Abstract
Background and Aims:
NAFLD strongly associates with cardiovascular disease (CVD) risk factors; however, the association between NAFLD and incident CVD, CVD-related mortality, incident cancer, and all-cause mortality is unclear.
Approach and Results:
We included 10,040 participants from the Framingham Heart Study, the Coronary Artery Risk Development in Young Adults Study, and the Multi-ethnic Study of Atherosclerosis to assess the longitudinal association between liver fat (defined on CT) and incident CVD, CVD-related mortality, incident cancer, and all-cause mortality. We performed multi-variable-adjusted Cox regression models including age, sex, diabetes, systolic blood pressure, alcohol use, smoking, HDL, triglycerides, and body mass index at baseline or time-varying covariates. The average age was 51.3 ± 3.3 years and 50.6% were women. Hepatic steatosis was associated with all-cause mortality after 12.7 years of mean follow-up when adjusting for baseline CVD risk factors, including body mass index (HR: 1.21, 1.04–1.40); however, the results were attenuated when utilizing time-varying covariates. The association between hepatic steatosis and incident CVD was not statistically significant after we accounted for body mass index in models considering baseline covariates or time-varying covariates. We observed no association between hepatic steatosis and CVD-related mortality or incident cancer.
Conclusions:
In this large, multicohort study of participants with CT-defined hepatic steatosis, accounting for change in CVD risk factors over time attenuated associations between liver fat and overall mortality or incident CVD. Our work highlights the need to consider concurrent cardiometabolic disease when determining associations between NAFLD and CVD and mortality outcomes.
INTRODUCTION
NAFLD is the most common liver disorder in the world, with estimates of prevalence in the US and Europe ranging from 10% to 46%[1-3] and global estimate of prevalence of 24%.[4] The incidence of NAFLD is rising along with an increase in NAFLD risk factors such as obesity, insulin resistance, hyperlipidemia, and hypertension.[5] Whereas the majority of individuals with NAFLD have good long-term prognoses, up to 20% of patients may develop cirrhosis and hepatic complications, with NAFLD poised to become the leading indication for liver transplantation in the US.[6-8] Despite these trends, the leading causes of death in individuals with NAFLD are because of cardiovascular disease (CVD) and extrahepatic malignancy.[9]
NAFLD is closely related to CVD risk factors including hypertension, hyperlipidemia, obesity, and insulin resistance.[10-12] The relations between NAFLD and CVD are complex and bidirectional. CVD risk factors are associated with risk of NAFLD and NASH, the presence of NAFLD is also strongly associated with the development of metabolic syndrome.[11,13] However, the association between NAFLD and incident CVD is unclear, as study results are mixed. In cohort studies from the US and Europe with NAFLD diagnosis based on diagnostic codes, NAFLD was not associated with myocardial infarction or stroke after adjusting for CVD risk factors.[14,15] A recent meta-analysis of observational studies demonstrated an association between NAFLD and CVD events, but there was significant heterogeneity in CVD risk factor reporting, making it unclear whether the relation between NAFLD and CVD events would persist after appropriate adjustment for other CVD risk factors.[16] Several meta-analyses with varying methods of NAFLD ascertainment demonstrated mixed results in the association between NAFLD and both CVD-related and all-cause mortality.[17,18]
NAFLD is also associated with increased risk of HCC, even in the absence of cirrhosis.[19,20] However, the association between NAFLD and other cancers is only newly being studied, and results are conflicting. A recent US community-based cohort study reported that NAFLD was associated with higher risk of developing extrahepatic cancers after adjusting for obesity.[21] Conversely, a recent population-based longitudinal study from Japan reported that NAFLD was only associated with the increased risk of gastric or colorectal cancer in combination with obesity.[22]
Thus, we conducted a large, longitudinal study of data from 3 US-based cohort studies across diverse geographic and racial/ethnic backgrounds to evaluate the association between hepatic steatosis and incident CVD, CVD-related mortality, cancer, and all-cause mortality.
METHODS
Study sample
We included participants from 3 longitudinal cohort studies, the Framingham Heart Study (FHS), the Coronary Artery Risk Development in Young Adults (CARDIA) study, and the Multi-ethnic Study of Atherosclerosis (MESA). Detailed study designs of all the 3 cohorts have been published.[23-26] Briefly, the FHS is a single-center, community-based cohort study that began in 1948 and includes multiple generational cohorts of participants living in Framingham, MA. We included 2572 participants from the Offspring Cohort and the Third Generation Cohort who underwent CT scans as part of the multidetector CT substudy between the years 2002 and 2005.
CARDIA is a multicenter, community-based cohort study that began in 1985 and included 5115 participants aged 18–30 years from 4 different field centers (including Birmingham, AL, Chicago, IL, Minneapolis, MN, and Oakland, CA). We included 2577 participants who underwent a CT scan in the year 25 exam between July 2010 and August 2011.
