Abstract
Cancers show a metabolic shift towards aerobic glycolysis. By “corrupting” their microenvironment, carcinoma cells are able to obtain energy substrates to “fuel” their mitochondrial metabolism and cell growth in an autophagy-associated, paracrine manner. However, the metabolic changes and role of normal fibroblasts in this process remain unclear. We devised a novel, indirect co-culture system to elucidate the mechanisms of metabolic coupling between stromal cells and oral squamous cell carcinoma (OSCC) cells. Here, we showed that normal oral fibroblasts (NOFs) and OSCC become metabolically coupled through several processes before acquiring an activated phenotype and without inducing senescence. We observed, for the first time, that NOFs export mitochondria towards OSCCs through both direct contact and via indirect mechanisms. NOFs are activated and are able to acquire a cancer-associated fibroblasts metabolic phenotype when co-cultivation with OSSC cells, by undergoing aerobic glycolysis, secreting more reactive oxygen species (ROS), high l-lactate and overexpressing lactate exporter MCT-4, leading to mitochondrial permeability transition pore (mPTP) opening, hypoxia, and mitophagy. On the other hand, Cav-1-low NOFs generate l-lactate to “fuel” mitochondrial metabolism and anabolic growth of OSCC. Most interestingly, the decrease in AMPK activity and PGC-1α expression might involve in regulation of ROS that functions to maintain final energy and metabolic homeostasis. This indicated, for the first time, the existence of ATP and ROS homeostasis during carcinogenesis. Our study suggests that an efficient therapeutical approach has to target the multiple mechanisms used by them to corrupt the normal surrounding stroma and metabolic homeostasis.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03209-y) contains supplementary material, which is available to authorized users.
Keywords: Metabolic reprogramming, OSCC, NOF, ROS, Mitochondrial transfer, l-Lactate
Introduction
Oral squamous cell carcinoma (OSCC) is one of the most aggressive tumors and comprises 80–90% of all malignant neoplasms of the oral cavity [1]. Due to high recurrence, intrusive loco-regional invasion and metastasis, prognosis of OSCC remains poor, despite progress in surgery, radiotherapy, chemotherapy, and even immunotherapy.
Increasing evidence indicates that cancer is primarily a metabolic disease with deregulated cellular energetics and glycolysis. The previous studies [2] have linked disturbances in tumor cells to abnormalities in structure and function of their mitochondria. It has been established that cancer cells divert energy metabolism away from oxidative towards glycolytic metabolism even in the presence of oxygen. Moreover, stromal compartment of carcinomas was also suggested to play a role in their metabolism, particularly in breast cancer. The reverse Warburg effect [3] as this phenomenon of stroma contribution to the tumor metabolism became known was based on the observation that tumors displayed a metabolic shift towards aerobic glycolysis by “corrupting” the cancer-associated fibroblasts (CAFs) and turning them into factories producing energy-rich metabolites that can be used by cancer cells. The previous research has shown stromal activation in OSCC as well. CAFs derived from OSCC demonstrated phenotypic diversity [4–6] as well as complex changes in their secretory activity. Our previous studies showed that CAF-N, a subtype with a transcriptome and secretome closer to normal fibroblasts, were able to support a more aggressive local invasion of OSCC cells and subcutaneous tumors in mice mode [7]. Of note, research focused on the metabolic interactions between CAFs-N and OSCC could not show [8], however, the same mechanism of low Cav-1-driven autophagy in OSCC–CAFs as initially reported for breast cancer CAFs [9]. In contrast, high Cav1-CAFs had a negative prognostic value in tongue squamous carcinoma [10]. Therefore, the metabolic coupling between cancer cells and stroma is still controversial in OSCC and its pathological significance for OSCC progression remains unclear.
Here, we tested the hypothesis that OSCC cells are able to induce metabolic changes in the surrounding normal oral fibroblasts (NOFs) by subverting their metabolism for further progression of carcinoma. To investigate this, we have constructed an indirect co-culture model to elucidate the metabolic coupling and its effects on each cell type. We used OSCC–NOF co-injections in immunodeficient animal models and direct in vitro co-cultures. By exploring the role of NOFs as key players in metabolic reprogramming and co-evolution in oral cancers, we aimed to a better understanding of mechanisms that lead to tumor progression in OSCC and provide evidence for novel treatment strategies for OSCC directed towards neutralizing the metabolic reprogramming.
Materials and methods
Cell isolation and culture
A total of six pairs of primary oral fibroblasts, cancer-associated fibroblast and normal oral fibroblasts, and primary cancerous epithelial cells isolated from patients with histologically confirmed OSCC were used in this study (n = 6), although not all assays could be performed on all isolated cell strains. Written consent was obtained from all patients. CAFs were isolated from the stromal compartment of tumors, while NOFs from the morphologically normal oral mucosa harvested more than at least 5 cm from the surgical margin. Normal oral epithelial cells (NOKs) were isolated from healthy young people’s normal gingival tissue who underwent wisdom tooth extraction. Fresh OSCC tissue samples were collected following protocols approved by the Committee for Ethics in Health Research of West Norway (REK nr. 2010/481). They were minced into small pieces in tissue culture dishes, allowed to adhere, and cultured in FAD medium, which was 3:1 mixture of Dulbecco’s modified Eagle’s medium (Sigma, St. Louis, MO, USA) and Ham’s F12 (Sigma, cat. no. D8437), supplemented with 10% fetal bovine serum (FBS, Invitrogen, cat. no. 10270-106), 25 µg/mL bovine pituitary extract (BPE) (Invitrogen, cat. no. 13028-014), 0.4 µg/mL hydrocortisone (Sigma, cat no. H0888), 5 µg/mL insulin (Invitrogen, cat. no. 51300-044), 20 µg/mL transferrin (Invitrogen, cat. no. 51300-044), 50 µg/mL l-ascorbic acid (Sigma, cat. no. A7631), and 20 µg/mL l-glutamine (Invitrogen, cat no. 25030-024). Outgrowths of cells arising from the tumor pieces were morphologically assessed and dissociated using clonal rings. The outgrowing cells with epithelial morphology were subsequently propagated in plastic surfaces (Nunc. Denmark) with no feeding layers and grown in serum-free keratinocyte specific medium (KSFM, Invitrogen, cat. no. 17005-034) supplemented with 1 ng/mL human recombinant epidermal growth factor (Invitrogen, cat. no. 10450-013), 25 µg/mL BPE (Invitrogen, cat. no. 13028-014). The outgrowth of cells with fibroblast morphology were subsequently propagated on plastic surfaces in DMEM medium supplemented with 10% FBS. The cells were regularly assessed morphologically, and the ones that had an undesired morphology (epithelial in the fibroblast dishes or fibroblast-like in the keratinocyte dishes) were removed by scraping or serial trypsinization. At the second passage, the cells were FACS and analyzed for lineage-specific markers. All cells were grown under the standard cell-culture conditions: a humidified incubator at 37 °C and 5% CO2/95% air. At 60–70% confluence, the cells were released using 0.25% trypsin–EDTA (Sigma, cat. no. T3924). Clinical data on all patients was retrieved from the clinical hospital records of Haukeland University Hospital. The commercially human (Caucasian) dysplastic oral keratinocyte (DOK) cell line has been used in this study. DOK was established from a tongue dysplasia. It was reported not to form tumors in nude mice, and considered to have a transformed but not fully malignant phenotype. DOK cells were routinely grown in FAD medium on plastic surfaces without feeding layers. The passages of at which the cells were used in the study were: for NOKs 2–4 passages; for NOFs and CAFs for 3–6 passages; for OSCCs for 4–10 passages.
Characterization of primary cells
Cells were trypsinized, washed in PBS and adjusted to a concentration of 5 × 106 cells/500 µL in ice cold PBS with 2% FBS and 1% HEPES. Antibodies including the PE-conjugated lineage-specific antibodies CD31 (BD Pharmingen™, cat. no. 555446) and CD140b (PDGFRB) (BD Pharmingen™, cat. no. 558821), APC-conjugated lineage-specific antibodies CD146 (Miltenyi Biotec, cat. no. 130-092-849), and EpCAM (BD Biosciences, cat. no. 347200) were added and incubated for 45 min incubation in the dark at 4 °C. The cells were analyzed by Accuri™ C6 flow cytometer. For each sample, 10,000 particles were analyzed. Data analysis was performed using the Accuri™ C6 software.
Indirect co-culture system
Six-well transwell chambers with pore size 0.4 µm (Corning, cat. no. 3450) were used for epithelial–stromal indirect co-culture experiments. NOFs or CAFs were either plated onto the membranes or on the bottom of the wells at a concentration of 1 × 105 cells/well. NOK or OSCC (1 × 105 cells/transwell) were seeded either on the bottom of the wells or on the membrane, respectively, at the same cell density. The incubation was performed in the individual medium (OSCC/NOKs cultured in KSFM with 1 ng/mL human recombinant epidermal growth factor (EGF) and 25 µg/mL BPE, while CAFs and NOFs in DMEM with 10% FBS at 37 °C and 5% CO2/95% air for 48 h. Half of the medium was changed in 24 h.
