Abstract
Objectives
Geriatric health screening in cats is highly recommended. However, information about normal and abnormal findings is scarce, especially regarding the eyes of ageing cats. This prospective study examined the influence of the ageing process on vision and ocular structures in older cats.
Methods
A total of 209 cats (aged 9–24 years) underwent physical examination, vision assessment, slit lamp biomicroscopy and ophthalmoscopy. Systolic blood pressure (SBP) measurement, Schirmer tear test (STT) reading and rebound tonometry were performed. Systemic disease was not a criterion for exclusion.
Results
Vision was good in 157/209 cats (75.1%) and impaired in 52/209 cats (24.9%). Increasing age and the occurrence of vision impairment were not statistically associated (P = 0.053). Retinal oedema, retinal haemorrhage and/or retinal detachment (19 cats) and glaucoma (12 cats) were the most common findings in vision impaired cats. Increasing age was significantly associated with the occurrence of lenticular sclerosis (P = 0.01) and attenuated retinal vessels (P = 0.02). Increasing age and SBP were significantly associated with the occurrence of retinal detachment, haemorrhage and oedema (P <0.001 each). In cats without evidence of hypertensive ocular damage, younger cats had a tendency for higher SBP values than older cats, although this difference was not significant. Mean intraocular pressure (IOP) was 16.5 ± 5.0 mmHg. Age did not significantly affect the IOP values (P = 0.54). Mean STT was 15.8 ± 4.8 mm/min. The STT was found to increase with age (P = 0.025).
Conclusions and relevance
Although vision impairment is not a clinical sign of old age in cats, age-related changes may contribute to vision-threatening diseases. This study contributes to preventive healthcare by examining the influence of the ageing process on vision and ocular structures in older cats.
Introduction
The increasing life span of cats and the considerable proportion of older cats in the pet population requires improved healthcare in older feline patients.1,2 Routine health screening, including a physical examination, laboratory tests, blood pressure measurements and a routine ophthalmological examination, is recommended in elderly feline patients.3–6 The classification of age-related changes and age-related disease is a challenge in veterinary practice because pertinent studies are scarce.2,7,8
This is particularly true regarding the eyes of ageing cats. Previous studies focus on single ocular structures such as the lens, 9 case series about pathological changes such as entropion, 10 ocular tumours,11–14 aqueous humour misdirection syndrome 15 or hypertensive retinopathy.16–18 To the authors’ knowledge, there is no study regarding the occurrence and the influence of age on ocular changes in the eyes of older cats. Whereas there are several studies on systolic blood pressure (SBP) in adult cats,19–21 there is a paucity of information about normal SBP in older cats, especially regarding the ageing feline eye.7,22 Intraocular pressure (IOP) in older cats was determined only in one study using applanation tonometry. 5 Only one study measured the Schirmer tear test (STT) in older cats. 7 Therefore, the aim of the present study was to contribute to preventive healthcare by determining normal values of SBP, STT and IOP, examining vision and the occurrence of ocular changes, as well as assessing how age influences all of these parameters in older cats.
Materials and methods
Study population
A total of 209 cats were examined over a 28 month period in the Small Animal Clinic at the Freie Universität of Berlin, Germany. The study was conducted according to the guidelines of the Association for Research in Vision and Ophthalmology (ARVO). Owner consent was given prior to all examinations. Systemic disease was not a criterion for exclusion. No further examinations were conducted when the handling was too stressful for the cat or the clinical condition would have led to the corruption of the results. The cats were allocated into three age groups following the American Association of Feline Practitioners (AAFP) senior care guidelines: middle-aged cats (9–10 years), senior cats (11–14 years) and geriatric cats (⩾15 years). 4
Procedures
All procedures were part of routine preventive healthcare in older cats.3–5 SBP was measured using the Doppler technique and following the corresponding American College of Veterinary Internal Medicine (ACVIM) recommendations. 23 SBP ⩽160 mmHg was considered normal. SBP >160 mmHg with known target organ injury was considered systemic hypertension.
Vision was evaluated based on history and standard vision testing (orientation skills, menace response, dazzle reflex and when necessary cotton ball test and visual placing). If at least one eye was blind or had questionable or reduced vision, the animal’s vision was classified as ‘impaired’.
A standardised STT strip (Schirmer Tear Test; Intervet) was placed in the lateral conjunctival sac for 60 s.
Slit lamp biomicroscopy was performed with the KOWA SL-15 (KOWA).
Rebound tonometry was performed using the Tonovet (Icare) following recommendations from the literature. 24
After tonometry, mydriasis was induced using 0.5% tropicamide (Mydrum; Bausch & Lomb). The lenses were examined in complete mydriasis by using the slit lamp. The vitreous, fundus and optic disc were examined using the Panoptic indirect monocular ophthalmoscope (Wellch Allyn).
Statistical analysis
Descriptive statistics were used to assess the validity and plausibility of the data.
Normal distribution of metric parameters was determined using the Kolmogorow-Smirnov test and histograms with marked curves of standard distribution and Q-Q diagrams.
The SBP range was determined by excluding animals with hypertensive changes of the anterior chamber, vitreous, retina and retinal vessels, as well as those on antihypertensive medication.
