Abstract
DNA damage response (DDR) relies on swift and accurate signaling to rapidly identify DNA lesions and initiate repair. A critical DDR signaling and regulatory molecule is the posttranslational modification poly(ADP-ribose) (PAR). PAR is synthesized by a family of structurally and functionally diverse proteins called poly(ADP-ribose) polymerases (PARPs). Although PARPs share a conserved catalytic domain, unique regulatory domains of individual family members endow PARPs with unique properties and cellular functions. Family members PARP-1, PARP-2, and PARP-3 (DDR–PARPs) are catalytically activated in the presence of damaged DNA and act as damage sensors. Family members tankyrase-1 and closely related tankyrase-2 possess SAM and ankyrin repeat domains that regulate their diverse cellular functions. Recent studies have shown that the tankyrases share some overlapping functions with the DDR–PARPs, and even perform novel functions that help preserve genomic integrity. In this review, we briefly touch on DDR–PARP functions, and focus on the emerging roles of tankyrases in genome maintenance. Preservation of genomic integrity thus appears to be a common function of several PARP family members, depicting PAR as a multifaceted guardian of the genome.
Keywords: PAR, poly(ADP-ribose); PARP, poly(ADP-ribose) polymerase; Tankyrase; DDR, DNA damage response
Introduction
Maintaining the integrity of the human genome and passing healthy genetic information to offspring are essential life functions. It is therefore of critical importance to preserve the sequence of all 3 × 109 base pairs, a sizable task considering that the genome incurs an estimated 105 DNA lesions per day [1]. DNA can sustain damage from several chemical and environmental sources resulting in many types of lesions including chemical modifications of bases, single strand breaks (SSBs), and double strand breaks (DSBs). In addition, errors made by DNA processing enzymes can result in mismatched DNA bases and inclusion of ribonucleotides. Repair must also be performed in concert with other genome manipulations such as DNA replication and its associated processing machinery, requiring logistical precision and extensive chromatin remodeling. Failure to correct DNA damage can lead to cancer-causing mutations or cell death. Cells therefore invest a significant amount of energy in preserving genome integrity, and rely on complex signaling pathways to coordinate repair.
DNA damage is identified and repaired using several specialized repair pathways collectively referred to as the DNA damage response (DDR) [2]. DDR relies on swift and accurate signaling to recruit the appropriate machinery to sites of damage. One of the most important DDR signaling molecules is poly(ADP-ribose) (PAR), a transient posttranslational modification (PTM) that functions as a stress sensor and a facilitator of DNA repair [3] (Fig. 1a). However, in the event of prolonged or catastrophic DNA damage, PAR accumulation can induce a specialized mode of cell death called parthanatos [4]. Cellular PAR levels must therefore be tightly controlled. As such, cells have evolved mechanisms for rapid modification of target proteins with PAR (termed PARylation), accurate recognition of the PAR moiety by PAR-binding proteins, and subsequent swift processing and PAR turnover [5]. This “PAR code” is essential for sustaining life, and as such is constantly being written, read, and erased.
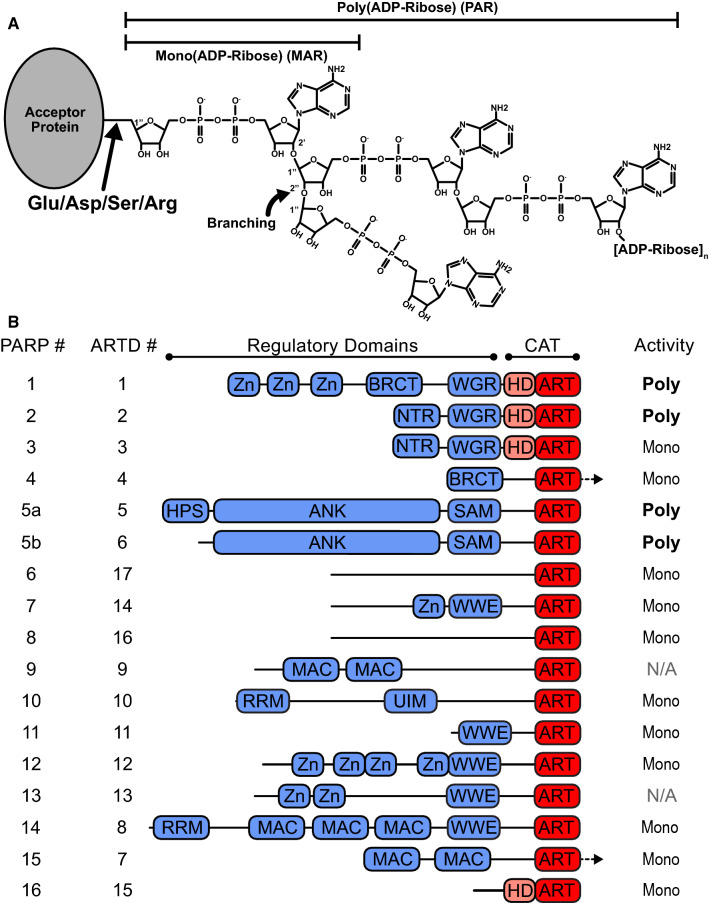
Fig. 1.
ADP-ribose and PARP superfamily overview. a Representation of mono- and poly(ADP-ribose) (MAR and PAR, respectively) as posttranslational modifications (PTMs). b Domain schematic of PARP superfamily members. PARP catalytic (CAT) regions are shown in pink/red, regulatory domains are shown in blue. Family members are identified by their current “PARP” numbering as well as their “ARTD” classification (ADP-ribosyltransferases diphtheria toxin-like) numbering. Arrows indicate regions that extend C-terminal to the CAT domains
The PAR code is written by a family of structurally and functionally diverse enzymes called poly(ADP-ribose) polymerases (PARPs), also known as ADP-ribosyl transferase diphtheria toxin-like (ARTD) proteins. PARPs use nicotinamide adenine dinucleotide (NAD+) as a substrate to posttranslationally modify themselves (automodification) and target proteins (heteromodification) with PAR. Although PARP family members possess conserved PARP catalytic domains (CAT), the unique regulatory domains of individual family members confer distinct biochemical properties and diverse cellular functions (Fig. 1b). PARP family members 1, 2, and 3, the so-called DDR–PARPs, function as DNA damage sensors. The DDR–PARPs play important roles in single and double strand break repair pathways (SSBR and DSBR, respectively) (reviewed in [6, 7]), and are emerging as critical regulators of multiple additional aspects of genome maintenance [8]. Small-molecule inhibition of PARP function (PARPi) in DDR is a potent therapeutic strategy for the treatment of cancer [9, 10]. As a result, there has been considerable interest in elucidating the regulatory mechanisms of the DDR–PARPs. However, although biological functions have been identified for other family members, much less is known about their regulation.
The PARP enzymes commonly known as tankyrase-1 (PARP-5a/ARTD5) and closely related tankyrase-2 (PARP-5b/ARTD6) possess regulatory domains that are unique in the PARP family [11], and regulate a growing list of seemingly unrelated cellular functions. Although the tankyrases are typically known as regulators of telomere length and Wnt signaling [12, 13], recent findings have demonstrated that the tankyrases share some overlapping functions with the DDR–PARPs, and even perform novel functions that help ensure genetic fidelity. Preservation of genomic integrity thus appears to be a common function of several PARP family members, suggesting that PAR functions as a multifaceted guardian of the genome. In this review, we briefly touch on the functions of the DDR–PARPs, and focus on the emerging roles of the tankyrases in DDR and other functions that ensure genomic fidelity.
The PARP superfamily
PARP catalytic activity
PAR was first discovered in the early 1960s in rat liver nuclear extract [14]. The structure of PAR was later shown to consist of chains of ADP-ribose linked with ribose–ribose glycosidic bonds. During synthesis, the growing PAR chain can be extended by adding ADP-ribose to the terminal adenosine ribose through 1″–2″ glycosidic linkages, or branches can be created by 1″–2″ linkages [15] (Fig. 1a). The heterogeneous polymers are attached to target proteins using glutamate, aspartate, lysine, and serine residues as common PAR acceptors [16].
Of all of the known roles of PAR, the best studied role is that of a stress signal. Specifically, PAR acts as a critical survival factor by flagging the location of DNA damage. Double knockout of the two main DDR PARPs 1 and 2 is embryonically lethal in mice, demonstrating that PARPs perform overlapping crucial functions in maintaining genomic stability and cell survival [17]. Alternatively, catastrophic DNA damage causes PAR accumulation that can induce a specialized mode of cell death called parthanatos [4]. Prolonged PAR synthesis depletes NAD+ stores, halting synthesis of ATP and causing the release of cell death effector apoptosis-inducing factor (AIF) from the mitochondria [18]. PAR accumulation in the nucleus leads to PAR translocation to the cytosol, also aiding the release of AIF from mitochondria [19]. Once released into the cytosol, AIF translocates to the nucleus and induces chromatin condensation and DNA fragmentation, resulting in cell death [4]. Within the nervous system, parthanatos-mediated death of neuronal cells is believed to be the causative agent of some neurodegenerative disorders [4]. Proper maintenance of PAR levels is thus extremely important for cell survival, and PAR synthesis is tightly regulated.
PARP CAT domains synthesize PAR using a histidine–tyrosine–glutamate (HYE) catalytic triad within a conserved ADP-ribosyltransferase (ART) fold [20] (Fig. 1b). Although the ART fold is expected to have high structural similarity in the PARP family, substitutions within the HYE motif render most PARP enzymes unable to actually synthesize PAR. For example, mutating the Glu residue to Gln (E988Q in PARP-1) yields mono(ADP-ribosyl) transferase (MART) activity [21], and mutating the His residue is predicted to inhibit NAD+ binding entirely [22]. PARPs 1, 2, and tankyrase-1 and tankyrase-2 synthesize PAR, while PARPs 3, 4, 6–8, 10–12, and 14–16 synthesize mono-ADP-ribose (MAR) [20]. The other PARP family members, 9 and 13, have either extremely low MART activity or are completely inactive, respectively [20]. Polymers generated by different PARP family members also show unique length and branching, with PARP-1 forming large, branched polymers consisting of up to 200 ADP-ribose units and TNKS forming linear polymers consisting of up to 20 units [23]. The DDR–PARP CAT domains possess an additional autoinhibitory subdomain (Fig. 1b), termed the helical domain (HD). The HDs function in concert with additional PARP regulatory domains to regulate PAR activity [24, 25].
