Abstract
Objectives
The aim of this study was to perform neonatal clinical assessments at birth to identify newborn kittens at risk according to type of delivery, thus allowing immediate intervention and increasing their chances of survival.
Methods
This study compared Apgar scores, reflexes and clinical parameters (temperature, weight, blood glucose and peripheral oxygen saturation [SpO2]) between eutocic neonates and those delivered by emergency cesarean section. The animals were evaluated at birth and after 10 and 60 mins.
Results
Thirty-two neonates were evaluated, with 19 animals in the eutocic group (EG) and 13 animals in the cesarean group (CG). When comparing groups, CG neonates had significantly lower Apgar scores (P <0.0001), lower SpO2 (P = 0.0535), higher blood glucose (P = 0.0009), reduced reflexes (P <0.0001) and lower respiratory rates (P <0.0001) at birth and after 10 and 60 mins than EG neonates. Apgar scores positively correlated with parameters such as heart rate, reflex score, SpO2 and weight. The mortality rate in evaluated newborns was 15.6% (5/32). The early mortality rate (0–2 days old) was 80% (4/5) and the late mortality rate (3–30 days old) was 20% (1/5).
Conclusions and relevance
This study showed lower vitality in cats delivered by emergency cesarean section than in those delivered through eutocic birth. In general, neonates delivered by cesarean section have greater depression and low vitality at birth and may require advanced resuscitation procedures. The evaluations carried out in this study identified newborns with low vitality and those requiring advanced resuscitation, thus allowing immediate intervention. Apgar and reflex scores for feline neonates were suggested. Newborn-specific clinical assessment with these feline vitality scores allows the identification of at-risk neonates. Care immediately after birth increases the chance of survival among these patients.
Keywords: Feline neonate, Apgar score, cats, cesarean section, mortality
Introduction
The neonatal mortality rate of cats is high, ranging from 9% to 16% before weaning, reaching even higher rates during delivery, immediately after delivery and in the first days of life.1–4
Birth is a critical period of adaptation to extrauterine life and a major challenge to neonatal survival. Feline neonates need to make an adequate fetal–neonatal transition, which includes the shift to effective lung breathing.5,6 Owing to dystocias, prolonged deliveries and anesthetic depression induced during cesarean sections, newborns may experience exacerbated asphyxia, failure to adapt to changes in respiration, severe hypoxia, respiratory distress syndrome, bradycardia and neonatal depression (low vitality), all of which increase the chance of death in the transition period and during the first few days of life.7–12
Thus, birth assessment methods that identify at-risk newborns are crucial for enabling immediate intervention and monitoring of the newborn’s clinical evolution after resuscitation maneuvers. However, studies on clinical evaluations and complementary examinations of cats at birth are scarce. Knowledge of the neonatal particularities of kittens and their physiological parameters at birth is essential for the proper clinical management of these patients.
The Apgar score is an assessment index routinely used in medicine to facilitate clinical assessment at the time of delivery, to immediately identify the clinical condition of the neonate at birth and to direct interventions or to monitor the effectiveness of neonatal resuscitation maneuvers.12–14 Apgar scores should be used in conjunction with other analytical methods to assess newborn vitality and viability. 14
This study aimed to evaluate and compare neonatal vitality in cats delivered by eutocic birth and cesarean section via Apgar scores and neonatal reflexes. Blood glucose, body temperature, peripheral oxygen saturation (SpO2) and weight were also assessed; these measurements supported the use of an Apgar score specific to feline neonates.
Material and methods
This study was carried out by the Veterinary Neonatology Research Group of the School of Veterinary Medicine and Animal Science at Universidade Estadual Paulista (UNESP) in Botucatu, Brazil (dgp.cnpq.br/dgp/espelhogrupo/2627734671789524). The animals were included in the study only after approval by the institution’s Research Ethics Committee (protocol 0024/2021) and it was carried out in accordance with the animal welfare standards and only after receiving authorization from the animals’ guardians by collecting signatures indicating their informed consent.
The inclusion criterion was that the neonates must have been full-term. Neonates that presented with congenital malformations were excluded.
This study included seven females of different breeds, from 6 months to 3 years of age, and their respective newborns. The cats were treated at the Small Animal Reproduction Services Center of the FMVZ UNESP Veterinary Hospital or in catteries. Thirty-two neonates were evaluated, with 19 animals in the eutocic birth group (EG) and 13 animals in the cesarean section group (CG).
All eutocic births were from catteries. Eutocic births were defined as births in which kittens were delivered spontaneously without any kind of assistance, including obstetric assistance. The mother performed the entire newborn resuscitation process (removed the newborn from the fetal envelopes and performed respiratory stimuli through vigorous licking and umbilical manipulation). The processes were observed without intervention, and, soon after, neonatal evaluations were started.
The EG and CG evaluations were carried out by the same evaluators. Emergency cesarean section deliveries were defined as births in which the mother was unresponsive to obstetric maneuvers for the correction of dystocia or in which a maternal abdominal ultrasound revealed fetal distress. Dystocias were diagnosed by the maternal clinical assessment associated with complementary examinations, such as radiography and abdominal ultrasound. The causes of dystocia varied between litters, including maternal origin (narrowing of the pelvis due to trauma, primary or secondary uterine inertia or inadvertent exposure to progestins) and fetal origin (exaggerated fetal size, inadequate fetal positioning).
