Abstract
The Tasmanian devil (Sarcophilus harrisii) is the only mammalian species known to be affected by multiple transmissible cancers. Devil facial tumours 1 and 2 (DFT1 and DFT2) are independent neoplastic cell lineages that produce large, disfiguring cancers known as devil facial tumour disease (DFTD). The long-term persistence of wild Tasmanian devils is threatened due to the ability of DFTD cells to propagate as contagious allografts and the high mortality rate of DFTD. Recent studies have demonstrated that both DFT1 and DFT2 cancers originated from founder cells of the Schwann cell lineage, an uncommon origin of malignant cancer in humans. This unprecedented finding has revealed a potential predisposition of Tasmanian devils to transmissible cancers of the Schwann cell lineage. In this review, we compare the molecular nature of human Schwann cells and nerve sheath tumours with DFT1 and DFT2 to gain insights into the emergence of transmissible cancers in the Tasmanian devil. We discuss a potential mechanism, whereby Schwann cell plasticity and frequent wounding in Tasmanian devils combine with an inherent cancer predisposition and low genetic diversity to give rise to transmissible Schwann cell cancers in devils on rare occasions.
Keywords: Tasmanian devil, Devil facial tumour disease, Transmissible cancer, Schwann cell, Nerve sheath tumour, Tumour microenvironment
Introduction
Transmissible cancers are neoplastic cell lineages that have acquired the ability to spread between individuals as contagious allografts [1–4]. Transmissible cancers originate within a single founder animal are clonal and genetically distinct from the host in all subsequent cases of the disease. Several isolated cases of cancer transmission have been observed in humans (e.g., [5, 6]), but epidemics involving wide-spread and continuous horizontal cancer transmission are rare in nature. As of 2019, only nine transmissible cancers affecting eight animal species have been identified. These involve dogs (Canis lupus familiaris) [3], Tasmanian devils (Sarcophilus harrisii) [2, 7], and six mollusc species (Mys arenaria, Mytilus trossulus, Mytilus chilensis, Mytilis edulis, Cerastoderma edule, and Polititapes aureus) [4, 8, 9]. The rarity of transmissible cancers is most likely a consequence of highly evolved physical barriers, allogeneic defences, and anti-cancer immune responses that have been shaped throughout evolution to protect against negative effects of ‘non-self’ and oncogenic threats. Indeed, allogeneic defences are evident in species as primitive as the basal metazoans and potentially evolved to prevent parasitism of transferred somatic cells [10, 11]. Transmissible cancers emerge when key enabling factors combine to overcome these barriers and permit cancer cell transfer from one individual to another. Key enabling factors include mechanisms for ongoing transmission (e.g., biting and coitus), strategies to overcome allogeneic barriers (e.g., immune suppression), and a host environment receptive for the growth and expansion of the transmitted cancer cells. Prior to the mid-1990s, transmissible cancers were known to have emerged naturally only in Canine Transmissible Venereal Tumour (CTVT), an ancient venereal sarcoma transmitted between dogs during coitus [3]. Eight transmissible cancers have since been described; two nerve sheath tumours in Tasmanian devils [12, 13] and six haemic neoplasias in marine mollusc species [4, 8, 9]. These discoveries suggest that cancer transmission could occur more readily in nature than previously thought.
Investigations into transmissible cancers have the potential to provide insights into mechanisms by which nature’s most robust barriers against infection can be overcome to cause disease. The first transmissible cancer to be identified in a mammalian species, CTVT, has been endemic in dog populations for thousands of years [14, 15]. Consequently, the age of the clonal CTVT cells has provided an impediment to investigating the genesis of this cancer. CTVT cells underwent significant evolution after emergence to give rise to a well-adapted transmissible cancer that has undergone global spread aided by human migration [14]. In otherwise healthy dogs, CTVT has a low capacity to become metastatic, can spontaneously regress, and exhibits high sensitivity to chemotherapeutic agents such as Vincristine [16–18]. As a result, this transmissible cancer does not pose a significant threat to the long-term survival of the dog population.
In contrast to CTVT, Tasmanian devil populations have been considerably impacted by two fatal transmissible cancers that are known collectively as devil facial tumour disease (DFTD) [7, 19]. The first of these cancers was observed in 1996 and was initially termed DFTD due to its propensity to affect the facial area [19]. Since the discovery of a second independent facial tumour in 2014, the cancers have frequently been referred to as DFT1 and DFT2 [7]. The recently emerged nature of DFT1 and DFT2 has provided a unique opportunity for studies into the requirements for cancer transmission in mammalian species. Parallels drawn between DFT1 and DFT2 suggest that these cancers did not emerge by chance, but due to a combination of factors that predispose devils to transmissible cancers of this type [13, 20]. Most striking of these similarities was the discovery that both DFT1 and DFT2 arose from founder cells of the Schwann cell lineage [12, 13]. Insights into the molecular nature of DFT1 and DFT2, Schwann cells, and nerve sheath tumours are providing a greater understanding of how DFTD tumours emerge in Tasmanian devils.
Devil facial tumour disease (DFT1 and DFT2)
The Tasmanian devil is a marsupial scavenger and hunter unique to the Australian island state of Tasmania. Aptly named by early European settlers for their unearthly shrieks and seemingly cantankerous nature, devils are the apex mammalian predator in Tasmania. Consequently, devils play an essential role in the Tasmanian ecosystem [19]. In the mid-1990s, the Tasmanian devil population was estimated at approximately 150,000 individuals [19, 21]. However, the emergence of DFT1 has reduced numbers in affected populations by an average of 77% [22]. The first observed case of DFT1 was in 1996 near Mount William National Park (wukalina) in Tasmania’s northeast (Fig. 1). Within 20 years, the disease spread across the majority of Tasmania [19, 22]. DFT1 cancers present as large, disfiguring masses that severely impact body condition, frequently metastasise to internal organs and are usually fatal within 6–12 months of lesion appearance [23, 24]. The cancers are usually found in and around the oral cavity [23] and are primarily transmitted by devil-to-devil biting, a common interaction of devils during feeding and mating [2, 25, 26]. In populations where DFT1 is endemic, devils tend to become infected from around 2 years of age and older devils are rarely found [27, 28]. This reduction in mature devil numbers has led to increased precocial breeding of females in affected populations, which may allow maintenance of these populations at low numbers [22, 27, 28]. There is no evidence for extinction of local populations as a result of DFT1 infection, and recent observations of DFT1-driven genetic selection have ignited predictions that devils could evolve to resist DFT1 [29, 30]. However, with the combined influence of population decline and other factors such as vehicle strike, additional diseases, and loss of genetic diversity [31], it is likely that devil populations will remain endangered for the foreseeable future.
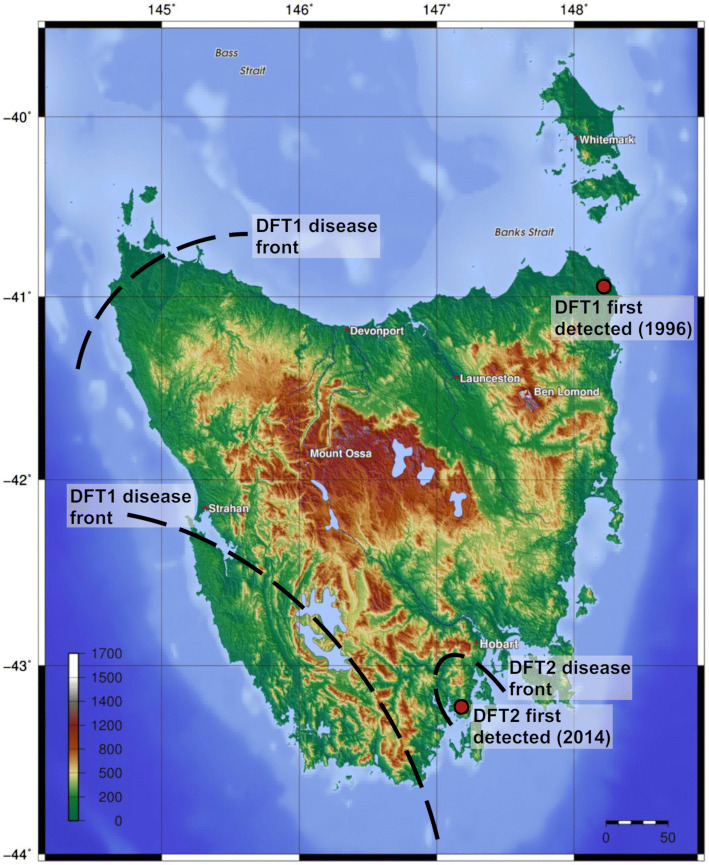
Fig. 1.
Topographical map demonstrating distribution and spread of DFTD. DFT1 was first observed at Waterhouse Point near Mount William National Park (wukalina) in 1996 and has spread from east to west across the majority of the state. Initially, DFT1 spread south and west to almost half the of the known devil habitat. Geographical barriers such as mountains (shaded brown) and rivers then impeded the spread. Consequently, only the far north-west and south-west of Tasmania are believed to be DFT1-free. Ultimately, DFT1 will reach the far north-west. Despite evidence for pockets of devil populations, it is unlikely that DFT1 will reach the south-west of Tasmania, as this area is rugged and unsuitable devil habitat. DFT2 was first observed in the D’Entrecasteaux Peninsula region in 2014. DFT2 currently remains localised to this region [7], as the area is surrounded by mountain ranges and the sea. Heat map represents topography in meters and distance scale is in kilometres. The topographical map was sourced from Wikimedia Commons (https://upload.wikimedia.org/wikipedia/commons/2/21/Topography_of_Tasmania.jpg) and author (AP) overlaid the distribution and spread of DFT1 and DFT2
The initial studies into the nature of DFT1 identified unique chromosomal rearrangements that were consistent between different tumours and distinguishable from host tissues [2]. It was apparent that the tumour cells were clonal in origin, giving rise to the ‘allograft theory’. This theory proposed that DFT1 was a transmissible cancer spread as an allograft during biting behaviours of devils [2]. A devil with a pericentric inversion of host chromosome 5 provided the most compelling evidence for horizontal DFT1 transmission. The DFT1 cancer affecting this devil did not share this genetic abnormality, suggesting that the tumour must have arisen elsewhere [2]. Other studies confirmed the allograft theory through microsatellite and major histocompatibility complex class I (MHC-I) genotyping of host and DFT1 tissues [32]. An explanation for DFT1 transmission was provided in 2013 when it was discovered that DFT1 cells lack surface MHC-I molecules, which are required for recognition of allogeneic cells by the immune system [33]. Indeed, components of MHC-I processing pathways including β2-microglobulin (β2m) and the transporters associated with antigen processing (TAP) -1 and -2, are suppressed by epigenetic silencing of MHC-I in DFT1, preventing MHC-I exposure at the cell surface [33]. To characterize mechanisms that lead to MHC-I silencing in human cancers, a genome-wide CRISPR/Cas9 screen of the MHC-I negative human erythroleukaemic cell line, K562, was undertaken [34]. The polycomb repressive complex 2 (PRC2) was shown to cause transcriptional silencing of the MHC-I antigen-processing pathway. Reversal of PRC2 inhibition was also found to induce expression of multiple MHC-I genes in DFT1 cells. This led the authors to suggest that epigenetic silencing of the MHC-I processing pathway in DFT1 is related to the PRC2. Currently, MHC-I loss provides the basis for understanding the transmission of DFT1 across genetically diverse devil populations.
In comparison with DFT1, DFT2 was first observed in 2014 and almost immediately was determined to be another transmissible cancer [7]. At the gross level, DFT2 appears almost identical to DFT1. However, the tumours were serendipitously determined to be distinct when standard immunohistochemical detection of periaxin (PRX), a diagnostic marker of DFT1 tumours [12, 35], was negative in two tumours obtained from the D’Entrecasteaux Peninsula region of southern Tasmania (Fig. 1) [7]. Subsequent analysis of the two tumours and further tumours from this region revealed chromosomal rearrangements, microsatellite genotypes, and MHC-I genotypes that were distinct from DFT1 and host tissues, but identical across all the tumours [7]. The presence of a Y chromosome in DFT2 cells confirmed that the tumour arose independently from DFT1, which harbours genetic material from two X chromosomes and lacks a Y chromosome [7, 36]. DFT2 currently remains localised to the semi-isolated D’Entrecasteaux Peninsula region. It is unclear how this cancer will impact populations of devils already decimated by DFT1 if the disease spreads to other regions of Tasmania [37]. Nonetheless, the discovery of a transmissible tumour at an early stage of evolution [20] has provided a unique tool for investigating how cancers become transmissible in the Tasmanian devil.
