Abstract
Objectives
The objective was to evaluate the clinical efficiacy of a constant rate infusion of heated fluid as the sole means of preventing intraoperative hypothermia in cats.
Methods
This randomised, prospective, clinical study was conducted at a university teaching veterinary hospital. Female cats (American Society of Anesthesiologists [ASA] grade I) undergoing elective surgery by laparotomy under general anaesthesia (acepromazine 0.05 mg/kg SC; morphine 0.2 mg/kg IV; propofol IV titrated, isoflurane 2% in 100% oxygen) were randomised in two groups. Both groups were infused with fluid (NaCl 0.9%, 5 ml/kg/h) either at room temperature (control group) or prewarmed at 43°C (warmed group) using an Astoflo Plus eco (Stihler Electronic) fluid heating device. No other heating device was used. Temperature, heart rate, respiratory rate and SpO2 were evaluated after induction (T0) and every 15 mins for 1 h (T15, T30, T45, T60). Mean arterial blood pressure was recorded every 30 mins (T0, T30 and T60).
Results
Thirty-four female cats (ASA grade I) were enrolled in the study. There was no difference in age, weight, propofol dose or room temperature (22.4 ± 1.1°C vs 22.0 ± 1.5°C; P = 0.363) between control and warmed groups, respectively. In both groups, oesophageal temperature significantly decreased during anaesthesia (P <0.0001). The temperature decrease after 1 h was −3.6 ± 0.7°C in the warmed group and was not significantly different from the control group (−3.4 ± 0.7°C; P = 0.307). The slopes of the temperature decrease did not significantly differ between the two groups (–0.058 ± 0.013°C/min vs −0.060 ± 0.010°C/min for the control and warmed groups, respectively; P = 0.624).
Conclusions and relevance
This study provides clinical evidence that a constant rate infusion of heated fluid alone fails to prevent intraoperative hypothermia in cats. The low infusion rate (5 ml/kg/h) could partly explain the ineffectiveness of this active warming device in minimising or delaying the onset of intraoperative hypothermia.
Introduction
As a result of redistribution and the imbalance between heat production and heat losses, hypothermia is recognised as a frequent side effect of general anaesthesia, both in human and veterinary medicine. Defined as a decrease in body temperature below 37°C,1,2 hypothermia is observed in more than 97% of anaesthetised cats. 3 Intraoperative hypothermia has numerous causes and predisposing factors, including a large surface area-to-mass ratio, redistribution, anaesthesia per se, as well as intraoperative causes such as open abdominal procedures or the use of cold intravenous (IV) fluids. Hypothermia is also considered to be associated with coagulopathy, arrhythmias, increased rate of wound breakdown, infections, impaired renal and hepatic functions,1,2,4 and death, although to our knowledge this has not been clearly demonstrated in cats. Thus, prevention of hypothermia remains a major issue in veterinary anaesthesia.
Passive and active warming methods have been used to prevent iatrogenic hypothermia, but mainly in dogs. 5 Few data are available for cats.6,7 In children, constant rate infusion (CRI) of heated fluids has been successfully proposed to prevent intraoperative hypothermia.8,9 In two recent in vitro veterinary studies,10,11 no beneficial thermodynamic effect was found when comparing the use of prewarmed and room temperature IV fluids. However, one study showed that warmed IV infusions have a significant influence on the reduction of heat loss in cats. 7 CRI of heated fluids is now a widespread recommendation for good clinical practice in veterinary practice. But, surprisingly, even if some in vitro studies and a few in vivo studies have pointed out this method to be promising, there is not, as yet, solid evidence that infusion of prewarmed fluids has a clinical impact on prevention of hypothermia during anaesthesia.
To the best of our knowledge, no in vivo study using CRI of heated fluids as the sole active warming method during general anaesthesia has been conducted in cats. We hypothesised that continuous infusion of warmed fluids alone at a standard rate would help to prevent intraoperative hypothermia.
