SUMMARY
Mutations in the gene DNMT3A have been identified in various hematopoietic conditions, including clonal hematopoiesis, myelodysplastic syndrome, and acute myeloid leukemia. The clinical significance of this early mutation and the resultant enhanced clonal fitness have been a focus for therapeutic intervention.
In this issue of Clinical Cancer Research, Venugopal and colleagues investigate the response to DNA-damaging chemotherapeutic agents in various model systems of DNMT3A-mutant leukemia and identify defects in DNA damage response and repair (1). DNMT3A is a de novo DNA methyltransferase that is required for normal hematopoietic stem cell differentiation, lineage determination, and mature blood cell formation (2). Investigations into the biochemistry of cytosine methylation have revealed that replication fidelity of methylcytosine (5mC) is performed by DNMT1 [rarely mutated in myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML)] whereas the genes responsible for dynamic de novo cytosine methylation (DNMT3A or DNMT3B) or active demethylation (TET2) through hydroxymethlycytosine (5hmC) conversion are recurrently observed. Given the high prevalence of disease alleles affecting DNA methylation in MDS and AML (i.e., DNMT3A, TET2, IDH1/2), many investigators have attempted to profile DNA methylation in patients with AML either for deeper biologic insight or to inform therapeutic intervention (3). Disconcertingly, the direct mechanisms for how abnormal DNA methylation causes clonal outgrowth remains unclear. In this new work from Venugopal and colleagues, the authors show in multiple systems including transgenic mice, AML cell lines, and isogenic US02 cells, that DNMT3A R882 mutations sensitize cells to DNA-damaging agents leading to replication fork collapse.
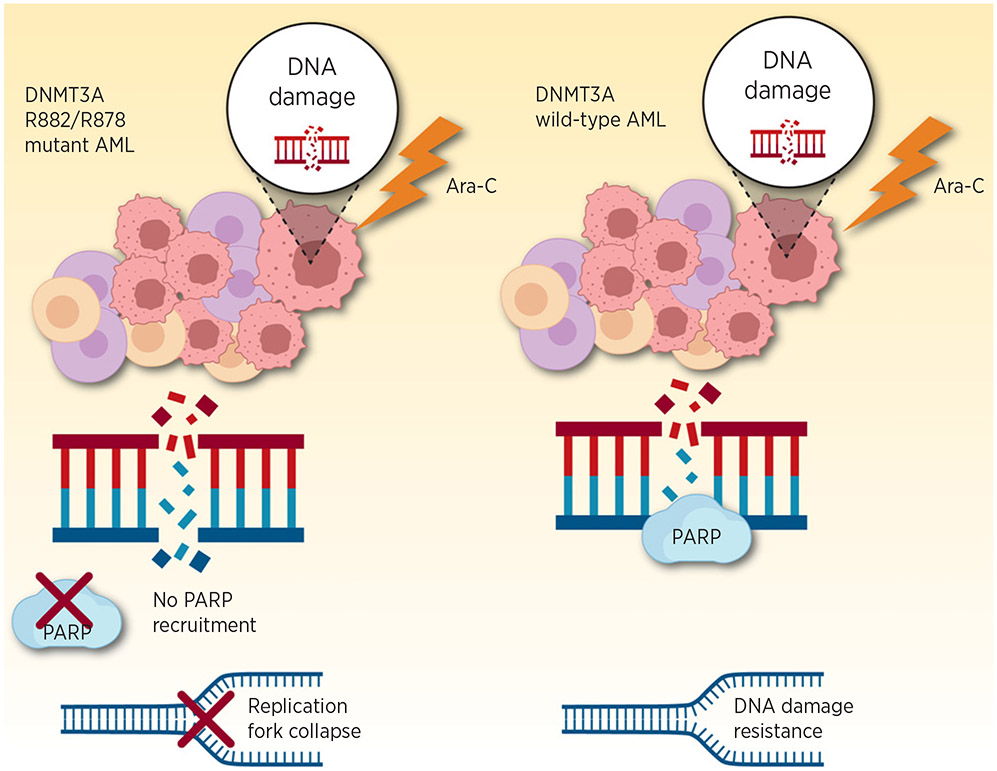
For many solid tumors, specifically those harboring a mutation in DNA damage response pathway (e.g., BRCA1, BRCA2), intentional and therapeutic synthetic lethality in tumor cells have led to use of the single-strand DNA repair enzyme PARP. One key and very distinct mechanism shown in this article is the inability of DNMT3A R882 to recruit PARP resulting in replication fork arrest and lack of sensitivity or synergy with PARP inhibition (Fig. 1). In essence, these data suggest that DNMT3A R882 mutations confer functional PARP loss of function. Mechanistically, the precise interaction preventing PARP recruitment is unclear, but functionally and from an experimental therapeutic perspective, these data are provocative, perhaps lending credence away from PARP inhibition in DNMT3A-mutant setting owing to overlapping mechanistic defects. In fact, retrospective work in clonal hematopoiesis suggests that PARP inhibitors may predispose toward clonal selection, perhaps due to similar selective pressure (4). Patients previously treated with PARP inhibition for solid tumors were systematically evaluated for clonal hematopoiesis. Such therapy was associated with nearly a 2-fold increased risk of clonal hematopoiesis, specifically with mutations in genes related to DNA damage response such as PPM1D, CHEK2, and TP53. Provocatively, in this current study, DNMT3A R882-mutant cells progress through cell cycle despite the accumulation of DNA damage. It might be reasonable to suspect resultant clonal evolution with secondary mutations as observed with PARP inhibition. On the contrary, there is the competing observation that DNMT3A mutations persist in clonal hematopoiesis, often for decades without hematologic sequelae in the vast majority of such patients. It is important to note, however, that most commonly DNMT3A mutations related to clonal hematopoiesis are, in fact, not at the R882 codon and the role of DNMT3A in clonal hematopoiesis may be completely distinct from that in acute leukemia.
