Abstract
The degradation of maternally provided molecules is a very important process during early embryogenesis. However, the vast majority of studies deals with mRNA degradation and protein degradation is only a very little explored process yet. The aim of this article was to summarize current knowledge about the protein degradation during embryogenesis of mammals. In addition to resuming of known data concerning mammalian embryogenesis, we tried to fill the gaps in knowledge by comparison with facts known about protein degradation in early embryos of non-mammalian species. Maternal protein degradation seems to be driven by very strict rules in terms of specificity and timing. The degradation of some maternal proteins is certainly necessary for the normal course of embryonic genome activation (EGA) and several concrete proteins that need to be degraded before major EGA have been already found. Nevertheless, the most important period seems to take place even before preimplantation development—during oocyte maturation. The defects arisen during this period seems to be later irreparable.
Keywords: Ubiquitin, Proteasome system, Maternal to zygotic transition, Ubiquitin ligase, Autophagy, Embryonic genome activation
Introduction
The early embryonic development is initially driven by maternally inherited mRNAs, proteins and other molecules (such as NTPs). As development proceeds, the stored stocks are degraded and replaced by embryonally synthesized molecules. There are plenty of articles dealing with maternal RNA processing during early embryonic development (summarized in [1, 2]). The degradation of maternal mRNAs is a gradual process, which peaks around the major wave of embryonic genome activation (EGA) and results in the degradation of the vast majority of maternal mRNAs at this time [3]. On the other hand, the way of maternally inherited protein degradation is not well described, especially in mammals. Protein degradation is relatively well described during oocyte maturation and fertilization ([4–6] and others) and several proteins such as securin and cyclin B1 are known to be degraded during oocyte maturation [5, 7]. However there is much fewer studies dealing with protein degradation during early embryogenesis.
During the pre-EGA stages of preimplantation development, the embryos are transcriptionally silent. This indicates that some of the inherited mRNA must be unusually stable [8], so that it persists even until the EGA stage. Therefore, embryonic life regulation is driven mostly at the post-transcriptional level with a high impact on the balance of protein synthesis/storage/degradation.
Due to the strict post-transcriptional regulation of stored maternal mRNA, mRNA and protein expression often do not correlate with each other (Stat1, G10 in Xenopus or Nanog in rabbit) [9, 10]. The onset of transcription of a concrete gene often coincides with a decrease in protein level as a result of its maternal mRNA degradation [9]. The levels of other proteins increase during the mid-blastula transition (MBT; an equivalent of EGA in mammals) in Xenopus [9]. Surprisingly, this increase is often caused not only by translation from embryonic but also from maternal mRNA. The maternal mRNA might be translationally silent, and its activation can be postponed to later stages of the development (e.g. fibronectin in Xenopus) [2, 11–13]. Besides maternal mRNAs and proteins, oocyte also contains metabolites necessary for several embryonic divisions. For example, maternal supply of deoxyribonucleotides (dNTPs) is important for increasing DNA content during the fast embryonic cell cycles in Drosophila [14]. Almost one-third of required dNTPs are of maternal origin, the rest must be synthesized de novo. The shift from using of maternally deposited to newly synthesized dNTPs is an important step leading to maternal-to-zygotic transition [15]. For successful EGA and histone modification, the presence of enzymes of tricarboxylic acid cycle localized to the nucleus is important in mouse embryos [16].
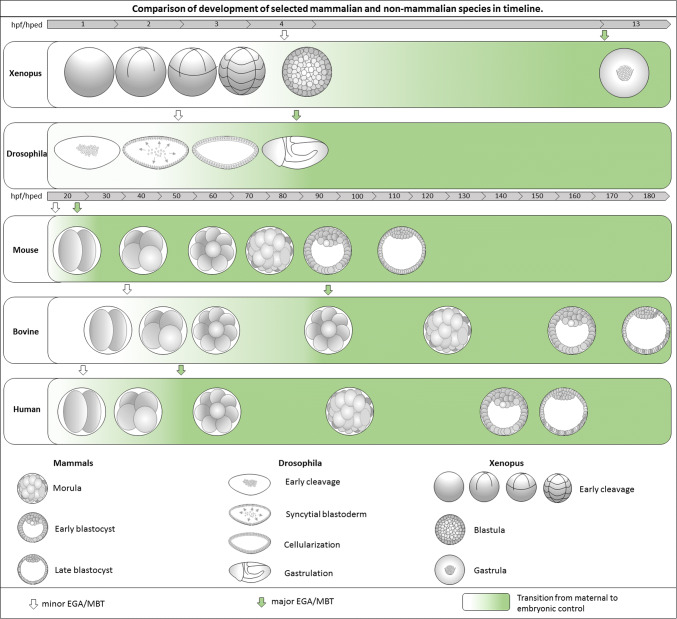
The preimplantation development of mammals is in many ways very specific and differs from the early embryogenesis of non-mammalian species [17]. The meiotic division of the oocyte is only completed after fertilization, and the cleavage of the early embryo is really slow in comparison to other animals (Fig. 1). In the time it takes mammalian embryos to go from fertilization to implantation, some non-mammalian species have already formed most of their organ primordia. As far as the developmental stage is concerned, the EGA occurs very early in mammalian embryos—the major wave occurs at the 2-cell to 16-cell stage for mammalian species [18–20]. Nevertheless, with respect to the time since fertilization, EGA occurs significantly later in mammals (compare Figs. 2, 3). At the 8-cell stage (at the same developmental stage for all mammalian species) the mammalian embryo undergoes a process called compaction (Figs. 2, 3). During this process, blastomeres that used to loosely attach to each other start to express adhesive molecules such as E cadherin, and the embryo becomes a compact structure. Asymmetry appears after the 8-cell stage with the formation of polar and apolar cells [21, 22], but can still be altered when needed [23, 24]. The cells are still able to adapt to a new environment (reviewed in [25]). The definitive formation of two distinct cell lineages comes at the blastocyst stage with the formation of the inner cell mass (ICM) and trophectoderm (TE). Afterward the blastocyst hatches, which is taken to be the final step of preimplantation development.
Fig. 1.
Comparison of development of selected mammalian and non-mammalian species in timeline. The figure shows progression of development during time after fertilization in Xenopus, Drosophila, mouse, bovine and human embryos. While the fast developing species, Xenopus and Drosophila, undergoes the first divisions in rapid manner and during several hours are able to develop to the gastrula stage, mammalian embryos divide slowly, approximately every 12–24 h and reach the blastocyst stage after a few days [17]. hpf hours post fertilization, hped hours post egg deposition, EGA embryonic genome activation, MBT mid-blastula transition
Fig. 2.
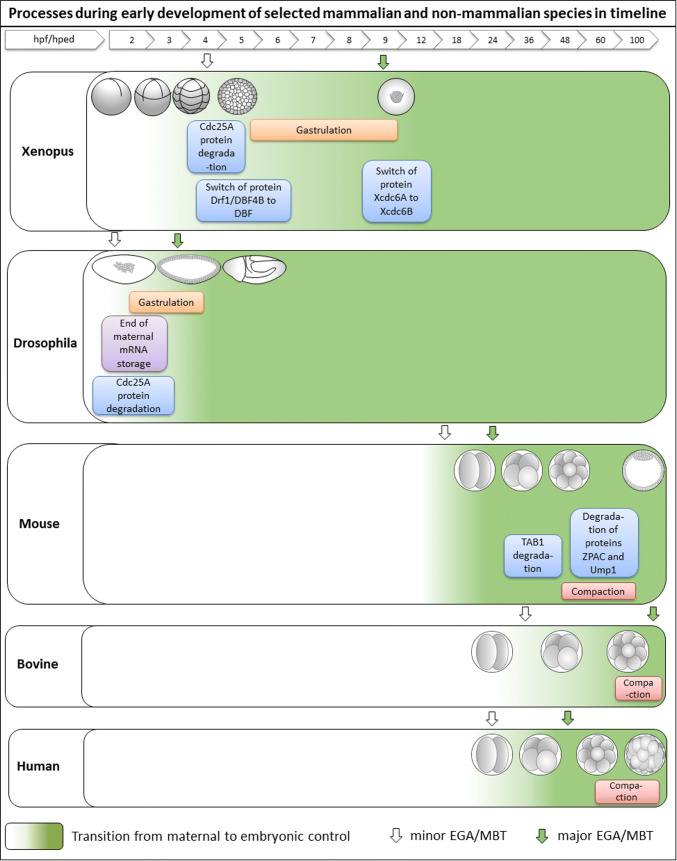
Processes during early development of selected mammalian and non-mammalian species in timeline. The figure shows important events during the early development of Xenopus, Drosophila, mouse, bovine and human embryos compared to each other in timeline. These important processes occur very soon after fertilization in Xenopus and Drosophila. Similar processed occur later in mammalian embryos, but these embryos are in a lower developmental stages that non-mammalian species at this time. Green shading density illustrate the maternal to zygotic transition of developmental control. The white arrow marks minor EGA (mouse, bovine, human) or minor MBT (Xenopus, Drosophila). The green arrow marks major EGA (mouse, bovine, human) or major MBT (Xenopus, Drosophila). hpf hours post fertilization, hped hours post egg deposition, EGA embryonic genome activation, MBT mid-blastula transition
Fig. 3.
Processes during early development of selected mammalian and non-mammalian species according to the cell cycle. The figure shows important events occurring during the early development of Xenopus, Drosophila, mouse, bovine and human embryos compared to each other during the cell cycle progression, focused specially on maternal protein level changes. Although some processes occur very quickly after fertilization in Drosophila and Xenopus, these embryos are in higher developmental stages than embryos of mammals, where the similar processes occur in earlier developmental stages actually. Green shading density illustrate the maternal to zygotic transition of developmental control. The white arrow marks minor EGA (mouse, bovine, human) or minor MBT (Xenopus, Drosophila). The green arrow marks major EGA (mouse, bovine, human) or major MBT (Xenopus, Drosophila). hpf hours post fertilization, hped hours post egg deposition, EGA embryonic genome activation, MBT mid-blastula transition
In this review, we summarize current knowledge on protein degradation during preimplantation development in mammals. Since a much larger dataset is available on the early embryogenesis of non-mammalian species, we also use this information and try to find a way to transfer this knowledge from non-mammalian to mammalian species.
Protein degradation pathways involved in preimplantation development
The degradation pathways of maternal proteins during early embryogenesis are to a large extent unknown. The most attention is paid to the ubiquitin–proteasome system (UPS) and autophagy, nevertheless other minor pathways are involved as well. For example in Xenopus, miRNAs are involved in protein degradation during early embryogenesis [26].
In particular UPS is a highly branched pathway comprising of a lot of different enzymes, many of them involved during preimplantation development. Results to date suggest that maternal protein degradation in embryos is not a mass process, but the degradation of each protein is instead controlled separately. Although the hypotheses and experiments can be largely based on the knowledge gained in somatic cells, there are clearly many specifics in the usage of an enzyme in early embryos for the degradation of a particular protein, its timing or localization.
Ubiquitin–proteasome system
UPS is a highly specific degradation pathway that primarily deals with the degradation of endogenous proteins. The proteins targeted for degradation are marked using the small protein ubiquitin that is reversibly linked by a covalent bond to targeted protein [27]. The proteins are polyubiquinated, which directs them to be degraded by the 26S proteasome. The ubiquitination of proteins is performed by three enzyme complexes: E1—ubiquitin activating enzyme; E2—ubiquitin-conjugating enzyme and E3—ubiquitin ligase [27]. While there is only one type of E1 enzyme and a little over 40 types of E2 enzymes, there are more than 500 types of E3 ligases [28]. The plurality of E3 ligases enables the polyubiquitination of different protein targets. The ubiquitinated proteins are rapidly degraded by the proteasome system.
The protein degradation using UPS regulates many cellular functions, including H2B methylation and chromatin remodelling [29, 30], cell cycle progression, the continuous running of transcription [31] and also its termination [32], without which the cell division does not occur. Moreover ubiquitination without the activation of UPS is also involved in DNA repair, autophagy, transcription and vesicle processing [33]. It is thought that a great deal of maternal protein degradation is mediated by the ubiquitin-proteolytic system [34–37] and the activity of UPS reflects the embryo quality [35]. Thus the role of the ubiqutin-proteasome pathway (UPS) in preimplantation development seems to be undeniable. The involvement of UPS in oocyte maturation and early preimplantation development has been shown by mass spectrometry (MS) [38–40]. MS is an ideal method for finding candidate proteins to be degraded during normal development. It seems that in a certain moment, degradation of only a few proteins is needed. This results were shown by Yang et al. [38] who identified just one protein, which is degraded by examined enzyme at specific time, between thousands of potential proteins analysed using MS. Thus, in spite of high material requirements, MS provide valuable insights into protein processing during embryogenesis.
