Abstract
Adipose tissue is located in discrete depots that are differentially associated with elevated risk of metabolic complications, with fat accretion in visceral depots being most detrimental to metabolic health. Currently, the regulation of specific adipose depot expansion, by adipocyte hypertrophy and hyperplasia and consequently fat distribution, is not well understood. However, a growing body of evidence from in vitro investigations indicates that mature adipocytes secrete factors that modulate the proliferation and differentiation of progenitor, adipose-derived stem cells (ADSCs). It is therefore plausible that endocrine communication between adipocytes and ADSCs located in different depots influences fat distribution, and may therefore contribute to the adverse health outcomes associated with visceral adiposity. This review will explore the available evidence of paracrine and endocrine crosstalk between mature adipocytes and ADSCs that affects adipogenesis, as a better understanding of the regulatory roles of the extracellular signalling mechanisms within- and between adipose depots may profoundly change the way we view adipose tissue growth in obesity and related comorbidities.
Keywords: Adipose, Adipocytes, Adipose-derived stem cells, Adipogenesis, Paracrine, Endocrine
Introduction
The increasing prevalence of obesity has become a major public health problem worldwide [1]. This is of particular concern as obesity is a major risk factor for type 2 diabetes, dyslipidaemia, hypertension, cardiovascular disease and certain types of cancers [2, 3]. The rise in obesity and related health complications has prompted renewed interest in adipose tissue biology, as a detailed understanding of the cellular and molecular mechanisms that govern adipose tissue expansion, distribution, and function may be the foundation for the development of new therapeutic strategies aimed at reducing obesity-related disorders.
Adipose tissue accounts for 5–50% of human body weight and is mainly found in subcutaneous and visceral depots, although depots are also located in bone marrow which can constitute up to 10% of total body fat [4]. The tissue is complex and multicellular, consisting of adipocytes, adipose progenitor cells, fibroblasts, vascular endothelial cells, resident macrophages and a variety of other immune cells. Adipose tissue plays a key role in whole-body energy homeostasis and lipid metabolism. In times of sufficient energy supply, adipocytes act as energy stores by efficiently sequestering excess glucose and fatty acids from the circulation to be stored in intracellular lipid droplets. This lipid accrual increases the diameter of adipocytes and contributes to adipose tissue expansion. Conversely, lipids stored in adipocytes can be released through lipolytic pathways in times of energy insufficiency or when energy expenditure is increased, with the liberated glycerol and fatty acids being distributed via the blood throughout the body. Hence, adipose tissue is dynamically remodelled to accommodate the redistribution of lipids in response to fluctuations in nutrient supply [5]. The processes of glucose and fatty acid uptake, lipogenesis, and lipolysis are tightly controlled by a complex regulatory network, which has been extensively studied [6].
When individuals become obese, the increased energy supply is initially met by increased intracellular lipid accumulation and adipocyte hypertrophy in subcutaneous adipose tissues. However, large, hypertrophic adipocytes eventually face limits of expansion and increasing hypoxia due to inadequate vascularisation of the enlarging adipose tissue. This results in adipocyte dysfunction, where excess free fatty acid and proinflammatory cytokine release may promote dyslipidaemia, chronic inflammation and insulin resistance [7]. Consequently, to compensate for the limited capacity of highly hypertrophic adipocytes to safely store lipids, new adipocytes are formed from adipocyte progenitor cells by de novo adipogenesis to increase the storage capacity of adipose tissue. However, a continued energy surplus ultimately results in the inability of progenitor cells to adequately proliferate and differentiate, contributing to pathological adipocyte hypertrophy and tissue dysfunction [8].
The location of the increased adiposity is an important factor affecting the metabolic complications of obesity. While subcutaneous adipose tissue expansion is associated with relatively benign storage of excess lipids, expansion of visceral adipose depots is more closely linked to adverse metabolic outcomes [9, 10]. Moreover, subcutaneous and visceral adipocytes demonstrate distinct differences in phenotype, gene expression, lipolysis rates and secretion profiles [11, 12]. As subcutaneous adipose tissue of obese individuals approaches its capacity for adipocyte enlargement and progenitor cell differentiation, the excess energy is stored in expanding visceral adipose depots. This limits the potentially cytotoxic effects of ectopic lipid deposition, where excess lipids are deposited in non-adipocyte cells in the liver, muscles and pancreas [13]. However, the mechanisms whereby visceral adipose depot expansion responds to the status of subcutaneous adipose tissue are not well understood.
In addition to its role in systemic lipid metabolism, adipose tissue is increasingly recognised as an important endocrine organ. Adipocytes secrete a wide range of growth factors and hormones (termed adipokines) that may act locally by autocrine or paracrine signalling, or may be circulated to relay information to other metabolically active organs via endocrine mechanisms [14]. Adipokines act by binding to specific receptors on target cells, which initiates intracellular signalling pathways and a cellular response. Well-characterized adipokines include leptin, adiponectin, adipsin, and chemerin, amongst others [15–18]. Proteomic studies suggest that adipocytes secrete at least several hundred distinct proteins [19, 20] and, for the vast majority, the target tissue and biological action of the secreted proteins remain unknown. Further studies on adipokine signalling are warranted, as a growing body of evidence has implicated impairment of the synthesis, secretion, and function of adipokines with the development of obesity and its related complications [21, 22].
While mature adipocytes are terminally differentiated, they are thought to have a constant turn-over rate [23], with new adipocytes arising from the differentiation of progenitor cells. These adipocyte progenitors are fibroblast-like stem cells that can be found in the stromal-vascular fraction of adipose tissue (which also contains fibroblasts, vascular endothelial cells and various immune cells). Techniques for the isolation and in vitro expansion of these adipose-derived stem cells (ADSCs) have been well established, and ADSCs can be readily induced to undergo adipogenic differentiation and maturation via stimulation with a cocktail of appropriate reagents [24–26]. However, the exact mechanisms governing adipogenesis in vivo are not well understood [27].
To date, several cell culture-based studies have reported possible paracrine interactions between mature adipocytes and ADSCs that influence the proliferation and/or differentiation of the progenitor cells. These studies range from investigations of immortalised cell lines, such as the murine 3T3-L1 preadipocyte cell line, to primary adipocytes and ADSCs that were obtained and purified from animal or human adipose tissue, without the need for immortalisation. More recently, the use of three-dimensional (3D) co-cultures of different cells and microfluidic perfusion cell culture systems has facilitated the study of paracrine interactions between adipocytes and other cells, in a setting that better approximates the in vivo adipose tissue milieu than conventional two-dimensional (2D) cultures. While these studies suggest that paracrine communication exists between adipocytes and ADSCs within the same adipose depot, it is currently unclear whether factors secreted by adipocytes may also influence ADSCs resident in distant adipose depots, via endocrine mechanisms. Such endocrine factors, secreted by hypertrophic subcutaneous adipocytes, may contribute to the delayed onset of visceral adipose depot expansion seen in obesity. A better understanding of the autocrine, paracrine and endocrine communication that regulate adipogenesis may provide profound insights into the expansion visceral adiposity and the pathobiology of obesity-related comorbidities.
This review focusses on studies of intra- and inter-adipose communication between mature adipocytes and ADSCs (summarised in Table 1). We will review various lines of evidence demonstrating paracrine signalling from adipocytes that influence differentiation of surrounding ADSCs, as well as discuss the possibility that comparable endocrine signalling mechanisms may contribute to communication between cells in different adipose depots.
Table 1.
