Abstract
Propofol emulsion containing benzyl alcohol preservative (BA) was evaluated in cats. Eight (PB) received 1% propofol containing 2% benzyl alcohol and eight (PC) preservative-free propofol. In phase 1, cats were anaesthetised (8 mg/kg) three times at 48 h intervals. In phase 2, cats underwent three anaesthetic procedures at 48 h intervals where anaesthesia was maintained until 24 mg/kg had been administered. Clinical examination and haematological and biochemical analyses were performed regularly. Cardiorespiratory function was monitored throughout anaesthesia. Neurological examination was performed daily for 7 days after phase 2. All cats were euthanased 7 days after phase 2 and examined post mortem to determine any organ toxicity and to comply with regulatory requirements. Anaesthesia was as expected for propofol in cats and no clinically relevant differences between PB and PC were detected. The addition of BA has no additional effect when propofol is used at normal-to-high clinical doses in healthy cats.
Introduction
Propofol has been used successfully in clinical practice since its introduction into veterinary anaesthesia in the 1980s and is currently the most popular short-acting intravenous anaesthetic induction agent in dogs and cats.1–3 In most species propofol is rapidly metabolised and is well known for its high quality of anaesthesia: smooth induction, good relaxation and, in particular, a rapid, complete and excitement-free recovery. In common with most anaesthetics, propofol causes dose-dependent cardiovascular depression and significant respiratory depression.2,4
Propofol (di-isopropyl phenol) is an oil that is not water soluble. It is formulated for anaesthetic use in a lipid-based oil-in-water emulsion containing soybean oil and egg phospholipid. This emulsion supports bacterial growth; most propofol emulsions are marketed without preservative, necessitating the disposal of unused material within a few hours of opening the ampoule or vial. 5
The addition of a preservative to the propofol emulsion would allow opened vials to be retained for a longer period, thereby reducing wastage. A new propofol formulation containing the original lipid emulsion with 2% benzyl alcohol added as a preservative has been recently introduced to the market (PropoFlo Plus and PropoFlo 28; Abbott Laboratories). Benzyl alcohol has been used for many years as a preservative in a number of medicinal and food products.
The cat, however, is likely to be particularly vulnerable to benzyl alcohol toxicity,6,7 as it is deficient in glucuronidating metabolic pathways, which are involved in benzyl alcohol metabolism. 8 In addition, cats do not deal with propofol itself as well as dogs and other species. Recovery is slower, the cumulative effects are greater, and blood dyscrasias are well recognised hazards of propofol anaesthesia in cats.4,9
This investigation was conducted to evaluate the potential toxicity in cats that might be caused by the benzyl alcohol preservative. The effects of anaesthesia with propofol emulsion containing 2% benzyl alcohol preservative were compared with those of propofol emulsion alone. Maximum expected propofol dosages and the shortest time intervals between multiple anaesthetics likely to be encountered under clinical conditions were studied in order to assess the worst case scenario.
The study was approved by the testing laboratory’s Institutional Animal Care and Use Committee.
Materials and methods
Pilot study
Preliminary data were collected in a pilot study to assess the potential toxicity of a propofol (10 mg/ml) emulsion formulation containing 20 mg/ml benzyl alcohol in order to design the protocol for the main study. Sixteen healthy cats (eight male, eight female, weighing 3–4 kg, aged 6–12 months) were treated with benzyl alcohol doses up to 19.5 mg/kg (single dose) or 13.2 mg/kg followed by three 4.4 mg/kg doses on six occasions over 2 weeks (repeat dose) of either a propofol formulation containing 20 mg/ml benzyl alcohol or an unpreserved propofol emulsion. Clinical and physiological observations and clinical pathology measurements were made.
Main study
Animals
Sixteen healthy domestic short hair cats (eight female, eight male) weighing 1.9–2.7 kg and aged 4–5 months were included. They were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (US National Research Council 1996) and were housed individually in stainless steel cages with shelving perches, a litter tray and numerous toys. Room temperature was maintained between 19°C and 21°C (relative humidity 40–65%) and a 12 h light/dark cycle was provided. The cats were fed daily with Certified Purina Feline Diet 5003 and drinking water was available ad libitum. All cats were well handled and familiarised with the procedures before the study began.
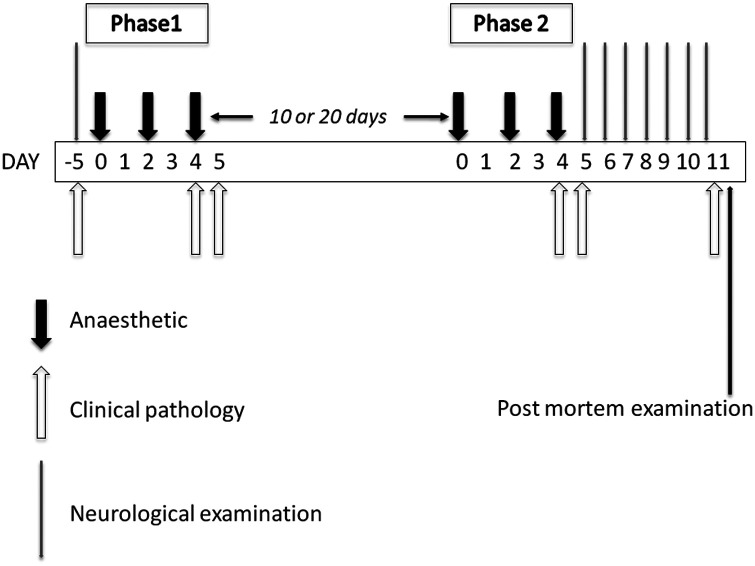
The cats were divided into two groups, with four males and four females each. Group PB received preserved propofol emulsion containing 10 mg propofol and 20 mg benzyl alcohol per millilitre (Multidose PropoFlo; Abbott Animal Health). Group PC, the control group, received unpreserved propofol emulsion, containing 10 mg/ml propofol only (PropoFlo; Abbott Laboratories). Prior to each anaesthetic procedure, food, but not water, was withheld overnight, and, under manual restraint alone, a catheter (22 g) was placed aseptically into a cephalic vein and secured with tape. The complete study protocol is illustrated in Figure 1.
