Abstract
In most vertebrates, the yolk sac (YS) represents the very first tissue where blood cells are detected. Therefore, it was thought for a long time that it generated all the blood cells present in the embryo. This model was challenged using different animal models, and we now know that YS hematopoietic precursors are mostly transient although their contribution to the adult system cannot be excluded. In this review, we aim at properly define the different waves of blood progenitors that are produced by the YS and address the fate of each of them. Indeed, in the last decade, many evidences have emphasized the role of the YS in the emergence of several myeloid tissue-resident adult subsets. We will focus on the development of microglia, the resident macrophages in the central nervous system, and try to untangle the recent controversy about their origin.
Keywords: Yolk Sac, Primitive hematopoiesis, Definitive hematopoiesis, Microglia, Hematopoietic stem cells
Introduction
The adult hematopoietic tissue is constantly replenished from hematopoietic stem cells (HSCs) that, in most vertebrates, reside in the bone marrow. These HSCs, which are mostly quiescent [1, 2] allow full reconstitution of the blood tissue through differentiating into many multipotent progenitors to repopulate all blood lineages in circulation and in tissues [3, 4]. However, in the two last decades, evidence pointed to the existence of self-renewing tissue-resident macrophage subsets. These different macrophage populations (microglia in the brain, Kupffer cells in the liver, Langerhans cells in the epidermis, among others) are formed during embryogenesis and persist throughout adulthood (for a complete review, see [5]). Fate-mapping analyses have shown that these populations probably, for most of them, originate from yolk sac (YS) hematopoiesis.
In all vertebrates, HSCs are born from a hemogenic endothelium that lies on the ventral side of the embryonic aorta [6–9], although they are not the first blood cells generated in the embryo. In amniotes, the first hematopoietic site is the YS; while in anamniotes vertebrates, hematopoiesis starts in the rostral blood island and intermediate cell mass, and in the ventral blood island, in fish and xenopus, respectively [10, 11]. Following these observations, it was first believed that the YS produces HSCs, as they can be detected in this tissue in early embryos [12]. However, the use of quail-chicken chimeras in the 80 s showed that HSCs were exclusively intra-embryonic derived [13, 14], which was later confirmed in mammals [15]. Of note, despite being strongly supported by a wealth of molecular, cellular and functional data, this paradigm has been constantly challenged over the years. Central to this was the use of new tamoxifen-inducible fate-mapping mouse models, some of which concluded to a possible contribution of YS-derived cells to the pool of adult HSCs [12, 16, 17]. However, in light of recent evidence pointing to the unexpected long persistence of tamoxifen in vivo [18] and the possible simultaneous labeling of AGM-derived HSCs in such settings, the relevance of these findings remains unclear. Finally, recent data point to the existence of additional waves of multipotent progenitors with HSC characteristics [19, 20].
During development, the YS is formed after gastrulation as an extraembryonic splanchnopleura, resulting from the association of two endodermal and mesodermal cell sheets. In all amniotes, the YS plays a role in transporting nutriments from the yolk to the embryo (in reptiles and birds), or from the mother to the embryo, as it contributes to the establishment of the primitive cord blood before it degenerates (in placental mammals). One noticeable exception is the mouse, as the embryo wraps himself in the YS at mid-gestation. In teleosts and amphibians, there is no such structure or tissue as the YS, as there are no extraembryonic tissues. However, there are functional equivalents that produce the first hematopoietic cells/progenitors.
In this review, we will focus on the different types of hematopoietic cells/progenitors that are produced in the YS, their origin and their fate during embryogenesis, with mammals as our main model. The YS will appear as a hematopoietic tissue, far more complex as what can be thought.
Yolk sac hematopoiesis
Hemangioblasts
During mouse embryogenesis, the YS is the first tissue to produce hematopoietic cells. These first blood cells can be found in very close association with vascular progenitors in structures identified as blood islands. These mouse blood islands, first described back to 1920 in the avian embryo [21], can be detected as early as embryonic day (E) 7.5, as individual structures looking like spheres of erythrocyte progenitors surrounded by vascular progenitors. Over development, the different blood islands will merge to form a primitive extra-embryonic vascular plexus, whose lumen is then filled with primitive erythrocytes (EryP). At E8.25 (5-somite stage), when the YS vascular system is connected to the intra-embryonic circulation [22], the EryP can now be pumped by the beating heart and circulate in the embryo.
Because of the spatio-temporal proximity between blood and vascular progenitors in blood islands, it has long been proposed that both cell types derive from a common progenitor: the hemangioblast. The existence for such progenitors has first been supported by the co-expression of blood and endothelial markers in single cells of the YS [23, 24]. The clonal contribution of a hemangioblast to a single blood island is still controversial, as lineage-tracing experiments have shown that each so-called hemangioblast progenitor can contribute to multiple blood islands [25]. Moreover, using the same set-up, a dual contribution to both endothelial and hematopoietic fate from the same progenitor was rarely observed. It is important to note here that these experiments were performed from tetrachimeric blastocysts, where different grafted ES cells were carrying a different fluorescent protein. These analyses would now benefit from the use of new experimental approaches, such as the Brainbow technology [26] combined to the adequate CRE recombinase reporter lines, to examine the validity of the hemangioblast hypothesis in vivo.
However, at least in an in vitro setting, the use of embryonic stem cells has allowed to identify a specific subset of progenitors that fulfill the criteria for the hemangioblast: differentiation into blood and vascular cells at the single-cell level. Such blast colony-forming cells were first identified as adherent cells expressing Flk1/Kdr, CD31 and Tie-2 [27]. Later, it was shown that they also co-expressed Brachyury, which served as a marker for isolating them from Brachyury-GFP transgenic mouse embryos [28]. Hemangioblasts could be purified from E7–7.5 embryos and cultured in conditions that allowed both endothelial and blood development. The clones that grew gave rise to adherent and non-adherent cells, comprising endothelial and vascular smooth muscle cell lineages and hematopoietic cells, respectively. Notably, the authors noted a heterogeneity among the hematopoietic progeny, and among the different hemangioblast clones, as they could obtain either primitive erythroid potential and/or monocyte/macrophage potential as well as very few clones with the ability to develop into C-myb-positive “definitive” hematopoietic progenitors [28]. We will discuss these different classes of hematopoietic subsets in the following chapters.