The STT range was found by excluding cats with ocular discharge as well as those with changes in the conjunctiva and in the cornea.
The range of IOP was determined by excluding animals with changes in the cornea and the anterior chamber.
The IOP difference between the right eye (OD) and the left eye (OS) was calculated. Mean STT and mean IOP were calculated by averaging the sum of OD and OS values. The STT and IOP values determined in this study were compared with known reference intervals using the one-sample t-test.7,24 The mean STT reference value was calculated using the average results OD and OS, published elsewhere. 7
Linear regression analysis was used to evaluate the influence of age on SBP, IOP and STT. The 97.5% confidence percentile (typical maximum value) was plotted in the SBP chart to show the tendencies of maximum SBP values in animals without hypertensive changes in the three age groups.
The influence of age and SBP on ocular changes and the associations between age and the occurrence of hypertensive retinopathy, uveitis and glaucoma were evaluated using logistic regression models. The probability of the occurrence of either ocular changes, hypertensive retinopathy, uveitis or glaucoma was used as dependent factors, while age and SBP were included as independent factors.
Data analyses were performed using commercial data analysis software (IBM SPSS Statistics version 22). The significance level was P <0.05.
Results
The study included 209 cats between 9 and 24 years of age (mean 13.0 ± 2.6 years). The population consisted of 122 males (118 castrated and four intact) and 87 females (81 spayed and six intact). A total of 74/209 cats had outdoor access, 124/209 were strictly indoor, 11/209 cats had unknown housing regimens. Fourteen pedigree breeds were included, while the majority (157/209) were domestic shorthair cats.
SBP was measured in 164 cats. A total of 91/164 animals met our criteria to determine SBP values. Mean SBP was 153.9 ± 29.3 mmHg. SBP did not increase with age (P = 0.4), but the 97.5% confidence percentile of the SBP decreased with age (Figure 1).
Figure 1.
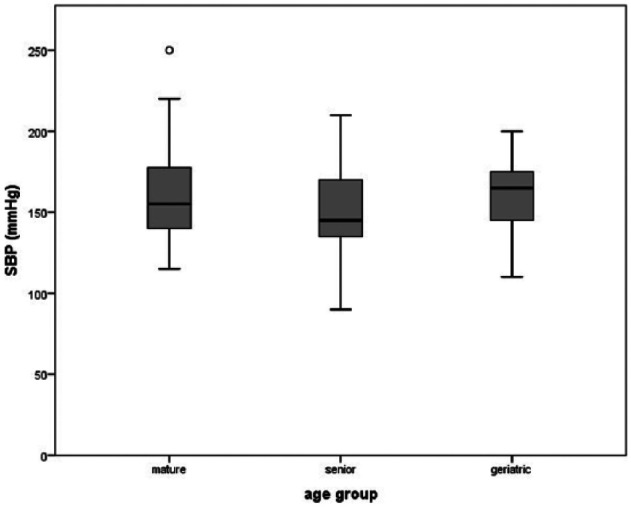
Mean systolic blood pressure (SBP) in 91 cats without hypertensive ocular changes. Of these, 23/91 cats (25.3%) were mature (9–10 years), 50/91 cats (54.9 %) were senior (11–14 years) and 18/91 cats (19.8 %) were geriatric (⩾15 years). The 97.5% confidence percentile (typical maximum value) is plotted on the chart to see the tendency of decreasing maximum SBP values in older cats without hypertensive ocular changes
Vision was good in 157 cats (75.1%) and impaired in 52 cats (24.9%). The most common ocular causes of vision impairment were retinal oedema, haemorrhage and/or detachment (19 cats), followed by glaucoma (12 cats). Increasing age and impaired vision were not statistically associated with each other (P = 0.053).
The STT was performed in 157 cats. A total of 79/157 animals met our criteria to determine STT values. The results are shown in Table 1.
Table 1.
Schirmer tear test (STT) values in 79 cats without ocular discharge and without changes in the conjunctiva and the cornea, and intraocular pressure (IOP) values in 147 cats without changes in the cornea and anterior chamber. Influence of age and comparison to known reference values (test value) with the one sample t-test are shown
IOP was measured in 195 cats. A total of 147/195 animals met our criteria to determine IOP values. The results are shown in Table 1. The IOP difference between OD and OS was 2.8 ± 2.8 mmHg. There was no statistical association between increasing age and IOP difference (P = 0.39).
Findings in ocular structures are presented in Tables 2–4.
Table 2.
