Abstract
Stem cells can differentiate to diverse cell types in our body, and they hold great promises in both basic research and clinical therapies. For specific stem cell types, distinctive nutritional and signaling components are required to maintain the proliferation capacity and differentiation potential in cell culture. Various vitamins play essential roles in stem cell culture to modulate cell survival, proliferation and differentiation. Besides their common nutritional functions, specific vitamins are recently shown to modulate signal transduction and epigenetics. In this article, we will first review classical vitamin functions in both somatic and stem cell cultures. We will then focus on how stem cells could be modulated by vitamins beyond their nutritional roles. We believe that a better understanding of vitamin functions will significantly benefit stem cell research, and help realize their potentials in regenerative medicine.
Keywords: Vitamin, Cell culture, Vitamin A, Vitamin B3, Vitamin C, Vitamin E, Embryogenesis, Stem cells
Introduction
Vitamins are natural organic compounds that play essential roles in normal physiological functions in minimum amounts, but the host either cannot synthesize them, or cannot produce an adequate amount to meet the normal physiological demands [1]. The word vitamin comes from the Latin word “vita” meaning “life”, which reflects its essential roles in the survival and well-being of humans [2]. Vitamins are involved in diverse cellular functions, and their deficiency often leads to serious symptoms to people, sometimes even death [3]. Since the discovery of vitamin A in 1912, 13 vitamins have been identified based on their essential roles in human health [4]. Most vitamins can be obtained through balanced food intake, and vitamin supplements are also widely used in healthcare practices. In the 1950s, people found that vitamin supplements are also essential for in vitro cell culture due to their nutritional functions [5, 6]. Recently, various vitamins are shown to possess regulatory mechanisms on the cellular level, especially in stem cells [7].
Stem cells are a special group of cells that can proliferate extensively and have the potential to generate various cell types in the human body [8]. Embryonic stem cells (ESCs) are pluripotent and can differentiate to all cell types. ESCs only transiently exist during embryogenesis, and finally give rise to all the cells in an embryo. Adult stem cells possess limited potential to differentiate to specific cell types, and can be classified into multipotent and unipotent stem cells [9]. They are responsible for the daily maintenance and repair of tissues [10]. With somatic reprogramming technologies, stem cells can now be generated from somatic cells with defined factors [11]. Stem cells are widely used in basic research to understand embryogenesis and homeostasis, to model diseases, and are also important source materials for cell therapies in regenerative medicine [12]. Most stem cell-related studies and applications involve cell culture systems, which provide essential components for specific cell types to survive and properly exert their normal functions.
A typical cell culture system normally contains ten categories of components, including water, inorganic salts, growth factors, amino acids, buffering reagent, energetic substrates, extracellular matrix, vitamins, vitamin-like organic factors and the cell culture atmosphere. Functional stem cells require a culture system in which all components are suitably balanced. To realize the great potentials of stem cells in regenerative medicine, people often modulate and optimize cell culture components to improve stem cell functions. Regulation of signal transduction pathways with growth factors has traditionally been the main approach [13, 14]. However, nutritional regulation is emerging as a viable target for stem cell modulation, which could affect not only cell survival but also pluripotency and cell fates [15–17]. As an essential part of cell culture, the important roles of vitamins are manifested in our daily use of cell culture in basic research and clinical applications. This article will try to review how vitamins are utilized in stem cell applications. We will first introduce the general vitamin requirements in cell culture. Then we will focus on vitamin A, vitamin B3, vitamin C and vitamin E, and discuss how they are utilized in stem cell applications [18–21].
A brief background on vitamins in the human body
Human vitamins are generally categorized into two classes, nine water-soluble vitamins and four fat-soluble vitamins (Table 1) [22]. Water-soluble vitamins include 8 members of the B type vitamins and vitamin C, and fat-soluble vitamins include vitamins A, D, E and K. All the vitamins can be obtained from food to fulfill the nutritional needs (Table 1). Some vitamins can be synthesized in the human body, but at a very low rate (Table 2) [23, 24]. In this review, we will briefly summarize some key vitamin-dependent processes and the role these vitamins play in stem cell biology.
Table 1.
| Vitamin | Names | Daily dose | Cellular function |
|---|---|---|---|
| Vitamin B1 | Thiamine | 1.2 mg | Glycolysis, non-oxidative phase of pentose pathway |
| Vitamin B2 | Riboflavin | 1.2 mg | Coenzyme in carbohydrate and lipid metabolism; activation of B6 and B9; antioxidant |
| Vitamin B3 | Niacin, nicotinamide, nicotinamide riboside | 15 mg | Coenzyme in carbohydrate, amino acid and lipid metabolism |
| Vitamin B5 | Pantothenic acid | 5 mg | Coenzyme in carbohydrate and lipid metabolism, lipid biosynthesis |
| Vitamin B6 | Pyridoxine, pyridoxamine, pyridoxal | 1.5 mg | Coenzyme in glycogenolysis and amino acid metabolism |
| Vitamin B7 | Biotin | 30 µg | Lipid synthesis; leucine catabolism; conversion of amino acids and propionate to glucose in liver; gluconeogenesis |
| Vitamin B9 | Folic Acid | 400 µg | Coenzyme in nucleotide synthesis, methylation of chromatin, DNA, RNA, histone and transcription factors, amino acid metabolism |
| Vitamin B12 | Cobalamin | 2.4 µg | Coenzyme in folate and homocysteine metabolism |
| Vitamin C | Ascorbic acid | 85 mg | Antioxidant; coenzyme in collagen synthesis |
| Vitamin A | Retinoic acid, retinol, all-trans-RA | 800 µg | Vision, cell differentiation, reproduction |
| Vitamin D | Cholecalciferol, ergocalciferol | 15 µg | Mg, Ca and P absorption |
| Vitamin E | Tocopherols, tocotrienols | 15 mg | Antioxidant, cell membrane integrity |
| Vitamin K | Phylloquinones, menaquinones | 115 µg | Protein synthesis in blood coagulation |
Table 2.
| Vitamin | Concentration in blood | Concentration in DMEM/F12 | Endogenous source | Application in stem cells |
|---|---|---|---|---|
| Vitamin B1 | 66–200 nM | 6.44 µM | Gut bacteria | In all the base medium; used for somatic and stem cell culture |
| Vitamin B2 | 174–471 nM | 0.58 µM | Colon bacteria | In all the base medium; used for somatic and stem cell culture |
| Vitamin B3 | 81–213 nM | 16.6 µM |
Biosynthesis from tryptophan Colon bacteria |
In all the base medium; used for somatic and stem cell culture |
| Vitamin B5 | 0.5–1.9 µM | 4.7 µM | Colon bacteria | In all the base medium; used for somatic and stem cell culture |
| Vitamin B6 | 15–73 nM | 9.8 µM | Colon bacteria | In all the base medium; used for somatic and stem cell culture |
| Vitamin B7 |
> 400 ng/L 1.64 nM |
14.3 nM | Hindgut bacteria | In most base medium, such as BME, α-MEM, Ham’s F12 and DMEM/F12; Used for somatic and stem cell culture |
| Vitamin B9 | > 3.0 nM | 6 µM | Gut bacteria | In all the base medium; used for somatic and stem cell culture |
| Vitamin B12 | 118–716 pM | 50 nM | Gut bacteria; not clear whether B12 can across colon | In most base medium, such as α-MEM, Ham’s F12 and DMEM/F12; Used for somatic and stem cell culture |
| Vitamin A | 1.4–3.2 µM | – | – | Retinol promotes self-renewal and pluripotency; Retinoic acid mainly drives cell differentiation by modulating epigenetics |
| Vitamin C | 25–85 µM | – | In kidney and liver (except high primates) | Supports cell reprogramming, survival and collagen production; Reduces ROS |
| Vitamin D | 30–100 μg/L | – | Precursor synthesized in the sebaceous glands of the skin | Leukocyte production and differentiation |
| Vitamin E | 20–35 µM | – | – | Component of B-27 supplement and chemically defined lipid concentrate; Protects stem cells and progenitor cells against oxidative stress; Affects ESC differentiation through ROS levels |
| Vitamin K | 0.22–2.22 nM | – | Menaquinones synthesized by bacteria in the large intestine | Promotes the differentiation of dental pulp stem cells (DPSCs) to osteoblast in vitro |
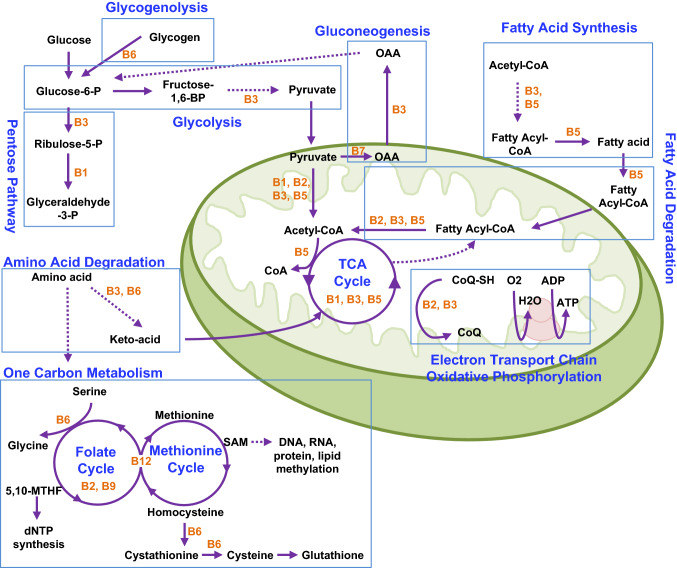
All the water-soluble vitamins are coenzymes for important metabolic enzymes that are essential for cellular functions. Their essential roles in metabolic pathways are illustrated in Fig. 1. Vitamins B1, B3, B6 and B7 are involved in glucose metabolism that includes glycolysis, pentose pathway, glycogenolysis and gluconeogenesis. Fatty acid synthesis and degradation require vitamins B2, B3 and B5. Meanwhile, amino acid degradation requires vitamins B3, B6, B9 and B12. The TCA cycle and oxidative phosphorylation take place in mitochondria, and utilize vitamins B1, B2, B3, B5 and B7 in specific steps. Often times, multiple vitamins are involved in the same metabolic process. For example, When acetyl-CoA is generated from pyruvate by pyruvate dehydrogenase, four of the five coenzymes involved in this step are vitamins, including vitamins B1, B2, B3 and B5 [25, 26]. Any deficiency in these vitamins could lead to malfunction of the TCA cycle.
Fig. 1.
Vitamin B in metabolism. B vitamins are essential for the major metabolic pathways. Specific vitamins (orange fonts) are highlighted in multiple metabolic processes (blue fonts). OAA, oxaloacetic acid
Besides type B vitamins, other vitamins’ functions are more diverse. Vitamin C is the only water-soluble vitamin that does not belong to the vitamin B family, and it is known to regulate collagen synthesis by acting as a cofactor for prolyl hydroxylases, reducing its iron center [27–29]. In addition, vitamin C is an antioxidant that suppresses the production of reactive oxygen species (ROS). It is well known for its role in the prevention of scurvy [30, 31]. Vitamin A family members have distinctive functions, including the prevention of night blindness. At the molecular level, vitamin A functions through antioxidation and transcriptional regulation [32, 33]. Vitamin D is a hormone that binds to nuclear receptors to regulate transcription, and it is best known for its role in calcium absorption [34]. Vitamin E is a potent fat-soluble antioxidant. Some vitamin E isoforms were also reported to modulate signal transduction [35]. Vitamin K is a cofactor for γ-glutamyl carboxylase that is essential for blood clotting [36, 37].
Essential vitamins in regular cell culture
Because of vitamins’ important functions, they are essential not only for the whole organism but also for individual cells. However, the vitamin dependency of the human body is often different from cells in culture media. The importance of individual vitamins is gradually discovered through the years. In 1950, Morgan and colleagues first showed that cell survival was improved by a vitamin mixture in serum-free synthetic medium [38]. In 1955, Eagle systematically analyzed the impact of individual vitamins on the growth of both mouse fibroblasts and Hela cells [39]. Six vitamins were shown essential for cell proliferation of both cell lines. They all belong to the B vitamin complex, including B1, B2, B3, B5, B6 and B9. The medium was named Basal Medium Eagle (BME), which is the first synthetic medium with defined vitamin functions. In the following years, Minimum Essential Medium (MEM) and Dulbecco’s Modified MEM (DMEM) were developed with increased amino acid or vitamin concentrations, but the vitamin composition still remained at six [40, 41].
Although the above basic media can support short-term proliferation of a few cell lines, their capacity is insufficient for many other lines in long-term culture. To support clonal growth and long-term culture of Chinese hamster cell lines, Ham developed the Ham’s F-10 and F-12 media that contain additional B7 and B12 [42, 43]. B7 was important for the cell growth and viability of a variety of cell types [44], while vitamin B12 was found to be essential for lipid metabolism [45, 46]. People later found that cell growth is improved when DMEM and Ham’s F12 are mixed in a 1:1 ratio, and this medium was named DMEM/F12 [47]. The eight B vitamins (B1, B2, B3, B5, B6, B7, B9 and B12) in DMEM/F12 are also present in a variety of other basic media such as RMPI, IMDM and α-MEM [48]. These vitamins are generally considered as essential vitamins for most cells cultured in vitro. DMEM/F12 is the most commonly used base medium for human embryonic stem cells [17, 49–51], so we will use DMEM/F12 as a reference to discuss vitamin formula and concentration effect in this review.
When comparing vitamin composition in blood and in DMEM/F12, there are two obvious discrepancies (Table 2). First, all B vitamins are present in DMEM/F12, but not other vitamins including vitamins A, C, D, E and K. Considering all B vitamins are coenzymes for essential metabolic processes, it is understandable that they are required for cell culture. It also implies that the non-B vitamins are probably not required for most cell types. It is also possible that those vitamins could be provided through serum or medium supplements such as B27. Second, all individual vitamins are provided at significantly higher concentrations in DMEM/F12 than in blood (Table 2). It indicates that cells in culture have differential reliance on vitamins.
