Abstract
Plasma membranes are heterogeneous and laterally compartmentalized into distinct microdomains. These membrane microdomains consist of special lipids and proteins and are thought to act as signaling platforms. In plants, membrane microdomains have been detected by super-resolution microscopy, and there is evidence that they play roles in several biological processes. Here, we review current knowledge about the lipid and protein components of membrane microdomains. Furthermore, we summarize the dynamics of membrane microdomains in response to different stimuli. We also explore the biological functions associated with membrane microdomains as signal integration hubs. Finally, we outline challenges and questions for further studies.
Keywords: Microdomain marker proteins, Lipid, Assembly, Motion, Stress
Introduction
The fluid mosaic model for biomembranes proposed by Singer and Nicholson was a major conceptual breakthrough because it suggested that amphiphilic proteins resided within the bilayer and that membrane organization was dynamic [1, 2]. Shortly, after the fluid mosaic model was presented, detergent-soluble and detergent-resistant membrane (DSM and DRM) fractions were isolated, indicating that distinct membrane subpopulations were present in biological membranes [3]. Subsequently, a large body of evidence suggested that cellular membranes are laterally heterogeneous [4–6]. In 1988, Simons and van Meer postulated the membrane raft (or lipid raft) hypothesis, which introduced the broader concept of membrane lateral heterogeneity, proposing that self-assembly of certain lipids can promote protein aggregation and the formation of molecular complexes [7]. This concept was confirmed by observations of lateral segregation of proteins and lipids into large-scale lateral microdomains in biomimetic model membranes [8]. Subsequently, the existence of lateral sub-compartments and division of the plasma membrane (PM) into distinct microdomains was established [9–12]. In recent years, novel biochemical and biophysical technologies have been used to visualize and study membrane heterogeneity, yielding new insights into the organization of the PM and lateral heterogeneity [13].
The first evidence for a laterally heterogeneous membrane was the solubility of membrane lipids and proteins in nonionic detergents. Brown and Rose [14] used a simple detergent extraction method to demonstrate that DRMs contained glycosphingolipids, cholesterol, and glycosylphosphatidylinositol (GPI)-anchored proteins and were insoluble in cold detergent. However, it has been argued that DRMs do not reflect the native composition and organization of membrane microdomains in living cells. For example, the protein composition of DRMs varies widely depending on the detergent used for isolation [15]. Nevertheless, as an initial approach, detergent extraction has been a useful and valuable tool for enriching DRMs and associated proteins for further membrane microdomain-associated study [16]. In parallel with studies of DRMs isolated from cells, artificial membrane models have been developed and used to study liquid–liquid phase separation, providing insights into the physical mechanism of membrane microdomain formation [17]. The direct visualization of membrane microdomains in living cells has also provided convincing evidence for the compartmentalization of the PM [18, 19].
Although membrane microdomains have been studied intensely, several aspects of microdomains remain a matter of debate. Here, we review the components and organization of microdomains in plants. We also summarize the dynamics of membrane microdomains studied by techniques such as single-particle tracking (SPT). Finally, we focus on our current understanding of the biological function of membrane microdomains in plant cells and outline challenges remaining in the membrane microdomain field.
Components and organization of plant membrane microdomains
The PM is highly asymmetric and laterally compartmentalized with differences in lipid and protein components. Microdomains are lipid-order domains, enriched in sterols and sphingolipids, which exhibit self-assembly and can recruit specific proteins into such regions (Fig. 1). Interactions between lipid and protein components serve to maintain microdomain organization.
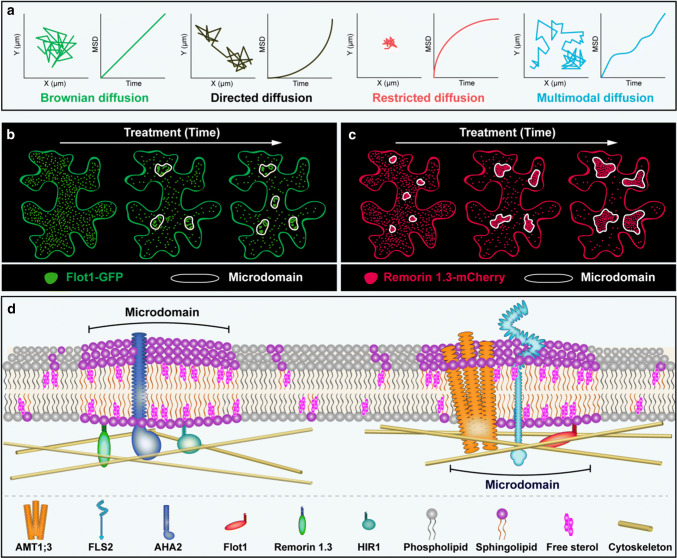
Fig. 1.
Schematic representation of membrane microdomains in the PM of plant cells. Microdomains of different sizes coexist in the PM and consist of sphingolipids, sterols, and special proteins. Flotillins (e.g., Flot1), Remorins (e.g., Remorin1.3), and HIRs (e.g., HIR1) are only located in microdomains and can be regarded as marker proteins for different microdomains. In response to stimuli, signaling proteins such as AMT1;3, FLS2 and AHA2 are recruited into distinctive microdomains to deliver signals
Specific lipids are present in plant microdomains
Large-scale analysis of membrane lipids, or lipidomics, has been enabled by mass spectrometry (MS)-based techniques and software tools for analyzing large datasets [20–22]. The study of plant DRMs by lipidomics revealed enrichment of special sterols and sphingolipids in plant microdomains [23, 24]. However, sterol and sphingolipid species in plant microdomains differ from those in animals and fungi [25]. Cells in higher plants contain a vast array of special phytosterols; for example, 61 sterols and pentacyclic triterpenes had been identified in maize (Zea mays) seedlings [26]. Furthermore, different plant cells contain different major phytosterols. For instance, sitosterol is the most abundant sterol in PMs isolated from soybean (Glycine max) and leek (Allium porrum) seedlings, whereas stigmasterol predominates in PMs from oat (Avena sativa) and maize roots [24, 27–29]. Using sphingolipidomic approaches, more than 200 sphingolipid species were identified in plant and they were very different from those in animals [30, 31]. For example, sphingomyelin as the major phosphosphingolipid in animals is absent in plants. Glycosyl inositol phosphorylceramides (GIPCs) are the major class of sphingolipids in plants, but they are not detected in animals [30, 32, 33]. Studies showed that almost all structural phospholipids, such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidic acid (PA), are markedly depleted in plant DRMs relative to the entire PM [23, 24]. However, polyphosphoinositides (PI4P and PI4,5P2), as kinds of glycerolipids, were enriched in DRMs in comparison with the whole PM, implying that PIPs may be present inside microdomains in plant [34]. Moreover, this hypothesis was verified by the visualization of nanodomain-like clustering using immunogold labeling [34].
Visualizing the physical phase behavior and distribution of microdomains is important for understanding their function. Several sensitive fluorophores, whose emission spectra are regulated by the polarity and hydration of their surrounding environment, have been successfully used to monitor microdomains in animal cells. These lipophylic dyes include NBD (Nitrobenzoxadiazole), DPH (1,6-diphenyl-1,3,5-hexatriene), Prodan, di-4-ANEPPDHQ, filipin, BODIPY LacCer, and Pyr-met-Chol [35–41]. However, only a few have been successfully used in plant cells because of the presence of cell walls. Increased PM fluidity upon temperature increase was demonstrated in BY-2 cells using DPH [42]. In addition, Liu et al. visualized the formation of sterol-rich domains at the growing tip of Picea meyeri pollen tubes using filipin fluorescence and di-4-ANEPPDHQ, which can also be used to visualize the microdomains in Araibidopsis root cells [43–45]. These dyes are robust tool to monitor the status of microdomains. Besides chemical dyes, proteins that can interact with specific lipids have also been used to analyze lipid distribution in the PM. In the late 1990s, two independent groups developed a powerful biosensor for PI(4,5)P2 by expressing GFP fused with the pleckstrin homology (PH) domain of human phospholipase C delta 1 (PLCδ1) that specifically binds PI(4,5)P2 [46, 47]. Under normal growth conditions, fluorescence of PH–PLCδ1–GFP is not localized to the PM in Arabidopsis thaliana or BY2 cells that may be due to the small amount of PI(4,5)P2 present in plant cells [48]. However, upon treatment with a PLC inhibitor or in response to salt stress, GFP fluorescence is detected in the PM with the increasing of PI(4,5)P2, demonstrating the usefulness of this methodology in plants [48].