Findings in the anterior segment of the eye in 209 older cats (multiple mentions are possible)
| Ocular structure | Number of affected cats |
|---|---|
| Globe and orbit | |
| Enophthalmos | 15 |
| Buphthalmos* | 7 |
| Extirpated globe | 2 |
| Exophthalmos | 2 |
| Strabismus | 1 |
| Eyelids | |
| Entropion | 8 |
| Blepharospasm | 8 |
| Swelling | 6 |
| Ptosis | 5 |
| Pigmented moles | 5 |
| Incomplete eyelid closing | 4 |
| Alopecia | 3 |
| Eyelid tumour | 2 |
| Conjunctiva | |
| Conjunctivitis | 28 |
| Paleness | 12 |
| Other | 3 |
| Third eyelid | |
| Prolapse | 4 |
| Active protrusion | 1 |
| Cornea | |
| Superficial ulcers | 12 |
| Keratitis | 8 |
| Oedema † | 6 |
| Scars | 6 |
| Keratitic precipitates* | 4 |
| Sequestra | 3 |
| Sclera | |
| Prominent episcleral vessels | 6 |
| Other | 2 |
| Anterior chamber* ‡ | |
| Iris and ciliary body | |
| Atrophy | 48 |
| Iris pigment changes | 38 |
| Iris discolouration § | 9 |
| Others* ‡ | 1 |
| Pupil | |
| Anisocoria ¶ | 16 |
| Bilateral mydriasis | 7 |
| D-shape or reverse D-shape pupil | 5 |
See Table 6
See Table 6; the other cause for corneal oedema was keratitis
See Table 8
Seven cases with uveitis (Table 6); one other cat showed a flesh-coloured discolouration without uveitis caused by lymphoma, in another cat the cause of the flesh-coloured discolouration was unknown
Causes are glaucoma (n = 3), Horner syndrome (n = 2), extraocular tumour (n = 2), uveitis (n = 1), hypertensive retinopathy (n = 1), lens luxation (n = 1), synechia (n = 1), unknown (n = 5)
Table 3.
Features of iris pigment changes in 38 cats (multiple mentions are possible)
| Number of affected cats |
|||
|---|---|---|---|
| Unilateral | Bilateral | ||
| Colour | Light coloured | 12 | 7 |
| Dark | 19 | 7 | |
| Distribution | Localised | 19 | 5 |
| Multifocal | 14 | 4 | |
| Diffuse | 2 | 3 | |
| Elevation | Elevated | 3 | 0 |
Table 4.
Findings in the posterior segment of the eye in 209 older cats (multiple mentions are possible)
| Ocular structure | Number of affected cats |
|---|---|
| Lens | |
| Subluxation* | 2 |
| Anterior luxation* | 2 |
| Lenticular sclerosis | 170 |
| Cortical linear opacities | 31 |
| Lens suture opacities | 26 |
| Plane cortical cataract | 15 |
| Lens capsule cataract | 14 |
| Fibrin or pigment on anterior lens capsule* |
7 |
| Nuclear cataract | 1 |
| Vitreous | |
| Haemorrhage † | 8 |
| Other ‡ | 6 |
| Fundus | |
| Halo | 36 |
| Retinal detachment* † | 19 |
| Retinal haemorrhage* † | 18 |
| Attenuated retinal vessels | 17 |
| Tortuositas vasorum | 15 |
| Retinal edema † | 15 |
| Retinal degeneration | 3 |
| Chorioretinitis (scars) | 1 |
| Edema of optic nerve head † | 1 |
| Other | 4 |
Increasing age was not statistically associated with any of the following variables (univariate logistic regression): entropion (P = 0.173), ptosis (P = 0.228), pigmented moles of the eyelid (P = 0.509), conjunctivitis (P = 0.383), corneal changes (P = 0.851), scleral changes (P = 0.281), changes of the anterior chamber (P = 0.168), pupil changes (P = 0.909), changes of the lens position (P = 0.269), the lens capsule (P = 0.343), the lens cortex (P = 0.442), vitreous changes (P = 0.077), tortuous retinal vessels (P = 0.898) and peripapillary halo (P = 0.969).
Increasing age and the occurrence of bilateral enophthalmos were statistically associated (P = 0.01). Seven out of nine cats with this condition were underweight, and the enophthalmos was considered pain-related in one cat.
We found a strong statistical association between increasing age and the occurrence of iris atrophy and concurrent iris pigment changes (P = 0.002), but not with iris atrophy (P = 0.101) or iris pigment changes only (P = 0.247) (univariate multinomial logistic regression). However, there was a trend for iris atrophy being more likely associated with older age (OR 1.127, confidence interval 0.977–1.301), and iris pigment changes being more likely associated with younger age (OR 0.903, confidence interval 0.760–1.073). Two out of three elevated iris pigment changes were histopathologically confirmed as iris melanomas.
Increasing age and the occurrence of lenticular sclerosis (P = 0.01) and attenuated retinal vessels (P = 0.02) (univariate binary logistic regression each) were statistically associated. Age and SBP combined were strongly associated with the development of retinal oedema, haemorrhage and detachment (P <0.001, multivariate multinomial logistic regression). Mean SBP was 181.3 ± 43.6 mmHg in cats with retinal haemorrhage and/or oedema but no signs of retinal detachment. In cats with retinal detachment, mean SBP was 215.9 ± 49.1 mmHg.
Hypertensive retinopathy
The risk of the occurrence of hypertensive retinopathy increased with age (P = 0.005, OR = 1.26, confidence interval 1.073–1.477). Clinical signs of hypertensive retinopathy in 26 cats are shown in Table 8. In 6/18 cats with retinal oedema and/or haemorrhage, SBP was ⩽160 mmHg; in one cat with 170 mmHg, unilateral haemorrhage was presumably caused by an ipsilateral oral neoplasia; in two cats, SBP was not measured. Therefore, these animals were not diagnosed with hypertensive retinopathy.
