Abstract
Faithful chromosome segregation during mitosis in eukaryotes requires attachment of the kinetochore, a large protein complex assembled on the centromere of each chromosome, to the spindle microtubules. The kinetochore is a structural interface for the microtubule attachment and provides molecular surveillance mechanisms that monitor and ensure the precise microtubule attachment as well, including error correction and spindle assembly checkpoint. During mitotic progression, the kinetochore undergoes dynamic morphological changes that are observable through electron microscopy as well as through fluorescence microscopy. These structural changes might be associated with the kinetochore function. In this review, we summarize how the dynamics of kinetochore morphology are associated with its functions and discuss recent findings on the switching of protein interaction networks in the kinetochore during cell cycle progression.
Keywords: Kinetochore, Centromere, Cell cycle, Mitosis, Phosphorylation, Protein-protein interaction
Introduction
Faithful chromosome segregation is essential for self-replication in living organisms. Failure of these processes causes chromosomal instability, resulting in aneuploidy and subsequent genetic disorders. Accurate chromosome segregation in eukaryotic cells is achieved by precise bipolar attachment of the spindle microtubules to each sister chromatid. The microtubules-sister chromatids linkage is enabled by the kinetochore, a large protein complex assembled on the specific genomic region in chromosomes named centromere [1–3]. The kinetochore also provides a binding platform for spindle assembly checkpoint (SAC) proteins to monitor and correct erroneous microtubule attachment to the kinetochore [1, 4–6]. Given that the kinetochore plays these crucial roles, deciphering the mechanism underlying assembly of the complex is significant for understanding the process behind precise chromosome segregation.
The kinetochore consists of more than a hundred proteins in vertebrates [1, 5, 7, 8]. Studies using electron microscopy (EM) or fluorescence microscopy have indicated that morphology of the outer kinetochore structure changes dynamically during mitotic progression [9–13]. The changes occur upon microtubules attachment to the kinetochore, which alters localization of multiple kinetochore proteins, including SAC proteins. The pulling force produced from microtubules to the kinetochore distorts the shapes of centromeric chromatin and inner kinetochore. It is suggested that the shape changes are important for the stable bipolar microtubule attachment and for SAC signal silencing [4, 14–16].
The microtubules and centromeric chromatin are connected by two major protein interaction networks in the kinetochore: constitutive centromere-associated network (CCAN) and the Knl1 complex–Mis12 complex–Ndc80 complex network (KMN) [2, 8, 17, 18]. CCAN is a 16-subunit complex in vertebrates and is associated with centromeric chromatin throughout the cell cycle. KMN is recruited onto CCAN in M-phase. As the Ndc80 complex (Ndc80C) of KMN directly binds to the microtubules together with other microtubule-binding proteins [19], the CCAN–KMN interaction forms a key step in building the microtubule–kinetochore interaction surface. We recently observed dynamic interaction changes between centromere, CCAN and KMN during cell cycle progression [20, 21]. In this review, we discuss the changes in the kinetochore morphology and the dynamics of the protein interaction network that provides plasticity to the kinetochore during mitotic progression.
The centromere and kinetochore
The centromere is a specific genomic region in the chromosome recognizable by a primary constriction [3]. In most species, except for in budding yeast, the centromere position is specified by sequence-independent epigenetic mechanisms [3, 22]. A key epigenetic marker for centromere specification is the centromere protein (CENP)-A, a centromeric histone H3 variant. CENP-A assembles a centromere-specific nucleosome with histone H4 and H2A/B [3, 22], forming the centromeric chromatin with interspersed H3-containing nucleosomes (Fig. 1).
Fig. 1.

The kinetochore in M-phase. a Mitotic chromosome segregation and a trilaminar structural model for the kinetochore. The kinetochore is a large protein complex built on the centromere of each sister chromatid (chromosome). The kinetochores attach to microtubules emanating from each opposing spindle pole, segregating chromatids. Electron microscopy studies revealed that the kinetochore has three layers in its structure: electron-dense inner and outer plates, and an electron-translucent middle layer. b A model of basic kinetochore structure. The main structure of the kinetochore is formed of constitutive centromere-associated network (CCAN) composed of 16 protein subunits and the KMN (Knl1, Mis12, and Ndc80 complexes: Knl1C, Mis12C, Ndc80C) network (KMN). CCAN interacts with the centromeric chromatin epigenetically marked with the CENP-A nucleosome during the entire cell cycle. CCAN recruits KMN, which directly binds to microtubules, in M-phase, forming a linkage between the centromere and microtubules
A large number of proteins are assembled onto the centromere, building the kinetochore that provides a bridge between centromeric chromatin and microtubules during mitosis. EM studies have revealed that the vertebrate kinetochore forms a trilaminar structure on the centromere, with electron-dense inner and outer plates, and an electron-translucent middle layer (Fig. 1a) [11, 23–27].
Although the composition of molecular substances in each layer needs to be investigated further, CENP-A and CCAN are known to be components of the inner plate [28]. CCAN binds to the CENP-A-containing centromeric chromatin throughout the cell cycle [8, 17, 22]. In the M-phase, CCAN recruits KMN [18, 29–31]. An EM study demonstrated that Ndc80C of KMN is a component of the outer plate [32]. Owing to its microtubule-binding ability, Ndc80C builds a microtubule–kinetochore interaction surface for a stable end-on attachment together with other microtubule-binding proteins (Fig. 1B) [29, 33–35].
Dynamic morphological changes in the kinetochore during M-phase
Kinetochore expansion
In addition to the trilaminar structure, EM studies have also depicted that an expanded fibrous structure—known as the fibrous corona—radiates from the distal surface of the outer plate in the kinetochore without attached microtubules (Fig. 2a) [10, 24, 26, 36–40]. This prominent fibrous structure extending over 100 nm to the cytoplasm is diminished in the kinetochores that attach to microtubules, suggesting that the kinetochore morphology alters dynamically upon kinetochore–microtubule attachment during M-phase [10, 41]. The corona includes proteins such as motor proteins (CENP-E, dynein), Rod–Zw10–Zwilch complex (RZZ), SAC proteins, and microtubule-associating proteins, and these components demonstrate equivalent dynamic morphological changes in light microscopic studies [12, 39, 40, 42–47]. In cells treated with drugs inhibiting microtubules polymerization, the corona forms a large crescent-like structure on the centromere or expands further, resulting in a ring surrounding the sister centromeres [12, 43, 45–47].
Fig. 2.
Kinetochore expansion and compaction during M-phase. a Fibrous corona. An expanded structure, called “fibrous corona” is formed on the outer kinetochore at unattached kinetochores. Once microtubules attach to the kinetochore, most of the components of the expansion are removed in a dynein-dependent manner. b Rod–Zw10–Zwilch complex (RZZ). RZZ forms a dimer with the antiparallel arrangement of Rod. Spindly and Mps1 kinase activity promote oligomerization of RZZ. Farnesylation of Spindly C terminus is crucial for the RZZ oligomerization by suppression of the inhibitory effect of Spindly N terminus
The expanded crescent is also observed during early prophase in unperturbed cells and then becomes compact after microtubule end-on attachment by removal of the corona proteins dependent on dynein (Fig. 2a) [12, 46, 48–51]. These observations indicate that the outer kinetochore morphology alters dynamically during mitotic progression. The expansion and compaction of the outer kinetochore play important roles in robust spindle assembly [12, 13, 45]. The expanded shape in unattached kinetochores facilitates lateral microtubule attachment that later promotes efficient end-on attachment. Further, the microtubule attachment induces compaction of the outer kinetochore; therefore, the structural change might reduce the risk of binding to microtubules from the opposite pole. Disruption of compaction leads to erroneous microtubule attachment, such as merotelic attachment, as well as chromosome segregation error [47].
RZZ and Spindly oligomerize to form expanded kinetochore
What makes the expanded fibrous structures had been a long-standing question. Recent studies have demonstrated that oligomerization of the RZZ complex and Spindly protein contributes to the formation of the meshwork for expanded outer kinetochore [43, 44, 47].
The RZZ complex consists of three subunits that were originally identified in Drosophila: Rod (Rough Deal), Zw10 (Zest-White 10), and Zwilch, and is conserved in metazoans (Fig. 2b) [52–59]. Based on size exclusion column chromatography analyses, it was suggested that the RZZ complex contains two copies of each subunits [52, 59]. Indeed, structural study on human RZZ demonstrated the 2:2:2 stoichiometric RZZ complex [60]. In the complex, two molecules of Rod, the largest subunit, interact in an antiparallel configuration with beta-propellers in its N terminus at opposite ends (Fig. 2b) [60]. Spindly, which was originally discovered in Drosophila as a dynein adaptor detected on the kinetochore in mitosis, has functions in chromosome alignment and SAC signaling [61–64]. Human Spindly is farnesylated at its C terminus that is essential for its kinetochore interaction and binding with RZZ via the beta-propellers of Rod [60, 65, 66].