Specific proteins are present in plant microdomains
Proteomic analysis of DRMs revealed two classes of proteins in microdomains. The first is found in both DRM and DSM, and includes the transmembrane proteins SLOW ANION CHANNEL1 (SLAC1) and HOMOLOG 3 (SLAH3), the RESPIRATORY BURST OXIDASE HOMOLOG D (RBOHD), and the functional tetrameric aquaporin PIP2;1 [49–51]. These proteins move in and out of microdomains, presumably functioning in signaling in response to specific stimuli. Keinath et al. [52] employed quantitative mass spectrometry analysis of proteins in DRMs of Arabidopsis suspension cells to investigate protein dynamics in response to the bacterial pathogen-associated molecular pattern (PAMP) flagellin (flg22). Detergent-resistant membrane protein composition changed significantly following PAMP treatment, with proton ATPases and receptor-like kinases, including the flagellin receptor FLS2, notably affected. This shows that microdomains can integrate signal transduction by recruiting signaling proteins into such microdomain signal hubs.
The second class of proteins can be defined as marker proteins for microdomains because they are detected only in DRMs and not in DSMs; this group includes SPFH (stomatin/prohibitin/flotillin/HflK/C) domain proteins and Remorins. SPFH proteins are evolutionarily conserved across prokaryotes and eukaryotes and have been identified in many plant species, such as rice (Oryza sativa), Petunia species, and Arabidopsis [53–55]. The Flotillin-like protein family has been extensively studied in plants. The first Flotillin-like protein, GmNOD53b, was isolated from soybean, and other GmNod53b-like proteins have since been identified in the peribacteroid membrane of soybean and pea (Pisum sativum) nodules [56, 57]. In the model plant Arabidopsis, the Flotillin-like protein AtFlot1 was identified from DRMs [55]. Members of the newly annotated PID (proliferation, ion, and death) protein superfamily consisting of prohibitins, stomatins, and a group of plant defense response genes can also be considered plant SPFH proteins [58]. Additionally, three HIR (hypersensitive induced reaction) proteins displaying considerable similarity to prohibitins and stomatins are thought to belong to the SPFH domain protein family in plants [59]. Using phylogenetic analysis, Arabidopsis SPFH domain proteins can be classified into five groups: two stomatins, seven prohibitins, three Flotillins, one erlin-like protein, and four HIR-type proteins. In contrast to SPFH domain proteins, Remorins are plant-specific proteins consisting of 16, 8, and 19 members in Arabidopsis, Populus trichocarpa, and rice (Oryza sativa), respectively [60]. Phylogenetically, Remorins are subdivided into six groups based on differences in their N-terminal regions [60]. These marker proteins of microdomains are essential for distinguishing and organizing of distinct microdomains.
Methods for observing microdomain components
Observing membrane microdomains is the most direct way to elucidate their structure. As lipid dyes are unable to easily penetrate plant cell walls, observing microdomain lipids using chemical dyes is difficult [61]. Filipin, which forms fluorescent complexes with sterols, is one of the major fluorescent dyes to label the sterols in plants. Using the freeze-fracture technique, filipin–sterol complexes of 25–30 nm have been observed, corresponding well with the size of microdomains [25]. Since microdomains are dynamic and affected by specific factors, live-cell imaging represents a superior investigation method. Proteins fused with fluorescent reporter proteins are easier to study than labeled lipids; hence, observing microdomain marker proteins distribution and dynamics has become a popular way of monitoring the distribution and dynamics of microdomains. Previously, the diffraction limits of light were a major obstacle for observing microdomains with a size smaller than 200 nm. Nevertheless, advances in microscopy techniques, e.g., super-resolution photoactivated localization microscopy (PALM), stochastic optical reconstruction microscopy (STORM), structured illumination microscopy (SIM), and stimulated emission depletion (STED), have enabled more detailed and high-resolution analysis of microdomains [62]. Demir et al. [49] assessed the size of Remorin patches by observing eGFP::StRem1.3 in stable transgenic Arabidopsis by STED, showing that the diameters of StRem1.3-positive spots are about 97 nm. Structured illumination microscopy revealed that the microdomain-resident protein Flot1 forms linear or circular aggregates after flg22 treatment, demonstrating that microdomains are dynamically rearranged in plant immunity [63]. Besides super-resolution microscopy, the total internal reflection fluorescent microscope (TIRFM) is another robust tool for detecting the distribution and dynamics of microdomains. For instance, observing CFP- and YFP-labeled Remorins showed that different microdomains coexist in the PM [64].
Lipids and proteins cooperatively regulate the organization of plant microdomains
Both specific lipids and proteins are important for the organization of microdomains. Artificial model membrane studies have shown that the length and degree of fatty acid chain unsaturation control the melting temperature of lipids, and some components of the PM with different melting temperatures may segregate into localized discrete areas [25]. Furthermore, several plant sterols modulate thickness, elasticity, and water permeability of PM bilayers [65]. Moreover, when cholesterol, one of the major lipids in microdomains, is embedded in model membranes, they interact favorably with saturated lipids, whereas repulsive interactions occur between saturated and disordered phospholipids, and between cholesterol and disordered phospholipids [66]. These findings show that biophysical properties of specific lipids in microdomains play an important role in the self-assembly of microdomains.
Proteins are also crucial for the organization of microdomains. Remorins and Flotillins are hypothesized to function as scaffolds for organizing receptor-like kinases (RLKs) in microdomains, e.g., phosphorylated OsRemorin4.1 can directly interact with OsSERK1 to restrain OsSERK1 in microdomains [67]. The lipid composition of microdomains can disrupt the localization of microdomain marker proteins; for example, Remorins dissociate from microdomains after treatment with MβCD that depletes sterols [68]. Moreover, studies have proven that biophysical and other interactions between proteins or with membrane lipids contribute to the compartmentalization of the PM [69, 70]. For instance, Gronnier et al. showed that PI4P and sterols were necessary for StREM1.3 targeting to PM microdomains by REM-CA, an unconventional C-terminal structural lipid-binding motif. Furthermore, the interaction between REM-CA and lipids mediated plant PM-microdomain organization [70]. Whether the proteins in microdomains can affect the lipid composition or phase behavior remains elusive, although studies of the interactions between membrane-spanning transmembrane domains (TMDs) and lipids indicate that TMDs can affect the behavior of local lipids [71].
In addition to lipids and proteins, the cytoskeleton is also important for microdomain formation. In animal cells, it is generally accepted that the actin cytoskeleton controls at least one layer of membrane compartmentalization. This is achieved via membrane-adjacent actin filaments and cytoskeleton-bound proteins that span the membrane. In plants, a range of evidence supports the cytoskeleton regulating microdomain organization. In Arabidopsis, a DRM proteomic experiment showed that microtubules control the density and size of membrane microdomains [72]. Recently, TIRFM observation revealed that oligomerization of HIR1 at the PM was dependent on the integrity of microtubules rather than actin, indicating that the mechanism of cytoskeleton-regulated microdomain organization is different in plant cells than in animal cells [73].
Dynamics of membrane microdomains in plants
Recent observations of microdomains in live cells have provided unprecedented information on their dynamics. Our knowledge of the special proteins and lipids in microdomains has allowed monitoring of those components to reveal the dynamics of microdomains as a whole. Quantifying the local and global diffusion behavior of specific proteins and lipids in mammalian cells has revealed Brownian diffusion, directed flow, hop diffusion across membrane picket fences, immobile obstacles, and transient confinement in dynamic membrane microdomains [74]. The diffusion behavior of proteins is crucial in signal transmission. Diffusion of the PM protein BRI1 was quantified using TIRFM and FCS in Arabidopsis roots [75], with lateral diffusion behavior classified into four categories: pure Brownian diffusion, directed diffusion, pure restricted (or confined) diffusion, and diffusion with combinations of Brownian and restricted modes (Fig. 2a). Therefore, it appears that proteins moving within a cellular lipid membrane experience multiple sources of resistance that further influence their motion.
Fig. 2.
Dynamics and organization of membrane microdomains. a Different trajectories and diffusion modes of proteins in the PM. b Flg22 treatment results in linear and circular aggregation of Flot1 in the PM. c PVX treatment causes Remorin 1.3-formed microdomains to become larger in size. d The cortical cytoskeleton restricts the dynamics of microdomain proteins and also affects their assembly
Dynamics of membrane microdomain marker proteins
As reported previously, microdomain marker proteins, including Flotillins, Remorins, and HIRs, are significant components and can label distinct microdomains. Moreover, biophysical and microscopy technologies have shown that the dynamic behaviors of proteins, which are dependent on subcellular environment, are closely related to protein biological functions. Therefore, dynamic analysis of microdomain marker proteins can shed light on the underlying mechanisms of cell signal transduction.