Table 8.
Age, breed, sex, clinical signs, systolic blood pressure (SBP) and antihypertensive medication in 26 cats with hypertensive retinopathy
| Cat reference number | Age (years) | Breed | Sex | Clinical signs of hypertensive retinopahy | SBP (mmHg) | Antihypertensive medication |
|---|---|---|---|---|---|---|
| 32 | 12 | BSH | sf | OD multifocal retinal haemorrhage | 140* | Ramipril, amlodipine |
| 38 | 13 | DSH | cm | OU focal retinal haemorrhage, oedema optic nerve head OD partial retinal detachment OS focal vitreal haemorrhage, full retinal detachment |
280 | – |
| 47 | 13 | DSH | cm | OU multifocal retinal haemorrhage OD partial retinal detachment |
155* | Amlodipine |
| 48 | 15 | DSH | sf | OU partial retinal detachment OD focal retinal haemorrhage OS multifocal retinal haemorrhage and oedema |
145* | Benazepril, amlodipine |
| 59 | 13 | DSH | cm | OS focal retinal oedema | 170 | – |
| 64 | 14 | DSH | sf | OU focal retinal oedema OS multifocal retinal detachment |
165 | – |
| 74 | 16 | DSH | sf | OS focal retinal detachment | 170 | – |
| 76 | 16 | DSH | cm | OD multifocal retinal detachment OS hyphema |
250 | – |
| 92 | 12 | Persian | sf | OU multifocal vitreal haemorrhage, retinal oedema | 250 | – |
| 99 | 17 | DSH | cm | OD diffuse retinal haemorrhage OS focal retinal haemorrhage |
190 | – |
| 105 | 13 | DSH | cm | OU multifocal retinal oedema | 280† | Ramipril |
| 113 | 13 | DSH | sf | OU multifocal retinal haemorrhage OD flare, full retinal detachment |
240 | – |
| 122 | 14 | DSH | sf | OU focal retinal haemorrhage and detachment OS mydriasis |
240 | – |
| 134 | 15 | Maine Coon | cm | OU focal vitreal haemorrhage, retinal oedema OD partial retinal detachment |
180 ‡ | Amlodipine |
| 137 | 16 | DSH | cm | OU partial retinal detachment | 200 | – |
| 147 | 13 | DSH | m | OS focal retinal haemorrhage | 190 | – |
| 160 | 14 | DSH | cm | OU multifocal retinal oedema OD hyphaema, iris haemorrhage, OS linear vitreal haemorrhage |
250 § | Imidapril |
| 167 | 15 | DSH | cm | OU mydriasis OD multifocal retinal detachment OS hyphaema |
250 | – |
| 170 | 16 | DSH | cm | OS multifocal retinal oedema and haemorrhage | 170 | – |
| 173 | 15 | DSH | f | OU mydriasis, vitreal haemorrhage OD multifocal retinal haemorrhage and partial retinal detachment |
290 | – |
| 176 | 18 | Siamese | cm | OD hyphaema, fibrin in anterior chamber OS vitreal haemorrhage, multifocal retinal haemorrhage and partial retinal detachment |
150* | Ramipril, amlodipine |
| 184 | 12 | DSH | m | OU mydriasis, vitreal haemorrhage OD full retinal detachment OS partial retinal detachment |
280 | – |
| 203 | 15 | DSH | sf | OD multifocal retinal haemorrhage OS hyphaema |
200 | – |
| 204 | 17 | Siberian Cat | cm | OU multifocal retinal haemorrhage and partial retinal detachment | 205 | – |
| 205 | 18 | DSH | sf | OU full retinal detachment OD multifocal retinal haemorrhage OS vitreal haemorrhage |
260 | – |
| 216 | 10 | DSH | f | OS partial retinal detachment | 210 | – |
Hypertensive retinopathy was diagnosed and treated prior to this study
Hypertrophic cardiomyopathy secondary to hyperthyreosis was diagnosed and treated prior to this study
Systemic hypertension was diagnosed and treated prior to this study
Chronic renal disease was diagnosed and treated prior to this study
BSH = British Shorthair; DSH = domestic shorthair; f = entire female; m = entire male; cm = castrated male; OD = right eye; OS = left eye; OU = both eyes; sf = spayed female
Uveitis and glaucoma
There was no statistical association between increasing age and the occurrence of uveitis (P = 0.843) and glaucoma (P = 0.696) (univariate binary logistic regression each). Clinical signs and causes of uveitis and glaucoma are shown in Tables 5–7.
Table 5.