Figure 1.
Schematic diagram illustrating the schedule of treatment
Phase 1
In both groups, each cat was anaesthetised three times at 48 h intervals with the respective propofol emulsion, PB or PC. On each occasion 8 mg/kg propofol was administered intravenously (IV) as a single dose; over 40 s on the first occasion, over 20 s on the second occasion and over 10 s on the third.
Phase 2
Ten (eight cats, four in each group) to 20 days (eight cats, four in each group) after phase 1, each cat was again anaesthetised three times, with 48 h between anaesthetic procedures. Again, group PB received preserved propofol and group PC received propofol without preservative. On each occasion a single dose of 8 mg/kg propofol was given IV over 10 s, followed by incremental bolus doses of 2 mg/kg as required (based on increases in muscle tone, respiratory rate, heart rate or jaw or eye movements) to maintain anaesthesia, until a further 16 mg/kg had been administered, leading to a total delivery of 24 mg/kg on each of the three occasions. Administration of the full 24 mg/kg took place over 45–120 min, as required. Lactated Ringer’s solution was administered IV at approximately 10 ml/kg/h throughout anaesthesia.
Anaesthesia and monitoring
Immediately prior to catheter placement and induction of anaesthesia, heart (HR) and respiratory (RR) rates were measured by auscultation and observation of the chest wall excursion, respectively. Immediately after induction, the trachea was intubated and the cats allowed to breath room air spontaneously. Each anaesthetised cat was placed on an insulated surface and covered with a towel to help maintain body temperature. During anaesthesia mucous membrane colour, HR and RR were recorded at 5 min intervals, as well as indirect blood pressure [mean arterial blood pressure (MABP) is reported] by oscillometry, oxygen haemoglobin saturation by lingual pulse oximetry (SpO2), end tidal carbon dioxide (ETCO2) measured using sidestream capnography and body temperature by rectal probe (SurgiVet Advisor; Smiths Medical PM). The endotracheal tube was removed when signs of swallowing were first seen after the last dose of propofol. Duration of anaesthesia was recorded in minutes. Recovery times to lifting head, achieving sternal recumbency and to standing were recorded in minutes from induction (phase 1) or from the last incremental dose (phase 2). Quality of recovery from anaesthesia was awarded a 0–3 score (see Table 1). Any adverse reactions such as apnoea (>90 s), bradycardia (<50 per min), hypotension [mean blood pressure (BP) <60 mmHg], hypoxaemia (SpO2 <90%) or seizures were recorded. All anaesthetic administration and assessments were carried out by personnel who did not know whether PB or PC was administered on each occasion.
Table 1.
Recovery scores
| 0 | Perfect: simply recovers consciousness, rolls into sternal and stands. No ataxia, normal cognition. |
| 1 | Good: recovers consciousness, one or two attempts to roll into sternal and stand. Some ataxia, normal cognition. |
| 2 | Moderate: recovers consciousness, rolls into sternal and stands with much ataxia, many extra attempts to roll into sternal and stand. Some hyperaesthesia, tremor. |
| 3 | Poor: recovers consciousness, many attempts to roll into sternal, and numerous crashing attempts to stand with a lot of ataxia; much hyperaesthesia, tremor. |
Clinical observations
All cats were observed twice daily for signs of any abnormality. Detailed clinical examination of each cat was performed during the pretest period within a few days prior to the first anaesthetic treatment, on the first day of dosing for each phase and 8–10 h after recovery from anaesthesia. Neurological examinations (Table 2) were carried out by an experienced clinician during the pretest period and daily for 1 week after the last dose of each phase.
Table 2.
Neurological examination
| At each examination made daily for 7 days after phase 2. |
| Each animal to be scored for the severity of each factor. |
| Only abnormal signs recorded (slight, moderate, severe). |
| 1 Aggression |
| 2 Hyperaesthesia |
| 3 Ease of handling (easy, difficult, impossible) |
| 4 Tremors |
| 5 Salivation |
| 6 Weakness |
| 7 Excitement |
| 8 Any other abnormal behaviour (including decreased consciousness, somnolence) |
Individual body weights were recorded weekly from the pretest period and on dosing days. Food consumption was recorded throughout the study.
Clinical pathology and post-mortem examination
Jugular venous blood was taken from all cats for haematology, coagulation evaluation, serum chemistry and blood gas analysis. Samples were withdrawn prior to the start of the study (pre), shortly after extubation at the end of each phase (extub), 24 h after the last dose in each phase (24 h), and immediately prior to euthanasia and post mortem examination (post). Overnight urine samples for routine urinalysis were collected from litter trays containing non-absorbent litter (NOSORB Catco) at the same time points. All cats were euthanased by pentobarbitone overdose 7 days after the last phase 2 anaesthetic procedure and a complete post-mortem examination was performed on each animal (Table 3).