Mitochondrial membrane potential and mitochondrial volume measurement
All the cells were seeded in six-well plate in their own medium for 2–3 days until they reached 50–70% confluency. The cells were then co-incubated with MitoTracker Green (MTG) (Invitrogen, cat. no. M7514) and tetramethylrhodamine, ethyl ester (TMRE) (Abcam, cat. no. ab113852), for 45 min at 37 °C in the dark. Cells treated with 100 µM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) (Abcam, cat. no. ab113852), a mitochondrial uncoupler for 10 min before incubation were used as negative control. For each sample, 40,000 particles were analyzed. Data analysis was performed using the Accuri™ C6 software.
Confocal microscopy for living cells and direct mitochondrial transfer
For mitochondrial morphology, 500 nM MTG and 200 nM TMRE were added for 45 min, and then washed for twice with phosphate-buffered saline (PBS). Cells were then incubated in PBS containing Hoechst 33324 1:5000 (Thermo Fisher Scientific, cat. no. H3570) for 15 min for nuclei staining. 5 nM TMRE was kept in FluoroBrite™ DMEM/no phenol red (Invitrogen, cat. no. A18967-01) with 10% FBS while imaging. The latter step allows adequate equilibration of the TMRE dye within the mitochondria. MTG stains mitochondria green, whereas TMRE stains mitochondria red under appropriate excitation wavelength. The double-staining approach facilitates proper identification of fluorescence intensity from mitochondria at different z-planes. 5 nM TMRE was kept in the medium while imaging.
To study mitochondrial transfer, we used 500 nM MitoTracker Deep Red (MTDR) (Invitrogen, cat. no. M22426) for fibroblasts and 500 nM MTG for epithelial cells. Cells were imaged lively by confocal microscope (Leica TCS SP2 AOBS, Germany) with a 40 × oil immersion objective. The cells were kept at 37 °C in a 5% CO2 humidified microscope stage chamber. MTG was subjected to 488-nm argon laser excitation, and emission was recorded through a bandpass 500–550-nm filter. TMRE and MTDR were subjected to 543-nm helium–neon laser. For Hoechst, excitation was at 405 nm, and a 420–480 nm bandpass filter was used.
Assay for reactive oxygen stress (ROS) production
We used a cell permeant reagent 2′,7′-dichlorofluorescin diacetate (DCFDA) (Abcam, cat. no. ab113851) to measure cellular ROS production. Cells were seeded in six-well plates 48 h before analysis. They were then co-incubated with 30 µM DCFDA for 10 min at 37 °C in the dark. Cells incubated in PBS with 10% FBS were used as negative control. For each sample, 40,000 particles were analyzed. Data analysis was performed using the Accuri™ C6 software.
Flow cytometric analysis for fibroblasts activation and senescence
Cells were trypsinized, washed in PBS, and adjusted to a concentration of 5 × 106 cells/500 µL in ice cold PBS with 2% FBS and 1% HEPES. Primary antibodies including anti-alpha-smooth muscle actin antibody (alpha-SMA) (Abcam, cat. no. ab32575); anti-fibroblast activation protein, alpha antibody (FAP) (Abcam, cat. no. ab28244); and cellular senescent marker anti-p16INK4A antibody (Abcam, cat. no. ab211542) and isotope control were added and incubated for 45 min in the dark at 4 °C. After washing, cells were incubated with Alexa Fluor 488-conjugated anti-rabbit secondary antibody, and then, cells were analyzed by Accuri™ C6 flow cytometer. For each sample, 10,000 live particles were analyzed. Data analysis was performed using the Accuri™ C6 software.
Adenosine triphosphate (ATP) and l-lactate production measurement
Cells were seeded in 96 wells plate for 48 h before analysis. ATP production was assessed using the Luminescent ATP Detection Assay Kit (Abcam, cat. no. ab113849); the kit irreversibly inactivates ATP-degrading enzymes (ATPases) during the lysis step and measures the luminescent signal corresponding to the endogenous levels of ATP. Three-to-six duplicates were measured for each sample. Luminescence intensity was monitored in Luminescent microplate Reader (InfiniteF50, TECAN).
l-Lactate generation was analyzed by colorimetric l-Lactate Assay Kit (Abcam, cat. no. ab65331) according to the manufacturer’s instructions. End point lactate concentration was determined in a 96-well plate by measuring the initial velocity (2 min) of the balance between NAD+ and NADH by lactate dehydrogenase. Immediately following the extracellular flux assay, the plate was measured at OD 450 nm in a microplate reader (VICTOR™ XLight, PerkinElmer).
Tumor xenografting in NOD/SCID IL2rγ (null) mice
NOD/SCID 12-week-old mice were used. A total number of 104 DOKs, suspended in 50 μl of growth factor-reduced matrigel (BD Biosciences), were inoculated alone (n = 6) or together with 105 NOFs of orthotopically in the tongue or subcutaneously on the back of mice. Tumor incidence and development (volume) was assessed at every 7 days. All mice were sacrificed 40 days after inoculation and tumor tissues were harvested for histologic assessment.
Indirect mitochondrial transfer assay
For detection of indirect mitochondrial transfer, fibroblasts and OSCC cells were stained with 500 nM MTDR and 500 nM MTG, respectively, for 12 min at 37 °C in the dark. Following that, the cells were seeded in co-culture in six-well plates with a transwell insert in their own medium for 48 h before analysis. We analyzed 40,000 particles for each sample. Data analysis was performed using the Accuri™ C6 software.
Immunohistochemistry staining (IHC)
The resected tissues were fixed in 10% formalin and embedded in paraffin blocks. Each block was cut into serial 4-μm-thick sections for staining with H&E, while the representative one was selected to perform immunohistochemistry for MCT-1(1:500, Abcam, cat. no. ab85021). Immunohistochemistry was performed using the EnVision + System-HRP (Dako Japan, Tokyo). Briefly, the sections were mounted on charged glass slides, deparaffinized, and rehydrated through a graded ethanol series. Antigens were heated in citric acid buffer solution (pH 6.0) at 95 °C for 30 min. After the blocking of endogenous peroxidase activity with 0.03% hydrogen peroxide, the sections were incubated with the primary antibodies MCT-1 at 4 °C overnight. And then reacted with second antibody (Dako, Envision + System-HRP labeled polymer anti-mouse, cat no. K4001, US) for 60 min at room temperature. Specific antigen–antibody reactions were visualized with diaminobenzidine chromogen applied for 10 min. Slides were counterstained with hematoxylin, dehydrated and mounted. Images were captured by Leica Microscope (Leica MC170 HD, Germany).
Western blotting
Protein concentration was determined using BCA protein assay (Thermo Fisher Scientific, cat. no. 23227). For western blotting, total cell protein was mixed with 10% SDS polyacrylamide gel (Invitrogen, cat. no. 18010870), and resolved using a NC membrane (Amersham, cat. no. A14117266). Membranes were blocked with 5% non-fat dry milk or 5% bovine serum albumin (BSA) in TBST for 1 h at room temperature. Membranes were then incubated overnight at 4 °C with rabbit polyclonal IgG anti-Caveolin-1 (1:1000, Abcam, cat. no. ab2910), rabbit polyclonal IgG anti-MCT-4 (1:1000, Proteintech, cat. no. 22787-1-AP), rabbit polyclonal anti-HIF-1α (1:1000, Abcam, cat. no. ab51608), rabbit polyclonal anti-BNIP3 (Abcam, cat. no. ab10962), mouse-monoclonal anti-PGC1 (1:1000, Abcam, cat. no. ab77210), rabbit polyclonal anti AMPKα/phospho-AMPKα (1:1000, CST, 2352S/2531S), mouse polyclonal anti VDAC1 (1:1000, Abcam, cat. no. Ab14734), rabbit polyclonal anti ANT1 (1:1000, Abcam, ab102032), rabbit polyclonal anti-MCT-1(1:1000, Abcam, ab85021), mouse polyclonal anti p16 (1:1000, BD Pharmingen™, cat no. 551153), and mouse polyclonal IgG Anti-GAPDH (1:1000, Invitrogen, cat. no. MA5-15738) as a control. After washing in TBST, membranes were incubated with donkey anti-mouse-monoclonal antibody or swine anti-rabbit monoclonal antibody conjugated to horseradish peroxidase secondary antibody (Jackson Immunoresearch, 1:2500), for 1 h at room temperature. Super signal west Pico chemiluminescent substrate (Thermo Fisher Scientific, USA) was used as enzyme–substrate according to manufacturer`s recommendations. The membranes were visualized in SynGene scanner (VWR, USA).
β-Galactosidase staining assay
β-Galactosidase activity was detected using a Senescence β-Galactosidase Staining Kit (Cell Signaling, #9860) according to the manufacturer’s instructions. NOFs were seeded into six-well plates (with cover slip) in complete media with or without co-culture with NOKs or OSCCs in the transwell insert (with cover slip). After 48 h, the cells were fixed with 1 × Fixative Solution and incubated overnight at 37 °C in a dry incubator without CO2, with the β-galactosidase staining solution. Afterwards, cells were observed under the microscope.
Statistical analysis
Average values were analyzed by the GraphPad Prism 6 software,and the data expressed as mean ± standard error of the mean (StEM). Wilcoxon matched signed-rank test was used to compare difference between the two groups in different pairs and paired t test was used to identify the difference the two groups in one paired sample. Differences were considered statistically significant when p < 0.05.
