Abstract
Objectives
The objectives of this study were to determine the reference interval for screening blood glucose in senior cats, to apply this to a population of obese senior cats, to compare screening and fasting blood glucose, to assess whether screening blood glucose is predicted by breed, body weight, body condition score (BCS), behaviour score, fasting blood glucose and/or recent carbohydrate intake and to assess its robustness to changes in methodology.
Methods
The study included a total of 120 clinically healthy client-owned cats aged 8 years and older of varying breeds and BCSs. Blood glucose was measured at the beginning of the consultation from an ear/paw sample using a portable glucose meter calibrated for cats, and again after physical examination from a jugular sample. Fasting blood glucose was measured after overnight hospitalisation and fasting for 18–24 h.
Results
The reference interval upper limit for screening blood glucose was 189 mg/dl (10.5 mmol/l). Mean screening blood glucose was greater than mean fasting glucose. Breed, body weight, BCS, behaviour score, fasting blood glucose concentration and amount of carbohydrate consumed 2–24 h before sampling collectively explained only a small proportion of the variability in screening blood glucose.
Conclusions and relevance
Screening blood glucose measurement represents a simple test, and cats with values from 117–189 mg/dl (6.5–10.5 mmol/l) should be retested several hours later. Cats with initial screening blood glucose >189 mg/dl (10.5 mmol/l), or a second screening blood glucose >116 mg/dl (6.4 mmol/l) several hours after the first, should have fasting glucose and glucose tolerance measured after overnight hospitalisation.
Introduction
Diabetes occurs in 1 in 50 to 1 in 400 cats, depending on the population studied, and risk factors include age, male sex, obesity and breed. Burmese cats are over-represented in Europe and Australasia1,2 and so are Russian Blue, Maine Coon and Siamese in the USA. 3 The terms impaired glucose tolerance and impaired fasting glucose refer to an intermediate stage between normal glucose homeostasis and diabetes, now referred to as pre-diabetes.4,5 At least 60% of humans who develop diabetes have either impaired glucose tolerance or impaired fasting glucose identified in the previous 5 years, 6 and up to 33% of people with impaired glucose tolerance develop diabetes over a 2 year period. 7 If identified before progression to diabetes, these individuals usually return to reasonable glycaemic control with weight loss, dietary management and exercise. As in humans, progression from impaired fasting glucose and impaired glucose tolerance to diabetes also occurs in cats. Of cats in diabetic remission with moderate impaired fasting glucose (⩾135–⩽151 mg/dl; ⩾7.5–⩽8.4 mmol/l) or moderate glucose intolerance, 75% and 38%, respectively, developed diabetes within 9 months of testing. 8 Impaired fasting glucose is rarely diagnosed in cats, and mild increases in blood glucose are mostly attributed to stress hyperglycaemia associated with the veterinary visit or with illness.6,9
As in humans, it is likely advantageous to differentiate cats with transient hyperglycaemia associated with stress or eating, from cats with persistent mild increases in glucose (prediabetic), so appropriate therapy can be implemented to prevent progression to overt diabetes. Measurement of fasting glucose concentration is the gold standard in human medicine used to diagnose impaired fasting glucose. However, fasting can be problematic in client-owned cats because the postprandial period in some cats fed twice daily can exceed 14 h, and 24 h in some cats fed once daily. 10 Although owners can fast cats at home, it does not resolve the effect of stress of travel to the clinic on blood glucose concentrations. Therefore, measurement of fasting blood glucose at home or in the clinic may not be a practical screening test for some pet cats.
Casual blood glucose, defined as blood glucose measured unrelated to time of eating or type of food, is used as a screening test to diagnose diabetes in humans. 11 In humans, the lower cutpoint for diabetes for casual blood glucose is higher4,11 than for fasting glucose (>200 mg/dl; 11.1 mmol/l vs >126 mg/dl; 7.0 mmol/l), and requires the presence of classical signs for definitive diagnosis. It is recommended that casual blood glucose concentrations above fasting values, but unaccompanied by diabetic signs, are confirmed by measurement of fasting blood glucose and glucose tolerance testing because of the potential for confounding factors to increase blood glucose, 4 such as ‘white coat’ hyperglycaemia associated with stress.12,13
In cats, stress hyperglycaemia is a major factor confusing diagnosis of diabetes, and struggling can increase blood glucose by as much as 180 mg/dl (10 mmol/l). 6 Cats without clinical signs of diabetes but blood glucose concentrations above the upper cutpoint for normal could have prediabetes or subclinical diabetes, or a response to stress or eating. Where other risk factors are present, such as senior age and obesity, it would be prudent to determine whether the hyperglycaemia is persistent and consistent with abnormal glucose homeostasis, or transient and consistent with stress-induced hyperglycaemia or eating.
Measurement of blood glucose in capillary blood from the ear or pad using a portable glucose meter requires less restraint than for venepuncture, which may help reduce the effect of stress on measurement of blood glucose.14–16 Numerous portable blood glucose meters calibrated for human blood have been used in cats17–19 and although precise, have lower accuracy than meters calibrated for feline blood, and typically under-report blood glucose by 8–18 mg/dl (0.4–1.0 mmol/l). 20 The availability of a portable glucose meter calibrated for feline blood methodology (AlphaTRAK Blood Glucose Monitoring System; Zoetis) and requiring only a small sample volume (0.3 µl), 21 facilitates more accurate measurement of blood glucose in cats.
The aims of this study were: (i) to establish the reference interval for a screening blood glucose test in client-owned cats aged 8 years and older, measured on entry to the clinic from a pad or paw sample using a glucose meter calibrated for cat blood (screening blood glucose); (ii) to apply this in a population of obese cats; (iii) to determine whether selected variables account for variability in screening blood glucose (breed, body weight, body condition score, behaviour score, fasting blood glucose, recent carbohydrate intake); and (iv) to determine whether blood glucose is affected by changes in screening blood glucose methodology that could be expected in veterinary practice, such as using a jugular sample or external laboratory.
Materials and methods
The protocol for these studies and the care and handling of these animals were approved by the Animal Experimentation Ethics Committee of the University of Queensland approval number SVS/040/10/NC/ABBOTT.
