Abstract
Objectives
The aim of the study was to investigate feline morbillivirus (FmoPV) frequency, phylogeny and associated pathology in cats in Istanbul, Turkey.
Methods
Samples from sick (n = 96) and dead (n = 15) cats were analysed using reverse transcription PCR. Blood and urine analyses and histopathology were also performed.
Results
FmoPV RNA was detected in six cats (5.4%), including three sick (in the urine) and three dead cats (tissues). A significantly greater proportion of FmoPV RNA-positive cats had street access compared with non-infected cats. Blood samples from the morbillivirus-positive cats were negative for morbillivirus RNA. Tubular parenchymal cells, lymphoid and plasma cells in kidney and hepatocytes, lymphoid and plasma cells in liver from dead cats were also positive by immunohistochemistry for the viral N protein. Two FmoPV-positive cats were also positive for feline coronavirus RNA and one cat for feline immunodeficiency virus RNA and feline leukaemia virus proviral DNA. Phylogenetic analysis of the six FmoPV-positive cats showed that the strains were grouped into cluster D and had high similarity (98.5–100%) with strains from Japan and Germany. In the three FmoPV RNA-positive sick cats, respiratory, urinary and digestive system signs were observed as well as weight loss, fever and depression in some cats. Similar clinical signs were also seen in the morbillivirus RNA-negative sick cats. FmoPV RNA-positive cats had lower median red blood cell count, haemoglobin, albumin, albumin/globulin and urobilinogen and higher alanine transaminase, alkaline phosphatase and bilirubin compared with non-infected cats. Significant histopathology of FmoPV RNA-positive dead cats included tubulointerstitial nephritis characterised by severe granular and vacuolar degeneration of the epithelial cells of the cortical and medullary tubules as well as mononuclear cell infiltrates. Widespread lymphoid cell infiltrates were detected in the renal cortex and medullary regions of the kidneys. Cellular infiltration, cholangiohepatitis and focal necrosis in the liver were also found. Although virus-infected cells were found in the kidney and liver of FmoRV RNA-positive cats, tubulointerstitial nephritis, cholangiohepatitis and focal necrosis seen in FmoRV RNA-positive cats were similar to those observed in FmoRV RNA-negative cats.
Conclusions and relevance
This is the first study to show the presence of FmoPV infection in cats in Turkey. Sick cats, particularly those with kidney disease, should be tested for this virus. The genotypes found in this study were similar to previously reported strains, indicating that circulating morbilliviruses in Turkey are conserved.
Introduction
Morbilliviruses cause significant infections in human and animals such as measles, peste des petit ruminants and canine distemper. Feline morbillivirus (FmoPV) has recently gained importance as there is increasing evidence of disease associations with tubular interstitial nephritis and chronic kidney disease in cats.1,2 The morbilliviruses are enveloped, negative-sense non-segmented single-stranded RNA viruses classified in the family of Paramyxoviridae. The genome size of FmoPV is 16,050 bases and encodes both non-structural and structural proteins (N, P/V/C, M, F, H and L). 2 L protein gene is widely used in molecular diagnosis because it has the most sequence conservation in the genome.2,3
FmoPV is a new morbillivirus in domestic cats that was first isolated in Hong Kong in 2012. 2 While morbilliviruses are distributed in other animals worldwide, FmoPV has only been detected in cats in Hong Kong, Japan, Italy, Germany and the USA,1–6 but no studies have been previously carried out in Turkey. Therefore, this study was performed to investigate the frequency, pathology and phylogeny of FmoPV in cats in Istanbul, Turkey.
Materials and methods
Study population and samples
Cats submitted to four different veterinary clinics located in Istanbul and to the Veterinary Faculty of Istanbul University, Turkey between October 2014 and September 2015 were analysed. A total of 110 cats were investigated including cats that had died (n = 15), unhealthy cats (n = 68) and healthy cats (n = 27). Dead cats were necropsied at the University of Istanbul and samples from the internal organs were taken for histopathological and virological analyses. All cats were clinically examined and sex, breed and age were recorded (Table 1). On clinical examination, fever, behavioural changes (insidious onset, depression) and clinical signs related to organ systems were recorded; specifically, urinary, circulatory (lymphadenopathy, anaemia, icterus), gastrointestinal (anorexia, weight loss, stomatitis, enteritis, abdominal distension, vomiting, ascites) and respiratory (pneumonia, dyspnoea). The unhealthy cats consisted of individuals with urinary tract, respiratory or gastrointestinal clinical signs and also fever, depression, dullness and/or weight loss. Blood and urine samples were collected from 95 unhealthy and healthy cats and sent immediately to the University of Istanbul in cold storage for FmoPV reverse transcription PCR (RT-PCR) analysis. In addition, serum samples were tested by rapid tests for the presence of antibodies to feline coronavirus (FCoV; Bionote, Antibody), feline immunodeficiency virus (FIV; Bionote, Antibody) and feline leukaemia virus antigen (FeLV; Bionote, Antigen) and analysed for haematological and biochemical changes.
