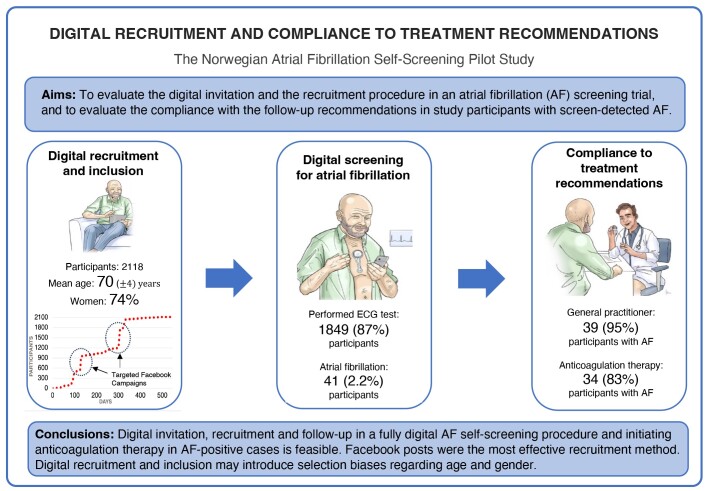
Abstract
Aims
Atrial fibrillation (AF) is prevalent, undiagnosed in approximately one-third of cases, and is associated with severe complications. Guidelines recommend screening individuals at increased risk of stroke. This report evaluated the digital recruitment procedure and compliance with the follow-up recommendations in participants with screen-detected AF in the Norwegian Atrial Fibrillation self-screening pilot study.
Methods and results
Norwegians ≥65 years were invited through Facebooks posts, web pages, and newspapers to participate in the study. Targeted Facebook posts promoted over 11 days reached 84 208 users and 10 582 visitors to the study homepage. This accounted for 51% of the total homepage visitors (n = 20 704). A total of 2118 (10%) of the homepage visitors provided digital consent to participate after they met the inclusion criteria. The mean (standard deviation) age of the participants was 70 (4) years, and the majority [n = 1569 (74%)] were women. A total of 1849 (87%) participants completed the electrocardiogram self-screening test, identifying AF in 41 (2.2%) individuals. Of these, 39 (95%) participants consulted a general practitioner, and 34 (83%) participants initiated anticoagulation therapy.
Conclusion
Digital recruitment and inclusion in digital AF screening with a high rate of initiation of anticoagulation therapy in AF positive screening cases are feasible. However, digital recruitment and inclusion may introduce selection bias with regard to age and gender. Larger studies are needed to determine the efficacy and cost-effectiveness of a fully digital AF screening.
Trial registration
Clinical trials: NCT04700865
Keywords: Atrial fibrillation, Screening, Digital recruitment, Anticoagulation, Compliance
Graphical Abstract
Graphical Abstract.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide.1–3 Atrial fibrillation is associated with significant mortality, morbidity, and health care costs.1–3 One-third of AF cases may go undiagnosed due to its subclinical or asymptomatic nature.3 The European Society of Cardiology recommends opportunistic screening for AF in individuals at increased risk of stroke due to the proved efficacy of anticoagulation therapy in stroke prevention.4
Several trials have demonstrated an increased detection rate of AF through screening, but conventional AF screening may be time- and resource-intensive.5–8 A digital approach may shorten recruitment time, increase protocol adherence, and ensure representative sampling of study participants. Recently, we reported excellent feasibility of a fully digital self-screening procedure for AF by a patch electrocardiogram (ECG) device in the Norwegian Atrial Fibrillation self-screening pilot study.9 However, the utility of digital screening for AF depends on several factors, including the efficacy of the recruitment process and the adherence to treatment advice among those with screen-detected AF.10
In this report, we evaluated the digital recruitment procedure and the compliance with the follow-up recommendations in participants diagnosed with AF in the Norwegian Atrial Fibrillation self-screening pilot study.
