Abstract
Nerve-induced muscle contraction regulates the BDNF/TrkB neurotrophic signalling to retrogradely modulate neurotransmission and protect the neuromuscular junctions and motoneurons. In muscles with amyotrophic lateral sclerosis, this pathway is strongly misbalanced and neuromuscular junctions are destabilized, which may directly cause the motoneuron degeneration and muscular atrophy observed in this disease. Here, we sought to demonstrate (1) that physical exercise, whose recommendation has been controversial in amyotrophic lateral sclerosis, would be a good option for its therapy, because it normalizes and improves the altered neurotrophin pathway and (2) a plausible molecular mechanism underlying its positive effect. SOD1-G93A mice were trained following either running or swimming-based protocols since the beginning of the symptomatic phase (day 70 of age) until day 115. Next, the full BDNF pathway, including receptors, downstream kinases and proteins related with neurotransmission, was characterized and motoneuron survival was analysed. The results establish that amyotrophic lateral sclerosis-induced damaging molecular changes in the BDNF/TrkB pathway are reduced, prevented or even overcompensated by precisely defined exercise protocols that modulate TrkB isoforms and neurotransmission regulatory proteins and reduce motoneuron death. Altogether, the maintenance of the BDNF/TrkB signalling and the downstream pathway, particularly after the swimming protocol, adds new molecular evidence of the benefits of physical exercise to reduce the impact of amyotrophic lateral sclerosis. These results are encouraging since they reveal an improvement even starting the therapy after the onset of the disease.
Keywords: ALS, Skeletal muscle, Exercise, NMJ, Neurotransmission, Motoneuron loss
Introduction
Amyotrophic lateral sclerosis (ALS) is the most frequent chronic motoneuron disease characterized by progressive motor weakness, atrophy and selective motoneuron loss. In spite of the frequency of the disease, its pathogenesis remains unknown, though neuromuscular junction (NMJ) degeneration and synaptic molecular alterations precede and may be responsible for the motoneuron loss [1–4]. Abnormal muscle cell metabolism and trophism, synaptic molecular changes and axonal transport disruption have been proposed to explain NMJ alteration in ALS [4–9]. These and other evidences [10] indicate that skeletal muscles promote a retrograde signalling pathway necessary to keep the synapses and, consequently, motoneurons healthy.
The brain-derived neurotrophic factor/tropomyosin-related kinase B (BDNF/TrkB) signalling, which is modulated by muscle activity [11–14], is one of the most implicated in the maintenance of neurons and synapses in the brain, but also in the NMJ of the peripheral nervous system [12, 15–18]. There are different isoforms of this receptor which are generated by alternative splicing. It results in full-length receptors (TrkB.FL) with strong survival effects for nervous cells and truncated receptors (TrkB.T1), without intracellular tyrosine kinase domain. At the NMJ, the ratio between TrkB.FL and TrkB.T1 modulates the BDNF triggering of a signalling pathway that influences presynaptic protein kinase C (PKC) activity and, therefore, the acetylcholine release by affecting the phosphorylation state of different proteins directly related with neurotransmission such as Munc18-1 and SNAP-25 [12, 19–21]. Recently, we have demonstrated that this signalling is perturbed in SOD1-G93A mice muscles even before disease onset, especially regarding neurotrophin levels and neurotrophin receptors misbalance [4]. Interestingly, the suppression of the expression of both p75 neurotrophin receptor (p75NTR) [22], which is a low affinity transmembrane receptor, and TrkB.T1 [23, 24] BDNF receptors is advantageous in ALS in animal models.
There are many evidences that BDNF is involved in exercise-mediated neuroprotective actions in the brain [25–28]. Therefore, it also may play a role in the benefits derived from moderate exercise in ALS, when it can trigger its signalling properly. However, despite increasing evidences that sustained moderate exercise is neuroprotective in ALS [29–34], its recommendation is still controversial, probably due to differential effects depending on exercise types, protocols and intensities [32, 35–37] that induce a differential activation of motoneuron subpopulations [38, 39], being the small motoneuron innervating slow muscle fibres more resistant to ALS [40–42].
Here, we aimed to investigate the molecular effect of two well-defined training protocols. On the one hand, we use a running-based training which is a low-amplitude and frequency exercise that preferentially triggers slow motor units integrated by small motoneurons, and on the other hand, a swimming-based training which is a high-amplitude and frequency exercise that, in addition, recruits fast motor units integrated by large motoneurons [39, 43]. We hypothesize that accurately defined physical exercise protocols can prevent the profound alterations observed in presymptomatic and symptomatic ALS muscles in the BDNF/TrkB downstream pathway, directly involved in the acetylcholine release modulation to avoid the development of the disease [4].
Results show that many changes from the ALS molecular phenotype are reduced or prevented by the swimming- and running-based training protocols. In addition to unveil the differences among the protocols, this study adds new evidence that exercise is beneficial until the late stages of ALS disease and reveals the molecular changes that are not prevented by training, pointing to a central role of the BDNF/TrkB downstream signalling in the pathogenesis of the disease.
Materials and methods
Animals
Transgenic male B6SJL-Tg(SOD1*G93A)1Gur/J (Stock No. 002726) mice from The Jackson Laboratory (Bar Harbor, ME, USA) were crossed with wild-type (WT) B6/SJL females (Janvier, le Genest, France) and only male descendants were used for comparison (1) to reduce variability in the results because these mice present gender differences in the onset of the disease, life expectancy and response to exercise [37, 44] and (2) because incidence and prevalence of ALS are greater in men than in women [45]. Mice were kept in the animal facility under standard conditions: constant temperature (22 ± 2 °C), relative humidity (50 ± 10%), a 12-h light/dark schedule and unrestricted access to food and water, in accordance with the guidelines of the European Community’s Council Directive of 24 November 1986 (86/609/EEC) for the humane treatment of laboratory animals. All experimental procedures which included minimizing the number of animals and their suffering were reviewed and approved by the Animal Research Committee of the Universitat Rovira i Virgili and by the Institutional Animal Care and Use Committee protocols of the University of Paris Descartes and followed the national authority (Ministère de la Recherche et de la Technologie, France) guidelines for the detention, use and the ethical treatment of laboratory animals based on European Union Directive 2010/63/EU.
ALS onset was defined as the time corresponding to the first observation of myotonia symptoms in the mice hind limb and the disease progression was assessed by a trained observer who evaluated myotonia symptoms and weighing. All the experiments using mice were performed in a blind systematic fashion to minimize bias.
Training protocol
ALS mice were trained from 70 until 115 days of age, 30 min a day, 5 days a week as previously described [43]. Five ALS mice were submitted to a moderate running-based training on a speed-regulated treadmill (max.13 m min−1) (Run ALS) which is a low-amplitude and frequency exercise that preferentially triggers slow motor units integrated by small motoneurons; and five ALS mice were submitted to a high-frequency and amplitude swimming-based training in an adjustable-flow swimming pool (max.5L min−1) (Swim ALS) which is a high-amplitude and frequency exercise that also recruits fast motor units integrated by large motoneurons [43]. These groups were compared to five Untrained ALS mice and five Untrained WT mice that only displayed an exploratory activity during the training time of the first and second groups. Sample size was calculated using previously established criteria [46, 47] to optimize the number of animals used.
Western blotting
The animals were euthanized 4 h after the training was complete (P115). Then, the plantaris muscles were dissected and immediately frozen in liquid nitrogen. The plantaris muscles were used because they are fast-twitching extensors of the ankle that are preferentially affected in ALS due to the vulnerability of the fast IIb fibres in ALS. Therefore, the plantaris muscles are a good model to study skeletal muscle degeneration in this disease [48]. Muscles from both hind limbs of the same animal were processed and analysed together.
Western blotting procedure was performed as previously described [12, 19, 49]. Primary and secondary antibodies conjugated to horseradish peroxidase (HRP) are specified in Table 1. Primary antibodies were omitted from some samples during the procedure as controls, and they did not reveal bands of the appropriate molecular weight. All antibodies specificity had been previously determined [12, 20, 21, 49, 50].
Table 1.