Cellular origins of DFT1 and DFT2
Early investigations into DFT1 and DFT2 focussed on determining the founder cell type that gave rise to these tumours [12, 13]. Immunohistochemical studies revealed that DFT1 cells were positive for markers of neuroectodermal cells including vimentin, S-100, melan A, chromogranin A, and synaptophysin [38]. Investigation of the DFT1 transcriptome confirmed this finding and identified a gene expression profile that was most similar to peripheral nerve and brain, and distinct from other tissue types including spleen, heart, lung, liver, skin, testis, and kidney [12]. DFT1 tumour sections were also demonstrated to express a range of proteins associated with Schwann cell differentiation and myelination, including myelin binding protein (MBP), peripheral myelin protein 22 (PMP22), myelin protein zero (MPZ), nestin (NES), and nerve growth factor receptor (NGFR) [12]. This finding suggested a Schwann cell origin for DFT1 cancers. The myelin protein PRX provided an excellent diagnostic marker for DFT1 due to its high specificity of expression by DFT1 cells [12, 35].
Immunohistochemical analysis of DFT2 cells demonstrated a similar expression of neuroectodermal markers such as vimentin, neural-specific enolase (NSE), and S-100, but a lack of myelin-specific proteins such as PRX [7, 20]. Although PRX was absent, further analysis of DFT2 tumours revealed expression of other Schwann cell lineage markers including SRY-box 10 (SOX10), NES, and NGFR [13]. Total gene expression patterns of DFT2 tumours were also found to be similar to DFT1 and peripheral nerve tissues, and different to a range of normal tissues including spleen, brain, heart, and testis. Together, these findings suggested that DFT2 also arose from the Schwann cell lineage, with the absence of PRX perhaps indicating a difference in the state of differentiation of DFT2 cells relative to DFT1 [13]. This was an unexpected finding due to the rarity of nerve sheath tumours in humans [39].
The origin of both DFT1 and DFT2 from Schwann cells indicates a predisposition of devils to transmissible cancers of this specific cell lineage. However, investigation of this predisposition in devils has been hampered by difficulties in developing stable Schwann cell lines. This difficulty is further exacerbated because the source of the primary material (neonatal devils) is rare, due to the endangered status of the population. Studies into the nature of Schwann cells in other species will instead provide clues as to how Schwann cells transformed into transmissible cancers in Tasmanian devils.
Schwann cell functions and DFTD
Myelinating Schwann cells
As the principal glia of the peripheral nervous system, Schwann cells have the essential function of producing the myelin sheath, a lipid-rich substance that wraps around nerves and is required for the rapid conduction of action potentials along neuronal axons. Schwann cells originate in the ectoderm and differentiate from neural crest stem cells through three developmental stages; from Schwann cell precursors, to immature Schwann cells, and finally to mature Schwann cells [40, 41]. Mature Schwann cells exist as both myelinating and non-myelinating (Remak) Schwann cells, which differ via activation of key transcription factors required for myelination such as early growth response protein 2 (EGR2; also known as Krox20) [41, 42]. EGR2 is required for expression of key myelin proteins including PRX, MPZ, MBP, and PMP22 [43]. During development, immature Schwann cells are randomly associated with axons that determine the state of EGR2 activation via paracrine and juxtacrine neuregulin-1 (NRG1)-ErbB2/3 signalling [44, 45]. Larger axons are dependent on myelination and promote EGR2 activation and differentiation of myelinating Schwann cells. In comparison, smaller neurons promote the differentiation of non-myelinating Remak Schwann cells, which maintain nerve integrity by wrapping multiple small axons in membrane protrusions (Remak bundles) [41, 46]. The ErbB2/3-EGR2 pathway exhibits high plasticity and can be up- or down-regulated in both Remak and myelinating Schwann cells via regulation of NRG1 isoform expression and concentration [44–46]. Indeed, transplantation of Remak Schwann cells onto larger neurons leads to activation of ERG2-mediated myelination, demonstrating the high plasticity of this pathway [47].
The function of Schwann cells is likely to be conserved in Tasmanian devils due to the necessity of myelination for nerve integrity. Indeed, devil peripheral nerve samples have been demonstrated to express a range of myelin genes, indicating that these functions are intact [12, 13]. Recent studies have indicated that DFT1 cells are phenotypically similar to myelinating Schwann cells due to overactivation of ERBB3 signalling and high expression of a range of myelin-specific proteins [13, 48]. Compared to DFT2, DFT1 cells also express high levels of genes associated with channel activity, which is required for communication between axons and myelinating Schwann cells [13]. Myelinating Schwann cells play vital roles in maintaining axons and respond to external cues such as damage-associated molecules and metabolic factors, to provide appropriate nerve support [49, 50]. Interaction between ATP released from axons and purinergic P2X receptors (ligand-gated ion channels) in Schwann cells are thought to be critical for proper myelination and to promote nerve regeneration upon injury [49, 51]. In DFT1, maintenance of these functions could allow the cancer cells to remain responsive to changes within the tumour microenvironment.
Repair Schwann cells
A second essential role of Schwann cells involves regulation of the regenerative response to peripheral nerve damage. This process, frequently referred to as Wallerian degeneration, involves the trans-differentiation of myelinating and Remak Schwann cells located distal to an injury into mesenchymal-like cells that migrate to the site of damage and participate in several sequential functions to repair damaged nerves [52–54]. These functions include release of growth factors to support neuron survival and regrowth [55–57], production of inflammatory factors that recruit and activate innate immune cells for wound healing [54, 58, 59], formation of tracks called Bands of Bünger that guide axons back to their targets [52, 53], activation of autophagic pathways to remove myelin debris [60, 61], and finally, remyelination [52, 62]. Various factors have been implicated in stimulating this phenotypic shift in Schwann cells, including damage-associated molecules such toll-like receptor (TLR) ligands and ATP [49, 50, 63], and cytokines such as transforming growth factor-β (TGFβ) and interleukin-1β (IL1β) [54, 64]. The transcription factor c-Jun is a critical mediator of the trans-differentiation of a mature Schwann cell into a repair Schwann cell and at high levels initiates the down-regulation of EGR2-mediated myelination to promote this response [53, 56, 65, 66]. Other signalling events that are required for an effective Schwann cell repair response include activation of merlin-mediated Hippo signalling, and reversal of epigenetic regulation by the histone methyltransferase PRC2 [67–69].
Another important signalling event during the Schwann cell repair response is activation of the transcription factor signal transducer and activator of transcription 3 (STAT3) [70]. Chronic loss of axonal contact within the distal portion of injured nerves decreases the regenerative capacity of repair Schwann cells. Reduced production of growth factors such as bone-derived neurotrophic factor (BDNF) and glial call-derived neurotrophic factor (GDNF) promotes death of these cells [71, 72]. STAT3 is activated by interleukin-6 (IL6)/glycoprotein 130 (gp130) and neuregulin-1 (NRG1)/ErbB2/3 signalling pathways during this loss of axonal innervation to support the long-term survival of repair Schwann cells [70, 73, 74].
Both DFT1 and DFT2 cells display activation of pathways that are up-regulated during the repair Schwann cell response. Although DFT1 cells predominantly exhibit a myelinating phenotype, they also display overactivation of the NRG1-ErbB2/3-STAT3-signalling pathway via copy number gains to ERBB3 [20, 48]. Pharmacological inhibitors of this pathway such as sapitinib-killed DFT1 cells in vitro and arrested DFT1 growth in a mouse model, suggesting that ErbB2/3-STAT3 signalling is a key driver of DFT1 survival [48]. Components of ErbB2/3-STAT3 signalling pathways were also suppressed in DFT1 cells treated with imiquimod, a drug that induces DFT1 cell death via overload of mitochondrial and ER stress responses [75, 76]. In comparison, DFT2 cells demonstrate activation of a wide range of mesenchymal genes and deactivation of myelination pathways, a similar phenotype to repair Schwann cells [13]. Further research is required to understand how this mesenchymal phenotype contributes to DFT2 tumorigenesis. As in human cancers, a potential scenario is that strong activation of mesenchymal pathways enhances proliferation, migration, invasion, ‘stemness’, and drug resistance in DFT2 tumours [77, 78]. Another possibility is that inflammatory factors released through these pathways aid repression or modulation of anti-cancer immune responses. A similar scenario has been observed in human lung cancer, neurofibroma, and melanoma, where factors in the tumour microenvironment promote the trans-differentiation of resident Schwann cells into a mesenchymal repair-like phenotype [79, 80]. These tumour-associated Schwann cells promote tumour progression via release of inflammatory factors and chemokines such as CXCL5 that further promote cancer EMT and modulate immunosuppression [79–81].
Schwann cell phenotypes and DFTD emergence
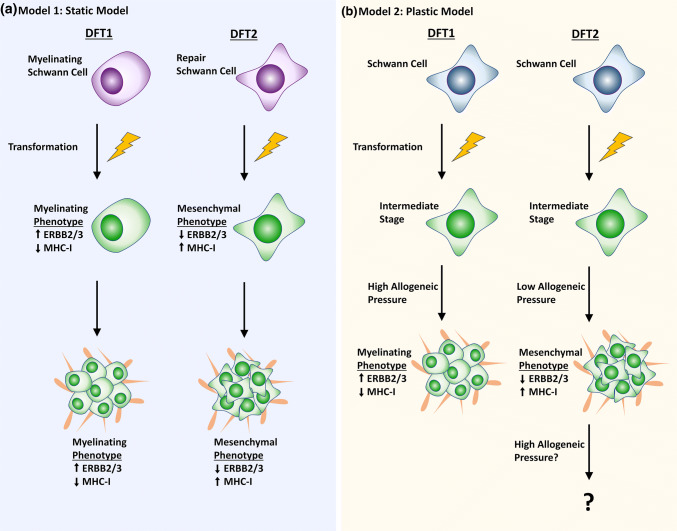
DFT1 and DFT2 cancers exhibit phenotypic differences, despite sharing a similar Schwann cell origin. We propose two models that could explain how DFT1 and DFT2 tumours with distinct phenotypes arose from the same cell type (Fig. 2). In the first model, DFT1 and DFT2 tumours arose from Schwann cells at different functional stages and have maintained key pathways that were activated at the time of transformation (Fig. 2a). For DFT2 cells, transformation could have occurred during peripheral nerve injury, accounting for deactivation of myelination pathways and activation of a mesenchymal signature [13]. In comparison, activation of ErbB2/3-ERG2-mediated pathways in DFT1 is consistent with transformation of this tumour from a Schwann cell participating in myelination, with STAT3 activation indicating that the tumour could have arisen during the late stages of nerve repair [13, 48]. While possible, this model assumes that DFTD phenotypes are static. This is perhaps an unlikely property of the tumours given the high plasticity of Schwann cells during normal nerve maintenance.
Fig. 2.
Models of DFTD emergence in Tasmanian devils. a Static model of emergence: DFTD cells arose from founder Schwann cells at different states of differentiation, giving rise to tumours with different phenotypes. In DFT1 tumours, the ‘myelinating’ phenotype, driven by ERBB2/3 signalling, enabled MHC-I down-regulation to permit tumour transfer across genetically dissimilar hosts. In DFT2 tumours, suppression of ERBB2/3 signalling via the mesenchymal ‘repair’ phenotype meant that alternative mechanisms of immune evasion, such as expression of non-polymorphic and non-classical MHC-I, were required. b Plastic model of emergence: Founder Schwann cells transformed into tumour cells. In DFT1, spread of the tumour into genetically diverse populations gave rise to rise to a ‘myelinating’ phenotype and low MHC-I expression driven by ERBB2/3 signalling. In DFT2, low allogeneic pressure gave rise to a mesenchymal phenotype with low ERBB2/3 signalling and high expression of non-polymorphic and non-classical MHC-I. It is not yet known how DFT2 tumours will change upon spread into genetically diverse populations of devils
Our second model of DFTD emergence proposes that DFT1 and DFT2 tumours assumed a phenotype post-transformation that was most fitting for immune evasion and survival under certain conditions (Fig. 2b). In this model, DFT1 and DFT2 cells benefit from Schwann cell plasticity and adopt a phenotype in response to external cues provided by host cells in the tumour microenvironment. Recent studies of MHC-I regulation in DFT1 and DFT2 provide support for this second model. In DFT1, high ERBB3/STAT3 signalling is thought to contribute to the observed down-regulation of MHC-I at the cell surface [33, 48]. Comparatively, DFT2 tumours express MHC-I, but achieve immune evasion by displaying alleles expressed by the infected host devil (i.e., non-polymorphic classical MHC-I alleles), or suppressive non-classical MHC-I alleles [82]. So far, this has been an effective method of immune evasion given that DFT2 is currently localised to a semi-isolated peninsula, where genetic diversity among devils is likely to be low [7, 37]. Early evidence from a small number of DFT2 tumours affecting hosts with different classical MHC-I alleles has revealed that MHC-I is down-regulated or lost in these DFT2 cancers, presumably in response to increased allogeneic immune pressure [82]. Given that ERBB3 signalling likely contributes to both MHC-I down-regulation and EGR2-mediated myelination in DFT1 [48], and that EGR2-mediated myelination is mutually exclusive to the repair phenotype in other models [66, 83], an increased requirement for MHC-I suppression in DFT2, as it spreads into genetically diverse populations of devils could result in the cancer adopting a similar phenotype to DFT1. Similarly, DFT1 may have existed with a similar phenotype to DFT2 prior to the commencement of its spread across Tasmania in the late 1990s.