The amount of proteasomes is mainly regulated at the transcriptional level of its subunits (reviewed in [41]). A decrease in proteasomal activity results in an increased mRNA expression of proteasomal genes and consequently in de novo proteasome formation [42]. However, transcription is inactive during the early stages of preimplantation development, and so the expression of proteasomal genes is not the way proteasome activity is regulated in embryos.
In mice, the polysomal maternal mRNA of genes responsible for ubiquitination is equally present in matured oocytes and one-cell embryos [43]. The embryonic expression of genes involved in protein ubiquitination is activated at the 8-cell stage in bovines; at the same time, the genes required for translation initiation and ribosome biogenesis are activated [44]. The major wave of embryonic genome activation takes place at the late 8-cell stage in cows. When experiments were focused on the expression of SCF complex members (Skp1-Cul-F box complex, one of the most common E3 ligases), it was found that the mRNA transcription of Cullin1 and Skp1 starts already at the early 8-cell (i.e. before major EGA) and of Rbx1 at the late 8-cell stage (EGA stage) [45]. The level of proteins related to UPS sharply increases in mouse zygotes [39], the Cullin1 protein level is the highest at the late 8-cell and morula stage, the level of SKP1 is the highest at the 4-cell stage [45]. This shows that the maternal mRNA of genes involved in UPS-based protein degradation is stored and translated until the major wave of EGA, and the expression of embryonic mRNA of these genes is initiated even before the major EGA, so that there is no gap in their protein synthesis. Interestingly, there are large interspecies differences in the expression of UPS genes mRNAs, as was shown by Mtango and Latham [35] after a comparison of mouse and rhesus monkey oocytes and embryos, and also after comparison with our results [45]. It would be very interesting to compare the expression of these genes at the protein level.
Polyubiquinated proteins are accumulated in the embryo around the major EGA—i.e. in mice from the 2-cell stage with rapid degradation at the 4-cell stage [46]. This degradation is consistent with the increase in the chymotrypsin-like activity of the proteasome at the 2-cell stage in mice [46]. However, it has been found that SCF complex activity does not significantly change during preimplantation development [45]. In preimplantation embryos, proteasomes and ubiquinated proteins are preferentially localized to the nucleus [47–49], active SCF complex (Cullin1 and Skp1 complex interaction) and Cullin 1 are mainly localized to the cytoplasm [45, 50]. At the blastocyst stage, ubiquitination is mainly localized to the trophectoderm. Sutovsky et al. [51] found that in bovine and murine blastocysts the diffuse cytoplasmic labelling of ubiquitin was present in both the TE and ICM, however in the TE, large granules of ubiquitin were accumulated, whilst these granules were not present in the ICM. Similarly in our laboratory, almost no SCF complex activity in the inner cell mass and high SCF complex activity in the TE was found [45]. On the other hand, the murine deubiquitinase Dub-2 is required for the development of the ICM and hatching of the blastocyst [52], and the localization of proteasomes seems to be the same in ICM and TE (nevertheless, this was observed using ivf-discarded triploid blastocysts; [47]). This indicates that UPS—mediated protein degradation is needed throughout the whole of preimplantation development from oocyte maturation to blastocyst formation. However, the two parts of the UPS—ubiquitination and proteasomal degradation—do not always correlate with each other, and the regulation of protein degradation is driven mainly by the proteasomal activity level rather than the level of ubiquitination in preimplantation embryos.
Moreover, the interaction of a protein with E3-ubiquitin ligase does not necessarily mean its degradation. For example, RECQL4 (RecQ like helicase 4) is ubiquinated by DDB1-CUL4A (DNA damage-binding protein 1-cullin 4A) E3 ubiquitin ligase, which triggers RECQL4 to mediate the repair of double-strand breaks in DNA instead of RECQL4 being degraded [53]. Further, it is associated with the ubiquitin ligases UBR1/UBR2, nevertheless this interaction does not even cause the ubiquitination of RECQL4 [54].
Deubiquitinating enzymes
Deubiquitinating enzymes (DUBs) are thiol proteases that remove the bound ubiquitin from its substrates (proteins, polyubiquitin chains) and thus reverses ubiquitination and enables the renewal of free monoubiquitin ([55, 56] reviewed in [57]). In this way, DUBs not only control (prevent) the substrate degradation, but also recycling of the ubiquitins for further use. It has been shown that silencing or inhibition of a DUB causes affected preimplantation development [58, 59] and others. The deterioration of their preimplantation development is likely mainly caused by the lack of free monoubiquitin (reviewed in [60]). The silencing of DUB USP36 causes decreased mRNA translation in murine morulas. USP36 is a nucleolar protein that is necessary for ribosome biogenesis and RNA processing [59]. To the best of our knowledge, there are no data on USP36 silencing in other mammalian species, however such an effect will likely occur earlier in development, according to the stage at which nucleoli are formed (e.g. at the 8-cell stage in cattle). DUBs certainly play an important role during fertilization in anti-polyspermy defence, specifically the Ubiquitin C-terminal hydrolases (UCHLs) [58]. UCHLs are the most important subgroup of DUBs. Further, there are four other families of DUBs: ubiquitin-specific proteases (USPs), ovarian-tumour domain (OTU DUBs), Machado–Joseph domain (MJD DUBs) and a Jab1/MPN metalloenzyme (JAMM) zinc-dependent metalloprotease (reviewed in [60]), whose role during preimplantation development has however not been elucidated yet.
UCHLs
As for oocytes and early embryos, UCHL1 and 3 are the most important members of the UCHL family. UCHL 1 and 3 are necessary for normal oocyte maturation and fertilization and the mechanism of their action seems to be highly conserved (reviewed in [60]). UCHL1 is highly expressed in porcine and bovine oocytes and is localized to the oocyte cortex [58, 61, 62]. This is likely because of its need for cortical granule maturation as part of the later polyspermy block [58]. It is further thought to regulate the level of maternal protein Mater [63]. UCHL3 is localized to the spindle and is probably involved in correct cumulus expansion [60]. Both UCHL1 and 3 are involved in correct polar body extrusion, which ensures leaving enough cytoplasm for the growing oocyte [60, 64]. The incorrect expression of UCHLs during oogenesis and fertilization causes abnormal embryo development, most defects are seen especially during morula compaction and blastocyst formation [64]. A potential role in early embryogenesis may also be played by UCHL2 (mostly referred to as BRCA1-associated protein 1; BAP1), which is needed for the removal of ubiquitin from histone H2B [65]. However, its role during preimplantation embryo development has not been studied at all yet.
Ring finger protein 114 (RNF114)
RNF114 is an E3 ubiquitin ligase that is predominantly expressed in oocytes and early embryos, and it is one of the most abundant proteins during the late stage of oocyte maturation [40, 66]. Recently, Yang et al. [38] found that RNF114 plays a crucial role in murine preimplantation development through the degradation of TAB1 (TGF-beta activated kinase 1 (MAP3K7) binding protein 1). Embryos with silenced RNF114 arrest at the two-cell stage (i.e. the stage of major EGA), which is likely caused by a defect in NFkB pathway activation during major EGA after TAB1 accumulation [38]. Interestingly, the authors studied more than 9000 proteins, and TAB1 was shown to be the only target of RNF114, whose degradation is necessary for normal preimplantation development [38]. This suggests that the degradation of individual proteins rather than all maternal proteins is important for the initiation of EGA.
specific proteasome assembly chaperone (ZPAC)
ZPAC is a protein that is specifically expressed in murine gonads, germ cells and early embryos, and not expressed in other somatic cells [46, 67]. This is probably because of an increased demand for protein degradation. The expression of ZPAC-like protein in other species has not been found to date [46]. The expression of ZPAC is increased at major EGA and is involved in the degradation of maternal proteins, as it interacts with and stabilizes proteasome assembly chaperone UMP1 (ubiquitin-mediated-proteolysis 1) and promotes 20S proteasome biogenesis [46]. The expression of these proteins is interconnected in the above-mentioned tissues and cells, as the silencing of one of them results in depleting the other [46]. ZPAC replaces PAC proteins that work as proteasome chaperones in somatic cells, probably because of the high abundance of β-precursors of the proteasome in quickly developing cells such as embryos [42, 46, 68]. Its protein level is highest from 12 to 36 h post fertilization (hpf; peak at 24 hpf) and is almost undetectable after the 8-cell stage in mice. Its mRNA becomes undetectable as soon as 36 hpf [46]. The UMP1 protein remains highly expressed until 48 hpf, but its level sharply decreases thereafter and is also almost undetectable from the 8-cell stage onwards [46]. This is a very early degradation compared to other maternal factors such as MATER that are present until the blastocyst stage. Both of these proteins are degraded in proteasomes, have a short life-time and are degraded and de novo synthesized even during early embryogenesis [46]. The majority of ZPAC knock-down and Ump1-knockdown embryos arrested between the 1- and 2-cell stage, i.e. just before the initiation of major EGA in mice because of the accumulation of β-subunits of the 20S proteasome [46, 67]. Both of these inhibitions cause a decrease in proteasomal activity and an accumulation of polyubiquinated proteins in the treated embryos [46]. ZPAC is also needed for normal spermatogenesis in mice [67, 69].
SCF complex
An SCF complex is an E3 ubiquitin ligase that is formed of Cullin1, SKP1, RBX1 and an F box protein. The F box determines the specificity of the complex to its substrate. There are also other types of similar ligases based on Cullin 2, 3, 4A, 4B, 5, 7 and 9 that are called Cullin 2–9 based CRL (Cullin-RING ubiquitin ligases) complexes, or often Cullin 2–9 based SCF complexes [70, 71]. Of these, CRL4 is known to be involved in mammalian preimplantation development [72–76].
SCF complexes are probably involved to a large extent in the protein degradation of maternal proteins, since there are a large number of SCF complexes in mouse oocytes and zygotes [39, 40], it is active throughout preimplantation development, and the embryonic expression of its invariant members is activated no later than the major EGA stage [45]. Moreover, the inhibition of SCF ligase activity causes an increase in total protein level [77]. It is certain that an SCF complex is responsible for the degradation of CDC6 (cell division cycle 6) before GVBD (germinal vesicle breakdown), CPEB (cytoplasmic polyadenylation element-binding protein) during oocyte maturation ([78, 79], CDC25A [M-phase inducer phosphatase 1]) in Xenopus around the major wave of MBT or DRF1/DFB4B (DBF4 zinc finger B) before the major MBT in Xenopus [80–84]. CRL4DCAF1 is responsible for protein phosphatase 2A degradation that is necessary for successful GVBD during murine oocyte maturation [85]. The processing of CDC6 and DRF1/DFB4B proteins is discussed in detail in the Specific protein degradation chapter. CRL4A is responsible for degradation of SUV39H1 (Suppressor Of Variegation 3–9 Homolog 1) and CDT1 (Chromatin Licensing and DNA Replication Factor 1) during early murine development and its malfunction during murine oocyte maturation causes early embryonic arrest and female infertility [72–76]. Normal function of CRL4 is involved in control of correct expression of cell fate genes like Cdx2, Nanog or Oct4 [72, 74].
Other UPS—related proteins involved in preimplantation development
In addition to the above-mentioned genes, several other proteins related to UPS were found to be necessary for normal preimplantation development. These are mainly E3 ubiquitin ligases (RNF20, MDM2 and Anaphase promoting complex/Cyclosome (APC/C) and the ubiquitin conjugation enzyme UBE2A/HR6A. RNF20 is responsible for H2B monoubiqutination. After the silencing of Rnf20, the ubiquitin ligase of H2B, the majority of embryos arrest at the morula stage and only one third of them develop to the blastocyst stage [86]. This arrest is however not related to protein degradation defects, but to the decrease in H2B monoubiquitination level. MDM2 causes the degradation of P53, and its knockout causes embryonic lethality due to P53 accumulation [87–89]. Nevertheless, this arrest occurs during the postimplantation period of development around E5.5 (embryonic day; [88, 89]). Anaphase promoting complex/cyclosome (APC/C) prevents securin degradation in mice oocytes and zygotes and causes arrest at the metaphase phase of the cell cycle [90, 91]. APC/C is known to be responsible for cyclin B and securin degradation, which is needed for the cell cycle transition to anaphase [92]. UBE2A/HR6A seems to play the most important role during preimplantation development as the embryos of a UBE2A/HR6A-deficient mother arrest at the 2-cell stage or earlier in mice [93], however the reason for this arrest is unknown. The early arrest of embryos shows that Ube2a/HR6A belongs to the Maternal effect genes group, like the Mater or Filia gene.