Table 1.
Signalment, clinical signs and test results of all cats analysed in this study
| n | FMoPV PCR positivity |
FCoV-Ab positivity | Urinary signs | Residue in urine | Struvite | Lack of appetite | Diarrhoea | Fever | Depression | Respiratory signs | Weight loss | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 0–<1 | 25 | 1 | 15 | 4 | 7 | 1 | 3 | 4 | 3 | 3 | 2 | 0 |
| 1–3 | 52 | 1 | 31 | 8 | 11 | 2 | 4 | 3 | 4 | 6 | 0 | 8 | |
| 4–6 | 12 | 1 | 9 | 6 | 4 | 0 | 3 | 1 | 1 | 5 | 2 | 1 | |
| 7–9 | 9 | 1 | 5 | 3 | 4 | 5 | 1 | 2 | 1 | 2 | 3 | 2 | |
| 10+ | 12 | 2 | 8 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 4 | |
| Gender | Male | 55 | 4 | 43 | 16 | 16 | 6 | 8 | 7 | 6 | 8 | 6 | 10 |
| Female | 55 | 2 | 25 | 7 | 12 | 3 | 5 | 4 | 4 | 9 | 2 | 5 | |
| Breed | Cross | 72 | 6 | 41 | 11 | 15 | 1 | 7 | 7 | 8 | 12 | 7 | 11 |
| Pure | 38 | 0 | 27 | 12 | 13 | 8 | 6 | 4 | 2 | 5 | 1 | 4 | |
| Health status | Healthy | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unhealthy | 68 | 2 | 56 | 21 | 22 | 9 | 7 | 5 | 5 | 10 | 1 | 10 | |
| Dead | 15 | 4 | 12 | 2 | 6 | 0 | 6 | 6 | 5 | 7 | 7 | 5 | |
| Lifestyle | Household | 80 | 5 | 57 | 17 | 22 | 9 | 6 | 5 | 3 | 8 | 2 | 8 |
| Street | 30 | 1 | 11 | 6 | 6 | 0 | 7 | 6 | 7 | 9 | 6 | 7 |
FmoPV = feline morbillivirus; FCoV-Ab = feline coronavirus antibody
Haematological and biochemical analyses
All blood and urine samples were processed within 30 mins of extraction at the veterinary clinics. Blood samples were analysed for a complete blood haemogram-histogram (18 parameters) using a veterinary-specific Genius KT-6200 VET haematology auto-analyser using the blood analysing kit. Samples were also analysed using the kits supplied by Vet-Scan (Abaxis) (n = 29) and Reflotron (Roche) (n = 23) for comprehensive blood biochemistry (14 parameters). Haematological and biochemical tests included total white blood cell (WBC) count, lymphocyte and red blood cell (RBC) count, haematocrit (HCT) and haemoglobin (HGB) and total protein (TP), total bilirubin (TBIL), albumin (ALB), globulin (GLOB), glucose (GLU), creatinine (CRE), alkaline phosphatase (ALP), alanine transaminase (ALT), amylase (AMY), urea, blood urea nitrogen (BUN) as well as minerals such as Ca, P, Na and K.
Urine samples were analysed by DIRUI H 100 auto-analyser with DIRUI H10 and H11 kits. Urine analyses included urobilinogen, bilirubin, ketone, blood, protein, nitrite, leukocyte, glucose, specific gravity and pH. Microscopy for urine sediments was performed to correlate the auto-analyser findings and to differentiate the sediment cells and crystals where present. Microscopy of the urine sediment included leukocyte count, bacterial count and motile or non-motile differentiation, other microorganisms such as parasites, epithelial cell differentiation as transitional epithelials, renal tubular epithelials, crystals such as struvite, oxalate, calcium carbonate, cystine, tyrosine or calcium phosphate.
RNA extraction and cDNA synthesis
Total RNA was extracted from 95 urine samples from healthy and unhealthy cats, 15 kidney samples from dead cats and blood samples from the FmoPV-positive cats and cats living together with FmoPV-positive cats. The QIAamp Viral RNA mini kit (Qiagen), RNeasy mini kit (Qiagen) and RNA blood kit (Qiagen) were used for urine, tissue and blood samples, respectively, as described by the manufacturer. The amount of RNA was measured using a NanoDrop spectrophotometer (NanoDrop 1000c; Thermo Scientific). Approximately 600 ng of RNA was used for reverse transcription to make cDNA, as described previously. 7
Detection, sequencing and phylogenetic analysis of FmoPV
Commercially (Sentegen Technology, Turkey) synthesised primers targeting the L gene 3 were used for the nested RT-PCR. The first step consisted of a total volume of 25 µl containing 12.5 µl GoTaq Colorless Master Mix (Promega), 0.5 µl FmoPV-F1 primer (5 pMol/µl), 0.5 µl FmoPV-R1 primer (5 pMol/µl), 6.5 µl nuclease-free water and 5 µl cDNA. The mixture was placed in a thermal cycler (Biorad, Chromo-4) and the polymerase activated by incubation at 95°C for 2 mins. Cycling conditions were 95°C for 30 s, 53°C for 30 s, 72°C for 30 s for 35 cycles. For the second step, the same protocol was used but with FmoPV-F2 and FmoPV-R2 primers and an annealing temperature of 55°C. Nuclease-free water was used as negative control in place of template. After PCR, the presence of the 401 bp product was analysed by agarose gel (1.5%) electrophoresis.