Studies in human cancer also support our second model of DFTD emergence. Single-cell analysis of 28 glioblastoma tumours has recently revealed four distinct and highly plastic cellular states driven by gene amplifications and the tumour microenvironment [84]. In DFTD, genomic studies have similarly revealed amplifications to ERBB3 in DFT1 and PDGFRA in DFT2 [20, 85], which have known roles in driving myelination and mesenchymal pathways, respectively [44, 86]. While ERBB3 signalling is closely linked to the myelinating phenotype present in DFT1, PDGFRA signalling has not been directly implicated in Schwan cell repair pathways. However, this gene does drive epithelial to mesenchymal transitions (EMT) in human gliomas [84, 86], and could give rise to a mesenchymal phenotype in DFT2 tumours that is similar to the repair phenotype. This difference in the mesenchymal state of DFT1 and DFT2 tumours reflects evidence from other cancer studies that suggests that cancers exist in different states of differentiation based on relative activation of EMT pathways [77, 87–89]. Indeed, human tumours often exist in an intermediate EMT state, with characteristics of both epithelial and mesenchymal cells [88–90]. Furthermore, it is now clear that human tumour cells can also undergo mesenchymal to epithelial (MET) transitions, allowing metastasising cells to establish tumours at distant sites via re-activation of certain epithelial proteins [88, 91, 92]. DFT1 and DFT2 cells display distinct morphology in cell culture and via histology, which could reflect the increased activation of mesenchymal pathways in DFT2 cells (Fig. 3).
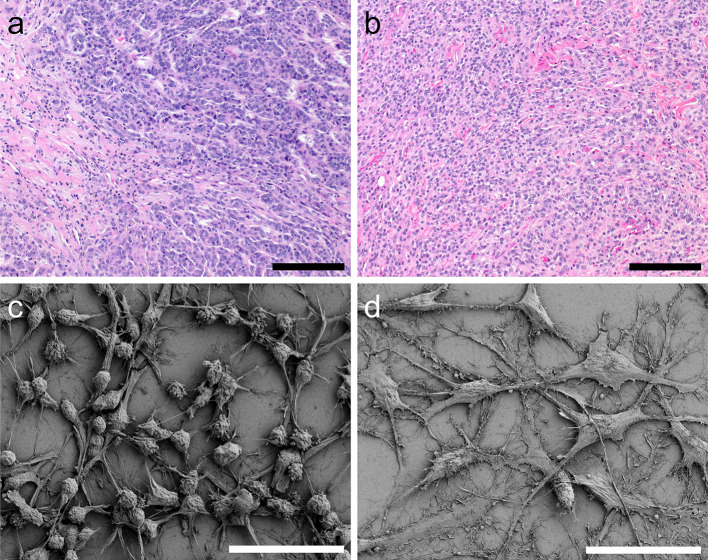
Fig. 3.
Morphology of DFT1 and DFT2 tumour sections and cell cultures. a, b Haematoxylin and eosin staining of a DFT1 (TD553) showing the characteristic pleomorphic round cells arranged in a distinct bundle (top right) and b DFT2 (TD523) with sheets of pleomorphic cells arranged in a solid pattern tumour sections. Scale bars represent 100 µm. c, d Scanning electron microscopy of c a representative DFT1 cell line (C5065) showing characteristic round cell bodies and short projections and d representative DFT2 cell line (RV) displaying flattened cell bodies and long projections. Scale bars represent 50 µm
DFTD and nerve sheath tumours
Knowledge of nerve sheath tumours in other animal species could provide insight into the emergence of DFTD cancers in Tasmanian devils. Relative to humans, benign and malignant nerve sheath tumours are perceived as rare in animal species. These tumours have most frequently been reported in the veterinary literature in animals that tend to undergo regular monitoring through routine veterinary care, such as dogs, cows, and horses [93–95]. Nerve sheath tumours arising in these species can be benign or malignant, but very few studies have been performed to understand the factors driving their genesis [95]. As a result, the human nerve sheath literature provides a better source of information for understanding DFTD cancers in devils. Although animal species such as dogs exhibit biting, licking, and fighting behaviours that could pose as mechanisms of nerve sheath tumour transfer, to our knowledge, there have been no reports of transmission of these cancers in any other animal species.
Human nerve sheath tumours are classified by the neoplastic proliferation of cells with Schwannian differentiation [96]. These cancers present frequently as benign Schwannomas or neurofibromas, and on rarer occasions as malignant peripheral nerve sheath tumours (MPNSTs), perineuriomas, granular cell tumours, or histologically indistinct ‘hybrid’ tumours with diagnostic characteristics of multiple nerve sheath tumour types [97, 98]. Most nerve sheath tumours have a low capacity to become malignant. This is especially the case for schwannomas, which arise directly from Schwann cells in the periphery of the nerve, consist solely of Schwann cells and are often asymptomatic [97]. In comparison, neurofibromas are heterogeneous benign tumours that arise from the centre of the nerve fibre and are composed of a variety of cell types including Schwann cells, perineural cells, vascular cells, fibroblasts, and inflammatory cells [97, 98]. Unlike Schwann cells, neurofibromas can give rise to MPNSTs, a rare form of malignant sarcoma affecting around 1 in 10 million people per year in the USA [39]. Prognosis is poor for patients affected by MPNSTs, with most of these cancers being of a high grade and particularly prone to recurrence [98, 99].
DFT1 and DFT2 cells have genomic aberrations that are similar to those that drive neoplasia in human Schwannomas, neurofibromas, and MPNSTs (Table 1) [20]. In humans, Schwannomas most frequently arise sporadically and are associated with loss-of-function mutations to the tumour-suppressor gene neurofibromin 2 (NF2) [97, 98, 100]. In a smaller portion of cases, schwannomas are associated with the autosomal dominant disorder neurofibromatosis 2, which is characterised by germline mutations to NF2 [100, 101]. NF2 encodes the protein merlin, a key initiator of the anti-proliferative Hippo-signalling pathway that inhibits oncogenic YAP1 and TAZ transcription factors [102, 103]. In DFT1, the genomic locus on chromosome 2 that encodes NF2 has undergone significant rearrangement [20, 85]. However, the NF2 gene lacks somatic changes and is highly expressed in both DFT1 and DFT2 transcriptomes, suggesting that it is functional [13, 20]. Instead, aberrations in other Hippo-signalling components might alter this anti-proliferative pathway in DFT1 and DFT2. Of 2883 single-nucleotide variants (SNVs) and 410 indels in DFT1, and 3591 SNVs and 573 indels in DFT2, only 18 variants in DFT1 and 19 variants in DFT2 are non-synonymous [20]. Of these non-synonymous variants, just one each in DFT1 and DFT2 were predicted to be loss-of-function changes, affecting the genes WWC3 and MPDZ, respectively [20]. Both WWC3 and MPDZ aid the sequestration of YAP1/TAZ in the cytoplasm to promote Hippo-mediated suppression of cell proliferation [104, 105]. In a similar manner to NF2 inactivation, mutation of WWC3 and MPDZ in DFT1 and DFT2 may result in overactivation of YAP1/TAZ, inhibiting Hippo signalling and driving DFTD proliferation. In addition, interaction of MPDZ with CAMK2A in humans is associated with synaptic plasticity, and disruption of this pathway can attenuate stress-induced p38 MAPK activity [106]. A subset of DFT1 cells contains an in-frame CAMK2A-NEURL1B gene fusion. The convergent alterations of pathways involving CAMK2A (DFT1) and MPDZ (DFT2) suggest that functional disruption of synaptic signalling pathways could be important for DFTD synapse formation and motility.
Table 1.
| Gene | Function | Mutation in DFT1a | Expression in DFT1b | Mutation in DFT2a | Expression in DFT2b |
|---|---|---|---|---|---|
| NF1 | Negative regulation of RAS signalling | nd | High | nd | High |
| NF2 | Positive regulation of Hippo signalling | nd | High | Germline missense variant, impact unknown | High |
| SMARCB1 | Chromatin remodelling | nd | High | nd | High |
| LZTR1 | Transcriptional regulation | Heterozygous SV, impact unknown | Moderate | nd | Moderate |
| WWC3 | Positive regulation of Hippo signalling | Truncating SNV; copy number loss, predicted LOF | Moderate | Heterozygous copy number loss | High |
| MPDZ | Positive regulation of Hippo signalling | nd | High | Truncating SV, frame-shift; copy number loss, predicted LOF | Low |
| YAP1 | Proliferative transcription factor inhibited by Hippo signalling | nd | High | nd | High |
| TAZ | Proliferative transcription factor inhibited by Hippo signalling | nd | Moderate | nd | High |
| ERBB3 | RTK signalling | Copy number gain; three germline missense variants, impact unknown | Moderate | Three germline missense variants, impact unknown | High |
| PDGFRA | RTK signalling | nd | Low | Copy number gain | High |
| PDGFRB | RTK signalling | Copy number gain | High | nd | High |
| PDGFA | RTK signalling | Copy number gain | High | nd | High |
| PDGFB | RTK signalling | Copy number gain; germline missense variant, impact unknown | High | Germline missense variant, impact unknown | High |
| NRAS | RAS signalling | nd | Not found | nd | Not found |
| KRAS | RAS signalling | nd | High | nd | High |
| HRAS | RAS signalling | Not found | Not found | Not found | Not found |
| MRAS | RAS signalling | nd | Moderate | nd | Moderate |
| CDKN2A | Cell cycle regulation | Not found | Not found | Not found | Not found |
| TP53 | Cell cycle regulation, pro-apoptotic | nd | High | nd | High |
| TP73 | Cell cycle regulation, pro-apoptotic | Copy number gain | Moderate | Homozygous copy number loss, predicted LOF | Moderate |
| SUZ12 | Subunit of the PRC2 complex | nd | High | nd | High |
| EED | Subunit of the PRC2 complex | nd | Moderate | nd | Moderate |
| EZH2 | Subunit of the PRC2 complex | Heterozygous SV; copy number loss, impact unknown | High | nd | Moderate |
Genes unannotated by Ensembl in Devil_ref v7.0 are denoted as ‘Not found’
nd none detected, SV structural variant, SNV single-nucleotide variant, LOF loss-of-function
aGene aberrancies reported by Stammnitz et al. [20] in one or more DFT1 or DFT2 tumours
bGene expression in DFT1 and DFT2 cell lines as measured by Patchett et al. [13]. Expression was classed as high, moderate, or low based on position in the top, middle, or bottom third of all genes ranked by RPKM-normalised read count
Schwannoma development in humans is also a characteristic of the condition Schwannomatosis, an autosomal dominant disorder involving inactivation of either SWI/SNF-related matrix-associated regulator of chromatin B1 (SMARCB1) or leucine zipper-like transcriptional regulator 1 (LZTR1) [107, 108]. In DFT1, rearrangement of chromosome 2 has resulted in an out-of-frame fusion of a single allele of the LZTR1 gene with the potassium calcium-activated channel N1 (KCNN1) gene [20]. LZTR1 encodes a transcription factor that promotes ubiquitination and regulation of the oncoprotein RAS [109]. Although DFT1 tumours express moderate-to-high levels of RAS genes including KRAS and MRAS (Table 1) [13], the impact of the heterozygous LZTR1 fusion on RAS activation in DFT1 is currently unknown.
Neurofibromas are usually caused by sporadic loss-of-function mutations to the tumour-suppressor gene neurofibromin 1 (NF1), a negative regulator of the proliferative protein RAS [97, 98]. In some cases, neurofibromas are associated with the autosomal dominant condition neurofibromatosis 1, which is caused by germline mutation to NF1 [97, 110]. Patients with neurofibromatosis 1 are also at greater risk of developing MPNSTs, with around half of these sarcomas diagnosed in these patients [111]. Neither DFT1 or DFT2 exhibit somatic mutations to NF1, and expression of this gene is high in both tumours, suggesting that it is functional (Table 1) [13, 20]. However, DFT1 and DFT2 tumours do exhibit genomic aberrations that are similar to other common changes in MPNSTs including overexpression of receptor tyrosine kinases (RTKs) [20, 48, 112–114] and inactivation of proteins involved in cell cycle checkpoints [20, 115–117]. Several genes involved in RTK-signalling exhibit copy number gains in DFT1 (ERBB3, PDGFA, PDGFB, and PDGFRB) and DFT2 (PDGFRA) [20, 85]. As in human nerve sheath cancers [113, 118–121], it is plausible that these pathways contribute to DFTD tumorigenesis by increasing proliferation, migration, and invasion. In other models, RTK signalling has been shown to positively regulate YAP1/TAZ activity and RAS signalling [122–125]. As YAP1/TAZ signalling also promotes RTK activation and might be overactivated in DFT1 and DFT2 [20, 123, 126, 127], cross-talk between ERBB3, PDGF, and YAP1/TAZ signalling could represent a key positive feedback loop controlling tumorigenesis in these cells.