Autophagy
Autophagy is, after UPS, the second most known pathway for degrading proteins, but also other molecules like lipids or small organelles present in the cytoplasm, and it is involved in the degradation of maternal mRNA during early embryogenesis [94–96]. Besides the degradation of unnecessary protein, protein degradation by autophagy is also needed for the recycling of amino acids that are necessary for de novo protein synthesis [97]. Autophagy degrades its substrates using lysosomes. It starts with the engulfment of part of the cytoplasm into an autophagosome. The autophagosome then fuses with the lysosome, in which proteins are broken down into amino acids. The regulation of autophagy is driven mainly by mTOR and ATG proteins (reviewed in [98, 99]). Murine Atg5−/− embryos arrest during the preimplantation phase of development [97], Beclin1 (ortholog of Arg6)−/− embryos arrest after implantation at E7.5 [100] and the inhibition of autophagy causes a significant decrease in blastocyst number in the treated group [101]. Autophagy is also involved in the regulation of pluripotency-associated proteins OCT4, NANOG and SOX2 [101, 102], which together suggest that autophagy plays a crucial role during early embryogenesis. The genes involved in the autophagy pathway are conserved from yeast to humans (reviewed in [103]). The autophagy pathway needs to be started shortly after fertilization, and in mice is active until the late 1-cell stage [97]. Interestingly, this fertilization-induced activation of autophagy is not dependent on mTORC1 [104] (mTOR is a member of two complexes, mTORC1 and mTORC2 [105]). During the middle 2-cell stage it becomes reactivated and is active until the 8-cell stage. After that, the autophagy activity decreases to the basal level and is only reactivated after birth [103]. Nevertheless, autophagy is clearly also important during the 8-cell to implantation period of development [106–108].
A higher rate of autophagy seems to correlate with a higher embryo quality. It was shown that mouse embryos with higher autophagic activity at 4-cell are of higher quality [109], LC3 (autophagosome marker)-induced autophagy can improve porcine oocyte quality [110], the induction of autophagy in bovine preimplantation embryos increases their developmental competence and on the other hand the inhibition of autophagy leads to a decrease in developmental competence [111]. Autophagy further improves the blastocyst survival rate during implantation [108]. On the other hand, a higher autophagy of oocytes and embryos can result from a poor maturation or developmental condition and is known as pro-death autophagy [107, 112–116]. Autophagy (not only) during preimplantation development is driven by poly(ADP-ribosyl)ation (PARylation) and PARylation inhibition leads to a decreased autophagic degradation of ubiquinated aggregates, and consequently to a decreased developmental competence of porcine and murine preimplantation embryos, especially at the blastocyst stage [106, 107]. Both the inhibition and induction of autophagy during the earliest stages of preimplantation development (from 1- to 2-cell stage or longer) decreases the number of blastomeres and increases apoptosis in blastocysts in mice. Nevertheless, the induction of autophagy does not influence the number of embryos reaching the blastocyst stage, in contrast to the inhibition of autophagy [101]. This is probably caused by the overexpression of Cdx2 (caudal type homeobox 2), Nanog and Sox2 mRNA in the treated blastocysts. This was true after both the induction and inhibition of autophagy, yet the increase was more significant after the induction of apoptosis, and in addition Pou5fl (Oct3/4) was also overexpressed [101]. However, the induction/inhibition of autophagy after reaching the 2-cell stage does not affect the development of embryos [101]. The normal operation of autophagy during the division from the 1-cell to the 2-cell stage thus seems to be the most important period of preimplantation development for the production of a normal blastocyst. Surprisingly in pigs, the number of embryos reaching the blastocyst stage was not affected after the inhibition of autophagy from the 1-cell to morula stage, but the number significantly decreased after inhibition from the morula to the blastocyst stage [107]. The authors suggest that only UPS based degradation and not autophagy may be involved in the maternal protein degradation in early stages of porcine preimplantation development [107, 117]. Nevertheless, the development of pig embryos arising from oocytes with an affected autophagy pathway significantly decreases the number and quality of blastocysts [116].
The autophagic activity in embryos declines with maternal age [109] and the level of autophagy in 2–4-cell-stage cloned porcine embryos influences major EGA initiation [96]. Interestingly, the activity of autophagy is dependent on the origin of the embryos. The highest activity of autophagy in porcine cloned embryos was found at the 2-cell stage, however the same was true for 4-cell-stage parthenogenetically activated embryos and 1-cell-stage IVFderived embryos [96, 107]. This is also true for the relocalization of the LC3 autophagosome marker from nuclei to the cytoplasm that coincides with a decrease in autophagic activity [96, 107].
Further, autophagy is interconnected with ubiquitination, as it degrades protein complexes that were imperfectly degraded using UPS or in organelle degradation [103]. How ubiquitination is further processed depends on the type of ubiquitin (Ub) chain binding. Chains of 4 or more Ubs on lysine 48 results in processing by UPS, the binding of Ubs on lysine 63 (K63) results in autophagy, the endocytosis of membrane proteins or DNA repair [33, 118, 119]. The timing of K48 and K63 ubiquitination in the peri-fertilization time of C. elegans is different. While UPS-related ubiquitination is present especially before the MII stage, K63 ubiquitination starts from the MII stage onwards and is connected not only to autophagy but also endocytosis [119, 120]. During the degradation of damaged mitochondria, autophagy collaborates with the E3 ubiquitin ligases Parkin and MUL1, which ubiquitinates the mitochondrial membrane protein. In this way, autophagy becomes selective, as the ubiquitination targets the marked mitochondria for lysosomal degradation [121]. The selective degradation is otherwise typical, especially for starvation (reviewed in [60]). Ubiquitination and autophagy also collaborate during paternal mitophagy after fertilization [121–123]. The selective degradation of ubiquitinated proteins is mediated through protein P62, which is a receptor for ubiquitinated proteins for autophagy (reviewed in [124]).
In conclusion, autophagy and ubiquitination clearly intersect with each other to correctly degrade/store maternal proteins during preimplantation development. The activity of both these pathways reflects the quality of the embryo [35, 103]. Similarly to UPS-related pathways, autophagy (autophagosomes) is mainly localized to the trophectoderm at the blastocyst stage [45, 51, 108, 125]. However, autophagy during preimplantation development is to a large extent related to the degradation of targets other than proteins (maternal mRNA, lipids, epigenetic changes etc.) [95, 96].
Endocytosis
Endocytosis is involved in plasma membrane proteins degradation. The selected proteins are first loaded into an intraluminal vesicle and then form multivesicle bodies that fuse with lysosomes [33, 126, 127]. It may be triggered by monoubiquitination or K63 ubiquitination (which also triggers autophagy) [33, 127]. In C. elegans, endocytosis is involved in the selective degradation of plasma membrane proteins after fertilization (e.g. caveolin 1, receptor mediated endycytosis 2 RME-2, chitin synthase 1 CHS-1, EGG-1) [128–132]. In mammals, the role of endocytosis in maternal plasma membrane protein degradation has not been demonstrated to date, but its role seems to be undeniable, as it is one of the earliest activated pathways in the preimplantation embryo [133].
Ornithine decarboxylase
Ornithine decarboxylase (ODC1) is a crucially important enzyme for the synthesis of polyamines [134]. Its inhibition causes implantation defects and entry of the murine blastocyst into an embryonic diapause [135]. ODC1 expression is also important during peri-implantation stages in species that do not normally undergo embryonic diapause [136]. The ODC1−/− offspring of ODC1 ± parents are able to implant, but die soon thereafter. This is likely related to the fact that ODC1 expression is needed for ICM expansion [137]. The enzyme is unusual in its specific way of targeting for proteasomal degradation. Instead of being marked with ubiquitin, ODC1 interacts with ornithine decarboxylase antizyme (OAZ), which directs it to be degraded by the 26S proteasome. An intact COOH-terminal part of the protein is needed for the degradation (reviewed in [138]). It seems that ODC1 can be also degraded in an OAZ/COOH-terminal/ubiquitin-independent manner by the 20S proteasome, when not bound to NAD(P)H quinone oxidoreductase (reviewed in [138]). In Xenopus, the maternal protein is stable and is not degraded before MBT [139]. No such data are available for mammalian preimplantation embryos.
Timing of protein degradation during early embryogenesis
The first extensive protein degradation occurs even before fertilization, and enables the resumption of meiosis and transition to mitosis. This is when the degradation occurs of proteins like cytoplasmic polyadenylation element-binding protein 1 (CPEB1; involved in translational control) in Xenopus, bovine and mouse oocytes [140–143], ELAVL2 in mouse oocytes (translational control) [6], or MEI1 and 2 (microtubule severing complex) in C. elegans (reviewed in [144]). CPEB is degraded with E3 ubiquitin ligase SCFβ−TrCP [80]. MEI1 and 2 are degraded by Cul3-based ubiquitin ligase [145, 146]. The Skp1-Cullin1-Fbox (SCF) complex ligases are suggested to be involved in protein degradation during bovine oocyte maturation and preimplantation development [45, 77, 125], since after the inhibition of SCF-ligases activity during oocyte maturation, an increase in total protein level occurs (see chapter SCF ligases). Interestingly, this inhibition largely influences further preimplantation development even after the transfer of the MII oocytes to standard culture medium for fertilization [77]. The quality of the embryo likely depends on the correct course of protein degradation during oocyte maturation [35, 77]. UPS is also involved in cumulus cell expansion, as the inhibition of UCHL3 or SCF ligases during oocyte maturation prevents cumulus cell expansion [60, 77]. The embryo arising from an oocyte with a defective process of protein storage/degradation may not be able to overcome this handicap, even if its protein processing is appropriate during preimplantation development [35]. Interestingly, the inhibition of UPS using the proteasome inhibitor MG132 in oocytes leads to an increased quality of cloned mammalian embryos [147–152]. The treatment prevents the degradation of cyclin B, maintains MPF activity and cause premature chromosome condensation, which enables a better availability of remodelling and reprogramming of the somatic nucleus and thus better reorganization of chromatin [150, 153]. Furthermore, it likely prevents the inappropriate degradation of proteins essential for initiation of EGA in cloned embryos and regulates the expression of genes involved in histone acetylation [153].
The next extensive protein degradation comes after fertilization of the oocyte. This early degradation depends on both maternally derived UPS and autophagy [49, 97, 104]. After the inhibition of proteasomal activity, polyubiquinated proteins become accumulated after fertilization [154]. UPS is likely involved in the proper reprogramming of the differentiated oocyte into a totipotent zygote after fertilization and its dysfunction is likely the reason for the deteriorated development of somatic cell nuclear transfer embryos [154]. The mouse zygote is enriched in proteins involved in the ubiquitin–proteasome pathway in comparison to MII oocytes, and the amount of proteins in zygotes is decreased compared to MII [39]. In murine MII oocytes, proteins related to ubiquitination and protein degradation amount to 9% [155]. This points to the rapid degradation of maternal proteins after fertilization in mice mentioned in the Ubiquitin proteasome pathway chapter. Moreover, a large set of F-box proteins is present in murine MII and zygotes, which suggests the involvement of an SCF complex in maternal protein degradation in mice [39]. Nevertheless, F-box proteins are involved in many other processes such as epigenetic regulation or reprogramming and its overexpression during the perifertilization period is likely also connected to these UPS-unrelated functions [39]. These results point to the involvement of UPS in post-fertilization protein degradation.
Further degradation is not thoroughly described, and is thought to not be so massive. Instead individual proteins are degraded at specific time points. We suppose that there is a smaller wave of maternal protein degradation before/at the major EGA stage, similarly to such degradation in Xenopus (discussed in the Specific protein degradation chapter). The degradation of maternal proteins is the easiest to explore in genes that are only expressed from maternally derived reserves. For example, the maternal protein MATER (NLRP5) is stored until the blastocyst stage and hatched blastocyst stage, when it is degraded in mice and bovines, respectively [156, 157]. Gao et al. [158] detected a decrease in the protein amount of maternal factors MATER (NLRP5), Floped or TLE6 even at the 8-cell stage. We suppose that the discrepancy in results is caused by the different method used, as Gao et al. used mass spectrometry (MS) and Pennetier and Ohsugi and their colleagues used western blot analysis [156–158]. Murine ZPAC and UMP1 are among the earliest degraded maternal proteins. Their degradation occurs at the 8-cell stage [46] and is discussed in the ZPAC chapter. The degradation of TAB1 occurs even earlier (2-cell stage). This degradation is necessary for the normal course of major EGA [38].
