Abstract
This study was conducted to validate an ultrasound-guided technique to block the sciatic nerve in cats. An anatomical study was first carried out in four feline cadavers to evaluate the feasibility of the glutea (cranial and caudal), femoris and poplitea ultrasonographical approaches for the sciatic nerve block. The results showed that the femoris approach was optimal because the region was free of vascular and bony structures, and the needle was easily visualised in-plane. Then, the efficacy of the femoris ultrasonographical approach to block the sciatic nerve was tested in six healthy adult experimental cats. A dose of 2 mg/kg lidocaine 2% diluted in saline to a final volume of 1 ml was administered in all cats. The blockade was successful in all cases and the cats recovered uneventfully. This study shows the usefulness of the femoris approach in performing an ultrasound-guided blockade of the sciatic nerve in cats.
Introduction
The blockade of peripheral nerves produces complete inhibition of nociceptive input preventing the development of central sensitisation. 1 The analgesia provided by these techniques also reduces the required dose of anaesthetic agents and opioids, decreasing the incidence of side effects from the use of these drugs.2,3 Until recently, peripheral nerve block techniques were not commonly performed in small animals, but recent studies suggest an increased interest.4–6 Description and validation of these techniques in cats is scarce.7,8 Peripheral nerve blocks have traditionally been performed using landmarks or peripheral nerve electrostimulation to locate the target nerves. The recent development of high resolution, electronic transducers has enabled the development of ultrasound (US)-guided techniques to block peripheral nerves, which may improve the success rate of the block and the safety of these techniques. 9 US-guided peripheral nerve block techniques are useful for optimising needle position in relation to the target nerve and to observe, in real time, the spread of local anaesthetic (LA) solution around the nerve. 9 Furthermore, employing US-guided techniques may reduce the dose of LA required to produce a clinically effective nerve block. 10 This advantage could be of particular importance in the cat as this species has an increased risk of toxicity to LAs as a result of its lower capacity for hepatic glucuronidation. 11
The blockade of the sciatic nerve (ScN) has been recommended in dogs for surgical procedures carried out on the foot and tarsus, as it produces complete analgesia of the lateral aspect of the stifle, together with the distal parts of the pelvic limb. 4 The utility of US-guided techniques to perform the blockade of the ScN has been widely described in humans9,12 and, more recently, in the dog.5,6 The normal ultrasonographical appearance of the feline ScN has recently been described using the glutea cranial, glutea caudal, femoris and poplitea approaches. 7 However, to our knowledge there are no descriptions of an US-guided technique to block this nerve in the cat.
The objective of this study was to evaluate the usefulness of an US-guided technique to block the ScN in cats. The study was conducted in two stages. Firstly, an anatomical study was performed to establish the feasibility and accuracy of the glutea cranial, glutea caudal, femoris and poplitea ultrasonographical approaches to the ScN in order to select the optimal approach to perform its blockade. Secondly, an in vivo study was performed to examine the efficacy of the selected approach in experimental cats.
Materials and methods
Animals
The project was approved by the Local Animal Care and Ethics Committee. The study was designed to be carried out in two phases. In the first phase, eight pelvic limbs from four fresh adult feline cadavers (two males and two females) with a mean weight of 3.3 kg (range 2.9–4.5 kg) were used to determine the optimal approach to perform the block of the ScN. The cats were obtained from the Local Zoonoses and Public Health Service and were humanely euthansed for reasons unrelated to pelvic limb lameness. In the second phase, six healthy adult experimental male cats with a mean weight of 4.1 kg (range 3.8–5 kg) were employed to evaluate in vivo the efficacy of an US-guided technique to block the ScN. The animals were handled following the guidelines for humane care of experimental animals.
Procedures
Phase I: US-guided ink injection in vitro
Eight pelvic limbs from four fresh feline cadavers were used. The cadavers were positioned in lateral recumbency. The skin was clipped and cleaned from the sacroiliac region to just below the stifle and coupling gel was applied. The limbs were scanned immediately after euthanasia using a linear transducer set at a frequency of 13MHz (Mylab70). Two focal points were selected at depths of 0.5 cm and 1.5 cm to perform the scans of the ScN and guide the injections. The glutea cranial, glutea caudal, femoris and poplitea ultrasonographic approaches were tested. A total of two approaches were performed on each pelvic limb. The glutea cranial and femoris approaches were evaluated on the left pelvic limb while the glutea caudal and poplitea approaches were tested on the right pelvic limb.