Results
Patients’ clinical and pathological characteristics and primary cells generation
All patients were male Caucasian with age range 55–74 years, clinical TNM stages III/IV, and different histopathological degree of differentiation (Table S1). Lineage specificity of each strain of primary cells, NOF, CAF, NOK, and OSCC was confirmed by flow cytometry, which showed that 96.2% of NOFs (Fig. S1, Panel A) and 99.4% of CAFs (Fig. S1, Panel B) stained positively with CD140b that recognizes the platelet derived growth factor b receptor (PDGFBR), a mesenchymal lineage marker. No expression of epithelial-specific antigen (ESA), an epithelial cell marker, CD146 and CD31, pericyte, and endothelial markers was detected in neither NOFs (Fig. S1, Panel A) nor CAFs (Fig. S1, Panel B). The purity of epithelial cells was 84.5% ESA positive cells for NOKs (Fig. S1, Panel C) with and 99.9% for OSCCs (Fig. S1, Panel D), while 12.6% of NOKs (Fig. S1, Panel C) and 8.6% of OSCCs (Fig. S1, Panel D) were stained positively with CD140b. No expression of CD146 and CD31 was detected in either NOKs (Fig. S1, Panel C) or in OSCCs (Fig. S1, Panel C.)
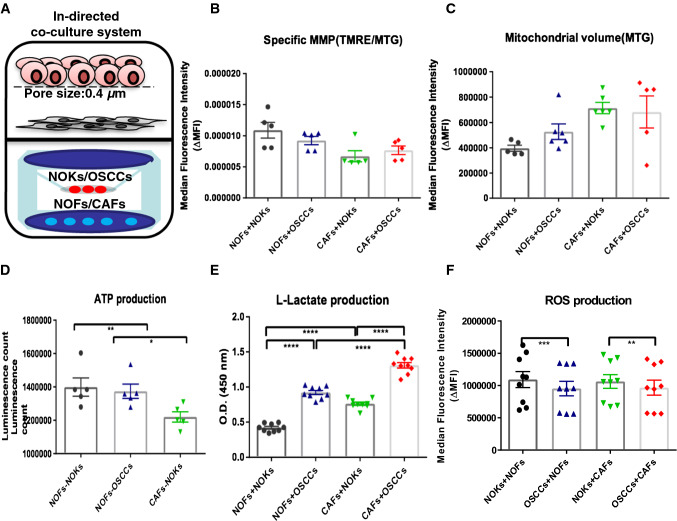
Cancer–stromal units exhibit increased l-lactate production but similar ATP and ROS generation when compared to normal epithelial–stromal units
To understand the behavior of a tumor as it happens in vivo, as a whole organ, we have further analyzed the sum of mitochondrial function and metabolic regulation of epithelial and stromal cell compartments together and called it epithelium-stroma unit (EMU) (Fig. 1a). This unit consisted of either NOKs or OSCC placed in the transwell insert (mimicking the epithelial compartment of the unit) and NOFs or CAFs placed at the chamber of six-well plates (mimicking the stromal compartment of the unit) and co-cultured indirectly for 48 h. The following combinations were studied: (a) NOFs + NOKs, (b) NOFs + OSCC, (c) CAFs + NOKs, and (d) CAFs + OSCC. Specific MMP was highest in NOFs + NOKs and fell gradually, the lowest being in CAFs + OSSCs (Fig. 1b), despite an increase in mitochondrial volume (Fig. 1c). Interestingly, we found that overall ATP and ROS production remained similar. The equivalent level of ATP production between OSCC and NOKs co-cultured units suggests reprogramming of the energy balance with ATP generated from stromal cells being used by the cancer cells (Fig. 1d). l-Lactate production was significantly elevated in OSCC–stromal units, compared to NOKs–stromal units (Fig. 1e). In addition, similar level of ROS was produced between OSCCs–stromal units and NOKs–stromal units (Fig. 1f). These finding suggest the presence of cancer–stromal metabolic coupling and reprogramming that reaches to a final ATP and ROS homeostasis.
Fig. 1.
Mitochondrial volume, MMP, ATP, l-lactate, and ROS generation in epithelial–stromal unit (indirect keratinocytes–fibroblasts co-culture system). a Graphic representation of the indirect co-culture model system used in the study. Either NOFs or CAFs (representing the stromal compartment) were co-cultured with NOKs or OSCCs (representing the epithelial compartment) in six-well plates with transwell membranes with pore size 0.4 μm. Flow cytometric analysis of different epithelial–stromal units for specific MMP (b) and their mitochondrial volume (c). Increase of mitochondrial volume but decrease of MMP was observed from NOFs + NOKs unit, NOFs + OSCCs unit, CAFs +NOKs unit, CAFs + OSCCs unit. d Measurement of intracellular ATP in four different epithelial–stromal unit using luminescent assay showed no significant differences among these four units. e, f Measurement of l-lactate and ROS activity in these different epithelial–stromal units showed statistically significant increased lactate production in the units containing OSCC cells and no significant differences in ROS activity among the four units. The data points were presented from six different patient sets in b–d. The data points were presented from the replicates on the averages of the six different patient combinations in e and f. All points represent mean values and SEM. Independent experiments were performed more than three times. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; Wilcoxon signed-rank test was used in b–d; paired t test was used in e and f
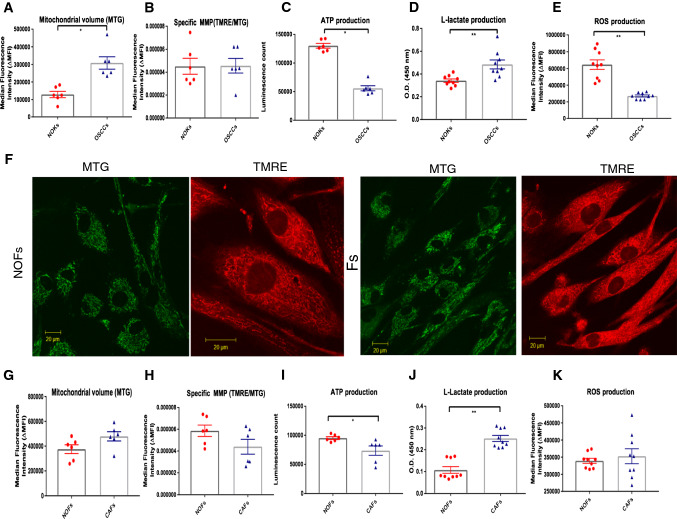
OSCC cells display high l-lactate generation, decreased mitochondrial activities, intracellular ATP depletion, and less oxidative stress than their normal counterparts
Next, we investigated metabolic differences between the two epithelial cell types, NOKs and OSCC. OSCC and NOKs were double stained with MTG and TMRE. Fluorescence intensity measured by flow cytometry indicated lower specific MMP or MMP per mitochondrial mass with significant higher mitochondrial volume in OSCC compared with NOKs (Fig. 2a, b).
Fig. 2.
Comparison of mitochondrial volume, MMP, ATP, l-lactate and ROS generation between NOKs and OSCCs AND between NOFs and CAFs. a, b Flow cytometric analysis of NOKs and OSCCs for their mitochondrial volume (b) and specific MMP (c). Although there was increased level of mitochondrial volume detected in OSCCs compared to NOKs, the specific MMP displayed the same level. c Measurement of the intracellular ATP showed statistical significant higher levels in NOKs compared to OSCCs. d, e Measurement of l-lactate production and ROS activity showed increased l-lactate and less ROS levels in OSCCs compared to NOKs. f Representative images of mitochondrial morphology stained with MTG and TMRE in NOFs and CAFs (magnification × 40, scale bar = 20 μm). Flow cytometric analysis of NOFs and CAFs for their mitochondrial volume (g) and specific MMP (h) showed increased mitochondrial volume and less specific MMP in CAFs compared to NOFs. i Measurement of intracellular ATP production showed significantly higher production in NOFs than in CAFs. j, k Measurement of l-lactate and ROS activity shows slightly higher levels of both in CAFs than in NOFs. The data points were presented from six different patient sets. All points represent mean values and SEM. Independent experiments were performed more than three times. *p < 0.05, **p < 0.01; Wilcoxon signed-rank test was used
To assess whether these findings were reflected by their energy production, we measured intercellular ATP generation in these cells. OSCC showed significantly decreased ATP production compared to NOKs (Fig. 2c). Measurement of l-lactate and ROS production by flow cytometry and absorbance colorimetric assay, respectively, showed that OSCC alone were highly glycolytic with a high l-lactate generation (Fig. 2d). OSCC were also shown to produce lower levels of ROS than NOKs (Fig. 2e). In our system, OSCC were shown to produce lower levels of ROS than NOKs (Fig. 2e) and this could be due to the very high numbers of senescent cells in the NOK cultures as assessed by the SA Beta Gal staining (Fig. S3A, B)
CAFs display also high l-lactate generation, impaired mitochondrial function, intracellular ATP depletion, and similar mitochondrial morphology and senescence levels
We also examined mitochondrial structure and activities, and metabolic alterations in CAFs and NOFs stained with MTG and TMRE in monocultures. Microscopy of NOFs revealed an extensive network of mitochondria throughout the cell, with long and highly branched individual mitochondria similar in appearance to those present in CAFs (Fig. 2f). We found a higher mitochondrial volume and lower specific MMP in CAFs compared with NOFs, but this was not significant (Fig. 2g, h). CAFs also showed significantly decreased ATP production (Fig. 2i) and l-lactate production compared with NOFs (Fig. 2k) when cultured alone. In addition, CAFs showed slightly higher levels of ROS than NOFs; however, the difference did not reach the significance level (Fig. 2k). These results suggest that unlike NOFs, CAFs display aerobic glycolysis and lower ATP production. Western blotting for p16INK4A antibody, a marker of senescence, and Senescence β-Galactosidase Staining showed no positivity for CAFs or NOFs, in contrast to NOK cells (Fig. S3).