In this study, after optimisation, a positive control in a room separated from the test samples was used in order to avoid contamination. As negative control, RNase-free water was added to a PCR reaction instead of template. In order to assure extraction efficiency, primers for the feline glyceraldehyde-3-phosphate dehydrogenase (G3PDH) gene were used.
All sequencing was performed by the Sanger dideoxy termination method in duplicate by a commercial company (Medsantek, Istanbul, Turkey). Multiple alignments of L gene sequences were made using Clustal W (Geneious 6.1). Phylogenetic analysis was carried out using neighbour-joining trees based on the genetic distance model of Tamura-Nei principle 8 and compared with L sequences of FmoPV strains and feline paramyxovirus (FPaV) strains deposited in GenBank.
Detection of FIV, FeLV, FCoV and feline panleukopenia in morbillivirus-positive cats by RT-PCR and real-time RT-PCR
All FmoPV RNA-positive samples were analysed by real-time RT-PCR and conventional PCR for the presence of FCoV RNA, 9 FIV RNA, 10 FeLV DNA 11 and feline parvovirus DNA. 12
Necropsy and histopathology
Systemic necropsy was performed on 15 dead cats and examined grossly at the Department of Pathology, University of Istanbul. Tissue samples were taken from all of the organs and fixed in 10% neutral buffered formalin. After routine processing they were embedded in paraffin. Sections (4–5 µm thickness) were cut and stained with haematoxylin and eosin, and additional sections of liver and kidney were stained with Congo Red. All sections were evaluated by light microscopy.
Immunohistochemistry
In order to detect FmoPV antigen in tissues, 5 μm thick formalin-fixed liver, kidney and lymph node tissues were used. Tissue sections were de-paraffinised and rehydrated. The sections were boiled twice in 0.01 M sodium citrate buffer (pH 6.0) in a microwave oven for 10 mins to unmask the epitopes. Endogenous peroxidase activity was blocked with 3% v/v H2O2 in methanol for 30 mins and the sections were blocked with normal rabbit serum/PBS solution at a dilution of 1:10 at room temperature for 1 h. After washing, slides were then incubated with 1:250 dilution of guinea pig anti-morbillivirus N protein serum overnight at 4°C (kindly provided by Kwok-Yung Yuen, Department of Microbiology, University of Hong Kong, Pok Fu Lam, Hong Kong). After washing, the SensiTek HRP (Anti-Polyvalent) (ready to use) (ScyTek Laboratories, UT) kit was applied according to the manufacturer’s instructions. As a chromogen, 3,3’- diaminobenzidine (DAB) substrate (Abcam 64238) was used to detect the immunoreactive sites. The sections were counter-stained with Mayer’s hematoxylin and mounted in Entellan for examination by light microscopy. Positive control sections were obtained from a cat with positive PCR results and gross lesions. For negative control, guinea pig sera without antibody to morbillivirus was used. Tissues from FmoPV RNA-negative cats were also analysed to compare with FmoPV-positive cat tissue.
Statistical analysis
The distributions of haematological and biochemical parameters were analysed and the proportion of cats with abnormal values was estimated. The non-parametric Kruskal–Wallis test was used to compare medians in FmoPV-infected and non-infected cats. Significance was taken as P <0.10.
Results
RT-PCR and real-time RT-PCR
In total, six of 110 cats (5.4%) were PCR positive for FmoPV RNA, including in three urine samples from live unhealthy cats and in three kidney samples from dead cats out of 110 analysed by RT-PCR (Table 1). The blood was taken from the FmoPV RNA-positive live cats immediately after detection of FmoPV RNA in the urine. However, FmoPV RNA was not amplified from the blood of any of the FmoPV RNA urine-positive cats. One positive cat died 6 months after sample submission to the veterinary clinic. Out of the 15 cats analysed by post mortem, four had detectable FmoPV RNA. One cat had FmoPV RNA in kidney, lung, spleen, intestine, liver and lymph node, one cat had FmoPV RNA in the kidney and lymph node and two cats had FmoPV RNA only in the kidney. When cats living with the morbillivirus PCR-positive cats that had died were analysed for FmoPV RNA, none were positive.