Vitamin-like nutrients for cell culture
In addition to essential vitamins, DMEM/F12 and many other basic media also contain minute amount of some organic compounds that are required to be supplemented to the organism from food sources (Table 3). We will brief some of them here.
Table 3.
| Names | Solubility | Function | Endogenous source |
|---|---|---|---|
| Choline | Hydrophilic | Lipid transport and metabolism, neurotransmission, methyl group donor | Choline de novo synthesized through S-adenosylmethionine (SAM)-dependent methylation of phosphatidylethanolamine by phosphatidylethanolamine N-methyltransferase (PEMT). This process occurs mostly in liver |
| myo-inositol, Inositol | Hydrophilic | Signal transduction and osmoregulation | Inositol can be de novo synthesized from glucose, and the biosynthesis occurs in brain, liver, and kidney |
Choline can be biosynthesized from serine [52], and it was first demonstrated as essential for cell survival and proliferation in Eagle’s original vitamin study [39]. Choline is essential for the generation of phosphatidylcholine (PC) that is crucial for lipid transport and plasma membrane integrity. Choline is also used to generate acetylcholine that is important for neurotransmission [39, 53]. Choline can serve as methyl donor in one-carbon transfer pathways, and contribute to DNA modulation and histone epigenetic modification with the help of vitamins B9 and B12 [54].
Inositol can be naturally synthesized by the human body from glucose in many tissues [55, 56], and Myo-inositol was also identified by Eagle as an essential factor for cell survival and proliferation in a wide variety of human cells, both malignant and nonmalignant [57]. Myo-inositol is the main source of phosphatidylinositol that mediates cell signal transduction, neurotransmission and osmoregulation [58, 59].
Besides choline and inositol, essential fatty acids, such as omega (ω)-3 and 6, are often found in culture media. They are metabolized to form eicosanoids that affect lipid homeostatic processes as well as the inflammatory response [60–63]. These lipids usually bind to albumin, and can be supplemented to cells through albumin without notice.
Vitamin dependence in cell culture
Cells in culture, including somatic and stem cells, have different vitamin dependency in comparison with the human body as a whole. We believe that such difference is caused by the inherent difference between the human body and artificial cell culture systems. First, not all vitamins that are needed for the human body will be essential for cell culture. The deficiency of some vitamins often affects just one or a few specific organs in the human body. For example, vitamin K deficiency usually affects blood clotting but no other physiological functions [37, 64]. When it comes to cell culture, a vitamin may not be required for general cell culture if it is needed for the survival and proliferation of a specific cell type. Second, the human body usually has specific organs to produce and store vitamins, which allows people to tolerate temporary vitamin deficiency. However, there is no endogenous backup mechanisms to complement vitamin needs in cell culture, and all essential vitamins have to be provided. If an essential vitamin is not provided in culture, severe symptoms often emerge quickly in cells. For this reason, cell culture platforms have led to novel discoveries of vitamin functions in recent years. Third, some nutrients are not essential for the body, because specific organs can produce sufficient amounts for all the cells in the body. However, in cell culture, these nutrients are considered vitamin-like for cell culture, because they have to be provided for normal cellular functions in the medium. Fourth, cell culture is an artificial system, and nutrient concentrations in cell culture can be modulated as needed. Often times, nutrients can be tested and studied at concentrations that do not exist in physiological conditions. Some novel vitamin-dependent phenomena could only be identified in cell culture, in artificial conditions.
Differential vitamin dependence exists not only between individual cells and the whole organism, but also among different cell types. Vitamins affect metabolism similarly in both somatic and stem cells, but they could have additional impacts on stem cells. In somatic cells, modulation of specific vitamins will not change the cell identity. However, such changes might lead to loss of stemness or cell fate changes in stem cells. A few vitamins have gathered intensive interest in stem cell applications, and we will discuss them in more details here.
Vitamin A
Vitamin A was the first vitamin discovered, and is actually a group of compounds also known as retinoids, including retinol, retinal and retinoic acid (RA) (Fig. 2). Vitamin A compounds are usually found in food of animal origin, while their precursor, carotenoid, is present in plants. Humans can synthesize vitamin A from carotenoids such as β-carotenes, a lipid-soluble pigment responsible for the vivid colors in plants. β-Carotenes can be converted into two retinals by β-carotene 15,15′-deoxygenase [65]. Retinal is then reduced to retinol by retinaldehyde reductase, using NAPDH (vitamin B3) as a cofactor. Retinol either is esterified by acyltransferases LRAT (lecithin-retinol acyltransferase) and ARAT (retinol acetyltransferase) into retinyl palmitate for storage, or is oxidized into retinoic acid by aldehyde dehydrogenase (ALDH) [66]. In human cells, retinal and retinol are interconvertible; however, the conversion to retinoic acid is irreversible [67].
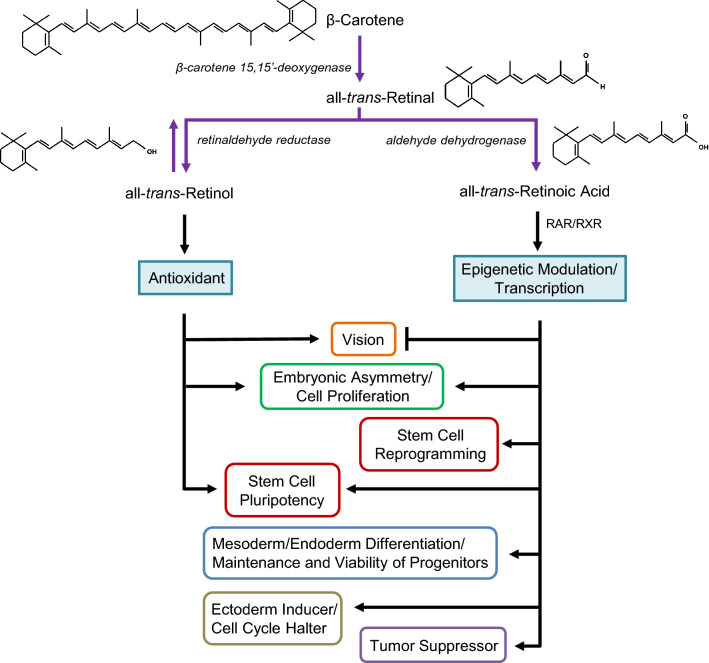
Fig. 2.
Vitamin A metabolism and function. Vitamin A is a group of compounds derived from β-carotene. In humans, its alcohol isoform has been reported to be beneficial for visual health. It also acts as an antioxidant and promotes the pluripotency of stem cells. The acid form, all-trans-retinoic acid, can interfere with the effect of retinol in vision. Retinoic acid is a determinant for embryonic development, and promotes cell proliferation. It can also promote reprogramming of stem cells and differentiation of progenitors and act as a tumor suppressor
Although belonging to the same family, retinal, retinol and retinoic acid play quite different roles in the human body. For example, retinal is incorporated into the light sensitive receptor rhodopsin in the retina, and prevents night blindness. In contrast, retinoic acid can cause night blindness by suppressing retinal production through the transcriptional inhibition of ocular retinol dehydrogenases [33].
Vitamin A family members also play distinctive roles in embryogenesis and stem cells in culture. Retinol and retinal are readily oxidized in culture, so they can act as antioxidants to promote cell survival and growth [32, 68]. Retinol has been reported to help maintain the pluripotency and self-renewal of hESCs [69], mESCs [70] and other progenitor cells [71–73]. In contrast, retinoic acid is a strong cell fate modulator, which will be discussed in more detail below.
As a lipid-soluble compound, retinoic acid can diffuse into the cytoplasm, bind to its nuclear receptor, and initiate nuclear translocation and downstream regulation. Retinoic acid initiates the dimerization of retinoic acid receptor (RAR) and retinoid X receptor (RXR). The heterodimer then either directly regulates gene expression through a DNA response element, or indirectly modulates transcription through intermediate transcription factors [74]. Over 500 genes are influenced by the action of retinoic acid, and many of the genes are involved in stem cell differentiation and metabolism [74]. It was shown to be an inducer that initiates differentiation in ESCs, and also a modulator in lineage specific differentiation [75].
Retinoic acid modulates stem cell pluripotency and differentiation through the expression of mRNA and microRNA [21, 76]. It alters the expression of genes involved in DNA methylation, histone acetylation and histone methylation. In hESCs, the average level of DNA methylation is increased by RA, promoting stem cell differentiation [77]. RA also affect histone modifications, including acetylation of H3, H4 and H3K in hESCs and mESCs, which leads to stem cell differentiation [78, 79]. RA suppresses methylation in H3K27 while promoting methylation in H3K4 in mESCs and neuroblastoma, both stimulating cell differentiation [78]. At the same time, retinoic acid targets genes in metabolism, cell proliferation and pluripotency. It usually suppresses pluripotency gene expression, and promotes ectodermal differentiation in ESCs upon the exit of self-renewal [21, 80, 81]. Retinoic acid is used to promote neural differentiation through MAPK and integrin pathways [82].
Retinoic acid’s roles during embryogenesis has been well documented. Retinoic acid promotes the expression of genes involved in the development of central nervous system, embryonal circulatory as well as heart asymmetry [83]. Vitamin A-deficient embryos presented various congenital malformations, such as absence of eyes as well as deficiencies in the central nervous system, skin, lungs and heart [84–86].
Retinoic acid also plays critical roles in cell fate determination in later stage of embryogenesis. For example, in heart development, retinoic acid is involved in cardiac differentiation. It modulates vascularization by suppressing the gene expression of N-cadherin, Msx1 and TGFβ pathways; It affects heart asymmetry through the inhibition of Nodal, Snail and Pitx2 genes; It also promotes cell proliferation and enhances BMP2 pathway by affecting the cardiogenesis transcription factor GATA4 [87–94]. Based on retinoic acid’s function in embryogenesis, it has been used to generate atrial cardiomyocytes [20]. In hematopoiesis, retinoic acid enhances the ex vivo maintenance and viability of transplantable hematopoietic stem cells [95]. Retinoic acid suppresses the proliferation of dormant hematopoietic stem cells (HSCs), and prevents HSC differentiation to downstream cell types [96, 97]. As a result, retinoic acid helps maintain the multipotency of HSCs, being enriched in these cells compared to other multipotent progenitors [97–99]. Furthermore, retinoic acid is also involved in germline differentiation. Due to its interaction with BMP and NOTCH pathways, retinoic acid’s targets are involved in four main developmental stages of fetal germ cell development [82, 93, 100]. Retinoic acid increases the expression of germline markers VASA, SCP3, TEKT1 and GDF9 [101], and promotes the generation of tailed male gamete-like cells that could generate offspring in mice [102].
Enzymes involved in retinoid acid production play essential roles in embryogenesis. The oxidation of retinol to retinal is the rate-limiting step in RA production, and the enzymes RDH10 (short-chain dehydrogenase in charge of the second oxidation of retinol) and DHRS3 (short-chain dehydrogenase reductase in charge of reducing retinal to retinol) are key in this process. Knockouts of these enzymes result in developmental defects in craniofacial, heart and limb patterning. RDH10-K.O. is lethal between E10.5 and E14.5, and DHRS3-K.O. is lethal between E17.5 and E18.5 [103–105]. Retinaldehyde dehydrogenase, which facilitates the generation of retinoic acid from all-trans retinal, is a key enzyme involved in cell fate determination [20, 66].
Although retinoic acid leads to ESC differentiation, it is also paradoxically a potent promoter for somatic reprogramming. Somatic cells can be reprogrammed to induced pluripotent stem cells (iPSCs) by the overexpression of transcription factors, such as OCT4, KLF4, MYC and SOX2 [11, 106, 107]. The activation of retinoic acid pathway accelerates reprogramming, while its removal suppresses reprogramming efficiency [108, 109]. The activation of retinoic acid pathway is essential component in chemically induced reprogramming without overexpressing transcription factors [110, 111]. Short-term treatment with retinoic acid is reported to promote pluripotency of iPSCs by inhibiting the canonical Wnt pathway, while positively modulating AKT/mTOR signaling [112]. Additionally, retinol and RA promote the transcription of Ten-eleven translocation (Tet) proteins in naïve pluripotent stem cells, and the regulation of Tet proteins by vitamin A is independent of vitamin C, a known modulator of enzymatic activities (see more discussions in “Vitamin C” section) [113]. In addition, retinoic acid signaling is found to maintain the dormancy of HSCs through cell cycle regulation [97].
Vitamin B3
Similar to vitamin A, vitamin B3 is also a family of compounds including niacin (nicotinic acid), nicotinamide (NAM) and nicotinamide riboside (NR). They are precursors of nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) that serve as cofactors or substrates in a wide range of metabolic reactions [114, 115], so they are implicated in all metabolic processes that utilize NAD or NADP (Fig. 1). Because of NAD’s importance in metabolism, there are both de novo and salvage pathways for NAD synthesis from niacin, nicotinamide and NR (Fig. 3). Nicotinamide is usually maintained at around 100–200 nM range in blood, while 16.6 μM nicotinamide is supplied in DMEM/F12, which is sufficient to sustain nutritional requirement of cells in vitro (Table 2).
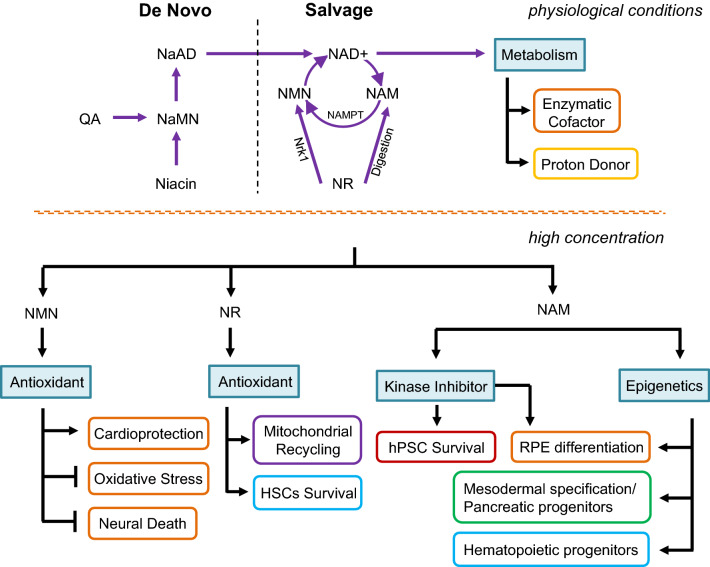
Fig. 3.