Methods
Study design
The Norwegian Atrial Fibrillation self-screening pilot study was a fully digital, prospective cohort study conducted at Sorlandet Hospital Arendal, Norway, between 1 January 2021 and 6 June 2022.9
Study population
Men and women ≥65 years from the general population of Norway (inhabitants ≥65 years per 01.01.2021: 965 742), with at least one additional risk factor for stroke according to the congestive heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65–74 and sex category (female) (CHA2DS2-VASc) risk score (heart failure, hypertension, age ≥75 years, diabetes mellitus, previous stroke/transient ischaemic attack, vascular disease, female gender) were invited to participate in the study.4
Recruitment
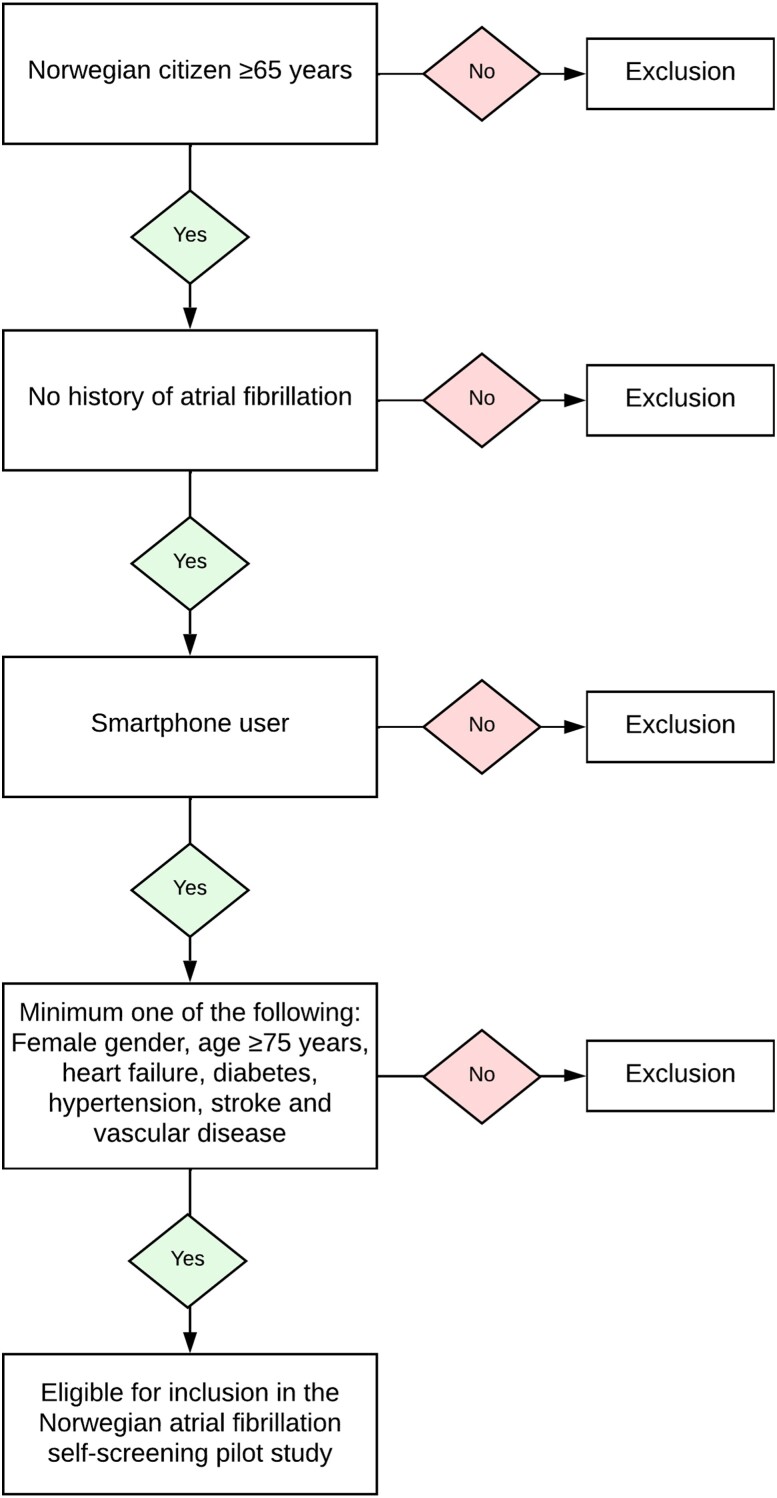
Potential participants were openly invited to visit the study homepage after study promotion in newspapers, radio, at the web page of the local hospital, and through Facebook posts (targeting men and women ≥65 years residing in Norway). Information about the study and access to a digital inclusion/exclusion test were available on the study homepage. The inclusion/exclusion test was based on the CHA2DS2-VASc risk score (Figure 1). Individuals who self-reported a history of AF or no access to a smartphone were excluded. Individuals passing the inclusion/exclusion test (i.e. CHA2DS2-VASc score ≥2 and no exclusion criteria) were forwarded to sign a digital consent for study participation using the Norwegian BankID system. BankID (www.bankid.no) is a personal electronic identification method designed for secure online authentication and digital signing. BankID is generally available and is used for all digital communication with public authorities in Norway. Participants self-reported their baseline characteristics, symptoms, and medication use in a digital questionnaire. Google Analytics identified unique users visiting the study's homepage, while Facebook Metrics tracked unique users interacting with the Facebook posts. In both instances, unique users were identified based on their internet protocol (IP) addresses and types of devices.