In addition, when all FmoPV RNA-positive cats (n = 6) were analysed for the presence of FCoV RNA, FIV RNA, FeLV DNA and feline parvovirus DNA, two cats were positive for FCoV RNA and one cat for FIV RNA and FeLV proviral DNA (Table 1).
Antibodies to FCoV, FIV and FeLV
Among the 95 live cats tested for FCoV, FIV and FeLV, 68 cats (71.5%) were found to be positive for antibodies to FCoV, and two cats (2.1%) for antibodies to FIV and for FeLV antigen. Among FmoPV RNA-positive cats, only two cats were found to be positive for antibodies to FCoV.
Signalment and clinical signs
FmoPV positivity was detected in all age groups ranging from 2 months to 15 years of age (Table 1). Four of the FmoPV RNA-positive cats were male and two were female. They were all mixed breed (Table 1). A significantly greater proportion of FmoPV RNA-positive cats had street access compared with non-infected ones.
Clinical examination of the live, unhealthy cats (n = 68) and post-mortemed cats (n = 15) prior to dying revealed urinary tract signs (renal disorders, residue in urine) in 28 (33.7%), respiratory signs in eight (9.6%), gastrointestinal signs (anorexia, diarrhoea, vomiting) in 11 (13.2%), depression or/and dullness in 17 (20.4%), fever in 10 (12%) and weight loss in 15 (18%) cats. In FmoPV RNA-positive cats, respiratory, urinary and digestive system signs were observed as well as weight loss, fever and depression in some cats (Table 2).
Table 2.
Signalment, clinical, haematological and urological findings of the three morbillivirus-positive live cats
| Signalment and clinical findings | Haematological findings | Urological findings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat 1* | Cat 2 | Cat 3 | Cat 1* | Cat 2 | Cat 3 | Cat 1* | Cat 2 | Cat 3 | |||
| Age (years) | 4 | 12 | 0.2 | White blood cells | H | N | H | Ketone | NP | NP | NP |
| Gender | M | F | F | Lymphocytic count | N | L | H | Blood | NP | NP | NP |
| Breed | Mix | Mix | Mix | Monocyte | N | N | H | Protein | H | NP | NP |
| Health status | Death | Unhealthy | Unhealthy | Granulocyte | H | H | H | Bilirubin | H | NP | NP |
| Household | No | No | Yes | Red blood cells | N | N | L | Nitrite | P | NP | NP |
| Renal disorder | No | No | No | Haemoglobin | N | N | L | Leukocyte | P | P | NP |
| Urological signs | Yes | No | Yes | Haematocrit | N | N | N | Glucose | P | NP | NP |
| Respiratory signs | Yes | No | No | Albumin | N | N | N | pH | 6 | 6 | 5.5 |
| Allergy | No | Yes | No | Globulin | H | N | N | Residue in urine | P | P | P |
| Anorexia | Yes | No | No | Alb/glob ratio | N | N | N | Microleukocyte | P | P | P |
| Diarrhoea | No | Yes | No | Total protein | H | N | N | Bacteria | P | P | P |
| Vomiting | No | Yes | No | BUN | N | N | N | Trans epithelial | P | N | P |
| Weight loss | Yes | No | No | Urea | H | H | N | Renal epithelia | NP | NP | NP |
| Fever | Yes | No | No | Creatinine | H | H | H | Struvite | NP | NP | NP |
| Depression | Yes | No | No | ALT | H | N | H | Oxalate | NP | P | NP |
| Hair loss, dullness | No | No | No | ALP | H | N | H | Cystine | NP | NP | NP |
| Dermatitis | No | No | No | Bilirubin | H | N | H | Tyrosine | NP | NP | NP |
| Stomatitis | No | No | No | Ca phosphate | NP | NP | NP | ||||
Cat 1 died later
M = male; F = female; H = high; L = low; N = normal; P = present; NP = not present; Alb/glob ratio: albumin:globulin ratio; BUN = blood urea nitrogen; ALT = alanine aminotransferase; ALP = alkaline phosphatase
Haematological and biochemical analyses
There were no statistical differences in the proportion of cats with haematological alterations. In live unhealthy cats, leukocytosis was seen in 10 cats, leukopenia in five cats, high RBC count in 12 cats, low RBC count in five cats, high HGB level in seven cats, low HGB level in 10 cats, high HCT level in six cats and low HCT level in 21 cats. In same group of cats, high ALT was seen in 10 cats, high ALP in six cats, high bilirubin level in seven cats, low ALB level in three cats, low globulin level in three cats, low BUN in four cats, high BUN in seven cats, low urea in one cat, high urea in five cats and high CRE level in two cats.