YAP1/TAZ overactivation is common in MPNSTs and has been demonstrated to drive oncogenic transformation of Schwann cells in animal models by giving rise to common aberrations including loss-of-function mutations to the cell cycle regulator TP53 [126]. Although TP53 is not mutated in DFT1 or DFT2, DFT2 tumours demonstrate homozygous deletion of TP73, a transcription factor related to TP53 that associates with YAP1 to positively regulate apoptosis in response to DNA damage [20, 128, 129]. MPNSTs also frequently exhibit inactivating somatic mutations of polycomb proteins SUZ12 (SUZ12) and EED (EED). These genes are members of the histone methyltransferase PRC2, which trimethylates histone H3 on lysine 27 (H3K27me3) to regulate target gene repression [116, 130, 131]. It has been hypothesised that loss of PRC2 in cancer leads to a reduction in the threshold of transcriptional activation for target genes such as growth factors and proteins involved in immune evasion [132, 133]. Furthermore, PRC2 loss may contribute to epigenetic suppression of MHC-I antigen presentation pathways in nerve sheath tumours [133]. In a subset of DFT1 tumours, EZH2, which encodes the third member of the PRC2 complex, has undergone an in-frame fusion with the gene ETNK2 [20]. However, rather than contributing to MHC-I suppression, studies have suggested that PCR2 loss in DFT1 cells potentiates MHC-I up-regulation in response to interferon-gamma [34]. Additional studies are required to determine how the ETNK2–EZH2 fusion contributes to MHC-I regulation in DFT1.
Factors predisposing devils to transmissible Schwann cell cancers
The identification of two transmissible Schwann cell cancers in Tasmanian devils within 20 years highlights the susceptibility of this species to cancers of this nature [13]. Transmissible cancers must overcome robust allogeneic and oncogenic defences to survive across genetically distinct animals. Consequently, the susceptibility of devils to cancer transmission is potentially due to a combination of factors acting in unison to allow DFTD emergence. No exogenous pathogens and carcinogens have been associated with DFT1 or DFT2 to date, but several endogenous factors have been described [20, 36]. The potential contributions of these factors to DFTD emergence are discussed below.
Inherent predisposition and immune function
Since its emergence in 1996, DFT1 has become the primary cause of mortality in wild Tasmanian devils. Interestingly, non-DFTD neoplasia is a major cause of mortality and morbidity of devils in captivity. A recent study reported that over an 8-year period in Tasmania, non-DFTD cancers accounted for 43% of deaths in captivity [134]. Of these cancer-related mortalities, cutaneous lymphomas, cutaneous round cell tumours, squamous cell carcinomas, and adenocarcinomas were most common. No cancers of neural origin were recorded, although neurodegenerative conditions leading to hindlimb paralysis, including leucoencephalomyelopathy and spinal Wallerian degeneration, were the second leading cause of mortality [134]. Historical data from the San Diego Zoo support the susceptibility of devils to cancer, with a reported incidence at necropsy that was twice any other measured species and ten times higher than average [135, 136]. Together, these studies indicate that devils could be genetically predisposed to the emergence of cancers, including DFTD. Indeed, both DFT1 and DFT2 arose from devils with similar ‘eastern’ genotypes, and candidate germline alleles with a potential role in DFTD predisposition have been identified in these tumours [20]. However, these alleles were not associated with variants of known inherited cancer risk in humans, and further investigation is required to determine if they play a role in DFTD susceptibility [20]. Other heritable factors that may play a role in the emergence of cancers in Tasmanian devils include their unusual telomere organisation (extreme length dimorphism), which could predispose cells to chromosomal rearrangements [20, 137].
Aberrancies among pathways involved in cancer prevention, such as immune function, responses to DNA damage and cell cycle checkpoints are ideal candidates for an increased cancer predisposition in devils. Given the transmissible nature of DFT1 and DFT2, the immune system of the Tasmanian devil has been investigated for defects that could explain the emergence of these unusual cancers. Marsupials were traditionally thought of as having weak immune systems a concept that was supported by the detection of weak mixed-lymphocyte responses in the short-tailed opossum (Monodelphis domestica) [138] and poor antigen-specific responses in the koala (Phascolarctos cinereus) [139]. However, this concept has been challenged by studies in the Tasmanian devil, which have so far failed to reveal significant insufficiencies among immune responses. Tasmanian devils have all the expected primary and secondary lymphoid organs and a full complement of leukocytes [140, 141]. Important immune functions including toll-like receptor (TLR) activation, phagocytosis, leukocyte proliferation, allogeneic detection, antibody production, and cytotoxicity are also effective [141–144], and rapid and potent recall responses to antigenic challenge have been observed [143, 145]. Given that DFT1 cells lack MHC-I expression [33], the function of natural killer (NK) cells, which detect and kill aberrant cells lacking MHC-I, remains under investigation. NK cells have been difficult to assess in the Tasmanian devil due to a lack of available reagents for detecting this cell subset. However, evidence for rapid antibody-dependent and mitogen-induced killing of human tumour cells suggests that these cells are also present and functional in Tasmanian devils [146, 147].
Although devils have a functional immune system, there is evidence for a decline in immune function once they reach adulthood. Adult devils exhibit reduced lymphocyte abundance and T-cell receptor (TCR) diversity relative to juveniles, which could affect host defences and perhaps increase their susceptibility to cancer transmission [148, 149]. Despite this age-related decline in immune function, a proportion of adult devils can activate immune responses against DFT1 tumours. This has been demonstrated through monitoring programs that have detected a small number of wild devils with increased levels of DFT1-specific antibody and spontaneous DFT1 regressions [150, 151]. Studies in captivity have also demonstrated immune-mediated rejection of DFT1 tumours after immunotherapy [152]. Although it is unknown whether natural immune responses against DFT1 are protective against subsequent DFT1 encounters, these findings suggest that the genesis of DFTD in devils is not limited to reduced immune function. Instead, the emergence of DFT1 and DFT2 as successful transmissible cancers was likely influenced by the acquisition of active mechanisms of immune evasion by the cancer cells. In support of this, modulation of MHC-I expression appears to be critical to survival of DFT1 and DFT2 cells under different conditions [33, 82]. In addition, TCR diversity is markedly decreased after DFT1 infection, suggesting that DFT1 cells directly alter their immune landscape [148]. Other strategies used by DFT1 and DFT2 to modulate immune responses could involve expression of immunosuppressive cytokines and inhibitory immune checkpoint molecules [153–155].
Low genetic diversity
A lack of genetic diversity among devil populations has long been implicated as a potential mechanism contributing to DFTD emergence [32]. Historical evidence suggests that devils have undergone population fluctuations and bottlenecks, with factors such as climate events, disease, increased human density, and historical culling postulated to have played a role [19, 156, 157]. These fluctuations have resulted in low diversity among devils which is detectable through genomic sequencing and analysis of MHC-I and microsatellite genotypes [31, 32, 158–160]. A failure of the immune system to reject allografts has been observed in the cheetah, which suffered severe inbreeding and reduced genetic diversity following a previous population bottleneck [161]. In devils, early experiments revealed low mixed-lymphocyte responses between devils from similar locations, suggesting that a lack of diversity could account for DFT1 tolerance [32, 162]. To determine if this was the case, allogeneic skin transplants were performed between devils [162]. Within 2 weeks, all allografts displayed extensive immune infiltration and were subsequently rejected. These findings convincingly demonstrated that Tasmanian devils are capable of allorecognition, and it was proposed that low genetic diversity could not fully account for DFT1 transmission among devils.
The discovery of DFT2 in 2014 revealed an alternative role for low genetic diversity in the emergence of DFTD cancers. As discussed above, DFT2 cells predominately express MHC-I alleles that are common in the population where they emerged, allowing the cancer to avoid allogeneic detection in any host also expressing these MHC-I alleles [82]. Low genetic diversity increases the number of hosts with these common MHC-I alleles, thus providing a larger pool of individuals for the tumour to initially infect. This may in turn increase the chance that successful tumour variants will evolve that can spread into genetically diverse devils (i.e., through MHC-I loss). While these findings suggest that low genetic diversity is important to the initial emergence of a transmissible cancer, the evolution of MHC-I down-regulation appears to be fundamental to the subsequent transmission of these tumours into genetically diverse populations [33, 82]. In dogs, CTVT cells also exhibit low MHC-I expression that is thought to prevent allorecognition, but is likely sufficient for inactivation of NK responses [3, 163]. MHC-I down-regulation can be transient in CTVT, and induced expression of this molecule supports host survival by enabling tumour rejection [164, 165].
The role of low genetic diversity in the emergence of DFT1 and DFT2 could explain the recent appearance of these cancers. Low genetic diversity in devil populations is believed to have arisen before or during the mid-Holocene [156, 157]. However, population fluctuations that occurred as recent as the mid-1900s could have further reduced genetic diversity in devil populations to allow transmissible cancers to emerge [156]. Similarly, regrowth of the population after these latest bottlenecks could have given rise to a common genotype predisposing devils to cancer. Understanding the contribution of low genetic diversity to DFTD emergence will be important for mitigating the risk of further transmissible cancers emerging in Tasmanian devils.
Devil behaviour
For a cancer to become transmissible, it must acquire an effective route of tumour cell transfer. In the Tasmanian devil, DFTD cancers appear on external surfaces as large, often ulcerated and friable tumours that are accessible for contact-dependent transmission [23]. Devil-to-devil biting provides an uncontrolled means of contact for this cell transfer to occur. Biting is a common behaviour of devils, particularly during mating interactions [25, 26]. Tumour transfer is thought to occur when a healthy devil bites the tumour of an infected devil and the cells become established in existing wounds within the oral cavity of the new host. Cancer cells may also be transferred when an infected devil bites a healthy devil and inoculates cancer cells into the bite wound of the recipient [26]. Wounds provide a break in the protective epithelium of the skin or oral cavity, thus allowing the tumour cells to overcome a major barrier to infection. Furthermore, wounds provide an ideal immunomodulatory environment for tumour establishment. A key step in the wound healing responses involves the release of growth factors and cytokines that promote tissue healing and growth [166]. Similar events are involved in tumour establishment, with recruited fibroblasts and innate cells playing key roles in building the tumour stroma and establishing a suppressive tumour microenvironment [167–169]. Transforming growth factor-β (TGFβ) is a key suppressive cytokine released by cells at the site of wounding that activates mesenchymal transcription factors and repair pathways within Schwann cells and has many tumour-promoting effects [54, 170]. Previous studies have detected TGFβ expression within DFT1 tumours, suggesting that this cytokine could be important to DFTD tumorigenesis [153].
In humans, chemicals and conditions that cause tissue damage contribute to mutagenesis by promoting the release of inflammatory mediators, growth factors and damaging molecules such as reactive oxygen and reactive nitrogen species [171]. These agents prevent tumour suppression, promote cell proliferation and cause DNA damage, increasing the chance of tumour development. It has been hypothesised that tumours are “wounds that do not heal” [168], with repetitive injury or unresolved inflammation leading to an unchecked wound healing process that promotes cancer growth and survival [169]. This situation could apply to the Tasmanian devil, where consistent wounding through bite injuries and scavenging on sharp bone fragments has potential to produce repeated inflammation among Schwann cells of the innervated sensory vibrissae. The high plasticity and proliferative nature of Schwann cells during injury might leave these cells vulnerable to oncogenic transformation. Indeed, the YAP1/TAZ and ERBB3 pathways that have been implicated as potential drivers of DFT1 and DFT2 tumorigenesis are also regulated during the Schwann cell repair response during injury [20, 48, 67, 70]. These pathways are driven by inflammatory factors such as TGFβ and IL1β, which act in wound and cancer microenvironments to modulate both cancer cells and immune responses [54, 64, 170].
We have previously proposed that a vulnerability of Schwann cells to undergo oncogenic transformation could be exacerbated in Tasmanian devils due to the high frequency of peripheral nerve injury in this species [13]. As biting also accounts for the transmission of DFTD cancers, it is possible that this combination of frequent wounding and Schwann cell plasticity underlies both the genesis and persistence of DFTD cancers in Tasmanian devils (Fig. 4). Other contributing factors, such as an inherent cancer susceptibility, low genetic diversity, and successful evolution of tumorigenic mechanisms, could be fundamental to progression through the different stages of DFTD evolution. This model suggests that the emergence of DFTD cancers in the Tasmanian devils is the consequence of a ‘perfect storm’ of factors that in combination overcome robust defences against cancer transmission to allow for successful propagation of DFTD tumours [172]. The simultaneous occurrence of similar factors in other species is likely to be unusual, perhaps explaining the rarity of cancer transmission within mammalian species.
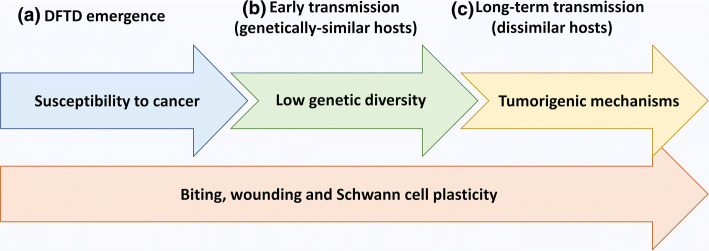
Fig. 4.
