Abstract
The microbiome plays a critical role in the process of conception and the outcomes of pregnancy. Disruptions in microbiome homeostasis in women of reproductive age can lead to various pregnancy complications, which significantly impact maternal and fetal health. Recent studies have associated the microbiome in the female reproductive tract (FRT) with assisted reproductive technology (ART) outcomes, and restoring microbiome balance has been shown to improve fertility in infertile couples. This review provides an overview of the role of the microbiome in female reproductive health, including its implications for pregnancy outcomes and ARTs. Additionally, recent advances in the use of microbial biomarkers as indicators of pregnancy disorders are summarized. A comprehensive understanding of the characteristics of the microbiome before and during pregnancy and its impact on reproductive health will greatly promote maternal and fetal health. Such knowledge can also contribute to the development of ARTs and microbiome-based interventions.
Keywords: Microbiome, Pregnancy, Female reproductive health, Assisted reproductive technology, Microbial biomarker
Introduction
With rapid social and economic development, female reproductive health has received increasing attention. There have been various proactive lifestyle recommendations to promote reproductive health, such as engaging in regular exercise and maintaining a balanced diet that includes high-fiber food [1,2]. Recent studies have indicated that the microbiome also plays a crucial role in successful pregnancy and favorable pregnancy outcomes [3,4]. Microbiome refers to the community of all commensal, symbiotic, and pathogenic microorganisms within a body or other environment, and their habitat [5]. Previous studies have shown that the microbiome plays an essential role in the healthy development of the human body and the onset of various diseases [6–8]. Microbiomes in different parts of the human body exhibit different dynamic patterns throughout different life stages, with pregnancy being one of the most critical life stages [9–12]. Previous studies focusing on mothers and babies during the perinatal period have shown frequent microbial interaction and transmission across various body sites, indicating the importance of microbial homeostasis, particularly in the gut and reproductive tract, for the health of both mothers and infants during this critical time window [9,13]. Emerging studies also suggest that the depletion of certain bacteria in early life may lead to significant health issues in childhood [14–16]. Therefore, investigating the characteristics of the microbiome before and during pregnancy, as well as understanding how the microbial variations impact pregnancy health and delivery outcomes, not only contributes to the development of risk prediction models, but also provides targets for microbiome-based interventions to improve reproductive health.
In modern times, unhealthy lifestyles have remarkably increased the incidence of pregnancy complications. These illnesses during pregnancy have a noticeable impact on healthy reproduction. Over the past few decades, the prevalence of infertility has led to the growth of assisted reproductive technologies (ARTs) [17], which provide hope to many couples facing reproductive difficulties. ART refers to any fertility-related treatment in which eggs or embryos are manipulated, with in vitro fertilization (IVF) being the main type [18]. The IVF process typically involves retrieving eggs and sperm, fertilizing the eggs, culturing and screening the embryos, and finally transferring an appropriate embryo into the uterus [18]. Recently, a lot of studies have associated the microbiome in the female reproductive tract (FRT) with ART outcomes and provided some interesting observations [19–23]. However, due to inconsistent conclusions among different studies, the progress of ARTs remains limited. A comprehensive understanding of the role of the microbiome in the ART process is crucial and urgently needed.
Pregnancy is a complex process during which several critical changes occur in a woman’s body, including alterations in immune response, hormonal balance, microbial pattern, and metabolism, to support or adapt to the growth and development of the fetus in the uterus [9,11,24]. If these dynamic patterns during pregnancy are disrupted, it can lead to a variety of pregnancy complications such as gestational diabetes mellitus (GDM), preeclampsia (PE), and adverse pregnancy outcomes including premature labor, low birth weight, and macrosomia [25–28]. For example, many studies have confirmed a close association between unfavorable maternal health and the perturbation of metabolites originating from disrupted microbiota during pregnancy [29]. Other observations have linked increased microbial diversity in the reproductive tract to preterm birth, highlighting the potential of the microbiome as an indicator of reproductive health [30,31]. These disorders not only have a significant impact on maternal health, but also have a detrimental effect on the fetus and future infant [32]. Using specific microbes as markers can predict the onset of diseases to a large extent and allow for early intervention [33–35]. At the same time, microbiome-targeted therapies [36], such as probiotic intervention and vaginal microbiota transplantation (VMT), show promise for improving reproductive health and pregnancy outcomes [33,37–39]. Probiotics can enhance the microbial environment and may relieve complications, while VMT aims to restore a normal vaginal microbiome using donor samples, much like fecal microbiota transplantation (FMT) for the gut [39]. These interventions highlight the pressing need to better understand microbial balance in the FRT.
It is of great significance to restore the maternal gut and reproductive tract microbiomes through appropriate interventions to improve maternal health and pregnancy outcomes and promote the healthy development of newborns after delivery [39–42]. More importantly, it is crucial to understand the changes in the microbiome before and after conception and their potential associations and influences on maternal and fetal health. This review provides an overview of the impact of gut and reproductive tract microbiota on female reproductive health before and during pregnancy, and summarizes recent advancements in the microbial characteristics in the FRT. In particular, we emphasize the significance of the microbiome in the IVF process and explore its predictive role in pregnancy complications.
Impact of lifestyle on gut microbiome and reproductive health
The microbiome is believed to be involved throughout gestation and exerts persistent influences on both mothers and fetuses [9]. Although most host–microbe interactions between mother and offspring occur during pregnancy and delivery, the prepregnancy microbiome of females may also play a vital role in fertility and gestation maintenance [3,4,43]. A number of studies have indicated that maternal conditions before pregnancy persist into the pregnancy and have a tremendous effect on conception and the health of future children [44–46]. Factors such as stress, drug use, smoking, and alcohol consumption, which are known to affect human health, also affect the microbiome of different body sites [46–48].
The intestine is a delicate ecosystem housing trillions of microbes [7]. These microbes interact with each other and play an important role in maintaining gut homeostasis and normal neurodevelopment [6,49,50]. Dysbiosis of the gut microbiota before and during pregnancy is closely associated with some pregnancy complications and pediatric diseases, such as GDM, asthma, allergies, wheezing, and obesity [51–55]. Among these, obesity is one of the most common metabolic disorders that can disturb the gut microbiome and lead to long-term impacts on both mothers and their offspring [56,57]. Obesity induces excessive accumulation of adipose tissue, which leads to chronic inflammation and disrupts metabolic homeostasis [58,59]. It is widely associated with GDM and other diseases [60]. Some studies have demonstrated that mothers with excessive weight gain have a higher chance of giving birth to obese offspring [55,61]. Other studies have also showed that the prepregnancy body mass index (BMI) of mothers is correlated with the healthy condition of their infants, suggesting that the relationship between gut microbiome and reproductive health may begin before conception [62,63]. Nevertheless, the underlying mechanism between the prepregnancy microbiome and postnatal health needs further explanation.
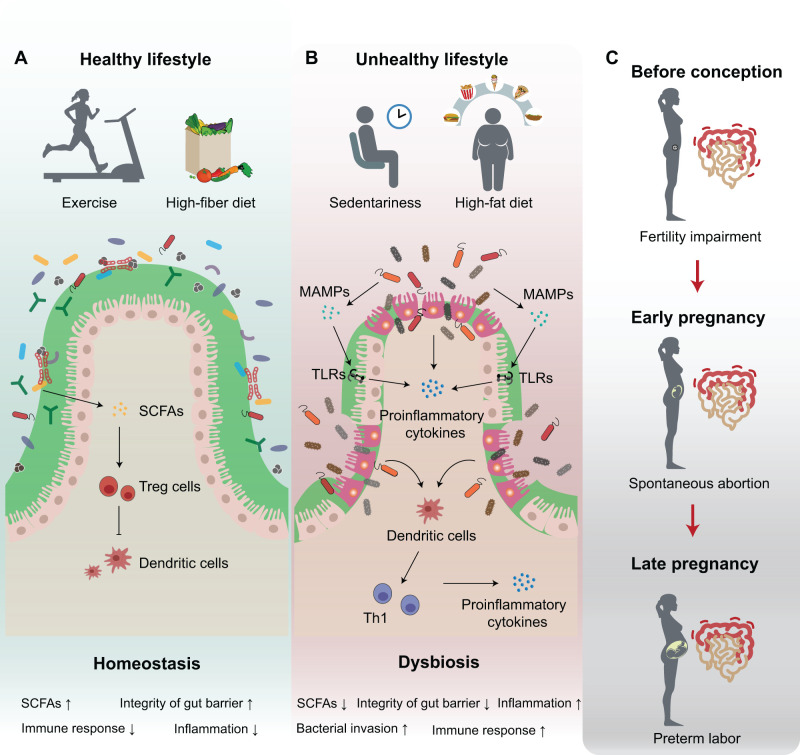
An active lifestyle is recommended for all women, whether currently pregnant or planning for conception [1,64,65]. Numerous studies have illustrated that regular exercise and a balanced diet are powerful contributors to overall health [1,64,66]. The abundance of Akkermansia and some butyrate-producing bacterial taxa, such as Faecalibacterium prausnitzii, Roseburia hominis, and Lachnospiraceae, is increased in women with active lifestyles [67–69]. Such bacteria can produce short-chain fatty acids (SCFAs), which are a common type of bacterial metabolites including acetate, propionate, and butyrate. SCFAs provide more energy to the host and reduce the inflammatory responses in the colonic epithelium [67–69], thereby helping expectant mothers stay in good health (Figure 1A). In contrast, dysbiosis of the maternal gut microbiota enhances the inflammatory responses, increasing the risk of fetal rejection in early pregnancy [70]. Most SCFAs are produced by bacteria from the fermentation of dietary fiber and resistant starch [71]. High fiber can be found in most plant foods such as fruits, vegetables, and grains. A high-fiber diet is believed to increase SCFA levels and improve human health [66]. A recent study involving 120 pairs of GDM women and matched controls from the first trimester to the third trimester indicated that the healthy status of women suffering from GDM was modified with the intake of a high-fiber diet [25]. Parallel changes in the gut microbiome, including Escherichia coli, Fusobacterium mortiferum, Bacteroides massiliensis, and Bifidobacterium dentium, were also observed [25].
Figure 1.