Materials and methods
In vivo study
Female cats presented to our veterinary university hospital between April and June 2014 for elective surgery (ovariectomy or ovariohysterectomy) and considered to be American Society of Anaesthesiologists (ASA) grade I, on the basis of a physical examination, were included in this randomised prospective study. A table of randomisation was previously established using the aleatory function of Excel software (Microsoft Excel 2010). Cats were cared for according to institutional guidelines. Procedures were approved by our local ethical committee (SSA_2016_007) and informed consent was obtained from the cats’ owners prior to enrolment. An a priori statistical approach was performed to determine minimal sample size to show a significant clinical difference of 2°C. Using α = 0.05, β = 0.8, SD = 1 and a mean temperature of 36°C and 34°C in warmed and control groups, respectively, the test of power (XLSTAT v2012; Addinsoft, France) indicated a minimal number of seven animals in each group.
After a preoperative physical examination, including rectal temperature (Predictor; Omega Pharma), cats were premedicated with acepromazine (0.05 mg/kg SC [Calmivet; Vetoquinol]) and morphine (0.2 mg/kg IV [Morphine Chlorhydrate; Laboratoire Aguettant]). Twenty minutes later, general anaesthesia was induced with propofol (Propovet; Zoetis) and maintained with 2% isoflurane (Vetflurane; Virbac) in 100% oxygen (200 ml/kg/min) using a non-rebreathing circuit (Bain circuit). During anaesthesia, all cats were administered a 5 ml/kg/h CRI of isotonic crystalloid fluids (NaCl 0.9%) through a syringe pump (Vial Program 2; Becton Dickinson) to prevent arterial hypotension and compensate for fluid losses. Cats were randomised into two groups. They received either fluids at room temperature, around 22°C (control group) or fluids initially heated up to 43°C using an Astoflo Plus eco (Stihler Electronic) fluid heating device (warmed group). The infusion set was fully threaded through a tube-like structure, named the heating profile by the manufacturer, which actively warmed the fluid along its length. The heating profile had integrated temperature sensors that guaranteed optimum warming right up to a three-way valve connected to the cats’ IV catheters. Cats were insulated from the table with disposable undersheets. No other heating device was used during the period of observation. For ethical reasons, observation was deliberately limited to the first hour.
Body temperature (T°) was recorded regularly for 60 mins using an oesophageal thermometer (MM8; Kontron Medical) placed at the level of the fifth intercostal space. We also evaluated heart rate (HR), respiratory rate (RR), pulse oximetry (SpO2) (MM8; Kontron Medical) and non-invasive mean arterial blood pressure (MAP) (PetMap; Ramsey Medical). Parameters were recorded at the same time, starting immediately after induction (T0) and then every 15 mins (T15, T30, T45 and T60) for HR, RR, and SpO2, and every 30 mins for MAP (T30, T60). In case of symptomatic and durable hypotension (MAP <60 mmHg), supportive therapy was instituted (IV bolus of crystalloid fluids 5 ml/kg and ephedrine 0.1–0.2 mg/kg IV, or both). Similarly, rescue doses of morphine would be added if necessary. Treatments would be left to the judgement of the anaesthetist, in accordance with good clinical practice of our institution.
Changes in parameter values were calculated as the difference between measured values at T60 and T0 (ΔT60–T0).
Occurrence of side effects (local inflammation, venous pain, burn) was observed in the warmed group for 12 h postoperatively.
In vitro study
We planned further in vitro measurements to evaluate the temperature of fluids warmed with Astoflo Plus eco fluid heating device (Stihler Electronic) using an infrared thermometer (Raynger MX4; Raytek). The temperature of fluids was measured at two points: immediately upstream and downstream of the three-way valve connected to the infusion set, itself enclosed in the heating profile. Fluids were infused at 15 ml/h, corresponding to a standard rate during general anaesthesia (ie, 5 ml/kg/h) for a cat with a mean body weight of 3 kg. This way, 30 pairs of data regarding temperature were generated over 3 days (10 per day).