Table 1.
Proteins degraded in the time of EGA
| Protein | Function | Organism | References |
|---|---|---|---|
| TAB1 | Regulator of MAP3K7/TAK1, NFKB, MAPK14/p38alpha | Mouse | [38] |
| CDC25A | Induces progression of mitosis; tyrosine protein phosphatase | Xenopus, Drosophila | [81, 82, 185] |
| CDC6 | Controls progression of DNA replication | Xenopus | [9] |
| Treslin | Regulates DNA replication | Xenopus | [9] |
| RECQL4 | DNA dependent ATPase | Xenopus | [9] |
| TOPBP1 | Involved in DNA replication | Xenopus | [9] |
| DRF1/DBF4B | Plays crucial role DNA replication control, Cdc7 subunit | Xenopus | [168] |
The degradation of these proteins in necessary for normal course of EGA
Specific protein degradation
Even though there is a large decrease in protein number from MII stage oocytes to zygotes in mouse oocytes [39], not all proteins are degraded before the initiation of EGA. Some of the proteins are stored throughout the whole preimplantation development [157, 159, 160]; reviewed in [161]. In Xenopus, a stable amount of maternally stored proteins is stored long after the MBT, until beating heart [162].
Maternal proteins often form complexes that persist to preimplantation development and are then involved in the regulation of embryogenesis even after major EGA (reviewed in [161]). These are mainly functional complexes of proteins such as Zona pellucida sperm-binding proteins (ZPs) or SCMC (subcortical maternal complex; formed of MATER, FLOPED, TLE6, Filia, PADI6) and likely other proteins [163]. The expression of these genes is exclusively maternal, and mRNAs of its members are degraded at EGA. However, the proteins are stored until the blastocyst stage (reviewed in [164]). Another case of protein modification is the temporary masking of the protein CENPE during oocyte maturation, and it is possible that other proteins are also modified or form complexes to enable their storage to preimplantation development [165]; reviewed in [161]. Some cell cycle regulating proteins (like stem-loop binding protein or centromeric protein F) that are expressed in a cell-cycle dependent manner are expressed constantly in oocytes and early embryos [166, 167].
On the other hand, some maternal proteins need to be degraded, so that the embryo can take control over their development. The list of proteins that needs to be degraded before the major wave of EGA/MBT is shown in Table 1. Inmouse, the degradation of the TAB1 protein is needed for the normal course of major EGA [38]. Degradation of SUV39H1 by Cullin Ring Ligase 4A (CRL4A) is necessary for normal preimplantation development [74]. SUV39H1 is degraded by CRL4ADCAF13. The SUV39H1-enriched embryos develop normally to 8-cell but are not able to compact and form blastocyst [74]. CRL4 is necessary also for CDT1 degradation. The CDT1 enriched murine embryos arrest already at 1–2-cell, i. e. before the major EGA. Nevertheless, this degradation seems to be cell cycle specific rather than EGA-specific. Moreover, several other unidentified substrates seems to be degraded by CRL4 enzymes during preimplantation development too [74, 76]. Even though it seems that in general maternal protein degradation is not needed for embryonic genome activation, Shin et al. [5] have shown that the transient inhibition of the ubiquitin proteasome pathway in murine preimplantation embryos using MG132 delays embryonic genome activation. This delay occurs regardless of whether the embryo is arrested or developing. This suggests that the degradation of maternal protein is highly specific and the degradation of one is just as important as keeping the others.
In Xenopus, five proteins that need to be degraded at major EGA were identified: TOPBP1 (a human ortholog of Xenopus Cut5), RECQ4, Treslin, DRF1 (Dumbbell-forming 4; a homolog of mammalian DBF4B) and XCDC6 (cell division control protein; [9, 168]). All these genes are involved in replication control. DRF1/DBF4B, being part of CDC7, is essential for the activation of the replication origin complex that the others are part of. CDC6 is one of the first proteins loaded into the complex. TOPBP1/CUT5, RECQ4 (or RECQL4 in mammals) and Treslin are part of the replication pre-initiation complex and are loaded to the replication origin in the second step (reviewed in [169]). Thus, the need for degradation of these proteins is likely connected to the lengthening of the cell cycle after the MBT and less a need for replication initiation. Whether the degradation of these genes is needed for the normal course of major EGA in mammals is to the best of our knowledge unknown. Certainly, these genes are important for mammalian preimplantation development as well. The knockouts are mostly lethal at peri-implantation stages in mammals, however the development of lower animals is enabled far beyond the MBT stage [170–177]. The only exception is Drf1/DBF4B. Drf1−/− mice are viable, only develop lymphopenia [178] and mutant cell cultures show affected cytoskeletal remodelling [179]. A complete knockout of Drf1/DBF4B was found as a rare sequence variant in humans [180] which further confirms that embryonic development is possible without Drf1/DBF4B expression even though it participates in the manifestation of disease. This similarity of null phenotypes in these genes suggests that the initiation of embryonic protein synthesis is as important as the degradation of the maternal protein. The differences between mammalian and non-mammalian species are likely due to the difference in developmental speed and must be taken into account when data are transferred between different model organisms. Taken together, it is clear that all the above-mentioned genes are needed for the normal preimplantation development of mammals, however there is no evidence on whether the degradation of maternal protein before major EGA is as necessary as in the Xenopus embryo. Certainly, these genes together form a group of proteins that play a similar role during the earliest phases of development and are processed in a similar manner. The necessity of their degradation is further supported by the overexpression experiments. Embryos overexpressing XCdc6 exhibit a high level of apoptosis [9]. Embryos that combined the overexpression of Cut5, RecQ4, Treslin and Drf1 do not lengthen cell cycles (dependently on protein level) and arrest at stage 17 (i.e. at 17th division) [168]. After the overexpression of Drf1/DBF4B, the viability of the Xenopus embryos decreases after stage 11 [83]. Interestingly, DBF4 overexpression in pre-MBT embryos can suppress the activation of Chk1, but the overexpression is not lethal, in contrast to Drf1 overexpression [83]. Thus, it would be extremely interesting to study the whole group thoroughly and complexly during mammalian preimplantation development.
In Xenopus, two of the above-mentioned proteins—DRF1/DBF4B and CDC6—have two distinct variants expressed from two different genes with a switch between those variants around the major MBT. DRF1/DBF4B is replaced by DBF4 before the major wave of MBT and cannot be detected afterwards (Figs. 2, 3). Consequently DRF1/DBF4B is present mainly in embryonic cells and DBF4 is found mainly in adult cells [181, 182]. With Cdc6, the Xcdc6A, and Xcdc6B variants were found. Only Xcdc6A is functional at early embryonic stages (Xcdc6B is present in early stages, however not functional). It switches around the major MBT stage from XCdcd6A to XCdcd6B and Xcdc6A is undetectable after stage 12 (Fig. 3) and only Xcdc6B is present in somatic cells [183]. Interestingly, Xcdc6A is not expressed in a cell-cycle dependent manner like the somatic Xcdc6B is [183]. Tikhmyanova and Coleman [183], however, suppose that the CDCD6A/B isoforms may be restricted to Xenopus since they did not find the isoforms in humans, fruit flies, puffer fish and worms in the available genomic databases, and their regulation significantly differs in different organisms [183]. In mammals, a similar switch was found in bovine embryos in Cullin 1, where the maternal variant of Cullin 1 mRNA switches to the embryonic (adult) Cullin 1 just before the major wave of EGA [45, 50]. The time of protein variant switch is unfortunately unknown. In Xenopus embryos, DRF1/DBF4B is degraded by SCFβ−TRCP (Skp1–Cullin 1–Fbox complex where β-TRCP represents the F-box protein) at the major MBT. This ensures a DRF1/DBF4B shortage and consequently a lengthening of cell cycles [83]. Thus, we suppose that the switch between DBF4 and Cullin 1 variants might be interconnected and it would be really interesting to find out whether there is a similar switch between DRF1/DBF4B and DBF4 in mammals and study the link between the maternal to embryonic Cullin 1 switch and DRF1/DBF4B degradation. The way that XCDC6 degrade at the major wave of MBT is to the best of our knowledge unknown. However, the degradation of CDC6 before GVBD is UPS-dependent and is mediated by SCFFBXW7 [84]. (The decrease in CDC6 protein level in prophase arrest is caused by a decrease in its translation and is UPS independent.)
Similarly to the above-described proteins involved in replication control, CDC25A is degraded in Xenopus embryos around the minor MBT by SCFβ−TRCP (Figs. 2, 3) [81, 82]. CDC25A is a mitotic progression inducer and is involved in the resumption of meiosis during mouse oocyte maturation [184]. In Drosophila, a steep decrease in CDC25A level in cycles 10–13 causes cell cycle lengthening [185]. This is partly caused by CHK1 inhibition, which in Xenopus mediates the degradation of DRF1 by SCFβ−TRCP [83]. In Drosophila, the inhibition of CHK1 is sufficient for the prevention of cell cycle lengthening, which is not true for Xenopus embryos [83, 186].
The necessity of UPS for EGA was also found in mammalian species, as the deactivation of UPS or its part delays the initiation of both the minor and major wave of EGA [5, 38, 46, 67, 77, 154]. However, specific proteins that need to be degraded have not yet been found in mammals. Higuchi et al. [154] suppose that the delay is caused by the delayed recruitment of Pol II into the embryo pronuclei. According to their results, the delayed initiation of DNA replication does not seem to cause the delay in major EGA initiation [154]. Together with the results of Collart et al. [168], Sun et al. [9], Farrell et al. [185] and others, this suggests that for the normal course of EGA and further development, the replication rate decrease around major EGA and consequent slowing of development speed is especially important.
Concluding remarks
In this review, we summarized current knowledge of the way proteins are processed during preimplantation development. The degradation of maternal proteins is slower than the degradation of maternal RNAs with a large impact on specificity, timing and likely also localization of the degradation. It seems that the most important period for normal preimplantation development in terms of protein degradation occurs already during oocyte maturation. Normal maternal protein degradation during proper preimplantation development is definitely important as well, however without correct protein processing during oocyte maturation, the embryos are not able to develop normally, even under standard conditions. Both autophagy and UPS are necessary for normal preimplantation development. However, the extent to which each of these pathways takes part in maternal protein degradation has not been determined yet.
Since there is limiting data available concerning mammals, the data were also based on a comparison with non-mammalian species and the timing of individual events related to maternal protein degradation in several species was compared. For this purpose, the preparation of a theoretical model that will facilitate the transfer of knowledge from one model organism to the others will be highly beneficial. For a proper understanding of protein processing during preimplantation development, it will be necessary to concentrate especially on the time period around the major wave of EGA. This period is not well described in mammals, and based on the results obtained with lower organisms, it seems to be to a large extent driven by protein degradation. During this period, it is likely that no general or extensive degradation will occur (such as during oocyte maturation or fertilization). We suppose there is a specific degradation of some groups of proteins or even of individual proteins. Identifying such proteins will however be a very challenging and time-consuming work, when we take into account the identification by Yang et al. [38] of just one protein whose degradation is necessary for the normal course of major EGA.
