Abstract
FA-SAT is a highly conserved satellite DNA sequence transcribed in many Bilateria species. To disclose the cellular and functional profile of FA-SAT non-coding RNAs, a comprehensive experimental approach, including the transcripts location in the cell and in the cell cycle, the identification of its putative protein interactors, and silencing/ectopic expression phenotype analysis, was performed. FA-SAT non-coding RNAs play a nuclear function at the G1 phase of the cell cycle and the interactomic assay showed that the PKM2 protein is the main interactor. The disruption of the FA-SAT non-coding RNA/PKM2 protein complex, by the depletion of either FA-SAT or PKM2, results in the same phenotype—apoptosis, and the ectopic overexpression of FA-SAT did not affect the cell-cycle progression, but promotes the PKM2 nuclear accumulation. Overall, our data first describe the importance of this ribonucleoprotein complex in apoptosis and cell-cycle progression, what foresees a promising novel candidate molecular target for cancer therapy and diagnosis.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03234-x) contains supplementary material, which is available to authorized users.
Keywords: FA-SAT, Non-coding RNA, Satellite RNA, PKM2, Apoptosis
Introduction
Satellite DNAs (satDNAs) are highly repetitive sequences that are traditionally located at the heterochromatin—i.e., the (peri)centromeric and (sub)telomeric regions—and belong to the “dark matter” of the genomes [1–3]. Recently, satellite transcripts have been associated with important roles in different cellular processes, biological contexts, and diseases, such as cancer [4–8]. However, for most genomes, the functionality of the majority of these satellite RNAs remains unknown or unclear [7, 9].
FA-SAT is a satDNA sequence highly conserved in several Bilateria species, including Homo sapiens (HSA, human), and its transcription was detected in all these genomes [3]. This satDNA was first described as the major satDNA sequence of Felis catus (FCA, domestic cat) with a monomeric unit of 483 bp, a G + C content of 64% [10], representing 2% of the cat’s genome, with more than 100,000 copies of its monomeric unit [11]. FA-SAT is primarily located at the telomeres, but it is also present at the centromeres of the cat’s chromosomes [12, 13], showing also an interspersed genomic architecture [3]. More recently, our group proved that DNA methylation of FA-SAT is involved in the transcription regulation of this satDNA; however, in cancer, other mechanisms also seem to contribute to the transcriptional status of this sequence [14].
The fact that FA-SAT is the most conserved satDNA described so far may anticipate an important function for these satellite transcripts [3]. With this premise in mind, we conceived an experimental design with the goal to disclose the function of FA-SAT ncRNAs in mammalian cells. In a first approach, we defined the cellular profile of FA-SAT transcripts as being cell-cycle dependent, namely, at the G1 phase and with a putative nuclear function. To unveil the FA-SAT ncRNA protein interactor(s), we carried out an interatomic study that revealed the PKM2 protein (Pyruvate Kinase Muscle Isozyme) as the most promising interactor. PKM2 is a moonlight protein that acts as a pyruvate kinase at the cytoplasm and as a kinase at the nucleus [15–18]. In addition, a functional analysis was carried out and revealed that the depletion phenotype of either FA-SAT or PKM2 is apoptosis. In addition, the ectopic expression of FA-SAT does not affect cell proliferation, but an accumulation was detected of PKM2 nuclear location. FA-SAT satellite RNAs seem to recruit the nuclear PKM2 protein to accomplish its cellular function in the nucleus.
Materials and methods
Cell culture, transfection, and ectopic expression of lentiviral transduction
Felis catus (FCA) primary cell culture and the HeLa cell line were grown in DMEM supplemented with 13% AmnioMax C-100 Basal Medium, 2% AmnioMax C-100 supplement, 10% FBS, 100 U/ml/100 µg/ml Penicillin/Streptomycin antibiotic mixture, and 200 mM l-glutamine (all from Gibco, Thermo Fisher Scientific). FCA primary cell culture was established by our group and was derived from a normal mammary biopsy of a female F. catus individual, being the sample collected in accordance with the EU Directive 2010/63/EU and approved by the “Universidade de Trás-os-Montes e Alto Douro” (approval reference POCI/CVT/62940/04). All methods and experimental protocols carried out are in accordance with the guidelines and regulations of the University of Trás-os-Montes and Alto Douro. To deplete FA-SAT, 50 nM of a customized Antisense LNA™ GapmeR (5′-FAM TGATGCTGTCAGACGT, FA-SAT_LNA, Exiqon) was used. An LNA GapmeR negative control (CTR_LNA, Exiqon) was used to exclude off-target effects. PKM2 was depleted with siPKM2 (siRNA1 and siRNA3) from MISSION® siRNA (Sigma-Aldrich). For all transfections, a mock control (only with the transfection reagent) was used to exclude cytotoxicity. LNA GapmerRs and siRNA transient transfections were carried out using Lipofectamine® RNAiMAx Transfection Reagent (Invitrogen, Thermo Fisher Scientific) according to the manufacturer’s instructions. For the ectopic expression of FA-SAT, this sequence was cloned into a lentiviral vector with an H1 promoter (pLVTHM) and was sequenced. pLVTHM was a gift from Didier Trono (Addgene plasmid #12247) [19]. The lentivirus was produced through the transfection of HEK 293 T with pMd2.G, Pax2 and lentiviral vector (with and without the FA-SAT sequence) using Lipofectamine® 2000 Transfection Reagent (Invitrogen, Thermo Fisher Scientific). After 48 h, the medium containing the infectious lentivirus was collected, and the supernatant was cleared of cell debris. For the cell transduction, 8 µg/ml polybrene was used (Sigma-Aldrich). A control lentivirus without the sequence was used in all the experiments.
