Abstract
Wnt ligands signal through canonical or non-canonical signaling pathways. Although both routes share common elements, such as the Fz2 receptor, they differ in the co-receptor and in many of the final responses; for instance, whereas canonical Wnts increase β-catenin stability, non-canonical ligands downregulate it. However, both types of ligands stimulate tumor cell invasion. We show here that both the canonical Wnt3a and the non-canonical Wnt5a stimulate Fz2 tyrosine phosphorylation, Fyn binding to Fz2, Fyn activation and Fyn-dependent Stat3 phosphorylation. Wnt3a and Wnt5a require Src for Fz2 tyrosine phosphorylation; Src binds to canonical and non-canonical co-receptors (LRP5/6 and Ror2, respectively) and is activated by Wnt3a and Wnt5a. This Fz2/Fyn/Stat3 branch is incompatible with the classical Fz2/Dvl2 pathway as shown by experiments of over-expression or depletion. Fyn is necessary for transcription of genes associated with invasiveness, such as Snail1, and for activation of cell invasion by both Wnt ligands. Our results extend the knowledge about canonical Wnt pathways, demonstrating additional roles for Fyn in this pathway and describing how this protein kinase is activated by both canonical and non-canonical Wnts.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03221-2) contains supplementary material, which is available to authorized users.
Keywords: Canonical Wnt, Non-canonical Wnt, Src, Fyn, Stat3
Introduction
Wnt proteins induce the activation of several signaling pathways essential in different physiological processes. They have been classified either canonical or non-canonical, according to their effect on β-catenin. Canonical Wnts, such as Wnt3a, enhance β-catenin stability, translocate β-catenin to the nucleus and increase β-catenin transcriptional activity [1]. In contrast, the non-canonical Wnts, such as Wnt5a, activate β-catenin-independent pathways involving the small GTPases Rho and Rac1, Vangl2 and the protein kinases Jnk2, ERK2 and Ca2+/Calmodulin-dependent kinase [2].
Although many responses to the activation of canonical and non-canonical pathways are different, they also share common elements. For instance, both Wnt3a and Wnt5a bind to the transmembrane protein Frizzled (Fz), the common receptor for both ligands. However, they also require a different co-receptor: LRP5/6 for canonical ligands and Ror2 for non-canonical Wnts [1, 3, 4]. Both Wnts activate CK1ε through the action of the Fz-associated PR61ε/Phosphatase 2A (PP2A) [5–8]. CK1ε binds to the receptor complex through its direct interaction with the non-canonical co-receptor Ror2, or indirectly to LRP5/6 through its association with p120-catenin and cadherin [8–10]. In both pathways, CK1ε activity is required for the association of Dvl2 with Fz [1, 10]. In the case of canonical Wnt, the interaction of Dvl2 with Fz2–LRP5/6 enables further reactions, such as the recruitment of CK1γ and the subsequent LRP5/6 phosphorylation on Thr1479, the binding of Axin and its associated protein kinases CK1α and GSK3 to LRP5/6, a further LRP5/6 phosphorylation at Ser1490 and the inhibition of GSK3 activity for β-catenin Ser37. Consequently, Dvl2 binding to Fz2/LRP5/6 is required for the inhibition of β-catenin proteasomal degradation by Wnt ligands [1, 10]. For non-canonical Wnts, the processes downstream of Dvl2 recruitment have not been well characterized, although they involve the activation of the Rac1 small GTPase and Jun kinase [2, 11], two elements also activated by canonical Wnts [12].
One of the final non-canonical Wnt responses is β-catenin downregulation, an effect that requires transcription of the β-catenin E3 ubiquitin ligase Siah2 [13]. Therefore, Wnt3a and Wnt5a promote opposite effects on β-catenin stability: whereas canonical Wnt increase it, non-canonical Wnts downregulate this protein. However, both types of ligands stimulate cell invasion indicating that β-catenin-dependent transcription neither explains nor is entirely responsible for this cellular property.
In 2014, it was found that Wnt5a acting through Fz2 stimulates Stat3 transcriptional activity as well as the expression of genes associated with epithelial-to-mesenchymal transcription and cell invasion [14]. As this activation is not sensitive to inhibitors of β-catenin transcriptional activity, it was, therefore, classified as a non-canonical response. Activation of Stat3 is dependent on Fz2 phosphorylation at Tyr552 by an unidentified kinase; modification of this residue promotes Fyn binding to Fz2, Fyn stimulation and Stat3 phosphorylation at Tyr705 and activation [14]. Here, we have further investigated this signaling pathway by incorporating other elements, such as Ror2 and Src. We now demonstrate that Src, Fyn and Stat3 are stimulated not only by non-canonical but also by canonical Wnts defining a novel signaling pathways. Strikingly, Src and Fyn and are required for the full response to both canonical and non-canonical ligands.
Results
Canonical and non-canonical Wnt ligands stimulate Stat3 phosphorylation
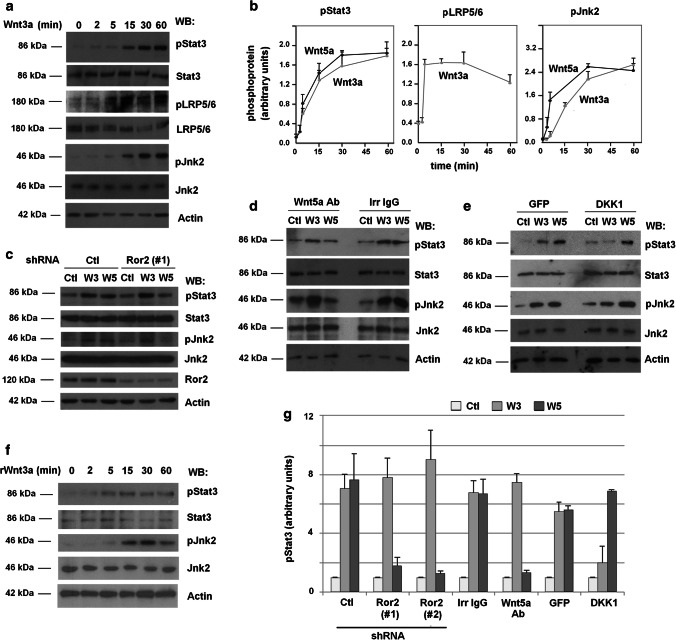
Confirming previous observations [14], we verified that Wnt5a stimulates Stat3 phosphorylation at Tyr705 (pStat3) in the human embryonic kidney HEK293T cells, which are widely used for Wnt studies (Suppl. Figure 1a). This effect of Wnt5a was dependent on the non-canonical Wnt co-receptor Ror2 as down-regulation of this pseudo-kinase with two distinct shRNAs prevents both stimulation of pStat3 and activation of Jnk2 (Suppl. Figure 1b). Unexpectedly, Stat3 phosphorylation was also stimulated by Wnt3a, an activator of the canonical pathway (Fig. 1a), with kinetics similar to that of Wnt5a (Fig. 1b). Wnt3a promoted a concomitant Jnk2 activation and a faster Ser1490 phosphorylation in LRP5/6 (Fig. 1a, b). The increase in pStat3 by Wnt3a was not due to an artefactual activation of the non-canonical pathway, as it was not affected by Ror2 depletion in contrast to the stimulation of pStat3 by Wnt5a (Fig. 1c, g and Suppl. Figure 1b). Moreover, while the effects of Wnt3a on pStat3 and pJnk2 were not inhibited by a Wnt5a blocking antibody, they were hampered by an antagonist of canonical Wnts, DKK1 [15] (Fig. 1d, e, g). As expected, DKK1 did not interfere with Wnt5a–stimulated pStat3. Finally, both recombinant Wnt3a and recombinant Wnt5a produced the same stimulation of pStat3 as the L-fibroblasts conditional medium (Fig. 1f and Suppl. Figure 1c). In sum, these results demonstrate that Wnt3a stimulates Stat3 phosphorylation through the Fz2/LRP5/6 receptor complex. To further corroborate this result, a similar pStat3 up-regulation was obtained by transfection of a constitutive-active mutant of LRP6 (CA-LRP6) that potently stimulates canonical Wnt signaling [16] (see below).
Fig. 1.
