Abstract
Neurovascular coupling (NVC) is the mechanism whereby an increase in neuronal activity causes an increase in local cerebral blood flow (CBF) to ensure local supply of oxygen and nutrients to the activated areas. The excitatory neurotransmitter glutamate gates post-synaptic N-methyl-d-aspartate receptors to mediate extracellular Ca2+ entry and stimulate neuronal nitric oxide (NO) synthase to release NO, thereby triggering NVC. Recent work suggested that endothelial Ca2+ signals could underpin NVC by recruiting the endothelial NO synthase. For instance, acetylcholine induced intracellular Ca2+ signals followed by NO release by activating muscarinic 5 receptors in hCMEC/D3 cells, a widely employed model of human brain microvascular endothelial cells. Herein, we sought to assess whether also glutamate elicits metabotropic Ca2+ signals and NO release in hCMEC/D3 cells. Glutamate induced a dose-dependent increase in intracellular Ca2+ concentration ([Ca2+]i) that was blocked by α-methyl-4-carboxyphenylglycine and phenocopied by trans-1-amino-1,3-cyclopentanedicarboxylic acid, which, respectively, block and activate group 1 metabotropic glutamate receptors (mGluRs). Accordingly, hCMEC/D3 expressed both mGluR1 and mGluR5 and the Ca2+ response to glutamate was inhibited by their pharmacological blockade with, respectively, CPCCOEt and MTEP hydrochloride. The Ca2+ response to glutamate was initiated by endogenous Ca2+ release from the endoplasmic reticulum and endolysosomal Ca2+ store through inositol-1,4,5-trisphosphate receptors and two-pore channels, respectively, and sustained by store-operated Ca2+ entry. In addition, glutamate induced robust NO release that was suppressed by pharmacological blockade of the accompanying increase in [Ca2+]i. These data demonstrate for the first time that glutamate may induce metabotropic Ca2+ signals in human brain microvascular endothelial cells. The Ca2+ response to glutamate is likely to support NVC during neuronal activity, thereby reinforcing the emerging role of brain microvascular endothelial cells in the regulation of CBF.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03284-1) contains supplementary material, which is available to authorized users.
Keywords: Glutamate, Neurovascular coupling, Brain microvascular endothelial cells, Group 1 metabotropic glutamate receptors, Ca2+ signaling, Nitric oxide
Introduction
Lying at the interface between circulation and vascular tissues, the endothelium serves as a signal transduction platform that integrates hemodynamic forces and blood-borne signals to regulate multiple vascular processes, including vascular tone and permeability as well as vascular structure [1–4]. Appropriate control of local blood flow through resistance arteries is critical to ensure the proper supply of oxygen and nutrients, as well as the removal of catabolic waste, and to maintain blood pressure within the physiological range [5, 6]. Vascular endothelial cells respond to vasodilatory autacoids by releasing diffusible mediators, such as nitric oxide (NO) and prostacyclin (PGI2), and/or by undergoing membrane hyperpolarization that spreads to medial smooth muscle cells via myoendothelial gap junctions (MEGJs) to suppress contractility, according to a mechanism termed endothelium-dependent hyperpolarization (EDH) [3, 7, 8]. An increase in endothelial intracellular Ca2+ concentration ([Ca2+]i) represents the signal which recruits the most effective vasorelaxing pathways in the vascular wall [3, 7, 9]. For instance, endothelial Ca2+ signals stimulate NO release by engaging the Ca2+-dependent calmodulin (CaM) to displace endothelial nitric oxide (NO) synthase (eNOS) from caveolin-1, whereas PGI2 is synthesized by cyclooxygenase which acts on the arachidonic acid cleaved from membrane phospholipids by the Ca2+-dependent phospholipase A2 (PLA2) [3, 8].
Physiologically, extracellular autacoids bind to their cognate Gq-protein-coupled receptors (GqPCRs), thereby stimulating phospholipase Cβ (PLCβ) to cleave phosphatidylinositol 4,5-bisphosphate (PIP2), a minor (≈ 1%) membrane phospholipid, into the intracellular second messengers, inositol-1,4,5-trisphosphate (InsP3) and diacylglycerol (DAG) [2, 4]. InsP3, in turn, elicits massive Ca2+ release from the endoplasmic reticulum (ER) through InsP3 receptors (InsP3Rs), followed by Ca2+ influx via a store-operated Ca2+ entry (SOCE) pathway on the plasma membrane [9, 10]. Endothelial SOCE is mainly mediated by the physical interaction between STIM1, a sensor of ER Ca2+ concentration, and Orai1, which provides the pore-forming subunit of store-operated channels [11, 12]. Vascular endothelial cells also express the STIM and Orai paralogues, STIM2, Orai2, and Orai3 [11, 13, 14]. STIM2 is likely to trigger SOCE in STIM1-deficient endothelial cells [14], whereas Orai2 acts as a negative modulator of Orai1 [15], as recently demonstrated in other cell types [16, 17]. In addition, endogenous Ca2+ release may be supported by endolysosomal Ca2+ release through nicotinic acid adenine dinucleotide phosphate (NAADP)-gated two-pore channels 1 and 2 (TPC1-2), which trigger InsP3-induced ER Ca2+ mobilization through the Ca2+-induced Ca2+ release (CICR) process in response to extracellular stimulation [14, 18]. Endothelial Ca2+ signals control the vascular tone by driving NO release in response to a multitude of autacoids, including acetylcholine [19, 20], ATP [21], bradykinin [22], histamine [23], and thrombin [24], throughout peripheral circulation. Surprisingly, endothelial Ca2+ signaling has barely been regarded as an active participant in neurovascular coupling (NVC) [8, 25, 26], the mechanism by which neuronal activity induces vasorelaxation of cortical microvessels to redirect cerebral blood flow (CBF) to activated areas [27, 28].
NVC is crucial to maintain the homeostasis of the brain internal milieu and to sustain normal brain function; moreover, several vascular-based functional brain imaging techniques, such as functional magnetic resonance imaging (fMRI), rely on NVC to infer changes in neuronal activity [27–29]. Glutamate, the major excitatory neurotransmitter in the brain, triggers NVC by stimulating post-synaptic N-methyl-d-aspartate (NMDA) receptors (NMDARs) to mediate extracellular Ca2+ entry, thereby engaging the Ca2+/CaM-dependent neuronal NOS (nNOS) [27, 28, 30]. NO may directly stimulate vasorelaxation of adjacent microvessels in hippocampus and cerebellum [31, 32], while it permits the vasodilatory response to astrocyte-derived vasoactive mediators, such as epoxyeicosatrienoic acids (EETs) and prostaglandin E2 (PGE2), in the somato-sensory cortex [33–38]. Pharmacological blockade of group 1 metabotropic glutamate receptors (mGluRs), i.e., mGluR1 and mGluR5, which are GqPCRs coupled to PLCβ and InsP3-dependent Ca2+ release, also attenuates the hemodynamic response to sensory stimulation in vivo [39–42]. The earlier model according to which mGluRs were mainly located in perisynaptic astrocytes was later discounted by the discovery that mGluR5 downregulates to barely detectable levels in adult astrocytes and that the genetic deletion of type 2 InsP3R, the principal InsP3R isoform in glial cells, does not inhibit NVC [43–45]. Therefore, the exact mechanism whereby group 1 mGluRs control NVC remains unclear [27, 46]. Conversely, group 2 mGluRs include the mGluR2 and mGluR3, which are Gi/o coupled receptors and inhibit adenylate cyclase (AC). Finally, group 3 mGluRs comprise mGluR4, mGluR7, and mGluR8, which are also negatively coupled to AC, and mGluR6, which stimulates a cGMP phosphodiesterase [47]. Group 2 and group 3 mGluRs mainly exhibit a pre-synaptic location and inhibit neurotransmitter (glutamate or GABA) release [47]. Therefore, their role in NVC is less clear.
Intriguingly, a series of recent studies demonstrated that synaptically released glutamate could induce NO release directly from brain microvascular endothelial cells [48]. For instance, glutamate has been shown to elicit NO release within rodent brain microvasculature by activating endothelial NMDARs in cortical microvessels [49, 50] and group 1 mGluRs in mouse brain microvascular endothelial cells [51]. These results strongly support the observation that long-term synaptic plasticity requires endothelial-derived NO at the Schaffer collateral to CA1 synapse in mouse hippocampal slices [52], and that synaptic glutamate induces vascular NO release in response to whisker stimulation in the somato-sensory cortex in vivo [48]. It has recently been demonstrated that acetylcholine generates an intracellular Ca2+ signal which drives NO release in hCMEC/D3 cells, a widely employed human brain microvascular endothelial cell line [53]. Acetylcholine-induced NO synthesis was initiated by endogenous Ca2+ release through type 3 InsP3R (InsP3R3) and endolysosomal TPC1-2, was sustained by SOCE [53], and triggered a robust hemodynamic response in the somato-sensory cortex in vivo [48]. Early investigations reported that mGluR1 and mGluR5 are expressed in human brain microvascular cells [54] and in human meningeal microvasculature, as well as in the parenchymal microvasculature [55], but their functional role remains unclear.
Herein, we exploited a multidisciplinary approach to assess whether and how group 1 mGluRs induce Ca2+-dependent NO release in hCMEC/D3 cells. We provided the evidence that glutamate causes a dose-dependent increase in [Ca2+]i by activating mGluR1 and, at a larger extent, mGluR5. Glutamate-induced Ca2+ signal is supported by InsP3- and NAADP-dependent intracellular Ca2+ release and is prolonged by SOCE. Finally, the metabotropic Ca2+ response to glutamate leads to rapid NO release, which is abolished by inhibition of endogenous Ca2+ release. These findings reinforce the emerging view that brain microvascular endothelial cells may be recruited by neuronal activity to control NVC.
Materials and methods
Cell culture
Human brain endothelial cells (hCMEC/D3) were obtained from Institut National de la Santé et de la Recherche Médicale (INSERM, Paris, France). hCMEC/D3 cells cultured between passage 25 and 35 were used. As described in [53], the cells were seeded at a concentration of 27,000 cells/cm2 and grown in tissue culture flasks coated with 0.1 mg/mL rat tail collagen type 1, in the following medium: EBM-2 medium (Lonza, Basel, Switzerland) supplemented with 5% fetal bovine serum (FBS), 1% Penicillin–Streptomycin, 1.4 μM hydrocortisone, 5 μg/mL ascorbic acid, 1/100 chemically defined lipid concentrate (Invitrogen), 10 mM HEPES, and 1 ng/mL basic FGF (bFGF). The cells were cultured at 37 °C, 5% CO2 saturated humidity.
Solutions
Physiological salt solution (PSS) had the following composition (in mM): 150 NaCl, 6 KCl, 1.5 CaCl2, 1 MgCl2, 10 Glucose, 10 Hepes. In Ca2+-free solution (0Ca2+), Ca2+ was substituted with 2 mM NaCl, and 0.5 mM EGTA was added. Solutions were titrated to pH 7.4 with NaOH. In Mn2+-quenching experiments, 200 μM MnCl2 was added to the 0Ca2+ external solution. The osmolality of PSS as measured with an osmometer (Wescor 5500, Logan, UT) was 338 mmol/kg.
[Ca2+]i and NO measurements
We utilized the Ca2+ imaging set-up that we have described elsewhere [56]. hCMEC/D3 cells were loaded with 4 µM fura-2 acetoxymethyl ester (Fura-2/AM; 1 mM stock in dimethyl sulfoxide) in PSS for 1 h min at 37 °C and 5% CO2. After washing in PSS, the coverslip was fixed to the bottom of a Petri dish and the cells observed by an upright epifluorescence Axiolab microscope (Carl Zeiss, Oberkochen, Germany), usually equipped with a Zeiss × 40 Achroplan objective (water immersion, 2.0 mm working distance, 0.9 numerical aperture). The cells were excited alternately at 340 and 380 nm, and the emitted light was detected at 510 nm. A first neutral density filter (1 or 0.3 optical density) reduced the overall intensity of the excitation light and a second neutral density filter (optical density = 0.3) was coupled to the 380 nm filter to approach the intensity of the 340 nm light. A round diaphragm was used to increase the contrast. The excitation filters were mounted on a filter wheel (Lambda 10, Sutter Instrument, Novato, CA, USA). Custom software, working in the LINUX environment, was used to drive the camera (Extended-ISIS Camera, Photonic Science, Millham, UK) and the filter wheel, and to measure and plot on-line the fluorescence from 30 to 45 rectangular “regions of interest” (ROI) enclosing 20–30 single cells. Each ROI was identified by a number. Adjacent ROIs never superimposed. [Ca2+]i was monitored by measuring, for each ROI, the ratio of the mean fluorescence emitted at 510 nm when exciting alternatively at 340 and 380 nm [ratio (F340/F380)]. An increase in [Ca2+]i causes an increase in the ratio [14]. Ratio measurements were performed and plotted on-line every 3 s. The experiments were performed at room temperature (22 °C) [57].