Lifestyle influences gut microbiome and is associated with reproductive healthA. The impact of healthy lifestyle on gut microbiome and immunity. Active lifestyles such as regular exercise and a high-fiber diet can balance the gut microbiome homeostasis, maintain the gut barrier integrity, and increase the production of SCFAs, which reduce the production of proinflammatory cytokines and down-regulate immune responses and thereby help maintain reproductive health. B. The impact of unhealthy lifestyle on the gut microbiome and immunity. Unhealthy lifestyles such as sedentariness and a high-fat diet disrupt the gut microbiome, which further impairs the gut barrier integrity. The evasion of pathogens stimulates immune cells to produce more proinflammatory cytokines, which subsequently activates the immune responses and potentially induces various diseases related to reproductive health. C. Persistent inflammation in the intestine at different gestational stages may induce adverse pregnancy outcomes such as fertility impairment, spontaneous abortion, and preterm labor. MAMP, microbe-associated molecular pattern; SCFA, short-chain fatty acid; Th1, T helper type 1; Treg, regulatory T; TLR, Toll-like receptor.
SCFAs are also one type of the most important bacterial metabolites related to the health of mothers and their offspring [72,73]. A high concentration of SCFAs in obese mother’s gut affects maternal weight gain and glucose metabolism, and is associated with some metabolic disorders such as hypertension and GDM, which may lead to persistent perturbation of the offspring’s microbiome and metabolome [72,74]. Moreover, a high level of SCFAs in the female vagina is associated with infertility, indicating the importance of metabolic balance in the preparation of pregnancy [75]. SCFAs directly or indirectly influence the production of various T lymphocytes, producing a tolerogenic immune environment in infants [76]. One study found that acetic supplementation increased the production of CD4+ T cells and the expression of forkhead box P3 (Foxp3), promoting the formation of regulatory T (Treg) cells in early life [77] (Figure 1A). Other studies have also confirmed that SCFAs regulate the function of dendritic cells and Treg cells, reducing inflammatory responses and the development of asthma in offspring [78–81]. The microbiome and its metabolites are widely involved in human health. Apart from SCFAs, other bacterial metabolites such as bile acids (BAs), bioactive lipids or peptides, advanced glycation end products (AGEs), trimethylamine N-oxide (TMAO), and imidazole propionate (ImP), have been proven to play essential roles in immune homeostasis. They are closely involved in the regulation of most life activities, exerting either positive or negative influences on mothers and fetuses, the disturbance of which often induces inflammation and specific gastrointestinal disorders [82,83]. Such observations underscore the important role of microbiome homeostasis in women under reproductive age.
Since alteration of gut microbiome is associated with long-term changes in immune response, maintaining gut microbiome homeostasis before and during pregnancy is very important to reproductive health. Consequently, to maintain microbiome homeostasis and stay in good reproductive health, an active lifestyle is necessary for every mother-to-be. For example, bad habits such as being sedentary and a high-fat diet, which can disturb gut microbiome homeostasis and cause an increasing risk of gut inflammation, should be avoided [64]. A high-fat diet is linked to gut microbiota dysbiosis and inflammatory responses [84,85]. Gut microbiome alteration may increase inflammation through microbe-associated molecular patterns (MAMPs) [86]. MAMPs are small molecular motifs conserved within a class of microbes that can be recognized by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) in the epithelial cells [86–88]. The activation of TLRs leads to an up-regulation of proinflammatory cytokines [82,87] (Figure 1B). Gut microbiota dysbiosis and inflammation in the colonic epithelium are related to gut leak, which damages the integrity of the intestinal barrier and leads to microbes and bacterial metabolites entering host circulation, inducing infections and diseases [85] (Figure 1B). For example, several studies have indicated that the fertility of women may be impaired when they suffer from inflammatory bowel disease (IBD) [89]. Other studies have also observed a higher risk of pregnancy complications such as spontaneous abortion and preterm labor in women with gut inflammation [90], suggesting an immune interaction between gut and FRT (Figure 1C).
In addition, the microbiota dysbiosis caused by drug abuse before pregnancy also persists for a long time and is very likely to be transmitted to offspring through vertical transmission [53,91]. Recent studies have demonstrated that the inheritance of resistance genes associated with the microbiome, either before or during pregnancy, can lead to antibiotic resistance in offspring and thus increase the risk of asthma and atopic diseases [52,91,92]. However, as there is a great difference in gut microbiome and immune modulation between early pregnancy and late pregnancy, the long-term influences of gut microbiota dysbiosis and the causal relationship between gut microbiome and pregnancy outcomes require further investigation.
Reproductive tract microbiome of women of reproductive age
The microbiome in the FRT plays an important role in the initiation of conception and exerts influences on pregnancy outcomes, gynecological issues, and fetal development [11,22,31,93,94]. Different from the microbiome in the gut, which is influenced by lifestyle and daily diet, the microbiome in the FRT is more closely related to physiological state, hormone secretion, sexual activities, and hygiene practices [95].
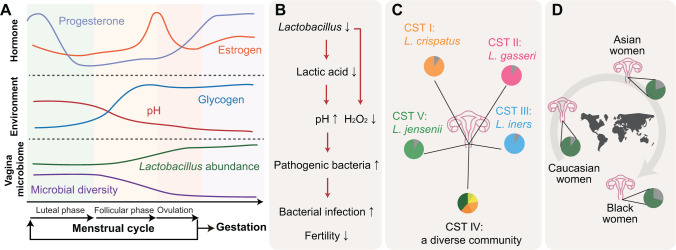
The vagina is the most widely studied site in the FRT. A healthy female vagina is predominantly colonized by Lactobacillus spp. from puberty onwards [31,96]. The normal menstrual cycle in healthy women of reproductive age comprises the follicular phase, ovulation, and the luteal phase, during which the level of hormones and the abundance of microbiome in the vagina fluctuate [38,97]. The cyclical change of hormones and microbiome provides a suitable environment for conception. Estrogen, one of the most important hormones in women’s reproduction, rises during the follicular phase and increases to the highest level at ovulation [95,98] (Figure 2A). Previous studies have indicated that exercise and diet have a significant impact on the levels of estrogen in the body [99–102]. Regular physical activity, especially weight-bearing and resistance exercises, can help increase estrogen levels in females [101,103]. On the other hand, a diet high in processed foods and low in fruits, vegetables, and whole grains can lead to a decrease in estrogen levels [99,100,102]. Maintaining a healthy diet and exercise routine can help regulate estrogen levels and provide numerous health benefits. The rise of estrogen causes glycogen deposits in the vaginal epithelium and thus contributes to the expansion of Lactobacillus spp. in the vagina [104] (Figure 2A). In contrast, progesterone does not reach its peak until luteal phase [38]. The high levels of progesterone help thicken the lining of the uterus in preparation for fertilization [105,106]. If there is no fertilized egg, progesterone levels drop and women enter the next menstrual cycle [107].
Figure 2.
Microbial characteristics in the FRTA. Changes in hormones and microbiome before and during pregnancy. As the follicular phase begins, the levels of estrogen and progesterone rise, though the peaks of the two important hormones occur at different stages. Fluctuations in the hormones lead to changes in the vaginal environment and microbiota: an increase in the abundance of Lactobacillus spp. and a decrease in the vaginal pH and microbial diversity, which gradually makes the vaginal context more favorable for conception. It should be noted that the correct order of a complete menstrual cycle is the follicular phase, ovulation, and the luteal phase. B. The role of Lactobacillus on fertility. The decrease of Lactobacillus is associated with lower lactic acid and H2O2 levels in the vagina, which further leads to the rise of environmental pH and overgrowth of pathogenic bacteria. The expansion of pathogenic bacteria in the vagina often induces BV and infertility. C. Five CSTs in the vagina. Each CST is dominated by different strains. Among them, four CSTs (CST I, CST II, CST III, and CST V) are dominated by Lactobacillus spp., while CST IV consists of a diverse microbial community. D. Ethnic variations of Lactobacillus proportions in the vagina based on limited studies. The vaginal microbiota of most healthy Caucasian women from North America is dominated by Lactobacillus spp. [111,117], while Asian and black women appear to have a lower proportion of Lactobacillus spp. [111,117]. Nevertheless, further confirmation of this observation is still needed. FRT, female reproductive tract; BV, bacterial vaginosis; CST, community state type.
During pregnancy, the proportion of Lactobacillus spp. further increases, reducing the microbial diversity in the vagina (Figure 2A). Lactobacillus spp. produce lactic acid [31], the accumulation of which results in a lower pH, creating an unfavorable environment for the proliferation of pathogenic bacteria and other less beneficial bacterial species [95,108]. The abundance of Lactobacillus spp. is also associated with the levels of hydrogen peroxide (H2O2), which has the capability to inhibit the growth of anaerobic bacteria and enhance the competitiveness of Lactobacillus spp. [31,95] (Figure 2B). The perturbation of the vagina microbiome may disturb the homeostasis of the vaginal microenvironment. Previous studies have demonstrated that pregnant women with a higher microbial diversity in the vagina have an increased risk of miscarriage and preterm birth [109,110]. In contrast, women with a dominance of Lactobacillus spp. experience less infection and lower levels of inflammation [75]. Studies have also implied that infants acquire their initial microbiome from the maternal vagina and babies delivered via the vagina experience fewer diseases such as asthma and allergies [9,13–15], indicating the importance of inheriting a beneficial maternal microbiome.
However, the populations of lactobacilli exhibit a high intraspecies diversity, and not all strains of Lactobacillus in the vagina provide equivalent protective effects on fertilization and pregnancy outcomes [31]. According to recent studies based on large populations, there are five microbial clusters, namely community state types (CSTs), in the healthy female vagina [30,111]. CST I, CST II, CST III, and CST V are dominated by species Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii, respectively [30,31,111] (Figure 2C). Among them, CST I with the dominance of L. crispatus provides the most protective benefits to the host health, while CST III dominated by L. iners offers the least [31]. The other two communities (CST II and CST V) are moderate and are much closer to the former [31]. The different effects among Lactobacillus strains may originate from their metabolic capabilities [31]. For example, among the four bacteria, only L. iners has no capability to produce D-lactic acid and H2O2, which are thought to have the ability to inhibit the overgrowth of anaerobic bacteria and are involved in the modulation of the immune system [112–114]. Nevertheless, the deeper reasons behind the problem may need further exploration. Another reason why CST III (L. iners dominant community) is not favorable in the vaginal microenvironment is that this community is less stable and often transitioned to CST IV [30,31]. CST IV is a community that lacks lactobacilli and harbors a higher ratio of strictly anaerobic genera including Prevotella, Dialister, Atopobium, Gardnerella, and Sneathia [31] (Figure 2C). Such a community is characterized by a much higher vaginal pH (> 4.5) and is often associated with the occurrence of bacterial vaginosis (BV), which has associations with infertility and spontaneous abortion [31,95].