Canonical and non-canonical ligands promote Stat3 phosphorylation through LRP5/6 and Ror2 co-receptors, respectively. a HEK293T cells were stimulated with control or Wnt-3a-conditioned medium for the indicated times. Cells were lysed and proteins were analyzed by WB with specific antibodies. LRP5/6, Jnk2 and Stat3 phosphorylation was determined with anti-phospho antibodies against LRP6 (Ser1490), Jnk (Thr183/Tyr185, Thr221/Tyr223) or Stat3 (Tyr705). b A quantification of three independent experiments carried out as in (a) and Suppl. Figure 1a is represented. c HEK293T cells depleted of Ror2 with a specific shRNA were stimulated with control, Wnt3a- or Wnt5a-conditioned medium for 30 min. Cells were lysed and Jnk2 and Stat3 phosphorylation was determined as above. d, e Control, Wnt3a- or Wnt5a-conditioned medium was incubated with a Wnt5a ab or an irrelevant IgG (both 8 μg/ml) (d) for 1 h or was alternatively supplemented with DKK1 or GFP as control (e). HEK293T cells were treated for 30 min with the indicated medium; cell extracts were prepared and analyzed by WB with the indicated antibodies. f Cells were incubated for the indicated times with recombinant Wnt3a (50 ng/ml) and cell extracts analyzed by WB. g Signals from the different experiments performed as in panels (c–e) and Suppl. Figures 1b–d were densitometered and represented. The mean ± SD of three different experiments is shown
Fyn tyrosine kinase is stimulated by Wnt3a and is required for Stat3 phosphorylation
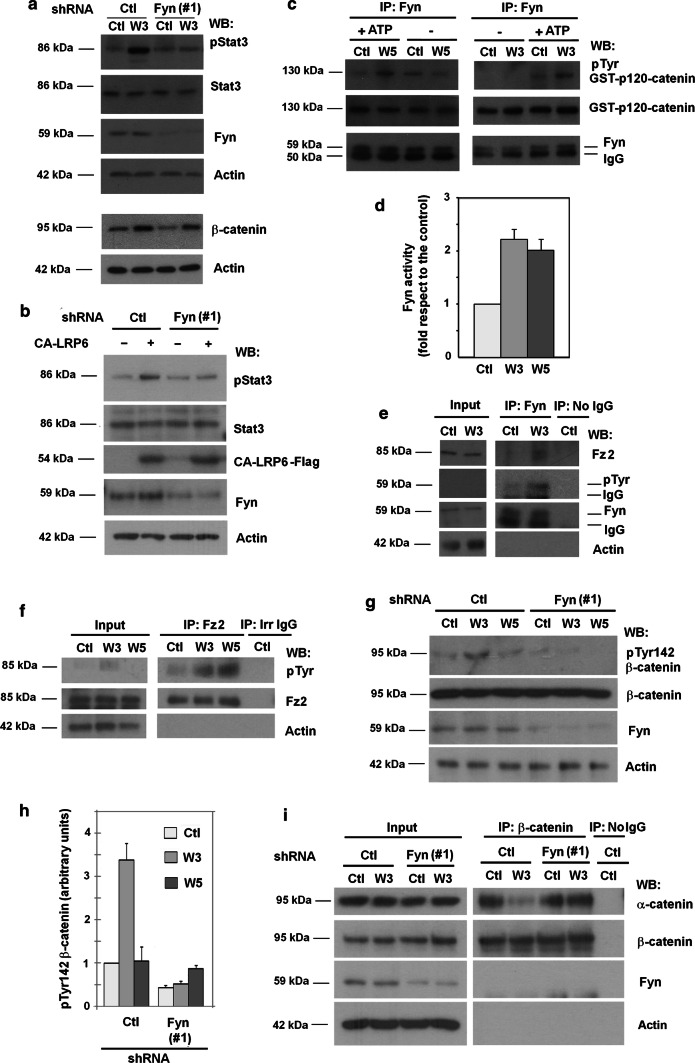
Fyn is required for Stat3 phosphorylation upon Wnt5a stimulation [14]. Decreasing in Fyn protein levels using two specific shRNAs prevented the Wnt5a-induced up-regulation in pStat3 but did not affect Ror2 and Fz2 receptors (Suppl. Figure 2a; quantification in Suppl. Figure 2b). In contrast, Fyn down-modulation did not alter the increase in pJnk2 (Suppl. Figure 2a) or in Rac1 activity (Suppl. Figure 2c), two responses to Wnt5a that have been placed downstream of Dvl2 phosphorylation and Dvl2 binding to the Fz2/Ror2 complex. Fyn depletion also prevented the Wnt3a-induced increase in pStat3 (Fig. 2a, and Suppl Figure 2b, d); in contrast, it did not alter another classical response to canonical Wnt, β-catenin stabilization (Fig. 2a). Notably, transfection of CA-LRP6, a potent activator of the canonical Wnt pathway also stimulated Stat3 phosphorylation, and this up-regulation was sensitive to Fyn depletion (Fig. 2b and Suppl Fig. 2b).
Fig. 2.
Fyn kinase is activated by Wnt3a and Wnt5a ligands. a HEK293T cells depleted of Fyn with shRNA(#1) were stimulated with control or Wnt3a-conditioned medium for 30 min to determine Stat3 phosphorylation (top), or during 16 h to analyze β-catenin levels by WB (bottom). b Cells were transfected with a CA-LRP6 expressing plasmid or the corresponding control and analyzed after 48 h by WB. c Fyn was immunoprecipitated from total cell extracts and the immunocomplex was incubated with 8 pmol of recombinant GST–p120-catenin in Fyn phosphorylation conditions. Phosphorylation of Tyr in GST-p120-catenin was analyzed by WB with a general phospho Tyr antibody. d Signal was densitometered, normalized with respect to the GST-p120-catenin and represented. The quantification of three different experiments is shown (mean ± SD). e Fyn was immunoprecipitated from total HEK293T cell extracts treated with control or Wnt3a- conditioned medium for 30 min, and tyrosine-phosphorylated Fyn or associated Fz2 were detected by WB. f Fz2 was immunoprecipitated from total HEK293T cell extracts treated with control, Wnt3a- or Wnt5a-conditioned medium for 30 min, and tyrosine-phosphorylated Fz2 or associated Fyn was detected by WB. g HEK293T cells were transfected with Fyn or control shRNAs and stimulated with control, Wnt3a or Wnt5a-conditioned medium for 30 min. Cells were lysed and analyzed by WB with specific antibodies. The densitometric analysis of the experiments is shown in h. i shRNA-transfected cells were treated with Wnt3a for 30 min; β-catenin was immunoprecipitated from cell extracts and associated α-catenin was determined by WB
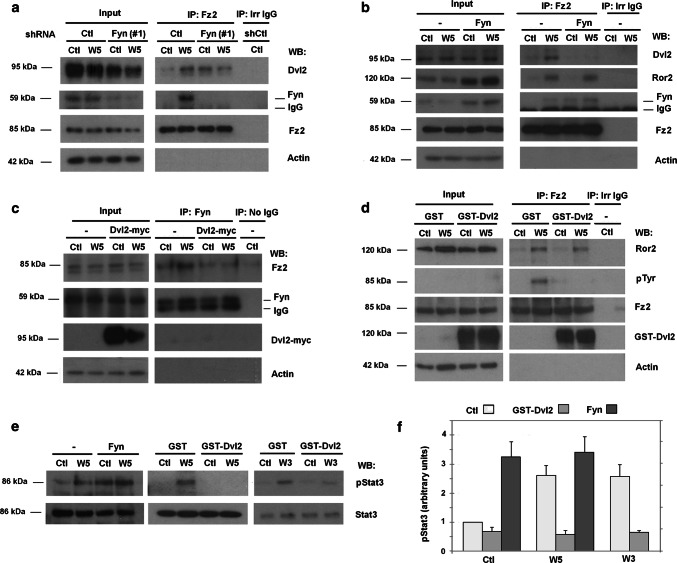
In contrast to the effect of Fyn, down-regulation of Dvl2 did not decrease Wnt3a-or Wnt5a-induced Stat3 phosphorylation; actually it slightly increased the basal phosphorylation (Suppl. Figure 2b, e). Conversely, Dvl2 was required for the up-regulation in Jnk2 phosphorylation caused by both Wnts (Suppl. Figure 2e). Therefore, pStat3 stimulation either by Wnt5a or Wnt3a is independent of Dvl2 but requires Fyn.
To test whether Fyn activity was stimulated by both Wnt3a and Wnt5a, Fyn was immunoprecipitated from cells extracts and its activity assayed in vitro on recombinant p120-catenin, a known tyrosine kinase substrate. Indeed, immunoprecipitated Fyn showed higher activity when purified from cells treated with Wnt3a or Wnt5a than from untreated cells (Fig. 2c, see also a quantification in Fig. 2d). As Fyn activation is associated with its autophosphorylation at Tyr residues, our finding that pTyr increased in Fyn following stimulation by Wnt3a (Fig. 2e) or Wnt5a (Suppl. Figures 3a, b) provided further evidence for Fyn being activated by both canonical and non-canonical Wnts.
Stimulation of Fyn by Wnt5a has been related to Fz2 phosphorylation at Tyr552 and Fyn interaction with Fz2 [14]. Both Wnt3a and Wnt5a up-regulated Fz2 tyrosine phosphorylation (Fig. 2f) and increased the levels of Fz2 that co-immunoprecipitated with Fyn (Fig. 2e and Suppl. Figure 3a; see a quantification in Suppl Fig. 3b). As Fyn also interacts with p120-catenin [17], which binds to the canonical Wnt receptor complex, we analyzed whether p120-catenin was involved in the Fyn/Fz2 association. As shown in Suppl. Figure 3c, p120-catenin depletion did not alter the amount of Fz2 that co-immunoprecipitated with Fyn suggesting a direct interaction between Fyn and Fz2.
