Abstract
Immunotherapy is considered as an effective method for cancer treatment owing to the induction of specific and long-lasting anti-cancer effects. Immunotherapeutic strategies have shown significant success in human malignancies, particularly in prostate cancer (PCa), a major global health issue regarding its high metastatic rates. In fact, the first cancer vaccine approved by FDA was Provenge, which has been successfully used for treatment of PCa. Despite the remarkable success of cancer immunotherapy in PCa, many of the developed immunotherapy methods show poor therapeutic outcomes. Immunosuppression in tumor microenvironment (TME) induced by non-functional T cells (CD4+ and CD8+), tolerogenic dendritic cells (DCs), and regulatory T cells, has been reported to be the main obstacle to the effectiveness of anti-tumor immune responses induced by an immunotherapy method. The present review particularly focuses on the latest findings of the immune checkpoints (ICPs), including CTLA-4, PD-1, PD-L1, LAG-3, OX40, B7-H3, 4-1BB, VISTA, TIM-3, and ICOS; these checkpoints are able to have immune modulatory effects on the TME of PCa. This paper further discusses different approaches in ICPs targeting therapy and summarizes the latest advances in the clinical application of ICP-targeted therapy as monotherapy or in combination with other cancer therapy modalities in PCa.
Keywords: Monoclonal antibody, Co-stimulatory, Co-inhibitory, Biomarkers, Clinical trials
Introduction
Prostate cancer (PCa) is the most prevalent cancer among men and the second leading cause of cancer-related death in men worldwide. PCa-related mortality is estimated at 385,560 global deaths in 2020. Currently, approximately 50% of PCa patients are diagnosed with metastatic disease, and those with localized tumor show fairly rapid progression-to-advanced stages of the disease characterized by metastasis to long distant organs [1]. The relatively high incidence of PCa in men and its poor prognosis mark this deadly disease as an important global health issue.
Treatment strategies for localized or early stage PCa include radical dissection and androgen deprivation therapy (ADT), both employed as effective initial backbones of the treatment. Despite the success in ADT, treatment failures have been observed in many cases due to the development of resistance to ADT. The standard treatment alternatives for PCa patients showing resistance to ADT are radiotherapy (RT), chemotherapy, and endocrine drugs (such as abiraterone acetate), all of which have shown limited therapeutic efficacy [2]. In addition to the standard treatment modulates, the new immunotherapy approaches have shown great success in the treatment of PCa.
Therapeutic anti-cancer vaccines, including dendritic cell (DC)-based, whole cell-based, and vector-based vaccines, are the main type of immunotherapeutic strategies used for the treatment of PCa. Sipuleucel-T (Provenge®) is the first and only autologous DC-based therapeutic vaccine approved by FDA for the treatment of symptomatic metastatic PCa. Provenge induces T-cell immune responses against prostate tumor cells, expressing the specific antigen of prostatic acid phosphatase (PAP) [3]. The administration of Provenge in several clinical trials of PCa has demonstrated modest clinical benefits (mixed responses) in the improvement of overall survival (OS) and induction of immunological responses [4]. GVAX®, another vaccine developed for PCa, is a whole cell-based vaccine which is the genetically modified autologous or allogeneic prostate tumor cells expressing granulocyte–macrophage colony-stimulating factor (GM-CSF). GVAX has shown OS benefits in phase I and II of clinical trials; however, its clinical evaluation in phase III of trial was terminated due to unsuccessful results and increased mortality in the vaccine arm. Finally, PSA-TRICOM (PROSTVAC®) is a vector-based vaccine targeting prostate-specific antigen (PSA). This vaccine is composed of PSA transgene and three co-stimulatory molecules including CD80, lymphocyte function-associated antigen 3 (LFA-3), and intercellular adhesion molecule (ICAM-1) [3]. PROSTVAC was reported to significantly prolong OS in metastatic castration-resistant prostate cancer (mCRPC) in a phase II study; however, it failed to generate clinical benefits in Phase III of the trials in patients with mCRPC. PROSTVAC is currently being evaluated in combination regimens in clinical trials [5]. Despite the success of the previously developed anti-cancer vaccines in priming anti-tumor T-cell responses in PCa, many of the developed vaccines have not been able to find their way into clinical applications due to their poor therapeutic efficacy in patients. The failure of therapeutic vaccinations could mainly be ascribed to their inability to overcome immunosuppression in tumor microenvironment (TME) [6, 7].
Immunosuppressive TME plays a key role in tumor progression, and it is the main obstacle to effective cancer immunotherapy [8, 9]. Previous studies have shown that tumor-targeted delivery of immunomodulatory agents (such as Toll-like receptor ligands) significantly enhances the trafficking and function of anti-tumor immune responses in animal cancer models [10, 11]. These findings suggest that overcoming immune suppression in TME is a key to a successful immunotherapy of cancer [12]. An increasing number of studies have reported that the main reason for PCa progression is the defective and immunosuppressive function of immune cells in TME [7, 8]. FOXP3+ regulatory T (Treg) cells and non-functional CD4+ and CD8+ T cells have been observed to play important roles in the suppression of anti-tumor immunity and promoting cancer progression in many types of human malignancies. Ebelt et al. confirmed that PCa tumors were infiltrated with PD-1+, B7-H1+, and FOXP3+ immunosuppressive lymphocytes. This was able to establish immunosuppression in TME and negatively impact anti-tumor immune responses [13]. Additionally, Miller et al. reported the high prevalence of ICOS+ FOXP3+ Treg cells in peripheral blood (PB) and inside the tumor of PCa patients compared with the normal donors [14]. In a recent study, ICOS+ tumor-infiltrated Treg cells in PCa had stronger suppressive activity compared with non-ICOS-expressing Treg cells (ICOSNEG Treg cells) [8]. It has been also suggested that non-functional prostate-infiltrating CD8+ T cells can up-regulate their negative co-inhibitory markers and down-regulate the positive co-stimulatory molecules, leading to the generation of anergic state or tumor tolerance, which, in turn, results in the suppression of anti-tumor immune responses [15]. Prostate preclinical studies have suggested that lymphocyte activation gene-3 (LAG-3) among the crucial co-inhibitory molecules overexpressed on CD8+ T cell upon antigen stimulation, regulating T-cell tolerance to tumor antigens [16].
Immune checkpoints (ICPs) are co-inhibitory and co-stimulatory molecules mainly expressed on T cells. Essential for human survival, these regulatory ICPs maintain the immune hemostasis in normal physiological conditions [17]. B7 superfamily molecules, including cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), programmed death protein-1 (PD-1), and its ligand (PD-L1) are the best known ICPs. Recent studies have identified several other ICPs which are expressed either on T cells or tumor cells and are able to suppress anti-cancer immune responses. A growing body of research has shown that cancer cells alter the expression and function of ICPs to evade anti-tumor immune responses.
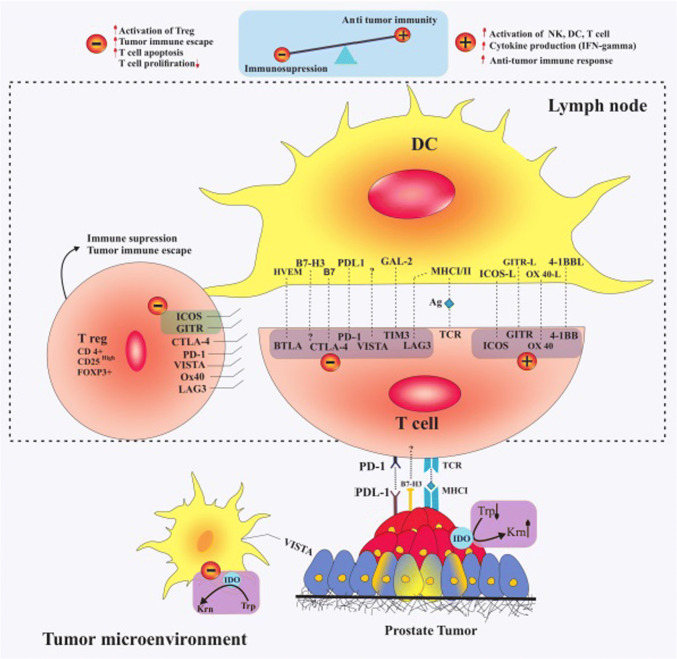
The key roles of ICPs in the initiation and progression of cancer have been well documented in previous studies. Analysis of immune cells trafficking in TME shows that the preponderance of tumor-infiltrated immune cells is immunosuppressed, supporting instead of fighting against cancer. Further characterization of tumor-infiltrated immune cells indicates that these cells up-regulate negative co-inhibitory ICPs and down-regulate positive co-stimulatory ICPs. These findings suggest that alteration in the expression of ICPs is the main mechanism by which anti-tumor cytotoxic functions of T cells are suppressed and gain a tumor supportive action [15]. It has further been shown that ICPs play an important role in multiple drug resistance (MDR) in human malignancies such as PCa [7]. Figure 1 summarizes the role and position of potential targets in lymph-node and prostate TME.
Fig. 1.
Potential ICP targets in PCa and their interplay with specific ligand. The interaction among negative co-inhibitory ICPs, including PD-1, CTLA-4, LAG-3, and V-domain immunoglobulin-containing suppressor of T-cell activation (VISTA), T-cell immunoglobulin mucin 3 (TIM-3), B- and T-lymphocyte attenuator (BTLA), and B7-H3 by their specific ligand or receptor activates immunosuppression in TME. Induction of OX40, 4-1BB, ICOS, and glucocorticoid-induced TNFR family-related gene (GITR) signaling pathways in effector T cells stimulates anti-tumor immune responses. Overexpression of ICOS and GITR on Treg cells has immune inhibitory roles in PCa. Overexpression of indoleamine-2, 3-dioxygenase (IDO) on tumor tissue and infiltrated DCs induces immunosuppression and Treg cell activation
Given the profound role of ICPs in the induction of tumor immunosuppression, blocking co-inhibitory ICPs and/or activation of co-stimulatory ICPs can be a promising strategy for overcoming immunosuppression in TME. Over the past 2 decades, several types of monoclonal antibodies (mAbs) against ICPs have been developed and approved by the FDA for treatment of various human malignancies including PCa. Despite the success in the development and clinical use of ICP inhibitors, the majority of PCa patients show resistance to ICP-based monotherapies [18]. Therefore, to achieve the optimal therapeutic efficacy of ICP inhibitors in PCa patients, it is necessary to insightfully select ICPs through an in-depth understanding of their role and networking. This review aims to discuss the significant roles played by ICPs in PCa progression and its resistance to the presented therapies based on preclinical and clinical evidence. Finally, it reviews the potential of ICPs as biomarkers for a better planning of treatment for PCa patients.
