | 21.25 | 5.57 | 12 |
| Superficial fibular nerve | 22.82 | 35.80 | 11.30 | 22.85 | 7.13 | 10 |
| Tibial nerve | 30.89 | 44.00 | 23.20 | 29.50 | 6.99 | 11 |
| Saphenous nerve | 25.16 | 45.80 | 12.20 | 24.40 | 8.09 | 11 |
Discussion
In this study we describe the anatomical location of certain pelvic limb nerves in relation to externally palpable landmarks and propose techniques to perform analgesia of the distal limb, from the level of the distal crus (‘distal crus block’) and from the level of the tarsus (‘distal pes block’). In the dye injection study using these injection techniques, all tested nerves targeted for the regional analgesia of the feline distal pelvic limb were successfully stained with NMB. This detailed description of block methods and associated diagrams, we believe, will allow veterinarians to perform a successful nerve blockade of the feline distal pelvic limb. We propose the ‘distal crus block’ be used for procedures of the distal tibia and tarsal region and the ‘distal pes block’ be used for procedures of the metatarsus and phalanges. However, whether or not these described techniques result in a successful nerve blockade needs to be clinically tested.
Peripheral nerve blocks are ideal for pre-emptive analgesia as they inhibit the transmission of nociceptive signals by reversibly blocking voltage-dependent sodium channels.23,24 Additionally, this should help avoid cellular wind up and subsequent central sensitization, two processes that appear to contribute to postoperative pain in clinical cases. 25 Local anesthetics have been used as part of multimodal analgesia for perioperative pain management in cats. 3 A recent report showed that the use of pelvic limb peripheral nerve blocks (lumbar plexus and sciatic nerve) in cats resulted in lower intraoperative requirement of antinociceptive drugs and there were no neurological complications. 4 Although these proximal limb blocks desensitize the majority of the limb, they are particularly utilized for analgesia of the thigh, stifle and crus. 4 Compared with more distally placed nerve blocks, we suggest these proximal blocks may be considered more technically difficult to perform, requiring specialized equipment for accurate drug deposition (ultrasound or peripheral nerve stimulator guidance), and are more invasive based on depth of needle placement.14–16 In our study we chose to describe peripheral nerve blocks to achieve analgesia specifically of the distal pelvic limb, given this region of the limb is not infrequently the location of painful conditions and procedures. These relatively distal peripheral nerve blocks can also be considered technically easy to accomplish and do not require specialized equipment to perform accurately.
Clinical research studies are needed to determine how limb use may be affected by the described blocks and use of different duration local anesthetics, particularly with regard to the CF n. block, which may be anticipated to also disrupt motor innervation to the craniolateral crus muscles. In current practice there is little clinical concern for negative ambulatory side effects in hospitalized cats. However, in the theoretical scenario of a cat receiving an extended-release local anesthetic for pain relief to the distal pelvic limb and being discharged from hospital with this drug still in effect, the potential for ambulatory dysfunction in the home environment is a possibility.
Bupivacaine is known to cause cardiac and neurological side effects, and its intravascular injection should be avoided in cats. 2 There is a possibility of entering a vessel for most nerve blocks and in our study, the dorsal pedal artery for the DpF n. block, the caudal branch of the lateral saphenous vein for the T n. block, and the cranial branch of the medial saphenous vein and cranial branch of the saphenous artery for the Sa n. block are closely related to our injection sites. Although we did not detect any vascular trauma or intravascular injection, precautions should be taken to avoid this, including syringe aspiration to confirm an absence of intravascular needle position before injection is performed. From our observations during dissections, we believe that the highest risk for intravascular injection is into the dorsal pedal artery, which courses in association with the DpF n. When targeting the DpF n., palpation of the dorsal pedal artery pulse may help identify its location and increase awareness of this vessel. Further, there is a possibility of intraneural injection of the CF n. if directly penetrated. Thus, the pressure achieved during injection should be considered. If the pressure feels excessive, the needle tip maybe in periosteum or it may be located intraneurally and the needle should be withdrawn 1–2 mm and the injection attempted again.