The ScN was visualised on a transversal view in all the approaches. The anatomic landmarks employed to localise the ScN at the glutea cranial approach were the trochanter major and the ala ossis ilii (Figure 1). The trochanter major and the tuber ischiadicum were employed as the landmarks for the glutea caudal approach. The trochanter major, the os femoris and the condylus lateralis of the os femoris were the landmarks for the femoris and poplitea approaches. 7 The orientation marker of the transducer was positioned cranially for all the approaches with the exception of the glutea cranial approach where the mark was placed laterally. The needle was inserted parallel to the long axis of the US transducer (in-plane technique) or across the short axis of the transducer (out-of-plane technique) according to the approach tested. Once the ScN was identified, 1 ml of blue ink was injected by US-guidance using an atraumatic peripheral nerve block needle (Stimuplex D 0.71 • 40 mm 30°; B Braun Melsungen AG) inserted close to the nerve. Images of the ink distribution around the nerve were recorded. The limbs from two cadavers were immediately dissected to study the distribution of the ink injected. The other two cadavers were frozen at -20°C for 24 h, and, then, kept at -80°C for a further 24 h. Transverse cryosections of 0.3 cm in thickness were made at the level of the injection site using a high-speed bandsaw to perform a cross-sectional study of the distribution of the ink around the ScN.
Figure 1.
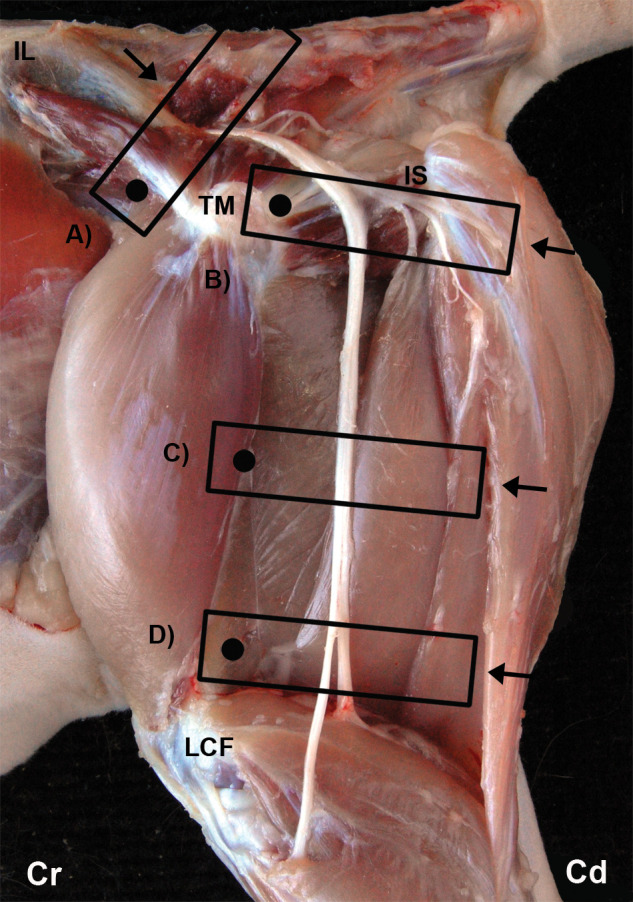
The glutea cranial (A), glutea caudal (B), femoris (C) and poplitea (D) US approaches. The position of the transducer is represented by a rectangle and the orientation marker of the transducer by a black dot. The arrow represents the pathway of the needle. IL = ala ossis ilii, TM = trochanter major of os femoris, IS = tuber ischiadicum, LCF = condylus lateralis of os femoris, Cr = cranial, Cd = caudal