Animals
Client-owned, neutered cats 8 years and older (mean 10.9 years, range 8–18 years; n = 134, Table 1) presenting for a routine health check at local veterinary clinics in Brisbane were assessed. Other than for excess body condition in some cats, cats enrolled in the study were assessed as healthy based on results of a clinical examination, patient history, haematological and biochemical assays, total thyroxine and feline pancreatic lipase immunoreactivity (fPLI). Body condition score (BCS) was recorded on a scale of 1–9, and cats with scores between 4 and 9 were included. 22 Exclusions (n = 14) were due to chronic renal failure (n = 2), hyperthyroidism (n = 6), suspected pancreatitis based on increased fPLI (>3.5 µg/l; n = 1), gastrointestinal neoplasia detected at initial examination (n = 1), inability to sample from an ear or paw pad (n = 1) and BCS was ⩽3/9 (n = 2) or not recorded (n = 1). Reference intervals were established using cats with ideal BCSs (4–5/9, n = 49), and then applied in a population of otherwise healthy overweight (6–7/9, n = 45), and obese (8–9/9, n = 26) cats. The mean body weight of the 120 cats was 5.5 kg (range 2.9–10.0 kg, Table 1).
Table 1.
Means, standard deviations (SDs) and ranges for body condition score (BCS), body weight and age, and distributions of sex, breed and behaviour score during screening blood sampling for healthy cats ⩾8 years of age
| BCS/9 (mean ± SD) (range) |
Body weight (kg) mean ± SD (range) |
Age (years) mean ± SD (range) |
Sex (F/M) | Burmese and Burmese cross/non-Burmese (B/NB) | Behaviour score % ⩾2 (range) * |
|
|---|---|---|---|---|---|---|
| All cats n = 120 † |
6.2 ± 1.5 (4–9) |
5.5 ± 1.6 (2.9–10.0) |
10.9 ± 2.4 (8–18) |
62 F 58 M |
29 B 91 NB |
34% (0–4) |
| Ideal body condition cats (BCSs 4 or 5) n = 49 † |
4.7 ± 0.5 (4–5) |
4.3 ± 0.7 (2.9–5.5) |
11.4 ± 2.6 (8–18) |
22 F 27 M |
12 B 37 NB |
35% (0–4) |
| Overweight cats (BCSs 6 or 7) n = 45 † |
6.6 ± 0.5 (6–7) |
5.5 ± 1.0 (4.1–7.3) |
10.8 ± 2.3 (8–17) |
24 F 21 M |
15 B 30 NB |
26% (0–3) |
| Obese cats (BCSs 8 or 9) n = 26 † |
8.3 ± 0.5 (8–9) |
7.4 ± 1.2 (5.6–10.0) |
10.2 ± 2.2 (8–16) |
16 F 10 M |
2 B 24 NB |
46% (0–3) |
For all groups, the median score was 1
For body weights, ages and behaviour scores, numbers of cats are less than shown for some groups as these data were not recorded for all cats
F = female; M = male
The following definitions for blood glucose measurement methods were used: ‘screening’ (measurement any time after eating and on entry to the consultation room prior to history taking and physical examination, from an ear or paw pad blood sample, using a portable glucose meter calibrated for cat blood) and ‘fasting’ (after overnight hospitalisation and withholding food for 18–24 h, from ear or paw pad blood sample, and measurement by glucose meter). To determine the robustness of screening blood glucose to some variations in sampling and measurement that could be expected in a busy veterinary practice, blood glucose was also measured using a glucose meter from a venepuncture sample obtained any time after eating and after history taking and physical examination, with blood dropped directly from the syringe after removal of the needle (traditional meter). It was then placed in a tube containing the anticoagulant sodium fluoride/potassium oxalate (FO) and measured by meter (traditional meter FO) and by an external laboratory in plasma (traditional laboratory FO).
Experimental protocol
After entry of the client and their cat into the consulting room for screening blood glucose measurement, a drop of blood was obtained from the pinna margin (n = 106) or edge of the pisiform pad (n = 11) using a lancet (AlphaTRAK lancing device; Zoetis). The method of sampling (pinna or pad) was not recorded for three cats. Whenever possible, the cat was positioned with minimal restraint with its forepaws on the shoulder of the client. To increase perfusion, the ear/paw was warmed by holding a cotton wool ball soaked in warm water and squeezed dry to the area for approximately 10 s. The drop of blood was analysed immediately using the portable glucose meter using glucose oxidase methodology (AlphaTRAK 2; Zoetis; screening blood glucose). Behaviour in cats was assessed by the clinician at the time of the screening blood glucose measurement and an integer score from 0–4 allocated based on previously determined criteria, 6 where 0 indicates no behaviours suggesting stress and 4 indicates behaviours suggesting extreme stress. Behaviours assessed to determine each cat’s score were escape attempts including struggling, vocalisation, hypersalivation, mouth breathing, aggression and immobility.
The medical history, indoor or outdoor access, body weight (in kg), BCS and feeding (timing, and type and amount of food fed) were recorded and a physical examination was performed. For 105 of the 120 study cats, a venous blood sample was taken at the end of the consultation and measured with the meter (traditional meter). For 48 of these, the sample was then placed into a tube containing the anticoagulant FO and blood glucose concentration measured with the meter after using a syringe to aspirate a droplet (traditional meter FO), and the same tube was then sent to a commercial veterinary laboratory (IDEXX Laboratories, Brisbane, Australia) for blood glucose analysis with an automated serum chemistry analyser (Beckman Coulter AU 600) which used the hexokinase reaction (traditional laboratory FO). For the portable glucose meter, the previously established interassay precision was 2% (coefficient of variation) and intra-assay 3.3%. 23 For the laboratory method, within-run precision was <3% and total precision <3%.
Study cats that were compliant and had an owner who gave consent (n = 74) were subsequently hospitalised overnight, and samples collected for fasting blood glucose and a simplified glucose tolerance test conducted. This was undertaken on the day after the cat’s first study visit for 51 cats and fasting values from only these cats were used in statistical analyses. Cats were fasted and glucose was measured at 0 h (fasting blood glucose) and again 2 h after receiving 0.5 g/kg glucose IV, using the same pinna/pad technique and portable glucose meter. Results from these samples are reported in a separate publication 23 but comparisons between screening blood glucose and fasting blood glucose, and some individual glucose tolerance test results, are reported here.