Acknowledgements
Supported by Grant GACR 13-24730P and Internal Grant Agency of the Czech University of Life Sciences CIGA 20172013. This work was supported by IAPG institutional support RVO: 67985904. JK was supported by the Danish Council for Independent Research/Natural Sciences (FNU) 8021-00048B. The authors would like to thank B. J. Watson-Jones for reading the manuscript and language correction.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Svoboda P, Fulka H, Malik R. Clearance of parental products. Adv Exp Med Biol. 2017;953:489–535. doi: 10.1007/978-3-319-46095-6_10. [DOI] [PubMed] [Google Scholar]
- 2.Sha QQ, Zhang J, Fan HY. A story of birth and death: mRNA translation and clearance at the onset of maternal-to-zygotic transition in mammals. Biol Reprod. 2019;101:579–590. doi: 10.1093/biolre/ioz012. [DOI] [PubMed] [Google Scholar]
- 3.Yokoi H, Natsuyama S, Iwai M, Noda Y, Mori T, Mori KJ, Fujita K, Nakayama H, Fujita J. Nonradioisotopic quantitative RT-PCR to detect changes in messenger-RNA levels during early mouse embryo development. Biochem Biophys Res Commun. 1993;195:769–775. doi: 10.1006/bbrc.1993.2112. [DOI] [PubMed] [Google Scholar]
- 4.Karabinova P, Kubelka M, Susor A. Proteasomal degradation of ubiquitinated proteins in oocyte meiosis and fertilization in mammals. Cell Tissue Res. 2011;346:1–9. doi: 10.1007/s00441-011-1235-1. [DOI] [PubMed] [Google Scholar]
- 5.Shin SW, Tokoro M, Nishikawa S, Lee HH, Hatanaka Y, Nishihara T, Amano T, Anzai M, Kato H, Mitani T, et al. Inhibition of the ubiquitin–proteasome system leads to delay of the onset of ZGA gene expression. J Reprod Dev. 2010;56:655–663. doi: 10.1262/jrd.10-104m. [DOI] [PubMed] [Google Scholar]
- 6.Chalupnikova K, Solc P, Sulimenko V, Sedlacek R, Svoboda P. An oocyte-specific ELAVL2 isoform is a translational repressor ablated from meiotically competent antral oocytes. Cell Cycle. 2014;13:1187–1200. doi: 10.4161/cc.28107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huo LJ, Zhong ZS, Liang CG, Wang Q, Yin S, Ai JS, Yu LZ, Chen DY, Schatten H, Sun QY. Degradation of securin in mouse and pig oocytes is dependent on ubiquitin–proteasome pathway and is required for proteolysis of the cohesion subunit, Rec8, at the metaphase-to-anaphase transition. Front Biosci. 2006;11:2193–2202. doi: 10.2741/1961. [DOI] [PubMed] [Google Scholar]
- 8.Bachvarova R. Synthesis, turnover, and stability of heterogeneous RNA in growing mouse oocytes. Dev Biol. 1981;86:384–392. doi: 10.1016/0012-1606(81)90196-2. [DOI] [PubMed] [Google Scholar]
- 9.Sun L, Bertke MM, Champion MM, Zhu G, Huber PW, Dovichi NJ. Quantitative proteomics of Xenopus laevis embryos: expression kinetics of nearly 4000 proteins during early development. Sci Rep. 2014;4:4365. doi: 10.1038/srep04365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson GRW, Brahmasani SR, Yelisetti UM, Konijeti S, Katari VC, Sisinthy S. Candidate gene expression patterns in rabbit preimplantation embryos developed in vivo and in vitro. J Assist Reprod Genet. 2014;31:899–911. doi: 10.1007/s10815-014-0233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee G, Hynes R, Kirschner M. Temporal and spatial regulation of fibronectin in early Xenopus development. Cell. 1984;36:729–740. doi: 10.1016/0092-8674(84)90353-2. [DOI] [PubMed] [Google Scholar]
- 12.Jansova D, Tetkova A, Koncicka M, Kubelka M, Susor A. Localization of RNA and translation in the mammalian oocyte and embryo. PLoS ONE. 2018;13:e0192544. doi: 10.1371/journal.pone.0192544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tetkova A, Jansova D, Susor A. Spatio-temporal expression of ANK2 promotes cytokinesis in oocytes. Sci Rep. 2019;9:13121. doi: 10.1038/s41598-019-49483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B, Winkler F, Herde M, Witte CP, Großhans J. A link between deoxyribonucleotide metabolites and embryonic cell-cycle control. Curr Biol. 2019;29:1187–1192. doi: 10.1016/j.cub.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Djabrayan NJV, Smits CM, Krajnc M, Stern T, Yamada S, Lemon WC, Keller PJ, Rushlow CA, Shvartsman SY. Metabolic regulation of developmental cell cycles and zygotic transcription. Curr Biol. 2019;29:1193–1198. doi: 10.1016/j.cub.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagaraj R, Sharpley MS, Chi F, Braas D, Zhou Y, Kim R, Clark AT, Banerjee U. Nuclear localization of mitochondrial TCA cycle enzymes as a critical step in mammalian zygotic genome activation. Cell. 2017;168:210–223. doi: 10.1016/j.cell.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert SF. Developmental biology. 6. Sunderland: Sinauer Associates; 2000. [Google Scholar]
- 18.Tomanek M, Kopecny V, Kanka J. Genome reactivation in developing early pig embryos: an ultrastructural and autoradiographic analysis. Anat Embryol (Berl) 1989;180:309–316. doi: 10.1007/BF00315889. [DOI] [PubMed] [Google Scholar]
- 19.Kanka J. Gene expression and chromatin structure in the pre-implantation embryo. Theriogenology. 2003;59:3–19. doi: 10.1016/s0093-691x(02)01267-0. [DOI] [PubMed] [Google Scholar]
- 20.Kanka J, Bryova A, Duranthon V, Oudin JF, Peynot N, Renard JP. Identification of differentially expressed mRNAs in bovine preimplantation embryos. Zygote. 2003;11:43–52. doi: 10.1017/s0967199403001060. [DOI] [PubMed] [Google Scholar]
- 21.Johnson MH, Ziomek CA. The foundation of two distinct cell lineages within the mouse morula. Cell. 1981;24:71–80. doi: 10.1016/0092-8674(81)90502-x. [DOI] [PubMed] [Google Scholar]
- 22.Johnson MH, Ziomek CA. Induction of polarity in mouse 8-cell blastomeres: specificity, geometry, and stability. J Cell Biol. 1981;91:303–308. doi: 10.1083/jcb.91.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiiragi T, Solter D. First cleavage plane of the mouse egg is not predetermined but defined by the topology of the two apposing pronuclei. Nature. 2004;430:360–364. doi: 10.1038/nature02595. [DOI] [PubMed] [Google Scholar]
- 24.Motosugi N, Bauer T, Polanski Z, Solter D, Hiiragi T. Polarity of the mouse embryo is established at blastocyst and is not prepatterned. Genes Dev. 2005;19:1081–1092. doi: 10.1101/gad.1304805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wennekamp S, Mesecke S, Nédélec F, Hiiragi T. A self-organization framework for symmetry breaking in the mammalian embryo. Nat Rev Mol Cell Biol. 2013;14:452–459. doi: 10.1038/nrm3602. [DOI] [PubMed] [Google Scholar]
- 26.Lund E, Sheets MD, Imboden SB, Dahlberg JE. Limiting Ago protein restricts RNAi and microRNA biogenesis during early development in Xenopus laevis. Genes Dev. 2011;25:1121–1131. doi: 10.1101/gad.2038811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs O. The role of ubiquitin–proteasome system in transforming growth factor-β signaling and its importance in tumorigenesis. Klin Onkol. 2005;18:199–206. [Google Scholar]
- 29.Muratani M, Tansey WP. How the ubiquitin–proteasome system controls transcription. Nat Rev Mol Cell Biol. 2003;4:192–201. doi: 10.1038/nrm1049. [DOI] [PubMed] [Google Scholar]
- 30.Osley MA. H2B ubiquitylation: the end is in sight. Biochim Biophys Acta. 2004;1677:74–78. doi: 10.1016/j.bbaexp.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Lipford JR, Smith GT, Chi Y, Deshaies RJ. A putative stimulatory role for activator turnover in gene expression. Nature. 2005;438:113–116. doi: 10.1038/nature04098. [DOI] [PubMed] [Google Scholar]
- 32.Gillette TG, Gonzalez F, Delahodde A, Johnston SA, Kodadek T. Physical and functional association of RNA polymerase II and the proteasome. Proc Natl Acad Sci USA. 2004;101:5904–5909. doi: 10.1073/pnas.0305411101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 34.DeRenzo C, Seydoux G. A clean start: degradation of maternal proteins at the oocyte-to-embryo transition. Trends Cell Biol. 2004;14:420–426. doi: 10.1016/j.tcb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Mtango NR, Latham KE. Ubiquitin proteasome pathway gene expression varies in rhesus monkey oocytes and embryos of different developmental potential. Physiol Genom. 2007;31:1–14. doi: 10.1152/physiolgenomics.00040.2007. [DOI] [PubMed] [Google Scholar]
- 36.Verlhac MH, Terret ME, Pintard L. Control of the oocyte-to-embryo transition by the ubiquitin–proteolytic system in mouse and C. elegans. Curr Opin Cell Biol. 2010;22:758–763. doi: 10.1016/j.ceb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Suzumori N, Burns KH, Yan W, Matzuk MM. RFPL4 interacts with oocyte proteins of the ubiquitin–proteasome degradation pathway. Proc Natl Acad Sci USA. 2003;100:550–555. doi: 10.1073/pnas.0234474100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, Zhou C, Wang Y, Liu W, Liu C, Wang L, Liu Y, Shang Y, Li M, Zhou S, et al. The E3 ubiquitin ligase RNF114 and TAB1 degradation are required for maternal-to-zygotic transition. EMBO Rep. 2017;18:205–216. doi: 10.15252/embr.201642573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Kou Z, Jing Z, Zhang Y, Guo X, Dong M, Wilmut I, Gao S. Proteome of mouse oocytes at different developmental stages. Proc Natl Acad Sci USA. 2010;107:17639–17644. doi: 10.1073/pnas.1013185107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang P, Ni X, Guo Y, Guo X, Wang Y, Zhou Z, Huo R, Sha J. Proteomic-based identification of maternal proteins in mature mouse oocytes. BMC Genom. 2009;10:348. doi: 10.1186/1471-2164-10-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livneh I, Cohen-Kaplan V, Cohen-Rosenzweig C, Avni N, Ciechanover A. The life cycle of the 26S proteasome: from birth, through regulation and function, and onto its death. Cell Res. 2016;26:869–885. doi: 10.1038/cr.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meiners S, Heyken D, Weller A, Ludwig A, Stangl K, Kloetzel PM, Krüger E. Inhibition of proteasome activity induces concerted expression of proteasome genes and de novo formation of Mammalian proteasomes. J Biol Chem. 2003;278:21517–21525. doi: 10.1074/jbc.M301032200. [DOI] [PubMed] [Google Scholar]
- 43.Potireddy S, Vassena R, Patel BG, Latham KE. Analysis of polysomal mRNA populations of mouse oocytes and zygotes: dynamic changes in maternal mRNA utilization and function. Dev Biol. 2006;298:155–166. doi: 10.1016/j.ydbio.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 44.Graf A, Krebs S, Heininen-Brown M, Zakhartchenko V, Blum H, Wolf E. Genome activation in bovine embryos: review of the literature and new insights from RNA sequencing experiments. Anim Reprod Sci. 2014;149:46–58. doi: 10.1016/j.anireprosci.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Benesova V, Kinterova V, Kanka J, Toralova T. Characterization of SCF-complex during bovine preimplantation development. PLoS ONE. 2016;11:e0147096. doi: 10.1371/journal.pone.0147096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin SW, Shimizu N, Tokoro M, Nishikawa S, Hatanaka Y, Anzai M, Hamazaki J, Kishigami S, Saeki K, Hosoi Y, et al. Mouse zygote-specific proteasome assembly chaperone important for maternal-to-zygotic transition. Biol Open. 2013;2:170–182. doi: 10.1242/bio.20123020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wójcik C, Benchaib M, Lornage J, Czyba JC, Guerin JF. Localization of proteasomes in human oocytes and preimplantation embryos. Mol Hum Reprod. 2000;6:331–336. doi: 10.1093/molehr/6.4.331. [DOI] [PubMed] [Google Scholar]