While multiple studies have confirmed the negative effects of CST IV on reproductive health, the relationship is not absolute, as most published studies have primarily focused on Western populations. Limited literature focusing on black women and Asian populations has revealed that the proportion of Lactobacillus spp. in a healthy vagina may vary depending on ethnicity [111,115–121]. For example, cross-population studies conducted by Zhou et al. and Ravel et al. demonstrated that healthy black women from North America harbor the highest proportion of non-Lactobacillus species in the vagina, followed by Asian and Caucasian women [111,117] (Figure 2D). Whether this observation on different populations is universal remains unknown. The underlying mechanism of variation of microbial composition in the female vagina across different ethnic groups and its further influences need more evaluation. Nonetheless, the divergence in the vaginal microbiome community in different races indicates that some gynecological disorders are associated with global microbiota dysbiosis rather than the emergence or absence of a single microbe. There is still a long way to go to thoroughly understand the exact relationship between vaginal microbiome and reproductive health.
Reproductive tract microbiome and ARTs
The decline in fertility in both males and females and the trend of delaying pregnancy to a later age have promoted the development of ARTs [17]. However, the moderate success rate of pregnancy and the unclear mechanisms behind it are hindering the wide use of ARTs [122]. In addition, some studies observe a higher risk of adverse outcomes in ART-conceived babies (and animals) than in naturally conceived ones [123–126], pushing us to update our understanding in this area.
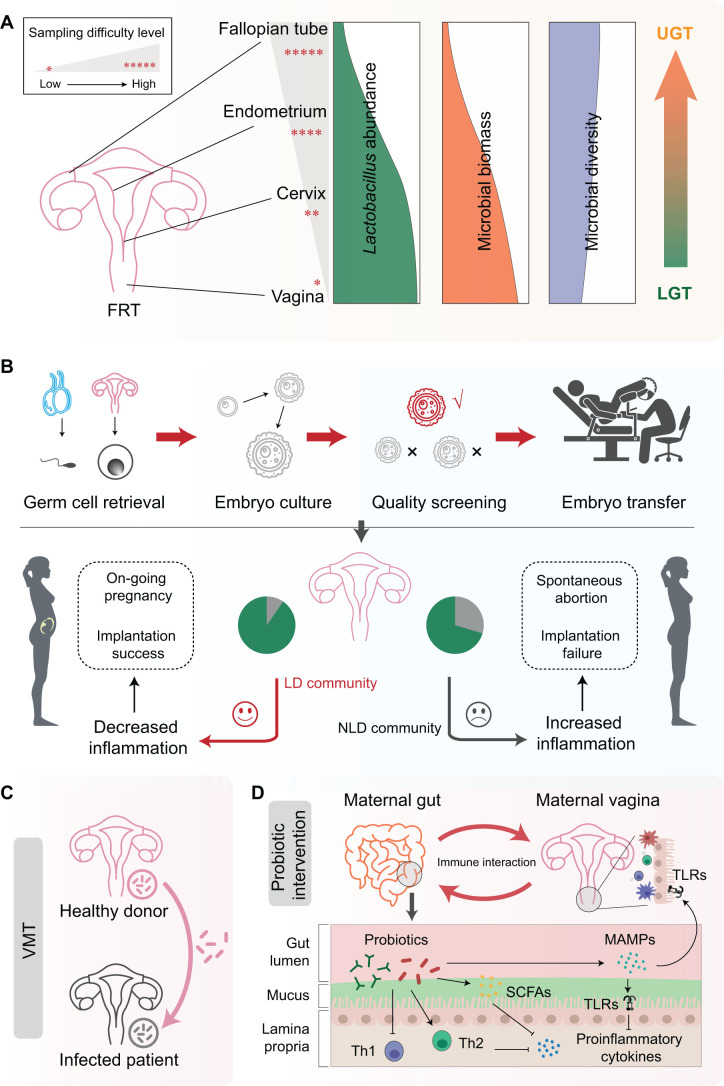
The role of the microbiome in the FRT and its related modulation of the immune system on the success of pregnancy have been highlighted in recent years, promoting advances in exploring the impact of microbiome on ARTs [19,20,127]. The most abundant bacteria in the healthy FRT are Lactobacillus spp.; however, the proportion of Lactobacillus spp. varies between the lower genital tract (LGT; vagina and cervix) and the upper genital tract (UGT; uterus, fallopian tubes, and ovaries) [93,98,128]. For example, during a normal pregnancy, there are about 99% of bacteria belonging to Lactobacillus in the vagina, but the proportion of such lactic acid-producing bacteria is much lower in the cervix, endometrium, and fallopian tubes [93,128] (Figure 3A). Studies have also indicated that the microbial biomass in the UGT largely decreases, but the microbial diversity slightly increases compared to that in the LGT [20,93,98,128] (Figure 3A). Although different locations of the FRT have distinct microbial compositions, the protective effect of Lactobacillus spp. on ART outcomes seems comparable. Previous studies have linked the vaginal microbiome to ART outcomes [129–137]. Vagina microbiota dysbiosis, which is mainly characterized by a shift in microbial community from other CSTs to CST IV, can induce BV [20,31]. In women receiving ART, BV is associated with implantation failure and spontaneous abortion [138]. In contrast, women with a high abundance of Lactobacillus spp. in the vagina are more likely to become pregnant during ART [19].
Figure 3.
Microbiome in ARTs and interventionsA. Difference of microbiomes in FRT. The proportion of Lactobacillus spp. and microbial biomass sharply decrease from LGT to UGT, while the alpha diversity of microbiota and sampling difficulty increase. B. General flow of IVF and the impact of different microbial communities on ART outcomes. The IVF process typically involves retrieving eggs and sperm, fertilizing the eggs, culturing and screening the embryos, and finally transferring an appropriate embryo into the maternal uterus. Different microbial communities are associated with different outcomes. Specifically, an LD community is considered to be related to decreased inflammation and more favorable ART outcomes. C. Restoration of vagina microbiome using VMT. VMT is a novel treatment strategy aiming at modifying vaginal microbiota composition and treating vaginal dysbiosis and related conditions. It involves transferring vaginal fluid from a healthy donor to a recipient to reestablish a normal vaginal microbiome. D. Probiotic intervention and immune interaction between the gut and vagina. Probiotics, especially lactic acid-producing bacteria, can positively modulate immune responses in the gut and vagina. This includes stimulating Th2 cells and suppressing Th1 cells, altering cytokine profiles, and modulating tolerance to commensal bacteria, which helps create an optimal immune environment in the vagina that supports healthy microbiota. ART, assisted reproductive technology; IVF, in vitro fertilization; LD, Lactobacillus-dominated; LGT, lower genital tract; NLD, non-Lactobacillus-dominated; UGT, upper genital tract; Th2, T helper type 2; VMT, vagina microbiota transplantation.
Like in the vagina, the most prevalent colonizer in the cervix is Lactobacillus spp. (97%–99%). Apart from this, the cervix also harbors a small proportion of non-Lactobacillus bacteria [128], such as Gardnerella, Streptococcus, Prevotella, and Pseudomonas, while the total biomass in the cervix is much lower than that in the vagina [139,140] (Figure 3A). Different from the vagina and the cervix, the UGT, such as the uterine cavity and fallopian tubes, contains a much lower biomass of microbes [20,98,141] (Figure 3A). In addition, the more invasive and complicated sampling process increases the risk of contamination, posing great challenges to exploring the role of the microbiome in such locations [38]. Despite its controversy, some studies have examined microbes in the UGT. For example, the endometrial microbiota is thought to consist of 30%–70% Lactobacillus and other bacteria such as Gardnerella, Bifidobacterium, Flavobacterium, Pseudomonas, Streptococcus, Prevotella, and Atopobium, which vary dependent on the populations [128,134,142,143]. Some studies have indicated that the cervical and endometrial microbiomes might be involved in ART outcomes, influencing the implantation of embryo and fetal development [20,21].
Similar to that in the vagina, Lactobacillus spp. provide a protective effect on the cervix and endometrium as shown in most studies [22,23,137,142–152]. Decreased Lactobacillus abundance and increased microbial diversity in the cervical microbiota are positively associated with adverse ART outcomes [22,23,137,143–150]. The increase of non-Lactobacillus bacteria such as Gardnerella may be associated with human papillomavirus (HPV) risk in women, inducing inflammation and cervical cancer [151]. Studies including hundreds of women undergoing ART have also demonstrated that compared with Lactobacillus-dominant (LD) microbiota, women with non-Lactobacillus-dominant (NLD) communities in endometrium exhibit lower rates of implantation, pregnancy, ongoing pregnancy, and live birth [22,146]. Increased endometrial blooms of non-Lactobacillus bacteria, such as Gardnerella, Streptococcus, Staphylococcus, and Enterobacteriaceae, are associated with chronic endometritis and endometriosis, which increases the risk of infertility and implantation failure [142,143,152].
Microbes in the fallopian tubes or ovaries exhibit much lower Lactobacillus abundance and much higher inter-individual variability, and their relationship to ART outcomes is controversial [20,93] (Figure 3A). For example, some studies claimed that extremely low or even no Lactobacillus spp. existed in the fallopian tubes or ovaries [128,153], while other studies detected an LD community and associated the presence of Propionibacterium and Actinomyces in such locations with adverse reproductive outcomes [154,155]. However, due to the lack of sufficient evidence, further studies should be conducted to investigate the causal relationship between ART outcomes and the microbiome in the fallopian tubes or ovaries.