Age, breed, sex, presumed cause of uveitis and information regarding the presence of glaucoma in 13 cats
| Cat reference number | Age (years) | Breed | Sex | Presumed cause of uveitis | Glaucoma |
|---|---|---|---|---|---|
| 7 | 10 | Chartreux | sf | Amelanotic iris melanoma | Yes |
| 76 | 16 | DSH | cm | Hypertensive retinopathy | Yes |
| 113 | 13 | DSH | sf | Hypertensive retinopathy | No |
| 146 | 14 | Persian | sf | Benign melanoma of the ciliary body | Yes |
| 154 | 10 | DSH | sf | Cornea perforation | Yes |
| 156 | 10 | DSH | cm | Toxoplasmosis (IgG = 1:512, IgM <1:32) | Yes |
| 158 | 14 | DSH | cm | Infection with felne immunodeficiency virus | Yes |
| 181 | 16 | DSH | cm | Extraocular neoplasia | No |
| 195 | 14 | DSH | cm | Unknown | Yes |
| 203 | 15 | DSH | sf | Hypertensive retinopathy | Yes |
| 210 | 12 | DSH | sf | Extraocular tumor in kidneys and lung | Yes |
| 220 | 14 | Persian | sf | Cornea perforation | No |
| 221 | 13 | DSH | cm | Toxoplasmosis (IgG = 1:256, IgM <1:32) | Yes |
DSH = domestic shorthair; cm = castrated male; sf = spayed female
Table 6.
Clinical signs of uveitis (13 cats) and glaucoma (13 cats) (multiple mentions are possible)
| Clinical sign in ocular structure | Number of affected cats |
|---|---|
| Globe | |
| Buphthalmos | 7 |
| Conjunctiva | |
| Conjunctivitis (redness, swelling) | 7 |
| Cornea | |
| Keratitic precipitates | 4 |
| Oedema | 3 |
| Anterior chamber | |
| Fibrin | 4 |
| Shallow anterior chamber | 3 |
| Hyphaema | 3 |
| Flare | 2 |
| Mass | 2 |
| Iris | |
| Discolouration | |
| Rubeosis iridis | 5 |
| Flesh-coloured (lymphoma [n = 1], toxoplasmosis [n = 1]) | 2 |
| Synechia | 1 |
| Pupil | |
| Unilateral mydriasis | 3 |
| Distorted pupil | 3 |
| Unilateral miosis | 1 |
| Lens | |
| Subluxation | 2 |
| Anterior luxation | 1 |
| Cortical cataract (three mature, two incipient) | 5 |
| Pigment on anterior lens capsule | 3 |
| Fibrin on anterior lens capsule | 1 |
| Anterior capsule cataract (incipient) | 1 |
| Vitreous | |
| Opacity | 2 |
| Retina | |
| Detachment | 3 |
| Haemorrhage | 1 |
Table 7.
Age, breed, sex and presumed cause of glaucoma in 13 cats
| Cat reference number | Age (years) | Breed | Sex | Presumed cause | Number of affected cats |
|---|---|---|---|---|---|
| See Table 5 | Uveitis | 10 | |||
| 161 | 17 | DSH | sf | Uveal neoplasia | 1 |
| 172 | 14 | DSH | cm | Iris neoplasia with renal involvement | 1 |
| 163 | 14 | DSH | sf | Unknown | 1 |
DSH = domestic shorthair; cm = castrated male; sf = spayed female
Discussion
This study addressed the need for information about the ageing feline eye as a contribution to preventive healthcare in older cats by examining 209 cats ⩾9 years of age. We determined ranges of SBP that are unlikely to affect the ageing feline eye, as well as normal values for the STT and IOP in ageing subjects. This is the first study that has examined the occurrence of ocular changes in older cats. Overall, the cats were examined once, and animals younger than 9 years old were not included. Therefore, it was not always possible to determine the onset of the ophthalmological changes. However, it was possible to determine the statistical association between increasing age and ocular changes, SBP, STT and IOP values.
Mean SBP (153.9 ± 29.9 mmHg) in the present study was higher compared with two other studies (133.6 ± 21.5 mmHg and 134.4 ± 6.7 mmHg).7,22 Both studies examined healthy client-owned cats with the indirect Doppler technique.7,22 In the present study, systemic disease was not a criterion for exclusion. Therefore, these values are not exactly comparable. Although we tried to limit the white-coat effect, it is possible that SBP increased temporarily in some cats.
In this study, there was no significant change in SBP associated with age (P = 0.4), but there was a trend for lower SBP in older cats without evidence of hypertensive ocular damage, with the 97.5% confidence percentile of the SBP decreasing with age (Figure 1). Some authors report an increased blood pressure with increased age in feline patients.7,20,21 In other studies, age was not associated with increasing blood pressure.19,22,25 The decreasing maximum values of SBP in the higher age groups of the present study could be an indication that younger cats have better ocular protection mechanisms against higher SBP than older cats.
A total of 75% of the cats in the present study showed good vision. The most common causes of vision impairment were retinal oedema, haemorrhage and/or detachment and glaucoma. Increasing age was statistically associated with the occurrence of these retinal findings, but not with the presence of glaucoma. This conforms with one other publication where the most common finding in cats with vision loss was retinal detachment. 18 Furthermore, glaucoma is considered to be one of the most frequent causes of irreversible blindness in cats. 15 The results of the present study show that vision impairment per se is not a sign of old age in the cat. However, age-related changes of the eye may be a contributing factor for vision impairment.