Anesthetic induction was performed with propofol, followed by lumbosacral epidural anesthesia with lidocaine and anesthetic maintenance with isoflurane diluted in oxygen. The queens were placed in dorsal decubitus, performing trichotomy in the abdominal region, followed by antisepsis. After the pre-retroumbilical incision at the linea alba, the body of the uterus was exposed and incised. Immediately after removing the newborn from the uterus, the surgeon removed the fetal envelopes to keep the airway free for the beginning of breathing. Fluids from the nostrils and cavity were quickly cleaned away with the aid of a compress. Then, tactile stimulation was performed by vigorous massage with a dry and heated compress on the back and chest, in order to dry the newborn and stimulate breathing. Then, the newborn was handed over to the team to continue these procedures in a warm environment, and soon after the beginning of neonatal breathing, the assessments were carried out.
Neonatal assessments
In the first 5 mins after birth, neonates from both groups were given colored neck ribbons for the purposes of identification and assessed for Apgar score, reflexes, blood glucose, body temperature, SpO2 and weight. Neonatal assessments were repeated after 10 mins and 60 mins. Total mortalities were recorded and categorized as early (0–2 days old) or late (3–30 days old).
Owing to the scarcity of Apgar score studies in cats, the assessment in this study was performed based on the Apgar score index described for dogs, following adaptations of what was proposed by Lourenço 12 and Vassalo et al. 14
Five parameters are evaluated in the Apgar score: mucosal color, heart and respiratory rates, muscle tone and reflex irritability. Heart rate auscultation was performed with a stethoscope on the left side of the chest (Figure 1a). Respiratory rate was assessed by observing the movements of the chest (Figure 1b). Mucosal coloration was assessed by observing the oral mucosa (Figure 1c). Muscle tone was determined with the newborn in the supine position, observing their active movements (Figure 1d). Reflex irritability was assessed after a painful stimulus (pressure) was applied to the interdigital space (Figure 1e). Each parameter received a score of 0, 1 or 2, according to the presentation of the neonate (Table 1); the sum of these parameters was used to identify neonatal vitality and viability. Apgar scores were interpreted as follows: scores of 0–3 indicated weak vitality, 4–6 indicated moderate vitality and 7–10 indicated normal vitality.
Figure 1.
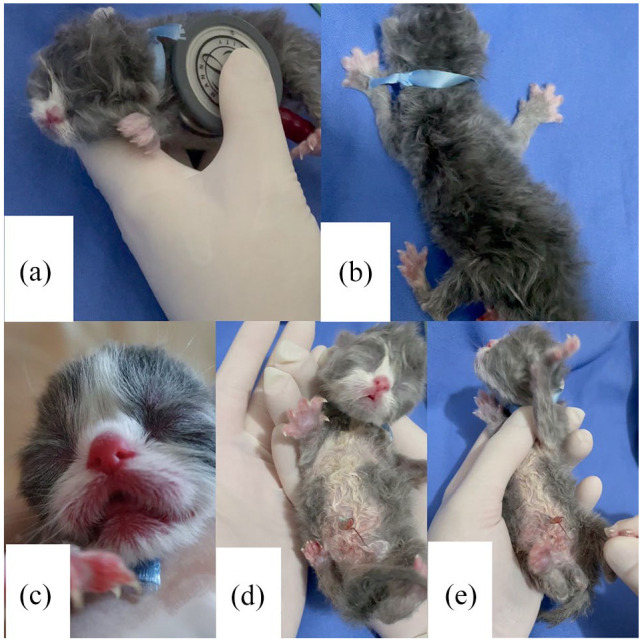
Apgar score assessment of (a) heart rate, (b) respiratory rate, (c) mucosal color, (d) muscle tone and (e) reflex irritability
Table 1.
Apgar scores for canine neonates
| Parameter/score | 0 | 1 | 2 |
|---|---|---|---|
| Mucosal coloration | Cyanotic | Pale | Pink |
| Heart rate | <100 bpm | <180 bpm | 200–260 bpm |
| Reflex irritability | Absent | Some movement | Vigorous |
| Muscle tone | Flaccid | Some limb flexion | Active movement |
| Respiratory rate | Absent or <6 mpm |
Weak and irregular, <15 mpm |
Regular and rhythmic, 15–40 mpm |
An overall score of 7–10 is considered adequate and represents newborns with good vitality and in favorable clinical condition. A score of 4–6 indicates that resuscitation maneuvers may be necessary, and a score <3 is an indication for emergency care.14,15
Newborn cats with low Apgar scores at birth underwent advanced resuscitation procedures to increase their chances of survival. 16 This treatment consisted of clearing the neonate’s airways with a nasal aspirator, drying the neonate with the aid of a heated compress, stimulating the cardiovascular system via the Jen Chung acupuncture point (at governor vessel [GV] 26), administering pulmonary ventilation (with a specific pulmonary expander for canine and feline neonates [One Puff Puppy]), providing 100% oxygen by mask and, when necessary, administering 24 mg/ml aminophylline (0.2 ml/100 g of weight, sublingually) and 0.1 mg/ml epinephrine (1 mg/ml diluted in 1:10 solution [0.01–0.03 ml/100 g of weight, IV or IO]). The neonatal environment was also warmed to 30–32°C with a space heater or an incubator to maintain the newborn’s body temperature; in the CG the queens were also warmed up with the kittens.
Reflexes were evaluated for the degree of neonatal depression (vitality). The main neonatal reflexes present at birth are sucking, rooting and righting. The sucking reflex was evaluated by inserting the tip of the examiner’s smallest digit into the mouth of the neonate and observing the suction force (Figure 2a). Then, the search reflex was examined by placing a hand close enough to the newborn’s face to trigger the reflex and waiting for an immediate search for the teat (Figure 2b). Finally, the vestibular righting reflex was evaluated by placing the neonate in a supine position on a soft, warm surface and waiting for it to return immediately to a prone position (Figure 2c). Each reflex received a score of 0, 1 or 2 (absent, weak or strong response, respectively) and the sum was used to categorize neonatal vitality. Scores were interpreted as demonstrating weak (0–2), moderate (3–4) or normal (5–6) vitality. This reflex evaluation followed the scheme proposed by Vassalo et al (Table 2). 14
Figure 2.