The DNA damage-dependent PARPs
PARP family members possess diverse regulatory domains that endow individual PARPs with their own unique functions. The PARP-1 regulatory domains consist of three zinc fingers, a BRCA C-terminal (BRCT) domain, and a Trp–Gly–Arg (WGR) domain that are critical for its function in DDR. In its native state, in the absence of damaged DNA, the HD subdomain of the CAT blocks productive binding of NAD+ to the ART active site [24, 26]. In the presence of damaged DNA, the zinc fingers and WGR domain bind the damaged strands, causing all of the domains to collapse onto each other and the CAT [25, 27]. The collapsed architecture promotes inter-domain contacts that distort the HD, activating PARylation via an allosteric mechanism [24, 25, 27]. Utilizing this mechanism, the regulatory domains of PARP-1 facilitate PARylation in the presence of several types of damaged DNA including but not limited to nicks, gaps, blunt ends, and 5′ or 3′ extensions [28].
The other DDR PARPs, PARP-2 and PARP-3, lack zinc fingers, and instead engage DNA primarily through their WGR domains [29]. PARylation is activated via allosteric mechanisms similar to that of PARP-1, but selectively triggered in the presence of 5′-phosporylated (5′P) DNA fragments [28]. Selective activation by 5′P DNA, an intermediate formed at later stages during repair, suggests that PARPs 2 and 3 act during later stages of the repair process, while PARP-1 acts at an earlier stage. Thus, the unique regulatory domains of even similar PARPs with similar functions can endow distinct functions within DDR.
PARP-1 is the best studied PARP, and serves important functions in both SSBR and DSBR. In SSBR, PARP-1 signaling acts as a beacon for the recruitment of additional DDR machinery (reviewed in [30]). The role of DDR–PARPs in DSBR is not as well understood, a gap that may be attributed to the complexity of the multiple DBSR strategies. When a single strand incurs damage, the SSBR pathway uses the intact strand as a template to accurately repair damage. If both strands are broken, the DSBR pathway must either use sister chromatids as a template for repair, or risk attempting repair without a template. In the presence of sister chromatids during S phase, homologous recombination (HR) uses the intact copy of the DNA strand as a template when repairing the broken strand. Alternatively, if a homologous template is not available, non-homologous end joining (NHEJ) can be used to join broken strands that have very small regions of homology, termed microhomology, or no homology at all [31]. Several members of both pathways either directly bind PAR or interact with the PARP-1 BRCT domain [32–35]. Yet the specific role of PARP-1 in these processes has remained somewhat elusive. PARP-1 has been shown to promote HR in some studies [36, 37], yet other studies show that PARP-1 activity or PAR accumulation can inhibit HR [38, 39], or show that PARP-1 inactivation has no effect on HR [40]. The role of the DDR–PARPs in canonical NHEJ (C-NHEJ) is also not well understood. However, PARP-1 competes with C-NHEJ components for binding to DSBs, and appears to regulate an alternative NHEJ (alt-NHEJ) pathway that operates when C-NHEJ is deficient [41, 42]. Although the exact roles of the DDR–PARPs in HR and NHEJ are still an active area of study, it is evident that PAR plays a key role in regulating DSBR.
In addition to the functions described above, DDR–PARPs also facilitate DDR by regulating chromatin remodeling and managing stalled replication forks [43–46]. PARPs can even function as motif-dependent transcription factors for the expression of some proteins, including DDR machinery [47–49]. Although the “DDR–PARP” classification is currently limited to PARPs 1–3, tankyrase has recently been linked to several aspects of DDR. Tankyrase shares some overlapping functions with PARPs 1–3, and even performs several distinct functions that preserve genomic integrity, suggesting that tankyrase is emerging as a bona fide DDR–PARP in its own right.
Tankyrase regulatory domains
Tankyrase-1 (TNKS1) and closely related tankyrase-2 (TNKS2) regulate several seemingly unrelated cellular functions including Wnt signaling, telomere maintenance, Golgi trafficking, and apoptosis [12, 13, 50, 51]. The highly conserved TNKS1 and TNKS2 possess regulatory domains that allow them to engage multiple binding partners (Fig. 2, inner ring) from a growing list of signaling pathways [52, 53] (Fig. 2, outer ring). As such, TNKS1 and TNKS2 are found in several cellular locations including mitotic spindle poles, the Golgi apparatus, stress granules, and, although they lack nuclear localization signals, they also localize to telomeres [11, 54–56] (Fig. 2). TNKS1 and TNKS2 share 83% sequence identity [52, 57] yet demonstrate different tissue expression patterns, suggesting that they may perform distinct functions. Both tankyrases are ubiquitously expressed in mammalian tissue, yet TNKS1 is highly expressed in the testes while TNKS2 is highly expressed in the ovaries, prostate, thymus, skeletal muscle, and placenta [11, 57]. Nevertheless, studies have shown that TNKS1 and TNKS2 interact with the same binding partners. Furthermore, embryonic lethality results only from simultaneous knockout of TNKS1 and TNKS2, suggesting that the molecules share overlapping functions [58–60]. Because of their similarity and overlapping functions, tankyrases 1 and 2 will simply be referred to here as TNKS unless otherwise noted.
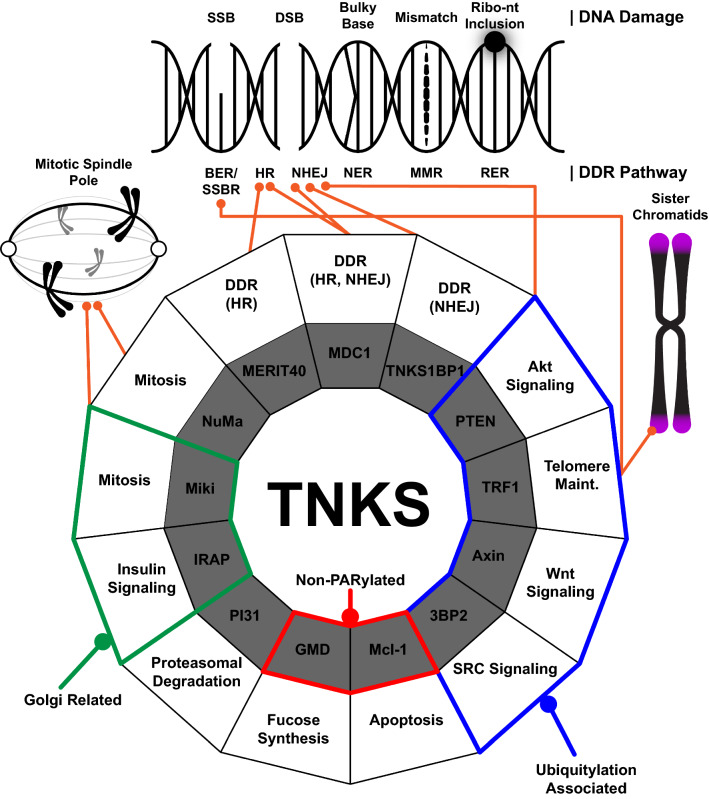
Fig. 2.
TNKS-binding partners and their respective functions. Types of DNA damage and their respective repair pathways (top), and wheel depicting the many known functions of TNKS (bottom) are shown. The types of DNA damage depicted include single and double strand breaks (SSB and DSB, respectively), bulky base, mismatch, and ribonucleotide (Ribo-nt) inclusion. Repair pathways include base excision repair (BER), single strand break repair (SSBR), homologous recombination (HR), non-homologous end joining (NHEJ), nucleotide excision repair (NER), mismatch repair (MMR), and ribonucleotide excision repair (RER). Binding partners are represented by the inner ring of the wheel (in gray), and their associated functions are represented in the outer ring of the wheel (in white). Non-PARylated binding partners are outlined in red. Related TNKS functions are grouped into Golgi-related (green outline), ubiquitylation associated (blue outline), and preservation of genomic integrity (orange lines)
TNKS regulatory domains include an N-terminal histidine, proline, and serine-rich (HPS) domain, an ankyrin repeat domain, and a sterile alpha motif (SAM) domain [11] (Fig. 3a). The HPS is present in TNKS1 but not in TNKS2; it currently has no known function and is expected to be unstructured based on the low complexity of the amino acid sequence in this region. SAM domains are abundantly found in the proteome and demonstrate significant diversity of function including RNA binding, kinase docking, and homo- and heteropolymerization [61, 62]. The TNKS1 SAM domain allows it to form helical polymers with itself or with TNKS2 [52, 63–65]. Although the mechanisms regulating polymer assembly and disassembly are not well understood, disruption of polymer formation lowers TNKS PARP activity, alters subcellular localization, and interferes with TNKS function [64, 65]. Disruption of polymerization additionally reduces TNKS interaction with Wnt signaling component Axin1, which has also been shown to form homopolymers through its DIX domain [66] (Fig. 4c). SAM-mediated polymerization may therefore facilitate multivalent interactions with Axin1, potentially increasing binding affinity through avidity [64].