List of primary and secondary antibodies used
| Target | Source | Reference | Dilution | Target | Source | Reference | Dilution |
|---|---|---|---|---|---|---|---|
| BDNF | Rb pAb | Sc-20981 | 1/500 | PKA Cβ | Rb pAb | Sc-904 | 1/1000 |
| NT4 | Rb pAb | Sc-545 | 1/500 | PKA RIα | Ms mAb | Sc-136231 | 1/1000 |
| p75NTR | Rb pAb | 07-476 | 1/800 | PKA RIβ | Rb pAb | Sc-907 | 1/1000 |
| TrkB | Ms mAb | Sc-377218 | 1/1000 | PKA RIIα | Rb pAb | Sc-909 | 1/1000 |
| pTrkB (Tyr816) | Rb pAb | ABN1381 | 1/1000 | PKA RIIβ | Ms mAb | Sc-376778 | 1/1000 |
| PDK1 | Ms mAb | Sc-17765 | 1/1000 | Munc18-1 | Rb mAb | 13414 | 1/1000 |
| pDPK1 (Ser241) | Rb pAb | #3061 | 1/1000 | pMunc18-1 (Ser313) | Rb pAb | Ab138687 | 1/1000 |
| cPKCα | Rb pAb | Sc-208 | 1/800 | SNAP-25 | Rb mAb | #5309 | 1/1000 |
| pcPKCα (Ser657) | Rb pAb | 06-822 | 1/1000 | pSNAP-25 (Ser187) | Rb pAb | Ab169871 | 1/1000 |
| cPKCβI | Rb pAb | Sc-209 | 1/1000 | pSNAP-25 (Thr138) | Rb pAb | Orb163730 | 1/1000 |
| pcPKCβI (Thr642) | Rb pAb | Ab75657 | 1/1000 | ChAT | Gt pAb | AB144 | 1/800 |
| nPKCε | Rb pAb | Sc-214 | 1/1000 | HRP-conjugated | Dk a-Rb pAb | 711-035-152 | 1/10.000 |
| pnPKCε (Ser729) | Rb pAb | Sc-12355 | 1/1000 | HRP-conjugated | Rb a-Ms pAb | A9044 | 1/10.000 |
| PKA Cα | Rb pAb | Sc-903 | 1/1000 | Alexa fluor 568 | Dk a-Gt pAb | A-11057 | 1/500 |
Immunohistochemistry
The spinal cord of the P115 mice was dissected after the animals were anaesthetized by intraperitoneal injection of 3.5% chloral hydrate and perfused transcardially with buffered saline and 4% paraformaldehyde. Then, the spinal cords were post‐fixed in 4% PFA and rinsed two times in PBS azide 0.01% buffer. The L1–L5 lumbar region of the spinal cord was sectioned with a vibrating blade microtome (VT‐1000S, Leica Microsystems SAS, Nanterre, France) at 50 μm thickness. One out of every six sections was subsequently processed for immunostaining on free-floating sections (an average of 7 sections per animal were studied). The immunohistochemical analysis was based on detection of choline acetyltransferase (ChAT) to stain motoneurons (Table 1). Moreover, DAPI was also used to stain cell nuclei. Sections were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) and collected with a CMOS camera (ORCA Flash 2.8, Hamamatsu Photonics France, Massy, France) mounted on a Zeiss AxioObserver microscope (Z1, Carl Zeiss SAS, Le Pecq, France) using the ZEN 2012 software (Carl Zeiss SAS). The staining specificity was checked by performing the incubation in the absence of the primary antibodies. All counts were performed using the ZEN 2012 software (Carl Zeiss SAS) and involved the measurement of at least 100 motoneurons per animal and ≥ 3 animals per group.
Statistical analysis
All values are expressed as mean ± standard deviation (SD) within each group. Statistical significance of the differences between the experimental groups was evaluated under a non-parametric Kruskal–Wallis test followed by Dunn’s post hoc test (GraphPad Prism software, San Diego, USA). The criterion for statistical significance was: *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
Previous results show that the BDNF-NT4/TrkB signalling at the NMJ is strongly affected (at its three levels of study: diffusible molecules and receptors, targeted kinases and phosphorylated exocytotic proteins) in SOD1-G93A mice plantaris muscles since the presymptomatic stage of the disease [4]. Nerve-induced muscle contraction regulates this neurotrophic signalling to retrogradely modulate neurotransmission [12, 51] and protect NMJ and motoneurons [16]. Therefore, here we aimed to investigate whether running and swimming-based training protocols could prevent the molecular misbalances to provide evidence that physical exercise is effective to slow down ALS progression in SOD1-G93A, in accordance with the improved survival and reduced phenotype severity found by Deforges et al., 2009 [38].
Neurotrophins and TrkB signalling
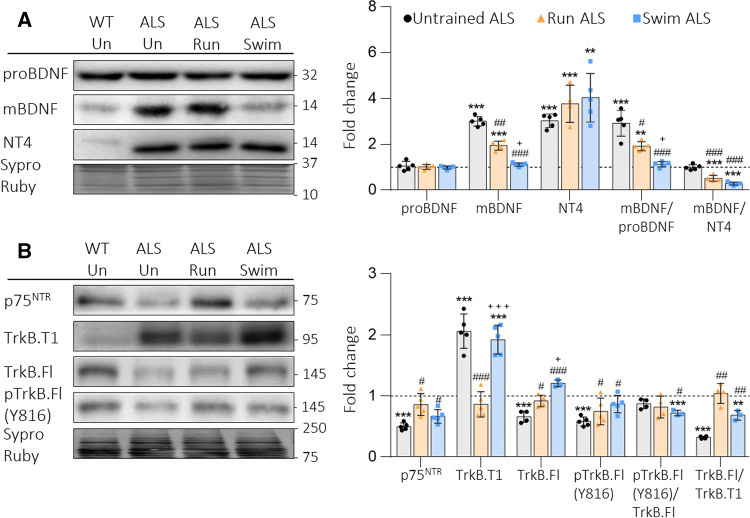
In concordance with previous results, in Fig. 1a, we show that mBDNF and neurotrophin-4 (NT4) are significantly increased in ALS muscles. The running protocol reduces, while swimming fully prevents, this strong increase of mBDNF. However, NT4 is even more upregulated in trained ALS animals than in Untrained ALS mice. Additionally, proBDNF remains unchanged after both training. Therefore, the mBDNF/proBDNF ratio decreases in Run and is fully normalized in Swim ALS animals, whereas the mBDNF/NT4 ratio is strongly reduced in both training protocols (Fig. 1a).
Fig. 1.
BDNF, NT4 and receptors in plantaris muscles of trained ALS mice. a Run reduces and swim normalizes upregulated mBDNF levels, while any of them normalizes NT4. proBDNF is not modified. b Run completely avoids all the alterations present in Untrained ALS animals. Swim avoids protein alterations except TrkB.T1. Data are mean value ± SD, *p < 0.05, **p < 0.01, ***p < 0.001. *Versus Untrained WT mice; #versus Untrained ALS; +versus Run ALS mice
We next analysed the receptors p75NTR and TrkB (TrkB.T1 and TrkB.FL). Figure 1b shows that both protocols significantly increase p75NTR in relation with Untrained ALS mice to achieve Untrained WT values. Moreover, running fully prevents TrkB.T1 increase and TrkB.FL and pTrkB.Fl decrease. On the other hand, swimming normalizes TrkB.Fl and pTrkB.FL, but does not reduce TrkB.T1 (Fig. 1b). Consequently, running maintains the pTrkB.FL/TrkB.FL ratio and recovers the TrkB.FL/TrkB.T1 ratio, while swimming reduces the pTrkB.FL/TrkB.FL ratio and partially recovers the TrkB.FL/TrkB.T1 ratio, in relation to the Untrained WT values.
Altogether, the first conclusion is that the two training protocols have differential influence over molecular changes of ALS to maintain the protein levels inside the control values to limit disease progression. Interestingly, the alterations that the trainings could not prevent are the ones that were already found at the presymptomatic stage [4].