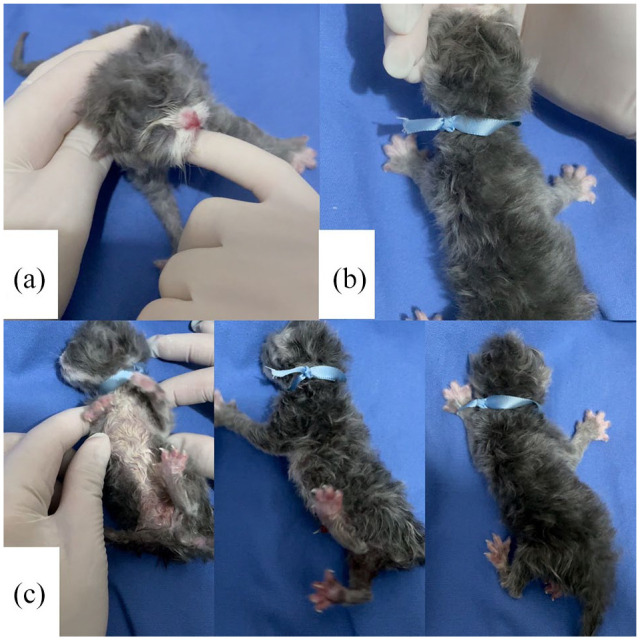
Assessment of neonatal reflexes: (a) sucking reflex, (b) rooting reflex and (c) righting reflex
Table 2.
System used to evaluate neonatal reflexes
| Reflex/score | 0 | 1 | 2 |
|---|---|---|---|
| Sucking | Absent | Weak | Strong |
| Rooting | Absent | Slow muzzle fitting inside the evaluator’s hand | Immediate fitting muzzle within the evaluator’s hand |
| Righting | Absent (continues in initial position) | Slow body repositioning | Immediate body repositioning |
Vassalo et al 14
Body temperature was measured rectally using a digital thermometer (Figure 3), and weight was assessed in grams using a digital scale (Figure 4). Blood glucose was measured with a portable glucometer (On Call Plus) by using 21 G needles to collect a drop of blood from the neonate’s pad after site antisepsis with 70% alcohol (Figure 5). Peripheral oxygen saturation was assessed using the R40VET Rzvet oximetry monitor in the femoral artery region of newborn cats (Figure 6).
Figure 3.
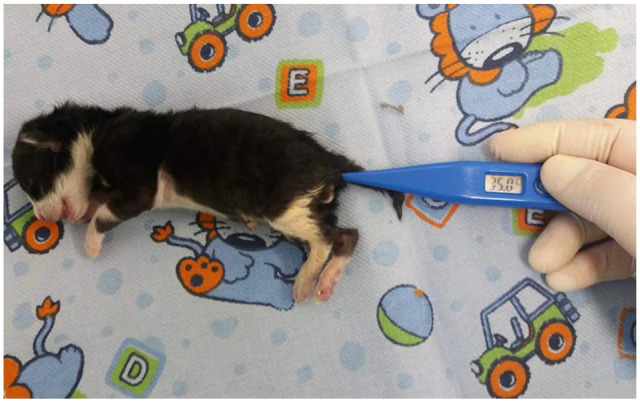
Body temperature measurement in a newborn cat
Figure 4.

Weight assessment in a newborn cat
Figure 5.
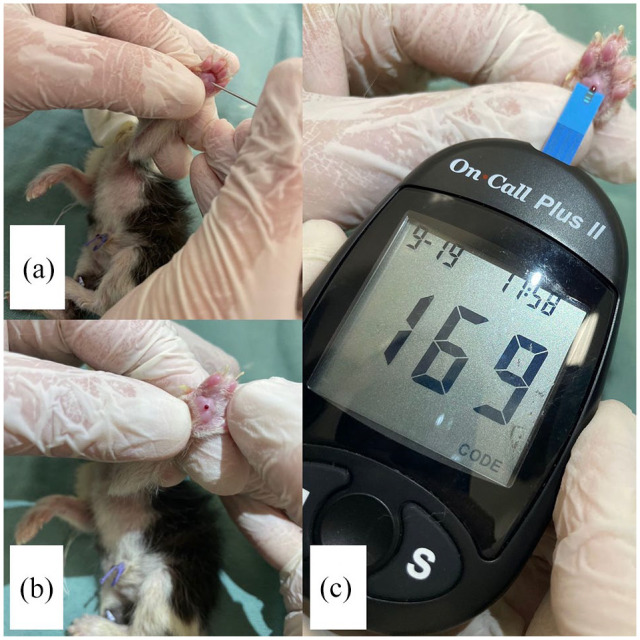
Blood glucose measurement in a newborn cat: (a) pad pricked with needle, (b) formation of blood droplet for measurement by the (c) portable glucometer
Figure 6.