Flotillins, representing significant members of SPFH marker proteins, are highly mobile and heterogeneously distributed at the PM and are required for signal transmission [76]. Using SPT, Li et al. [45] revealed that Flotillin1 (Flot1) is heterogeneous and highly dynamic in the membranes of Arabidopsis roots. Similar observations have recently been reported for Flot1 in Arabidopsis cotyledon epidermal cells [63]. Flot1 dynamics were markedly decreased following MβCD treatment to deplete sterol and disturb microdomains [45]. Interestingly, MβCD treatment also changed the early flg22 signaling cascade, indicating that microdomains may be necessary for related signal transduction [77]. Examination of Flot1 using super-resolution microscopy SIM further supported the idea that the dynamics of proteins on the plasma membrane are related to signal transduction. After treatment with flg22, Flot1 formed linear or circular aggregates of GFP-Flot1 spots, demonstrating that flg22 may contribute to the endocytosis of Flot1 [63] (Fig. 2b). These findings are in line with the suggestion that pathogens might trigger innate immune responses by regulating the endocytosis of proteins in plants.
Remorins are another well-established group of marker proteins for membrane microdomains that dynamically form or disintegrate under different environmental conditions or developmental stages [64]. For example, a mass spectrometry-based quantitative phosphoproteomics approach was used to demonstrate increased phosphorylation of REM1.3 compared with the control following flg22 treatment, which alters the mobility of REM1.3, indicating that flg22 treatment induces defense signaling activation and by inference basal defense [52]. Using SPT, Bucherl et al. [78] monitored the lateral mobility of REMs at the PM and found that REM6.1 and REM6.2 exhibited a different lateral displacement within the PM in response to ligand binding. Similarly, Keinath et al. [52] further supported the idea that flg22 can affect lateral mobility of REM1.2 and REM1.3, suggesting that microdomains may undergo lateral movements under environmental stress. Potato virus X (PVX) treatment can also affect dynamic lateral segregation of REM1.3 in the PM and plasmodesmata subcompartments by phosphorylating REM1.3 [79].
HIRs participate in the development of spontaneous hypersensitive response lesions in leaves, in response to pathogen attack [80]. Recently, Ishikawa et al. [81] demonstrated that the protein abundance of Flotillin homolog and HIR3 was markedly decreased in DRMs of cells overexpressing BAX INHIBITOR-1 (BI-1). Using one-dimensional blue native (BN)-PAGE separation, Lv et al. [73] showed that levels of the AtHIR1 complex increased remarkably upon perception of flg22. In addition, the diffusion of AtHIR1 increased significantly when cholesterol was depleted, suggesting that membrane microdomains are a prerequisite for lateral stability and function in confinement of AtHIR1 at the PM [73]. These findings demonstrate that microdomains provide a platform for efficient execution of highly specific signaling events.
Microdomains can vary in size, and nanodomains, microdomains, and mesodomains have been reported [61]. Importantly, small domains can merge into larger domains via interactions between their individual components. FLS2, a RLK, has been identified in microdomains in Arabidopsis leaves [78]. In response to its ligand, flg22, small particles of FLS2 will form larger particles at the PM [77]. Moreover, the microdomain marker protein Flot1 also aggregates after flg22 treatment [63] (Fig. 2b). A recent study also showed that phosphorylated EOS-REM1.3-formed microdomains become larger in size after PVX infection [79] (Fig. 2c). Together, these reports highlight the environment stimulus are regulator for domain size in plants.
Dynamics of other PM proteins related to membrane microdomains
PM proteins exhibit highly dynamic behaviors such as diffusion within the PM, recycling from the PM to sorting endosomes, and interconversion between multiple conformations in equilibrium [62]. Emerging evidence also shows that some PM proteins can move into mobile or immobile microdomains to transmit signals, undergoing tethered motion or traversing wider regions [77]. For example, dual-color VA-TIRFM revealed that Arabidopsis Phot1 and AtRem1.3 display increased codiffusion in blue light, in a time-dependent manner, indicating that phot1 is associated with AtRem1.3-labeled microdomains. Single-particle tracking analysis demonstrated that Phot1–GFP proteins move faster and diffuse into wider regions under blue light treatment, suggesting that membrane microdomains are involved in Phot1 dynamics at the PM [82]. In addition, under salt stress or brassinosteroids (BR) treatment, cross-correlations of Arabidopsis PIP2;1 and BRI1 with the membrane microdomain marker AtFlot1 are shown to increase significantly, leading to the conclusion that salt stress or BR stimulus may promote the recruitment of PIP2;1 and BRI1 into functional membrane microdomains [51, 75]. Similar to previous observations, Baral et al. [83] reported that GPI-anchored proteins (or lipids) appeared as dynamic and well-demarcated cortical-associated foci embedded within diffuse and weaker background fluorescence. Thus, these results demonstrate that information on lateral diffusion of PM proteins and lipid-anchored proteins is helpful for understanding signal transduction pathways.
Internalized membrane proteins may use different endocytic routes in plants, including clathrin-dependent endocytosis (CME) and clathrin-independent endocytosis (CIE) [84]. CME was involved in several biological processes, such as plant immunity and borate transport [85, 86]. Except that, recent study showed that the transcriptional expression of Flots was regulated by several stresses, indicating that Flots-associated endocytosis may play a vital role in stress response in plant [87]. For instance, treatment with the CIE inhibitor and sterol-depleting agent MβCD can significantly affect endocytosis of flg22-activated FLS2–GFP, indicating that microdomains are crucial for flg22-dependent FLS2 endocytic pathways [77]. Internalization of Arabidopsis ammonium transporter 1;3 (AMT1;3) is partially associated with the Flot1-associated endocytic pathway, indicating involvement of membrane microdomains in the regulation of signal transduction via endocytosis [88]. Similar studies in Arabidopsis roots showed that clathrin- and membrane microdomain-associated endocytic pathways cooperatively regulate RbohD dynamics [50]. Importantly, Słupianek et al. [89] found that CIE pathway also present in woody plant, indicating that CIE pathway plays an important role in plant kingdom. These findings suggest that membrane microdomains serve as signaling platforms that mediate cargo protein sorting and protein–protein interactions in a variety of contexts.
Cytoskeleton and cell wall influencing membrane microdomain dynamics
The environment or internal factors can affect the movement of membrane proteins and other macromolecules [90]. Cytoskeleton is an important internal factor for controlling movement of components in microdomains. In animal cells, most lipids and proteins have uneven distribution in the PM due to molecular interactions and cytoskeleton corralling that generates homogeneities of varying size and stability, demonstrating that the formation and mobility of microdomains are controlled by proteins linked to the actin cytoskeleton [91]. In plants, the cortical cytoskeleton and cell wall mainly influence the formation and/or stability of PM microdomains (Fig. 2d). It, thus, appears that membrane proteins moving in the PM experience multiple sources of resistance that influence their dynamics. Studies using a combination of live-cell imaging techniques have reported that the cortical cytoskeleton influences the formation and/or stability of PM microdomains in plants [78, 92]. For instance, dual-color channel analysis demonstrated that the AtHIR1 protein can be effectively restricted to a region defined by microtubules and some AtHIR1 protein can directly bind to microtubules [73]. In addition to contributing to PM microdomain formation, cytoskeleton components also affect lateral mobility of PM proteins in plant cells. The mobility of microdomain marker proteins tends to increase upon microtubule depolymerization, indicating that the cortical cytoskeleton forms barriers curtailing the movement of microdomain marker proteins, presumably via interactions between microdomain-associated proteins and the cytoskeleton [73]. The exact mechanisms underlying the regulation of microdomain marker protein dynamics by the actin or microtubule cytoskeleton in plants are currently unclear. However, the cytoskeleton is known to be involved in the maintenance of the cell surface continuum and membrane remodeling [93].
Besides cytoskeleton effects on protein diffusion, the cell wall can also restrict PM protein movements to regulate protein interactions in various processes [78]. Presence of the cell wall or decreased distance between the PM and the cell wall can make mobile PM proteins nearly immobile [90]. A study using single-molecule fluorescence imaging reported that the cellulose deposition pattern in the cell wall affects the trajectory and speed of PM protein diffusion [94]. Furthermore, fluorescence recovery after photobleaching (FRAP) studies have revealed that protein projected into cell wall space has a higher mobile fraction in fresh protoplasts than after cell wall regrowth. Conversely, the dynamics of proteins were same in fresh protoplasts and those with regrown cell walls when we project these proteins into the cell. All of these suggested that the cell wall had a crucial role in immobilizing PM proteins that project into the cell wall space [94]. Although some microdomain proteins (e.g., REMs and HIRs) bind to the inner leaflet of PMs and therefore cannot directly interact with the extracellular cell wall, the cell wall can influence the lateral PM mobility of outer leaflet proteins (e.g. GPIs). Thus, these observations highlight the strong interdependence between the cell wall and PM proteins with extracellular domains and that cell wall organization universally influences protein diffusion.
