Abstract
The mechanisms that synchronize the biorhythms of the mammalian retina with the light/dark cycle are independent of those synchronizing the rhythms in the central pacemaker, the suprachiasmatic nucleus. The identity of the photoreceptor(s) responsible for the light entrainment of the retina of mammals is still a matter of debate, and recent studies have reported contradictory results in this respect. Here, we suggest that cryptochromes (CRY), in particular CRY 2, are involved in that light entrainment. CRY are highly conserved proteins that are a key component of the cellular circadian clock machinery. In plants and insects, they are responsible for the light entrainment of these biorhythms, mediated by the light response of their flavin cofactor (FAD). In mammals, however, no light-dependent role is currently assumed for CRY in light-exposed tissues, including the retina. It has been reported that FAD influences the function of mammalian CRY 2 and that human CRY 2 responds to light in Drosophila, suggesting that mammalian CRY 2 keeps the ability to respond to light. Here, we hypothesize that CRY 2 plays a role in the light entrainment of retinal biorhythms, at least in diurnal mammals. Indeed, published data shows that the light intensity dependence and the wavelength sensitivity commonly reported for that light entrainment fits the light sensitivity and absorption spectrum of light-responsive CRY. We propose experiments to test our hypothesis and to further explore the still-pending question of the function of CRY 2 in the mammalian retina.
Keywords: Circadian rhythms, Flavin cofactor, Retina, Magnetoreception, Radical pair mechanism
Introduction
Most biological processes exhibit circadian rhythmicity. Throughout the organism, these biorhythms are regulated by an auto-regulatory transcriptional–translational feedback loop that runs at the level of each cell and is coordinated by the central clock of the suprachiasmatic nucleus (SCN) [1]. Endogenous biorhythms are influenced by diverse environmental factors. Light is a major factor for the synchronization and resetting of endogenous rhythms with the light/dark cycle (light entrainment). In mammals, the retina constitutes the only input pathway of light both to the central pacemaker in the SCN as well as to peripheral pacemakers of the biological clock [2]. Indeed, recent studies have shown that peripheral clocks can be light entrained by inputs directly issued from the retina, independently of the SCN [2, 3]. Recent studies have also focused on the skin, the other organ exposed to light, but observations are contradictory. While one study reported that light entrainment of skin biorhythms depends on the retina via a neural pathway and is independent of the SCN [4], another has reported that entrainment directly depends on the UV-sensitive neuropsin (OPN5) photopigments that skin cells contain [5].
The retina was the first organ in which local, autonomous, biorhythms were observed [6]. Retinal biorhythms regulate many eye functions, including visual and non-visual (i.e. circadian) light reception [7]. Many retinal physiologic functions oscillate with a 24-h period, e.g. transcription and translation of photoreceptor genes, neurotransmitter synthesis and release, inter-photoreceptor coupling, disk shedding in rods, and the amplitude of the electroretinogram (reviewed in Buhr et al. [8], and Felder-Schmittbuhl et al. [9]). The light entrainment of retinal biorhythms is directly achieved by photoreceptors of the retina, independently of the SCN [9, 10]. Similar SCN-independent rhythmicity exists in the cornea and the retinal pigmented epithelium [11]. While the receptor responsible for the light entrainment of the SCN is melanopsin (OPN4), a pigment localized in intrinsically photosensitive retinal ganglion cells (ipRGCs), the precise identity of the receptor(s) responsible for the light entrainment of the retinal biorhythms remains debated [8, 9, 11, 12]. Here, following the underexplored previous suggestion from Buhr et al. [8], we propose that cryptochrome (CRY), and in particular CRY 2, acts as a light-receptor mediating retinal light entrainment in mammals.
State of the art
Light entrainment of retinal biorhythms
Different studies have addressed the question of the identity of the photoreceptor responsible for the light entrainment of retinal biorhythms by monitoring the expression of the clock gene Per2 in mouse retinal explants [8, 11–13]. By exposing the explants to phase-shifting pulses of white light at an intensity of > 1014 photons/cm2/s, Ruan et al. [13] reported that, like the SCN biorhythms, the retinal biorhythms show, respectively, a phase delay, or a phase advance, when exposed in the first hours, or the last hours, of the subjective night. Moreover, they reported the dopaminergic circuitry to be crucially involved in the light resetting of retinal biorhythms, suggesting the involvement of amacrine cells and the upstream ipRGCs from which they receive inputs (Fig. 1) [8].
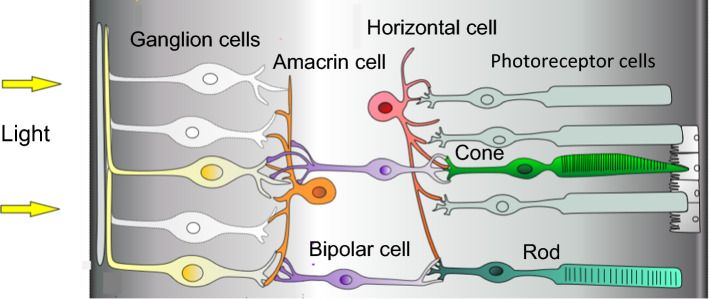
Fig. 1.
Cellular organization of the mammalian retina. The distribution of photoreceptor cells is species-dependent
Using transgenic mice, Buhr et al. [8] showed that neither rods or cones nor OPN4 containing ganglion cells were required for the light entrainment of retinal biorhythms under antiphasic 12 h/12 h light/dark cycles. During the whole light phase, light was provided by LED (between 417 and 530 nm) at a constant intensity of 5 W/m2, which corresponds to about 1.4 × 1015 photons/cm2/s at 470 nm. In a follow-up paper [11], using antiphasic 9 h/15 h light/dark cycles with a light intensity of about 1014 photons/cm2/s (about 1013 photons/cm2/s at 370 nm), the authors showed, respectively, that 370 nm UV light and 417 nm and 475 nm blue light can photoentrain retinal biorhythms but 530 nm green light cannot. Furthermore, they reported that, for a light pulse of 3 h duration, the minimal intensity needed to cause a phase shift was between 1013 and 1014 photons/cm2/s at 417 nm. Using knockout mice, they demonstrated that neither short-wavelength-sensitive cone opsins (OPN1SW), nor the orphan opsin encephalopsin (OPN3) were required, and they identified neuropsin (OPN5) as the responsible photoreceptor [11]. Contrasting these findings, Calligaro et al. [12] found that rods play a crucial role in the light-induced phase-shift of retinal biorhythms when exposed to light pulses reduced to only 15 min of duration (intensity of between 1013 and 1014 photons/cm2/s) at wavelengths up to 520 nm. Using a candidate gene approach similar to Buhr et al. [11] they then demonstrated the absence of a light-induced phase shift in rodless mice. They did, however, find a residual phase shift in rodless retinas when using UV light (395 nm), which they attribute to OPN5 and/or OPN1SW [12].