Targeting ICPs in PCa
CTLA-4
CTLA-4 (CD152) is the most well-known immune checkpoint (ICP) which is recognized as a transmembrane glycoprotein member of CD28/B.7 family and a homolog of CD28. CTLA-4 is constitutively expressed on Treg cells and can also be expressed on naïve T cells when they interact with DCs in an immunosuppressive microenvironment [19]. Upon the binding of T-cell receptor (TCR) to the targeted antigen presented on major histocompatibility complex (MHC), CD28 provides the second activation signal through binding to CD80 (B7.1) and CD86 (B7.2) on antigen-presenting cells (APCs). In the presence of CTLA-4 receptors, CD28 binding to B7 molecules is neutralized due to the higher affinity of CTLA-4 to B7 molecules compared with CD28 [20]. Ligation of B7 by CTLA-4 is a known mechanism for peripheral tolerance which suppresses the activation of T cells by DCs presenting self-antigen [19]. Given the immunosuppressive effects of CTLA-4 receptor, blocking CTLA-4 activity can improve the CD4+ T-helper-dependent immune responses. CTLA-4 is constitutively expressed on Treg cells and plays a crucial role in their biological function; therefore, the inhibition of CTLA-4 reduces the immunosuppressive function of Treg cells [21]. It is well known that Treg cells can be activated and suppress anti-cancer immune responses in many types of human malignancies including PCa. Inhibition of Treg cells using mAb against CTLA-4 is considered as a promising approach to PCa immunotherapy. Preclinical and clinical studies have suggested that CTLA-4 targeting can enhance the therapeutic efficacy of PCa vaccines [22, 23]. Furthermore, CTLA-4 targeting is reported to be advantageous when combined with ADT and RT. Ipilimumab and tremelimumab are two humanized anti-CTLA-4 mAbs widely employed in PCa patients [24].
Ipilimumab (Yervoy®) is an IgG1 mAb that blocks CTLA-4, thereby enhancing T-cell function and anti-tumor immune responses. It is an FDA approved mAb which was first used in 2011 for the treatment of metastatic melanoma [25]. The results of phase III study of ipilimumab as a monotherapy in metastatic castration-resistant prostate cancer (mCRPC) patients without prior chemotherapy showed that progression-free survival (PFS) improvement and reduced PSA occurred in response to 10 mg/kg ipilimumab with manageable immune-related adverse events (ir AEs); however, ipilimumab did not enhance OS in these patients [26].
The results of previous preclinical studies in PCa models and clinical studies in metastatic melanoma and hormone-refractory PCa patients suggest that ipilimumab can be used both alone and in combination with ADT or therapeutic vaccines in patients with mCRPC [27]. The first clinical trial, which used ipilimumab in combination with ADT, reported that ipilimumab could be safely employed in combination therapy in PCa patients. The results of these clinical trials show that combination therapy with ipilimumab significantly reduces the level of PSA in PCa patients. Reduction in the level of PSA indicates the potent anti-cancer effects of ipilimumab therapy in PCa patients [28]. Currently, there are new ongoing phase I and II clinical studies on ipilimumab combined with abiraterone acetate and prednisone in mCRPC patients.
The superiority of ipilimumab therapy over other immunotherapy approaches has been confirmed by the results of some clinical studies reporting that a DC-based therapeutic cancer vaccine (Provenge) showed survival benefits without affecting the level of PSA in PCa patients [29]. A synergistic anti-tumor effect was observed in PCa patients receiving Provenge in combination with ipilimumab. A significantly higher level of IgG and IgM specific for the PAP protein was detected in the serum of patients treated with combination therapy as compared with patients who received Provenge alone [30]. The superior anti-cancer effects of ipilimumab and Provenge combination therapy can be attributed to the better function of anti-cancer T cells induced by Provenge in the presence of a CTLA-4 inhibitory agent which prevents the suppression of cancer-specific T cells activated by the therapeutic vaccine [30]. In this regard, the previously conducted clinical trials have also shown the safety and tolerability of ipilimumab combined with GVAX [23] and PROSTVAC vaccine in mCRPC patients [31].
Ipilimumab has further been used in combination with RT in PCa patients. The results of clinical studies have indicated that the treatment of PCa patients with RT in combination with ipilimumab may result in enhanced anti-tumor effects. In a Phase I/II clinical study, PSA was reduced and tumor responses were detected in response to 3 and 10 mg/kg of ipilimumab both alone and in combination with bone-directed RT in all cohorts. Among different cohorts of this trial, 10 mg/kg ipilimumab alone and in combination with RT showed potent clinical anti-tumor activity in mCRPC patients with manageable AEs given 4% complete response, 21% stable disease (SD), and 16% PSA50 response rate [32]. Based on the anti-tumor activity reported in this study and the synergistic anti-tumor effect of CTLA-4 blockade with RT in previous preclinical studies and two case reports of metastatic melanoma, it seems that combination of 10 mg/kg ipilimumab and RT merits additional evaluation in mCRPC patients [33]. The reason behind the synergistic anti-cancer effects of ipilimumab and RT may be the ability of RT to induce immunogenic cell death (ICD) and activate anti-cancer immune responses. Indeed, ipilimumab is capable of improving the function of anti-cancer immune responses induced by RT through blocking CTLA-4 on Treg cells. Previous studies have well documented that Treg cells are abundant in TME and play a key role in suppressing anti-tumor T-cell response. It has been proposed a higher percentage of tumor-infiltrated Treg cells is correlated with poor prognosis in PCa [14]. The other phase III trial, providing further affirmative evidence, assessed the therapeutic effects of ipilimumab administered following RT in patients who had received unsuccessful docetaxel chemotherapy prior to RT [34]. This trial planned to evaluate whether RT plus ipilimumab increased the survival of advanced PCa patients compared to those treated with RT alone. While the median OS of the patients treated with ipilimumab was not significantly different from that of placebo group, a significantly lower level of PSA was observed in the serum of patients receiving ipilimumab [34]. Patients treated with ipilimumab following RT showed improved PFS, which might be a reflection of immune-mediated tumor-specific responses against cancer cells. Besides, this study corroborated the notion that administration of ipilimumab can have clinical benefits in certain subtypes of PCa patients with favorable prognostic features, particularly those with non-raised or mildly raised alkaline phosphatase and without visceral metastases and anemia [34]. Further studies are required to develop specific biomarkers, identify different subtypes of PCa, and evaluate the therapeutic efficacy of combination therapy with ipilimumab and RT in these patients.
Some clinical studies have reported the overexpression of other immune suppressive checkpoint molecules in tumor following treatment with ipilimumab. These observations suggest that the therapeutic efficacy of ipilimumab can be compromised through the up-regulation of other negative checkpoint molecules during treatment. To describe the immune profile in PCa patients receiving ipilimumab prior to surgery, Gao et al. analyzed post-treatment and baseline tissue and blood samples for the presence of immune cells and expression of ICPs. They observed high levels of T cells (CD4+, CD8+) and macrophages which overexpressed PD-L1 and V-domain immunoglobulin suppressor of T-cell activation (VISTA) in the tumor tissues of both localized and metastatic PCa following ipilimumab therapy [35, 36]. These observations suggest that the induction of the expression of inhibitory molecules such as PD-L1 and VISTA may cause resistance to ipilimumab therapy in certain groups of PCa patients. Additionally, phenotype-based analysis of known markers on the immune cells isolated from mCRPC patients treated with the combination of ipilimumab and a therapeutic vaccine (PROSTVAC) showed that prolonged OS was associated with the presence of certain immune cell subsets, including lower PD-1+ Tim-3NEG CD4 T cells and higher CTLA-4NEG Treg and PD-1NEGTim-3+ CD8 T cells prior to immunotherapy [37]. These studies opened a new insight to the role of other ICPs including PD-1, VISTA, and TIM-3 in response to CTLA-4-based immunotherapy; they also suggested that the co-administration of ipilimumab and mAb against these induced negative checkpoints might be useful in specific subgroups of PCa patients.
In a case study, Cabel et al. reported two cases of mCRPC patients with complete long-term remissions after ipilimumab therapy. Interestingly, immunohistochemical analysis of archived biopsy sample revealed high levels of CD3+, CD8+, and Treg FOXP3+ T cells in one of the patients [38]. In another study, it was reported that tumor-infiltrating lymphocytes (TIL) in biopsy samples collected prior to ipilimumab therapy were not able to be predictive biomarkers, while the high density of TIL in post-treatment samples was associated with greater clinical activity of ipilimumab [39].
Tremelimumab (CP-675,206) is also a humanized IgG2 mAb specific to CTLA-4, which was first developed for patients with metastatic melanoma [40]. Tremelimumab was marketed by Astra Zeneca in 2015 and has since been assessed in the treatment of different cancer types, including melanoma, mesothelioma, pancreatic, and hepatocellular carcinomas [40]. Following the clinical benefits observed in the OS of melanoma patients treated with tremelimumab as a monotherapy, other clinical studies evaluated the therapeutic efficacy of this drug in combination with other anti-cancer therapies, including immunotherapy strategies such as DC vaccination, anti-CD40 mAb, Toll-like receptor-9 agonist (PF-3512676), and interferon-α-2b. Tremelimumab can be a valuable tool in immune combinational therapies, known to give the most optimal results [41]. In case of melanoma cancer, combination of tremelimumab with DC vaccination increased OS to more than 10 months [41]. According to the clinical studies on different cancer types, tremelimumab contributes to the activation and increase in memory T cell [42]. Tremelimumab is still in clinical development in other cancers. In the first phase I clinical trial of this antibody in 2012, tremelimumab was administered with short-term ADT in men with PSA-recurrent PCa. The favorable reduction in the PSA level of serum suggested the future examination of this combination therapy in men with high-risk PCa recurrence to delay disease progression [43]. Clinical findings suggest dermatitis and diarrhea as dose-limiting toxicities and hyperthyroidism as non-dose-limiting toxicity of tremelimumab [41]. Another recruiting clinical trial is testing tremelimumab in combination with durvalumab in mCRPC (NCT02788773). Table 1 summarizes the completed clinical trials of anti-CTLA-4 mAbs.
Table 1.
Completed clinical trials of anti-CTLA-4
| mAb alone/combination | Phase | Setting | Result | NCT |
|---|---|---|---|---|
| Ipilimumab | Pilot trial | Hormone-refractory PCa |
PSA decline: 16% (2 /12) Safe and well tolerated |
– |
| Ipilimumab | III | mCRPC |
PFS improvement (5.6 vs 3.8 months in placebo) PSA declines (23% vs 8% in placebo) Median OS: no change |
NCT01057810 |
| Ipilimumab + ADT | II | Locally advanced PCa |
PSA decline in: ADT plus Ipilimumab: 55% ADT alone: 38% |
NCT01377389 |
| Ipilimumab + ADT | II | Localized PCa candidate for radical prostatectomy |
Increase in T cells (CD4+, CD8+) Overexpression of PD-L1+ and VISTA+ on immune cell |
NCT01194271 |
| Ipilimumab + ADT | I | Advanced PCa |
Undetectable PSA Grade > = 3 irAE % (3/11) |
NCT00170157 |
| Tremelimumab + ADT | II | PSA-recurrent PCa | Prolonged PSA doubling time | - |
| Ipilimumab + GVAX | II | mCRPC | 3 mg/kg: well tolerated and safe | NCT01510288 |
| Ipilimumab + Prostvac | I | mCRPC |
Safe and well tolerated PSA declines: 58% (14 /24) Median OS: 31.6 months |
NCT00113984 |
| Ipilimumab + Provenge | I | mCRPC |
Increase in IgG and IgG–IgM Median PSA: 3.8 (range: 0.6–7.47) |
NCT01832870 |
| ipilimumab ± RT | I/II | mCRPC patients receiving prior ADT |
Disease control in 10 mg/kg PSA decline in 10 mg/kg: 16% (8/50) |
NCT00323882 |
| Ipilimumab after RT | III | mCRPC patients receiving prior docetaxel |
Median OS: no change PSA decline Immune activity |
NCT00861614 |
PD-1/PD-L1
The PD-1/PD-L1 pathway is another critical pathway in ICP regulatory system, which comprises PD-1, mainly expressed on T cells, and its ligands, PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273), mainly expressed on cancer cells and DCs. Under normal physiological conditions, PD-L1 and PD-1 suppress T-cell activity to prevent autoimmunity. This pathway mainly mediates immune tolerance; therefore, tumor cells suppress anti-tumor immune response through blocking the effector functions of cancer-specific cytotoxic T lymphocytes (CTL) [2]. Similar to CTLA-4, PD-1 is highly expressed on Treg cells, promoting their proliferation and suppressive activity [2]. When PD-1 is expressed on effector T cells (CD4 and CD8); it negatively regulates their proliferation and survival and induces their differentiation into suppressive T cells upon binding to PD-L1. In addition to T cells, PD-1 is expressed on non-T-cell subsets such as B cells, professional APCs, and natural killer (NK) cells [44]. In contrast to PD-1, PD-1 ligands have various expression profiles. PD-L1 is expressed both on non-hematopoietic cells such as epithelial and endothelial cells and hematopoietic cells, including B cell, T cells, DCs, macrophages, and bone marrow-derived mast cells [44]. The PD-L2 expression is mostly restricted to the activated DCs, macrophages, peritoneal B1 cells, and bone marrow-derived mast cells [45]. PD-L1 expression on cancer cells is potentially under control of onco-signaling pathways and activation of inflammatory cytokines [46]. PD-L1 is also capable of interacting with non-PD-1 receptors on T cells, including CTLA-4, CD28, and CD80, thereby suppressing T-cell proliferation [47]. CD80 is consistently expressed on anergic T cells and overexpressed when exposed to the antigen. Therefore, it serves as another receptor for PD-L1 to deliver cell proliferation inhibitory signals. PD-L1 overexpression has been known as a common mechanism for immune evasion of human cancers such as PCa and a dynamic biomarker in PCa. Up-regulated PD-L1/PD-L2 expression on blood DCs is considered as a poor prognosis marker [2].