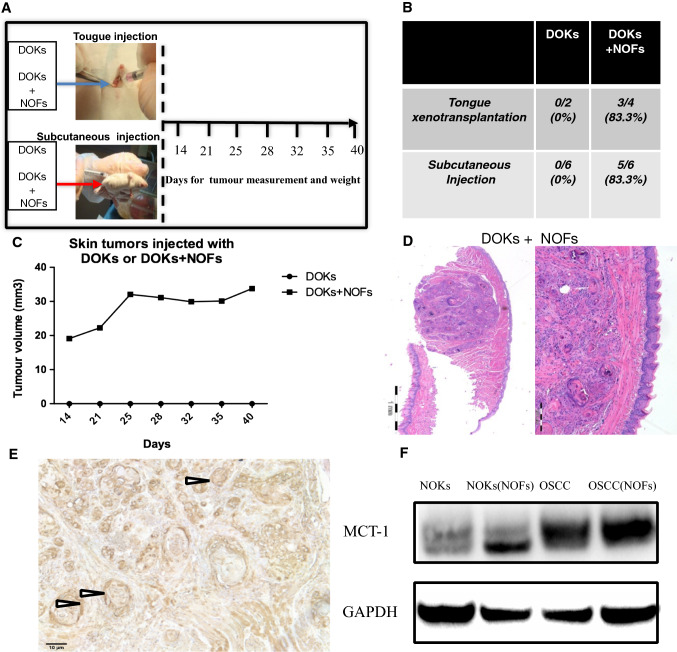
NOFs support tumor formation and expression of l-lactate transporter MCT-1 by early neoplastic oral keratinocytes in a mouse xenograft model
Given the growing recognition of “energy transfer” or “metabolic coupling” between tumor stroma and epithelial cancer cells as being important for carcinogenesis [11], we tested this in an animal model and looked for signs of metabolic coupling between neoplastic cells and fibroblasts. DOK cells, a model of early oral neoplasia, were previously shown not to be tumorigenic in nude mice when injected alone, but could form tumors when co-injected with CAF in NOD/SCID mice [7]. We published evidence for CAF’s supporting tumor formation and invasion of DOK cells [7] by showing that: (a) CAFs were able to support tumor formation by DOK in NOD/SCID/Ilγ2 (null) mice; (b) some CAF strains were better than others at supporting an invasive growth of DOK cells; and (c) the depth of invasion of a malignant oral keratinocyte cell line was significantly higher when both subsets of CAFs were embedded into a 3D collagen biomatrix than NOFs. Here, we injected DOKs alone or combined with NOFs into NOD/SCID IL2rγ (null) mice using both subcutaneous and tongue xenotransplantation (Fig. 3a). Following subcutaneous injection, tumor growth was not observed when DOKs were inoculated alone, but 83.3% of the injected mice (5 of 6) developed tumors when DOKs were co-inoculated with NOFs (Fig. 3b). Similarly, no tumors could be measured in the tongues of mice injected with DOKs alone (Fig. 3c), but well-circumscribed tumors were detected as early as 14 days after co-inoculation with NOFs (Fig. 3d). We detected strong membrane and cytoplasm expression of the l-lactate transporter MCT-1 in the cells of the cancer islands of the tumor xenografts (Fig. 3e). Since no tumors were formed by DOK alone, we could not compare MCT-1 expression in presence and absence of fibroblasts in vivo. However, in vitro, we could show that MCT-1 levels were increased by co-culture with fibroblasts (Fig. 3f).
Fig. 3.
Tumor formation by DOKs xenotransplated alone or with NOFs. a Flowchart for tongue and skin xenotransplantation in NOD/SCID IL2rγ (null) mice of DOK cells with and without NOFs. Tumor formation and measurement were checked once a week from day 14 until day 40. b Quantification of the incidence of tumors induced by xenotransplantation of DOK alone or with NOFs, showing that DOKs can only generate tumors in combination with NOFs. c Growth rates of subcutaneous tumors induced by DOKs xenotransplanted alone and with NOFs. d H&E staining of representative tongue tumors harvested at day 42 after orthotopic tongue injections with 103 DOKs with 105 NOFs, showing well differentiated squamous cell carcinoma lesions with typical keratin pearl formation. e Representative image of an immunohistochemically stained a tongue tumor showing strong expression of MCT-1 by epithelial cancer islands (like the island pointed by the arrow). Scale bar = 10 μm
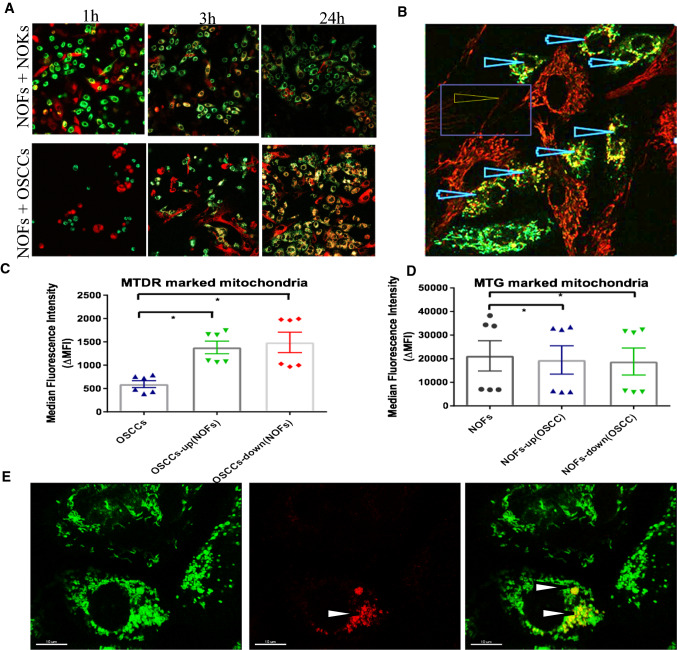
NOFs export mitochondria towards OSCCs through direct contact via tunneling nanotubes (TNT) as well as via indirect mechanisms
We next investigated the possibilities for metabolic coupling between oral cancer cells and stroma in the in vitro models. Mitochondrial transfer has been previously identified to occur between endothelial cells and cancer cells [12], but not between normal stromal cells and cancer cells. To address this, we used an in vitro co-culture system and confocal microscopy to visualize mitochondrial transfer. Before seeding, NOFs were stained with MTDR and NOKs/OSCC were stained with MTG. After 3 h of direct co-culture, OSCC cells containing both green and red-marked mitochondria (Fig. 4a) and dynamic transfer of mitochondria from NOF to OSCC cells was recorded (Movie S1). After 24 h, the majority of OSCC cells contained double-stained mitochondria, while only few of the NOFs showed double staining, indicating that mitochondrial transfer occurred preferentially from NOFs to OSCC (Fig. 4a). Fluorescence confocal microscopy could visualize that the mitochondrial transfer occurred through direct cellular extensions under the form of tunneling nanotubes (TNTs), from NOFs to OSCC (Fig. 4b).
Fig. 4.