Primitive hematopoiesis in the YS
Primitive hematopoiesis consists of the wave of blood cells that are specified the first during embryogenesis. However, since this initial definition, several layers of complexity and new concepts have been added to this terminology. Primitive hematopoiesis also refers to cells that possess different characteristics than their adult counterparts, as well as to cells that do not contribute to the adult hematopoietic tissue, compared to definitive hematopoiesis, which depends solely on HSCs. Finally, we have introduced the idea that primitive hematopoiesis consists of the specification of unipotent hematopoietic precursors; whereas, definitive hematopoiesis initiates with the specification of multipotent precursors [29, 30], independent of previously admitted criteria. This last definition seems to correlate with the identity of the direct precursors of each hematopoietic wave. Indeed, while primitive hematopoietic progenitors derive directly from a hemangioblast, definitive hematopoietic progenitors differentiate from a hemogenic endothelium.
Primitive red blood cells
Primitive erythrocytes represent the best studied example of primitive hematopoiesis. They are the first hematopoietic cells to enter circulation at the onset of heart beating, at around E8.25 [22]. As their definitive counterparts, primitive erythrocytes play an important role in the transport of O2 molecules in the developing embryo. However, two main characteristics differentiate them from their adult counterparts. First, they express different hemoglobin chains, such as βH1, and ε chains, when adult erythrocytes mainly express α and β hemoglobins [31, 32]. Although embryonic and adult hemoglobins share the same structure, the former display higher avidity for O2 molecules, therefore allowing for better oxygen capture from the maternal bloodstream. The second main difference resides in the presence of an intact nucleus in circulating primitive erythrocytes [33]. This nucleated phase is transient, however, and like any other red blood cells, primitive erythrocytes will eventually expel their nuclei, at mid-gestation stage. Primitive erythrocyte enucleation appears to occur within the blood circulation, implying that it happens outside the structure called erythroblastic island, and, therefore, in the absence of a central macrophage [34]. Alternatively, resident macrophages encountered while transiting by the fetal liver and spleen could also contribute to enucleation of primitive erythrocytes [35, 36].
As explained before, primitive erythrocytes are thought to derived directly from the hemangioblast, or the hematopoietic-prone extra-embryonic lateral plate mesoderm. For instance, in the zebrafish, the primitive erythroid precursors can be detected as early as 11–12 h post-fertilization. At this stage, two bilateral stripes of undifferentiated lateral plate mesoderm start to express gata1, as well as other genes important for the transition from mesoderm to hematopoiesis, such as runx1, lmo2, tal1, the expression of which is conserved in the YS blood islands of mammals [11].
Primitive megakaryocytes
Limiting dilution assays scoring for hematopoietic progenitors in the early YS have revealed the existence of unipotent progenitors committed towards the thrombocyte lineage [37, 38]. Like their adult counterparts, primitive megakaryocytes differentiate into platelets that can be first detected in circulation at around E10.5. They seem to emerge rapidly, as attested by their presence in the YS as early as E7.25, but the identity of their direct progenitors is still unknown. Whether they share a common precursor with primitive erythrocytes has not been investigated, although it is likely that they also derive from a hemangioblast-like subset. YS primitive megakaryocytes differ from adult megakaryocytes by their size and polyploidy; indeed, they measure less than 10um (compared to around 20um for adult bone marrow megakaryocytes) and their DNA content remains below 4 N, compared to up to 16 N for adult BM cells. Megakaryocytes also appear to lack expression of the pan-leukocytic marker CD45 until the fetal liver stage [39]. Of note, although the primitive and definitive megakaryocyte lineages share a core developmental program, they also seem to have different molecular requirements, as primitive megakaryopoiesis occurs independent of the MPL–THPO pathway [40].
Primitive macrophages
The existence of yolk sac macrophages has been first evidenced by Naito and Takahashi [41–43]. In their seminal papers, they reported the existence of two distinct waves of macrophages in the early YS between E7 and E10. According to their data, the two waves could be discriminated based on the hematopoietic process that would generate them. The first wave of (or fetal) macrophages would differentiate by bypassing the monocytic stage, while the development of macrophages from the second wave would follow the classical cellular pathway of myelopoiesis, as known to occur in the adult BM. For these reasons, they termed these two waves primitive and definitive macrophages. The existence of macrophage unipotent progenitors was later confirmed by performing limiting dilution assays in methylcellulose [44].
We revisited this model a few years later, aided by new cell isolation protocols, in vitro cell culture assays and direct imaging of the YS. By characterizing the hematopoietic cells in the YS, we found two waves of macrophages, as described by Naito and Takahashi. However, we found that the population previously referred to as primitive macrophages was not generated in situ in the YS, but was in fact of maternal origin [30], i.e., macrophages derived from the mother and invading the extra-embryonic tissues. Whether or not their presence in the YS is linked to placentation has not been elucidated. However, the life span of these macrophages appears to be limited, as they were never found in the embryo proper. Importantly, maternal macrophages displayed a differentiated cell morphology, thus correlating with observations made by Naito and Takahashi that they were bypassing the monocytic stage.
On the other hand, we could isolate cKit-positive cells from the E7.5 YS that would exclusively give rise to macrophage colonies in vitro [30]. Both the hematopoietic potential and the kinetic of appearance of these cells were comparable to that of a population of murine embryonic myeloid progenitors previously identified by Palis and colleagues [44]. These macrophage precursors developed into macrophages through a monocytic intermediate and expressed genes related to the monocyte/macrophage lineage, such as Lysozyme M, Myeloperoxidase, or c-fms. This population represents the primitive macrophages, i.e., the very first macrophages produced by the embryo in the early YS. The precise origin of these primitive macrophages is currently unknown. This lineage has been described in other species, such as Zebrafish, Xenopus and avian embryos. In Zebrafish, they derive from a distinct territory compared to primitive erythrocytes (anterior versus posterior lateral plate mesoderm, respectively), therefore, excluding the possibility of a common precursor to both lineages [45]. It could be, therefore, speculated that both mouse lineages also arise independently in the YS.