Factors hypothesised to contribute to DFTD emergence in Tasmanian devils. a Inherent susceptibility to cancer combines with frequent Schwann cell wounding from biting to give rise to a DFTD tumour in a founder Tasmanian devil. b Biting behaviours allow transmission of the cancer cells into the wounds of new hosts. Low genetic diversity in the founder population combines with the plastic nature of Schwann cells to prevent allogeneic rejection of the DFTD allograft. c Biting behaviours allow transmission of the cancer cells into the wounds of genetically diverse devils. The cancer cells evolve tumorigenic mechanisms, such as loss of MHC-I, which combine with the plastic nature of Schwann cells to avoid allogeneic responses
Conclusion
The emergence of two transmissible Schwann cell cancers in the Tasmanian devil was unexpected due to the rarity of cancer transmission in nature and malignant Schwann cell cancers in humans. Accumulating evidence suggests that these cancers are the consequence of key enabling factors that combined on two occasions to give rise to founder DFTD tumours that were able to be transmitted among devils. A lack of fossil or anecdotal evidence for DFTD-like cancers in devils prior to 1996 suggests that the emergence of these cancers may be a recent phenomenon, perhaps influenced by recent population ‘bottlenecks’ that have impacted the genetic diversity of the species. Alternatively, these diseases could be a downstream consequence of previous disease-associated selection events that have channelled the devil's genetic architecture into an increased predisposition for transmissible Schwann cell cancers. It is possible that long-term genetic selection and loss of diversity imposed by DFT1 and DFT2 could similarly leave the species vulnerable to further transmissible cancers or other disease threats in the future. Strategies for genetic rescue and management of devil populations have potential to reduce this risk. Meanwhile, continued investigations into the nature of DFT1 and DFT2 will be driven by knowledge of Schwann cell function and nerve sheath tumours in other species. These studies will direct the identification of molecular targets and inform the development of DFTD interventions such as vaccines, which could mitigate the threat of transmissible Schwann cell cancers in Tasmanian devil populations.
Acknowledgements
The authors wish to thank Narelle Phillips for immunohistochemistry, Karsten Goemann for scanning electron microscopy and Jocelyn Darby, Ruth Pye and Cesar Tovar for useful discussion. Research support was provided by the Australian Research Council (DP130100715, DE180100484, DP180100520) and the University of Tasmania Foundation through funds raised by the Save the Tasmanian Devil Appeal.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Metzger MJ, Goff SP. A sixth modality of infectious disease: contagious cancer from devils to clams and beyond. PLoS Pathog. 2016;12(10):e1005904. doi: 10.1371/journal.ppat.1005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearse AM, Swift K. Allograft theory: transmission of devil facial-tumour disease. Nature. 2006;439(7076):549. doi: 10.1038/439549a. [DOI] [PubMed] [Google Scholar]
- 3.Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA. Clonal origin and evolution of a transmissible cancer. Cell. 2006;126(3):477–487. doi: 10.1016/j.cell.2006.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metzger MJ, Reinisch C, Sherry J, Goff SP. Horizontal transmission of clonal cancer cells causes leukemia in soft-shell clams. Cell. 2015;161(2):255–263. doi: 10.1016/j.cell.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matser YAH, Terpstra ML, Nadalin S, Nossent GD, de Boer J, van Bemmel BC, van Eeden S, Budde K, Brakemeier S, Bemelman FJ. Transmission of breast cancer by a single multiorgan donor to 4 transplant recipients. Am J Transplant. 2018;18(7):1810–1814. doi: 10.1111/ajt.14766. [DOI] [PubMed] [Google Scholar]
- 6.Gartner HV, Seidl C, Luckenbach C, Schumm G, Seifried E, Ritter H, Bultmann B. Genetic analysis of a sarcoma accidentally transplanted from a patient to a surgeon. N Engl J Med. 1996;335(20):1494–1496. doi: 10.1056/nejm199611143352004. [DOI] [PubMed] [Google Scholar]
- 7.Pye RJ, Pemberton D, Tovar C, Tubio JM, Dun KA, Fox S, Darby J, Hayes D, Knowles GW, Kreiss A, Siddle HV, Swift K, Lyons AB, Murchison EP, Woods GM. A second transmissible cancer in Tasmanian devils. Proc Natl Acad Sci USA. 2016;113(2):374–379. doi: 10.1073/pnas.1519691113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metzger MJ, Villalba A, Carballal MJ, Iglesias D, Sherry J, Reinisch C, Muttray AF, Baldwin SA, Goff SP. Widespread transmission of independent cancer lineages within multiple bivalve species. Nature. 2016;534(7609):705–709. doi: 10.1038/nature18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yonemitsu MA, Giersch RM, Polo-Prieto M, Hammel M, Simon A, Cremonte F, Aviles FT, Merino-Veliz N, Burioli EA, Muttray AF, Sherry J, Reinisch C, Baldwin SA, Goff SP, Houssin M, Arriagada G, Vazquez N, Bierne N, Metzger MJ. A single clonal lineage of transmissible cancer identified in two marine mussel species in South America and Europe. Elife. 2019 doi: 10.7554/eLife.47788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakkis FG, Dellaporta SL, Buss LW. Allorecognition and chimerism in an invertebrate model organism. Organogenesis. 2008;4(4):236–240. doi: 10.4161/org.4.4.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Busquets X, Burger MM. Cell adhesion and histocompatibility in sponges. Microsc Res Tech. 1999;44(4):204–218. doi: 10.1002/(sici)1097-0029(19990215)44:4<204::Aid-jemt2>3.0.Co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Murchison EP, Tovar C, Hsu A, Bender HS, Kheradpour P, Rebbeck CA, Obendorf D, Conlan C, Bahlo M, Blizzard CA, Pyecroft S, Kreiss A, Kellis M, Stark A, Harkins TT, Marshall Graves JA, Woods GM, Hannon GJ, Papenfuss AT. The Tasmanian devil transcriptome reveals Schwann cell origins of a clonally transmissible cancer. Science. 2010;327(5961):84–87. doi: 10.1126/science.1180616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patchett AL, Coorens THH, Darby J, Wilson R, McKay MJ, Kamath KS, Rubin A, Wakefield M, McIntosh L, Mangiola S, Pye RJ, Flies AS, Corcoran LM, Lyons AB, Woods GM, Murchison EP, Papenfuss AT, Tovar C. Two of a kind: transmissible Schwann cell cancers in the endangered Tasmanian devil (Sarcophilus harrisii) Cell Mol Life Sci. 2019 doi: 10.1007/s00018-019-03259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baez-Ortega A, Gori K, Strakova A, Allen JL, Allum KM, Bansse-Issa L, Bhutia TN, Bisson JL, Briceno C, Castillo Domracheva A, Corrigan AM, Cran HR, Crawford JT, Davis E, de Castro KF, BdN A, de Vos AP, Delgadillo Keenan L, Donelan EM, Espinoza Huerta AR, Faramade IA, Fazil M, Fotopoulou E, Fruean SN, Gallardo-Arrieta F, et al. Somatic evolution and global expansion of an ancient transmissible cancer lineage. Science. 2019 doi: 10.1126/science.aau9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murchison EP, Wedge DC, Alexandrov LB, Fu B, Martincorena I, Ning Z, Tubio JMC, Werner EI, Allen J, De Nardi AB, Donelan EM, Marino G, Fassati A, Campbell PJ, Yang F, Burt A, Weiss RA, Stratton MR. Transmissible [corrected] dog cancer genome reveals the origin and history of an ancient cell lineage. Science. 2014;343(6169):437–440. doi: 10.1126/science.1247167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frampton D, Schwenzer H, Marino G, Butcher LM, Pollara G, Kriston-Vizi J, Venturini C, Austin R, de Castro KF, Ketteler R, Chain B, Goldstein RA, Weiss RA, Beck S, Fassati A. Molecular signatures of regression of the canine transmissible venereal tumor. Cancer Cell. 2018;33(4):620–633.e626. doi: 10.1016/j.ccell.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amber EI, Henderson RA, Adeyanju JB, Gyang EO. Single-drug chemotherapy of canine transmissible venereal tumor with cyclophosphamide, methotrexate, or vincristine. J Vet Intern Med. 1990;4(3):144–147. doi: 10.1111/j.1939-1676.1990.tb00887.x. [DOI] [PubMed] [Google Scholar]
- 18.Murchison EP. Clonally transmissible cancers in dogs and Tasmanian devils. Oncogene. 2008;27(Suppl 2):S19–30. doi: 10.1038/onc.2009.350. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins CE, Baars C, Hesterman H, Hocking G, Jones ME, Lazenby B, Mann D, Mooney N, Pemberton D, Pyecroft S. Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biol Conserv. 2006;131(2):307–324. doi: 10.1016/j.biocon.2006.04.010. [DOI] [Google Scholar]
- 20.Stammnitz MR, Coorens THH, Gori KC, Hayes D, Fu B, Wang J, Martin-Herranz DE, Alexandrov LB, Baez-Ortega A, Barthorpe S, Beck A, Giordano F, Knowles GW, Kwon YM, Hall G, Price S, Pye RJ, Tubio JMC, Siddle HVT, Sohal SS, Woods GM, McDermott U, Yang F, Garnett MJ, Ning Z, et al. The origins and vulnerabilities of two transmissible cancers in Tasmanian Devils. Cancer Cell. 2018;33(4):607–619.e615. doi: 10.1016/j.ccell.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCallum H. Tasmanian devil facial tumour disease: lessons for conservation biology. Trends Ecol Evol. 2008;23(11):631–637. doi: 10.1016/j.tree.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Lazenby BT, Tobler MW, Brown WE, Hawkins CE, Hocking GJ, Hume F, Huxtable S, Iles P, Jones ME, Lawrence C, Thalmann S, Wise P, Williams H, Fox S, Pemberton D. Density trends and demographic signals uncover the long-term impact of transmissible cancer in Tasmanian devils. J Appl Ecol. 2018;55(3):1368–1379. doi: 10.1111/1365-2664.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loh R, Bergfeld J, Hayes D, O'Hara A, Pyecroft S, Raidal S, Sharpe R. The pathology of devil facial tumor disease (DFTD) in Tasmanian Devils (Sarcophilus harrisii) Vet Pathol. 2006;43(6):890–895. doi: 10.1354/vp.43-6-890. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Aravena M, Jones ME, Carver S, Estay S, Espejo C, Storfer A, Hamede RK. Sex bias in ability to cope with cancer: Tasmanian devils and facial tumour disease. Proc Biol Sci. 2018 doi: 10.1098/rspb.2018.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamede RK, Mccallum H, Jones M. Seasonal, demographic and density-related patterns of contact between Tasmanian devils (Sarcophilus harrisii): implications for transmission of devil facial tumour disease. Austral Ecol. 2008;33(5):614–622. doi: 10.1111/j.1442-9993.2007.01827.x. [DOI] [Google Scholar]
- 26.Hamede RK, McCallum H, Jones M. Biting injuries and transmission of Tasmanian devil facial tumour disease. J Anim Ecol. 2013;82(1):182–190. doi: 10.1111/j.1365-2656.2012.02025.x. [DOI] [PubMed] [Google Scholar]
- 27.Jones ME, Cockburn A, Hamede R, Hawkins C, Hesterman H, Lachish S, Mann D, McCallum H, Pemberton D. Life-history change in disease-ravaged Tasmanian devil populations. Proc Natl Acad Sci USA. 2008;105(29):10023–10027. doi: 10.1073/pnas.0711236105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachish S, McCallum H, Jones M. Demography, disease and the devil: life-history changes in a disease-affected population of Tasmanian devils (Sarcophilus harrisii) J Anim Ecol. 2009;78(2):427–436. doi: 10.1111/j.1365-2656.2008.01494.x. [DOI] [PubMed] [Google Scholar]