Biological functions of plant membrane microdomains
The organization and dynamics of microdomains are affected by different stimuli, implying that microdomains may play a role in signal transduction. An increasing number of studies in mammalian cells have demonstrated that the formation of microdomains is associated with many biological processes, such as differentiation and apoptosis, Ras signaling, and cancer [95, 96]. In plants, the species and content of microdomain proteins change in response to numerous stimuli, demonstrating that microdomains are also associated with signal transduction in plant cells [97]. Furthermore, a plethora of highly distinct microdomains coexist in the PM, indicating that different microdomains may perform distinct functions, playing important roles in plant development and responses to biotic and abiotic stresses.
Membrane microdomains participate in plant growth and development
The uneven distribution of lipids within the PM is necessary for morphogenesis in some organisms [16]. The tip growth domain is a polar microdomain crucial for growth of pollen tubes and root hairs. Microdomains have been visualized in the growing tip of Picea meyeri pollen tubes by staining with di-4-ANEPPDHQ or filipin, and also identified in NADPH oxidase-dependent reactive oxygen species (ROS) signaling, demonstrating that polar sterol location is important for pollen tube tip growth [43]. Sterol composition of microdomains is closely related to the polar location of proteins. Smt1 mutants, defective in major membrane sterol synthesis, display clear polarity defects, with the polar locations of PIN1 and PIN3 changed [98]. The cyclopropylsterol isomerase1-1 (cpi1-1) mutant also affects PIN2 polar location and root gravitropism because of defective sterol biosynthesis [99]. These sterol synthesis mutations cause dwarfism with short root length and can be embryo lethal, indicating that microdomain integrity is essential for plant growth and development.
Compared with the bulk of PM, plasmodesmata membranes are enriched in sterols and sphingolipids with very long chain saturated fatty acids [100], implying that the plasmodesmata contains several microdomains. Using chemical fenpropimorph to inhibit the sterol biosynthesis in Arabidopsis, two GPI-anchored plasmodesmata proteins PDCB1 and PDBG2 were mislocalized which affect the callose deposition [100]. Moreover, Zavaliev et al. [101] showed that GPI modification of BG_pap and PDCB1 was essential for them targeting to plasmodesmata. All of these results demonstrated that microdomains were necessary for the function of plasmodesmata.
In addition to lipids in microdomains, the microdomain marker proteins are also crucial for maintaining plant development. Overexpression REM1.3 slightly accelerates senescence of tomato (Solanum lycopersicum) indicating that Remorins play important roles in plant development [102]. In 2013, Li et al. cloned a Remorin gene from Populus deltoides (Marsh.) and first demonstrated the molecular mechanism of PdREM. In PdREM antisense-expressing transgenic poplar, the expression of secondary wall biosynthesis-associated and microfibril angle (MFA)-associated genes was significantly increased, indicating that PdREM can inhibit secondary cell wall expansion to inversely regulate vascular growth [103]. GSD1, a member of Remorin family group 6, is associated with grain setting in rice. Overexpression of GSD1 leads to reduction of grain setting rate; GSD1 directly interacts with ACTIN1 and colocalizes with PDCB1 (a callose binding protein) to affect PD conductance [104]. In addition, Flot1 amiRNA mutant seedlings display retarded development and defects in endocytosis [45], indicating that Flot1 is more than just a marker protein for microdomains and is essential for plant development.
Membrane microdomains are involved in pathogen defense
Proteomic and gene expression analyses show that microdomains are also associated with plant–microbe interactions [105–107]. A recent study found that FLS2 forms nanoclusters within microdomains [78]. Moreover, our study showed that the sterol synthesis mutant smt is affected in plant immunity induced by flg22 [77]. These results indicate that microdomains play key roles in pathogen defense in plants.
In 2009, Raffaele et al. [102] showed that REM1.3 directly interacts with TGBp1 (triple gene block protein-1) to inversely regulate the movement of PVX in tomato plants. This was the first demonstration of the function of a Remorin family member protein in plant–microbe interaction. Later, the MtSYMREM1 (Medicago truncatula symbiotic remorin 1) gene, encoding a Remorin of phylogenetic group 2, was found to be highly up-regulated in nodules in response to nod factors [108]. In further study, MtSYMREM1 physically interacts with three symbiotic RLKs, NFP, LYK3, and DMI2, to mediate rhizobial infection and nodule development [108]. This mechanism is also applicable in Lotus japonicus [109]. For plant–fungal interaction, ZmREM6.3 was first reported to be associated with northern leaf blight by quantitative disease resistance analysis [110]. However, little is known about the functions of Remorin proteins in the model plant Arabidopsis. In 2014, two Arabidopsis Remorin proteins belonging to group 4 were identified. Double mutants of AtRem4.1/4.2 were resistant to beet curly top virus and beet severe curly top virus, while seedlings overexpressing these genes were more susceptible [111]. Moreover, AtREM4.1 can be phosphorylated by interacting with SnRK1, indicating that AtRem4s regulates geminivirus infection through a SnRK1-mediated signaling pathway [111]. Interestingly, several studies have shown that Remorins participate in plant immunity in Arabidopsis. RIN4, a regulator of both branches of plant immunity is localized in Remorin1.2-labeled microdomains by interacting with Remorin1.2 in Arabidopsis [112]. However, the function of Remorin1.2 in this cellular process is still unknown.
The functions of Flotillin proteins were demonstrated in M. truncatula during symbiotic bacterial infection. Of seven Flotillin proteins identified, Flot2 and Flot4 are necessary for nodule formation and initiation of infection threads, as demonstrated by silencing Flot2 and Flot4 [76]. Furthermore, it was shown that Flot1 amiRNA mutant lines were insensitive to flg22, and flg22 can induce GFP–Flot1 endocytosis leading to degradation of GFP–Flot1 [63]. This indicated that Flot1 is associated with flg22-induced signaling transduction in Arabidopsis, even though the mechanism is unknown.
The other microdomain marker proteins, HIRs, have also been shown to participate in plant pathogen defense. The expression of HIR genes was increased in disease lesion mimic maize and barley (Hordeum vulgare) mutants, suggesting that HIR genes are associated with the hypersensitive response (HR) [59]. Further studies showed that overexpression of CaHIR1 (A Capsicum annuum hypersensitive induced reaction protein1) in transgenic Arabidopsis plants also enhances resistance to the hemi-biotrophic Pseudomonas syringae pv. Tomato (Pst) and the biotrophic Hyaloperonospora parasitica., but increases sensitivity to osmotic stress [113]. In wheat (Triticum aestivum), expression of TaHIR1, TaHIR3, and TaHIR4 is increased by leaf rust, and mutants of TaHIR1 and TaHIR3 are more sensitive to the avirulent stripe rust pathotype CYR23 [114]. These findings indicate that HIR proteins regulate hypersensitive cell death in response to different external stimuli. HIR1 can physically interact with LRR1 (leucine-rich repeat) proteins, whose gene expression is induced by pathogen attack and PAMP in Capsicum annuum and rice [115, 116]. However, the function of CaLRR1 is different from that of OsLRR1 in the HIR1-mediated cell death responses. CaLRR1 can suppress the HR induced by CaHIR1, whereas overexpression of OsLRR1 enhances resistance to Pseudomonas syringae (Pst DC3000) [116]. This may be because of different clusters of CaHIR1 and OsLRR1. The pathogen Xanthomonas campestris pv. vesicatoria releases virulence factor Fha1 (filamentous hemagglutinin-like protein), which can directly interact with CaHIR1 to induce disease-associated cell death, suppressing pathogenesis-related gene expression. There are four members of the HIR family in Arabidopsis that form homo- or hetero-oligomers similar to other SPFH proteins. AtHIR1 and AtHIR2 can form a complex with RPS2 which is a resistance gene of Arabidopsis thaliana. The Athir2-1 and Athir3-1 mutants are susceptible to Pst DC3000 AvrRpt2 but not to Pst DC3000, and overexpression of AtHIR1 and AtHIR2 enhances resistance to Pst DC3000 [80]. All of these findings suggest that AtHIR proteins play an important role in RPS2-mediated effector-triggered immunity.