Conclusion on primitive hematopoiesis
During development, the first primitive hematopoietic cells are produced in the YS. They arise from monopotent progenitors and have distinct properties compared to their definitive counterparts (Table 1). This fast differentiation will allow for the rapid production of all the blood cells that are necessary for the development of the early embryo. The exact origin of these primitive cells is difficult to assess as very few progenitors can be sorted from the very early YS stages. However, in vitro studies using embryonic stem cells have raised the possibility that primitive erythroblast (βH1-GFP) could derive from a Tie2+cKit+ hemogenic endothelium [46]. This was corroborated in vivo, by showing that these two markers co-localized with βH1 in the E7.5 YS [46]. Further studies will be necessary to reinforce this hypothesis as the expression of Tie2 and cKit is largely shared by a variety of endothelial, hematopoietic and mesodermal progenitors. Moreover, it will be necessary to also re-assess the precise origin of the other primitive lineages. We will now discuss how the YS produces “definitive” hematopoietic cells, and if/how they contribute to the embryonic and/or adult tissues.
Table 1.
Primitive versus definitive hematopoiesis in the mouse model
| Primitive lineage | Transient-definitive lineage | Definitive lineage | References | |
|---|---|---|---|---|
| Erythrocytes | Nucleated when released in blood flow, before enucleation. Diameter: 12mm Embryonic hemoglobins (βH1, ε, ζ) | Enucleation before release into circulation Diameter: 10mm Fetal hemoglobins (βH1 and β1-adult) | Enucleation before release into circulation Diameter: 8mm Adult hemoglobin (β-major) | Craig and Russel [31]; Wong et al. [32]; McGrath et al. [22]; Kingsley et al. [34], McGrath et al. [48] ) |
| Megakaryocytes | Average size: 9 um Polyploidy < 4N | No information | Average size: 20 um Polyploidy > 16N | Tober et al. [37]; Xu et al. [38] |
| Macrophages | Maternal-derived macrophages: Not produced by the YS. Initially described as primitive macrophages by-passing the monocytic stage | Takahashi et al. [43]; Naito et al. [42]; Naito et al. [41]; Bertrand et al. [30] | ||
| YS-derived macrophages: CD45+ Mac1+ CSF1R+ CX3CR1+ F4/80+ Derive from a monopotent (Mϕ-restricted) progenitors, through a monocytic stage Proliferative capacity Colonize all embryonic tissues | EMP-derived macrophages: CD45+ Mac1+ CSF1R+ CX3CR1+ F4/80+ Produced in the fetal liver environment | BM-derived macrophages: CD45+ Mac1+, CSF1R+ CX3CR1+ F4/80+ Derive from a Common Myeloid Progenitor, through a monocytic stage No proliferative capacity Colonize tissues upon inlfammation | Palis et al. [44]; Bertrand et al. [29]; Bertrand et al. [30]; Ovchinnikov [66] |
Transient-definitive hematopoiesis in the YS
Multipotent erythromyeloid precursors in the YS
The existence of erythromyeloid progenitors has been widely documented in the adult bone marrow, where common myeloid precursors give rise to erythroid, megakaryocytic and myeloid lineages at the single-cell level [47]. However, in the bone marrow, these cells derive from HSCs, which is not the case in the YS.
YS-derived multipotent erythromyeloid precursors (EMPs) have been first identified through in vitro differentiation assays in methylcellulose culture. When Palis and colleagues evaluated the hematopoietic potential of progenitor cells isolated from the YS, not only did they obtain colonies of macrophages (see previous section), but also they observed mixed colonies containing erythrocytes and myeloid cells [44]. These colonies were big and seemed to arise from highly proliferative progenitors, hence their qualification as HPP-CFC, high-proliferative potential colony-forming-cells. Interestingly, erythrocytes in these colonies were not of primitive origin, as they did not express embryonic globins but rather a unique combination of hemoglobins (see Table 1). Later, we purified HPP-CFCs based on their expression of multiple surface markers. At E10–E10.5 in the mouse embryo, we showed that all EMPs were contained in the CD45locKit+AA4.1+ YS cell subset [29]. Interestingly, this phenotype was identical to the first HSCs identified in the intra-embryonic AGM region [29]. However, both populations displayed different differentiation capacity when plated as single cells. Indeed, AGM-derived cells differentiated into B cells, erythrocytes and myeloid cells; whereas, YS-derived cells only produced red blood cells and myeloid cells, in similar conditions [29]. While at the time we could only separate these two populations of hematopoietic progenitors based on their differentiation capacity in vitro, we found that these two cell subsets expressed different transcription factors. Eventually, Palis and colleagues found that EMPs could be purified based on their expression of CD16/32, which was not expressed by HSCs in the AGM region [48]. They also showed that EMPs could differentiate into erythrocytes expressing a specific hemoglobin combination [48] (see Table 1).
We established that during development, EMPs appear as Kit + precursors in the YS as early as E7.5–E8 [30], which was consistent with the emergence of HPP-CFCs [44]. Lately, it was revealed that EMPs, like HSCs, also derive from hemogenic endothelial cells, and similarly depend on the transcription factor Runx1 [49]. However, they developmental programs differ by their requirements for Notch signaling. Whereas Notch is essential for the emergence of HSCs in the intra-embryonic aorta [50, 51], it is dispensable for the generation of EMPs in the mouse YS [51] and in zebrafish [52]. Altogether, these findings show that EMPs do not arise from an arterial-derived hemogenic endothelium, but rather from a non-specified or a venous endothelium. This origin appears to be conserved across the vertebrate phylum as EMPs also derive from a venous hemogenic endothelium in the zebrafish embryo [52, 53]. Moreover, HSCs, but not EMPs, express Gata3 (a Notch target gene) [29], and Hlf, as shown recently [54].
We and others have established that EMPs are the first hematopoietic precursors to seed the fetal liver during mouse development. It is only in this particular microenvironment that they differentiate into definitive erythroid cells as well as myeloid cells—neutrophils and macrophages [30, 48].
Altogether, EMPs are very close to HSCs in terms of ontogeny, development and phenotype. The main differences identified so far reside in their absence of lymphoid potential, as well as their inability to self-renew. Indeed, YS progenitors show no capacity for long-term reconstitution of lethally irradiated hosts, whereas AGM progenitors do [15]. Importantly, these similarities/differences have created a pressing need for a better definition of primitive versus definitive hematopoiesis (see Box1 for definitions). Through many aspects, EMPs do not meet the criteria of primitive hematopoiesis, as discussed in the previous section. However, like HSCs, they are multipotent, they are specified from a hemogenic endothelium, and their generation is controlled by the transcription factor Cmyb. Therefore, if we limit definitive hematopoiesis to these criteria, EMPs fit to the definition, and because of their non-contribution to the adult hematopoietic system, many developmental hematologists now refer to this population as transient-definitive hematopoietic progenitors. However, this issue appears more complicated and will be discussed in Box2.