Vitamin B3 function in metabolism and signal transduction. NAD+ is synthesized in humans by de novo and salvage pathways. In the salvage pathway, the enzyme nicotinamide phosphoribosyltransferase (NAMPT) converts NAM (nicotinamide) to NMN (nicotinamide mononucleotide), which is then metabolized to NAD+. NAMPT is the rate-limiting step in this process and is a crucial factor to maintain NAD+ levels. Nrk1 can directly phosphorylate NR (nicotinamide riboside) to NMN, bypassing NAMPT, and NR can also be digested into NAM. In the de novo pathway, QA (quinolinic acid) and niacin are metabolized into NaMN (nicotinic acid mononucleotide) which can be further catalyzed into NaAD (nicotinic acid adenine dinucleotide). At high concentration, NAM functions as inhibitors in sirtuin, PARP and kinase pathways
Nicotinamide has been utilized in clinical applications (Table 4). Nicotinamide ameliorates age-related macular degeneration phenotypes [116]. It prevents hepatosteatosis in obese mice while improving glucose metabolism and increasing health span in mice [117]. These therapeutic effects imply that nicotinamide could be involved in functions beyond nutritional regulation.
Table 4.
| Conditions | Dose of nicotinamide |
|---|---|
| Acne | 750 mg/day |
| Vitamin B3 deficiency (Pellagra) | 300–500 mg/day |
| Diabetes | 1.2 g/mL/day |
| Hyperphosphatemia | 0.5–1.75 g/day |
| Larynx cancer | 60 mg/kg of niacinamide/h before inhaling carbogen |
| Skin cancer (other than melanoma) | 0.5 g niacinamide/day |
| Osteoarthritis | 3 g/day |
Compared to regular culture for somatic cells, a higher concentration of nicotinamide (5–10 mM) are often used in stem cell manipulations [19]. Nicotinamide in medium can easily cross plasma membrane and translocate into cytoplasm [19]. Nicotinamide was reported to promote cell survival of hESCs. In differentiation, it promotes cardiomyocyte differentiation, and facilitates the generation of endocrine pancreatic cells [118, 119]. Nicotinamide is also used in the maintenance of somatic stem cells [120], as well as organoid culture of different cell types [121–123]. It is used in the expansion of hematopoietic progenitors [124].
Nicotinamide is involved in various stem cell applications, but its exact molecular mechanism in each process is still unclear. At high concentration, nicotinamide can inhibit the activities of sirtuins, a family of protein deacetylases that regulate epigenetic modification and potential cell fates [125]. Nicotinamide is used to enrich CD34+ hematopoietic progenitors as a SIRT1 specific inhibitor [124]. At the same time, nicotinamide is also an inhibitor of poly(ADP-ribose) polymerase (PARP) that is involved in cell death [126, 127]. It is thought to improve cell survival by inhibiting apoptosis.
Recently, nicotinamide was identified as a kinase inhibitor at high concentration (millimolar range) [19]. Nicotinamide targets multiple kinases that are involved in cell survival and pluripotency. It binds and inhibits ROCK kinases, and it suppresses cell death caused by ROCK activation after cell individualization. Nicotinamide is also an inhibitor of casein kinase 1 (CK1). The inhibition of CK1 leads to the exit of self-renewal, and also promotes differentiation towards retinal pigment epithelium [19]. It is foreseeable that nicotinamide could be involved in additional stem cell regulations as a modulator in sirtuin, PARP and kinase pathways.
The concentration-dependent phenomena also exist in some other nicotinamide derivatives, such as nicotinamide mononucleotide (NMN) and NR. Recent studies show that NMN and NR have functions beyond NAD synthesis. With elevated concentration, NMN reverses vascular dysfunction and oxidative stress, and promotes cardioprotection via glycolysis and acidic pH [128, 129]. NMN also protects against cognitive impairment and neuronal death induced by the inhibition of long-term potentiation (LTP) after Aβ1–42 oligomer treatment [130]. NR at elevated concentration increases mitochondrial recycling and cell survival in hematopoietic stem cells [131]. It also prevents aging, and extends life span [132]. It is intriguing why nicotinamide derivatives have such concentration-dependent effect. It would be interesting to explore potential connections in these biological processes.
Vitamin C
Vitamin C, or l-ascorbic acid (AA/LAA), is soluble in water due to its sugar-like structure. Although ascorbic acid is found at equal amounts in both isomeric states, l and d-ascorbic acid, only LAA is chemically active. Ascorbic acid can be synthesized in plants and the majority of animals (Fig. 4, adapted from Linster’s and Schaftingen’s review) [133]. In vertebrates, the last step of ascorbic acid biosynthesis from glucose is the formation of 2-keto-gulonolactone which spontaneously enolizes into ascorbic acid. The enzyme for this step, l-gulonolactone oxidase, is found inactive in high primates, including humans, so human beings have to take vitamin C from food sources [133, 134]. LAA is not stable in nature due to its hydrogen ion, and acidic pH will increase its stability. When exposed to light, it gets oxidized to dehydroascorbic acid (DHA) [135]. In practice, more stable LAA derivatives are used in cell culture, such as magnesium ascorbyl phosphate (MAP) and ascorbyl 6 palmitate (AA6P) [136–138].
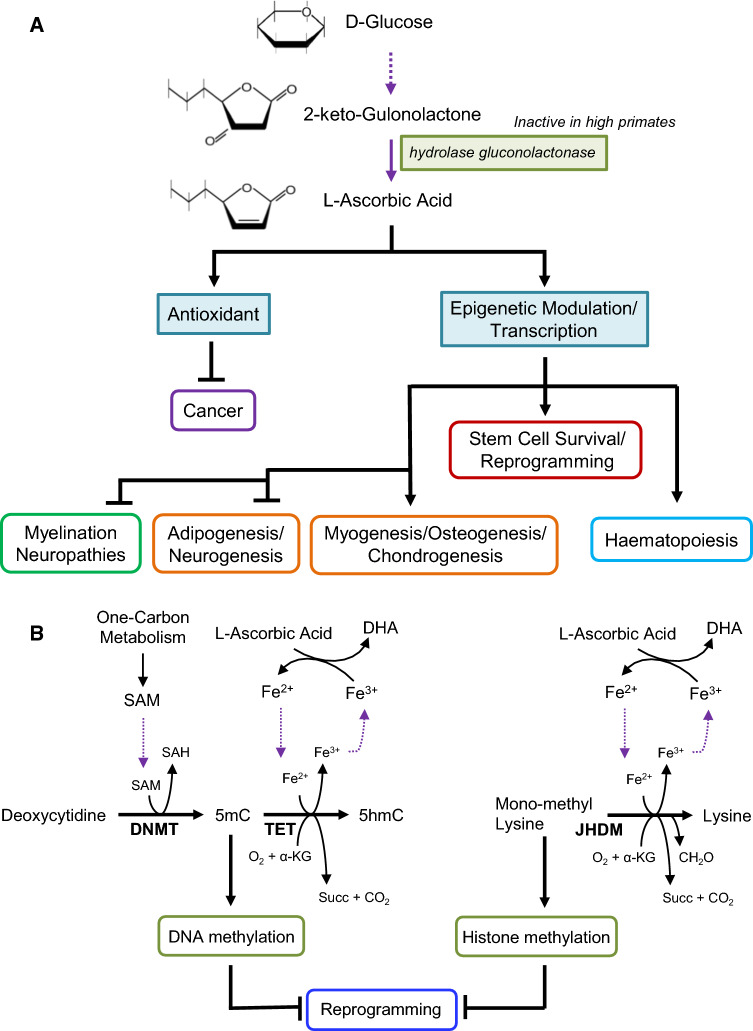
Fig. 4.
a Vitamin C regulation in stem cells. Vitamin C, commonly referring to as l-ascorbic acid, cannot be synthesized by humans due to the lack of the hydrolase gluconolactonase enzyme. Similar to vitamin A, it acts as an antioxidant and tumor suppressor, and increases the myelination of neurons. Its effect on chromatin remodeling and other epigenetics marks allows it to affect reprogramming of pluripotent stem cells, but it is also necessary for the culture of both embryonic and mesenchymal stem cells. It promotes hematopoiesis differentiation and promotes mesoderm lineages including cardiomyocytes, bone and cartilage. The effect of vitamin C on adipocyte differentiation depends on the platform and concentration. b Vitamin C regulation of reprogramming. l-Ascorbic acid facilitates the reaction that converts ferric ions (Fe3+) to ferrous ions (Fe2+). Fe2+ is required for the activity of both Tet proteins and JHDM histone demethylases, and it is oxidated into Fe3+ when α-KG and O2 are converted into succinate and CO2 during DNA and histone demethylation
Vitamin C is a potent antioxidant and reduces reactive oxygen species (ROS), and it participates in various biological processes [139]. In addition, vitamin C acts as a kinase inhibitor. When it is oxidized into dehydroascorbic acid (DHA), it inhibits IκBα Kinase β and modulates NF-κB signaling [140, 141]. Vitamin C also reduces ferric to ferrous iron, and increases its absorption in the intestine [142].
High doses of vitamin C can actually promote an oxidative state in cancer cells, acting as a potential anti-cancer therapy [143–145]. It is proposed that the anti-cancer effect may be due to induction of ferroptosis, a form of programmed cell death related to vitamin E deficiency and lipid peroxidation [146–148]. High doses of ascorbic acid was reported to regress Charcot-Marie-Tooth disease in mice, a neuropathy with impairment in the myelination of peripheral nerves, due to the myelination effect of ascorbic acid [149–152]. The lack of Vitamin C is the trigger of a well-known avitaminosis called scurvy, which if prolonged in time can be fatal due to hemorrhages and impaired wound healing [31].
Vitamin C plays critical roles in promoting PSC survival and derivation. When hESCs are transitioned from mTeSR medium to albumin-free and more defined condition, cells die in the absence of vitamin C after a few days [50]. At the same time, vitamin C also regulates the homeostasis of the extracellular matrix [18]. It affects the folding and deposition of collagen proteins, which may have contributed to its effect on hESC attachment and survival [27, 50, 153]. During reprogramming, ascorbic acid promotes reprogramming in human and mouse cells [50, 154]. Vitamin C reduces cell senescence during reprogramming by suppressing p53 [155, 156]. It was shown to act through a mechanism independent from its antioxidant role, and accelerates transcriptional changes during reprogramming [154, 157]. Vitamin C also influences cell survival in reprogramming through epigenetic modulation. It is a cofactor for polyhydroxylates and demethylases [158], and promotes demethylase activity on shore CpG islands involved in tissue-specific DNA methylation and reprogramming [159, 160].
Besides its use for the maintenance of pluripotent stem cells, vitamin C also impacts the differentiation of multiple cell lineages. Vitamin C triggers mesoderm differentiation of mouse embryonic stem cells [161]. It promoted myogenesis and osteogenesis, and inhibited adipogenesis. Vitamin C inhibits neurogenesis to favor myogenesis through the activation of the p38 MAPK/CREB pathway and chromatin remodeling [161, 162]. It also promotes cardiac differentiation and increases the proliferation of cardiac progenitor cells by enhancing collagen synthesis [163].
In addition to ESCs, vitamin C also regulates mesenchymal stem cell growth and differentiation [164–166]. It suppresses hypoxia inducible factor 1 (HIF1α) activity through two parallel pathways. Vitamin C suppresses HIFα transcription, while activating HIF1α hydroxylase to breakdown HIF1α. Inhibition of HIF1α leads to mitochondrial activation, affecting cell proliferation and metabolism [167]. MSCs cultured with vitamin C show upregulation of Oct4 and Sox2, without affecting the expression of MSC markers such as CD105 and CD13 [168, 169]. Vitamin C in combination with TGFβ treatment was shown to promote MSC differentiation toward vascular smooth muscle cell types [170, 171]. Vitamin C also facilitates osteogenic differentiation by increasing collagen secretion, since it is used as a cofactor for enzymes that hydroxylate proline and lysine in pro-collagen [171–174]. Vitamin C also enhances chondrogenic differentiation [175], and protects chondrocytes from oxidative stress due to hydrogen peroxide (H2O2) [176].
Vitamin C is also beneficial to hematopoietic differentiation and it has been used to promote the maturation of T cells and NK cells from HSC-derived progenitors [177, 178]. Ascorbic acid is used to generate hematopoietic stem cell progenitors (hemangioblasts) from hESCs [179]. Ascorbic acid concentration is high in human and mouse hematopoietic stem cells (HSCs), and declines upon differentiation. With the accumulation of intracellular ascorbic acid, HSC frequency is limited, while leukemogenesis is suppressed [180, 181].