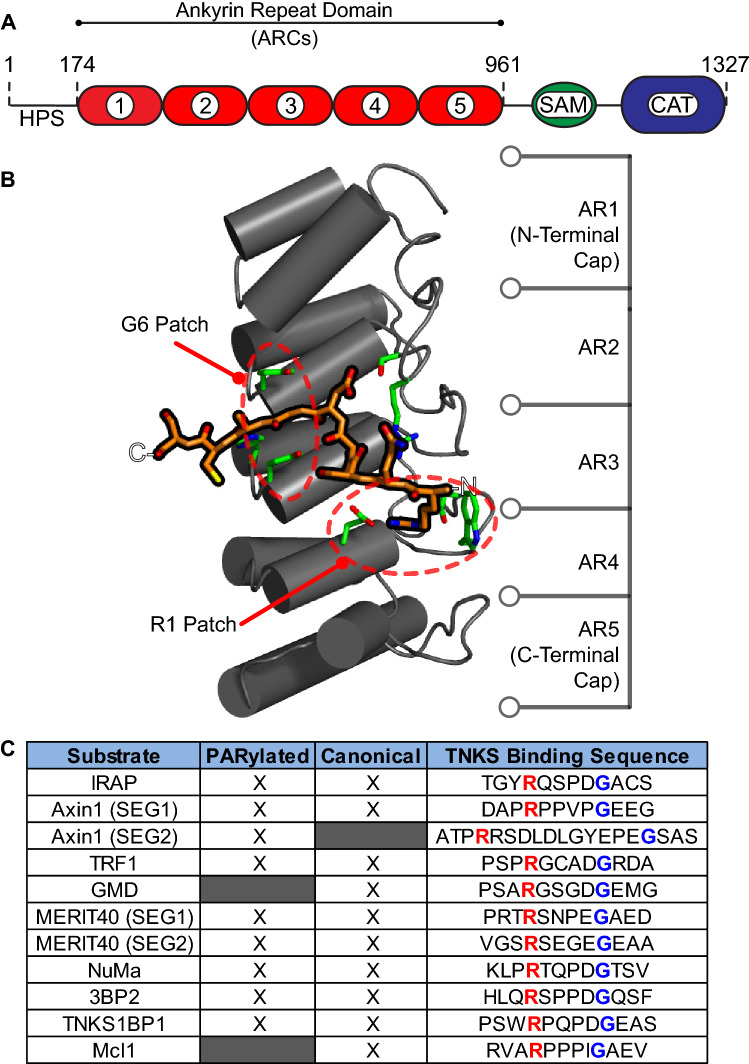
Fig. 3.
TNKS interaction with TNKS-binding motifs. a Schematic of TNKS domains with human tankyrase-1 amino acid numbering shown. b Structure of a representative ankyrin repeat cluster (ARC) (in gray) bound to a peptide representing the binding partner IRAP (orange, PDB #5JHQ [70]). The ARC consists of five ankyrin repeats (AR). Residues that interact with the IRAP peptide are shown in green. The patches that coordinate binding to the R1 or G6 residues of the TNKS-binding motif are circled in red. c Table showing the TNKS-binding motifs of several TNKS-binding partners. Peptides with the canonical binding motif are noted with an “X” in the “Canonical” column. Binding partners that are modified with PAR are noted with an “X” in the “PARylated” column. The amino acid sequences of the binding motifs are shown, with the residues that bind at position R1 or G6 in red or blue text, respectively
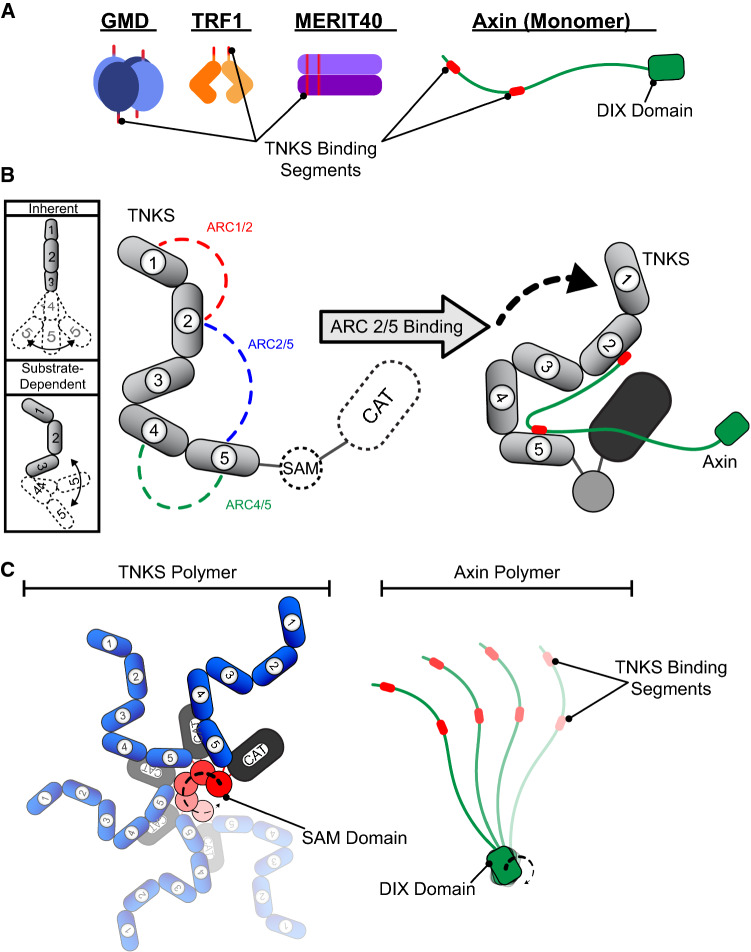
Fig. 4.
TNKS interaction with structurally dissimilar binding partners. a Structural heterogeneity of TNKS-binding partners. The schematics indicate the varying multimeric quaternary structures of TNKS-binding partners. Each unique TNKS-binding motif is indicated, and all TNKS-binding motifs are drawn in red. b (left) The expected flexibility of the ankyrin repeat domain and the relative orientations of ARCs is shown schematically. The five ARCs are numbered 1–5. (middle) ARC pairs that function together in binding to Axin1 are shown in the context of the complete TNKS domain structure. (right) A proposed model for the roles of TNKS structural characteristics in binding partner interaction and potential delivery to the catalytic domain (CAT). c Schematic of TNKS and Axin polymers, illustrating the potential for multivalent contacts between the polymeric proteins
The ankyrin repeat domain consists of 25 copies of the ankyrin repeat, a 30–34 amino acid structural motif that forms a helix-turn-helix loop structure [67, 68]. Ankyrin repeats typically stack to form continuous, curved solenoid structures, creating large solvent-exposed surfaces that are commonly used for protein–protein interactions [67]. However, the TNKS ankyrin repeat domains are segmented into five ankyrin repeat clusters (ARCs) [69] (Fig. 3a). Segmentation was first suggested by the observation that the ankyrin repeat domain possessed multiple binding sites [69]. Subsequent computational identification of a conserved LLEAAR/K motif helped to roughly divide the ankyrin repeats into five subdomains [52]. Structural analysis later demonstrated that ARCs consist of three central, canonical ankyrin repeats that are capped by ankyrin repeat variants at their N- and C-termini [53, 70] (Fig. 3b). These ankyrin repeat variants and inter-ARC linkers affect the relative positioning and flexibility between each ARC. As a result, ARCs 1-3 adopt a rigid “C” conformation, while ARCs 4 and 5 are more flexible (Fig. 4b, left) [71].
The central three ankyrin repeats form a pocket capable of accommodating an octapeptide, and establishing key conserved interactions with consensus TNKS-binding motifs found within target proteins (Fig. 3b). The TNKS-binding motif was originally identified as a hexapeptide “RxxPDG” motif where “x” is any amino acid [72]; however, as many as eight residues can contribute to binding in canonical motifs [53, 70, 73] (Fig. 3c). Structural analyses of ARC–peptide interactions have shown that the most important contacts for binding involve the arginine residue at position 1 (R1) and the glycine at position 6 (G6). R1 is bound to the “arginine cradle” within the ARC pocket, which consists of residues that engage both the charged guanidinium group of R1 and pack against the nonpolar region of its side chain [53, 70, 73]. G6 is arranged between two aromatic residues in a region of the binding pocket referred to as the “aromatic glycine sandwich”. The aromatic residues function as a size gate, and are arranged in such a way that only glycine, the smallest amino acid, can bind in that position [53]. The ARCs are structurally similar, however, substitutions within the R1- and G6-patches can significantly hinder ARC-binding capacities. For example, multiple substitutions in both R1- and G6-coordinating patches in ARC3 completely ablate binding, and substitutions within the G6-coordinating patch greatly reduce the peptide-binding capacity of ARC1 [53, 71].
In contrast to R1 and G6 binding, fewer rules appear to apply at positions 2–5 and 7–8 [53]. Specifically, interactions between the ARCs and main chain amino and carbonyl groups facilitate binding without engaging side chains [53, 70, 73] (Fig. 3c). Some specific amino acid side chains that interact with the ARC-binding pocket can either disrupt or further stabilizing binding. The side chain of the aspartate residue at position 5 within the consensus TNKS-binding motif (D5), for example, forms stabilizing interactions with the ARC pocket, yet this residue is not strictly required for binding [53]. Interestingly, binding partner Axin1 possesses two TNKS-binding motifs separated by a linker sequence, one canonical motif and a second non-canonical motif that spans 13 residues. Both canonical and non-canonical motifs are stabilized by similar interactions within the binding pocket [70], demonstrating that ARCs can tolerate length as well as sequence variations within the TNKS-binding motif.
In addition to motif sequence variation, binding partners often possess multiple TNKS-binding motifs (Fig. 4a). Binding partners can possess multiple consecutive TNKS-binding motifs, form homomultimers, or both, as in the case of Axin1 and Mediator of Rap80 Interactions and Targeting 40kD (MERIT40) [53, 70]. Some binding partners can engage multiple ARCs [53, 69, 71, 74]; however, the possible roles of multivalent binding in TNKS regulation have remained enigmatic. Binding multiple ARCs may function as a means of creating high-affinity multivalent interactions. For example, E3 ligase RNF146 possesses five TNKS-binding motifs [74]. The individual binding motifs demonstrate weak TNKS interaction, but together they allow RNF146 to form a robust 1:1 complex with TNKS [74]. Alternatively, multivalent binding may facilitate PARylation. The bipartite TNKS-binding domain of Axin1 engages only specific ARC pairs. These pairs include ARCs 1/2, 4/5, and, surprisingly, ARC pair 2/5 (Fig. 4b). Axin1 binding to ARC pair 2/5 causes TNKS to dynamically sample more compact conformations that may orient Axin1 closer to the CAT [71], potentially facilitating PARylation (Fig. 4b, right). The ankyrin repeat domain thus functions as a heteromultivalent binding platform that can alter its conformation to facilitate binding. Such plasticity and tolerance of sequence and structural variation may explain how TNKS is able to coordinate binding with a vast array of dissimilar binding partners.