Figure 1.
Graphical depiction of differential DNA damage response of DNMT3A-mutant leukemia (left) and DNMT3A wild-type leukemia (right) in response to cytarabine (Ara-C). While the wild-type leukemia cells are able to recruit PARP to repair single-strand DNA breaks, the DNMT3A-mutant leukemia is unable to do so. The result is DNA replication form collapse and increased sensitivity to the chemotherapeutic agent. Adapted from an image created with BioRender.com.
The findings that even lower doses of DNA-damaging agents such as cytarabine or cladribine can be highly effective in arresting replication forks in DNMT3A-mutant cells warrants pause. Prior efforts concerning anthracycline resistance is rather distinct, with prior findings that anthracycline dose intensification strategies improve survival in patients with DNMT3A-mutant AML. In younger individuals (age <60) with AML were randomized on in two large cooperative group clinical trial (E1900 and HOVON) to receive what was the standard dose at the time (45 mg/m2) versus a dose intensified anthracycline at 90 mg/m2. The patients were not selected on the basis of any genomic features. Subsequent studies have gone on to suggest that DNMT3A-mutant patients as well as other genetic AML backgrounds may derive subtype-specific benefit (5). However, these data have been difficult to reconcile with two observations: (i) DNMT3A mutations are far more common in older individuals—thus these young patients with DNMT3A AML may not truly be reflective of all DNMT3A-mutant AML, and (ii) preclinical data have suggested DNMT3A may functionally confer anthracycline-specific resistance. Notably in these dose intensification studies, all patients received the same 7-day cytarabine continuous infusion, which Venugopal and colleagues suggest may have more of the biologically effective role. Moreover, consolidation therapy commonly consists of high-dose cytarabine, with use also in induction phase for second-line induction therapy and in primary induction failure. In essence, cytarabine has been and continues to be a pillar of present-day AML therapy.
However, a competing interest in therapeutic interventions is often complicated by advanced patient age and medical comorbidities that might prevent such high-dose therapies from being safely administered. The standard of care for older individuals with AML or patients who cannot tolerate high-dose induction chemotherapy has been supportive care alone or hypomethylating agents (HMA), either 5-azacytidine or decitabine. Despite the less myeloablative effects of these agents, HMA monotherapy results in partial or complete response in approximately 20% of patients. Compared with supportive care and low-dose cytarabine, for older individuals HMAs has a survival benefit, but only with post hoc analysis done after completion of primary analysis. These responses are rarely durable and alternative strategies in older individuals with AML have been sorely needed. This article presents compelling evidence that alterations in cytarabine dose or administration schedule aimed at maximizing effects on stalling replication forks, potentially in combination with other targeted molecules and/or HMAs, may prove useful in reaching therapeutic endpoints while reducing toxicity. Nonetheless, it is important to emphasize that standard regimen already include 7 days of continuous infusion cytarabine so it remains to be seen what are the realistic expectations of modified cytarabine dosing strategies.
Footnotes
Author’s Disclosures
No disclosures were reported.
References
- 1.Venugopal K, Feng Y, Nowialis P, Xu H, Shabashvili DE, Berntsen CM, et al. DNMT3A harboring leukemia-associated mutations directs sensitivity to DNA damage at replication forks. Clin Cancer Res 2022;28: 756–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet 2011;44: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueroa ME, Skrabanek L, Li Y, Jiemjit A, Fandy TE, Paietta E, et al. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood 2009;114:3448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton KL, Moukarzel LA, Ptashkin R, Gao T, Patel M, Caltabellotta N, et al. The impact of poly ADP ribose polymerase (PARP) inhibitors on clonal hematopoiesis. J Clin Oncol 38:15s, 2020. (suppl; abstr 1513). [Google Scholar]
- 5.Luskin MR, Lee JW, Fernandez HF, Abdel-Wahab O, Bennett JM, Ketterling RP, et al. Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood 2016;127:1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]