Abstract
Metallomics is a rapidly evolving field of bio-metal research that integrates techniques and perspectives from other “-omics” sciences (e.g. genomics, proteomics) and from research vocations further afield. Perhaps the most esoteric of this latter category has been the recent coupling of biomedicine with element and isotope geochemistry, commonly referred to as isotope metallomics. Over the course of less than two decades, isotope metallomics has produced numerous benchmark studies highlighting the use of stable metal isotope distribution in developing disease diagnostics—e.g. cancer, neurodegeneration, osteoporosis—as well as their utility in deciphering the underlying mechanisms of such diseases. These pioneering works indicate an enormous wealth of potential and provide a call to action for researchers to combine and leverage expertise and resources to create a clear and meaningful path forward. Doing so with efficacy and impact will require not only building on existing research, but also broadening collaborative networks, bolstering and deepening cross-disciplinary channels, and establishing unified and realizable objectives. The aim of this review is to briefly summarize the field and its underpinnings, provide a directory of the state of the art, outline the most encouraging paths forward, including their limitations, outlook and speculative upcoming breakthroughs, and finally to offer a vision of how to cultivate isotope metallomics for an impactful future.
Keywords: Stable metal isotopes, Metallomics, Isotope metallomics, Geochemistry, Biomarkers, Disease diagnostics, Neurodegeneration
Introduction
The metallome as a research entity came into being two decades ago when the term was first coined in reference to the equilibrium concentrations and distribution of metal ions (and/or their total contents) in a cellular compartment, cell or organism [1]. This definition was subsequently expanded to denote the entirety of metal(loid) species present in a cell or tissue type [2]. Not to be left without a formal title, research aimed at studying the metallome and its relationship with other –omes (ensembles of like biological entities) and the natural environment was quickly coined Metallomics [3]. Further information on the linkages between the metallome and the various other biological –omes, the functional importance of metals in the body (e.g. as enzymatic cofactors) and further back history into the field are well covered in Mounicou et al. [4], Banci [5], Arruda [6], and Tanaka and Hirata [7]. While the metallome is inherently linked to the genome and proteome, it is distinct from both in that it is directly and significantly influenced by external factors, such as environmental forcings and availability, which themselves are fundamentally governed by geological processes [1]. From this vantage point, it is easy to understand why in the years following its inception as a field, bridges between metallomics and the geosciences were built to allow researchers from either camp to leverage the expertise and perspectives of the other. Perhaps, the two greatest examples of such bridging subfields are medical geology, which—broadly speaking—explores the public health impacts of geologic materials and processes (but generally without direct involvement in medical research) [8, 9], and isotope metallomics, which references the use of stable metal isotope geochemistry techniques in the health and medical sectors, i.e. exploring stable metal isotopes in biological contexts and as medical biomarkers [10–12].
Isotope metallomics is the focus of the current work because it is not separate from medical geology, but rather encompasses and expands upon it by exploring the geologic history behind element/metal availability in biospheres, quantifying their distribution in eco-bio-systems, and using state-of-the-art analytical instrumentation to understand and quantify the natural isotopic distribution of these same metals within these interconnected systems. The added perspective of natural stable metal isotope ratios yields new and independent means of understanding environmental inputs, while also commenting on underlying mechanisms both in the geological and biological realms. Exemplary in the latter context, natural stable metal isotope ratios have been used to: (1) unveil previously unaccounted for erythropoietic pathways for Fe and Cu [13]; (2) provide a novel quantitative description of Ca mineralization and de-mineralization in humans and highlight its diagnostic value in early osteoporosis detection in blood and urine samples (i.e. significantly prior to dual-energy X-ray absorptiometry, or DXA) [14]; and (3) potentially constrain the speciation of Cu in the amyloid beta (Aβ) plaques associated with Alzheimer’s disease (AD), along with confirming the increased production of Cu(I) associated with Aβ plaques [15]. Such examples clearly define the added value of natural stable metal isotopes in medical research; however, linking excursions in stable isotope compositions of biological materials with underlying biological function(s) is of course very complex. While most studies offer hypotheses in this regard, each topic and the field more generally will greatly benefit from continued research that will delineate where and when stable metal isotope ratios may act as empirical biomarkers of change, and when they can make the non-trivial leap to a marker of biological mechanism(s).
Nearly two decades on, isotope metallomics is coming of age at a time when methodological and analytical advancements have thrust us along new frontiers in terms of what can be accomplished. Among them, areas of research involving cell line cultivation and analysis (compartmental analysis), in situ (i.e. spatially resolved) elemental and isotopic mapping of biological materials, and the development of scalable techniques and workflows have all positioned themselves at the forefront of the field by pushing technical and analytical limits, as well as by capitalizing on the merger of expertise, instrumentation, and the respective ethos of both geo- and medical sciences. Here, we first briefly outline the underpinnings and history of isotope metallomics, and provide a roadmap of the field through references to comprehensive reviews and benchmark studies. Following this, we highlight promising contemporary approaches, each with a brief synopsis, including limitations and future outlook. Finally, we leverage this reflection to offer a speculative look at what may await us along the research horizon.
Stable isotope fractionation in biological materials, a crash course
Stable isotopes are atoms of the same element with different numbers of neutrons and therefore different masses that are stable through time (i.e. not radioactive). These differences in mass lead to an energy change when one isotope substitutes for another in a chemical bond [16–18]. Mass-dependent stable isotope fractionation, such as that seen for metals in biological processes, is—at its simplest—the redistribution of the isotopes of a given element as a function of their mass in a binary chemical process. Based largely on the pioneering work of Bigeleisen and Mayer [17] and Urey [18], a number of physical and theoretical descriptions of mass-dependent isotope fractionation exist [19–21], with all nominally focused on application in the geosciences. Perhaps the most accessible descriptions for the non-geochemist can be found in two complementary papers, Young et al. [22, 23], with the former discretely detailing and mathematically describing the two flavours of mass-dependent isotope fractionation, equilibrium and kinetic, and the latter paper extensively covering the physics of equilibrium isotope fractionation and providing a mathematical formalization of two-phase (binary) isotope fractionation that clearly details its dependence on temperature, the mass difference between isotope pairs, and the difference in force constants in each phase (i.e. bond stiffness) [23].
Very briefly, equilibrium isotope fractionation is the redistribution of the isotopes of an element during an exchange reaction between phases at equilibrium, and kinetic isotope fractionation is that which occurs during a unidirectional chemical process. Both processes produce similar results when the threshold of analytical precision and/or natural background variation is at or above ~ 0.01% (0.1‰, or 100 ppm) [22]. Slightly decreased analytical resolution—and especially heightened natural variation between samples—are often encountered in biological materials relative to geological ones, and therefore within the context of isotope metallomics and this review, broadly speaking they can be (and are) collectively referred to as isotope fractionation.
Isotope metallomics owes its applicability in medicine to the fact that isotope fractionation is subject to the laws of physics regardless of medium—geological, biological, or otherwise. Since most biological processes of interest occur within the same temperature range (approximately 25–37 °C in mammals), for a given element, isotope fractionation—e.g. that between bio-minerals and/or ligands in the body—is largely determined by the availability of bond sites and the relative difference in characteristic bond stiffness between them. In general, the heavier isotopes of an element will concentrate in compounds where that element forms the strongest bonds [21, 23], and lighter isotopes travel (e.g. diffuse) faster and form weaker bonds, leading to light isotope enrichment in kinetic reaction products [18, 20–22], such as during movement up a food chain (e.g. from plants to humans) [14, 24, 25]. Relevant examples where isotope fractionation(s) can be induced—and potentially measured—include:
When there is a substantial change in the isotopic composition of a major biological input, such as (1) diet and/or (2) intestinal absorption, where light (or heavy isotopes) may be preferentially absorbed due to bond strength differentials [26, 27].
When an appreciable change in internal bonding environment takes place, such as in the formation of tumours/lesions associated with various diseases [15, 28–31].
When a substantial bodily reservoir with an isotopically distinct signature (again due to bonding environment) flushes into another, such as Ca flux into biological fluids (e.g. blood, urine) during bone loss [14, 32, 33].
Lastly, it is worth noting that in isotope metallomics, it is commonly the stable metal isotopes of “non-traditional” isotope systems (elements heavier than S) that are being investigated. Due to physics, i.e. the lower % mass difference between isotopes, heavier elements (with heavier isotopes) tend to encounter lower magnitude isotope fractionations both in biological and geological settings [23]. However, ranges in bonding environments—that is, the relative range in bond strength and availability observed—function independent of element masses and therefore the two do not scale proportionately. As a consequence, metal isotope fractionations for heavier elements within a single biological system often cover the entire spectrum (or more) of that observed in geological settings [34, 35]. For example, Li isotopes in mammals (e.g. sheep) vary by ~ 40 ‰ [36], lower in comparison to a range of 60+ ‰ in terrestrial geologic samples [37]. Meanwhile, Cu and Zn isotopes vary by only around 1‰ in nearly all geochemical settings [38], and this range is matched or exceeded in biological systems [31, 34, 35]. This offers a relatively high signal-to-noise ratio: exogenous geological inputs (e.g. local soil/fluids/rocks) are often more stalwart in terms of isotope compositions for heavier metals such as Cu and Zn (among others), and therefore their isotope fractionations in biological systems can act as statistically significant biomarkers of change. These biomarkers can act in the strictly empirical sense—as an “early alarm”—or can be linked by inference to underlying biological mechanisms. It is worth discretizing the two, as the former case implies the use of stable metal isotopes simply as diagnostic indicators of disease, while the latter implies their use as biomarkers of disease mechanisms.
A brief history of isotope metallomics
Although the individual importance of isotope geochemistry, and that of metals in the biological sphere (e.g. bioinorganic chemistry), have been recognized and studied as contemporary sciences since at least the mid-twentieth century, the importance and potential of combining them are relatively recent developments reaching back no more than about two decades [5, 12]. Around the turn of the millennium, only a couple of readily available works were published that today would be considered isotope metallomics articles (as defined by Albarède et al. [12]), namely Skulan and DePaolo [39] investigating Ca isotope fractionation in animal tissues, and Walczyk and von Blanckenburg [40] characterizing Fe isotopes in human blood. These early works highlighted the raw potential of using stable metal isotopes to better understand biological systems and paved the way for future growth in this area of research. The same period of time saw the development of multi-collector inductively coupled mass spectrometry (MC-ICP-MS), with significantly reduced analytical error for isotope ratios (e.g. relative to quadrupole ICP-MS, or Q-ICP-MS, due to simultaneous isotope data collection), and increased sample throughput (relative to thermal ionization mass spectrometry, or TIMS). This era also saw the development of improved chemical separation techniques—e.g. ion exchange chromatography, or IEC—and together these advancements made it possible to more easily and reliably measure stable isotope ratios for elements heavier than sulphur [41]. Within just a few years, new research emerged exploring metals in biological systems, still including Ca [42–44] and Fe [45–48], while expanding to include Zn [25, 49–51], and later Sr [52], Cu [13, 34], and Mg [53, 54] (a non-exhaustive list). Current investigations tend to focus on Ca, Fe, Cu, and Zn. In the case of Ca, this is largely because it is a major metal constituent of the human body (e.g. in bone) and is involved in numerous biological processes [14, 32]. In the case of the latter three metals (Fe, Cu, Zn), they are often found as enzymatic cofactors and are therefore inherent to both normal and diseased bodily function, and from a pragmatic standpoint (with varying efficacy) they can be extracted and isolated from various matrices using a single purification scheme [34, 55].