- 29.Wells K, Hamede RK, Jones ME, Hohenlohe PA, Storfer A, McCallum HI. Individual and temporal variation in pathogen load predicts long-term impacts of an emerging infectious disease. Ecology. 2019;100(3):e02613. doi: 10.1002/ecy.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epstein B, Jones M, Hamede R, Hendricks S, McCallum H, Murchison EP, Schonfeld B, Wiench C, Hohenlohe P, Storfer A. Rapid evolutionary response to a transmissible cancer in Tasmanian devils. Nat Commun. 2016;7:12684. doi: 10.1038/ncomms12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grueber CE, Fox S, McLennan EA, Gooley RM, Pemberton D, Hogg CJ, Belov K. Complex problems need detailed solutions: Harnessing multiple data types to inform genetic management in the wild. Evol Appl. 2019;12(2):280–291. doi: 10.1111/eva.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddle HV, Kreiss A, Eldridge MD, Noonan E, Clarke CJ, Pyecroft S, Woods GM, Belov K. Transmission of a fatal clonal tumor by biting occurs due to depleted MHC diversity in a threatened carnivorous marsupial. Proc Natl Acad Sci USA. 2007;104(41):16221–16226. doi: 10.1073/pnas.0704580104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddle HV, Kreiss A, Tovar C, Yuen CK, Cheng Y, Belov K, Swift K, Pearse AM, Hamede R, Jones ME, Skjodt K, Woods GM, Kaufman J. Reversible epigenetic down-regulation of MHC molecules by devil facial tumour disease illustrates immune escape by a contagious cancer. Proc Natl Acad Sci USA. 2013;110(13):5103–5108. doi: 10.1073/pnas.1219920110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burr ML, Sparbier CE, Chan KL, Chan YC, Kersbergen A, Lam EYN, Azidis-Yates E, Vassiliadis D, Bell CC, Gilan O, Jackson S, Tan L, Wong SQ, Hollizeck S, Michalak EM, Siddle HV, McCabe MT, Prinjha RK, Guerra GR, Solomon BJ, Sandhu S, Dawson SJ, Beavis PA, Tothill RW, Cullinane C, et al. An evolutionarily conserved function of polycomb silences the MHC class I antigen presentation pathway and enables immune evasion in cancer. Cancer Cell. 2019 doi: 10.1016/j.ccell.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tovar C, Obendorf D, Murchison EP, Papenfuss AT, Kreiss A, Woods GM. Tumor-specific diagnostic marker for transmissible facial tumors of Tasmanian devils: immunohistochemistry studies. Vet Pathol. 2011;48(6):1195–1203. doi: 10.1177/0300985811400447. [DOI] [PubMed] [Google Scholar]
- 36.Murchison EP, Schulz-Trieglaff OB, Ning Z, Alexandrov LB, Bauer MJ, Fu B, Hims M, Ding Z, Ivakhno S, Stewart C, Ng BL, Wong W, Aken B, White S, Alsop A, Becq J, Bignell GR, Cheetham RK, Cheng W, Connor TR, Cox AJ, Feng ZP, Gu Y, Grocock RJ, Harris SR, et al. Genome sequencing and analysis of the Tasmanian devil and its transmissible cancer. Cell. 2012;148(4):780–791. doi: 10.1016/j.cell.2011.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.James S, Jennings G, Kwon YM, Stammnitz M, Fraik A, Storfer A, Comte S, Pemberton D, Fox S, Brown B. Tracing the rise of malignant cell lines: distribution, epidemiology and evolutionary interactions of two transmissible cancers in Tasmanian devils. Evol Appl. 2019;12(9):1772–1780. doi: 10.1111/eva.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loh R, Hayes D, Mahjoor A, O'Hara A, Pyecroft S, Raidal S. The immunohistochemical characterization of devil facial tumor disease (DFTD) in the Tasmanian Devil (Sarcophilus harrisii) Vet Pathol. 2006;43(6):896–903. doi: 10.1354/vp.43-6-896. [DOI] [PubMed] [Google Scholar]
- 39.Ng VY, Scharschmidt TJ, Mayerson JL, Fisher JL. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res. 2013;33(6):2597–2604. [PubMed] [Google Scholar]
- 40.Woodhoo A, Sommer L. Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia. 2008;56(14):1481–1490. doi: 10.1002/glia.20723. [DOI] [PubMed] [Google Scholar]
- 41.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6(9):671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 42.Decker L, Desmarquet-Trin-Dinh C, Taillebourg E, Ghislain J, Vallat JM, Charnay P. Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. J Neurosci. 2006;26(38):9771–9779. doi: 10.1523/jneurosci.0716-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371(6500):796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- 44.Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47(5):681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Syed N, Reddy K, Yang DP, Taveggia C, Salzer JL, Maurel P, Kim HA. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J Neurosci. 2010;30(17):6122–6131. doi: 10.1523/jneurosci.1681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birchmeier C, Nave KA. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia. 2008;56(14):1491–1497. doi: 10.1002/glia.20753. [DOI] [PubMed] [Google Scholar]
- 47.Aguayo AJ, Charron L, Bray GM. Potential of Schwann cells from unmyelinated nerves to produce myelin: a quantitative ultrastructural and radiographic study. J Neurocytol. 1976;5(8):565–573. doi: 10.1007/bf01175570. [DOI] [PubMed] [Google Scholar]
- 48.Kosack L, Wingelhofer B, Popa A, Orlova A, Agerer B, Vilagos B, Majek P, Parapatics K, Lercher A, Ringler A, Klughammer J, Smyth M, Khamina K, Baazim H, de Araujo ED, Rosa DA, Park J, Tin G, Ahmar S, Gunning PT, Bock C, Siddle HV, Woods GM, Kubicek S, Murchison EP, et al. The ERBB-STAT3 axis drives Tasmanian Devil facial tumor disease. Cancer Cell. 2019;35(1):125–139.e129. doi: 10.1016/j.ccell.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Negro S, Bergamin E, Rodella U, Duregotti E, Scorzeto M, Jalink K, Montecucco C, Rigoni M. ATP released by injured neurons activates schwann cells. Front Cell Neurosci. 2016;10:134. doi: 10.3389/fncel.2016.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Man LL, Liu F, Wang YJ, Song HH, Xu HB, Zhu ZW, Zhang Q, Wang YJ. The HMGB1 signaling pathway activates the inflammatory response in Schwann cells. Neural Regen Res. 2015;10(10):1706–1712. doi: 10.4103/1673-5374.167773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ino D, Sagara H, Suzuki J, Kanemaru K, Okubo Y, Iino M. Neuronal regulation of schwann cell mitochondrial Ca(2+) signaling during myelination. Cell Rep. 2015;12(12):1951–1959. doi: 10.1016/j.celrep.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 52.Gomez-Sanchez JA, Pilch KS, van der Lans M, Fazal SV, Benito C, Wagstaff LJ, Mirsky R, Jessen KR. After nerve injury, lineage tracing shows that myelin and remak schwann cells elongate extensively and branch to form repair schwann cells, which shorten radically on remyelination. J Neurosci. 2017;37(37):9086–9099. doi: 10.1523/jneurosci.1453-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75(4):633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clements MP, Byrne E, Camarillo Guerrero LF, Cattin AL, Zakka L, Ashraf A, Burden JJ, Khadayate S, Lloyd AC, Marguerat S, Parrinello S. The wound microenvironment reprograms Schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron. 2017;96(1):98–114.e117. doi: 10.1016/j.neuron.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci. 2000;12(12):4171–4180. doi: 10.1111/j.1460-9568.2000.01312.x. [DOI] [PubMed] [Google Scholar]
- 56.Fontana X, Hristova M, Da Costa C, Patodia S, Thei L, Makwana M, Spencer-Dene B, Latouche M, Mirsky R, Jessen KR, Klein R, Raivich G, Behrens A. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J Cell Biol. 2012;198(1):127–141. doi: 10.1083/jcb.201205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, Wright M, Vyas A, Hoke A. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp Neurol. 2013;247:272–281. doi: 10.1016/j.expneurol.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002;22(15):6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parfejevs V, Debbache J, Shakhova O, Schaefer SM, Glausch M, Wegner M, Suter U, Riekstina U, Werner S, Sommer L. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat Commun. 2018;9(1):236. doi: 10.1038/s41467-017-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Griffith M, Hantke J, Macias-Camara N, Azkargorta M, Aurrekoetxea I, De Juan VG, Jefferies HB, Aspichueta P, Elortza F, Aransay AM, Martinez-Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A, Jessen KR. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol. 2015;210(1):153–168. doi: 10.1083/jcb.201503019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jang SY, Shin YK, Park SY, Park JY, Lee HJ, Yoo YH, Kim JK, Park HT. Autophagic myelin destruction by Schwann cells during Wallerian degeneration and segmental demyelination. Glia. 2016;64(5):730–742. doi: 10.1002/glia.22957. [DOI] [PubMed] [Google Scholar]
- 62.Kim S, Maynard JC, Strickland A, Burlingame AL, Milbrandt J. Schwann cell O-GlcNAcylation promotes peripheral nerve remyelination via attenuation of the AP-1 transcription factor JUN. Proc Natl Acad Sci USA. 2018;115(31):8019–8024. doi: 10.1073/pnas.1805538115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korimova A, Klusakova I, Hradilova-Svizenska I, Kohoutkova M, Joukal M, Dubovy P. Mitochondrial damage-associated molecular patterns of injured axons induce outgrowth of schwann cell processes. Front Cell Neurosci. 2018;12:457. doi: 10.3389/fncel.2018.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen G, Luo X, Wang W, Wang Y, Zhu F, Wang W. Interleukin-1beta promotes schwann cells de-differentiation in Wallerian degeneration via the c-JUN/AP-1 pathway. Front Cell Neurosci. 2019;13:304. doi: 10.3389/fncel.2019.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fazal SV, Gomez-Sanchez JA, Wagstaff LJ, Musner N, Otto G, Janz M, Mirsky R, Jessen KR. Graded elevation of c-Jun in schwann cells in vivo: gene dosage determines effects on development, remyelination, tumorigenesis, and hypomyelination. J Neurosci. 2017;37(50):12297–12313. doi: 10.1523/jneurosci.0986-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen KR. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181(4):625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mindos T, Dun XP, North K, Doddrell RD, Schulz A, Edwards P, Russell J, Gray B, Roberts SL, Shivane A, Mortimer G, Pirie M, Zhang N, Pan D, Morrison H, Parkinson DB. Merlin controls the repair capacity of Schwann cells after injury by regulating Hippo/YAP activity. J Cell Biol. 2017;216(2):495–510. doi: 10.1083/jcb.201606052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma KH, Hung HA, Svaren J. Epigenomic regulation of schwann cell reprogramming in peripheral nerve injury. J Neurosci. 2016;36(35):9135–9147. doi: 10.1523/jneurosci.1370-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hung HA, Sun G, Keles S, Svaren J. Dynamic regulation of Schwann cell enhancers after peripheral nerve injury. J Biol Chem. 2015;290(11):6937–6950. doi: 10.1074/jbc.M114.622878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benito C, Davis CM, Gomez-Sanchez JA, Turmaine M, Meijer D, Poli V, Mirsky R, Jessen KR. STAT3 controls the long-term survival and phenotype of repair schwann cells during nerve regeneration. J Neurosci. 2017;37(16):4255–4269. doi: 10.1523/jneurosci.3481-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eggers R, Tannemaat MR, Ehlert EM, Verhaagen J. A spatio-temporal analysis of motoneuron survival, axonal regeneration and neurotrophic factor expression after lumbar ventral root avulsion and implantation. Exp Neurol. 2010;223(1):207–220. doi: 10.1016/j.expneurol.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 72.Weinberg HJ, Spencer PS. The fate of Schwann cells isolated from axonal contact. J Neurocytol. 1978;7(5):555–569. doi: 10.1007/BF01260889. [DOI] [PubMed] [Google Scholar]
- 73.Lee HK, Jung J, Lee SH, Seo SY, Suh DJ, Park HT. Extracellular signal-regulated kinase activation is required for serine 727 phosphorylation of STAT3 in Schwann cells in vitro and in vivo. Korean J Physiol Pharmacol. 2009;13(3):161–168. doi: 10.4196/kjpp.2009.13.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee HK, Seo IA, Suh DJ, Hong JI, Yoo YH, Park HT. Interleukin-6 is required for the early induction of glial fibrillary acidic protein in Schwann cells during Wallerian degeneration. J Neurochem. 2009;108(3):776–786. doi: 10.1111/j.1471-4159.2008.05826.x. [DOI] [PubMed] [Google Scholar]
- 75.Patchett AL, Wilson R, Charlesworth JC, Corcoran LM, Papenfuss AT, Lyons BA, Woods GM, Tovar C. Transcriptome and proteome profiling reveals stress-induced expression signatures of imiquimod-treated Tasmanian devil facial tumor disease (DFTD) cells. Oncotarget. 2018;9(22):15895–15914. doi: 10.18632/oncotarget.24634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patchett AL, Darby JM, Tovar C, Lyons AB, Woods GM. The immunomodulatory small molecule imiquimod induces apoptosis in devil facial tumour cell lines. PLoS ONE. 2016;11(12):e0168068. doi: 10.1371/journal.pone.0168068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 78.Aiello NM, Kang Y. Context-dependent EMT programs in cancer metastasis. J Exp Med. 2019;216(5):1016–1026. doi: 10.1084/jem.20181827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shurin GV, Kruglov O, Ding F, Lin Y, Hao X, Keskinov AA, You Z, Lokshin AE, LaFramboise WA, Falo LD, Jr, Shurin MR, Bunimovich YL. Melanoma-induced reprogramming of Schwann cell signaling aids tumor growth. Cancer Res. 2019;79(10):2736–2747. doi: 10.1158/0008-5472.Can-18-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Y, Shurin GV, Zhong H, Bunimovich YL, Han B, Shurin MR. Schwann cells augment cell spreading and metastasis of lung cancer. Cancer Res. 2018;78(20):5927–5939. doi: 10.1158/0008-5472.Can-18-1702. [DOI] [PubMed] [Google Scholar]