Direct and indirect mitochondrial transfer from fibroblasts to epithelial cells. a Before co-cultivation, NOFs were stained with MTDR (red mitotracker), while NOKs and OSCCs were stained with MTG (green mitotracker). Confocal microscopy for live cells showing mitochondrial transfer from NOFs (red colour) to NOKs and OSCCs (green colour) at different time points (1 h, 3 h, 24 h). Already after 3 h of direct co-culture, mitochondrial transfer was observed from NOFs into epithelial cells (yellow colour), but predominantly in OSCCs. b Confocal microscopy for live cells showing mitochondrial transfer from NOFs to OSCCs through tunneling nanotubes (pointed by the arrow). Presence of mitochondria in TNTs protruding from NOFs into OSCCs and NOKs could be detected after 24 h. Again, mitochondria-containing TNTs were observed predominantly between NOFs and OSCCs (magnification × 40). c, d Quantification of the mitochondrial transfer after co-culture at distance (indirect transfer) by flow cytometry. NOFs were stained with MTDR and OSCC were stained with MTG, then co-cultured for 48 h in transwell. There was a significant increase of MTDR in OSCCs after co-culture with NOFs, suggesting presence of indirect mitochondrial transfer from NOFs to OSCCs (c). Less MTG signal could be found in NOFs after indirect co-cultured with MTG marked OSCCs (d). e Confocal microscopy showing mitochondrial transfer between NOF to NOK and OSCC after co-culture at distance, in transwells. Before seeding, NOFs were stained with MTDR and NOKs or OSCCs were stained with MTG. After 48 h of indirect co-cultivation, epithelial cells (mainly OSCCs) with both green and red mitochondria were distinguished (pointed by the arrow). Double-stained cells (i.e., with green and red fluorescent mitochondria) reflect mitochondrial exchange. Scale bar = 10 μm. The data points were presented from six different patient sets. All points represent mean values and SEM. Independent experiments were performed more than three times *p < 0.05; Wilcoxon signed-rank test was used
While direct co-culture system permits investigation of direct interactions of two cell types by descriptive methods, it obscures the use of more quantitative methods that need pure cell subpopulations, difficult to obtain after direct co-culture. For more robust and standardized method of recovering pure cell populations after co-cultures, we designed a non-contact co-culture system using a transwell system, still able to closely mimic the in vivo interactions with the tumor microenvironment, but without direct contact between the two cell types. NOKs/OSCC stained with MTG were seeded on the insert membrane, and NOFs stained with MTDR were cultured on the bottom. Mitochondrial uptake could then be analyzed by flow cytometry after 48 h co-cultivation. Of note, mitochondrial transfer was still observed, even in the absence of direct cell–cell contact. The mitochondrial uptake in the non-contact co-cultures was less pronounced than that observed after direct co-culture, but still increased up to 23 ± 6.8% for OSCC and 4 ± 1.7% for NOKs (Fig. 4c; Fig. S2A). In the transwell system, no mitochondrial transfer into NOFs, neither originating from OSCC nor from NOKs, was detectable (Fig. 4d; Fig. S2B). To understand whether position or gravity played a role, we seeded NOFs into upper and NOKs/OSCC in the lower chamber and obtained similar results: mitochondrial transfer from NOFs to OSCC occurred in 22 ± 6.3% (Fig. S2A). However, there was no significant increase in MTDR in NOKs after co-culture with NOFs, while a slight decrease in MTG was found in NOKs after co-culture (Fig. S2C). To further examine the mitochondrial transfer between OSCC and NOFs, we used the indirect co-culture system and confocal microscopy. After 48 h of indirect co-cultivation, several OSCC cells with both green and red mitochondria were observed (Fig. 4E).
NOFs are metabolically reprogrammed after co-cultivation with OSCCs by undergoing aerobic glycolysis, secreting high l-lactate and overexpressing lactate exporter MCT-4
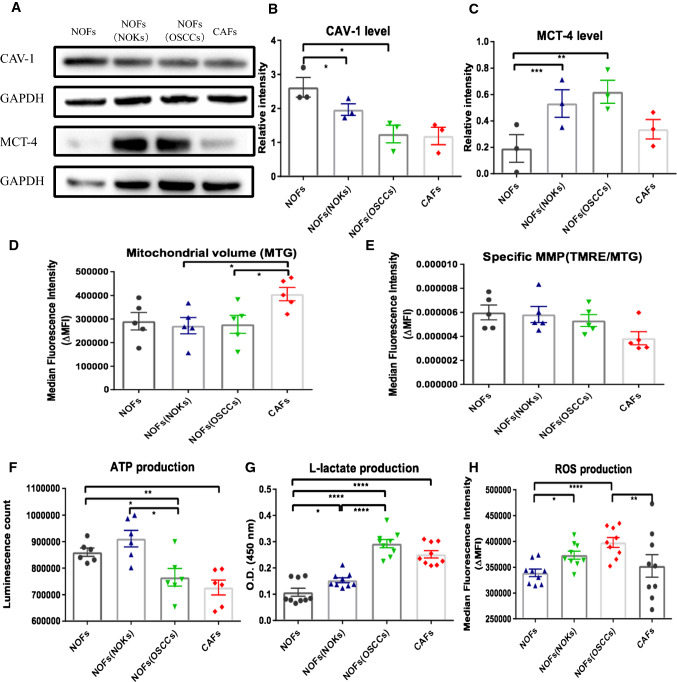
Given our observations of mitochondrial transfer from NOFs to OSCC, directly via TNTs and indirectly at distance in transwell system, we next asked if indirect co-culture of NOFs to OSCC could involve alterations of the redox state, mitochondrial activity, energy balance, and intra-tumoral metabolic interactions, as this has not been studied previously. First, we determined how NOFs can be altered metabolically when co-cultured with OSCC or NOKs in the transwell system. Cav-1 has been reported as a novel biomarker associated with tumor progression and metastasis [13]. Loss of Cav-1 appears to be associated to metabolic alterations in CAFs and increased mitophagy and aerobic glycolysis in breast cancer [10, 14]. Our immunoblot analysis showed lower Cav-1 expression in CAFs compared to NOFs. Furthermore, Cav-1 was downregulated in NOFs when co-incubated with OSCC, but not with NOKs (Fig. 5a, b). Flow cytometric analysis showed that specific MMP was highest in NOFs monolayer and gradually decreased in NOFs co-cultured with NOKs and OSCC and was lowest in CAFs monolayer despite similar amounts of mitochondrial volume (Fig. 5d, e). These findings suggest that OSCC could trigger NOFs to switch into a metabolic phenotype resembling CAFs with loss of Cav-1 and impaired mitochondrial activity.
Fig. 5.
Alterations in Cav-1 and MTC-4 expression, mitochondrial volume, MMP, ATP, l-lactate, and ROS generation induced in fibroblasts by co-cultured with epithelial cells. a Representative images for detection of Cav1, MCT-4, and corresponding GAPDH by western blot. b, c Quantification of western blots for CAV-1 and MCT-4 expression showing significant decrease CAV-1 and up-regulation of MCT-4 in NOF when co-cultured with epithelial cells at distance, in transwells. Flow cytometric analysis of mitochondrial volume (d) and specific MMP (e) for fibroblasts alone or in epithelial–stromal units, showing a similar MTG and trendency of MMP drop after co-cultivation. f Luminescent analysis for ATP generation showing increased ATP production in NOFs co-cultured with NOKs compared to NOFs alone and decreased ATP level in NOFs seeded with OSCCs. g, h Measurement of l-lactate generation and flow cytometric analysis of ROS production showing gradually upregulated l-lactate production and ROS level from NOFs alone, to NOFs co-cultured with NOKs, and NOFs co-cultured with OSCCs. The data points were presented from six different patient sets in d–f. The data points were presented from the replicates on the averages of the six different patient combinations in g and h. All points represent mean values and SEM. Independent experiments were performed more than three times. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; Wilcoxon signed-rank test was used in d–f; paired t test was used in b, c, g and h
Since l-lactate is a critical fuel required to provide continuous energetic support for tumor epithelial cells, we next examined the changes in ATP generation and l-lactate production. We found that NOFs showed slightly lower level of intracellular ATP when co-cultured with OSCC than with NOKs (Fig. 5f), but produced significantly higher levels of l-lactate when co-cultured with OSCC compared to co-culture with NOKs (Fig. 5g). Analysis of redox status showed increasing production of ROS in NOFs from the lowest amount in monoculture, to increased generation when in co-cultured with epithelial cells, particularly with OSCC (Fig. 5h).
Meanwhile, MCT-4, as a monocarboxylate transporter, has been implicated in lactate efflux from CAF to cancer cells and oxidative stress in breast cancer CAFs. Therefore, we set out to determine if a similar compartmentalization of MCT transporters occurs in cells of the stroma. We found a slightly lower level of MCT-4 in NOKs than in CAFs in monolayer. MCT-4 was upregulated in NOFs when co-cultured with epithelial cells, indicating again that OSCC are able to convert the NOF phenotype towards a CAF-like metabolic phenotype (Fig. 5a, c).
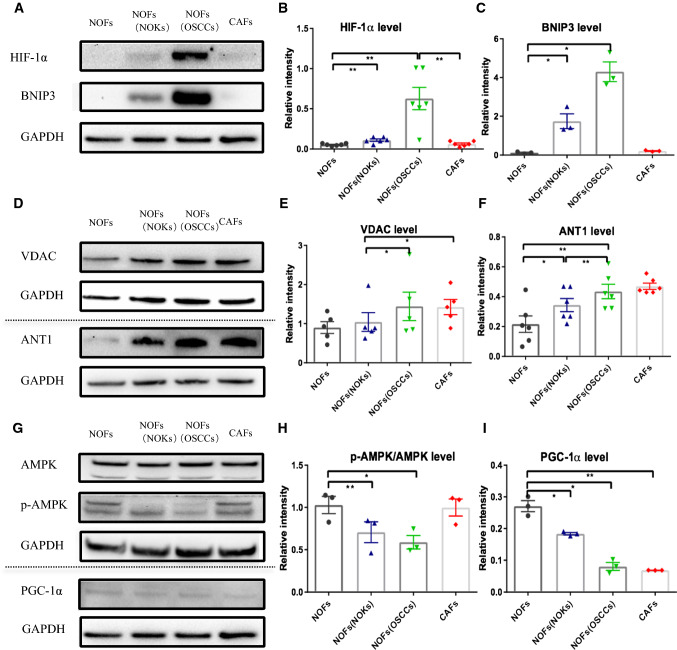
NOFs are under hypoxia-like condition when co-cultured with OSCC, accompanied by mitophagy and mitochondrial permeability transition pore (mPTP) opening via a decrease in activation of AMPK–PGC-1α axis
Next, we wanted to understand the mechanism(s) of increased ROS in NOFs. Since it is well known that MCT-4 is involved in inducing mitophagy [15] we investigated mitophagy in our co-culture system. We found increasing expression of BNIP3, a classic biomarker of mitophagy, in NOFs co-cultured with OSCC, rather than with NOKs, while the expression was low in both NOFs and CAFs grown alone (Fig. 6a, b). In addition, MCT-4 is regulated by catabolic transcription factors such as hypoxia-inducible factor 1α (HIF-1α) [16], which is also an important inducer of mitochondrial ROS production and mitophagy. We found a higher level of HIF-1α protein in NOFs after indirect co-culture with OSCC than in NOFs alone or in NOFs co-cultured with NOKs (Fig. 6a, c).