NO was measured as described in [53]. Briefly, hCMEC/D3 cells were loaded with the membrane-permeable NO-sensitive dye 4-Amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) diacetate (10 µM) for 60 min at room temperature and washed in PSS for 15 min. DAF-FM fluorescence was measured using the same equipment described for Ca2+ recordings but with a different filter set, i.e., excitation at 480 nm and emission at 535 nm wavelength (emission intensity was shortly termed “NOi”). The changes in DAF-FM fluorescence induced by glutamate were recorded and plotted on-line every 5 s. Again, off-line analysis was performed using custom-made macros developed by Microsoft Office Excel software. The experiments were performed at room temperature (22 °C). DAF-FM fluorescence remained constant during 1 h recording at the sampling rate and light intensity employed in the present investigation (not shown).
RNA isolation and real-time RT-PCR (qRT-PCR)
Total RNA was extracted from hCMEC/D3 cells using the QIAzol Lysis Reagent (QIAGEN, Italy). Reverse transcription and qRT-PCR were performed as previously described [14] using specific primers (intron-spanning primers). The specific intron-spanning primers and the molecular weight of the amplicon (in parentheses) were indicated below: mGluR1, sense, 5′-GTCCACACGGAAGGGAATTATG-3′; antisense, 5′-GAGTTTGCGCAAGAGTCGGT-3′ (144 bp); mGluR5, sense, 5′-GCACACAGAAGGCAACTATG-3′; antisense, 5′-TTGGGCAAGTGACTTGTGAG-3′ (159 bp); B2 M, Hs_B2M_1_SG QuantiTect Primer Assay QT00088935 (Qiagen, Italia) (98 bp).
The qRT-PCR reactions were normalized using β-2-microglobulin (B2 M) as housekeeping gene. The triplicate threshold cycle (Ct) values for each sample were averaged resulting in mean Ct values for both the gene of interest and the housekeeping genes. The gene Ct values were then normalized to the housekeeping gene by taking the difference: ΔCt = Ct[gene] − Ct[housekeeping], with high ΔCt values reflecting low mRNA expression levels. Melting curves were generated to detect the melting temperatures of specific products immediately after the PCR run. The molecular weight of the PCR products was compared to the DNA molecular weight marker VIII (Roche Molecular Biochemicals, Italy).
Immunoblotting
Cells were homogenized using a Dounce homogenizer in a solution containing: 250 mM Sucrose, 1 mM EDTA, 10 mM Tris–HCl, pH 7.6, 0.1 mg/ml PMSF, 100 mM β-mercaptoethanol, protease, and phosphatase inhibitor cocktails (P8340 and P5726, P0044, Sigma-Aldrich Inc.). 30 μg of solubilized proteins were subjected to 7.5% SDS-polyacrylamide gel electrophoresis and blotted to the Hybond-P PVDF Membrane (GE Healthcare, Italy). Membranes were blocked for 1 h with Tris-buffered saline (TBS) containing 3% BSA and 0.1% Tween (blocking solution) and then incubated overnight at 4 °C with the following antibodies diluted in the TBS and 0.1% Tween: anti-mGluR1 (AGC-006; 1: 200, dilution), anti-mGluR5 (AGC-007; 1:200, dilution) from Alomone labs, Jerusalem BioPark (JBP), Jerusalem, Israel. After 3 washing with TBS and 0.1% Tween, membranes were incubated for 1 h with goat anti-rabbit IgG antibody, peroxidase conjugated (AP132P, Millipore part of Merck S.p.a., Vimodrone, Italy), diluted 1:10,000 in blocking solution. The bands were detected with ECL™ Select western blotting detection system (GE Healthcare Europe GmbH, Italy). Prestained molecular weight markers (ab116028, Abcam, Cambridge, UK) were used to estimate the molecular weight of the bands. Blots were stripped with the method of Yeung and Stanley [58] and re-probed with anti β-2-microglobulin antibody (B2 M) (Abcam) as housekeeping. The antibody was diluted 1:10,000 in blocking solution.
Protein content
Protein contents of all the samples were determined by the Bradford’s method [59] using bovine serum albumin (BSA) as standard.
Statistics
All the data have been collected from hCMEC/D3 cells deriving from at least three coverslips from three independent experiments. The amplitude of Ca2+ and NO signals induced by each agonist was measured as the difference between the ratio at the peak of intracellular Ca2+ mobilization and the mean ratio of 1 min baseline before the peak. Pooled data are given as mean ± SE and statistical significance (P < 0.05) was evaluated by the Student’s t test for unpaired observations as indicated. Data are presented as mean ± SE, while the number of cells analysed is indicated within/above the histogram bars.
Chemicals
Fura-2/AM and DAF-FM were obtained from Molecular Probes (Molecular Probes Europe BV, Leiden, The Netherlands). (RS)-α-methyl-4-carboxyphenylglycine (MCPG) was supplied by Abcam Biochemicals (Cambridge, UK). CPCCOEt, MTEP hydrochloride (MTEP), CHPG, YM-58483/BTP-2, and NED-19 were purchased from Tocris (Bristol, UK). All the chemicals were of analytical grade and obtained from Sigma Chemical Co. (St. Louis, MO, USA).
Results
Glutamate induces a dose-dependent increase in [Ca2+]i in hCMEC/D3 cells
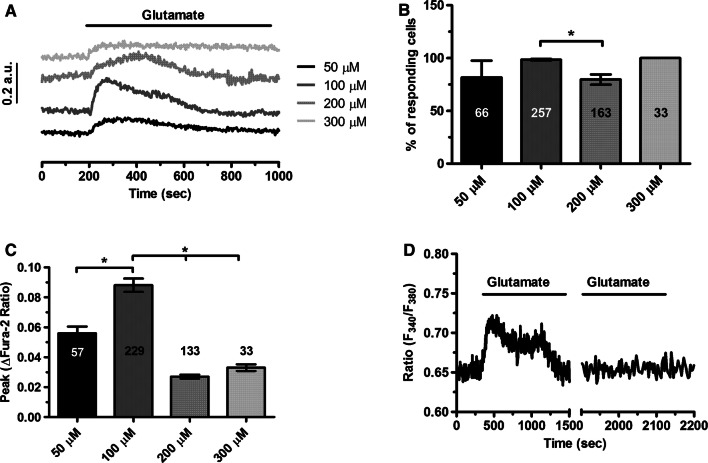
To assess whether glutamate induces intracellular Ca2+ signals, hCMEC/D3 cells were loaded with the Ca2+-sensitive fluorochrome, Fura-2/AM, as shown in [53]. Unlike bEND5 cells, a mouse brain microvascular endothelial cell line [51], hCMEC/D3 did not exhibit any spontaneous Ca2+ activity in the absence of external stimulation (data not shown). The extracellular application of glutamate induced a discernible increase in [Ca2+]i which consisted in an initial Ca2+ peak followed by a plateau level of intermediate amplitude above resting [Ca2+]i (Fig. 1a). Thereafter, the Ca2+ signal declined to the baseline despite for the continuous presence of the agonist in the bath (Fig. 1a). Glutamate (100 µM) failed to induce an additional increase in [Ca2+]i upon 15 min washout (Fig. 1d), which is indicative of receptor desensitization. The percentage of responding cells did not significantly change throughout the concentration range that we probed (Fig. 1a, b), i.e., 50–300 µM, but the peak Ca2+ response was attained at 100 µM as the Ca2+ signal desensitized at higher doses (Fig. 1a, c). Overall, these data demonstrate for the first time that glutamate is able to increase the [Ca2+]i in a human model of brain microvascular endothelial cells at physiological doses [60, 61]. As 100 µM proved to be the most effective dose to induce the glutamate-evoked Ca2+ signal, we employed this concentration throughout the remainder of the investigation.
Fig. 1.
Glutamate evokes a dose-dependent increase in [Ca2+]i in hCMEC/D3 cells. a Glutamate caused a dose-dependent increase in [Ca2+]i which achieved its peak at 100 M. b Mean ± SE of the percentage of hCMEC/D3 cells displaying glutamate-induced Ca2+ responses at different agonist concentrations (from 50 to 300 µM). The asterisk indicates p < 0.05. c Mean ± SE of the amplitude of glutamate-induced Ca2+ responses measured in hCMEC/D3 cell at different agonist concentrations (from 50 to 300 µM). The asterisk indicates that p < 0.05. d Glutamate (100 µM) failed to induce an additional increase in [Ca2+]i upon 15 min washout which is indicative of receptor desensitization
mGluR1 and mGluR5 trigger the Ca2+ response to glutamate in hCMEC/D3 cells
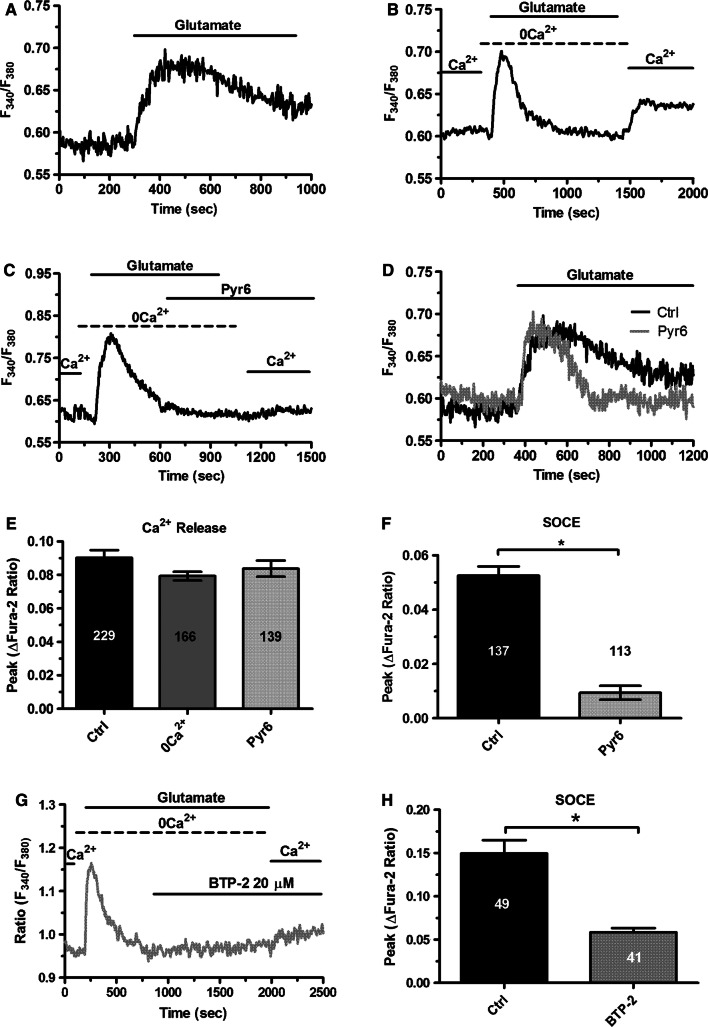
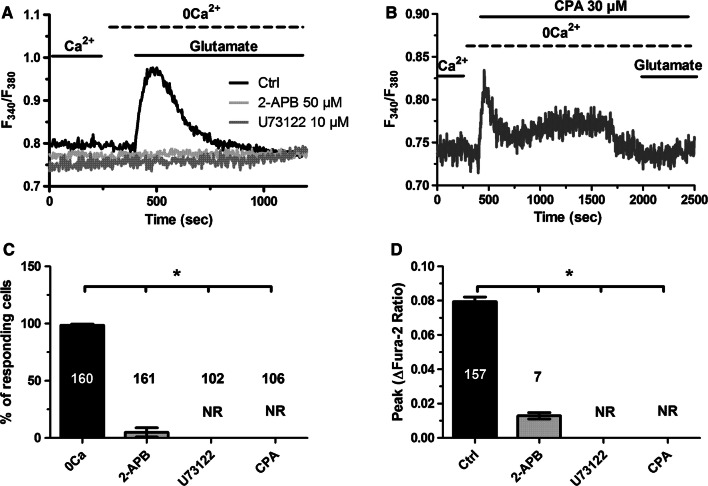
The Ca2+ response to glutamate described in Fig. 1 was recorded in the absence of extracellular glycine or d-serine, which unmask endothelial NMDAR activation in mouse middle cerebral arteries [49, 50]. Group 1 mGluRs represent, therefore, the most suitable target for glutamate to induce Ca2+ signaling in hCMEC/D3 cells. To corroborate this hypothesis, we first challenged hCMEC/D3 cells with glutamate (100 µM) in the absence of extracellular Ca2+ (0Ca2+). As shown in Fig. 2a–c, removal of extracellular Ca2+ did not affect the initial Ca2+ peak, as it would be expected in the case of NMDAR activation [62], although it curtailed the duration of the Ca2+ response. This finding strongly suggests that glutamate-evoked Ca2+ signals are initiated by group 1 mGluRs, which are coupled to Gq and able to engage PLCβ, thereby inducing InsP3-dependent Ca2+ release from the ER [47, 63]. The subsequent re-addition of extracellular Ca2+, in the absence of glutamate to prevent the opening of receptor-operated channels, resulted in a second increase in [Ca2+]i (Fig. 2b), which was indicative of SOCE recruitment [51, 53, 56]. Accordingly, as glutamate was removed from the bath 100 s before restoration of extracellular Ca2+ levels, the only physiological stimulus responsible for Ca2+ entry was ER Ca2+ store depletion. As widely discussed elsewhere [64, 65], neither ionotropic receptors, i.e., NMDARs [49, 50], nor second messenger-operated channels, e.g., Transient Receptor Potential (TRP) Vanilloid 4 (TRPV4) [26], can be gated in the absence of