To sum up the findings to date, the photoreceptor responsible for the light entrainment of retinal biorhythms is thought to be one or several opsins (Table 1) [9]. On the one hand, the precise identity of the receptor(s) remains a matter of debate, and the conflicting evidence is not easy to reconcile [12]. The OPN5 proposed by Buhr et al. [11] has a sensitivity that mainly covers the UV spectrum (maximal absorption at 380 nm, Fig. 2), both in rodents and in humans [18]. Buhr et al. [11] and Calligaro et al. [12], however, observed a phase shift at 470 nm blue light, which is outside the absorption spectrum of that opsin (Fig. 2), even in mice [18]. As noted by the authors themselves and by Felder-Schmittbuhl et al. [9], the role of OPN5 in the entrainment of retinal rhythms in diurnal mammals thus remains uncertain. Furthermore, the lens is much less UV-transparent in diurnal than in nocturnal mammals [20], and the expression of OPN5 in the retina of humans and non-human primates remains controversial [12, 18, 21]. On the other hand, the reported involvement of rods in retinal light entrainment by Calligaro et al. [12], as well as these authors’ proposal of a contribution of OPN1SW containing cones, raises the question (stated by the authors themselves) of why the light response of retinal biorhythms requires light intensities that are several orders of magnitude above the sensitivity threshold of rod opsins (rhodopsin, or OPN2, ~ 107 photons/cm2/s) and cone opsins (~ 108 photons/cm2/s), respectively [22]. Finally and most puzzlingly, Calligaro et al. [12] reported a phase shift of retinal biorhythms at 520 nm, while Buhr et al. [11] could not light entrain these biorhythms with 530 nm green light. Even though the experimental paradigms of the studies mentioned were different (light entrainment vs pulse-induced phase shifting), the discrepancies between their respective results raise questions.
Table 1.
Mammalian opsin photoreceptors and their expression pattern, functional role and approximative maximal wavelength sensitivity
| Abbreviation | Name | Location | Main role | Maximal sensitivity | ||
|---|---|---|---|---|---|---|
| Eye | Other | Mouse | Human | |||
| OPN1 | ||||||
| OPN1LW | L-cone opsin | Cones | Visuala | 560 nm | ||
| OPN1MW | M-cone opsin | Cones | Visuala | 510 nm | 530 nm | |
| OPN1SW | S-cone opsin | Cones | Skinb | Visuala | 360 nm | 420 nm |
| OPN2 | Rhodopsin | Rods | Skinb | Visuala | 500 nm | |
| OPN3 | Encephalopsin |
Rods, cones Plexiform layers Ganglion cells |
Skinb Brainc |
Non-visual | ||
| OPN4 | Melanopsin | ipRGCs | Non-visuald | 480 nm | ||
| OPN5 | Neuropsin | RGCs | Skinb | Non-visual | 380 nm | |
| Go/RGR opsins | ||||||
| RRH | Peropsin | RPE | Non-visual | |||
| RGR | Retinal G protein-coupled receptor | RPE | Non-visual | |||
All three different types of OPN1 exist only in trichromats, such as humans (mouse, e.g., have only M- and S-cone opsins)
LW long wavelength, MW middle wavelength, SW short wavelength, RPE retinal pigmented epithelium
aRods and cones also have non-visual functions (pupillary light response, ipRGCs-mediated light response)
bMelanocytes and keratinocytes
cCortex, cerebellum, diencephalon
dThe main function of OPN4 is circadian photoreception, but it also takes part in the pupillary light response and incidentally in visual photoreception [11, 14–18]
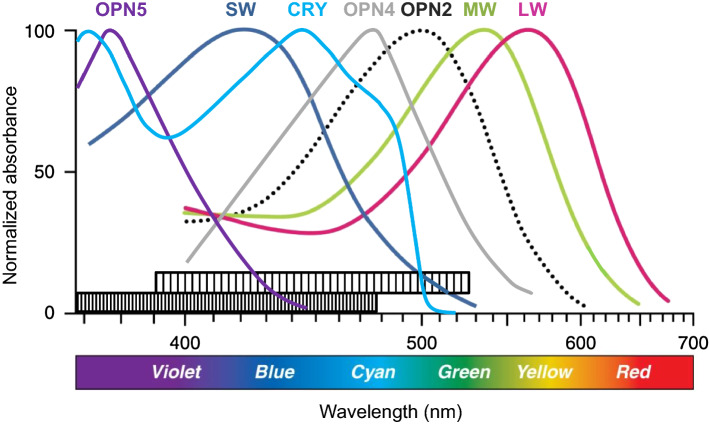
Fig. 2.
Absorption spectrum of the main opsins of the human retina and cryptochrome (CRY) in its oxidized, ground state (in mouse, OPN1SW has a maximal sensitivity at about 360 nm). The bar at the bottom of the graph displays the range of wavelength sensitivity of retinal biorhythms (densely hatched: reported in experiments using light-entrainment; less densely hatched: reported in experiments using light-induced phase shifting). LW: OPN1LW; MW: OPN1MW; SW: OPN1SW [8, 11, 12, 14, 18, 19]
Cryptochrome photopigments
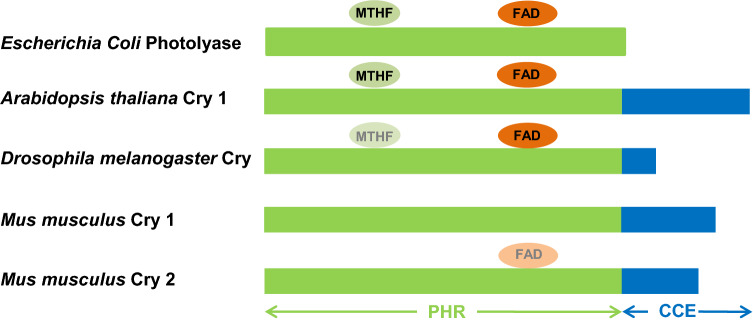
CRY are flavoproteins that are highly conserved from bacteria to humans (Fig. 3). They are between 500 and 700 amino acids in size depending on the phylum or class considered. The N-terminal domain is highly conserved from their photolyase ancestor (the photolyase homology domain, or PHR), while the 40–250 amino acid long C-terminal α-helical domain is less conserved. The C-terminal domain seems essential for CRY activity, whether or not it depends on light [23]. Animal CRY are classified into two main functional groups, type I and type II CRY. Type I CRY, also known as Drosophila-type CRY, are photoreceptive, while type II, or vertebrate-type, CRY are generally thought to only fulfill non-light-dependent functions [24]. In all organisms, CRY are key components of the cellular clock, where they are part of the negative limb of the autoregulatory transcription–repression feedback loop. In mammals, they bind to the protein PER (period) to repress CLOCK/BMAL1-dependent transcription [25].