Cell treatments and stresses
Heat shock was performed in FCA primary cells at 42 °C during 3 h. For the starvation stress, the cells were grown in an FBS-free (0%) medium during 5 days. For the confluence stress, cells were grown as usual until reaching 95–100% of confluence, remaining confluent during 5 days. Both for confluence and starvation stresses, the culture medium was replaced every 2 days. Normal growing conditions were used as control for all the stresses. All these treatments were performed in slides for RNA-FISH and in flasks for RNA isolation.
RNA-FISH and RNA-FISH/IF
The RNA-FISH procedure was performed using cells that grown on Superfrost Excell microscope slides (Thermo Scientific) at a concentration of 100,000 cells/ml at 37 °C overnight. In brief, the cells were washed in 1 × PBS, fixed for 20 min at RT with 2% (m/v) paraformaldehyde in PBS, and permeabilized with 4% (v/v) Triton X-100 with 100 µg/ml digitonin or 4% (v/v) Tween-20 (the latter only for the PCNA antibody) in PBS supplemented with 200 mM of ribonucleoside Vanadyl Complex (RVC, Sigma-Aldrich) for 15–20 min. Before the hybridization step, the cells were dehydrated in sequential ethanol baths (70%, 90%, and 100%). The cells were hybridized overnight at 37 °C (in accordance with other authors, e.g., [20]) with the FA-SAT probe, resulting from PCR amplification of the FA-SAT cloned sequence were labelled with digoxigenin-11-dUTP (Roche Applied Science). The most stringent wash was carried out in 0.1 × SSC at 42 °C. Cells were incubated with blocking buffer (10% FBS in PBS) for 30 min. When the RNA-FISH protocol was combined with IF, incubation with the primary antibody was performed for 1 h. Cells where then incubated with the secondary antibody. Thereafter, the cells were mounted with coverslips and counterstained with Vectashield mounting medium containing 4′-6-diamidino-2-phenylindole (DAPI) (Vector Laboratories). The treatments with RNase A (0.1 mg/ml, Sigma-Aldrich), RNase T1 (100 U/ml, Thermo Scientific), and DNase I (0.12 U/µl, Thermo Scientific) were performed for 1 h at 37 °C after the permeabilization step of the RNA-FISH protocol.
Antibodies
Cell signaling: anti-PSMD2 polyclonal rabbit antibody (IF 1:25, 14141) and anti-PTBP1 polyclonal rabbit antibody (IF 1:25, 8776). Millipore: anti-PCNA monoclonal mouse antibody (IF 1:100, NA03, Calbiochem), anti-cyclin D1 monoclonal mouse (IF 1:50, 05-815), anti-Cdc25 monoclonal mouse (IF 1:100, TC-15 clone, 05-507SP), anti-cyclin A polyclonal rabbit antibody (IF 1:75, 06-138), anti-phospho-histone H3 (Ser10) polyclonal rabbit antibody (IF 1:200, 06-570), anti-PKM2 polyclonal rabbit antibody (IF 1:50, WB 1:300, ABS245), anti-ROCK2 polyclonal rabbit antibody (IF 1:50, ABT353), anti-FUS monoclonal rabbit antibody (IF 1:100, clone EPR5813, MABE587), anti-YB1 polyclonal rabbit antibody (IF 1:200, ABE187), anti-hnRNP U monoclonal mouse antibody (IF 1:400, clone 3G6, 05-1516), anti-Myc polyclonal rabbit antibody (WB 1:250, 06-340), anti-p53 monoclonal mouse antibody (WB 1:150, CBL404), and anti-rabbit polyclonal FITC antibody (1:200, AP132F). Thermo Scientific: anti-fibrillarin monoclonal mouse (1:100, MA3-16771). Sigma-Aldrich: anti-α-tubulin (IF 1:1500, clone B-5-1-2, T5168), anti-digoxigenin-50-TAMRA (1:200, 11207750910). Zymed: anti-mouse monoclonal FITC (1:200, 81-6511). Advansta: Goat-anti-mouse IgG (H + L), HRP conjugate (1:10,000, R-05071-500), Goat-anti-rabbit IgG (H + L), HRP conjugate (1: 10,000, R-05072-500).