Nevertheless, as the field of developmental immunology is evolving rapidly, it seems more important than ever for investigators to follow a unified nomenclature. Lately, for example, the terminology of the YS-derived myeloid lineages has been a point of confusion, creating difficulties in the interpretation of many sets of data. Indeed, from the literature it appears that several groups and investigators now refer to primitive macrophages as “early EMPs” or “EMP1” and to bona fide EMPs as “late EMPs” or “EMP2”. From a functional point of view, this terminology is incorrect since primitive macrophage progenitors lack any erythroid potential. In addition, this usage is misleading, as it suggests a certain relationship between the two populations. For these reasons, as well as to avoid confusion with another incorrect notion that primitive macrophages constitute the progeny of the EMP1 population, we would strongly advise to refrain using this new terminology.
The particular case of HSC-independent lymphoid progenitors
Over the course of probing for hematopoietic cell activity in the early murine embryo, several studies have reported the existence of lymphoid-primed progenitors that would arise independently from and prior to HSCs. Lineage-tracing experiments, as well as more classical in vivo adoptive transfers and in vitro differentiation assays, have shown that these progenitors give rise to innate B1a cells [55, 56], as well as γδ T cells that will later colonize the epidermis [57, 58]. In the case of innate B-1 lymphocytes, cells exhibiting B-1 cell differentiation potential have been found in the YS of murine HSC-deficient embryos, and in that of embryos with defective blood circulation, therefore excluding any contribution from circulating intraembryonic-derived progenitors [56, 58]. It should be noted, however, these transplantable B-1 progenitor cells exhibited low differentiation efficiency in vivo. In addition, lineage-tracing using Cdh5-CREert2 at very early stages (E7.5) revealed that these progenitors also arise from a hemogenic endothelium in the YS [59]. However, due to technical limitations inherent to the Cre-lox technology (as discussed before), labeling and subsequent fate-mapping of intra-embryonic progenitors cannot be excluded in these experiments, thus leaving open the question of the precise origin of these lymphoid progenitors [18]. For more insights into the ontogeny of innate-like B lymphocytes, please refer to this recent review from Ghosn and colleagues [60].
Of note, the existence of HSC-independent T-cell progenitors have also been documented in the zebrafish embryo. According to Tian and colleagues, the hemogenic endothelium in the aorta produces restricted T-cell progenitors and definitive HSCs at the same time, in a very narrow spatio-temporal window [61].
Fate of YS hematopoietic progenitors
In the previous part, we defined the different hematopoietic lineages that are born in the YS microenvironment. Here, we will summarize the fate of these hematopoietic cells, with a particular emphasis on the establishment of tissue-resident myeloid cells.
Primitive erythrocytes anucleate in circulation
We previously discussed that primitive erythrocytes are produced concomitantly to the primitive extraembryonic vascular plexus. Therefore, as soon as the heart starts beating, towards E8.25 [22], these primitive red blood cells are released in circulation, even though their differentiation is not totally over, as they are still nucleated. Kingsley and colleagues have shown that in the mouse embryo, the enucleation step occurs in circulation, between E12.5 and E16.5 [34]. Whether or not macrophages are directly involved in this process is yet to be proved, as they are absolutely necessary for the final maturation of definitive erythrocytes in the fetal liver and bone marrow [62, 63]. However, a contribution from fetal liver macrophages (as well as placenta-resident macrophages to a lesser extend) cannot be excluded as they are able to phagocytose the nuclei of fluorescently marked primitive erythrocytes (using the ε-globin-H2B-CFP line) [64]. This wave of erythropoiesis is then progressively replaced by fetal and definitive adult erythropoiesis but mature primitive red blood cells can be detected in blood circulation up to 5–6 days after birth [34].
Primitive macrophages colonize all embryonic tissues
Csf1r, the receptor for Csf1 (also known as M-CSF) and for Interleukin-34, is exclusively expressed by cells from the monocytic/macrophage lineage [65]. By establishing the Csf1r-GAL4/UAS-ECFP transgenic mouse, Ovchinnikov and colleagues showed that macrophages already reach all tissues as early as E9, including the brain rudiment and the epidermis, among others [66]. At E9, we previously showed that EMPs have not started their differentiation into any lineage yet [30]; therefore, these early macrophages can only represent the progeny of the primitive macrophages lineage. It was long believed that their only roles were to phagocytose dead cells, and remodel tissues as development progresses.
EMPs colonize the FL to differentiate
At E10.5, both EMPs and HSCs are found in the same phenotypic subset of the AGM. As it is now established that EMPs are only produced in the YS, these observations indicate that EMPs have the ability to colonize the embryo [29]. Indeed, their presence has been detected in the embryonic blood circulation, as well as in the FL before it is colonized by HSCs [67]. McGrath and colleagues showed that EMPs likely contribute to the first embryonic neutrophils as early as E11.5 [67], confirming our earlier findings that EMPs could differentiate in the FL microenvironment. Indeed, we had previously shown that FL organ culture colonized by freshly isolated YS-derived EMPs differentiated into myeloid cells (Gr1+Mac1+ neutrophils) but not into lymphoid B cells [30]. Although this wave is transient, it will likely provide the embryo with a large array of myeloid cells, as well as definitive erythrocytes.
Origin of tissue-resident macrophages: the particular example of microglia
Across the vertebrate phyla, the brain is the first organ to be colonized by macrophages during embryogenesis. The detection of macrophages as soon as they begin to invade the brain at early developmental stages has been well documented in various species, including the mouse, the chick and the zebrafish. Once established, these macrophages differentiate locally into microglia, which exhibit fundamental roles during brain and spinal cord development and homeostasis, both in the steady state and disease. The origin of microglia, whose maintenance in the central nervous system relies on local progenitors rather than adult HSCs [68, 69], has been largely debated over the past two decades. Recently, by uncovering the fetal origin of murine microglial cells, such studies have paved the way to the new paradigm in immunology that most tissue-resident macrophages originate from embryonic precursors, and have, thus, contributed to changing the landscape of the field of developmental hematopoiesis.