- 81.Choi K, Komurov K, Fletcher JS, Jousma E, Cancelas JA, Wu J, Ratner N. An inflammatory gene signature distinguishes neurofibroma Schwann cells and macrophages from cells in the normal peripheral nervous system. Sci Rep. 2017;7:43315. doi: 10.1038/srep43315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caldwell A, Coleby R, Tovar C, Stammnitz MR, Kwon YM, Owen RS, Tringides M, Murchison EP, Skjodt K, Thomas GJ, Kaufman J, Elliott T, Woods GM, Siddle HV. The newly-arisen Devil facial tumour disease 2 (DFT2) reveals a mechanism for the emergence of a contagious cancer. Elife. 2018;7:e35314. doi: 10.7554/eLife.35314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D'Antonio M, Mirsky R, Jessen KR. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol. 2004;164(3):385–394. doi: 10.1083/jcb.200307132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, Richman AR, Silverbush D, Shaw ML, Hebert CM, Dewitt J, Gritsch S, Perez EM, Gonzalez Castro LN, Lan X, Druck N, Rodman C, Dionne D, Kaplan A, Bertalan MS, Small J, Pelton K, Becker S, Bonal D, Nguyen QD, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178(4):835–849.e821. doi: 10.1016/j.cell.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor RL, Zhang Y, Schoning JP, Deakin JE. Identification of candidate genes for devil facial tumour disease tumourigenesis. Sci Rep. 2017;7(1):8761. doi: 10.1038/s41598-017-08908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang L, Zhang W, Li Y, Alvarez A, Li Z, Wang Y, Song L, Lv D, Nakano I, Hu B, Cheng SY, Feng H. SHP-2-upregulated ZEB1 is important for PDGFRalpha-driven glioma epithelial-mesenchymal transition and invasion in mice and humans. Oncogene. 2016;35(43):5641–5652. doi: 10.1038/onc.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang J, Tian XJ, Zhang H, Teng Y, Li R, Bai F, Elankumaran S, Xing J. TGF-beta-induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci Signal. 2014;7(345):ra91. doi: 10.1126/scisignal.2005304. [DOI] [PubMed] [Google Scholar]
- 88.Hong T, Watanabe K, Ta CH, Villarreal-Ponce A, Nie Q, Dai X. An Ovol2-Zeb1 mutual inhibitory circuit governs bidirectional and multi-step transition between epithelial and mesenchymal states. PLoS Comput Biol. 2015;11(11):e1004569. doi: 10.1371/journal.pcbi.1004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D, Moers V, Lemaire S, De Clercq S, Minguijon E, Balsat C, Sokolow Y, Dubois C, De Cock F, Scozzaro S, Sopena F, Lanas A, D'Haene N, Salmon I, Marine JC, Voet T, Sotiropoulou PA, Blanpain C. Identification of the tumour transition states occurring during EMT. Nature. 2018;556(7702):463–468. doi: 10.1038/s41586-018-0040-3. [DOI] [PubMed] [Google Scholar]
- 90.Jolly MK, Tripathi SC, Jia D, Mooney SM, Celiktas M, Hanash SM, Mani SA, Pienta KJ, Ben-Jacob E, Levine H. Stability of the hybrid epithelial/mesenchymal phenotype. Oncotarget. 2016;7(19):27067–27084. doi: 10.18632/oncotarget.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aokage K, Ishii G, Ohtaki Y, Yamaguchi Y, Hishida T, Yoshida J, Nishimura M, Nagai K, Ochiai A. Dynamic molecular changes associated with epithelial-mesenchymal transition and subsequent mesenchymal-epithelial transition in the early phase of metastatic tumor formation. Int J Cancer. 2011;128(7):1585–1595. doi: 10.1002/ijc.25500. [DOI] [PubMed] [Google Scholar]
- 92.Hamilton G, Rath B. Mesenchymal-epithelial transition and circulating tumor cells in small cell lung cancer. Adv Exp Med Biol. 2017;994:229–245. doi: 10.1007/978-3-319-55947-6_12. [DOI] [PubMed] [Google Scholar]
- 93.Boos GS, Bassuino DM, Wurster F, Castro NB, Watanabe TT, Silva GS, Sonne L, Driemeier D. Retrospective canine skin peripheral nerve sheath tumors data with emphasis on histologic, immunohistochemical and prognostic factors. Pesq Vet Bras. 2015;35(12):965–974. doi: 10.1590/S0100-736X2015001200005. [DOI] [Google Scholar]
- 94.Schoniger S, Valentine BA, Fernandez CJ, Summers BA. Cutaneous schwannomas in 22 horses. Vet Pathol. 2011;48(2):433–442. doi: 10.1177/0300985810377072. [DOI] [PubMed] [Google Scholar]
- 95.Stoica G, Tasca SI, Kim HT. Point mutation of neu oncogene in animal peripheral nerve sheath tumors. Vet Pathol. 2001;38(6):679–688. doi: 10.1354/vp.38-6-679. [DOI] [PubMed] [Google Scholar]
- 96.Fletcher CD, Bridge JA, Hogendoorn PC, Mertens F. WHO Classification of tumours of soft tissue and bone. Lyon: IARC Press; 2013. [Google Scholar]
- 97.De Luca-Johnson J, Kalof AN. Peripheral nerve sheath tumors: an update and review of diagnostic challenges. Diagn Histopathol. 2016;22(11):447–457. doi: 10.1016/j.mpdhp.2016.10.008. [DOI] [Google Scholar]
- 98.Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123(3):295–319. doi: 10.1007/s00401-012-0954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stucky CC, Johnson KN, Gray RJ, Pockaj BA, Ocal IT, Rose PS, Wasif N. Malignant peripheral nerve sheath tumors (MPNST): the mayo clinic experience. Ann Surg Oncol. 2012;19(3):878–885. doi: 10.1245/s10434-011-1978-7. [DOI] [PubMed] [Google Scholar]
- 100.Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26(1):93–97. doi: 10.1097/00129492-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 101.Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C, Plougastel B, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363(6429):515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 102.Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19(1):27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8(1):27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 104.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19(6):831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 105.Han Q, Lin X, Zhang X, Jiang G, Zhang Y, Miao Y, Rong X, Zheng X, Han Y, Han X, Wu J, Kremerskothen J, Wang E. WWC3 regulates the Wnt and Hippo pathways via Dishevelled proteins and large tumour suppressor 1, to suppress lung cancer invasion and metastasis. J Pathol. 2017;242(4):435–447. doi: 10.1002/path.4919. [DOI] [PubMed] [Google Scholar]
- 106.Krapivinsky G, Medina I, Krapivinsky L, Gapon S, Clapham DE. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron. 2004;43(4):563–574. doi: 10.1016/j.neuron.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 107.Piotrowski A, Xie J, Liu YF, Poplawski AB, Gomes AR, Madanecki P, Fu C, Crowley MR, Crossman DK, Armstrong L, Babovic-Vuksanovic D, Bergner A, Blakeley JO, Blumenthal AL, Daniels MS, Feit H, Gardner K, Hurst S, Kobelka C, Lee C, Nagy R, Rauen KA, Slopis JM, Suwannarat P, Westman JA, et al. Germline loss-of-function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas. Nat Genet. 2014;46(2):182–187. doi: 10.1038/ng.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hadfield KD, Newman WG, Bowers NL, Wallace A, Bolger C, Colley A, McCann E, Trump D, Prescott T, Evans DG. Molecular characterisation of SMARCB1 and NF2 in familial and sporadic schwannomatosis. J Med Genet. 2008;45(6):332–339. doi: 10.1136/jmg.2007.056499. [DOI] [PubMed] [Google Scholar]
- 109.Bigenzahn JW, Collu GM, Kartnig F, Pieraks M, Vladimer GI, Heinz LX, Sedlyarov V, Schischlik F, Fauster A, Rebsamen M, Parapatics K, Blomen VA, Muller AC, Winter GE, Kralovics R, Brummelkamp TR, Mlodzik M, Superti-Furga G. LZTR1 is a regulator of RAS ubiquitination and signaling. Science. 2018;362(6419):1171–1177. doi: 10.1126/science.aap8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM, Fountain JW, Brereton A, Nicholson J, Mitchell AL, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249(4965):181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- 111.Uusitalo E, Rantanen M, Kallionpaa RA, Poyhonen M, Leppavirta J, Yla-Outinen H, Riccardi VM, Pukkala E, Pitkaniemi J, Peltonen S, Peltonen J. Distinctive cancer associations in patients with neurofibromatosis type 1. J Clin Oncol. 2016;34(17):1978–1986. doi: 10.1200/jco.2015.65.3576. [DOI] [PubMed] [Google Scholar]
- 112.Torres KE, Liu J, Young E, Huang KL, Ghadimi M, Lusby K, Lazar AJ, Lev D. Expression of 'drugable' tyrosine kinase receptors in malignant peripheral nerve sheath tumour: potential molecular therapeutic targets for a chemoresistant cancer. Histopathology. 2011;59(1):156–159. doi: 10.1111/j.1365-2559.2011.03867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ki DH, He S, Rodig S, Look AT. Overexpression of PDGFRA cooperates with loss of NF1 and p53 to accelerate the molecular pathogenesis of malignant peripheral nerve sheath tumors. Oncogene. 2017;36(8):1058–1068. doi: 10.1038/onc.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tabone-Eglinger S, Bahleda R, Cote JF, Terrier P, Vidaud D, Cayre A, Beauchet A, Theou-Anton N, Terrier-Lacombe MJ, Lemoine A, Penault-Llorca F, Le Cesne A, Emile JF. Frequent EGFR positivity and overexpression in high-grade areas of human MPNSTs. Sarcoma. 2008;2008:849156. doi: 10.1155/2008/849156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nielsen GP, Stemmer-Rachamimov AO, Ino Y, Moller MB, Rosenberg AE, Louis DN. Malignant transformation of neurofibromas in neurofibromatosis 1 is associated with CDKN2A/p16 inactivation. Am J Pathol. 1999;155(6):1879–1884. doi: 10.1016/s0002-9440(10)65507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brohl AS, Kahen E, Yoder SJ, Teer JK, Reed DR. The genomic landscape of malignant peripheral nerve sheath tumors: diverse drivers of Ras pathway activation. Sci Rep. 2017;7(1):14992. doi: 10.1038/s41598-017-15183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Menon AG, Anderson KM, Riccardi VM, Chung RY, Whaley JM, Yandell DW, Farmer GE, Freiman RN, Lee JK, Li FP. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci USA. 1990;87(14):5435–5439. doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eckert JM, Byer SJ, Clodfelder-Miller BJ, Carroll SL. Neuregulin-1 beta and neuregulin-1 alpha differentially affect the migration and invasion of malignant peripheral nerve sheath tumor cells. Glia. 2009;57(14):1501–1520. doi: 10.1002/glia.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perrone F, Da Riva L, Orsenigo M, Losa M, Jocolle G, Millefanti C, Pastore E, Gronchi A, Pierotti MA, Pilotti S. PDGFRA, PDGFRB, EGFR, and downstream signaling activation in malignant peripheral nerve sheath tumor. Neuro Oncol. 2009;11(6):725–736. doi: 10.1215/15228517-2009-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stonecypher MS, Byer SJ, Grizzle WE, Carroll SL. Activation of the neuregulin-1/ErbB signaling pathway promotes the proliferation of neoplastic Schwann cells in human malignant peripheral nerve sheath tumors. Oncogene. 2005;24(36):5589–5605. doi: 10.1038/sj.onc.1208730. [DOI] [PubMed] [Google Scholar]
- 121.Aoki M, Nabeshima K, Koga K, Hamasaki M, Suzumiya J, Tamura K, Iwasaki H. Imatinib mesylate inhibits cell invasion of malignant peripheral nerve sheath tumor induced by platelet-derived growth factor-BB. Lab Invest. 2007;87(8):767–779. doi: 10.1038/labinvest.3700591. [DOI] [PubMed] [Google Scholar]
- 122.Smoot RL, Werneburg NW, Sugihara T, Hernandez MC, Yang L, Mehner C, Graham RP, Bronk SF, Truty MJ, Gores GJ. Platelet-derived growth factor regulates YAP transcriptional activity via Src family kinase dependent tyrosine phosphorylation. J Cell Biochem. 2018;119(1):824–836. doi: 10.1002/jcb.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.He C, Lv X, Hua G, Lele SM, Remmenga S, Dong J, Davis JS, Wang C. YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression. Oncogene. 2015;34(50):6040–6054. doi: 10.1038/onc.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Satoh T, Fantl WJ, Escobedo JA, Williams LT, Kaziro Y. Platelet-derived growth factor receptor mediates activation of ras through different signaling pathways in different cell types. Mol Cell Biol. 1993;13(6):3706–3713. doi: 10.1128/mcb.13.6.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goodearl A, Viehover A, Vartanian T. Neuregulin-induced association of Sos Ras exchange protein with HER2(erbB2)/HER3(erbB3) receptor complexes in Schwann cells through a specific Grb2-HER2(erbB2) interaction. Dev Neurosci. 2001;23(1):25–30. doi: 10.1159/000048693. [DOI] [PubMed] [Google Scholar]