Fig. 3.
Schematic representation of the structures of photolyase of bacteria (Escherichia coli) and representative cryptochromes (CRY) of plant (Arabidopsis thaliana), insects (type I CRY of Drosophila melanogaster), and mammals (type II CRY of mice, Mus musculus), with approximate binding sites of 5-methyltetrahydrofolate (MTHF) (equivocal in Drosophila) and flavin adenine dinucleotide (FAD) cofactors (equivocal in mammalian CRY 2). PHR photolyase homology region domain, CCE C-terminal extension domain [23–25]
In plants and insects, CRY act as (non-opsin) photopigments that entrain the biological clock directly through the light response (photoreduction) of their flavine adenine dinucleotide (FAD) cofactor (cf. Fig. 2). The absorption spectrum of FAD in its ground, fully oxidized state is quite broad, with two maxima, one in the visible range around 450 nm and another in the UV range around 370 nm (Fig. 2). At 400 nm, the absorption is around 60% of the maximal absorption (Fig. 2) [19]. Only FADH°, the neutral radical form of the flavin cofactor in the active form of plant CRY and of the particular animal type IV Cry (CRY 4, see below) that mammals do not express, absorbs light up to 650 nm [19, 26]. The light sensitivity threshold of CRY has been mostly studied in plant CRY 2 where it is expressed as the photosynthetic photon flux density in micromol/m2/s (1 µmol/m2/s = 6 × 1013 photons/cm2/s). When measured based on the phosphorylation response of CRY 2, this threshold lies around 0.1 µmol/m2/s for 30 min of 450 nm light illumination [27]. By studying its light-induced degradation, Weidler et al. [28] showed that plant CRY 2 responds to 0.01 µmol/m2/s under 2 h of blue light illumination, with saturation at about 5 µmol/m2/s. They also showed that CRY-dependent signaling response in plants can integrate light energy over time, with 2 h blue light causing about 65% and 80% of CRY degradation at 0.01 and 0.1 µmol/m2/s, respectively. To our knowledge, 0.01 µmol/m2/s is the lowest fluence studied to date in plant CRY. As the light response of CRY relies on the interaction between the FAD cofactor and its binding site in the highly conserved PHR domain, that threshold can be expected to not show large variations with CRY type [24]. In this respect, Zoltowski et al. [26] showed that point mutations in the FAD binding site cause variation of no more than a factor 2 in the light sensitivity of the reaction constant of FAD photoreduction, unless they concern the tryptophan (Trp) residues of the Trp triad that is involved in the light-response of CRY. This difference in light sensitivity is of the same order as the variation observed between the highly sensitive type IV CRY and plant CRY [26].
Cryptochromes of mammals
In mammals, two type II CRY exist, CRY 1 and CRY 2. CRY 1 is the prominent subtype in most cells and tissues, where it plays an important and well-documented role in the cellular clock feedback loop (review in [29]). We know much less about the function of mammalian CRY 2. In the retina, its function is considered marginal compared to CRY 1, and only a partial role could be identified in photopic electroretinogram rhythms [30]. Furthermore, the expression pattern of CRY 2 in the mammalian retina has been a matter of debate. While Thompson et al. [31] initially found CRY 2 to be preferentially localized in retinal ganglion cells, subsequent studies reported CRY 2 to also be located in amacrine, horizontal and bipolar cells, as well as in cones (see Fig. 1) [9]. A recent study challenged these conclusions, demonstrating the lack of specificity in two commercial CRY 2 antibodies using tissue from CRY 2 knockout mice as negative controls [30].
Based on the known light dependence of the circadian function of CRY in plants and the high structural homology between type II CRY of vertebrates and plant CRY, including the Trp triad [32], it has been proposed that CRY of the retina are responsible for circadian photoreception in vertebrates [33]. To investigate the role of CRY in nonvisual photoreception in mice, Van Gelder et al. [34] studied the pupillary constriction reflex in response to blue light (470 nm). Mice with severe degeneration of the photoreceptors (rd/rd mice) retained substantial photic sensitivity for pupillary responses and the light-induced phase shift of circadian rhythms. The authors showed that pupillary responses of Cry 1 and Cry 2 knockout, rd/rd mice were almost abolished in comparison to rd/rd mice, suggesting that CRY may function as retinal photopigments. However, the same authors later showed that mutations in other essential circadian clock genes, mPeriod and Bmal1 also induced a significant lower pupillary light sensitivity, which demonstrated that the effect of CRY loss on nonvisual photoreception was due to a non-specific loss of the circadian clock [7]. These results thus do not argue in favour of a direct photoreceptive role of CRY in mammals, at least not with respect to the pupillary response and a central clock phase shift (review in Felder-Schmittbuhl et al. [9]).