Pregnancy is a very complicated process, during which women undergo great changes in hormones, microbiome, metabolites, and immune responses [9,20]. Therefore, to promote the success of IVF, many factors need to be considered. The success of conception is believed to be associated with the development of tolerance by the maternal immune system towards the fetus [156]. The beginning of pregnancy is accompanied by great changes in both innate immunity and adaptive immunity [127,157,158]. Changes in immune responses are synchronized with corresponding hormonal fluctuations and microbiome alterations, accepting or rejecting the implantation of the embryo during ART [157] (Figure 3B). For example, during normal pregnancy, the levels of estrogen and progesterone increase, which leads to further reduction of the production of proinflammatory mediators such as nitric oxide (NO), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) [157]. The increase of progesterone forms an LD community in FRT, which not only increases the production of lactic acid, but also down-regulates the expression of TLR4, suppressing inflammatory responses in cervicovaginal epithelial cells [75,156]. The LD community in FRT at early pregnancy can be a beneficial factor in preventing fetal rejection during ART [20,21]. In contrast, the NLD community is frequently associated with extensive inflammatory responses, leading to embryo transfer failure in many ART cases [20,21]. Despite such importance of microbiome in the FRT, the ART outcomes can be associated with extensive factors beyond microbiota, such as embryo types (frozen or fresh) and quality, maternal age, hormonal level, and standardized approach [95]. Further studies are needed to enhance our current understanding of the underlying immune mechanism behind the associations between the microbiome and reproductive outcomes in ARTs.
Numerous diseases have been associated with the microbiome [35]. Considering the vital role of the FRT microbiome in reproductive health, some studies have tried to restore vaginal microbiota to alleviate the impact of gynecological disorders on reproduction [37,39–42,159–162]. One solution is microbiota transplantation. Microbiota transplantation is a strategy to transfer microbiota from a healthy donor to the patient to change the recipient’s microbial composition and confer a health benefit. FMT has been used to treat many diseases such as obesity, recurrent Clostridium difficile infection, and IBD, achieving favorable outcomes [163,164]. Recently, several studies have proposed using VMT, which is similar to FMT, to reconstruct the vaginal microbial community [39,42,162] (Figure 3C). A full long-term remission has been reported in some women with FRT infection, indicating that the restoration of vaginal microenvironment is beneficial to the reproductive health.
Apart from VMT, some studies have suggested the existence of microbiome–gut–vagina axis, leading to the intervention using probiotics to rehabilitate gut microbiota homeostasis and thereby improve reproductive health [37,40,41,159–161]. Strains in most probiotic supplements such as Lactobacillus spp. are involved in balancing SCFA levels and regulating the balance of T helper type 1 (Th1) and Th2 cells [165–168] (Figure 3D). The elevated production of SCFAs and decreased Th1/Th2 cell ratio suppress the production of proinflammatory cytokines and enhance the integrity of the uterine barrier, further reducing the risk of many reproductive disorders, including infertility, BV, endometrial diseases, polycystic ovary syndrome (PCOS), GDM, PE, and preterm labor both in IVF and natural conception [41,161,166,169,170]. Probiotic-derived MAMPs also have the ability to modulate the expression of TLRs and alleviate inflammatory responses in epithelial cells [159,160,171,172] (Figure 3D). Intervention with probiotics in females has been proven to restore the microbiome in both the gut and FRT, improving the health of mothers and offspring [41,173]. Nevertheless, there still are some failure cases in probiotic intervention, suggesting the urgent need to evaluate the safety of probiotics for pregnant women and to explore the molecular mechanism underlying microbiome in reproductive health [40,174].
Microbial biomarkers and pregnant complications
A number of studies have highlighted the importance of microbiome in the health of pregnant women and their unborn children [9,11,55,175,176]. Previous investigations have demonstrated the changes in the microbiome and immune responses at different body sites during normal pregnancy [9,177]. In early pregnancy, commencing from conception to the end of the 13th week of pregnancy, the microbiome and immunity in both the gut and vagina are similar to that in healthy unpregnant women [9,178]. While in the third trimester, which ranges from the 27th week to delivery, the microbial profiles and immune responses resemble those of women suffering from obesity [177]. Studies indicated that the increase of Enterobacteriaceae, Streptococcus, and Lactobacillus, as well as high levels of insulin insensitivity and blood glucose, helps mothers harvest more energy, thus supporting the growth of fetuses [172,177]. Nevertheless, an unhealthy lifestyle during pregnancy disturbs the maternal microbiome, which is associated with poor maternal conditions and leads to the occurrence of pregnancy complications, further influencing the development of infants [13,25,26,175].
With current knowledge and case-control studies, bacterial biomarkers can be identified to promote the prevention and early diagnosis of many pregnancy complications. Previous studies have associated the microbiomes in the female gut and reproductive tract with some obstetric and gynecological diseases, such as recurrent miscarriage (RM) [179], repeat implantation failure (RIF) [22,134,146,148,180], PCOS [29,181–192], GDM [25,175,193–199], gestational hypertension [200], PE [27,201–209], preterm labor, and preterm premature rupture of membrane (PPROM) [26,210–214]. While some studies reported consistent observations for certain diseases, other studies reached controversial conclusions. For example, GDM is one of the most common metabolic syndromes during the second trimester, which is often associated with dysbiosis of the gut microbiota and metabolic alteration [175,215]. Crusell et al. demonstrated that mothers with GDM exhibited a higher abundance of Faecalibacterium and Anaerotruncus and a decreased abundance of Clostridium and Veillonella in the gut [193]. In contrast, Cortez et al. indicated that the gut microbial differences were insignificant between GDM and healthy pregnant women [194]. Differences in ethnicity and environments can be one of the reasons that result in such controversial observations, while further associations remain explored. A prospective study conducted by Sun et al. demonstrated significant differences in the gut microbiome, including Ruminococcus bromii, Alistipes putredinis, and Bacteroides ovatus, between GDM women and their healthy controls [25]. Furthermore, they indicated that the microbial characteristics at early pregnancy can be used to predict the occurrence of GDM in the future [25]. Nevertheless, regarding preterm labor, a more uniform relationship is observed. Most studies have confirmed the protective effect of L. crispatus in the vagina, while L. iners is one of the most common taxa associated with low gestational age [26,210–214].
Previous studies have indicated that persistent infections within the vagina or uterine cavity might increase the risk of pregnancy loss and lead to unfavorable outcomes [216,217]. In addition, communication of microbes between the vagina and uterine cavity is associated with reproductive health. A recent study has demonstrated that some bacteria in the vagina, such as Clostridium perfringens and Prevotella bivia, may ascend to the uterine cavity and induce inflammatory responses [94]. In contrast, other microbes such as Lactobacillus murinus can provide a protective effect during microbial translocation between the vagina and the uterus [94]. Such explicit bacterial biomarkers could largely reduce adverse outcomes during pregnancy in clinical settings. Recent studies have also implied that the microbiomes at different body sites might experience disturbance when mothers are under unfavorable conditions, highlighting the close relationship between the microbiome and reproductive health. Wang et al. showed that significant perturbance of microbiomes in the maternal gut, vagina, and oral cavity was observed in GDM women, and the microbial interactions of key bacteria with others were closely associated with maternal health [175]. In addition, concordance of microbial dysbiosis was also found between mothers and their future babies [175], indicating the predictability of microbiome during pregnancy on the health of mothers and infants. Pregnant women with periodontal infections were also found to have a higher risk of hypertension or preterm labor during pregnancy [200,218,219]. Detection of a high proportion of pathogens, such as Porphyromonas gingivalis, in both subgingival plaque and genital tract suggested that bacteria may enter maternal circulation and further influence maternal health and fetal development [200,218].
The human body is a balanced ecosystem, and alterations in the microbiome are associated with various changes in metabolites and immune responses [11,50], making it a mirror of human health and predicting future health for both mothers and offspring. However, the use of microbial biomarkers to predict and prevent pregnancy complications has some limitations. Sampling the reproductive tract is considerably more challenging compared with other sites such as the oral cavity and stool, which introduces risks of contamination. In addition, various confounding factors, including hormone changes and diet influences during pregnancy, may also introduce bias into taxonomic profiling. Moreover, it is important to note that most previous studies on microbial biomarkers were observational, establishing associations rather than causation. Consequently, caution must be taken to avoid unintended outcomes when using microbial biomarkers. Confirming the causal relationships between the microbiome and various diseases is still necessary before adopting microbiome-based therapies for pregnancy complications and childhood disorders. To achieve this, the underlying mechanisms of specific probiotic strains and microbial communities should be further explored in animal models. Large clinical cohorts focusing on major diseases are also needed to establish causal relationships in humans. In summary, although microbiome research presents promising possibilities for understanding, diagnosing, and treating disorders of the FRT, cautious interpretation and understanding causal relationships are important to meaningful clinical translation. Robust basic, translational, and clinical research will be essential to fully realize the potential benefits of microbiome-based therapies for maternal and infant health.
Conclusion and perspectives
This review summarizes the long-term impacts of various lifestyles on the microbiome and reproductive health. It highlights the association between healthy habits and the microbiome before and during pregnancy and emphasizes the important role of the FRT microbiome in predicting pregnancy outcomes. Further understanding of the microbiome during pregnancy will greatly advance biological and medical research, increase the success rate of ARTs, and reduce the risk of adverse pregnancy outcomes.
CRediT author statement
Liwen Xiao: Writing – original draft, Visualization. Zhenqiang Zuo: Writing – review & editing, Visualization. Fangqing Zhao: Conceptualization, Supervision, Writing – review & editing. All authors have read and approved the final manuscript.
Competing interests
The authors have declared no competing interests.
Acknowledgments
This work was supported by grants from the National Key R&D Program of China (Grant Nos. 2022YFA1303900, 2021YFA1301000, and 2022YFC2704702), the National Natural Science Foundation of China (Grant Nos. 32001082 and 32025009), and the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDB38020300).
Contributor Information
Liwen Xiao, CAS Key Laboratory of Systems Biology, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou 310024, China; Beijing Institutes of Life Science/Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China.
Zhenqiang Zuo, Beijing Institutes of Life Science/Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China.
Fangqing Zhao, CAS Key Laboratory of Systems Biology, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou 310024, China; Beijing Institutes of Life Science/Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China.