MESA is a multicenter community-based cohort study that began in 2000, including 6814 participants aged 45–84 years. Participants were enrolled across 6 different field centers (including Columbia University in New York City, NY, John Hopkins University in Baltimore, MD, Northwestern University in Chicago, IL, UCLA in Los Angeles, CA, The University of Minnesota in Minneapolis and St. Paul, MN, and Wake Forest University in Winston-Salem, NC). We included 4891 MESA cohort participants who underwent CT coronary (chest) scans during exam 1 between July 2000 and August 2002.
In all individual cohorts, we excluded participants who had heavy alcohol use (defined as ≥ 7 drinks per week for women and as ≥ 14 drinks per week for men), missing covariates, and missing interpretable liver CT data. For the analyses of incident CVD and CVD-related mortality, we excluded participants with prevalent CVD at the time of liver fat assessment. For the analyses of incident cancer and cancer-related mortality, we excluded participants with prevalent cancer (other than nonmelanoma skin cancers). CARDIA participants were not included in the cancer analyses because of the lack of cancer outcome data.
The Institutional Review Boards of each institution for the individual studies approved the original cohort studies. Participants provided signed consent for participation in each study. The Institutional Review Board from Boston University Medical Center (IRB-Boston University H-40090) approved the present study. All research was conducted in accordance with both the Declaration of Helsinki and Istanbul.
CT assessment of liver fat
We derived liver fat data from CT scans performed in each cohort study as described.[27-29] In the multidetector CT substudy of the FHS cohort, participants underwent abdominal CT scan [LightSpeed Ultra; General Electric (GE), Milwaukee, WI] with a radiopaque phantom (Image Analysis, Lexington, KY) placed under each participant. The liver phantom ratio was calculated as the ratio between the average liver attenuation [in Hounsfield units (HU)] and the phantom HU.[30] In the CARDIA cohort, participants underwent noncontrast abdominal CT scans with GE scanners (750HD 64 and LightSpeed VCT 64, Milwaukee, WI) in the Birmingham and Oakland centers or Siemens scanners (Sensation 64, Siemens Medical Solutions, Erlangen, Germany) in the Chicago and Minneapolis centers. In the MESA cohort, participants underwent noncontrast cardiac CT scans using GE scanners (Imatron C150 or LightSpeed, Milwaukee, WI) in the New York, Chicago, and Los Angeles centers and the Siemens scanner (Volume Zoom, Erlangen, Germany) in the Baltimore, Minneapolis, and Winston-Salem centers. In both the CARDIA and MESA studies, liver attenuation was quantified in HU. Protocol for measurement of liver attenuation and liver phantom ratio for each study is described in Supplementary Table 1[28,30,31] (http://links.lww.com/HEP/B798).
The primary exposure was continuous liver fat, and the secondary exposure was dichotomous hepatic steatosis. For the FHS cohort, continuous liver fat was defined as—liver phantom ratio (as the liver phantom ratio decreases with increasing liver fat), and hepatic steatosis was defined by liver phantom ratio ≤ 0.33, which has previously been shown to be sensitive and specific for hepatic steatosis in the FHS cohort.[27] For both the CARDIA and MESA cohorts, continuous liver fat was defined as liver attenuation in HU, and hepatic steatosis was defined as liver attenuation HU <51, with a further subgroup of moderate-to-severe hepatic steatosis (defined as a liver attenuation <40 HU).[7,32]
Covariates
Baseline demographic, clinical, and laboratory variables were obtained per protocol for each cohort study. We attained the following variables: age, sex, number of alcoholic drinks per week, current smoking (defined as smoking at least 1 cigarette per day during the previous year), continuous systolic blood pressure (mm Hg), diabetes (defined as fasting plasma glucose ≥ 126 mg/dL, hemoglobin A1c ≥ 6.5%, or treatment with an oral hypoglycemic agent or insulin), serum triglycerides, serum HDL cholesterol, and body mass index (BMI) in kg/m2.
Outcomes
Outcomes were obtained from adjudicated event data from each cohort. The primary outcome was incident CVD (myocardial infarction, non-myocardial infarction, acute coronary syndrome, coronary revascularization, heart failure, stroke, and transient ischemic attack). The secondary outcomes were CVD-related mortality, all-cause mortality, and incident cancer (excluding nonmelanoma skin cancers such as squamous cell carcinoma or basal cell carcinoma, nonmalignant neoplasms, and benign neoplasms).
Statistical analysis
We assessed the baseline clinical characteristics for each cohort, and then combined the estimates of these characteristics. Continuous predictors were standardized by dividing the pooled SD from the 3 cohorts. We performed a 2-stage meta-analysis. We compared the characteristics of participants who developed primary and secondary outcomes versus those who did not using t tests for continuous variables and chi-squared tests for categorical variables. We used Cox regression to determine the longitudinal association between continuous liver fat (per unit decrease in liver attenuation) and at least mild hepatic steatosis with the outcomes of incident CVD, CVD-related mortality, incident cancer, cancer-related mortality, and all-cause mortality. We tested the proportional assumption and nonlinear effects. We combined log HR estimates using fixed effect model meta-analysis. We calculated the Cochran Q and I2 to evaluate the heterogeneity across cohorts. We considered heterogeneity shown if I2 was >50%, and DerSimonian and Laird random-effects model meta-analysis was then used for combined estimates. The random-effects model results were reported for CVD-related mortality. We created 3 models to adjust for baseline covariates. Model 1 included age and sex. Model 2 added known CVD risk factors of diabetes, continuous systolic blood pressure, alcohol use, current smoking, serum HDL, and serum triglycerides. Model 3 further adjusted for BMI as a continuous variable, as BMI is known to weakly correlate with hepatic steatosis.[27] We also performed the Cox regression analysis as detailed above substituting time-varying rather than baseline covariates for age, diabetes, continuous systolic blood pressure, alcohol use, current smoking, serum HDL, serum triglycerides, and BMI for all the 3 models. All results were expressed as HR with 95% CI. Statistical significance was defined as a 2-sided p-value < 0.05.