Fig. 6.
Assessment of the hypoxia marker HIF-1α, and mitophagy marker BNIP3, mPTP components VDAC and ANT1, mitochondrial biogenesis factor PGC-1, as well as p-AMPK in NOFs after co-culture with epithelial cells. Representative images (a) and quantification for western blotting with for hypoxia marker HIF-1α (b) and mitophagy marker BNIP3 (c). Representative images (d) and quantification for mPTP components VDAC (e) and ANT1 (f). Representative images (g) and quantification for mitochondrial biogenesis factor PGC-1α (i) and p-AMPK (h). All points represent mean values and SEM. Independent experiments were performed more than three times. *p < 0.05; **p < 0.01; paired t test was used
Activation of the mPTP has been suggested to cause changes in the intra- and inter mitochondrial redox environment which, in turn, leads to increased ROS production [17]. Consistent with the previous observations, we found that expression levels of both voltage-dependent anion channel (VDAC), and ANT1, a presumed component of the mPTP complex, were increased both in the NOFs co-cultured with OSCC and with NOKs. These proteins were also more highly expressed in CAFs alone than in NOFs (Fig. 6d–f).
Recently, the reports demonstrated that AMPK–PGC-1α-dependent control of ROS regulates HIF-1α stabilization, and ROS promote the glycolytic metabolism in cells lack of AMPK signaling [18]. Therefore, we asked if AMPK–PGC-1α axis is activated in NOF under OSCC influence. Interestingly, NOFs cultured with OSCC displayed reduced AMPK activation when compared with all the other groups, as determined by reduced phosphorylation of the AMPKα catalytic subunit at Thr-172 (Fig. 6g, h). Transcriptional co-activator PGC-1α is a key regulator of mitochondrial biogenesis and antioxidant gene expression in response to oxidative stress [19]. Given that PGC-1α is a downstream effector of AMPK [20], we next examined the levels of PGC-1α (Fig. 6i). Following a similar pattern to AMPKα, NOFS showed decreased levels of PGC-1α when co-cultured with epithelial cells, particularly with OSCC.
Fibroblast activation and conversion into a CAF phenotype by co-culture with OSCC occurred after the metabolical reprogramming and was not accompanied by induction of cellular senescence
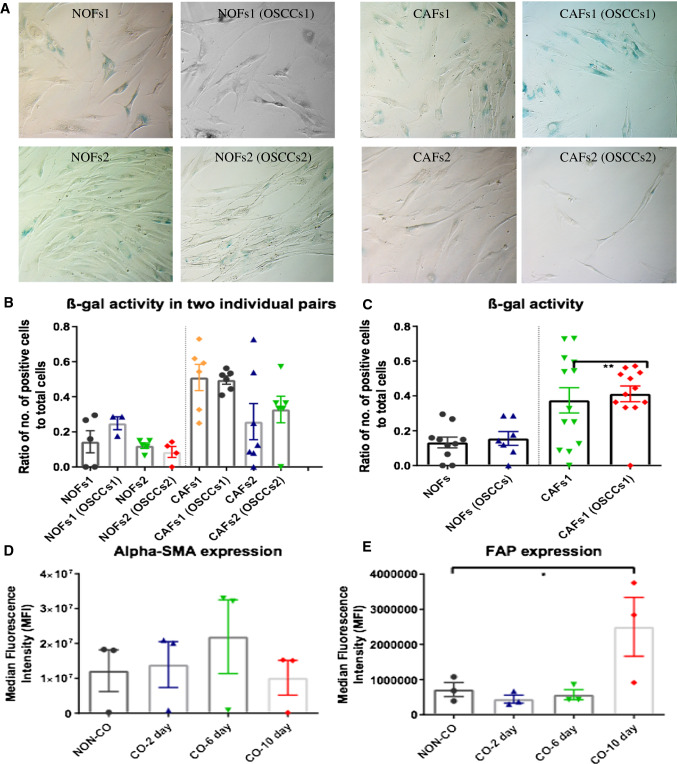
Accumulating evidence shows that CAFs in cancer including OSCCs contain a high percentage of senescent cells [21, 22] that display many features of glycolysis and the pentose phosphate pathway [23, 24]. To investigate if this phenomenon is induced by cancer cells in NOFs and to explore whether the metabolic changes and autophagy of NOFs observed in our co-culture system were induced via senescence, we performed the senescence associated β-galactosidase staining in two pairs of NOFs and CAFs after co-cultured with OSCCs and western blotting with p16INK4A antibody). No significant changes were found in terms of the level of β-galactosidase activity (Fig. 7a–c) and protein level for p16INK4A expression (Fig. S4) in NOFs and CAFs after cocultured with OSCCs. These data indicate that OSCCs did not increase the level of cell senescence; therefore, the mitochondrial reprogramming between NOFs and OSCCs seemed not to be related to senescence in our model system.
Fig. 7.
Assessment of senescence β-galactosidase staining for NOFs and CAFs alone or in co-culture with OSCCs and expression level of alpha-SMA and FAP, p16INK4 in NOFs before and after co-cultured with OSCCs over the time frame of 10 days. Representative images (a) and quantification for senescence β-galactosidase staining for two individual pairs of NOFs and CAFs before and after co-cultured with OSCCs (b). c was calculated from the mean of two individual pairs in b (magnification × 20). Flow cytometric analysis for the expression level of fibroblast activation markers alpha-SMA (d) and FAP (e) in NOFs before and after co-cultured with OSCCs over the time frame of 10 days. All points represent mean values and SEM. Independent experiments were performed more than three times. *p < 0.05; paired t test was used
Considering that the observation of metabolic reprogramming in our model system was noticed to occur as early as 48 h after co-culture, we then investigated whether the metabolic changes in our co-culture system precedes fibroblast activation. We performed flow cytometric analysis for two well known markers for CAF activation: alpha-smooth muscle actin (α-SMA) and fibroblast activation protein (FAP) in NOFs alone and co-cultured with OSCCs for a serial timepoints of 2 days, 6 days, and 10 days. The expression of α-SMA increased when NOFs were cocultured with OSCCs for 6 days, but this was not significant and declined again by day 10 (Fig. 7d). Meanwhile, the FAP expression showed a significant elevation after 10 day co-culture (Fig. 7e).
These data points to a sequence of epithelial–mesenchymal interaction events in our model system in which the metabolic changes and reprogramming occur early, before fibroblasts transition into a CAF phenotype and without concomitant cellular senescence.
Discussion
Tumor microenvironment has received renewed attention in the last decade for its role in supporting and even promoting the invasive cancerous phenotype. While mitochondria are the major site for oxygen-dependent cellular energy (ATP) production, cancer cells have the capacity to ferment glucose into lactate even in the presence of oxygen, a process known as aerobic glycolysis. More recently, cancer cells were also shown to subvert their local environment to obtain a supply of fuels to drive cellular growth. Understanding how the cancer cells control energy balance and influence stromal cells in the environment is vital for cancer treatment. Tumorigenesis is a complex and dynamic process encircled by extracellular matrix (ECM) and stromal cells. Researchers have an understanding of most metabolic characteristics of cancer and associated stromal cell separately; however, the change in the entire tumor, or EMU as we named the experimental model of the tumor, containing both epithelial tumor cells and tumor stroma, remains unknown. To understand more about the metabolic and energy changes that are driven by the presence of cancer, and to be able to quantify the extent of these changes in each of the cell type investigated, we constructed an indirect co-culture system to imitate the cancer–stroma interactions while being able to efficiently and conveniently separate the cell types after their interaction.
By different combinations of epithelial cells and stromal cells, we could pinpoint a continuous loss of mitochondrial activity as indicated by decreased MMP and a consequential mitochondrial accumulation with tumor progression, but interestingly, the levels of ATP and ROS remained constant, indicating, for the first time, the existence of ATP and ROS homeostasis during carcinogenesis. The previous studies on l-lactate showed a key role in tumor cell migration, as well as in metastasis and self-sufficient metabolism. Other studies did also show that lactate helps to create an acidic microenvironment outside cancer cells, supports the spread of cancer cells [25]. Our study demonstrated an elevated generation of l-lactate in EMUs containing OSCCs, with NOFs or CAFs being the main producers, indicating that the energy producer is the tumor stroma. Several studies have proposed that senescence can lead to mitochondrial dysfunction and a shift towards aerobic glycolysis in CAFs, which in turn can support tumorigenesis [16, 26]. Here, in our model system and based on the and senescence β-galactosidase activity, we could not confirm that, since we did not observe an increase number of senescent cells in NOFs in the presence of NOKs or OSCCs. These data indicated that the mitochondrial reprogramming between NOFs and OSCCs was not induced by the activation of senescence in our model system, distinct from the previous reports [26]. This does not exclude that OSCC can induce NOF into senescence, but our data indicate that if this occurs, it is subsequent to metabolic reprogramming and activation into CAF phenotype and that senescence might be the ‘terminal differentiated’ state of CAFs. Unrevealing this kinetic of changes from NOFs to CAFs might have major implication for our understanding of CAF activation and on how targeting specific metabolic pathways could be employed in the treatment of OSCC, since these findings indicate that metabolic reprogramming may act as the driver of fibroblast activation as suggested recently [27].