Phase II: US-guided feline ScN block in vivo
In this part of the study, the ScN was located by the femoris approach at the mid-thigh level, as the results from phase I showed that this approach was the optimal to block the ScN under US-guidance. Six experimental cats sedated by intramuscular (IM) administration of medetomidine (Domitor; Pfizer) 30 μg/kg and butorphanol (Turbogesic; Fort Dodge) 0.2 mg/kg were used. The US-guided location of the ScN was always performed by the same investigators (PH, AA). The administration of LA to block the nerve was performed by a single operator (PH). The cats were placed in lateral recumbency and the skin was clipped and aseptically prepared. Then, a stab skin incision of 2 mm was performed in the caudal skin surface of the thigh using a surgical blade (11 Sovereign; Paramount Surgimed) to facilitate the insertion of the atraumatic peripheral nerve block needle. The needle was inserted using an in-plane technique. A dose of 2 mg/kg of lidocaine 2% (B Braun) diluted in saline to a final volume of 1 ml was injected around the ScN when the tip of the needle was seen close to the nerve. Multiple small volume injections were made around the ScN to elicit the appearance of a ‘doughnut’ sign. At the end of the procedure, sedation was reversed by IM administration of atipamezole (Antisedan; Pfizer) 75 μg/kg. The success of the ScN blockage was determined by the assessment of the motor and sensory function. Motor blockade was considered positive when proprioception was absent and the cats exhibited inability to flex the stifle and a paralysed foot. Sensory blockade was considered clinically effective when response to deep pain was