We further performed a sensitivity analysis using Cox regression to determine the longitudinal association between the subgroup of moderate-to-severe hepatic steatosis and the outcomes. Statistical significance was defined as a 2-sided p-value < 0.05. Statistical analysis was performed with SAS software (version 9.4, Cary, NC).
RESULTS
Baseline characteristics
We included a total of 10,040 participants across all the 3 cohorts in our study (FHS n = 2572, CARDIA n=2577, MESA n=4891), with a total follow-up of 127,481 person-years and a mean follow-up time of 12.7 years. Overall, the average age was 51.3 ± 3.3 years and 50.6% were women. There were 58.5% non-Hispanic White participants, 27.5% Black participants, and 10.5% Hispanic participants. The mean BMI was 28.7±3.4 kg/m2 and 11.5% had diabetes. A total of 1808 (18.0%) participants had hepatic steatosis, with a further 938 (9.8%) having moderate-to-severe hepatic steatosis. Full baseline characteristics for individual cohorts and combined cohorts is listed in Table 1.
TABLE 1.
Baseline characteristics by individual study cohort and combined cohorts
| Clinical characteristic | CARDIA (n = 2577) | MESA (n = 4891) | FHS (n = 2572) | Overall (n = 10040) |
|---|---|---|---|---|
| Age (y) | 50.1 ± 3.66 | 61.8 ± 10.2 | 51.0 ± 10.7 | 51.3 ± 3.28 |
| Women, N (%) | 1443 (56.0) | 2324 (47.5) | 1313 (51.0) | 5080 (50.6) |
| Race White, N (%) | 1302 (50.5) | 1995 (40.8) | 2572 (100) | 5869 (58.5) |
| BMI (kg/m2) | 30.7 ± 7.23 | 28.7 ± 5.47 | 27.7 ± 5.41 | 28.7 ± 3.40 |
| Alcohol (drinks/wk) | 1.96 ± 3.00 | 1.47 ± 2.56 | 2.77 ± 3.29 | 1.96 ± 1.68 |
| Current smoking, N (%) | 545 (24.9) | 674 (13.8) | 283 (11.0) | 1502 (15.0) |
| Diabetes, N (%) | 384 (14.9) | 614 (12.6) | 152 (5.9) | 1150 (11.5) |
| Fasting glucose (mg/dL) | 100 ± 29 | 97 ± 30 | 99 ± 21 | 99 ± 15 |
| Systolic blood pressure (mm Hg) | 120 ± 16 | 126 ± 21 | 121 ± 16 | 122 ± 10 |
| Diastolic blood pressure (mm Hg) | 75 ± 11 | 72±10 | 75 ± 9 | 74 ± 6 |
| Hypertension, N (%) | 996 (38.6) | 2145 (43.9) | 738 (28.7) | 3879 (38.6) |
| Triglycerides (mg/dL) | 116.1 ± 92.1 | 129.5 ± 85.5 | 125.4 ± 84.3 | 124.0 ± 50.3 |
| HDL Cholesterol (mg/dl) | 56.1 ± 16.8 | 50.0 ± 14.3 | 52.3 ± 15.6 | 52.5 ± 8.94 |
| Prevalent CVD, N (%) | 87 (3.4) | 0 | 88 (3.4) | 175 (1.7) |
| Prevalent cancer, N (%) | — | 3 (0.1) | 128 (5.0) | 131 (1.3) |
| Liver attenuation mean (HU) | 55.3 ± 11.9 | 61.7 ± 12.3 | 65.7 ± 9.59 | 61.6 ± 6.38 |
| At least mild hepatic steatosis, N (%) | 633 (24.6) | 733 (15.0) | 442 (17.2) | 1808 (18.0) |
| Moderate-to-severe hepatic steatosis, N (%) | 264 (10.2) | 281 (5.7) | 442 (17.2) | 987 (9.8) |
Note: At least mild hepatic steatosis is defined as liver attenuation < 51 HU in CARDIA and MESA and as liver phantom ratio (LPR) < 0.33 in FHS. Moderate-to-severe 2 hepatic steatosis is defined as liver attenuation <40 HU in CARDIA and MESA.
Abbreviations: BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; CVD, cardiovascular disease; FHS, Framingham Heart Study; MESA, Multi-ethnic Study of Atherosclerosis.