In addition, to showing that OSCC cells are able to alter the metabolism of NOFs and to reprogram it to become similar to that of CAFs. We show for the first time the evidence for unidirectional and selective mitochondrial transfer from NOFs to OSCC. The observed cell-to-cell mitochondrial transfer from NOFs to OSCC through TNTs is similar to the phenomenon proposed to occur between endothelial cells and cancer cells [28]. Surprisingly, we also detected and quantified a mitochondrial transfer from NOFs to OSCC when the cells were not in direct contact but interacted through the transwell membrane. Technical detection errors due to dye diffusion were excluded by carefully designed experiments and controls. Plausible explanations of mitochondrial transfer could be exosome-cell fusion, or even intercellular cytoplasm bridges membrane nanotubes been proposed before [12], but were not further investigated here. Additional future studies are required to investigate this and confirm its occurrence in vivo.
Furthermore, the current two-component model identified the metabolically coupling in cancer cells and NOFs. As the main supplier of cellular ROS, mitochondrial dysfunction will result in the accumulation of ROS. In our study, the level of ROS was significantly increased in stromal cells after co-culture with OSCC. While the opposite effect was observed in the OSCC cells, indicating that the microenvironment has been reprogrammed for the benefit of cancer cells.
The previous reports have shown that normal keratinocytes could generate less ROS than OSCCs [29]. Conversely, we detected higher ROS production in NOKs than OSCCs. As it has been suggested previously, normal oral keratinocytes can undergo senescence very rapidly in the suboptimal culture system [28]. In the present study, NOKs alone contained many senescent cells, and they reversed their senescence in the presence of fibroblasts, as shown by the β-galactosidase staining (Fig. S3). This could be explained by the still inadequate cell-culture conditions for this type of cells that indeed does not manage to keep primary NOK cells in culture for more than several passages. The high percentage of senescent cells could well justify why they usually perish in culture after few passages. The fibroblasts may deliver to them the necessary growth factors that are lacking from the culture media, impairing thus their premature senescence in culture. Thus, we cannot exclude the fact that the presence of this subpopulation in the in vitro system, which is not present in vivo in healthy tissues, might make this model system less representative of normal oral keratinocytes in vivo. Better suited in vitro culture systems for NOKs should be further optimized.
Lactate is formed and diffusing across membrane via monocarboxylate transporters (MCTs) whenever glycolysis is active, followed by taking up as a fuel. The widely accepted hypothesis points out that lactate can swimmingly substitute glucose as a fuel for virtually all cells of the body to be the key intermediate metabolite in whole body metabolism [30]. Our experiments corroborate this hypothesis; the phenomenon of lactate fleetly accumulating into normal stromal cells appeared after co-culture with cancer cells, which turned them into an infinite energy pool. Further detection of higher expression of MCT-4 (a biomarker of oxidative stress with the function of exporting lactate) in relevant stromal cell reminds us the energy conversion and metabolic evolution between cancer cell and surrounding malignant infiltrates.
The previous studies suggested that loss of stromal Cav-1 acts as an important marker of poor prognosis and may be the crucial factor in promoting the metastatic behavior of cancer cells in breast and prostate carcinomas [31, 32]. Consistently, our study demonstrated that CAFs are distinct from NOFs in terms of low expression level of Cav-1. More interestingly, further examination of changes induced by OSCC in the stromal cells and the mechanisms of metabolic reprogramming showed gradual loss of Cav-1 in the NOFs under co-culture system with OSCC, rather than with NOKs. With ambiguous identity as suppressor or promoting tumor, Cav-1, located in chromosome 7, is involved in diverse cellular process, such as vesicular transport and signal transduction [20, 33]. Loss of Cav-1 has been linked to a pseudo-hypoxia state induced by cancer cells. The higher expression of HIF-1α that we detected in NOFs after co-culture with OSCC but not in other co-culture systems indicates that OSCC cells might also construct a hypoxia state in the microenvironment to induce ROS production and further mitochondrial dysfunction or DNA damage. More interestingly, higher expression of BNIP3, one protein related to the BH3-only family, which could regulate mitophagy in response to hypoxia, was detected only in the units of NOFs–OSCC. This finding further indicates a unidirectional metabolic and energy reprogramming induced by OSCC cells into the stromal cells.
The opening of mPTP is a phenomenon known in the field of mitochondrial research for many decades, and it was described for the first time in details by Haworth and Hunter in 1979 [34]. At higher ROS levels, longer mPTP openings may release ROS to a toxic level which can lead to damage to mitochondria and cells [35]. Some candidates were considered to serve as the core of the pore, including mitochondrial VDAC in mitochondrial outer membrane and ANT1, distributed in the mitochondrial inner membrane. Consistently, our present study identified activation of mPTP, as showed by higher protein expression for both VDAC and ANT1. This activation could be induced from over production of ROS and further damage the cells and mitochondria itself to switch on mitophagy. This study is the first one to indicate that cancer cells can induce longer mPTP openings in the adjacent stromal cells.
It is interesting that we detected a decrease in activation of AMPK in NOF after co-culture. An emerging role for AMPK is as a cellular energy sensor responding to low ATP levels. AMPK activation positively regulates signaling pathways that replenish cellular ATP supplies and negatively regulates ATP-consuming biosynthetic processes. Our findings of increased ATP and low p-AMPK and PGC-1a would corroborate this line of evidence.
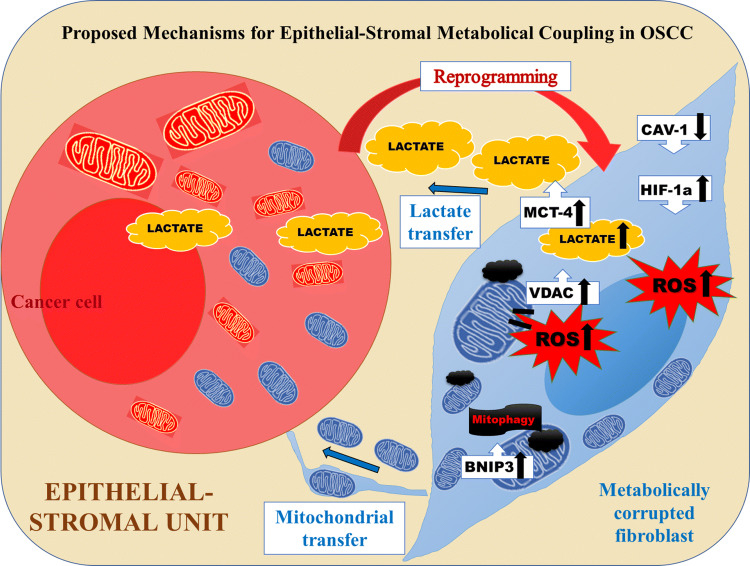
In summary, our data demonstrate that OSCC cells are able to induce a unidirectional mitochondrial exchange and reprogramming in normal fibroblasts. We show here that, OSCC are able to metabolically reprogramme NOF, via Cav-1 downregulation, MCT-4 increase, increased l-lactate production and oxidative stress via induced hypoxia, mitophagy and mPTP opening. The decrease in AMPK activity and PGC-1α expression might involve in regulation of ROS that functions to maintain final energy and metabolic homeostasis (Fig. 8). These metabolic alterations can be further exploited for metabolically addressed therapy targeted at both epithelial and stromal tumor compartments.
Fig. 8.
Summary. Graphic representation of the metabolic coupling existent in the epithelial stromal unit in OSCC as revealed by the findings of this study. In summary, our data demonstrate that OSCC cells are able to induce a unidirectional mitochondrial exchange and reprogramming in normal surrounding fibroblasts. We show here that, OSCC are able to metabolically reprogramme NOF, via Cav-1 downregulation, MCT-4 increase, increased l-lactate production and oxidative stress via induced hypoxia, mitophagy and mPTP opening. The decrease in AMPK activity and PGC-1α expression might involve in regulation of ROS that functions to maintain final energy and metabolic homeostasis
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Hanne Linda Nakkestad for excellent technical assistance. We also thank Molecular Imaging Centre (MIC) for outstanding facilities.