absent. Deep pain was elicited by pinching the dorsal and plantar skin areas in the hind limbs with forceps. The forceps were progressively closed for a maximum time of 10 s until a pain-related response was noted or until the first ratchet notch was locked. The animals were also evaluated for 72 h to assess potential complications, such as haematomas or nerve injuries after the blocks.
Results
Phase I: US-guided ink injection in vitro
The ScN was correctly localised by US at all the tested ultrasonographical approaches. This was confirmed by observing the spread of ink around the ScN in the dissected cadavers (Figures 2 and 3), as well as in the transverse cryosections (Figure 4a–d). No evidence of intraneural injection was observed in any case. The femoris approach was considered to be the optimal ultrasonographical approach to block the ScN in the cat. This acoustic window was devoid of bony and vascular structures which could impair the trajectory of the needle to the nerve. Additionally, the needle could be easily inserted using an in-plane technique.
Figure 2.
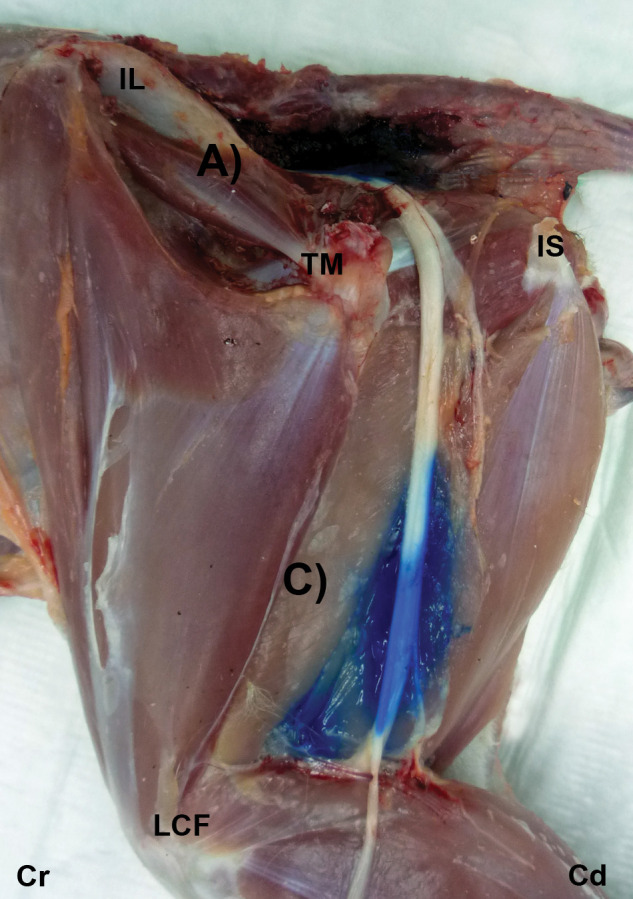
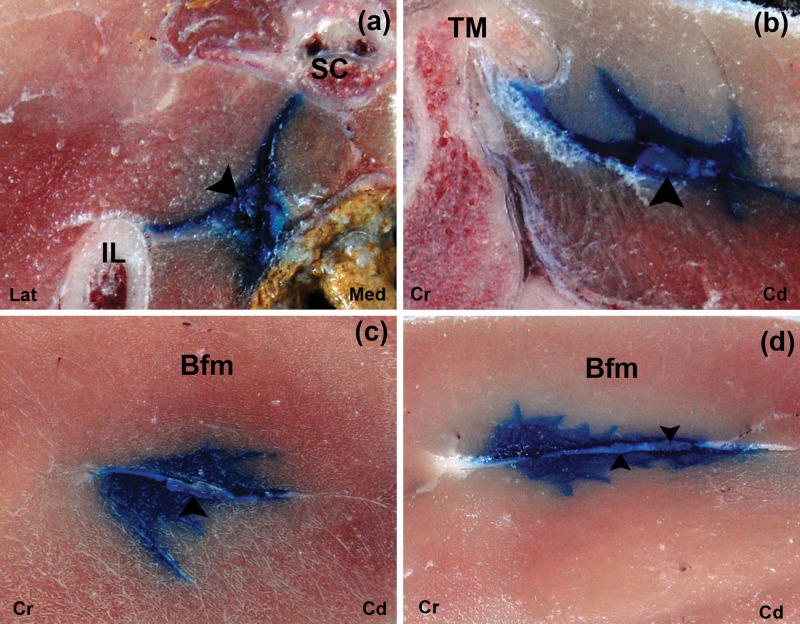
Gross dissection images after US-guided injection of 1 ml ink at the glutea cranial (A) and femoris (C) approaches. The ink is observed around the ScN. IL = ala ossis ilii, TM = trochanter major of os femoris, IS = tuber ischiadicum, LCF = condylus lateralis of os femoris, Cr = cranial, Cd = caudal
Figure 3.
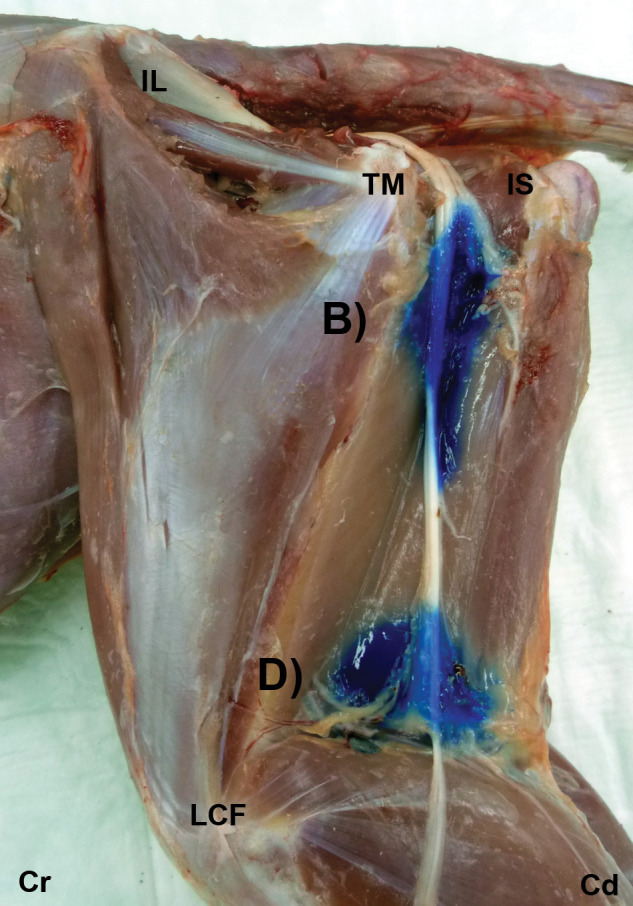
Gross dissection images after US-guided injection of 1 ml ink at the glutea caudal (B) and poplitea (D) approaches. The ink is observed around the ScN. IL = ala ossis ilii, TM = trochanter major of os femoris, IS = tuber ischiadicum, LCF = condylus lateralis of os femoris, Cr = cranial, Cd = caudal
Figure 4.