Our proposed injection techniques do not include the blockade of the distal caudal cutaneous sural nerve, which innervates the skin of the caudolateral crus and caudolateral tarsus. Descriptions of this nerve’s specific distribution in the cat are lacking in the literature. The focus of this study was on carefully defining the anatomy of accurate nerve block techniques for distal pelvic limb analgesia with regard to the nerves routinely described in the literature to be blocked. For our described distal pes block the distribution of the distal caudal cutaneous sural nerve was not considered to be in the field of interest. If, however, the caudolateral surface of the distal crus and tarsus is to be included in a surgical field then blockade of the distal caudal cutaneous sural nerve, as part of our described distal crus block, would be indicated, to include comprehensive cutaneous desensitization. The distal caudal cutaneous sural nerve passes in association with the lateral saphenous vein, over the caudolateral surface of the lateral head of the gastrocnemius muscle and through the fossa bound cranially by the distal caudal surface of the tibia and caudally by the common calcaneal tendon (see supplementary material). It was identifiable at the level of the musculotendinous junction of the common calcanean tendon. Although we did not specifically test this approach, we suggest the distal caudal cutaneous sural nerve would be readily blocked by the subcutaneous injection of local anesthetic using the same method described for the T n. but from a lateral approach and with a small volume of bupivacaine, such as 0.01ml/kg. Alternatively, and perhaps more practically in many instances, subcutaneous infiltration of local anesthetic at the skin incision may provide sufficient postoperative pain management to cover the in-hospital period.
Staining of ⩾2 cm along the nerve was considered sufficient to produce a clinically effective neural blockade in a frog sciatic nerve study. 26 In another study, nerve staining >4 cm was considered excessive and a target of 2–4 cm was aimed for. 27 In our study, most of the nerves were stained over a length of 2–4 cm and we believe the volume we used for dye testing should be appropriate for clinical application. However, as some individual nerves were stained less than the length considered sufficient for an effective block, this might result in a partial block of the nerve. In vivo studies, such as have been performed in dogs recently, 22 should be performed to test our proposed block methods and volumes used.
Additional limitations of this study, similar to our previous report, 21 include the use of cadavers and the use of NMB stain for injections. The distribution of injectate in a clinical patient may be different than that in cadavers because of the possible uptake of the local anesthetic solution by the lymph and blood vessels. 28 We used NMB as a substitute for local anesthetic solution, but NMB distribution may not reflect the action of local anesthetics in vivo, where the action on the nerve fiber depends on several factors, including drug lipid solubility, tissue pH and molecular weight. 29 Another possible limitation is that the relatively narrow range of body weights of the cadavers may affect the interpretation of any correlation between body weight and the various measurements of bone landmarks and nerve locations.
Conclusions
All selected nerves targeted for the regional analgesia of the feline distal pelvic limb were successfully stained with NMB using the described injection techniques. To assess the utility and effectiveness of these nerve block injection techniques, they should be evaluated in clinical patients.
Supplemental Material
An image of the location of the caudal cutaneous sural nerve
Acknowledgments
We are grateful to Alice MacGregor Harvey for the medical illustrations, and to Nathan Latil for the photography. Figure illustrations are courtesy of NC State University.
Footnotes
Supplementary material: An image of the location of the caudal cutaneous sural nerve.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Internally funded by the Comparative Pain Research Laboratory.
Accepted: 6 December 2016
References
- 1. Lascelles D, Waterman A. Analgesia in cats. In Pract 1997; 19: 203–213. [Google Scholar]
- 2. Robertson SA, Taylor PM. Pain management in cats – past, present and future. Part 2. Treatment of pain – clinical pharmacology. J Feline Med Surg 2004; 6: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steagall PV, Monteiro-Steagall BP. Multimodal analgesia for perioperative pain in three cats. J Feline Med Surg 2013; 15: 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vettorato E, Corletto F. Retrospective assessment of peripheral nerve block techniques used in cats undergoing hindlimb orthopaedic surgery. J Feline Med Surg 2016; 18: 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Melzack R, Coderre TJ, Katz J, et al. Central neuroplasticity and pathological pain. Ann N Y Acad Sci 2001; 933: 157–174. [DOI] [PubMed] [Google Scholar]