Statistical approach
Results are presented as mean ± SD. The normality of the data was assessed with the Shapiro–Wilk test and homogeneity of variance was confirmed using the Fisher–Snedecor test. All statistical comparisons were performed using the parametric approach. Intergroup comparisons were performed using the unpaired bilateral Student’s t-test (including Aspin–Welch correction, where necessary) or one-way ANOVA when required. To evaluate the time influence within a group, ANOVA for repeated measures was performed, followed by a bilateral Bonferroni-Dunnett post-hoc test using T0 values as control. A P value ⩽0.05 was considered significant. Each statistical analysis was performed using commercial software (XLSTAT v2012; Addinsoft).
Results
In vivo results
Thirty-four female cats were included in the in vivo part of the study. All the cats were attributed ASA grade I status.
There was no significant difference regarding preoperative clinical parameters between the control (n = 17) and warmed (n = 17) groups, respectively: age (13.6 ± 8.5 vs 14.8 ± 10.8 months; P = 0.714); weight (3.0 ± 0.6 vs 2.9 ± 0.7 kg; P = 0.665), HR (191.3 ± 27.6 vs 188.9 ± 24.9 beats per min [bpm]; P = 0.791); RR (57.2 ± 23.4 vs 54.8 ± 18.8 movements per min [mpm]; P = 0.749); and rectal temperature (38.9 ± 0.5 vs 38.9 ± 0.6°C; P = 0.829).
Total duration of anaesthesia (109 ± 22 vs 107 ± 24 mins; P = 0.749) was not significantly different for the control and warmed groups, respectively. During anaesthesia, parameter changes failed to be significantly different between the two groups: ΔT60–T0HR = 2.1 ± 19.1 vs 8.2 ± 30.7 bpm (P = 0.487); ΔT60–T0RR = −3.3 ± 13.9 vs −1.5 ± 15.1 mpm (P = 0.725); ΔT60–T0SpO2 = −0.5 ± 1.8 vs 0.2 ± 1.6% (P = 0.201) in the control and warmed groups, respectively. MAP values slightly increased from T0 to T30 and T60 in the two groups (59.8 ± 9.8, 76.8 ± 27.3, 73.4 ± 14.7 mmHg vs 68.6 ± 16.7, 66.6 ± 12.3, 85.2 ± 23.1 mmHg in the control and warmed groups, respectively). MAP values at T60 were significantly increased compared with T0 in control (P = 0.002) and warmed (P = 0.024) groups.
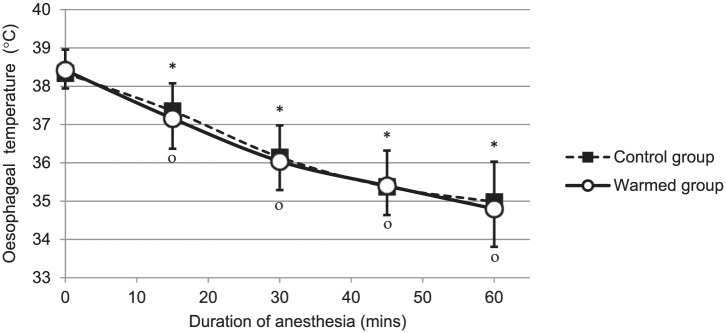
Room temperature (22.4 ± 1.1°C vs 22.0 ± 1.5°C; P = 0.363) was not significantly different for the control and warmed groups, respectively. In the two groups, body temperature significantly (P <0.0001) decreased during surgical procedure performed under general anaesthesia (Figure 1). The temperature decrease after 1 h of general anaesthesia (ΔT60–T0T°) was −3.6 ± 0.7°C in the warmed group and failed to be significantly different from the control group (–3.4 ± 0.7°C; P = 0.307). The slope of the temperature decrease during the first hour of anaesthesia was, respectively, −0.058 ± 0.013°C/min and −0.060 ± 0.010°C/min for the control and warmed groups and did not significantly differ between the two groups (P = 0.624).
Figure 1.