Microscopy and cell-imaging tools
Confocal fluorescence images were acquired on an LSM 510 META with a Zeiss Axio Imager Z1 microscope and LSM 510 software (version 4.0 SP2). The same microscope settings were applied for all images to normalize the results. The lasers used were: argon (488 nm) set at 12.9%, helium–neon (543 nm) set at 50.8% and diode (405 nm) set at 9.9%. The pinhole was set to 96 mm (1.02 airy units) for argon laser, 102 mm (0.98 airy units) for helium–neon laser, and 112 mm for the diode laser using a 63 × objective. Images were captured at a scan speed of 4 with 1 µm thick Z sections, deconvolutioned with 3D deconvolution tool of the AutoQuant X3 software (Media Cybernetics) and processed in TIFF images with ImageJ (1.47v). The three dimensional Iso-Surfaces (for measuring volumes and counting objects) and ortho slices (that creates perpendicular or parallel angles to the volume) of the confocal images were produced in Image Pro Premier 3D (version 9.3, Media Cybernetics). For transfected cells live imaging, a Zeiss Axiovert 200 microscope with P.A.L.M. image browser was used. All the images were prepared at the contrast and color optimization (at whole image) using Adobe Photoshop (version 7.0). The co-localization analysis of FA-SAT RNA with putative protein interactors was performed with co-localization analysis tool of the Autoquant X3 software (Media Cybernetics). In each cell, each co-localization spot was analyzed by Pearson’s correlation coefficient and Manders’ overlap coefficient (Bolte and Cordelieres 2006) (a minimum of 25 co-localization spots or 15 cells was analyzed). The quantification of FA-SAT RNA or PKM2 nuclear signals was performed in both Autoquant X3 (Media Cybernetics) and Image Pro Premier 3D (version 9.3, Media Cybernetics) software’s with similar results. We present the data obtained from the Autoquant X3—counting and tracking tool. Only the separated signals were considered, i.e., each cluster was considered as one single signal (a minimum of 10 cells was analyzed).
Bioinformatic prediction of the FA-SAT ncRNA/PKM2-binding propensity
The PKM2 (Uniprot, protein identifier: P14618-1) and FA-SAT ncRNA (monomer or dimer) (GenBank, sequence accession number: X06372.1)-binding propensity prediction was performed using catRAPID method and the Global Score algorithm, selecting the Signal Localization option [21].
Isolation of DNA, RNA, and proteins
Genomic DNA isolation was performed using Quick-Gene DNA Tissue Kit S (Fujifilm Life Science), following the manufacturer’s instructions. RNA was isolated using the mirVana Isolation Kit (Ambion, Thermo Fisher Scientific) following the manufacturer’s recommendations. For RNA level analysis, the RNA was purified using the TURBO DNA-free™ Kit (Ambion, Thermo Fisher Scientific). For the DNA and RNA quantification, the Nanodrop 1000 (Thermo Fisher Scientific) was used. The total proteins’ extraction was performed with Qproteome Mammalian Protein Prep Kit (Qiagen) according to manufacturer’s protocol. Total proteins were quantified using the GRS Protein Quantification Kit (Bradford) (Grisp). The total RNAs’ polls from human brain (cat. no. 636530, Takara Bio USA, Inc), small intestine (cat. no. 636539, Takara Bio USA, Inc), and mammary gland (cat. no. 636576, Takara Bio USA, Inc) were used for the assessment of FA-SAT expression in different tissues.
Flow cytometry
For apoptosis analysis, the Cell Staining Buffer and APC Annexin Apoptosis Detection Kit with PI (Biolegend) was used following the manufacturer’s protocol. The cells were analyzed in the BD Accuri™ C6 Plus (BD Biosciences). Early apoptosis was considered positive for annexin-V staining, and late apoptosis was considered positive for annexin-V and PI staining.
3′ RACE
For the poly-A tail presence and size of FA-SAT transcripts analysis, the 5′/3′ RACE Kit, 2nd Generation (Roche) was used, following the manufacturer’s protocol. The FCA total RNA was reverse transcribed using the same kit. The cDNA was subjected to PCR using the PCR anchor primer from the kit and the FA-SAT forward primer (5′-GTCCCTTGTGCCCTCAACTC-3′). The PCR products were isolated in an agarose gel.