Extraembryonic origin of microglia in the vertebrate embryo
The concept of an extraembryonic origin of microglia emerged in the 1990s, with investigators documenting the ontogeny of brain macrophages through meticulous embryological observations. These early studies revealed that macrophages in various vertebrate species begin to invade the brain early during development, within a time window that coincides with the appearance of primitive macrophages in the YS. This view was further supported by findings from avian YS chimera and parabiotic experiments which indicated that most of the early avian CNS macrophages have a YS origin [70, 71]. Moreover, the detection in the mouse embryo of microglial progenitors exhibiting high proliferative potential prompted the hypothesis that adult microglia might be derived from these initial residents [72]. However, despite the elegance of the idea, experimental evidence was lacking at the time and it took a decade and new advances in cell lineage-tracing methods to shed light on the fate of these early microglia. In 2010, Ginhoux and colleagues were the first to validate the theory of the YS origin for adult microglia. In their seminal study, the group made use of a Runx1:Mer-cre-Mer knock-in allele, allowing for the permanent genetic labeling of Runx1-expressing hematopoietic cell progenitors in the mouse model. They showed that treatment with tamoxifen between E7 and E7.5 resulted in 30% of the adult microglia population being marked. As this time window encompasses YS hematopoiesis, these findings firmly supported a direct developmental relationship between YS-derived progenitors and microglia in the adult mouse [73]. Another important conclusion from this study was to rule out the contribution of embryonic hematopoietic stem cells to microglia ontogeny, as injection of tamoxifen between E8.5 and E10, which efficiently labeled circulating monocytes, did not mark adult microglia [73]. This paper was soon followed by several studies employing newly generated inducible fate-mapping models such as Csf1rMerCreMer and Cx3cr1CreERT2, and which all reached similar conclusions in delineating E7–E8 as the time window of appearance of microglial progenitors in the mouse embryo [74–80].
Precise origin of microglia? YS, yes, but primitive macrophages or EMPs?
Although the combined results and conclusions of many such fate-mapping investigations convincingly identified the YS as the source of adult microglia in the mouse, one important question that remained a hotly debated topic was the cellular identity of the YS-derived microglial precursor. While Ginhoux et al. initially proposed that primitive macrophages serve as the embryonic precursors of microglia in the mouse [73], this was later challenged by several reports claiming EMPs as their direct cells of origin [76, 81]. At the heart of the controversy was the fact that none of the reporter mice, used to permanently label YS-derived hematopoietic progenitors, was specific for either primitive macrophages or EMPs. As both populations express Runx1, Csf1r and Cx3cr1 and temporally overlap during YS hematopoiesis, this complicated result interpretation (see Fig. 1). Although a pattern emerged from these studies that suggested a strong correlation between labeling of adult microglia and CRE induction at a stage when the first wave of YS-derived myeloid cells begins to appear, other approaches were needed to characterize in more details the nature of the microglial progenitor. We tackled this question using the zebrafish embryo as a model of investigation, taking advantage of the distinct anatomical locations of each hematopoietic wave [82]. A key aspect of our experiment was the use of a constitutive fate-mapping strategy that unambiguously distinguishes primitive (primitive macrophages) versus definitive (EMPs and HSCs) hematopoietic waves, based on the hemogenic nature of their precursors [6, 53]. This cell-specific lineage-tracing approach, in combination with analyses of hematopoietic mutant lines, allowed us to reinvestigate the microglial potential of primitive macrophages, EMPs and HSCs in vivo [6, 53]. Interestingly, these studies found that the zebrafish microglia network is established without any contribution from EMPs. Instead, microglia in the zebrafish embryo are exclusively generated by primitive macrophages [6, 53]. Because microglia ontogeny in both mouse and zebrafish embryos occurs in a C-myb/Myb-independent manner [79], it was tempting to speculate that primitive macrophages also served as the source of murine microglia, owing to the well-established dispensability of this transcription factor for primitive macrophage development in vertebrates. However, as it remains unclear whether all the macrophage potential of the EMPs relies on Myb, the question of the identity of the microglial precursor remained open. In our view, unequivocal evidence that the unique lineage relationship between embryonic microglia and primitive macrophages was phylogenetically conserved came from a recent study in the mouse addressing the requirement for Kit-ligand (Kitlg) in the different embryonic hematopoietic niches [83]. While Kitlg-deficient embryos possess functional primitive macrophages, they display severe defects in the normal expansion of YS-derived EMPs, resulting in a reduced, phenotypically and functionally impaired EMP pool. Interestingly, at E14.5 strongly reduced numbers of macrophages were found in the skin, lungs, limb buds and fetal liver of Kitlg-deficient embryos. In striking contrast, microglia cell numbers in the brain remained unaffected [83]. Together, these experiments provide compelling evidence that during mouse embryogenesis, primitive macrophages, and not EMPs, are the true source of microglia. Likewise, this important study also showed the role of EMP-derived macrophages (fetal monocytes) in the ontogeny of other tissue-resident macrophage subsets (Langerhans cells, lung macrophages, among others). Microglia ontogeny in the mouse embryo, thus, closely parallels that of zebrafish embryos, with Kitlg-independent primitive macrophages serving as the unique source of microglia in the embryo.
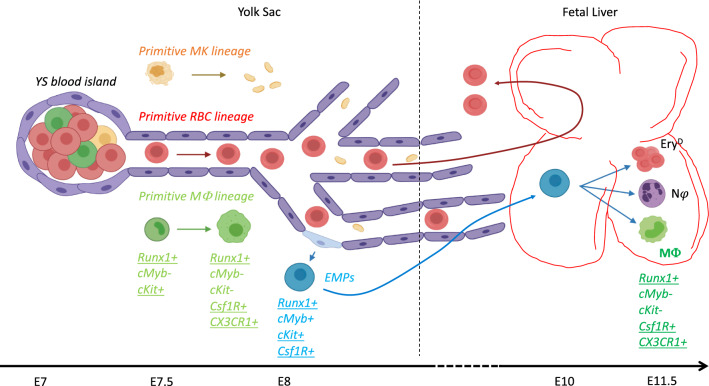
Fig. 1.