Evidence of paracrine/endocrine interactions between mature adipocytes and adipose-derived stem cells (ADSCs)
| Refs. | Experimental model | Source of adipocytes | Source of ADSCs | Experimental approach | Effect of adipocytes on ADSCs | Candidate factor? |
|---|---|---|---|---|---|---|
| [29] | Rats | scWAT; vWAT; epWAT | scWAT; vWAT; epWAT | Direct co-culture | Differentiation ↑ | No |
| [32] | Obese vs. lean rats | scWAT; vWAT | scWAT; vWAT | Direct co-culture |
Obese: differentiation ↑ Lean: differentiation ↑ |
No |
| [32] | Old vs. young rats | epWAT | epWAT | Direct co-culture |
Young: differentiation ↑ Old: differentiation ↑ |
No |
| [33] | Rats | vWAT | vWAT | Adipocyte-conditioned media | Differentiation ↑ | 53 kDa protease-sensitive factor |
| [34] | Rats | scWAT | scWAT | Adipose tissue-conditioned media |
Differentiation ↑ Proliferation ↓ |
No |
| [36] | Human subjects | scWAT | scWAT | Direct co-culture, 3D co-culture | Differentiation ↑ | No |
| [38] | Human subjects | scWAT | scWAT | Adipose tissue-conditioned media | Differentiation ↑ | bFGF, leptin, IL-6, adiponectin, CCL5 |
| [39] | Human subjects | scWAT | scWAT | Co-culture with porous insert | No effect on differentiation | N/a |
| [40] | Human subjects | scWAT | scWAT | Co-culture with porous insert | Differentiation ↓ | Ang II |
| [45] | Obese vs. lean human subjects | scWAT | scWAT | Co-culture with porous insert |
Obese: proliferation ↑ Lean: proliferation ↑ |
No |
| [47] | Human subjects | scWAT | scWAT | Adipocyte-conditioned media |
Proliferation ↑ No effect on differentiation |
no |
| [50] | Human subjects | scWAT | scWAT | Direct co-culture | Secretion of VEGF, G-CSF, bFGF ↑ | N/a |
| [52] | Human subjects | Differentiated ADSCs | scWAT | Microfluidics chamber | Differentiation ↑ | No |
| [53] | T2D vs ND human subjects | BM | BM | Adipocyte-conditioned media |
T2D: differentiation ↑ ND: differentiation ↑ |
MCP-1 |
| [59] | 3T3-L1 cell line | 3T3-L1 adipocytes | 3T3-L1 preadipocytes | Microfluidics chamber | Differentiation ↑ | No |
| [60] | 3T3-L1 cell line | 3T3-L1 adipocytes | 3T3-L1 preadipocytes | Adipocyte-conditioned media | Differentiation ↑ | Cav-1 |
| [61] | 3T3-L1 cell line | 3T3-L1 adipocytes | 3T3-L1 preadipocytes | Co-culture with porous insert; + inflammation and/or excess insulin | Standard conditions: no effect on differentiation Inflammation and/or excess insulin: differentiation ↓ | No |
| [63] | Mice | N/a (in vivo) | 3T3-F442A preadipocytes | Subcutaneous implantation of ADSCs | Differentiation ↑ | No |
| [64] | Mice | N/a (in vivo) | Human scWAT | Subcutaneous implantation of ADSCs | Differentiation ↑ | No |
| [65] | Mice | N/a (in vivo) | Human scWAT | Subcutaneous implantation of ADSCs | Differentiation ↑ | No |
| [66] | Mice | N/a (in vivo) | scWAT | Adipocyte-specific overexpression of ADAMTS1 |
Differentiation ↓ Proliferation ↑ |
ADAMTS1 |
| [68] | Mice | scWAT | scWAT; BM | Adipose tissue-conditioned media |
ADSCs: differentiation ↑ BM-MSCs: differentiation ↑ |
3-5 kDa factor |
| [69] | Human subjects | scWAT | BM | 3D co-culture; + excess glucose | Viability ↑; clonogenicity ↑ | No |
| [70] | Mice | scWAT; vWAT | scWAT; vWAT; 3T3-L1 preadipocytes | Co-culture with porous insert; adipocyte-conditioned media | Differentiation ↓ |
Slc27a1a, Vima, Cpa, Ecm1a Got2b, Cpqb, Il1rl1b, Lgals3 bpb |
ADAMTS1 ADAM metallopeptidase with thrombospondin motif type 1, ADSC adipose-derived stem cell, Ang II angiotensin II, bFGF basic fibroblast growth factor, CCL5 chemokine ligand 5, BM bone marrow, BM-MSC bone marrow mesenchymal stem cell, Cav-1 caveolin 1, Cp ceruloplasmin, Cpq carboxypeptidase Q, Ecm1 extracellular matrix protein-1, epWAT epididymal white adipose tissue, Got2 aspartate aminotransferase, IL-6 interleukin 6, Il1rl1 interleukin-1 receptor-like 1, kDa kiloDalton, Lgals3bp galectin-3-binding protein, MCP-1 monocyte chemoattractant protein 1, ND non-diabetic, Ref. reference, scWAT subcutaneous white adipose tissue, Slc27a1 long-chain fatty acid transport protein 1, T2D Type 2 diabetic, Vim vimentin, vWAT visceral white adipose tissue
aPromotes ADSC differentiation
bInhibits ADSC differentiation
Paracrine interactions in primary rodent cells
In periods of energy excess, mature adipocytes accumulate lipids to become hypertrophic, but progressive expansion can result in cellular stress and insulin resistance. This is normally limited by hyperplasia and differentiation of progenitor ADSCs to produce sufficiently abundant mature adipocytes to store the excess energy. It is therefore easily conceivable that the regulation of ADSC proliferation and differentiation could be controlled by the energetic state of the adipose depot as a whole or by the increasingly hypertrophic adipocytes within it. However, the proliferation and differentiation of cells are usually mutually exclusive and therefore it is likely that signals from enlarged adipose depots, containing mature adipocytes, to ADSCs would be either proliferative or stimulate differentiation. Initial investigations therefore strived to examine whether mature adipocytes or other cells within adipose tissue could evoke a cellular response in ADSCs by inducing differentiation or proliferation. Furthermore, adiposity is known to increase with age, which is associated with insulin resistance [28]. It is plausible that this might be due to a diminished response of ADSCs to compensate for adipocyte hypertrophy during the period of positive energy balance in later life, possibly as a result of dysfunctional communication between enlarged adipocytes and progenitor cells. Investigators have therefore also investigated the effects of age on the secretome of mature adipocytes, examining the effects of adipocyte-conditioned media on the propensity of ADSCs to differentiate.
In a landmark study, Shillabeer et al. [29] investigated the effect of adipose tissue on the differentiation of cultured ADSCs, obtained from three adipose depots. Epididymal, retroperitoneal and inguinal fat depots of healthy adult Sprague–Dawley rats were dissected, shredded and co-cultured with ADSCs obtained from stromal-vascular fractions from the corresponding fat depots. The authors found that direct co-culture of ADSCs with cells liberated from adipose tissue induced a marked (30–40 fold) increase in intracellular lipid accrual in the progenitor cells, compared to ADSCs cultured alone. Moreover, incubation with adipose tissue induced an increase in glycerol 3-phosphate dehydrogenase (GPDH) activity, a key enzyme in triglyceride biosynthesis and marker of adipogenic differentiation [30], establishing that lipid droplet expansion within ADSCs was due to differentiation rather than merely uptake of lipids from the culture medium. Co-culture of ADSCs with isolated, tissue-liberated mature adipocytes was found to result in similar levels of ADSC differentiation as incubation with whole tissue, indicating that the adipogenic effect was specific to mature adipocytes and not dependent on other cell types found within the tissue. Adipogenic conversion was consistently demonstrated regardless of the site of origin of the ADSCs or mature adipocytes. Mature adipocytes did not influence the proliferation of ADSCs within the 6-day study period. Notably, this adipogenic effect could not be ascribed to the release of free fatty acids by damaged adipocytes, suggesting that mature adipocytes release factors that stimulate ADSC differentiation and maturation.
Follow-up studies established that the paracrine effects of adipocytes in co-culture with ADSCs are influenced by factors such as the corpulence and age of the donor animals. For example, ADSCs from normal-weight adult Sprague–Dawley rats that were co-incubated with mature adipocytes from genetically obese rats underwent robust adipogenic differentiation, whereas this effect was considerably diminished when the adipocytes were isolated from lean rats [31]. The authors speculated that lower levels of differentiation factor(s) were being released by lean- than obese-derived adipocytes. Similarly, the co-culture of ADSCs and mature adipocytes isolated from epididymal tissue of old (20 months) rats resulted in a lower degree of ADSC differentiation than when the ADSCs and adipocytes were obtained from young (3 months) rats [32]. Cross-over experiments (ADSCs from old rats co-cultured with mature adipocytes from young rats, and vice versa) demonstrated that this effect was not due to a reduced adipogenic potential of ADSCs from aged rats, but rather to a reduced production and secretion of differentiation-promoting factor(s) from old adipocytes.
Experimental approaches that used adipocyte-conditioned media (cell-free media enriched with adipocyte-secreted factors), as opposed to direct co-culture of ADSCs with mature adipocytes, negated possible juxtacrine, contact-dependent interactions between the two cell populations and examined paracrine and endocrine stimulation of adipogenesis. Whereas rat ADSCs from epididymal or retroperitoneal fat depots that were cultured in basal growth media did not undergo spontaneous adipogenic differentiation, cells cultured in adipocyte-derived conditioned media rapidly differentiated and accrued lipid droplets in a linear dose-dependent manner [33]. Ultrafiltration and fractionation of the conditioned media revealed that the adipogenic factor contained therein is a papain- and chymotrypsin-sensitive protein with an approximate molecular weight of 53 kDa, which the authors designated adipocyte differentiation factor (ADF). While the identity of ADF is yet to be confirmed, ADF may act as an important paracrine hormone that regulates adipogenic processes in vivo.