Membrane microdomains are associated with abiotic stress and hormone signal transduction
Several proteomics studies have shown that the proteins in DRMs can be regulated by abiotic stress or hormone treatment. Wang et al. [75] found that BR signal was inhibited in Arabidopsis after MβCD treatment. Moreover, Bücherl et al. [78] also found that BRI1 forms nanoclusters within microdomains. These results show that microdomains are associated with abiotic stress and hormone signal transduction.
Microdomain marker proteins also play crucial roles in plant resistance to abiotic stress. The expression of MiREM cloned from Morus indica is significantly increased under abiotic stress and treatment with phytohormones such as SA (Salicylic acid), ABA (abscisic acid), and BR; heterologous expression of MiREM in Arabidopsis enhances drought tolerance and NaCl tolerance [117]. In foxtail millet, SiREM6, a member of group 6 targeted by SiARDP, is associated with resistance to high salt stress [118]. Interestingly, in a recent study, Gui et al. [67] showed that OsREM4.1 is ABA inducible and the phenotype of OsREM4.1 overexpression lines resembles the phenotype of the BRI1 mutant. Further study demonstrated that OsREM4.1 interacts directly with OsSERK1 in the ABA signaling pathway, but OsSERK1 is released from the OsSERK1–OsREM1.4 complex to form an OsSERK1–OsBRI1 complex in the BR signaling pathway [67]. These findings shed light on the mechanism of OsREM4.1-mediated crosstalk in ABA and BR signaling through interaction with OsSERK1 in rice.
Besides Remorins, Flot1-associated endocytosis is also important for abiotic stress and hormone signal transduction. The molecular movement and localization of PM proteins revealed enhanced PIP2:1 and BRI1 internalization through the Flot1-associated endocytosis pathway after NaCl and BR treatment [51, 75, 119]. A recent study also showed that OsFlot and OsHIR3 mutant lines are more resistant to oxidative stress- and SA-induced cell death compared to wild-type rice [81].
Conclusions and perspective
Increasing evidence suggests that cellular membranes are heterogeneous and can be laterally compartmentalized. Studies in plant cells using advanced microscopy approaches have shown that different microdomains coexist in the PM and are required for different biological processes. Moreover, the components of microdomains can interact with each other to maintain microdomain organization. The cell wall and cytoskeleton both play important roles in microdomain organization and dynamics; however, the underlying mechanisms remain to be elucidated. The spatiotemporal dynamics of microdomains clearly play a role in signaling in response to different stimuli. Interestingly, microdomains act as signal integration hubs and are essential for plant development and responses to biotic and abiotic stresses.
Although there has been progress in the study of microdomains, exploring the relationship between the functions and principles of microdomain organization and dynamics remains challenging due to limitations in observing and detecting microdomains in plant cells. Although the development of microscopy technology has made observing microdomain protein dynamics easier, it is still difficult to detect the movement of special lipids in microdomains because of the lack of specific lipid dyes that can penetrate the plant cell wall. Moreover, chemicals need to be developed to replace MβCD, which has some nonspecific side effects in addition to depleting sterols from cellular membranes.
Several studies have shown that the lipids in the outer leaflet and inner leaflet of the PM are asymmetric in mammalian cells, whereas such evidence is still lacking in plants. Uncovering the mechanisms concerning how the cell wall and cytoskeleton affect the organization and dynamics of microdomains is another challenge in plant cells. Although different microdomains may perform various functions by sorting specific signal proteins, the formation mechanism of such signal hub microdomains and principles for sorting different signal proteins to specific microdomains remain to be elucidated. In the future, it will be necessary to use multidisciplinary techniques to elucidate the molecular principles of membrane microdomains.
Acknowledgements
We thank lab members for helpful discussions and critical reading of the paper. This work was supported by the State ‘13.5’ Key Research Program of China (No. 2016YFD0600102), the National Natural Science Foundation of China (31530084, 31761133009, 31670182, 31401149), and the Fundamental Research Funds for the Central Universities (2019ZY29, 2017ZY10) and the Program of Introducing Talents of Discipline to Universities (111 project, B13007).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meng Yu and Yaning Cui contributed equally to this work.
Contributor Information
Ruili Li, Email: liruili@bjfu.edu.cn.
Jinxing Lin, Email: linjx@ibcas.ac.cn.
References
- 1.Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Fischman DA, Steck TL. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1:233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]
- 4.Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 5.Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedrichson T, Kurzchalia TV. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature. 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- 7.Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 8.Simons K, Vaz WLC. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson K, Mouritsen OG, Anderson RGW. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 10.Malinsky J, Opekarova M, Tanner W. The lateral compartmentation of the yeast plasma membrane. Yeast. 2010;27:473–478. doi: 10.1002/yea.1772. [DOI] [PubMed] [Google Scholar]
- 11.Mongrand S, Stanislas T, Bayer EMF, Lherminier J, Simon-Plas F. Membrane rafts in plant cells. Trends Plant Sci. 2010;15:656–663. doi: 10.1016/j.tplants.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Bio. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 13.Sezgin E, Levental I, Mayor S, Eggeling C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Bio. 2017;18:361–374. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 15.Shogomori H, Brown DA. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol Chem. 2003;384:1259–1263. doi: 10.1515/BC.2003.139. [DOI] [PubMed] [Google Scholar]
- 16.Malinsky J, Opekarova M, Grossmann G, Tanner W. Membrane microdomains, rafts, and detergent-resistant membranes in plants and fungi. Annu Rev Plant Bio. 2013;64:501–529. doi: 10.1146/annurev-arplant-050312-120103. [DOI] [PubMed] [Google Scholar]
- 17.Sezgin E, Schwille P. Model membrane platforms to study protein-membrane interactions. Mol Membr Biol. 2012;29:144–154. doi: 10.3109/09687688.2012.700490. [DOI] [PubMed] [Google Scholar]
- 18.Day CA, Kenworthy AK. Tracking microdomain dynamics in cell membranes. Biochim Biophys Acta. 2009;1788:245–253. doi: 10.1016/j.bbamem.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagerholm BC, Weinreb GE, Jacobson K, Thompson NL. Detecting microdomains in intact cell membranes. Annu Rev Phys Chem. 2005;56:309–336. doi: 10.1146/annurev.physchem.56.092503.141211. [DOI] [PubMed] [Google Scholar]
- 20.Yang K, Cheng H, Gross RW, Han X. Automated lipid identification and quantification by multidimensional mass spectrometry-based shotgun lipidomics. Anal Chem. 2009;81:4356–4368. doi: 10.1021/ac900241u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzog R, Schwudke D, Schuhmann K, Sampaio JL, Bornstein SR, Schroeder M, Shevchenko A. A novel informatics concept for high-throughput shotgun lipidomics based on the molecular fragmentation query language. Genome Biol. 2011;12:R8. doi: 10.1186/gb-2011-12-1-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haimi P, Uphoff A, Hermansson M, Somerharju P. Software tools for analysis of mass spectrometric lipidome data. Anal Chem. 2006;78:8324–8331. doi: 10.1021/ac061390w. [DOI] [PubMed] [Google Scholar]
- 23.Mongrand S, Morel J, Laroche J, Claverol S, Carde JP, Hartmann MA, Bonneu M, Simon-Plas F, Lessire R, Bessoule JJ. Lipid rafts in higher plant cells: purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. J Biol Chem. 2004;279:36277–36286. doi: 10.1074/jbc.M403440200. [DOI] [PubMed] [Google Scholar]