Dietary carbohydrate calculations
Carbohydrate consumption 2–24 h and 2–12 h prior to the screening blood glucose measurement was estimated. Carbohydrate consumed in the last 2 h before sampling was not included in the calculations as blood glucose concentration would have been minimally affected by this. 24 Owners were informed that dietary data would be collected from them and, when possible, they were emailed the data capture form prior to the consultation. Owners recorded food, amount fed and timing of feedings. Dietary composition data supplied by the various food manufacturers were used to calculate the amount of carbohydrate (nitrogen-free extract) in grams consumed. Estimates were not generated for the cats with owners who provided incomplete or unreliable data on the food, amounts and timing of foods given/eaten by the cat (n = 55 of the 120 study cats).
The nitrogen-free extract consumption of cats fed ad libitum (defined as food always or nearly always present, and in excess of what could be consumed; n = 39 cats) was calculated using estimated daily metabolisable energy requirements. Two methods were used:
Daily consumption was estimated at 60 kcal/kg/24 h or 30 kcal/kg/12 h.
Using the following formula24,25: daily energy requirement = 151.8 × (body weight [kg])0.4 − 87.5. The exponent of 0.4 and the constants 151.8 and 87.5 are reported to provide an accurate estimate of daily energy requirements/kg body weight in cats of various body conditions from lean to obese.25,26
Carbohydrate intake for the period (ie, for 12 or 24 h) was then calculated for each cat as estimated daily energy intake for the period multiplied by the percentage of the dietary energy that was supplied by carbohydrate. For cats meal-fed once daily (n = 26 cats), the time when food was replenished was used to calculate the cats’ estimated consumption, assuming that 50% of daily energy provided was eaten in the 12 h following feeding, and 100% of daily energy provided was eaten in 24 h, if bowls were replenished once daily. For cats fed twice or more times daily, the amount fed (data provided by the owner) was used for calculation of carbohydrate intake in 12 and 24 h. These owners accurately kept a record of how much the cats were fed and at what time.
Statistical analyses
Reference intervals were determined after Box-Cox transformation and exclusion of outliers (Reference Interval Draft Version, 2005, University of Cincinnati). Confidence intervals (CIs) for each reference interval limit were calculated using 1000 bootstrap resamplings. The upper cutpoints were defined as the upper limits of the 95% reference intervals. Medians and interquartile ranges were compared between Burmese and non-Burmese cats, and where absolute differences between the medians and interquartile ranges were <50% and <100% of the lowest median and interquartile range, respectively, the subgroups of cats (ie, Burmese and non-Burmese) were pooled, an approach that has been used previously. 27
All other analyses were performed using a statistical software program (Stata, version 14, StataCorp, College Station, Texas, USA). Means were compared between screening and fasting samples using cats with a BCS of 4 or 5 that had fasting blood glucose assessed on the day after their first study visit, using the paired t-test, calculated using Stata’s -ttest- command.
The amount of variability in screening blood glucose that was accounted for by potential determinants of screening blood glucose concentration was assessed using multivariable linear regression models, simultaneously fitting breed (Burmese/Burmese cross or non-Burmese), body weight, BCS, behaviour score during screening blood sampling, fasting blood glucose concentration (as assessed at the cat’s subsequent hospitalisation, using only the cats that had fasting blood glucose assessed on the day after their first study visit) and amount of carbohydrate consumed in either the 2–24 h or 2–12 h before screening sampling. Behaviour score was fitted as a categorical variable with four categories (0, 1, 2, 3 and 4 combined). All six variables were recorded for only 29 of the 120 study cats but breed, BCS, behaviour score and fasting blood glucose concentration were all recorded for 49 cats. Models were fitted both including and not including body weight and amount of carbohydrate consumed. The overall P values for behaviour score were assessed using the multiple Wald test. One cat had elevated screening and fasting blood glucose concentrations (238 and 221 mg/dl; 13.2 and 12.3 mmol/l, respectively). A further cat had lower screening but elevated fasting blood glucose concentrations (155 and 223 mg/dl; 8.6 and 12.4 mmol/l, respectively). Regression analyses were performed with and without these cats. As results were similar, only those with these cats included are reported. With preliminary univariable linear regression analyses, histograms of residuals were used to identify whether the residuals had a normal distribution. Residual-vs-predictor plots were used to assess homoscedasticity of residuals. Residuals were not normally distributed and/or were heteroscedastic in models that included the cat with elevated screening and fasting blood glucose concentrations, so the bootstrap method with 1000 replications was used for multivariable regression analyses. Normal-based CIs were used.
To determine the robustness of the screening blood glucose to some changes in methodology that could be expected in veterinary practice, we compared screening blood glucose concentrations with those obtained following some changes in sampling and measurement. Agreement was assessed using Lin’s concordance correlation coefficient with 95% CIs based on the z-transformation, Pearson’s correlation coefficient with 95% CIs based on Fisher’s transformation, comparison between the reduced major axis line and the line of perfect concordance, and 95% limits of agreement, using Stata’s -concord- and -ci2- commands. Mean glucose concentrations were also compared, using paired t-tests, calculated with Stata’s -ttest- command.
Means and standard deviations are reported, unless otherwise indicated, and P <0.05 was considered significant.
Results
Reference intervals for screening and fasting blood glucose
Distributions of screening, traditional and fasting blood glucose concentrations for the 71 cats with all three measures are shown in Figure 1. In this study of senior cats, using those with an ideal BCS (4–5/9; n = 49), the reference interval for screening blood glucose was 67–189 mg/dl (3.7–10.5 mmol/l; 90% CI for upper limit 164–212 mg/dl; 9.1–11.8 mmol/l). This upper limit for screening blood glucose was 73 mg/dl (4.1 mmol/l) higher than for fasting blood glucose (116 mg/dl; 6.4 mmol/l; 90% CI for upper limit 107–120 mg/dl; 5.9–6.7 mmol/l) calculated using the 28 of these cats that had fasting values. For 35% (17/49) of the study cats with an ideal BCS, screening blood glucose concentration was above that upper limit for fasting blood glucose. Using only the 20 cats with an ideal BCS and that had fasting blood glucose assessed on the day after their first study visit, mean blood glucose concentrations were significantly higher (P = 0.001) for screening samples (121 ± 39 mg/dl [6.7 ± 2.1 mmol/l]) than fasting samples (96 ± 32 mg/dl [5.3 ± 1.8 mmol/l]), with a difference of 25 mg/dl (95% CI 12–39 mg/dl) (1.4 mmol/l; 95% CI 0.7–2.2 mmol/l). Behaviour scores were low (63% of cats scored 0 or 1 and a further 25% scored 2), and no cats were observed to struggle (Table 1).