Isotope metallomics is now reaching a stage in its life cycle where individual articles abound, and retrospectives and summaries of past works are viable and in demand. Table 1 provides—to the best of our knowledge—the first comprehensive directory of research articles, reviews, and books in isotope metallomics and related fields, with the aim of providing a reference point from which readers can navigate to any subject or sub-field of interest, however broad or esoteric.
Table 1.
A chronological reference list of articles relevant to isotope metallomics
| Article | Focus and notable information | References |
|---|---|---|
| Haraguchi (2004) | The original article coining and describing metallomics as a discrete field of study related to biometals, and intrinsically linked to genomics and proteomics. Notably includes: a table of typical metalloenzymes and metalloproteins, along with their corresponding biological function(s); a schematic diagram of the biological system, its various "-omes" and their inter-relationships; recognizable sub-disciplines within metallomics and their associated analytical techniques | [3] |
| Mounicou et al. (2009) | The canonical summary/review of metallomics, largely focusing on its relationship to other "-omics", areas where metallomics-based research can be applied, and to avenues of analyses. Notably includes: relationship trees for (1) metal species in biological environments, (2) experimental approaches, and (3) element-specific detection protocols; numerous analytical and workflow diagrams | [4] |
| Banci (2013) | Book comprehensively describing metals in biological systems. Notably includes: a historical account of metallomics, with definitions/classifications of various "-omes"; entire chapters dedicated to the element-specific descriptions of homeostasis, uptake, trafficking, etc. for Na, K, Mg, Ca, Mn, Fe, Co, Ni, Cu, Zn, and Mo | [5] |
| Fujii et al. (2014) | Research article covering density functional theory (DFT) estimates of isotopic fractionation among species relevant in biology for Fe, Ni, Cu, and Zn. Note: DFT theoretical estimates can be found for Ca in Moynier and Fujii [58] | [59] |
| Albarède (2015) | Review highlighting the virtues of isotope systems such as that for Ca, Fe, and Cu as medical diagnostic tools, and their use in constraining biological processes/mechanisms | [10] |
| Costas-Rodríguez et al. (2016) | Ca, Cu, Fe and Zn isotopic composition and variations in biofluids, and their potential as prognostic/diagnostic tools. Notably includes: purification protocols for Ca, Cu, Fe and Zn for subsequent analysis via MC-ICP-MS; isotopic compositions of common standards and reference materials for Ca, Cu, Fe and Zn; biofluid and tissue isotopic compositions for Ca, Cu, Fe and Zn; biofluid and tissue isotopic compositions for Fe and Zn in mice, sheep and pigs (Fe only); element-specific forecast for prognostics/diagnostics for Ca, Cu, Fe and Zn, including benchmark examples | [69] |
| Albarède et al. (2016) | One of two follow-ups to Albarède (2015), focusing on Cu, Zn and S isotopes in medicine and applicability to cancer diagnostics via blood analysis. Notably includes: theoretical fractionation factors for Zn and Cu; stability constants for Cu and Zn chelation by relevant carboxylates; schematics of Zn, Cu and S trafficking in a generalized cell; Cu and S isotope results in cancer | [11] |
| Albarède et al. (2017) | Largely an expansion of Albarède (2015), Albarède et al. (2016), Costas-Rodriguez et al. (2016) and other works. Notably includes: an overview of isotope fractionation; a compilation of blood and tissue isotopic compositions for Ca, Zn and Cu in mice, sheep and humans; element-specific biochemistry, homeostasis and medical utility of isotopes for Ca, Fe, Zn, Cu and S | [12] |
| Pozebon et al. (2017) | A (follow-up) review dedicated solely to the use of LA-ICP-MS on biological samples, with a focus on element quantification. Notably includes: a massive compilation (~ 12 pages) of brief summaries of analyses in biological tissues to date across the periodic table; a section covering isotope ratio measurements via LA-ICP-MS to date; a critique of the current state of the art, its limitations and outlook | [80] |
| Jaouen and Pons (2017) | Overview and forecast of the use of non-traditional stable isotopes for bioarchaeology, itself an offshoot of metallomics. Notably includes: a periodic table of elements broken down by historical use and application; abundances, metal type and common delta notations for Ca, Cu, Fe, Mg, Sr and Zn; a diagram of basic internal and external factors influencing isotope compositions for H, C, N, O, Mg, S, Ca, Fe, Cu, Zn and Sr; diagrams of trophic level effects on isotope compositions | [25] |
| Arruda (2018) | Book covering numerous analytical techniques and advances in metallomics. Notably includes: a biological periodic table outlining essential metal and non-metal elements; a chapter (Ch. 2) dedicated to neurodegeneration and numerous mental diseases; a chapter (Ch. 7) dedicated to state-of-the-art bio-imaging techniques, including laser ablation (LA)-ICP-MS; a chapter (Ch. 10) dedicated to element and species specific analyses at the cellular and sub-cellular level | [6] |
| Paredes et al. (2018) | An in-depth investigation of isotope analysis of U, Cu and Zn at nanogram levels in cultured human cells. Notably includes: a detailed schematic for a stable high-efficiency introduction system; a careful review of procedural blank contributions and conditions needed for “fit-for-purpose” data outcomes | [76] |
| Tanaka and Hirata (2018) | Review exclusively detailing stable metal isotopes—Ca, Fe, Cu and Zn—in biological materials as metabolic tracers. Notably includes: schematics for possible linkages between Fe and Cu metabolism(s) and their isotopes; a large compilation of Fe, Cu and Zn isotope compositions in human tissue samples | [7] |
| Lobo et al. (2018) | Critical review of isotopic analysis in biological samples via LA-ICP-MS. Notably includes: a table of elements for which isotope ratios have been measured via LA-ICP-MS; sections outlining the utility various isotope systems in disease diagnostics, evolution and paleoenvironment reconstruction | [93] |
Promising future directions
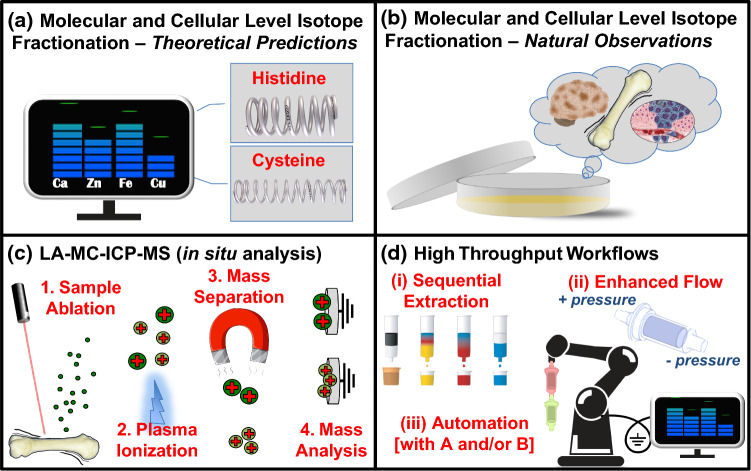
It has become quite clear that isotope metallomics is a field of research presenting numerous opportunities in the medical sector, and these have been partially realized through numerous methodological and analytical approaches. It is sensible to take stock of emerging methodologies and analytical platforms that show the most potential in terms of returning meaningful and impactful results through current and future advancements. In this respect, three main areas of growth come to the forefront: (1) theoretical and natural isotope fractionation at the molecular and cellular level; (2) in situ, spatially resolved methods for quantifying stable metal isotope compositions in biological materials; and (3) the development of integrated and scalable techniques and workflows (Fig. 1).
Fig. 1.
Future-shaping research areas: a theoretical predictions of isotope fractionation at the molecular and cellular level; b measurements of natural stable isotope fractionation at the molecular and cellular level; c in situ analyses in soft tissues (e.g. LA-MC-ICP-MS); d high-throughput techniques and scalable workflow
Theoretical isotope fractionation at the molecular and cellular level
In essence, theoretical estimates of isotope fractionation in biological relevant environments, and their contextual placement within isotope metallomics, can be largely traced back to a select few publications: Mg [56, 57]; Ca [58]; Fe, Ni, Cu, and Zn [59]. Though other relevant estimates can be located (with some difficulty) [60], these few publications represent the majority of our understanding of theoretical isotope fractionation for stable metal isotopes in biological environments. Small though it may be, in tandem with measurements on natural samples, these calculations allow us to interpret our observations in a biological context and constrain underlying biological processes that manifest isotope fractionations. A topical example is the use of Zn isotopes in mouse models of AD, where a shift towards heavier Zn isotopic composition in the brains of AD mice is interpreted as a shift from cysteine-rich Zn binding to histidine- and glutamate-rich binding, with the latter two both preferentially sequestering heavy Zn isotopes in accordance with theoretical calculations [30].
Limitations, outlook, and future advances
In brief, without theoretical calculations that can prescribe the baseline isotope fractionations that are likely to be encountered in measurements of biological materials within a given context, and delineate between likely bonding environments, we are limited in our ability to interpret data beyond empirical relationships. This undermines our ability to link observations back to biological processes, and therefore stifles our ability to arrive at scientifically rigorous and medically valuable conclusions, especially with regard to underlying pathological mechanisms. Clearly, the main limitation here lies in the motivation and expertise to conduct these calculations, as to date this has been the effort of a rarefied few.
The outlook is positive, however, as the necessity of these calculations, coupled with increased awareness and interest in isotope metallomics, ensures that this will (read: must) be an area of focus in the immediate future. Broadening the network of researchers generating theoretical estimates of isotope fractionation, and further data vetting (e.g. separate research entities computing the same estimates), will validate existing literature in this area and bring clarity to where and why theoretical estimates and natural measurements differ.
Theoretical estimates of biological isotope fractionation, coupled with natural observations, will allow us to peer behind the veil of human bodily function and diseases and characterize the biological mechanisms that are at their roots, and thus move more confidently from empirical diagnostic tools to direct biomarkers of biological mechanisms. Specific areas of focus could include:
An expansion of theoretical estimates to include metal isotope redistribution between full proteins. Currently, most calculations are conducted at amino acid level, as this provides less calculation-intensive approximations [59]. However, such calculations make the strong assumption that electromagnetic interactions between metals and proteins can be simplified by constituent amino acids. Computing isotope effects for metals bound to full proteins can provide a means to further understand and quantify the biological mechanism(s) at play [61].
The comparison of theoretical isotope fractionation to cell culture studies targeting sub-cellular isotope fractionation in neuroblasts, AD-associated proteins (e.g. Aβ and tau), and reactive oxygen species (ROS). Such investigations will play a pivotal role in uncovering the mechanisms of AD and other neurodegenerative diseases [62, 63].
Comparative analyses of theoretical and empirical observations of metal isotope fractionation during aggregation of widely implicated proteins for neurodegenerative diseases, e.g. superoxide dismutase 1 (SOD1) and TAR DNA-binding protein of 43 kDa (TDP-43) [64, 65].