In the three FmoPV RNA-positive unhealthy cats, leukocytosis was seen in one cat, leukopenia in one cat, and low RBC and low HGB levels in one cat; HCT levels were normal in all three cats. In the post-mortemed cats, prior to dying, after clinical examination, high GLOB, total protein, urea, CRE, ALT, ALP and bilirubin were measured. In one cat (positive for morbillivirus RNA) before dying, high urea, CRE, ALT, ALP and bilirubin were detected (Table 2).
FmoPV RNA-positive cats had lower median RBCs, HGB, ALB, ALB/GLOB and urobilinogen and higher ALT, ALP and bilirubin compared with non-infected cats.
Urine analyses
In live unhealthy cats, there were macroscopic sediments in 31 cats, bilirubinuria in 11 cats, ketonuria in 15 cats, haematuria in 22 cats, proteinuria in 62 cats, nitrituria in nine cats, glucosuria in 10 cats, high specific gravity in 56 cats, acidic urine in four cats, basic urine in 17 cats, urobilinuria in two cats, crystaluria in 47 cats; bilirubin crystals in seven cats, struvite in 27 cats, oxalate in three cats, calcium carbonate in eight cats, cystine in four cats and tyrosine crystals in two cats; 25–50 leukocytes/μl were seen in urine.
In dead cats, proteinuria, bilirubinuria, nitrituria, glucosuria, bacteriuria, oxalate crystaluria were seen in one cat and struvite crystaluria associated with high pH level in the other cat; 250–500 leukocytes/μl in urine, concentrated urine, sedimented urine and increased transitional epithelium were detected.
In the three FmoPV RNA-positive unhealthy cats, proteinuria, bilirubinuria, nitrituria and glucosuria were observed in one cat, bacteriuria and residue in the urine was seen for all three cats, epithelial cells and leukocytes were seen in the urine of two cats and oxalate crystaluria was seen in one cat (Table 2).
Gross pathology of morbillivirus-positive cats
Four cats that were positive for FmoPV RNA by RT-PCR had fibrin strands on the liver surface. Purulent exudate was also seen in the lumen of the bile ducts. The kidneys were firm but pale in appearance, and there were widespread haemorrhages under the capsule of the spleen. Oedema and hepatisation were remarkable in some parts of the lobules of the lungs. Icterus was seen in two FmoPV RNA-positive cats that were also positive for FCoV RNA.
Histopathology of FmoPV infection
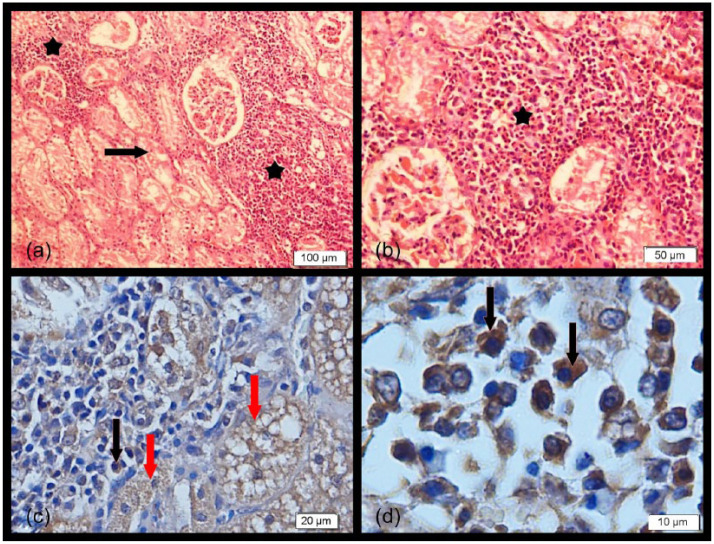
Histological examination of the kidneys of seven cats (C6, C7, C8, C11, C12, C14, C15) revealed tubulointerstitial nephritis that was characterised by severe granular and vacuolar degeneration in the epithelial cells of the cortical and medullary tubules and also prominent and widespread mononuclear cell infiltrates composed of lymphocytes and plasma cells in the intertubular area (Table 3 and Figure 1a,b). Although virus-infected cells were found in the kidney, distinctive histopathological lesions were not found.
Table 3.