Both Spindly and RZZ are proteins that reside on the expanded crescents [43, 44, 47, 56, 64]. RZZ localization to the kinetochore depends on Knl1 and Bub1, a KMN subunit and SAC protein, respectively, followed by recruitment of Spindly [60, 67–71]. Depletion of the Spindly or RZZ components introduces defects in SAC signaling and also prevents formation of the expanded crescent structure on outer kinetochores in nocodazole-treated cells [43, 44, 47], suggesting that these proteins are responsible for kinetochore expansion.
Given that RZZ dimer structure is similar to that of the membrane coat protein complex, which self-assembles to forms oligomers, RZZ has been suggested to be oligomerized [60]. However, purified recombinant human RZZ components did not form oligomers, in vitro [47, 60]. However, the RZZ complex in a mixture with farnesyled Spindly oligomerizes into filamentous structures [47], suggesting that Spindly interaction with RZZ is crucial for RZZ oligomerization (Fig. 2b). Farnesylation is necessary for kinetochore expansion [47]. Furthermore, Sacristan et al. [47] demonstrated that an auto-inhibitory role of the N-terminal region of Spindly in RZZ oligomerization (Fig. 2b). The Spindly N-terminal region is arranged in an intramolecular inhibitory conformation to prevent RZZ-Spindly oligomerization [47]. During mitosis, Spindly has to be released from the inhibitory configuration to form the expanded crescent. One of the key factors that regulate the process is a mitotic kinase, Mps1 that is necessary for outer kinetochore expansion. Spindly ∆N induces the kinetochore expansion under an Mps1-inhibited condition (Fig. 2b) [43, 47]. Although the molecular mechanisms underlying the prevention of Spindly autoinhibition enabled by Mps1 are yet to be studied, Rod is one of the Mps1 substrates observed to be involved in the expansion [43]. Rod has two Mps1 phosphorylation sites—Thr13 and Ser15 of human Rod [43]. While Rod with Alanine substitution in these sites forms the RZZ complex, localizes to the kinetochore, and recruits Spindly and other crescent proteins in human cells, the outer kinetochores do not undergo expansion in nocodazole-treated cells expressing the Rod phospho-dead mutant [43].
These findings indicate that post-translational modifications of the RZZ complex and Spindly play key roles in the kinetochore expansion. In addition to Spindly farnesylation and Mps1-mediated phosphorylation for the RZZ complex, Cdk1 activity is also involved in the expansion by tethering of RZZ onto outer kinetochore [44, 47].
As suggested by light and electron microscopy studies, the expanded outer kinetochores composed of Spindly-RZZ oligomers facilitate lateral attachment of microtubules to establish robust kinetochore–microtubule attachment [38, 47]. This is consistent with a prediction by a mathematical model [45]. The expanded Spindly-RZZ structure is also engaged in SAC signal maintenance, interacting with the Mad1–Mad2 complex [43, 68, 70, 72–75]. After end-on attachment is established, the expanded crescent structure is released from the kinetochores by poleward transport of dynein [12, 48–51]. Spindly has dynein–dynactin binding domains in its N-terminal region [5, 64, 66, 71]. Deletion or mutations in the domains prevent dynein recruitment to the kinetochores, even though the expanded crescent structures still form at the kinetochores, indicating that dynein is not necessary for outer kinetochore expansion [47, 64, 66, 71]. However, the expanded outer kinetochores, with the Spindly mutants that do not bind to dynein, are maintained even in metaphase. The cells with the persistent expanded crescent structures cause microtubule malattachments and subsequent chromosome mis-segregation, indicating that temporal morphological dynamics of the outer kinetochore in conjunction with mitotic progression is essential for correct microtubule–kinetochore attachment, ensuring faithful chromosome segregation [45, 47, 64].
Kinetochore stretches
Inter- and intra-kinetochore stretch
Once microtubules emanating from opposing spindle poles capture the kinetochores on sister chromatids, the sister kinetochore distance is increased [76]. This structural change is called centromere stretch or inter-kinetochore stretch (Fig. 3). The change in inter-kinetochore distance indicates tension applied on the kinetochores by microtubule-pulling force, as treatment of cells with Taxol, which stabilizes microtubules and inhibits microtubule dynamics, prevents the inter-kinetochore stretch even with kinetochore-attached microtubules [76, 77].
Fig. 3.
Kinetochore stretches. When the spindle microtubules attach to sister kinetochores, the distance between inner kinetochore proteins of a sister kinetochore is stretched out (centromere stretch or inter-kinetochore stretch: dinnner). The kinetochore itself is stretched by microtubule attachment (intra-kinetochore stretch). The intra-kinetochore distance is defined by half of the length difference between dinnner and douter (distance between the outer kinetochore proteins of a sister kinetochore)
In addition to the increase in the distance between the sister kinetochores, the kinetochore itself stretches in length between inner and outer kinetochore proteins upon microtubule attachment (intra-kinetochore stretch) (Fig. 3) [77, 78]. The intra-kinetochore distance is measured by colocalization analysis of two-color fluorescence proteins (outer and inner kinetochore proteins labeled with different colors) in living and fixed cells (Fig. 3) [77, 78]. To calculate intra-kinetochore distance, the distance between inner or outer kinetochore proteins in the sister kinetochores are measured, respectively. The distance between the inner proteins is subtracted from that between the outer proteins, and half of this value is defined as the intra-kinetochore distance (Fig. 3). This method allows correction of errors due to chromatic aberration [77–80].
The bipolar attachment of microtubules causes both inter- and intra-kinetochore deformation. However, they do not appear to be a simple two-linked Hookean spring, as the weak treatment of cells with nocodazole, which inhibits microtubule polymerization, allows inter-kinetochore stretch, while it does not induce intra-kinetochore stretch [78]. Moreover, weak Taxol treatment leads to intra-kinetochore stretch, while it prevents inter-kinetochore stretch [77]. These findings suggest that the microtubule-pulling force might not simply induce the intra-kinetochore stretch, and there might be other mechanisms involved in the intra-kinetochore stretch, such as conformational changes in the kinetochore.
Kinetochore stretch and error correction
The deformation of kinetochore is proposed to regulate error correction of microtubule malattachments [14, 15]. Aurora B kinase (AurB) is an important kinase controlling the error correction machinery [29, 34, 81–85]. AurB is a catalytic subunit of chromosome passenger complex (CPC) along with other subunits (INCENP, Borealin and Survivin) [86–95]. CPC is involved in various regulatory processes in mitotic progression and correlated with its function, its localization alters dynamically during mitotic progression from chromosome arms to spindle midzone via centromeres.
CPC localizes to centromeres between prometaphase and metaphase and plays a key role in the error correction. The kinase activity of AurB in CPC forms a gradient centered at the inner centromere (Fig. 4) [14, 96–100]. In the absence of microtubule-binding or tension due to malattachment of microtubule–kinetochore, AurB substrates in the kinetochore are placed within or near to the AurB kinase gradient, leading to their phosphorylation. Among those substrates, KMN components, which provides a microtubule-binding surface of the kinetochore, are major AurB substrates in the outer kinetochore [100]. Ndc80 (also called Hec1 in vertebrates) is a critical substrate of AurB for error correction in KMN (Fig. 4). Ndc80 directly binds to microtubules through a globular domain in its N terminus [29, 33–35]. AurB phosphorylates the Ndc80 N-terminal tail preceding the globular domain, and the phosphorylated Ndc80 does not bind properly to microtubules [29, 34, 100–102]. When kinetochores attach incorrectly to microtubules, AurB phosphorylates Ndc80 and destabilizes the Ndc80–microtubule interaction [29, 33, 34, 84, 103–105]. Once the precise bi-polar attachment is established, AurB substrates in the outer kinetochore are physically separated from the AurB activity gradient and are dephosphorylated by protein phosphatase counteracting AurB, such as protein phosphatase 1 (PP1) (Fig. 4) [102, 105–110]. The dephosphorylated Ndc80 stabilizes the microtubule–kinetochore attachment to enable proper chromosome segregation (Fig. 4) [29, 34, 100–102].
Fig. 4.