agonist binding to their cognate receptors. In addition, hCMEC/D3 cells express very low levels of TRP Canonical 7 (TRPC7) channel, which is gated by DAG [14]. However, 1-oleoyl-2-acetyl-sn-glycerol (OAG), a membrane-permeable analogue of DAG, failed to induce sizeable Ca2+ signals in hCMEC/D3 cells [14]. This finding has been confirmed in Supplementary Figure 1A. Collectively, these pieces of evidence strongly support the view that SOCE mediates glutamate-induced extracellular Ca2+ entry in hCMEC/D3 cells. A previous report demonstrated that hCMEC/D3 cells express STIM2, but not STIM1, as well as all Orai isoforms. However, SOCE was sensitive to Pyr6 [14], which is a selective Orai1 inhibitor [66–68]. These data, therefore, strongly support the notion that SOCE is mediated by STIM2 and Orai1 in hCMEC/D3 cells. In agreement with this model, glutamate-induced extracellular Ca2+ entry was suppressed by Pyr6 (10 µM, 10 min) (Fig. 2c, f). In addition, Pyr6 (10 µM, 10 min) curtailed the Ca2+ response to glutamate without affecting the initial peak (Fig. 2d, e), thereby mimicking the Ca2+ signal recorded in the absence of extracellular Ca2+. To further support the involvement of Orai1 in glutamate-evoked Ca2+ entry, we probed the effect of two other specific Orai1 inhibitors, S66 [69, 70] and BTP-2 [69, 71]. Unfortunately, S66 (10 μM) induced intracellular Ca2+ oscillations even in the absence of extracellular Ca2+ (0Ca2+) (Supplementary Figure 2), which might reflect previously unreported off-target effects. Conversely, BTP-2 (10 μM) did not elicit any increase in [Ca2+]i (Supplementary Figure 3B). Our preliminary experiments confirmed that BTP-2 (10 μM, 20 min) suppressed SOCE induced by previous depletion of the ER Ca2+ pool with CPA (10 μM) (Supplementary Figure 3). Furthermore, BTP-2 (10 μM, 20 min) also inhibited glutamate-evoked Ca2+ entry (Fig. 2g, h), which reinforces the hypothesis that Orai1 mediates glutamate-dependent SOCE in hCMEC/D3 cells.
Fig. 2.
The Ca2+ response to glutamate requires endogenous Ca2+ release and Orai1-mediated Ca2+ entry. a Glutamate (100 µM) induced a biphasic increase in [Ca2+]i in the presence of extracellular Ca2+ in hCMEC/D3 cells. b Removal of extracellular Ca2+ (0Ca2+) did not affect the initial Ca2+ peak, although it curtailed the duration of the Ca2+ response. Restoration of extracellular Ca2+ upon removal of glutamate resulted in a second bump in [Ca2+]i, which was indicative of SOCE. c Pyr6 (10 µM, 10 min), a selective inhibitor of Orai1, prevented glutamate-induced Ca2+ entry in hCMEC/D3 cells. d Preincubating the cells with Pyr6 (10 µM, 10 min) did not affect the magnitude of the Ca2+ response to glutamate, but curtailed the plateau phase. e Bar histogram shows the mean ± SE of the amplitude of the Ca2+ release in control (Ctrl) cells, under 0Ca2+ condition and upon treatment with Pyr6 (10 µM, 10 min). f Bar histogram shows the mean ± SE of SOCE amplitude in control cells and upon treatment with Pyr6 (10 µM, 10 min). g BTP-2 (20 µM, 20 min), a selective inhibitor of Orai1, prevented glutamate-induced Ca2+ entry in hCMEC/D3 cells. h Bar histogram shows the mean ± SE of SOCE amplitude in control (Ctrl) cells and upon treatment with BTP-2 (20 µM, 20 min)
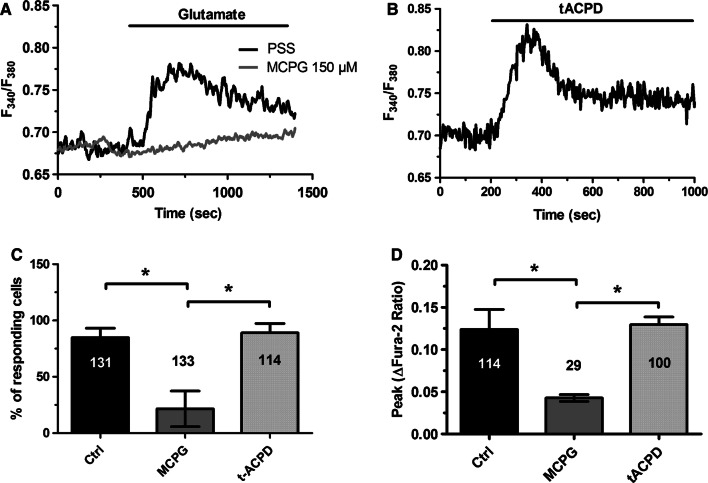
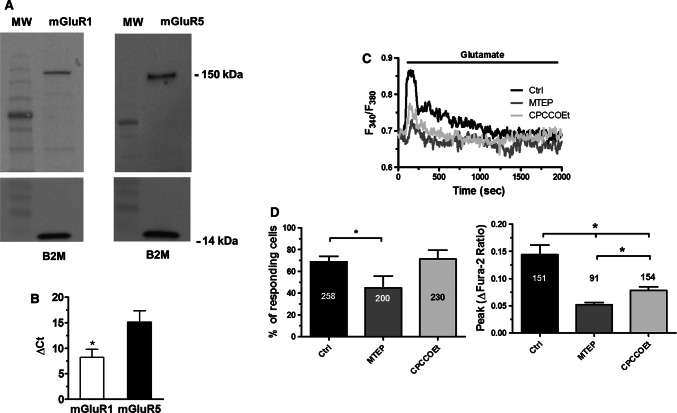
Taken together, these results indicate that group 1 mGluRs drive the Ca2+ response to glutamate in hCMEC/D3 cells. In agreement with this hypothesis, we next found that glutamate-evoked Ca2+ signals were abolished by MCPG (150 µM, 20 min) (Fig. 3a, c, d), a broad-spectrum group 1 mGluR antagonist [32, 51, 62]. Conversely, the Ca2+ response to glutamate was phenocopied by trans-1-amino-1,3-cyclopentanedicarboxylic acid (trans-ACPD or tACPD; 100 µM), a selective agonist of group 1 mGluRs [72] (Fig. 3b–d). In agreement with these observations, Western blot analysis confirmed that both mGluR1 and mGluR5 proteins were broadly expressed in hCMEC/D3 cells. Immunoblots showed a major band of about 150 kDa for both mGluR1 and mGluR5 (Fig. 4a), which is in the size range indicated by the manufacturer. Moreover, qRT-PCR analysis, carried out using the specific primers described in Materials and methods, found that both mGluR1 and mGluR5 transcripts were expressed in hCMEC/D3 cells, although the latter was less abundant (Fig. 4b). In agreement with these recordings, the Ca2+ response to glutamate (100 µM) was sensitive to both CPCCOEt (100 µM, 10 min) and MTEP (100 µM, 10 min) (Fig. 4c), which, respectively, block mGluR1 and mGluR5 [39–42]. Accordingly, MTEP significantly (p < 0.05) reduced the percentage of responding cells (Fig. 4d, left panel) and the peak Ca2+ response (Fig. 4d, right panel), whereas CPCCOEt only attenuated the amplitude of the initial Ca2+ peak (Fig. 4d). Notably, the inhibitory effect of MTEP was slightly stronger as compared to CPCCOEt (Fig. 4d, right panel). To confirm the hypothesis that mGluR5 was tightly coupled to intracellular Ca2+ signalling in hCMEC/D3 cells, we exploited the selective mGluR5 agonist CHPG [73]. As shown in Supplementary Figure 4, CHPG (25 μM) induced an increase in [Ca2+]i in 65 out of 65 hCMEC/D3 cells. Unfortunately, no specific mGluR1 agonist is available and we could not assess mGluR1 capability to elicit intracellular Ca2+ signals in hCMEC/D3 cells. Taken together, these findings demonstrate that mGluR1 and mGluR5 mediate glutamate-evoked Ca2+ signals in hCMEC/D3 cells.
Fig. 3.
The Ca2+ response to glutamate is mediated by metabotropic glutamate receptors (mGluRs). a Glutamate (100 μM) caused a rapid increase in [Ca2+]i which was inhibited by MCPG (150 µM, 20 min), a broad-spectrum group 1 mGluR antagonist. b Trans-ACPD (100 μM), a selective agonist of group 1 mGluRs, mimicked the Ca2+ response to glutamate. c Bar histogram shows the mean ± SE of the percentage of responding cells under the designated treatments. The asterisk indicates p < 0.05. d Bar histogram shows the mean ± SE of the amplitude of the Ca2+ response under the designated treatments. The asterisk indicates p < 0.05
Fig. 4.
The Ca2+ response to glutamate is mediated by mGluR1 and mGluR5. a Expression of mGluR1 and mGluR5 proteins in hCMEC/D3 cells. Blots representative of four independent experiments were shown. Lanes were loaded with 30 µg of proteins, probed with affinity purified antibodies, and processed as described in “Materials and methods”. The same blots were stripped and re-probed with anti-beta-2-microglobulin (B2M) polyclonal antibody, as housekeeping. Major bands of the expected molecular weights were indicated. b Expression of mGluR1 and mGluR5 transcripts in hCMEC/D3 cells. Reverse transcription polymerase chain reaction of total RNA was performed using specific primers as indicated in Materials and methods. c Glutamate (100 μM) caused a rapid increase in [Ca2+]i which was reduced by CPCCOEt (100 µM, 10 min) and MTEP (100 µM, 10 min), which, respectively, block mGluR1 and mGluR5. d Left panel, bar histogram shows the mean ± SE of the percentage of responding cells in control conditions and upon treatment with MTEP (100 µM, 10 min) and CPCCOEt (100 µM, 10 min). Right panel, bar histogram shows the mean ± SE of the amplitude of the response under the designated treatments. The asterisk indicates that p < 0.05
The PLCβ/InsP3 signalling pathway sustains the intracellular Ca2+ response to glutamate in hCMEC/D3 cells
As anticipated earlier, glutamate-induced endogenous Ca2+ release in the absence of extracellular Ca2+ is likely to be supported by ER-embedded InsP3Rs, as mGluR1 and mGluR5 are coupled to Gq [47, 63]. In agreement with this hypothesis, the intracellular Ca2+ response to glutamate (100 µM) was suppressed by U73122 (10 µM, 30 min) (Fig. 5a, c, d), an aminosteroid which selectively blocks PLC in brain microvascular endothelial cells [51, 53, 56], and by 2-aminoethoxydiphenyl borate (2-APB; 50 µM, 30 min), which selectively inhibits InsP3Rs in the absence of extracellular Ca2+ at this concentration [74, 75] (Fig. 5a, c, d). In addition, glutamate-induced endogenous Ca2+ release was abrogated by depleting the ER Ca2+ store with cyclopiazonic acid (CPA; 10 µM), which is widely employed to impair Sarco-Endoplasmic Reticulum Ca2+-ATPase (SERCA) activity. As reported elsewhere [53], CPA caused a transient increase in [Ca2+]i, which was due to passive Ca2+ efflux through ER leakage channel followed by Ca2+ removal from the cytosol (Fig. 5b). The subsequent addition of glutamate (100 µM) failed to induce any detectable elevation in [Ca2+]i due to previous emptying of the ER Ca2+ store. Taken together, these data confirmed that the PLCβ/InsP3 signalling pathway sustains glutamate-induced endogenous Ca2+ release in hCMEC/D3 cells, as suggested by the role of mGluR1 and mGluR5 in the onset of the signal.