Clinical and histopathological findings of dead cats. Kidney, liver and the lymph node from the samples (C3, C8, C11 and C11) were positive on immunocytochemistry stained with anti-morbillivirus guinea pig serum
| Sample ID | Presence of FmoPV RNA | Antibody to FCoV | Clinical findings | IHC findings (staining with anti-morbillivirus guinea pig serum) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | U | R | F | W | Di | De | Kidney | Liver | Lymph node | |||
| C1 | + | + | - | - | - | - | - | + | - | - | - | |
| C2 | + | + | - | - | - | - | + | + | - | - | - | |
| C3 | + | + | - | - | + | + | + | - | - | + | + | + |
| C4 | - | - | - | + | + | - | - | - | - | - | - | |
| C5 | + | - | - | + | - | - | + | - | - | - | - | |
| C6 | + | + | - | - | - | + | + | + | - | - | - | |
| C7 | - | - | - | + | + | - | - | - | - | - | - | |
| C8 | + | - | - | + | + | - | - | + | - | + | + | + |
| C9 | - | + | - | - | - | - | - | - | - | - | - | |
| C10 | - | - | - | - | - | - | - | - | - | - | - | |
| C11 | + | - | - | - | - | + | + | - | + | + | + | + |
| C12 | + | + | - | + | - | + | + | + | - | - | - | |
| C13 | - | - | - | - | - | - | - | - | - | - | - | |
| C14 | + | + | - | - | - | - | + | + | - | - | - | |
| C15 | + | + | + | + | + | + | + | - | + | + | + | + |
R = respiratory signs; F = fever; W = weight loss; Di = diarrhoea; De = depression; NE = not examined; A = anorexia; U = urinary signs; IHC = immunohistochemistry; FmoPV = feline morbillivirus; FCoV = feline coronavirus
Figure 1.
(a,b) Prominent mononuclear cell infiltrates composed of lymphocytes and plasma cells (stars) and severe granular and vacuolar degeneration in the epithelial cells of tubules (arrow) (haematoxylin and eosin). (c,d) Kidney sections showing intensely positive intracytoplasmic staining of tubular epithelial cells (red arrows) and mononuclear cells (black arrows) (immunohistochemistry)
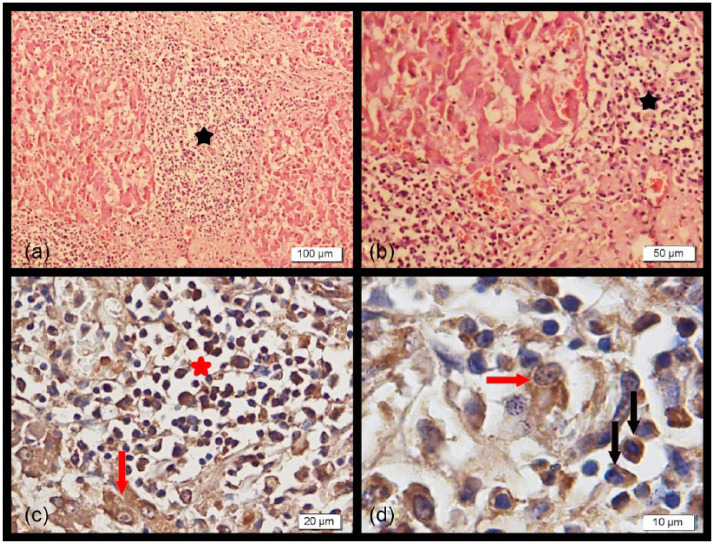
In the livers of five cats (C7, C8, C9, C14 and C15), large areas of granular degeneration were prominent. There was distinct mononuclear cell infiltration in the portal area spreading into the parenchyma and excess fibrous connective tissue proliferation was prominent (Table 3 and Figure 2a,b). Severe infiltration of neutrophils in the bile ducts was also seen. Coagulative necrosis was seen in some sections, and amyloid deposits were sometimes observed between the degenerative parenchymal cells. All the histological findings revealed widespread cholangiohepatitis in the liver. Cholangiohepatitis was prominent in most of the cats (C2, C7, C8, C9, C13, C14, C15) (Table 3). Similar lesions were also seen in morbillivirus RNA-negative cats.
Figure 2.
(a,b) Distinct mononuclear cell infiltration (stars) and fibrosis in the portal fields spreading into the liver parenchyma (haematoxylin and eosin). (c,d) Liver sections showing intensely positive intracytoplasmic staining of hepatocytes (red arrows) and mononuclear cells (red star and black arrows) (immunohistochemistry)
In addition, there was lymphoid hyperplasia that was characterised with a prominent enlargement in the cortical lymph follicle in some of the lymph nodes that were examined.
Immunohistochemistry
On immunostaining of tissues from cats found to be positive for FmoPV by RT-PCR, intracytoplasmic staining was observed in the renal tubular parenchymal cells localised in the cortical and medullary parts of the kidney. Also, there were positively stained mononuclear cells (lymphoid and plasma cells) infiltrated in the tubular and interstitial areas (Figure 1c,d). Intensive intracytoplasmic staining of hepatocytes and mononuclear cells (lymphoid and plasma cells) was seen in the liver sections (Figure 2c,d). The lymphoid cells and some macrophages in the cortical and medullary regions of the lymph nodes were also positively stained. No or faint staining was observed in the cat tissues that were negative for FmoPV RNA by RT-PCR.