In this study, mean STT was 15.8 ± 4.8 mm/min. STT values increased with age (P = 0.025). These results differ from the findings of another study in cats >6 years of age, where mean STT was 13.7 mm/min and STT did not change with age. 7 Lower and higher STT readings are reported in younger cats (14.3 ± 4.7 mm/min, 16.2 ± 6.2 mm/min, 20.4 ± 5.5 mm/min).26–28 Age-related reduced tear production is reported in humans with a higher prevalence in women, as well as in dogs of various breeds, sex and ages of onset of clinical signs.29–31 Dry eye disease is multifactorial: osmolarity is always increased, while tear volume, proteins and in some cases lipids are reduced. 32 A new study found a weak correlation between age, tear film break-up time and tear film osmolarity in cats. Both test values decreased in cats ⩾7 years. 33 The present study supports the opinion that age-related reduced aqueous tear production does not occur in the cat. Other tests were not within the scope of this study, but could be valuable regarding further information about dry eye disease.
In the present study, IOP values did not decrease with age. The mean IOP was 16.5 ± 5.0 mmHg. This value was significantly lower than the mean IOP (20.74 mmHg) obtained by Rusanen et al using rebound tonometry in healthy cats (mean age 42 months) (P <0.001), 24 and was equal to the values obtained by von Spiessen et al (16.7 ± 3 mmHg). 34 Only one other study evaluated IOP in older cats (mean age 12.3 ± 2.9 years) using applanation tonometry. The mean IOP was 12.3 ± 4 mmHg and IOP decreased with increasing age. 5 The discrepancy between the values obtained with rebound and applanation tonometry can easily be explained by the different reliability of the tonometers. Applanation tonometry is considered to underestimate IOP values. 35 Therefore, because of the similarity to the IOP values determined by von Spiessen et al 34 the results of the present study can be considered reliable and applicable in routine healthcare for older cats.
In the present study, the most common ocular changes were found in the iris, the lens and the fundus.
Iris atrophy and pigment changes were common. Although not statistically significant, we found it interesting that animals with iris atrophy were likely older, and animals with iris pigment changes were likely younger. Iris atrophy is reported in older dogs, and is also considered an age-related change in cats.4,36,37
Some authors consider non-neoplastic changes in iris pigmentation to be age-related changes in healthy senior cats. 4 However, non-neoplastic changes in iris pigmentation may eventually progress into neoplasia.11,38 Dyscoria and abnormal pupillary light reflex are reliable indicators to distinguish iris melanoma from melanosis iridis. 39 Extrascleral extension, choroidal invasion, higher mitotic index and necrosis are associated with an increased rate of metastasis in iris melanoma. 40 Therefore, our study supports the opinion that although non-neoplastic changes in iris pigmentation are seen frequently in older cats, they should not be considered normal age-related findings and must be monitored closely.
Lenticular sclerosis was the most common finding of the present study. Increasing age and the occurrence of lenticular sclerosis were strongly associated. This is consistent with other publications.4,9 In one study of 2000 normal cats, 50 cats with diabetes and 100 cats after dehydration crisis termed the age at which cataract prevalence was 50% (C50). C50 for lenticular sclerosis was 14.6 ± 4.1, which is higher than the mean age (13.2 ± 2.6 years) in our study. 9 Unlike these authors, we dilated the cat’s pupils, which may contribute to better detection of lenticular sclerosis.
In the present study, small linear cortical opacities were found in 31 cats. The association between increasing age and the occurrence of cortical changes could not be proven. C50 for cataracts in the study of Williams et al was 12.7 ± 3.4 years in the normal cats. Most of the lens opacities were small linear opacities of the posterior cortex. In cats with diabetes and after dehydration crisis, cataracts developed earlier. (C50 was 5.6 ± 1.9 and 9.9 ± 2.5 years). 9 It cannot be ruled out that prior dehydration crisis affected the data in the present study.
The occurrence of attenuated retinal vessels and increasing age were statistically associated in the present study. In people, age affects the hyalinisation of retinal vessels and a decreased density of the choriocapillaris. 41 Maggio et al 18 concluded that vascular narrowing, which occurs in human patients, may also develop in the earlier stages of hypertensive retinopathy in cats. 18 Multifocal cerebral arteriosclerosis with focal haemorrhage was also found in the brains of cats with neurologic signs of systemic hypertension. 16 It is possible that attenuated retinal vessels are early signs of age-related vascular changes that could later affect the protection mechanisms of cats against higher SBP.
Both age and SBP combined were strongly associated with the occurrence of retinal oedema, haemorrhage and detachment in the present study. The mean SBP in cats with retinal oedema and/or haemorrhage was 181.3 ± 43.6 mmHg and 215.9 ± 49.1 mmHg in cats with retinal detachment. In 6/18 cats with retinal oedema and/or haemorrhage, the SBP was ⩽160 mmHg (140–160 mmHg). Therefore, they were not diagnosed with hypertensive retinopathy. Retinal oedema, haemorrhage and detachment are common signs of hypertensive retinopathy.17,18 Increased blood pressure causes vasoconstriction of retinal arterioles and ultimately degenerative damage of the endothelium and the smooth muscle layer, leaking blood and serum into the retinal tissue. 42 Hypertension may also lead to occlusion of the choriocapillaris, ultimately resulting in ischaemia of the outer retina, exudations in the subretinal space and complete retinal detachment – a sign of hypertensive choroidopathy. 43 Karck et al 22 found a positive correlation of SBP with the grade of fundus change (retinal detachment being the highest grade) and with age. 22 Furthermore, sudden increases of SBP (spikes) are also suggested to cause retinal damage, and retinal haemorrhage and oedema also occurred in anaemic cats.18,44
The results of the present study show that age and SBP combined have the strongest influence on the occurrence of retinal oedema, haemorrhage and detachment. Ocular age-related changes, such as attenuated retinal vessels, may favour the development of hypertensive retinopathy. Furthermore, the occurrence of retinal detachment may require higher SBP values than retinal oedema or haemorrhage. Retinal oedema and haemorrhage may also occur in cats with an SBP <160 mmHg. This may also be caused by vasculitis due to systemic disease, but SBP spikes and generally hypertensive retinopathy should be considered.