Figure 1.
Inclusion/exclusion criteria in the Norwegian Atrial Fibrillation self-screening pilot study.
The electrocardiogram self-screening test
All included participants received an ECG247 Smart Heart Sensor by post. ECG247 Smart Heart Sensor (Appsens AS, Lillesand, Norway, www.ecg247.com) is a reusable, single-lead, wireless, and patch ECG sensor.11 The ECG screening tests were performed at home by the participants guided by instructions in the smartphone application. Video instructions were available at the manufacturer’s homepage (www.ecg247.com) and phone support was available from the study centre. A minimum test period of 3 days was recommended. A cardiology resident assessed all test results remotely, and all participants received a digital report in the ECG247 application. An external independent Data Monitoring Committee verified all arrhythmias.
Follow-up of participants with atrial fibrillation
All participants diagnosed with AF received information about the diagnosis via a personal phone call in addition to the digital report in the ECG247 application. Participants with diagnosed AF were strongly advised to contact their general practitioner (GP) for further diagnostic assessment and treatment. Anticoagulation therapy was recommended in participants with AF. All participants with diagnosed AF received a digital questionnaire regarding assessment and treatment 3–6 months after the screening procedure.
Outcomes
The reported outcomes were: (i) the effectiveness of the open digital recruitment of study participants and (ii) the compliance with the follow-up recommendations in participants diagnosed with AF. The effectiveness of the recruitment process was defined as: (i) the proportion of reached Facebook users who visited the study homepage, (ii) the proportion of study homepage visitors who performed the inclusion/exclusion test, and (iii) the proportion of eligible individuals who signed digital consent for study participation. Compliance with follow-up recommendations was defined as: (i) the proportion of AF positive study participants who counselled a GP and (ii) the proportion of AF positive study participants who initiated anticoagulation therapy.
Statistics
Continuous variables are presented as mean (standard deviation, SD) or median (interquartile range, IQR) and differences between groups were analysed using Mann–Whitney non-parametric test. Categorical variables are presented as numbers and percentages. A P-value of <0.05 was regarded statistically significant. The analyses were performed using STATA, version 17 (StataCorp, College Station, TX, USA).
Patient and public involvement
A user representative was consulted in the preparation of the study protocol, and feedback from participants was used to adjust the study procedure within the protocol frames.
Ethics
The study was approved by the Regional Committee for Medical and Health Research Ethics (REK 147963). All participants signed informed consent for study participation.
Results
Recruitment
Two Facebook posts targeting Norwegians ≥65 years promoted over 11 days in total reached 84 208 unique users, corresponding to 8.7% of the total population ≥65 years. Each user was exposed to these posts 2.5 times on average, resulting in 186 540 total exposures. Of these exposures, 140 001 (75%) targeted women, 44 254 (24%) targeted men, and 2285 (1%) targeted users with uncategorized gender. The Facebook exposures resulted in 10 582 (13%) click-throughs to the study homepage. Women accounted for 7986 (75%) and men for 2428 (23.7%) of the click-throughs. Gender was not identified in 168 (1.3%) users. The click-through rate from the targeted Facebook posts to the study homepage did not differ between women and men (5.7 vs. 5.5%). The geographical click-through rates varied across Norway and was highest in the area close to the study centre (southern Norway/Agder county; 9%) and lowest in northern Norway (Finnmark county; 3%).