Origin and fate of YS-derived erythro-myeloid progenitors. Shortly after gastrulation, blood islands form in the YS. They will give rise (through hemangioblast progenitors?) to the three primitive lineages: primitive megakaryocytes (MK), primitive Red Blood Cells (RBCs) and primitive macrophages (MΦ). Later, by E8 at the onset of blood circulation, hemogenic endothelial cells give rise to erythro-myeloid progenitors (EMPs). These newly generated progenitors will colonize the fetal liver where they can differentiate into definitive erythrocytes as well as myeloid cells. The expression of several markers is indicated as they have been used to understand the ontogeny of adult tissue-resident cells. Underlined markers have been used for direct cell fate-mapping. The figure illustrates that these markers are shared between the different waves and clearly overlap in time. (This figure has been realized through the BioRender web interface)
Multiple waves of microglia?
Despite these great advances in our understanding of microglia ontogeny, the controversy remains in the field as whether microglia could be derived from more than one source, as it has been found for tissue-resident macrophages of the skin, testis or heart [78, 84, 85]. Indeed, identification of the microglial potential of YS-derived primitive macrophages in the murine embryo does not necessarily exclude a partial contribution of EMPs to the adult microglia network. Interestingly, in the mouse, the existence of a second microglia population with a distinct origin from early microglia was recently reported by Capecchi and colleagues [86]. This population, which is detected in the brain at E13 and accounts for about 30% of total microglia in the adult, can be traced back to Hoxb8 + cells that emerge in the embryo in a spatial and temporal fashion reminiscent of EMPs [86]. Whether this microglia subset originates from EMPs remains, however, to be determined. The identification of molecular markers that unambiguously define EMPs and could serve for the generation of novel EMP-specific CRE driver lines may help to solve this question. Going forward, it would also be interesting to determine the status of the Hoxb8 + microglia subset in Kitlg-deficient mice [83]. Because this mutation is embryonically lethal, such experiments will, however, need to rely on in utero rescuing of Kitlgnull embryos. Nevertheless, while it will be exciting to clarify the existence of this second microglia wave in the mouse, firm evidence that brain colonization by microglia progenitors can happen at multiple stages of embryonic life has been provided by the zebrafish model. Indeed, we and others have found that embryonic microglia in zebrafish are transient, as they are fully replaced at late larval stages by a definitive population that persists through adulthood [53, 87]. Interestingly, through fate-mapping analyses and grafts, we identified cmyb-dependent embryonic HSCs as the source of adult microglia [53]. Thus, in zebrafish, the microglial network is established through two successive waves of progenitors. This is in contrast to the mouse, where embryonic microglia remain into adulthood, without any contribution from HSCs. These findings have paved the way to new investigations aimed at exploring whether the distinct lineage histories between mouse and zebrafish adult microglia may confer different phenotypic and functional properties to each population.
Conclusion
The YS is the first tissue to produce blood cells, and was such originally thought as the place where all blood cells, including definitive HSCs, were generated. However, multiple approaches in different animal models have shown that multipotent, self-renewing HSCs are born intra-embryonically, while the YS first produces monopotent, then oligopotent non-self-renewing progenitors. Nevertheless, many fate-mapping experiments have pointed to the origin of several adult tissue-resident macrophages subsets in YS hematopoiesis, which is possible as YS-derived macrophages colonize many tissues where they will settle, independent of any bone marrow input during steady-state adult life. Indeed, many studies have shown that these populations of tissue-resident macrophages can self-renew locally. However, in the mouse model, their precise cellular origin is still controversial as the fate-mapping tools developed so far always target several waves of hematopoiesis, due to the temporal overlapping of primitive and definitive hematopoiesis, and because all these progenitors share multiple markers. Therefore, it will be necessary to better characterize these subsets to unravel new specific markers. In this context, single cell RNA sequencing holds great promises [88, 89] and will likely contribute to the identification of new hematopoietic subsets in the embryo. Ultimately, this will result in the emergence of novel concepts and great advances in our knowledge of tissue-resident macrophages in the adult.
Acknowledgements
V.W. is an investigator of WELBIO and is also supported by grants from the Fonds National de la Recherche Scientifique (FNRS) and The Minerve Foundation. J.Y.B. is funded by the swiss national fund (#310030_184814).
Box 1: a few definitions about developmental hematopoiesis
Monopotent blood progenitor: blood precursor endowed with a single differentiation capacity.
Multipotent blood progenitor: blood progenitor that can give rise to multiple lineages after differentiation.
Hematopoietic stem cells: multipotent self-renewable hematopoietic precursors. They are the only ones with the ability to reconstitute all blood lineages after transplantation. In the adult animal they are located mainly in the bone marrow, whereas their niche is the fetal liver during embryogenesis in mammals. These cells are specified during a very short time window during embryogenesis from the main arteries, through a process called endothelial-to-hematopoiesis transition (EHT).
Hemogenic endothelium: it consists of a limited number of endothelial cells that undergo EHT to give rise to hematopoietic progenitors.
Hemangioblast: mesoderm-derived precursor endowed with endothelial and hematopoietic potential at the single-cell level.
Primitive hematopoiesis: historically, represents the very first wave of hematopoiesis in the embryo. The blood cells produced from this wave are specified from mesoderm immediately after gastrulation. This wave does not originate from hematopoietic stem cells.
Definitive hematopoiesis: historically, represents the process that generates blood cells in the adult, and by extension all hematopoietic phenomena that concern HSCs.
Box2: time to revisit nomenclature in developmental hematopoiesis?