Other studies focussed on the combined effect of all secreted factors from the heterogeneous cells in adipose tissue explants, as opposed to secreted factors from isolated mature adipocytes. Conditioned media derived from adipose tissue explants of inguinal fat depots from young (3 weeks old), lean rats added to cultured ADSCs, isolated from the same fat depot, inhibited the proliferation and colony-forming efficiency of ADSCs [34]. Adipose explant-derived conditioned media also promoted ADSC differentiation and increased the expression of the adipogenesis markers C/EBPβ, PPARγ2, ADIPOQ (adiponectin) and LPL (lipoprotein lipase) in these ADSCs. The twofold inhibition of cell proliferation and stimulation of cell differentiation described in this study is not unexpected, as proliferation and differentiation of ADSCs have been inversely related [35].
Paracrine interactions in primary human cells
The differentiation-promoting effect of mature adipocytes on ADSCs, observed either directly in co-culture experiments or via secreted factors in adipocyte-conditioned media, has also been found in cultured human cells. Stacey et al. [36] compared the adipogenic differentiation of human subcutaneous ADSCs either co-cultured with isolated adipocytes or treated with standard adipogenic differentiation media, the conventional method of differentiating ADSCs into adipocytes in vitro [37]. Adipogenesis was substantially greater in cells co-cultured with adipocytes than in cells cultured in standard adipogenic media, and measurements of glycerol, leptin and adiponectin release indicated that ADSCs in co-culture differentiated at an earlier time point. It is noteworthy that this investigation found that the most common means of achieving adipogenesis in vitro (with adipogenic media) was less supportive of ADSC differentiation than co-culture conditions, even in the absence of supplemented pro-adipogenic compounds. These observations were extended to cell populations cultured in 3D in vitro environments rather than 2D environments; in general, production of adipogenesis markers was higher in 3D cell cultures than 2D cultures regardless of treatment conditions. In 3D culture, co-culture again stimulated greater adipogenesis than the use of adipogenic media alone. It can be argued that co-culture of different cell populations scaffolded in 3D is more physiologically relevant, because it better approximates the in vivo environment of adipose tissue [36]. Furthermore, this also highlights insufficiencies in the differentiation of ADSCs by standard in vitro differentiation media and a lack of a full understanding of the process of adipogenesis and its regulation.
Adipogenic effects have also been described for media conditioned with adipose tissue explants containing a heterogeneous cell population, obtained from human subjects [38]. Explant-conditioned media (enriched with secreted factors from a mixture of different adipose-resident cells) effectively induced lipid accumulation and upregulation of adipogenic markers in human ADSCs in a dose-dependent manner, with higher concentrations of conditioned media resulting in increased stimulation of adipogenesis. Moreover, cells treated with explant-conditioned media demonstrated accelerated and higher lipid accumulation than cells treated with control adipogenic media. Assessments of the cytokine composition of explant-derived conditioned media found that it contained numerous cytokines at physiologically active concentrations, including leptin, adiponectin, bFGF, CCL5 and IL-6, which may modulate lipid accumulation in treated ADSCs [38].
In contrast to these findings, inhibition of ADSC differentiation by mature adipocytes has also been reported. Co-culture of human ADSCs with mature adipocytes in cell culture dishes with porous transwell inserts failed to induce differentiation of ADSCs, even after an extended co-incubation period of 20 days [39]. Similarly, the presence of co-cultured mature adipocytes fully suppressed adipogenesis in human ADSCs that have been supplemented with adipogenic media [40]. ADSCs treated with adipogenic media successfully underwent differentiation in the absence of co-cultured adipocytes, indicating that the observed inhibitory effect was not due to diminished adipogenic potential of these cells. The authors specifically examined the contribution of angiotensin II (Ang II), a vasoactive peptide that has been previously implicated in the modulation of adipogenesis [41]. Mature adipocytes secrete the Ang II precursor angiotensinogen, and may therefore serve as an endogenous source of Ang II [42]. It was demonstrated that the suppression of adipogenesis in ADSCs co-cultured with isolated adipocytes was abolished by blockade of the Ang II type I receptor, which resulted in rapid lipid accrual and maturation of ADSCs [40]. This inhibition of ADSC differentiation by mature adipocytes suggests a paracrine negative feedback loop, whereby increased secretion of Ang II by mature adipocytes suppresses further recruitment of ADSCs, and points to a modulatory role of the renin–angiotensin system on adipose tissue function [43]. While the biological relevance of this is currently unclear, adipose Ang II may act in the regulation of both the regional blood flow to adipose tissue and adipose hyperplasia [44].
Adipocyte function and dysfunction can also influence the proliferation of ADSCs. Considine et al. [45] used a double chamber co-culture system to investigate paracrine interactions between primary ADSCs and mature adipocytes, isolated from lipoaspirates of subcutaneous adipose tissue from both lean and obese subjects. Porous transwell inserts allowed for diffusion of secreted factors between mature adipocytes and ADSCs while maintaining the physical separation of the two cultures. It was found that mature adipocytes from both lean and obese subjects secreted factors that promoted the proliferation of ADSCs, where the rate of proliferation increased with both prolonged exposure to mature adipocytes and increasing numbers of mature adipocytes, indicating a time– and dose–response. Proliferative rates of the ADSCs were markedly higher in the presence of adipocytes from obese subjects compared to adipocytes from lean subjects. This indicates a greater growth-stimulating potential of adipocytes from obese individuals, which could contribute to the elevated adipose tissue hyperplasia initially seen in obesity [46].
Later studies substantiated the pro-proliferative effects reported by Considine et al. [45]. Human ADSCs isolated from abdominal subcutaneous adipose tissue, and subsequently exposed to human adipocyte-conditioned media, demonstrated greater rates of proliferation than ADSCs cultured in basal media alone [47]. Conditioned media did not stimulate adipogenic differentiation in this experimental setting, which may be linked to the inverse relationship between ADSC proliferation and differentiation [35]. The authors also investigated the growth responsiveness of ADSCs to purified adipokines known to be secreted from mature adipocytes. While adiponectin supplementation had no effect on ADSC proliferation, addition of the adipokines leptin, VEGF-A, LPA and IL-6 increased the proliferation of ADSCs, mirroring effects previously described in rodent ADSCs and cell lines [48, 49]. Gene expression analysis confirmed that the ADSCs express receptors for leptin, LPA and IL-6, indicating that it was highly likely that there is specific binding by ADSCs of these factors released into the adipose tissue microenvironment.
In line with the modulatory effects of adipocytes on ADSC proliferation and differentiation, co-culture also affects the secretion profiles of the cells. Blaber et al. [50] employed a cytokine panel to analyse the secretion profiles of human subcutaneous ADSCs, isolated subcutaneous mature adipocytes, and a co-culture of both these cell types. It was reported that co-culture of ADSCs and mature adipocytes resulted in significantly increased concentrations of three cytokines, namely VEGF-A, granulocyte colony stimulating factor (G-CSF), and basic fibroblast growth factor (bFGF) in the growth media. This finding further supports the existence of paracrine signalling between adipocytes and ADSCs that enhances the secretion of these cytokines by either or both cell types.
Autocrine/paracrine communication has also been described within populations of differentiating ADSCs, whereby adipocyte-differentiated cells stimulate naïve ADSCs in the microenvironment toward further adipogenesis. This has been demonstrated with microfluidic perfusion cell culture chambers, which enable tight control of the cellular environment during differentiation as the media composition remains constant over the entire experiment [51]. Human ADSCs obtained from abdominal subcutaneous lipoaspirates were cultured in a microfluidics chamber, with ADSC differentiation controlled by perfusion with either standard adipogenic media, a mixture of adipogenic media and differentiating ADSC-conditioned media, or basal media [52]. This conditioned media was prepared by stimulating adipogenesis in ADSCs and subsequently collecting and pooling culture supernatants at several time points; hence, the conditioned media contained factors secreted by a mixture of cells across various stages of differentiation. Of note, ADSCs perfused with adipogenic media were only exposed to exogenous adipogenic stimuli, as the perfusion conditions rapidly removed released factors secreted by differentiating ADSCs, whereas conditioned media provided cells with both adipogenic stimuli and cell-secreted factors. It was found that conditioned media increased lipid accrual and upregulation of adipogenesis markers to a greater extent than adipogenic media alone, as a result of autocrine/paracrine stimulation. Perfusion with conditioned media for a short period of 12 hours was sufficient to induce expression of CEBPB and CEBPD (early markers of adipogenesis) in ADSCs, indicating that the pro-adipogenesis factors, released in response to adipogenic media, act very early in the adipogenic process upstream of the transcription factors C/EBPβ and C/EBPδ.