- 48.Evsikov AV, de Vries WN, Peaston AE, Radford EE, Fancher KS, Chen FH, Blake JA, Bult CJ, Latham KE, Solter D, Knowles BB. Systems biology of the 2-cell mouse embryo. Cytogenet Genome Res. 2004;105:240–250. doi: 10.1159/000078195. [DOI] [PubMed] [Google Scholar]
- 49.Huo LJ, Fan HY, Zhong ZS, Chen DY, Schatten H, Sun QY. Ubiquitin–proteasome pathway modulates mouse oocyte meiotic maturation and fertilization via regulation of MAPK cascade and cyclin B1 degradation. Mech Dev. 2004;121:1275–1287. doi: 10.1016/j.mod.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Kepkova KV, Vodicka P, Toralova T, Lopatarova M, Cech S, Dolezel R, Havlicek V, Besenfelder U, Kuzmany A, Sirard MA, Laurincik J, Kanka J. Transcriptomic analysis of in vivo and in vitro produced bovine embryos revealed a developmental change in cullin 1 expression during maternal-to-embryonic transition. Theriogenology. 2011;75:1582–1595. doi: 10.1016/j.theriogenology.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 51.Sutovsky P, Motlik J, Neuber E, Pavlok A, Schatten G, Palecek J, Hyttel P, Adebayo OT, Adwan K, Alberio R, et al. Accumulation of the proteolytic marker peptide ubiquitin in the trophoblast of mammalian blastocysts. Cloning Stem Cells. 2001;3:157–161. doi: 10.1089/153623001753205115. [DOI] [PubMed] [Google Scholar]
- 52.Baek KH, Lee H, Yang S, Lim SB, Lee W, Lee JE, Lim JJ, Jun K, Lee DR, Chung Y. Embryonic demise caused by targeted disruption of a cysteine protease Dub-2. PLoS ONE. 2012;7:e44223. doi: 10.1371/journal.pone.0044223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu H, Shamanna RA, de Freitas JK, Okur M, Khadka P, Kulikowicz T, Holland PP, Tian J, Croteau DL, Davis AJ, et al. Cell cycle-dependent phosphorylation regulates RECQL4 pathway choice and ubiquitination in DNA double-strand break repair. Nat Commun. 2017;8:2039. doi: 10.1038/s41467-017-02146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin J, Kwon YT, Varshavsky A, Wang W. RECQL4, mutated in the Rothmund–Thomson and RAPADILINO syndromes, interacts with ubiquitin ligases UBR1 and UBR2 of the N-end rule pathway. Hum Mol Genet. 2004;13:2421–2430. doi: 10.1093/hmg/ddh269. [DOI] [PubMed] [Google Scholar]
- 55.Larsen CN, Krantz BA, Wilkinson KD. Substrate specificity of deubiquitinating enzymes: ubiquitin C-terminal hydrolases. Biochemistry. 1998;37:3358–3368. doi: 10.1021/bi972274d. [DOI] [PubMed] [Google Scholar]
- 56.Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004;1695:189–207. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Wilkinson KD. DUBs at a glance. J Cell Sci. 2009;122:2325–2329. doi: 10.1242/jcs.041046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Susor A, Liskova L, Toralova T, Pavlok A, Pivonkova K, Karabinova P, Lopatarova M, Sutovsky P, Kubelka M. Role of ubiquitin C-terminal hydrolase-L1 in antipolyspermy defense of mammalian oocytes. Biol Reprod. 2010;82:1151–1161. doi: 10.1095/biolreprod.109.081547. [DOI] [PubMed] [Google Scholar]
- 59.Fraile JM, Campos-Iglesias D, Rodríguez F, Astudillo A, Vilarrasa-Blasi R, Verdaguer-Dot N, Prado MA, Paulo JA, Gygi SP, Martín-Subero JI, et al. Loss of the deubiquitinase USP36 destabilizes the RNA helicase DHX33 and causes preimplantation lethality in mice. J Biol Chem. 2018;293:2183–2194. doi: 10.1074/jbc.M117.788430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mtango NR, Latham KE, Sutovsky P. Deubiquitinating enzymes in oocyte maturation, fertilization and preimplantation embryo development. Adv Exp Med Biol. 2014;759:89–110. doi: 10.1007/978-1-4939-0817-2_5. [DOI] [PubMed] [Google Scholar]
- 61.Ellederova Z, Halada P, Man P, Kubelka M, Motlik J, Kovarova H. Protein patterns of pig oocytes during in vitro maturation. Biol Reprod. 2004;71:1533–1539. doi: 10.1095/biolreprod.104.030304. [DOI] [PubMed] [Google Scholar]
- 62.Massicotte L, Coenen K, Mourot M, Sirard MA. Maternal housekeeping proteins translated during bovine oocyte maturation and early embryo development. Proteomics. 2006;6:3811–3820. doi: 10.1002/pmic.200500803. [DOI] [PubMed] [Google Scholar]
- 63.Koyanagi S, Hamasaki H, Sekiguchi S, Hara K, Ishii Y, Kyuwa S, Yoshikawa Y. Effects of ubiquitin C-terminal hydrolase L1 deficiency on mouse ova. Reproduction. 2012;143:271–279. doi: 10.1530/REP-11-0128. [DOI] [PubMed] [Google Scholar]
- 64.Mtango NR, Sutovsky M, VandeVoort CA, Latham KE, Sutovsky P. Essential role of ubiquitin C-terminal hydrolases UCHL1 and UCHL3 in mammalian oocyte maturation. J Cell Physiol. 2012;227:2022–2029. doi: 10.1002/jcp.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scheuermann JC, Gutiérrez L, Müller J. Histone H2A monoubiquitination and Polycomb repression: the missing pieces of the puzzle. Fly (Austin) 2012;6:162–168. doi: 10.4161/fly.20986. [DOI] [PubMed] [Google Scholar]
- 66.Liu C, Ma Y, Shang Y, Huo R, Li W. Post-translational regulation of the maternal-to-zygotic transition. Cell Mol Life Sci CMLS. 2018;75:1707–1722. doi: 10.1007/s00018-018-2750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimizu N, Ueno K, Kurita E, Shin SW, Nishihara T, Amano T, Anzai M, Kishigami S, Kato H, Mitani T, Hosoi Y, et al. Possible role of ZPAC, zygote-specific proteasome assembly chaperone, during spermatogenesis in the mouse. J Reprod Dev. 2014;60:179–186. doi: 10.1262/jrd.2014-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramos PC, Dohmen RJ. PACemakers of proteasome core particle assembly. Structure. 2008;16:1296–1304. doi: 10.1016/j.str.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Zuo EW, Yang XG, Lu YQ, Xie L, Shang JH, Li D, Yang H, Hu LL, Zhao HM, Lu SS, et al. ZPAC is required for normal spermatogenesis in mice. Mol Reprod Dev. 2015;82:747–755. doi: 10.1002/mrd.22507. [DOI] [PubMed] [Google Scholar]
- 70.Bosu DR, Kipreos ET. Cullin-RING ubiquitin ligases: global regulation and activation cycles. Cell Div. 2008;3:7. doi: 10.1186/1747-1028-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou L, Zhang W, Sun Y, Jia L. Protein neddylation and its alterations in human cancers for targeted therapy. Cell Signal. 2018;44:92–102. doi: 10.1016/j.cellsig.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu C, Zhang YL, Pan WW, Li XM, Wang ZW, Ge ZJ, Zhou JJ, Cang Y, Tong C, Sun QY, Fan HY. CRL4 complex regulates mammalian oocyte survival and reprogramming by activation of TET proteins. Science. 2013;342:1518–1521. doi: 10.1126/science.1244587. [DOI] [PubMed] [Google Scholar]
- 73.Xu YW, Cao LR, Wang M, Xu Y, Wu X, Liu J, Tong C, Fan HY. Maternal DCAF2 is crucial for maintenance of genome stability during the first cell cycle in mice. J Cell Sci. 2017;130:3297–3307. doi: 10.1242/jcs.206664. [DOI] [PubMed] [Google Scholar]
- 74.Zhang YL, Zhao LW, Zhang J, Le R, Ji SY, Chen C, Gao Y, Li D, Gao S, Fan HY. DCAF13 promotes pluripotency by negatively regulating SUV39H1 stability during early embryonic development. EMBO J. 2018;37:e9898. doi: 10.15252/embj.201898981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, Zhang YL, Zhao LW, Guo JX, Yu JL, Ji SY, Cao LR, Zhang SY, Shen L, Ou XH, Fan HY. Mammalian nucleolar protein DCAF13 is essential for ovarian follicle maintenance and oocyte growth by mediating rRNA processing. Cell Death Differ. 2019;26:1251–1266. doi: 10.1038/s41418-018-0203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Zhao LW, Shen JL, Fan HY, Jin Y. Maternal DCAF13 regulates chromatin tightness to contribute to embryonic development. Sci Rep. 2019;9:6278. doi: 10.1038/s41598-019-42179-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kinterova V, Kanka J, Petruskova V, Toralova T. Inhibition of Skp1-Cullin-F-box complexes during bovine oocyte maturation and preimplantation development leads to delayed development of embryos. Biol Reprod. 2019;100:896–906. doi: 10.1093/biolre/ioy254. [DOI] [PubMed] [Google Scholar]
- 78.Chen J, Melton C, Suh N, Oh JS, Horner K, Xie F, Sette C, Blelloch R, Conti M. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 2011;25:755–766. doi: 10.1101/gad.2028911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sousa Martins JP, Liu X, Oke A, Arora R, Franciosi F, Viville S, Laird DJ, Fung JC, Conti M. DAZL and CPEB1 regulate mRNA translation synergistically during oocyte maturation. J Cell Sci. 2016;129:1271–1282. doi: 10.1242/jcs.179218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Setoyama D, Yamashita M, Sagata N. Mechanism of degradation of CPEB during Xenopus oocyte maturation. Proc Natl Acad Sci USA. 2007;104:18001–18006. doi: 10.1073/pnas.0706952104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shimuta K, Nakajo N, Uto K, Hayano Y, Okazaki K, Sagata N. Chk1 is activated transiently and targets Cdc25A for degradation at the Xenopus midblastula transition. EMBO J. 2002;21:3694–3703. doi: 10.1093/emboj/cdf357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kanemori Y, Uto K, Sagata N. Beta-TrCP recognizes a previously undescribed nonphosphorylated destruction motif in Cdc25A and Cdc25B phosphatases. Proc Natl Acad Sci USA. 2005;102:6279–6284. doi: 10.1073/pnas.0501873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Collart C, Smith JC, Zegerman P. Chk1 inhibition of the replication factor Drf1 guarantees cell-cycle elongation at the Xenopus laevis mid-blastula transition. Dev Cell. 2017;42:82–96.e3. doi: 10.1016/j.devcel.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daldello EM, Le T, Poulhe R, Jessus C, Haccard O, Dupré A. Control of Cdc6 accumulation by Cdk1 and MAPK is essential for completion of oocyte meiotic divisions in Xenopus. J Cell Sci. 2015;128:2482–2496. doi: 10.1242/jcs.166553. [DOI] [PubMed] [Google Scholar]
- 85.Yu C, Ji SY, Sha QQ, Sun QY, Fan HY. CRL4-DCAF1 ubiquitin E3 ligase directs protein phosphatase 2A degradation to control oocyte meiotic maturation. Nat Commun. 2015;6:8017. doi: 10.1038/ncomms9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ooga M, Suzuki MG, Aoki F. Involvement of histone H2B monoubiquitination in the regulation of mouse preimplantation development. J Reprod Dev. 2015;61:179–184. doi: 10.1262/jrd.2014-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jin XL, Chandrakanthan V, Morgan HD, O’Neill C. Preimplantation embryo development in the mouse requires the latency of TRP53 expression, which is induced by a ligand-activated PI3 kinase/AKT/MDM2-mediated signaling pathway. Biol Reprod. 2009;80:286–294. doi: 10.1095/biolreprod.108.070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 89.de Oca M, Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 90.Baran V, Brzakova A, Rehak P, Kovarikova V, Solc P. PLK1 regulates spindle formation kinetics and APC/C activation in mouse zygote. Zygote. 2016;24:338–345. doi: 10.1017/S0967199415000246. [DOI] [PubMed] [Google Scholar]
- 91.Solc P, Kitajima TS, Yoshida S, Brzakova A, Kaido M, Baran V, Mayer A, Samalova P, Motlik J, Ellenberg J. Multiple requirements of PLK1 during mouse oocyte maturation. PLoS ONE. 2015;10:e0116783. doi: 10.1371/journal.pone.0116783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 93.Roest HP, Baarends WM, de Wit J, van Klaveren JW, Wassenaar E, Hoogerbrugge JW, van Cappellen WA, Hoeijmakers JHJ, Grootegoed JA. The ubiquitin-conjugating DNA repair enzyme HR6A is a maternal factor essential for early embryonic development in mice. Mol Cell Biol. 2004;24:5485–5495. doi: 10.1128/MCB.24.12.5485-5495.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu YN, Shen XH, Lee SE, Kwon JS, Kim DJ, Heo YT, Cui XS, Kim NH. Autophagy influences maternal mRNA degradation and apoptosis in porcine parthenotes developing in vitro. J Reprod Dev. 2012;58:576–584. doi: 10.1262/jrd.2012-005. [DOI] [PubMed] [Google Scholar]