Cross-sectional images after US-guided injection of 1 ml ink at the glutea cranial (a), glutea caudal (b), femoris (c) and poplitea (d) approaches. The ink is observed around the ScN (arrow head). IL = ala ossis ilii, TM: = trochanter major of os femoris, Bfm = biceps femoris muscle, Sc = os sacrum, Lat = lateral, Med = medial, Cr = cranial, Cd = caudal
The poplitea approach was considered unreliable to perform a complete ScN block as the nerve bifurcates in this area into its two components (peroneus communis and tibialis nerves). The glutea cranial approach was difficult to perform owing to the os ilium and the os sacrum producing a wide acoustic shadow and a narrow acoustic window to access the ScN. Furthermore, it was only possible to position the needle out-of-plane. This approach was considered dangerous because of the presence of the glutea caudalis artery and vein in the area. The glutea caudal approach was found to be suboptimal in performing the ScN block in the cat owing to the limitations imposed by the trochanter major and the tuber ischiadicum which produced a large acoustic shadow, decreasing the visibility of the nerve in this area. It was possible to position the needle in-plane but it was difficult to displace it around the ScN.
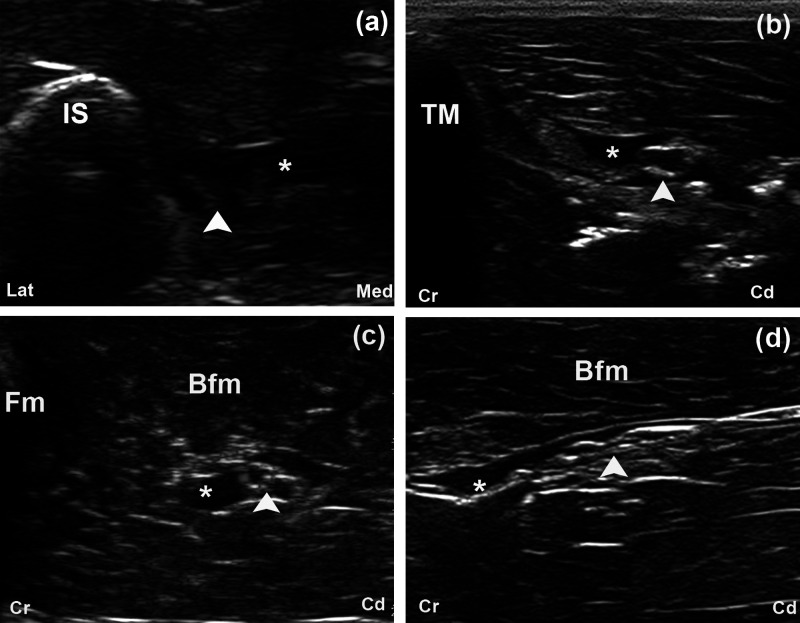
A complete perineural infiltration, the ‘doughnut’ sign, was observed in all cases at the glutea cranial, glutea caudal and femoris approaches (Figure 5a–c). This sign could not be produced at the poplitea approach (Figure 5d). The longest nerve stains with a mean length of 5.0 ± 0.34 cm were observed at the femoris approach. The length of the nerve stains were 4.2 ± 0.34 cm, 2.2 ± 0.34 cm and 2.2 ± 0.34 cm for the glutea caudal, poplitea and glutea cranial approaches, respectively.
Figure 5.