Paracrine communication between adipocytes and adipocyte progenitor cells has also been described for cells residing in the bone marrow niche [53]. Bone marrow was collected from femoral heads of diabetic and non-diabetic patients undergoing hip surgery, from which mature adipocytes and bone marrow mesenchymal stem cells (BM-MSCs) were isolated and separately cultured. Conditioned media was subsequently harvested from mature bone marrow adipocyte cultures of diabetic- and non-diabetic patients. BM-MSCs of non-diabetic subjects were exposed to standard adipogenic media, or adipogenic media supplemented with either conditioned media from diabetic adipocytes or conditioned media from non-diabetic adipocytes. It was found that diabetic conditioned media induced adipogenic differentiation to a greater degree than non-diabetic conditioned media or standard adipogenic media alone. Furthermore, whereas stimulation with osteogenic media alone or osteogenic media supplemented with non-diabetic conditioned media resulted in increased BM-MSC differentiation into alkaline phosphatase-positive osteoblasts, this osteogenic stimulation was negated with supplementation with diabetic conditioned media. These results suggest a diabetic paracrine loop in bone marrow whereby bone marrow adipocytes of diabetic patients promote the adipogenic differentiation of BM-MSCs at the expense of osteogenesis [53]. A comparison of the secretomes of mature adipocytes from diabetic and non-diabetic patients revealed, amongst other alterations, a prominently increased secretion of monocyte chemoattractant protein 1 (MCP-1) by diabetic adipocytes. This protein has been previously implicated in adipogenesis [54] and higher circulating levels of MCP-1 have been associated with insulin resistance [55]. Antagonism of MCP-1 signalling moderately decreased the observed adipogenic potential of standard adipogenic media and non-diabetic conditioned media, but severely inhibited adipogenesis induced by diabetic conditioned media [53]. This implicates MCP-1 as an important paracrine factor in bone marrow adipose tissue, which may contribute to dysregulated bone marrow adiposity in diabetes.
Paracrine interactions in immortalised cell lines
In addition to those that have been described in primary rodent and human cells, paracrine interactions have also been demonstrated in immortalised cell lines. The most commonly used cell line in the study of adipogenesis is the murine 3T3-L1 cell line, a well-established preadipocyte (adipocyte lineage committed precursor cell) model originally derived from Swiss 3T3 mouse embryos [56]. The fibroblastic 3T3-L1 cells are readily induced to undergo de novo differentiation to adipocytes under appropriate culture conditions and chemical stimulation [57]. The main advantages of using 3T3-L1 preadipocytes over primary cells include the ease of culturing, the sustained proliferation after successive passages and the homogeneity of the cell population, which provides consistent responses to changes in experimental conditions. Consequently, 3T3-L1 cells are routinely used in co-culture experiments to dissect paracrine and endocrine interactions between different cell types, such as those between macrophages and adipocytes [58].
Several studies have demonstrated paracrine communication between naïve and differentiated 3T3-L1 preadipocytes. Lai et al. [59] cultured 3T3-L1 cells in microfluidic cell chambers, where the controlled application of an adipogenic gradient developed a spatially defined co-culture of preadipocytes and adipocytes. It was found that mature adipocytes stimulated the adipogenic conversion of surrounding preadipocytes, even in the absence of exogenous adipogenic agents. This stimulatory effect was restricted to large hypertrophied adipocytes, as small, newly formed adipocytes did not demonstrate a similar differentiation-inducing potential. This supports the hypothesis that enlargement of adipocytes triggers the release of paracrine factors that promote the differentiation of preadipocytes; however, the cellular mechanism coupling adipocyte enlargement with the secretion of differentiation factors remains to be identified.
A recent study identified caveolin-1 (Cav-1), a scaffolding protein and component of plasma membrane caveolae, as a potential adipogenic differentiation factor [60]. It was shown that differentiated 3T3-L1 adipocytes, but not naïve preadipocytes, secreted Cav-1 within microvesicles and exosomes, via an ERK1/2-dependent mechanism. It was found that secreted Cav-1 was taken up by naïve preadipocytes, which enhanced the differentiation of these cells when cultured in adipogenic media. The specific role of Cav-1 in adipogenesis was examined by overexpressing Cav-1 in transgenic 3T3-L1 preadipocytes: intracellular Cav-1 enhanced adipogenic differentiation of preadipocytes overexpressing Cav-1 compared to cells transfected with an empty vector [60]. Furthermore, adipocyte hypertrophy, induced by excess exogenous glucose and fatty acids, markedly enhanced Cav-1 levels and secretion in mature 3T3-L1 adipocytes. Cav-1-containing microvesicles and exosomes were also found to be secreted by mouse adipocytes isolated from inguinal and epididymal adipose depots, while adipocytes from obese mice demonstrated greater Cav-1 release than from lean mice. Hence, obesity and adipocyte hypertrophy may enhance Cav-1 secretion which promotes adipogenesis in progenitor cells, although the mechanism whereby obesity and hypertrophy regulate Cav-1 secretion remains unknown.
Paracrine communication between adipocytes and ADSCs may be substantially disrupted in pathophysiological conditions, such as chronic inflammation and insulin resistance. Wei et al. [61] demonstrated that, when naïve 3T3-L1 preadipocytes were chemically stimulated to differentiate while being co-cultured with differentiated 3T3-L1 cells, the presence of the mature adipocytes did not significantly affect adipogenesis. However, prior exposure of the mature adipocytes to high concentrations of either lipopolysaccharide or insulin (inducing inflammation or insulin resistance, respectively) subsequently inhibited differentiation of the co-cultured preadipocytes. Hence, conditions of chronic inflammation or insulin resistance may modulate paracrine signalling between adipocytes and progenitor cells, and thereby contribute to adipose tissue dysfunction.
In vivo animal experiments
The high proliferation and differentiation potential of ADSCs has garnered considerable attention in the growing fields of regenerative medicine and tissue engineering, which aim to repair or regenerate a body defect by implanting progenitor cells at the site, often in combination with an artificial cellular scaffold and relevant growth factor(s) [62]. This tissue engineering approach may also be used for the regeneration of adipose tissue, where the introduced adipose progenitor cells are expected to undergo in vivo-induced adipogenesis. For example, an early study demonstrated that nude mice which were subcutaneously injected with cultured preadipocytes from the 3T3-F442A cell line developed mature fat tissue at the injection site, suggesting that the necessary adipogenic factors were derived from the host [63]. This effect has also been demonstrated with human ADSCs isolated from lipoaspirates, which underwent in vivo adipogenesis following subcutaneous implantation into the backs of nude mice [64, 65]. Immunohistochemical examination confirmed that the newly formed adipose tissue was composed of human matured adipocytes. Moreover, the survival and differentiation efficiency of implanted ADSCs were enhanced by co-implantation with bFGF [64]. While it is unclear to what extent the in vivo differentiation of implanted ADSCs into mature adipocytes is directed by the local microenvironment of the implantation site, it is likely that a multitude of paracrine/endocrine factors secreted by host adipose tissue may contribute to this effect. One such paracrine factor is ADAMTS1 (a disintegrin and metalloproteinase with thrombospondin motif 1), that is predominantly expressed and secreted by adipocytes and has been shown to affect adipogenesis in in vivo animal experiments [66].
Glucocorticoids are known to upregulate adipocytic genes and promote ADSC differentiation [67]. To identify glucocorticoid-responsive genes that may modulate these effects, Wong et al. [66] analysed microarrays performed on cultured mouse preadipocytes treated with the synthetic glucocorticoid dexamethasone. ADAMTS1 was found to be downregulated by dexamethasone in the arrays and similarly reduced in subcutaneous adipose tissue of mice after treatment with the glucocorticoid [66]. To investigate the function of ADAMTS1 in vivo, transgenic mice were generated with cell type specific overexpression of ADAMTS1 in adipocytes, driven by the adipocyte fatty acid binding protein 4 (FABP4) promoter. It was found that ADAMTS1 transgenic mice had smaller adipose depots than wild-type mice. Moreover, de novo adipocyte formation was monitored by injecting the fluorescent dye EdU, which labels synthesising DNA, into ADAMTS1 transgenic and wild-type mice. Transgenic mice had significantly fewer EdU+ adipocytes, but more EdU+ ADSCs, than mice that are not overexpressing ADAMTS1, which suggests that adipocyte-secreted ADAMTS1 suppresses differentiation and promotes progenitor cell proliferation in vivo. Paracrine effects were demonstrated in vitro by means of the addition of purified recombinant ADAMTS1 to cultured ADSCs, indicating the likelihood of direct communication in vivo, rather than systemic effects. Further characterisation of this interaction indicated that extracellular ADAMTS1 activated the Wnt/β-catenin pathway in ADSCs, which suppresses ADSC differentiation. It is of note that both glucocorticoid exposure and a high fat diet reduced in vivo ADAMTS1 expression in adipocytes, resulting in increased adipogenesis and enlarged adipose depots. Therefore, this protein may act in a paracrine pathway that regulates the expansion of adipose tissue in response to systemic cues.