One of the most perplexing aspects of TNKS regulation is that although binding is required for PARylation, not all TNKS-binding partners are modified. Binding partner GDP-mannose-4,6-dehydratase (GMD), a component of de novo fucose synthesis, engages TNKS in the cytosol during interphase, but is not an acceptor of PAR [75]. The effect of this interaction on GMD activity is unknown, however, GMD binding increases the stability of TNKS and decreases its catalytic activity [75]. Non-PARylated-binding partner Mcl-1, a Bcl-2 family protein, has two splicing variants, both of which bind TNKS [51]: an anti-apoptotic long variant (Mcl-1L) and a proapoptotic short variant (Mcl-1S). Despite not being acceptors of PAR, overexpression of TNKS decreases expression of both Mcl-1 variants, and antagonizes their pro- or anti-apoptotic functions [51]. These interactions suggest that TNKS can regulate some cellular functions even in the absence of PAR signaling. Furthermore, increased concentrations of either Mcl-1 splicing variant result in decreased heteromodification of TRF1 in vitro [51], suggesting that binding partners can modulate TNKS catalytic activity.
TNKS thus appears to be capable of promiscuous binding while simultaneously deciding which binding partners do and do not get modified. However, binding partners demonstrate such remarkable sequence and structural disparity that no pattern has yet emerged that can identify a unifying regulatory mechanism. Although the factors that govern TNKS:binding partner interaction are not fully characterized, several biological TNKS functions have been identified in DDR and beyond.
TNKS functions
TNKS functions in DDR
TNKS-mediated PARylation of binding partners imparts a substantial negative charge that alters their behavior or facilitates their interaction with PAR-binding proteins. In some instances, PARylation facilitates interaction with PAR-binding E3 ligase RNF146, leading to ubiquitylation and subsequent degradation of target proteins [76–78]. TNKS forms a complex with RNF146, suggesting a coupling between TNKS-mediated PARylation and ubiquitylation [74, 77]. RNF146 also mediates interaction with members of DDR pathways and histones, and affects SSBR regulation, suggesting additional downstream roles in DDR [79]. The fate of each PARylated protein and its impact on cellular function, however, appear to be binding partner specific.
A recent study has found that TNKS is required for SSBR at sites of telomeric damage [80]. Inhibition of TNKS catalytic activity completely abolishes the recruitment of SSBR proteins XRCC1 and Polβ to sites of telomeric damage, and sensitizes cells to oxidative damage at telomeres and XRCC1 inhibition. Furthermore, PARP-1/2 inhibition does not have the same effect on telomere SSBR, indicating a specific role for TNKS [80].
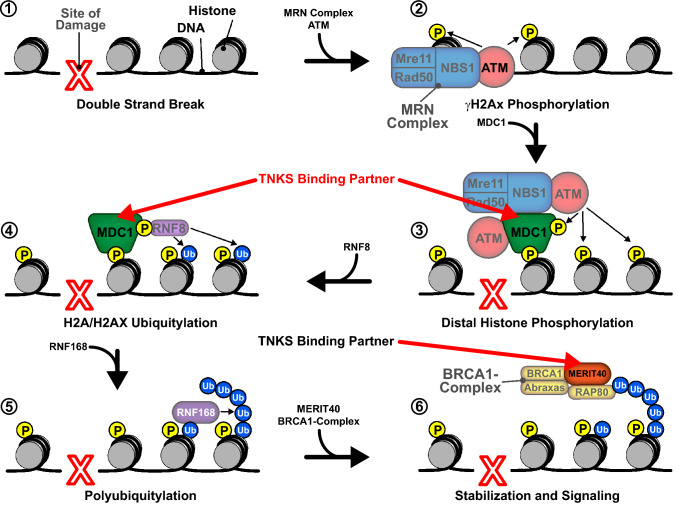
TNKS has also been shown to regulate DSBR through interactions with several binding partners (Fig. 5). One binding partner, mediator of DNA damage checkpoint protein 1 (MDC1), is a scaffolding protein that aids in slowing the cell cycle in response to DNA damage and recruiting proteins involved in HR and NHEJ [81]. MDC1 assembles at sites of DSBs in an ataxia telangiectasia mutated (ATM)-dependent manner, and facilitates signaling through ubiquitin ligases and phosphatidylinositol 3-kinase (PI3 K), with subsequent recruitment of checkpoint mediators 53BP1 and BRCA1 [82, 83] (Fig. 5, Steps 3–4). Both TNKS1 and TNKS2 are recruited to DSBs in an MDC1-dependent manner and loss of TNKS function results in defects in HR [84]. Furthermore, MDC1 also recruits chromatin remodeler p400 to DSBs, possibly linking TNKS to both DDR and alterations of chromatin structure [85].
Fig. 5.
A model for recruitment of TNKS-binding partners in the DSBR pathway. Following a double strand break (1), the MRN complex and ATM colocalize to the site of damage (2). MDC1 binds, recruiting additional ATM molecules and resulting in the phosphorylation of distal histones (3). MDC1 also recruits RNF8, which ubiquitylates histones H2A and H2AX (4). RNF168 then binds the ubiquitylated histones, yielding polyubiquitylated histones (5). The BRCA1 complex is then recruited through an interaction between RAP80 and polyubiquitin (6). MERIT40 functions as a scaffold that stabilizes the BRCA1 complex, facilitating signaling for downstream repair factors. TNKS-binding partners MDC1 and MERIT40 are designated by red arrows
Another TNKS-binding partner involved in DSBR is scaffolding protein MERIT40. MERIT40 aids in the assembly of the BRCA1A complex (consisting of Rap80, BRCA1, BRCC36, and CCDC98) at DSBs, facilitating DSBR and cell cycle checkpoint functions [86, 87] (Fig. 5, Step 6). Knockdown of either TNKS1 or TNKS2 results in a significant decrease in recruitment of the MERIT40 and BRCA1A complex, suggesting a critical role for TNKS in recruiting scaffolding proteins at sites of DSBs [84]. A previous study demonstrated that TNKS PARylates MERIT40; however, TNKS catalytic function was not required for TNKS:MERIT40 interaction [53, 84]. It thus appears that TNKS function in DDR may not always require PARylation.
Finally, TNKS-binding partner and tumor suppressor phosphatidylinositol (3,4,5)-trisphosphate phosphatase and tensin homolog deleted from chromosome 10 (PTEN) also facilitates DSBR (Fig. 2) [88]. PTEN inhibits the Akt pathway, promoting cell survival and growth, and facilitating NHEJ (reviewed in [89]). Loss of PTEN function contributes to tumorigenesis, and indeed PTEN is one of the most frequently mutated proteins in cancer [88]. The stability and localization of PTEN are controlled by many PTMs including TNKS-mediated PARylation [90]. PARylated PTEN is ubiquitylated by RNF146 and subsequently degraded [90]. Depletion of both TNKS1 and TNKS2 stabilizes PTEN, subsequently inhibiting the Akt pathway [90]. Although the direct connection between TNKS and PTEN-mediated DSRB requires further exploration, PTEN modulation may be an additional means through which TNKS regulates DDR.
Preservation of genome integrity
TNKS is emerging as a critical factor for ensuring genomic integrity. The first TNKS function to be described was maintenance of telomere length [11]. Telomeres are repetitive non-coding “TTAGGG” sequences that prevent chromosome termini from fraying, being degraded through incomplete replication, or being inadvertently recognized as damaged DNA [91]. However, telomeres themselves are shortened every cell cycle, and progressive shortening can lead to senescence after several rounds of replication [92]. A six-subunit protein complex called shelterin is associated with mammalian telomeres (reviewed in [93]). In addition to protecting telomeres from being recognized as damaged DNA, the shelterin complex also regulates telomerase, an enzyme that lengthens telomeres [94]. Shelterin component telomeric repeat-binding factor 1 (TRF1) binds to telomeric repeats and prevents telomerase from binding [94]. TNKS binds and PARylates TRF1 causing it to dissociate from telomeres followed by degradation through the ubiquitin–proteasome pathway, subsequently making telomeres more accessible to telomerase [11, 95]. Through this mechanism, overexpression of TNKS has been shown to elongate telomeres and, conversely, TNKS inhibition and TNKS depletion have been shown to shorten telomeres [13, 96, 97]. Disruption of TNKS:TRF1 binding also negatively affects SSBR, which suggests a role for shelterin complex members outside of telomere maintenance [80]. Interestingly, mouse TRF1 does not contain a TNKS-binding motif and mouse TNKS does not localize to telomeres, suggesting that mouse telomeres are regulated through alternative means [72, 98].
Despite being a successful strategy for preserving genetic content at chromosome ends, the homologous and repetitive nature of telomeres make them excellent candidates for fusion by NHEJ. Telomeres must therefore be capped to avoid being processed as DSBs, which would result in end-to-end chromosome fusion [99]. Capping requires shelterin complex member telomeric repeat-binding factor 2 (TRF2), and paradoxically, NHEJ components Ku70, Ku80, and DNA-PKcs [100, 101]. Inhibition of TNKS activity has been demonstrated to lower cellular DNA-PKcs levels, resulting in DSBR defects including end-to-end chromosome fusions [102]. However, TNKS does not appear to regulate DNA-PKcs directly, rather it acts through binding partner TNKS-binding protein 1 of 182 kDa (TNKS1BP1 or TAB182) [69]. TNKS1BP1 both interacts with and promotes the interaction between DNA-PKcs and PARP-1, facilitating DNA-PKcs PARylation and demonstrating cooperation between multiple PARPs [103]. Consistently, TNKS1BP1 depletion sensitized cells to DNA damage, and also elicited deficiency in DSBR [103]. In addition to TNKS, PARP-2 binds to TRF2 and regulates its DNA-binding activity by PARylating the TRF2 dimerization domain [104]. Loss of PARP-2 results in chromosome breaks and shortening of telomeres [104], suggesting overlapping functions between PARP-2 and TNKS at telomeres.