In mammals, the clock function of CRY is therefore assumed to be independent of light, even in the retina [24]. Indeed, so far in vertebrates, only one light-dependent function is assumed for retinal CRY, specifically the magnetic compass sense in birds (review in [35]). The assumed mechanism relies on radical pairs formed upon photoexcitation of CRY, the spin state of which is influenced by weak magnetic fields (MF). The spin states of these chemical intermediates, in turn, influence the rate of CRY activation, which is transduced into neuronal impulses through an as yet unknown pathway. Even though sensitivity to weak MF could hitherto only be demonstrated in the plant Arabidopsis thaliana [36] and fruit fly (Drosophila melanogaster) CRY [37], a wealth of evidence supports the involvement of CRY in the ability of birds to orient to the geomagnetic field [35]. From recent data, the magnetic sense in birds is based on a type IV CRY (CRY 4) localized in cones of the avian retina [38]. Mammals do not possess an ortholog of CRY4, and although a magnetic compass sense has also been attested in them [39], no solid indication exists to date that supports the involvement of CRY [40]. Of note, the CRY-based compass sense relies on the anisotropic part of the response of CRY to the MF, i.e. the one that depends on the relative orientation of the MF. That anisotropic response, however, only represents a minor part of the total MF response of CRY, which indeed is mainly isotropic [35].
Light responsiveness of mammalian CRY 2
Even though no light dependence is assumed for the function of mammalian type II CRYs, some data suggest that CRY 2 of mammals is light responsive. Such responsiveness does not per se imply light dependence of a CRY function, but it does support the possibility of it.
Binding of the FAD cofactor to CRY 2
A prerequisite for CRY to fulfill a light-dependent function is that it binds the flavin cofactor FAD. Although that question remains debated [41, 42], the studies reviewed hereunder seem to rule out light-dependent functions in CRY 1, but support that possibility in CRY 2.
In mice, the ability of CRY 1 to form the heterodimer CRY1/PER2 that represses the CLOCK/BMAL1-dependent transcription has been shown not to depend on FAD, in contrast to that of Drosophila CRY [25]. In line with that in vitro observation, the FAD binding pocket of vertebrate CRY 1 is unoccupied when it is either in its ground state or in its active, heterodimeric, form [32, 43].
Contrary to CRY 1, the crystal structure of mammalian CRY 2 supports the view that the FAD binding pocket has an effective, dynamic, affinity for FAD [44], suggesting that the FAD cofactor plays a role in its function. While noting that this affinity is only modest for the ground, oxidized, state of FAD, the authors point out that it depends on the redox state of FAD, and could thus be higher for FAD in its active, reduced, form [44]. Indeed, Hirano et al. [45] reported that FAD binds to human CRY 2 (hsCRY 2) and that this is affected by a single point mutation located in the phosphate loop of the FAD-binding domain that is responsible for a heritable sleep phenotype (familial advanced sleep phase). By subsequently studying that mutation in transgenic mice, they showed that it caused a reduction of the response (phase shift) of the circadian biorhythms to a light pulse [45].
Light response of human CRY 2 in Drosophila
Light responses of hsCRY have been reported in three different studies using transgenic Drosophila. First, photoreduction of hsCRY 1 and 2 has been documented in Drosophila cells using fluorescence and electron paramagnetic resonance spectroscopic techniques [46]. Additionally, two studies have reported that hsCRY restore CRY-dependent behavioral responses to weak MF. As discussed above from the radical pair mechanism, such responses are presumed to rely on the light responsiveness of the CRY molecules [35]. Foley et al. [47] showed that hsCRY 2 rescues light-dependent magnetoreception in CRY-deficient flies. Furthermore, Fedele et al. [48] reported blue light (450 nm ± 20 nm) dependent disruption of biorhythms in flies exposed to static or extremely low-frequency MF in the microtesla range, but only in flies expressing either native Drosophila CRY or hsCRY 2 and not hsCRY 1 (in the absence of Drosophila CRY). These two last observations illustrate the difficulties of extrapolating from in vitro to in vivo, which might explain the discrepancies between the reported FAD binding to type II CRY in living cells [46], that was absent in purified CRY molecules [42].
Discussion
The identity of the photoreceptor(s) responsible for the synchronization of biorhythms of the mammalian retina with the light/dark cycle remains debated. The light entrainment appears to be effective from the UVA spectrum (≥ 370 nm) up to over 500 nm [8, 11, 12]. According to the phase-shifting effect of light pulses observed both by Buhr et al. [11] and Calligaro et al. [12], the intensity threshold for that entrainment (respectively, at 417 nm and 465 nm) lies between 1013 and 1014 photons/cm2/s [11, 12]. Notwithstanding differences in the respective methodologies used (light entrainment vs pulse-induced phase shifting), two main contradictions emerge between the findings of these two research teams. Firstly, while Calligaro et al. [12] reported a role for rods, Buhr et al. [8] excluded it. Secondly, while Calligaro et al. [12] reported a response (phase shifting) to 520 nm light, Buhr et al. [11] did not observe any response (light entrainment) at 530 nm. Calligaro et al. [12] considered this last discrepancy as “puzzling”, i.e. as having no satisfactory explanation, even though it resulted from different experimental paradigms (pulse-induced phase-shifting vs light entrainment).
The dependence on rods reported by Calligaro et al. [12] was shown for a single dephasing light pulse between 15 min and 3 h of duration at an intensity 1 log lower than the one used by Buhr et al. [8] for the whole 12 h light phase of the light/dark cycle that they reported to light entrain retinal biorhythms in rodless mice. These results can be compared with the intensity- and duration-thresholds of the different activation pathways of ipRGCs for the light entrainment of central biorhythms. Indeed, while the “extrinsic” activation of ipRGCs by rods and cones is already effective as from intensities of 109 to 1011 photons/cm2/s, as attested by rod-dependent phase shift [22], their “intrinsic”, OPN4-dependent (rod-independent), activation is only effective above 1012 photons/cm2/s at 480 nm, with saturation at about 3 × 1013 photons/cm2/s [49, 50]. It is noteworthy here that OPN4-dependent activation is characterized by a long response latency, and the ability of photon energy integration over time [51]. The observations by Calligaro et al. [12] and Buhr et al. [8] might thus be reconciled, for example, by supposing that the light entrainment of the retinal biorhythms is achieved by (a) specific photoreceptor cell(s) that require(s) extrinsic activation by rods below a certain light intensity and a certain duration of illumination, but that can work independently of extrinsic activation above that intensity and/or duration, as in the case of ipRGCs-dependent photo-response [52].