Fig. 5.
Glutamate-induced endogenous Ca2+ mobilization requires ER-dependent Ca2+ release through InsP3Rs. a The Ca2+ response to glutamate (100 µM) was inhibited by U73122 (10 µM, 30 min), a selective PLC blocker. Moreover, the Ca2+ signal was inhibited by blocking InsP3Rs with 2-APB (50 µM, 30 min). b Emptying the ER Ca2+ pool with CPA (10 µM), a selective SERCA inhibitor, prevented the Ca2+ response to glutamate. As expected, CPA elicited a transient elevation in [Ca2+]i due to the passive depletion of the ER Ca2+ pool followed by Ca2+ clearing through the plasma membrane and by mitochondria. c Bar histogram shows the mean ± SE of the percentage of responding cells under the designated treatments. The asterisk indicates that p < 0.05. d Bar histogram shows the mean ± SE of the amplitude of the Ca2+ response under the designated treatments. The asterisk indicates that p < 0.05. NR no response
NAADP-induced Ca2+ mobilization contributes to glutamate-induced intracellular Ca2+ release in hCMEC/D3 cells
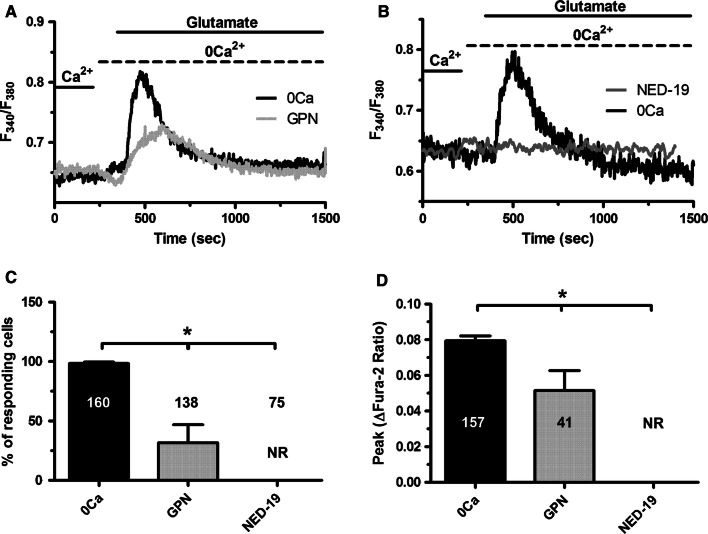
We recently reported that acetylcholine-induced intracellular Ca2+ release in hCMEC/D3 cells was supported by NAADP-dependent EL Ca2+ mobilization through TPC1-2 [53]. Likewise, Glycyl-l-phenylalanine 2-naphthylamide (GPN; 200 µM), a cathepsin C substrate that mobilizes lysosomal Ca2+ by osmotic rupture of the acidic vesicles [76, 77], caused a transient increase in [Ca2+]i in the absence of extracellular Ca2+ (0Ca2+), thereby preventing the subsequent glutamate-induced endogenous Ca2+ release (Fig. 6a, c, d). Furthermore, the intracellular Ca2+ response to glutamate (100 µM) was prevented by NED-19 (100 µM, 30 min) (Fig. 6b–d), a selective TPC1-2 antagonist [78, 79]. Collectively, these findings demonstrated that NAADP-gated EL TPC1-2 contribute to glutamate-induced endogenous Ca2+ release in hCMEC/D3 cells.
Fig. 6.
NAADP-induced intracellular Ca2+ mobilization contributes to glutamate-induced endogenous Ca2+ release in hCMEC/D3 cells. a GPN (200 μM), a lysosomotropic agent that is widely used to deplete the EL Ca2+ store, prevented the Ca2+ response to glutamate (100 μM). b Glutamate-induced increase in [Ca2+]i in the absence, but not in the presence, of NED-19 (100 μM, 30 min), a selective TPC inhibitor. Glutamate was administered at 100 μM. c Bar histogram shows the mean ± SE of the percentage of responding cells under the designated treatments. The asterisk indicates p < 0.05. d Bar histogram shows the mean ± SE of the amplitude of the Ca2+ response under the designated treatments. The asterisk indicates p < 0.05. NR no response
Glutamate-induced intracellular Ca2+ signaling drives NO release in hCMEC/D3 cells
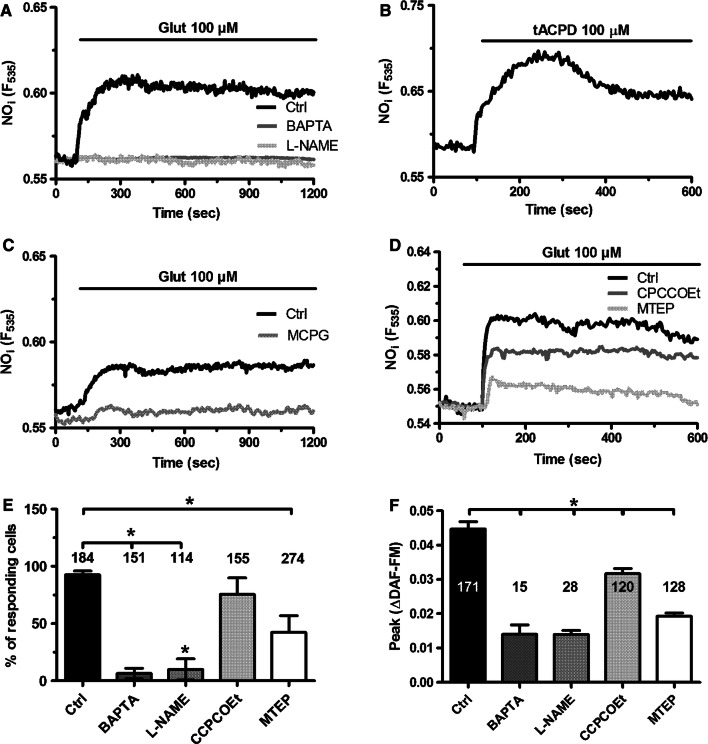
To assess whether and how glutamate induces Ca2+-dependent NO release, we loaded hCMEC/D3 cells with the NO-sensitive fluorophore, DAF-FM, as described in [53]. Glutamate (100 µM) caused an immediate increase in DAF-FM fluorescence that was inhibited by pretreating the cells with L-NAME (100 µM, 1 h) (Fig. 7a), a widely employed NOS inhibitor, or BAPTA (30 µM, 2 h) (Fig. 7a), a membrane-permeant buffer of intracellular Ca2+ levels [51, 53]. Furthermore, glutamate-induced NO release was phenocopied by tACPD (100 µM) (Fig. 7b) and blocked by MCPG (150 µM, 20 min) (Fig. 7c). In further agreement with the Ca2+ imaging data, MTEP (100 µM, 10 min) and CPCCOEt (100 µM, 10 min) significantly (p < 0.05) reduced NO production in hCMEC/D3 cells challenged with glutamate (Fig. 7d). The statistical analysis of NO release under each of these conditions is presented in Fig. 7e, f. These data, therefore, demonstrate that mGluR1 and mGluR5 drive NO release by recruiting eNOS in a Ca2+-dependent manner also in human brain microvascular endothelial cells.
Fig. 7.
Glutamate-induced NO release in hCMEC/D3 cells through the stimulation of mGluR1 and mGluR5. a Glutamate (100 µM) caused a robust increase in DAF/FM fluorescence in hCMEC/D3 cells, that was strongly reduced by either L-NAME (100 µM, 2 h), an inhibitor of NO synthase, or BAPTA (30 µM, 2 h), a membrane-permeable intracellular Ca2+ chelator. b Glutamate induced a massive increase in NO production, which was inhibited by MCPG (150 µM, 20 min). cTrans-ACPD (100 µM) induced robust NO release in hCMEC/D3 cells, thereby phenocopying the response to glutamate. d Glutamate-induced NO release was reduced by CPCCOEt (100 µM, 10 min) and MTEP (100 µM, 10 min). e Bar histogram shows the mean ± SE of the percentage of responding cells under the designated treatments. The asterisk indicates p < 0.05 as compared to control cells. f Bar histogram shows the mean ± SE of the amplitude of the response under the designated treatments. The asterisk indicates that p < 0.05 as compared to control cells
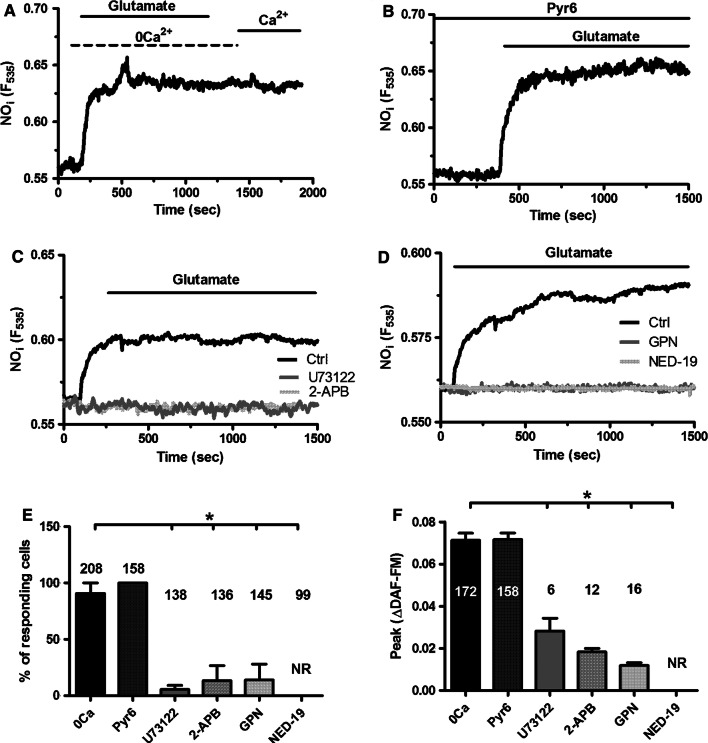
Subsequently, we found that glutamate-induced NO release occurred also in the absence of extracellular Ca2+ (0Ca2+), whereas Ca2+ restitution to the perfusate did not cause any detectable increase in DAF-FM fluorescence (Fig. 8a). Furthermore, pharmacological blockade of SOCE with Pyr6 (10 µM, 10 min) did not reduce glutamate-induced NO release (Fig. 8b). Likewise, suppressing SOCE with BTP-2 (20 μM, 20 min) did not affect glutamate-induced NO production (Supplementary Figure 5). As expected, OAG failed to increase DAF-FM fluorescence in hCMEC/D3 cells (Supplementary Figure 1B–D). Furthermore, glutamate-induced NO release was not affected by simultaneously blocking SOCE with Pyr6 (10 μM, 10 min) and TRPC7 with La3+ (100 μM, 20 min) (Supplementary Figure 6). These data strongly suggest that glutamate drives NO production through the endogenous Ca2+ release. Accordingly, glutamate failed to increase DAF-FM fluorescence in the presence of U73122 (10 µM, 30 min) and 2-APB (50 µM, 30 min) (Fig. 8c). Moreover, glutamate-induced NO release was abrogated following depletion of the EL Ca2+ pool with GPN (200 µM, 30 min) (Fig. 8d) and upon pharmacological blockade of TPC1-2 channels with NED-19 (100 µM, 30 min) (Fig. 8d). Taken together, these findings demonstrated that InsP3 and NAADP sustain glutamate-induced NO release in hCMEC/D3 cells. The statistical analysis of these data has been reported in Fig. 8e, f.
Fig. 8.