Serine-threonine kinases
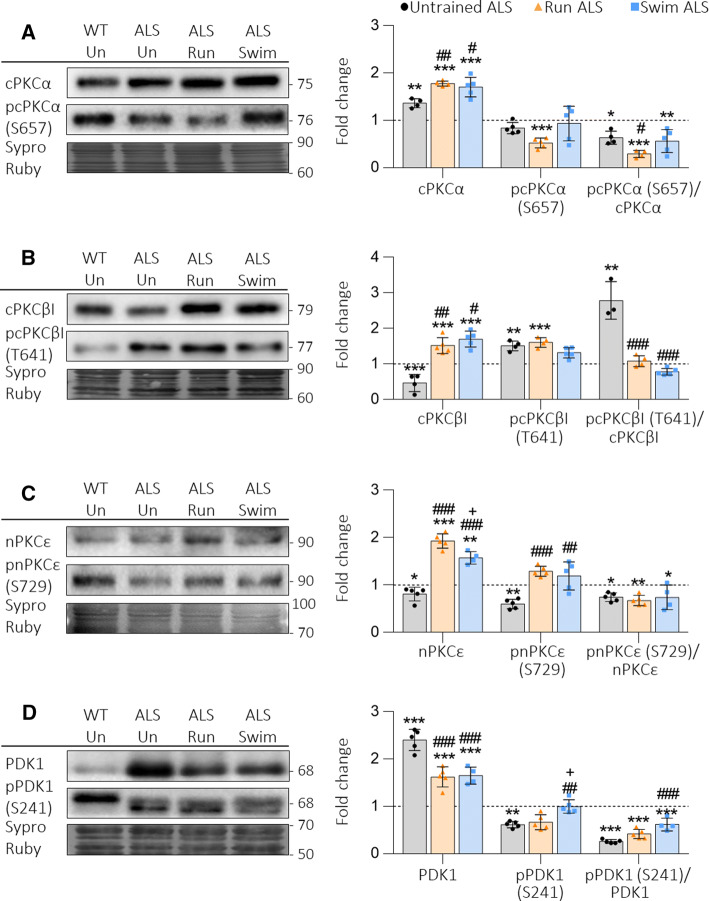
We also investigated how exercise affects the ubiquitous cPKCα, the exclusive presynaptic cPKCβI and nPKCε, the priming kinase phosphoinositide-dependent kinase-1 (PDK1) (Fig. 2) and the different catalytic and regulatory subunits of the protein kinase A (PKA) (Fig. 3). Both protocols increase above the Untrained WT value the total levels of cPKCα, cPKCβI and nPKCε, independently of their affectation in Untrained ALS. Phosphorylated pcPKCα, which is unaffected in Untrained ALS, is decreased after running and so it is the pcPKCα/cPKCα ratio, indicating that cPKCα is downregulated. On the other hand, in Swim ALS, the pcPKCα/cPKCα ratio also decreases because of total protein increase (Fig. 2a). Regarding cPKCβI, swimming reduces pcPKCβI increased levels and normalizes the extremely high pcPKCβI/cPKCβI ratio. In Run ALS it is also fully normalized because of total protein increase (Fig. 2b). Finally, nPKCε and pnPKCε increase proportionally due to training. Because of that, the pnPKCε/nPKCε ratio does not change in relation with Untrained ALS but, as there is more nPKCε available, its activity can increase (Fig. 2c). These changes in the balance of PKC isoforms may be related with the imbalance of the TrkB isoforms directly or through the effect of other presynaptic metabotropic receptors that modulate TrkB such as muscarinic and purinergic receptors [18, 52–54].
Fig. 2.
cPKCα, cPKCβI, nPKCε and PDK1 in plantaris muscles of trained ALS mice. a–c Physical activity increases total PKC levels in ALS animals, the values being always above Untrained WT and ALS values. Regarding the phosphorylation ratios, only the pcPKCβI/cPKCβI is normalized. d Neither running nor swimming normalizes PDK1 or pPDK1 levels. Consequently, also the ratios are still decreased as in Untrained ALS animals. Data are mean value ± SD, *p < 0.05, **p < 0.01, ***p < 0.001. *Versus Untrained WT mice; #versus Untrained ALS; +versus Run ALS mice
Fig. 3.
Catalytic and regulatory PKA subunits in plantaris muscles of trained ALS mice. a Catalytic Cα still increases despite physical trainings, while Cβ is not modified. b Regulatory RIα decreases and RIIβ increases despite physical training, while RIβ is reduced by run. Data are mean value ± SD, *p < 0.05, **p < 0.01, ***p < 0.001. *Versus Untrained WT mice; #versus Untrained ALS; +versus Run ALS mice. RIβ image is made using images taken from the same gel
Furthermore, both types of training slightly increase the pPDK1/PDK1 ratio observed in Untrained ALS muscles by reducing total PDK1 and increasing pPDK1 (Fig. 2d), indicating the possibility of being more active, especially after swimming, which is in accordance with the recovery of exclusive presynaptic PKC activity.
We also analysed the two catalytic PKA subunits (Cα, Cβ) and the four regulatory subunits (RIα, RIβ, RIIα and RIIβ). Results show that both protocols normalize the ALS-induced increase of Cα, while Cβ remains unchanged (Fig. 3a). On the other hand, any protocol prevents neither the reduction of RIα nor the increase of RIIβ regulatory subunits caused by ALS. Finally, RIβ, which is unchanged in Untrained ALS, is slightly reduced in Run ALS (Fig. 3b).
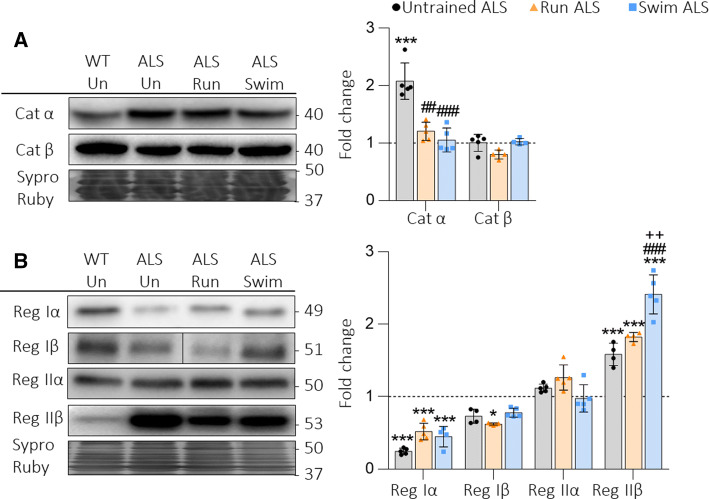
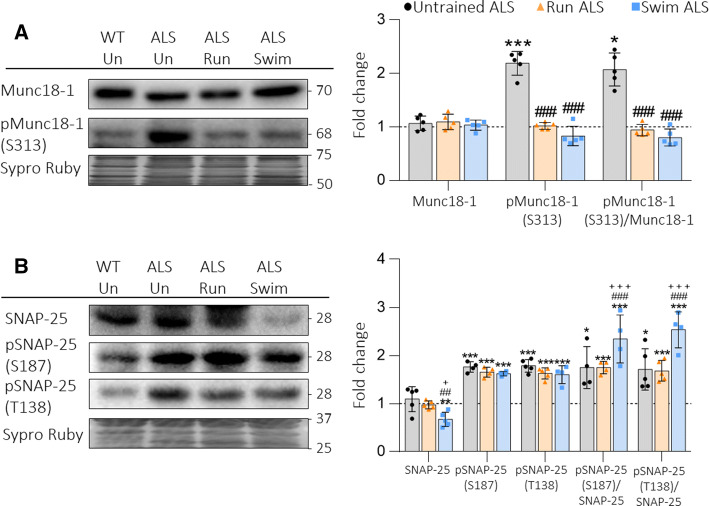
Snare/SM proteins
ALS strongly enhances the phosphorylation of two key proteins of the synaptic vesicle exocytotic machinery, Munc18-1 from the regulatory Sec1/Munc18-like (SM) family and the synaptosomal-associated protein 25 (SNAP-25) a component of the soluble NSF attachment protein receptor (Snare) [4]. Figure 4 shows that both Run and Swim protocols fully prevent the increase of pMunc18-1 and, in concordance, normalize the pMunc18-1/Munc18-1 ratio (Fig. 4a). However, the training does not reduce either pSNAP-25 S187 (PKC target) or T138 (PKA target). Therefore, the ratio pSNAP-25/SNAP-25 (for both phosphorylation sites) is as increased (Run ALS) or even significantly higher (Swim ALS) as it is in Untrained ALS (Fig. 4b).