As mentioned by Calligaro et al. [12], a role for rods would imply some process downstream of the photoreception by OPN2 in order to reconcile the intensities required for the light entrainment of retinal biorhythms with the much lower sensitivity threshold of that photopigment. In this respect, however, a recent observation has been made of an OPN2-dependent effect (dopamine release in the retina) that shows a bimodal behavior, with an inhibitory effect at a low light intensity and a stimulating effect at intensities above about 3 × 1014 photons/cm2/s [53]. Other questions about a role for OPN2 arise, notably, from the absence of response reported by Buhr et al. [11] to 530 nm light, and the report by the same authors of retinal light entrainment in rodless mice [8]. As mentioned above, a role for OPN5 in responses at 465 nm and above is incompatible with its wavelength sensitivity both in rodents and humans [18]. Furthermore, as noted by Calligaro et al. [12], recent studies failed to detect OPN5 expression in the retina of human and non-human primates [18, 21]. Finally, as suggested by Calligaro et al. [12], a role for OPN1SW and thus of SW cones should again imply the involvement of some downstream process, such as in the above-discussed role of rods depending on intensity. However, such a role for OPN1SW was excluded by Buhr et al. [8, 11] who also excluded a role for OPN4, of which the sensitivity is moreover nearly null at 395 nm. Finally, OPN3 has been ruled out by Buhr et al. [11]. Could the photoreceptor mediating the response be of non-opsin nature?
Here, we have reviewed data and arguments of different nature in support of the light responsiveness of mammalian CRY 2 and suggest a role for it in the light entrainment of retinal biorhythms (Fig. 4). Although it has already been evoked by Buhr et al. [8], this hypothesis has not yet been developed or studied. The hypothesis is confronted with many unknowns that need to be addressed because most of the supporting arguments are indirect. On the one hand, it is unknown whether actual binding of FAD to CRY per se implies that it fulfills a light-dependent function. For example, from a study on both CRY 1 and CRY 2, Hirano et al. [41] concluded that FAD stabilizes CRY and influences their clock function, simply because FAD competes with the ubiquitin ligase FBXL3 that labels CRY for degradation. On the other hand, as noted by Fedele et al. [48] and in line with the adaptability to cell environment that has been observed for the function of Drosophila CRY [54], the observations of a light response of hsCRY in transgenic Drosophila [47, 48], raises the possibility that the particular molecular environment of Drosophila cells has provided the biochemical environment or molecular interactors that allow mammalian CRY to respond to light. Alternatively, the difference between in vitro and in vivo could be critical, i.e. that FAD can bind to CRY 2 only in vivo. Finally, light responsiveness of CRY 2 might not automatically imply that its function in the retina does depend on light. Noteworthy here, although Hoang et al. [46] did report a light response for both hsCRY 1 and 2 in transgenic Drosophila, Fedele et al. [48] reported that only hsCRY 2, thus not hsCRY 1, could restore the (light-dependent) clock function of CRY in them.
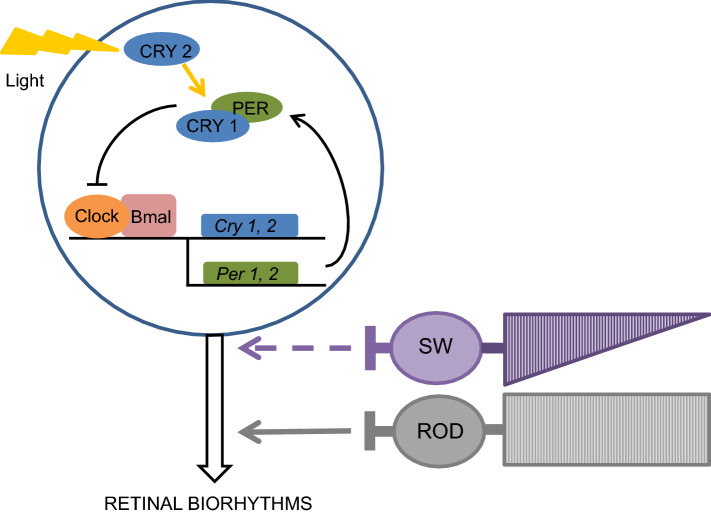
Fig. 4.
Model of the hypothesis of the involvement of CRY 2 in the light-entrainment of the retinal biorhythms, in association with inputs from rods and, possibly, short-wavelength cones (SW). In retinal cells that express CRY 2, the biorhythms are directly synchronized with the light/dark phase due to the action of light on the activational state of CRY 2. In retinal cells, at the contrary to cells of other organs, only the photoactivated state of CRY 2 can play a role in the clock feedback loop. PER: period; CLOCK, BMAL1: transcription factors (only a part of the molecular clock machinery is shown) [9, 12, 24, 29]
Two characteristics of the light response reported for retinal biorhythms support the hypothesis of the involvement of CRY in their light entrainment. These are the large wavelength sensitivity range of these biorhythms and the relatively high light intensities that are required for their entrainment. First, the wavelength sensitivity of the oxidized form (ground state) of CRY covers the whole wavelength sensitivity range that has been commonly reported by Buhr et al. [8, 11] and Calligaro et al. [12] (Fig. 2). Second, the intensity threshold reported for the activation of CRY at 450 nm [27, 28] is close to that reported by both Buhr et al. [11] and Calligaro et al. [12] for light-induced phase shift at 417 nm and 465 nm, respectively. If CRY2 plays a role in the light entrainment of retinal biorhythms, different downstream mechanisms could transduce the photoactivation of CRY 2, such as an increased binding affinity to PER and/or CLOCK/BMAL1 (compared to CRY 1 that affinity is low for CRY 2 in its oxidized form [55]) or a light-induced interaction with another molecular partner, like in Drosophila [24]. Comparing the circadian light responses of the mammalian retina [8, 11–13] with the CRY-based circadian light responses in Drosophila, one makes two interesting observations. First, in Drosophila, like in mice, a light pulse can cause a phase shift as from 15 min of duration [56]. Second, the phase shift caused by blue light in Drosophila shows an inverse relationship between the intensity and the duration of the light pulse required for that shift. Vinayak et al. [57] indeed reported that, as in plants [28], CRY-dependent signaling in Drosophila could integrate light intensity over time, with phase shifting requiring about 1016 photons/cm2/s for a 10 min pulse of blue light and only about 1014 photons/cm2/s for a 120 min pulse. This resembles the abovementioned phenomenon of light energy integration that is known for the OPN4-dependent light entrainment of central biorhythms [51], and that Calligaro et al. [12] also noted, yet to a lesser extent, for the retinal biorhythms.