Several studies have shown that blocking PD-1/PD-L1 axis potentially re-activates cytotoxic CD8+ T cells so as to detect and eliminate tumor cells [2, 48]. There exist a small proportion of PCa patients that respond to anti-PD-1/PD-L1 checkpoint therapies; however, there are not sufficient data to warrant the treatment of PCa patients with these drugs. A clinical study reported that targeting PD-1/PD-L1 was superior to blocking CTLA-4 in terms of inducing anti-cancer immunity and improving OS in cancer patients [49]. It has been reported that the suppression of PD-1/PD-L1 induces stronger anti-tumor immune response, better anti-cancer effects, and higher OSR in patients in comparison with CTLA-4-targeted therapy. Besides, the irAEs reported with anti-CTLA-4 mAbs are less severe in patients treated with anti-PD-1/PD-L1 mAbs [50, 51]. Therefore, targeting PD-1/PD-L1 is considered as a promising strategy for immunotherapy of PCa patients. Tables 2 and 3 list the completed clinical trials on PD-1 and PD-L1 inhibitors, respectively.
Table 2.
Completed clinical trials of anti-PD-1
| mAb alone/combination | Phase | Setting | Result | NCT |
|---|---|---|---|---|
| Nivolumab | I | mCRPC | No response | NCT00730639 |
| Nivolumab + Ipilimumab | II | AR-V7+ mCRPC |
PSA50 Response: 13% (2/15) ORR: 25% Durable PFS: 20% |
NCT02601014 |
| Nivolumab + cabozantinib | I | mCRPC | Well tolerated | NCT02496208 |
| Pembrolizumab + enzalutamide | II |
Enzalutamide-resistant PCa |
PSA Response: 20% | NCT02312557 |
| Pembrolizumab | Ib | mCRPC |
ORR: 13% Response duration: 59 weeks Median OS: 8 months |
NCT02054806 |
| Pembrolizumab | II | PCa with somatic MMR mutation | ORR: 100% (1/1) | NCT01876511 |
Table 3.
Completed clinical studies of PD-L1 inhibitors
| mAb alone/combination | Phase | Setting | Result | NTC |
|---|---|---|---|---|
| Avelumab ± enzalutamide | I | mCRPC patients whose diseases progressed on ADT |
Well tolerated Prolonged PSA doubling time: 17% (3/17) SD: 80% (4/ 5) |
- |
| Atezolizumab | Ia | mCRPC patients who had received enzalutamide and/or Provenge |
PSA50 response rate: 13% (2 /15) Median survival: 15.8 months Median PFS: 3.4 months OSR: 12 months (55.6%) |
NCT01375842 |
| Durvalumab + olaparib | II | mCRPC patients who had received either abiraterone or enzalutamide |
PSA50 response: 44% (7/16) BRCA2 mutation: 35% (6/17) PSA50 response in BRCA2+ cases: 83% (5/6) Median PFS: 7.8 months |
NCT02484404 |
Nivolumab (Opdivo®) is a full human IgG4 antibody specifically targeting PD-1 expressed on the immune cells mainly on T cells. This antibody is currently applied in the treatment of several types of human malignancies including PCa. Nivolumab was approved by the FDA in 2014 for treatment of unresectable or metastatic melanoma based on the therapeutic outcomes observed in the clinical trials of patients with metastatic melanoma [20]. Consequently, nivolumab was approved for the treatment of a few other cancers, including squamous cell lung cancer, renal cell carcinoma (RCC), and Hodgkin lymphoma. In 2017, the FDA approved nivolumab as an adjuvant therapy for the treatment of metastatic cancers with complete resection [20]. Finally, in 2018, the FDA granted approval of nivolumab administration in combination with ipilimumab for advanced RCC [20]. In the first phase 1 trial of nivolumab for the assessment of the safety and activity of this antibody, 17 castration-resistant prostate cancer (CRPC) patients received nivolumab; however, none showed objective response compared to what had been observed in other cancers, including melanoma, RCC, and non-small cell lung cancer (NSCLC) [49]. In a case report study, nivolumab was reported to effectively reduce the PSA level in a 65-year-old male with advanced CRPC, who had failed in many lines of therapy [52]. Nivolumab is currently being tested in clinical trials for the treatment of localized or metastatic PCa as a monotherapy (NCT03040791).
Some mCRPC studies have demonstrated the clinical benefits of PD-1 and CTLA-4 inhibitor combinations [53, 54]. The first phase II trial of nivolumab and ipilimumab combinational therapy in PCa was conducted in androgen receptor splice variant-7 (AR-V7) positive mCRPC patients who showed resistance to anti-androgens [55]. AR-V7-positive mCRPC patients with high levels of DNA mismatch repair (dMMR) mutations showed better therapeutic outcomes compared with DNA repair proficient patients. The results of this trial revealed the safety and feasibility of nivolumab and ipilimumab combination therapy [53]. Another recruiting phase I study is currently evaluating the therapeutic efficacy of cabozantinib (Tyrosine kinase inhibitor) in combination with both nivolumab and ipilimumab in patients with genitourinary tumors (NCT02496208). The primary results of this investigation have demonstrated that the combination of cabozantinib and nivolumab is well tolerated. Other clinical trials using nivolumab in PCa include nivolumab plus PROSTVAC (NCT02933255), nivolumab plus ipilimumab (NCT02985957) and (NCT03061539), and nivolumab plus ipilimumab and enzalutamide (NCT02601014).
Pembrolizumab or lambrolizumab (Keytruda®) is a highly selective humanized IgG 4 mAb against PD-1 approved by the FDA in 2014 for the second-line treatment of metastatic malignant melanoma [20]. Pembrolizumab received FDA accelerated approval in 2017 for patients with metastatic solid tumors harboring dMMR genes whose tumors had progressed following systemic chemotherapy [2]. This antibody was approved for cervical cancer in 2018 [20] and has since been evaluated as a single agent for monotherapy in different types of human cancers. To assess the safety and efficacy of pembrolizumab monotherapy, mCRPC patients with positive and measurable PD-L1 expression (> 1%) were included in a KEYNOTE-28 study. Among the patients, 13% showed objective response and 39% had SD [56]. Pembrolizumab has further been reported to have therapeutic efficacy in mCRPC patients showing resistance to enzalutamide. Pembrolizumab and enzalutamide combination therapy sensitized mCRPC patients to ADT [50, 57]. Other studies are currently evaluating the therapeutic efficacy of pembrolizumab in combination with enzalutamide (NCT02312557) and other anti-cancer drugs (docetaxel and olaparib) in mCRPC (NCT02861573).
Some preclinical studies have shown the benefits of combination therapy with prostate vaccines and pembrolizumab, resulting in their progress to clinical trial studies [58]. ADXS31-142, a live listeria-based vaccine, was designed to induce PSA-specific immune response [59]. Currently, a KEYNOTE-phase I/II trial is evaluating the safety and anti-tumor activity of ADXS31-142 as a monotherapy and in combination with pembrolizumab in patients with mCRPC (NCT0232555). Another clinical trial in mCRPC is currently assessing the safety and efficacy of combinational therapy with pembrolizumab and a DNA-based vaccine (pTVG-HP) reported to induce PD-1-regulated PAP-specific CTL responses against PAP-expressing PCa (NCT02499835) [60].
Recent studies have suggested that anti-PD-1 therapy might enhance the therapeutic efficacy of RT in PCa. It is well known that RT induces ICD in cancer cells and releases tumor-specific antigens from dead cells as well as certain danger signals able to stimulate tumor-specific immune responses [61]. Nevertheless, the anti-tumor effect of tumor-specific CTL is hampered by FOXP3+, PD-1+ tumor-infiltrating T lymphocytes [62]. Therefore, the administration of anti-PD-1 antibodies in the patients undergoing RT is expected to surmount the immunosuppressive status induced by PD-1+ TIL and enhance the therapeutic efficacy of RT. Recently, a phase II clinical study has commenced evaluating the safety and efficacy of radium-223 therapy in combination with pembrolizumab in mCPRC patients (NCT03093428).
Avelumab (Bavencio®) is a fully humanized mAb, which binds to PD-L1 and blocks PD-L1 interaction with PD-1 and CD80. Avelumab has received accelerated approval regarding the treatment of patients with metastatic urothelial cancer whose disease has progressed following chemotherapy [20]. In a phase I clinical trial, 18 patients with mCRPC, whose disease had progressed on prior ADT, were treated with avelumab. The results showed that avelumab was well tolerated in mCRPC patients. Nevertheless, avelumab did not show significant clinical response in these patients [2]. Current ongoing studies are using avelumab as monotherapy in mCRPC (NCT01772004) and in neuroendocrine phenotype (NCT03179410) patients. Avelumab is also being utilized in combination with talazoparib (NCT03330405) in mCRPC to evaluate safety and OSR.
Atezolizumab (Tecentriq®) is another humanized IgG1 mAb against PD-L1. This mAb received accelerated approval in terms of treating advanced cases of urothelial cancer in 2016 and full approval for NSCLC in 2018 [20]. Atezolizumab has shown clinical efficacy in many types of solid tumors. The first clinical use of atezolizumab in PCa was in a phase I trial, which enrolled mCRPC patients who had received prior enzalutamide and/or Provenge for the evaluation of the safety and activity of this antibody. The results of this study revealed that atezolizumab was well tolerated in patients and showed long-term disease control with 12-month OSR. Biomarker analyses of patients who had experienced immune-related response criteria showed an activated immune response, implying increased CD8+ expression and expanded T-cell clones [63]. This observation can be ascribed to a previous study which reported high levels of PD-L1/PD-L2 positive DCs in enzalutamide-resistant mCRPC patients versus enzalutamide-naive patients [64]. Another phase III clinical trial has been recently planned to evaluate the efficacy and safety of atezolizumab in combination with enzalutamide in mCRPC patients (NCT03016312). Atezolizumab is also being investigated in clinical trials regarding its efficacy in the patients treated with RT. RT has been shown to induce higher levels of PD-L1 expression on tumor and DCs, suggesting that the combination of RT with an anti-PD-L1 mAb can improve the anti-cancer effects of RT, known to induce ICD [61, 65]. A phase I clinical trial is evaluating the safety and tolerability of atezolizumab plus radium-223 dichloride in mCRPC patients with bone metastasis (NCT02814669) [2]. Another phase I study is assessing the safety of different doses of atezolizumab in combination with Provenge vaccine in asymptomatic or minimally symptomatic mCRPC patients (NCT03024216).