RNA pull down and SWATH-MS
FA-SAT ncRNA was isolated from FCA RNA and biotin labelled in the forward primer (5′-Biotin-GTCCCTTGTGCCCTCAACTC-3′). For the sequence binding, Dynabeads® MyOne™ Streptavidin T1 were used (Invitrogen, Thermo Fisher Scientific), following the manufacturer’s instructions. The FCA total protein extract was isolated under normal proliferative conditions and confluence stress; the protein extracts were incubated with the beads–FA-SAT complex for 16 h with gentle rotation. For the washes, B&W buffer and DynaMag™-Spin Magnet (Invitrogen, Thermo Fisher Scientific) were used. Negative controls were performed to eliminate false interactors—beads without the FA-SAT RNA were incubated with the different protein extracts. After the protein–FA-SAT–bead complex washes, the protein was released from this complex and was identified and quantified by Short GeLC-SWATH [22] (see detailed information in Supplementary Data). The RNA pull-down assay was performed in n = 1, and for comparisons between conditions, protein abundances were normalized for the respective control. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [23] partner repository with the data set identifier PXD010081. The identification of nuclear proteins (GO category: nucleus) was performed by Gene Ontology (GO) analysis using DAVID [24, 25]. Reactome (version 57) [26] was used to perform pathway enrichment analysis using the list of interactors identified in the present study. For all of the interactors uploaded in Reactome, their known interactors were also imported (available in IntAct) to include the entire network of interactions, thus increasing the analysis background. Pathways were considered enriched for FDR analysis below 5%. Eight interactor proteins were validated using the RNA-FISH/IF protocol. The images were acquired by confocal microscopy, and co-localization analysis was performed with AutoQuant X3 software (Media Cybernetics) using Pearson’s Correlation and Manders’ Overlap Coefficients.
RNA immunoprecipitation (RIP) assays
RIP assay was performed using the Pierce Agarose ChIP kit (Thermo Scientific) using the normal Rabbit IgG as a negative control and PKM2 antibody (10 mg/ml) to capture the target protein/RNA. To this end, before the immunoprecipitation, the DNA was degraded using the TURBO DNA-free™ Kit (Ambion, Thermo Fisher Scientific). After the immunoprecipitation protocol, the RNA was purified using the mirVana Isolation Kit (Ambion, Thermo Fisher Scientific) and the residual DNA present was digested using the TURBO DNA-free™ Kit (Ambion, Thermo Fisher Scientific). The samples were analyzed by real-time RT-qPCR, with specific primers for FA-SAT detection (as described in [3] and in Supplementary Table 1), and the data treatment for the fold enrichment in relation to the negative control was performed.
RNA expression analysis by real-time reverse transcriptase quantitative polymerase chain reaction (RT-qPCR)
For FA-SAT ncRNA, PKM2, and MYC RNA quantification (primers in Supplementary Table 1), the standard curve method was used that was previously validated for the quantification of repetitive transcripts by [3]. Standard curve parameters are referred in Supplementary Table 2. For the expression quantification, Verso 1-Step RT-qPCR kit, SYBR Green, ROX (Thermo Scientific) was used. The absolute RNA quantification of the unknown RNA was obtained by interpolating its CT value against the standard curve. In all the PCR reactions, 80 ng of RNA were used. The Verso One-Step RT-PCR kit was used in the reaction, which uses the SYBR Green dye (Thermo Scientific), following the recommendations of the manufacturer. The reactions were carried out in a 48-well optical plate (StepOne real-time PCR system, Applied Biosystems, Thermo Fisher Scientific) at 50 °C for 15 min and 95 °C for 15 min, followed by 40 cycles of 95 °C for 15 s, 59 °C for 45 s and 72 °C for 1 min for FA-SAT ncRNA. In respect to PKM2 and MYC, the cycles were: 95 °C for 15 s, 60 °C for 1 min. Subsequently, a melt curve was generated to evaluate the primer specificity. All reactions were performed in triplicate, and negative controls (without RNA and without reverse transcription Enzyme) were also included in the plate. The data were analyzed using the same parameters on the StepOne software (version 2.2.2, Applied Biosystems, Thermo Fisher Scientific).
Western blotting
For western blotting of PKM2, MYC, and P53, 40 µg of each protein sample was mixed with 5 × SDS loading buffer and denatured at 95 °C for 5 min. The proteins were loaded and separated into a NuPAGE™ Novex™ 10% Bis–Tris Protein Gel (Invitrogen, Thermo Fisher Scientific) with MOPs running buffer before transference to a PVDF membrane using the iBlot® Transfer Stack, PVDF and iBlot® Gel Transfer Device (Thermo Fisher Scientific). The membrane was blocked with 5% dry milk in TBS-T for 1 h, incubated with the primary antibodies in 1% dry milk in TBS-T (overnight at 4 °C with agitation), and then with the horseradish peroxidase-coupled secondary antibodies (1 h, room temperature). The results were visualized with chemiluminescent HRP substrate (WesternBright™ ECL kit; Advansta). The membranes were stripped with GRS Stripping Solution (Grisp) to remove the previous antibody.