Fyn plays an additional role in canonical Wnt by phosphorylating Tyr142 in β-catenin, which disrupts β-catenin interaction with α-catenin and facilitates its binding to Bcl9-2, a switch necessary for β-catenin transcriptional activity [17, 18]. β-catenin associates with canonical co-receptor LRP5/6 through the common interaction of β-catenin and LRP5/6 with N-cadherin [10] but it does not interact with Ror2. As it would be expected given that Fyn binds to Fz2 and is activated by Wnt3a, Wnt3a induced a Fyn-dependent increase in β-catenin phosphorylation at Tyr142 (Fig. 2g; quantification in Fig. 2h). This stimulation was not observed with Wnt5a. In line with the role of Fyn in this phosphorylation, the loss of β-catenin/α-catenin interaction caused by Wnt3a was prevented in Fyn-depleted cells (Fig. 2i).
Src phosphorylates Fz2 and increases Fz2/Fyn association
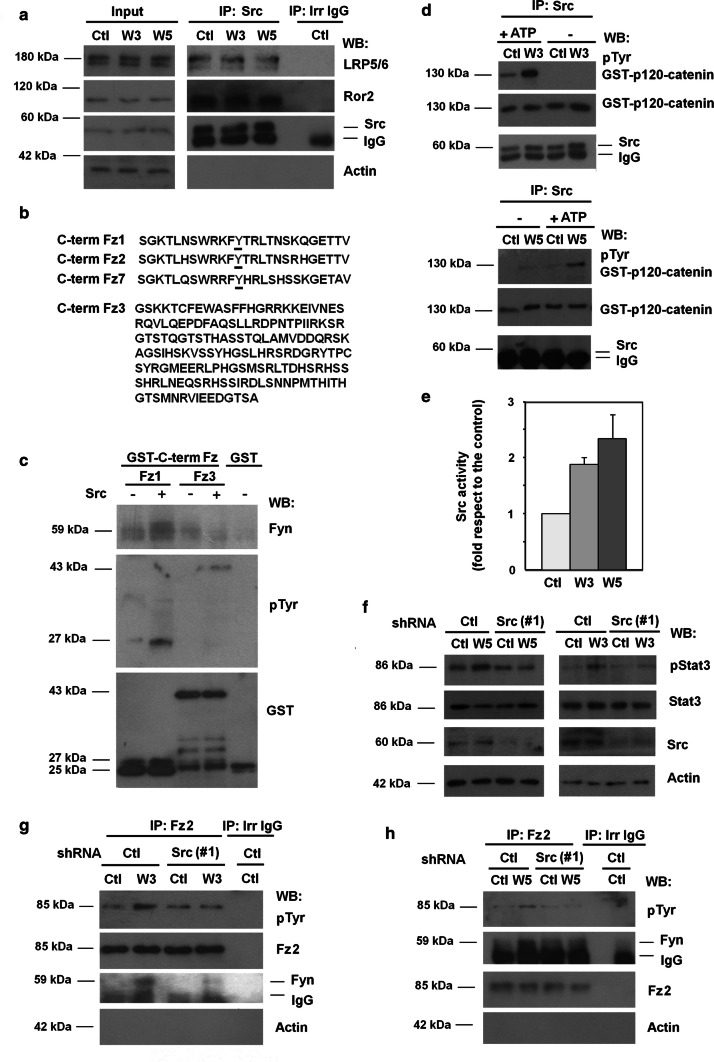
The protein kinase responsible for phosphorylating Tyr552 in Fz2, which enhances its interaction with Fyn, has not yet been identified. We speculated that this Tyr kinase should interact with the receptor complexes for both canonical and non-canonical Wnts. It has been reported that Src tyrosine kinase binds to these two proteins, LRP5/6 and Ror2 [19, 20]. We first verified these two interactions; indeed both LRP5/6 and Ror2 co-immunoprecipitated with Src regardless of Wnt activation (Fig. 3a). A pull-down assay performed with GST–Ror2 confirmed that Src interacted with this protein (Suppl. Figure 4). However, we were unable to pull down the tyrosine kinase with recombinant GST–LRP5/6, suggesting that binding to Src requires a previous modification of the LRP5/6 co-receptor.
Fig. 3.
Wnt3a- and Wnt5a-dependent Src activation promotes Tyr552 Fz2 phosphorylation and binding to Fyn. a HEK293T cells were treated with control, Wnt3a- or Wnt5a-conditioned medium for 30 min; Src was immunoprecipitated from total cell extracts, and associated co-receptors Ror2 and LRP5/6 were analyzed by WB. b Comparison of amino acid sequences of the C-terminal region of human Fz1, Fz2, Fz7 and Fz3. Underlined amino acid corresponds to Tyr552. c 4 pmol of recombinant GST-Fz1 and GST-Fz3 were in vitro phosphorylated by Src kinase. A pull-down assay was performed and associated Fyn and tyrosine-phosphorylated Fz was analyzed. d Src was immunoprecipitated from total cell extracts, and the immunocomplex was incubated with 8 pmol of recombinant GST–p120-catenin in Src phosphorylation conditions. Tyr phosphorylation in GST–p120-catenin was analyzed by WB as above. e Signal was densitometered, normalized with respect to the GST–p120-catenin and represented. The quantification of three different experiments is shown (mean ± SD). f–h HEK293T were transfected with shRNA control or shRNA Src(#1) and treated with control, Wnt3a- or Wnt5a-conditioned medium for 30 min, lysed and Stat3 phosphorylation was analyzed by WB (f). Fz2 was immunoprecipitated from total cell extracts treated with control, Wnt3a- (g) or Wnt5a-conditioned (h) medium; Fz2 tyrosine phosphorylation and Fyn recruitment to Fz2 were analyzed by WB
Tyr552 is present in the C-terminal tail of Fz1, -2 and -7 but not in other members of this family, even though they contain more extended C-terminal domains (see Fz3 in Fig. 3b). The sequences of Fz1, -2 and -7 in this domain are very similar and only include this Tyr (Fig. 3b). Recombinant Src efficiently phosphorylated a GST–Fz1 C-tail fusion protein (Fig. 3c); more importantly, this modification upregulated the association of Fyn with this fusion protein (Fig. 3c). In contrast, phosphorylation of GST–Fz3 by Src was less efficient and did not stimulate Fyn binding (Fig. 3c).
The activity of immunoprecipitated Src on recombinant p120-catenin was higher when purified from Wnt3a- or Wnt5a-treated cells (Fig. 3d, e). Moreover, down-regulation of Src protein using two specific shRNAs blocked Stat3 phosphorylation induced by Wnt3a or Wnt5a (Fig. 3f and Suppl. Figure 5a, b), and prevented both Tyr phosphorylation of Fz2 and Fz2 interaction with Fyn (Fig. 3g, h). Therefore, these results indicate that Wnt5a and Wnt3a induce the activation of the co-receptor-bound Src; this phosphorylates Fz2, thereby enhancing the association with Fyn, its activation and phosphorylation of Stat3.
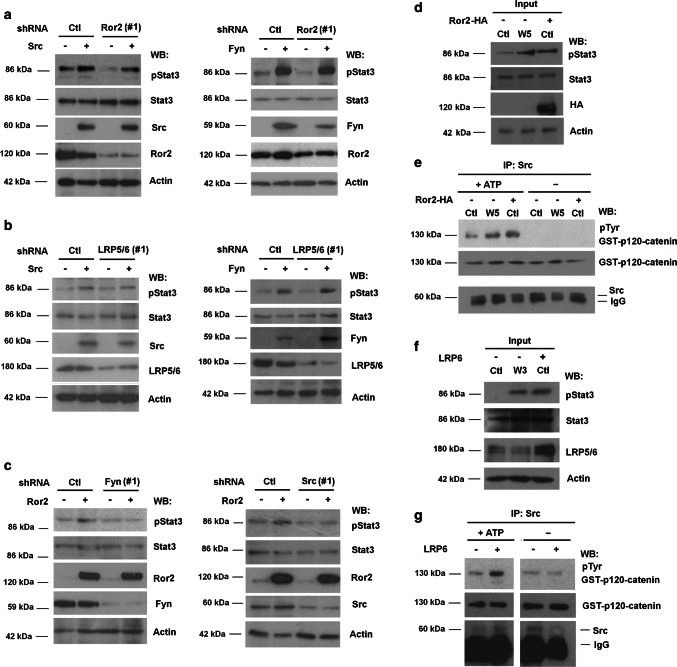
Other results also support the action of Src as an effector of Stat3 phosphorylation. Ectopic expression of Src or Fyn increased Stat3 phosphorylation even in cells with down-regulated Ror2 or LRP5/6 (Fig. 4a, b, quantification in Suppl. Figure 5c). Ror2 over-expression also upregulated pStat3 (Fig. 4c, d). Similar to the previously presented data with transfected CA-LRP6 (see Fig. 2b), pStat3 stimulation by Ror2 was dependent on Src and Fyn, as depletion of any of these kinases severely compromised it (Fig. 4c and Suppl Figure 5c). Ror2-induced Stat3 phosphorylation was associated with the activation of Src activity since this protein kinase was stimulated to the same extent by Ror2 ectopic expression as by Wnt5 (Fig. 4d, e). Similar results were obtained when the Wnt3a co-receptor LRP5/6 was ectopically over-expressed in HEK293T cells: as this co-receptor also stimulated both pStat3 and Src activity (Fig. 4f, g).