The decrease in oesophageal temperature in cats undergoing abdominal surgery. All cats were anaesthetised and subjected to a constant rate infusion of NaCl 0.9% at room temperature (control group, n = 17, black squares) or warmed to 43°C (warmed group, n = 17, open circles) using an Astoflo Plus eco (Stihler Electronic) fluid heating device. Symbols indicate a P value <0.05 compared with values measured at T0 using ANOVA for repeated measures followed by a bilateral Bonferroni–Dunnett post-hoc test in control (*) and warmed (°) groups, respectively
Concurrently, no side effects (local inflammation, venous pain, burn) were observed in the warmed group during the early postoperative period (12 h postoperative).
In vitro results
At the end of the heating profile and just before the three-way valve, the fluid temperature was 39.9 ± 1.1°C and significantly decreased at 31.6 ± 0.5°C (P <0.001) just after the three-way valve.
Discussion
Our primary results suggest that continuous infusion of warmed fluids at 5 ml/kg/h as the sole active warming method fails to prevent hypothermia in cats undergoing an abdominal surgical procedure. These in vivo results suggest that a warm infusion alone is unable to provide a sufficient calorific input to avoid intraoperative hypothermia in cats in our clinical anaesthetic conditions.
Our clinical trial was initially conducted in vivo and designed to prove that continuous infusion of heated fluids alone at a standard rate will help to prevent intraoperative hypothermia in cats. Unfortunately, that was not the case. Some in vitro tests were then performed after the in vivo phase, in order to explain the unexpected outcome. The in vitro results show that calorific input is reduced by the presence of a three-way valve between the infusion set inserted in the heating profile and the IV catheter. Indeed, the prewarmed fluid undergoes drastic heat loss (around 8°C) during its passage through the valve. This reduced calorific input could explain the ineffectiveness of this active internal warming method in preventing or reducing intraoperative hypothermia. In addition to the three-way valve, the low flow rate also played a role in heat dissipation. This was confirmed by the discrete decrease in temperature recorded between the heating device and the catheter region immediately upstream of the three-way valve. Taken together, these results show that infusion of heated fluids used as the sole active warming method to prevent or reduce hypothermia is unable to reduce or delay significantly the onset of intraoperative hypothermia in cats compared with infusion of fluids at room temperature.
Indeed, the low infusion rate used in cats and, subsequently, the low calorific input provided, can entirely explain the ineffectiveness of this active internal warming method in preventing or reducing intraoperative hypothermia. Change in mean body temperature (ΔMBT) is one of several methods used to quantify the effects of administering warmed IV fluids. 12 ΔMBT is defined as: [(Tf − Tpt)(Sf)(Vf)]/(Spt)(Wt), with Tf corresponding to the temperature of infused fluid (°C), Tpt to the core temperature of the patient (°C), Sf to the specific heat of the fluid (1.0 kcal/l°C for saline), Vf to the volume of fluid infused (kg), Spt to the specific heat of the patient (0.83 kcal/l°C) and Wt to patient weight (kg). In our study, ΔMBT after 1 h of anaesthesia was approximately −3.5°C. According to this formula, for a cat with a mean body weight of 3 kg and a total flow rate of 15 ml for 1 h, the temperature of the infused fluid needed to prevent a decrease of 3.5°C in body temperature should be above 43°C. Our in vivo results seem in accordance with this formula, as well as the in vitro results of two recent veterinary studies, which concluded that there is no thermodynamic benefit to using heated IV fluids compared with room temperature IV fluids at low rate for small animals (⩽60 ml/h).10,11
The lack of significant clinical effect observed in our study may be also explained, in part, by the small size of groups. However, the a priori statistical approach to showing a significant clinical difference indicates a minimum number of seven animals in each group. Moreover, two previous studies have shown significant results concerning hypothermia and its prevention in a group of eight dogs and between two groups of 15 cats.5,7 With 34 cats (two groups of 17 cats) included in our study, we could expect a statistically significant result if one existed. In the study by Steinbacher et al, 7 warmed IV infusions have a significant influence on the reduction of perioperative heat loss in cats, but major differences can be noted compared with our study. First, in the study by Steinbacher et al, 7 fluid rate is greater (10 ml/kg/h). Second, the type of procedure (ie, diagnostic or surgical) requiring general anaesthesia in cats is not defined. Finally, the random use of a warm-water blanket in addition to warm fluid infusion precludes any conclusion regarding prewarmed fluid infusion as a sole method for prevention of hypothermia. Steinbacher et al concluded that other additional methods to prevent heat loss are necessary to keep the cats in a normothermic range. 7 Moreover, their study underlines the significant influence of room temperature, which should optimally be ⩾26°C.