- 126.Wu LMN, Deng Y, Wang J, Zhao C, Wang J, Rao R, Xu L, Zhou W, Choi K, Rizvi TA, Remke M, Rubin JB, Johnson RL, Carroll TJ, Stemmer-Rachamimov AO, Wu J, Zheng Y, Xin M, Ratner N, Lu QR. Programming of Schwann cells by Lats1/2-TAZ/YAP signaling drives malignant peripheral nerve sheath tumorigenesis. Cancer Cell. 2018;33(2):292–308.e297. doi: 10.1016/j.ccell.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boin A, Couvelard A, Couderc C, Brito I, Filipescu D, Kalamarides M, Bedossa P, De Koning L, Danelsky C, Dubois T, Hupe P, Louvard D, Lallemand D. Proteomic screening identifies a YAP-driven signaling network linked to tumor cell proliferation in human schwannomas. Neuro Oncol. 2014;16(9):1196–1209. doi: 10.1093/neuonc/nou020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, Mantovani F, Damalas A, Citro G, Sacchi A, Del Sal G, Levrero M, Blandino G. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol Cell. 2005;18(4):447–459. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 129.Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, Oren M, Sudol M, Cesareni G, Blandino G. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276(18):15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- 130.Zhang M, Wang Y, Jones S, Sausen M, McMahon K, Sharma R, Wang Q, Belzberg AJ, Chaichana K, Gallia GL, Gokaslan ZL, Riggins GJ, Wolinksy JP, Wood LD, Montgomery EA, Hruban RH, Kinzler KW, Papadopoulos N, Vogelstein B, Bettegowda C. Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat Genet. 2014;46(11):1170–1172. doi: 10.1038/ng.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee W, Teckie S, Wiesner T, Ran L, Prieto Granada CN, Lin M, Zhu S, Cao Z, Liang Y, Sboner A, Tap WD, Fletcher JA, Huberman KH, Qin LX, Viale A, Singer S, Zheng D, Berger MF, Chen Y, Antonescu CR, Chi P. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat Genet. 2014;46(11):1227–1232. doi: 10.1038/ng.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Riising EM, Comet I, Leblanc B, Wu X, Johansen JV, Helin K. Gene silencing triggers polycomb repressive complex 2 recruitment to CpG islands genome wide. Mol Cell. 2014;55(3):347–360. doi: 10.1016/j.molcel.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 133.Wojcik JB, Marchione DM, Sidoli S, Djedid A, Lisby A, Majewski J, Garcia BA. Epigenomic reordering induced by polycomb loss drives oncogenesis but leads to therapeutic vulnerabilities in malignant peripheral nerve sheath tumors. Cancer Res. 2019;79(13):3205–3219. doi: 10.1158/0008-5472.Can-18-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peck SJ, Michael SA, Knowles G, Davis A, Pemberton D. Causes of mortality and severe morbidity requiring euthanasia in captive Tasmanian devils (Sarcophilus harrisii) in Tasmania. Aust Vet J. 2019;97(4):89–92. doi: 10.1111/avj.12797. [DOI] [PubMed] [Google Scholar]
- 135.Abegglen LM, Caulin AF, Chan A, Lee K, Robinson R, Campbell MS, Kiso WK, Schmitt DL, Waddell PJ, Bhaskara S, Jensen ST, Maley CC, Schiffman JD. Potential mechanisms for cancer resistance in elephants and comparative cellular response to DNA damage in humans. JAMA. 2015;314(17):1850–1860. doi: 10.1001/jama.2015.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Griner LA. Neoplasms in Tasmanian devils (Sarcophilus harrisii) J Natl Cancer Inst. 1979;62(3):589–595. doi: 10.1093/jnci/62.3.589. [DOI] [PubMed] [Google Scholar]
- 137.Bender HS, Murchison EP, Pickett HA, Deakin JE, Strong MA, Conlan C, McMillan DA, Neumann AA, Greider CW, Hannon GJ, Reddel RR, Graves JA. Extreme telomere length dimorphism in the Tasmanian devil and related marsupials suggests parental control of telomere length. PLoS ONE. 2012;7(9):e46195. doi: 10.1371/journal.pone.0046195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stone WH, Brunn DA, Foster EB, Manis GS, Hoffman ES, Saphire DG, VandeBerg JL, Infante AJ. Absence of a significant mixed lymphocyte reaction in a marsupial (Monodelphis domestica) Lab Anim Sci. 1998;48(2):184–189. [PubMed] [Google Scholar]
- 139.Wilkinson R, Kotlarski I, Barton M. Koala lymphoid cells: analysis of antigen-specific responses. Vet Immunol Immunopathol. 1992;33(3):237–247. doi: 10.1016/0165-2427(92)90184-R. [DOI] [PubMed] [Google Scholar]
- 140.Howson LJ, Morris KM, Kobayashi T, Tovar C, Kreiss A, Papenfuss AT, Corcoran L, Belov K, Woods GM. Identification of dendritic cells, B cell and T cell subsets in Tasmanian devil lymphoid tissue; evidence for poor immune cell infiltration into devil facial tumors. Anat Rec (Hoboken) 2014;297(5):925–938. doi: 10.1002/ar.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woods GM, Kreiss A, Belov K, Siddle HV, Obendorf DL, Muller HK. The immune response of the Tasmanian devil (Sarcophilus harrisii) and devil facial tumour disease. EcoHealth. 2007;4(3):338–345. doi: 10.1007/s10393-007-0117-1. [DOI] [Google Scholar]
- 142.Kreiss A, Fox N, Bergfeld J, Quinn SJ, Pyecroft S, Woods GM. Assessment of cellular immune responses of healthy and diseased Tasmanian devils (Sarcophilus harrisii) Dev Comp Immunol. 2008;32(5):544–553. doi: 10.1016/j.dci.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 143.Kreiss A, Wells B, Woods GM. The humoral immune response of the Tasmanian devil (Sarcophilus harrisii) against horse red blood cells. Vet Immunol Immunopathol. 2009;130(1–2):135–137. doi: 10.1016/j.vetimm.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 144.Patchett AL, Latham R, Brettingham-Moore KH, Tovar C, Lyons AB, Woods GM. Toll-like receptor signaling is functional in immune cells of the endangered Tasmanian devil. Dev Comp Immunol. 2015;53(1):123–133. doi: 10.1016/j.dci.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 145.Patchett AL, Tovar C, Corcoran LM, Lyons AB, Woods GM. The toll-like receptor ligands Hiltonol((R)) (polyICLC) and imiquimod effectively activate antigen-specific immune responses in Tasmanian devils (Sarcophilus harrisii) Dev Comp Immunol. 2017;76:352–360. doi: 10.1016/j.dci.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 146.Brown GK, Kreiss A, Lyons AB, Woods GM. Natural killer cell mediated cytotoxic responses in the Tasmanian devil. PLoS ONE. 2011;6(9):e24475. doi: 10.1371/journal.pone.0024475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brown GK, Tovar C, Cooray AA, Kreiss A, Darby J, Murphy JM, Corcoran LM, Bettiol SS, Lyons AB, Woods GM. Mitogen-activated Tasmanian devil blood mononuclear cells kill devil facial tumour disease cells. Immunol Cell Biol. 2016;94(7):673–679. doi: 10.1038/icb.2016.38. [DOI] [PubMed] [Google Scholar]
- 148.Cheng Y, Makara M, Peel E, Fox S, Papenfuss AT, Belov K. Tasmanian devils with contagious cancer exhibit a constricted T-cell repertoire diversity. Commun Biol. 2019;2:99. doi: 10.1038/s42003-019-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cheng Y, Heasman K, Peck S, Peel E, Gooley RM, Papenfuss AT, Hogg CJ, Belov K. Significant decline in anticancer immune capacity during puberty in the Tasmanian devil. Sci Rep. 2017;7:44716. doi: 10.1038/srep44716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pye R, Hamede R, Siddle HV, Caldwell A, Knowles GW, Swift K, Kreiss A, Jones ME, Lyons AB, Woods GM. Demonstration of immune responses against devil facial tumour disease in wild Tasmanian devils. Biol Lett. 2016 doi: 10.1098/rsbl.2016.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Margres MJ, Ruiz-Aravena M, Hamede R, Jones ME, Lawrance MF, Hendricks SA, Patton A, Davis BW, Ostrander EA, McCallum H, Hohenlohe PA, Storfer A. The genomic basis of tumor regression in Tasmanian devils (Sarcophilus harrisii) Genome Biol Evol. 2018;10(11):3012–3025. doi: 10.1093/gbe/evy229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tovar C, Pye RJ, Kreiss A, Cheng Y, Brown GK, Darby J, Malley RC, Siddle HV, Skjodt K, Kaufman J, Silva A, Baz Morelli A, Papenfuss AT, Corcoran LM, Murphy JM, Pearse MJ, Belov K, Lyons AB, Woods GM. Regression of devil facial tumour disease following immunotherapy in immunised Tasmanian devils. Sci Rep. 2017;7:43827. doi: 10.1038/srep43827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Morris K, Belov K. Does the devil facial tumour produce immunosuppressive cytokines as an immune evasion strategy? Vet Immunol Immunopathol. 2013;153(1–2):159–164. doi: 10.1016/j.vetimm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 154.Flies AS, Lyons AB, Corcoran LM, Papenfuss AT, Murphy JM, Knowles GW, Woods GM, Hayball JD. PD-L1 is not constitutively expressed on Tasmanian devil facial tumor cells but is strongly upregulated in response to IFN-gamma and can be expressed in the tumor microenvironment. Front Immunol. 2016;7:581. doi: 10.3389/fimmu.2016.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Flies AS, Blackburn NB, Lyons AB, Hayball JD, Woods GM. Comparative analysis of immune checkpoint molecules and their potential role in the transmissible Tasmanian devil facial tumor disease. Front Immunol. 2017;8:513. doi: 10.3389/fimmu.2017.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Morris K, Austin JJ, Belov K. Low major histocompatibility complex diversity in the Tasmanian devil predates European settlement and may explain susceptibility to disease epidemics. Biol Lett. 2013;9(1):20120900. doi: 10.1098/rsbl.2012.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bruniche-Olsen A, Jones ME, Austin JJ, Burridge CP, Holland BR. Extensive population decline in the Tasmanian devil predates European settlement and devil facial tumour disease. Biol Lett. 2014;10(11):20140619. doi: 10.1098/rsbl.2014.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miller W, Hayes VM, Ratan A, Petersen DC, Wittekindt NE, Miller J, Walenz B, Knight J, Qi J, Zhao F, Wang Q, Bedoya-Reina OC, Katiyar N, Tomsho LP, Kasson LM, Hardie RA, Woodbridge P, Tindall EA, Bertelsen MF, Dixon D, Pyecroft S, Helgen KM, Lesk AM, Pringle TH, Patterson N, et al. Genetic diversity and population structure of the endangered marsupial Sarcophilus harrisii (Tasmanian devil) Proc Natl Acad Sci USA. 2011;108(30):12348–12353. doi: 10.1073/pnas.1102838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jones ME, Paetkau D, Geffen E, Moritz C. Genetic diversity and population structure of Tasmanian devils, the largest marsupial carnivore. Mol Ecol. 2004;13(8):2197–2209. doi: 10.1111/j.1365-294X.2004.02239.x. [DOI] [PubMed] [Google Scholar]
- 160.Siddle HV, Marzec J, Cheng Y, Jones M, Belov K. MHC gene copy number variation in Tasmanian devils: implications for the spread of a contagious cancer. Proc Biol Sci. 2010;277(1690):2001–2006. doi: 10.1098/rspb.2009.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.O'Brien SJ, Roelke ME, Marker L, Newman A, Winkler CA, Meltzer D, Colly L, Evermann JF, Bush M, Wildt DE. Genetic basis for species vulnerability in the cheetah. Science. 1985;227(4693):1428–1434. doi: 10.1126/science.2983425. [DOI] [PubMed] [Google Scholar]
- 162.Kreiss A, Cheng Y, Kimble F, Wells B, Donovan S, Belov K, Woods GM. Allorecognition in the Tasmanian devil (Sarcophilus harrisii), an endangered marsupial species with limited genetic diversity. PLoS ONE. 2011;6(7):e22402. doi: 10.1371/journal.pone.0022402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cohen D, Shalev A, Krup M. Lack of beta 2-microglobulin on the surface of canine transmissible venereal tumor cells. J Natl Cancer Inst. 1984;72(2):395–401. [PubMed] [Google Scholar]
- 164.Hsiao YW, Liao KW, Hung SW, Chu RM. Effect of tumor infiltrating lymphocytes on the expression of MHC molecules in canine transmissible venereal tumor cells. Vet Immunol Immunopathol. 2002;87(1–2):19–27. doi: 10.1016/S0165-2427(02)00026-0. [DOI] [PubMed] [Google Scholar]
- 165.Yang TJ, Chandler JP, Dunne-Anway S. Growth stage dependent expression of MHC antigens on the canine transmissible venereal sarcoma. Br J Cancer. 1987;55(2):131–134. doi: 10.1038/bjc.1987.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 167.Foster DS, Jones RE, Ransom RC, Longaker MT, Norton JA. The evolving relationship of wound healing and tumor stroma. JCI Insight. 2018 doi: 10.1172/jci.insight.99911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. doi: 10.1056/nejm198612253152606. [DOI] [PubMed] [Google Scholar]
- 169.Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35(4):467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer. 2013;13(11):788–799. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 172.Ujvari B, Gatenby RA, Thomas F. The evolutionary ecology of transmissible cancers. Infect Genet Evol. 2016;39:293–303. doi: 10.1016/j.meegid.2016.02.005. [DOI] [PubMed] [Google Scholar]