Fig. 4.
The SNARE/SM Munc18-1 and SNAP-25 in plantaris muscles of trained ALS mice. a Run and swim normalize upregulated pMunc18-1, while Munc18-1 is never modified. The ratio pMunc18-1/Munc18-1 is also normalized after both protocols. b SNAP-25 is decreased by swim and pSNAP-25 (S187) and (T138) are significatively increased despite the trainings. Data are mean value ± SD, *p < 0.05, **p < 0.01, ***p < 0.001. *Versus Untrained WT mice; #versus Untrained ALS; +versus Run ALS mice
Altogether, it seems that exercise preferentially modulates proteins that regulate exocytosis rather than the SNARE proteins directly implicated on it, probably in relation with the selective modulation of cPKCβI.
Motoneuron survival
Finally, we aimed to investigate whether running- and swimming-based training protocols could prevent motoneuron death in relation with the improvement they provoke over the BDNF/TrkB neurotrophic signalling pathway. Because ALS preferentially affects larger motoneurons innervating faster muscle fibres [4, 38, 41], in Fig. 5 we analysed the changes in the proportion between fast (larger) and slow (smaller) motoneurons in the spinal cord of the symptomatic untrained and trained ALS mice (115 days old, which represents the end stage of the disease in Untrained ALS) compared with WT Untrained animals (Fig. 5).
Fig. 5.
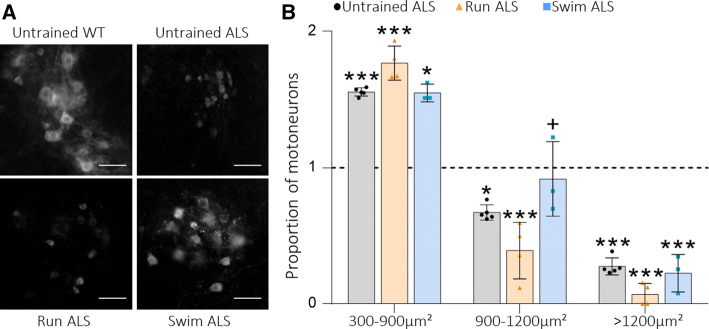
Motoneuron population proportions change depending on the training protocol. a Representative images of motoneurons identified with ChAT immunolabeling in the ventral horn of the lumbar spinal cord (scale bar: 50 μm). b Proportion of motoneuron populations in relation to the WT untrained group by soma area at P115. Data are mean ± S.D ***P < 0.001, **0.001 < P < 0.01, *0.01 < P < 0.05), *Versus Untrained WT mice; +versus Run ALS mice
In Untrained and Run ALS mice, the proportion of smaller (and mainly slow) α motoneurons (300–900 μm2 of area) becomes significatively higher, while the proportion of bigger (and mainly fast) motoneurons strongly decreases (900–1200 μm2 of area). These results indicate that big motoneurons are lost in both groups in comparison with the Untrained WT mice. On the other hand, in Swim ALS mice, the proportion of small motoneurons increases like in Untrained and Run ALS groups but less significantly, while the proportion of big motoneurons (900–1200 μm2 of area) remains equivalent to the control population, indicating that the loss of this population of motoneurons is prevented in these animals. However, the proportion of the biggest motoneurons (> 1200 μm2 of area) is significatively decreased in the three groups (Fig. 5a, b), indicating that they are lost despite the training, while the proportion of sensitive γ motoneurons (< 300 μm2 of area) increases (data not shown). Altogether, it confirms that ALS specially damages fast motoneurons, corresponding to somas bigger than 900 µm2, but that a swimming-based protocol started after disease onset can reduce it while running exacerbates the pattern of Untrained ALS.
Discussion
Because the impairment of the NMJ is crucial in ALS onset and progression, strategies that structurally and functionally preserve it would improve the health of the neuromuscular cell partners. Indeed, in SOD1-G93A mice, maintaining neuromuscular activity extends motor units survival along ALS progression in partially denervated muscles [55] and, in ALS patients, moderate exercise ameliorates the disease symptoms and delays the progression [29, 56–58]. This may be, among others, due to neurotrophic factors expression [59], which prevents motoneuron degeneration, muscle denervation and atrophy [60–62].
The BDNF/TrkB signalling is one of the most implicated pathways in the NMJ stability and it is essential for neurotransmission [12, 15, 16, 63, 64]. Recently, we have demonstrated that this signalling is altered in the muscles of SOD1-G93A mice even before disease onset [4], suggesting that its dysfunctionality contributes to motor impairment in ALS. Now, the present results add new evidence that the two training protocols that have been analysed are capable of inducing activity-dependent adaptations to reduce or fully prevent most of the profound molecular alterations observed in the BDNF/TrkB pathway in Untrained ALS mice.
Neurotrophins and TrkB signalling
Exercise training, through synaptic activity and muscle contraction, increases BDNF in spinal cord and skeletal muscle of rodents and humans [12, 59, 65–67]. This normal situation, which is beneficial not only for the peripheral nervous system [68] but also for the central nervous system [66, 67], is altered in ALS disease, where mBDNF is significantly increased [4, 69] despite the reduction of neuromuscular activity. Contrarily, we show that running and swimming reduce or completely normalize the BDNF accumulation, respectively. These apparent contradictions, together with previous results showing that exogenous BDNF therapy is not beneficial for ALS [70, 71], drives us to the hypothesis that, in ALS, BDNF and other neurotrophins are synthesized by myocytes affected by ALS in an attempt to ameliorate NMJ activity. However, it is not useful because of TrkB.FL downregulation and TrkB.T1 upregulation [4, 72, 73]. Indeed, the adverse effect of TrkB.T1 on ALS mice has been extensively demonstrated [24, 74] and it seems that its overpresence limits the BDNF action because of its negative effect over TrkB.FL that results in pTrkB.FL not transducing the intracellular signalling [74]. In contrast, in trained animals, TrkB.FL is able to trigger the pathway that results in the recovery of the downstream signalling. Indeed, a similar modulation of TrkB receptors has been reported by physical training in WT mice spinal cord [75] and the same effect is found in the WT diaphragm when muscle contraction is increased [12]. Furthermore, in the first stages of ALS, activated microglia synthesize BDNF, which exerts anti-inflammatory and protective effects. However, at later stages, neither toxic microglia nor astrocytes express BDNF [76, 77]. Because this occurs in the central nervous system, it is probably also directly related with the loss of motoneurons in this disease.
In accordance with previous studies [78], we found that p75NTR is reduced in the NMJ of Untrained ALS animal limb muscles and it is reestablished after training. Indeed, it is possible that exercise enhances p75NTR levels to potentiate its myogenic effect as it plays a crucial role in muscle repair [79, 80], it is co-expressed with embryonic myosin heavy chain, indicating muscle fibre regeneration [78] and regulates neuronal survival and differentiation by interacting with Trk receptors [81]. Altogether, it seems that p75NTR could be a marker of improvement in the skeletal muscles.
Interestingly, the only alteration that training could not prevent at all is the great increase of NT4 which is observed yet in the presymptomatic stage [4, 69]. Indeed, it has been reported that NT4 prevented motoneuron death [82] and that avoids the disassembly of postsynaptic acetylcholine receptors (AChR) clusters [83]. Thus, it seems that NT4 could be postsynapticaly synthesized to further increase after exercise and modulate the presynaptic activity in ALS muscles when TrkB receptors are correctly balanced. This would have the final objective to better preserve motoneurons.