Sequencing and phylogenetic analysis
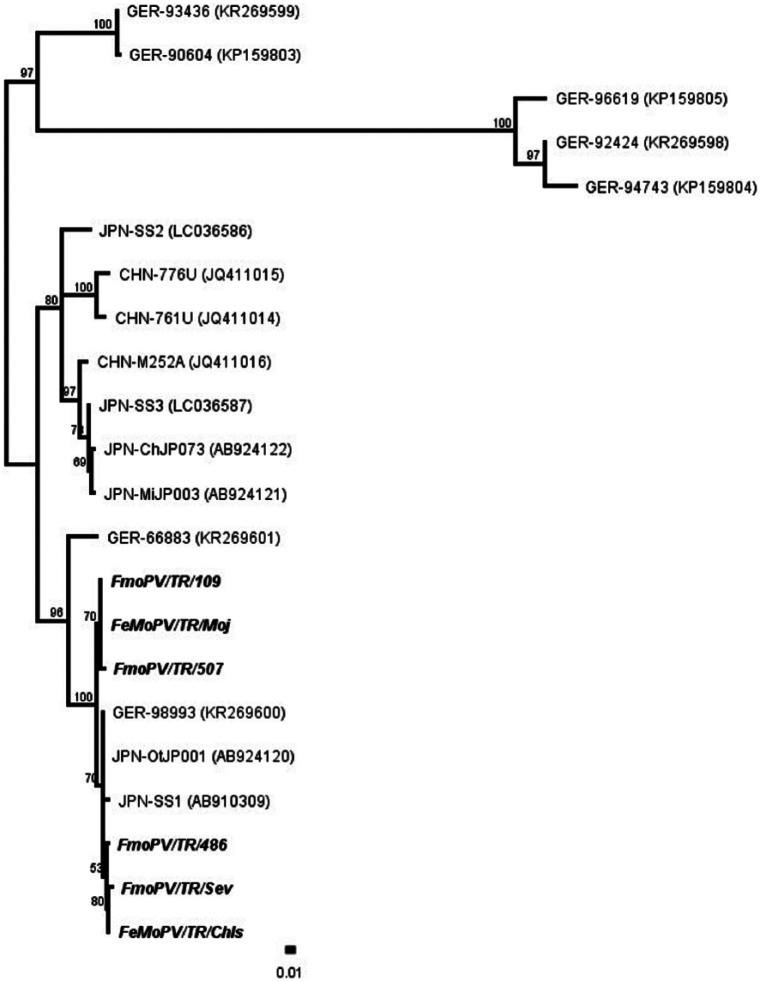
A phylogenetic tree was generated by using 16 known FMoPV L gene sequences from four clusters (A, B, C, D) of FmoPV 1 and the six FmoPV L gene sequences from the six infected cats. The six positive samples showed 98.5–100% identity to each other and all grouped in Cluster D, showing a high degree of homology to four other strains from Japan (AB924120; AB910309) and Germany (KR269601, KR269600) (Figure 3).
Figure 3.
Phylogenetic tree generated by using morbillivirus sequences from six positive cats (109, Moj, 507, 486, Sev, Chls) detected in this study. Clusters A, B, C and D
Discussion
Cats are exposed to many different viruses that can cause serious health problems in cat populations.2,9,13 FmoPV is a newly identified morbillivirus in domestic cats and was first isolated in Hong Kong in 2012. 2 This study describes the first report of FmoPV RNA in cats in Istanbul, Turkey.
FmoPV frequency in the present study is similar to that found by Sieg and others 1 and Furuya and others, 3 but lower than that reported by Woo et al. 2 Differences could be associated with cat lifestyle (household or stray cats), 3 and in the present study FmoPV was significantly associated with street access. However, samples from the cats living together with the FmoPV-positive cats were negative for FmoPV RNA. This result may be explained if cat-to-cat transmission is not easy, or FmoPV could have a long incubation period before viraemia and viral shedding.
When the six FmoPV L gene sequences from the positive cats were analysed with previously reported sequences, two clusters from Germany (KR269599, KP159803, KP159805, KR269598, KP159804) were quite different from Chinese, Japanese, Turkish and two German cat sequences. The sequences of these two clusters describe different feline paramyxoviruses by Sieg et al. 1 The FmoPV sequences from Turkish cats are more closely related to Japan-OtJP001 (AB924120), Japan-SS1 (AB910309) and Germany-98993 (KR269600). The similarity percentages may change when different primers targeting different genes such as H gene are used, as reported by others.5,6 Therefore, it is important to use different primers targeting different regions of the virus to analyse the diversity of the strains detected. In this study, the primers targeting L gene were used, as reported by Furuya and others. 3 Different primers could have been used, but there were few studies published and not many strains reported in GenBank when this study was performed.
Clinical findings observed in morbillivirus-positive cats were similar to findings in negative cats. It seems difficult to clinically distinguish morbillivirus-infected cats from the negatives. Also, findings of urine analyses, haematological and biochemical analyses of morbillivirus-positive cats did not differ from the findings in negative cats.