- 95.Shen X, Zhang N, Wang Z, Bai G, Zheng Z, Gu Y, Wu Y, Liu H, Zhou D, Lei L. Induction of autophagy improves embryo viability in cloned mouse embryos. Sci Rep. 2015;5:17829. doi: 10.1038/srep17829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chi D, Zeng Y, Xu M, Si L, Qu X, Liu H, Li J. LC3-dependent autophagy in Pig 2-cell cloned embryos could influence the degradation of maternal mRNA and the regulation of epigenetic modification. Cell Reprogramming. 2017;19:354–362. doi: 10.1089/cell.2017.0016. [DOI] [PubMed] [Google Scholar]
- 97.Tsukamoto S, Kuma A, Murakami M, Kishi C, Yamamoto A, Mizushima N. Autophagy is essential for preimplantation development of mouse embryos. Science. 2008;321:117–120. doi: 10.1126/science.1154822. [DOI] [PubMed] [Google Scholar]
- 98.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 100.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee SE, Hwang KC, Sun SC, Xu YN, Kim NH. Modulation of autophagy influences development and apoptosis in mouse embryos developing in vitro. Mol Reprod Dev. 2011;78:498–509. doi: 10.1002/mrd.21331. [DOI] [PubMed] [Google Scholar]
- 102.Cho YH, Han KM, Kim D, Lee J, Lee SH, Choi KW, Kim J, Han YM. Autophagy regulates homeostasis of pluripotency-associated proteins in hESCs. Stem Cells. 2014;32:424–435. doi: 10.1002/stem.1589. [DOI] [PubMed] [Google Scholar]
- 103.Tsukamoto S, Tatsumi T. Degradation of maternal factors during preimplantation embryonic development. J Reprod Dev. 2018;64:217–222. doi: 10.1262/jrd.2018-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamamoto A, Mizushima N, Tsukamoto S. Fertilization-induced autophagy in mouse embryos is independent of mTORC1. Biol Reprod. 2014;91:7. doi: 10.1095/biolreprod.113.115816. [DOI] [PubMed] [Google Scholar]
- 105.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Imamura T, Neildez TMA, Thenevin C, Paldi A. Essential role for poly(ADP-ribosyl)ation in mouse preimplantation development. BMC Mol Biol. 2004;5:4. doi: 10.1186/1471-2199-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee HR, Gupta MK, Kim DH, Hwang JH, Kwon B, Lee HT. Poly(ADP-ribosyl)ation is involved in pro-survival autophagy in porcine blastocysts. Mol Reprod Dev. 2016;83:37–49. doi: 10.1002/mrd.22588. [DOI] [PubMed] [Google Scholar]
- 108.Lee JE, Oh HA, Song H, Jun JH, Roh CR, Xie H, Dey SK, Lim HJ. Autophagy regulates embryonic survival during delayed implantation. Endocrinology. 2011;152:2067–2075. doi: 10.1210/en.2010-1456. [DOI] [PubMed] [Google Scholar]
- 109.Tsukamoto S, Hara T, Yamamoto A, Kito S, Minami N, Kubota T, Sato K, Kokubo T. Fluorescence-based visualization of autophagic activity predicts mouse embryo viability. Sci Rep. 2014;4:4533. doi: 10.1038/srep04533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee HR, Kim DH, Kim MG, Lee JS, Hwang JH, Lee HT. The regulation of autophagy in porcine blastocysts: regulation of PARylation-mediated autophagy via mammalian target of rapamycin complex 1 (mTORC1) signaling. Biochem Biophys Res Commun. 2016;473:899–906. doi: 10.1016/j.bbrc.2016.03.148. [DOI] [PubMed] [Google Scholar]
- 111.Song BS, Yoon SB, Kim JS, Sim BW, Kim YH, Cha JJ, Choi SA, Min HK, Lee Y, Huh JW, et al. Induction of autophagy promotes preattachment development of bovine embryos by reducing endoplasmic reticulum stress. Biol Reprod. 2012;87:1–11. doi: 10.1095/biolreprod.111.097949. [DOI] [PubMed] [Google Scholar]
- 112.Xu YN, Cui XS, Sun SC, Lee SE, Li YH, Kwon JS, Lee SH, Hwang KC, Kim NH. Mitochondrial dysfunction influences apoptosis and autophagy in porcine parthenotes developing in vitro. J Reprod Dev. 2011;57:143–150. doi: 10.1262/jrd.10-110h. [DOI] [PubMed] [Google Scholar]
- 113.Kang MH, Das J, Gurunathan S, Park HW, Song H, Park C, Kim JH. The cytotoxic effects of dimethyl sulfoxide in mouse preimplantation embryos: a mechanistic study. Theranostics. 2017;7:4735–4752. doi: 10.7150/thno.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shin KT, Guo J, Niu YJ, Cui XS. The toxic effect of aflatoxin B1 on early porcine embryonic development. Theriogenology. 2018;118:157–163. doi: 10.1016/j.theriogenology.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 115.Tang L, Yang S, Wang H, Gu H, Xia X, Feng Y, Yang Z, Zhao S, Su C, Su Z, et al. Nucleoside reverse transcriptase inhibitor-induced rat oocyte dysfunction and low fertility mediated by autophagy. Oncotarget. 2018;9:3895–3907. doi: 10.18632/oncotarget.23243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin T, Oqani RK, Lee JE, Kang JW, Kim SY, Cho ES, Jeong YD, Baek JJ, Jin DI. α-Solanine impairs oocyte maturation and quality by inducing autophagy and apoptosis and changing histone modifications in a pig model. Reprod Toxicol. 2018;75:96–109. doi: 10.1016/j.reprotox.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 117.Sutovsky P, Manandhar G, Laurincik J, Letko J, Caamaño JN, Day BN, Lai L, Prather RS, Sharpe-Timms KL, Zimmer R, et al. Expression and proteasomal degradation of the major vault protein (MVP) in mammalian oocytes and zygotes. Reproduction. 2005;129:269–282. doi: 10.1530/rep.1.00291. [DOI] [PubMed] [Google Scholar]
- 118.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tan JMM, Wong ESP, Kirkpatrick DS, Pletnikova O, Ko HS, Tay SP, Ho MWL, Troncoso J, Gygi SP, Lee MK, et al. Lysine 63-linked ubiquitination promotes the formation and autophagic clearance of protein inclusions associated with neurodegenerative diseases. Hum Mol Genet. 2008;17:431–439. doi: 10.1093/hmg/ddm320. [DOI] [PubMed] [Google Scholar]
- 120.Sato M, Konuma R, Sato K, Tomura K, Sato K. Fertilization-induced K63-linked ubiquitylation mediates clearance of maternal membrane proteins. Development. 2014;141:1324–1331. doi: 10.1242/dev.103044. [DOI] [PubMed] [Google Scholar]
- 121.Rojansky R, Cha MY, Chan DC. Elimination of paternal mitochondria in mouse embryos occurs through autophagic degradation dependent on PARKIN and MUL1. eLife. 2016;5:e17896. doi: 10.7554/eLife.17896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hajjar C, Sampuda KM, Boyd L. Dual roles for ubiquitination in the processing of sperm organelles after fertilization. BMC Dev Biol. 2014;14:6. doi: 10.1186/1471-213X-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Song WH, Yi YJ, Sutovsky M, Meyers S, Sutovsky P. Autophagy and ubiquitin–proteasome system contribute to sperm mitophagy after mammalian fertilization. Proc Natl Acad Sci USA. 2016;113:E5261–5270. doi: 10.1073/pnas.1605844113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Komatsu M, Ichimura Y. Selective autophagy regulates various cellular functions. Genes Cells Devoted Mol Cell Mech. 2010;15:923–933. doi: 10.1111/j.1365-2443.2010.01433.x. [DOI] [PubMed] [Google Scholar]
- 125.Benesova V, Kinterova V, Kanka J, Toralova T. Potential involvement of SCF-complex in zygotic genome activation during early bovine embryo development. Methods Mol Biol. 2017;1605:245–257. doi: 10.1007/978-1-4939-6988-3_17. [DOI] [PubMed] [Google Scholar]
- 126.Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- 127.Traub LM, Lukacs GL. Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J Cell Sci. 2007;120:543–553. doi: 10.1242/jcs.03385. [DOI] [PubMed] [Google Scholar]
- 128.Sato M, Sato K. Dynamic regulation of autophagy and endocytosis for cell remodeling during early development. Traffic. 2013;14:479–486. doi: 10.1111/tra.12050. [DOI] [PubMed] [Google Scholar]
- 129.Sato K, Sato M, Audhya A, Oegema K, Schweinsberg P, Grant BD. Dynamic regulation of caveolin-1 trafficking in the germ line and embryo of Caenorhabditis elegans. Mol Biol Cell. 2006;17:3085–3094. doi: 10.1091/mbc.E06-03-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- 131.Kadandale P, Stewart-Michaelis A, Gordon S, Rubin J, Klancer R, Schweinsberg P, Grant BD, Singson A. The egg surface LDL receptor repeat-containing proteins EGG-1 and EGG-2 are required for fertilization in Caenorhabditis elegans. Curr Biol. 2005;15:2222–2229. doi: 10.1016/j.cub.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 132.Audhya A, McLeod IX, Yates JRIII, Oegema K. MVB-12, a Fourth subunit of metazoan ESCRT-I, functions in receptor downregulation. PLoS ONE. 2007;2:9. doi: 10.1371/journal.pone.0000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zuo Y, Su G, Wang S, Yang L, Liao M, Wei Z, Bai C, Li G. Exploring timing activation of functional pathway based on differential co-expression analysis in preimplantation embryogenesis. Oncotarget. 2016;7:74120–74131. doi: 10.18632/oncotarget.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller-Fleming L, Olin-Sandoval V, Campbell C, Ralser M. Remaining mysteries of molecular biology: the role of polyamines in the cell. J Mol Biol. 2015;427:3389–3406. doi: 10.1016/j.jmb.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 135.Fenelon JC, Murphy BD. Inhibition of polyamine synthesis causes entry of the mouse blastocyst into embryonic diapause. Biol Reprod. 2017;97:119–132. doi: 10.1093/biolre/iox060. [DOI] [PubMed] [Google Scholar]
- 136.Lenis YY, Johnson GA, Wang X, Tang WW, Dunlap KA, Satterfield MC, Wu G, Hansen TR, Bazer FW. Functional roles of ornithine decarboxylase and arginine decarboxylase during the peri-implantation period of pregnancy in sheep. J Anim Sci Biotechnol. 2018;9:10. doi: 10.1186/s40104-017-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pendeville H, Carpino N, Marine JC, Takahashi Y, Muller M, Martial JA, Cleveland JL. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol Cell Biol. 2001;21:6549–6558. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pegg AE. Regulation of ornithine decarboxylase. J Biol Chem. 2006;281:14529–14532. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- 139.Osborne HB, Duval C, Ghoda L, Omilli F, Bassez T, Coffino P. Expression and post-transcriptional regulation of ornithine decarboxylase during early Xenopus development. Eur J Biochem. 1991;202:575–581. doi: 10.1111/j.1432-1033.1991.tb16410.x. [DOI] [PubMed] [Google Scholar]
- 140.Reverte CG, Ahearn MD, Hake LE. CPEB degradation during Xenopus oocyte maturation requires a PEST domain and the 26S proteasome. Dev Biol. 2001;231:447–458. doi: 10.1006/dbio.2001.0153. [DOI] [PubMed] [Google Scholar]
- 141.Mendez R, Barnard D, Richter JD. Differential mRNA translation and meiotic progression require Cdc2-mediated CPEB destruction. EMBO J. 2002;21:1833–1844. doi: 10.1093/emboj/21.7.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hodgman R, Tay J, Mendez R, Richter JD. CPEB phosphorylation and cytoplasmic polyadenylation are catalyzed by the kinase IAK1/Eg2 in maturing mouse oocytes. Development. 2001;128:2815–2822. doi: 10.1242/dev.128.14.2815. [DOI] [PubMed] [Google Scholar]
- 143.Uzbekova S, Arlot-Bonnemains Y, Dupont J, Dalbiès-Tran R, Papillier P, Pennetier S, Thélie A, Perreau C, Mermillod P, Prigent C, et al. Spatio-temporal expression patterns of aurora kinases A, B, and C and cytoplasmic polyadenylation-element-binding protein in bovine oocytes during meiotic maturation. Biol Reprod. 2008;78:218–233. doi: 10.1095/biolreprod.107.061036. [DOI] [PubMed] [Google Scholar]