Microtubule attachment and spindle assembly checkpoint. Aurora B kinase (AurB) localizes in inner centromere and forms a gradient of kinase activity centered at the centromere. AurB phosphorylates proteins in the kinetochore. Ndc80 is an AurB substrate that resides in the outer kinetochore. Without the correct bipolar microtubule attachment, Ndc80 remains in the AurB activity gradient and is phosphorylated, leading to unstable attachment to the microtubules. The unattached and malattached kinetochores activate spindle assembly checkpoint (SAC) signals. Correct and stable microtubule–kinetochore attachment silences SAC signals and further stabilizes the attachment by pulling out Ndc80 from the AurB kinase active zone through the intra-kinetochore stretch. Although the kinetochore stretch enables stable microtubule attachment, the stretch itself is not necessary; however, a stable microtubule attachment is sufficient for SAC silencing
Distance of kinetochore substrates from the AurB active zone is a significant factor for their dephosphorylation. However, the removal of tension after dephosphorylation of substrates does not result in rephosphorylation [101], suggesting that additional mechanisms are necessary to sustain dephosphorylation.
SAC silencing and microtubule attachment
The bipolar attachment induces the kinetochore stretches, which further stabilize the microtubule–kinetochore attachment (Fig. 3). Since the inter-kinetochore stretch cab easily be observed under a light microscope, it is often used as an indicator of the bioriented stable microtubule–kinetochore attachment that is necessary for SAC silencing in anaphase onset. However, it was observed through detailed live-cell imaging analyses that the inter-kinetochore stretch itself is not necessary for SAC silencing; instead, the intra-kinetochore stretch is necessary for it [77, 78]. As mentioned above, it has been proposed that the inter- and intra-kinetochores are not two-linked Hookean springs. Inter- and intra-kinetochore stretches can be separated using microtubule-targeting drugs [77, 78]. The mitotic duration is much longer when the inter-, but not the intra-kinetochore stretch occurs, compared to that in an unperturbed control condition, suggesting that SAC silencing is ineffective without the intra-kinetochore stretch [78]. In contrast, during only intra-kinetochore stretch, without an increase in the inter-kinetochore distance in mitotic cells, the cells progress enter into anaphase with the same mitotic duration as the control cells, indicating that the SAC signal is silenced without the inter-kinetochore stretch [77]. These observations indicate that the intra-kinetochore stretch without inner-kinetochore stretch is sufficient for SAC inactivation.
Provided that the SAC monitors the bipolar microtubule–kinetochore attachment, SAC machinery has to sense some changes in the kinetochore upon the bipolar microtubule–kinetochore attachment for SAC silencing. As the intra-kinetochore stretch is involved in SAC silencing, the change in intra-kinetochore distance might directly trigger SAC silencing. However, this does not appear to be the case. The bipolar microtubule–kinetochore attachment stretches the kinetochore by microtubule-pulling force, separating outer kinetochore proteins form the centromere. This facilitates dephosphorylation of AurB substrates in the outer kinetochore. As mentioned above, dephosphorylated Ndc80 stabilizes microtubule–kinetochore attachment [29, 33, 34]. When an Ndc80 mutant, with all potential AurB phosphorylation sites substituted with alanine, was expressed in human cells, the cells silenced the SAC and exit from metaphase in the absence of bi-oriented sister and microtubule-pulling force [111, 112], suggesting that the intra-kinetochore stretch might not play a direct role in SAC silencing; yet, the stable microtubule–kinetochore interaction is sufficient for it (Fig. 4). Although the intra-kinetochore stretch is not a direct cue for SAC inactivation, this stretch is an event occurring upstream of SAC silencing, since stable microtubule–kinetochore attachment requires Ndc80 dephosphorylation induced by the intra-kinetochore stretch (Fig. 4). It is important to clarify next how the stable microtubule–kinetochore attachment induces changes in the kinetochore structure and how these changes lead to SAC silencing.
Dynamic interaction between CCAN and centromere
CCAN and centromere interaction interface
The kinetochore bridges centromeric chromatin with microtubules. One of the kinetochore components associated with the centromeric chromatin is CCAN, which is a 16-protein complex and constitutively localizes to the centromere during cell cycle progression (Fig. 1b) [8, 17]. It has been demonstrated that CCAN has three interfaces for the centromeric chromatin binding: CENP-T, CENP-N and CENP-C (Fig. 5). CENP-T has a histone-hold domain in its C terminus and forms a complex with three other histone-hold proteins (CENP-W, -S, and -X) to assemble a nucleosome-like structure (Fig. 5). This complex directly binds to centromeric DNA. However, the mechanism underlying binding of CENP-T complex to the centromeric DNA in cells remains to be determined, though DNA rounds up the nucleosome-like CENP-T/W/S/X complex in vitro, and it is suggested that CENP-T associates with H3 nucleosomes on centromeres [113, 114]. CENP-N and CENP-C bind to the CENP-A nucleosome directly in vitro, and it is proposed that these factors recognize centromere chromatin (Fig. 5) [115–118].
Fig. 5.

CCAN-centromere interaction interfaces. CENP-T interacts with centromere chromatin containing histone H3 nucleosomes. CENP-N and CENP-C bind to the CENP-A nucleosome. CENP-N interacts with CENP-A L1 loop (also known as RG loop because of the presence of conserved Arg and Gly residues). CENP-C binds to the CENP-A C-terminal tail and the histone H2A/B acidic patch in the CENP-A nucleosome
Recent studies using Cryo-EM have determined the structures of the human CENP-A nucleosome with CENP-N [119–122]. The CENP-N N-terminal region binds to the CENP-A nucleosome surface as well as to the DNA. CENP-N interacts with CENP-A via L1-loop (also known as RG loop, because of the presence of conserved Arg and Gly residues) of CENP-A that is unique to CENP-A, though it is absent from the canonical histone H3 [123]. The RG loop interaction of CENP-N is necessary for CENP-N recruitment to the centromere in cells [120] (Fig. 5). In addition, basic residues in the N-terminal region of CENP-N interact with nucleosomal DNA and appear to stabilizes the DNA segments at the entrance and exit of the CENP-A nucleosome that have been shown to be flexible [123].
CENP-C forms another contact surface with the CENP-A nucleosome. CENP-C has the evolutionarily conserved motif called “CENP-C motif” in its C-terminal region (Fig. 5) [115, 118]. Human CENP-C has another contact sequence in the central region called “central domain”, which shows sequence similarity to the CENP-C motif [115, 118, 124]. These sequences directly bind to the CENP-A nucleosome [21, 115, 118, 124]. Structural analysis using a short peptide, including the CENP-C motif, revealed that CENP-C motif binds to the C-terminal tail of CENP-A and the acidic patch of histone H2A/2B in the CENP-A nucleosome [118].
Cell cycle-dependent interaction changes between CCAN-CENP-A interaction
Although the CENP-C motif stably binds the CENP-A nucleosome in vitro, recent studies have revealed that the interaction is dynamic during cell cycle progression in cells [21, 125]. The CENP-C C-terminal region, including the CENP-C motif, interacts with centromeric chromatin containing the CENP-A nucleosome only in M-phase, and not in interphase in human RPE-1 and chicken DT40 cells (Fig. 6) [21, 125]. This mitotic interaction requires phosphorylation at a CDK1 consensus site (Thr 651 and Thr734 for chicken and human CENP-C, respectively) adjacent to and upstream of the CENP-C motif in both human and chicken CENP-C (Fig. 6) [21]. The site is directly phosphorylated by CDK1 and the phosphorylation positively regulates the interaction between the CENP-C motif and CENP-A nucleosome in vitro [21]. The CDK1-mediated phospho-regulation of CENP-C could be significant for proper kinetochore assembly in M-phase [21]. Since the phosphorylation site does not include the CENP-C motif peptide used in current structural studies and modelling [118, 122], use of the longer CENP-C C-terminal region including the phospho-residue is crucial in future structural analyses for better understanding of the molecular basis of the CENP-C–CENP-A nucleosome interaction.
Fig. 6.

Cell cycle-dependent switching of interaction between CENP-A and CENP-C. As a CCAN component, CENP-C constitutively localizes to the centromere throughout the cell cycle. During interphase, the CENP-C localization relies on the CENP-H complex (CENP-H, -I, -K, and -M) and the CENP-L-N complex. During M-phase, in addition to the contacts, CENP-C binds directly to the CENP-A nucleosome. The M-phase interaction requires threonine phosphorylation by CDK1 (Thr651 in chicken CENP-C and Thr734 in human CENP-C)
Localization hierarchy of CENP-C to centromeres changes between interphase and M-phase [126, 127]. In interphase, centromere localization of CENP-C requires the CENP-H complex, as centromeric CENP-C signals are not detected in CENP-H- or CENP-K (a member of the CENP-H complex)-depleted interphase cells. However, CENP-C localizes to mitotic kinetochore in the CENP-H- or CENP-K-depleted cells, suggesting that CENP-C directly binds to centromere during M-phase, while it localizes to centromeres via the CENP-H complex in interphase cells. This change is well explained by the CDK1-mediated CENP-C interaction with the CENP-A nucleosome during M-phase.