The development of theoretical models for induced pluripotent stem cell (iPSC) lines, and the further development of their natural counterparts, which mirror human diseased cells and tissues. Such developments will greatly enhance our ability to theoretically and experimentally constrain stable isotope fractionations associated with diseases and their therapies at large (e.g. cancer) [66].
Combined theoretical and empirical observations of isotope fractionation along metal-specific pathways, such as Cu along affinity gradients towards its final cellular compartmentalization, providing a clearer understanding of disease mechanisms while de-convolving the complexities of Cu isotope fractionation in biological systems [67].
Theoretical and empirical characterization of metal isotope fractionation between well-known bodily reservoirs, such as the metalloproteins SOD and metallothionein (MT) [68].
Natural isotope fractionation at the molecular and cellular level
All recent reviews in the field of isotope metallomics have highlighted the importance of investigating stable metal isotope fractionation at the protein (molecular) and cellular level [11, 12, 69]. This consistent recurrence is largely fuelled by the fact that amino acid, protein-, and cell-specific data are needed to better constrain the biological controls on isotope fractionation, as most measurements to date—in biofluids, hard and soft tissues—are in essence still bulk measurements, since each tissue is a melange of organic structures.
Work in this area is already underway, with the last 5–10 years seeing a growing number of cell-specific investigations, largely aimed at determining Fe, Cu, and Zn isotope fractionation at the cellular level. Iron isotope fractionation in an intestinal cell line (Caco-2) has been investigated to understand cellular Fe uptake and transport mechanisms, adding independent constraints on light Fe isotope enrichment during both absorption and transport [70]. Copper and Zn have been studied in single-strain bacteria to understand adsorption vs. intracellular uptake and fractionation mechanisms (e.g. Cu reduction, Zn complexation vs. free Zn) [71, 72]. Such studies are relevant because: (1) bacteria are myriad in the gut microbiota and influence intestinal absorption, and (2) because key metal-binding proteins (such as MT) are relatively conserved between fungi, plants, and animals. Zinc isotopes have been studied in harvested breast cancer cells, with tumours sequestering light Zn isotopes, suggesting possible Zn delivery via metallothionein, and furthermore implicating Zn isotopes as a potentially viable early biomarker for breast cancer [29]. The largest number of cell-specific isotope metallomics studies to date have focused on Cu isotopes, including: (1) heavy Cu isotope enrichment in several human cell lines (HepG2) induced by hypoxic tumour conditions relevant to hepatocellular carcinoma (HCC) [73]; (2) evidence of heavy Cu isotope enrichment in a yeast proxy for human HCC associated with lower Cu reductase activity in tumours [74]; (3) Cu isotope fractionation associated with oxidative stress in a human cell line, demonstrating heavy Cu isotope enrichment with increased oxidative conditions, with strong implications for numerous diseases, e.g. cancer, liver, and neurodegenerative diseases [75]; (4) Cu isotope fractionation associated with oxidative chelation of Cu by cytosolic lactate in breast cancer [28]; (5) Cu isotope fractionation at the cellular and subcellular level in neuron-like human cell cultures, revealing that neuronal differentiation affects the Cu isotope fractionation associated with cellular Cu uptake (this study is also accompanied by Cu mapping via LA-ICP-MS) [62]; (6) Cu isotope compositions between two metalloproteins (SOD and MT) responsible for oxidative stress mediation and with implications for AD diagnostics [68]; and (7) from a methodological point of view, high precision nanogram-level U, Cu and Zn isotope ratio determination in cultured human cells [76].
Limitations, outlook, and future advances
Somewhat similar to the problems facing theoretical calculations, the key issues currently limiting cell culture studies are the necessary intellectual and logistical requirements needed to bring such studies to fruition, the combination of which has created an academic bottleneck. The current (and short) catalogue of stable metal isotope fractionation cell culture experiments comes largely from only two laboratories (Ghent University and ENS Lyon). It takes appreciable time, intellectual and logistical resources to effectively conduct these experiments under requisite controlled laboratory conditions, to ensure that sufficient numbers of cells can be harvested to provide metal concentrations at high enough levels for subsequent analyses, to reliably measure their isotopic compositions (generally via MC-ICP-MS), and finally to collate all results—medical and geochemical—into a coherent interpretation.
The outlook for this area is promising and it is tempting to speculate that the next decade will see the incorporation of MC-ICP-MS facilities for stable metal isotope analysis in this research sector, and in the medical sector more broadly. In terms of stable metal isotope fractionation, the horizon of this research area largely goes hand in hand with that of theoretical estimates, as they are enmeshed in terms of their collective ability to constrain underlying biological mechanisms of disease. However, even without theoretical estimates of molecular and cellular isotope fractionation, we can measure isotope fractionations in cell line studies, develop empirical correlations, and use these correlations in tandem with existing medical literature and the basic tenets of isotope fractionation to make strong inferences as to the underlying cellular and subcellular processes at work [23]. This is of course a more qualitative approach, but is still of great utility.
In situ elemental and isotopic analysis in biological materials (LA-MC-ICP-MS)
Although there exist other means of attaining spatially resolved elemental and isotopic abundances, e.g. secondary ion mass spectrometry (SIMS), X-ray fluorescence (XRF) imaging and single-collector laser ablation ICP-MS (LA-ICP-MS), multi-collector laser ablation ICP-MS (LA-MC-ICP-MS) has established itself as the clear frontrunner for analysing stable isotope ratios in biological materials [77, 78]. The reasons for this primarily include: (1) high sensitivity, low detection limits (ppm level), and lower analytical error relative to LA-ICP-MS (simultaneous isotope data collection); (2) high spatial resolution down to the micron (μm) scale; (3) relatively high accessibility/availability; (4) high-throughput capacity that increases potential research productivity while driving down operational costs; and (5) the ability to map numerous element (metal) species simultaneously. Relevant reviews in Table 1 comprehensively detail advances in element quantification and conventional stable isotope analysis via LA-ICP-MS and LA-MC-ICP-MS, and therefore emphasis is given here to benchmark examples of stable metal isotope analysis using the latter means. However, much of the technical development work that has led to the possibility of stable metal isotope analysis via LA-MC-ICP-MS has been done in geological samples and standards (e.g. rocks and minerals, alloys), and/or for element quantification via LA-ICP-MS, so it would be remiss not to briefly mention important milestones. Hereafter, unless otherwise noted, references include studies involving LA-MC-ICP-MS.
The very first study to quantitatively map metals in biological materials was published over 15 years ago, in Kindness et al. [79] (LA-ICP-MS), where Cu and Zn concentrations were simultaneously mapped with high spatial resolution (μm- to sub-mm scale) and precision (2–6% for Cu) in thin sections of sheep liver, detailing heterogeneous distribution, mainly for Cu. Since then, an extensive amount of LA-ICP-MS and LA-MC-ICP-MS development and analysis have been done, and this is well covered in Table 1 of Pozebon et al. [80] and Table 1 herein. Some of the first stable metal isotope ratio analyses via LA-MC-ICP-MS were conducted for Fe and Cu isotopes in naturally occurring minerals commonly found in geologic ores (e.g. Cu oxides and sulphides) [81–84]. Many of the lessons learned from these early investigations and subsequent studies [85–87], namely how to avoid and/or correct for isotope fractionation induced by sample heterogeneity and/or the analytical protocol itself, and how to develop matrix-matched (i.e. like-for-like) standards, have been instrumental to the application of isotope analysis in biological samples by LA-MC-ICP-MS. Likewise, related work using LA-ICP-MS aimed at spatially quantifying isotopically labelled tracers (i.e. isotope doping in unnatural proportions) in biological media have further enhanced our ability to apply such techniques to natural stable isotope analyses, including: imaging Zn isotope ratios in rat brains [88]; mapping Zn isotope ratios in planktonic crustaceans [89]; imaging Fe, Cu, and Zn in mouse brains with Alzheimer’s disease (AD) [90]; and imaging Fe (and ferroportin, FPN) in human hippocampus tissue with AD [91]. This last study, by Cruz-Alonso et al. [91], is of particular note as the simultaneous imaging of Fe and FPN renders this a metallomics–proteomics hybrid imaging technique.
Standing on the shoulders of almost two decades of technique development, this area of research has now entered a phase where exploratory studies are being published on stable metal isotope analysis in biological media. Of particular note, Resano et al. [77] successfully determined Cu isotope compositions of dried urine spots via LA-MC-ICP-MS, and these results strongly suggest that the further development of such analysis could serve as a biomarker for Wilson’s disease (WD), because untreated WD patients displayed isotopically light Cu in their urine relative to normal and treated patients. Indeed, similar approaches may be useful in diseases such as Parkinson’s, where Cu dyshomeostasis is implicated [92]. Furthermore, Tacail et al. [78] developed an LA-MC-ICP-MS technique for Ca isotope determination in biological apatite (i.e. bone mineral) with high potential for detecting changes in dietary and/or physiological conditions; this article also conveniently catalogues numerous previous works in stable isotope ratio determination via LA-MC-ICP-MS in its “Introduction”.
Limitations, outlook, and future advances
As is reiterated in nearly every publication on stable metal isotope analysis via LA-MC-ICP-MS, the major limitation of this area of research is overcoming various sources of analytical error, namely instrumental and methodological sources of isotope fractionation that distort or entirely overprint actual sample isotope signatures. Chief among these factors is the use of matrix-matched standards, i.e. standard materials with known elemental isotope compositions that are compositionally, morphologically, and texturally similar to the samples being analysed [78]. The importance of matrix matching is based on the idea that the closer the standard properties are to that of the samples, the more thermal effects, ablation physics, particle size distribution, plasma load, and differential ionization can be cancelled out (see relevant references in Table 1). Matrix-matched standards with known and/or certified isotopic compositions allow for the correction of instrumental/methodological sources of error and provide reference points to be reproduced to validate sample data sets. Additionally, LA-MC-ICP-MS provides a transient signal to the mass spectrometer that requires additional data processing, and transient signals sometimes come with deteriorated precision relative to solution-based MC-ICP-MS; however, the consequences of this are generally less paramount than those associated with matrix issues.
Once a collective effort is put in place to standardize, and thus commercialize/industrialize these techniques, the outlook and applicability of this research area will likely see significant growth, as the spatial resolution and tremendous high-throughput capabilities of these techniques are well suited to the demands of biological sample analysis (e.g. clinical work), and both features (spatial resolution, high throughput) are at the top of both scientific and practical priorities.
Future advances in this area will include the further development and wider-spread availability/use of recent technical advances that attenuate the impact of differential standard-to-sample matrices, including: shorter wavelength lasers for narrower particle size distributions [93]; shorter pulse times via femtosecond (vs. nanosecond) lasers for reducing thermal effects and particle size distribution, thereby increasing isotope ratio precision and accuracy [84, 93, 94]; and laser ablation cells designed to minimize signal variability caused by point-specific cell parameters (e.g. the HelEx ablation cell) [78, 86]. With the aid of such technical advancements, future research will likely see spatially resolved mapping of stable metal isotopes at the μm- to sub-mm scale in biological materials, such that we will be able to determine, in situ, the isotope fractionation between spatially adjacent normal and diseased cells, a feat that is currently not possible. An exemplary use of such future technology could be the determination of Zn (and Cu) isotope compositions between senile plaques and adjacent normal brain tissue(s) in AD, and/or for Cu (and S) isotope compositions between normal and tumorous cells in various cancers. Parallel advances in complementary technologies such as single-cell genomics and proteomics offer future opportunities to correlate spatially resolved isotopic changes with detailed molecular information.