Serine-threonine kinases and SNARE/SM targets
We previously demonstrated that induced muscle contraction retrogradely upregulates presynaptic nPKCε and cPKCβI through BDNF/TrkB signalling in WT animals and that they regulate neuromuscular transmission by controlling Munc18-1 and SNAP-25 phosphorylation [12, 19, 20, 49, 84]. Particularly, Munc18-1 phosphorylation is regulated by both cPKCβI and nPKCε at the NMJ [20], while SNAP-25 phosphorylation is exclusively regulated by nPKCε [19]. Here, we prove that in ALS the differential effects of the training over cPKCβI and nPKCε is directly related with the results seen in pMunc18-1 and pSNAP-25 modulation. Thus, the normalization of pMunc18-1/Munc18-1 ratio coincides with the normalization of the activity balance between cPKCβI and nPKCε. On the other hand, SNAP-25 phosphorylation in the residue S187 is still upregulated despite the training because the phosphorylating efficacy of nPKCε is increased enough to guarantee an optimal recruitment of ready-releasable vesicles [85] that may help to maintain neurotransmission. Alternatively, dephosphorylation of pSNAP-25 may be impaired in ALS, as even in untrained muscles pSNAP-25 is elevated despite nPKCε minor activity in Untrained ALS mice.
Regarding PKA, both trainings normalize the ALS-induced increase of Cα subunit, while the complex change in the stoichiometry of the regulatory subunits RIα and RIIβ persists. Adenosine receptor A2 (A2AR) has neuroprotective effects mediated by the PKA signalling [86]. Indeed, PKA activation neuroprotected motoneurons, while its inhibition did not [87]. Altogether, it seems that an increased PKA activity has a neuroprotective effect. Interestingly, this coincides with the found high levels of pSNAP-25 T138, pointing to the change in regulatory subunits as a major change in ALS that cannot be normalized by training. Therefore, it seems that the potentiation of the PKA pathway could be of great interest in the treatment of ALS patients.
Run and swim have different effects
Since ALS preferentially affects fast-twitching muscles, it is conceivable that the effect of exercise depends on its type, the protocol followed and the intensity [32, 35–38] and that these differences would also be found in the molecular field. Recently, we differentiated the molecular changes in BDNF/TrkB signalling that could be explained or not by the fast-to-slow transition [4] described in ALS plantaris muscles [38, 41]. Deforges et al. [38] shows that, while running exacerbated the fast-to-slow functional transition, swimming preserved the normal phenotype in the plantaris. However, our molecular results reveal that the changes in ALS that presumably reflect the fast-to-slow transition (see Table 2 for protein classification) are always equally modulated by both protocols. On the one hand, some of them are unaffected by the trainings, suggesting that they are unavoidable, perhaps because they are directly related with the pathogenesis of the disease or because they are a strategy to increase motoneuron survival, such as we hypothesized for NT4 or PKA activity. On the other hand, some molecules are normalized by both protocols, which could be related to the coincident benefits of both protocols such as the avoidance of muscular atrophy [38].
Table 2.
Classification of proteins
| Effect | ||||
|---|---|---|---|---|
| No | Equal | Swim | Run | |
| Fast-to-slow transition | NT4 | TrkB.FL | ||
| cPKCα | pTrkB.FL (Tyr816) | |||
| PKA RIIβ | cPKCβI | |||
| pSNAP-25 (Ser187) | pnPKCε (Ser729) | |||
| PKA Cα | ||||
| No Fast-to-slow transition | PKA RIα | nPKCε | mBDNF | TrkB.T1 |
| pSNAP-25 (Thr138) | PDK1 | pDPK1 (Ser241) | ||
| pMunc18-1 (Ser313) | pcPKCβI (Thr642) | |||
| p75NTR | ||||
| No change in ALS Untrained | pcPKCα (Ser657) | SNAP-25 | PKA RIβ | |
| PKA Cβ | ||||
| PKA RIIα | ||||
| Munc18-1 | ||||
Depending on (1) the changes that follow in ALS mice untrained (rows) and (2) which training protocol has effect on them (columns). The proteins that followed a fast-to-slow transition acquired values similar to the soleus of WT mice and the ones that did not follow a fast-to-slow transition did not. Finally, some proteins do not change in ALS mice untrained
Also, there are molecules that do not follow a fast-to-slow transition (Table 2). Among them, the specific effect of swimming over mBDNF and phosphorylated PKCβI and PDK1 coincides with the functional benefits described by Deforges et al. Despite that TrkB.T1 is still upregulated after swimming, the ratio TrkB.FL/TrkB.T1 is strongly increased, while the ratio mBDNF/proBDNF is restored. Therefore, the triggering and the function of the downstream kinases of the signalling are reestablished. As a result, NMJ and the neuromuscular cell partners are better preserved and communicated, which is reflected by the decrease of motoneuron loss, from a 49 and 45% in untrained and running animals, respectively, to only a 28% in swimming animals [38]. As motoneuron death precedes ALS onset [1, 2], their preservation delays it, increases mean survival of the animals and maintains body weight and motor function for longer [33, 36–38, 88]. Indeed, previous studies found that swimming maintains the population of fast myofibres and improves motor performance [38]. Altogether, here we found that swimming softens the drastic loss of big and fast motoneurons by retrogradely modulating presynaptic signalling, which reinforces the idea that neuroprotection is bidirectional. However, swimming exclusively preserves big motoneurons, with a soma area between 900 and 1200 μm2. This may be due to the higher requirements of the biggest ones (> 1200 μm2), which are more susceptible to oxidative stress and excitotoxicity, which is promoted by physical training [5, 89–91]. Indeed, running is a high-impact exercise which only recruits small motoneurons and generates more oxidative stress than swimming, which is a low-impact exercise that recruits both small and big motoneurons. Therefore, it is comprehensible that, despite both protocols induce molecular changes at the motoneuron terminal, swimming prevents motoneuron loss while running does not. In accordance with the benefits of low-impact exercise, [87, 92] found that voluntary physical activity motivated by the change of the environment induces similar benefits to those of swimming, probably in relation with less oxidative stress production in comparison with forced running protocols. Altogether, this adds new evidence of how susceptible is to determine the effect of physical training and justifies the controversy around it.
The molecules that do not follow a fast-to-slow transition but are modulated equally by both protocols and the ones that do not change could participate in the benefits shared by both running and swimming or may be part of the unavoidable molecular alterations induced by the pathology. In fact, both SNAP-25 phosphorylations (S187 and T138) are unsensitive to any activity training despite that the first one follows the fast-to-slow transition and the second one does not. However, vesicle refilling and ready-releasable vesicle pool size are controlled by them [85, 93], and therefore their upregulation can be a requirement for the maintenance of useful neurotransmission and NMJ structure.
Concluding remarks
In summary, many molecular changes in ALS muscles are prevented or reduced and sometimes overcompensated by the training protocols (for a summary see the Graphical abstract in Fig. 6). They partially prevent the neurotrophic signalling alteration, directly related with neurotransmission and neuromuscular activity protection. Indeed, these molecular changes are contemporary with improvements in animals’ condition, especially regarding swimming, where motoneuron loss is reduced [38]. Altogether, despite the controversial opinions on physical exercise as therapy in ALS, it seems that it is always beneficial, but with precise exercise-dependent outcomes. Thus, this study contributes to the understanding of the variable role of exercise in ALS and adds new evidence that both run and swim trainings are beneficial as they diminish the profound molecular alterations observed in the BDNF/TrkB pathway in Untrained ALS mice muscles. Altogether, despite being still far of clinic application, further investigation in this field would be useful as the differences among protocols are essential to target specific molecular changes.
Fig. 6.