It has also been suggested that CENP-N interaction to the centromere (CENP-A nucleosome) is dynamic during cell cycle progression. CENP-N is loaded onto the centromere and its levels are increased during S-phase [128, 129]. The CENP-N levels at the centromeres peak prior to G2/M-phase and decrease in M-phase [122, 129]. Whereas structural change in centromere chromatin is one phenomenon that controls the CENP-N levels, regulation of CENP-N dynamics during cell cycle progression remains a matter of intrigue. Additionally, it is important to address the arrangement of CENP-C and CENP-N on the CENP-A nucleosome. Since CENP-C and CENP-N recognize different surfaces of the CENP-A nucleosome, current structural models suggest that CENP-N and CENP-C bind to a CENP-A nucleosome simultaneously [119, 120]. This idea is supported by a recent study that determined the structure of the CENP-A nucleosome bound with CENP-C and CENP-N using Cryo-EM [122]. However, since these studies used a short peptide for CENP-C, there is large scope for future efforts to understand how CENP-C and CENP-N are arranged on a CENP-A nucleosome in centromeric chromatin. Building a CENP-A nucleosome bound to a larger CENP-C fragment including the CENP-A-binding region and the post-translational modification is necessary to clarify how CCAN recognizes the CENP-A nucleosome.
Two CCAN pathways to recruit KMN onto the kinetochore: changes in KMN–CCAN interaction during M-phase progression
In contrast to CCAN, which constitutively associates with the centromere throughout the cell cycle, KMN starts localizing to the centromere at the onset of G2/M-phase transition and remains localized to the centromere during M-phase by binding to CCAN [2, 18, 130]. Since Ndc80C in KMN binds to microtubules along with other microtubule-binding proteins [19], KMN–CCAN interaction establishes a linkage between microtubules and the centromere.
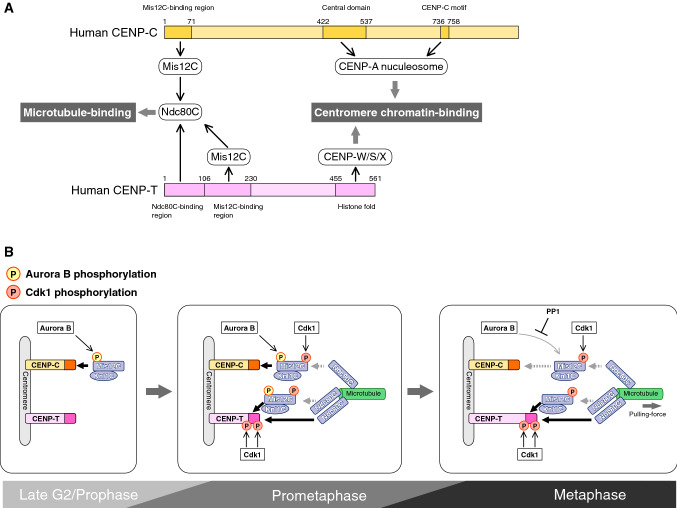
Two CCAN components, CENP-C and CENP-T, provide KMN recruiting platforms in CCAN (Figs. 1, 7). As both CENP-C and CENP-T directly bind to centromeric chromatin [113, 118, 131, 132], a two-pathway model (CENP-C- and CENP-T-pathways) is proposed for bridging of centromeres and microtubules during mitosis (Fig. 7a) [1, 133, 134].
Fig. 7.
Two-pathway links between centromere chromatin and spindle microtubules. a Two-pathway: CENP-C and CENP-T pathways. CENP-C has a Mis12 complex-binding (Mis12C-binding) region in its N terminus and two CENP-A nucleosome-binding domains in its middle and C-terminal regions (central domain and CENP-C motif, respectively). CENP-T binds to the Ndc80 complex (Ndc80C) and Mis12C via its N-terminal region. The CENP-T C terminus has a histone fold domain, which forms a nucleosome-like structure with CENP-W, -S, and -X, binding to centromere chromatin. Both CENP-C and CENP-T recruit to Ndc80C, which binds to spindle microtubules directly or indirectly through Mis12C, and also interacts with the centromere chromatin, forming bridges between the centromere and the microtubules. b Dynamic interaction of the KMN network with the two pathways. Two CCAN components, CENP-C and CENP-T, interact with and recruit the KMN network to the centromere in M-phase. The multiple phosphoregulations by CDK1 and AurB induce changes in the interaction between CCAN and the KMN network during M-phase progression, making the CENP-T pathway the major load-bearing KMN scaffold
CENP-C is conserved and essential for chromosome segregation in most model organisms [135–137]. In addition to the centromeric chromatin interacting motifs, CENP-C also has the Mis12C-binding domain in its N-terminal end [138–141], which directly binds to Mis12C in vitro and further recruits Ndc80C on to CENP-C [140, 141]. The CENP-C–Mis12C interaction is regulated by AurB-mediated phosphorylation of Mis12C [100, 141, 142] (Fig. 7B). Structural analyses of the Mis12C–CENP-C complex demonstrated that a Mis12C subunit, Dsn1, has a conserved basic domain which plays an auto-inhibitory role in the Mis12C–CENP-C interaction by masking the CENP-C-binding surface from Mis12C [141, 143]. AurB phosphorylates the basic domain and prevents autoinhibition of Mis12C–CENP-C interaction, leading to their stable binding and Mis12C localization to the centromere during mitosis [100, 141, 142].
CENP-T forms another platform to recruit KMN onto CCAN. In contrast to CENP-C, which binds to Mis12C to further recruit Ndc80C, the CENP-T N-terminal region directly interacts with Ndc80C (Fig. 7a) [131, 138, 144, 145]. Human CENP-T has two Ndc80C-binding sites, whereas chicken CENP-T and the budding yeast CENP-T homologue Cnn1 have one site [131, 145, 146]. In addition, the CENP-T N-terminal region directly binds to Mis12C, which recruits another Ndc80C (Fig. 7a) [134, 138, 144, 145]. Phosphorylation also plays a key role in the regulation of the CENP-T–KMN interaction. The Ndc80C-binding region of CENP-T interacts with the Spc24–Spc25 sub-complex in Ndc80C (Fig. 7b) [131]. This interaction is stabilized by phosphorylation at Cdk1 consensus motifs in the CENP-T N-terminal region; human CENP-T has two Cdk1 consensus motifs, whereas chicken CENP-T has one motif [131, 144, 145]. Phosphorylation at these sites is necessary for Ndc80C recruitment to the CENP-T-pathway in mitotic human cells [144, 145]. The CENP-T–Mis12C interaction is also regulated by Cdk1 [144, 145]. Cdk1 phosphorylates Thr195 and Ser201 in human CENP-T and facilitates CENP-T–Mis12C interaction [144, 145]. Besides the phosphoregulation, the CENP-T–Mis12C interaction requires the direct binding of Ndc80C to the CENP-T N-terminal region; deletion of the Ndc80-binding region of CENP-T or knock-down of Ndc80C components hinders the CENP-T–Mis12C interaction [134, 144]. Further studies are necessary to decipher the multi-layer regulation of the CENP-T–Mis12C interaction.
Evidences of the direct binding of KMN to CENP-C and CENP-T N-terminal regions are provided by the artificial tethering of either the CENP-C or CENP-T N-terminal fragment to a non-centromeric locus in human and chicken cells [134, 138, 144]. The artificially tethered CENP-C or CENP-T N-terminal fragment recruits KMN on a non-centromeric locus. Notably, neither the CENP-C nor the CENP-T N-terminal fragments recruit other CCAN components at the tethering locus. Even in the absence of other CCAN components, KMN recruited onto the non-centromeric site is sufficient for forming a functional kinetochore in chicken DT40 cells, which is able to ensure normal chromosome segregation after removal of the native centromere on chicken chromosome Z [134].
The native kinetochores have both CENP-C and CENP-T (Fig. 1). Given the stable interactions between KMN and CENP-C or CENP-T in vitro, they seem to firmly bind to KMN to form two stringent pathways in M-phase. However, in fact, our recent studies using chicken DT40 cells revealed that the KMN–CCAN interaction is dynamic during M-phase progression (Fig. 7b) [20].