High-throughput techniques and integrated workflows
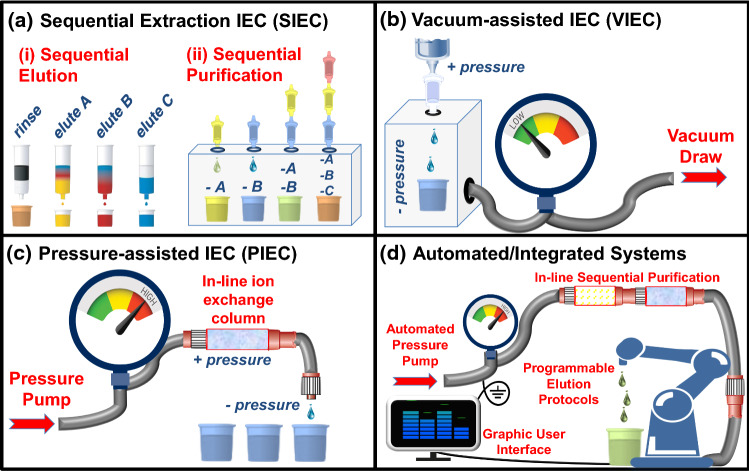
Novel stable metal isotope measurements within a given study generally lies within the low tens of samples (rarely hundreds), especially when conventional techniques are employed, i.e. hot plate sample dissolution and manual ion exchange chromatography (IEC) [35, 62, 70]. In the medical sector, however, much higher sample cohorts—often in the thousands to account not only for different participants, but also temporal collection of samples—are a natural consequence of clinical research studies and are required for robust statistics, especially in regard to prognostic, diagnostic, and/or therapeutic tools. Although often contextually marginalized as an area of future development and focus in isotope metallomics, sample throughput is perhaps the single largest obstacle in the way of successfully merging isotope geochemistry and medical applications and is therefore emphasized here (Figs. 1d, 2).
Fig. 2.
Strategies for high-throughput IEC: a sequential element extraction (SIEC); b vacuum-assisted IEC (VIEC); c pressure-assisted IEC (PIEC); and d automated/hyphenated systems
By far, the most common means of generating samples for isotope measurements has been and continues to be through conventional “wet” isotope chemistry, where the common workflow includes (1) sample digestion/dissolution via strong acid attack, (2) element isolation via ion exchange chromatography (IEC) using gravity-driven column chemistry, and (3) analysis of purified solutions via MC-ICP-MS. The first and last steps in this generalized workflow are relatively quick (from a geochemistry perspective, but relatively slow from a biomedical perspective), from less than a day up to a few days (depending on samples and instrument temperament); it is the step in the middle—IEC—that is truly rate limiting.
Ion exchange chromatography (IEC), a crash course
Conventional IEC includes cleaning and packing plastic (usually polypropylene, PP) or poly-fluoroalkyl (PFA) columns with cationic or anionic resin which is held in place by a frit (a porous membrane, usually PFA or PP) that allows fluids to pass through under the force of gravity; this traditional technique is hereafter referred to as gravity-assisted IEC, or GIEC. Columns are vertically fixed in a column rack, and often pre-cleaned by consecutively passing through reagents that will be used during IEC (2–3 × in most cases). Samples are loaded onto columns in a prescribed reagent solution (eluent) that allow the element(s) of interest to strongly bind to the resin (via ionic bonding), while matrix elements are flushed out during loading and/or subsequent rinsing steps. Once all matrix elements have been rinsed off the columns, collection beakers (usually PFA) are placed underneath the columns and another prescribed reagent is used to quantitatively elute the element of interest (the eluate). Occasionally, this solution can go straight to the MC-ICP-MS for analysis, but often it is evaporated to dryness and reconstituted in a dilute acid that is compatible with the sample introduction system of the instrument (commonly as element nitrates in ~ 2% HNO3 solution). In the ideal scenario, samples are loaded and all matrix elements are rinsed off in one eluent, then the isolated element of interest is completely eluted in a second eluent, colloquially referred to as on/off or stick/no-stick separation chemistry. The appropriate IEC protocol for a given project is determined based on sample type and the average concentration of the element of interest within them, for example low element concentration samples require the lowest procedural blanks (contamination). In such cases, overall efficiency is a secondary priority and vice versa for samples with copious amounts of the element of interest.
Flow rates of reagents during GIEC are virtually unreported in the literature, but vary from tens to low hundreds of microliters per minute (μL/min), with custom-made PFA columns often being the slowest [35, 38, 95–98]. Since cleaning, rinsing, and elution steps often require up to tens of millilitres of reagents(s), GIEC can take hours to days to complete a single column protocol, and sometimes up to a week or more for multi-stage, multi-column protocols. It is also worth noting that flow rates in GIEC often vary within the confines of a single protocol (and between columns), due to the type, amount, and packing of resin, column diameter, reagent viscosity, frit characteristics, and other variables. These conflating issues can lead to significant variations in chemical separation efficiency both within a given protocol and even within the same column.
Strategies for high-throughput IEC
The procedural bottleneck created via the use of conventional GIEC columns, along with its exceedingly low sample throughput power, has led a number of isotope geochemists to pursue various means of expediting the IEC process, each with their own advantages and disadvantages, all of which are graphically illustrated in Fig. 2 and briefly detailed here with extensive referencing to literature examples.
Sequential element extraction IEC
The most straightforward strategy employed to augment overall IEC time is to sequentially extract more than one element from the same GIEC protocol (hereafter SGIEC), and this strategy has been employed from very early on (Fig. 2a). Notably, the SGIEC technique developed by Maréchal et al. [41] has largely laid the groundwork for subsequent techniques aimed at recovering purified Fe, Cu, and Zn solutions from a single protocol, both in geological and biological samples [13, 34, 38, 98], with one of the most recent iterations of this SGIEC strategy extracting Fe, Cu, Zn, and Cd from blood samples with a single gravity column for subsequent analyses via MC-ICP-MS [55]. This protocol—among others (e.g. Moynier and LeBorgne [95]; Lanping et al. [99])—was further refined for the lowest possible sample, resin, and acid volumes needed for effective separation of the elements of interest and subsequent analyses. This strategy—sometimes referred to as miniaturization—is essentially protocol optimization taken to its limits and is common to most protocols aiming for high throughput, and therefore is noted here but not further elaborated upon.
In addition to reduced procedural time relative to GIEC, the main advantages of SGIEC are: (1) the reduction in the requisite sample mass needed, as essentially the maximum mass (or volumes, e.g. blood) needed is equivalent to the minimum mass required to reliably analyse the least abundant element; and (2) the reduction in the mass/volume of acids needed, often lowering contamination (blanks); and (3) the reduction in preparation time and consumables, which reduces overhead costs. Finally, a defining advantage sometimes allotted by lower flow rates, e.g. in high aspect ratio PFA columns, is that this can garner better element isolation from matrix elements and/or other eluted elements of interest [96].
The main disadvantages of SGIEC are: (1) sequential extraction protocols are counter-productive when only a single element is targeted; (2) the element combinations possible are beholden to resin properties and are therefore finite; (3) sequential extraction techniques often require higher overall reagent volumes for all but the first element eluted, and therefore can induce higher blanks for later eluted elements (as more reagent has passed through the column prior to elution); and (4) sequential extraction techniques sometimes require a second purification step (such as a repeat SGIEC column pass) for one or more elements, because priority may be given to multi-element extraction over complete single-element isolation.
Vacuum-assisted IEC
Vacuum-assisted IEC (VIEC) is accomplished by creating a pressure differential between the top and bottom of the column, where reagent solutions at higher pressure (top) are pulled to the lower pressure end (bottom). In practice, most studies generate this pressure difference by using an external vacuum pump (usually electric, but Venturi pumps are possible) and a vacuum box, where the columns are fit into the top of the vacuum box creating an airtight seal, with the uppermost portion of the column in open air (for adding sample and reagent solutions) and the bottom portion sealed inside the box where the pressure is decreased to a prescribed level by an attached vacuum hose (Fig. 2b).
To date, there is only a relatively small body of work utilizing VIEC, much of which employs sequential element extraction via VIEC (SVIEC). One of the earliest studies, Pourmand and Dauphas [100] explored the element behaviour of 60 different elements in a SVIEC setup. Along with providing a wealth of information for many elements in a single article, this study provides strong evidence for the use of such techniques for the purification of Ca, Lu, Hf, U, and Th for subsequent isotope analysis via MC-ICP-MS [100]. Similarly, SVIEC techniques for Np, Am, Th, U, Pu, and Cm separation have been developed with applicability to urine samples [101, 102]; Pu has been successfully separated using VIEC in water samples [103]; and a VIEC method for Sr purification from environmental samples has been established [104]. This latter study, Wall et al. [104], includes the added innovation that the eluted Sr solution is ready for MC-ICP-MS analysis without the need of evaporation and re-constitution, a workflow modification that single-handedly reduces procedural times by up to 1 day. Of particular note regarding single-element VIEC, Lanping et al. [99] developed a single-step Ca separation protocol that can be applied to both geological and biological samples.
A major advantage of (S)VIEC relative to GIEC is that essentially the same protocol can be adapted while dramatically decreasing processing times, thereby reducing sample processing times from days or weeks down to hours. The other key advantage of VIEC is that it protects the purified element solutions, both by sequestering them in an isolated box (the vacuum box) and by having the external force located downstream of the IEC columns themselves, mitigating exogenous contamination.
The main disadvantages of VIEC are that it requires a significant amount of initial setup relative to GIEC, including: (1) the purchasing of all auxiliary components (vacuum box, vacuum pump, tubes and fittings); (2) vacuum/flow rate calibration; and (3) dedicated laboratory space that can accommodate an electric vacuum (or Venturi) pump, which can be a significant source of laboratory contamination (not to mention noise), or an external pump location (as pumps generally cannot be located in clean rooms) and additional vacuum lines plumbed to the laboratory.
Enhanced pressure-assisted IEC
In pressure-assisted IEC, PIEC, an external positive pressure is applied to one end of a column or cartridge (inlet, “top”) that forces eluents through the column and out the other end (outlet, “bottom”) (Fig. 2c). At present, there are two prominent and commercially available HPIEC varieties that have been used for metals separation—the Dionex™ High performance ion chromatography (HPIC) systems from Thermo Fisher Scientific and prepFAST-MC™ automated chromatography systems from Elemental Scientific (ESI®). Regardless of brand, the general workflow is relatively simple and conserved: a piston-driven pump or syringe pushes reagent fluids (usually via pressurized air buffers) through a network of chemically resistive circuitry (e.g. PFA), including an in-line IEC column (often called a cartridge) and all or most steps are automated and software driven (Fig. 2d). The in-line circuitry is connected to eluent reservoirs for cleaning, rinsing and elution, and an autosampler serves the dual function of drawing up sample solutions for introduction to the column and depositing eluates into collection beakers.