- 24.Laloi M, Perret AM, Chatre L, Melser S, Cantrel C, Vaultier MN, Zachowski A, Bathany K, Schmitter JM, Vallet M, et al. Insights into the role of specific lipids in the formation and delivery of lipid microdomains to the plasma membrane of plant cells. Plant Physiol. 2007;143:461–472. doi: 10.1104/pp.106.091496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cacas JL, Furt F, Le Guedard M, Schmitter JM, Bure C, Gerbeau-Pissot P, Moreau P, Bessoule JJ, Simon-Plas F, Mongrand S. Lipids of plant membrane rafts. Prog Lipid Res. 2012;51:272–299. doi: 10.1016/j.plipres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Guo DA, Venkatramesh M, Nes WD. Developmental regulation of sterol biosynthesis in Zea mays. Lipids. 1995;30:203–219. doi: 10.1007/BF02537823. [DOI] [PubMed] [Google Scholar]
- 27.Travis RL, Berkowitz RL. Characterization of soybean plasma membrane during development: free sterol composition and concanavalin a binding studies. Plant Physiol. 1980;65:871–879. doi: 10.1104/pp.65.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norberg P, Liljenberg C. Lipids of plasma membranes prepared from oat root cells: effects of induced water-deficit tolerance. Plant Physiol. 1991;96:1136–1141. doi: 10.1104/pp.96.4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grandmougin A, Bouvier-Nave P, Ullmann P, Benveniste P, Hartmann MA. Cyclopropyl sterol and phospholipid composition of membrane fractions from maize roots treated with fenpropimorph. Plant Physiol. 1989;90:591–597. doi: 10.1104/pp.90.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pata MO, Hannun YA, Ng CKY. Plant sphingolipids: decoding the enigma of the Sphinx. New Phytol. 2010;185:611–630. doi: 10.1111/j.1469-8137.2009.03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cacas JL, Bure C, Furt F, Maalouf JP, Badoc A, Cluzet S, Schmitter JM, Antajan E, Mongrand S. Biochemical survey of the polar head of plant glycosylinositolphosphoceramides unravels broad diversity. Phytochemistry. 2013;96:191–200. doi: 10.1016/j.phytochem.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Sperling P, Heinz E. Plant sphingolipids: structural diversity, biosynthesis, first genes and functions. Bba-Mol Cell Biol L. 2003;1632:1–15. doi: 10.1016/s1388-1981(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 33.Cacas JL, Bure C, Grosjean K, Gerbeau-Pissot P, Lherminier J, Rombouts Y, Maes E, Bossard C, Gronnier J, Furt F, et al. Revisiting plant plasma membrane lipids in tobacco: a focus on sphingolipids. Plant Physiol. 2016;170:367–384. doi: 10.1104/pp.15.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furt F, Konig S, Bessoule JJ, Sargueil F, Zallot R, Stanislas T, Noirot E, Lherminier J, Simon-Plas F, Heilmann I, et al. Polyphosphoinositides are enriched in plant membrane rafts and form microdomains in the plasma membrane. Plant Physiol. 2010;152:2173–2187. doi: 10.1104/pp.109.149823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Meer G, Stelzer EH, Wijnaendts-van-Resandt RW, Simons K. Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells. J Cell Biol. 1987;105:1623–1635. doi: 10.1083/jcb.105.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lentz BR, Burgess SW. A dimerization model for the concentration dependent photophysical properties of diphenylhexatriene and its phospholipid derivatives. DPHpPC and DPHpPA. Biophys J. 1989;56:723–733. doi: 10.1016/S0006-3495(89)82720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kusube M, Tamai N, Matsuki H, Kaneshina S. Pressure-induced phase transitions of lipid bilayers observed by fluorescent probes Prodan and Laurdan. Biophys Chem. 2005;117:199–206. doi: 10.1016/j.bpc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Jin L, Millard AC, Wuskell JP, Dong X, Wu D, Clark HA, Loew LM. Characterization and application of a new optical probe for membrane lipid domains. Biophys J. 2006;90:2563–2575. doi: 10.1529/biophysj.105.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beknke O, Tranum-Jensen J, van Deurs B. Filipin as a cholesterol probe. I. Morphology of filipin-cholesterol interaction in lipid model systems. Eur J Cell Biol. 1984;35:189–199. [PubMed] [Google Scholar]
- 40.Singh RD, Liu Y, Wheatley CL, Holicky EL, Makino A, Marks DL, Kobayashi T, Subramaniam G, Bittman R, Pagano RE. Caveolar endocytosis and microdomain association of a glycosphingolipid analog is dependent on its sphingosine stereochemistry. J Biol Chem. 2006;281:30660–30668. doi: 10.1074/jbc.M606194200. [DOI] [PubMed] [Google Scholar]
- 41.Le Guyader L, Le Roux C, Mazeres S, Gaspard-Iloughmane H, Gornitzka H, Millot C, Mingotaud C, Lopez A. Changes of the membrane lipid organization characterized by means of a new cholesterol-pyrene probe. Biophys J. 2007;93:4462–4473. doi: 10.1529/biophysj.107.112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konigshofer H, Tromballa HW, Loppert HG. Early events in signalling high-temperature stress in tobacco BY2 cells involve alterations in membrane fluidity and enhanced hydrogen peroxide production. Plant Cell Environ. 2008;31:1771–1780. doi: 10.1111/j.1365-3040.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 43.Liu P, Li RL, Zhang L, Wang QL, Niehaus K, Baluska F, Samaj J, Lin JX. Lipid microdomain polarization is required for NADPH oxidase-dependent ROS signaling in Picea meyeri pollen tube tip growth. Plant J. 2009;60:303–313. doi: 10.1111/j.1365-313X.2009.03955.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhao X, Li R, Lu C, Baluska F, Wan Y. Di-4-ANEPPDHQ, a fluorescent probe for the visualisation of membrane microdomains in living Arabidopsis thaliana cells. Plant Physiol Biochem. 2015;87:53–60. doi: 10.1016/j.plaphy.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Li R, Liu P, Wan Y, Chen T, Wang Q, Mettbach U, Baluska F, Samaj J, Fang X, Lucas WJ, et al. A membrane microdomain-associated protein, Arabidopsis Flot1, is involved in a clathrin-independent endocytic pathway and is required for seedling development. Plant Cell. 2012;24:2105–2122. doi: 10.1105/tpc.112.095695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 47.Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Leeuwen W, Vermeer JE, Gadella Jr TW, Munnik T. (2007) Visualization of phosphatidylinositol 4,5-bisphosphate in the plasma membrane of suspension-cultured tobacco BY-2 cells and whole Arabidopsis seedlings. Plant J 52:1014–1026 [DOI] [PubMed]
- 49.Demir F, Horntrich C, Blachutzik JO, Scherzer S, Reinders Y, Kierszniowska S, Schulze WX, Harms GS, Hedrich R, Geiger D, et al. Arabidopsis nanodomain-delimited ABA signaling pathway regulates the anion channel SLAH3. Proc Natl Acad Sci USA. 2013;110:8296–8301. doi: 10.1073/pnas.1211667110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao H, Fan L, Chen T, Li R, Li X, He Q, Botella MA, Lin J. Clathrin and membrane microdomains cooperatively regulate rbohd dynamics and activity in Arabidopsis. Plant Cell. 2014;26:1729–1745. doi: 10.1105/tpc.113.122358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Wang X, Yang Y, Li R, He Q, Fang X, Luu DT, Maurel C, Lin J. Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell. 2011;23:3780–3797. doi: 10.1105/tpc.111.091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keinath NF, Kierszniowska S, Lorek J, Bourdais G, Kessler SA, Shimosato-Asano H, Grossniklaus U, Schulze WX, Robatzek S, Panstruga R. PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J Biol Chem. 2010;285:39140–39149. doi: 10.1074/jbc.M110.160531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi A, Kawasaki T, Wong HL, Suharsono U, Hirano H, Shimamoto K. Hyperphosphorylation of a mitochondrial protein, prohibitin, is induced by calyculin A in a rice lesion-mimic mutant cdr1. Plant Physiol. 2003;132:1861–1869. doi: 10.1104/pp.103.021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahn CS, Lee JH, Reum Hwang A, Kim WT, Pai HS. Prohibitin is involved in mitochondrial biogenesis in plants. Plant J. 2006;46:658–667. doi: 10.1111/j.1365-313X.2006.02726.x. [DOI] [PubMed] [Google Scholar]
- 55.Borner GH, Sherrier DJ, Weimar T, Michaelson LV, Hawkins ND, Macaskill A, Napier JA, Beale MH, Lilley KS, Dupree P. Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol. 2005;137:104–116. doi: 10.1104/pp.104.053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winzer T, Bairl A, Linder M, Linder D, Werner D, Muller P. A novel 53-kDa nodulin of the symbiosome membrane of soybean nodules, controlled by Bradyrhizobium japonicum. Mol Plant Microbe Interact. 1999;12:218–226. doi: 10.1094/MPMI.1999.12.3.218. [DOI] [PubMed] [Google Scholar]