Centromere localization of Mis12C is observed in a subset of interphase cells and all mitotic cells [130]. Our recent studies on Mis12C localization analyses using various DT40 mutant cell lines have revealed how Mis12C is recruited onto two different platforms in CCAN [20]. In late G2 phase and prophase, the Mis12C localization depends on CENP-C, and does not depend on CENP-T [20, 142]. However, in the subsequent prometaphase, Mis12C starts binding to CENP-T in addition to CENP-C, resulting in an increase in levels of Mis12C on centromeres [20]. Thereafter, the levels of CENP-T-binding Mis12C are constant in metaphase and anaphase, while those of CENP-C-binding Mis12C reduce [20]. On the other hand, the Ndc80C levels are undetectable in prophase and peak in prometaphase [20]. Notably, centromeric localization of a majority of Ndc80C molecules depend significantly on CENP-T rather than on CENP-C. Ndc80C remains attached to CENP-T from metaphase to anaphase, though its levels are reduced [20]. These dynamic changes in KMN–CCAN interaction results in binding of the majority of KMN to CENP-T in metaphase and anaphase (Fig. 7b), indicating that CENP-T is a major KMN-binding scaffold in the native kinetochores in chicken DT40 cells. Consistent with this observation, the KMN-binding of CENP-C is dispensable, while that of CENP-T is essential for DT40 cell viability [20].
These dynamic changes in KMN–CCAN interaction during mitosis are regulated by phosphorylation and dephosphorylation of KMN and CCAN components. AurB-mediated phosphorylation of the Dsn1 basic motif facilitates the CENP-C–Mis12C interaction, leading to CENP-C-dependent Mis12C localization in G2 phase and prophase, while it decreases in late mitosis [20, 100, 141, 142]. In correlation with high Cdk1 activity in prometaphase and metaphase, the CENP-T N-terminal region must be highly phosphorylated to induce the direct binding of Ndc80C and Mis12C to CENP-T. We have showed that Cdk1 also phosphorylates the Dsn1 C-terminal region, which binds to Ndc80C. However, unlike CENP-T phosphorylation, Dsn1 phosphorylation reduces the Ndc80C–Mis12C interaction [20]. These phosphorylation profiles account for the low levels of Ndc80C on CENP-C even when Mis12C is present (Fig. 7b) [100, 141, 142]. Once the bipolar-attachment is established, AurB substrates in the outer kinetochore are dephosphorylated in a PP1-dependent manner [102, 105–110]. Dsn1 basic domain could also be dephosphorylated at this stage, presumably leading to reduction of binding-affinity between CENP-C and Mis12C in metaphase and anaphase; the levels of CENP-C binding Mis12C reduce in metaphase and anaphase (Fig. 7b) [20]. On the other hand, Cdk1 substrates should remain phosphorylated until mitotic exit in general [147]. The Cdk1-dependent interaction between KMN and CENP-T can be maintained through M-phase, indicating that CENP-T functions as the major load-bearing pathway for faithful chromosome segregation (Fig. 7b).
The proposition that CENP-T forms the major link between centromeres and microtubules is further supported by studies using supper-resolution microscopy for analysing detailed localization of kinetochore proteins in mitotic human cells [148]. They demonstrate that the CENP-C C terminus is placed near the surface of the centromeric chromatin, as expected, due to its binding to CENP-A. The middle part of CENP-C stretches away from the centromere, while its N terminus turns back inside, close to the centromere surface [148]. In contrast, CENP-T forms an elongated structure in the kinetochore; the CENP-T C terminus, which binds to DNA, localizes close to the centromeric chromatin, whereas its KMN-binding region in the N terminus stretches outside of the kinetochore, where Ndc80C is placed [148]. This observation suggests that while the microtubule-pulling force is applied on CENP-T, it is applied insignificantly or not applied on CENP-C in human cells, consistent with the proposition that CENP-T functions as the major load-bearing pathway. A tension sensor probe also suggests that a major microtubule-pulling force is applied on CENP-T and less on CENP-C in chicken DT40 cells [20].
Conclusion
The kinetochore is a dynamic molecular complex observed during the cell cycle progression and the dynamic changes in the kinetochore structure appear to be critical for faithful chromosome segregation. The pioneering EM studies captured the expansion and compaction of the kinetochore structures that is further confirmed by fluorescent microscopy observations. Molecular genetics and biochemical reconstitution continue to reveal the elaborate protein interaction network of kinetochore proteins and their dynamic features in detail. Since the changes in the protein–protein interactions and the structures are significant for kinetochore functions, further studies on regulations of the kinetochore protein interaction network will provide a comprehensive understanding of regulatory mechanisms ensuring faithful chromosome segregation.
Acknowledgements
We thank all members in the Fukagawa lab for helpful discussions. This work was supported by JSPS KAKENHI Grant Numbers 15H05972, 16H06279, and 17H06167 to TF, JSPS KAKENHI Grant Number 16K18491 to MH.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Masatoshi Hara, Email: mahara@fbs.osaka-u.ac.jp.
Tatsuo Fukagawa, Email: tfukagawa@fbs.osaka-u.ac.jp.
References
- 1.Cheeseman IM. The kinetochore. Cold Spring Harb Perspect Biol. 2014;6:a015826–a015826. doi: 10.1101/cshperspect.a015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musacchio A, Desai A. A molecular view of kinetochore assembly and function. Biology. 2017;5(6):5. doi: 10.3390/biology6010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukagawa T, Earnshaw WC. The centromere: chromatin foundation for the kinetochore machinery. Dev Cell. 2014;30:496–508. doi: 10.1016/j.devcel.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musacchio A. The molecular biology of spindle assembly checkpoint signaling dynamics. Curr Biol. 2015;25:R1002–R1018. doi: 10.1016/j.cub.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 5.Sacristan C, Kops GJPL. Joined at the hip: kinetochores, microtubules, and spindle assembly checkpoint signaling. Trends Cell Biol. 2015;25:21–28. doi: 10.1016/j.tcb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Sarangapani KK, Asbury CL. Catch and release: how do kinetochores hook the right microtubules during mitosis? Trends Genet. 2014;30:150–159. doi: 10.1016/j.tig.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagpal H, Fukagawa T. Kinetochore assembly and function through the cell cycle. Chromosoma. 2016;125:645–659. doi: 10.1007/s00412-016-0608-3. [DOI] [PubMed] [Google Scholar]
- 8.Pesenti ME, Weir JR, Musacchio A. Progress in the structural and functional characterization of kinetochores. Curr Opin Struct Biol. 2016;37:152–163. doi: 10.1016/j.sbi.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Thrower DA, Jordan MA, Wilson L. Modulation of CENP-E organization at kinetochores by spindle microtubule attachment. Cell Motil Cytoskeleton. 1996;35:121–133. doi: 10.1002/(SICI)1097-0169(1996)35:2<121::AID-CM5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 10.Rieder CL. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int Rev Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BF, Hsieh CE, Mattheyses AL, Rieder CL. A new look at kinetochore structure in vertebrate somatic cells using high-pressure freezing and freeze substitution. Chromosoma. 1998;107:366–375. doi: 10.1007/s004120050320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman DB, Pearson CG, Yen TJ, et al. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol Biol Cell. 2001;12:1995–2009. doi: 10.1091/mbc.12.7.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynne DJ, Funabiki H. Kinetochore function is controlled by a phospho-dependent coexpansion of inner and outer components. J Cell Biol. 2015;210:899–916. doi: 10.1083/jcb.201506020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krenn V, Musacchio A. The Aurora B kinase in chromosome bi-orientation and spindle checkpoint signaling. Front Oncol. 2015;5:225. doi: 10.3389/fonc.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lampson MA, Cheeseman IM. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011;21:133–140. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maresca TJ, Salmon ED. Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal. J Cell Sci. 2010;123:825–835. doi: 10.1242/jcs.064790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara M, Fukagawa T. Critical foundation of the kinetochore: the constitutive centromere-associated network (CCAN) Prog Mol Subcell Biol. 2017;56:29–57. doi: 10.1007/978-3-319-58592-5_2. [DOI] [PubMed] [Google Scholar]