Evidence of endocrine signalling?
This review highlights the growing body of evidence of paracrine signalling between mature adipocytes and ADSCs that influence adipogenesis. However, a salient feature of the majority of the reviewed studies is that both the adipocytes and progenitor cells are sourced from similar or the same fat depot. Hence, the plausibility of paracrine communication under physiological conditions is supported by the close proximity of these cell populations in adipose tissue. It can be speculated that comparable endocrine communication may also exist between more remote adipose tissues, such as between subcutaneous adipose stores, visceral fat depots and/or bone marrow adipose tissue. Such endocrine signalling might modulate the development of pathological states such as visceral adipose tissue expansion and hypertrophy.
Wu et al. [68] investigated the adipogenic potential of conditioned media enriched with factors secreted by mouse subcutaneous adipose tissue fragments. ADSCs, sourced from the same tissue fragments, were stimulated to differentiate when treated with explant-conditioned media, even in the absence of exogenous adipogenic compounds. In parallel, treatment of BM-MSCs with the conditioned media similarly induced spontaneous adipogenesis in these progenitor cells. Hence, factors secreted by adipose tissue fragments could stimulate the differentiation of progenitor cells that would be distantly located in vivo. Subsequently, ultracentrifugation was used to characterise the adipogenic factors of the conditioned media, which refined the molecular weight of the active components to be between 3 and 5 kDa. While the identities of these adipogenic factors are still unclear, follow-up studies may reveal one or more small molecules in the adipose tissue secretome that modulate differentiation of ADSCs.
Possible endocrine interactions between subcutaneous adipocytes and BM-MSCs have also been demonstrated in a 3D co-culture system during investigations on the effects of adipocytes and hyperglycaemia on BM-MSC viability [69]. Unlike BM-MSCs in monoculture, BM-MSCs in co-culture with mature adipocytes originally derived from human subcutaneous adipose tissue maintained a high viability and clonogenicity in high glucose conditions. This protective effect of adipocyte-secreted factors on BM-MSCs corresponded with significant gene expression changes in both adipocytes and BM-MSCs in co-culture, compared to either cell type in monoculture. These results are suggestive of bi-directional crosstalk between adipocytes and BM-MSCs. Hence, under hyperglycaemic conditions, an increased number of adipocytes in the bone marrow may exert compensatory signalling to maintain BM-MSC function and possibly delay the onset of osteoporosis [69].
Plausible inter-adipose depot communication may also be inferred from a comprehensive investigation of the crosstalk between adipocytes and ADSCs combined with fractionation approaches [70]. In this study, mature adipocytes from both subcutaneous and visceral mouse depots were co-cultured with subcutaneous primary ADSCs, visceral ADSCs or 3T3-L1 preadipocytes to investigate the effect of secreted factors. It was found that co-culture of adipocytes from either depot had an inhibitory effect on the differentiation potential of subcutaneous, visceral or 3T3-L1 preadipocytes. This suppression of adipogenesis could be fully reversed by heat inactivation. Subsequent proteomics and fractionation approaches identified a spectrum of factors that either positively or negatively affected adipocyte maturation in ADSCs; among the secreted factors, long-chain fatty acid transport protein 1 (Slc27a1), vimentin (Vim), ceruloplasmin (Cp), and extracellular matrix protein-1 (Ecm1) promoted adipocyte differentiation, whereas secreted aspartate aminotransferase (Got2), carboxipeptidase Q (Cpq), interleukin-1 receptor-like 1 (Il1rl1), and galectin-3-binding protein (Lgals3 bp) decreased adipocyte differentiation. Although the investigators did not comment on this, it is noteworthy that the complex regulatory effects of adipocytes on ADSCs described by Challa et al. were not limited to cells from the same depot of origin and could plausibly represent endocrine mechanisms. Future studies may clarify the in vivo contribution of these identified factors and whether the maintenance of adipose tissue homeostasis is also influenced by distant, endocrine communication between different adipose depots.
It is increasingly recognised that adipose progenitor cells are highly heterogeneous, existing in distinct but unstructured subpopulations of cells. Recent reports suggest that different subpopulations of ADSCs may influence adipogenic differentiation in a paracrine manner [71]. Using single-cell transcriptomics, investigators identified a CD142+ population of mouse ADSCs that were refractory to adipogenesis and negatively regulated the adipogenic capacity of other mouse ADSCs. Co-cultures of these adipogenesis-regulatory cells (Aregs) with other ADSCs demonstrated that the adipogenesis-suppressive effects of Aregs were maintained in the absence of direct cell contact, suggesting a paracrine mechanism of action. Moreover, loss-of-function genetic screening highlighted three proteins that may mediate the anti-adipogenic effect of Aregs, namely receptor transport protein 3 (RTP3), serine peptidase inhibitor Kazal type 2 (SPINK2) and/or fibroblast growth factor 12 (FGF12). While Aregs constitute a small minority of the total ADSC population, visceral adipose depots were found to contain a significantly higher proportion of Aregs compared to subcutaneous adipose tissue, and genetically obese mice have considerably more Aregs than lean mice. Hence, paracrine signalling from these differentiation-modulatory Aregs may contribute to the different responses of subcutaneous and visceral adipose depots in obesity.
While this review focusses on paracrine and endocrine communication between adipocytes and ADSCs, it has been well established that adipocytes exert endocrine effects on various other cells throughout the body. For example, adipocyte-secreted circulating adiponectin directly influences bone cell function by stimulating osteoblast growth and inhibiting osteoclastogenesis [72]. Similarly, adipocytes suppress the differentiation of skeletal muscle myocytes and modulate insulin-stimulated glucose uptake in skeletal muscle [73, 74]. Several known adipokines have either vasorelaxing or vasoconstrictive effects on smooth muscle cells in arteries, contributing to the regulation of vascular tone [75]. Adipocytes are also key regulatory cells in the control of adipose tissue inflammation, via the crosstalk between adipocytes, macrophages and other immune cells [76]. Hence, it is plausible that similar endocrine communication mechanisms exist between adipocytes and distant ADSCs, residing in different adipose depots. This inter-depot crosstalk may affect adipose depot size and function, and influence fat distribution throughout the body. Impairment of this endocrine signalling may also be an important contributing factor in the systemic metabolic disturbances associated with obesity.
For adipocytes to mediate inter-depot endocrine communication, the secreted factors should be released by adipocytes in a regulated manner, the serum concentrations of the factors must reach physiologically active levels, and the target cells need to be receptive to the endocrine signals, i.e. express cell-surface receptors for circulating adipose-secreted factors. Consequently, studies of the adipose tissue secretomes under various conditions are of great interest. Lim et al. [77] characterised the secreted proteomes of immortalised 3T3-L1 adipocytes as well as primary adipocytes from rat subcutaneous adipose tissue, identifying 97 and 203 secreted proteins, respectively. Similarly, analyses of in vitro-differentiated subcutaneous adipocytes from healthy women identified 347 protein released into conditioned media [78]. Differential secretion profiles have also been described between obese and lean adipose tissue: a total of 87 secreted proteins were identified in conditioned media from adipocytes of obese mice, compared to only 31 from lean mice [79]. Extracellular proteins that were uniquely detected in obese adipose tissue include inflammatory molecules, collagens, proteases and extracellular matrix proteins. By characterising and comparing the secretomes of rat adipose tissue from subcutaneous, visceral and epididymal depots, Roca-Rivada et al. [12] identified 45 secreted proteins that significantly diverged between the different adipose tissue sources, including known adipokines and novel released proteins. These marked secretome differences support the differential functioning of adipose tissue corresponding to its anatomical location. This is further supported by a follow-up study of human obese subcutaneous and visceral adipose secretomes, which also demonstrated distinct secretion profiles between the depots [20].