Glutamate-induced intracellular Ca2+ signaling drives NO release in hCMEC/D3 cells. a Glutamate-induced NO release was not prevented by removal of extracellular Ca2+ (0Ca2+). Glutamate was administered at 100 μM. b Glutamate-induced NO release was not prevented by SOCE inhibition with Pyr6 (10 µM, 10 min). Glutamate was administered at 100 μM. c Glutamate-induced NO release was abolished by U73122 (10 µM, 30 min) and by 2-APB (50 µM, 30 min). Glutamate was administered at 100 μM. d Glutamate-induced NO release was inhibited by GPN (200 μM) and NED-19 (100 μM, 30 min). Glutamate was administered at 100 μM. e Bar histogram shows the mean ± SE of the percentage of responding cells under the designated treatments. The asterisk indicates p < 0.05. f Bar histogram shows the mean ± SE of the amplitude of the response under the designated treatments. The asterisk indicates that p < 0.05. NR no response
Discussion
Herein, we showed for the first time that glutamate induces a transient increase in [Ca2+]i in human brain microvascular endothelial cells. The Ca2+ response to glutamate is triggered by mGluR1 and mGluR5, initiated by endogenous Ca2+ release driven by the Ca2+ releasing messengers, InsP3 and NAADP, and sustained by SOCE. Glutamate-induced intracellular Ca2+ signalling, in turn, causes a robust NO release, which could play a key role in the slower component of NVC. These data, therefore, lend further support to the emerging notion that neuronal activity may be sensed by perisynaptic microvessels [38, 42, 49, 80, 81] and that brain microvascular endothelial cells fulfil a crucial function in the hemodynamic response to synaptic activity [8, 29].
mGluR1 and mGluR5 trigger the Ca2+ response to glutamate in hCMEC/D3 cells
Early studies demonstrated that group 1 mGluRs mediate glutamate-induced decrease in blood–brain barrier (BBB) permeability by inducing the dephosphorylation of vasodilator-stimulated phosphoprotein (VASP) [54]. Notably, disassembly of adherent junctions between adjacent vascular endothelial cells may also be triggered by endothelial Ca2+ signals [82]. A recent investigation demonstrated that glutamate evokes metabotropic Ca2+ signals in mouse brain microvascular endothelial cells, thereby resulting in massive NO production [51]. Therefore, we decided to assess whether group 1 mGluRs were expressed and able to increase the [Ca2+]i in the human cerebrovascular endothelial cell line hCMEC/D3 [83–86]. Glutamate induced a dose-dependent increase in [Ca2+]i in hCMEC/D3 cells, which attained a peak at 100 µM and consisted in a rapid Ca2+ transient which then declined to a plateau level before returning to the baseline. This Ca2+ waveform was strikingly different from the repetitive [Ca2+]i oscillations induced by glutamate in bEND5 cells [51], as more widely illustrated below, and was indicative of receptor desensitization during the prolonged exposure to the agonist. Accordingly, the Ca2+ response to glutamate did not resume upon 15 min of washout. The following pieces of evidence indicate that glutamate-evoked Ca2+ signals in hCMEC/D3 cells are triggered by group 1 mGluRs. First, NMDARs-induced Ca2+ entry in microvascular endothelial cells cannot be elicited by physiological doses of glutamate, such as those employed in the present investigation [60, 61], in the absence of its co-agonists d-serine or glycine [49, 81]. Second, the Ca2+ response to glutamate arose in the absence of extracellular Ca2+, i.e., a condition which prevents NMDAR signaling [62], whereas GqPCRs are still able to release endogenous Ca2+ in an InsP3-dependent manner [51, 53]. Third, glutamate-induced increase in [Ca2+]i was inhibited by MCPG and phenocopied by tACPD, which, respectively, inhibit [32, 51, 62] and activate [72] group 1 mGluRs. Fourth, mGluR1 and mGluR5 transcripts and proteins were expressed in hCMEC/D3 cells, as previously demonstrated in primary human brain microvascular endothelial cells [54] and in human cortical microvessels [55]. Moreover, pharmacological blockade of mGluR1 and mGluR5 with CPCCOEt and MTEP, respectively, impaired the Ca2+ response to glutamate, although only MTEP significantly reduced also the percentage of responding cells. In addition, the extent of inhibition of the Ca2+ response to glutamate by MTEP was larger as compared to CPCCOEt. These observations led us to conclude that mGluR1 and mGluR5 drive glutamate-induced elevation in [Ca2+]i in hCMEC/D3 cells, although the contribution of mGluR5 is seemingly larger. Accordingly, specific mGluR5 activation with CHPG induced a Ca2+ signal in 100% of the recorded cells. Intriguingly, mGluR5 represents also the main isoform whereby glutamate triggers intracellular Ca2+ waves in rodent astrocytes both in vitro and in vivo [36, 63, 87]. Despite the fact that mGluR5 are less expressed as compared to mGluR1 transcripts in hCMEC/D3 cells, the finding that mGluR5 plays a pivotal role in the Ca2+ response to glutamate is not surprising. Accordingly, it has long been known that, when mGluR1 and mGluR5 are co-expressed in brain neurons, mGluR1 induces lower PIP2 hydrolysis, and, therefore, InsP3-dependent signaling, as compared to mGluR5 [88, 89]. In addition, recent work provided the evidence that only small clusters of InsP3Rs located beneath the plasma membrane are licensed to respond to extracellular stimuli [90]. One could speculate that most of these InsP3R clusters are packed in close proximity of mGluR5 rather than mGluR1 in hCMEC/D3 cells. Therefore, the desensitization of the Ca2+ signal occurring at higher doses of glutamate and during prolonged stimulation could be ascribed either to the prolonged phosphorylation of the intracellular COOH-terminus at position Ser839 of mGluR5 [91], which is the major receptor isoform involved in the onset of the signal, or the receptor internalization by G protein-coupled receptor kinase 2 [92], as observed in other brain cell types.
The role of InsP3, NAADP, and SOCE in glutamate-induced Ca2+ signals
The following pieces of evidence indicate that the Ca2+ response to glutamate is supported by InsP3- and NAADP-dependent intracellular Ca2+ release and prolonged by SOCE. First, glutamate-induced Ca2+ signals were abrogated by U73122, a selective PLC blocker, and by 2-APB, which specifically targets InsP3Rs under the conditions employed in the present investigation. Accordingly, the effect of 2-APB, which could also target Orai and TRP channels at 50 μM [67], has been probed upon removal of extracellular Ca2+, when extracellular Ca2+ entry cannot occur. InsP3R3 presents the lowest affinity to InsP3 and Ca2+ as compared to InsP3R1 and InsP3R2 and lacks the Ca2+-induced inhibition observed at high Ca2+ concentrations nearby the receptor [93, 94]. Therefore, InsP3R3 functions as anti-oscillatory unit and maintains transient Ca2+ signatures [93, 94]. Conversely, bEND5 cells express InsP3R1 and InsP3R2, while they lack InsP3R3: this subtle difference in the Ca2+ toolkit could explain why glutamate initiates long-last intracellular Ca2+ oscillations in this cell type [51]. Second, depletion of the ER Ca2+ pool with CPA fully suppressed the intracellular Ca2+ response to glutamate. Third, glutamate-induced increase in [Ca2+]i was eradicated by depleting the EL Ca2+ pool with GPN and upon pharmacological blockade of TPC1-2 with NED-19. Notably, NAADP and InsP3 interact to sustain the endogenous Ca2+ response to glutamate also in rodent hippocampal neurons [95] and astrocytes [96] and in mouse brain microvascular endothelial cells [51]. Moreover, NAADP and InsP3 also cooperate to trigger acetylcholine-induced Ca2+ and NO release in hCMEC/D3 cells [53] and to trigger the endothelial Ca2+ activity induced by multiple agonists throughout the vascular bed [24, 97, 98]. According to the so-called “trigger hypothesis”, extracellular stimuli evoke NAADP-mediated spatially restricted EL Ca2+ signals which are then globalized into a cytosolic Ca2+ wave by the recruitment of juxtaposed InsP3Rs through the Ca2+-induced Ca2+ release (CICR) process [76, 99]. RyRs, which are also engaged by NAADP-dependent EL Ca2+ release [99], are absent in hCMEC/D3 cells [53], and are not involved in the Ca2+ response to glutamate. The peak Ca2+ signal was not affected in the absence of extracellular Ca2+, although its duration was remarkably curtailed (see Fig. 2a, b). These effects were mimicked by Pyr6, thereby suggesting that SOCE was engaged by ER Ca2+ depletion to prolong glutamate-induced increase in [Ca2+]i. The role of SOCE was further supported by the evidence that glutamate-induced extracellular Ca2+ entry was sensitive to BTP-2. Moreover, glutamate-induced extracellular Ca2+ entry could arise upon ER Ca2+ depletion and in the absence of the agonist from the bath, which suggests that Ca2+ influx does not require ligand or second messenger binding to the Ca2+ permeable pathway [64, 65]. We, therefore, hypothesize that SOCE prolongs the duration of the Ca2+ response to glutamate, as observed when hCMEC/D3 cells are challenged with acetylcholine [53] and in vascular endothelial cells upon GqPCR stimulation [9, 11]. Our previous work provided the evidence that SOCE was mediated by STIM2 and Orai1 in hCMEC/D3 cells, as STIM1 was not expressed [53] and Pyr6 is regarded as a selective Orai1 blocker [66, 67]. Herein, we further showed that glutamate-evoked Ca2+ entry is sensitive to BTP-2, another established Orai1 inhibitor [69, 71]. Indeed, although BTP-2 may also target TRPC5 [100], this channel is not expressed in hCMEC/D3 cells [14]. Previous work also showed that hCMEC/D3 cells express Orai2 and Orai3 [14]. However, Orai2 has been shown to serve as negative modulator of Orai1 in brain microvascular endothelial cells [15], a finding that has been confirmed also in mouse enamel cells [16] and T cells [17]. Moreover, a number of studies argued against the contribution of Orai3 to endothelial SOCE [11, 101, 102], as this Orai isoform has hitherto been implicated only in leukotriene C4-induced Ca2+ entry in vascular endothelial cells [103].
Glutamate-induced Ca2+ signals drive NO release in hCMEC/D3 cells
It has recently been shown that glutamate-induced metabotropic Ca2+ oscillations promote NO release in bEND5 cells [18]. The present investigation revealed that mGluR1 and, at a larger extent, mGluR5 elicited NO release through NAADP- and InsP3-induced intracellular Ca2+ signals also in hCMEC/D3 cells. Accordingly, glutamate-induced NO release was phenocopied by t-ACPD and suppressed by any of the following treatments: (1) unspecific inhibition of group 1 mGluRs with MCPG; (2) pharmacological blockade of mGluR1 and mGluR5 with CPCCOEt and MTEP, respectively; and (3) preventing the increase in [Ca2+]i with BAPTA or through pharmacological blockade of the InsP3- and NAADP-signaling pathways. Conversely, glutamate-induced NO release was not impaired by removal of extracellular Ca2+ or pharmacological blockade of SOCE, which suggests that SOCE does not drive eNOS recruitment. This finding was somehow unexpected as SOCE is routinely required to sustain NO production in vascular endothelial cells [9, 21], including mouse [56] and human [53] brain endothelial cells challenged with acetylcholine. It is, therefore, likely that the eNOS pool recruited by glutamate is physically closer to InsP3R3 and TPC1-2 rather than Orai1 in hCMEC/D3 cells and is selectively engaged by endogenously released Ca2+. In vascular endothelial cells, the vast majority of eNOS is localized to plasma membrane caveolae [104], which are apposed to ER cisternae and could be easily invested by InsP3-induced ER Ca2+ release [105]. As functionally different sources of eNOS exist in vascular endothelium [104, 106], it is conceivable that acetylcholine and glutamate impinge on two distinct eNOS pools, one that is regulated by Orai1 and a second pool that is activated by InsP3R3.