- 18.Varma D, Salmon ED. The KMN protein network—chief conductors of the kinetochore orchestra. J Cell Sci. 2012;125:5927–5936. doi: 10.1242/jcs.093724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monda JK, Cheeseman IM. The kinetochore-microtubule interface at a glance. J Cell Sci. 2018;131:jcs214577. doi: 10.1242/jcs.214577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara M, Ariyoshi M, Okumura E-I, et al. Multiple phosphorylations control recruitment of the KMN network onto kinetochores. Nat Cell Biol. 2018;20:1378–1388. doi: 10.1038/s41556-018-0230-0. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe R, Hara M, Okumura E-I, et al. CDK1-mediated CENP-C phosphorylation modulates CENP-A binding and mitotic kinetochore localization. J Cell Biol. 2019;218:4042–4062. doi: 10.1083/jcb.201907006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinley KL, Cheeseman IM. The molecular basis for centromere identity and function. Nat Rev Mol Cell Biol. 2016;17:16–29. doi: 10.1038/nrm.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinkley BR, Stubblefield E. The fine structure of the kinetochore of a mammalian cell in vitro. Chromosoma. 1966;19:28–43. doi: 10.1007/bf00332792. [DOI] [PubMed] [Google Scholar]
- 24.Jokelainen PT. The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells. J Ultrastruct Res. 1967;19:19–44. doi: 10.1016/s0022-5320(67)80058-3. [DOI] [PubMed] [Google Scholar]
- 25.Rieder CL. The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma. 1981;84:145–158. doi: 10.1007/bf00293368. [DOI] [PubMed] [Google Scholar]
- 26.McEwen BF, Arena JT, Frank J, Rieder CL. Structure of the colcemid-treated PtK1 kinetochore outer plate as determined by high voltage electron microscopic tomography. J Cell Biol. 1993;120:301–312. doi: 10.1083/jcb.120.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEwen BF, Dong Y. Contrasting models for kinetochore microtubule attachment in mammalian cells. Cell Mol Life Sci. 2010;67:2163–2172. doi: 10.1007/s00018-010-0322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maiato H, DeLuca J, Salmon ED, Earnshaw WC. The dynamic kinetochore–microtubule interface. J Cell Sci. 2004;117:5461–5477. doi: 10.1242/jcs.01536. [DOI] [PubMed] [Google Scholar]
- 29.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 30.Cheeseman IM, Desai A. Molecular architecture of the kinetochore–microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 31.Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol. 2013;14:25–37. doi: 10.1038/nrm3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLuca JG, Dong Y, Hergert P, et al. Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol Biol Cell. 2005;16:519–531. doi: 10.1091/mbc.e04-09-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciferri C, Pasqualato S, Screpanti E, et al. Implications for kinetochore–microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133:427–439. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeLuca JG, Gall WE, Ciferri C, et al. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 35.Wei RR, Al-Bassam J, Harrison SC. The Ndc80/HEC1 complex is a contact point for kinetochore–microtubule attachment. Nat Struct Mol Biol. 2007;14:54–59. doi: 10.1038/nsmb1186. [DOI] [PubMed] [Google Scholar]
- 36.Cassimeris L, Rieder CL, Rupp G, Salmon ED. Stability of microtubule attachment to metaphase kinetochores in PtK1 cells. J Cell Sci. 1990;96(Pt 1):9–15. doi: 10.1242/jcs.96.1.9. [DOI] [PubMed] [Google Scholar]
- 37.Dong Y, Vanden Beldt KJ, Meng X, et al. The outer plate in vertebrate kinetochores is a flexible network with multiple microtubule interactions. Nat Cell Biol. 2007;9:516–522. doi: 10.1038/ncb1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao X, Anderson KL, Cleveland DW. The microtubule-dependent motor centromere-associated protein E (CENP-E) is an integral component of kinetochore corona fibers that link centromeres to spindle microtubules. J Cell Biol. 1997;139:435–447. doi: 10.1083/jcb.139.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooke CA, Schaar B, Yen TJ, Earnshaw WC. Localization of CENP-E in the fibrous corona and outer plate of mammalian kinetochores from prometaphase through anaphase. Chromosoma. 1997;106:446–455. doi: 10.1007/s004120050266. [DOI] [PubMed] [Google Scholar]
- 41.Magidson V, He J, Ault JG, et al. Unattached kinetochores rather than intrakinetochore tension arrest mitosis in taxol-treated cells. J Cell Biol. 2016;212:307–319. doi: 10.1083/jcb.201412139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson VL, Scott MIF, Holt SV, et al. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J Cell Sci. 2004;117:1577–1589. doi: 10.1242/jcs.01006. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Rodriguez J-A, Lewis C, McKinley KL, et al. Distinct roles of RZZ and Bub1-KNL1 in mitotic checkpoint signaling and kinetochore expansion. Curr Biol. 2018;28:3422–3429.e5. doi: 10.1016/j.cub.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira C, Reis RM, Gama JB, et al. Self-assembly of the RZZ complex into filaments drives kinetochore expansion in the absence of microtubule attachment. Curr Biol. 2018;28:3408–3421.e8. doi: 10.1016/j.cub.2018.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magidson V, Paul R, Yang N, et al. Adaptive changes in the kinetochore architecture facilitate proper spindle assembly. Nat Cell Biol. 2015;17:1134–1144. doi: 10.1038/ncb3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wynne DJ, Funabiki H. Heterogeneous architecture of vertebrate kinetochores revealed by three-dimensional superresolution fluorescence microscopy. Mol Biol Cell. 2016;27:3395–3404. doi: 10.1091/mbc.E16-02-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sacristan C, Ahmad MUD, Keller J, et al. Dynamic kinetochore size regulation promotes microtubule capture and chromosome biorientation in mitosis. Nat Cell Biol. 2018;20:800–810. doi: 10.1038/s41556-018-0130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howell BJ, McEwen BF, Canman JC, et al. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J Cell Biol. 2001;155:1159–1172. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wojcik E, Basto R, Serr M, et al. Kinetochore dynein: its dynamics and role in the transport of the Rough deal checkpoint protein. Nat Cell Biol. 2001;3:1001–1007. doi: 10.1038/ncb1101-1001. [DOI] [PubMed] [Google Scholar]
- 50.Mische S, He Y, Ma L, et al. Dynein light intermediate chain: an essential subunit that contributes to spindle checkpoint inactivation. Mol Biol Cell. 2008;19:4918–4929. doi: 10.1091/mbc.e08-05-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sivaram MVS, Wadzinski TL, Redick SD, et al. Dynein light intermediate chain 1 is required for progress through the spindle assembly checkpoint. EMBO J. 2009;28:902–914. doi: 10.1038/emboj.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karess R. Rod–Zw10–Zwilch: a key player in the spindle checkpoint. Trends Cell Biol. 2005;15:386–392. doi: 10.1016/j.tcb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Karess RE, Glover DM. rough deal: a gene required for proper mitotic segregation in Drosophila. J Cell Biol. 1989;109:2951–2961. doi: 10.1083/jcb.109.6.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams BC, Karr TL, Montgomery JM, Goldberg ML. The Drosophila l(1)zw10 gene product, required for accurate mitotic chromosome segregation, is redistributed at anaphase onset. J Cell Biol. 1992;118:759–773. doi: 10.1083/jcb.118.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Starr DA, Williams BC, Li Z, et al. Conservation of the centromere/kinetochore protein ZW10. J Cell Biol. 1997;138:1289–1301. doi: 10.1083/jcb.138.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scaërou F, Starr DA, Piano F, et al. The ZW10 and Rough Deal checkpoint proteins function together in a large, evolutionarily conserved complex targeted to the kinetochore. J Cell Sci. 2001;114:3103–3114. doi: 10.1242/jcs.114.17.3103. [DOI] [PubMed] [Google Scholar]
- 57.Basto R, Gomes R, Karess RE. Rough deal and Zw10 are required for the metaphase checkpoint in Drosophila. Nat Cell Biol. 2000;2:939–943. doi: 10.1038/35046592. [DOI] [PubMed] [Google Scholar]
- 58.Chan GK, Jablonski SA, Starr DA, et al. Human Zw10 and ROD are mitotic checkpoint proteins that bind to kinetochores. Nat Cell Biol. 2000;2:944–947. doi: 10.1038/35046598. [DOI] [PubMed] [Google Scholar]