Figure 1.
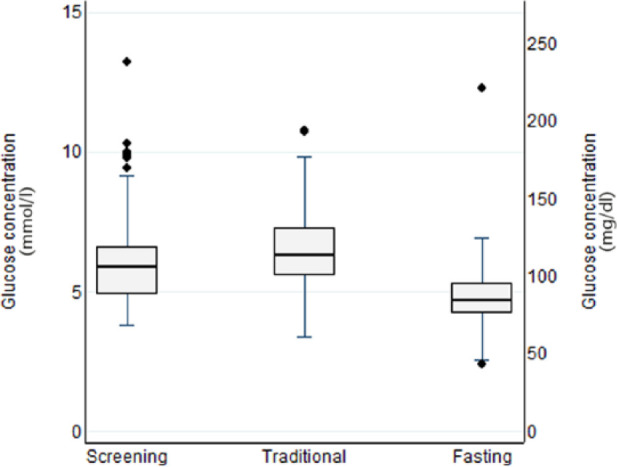
Box and whisker plot showing blood glucose concentrations in screening samples (collected immediately after entry to the consultation room from pinna or pad) from the cat’s first study visit, traditional method (jugular venous sample collected after history and taking and physical examination) and after fasting (sampled from pinna or pad after overnight hospitalisation) from 71 cats 8 years of age or older that had all three samples collected. All samples were tested using a portable glucose meter. (Medians are shown as thick horizontal lines; interquartile ranges and 25th and 75th percentiles are shown by boxes; values more than 1.5 interquartile ranges above the 75th percentile or below the 25th percentiles are each shown as diamonds; error bars show ranges for all less extreme values)
Determinants of screening blood glucose concentration
We investigated the extent to which screening blood glucose concentration was accounted for by breed, body weight, BCS, behaviour score during screening blood sampling, fasting blood glucose concentration and carbohydrate intake before sampling. These six variables collectively explained only a small proportion of the variability in the screening blood glucose (R2 36%, adjusted R2 10%). This model was not significantly better than the null model (P = 0.893), and none of these variables were significantly associated with screening blood glucose after adjustment for the other five variables (Table 2). Results were similar when amounts of carbohydrate consumed from 2–12 h were used instead of 2–24 h before sampling. From the four-variable model with breed, BCS, behaviour score and fasting blood glucose concentration fitted, screening blood glucose concentration was also not significantly associated with fasting blood glucose concentration (Table 2).
Table 2.
Regression coefficients (estimated changes in screening blood glucose in mg/dl [mmol/l]), 95% confidence intervals for estimates and P values for the associations between screening blood glucose and each of breed, body weight, body condition score (BCS), behaviour score during screening blood sampling, fasting blood glucose concentration and carbohydrate intake from 2–24 h before sampling for healthy cats ⩾8 years of age
| Variable and category | Adjusted regression coefficient * | 95% confidence interval | P |
|---|---|---|---|
| Breed (Burmese/Burmese cross relative to non-Burmese) |
−8.0
†
(−0.4) |
−58.0 to 42.0 (−3.2 to 2.3) |
0.754 |
| Body weight (kg) | −7.1
‡
(−0.4) |
−31.4 to 17.3 (−1.7 to 1.0) |
0.570 |
| BCS | 9.9 (0.6) |
−15.8 to 35.7 (−0.9 to 2.0) |
0.450 |
| Behaviour score: | 0.260 § | ||
| 1 relative to 0 | 31.1 (1.7) |
−7.7 to 69.8 (−0.4 to 3.9) |
0.116 |
| 2 relative to 0 | 15.1 (0.8) |
−25.2 to 55.5 (−1.4 to 3.1) |
0.463 |
| 3 or 4 relative to 0 | 50.6 (2.8) |
−8.9 to 110.1 (−0.5 to 6.1) |
0.095 |
| Fasting blood glucose concentration (mmol/l) | 11.8
¶
∞
(0.7) |
−13.3 to 37.0 (−0.7 to 2.1) |
0.355 |
| Carbohydrate intake from 2–24 h before sampling (g) | −0.2
#
(0.0) |
−4.8 to 4.4 (−0.3 to 0.2) |
0.925 |
Adjusted for the other five variables in the model
Screening blood glucose concentration was estimated to be 8 mg/dl (0.4 mmol/l) lower in Burmese/Burmese cross relative to non-Burmese (95% confidence interval 58.0 mg/dl [3.2 mmol/l] lower to 42.0 mg/dl [2.3 mmol/l] higher; P = 0.754)
For each additional kg body weight, screening blood glucose concentration was estimated to be 7.1 mg/dl (0.4 mmol/l) lower (95% confidence interval 31.4 mg/dl [1.7 mmol/l] lower to 17.3 mg/dl [1.0 mmol/l] higher; P = 0.570)
Overall P value for behaviour score
For each 18 mg/dl (1 mmol/l) increase in fasting blood glucose concentration, screening blood glucose concentration was estimated to be 11.8 mg/dl (0.7 mmol/l) higher (95% confidence interval 13.3 mg/dl [0.7 mmol/l] lower to 37.0 mg/dl [2.1 mmol/l] higher; P = 0.355)
After adjusting only for breed, BCS and behaviour score, for each 18 mg/dl (1 mmol/l) increase in fasting blood glucose concentration, screening blood glucose concentration was estimated to be 10.1 mg/dl (0.6 mmol/l) higher (95% confidence interval 7.4 mg/dl [0.4 mmol/l] lower to 27.6 mg/dl [1.5 mmol/l] higher; P = 0.259)
For each 1 g increase in carbohydrate intake, screening blood glucose concentration was estimated to be 0.2 mg/dl (0.0 mmol/l) lower (95% confidence interval 4.8 mg/dl [0.3 mmol/l] lower to 4.4 mg/dl [0.2 mmol/l] higher; P = 0.925)
The reference interval upper limit for screening blood glucose obtained from cats with ideal BCSs (189 mg/dl or 10.5 mmol/l) was applied to the population of obese cats (range 8–9/9; n = 26). No obese cats were above the upper limit. One cat (with a BCS of 5/9) had both a screening blood glucose and fasting blood glucose above the screening blood glucose cutpoint (screening blood glucose was 238 mg/dl; 13.2 mmol/l and fasting blood glucose was 221 mg/dl; 12.3 mmol/l, respectively). However, the traditional blood glucose measured 20 mins after screening was normal (117 mg/dl; 6.5 mmol/l) and glucose tolerance the following day was normal with a blood glucose value at 2 h of 140 mg/dl (7.8 mmol/l), which was below the upper cutpoint for our laboratory (178mg/dl; 9.9 mmol/l). 23
Robustness of screening blood glucose to variations in methodology
Because veterinarians in practice usually take a jugular blood sample after history taking and physical examination (‘traditional sampling’) rather than using a screening sample, and measure blood glucose either directly from the syringe by meter (traditional meter), or place the sample in a tube with anticoagulant (in our study, FO) and measure with meter (traditional meter FO) prior to sending it to an external laboratory (traditional laboratory FO), we investigated how well screening blood glucose concentrations agreed with measurements of blood glucose after some changes in methods of sampling and measurement that could be expected in a busy veterinary practice (Table 3).