Durvalumab (Imfinzi®), another anti-PD-L1 humanized mAb, received FDA approval for locally advanced or metastatic urothelial carcinoma in patients whose disease progressed after prior platinum-based chemotherapy. This mAb has further been approved for the treatment of stage III NSCLC patients following an unsuccessful chemotherapy. Durvalumab has also received accelerated approval for second-line treatment of progressive metastatic urothelial carcinoma [20]. It has further been proposed that the combination of anti-PD-L1 therapy with olaparib, a poly (ADP-ribose) polymerase inhibitor (PARPi), significantly enhances the therapeutic efficacy of PARPi-therapy. Olaparib has demonstrated anti-tumor activity in sporadic mCRPC patients with dMMR mutation. The single use of olaparib revealed overall PSA50 response rate of 20% (6/30) [66]. Another phase II trial evaluated the therapeutic efficacy of olaparib and durvalumab combination therapy in 19 mCRPC patients who had previously received either enzalutamide or abiraterone. The results of this study showed that this combination therapy was well tolerated in the patients. Somatic and germline genetic sequencing of 17 patients revealed that 35% harbored a dMMR mutation of BRCA (2:4 cases were somatic and two had germline mutation). BRCA2 is among the well-known gene mutations increasing the risk of PCa development in men [67]. In this research, the overall PSA50 response rate was 44%, and among the BRCA2-positive mutation patients, the PSA50 response rate was 83% [68]. For further assessment of the safety and efficacy of olaparib and durvalumab combinational therapy, this study is currently expanding to 50 mCRPC patients. The enhanced PSA response rate of patients treated with combination of durvalumab and olaparib compared to olaparib monotherapy can be explained by a study reported by Jiao et al. In their study, PARP inhibition alone up-regulated PD-L1 expression in breast cancer cell line and animal tumor models and induced cancer associated-immunosuppression [69].
Emerging ICP targets in PCa
Despite encouraging results, the overall clinical responses to anti-PD-1/PD-L1 and anti-CTLA-4 therapy remain unsatisfactory in certain types of PCa patients. The lack of significant clinical response to PD-1/PD-L1- or CTLA-4-targeted therapy in PCa might be associated with the fact that certain other ICPs are involved in tumor-induced immunosuppression in this type of human malignancy. Therefore, over the past decade, some new ICPs have been identified and targeted in the treatment of PCa. Table 4 summarizes the new emerging ICPs studied in regard to PCa.
Table 4.
Novel ICP targets in PCa therapy
| ICPs | Receptor expression | Clinico-pathologic association with marker overexpression | Clinical development in PCa | References |
|---|---|---|---|---|
| LaG-3 | PB T cell, TIL | Malignancy | Phase II and I | [71, 94] |
| 4-1BB | T cell, NK cell, DC | Survival and CTL response | Preclinical | [73, 91] |
| OX-40 | T cell, NK cell, NKT cell, neutrophil | - | Phase I/II | [18, 84] |
| B7-H3 | Tumor cell, DC | High expression: PCa progression, resistance to ADT | Phase I | [86, 95] |
| VISTA | Infiltrated DC, naïve T cell, FOXP3+CD4+Treg cell | High expression: resistance to anti-CTLA-4 | Preclinical | [35, 96] |
| TIM-3 | Infiltrated T cell, T reg cell, certain tissues | High expression: advance disease stage, poor prognosis marker | Preclinical | [97, 98] |
| ICOS |
T cell Treg cell |
High expression: effective response to anti-CTLA-4 High expression: inducing immunosuppression |
Preclinical | [99, 100] |
LAG-3
LAG-3 (CD223) is an ICP molecule expressed on activated CD4+ and CD8+ T cells, Treg, NK cells, and B cells. LAG-3 is considered as a promising target for cancer therapy [70, 71]. This ICP molecule is located in proximity to CD4 receptor and binds to major histocompatibility complex class II (MHCII) molecule with strong affinity. L-sectin and galectin-3, expressed in TME, are two other putative ligands for LAG-3 [72]. Based on evidence, LAG-3 has dual functions in the immune system. LAG-3 activates DCs through interaction with MHCII on immature DCs, leading to their maturation [72]. On the other hand, LAG-3, expressed on T cells, is a negative regulatory receptor, directly competing with CD4 in terms of binding to MHCII on APCs. This triggers an inhibitory condition that interferes with effector T-cell activation and increases the suppressive activity of Treg cells [70, 71]. It has been reported that inflammatory conditions induce the overexpression of LAG-3 on the surface of activated CD4+, CD8+, and NK cells [73]. Targeting LAG-3, also up-regulated in anergic T cells, has been suggested to be a promising strategy for the reversal of anergic state in T cells [74]. The high expression of LAG-3 on TILs is further observed to correlate with histological signs of malignancy in PCa [21]. Over the last decade, several approaches have been used to target LAG-3 in cancer therapy. These approaches include the stimulation of DCs by LAG-3 soluble fusion protein and activation of T cells by anti-LAG-3 antibodies [71, 75]. IMP321 (Eftilagimod alpha) is a soluble LAG-3 immunoglobulin investigated as a vaccine adjuvant or a protein drug in combination with chemotherapy in three completed clinical trials to treat RCC, melanoma, and metastatic breast cancers [76, 77]. The encouraging outcomes of the studies reporting the significant anti-tumor effects of LAG-3-targeted therapy have led to the initiation of other clinical trials.
Several studies have shown that the blockade of LAG-3 induces effector T-cell response and reduces the immunosuppression in TMEs. Up to now, different humanized antagonistic IgG4 mAbs, including BMS-986016 (Relatlimab), LAG525, REGN3767, INCAGN02385, and TSR-033 have been developed. Anti-LAG-3 mAbs bind to LAG-3 on TILs and prevent its binding to MHCII [78]. Preclinical and clinical studies have shown that the blockade of LAG-3 on T cells can effectively enhance the therapeutic efficacy of PD-1 inhibitors. The combination of relatlimab with nivolumab depicted compelling clinical responses in melanoma patients who did not respond to PD-1-targeted therapy [79]. The superior anti-cancer efficacy of co-targeting LAG-3 and PD-1 pathways suggests that these two ICPs might be co-expressed on anergic T cells and synergistically support cancer immune evasion. Besides, LAG-3 is a direct modulator of PD-1+ cells activity [80]. Consequently, dual blockade of PD-1 and LAG-3 may lead to better clinical responses in PCa [81]. LAG525 and REGN3767 are currently being employed in phase II clinical trial to investigate their efficacy in combination with anti-PD-1 in advanced CRPC (NCT03365791) and advanced cancers (NCT03005782), respectively. MGD013 and FS118 are first-in-class bispecific proteins that block PD-1/LAG-3 and anti-PD-L1/LAG-3, respectively. These dual-affinity proteins are currently undergoing phase I clinical trial in patients with metastatic solid tumors.
OX40/OX40L
OX40 (CD134), belonging to tumor necrosis factor receptor (TNFR) superfamily, is yet another attractive ICP molecule for cancer-targeted therapy on PCa. This ICP is a co-stimulatory molecule transiently expressed on activated T cells, NK cells, NKT cells, and neutrophils, and overexpressed on human Treg cells. The study on the expression and function of OX40 on TILs has revealed that the stimulation of its signaling potentially induces the effector activity of CD8+ and CD4+ T cells and depletes tumor-infiltrating FOXP3+ Treg cells expressing OX40 [18]. Preclinical studies have shown that monotherapy with OX40 agonists results in tumor regression, significantly increasing OS in mice cancer models [82]. These studies have further reported that the administration of OX40 agonists in immunized tumor-bearing mice increases the proliferation of T cells, production of cytokines, and generation of memory T cells [82]. OX40L-Fc and four other anti-OX40 agonist antibodies are currently being examined in human clinical studies in metastatic cancers [18].
9B12 is a murine agonistic anti-human OX40 mAb, which was applied in phase I clinical study to patients with advanced solid tumors refractory for conventional treatment (NCT01644968). The results showed that this antibody was well tolerated and increased the proliferation of FOXP3NEG CD4+ and CD8+ T cells. This specific study introduced OX40 as a potent immunogenic target for cancer patients in the late stage [82]. Some preclinical studies have reported that OX40 agonists enhance the therapeutic efficacy of RT and chemotherapy (such as cyclophosphamide) in animal cancer models [82]. RT and chemotherapy can induce ICD, able to activate DCs, and initiate immune responses [83]. Therefore, as reported in the aforementioned study, OX40 agonists are expected to enhance the induction of anti-cancer immune responses and lead to better therapeutic outcomes in combination therapy as compared with monotherapy. Following the encouraging results in preclinical studies, a phase I/II trial tested 9B12 in combination with cyclophosphamide and radiation in patients with progressive metastatic and chemotherapy-resistant PCa (NCT01303705). Anti-OX40 was administered intravenously, and the combination therapy showed manageable safety and tolerability. Among nine patients, 44% had reduced transient PSA and 55% demonstrated radiographically stable bone and lymph-node metastasis during the study. Immunological data obtained from PB analysis showed about 2–threefold increase in FOXP3NEG CD4+, CD8+, and NK cells [84].
Additionally, co-treatment of TRAMP-C1 mouse prostate tumor model with anti-OX40 and anti-CTLA-4 enhanced anti-tumor activity and survival in PCa models [18]. Preclinical studies have reported that anti-OX40 and anti-CTLA-4 have similar abilities to stimulate T cells in both basic and tumor immunology models [82]: anti-OX40 by co-stimulation and anti-CTLA-4 by blocking inhibition. Presently, several ongoing clinical studies are investigating the therapeutic efficacy of different anti-OX40 murine mAbs such as MEDI0562 as a monotherapy (NCT02219724) or MEDI6469 in combination with anti-PD-L1, and anti-CTLA-4 (NCT02205333) in patients with advanced solid tumors.
B7-H3
B7-H3, a member of B7 superfamily, is another important ICP targeted in PCa. B7 superfamily ligands are expressed on lymphoid and non-lymphoid tissues. While B7 superfamily ligands generally deliver co-stimulatory signals to DCs, B7-H3 ligation has been observed to have dual effects (co-inhibitory or co-stimulatory) on T cells [85]. Although further studies are required to fathom the contradictory roles of B7-H3 in tumor biology, there is a growing body of evidence, showing that B7-H3 overexpression is correlated with poor prognosis in various solid tumors such as breast, pancreatic, NSCLC, squamous cell carcinoma (SCC), and PCa [31, 85, 86]. This evidence suggests that B7-H3 can be a promising target for the targeted therapy of PCa and a valid biomarker in PCa treatment, particularly in those resistant to ADT. 8H9, enoblituzumab, and orlotamab are mAbs developed for B7-H3-based targeted therapy in cancer [85]. Enoblituzumab (MGA271) is an anti-B7-H3 humanized mAb. Combination therapy with enoblituzumab and ipilimumab/pembrolizumab is being assessed in a phase I clinical trials in refractory cancers with B7-H3 expression such as melanoma, NSCLC, and PCa (NCT02381314, NCT02475213). Orlotamab (MGD009) or human dual-affinity re-targeting (DART®) protein is the other developed mAb that targets both B7-H3 and CD3. Currently, phase I dose escalation study of orlotamab is being conducted in patients with unresectable or metastatic B7-H3-expressing tumors (NCT02628535).