Incident CVD and CVD-related mortality
A total of 1116 participants developed incident CVD, and 333 died from CVD-related causes over the 127,495 person-years of follow-up. In the combined cohort utilizing baseline covariates, higher liver fat was associated with incident CVD in the age-adjusted and sex-adjusted model (HR: 1.21, 95% CI, 1.15–1.28) and multivariable model (HR: 1.07, 1.00–1.14), but not when further adjusted for BMI. When we considered dichotomous hepatic steatosis, results were overall similar. At least mild hepatic steatosis was associated with incident CVD in both the age-adjusted and sex-adjusted model (HR: 1.55, 1.34–1.80) and the multivariable model (HR: 1.18, 1.01–1.38) using baseline covariates, but results were attenuated and no longer significant when we additionally adjusted for BMI. (Table 3). When utilizing time-varying covariates instead of baseline covariates, liver fat and at least mild hepatic steatosis were associated with incident CVD in the age-adjusted and sex-adjusted model, but not in the multivariable models.
TABLE 3.
At least mild hepatic steatosis and incident cardiovascular disease, cardiovascular disease-related mortality, all-cause mortality, and incident cancer in the combined cohort
| Baseline covariates | Incident CVD HR (95% CI) |
CVD-related mortality HR (95% CI) |
All-cause mortality HR (95% CI) |
Incident cancer HR (95% CI) |
|---|---|---|---|---|
| Model | ||||
| Age-sex adjusted | 1.55 (1.34–1.80) | 1.94 (1.10–3.41) | 1.36 (1.19–1.57) | 0.88 (0.74–1.05) |
| Multivariable | 1.18 (1.01–1.38) | 1.57 (0.94–2.62) | 1.28 (1.11–1.47) | 0.88 (0.73–1.05) |
| Multivariable + BMI | 1.09 (0.93–1.28) | 1.50 (0.82–2.74) | 1.21 (1.04–1.40) | 0.81 (0.68–0.98) |
| Time-varying covariates | ||||
| Model | ||||
| Age-sex adjusted | 1.56 (1.34–1.81) | 1.93 (1.10–3.37) | 1.34 (1.17–1.54) | 0.89 (0.74–1.06) |
| Multivariable | 1.15 (0.96–1.39) | 1.38 (0.69–2.76) | 1.14 (0.95–1.37) | 0.84 (0.68–1.04) |
| Multivariable + BMI | 1.08 (0.89–1.30) | 1.35 (0.62–2.94) | 1.14 (0.95–1.37) | 0.78 (0.63–0.97) |
Note: At least mild hepatic steatosis is defined as HU <51 or LPR ≤0.33. The HR for incident CVD, all-cause mortality, and incident cancer are from fixed effects models. The HR for CVD-related mortality is from random-effects models. Bold values are statistically significant with p<0.05.
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; HU, Hounsfield unit.
Continuous liver fat and at least mild hepatic steatosis were both associated with CVD-related mortality in the age-adjusted and sex-adjusted model when utilizing baseline covariates (continuous liver fat: HR: 1.17, 1.05–1.31; at least mild hepatic steatosis: HR: 1.94, 1.10–3.41) and time-varying covariates (continuous liver fat: HR: 1.17, 1.04–1.30; at least mild hepatic steatosis: HR: 1.93, 1.10–3.37), but the associations did not persist in the multivariable models (Tables 2, 3). The results for individual cohorts are available in Supplementary Table 2 (http://links.lww.com/HEP/B798).
TABLE 2.
Continuous liver fat (SD) and incident cardiovascular disease, cardiovascular disease-related mortality, all-cause mortality, and incident cancer in the combined cohort
| Baseline covariates | Incident CVD HR (95% CI) |
CVD-related mortality HR (95% CI) |
All-cause mortality HR (95% CI) |
Incident cancer HR (95% CI) |
|---|---|---|---|---|
| Model | ||||
| Age-sex adjusted | 1.21 (1.15–1.28) | 1.17 (1.05–1.31) | 1.12 (1.06–1.19) | 1.01 (0.95–1.08) |
| Multivariable | 1.08 (1.01–1.14) | 1.06 (0.94–1.19) | 1.09 (1.03–1.15) | 1.02 (0.95–1.09) |
| Multivariable + BMI | 1.04 (0.97–1.11) | 1.01 (0.89–1.14) | 1.06 (1.00–1.12) | 0.98 (0.92–1.05) |
| Time-varying covariates | ||||
| Model | ||||
| Age-sex adjusted | 1.21 (1.15–1.29) | 1.17 (1.04–1.30) | 1.11 (1.06–1.18) | 1.04 (0.98–1.11) |
| Multivariable | 1.06 (0.98–1.14) | 0.99 (0.84–1.17) | 1.04 (0.97–1.12) | 1.04 (0.91–1.19) |
| Multivariable + BMI | 1.02 (0.95–1.11) | 0.96 (0.81–1.14) | 1.04 (0.97–1.12) | 1.01 (0.90–1.13) |
Note: Continuous liver fat is reported as SD of mean liver attenuation. The HR are from fixed effects models. The results for each model are presented for both analyses including baseline covariates and time-varying covariates. Bold values are statistically significant with p<0.05.