Fig. 4.
Overexpression of Ror2 and LRP6 co-receptors induces Src activation and Stat3 phosphorylation. HEK293T depleted of Ror2 (a), LRP5/6 (b), Fyn or Src (c) using specific shRNAs or non-targeting shRNAs as control and overexpressing Src, Fyn or Ror2 when indicated were lysed and Stat3 phosphorylation was analyzed by WB. Signal was densitometered, and represented in Suppl. Figure 5. HEK293T cells treated with control, Wnt-conditioned medium or overexpressing (d, e) Ror2-HA or (f, g) LRP6, were lysed and pStat3 levels (d, f) were determined by WB with a specific antibody. (e, g) Src was immunoprecipitated from whole extracts and the immunocomplex was incubated with 8 pmol of recombinant GST–p120-catenin in Src phosphorylation conditions. Tyr phosphorylation in GST–p120-catenin was analyzed by WB with a general phosphoTyr antibody
We reasoned that this activation of Src by both proteins might be the consequence of clustering of the Src-binding co-receptors, as has been described for integrins [21]. Over-expressed Ror2 aggregated: Ror2-HA was detected in anti-Flag immunocomplexes when both Ror2-HA and Ror2-Flag were ectopically expressed (Suppl. Figure 6a). Similarly, CA-LRP6-Flag also associated with the endogenous LRP5/6 (Suppl. Figure 6b). Formation of these complexes was differentially sensitive to the addition of the CK1 inhibitor PF5006739 (PF), which decreased the formation of Ror2 aggregates but not of LRP5/6 complexes (Suppl. Figs. S6a and b); accordingly, this inhibitor blocked Wnt5a- but not Wnt3a-induced Stat3 phosphorylation (Suppl. Fig. S6c).
Fyn and Dvl2 define two different branches in canonical and non-canonical signaling
As shown above, Dvl2 is not required for Stat3 phosphorylation; notably, Dvl2 down-regulation increased basal pStat3 (see Suppl Fig. 2e). Moreover, when analyzing the effect of down-modulation of Src, we detected a higher binding of Dvl2 to Fz2 in the absence of Wnt (Suppl. Figure 7a, b). We, thus, tested the possibility that the Src/Fyn branch and the Dvl2-dependent branch of both canonical and non-canonical Wnt might mutually interfere.
Fyn downregulation also increased basal Dvl2 binding to Fz2 (Fig. 5a, compare lanes 1 and 3 in Fz2 immunoprecipitate; see also Suppl. Figure 7b). Inversely, Fyn over-expression prevented Wnt5a-induced Dvl2 interaction with Fz2 receptor (Fig. 5b, Suppl. Figure 7b) without affecting the association of Fz2 with the Ror2 co-receptor. Over-expression of Dvl2 promoted the opposite effect: it prevented activation of the Fyn/Stat3 branch. Specifically, ectopic expression of Dvl2-myc inhibited the stimulation by Wnt5a or Wnt3a of Fyn/Fz2 binding as it decreased the amount of Fz2 coimmunoprecipitated with Fyn upon Wnt stimulation (Figs. 5c and Suppl. Figure 7c, d). Further, transfection of another form of Dvl2, GST-Dvl2, interfered with Fz2 phosphorylation induced by Wnt5a (Fig. 5d and Suppl. Figure 7d). In accordance with these results, ectopic transfection of Fyn promoted Stat3 phosphorylation even in the absence of stimulus, whereas that of Dvl2 prevented the increase in pStat3 caused by Wnt5a or Wnt3a (Fig. 5e, f).
Fig. 5.
Dvl2 and Fyn compete for its binding to Fz2. a HEK293T depleted of Fyn using shRNA(#1) or a non-targeting shRNA as control, or b overexpressing Fyn was treated with control or Wnt5a-conditioned medium for 30 min. Fz2 was immunoprecipitated from total cell extracts and the protein complexes were analyzed by WB. c, d HEK293T cells overexpressing Dvl2-myc or GST-Dvl2 were stimulated with control or Wnt5a-conditioned medium for 30 min. Fyn (c) or Fz2 (d) were immunoprecipitated from cell extracts and Tyr phosphorylation in Fz2 (d) and associated proteins (c, d) were assessed by WB. e Stat3 phosphorylation was analyzed in HEK293T cells transfected with Dvl2 or Fyn and stimulated with control, Wnt3a- or Wnt5a-conditioned medium. f Signals from results of experiments performed as in e was densitometered, and represented. The quantification of three different experiments is shown (mean ± SD)
Fyn is needed for a full transcriptional activation by Wnt3a
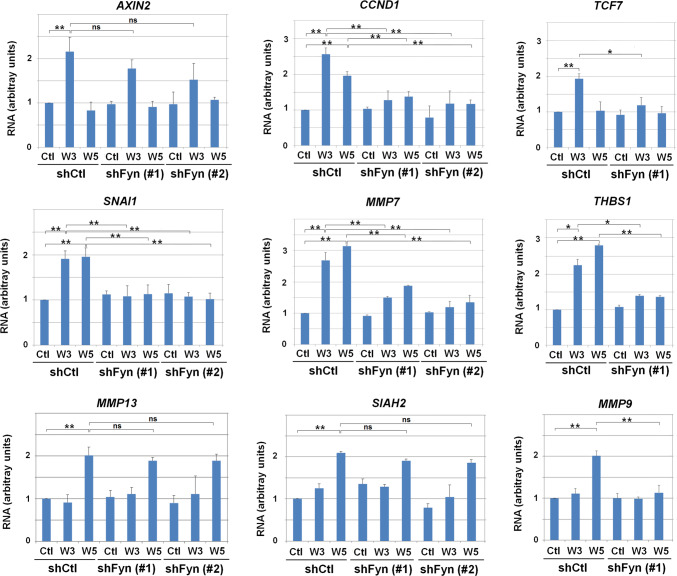
We next assessed the relevance of Fyn for the activation of Wnt5a- and Wnt3a-target genes. For that, we used Fyn down-modulated cells, previously described in Figs. 2, 4 and Suppl. Figure 2. We analyzed the transcription of nine genes known to be induced by the canonical or non-canonical pathways (Fig. 6). These genes were classified in three categories. (a) Genes whose transcription was stimulated by Wnt3a (such as AXIN2, CCND1 and TCF7) (upper row) and, in the case of CCND1, also by Wnt5a to a lesser extent. (b) Genes activated specifically by Wnt5a (such as MMP13, SIAH2 and MMP9) (lower row). (c) Genes induced by both Wnts (such as SNAI1, MMP7 and THBS1) (middle row). Six of these nine genes were dependent on Fyn (Fig. 6) Strikingly, all the genes studied whose transcription was stimulated by both Wnt3a and Wnt5a were sensitive to Fyn downregulation. These results indicate that a high proportion of genes transcriptionally activated by canonical and non-canonical Wnts are sensitive to Fyn depletion, particularly those stimulated by both types of factors.
Fig. 6.
Fyn is required for the expression of canonical and non-canonical Wnt target genes. RNA was isolated from control, Fyn#1- and Fyn#2-depleted HEK293T cells stimulated overnight with control, Wnt3a- or Wnt5a-conditioned medium. Expression of AXIN2, TCF7, CCND1, MMP7, SNAI1, THBS1, MMP13, SIAH2 and MMP9 was assessed by quantitative PCR coupled to retrotranscription. Results are presented as mean ± SD from three independent experiments performed in triplicate. *p < 0.05; **p < 0.01
Fyn is required for the activation of cellular functions by Wnt3a and Wnt5a
We next analyzed whether the Wnt3a-induced Stat3 phosphorylation was also observed in other cellular systems. Similar to Wnt5a, Wnt3a also stimulated cell invasion of the colon cancer cell lines SW620 and T47D (Suppl. Figure 8a). In both cell lines, Wnt3a and Wnt5a upregulated Stat3 phosphorylation with similar kinetics (Suppl. Figure 8b, c). Similar results were obtained in murine mesenchymal stem cells (MSC). Fyn or Src downregulation (Suppl. Figure 9a) prevented the increase in Stat3 phosphorylation caused by both Wnt5a and Wnt3a in these cells (Suppl. Figures 9b and c).