- 57.Saalbach G, Erik P, Wienkoop S. Characterisation by proteomics of peribacteroid space and peribacteroid membrane preparations from pea (Pisum sativum) symbiosomes. Proteomics. 2002;2:325–337. doi: 10.1002/1615-9861(200203)2:3<325::aid-prot325>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 58.Nadimpalli R, Yalpani N, Johal GS, Simmons CR. Prohibitins, stomatins, and plant disease response genes compose a protein superfamily that controls cell proliferation, ion channel regulation, and death. J Biol Chem. 2000;275:29579–29586. doi: 10.1074/jbc.M002339200. [DOI] [PubMed] [Google Scholar]
- 59.Rostoks N, Schmierer D, Kudrna D, Kleinhofs A. Barley putative hypersensitive induced reaction genes: genetic mapping, sequence analyses and differential expression in disease lesion mimic mutants. Theor Appl Genet. 2003;107:1094–1101. doi: 10.1007/s00122-003-1351-8. [DOI] [PubMed] [Google Scholar]
- 60.Raffaele S, Mongrand S, Gamas P, Niebel A, Ott T. Genome-wide annotation of remorins, a plant-specific protein family: evolutionary and functional perspectives. Plant Physiol. 2007;145:593–600. doi: 10.1104/pp.107.108639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konrad SS, Ott T. Molecular principles of membrane microdomain targeting in plants. Trends Plant Sci. 2015;20:351–361. doi: 10.1016/j.tplants.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 62.Wang L, Xue Y, Xing J, Song K, Lin J. Exploring the spatiotemporal organization of membrane proteins in living plant cells. Annu Rev Plant Biol. 2018;69:525–551. doi: 10.1146/annurev-arplant-042817-040233. [DOI] [PubMed] [Google Scholar]
- 63.Yu M, Liu H, Dong Z, Xiao J, Su B, Fan L, Komis G, Samaj J, Lin J, Li R. The dynamics and endocytosis of Flot1 protein in response to flg22 in Arabidopsis. J Plant Physiol. 2017;215:73–84. doi: 10.1016/j.jplph.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Jarsch IK, Konrad SS, Stratil TF, Urbanus SL, Szymanski W, Braun P, Braun KH, Ott T. Plasma membranes are subcompartmentalized into a plethora of coexisting and diverse microdomains in Arabidopsis and Nicotiana benthamiana. Plant Cell. 2014;26:1698–1711. doi: 10.1105/tpc.114.124446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hodzic A, Rappolt M, Amenitsch H, Laggner P, Pabst G. Differential modulation of membrane structure and fluctuations by plant sterols and cholesterol. Biophys J. 2008;94:3935–3944. doi: 10.1529/biophysj.107.123224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Saez AJ, Schwille P. Stability of lipid domains. FEBS Lett. 2010;584:1653–1658. doi: 10.1016/j.febslet.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 67.Gui J, Zheng S, Liu C, Shen J, Li J, Li L. OsREM4.1 Interacts with OsSERK1 to coordinate the Interlinking between abscisic acid and brassinosteroid signaling in rice. Dev Cell. 2016;38:201–213. doi: 10.1016/j.devcel.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 68.Konrad SS, Popp C, Stratil TF, Jarsch IK, Thallmair V, Folgmann J, Marin M, Ott T. S-acylation anchors remorin proteins to the plasma membrane but does not primarily determine their localization in membrane microdomains. New Phytol. 2014;203:758–769. doi: 10.1111/nph.12867. [DOI] [PubMed] [Google Scholar]
- 69.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 70.Gronnier J, Crowet JM, Habenstein B, Nasir MN, Bayle V, Hosy E, Platre MP, Gouguet P, Raffaele S, Martinez D, et al. Structural basis for plant plasma membrane protein dynamics and organization into functional nanodomains. Elife. 2017;6:e26404. doi: 10.7554/eLife.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ernst AM, Contreras FX, Brugger B, Wieland F. Determinants of specificity at the protein-lipid interface in membranes. FEBS Lett. 2010;584:1713–1720. doi: 10.1016/j.febslet.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 72.Szymanski WG, Zauber H, Erban A, Gorka M, Wu XN, Schulze WX. Cytoskeletal components define protein location to membrane microdomains. Mol Cell Proteomics. 2015;14:2493–2509. doi: 10.1074/mcp.M114.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lv X, Jing Y, Xiao J, Zhang Y, Zhu Y, Julian R, Lin J. Membrane microdomains and the cytoskeleton constrain AtHIR1 dynamics and facilitate the formation of an AtHIR1-associated immune complex. Plant J. 2017;90:3–16. doi: 10.1111/tpj.13480. [DOI] [PubMed] [Google Scholar]
- 74.Owen DM, Williamson D, Rentero C, Gaus K. Quantitative microscopy: protein dynamics and membrane organisation. Traffic. 2009;10:962–971. doi: 10.1111/j.1600-0854.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- 75.Wang L, Li H, Lv X, Chen T, Li R, Xue Y, Jiang J, Jin B, Baluska F, Samaj J, et al. Spatiotemporal dynamics of the BRI1 receptor and its regulation by membrane microdomains in living Arabidopsis cells. Mol Plant. 2015;8:1334–1349. doi: 10.1016/j.molp.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Haney CH, Long SR. Plant Flotillins are required for infection by nitrogen-fixing bacteria. Proc Natl Acad Sci USA. 2010;107:478–483. doi: 10.1073/pnas.0910081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cui Y, Li X, Yu M, Li R, Fan L, Zhu Y, Lin J. Sterols regulate endocytic pathways during flg22-induced defense responses in Arabidopsis. Development. 2018;145(19):dev165688. doi: 10.1242/dev.165688. [DOI] [PubMed] [Google Scholar]
- 78.Bucherl CA, Jarsch IK, Schudoma C, Segonzac C, Mbengue M, Robatzek S, MacLean D, Ott T, Zipfel C. Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains. Elife. 2017;6:e25114. doi: 10.7554/eLife.25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perraki A, Gronnier J, Gouguet P, Boudsocq M, Deroubaix AF, Simon V, German-Retana S, Legrand A, Habenstein B, Zipfel C, et al. REM1.3’s phospho-status defines its plasma membrane nanodomain organization and activity in restricting PVX cell-to-cell movement. PLoS Pathog. 2018;14:e1007378. doi: 10.1371/journal.ppat.1007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qi Y, Tsuda K, le Nguyen V, Wang X, Lin J, Murphy AS, Glazebrook J, Thordal-Christensen H, Katagiri F. Physical association of Arabidopsis hypersensitive induced reaction proteins (HIRs) with the immune receptor RPS2. J Biol Chem. 2011;286:31297–31307. doi: 10.1074/jbc.M110.211615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishikawa T, Aki T, Yanagisawa S, Uchimiya H, Kawai-Yamada M. Overexpression of BAX INHIBITOR-1 links plasma membrane microdomain proteins to stress. Plant Physiol. 2015;169:1333–1343. doi: 10.1104/pp.15.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xue Y, Xing J, Wan Y, Lv X, Fan L, Zhang Y, Song K, Wang L, Wang X, Deng X, et al. Arabidopsis blue light receptor phototropin 1 undergoes blue light-induced activation in membrane microdomains. Mol Plant. 2018;11:846–859. doi: 10.1016/j.molp.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Baral A, Irani NG, Fujimoto M, Nakano A, Mayor S, Mathew MK. Salt-induced remodeling of spatially restricted clathrin-independent endocytic pathways in Arabidopsis root. Plant Cell. 2015;27:1297–1315. doi: 10.1105/tpc.15.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Claus LAN, Savatin DV, Russinova E. The crossroads of receptor-mediated signaling and endocytosis in plants. J Integr Plant Biol. 2018;60:827–840. doi: 10.1111/jipb.12672. [DOI] [PubMed] [Google Scholar]
- 85.Mbengue M, Bourdais G, Gervasi F, Beck M, Zhou J, Spallek T, Bartels S, Boller T, Ueda T, Kuhn H, et al. Clathrin-dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proc Natl Acad Sci USA. 2016;113:11034–11039. doi: 10.1073/pnas.1606004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoshinari A, Hosokawa T, Amano T, Beier MP, Kunieda T, Shimada T, Hara-Nishimura I, Naito S, Takano J. Polar localization of the borate exporter BOR1 requires AP2-Dependent endocytosis. Plant Physiol. 2019;179:1569–1580. doi: 10.1104/pp.18.01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kroumanova K, Kocourkova D, Danek M, Lamparova L, Pospichalova R, Malinska K, Krckova Z, Burketova L, Valentova O, Martinec J, et al. Characterisation of Arabidopsis Flotillins in response to stresses. Biol Plant. 2019;63:144–152. [Google Scholar]