Table 3.
Post-mortem examination
| All cats 7 days after phase 2. |
| Macroscopic examination and histology of: |
| Brain (cerebrum and medulla/pons) |
| Eyes (including optic nerve) |
| Heart |
| Kidneys |
| Liver |
| Skeletal muscle |
| Spinal cord |
| Thymus |
| Injection sites |
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.0d. Parametric data from the two groups were compared using analysis of variance (ANOVA) and changes with time within groups by using repeated measures ANOVA. Non-parametric data (recovery scores) were compared using the χ2 test P<0.05 was regarded as significant.
Results
Pilot study
One animal died as a result of propofol-related apnoea during the initial injection of the 19.5 mg/kg dose of the preserved formulation. Thereafter, respiratory support was given whenever high propofol doses caused apnoea. In the repeat-dose portion of the study, a single animal on the preserved formulation showed transient clinical signs (depression and cringing) on one occasion. There was no further mortality or morbidity in the study. It was concluded that the presence of 2% benzyl alcohol in a formulation of propofol does not cause significant morbidity and mortality in cats under excessive and repeat dose conditions.
Phase 1
With the exception of the hypoxaemia described below, anaesthesia was uneventful in all three anaesthetic procedures in each cat. Propofol anaesthesia increased HR and decreased RR from baseline during all three anaesthetic periods in both groups, but there were no significant differences between groups (Table 4). Mean PC group MABP ranged from 91–108 mmHg during anaesthesia and mean PB group MABP ranged from 87–126 mmHg. MABP was higher in the third anaesthetic period in the PB group but there were no changes in the PC group; there were no significant differences between the two groups (Table 4). SpO2 was below 90% in both groups during each anaesthetic period but there were no differences between the three anaesthetic periods in either group or between the groups (Table 4). The mean ETCO2 in the PC group ranged between 3.9 kPa and 4.7 kPa (29–35 mmHg) and between 4.4 kPa and 4.9 kPa (33–37 mmHg) in the PB group during anaesthesia. ETCO2 was significantly lower in the PC group during the second and third anaesthetic procedures compared with the first, but did not change over the three procedures in the PB group. ETCO2 was lower in the PC group than in the PB group (Table 4). Body temperature decreased during all three anaesthetic periods in both groups, but there were no significant differences between groups (Table 4). The lowest temperature recorded in either group was 36.5°C. There were some small differences between the groups in recovery time and these are shown in Table 5. Most recoveries were scored as 1 or 0 (good or perfect) and there were no significant differences between anaesthetic procedures in either group or between the two groups.
Table 4.
Physiological data, phase 1
| Heart rate (beats per min) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First anaesthetic |
Second anaesthetic |
Third anaesthetic |
|||||||||||||
| PC | Pre | 5 min | Extub | stand | 8h | Pre | 5 min | Extub | Stand | 8h | Pre | 5 min | Extub | Stand | 8h |
| Mean | 118 | 213 | 161 | 138 | 124 | 136 | 229 | 134 | 139 | 164 | 132 | 209 | 152 | 138 | 140 |
| SD | 14 | 36 | 43 | 22 | 22 | 12 | 19 | 5 | 18 | 17 | 8 | 28 | 19 | 10 | 7 |
| PB | |||||||||||||||
| Mean | 122 | 225 | 157 | 146 | 118 | 124 | 239 | 140 | 135 | 171 | 126 | 222 | 149 | 147 | 134 |
| SD | 5 | 27 | 30 | 29 | 19 | 8 | 49 | 13 | 12 | 10 | 12 | 17 | 10 | 12 | 8 |
No significant differences between groups
PC and PB: significant increase during anaesthesia
PC and PB: no significant difference between first, second and third anaesthetics
Table 5.