Statistics and reproducibility
All data are presented as mean ± standard deviation. Statistical significance was determined using two-tailed Student’s t test for the comparison between two independent samples and analysis of variance (ANOVA) tests when more than two groups were under analysis. ns (non-significant) for p > 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Results
Expression profile of FA-SAT satellite RNAs
The first approach used to disclose the FA-SAT ncRNA cellular pattern was performed in cat primary proliferative cells (the species with a higher amount of FA-SAT transcripts [3]) and allowed to identify the transcripts localization in the cells by RNA-FISH combined with immunofluorescence techniques. FA-SAT transcripts are observed as spot-like and cluster-type FISH signals, consistently located in the nucleus and with a scattered distribution; however, they can also be found in the nucleolus with an accumulation at its periphery as revealed by the immune identification of the nucleolus with an anti-fibrillarin antibody (Fig. 1a, b). FA-SAT RNA-FISH followed by DNA-FISH (Fig. 1c) showed that the FISH signals from RNA and DNA are clearly different, with some expected co-localization. Furthermore, RNase A, RNase T1, and DNase I treatments (Supplementary Fig. 1A) validated that the RNA-FISH signals are solely produced by the FA-SAT satellite RNAs hybridization and confirmed that these satellite transcripts are single-stranded RNA polynucleotides [27].
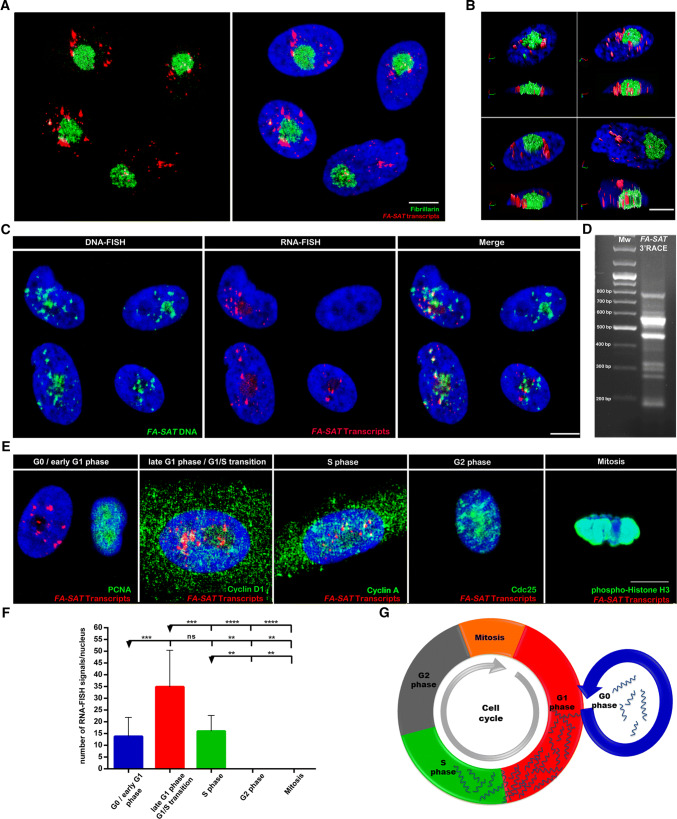
Fig. 1.
FA-SAT satellite RNAs profile in normal proliferating cat cells. a Nuclear localization of FA-SAT ncRNA by RNA-FISH (red) with the nucleolus fibrillarin detection by immunofluorescence (IF, green) in projections of several plans (confocal image). b 3D analysis of the FA-SAT ncRNA nuclear distribution in the cells shown in a in iso-surfaces and with orthogonal slices. This image reveals the presence of FA-SAT RNA at the nucleolus and its periphery. c Sequential RNA-FISH of FA-SAT ncRNA (red) and DNA-FISH of FA-SAT DNA (green) and merged image of both obtained by confocal microscopy (projections of several plans). The co-localization of some RNA signals with the DNA is evident and could be nascent RNAs. It is possible to observe cells without FA-SAT RNA. dFA-SAT 3′ RACE-amplified transcripts consisting of a ladder of bands and a smear corresponding to different fragments of the FA-SAT repeating units (the majority are up to two repeating units). The molecular weight (MW) is presented in the first lane and the FA-SAT 3′ RACE result is presented in the second lane. e Cell-cycle distribution of FA-SAT ncRNA by RNA-FISH (red) conjugated with IF with cell-cycle-specific antibodies; for the identification of the G0 phase, PCNA-negative cells were considered; for the G1/S transition, cyclin D1-positive cells were analyzed; cyclin A-positive cells are in the S phase; Cdc25-positive cells are considered in the G2 phase; phospho-histone H3 (ser10) are cells in mitosis. All the images are projections of several plans (confocal images). In these representative images, the distribution of the FA-SAT RNA in G0 phase, G1/S transition and S phase is observed. The DNA is in blue (DAPI) in all the presented images. All the scale bars represent 10 µm. f Graphical view of the FA-SAT ncRNA signal quantification in each cell-cycle phase. Only the separated signals were considered—i.e., each cluster was considered as one single signal (a minimum of 10 cells were analyzed). The highest amount of FA-SAT transcripts was observed in the G1/S transition. g Schematic representation of FA-SAT ncRNA distribution throughout the cell cycle. The cell-cycle phase duration was not considered. The data are presented as the mean ± SD. ns non-significant. p > 0.05, **p ≤0.01, ***p ≤0.001, ****p ≤ 0.0001, as determined by one-way ANOVA
To determine whether FA-SAT satellite RNAs are polyadenylated, we performed a 3′ rapid amplification of cDNA ends (3′ RACE). The 3′ RACE analysis (Fig. 1d) revealed a ladder of bands and a smear, as expected for satellite transcripts originated from a tandemly repeated sequence [28]. This ladder of bands corresponds to different numbers of copies or fragments of the FA-SAT repeating units (either the 483-bp monomer, or incomplete monomer or dimer fragments). This result suggests that this satellite sequence forms polyadenylated products, mainly consisting of up to two repeating units.