- 88.Wang Q, Zhao Y, Luo W, Li R, He Q, Fang X, Michele RD, Ast C, von Wiren N, Lin J. Single-particle analysis reveals shutoff control of the Arabidopsis ammonium transporter AMT1;3 by clustering and internalization. Proc Natl Acad Sci USA. 2013;110:13204–13209. doi: 10.1073/pnas.1301160110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Slupianek A, Kasprowicz-Maluski A, Myskow E, Turzanska M, Sokolowska K. Endocytosis acts as transport pathway in wood. New Phytol. 2019;222:1846–1861. doi: 10.1111/nph.15637. [DOI] [PubMed] [Google Scholar]
- 90.Li X, Luu DT, Maurel C, Lin J. Probing plasma membrane dynamics at the single-molecule level. Trends Plant Sci. 2013;18:617–624. doi: 10.1016/j.tplants.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 91.Viola A, Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat Rev Immunol. 2007;7:889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]
- 92.Szymanski DB, Cosgrove DJ. Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr Biol. 2009;19:R800–R811. doi: 10.1016/j.cub.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 93.McKenna JF, Tolmie AF, Runions J. Across the great divide: the plant cell surface continuum. Curr Opin Plant Biol. 2014;22:132–140. doi: 10.1016/j.pbi.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 94.Martiniere A, Lavagi I, Nageswaran G, Rolfe DJ, Maneta-Peyret L, Luu DT, Botchway SW, Webb SE, Mongrand S, Maurel C, et al. Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proc Natl Acad Sci USA. 2012;109:12805–12810. doi: 10.1073/pnas.1202040109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sorci-Thomas MG, Thomas MJ. Microdomains, inflammation, and atherosclerosis. Circ Res. 2016;118:679–691. doi: 10.1161/CIRCRESAHA.115.306246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goldfinger LE, Michael JV. Regulation of Ras signaling and function by plasma membrane microdomains. Biosci Trends. 2017;11:23–40. doi: 10.5582/bst.2016.01220. [DOI] [PubMed] [Google Scholar]
- 97.Takahashi D, Kawamura Y, Uemura M. Detergent-resistant plasma membrane proteome to elucidate microdomain functions in plant cells. Front Plant Sci. 2013;4:27. doi: 10.3389/fpls.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Willemsen V, Friml J, Grebe M, van den Toorn A, Palme K, Scheres B. Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell. 2003;15:612–625. doi: 10.1105/tpc.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Men S, Boutte Y, Ikeda Y, Li X, Palme K, Stierhof YD, Hartmann MA, Moritz T, Grebe M. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat Cell Biol. 2008;10:237–244. doi: 10.1038/ncb1686. [DOI] [PubMed] [Google Scholar]
- 100.Grison MS, Brocard L, Fouillen L, Nicolas W, Wewer V, Dormann P, Nacir H, Benitez-Alfonso Y, Claverol S, Germain V, et al. Specific membrane lipid composition is important for plasmodesmata function in Arabidopsis. Plant Cell. 2015;27:1228–1250. doi: 10.1105/tpc.114.135731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zavaliev R, Dong X, Epel BL. Glycosylphosphatidylinositol (GPI) modification serves as a primary plasmodesmal sorting signal. Plant Physiol. 2016;172:1061–1073. doi: 10.1104/pp.16.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raffaele S, Bayer E, Lafarge D, Cluzet S, German Retana S, Boubekeur T, Leborgne-Castel N, Carde JP, Lherminier J, Noirot E, et al. Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs potato virus X movement. Plant Cell. 2009;21:1541–1555. doi: 10.1105/tpc.108.064279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li S, Su X, Zhang B, Huang Q, Hu Z, Lu M. Molecular cloning and functional analysis of the Populus deltoides remorin gene PdREM. Tree Physiol. 2013;33:1111–1121. doi: 10.1093/treephys/tpt072. [DOI] [PubMed] [Google Scholar]
- 104.Gui J, Liu C, Shen J, Li L. Grain setting defect1, encoding a remorin protein, affects the grain setting in rice through regulating plasmodesmatal conductance. Plant Physiol. 2014;166:1463–1478. doi: 10.1104/pp.114.246769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Colebatch G, Desbrosses G, Ott T, Krusell L, Montanari O, Kloska S, Kopka J, Udvardi MK. Global changes in transcription orchestrate metabolic differentiation during symbiotic nitrogen fixation in Lotus japonicus. Plant J. 2004;39:487–512. doi: 10.1111/j.1365-313X.2004.02150.x. [DOI] [PubMed] [Google Scholar]
- 106.El Yahyaoui F, Kuster H, Ben Amor B, Hohnjec N, Puhler A, Becker A, Gouzy J, Vernie T, Gough C, Niebel A, et al. Expression profiling in Medicago truncatula identifies more than 750 genes differentially expressed during nodulation, including many potential regulators of the symbiotic program. Plant Physiol. 2004;136:3159–3176. doi: 10.1104/pp.104.043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Coaker GL, Willard B, Kinter M, Stockinger EJ, Francis DM. Proteomic analysis of resistance mediated by Rcm 2.0 and Rcm 5.1, two loci controlling resistance to bacterial canker of tomato. Mol Plant Microbe Interact. 2004;17:1019–1028. doi: 10.1094/MPMI.2004.17.9.1019. [DOI] [PubMed] [Google Scholar]
- 108.Lefebvre B, Timmers T, Mbengue M, Moreau S, Herve C, Toth K, Bittencourt-Silvestre J, Klaus D, Deslandes L, Godiard L, et al. A remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proc Natl Acad Sci USA. 2010;107:2343–2348. doi: 10.1073/pnas.0913320107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Toth K, Stratil TF, Madsen EB, Ye J, Popp C, Antolin-Llovera M, Grossmann C, Jensen ON, Schussler A, Parniske M, et al. Functional domain analysis of the Remorin protein LjSYMREM1 in Lotus japonicus. PLoS One. 2012;7:e30817. doi: 10.1371/journal.pone.0030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jamann TM, Luo X, Morales L, Kolkman JM, Chung CL, Nelson RJ. A remorin gene is implicated in quantitative disease resistance in maize. Theor Appl Genet. 2016;129:591–602. doi: 10.1007/s00122-015-2650-6. [DOI] [PubMed] [Google Scholar]
- 111.Son S, Oh CJ, An CS. Arabidopsis thaliana remorins interact with SnRK1 and play a role in susceptibility to beet curly top virus and beet severe curly top virus. Plant Pathol J. 2014;30:269–278. doi: 10.5423/PPJ.OA.06.2014.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu J, Elmore JM, Fuglsang AT, Palmgren MG, Staskawicz BJ, Coaker G. RIN4 functions with plasma membrane H+ -ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol. 2009;7:e1000139. doi: 10.1371/journal.pbio.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jung HW, Lim CW, Lee SC, Choi HW, Hwang CH, Hwang BK. Distinct roles of the pepper hypersensitive induced reaction protein gene CaHIR1 in disease and osmotic stress, as determined by comparative transcriptome and proteome analyses. Planta. 2008;227:409–425. doi: 10.1007/s00425-007-0628-6. [DOI] [PubMed] [Google Scholar]
- 114.Duan Y, Guo J, Shi X, Guan X, Liu F, Bai P, Huang L, Kang Z. Wheat hypersensitive-induced reaction genes TaHIR1 and TaHIR3 are involved in response to stripe rust fungus infection and abiotic stresses. Plant Cell Rep. 2013;32:273–283. doi: 10.1007/s00299-012-1361-6. [DOI] [PubMed] [Google Scholar]
- 115.Jung HW, Hwang BK. The leucine-rich repeat (LRR) protein, CaLRR1, interacts with the hypersensitive induced reaction (HIR) protein, CaHIR1, and suppresses cell death induced by the CaHIR1 protein. Mol Plant Pathol. 2007;8:503–514. doi: 10.1111/j.1364-3703.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 116.Zhou L, Cheung MY, Zhang Q, Lei CL, Zhang SH, Sun SS, Lam HM. A novel simple extracellular leucine-rich repeat (eLRR) domain protein from rice (OsLRR1) enters the endosomal pathway and interacts with the hypersensitive-induced reaction protein 1 (OsHIR1) Plant Cell Environ. 2009;32:1804–1820. doi: 10.1111/j.1365-3040.2009.02039.x. [DOI] [PubMed] [Google Scholar]
- 117.Checker VG, Khurana P. Molecular and functional characterization of mulberry EST encoding remorin (MiREM) involved in abiotic stress. Plant Cell Rep. 2013;32:1729–1741. doi: 10.1007/s00299-013-1483-5. [DOI] [PubMed] [Google Scholar]
- 118.Yue J, Li C, Liu Y, Yu J. A remorin gene SiREM6, the target gene of SiARDP, from foxtail millet (Setaria italica) promotes high salt tolerance in transgenic Arabidopsis. PLoS One. 2014;9:e100772. doi: 10.1371/journal.pone.0100772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cui Y, Zhang X, Yu M, Zhu Y, Xing J, Lin J. Techniques for detecting protein-protein interactions in living cells: principles, limitations, and recent progress. Sci China Life Sci. 2019;62:619–632. doi: 10.1007/s11427-018-9500-7. [DOI] [PubMed] [Google Scholar]