The majority of the reviewed studies that characterised the adipocyte-secreted factors which could mediate paracrine effects on adipogenesis concluded that the active secreted factors are proteins, as these factors were found to be sensitive to proteases [33] or to be heat-labile [70]. However, it is plausible that non-peptide secreted factors, such as free fatty acids and extracellular vesicles, also contribute to the regulation of adipogenesis. ADSC differentiation can be stimulated by long chain poly-unsaturated fatty acids, via activation of PPAR-γ [80], and linoleic acid and arachidonic acid were found to promote triglyceride accrual in human ADSCs [81, 82]. Moreover, paracrine and endocrine signalling may be mediated by adipose-secreted microvesicles and/or exosomes [83]. In a recent study, exosomes isolated from rat subcutaneous adipose tissue fragments by differential ultracentrifugation were able to dose-dependently stimulate adipogenesis in ADSCs from the same tissue [84]. Similarly, exosomes purified from differentiated 3T3-L1 adipocytes cultured in hypoxic conditions [85] promoted lipid accrual in recipient ADSCs, in contrast to those produced under normoxic conditions. The total amount of exosome-associated proteins increased three- to four-fold following adipocyte hypoxia, and these exosomes were enriched in proteins related to de novo lipogenesis. Characterisation of exosomes isolated from primary human ADSCs differentiated to adipocytes identified 884 exosome-associated proteins, which were enriched for molecular pathways relating to cell signalling and membrane-mediated processes, supporting the role of exosomes in signalling and inter-organ crosstalk [86]. These studies highlight the potential importance of exosome-mediated crosstalk within- and between adipose depots in the regulation of adipogenesis and adipose tissue expansion.
While this review focusses on the communication between white adipocytes and ADSCs, thermogenic fat cells (brown and beige adipocytes) are increasingly recognised as important modulators of metabolism in rodent and human physiology [87]. Comparable studies of possible crosstalk between progenitor cells and mature brown/beige adipocytes are therefore of interest. To date, numerous endocrine factors have been identified that modulate adipose tissue browning or brown/beige adipocyte activity [88]. The recognition of these circulating factors, derived from distinct organs and tissues, support the role of inter-organ crosstalk in the regulation of brown and beige adipose tissue. Moreover, brown adipocytes secrete a large variety of extracellular proteins, with the secretory profiles substantially changing following β-adrenergic stimulation and activation [89]. Several brown adipocyte-derived autocrine/paracrine factors have been demonstrated to affect thermogenic adipocyte recruitment and activity, including adenosine [90], bone morphogenic protein (BMP) 7 [91], BMP8 [92] and endothelin-1 [93]. These and other secreted factors have been comprehensively reviewed elsewhere [88, 94]. However, to the best of our knowledge, there is currently no experimental evidence for direct paracrine or endocrine communication between progenitor cells and brown/beige adipocytes. Co-culture experiments and investigations examining how conditioned media from mature brown/beige adipocytes affect progenitor cell proliferation and differentiation are therefore warranted.
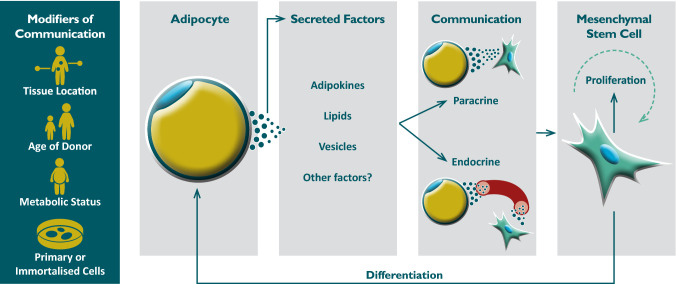
Taken together, the reviewed studies provide compelling evidence for the role of paracrine and endocrine interactions between white adipocytes and ADSCs that influence adipogenesis (Fig. 1). However, investigations of paracrine/endocrine signalling between adipocytes and ADSCs often reported contradicting effects. While the majority of studies observed the promotion of adipogenesis [29, 31, 33, 34, 36, 38, 52, 53, 59, 60, 68], other studies found that adipocytes had no effect on ADSC differentiation [39, 47] or inhibited differentiation of ADSCs cultured in adipogenic media [40, 61, 70]. Similarly, adipocytes were reported to either promote [45, 47, 66] or inhibit [34] ADSC proliferation, or had no observable effects on mitogenesis [29]. The divergent in vitro effects of adipocyte-secreted factors on ADSCs likely reflect the complexity of the underlying interactions, which may involve bi-directional crosstalk via multiple stimulatory and inhibitory factors. The different observations may also be due to the wide range of different experimental models used in these studies. For example, while several of the investigations employed the 3T3-L1 preadipocyte cell line, many aspects of the complex intracellular signalling pathways that regulate adipogenesis in vivo are not recapitulated in these cells [95]. Furthermore, the characteristics of the donor animals or subjects from whom primary cells are sourced can significantly affect the observed interactions, including donor age [32] and metabolic status, such as obesity [31, 45].
Fig. 1.
Factors which affect adipocyte-progenitor cell communication
While mice and rats are commonly used to model human metabolic disturbances and obesity, there are notable differences in adipose tissue distribution and function between rodents and humans [96]. Results obtained from murine studies may therefore not be fully applicable to human physiology, and should be interpreted with caution. For example, the rodent adipokine resistin is expressed by mature adipocytes in mice, where elevated levels are closely linked to obesity and insulin resistance [97]. In contrast, human resistin is mainly secreted by macrophages and is increased in inflammatory conditions [98]. The stark differences in the physiological roles of resistin in rodents and humans illustrate the difficulty in drawing clear parallels between rodent and human adipose biology.
It should also be noted that the current evidence for adipocyte—progenitor cell communication, as reviewed here, is almost exclusively based on in vitro results or animal studies. Hence, it is not yet established whether analogous in vivo paracrine/endocrine signalling may be found in humans, or whether it contributes to human health and disease. Under physiological conditions, ADSCs reside in complex depot-specific microenvironments, where they are exposed to fluctuating levels of a multitude of pro- and anti-adipogenic signals at any given time. It is therefore difficult to dissect the actions of adipocyte-derived factors on ADSCs from factors secreted by other endocrine organs, as well as from the effects of adipose tissue innervation by peripheral nerves [99]. Further studies are required to understand how complex adipogenic signals from multiple sources are integrated in a physiological milieu. Recently developed transgenic models that can track ADSCs and adipogenesis in vivo are well suited to study adipose tissue remodelling [100, 101]. While only a few studies have investigated adipogenesis in humans in vivo, a recent examination of subjects that underwent allogenic bone marrow transplantation found that approximately 10% of adipose tissue-resident adipocytes are derived from BM-MSCs [102]. Human adipocyte turnover has been measured by analysing of the integration of 14C into genomic DNA, demonstrating that an average of 10% of adipocytes are renewed annually, regardless of age or level of adiposity [103]. These and other studies are shedding new light on the complex dynamics of in vivo adipogenesis in humans.
While paracrine/endocrine communication between adipocytes and ADSCs that influence adipogenesis are difficult to study and are likely to be highly complex, further studies are warranted to establish whether these interactions are relevant to in vivo adipose tissue function. Endocrine signalling mechanisms may play a significant role in the delayed onset of visceral adipose depot growth when compared to subcutaneous adipose expansion. Ultimately, a deeper understanding of the regulation of adipose development may provide novel insights into the pathobiology of obesity, and could uncover new therapeutic targets for the treatment of obesity-related disorders.