One of the earliest studies utilizing PIEC, Meynadier et al. [105], developed a protocol focused on purifying Sr in river water samples for subsequent isotope analysis (via thermal ionization mass spectrometry, TIMS). Though not explicitly addressed, the details of this work hinted at the viability of PIEC for sequential extraction of Ca and Sr purification, foreshadowing future work. Indeed, more recently a sequential extract PIEC (SPIEC) method has been developed for the separation of both Ca and Sr from various sample types, including seawater, rock, and biological hard tissues (bone ash and llama bone), for subsequent isotope analysis via MC-ICP-MS [106], along with additional methods for Ca (and Mg) separation via PIEC [107–109]. Along with the development of other SPIEC protocols for Sr–Pb–Nd and Sr–Pu–Am [110, 111], recent years have seen the development of PIEC protocols for Cu purification in biological samples for subsequent isotope analysis, namely Enge et al. [112] and Yuan et al. [113], with the latter study further including an SPIEC protocol for Sr–Nd, and in a novel leap forward, using a custom-built pressure-driven apparatus.
An inherent feature of most PIEC protocols is automation, and this generates numerous advantages for PIEC protocols over all previously described IEC methods. Automation removes the human component of IEC, mitigating sources of methodological error and limiting the potential sources of contamination that arise during manual IEC (i.e. both GIEC and VIEC), much of which in fact comes from laboratory gloves [114, 115]. Furthermore, reported sample processing times via PIEC are approximately 2–5 × faster than GIEC methods, and overall efficiency is even greater than these face value estimates, given that it is possible to conduct manual work contemporaneously.
The two main disadvantages of PIEC are the initial costs and calibrations necessary to establish a method. The average cost of a commercially available PIEC system (plus peripherals) pales in comparison to the average price of an MC-ICP-MS; however, automated platforms are often considered non-essential and thus their priority scales proportionately. Furthermore, commercial (and custom) PIEC systems are complex and it can take considerable time (6–12 mo.) to on-board equipment, validate methods, and generate reliable and reproducible results. That being said, the dearest and perhaps most overlooked cost of any laboratory is personnel, and therefore a strong argument can be made that PIEC systems quickly pay for themselves.
Limitations, outlook, and future advances
At present, the largest—and nearly singular—limitation of high-throughput IEC is that every conceivable protocol is beholden to the properties of the chromatographic resin being used. Since the properties of most presently available resins degrade significantly after flow rates exceeding ~ 1–2 mL/min during elution steps (~ 6 mL/min for cleaning and rinsing), improvements in IEC protocol efficiency using such resins are inherently limited. However, the vast majority of IEC protocols currently used for isotope geochemistry generally do not exceed a few hundred μL/min, and thus there is ample room to further reduce IEC times.
We tentatively speculate that the next frontier in this research space will be the use of ion exchange membranes during element purification for subsequent isotope analysis. Currently, solid-state ion exchangers are being developed largely for use in water purification and/or heavy metal extraction [116], and their potential has yet to be realized in the realms of isotope geochemistry and isotope metallomics. Relative to traditional beaded resin chemistry, ion exchange membranes have multiple significant advantages, including: (1) binding capacities in ion exchange membranes are generally far superior to that of beaded resins [117]; (2) binding capacities are in general independent of flow rates, and thus volumetric throughput can be up to an order of magnitude higher [117, 118]; and (3) the size and shape of membranes do not affect their properties (unlike resin-packed columns), and therefore their application is easily scalable to commercial/industrial levels.
Towards a collectively meaningful future
There are very few research fields that are more inter-disciplinary than isotope metallomics. Future progress will require sharing techniques, standards and other methodological aspects, so that the relative strengths of individual fields can be leveraged towards collective goals, ultimately allowing isotope geochemistry techniques (e.g. IEC and MC-ICP-MS analyses) to find their way into medical research facilities, and vice versa allowing biomedical approaches and practices (e.g. size exclusion chromatography, cell culture experiments) to be integrated into isotope geochemistry labsoratories.
Theoretical, molecular, and cellular level isotope metallomics research is conducted either via computer or already in medically oriented laboratories, and therefore few pragmatic changes are needed for cross-facility integration. The transplantation of in situ methods (e.g. LA-MC-ICP-MS) into medical facilities is largely limited by the infrastructure and intellectual requirements needed to effectively couple a laser system and an MC-ICP-MS instrument, and these limitations are mirrored when considering the integration of conventional medical practices into isotope geochemistry laboratories (e.g. cell culture experiments). The effective cross-pollination of these practices hinges on structural and cultural inclusivity within the geological and medical fields. The continued pursuit of research breakthroughs and cross-sectoral collaboration in isotope metallomics will facilitate and catalyse these changes.
For high-throughput techniques and integrated workflows there exist a more direct and clear path forward. This entails placing value on the translatability of IEC and instrument protocols into the medical sector (e.g. MC-ICP-MS), and developing a culture of mutual respect and understanding between fields. As researchers in the medical sector are already acquainted with large sample cohorts and the handling of sensitive and/or dangerous materials, many aspects of isotope metallomics research [in practice] are already a part of the skillset.
With that in mind, isotope geochemistry protocols can be adapted or engineered in such a way that they can be integrated into pathology clinics and medical research facilities. Broadly speaking, this includes considering the common practices and equipment of medical facilities and (where possible) constructing protocols than can be easily slotted into such environments. For example, while developing an IEC/MC-ICP-MS protocol for metal isotopes in biological materials, a viable strategy might include: (1) prioritizing simple element specific on/off IEC protocols and mitigating use of caustic reagents, thus allowing for the use of cost-efficient consumables (e.g. PP beakers instead of expensive PFA vials) and engendering fewer laboratory safety restrictions; (2) adapting/augmenting protocols to remove time-consuming steps such as evaporation and re-constitution; (3) tailoring IEC protocols such as sample masses and reagent volumes to specific sample types routinely used in medical diagnostics, e.g. blood and urine; and (4) emphasizing complete element purification, such that subsequent MC-ICP-MS analysis can be done with the minimum amount of mathematical corrections. Lastly and importantly, fostering inter-disciplinary and inter-laboratory communication and research will allow us to better construct clear, coherent, and broadly accessible approaches.
Concluding remarks
The utility of isotope metallomics, and more pointedly that of stable metal isotopes in the biomedical sector, has only very recently gone from an open-ended question to a mounting consensus, owing to nearly 20 years of research establishing the foundation of the field, and to a handful of breakthrough studies along the way that have brought attention, motivation, and funding to this research area. Of note, Ca isotopes have proven very useful as a diagnostic tool for bone loss, e.g. osteoporosis, and their biomarker manifestation predates that of traditional imaging techniques [119]; Fe isotopes have uncovered missing erythropoietic pathways in the human body [13]; Cu isotopes have proven useful as biomarkers for various cancers [28, 120], tissue disorders [121], genetic disorders [122], and neurodegenerative diseases [115]; and Zn isotopes have shown utility as a biomarker for prion disease and other neurodegenerative disorders such as AD [30, 123]. This body of work shows great promise, and continued research along these horizons will aid in the determination of when and where stable metal isotopes can be used as emperical diagnostic tools, and beyond that where they may be used as diagnostic indicators of underlying biological mechanisms of disease.
Isotope metallomics has been built on the tenacity and hard work of a surprisingly small enclave of researchers the world over, with the number of dedicated research groups being in the single digits. Taking this into account, the number of ground-breaking studies that have come out of this burgeoning field in such a short period of time attests both to its incredible potential and to the notion that this is only a glimpse of all that is possible with continued growth, collaboration, and a clear vision moving forward. The future horizon of isotope metallomics will be defined by the united efforts of medical and geo-scientists.
Acknowledgements
The authors wish to thank Olivier Alard for his expert feedback regarding LA-MC-ICP-MS techniques. We would also like to thank our two anonymous reviewers, whose comments greatly benefited the manuscript. This research was supported by Macquarie University Deputy Vice Chancellor of Research (DVCR) discretionary funding.
Abbreviations
- Aβ
Amyloid beta
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- Caco-2
Heterogeneous human epithelial colorectal adenocarcinoma cell line
- DXA
Dual-energy X-ray absorptiometry
- FPN
Ferroportin
- GIEC
Gravity-driven ion exchange chromatography
- HCC
Hepatocellular carcinoma
- IEC
Ion exchange chromatography
- ICP-MS
Inductively coupled mass spectrometry
- HepG2
Human liver cells from hepatocellular carcinoma patient
- HPIC
High-performance ion chromatography