Besides its antioxidant activity, vitamin C mainly acts as an enzyme cofactor for the demethylation of DNA and histone in stem cells (Fig. 4b). Changes in DNA and histone methylation are often associated with stem cell differentiation and reprogramming [182–184]. The methylation on the fifth position of the pyrimidine ring of cytosine (5mC) is the most common DNA modification, and its demethylation to 5-hydroxymethylcytosine (5hmC) is catalyzed by Tet proteins [185–187]. On the other hand, histone demethylation is carried out by histone demethylases such as the Jumonji-C domain-containing family (JHDMs) [184, 188, 189]. Both Tet and JHDM proteins are vitamin C-dependent, Fe2+/alpha-ketoglutarate-dependent hydroxylases (Fe2+/α-KGDDs). During demethylation, Fe2+/α-KGDD catalyzes the reaction that converts α-ketoglutarate (α-KG) and O2 into succinate and CO2. Fe2+/α-KGDD activity requires Fe2+ that is oxidized to Fe3+ in the process [148, 181, 190–192]. Vitamin C reduces Fe3+ back to Fe2+ which could then be utilized by Tet or JHDM in demethylation again, while vitamin C itself is oxidized into dehydroascorbic acid (DHA) [113, 193]. Vitamin C influences the biological outcome of Tet-mediated DNA demethylation, and promotes the demethylation of histones such as H3, H3K9, H3K36 and H3K27 [194]. Collectively, vitamin C enhances the efficiency of somatic programming [154]. In addition, vitamin C also impacts stem cell differentiation. Vitamin C improves HSC differentiation by modulating Tet activity [180, 181], and it also increases the expression of key genes in dopaminergic neurons in the fetal brain [195], as well as trophectoderm genes like Cdx2, Eomes and Elf2 in the differentiation of mouse embryonic stem cells [196].
Vitamin E
Since the discovery of α-tocopherol in 1922 [197], vitamin E has been extensively studied and become one of the most commonly consumed vitamins. There are eight known natural isoforms of vitamin E, including four tocopherols and four tocotrienols, each designated as α, β, γ and δ based on the position of methyl groups on the chromanol ring [198–200]. Vitamin E exists in almost all the tissues in the human body, with highest levels in the adipose tissue and adrenal gland [200]. Early studies on vitamin E mostly focused on α-tocopherol, the most abundant vitamin E isoform [200]. Compared to the other isoforms, α-tocopherol has higher bioavailability and longer retention time, due to its preferential incorporation into lipoproteins by alpha-tocopherol transfer protein (α-TTP) in the liver [199, 201]. It is also the isoform commonly provided in dietary supplements [199]. In recent years, non-α-tocopherols have received increasing attention, and the tocotrienols are reported to be superior over tocopherols in many clinical applications [201–203]. Synthetic forms of vitamin E and its chemically modified analogs, such as trolox [204], tocoflexol [205] and esters of vitamin E [206–209] are also widely used for improved bioavailability and stability.
Vitamin E is a lipid soluble, chain-breaking antioxidant, capable of neutralizing free radicals and terminating chain reactions in the oxidation of polyunsaturated fatty acids. It is one of the major antioxidants in the human plasma [210]. Due to its lipid solubility, vitamin E effectively protects against oxidative damage from lipid peroxidation in the membrane as well as in lipid vesicles, but is less effective against damage from aqueous free peroxyl radicals [210, 211].
In addition to its antioxidant role, vitamin E also modulates cellular signal transduction through kinases, phosphatases, lipid mediators and transcription factors [35, 212]. α-Tocopherol inhibits protein kinase C (PKC), while other vitamin E isoforms were reported to have no influence or opposing effect [213–215]. Regulation of PKC by vitamin E leads to changes in cell proliferation, adhesion, gene expression and downstream signal transduction [35, 213, 216, 217]. Another important target of vitamin E is protein kinase B (PKB/AKT), which plays a key role in cell survival. Vitamin E may activate or inhibit PI3K/AKT pathway and cell survival in a cell type-specific manner [218–221]. Other signaling pathways regulated by vitamin E include ERK [219], p38 MAPK [222] and Wnt signaling [223]. Due to its influence on membrane composition, vitamin E can not only directly or indirectly activate/inhibit its targets, but also change specific structural features of the plasma membrane (such as lipid rafts), which may be involved in the membrane translocation or activation of signaling molecules [212].
Vitamin E was frequently used in primary cell culture to prevent cell death and preserve cell function after exposure to stress conditions, and both antioxidant and signal transduction modulating mechanisms may be involved. For example, vitamin E treatment during enzymatic dissociation protected rat mammary epithelial cells against oxidative damage and improved survival [224]. γ-Tocotrienol was reported to enhance AKT phosphorylation in intestinal tissue following total body irradiation, thereby protecting the tissue against damage by radiation [221]. Low micromolar concentrations of α-tocopherol suppressed the rise of metalloproteinase 1 (MMP-1) expression in UVA-irradiated fibroblasts, suggesting a photoprotective effect [225]. In an endothelial cell model for type I diabetes, 20 mg/L α-tocopherol showed protective effects against endothelial dysfunction caused by hyperglycemia [226]. In some studies, high concentrations (200–2500 µmol/L) of vitamin E were used for cell culture [227–229], far exceeding the reported plasma vitamin E levels ranging from 15 to 27 µmol/L [230–233].
Commercial cell culture supplements containing vitamin E are available. The B-27 supplement is widely used for neuronal cell culture [234, 235], and chemically defined lipid concentrate is used to support mammalian and insect cell culture in place of fetal bovine serum [236]. The isoform of vitamin E supplied in these supplements are α-tocopherol or α-tocopherol acetate in low micromolar concentrations.
As a potent antioxidant, vitamin E was reported to be protective for stem cells and progenitor cells which are sensitive to oxidative stress. Treatment with α-tocopherol protected mesenchymal stem cells (MSCs) against H2O2-induced apoptosis and promoted MSC survival via the AKT pathway [220, 237]. Similarly, trolox was reported to enhance the proliferation of human dental pulp stem cells under oxygen tension [238]. α-tocopherol also promoted the survival of cultured human neural progenitors, and the effect was abolished by inhibitors of PI3K/AKT and Src signaling [239]. This is consistent with in vivo studies using mouse models, in which vitamin E deficiency or impairment of its uptake resulted in neural tube defects [240, 241].
In addition to affecting cell survival, vitamin E was also reported to affect differentiation of stem cells as a free radical scavenger. Reactive oxygen species (ROS) were proposed to participate in cellular signaling and regulate embryonic stem cell (ESC) differentiation, and vitamin E typically antagonizes the ROS effects. Arachidonic acid, the precursor of prostaglandins and leukotrienes, was reported to promote the generation of vascular progenitor cells from mouse ESC embryoid bodies. ROS was elevated in the process, and trolox treatment from day 3 to day 10 abolished the effect of arachidonic acid on differentiation [242]. In another study, electrical field treatment stimulated endothelial differentiation of mouse ESCs through a mechanism involving ROS, and trolox treatment inhibited its effect [243]. In cardiac differentiation from mouse ESCs, treatment with valproic acid from day 3 to 7 was reported to inhibit embryoid body growth and suppress cardiomyocyte differentiation while increasing ROS. Co-administration of trolox antagonized the inhibitory effect and restored cardiomyocyte differentiation [244]. In contrast, icariin treatment from day 5 to 16 of cardiac differentiation, which elevated ROS and induced ERK/p38 phosphorylation, significantly enhanced cardiac differentiation, and vitamin E treatment decreased the promoting effect by half [245]. Similarly, elevated intracellular ROS by cardiotrophin-1 (CT-1, from day 7 on) is associated with improved cardiomyocyte differentiation and increased Ki-67 expression, suggesting better cardiomyocyte proliferation. Vitamin E abolished these effects as well through a mechanism involving Jak/Stat and ERK pathways [246]. Taken together, vitamin E can play regulatory roles during ESC differentiation toward multiple lineages, potentially through a mechanism involving ROS generation and activation of relevant signaling pathways. The exact effect may depend on the setting of differentiation and the timing of treatment.
The functions of vitamin E are summarized in Fig. 5.
Fig. 5.
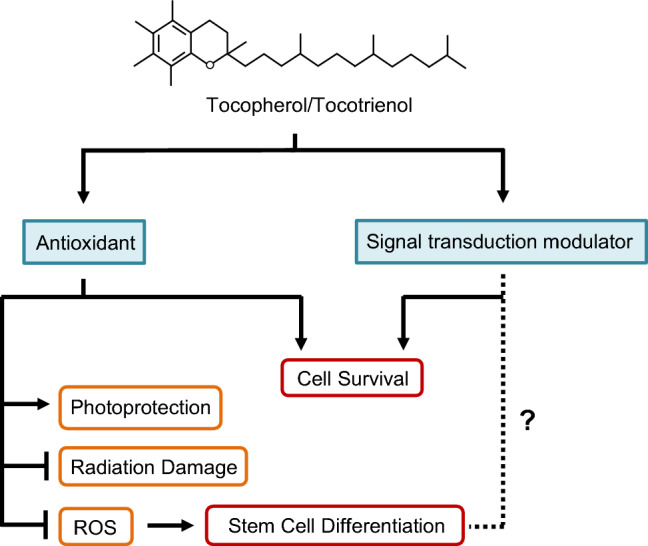
Vitamin E in stem cell culture. Vitamin E is a potent lipid-soluble antioxidant, and is capable of protecting stem cells and progenitor cells against oxidative damage. In addition, vitamin E can modulate signaling pathways, including the PI3K/AKT pathway, to promote survival and proliferation of cells in culture. The impact of vitamin E on ESC differentiation is mainly mediated through ROS levels. Whether vitamin E can directly modulate signal transduction events involved in cell fate determination has not been reported
Coordinated vitamin actions in stem cell regulation
Each vitamin has its distinctive role in biochemical processes, and many of them work together to carry out critical cellular functions. For example, the generation of acetyl-CoA requires B1, B2, B3 and B5, which is essential for both somatic and stem cells. Many other biological processes also demand collaborative actions of multiple vitamins, and some of them are especially important to stem cells.
Epigenetic regulation is essential for self-renewal and cell fate determination [247]. DNA and histone methylation are a key modification, and it is responsive to nutrition and metabolic changes. Appropriate epigenetic regulation is essential for pregnancy and embryonic development. Vitamin B12, B9, and B6 are key coenzymes in one carbon metabolism and can synergistically influence DNA and histone methylation [248, 249]. One carbon metabolism involves the donation of carbon units from amino acids for utilization in various biochemical reaction. In the folate (B9) cycle, a carbon unit produced from the conversion of serine to glycine is transferred to tetrahydrofolate (THF) by serine hydroxymethyltransferase (SHMT), a vitamin B6-dependent enzyme. The resulting 5,10-methylene-THF is important for nucleotide synthesis. In the methionine cycle, vitamin B12 serves as a coenzyme in the conversion of homocysteine to methionine by accepting a carbon unit from the folate cycle. Methionine is further converted to S‑adenosylmethionine (SAM) [250, 251], which is the main methyl group donor for the methylation of proteins, DNA, RNA and lipids [185].
The combined actions of vitamins are also reflected in multiple stem cell media containing vitamin combinations (Table 2), and are utilized in some stem cell protocols [252].
Concluding remarks
Vitamins are deeply involved in various basic metabolic and signaling processes, and many of them are required for normal functions in specific stem cells. Besides the conventional approach of stem cell modulation through growth factor signaling pathways, vitamin modulation could become a critical approach to improve stem cell maintenance and downstream differentiation. Studies on vitamins such as A, B3 and C have shown that vitamin-dependent pathways are effective targets in stem cell manipulation. However, most vitamins have not been systematically explored in different stem cell studies. Considering that specific cell types rely on distinctive combinations of vitamins, it is possible that more stem cell applications could be developed using different vitamin formulations in media. At the same time, stem cell culture also provides a unique platform to study vitamin function in human embryogenesis. Following the recent discoveries of vitamin-related molecular mechanisms, more novel mechanisms could be identified in stem cell models. We believe that vitamin study in stem cell research will lead to new modulations to improve stem cell applications, and help realize their great potentials in basic research and regenerative medicine.