TNKS also preserves genomic integrity by helping to resolve sister chromatid cohesion (Fig. 2). Following DNA replication, sister chromatids are physically connected to each other via complexes called cohesins. This cohesion opposes the force of microtubules that assemble on the mitotic spindle assembly, facilitating HR and maintaining proper orientation until anaphase when the chromosomes are sorted into separate daughter cells [105]. Faulty cohesion and release mechanisms can lead to mitotic defects, aneuploidy, and chromosome instability that can potentially result in cancer [105–109]. Three pathways have been identified that dissociate cohesins at centromeres, chromosome arms, and telomeres. TNKS knockdown stalled mitosis at metaphase, with chromosomes aligned properly at the metaphase plate but not progressing through anaphase [110, 111]. Under these conditions, sister chromatids showed separation at centromeres and chromosome arms, but not at telomeres [110], suggesting that TNKS is directly involved in telomere chromatid dissociation.
The precise mechanism underlying TNKS role in sister chromatid dissociation is not well understood, but it appears to involve a cell cycle-dependent pattern of TNKS ubiquitylation. Active TNKS is normally recognized by RNF146 and tagged for degradation through the addition of K48-linked polyubiquitin chains [112, 113]. However, during the S and G2 phases of the cell cycle, RNF8 adds K63-linked polyubiquitin chains, followed by removal by deubiquitinase complex ABRO1/BRCC36 at the end of mitosis [114]. Interestingly, RNF8 is recruited to sites of DSBR in an ATM-dependent manner along with TNKS-binding partner MDC1 (Fig. 5, Step 4). RNF8 then ubiquitylates histones, also with K63-linkage, generating binding platforms for other DSBR machinery.
Finally, TNKS is a critical component of mitosis. The mitotic spindle apparatus is a microtubule assembly that aids in the separation of chromosomes during mitosis. Proper segregation of genetic material into daughter cells requires precision and coordination, a great amount of physical force [115], and TNKS-mediated PARylation of multiple components [72, 115, 116] (Fig. 2). Microtubule crosslinking protein Nuclear Mitotic Apparatus Protein (NuMA) is a TNKS-binding partner that stabilizes microtubules at spindle poles and acts as a load bearer for the mechanical force generated during separation [117]. NuMA recruits TNKS to spindle poles during mitosis, resulting in NuMA PARylation [118]. In addition, TNKS regulates another mitotic component: Mitotic Kinetics Regulator (Miki). In interphase, Miki is anchored to the Golgi. TNKS-mediated Miki PARylation during the G2/M phase of the cell cycle promotes migration of Miki to centrosomes and mitotic spindles [116]. Although the exact function of TNKS at spindle poles is not well characterized, the importance of TNKS can be inferred from the effects of TNKS depletion, which results in mitotic arrest in anaphase, aberrant prometaphase progression, and spindle defects [116, 119].
TNKS and the DDR–PARPS 1–3 support multiple, sometimes overlapping, aspects of DDR, and TNKS additionally regulates telomere maintenance and mitosis. Preservation of genomic integrity thus appears to be a common function of multiple PARPs, suggesting that TNKS should officially be considered as a PARP enzyme contributing to the DDR. Pharmacological inhibition of DDR–PARPs, specifically PARP-1, has been the subject of intense study for more than a decade due to its applications in cancer treatment. Similarly, disrupting TNKS function is also emerging as a viable cancer therapeutic.
PARP inhibition therapy
PARP inhibition (PARPi) is a potent therapeutic strategy for BRCA-deficient breast and ovarian cancers. BRCA, a tumor suppressor, is a key component of the HR pathway, and BRCA-deficient cells cannot repair DNA through HR. Instead, these cells rely on the ability of PARP-1 to sense damage and activate alternative repair pathways. Although loss of either BRCA or PARP-1 function individually is tolerated, loss of PARP function through PARPi in BRCA-deficient cells renders them incapable of repairing DNA, causing accumulation of DNA damage and cell death in a phenomenon termed “synthetic lethality” [9, 10]. Three PARP inhibitors, olaparib, rucaparib, and niraparib, have been FDA approved for use in cancer treatment [120]. While olaparib and rucaparib are approved for use in treatment of BRCA-deficient ovarian cancers, niraparib is approved for use as a maintenance therapy for recurrent epithelial, ovarian, fallopian tube, or primary peritoneal cancer that respond to platinum-based chemotherapeutic drugs [121].
Inhibition of TNKS catalytic activity (TNKSi) likewise has promising implications in the treatment of cancer and other diseases. In addition to TNKS being overexpressed and associated with poor prognosis in some cancers [122–126], TNKS regulates a number of different pathways that have been implicated in cancer progression. Of the many functions of TNKS, its roles in telomere maintenance and Wnt signaling have the greatest therapeutic implications. Inhibition of telomere lengthening, a strategy that has been shown to stop cancer cell proliferation and induce apoptosis [127, 128], is an attractive treatment option that would be predicted to have minimal effect on healthy cells. Indeed, treatment of gastric cancer cells with TNKSi significantly shortens telomeres and sensitizes telomeres to DNA-damaging agents [80, 129]. Alternatively, aberrant Wnt signaling is found in several types of cancer, and Wnt signaling component APC is one of the most mutated genes in all human cancer [130, 131]. TNKSi-mediated deactivation of Wnt signaling, either alone or in combination with other chemotherapeutic agents, has been shown to reduce cell proliferation and induce apoptosis in several types of cancer including colorectal, brain, lung, and breast [132–143].
TNKS inhibition therefore appears to be a multi-functional treatment option for several types of cancer, and can even treat some types of BRCA-deficient cancer through synthetic lethality [144]. Nevertheless, there are several risk factors associated with TNKSi that have not been addressed in most studies to date. The inherent flaw in the current TNKSi strategy is that inhibiting TNKS catalytic activity may affect all TNKS functions, not just the individual functions that are the focus of investigation. Indeed, studies have shown that TNKS inhibitor XAV939 simultaneously inhibits Wnt signaling, shortens telomeres, and disrupts mitosis [135, 145]. Disruption of TNKS-mediated PARylation has also been shown to reduce insulin sensitivity, increase blood glucose, and is the underlying cause of the genetic disease cherubism [53, 146, 147]. TNKSi may consequently be expected to have several undesirable off-target effects. Nevertheless, rucaparib, the least selective FDA-approved PARP inhibitor, is also a potent inhibitor of TNKS [148]. Inhibition of TNKS activity may therefore be tolerated over the course of treatment, or, alternatively, some of the clinical benefits of PARPi may be due to loss of TNKS function. A more complete understanding of TNKS regulation may highlight novel inhibition strategies, but in the immediate future it will be important for TNKS inhibition studies to monitor the effects of PARPi and TNKSi on off-target TNKS functions.
In summary, several PARP family members contribute to the mechanisms that maintain genome integrity and mediate the cellular response to DNA damage. TNKS has long been appreciated for its roles in the regulation and organization of cellular structures such as telomeres and the mitotic spindle, which are important facets of genome integrity. Recent studies have also implicated TNKS in the regulation of specific DNA repair pathways, suggesting that TNKS should also be considered as a DDR–PARP. The inclusion of TNKS as a DDR–PARP adds to the diversity of functions that PARPs and PARylation play in the cellular response to DNA damage, further supporting the role of PARPs as multifaceted guardians of the genome.