4-1BB/4-1BBL
4-1BB and 4-1BBL play important roles in the regulation of immune responses. 4-1BB receptor (CD137), another member of TNFR superfamily, is a co-stimulatory receptor expressed on stimulated CD4+ and CD8+ T cells, activated NK cells, and DCs. 4-1BB binds to 4-1BBL which is expressed on activated DCs, macrophages and B cells [73]. 4-1BB signaling in T cells enhances their survival through the up-regulation of anti-apoptotic pathways and induces the release of pro-inflammatory cytokines such as IFN-γ and IL-2 [87, 88]. Additionally, 4-1BB ligation stimulates TCR signaling and increases the cytotoxicity of CD8+ T cells. Similarly, in NK cells, 4-1BB stimulation triggers cell proliferation, IFN-γ production, and cytolytic effect. 4-1BB ligation further increases the secretion of perforin and granzyme [89]. Moreover, 4-1BB ligation accelerates DCs maturation by the overexpression of CD80 and CD86 co-stimulatory molecules, and results in increased cell survival rate and production of IL-6 and IL-12 [90]. Previous studies have shown that the down-regulation of 4-1BB in cancer results in T-cell apoptosis and cancer immune evasion [88]. On the other hand, immunotherapy with 4-1BB agonists has been observed to induce potent type 1 inflammatory and cytotoxic T-cell responses within TME [88]. In line with these findings, 4-1BB agonistic mAbs have been shown to induce tumor rejection in multiple murine models, in a study by Youlin et al., combination therapy of PCa-bearing mice with 4-1BBL expressing cellular vaccine and anti-CTLA-4 mAb resulted in the induction of CTL responses and regression of established tumors [91].
Urelumab and utomilumab are two 4-1BB agonistic mAbs reported to be very effective for cancer immunotherapy in murine cancer models [6]. Urelumab is the first generation humanized IgG 4 mAb agonist utilized in clinical trials for patients with advanced solid tumors and hematologic cancers. The clinical development of urelumab is limited, because it induces liver inflammation [92]. Utomilumab is the second generation humanized IgG2 mAb agonist with less liver toxicity compared to urelumab. A Phase 1b trial evaluated the therapeutic efficacy of utomilumab and pembrolizumab combination therapy in cancer patients, confirming the safety of utomilumab. Additionally, this combination demonstrated efficient and long-lasting anti-tumor activity over a broad range of solid tumors, including RCC, thyroid cancer, lung cancer, and SCC of the head and neck [93]. Utomilumab is currently being examined in several clinical trials for the treatment of patients with advanced solid tumors.
VISTA
VISTA is a new and promising ICP molecule for targeting in PCa immunotherapy and a co-inhibitory ICP that controls peripheral tolerance. Besides, in cooperation with co-inhibitory receptors such as PD-1, CTLA-4, LAG-3, and TIM-3, VISTA regulates immune response against infections and cancer [101]. This ICP molecule is constitutively expressed on CD11b+ myeloid DCs, naïve T cells, and FOXP3+ CD4+ Treg cells [96]. On the surface of APCs, VISTA is an inhibitory ligand suppressing the proliferation and cytokine production of both CD4+ and CD8+ T cells. Moreover, CD4+ T cells express VISTA to the suppress activation of T-cell function in a T-cell autonomous manner [96]. Preclinical studies have shown that the blockade of VISTA using VISTA-targeted mAbs improves T-cell responses and anti-tumor immunity [102]. Anti-VISTA mAbs have been reported to induce tumor-specific T-cell responses and enhance the function of TILs in the TME. It has further been shown that anti-VISTA monotherapy significantly suppresses both inducible and transplantable cancers in mice melanoma models [103]. The importance of targeting VISTA in PCa was first shown by Gor et al.; they reported that VISTA was overexpressed on T cells and macrophages in mCRPC patients who did not respond to ipilimumab. This observation suggested that the overexpression of VISTA might be a reason behind the poor therapeutic efficacy of ipilimumab in mCRPC patients [35]. Many important questions still remain as to the importance of VISTA expression in PCa patients and its significance in mediating resistance to ICP-targeted immunotherapies. Anti-VISTA mAbs are also being examined as monotherapy or combination therapy with cancer vaccines in various types of solid tumors [104].
TIM-3
TIM-3 is a new emerging inhibitory ICP considered as a promising therapeutic target for cancer immunotherapy. This inhibitory ICP induces cancer progression by stimulating T-cell exhaustion [98]. Furthermore, it is a glycoprotein of immunoglobulin superfamily with extracellular immunoglobulin and mucin domains that serve as a marker for IFN-γ to generate fully differentiated CD4+ T-helper-1 lymphocytes. TIM-3 is expressed on certain tissues (liver, small intestine, thymus, kidney, spleen, lung, muscle, and brain) and activated T cells [98]. Galectin is known as the most prominent ligand of TIM-3. There exist other soluble ligands for TIM-3, including phosphatidyl serine (PTS) and high mobility group box 1 (HMGB1) [73, 105]. The interaction between TIM-3 and galectin-9 induces peripheral tolerance by the inhibition of Th1 and Th17 immune response. Although TIM-3 does not directly contribute to T-cell differentiation, it plays a crucial role in Th1 trafficking [98].
TIM-3 was observed to be overexpressed on T cells in certain types of cancer, including melanoma, NSCLC, ovarian cancers, and PCa [97, 98]. The biological significance of TIM-3 in PCa was first highlighted in 2014 when a high level of PD-1NEG Tim-3+ CD8+ T cells was reported in mCRPC patients treated with ipilimumab-based therapy [37]. In line with this study, it was found that PCa patients with low levels of PSA-specific CD8+ T cell showed higher expressions of TIM-3 and CD38 markers on these T cells, implying the dysfunctionality of these T cells [106]. The importance of TIM-3 as a therapeutic target in PCa was underscored by a study in which TIM-3 was highly expressed on CD4+ and CD8+ T cells in both PB and tumor tissue samples of patients with PCa; moreover, overexpression of TIM-3 was correlated with poor prognosis in these patients [98]. These observations corroborate the notion that TIM-3 is a promising target for immunotherapy in PCa. The main approach to targeting TIM-3 is the development of anti-TIM-3 mAbs including Sym023, TSR022, MBG453, and LY3321367. Phase I and phase Ib/II clinical trials have been initiated to assess the safety and therapeutic efficacy of the developed anti-TIM-3 mAbs in patients with advanced solid tumors.
ICOS/ICOSL
ICOS is another ICP molecule considered as a potential target for the immunotherapy of PCa. This ICP molecule belongs to B7/CD28 superfamily and gets induced upon the activation of T cells [107]. ICOS/ICOSL signal plays a dual role in anti-cancer immune responses owing to its expression on both CD4+ T and Treg cells [99]. Therefore, antagonistic or agonistic antibody therapy against ICOS is selected based on the immunological criteria and planned in a case-by-case manner with regards to each patient. ICOS expressed on activated T cells functions as a stimulatory and plays a crucial role in CD4+ T-cell responses and regulation of cytokine production (IFN-γ stimulation). It has further been reported that ICOS/ICOSL signaling pathway is required for an efficient therapeutic effect of anti-CTLA-4 agents [107]. Other studies have reported an increase in the percentage of effector CD4+ ICOShigh cells in patients with melanoma and bladder cancer and responding effectively to anti-CTLA-4 agents. Enhanced survival rates in small subsets of melanoma patients taking ipilimumab were correlated with an increase in the level of ICOShigh Tcells. Furthermore, in bladder cancer patients, anit-CTLA-4 therapy enhanced the ratio of effector CD4+ ICOShigh T cells/ICOShigh Treg cells in PB and tumor tissues [99]. According to research, the increased level of CD4+ ICOShigh T cells serves as a pharmacodynamics biomarker of anti-CTLA-4 therapy [100]. Altogether, these findings accentuate the importance of ICOS signaling in response to anti-CTLA-4 immunotherapy and suggest that ICOS agonistic mAbs might be useful in regard to patients treated with anit-CTLA-4 mAbs. JTX-2011 and GSK3359609 are two anti-ICOS agonistic mAbs that target and stimulate their proliferation of ICOS+ infiltrating CD4+ T cells and induce CTL responses against cancer cells [107]. Currently, clinical trials are in progress to evaluate the safety and therapeutic efficacy of these antibodies employed in monotherapy or combination therapy with anti-PD-1/PD-L1 (NCT02723955) and anti-CTLA-4 (NCT02904226) mAbs.
In contrast to the stimulatory function of ICOS on CD4+ T cell, high expression of ICOS on Treg infiltration cells is correlated with a poor prognosis. There exist myriad Treg cells in the PB and tumor tissues of early stage PCa patients. It was reported that the high proportion of these Treg cells were ICOS, GITR, and FOXP3 positive [14]. Moreover, anti-ICOS antagonistic mAbs enhanced the anti-tumor immune responses induced by a cancer vaccine (GVAX) in PCa patients [99]. These findings suggest that the depletion of ICOS+ Treg cells by antagonistic anti-ICOS mAbs might be a promising strategy for ameliorating the therapeutic efficacy of cancer vaccines in PCa patients. MEDI-570 is a humanized anti-ICOS IgG1 mAb preventing the interaction between ICOS expressed on TILs and ICOSL expressed on plasmacytoid DCs. Blocking ICOS activation inhibits the proliferation of DCs, accumulation of ICOS+ Treg cells, and secretion of IL-10 through infiltrating CD4+ T cells [99]. MEDI-570 is under investigation in a phase I clinical trial in patients with lymphoid malignancies (NCT02520791).
Potential predictive biomarkers
Following the encouraging results pertaining to the ICP-targeted therapy of cancer, some ICPs are emerging as potential biomarkers for personalized treatment of cancer patients. Among the different predictive biomarkers, PD-L1 overexpression and high somatic mutational load are the most potential biomarkers, which have been found to be predictive for the possible benefits of anti-CTLA-4 and anti-PD-1/PD-L1 therapy in many types of human malignancies such as PCa.
PD-L1 expression
PD-L1 expression on tumor cells has been found to be a valuable prognostic biomarker for the prediction of therapeutic response to anti-PD-1/PD-L1 therapies in PCa [39]. Overexpression of PD-L1 on tumor cells is an important mechanism for them to escape from the host immune system, ensuing poor prognosis in different tumor types [108]. Studies have shown that moderate-to-high expressions of PD-L1 are correlated with higher Gleason score, higher proliferation rates (Ki-67), and androgen receptor expression in primary PCa patients following radical prostatectomy [108]. In a phase I study, Topalian et al. showed that none of the 17 mCRPC patients, treated with nivolumab, had significant clinical responses [49]. In this study, the optional sampling of biopsies from patients with different cancers showed that patients with negative PD-L1 expression had no objective response [49]. These finding suggest that PD-L1 expression significantly affects the therapeutic efficacy of PD-1/PD-L1 inhibitors [109]. Some recent studies report that the level of PD-L1 expression in mCRPC patients is higher than that in patients with primary PCa (32.1% vs 7.7%). These findings highlight the biological significance associated with the overexpression of PD-1/PD-L1 receptors in patients with advanced forms of PCa and suggest that PD-1/PD-L1-targeted therapy can be beneficial in these patients [110-112].