Phase 1: recovery time (min)
| First anaesthetic |
Second anaesthetic |
Third anaesthetic |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PC | Head | Stern | Stand | Head | stern | Stand | Head | Stern | Stand |
| Mean | 7 | 13 | 37 | 4 | 10 | 35 | 5 | 8 | 39 |
| SD | 3 | 7 | 11 | 2 | 6 | 5 | 3 | 1 | 6 |
| PB | |||||||||
| Mean | 3 | 11 | 33 | 4 | 10 | 36 | 7* | 11 | 48† |
| SD | 2 | 8 | 7 | 2 | 4 | 5 | 4 | 4 | 13 |
Group comparison: first anaesthetic head: PC longer than PB, otherwise no significant differences between groups
PC: no significant differences between first, second and third anaesthetics
PB: third anaesthetic recovery to head longer than first and second
Third anaesthetic recovery to stand longer than first and second
PC = control group — unpreserved propofol, SD = standard deviation, PB = group receiving propofol + 2% benzyl alcohol
| Respiratory rate (breaths per min) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First anaesthetic |
Second anaesthetic |
Third anaesthetic |
|||||||||||||
| PC | Pre | 5 min | Extub | Stand | 8h | Pre | 5 min | Extub | Stand | 8h | Pre | 5 min | Extub | Stand | 8h |
| Mean | 47 | 38 | 59 | 57 | 53 | 50 | 38 | 50 | 46 | 55 | 54 | 40 | 55 | 50 | 57 |
| SD | 4 | 7 | 23 | 10 | 14 | 5 | 6 | 11 | 7 | 9 | 11 | 4 | 7 | 4 | 6 |
| PB | |||||||||||||||
| Mean | 48 | 34 | 61 | 56 | 53 | 54 | 34 | 48 | 50 | 49 | 50 | 39 | 50 | 47 | 52 |
| SD | 7 | 4 | 11 | 16 | 12 | 8 | 6 | 8 | 8 | 6 | 4 | 18 | 12 | 7 | 8 |
No significant differences between groups
PC and PB: significant decrease during anaesthesia
PC and PB: no significant difference between first, second and third anaesthetics
| Body temperature (°C) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First anaesthetic |
Second anaesthetic |
Third anaesthetic |
|||||||||||||
| PC | Pre | 5 min | Extub | Stand | 8h | Pre | 5 min | Extub | Stand | 8h | Pre | 5 min | Extub | Stand | 8h |
| Mean | 37.5 | 36.8 | 37.3 | 37.4 | 36.9 | 37.5 | 37.4 | 36.9 | 37.2 | 37.0 | 37.5 | 37.2 | 37.4 | 36.9 | 37.5 |
| SD | 0.7 | 0.8 | 0.7 | 0.7 | 1.3 | 0.6 | 0.7 | 0.8 | 0.5 | 0.2 | 0.6 | 0.9 | 0.4 | 0.8 | 0.6 |
| PB | |||||||||||||||
| Mean | 37.7 | 37.1 | 37.4 | 37.5 | 37.3 | 38.0 | 37.3 | 37.2 | 37.1 | 36.9 | 37.7 | 37.7 | 36.8 | 36.5 | 37.8 |
| SD | 0.8 | 0.6 | 0.4 | 0.7 | 1.6 | 0.4 | 0.5 | 0.6 | 0.5 | 0.3 | 0.5 | 0.8 | 0.5 | 0.3 | 0.3 |
No significant differences between groups
PC and PB: significant decrease during anaesthesia
PC and PB: no significant difference between first, second and third anaesthetics
| Mean arterial blood pressure (mmHg) | |||||||
|---|---|---|---|---|---|---|---|
| First anaesthetic | Second anaesthetic | Third anaesthetic | First anaesthetic | Second anaesthetic | Third anaesthetic | ||
| PC | PB | ||||||
| Mean | 96 | 91 | 108 | Mean | 87 | 102 | 126* |
| SD | 23 | 33 | 36 | SD | 15 | 29 | 23 |
No significant differences between groups
PC: no significant differences between first, second and third anaesthetics
PB: third anaesthetic blood pressure greater than first and second
| SpO2 (% saturation) | |||||||
|---|---|---|---|---|---|---|---|
| First anaesthetic | Second anaesthetic | Third anaesthetic | First anaesthetic | Second anaesthetic | Third anaesthetic | ||
| PC | PB | ||||||
| Mean | 87 | 76 | 84 | mean | 83 | 79 | 84 |
| SD | 9 | 12 | 12 | SD | 4 | 7 | 7 |
No significant differences between groups
PC and PB: no significant differences between first, second and third anaesthetics
| ETCO2 (kPa) | |||||||
|---|---|---|---|---|---|---|---|
| First anaesthetic | Second anaesthetic | Third anaesthetic | First anaesthetic | Second anaesthetic | Thrid anaesthetic | ||
| PC | PB | ||||||
| Mean | 4.7 | 3.9* | 3.7 | mean | 4.4 | 4.9* | 4.7 |
| SD | 0.4 | 0.7 | 0.8 | SD | 0.9 | 0.7 | 0.8 |
Group comparison: PC<PB
Second anaesthetic, PC<PB AQ3: Please indicate * in the table body
PC: first anaesthetic ETCO2 greater than second and third
PB: no significant differences between first, second and third anaesthetics
Eight cats per group, three anaesthetic periods, 48 h apart
Recorded at 5 min intervals during anaesthesia
Pre = before anaesthesia, 5 min = 5 min after induction, extub = at extubation, 8 h = 8 h after induction, PC = control group — unpreserved propofol, SD = standard deviation, PB = group receiving propofol + 2% benzyl alcohol
Phase 2
As in phase 1, hypoxaemia was noted in both groups (SpO2 below 90%). In the second anaesthetic procedure ventilatory assistance was required in one male cat in each treatment group using an Ambu bag and air. Both cats continued to be dosed as per protocol and recovered uneventfully. Propofol anaesthesia increased HR and decreased RR from baseline throughout all three anaesthetic periods in both groups, but there were no significant differences between groups (Table 6). MABP decreased during each anaesthetic period in both groups, but there were no significant differences between the three anaesthetic procedures in either group or between the two groups (Table 6). Mean SpO2 ranged from 77% to 88% in the PC group and from 77% to 91% during anaesthesia in the PB group, but there were no differences between the three anaesthetic procedures in either group or between the groups (Table 6). ETCO2 increased during anaesthesia in all three anaesthetic periods in PB but not in PC; there were no significant differences between the groups. The mean ETCO2 in the PC group ranged from 3.5 kPa to 4.5 kPa (26–34 mmHg); there were no significant differences between the three anaesthetic procedures. The mean ETCO2 in the PB group ranged from 3.2 kPa to 5.3 kPa (24–40 mmHg) and was higher at some time points during the first procedure compared with the third (Table 6). Body temperature decreased during all three anaesthetic periods in both groups but there were no significant differences between groups (Table 6). The lowest temperature recorded in either group was 37.3°C. There were no differences in any recovery times between the three anaesthetic procedures in either group or between the two groups (Table 7). Most recoveries were scored as 1 or 0 (good or perfect) and there were no significant differences in quality between anaesthetic procedures in either group or between the two groups. Recovery to all stages was slower in phase 2 than in phase 1.