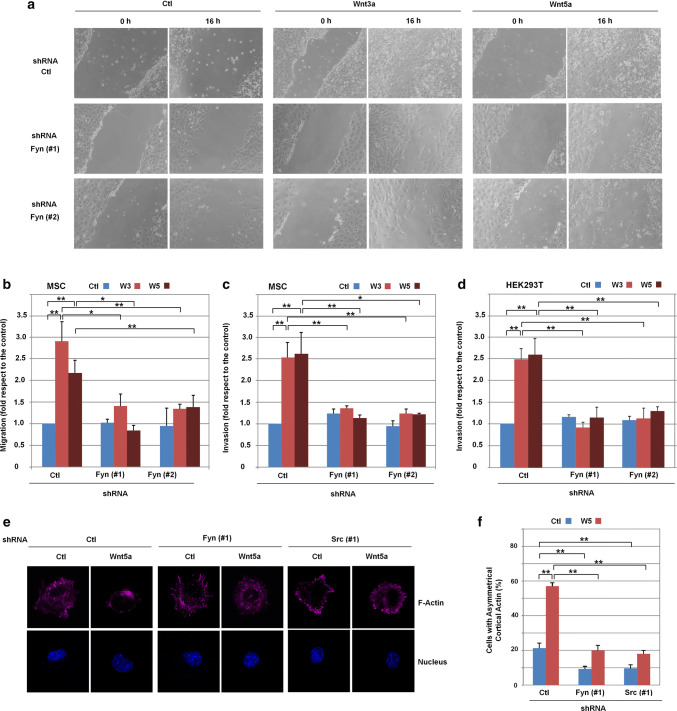
Finally, we assessed the relevance of Src/Fyn in several cellular responses to canonical and non-canonical Wnts. Both Wnt3a and Wnt5a increased the migration and invasion of MSC (Fig. 7a–c) and HEK293T cells (Fig. 7d). For both factors, the stimulation of migration and invasion was sensitive to Fyn depletion indicating that the Src/Fyn signaling pathway is necessary for a full response to either Wnt5a- or Wnt3a.
Fig. 7.
Fyn is required for cellular migration, invasion and polarization in HEK293T cells and MSC. a Micrographs showing scratch healing after 16 h of incubation of the indicated cells with control, Wnt3a or Wnt5a-medium. Assays were performed as indicated in Methods. b Transfected MSC were seeded on Transwells and stimulated for 16 h with Wnt3a and Wnt5a when indicated. Cells that have migrated through the filter cells were fixed and stained with crystal violet, and optical density was quantified at 590 nm. Results are presented as mean ± range from two independent experiments. c, d, Transfected MSCs or HEK293T cells treated with control, Wnt3a or Wnt5a-conditioned medium were seeded in Transwell chambers containing 1 mg/mL collagen type I. After 16 h (c) or 36 h (d) of incubation, cells that have migrated through collagen were quantified a described. Results are presented as mean ± range from three independent experiments. e Polarized cortical actin was determined as indicated in Methods in MSC transfected with control, Fyn or Src shRNAs treated with Wnt5a when indicated. Cells were fixed and stained for F-actin and nucleus with Dapi. f The percentage of cells presenting polarized actin in e is shown. At least 100 GFP-positive cells were counted for each condition. Results are presented as mean ± SD from three independent experiments. *p < 0.05; **p < 0.01
As previously reported [22], Wnt5a promotes the polarization of cortical actin in MSC. This effect was not observed with Wnt3a. In these cells, depletion of either Src or Fyn prevented cortical actin polarization specifically induced by Wnt5a (Fig. 7e, f).
Discussion
Although the best characterized signaling pathways activated for canonical and non-canonical Wnts are different, both types of ligands produce some common responses, such as activation of cell invasion. While investigating common reactions induced by both type of ligands we realized that both canonical and non-canonical Wnts stimulate Stat3 phosphorylation, and thus investigated the signaling pathways initiated by these factors that lead to this response. We have confirmed that Wnt5a promotes Fz2 phosphorylation, Fyn binding to Fz2 and Fyn activation [14]. This pathway is dependent on the Wnt5a co-receptor Ror2 and does not require the downstream effector Dvl2. The canonical Wnt ligand Wnt3a also activates a similar pathway and increases Fz2 phosphorylation, Fyn activity and Stat3 phosphorylation. In this case, it works independently of Ror2 and Wnt3a responses are inhibited by a specific antagonist of the canonical pathway, DKK1, which blocks the activity of the LRP5/6 canonical Wnt co-receptor [15]. Wnt3a activation of Stat3 phosphorylation was mimicked by transfection of an activated form of LRP6.
We have also identified Src, a tyrosine kinase bound to both LRP5/6 and Ror2 co-receptors, as a kinase activated by canonical and non-canonical Wnts and responsible for phosphorylating Fz2 in both pathways, thereby allowing Fyn binding to Fz2 and Fyn activation.
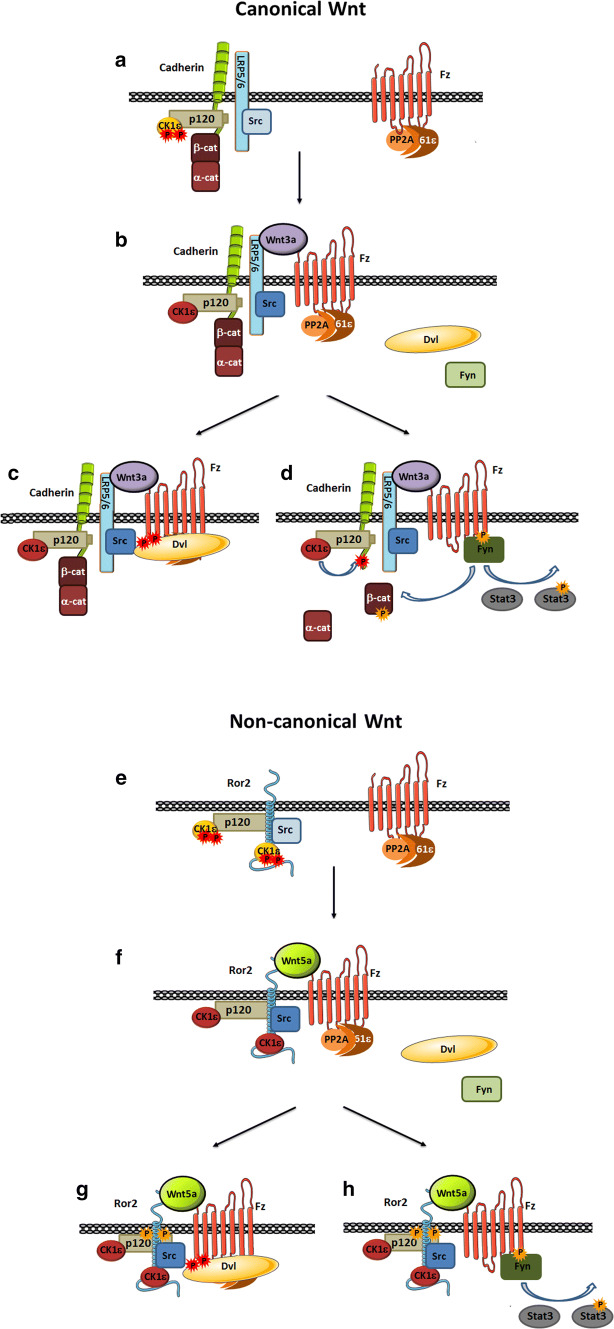
Accordingly, our current model of the two pathways is the following. In canonical Wnt, the co-receptor LRP5/6 associates directly or indirectly with Src [20] (see also Fig. 3a), and with CK1ε through cadherin and p120-catenin (Fig. 8a). Upon binding of Wnt3a, the formation of the Fz2/LRP5/6 complex enables the activation of CK1ε by PP2A as previously discussed [10]. Src is also activated (Fig. 8b), likely as a consequence of the receptor polymerization (which is not depicted in our model). Receptor polymerization, required for Wnt signaling [23], might promote intermolecular phosphorylation of Src protein. However, we cannot discard that a Fz-bound factor might be also relevant in Src activation. CK1ε promotes Dvl2 binding to Fz2 (Fig. 8c) either by phosphorylating Fz in the cytosolic tail, Dvl2 or both. This initiates other reactions such as Axin binding to LRP5/6, GSK3 inactivation and β-catenin stabilization. In parallel, Src phosphorylates Fz2 in Tyr 552, leading to Fyn binding to this residue and its activation. Consequently, Fyn phosphorylates Stat3 at Tyr705 (to activate it) as well as β-catenin at Tyr142 (breaking its interaction with α-catenin) [17] (Fig. 8d). Tyr142-phosphorylated β-catenin is transcriptionally active; it associates to Bcl9-2 and translocates to the nucleus [18]. It is likely that β-catenin is also released from its interaction with cadherin by other members of the complex as both Src [24] and CK1 [25] can disrupt this association.
Fig. 8.