The putative role of endothelial group 1 mGluRs in NVC
The following pieces of evidence recently hinted at an unexpected role of brain microvascular endothelial cells in NVC [8, 27, 29]. First, the hemodynamic response to neuronal activity is often initiated by cortical capillaries, which are enwrapped by contracting pericytes and deliver a retrograde vasorelaxing signal to upstream arterioles and pial arteries to irrigate the activated area [32, 38, 42, 80]. According to this model, brain microvascular endothelial cells are placed in the ideal position to sense neuronal activity and directly control CBF [8, 27, 29]. Second, discrete interruption of endothelial signaling dampens stimulus-evoked retrograde propagation of vasodilation in pial arteries, whereas wide-field disruption of the endothelial monolayer significantly attenuates the hemodynamic signal [107]. Third, activation of endothelial GqPCRs by neuronal activity at capillary level was recently shown to modulate the onset and retrograde propagation of the hemodynamic signal in a Ca2+-dependent manner [26]. These observations indicated that brain microvascular endothelial cells endowed with NMDARs, and/or mGluRs, were able to detect and react to synaptically released glutamate. Consistently, it was first shown that neuronal activity stimulated perisynaptic astrocytes to release d-serine, thereby inducing cortical arteriole vasodilation by activating endothelial NMDARs and recruiting eNOS in mouse brain [49, 50, 81]. Subsequently, glutamate was found to induce metabotropic Ca2+ signals and NO release in mouse brain microvascular endothelial cells [51]. The findings reported in the present investigation lend further support to the notion that brain microvascular endothelium actively participates in NVC and suggest an alternative mechanism to understand the role played by group 1 mGluRs in functional hyperemia. Neuronal (and endothelial) ionotropic NMDARs trigger NVC by inducing fast NO release, which directly or indirectly elicits the rapid component of the vasorelaxing response [31–34, 81]. Endothelial mGluRs could in turn support the slower component of the hemodynamic signal during prolonged (up to 1 min) synaptic stimulation either by directly vasorelaxing mural cells (i.e., vascular smooth muscle cells and pericytes) or facilitating EETs-induced vasodilation [31, 33, 34]. The finding that group 1 mGluRs are expressed in brain microvascular endothelial cells and elicit Ca2+-dependent NO release could help to understand the long known inhibitory effect of CPCCOEt and MTEP on NVC. Accordingly, although it has long been known that group 1 mGluRs somehow regulate NVC and drive the Ca2+-dependent release of EETs from astrocytes, how this occurs is matter of controversy [39–42]. Notably, a recent study demonstrated that synaptic glutamate induces astrocytic Ca2+ signals and arteriolar vasodilation by inducing endothelial-dependent NO release in vivo [48]. Therefore, we hypothesize that the effect exerted by CPCCOeT and MTEP on NVC should rather be ascribed to its inhibitory action on endothelial group 1 mGluRs, which triggers robust NO production and could, therefore, be responsible for astrocyte activation and EET release. This hypothesis, however, remains to be experimentally probed and will be the focus of future investigation.
Conclusion
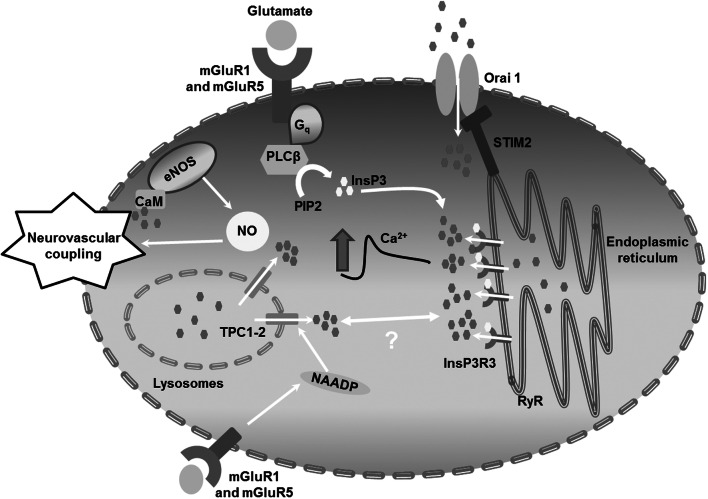
In conclusion, this investigation demonstrates for the first time that glutamate is able to induce Ca2+-dependent NO release by selectively activating mGluR1 and mGluR5 in human brain microvascular endothelial cells. The Ca2+ response to glutamate is initiated by endogenous Ca2+ release through InsP3R3 and NAADP-gated TPCs and sustained by SOCE (Fig. 9), although only endogenous Ca2+ mobilization drives NO production. These observations reinforce the view that the cellular and molecular mechanisms of NVC should be revisited by taking brain microvascular endothelial cells into account and propose an alternative model to explain the documented involvement of group 1 mGluRs in NVC.
Fig. 9.
Schematic representation of glutamate-induced Ca2+ and NO signals in hCMEC/D3 cells. The neurotransmitter glutamate binds to mGluR1 and mGluR5 to elicit an increase in [Ca2+]i in hCMEC/D3 cells. The Ca2+ response to glutamate is patterned by InsP3R3, TPC1-2, and SOCE, and results in eNOS recruitment and NO release. NO, in turn, is predicted to regulate neurovascular coupling (NVC) within brain microcirculation
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1. TRPC7 activation did not induce Ca2+and NO signals in hCMEC/D3 cells. A. 1-oleoyl-2-acetyl-sn-glycerol (OAG; 100 μM) failed to increase the [Ca2+]i in hCMEC/D3 cells. The Ca2+ tracing is representative of 63 cells from three independent experiments. B. OAG (100 μM) did not increase DAF-FM fluorescence in hCMEC/D3 cells, while glutamate (100 μM) elicited NO release. C. Bar histogram shows the mean ± SE of the percentage of hCMEC/D3 cells displaying NO release in the presence of OAG and glutamate. The asterisk indicates p < 0.05. NR = No response. D. Bar histogram shows the mean ± SE of the amplitude of OAG- and glutamate-induced NO release in hCMEC/D3 cells. The asterisk indicates p < 0.05. NR = No Response. (TIFF 353 kb)
Supplementary Fig. 2. The Orai1 specific inhibitor S66 triggers intracellular Ca2+oscillations in hCMEC/D3 cells. The specific Orai1 inhibitor S66 (20 μM) was administered following recovery of the intracellular Ca2+ response to glutamate (100 μM) under 0Ca2+ conditions to assess its effect on the subsequent restoration of extracellular Ca2+ levels. However, S66 immediately elicited repetitive Ca2+ spikes which prevented further examination of its effect on SOCE. This trace is representative of 71 recordings from three independent experiments. (TIFF 93 kb)
Supplementary Fig. 3. The Orai1 specific inhibitor BTP-2 impairs SOCE in hCMEC/D3 cells. A. Pharmacological depletion of the ER Ca2+ pool with the SERCA inhibitor CPA (10 μM) under 0Ca2+ conditions resulted in robust SOCE upon Ca2+ restitution to the bath. B. BTP-2 (20 µM), a selective inhibitor of Orai1, attenuated CPA-induced SOCE in hCMEC/D3 cells. C. Bar histogram shows the mean ± SE of the percentage of cells displaying SOCE under control (Ctrl) conditions and upon treatment with BTP-2 (20 µM, 20 min). D. Bar histogram shows the mean ± SE of SOCE amplitude under control (Ctrl) conditions and upon treatment with BTP-2 (20 µM, 20 min). The asterisk indicates p < 0.05. (TIFF 995 kb)
Supplementary Fig. 4. The mGluR5 specific agonist CHPG reliably induces intracellular Ca2+signals in hCMEC/D3 cells. CHPG (25 μM), a selective CHPG agonist, induced an increase in [Ca2+]i in hCMEC/D3 cells. The trace was representative of 65 cells from three independent experiments. (TIFF 195 kb)
Supplementary Fig. 5. BTP-2 did not affect glutamate-induced NO release in hCMEC/D3 cells. A. Glutamate (100 μM) induced a robust increase in DAF-FM fluorescence both in control (Ctrl) conditions and in the presence of BTP-2 (20 μM, 20 min). B. Bar histogram shows the mean±SE of the percentage of cells showing glutamate-induced NO release in the absence (Ctrl) and presence of BTP-2. C. Bar histogram shows the mean±SE of the amplitude of glutamate-induced NO release in the absence (Ctrl) and presence of BTP-2. (TIFF 1359 kb)
Supplementary Fig. 6. Pyr6 and La3+did not affect glutamate-induced NO release in hCMEC/D3 cells. A. Glutamate (100 μM) induced a robust increase in DAF-FM fluorescence both in control (Ctrl) conditions and in the presence of Pyr6 (10 μM, 10 min) plus La3+ (100 μM, 20 min). B. Bar histogram shows the mean±SE of the amplitude of glutamate-induced NO release in the absence (Ctrl) and presence of Pyr6 plus La3+. (TIFF 585 kb)
Acknowledgements
This research was funded by: Italian Ministry of Education, University and Research (MIUR): Dipartimenti di Eccellenza Program (2018–2022)—Dept. of Biology and Biotechnology “L. Spallanzani”, University of Pavia (F.M.), and by Fondo Ricerca Giovani from the University of Pavia (F.M.). P.S.F. was supported by MAECI (Ministero degli Affari Esteri e della Cooperazione Internazionale).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McCarron JG, Lee MD, Wilson C. The endothelium solves problems that endothelial cells do not know exist. Trends Pharmacol Sci. 2017;38(4):322–338. doi: 10.1016/j.tips.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moccia F, Tanzi F, Munaron L. Endothelial remodelling and intracellular calcium machinery. Curr Mol Med. 2014;14(4):457–480. doi: 10.2174/1566524013666131118113410. [DOI] [PubMed] [Google Scholar]
- 3.Khaddaj Mallat R, Mathew John C, Kendrick DJ, Braun AP. The vascular endothelium: a regulator of arterial tone and interface for the immune system. Crit Rev Clin Lab Sci. 2017;54(7–8):458–470. doi: 10.1080/10408363.2017.1394267. [DOI] [PubMed] [Google Scholar]
- 4.Moccia F, Guerra G. Ca(2+) signalling in endothelial progenitor cells: friend or foe? J Cell Physiol. 2016;231(2):314–327. doi: 10.1002/jcp.25126. [DOI] [PubMed] [Google Scholar]
- 5.Kerr P, Tam R, Plane F (2011) Endothelium. In: Fitridge R, Thompson M (eds) Mechanisms of vascular disease: a reference book for vascular specialists. University of Adelaide Press, Adelaide (AU) [PubMed]
- 6.Godo S, Shimokawa H. Endothelial functions. Arterioscler Thromb Vasc Biol. 2017;37(9):e108–e114. doi: 10.1161/ATVBAHA.117.309813. [DOI] [PubMed] [Google Scholar]
- 7.Garland CJ, Dora KA. EDH: endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol. 2017;219(1):152–161. doi: 10.1111/apha.12649. [DOI] [PubMed] [Google Scholar]
- 8.Guerra G, Lucariello A, Perna A, Botta L, De Luca A, Moccia F. The role of endothelial Ca(2+) signaling in neurovascular coupling: a view from the Lumen. Int J Mol Sci. 2018 doi: 10.3390/ijms19040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blatter LA. Tissue specificity: SOCE: implications for Ca2+ handling in endothelial cells. Adv Exp Med Biol. 2017;993:343–361. doi: 10.1007/978-3-319-57732-6_18. [DOI] [PubMed] [Google Scholar]
- 10.Moccia F, Dragoni S, Lodola F, Bonetti E, Bottino C, Guerra G, et al. Store-dependent Ca(2+) entry in endothelial progenitor cells as a perspective tool to enhance cell-based therapy and adverse tumour vascularization. Curr Med Chem. 2012;19(34):5802–5818. doi: 10.2174/092986712804143240. [DOI] [PubMed] [Google Scholar]
- 11.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103(11):1289–1299. doi: 10.1161/01.res.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Cubbon RM, Wilson LA, Amer MS, McKeown L, Hou B, et al. Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ Res. 2011;108(10):1190–1198. doi: 10.1161/circresaha.111.243352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachdeva R, Fleming T, Schumacher D, Homberg S, Stilz K, Mohr F, et al. Methylglyoxal evokes acute Ca(2+) transients in distinct cell types and increases agonist-evoked Ca(2+) entry in endothelial cells via CRAC channels. Cell Calcium. 2019;78:66–75. doi: 10.1016/j.ceca.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Zuccolo E, Laforenza U, Negri S, Botta L, Berra-Romani R, Faris P, et al. Muscarinic M5 receptors trigger acetylcholine-induced Ca(2+) signals and nitric oxide release in human brain microvascular endothelial cells. J Cell Physiol. 2019;234(4):4540–4562. doi: 10.1002/jcp.27234. [DOI] [PubMed] [Google Scholar]
- 15.Kito H, Yamamura H, Suzuki Y, Yamamura H, Ohya S, Asai K, et al. Regulation of store-operated Ca2+ entry activity by cell cycle dependent up-regulation of Orai2 in brain capillary endothelial cells. Biochem Biophys Res Commun. 2015;459(3):457–462. doi: 10.1016/j.bbrc.2015.02.127. [DOI] [PubMed] [Google Scholar]