- LA-ICP-MS
Laser ablation inductively coupled mass spectrometry
- LA-MC-ICP-MS
Laser ablation multi-collector inductively coupled mass spectrometry
- MC-ICP-MS
Multi-collector inductively coupled mass spectrometry
- MT
Metallothionein
- PFA
Poly-fluoralkyl
- PIEC
Pressure-assisted ion exchange chromatography
- PP
Polypropylene
- Q-ICP-MS
Quadrupole inductively coupled mass spectrometry
- SGIEC
Sequential gravity-driven ion exchange chromatography
- SVIEC
Sequential vacuum-assisted ion exchange chromatography
- SIMS
Secondary ion mass spectrometry
- SOD1
Superoxide dismutase (form 1)
- TIMS
Thermal ionization mass spectrometry
- VIEC
Vacuum-assisted ion exchange chromatography
- WD
Wilson’s disease
- XRF
X-ray fluorescence
Author contributions
BM, RC, and ST conceptualized manuscript content. BM wrote the manuscript. RC, DP, FM, and ST assisted in revision and editing.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Williams RJP. Chemical selection of elements by cells. Coord Chem Rev. 2001;216–217:583–595. doi: 10.1016/S0010-8545(00)00398-2. [DOI] [Google Scholar]
- 2.Szpunar J. Advances in analytical methodology for bioinorganic speciation analysis: metallomics, metalloproteomics and heteroatom-tagged proteomics and metabolomics. Analyst. 2005;130(4):442–465. doi: 10.1039/b418265k. [DOI] [PubMed] [Google Scholar]
- 3.Haraguchi H. Metallomics as integrated biometal science. J Anal Atomic Spectrometry. 2004;19(1):5. doi: 10.1039/b308213j. [DOI] [Google Scholar]
- 4.Mounicou S, Szpunar J, Lobinski R. Metallomics: the concept and methodology. Chem Soc Rev. 2009;38(4):1119–1138. doi: 10.1039/b713633c. [DOI] [PubMed] [Google Scholar]
- 5.Banci L. Metallomics and the cell. Netherlands: Springer; 2013. [Google Scholar]
- 6.Arruda MAZ. Metallomics, the science of biometals. Advances in experimental medicine and biology. Berlin: Springer International Publishing; 2018. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y-K, Hirata T. Stable isotope composition of metal elements in biological samples as tracers for element metabolism. Anal Sci. 2018;34:645–655. doi: 10.2116/analsci.18SBR02. [DOI] [PubMed] [Google Scholar]
- 8.Simpson S. Medical geology: coal control—tackling the health dangers of China’s "dirty" coal. Sci Am. 2002;286:20–21. doi: 10.1038/scientificamerican0202-20b. [DOI] [PubMed] [Google Scholar]
- 9.Bunnell JE. Medical geology: Emerging discipline on the ecosystem? Human Health interface. EcoHealth. 2004;1(1):15–18. doi: 10.1007/s10393-004-0068-8. [DOI] [Google Scholar]
- 10.Albarede F. Metal stable isotopes in the human body: a tribute of geochemistry to medicine. Elements. 2015;11(4):265–269. doi: 10.2113/gselements.11.4.265. [DOI] [Google Scholar]
- 11.Albarede F, Telouk P, Balter V, Bondanese VP, Albalat E, Oger P, Bonaventura P, Miossec P, Fujii T. Medical applications of Cu, Zn, and S isotope effects. Metallomics. 2016;8(10):1056–1070. doi: 10.1039/c5mt00316d. [DOI] [PubMed] [Google Scholar]
- 12.Albarède F, Télouk P, Balter V. Medical Applications of isotope metallomics. Rev Mineral Geochem. 2017;82(1):851–885. doi: 10.2138/rmg.2017.82.20. [DOI] [Google Scholar]
- 13.Albarede F, Telouk P, Lamboux A, Jaouen K, Balter V. Isotopic evidence of unaccounted for Fe and Cu erythropoietic pathways. Metallomics. 2011;3(9):926–933. doi: 10.1039/c1mt00025j. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer A, Muller M, Heuser A, Kolevica A, Gluer CC, Both M, Laue C, Hehn UV, Kloth S, Shroff R, Schrezenmeir J. Calcium isotope ratios in blood and urine: a new biomarker for the diagnosis of osteoporosis. Bone Rep. 2019;10:100200. doi: 10.1016/j.bonr.2019.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moynier F, Creech J, Dallas J, Le Borgne M. Serum and brain natural copper stable isotopes in a mouse model of Alzheimer's disease. Sci Rep. 2019;9(1):11894. doi: 10.1038/s41598-019-47790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindemann FA (2019) XII. Note on the vapour pressure and affinity of isotopes. Lond Edinb Dublin Philos Mag J Sci 38(223):173–181. 10.1080/14786440708635937
- 17.Bigeleisen J, Mayer MG. Calculation of equilibrium constants for isotopic exchange reactions. J Chem Phys. 1947;15:261–267. doi: 10.1063/1.1746492. [DOI] [Google Scholar]
- 18.Urey HC. The thermodynamic properties of isotopic substances. J Chem Soc. 1947;1947:562–581. doi: 10.1039/jr9470000562. [DOI] [PubMed] [Google Scholar]
- 19.Galimov E. The biological fractionation of isotopes. Orlando: Academic Press; 1985. [Google Scholar]
- 20.Criss RE. Principles of stable isotope distribution. Oxford: Oxford University Press; 1999. [Google Scholar]
- 21.Schauble EA. Applying stable isotope fractionation theory to new systems. Rev Mineral Geochem. 2004;55:65–111. doi: 10.2138/gsrmg.55.1.65. [DOI] [Google Scholar]
- 22.Young ED, Galy A, Nagahara H. Kinetic and equillibrium mass-dependent isotope fractionation laws in nature and their geochemical and cosmochemical significance. Geochim Cosmochim Acta. 2002;66:1095–1104. doi: 10.1016/S0016-7037(01)00832-8. [DOI] [Google Scholar]
- 23.Young ED, Manning CE, Schauble EA, Shahar A, Macris CA, Lazar C, Jordan M. High-temperature equilibrium isotope fractionation of non-traditional stable isotopes: experiments, theory, and applications. Chem Geol. 2015;395:176–195. doi: 10.1016/j.chemgeo.2014.12.013. [DOI] [Google Scholar]
- 24.Bullen TD, Eisenhaur A. Metal stable isotopes in low-temperature systems: a primer. Elements. 2009;5(6):349–352. doi: 10.2113/gselements.5.6.349. [DOI] [Google Scholar]
- 25.Jaouen K, Pons M-L. Potential of non-traditional isotope studies for bioarchaeology. Archaeol Anthropol Sci. 2017;9(7):1389–1404. doi: 10.1007/s12520-016-0426-9. [DOI] [Google Scholar]
- 26.Jaouen K, Gibert M, Lamboux A, Telouk P, Fourel F, Albarede F, Alekseev AN, Crubezy E, Balter V. Is aging recorded in blood Cu and Zn isotope compositions? Metallomics. 2013;5(8):1016–1024. doi: 10.1039/c3mt00085k. [DOI] [PubMed] [Google Scholar]
- 27.Jaouen K, Colleter R, Pietrzak A, Pons ML, Clavel B, Telmon N, Crubezy E, Hublin JJ, Richards MP. Tracing intensive fish and meat consumption using Zn isotope ratios: evidence from a historical Breton population (Rennes, France) Sci Rep. 2018;8(1):5077. doi: 10.1038/s41598-018-23249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Télouk P, Puisieux A, Fujii T, Balter V, Bondanese VP, Morel A-P, Clapisson G, Lamboux A, Albarède F. Copper isotope effect in serum of cancer patients: a pilot study. Metallomics. 2015;7:299–308. doi: 10.1039/C4MT00269E. [DOI] [PubMed] [Google Scholar]
- 29.Larner F, Woodley LN, Shousha S, Moyes A, Humphreys-Williams E, Strekopytov S, Halliday AN, Rehkamper M, Coombes RC. Zinc isotopic compositions of breast cancer tissue. Metallomics. 2015;7(1):112–117. doi: 10.1039/c4mt00260a. [DOI] [PubMed] [Google Scholar]
- 30.Moynier F, Foriel J, Shaw AS, BorgneLe M. Distribution of Zn isotopes during Alzheimer’s disease. Geochem Perspect Lett. 2017;2017:142–150. doi: 10.7185/geochemlet.1717. [DOI] [Google Scholar]
- 31.Moynier F, Fujii T, Shaw AS, Le Borgne M. Heterogeneous distribution of natural zinc isotopes in mice. Metallomics. 2013;5(6):693–699. doi: 10.1039/c3mt00008g. [DOI] [PubMed] [Google Scholar]
- 32.Morgan JLL, Skulan JL, Gordon GW, Romaniello SJ, Smith SM, Anbar AD. Rapidly assessing changes in bone mineral balance using natural stable calcium isotopes. Proc Natl Acad Sci USA. 2012;109(25):9989–9994. doi: 10.1073/pnas.1119587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heuser A, Frings-Meuthen P, Rittweger J, Galer SJG. Calcium Isotopes in human urine as a diagnostic tool for bone loss: additional evidence for time delays in bone response to experimental bed rest. Front Physiol. 2019;10:12. doi: 10.3389/fphys.2019.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balter V, Lamboux A, Zazzo A, Télouk P, Leverrier Y, Marvel J, Moloney AP, Monahan FJ, Schmidt O, Albarède F. Contrasting Cu, Fe, and Zn isotopic patterns in organs and body fluids of mice and sheep, with emphasis on cellular fractionation. Metallomics. 2013;5:1470–1482. doi: 10.1039/c3mt00151b. [DOI] [PubMed] [Google Scholar]
- 35.Mahan B, Moynier F, Jorgensen AL, Habekost M, Siebert J. Examining the homeostatic distribution of metals and Zn isotopes in Gottingen minipigs. Metallomics. 2018;10(9):1264–1281. doi: 10.1039/c8mt00179k. [DOI] [PubMed] [Google Scholar]
- 36.Balter V, Vigier N. Natural variations of lithium isotopes in a mammalian model. Metallomics. 2014;6(3):582–586. doi: 10.1039/c3mt00295k. [DOI] [PubMed] [Google Scholar]
- 37.Tang Y-J, Zhang H-F, Ying J-F. Review of the lithium isotope system as a geochemical tracer. Int Geol Rev. 2007;49(4):374–388. doi: 10.2747/0020-6814.49.4.374. [DOI] [Google Scholar]
- 38.Moynier F, Vance D, Fujii T, Savage P. The isotope geochemistry of zinc and copper. Rev Mineral Geochem. 2017;82(1):543–600. doi: 10.2138/rmg.2017.82.13. [DOI] [Google Scholar]
- 39.Skulan J, DePaolo DJ. Ca isotope fractionation between soft and mineralized tissues in vertebrates. Proc Natl Acad Sci USA. 1999;24:13709–13713. doi: 10.1073/pnas.96.24.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walczyk T, von Blanckenburg F. Natural Iron isotope variations in human blood. Science. 2002;295:2065–2066. doi: 10.1126/science.1069389. [DOI] [PubMed] [Google Scholar]
- 41.Maréchal CN, Télouk P, Albarède F. Precise anlaysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chem Geol. 1999;156:251–273. doi: 10.1016/S0009-2541(98)00191-0. [DOI] [Google Scholar]
- 42.Chu NC, Henderson GM, Belshaw NS, Hedges REM. Establishing the potential of Ca isotopes as proxy for consumption of dairy products. Appl Geochem. 2006;21:1656–1667. doi: 10.1016/j.apgeochem.2006.07.003. [DOI] [Google Scholar]
- 43.Skulan J, Bullen T, Anbar AD, Puzas E, Shackelford L, LeBlanc A, Smith SM. Natural calcium isotopic composition of urine as a marker of bone mineral balance. Clin Chem. 2007;53(6):1155–1158. doi: 10.1373/clinchem.2006.080143. [DOI] [PubMed] [Google Scholar]
- 44.Martin JE, Tacail T, Adnet S, Girard C, Balter V. Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chem Geol. 2015;415:118–125. doi: 10.1016/j.chemgeo.2015.09.011. [DOI] [Google Scholar]
- 45.Stenberg A, Malinovsky D, Rodushkin I, Andrén H, Pontér C, Öhlander B, Baxter DC. Separation of Fe from whole blood matrix for precise isotopic ratio measurements by MC-ICP-MS: a comparison of different approaches. J Anal Spectrom. 2003;18:23–28. doi: 10.1039/B210482B. [DOI] [Google Scholar]