Acknowledgements
Pascal laboratory research on PARP enzymes is supported by grants from the Canadian Institute of Health Research (BMA342854 and PJT374609).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoeijmakers JHJ. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 2.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei H, Yu X. Functions of PARylation in DNA damage repair pathways. Genom Proteom Bioinform. 2016;14:131–139. doi: 10.1016/j.gpb.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aredia F, Scovassi AI. Poly(ADP-ribose): a signaling molecule in different paradigms of cell death. Biochem Pharmacol. 2014;92:157–163. doi: 10.1016/j.bcp.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Schuhwerk H, Atteya R, Siniuk K, Wang ZQ. PARPing for balance in the homeostasis of poly(ADP-ribosyl)ation. Semin Cell Dev Biol. 2017;63:81–91. doi: 10.1016/j.semcdb.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck C, Robert I, Reina-San-Martin B, Schreiber V, Dantzer F. Poly(ADP-ribose) polymerases in double-strand break repair: focus on PARP1, PARP2 and PARP3. Exp Cell Res. 2014;329:18–25. doi: 10.1016/j.yexcr.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 10.Bryant HE, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 11.Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science. 1998;282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- 12.Huang S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 13.Smith S, De Lange T. Tankyrase promotes telomere elongation in human cells. Curr Biol. 2000;10:1299–1302. doi: 10.1016/S0960-9822(00)00752-1. [DOI] [PubMed] [Google Scholar]
- 14.Chambon P, Weill JD, Mandel P. Nicotinamide mononucleotide activation of a new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem Biophys Res Commun. 1963;11:39–43. doi: 10.1016/0006-291X(63)90024-X. [DOI] [PubMed] [Google Scholar]
- 15.Ruf A, Rolli V, de Murcia G, Schulz GE. The mechanism of the elongation and branching reaction of poly(ADP-ribose) polymerase as derived from crystal structures and mutagenesis. J Mol Biol. 1998;278:57–65. doi: 10.1006/jmbi.1998.1673. [DOI] [PubMed] [Google Scholar]
- 16.Crawford K, Bonfiglio JJ, Mikoč A, Matic I, Ahel I. Specificity of reversible ADP-ribosylation and regulation of cellular processes. Crit Rev Biochem Mol Biol. 2018;53:64–82. doi: 10.1080/10409238.2017.1394265. [DOI] [PubMed] [Google Scholar]
- 17.de Murcia JM, et al. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO J. 2003;22:2255–2263. doi: 10.1093/emboj/cdg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alano CC, et al. NAD + depletion is necessary and sufficient for PARP-1—mediated neuronal death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu S-W, et al. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci USA. 2006;103:18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vyas S, et al. Family-wide analysis of poly(ADP-ribose) polymerase activity. Nat Commun. 2015;5:4426. doi: 10.1038/ncomms5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsischky GT, Wilson BA, Collier RJ. Role of glutamic acid 988 of human poly-ADP-ribose polymerase in polymer formation: evidence for active site similarities to the ADP-ribosylating toxins. J Biol Chem. 1995;270:3247–3254. doi: 10.1074/jbc.270.7.3247. [DOI] [PubMed] [Google Scholar]
- 22.Kleine H, et al. Substrate-assisted catalysis by PARP10 limits its activity to mono-ADP-ribosylation. Mol Cell. 2008;32:57–69. doi: 10.1016/j.molcel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Rippmann JF, Damm K, Schnapp A. Functional characterization of the poly(ADP-ribose) polymerase activity of tankyrase 1, a potential regulator of telomere length. J Mol Biol. 2002;323:217–224. doi: 10.1016/S0022-2836(02)00946-4. [DOI] [PubMed] [Google Scholar]
- 24.Dawicki-McKenna JM, et al. PARP-1 activation requires local unfolding of an autoinhibitory domain. Mol Cell. 2015;60:755–768. doi: 10.1016/j.molcel.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langelier MF, Pascal JM. PARP-1 mechanism for coupling DNA damage detection to poly(ADP-ribose) synthesis. Curr Opin Struct Biol. 2013;23:134–143. doi: 10.1016/j.sbi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langelier MF, Zandarashvili L, Aguiar PM, Black BE, Pascal JM. NAD + analog reveals PARP-1 substrate-blocking mechanism and allosteric communication from catalytic center to DNA-binding domains. Nat Commun. 2018;9:844. doi: 10.1038/s41467-018-03234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langelier MF, Planck JL, Roy S, Pascal JM. Structural basis for DNA damage—dependent poly(ADP-ribosyl)ation by human PARP-1. Science. 2012;336:728–732. doi: 10.1126/science.1216338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langelier MF, Riccio A, Pascal JM. PARP-2 and PARP-3 are selectively activated by 5′ phosphorylated DNA breaks through an allosteric regulatory mechanism shared with PARP-1. Nucleic Acids Res. 2014;42:7762–7775. doi: 10.1093/nar/gku474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riccio AA, Cingolani G, Pascal JM. PARP-2 domain requirements for DNA damage-dependent activation and localization to sites of DNA damage. Nucleic Acids Res. 2015;44:1691–1702. doi: 10.1093/nar/gkv1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caldecott KW. DNA single-strand break repair. Exp Cell Res. 2014;329:2–8. doi: 10.1016/j.yexcr.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 31.Chang HHY, Pannunzio NR, Adachi N, Lieber MR. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol. 2017;18:495–506. doi: 10.1038/nrm.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagné JP, et al. Proteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexes. Nucleic Acids Res. 2008;36:6959–6976. doi: 10.1093/nar/gkn771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ariumi Y, et al. Suppression of the poly(ADP-ribose) polymerase activity by DNA-dependent protein kinase in vitro. Oncogene. 1999;18:4616–4625. doi: 10.1038/sj.onc.1202823. [DOI] [PubMed] [Google Scholar]
- 34.Boulton SJ. Combined functional genomic maps of the C. elegans DNA damage response. Science. 2002;295:127–131. doi: 10.1126/science.1065986. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Lu LY, Yang CY, Wang S, Yu X. The FHA and BRCT domains recognize ADP-ribosylation during DNA damage response. Genes Dev. 2013;27:1752–1768. doi: 10.1101/gad.226357.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hochegger H, et al. Parp-1 protects homologous recombination from interference by Ku and Ligase IV in vertebrate cells. EMBO J. 2006;25:1305–1314. doi: 10.1038/sj.emboj.7601015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugimura K, Takebayashi SI, Taguchi H, Takeda S, Okumura K. PARP-1 ensures regulation of replication fork progression by homologous recombination on damaged DNA. J Cell Biol. 2008;183:1203–1212. doi: 10.1083/jcb.200806068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ying S, Hamdy FC, Helleday T. Mre11-dependent degradation of stalled DNA replication forks is prevented by BRCA2 and PARP1. Cancer Res. 2012;72:2814–2821. doi: 10.1158/0008-5472.CAN-11-3417. [DOI] [PubMed] [Google Scholar]
- 39.Illuzzi G, et al. PARG is dispensable for recovery from transient replicative stress but required to prevent detrimental accumulation of poly(ADP-ribose) upon prolonged replicative stress. Nucleic Acids Res. 2014;42:7776–7792. doi: 10.1093/nar/gku505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y-G, Cortes U, Patnaik S, Jasin M, Wang Z-Q. Ablation of PARP-1 does not interfere with the repair of DNA double-strand breaks, but compromises the reactivation of stalled replication forks. Oncogene. 2004;23:3872–3882. doi: 10.1038/sj.onc.1207491. [DOI] [PubMed] [Google Scholar]
- 41.Audebert M, Salles B, Calsou P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem. 2004;279:55117–55126. doi: 10.1074/jbc.M404524200. [DOI] [PubMed] [Google Scholar]
- 42.Wang M, et al. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 2006;34:6170–6182. doi: 10.1093/nar/gkl840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahel D, et al. Poly(ADP-ribose)–dependent regulation of DNA repair by the chromatin remodeling Enzyme ALC1. Science. 2009;325:1240–1243. doi: 10.1126/science.1177321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langelier MF, Ruhl DD, Planck JL, Kraus WL, Pascal JM. The Zn3 domain of human poly(ADP-ribose) polymerase-1 (PARP-1) functions in both DNA-dependent poly(ADP-ribose) synthesis activity and chromatin compaction. J Biol Chem. 2010;285:18877–18887. doi: 10.1074/jbc.M110.105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryant HE, et al. PARP is activated at stalled forks to mediate Mre11-dependent replication restart and recombination. EMBO J. 2009;28:2601–2615. doi: 10.1038/emboj.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haince JF, et al. PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. J Biol Chem. 2008;283:1197–1208. doi: 10.1074/jbc.M706734200. [DOI] [PubMed] [Google Scholar]
- 47.Ko HL, Ren EC. Functional aspects of PARP1 in DNA repair and transcription. Biomolecules. 2012;2:524–548. doi: 10.3390/biom2040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jubin T, et al. Poly ADP-ribose polymerase-1: beyond transcription and towards differentiation. Semin Cell Dev Biol. 2017;63:167–179. doi: 10.1016/j.semcdb.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 49.Kraus WL, Lis JT. PARP goes transcription. Cell. 2003;113:677–683. doi: 10.1016/S0092-8674(03)00433-1. [DOI] [PubMed] [Google Scholar]
- 50.Chi NW, Lodish HF. Tankyrase is a golgi-associated mitogen-activated protein kinase substrate that interacts with IRAP in GLUT4 vesicles. J Biol Chem. 2000;275:38437–38444. doi: 10.1074/jbc.M007635200. [DOI] [PubMed] [Google Scholar]
- 51.Bae J, Donigian JR, Hsueh AJW. Tankyrase 1 interacts with Mcl-1 proteins and inhibits their regulation of apoptosis. J Biol Chem. 2003;278:5195–5204. doi: 10.1074/jbc.M201988200. [DOI] [PubMed] [Google Scholar]
- 52.De Rycker M, Venkatesan RN, Wei C, Price CM. Vertebrate tankyrase domain structure and sterile alpha motif (SAM)-mediated multimerization. Biochem J. 2003;372:87–96. doi: 10.1042/bj20021450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guettler S, et al. Structural basis and sequence rules for substrate recognition by tankyrase explain the basis for cherubism disease. Cell. 2011;147:1340–1354. doi: 10.1016/j.cell.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 54.Chang P, Coughlin M, Mitchison TJ. Tankyrase-1 polymerization of poly(ADP-ribose) is required for spindle structure and function. Nat Cell Biol. 2005;7:1133–1139. doi: 10.1038/ncb1322. [DOI] [PubMed] [Google Scholar]