Conclusions
Our results provide clinical evidence that the use of a fluid heating device alone cannot prevent or minimise hypothermia during general anaesthesia in cats, when a low-rate infusion is used. However, further in vivo studies appear to be necessary to evaluate the clinical efficacy of a point-of-care fluid-heating device in other clinical contexts requiring larger volumes of fluids in cats (eg, cats presenting with pre-existing hypotension and hypothermia in the emergency room). In any case, it could be useful to connect cats to the fluid warmer via a shorter infusion set, without a three-way valve between the infusion set threaded through the heating profile and the IV catheter, to minimise heat loss of the infused fluids.
Acknowledgments
We wish to acknowledge Mrs Séverine Dumond for her technical and nursing assistance.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 19 December 2016
References
- 1. Armstrong SR, Roberts BK, Aronsohn M. Perioperative hypothermia. J Vet Emerg Crit Care 2005; 15: 32–37. [Google Scholar]
- 2. Todd JM. Hypothermia. In: Silverstein DC, Hopper K. (eds). Small animal critical care medicine. 2nd ed. St Louis, MO: Saunders, 2015, pp 789–794. [Google Scholar]
- 3. Redondo JI, Suesta P, Gil L, et al. Retrospective study of the prevalence of postanaesthetic hypothermia in cats. Vet Rec 2012; 170: 206–209. [DOI] [PubMed] [Google Scholar]
- 4. Otto K. Therapeutic hypothermia applicable to cardiac surgery. Vet Anaesth Analg 2015; 42: 559–569. [DOI] [PubMed] [Google Scholar]
- 5. Clark-Price SC, Dossin O, Jones KR, et al. Comparison of three different methods to prevent heat loss in healthy dogs undergoing 90 minutes of general anesthesia. Vet Anaesth Analg 2013; 40: 280–284. [DOI] [PubMed] [Google Scholar]
- 6. Hale FA, Anthony JMG. Prevention of hypothermia in cats during routine oral hygiene procedures. Can J Vet 1997; 38: 297–299. [PMC free article] [PubMed] [Google Scholar]
- 7. Steinbacher R, Mosing M, Eberspächer E, et al. Der einsatz von infusionswärmepumpen vermindert perioperative hypothermie bei katzen [Article in German]. Tierärztl Prax 2010; 38: 15–22. [PubMed] [Google Scholar]
- 8. Hong-xia X, Zhi-jian Y, Hong Z, et al. Prevention of hypothermia by infusion of warm fluid during abdominal surgery. J Perianesth Nursing 2010; 25: 366–370. [DOI] [PubMed] [Google Scholar]
- 9. Campbell G, Alderson P, Smith AF, et al. Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev 2015; 4: CD009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee RA, Towle Millard HA, Weil AB, et al. In vitro evaluation of three intravenous fluid line warmers. J Am Vet Med Assoc 2014; 244: 1423–1428. [DOI] [PubMed] [Google Scholar]
- 11. Sorro JI, Towle Millard HA, Lee RA, et al. In vitro comparison of output fluid temperatures for room temperature and prewarmed fluids. J Small Anim Pract 2014; 55: 415–419. [DOI] [PubMed] [Google Scholar]
- 12. Chiang V, Hopper K, Mellena MS. In vitro evaluation of the efficacy of a veterinary dry heat fluid warmer. J Vet Emerg Crit Care 2011; 21: 639–647. [DOI] [PubMed] [Google Scholar]