Table 6.
Physiological data, phase 2
| Heart rate (beats per min) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First anaesthetic |
Second anaesthetic |
Third anaesthetic |
|||||||||||||
| PC | Pre | 60 min | Extub | Stand | 8h | Pre | 60 min | Extub | Stand | 8h | Pre | 60 min | Extub | Stand | 8h |
| Mean | 144 | 163 | 139 | 155 | 147 | 130 | 154 | 143 | 141 | 135 | 134 | 158 | 143 | 143 | 140 |
| SD | 15 | 14 | 8 | 12 | 10 | 18 | 24 | 20 | 18 | 11 | 11 | 21 | 12 | 12 | 14 |
| PB | |||||||||||||||
| Mean | 151 | 168 | 146 | 139 | 143 | 128 | 167 | 144 | 143 | 143 | 139 | 155 | 144 | 143 | 137 |
| SD | 10 | 18 | 15 | 14 | 19 | 15 | 28 | 12 | 6 | 9 | 11 | 20 | 14 | 8 | 5 |
No significant differences between groups
PC and PB: significant increase during anaesthesia
PC and PB: no significant difference between first, second and third anaesthetics
Table 7.
Phase 2: recovery time (min)
| First anaesthetic |
Second anaesthetic |
Third anaesthetic |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PC | Head | Stern | Stand | Head | Stern | Stand | Head | Stern | Stand |
| Mean | 11 | 14 | 53 | 9 | 13 | 55 | 8 | 13 | 55 |
| SD | 13 | 12 | 18 | 9 | 7 | 9 | 10 | 17 | 19 |
| PB | |||||||||
| Mean | 22 | 26 | 67 | 15 | 19 | 66 | 9 | 13 | 58 |
| SD | 17 | 16 | 12 | 12 | 12 | 10 | 12 | 13 | 17 |
No significant difference between groups
PC and PB: no significant differences between first, second and third anaesthetics
PC = control group — unpreserved propofol, PB = group receiving propofol + 2% benzyl alcohol
| Respiratory rate (breaths per min) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First anaesthetic |
Second anaesthetic |
Third anaesthetic |
|||||||||||||
| PC | Pre | 60 min | Extub | Stand | 8h | Pre | 60 min | Extub | Stand | 8h | Pre | 60 min | Extub | Stand | 8h |
| Mean | 42 | 23 | 45 | 46 | 51 | 56 | 23 | 45 | 45 | 50 | 54 | 43 | 49 | 47 | 43 |
| SD | 6 | 5 | 8 | 5 | 6 | 5 | 6 | 11 | 7 | 7 | 8 | 17 | 10 | 11 | 17 |
| PB | |||||||||||||||
| Mean | 42 | 23 | 44 | 48 | 47 | 48 | 25 | 41 | 44 | 46 | 47 | 24 | 44 | 48 | 44 |
| SD | 5 | 5 | 11 | 6 | 6 | 6 | 5 | 8 | 9 | 12 | 6 | 5 | 6 | 9 | 7 |
No significant differences between groups
PC and PB: significant decrease during anaesthesia
PC and PB: no significant difference between first, second and third anaesthetics
| Body temperature (°C) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First anaesthetic |
Second anaesthetic |
Third anaesthetic |
|||||||||||||
| PC | Pre | 60 min | Extub | Stand | 8h | Pre | 60 min | Extub | Stand | 8h | Pre | 60 min | Extub | Stand | 8h |
| Mean | 38.6 | 37.6 | 38.0 | 37.9 | 37.6 | 38.1 | 37.6 | 37.7 | 38.5 | 38.3 | 38.7 | 37.6 | 38.3 | 38.2 | 37.7 |
| SD | 1.0 | 0.3 | 0.8 | 0.6 | 0.6 | 0.5 | 0.6 | 0.7 | 1.0 | 1.3 | 0.8 | 0.5 | 1.5 | 1.0 | 1.0 |
| PB | |||||||||||||||
| Mean | 38.5 | 37.3 | 37.7 | 37.6 | 37.6 | 38.0 | 37.7 | 37.7 | 38.4 | 38.0 | 39.0 | 37.4 | 37.8 | 38.0 | 37.9 |
| SD | 1.2 | 0.5 | 0.7 | 0.7 | 1.0 | 0.7 | 0.4 | 0.9 | 1.3 | 1.1 | 1.1 | 0.7 | 1.5 | 0.5 | 1.0 |
No significant differences between groups
PC and PB: significant decrease during anaesthesia
PC and PB: no significant difference between first, second and third anaesthetics
| Mean arterial blood pressure (mmHg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First anaesthetic |
Second anaesthetic |
Third anaesthetic |
||||||||||
| PC | 5 min | 20 min | 40 min | 60 min | 5 min | 20 min | 40 min | 60 min | 5 min | 20 min | 40 min | 60 min |
| Mean | 75 | 61 | 62 | 63 | 90 | 68 | 63 | 59 | 67 | 64 | 59 | 60 |
| SD | 14 | 9 | 5 | 9 | 25 | 14 | 7 | 12 | 26 | 10 | 10 | 8 |
| PB | ||||||||||||
| Mean | 82 | 67 | 62 | 72 | 87 | 61 | 65 | 69 | 82 | 67 | 60 | 61 |
| SD | 22 | 14 | 11 | 10 | 22 | 12 | 7 | 10 | 14 | 14 | 5 | 8 |
No significant differences between groups
PC and PB: significant decrease during anaesthesia
PC and PB: no significant difference between first, second and third anaesthetics