The analysis of the RNA-FISH results (Fig. 1c) revealed that not all proliferative cells display satellite transcripts. This raised the possibility that FA-SAT ncRNA levels may change in a cell-cycle-dependent manner. To examine satellite transcription during the cell cycle, several cell-cycle phase-specific antibodies were combined with simultaneous RNA-FISH (Fig. 1e). These experiments revealed that FA-SAT transcripts accumulate mostly in late G1 and G1/S transition (Cyclin D1 positive cells). Transcripts were also observed in G0/early G1 (PCNA-negative cells, i.e., without cyclin labelling) and in S phase (Cyclin A-positive cells). It was not possible to detect satellite RNAs’ signals in the G2 or M phases (Fig. 1e–g). The data based on cell-cycle phase identification with specific antibodies suggest that FA-SAT transcription starts at the G0/early G1 phases and that the FA-SAT transcript levels increase until the G1/S transition, demonstrating that FA-SAT expression is cell-cycle dependent.
FA-SAT satellite RNAs interact with PKM2 protein in the nucleus
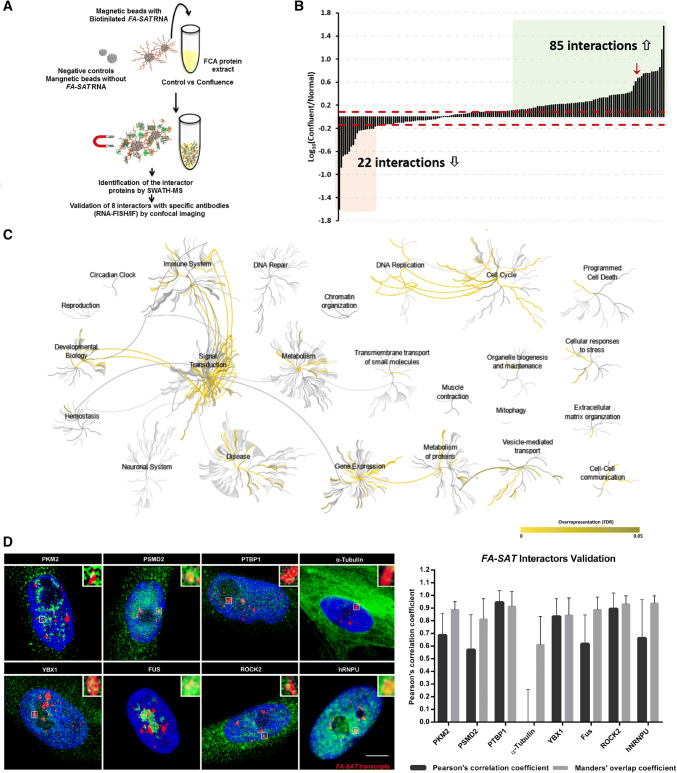
To elucidate the role of FA-SAT ncRNAs in the cells, a series of experiments were conceived with the goal of increase the expression of FA-SAT and hence facilitate the identification of the protein interactors of these ncRNAs. As it is well known, the expression of several satellite RNAs is unleashed by specific cellular stresses [29–34]. Therefore, confluence, heat shock, and serum starvation stresses were used to evaluate the expression of FA-SAT and only the confluence stress increased the FA-SAT transcripts level (Fig. 2a, b, Supplementary Fig. 2 and Table 3). This stress acts like a G0/G1-stage synchronization experiment [35, 36] (also confirmed by PCNA immuno-labelling, data not shown), avoiding the use of any synchronization agent that could interfere with the subsequent experiments. Next, the protein extracts from both control and confluent cells were used in the FA-SAT ncRNA pull-down experiments with synthesized biotinylated FA-SAT ncRNA fragments (Fig. 3a). Mass spectrometry was applied to identify and quantify the proteins that bound to these fragments and to the appropriate controls (i.e., pull-down experiments with both protein extracts using beads without the FA-SAT ncRNA). Proteins were considered as potential FA-SAT ncRNA interactors if all the following criteria were met: (1) being quantified with a coefficient of variation below 30% in the technical replicates; (2) having a 1.223-fold increase in relation to the respective negative control (this cut-off value was determined based on the analysis of the technical variation observed in this experiment, see Supplementary Fig. 3 for details); and (3) belonging to the Gene Ontology (GO) category “Nucleus” (considering the cellular localization of FA-SAT ncRNAs). A panel of 163 proteins (Supplementary Table 4) fulfilled these criteria and were considered putative interactors of the FA-SAT ncRNAs. The impact of the confluence stress in these interactions was further determined by calculating their ratio to the control condition (Fig. 3b), revealing that 22 of these interactions decreased in confluence, while 85 interactions increased. As can be seen in the reactome analysis of the putative FA-SAT ncRNA interactors (Fig. 3c and Supplementary Table 5), different cellular pathways and biological processes are assigned to these interactor proteins. Furthermore, some of the pathways recognized validated the previous cellular processes identified, where FA-SAT satellite RNAs may be involved, namely, transcription by RNA pol II, polyadenylation, and cell-cycle progression (specifically G1/S transition) (Supplementary Tables 4 and 5). Then, we selected eight protein interactors that were further validated by co-localization assays using confocal imaging. One of the proteins identified in the interatomic study, the PKM2 protein, revealed to be the most promising FA-SAT ncRNA interactor, because it is the one that presents a higher fold