Abbreviations: BMI, body mass index; CVD, cardiovascular disease.
All-cause mortality
A total of 1457 participants in the combined cohort died over a mean of 12.7 years. When utilizing baseline covariates, continuous liver fat associated with all-cause mortality in the age-adjusted and sex-adjusted model (HR: 1.12, 1.06–1.19], the multivariable model (HR: 1.09, 1.03–1.15), and the multivariable and BMI-adjusted model (HR: 1.06, 1.00–1.13) (Table 2; Figure 1). In addition, at least mild hepatic steatosis was also associated with all-cause mortality in all the 3 models (multivariable + BMI: HR: 1.21, 1.04–1.40) (Table 3). However, when utilizing time-varying covariates, there was a trend toward increased risk of mortality in the multivariable and multivariable + BMI model, but the association was only significant in the age-adjusted and sex-adjusted model [HR: 1.34, 1.17–1.54].
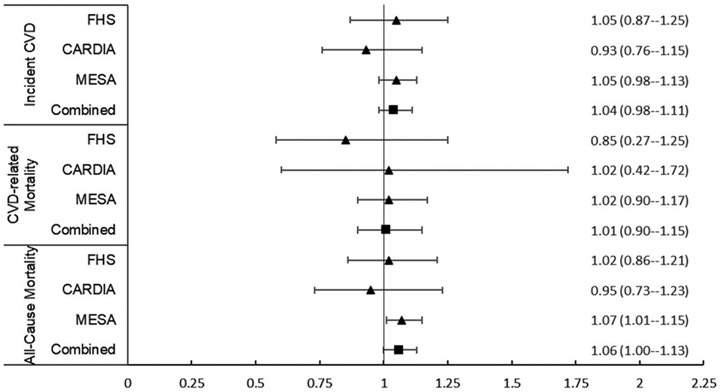
FIGURE 1.
Forest plot (HR, 95% CI) for continuous liver fat and incident cardiovascular disease, cardiovascular disease-related mortality, and all-cause mortality in the individual and combined cohorts in the multivariable and body mass index model. Continuous liver fat is measured per unit increase in SD. Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults Study; CVD, cardiovascular disease; FHS, Framingham Heart Study; MESA, Multi-Ethnic Study of Atherosclerosis.
Incident cancer
A total of 1084 participants developed cancer in the MESA and FHS cohorts. Continuous liver fat and at least mild hepatic steatosis, and moderate-to-severe hepatic steatosis were not associated with incident cancer in the individual cohorts or combined cohorts in the age-adjusted and sex-adjusted models, multivariable models, or when further adjusted for BMI (Tables 2, 3) when using either baseline or time-varying covariates.
Sensitivity analysis with moderate-to-severe hepatic steatosis
Moderate-to-severe hepatic steatosis was associated with incident CVD in the age-adjusted and sex-adjusted model (HR: 1.73, 1.43–2.08) only, and it was not associated with CVD-related mortality in any model. Moderate-to-severe hepatic steatosis was associated with all-cause mortality in the age-adjusted and sex-adjusted and multivariable models (HR: 1.18, 1.01–1.38), but not after including BMI. Finally, moder-ate-to-severe hepatic steatosis was not associated with incident cancer in any model. Full results are available in Supplemental Table 3 (http://links.lww.com/HEP/B798).
DISCUSSION
Principal findings
In our large prospective study of over 10,000 individuals from 3 longitudinal cohorts with 12.7 mean years of follow-up, we observed that associations between CT-defined liver fat and incident CVD were no longer statistically significant if we accounted for BMI or if we took into account time-varying covariates. Furthermore, after considering CVD risk factors, liver fat was not significantly associated with CVD-related mortality. Although the associations between continuous liver fat or at least mild hepatic steatosis and all-cause mortality we present when accounting for baseline CVD risk factors and BMI, when we considered time-varying covariates, the results were no longer statistically significant. Finally, we observed no significant associations between liver fat and incident cancer; however, the cancer event rate was relatively low in our younger-aged cohort. Taken together, in our sample, the associations between liver fat and CVD risk or all-cause mortality can largely be explained by comorbid cardiometabolic disease as liver fat did not significantly contribute to the outcomes after considering other cardiometabolic conditions.
Liver fat and incident CVD
Our results differ from prior studies, which have relied on imaging to define NAFLD. In a large Danish study, the highest quartile of liver fat content on CT scan was associated with increased risk of ischemic heart disease compared with the lowest quartile, but not after adjusting for statin therapy and BMI.[33] In the North American PROMISE trial, CT-derived hepatic steatosis was associated with increased risk of composite all-cause death, hospitalization for unstable angina, or myocardial infarction despite accounting for CV risk factors that included obesity or BMI.[34] Overall, these results are similar to our findings when adjusting for baseline covariates, but not when accounting for change in CV risk factors over time. In addition, although they did show a positive association with their primary outcome, the authors did not evaluate CVD events or CVD-related mortality alone. Participants enrolled in the trial were undergoing noninvasive testing for evaluation of chest pain or other symptoms suggestive of coronary artery disease and had relatively short follow-up, providing potential bias by increasing the number of individuals with NAFLD with underlying coronary artery disease compared with the general population.