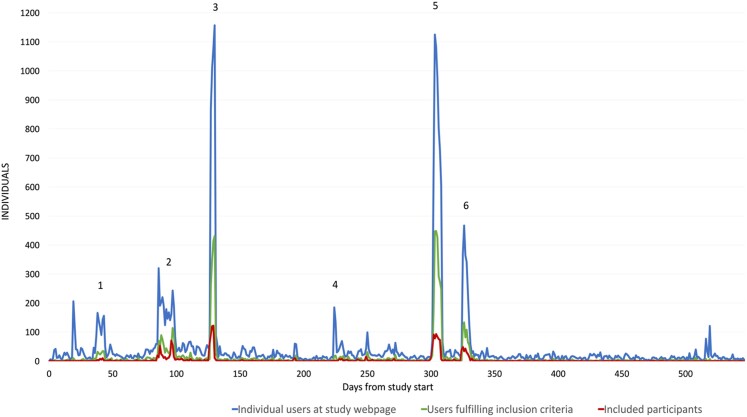
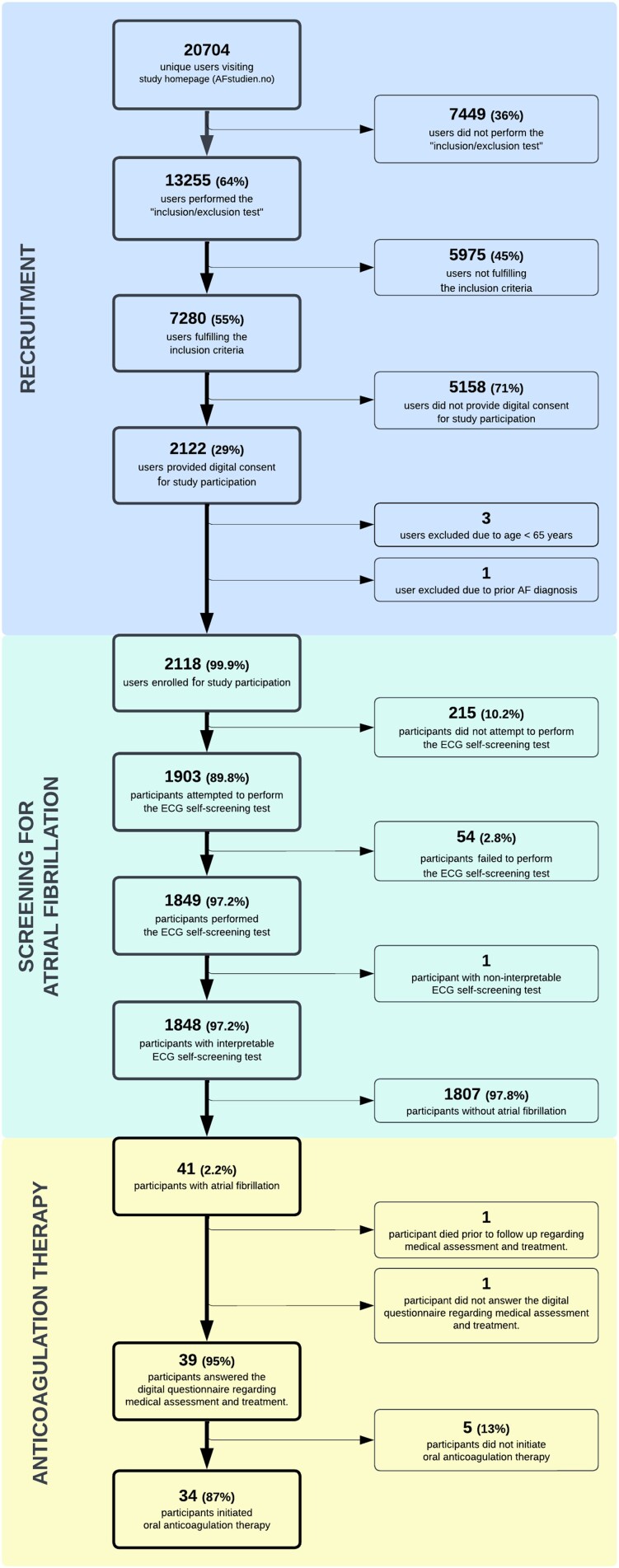
After promotion in local newspapers or radio, promotion at the local hospital webpage, and after an interview in a national newspaper for technology, 10 122 individuals visited the study homepage (Figure 2). Of the 20 704 unique users who visited the study homepage during the study period (546 days), 13 255 (64%) individuals performed the ‘inclusion/exclusion’ test, and 7280 (35%) individuals were eligible for study participation (Figure 3). A total of 2122 (29%) of the eligible individuals signed digital consent for study participation. We have no further information about individuals who did not pass the ‘inclusion/exclusion’ test or those who did not consent to study participation. Four individuals were excluded by the study staff after self-enrolment due to age <65 years or self-reported prior diagnosis of AF.