Multiple factors can influence the predictive ability of PD-L1 as a biomarker for the response to ICPs-targeted therapies [113]. It has been reported that the site of PD-L1 expression (tumor cells vs. infiltrating immune cells) is able to impact the response to PD-1-targeted therapy in CRPC [113]. In addition to the location of PD-L1 expression, genetic mutations in tumors, different levels of PD-L1 expression, TME composition, and their dynamic interactions can affect the therapeutic outcomes of PD-1/PD-L1-targeted therapy [113, 114]. PD-L1 expression in TME negatively influences T-cell infiltration and induces up-regulation of ICPs (CTLA-4 and PD-1) on T cells due to the high levels of immunosuppressive cytokines present in such TME [6]. Several studies have shown that blocking CTLA-4 or PD-L1 enhances the therapeutic efficacy of anti-cancer vaccines [115, 116]. These studies suggest that combination therapy with PD-1/PD-L1 inhibitors, anti-CTLA-4 mAb (ipilimumab), and Provenge vaccine may be a promising strategy for inducing effective anti-tumor immune responses in patients with advanced PCa.
The levels of PD-L1+ DCs significantly increased in the blood circulation of mCRPC patients whose disease progressed on enzalutamide therapy. It was suggested that the elevated levels of PD-L1 in DCs might be one of the enzalutamide resistance mechanisms in these [2]. Similarly, several lines of studies have shown that PD-L1 is highly expressed in enzalutamide-resistant CRPC [57, 64]. In a recent study, Heng et al. observed that PD-L1 expression was an independent and important prognostic biomarker in PCa patients who had received adjuvant hormonal therapy following radical prostatectomy. Their findings showed that the high PD-L1 expression level was correlated with bad therapeutic outcomes, suggesting that anti-PD-1/PD-L1 therapy might be beneficial for PCa patients after radical prostatectomy [117].
Despite all the observations pointing to the prognostic values of PD-L1 in PCa, this ICP may not be a valid prognostic biomarker for a few reasons. First, there are no uniformed cut-off expression levels and a standard technique for PD-L1 measurement [114]. Besides, PD-L1 expression data might be misinterpreted due to the dynamic status of TME [114]. Over the past few years, PD-L1 expression has been approved as a predictive marker for the responsiveness of advanced NSCLC, esophagus, stomach and gastroesophageal junction, cervix, and head and neck squamous cell carcinomas to pembrolizumab and the responsiveness of urothelial and breast carcinomas to atezolizumab [113, 118].
Mutations in PCa
Hypermutation in cancer genomes is known as another beneficial biomarker for the prediction of therapeutic response to ICP-targeted therapies in PCa. It has been revealed that PCa harboring dMMR mutation and microsatellite instability (MIS) show remarkable and long-lasting therapeutic responses to PD-1 checkpoint blockade. Pembrolizumab has been approved for the treatment of advanced PCa with dMMR/MSI-high mutation in any histological variants based on the significant therapeutic response of patients with dMMR CRPC to single-agent pembrolizumab [119].
Other studies have further reported that MMR mutations might be correlated with high-grade Gleason scores and certain histological variants of PCa, including ductal, intraductal, and small cell PCa [120]. Based on the case studies, in addition to MMR mutations, tumor mutational burden (> 10/Mb), biallelic CDK12 mutation, and PD-L1 3′UTR mutation are among the effective genetic biomarkers for the responsiveness of PCa patients to anti-PD-1/PD-L1 [111].
Indoleamine-2, 3-dioxygenase (IDO)
IDO is an intracellular immune modulator that activates Treg cells and myeloid-derived suppressor cells, suppresses T and NK cells, and promotes angiogenesis in TME. This modulatory enzyme normally exists in several normal tissues and the mature DCs of lymphoid tissues [121]. The high expression of IDO has been found to be associated with PCa progression and poor clinical responses to ICP-targeted therapies. Previous studies have reported that higher IDO expression is associated with lower survival rate in cancer patients. Research has also suggested that the expression of IDO in the urines of men is a diagnostic and potential biomarker for PCa progression [122]. It has been reported that the quantitative gene expression of IDO in PCa tissues is higher than that in benign prostatic hyperplasia (BPH). Besides, there is a correlation between IDO activity and kynurenine/tryptophan ratio in the serum of cancer patients [123]. This finding suggests that the plasma level of kynurenine and/or tryptophan can be considered to estimate IDO activity with no need for analyzing the tumor samples in cancer patients [122]. The significance of IDO as a potential biomarker for the enrollment of cancer patients in immunotherapeutic regimens was shown by studies reporting that IDO expression was also correlated with resistance to anti-CTLA-4 antibodies in mouse tumor models [124]. Thus, IDO expression could be a biomarker for predicting the therapeutic response to anti-CTLA-4-targeted therapy. These results further propose that combinational therapy with IDO inhibitors (indoximod), anti-CTLA-4 mAb, and DC-based vaccines (Provenge) could be an effective strategy for the treatment of PCa patients [121, 125].
Conclusion
Immunosuppression in TME is a major challenge to a successful cancer immunotherapy. ICPs have been found to play a key role in the establishment of tumor-induced immunosuppression in TME. Therefore, targeting ICPs is considered as a promising strategy for ameliorating the therapeutic efficacy of cancer immunotherapy modalities in solid tumors, particularly PCa. CTLA-4 and PD-1/PD-L1 are the most well-known targeted ICPs for overcoming immunosuppression in TME and induction of effective anti-cancer immune responses in PCa. Clinical studies show that anti-CTLA-4 and anti-PD-1/PD-L1 mAbs significantly improve the therapeutic outcomes of cancer vaccines in PCa. Moreover, CTLA-4 and PD-1/PD-L1 inhibition enhances the anti-tumor activity of RT and certain chemotherapeutic agents in PCa. The results of clinical studies in patients also show the safety and superiority associated with the therapeutic efficacy of combination therapy with anti-CTLA-4 mAbs and anti-PD-1/PD-L1 mAbs in PCa. Despite the encouraging results in PCa patients treated with anti-CTLA-4 mAbs and anti-PD-1/PD-L1 mAbs, resistance to CTLA-4 and PD-1/PD-L1-targeted therapies has been reported in several clinical studies. The overexpression of other ICPs (VISTA and TIM-3) is an important mechanism for the development of resistance to anti-CTLA-4 mAb and anti-PD1/PD-L1 mAb in PCa. These observations show the significance of other ICPs in mediating and sustaining immunosuppression in TME. On the whole, the findings discussed in the present review suggest that ICP-targeted therapy is a promising approach for the management of solid tumors, especially PCa. Nevertheless, further studies are required to elucidate the significance of newly introduced ICPs and explore the other possible ICPs with key roles in the establishment of immunosuppressive network in TME and development of resistance to ICP-targeted therapies. Moreover, the clinical application of combination therapy with ICP-targeted therapy and other cancer treatment modalities in PCa needs more clinical investigations regarding their safety and therapeutic efficacy in PCa.
Acknowledgements
This work is part of a dissertation (No. 128) by S. Jafari, submitted for Ph.D. degree and supported by Biotechnology Research Center, Tabriz University of Medical Sciences.
Compliance with ethical standards
Conflict of interest
None to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Isaacsson Velho P, Antonarakis ES. PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert Rev Clin Pharmacol. 2018;11(5):475–486. doi: 10.1080/17512433.2018.1464388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris E. Immunotherapeutics for the treatment of prostate cancer: a patent landscape based on key therapeutic mechanisms of actions. Pharm Pat Anal. 2018;7(1):47–57. doi: 10.4155/ppa-2017-0029. [DOI] [PubMed] [Google Scholar]
- 4.Vlachostergios PJ, Paddock M, Molina AM (2018) Molecular targeted therapies of prostate cancer. In: Precision molecular pathology of prostate cancer, Springer, New York, pp 523–546
- 5.Gulley JL, Borre M, Vogelzang NJ, Ng S, Agarwal N, Parker CC, Pook DW, Rathenborg P, Flaig TW, Carles J. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37(13):1051–1061. doi: 10.1200/JCO.18.02031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curran MA, Glisson BS. New hope for therapeutic cancer vaccines in the era of immune checkpoint modulation. Annu Rev Med. 2019;70:409–424. doi: 10.1146/annurev-med-050217-121900. [DOI] [PubMed] [Google Scholar]
- 7.Thakur A, Vaishampayan U, Lum L. Immunotherapy and immune evasion in prostate cancer. Cancers. 2013;5(2):569–590. doi: 10.3390/cancers5020569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mo L, Chen Q, Zhang X, Shi X, Wei L, Zheng D, Li H, Gao J, Li J, Hu Z. Depletion of regulatory T cells by anti-ICOS antibody enhances anti-tumor immunity of tumor cell vaccine in prostate cancer. Vaccine. 2017;35(43):5932–5938. doi: 10.1016/j.vaccine.2017.08.093. [DOI] [PubMed] [Google Scholar]
- 9.Tang M, Diao J, Cattral MS. Molecular mechanisms involved in dendritic cell dysfunction in cancer. Cell Mol Life Sci. 2017;74(5):761–776. doi: 10.1007/s00018-016-2317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine. 2008;26(39):5046–5057. doi: 10.1016/j.vaccine.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Molavi O, Ma Z, Hamdy S, Lai R, Lavasanifar A, Samuel J. Synergistic anti-tumor effects of CpG oligodeoxynucleotide and STAT3 inhibitory agent JSI-124 in a mouse melanoma tumor model. Immunol Cell Biol. 2008;86(6):506–514. doi: 10.1038/icb.2008.27. [DOI] [PubMed] [Google Scholar]
- 12.Molavi O, Ma Z, Hamdy S, Lavasanifar A, Samuel J. Immunomodulatory and anticancer effects of intra-tumoral co-delivery of synthetic lipid A adjuvant and STAT3 inhibitor, JSI-124. Immunopharmacol Immunotoxicol. 2009;31(2):214–221. doi: 10.1080/08923970802380452. [DOI] [PubMed] [Google Scholar]
- 13.Ebelt K, Babaryka G, Frankenberger B, Stief CG, Eisenmenger W, Kirchner T, Schendel DJ, Noessner E. Prostate cancer lesions are surrounded by FOXP3+, PD-1+ and B7–H1+ lymphocyte clusters. Eur J Cancer. 2009;45(9):1664–1672. doi: 10.1016/j.ejca.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Miller AM, Lundberg K, Özenci V, Banham AH, Hellström M, Egevad L, Pisa P. CD4+ CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177(10):7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 15.Gururajan M, Posadas EM, Chung LW. Future perspectives of prostate cancer therapy. Transl Androl Urol. 2012;1(1):19. doi: 10.3978/j.issn.2223-4683.2012.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self-and tumor-tolerance systems. J Clin Invest. 2007;117(11):3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, Vignali DA. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immun. 2016;44(5):1034–1051. doi: 10.1016/j.immuni.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria J-C, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. doi: 10.1016/j.ejca.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Riva A, Chokshi S. Immune checkpoint receptors: homeostatic regulators of immunity. Hepatol Int. 2018;12(3):223–236. doi: 10.1007/s12072-018-9867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Yang W, Huang Y, Cui R, Li X, Li B. Evolving roles for targeting CTLA-4 in cancer immunotherapy. Cell Physiol Biochem. 2018;47(2):721–734. doi: 10.1159/000490025. [DOI] [PubMed] [Google Scholar]