- 59.Williams BC, Li Z, Liu S, et al. Zwilch, a new component of the ZW10/ROD complex required for kinetochore functions. Mol Biol Cell. 2003;14:1379–1391. doi: 10.1091/mbc.e02-09-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mosalaganti S, Keller J, Altenfeld A, et al. Structure of the RZZ complex and molecular basis of its interaction with Spindly. J Cell Biol. 2017;216:961–981. doi: 10.1083/jcb.201611060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffis ER, Stuurman N, Vale RD. Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J Cell Biol. 2007;177:1005–1015. doi: 10.1083/jcb.200702062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan YW, Fava LL, Uldschmid A, et al. Mitotic control of kinetochore-associated dynein and spindle orientation by human Spindly. J Cell Biol. 2009;185:859–874. doi: 10.1083/jcb.200812167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barisic M, Sohm B, Mikolcevic P, et al. Spindly/CCDC99 is required for efficient chromosome congression and mitotic checkpoint regulation. Mol Biol Cell. 2010;21:1968–1981. doi: 10.1091/mbc.e09-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gassmann R, Holland AJ, Varma D, et al. Removal of Spindly from microtubule-attached kinetochores controls spindle checkpoint silencing in human cells. Genes Dev. 2010;24:957–971. doi: 10.1101/gad.1886810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holland AJ, Reis RM, Niessen S, et al. Preventing farnesylation of the dynein adaptor Spindly contributes to the mitotic defects caused by farnesyltransferase inhibitors. Mol Biol Cell. 2015;26:1845–1856. doi: 10.1091/mbc.E14-11-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moudgil DK, Westcott N, Famulski JK, et al. A novel role of farnesylation in targeting a mitotic checkpoint protein, human Spindly, to kinetochores. J Cell Biol. 2015;208:881–896. doi: 10.1083/jcb.201412085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gassmann R, Essex A, Hu J-S, et al. A new mechanism controlling kinetochore–microtubule interactions revealed by comparison of two dynein-targeting components: SPDL-1 and the Rod/Zwilch/Zw10 complex. Genes Dev. 2008;22:2385–2399. doi: 10.1101/gad.1687508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang G, Lischetti T, Hayward DG, Nilsson J. Distinct domains in Bub1 localize RZZ and BubR1 to kinetochores to regulate the checkpoint. Nat Commun. 2015;6:7162–7214. doi: 10.1038/ncomms8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian J, García-Gimeno MA, Beullens M, et al. An attachment-independent biochemical timer of the spindle assembly checkpoint. Mol Cell. 2017;68:715–730.e5. doi: 10.1016/j.molcel.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 70.Caldas GV, Lynch TR, Anderson R, et al. The RZZ complex requires the N-terminus of KNL1 to mediate optimal Mad1 kinetochore localization in human cells. Open Biol. 2015;5:150160. doi: 10.1098/rsob.150160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gama JB, Pereira C, Simões PA, et al. Molecular mechanism of dynein recruitment to kinetochores by the Rod–Zw10–Zwilch complex and Spindly. J Cell Biol. 2017;216:943–960. doi: 10.1083/jcb.201610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silió V, McAinsh AD, Millar JB. KNL1-Bubs and RZZ provide two separable pathways for checkpoint activation at human kinetochores. Dev Cell. 2015;35:600–613. doi: 10.1016/j.devcel.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 73.Kops GJPL, Kim Y, Weaver BAA, et al. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J Cell Biol. 2005;169:49–60. doi: 10.1083/jcb.200411118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buffin E, Lefebvre C, Huang J, et al. Recruitment of Mad2 to the kinetochore requires the Rod/Zw10 complex. Curr Biol. 2005;15:856–861. doi: 10.1016/j.cub.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 75.Basto R, Scaerou F, Mische S, et al. In vivo dynamics of the rough deal checkpoint protein during Drosophila mitosis. Curr Biol. 2004;14:56–61. doi: 10.1016/j.cub.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 76.Waters JC, Mitchison TJ, Rieder CL, Salmon ED. The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work. Mol Biol Cell. 1996;7:1547–1558. doi: 10.1091/mbc.7.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maresca TJ, Salmon ED. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol. 2009;184:373–381. doi: 10.1083/jcb.200808130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uchida KSK, Takagaki K, Kumada K, et al. Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol. 2009;184:383–390. doi: 10.1083/jcb.200811028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wordeman L, Steuer ER, Sheetz MP, Mitchison T. Chemical subdomains within the kinetochore domain of isolated CHO mitotic chromosomes. J Cell Biol. 1991;114:285–294. doi: 10.1083/jcb.114.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schittenhelm RB, Heeger S, Althoff F, et al. Spatial organization of a ubiquitous eukaryotic kinetochore protein network in Drosophila chromosomes. Chromosoma. 2007;116:385–402. doi: 10.1007/s00412-007-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Biggins S, Severin FF, Bhalla N, et al. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes Dev. 1999;13:532–544. doi: 10.1101/gad.13.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanaka TU, Rachidi N, Janke C, et al. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 83.Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Correcting improper chromosome-spindle attachments during cell division. Nat Cell Biol. 2004;6:232–237. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- 84.Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 85.Kelly AE, Funabiki H. Correcting aberrant kinetochore microtubule attachments: an Aurora B-centric view. Curr Opin Cell Biol. 2009;21:51–58. doi: 10.1016/j.ceb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carvalho A, Carmena M, Sambade C, et al. Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. J Cell Sci. 2003;116:2987–2998. doi: 10.1242/jcs.00612. [DOI] [PubMed] [Google Scholar]
- 88.Honda R, Körner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–3341. doi: 10.1091/mbc.e02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gassmann R, Carvalho A, Henzing AJ, et al. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166:179–191. doi: 10.1083/jcb.200404001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klein UR, Nigg EA, Gruneberg U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol Biol Cell. 2006;17:2547–2558. doi: 10.1091/mbc.e05-12-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Romano A, Guse A, Krascenicova I, et al. CSC-1: a subunit of the Aurora B kinase complex that binds to the survivin-like protein BIR-1 and the incenp-like protein ICP-1. J Cell Biol. 2003;161:229–236. doi: 10.1083/jcb.200207117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cooke CA, Heck MM, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987;105:2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaitna S, Mendoza M, Jantsch-Plunger V, Glotzer M. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol. 2000;10:1172–1181. doi: 10.1016/s0960-9822(00)00721-1. [DOI] [PubMed] [Google Scholar]
- 94.Sampath SC, Ohi R, Leismann O, et al. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 95.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 96.Samejima K, Platani M, Wolny M, et al. The inner centromere protein (INCENP) coil is a single α-helix (SAH) domain that binds directly to microtubules and is important for chromosome passenger complex (CPC) localization and function in mitosis. J Biol Chem. 2015;290:21460–21472. doi: 10.1074/jbc.M115.645317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zaytsev AV, Segura-Peña D, Godzi M, et al. Bistability of a coupled Aurora B kinase-phosphatase system in cell division. Elife. 2016;5:e10644. doi: 10.7554/eLife.10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang E, Ballister ER, Lampson MA. Aurora B dynamics at centromeres create a diffusion-based phosphorylation gradient. J Cell Biol. 2011;194:539–549. doi: 10.1083/jcb.201103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu D, Vader G, Vromans MJM, et al. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Welburn JPI, Vleugel M, Liu D, et al. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore–microtubule interface. Mol Cell. 2010;38:383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DeLuca KF, Lens SMA, DeLuca JG. Temporal changes in Hec1 phosphorylation control kinetochore–microtubule attachment stability during mitosis. J Cell Sci. 2011;124:622–634. doi: 10.1242/jcs.072629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheeseman IM, Anderson S, Jwa M, et al. Phospho-regulation of kinetochore–microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- 103.Guimaraes GJ, Dong Y, McEwen BF, DeLuca JG. Kinetochore–microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr Biol. 2008;18:1778–1784. doi: 10.1016/j.cub.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller MP, Asbury CL, Biggins S. A TOG protein confers tension sensitivity to kinetochore–microtubule attachments. Cell. 2016;165:1428–1439. doi: 10.1016/j.cell.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pinsky BA, Kung C, Shokat KM, Biggins S. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat Cell Biol. 2006;8:78–83. doi: 10.1038/ncb1341. [DOI] [PubMed] [Google Scholar]