Table 3.
Agreement (and comparisons of means) between screening blood glucose concentrations after various changes in methodology for sampling and measurement that could be expected in a busy veterinary practice. Screening blood glucose: pinna or paw sample on entry to the consulting room measured with a portable glucose meter was compared with traditional sampling (jugular blood sample after history taking and physical examination) and blood glucose measured either directly from the syringe by meter (traditional meter), or placed in a tube with anticoagulant (sodium fluoride/potassium oxalate [FO]) and measured with meter (traditional meter FO) and at an external laboratory (traditional laboratory FO)
| Methodologies compared | n | Lin’s concordance correlation coefficient (95% confidence interval) | Pearson’s correlation coefficient (95% confidence interval) | 95% limits of agreement mg/dl (mmol/l) * | Mean ±SD mg/dl (mmol/l) | Difference mg/dl (mmol/l)* |
95% confidence interval of difference mg/dl (mmol/l) and P value for difference |
|---|---|---|---|---|---|---|---|
| Screening vs traditional meter | 105 | 0.54 (0.40 to 0.66) |
0.57 (0.43 to 0.69) |
−60 to 44 (−3.4 to 2.4) |
106 ± 30 (5.9 ± 1.7) 115 ± 27 (6.4 ± 1.5) |
−8 (−0.5) | −13 to −3 (−0.7 to −0.2) P = 0.002 |
| Screening vs traditional meter FO | 50 | 0.55 (0.33 to 0.71) |
0.56 (0.33 to 0.72) |
−57 to 53 (−3.2 to 2.9) |
112 ± 32 (6.2 ± 1.8) 114 ± 27 (6.3 ± 1.5) |
−2 (−0.1) | −10 to 6 (−0.6 to 0.3) P = 0.551 |
| Screening vs traditional laboratory FO | 91 | 0.51 (0.36 to 0.63) |
0.58 (0.42 to 0.70) |
−42 to 60 (−2.3 to 3.4) |
106 ± 32 (5.9 ± 1.8) 97 ± 22 (5.4 ± 1.2) |
9 (0.5) | 4 to 15 (0.2 to 0.8) P = 0.001 |
| Traditional meter vs traditional meter FO | 49 | 0.98 (0.96 to 0.99) |
0.98 (0.97 to 0.99) |
−9 to 12 (−0.5 to 0.7) |
115 ± 28 (6.4 ± 1.5) 114 ± 27 (6.3 ± 1.5) |
1 (0.1) | 0 to 3 (0.0 to 0.2) P = 0.086 |
| Traditional meter vs traditional laboratory FO | 86 | 0.73 (0.66 to 0.80) |
0.95 (0.92 to 0.96) |
−2 to 36 (−0.1 to 2.0) |
114 ± 26 (6.3 ± 1.5) 95 ± 21 (5.3 ± 1.2) |
17 (0.9) | 15 to 19 (0.8 to 1.0) P <0.001 |
| Traditional meter FO vs traditional laboratory FO | 48 | 0.79 (0.70 to 0.85) |
0.96 (0.93 to 0.98) |
−1 to 30 (0 to 1.6) |
112 ± 25 (6.2 ± 1.4) 97 ± 21 (5.4 ± 1.2) |
14 (0.8) | 12 to 17 (0.7 to 0.9) P <0.001 |
First listed methodology minus second listed methodology.
Agreement between results from screening and the three other methods was low as indicated by low Lin’s concordance correlation coefficients (0.51–0.55) because they were not closely correlated (Pearson’s correlation coefficients 0.56–0.58), but accuracy was high; that is, on average, results from each method from the same cat were close to each other. Differences in concentrations between screening and the three other methods were widely spread. For example, for screening vs traditional meter, the 95% CI limits of agreement were −60 to +44 mg/dl (−3.4 to +2.4 mmol/l); these results indicate that for 95% of cats, the difference would fall between these values. Agreement was only a little better for each of traditional meter and traditional meter FO vs traditional laboratory FO (Lin’s concordance correlation coefficients 0.73 and 0.77). Concentrations were closely correlated (Pearson’s correlation coefficients 0.95 and 0.96) but accuracy was poor because laboratory results were generally lower than glucose meter results. Mean differences were 17 and 14 mg/dl (0.9 and 0.8 mmol/l) lower for laboratory results compared with traditional meter and traditional meter FO, respectively. Of note, when blood was dropped from the syringe with the needle attached, inconsistent and spurious results for blood glucose measurement were obtained, resulting from inconsistent mixing of red blood cells in plasma in the needle (personal communication, L Cozzi, Abbott Animal Health).
Discussion
In this study of healthy, client-owned, senior cats, we standardisd measurement of screening blood glucose and established the upper cutpoint of 189 mg/dl (10.5 mmol/l) for cats with ideal BCSs using a portable glucose meter validated for feline blood (AlphaTRAK 2; Zoetis). Screening blood glucose was defined in our study as measurement any time after eating and on entry to the consulting room, with blood sampling from an ear or paw pad, and measurement with a portable glucose meter calibrated for cats.