Transverse US images obtained after US-guided injection of 1 ml ink at the glutea cranial (a), glutea caudal (b), femoris (c) and poplitea (d) approaches in fresh cadavers. The ScN (arrow head) is surrounded by ink (*). The typical ‘doughnut’ sign is observed at the glutea cranial (a), glutea caudal (b) and femoris (c) approaches, but not at the poplitea approach (d). Fm = os femoris, TM = trochanter major of os femoris, IS = tuber ischiadicum, Sc = os sacrum, Bfm = biceps femoris muscle, Lat = lateral, Med = medial, Cr = cranial, Cd = caudal
Phase II: US guided feline ScN block in vivo
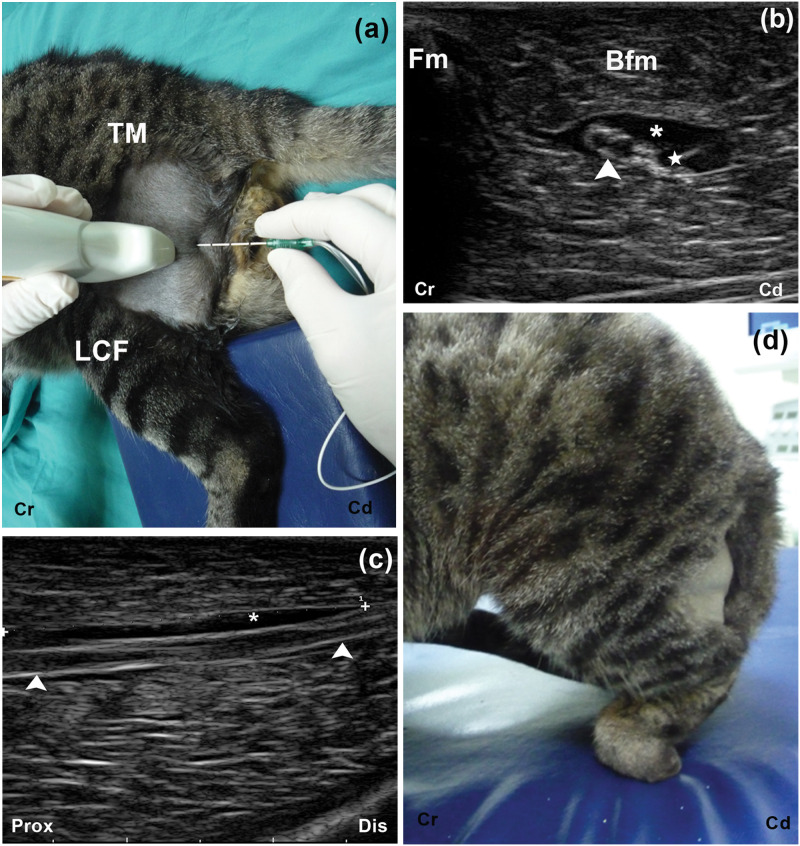
The blockade of the ScN by the femoris approach was easily and successfully performed in all cases (Figure 6a). The ‘doughnut’ sign was observed in five out of six cases (Figure 6b). In one case, the ScN was cranially displaced by the LA solution. The LA solution was distributed along the ScN at a mean length of 2.2 ± 0.37 cm (Figure 6c). Loss of proprioception and inability to flex the stifle and to perceive deep pain were confirmed in all the cats (Figure 6d). No complications were observed and the cats recovered uneventfully from the trials.
Figure 6.
Position of the US transducer and needle for the femoris approach to the ScN in experimental cats (a). Local anaesthetic (*) solution is surrounding the ScN (arrow head) showing the characteristic ‘doughnut’ sign. Tip of the needle (*) (b). Longitudinal US image showing the spread of local anaesthetic (*) along the ScN (arrow head) (c). Loss of proprioception after the injection (d).TM = trochanter major of the os femoris, LCF = condylus lateralis of os femoris, Fm = os femoris, Bfm = biceps femoris muscle, Lat = lateral, Med = medial, Cr = cranial, Cd = caudal, Prox = proximal, Dis = distal
Discussion
The block of the ScN is used for anaesthesia or analgesia during orthopaedic procedures of the stifle and distal parts of the hind limbs in dogs. 4 To our knowledge, this is the first study to investigate the use of a US-guided technique for the blockade of the ScN in cats. In dogs, several different approaches to the ScN block have been described.5,6 In this report, the glutea cranial, glutea caudal, femoris (midfemoral) and poplitea approaches were evaluated in vitro to validate an optimal US-guided technique to block the ScN in the cat. In the current study, the femoris approach at the mid-level of the femur was considered to be optimal to perform the ScN block owing to the fact that the nerve at this level is not accompanied by any other major nerve or vascular structures that could complicate or impair the block. Moreover, it was easier to maintain the needle in a more stable position by using this approach. 5 Another advantage of this approach is that the ScN can be approached at different levels because of the long length in which the nerve can be observed in this acoustic window. This could be useful in a clinical setting to avoid areas of skin lesion or infection in cats requiring this block. 5
In our study, the US-guided femoris approach allowed an easy and accurate ScN location and blockade in all the experimental cats. This was confirmed by the complete motor and sensory impairment observed after the blocks in all the cats. These results suggest that a ScN blockade may be achieved easily in cats using an US-guided technique alone without needing to confirm the accuracy of the nerve location by electrostimulation. In humans, the sole use of US to guide the blockade of the ScN using the midfemoral approach has been associated with a success rate of 95%. 13 In dogs, however, a success rate of 100% has been reported using the midfemoral approach. 5 On the contrary, only 67% of dogs were effectively blocked when a glutea cranial approach was employed to block the ScN. 6 The difficulty of the glutea cranial approach was also observed in our study.