ORCID
0000-0002-4544-2713 (Liwen Xiao)
0000-0003-0496-2437 (Zhenqiang Zuo)
0000-0002-6216-1235 (Fangqing Zhao)
References
- [1].Ferrari N, Joisten C.. Impact of physical activity on course and outcome of pregnancy from pre- to postnatal. Eur J Clin Nutr 2021;75:1698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sharma R, Biedenharn KR, Fedor JM, Agarwal A.. Lifestyle factors and reproductive health taking control of your fertility. Reprod Biol Endocrinol 2013;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hong X, Zhao J, Yin J, Zhao F, Wang W, Ding X, et al. The association between the pre-pregnancy vaginal microbiome and time-to-pregnancy: a Chinese pregnancy-planning cohort study. BMC Med 2022;20:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chadchan SB, Singh V, Kommagani R.. Female reproductive dysfunctions and the gut microbiota. J Mol Endocrinol 2022;69:R81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Berg G, Rybakova D, Fischer D, Cernava T, Verges MC, Charles T, et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome 2020;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shreiner AB, Kao JY, Young VB.. The gut microbiome in health and in disease. Curr Opin Gastroenterol 2015;31:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cani PD. Human gut microbiome: hopes, threats and promises. Gut 2018;67:1716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xiao L, Zhao F.. Microbial transmission, colonisation and succession: from pregnancy to infancy. Gut 2023;72:772–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Martino C, Dilmore AH, Burcham ZM, Metcalf JL, Jeste D, Knight R.. Microbiota succession throughout life from the cradle to the grave. Nat Rev Microbiol 2022;20:707–20. [DOI] [PubMed] [Google Scholar]
- [11].Yang J, Hou L, Wang J, Xiao L, Zhang J, Yin N, et al. Unfavourable intrauterine environment contributes to abnormal gut microbiome and metabolome in twins. Gut 2022;71:2451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang J, Jia Z, Zhang B, Peng L, Zhao F.. Tracing the accumulation of in vivo human oral microbiota elucidates microbial community dynamics at the gateway to the GI tract. Gut 2020;69:1355–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bolte EE, Moorshead D, Aagaard KM.. Maternal and early life exposures and their potential to influence development of the microbiome. Genome Med 2022;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Enav H, Backhed F, Ley RE.. The developing infant gut microbiome: a strain-level view. Cell Host Microbe 2022;30:627–38. [DOI] [PubMed] [Google Scholar]
- [15].Xiao L, Wang J, Zheng J, Li X, Zhao F.. Deterministic transition of enterotypes shapes the infant gut microbiome at an early age. Genome Biol 2021;22:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brodin P. Immune-microbe interactions early in life: a determinant of health and disease long term. 2022;376:945–50. [DOI] [PubMed] [Google Scholar]
- [17].Skakkebaek NE, Lindahl-Jacobsen R, Levine H, Andersson AM, Jorgensen N, Main KM, et al. Environmental factors in declining human fertility. Nat Rev Endocrinol 2022;18:139–57. [DOI] [PubMed] [Google Scholar]
- [18].Jain M, Singh M.. Assisted Reproductive Technology (ART) Techniques. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- [19].Diaz-Martinez MDC, Bernabeu A, Lledo B, Carratala-Munuera C, Quesada JA, Lozano FM, et al. Impact of the vaginal and endometrial microbiome pattern on assisted reproduction outcomes. J Clin Med 2021;10:4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tsonis O, Gkrozou F, Paschopoulos M.. Microbiome affecting reproductive outcome in ARTs. J Gynecol Obstet Hum Reprod 2021;50:102036. [DOI] [PubMed] [Google Scholar]
- [21].Dube R, Kar SS.. Genital microbiota and outcome of assisted reproductive treatment—a systematic review. Life (Basel) 2022;12:1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Moreno I, Garcia-Grau I, Perez-Villaroya D, Gonzalez-Monfort M, Bahceci M, Barrionuevo MJ, et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Villani A, Fontana A, Barone S, de Stefani S, Primiterra M, Copetti M, et al. Identifying predictive bacterial markers from cervical swab microbiota on pregnancy outcome in woman undergoing assisted reproductive technologies. J Clin Med 2022;11:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhou T, Xiao L, Zuo Z, Zhao F.. MAMI: a comprehensive database of mother–infant microbiome and probiotic resources. Nucleic Acids Res 2024;52:D738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sun Z, Pan XF, Li X, Jiang L, Hu P, Wang Y, et al. The gut microbiome dynamically associates with host glucose metabolism throughout pregnancy: longitudinal findings from a matched case-control study of gestational diabetes mellitus. Adv Sci (Weinh) 2023;10:e2205289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Elovitz MA, Gajer P, Riis V, Brown AG, Humphrys MS, Holm JB, et al. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat Commun 2019;10:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang J, Shi ZH, Yang J, Wei Y, Wang XY, Zhao YY.. Gut microbiota dysbiosis in preeclampsia patients in the second and third trimesters. Chin Med J (Engl) 2020;133:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gorczyca K, Obuchowska A, Kimber-Trojnar Z, Wierzchowska-Opoka M, Leszczynska-Gorzelak B.. Changes in the gut microbiome and pathologies in pregnancy. Int J Environ Res Public Health 2022;19:9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med 2019;25:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 2015;112:11060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].France M, Alizadeh M, Brown S, Ma B, Ravel J.. Towards a deeper understanding of the vaginal microbiota. Nat Microbiol 2022;7:367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Younes JA, Lievens E, Hummelen R, van der Westen R, Reid G, Petrova MI.. Women and their microbes: the unexpected friendship. Trends Microbiol 2018;26:16–32. [DOI] [PubMed] [Google Scholar]
- [33].Gao XS, Laven J, Louwers Y, Budding A, Schoenmakers S.. Microbiome as a predictor of implantation. Curr Opin Obstet Gynecol 2022;34:122–32. [DOI] [PubMed] [Google Scholar]
- [34].Sorbara MT, Pamer EG.. Microbiome-based therapeutics. Nat Rev Microbiol 2022;20:365–80. [DOI] [PubMed] [Google Scholar]
- [35].Xiao L, Zhang F, Zhao F.. Large-scale microbiome data integration enables robust biomarker identification. Nat Comput Sci 2022;2:307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zuo Z, Zhao F.. Gut microbiota-targeted interventions: from conventional approaches to genetic engineering. Sci Bull (Beijing) 2023;68:1231–4. [DOI] [PubMed] [Google Scholar]
- [37].Corbett GA, Crosby DA, McAuliffe FM.. Probiotic therapy in couples with infertility: a systematic review. Eur J Obstet Gynecol Reprod Biol 2021;256:95–100. [DOI] [PubMed] [Google Scholar]
- [38].Garcia-Velasco JA, Menabrito M, Catalan IB.. What fertility specialists should know about the vaginal microbiome: a review. Reprod Biomed Online 2017;35:103–12. [DOI] [PubMed] [Google Scholar]
- [39].Lev-Sagie A, Goldman-Wohl D, Cohen Y, Dori-Bachash M, Leshem A, Mor U, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med 2019;25:1500–4. [DOI] [PubMed] [Google Scholar]
- [40].Jarde A, Lewis-Mikhael AM, Moayyedi P, Stearns JC, Collins SM, Beyene J, et al. Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2018;18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Feng T, Liu Y.. Microorganisms in the reproductive system and probiotic's regulatory effects on reproductive health. Comput Struct Biotechnol J 2022;20:1541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Junca H, Pieper DH, Medina E.. The emerging potential of microbiome transplantation on human health interventions. Comput Struct Biotechnol J 2022;20:615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Salliss ME, Farland LV, Mahnert ND, Herbst-Kralovetz MM.. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update 2021;28:92–131. [DOI] [PubMed] [Google Scholar]
- [44].Triunfo S, Lanzone A.. Impact of maternal under nutrition on obstetric outcomes. J Endocrinol Invest 2015;38:31–8. [DOI] [PubMed] [Google Scholar]
- [45].Hawthorne G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25:77–90. [DOI] [PubMed] [Google Scholar]
- [46].Vizzini L, Popovic M, Zugna D, Vitiello B, Trevisan M, Pizzi C, et al. Maternal anxiety, depression and sleep disorders before and during pregnancy, and preschool ADHD symptoms in the NINFEA birth cohort study. Epidemiol Psychiatr Sci 2019;28:521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Capurso G, Lahner E.. The interaction between smoking, alcohol and the gut microbiome. Best Pract Res Clin Gastroenterol 2017;31:579–88. [DOI] [PubMed] [Google Scholar]
- [48].Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF.. The microbiome: stress, health and disease. Mamm Genome 2014;25:49–74. [DOI] [PubMed] [Google Scholar]
- [49].Yu Y, Zhao F.. Microbiota-gut-brain axis in autism spectrum disorder. J Genet Genomics 2021;48:755–62. [DOI] [PubMed] [Google Scholar]
- [50].Yu Y, Zhang B, Ji P, Zuo Z, Huang Y, Wang N, et al. Changes to gut amino acid transporters and microbiome associated with increased E/I ratio in Chd8+/− mouse model of ASD-like behavior. Nat Commun 2022;13:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wong WSW, Sabu P, Deopujari V, Levy S, Shah AA, Clemency N, et al. Prenatal and peripartum exposure to antibiotics and cesarean section delivery are associated with differences in diversity and composition of the infant meconium microbiome. Microorganisms 2020;8:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Patangia DV, Ryan CA, Dempsey E, Stanton C, Ross RP.. Vertical transfer of antibiotics and antibiotic resistant strains across the mother/baby axis. Trends Microbiol 2022;30:47–56. [DOI] [PubMed] [Google Scholar]
- [53].LZ S, Tarab M, SR A, DJ K, BZ A.. Essential pre-pregnancy and pregnancy interventions for improved maternal, newborn and child health. Reprod Health 2014;11:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Turjeman S, Collado MC, Koren O.. The gut microbiome in pregnancy and pregnancy complications. Curr Opin Endocr Metab Res 2021;18:133–8. [Google Scholar]
- [55].Gohir W, Ratcliffe EM, Sloboda DM.. Of the bugs that shape us: maternal obesity, the gut microbiome, and long-term disease risk. Pediatr Res 2015;77:196–204. [DOI] [PubMed] [Google Scholar]
- [56].Guzzardi MA, Ederveen THA, Rizzo F, Weisz A, Collado MC, Muratori F, et al. Maternal pre-pregnancy overweight and neonatal gut bacterial colonization are associated with cognitive development and gut microbiota composition in pre-school-age offspring. Brain Behav Immun 2022;100:311–20. [DOI] [PubMed] [Google Scholar]
- [57].Yogev Y, Visser GH.. Obesity, gestational diabetes and pregnancy outcome. Semin Fetal Neonatal Med 2009;14:77–84. [DOI] [PubMed] [Google Scholar]