Figure 2.
Individuals visiting the study homepage, individuals passing the ‘inclusion/exclusion test’ and individuals providing digital consent for study participation during the study period from 1 January 2021 to 6 June 2022. (1) Promotion in local newspapers/radio, (2) Promotion at hospital webpage, (3) Targeted Facebook posts, (4) Interview in national newspaper for technology, (5) Targeted Facebook posts, and (6) Interview in national newspaper for research.
Figure 3.
Study flowchart.
Study population
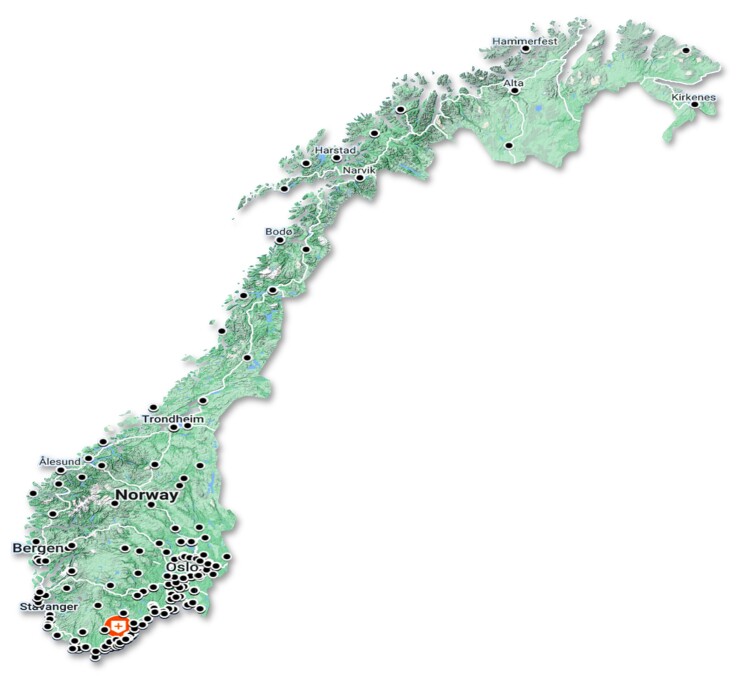
Self-reported clinical characteristics of the 2118 study participants are presented in Table 1. The mean age was 70 (4) years and the mean CHA2DS2-VASc risk score was 2.6 (0.8). The majority [n = 1569 (74%)] of the participants were women. The study participants were recruited from the whole country of Norway (Figure 4). However, a large proportion of the participants were recruited from the southern part of Norway [Agder county; 867 (41%) individuals vs. northern Norway/Finnmark county; 11 (0.5%) individuals].
Table 1.
Self-reported baseline clinical characteristics of study participants (n = 2118)
| Women, n (%) | 1569 (74) |
| Age (years), mean (SD) | 70 (4) |
| Age (years), median (IQR) | 70 (65–89) |
| Smoking, n (%) | 140 (7) |
| CHA2DS2-VASc risk score, mean (SD) | 2.6 (0.8) |
| CHA2DS2-VASc risk score, median (IQR) | 2 (2–3) |
| Heart failure, n (%) | 36 (2) |
| Hypertension, n (%) | 910 (43) |
| Diabetes mellitus, n (%) | 156 (7) |
| Previous stroke/TIA, n (%) | 64 (3) |
| Peripheral artery disease, n (%) | 19 (1) |
| Myocardial infarction, n (%) | 119 (6) |
| Chronic obstructive pulmonary disease, n (%) | 96 (5) |
| Anticoagulation therapy, n (%) | 25 (1) |
| Antiplatelet therapy, n (%) | 493 (23) |
| Antihypertensive therapya, n (%) | 523 (25) |
| Beta blocker, n (%) | 273 (13) |
aAngiotensin-converting enzyme inhibitors or angiotensin II receptor blockers.
IQR, interquartile range; TIA, transient ischaemic attack; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65–74 and sex category (female); SD, standard deviation.
Figure 4.
Geographical distribution of study participants. (Red marker: study centre. Black markers: >5 participants).