Acknowledgements
This work was supported by the Science and Technology Development Fund of Macau SAR (FDCT/131/2014/A3, FDCT/056/2015/A2 and FDCT/0059/2019/A1) and University of Macau Multiyear Research Grant (MYRG2018-00135-FHS).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Combs GF. The vitamins: fundamental aspects in nutrition and health. Can Vet J. 1992;40:813–814. [Google Scholar]
- 2.Funk C. The preparation from yeast and certain foodstuffs of the substance the deficiency of which in diet occasions polyneuritis in birds. J Physiol. 1912;43:395–400. doi: 10.1113/jphysiol.1912.sp001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fall CHD, Yajnik CS, Rao S, et al. Micronutrients and fetal growth. Am Soc Nutr Sci. 2003;133:1747S–1756S. doi: 10.1093/jn/133.5.1747S. [DOI] [PubMed] [Google Scholar]
- 4.Semba RD. The discovery of the vitamins. Int J Vitam Nutr Res. 2012;82:310–315. doi: 10.1024/0300-9831/a000124. [DOI] [PubMed] [Google Scholar]
- 5.Schnellbaecher A, Binder D, Bellmaine S, Zimmer A. Vitamins in cell culture media: stability and stabilization strategies. Biotechnol Bioeng. 2019;116:1537–1555. doi: 10.1002/bit.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arigony ALV, de Oliveira IM, Machado M, et al. The influence of micronutrients in cell culture: a reflection on viability and genomic stability. Biomed Res Int. 2013;2013:597282. doi: 10.1155/2013/597282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaner WS. The fat-soluble vitamins 100 years later: where are we now? J Lipid Res. 2013;54:1716–1718. doi: 10.1194/jlr.e039891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mcculloch EA, Till JE. The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res. 1960;13:115–125. doi: 10.2307/3570877. [DOI] [PubMed] [Google Scholar]
- 9.Singh VK, Saini A, Kalsan M, et al. Describing the stem cell potency: the various methods of functional assessment and in silico diagnostics. Front Cell Dev Biol. 2016 doi: 10.3389/fcell.2016.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;2:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Trounson A, Dewitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev. 2016;17:194–200. doi: 10.1038/nrm.2016.10. [DOI] [PubMed] [Google Scholar]
- 13.Thomson JA, ItsKovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 14.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 15.Xu C, Rosler E, Jiang J, et al. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005;23:315–323. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Schulz TC, Sherrer ES, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–4120. doi: 10.1182/blood-2007-03-082586.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 18.D’Aniello C, Cermola F, Patriarca EJ, Minchiotti G. Vitamin C in stem cell biology: impact on extracellular matrix homeostasis and epigenetics. Stem Cells Int. 2017;2017:1–16. doi: 10.1155/2017/8936156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng Y, Ren Z, Xu F, et al. Nicotinamide promotes cell survival and differentiation as kinase inhibitor in human pluripotent stem cells. Stem Cell Rep. 2018;11:1347–1356. doi: 10.1016/j.stemcr.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. 2000;80:1021–1054. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Gao Y, Yu M, et al. Retinoic acid induces embryonic stem cell differentiation by altering both encoding RNA and microRNA expression. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0132566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Combs GF, McClung JP (2017) The vitamins : fundamental aspects in nutrition and health
- 23.Hill MJ. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev Suppl. 1997;1:S43–S45. doi: 10.1097/00008469-199703001-00009. [DOI] [PubMed] [Google Scholar]
- 24.Yano M, Fujita A. Synthesis of vitamins by intestinal bacteria in man and the effect of cellulose. VI. Synthesis of folic acid. J Vitaminol (Kyoto) 1958;2:209–215. doi: 10.5925/jnsv1954.4.81. [DOI] [PubMed] [Google Scholar]
- 25.Stacpoole PW. The pyruvate dehydrogenase complex as a therapeutic target for age-related diseases. Aging Cell. 2012;11:371–377. doi: 10.1111/j.1474-9726.2012.00805.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhou ZH, McCarthy DB, O’Connor CM, et al. The remarkable structural and functional organization of the eukaryotic pyruvate dehydrogenase complexes. Proc Natl Acad Sci. 2002;98:14802–14807. doi: 10.1073/pnas.011597698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murad S, Grove D, Lindberg KA, et al. Regulation of collagen synthesis by ascorbic acid. Biochemistry. 1981;78:2879–2882. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasta JD, Raines RT. Human collagen prolyl 4-hydroxylase is activated by ligands for its iron center. Biochemistry. 2016;55:3224–3233. doi: 10.1021/acs.biochem.6b00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nytko KJ, Maeda N, Schläfli P, et al. Vitamin C is dispensable for oxygen sensing in vivo. Blood. 2011;117:5485–5493. doi: 10.1182/blood-2010-09-307637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebouche CJ. Ascorbic acid and carnitine biosynthesis. Am J Clin Nutr. 1991;54:1147S–1152S. doi: 10.1093/ajcn/54.6.1147s. [DOI] [PubMed] [Google Scholar]
- 31.Delanghe JR, Langlois MR, De Buyzere ML, et al. Vitamin C deficiency: more than just a nutritional disorder. Genes Nutr. 2011;6:341–346. doi: 10.1007/s12263-011-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson GR, Morgan BC, Werrbach-Perez K, Perez-Polo JR. Antioxidant effect of retinoic acid on PC12 rat pheochromocytoma. Int J Dev Neurosci. 1991;9:161–170. doi: 10.1016/0736-5748(91)90007-9. [DOI] [PubMed] [Google Scholar]
- 33.Law WC, Rando RR. The molecular basis of retinoic acid induced night blindness. Biochem Biophys Res Commun. 1989;161:825–829. doi: 10.1016/0006-291X(89)92674-0. [DOI] [PubMed] [Google Scholar]
- 34.Narbaitz R. Role of vitamin D in the development of the chick embryo. J Exp Zool Suppl. 1987;1:15–23. [PubMed] [Google Scholar]
- 35.Sylvester Paul W. Mechanisms mediating the antiproliferative and apoptotic effects of vitamin E in mammary cancer cells. Front Biosci. 2005;10:699–709. doi: 10.2741/1565. [DOI] [PubMed] [Google Scholar]
- 36.Stenflo J, Fernlund P, Egan W, Roepstorff P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc Natl Acad Sci. 1974;71:2730–2733. doi: 10.1073/pnas.71.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vermeer C. γ-Carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. Biochem J. 1990;266:625–636. doi: 10.1042/bj2660625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan JF, Morton HJ, Parker RC. Nutrition of animal cells in tissue culture. I. Initial studies on a synthetic medium. Proc Soc Exp Bio Med. 1949;73:1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- 39.Eagle BH. The minimum vitamin requirements of the L and HeLa cells in tissue culture, the production of specific vitamin deficiencies, and their cure. J Exp Med. 1955;102:595–600. doi: 10.1084/jem.102.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eagle H. Amino acid metabolism in mammalian cell cultures. Science. 1959;130:432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- 41.Dulbecco R, Freeman G. Plaque production by the polyoma virus. Virology. 1959;8:396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- 42.Ham RG. An improved nutrient solution for diploid Chinese hamster and human cell lines. Exp Cell Res. 1963;29:515–526. doi: 10.1016/S0014-4827(63)80014-2. [DOI] [PubMed] [Google Scholar]
- 43.Ham RG. Clonal growth of mammalian cells in a chemically defined, synthetic medium. Proc Natl Acad Sci. 1965;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dakshinamurti K, Chalifour L, Bhullar RP. Requirement for biotin and the function of biotin in cells in culture. Ann N Y Acad Sci. 1985;447:38–55. doi: 10.1111/j.1749-6632.1985.tb18424.x. [DOI] [PubMed] [Google Scholar]
- 45.Fehling C, Jägerstad M, Åkesson B, et al. Effects of vitamin B12 deficiency on lipid metabolism of the rat liver and nervous system. Br J Nutr. 1978;39:501–513. doi: 10.1079/bjn19780066. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi-Iñiguez T, García-Hernandez E, Arreguín-Espinosa R, Flores ME. Role of vitamin B12 on methylmalonyl-CoA mutase activity. J Zhejiang Univ Sci B. 2012;13:423–437. doi: 10.1631/jzus.B1100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnes D, Sato G. Growth of a human mammary tumour cell line in a serum-free medium. Nature. 1979;281:388–389. doi: 10.1038/281388a0. [DOI] [PubMed] [Google Scholar]
- 48.Yao T, Asayama Y. Animal-cell culture media: history, characteristics, and current issues. Reprod Med Biol. 2017;16:99–117. doi: 10.1002/rmb2.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akopian V, Andrews PW, Beil S, et al. Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. In Vitro Cell Dev Biol Anim. 2010;46:247–258. doi: 10.1007/s11626-010-9297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen G, Gulbranson DR, Hou Z, et al. Chemically defined conditions for human iPS cell derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hasegawa K, Yasuda S, Teo J-L, et al. Wnt signaling orchestration with a small molecule DYRK inhibitor provides long-term xeno-free human pluripotent cell expansion. Stem Cells Transl Med. 2012;1:18–28. doi: 10.5966/sctm.2011-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elwyn D, Weissbach A, Henry SS, Sprinson DB. The biosynthesis of choline from serine and related compounds. J Biol Chem. 1955;213:281–295. [PubMed] [Google Scholar]
- 53.Penry JT, Manore MM. Choline: an important micronutrient for maximal endurance-exercise performance? Int J Sport Nutr Exerc Metab. 2008;18:191–203. doi: 10.1123/ijsnem.18.2.191. [DOI] [PubMed] [Google Scholar]
- 54.Dominguez-Salas P, Moore SE, Cole D, et al. DNA methylation potential: dietary intake and blood concentrations of one-carbon metabolites and cofactors in rural African women. Am J Clin Nutr. 2013;97:1217–1227. doi: 10.3945/ajcn.112.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parthasarathy LK, Ratnam L, Seelan S, et al. Mammalian inositol 3-phosphate synthase: its role in the biosynthesis of brain inositol and its clinical use as a psychoactive agent BT—biology of inositols and phosphoinositides: subcellular biochemistry. In: Majumder AL, Biswas BB, et al., editors. Springer. Boston: US; 2006. pp. 293–314. [DOI] [PubMed] [Google Scholar]
- 56.Deranieh RM, He Q, Caruso JA, Greenberg ML. Phosphorylation regulates myo-inositol-3-phosphate synthase a novel regulatory mechanism of inositol biosynthesis. J Biol Chem. 2013;288:26822–26833. doi: 10.1074/jbc.M113.479121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eagle H, Oyama VI, Levy I, Freeman A. Myo-inositol as an essential growth factor for normal and malignant human cells in tissue culture. Science. 1956;123:845–847. doi: 10.1126/science.123.3202.845-a. [DOI] [PubMed] [Google Scholar]
- 58.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 59.Holub BJ. Metabolism and function of myo-inositol and inositol phospholipids. Annu Rev Nutr. 1986;6:563–597. doi: 10.1146/annurev.nu.06.070186.003023. [DOI] [PubMed] [Google Scholar]
- 60.Conquer JA, Tierney MC, Zecevic J, et al. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–1312. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 61.Lazzarin N, Vaquero E, Exacoustos C, et al. Low-dose aspirin and omega-3 fatty acids improve uterine artery blood flow velocity in women with recurrent miscarriage due to impaired uterine perfusion. Fertil Steril. 2009;92:296–300. doi: 10.1016/j.fertnstert.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 62.Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr. 2012;3:1–7. doi: 10.3945/an.111.000893.Omega-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:644–651. doi: 10.4088/JCP.v69n0418. [DOI] [PubMed] [Google Scholar]
- 64.Shearer MJ. Vitamin K. Lancet. 1995;345:229–234. doi: 10.1081/E-EDS-120022055. [DOI] [PubMed] [Google Scholar]
- 65.Olson JA, Hayaishi O. The enzymatic cleavage of β-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc Natl Acad Sci. 1965;54:1364–1370. doi: 10.1073/pnas.54.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kin R, Kam T, Deng Y, et al. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2015;2:1–14. doi: 10.1186/2045-3701-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharow KA, Temkin B, Asson-batres MANN. Retinoic acid stability in stem cell cultures. Int J Dev Biol. 2012;56:273–278. doi: 10.1387/ijdb.113378ks. [DOI] [PubMed] [Google Scholar]
- 69.Chen L, Yang M, Dawes J, Khillan JS. Suppression of ES cell differentiation by retinol (vitamin A) via the overexpression of Nanog. Differentiation. 2007;75:682–693. doi: 10.1111/j.1432-0436.2007.00169.x. [DOI] [PubMed] [Google Scholar]
- 70.Chen L, Khillan JS. A novel signaling by vitamin A/retinol promotes self renewal of mouse embryonic stem cells by activating PI3K/Akt signaling pathway via insulin-like growth factor-1 receptor. Stem Cells. 2010;28:57–63. doi: 10.1002/stem.251. [DOI] [PubMed] [Google Scholar]
- 71.Khillan JS. Vitamin A/retinol and maintenance of pluripotency of stem cells. Nutrients. 2014;6:1209–1222. doi: 10.3390/nu6031209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mosher KI, Schaffer DV. Proliferation versus differentiation: redefining retinoic acid’s role. Stem Cell Rep. 2018;10:1673–1675. doi: 10.1016/j.stemcr.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang S, Sun J, Pan S, et al. Retinol (vitamin A) maintains self-renewal of pluripotent male germline stem cells (mGSCs) from adult mouse testis. J Cell Biochem. 2011;112:1009–1021. doi: 10.1002/jcb.23029. [DOI] [PubMed] [Google Scholar]
- 74.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 75.Del Corral RD, Olivera-Martinez I, Goriely A, et al. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:1673–1675. doi: 10.1016/S0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- 76.Li X, Fok KL, Guo J, et al. Retinoic acid promotes stem cell differentiation and embryonic development by transcriptionally activating CFTR. Biochim Biophys Acta Mol Cell Res. 2018;1865:605–615. doi: 10.1016/j.bbamcr.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Cheong HS, Lee HC, Park BL, et al. Epigenetic modification of retinoic acid-treated human embryonic stem cells. BMB Rep. 2010;43:830–835. doi: 10.5483/BMBRep.2010.43(12).830. [DOI] [PubMed] [Google Scholar]