EMPs are referred to as transient definitive progenitors. However, using the term “definitive” similarly between HSCs and EMPs could be misleading, as it may imply that those progenitors are not transient and remain throughout life. Since it is widely accepted that HSCs are truly definitive (they seed the bone marrow prior to birth and regenerate immune cells throughout life), limiting the use of “definitive” for cells that are indeed derived from HSCs may thus better reflect the biology. As a consequence, mono- or bi- (i.e., mesoderm/hemangioblastderived cells) versus multi-potency (hemogenic endotheliumderived progenitors) could be addressed separately from the term “primitive”. Such approach would also leave room for additional progenitors that may be identified in the future, as well as for the transient lymphoid-primed progenitors that emerge from the hemogenic endothelium before the HSCs. However, perhaps the most appropriate designation would be to describe hematopoiesis as pre- (independent of) HSCs and post- (dependent of) HSCs, with the HSC-independent hematopoiesis compartment including monopotent (mesoderm/hemangioblast-derived) progenitors in the blood island and later multi-potent progenitors derived from the hemogenic endothelium. While these ideas can certainly serve as a starting point for discussion, deciding on a revised terminology will however require a large community effort.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Busch K, et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015;518(7540):542–546. doi: 10.1038/nature14242. [DOI] [PubMed] [Google Scholar]
- 2.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 3.Carrelha J, et al. Hierarchically related lineage-restricted fates of multipotent haematopoietic stem cells. Nature. 2018;554(7690):106–111. doi: 10.1038/nature25455. [DOI] [PubMed] [Google Scholar]
- 4.Sawai CM, et al. Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals. Immunity. 2016;45(3):597–609. doi: 10.1016/j.immuni.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44(3):439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand JY, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boisset JC, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464(7285):116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 8.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464(7285):112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 9.Lam EY, et al. Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood. 2010;116(6):909–914. doi: 10.1182/blood-2010-01-264382. [DOI] [PubMed] [Google Scholar]
- 10.Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102(6):787–796. doi: 10.1016/s0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 11.Davidson AJ, Zon LI. The 'definitive' (and 'primitive') guide to zebrafish hematopoiesis. Oncogene. 2004;23(43):7233–7246. doi: 10.1038/sj.onc.1207943. [DOI] [PubMed] [Google Scholar]
- 12.Weissman IPV, Gardner R. Fetal hematopoietic origins of the adult hematolymphoid system. In: Clarckson MPB, Till JE, editors. Differentiation of normal and neoplastic hematopoietic cellss. New York: Cold Spring Harbor Laboratory; 1978. pp. 33–47. [Google Scholar]
- 13.Dieterlen-Lievre F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J Embryol Exp Morphol. 1975;33(3):607–619. [PubMed] [Google Scholar]
- 14.Dieterlen-Lievre F, Beaupain D, Martin C. Origin of erythropoietic stem cells in avian development: shift from the yolk sac to an intraembryonic site. Ann Immunol (Paris) 1976;127(6):857–863. [PubMed] [Google Scholar]
- 15.Cumano A, et al. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15(3):477–485. doi: 10.1016/s1074-7613(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 16.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446(7139):1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, et al. Early ontogenic origin of the hematopoietic stem cell lineage. Proc Natl Acad Sci U S A. 2012;109(12):4515–4520. doi: 10.1073/pnas.1115828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senserrich J, et al. Analysis of Runx1 using induced gene ablation reveals its essential role in pre-liver HSC development and limitations of an in vivo approach. Stem Cell Rep. 2018;11(3):784–794. doi: 10.1016/j.stemcr.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaudin AE, et al. A transient developmental hematopoietic stem cell gives rise to innate-like B and T cells. Cell Stem Cell. 2016;19(6):768–783. doi: 10.1016/j.stem.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yvernogeau L, et al. In vivo generation of haematopoietic stem/progenitor cells from bone marrow-derived haemogenic endothelium. Nat Cell Biol. 2019;21(11):1334–1345. doi: 10.1038/s41556-019-0410-6. [DOI] [PubMed] [Google Scholar]
- 21.Sabin F. Studies on the origin of blood-vessels and of red corpuscules as seen in the living blastoderm of chicks during the second day of incubation. Carnegie Contrib Embryol. 1920;272:214–262. [Google Scholar]
- 22.McGrath KE, et al. Circulation is established in a stepwise pattern in the mammalian embryo. Blood. 2003;101(5):1669–1676. doi: 10.1182/blood-2002-08-2531. [DOI] [PubMed] [Google Scholar]
- 23.Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95(5):1671–1679. [PubMed] [Google Scholar]
- 24.Manaia A, et al. Lmo2 and GATA-3 associated expression in intraembryonic hemogenic sites. Development. 2000;127(3):643–653. doi: 10.1242/dev.127.3.643. [DOI] [PubMed] [Google Scholar]
- 25.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11(4):519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Livet J, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450(7166):56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 27.Choi K, et al. A common precursor for hematopoietic and endothelial cells. Development. 1998;125(4):725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 28.Huber TL, et al. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432(7017):625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand JY, et al. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc Natl Acad Sci U S A. 2005;102(1):134–139. doi: 10.1073/pnas.0402270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertrand JY, et al. Three pathways to mature macrophages in the early mouse yolk sac. Blood. 2005;106:3004–3011. doi: 10.1182/blood-2005-02-0461. [DOI] [PubMed] [Google Scholar]
- 31.Craig ML, Russell ES. A developmental change in hemoglobins correlated with an embryonic red cell population in the mouse. Dev Biol. 1964;10:191–201. doi: 10.1016/0012-1606(64)90040-5. [DOI] [PubMed] [Google Scholar]
- 32.Wong PM, et al. Adult hemoglobins are synthesized in murine fetal hepatic erythropoietic cells. Blood. 1983;62(6):1280–1288. [PubMed] [Google Scholar]
- 33.Baron MH. Concise Review: early embryonic erythropoiesis: not so primitive after all. Stem Cells. 2013;31(5):849–856. doi: 10.1002/stem.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kingsley PD, et al. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood. 2004;104(1):19–25. doi: 10.1182/blood-2003-12-4162. [DOI] [PubMed] [Google Scholar]
- 35.Isern J, et al. The fetal liver is a niche for maturation of primitive erythroid cells. Proc Natl Acad Sci U S A. 2008;105(18):6662–6667. doi: 10.1073/pnas.0802032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGrath KE, et al. Enucleation of primitive erythroid cells generates a transient population of "pyrenocytes" in the mammalian fetus. Blood. 2008;111(4):2409–2417. doi: 10.1182/blood-2007-08-107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tober J, et al. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109(4):1433–1441. doi: 10.1182/blood-2006-06-031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu MJ, et al. Evidence for the presence of murine primitive megakaryocytopoiesis in the early yolk sac. Blood. 2001;97(7):2016–2022. doi: 10.1182/blood.v97.7.2016. [DOI] [PubMed] [Google Scholar]
- 39.Cortegano I, et al. CD45 expression discriminates waves of embryonic megakaryocytes in the mouse. Haematologica. 2019;104(9):1853–1865. doi: 10.3324/haematol.2018.192559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potts KS, et al. Mouse prenatal platelet-forming lineages share a core transcriptional program but divergent dependence on MPL. Blood. 2015;126(6):807–816. doi: 10.1182/blood-2014-12-616607. [DOI] [PubMed] [Google Scholar]