Follow-up and initiation of oral anticoagulation
The performance and results of the ECG self-screening test have been described in detail previously.9 A total of 1849 (87%) participants completed the ECG self-screening test and AF was identified in 41 (2.2%) participants (Figure 3). Intermittent episodes of AF were detected in 36 (88%) of the 41 participants with AF. The mean ECG monitoring duration was 6.3 days (±3.6 days). A total of 39 (95%) participants diagnosed with screen-detected AF reported follow-up data. One participant with AF died before follow-up, and another declined to participate in the follow-up. All participants with follow-up information consulted a GP as recommended. Further assessment by cardiologists, including an echocardiographic examination, was performed in 30 (77%) of the 39 participants. Anticoagulation therapy was initiated in 34 (87%) of the 39 participants. None of the participants diagnosed with AF used anticoagulation therapy prior to the diagnosis. We have no information about the reasons for not initiating anticoagulation therapy in the remaining five individuals, but three of these participants were women between 65 and 75 years old with no other self-reported comorbidities (i.e. CHA2DS2-VASc risk score 2).
Discussion
Recruitment and performance of the Norwegian Atrial Fibrillation self-screening pilot study was fully digital. Targeted Facebook posts were by far the most effective recruitment method. Previously undiagnosed AF was identified in 41 (2.2%) of the 1849 individuals who completed the ECG self-screening test and 39 (95%) of them reported follow-up data. Anticoagulation therapy was initiated by general practitioners (GPs) in 34 (87%) of these cases.
Recruitment
Inclusion of a sufficient number of study participants in clinical studies may be challenging.12 Digital study recruitment appears to be both cost and time beneficial in this study. Similar findings were reported from a Swedish study of women with palpitations.13 The recruitment and inclusion rates were influenced by several factors, particularly the choice of media for study promotion. Social media proved to be an efficient method for inclusion in this study. The COVID-19 pandemic was an important reason for selecting this particular recruitment method in the current study, and the pandemic may also have accelerated digital literacy in the population in general.14 To the best of our knowledge, no other similar studies have used social media to recruit participants for an AF self-screening procedure using a continuous ECG recording system. The mHealth Screening to Prevent Strokes (mSToPS) and the eHealth-based Bavarian Alternative Detection of Atrial Fibrillation (eBRAVE-AF) trials included participants by targeted emails in different health care insurance populations.8,15 The smartwatch trials from Apple, Huawei and Fitbit included participants from their own customer platforms.5–7
The majority of the included participants were relatively healthy women, and the mean age was relatively low. The age and gender distribution may have contributed to the low AF detection rate. The use of Facebook probably introduced gender and age biases.16 More women than men use Facebook, and the proportion of Facebook users decline with increasing age.17 The Fitbit study also experienced a female skewness, but this was not evident in the Apple, Huawei, eBRAVE-AF, and mSToPS trials.5–8,15 Several trials have described a higher prevalence of AF in elderly and in men compared with females.18 Elderly males were challenging to reach by social media. However, men and women had a similar Click-Through-Rate from Facebook to the study homepage. Future studies should consider other invitation channels to include older participants and especially older men.
Digital platforms also require digital competence, and the use of social media may entail a risk of geographical and socioeconomic differences. Media attention in the local press may explain the relatively high proportion of participants living in the vicinity of the study centre. We did not collect sociodemographic data, but the baseline characteristics and the findings of relatively few cases of other arrhythmias indicate a rather healthy study population.9 Previous trials have shown a higher proportion of female participants and more individuals with higher education and income levels after recruitment by Facebook posts compared with traditional recruitment methods.16 Hence, clinical studies with digital invitation and inclusion strategies are at risk of losing participants with the greatest benefit of screening.
Hoare et al.19 have previously described several other barriers such as time commitment, procedural complexities, and concerns about health outcomes for participation in clinical trials. Only one out of ten individuals visiting the study homepage provided digital consent for study participation, indicating a significant disparity between the number needed to invite and included participants. Information regarding reasons for non-consent was not available in this study. Improved understanding of the reasons for non-participation seems necessary to increase the recruitment rate in future trials.