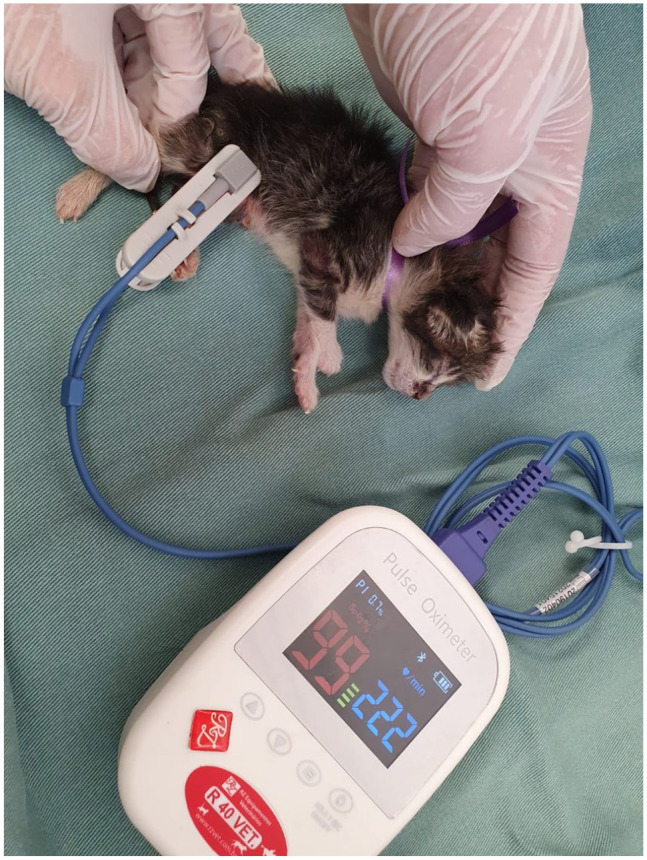
Peripheral oxygen saturation assessment in a newborn cat
Statistical analysis
The data were not normally distributed and therefore were log transformed and analyzed using parametric tests.
The statistical analysis of the parameters was performed using SAS software version 9.3 and conducting generalized linear models using the least squares method of the generalized linear model procedure. This model included the fixed effects of group (CG or EG), time (at birth and after 10 and 60 mins) and a group × time interaction. The model was followed up with Tukey’s mean comparison test. Pearson correlation coefficients of the variables were also calculated (using the Corr procedure).
All differences were considered significant at the 5% level (P <0.05).
Results
Eutocic group
The EG consisted of 19 newborn cats, with the following breed distribution: Maine Coon (n = 7), Persian (n = 8) and mixed breed (n = 4). All queens were monitored during delivery. The shortest duration of eutocia was 1 h and the longest was 5 h, with the interval between kittens ranging from 10 mins to 5 h.
There was no significant difference (P >0.05) between time points in terms of the Apgar score, reflex score, blood glucose, body temperature, SpO2 or weight for EG neonates. However, the neonatal parameters of heart rate (HR) and respiratory rate (RR) differed significantly (P <0.0001) between the time points: lower HR and RR were found at birth than at 60 mins after birth. On average, newborns in the EG had good vitality (normal vitality >7) and favorable clinical parameters.
Apgar scores positively correlated with HR (P <0.0001), reflex score (P <0.0001), SpO2 (P <0.0001) and weight (P = 0.0104) in EG neonates, with correlation coefficients of 0.70, 0.58, 0.67 and 0.33, respectively. HR also positively correlated with reflex score (P = 0.0240) and SpO2 (P = 0.0007), with correlation coefficients of 0.30 and 0.43, respectively.
Cesarean group
The CG consisted of 13 mixed-breed neonate cats. In CG neonates, Apgar scores, HR, RR, SpO2 and body temperature differed significantly between birth and 60 mins after birth (P <0.0001). Specifically, the vitality parameters were lower at birth than after 60 mins. There were no significant differences between times for reflex score, blood glucose or weight. Most CG neonates had moderate vitality at birth and evolved to normal vitality after 60 mins. Neonates that required advanced resuscitation procedures also demonstrated an improvement in clinical parameters after 60 mins.
Apgar scores positively correlated with HR (P = 0.0012), RR (P <0.0001), reflex score (P <0.0001) and weight (P = 0.0094) in CG neonates, with correlation coefficients of 0.50, 0.85, 0.65 and 0.41, respectively. RR also positively correlated with the HR (P = 0.0034) and reflex (P = 0.0002) parameters, with correlation coefficients of 0.46 and 0.56, respectively. Thus, in the CG it was observed that the lower the HR, reflex score, SpO2 and weight, the lower the Apgar score. Also, the lower the HR, the lower the reflex score and SpO2.
Comparison between groups
Significant differences (P <0.05) were found between the EG and CG, at the different time points.
Specifically, CG neonates had lower Apgar scores (P <0.0001) and SpO2 (P = 0.0535) and higher blood glucose (P = 0.0009) at birth than EG neonates. CG neonates had lower reflex scores (P <0.0001) and RR (P <0.0001) at birth, as well as after 10 and 60 mins than EG neonates. There were no significant differences between the groups in terms of HR, body temperature or weight. The means, SDs, statistical significance between timed assessments and groups, and minimum and maximum of the evaluated parameters are provided in Table 3.
Table 3.