Further illustrating the fact that the debate about a light-dependent role of CRY in mammalian retina is ongoing, Michael et al. [58] recently summarized different arguments in support of such a role. For example, transgenic mice that lacked both CRY and opsins (absence of photoreceptor cells, or vitamin A depletion) had substantially less circadian light responses compared to those lacking either only CRY, or only opsins [59–61].
Research avenues
How can the hypothesis be tested? A critical investigation of the role of CRY 2 in the light entrainment of retinal biorhythms would in principle require the use of CRY 2 knockout animals (ideally of a diurnal species). A full knockout will, however, disturb the intrinsic biorhythms themselves (CRY 2 knockout mice have a circadian phenotype [7, 30]). As the present hypothesis specifically addresses the light response of CRY 2, which itself relies on the FAD cofactor, one possible solution could be to use a mutant form of CRY 2 that is unable to bind FAD, such as the mutant form A260T that Hirano et al. [45] tested in mice. Another way to test the involvement of CRY could be to study retinal explants of diurnal mammals, which likely lack OPN5, under 360–370 nm light and at intensities above the intensity threshold of CRY, that is above about 1014 photons/cm2/s. Indeed, any response (phase shift) at such wavelength would suggest the involvement of CRY (cf. Fig. 2). For these experiments, it would be crucial that the expression pattern of both CRY 2 and OPN5 in the retina of diurnal mammals will be established using well-characterized specific antibodies. Additionally, the controversy about the role of rods [8, 12] might be further explored by studying the phase shift of biorhythms of retinal explants from rodless mice by testing a large range of pulse durations and light intensities, for example, between 1013 and 1016 photons/cm2/s. Indeed, this might uncover the possibility of a cutoff intensity and/or duration, below which rods are required and above which they are dispensable.
Conclusion
At the current state of knowledge, mammalian CRY 2 might have kept the ability to respond to light. We reviewed several observations that led us to suggest their involvement in the light entrainment of retinal biorhythms, plausibly in collaboration with rods [12], as recently suggested by Buhr et al. [8] and in line with the light dependence that had been formerly proposed for the function of CRY in the mammalian retina [33]. If future experiments support our hypothesis, they would help to answer the still pending question of CRY 2 function in the mammalian retina and might have consequences for studies on magnetic sensitivity in mammals [47, 48, 62].
Acknowledgements
We are indebted to Martha Daniel for proof reading the manuscript and thankful to three anonymous referees for helpful comments that improved the quality of the manuscript.
Author contributions
JV developed the hypothesis. All authors wrote the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare there is no conflict of interest regarding the publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Astiz M, Heyde I, Oster H. Mechanisms of communication in the mammalian circadian timing system. Int J Mol Sci. 2019;20:E343. doi: 10.3390/ijms20020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Husse J, Eichele G, Oster H. Synchronization of the mammalian circadian timing system: light can control peripheral clocks independently of the SCN clock: alternate routes of entrainment optimize the alignment of the body’s circadian clock network with external time. BioEssays. 2015;37:1119–1128. doi: 10.1002/bies.201500026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welz PS, Zinna VM, Symeonidi A, Koronowski KB, Kinouchi K, Smith JG, Guillén IM, Castellanos A, Furrow S, Aragón F, Crainiciuc G, Prats N, Caballero JM, Hidalgo A, Sassone-Corsi P, Benitah SA. BMAL1-driven tissue clocks respond independently to light to maintain homeostasis. Cell. 2019;177:1436–1447. doi: 10.1016/j.cell.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan SM, Chang YT, Chen CL, Wang WH, Pan MK, Chen WP, Huang WY, Xu Z, Huang HE, Chen T, Plikus MV, Chen SK, Lin SJ. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci USA. 2018;115:E6880–E6889. doi: 10.1073/pnas.1719548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buhr ED, Vemaraju S, Diaz N, Lang RA, Van Gelder RN. Neuropsin (OPN5) mediates local light-dependent induction of circadian clock genes and circadian photoentrainment in exposed murine skin. Curr Biol. 2019;29:1–10. doi: 10.1016/j.cub.2019.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tosini G, Menaker M. Circadian rhythms in cultured mammal retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 7.Owens L, Buhr ED, Tu DC, Lamprecht TL, Lee J, Van Gelder RN. Effect of circadian clock gene mutations on nonvisual photoreception in the mouse. Investig Ophthalmol Vis Sci. 2012;53:454–460. doi: 10.1167/iovs.11-8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhr ED, Van Gelder RN. Local photic entrainment of the retinal circadian oscillator in the absence of rods, cones, and melanopsin. Proc Natl Acad Sci USA. 2014;111:8625–8630. doi: 10.1073/pnas.1323350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felder-Schmittbuhl MP, Buhr ED, Dkhissi-Benyahya O, Hicks D, Peirson SN, Ribelayga CP, Sandu C, Spessert R, Tosini G. Ocular clocks: adapting mechanisms for eye functions and health. Investig Ophthalmol Vis Sci. 2018;59:4856–4870. doi: 10.1167/iovs.18-24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tosini G, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- 11.Buhr ED, Yue WW, Ren X, Jiang Z, Liao HW, Mei X, Vemaraju S, Nguyen MT, Reed RR, Lang RA, Yau KW, Van Gelder RN. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc Natl Acad Sci USA. 2015;112:13093–13098. doi: 10.1073/pnas.1516259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calligaro H, Coutanson C, Najjar RP, Mazzaro N, Cooper HM, Haddjeri N, Felder-Schmittbuhl MP, Dkhissi-Benyahya O. Rods contribute to the light-induced phase shift of the retinal clock in mammals. PLoS Biol. 2019;17:e2006211. doi: 10.1371/journal.pbio.2006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan GX, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6:e249. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peirson SN, Brown LA, Pothecary CA, Benson LA, Fisk AS. Light and the laboratory mouse. J Neurosci Methods. 2018;15(300):26–36. doi: 10.1016/j.jneumeth.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown TM. Using light to tell the time of day: sensory coding in the mammalian circadian visual network. J Exp Biol. 2016;219(Pt 12):1779–1792. doi: 10.1242/jeb.132167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haltaufderhyde K, Ozdeslik RN, Wicks NL, Najera JA, Oancea E. Opsin expression in human epidermal skin. Photochem Photobiol. 2015;91:117–123. doi: 10.1111/php.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spitschan M. Melanopsin contribution to non-visual and visual function. Curr Opin Behav Sci. 2019;30:67–72. doi: 10.1016/j.cobeha.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima D, Mori S, Torii M, Wada A, Morishita R, Fukada Y. UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS One. 2011;6:e26388. doi: 10.1371/journal.pone.0026388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Zhong D, Lin C. Searching for a photocycle of the cryptochrome photoreceptors. Curr Opin Plant Biol. 2010;13:578–586. doi: 10.1016/j.pbi.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hut RA, Scheper A, Daan S. Can the circadian system of a diurnal and a nocturnal rodent entrain to ultraviolet light ? J Comp Physiol A. 2000;186:707–715. doi: 10.1007/s003590000124. [DOI] [PubMed] [Google Scholar]
- 21.Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, Panda S. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;359:eaao0318. doi: 10.1126/science.aao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Güler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oztürk N, Song SH, Ozgür S, Selby CP, Morrison L, Partch C, Zhong D, Sancar A. Structure and function of animal cryptochromes. Cold Spring Harb Symp Quant Biol. 2007;72:119–131. doi: 10.1101/sqb.2007.72.015. [DOI] [PubMed] [Google Scholar]
- 24.Chaves I, Pokorny R, Byrdin M, Hoang N, Ritz T, Brettel K, Essen LO, van der Horst GT, Batschauer A, Ahmad M. The cryptochromes: blue light photoreceptors in plants and animals. Annu Rev Plant Biol. 2011;62:335–364. doi: 10.1146/annurev-arplant-042110-103759. [DOI] [PubMed] [Google Scholar]
- 25.Czarna A, Berndt A, Singh HR, Grudziecki A, Ladurner AG, Timinszky G, Kramer A, Wolf E. Structures of Drosophila cryptochrome and mouse cryptochrome1 provide insight into circadian function. Cell. 2013;153:1394–1405. doi: 10.1016/j.cell.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Zoltowski BD, Chelliah Y, Wickramaratne A, Jarocha L, Karki N, Xu W, Mouritsen H, Hore PJ, Hibbs RE, Green CB, Takahashi JS. Chemical and structural analysis of a photoactive vertebrate cryptochrome from pigeon. Proc Natl Acad Sci USA. 2019 doi: 10.1073/pnas.1907875116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q, Wang Q, Deng W, Wang X, Piao M, Cai D, Li Y, Barshop WD, Yu X, Zhou T, Liu B, Oka Y, Wohlschlegel J, Zuo Z, Lin C. Molecular basis for blue light-dependent phosphorylation of Arabidopsis cryptochrome 2. Nat Commun. 2017;8:15234. doi: 10.1038/ncomms15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weidler G, Zur Oven-Krockhaus S, Heunemann M, Orth C, Schleifenbaum F, Harter K, Hoecker U, Batschauer A. Degradation of Arabidopsis CRY2 is regulated by SPA proteins and phytochrome A. Plant Cell. 2012;24:2610–2623. doi: 10.1105/tpc.112.098210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustafson CL, Partch CL. Emerging models for the molecular basis of mammalian circadian timing. Biochemistry. 2015;54:134–149. doi: 10.1021/bi500731f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong JCY, Smyllie NJ, Banks GT, Pothecary CA, Barnard AR, Maywood ES, Jagannath A, Hughes S, van der Horst GTJ, MacLaren RE, Hankins MW, Hastings MH, Nolan PM, Foster RG, Peirson SN. Differential roles for cryptochromes in the mammalian retinal clock. FASEB J. 2018;32:4302–4314. doi: 10.1096/fj.201701165RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson CL, Bowes Rickman C, Shaw SJ, Kelly U, Sancar A, Rickman DW. Expression of the blue-light receptor cryptochrome in the human retina. Investig Ophthalmol Vis Sci. 2003;44:4515–4521. doi: 10.1167/iovs.03-0303. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Jing C, Selby CP, Chiou YY, Yang Y, Wu W, Sancar A, Wang J. Comparative properties and functions of type 2 and type 4 pigeon cryptochromes. Cell Mol Life Sci. 2018;75:4629–4641. doi: 10.1007/s00018-018-2920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Partch CL, Sancar A. Cryptochromes and circadian photoreception in animals. Methods Enzymol. 2005;393:726–745. doi: 10.1016/S0076-6879(05)93038-3. [DOI] [PubMed] [Google Scholar]
- 34.Van Gelder RN, Wee R, Lee JA, Tu DC. Reduced pupillary light responses in mice lacking cryptochromes. Science. 2003;299:222. doi: 10.1126/science.1079536. [DOI] [PubMed] [Google Scholar]
- 35.Hore PJ, Mouritsen H. The radical-pair mechanism of magnetoreception. Annu Rev Biophys. 2016;45:299–344. doi: 10.1146/annurev-biophys-032116-094545. [DOI] [PubMed] [Google Scholar]
- 36.Kattnig DR, Evans EW, Déjean V, Dodson CA, Wallace MI, Mackenzie SR, Timmel CR, Hore PJ. Chemical amplification of magnetic field effects relevant to avian magnetoreception. Nat Chem. 2016;8:384–391. doi: 10.1038/nchem.2447. [DOI] [PubMed] [Google Scholar]
- 37.Sheppard DM, Li J, Henbest KB, Neil SR, Maeda K, Storey J, Schleicher E, Biskup T, Rodriguez R, Weber S, Hore PJ, Timmel CR, Mackenzie SR. Millitesla magnetic field effects on the photocycle of an animal cryptochrome. Sci Rep. 2017;7:42228. doi: 10.1038/srep42228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Günther A, Einwich A, Sjulstock E, Feederlee R, Bolte P, Koch KW, Solov’yov IA, Mouritsen H. Double-cone localization and seasonal expression pattern suggest a role in magnetoreception for European robin cryptochrome 4. Curr Biol. 2018;28:211–223. doi: 10.1016/j.cub.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Begall S, Malkemper EP, Ceverny J, Nemec P, Burda H. Magnetic alignment in mammals and other animals. Mammal Biol. 2013;78:10–20. doi: 10.1016/j.mambio.2012.05.005. [DOI] [Google Scholar]