- 22.Fong L, Kwek SS, O'Brien S, Kavanagh B, McNeel DG, Weinberg V, Lin AM, Rosenberg J, Ryan CJ, Rini BI. Potentiating endogenous anti-tumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69(2):609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PubMed] [Google Scholar]
- 23.van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJA, van der Sluis TM, Gall HE, Harding TC, Jooss K, Lowy I. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 24.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the anti-tumor activity of anti–CTLA-4 antibodies. J Exp Med. 2009;206(8):1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron F, Whiteside G, Perry C. Ipilimumab. Drugs. 2011;71(8):1093–1104. doi: 10.2165/11594010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Beer T, Logothetis C, Sharma P, Gerritsen W, Ezzeddine R, Fairchild J, Gagnier P, Chin K, Cuillerot J. Randomized, double-blind, phase III trial to compare the efficacy of ipilimumab (Ipi) versus placebo in asymptomatic or minimally symptomatic patients (pts) with metastatic chemotherapy-naïve castration-resistant prostate cancer (CRPC) J Clin Oncol. 2011;29(15_suppl):TPS182. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 27.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13(6):1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 28.Tollefson M, Karnes RJ, Thompson RH, Granberg C, Hillman D, Breau R, Allison J, Kwon E, Blute M. 668 a randomized Phase II study of ipilimumab with androgen ablation compared with androgen ablation alone in patients with advanced prostate cancer. J Urol. 2010;183(4):e261. [Google Scholar]
- 29.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 30.Scholz M, Yep S, Chancey M, Kelly C, Chau K, Turner J, Lam R, Drake CG. Phase I clinical trial of sipuleucel-T combined with escalating doses of ipilimumab in progressive metastatic castrate-resistant prostate cancer. Immunol Target Ther. 2017;6:11. doi: 10.2147/ITT.S122497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modena A, Ciccarese C, Iacovelli R, Brunelli M, Montironi R, Fiorentino M, Tortora G, Massari F. Immune checkpoint inhibitors and prostate cancer: a new frontier? Oncol Rev. 2016;10(1):293. doi: 10.4081/oncol.2016.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slovin S, Higano C, Hamid O, Tejwani S, Harzstark A, Alumkal J, Scher H, Chin K, Gagnier P, McHenry M. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24(7):1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85(2):293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, Van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23(5):551. doi: 10.1038/nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E. Investigation of mechanisms of resistance to ipilimumab therapy with a pre-surgical trial in patients with high-risk, localized prostate cancer. New York: American Society of Clinical Oncology; 2017. [Google Scholar]
- 37.Jochems C, Tucker JA, Tsang K-Y, Madan RA, Dahut WL, Liewehr DJ, Steinberg SM, Gulley JL, Schlom J. A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates. Cancer Immunol Immunther. 2014;63(4):407–418. doi: 10.1007/s00262-014-1524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabel L, Loir E, Gravis G, Lavaud P, Massard C, Albiges L, Baciarello G, Loriot Y, Fizazi K. Long-term complete remission with Ipilimumab in metastatic castrate-resistant prostate cancer: case report of two patients. J Immunother Cancer. 2017;5(1):31. doi: 10.1186/s40425-017-0232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Comin-Anduix B, Escuin-Ordinas H, Ibarrondo FJ. Tremelimumab: research and clinical development. Oncol Targets Ther. 2016;9:1767. doi: 10.2147/OTT.S65802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calabrò L, Morra A, Fonsatti E, Cutaia O, Amato G, Giannarelli D, Di Giacomo AM, Danielli R, Altomonte M, Mutti L. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14(11):1104–1111. doi: 10.1016/S1470-2045(13)70381-4. [DOI] [PubMed] [Google Scholar]
- 43.McNeel DG, Smith HA, Eickhoff JC, Lang JM, Staab MJ, Wilding G, Liu G. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunther. 2012;61(7):1137–1147. doi: 10.1007/s00262-011-1193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chamoto K, Al-Habsi M, Honjo T (2017) Role of PD-1 in immunity and diseases. In: Emerging concepts targeting immune checkpoints in cancer and autoimmunity, Springer, New York, pp 75–97
- 45.Muenst S, Soysal SD, Tzankov A, Hoeller S. The PD-1/PD-L1 pathway: biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert Opin Ther Target. 2015;19(2):201–211. doi: 10.1517/14728222.2014.980235. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Jiang C, Jin L, Zhang X. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2015;27(3):409–416. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 47.Park J-J, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S. B7–H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116(8):1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2014;27(1):39–46. doi: 10.1093/intimm/dxu095. [DOI] [PubMed] [Google Scholar]
- 49.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, Cetnar JP, Ey FS, Bergan RC, Slottke R. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7(33):52810. doi: 10.18632/oncotarget.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dolan DE, Gupta S. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control. 2014;21(3):231–237. doi: 10.1177/107327481402100308. [DOI] [PubMed] [Google Scholar]
- 52.Basnet A, Khullar G, Mehta R, Chittoria N. A case of locally advanced castration-resistant prostate cancer with remarkable response to nivolumab. Clin Genitourin Cancer. 2017;15(5):e881–e884. doi: 10.1016/j.clgc.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apolo AB, Mortazavi A, Stein MN, Pal SK, Davarpanah NN, Parnes HL, Ning YM, Francis DC, Cordes LM, Monk P. A phase I study of cabozantinib plus nivolumab (CaboNivo) and ipilimumab (CaboNivoIpi) in patients (pts) with refractory metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors. New York: American Society of Clinical Oncology; 2017. [Google Scholar]
- 55.Boudadi K, Suzman DL, Luber B, Wang H, Silberstein J, Sullivan R, Dowling D, Harb R, Nirschl T, Dittamore RV. Phase 2 biomarker-driven study of ipilimumab plus nivolumab (Ipi/Nivo) for ARV7-positive metastatic castrate-resistant prostate cancer (mCRPC) New York: American Society of Clinical Oncology; 2017. [Google Scholar]
- 56.Hansen A, Massard C, Ott P, Haas N, Lopez J, Ejadi S, Wallmark J, Keam B, Delord J, Aggarwal R. Pembrolizumab for patients with advanced prostate adenocarcinoma: preliminary results from the KEYNOTE-028 study. London: European Society for Medical Oncology; 2016. [DOI] [PubMed] [Google Scholar]
- 57.Graff J, Alumkal J, Drake C, Thomas G, Redmond W, Farhad M, Slottke R, Beer T. First evidence of significant clinical activity of PD-1 inhibitors in metastatic, castration resistant prostate cancer (mCRPC) Ann Oncol. 2016;27(suppl_6):vi243–vi265. [Google Scholar]
- 58.Haas NB, Stein MN, Tutrone R, Perini R, Denker A, Mauro D, Fong L. Phase I-II study of ADXS31-142 alone and in combination with pembrolizumab in patients with previously treated metastatic castration-resistant prostate cancer (mCRPC): the KEYNOTE-046 trial. J Immunother Cancer. 2015;3(Suppl 2):P153. [Google Scholar]
- 59.Hayes S, Petit R, Stein M, Tutrone R, Mega A, Agarwal M, Fong L, Haas N (2017) ADXS-PSA immunotherapy increases the magnitude and quality of prostate cancer-antigen-specific T cell responses in patients with metastatic castration-resistant prostate cancer. In: Proceedings of the society for immunotherapy of cancer’s (SITC) 32nd annual meeting
- 60.Rekoske BT, Olson BM, McNeel DG. Anti-tumor vaccination of prostate cancer patients elicits PD-1/PD-L1 regulated antigen-specific immune responses. Oncoimmunol. 2016;5(6):e1165377. doi: 10.1080/2162402X.2016.1165377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Derer A, Frey B, Fietkau R, Gaipl US. Immune-modulating properties of ionizing radiation: rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol Immunther. 2016;65(7):779–786. doi: 10.1007/s00262-015-1771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nardone V, Botta C, Caraglia M, Martino EC, Ambrosio MR, Carfagno T, Tini P, Semeraro L, Misso G, Grimaldi A. Tumor infiltrating T lymphocytes expressing FoxP3, CCR7 or PD-1 predict the outcome of prostate cancer patients subjected to salvage radiotherapy after biochemical relapse. Cancer Biol Ther. 2016;17(11):1213–1220. doi: 10.1080/15384047.2016.1235666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JW, Shaffer DR, Massard C, Powles T, Harshman LC, Braiteh FS, Conkling PR, Sarkar I, Kadel EE, Mariathasan S. A phase Ia study of safety and clinical activity of atezolizumab (atezo) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) New York: American Society of Clinical Oncology; 2018. [Google Scholar]
- 64.Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, Zoubeidi A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget. 2015;6(1):234. doi: 10.18632/oncotarget.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vilalta M, Rafat M, Graves EE. Effects of radiation on metastasis and tumor cell migration. Cell Mol Life Sci. 2016;73(16):2999–3007. doi: 10.1007/s00018-016-2210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mateo J, Hall E, Sandhu S, Omlin A, Miranda S, Carreira S, Goodall J, Gillman A, Mossop H, Ralph C. Lba20antitumour activity of the Parp inhibitor olaparib in unselected sporadic castration-resistant prostate cancer (CRPC) in the Toparp trial. Ann Oncol. 2014;25(suppl_4):v1–v41. [Google Scholar]
- 67.Ostrander EA, Udler MS. The role of the BRCA2 gene in susceptibility to prostate cancer revisited. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1843–1848. doi: 10.1158/1055-9965.EPI-08-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karzai F, Madan RA, Owens H, Hankin A, Couvillon A, Cordes LM, Fakhrejahani F, Houston ND, Trepel JB, Chen C. Combination of PDL-1 and PARP inhibition in an unselected population with metastatic castrate-resistant prostate cancer (mCRPC) New York: American Society of Clinical Oncology; 2017. [Google Scholar]
- 69.Jiao S, Xia W, Yamaguchi H, Wei Y, Chen M-K, Hsu J-M, Hsu JL, Yu W-H, Du Y, Lee H-H. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711–3720. doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gianchecchi E, Fierabracci AF. Inhibitory Receptors and Pathways of Lymphocytes: The Role of PD-1 in Treg Development and Their Involvement in Autoimmunity Onset and Cancer Progression. Front Immunol. 2018;9:2374. doi: 10.3389/fimmu.2018.02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long L, Zhang X, Chen F, Pan Q, Phiphatwatchara P, Zeng Y, Chen H. The promising immune checkpoint LAG-3: from tumor microenvironment to cancer immunotherapy. Genes Cancer. 2018;9(5–6):176. doi: 10.18632/genesandcancer.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276(1):80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim ES, Kim JE, Patel MA, Mangraviti A, Ruzevick J, Lim M. Immune checkpoint modulators: an emerging antiglioma armamentarium. J Immunol Res. 2016;2016:4683607. doi: 10.1155/2016/4683607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savitsky D, Ward R, Riordan C, Mundt C, Jennings S, Connolly J, Findeis M, Sanicola M, Underwood D, Nastri H. INCAGN02385 is an antagonist antibody targeting the co-inhibitory receptor LAG-3 for the treatment of human malignancies. Cancer Res. 2018;78(13):3819–3819. [Google Scholar]