Acknowledgements
This work is based on the research supported wholly/in part by the National Research Foundation of South Africa (Grant Numbers 118565 and 118990), the South African Sugar Association (Project 257) and The Harry Crossley Foundation; WLH is supported by the Faculty of Medicine and Health Sciences, Stellenbosch University, South Africa.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gregg EW, Shaw JE. Global health effects of overweight and obesity. N Engl J Med. 2017;377:80–81. doi: 10.1056/NEJMe1706095. [DOI] [PubMed] [Google Scholar]
- 2.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/S1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 3.Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 4.Booth A, Magnuson A, Foster M. Detrimental and protective fat: body fat distribution and its relation to metabolic disease. Horm Mol Biol Clin Investig. 2014;17:13–27. doi: 10.1515/hmbci-2014-0009. [DOI] [PubMed] [Google Scholar]
- 5.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood IS, de Heredia FP, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68:370–377. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- 8.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 9.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 10.Neeland IJ, Ayers CR, Rohatgi AK, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obes Silver Spring Md. 2013;21:E439–E447. doi: 10.1002/oby.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev Off J Int Assoc Study Obes. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 12.Roca-Rivada A, Alonso J, Al-Massadi O, et al. Secretome analysis of rat adipose tissues shows location-specific roles for each depot type. J Proteom. 2011;74:1068–1079. doi: 10.1016/j.jprot.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Sam S, Mazzone T. Adipose tissue changes in obesity and the impact on metabolic function. Transl Res J Lab Clin Med. 2014;164:284–292. doi: 10.1016/j.trsl.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 14.McGown C, Birerdinc A, Younossi ZM. Adipose tissue as an endocrine organ. Clin Liver Dis. 2014;18:41–58. doi: 10.1016/j.cld.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Cook KS, Min HY, Johnson D, et al. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237:402–405. doi: 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 17.Scherer PE, Williams S, Fogliano M, et al. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 18.Goralski KB, McCarthy TC, Hanniman EA, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 19.Dahlman I, Elsen M, Tennagels N, et al. Functional annotation of the human fat cell secretome. Arch Physiol Biochem. 2012;118:84–91. doi: 10.3109/13813455.2012.685745. [DOI] [PubMed] [Google Scholar]
- 20.Roca-Rivada A, Bravo SB, Pérez-Sotelo D, et al. CILAIR-based secretome analysis of obese visceral and subcutaneous adipose tissues reveals distinctive ECM remodeling and inflammation mediators. Sci Rep. 2015;5:12214. doi: 10.1038/srep12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63:250–259. doi: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 24.Armani A, Mammi C, Marzolla V, et al. Cellular models for understanding adipogenesis, adipose dysfunction, and obesity. J Cell Biochem. 2010;110:564–572. doi: 10.1002/jcb.22598. [DOI] [PubMed] [Google Scholar]
- 25.Lee M-J, Fried SK. Optimal protocol for the differentiation and metabolic analysis of human adipose stromal cells. Methods Enzymol. 2014;538:49–65. doi: 10.1016/B978-0-12-800280-3.00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang QA, Scherer PE, Gupta RK. Improved methodologies for the study of adipose biology: insights gained and opportunities ahead. J Lipid Res. 2014;55:605–624. doi: 10.1194/jlr.R046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8:339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Shillabeer G, Forden JM, Lau DC. Induction of preadipocyte differentiation by mature fat cells in the rat. J Clin Invest. 1989;84:381–387. doi: 10.1172/JCI114177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pairault J, Green H. A study of the adipose conversion of suspended 3T3 cells by using glycerophosphate dehydrogenase as differentiation marker. Proc Natl Acad Sci USA. 1979;76:5138–5142. doi: 10.1073/pnas.76.10.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shillabeer G, Forden JM, Russell JC, Lau DC. Paradoxically slow preadipocyte replication and differentiation in corpulent rats. Am J Physiol-Endocrinol Metab. 1990;258:E368–E376. doi: 10.1152/ajpendo.1990.258.2.E368. [DOI] [PubMed] [Google Scholar]
- 32.Carraro R, Li ZH, Johnson JE, Gregerman RI. Adipocytes of old rats produce a decreased amount of differentiation factor for preadipocytes derived from adipose tissue islets. J Gerontol. 1992;47:B198–B201. doi: 10.1093/geronj/47.6.B198. [DOI] [PubMed] [Google Scholar]
- 33.Li Z-H, Carraro R, Gregerman RI, Lau DCW. Adipocyte differentiation factor (adf): a protein secreted by mature fat cells that induces preadipocyte differentiation in culture. Cell Biol Int. 1998;22:253–270. doi: 10.1006/cbir.1998.0237. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Qiao X, Yu M, et al. Secretory factors from rat adipose tissue explants promote adipogenesis and angiogenesis. Artif Organs. 2014;38:E33–E45. doi: 10.1111/aor.12162. [DOI] [PubMed] [Google Scholar]
- 35.Ailhaud G, Amri E, Bardon S, et al. The adipocyte: relationships between proliferation and adipose cell differentiation. Am Rev Respir Dis. 1990;142:S57–S59. doi: 10.1164/ajrccm/142.6_Pt_2.S57. [DOI] [PubMed] [Google Scholar]
- 36.Stacey DH, Hanson SE, Lahvis G, et al. In vitro adipogenic differentiation of preadipocytes varies with differentiation stimulus, culture dimensionality, and scaffold composition. Tissue Eng Part A. 2009;15:3389–3399. doi: 10.1089/ten.TEA.2008.0293. [DOI] [PubMed] [Google Scholar]
- 37.Janderová L, McNeil M, Murrell AN, et al. Human mesenchymal stem cells as an in vitro model for human adipogenesis. Obes Res. 2003;11:65–74. doi: 10.1038/oby.2003.11. [DOI] [PubMed] [Google Scholar]
- 38.Sarkanen J-R, Kaila V, Mannerström B, et al. Human adipose tissue extract induces angiogenesis and adipogenesis in vitro. Tissue Eng Part A. 2012;18:17–25. doi: 10.1089/ten.tea.2010.0712. [DOI] [PubMed] [Google Scholar]
- 39.Song K, Li W, Wang H, et al. Investigation of coculture of human adipose-derived stem cells and mature adipocytes. Appl Biochem Biotechnol. 2012;167:2381–2387. doi: 10.1007/s12010-012-9764-y. [DOI] [PubMed] [Google Scholar]
- 40.Janke J, Engeli S, Gorzelniak K, et al. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699–1707. doi: 10.2337/diabetes.51.6.1699. [DOI] [PubMed] [Google Scholar]
- 41.Ailhaud G, Fukamizu A, Massiera F, et al. Angiotensinogen, angiotensin II and adipose tissue development. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 2000;24(Suppl 4):S33–S35. doi: 10.1038/sj.ijo.0801501. [DOI] [PubMed] [Google Scholar]
- 42.Pinterova L, Krizanova O, Zorad S. Rat epididymal fat tissue express all components of the renin-angiotensin system. Gen Physiol Biophys. 2000;19:329–334. [PubMed] [Google Scholar]
- 43.Frigolet ME, Torres N, Tovar AR. The renin–angiotensin system in adipose tissue and its metabolic consequences during obesity. J Nutr Biochem. 2013;24:2003–2015. doi: 10.1016/j.jnutbio.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Townsend RR. The effects of angiotensin-II on lipolysis in humans. Metabolism. 2001;50:468–472. doi: 10.1053/meta.2001.21021. [DOI] [PubMed] [Google Scholar]
- 45.Considine RV, Nyce MR, Morales LM, et al. Paracrine stimulation of preadipocyte-enriched cell cultures by mature adipocytes. Am J Physiol-Endocrinol Metab. 1996;270:E895–E899. doi: 10.1152/ajpendo.1996.270.5.E895. [DOI] [PubMed] [Google Scholar]
- 46.Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5:299–311. doi: 10.1016/S0300-595X(76)80023-0. [DOI] [PubMed] [Google Scholar]
- 47.Maumus M, Sengenès C, Decaunes P, et al. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab. 2008;93:4098–4106. doi: 10.1210/jc.2008-0044. [DOI] [PubMed] [Google Scholar]
- 48.Valet P, Pagès C, Jeanneton O, et al. Alpha2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. A paracrine signal for preadipocyte growth. J Clin Invest. 1998;101:1431–1438. doi: 10.1172/JCI806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagoner B, Hausman DB, Harris RBS. Direct and indirect effects of leptin on preadipocyte proliferation and differentiation. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1557–R1564. doi: 10.1152/ajpregu.00860.2005. [DOI] [PubMed] [Google Scholar]
- 50.Blaber SP, Webster RA, Hill CJ, et al. Analysis of in vitro secretion profiles from adipose-derived cell populations. J Transl Med. 2012;10:172. doi: 10.1186/1479-5876-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blagovic K, Kim LY, Voldman J. Microfluidic perfusion for regulating diffusible signaling in stem cells. PLoS One. 2011;6:e22892. doi: 10.1371/journal.pone.0022892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hemmingsen M, Vedel S, Skafte-Pedersen P, et al. The role of paracrine and autocrine signaling in the early phase of adipogenic differentiation of adipose-derived stem cells. PLoS One. 2013 doi: 10.1371/journal.pone.0063638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferland-McCollough D, Masseli D, Spinetti G, et al. MCP-1 Feedback Loop Between adipocytes and mesenchymal stromal cells causes fat accumulation and contributes to hematopoietic stem cell rarefaction in the bone marrow of diabetic patients. Diabetes. 2018 doi: 10.2337/db18-0044. [DOI] [PubMed] [Google Scholar]