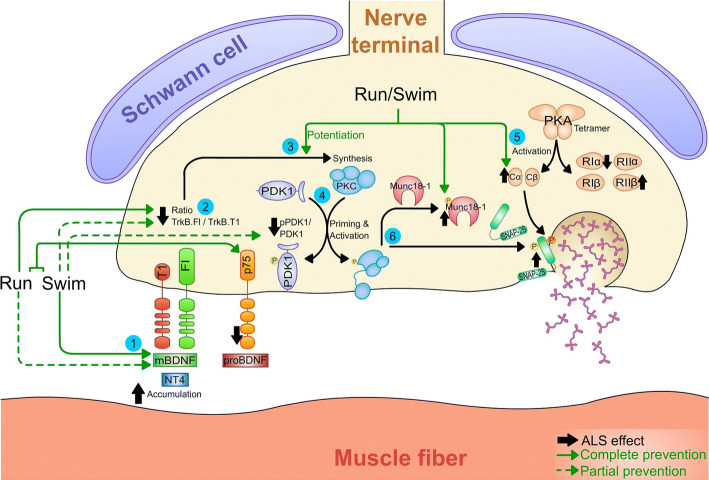
Graphical representation of the effect of physical training over the BDNF signalling in plantaris muscles with ALS. Despite that any training reduces the accumulation of NT4, both, and especially swimming, reduce mBDNF levels (1). However, the fall of the ratio TrkB.FL/TrkB.T1 is better prevented by running than by swimming (2). The PKC pathway is potentiated by both protocols (3) in contrast to PDK1, whose ratio is only partially normalized by swimming (4). On the other hand, the trainings exclusively modulate catalytic but not regulatory PKA subunits (5). In accordance with global serine/threonine kinase activity affectation, the target related with vesicle exocytosis SNAP-25 is upregulated; while the regulator protein Munc18-1 is normalized after the trainings (6). Altogether, many molecular changes in ALS muscles are prevented or reduced and sometimes overcompensated by the training protocols. Code: green solid arrows indicate a complete prevention of the alteration produced by ALS (represented with strong black arrows next to the proteins) in the studied system, while green dashed arrows indicate a partial prevention. Numbers inside blue circumferences are mean to focus attention into important steps explained in the legend of the figure
Acknowledgements
This work was possible with the financial support of Ministerio de Economía, Industria y Competitividad, the Agencia Estatal de Investigación (AEI) and the European Regional Development Fund (ERDF) (SAF2015-67143-P) and the support of the Universitat Rovira i Virgili (URV) (2014PFR-URV-B2-83 and 2017PFR-URV-B2-85) and the Catalan Government (2014SGR344 and 2017SGR704). VC has been supported by the Ministerio de Economía y Competitividad (MINECO) under the framework of the Sistema Nacional de Garantía Juvenil, the European Social Fund (ESF) and the Iniciativa de Empleo Juvenil (IEJ). LJ has been supported by Universitat Rovira i Virgili.
Abbreviations
- ALS
Amyotrophic lateral sclerosis
- BDNF
Brain-derived neurotrophic factor
- NMJ
Neuromuscular junction
- NT4
Neurotrophin-4
- p75NTR
p75 neurotrophin receptor
- PDK1
Phosphoinositide-dependent kinase-1
- PKA
Protein kinase A
- PKC
Protein kinase C
- SM
Sec1/Munc18-like
- SNAP-25
Synaptosomal-associated protein 25
- SNARE
SNAP (Soluble NSF Attachment Protein) receptor
- TrkB.T1
Truncated tropomyosin-related kinase B receptor
- TrkB.FL
Full-length tropomyosin-related kinase B receptor
- WT
Wild type
Author contributions
Data collection: LJ, EH, VC, MT. Quantitative analysis and statistics: LJ. Graphical abstract design: LJ. Data interpretation: LJ, EH, VC, OB, FC, JT, MAL, NG. Literature search: LJ, EH, VC, JT, MAL, NG. Manuscript preparation: LJ, EH, VC, JT, MAL, NG. Conception and design: JT, MAL, NG. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflicts of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maria A. Lanuza, Josep Tomàs and Neus Garcia contributed equally to this work.
Contributor Information
Josep Tomàs, Email: josepmaria.tomas@urv.cat.
Maria A. Lanuza, Email: mariaangel.lanuza@urv.cat
References
- 1.Moloney EB, de Winter F, Verhaagen J. ALS as a distal axonopathy: molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease. Front Neurosci. 2014;8:1–18. doi: 10.3389/fnins.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer LR, Culver DG, Tennant P, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Dadon-Nachum M, Melamed E, Offen D. The “Dying-Back” phenomenon of motor neurons in ALS. J Mol Neurosci. 2011;43:470–477. doi: 10.1007/s12031-010-9467-1. [DOI] [PubMed] [Google Scholar]
- 4.Just-Borràs L, Hurtado E, Cilleros-Mañé V, et al. Overview of Impaired BDNF signaling, their coupled downstream serine-threonine kinases and SNARE/SM complex in the neuromuscular junction of the amyotrophic lateral sclerosis model SOD1-G93A mice. Mol Neurobiol. 2019 doi: 10.1007/s12035-019-1550-1. [DOI] [PubMed] [Google Scholar]
- 5.Cleveland DW, Williamson TL. Slowing of axonal transport is a very early event in the toxicity ofALS–linked SOD1 mutants to motor neurons. Nat Neurosci. 1999;2:50–56. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Tu P, Abtahian F, et al. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J Cell Biol. 1997;139:1307–1315. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park KHJ, Vincent I. Presymptomatic biochemical changes in hindlimb muscle of G93A human Cu/Zn superoxide dismutase 1 transgenic mouse model of amyotrophic lateral sclerosis. Biochim Biophys Acta Mol Basis Dis. 2008;1782:462–468. doi: 10.1016/J.BBADIS.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupuis L, Gonzalez De Aguilar J-L, Echaniz-Laguna A, et al. Muscle mitochondrial uncoupling dismantles neuromuscular junction and triggers distal degeneration of motor neurons. PLoS One. 2009;4:1–12. doi: 10.1371/journal.pone.0005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt ERE, Pasterkamp RJ, van den Berg LH. Axon guidance proteins: novel therapeutic targets for ALS? Prog Neurobiol. 2009;88:286–301. doi: 10.1016/J.PNEUROBIO.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Boyer JG, Ferrier A, Kothary R. More than a bystander: the contributions of intrinsic skeletal muscle defects in motor neuron diseases. Front Physiol. 2013;4:1–45. doi: 10.3389/fphys.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews VB, Åström M-B, Chan MHS, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 12.Hurtado E, Cilleros V, Nadal L, et al. Muscle contraction regulates BDNF/TrkB signaling to modulate synaptic function through presynaptic cPKCα and cPKCβI. Front Mol Neurosci. 2017;10:1–22. doi: 10.3389/fnmol2017.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Udina E, Cobianchi S, Allodi I, Navarro X. Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Ann Anat Anat Anzeiger. 2011;193:347–353. doi: 10.1016/j.aanat.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantilla CB, Stowe JM, Sieck DC, et al. TrkB kinase activity maintains synaptic function and structural integrity at adult neuromuscular junctions. J Appl Physiol. 2014;117:910–920. doi: 10.1152/japplphysiol.01386.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadal L, Garcia N, Hurtado E, et al. Presynaptic muscarinic acetylcholine autoreceptors (M1, M2 and M4 subtypes), adenosine receptors (A1 and A2A) and tropomyosin-related kinase B receptor (TrkB) modulate the developmental synapse elimination process at the neuromuscular junction. Mol Brain. 2016;9:1–19. doi: 10.1186/s13041-016-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadal L, Garcia N, Hurtado E, et al. Presynaptic muscarinic acetylcholine receptors and TrkB receptor cooperate in the elimination of redundant motor nerve terminals during development. Front Aging Neurosci. 2017;9:1–7. doi: 10.3389/fnagi.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simó A, Cilleros-Mañé V, Just-Borràs L, et al. nPKCε mediates SNAP-25 phosphorylation of Ser-187 in basal conditions and after synaptic activity at the neuromuscular junction. Mol Neurobiol. 2019 doi: 10.1007/s12035-018-1462-5. [DOI] [PubMed] [Google Scholar]