- 55.Smith S, de Lange T. Cell cycle dependent localization of the telomeric PARP, tankyrase, to nuclear pore complexes and centrosomes. J Cell Sci. 1999;112:3649–3656. doi: 10.1242/jcs.112.21.3649. [DOI] [PubMed] [Google Scholar]
- 56.Leung AKL, et al. Article poly (ADP-Ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol Cell. 2011;42:489–499. doi: 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyons RJ, et al. Identification of a novel human tankyrase through its interaction with the adaptor protein Grb14. J Biol Chem. 2001;276:17172–17180. doi: 10.1074/jbc.M009756200. [DOI] [PubMed] [Google Scholar]
- 58.Chiang YJ, et al. Tankyrase 1 and Tankyrase 2 are essential but redundant for mouse embryonic development. PLoS One. 2008;3:1–10. doi: 10.1371/journal.pone.0002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sbodio JI, Lodish HF, Chi N-W. Tankyrase-2 oligomerizes with tankyrase-1 and binds to both TRF1 (telomere-repeat-binding factor 1) and IRAP (insulin-responsive aminopeptidase) Biochem J. 2002;361:451–459. doi: 10.1042/bj3610451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cook BD, Dynek JN, Chang W, Shostak G, Smith S. Role for the related poly(ADP-Ribose) polymerases tankyrase 1 and 2 at human telomeres. Mol Cell Biol. 2002;22:332–342. doi: 10.1128/MCB.22.1.332-342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim CA, Bowie JU. SAM domains: uniform structure, diversity of function. Trends Biochem Sci. 2003;28:625–628. doi: 10.1016/j.tibs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 62.Qiao F, Bowie JU. The many faces of SAM. Sci STKE. 2005;286:re7. doi: 10.1126/stke.2862005re7. [DOI] [PubMed] [Google Scholar]
- 63.De Rycker M, Price CM. Tankyrase polymerization is controlled by its sterile alpha motif and poly(ADP-ribose) polymerase domains. Mol Cell Biol. 2004;24:9802–9812. doi: 10.1128/MCB.24.22.9802-9812.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mariotti L, et al. Tankyrase requires SAM domain-dependent polymerization to support Wnt-Beta-catenin signaling. Mol Cell. 2016;63:498–513. doi: 10.1016/j.molcel.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riccio AA, McCauley M, Langelier MF, Pascal JM. Tankyrase sterile alpha motif domain polymerization is required for its role in Wnt signaling. Structure. 2016;24:1573–1581. doi: 10.1016/j.str.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwarz-Romond T, et al. The DIX domain of dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- 67.Mosavi LK, Cammett TJ, Desrosiers DC, Peng Z. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 2004;13:1435–1448. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Mahajan A, Tsai M-D. Ankyrin repeat: a unique motif mediating protein-protein interactions. Biochemistry. 2006;45:15168–15178. doi: 10.1021/bi062188q. [DOI] [PubMed] [Google Scholar]
- 69.Seimiya H, Smith S. The telomeric poly(ADP-ribose) polymerase, tankyrase 1, contains multiple binding sites for telomeric repeat binding factor 1 (TRF1) and a novel acceptor, 182-kDa tankyrase-binding protein (TAB182) J Biol Chem. 2002;277:14116–14126. doi: 10.1074/jbc.M112266200. [DOI] [PubMed] [Google Scholar]
- 70.Morrone S, Cheng Z, Moon RT, Cong F, Xu W. Crystal structure of a Tankyrase-Axin complex and its implications for Axin turnover and Tankyrase substrate recruitment. Proc Natl Acad Sci USA. 2012;109:1500–1505. doi: 10.1073/pnas.1116618109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eisemann T, et al. Tankyrase-1 ankyrin repeats form an adaptable binding platform for targets of ADP-ribose modification. Structure. 2016;24:1679–1692. doi: 10.1016/j.str.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 72.Sbodio JI, Chi NW. Identification of a tankyrase-binding motif shared by IRAP, TAB182, and human TRF1 but not mouse TRF1: NuMA contains this RXXPDG motif and is a novel tankyrase partner. J Biol Chem. 2002;277:31887–31892. doi: 10.1074/jbc.M203916200. [DOI] [PubMed] [Google Scholar]
- 73.Li B, et al. Crystal structure of a tankyrase 1–telomere repeat factor 1 complex. Acta Crystallogr F Struct Biol Commun. 2016;2:320–327. doi: 10.1107/S2053230X16004131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DaRosa PA, Klevit RE, Xu W. Structural basis for tankyrase-RNF146 interaction reveals noncanonical tankyrase-binding motifs. Protein Sci. 2018;27:1057–1067. doi: 10.1002/pro.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bisht KK, et al. GDP-Mannose-4,6-dehydratase is a cytosolic partner of tankyrase 1 that inhibits its poly(ADP-Ribose) polymerase activity. Mol Cell Biol. 2012;32:3044–3053. doi: 10.1128/MCB.00258-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Z, Chan CH, Xiao Z, Tan E. Ring finger protein 146/Iduna is a Poly(ADP-ribose) polymer binding and PARsylation dependent E3 ubiquitin ligase. Cell Adhes Migr. 2011;5:463–471. doi: 10.4161/cam.5.6.18356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DaRosa PA, et al. Allosteric activation of the RNF146 ubiquitin ligase by a poly(ADP-ribosyl)ation signal. Nature. 2014;17:223–226. doi: 10.1038/nature13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Z, et al. Recognition of the iso-ADP-ribose moiety in poly(ADP-ribose) by WWE domains suggests a general mechanism for poly(ADP-ribosyl)ation-dependent ubiquitination. Genes Dev. 2012;26:235–240. doi: 10.1101/gad.182618.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang HC, et al. Iduna is a poly(ADP-ribose) (PAR)-dependent E3 ubiquitin ligase that regulates DNA damage. Proc Natl Acad Sci USA. 2011;108:14103–14108. doi: 10.1073/pnas.1108799108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang L, et al. Tankyrase1-mediated poly(ADP-ribosyl)ation of TRF1 maintains cell survival after telomeric DNA damage. Nucleic Acids Res. 2017;45:3906–3921. doi: 10.1093/nar/gkx083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shibata A, et al. Role of ATM and the damage response mediator proteins 53BP1 and MDC1 in the Maintenance of G2/M checkpoint arrest. Mol Cell Biol. 2010;30:3371–3383. doi: 10.1128/MCB.01644-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panier S, Boulton SJ. Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol. 2014;15:7–18. doi: 10.1038/nrm3719. [DOI] [PubMed] [Google Scholar]
- 83.Huen MSY, Sy SMH, Chen J. BRCA1 and its toolbox for the maintenance of genome integrity. Nat Rev Mol Cell Biol. 2010;11:138–148. doi: 10.1038/nrm2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagy Z, et al. Tankyrases promote homologous recombination and check point activation in response to DSBs. PLoS Genet. 2016;12:1–26. doi: 10.1371/journal.pgen.1005791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Y, et al. The p400 ATPase regulates nucleosome stability and chromatin ubiquitination during DNA repair. J Cell Biol. 2010;191:31–43. doi: 10.1083/jcb.201001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shao G, et al. MERIT40 controls BRCA1-Rap80 complex integrity and recruitment to DNA double-strand breaks. Genes Dev. 2009;23:740–754. doi: 10.1101/gad.1739609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng L, Huang T, Chen J. MERIT40 facilitates BRCA1 localization and DNA damage repair. Genes Dev. 2009;23:719–728. doi: 10.1101/gad.1770609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naderali E, et al. Regulation and modulation of PTEN activity. Mol Biol Rep. 2018;45:2869–2881. doi: 10.1007/s11033-018-4321-6. [DOI] [PubMed] [Google Scholar]
- 89.Liu Q, Turner KM, Yung WKA, Chen K, Zhang W. Role of AKT signaling in DNA repair and clinical response to cancer therapy. Neuro Oncol. 2014;16:1313–1323. doi: 10.1093/neuonc/nou058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li N, et al. Poly-ADP ribosylation of PTEN by tankyrases promotes PTEN degradation and tumor growth. Genes Dev. 2015;29:157–170. doi: 10.1101/gad.251785.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Calado R, Dumitriu B. Telomere dynamics in mice and humans. Semin Hematol. 2013;50:165–174. doi: 10.1053/j.seminhematol.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deng Y, Chan S, Chang S. Telomere dysfunction and tumor suppression-the senescence connection. Nat Rev Cancer. 2008;8:450–458. doi: 10.1038/nrc2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 94.Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem. 2004;73:177–208. doi: 10.1146/annurev.biochem.73.071403.160049. [DOI] [PubMed] [Google Scholar]
- 95.Chang W, Dynek JN, Smith S. TRF1 is degraded by ubiquitin-mediated proteolysis after release from telomeres. Genes Dev. 2003;17:1328–1333. doi: 10.1101/gad.1077103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seimiya H, Muramatsu Y, Ohishi T, Tsuruo T. Tankyrase 1 as a target for telomere-directed molecular cancer therapeutics. Cancer Cell. 2005;7:25–37. doi: 10.1016/j.ccr.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 97.Donigian JR, De Lange T. The role of the poly(ADP-ribose) polymerase tankyrase1 in telomere length control by the TRF1 component of the shelterin complex. J Biol Chem. 2007;282:22662–22667. doi: 10.1074/jbc.M702620200. [DOI] [PubMed] [Google Scholar]