- [58].Monteiro R, Azevedo I.. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm 2010;2010:289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Li Z, Zhang B, Wang N, Zuo Z, Wei H, Zhao F.. A novel peptide protects against diet-induced obesity by suppressing appetite and modulating the gut microbiota. Gut 2022;72:686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007;30:2070–6. [DOI] [PubMed] [Google Scholar]
- [61].Singh S, Karagas MR, Mueller NT.. Charting the maternal and infant microbiome: what is the role of diabetes and obesity in pregnancy? Curr Diab Rep 2017;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Singh SB, Madan J, Coker M, Hoen A, Baker ER, Karagas MR, et al. Does birth mode modify associations of maternal pre-pregnancy BMI and gestational weight gain with the infant gut microbiome? Int J Obes (Lond) 2020;44:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Josefson JL, Hoffmann JA, Metzger BE.. Excessive weight gain in women with a normal pre-pregnancy BMI is associated with increased neonatal adiposity. Pediatr Obes 2013;8:e33-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Meander L, Lindqvist M, Mogren I, Sandlund J, West CE, Domellof M.. Physical activity and sedentary time during pregnancy and associations with maternal and fetal health outcomes: an epidemiological study. BMC Pregnancy Childbirth 2021;21:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Schoenaker DA, Soedamah-Muthu SS, Callaway LK, Mishra GD.. Pre-pregnancy dietary patterns and risk of gestational diabetes mellitus: results from an Australian population-based prospective cohort study. Diabetologia 2015;58:2726–35. [DOI] [PubMed] [Google Scholar]
- [66].Pretorius RA, Palmer DJ.. High-fiber diet during pregnancy characterized by more fruit and vegetable consumption. Nutrients 2020;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bressa C, Bailen-Andrino M, Perez-Santiago J, Gonzalez-Soltero R, Perez M, Montalvo-Lominchar MG, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One 2017;12:e0171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Munukka E, Ahtiainen JP, Puigbo P, Jalkanen S, Pahkala K, Keskitalo A, et al. Six-week endurance exercise alters gut metagenome that is not reflected in systemic metabolism in over-weight women. Front Microbiol 2018;9:2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Clauss M, Gerard P, Mosca A, Leclerc M.. Interplay between exercise and gut microbiome in the context of human health and performance. Front Nutr 2021;8:637010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Warning JC, McCracken SA, Morris JM.. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction 2011;141:715–24. [DOI] [PubMed] [Google Scholar]
- [71].Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F.. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016;165:1332–45. [DOI] [PubMed] [Google Scholar]
- [72].Zietek M, Celewicz Z, Szczuko M.. Short-chain fatty acids, maternal microbiota and metabolism in pregnancy. Nutrients 2021;13:1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Nyangahu DD, Jaspan HB.. Influence of maternal microbiota during pregnancy on infant immunity. Clin Exp Immunol 2019;198:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Priyadarshini M, Thomas A, Reisetter AC, Scholtens DM, Wolever TM, Josefson JL, et al. Maternal short-chain fatty acids are associated with metabolic parameters in mothers and newborns. Transl Res 2014;164:153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Delgado-Diaz DJ, Tyssen D, Hayward JA, Gugasyan R, Hearps AC, Tachedjian G.. Distinct immune responses elicited from cervicovaginal epithelial cells by lactic acid and short chain fatty acids associated with optimal and non-optimal vaginal microbiota. Front Cell Infect Microbiol 2019;9:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gray LE, O'Hely M, Ranganathan S, Sly PD, Vuillermin P.. The maternal diet, gut bacteria, and bacterial metabolites during pregnancy influence offspring asthma. Front Immunol 2017;8:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D.. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. 2015;348:589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol 2005;66:13–20. [DOI] [PubMed] [Google Scholar]
- [79].Correa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA.. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology 2016;5:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Millard AL, Mertes PM, Ittelet D, Villard F, Jeannesson P, Bernard J.. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin Exp Immunol 2002;130:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, et al. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem 2010;285:27601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].de Vos WM, Tilg H, Van Hul M, Cani PD.. Gut microbiome and health: mechanistic insights. Gut 2022;71:1020–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Megli CJ, Coyne CB.. Infections at the maternal-fetal interface: an overview of pathogenesis and defence. Nat Rev Microbiol 2022;20:67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun 2014;5:3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008;57:1470–81. [DOI] [PubMed] [Google Scholar]
- [86].Cerf-Bensussan N, Gaboriau-Routhiau V.. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol 2010;10:735–44. [DOI] [PubMed] [Google Scholar]
- [87].Negi S, Das DK, Pahari S, Nadeem S, Agrewala JN.. Potential role of gut microbiota in induction and regulation of innate immune memory. Front Immunol 2019;10:2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ingle RA, Carstens M, Denby KJ.. PAMP recognition and the plant-pathogen arms race. Bioessays 2006;28:880–9. [DOI] [PubMed] [Google Scholar]
- [89].Ronchetti C, Cirillo F, Di Segni N, Cristodoro M, Busnelli A, Levi-Setti PE.. Inflammatory bowel disease and reproductive health: from fertility to pregnancy—a narrative review. Nutrients 2022;14:1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ali MF, He H, Friedel D.. Inflammatory bowel disease and pregnancy: fertility, complications and treatment. Ann Gastroenterol 2020;33:579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].de Jonge L, Bos HJ, van Langen IM, de Jong-van den Berg LT, Bakker MK.. Antibiotics prescribed before, during and after pregnancy in the Netherlands: a drug utilization study. Pharmacoepidemiol Drug Saf 2014;23:60–8. [DOI] [PubMed] [Google Scholar]
- [92].Kuperman AA, Koren O.. Antibiotic use during pregnancy: how bad is it? BMC Med 2016;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Laniewski P, Ilhan ZE, Herbst-Kralovetz MM.. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol 2020;17:232–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wang J, Li Z, Ma X, Du L, Jia Z, Cui X, et al. Translocation of vaginal microbiota is involved in impairment and protection of uterine health. Nat Commun 2021;12:4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gunther V, Allahqoli L, Watrowski R, Maass N, Ackermann J, von Otte S, et al. Vaginal microbiome in reproductive medicine. Diagnostics (Basel) 2022;12:1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhang X, Zhai Q, Wang J, Ma X, Xing B, Fan H, et al. Variation of the vaginal microbiome during and after pregnancy in Chinese women. Genomics Proteomics Bioinformatics 2022;20:322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hawkins SM, Matzuk MM.. The menstrual cycle: basic biology. Ann N Y Acad Sci 2008;1135:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Punzon-Jimenez P, Labarta E.. The impact of the female genital tract microbiome in women health and reproduction: a review. J Assist Reprod Genet 2021;38:2519–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Goodman MT, Wilkens LR, Hankin JH, Lyu L-C, Wu AH, Kolonel1 LN.. Association of soy and fiber consumption with the risk of endometrial cancer. Am J Epidemiol 1997;146:294–306. [DOI] [PubMed] [Google Scholar]
- [100].Coffey DS. Similarities of prostate and breast cancer evolution, diet, and estrogens. Urology 2001;57:31–8. [DOI] [PubMed] [Google Scholar]
- [101].Copeland JL, Consitt LA, Tremblay MS.. Hormonal responses to endurance and resistance exercise in females aged 19–69 years. J Gerontol A Biol Sci Med Sci 2002;57:B158–65. [DOI] [PubMed] [Google Scholar]
- [102].Setchell KD, Lydeking-Olsen E.. Dietary phytoestrogens and their effect on bone evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr 2003;78:593S–609S. [DOI] [PubMed] [Google Scholar]
- [103].Ennour-Idrissi K, Maunsell E, Diorio C.. Effect of physical activity on sex hormones in women: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res 2015;17:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Tester R, Al-Ghazzewi FH.. Intrinsic and extrinsic carbohydrates in the vagina: a short review on vaginal glycogen. Int J Biol Macromol 2018;112:203–6. [DOI] [PubMed] [Google Scholar]
- [105].Csapo AI, Pinto-Dantas CA.. The effect of progesterone on the human uterus. Proc Natl Acad Sci U S A 1965;54:1069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Di Renzo GC, Giardina I, Clerici G, Brillo E, Gerli S.. Progesterone in normal and pathological pregnancy. Horm Mol Biol Clin Investig 2016;27:35–48. [DOI] [PubMed] [Google Scholar]
- [107].Owen JA Jr. Physiology of the menstrual cycle. Am J Clin Nutr 1975;28:333–8. [DOI] [PubMed] [Google Scholar]
- [108].O'Hanlon DE, Moench TR, Cone RA.. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One 2013;8:e80074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Freitas AC, Bocking A, Hill JE, Money DM, Group VR.. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome 2018;6:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zhang F, Zhang T, Ma Y, Huang Z, He Y, Pan H, et al. Alteration of vaginal microbiota in patients with unexplained recurrent miscarriage. Exp Ther Med 2019;17:3307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011;108:4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Mendes-Soares H, Suzuki H, Hickey RJ, Forney LJ.. Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. J Bacteriol 2014;196:1458–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].France MT, Mendes-Soares H, Forney LJ.. Genomic comparisons of Lactobacillus crispatus and Lactobacillus iners reveal potential ecological drivers of community composition in the vagina. Appl Environ Microbiol 2016;82:7063–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Antonio MA, Hawes SE, Hillier SL.. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis 1999;180:1950–6. [DOI] [PubMed] [Google Scholar]
- [115].Gupta VK, Paul S, Dutta C.. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front Microbiol 2017;8:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J 2007;1:121–33. [DOI] [PubMed] [Google Scholar]
- [117].Zhou X, Hansmann MA, Davis CC, Suzuki H, Brown CJ, Schutte U, et al. The vaginal bacterial communities of Japanese women resemble those of women in other racial groups. FEMS Immunol Med Microbiol 2010;58:169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Das Purkayastha S, Bhattacharya MK, Prasad HK, Upadhyaya H, Lala SD, Pal K, et al. Contrasting diversity of vaginal lactobacilli among the females of Northeast India. BMC Microbiol 2019;19:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Shi Y, Chen L, Tong J, Xu C.. Preliminary characterization of vaginal microbiota in healthy Chinese women using cultivation-independent methods. J Obstet Gynaecol Res 2009;35:525–32. [DOI] [PubMed] [Google Scholar]