- 20.Simó A, Just-Borràs L, Cilleros-Mañé V, et al. BDNF-TrkB signaling coupled to nPKCε and cPKCβI modulate the phosphorylation of the exocytotic protein Munc18-1 during synaptic activity at the neuromuscular junction. Front Mol Neurosci. 2018;11:207–227. doi: 10.3389/fnmol.2018.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurtado E, Cilleros V, Just L, et al. Synaptic activity and muscle contraction increases PDK1 and PKCβI phosphorylation in the presynaptic membrane of the neuromuscular junction. Front Mol Neurosci. 2017;10:1–13. doi: 10.3389/fnmol.2017.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner BJ, Cheah IK, Macfarlane KJ, et al. Antisense peptide nucleic acid-mediated knockdown of the p75 neurotrophin receptor delays motor neuron disease in mutant SOD1 transgenic mice. J Neurochem. 2003;87:752–763. doi: 10.1046/j.1471-4159.2003.02053.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhai J, Zhou W, Li J, et al. The in vivo contribution of motor neuron TrkB receptors to mutant SOD1 motor neuron disease. Hum Mol Genet. 2011;20:4116–4131. doi: 10.1093/hmg/ddr335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanpallewar SU, Barrick CA, Buckley H, et al. Deletion of the BDNF truncated receptor TrkB.T1 delays disease onset in a mouse model of amyotrophic lateral sclerosis. PLoS One. 2012;7:1–7. doi: 10.1371/journal.pone.0039946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berchtold NC, Chinn G, Chou M, et al. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/J.NEUROSCIENCE.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/S0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 27.Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–130. doi: 10.1016/J.BRAINRES.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 28.Wu C-W, Chang Y-T, Yu L, et al. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol. 2008;105:1585–1594. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]
- 29.Bello-Haas VD, Florence JM, Kloos AD, et al. A randomized controlled trial of resistance exercise in individuals with ALS. Neurology. 2007;68:2003–2007. doi: 10.1212/01.wnl.0000264418.92308.a4. [DOI] [PubMed] [Google Scholar]
- 30.Drory VE, Goltsman E, Reznik JG, et al. The value of muscle exercise in patients with amyotrophic lateral sclerosis. J Neurol Sci. 2001;191:133–137. doi: 10.1016/S0022-510X(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 31.Pinto AC, Alves M, Nogueira A, et al. Can amyotrophic lateral sclerosis patients with respiratory insufficiency exercise? J Neurol Sci. 1999;169:69–75. doi: 10.1016/S0022-510X(99)00218-X. [DOI] [PubMed] [Google Scholar]
- 32.Kaspar BK, Frost LM, Christian L, et al. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann Neurol. 2005;57:649–655. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- 33.Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. Regular exercise is beneficial to a mouse model of amyotrophic lateral sclerosis. Ann Neurol. 2003;53:804–807. doi: 10.1002/ana.10597. [DOI] [PubMed] [Google Scholar]
- 34.McCrate ME, Kaspar BK. Physical activity and neuroprotection in amyotrophic lateral sclerosis. Neuromol Med. 2008;10:108–117. doi: 10.1007/s12017-008-8030-5. [DOI] [PubMed] [Google Scholar]
- 35.Liebetanz D, Hagemann K, von Lewinski F, et al. Extensive exercise is not harmful in amyotrophic lateral sclerosis. Eur J Neurosci. 2004;20:3115–3120. doi: 10.1111/j.1460-9568.2004.03769.x. [DOI] [PubMed] [Google Scholar]
- 36.Mahoney DJ, Rodriguez C, Devries M, et al. Effects of high-intensity endurance exercise training in the G93A mouse model of amyotrophic lateral sclerosis. Muscle Nerve. 2004;29:656–662. doi: 10.1002/mus.20004. [DOI] [PubMed] [Google Scholar]
- 37.Veldink JH, Bär PR, Joosten EAJ, et al. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromusc Disord. 2003;13:737–743. doi: 10.1016/S0960-8966(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 38.Deforges S, Branchu J, Biondi O, et al. Motoneuron survival is promoted by specific exercise in a mouse model of amyotrophic lateral sclerosis. J Physiol. 2009;587:3561–3571. doi: 10.1113/jphysiol.2009.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elbasiouny SM, Schuster JE. The effect of training on motoneuron survival in amyotrophic lateral sclerosis: which motoneuron type is saved? Front Physiol. 2011 doi: 10.3389/fphys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frey D, Schneider C, Xu L, et al. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hegedus J, Putman CT, Tyreman N, Gordon T. Preferential motor unit loss in the SOD1 G93A transgenic mouse model of amyotrophic lateral sclerosis. J Physiol. 2008;586:3337–3351. doi: 10.1113/jphysiol.2007.149286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pun S, Santos AF, Saxena S, et al. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- 43.Grondard C, Biondi O, Pariset C, et al. Exercise-induced modulation of calcineurin activity parallels the time course of myofibretransitions. J Cell Physiol. 2008;2008:126–135. doi: 10.1002/jcp.21168. [DOI] [PubMed] [Google Scholar]
- 44.Ferrante RJ, Klein AM, Dedeoglu A, Beal MF. Therapeutic efficacy of EGb761 (Gingko biloba extract) in a transgenic mouse model of amyotrophic lateral sclerosis. J Mol Neurosci. 2001;17:89–96. doi: 10.1385/JMN:17:1:89. [DOI] [PubMed] [Google Scholar]
- 45.McCombe PA, Henderson RD. Effects of gender in amyotrophic lateral sclerosis. Gend Med. 2010;7:557–570. doi: 10.1016/j.genm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Dell RB, Holleran S, Ramakrishnan R. Sample size determination. ILAR J. 2002;43:207–213. doi: 10.1093/ilar.43.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snedecor GW, Cochran WG. Statistical methods applied to experiments in agriculture and biology. 8. Ames: Iowa State University Press; 1989. [Google Scholar]
- 48.Loeffler J-P, Picchiarelli G, Dupuis L, Gonzalez De Aguilar J-L. The role of skeletal muscle in amyotrophic lateral sclerosis. Brain Pathol. 2016;26:227–236. doi: 10.1111/bpa.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obis T, Hurtado E, Nadal L, et al. The novel protein kinase C epsilon isoform modulates acetylcholine release in the rat neuromuscular junction. Mol Brain. 2015;8:1–16. doi: 10.1186/s13041-015-0171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Besalduch N, Tomàs M, Santafé MM, et al. Synaptic activity-related classical protein kinase C isoform localization in the adult rat neuromuscular synapse. J Comp Neurol. 2010;518:211–228. doi: 10.1002/cne.22220. [DOI] [PubMed] [Google Scholar]
- 51.Mantilla CB, Zhan W-Z, Sieck GC. Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve. 2004;29:381–386. doi: 10.1002/mus.10558. [DOI] [PubMed] [Google Scholar]
- 52.Tomàs J, Garcia N, Lanuza MA, et al. Presynaptic membrane receptors modulate ACh release, axonal competition and synapse elimination during neuromuscular junction development. Front Mol Neurosci. 2017;10:132. doi: 10.3389/fnmol.2017.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pousinha PA, Diogenes MJ, Ribeiro JA, Sebastião AM. Triggering of BDNF facilitatory action on neuromuscular transmission by adenosine A2A receptors. Neurosci Lett. 2006;404:143–147. doi: 10.1016/j.neulet.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 54.Wiese S, Jablonka S, Holtmann B, et al. Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc Natl Acad Sci. 2007;104:17210–17215. doi: 10.1073/pnas.0705267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gordon T, Tyreman N, Li S, et al. Functional over-load saves motor units in the SOD1-G93A transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2010;37:412–422. doi: 10.1016/j.nbd.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 56.Lunetta C, Lizio A, Sansone VA, et al. Strictly monitored exercise programs reduce motor deterioration in ALS: preliminary results of a randomized controlled trial. J Neurol. 2016;263:52–60. doi: 10.1007/s00415-015-7924-z. [DOI] [PubMed] [Google Scholar]
- 57.Meyer R, Spittel S, Steinfurth L, et al. Patient-reported outcome of physical therapy in amyotrophic lateral sclerosis: observational online study. JMIR Rehabil Assist Technol. 2018 doi: 10.2196/10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merico A, Cavinato M, Gregorio C, et al. Effects of combined endurance and resistance training in Amyotrophic Lateral Sclerosis: a pilot, randomized, controlled study. Eur J Transl Myol. 2018;28:72–78. doi: 10.4081/ejtm.2018.7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gómez-Pinilla F, Ying Z, Opazo P, et al. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 60.Acsadi G, Anguelov RA, Yang H, et al. Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum Gene Ther. 2002;13:1047–1059. doi: 10.1089/104303402753812458. [DOI] [PubMed] [Google Scholar]