- 16.Eckstein M, Vaeth M, Aulestia FJ, Costiniti V, Kassam SN, Bromage TG, et al. Differential regulation of Ca(2+) influx by ORAI channels mediates enamel mineralization. Sci Signal. 2019 doi: 10.1126/scisignal.aav4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaeth M, Yang J, Yamashita M, Zee I, Eckstein M, Knosp C, et al. ORAI2 modulates store-operated calcium entry and T cell-mediated immunity. Nat Commun. 2017;8:14714. doi: 10.1038/ncomms14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuccolo E, Kheder DA, Lim D, Perna A, Nezza FD, Botta L, et al. Glutamate triggers intracellular Ca(2+) oscillations and nitric oxide release by inducing NAADP- and InsP3-dependent Ca(2+) release in mouse brain endothelial cells. J Cell Physiol. 2019;234(4):3538–3554. doi: 10.1002/jcp.26953. [DOI] [PubMed] [Google Scholar]
- 19.Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Sources of Ca2+ in relation to generation of acetylcholine-induced endothelium-dependent hyperpolarization in rat mesenteric artery. Br J Pharmacol. 1997;120(7):1328–1334. doi: 10.1038/sj.bjp.0701027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3(2):121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 21.Berra-Romani R, Avelino-Cruz JE, Raqeeb A, Della Corte A, Cinelli M, Montagnani S, et al. Ca(2)(+)-dependent nitric oxide release in the injured endothelium of excised rat aorta: a promising mechanism applying in vascular prosthetic devices in aging patients. BMC Surg. 2013;13(Suppl 2):S40. doi: 10.1186/1471-2482-13-S2-S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blatter LA, Taha Z, Mesaros S, Shacklock PS, Wier WG, Malinski T. Simultaneous measurements of Ca2+ and nitric oxide in bradykinin-stimulated vascular endothelial cells. Circ Res. 1995;76(5):922–924. doi: 10.1161/01.RES.76.5.922. [DOI] [PubMed] [Google Scholar]
- 23.Lantoine F, Iouzalen L, Devynck MA, Millanvoye-Van Brussel E, David-Dufilho M. Nitric oxide production in human endothelial cells stimulated by histamine requires Ca2+ influx. Biochem J. 1998;330(Pt 2):695–699. doi: 10.1042/bj3300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brailoiu GC, Gurzu B, Gao X, Parkesh R, Aley PK, Trifa DI, et al. Acidic NAADP-sensitive calcium stores in the endothelium: agonist-specific recruitment and role in regulating blood pressure. J Biol Chem. 2010;285(48):37133–37137. doi: 10.1074/jbc.C110.169763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, et al. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015;309(11):H1837–H1845. doi: 10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harraz OF, Longden TA, Hill-Eubanks D, Nelson MT. PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells. eLife. 2018 doi: 10.7554/elife.38689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468(7321):232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillman EM. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci. 2014;37:161–181. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lourenco CF, Ledo A, Barbosa RM, Laranjinha J. Neurovascular-neuroenergetic coupling axis in the brain: master regulation by nitric oxide and consequences in aging and neurodegeneration. Free Radic Biol Med. 2017;108:668–682. doi: 10.1016/j.freeradbiomed.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Lourenco CF, Santos RM, Barbosa RM, Cadenas E, Radi R, Laranjinha J. Neurovascular coupling in hippocampus is mediated via diffusion by neuronal-derived nitric oxide. Free Radic Biol Med. 2014;73:421–429. doi: 10.1016/j.freeradbiomed.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 32.Mapelli L, Gagliano G, Soda T, Laforenza U, Moccia F, D’Angelo EU. Granular layer neurons control cerebellar neurovascular coupling through an NMDA receptor/NO-dependent system. J Neurosci. 2017;37(5):1340–1351. doi: 10.1523/JNEUROSCI.2025-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Front Neuroenerg. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duchemin S, Boily M, Sadekova N, Girouard H. The complex contribution of NOS interneurons in the physiology of cerebrovascular regulation. Front Neural Circuits. 2012;6:51. doi: 10.3389/fncir.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Li C, Falck JR, Roman RJ, Harder DR, Koehler RC. Interaction of nitric oxide, 20-HETE, and EETs during functional hyperemia in whisker barrel cortex. Am J Physiol Heart Circ Physiol. 2008;295(2):H619–H631. doi: 10.1152/ajpheart.01211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6(1):43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 37.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431(7005):195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 38.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lecrux C, Toussay X, Kocharyan A, Fernandes P, Neupane S, Levesque M, et al. Pyramidal neurons are “neurogenic hubs” in the neurovascular coupling response to whisker stimulation. J Neurosci. 2011;31(27):9836–9847. doi: 10.1523/JNEUROSCI.4943-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sloan HL, Austin VC, Blamire AM, Schnupp JW, Lowe AS, Allers KA, et al. Regional differences in neurovascular coupling in rat brain as determined by fMRI and electrophysiology. Neuroimage. 2010;53(2):399–411. doi: 10.1016/j.neuroimage.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J Cereb Blood Flow Metab. 2008;28(1):111–125. doi: 10.1038/sj.jcbfm.9600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rungta RL, Chaigneau E, Osmanski BF, Charpak S. Vascular Compartmentalization of Functional Hyperemia from the Synapse to the Pia. Neuron. 2018;99(2):362–375. doi: 10.1016/j.neuron.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cauli B, Hamel E. Brain perfusion and astrocytes. Trends Neurosci. 2018;41(7):409–413. doi: 10.1016/j.tins.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Nortley R, Attwell D. Control of brain energy supply by astrocytes. Curr Opin Neurobiol. 2017;47:80–85. doi: 10.1016/j.conb.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18(7):419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lecrux C, Hamel E. Neuronal networks and mediators of cortical neurovascular coupling responses in normal and altered brain states. Philos Trans R Soc Lond B Biol Sci. 2016 doi: 10.1098/rstb.2015.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tran CHT, Peringod G, Gordon GR. Astrocytes integrate behavioral state and vascular signals during functional hyperemia. Neuron. 2018;100(5):1133–1148. doi: 10.1016/j.neuron.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 49.LeMaistre JL, Sanders SA, Stobart MJ, Lu L, Knox JD, Anderson HD, et al. Coactivation of NMDA receptors by glutamate and d-serine induces dilation of isolated middle cerebral arteries. J Cereb Blood Flow Metab. 2012;32(3):537–547. doi: 10.1038/jcbfm.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu L, Hogan-Cann AD, Globa AK, Lu P, Nagy JI, Bamji SX, et al. Astrocytes drive cortical vasodilatory signaling by activating endothelial NMDA receptors. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678x17734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuccolo E, Kheder DA, Lim D, Perna A, Nezza FD, Botta L, et al. Glutamate triggers intracellular Ca(2+) oscillations and nitric oxide release by inducing NAADP- and InsP3-dependent Ca(2+) release in mouse brain endothelial cells. J Cell Physiol. 2018 doi: 10.1002/jcp.26953. [DOI] [PubMed] [Google Scholar]
- 52.Hopper RA, Garthwaite J. Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J Neurosci. 2006;26(45):11513–11521. doi: 10.1523/JNEUROSCI.2259-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zuccolo E, Laforenza U, Negri S, Botta L, Berra-Romani R, Faris P, et al. Muscarinic M5 receptors trigger acetylcholine-induced Ca(2+) signals and nitric oxide release in human brain microvascular endothelial cells. J Cell Physiol. 2018 doi: 10.1002/jcp.27234. [DOI] [PubMed] [Google Scholar]
- 54.Collard CD, Park KA, Montalto MC, Alapati S, Buras JA, Stahl GL, et al. Neutrophil-derived glutamate regulates vascular endothelial barrier function. J Biol Chem. 2002;277(17):14801–14811. doi: 10.1074/jbc.M110557200. [DOI] [PubMed] [Google Scholar]
- 55.Gillard SE, Tzaferis J, Tsui HC, Kingston AE. Expression of metabotropic glutamate receptors in rat meningeal and brain microvasculature and choroid plexus. J Comp Neurol. 2003;461(3):317–332. doi: 10.1002/cne.10671. [DOI] [PubMed] [Google Scholar]
- 56.Zuccolo E, Lim D, Kheder DA, Perna A, Catarsi P, Botta L, et al. Acetylcholine induces intracellular Ca2+ oscillations and nitric oxide release in mouse brain endothelial cells. Cell Calcium. 2017;66:33–47. doi: 10.1016/j.ceca.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Bootman MD, Rietdorf K, Collins T, Walker S, Sanderson M. Ca2+-sensitive fluorescent dyes and intracellular Ca2+ imaging. Cold Spring Harbor Protoc. 2013;2013(2):83–99. doi: 10.1101/pdb.top066050. [DOI] [PubMed] [Google Scholar]
- 58.Yeung YG, Stanley ER. A solution for stripping antibodies from polyvinylidene fluoride immunoblots for multiple reprobing. Anal Biochem. 2009;389(1):89–91. doi: 10.1016/j.ab.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 60.Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258(5087):1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- 61.Lee KH, Kristic K, van Hoff R, Hitti FL, Blaha C, Harris B, et al. High-frequency stimulation of the subthalamic nucleus increases glutamate in the subthalamic nucleus of rats as demonstrated by in vivo enzyme-linked glutamate sensor. Brain Res. 2007;1162:121–129. doi: 10.1016/j.brainres.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 62.Lim D, Mapelli L, Canonico PL, Moccia F, Genazzani AA. Neuronal activity-dependent activation of astroglial calcineurin in mouse primary hippocampal cultures. Int J Mol Sci. 2018 doi: 10.3390/ijms19102997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim D, Iyer A, Ronco V, Grolla AA, Canonico PL, Aronica E, et al. Amyloid beta deregulates astroglial mGluR5-mediated calcium signaling via calcineurin and Nf-kB. Glia. 2013;61(7):1134–1145. doi: 10.1002/glia.22502. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez-Hernandez Y, Laforenza U, Bonetti E, Fontana J, Dragoni S, Russo M, et al. Store-operated Ca(2+) entry is expressed in human endothelial progenitor cells. Stem Cells Dev. 2010;19(12):1967–1981. doi: 10.1089/scd.2010.0047. [DOI] [PubMed] [Google Scholar]
- 65.Bird GS, DeHaven WI, Smyth JT, Putney JW., Jr Methods for studying store-operated calcium entry. Methods. 2008;46(3):204–212. doi: 10.1016/j.ymeth.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schleifer H, Doleschal B, Lichtenegger M, Oppenrieder R, Derler I, Frischauf I, et al. Novel pyrazole compounds for pharmacological discrimination between receptor-operated and store-operated Ca(2+) entry pathways. Br J Pharmacol. 2012;167(8):1712–1722. doi: 10.1111/j.1476-5381.2012.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev. 2015;95(4):1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moccia F, Zuccolo E, Poletto V, Turin I, Guerra G, Pedrazzoli P, et al. Targeting stim and orai proteins as an alternative approach in anticancer therapy. Curr Med Chem. 2016;23(30):3450–3480. doi: 10.2174/0929867323666160607111220. [DOI] [PubMed] [Google Scholar]
- 69.Azimi I, Bong AH, Poo GXH, Armitage K, Lok D, Roberts-Thomson SJ, et al. Pharmacological inhibition of store-operated calcium entry in MDA-MB-468 basal A breast cancer cells: consequences on calcium signalling, cell migration and proliferation. Cell Mol Life Sci. 2018;75(24):4525–4537. doi: 10.1007/s00018-018-2904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Majeed Y, Amer MS, Agarwal AK, McKeown L, Porter KE, O’Regan DJ, et al. Stereo-selective inhibition of transient receptor potential TRPC5 cation channels by neuroactive steroids. Br J Pharmacol. 2011;162(7):1509–1520. doi: 10.1111/j.1476-5381.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zitt C, Strauss B, Schwarz EC, Spaeth N, Rast G, Hatzelmann A, et al. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J Biol Chem. 2004;279(13):12427–12437. doi: 10.1074/jbc.M309297200. [DOI] [PubMed] [Google Scholar]