- [120].McClelland RS, Lingappa JR, Srinivasan S, Kinuthia J, John-Stewart GC, Jaoko W, et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect Dis 2018;18:554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gosmann C, Anahtar MN, Handley SA, Farcasanu M, Abu-Ali G, Bowman BA, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 2017;46:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Del Giudice F, Belladelli F, Chen T, Glover F, Mulloy EA, Kasman AM, et al. The association of impaired semen quality and pregnancy rates in assisted reproduction technology cycles: systematic review and meta-analysis. Andrologia 2022;54:e14409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Laprise SL. Implications of epigenetics and genomic imprinting in assisted reproductive technologies. Mol Reprod Dev 2009;76:1006–18. [DOI] [PubMed] [Google Scholar]
- [124].Massaro PA, MacLellan DL, Anderson PA, Romao RL.. Does intracytoplasmic sperm injection pose an increased risk of genitourinary congenital malformations in offspring compared to in vitro fertilization? A systematic review and meta-analysis. J Urol 2015;193:1837–42. [DOI] [PubMed] [Google Scholar]
- [125].Rexhaj E, Paoloni-Giacobino A, Rimoldi SF, Fuster DG, Anderegg M, Somm E, et al. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J Clin Invest 2013;123:5052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wang LY, Wang N, Le F, Li L, Lou HY, Liu XZ, et al. Superovulation induced changes of lipid metabolism in ovaries and embryos and its probable mechanism. PLoS One 2015;10:e0132638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ahmadi H, Fathi F, Karimi H, Amidi F, Mehdinejadiani S, Moeini A, et al. Altered TH1, TH2, TH17 balance in assisted reproductive technology conceived mice. J Reprod Immunol 2020;139:103117. [DOI] [PubMed] [Google Scholar]
- [128].Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun 2017;8:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Hyman RW, Herndon CN, Jiang H, Palm C, Fukushima M, Bernstein D, et al. The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transfer. J Assist Reprod Genet 2012;29:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Sirota I, Zarek SM, Segars JH.. Potential influence of the microbiome on infertility and assisted reproductive technology. Semin Reprod Med 2014;32:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Koedooder R, Singer M, Schoenmakers S, Savelkoul PHM, Morre SA, de Jonge JD, et al. The ReceptIVFity cohort study protocol to validate the urogenital microbiome as predictor for IVF or IVF/ICSI outcome. Reprod Health 2018;15:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Bernabeu A, Lledo B, Diaz MC, Lozano FM, Ruiz V, Fuentes A, et al. Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J Assist Reprod Genet 2019;36:2111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Haahr T, Humaidan P, Elbaek HO, Alsbjerg B, Laursen RJ, Rygaard K, et al. Vaginal microbiota and in vitro fertilization outcomes: development of a simple diagnostic tool to predict patients at risk of a poor reproductive outcome. J Infect Dis 2019;219:1809–17. [DOI] [PubMed] [Google Scholar]
- [134].Kitaya K, Nagai Y, Arai W, Sakuraba Y, Ishikawa T.. Characterization of microbiota in endometrial fluid and vaginal secretions in infertile women with repeated implantation failure. Mediators Inflamm 2019;2019:4893437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Koedooder R, Singer M, Schoenmakers S, Savelkoul PHM, Morre SA, de Jonge JD, et al. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod 2019;34:1042–54. [DOI] [PubMed] [Google Scholar]
- [136].Amato V, Papaleo E, Pasciuta R, Vigano P, Ferrarese R, Clementi N, et al. Differential composition of vaginal microbiome, but not of seminal microbiome, is associated with successful intrauterine insemination in couples with idiopathic infertility: a prospective observational study. Open Forum Infect Dis 2020;7:ofz525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wang R, Zhou G, Wu L, Huang X, Li Y, Luo B, et al. The microbial composition of lower genital tract may affect the outcome of in vitro fertilization-embryo transfer. Front Microbiol 2021;12:729744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Haahr T, Jensen JS, Thomsen L, Duus L, Rygaard K, Humaidan P.. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: a prospective study in IVF patients. Hum Reprod 2016;31:795–803. [DOI] [PubMed] [Google Scholar]
- [139].Pelzer ES, Willner D, Buttini M, Huygens F.. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Van Leeuwenhoek 2018;111:933–43. [DOI] [PubMed] [Google Scholar]
- [140].Chen S, Gu Z, Zhang W, Jia S, Wu Y, Zheng P, et al. Microbiome of the lower genital tract in Chinese women with endometriosis by 16s-rRNA sequencing technique: a pilot study. Ann Transl Med 2020;8:1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].He Q, Kwok LY, Xi X, Zhong Z, Ma T, Xu H, et al. The meconium microbiota shares more features with the amniotic fluid microbiota than the maternal fecal and vaginal microbiota. Gut Microbes 2020;12:1794266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Liu Y, Ko EY, Wong KK, Chen X, Cheung WC, Law TS, et al. Endometrial microbiota in infertile women with and without chronic endometritis as diagnosed using a quantitative and reference range-based method. Fertil Steril 2019;112:707–17.e1. [DOI] [PubMed] [Google Scholar]
- [143].Moreno I, Franasiak JM.. Endometrial microbiota-new player in town. Fertil Steril 2017;108:32–9. [DOI] [PubMed] [Google Scholar]
- [144].Salim R, Ben-Shlomo I, Colodner R, Keness Y, Shalev E.. Bacterial colonization of the uterine cervix and success rate in assisted reproduction results of a prospective survey. Hum Reprod 2002;17:337–40. [DOI] [PubMed] [Google Scholar]
- [145].Fanchin R, Harmas A, Benaoudia F, Lundkvist U, Olivennes Fo, Frydman R.. Microbial flora of the cervix assessed at the time of embryo transfer adversely affects in vitro fertilization outcome. Fertil Steril 1998;70:866–70. [DOI] [PubMed] [Google Scholar]
- [146].Moreno I, Codoner FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazan J, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol 2016;215:684–703. [DOI] [PubMed] [Google Scholar]
- [147].Franasiak JM, Scott RT.. Endometrial microbiome. Curr Opin Obstet Gynecol 2017;29:146–52. [DOI] [PubMed] [Google Scholar]
- [148].Hashimoto T, Kyono K.. Does dysbiotic endometrium affect blastocyst implantation in IVF patients? J Assist Reprod Genet 2019;36:2471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Kyono K, Hashimoto T, Kikuchi S, Nagai Y, Sakuraba Y.. A pilot study and case reports on endometrial microbiota and pregnancy outcome: an analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod Med Biol 2019;18:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Winters AD, Romero R, Gervasi MT, Gomez-Lopez N, Tran MR, Garcia-Flores V, et al. Does the endometrial cavity have a molecular microbial signature? Sci Rep 2019;9:9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Usyk M, Zolnik CP, Castle PE, Porras C, Herrero R, Gradissimo A, et al. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLoS Pathog 2020;16:e1008376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Baker JM, Chase DM, Herbst-Kralovetz MM.. Uterine microbiota: residents, tourists, or invaders? Front Immunol 2018;9:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Pelzer ES, Willner D, Buttini M, Hafner LM, Theodoropoulos Christina, et al. The fallopian tube microbiome implications for reproductive health. Oncotarget 2018;9:21541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Pelzer ES, Allan JA, Cunningham K, Mengersen K, Allan JM, Launchbury T, et al. Microbial colonization of follicular fluid: alterations in cytokine expression and adverse assisted reproduction technology outcomes. Hum Reprod 2011;26:1799–812. [DOI] [PubMed] [Google Scholar]
- [155].Franasiak JM, Scott RT Jr. Reproductive tract microbiome in assisted reproductive technologies. Fertil Steril 2015;104:1364–71. [DOI] [PubMed] [Google Scholar]
- [156].Munoz-Suano A, Hamilton AB, Betz AG.. Gimme shelter: the immune system during pregnancy. Immunol Rev 2011;241:20–38. [DOI] [PubMed] [Google Scholar]
- [157].Konstantinov SR, van der Woude CJ, Peppelenbosch MP.. Do pregnancy-related changes in the microbiome stimulate innate immunity? Trends Mol Med 2013;19:454–9. [DOI] [PubMed] [Google Scholar]
- [158].Ziegler SM, Feldmann CN, Hagen SH, Richert L, Barkhausen T, Goletzke J, et al. Innate immune responses to toll-like receptor stimulation are altered during the course of pregnancy. J Reprod Immunol 2018;128:30–7. [DOI] [PubMed] [Google Scholar]
- [159].Llewellyn A, Foey A.. Probiotic modulation of innate cell pathogen sensing and signaling events. Nutrients 2017;9:1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Monteagudo-Mera A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A.. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol 2019;103:6463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Younis N, Mahasneh A.. Probiotics and the envisaged role in treating human infertility. Middle East Fertil Soc J 2020;25:33. [Google Scholar]
- [162].Ma D, Chen Y, Chen T.. Vaginal microbiota transplantation for the treatment of bacterial vaginosis: a conceptual analysis. FEMS Microbiol Lett 2019;366:fnz025. [DOI] [PubMed] [Google Scholar]
- [163].He R, Li P, Wang J, Cui B, Zhang F, Zhao F.. The interplay of gut microbiota between donors and recipients determines the efficacy of fecal microbiota transplantation. Gut Microbes 2022;14:2100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Zhao Z, Ji X, Zhang T, Li Q, Marcella C, Wen Q, et al. Washed microbiota transplantation improves the fertility of patients with inflammatory bowel disease. Chin Med J (Engl) 2022;135:1489–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Markowiak-Kopec P, Slizewska K.. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020;12:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Han Y, Liu Z, Chen T.. Role of vaginal microbiota dysbiosis in gynecological diseases and the potential interventions. Front Microbiol 2021;12:643422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Lan Y, Li Y, Yang X, Lei L, Liang Y, Wang S.. Progesterone-induced blocking factor-mediated Th1/Th2 balance correlates with fetal arrest in women who underwent in vitro fertilization and embryo transfer. Clin Immunol 2021;232:108858. [DOI] [PubMed] [Google Scholar]
- [168].Druckmann R, Druckmann MA.. Progesterone and the immunology of pregnancy. J Steroid Biochem Mol Biol 2005;97:389–96. [DOI] [PubMed] [Google Scholar]