Conclusions
Routine ophthalmological examination as part of geriatric health examination in cats can give important cues for systemic illness and can prevent vision impairment. This study provides information about the occurrence of ocular changes in older cats and their association with age.
Footnotes
Author note: This paper was presented in part at the 59 Jahreskongress der Deutschen Gesellschaft für Kleintiermedizin, Berlin, 2013.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 16 October 2017
References
- 1. Gunn-Moore D. Considering older cats. J Small Anim Pract 2006; 47: 430–431. [DOI] [PubMed] [Google Scholar]
- 2. Bellows J, Center S, Daristotle L, et al. Aging in cats. J Feline Med Surg 2016; 11: 594–604. [Google Scholar]
- 3. Epstein M, Kuehn NF, Landsberg G, et al. AAHA senior care guidelines for dogs and cats. J Am Anim Hosp Assoc 2005; 41: 81–91. [DOI] [PubMed] [Google Scholar]
- 4. Pittari J, Rodan I, Beekman G, et al. American Association of Feline Practitioners: senior care guidelines. J Feline Med Surg 2009; 11: 763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kroll MM, Miller PE, Rodan I. Intraocular pressure measurements obtained as part of a comprehensive geriatric health examination from cats seven years of age or older. J Am Vet Med Assoc 2001; 219: 1406–1410. [DOI] [PubMed] [Google Scholar]
- 6. Stiles J, Kimmit B. Eye examination in the cat. J Feline Med Surg 2016; 18: 702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paepe D, Verjans G, Duchateau L, et al. Routine health screening: findings in apparently healthy middle-aged and old cats. J Feline Med Surg 2013; 15: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bellows J, Center S, Daristotle L, et al. Evaluating aging in cats. J Feline Med Surg 2016; 18: 551–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams DL, Heath MF. Prevalence of feline cataract: results of a cross-sectional study of 2000 normal animals, 50 cats with diabetes and 100 cats following dehydrational crises. Vet Ophthalmol 2006; 9: 341–349. [DOI] [PubMed] [Google Scholar]
- 10. Williams DL, Kim JY. Feline entropion: a case series of 50 affected animals (2003–2008). Vet Ophthalmol 2009; 12: 221–226. [DOI] [PubMed] [Google Scholar]
- 11. Patnaik AK, Mooney S. Feline melanoma: a comparative study of ocular, oral, and dermal neoplasms. Vet Pathol 1988; 25: 105–112. [DOI] [PubMed] [Google Scholar]
- 12. Attali-Soussay K, Jegou JP, Clerc B. Retrobulbar tumors in dogs and cats: 25 cases. Vet Ophthalmol 2001; 4: 19–27. [DOI] [PubMed] [Google Scholar]
- 13. Armour MD, Broome M, Dell’Anna G, et al. A review of orbital and intracranial magnetic resonance imaging in 79 canine and 13 feline patients (2004–2010). Vet Ophthalmol 2011; 14: 215–226. [DOI] [PubMed] [Google Scholar]
- 14. Nerschbach V, Eule JC, Eberle N, et al. Ocular manifestation of lymphoma in newly diagnosed cats. Vet Comp Oncol 2013; 14: 58–66. [DOI] [PubMed] [Google Scholar]
- 15. Czederpiltz JM, La Croix NC, van der Woerdt A, et al. Putative aqueous humor misdirection syndrome as a cause of glaucoma in cats: 32 cases (1997–2003). J Am Vet Med Assoc 2005; 227: 1434–1441. [DOI] [PubMed] [Google Scholar]
- 16. Littman MP. Spontaneous systemic hypertension in 24 cats. J Vet Intern Med 1994; 8: 79–86. [DOI] [PubMed] [Google Scholar]
- 17. Stiles J, Polzin D, Bistner S. The prevalence of retinopathy in cats with systemic hypertension and chronic renal failure or hyperthyroidism. J Am Anim Hosp Assoc 1994; 30: 564–572. [Google Scholar]
- 18. Maggio F, DeFrancesco TC, Atkins CE, et al. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985–1998). J Am Vet Med Assoc 2000; 217: 695–702. [DOI] [PubMed] [Google Scholar]
- 19. Sparkes AH, Caney SM, King MC, et al. Inter- and intraindividual variation in Doppler ultrasonic indirect blood pressure measurements in healthy cats. J Vet Intern Med 1999; 13: 314–318. [DOI] [PubMed] [Google Scholar]
- 20. Bodey AR, Sansom J. Epidemiological study of blood pressure in domestic cats. J Small Anim Pract 1998; 39: 567–573. [DOI] [PubMed] [Google Scholar]
- 21. Sansom J, Rogers K, Wood JL. Blood pressure assessment in healthy cats and cats with hypertensive retinopathy. Am J Vet Res 2004; 65: 245–252. [DOI] [PubMed] [Google Scholar]