- 72.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95(10):e73–e81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- 73.Le Duigou C, Kullmann DM. Group I mGluR agonist-evoked long-term potentiation in hippocampal oriens interneurons. J Neurosci. 2011;31(15):5777–5781. doi: 10.1523/JNEUROSCI.6265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kotova PD, Bystrova MF, Rogachevskaja OA, Khokhlov AA, Sysoeva VY, Tkachuk VA, et al. Coupling of P2Y receptors to Ca(2+) mobilization in mesenchymal stromal cells from the human adipose tissue. Cell Calcium. 2018;71:1–14. doi: 10.1016/j.ceca.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 75.Cardenas C, Liberona JL, Molgo J, Colasante C, Mignery GA, Jaimovich E. Nuclear inositol 1,4,5-trisphosphate receptors regulate local Ca2+ transients and modulate cAMP response element binding protein phosphorylation. J Cell Sci. 2005;118(Pt 14):3131–3140. doi: 10.1242/jcs.02446. [DOI] [PubMed] [Google Scholar]
- 76.Faris P, Shekha M, Montagna D, Guerra G, Moccia F. Endolysosomal Ca(2+) signalling and cancer hallmarks: two-pore channels on the move, TRPML1 Lags Behind! Cancers (Basel) 2018 doi: 10.3390/cancers11010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ronco V, Potenza DM, Denti F, Vullo S, Gagliano G, Tognolina M, et al. A novel Ca(2)(+)-mediated cross-talk between endoplasmic reticulum and acidic organelles: implications for NAADP-dependent Ca(2)(+) signalling. Cell Calcium. 2015;57(2):89–100. doi: 10.1016/j.ceca.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 78.Di Nezza F, Zuccolo E, Poletto V, Rosti V, De Luca A, Moccia F, et al. Liposomes as a putative tool to investigate NAADP signaling in vasculogenesis. J Cell Biochem. 2017 doi: 10.1002/jcb.26019. [DOI] [PubMed] [Google Scholar]
- 79.Pitt SJ, Reilly-O’Donnell B, Sitsapesan R. Exploring the biophysical evidence that mammalian two-pore channels are NAADP-activated calcium-permeable channels. J Physiol. 2016;594(15):4171–4179. doi: 10.1113/JP270936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, et al. Capillary K(+)-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017;20(5):717–726. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stobart JL, Lu L, Anderson HD, Mori H, Anderson CM. Astrocyte-induced cortical vasodilation is mediated by d-serine and endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2013;110(8):3149–3154. doi: 10.1073/pnas.1215929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moccia F, Berra-Romani R, Tanzi F. Update on vascular endothelial Ca2+ signalling: a tale of ion channels, pumps and transporters. World J Biol Chem. 2012;3(7):127–158. doi: 10.4331/wjbc.v3.i7.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Re F, Cambianica I, Sesana S, Salvati E, Cagnotto A, Salmona M, et al. Functionalization with ApoE-derived peptides enhances the interaction with brain capillary endothelial cells of nanoliposomes binding amyloid-beta peptide. J Biotechnol. 2011;156(4):341–346. doi: 10.1016/j.jbiotec.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 84.Salvati E, Re F, Sesana S, Cambianica I, Sancini G, Masserini M, et al. Liposomes functionalized to overcome the blood–brain barrier and to target amyloid-beta peptide: the chemical design affects the permeability across an in vitro model. Int J Nanomed. 2013;8:1749–1758. doi: 10.2147/IJN.S42783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Veszelka S, Toth A, Walter FR, Toth AE, Grof I, Meszaros M, et al. Comparison of a rat primary cell-based blood–brain barrier model with epithelial and brain endothelial cell lines: gene expression and drug transport. Front Mol Neurosci. 2018;11:166. doi: 10.3389/fnmol.2018.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bader A, Bintig W, Begandt D, Klett A, Siller IG, Gregor C, et al. Adenosine receptors regulate gap junction coupling of the human cerebral microvascular endothelial cells hCMEC/D3 by Ca(2+) influx through cyclic nucleotide-gated channels. J Physiol. 2017;595(8):2497–2517. doi: 10.1113/JP273150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci USA. 2013;110(48):E4678–E4687. doi: 10.1073/pnas.1310065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Casabona G, Knopfel T, Kuhn R, Gasparini F, Baumann P, Sortino MA, et al. Expression and coupling to polyphosphoinositide hydrolysis of group I metabotropic glutamate receptors in early postnatal and adult rat brain. Eur J Neurosci. 1997;9(1):12–17. doi: 10.1111/j.1460-9568.1997.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 89.Kettunen P, Krieger P, Hess D, El Manira A. Signaling mechanisms of metabotropic glutamate receptor 5 subtype and its endogenous role in a locomotor network. J Neurosci. 2002;22(5):1868–1873. doi: 10.1523/JNEUROSCI.22-05-01868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thillaiappan NB, Chavda AP, Tovey SC, Prole DL, Taylor CW. Ca(2+) signals initiate at immobile IP3 receptors adjacent to ER-plasma membrane junctions. Nat Commun. 2017;8(1):1505. doi: 10.1038/s41467-017-01644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim CH, Braud S, Isaac JT, Roche KW. Protein kinase C phosphorylation of the metabotropic glutamate receptor mGluR5 on Serine 839 regulates Ca2+ oscillations. J Biol Chem. 2005;280(27):25409–25415. doi: 10.1074/jbc.M502644200. [DOI] [PubMed] [Google Scholar]
- 92.Ribeiro FM, Ferreira LT, Paquet M, Cregan T, Ding Q, Gros R, et al. Phosphorylation-independent regulation of metabotropic glutamate receptor 5 desensitization and internalization by G protein-coupled receptor kinase 2 in neurons. J Biol Chem. 2009;284(35):23444–23453. doi: 10.1074/jbc.M109.000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87(2):593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18(5):1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Foster WJ, Taylor HBC, Padamsey Z, Jeans AF, Galione A, Emptage NJ. Hippocampal mGluR1-dependent long-term potentiation requires NAADP-mediated acidic store Ca(2+) signaling. Sci Signal. 2018 doi: 10.1126/scisignal.aat9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pereira GJ, Antonioli M, Hirata H, Ureshino RP, Nascimento AR, Bincoletto C, et al. Glutamate induces autophagy via the two-pore channels in neural cells. Oncotarget. 2017;8(8):12730–12740. doi: 10.18632/oncotarget.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Favia A, Desideri M, Gambara G, D’Alessio A, Ruas M, Esposito B, et al. VEGF-induced neoangiogenesis is mediated by NAADP and two-pore channel-2-dependent Ca2+ signaling. Proc Natl Acad Sci USA. 2014;111(44):E4706–E4715. doi: 10.1073/pnas.1406029111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zuccolo E, Dragoni S, Poletto V, Catarsi P, Guido D, Rappa A, et al. Arachidonic acid-evoked Ca2+ signals promote nitric oxide release and proliferation in human endothelial colony forming cells. Vasc Pharmacol. 2016;87:159–171. doi: 10.1016/j.vph.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 99.Galione A. A primer of NAADP-mediated Ca(2+) signalling: from sea urchin eggs to mammalian cells. Cell Calcium. 2015;58(1):27–47. doi: 10.1016/j.ceca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 100.He LP, Hewavitharana T, Soboloff J, Spassova MA, Gill DL. A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J Biol Chem. 2005;280(12):10997–11006. doi: 10.1074/jbc.M411797200. [DOI] [PubMed] [Google Scholar]
- 101.Zhou MH, Zheng H, Si H, Jin Y, Peng JM, He L, et al. Stromal interaction molecule 1 (STIM1) and Orai1 mediate histamine-evoked calcium entry and nuclear factor of activated T-cells (NFAT) signaling in human umbilical vein endothelial cells. J Biol Chem. 2014;289(42):29446–29456. doi: 10.1074/jbc.M114.578492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sundivakkam PC, Freichel M, Singh V, Yuan JP, Vogel SM, Flockerzi V, et al. The Ca(2+) sensor stromal interaction molecule 1 (STIM1) is necessary and sufficient for the store-operated Ca(2+) entry function of transient receptor potential canonical (TRPC) 1 and 4 channels in endothelial cells. Mol Pharmacol. 2012;81(4):510–526. doi: 10.1124/mol.111.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li J, Bruns AF, Hou B, Rode B, Webster PJ, Bailey MA, et al. Orai3 surface accumulation and calcium entry evoked by vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2015;35(9):1987–1994. doi: 10.1161/ATVBAHA.115.305969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shu X, Keller TCT, Begandt D, Butcher JT, Biwer L, Keller AS, et al. Endothelial nitric oxide synthase in the microcirculation. Cell Mol Life Sci. 2015;72(23):4561–4575. doi: 10.1007/s00018-015-2021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bundgaard M. The three-dimensional organization of smooth endoplasmic reticulum in capillary endothelia: its possible role in regulation of free cytosolic calcium. J Struct Biol. 1991;107(1):76–85. doi: 10.1016/1047-8477(91)90033-S. [DOI] [PubMed] [Google Scholar]
- 106.Biwer LA, Taddeo EP, Kenwood BM, Hoehn KL, Straub AC, Isakson BE. Two functionally distinct pools of eNOS in endothelium are facilitated by myoendothelial junction lipid composition. Biochim Biophys Acta. 2016;1861(7):671–679. doi: 10.1016/j.bbalip.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3(3):e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. TRPC7 activation did not induce Ca2+and NO signals in hCMEC/D3 cells. A. 1-oleoyl-2-acetyl-sn-glycerol (OAG; 100 μM) failed to increase the [Ca2+]i in hCMEC/D3 cells. The Ca2+ tracing is representative of 63 cells from three independent experiments. B. OAG (100 μM) did not increase DAF-FM fluorescence in hCMEC/D3 cells, while glutamate (100 μM) elicited NO release. C. Bar histogram shows the mean ± SE of the percentage of hCMEC/D3 cells displaying NO release in the presence of OAG and glutamate. The asterisk indicates p < 0.05. NR = No response. D. Bar histogram shows the mean ± SE of the amplitude of OAG- and glutamate-induced NO release in hCMEC/D3 cells. The asterisk indicates p < 0.05. NR = No Response. (TIFF 353 kb)
Supplementary Fig. 2. The Orai1 specific inhibitor S66 triggers intracellular Ca2+oscillations in hCMEC/D3 cells. The specific Orai1 inhibitor S66 (20 μM) was administered following recovery of the intracellular Ca2+ response to glutamate (100 μM) under 0Ca2+ conditions to assess its effect on the subsequent restoration of extracellular Ca2+ levels. However, S66 immediately elicited repetitive Ca2+ spikes which prevented further examination of its effect on SOCE. This trace is representative of 71 recordings from three independent experiments. (TIFF 93 kb)
Supplementary Fig. 3. The Orai1 specific inhibitor BTP-2 impairs SOCE in hCMEC/D3 cells. A. Pharmacological depletion of the ER Ca2+ pool with the SERCA inhibitor CPA (10 μM) under 0Ca2+ conditions resulted in robust SOCE upon Ca2+ restitution to the bath. B. BTP-2 (20 µM), a selective inhibitor of Orai1, attenuated CPA-induced SOCE in hCMEC/D3 cells. C. Bar histogram shows the mean ± SE of the percentage of cells displaying SOCE under control (Ctrl) conditions and upon treatment with BTP-2 (20 µM, 20 min). D. Bar histogram shows the mean ± SE of SOCE amplitude under control (Ctrl) conditions and upon treatment with BTP-2 (20 µM, 20 min). The asterisk indicates p < 0.05. (TIFF 995 kb)
Supplementary Fig. 4. The mGluR5 specific agonist CHPG reliably induces intracellular Ca2+signals in hCMEC/D3 cells. CHPG (25 μM), a selective CHPG agonist, induced an increase in [Ca2+]i in hCMEC/D3 cells. The trace was representative of 65 cells from three independent experiments. (TIFF 195 kb)
Supplementary Fig. 5. BTP-2 did not affect glutamate-induced NO release in hCMEC/D3 cells. A. Glutamate (100 μM) induced a robust increase in DAF-FM fluorescence both in control (Ctrl) conditions and in the presence of BTP-2 (20 μM, 20 min). B. Bar histogram shows the mean±SE of the percentage of cells showing glutamate-induced NO release in the absence (Ctrl) and presence of BTP-2. C. Bar histogram shows the mean±SE of the amplitude of glutamate-induced NO release in the absence (Ctrl) and presence of BTP-2. (TIFF 1359 kb)
Supplementary Fig. 6. Pyr6 and La3+did not affect glutamate-induced NO release in hCMEC/D3 cells. A. Glutamate (100 μM) induced a robust increase in DAF-FM fluorescence both in control (Ctrl) conditions and in the presence of Pyr6 (10 μM, 10 min) plus La3+ (100 μM, 20 min). B. Bar histogram shows the mean±SE of the amplitude of glutamate-induced NO release in the absence (Ctrl) and presence of Pyr6 plus La3+. (TIFF 585 kb)