Apgar score, reflex score, heart rate (HR), respiratory rate (RR), peripheral oxygen saturation (SpO2), blood glucose, body temperature and weight of the two groups of kittens at birth and after 10 and 60 mins
| Parameter | Group | At birth | 10 mins after birth | 60 mins after birth |
|---|---|---|---|---|
| Apgar (0–10) | CG | 7.0 ± 2.0 c (4–10) | 8.38 ± 1.6 bc (5–10) | 9.0 ± 1.5 ab (5–10) |
| EG | 8.84 ± 1.3 ab (6–10) | 9.89 ± 0.3 a (9–10) | 9.94 ± 0.2 a (9–10) | |
| Reflex (0–6) | CG | 1.3 ± 1.5 b (0–4) | 1.84 ± 2.2 b (0–6) | 2.69 ± 2.3 b (0–6) |
| EG | 4.68 ± 1.7 a (0–6) | 5.26 ± 0.9 a (4–6) | 5.47 ± 0.9 a (4–6) | |
| HR (bpm) | CG | 200.92 ± 42.4 b (120–268) | 231.07 ± 30.9 ab (164–272) | 250.31 ± 31.2 a (180–280) |
| EG | 208.42 ± 35.5 b (152–264) | 229.05 ± 21.3 ab (188–268) | 248.84 ± 18.8 a (224–288) | |
| RR (mpm) | CG | 48.76 ± 27.5 d (12–96) | 74.38 ± 30.7 cd (35–128) | 90.46 ± 25.4 bc (48–128) |
| EG | 94.52 ± 28.0 bc (40–148) | 110.26 ± 19.2 ab (75–156) | 117.47 ± 24.6 a (68–160) | |
| SpO2 (%) | CG | 84.46 ± 18.1 b (53–99) | 99.0 ± 0.0 a | 99.0 ± 0.0 a |
| EG | 93.73 ± 13.4 a (49–99) | 98.79 ± 0.9 a (95–99) | 97.89 ± 4.8 a (78–99) | |
| Glycemia (mg/dl) | CG | 129.0 ± 59.6 a (52–246) | – | 150.23 ± 63.8 a (70–300) |
| EG | 68.52 ± 36.9 b (26–151) | – | 101.79 ± 78.2 ab (30–296) | |
| Temperature (°C) | CG | 31.6 ± 2.3 b (28.2–35.4) | 33.8 ± 2.1 ab (28.2–35.7) | 35.1 ± 1.6 a (32.5–37.9) |
| EG | 31.9 ± 2.6 b (29–35.9) | 32.2 ± 2.1 b (29–35.4) | 33.6 ± 2.3 ab (29–35.9) | |
| Weight (g) | CG | 84.7 ± 11.9 b (74–110) | 84.7 ± 11.9 b (74–110) | 85.8 ± 12.3 ab (74–110) |
| EG | 102.1 ± 19.9 ab (59–132) | 101.9 ± 19.6 ab (59–132) | 103.1 ± 18.6 a (62–132) |
Data are mean ± SD (range). Different superscript letters indicate significant differences (P <0.05) between groups or time points
EG = eutocic group; CG = cesarean section group
A specific Apgar score for neonatal cats was proposed after observing the averages and range of parameters presented by newborn cats, as shown in Table 4. An evaluation of the neonatal reflex scores is available in Table 5.
Table 4.
Proposed modified Apgar scores for feline neonates
| Parameter/score | 0 | 1 | 2 |
|---|---|---|---|
| Mucosal coloration | Cyanotic | Pale | Pink |
| Heart rate | <100 bpm | <180 bpm | 200–280 bpm |
| Reflex irritability | Absent | Some movement | Vigorous |
| Muscle tone | Flabby | Some limb flexion | Active movement |
| Respiratory rate | Absent or <10 mpm | Weak and irregular, <40 mpm | Regular and rhythmic, 40–160 mpm |
Vitality score interpretations: weak (0–3), moderate (4–6) and normal (7–10)
bpm = beats per min; mpm = movements per min
Table 5.
Proposed modified neonatal reflex scores for feline neonates
| Reflex/score | 0 (absent) | 1 (weak) | 2 (strong) |
|---|---|---|---|
| Sucking | Absent | Weak intensity | Strong intensity or vacuum with tongue |
| Rooting | Absent | Slow search with muzzle and limbs inside evaluator’s hand | Immediate search with muzzle and limbs inside of evaluator’s hand |
| Righting | Absent (remained supine) | Slow body repositioning | Immediate body repositioning |
Vitality score interpretations: weak (0–2), moderate (3–4) and normal (5–6)
The total mortality rate in the evaluated neonates was 15.6% (5/32). The early (0–2 days old) mortality rate was 80% (4/5) and the late (3–30 days old) mortality rate was 20% (1/5). All the early kitten mortalities occurred in the CG due to hypoxia during dystocia and emergency cesarean section. The late kitten mortality occurred in the EG, with the cause of death being unknown.
Discussion
Previous studies have assessed Apgar scores in newborn dogs delivered by eutocic births or cesarean sections.14,17–20 However, studies in cats are scarce, and we found just one study on the evaluation of Apgar scores in kittens that were delivered only by eutocic births. 21
The pattern of lower Apgar scores at birth than after 60 mins align with existing findings. Newborns experience transient physiological hypoxemia resulting from reduced blood flow from the mother to the fetus, induced by uterine contractions during labor. Consequently, a decrease in the partial pressure of oxygen (pO2) and tissue hypoxia are observed, which can culminate in neonatal depression or transient neonatal low vitality. An evolution of these parameters can be observed within 60 mins after birth, as the newborn adapts to extrauterine life.12,14,20,22
Significantly lower HRs and RRs were also observed at birth than at 60 mins later, in both groups. Neonatal bradycardia can occur owing to myocardial hypoxia and is not mediated by the vagus nerve due to the immaturity of the autonomic nervous system. Therefore, these patients are predisposed to lower HRs during the physiological hypoxemia associated with birth. In addition, the compensatory response to hypoxia is reduced in neonates due to the immaturity of carotid chemoreceptors, structural immaturity of the lungs and reduced capacity for chest wall expansion. 23 The gradual evolution of the RRs and HRs is expected 10 and 60 mins after birth, with the onset of adequate lung breathing and increased myocardial oxygenation.12,14 However, neonates born with respiratory distress and intense bradycardia due to exacerbated perinatal asphyxia and consequent severe hypoxia require advanced resuscitation procedures to normalize their cardiorespiratory parameters and increase their chances of survival.