Our study results with time-varying analysis are similar to large-scale cohort studies that were limited by the definition of NAFLD (defined by diagnostic codes instead of imaging) or that lacked adjudicated CVD outcomes. In a European study of 17.7 million individuals with a shorter follow-up duration (mean: 2.1–5.5 y), a diagnosis of NAFLD based on diagnostic codes was associated with acute myocardial infarction and stroke when adjusting for age, sex, and smoking, but not after additionally adjusting for systolic blood pressure, diabetes, hypertension, statin use, or total cholesterol levels.[14] In a large midwestern US study, NAFLD based on diagnostic codes was not associated with incident CVD when 3 adjusting for diabetes, hypertension, and hyperlipidemia.[35] Our results suggest that a possible explanation for discordant results in the literature largely stems from how coexisting cardiometabolic disease risk factors are handled in models predicting outcomes. Persons with NAFLD often have a complex interplay between multiple cardiometabolic traits, which overall contribute strongly to their risk for incident CVD, CVD-related mortality, and overall mortality.
Liver fat, CVD-related mortality, and all-cause mortality
Several studies have also evaluated the association between NAFLD, CVD-related mortality, and all-cause mortality with mixed results. In the Third National Health and Nutrition Examination Survey (NHANES III), the presence of ultrasound-defined NAFLD was associated with an increased risk of CVD-related mortality in those aged 60–74, but not those above the age of 74 years.[36] Our study had fewer CVD-related deaths compared with CVD events (333 vs. 1166), reflecting the younger age of the cohort. Our null findings between hepatic steatosis and CVD-related mortality may not be applicable to an older population. Large meta-analyses evaluating NAFLD based on imaging or histology and CVD outcomes have differing results regarding CVD-related mortality. A meta-analysis of 16 observational studies reported that hepatic steatosis was associated with an increased risk of fatal CVD events,[16] but outcomes were not adjudicated, and individuals with prevalent CVD were not excluded from the study, likely increasing the risk of fatal CVD events within the individual study populations. However, another meta-analysis of 14 studies showed that NAFLD was associated with an increased risk of all-cause mortality, but not CVD-related mortality,[17] similar to our findings when only using baseline covariates. The meta-analysis also did not exclude those with prevalent CVD, and did include studies with participants with cirrhosis and potentially higher risk of overall mortality. The discrepancy in outcomes between our multicohort study and meta-analyses in the literature may be due to both the method of ascertaining hepatic steatosis using CT liver attenuation (rather than ultrasound findings), more robust CVD risk factor adjudication in our longitudinal cohorts, and differences in baseline versus time-varying covariates.
Liver fat and incident cancer
Several recent cohort studies have observed an association between NAFLD and extrahepatic malignancy, differing from our study results. A Japanese cohort study showed that there was an increased risk of gastric or colorectal cancer only among individuals with both NAFLD and obesity,[22] but a large US cohort study showed that there was an increased risk of extrahepatic cancers among individuals with NAFLD compared with those without NAFLD.[21] In a further subgroup analysis in the US study, the incident risk of cancer was higher in obese individuals with NAFLD compared with nonobese individuals without NAFLD. However, they did not compare obese to nonobese individuals with NAFLD, and it is not clear whether the higher incidence rate could be in part driven by BMI. Finally, in a Swedish population-based study, NAFLD based on histology was associated with an overall increased risk of extrahepatic cancer, and an individual increased risk of pancreatic cancer, kidney/bladder cancer, and melanoma, but not gastric, colorectal cancer, or other solid organ cancers.[37] Although the authors were able to conduct propensity score matching with metabolic syndrome, the lack of data regarding BMI and the inconsistency with administrative data leads to potential continued confounding, particularly with respect to obesity.
These studies showed different associations for individual types of cancer, which may be due to the different study populations with uncontrolled confounding factors and different methods of ascertainment of NAFLD. The event rate for cancer in our study is too low to corroborate the findings from these prior studies because of the young age of our cohort. However, future data from our cohort may be able to shed light on the association between NAFLD and incident cancer after accounting for BMI.
Implications
The results of our study indicate that even though liver fat is associated with incident CVD when accounting for baseline risk factors such as diabetes, hyperlipidemia, hypertension, smoking, and alcohol use, the association was nonsignificant after the addition of BMI. In addition, this association was attenuated when adjusting for time-varying risk factors. Neither BMI nor NAFLD are incorporated into current CVD risk models,[38] but if BMI were included, the addition of NAFLD would be unlikely to change the risk estimate. Further long-term studies will be necessary to determine whether individuals with NAFLD and certain categories of obesity would benefit from increased surveillance for the development of other cardiometabolic comorbidities and CVD. Finally, our study population included unselected individuals who had not sought medical care and were unaware of their diagnosis of NAFLD before study participation and imaging. Given the association between liver fat and all-cause mortality when adjusting for baseline CVD risk factors, the study highlights the importance of recognizing NAFLD as an important clinical entity for increased risk of death. However, an emphasis should still be placed on controlling other CVD risk factors as they likely play a stronger role in overall outcomes. Future studies should evaluate whether targeted therapy in individuals with NAFLD and adjusting for changes in liver fat over time may improve overall mortality.