An important advantage of using US-guided techniques to block a peripheral nerve is direct visualisation of the spread of LA during the injection around the target nerve. The presence of a complete perineural distribution of LA (‘doughnut’ sign) has been associated with a better outcome in terms of onset time, intensity, duration and a reduction in the required dose to achieve the block. 14 It has been reported previously that a volume of at least 1 ml is required to produce a ‘doughnut’ sign around the ScN in cats. 8 This volume produced a complete distribution of LA around the ScN in all the in vitro injections carried out by the glutea cranial, glutea caudal and femoris approaches. This sign was not observed after injecting the ink by the poplitea approach owing to the bifurcation of the ScN into its two nerve components at this level. In the experimental cats, the ScN blockade was clinically successful in all cases, despite the fact that the ‘doughnut’ sign was observed only in five out of six cases. This finding may reinforce recent reports supporting the idea that the presence of a complete perineural spread of LA may not be mandatory to produce a successful nerve block.8,15 This fact could be of importance in cats where LA toxicity is a major concern. Further research is necessary to investigate this issue.
In the current study, a dose of 2 mg/kg lidocaine was diluted in saline to produce a final volume of 1 ml. The final concentration of lidocaine given to the cats ranged between 0.76% and 1%, varying with the weight of the animals. This protocol was selected to maintain a constant administered volume of LA without increasing the risk of systemic toxicity, which may be produced by the administration of higher doses of lidocaine in the smallest cats. An important end point of this study was to confirm the production of a suitable ScN blockade in the experimental cats. The qualitative evaluation of the clinical effects produced by the administration of different concentrations of lidocaine was beyond the scope of this research. The use of a larger volume of diluted LA solution has been recommended to block major peripheral nerves to ensure that the length of nerve exposed to the solution is the adequate to achieve an effective nerve block. 16 It has been stated that the electrical activity of a peripheral nerve is blocked when the nerve is exposed to a LA solution along a critical length of 2 cm. 17 Our results revealed different spread averages between cadavers (5.0 ± 0.34 cm) and experimental cats (2.2 ± 0.37 cm). It has been previously reported that the distribution of an injectate in a clinical patient may be less than that observed in cadavers because of possible uptake of the solution by the lymphoid and blood circulation in live animals. 18 The differences found in this study between cadavers and live cats could also be explained by the different methods employed to evaluate the spread of injectate in the two phases of the study: gross anatomic dissection in cadavers and US in the experimental cats.
One of the limitations of the current study is the small number of live cats used. Therefore, further clinical studies are needed to confirm these results. Further research is also required to determine the onset time, duration and minimum volumes/doses required to obtain a clinically successful ScN block in this species.
Conclusions
The present study validates the utility of the US-guided midfemoral approach to block the ScN in cats. The use of this technique is useful in visualising the correct needle location and the distribution of LA during the blockade of the ScN. The dose and volume of LA employed in this study were sufficient to achieve successful blockade of the ScN in all the cats.
Footnotes
Funding: This research was supported by ‘Fundación Seneca’ – Agencia Regional de Ciencia y Tecnología (08784/PPC/08) Murcia (Spain) and CONACYT Consejo Nacional de Ciencia y Tecnología, México.
The authors do not have any potential conflicts of interest to declare.