increase when both experimental conditions were analyzed (control and confluence, Fig. 3b and Supplementary Table 4). In addition to the PKM2 protein, seven other protein interactors were chosen based on their high and low fold increases to perform co-localization analysis with confocal imaging, validating the previous approach. All proteins with high fold increase in the interactomic analysis showed to be co-localized with FA-SAT ncRNA, including the PKM2 interactor, as can be confirmed in Fig. 3d. As expected, the tubulin protein, which presented the lowest fold increase, was not co-localized with FA-SAT ncRNA.
Fig. 2.
FA-SAT ncRNA accumulation is naturally triggered in confluence stress. a Relative quantification of FA-SAT ncRNA under different stress conditions as follows: confluence, heat shock (HSK), and serum starvation (STV). For all the experiments, a control (normal growing conditions) was used as reference. FA-SAT ncRNA is upregulated under confluence stress. b RNA-FISH of FA-SAT transcripts (red) conjugated with immunofluorescence with anti-α-tubulin antibody labelling (green) in the control and confluence conditions in projections of several plans (confocal image). The DNA is in blue (DAPI). The scale bar represents 10 µm. A confluence figure in contrast-phase can be seen in Supplementary Fig. 2. The data are presented as the mean ± SD of three independent experiments. **p ≤0.01, ****p ≤0.0001 as determined by Student’s t test
Fig. 3.
FA-SAT ncRNA protein interaction analysis. a Schematic representation of FA-SAT ncRNA pull-down experiments. b Interaction changes of the 163 FA-SAT RNA interactors under normal vs confluent conditions. The levels of the interactor co-purified under confluence were compared with the normal conditions and the fold changes are presented in log10 scale. The dashed red lines represent the minimum fold-change values for biological regulation in the present analysis (according with Supplementary Fig. 3). The interactors with changes > 1.223-fold increase are in green and changes > 0.730-fold decrease are in red. c Pathway enrichment analysis of the FA-SAT ncRNA interactors. The analysis was performed in Reactome using the 163 putative FA-SAT ncRNA interactors. In all cases, their known interactors (available in IntAct) were imported to include the entire network and increase the analysis background. Pathways were considered enriched for FDR analysis below 5%. d Representative images of the co-localization assay of FA-SAT ncRNA (red) with 8 protein (green) that resulted from projections of several plans (confocal image). A co-localization spot was amplified 350% (top, right). The DNA is in blue (DAPI). Scale bar represents 10 µm. Graphic of the validation of the co-localization of the 8 proteins’ antibodies with FA-SAT ncRNA. Each co-localization spot in each nucleus was analyzed by Pearson’s correlation coefficient and Manders’ overlap coefficient (a minimum of 25 co-localization spots or 15 cells were analyzed)
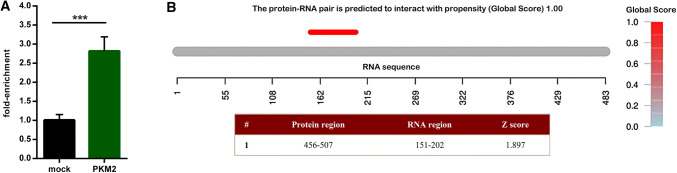
Based on these results, we decided to deeply study the FA-SAT ncRNA/PKM2 protein complex. Thus, the association between PKM2 and FA-SAT ncRNA was also assessed in an RIP (RNA Immunoprecipitation) assay in an in vivo cellular context, which demonstrated that the FA-SAT ncRNA associates with PKM2 protein with a significant fold enrichment (Fig. 4a). A coadjutant in silico analysis with an algorithm that integrates the local properties of the protein and RNA structures into an overall binding propensity [21, 37] was applied. Indeed, the FA-SAT ncRNA/PKM2 pair showed a higher propensity (algorithm global score of 1.00) to bind (Fig. 4b). In addition, the predicted binding region with PKM2 protein in the RNA secondary structure locates at the 151–202 nt position of the monomeric or dimeric FA-SAT ncRNA sequence. Furthermore, in the case of PKM2, the predicted binding site of FA-SAT ncRNA is in its C-terminal domain with a nuclear localization signal/sequence (NLS) (Fig. 4b and Supplementary Fig. 4).