In the present study, many mononuclear cells of the infiltrates in kidneys showed intensely positive intracytoplasmic staining for FmoPV N protein, indicating the presence of virally infected cells. In the absence of a comprehensive histopathological study of FmoPV infections, we have tried to provide a basic overview of our findings. In this context, widespread lymphoid cell infiltrates were seen in the renal cortex and medullary regions of the kidneys in both morbillivirus RNA-positive and negative cats. Therefore, in this study, it is difficult to associate these lesions with morbillivirus infection. In addition, lymphoid infiltrates were detected in the renal cortex of cats diagnosed with FCoV infection by PCR, but they were much scarcer in their localisation. This may indicate a difference in scale between FmoPV and FCoV on histopathology. However, this finding requires further supportive evidence by other authors in future studies.
Cholangiohepatitis and focal necrosis in the liver were found to be important histopathological findings in FmoPV infection. Similar findings were also detected in the livers of the FCoV RNA-positive cats. However, they were found to be much more prominent in the livers of the cats that were positive for both FCoV RNA and morbillivirus RNA. In those cases, beside PCR, immunohistochemistry should be performed in order to assess both diseases.
Immunohistochemical staining of kidney and lymph node sections of cats positive for FmoPV using anti-FmoPV N protein serum showed positive staining of mononuclear cells, similar to a previous study. 2 These authors also reported that FmoPV infection was related to tubulointerstitial nephritis, but in this study, similar histopathological findings in kidney of FCoV-positive cats were found. Therefore, experimental infection is needed to explain the relationship between FmoPV infection and nephritis in future analyses.
Conclusions
This is the first study that shows the presence of FmoPV infections in cats in Turkey. Sick cats, particularly those with kidney disease, should be tested for this virus. The genotypes found in this study were similar to previously reported strains, indicating that circulating morbilliviruses in Turkey are conserved.
Acknowledgments
We would like to thank to Professor Kwok-Yung Yuen and Rachel YY Fan for providing guinea pig anti-morbillivirus N protein antiserum for immunohistochemistry. Also thanks to University of Istanbul for funding this study.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was funded by the University of Istanbul (Project no: 22547).
Accepted: 30 November 2016
References
- 1. Sieg M, Heenemann K, Rückner A, et al. Discovery of new feline paramyxoviruses in domestic cats with chronic kidney disease. Virus Genes 2015; 51: 294–297. [DOI] [PubMed] [Google Scholar]
- 2. Woo PC, Lau SK, Wong BH, et al. Feline morbillivirus, a previously undescribed paramyxovirus associated with tubulointerstitial nephritis in domestic cats. Proc Natl Acad Sci U S A 2012; 109: 5435–5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Furuya T, Sassa Y, Omatsu T, et al. Existence of feline morbillivirus infection in Japanese cat populations. Arch Virol 2014; 159: 371–373. [DOI] [PubMed] [Google Scholar]
- 4. Lorusso A, Di Tommaso M, Di Felice E, et al. First report of feline morbillivirus in Europe. Vet Ital 2015; 51: 235–237. [DOI] [PubMed] [Google Scholar]
- 5. Sakaguchi S, Nakagawa S, Yoshikawa R, et al. Genetic diversity of feline morbilliviruses isolated in Japan. J Gen Virol 2014; 95: 1464–1468. [DOI] [PubMed] [Google Scholar]
- 6. Sharp CR, Nambulli S, Acciardo AS, et al. Chronic infection of domestic cats with feline morbillivirus, United States. Emerg Infect Dis 2016; 22: 760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yilmaz H, Turan N, Altan E, et al. First report on the phylogeny of bovine norovirus in Turkey. Arch Virol 2011; 156: 143–147. [DOI] [PubMed] [Google Scholar]
- 8. Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. J Mol Evol 1993; 10: 512–526. [DOI] [PubMed] [Google Scholar]
- 9. Dye C, Helps CR, Siddell SG. Evaluation of real-time RT-PCR for the quantification of FCoV shedding in the faeces of domestic cats. J Feline Med Surg 2008; 10: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pinches MDG, Diesel G, Helps CR, et al. An update on FIV and FeLV test performance using a Bayesian statistical approach. Vet Clin Path 2007; 36: 141–147. [DOI] [PubMed] [Google Scholar]
- 11. Pinches MDG, Helps CR, Gruffydd-Jones TJ, et al. Diagnosis of feline leukaemia virus infection by semi-quantitative real-time polymerase chain reaction. J Feline Med Surg 2007; 9: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buonavoglia C, Martella V, Pratella Tempesta M, et al. Evidence for evolution of canine parvovirus type 2 in Italy. J Gen Virol 2001; 82: 3021–3025. [DOI] [PubMed] [Google Scholar]
- 13. Tekelioglu BK, Berriatua E, Turan N, et al. A retrospective clinical and epidemiological study on feline coronavirus (FCoV) in cats in Istanbul, Turkey. Prev Vet Med 2015; 119: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]