Initiation of anticoagulation therapy
Detection of AF per se does not affect the risk of complications of AF. Appropriate initiation of anticoagulation therapy in the patients with AF and increased risk of stroke is crucial to obtain the benefits of AF screening. The mSToPS-trial described a higher rate of prescription of oral anticoagulation in the actively monitored group but did not report the initiation rate.15 Initiation of anticoagulation therapy was not reported in the Apple Heart Study, the Fitbit study or in the ReducinG stroke by screening for UndiAgnosed atRial fibrillation in elderly inDividuals (GUARD-AF) study.6,7,20 The Swedish STROKESTOP and the Danish Implantable loop recorder detection of atrial fibrillation to prevent stroke (LOOP) studies reported initiation of anticoagulation therapy in 93 and 91% of the participants with AF, respectively.21,22 However, these Scandinavian studies were conducted as traditional clinical studies with personal follow-up. In our opinion, an initiation rate of anticoagulation therapy by local GPs of 87% in our fully digital study is highly satisfactory. Notably, three of five participants who did not initiate anticoagulation therapy were women 65–74 years of age, denoting an intermediate risk where anticoagulation therapy consideration is warranted but not mandatory according to guidelines.4
Limitations
This study has several limitations. The study had a single-arm non-randomized design, and it was not possible to compare with standard care. No information regarding reasons for non-participation among qualified individuals was recorded. Google Analytics and Facebook Metrics may not provide an accurate count of unique users due to shared IP addresses and devices. Comparable data for radio, newspapers, and other recruitment methods was not available. Furthermore, we had no opportunity to validate the self-reported information of initiation of anticoagulation therapy. The study had no further follow-up, and we have no information regarding the adherence to the anticoagulation therapy or insights into the GPs perspectives on the study procedure. We did not collect data regarding time and costs for the manual procedures, and the cost-effectiveness of the screening method cannot be estimated.
Conclusions
The present study demonstrated the effectiveness of digital recruitment and inclusion for AF screening, and a high rate of initiation of anticoagulation therapy in AF positive screening cases by local GPs. However, open invitation via social media may introduce a selection bias with regard to age and gender. Larger, randomized studies with long-term follow-up are needed to settle the cost-effectiveness of fully digital AF screening.
Contributor Information
Edvard Liljedahl Sandberg, Department of Cardiology, Sorlandet Hospital, Arendal, Sykehusveien 1, 4838 Arendal, Norway; Institute of Clinical Medicine, University of Oslo, Kirkeveien 166, 0450 Oslo, Norway.
Sigrun Halvorsen, Institute of Clinical Medicine, University of Oslo, Kirkeveien 166, 0450 Oslo, Norway; Department of Cardiology, Oslo University Hospital Ullevaal, Kirkeveien 166, 0450 Oslo, Norway.
Trygve Berge, Institute of Clinical Medicine, University of Oslo, Kirkeveien 166, 0450 Oslo, Norway; Department of Medical Research and Department of Internal Medicine, Vestre Viken Hospital Trust, Baerum Hospital, Rud, Sogneprest Munthe-kaas vei 100, 1346 Gjettum, Norway.
Jostein Grimsmo, Department of Cardiac Rehabilitation, Lovisenberg Rehabilitation, Cathinka Guldbergs Hospital, Ragnar Strøms Veg 10, 2067 Jessheim, Norway; LHL (National Organization for Heart and Lung Diseases), Ragnar Strøms Veg 4, 5067 Jessheim, Norway.
Dan Atar, Institute of Clinical Medicine, University of Oslo, Kirkeveien 166, 0450 Oslo, Norway; Department of Cardiology, Oslo University Hospital Ullevaal, Kirkeveien 166, 0450 Oslo, Norway.
Bjørnar Leangen Grenne, Clinic of Cardiology, St. Olavs Hospital, Trondheim, Prinsesse Kristinas gate 3, 7030 Trondheim, Norway; Department of Circulation and Medical Imaging, Norwegian University of Science and Technology, Postboks 8905, 7491 Trondheim, Norway.
Jarle Jortveit, Department of Cardiology, Sorlandet Hospital, Arendal, Sykehusveien 1, 4838 Arendal, Norway.
Author contributions
E.L.S., S.H., T.B., J.G., D.A., B.L.G., and J.J. contributed to the study design and conception. E.L.S. and J.J. were responsible for analyses and interpretation of the data. E.L.S., S.H., and J.J. drafted, and T.B., J.G., D.A., and B.L.G. critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of this work, ensuring its integrity and accuracy.