Model of canonical and non-canonical Wnt signaling. a In the canonical Wnt pathway, LRP5/6 co-receptor is bound to inactive and Ser-phosphorylated (red dots) CK1ε (through p120-catenin and E-cadherin) and Src. b Upon Wnt3a stimulation, PP2A phosphatase, associated with Fz2 through the PR61ε regulatory subunit, gets closer to CK1ε, allowing CK1ε serine dephosphorylation and activation. Wnt3a also induces Src activation. c CK1ε promotes Dvl2 phosphorylation and recruitment to Fz2 leading to further responses of this pathway, whereas Src phosphorylates Fz2 Tyr552 (yellow dot) allowing Fyn recruitment and activation (d). Fyn phosphorylates Stat3 and also β-catenin Tyr142, releasing β-catenin from α-catenin and cadherin; activated Stat3 and β-catenin are now capable to act as transcriptional activators (e) The non-canonical pathway is initiated by Wnt5a that binds to the same receptor Fz2 and to the tyrosine co-receptor Ror2. Ror2 is directly bound to CK1ε and p120-catenin. Src also constitutively interacts with Ror2. f Similar as it occurs in canonical Wnt signaling, the non-canonical ligand promotes PP2A-mediated CK1ε activation and Src activation dependent on Ror2 dimerization. CK1ε and Src activities facilitate Dvl2 (g) and Fyn (h) recruitment to Fz2, respectively, and further downstream reactions
An issue to be explored in further studies might be to determine the relative relevance of initially α-catenin-bound and free β-catenin in the final β-catenin/Tcf4 active complexes. It is possible that the relative contribution of both initial β-catenin pools may be gene dependent, and that those genes more sensitive and activated by a lower number of β-catenin/Tcf4 complexes do not require the participation of β-catenin released from α-catenin. For such genes, Fyn would not be necessary for the formation of these transcriptionally active complexes. This might be the case for Axin2, which would be activated by lower levels of β-catenin than is Tcf7 and, in contrast to this, is stimulated by Wnt3 independently of Fyn.
The non-canonical Wnt co-receptor Ror2 binds directly to Src. It also interacts with CK1ε, both directly and through the common binding of both proteins to p120-catenin (8) (Fig. 8e). Wnt5a promotes the formation of the Fz2/Ror2 complex and the activation of CK1ε and Src (Fig. 8f) following a mechanism similar to that described for Wnt3a, with the subsequent binding of to Fz2 of both Dvl2 (Fig. 8g) and Fyn (Fig. 8h). Fyn/Fz2 interaction activates Fyn enzymatic activity and phosphorylates Stat3. Thus, the role played by Ror2 in non-canonical Wnt is performed by the complex LRP5/6–cadherin–p120-catenin in canonical signaling. Accordingly, as β-catenin is not associated with Ror2, Wnt5a-activated Fyn does not phosphorylate β-catenin at Tyr142 and does not mobilize this protein.
Another difference between the two pathways is the dependence on CK1 activity for Stat3 phosphorylation. For Wnt3a, this modification is totally independent on inhibitors of this kinase or on CK1ε depletion; in contrast, Wnt5a stimulation is sensitive to pharmacological inhibition of CK1. This different sensitivity correlates with the action of the CK1 inhibitor on co-receptor dimerization: it does not modify LRP5/6 clustering but prevents that of Ror2. This might be associated with Ror2 Ser phosphorylation by CK1ε, which stimulates Ror2 Tyr phosphorylation and clustering [26]. It is also possible that this CK1ε-independent oligomerization of the Wnt3a receptor Fz/LRP5/6 is due to the specific action of proteins, such as TMEM59, which specifically interact with the two components of this complex [27].
Stimulation of Stat3 phosphorylation and activity after v-Src cell transformation has been previously reported by several authors [28, 29]. Some of these authors suggested that v-Src is directly responsible for this modification [29]. However, according to our data, and probably related to the limited c-Src activation by Wnt5a or Wnt3a, the effect of the kinase on Stat3 phosphorylation is more indirect and is mediated by Fyn that amplifies the initial signal. Nonetheless, we cannot discard than in other cells systems where Src is massively activated or over-expressed it can bypass the requirement of Fyn and directly phosphorylate Stat3.
Our results also show competition between the two signaling branches triggered by both Wnt3a and Wnt5a, since binding of Dvl2 and Fyn to Fz2 seem to be incompatible. Several previous results have suggested that Src tyrosine kinases interfere with canonical Wnt signaling. For instance, Src activation blocks β-catenin-dependent transcription caused by LRP5/6 ectopic expression or by Wnt3a [20]. This effect has been attributed to the direct phosphorylation of LRP5/6 by Src. Moreover, genetic elimination of Csk, a tyrosine kinase required for the repression of the Src family of tyrosine kinases, decreases the expression of canonical Wnt target genes [30]. However, other authors have observed stimulation of Src kinase activity by canonical ligands and negative effects of Src inhibitors on their responses [31, 32]. A requirement for Stat3 in the activation of β-catenin transcriptional targets has also been previously described [33].
We consider likely that the full activation of the Wnt3a or Wnt5a cellular responses needs the Dvl2-dependent and Src/Fyn/Stat3-dependent branches. We have noticed that Stat3 phosphorylation is temporally delayed with respect to that of Dvl2 or LRP5/6 (Fig. 1b, see also [8]). It is possible that this delay is due to a slower activation of Src; in contrast to CK1ε, which only requires the formation of the Fz2-LRP5/6 or Fz2-Ror2 complexes, Src activation needs a more extensive receptor aggregation and possibly the recruitment of additional factors to the complex. According to our model, this late, Src-dependent Fz2 phosphorylation and Fyn binding would limit the Dvl2-dependent response and also facilitate Fyn activation, required for a better mobilization of β-catenin (as discussed above) as well as for Stat3 activation. Transcriptionally active Stat3 would help to activate genes containing Stat3-responsive elements in their promoters; it is likely that these genes also have other sequences for binding to β-catenin/Tcf4 (for those activated by to Wnt3a), or AP1 (for those activated by Wnt5a) [34]. Finally, as the activation of the Fyn/Stat3 branch requires Wnt signaling through Fz receptor family members Fz1,2 and 7 [14], which are the only ones containing Tyr552, it is likely that the relative levels of these receptors with respect to other Fz proteins might affect the contribution of both branches to the final response. This might explain the conflicting results reported for Wnt5a; for instance, Wnt5a stimulates invasion of prostate cancer cells acting through Fz2 receptors [35] but it promotes tumor suppression via binding to Fz5 [36]. The absence of Tyr552 in this receptor prevents the stimulation of the Fyn branch but not of Siah2 transcription (independent on Fyn, see Fig. 6), an effect that has been associated with inhibition of cell proliferation [37]. This potential regulatory role of the Fz receptors needs to be further investigated.
Materials and methods
Antibodies
The following antibodies were used in this study: CK1ε, p120-catenin, β-catenin, α-catenin, phospho-tyrosine, Rac1, EEA1 and Fyn (from BD Biosciences, 610445, 610134, 610153, 610194, 610000, 610921, 610650 and 610164, respectively; Franklin Lakes, NJ, USA); Ror2, Dvl2, and Stat3 (from Santa Cruz Biotechnology, sc-374174, sc-13974 and sc 8019, respectively; Santa Cruz, CA, USA); Dvl2, phospho-Stat3, LRP6 and phospho-LRP6 (Ser1490) (from Cell Signaling, 3216, 9145, 2560 and 2568, respectively; Danvers, MA, USA); Fz2, Jnk2 and phospho-Tyr142 in β-catenin (from Abcam, 52565, 178953 and 27798; Cambridge, UK); Flag and β-actin (from Sigma,F3165 and A5441 St. Louis, MO, USA); GST (from GE Healthcare, 27-4577-01; Salt Lake City, UT, USA); HA (Roche, 11867423001, clone 3F10; Basilea, Switzerland); phospho-Jnk2 and Src (Millipore, 07-175 and 05-184, respectively; Billerica, MA, USA); and Myc (Developmental Studies Hybridoma Bank, 9E 10, University of Iowa, IA, USA). LRP6 and phospho-LRP6 antibodies also recognize the close homologue LRP5 since the protein detected in HEK293T cells is sensitive to both LRP5- and LRP6-specific shRNAs; therefore, the detected protein was considered LRP5/6.
Cell culture
HEK293T, SW620 and T47D cells were obtained from IMIM Cell Bank (Barcelona, Spain). Generation of p120-catenin-depleted HEK293T has been previously described [8]. Assays were performed with cells at 60–70% confluency. The generation and use of murine mesenchymal stem cells (MSCs) has also been reported [38]. Control L fibroblasts or stably transfected with plasmids encoding Wnt3a or Wnt5a were obtained from the ATCC (ref. CRL-2648, CRL-2647 and CRL-2814, respectively; Manassas, VA, USA). Wnt3a-, Wnt5a- and control L fibroblasts were cultured in medium containing 0.4 mg/ml G-418. Conditioned medium was collected from the corresponding cells as recommended by the ATCC. Cells were treated with conditioned medium from control, Wnt3a- or Wnt5a-expressing cells. When indicated, conditioned medium was supplemented with a Wnt5a antibody (R&D System, AF645; Minneapolis, MN, USA) or with an irrelevant IgG (both 8 μg/ml); this Wnt5a antibody has been reported to block Wnt5a-dependent responses [39–41]. Alternatively, the conditioned medium was supplemented with DKK1 (prepared from cells transfected with pcDNA3-DKK1, kindly provided by Dr. A. Muñoz, CIB-CSIC, Madrid, Spain) or with GFP. In some experiments recombinant Wnt3a or Wnt5a (both from R&D System; Wnt3a, 5036-WN; Wnt5a, 645-WN) were added at 50 ng/ml to stimulate Stat3 phosphorylation. The CK1 inhibitor PF5006739 (Sigma PZ0246) (50 nM) was added to the cell medium 1 h before extract preparation.