- 78.Lee ER, Murdoch FE, Fritsch MK. High histone acetylation and decreased polycomb repressive complex 2 member levels regulate gene specific transcriptional changes during early embryonic stem cell differentiation induced by retinoic acid. Stem Cells. 2007;25:2191–2199. doi: 10.1634/stemcells.2007-0203. [DOI] [PubMed] [Google Scholar]
- 79.Urvalek AM, Gudas LJ. Retinoic acid and histone deacetylases regulate epigenetic changes in embryonic stem cells. J Biol Chem. 2014;289:19519–19530. doi: 10.1074/jbc.M114.556555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bar-El Dadon S, Reifen R. Vitamin A and the epigenome. Crit Rev Food Sci Nutr. 2017;57:2404–2411. doi: 10.1080/10408398.2015.1060940. [DOI] [PubMed] [Google Scholar]
- 81.Szarc vel Szic K, Ndlovu MN, Haegeman G, Vanden Berghe W. Nature or nurture: Let food be your epigenetic medicine in chronic inflammatory disorders. Biochem Pharmacol. 2010;80:1816–1832. doi: 10.1016/j.bcp.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 82.Janesick A, Wu SC, Blumberg B. Retinoic acid signaling and neuronal differentiation. Cell Mol Life Sci. 2015;72:1559–1576. doi: 10.1007/s00018-014-1815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zile MH. Vitamin A and embryonic development: an overview. Am Soc Nutr Sci. 1998;128:455–458. doi: 10.1093/jn/128.2.455S. [DOI] [PubMed] [Google Scholar]
- 84.Maden M. Vitamin A and the developing embryo. Postgrad Med J. 2001;77:489–491. doi: 10.1136/pmj.77.910.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hale F. Pigs born without eye balls. J Hered. 1933;24:105–106. doi: 10.1093/oxfordjournals.jhered.a103720. [DOI] [Google Scholar]
- 86.Kalter H, Warkany J. Experimental production of congenital malformations in mammals by metabolic procedure. Physiol Rev. 1959;39:69–115. doi: 10.1152/physrev.1959.39.1.69. [DOI] [PubMed] [Google Scholar]
- 87.Noma T, Glick A, Geiser A, et al. Molecular cloning and structure of the human transforming growth factor-β2 gene promoter. Growth Factors. 1991 doi: 10.3109/08977199109043910. [DOI] [PubMed] [Google Scholar]
- 88.Pendaries V, Verrecchia F, Michel S, Mauviel A. Retinoic acid receptors interfere with the TGF-β/Smad signaling pathway in a ligand-specific manner. Oncogene. 2003;22:8212–8220. doi: 10.1038/sj.onc.1206913. [DOI] [PubMed] [Google Scholar]
- 89.Brown JM, Robertson KE, Wedden SE, Tickle C. Alterations in Msx 1 and Msx 2 expression correlate with inhibition of outgrowth of chick facial primordia induced by retinoic acid. Anat Embryol (Berl) 1997;195:203–207. doi: 10.1007/s004290050039. [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez SMDC, Ferland LH, Robert B, Abdelhay E. Structural and functional analysis of mouse Msx1 gene promoter: sequence conservation with human MSX1 promoter points at potential regulatory elements. DNA Cell Biol. 1998;17:561–572. doi: 10.1089/dna.1998.17.561. [DOI] [PubMed] [Google Scholar]
- 91.Ghatpande SK, Zhou HR, Cakstina I, et al. Transforming growth factor β2 is negatively regulated by endogenous retinoic acid during early heart morphogenesis. Dev Growth Differ. 2010;52:433–455. doi: 10.1111/j.1440-169X.2010.01183.x. [DOI] [PubMed] [Google Scholar]
- 92.Zile MH. Vitamin A-not for your eyes only: requirement for heart formation begins early in embryogenesis. Nutrients. 2010;2:532–550. doi: 10.3390/nu2050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heller LC, Li Y, Abrams KL, Rogers MB. Transcriptional regulation of the Bmp2 gene. J Biol Chem. 2002;274:1394–1400. doi: 10.1074/jbc.274.3.1394. [DOI] [PubMed] [Google Scholar]
- 94.Shannon SR, Moise AR, Trainor PA, et al. New insights and changing paradigms in the regulation of vitamin A metabolism in development. Wiley Interdiscip Rev Dev Biol. 2017;6:1–28. doi: 10.1002/wdev.264.New. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Collins SJ. The role of retinoids and retinoic acid receptors in normal hematopoiesis. Leukemia. 2002;16:1896–1905. doi: 10.1038/sj.leu.2402718. [DOI] [PubMed] [Google Scholar]
- 96.Rönn RE, Guibentif C, Moraghebi R, et al. Retinoic acid regulates hematopoietic development from human pluripotent stem cells. Stem Cell Rep. 2015;4:269–281. doi: 10.1016/j.stemcr.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cabezas-Wallscheid N, Buettner F, Sommerkamp P, et al. Vitamin A-retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell. 2017;169:807–823.e19. doi: 10.1016/j.cell.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 98.Chanda B, Ditadi A, Iscove NN, Keller G. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell. 2013;155:215–227. doi: 10.1016/j.cell.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 99.Cabezas-Wallscheid N, Klimmeck D, Hansson J, et al. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell. 2014;15:507–522. doi: 10.1016/j.stem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 100.Li L, Dong J, Yan L, et al. Single-cell RNA-Seq analysis maps development of human germline cells and gonadal niche interactions. Cell Stem Cell. 2017;20:858–873.e4. doi: 10.1016/j.stem.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 101.Koshimizu U, Watanabe M, Nakatsuji N. Retinoic acid is a potent growth activator of mouse primordial germ cells in vitro. Dev Biol. 1995;168:683–685. doi: 10.1006/dbio.1995.1113. [DOI] [PubMed] [Google Scholar]
- 102.Nayernia K, Nolte J, Michelmann HW, et al. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;11:125–132. doi: 10.1016/j.devcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 103.Sandell LL, Sanderson BW, Moiseyev G, et al. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Farjo KM, Moiseyev G, Nikolaeva O, et al. RDH10 is the primary enzyme responsible for the first step of embryonic vitamin A metabolism and retinoic acid synthesis. Dev Biol. 2011;357:347–355. doi: 10.1016/j.ydbio.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Billings SE, Pierzchalski K, Tjaden NEB, et al. The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development. FASEB J. 2013;27:4877–4889. doi: 10.1096/fj.13-227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 107.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 108.Wang W, Yang J, Liu H, et al. Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proc Natl Acad Sci. 2011;108:18283–18288. doi: 10.1073/pnas.1100893108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang J, Wang W, Ooi J, et al. Signalling through retinoic acid receptors is required for reprogramming of both mouse embryonic fibroblast cells and epiblast stem cells to induced pluripotent stem cells. Stem Cells. 2015;33:1390–1404. doi: 10.1002/stem.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hou P, Li Y, Zhang X, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 111.Shu J, Wu C, Wu Y, et al. Induction of pluripotency in mouse somatic cells with lineage specifiers. Cell. 2013;153:963–975. doi: 10.4172/2157-7633.1000305.Improved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Angelis MT, Parrotta EI, Santamaria G, Cuda G. Short-term retinoic acid treatment sustains pluripotency and suppresses differentiation of human induced pluripotent stem cells. Cell Death Dis. 2018;9:1–13. doi: 10.1038/s41419-017-0028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hore TA, Von Meyenn F, Ravichandran M, et al. Retinol and ascorbate drive erasure of epigenetic memory and enhance reprogramming to naïve pluripotency by complementary mechanisms. Proc Natl Acad Sci. 2016;113:12202–12207. doi: 10.1073/pnas.1608679113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garten A, Petzold S, Barnikol-Oettler A, et al. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is constitutively released from human hepatocytes. Biochem Biophys Res Commun. 2010;391:376–381. doi: 10.1016/j.bbrc.2009.11.066. [DOI] [PubMed] [Google Scholar]
- 115.Mullangi R, Srinivas NR. Niacin and its metabolites: role of LC–MS/MS bioanalytical methods and update on clinical pharmacology. An overview. Biomed Chromatogr. 2011;25:218–237. doi: 10.1002/bmc.1522. [DOI] [PubMed] [Google Scholar]
- 116.Saini JS, Corneo B, Miller JD, et al. Nicotinamide ameliorates disease phenotypes in a human iPSC model of age-related macular degeneration. Cell Stem Cell. 2018;20:635–647. doi: 10.1016/j.stem.2016.12.015.Nicotinamide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mitchell SJ, Bernier M, Aon MA, et al. Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Cell Metab. 2018;27:667–676.e4. doi: 10.1016/j.cmet.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Parsons XH, Teng YD, Parsons JF, et al. Efficient derivation of human cardiac precursors and cardiomyocytes from pluripotent human embryonic stem cells with small molecule induction. J Vis Exp. 2011 doi: 10.3791/3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nostro MC, Sarangi F, Yang C, et al. Efficient generation of NKX6-1+ pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Rep. 2015;4:591–604. doi: 10.1016/j.stemcr.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Son MJ, Son M-Y, Seol B, et al. Nicotinamide overcomes pluripotency deficits and reprogramming barriers. Stem Cells. 2013;31:1121–1135. doi: 10.1002/stem.1368. [DOI] [PubMed] [Google Scholar]
- 121.Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 122.Bartfeld S, Bayram T, van de Wetering M, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148:126–136.e6. doi: 10.1053/j.gastro.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Urbischek M, Rannikmae H, Foets T, et al. Organoid culture media formulated with growth factors of defined cellular activity. Sci Rep. 2019;9:6193. doi: 10.1038/s41598-019-42604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peled T, Shoham H, Aschengrau D, et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012;40:342–355. doi: 10.1016/j.exphem.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 125.Bosch-Presegué L, Vaquero A. Sirtuin-dependent epigenetic regulation in the maintenance of genome integrity. FEBS J. 2015;282:1745–1767. doi: 10.1111/febs.13053. [DOI] [PubMed] [Google Scholar]
- 126.Kuchmerovska T, Shymanskyy I, Donchenko G, et al. Poly(ADP-ribosyl)ation enhancement in brain cell nuclei is associated with diabetic neuropathy. J Diabetes Complicat. 2004;18:198–204. doi: 10.1016/S1056-8727(03)00039-4. [DOI] [PubMed] [Google Scholar]
- 127.Avalos JL, Bever KM, Wolberger C. Mechanism of sirtuin inhibition by nicotinamide: altering the NAD+ cosubstrate specificity of a Sir2 enzyme. Mol Cell. 2005;17:855–868. doi: 10.1016/j.molcel.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 128.de Picciotto NE, Gano LB, Johnson LC, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nadtochiy SM, Wang YT, Nehrke K, et al. Cardioprotection by nicotinamide mononucleotide (NMN): involvement of glycolysis and acidic pH. J Mol Cell Cardiol. 2018;121:155–162. doi: 10.1016/j.yjmcc.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang X, Hu X, Yang Y, et al. Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 2016;1643:1–9. doi: 10.1016/j.brainres.2016.04.060. [DOI] [PubMed] [Google Scholar]
- 131.Vannini N, Campos V, Girotra M, et al. The NAD-booster nicotinamide riboside potently stimulates hematopoiesis through increased mitochondrial clearance. Cell Stem Cell. 2019;24:405–418.e7. doi: 10.1016/j.stem.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 132.Belenky P, Racette FG, Bogan KL, et al. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+ Cell. 2007;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 133.Linster CL, Van Schaftingen E. Vitamin C: biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 134.Stone I. The natural history of ascorbic acid in the evolution of the mammals and primates and its significance for present day man. J Orthomol Psychiatry. 1972;1:82–89. [Google Scholar]
- 135.Andrews FE, Driscoll PJ. Stability of ascorbic acid in orange juice exposed to light and air during storage. J Am Diet Assoc. 1977;71:140. [PubMed] [Google Scholar]
- 136.Austria R, Semenzato A, Bettero A. Stability of vitamin C derivatives in solution and topical formulations. J Pharm Biomed Anal. 1997;15:795–801. doi: 10.1016/S0731-7085(96)01904-8. [DOI] [PubMed] [Google Scholar]
- 137.Lee WJ, Kim SL, Choe YS, et al. Magnesium ascorbyl phosphate regulates the expression of inflammatory biomarkers in cultured sebocytes. Ann Dermatol. 2015;27:376–382. doi: 10.5021/ad.2015.27.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meves A, Stock SN, Beyerle A, et al. Vitamin C derivative ascorbyl palmitate promotes ultraviolet-B-induced lipid peroxidation and cytotoxicity in keratinocytes. J Investig Dermatol. 2002;119:1103–1108. doi: 10.1046/j.1523-1747.2002.19521.x. [DOI] [PubMed] [Google Scholar]
- 139.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA. 1989;86:6377–6381. doi: 10.1073/PNAS.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Carcamo JM, Pedraza A, Borquez-Ojeda O, et al. Vitamin C is a kinase inhibitor: dehydroascorbic acid inhibits IκBα kinase β. Mol Cell Biol. 2004;24:6645–6652. doi: 10.1128/mcb.24.15.6645-6652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bowie AG, O’Neill LAJ. Vitamin C inhibits NF-κB activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol. 2000;165:7180–7188. doi: 10.4049/jimmunol.165.12.7180. [DOI] [PubMed] [Google Scholar]
- 142.Hurrel R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91:1461–1467. doi: 10.3945/ajcn.2010.28674F.Am. [DOI] [PubMed] [Google Scholar]
- 143.Ma Y, Chapman J, Levine M, et al. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014 doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 144.Bram S, Froussard P, Guichard M, et al. Vitamin C preferential toxicity for malignant melanoma cells. Nature. 1980;284:629–631. doi: 10.1038/284629a0. [DOI] [PubMed] [Google Scholar]
- 145.Bishun N, Basu TK, Metcalfe S, Williams DC. The effect of ascorbic acid (vitamin C) on two tumor cell lines in culture. Oncology. 1978;35:160–162. doi: 10.1159/000225276. [DOI] [PubMed] [Google Scholar]