- 40.Begall Sabine, Burda Hynek, Malkemper Erich Pascal. Advances in the Study of Behavior. 2014. Magnetoreception in Mammals; pp. 45–88. [Google Scholar]
- 41.Hirano A, Braas D, Fu YH, Ptáček LJ. FAD regulates cryptochrome protein stability and circadian clock in mice. Cell Rep. 2017;19:255–266. doi: 10.1016/j.celrep.2017.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kutta RJ, Archipowa N, Johannissen LO, Jones AR, Scrutton NS. Vertebrate cryptochromes are vestigial flavoproteins. Sci Rep. 2017;7:44906. doi: 10.1038/srep44906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmalen I, Reischl S, Wallach T, Klemz R, Grudziecki A, Prabu JR, Benda C, Kramer A, Wolf E. Interaction of circadian clock proteins CRY1 and PER2 is modulated by zinc binding and disulfide bond formation. Cell. 2014;157:1203–1215. doi: 10.1016/j.cell.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 44.Xing W, Busino L, Hinds TR, Marionni ST, Saifee NH, Bush MF, Pagano M, Zheng N. SCF(FBXL3) ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature. 2013;496:64–68. doi: 10.1038/nature11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirano A, Shi G, Jones CR, Lipzen A, Pennacchio LA, Xu Y, Hallows WC, McMahon T, Yamazaki M, Ptáček LJ, Fu YH. A cryptochrome 2 mutation yields advanced sleep phase in humans. Elife. 2016;5:e16695. doi: 10.7554/eLife.16695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoang N, Schleicher E, Kacprzak S, Bouly JP, Picot M, Wu W, Berndt A, Wolf E, Bittl R, Ahmad M. Human and Drosophila cryptochromes are light activated by flavin photoreduction in living cells. PLoS Biol. 2008;6:e160. doi: 10.1371/journal.pbio.0060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foley LE, Gegear RJ, Reppert SM. Human cryptochrome exhibits light-dependent magnetosensitivity. Nat Commun. 2011;2:356. doi: 10.1038/ncomms1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fedele G, Edwards MD, Bhutani S, Hares JM, Murbach M, Green EW, Dissel S, Hastings MH, Rosato E, Kyriacou CP. Genetic analysis of circadian responses to low frequency electromagnetic fields in Drosophila melanogaster. PLoS Genet. 2014;10:e1004804. doi: 10.1371/journal.pgen.1004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reifler AN, Chervenak AP, Dolikian ME, Benenati BA, Meyers BS, Demertzis ZD, Lynch AM, Li BY, Wachter RD, Abufarha FS, Dulka EA, Pack W, Zhao X, Wong KY. The rat retina has five types of ganglion-cell photoreceptors. Exp Eye Res. 2015;130:17–28. doi: 10.1016/j.exer.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao X, Stafford BK, Godin AL, King WM, Wong KY. Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J Physiol. 2014;592:1619–1636. doi: 10.1113/jphysiol.2013.262782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mure LS, Hatori M, Zhu Q, Demas J, Kim IM, Nayak SK, Panda S. Melanopsin-encoded response properties of intrinsically photosensitive retinal ganglion cells. Neuron. 2016;90:1016–1027. doi: 10.1016/j.neuron.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lucas RJ, Lall GS, Allen AE, Brown TM. How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock. Prog Brain Res. 2012;199:1–18. doi: 10.1016/B978-0-444-59427-3.00001-0. [DOI] [PubMed] [Google Scholar]
- 53.Pérez-Fernández V, Milosavljevic N, Allen AE, Vessey KA, Jobling AI, Fletcher EL, Breen PP, Morley JW, Cameron MA. Rod photoreceptor activation alone defines the release of dopamine in the retina. Curr Biol. 2019;29(763–774):e5. doi: 10.1016/j.cub.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 54.Collins B, Mazzoni EO, Stanewsky R, Blau J. Drosophila cryptochrome is a circadian transcriptional repressor. Curr Biol. 2006;16:441–449. doi: 10.1016/j.cub.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 55.Rosensweig C, Reynolds KA, Gao P, Laothamatas I, Shan Y, Ranganathan R, Takahashi JS, Green CB. An evolutionary hotspot defines functional differences between cryptochromes. Nat Commun. 2018;9:1138. doi: 10.1038/s41467-018-03503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaladchibachi S, Negelspach DC, Fernandez F. Circadian phase-shifting by light: beyond photons. Neurobiol Sleep Circadian Rhythms. 2018;5:8–14. doi: 10.1016/j.nbscr.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vinayak P, Coupar J, Hughes SE, Fozdar P, Kilby J, Garren E, Yoshii T, Hirsh J. Exquisite light sensitivity of Drosophila melanogaster cryptochrome. PLoS Genet. 2013;9:e1003615. doi: 10.1371/journal.pgen.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michael AK, Fribourgh JL, Van Gelder RN, Partch CL. Animal cryptochromes: divergent roles in light perception, circadian timekeeping and beyond. Photochem Photobiol. 2017;93:128–140. doi: 10.1111/php.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Selby CP, Thompson C, Schmitz TM, Van Gelder RN, Sancar A. Functional redundancy of cryptochromes and classical photoreceptors for nonvisual ocular photoreception in mice. Proc Natl Acad Sci USA. 2000;97:14697–14702. doi: 10.1073/pnas.260498597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson CL, Blaner WS, Van Gelder RN, Lai K, Quadro L, Colantuoni V, Gottesman ME, Sancar A. Preservation of light signaling to the suprachiasmatic nucleus in vitamin A-deficient mice. Proc Natl Acad Sci USA. 2001;98:11708–11713. doi: 10.1073/pnas.201301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson CL, Selby CP, Van Gelder RN, Blaner WS, Lee J, Quadro L, Lai K, Gottesman ME, Sancar A. Effect of vitamin A depletion on nonvisual phototransduction pathways in cryptochromeless mice. J Biol Rhythms. 2004;19:504–517. doi: 10.1177/0748730404270519. [DOI] [PubMed] [Google Scholar]
- 62.Vanderstraeten J, Gailly P, Malkemper EP. Low-light dependence of the magnetic field effect on cryptochromes: possible relevance to plant ecology. Front Plant Sci. 2018;9:121. doi: 10.3389/fpls.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]