Cell transfection and selection of transfectants
Human shRNAs specific for ROR2 (TRCN0000001492, TRCN00000010625, considered #1 and #2, respectively, in this article), FYN (TRC0000003100, TRC0000003099), SRC (TRCN0000038150, TRCN0000038151), DVL2 (TRCN0000033342, TRCN0000033343), LRP5 (TRCN0000033399, TRCN0000033403) LRP6 (TRCN0000033408, TRCN0000033405) or a non-targeting control (#SHC000000001, #SHC000000002) were all obtained from Mission shRNA, Sigma. Fyn shRNAs correspond to sequences completely conserved in the murine gene (Fyn #1, TRC3100) or with only two mismatches (Fyn #2, TRC3099); the sequence targeted by Src #1 (TRC3850) presents only one difference in mouse with respect to human. When depleting LRP5 and 6, LRP5 #1 and LRP6 #1 were combined in LRP5/6 #1 as well as LRP5 #2 and LRP6 #2 in LRP5/6 #2. For transient expression of shRNA in HEK293T cells, the indicated shRNAs were transfected using PEI (Polyethylamine, Polysciences, Inc; Niles, IL, USA) and downregulation of the protein of interest was analyzed at 48–72 h after transfection by Western blot (WB). Transient overexpression was achieved by transfecting the indicated eukaryotic plasmid using PEI, using ectopic pcDNA3-v-Src, pcDNA3-Ror2-HA or pcDNA3-Ror2-Flag (kindly provided by Dr. Y. Minami, Kobe University, Japan), pcDNA3-LRP6 (a generous gift from Dr. J.M. Gonzalez-Sancho, Universidad Autónoma de Madrid, Spain), pCS2-CA-LRP6-flag-GFP (kindly provided by Dr. A. Kikuchi, Osaka University, Suita, Japan) or pEF-Bos-Fyn (kindly provided by Dr. A.C Carrera, CNB-CSIC, Madrid, Spain). Cells were analyzed at 24–48 h after transfection. pEBG2T-Dvl2 was generated by digestion of pcDNA3-Dvl2-Myc (provided by Dr. J.M. Gonzalez-Sancho) with XhoI and after Klenow treatment, with KpnI. pEBG2T vector was digested with NotI, filled with Klenow and cut with KpnI. The insert was cloned into the linearized vector. The construction was verified by sequencing.
Single-cell cortical actin polarization analysis
Matrigel (BD Biosciences, 354230) (40 µl) was deposited on a round coverslip (15-mm diameter) and incubated at 37 °C for 30 min to solidify the gel. MSC cells were trypsinized to a single-cell suspension and 5 × 104 cells in control or Wnt5a conditioned medium and 2% Matrigel (V/V) were seeded on top of the solidified gel. Cells were incubated for 2 h with the indicated media and analyzed by immunofluorescence. Cells were then fixed with 4% paraformaldehyde for 15 min, and incubated with PBS-0.2% Triton X-100 for 5 min. After blocking with 3% BSA in PBS for 30 min at room temperature, CytoPainter Phalloidin-iFluor 647 Reagent (Abcam, 176759) was added for 1 h at room temperature. Slides were washed three times with PBS and incubated for 10 min with DAPI for nucleus identification. Coverslips were mounted on glass slides with Fluoprep (BioMérieux, 75521; Marcy l’Etoile, France), and immunofluorescence was analyzed with a Leica confocal microscope (LEICA spectral confocal TCS-SL; Wetzlar, Germany).
Purification of recombinant proteins, pull-down and phosphorylation assays
Generation of the bacterial expression plasmid pGEX-6P encoding the GST protein fused to p120-catenin, to the C-tail of Fz1 or Fz3 or to cytosolic Ror2 has been previously described [7, 8, 24]. GST-fusion proteins were expressed in E. coli and purified by affinity chromatography on Glutathione-Sepharose as described [24]. When indicated, the GST–Fz1 and GST–Fz3 C-tails were phosphorylated using 300 miliunits of recombinant Src (Millipore, Src 1-530 active) in a final volume of 50 µl in the following conditions: 50 mM Tris–HCl pH 7.5, 10 mM MgCl2, 0.1 mM EDTA, 2 mM DTT, 2.5 mM β-glycerol-phosphate pH 7.0, and 100 μM ATP. Reactions were performed for 30 min at 30 °C. Pull-down assays were performed using recombinant GST protein as bait and purified protein or HEK293T cell extracts (500-700 μg) lysed with NP-40 0.5% lysis buffer. Binding assays were performed at 4 °C for 2 h, after which 20 μl of Glutathione-Sepharose beads were added at 4 °C for an additional 1 h. After washing, pulled-down proteins were analyzed by WB with specific antibodies.
Rac1 activity assay
Rac1 activity was determined in HEK293T cells using specific pull-down assays for the activated form of this protein. Active Rac1 was affinity precipitated using the Rac1 binding domain of PAK as described [42].
Immunoprecipitation assays
Cell extracts were prepared by homogenizing cells in 0.5% NP-40 lysis buffer (25 mM Tris–HCl, pH 7.6, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40), supplemented with protease and phosphatase inhibitors as described [9]. When indicated, CK1 inhibitor (PF-5006730, Sigma, PZ0246) (50 nM) was added prior to cell lysis. Extracts were left on ice for 10 min and centrifuged at 14,000×g for 10 min at 4 °C. Supernatants constituted the cell extracts. Proteins were immunoprecipitated from cell extracts (300–600 μg) using 1 μg/ml of the appropriate antibody or an irrelevant IgG for 16 h at 4 °C. Samples were incubated for 2 h with 20 μl of γ-bind G-Sepharose (GE-Healthcare). Immunoprecipitates were washed three times with PBS-0.1% NP-40 and bound proteins were analyzed by WB.
Src and Fyn kinase assays
Proteins were immunoprecipitated from HEK293T cell extracts with Src or Fyn mAbs for 4 h at 4 °C, and then collected using 20 μl of γ-bind G-Sepharose (GE-Healthcare). Immunoprecipitates were washed three times with 0.1% NP-40 and the immunocomplexes were incubated with recombinant GST–p120-catenin in phosphorylation conditions in the presence of ATP when indicated. Tyrosine phosphorylation on GST–p120-catenin was analyzed by WB with an anti-pTyr antibody.
RNA isolation and analysis
RNA was obtained using RNeasy Mini Kit (Qiagen, 74106; Austin, TX, USA), retrotranscribed and analyzed by quantitative PCR in triplicate as in [43] using Pumilio homolog (PUM1) as control. The list of the primers used is provided in the Table S1.
Collagen type I invasion assay
MSC (4 × 104) were resuspended in 150 μl DMEM 0.1% BSA; HEK293T (7 × 104) were resuspended in 75 μl DMEM 0.1% BSA 0,1% FBS, supplemented with 75 μl of control medium or Wnt3a- or Wnt5a-conditioned medium. Cells were seeded on a Transwell filter chamber (Costar 3422, Thermo Fisher Scientific, Waltham, MA, USA) coated with 1 mg/ml Collagen Type I (Corning, 354249; Belford, MA, USA) and incubated for 16 h (MSC) and 36 h (HEK293T). Control, Wnt3a- or Wnt5a-conditioned medium was used as chemoattractant for MSC cells and DMEM 10% FBS, for HEK293T cells. Non-invading cells were removed from the upper surface of the membrane, while cells that adhered to the lower surface were fixed with 100% methanol for 20 min and stained with Crystal Violet. Cells were eluted with 30% acetic acid and the OD was measured at 590 nm.
Migration assays
Boyden chambers. MSC (4 × 104) were resuspended in 150 μl DMEM 0.1% BSA and seeded on Transwell filter chamber (Costar 3422,) and incubated for 16 h. Control, Wnt3a- or Wnt5a-conditioned media were used as chemoattractant. Non-invading cells were removed from the upper surface of the membrane, while cells that adhered to the lower surface were fixed with methanol for 20 min and stained with Crystal Violet. Cells were eluted with 30% acetic acid and OD at 590 nm was determined. Wound healing. MSC were seeded into 24-well tissue culture plate at a density that after 24 h of growth, they reach confluence. A scratch was gently and slowly inflicted to the monolayer with a 10-μl pipette tip. Cells were gently washed twice with PBS to remove the detached cells and treated with control-, Wnt3a- or Wnt-5a-conditioned media for 16 h. Images were captured on a Leica inverted microscope (Leica Dmil Led).