The upper cutpoint for screening blood glucose was higher (73 mg/dl or 4.1 mmol/l higher) than that for fasting glucose concentration. In humans, inadequate fasting, for example less than 10 h, can increase glucose concentrations above fasting values. 28 In cats consuming a high carbohydrate diet (50% of metabolisable energy, 12.8 g per 100 kcal), blood glucose increased on average by 61 mg/dl (3.4 mmol/l) after a meal of 100% of daily energy requirements. 29 Measurement of fasting blood glucose requires food to be withheld at least for 14 h after a meal of 50% of daily energy requirements, and for 24 h after a meal of 100% daily energy requirements, 10 which may be not practical in client-owned cats. In our study, carbohydrate intake accounted for little of the variation in screening glucose, possibly because cats in our study consumed diets with moderate carbohydrate content (mean ± SD; 7.5 ± 2.4 g per 100 kcal) and most were fed ad libitum or twice daily. However, there were many limitations in this calculation of carbohydrate consumed. More detailed understanding of the timing of carbohydrate consumption, along with a more detailed knowledge of the amount and nature of the carbohydrate would be required to fully evaluate effects of carbohydrate consumption. These constraints limit confidence in the findings relating to the effect of carbohydrate intake on blood glucose, and this dietary effect should be further investigated in a future study.
At the time of blood sampling, overt behaviours that may be associated with stress also did not account for much of the variation in screening blood glucose concentrations. In a study of stress hyperglycaemia associated with a 5 min spray bath, struggling was the only behaviour associated with increased blood glucose concentration. In that study, glucose concentration increased on average by 74 mg/dl (4.1 mmol/l) within 10 mins, and as much as 194 mg/dl (10.8 mmol/l) in some cats, and the mean change was the same magnitude to the difference between fasting and screening blood glucose in our study. The increase was associated with increased plasma lactate and norepinephrine concentrations, and resolved within 90 mins in most cats. 6 However, there were limitations in our study when assessing the effect on blood glucose of behaviours previously shown to be associated with stress hyperglycaemia. Behaviour scores were low compared with cats being spray bathed, no cats were observed to struggle, our stressor was shorter and milder than in the 5 min spray bath, and in our study, non-compliant cats were excluded to facilitate successful blood collection. Because neither carbohydrate consumed nor the behavioural scores in the consulting room explained much of the variation in screening blood glucose, it is likely that this variation was, in part, the result of stress prior to entry into the consulting room; for example, stress associated with travel to the clinic. Based on continuing research in our laboratory using a continuous glucose monitor to identify when stress hyperglycaemia occurs, it is clear that travel to the clinic in some cats results in marked hyperglycaemia. However, not all cats in the current study had higher values for screening compared with fasting blood glucose, suggesting not all cats develop stress hyperglycaemia associated with a veterinary clinic visit, and further research is required to understand the triggers for stress hyperglycaemia.
There was no significant association between either body weight or BCS and screening blood glucose concentration, consistent with previous findings that although obesity is associated with impaired glucose tolerance, fasting blood glucose concentrations are not typically increased.30,31 This suggests that simply measuring fasting or screening blood glucose may not be sensitive in identifying cats with milder disturbances of glucose metabolism, and a glucose tolerance test should be considered if multiple risk factors for diabetes are present.
Importantly, the cutpoints were not robust to changes in methodology, and if the cutpoints determined in our study are to be used, the methodology for screening blood glucose needs to be adhered to. Compared with screening blood glucose, agreement was poor when blood glucose was measured after physical examination and jugular blood sampling using the meter with blood either dropped from the syringe or preserved with FO then tested. There was also poor agreement with screening when glucose was measured in blood from the jugular sample several hours later by an external laboratory. There were a number of differences in methodology between screening blood glucose and these traditional sampling methods, and the relative contribution of these differences is unknown. However, they highlight the importance of adhering to the methodology described. The glycolysis inhibitor, FO, does not completely inhibit glycolysis, and it artefactually decreases plasma glucose concentration because of movement of water from erythrocytes, resulting in plasma dilution and erythrocyte shrinkage.32,33 Therefore, if the cutpoints identified in our study are being used, the methodology needs to be the same, including collection of the sample from the ear or foot pad on entry to the clinic and before physical examination, and blood glucose measured using a portable glucose meter calibrated for feline blood immediately after collection.
A range of blood glucose cutpoints are used for diagnosing diabetes in cats, varying from 171–290 mg/dl (9.5 34 to 16 mmol/l), the latter being the renal threshold. 35 In humans, the cutpoint for diabetes (126 mg/dl; 7 mmol/l) was established, in part, based on the associations with renal and microvascular complications. 11 Individuals below this cutpoint but above fasting (100 mg/dl; 5.6 mmol/l) are considered prediabetic and at high risk of developing clinical diabetes. 11 Approximately 30% of adult humans have undiagnosed diabetes, increasing to 62% by the age of 65 years. 36 It is likely that many cats have undiagnosed subclinical diabetes, and most cats with prediabetes are undiagnosed. The blood glucose concentration cutpoint for diabetes in cats urgently needs to be established.
Based on the increased incidence of diabetes in cats 8 years of age or older, 37 we recommend all senior cats have a screening blood glucose measured at each health check. This is especially important in cats with one or more additional risk factors for diabetes such as obesity, Burmese breed, male sex or glucocorticoid therapy. Measuring blood glucose in capillary blood from the ear or paw is easy and rapid to perform,14,15,18,38 and is less labour intensive and better tolerated than traditional methods of venous sampling (personal observation of author, MRJ). In our study of 134 cats, a screening blood sample was easily obtained in all but one cat and, for that cat, sampling from an ear or paw pad was successfully performed at a later date.
If blood glucose during the screening test is higher than the upper cutpoint for fasting (116 mg/dl; 6.4 mmol/l) but lower than the upper cutpoint for screening (189 mg/dl; 10.5 mmol/l), we recommend the cat is retested 3–4 h later to determine whether the hyperglycaemia has normalised and was likely associated with stress or eating. If hyperglycaemia is persistent, the cat should be retested at home or after overnight fasting and hospitalisation, ideally with a glucose tolerance test. In one study, stress hyperglycaemia resolved within 90 mins after the end of the stressor in the majority of cats, although in 8/20 cats glucose concentrations were still above baseline and up to 258 mg/dl [14.3 mmol/l]), and in general these cats had higher peak glucose concentrations during the spray bath. 6 In the author’s (JR) experience, 3–4 h is sufficient for stress hyperglycaemia to resolve in the majority of cats if they are quietly housed, and the second sample from the ear or pad is collected with the cat in the cage or carry basket. There is lack of definitive research in this area, but stress hyperglycaemia is likely affected by the nature and duration of the stressor(s) and the individual cat.