- 46.Stenberg A, Malinovsky D, Öhlander B, Andrén H, Forsling W, Engström L-M, Wahlin A, Engström E, Rodushkin I, Baxter DC. Measurement of iron and zinc isotopes in human whole blood: preliminary application to the study of HFE genotypes. J Trace Elem Med Biol. 2005;19:55–60. doi: 10.1016/j.jtemb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Krayenbuehl PA, Walczyk T, Schoenberg R, von Blanckenburg F, Schulthess G. Heriditary hemochromatosis is reflected in the iron isotope composition of blood. Blood. 2005;105:3812–3816. doi: 10.1182/blood-2004-07-2807. [DOI] [PubMed] [Google Scholar]
- 48.Ohno T, Shinohara A, Kohge I, Chiba M, Hirata T. Isotopic analysis of Fe in human red blood cells by multiple collector-ICP-mass spectrometry. Anal Sci. 2004;20:617–621. doi: 10.2116/analsci.20.617. [DOI] [PubMed] [Google Scholar]
- 49.Stenberg A, Andrén H, Malinovsky D, Engström E, Rodushkin I, Baxter DC. Isotopic variations of Zn in Biological materials. Anal Chem. 2004;76:3971–3978. doi: 10.1021/ac049698f. [DOI] [PubMed] [Google Scholar]
- 50.Ohno T, Shinohara A, Chiba M, Hirata T. Precise Zn isotopic ration measurements of human red blood cell and hair samples by multiple collector-ICP-mass spectrometry. Anal Sci. 2005;21:425–427. doi: 10.2116/analsci.21.425. [DOI] [PubMed] [Google Scholar]
- 51.Balter V, Zazzo A, Moloney AP, Moynier F, Schmidt O, Monahan FJ, Albarede F. Bodily variability of zinc natural isotope abundances in sheep. Rapid Commun Mass Spectrom. 2010;24(5):605–612. doi: 10.1002/rcm.4425. [DOI] [PubMed] [Google Scholar]
- 52.Knudson KJ, Williams HM, Buikstra JE, Tomczak PD, Gordon GW, Anbar AD. Introducing δ88/86Sr analysis in archaeology: a demonstration of the utility of strontium isotope fractionation in paleodietary studies. J Archaeol Sci. 2010;37(9):2352–2364. doi: 10.1016/j.jas.2010.04.009. [DOI] [Google Scholar]
- 53.Martin JE, Vance D, Balter V. Natural variation of magnesium isotopes in mammal bones and teeth from two South African trophic chains. Geochim Cosmochim Acta. 2014;130:12–20. doi: 10.1016/j.gca.2013.12.029. [DOI] [Google Scholar]
- 54.Martin JE, Vance D, Balter V. Magnesium stable isotope ecology using mammal tooth enamel. Proc Natl Acad Sci USA. 2015;112(2):430–435. doi: 10.1073/pnas.1417792112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang SC, Welter L, Kolatkar A, Nieva J, Waitman KR, Huang KF, Liao WH, Takano S, Berelson WM, West AJ, Kuhn P, John SG. A new anion exchange purification method for Cu stable isotopes in blood samples. Anal Bioanal Chem. 2019;411(3):765–776. doi: 10.1007/s00216-018-1498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schott J, Mavromatis V, Fujii T, Pearce CR, Oelkers EH. The control of carbonate mineral Mg isotope composition by aqueous speciation: theoretical and experimental modeling. Chem Geol. 2016;445:120–134. doi: 10.1016/j.chemgeo.2016.03.011. [DOI] [Google Scholar]
- 57.Moynier F, Fujii T. Theoretical isotopic fractionation of magnesium between chlorophylls. Sci Rep. 2017;7:6973. doi: 10.1038/s41598-017-07305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moynier F, Fujii T. Calcium isotope fractionation between aqueous compounds relevant to low-temperature geochemistry, biology and medicine. Sci Rep. 2017;7:44255. doi: 10.1038/srep44255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujii T, Moynier F, Blichert-Toft J, Albarède F. Density functional theory estimation of isotope fractionation of Fe, Ni, Cu, and Zn among species relevant to geochemical and biological environments. Geochim Cosmochim Acta. 2014;140:553–576. doi: 10.1016/j.gca.2014.05.051. [DOI] [Google Scholar]
- 60.Tossell JA. Calculating the partitioning of the isotopes of Mo between oxidic and sulfidic species in aqueous solution. Geochim Cosmochim Acta. 2005;69(12):2981–2993. doi: 10.1016/j.gca.2005.01.016. [DOI] [Google Scholar]
- 61.Tennant A, Rauk A, Wieser ME. Computational modelling of the redistribution of copper isotopes by proteins in the liver. Metallomics. 2017;9(12):1809–1819. doi: 10.1039/c7mt00248c. [DOI] [PubMed] [Google Scholar]
- 62.Costas-Rodriguez M, Colina-Vegas L, Solovyev N, De Wever O, Vanhaecke F. Cellular and sub-cellular Cu isotope fractionation in the human neuroblastoma SH-SY5Y cell line: proliferating versus neuron-like cells. Anal Bioanal Chem. 2019 doi: 10.1007/s00216-019-01871-6. [DOI] [PubMed] [Google Scholar]
- 63.Chang KH, Lee-Chen GJ, Huang CC, Lin JL, Chen YJ, Wei PC, Lo YS, Yao CF, Kuo MW, Chen CM. Modeling alzheimer's disease by induced pluripotent stem cells carrying APP D678H mutation. Mol Neurobiol. 2019;56(6):3972–3983. doi: 10.1007/s12035-018-1336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blokhuis AM, Groen EJ, Koppers M, van den Berg LH, Pasterkamp RJ. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125(6):777–794. doi: 10.1007/s00401-013-1125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benkler C, O'Neil AL, Slepian S, Qian F, Weinreb PH, Rubin LL. Aggregated SOD1 causes selective death of cultured human motor neurons. Sci Rep. 2018;8(1):16393. doi: 10.1038/s41598-018-34759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rowe RG, Daley GQ. Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet. 2019 doi: 10.1038/s41576-019-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Banci L, Bertini I, Ciofi-Baffoni S, Kozyreva T, Zovo K, Palumaa P. Affinity gradients drive copper to cellular destinations. Nature. 2010;465(7298):645–648. doi: 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- 68.Larner F, McLean CA, Halliday AN, Roberts BR. Copper Isotope compositions of superoxide dismutase and metallothionein from post-mortem human frontal cortex. Inorganics. 2019;7(7):86. doi: 10.3390/inorganics7070086. [DOI] [Google Scholar]
- 69.Costas-Rodríguez M, Delanghe J, Vanhaecke F. High-precision isotopic analysis of essential mineral elements in biomedicine: natural isotope ratio variations as potential diagnostic and/or prognostic markers. TrAC Trends Anal Chem. 2016;76:182–193. doi: 10.1016/j.trac.2015.10.008. [DOI] [Google Scholar]
- 70.Flórez MR, Anoshkina Y, Costas-Rodríguez M, Grootaert C, Van Camp J, Delanghe J, Vanhaecke F. Natural Fe isotope fractionation in an intestinal Caco-2 cell line model. J Anal Atomic Spectrometry. 2017;32(9):1713–1720. doi: 10.1039/c7ja00090a. [DOI] [Google Scholar]
- 71.Navarrete JU, Borrok DM, Viveros M, Ellzey JT. Copper isotope fractionation during surface adsorption and intracellular incorporation by bacteria. Geochim Cosmochim Acta. 2011;75(3):784–799. doi: 10.1016/j.gca.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kafantaris F-CA, Borrok DM. Zinc isotope fractionation during surface adsorption and intracellular incorporation by bacteria. Chem Geol. 2014;366:42–51. doi: 10.1016/j.chemgeo.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bondanese VP, Lamboux A, Simon M, Lafont JE, Albalat E, Pichat S, Vanacker JM, Telouk P, Balter V, Oger P, Albarede F. Hypoxia induces copper stable isotope fractionation in hepatocellular carcinoma, in a HIF-independent manner. Metallomics. 2016;8(11):1177–1184. doi: 10.1039/c6mt00102e. [DOI] [PubMed] [Google Scholar]
- 74.Cadiou JL, Pichat S, Bondanese VP, Soulard A, Fujii T, Albarede F, Oger P. Copper transporters are responsible for copper isotopic fractionation in eukaryotic cells. Sci Rep. 2017;7:44533. doi: 10.1038/srep44533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Florez MR, Costas-Rodriguez M, Grootaert C, Van Camp J, Vanhaecke F. Cu isotope fractionation response to oxidative stress in a hepatic cell line studied using multi-collector ICP-mass spectrometry. Anal Bioanal Chem. 2018;410(9):2385–2394. doi: 10.1007/s00216-018-0909-x. [DOI] [PubMed] [Google Scholar]
- 76.Paredes E, Avazeri E, Malard V, Vidaud C, Ortega R, Nonell A, Isnard H, Chartier F, Bresson C. A new procedure for high precision isotope ratio determinations of U, Cu and Zn at nanogram levels in cultured human cells: What are the limiting factors? Talanta. 2018;178:894–904. doi: 10.1016/j.talanta.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 77.Resano M, Aramendía M, Rello L, Calvo ML, Bérail S, Pécheyran C. Direct determination of Cu isotope ratios in dried urine spots by means of fs-LA-MC-ICPMS. Potential to diagnose Wilson's disease. J Anal At Spectrom. 2013;28(1):98–106. doi: 10.1039/c2ja30262d. [DOI] [Google Scholar]
- 78.Tacail T, Télouk P, Balter V. Precise analysis of calcium stable isotope variations in biological apatites using laser ablation MC-ICPMS. J Anal At Spectrom. 2016;31(1):152–162. doi: 10.1039/c5ja00239g. [DOI] [Google Scholar]
- 79.Kindness A. Two-Dimensional mapping of copper and zinc in liver sections by laser ablation-inductively coupled plasma mass spectrometry. Clin Chem. 2003;49(11):1916–1923. doi: 10.1373/clinchem.2003.022046. [DOI] [PubMed] [Google Scholar]
- 80.Pozebon D, Scheffler GL, Dressler VL. Recent applications of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) for biological sample analysis: a follow-up review. J Anal At Spectrom. 2017;32(5):890–919. doi: 10.1039/c7ja00026j. [DOI] [Google Scholar]
- 81.Jackson SE, Günther D. The nature and sources of laser induced isotopic fractionation in laser ablation-multicollector-inductively coupled plasma-mass spectrometry. J Anal At Spectrom. 2003;18(3):205–212. doi: 10.1039/b209620j. [DOI] [Google Scholar]
- 82.Graham S, Pearson N, Jackson S, Griffin W, O'Reilly SY. Tracing Cu and Fe from source to porphyry: in situ determination of Cu and Fe isotope ratios in sulfides from the Grasberg Cu–Au deposit. Chem Geol. 2004;207(3–4):147–169. doi: 10.1016/j.chemgeo.2004.02.009. [DOI] [Google Scholar]
- 83.Kuhn H-R, Pearson NJ, Jackson SE. The influence of the laser ablation process on isotopic fractionation of copper in LA-MC-ICP-MS. J Anal At Spectrom. 2007;22(5):547. doi: 10.1039/b616232k. [DOI] [Google Scholar]
- 84.Ikehata K, Notsu K, Hirata T. In situ determination of Cu isotope ratios in copper-rich materials by NIR femtosecond LA-MC-ICP-MS. J Anal At Spectrom. 2008;23(7):1003. doi: 10.1039/b801044g. [DOI] [Google Scholar]
- 85.Lazarov M, Horn I. Matrix and energy effects during in-situ determination of Cu isotope ratios by ultraviolet-femtosecond laser ablation multicollector inductively coupled plasma mass spectrometry. Spectrochim Acta Part B At Spectrosc. 2015;111:64–73. doi: 10.1016/j.sab.2015.06.013. [DOI] [Google Scholar]