- 106.Liu D, Vleugel M, Backer CB, et al. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J Cell Biol. 2010;188:809–820. doi: 10.1083/jcb.201001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Emanuele MJ, Lan W, Jwa M, et al. Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. J Cell Biol. 2008;181:241–254. doi: 10.1083/jcb.200710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Francisco L, Wang W, Chan CS. Type 1 protein phosphatase acts in opposition to IpL1 protein kinase in regulating yeast chromosome segregation. Mol Cell Biol. 1994;14:4731–4740. doi: 10.1128/mcb.14.7.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hsu JY, Sun ZW, Li X, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 110.Vanoosthuyse V, Hardwick KG. A novel protein phosphatase 1-dependent spindle checkpoint silencing mechanism. Curr Biol. 2009;19:1176–1181. doi: 10.1016/j.cub.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Etemad B, Kuijt TEF, Kops GJPL. Kinetochore–microtubule attachment is sufficient to satisfy the human spindle assembly checkpoint. Nat Commun. 2015;6:8987–8988. doi: 10.1038/ncomms9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tauchman EC, Boehm FJ, DeLuca JG. Stable kinetochore–microtubule attachment is sufficient to silence the spindle assembly checkpoint in human cells. Nat Commun. 2015;6:10036–10039. doi: 10.1038/ncomms10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hori T, Amano M, Suzuki A, et al. CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell. 2008;135:1039–1052. doi: 10.1016/j.cell.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 114.Nishino T, Takeuchi K, Gascoigne KE, et al. CENP-T-W-S-X forms a unique centromeric chromatin structure with a histone-like fold. Cell. 2012;148:487–501. doi: 10.1016/j.cell.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carroll CW, Silva MCC, Godek KM, et al. Centromere assembly requires the direct recognition of CENP-A nucleosomes by CENP-N. Nat Cell Biol. 2009;11:896–902. doi: 10.1038/ncb1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carroll CW, Milks KJ, Straight AF. Dual recognition of CENP-A nucleosomes is required for centromere assembly. J Cell Biol. 2010;189:1143–1155. doi: 10.1083/jcb.201001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McKinley KL, Sekulic N, Guo LY, et al. The CENP-L-N complex forms a critical node in an integrated meshwork of interactions at the centromere-kinetochore interface. Mol Cell. 2015;60:886–898. doi: 10.1016/j.molcel.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kato H, Jiang J, Zhou B-R, et al. A conserved mechanism for centromeric nucleosome recognition by centromere protein CENP-C. Science. 2013;340:1110–1113. doi: 10.1126/science.1235532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pentakota S, Zhou K, Smith C, et al. Decoding the centromeric nucleosome through CENP-N. Elife. 2017;6:213. doi: 10.7554/eLife.33442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chittori S, Hong J, Saunders H, et al. Structural mechanisms of centromeric nucleosome recognition by the kinetochore protein CENP-N. Science. 2018;359:339–343. doi: 10.1126/science.aar2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tian T, Li X, Liu Y, et al. Molecular basis for CENP-N recognition of CENP-A nucleosome on the human kinetochore. Cell Res. 2018;28:374–378. doi: 10.1038/cr.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Allu PK, Dawicki-McKenna JM, Van Eeuwen T, et al. Structure of the human core centromeric nucleosome complex. Curr Biol. 2019;29:2625–2639.e5. doi: 10.1016/j.cub.2019.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tachiwana H, Kagawa W, Shiga T, et al. Crystal structure of the human centromeric nucleosome containing CENP-A. Nature. 2011;476:232–235. doi: 10.1038/nature10258. [DOI] [PubMed] [Google Scholar]
- 124.Guo LY, Allu PK, Zandarashvili L, et al. Centromeres are maintained by fastening CENP-A to DNA and directing an arginine anchor-dependent nucleosome transition. Nat Commun. 2017;8:15775. doi: 10.1038/ncomms15775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagpal H, Hori T, Furukawa A, et al. Dynamic changes in CCAN organization through CENP-C during cell-cycle progression. Mol Biol Cell. 2015;26:3768–3776. doi: 10.1091/mbc.E15-07-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fukagawa T, Mikami Y, Nishihashi A, et al. CENP-H, a constitutive centromere component, is required for centromere targeting of CENP-C in vertebrate cells. EMBO J. 2001;20:4603–4617. doi: 10.1093/emboj/20.16.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kwon M-S, Hori T, Okada M, Fukagawa T. CENP-C is involved in chromosome segregation, mitotic checkpoint function, and kinetochore assembly. Mol Biol Cell. 2007;18:2155–2168. doi: 10.1091/mbc.E07-01-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fang J, Liu Y, Wei Y, et al. Structural transitions of centromeric chromatin regulate the cell cycle-dependent recruitment of CENP-N. Genes Dev. 2015;29:1058–1073. doi: 10.1101/gad.259432.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hellwig D, Emmerth S, Ulbricht T, et al. Dynamics of CENP-N kinetochore binding during the cell cycle. J Cell Sci. 2011;124:3871–3883. doi: 10.1242/jcs.088625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kline SL, Cheeseman IM, Hori T, et al. The human Mis12 complex is required for kinetochore assembly and proper chromosome segregation. J Cell Biol. 2006;173:9–17. doi: 10.1083/jcb.200509158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nishino T, Rago F, Hori T, et al. CENP-T provides a structural platform for outer kinetochore assembly. EMBO J. 2013;32:424–436. doi: 10.1038/emboj.2012.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takeuchi K, Nishino T, Mayanagi K, et al. The centromeric nucleosome-like CENP-T-W-S-X complex induces positive supercoils into DNA. Nucleic Acids Res. 2014;42:1644–1655. doi: 10.1093/nar/gkt1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hara M, Fukagawa T. Where is the right path heading from the centromere to spindle microtubules? Cell Cycle. 2019;18:1199–1211. doi: 10.1080/15384101.2019.1617008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hori T, Shang W-H, Takeuchi K, Fukagawa T. The CCAN recruits CENP-A to the centromere and forms the structural core for kinetochore assembly. J Cell Biol. 2013;200:45–60. doi: 10.1083/jcb.201210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Drinnenberg IA, Akiyoshi B. Evolutionary lessons from species with unique kinetochores. Prog Mol Subcell Biol. 2017;56:111–138. doi: 10.1007/978-3-319-58592-5_5. [DOI] [PubMed] [Google Scholar]
- 136.Drinnenberg IA, Henikoff S, Malik HS. Evolutionary turnover of kinetochore proteins: a ship of theseus? Trends Cell Biol. 2016;26:498–510. doi: 10.1016/j.tcb.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Hooff JJ, Tromer E, van Wijk LM, et al. Evolutionary dynamics of the kinetochore network in eukaryotes as revealed by comparative genomics. EMBO Rep. 2017;18:1559–1571. doi: 10.15252/embr.201744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gascoigne KE, Takeuchi K, Suzuki A, et al. Induced ectopic kinetochore assembly bypasses the requirement for CENP-A nucleosomes. Cell. 2011;145:410–422. doi: 10.1016/j.cell.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Przewloka MR, Venkei Z, Bolanos-Garcia VM, et al. CENP-C is a structural platform for kinetochore assembly. Curr Biol. 2011;21:399–405. doi: 10.1016/j.cub.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 140.Screpanti E, De Antoni A, Alushin GM, et al. Direct binding of Cenp-C to the Mis12 complex joins the inner and outer kinetochore. Curr Biol. 2011;21:391–398. doi: 10.1016/j.cub.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Petrovic A, Keller J, Liu Y, et al. Structure of the MIS12 complex and molecular basis of its interaction with CENP-C at human kinetochores. Cell. 2016;167:1028–1040.e15. doi: 10.1016/j.cell.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim S, Yu H. Multiple assembly mechanisms anchor the KMN spindle checkpoint platform at human mitotic kinetochores. J Cell Biol. 2015;208:181–196. doi: 10.1083/jcb.201407074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dimitrova YN, Jenni S, Valverde R, et al. Structure of the MIND complex defines a regulatory focus for yeast kinetochore assembly. Cell. 2016;167:1014–1027.e12. doi: 10.1016/j.cell.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rago F, Gascoigne KE, Cheeseman IM. Distinct organization and regulation of the outer kinetochore KMN network downstream of CENP-C and CENP-T. Curr Biol. 2015;25:671–677. doi: 10.1016/j.cub.2015.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huis In't Veld PJ, Jeganathan S, Petrovic A, et al. Molecular basis of outer kinetochore assembly on CENP-T. Elife. 2016 doi: 10.7554/eLife.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Malvezzi F, Litos G, Schleiffer A, et al. A structural basis for kinetochore recruitment of the Ndc80 complex via two distinct centromere receptors. EMBO J. 2013;32:409–423. doi: 10.1038/emboj.2012.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vigneron S, Robert P, Hached K, et al. The master Greatwall kinase, a critical regulator of mitosis and meiosis. Int J Dev Biol. 2016;60:245–254. doi: 10.1387/ijdb.160155tl. [DOI] [PubMed] [Google Scholar]
- 148.Suzuki A, Badger BL, Wan X, et al. The architecture of CCAN proteins creates a structural integrity to resist spindle forces and achieve proper Intrakinetochore stretch. Dev Cell. 2014;30:717–730. doi: 10.1016/j.devcel.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]