Senior cats with an initial screening blood glucose >189 mg/dl (10.5 mmol/l), or a second screening blood glucose ⩾117 mg/dl (6.5 mmol/l), should have fasting blood glucose and glucose tolerance measured after an 18–24 h fast and overnight hospitalisation. Based on data in humans, 11 senior cats with persistent mild hyperglycaemia or glucose intolerance could be considered prediabetic and at risk of developing diabetes, especially if other risk factors are present. Cats in diabetic remission with persistent fasting blood glucose between 135 mg/dl (7.5 mmol/l) and 151 mg/dl (8.4 mmol/l) have an estimated 14 times higher odds of development of diabetes compared with those that have glucose less than 135 mg/dl (7.5 mmol/l). 8 Mild persistently increased fasting blood glucose is therefore likely to be an indicator or risk factor for diabetes in other groups of predisposed cats, such as senior obese or Burmese cats. A longitudinal study would be required to identify the percentage of cats with impaired fasting glucose or glucose intolerance that develop clinical diabetes within 1–3 years.
One study cat had conflicting blood glucose data with screening 238 mg/dl (13.2 mmol/l) and a behaviour score of 2; traditional meter 117 mg/dl (6.5 mmol/l); fasting 221 mg/dl (12.3 mmol/l) and a normal glucose tolerance test. In humans and cats, glucose tolerance testing is more sensitive at detecting abnormalities of glucose metabolism than measurement of fasting glucose. 8 While occasionally some humans have mild impaired fasting glucose and normal glucose tolerance, this is unusual. 39 In this cat, screening and fasting blood glucose were in the diabetic range 40 and but traditional blood glucose measured approximately 20 mins after screening glucose was normal, and glucose tolerance was normal when measured the next day, suggesting both screening and fasting samples were stress affected. No clinical signs of diabetes developed in the ensuing 4 years after testing, supporting this interpretation of results. When fasting glucose is increased, but glucose tolerance is normal, stress hyperglycaemia should be suspected and measurement of blood glucose at home be considered.
In prediabetic humans, changes in lifestyle that increase physical activity and decrease body weight can mitigate the risk of developing diabetes. 41 In senior cats with impaired fasting glucose or impaired glucose tolerance, especially if other risk factors are present, it would also be prudent to implement strategies to reduce risk. For example, implementing a weight loss protocol and increasing physical activity in obese cats, changing from a high carbohydrate to a low carbohydrate diet, and reducing or eliminating steroid administration. 42
Conclusions
We established cutpoints for screening blood glucose concentrations in a population of healthy cats aged ⩾8 years and demonstrated that cutpoints are not robust to changes in methodology. We recommend that screening blood glucose, as described in our study, be included in the health examination for all senior cats. It is simple to perform, is well tolerated, and has the benefit of only needing 0.3 µl of blood. Cats with risk factors for diabetes and abnormal results should be retested to confirm they are persistent. For cats with persistently increased fasting glucose (impaired fasting glucose) or impaired glucose tolerance, it would be prudent to implement management strategies to reduce the risk of future diabetes, especially in cats with reversible risk factors such as obesity and corticosteroid administration. In cats where stress hyperglycaemia is suspected, it may be advisable to measure blood glucose at home. Future studies are needed to determine the relative risk for diabetes of blood glucose concentrations persistently above the cutpoints identified in this study, and to better understand transient hyperglycaemia in cats associated with stress and eating.
Acknowledgments
The authors wish to thank the Cat Clinics, Greencross Veterinary Clinics, Small Animal Hospital UQ St Lucia, participating owners and cats, and Dr Magdalena Zabek for assisting with sampling of the cats and formatting the manuscript.
Footnotes
Presented at the American College of Veterinary Internal Medicine Annual Forum, June 2012.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Abbott Animal Health, Illinois, USA and David Galbraith provided funding towards the study.
Accepted: 30 November 2016
References
- 1. Baral RM, Rand J, Catt MJ, et al. Prevalence of feline diabetes mellitus in a feline private practice. J Vet Intern Med 2003; 17: 434. [Google Scholar]
- 2. Panciera DL, Thomas CB, Eicker SW, et al. Epizootiological patterns of diabetes-mellitus in cats – 333 cases (1980–1986). J Am Vet Med Assoc 1990; 197: 1504–1508. [PubMed] [Google Scholar]
- 3. Lund EM, Armstrong PJ, Kirk CA, et al. Prevalence and risk factors for obesity in adult cats from private US veterinary practices. Int J Appl Res Vet Med 2005; 3: 88–96. [Google Scholar]
- 4. Gavin JR, Alberti K, Davidson MB, et al. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2009; 32: S62–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inzucchi S, Bergenstal R, Fonseca V, et al. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33: S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rand JS, Kinnaird E, Baglioni A, et al. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med 2002; 16: 123–132. [DOI] [PubMed] [Google Scholar]
- 7. Heine RJ, Mooy JM. Impaired glucose tolerance and unidentified diabetes. Postgrad Med J 1996; 72: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gottlieb S, Rand J, Marshall R, et al. Glycemic status and predictors of relapse for diabetic cats in remission. J Vet Intern Med 2015; 29: 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laluha P, Gerber B, Laluhova D, et al. Stress hyperglycemia in sick cats: a retrospective study over 4 years. Schweiz Arch Tierheilkd 2004; 146: 375–383. [DOI] [PubMed] [Google Scholar]
- 10. Coradini M, Rand JS, Morton JM, et al. Delayed gastric emptying may contribute to prolonged postprandial hyperglycemia in meal-fed cats. J Vet Intern Med 2006; 20: 726–727. [Google Scholar]