| SpO2 (% saturation) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First anaesthetic |
Second anaesthetic |
Third anaesthetic |
||||||||||
| PC | 5 min | 20 min | 40 min | 60 min | 5 min | 20 min | 40 min | 60 min | 5 min | 20 min | 40 min | 60 min |
| Mean | 85 | 77 | 85 | 88 | 86 | 82 | 86 | 88 | 86 | 81 | 85 | 85 |
| SD | 7 | 11 | 5 | 3 | 5 | 7 | 4 | 5 | 9 | 11 | 10 | 7 |
| PB | ||||||||||||
| Mean | 81 | 77 | 85 | 85 | 85 | 82 | 87 | 84 | 85 | 80 | 91 | 87 |
| SD | 8 | 12 | 5 | 5 | 5 | 12 | 3 | 7 | 7 | 10 | 4 | 13 |
No significant differences between groups
PC and PB: significant decrease during anaesthesia
PC and PB: no significant difference between first, second and third anaesthetics
| ETCO2 (kPa) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First anaesthetic |
Second anaesthetic |
Third anaesthetic |
||||||||||
| PC | 5 min | 20 min | 40 min | 60 min | 5 min | 20 min | 40 min | 60 min | 5 min | 20 min | 40 min | 60 min |
| Mean | 3.5 | 4.4 | 4.3 | 4.1 | 3.2 | 3.6 | 4.0 | 3.6 | 3.7 | 4.1 | 3.9 | 4.5 |
| SD | 0.9 | 1.9 | 1.6 | 1.3 | 1.1 | 1.9 | 1.6 | 1.5 | 1.6 | 2.0 | 1.6 | 1.6 |
| PB | ||||||||||||
| Mean | 3.6 | 5.3 | 5.3 | 4.7 | 3.9 | 4.0 | 4.3 | 4.3 | 3.2 | 3.6 | 3.6 | 3.9 |
| SD | 1.6 | 1.3 | 1.20 | 1.3 | 0.8 | 1.9 | 1.2 | 1.7 | 1.3 | 1.2 | 0.4 | 0.5 |
No significant differences between groups
PC: no significant changes during anaesthesia; no significant difference between first, second and third anaesthetics
PB: significant changes during anaesthesia; significant differences between first, second and third anaestheticsat 25 and 50 min
Eight cats per group, three anaesthetic periods, 48 h apart
Recorded at 5 min intervals during anaesthesia
PC = control group — unpreserved propofol, Pre = before anaesthesia, 20–60 min = 20–60 min after induction, extub = at extubation, 8 h = 8 h after induction, SD = standard deviation, PB = group receiving propofol + 2% benzyl alcohol
Clinical signs and clinical pathology
In both groups the body weight of all cats increased throughout the study. The PC group increased from 2.3 ± 0.3 kg at the start of the study period to 2.9 ± 0.4 kg at post mortem. The PB group increased from 2.4 ± 0.2 to 3.0 ± 0.5 kg over the same period (P >0.05) . No clinical or neurological abnormalities were detected at any time.
During the entire study period there were minor changes in many of the haematological and biochemical measurements (reference values available as supplementary data). Most values remained within, or close to, the normal range and, with one exception, there were no differences between the groups. In both groups, compared with baseline, haematocrit decreased after anaesthesia, Heinz bodies increased, particularly after phase 1 (see Table 8), and fibrinogen was slightly increased. Triglyceride, cholesterol and bile acids increased transiently after anaesthesia (Table 9). Aspartate aminotransferase was higher in the PC group than in the PB group at most time points, but all values were well within the normal range (see supplementary data). One cat from the PB group died during handling for withdrawal of the final blood sample immediately prior to euthanasia. No relevant gross or histological abnormalities were observed and it was considered a traumatic accident unrelated to the administration of the anaesthetic.
Table 8.
Heinz bodies (103/μl)
| Phase 1 |
Phase 2 |
|||||
|---|---|---|---|---|---|---|
| PC | Day -5 | Day 4 | Day 5 | Day 4 | Day 5 | Day 11 |
| Mean | 14.7 | 20.1 | 109.4 | 33.5 | 20.0 | 29.1 |
| SD | 26.9 | 15.5 | 51.5 | 37.8 | 27.7 | 33.2 |
| PB | ||||||
| Mean | 8.6 | 18.8 | 90.1 | 27.4 | 18.5 | 15.4 |
| SD | 10.6 | 20.9 | 27.7 | 26.7 | 24.6 | 16.6 |
No significant difference between groups
PC and PB: significant increases with time
PC = control group — unpreserved propofol, PB = group receiving propofol + 2% benzyl alcohol
Table 9.