- 41.Naito M, et al. Development, differentiation, and phenotypic heterogeneity of murine tissue macrophages. J Leukoc Biol. 1996;59(2):133–138. doi: 10.1002/jlb.59.2.133. [DOI] [PubMed] [Google Scholar]
- 42.Naito M, et al. Development, differentiation, and maturation of fetal mouse yolk sac macrophages in cultures. J Leukoc Biol. 1989;46(1):1–10. doi: 10.1002/jlb.46.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi K, Yamamura F, Naito M. Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J Leukoc Biol. 1989;45(2):87–96. doi: 10.1002/jlb.45.2.87. [DOI] [PubMed] [Google Scholar]
- 44.Palis J, et al. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126(22):5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 45.Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126(17):3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- 46.Stefanska M, et al. Primitive erythrocytes are generated from hemogenic endothelial cells. Sci Rep. 2017;7(1):6401. doi: 10.1038/s41598-017-06627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akashi K, et al. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 48.McGrath KE, et al. A transient definitive erythroid lineage with unique regulation of the beta-globin locus in the mammalian embryo. Blood. 2011;117(17):4600–4608. doi: 10.1182/blood-2010-12-325357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen MJ, et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9(6):541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burns CE, et al. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19(19):2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hadland BK, et al. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood. 2004;104(10):3097–3105. doi: 10.1182/blood-2004-03-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertrand JY, et al. Notch signaling distinguishes 2 waves of definitive hematopoiesis in the zebrafish embryo. Blood. 2010;115(14):2777–2783. doi: 10.1182/blood-2009-09-244590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrero G, et al. Embryonic microglia derive from primitive macrophages and are replaced by cmyb-dependent definitive microglia in zebrafish. Cell Rep. 2018;24(1):130–141. doi: 10.1016/j.celrep.2018.05.066. [DOI] [PubMed] [Google Scholar]
- 54.Yokomizo T, et al. Hlf marks the developmental pathway for hematopoietic stem cells but not for erythro-myeloid progenitors. J Exp Med. 2019;216(7):1599–1614. doi: 10.1084/jem.20181399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi M, et al. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfbeta for their development. Proc Natl Acad Sci U S A. 2014;111(33):12151–12156. doi: 10.1073/pnas.1407370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshimoto M, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108(4):1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boiers C, et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13(5):535–548. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 58.Yoshimoto M, et al. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119(24):5706–5714. doi: 10.1182/blood-2011-12-397489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gentek R, et al. Epidermal gammadelta T cells originate from yolk sac hematopoiesis and clonally self-renew in the adult. J Exp Med. 2018;215(12):2994–3005. doi: 10.1084/jem.20181206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosn E, et al. Hematopoietic stem cell-independent hematopoiesis and the origins of innate-like B lymphocytes. Development. 2019;146(15):dev170571. doi: 10.1242/dev.170571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian Y, et al. The first wave of T lymphopoiesis in zebrafish arises from aorta endothelium independent of hematopoietic stem cells. J Exp Med. 2017;214(11):3347–3360. doi: 10.1084/jem.20170488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allen TD, Dexter TM. The essential cells of the hemopoietic microenvironment. Exp Hematol. 1984;12(7):517–521. [PubMed] [Google Scholar]
- 63.Lichtman MA. The ultrastructure of the hemopoietic environment of the marrow: a review. Exp Hematol. 1981;9(4):391–410. [PubMed] [Google Scholar]
- 64.Vacaru AM, et al. Analysis of primitive erythroid cell proliferation and enucleation using a cyan fluorescent reporter in transgenic mice. Genesis. 2013;51(11):751–762. doi: 10.1002/dvg.22420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sasmono RT, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101(3):1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 66.Ovchinnikov DA. Macrophages in the embryo and beyond: much more than just giant phagocytes. Genesis. 2008;46(9):447–462. doi: 10.1002/dvg.20417. [DOI] [PubMed] [Google Scholar]
- 67.McGrath KE, et al. Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 2015;11(12):1892–1904. doi: 10.1016/j.celrep.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ajami B, et al. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10(12):1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 69.Mildner A, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10(12):1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 70.Cuadros MA, et al. First appearance, distribution, and origin of macrophages in the early development of the avian central nervous system. J Comp Neurol. 1993;330(1):113–129. doi: 10.1002/cne.903300110. [DOI] [PubMed] [Google Scholar]
- 71.Kurz H, Christ B. Embryonic CNS macrophages and microglia do not stem from circulating, but from extravascular precursors. Glia. 1998;22(1):98–102. [PubMed] [Google Scholar]
- 72.Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117(2):145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 73.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeFalco T, et al. Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc Natl Acad Sci U S A. 2014;111(23):E2384–E2393. doi: 10.1073/pnas.1400057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Epelman S, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomez Perdiguero E, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoeffel G, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42(4):665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mossadegh-Keller N, et al. Developmental origin and maintenance of distinct testicular macrophage populations. J Exp Med. 2017;214(10):2829–2841. doi: 10.1084/jem.20170829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 80.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kierdorf K, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16(3):273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 82.Bertrand JY, Traver D. Hematopoietic cell development in the zebrafish embryo. Curr Opin Hematol. 2009;16(4):243–248. doi: 10.1097/MOH.0b013e32832c05e4. [DOI] [PubMed] [Google Scholar]
- 83.Azzoni E, et al. Kit ligand has a critical role in mouse yolk sac and aorta-gonad-mesonephros hematopoiesis. EMBO Rep. 2018;19:e45477. doi: 10.15252/embr.201745477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoeffel G, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209(6):1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Molawi K, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med. 2014;211(11):2151–2158. doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De S, et al. Two distinct ontogenies confer heterogeneity to mouse brain microglia. Development. 2018;145(13):dev152306. doi: 10.1242/dev.152306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu J, et al. Temporal-spatial resolution fate mapping reveals distinct origins for embryonic and adult microglia in zebrafish. Dev Cell. 2015;34(6):632–641. doi: 10.1016/j.devcel.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 88.Moignard V, et al. Decoding the regulatory network of early blood development from single-cell gene expression measurements. Nat Biotechnol. 2015;33(3):269–276. doi: 10.1038/nbt.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Popescu DM, et al. Decoding human fetal liver haematopoiesis. Nature. 2019;574(7778):365–371. doi: 10.1038/s41586-019-1652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]