- [169].Reid JN, Bisanz JE, Monachese M, Burton JP, Reid G.. The rationale for probiotics improving reproductive health and pregnancy outcome. Am J Reprod Immunol 2013;69:558–66. [DOI] [PubMed] [Google Scholar]
- [170].Lindsay KL, Walsh CA, Brennan L, McAuliffe FM.. Probiotics in pregnancy and maternal outcomes: a systematic review. J Matern Fetal Neonatal Med 2013;26:772–8. [DOI] [PubMed] [Google Scholar]
- [171].Teame T, Wang A, Xie M, Zhang Z, Yang Y, Ding Q, et al. Paraprobiotics and postbiotics of probiotic Lactobacilli, their positive effects on the host and action mechanisms: a review. Front Nutr 2020;7:570344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Borella F, Carosso AR, Cosma S, Preti M, Collemi G, Cassoni P, et al. Gut microbiota and gynecological cancers: a summary of pathogenetic mechanisms and future directions. ACS Infect Dis 2021;7:987–1009. [DOI] [PubMed] [Google Scholar]
- [173].Gomez Arango LF, Barrett HL, Callaway LK, Nitert MD.. Probiotics and pregnancy. Curr Diab Rep 2015;15:567. [DOI] [PubMed] [Google Scholar]
- [174].Sheyholislami H, Connor KL.. Are probiotics and prebiotics safe for use during pregnancy and lactation? A systematic review and meta-analysis. Nutrients 2021;13:2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Wang J, Zheng J, Shi W, Du N, Xu X, Zhang Y, et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018;67:1614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Wang J, Xiao L, Xiao B, Zhang B, Zuo Z, Ji P, et al. Maternal and neonatal viromes indicate the risk of offspring's gastrointestinal tract exposure to pathogenic viruses of vaginal origin during delivery. mLife 2022;1:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Liu ZZ, Sun JH, Wang WJ.. Gut microbiota in gastrointestinal diseases during pregnancy. World J Clin Cases 2022;10:2976–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012;150:470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Fan T, Zhong XM, Wei XC, Miao ZL, Luo SY, Cheng H, et al. The alteration and potential relationship of vaginal microbiota and chemokines for unexplained recurrent spontaneous abortion. Medicine (Baltimore) 2020;99:e23558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Patel N, Patel N, Pal S, Nathani N, Pandit R, Patel M, et al. Distinct gut and vaginal microbiota profile in women with recurrent implantation failure and unexplained infertility. BMC Womens Health 2022;22:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Kelley ST, Skarra DV, Rivera AJ, Thackray VG.. The gut microbiome is altered in a letrozole-induced mouse model of polycystic ovary syndrome. PLoS One 2016;11:e0146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Lindheim L, Bashir M, Munzker J, Trummer C, Zachhuber V, Leber B, et al. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): a pilot study. PLoS One 2017;12:e0168390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Liu R, Zhang C, Shi Y, Zhang F, Li L, Wang X, et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol 2017;8:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Insenser M, Murri M, Del Campo R, Martinez-Garcia MA, Fernandez-Duran E, Escobar-Morreale HF.. Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. J Clin Endocrinol Metab 2018;103:2552–62. [DOI] [PubMed] [Google Scholar]
- [185].Torres PJ, Siakowska M, Banaszewska B, Pawelczyk L, Duleba AJ, Kelley ST, et al. Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. J Clin Endocrinol Metab 2018;103:1502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Zeng B, Lai Z, Sun L, Zhang Z, Yang J, Li Z, et al. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): a pilot study. Res Microbiol 2019;170:43–52. [DOI] [PubMed] [Google Scholar]
- [187].Zhang J, Sun Z, Jiang S, Bai X, Ma C, Peng Q, et al. Probiotic Bifidobacterium lactis V9 regulates the secretion of sex hormones in polycystic ovary syndrome patients through the gut-brain axis. mSystems 2019;4:e00017-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Chu W, Han Q, Xu J, Wang J, Sun Y, Li W, et al. Metagenomic analysis identified microbiome alterations and pathological association between intestinal microbiota and polycystic ovary syndrome. Fertil Steril 2020;113:1286–98.e4. [DOI] [PubMed] [Google Scholar]
- [189].Zhou L, Ni Z, Cheng W, Yu J, Sun S, Zhai D, et al. Characteristic gut microbiota and predicted metabolic functions in women with PCOS. Endocr Connect 2020;9:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Dong S, Jiao J, Jia S, Li G, Zhang W, Yang K, et al. 16S rDNA full-length assembly sequencing technology analysis of intestinal microbiome in polycystic ovary syndrome. Front Cell Infect Microbiol 2021;11:634981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Lull K, Arffman RK, Sola-Leyva A, Molina NM, Aasmets O, Herzig KH, et al. The gut microbiome in polycystic ovary syndrome and its association with metabolic traits. J Clin Endocrinol Metab 2021;106:858–71. [DOI] [PubMed] [Google Scholar]
- [192].Yang YL, Zhou WW, Wu S, Tang WL, Wang ZW, Zhou ZY, et al. Intestinal flora is a key factor in insulin resistance and contributes to the development of polycystic ovary syndrome. Endocrinology 2021;162:bqab118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Crusell MKW, Hansen TH, Nielsen T, Allin KH, Ruhlemann MC, Damm P, et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018;6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Cortez RV, Taddei CR, Sparvoli LG, Angelo AGS, Padilha M, Mattar R, et al. Microbiome and its relation to gestational diabetes. Endocrine 2019;64:254–64. [DOI] [PubMed] [Google Scholar]
- [195].Crusell MKW, Brink LR, Nielsen T, Allin KH, Hansen T, Damm P, et al. Gestational diabetes and the human salivary microbiota: a longitudinal study during pregnancy and postpartum. BMC Pregnancy Childbirth 2020;20:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Kuang YS, Lu JH, Li SH, Li JH, Yuan MY, He JR, et al. Connections between the human gut microbiome and gestational diabetes mellitus. Gigascience 2017;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Ferrocino I, Ponzo V, Gambino R, Zarovska A, Leone F, Monzeglio C, et al. Changes in the gut microbiota composition during pregnancy in patients with gestational diabetes mellitus (GDM). Sci Rep 2018;8:12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Zhang W, Ma C, Xie P, Zhu Q, Wang X, Yin Y, et al. Gut microbiota of newborn piglets with intrauterine growth restriction have lower diversity and different taxonomic abundances. J Appl Microbiol 2019;127:354–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Sililas P, Huang L, Thonusin C, Luewan S, Chattipakorn N, Chattipakorn S, et al. Association between gut microbiota and development of gestational diabetes mellitus. Microorganisms 2021;9:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Swati P, Ambika Devi K, Thomas B, Vahab SA, Kapaettu S, Kushtagi P.. Simultaneous detection of periodontal pathogens in subgingival plaque and placenta of women with hypertension in pregnancy. Arch Gynecol Obstet 2012;285:613–9. [DOI] [PubMed] [Google Scholar]
- [201].Ponzetto A, Cardaropoli S, Piccoli E, Rolfo A, Gennero L, Kanduc D, et al. Pre-eclampsia is associated with Helicobacter pylori seropositivity in Italy. J Hypertens 2006;24:2445–9. [DOI] [PubMed] [Google Scholar]
- [202].Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G.. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J Periodontol 2007;78:670–6. [DOI] [PubMed] [Google Scholar]
- [203].Amarasekara R, Jayasekara RW, Senanayake H, Dissanayake VH.. Microbiome of the placenta in pre-eclampsia supports the role of bacteria in the multifactorial cause of pre-eclampsia. J Obstet Gynaecol Res 2015;41:662–9. [DOI] [PubMed] [Google Scholar]
- [204].Lv LJ, Li SH, Li SC, Zhong ZC, Duan HL, Tian C, et al. Early-onset preeclampsia is associated with gut microbial alterations in antepartum and postpartum women. Front Cell Infect Microbiol 2019;9:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Wang J, Gu X, Yang J, Wei Y, Zhao Y.. Gut microbiota dysbiosis and increased plasma LPS and TMAO levels in patients with preeclampsia. Front Cell Infect Microbiol 2019;9:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Chang Y, Chen Y, Zhou Q, Wang C, Chen L, Di W, et al. Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia. Clin Sci (Lond) 2020;134:289–302. [DOI] [PubMed] [Google Scholar]
- [207].Chen X, Li P, Liu M, Zheng H, He Y, Chen MX, et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 2020;69:513–22. [DOI] [PubMed] [Google Scholar]
- [208].Liu J, Yang H, Yin Z, Jiang X, Zhong H, Qiu D, et al. Remodeling of the gut microbiota and structural shifts in preeclampsia patients in South China. Eur J Clin Microbiol Infect Dis 2017;36:713–9. [DOI] [PubMed] [Google Scholar]
- [209].Parthiban PS, Mahendra J, Logaranjani A, Shanmugam S, Balakrishnan A, Junaid M, et al. Association between specific periodontal pathogens, Toll-like receptor-4, and nuclear factor-kB expression in placental tissues of pre-eclamptic women with periodontitis. J Investig Clin Dent 2018;9:e12265. [DOI] [PubMed] [Google Scholar]
- [210].Petricevic L, Domig KJ, Nierscher FJ, Sandhofer MJ, Fidesser M, Krondorfer I, et al. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci Rep 2014;4:5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Paramel Jayaprakash T, Wagner EC, van Schalkwyk J, Albert AY, Hill JE, Money DM, et al. High diversity and variability in the vaginal microbiome in women following preterm premature rupture of membranes (PPROM): a prospective cohort study. PLoS One 2016;11:e0166794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Kindinger LM, Bennett PR, Lee YS, Marchesi JR, Smith A, Cacciatore S, et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Brown RG, Al-Memar M, Marchesi JR, Lee YS, Smith A, Chan D, et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl Res 2019;207:30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Kosti I, Lyalina S, Pollard KS, Butte AJ, Sirota M.. Meta-analysis of vaginal microbiome data provides new insights into preterm birth. Front Microbiol 2020;11:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Sifnaios E, Mastorakos G, Psarra K, Panagopoulos ND, Panoulis K, Vitoratos N, et al. Gestational diabetes and T-cell (Th1/Th2/Th17/Treg) immune profile. In Vivo 2019;33:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Langel SN, Blasi M, Permar SR.. Maternal immune protection against infectious diseases. Cell Host Microbe 2022;30:660–74. [DOI] [PubMed] [Google Scholar]
- [217].Gibbs RS. The relationship between infections and adverse pregnancy outcomes: an overview. Ann Periodontol 2001;6:153–63. [DOI] [PubMed] [Google Scholar]
- [218].Cassini MA, Pilloni A, Condò SG, Vitali LA, Pasquantonio G, Cerroni L. Periodontal bacteria in the genital tract: are they related to adverse pregnancy outcomes? Int J Immunopathol Pharmacol 2013;26:931–9. [DOI] [PubMed] [Google Scholar]
- [219].Lopez NJ, Smith PC, Gutierrez J.. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J Periodontol 2002;73:911–24. [DOI] [PubMed] [Google Scholar]