- 54.Younce C, Kolattukudy P. MCP-1 induced protein promotes adipogenesis via oxidative stress, endoplasmic reticulum stress and autophagy. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2012;30:307–320. doi: 10.1159/000339066. [DOI] [PubMed] [Google Scholar]
- 55.Panee J. Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012;60:1–12. doi: 10.1016/j.cyto.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 57.Zebisch K, Voigt V, Wabitsch M, Brandsch M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal Biochem. 2012;425:88–90. doi: 10.1016/j.ab.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Huang W-C, Chang W-T, Wu S-J, et al. Phloretin and phlorizin promote lipolysis and inhibit inflammation in mouse 3T3-L1 cells and in macrophage-adipocyte co-cultures. Mol Nutr Food Res. 2013;57:1803–1813. doi: 10.1002/mnfr.201300001. [DOI] [PubMed] [Google Scholar]
- 59.Lai N, Sims JK, Jeon NL, Lee K. Adipocyte induction of preadipocyte differentiation in a gradient chamber. Tissue Eng Part C Methods. 2012;18:958–967. doi: 10.1089/ten.tec.2012.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang C-C, Chen C-Y, Wen H-C, et al. Caveolin-1 secreted from adipose tissues and adipocytes functions as an adipogenesis enhancer. Obesity. 2017;25:1932–1940. doi: 10.1002/oby.21970. [DOI] [PubMed] [Google Scholar]
- 61.Wei Y-T, Xia D-S, Yang W-K, et al. Secretion of adipocytes and macrophages under conditions of inflammation and/or insulin resistance and effect of adipocytes on preadipocytes under these conditions. Biochem Mosc. 2014;79:663–671. doi: 10.1134/S0006297914070086. [DOI] [PubMed] [Google Scholar]
- 62.Bacakova L, Zarubova J, Travnickova M, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—a review. Biotechnol Adv. 2018;36:1111–1126. doi: 10.1016/j.biotechadv.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 63.Green H, Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol. 1979;101:169–171. doi: 10.1002/jcp.1041010119. [DOI] [PubMed] [Google Scholar]
- 64.Kimura Y, Ozeki M, Inamoto T, Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24:2513–2521. doi: 10.1016/S0142-9612(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 65.Tsuji W, Inamoto T, Yamashiro H, et al. Adipogenesis induced by human adipose tissue-derived stem cells. Tissue Eng Part A. 2009;15:83–93. doi: 10.1089/ten.tea.2007.0297. [DOI] [PubMed] [Google Scholar]
- 66.Wong JC, Krueger KC, Costa MJ, et al. A glucocorticoid- and diet-responsive pathway toggles adipocyte precursor cell activity in vivo. Sci Signal. 2016;9:ra103. doi: 10.1126/scisignal.aag0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee M-J, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta. 2014;1842:473–481. doi: 10.1016/j.bbadis.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu L, Wang T, Ge Y, et al. Secreted factors from adipose tissue increase adipogenic differentiation of mesenchymal stem cells. Cell Prolif. 2012;45:311–319. doi: 10.1111/j.1365-2184.2012.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rinker TE, Hammoudi TM, Kemp ML, et al. Interactions between mesenchymal stem cells, adipocytes, and osteoblasts in a 3D tri-culture model of hyperglycemic conditions in the bone marrow microenvironment. Integr Biol Quant Biosci Nano Macro. 2014;6:324–337. doi: 10.1039/c3ib40194d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Challa TD, Straub LG, Balaz M, et al. Regulation of de novo adipocyte differentiation through cross talk between adipocytes and preadipocytes. Diabetes. 2015;64:4075–4087. doi: 10.2337/db14-1932. [DOI] [PubMed] [Google Scholar]
- 71.Schwalie PC, Dong H, Zachara M, et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature. 2018;559:103–108. doi: 10.1038/s41586-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 72.Williams GA, Wang Y, Callon KE, et al. In vitro and in vivo effects of adiponectin on bone. Endocrinology. 2009;150:3603–3610. doi: 10.1210/en.2008-1639. [DOI] [PubMed] [Google Scholar]
- 73.Kudoh A, Satoh H, Hirai H, et al. Preliminary evidence for adipocytokine signals in skeletal muscle glucose uptake. Front Endocrinol. 2018;9:295. doi: 10.3389/fendo.2018.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seo K, Suzuki T, Kobayashi K, Nishimura T. Adipocytes suppress differentiation of muscle cells in a co-culture system. Anim Sci J Nihon Chikusan Gakkaiho. 2018 doi: 10.1111/asj.13145. [DOI] [PubMed] [Google Scholar]
- 75.Maenhaut N, Van de Voorde J. Regulation of vascular tone by adipocytes. BMC Med. 2011;9:25. doi: 10.1186/1741-7015-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells. 2014;37:365–371. doi: 10.14348/molcells.2014.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lim J-M, Sherling D, Teo CF, et al. Defining the regulated secreted proteome of rodent adipocytes upon the induction of insulin resistance. J Proteome Res. 2008;7:1251–1263. doi: 10.1021/pr7006945. [DOI] [PubMed] [Google Scholar]
- 78.Lehr S, Hartwig S, Lamers D, et al. Identification and validation of novel adipokines released from primary human adipocytes. Mol Cell Proteomics MCP. 2012;11(M111):010504. doi: 10.1074/mcp.M111.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen X, Hunt D, Cushman SW, Hess S. Proteomic characterization of thiazolidinedione regulation of obese adipose secretome in Zucker obese rats. Proteomics Clin Appl. 2009;3:1099–1111. doi: 10.1002/prca.200900026. [DOI] [PubMed] [Google Scholar]
- 80.Krey G, Braissant O, L’Horset F, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol Baltim Md. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 81.Hutley LJ, Newell FM, Joyner JM, et al. Effects of rosiglitazone and linoleic acid on human preadipocyte differentiation. Eur J Clin Invest. 2003;33:574–581. doi: 10.1046/j.1365-2362.2003.01178.x. [DOI] [PubMed] [Google Scholar]
- 82.Massiera F, Saint-Marc P, Seydoux J, et al. Arachidonic acid and prostacyclin signaling promote adipose tissue development: a human health concern? J Lipid Res. 2003;44:271–279. doi: 10.1194/jlr.M200346-JLR200. [DOI] [PubMed] [Google Scholar]
- 83.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 84.Dai M, Yu M, Zhang Y, Tian W. Exosome-like vesicles derived from adipose tissue provide biochemical cues for adipose tissue regeneration. Tissue Eng Part A. 2017;23:1221–1230. doi: 10.1089/ten.tea.2017.0045. [DOI] [PubMed] [Google Scholar]
- 85.Sano S, Izumi Y, Yamaguchi T, et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun. 2014;445:327–333. doi: 10.1016/j.bbrc.2014.01.183. [DOI] [PubMed] [Google Scholar]
- 86.Hartwig S, De Filippo E, Göddeke S, et al. Exosomal proteins constitute an essential part of the human adipose tissue secretome. Biochim Biophys Acta BBA Proteins Proteom. 2018 doi: 10.1016/j.bbapap.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 87.Rui L. Brown and beige adipose tissues in health and disease. In: Terjung R, editor. Comprehensive physiology. Hoboken: Wiley; 2017. pp. 1281–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scheideler M, Herzig S, Georgiadi A. Endocrine and autocrine/paracrine modulators of brown adipose tissue mass and activity as novel therapeutic strategies against obesity and type 2 diabetes. Horm Mol Biol Clin Investig. 2017 doi: 10.1515/hmbci-2017-0043. [DOI] [PubMed] [Google Scholar]
- 89.Ali Khan A, Hansson J, Weber P, et al. Comparative secretome analyses of primary murine white and brown adipocytes reveal novel adipokines. Mol Cell Proteom. 2018;17:2358–2370. doi: 10.1074/mcp.RA118.000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gnad T, Scheibler S, von Kügelgen I, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516:395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- 91.Tseng Y-H, Kokkotou E, Schulz TJ, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whittle AJ, Carobbio S, Martins L, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klepac K, Kilić A, Gnad T, et al. The Gq signalling pathway inhibits brown and beige adipose tissue. Nat Commun. 2016;7:10895. doi: 10.1038/ncomms10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol. 2017;13:26–35. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 95.Poulos SP, Dodson MV, Hausman GJ. Cell line models for differentiation: preadipocytes and adipocytes. Exp Biol Med. 2010;235:1185–1193. doi: 10.1258/ebm.2010.010063. [DOI] [PubMed] [Google Scholar]
- 96.Chusyd DE, Wang D, Huffman DM, Nagy TR. Relationships between rodent white adipose fat pads and human white adipose fat depots. Front Nutr. 2016;3:10. doi: 10.3389/fnut.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 98.Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 99.Blaszkiewicz M, Willows JW, Johnson CP, Townsend KL. The importance of peripheral nerves in adipose tissue for the regulation of energy balance. Biology. 2019 doi: 10.3390/biology8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang QA, Scherer PE. The AdipoChaser mouse: a model tracking adipogenesis in vivo. Adipocyte. 2014;3:146–150. doi: 10.4161/adip.27656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wolfrum C, Straub LG. Lessons from cre-mice and indicator mice. In: Pfeifer A, Klingenspor M, Herzig S, editors. Brown adipose tissue. Cham: Springer International Publishing; 2019. pp. 37–54. [Google Scholar]
- 102.Rydén M, Uzunel M, Hård JL, et al. Transplanted bone marrow-derived cells contribute to human adipogenesis. Cell Metab. 2015;22:408–417. doi: 10.1016/j.cmet.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 103.Arner E, Westermark PO, Spalding KL, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]