This study demonstrated significantly lower Apgar scores and reflex scores (ie, low vitality) in newborn cats delivered by emergency cesarean section than in those delivered by eutocic birth. In addition, CG neonates also had lower SpO2 and RRs. Similar results have been found in several previous studies on neonatal dogs.14,17–19 This difference between groups may occur owing to the contact of CG neonates with anesthetics during a cesarean section, as inhaled agents cross the placenta and the fetal blood–brain barrier. 11 Prolonged delivery or dystocia that culminate in cesarean sections may also lead to exacerbated asphyxia, prominent hypoxemia and further cardiorespiratory depression.10,11,20,24 However, despite lower Apgar scores and reflex scores upon delivery, after resuscitation procedures CG neonates displayed clinical evolutions of the measured parameters and vitality after 60 mins.
When comparing our results with those in dogs born by emergency cesarean section under the same anesthetic protocol,14,20 we observed that cats born by emergency cesarean section have a slower clinical evolution. For example, suction remained weak in most CG neonates at 60 mins after birth. The sucking reflex is an essential assessment in neonates as kittens with weak or absent suction force due to dystocia or cesarean depression require veterinary or breeder assistance, such as orogastric tube feeding, in order to nurse in the first hour of life. Failure to provide adequate nursing assistance can lead to the neonatal triad (hypoglycemia, hypothermia and dehydration) and an increased risk of death.12,23 In general, in both the CG and EG groups, reflex scores were strongly correlated with Apgar scores, and kittens that had Apgar scores <6 displayed decreased rooting and sucking reflexes than kittens that received Apgar scores of 7–10.
The anesthetic protocol used in this study (anesthetic induction with propofol, followed by lumbosacral epidural anesthesia with lidocaine to reduce percentage inhalation anesthesia and by anesthetic maintenance with isoflurane) is described for cesarean anesthesia in female dogs and cats.23,25,26 This protocol has a positive impact on queens, who wake up faster and can care for their kittens. However, studies that compare anesthetic protocols and evaluate the anesthetic depression effects of these drugs in newborns (by assessing Apgar scores) are mainly described in dogs27,28 and are scarce in cats.
In this study, several cats delivered by emergency cesarean section, owing to previous dystocia, were apneic at birth due to anesthetic depression and required advanced resuscitation procedures, such as stimulating the cardiovascular system via the Jen Chung GV-26 acupuncture point and administering positive pressure pulmonary ventilation with the neonatal-specific canine/feline expander (One Puff Puppy) to start breathing. Thus, our findings highlight the importance of studies on anesthetic protocols in pregnant cats to reduce fetal–neonatal depression.
Despite the significant difference in Apgar scores between the groups, there was no significant difference in HRs. This lack of difference in HR contrasts with previous studies of dogs, which demonstrated reduced HR in pups delivered by cesarean section vs pups delivered via eutocic birth.12,16,29 The normal neonatal HR in kittens can be >200 beats per min (bpm). 6 Owing to neonatal immaturity and prominent hypoxemia in dystocia and cesarean sections, it was assumed that CG cats would also have lower HRs. Furthermore, unlike what has been observed in dogs, the mean HR of cats at birth was >200 bpm. Thus, we hypothesize that the feline species possibly present greater maturity of cardiac autonomus development, as well as of carotid chemoreceptors, and thus myocardial hypoxia can be more effectively compensated for.
This hypothesis is supported by the comparison of RRs between feline and canine neonates. Regardless of delivery method, the RR of cats was higher than that of dogs.12,16,20,29 While an average RR of up to 40 movements per min (mpm) is expected in newborn dogs,12,30 in newborn cats in this study, averages above 100 mpm were observed. It is possible that cats demonstrate greater maturity of the adaptive breathing reflex, carotid chemoreceptors or lung structures than dogs, or a greater expansion of the chest wall, to compensate for hypoxemia during parturition. However, further studies are needed to examine cardiorespiratory maturity in newborn cats compared with dogs. In cats, a higher SpO2 than that in dogs29,31 was observed in both groups, possibly due to their higher RR and HR.
Owing to the differences in vitality parameters between newborn cats and dogs, we suggest a novel, modified feline Apgar score. As few studies exist on the clinical evaluation of newborn cats, the routine use of canine Apgar scores in cats is common; however, there are various physiological and hemodynamic differences between these two species. Therefore, studies on specific clinical evaluations in cats facilitate the appropriate clinical management of these patients.
Similar to existing findings on blood glucose levels in neonatal puppies, we found elevated glucose concentrations in neonates delivered by cesarean section than in those delivered through eutocia. 32 This difference is likely due to the mobilization of hepatic glycogen stores in CG neonates due to the stress of asphyxia and/or hypoxemia that often accompanies dystocia and cesarean sections, 23 as well as the stress resulting from the advanced resuscitation procedures at birth or from the administration of epinephrine. 32 However, in neonates that did not experience excessive fetal stress, glucose levels have been directly linked to maternal glycemia.33,34
We also found a positive correlation between Apgar scores and neonatal weight. This aligns with previous studies on feline21,35 and canine 14 neonates. In other words, puppies with low birth weights may be more immature and debilitated than puppies with medium birth weights, presenting with lower Apgar scores, lower vitality and, consequently, a higher risk of death. 35 Neonatal weight assessment is essential, as newborns with low birth weight require greater attention from veterinarians and breeders to ensure adequate development and improved survival rates.6,14,35
Feline neonates in both groups were hypothermic at birth, probably due to their immature thermoregulatory systems and their contact with amniotic fluid, as damp kittens quickly lose heat to the environment.23,36 The CG neonates showed significantly higher body temperatures after 60 mins compared with those at birth. Possibly, this was due to CG neonates’ time in the incubator, which was maintained at 30–32°C. It is crucial to manage neonatal body temperatures, as hypothermia can cause bradycardia, hypoxia and low vitality, as well as reduce the effectiveness of resuscitation maneuvers.6,12,23
The total mortality rate was 15.6% in the evaluated newborns, which is similar to the values observed by Cave et al 1 and Fournier et al, 2 who had losses of 14% and 16%, respectively. Mortality in feline and canine neonates is relatively higher in the first days of life.3,4,37 In dogs, hypoxia during parturition is the leading cause of neonatal mortality, according for over 60% of deaths; approximately 90% of all deaths in hypoxemic puppies occur during the first 2 days. 37 In this study, the feline mortality rate was also highest over the first 2 days, corresponding to 80% of the losses. These deaths occurred due to hypoxia during dystocia and emergency cesarean section in neonates with low Apgar scores at birth.