Strengths and limitations
This study has several major strengths, including a large multicohort, community-based sample that is geographically and racially/ethnically diverse, representing 10 different cities within the US, increasing generalizability to the US population. The study design for each individual cohort is robust, with multiple physical examinations, laboratory measurements, and interviews to record accurate CVD risk factor information and exclude those who had prevalent CVD from our analysis. Each cohort also had robust event adjudication to more accurately measure CVD, cancer, and mortality events. In addition, one of the major strengths of the study is in the ascertainment of liver fat and hepatic steatosis. Unlike many large cohort studies, we were able to not only diagnose the presence of hepatic steatosis on CT scan, but quantify the degree of hepatic steatosis with both a measurement of continuous liver fat and a subgroup of moderate-to-severe hepatic steatosis based on previously authenticated methods. Ours is one of the first studies to evaluate continuous liver fat in relation to incident CVD and mortality outcomes.
Our study also has several limitations. There is a low number of CVD deaths and incident cancer in our combined cohort compared with other studies, likely reflective of the smaller overall number of participants and the younger age of our cohorts—it is unlikely that NAFLD would have a large effect on CVD-mortality with this age group, and it may be worth evaluating in the cohort at a later time. Although our baseline prevalence of diabetes and BMI is lower than in other NAFLD cohorts, it is similar to prior NHANES data reflecting the prevalence in our population between 2000 and 2010.[39-41] There was heterogeneity among the cohorts in CVD-related mortality, and despite reporting the random-effects model results, the cohorts may not have equal contribution to the combined HR. Although we were also able to evaluate the association between hepatic steatosis and incident cancer, our cancer event rate was low, and we only had data available in the MESA and FHS cohorts without specific cancer diagnoses. Although the CVD risk factors we include are the leading risk factors for all-cause mortality, there are still potential unmeasured confounders that we do not explore, such as other medical comorbidities and socioeconomic status. Although we are able to use liver attenuation in HU for each cohort, the dates of CT measurements and type of scanners used vary, increasing the possibility of differences in image acquisition and liver fat assessment. CT scans are also unable to identify the subgroup of individuals with NASH, analysis of which may show differing results when compared with all-inclusive NAFLD. Finally, although we are able to adjust for CVD risk factors that change over time, we could only adjust for baseline liver fat and hepatic steatosis as an attempt to include limited data available for time-varying liver fat would have resulted in decreased power of the study.
CONCLUSIONS
In this large, US multicohort study, we report that a diagnosis of NAFLD based on CT imaging is associated with increased risk of incident CVD only when accounting for baseline CVD risk factors other than BMI, and that liver fat confers additional risk in all-cause mortality regardless of baseline CVD risk factors including BMI. Although there is still a trend toward increased risk of CVD and all-cause mortality, the associations are no longer significant when adjusting for change in CVD risk factors over time, suggesting that these risk factors play a more clinically relevant role. The differing results when compared with studies that also use similar event adjudication and CT-defined NAFLD highlight the importance of including longer-term data that captures the change in CVD risk factors in future studies. Future studies should evaluate whether modifying cardiometabolic risk factors leads to reduced risk of liver and cardiovascular events in persons with NAFLD.
Supplementary Material
FUNDING INFORMATION
The Framingham Heart Study is supported in part by the National Heart, Lung, and Blood Institute contracts N01-HC-25195, HHSN268201500001, and 75N92019D00031. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268 201800005I, HHSN268201800006I, HHSN268201800 007I, and R01-HL098445 from the National Heart, Lung, and Blood Institute (NHLBI). The Multi-ethnic Study of Atherosclerosis (MESA) is supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020 D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020 D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). Michelle T. Long is supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases K23 DK113252, the Doris Duke Charitable Foundation Grant #2019085, Gilead Sciences Research Scholars Award, the Boston University School of Medicine Department of Medicine Career Investment Award, and the Boston University Clinical Translational Science Institute UL1 TR001430. Heidi S. Ahmed is supported in part by the National Institute of Health T32 DK 720142. Lisa B. VanWagner is supported in part by the National Heart, Lung, and Blood Institute K23 HL136891. Emelia J. Benjamin is supported by R01HL092577; American Heart Association AHA_18SFRN34110082.
Abbreviations:
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults Study
- CVD
cardiovascular disease
- FHS
Framingham Heart Study
- GE
General Electric
- HU
Hounsfield unit
- LPR
liver phantom ratio
- MESA
Multi-ethnic Study of Atherosclerosis.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepjournal.com.
CONFLICTS OF INTEREST
J. Jeffrey Carr received grants from Thera Technologies. Lisa B. VanWagner consults for Noble Insights, Slingshot Insights and Gerson Lehrman Group. He received grants from W.L. Gore and Associates. Yuankai Huo received grants from IBM. Michelle T. Long is employed by and owns stock in Novo Nordisk. The remaining authors have no conflict of interest to declare.