Abbreviations
- OSCC
Oral squamous cell carcinoma
- NOFs
Normal oral fibroblasts
- CAFs
Cancer-associated fibroblasts
- ROS
Reactive oxygen species
- mPTP
Mitochondrial permeability transition pore
- NOKs
Normal oral epithelial cells
- FBS
Fetal bovine serum
- BPE
Bovine pituitary extract
- KSFM
Serum-free keratinocyte specific medium
- DOK
Dysplastic oral keratinocyte
- EGF
Epidermal growth factor
- MTG
MitoTracker Green
- TMRE
Tetramethylrhodamine, ethyl ester
- FCCP
Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone
- PBS
Phosphate-buffered saline
- MTDR
MitoTracker Deep Red
- ROS
Reactive oxygen stress
- DCFDA
2′,7′-Dichlorofluorescin diacetate
- ATP
Adenosine triphosphate
- ATPases
ATP-degrading enzymes
- IHC
Immunohistochemistry staining
- EMU
Epithelium–stroma unit
- TNTs
Tunneling nanotubes
- mPTP
Mitochondrial permeability transition pore
- VDAC
Voltage-dependent anion channel
- ECM
Extracellular matrix
- MCTs
Monocarboxylate transporters
- Alpha-SMA
Alpha-smooth muscle actin
- FAP
Fibroblast activation protein
Author contributions
XL, DEC, ZYZ, and JZG: conceptualization; XL, DEC, ZYZ, and JZG: methodology; DS, ZYZ, ZJG, LAB, RD, and SR: investigation; ZYZ and ZJG: writing—original draft; XL, DEC, ZYZ, LAB, and LJL: writing—review and editing; DEC and LAB: funding acquisition; HD, HP, SS, and LJL: resources; XL and DEC: supervision.
Funding
This work was partly supported by the Research Council of Norway through its Centres of Excellence funding scheme (DEC, project number 22325), the Western Health Authority (DEC grant nr. 911902/2013).
Availability of data and material
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
No potential conflicts of interest were disclosed.
Ethics approval
The project was approved by the Committee for Ethics in Health Research of West Norway (REK nr. 2010/481); the study was performed in accordance with the Declaration of Helsinki. Tissues were acquired with written informed consent from all patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhuoyuan Zhang and Zhenjie Gao are the first co-authors and contributed equally.
Daniela Elena Costea and Xiao Liang are the last corresponding authors and contributed equally.
Contributor Information
Daniela Elena Costea, Email: Daniela.Costea@uib.no.
Xiao Liang, Email: Xiao.Liang@uib.no.
References
- 1.Johnson NW, Jayasekara P, Amarasinghe AA. Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontology. 2011;57:19–37. doi: 10.1111/j.1600-0757.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- 2.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 3.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 4.Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, Thomas GJ. Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer. 2004;90:822–832. doi: 10.1038/sj.bjc.6601611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29:273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenthal E, McCrory A, Talbert M, Young G, Murphy-Ullrich J, Gladson C. Elevated expression of TGF-beta1 in head and neck cancer-associated fibroblasts. Mol Carcinog. 2004;40:116–121. doi: 10.1002/mc.20024. [DOI] [PubMed] [Google Scholar]
- 7.Costea DE, Hills A, Osman AH, Thurlow J, Kalna G, Huang X, Pena Murillo C, Parajuli H, Suliman S, Kulasekara KK, Johannessen AC, Partridge M. Identification of two distinct carcinoma-associated fibroblast subtypes with differential tumor-promoting abilities in oral squamous cell carcinoma. Cancer Res. 2013;73:3888–3901. doi: 10.1158/0008-5472.CAN-12-4150. [DOI] [PubMed] [Google Scholar]
- 8.Jensen DH, Therkildsen MH, Dabelsteen E. A reverse Warburg metabolism in oral squamous cell carcinoma is not dependent upon myofibroblasts. J Oral Pathol Med. 2015;44:714–721. doi: 10.1111/jop.12297. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Tan SH, Ng S, Zhou J, Yang ND, Koo GB, McMahon KA, Parton RG, Hill MM, Del Pozo MA, Kim YS, Shen HM. Critical role of CAV1/caveolin-1 in cell stress responses in human breast cancer cells via modulation of lysosomal function and autophagy. Autophagy. 2015;11:769–784. doi: 10.1080/15548627.2015.1034411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vered M, Lehtonen M, Hotakainen L, Pirila E, Teppo S, Nyberg P, Sormunen R, Zlotogorski-Hurvitz A, Salo T, Dayan D. Caveolin-1 accumulation in the tongue cancer tumor microenvironment is significantly associated with poor prognosis: an in vivo and in vitro study. BMC Cancer. 2015;15:25. doi: 10.1186/s12885-015-1030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, Birbe RC, Howell A, Pavlides S, Gandara R, Pestell RG, Sotgia F, Philp NJ, Lisanti MP. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: mCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772–1783. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquier J, Guerrouahen BS, Al Thawadi H, Ghiabi P, Maleki M, Abu-Kaoud N, Jacob A, Mirshahi M, Galas L, Rafii S, Le Foll F, Rafii A. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med. 2013;11:94. doi: 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee M, Ben-Josef E, Thomas DG, Morgan MA, Zalupski MM, Khan G, Andrew Robinson C, Griffith KA, Chen CS, Ludwig T, Bekaii-Saab T, Chakravarti A, Williams TM. Caveolin-1 is associated with tumor progression and confers a multi-modality resistance phenotype in pancreatic cancer. Sci Rep. 2015;5:10867. doi: 10.1038/srep10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercier I, Casimiro MC, Wang C, Rosenberg AL, Quong J, Minkeu A, Allen KG, Danilo C, Sotgia F, Bonuccelli G, Jasmin J-F, Xu H, Bosco E, Aronow B, Witkiewicz AK, Pestell RG, Knudsen ES, Lisanti MP. Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 down-regulation and RB tumor suppressor functional inactivation: implications for the response to hormonal therapy. Cancer Biol Ther. 2014;7:1212–1225. doi: 10.4161/cbt.7.8.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I, Martinez-Outschoorn UE, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid Redox Signal. 2012;16:1264–1284. doi: 10.1089/ars.2011.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilde L, Roche M, Domingo-Vidal M, Tanson K, Philp N, Curry J, Martinez-Outschoorn U. Metabolic coupling and the Reverse Warburg Effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44:198–203. doi: 10.1053/j.seminoncol.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabinovitch RC, Samborska B, Faubert B, Ma EH, Gravel SP, Andrzejewski S, Raissi TC, Pause A, St-Pierre J, Jones RG. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell reports. 2017;21:1–9. doi: 10.1016/j.celrep.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 19.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Rabinovitch RC, et al. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017;21(1):1–9. doi: 10.1016/j.celrep.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Yang G, et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci USA. 2006;103(44):16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capparelli C, et al. CDK inhibitors (p16/p19/p21) induce senescence and autophagy in cancer-associated fibroblasts, “fueling” tumor growth via paracrine interactions, without an increase in neo-angiogenesis. Cell Cycle. 2012;11(19):3599–3610. doi: 10.4161/cc.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quijano C, et al. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle. 2012;11(7):1383–1392. doi: 10.4161/cc.19800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James EL, et al. Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. J Proteome Res. 2015;14(4):1854–1871. doi: 10.1021/pr501221g. [DOI] [PubMed] [Google Scholar]
- 25.Audet-Walsh E, Papadopoli DJ, Gravel SP, Yee T, Bridon G, Caron M, Bourque G, Giguere V, St-Pierre J. The PGC-1alpha/ERRalpha axis represses one-carbon metabolism and promotes sensitivity to anti-folate therapy in breast cancer. Cell Rep. 2016;14:920–931. doi: 10.1016/j.celrep.2015.12.086. [DOI] [PubMed] [Google Scholar]
- 26.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Para R, et al. Metabolic reprogramming as a driver of fibroblast activation in pulmonary fibrosis. Am J Med Sci. 2019;357(5):394–398. doi: 10.1016/j.amjms.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassona Y, Cirillo N, Lim KP, Herman A, Mellone M, Thomas GJ, Pitiyage GN, Parkinson EK, Prime SS. Progression of genotype-specific oral cancer leads to senescence of cancer-associated fibroblasts and is mediated by oxidative stress and TGF-beta. Carcinogenesis. 2013;34:1286–1295. doi: 10.1093/carcin/bgt035. [DOI] [PubMed] [Google Scholar]
- 29.Berridge MV, Crasso C, Neuzil J. Mitochondrial genome transfer to tumor cells breaks the rules and establishes a new precedent in cancer biology. Mol Cell Oncol. 2015;5:e1023929. doi: 10.1080/23723556.2015.1023929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, Gutkind JS. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell. 2012;11:401–414. doi: 10.1016/j.stem.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350(Pt 1):219–227. doi: 10.1042/bj3500219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shan T, Lu H, Ji H, Li Y, Guo J, Chen X, Wu T. Loss of stromal caveolin-1 expression: a novel tumor microenvironment biomarker that can predict poor clinical outcomes for pancreatic cancer. PLoS One. 2014;9:e97239. doi: 10.1371/journal.pone.0097239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folgueira MA, Maistro S, Katayama ML, Roela RA, Mundim FG, Nanogaki S, de Bock GH, Brentani MM. Markers of breast cancer stromal fibroblasts in the primary tumour site associated with lymph node metastasis: a systematic review including our case series. Biosci Rep. 2013;33:e00085. doi: 10.1042/BSR20130060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha TK, Her NG, Lee MG, Ryu BK, Lee JH, Han J, Jeong SI, Kang MJ, Kim NH, Kim HJ, Chi SG. Caveolin-1 increases aerobic glycolysis in colorectal cancers by stimulating HMGA1-mediated GLUT3 transcription. Cancer Res. 2012;72:4097–4109. doi: 10.1158/0008-5472.CAN-12-0448. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, He Y, Gao J, Fan L, Li Z, Yang G, Chen H. Caveolin-1 expression level in cancer associated fibroblasts predicts outcome in gastric cancer. PLoS One. 2013;8:e59102. doi: 10.1371/journal.pone.0059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.