It is important to be aware of the particularities of cats vs dogs so that we can distinguish key differences in their neonatal and adult parameters.
Conclusions
Cats delivered by emergency cesarean section experience neonatal depression and may require advanced resuscitation procedures. The clinical evaluations carried out in this study allowed us to identify newborns with low vitality, as well as those requiring advanced resuscitation procedures due to prominent hypoxemia; thus, we could immediately intervene. In addition, these evaluations allowed us to monitor the clinical evolution of the newborn after these procedures. It is essential that cat labor is overseen by a veterinarian and that clinical evaluation of the newborns is carried out using cat-specific vitality scores. The use of the modified feline Apgar score proposed in this study allows the provision of immediate assistance at birth and increases the chance of survival for these patients.
Acknowledgments
We would like to thank the Academic Excellence Program (PROEX) of the Coordination for the Improvement of Higher Education Personnel (CAPES) in Brazil, as well as the Department of Veterinary Surgery and Animal Reproduction at UNESP, Campus Botucatu, in São Paulo, Brazil. We also thank American Journal Experts (AJE) for the excellent work in the translation
Footnotes
Accepted: 31 January 2022
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Coordination for the Improvement of Higher Education Personnel (CAPES) – Academic Excellence Program (PROEX), Brazil.
Ethical approval: The work described in this manuscript involved the use of experimental animals and the study therefore had prior ethical approval from an established (or ad hoc) committee as stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Keylla Helena Nobre Pacífico Pereira  https://orcid.org/0000-0001-6474-4808
https://orcid.org/0000-0001-6474-4808
Kárita da Mata Fuchs  https://orcid.org/0000-0002-3352-8261
https://orcid.org/0000-0002-3352-8261
Fabiana Ferreira de Souza  https://orcid.org/0000-0003-4721-1801
https://orcid.org/0000-0003-4721-1801
References
- 1. Cave TA, Thompson H, Reid SWJ, et al. Kitten mortality in the United Kingdom: a retrospective analysis of 274 histopathological examinations (1986 to 2000). Vet Rec 2002; 17: 497–501. [DOI] [PubMed] [Google Scholar]
- 2. Fournier A, Masson M, Corbière F, et al. Epidemiological analysis of reproductive performances and kitten mortality rates in 5,303 purebred queens of 45 different breeds and 28,065 kittens in France. Reprod Domest Anim 2017; 2: 153–157. [DOI] [PubMed] [Google Scholar]
- 3. Sparkes AH, Rogers K, Henley WE, et al. A questionnaire-based study of gestation, parturition and neonatal mortality in pedigree breeding cats in the UK. J Feline Med Surg 2006; 3: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dolan ED, Doyle E, Tran HR, et al. Pre-mortem risk factors for mortality in kittens less than 8 weeks old at a dedicated kitten nursery. J Feline Med Surg 2021; 8: 730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lourenço MLG, Machado LHA. Características do período de transição fetal-neonatal e particularidades fisiológicas do neonato canino. Rev Bras Reprod Anim 2013; 37: 303–308. [Google Scholar]
- 6. Little S. Feline pediatrics: how to treat the small and the sick. Compend Contin Educ Vet 2011; 33: 1–6. [PubMed] [Google Scholar]
- 7. Moon PF, Massat BJ, Pascoe PJ. Neonatal critical care. Vet Clin North Am Small Anim Pract 2001; 31: 343–367. [DOI] [PubMed] [Google Scholar]
- 8. Indrebø A, Trangerud C, Moe L. Canine neonatal mortality in four large breeds. Acta Vet Scand 2007; 49 DOI: 10.1186/1751-0147-49-S1-S2. [Google Scholar]
- 9. Münnich A. The pathological newborn in small animals: the neonate is not a small adult. Vet Res Commun 2008; 32: 81–85. [DOI] [PubMed] [Google Scholar]
- 10. Batista M, Moreno C, Vilar J, et al. Neonatal viability evaluation by Apgar score in puppies delivered by cesarean section in two brachycephalic breeds (English and French bulldog). Anim Reprod Sci 2014; 146: 218–226. [DOI] [PubMed] [Google Scholar]
- 11. Bailin HG, Thomas T, Levy NA. Retrospective evaluation of feline dystocia: clinicopathologic findings and neonatal outcomes in 35 cases (2009–2020). J Feline Med Surg. Epub ahead of print 14 June 2021. DOI: 10.1177/1098612X211024154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lourenço MLG. Cuidados com neonatos e filhotes. In: Jericó MM, Kogika MM, De Andrade Neto JP. (eds). Tratado de medicina interna de cães e gatos. Rio de Janeiro: Guanabara Koogan, 2015, p 2431. [Google Scholar]
- 13. Apgar V. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg 1953; 32: 260–267. [PubMed] [Google Scholar]