- 22. Karck J, von Spiessen L, Rohn K, et al. Interrelation between the degree of a chronic renal insufficiency and/or systemic hypertension and ocular changes in cats [article in German]. Tierarztl Prax Ausg K Kleintiere Heimtiere 2013; 41: 37–45. [PubMed] [Google Scholar]
- 23. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–558. [DOI] [PubMed] [Google Scholar]
- 24. Rusanen E, Florin M, Hassig M, et al. Evaluation of a rebound tonometer (Tonovet) in clinically normal cat eyes. Vet Ophthalmol 2010; 13: 31–36. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi DL, Peterson ME, Graves TK, et al. Hypertension in cats with chronic renal failure or hyperthyroidism. J Vet Intern Med 1990; 4: 58–62. [DOI] [PubMed] [Google Scholar]
- 26. Cullen CL, Lim C, Sykes J. Tear film breakup times in young healthy cats before and after anesthesia. Vet Ophthalmol 2005; 8: 159–165. [DOI] [PubMed] [Google Scholar]
- 27. Davis K, Townsend W. Tear-film osmolarity in normal cats and cats with conjunctivitis. Vet Ophthalmol 2011; 14 Suppl 1: 54–59. [DOI] [PubMed] [Google Scholar]
- 28. Eördögh R, Schwendenwein I, Tichy A, et al. Clinical effect of four different ointment bases on healthy cat eyes. Vet Ophthalmol 2016; 19: 4–12. [DOI] [PubMed] [Google Scholar]
- 29. Smith JA, Albeitz J, Begley C, et al. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf 2007; 5: 93–107. [DOI] [PubMed] [Google Scholar]
- 30. Hartley C, Williams DL, Adams VJ. Effect of age, gender, weight, and time of day on tear production in normal dogs. Vet Ophthalmol 2006; 9: 53–57. [DOI] [PubMed] [Google Scholar]
- 31. Sanchez RF, Innocent G, Mould J, et al. Canine keratoconjunctivitis sicca: disease trends in a review of 229 cases. J Small Anim Pract 2007; 48: 211–217. [DOI] [PubMed] [Google Scholar]
- 32. Gipson IK, Argueso P, Beuermann R, et al. Research in dry eye: report of the Research Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf 2007; 5: 179–193. [DOI] [PubMed] [Google Scholar]
- 33. Sebbag L, Kass PH, Maggs DJ. Reference values, intertest correlations, and test-retest repeatability of selected tear film tests in healthy cats. J Am Vet Med Assoc 2015; 246: 426–435. [DOI] [PubMed] [Google Scholar]
- 34. von Spiessen L, Karck J, Rohn K, et al. Clinical evaluation of the Tonovet rebound tonometer in dogs and cats considering potential errors in handling [article in German]. Tierarztl Prax Ausg K Kleintiere Heimtiere 2013; 41: 213–220. [PubMed] [Google Scholar]
- 35. McLellan GJ, Kemmerling JP, Kiland JA. Validation of the TonoVet rebound tonometer in normal and glaucomatous cats. Vet Ophthalmol 2013; 16: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fischer CA. Geriatric ophthalmology. Vet Clin North Am Small Anim Pract 1989; 19: 103–123. [DOI] [PubMed] [Google Scholar]
- 37. Whitehead J. Geriatric practice. Vet Clin North Am 1971; 1: 299–312. [DOI] [PubMed] [Google Scholar]
- 38. Dubielzig R, Lindley D. The relationship between pigmented spots on the feline iris and diffuse iris melanoma [abstract]. Vet Pathol 1993; 30: 451. [Google Scholar]
- 39. Edwards S, Metzler A, Rajala-Schultz P, et al. Feline diffuse iris melanoma vs. melanosis: a retrospective case series [abstract]. Vet Ophthalmol 2015; 18: E27. [Google Scholar]
- 40. Wiggans KT, Reilly CM, Kass PH, et al. Histologic and immunohistochemical predictors of clinical behavior for feline diffuse iris melanoma. Vet Ophthalmol 2016; 19: 44–55. [DOI] [PubMed] [Google Scholar]
- 41. Grossniklaus HE, Nickerson JM, Edelhauser HF, et al. Anatomic alterations in aging and age-related diseases of the eye. Invest Ophthalmol Vis Sci 2013; 54: 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garner A, Ashton N, Tripathi R, et al. Pathogenesis of hypertensive retinopathy. An experimental study in the monkey. Br J Ophthalmol 1975; 59: 3–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hayreh SS, Servais GE, Virdi PS. Fundus lesions in malignant hypertension. VI. Hypertensive choroidopathy. Ophthalmology 1986; 93: 1383–1400. [DOI] [PubMed] [Google Scholar]
- 44. Cinquoncie S. Occurrence of acute pathologic ophthalmologic findings in cats with systemic disease [in German]. Doctorate Thesis, Freie Universität Berlin, 2015. [Google Scholar]