- 76.Brignone C, Escudier B, Grygar C, Marcu M, Triebel F (2009) A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res:1078–0432. CCR-1009–0068 [DOI] [PubMed]
- 77.Legat A, Maby-El Hajjami H, Baumgaertner P, Cagnon L, Maillard SA, Geldhof C, Iancu EM, Lebon L, Guillaume P, Dojcinovic D. Vaccination with LAG-3Ig (IMP321) and peptides induces specific CD4 and CD8 T-cell responses in metastatic melanoma patients—report of a phase I/IIa clinical trial. Clin Cancer Res. 2016;22(6):1330–1340. doi: 10.1158/1078-0432.CCR-15-1212. [DOI] [PubMed] [Google Scholar]
- 78.Ruffo E, Wu RC, Bruno TC, Workman CJ, Vignali DA (2019) Lymphocyte-activation gene 3 (LAG3): the next immune checkpoint receptor. In: Seminars in immunology. Elsevier, Amsterdam, p 101305 [DOI] [PMC free article] [PubMed]
- 79.Elia AR, Caputo S, Bellone M. Immune checkpoint-mediated interactions between cancer and immune cells in prostate adenocarcinoma and melanoma. Front Immunol. 2018;9:1786. doi: 10.3389/fimmu.2018.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Woo S-R, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee J, Ahn E, Kissick HT, Ahmed R (2015) Reinvigorating exhausted T cells by blockade of the PD-1 pathway. In: Forum on immunopathological diseases and therapeutics, vol 1–2. Begel House Inc., New York [DOI] [PMC free article] [PubMed]
- 82.Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, Walker J, Gonzalez I, Meeuwsen T, Fox BA. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73(24):7189–7198. doi: 10.1158/0008-5472.CAN-12-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rapoport BL, Anderson R. Realizing the clinical potential of immunogenic cell death in cancer chemotherapy and radiotherapy. Int J Mol Sci. 2019;20(4):959. doi: 10.3390/ijms20040959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kovacsovics-Bankowski M, Chisholm L, Vercellini J, Crittenden M, Lary S, Curti B, Weinberg A. Phase I/II clinical trial of anti-OX40, radiation and cyclophosphamide in patients with prostate cancer: immunological analysis. J Immunother Cancer. 2013;1(1):P255. [Google Scholar]
- 85.Li G, Quan Y, Che F, Wang L. B7–H3 in tumors: friend or foe for tumor immunity? Cancer Chemother Pharmacol. 2018;81(2):245–253. doi: 10.1007/s00280-017-3508-1. [DOI] [PubMed] [Google Scholar]
- 86.Ross A, Benzon B, Zhao S, Takhar M, Haffner M, Erho N, Hurley P, Tosoian JJ, Alshalalfa M, Glavaris S. The relationship of B7H3 expression to androgen and prostate cancer outcomes in a large natural history cohort of men undergoing prostatectomy. New York: American Society of Clinical Oncology; 2016. [Google Scholar]
- 87.Lee H-W, Park S-J, Choi BK, Kim HH, Nam K-O, Kwon BS. 4–1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169(9):4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 88.Chester C, Ambulkar S, Kohrt HE. 4–1BB agonism: adding the accelerator to cancer immunotherapy. Cancer Immunol Immunther. 2016;65(10):1243–1248. doi: 10.1007/s00262-016-1829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP. 4–1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186(1):47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuang Y, Weng X, Liu X, Zhu H, Chen Z, Chen H. Effects of 4–1BB signaling on the biological function of murine dendritic cells. Oncol Lett. 2012;3(2):477–481. doi: 10.3892/ol.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Youlin K, Li Z, Xiaodong W, Xiuheng L, Hengchen Z. Combination immunotherapy with 4–1BBL and CTLA-4 blockade for the treatment of prostate cancer. Clin Dev Immunol. 2012;2012:439235. doi: 10.1155/2012/439235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bartkowiak T, Curran MA. 4–1BB agonists: multi-potent potentiators of tumor immunity. Front Oncol. 2015;5:117. doi: 10.3389/fonc.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tolcher AW, Sznol M, Hu-Lieskovan S, Papadopoulos KP, Patnaik A, Rasco DW, Di Gravio D, Huang B, Gambhire D, Chen Y. Phase Ib study of utomilumab (PF-05082566), a 4–1BB/CD137 agonist, in combination with pembrolizumab (MK-3475) in patients with advanced solid tumors. Clin Cancer Res. 2017;23(18):5349–5357. doi: 10.1158/1078-0432.CCR-17-1243. [DOI] [PubMed] [Google Scholar]
- 94.Norström MM, Rådestad E, Sundberg B, Mattsson J, Henningsohn L, Levitsky V, Uhlin M. Progression of benign prostatic hyperplasia is associated with pro-inflammatory mediators and chronic activation of prostate-infiltrating lymphocytes. Oncotarget. 2016;7(17):23581. doi: 10.18632/oncotarget.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Benzon B, Zhao S, Haffner M, Takhar M, Erho N, Yousefi K, Hurley P, Bishop J, Tosoian J, Ghabili K. Correlation of B7–H3 with androgen receptor, immune pathways and poor outcome in prostate cancer: an expression-based analysis. Prostate Cancer Prostatic Dis. 2017;20(1):28. doi: 10.1038/pcan.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu L-F, Gondek D, Wang Y, Fava RA. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS ONE. 2012;7(2):e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piao Y, Jin X. Analysis of Tim-3 as a therapeutic target in prostate cancer. Tumor Biol. 2017;39(7):1010428317716628. doi: 10.1177/1010428317716628. [DOI] [PubMed] [Google Scholar]
- 99.Amatore F, Gorvel L, Olive D. Inducible Co-Stimulator (ICOS) as a potential therapeutic target for anti-cancer therapy. Expert Opin Ther Target. 2018;22(4):343–351. doi: 10.1080/14728222.2018.1444753. [DOI] [PubMed] [Google Scholar]
- 100.Tang DN, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, Allison JP, Sharma P. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res. 2013;1(4):229–234. doi: 10.1158/2326-6066.CIR-13-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li N, Xu W, Yuan Y, Ayithan N, Imai Y, Wu X, Miller H, Olson M, Feng Y, Huang YH. Immune-checkpoint protein VISTA critically regulates the IL-23/IL-17 inflammatory axis. Sci Rep. 2017;7(1):1485. doi: 10.1038/s41598-017-01411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, Noelle RJ, Wang L. VISTA regulates the development of protective anti-tumor immunity. Cancer Res. 2014;74(7):1933–1944. doi: 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Assal A, Kaner J, Pendurti G, Zang X. Emerging targets in cancer immunotherapy: beyond CTLA-4 and PD-1. Immunotherapy. 2015;7(11):1169–1186. doi: 10.2217/imt.15.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Madan RA, Gulley JL. Prostate cancer: better VISTAs ahead? Potential and pitfalls of immunotherapy. Nat Rev Urol. 2017;14(8):455. doi: 10.1038/nrurol.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318(5853):1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 106.Japp AS, Kursunel MA, Meier S, Mälzer JN, Li X, Rahman NA, Jekabsons W, Krause H, Magheli A, Klopf C. Dysfunction of PSA-specific CD8+ T cells in prostate cancer patients correlates with CD38 and Tim-3 expression. Cancer Immunol Immunther. 2015;64(11):1487–1494. doi: 10.1007/s00262-015-1752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Omar HA, Tolba MF. Tackling molecular targets beyond PD-1/PD-L1: novel approaches to boost patients’ response to cancer immunotherapy. Crit Rev Oncol Hematol. 2019;135:21–29. doi: 10.1016/j.critrevonc.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 108.Gevensleben H, Dietrich D, Golletz C, Steiner S, Jung M, Thiesler T, Majores M, Stein J, Uhl B, Müller S. The immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancer. Clin Cancer Res. 2016;22(8):1969–1977. doi: 10.1158/1078-0432.CCR-15-2042. [DOI] [PubMed] [Google Scholar]
- 109.Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fay AP, Antonarakis ES. Blocking the PD-1/PD-L1 axis in advanced prostate cancer: Are we moving in the right direction? Ann Transl Med. 2019;7(Suppl 1):S7. doi: 10.21037/atm.2019.01.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manogue C, Cotogno P, Ledet E, Lewis B, Wyatt AW, Sartor O. Biomarkers for programmed death-1 inhibition in prostate cancer. Oncologist. 2018;24(4):444–448. doi: 10.1634/theoncologist.2018-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haffner MC, Guner G, Taheri D, Netto GJ, Palsgrove DN, Zheng Q, Guedes LB, Kim K, Tsai H, Esopi DM. Comprehensive evaluation of programmed death-ligand 1 expression in primary and metastatic prostate cancer. Am J Pathol. 2018;188(6):1478–1485. doi: 10.1016/j.ajpath.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fujii T, Naing A, Rolfo C, Hajjar J. Biomarkers of response to immune checkpoint blockade in cancer treatment. Crit Rev Oncol Hematol. 2018;130:108–120. doi: 10.1016/j.critrevonc.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 114.Song Y, Li Z, Xue W, Zhang M. Predictive biomarkers for PD-1 and PD-L1 immune checkpoint blockade therapy. Immunotheraphy. 2019;11(6):515–529. doi: 10.2217/imt-2018-0173. [DOI] [PubMed] [Google Scholar]
- 115.Fong L, Carroll P, Weinberg V, Chan S, Lewis J, Corman J, Amling CL, Stephenson RA, Simko J, Sheikh NA. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst. 2014;106(11):268. doi: 10.1093/jnci/dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mougel A, Terme M, Tanchot C. Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front Immunol. 2019;10:467. doi: 10.3389/fimmu.2019.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li H, Wang Z, Zhang Y, Sun G, Ding B, Yan L, Liu H, Guan W, Hu Z, Wang S. The immune checkpoint regulator PDL1 is an independent prognostic biomarker for biochemical recurrence in prostate cancer patients following adjuvant hormonal therapy. J Cancer. 2019;10(14):3102. doi: 10.7150/jca.30384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.FDA US Hematology/Oncology (Cancer) Approvals and safety notifications. https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications. Accessed 13 Sep 2019
- 119.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Antonarakis ES (2019) A new molecular taxonomy to predict immune checkpoint inhibitor sensitivity in prostate cancer. Oncol Theoncol. 2018–0819 [DOI] [PMC free article] [PubMed]
- 121.Bilir C, Sarisozen C. Indoleamine 2, 3-dioxygenase (IDO): only an enzyme or a checkpoint controller? J Oncol Sci. 2017;3(2):52–56. [Google Scholar]
- 122.Banzola I, Mengus C, Wyler S, Hudolin T, Manzella G, Chiarugi A, Boldorini R, Sais G, Schmidli TS, Chiffi G. Expression of indoleamine 2, 3-dioxygenase induced by iFn-γ and TnF-α as potential biomarker of prostate cancer progression. Front Immunol. 2018;9:1051. doi: 10.3389/fimmu.2018.01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Badawy AA, Guillemin G. The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2, 3-dioxygenase: time for appraisal. Int J Tryptophan Res. 2019;12:1178646919868978. doi: 10.1177/1178646919868978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2, 3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210(7):1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moon YW, Hajjar J, Hwu P, Naing A. Targeting the indoleamine 2, 3-dioxygenase pathway in cancer. J Immunother Cancer. 2015;3(1):51. doi: 10.1186/s40425-015-0094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]