Statistical analysis
All results shown were representative from at least three independent experiments. Data are presented as mean ± SD. When appropriate, statistical analyses were conducted using Excel software (Microsoft, Redmond, WA, USA) and data were analyzed for significance using the unpaired t test. p values < 0.05 are symbolized with one asterisk, and p < 0.01, with two asterisks.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Drs. A. Muñoz, Y. Minami, J.M. González-Sancho, A. Kikuchi, and A.C. Carrera for kindly providing reagents. This research was funded by Grants from the Ministerio de Economía y Competitividad (MINECO) co-funded by Fondo Europeo de Desarrollo Regional-FEDER-UE to MD (BFU2015-65153-R) and AGH (SAF2016-76461-R). We also appreciate support from ICREA Academia and Instituto de Salud Carlos III (PIE15/00008). GF was recipient of a predoctoral fellowship from FPI (MINECO).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aida Villarroel and Beatriz del Valle-Pérez contributed equally to this work.
Contributor Information
Antonio Garcia de Herreros, Email: agarcia@imim.es.
Mireia Duñach, Email: mireia.dunach@uab.es.
References
- 1.MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect Biol. 2013;4:a007880. doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, function and implications in disease. Acta Physiol. 2012;204:17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- 3.Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, et al. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated receptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green J, Nusse R, van Amerongen R. The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb Perspect Biol. 2014;6:a009175. doi: 10.1101/cshperspect.a009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swiatek W, Tsai IC, Klimowski L, Pepler A, Barnette J, Yost HJ, et al. Regulation of casein kinase I epsilon activity by Wnt signaling. J Biol Chem. 2004;279:13011–13017. doi: 10.1074/jbc.M304682200. [DOI] [PubMed] [Google Scholar]
- 6.Bryja V, Schulte G, Rawal N, Grahn A, Arenas E. Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J Cell Sci. 2007;120:586–595. doi: 10.1242/jcs.03368. [DOI] [PubMed] [Google Scholar]
- 7.Vinyoles M, Del Valle-Pérez B, Curto J, Padilla M, Villarroel A, Yang J, et al. Activation of CK1ε by PP2A/PR61ε is required for the initiation of Wnt signaling. Oncogene. 2017;36:429–438. doi: 10.1038/onc.2016.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curto J, Del Valle-Pérez B, Villarroel A, Fuertes G, Vinyoles M, Peña R, et al. CK1ε and p120-catenin control Ror2 function in noncanonical Wnt signaling. Mol Oncol. 2018;12:611–629. doi: 10.1002/1878-0261.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casagolda D, Del Valle-Pérez B, Valls G, Lugilde E, Vinyoles M, Casado-Vela J, et al. A p120-catenin-CK1epsilon complex regulates Wnt signaling. J Cell Sci. 2010;123:2621–2631. doi: 10.1242/jcs.067512. [DOI] [PubMed] [Google Scholar]
- 10.Duñach M, Del Valle-Pérez B, García de Herreros A. p120-catenin in canonical Wnt signalling. Crit Rev Biochem Mol Biol. 2017;52:327–339. doi: 10.1038/nrc1752. [DOI] [PubMed] [Google Scholar]
- 11.Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO. 2010;29:41–54. doi: 10.1038/emboj.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topol L, Jiang X, Choi H, Garret-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, MacBeath GA. A noncanonical Frizzled2 pathway regulates epithelial–mesenchymal transition and metastasis. Cell. 2014;159:844–856. doi: 10.1016/j.cell.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP, et al. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem. 1999;274:19465–19472. doi: 10.1074/jbc.274.27.19465. [DOI] [PubMed] [Google Scholar]
- 16.Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, et al. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/S1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 17.Piedra J, Miravet S, Castaño J, Pálmer HG, Heisterkamp N, García de Herreros A, et al. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes Dev. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akbarzadeh S, Wheldon LM, Sweet SM, Talma S, Mardakheh FK, Heath JK. The deleted in brachydactyly B domain of ROR2 is required for receptor activation by recruitment of Src. PLoS One. 2008;3:e1873. doi: 10.1371/journal.pone.0001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Q, Su Y, Wesslowski J, Hagemann AI, Ramialison M, Wittbrodt J, et al. Tyrosine phosphorylation of LRP6 by Src and Fer inhibits Wnt/β-catenin signalling. EMBO Rep. 2014;15:1254–1267. doi: 10.15252/embr.201439644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil J. Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc Natl Acad Sci USA. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gon H, Fumoto K, Ku Y, Matsumoto S, Kikuchi A. Wnt5a signaling promotes apical and basolateral polarization of single epitelial cells. Mol Biol Cell. 2013;24:3764–3774. doi: 10.1091/mbc.E13-07-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, et al. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/S1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roura S, Miravet S, Piedra J, García de Herreros A, Duñach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 25.Dupre-Crochet S, Figueroa A, Hogan C, Ferber EC, Bialucha CU, Adams J, et al. Casein kinase 1 is a novel negative regulator of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2007;27:3804–3816. doi: 10.1128/MCB.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kani S, Oishi I, Yamamoto H, Yoda A, Suzuki H, Nomachi A, et al. The receptor tyrosine kinase Ror2 associates with and is activated by casein kinase epsilon. J Biol Chem. 2004;279:50102–50109. doi: 10.1074/jbc.M409039200. [DOI] [PubMed] [Google Scholar]
- 27.Gerlach JP, Jordens I, Tauriello DVF, Land-Kuper I, Bugter J, Noordstra I, et al. TMEM59 potientiates Wnt signaling by promoting signalosome formation. Proc Natl Acad Sci USA. 2018;115:3996–4005. doi: 10.1073/pnas.1721321115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, et al. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–83. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 29.Cao X, Tay A, Guy GR, Tan YH. Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol. 1996;16:1595–1603. doi: 10.1128/MCB.16.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imada S, Murata Y, Kotani T, Hatano M, Sun C, Konno T, et al. Role of Src family kinases in regulation of intestinal epithelial homeostasis. Mol Cell Biol. 2016;36:2811–2823. doi: 10.1128/MCB.00311-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280:41342–41351. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- 32.Yokoyama N, Malbon CC. Dishevelled-2 docks and activates Src in a Wnt-dependent manner. J Cell Sci. 2009;122:4439–4451. doi: 10.1242/jcs.051847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fragoso MA, Patel AK, Nakamura RE, Yi H, Surapaneni K, Hackam AS. The Wnt/β-catenin pathway cross-talks with STAT3 signaling to regulate survival of retinal pigment epithelium cells. PLoS One. 2012;7:e46892. doi: 10.1371/journal.pone.0046892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishita M, Itsukushima S, Nomachi A, Endo M, Wang Z, Inaba D, et al. Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol Cell Biol. 2010;30:3610–3619. doi: 10.1128/MCB.00177-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, Matsubara A, et al. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29:2036–2046. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- 36.Thiele S, Göbel A, Rachner TD, Fuessel S, Froehner M, Muders MH, et al. WNT5A has anti-prostate cancer effects in vitro and reduces tumor growth in the skeleton in vivo. J Bone Miner Res. 2015;30:471–480. doi: 10.1002/jbmr.2362. [DOI] [PubMed] [Google Scholar]
- 37.Ren D, Dai Y, Yang Q, Zhang X, Guo W, Ye L, et al. Wnt5a induces and maintains prostate cancer cells dormancy in bone. J Exp Med. 2019;216:428–449. doi: 10.1084/jem.20180661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alba-Castellón L, Olivera-Salguero R, Mestre-Farrera A, Peña R, Herrera M, Bonilla F, et al. Snail1-dependent activation of cancer-associated fibroblast controls Epithelial tumor cell invasion and metastasis. Cancer Res. 2016;76:6205–6217. doi: 10.1158/0008-5472.CAN-16-0176. [DOI] [PubMed] [Google Scholar]
- 39.Murdoch B, Chadwick K, Martin M, Shojaei F, Shah KV, Gallacher L, et al. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc Natl Acad Sci USA. 2003;100:3422–3427. doi: 10.1073/pnas.0130233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- 41.Baarsma HA, Skronska-Wasek W, Mutze K, Ciolek F, Wagner DE, John-Schuster G, et al. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J Exp Med. 2017;214:143–163. doi: 10.1084/jem.20160675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valls G, Codina M, Miller RK, Del Valle-Pérez B, Vinyoles M, Caelles C, et al. Upon Wnt stimulation, Rac1 activation requires Rac1 and Vav2 binding to p120-catenin. J Cell Sci. 2012;125:5288–5301. doi: 10.1242/jcs.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solanas G, Porta-de-la-Riva M, Agustí C, Casagolda D, Sánchez-Aguilera F, Larriba MJ, et al. E-cadherin controls beta-catenin and NF-kappaB transcriptional activity in mesenchymal gene expression. J Cell Sci. 2008;121:2224–2234. doi: 10.1242/jcs.021667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.