Clinical pathology data
| Triglyceride (mmol/l) | ||||||
|---|---|---|---|---|---|---|
| Phase 1 |
Phase 2 |
|||||
| PC | Day -5 | Day 4 | Day 5 | Day 4 | Day 5 | Day 11 |
| Mean | 0.4 | 2.0 | 1.5 | 0.9 | 1.3 | 0.4 |
| SD | 0.1 | 0.7 | 0.4 | 0.6 | 0.6 | 0.1 |
| PB | ||||||
| Mean | 0.4 | 1.8 | 1.7 | 0.9 | 1.2 | 0.4 |
| SD | 0.1 | 0.4 | 0.8 | 0.7 | 0.7 | 0.1 |
No significant difference between groups
PC and PB: significant changes with time
| Cholesterol (mmol/l) | ||||||
|---|---|---|---|---|---|---|
| Phase 1 |
Phase 2 |
|||||
| PC | Day -5 | Day 4 | Day 5 | Day 4 | Day 5 | Day 11 |
| Mean | 2.7 | 3.1 | 3.5 | 2.9 | 3.5 | 2.8 |
| SD | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.6 |
| PB | ||||||
| Mean | 2.3 | 2.9 | 3.4 | 2.8 | 3.5 | 2.7 |
| SD | 0.3 | 0.3 | 0.4 | 0.3 | 0.6 | 0.5 |
No significant difference between groups
PC and PB: significant changes with time
| Bile acid (µmol/l) | ||||||
|---|---|---|---|---|---|---|
| Phase 1 |
Phase 2 |
|||||
| PC | Day -5 | Day 4 | Day 5 | Day 4 | Day 5 | Day 11 |
| Mean | 0.5 | 0.9 | 9.3 | 0.7 | 8.8 | 0.7 |
| SD | 0.2 | 0.2 | 3.4 | 0.3 | 4.4 | 0.4 |
| PB | ||||||
| Mean | 0.8 | 0.6 | 8.9 | 0.7 | 5.7 | 0.5 |
| SD | 1.1 | 0.1 | 5.4 | 0.5 | 3.9 | 0.2 |
No significant difference between groups
PC and PB: significant changes with time
PC = control group — unpreserved propofol, PB = group receiving propofol + 2% benzyl alcohol
Post-mortem examination of the study subjects revealed the finding of long-standing renal adenoma in the PB group cat that died during the final blood sampling. No other gross or histological abnormalities were detected at post-mortem examination.
Discussion
The main study was conducted in light of the data from the pilot study which indicated that addition of benzyl alcohol 2% did not cause additional morbidity over propofol alone. In the pilot study, the death of one cat from apnoea after effective propofol overdose demonstrates that propofol itself was the limiting factor. In the main study the effects of propofol anaesthesia in both groups were consistent with previous reports in cats;2,4 there was no evidence of any additional effect of benzyl alcohol over those of propofol alone. In particular, there was no evidence of any cumulative effect of repeat doses, suggesting that, at least at the dosing schedule used here, there was insufficient benzyl alcohol accumulation to produce any toxic effect.
The cardiovascular effects in both groups resulted in a lower arterial BP than in normal, conscious cats, 10 and an increased HR. The increase in HR contrasts with previous reports2,4 but may reflect excitement at induction in young, unpremedicated animals. Respiratory depression was evident in the marked hypoxaemia which developed, underlining the normal recommendation to supplement inspired air with oxygen whenever propofol is used for anaesthesia. The reported ETCO2 was not particularly high, which appears inconsistent with respiratory depression. However, the sidestream capnograph that was used (Surgivetadvisor) samples at 150 ml/min and most probably led to contamination of the sampled expired gas with external air, so that the recorded data were not a true reflection of alveolar CO2. Tidal volume in a 2–3 kg bodyweight cat is unlikely to be much above 30 ml, which, with the RR around 20 per minute (one breath per 3 s) during anaesthesia, means that a sampling rate of 7.5 ml/s will draw one quarter of the tidal volume — inevitably more than just end-expired gas. Use of a mainstream sampling unit would circumvent this problem.
Benzyl alcohol has been used for many years as a preservative for both food and injectable medicinal products. Its potential for toxicity became clear when reports of the potentially fatal human neonatal ‘gasping syndrome’ were shown to be a result of excessive benzyl alcohol accumulation after administration of substantial volumes of intravenous electrolyte solutions preserved with benzyl alcohol.11,12 The main cause of toxicity appears to be acidosis, but neurological signs are a prominent feature of the disease.
The potential for benzyl alcohol toxicity in the cat is greater than in other species, such as the dog, as cats have a low capacity for glucuronic acid conjugation. Detoxification of benzyl alcohol is generally through oxidation to benzoic acid and then conjugation to benzyl glucuronide and hippuric acid. 13 The cat is unusual in that it is relatively deficient in pathways of glucuronidation, produces little glucuronide and thus has limited capacity to metabolise benzoic acid. 8 Neurological signs of benzyl alcohol toxicity develop within a few hours of administration and include profuse salivation, excitation, hyperaesthesia, ataxia, dilated, fixed pupils and seizures, progressing to coma and death within 1 to 5 days.6,7
The dose, speed of injection and concentration of benzyl alcohol affect the likelihood of toxicity, and there are no specific studies investigating these effects in cats. Cullison et al 7 reported that cats given two subcutaneous doses several hours apart that totaled 800–1350 mg/kg benzyl alcohol diluted in lactated Ringer’s solution developed neurological signs and died within 24 h. Bedford and Clarke 6 reported the same effects from oral consumption of similar doses. The present study used a normal clinical dose of propofol in phase 1, and threetimes the normal clinical dose in phase 2. In this study, the lack of any sign of abnormality beyond the effect of propofol alone suggests that the dose of benzyl alcohol that can be administered in propofol containing 20 mg/ml (2%) benzyl alcohol is limited by the effects of the propofol, not by the preservative. In this investigation, 48 mg/kg benzyl alcohol were given over 45–120 min with no effect, and it is unlikely that this rate of administration would be exceeded under any clinical conditions. A prolonged infusion would inevitably lead to a higher dose, but even when 24 mg/kg was repeated three times at 48-h intervals there was no sign of accumulation.