- 146.Hinman A, Holst CR, Latham JC, et al. Vitamin E hydroquinone is an endogenous regulator of ferroptosis via redox control of 15-lipoxygenase. PLoS One. 2018;13:1–22. doi: 10.1371/journal.pone.0201369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stockwell BR, Angeli JPF. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1002/cncr.27633.Percutaneous. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cimmino L, Neel BG, Aifantis I. Vitamin C in stem cell reprogramming and cancer. Trends Cell Biol. 2018;28:698–708. doi: 10.1016/j.tcb.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rahman F, Fontes M. Ascorbic acid, myelination and associated disorders. PharmaNutrition. 2013;1:98–100. doi: 10.1016/j.phanu.2013.04.003. [DOI] [Google Scholar]
- 150.Podratz JL, Rodriguez E, Windebank AJ. Role of the extracellular matrix in myelination of peripheral nerve. Glia. 2001;35:35–40. doi: 10.1002/glia.1068. [DOI] [PubMed] [Google Scholar]
- 151.Passage E, Norreel JC, Noack-Fraissignes P, et al. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot–Marie–Tooth disease. Nat Med. 2004;10:396–401. doi: 10.1038/nm1023. [DOI] [PubMed] [Google Scholar]
- 152.Pareyson D, Reilly MM, Schenone A, et al. Ascorbic acid in Charcot–Marie–Tooth disease type 1A (CMT-TRIAAL and CMT-TRAUK): a double-blind randomised trial. Lancet Neurol. 2011;10:320–328. doi: 10.1016/S1474-4422(11)70025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chojkier M, Houglum K, Solis-Herruzo J, Brenner DA. Stimulation of collagen gene expression by ascorbic acid in human fibroblasts. J Biol Chem. 1989;246:16957–16962. [PubMed] [Google Scholar]
- 154.Esteban MA, Wang T, Qin B, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6:71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 155.Zhao Y, Yin X, Qin H, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3:475–479. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 156.Marión RM, Strati K, Li H, et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shi Y, Zhao Y, Deng H. Powering reprogramming with vitamin C. Cell Stem Cell. 2010;6:1–2. doi: 10.1016/j.stem.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 158.Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet. 2007;8:829. doi: 10.1038/nrg2218. [DOI] [PubMed] [Google Scholar]
- 159.Chung TL, Brena RM, Kolle G, et al. Vitamin C promotes widespread yet specific DNA demethylation of the epigenome in human embryonic stem cells. Stem Cells. 2010;28:1848–1855. doi: 10.1002/stem.493. [DOI] [PubMed] [Google Scholar]
- 160.Irizarry RA, Ladd-Acosta C, Wen B, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rahman F, Bordignon B, Culerrier R, et al. Ascorbic acid drives the differentiation of mesoderm-derived embryonic stem cells. Involvement of p38 MAPK/CREB and SVCT2 transporter. Mol Nutr Food Res. 2017 doi: 10.1002/mnfr.201600506. [DOI] [PubMed] [Google Scholar]
- 162.Takahashi T, Lord B, Schulze PC, et al. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107:1912–1916. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- 163.Cao N, Liu Z, Chen Z, et al. Ascorbic acid enhances the cardiac differentiation of induced pluripotent stem cells through promoting the proliferation of cardiac progenitor cells. Cell Res. 2012;22:219–236. doi: 10.1038/cr.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chepda T, Cadau M, Girin P, et al. Monitoring of ascorbate at a constant rate in cell culture: effect on cell growth. In Vitro Cell Dev Biol Anim. 2002;37:26. doi: 10.1290/1071-2690(2001)037<0026:moaaac>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 165.Hata R-I, Senoo H. l-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J Cell Physiol. 1989;138:8–16. doi: 10.1002/jcp.1041380103. [DOI] [PubMed] [Google Scholar]
- 166.Senoo H, Hata R. Extracellular matrix regulates and l-ascorbic acid 2-phosphate further modulates morphology, proliferation, and collagen synthesis of perisinusoidal stellate cells. Biochem Biophys Res Commun. 1994;200:999–1006. doi: 10.1006/bbrc.1994.1549. [DOI] [PubMed] [Google Scholar]
- 167.Fujisawa K, Hara K, Takami T, et al. Evaluation of the effects of ascorbic acid on metabolism of human mesenchymal stem cells. Stem Cell Res Ther. 2018;9:1–12. doi: 10.1186/s13287-018-0825-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Potdar PD, D’Souza SB. Ascorbic acid induces in vitro proliferation of human subcutaneous adipose tissue derived mesenchymal stem cells with upregulation of embryonic stem cell pluripotency markers Oct4 and SOX 2. Hum Cell. 2010;23:152–155. doi: 10.1111/j.1749-0774.2010.00095.x. [DOI] [PubMed] [Google Scholar]
- 169.Choi K-M, Seo Y-K, Yoon H-H, et al. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J Biosci Bioeng. 2008;105:586–594. doi: 10.1263/jbb.105.586. [DOI] [PubMed] [Google Scholar]
- 170.Narita Y, Yamawaki A, Kagami H, et al. Effects of transforming growth factor-beta 1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res. 2008;333:449–459. doi: 10.1007/s00441-008-0654-0. [DOI] [PubMed] [Google Scholar]
- 171.Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–477. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 172.Xiao G, Wang D, Benson MD, et al. Role of the α2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273:32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 173.Franceschi RT, Iyer BS, Cui Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J Bone Miner Res. 1994;9:843–854. doi: 10.1002/jbmr.5650090610. [DOI] [PubMed] [Google Scholar]
- 174.Castrén E, Sillat T, Oja S, et al. Osteogenic differentiation of mesenchymal stromal cells in two-dimensional and three-dimensional cultures without animal serum. Stem Cell Res Ther. 2015;6:1–13. doi: 10.1186/s13287-015-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Barlian A, Judawisastra H, Alfarafisa NM, et al. Chondrogenic differentiation of adipose-derived mesenchymal stem cells induced by L-ascorbic acid and platelet rich plasma on silk fibroin scaffold. PeerJ. 2018;6:e5809. doi: 10.7717/peerj.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chang Z, Huo L, Li P, et al. Ascorbic acid provides protection for human chondrocytes against oxidative stress. Mol Med Rep. 2015;12:7086–7092. doi: 10.3892/mmr.2015.4231. [DOI] [PubMed] [Google Scholar]
- 177.Huijskens MJAJ, Walczak M, Sarkar S, et al. Ascorbic acid promotes proliferation of natural killer cell populations inculture systems applicable for natural killer cell therapy. Cytotherapy. 2015;17:613–620. doi: 10.1016/j.jcyt.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 178.Huijskens MJAJ, Walczak M, Koller N, et al. Technical Advance: ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J Leukoc Biol. 2014;96:1165–1175. doi: 10.1189/jlb.1TA0214-121RR. [DOI] [PubMed] [Google Scholar]
- 179.Kennedy M, Keller G. Development of hemangioblast defines the onset of hematopoiesis in ES cell differentiation cultures. Blood. 2007;12:3521–3528. doi: 10.1182/blood-2006-09-047704.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Agathocleous M, Meacham CE, Burgess RJ, et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature. 2017;549:476–481. doi: 10.1097/CCM.0b013e31823da96d.Hydrogen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Minor EA, Court BL, Young JI, Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem. 2013;288:13669–13674. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen J, Guo L, Zhang L, et al. Vitamin C modulates TET1 function during somatic cell reprogramming. Nat Genet. 2013;45:1504–1509. doi: 10.1038/ng.2807. [DOI] [PubMed] [Google Scholar]
- 183.Shi DQ, Ali I, Tang J, Yang WC. New insights into 5hmC DNA modification: generation, distribution and function. Front Genet. 2017;8:1–11. doi: 10.3389/fgene.2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee Chong T, Ahearn EL, Cimmino L. Reprogramming the epigenome with vitamin C. Front Cell Dev Biol. 2019;7:1–13. doi: 10.3389/fcell.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism, and DNA methylation. J Nutr Biochem. 2012;23:853–859. doi: 10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martin EM, Fry RC. Environmental influences on the epigenome: exposure-associated DNA methylation in human populations. Annu Rev Public Health. 2018;39:309–333. doi: 10.1146/annurev-publhealth-040617-014629. [DOI] [PubMed] [Google Scholar]
- 187.Clare CE, Brassington AH, Kwong WY, Sinclair KD. One-carbon metabolism: linking nutritional biochemistry to epigenetic programming of long-term development. Annu Rev Anim Biosci. 2019;7:263–287. doi: 10.1146/annurev-animal-020518-115206. [DOI] [PubMed] [Google Scholar]
- 188.Wang T, Chen K, Zeng X, et al. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell. 2011;9:575–587. doi: 10.1016/j.stem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 189.Tsukada YI, Fang J, Erdjument-Bromage H, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 190.Ito S, Shen L, Dai Q, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1016/0031-6989(87)90091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wu H, Zhang Y. Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell. 2014;156:45–68. doi: 10.1016/j.cell.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang Q, Liang X, Sun X, et al. AMPK/α-ketoglutarate axis dynamically mediates DNA demethylation in the Prdm16 promoter and brown adipogenesis. Cell Metab. 2016;24:542–554. doi: 10.1016/j.cmet.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yin R, Mao SQ, Zhao B, et al. Ascorbic acid enhances tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc. 2013;135:10396–10403. doi: 10.1021/ja4028346. [DOI] [PubMed] [Google Scholar]
- 194.Clifton IJ, McDonough MA, Ehrismann D, et al. Structural studies on 2-oxoglutarate oxygenases and related double-stranded β-helix fold proteins. J Inorg Biochem. 2006;100:644–669. doi: 10.1016/j.jinorgbio.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 195.He X-B, Kim M, Kim S, et al. Vitamin C facilitates dopamine neuron differentiation in fetal MIdbrain through TET1- and JMJD3-dependent epigenetic control manner. Stem Cells. 2015;33:1320–1332. doi: 10.1002/stem.1932.VITAMIN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Koh KP, Yabuuchi A, Rao S, et al. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8:200–213. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Evans HM, Bishop KS. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science. 1922;56:650–651. doi: 10.1126/science.56.1458.650. [DOI] [PubMed] [Google Scholar]
- 198.Brigelius-Flohé R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. doi: 10.1096/fasebj.13.10.1145. [DOI] [PubMed] [Google Scholar]
- 199.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Torquato P, Ripa O, Giusepponi D, et al. Analytical strategies to assess the functional metabolome of vitamin E. J Pharm Biomed Anal. 2016;124:399–412. doi: 10.1016/j.jpba.2016.01.056. [DOI] [PubMed] [Google Scholar]
- 201.Peh HY, Tan WSD, Liao W, Wong WSF. Vitamin E therapy beyond cancer: tocopherol versus tocotrienol. Pharmacol Ther. 2016;162:152–169. doi: 10.1016/j.pharmthera.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 202.Aggarwal V, Kashyap D, Sak K, et al. Molecular mechanisms of action of tocotrienols in cancer: recent trends and advancements. Int J Mol Sci. 2019;20:656. doi: 10.3390/ijms20030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ling MT, Luk SU, Al-Ejeh F, Khanna KK. Tocotrienol as a potential anticancer agent. Carcinogenesis. 2011;33:233–239. doi: 10.1093/carcin/bgr261. [DOI] [PubMed] [Google Scholar]
- 204.Davies MJ, Forni LG, Willson RL. Vitamin E analogue Trolox C. E.s.r. and pulse-radiolysis studies of free-radical reactions. Biochem J. 1988;255:513–522. [PMC free article] [PubMed] [Google Scholar]
- 205.Compadre CM, Singh A, Thakkar S, et al. Molecular dynamics guided design of tocoflexol: a new radioprotectant tocotrienol with enhanced bioavailability. Drug Dev Res. 2014;75:10–22. doi: 10.1002/ddr.21162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Neuzil J, Weber T, Terman A, et al. Vitamin E analogues as inducers of apoptosis: implications for their potential antineoplastic role. Redox Rep. 2004;6:143–151. doi: 10.1179/135100001101536247. [DOI] [PubMed] [Google Scholar]
- 207.Zf M. Final report on the safety assessment of tocopherol, tocopheryl acetate, tocopheryl linoleate, tocopheryl linoleate/oleate, tocopheryl nicotinate, tocopheryl succinate, dioleyl tocopheryl methylsilanol, potassium ascorbyl tocopheryl phosphate, and tocophe. Int J Toxicol. 2002;21:51–116. doi: 10.1080/10915810290169819. [DOI] [PubMed] [Google Scholar]
- 208.Lee E, Choi M-K, Lee Y-J, et al. Alpha-tocopheryl succinate, in contrast to alpha-tocopherol and alpha-tocopheryl acetate, inhibits prostaglandin E 2 production in human lung epithelial cells. Carcinogenesis. 2006;27:2308–2315. doi: 10.1093/carcin/bgl073. [DOI] [PubMed] [Google Scholar]
- 209.Li Z-T, Wang L-M, Yi L-R, et al. Succinate ester derivative of δ-tocopherol enhances the protective effects against (60)Co γ-ray-induced hematopoietic injury through granulocyte colony-stimulating factor induction in mice. Sci Rep. 2017;7:40380. doi: 10.1038/srep40380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Frei B, Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci. 1988;85:9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dhitavat S, Rivera ER, Rogers E, Shea TB. Differential efficacy of lipophilic and cytosolic antioxidants on generation of reactive oxygen species by amyloid-β. J Alzheimer’s Dis. 2001;3:525–529. doi: 10.3233/JAD-2001-3602. [DOI] [PubMed] [Google Scholar]
- 212.Zingg J-M. Vitamin E: a role in signal transduction. Annu Rev Nutr. 2015;35:135–173. doi: 10.1146/annurev-nutr-071714-034347. [DOI] [PubMed] [Google Scholar]
- 213.Boscoboinik D, Szewczyk A, Henseys C, Ami A. Inhibition of cell proliferation by a-tocopherol. role of protein kinase C. J Biol Chem. 1991;266:6188–6194. [PubMed] [Google Scholar]
- 214.Ricciarelli R, Tasinato A, Clément S, et al. Alpha-tocopherol specifically inactivates cellular protein kinase C alpha by changing its phosphorylation state. Biochem J. 1998;334(Pt 1):243–249. doi: 10.1042/bj3340243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin E isoforms differentially regulate intercellular adhesion molecule-1 activation of PKCα in human microvascular endothelial cells. PLoS One. 2012;7:e41054–e41054. doi: 10.1371/journal.pone.0041054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Boscoboinik D, Szewczyk A, Azzi A. α-Tocopherol (vitamin E) regulates vascular smooth muscle cell proliferation and protein kinase C activity. Arch Biochem Biophys. 1991;286:264–269. doi: 10.1016/0003-9861(91)90039-L. [DOI] [PubMed] [Google Scholar]