REFERENCES
- 1.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. [DOI] [PubMed] [Google Scholar]
- 3.Harrison SA, Gawrieh S, Roberts K, Lisanti CJ, Schwope RB, Cebe KM, et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol. 2021;75:284–91. [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 5.Corey KE, Kaplan LM. Obesity and liver disease: the epidemic of the twenty-first century. Clin Liver Dis. 2014;18:1–18. [DOI] [PubMed] [Google Scholar]
- 6.Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeb I, Li D, Nasir K, Katz R, Larijani VN, Budoff MJ. Computed tomography scans in the evaluation of fatty liver disease in a population based study: the multi-ethnic study of atherosclerosis. Acad Radiol. 2012;19:811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younossi ZM, Stepanova M, Younossi Y, Golabi P, Mishra A, Rafiq N, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69:564–8. [DOI] [PubMed] [Google Scholar]
- 9.Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021. doi: 10.1016/S0140-6736(20)32511-3 [DOI] [PubMed] [Google Scholar]
- 10.Targher G, Byrne CD. Nonalcoholic fatty liver disease, cardiovascular outcomes, and mortality in patients undergoing a coronary angiogram. Hepatology. 2016;64:684–5. [DOI] [PubMed] [Google Scholar]
- 11.Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66:390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D, Touros A, Kim WR. Nonalcoholic fatty liver disease and metabolic syndrome. Clin Liver Dis. 2018;22:133–40. [DOI] [PubMed] [Google Scholar]
- 13.Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936–44. [DOI] [PubMed] [Google Scholar]
- 14.Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367:l5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellinger JL, Pencina KM, Massaro JM, Hoffmann U, Seshadri S, Fox CS, et al. Hepatic steatosis and cardiovascular disease outcomes: an analysis of the Framingham Heart Study. J Hepatol. 2015;63:470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Zhong GC, Tan HY, Hao FB, Hu JJ. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis. Sci Rep. 2019;9:11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: a systematic review and meta-analysis. Sci Rep. 2016;6:33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156:477–91 e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen AM, Hicks SB, Mara KC, Larson JJ, Therneau TM. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity—a longitudinal cohort study. J Hepatol. 2019;71:1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamaguchi M, Hashimoto Y, Obora A, Kojima T, Fukui M. Non-alcoholic fatty liver disease with obesity as an independent predictor for incident gastric and colorectal cancer: a population-based longitudinal study. BMJ Open Gastroenterol. 2019;6:e000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, et al. The third generation cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–35. [DOI] [PubMed] [Google Scholar]
- 24.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–1. [DOI] [PubMed] [Google Scholar]
- 25.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988; 41:1105–6. [DOI] [PubMed] [Google Scholar]
- 26.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. [DOI] [PubMed] [Google Scholar]
- 27.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanWagner LB, Ning H, Lewis CE, Shay CM, Wilkins J, Carr JJ, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tota-Maharaj R, Blaha MJ, Zeb I, Katz R, Blankstein R, Blumenthal RS, et al. Ethnic and sex differences in fatty liver on cardiac computed tomography: the multi-ethnic study of atherosclerosis. Mayo Clin Proc. 2014;89:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speliotes EK, Massaro JM, Hoffmann U, Foster MC, Sahani DV, Hirschhorn JN, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol. 2008;23:894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeb I, Katz R, Nasir K, Ding J, Rezaeian P, Budoff MJ. Relation of nonalcoholic fatty liver disease to the metabolic syndrome: the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2013;7:311–8. [DOI] [PubMed] [Google Scholar]
- 32.Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–2. [DOI] [PubMed] [Google Scholar]
- 33.Lauridsen BK, Stender S, Kristensen TS, Kofoed KF, Køber L, Nordestgaard BG, et al. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur Heart J. 2018;39: 385–93. [DOI] [PubMed] [Google Scholar]
- 34.Meyersohn NM, Mayrhofer T, Corey KE, Bittner DO, Staziaki PV, Szilveszter B, et al. Association of hepatic steatosis with major adverse cardiovascular events, independent of coronary artery disease. Clin Gastroenterol Hepatol. 2021;19:1480–8 e1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67:1726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golabi P, Paik J, Reddy R, Bugianesi E, Trimble G, Younossi ZM. Prevalence and long-term outcomes of non-alcoholic fatty liver disease among elderly individuals from the United States. BMC Gastroenterol. 2019;19:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon TG, Roelstraete B, Sharma R, Khalili H, Hagström H, Ludvigsson JF. Cancer risk in patients with biopsy-confirmed nonalcoholic fatty liver disease: a population-based cohort study. Hepatology. 2021;74:2410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 39.Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 41.Zhang N, Yang X, Zhu X, Zhao B, Huang T, Ji Q. Type 2 diabetes mellitus unawareness, prevalence, trends and risk factors: National Health and Nutrition Examination Survey (NHANES) 1999–2010. J Int Med Res. 2017;45:594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.