Propofol is less well tolerated in cats than in dogs or other species. Metabolism and excretion is slower in cats than in dogs, and repeated doses or infusions lead to prolonged recovery from anaesthesia. 4 The longer duration of recovery in phase 2 is a reflection of this. Andress et al 9 reported oxidative injury to feline red blood cells and development of Heinz bodies after three daily doses of propofol, and generalised malaise, anorexia and diarrhoea after 5 days of daily dosing. An inter-dosing interval of 48 h, as in the present study, appears sufficient to prevent much of this effect, but Heinz bodies were still increased, particularly after phase 1, further confirming that repeated propofol anaesthesia should be limited in cats, regardless of the presence of benzyl alcohol.
It was extremely unfortunate that one cat died in the pilot study and one in the main study during withdrawal of the final blood sample. In the pilot study this was completely consistent with the recognised respiratory depressant effects of propofol overdose; in the main study it appears that death was entirely caused by themechanical effects of restraint; neither death affects the conclusions of the investigation. It is also unlikely that the renal adenoma had any significant effect, but it is curiously coincidental that this was found in the second cat.
In conclusion, this study demonstrates that the addition of benzyl alcohol preservative 2% does not affect the behaviour of propofol emulsion when used at normal-to-high clinical doses in healthy cats. Inevitably, it does not address the potential effects in debilitated animals, or where prolonged infusions are necessary. However, in view of the limitations of the use of propofol in cats, addition of benzyl alcohol does not appear to cause any further restriction.
Supplemental Material
Clinical pathology reference values and tables of blood chemistry data collected between phase 1 and phase 2
Acknowledgments
The authors acknowledge the work and efforts of the technical staff at WIL Research Laboratories, LLC and Gwendolyn Maginnis, DVM for assistance in proofing the manuscript.
Footnotes
Supplementary data: Clinical pathology reference values and tables of blood chemistry data collected between phase 1 and phase 2 are available as supplementary data.
Funding: The study was funded by Abbott Laboratories.
Dr PM Taylor acts as an independent consultant in veterinary anaesthesia to Abbott Animal Health. Dr E Cozzi is an employee of Abbott Laboratories.
Accepted: 31 January 2012
References
- 1. Watkins SB, Hall LW, Clarke KW. Propofol as an intravenous anaesthetic agent in dogs. Vet Rec 1987; 120: 326–329. [DOI] [PubMed] [Google Scholar]
- 2. Brearley JC, Kellagher REB, Hall LW. Propofol anaesthesia in cats. J Small Anim Pract 1998; 29: 315–322. [Google Scholar]
- 3. Brodbelt D. The confidential enquiry into perioperative small animal fatalities [PhD]. London: University of London, 2006. [Google Scholar]
- 4. Pascoe PJ, Ilkiw JE, Frischmeyer KJ. The effect of the duration of propofol administration on recovery from anesthesia in cats. Vet Anaesth Analg 2006; 33: 2–7. [DOI] [PubMed] [Google Scholar]
- 5. Strachan FA, Mansel JC, Clutton RE. A comparison of microbial growth in alfaxalone, propofol and thiopental. J Small Anim Pract 2008; 49: 186–190. [DOI] [PubMed] [Google Scholar]
- 6. Bedford PG, Clarke EG. Experimental benzoic acid poisoning in the cat. Vet Rec 1972; 90: 53–58. [DOI] [PubMed] [Google Scholar]
- 7. Cullison RF, Menard PD, Buck WB. Toxicosis in cats from use of benzyl alcohol in lactated Ringer’s solution. J Am Vet Med Assoc 1983; 182: 61. [PubMed] [Google Scholar]
- 8. Wilcke JR. Idiosyncracies of drug metabolism in cats. Effects on pharmacotherapeutics in feline practice. Vet Clin North Am Small Anim Pract 1984; 14: 1345–1354. [DOI] [PubMed] [Google Scholar]
- 9. Andress JL, Day TK, Day D. The effects of consecutive day propofol anesthesia on feline red blood cells.Vet Surg 1995; 24: 277–282. [DOI] [PubMed] [Google Scholar]
- 10. Bodey AR, Sansom J. Epidemiological study of blood pressure in domestic cats. J Small Anim Pract 1998. 39: 567–573. [DOI] [PubMed] [Google Scholar]
- 11. Gershanik J, Boecler B, Ensley H, McCloskey S, George W. The gasping syndrome and benzyl alcohol poisoning. N Engl J Med 1982; 307: 1384–1388. [DOI] [PubMed] [Google Scholar]
- 12. Anderson CW, Ng KJ, Andresen B, Cordero L. Benzyl alcohol poisoning in a premature newborn infant. Am J Obstet Gynecol 1984; 148: 344–346. [DOI] [PubMed] [Google Scholar]
- 13. Bridges JW, French MR, Smith RL, Williams RT. The fate of benzoic acid in various species. Biochem J 1970; 118: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical pathology reference values and tables of blood chemistry data collected between phase 1 and phase 2