- 98.Scherthan H, et al. Mammalian meiotic telomeres: protein composition and redistribution in relation to nuclear pores. Mol Biol Cell. 2000;11:4189–4203. doi: 10.1091/mbc.11.12.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bailey SM, Cornforth MN. Telomeres and DNA double-strand breaks: ever the twain shall meet? Cell Mol Life Sci. 2007;64:2956–2964. doi: 10.1007/s00018-007-7242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bailey SM, et al. DNA double-strand break repair proteins are required to cap the ends of mammalian chromosomes. Proc Natl Acad Sci USA. 1999;96:14899–14904. doi: 10.1073/pnas.96.26.14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van Steensel B, Smogorzewska A, De Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/S0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 102.Dregalla RC, et al. Regulatory roles of tankyrase 1 at telomeres and in DNA repair: suppression of T-SCE and stabilization of DNA-pkcs. Aging (Albany NY) 2010;2:691–708. doi: 10.18632/aging.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zou LH, et al. TNKS1BP1 functions in DNA double-strand break repair though facilitating DNA-PKcs autophosphorylation dependent on PARP-1. Oncotarget. 2015;6:7011–7022. doi: 10.18632/oncotarget.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jaco I. Functional interaction between poly(ADP- ribose) polymerase 2 (PARP-2) and TRF2: PARP activity negatively regulates TRF2. Mol Cell Biol. 2015;2:1595–1607. doi: 10.1128/MCB.24.4.1595-1607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peters JM, Nishiyama T. Sister chromatid cohesion. Cold Spring Harb Perspect Biol. 2012;4:1–18. doi: 10.1101/cshperspect.a011130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sajesh BV, Lichtensztejn Z, McManus KJ. Sister chromatid cohesion defects are associated with chromosome instability in Hodgkin lymphoma cells. BMC Cancer. 2013;13:391. doi: 10.1186/1471-2407-13-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barber TD, et al. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci USA. 2008;105:3443–3448. doi: 10.1073/pnas.0712384105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoque MT, Ishikawa F. Cohesin defects lead to premature sister chromatid separation, kinetochore dysfunction, and spindle-assembly checkpoint activation. J Biol Chem. 2002;277:42306–42314. doi: 10.1074/jbc.M206836200. [DOI] [PubMed] [Google Scholar]
- 109.Hsiao SJ, Smith S. Sister telomeres rendered dysfunctional by persistent cohesion are fused by NHEJ. J Cell Biol. 2009;184:515–526. doi: 10.1083/jcb.200810132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dynek JN. Resolution of sister telomere association is required for progression through mitosis. Science. 2004;304:97–100. doi: 10.1126/science.1094754. [DOI] [PubMed] [Google Scholar]
- 111.Kim MK, Smith S, Bloom KS. Persistent telomere cohesion triggers a prolonged anaphase. Mol Biol Cell. 2014;25:30–40. doi: 10.1091/mbc.e13-08-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Callow MG, et al. Ubiquitin ligase RNF146 regulates tankyrase and Axin to promote Wnt signaling. PLoS One. 2011;6:e22595. doi: 10.1371/journal.pone.0022595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Y, et al. RNF146 is a poly(ADP-ribose)directed E3 ligase that regulates axin degradation and Wnt signalling. Nat Publ Group. 2011;13:623–629. doi: 10.1038/ncb2222. [DOI] [PubMed] [Google Scholar]
- 114.Tripathi E, Smith S. Cell cycle-regulated ubiquitination of tankyrase 1 by RNF8 and ABRO1/BRCC36 controls the timing of sister telomere resolution. EMBO J. 2016;36:1–17. doi: 10.15252/embj.201695135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maiato H, Gomes A, Sousa F, Barisic M. Mechanisms of chromosome congression during mitosis. Biology (Basel) 2017;6:E13. doi: 10.3390/biology6010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ozaki Y, et al. Poly-ADP ribosylation of Miki by tankyrase-1 promotes centrosome maturation. Mol Cell. 2012;47:694–706. doi: 10.1016/j.molcel.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 117.Elting MW, Prakash M, Udy DB, Dumont S. Mapping load-bearing in the mammalian spindle reveals local kinetochore-fiber anchorage that provides mechanical isolation and redundancy. bioRxiv. 2017;27:1–30. doi: 10.1016/j.cub.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chang W, Dynek JN, Smith S. NuMA is a major acceptor of poly(ADP-ribosyl)ation by tankyrase 1 in mitosis. Biochem J. 2005;184:177–184. doi: 10.1042/BJ20050885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chang P, Coughlin M, Mitchison TJ. Interaction between Poly(ADP-ribose) and NuMA contributes to mitotic spindle pole assembly. Mol Biol Cell. 2009;20:4575–4585. doi: 10.1091/mbc.e09-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim G, et al. FDA approval summary: olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res. 2015;21:4257–4261. doi: 10.1158/1078-0432.CCR-15-0887. [DOI] [PubMed] [Google Scholar]
- 121.Scott LJ. Niraparib: first global approval. Drugs. 2017;77:1029–1034. doi: 10.1007/s40265-017-0752-y. [DOI] [PubMed] [Google Scholar]
- 122.Xu D, et al. Telomerase activity in plasma cell dyscrasias. Br J Cancer. 2001;84:621–625. doi: 10.1054/bjoc.2000.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gelmini S, et al. Tankyrase, a positive regulator of telomere elongation, is over expressed in human breast cancer. Cancer Lett. 2004;216:81–87. doi: 10.1016/j.canlet.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 124.Gelmini S, et al. Distribution of Tankyrase-1 mRNA expression in colon cancer and its prospective correlation with progression stage. Oncol Rep. 2006;16:1261–1266. [PubMed] [Google Scholar]
- 125.Gelmini S, et al. Tankytase-1 mRNA expression in bladder cancer and paired urine sediment: preliminary experience. Clin Chem Lab Med. 2007;45(7):862–866. doi: 10.1515/CCLM.2007.133. [DOI] [PubMed] [Google Scholar]
- 126.Tang B, et al. Expression of TNKS1 is correlated with pathologic grade and Wnt/β-catenin pathway in human astrocytomas. J Clin Neurosci. 2012;19:139–143. doi: 10.1016/j.jocn.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 127.Hodes R. Molecular targeting of cancer: telomeres as targets. Proc Natl Acad Sci USA. 2001;98:7649–7651. doi: 10.1073/pnas.151267698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ahmed A, Tollefsbol T. Telomeres, telomerase, and telomerase inhibition: clinical implications for cancer. J Am Geriatr Soc. 2003;51:116–122. doi: 10.1034/j.1601-5215.2002.51019.x. [DOI] [PubMed] [Google Scholar]
- 129.Zhang HAO, Yang M, Zhao J, Chen L, Yu S. Inhibition of tankyrase 1 in human gastric cancer cells enhances telomere shortening by telomerase inhibitors. Oncol Rep. 2010;24:1059–1065. doi: 10.3892/or.2010.1059. [DOI] [PubMed] [Google Scholar]
- 130.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4:1–13. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Novellasdemunt L, Antas P, Li VSW. Targeting Wnt signaling in colorectal cancer. Am J Physiol Cell Physiol. 2015;309:C511–C521. doi: 10.1152/ajpcell.00117.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Renna C, Salaroli R, Cocchi C, Cenacchi G. XAV939-mediated ARTD activity inhibition in human MB cell lines. PLoS One. 2015;10:1–14. doi: 10.1371/journal.pone.0124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Scarborough HA, et al. AZ1366: an inhibitor of tankyrase and the canonical Wnt pathway that limits the persistence of non-small cell lung cancer cells following EGFR inhibition. Clin Cancer Res. 2017;23:1531–1541. doi: 10.1158/1078-0432.CCR-16-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thomson DW, et al. Discovery of a highly selective Tankyrase inhibitor displaying growth inhibition effects against a diverse range of tumor derived cell lines. J Med Chem. 2017;60:5455–5471. doi: 10.1021/acs.jmedchem.7b00137. [DOI] [PubMed] [Google Scholar]
- 135.Lupo B, et al. Tankyrase inhibition impairs directional migration and invasion of lung cancer cells by affecting microtubule dynamics and polarity signals. BMC Biol. 2016;14:5. doi: 10.1186/s12915-016-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu X, Luo F, Li J, Zhong X, Liu K. Tankyrase 1 inhibitor XAV939 increases chemosensitivity in colon cancer cell lines via inhibition of the Wnt signaling pathway. Int J Oncol. 2016;48:1333–1340. doi: 10.3892/ijo.2016.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang H, et al. Tankyrase inhibitor sensitizes lung cancer cells to endothelial growth factor receptor (EGFR) inhibition via stabilizing angiomotins and inhibiting yap signaling. J Biol Chem. 2016;291:15256–15266. doi: 10.1074/jbc.M116.722967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Arqués O, et al. Tankyrase inhibition blocks Wnt/b-catenin pathway and reverts resistance to PI3 K and AKT inhibitors in the treatment of colorectal cancer. Clin Cancer Res. 2016;22:644–656. doi: 10.1158/1078-0432.CCR-14-3081. [DOI] [PubMed] [Google Scholar]
- 139.Bao R, et al. Inhibition of tankyrases induces axin stabilization and blocks Wnt signalling in breast cancer cells. PLoS One. 2012;7:e48670. doi: 10.1371/journal.pone.0048670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Casás-Selves M, et al. Tankyrase and the canonical Wnt pathway protect lung cancer cells from EGFR inhibition. Cancer Res. 2012;72:4154–4164. doi: 10.1158/0008-5472.CAN-11-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tian X, et al. XAV939, a tankyrase 1 inhibitior, promotes cell apoptosis in neuroblastoma cell lines by inhibiting Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res. 2013;32:1–9. doi: 10.1186/1756-9966-32-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stratford EW, et al. The tankyrase-specific inhibitor JW74 affects cell cycle progression and induces apoptosis and differentiation in osteosarcoma cell lines. Cancer Med. 2014;3:36–46. doi: 10.1002/cam4.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lau T, et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013;73:3132–3144. doi: 10.1158/0008-5472.CAN-12-4562. [DOI] [PubMed] [Google Scholar]
- 144.Mccabe N, et al. Targeting Tankyrase 1 as a therapeutic strategy for BRCA-associated cancer. Oncogene. 2009;28:1465–1470. doi: 10.1038/onc.2008.483. [DOI] [PubMed] [Google Scholar]
- 145.Kulak O, et al. Disruption of Wnt/beta-catenin signaling and telomeric shortening are inextricable consequences of tankyrase inhibition in human cells. Mol Cell Biol. 2015;35:2425–2435. doi: 10.1128/MCB.00392-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo HL, et al. The Axin/TNKS complex interacts with KIF3A and is required for insulin-stimulated GLUT4 translocation. Cell Res. 2012;22:1246–1257. doi: 10.1038/cr.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Levaot N, et al. 3BP2-deficient mice are osteoporotic with impaired osteoblast and osteoclast functions. J Clin Inves. 2011;131:3244–3257. doi: 10.1172/JCI45843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thorsell AG, et al. Structural basis for potency and promiscuity in poly(ADP-ribose) polymerase (PARP) and tankyrase inhibitors. J Med Chem. 2017;60:1262–1271. doi: 10.1021/acs.jmedchem.6b00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Coster G, Goldberg M. The cellular response to DNA damage: a focus on MDC1 and its interacting proteins. Nucleus. 2010;1:166–178. doi: 10.4161/nucl.11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vikrant, Sawant UU, Varma AK. Role of MERIT40 in stabilization of BRCA1 complex: a protein–protein interaction study. Biochem Biophys Res Commun. 2014;446:1139–1144. doi: 10.1016/j.bbrc.2014.03.073. [DOI] [PubMed] [Google Scholar]