Funding
This work was supported by Sorlandet Hospital (PhD scholarship to E.L.S.), Pfizer (research grant to Sorlandet Hospital), Bristol Myers Squibb (research grant to Sorlandet Hospital), Boehringer Ingelheim Norway (research grant to Sorlandet Hospital), and the Norwegian Atrial Fibrillation Research Network (https://www.afib.no/) (research grant to Sorlandet Hospital). The funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript. The manufacturer of the ECG devices did not contribute financial support for the study.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author (E.L.S.).
References
- 1. Bakhai A, Sandberg A, Mittendorf T, Greiner W, Oberdiek AMS, Berto P, et al. Patient perspective on the management of atrial fibrillation in five European countries. BMC Cardiovasc Disord 2013;13:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ball J, Carrington MJ, McMurray JJ, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol 2013;167:1807–1824. [DOI] [PubMed] [Google Scholar]
- 3. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 5. Guo Y, Wang II H, Zhang II H, Liu T, Liang Z, Xia Y, et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–2375. [DOI] [PubMed] [Google Scholar]
- 6. Lubitz SA, Faranesh AZ, Selvaggi C, Atlas SJ, McManus DD, Singer DE, et al. Detection of atrial fibrillation in a large population using wearable devices: the Fitbit Heart Study. Circulation 2022;146:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rizas KD, Freyer L, Sappler N, von Stülpnagel L, Spielbichler P, Krasniqi A, et al. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat Med 2022;28:1823–1830. [DOI] [PubMed] [Google Scholar]
- 9. Sandberg EL, Halvorsen S, Berge T, Grimsmo J, Atar D, Fensli R, et al. Fully digital self-screening for atrial fibrillation with patch electrocardiogram. Europace 2023;25:euad075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salmasi S, Loewen PS, Tandun R, Andrade JG, De Vera MA. Adherence to oral anticoagulants among patients with atrial fibrillation: a systematic review and meta-analysis of observational studies. BMJ Open 2020;10:e034778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandberg EL, Grenne BL, Berge T, Grimsmo J, Atar D, Halvorsen S, et al. Diagnostic accuracy and usability of the ECG247 smart heart sensor compared to conventional Holter technology. J Healthc Eng 2021;2021:5230947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacques RM, Ahmed R, Harper J, Ranjan A, Saeed I, Simpson RM, et al. Recruitment, consent and retention of participants in randomised controlled trials: a review of trials published in the National Institute for Health Research (NIHR) Journals Library (1997–2020). BMJ Open 2022;12:e059230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schenck-Gustafsson K, Carnlöf C, Jensen-Urstad M, Insulander P. Improving efficiency of clinical studies using a total digital approach: Prospective Observational Study. JMIR Form Res 2021;5:e18385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keesara S, Jonas A, Schulman K. Covid-19 and health care's digital revolution. N Engl J Med 2020;382:e82. [DOI] [PubMed] [Google Scholar]
- 15. Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS Randomized Clinical Trial. JAMA 2018;320:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akers L, Gordon JS. Using Facebook for large-scale online randomized clinical trial recruitment: effective advertising strategies. J Med Internet Res 2018;20:e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pew Research Center . Social Media Use in 2021. Washington DC: Pew Research Center; 2021. [Google Scholar]
- 18. Kjerpeseth LJ, Igland J, Selmer R, Ellekjær H, Tveit A, Berge T, et al. Prevalence and incidence rates of atrial fibrillation in Norway 2004–2014. Heart 2021;107:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoare S, Thomas GPA, Powell A, Armstrong N, Mant J, Burt J. Why do people choose not to take part in screening? Qualitative interview study of atrial fibrillation screening nonparticipation. Health Expect 2023;26:2216–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singer DE, Atlas S, Go AS, Lopes RD, Lubitz S, McManus D, et al. A randomized clinical trial of screening for atrial fibrillation with a 14-day patch monitor: analysis of ECG recordings from the GUARD-AF study. J Am Coll Cardiol 2022;79:28. [Google Scholar]
- 21. Kemp Gudmundsdottir K, Fredriksson T, Svennberg E, Al-Khalili F, Friberg L, Frykman V, et al. Stepwise mass screening for atrial fibrillation using N-terminal B-type natriuretic peptide: the STROKESTOP II study. Europace 2020;22:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Svendsen JH, Diederichsen SZ, Højberg S, Krieger DW, Graff C, Kronborg C, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet 2021; 398:1507–1516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author (E.L.S.).