- 86.Zheng X-Y, Beard BL, Lee S, Reddy TR, Xu H, Johnson CM. Contrasting particle size distributions and Fe isotope fractionations during nanosecond and femtosecond laser ablation of Fe minerals: implications for LA-MC-ICP-MS analysis of stable isotopes. Chem Geol. 2017;450:235–247. doi: 10.1016/j.chemgeo.2016.12.038. [DOI] [Google Scholar]
- 87.Chernonozhkin SM, Costas-Rodríguez M, Claeys P, Vanhaecke F. Evaluation of the use of cold plasma conditions for Fe isotopic analysis via multi-collector ICP-mass spectrometry: effect on spectral interferences and instrumental mass discrimination. J Anal At Spectrom. 2017;32(3):538–547. doi: 10.1039/c6ja00428h. [DOI] [Google Scholar]
- 88.Urgast DS, Hill S, Kwun IS, Beattie JH, Goenaga-Infante H, Feldmann J. Zinc isotope ratio imaging of rat brain thin sections from stable isotope tracer studies by LA-MC-ICP-MS. Metallomics. 2012;4(10):1057–1063. doi: 10.1039/c2mt20119d. [DOI] [PubMed] [Google Scholar]
- 89.Flórez MR, Aramendía M, Resano M, Lapeña AC, Balcaen L, Vanhaecke F. Isotope ratio mapping by means of laser ablation-single collector-ICP-mass spectrometry: Zn tracer studies in thin sections of Daphnia magna. J Anal Atom Spectrom. 2013;28(7):1005. doi: 10.1039/c3ja50087j. [DOI] [Google Scholar]
- 90.Feng L, Wang J, Li H, Luo X, Li J. A novel absolute quantitative imaging strategy of iron, copper and zinc in brain tissues by isotope dilution laser ablation ICP-MS. Anal Chim Acta. 2017;984:66–75. doi: 10.1016/j.aca.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 91.Cruz-Alonso M, Fernandez B, Navarro A, Junceda S, Astudillo A, Pereiro R. Laser ablation ICP-MS for simultaneous quantitative imaging of iron and ferroportin in hippocampus of human brain tissues with Alzheimer's disease. Talanta. 2019;197:413–421. doi: 10.1016/j.talanta.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 92.Okita Y, Rcom-H'cheo-Gauthier AN, Goulding M, Chung RS, Faller P, Pountney DL. Metallothionein, copper and alpha-synuclein in alpha-synucleinopathies. Front Neurosci. 2017;11:114. doi: 10.3389/fnins.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lobo L, Pereiro R, Fernández B. Opportunities and challenges of isotopic analysis by laser ablation ICP-MS in biological studies. TrAC Trends Anal Chem. 2018;105:380–390. doi: 10.1016/j.trac.2018.05.020. [DOI] [Google Scholar]
- 94.Poitrasson F, d'Abzac F-X. Femtosecond laser ablation inductively coupled plasma source mass spectrometry for elemental and isotopic analysis: are ultrafast lasers worthwhile? J Anal At Spectrom. 2017;32(6):1075–1091. doi: 10.1039/c7ja00084g. [DOI] [Google Scholar]
- 95.Moynier F, Le Borgne M. High precision zinc isotopic measurements applied to mouse organs. J Vis Exp. 2015;99:e52479. doi: 10.3791/52479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sossi PA, Halverson GP, Nebel O, Eggins SM. Combined separation of Cu, Fe and Zn from rock matrices and improved analytical protocols for stable isotope determination. Geostand Geoanaly Res. 2015;39(2):129–149. doi: 10.1111/j.1751-908X.2014.00298.x. [DOI] [Google Scholar]
- 97.Moore RE, Larner F, Coles BJ, Rehkamper M. High Precision zinc stable isotope measurement of certified biological reference materials using the double spike technique and multiple collector-ICP-MS. Anal Bioanal Chem. 2017;409(11):2941–2950. doi: 10.1007/s00216-017-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moynier F, Albarède F, Herzog GF. Isotopic composition of zinc, copper, and iron in lunar samples. Geochimica Cosmochim Acta. 2006;70(24):6103–6117. doi: 10.1016/j.gca.2006.02.030. [DOI] [Google Scholar]
- 99.Lanping F, Zhou L, Yang L, Zhang W, Wang Q, Shuoyun T, Hu Z. A rapid and simple single-stage method for Ca separation from geological and biological samples for isotopic analysis by MC-ICP-MS. J Anal At Spectrom. 2018;33(3):413–421. doi: 10.1039/c7ja00370f. [DOI] [Google Scholar]
- 100.Pourmand A, Dauphas N. Distribution coefficients of 60 elements on TODGA resin: application to Ca, Lu, Hf U and Th isotope geochemistry. Talanta. 2010;81(3):741–753. doi: 10.1016/j.talanta.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 101.Maxwell SL. Rapid analysis of emergency urine and water samples. J Radioanal Nucl Chem. 2008;275(3):497–502. doi: 10.1007/s10967-007-7084-4. [DOI] [Google Scholar]
- 102.Vasile M, Jacobs K, Bruggeman M, Van Hoecke K, Dobney A, Verrezen F. On the sequential separation and quantification of (237)Np, (241)Am, thorium, plutonium, and uranium isotopes in environmental and urine samples. Appl Radiat Isot. 2018;134:455–460. doi: 10.1016/j.apradiso.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 103.Liu B, Shi K, Ye G, Li Y, Wu W. Rapid determination of plutonium in water samples using vacuum box system separation and low background liquid scintillation counter measurement. Anal Methods. 2015;7(23):9992–9999. doi: 10.1039/c5ay02232k. [DOI] [Google Scholar]
- 104.Wall AJ, Capo RC, Stewart BW, Phan TT, Jain JC, Hakala JA, Guthrie GD (2016) High-throughput method for strontium isotope analysis by multi-collector-inductively coupled plasma-mass spectrometer. In: NETL Technical Report Series; US Department of Energy, National Energy Technology Laboratory: Pittsburg, p 76
- 105.Meynadier L, Gorge C, Birck J-L, Allègre CJ. Automated separation of Sr from natural water samples or carbonate rocks by high performance ion chromatography. Chem Geol. 2006;227(1–2):26–36. doi: 10.1016/j.chemgeo.2005.05.012. [DOI] [Google Scholar]
- 106.Romaniello SJ, Field MP, Smith HB, Gordon GW, Kim MH, Anbar AD. Fully automated chromatographic purification of Sr and Ca for isotopic analysis. J Anal At Spectrom. 2015;30(9):1906–1912. doi: 10.1039/c5ja00205b. [DOI] [Google Scholar]
- 107.Schmitt A-D, Gangloff S, Cobert F, Lemarchand D, Stille P, Chabaux F. High performance automated ion chromatography separation for Ca isotope measurements in geological and biological samples. J Anal At Spectrom. 2009;24(8):1089. doi: 10.1039/b903303c. [DOI] [Google Scholar]
- 108.Husson JM, Higgins JA, Maloof AC, Schoene B. Ca and Mg isotope constraints on the origin of Earth’s deepest δ13C excursion. Geochim Cosmochim Acta. 2015;160:243–266. doi: 10.1016/j.gca.2015.03.012. [DOI] [Google Scholar]
- 109.Blättler CL, Higgins JA. Testing Urey's carbonate–silicate cycle using the calcium isotopic composition of sedimentary carbonates. Earth Planet Sci Lett. 2017;479:241–251. doi: 10.1016/j.epsl.2017.09.033. [DOI] [Google Scholar]
- 110.Retzmann A, Zimmermann T, Profrock D, Prohaska T, Irrgeher J. A fully automated simultaneous single-stage separation of Sr, Pb, and Nd using DGA Resin for the isotopic analysis of marine sediments. Anal Bioanal Chem. 2017;409(23):5463–5480. doi: 10.1007/s00216-017-0468-6. [DOI] [PubMed] [Google Scholar]
- 111.Sadi BB, Rinaldo C, Spencer N, Li C. An ion chromatographic separation method for the sequential determination of 90Sr, 241Am and Pu isotopes in a urine sample. J Radioanal Nucl Chem. 2018;316(1):179–189. doi: 10.1007/s10967-018-5758-8. [DOI] [Google Scholar]
- 112.Enge TG, Field MP, Jolley DF, Ecroyd H, Kim MH, Dosseto A. An automated chromatography procedure optimized for analysis of stable Cu isotopes from biological materials. J Anal At Spectrom. 2016;31(10):2023–2030. doi: 10.1039/c6ja00120c. [DOI] [Google Scholar]
- 113.Yuan H, Liu X, Bao Z, Chen K, Zong C. A Fast separation method for isotope analysis based on compressed nitrogen gas and ion-exchange chromatography technique—a case study of Sr-Nd isotope measurement. J Earth Sci. 2018;29(1):223–229. doi: 10.1007/s12583-017-0944-0. [DOI] [Google Scholar]
- 114.Garçon M, Sauzéat L, Carlson RW, Shirey SB, Simon M, Balter V, Boyet M. Nitrile, latex, neoprene and vinyl gloves: a primary source of contamination for trace element and Zn Isotopic analyses in geological and biological samples. Geostand Geoanaly Res. 2017;41(3):367–380. doi: 10.1111/ggr.12161. [DOI] [Google Scholar]
- 115.Sauzeat L, Bernard E, Perret-Liaudet A, Quadrio I, Vighetto A, Krolak-Salmon P, Broussolle E, Leblanc P, Balter V. Isotopic evidence for disrupted copper metabolism in amyotrophic lateral scleros. iScience. 2018;6:264–271. doi: 10.1016/j.isci.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chitpong N, Husson SM. Nanofiber ion-exchange membranes for the rapid uptake and recovery of heavy metals from water. Membranes (Basel) 2016;6:4. doi: 10.3390/membranes6040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.He ZY, Nie HL, Branford-White C, Zhu LM, Zhou YT, Zheng Y. Removal of Cu2+ from aqueous solution by adsorption onto a novel activated nylon-based membrane. Bioresour Technol. 2008;99(17):7954–7958. doi: 10.1016/j.biortech.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 118.Sartorius (2019) Sartobind(R) membrane adsorbers: a separation technology based on microporous membrane ion exchangers. Sartorius SL-6075-e00063
- 119.Eisenhauer A, Müller M, Heuser A, Kolevica A, Glüer C-C, Both M, Laue C, Hehn UV, Kloth S, Shroff R, Schrezenmeir J. Calcium isotope ratios in blood and urine: a new biomarker for the diagnosis of osteoporosis. Bone Rep. 2019;10:100200. doi: 10.1016/j.bonr.2019.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Balter V, Nogueira da Costa A, Bondanese VP, Jaouen K, Lamboux A, Sangrajrang S, Vincent N, Fourel F, Telouk P, Gigou M, Lecuyer C, Srivatanakul P, Brechot C, Albarede F, Hainaut P. Natural variations of copper and sulfur stable isotopes in blood of hepatocellular carcinoma patients. Proc Natl Acad Sci USA. 2015;112(4):982–985. doi: 10.1073/pnas.1415151112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Costas-Rodríguez M, Anoshkina Y, Lauwens S, Van Vlierberghe H, Delanghe J, Vanhaecke F. Isotopic analysis of Cu in blood serum by multi-collector ICP-mass spectrometry: a new approach for the diagnosis and prognosis of liver cirrhosis? Metallomics. 2015;7(3):491–498. doi: 10.1039/c4mt00319e. [DOI] [PubMed] [Google Scholar]
- 122.Aramendía M, Rello L, Resano M, Vanhaecke F. Isotopic analysis of Cu in serum samples for diagnosis of Wilson's disease: a pilot study. J Anal At Spectrom. 2013;28(5):675. doi: 10.1039/c3ja30349g. [DOI] [Google Scholar]
- 123.Buchl A, Hawkesworth CJ, Ragnarsdottir KV, Brown DR. Re-partitioning of Cu and Zn isotopes by modified protein expression. Geochem Trans. 2008;9:11. doi: 10.1186/1467-4866-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]