- 144.Bowerman B, Kurz T. Degrade to create: developmental requirements for ubiquitin-mediated proteolysis during early C. elegans embryogenesis. Development. 2006;133:773–784. doi: 10.1242/dev.02276. [DOI] [PubMed] [Google Scholar]
- 145.Kurz T, Pintard L, Willis JH, Hamill DR, Gönczy P, Peter M, Bowerman B. Cytoskeletal regulation by the Nedd8 ubiquitin-like protein modification pathway. Science. 2002;295:1294–1298. doi: 10.1126/science.1067765. [DOI] [PubMed] [Google Scholar]
- 146.Furukawa M, He YJ, Borchers C, Xiong Y. Targeting of protein ubiquitination by BTB-Cullin 3-Roc1 ubiquitin ligases. Nat Cell Biol. 2003;5:1001–1007. doi: 10.1038/ncb1056. [DOI] [PubMed] [Google Scholar]
- 147.Gao S, Han Z, Kihara M, Adashi E, Latham EK. Protease inhibitor MG132 in cloning: no end to the nightmare. Trends Biotechnol. 2005;23:66–68. doi: 10.1016/j.tibtech.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 148.Yu Y, Yong J, Li X, Qing T, Qin H, Xiong X, You J, Ding M, Deng H. The proteasomal inhibitor MG132 increases the efficiency of mouse embryo production after cloning by electrofusion. Reproduction. 2005;130:553–558. doi: 10.1530/rep.1.00758. [DOI] [PubMed] [Google Scholar]
- 149.Nakajima N, Inomata T, Ito J, Kashiwazaki N. Treatment with proteasome inhibitor MG132 during cloning improves survival and pronuclear number of reconstructed rat embryos. Cloning Stem Cells. 2008;10:461–468. doi: 10.1089/clo.2008.0038. [DOI] [PubMed] [Google Scholar]
- 150.Le Bourhis D, Beaujean N, Ruffini S, Vignon X, Gall L. Nuclear remodeling in bovine somatic cell nuclear transfer embryos using MG132-treated recipient oocytes. Cell Reprogramming. 2010;12:729–738. doi: 10.1089/cell.2010.0035. [DOI] [PubMed] [Google Scholar]
- 151.You J, Lee E, Bonilla L, Francis J, Koh J, Block J, Chen S, Hansen PJ. Treatment with the proteasome inhibitor MG132 during the end of oocyte maturation improves oocyte competence for development after fertilization in cattle. PLoS ONE. 2012;7:e48613. doi: 10.1371/journal.pone.0048613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.You J, Lee J, Kim J, Park J, Lee E. Post-fusion treatment with MG132 increases transcription factor expression in somatic cell nuclear transfer embryos in pigs. Mol Reprod Dev. 2010;77:149–157. doi: 10.1002/mrd.21115. [DOI] [PubMed] [Google Scholar]
- 153.Shen K, Li X, Dai X, Wang P, Li S, Xiong Z, Chen P, Liu Q, Shi D. Effects of MG132 on the in vitro development and epigenetic modification of Debao porcine somatic cell nuclear transfer embryos. Theriogenology. 2017;94:48–58. doi: 10.1016/j.theriogenology.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 154.Higuchi C, Shimizu N, Shin SW, Morita K, Nagai K, Anzai M, Kato H, Mitani T, Yamagata K, Hosoi Y, et al. Ubiquitin-proteasome system modulates zygotic genome activation in early mouse embryos and influences full-term development. J Reprod Dev. 2018;64:65–74. doi: 10.1262/jrd.2017-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yurttas P, Morency E, Coonrod SA. Use of proteomics to identify highly abundant maternal factors that drive the egg-to-embryo transition. Reproduction. 2010;139:809–823. doi: 10.1530/REP-09-0538. [DOI] [PubMed] [Google Scholar]
- 156.Pennetier S, Perreau C, Uzbekova S, Thélie A, Delaleu B, Mermillod P, Dalbiès-Tran R. MATER protein expression and intracellular localization throughout folliculogenesis and preimplantation embryo development in the bovine. BMC Dev Biol. 2006;6:26. doi: 10.1186/1471-213X-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ohsugi M, Zheng P, Baibakov B, Li L, Dean J. Maternally derived FILIA-MATER complex localizes asymmetrically in cleavage-stage mouse embryos. Development. 2008;135:259–269. doi: 10.1242/dev.011445. [DOI] [PubMed] [Google Scholar]
- 158.Gao Y, Liu X, Tang B, Li C, Kou Z, Li L, Liu W, Wu Y, Kou X, Li J, et al. Protein expression landscape of mouse embryos during pre-implantation development. Cell Rep. 2017;21:3957–3969. doi: 10.1016/j.celrep.2017.11.111. [DOI] [PubMed] [Google Scholar]
- 159.Toralova T, Benesova V, Vodickova Kepkova K, Vodicka P, Susor A, Kanka J. Bovine preimplantation embryos with silenced nucleophosmin mRNA are able to develop until the blastocyst stage. Reproduction. 2012;144:349–359. doi: 10.1530/REP-12-0033. [DOI] [PubMed] [Google Scholar]
- 160.Svarcova O, Laurincik J, Avery B, Mlyncek M, Niemann H, Maddox-Hyttel P. Nucleolar development and allocation of key nucleolar proteins require de novo transcription in bovine embryos. Mol Reprod Dev. 2007;74:1428–1435. doi: 10.1002/mrd.20727. [DOI] [PubMed] [Google Scholar]
- 161.Li L, Lu X, Dean J. The maternal to zygotic transition in mammals. Mol Aspects Med. 2013;34:919–938. doi: 10.1016/j.mam.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Peshkin L, Wühr M, Pearl E, Haas W, Freeman RM, Jr, Gerhart JC, Klein AM, Horb M, Gygi SP, Kirschner MW. On the relationship of protein and mRNA dynamics in vertebrate embryonic development. Dev Cell. 2015;35:383–394. doi: 10.1016/j.devcel.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lu X, Gao Z, Qin D, Li L. A maternal functional module in the mammalian oocyte-to-embryo transition. Trends Mol Med. 2017;23:1014–1023. doi: 10.1016/j.molmed.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 164.Bebbere D, Masala L, Albertini DF, Ledda S. The subcortical maternal complex: multiple functions for one biological structure? J Assist Reprod Genet. 2016;33:1431–1438. doi: 10.1007/s10815-016-0788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Duesbery NS, Choi T, Brown KD, Wood KW, Resau J, Fukasawa K, Cleveland DW, Vande Woude GF. CENP-E is an essential kinetochore motor in maturing oocytes and is masked during mos-dependent, cell cycle arrest at metaphase II. Proc Natl Acad Sci USA. 1997;94:9165–9170. doi: 10.1073/pnas.94.17.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Allard P, Champigny MJ, Skoggard S, Erkmann JA, Whitfield ML, Marzluff WF, Clarke HJ. Stem-loop binding protein accumulates during oocyte maturation and is not cell-cycle-regulated in the early mouse embryo. J Cell Sci. 2002;115:4577–4586. doi: 10.1242/jcs.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Toralova T, Susor A, Nemcova L, Kepkova K, Kanka J. Silencing CENPF in bovine preimplantation embryo induces arrest at 8-cell stage. Reproduction. 2009;138(5):783–791. doi: 10.1530/REP-09-0234. [DOI] [PubMed] [Google Scholar]
- 168.Collart C, Allen GE, Bradshaw CR, Smith JC, Zegerman P. Titration of four replication factors is essential for the Xenopus laevis midblastula transition. Science. 2013;341:893–896. doi: 10.1126/science.1241530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fisher D. Control of DNA replication by cyclin-dependent kinases in development. Results Probl Cell Differ. 2011;53:201–217. doi: 10.1007/978-3-642-19065-0_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ichikawa K, Noda T, Furuichi Y. Preparation of the gene targeted knockout mice for human premature aging diseases, Werner syndrome, and Rothmund–Thomson syndrome caused by the mutation of DNA helicases. Nihon Yakurigaku Zasshi Folia Pharmacol Jpn. 2002;119:219–226. doi: 10.1254/fpj.119.219. [DOI] [PubMed] [Google Scholar]
- 171.Hoki Y, Araki R, Fujimori A, Ohhata T, Koseki H, Fukumura R, Nakamura M, Takahashi H, Noda Y, Kito S, et al. Growth retardation and skin abnormalities of the Recql4-deficient mouse. Hum Mol Genet. 2003;12:2293–2299. doi: 10.1093/hmg/ddg254. [DOI] [PubMed] [Google Scholar]
- 172.Wu J, Capp C, Feng L, Hsieh T. Drosophila homologue of the Rothmund–Thomson syndrome gene: essential function in DNA replication during development. Dev Biol. 2008;323:130–142. doi: 10.1016/j.ydbio.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jeon Y, Ko E, Lee KY, Ko MJ, Park SY, Kang J, Jeon CH, Lee H, Hwang DS. TopBP1 deficiency causes an early embryonic lethality and induces cellular senescence in primary cells. J Biol Chem. 2011;286:5414–5422. doi: 10.1074/jbc.M110.189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sansam CL, Cruz NM, Danielian PS, Amsterdam A, Lau ML, Hopkins N, Lees JA. A vertebrate gene, TICRR, is an essential checkpoint and replication regulator. Genes Dev. 2010;24:183–194. doi: 10.1101/gad.1860310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yao L, Chen J, Wu X, Jia S, Meng A. Zebrafish cdc6 hypomorphic mutation causes Meier–Gorlin syndrome-like phenotype. Hum Mol Genet. 2017;26:4168–4180. doi: 10.1093/hmg/ddx305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.El Dika M, Laskowska-Kaszub K, Koryto M, Dudka D, Prigent C, Tassan JP, Kloc M, Polanski Z, Borsuk E, Kubiak JZ. CDC6 controls dynamics of the first embryonic M-phase entry and progression via CDK1 inhibition. Dev Biol. 2014;396:67–80. doi: 10.1016/j.ydbio.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 177.Zhou ZW, Liu C, Li TL, Bruhn C, Krueger A, Min WK, Wang ZQ, Carr AM. An essential function for the ATR-activation-domain (AAD) of TopBP1 in mouse development and cellular senescence. PLoS Genet. 2013;9:e1003702. doi: 10.1371/journal.pgen.1003702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Eisenmann KM, West RA, Hildebrand D, Kitchen SM, Peng J, Sigler R, Zhang J, Siminovitch KA, Alberts AS. T cell responses in mammalian diaphanous-related formin mDia1 knock-out mice. J Biol Chem. 2007;282:25152–25158. doi: 10.1074/jbc.M703243200. [DOI] [PubMed] [Google Scholar]
- 179.Peng J, Wallar BJ, Flanders A, Swiatek PJ, Alberts AS. Disruption of the Diaphanous-related formin Drf1 gene encoding mDia1 reveals a role for Drf3 as an effector for Cdc42. Curr Biol. 2003;13:534–545. doi: 10.1016/s0960-9822(03)00170-2. [DOI] [PubMed] [Google Scholar]
- 180.Cheng Y, Quinn JF, Weiss LA. An eQTL mapping approach reveals that rare variants in the SEMA5A regulatory network impact autism risk. Hum Mol Genet. 2013;22:2960–2972. doi: 10.1093/hmg/ddt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Silva T, Bradley RH, Gao Y, Coue M. Xenopus CDC7/DRF1 complex is required for the initiation of DNA replication. J Biol Chem. 2006;281:11569–11576. doi: 10.1074/jbc.M510278200. [DOI] [PubMed] [Google Scholar]
- 182.Takahashi TS, Walter JC. Cdc7-Drf1 is a developmentally regulated protein kinase required for the initiation of vertebrate DNA replication. Genes Dev. 2005;19:2295–2300. doi: 10.1101/gad.1339805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tikhmyanova N, Coleman TR. Isoform switching of Cdc6 contributes to developmental cell cycle remodeling. Dev Biol. 2003;260:362–375. doi: 10.1016/s0012-1606(03)00253-7. [DOI] [PubMed] [Google Scholar]
- 184.Solc P, Saskova A, Baran V, Kubelka M, Schultz RM, Motlik J. CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev Biol. 2008;317:260–269. doi: 10.1016/j.ydbio.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Farrell JA, Shermoen AW, Yuan K, O’Farrell PH. Embryonic onset of late replication requires Cdc25 down-regulation. Genes Dev. 2012;26:714–725. doi: 10.1101/gad.186429.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sibon OC, Stevenson VA, Theurkauf WE. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature. 1997;388:93–97. doi: 10.1038/40439. [DOI] [PubMed] [Google Scholar]