- 11. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009; 32: S62–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reeve-Johnson MK, Rand JS, Anderson S, et al. Human white coat hypertension: a community based feline model. J Vet Pharmacol Ther 2012; 35: 176–177. [Google Scholar]
- 13. Lardinois C. White coat hyperglycemia. Arch Family Med 1994; 3: 461–464. [DOI] [PubMed] [Google Scholar]
- 14. Casella M, Wess G, Reusch CE. Measurement of capillary blood glucose concentrations by pet owners: a new tool in the management of diabetes mellitus. J Am Anim Hosp Assoc 2002; 38: 239–245. [DOI] [PubMed] [Google Scholar]
- 15. Sieber-Ruckstuhl NS, Alt N, Reusch CE. Measurement of blood sugar levels using capillary blood from the ear in diabetic dogs and cats. Comment on the GST-VETLINE discussion of November 2003. Schweiz Arch Tierheilkd 2004; 146: 92–93. [DOI] [PubMed] [Google Scholar]
- 16. Thompson MD, Taylor SM, Adams VJ, et al. Comparison of glucose concentrations in blood samples obtained with a marginal ear vein nick technique versus from a peripheral vein in healthy cats and cats with diabetes mellitus. J Am Anim Hosp Assoc 2002; 221: 389–392. [DOI] [PubMed] [Google Scholar]
- 17. Casella M, Hassig M, Reusch CE. Home-monitoring of blood glucose in cats with diabetes mellitus: evaluation over a 4-month period. J Feline Med Surg 2005; 7: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wess G, Reusch C. Capillary blood sampling from the ear of dogs and cats and use of portable meters to measure glucose concentration. J Small Anim Pract 2000; 41: 60–66. [DOI] [PubMed] [Google Scholar]
- 19. Zeugswetter F, Benesch T, Pagitz M. Validation of the portable blood glucose meter FreeStyle Freedom for the use in cats. Wien Tierarztl Monatsschr 2007; 94: 143–148. [Google Scholar]
- 20. Wess G, Reusch C. Assessment of five portable blood glucose meters for use in cats. Am J Vet Res 2000; 61: 1587–1592. [DOI] [PubMed] [Google Scholar]
- 21. Zini E, Moretti S, Tschuor F, et al. Evaluation of a new portable glucose meter designed for the use in cats. Schweiz Arch Tierheilkd 2009; 151: 448–451. [DOI] [PubMed] [Google Scholar]
- 22. Laflamme D. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 1997; 25: 13–18. [Google Scholar]
- 23. Reeve-Johnson M, Rand J, Vankan D, et al. Diagnosis of prediabetes in cats: glucose concentration cut points for impaired fasting glucose and impaired glucose tolerance. Domest Anim Endocrinol 2016; 57: 55–62. [DOI] [PubMed] [Google Scholar]
- 24. Coradini M, Rand JS, Morton JM, et al. Effects of two commercially available feline diets on glucose and insulin concentrations, insulin sensitivity and energetic efficiency of weight gain. Br J Nutr 2011; 106: S64–S77. [DOI] [PubMed] [Google Scholar]
- 25. Earle KE, Smith PM. Digestible energy-requirements of adult cats at maintenance. J Nutr 1991; 121: S45–S46. [DOI] [PubMed] [Google Scholar]
- 26. Coradini M, Rand JS, Morton JM, et al. Effects of two commercially available feline diets on glucose and insulin concentrations, insulin sensitivity and energetic efficiency of weight gain (vol 106, pg S64, 2011). Br J Nutr 2012; 107: 1402–1402. [DOI] [PubMed] [Google Scholar]
- 27. Flint M, Morton JM, Limpus CJ, et al. Development and application of biochemical and haematological reference intervals to identify unhealthy green sea turtles (Chelonia mydas). Vet J 2010; 185: 299–304. [DOI] [PubMed] [Google Scholar]
- 28. Harris M. Classification and diagnosis of diabetes-mellitus and other categories of glucose-intolerance. Diabetes 1979; 28: 1039–1057. [DOI] [PubMed] [Google Scholar]
- 29. Farrow H, Rand J, Morton JM, et al. Effect of dietary carbohydrate, fat and protein on postprandial glycemia and energy intake in cats J Vet Intern Med 2013; 27: 1121–1135. [DOI] [PubMed] [Google Scholar]
- 30. Appleton DJ, Rand JS, Priest J, et al. Determination of reference values for glucose tolerance, insulin tolerance, and insulin sensitivity tests in clinically normal cats. Am J Vet Res 2001; 62: 630–636. [DOI] [PubMed] [Google Scholar]
- 31. Hoenig M. The cat as a model for human nutrition and disease. Curr Opin Clin Nutr Metabolic Care 2006; 9: 584–588. [DOI] [PubMed] [Google Scholar]
- 32. Waring WS, Evans LE, Kirkpatrick CT. Glycolysis inhibitors negatively bias blood glucose measurements: potential impact on the reported prevalence of diabetes mellitus. J Clin Pathol 2007; 60: 820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ceron JJ, Martinez-Subiela S, Hennemann C, et al. The effects of different anticoagulants on routine canine plasma biochemistry. Vet J 2004; 167: 294–301. [DOI] [PubMed] [Google Scholar]
- 34. Rios L, Ward C. Feline diabetes mellitus: diagnosis, treatment, and monitoring. Comp Cont Educ Vet 2008; 30: 626–639. [PubMed] [Google Scholar]
- 35. Kruth S, Cowgill L. Renal glucose transport in the cat [abstract]. Proceedings of the American College of Veterinary Internal Medicine Scientific Forum; May 1982; San Diego, USA, p 78. [Google Scholar]
- 36. Cowie CC, Engelgau MM, Rust KF, et al. Prevalence of diabetes and impaired fasting glucose in adults in the US population – National Health and Nutrition Examination Survey 1999–2002. Diabetes Care 2006; 29: 1263–1268. [DOI] [PubMed] [Google Scholar]
- 37. Rand JS, Marshall RD. Diabetes mellitus in cats. Vet Clin N Am Small Anim Pract 2005; 35: 211–224. [DOI] [PubMed] [Google Scholar]
- 38. Zini E. An update on the pathogenesis of diabetes mellitus in cats and on the methods used to monitor glucose levels. Veterinaria 2009; 23: 51–60. [Google Scholar]
- 39. Gavin JR, Alberti K, Davidson MB, et al. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997; 20: 1183–1197. [DOI] [PubMed] [Google Scholar]
- 40. Crenshaw KL, Peterson ME. Pretreatment clinical and laboratory evaluation of cats with diabetes mellitus: 104 cases (1992–1994). J Am Vet Med Assoc 1996; 209: 943–949. [PubMed] [Google Scholar]
- 41. Kopelman PG. Obesity as a medical problem. Nature 2000; 404: 635–643. [DOI] [PubMed] [Google Scholar]
- 42. Rand JS, Fleeman LM, Farrow HA, et al. Canine and feline diabetes mellitus: nature or nurture? J Nutr 2004; 134: 2072S–2080S. [DOI] [PubMed] [Google Scholar]