Accepted: 7 March 2012
References
- 1. Lemke KA. Understanding the pathophysiology of perioperative pain. Can Vet J 2004; 45: 405–413. [PMC free article] [PubMed] [Google Scholar]
- 2. Davies AF, Segar EP, Murdoch J, et al. Epidural infusion or combined femoral and sciatic nerve blocks as perioperative analgesia for knee arthroplasty. Br J Anaesth 2004; 93: 368–374. [DOI] [PubMed] [Google Scholar]
- 3. Egger C, Love L. Local and regional anesthesia techniques, part 1: overview and five simple techniques. Vet Med 2009; 104: 24–39. [Google Scholar]
- 4. Campoy L. Fundamentals of regional anesthesia using nerve stimulation in the dog. In: Gleed RD, Ludders JW. (eds). Recent advances in veterinary anesthesia and analgesia: companion animals. Ithaca, NY: International Veterinary Information Service, 2008, http://www.ivis.org/advances/Anesthesia_Gleed/campoy/chapter.asp?LA=1 (accessed 10 February 2011). [Google Scholar]
- 5. Echeverry DF, Gil F, Laredo F, et al. Ultrasound-guided block of the sciatic and femoral nerves in dogs: A descriptive study. Vet J 2010; 186: 210–215. [DOI] [PubMed] [Google Scholar]
- 6. Shilo Y, Pascoe PJ, Cissell D, et al. Ultrasound-guided nerve blocks of the pelvic limb in dogs. Vet Anaesth Analg 2010; 37: 460–470. [DOI] [PubMed] [Google Scholar]
- 7. Haro P, Soler M, Gil F, et al. Ultrasonographic study of the feline sciatic nerve. J Feline Med Surg 2011; 13: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haro P, Laredo FG, Soler M, et al. Relevancia del signo de donut en el bloqueo ecoguiado del nervio ciático en el gato: Estudio piloto. Proceedings of the 7th Congreso Nacional de la Sociedad Española de Anestesia y Analgesia Veterinaria; 2011 May 6–7. Murcia, 2011, p 64. [Google Scholar]
- 9. Marhofer P, Greher M, Kapral S. Ultrasound guidance in regional anaesthesia. Br J Anaesth 2005; 94: 7–17. [DOI] [PubMed] [Google Scholar]
- 10. Casati A, Baciarello M, Di Cianni S, et al. Effects of ultrasound guidance on the minimum effective anaesthetic volume required to block the femoral nerve. Br J Anaesth 2007; 98: 823–827. [DOI] [PubMed] [Google Scholar]
- 11. Robertson SA. Managing pain in feline patients. Vet Clin North Am Small Anim Pract 2008; 38: 1267–1290. [DOI] [PubMed] [Google Scholar]
- 12. Sandhu NS, Harmon D. Ultrasound-guided sciatic nerve block. In: Harmon D, Friselle HP, NavParkash SS, et al. (eds). Perioperative diagnostic and interventional ultrasound. Philadelphia, PA: Saunders Elsevier, 2008, pp 164–173. [Google Scholar]
- 13. Domingo-Triadó V, Selfa S, Martínez F, et al. Ultrasound guidance for lateral midfemoral sciatic nerve block: a prospective, comparative, randomized study. Anesth Analg 2007; 104: 1270–1274. [DOI] [PubMed] [Google Scholar]
- 14. Kumar PA, Gentry WB, Arora H. Ultrasound guidance in regional anaesthesia. J Anesth Clin Pharmacol 2007; 23: 121–128. [Google Scholar]
- 15. Eichenberger U, Stockl S, Marhofer P, et al. Minimal local anesthetic volume for peripheral nerve block: A new ultrasound-guided, nerve dimension-based method. Reg Anesth Pain Med 2009; 34: 242–246. [DOI] [PubMed] [Google Scholar]
- 16. Nakamura T, Popitz-Bergez F, Birknes J, Strichartz G. The critical role of concentration for lidocaine block of peripheral nerve in vivo—studies of function and drug uptake in the rat. Anesthesiology 2003; 99: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 17. Raymond SA, Steffensen SC, Gugino LD, Strichartz GR. The role of length of nerve exposed to local anesthetics in impulse blocking action. Anesth Analg 1989; 68: 563–570. [PubMed] [Google Scholar]
- 18. Kuli K, Baer GA, Samarutel J, et al. Distribution of local anesthetic solution in retromediastinal block: Preliminary experimental results. Reg Anesth 1997; 22: 308–312. [PubMed] [Google Scholar]