- 6. Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg 2006; 102: 248–257. [DOI] [PubMed] [Google Scholar]
- 7. Hunt JR, Knowles TG, Lascelles BD, et al. Prescription of perioperative analgesics by UK small animal veterinary surgeons in 2013. Vet Rec 2015; 176: 493. [DOI] [PubMed] [Google Scholar]
- 8. Paparella SF. A serious threat to patient safety: the unintended misuse of fentanyl patches. J Emerg Nurs 2013; 39: 245–247. [DOI] [PubMed] [Google Scholar]
- 9. Portillo J, Kamar N, Melibary S, et al. Safety of liposome extended-release bupivacaine for postoperative pain control. Front Pharmacol 2014; 5: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lonner J. Role of liposomal bupivacaine in pain management after total joint arthroplasty. J Surg Orthop Adv 2014; 23: 37–41. [DOI] [PubMed] [Google Scholar]
- 11. Lascelles BD, Rausch-Derra LC, Wofford JA, et al. Pilot, randomized, placebo-controlled clinical field study to evaluate the effectiveness of bupivacaine liposome injectable suspension for the provision of post-surgical analgesia in dogs undergoing stifle surgery. BMC Vet Res 2016; 12: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lascelles BDX, Kirkby Shaw K. An extended release local anaesthetic: potential for future use in veterinary surgical patients? Vet Med Sci 2016; 2: 229–238. DOI: 10.1002/vms3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haro P, Laredo F, Gil F, et al. Ultrasound-guided block of the feline sciatic nerve. J Feline Med Surg 2012; 14: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gurney MA, Leece EA. Analgesia for pelvic limb surgery. A review of peripheral nerve blocks and the extradural technique. Vet Anaesth Analg 2014; 41: 445–458. [DOI] [PubMed] [Google Scholar]
- 15. Haro P, Laredo F, Gil F, et al. Validation of the dorsal approach for the blockade of the femoral nerve using ultrasound and nerve electrolocation in cats. J Feline Med Surg 2016; 18: 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haro P, Laredo F, Gil F, et al. Ultrasound-guided dorsal approach for femoral nerve blockade in cats: an imaging study. J Feline Med Surg 2013; 15: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lemke KA, Dawson SD. Local and regional anesthesia. Vet Clin North Am Small Anim Pract 2000; 30: 839–857. [DOI] [PubMed] [Google Scholar]
- 18. Skarda RT, Tranquilli WJ. Local and regional anesthetic and analgesic techniques: cats. In: Tranquilli WJ, Thurmon JC, Grimm KA. (eds). Lumb & Jones’ veterinary anesthesia and analgesia. 4th ed. Ames, IA: Blackwell Publishing, 2007, pp 595–604. [Google Scholar]
- 19. Pearce CJ, Hamilton PD. Current concepts review: regional anesthesia for foot and ankle surgery. Foot Ankle Int 2010; 31: 732–739. [DOI] [PubMed] [Google Scholar]
- 20. Migues A, Slullitel G, Vescovo A, et al. Peripheral foot blockade versus popliteal fossa nerve block: a prospective randomized trial in 51 patients. J Foot Ankle Surg 2005; 44: 354–357. [DOI] [PubMed] [Google Scholar]
- 21. Enomoto M, Lascelles BD, Gerard MP. Defining the local nerve blocks for feline distal thoracic limb surgery: a cadaveric study. J Feline Med Surg 2016; 18: 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trumpatori BJ, Carter JE, Hash J, et al. Evaluation of a midhumeral block of the radial, ulnar, musculocutaneous and median (RUMM block) nerves for analgesia of the distal aspect of the thoracic limb in dogs. Vet Surg 2010; 39: 785–796. [DOI] [PubMed] [Google Scholar]
- 23. Buback JL, Boothe HW, Carroll GL, et al. Comparison of three methods for relief of pain after ear canal ablation in dogs. Vet Surg 1996; 25: 380–385. [DOI] [PubMed] [Google Scholar]
- 24. Naja ZM, El-Rajab M, Ziade F, et al. Preoperative vs postoperative bilateral paravertebral blocks for laparoscopic cholecystectomy: a prospective randomized clinical trial. Pain Pract 2011; 11: 509–515. [DOI] [PubMed] [Google Scholar]
- 25. Lascelles BD, Cripps PJ, Jones A, et al. Post-operative central hypersensitivity and pain: the pre-emptive value of pethidine for ovariohysterectomy. Pain 1997; 73: 461–471. [DOI] [PubMed] [Google Scholar]
- 26. Raymond SA, Steffensen SC, Gugino LD, et al. The role of length of nerve exposed to local anesthetics in impulse blocking action. Anesth Analg 1989; 68: 563–570. [PubMed] [Google Scholar]
- 27. Campoy L, Martin-Flores M, Looney AL, et al. Distribution of a lidocaine-methylene blue solution staining in brachial plexus, lumbar plexus and sciatic nerve blocks in the dog. Vet Anaesth Analg 2008; 35: 348–354. [DOI] [PubMed] [Google Scholar]
- 28. Kull K, Baer GA, Samarutel J, et al. Distribution of local anesthetic solution in retromediastinal block. Preliminary experimental results. Reg Anesth 1997; 22: 308–312. [PubMed] [Google Scholar]
- 29. Portela DA, Otero PE, Tarragona L, et al. Combined paravertebral plexus block and parasacral sciatic block in healthy dogs. Vet Anaesth Analg 2010; 37: 531–541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An image of the location of the caudal cutaneous sural nerve