- 14. Vassalo FG, Simões CRB, Sudano MJ, et al. Topics in the routine assessment of newborn puppy viability. Top Comp Anim Med 2015; 30: 16–21. [DOI] [PubMed] [Google Scholar]
- 15. Veronesi MC, Panzani S, Faustini M, et al. An Apgar scoring system for routine assessment of newborn puppy viability and short-term survival prognosis. Theriogenology 2009; 72: 401–407. [DOI] [PubMed] [Google Scholar]
- 16. Camargo PLD, Ferreira H, Machado CEG, et al. Estudo clínico da via intramedular como alternativa para infusão de fluídos em cães jovens. Braz J Vet Res Anim Sci 1996; 33: 235–238. [Google Scholar]
- 17. Crissiuma AL, Labarthe NV, Soares AMB, et al. Aspectos cardiorespiratórios e ácidos básicos do período de transição fetal-neonatal em cães. Rev Clín Vet 2005; 57: 14–16. [Google Scholar]
- 18. Lucio CF, Silva LCG, Rodrigues JA, et al. Acid–base changes in canine neonates following normal birth or dystocia. Reprod Domest Anim, 2009; 44: 208–210. [DOI] [PubMed] [Google Scholar]
- 19. Silva LCG, Lucio CF, Veiga GAL, et al. Neonatal clinical evaluation, blood gas and radiographic assessment after normal birth, vaginal dystocia or caesarean section in dogs. Reprod Domest Anim 2009; 44: 160–163. [DOI] [PubMed] [Google Scholar]
- 20. Pereira KHNP, Hibaru VY, Mata FKD, et al. Use of cardiac troponin I (cTnI) levels to diagnose severe hypoxia and myocardial injury induced by perinatal asphyxia in neonatal dogs. Theriogenology 2022; 180: 146–153. [DOI] [PubMed] [Google Scholar]
- 21. Axelsson R. APGAR score as a method for prediction of survival prognosis in newborn puppies and kittens. Second cycle, A2E. https://stud.epsilon.slu.se/14800/7/axelsson_r_190306.pdf (2019, accessed February 16, 2022). [Google Scholar]
- 22. Siristatidis C, Salamalekis E, Kassanos D, et al. Evaluation of fetal intrapartum hypoxia by middle cerebral and umbilical artery Doppler velocimetry with simultaneous cardiotocography and pulse oximetry. Arch Gynecol Obstet 2004; 270: 265–270. [DOI] [PubMed] [Google Scholar]
- 23. Peterson ME, Kutzler MA. Small animal pediatrics: the first 12 months of life. St Louis, MO: Saunders/Elsevier, 2011. [Google Scholar]
- 24. Traas AM. Resuscitation of canine and feline neonates. Theriogenology 2008; 70: 343–348. [DOI] [PubMed] [Google Scholar]
- 25. Pascoe PJ, Moon PF. Periparturient and neonatal anesthesia. Vet Clin North Am Small Anim Pract 2001; 31: 315–341. [DOI] [PubMed] [Google Scholar]
- 26. Kushnir Y, Epstein A. Anesthesia for pregnant bitch and cat. Isr J Vet Med 2012; 67: 19–23. [Google Scholar]
- 27. Cramer KGMD, Joubert KE, Nöthling JO. Puppy survival and vigor associated with the use of low-dose medetomidine, propofol induction and maintenance of anesthesia by inhalation of sevoflurane gas for cesarean section in the bitch. Theriogenology 2017; 96: 10–15. [DOI] [PubMed] [Google Scholar]
- 28. Vilar JM, Batista M, Pérez R, et al. Comparison of 3 anesthetic protocols for elective cesarean in dogs: effects on bitch and newborn pups. Anim Reprod Sci 2018: 190: 53–62. [DOI] [PubMed] [Google Scholar]
- 29. Almeida LLD. Estado oxidativo de neonatos e fêmeas caninas no periparto vaginal eutócico ou cesariana eletiva. MSc thesis, University of São Paulo, 2018. [Google Scholar]
- 30. McMichael M. Pediatric emergencies. Vet Clin North Am Small Anim Pract 2005; 35: 421–434. [DOI] [PubMed] [Google Scholar]
- 31. Almeida LLD, Abreu RAD, Brito MM, et al. Both spontaneous vaginal delivery and elective cesarean section influence neonatal redox status in dogs. Vet Rec. Epub ahead of print 8 November 2021. DOI: 10.1002/vetr.1082. [DOI] [PubMed] [Google Scholar]
- 32. Doebeli A, Michel E, Bettschart R, et al. Apgar score after induction of anesthesia for canine cesarean section with alfaxalone versus propofol. Theriogenology 2013; 80: 850–854. [DOI] [PubMed] [Google Scholar]
- 33. Van Rheenen PF, Sebastian G, Brabin BJ. Delayed umbilical cord clamping for reducing anaemia in low birth weight infants: implications for developing countries. Ann Trop Paediatr 2006; 26: 157–167. [DOI] [PubMed] [Google Scholar]
- 34. Davidson AP. Neonatal resuscitation. Vet Clin North Am Small Anim Pract 2014; 44: 191–204. [DOI] [PubMed] [Google Scholar]
- 35. Socha P, Lengling R, Bonecka J, et al. Obstetric and newborn parameters in the Maine Coon cats. Pol J Vet Sci 2019; 22: 439–443. [DOI] [PubMed] [Google Scholar]
- 36. Prats A, García F, Dumon C, et al. Neonatologia e pediatria canina e felina. Madrid: Interbook, 2005. [Google Scholar]
- 37. Münnich A, Küchenmeister U. Causes, diagnosis and therapy of common diseases in neonatal dogs in the first days of life: pillars of the practical approach. Reprod Domest Anim 2014; 49: 64–74. [DOI] [PubMed] [Google Scholar]