- 61.Manabe Y, Nagano I, Gazi MSA, et al. Adenovirus-mediated gene transfer of glial cell line-derived neurotrophic factor prevents motor neuron loss of transgenic model mice for amyotrophic lateral sclerosis. Apoptosis. 2002;7:329–334. doi: 10.1023/A:1016123413038. [DOI] [PubMed] [Google Scholar]
- 62.Sun W, Funakoshi H, Nakamura T. Overexpression of HGF retards disease progression and prolongs life span in a transgenic mouse model of ALS. J Neurosci. 2002;22:6537–6548. doi: 10.1523/JNEUROSCI.22-15-06537.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santafé MM, Garcia N, Tomàs M, et al. The interaction between tropomyosin-related kinase B receptors and serine kinases modulates acetylcholine release in adult neuromuscular junctions. Neurosci Lett. 2014;561:171–175. doi: 10.1016/j.neulet.2013.12.073. [DOI] [PubMed] [Google Scholar]
- 64.Garcia N, Tomàs M, Santafe MM, et al. Localization of brain-derived neurotrophic factor, neurotrophin-4, tropomyosin-related kinase b receptor, and p75NTR receptor by high-resolution immunohistochemistry on the adult mouse neuromuscular junction. J Peripher Nerv Syst. 2010;15:40–49. doi: 10.1111/j.1529-8027.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- 65.Gómez-Pinilla F, Ying Z, Roy RR, et al. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 66.Cuppini R, Sartini S, Agostini D, et al. Bdnf expression in rat skeletal muscle after acute or repeated exercise. Arch Ital Biol. 2007;145:99–110. [PubMed] [Google Scholar]
- 67.Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61:533–541. [PubMed] [Google Scholar]
- 68.Li X, Wu Q, Xie C, et al. Blocking of BDNF-TrkB signaling inhibits the promotion effect of neurological function recovery after treadmill training in rats with spinal cord injury. Spinal Cord. 2018 doi: 10.1038/s41393-018-0173-0. [DOI] [PubMed] [Google Scholar]
- 69.Küst BM, Copray JCVM, Brouwer N, et al. Elevated levels of neurotrophins in human biceps brachii tissue of amyotrophic lateral sclerosis. Exp Neurol. 2002;177:419–427. doi: 10.1006/exnr.2002.8011. [DOI] [PubMed] [Google Scholar]
- 70.Henriques Neurotrophic growth factors for the treatment of amyotrophic lateral sclerosis: where do we stand? Front Neurosci. 2010;4:1–14. doi: 10.3389/fnins.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 72.Eide FF, Vining ER, Eide BL, et al. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gonzalez M, Ruggiero FP, Chang Q, et al. Disruption of Trkb-mediated signaling induces disassembly of postsynaptic receptor clusters at neuromuscular junctions. Neuron. 1999;24:567–583. doi: 10.1016/S0896-6273(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 74.Dorsey SG, Lovering RM, Renn CL, et al. Genetic deletion of trkB.T1 increases neuromuscular function. Am J Physiol Cell Physiol. 2011;302:141–153. doi: 10.1152/ajpcell.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skup M, Dwornik A, Macias M, et al. Long-term locomotor training up-regulates TrkB(FL) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp Neurol. 2002;176:289–307. doi: 10.1006/exnr.2002.7943. [DOI] [PubMed] [Google Scholar]
- 76.Brambilla L, Martorana F, Guidotti G, Rossi D. Dysregulation of astrocytic HMGB1 signaling in amyotrophic lateral sclerosis. Front Neurosci. 2018 doi: 10.3389/fnins.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao B, Zhao W, Beers DR, et al. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol. 2012;237:147–152. doi: 10.1016/j.expneurol.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J-X, Brännström T, Andersen PM, Pedrosa-Domellöf F. Distinct changes in synaptic protein composition at neuromuscular junctions of extraocular muscles versus limb muscles of ALS donors. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0057473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scott ALM, Ramer MS. Schwann cell p75NTR prevents spontaneous sensory reinnervation of the adult spinal cord. Brain. 2010;133:421–432. doi: 10.1093/brain/awp316. [DOI] [PubMed] [Google Scholar]
- 80.Bussmann KA, Sofroniew M. Re-expression of p75NTR by adult motor neurons after axotomy is triggered by retrograde transport of a positive signal from axons regrowing through damaged or denervated peripheral nerve tissue. Neuroscience. 1999;91:273–281. doi: 10.1016/S0306-4522(98)00562-4. [DOI] [PubMed] [Google Scholar]
- 81.Meeker R, Williams K. Dynamic nature of the p75 neurotrophin receptor in response to injury and disease. J Neuroimmune Pharmacol. 2014;9:615–628. doi: 10.1007/s11481-014-9566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaal EC, Joosten EA, Bär PR. Prevention of apoptotic motoneuron death in vitro by neurotrophins and muscle extract. Neurochem Int. 1997;31:193–201. doi: 10.1016/S0197-0186(96)00148-9. [DOI] [PubMed] [Google Scholar]
- 83.Belluardo N, Westerblad H, Mudó G, et al. Neuromuscular junction disassembly and muscle fatigue in mice lacking neurotrophin-4. Mol Cell Neurosci. 2001;18:56–67. doi: 10.1006/mcne.2001.1001. [DOI] [PubMed] [Google Scholar]
- 84.Obis T, Besalduch N, Hurtado E, et al. The novel protein kinase C epsilon isoform at the adult neuromuscular synapse: location, regulation by synaptic activity-dependent muscle contraction through TrkB signaling and coupling to ACh release. Mol Brain. 2015;8:1–16. doi: 10.1186/s13041-015-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagy G, Matti U, Nehring RB, et al. Protein kinase C-dependent phosphorylation of synaptosome-associated protein of 25 kDa at Ser187 potentiates vesicle recruitment. J Neurosci. 2002;22:9278–9286. doi: 10.1523/JNEUROSCI.22-21-09278.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lai C-Y, Liu Y-J, Lai H-L, et al. The D2 dopamine receptor interferes with the protective effect of the A2A adenosine receptor on TDP-43 mislocalization in experimental models of motor neuron degeneration. Front Neurosci. 2018 doi: 10.3389/fnins.2018.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bilak M, Wu L, Wang Q, et al. PGE2 receptors rescue motor neurons in a model of amyotrophic lateral sclerosis. Ann Neurol. 2004;56:240–248. doi: 10.1002/ana.20179. [DOI] [PubMed] [Google Scholar]
- 88.Carreras I, Yuruker S, Aytan N, et al. Moderate exercise delays the motor performance decline in a transgenic model of ALS. Brain Res. 2010;1313:192–201. doi: 10.1016/j.brainres.2009.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williams TL, Day NC, Ince PG, et al. Calcium-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors: a molecular determinant of selective vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1997;42:200–207. doi: 10.1002/ana.410420211. [DOI] [PubMed] [Google Scholar]
- 90.Alexianu ME, Robbins E, Carswell S, Appel SH. 1Alpha, 25 dihydroxyvitamin D3-dependent up-regulation of calcium-binding proteins in motoneuron cells. J Neurosci Res. 1998;51:58. doi: 10.1002/(SICI)1097-4547(19980101)51:1<58::AID-JNR6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 91.Menzies FM, Grierson AJ, Cookson MR, et al. Selective loss of neurofilament expression in Cu/Zn superoxide dismutase (SOD1) linked amyotrophic lateral sclerosis. J Neurochem. 2004;82:1118–1128. doi: 10.1046/j.1471-4159.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- 92.Gerber YN, Sabourin J-C, Hugnot J-P, Perrin FE. Unlike physical exercise, modified environment increases the lifespan of SOD1G93A mice however both conditions induce cellular changes. PLoS One. 2012 doi: 10.1371/journal.pone.0045503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leenders AGM, Sheng Z-H. Modulation of neurotransmitter release by the second messenger-activated protein kinases: implications for presynaptic plasticity. Pharmacol Ther. 2005;105:69–84. doi: 10.1016/j.pharmthera.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]