- 217.Ferri P, Cecchini T, Ambrogini P, et al. α-tocopherol affects neuronal plasticity in adult rat dentate gyrus: the possible role of PKCδ. J Neurobiol. 2006;66:793–810. doi: 10.1002/neu.20255. [DOI] [PubMed] [Google Scholar]
- 218.Samant GV, Sylvester PW. γ-Tocotrienol inhibits ErbB3-dependent PI3K/Akt mitogenic signalling in neoplastic mammary epithelial cells. Cell Prolif. 2006;39:563–574. doi: 10.1111/j.1365-2184.2006.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shin-Kang S, Ramsauer VP, Lightner J, et al. Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free Radic Biol Med. 2011;51:1164–1174. doi: 10.1016/j.freeradbiomed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 220.Bhatti FU, Mehmood A, Latief N, et al. Vitamin E protects rat mesenchymal stem cells against hydrogen peroxide-induced oxidative stress in vitro and improves their therapeutic potential in surgically-induced rat model of osteoarthritis. Osteoarthr Cartil. 2017;25:321–331. doi: 10.1016/j.joca.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 221.Garg S, Sadhukhan R, Banerjee S, et al. Gamma-tocotrienol protects the intestine from radiation potentially by accelerating mesenchymal immune cell recovery. Antioxidants (Basel, Switzerland) 2019;8:57. doi: 10.3390/antiox8030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wilankar C, Khan NM, Checker R, et al. Gamma-tocotrienol induces apoptosis in human T cell lymphoma through activation of both intrinsic and extrinsic pathways. Curr Pharm Des. 2011;17:2176–2189. doi: 10.2174/138161211796957463. [DOI] [PubMed] [Google Scholar]
- 223.Ahmed RA, Alawin OA, Sylvester PW. γ-Tocotrienol reversal of epithelial-to-mesenchymal transition in human breast cancer cells is associated with inhibition of canonical Wnt signalling. Cell Prolif. 2016;49:460–470. doi: 10.1111/cpr.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lin TP, Hom Yun Kit, Richards J, Nandi S. Effects of antioxidants and reduced oxygen tension on rat mammary epithelial cells in culture. In Vitro Cell Dev Biol Anim. 1991;27A:191–196. doi: 10.1007/BF02630915. [DOI] [PubMed] [Google Scholar]
- 225.Offord EA, Gautier J-C, Avanti O, et al. Photoprotective potential of lycopene, β-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radic Biol Med. 2002;32:1293–1303. doi: 10.1016/S0891-5849(02)00831-6. [DOI] [PubMed] [Google Scholar]
- 226.Dhein S, Kabat A, Olbrich A, et al. Effect of chronic treatment with vitamin E on endothelial dysfunction in a type I in vivo diabetes mellitus model and in vitro. J Pharmacol Exp Ther. 2003;305:114–122. doi: 10.1124/jpet.102.045740. [DOI] [PubMed] [Google Scholar]
- 227.Steiner M, Li W, Ciaramella JM, et al. Dl-alpha-tocopherol, a potent inhibitor of phorbol ester induced shape change of erythro- and megakaryoblastic leukemia cells. J Cell Physiol. 1997;172:351–360. doi: 10.1002/(SICI)1097-4652(199709)172:3<351::AID-JCP9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 228.Pazdro R, Burgess JR. Differential effects of α-tocopherol and N-acetyl-cysteine on advanced glycation end product-induced oxidative damage and neurite degeneration in SH-SY5Y cells. Biochim Biophys Acta Mol Basis Dis. 2012;1822:550–556. doi: 10.1016/j.bbadis.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 229.Faedmaleki F, Shirazi FH, Ejtemaeimehr S, et al. Study of silymarin and vitamin E protective effects on silver nanoparticle toxicity on mice liver primary cell culture. Acta Med Iran. 2016;54:85–95. [PubMed] [Google Scholar]
- 230.Tangney CC, Shekelle RB, Raynor W, et al. Intra- and interindividual variation in measurements of beta-carotene, retinol, and tocopherols in diet and plasma. Am J Clin Nutr. 1987;45:764–769. doi: 10.1093/ajcn/45.4.764. [DOI] [PubMed] [Google Scholar]
- 231.Winklhofer-Roob BM, van’t Hof MA, Shmerling DH. Reference values for plasma concentrations of vitamin E and A and carotenoids in a Swiss population from infancy to adulthood, adjusted for seasonal influences. Clin Chem. 1997;43:146–153. doi: 10.1093/clinchem/43.1.146. [DOI] [PubMed] [Google Scholar]
- 232.High KP, Legault C, Sinclair JA, et al. Low plasma concentrations of retinol and α-tocopherol in hematopoietic stem cell transplant recipients: the effect of mucositis and the risk of infection. Am J Clin Nutr. 2002;76:1358–1366. doi: 10.1093/ajcn/76.6.1358. [DOI] [PubMed] [Google Scholar]
- 233.Borel P, Moussa M, Reboul E, et al. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J Nutr. 2007;137:2653–2659. doi: 10.1093/jn/137.12.2653. [DOI] [PubMed] [Google Scholar]
- 234.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented neurobasal™, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 235.Brewer GJ, Cotman CW. Survival and growth of hippocampal neurons in defined medium at low density: advantages of a sandwich culture technique or low oxygen. Brain Res. 1989;494:65–74. doi: 10.1016/0006-8993(89)90144-3. [DOI] [PubMed] [Google Scholar]
- 236.Spector AA, Mathur SN, Kaduce TL. Lipid nutrition and metabolism of cultured mammalian cells. Prog Lipid Res. 1980;19:155–186. doi: 10.1016/0163-7827(80)90003-X. [DOI] [PubMed] [Google Scholar]
- 237.Bhatti FUR, Kim SJ, Yi A-K, et al. Cytoprotective role of vitamin E in porcine adipose-tissue-derived mesenchymal stem cells against hydrogen-peroxide-induced oxidative stress. Cell Tissue Res. 2018;374:111–120. doi: 10.1007/s00441-018-2857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.El Alami M, Viña-Almunia J, Gambini J, et al. Activation of p38, p21, and NRF-2 mediates decreased proliferation of human dental pulp stem cells cultured under 21% O2. Stem Cell Rep. 2014;3:566–573. doi: 10.1016/j.stemcr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Malakoutikhah M, Satarian L, Kiani S, Javan M. Alpha-tocopherol increases the proliferation of induced pluripotent stem cell derived neural progenitor cells. Physiol Pharmacol. 2015;19:90–98. [Google Scholar]
- 240.Wu Y, Viana M, Thirumangalathu S, Loeken MR. AMP-activated protein kinase mediates effects of oxidative stress on embryo gene expression in a mouse model of diabetic embryopathy. Diabetologia. 2012;55:245–254. doi: 10.1007/s00125-011-2326-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Santander N, Lizama C, Parga MJ, et al. Deficient vitamin E uptake during development impairs neural tube closure in mice lacking lipoprotein receptor SR-BI. Sci Rep. 2017;7:5182. doi: 10.1038/s41598-017-05422-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Huang Y-H, Sharifpanah F, Becker S, et al. Impact of arachidonic acid and the leukotriene signaling pathway on vasculogenesis of mouse embryonic stem cells. Cells Tissues Organs. 2016;201:319–332. doi: 10.1159/000445680. [DOI] [PubMed] [Google Scholar]
- 243.Sauer H, Bekhite MM, Hescheler J, Wartenberg M. Redox control of angiogenic factors and CD31-positive vessel-like structures in mouse embryonic stem cells after direct current electrical field stimulation. Exp Cell Res. 2005;304:380–390. doi: 10.1016/j.yexcr.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 244.Na L, Wartenberg M, Nau H, et al. Anticonvulsant valproic acid inhibits cardiomyocyte differentiation of embryonic stem cells by increasing intracellular levels of reactive oxygen species. Birth Defects Res Part A Clin Mol Teratol. 2003;67:174–180. doi: 10.1002/bdra.10030. [DOI] [PubMed] [Google Scholar]
- 245.Wo Y, Zhu D, Hu Y, et al. Reactive oxygen species involved in prenylflavonoids, icariin and icaritin, initiating cardiac differentiation of mouse embryonic stem cells. J Cell Biochem. 2008;103:1536–1550. doi: 10.1002/jcb.21541. [DOI] [PubMed] [Google Scholar]
- 246.Sauer H, Neukirchen W, Rahimi G, et al. Involvement of reactive oxygen species in cardiotrophin-1-induced proliferation of cardiomyocytes differentiated from murine embryonic stem cells. Exp Cell Res. 2004;294:313–324. doi: 10.1016/j.yexcr.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 247.Boland MJ, Nazor KL, Loring JF. Epigenetic regulation of pluripotency and differentiation. Circ Res. 2014;115:311–324. doi: 10.1161/CIRCRESAHA.115.301517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Depeint F, Bruce WR, Shangari N, et al. Mitochondrial function and toxicity: role of B vitamins on the one-carbon transfer pathways. Chem Biol Interact. 2006;163:113–132. doi: 10.1016/j.cbi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 249.Malavolta M. Molecular basis of nutrition and aging. London: Academic Press; 2016. [Google Scholar]
- 250.Ragsdale SW. Catalysis of methyl group transfers involving tetrahydrofolate and B(12) Vitam Horm. 2008;79:293–324. doi: 10.1016/S0083-6729(08)00410-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yamazoe H, Kobori M, Murakami Y, et al. One-step induction of neurons from mouse embryonic stem cells in serum-free media containing vitamin B12 and heparin. Cell Transpl. 2006;15:135–145. doi: 10.3727/000000006783982061. [DOI] [PubMed] [Google Scholar]
- 253.Bannister DW, O’Neill IE, Whitehead CC. The effect of biotin deficiency and dietary protein content on lipogenesis, gluconeogenesis and related enzyme activities in chick liver. Br J Nutr. 1983;50:291–302. doi: 10.1079/BJN19830097. [DOI] [PubMed] [Google Scholar]
- 254.Mock DM, Stratton SL, Horvath TD, et al. Urinary excretion of 3-hydroxyisovaleric acid and 3-hydroxyisovaleryl carnitine increases in response to a leucine challenge in marginally biotin-deficient humans. J Nutr. 2011;141:1925–1930. doi: 10.3945/jn.111.146126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Huskisson E, Maggini S, Ruf M. The role of vitamins and minerals in energy metabolism and well-being. J Int Med Res. 2007;35:277–289. doi: 10.1177/147323000703500301. [DOI] [PubMed] [Google Scholar]
- 256.Cascante M, Centelles JJ, Veech RL, et al. Role of thiamin (vitamin B-1) and transketolase in tumor cell proliferation. Nutr Cancer. 2000;36:150–154. doi: 10.1207/S15327914NC3602_2. [DOI] [PubMed] [Google Scholar]
- 257.Shi L, Tu BP. Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr Opin Cell Biol. 2015;33:125–131. doi: 10.1016/j.ceb.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Janssen JJE, Grefte S, Keijer J, de Boer VCJ. Mito-nuclear communication by mitochondrial metabolites and its regulation by B-vitamins. Front Physiol. 2019;10:78. doi: 10.3389/fphys.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Xuemei L, Jing Y, Bei X, et al. Retinoic acid improve germ cell differentiation from human embryonic stem cells. Iran J Reprod Med. 2013;11:905–912. [PMC free article] [PubMed] [Google Scholar]
- 260.Rasouli-Ghahroudi AA, Akbari S, Najafi-Alishah M, Bohloli M. The effect of vitamin K2 on osteogenic differentiation of dental pulp stem cells: an in vitro study. Regen Reconstr Restor. 2017;2:26–29. doi: 10.22037/RRR.V2I1.18536. [DOI] [Google Scholar]
- 261.Cortes M, Chen MJ, Stachura DL, et al. Developmental vitamin D availability impacts hematopoietic stem cell production. Cell Rep. 2016;17:458–468. doi: 10.1002/jmri.25711.PET/MRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Trüeb RM. Serum biotin levels in women complaining of hair loss. Int J Trichology. 2016;8:73–77. doi: 10.4103/0974-7753.188040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cuerq C, Peretti N, Chikh K, et al. Overview of the in vitro stability of commonly measured vitamins and carotenoids in whole blood. Ann Clin Biochem. 2014;52:259–269. doi: 10.1177/0004563214542471. [DOI] [PubMed] [Google Scholar]
- 264.El-Heis S, Crozier SR, Robinson SM, et al. Higher maternal serum concentrations of nicotinamide and related metabolites in late pregnancy are associated with a lower risk of offspring atopic eczema at age 12 months. Clin Exp Allergy. 2016;46:1337–1343. doi: 10.1111/cea.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kathman JV, Kies C. Pantothenic acid status of free living adolescent and young adults. Nutr Res. 1984;4:245–250. doi: 10.1016/S0271-5317(84)80009-3. [DOI] [Google Scholar]
- 266.Puebla C, Cisterna BA, Salas DP, et al. Linoleic acid permeabilizes gastric epithelial cells by increasing connexin 43 levels in the cell membrane via a GPR40- and Akt-dependent mechanism. Biochim Biophys Acta. 2016;1861:439–448. doi: 10.1016/j.bbalip.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Belal SA, Sivakumar AS, Kang DR, et al. Modulatory effect of linoleic and oleic acid on cell proliferation and lipid metabolism gene expressions in primary bovine satellite cells. Anim Cells Syst (Seoul) 2018;22:324–333. doi: 10.1080/19768354.2018.1517824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Figueroa V, Sáez PJ, Salas JD, et al. Linoleic acid induces opening of connexin26 hemichannels through a PI3K/Akt/Ca2+-dependent pathway. Biochim Biophys Acta Biomembr. 2013;1828:1169–1179. doi: 10.1016/j.bbamem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 269.Walocko FM, Eber AE, Keri JE, et al. The role of nicotinamide in acne treatment. Dermatol Ther. 2017;30:e12481. doi: 10.1111/dth.12481. [DOI] [PubMed] [Google Scholar]
- 270.Bains P, Kaur M, Kaur J, Sharma S. Nicotinamide: mechanism of action and indications in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:234–237. doi: 10.4103/ijdvl.IJDVL_286_17. [DOI] [PubMed] [Google Scholar]
- 271.Gale EAM, Group* TENDIT (ENDIT) Intervening before the onset of Type 1 diabetes: baseline data from the European Nicotinamide Diabetes Intervention Trial (ENDIT) Diabetologia. 2003;46:339–346. doi: 10.1007/s00125-003-1033-8. [DOI] [PubMed] [Google Scholar]
- 272.Lenglet A, Liabeuf S, Guffroy P, et al. Use of nicotinamide to treat hyperphosphatemia in dialysis patients. Drugs R D. 2013;13:165–173. doi: 10.1007/s40268-013-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Janssens GO, Rademakers SE, Terhaard CH, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol. 2012;30:1777–1783. doi: 10.1200/JCO.2011.35.9315. [DOI] [PubMed] [Google Scholar]
- 274.Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8:13–14. [PMC free article] [PubMed] [Google Scholar]
- 275.Jonas WB, Rapoza CP, Blair WF. The effect of niacinamide on osteoarthritis: a pilot study. Inflamm Res. 1996;45:330–334. doi: 10.1007/BF02252945. [DOI] [PubMed] [Google Scholar]