Fig. 4.
FA-SAT satellite RNA/PKM2 association. aFA-SAT ncRNA detection by real-time RT-qPCR of the RIP (RNA immunoprecipitation) samples from the FCA RNA incubation with anti-PKM2 antibody. The results are presented as fold enrichment using as reference the negative control (mock). The data are presented as the mean ± SD of three independent experiments. b Output of the signal localization of PKM2 and FA-SAT ncRNA monomer binding propensity prediction presenting the Global Score value, signal localization plot, representing the localization of the signal along the RNA sequence colored according to the Global Score value, and a table with the information of the binding locals (catRAPID method, Global Score algorithm). ***p ≤0.001 as determined by Student’s t test
FA-SAT ncRNA or PKM2 depletion exhibit similar phenotype—apoptosis
To disclose the role of the ribonucleoprotein complex FA-SAT ncRNA/PKM2, we designed a series of functional assays in cat and human cells. But first, to verify the FA-SAT transcription in different human tissues, we analyzed its expression in different tissues as brain, small intestine and mammary gland (Supplementary Fig. 5). In addition, in a preliminary RIP assay, the FA-SAT ncRNA association with PKM2 was proven to occur also in in vivo human cells (Supplementary Fig. 6).
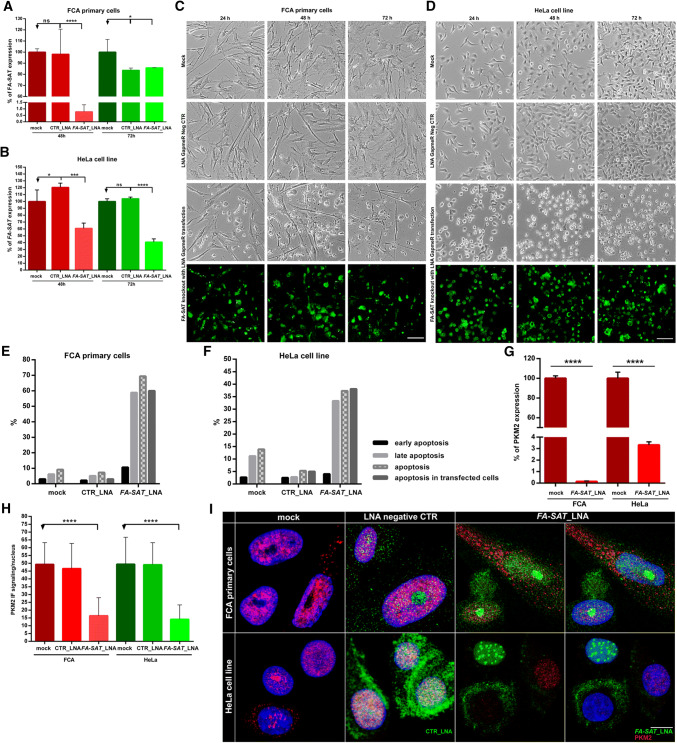
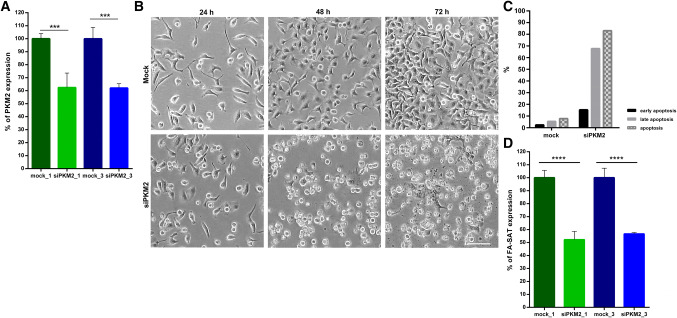
Regarding the functional analysis, a first approach was performed depleting the FA-SAT transcripts with locked nucleic acid (LNA) GapmeR (FA-SAT_LNA) in both cell lines (in cat proliferative primary cells and in the HeLa human immortal cell line) in a time scale of 24, 48 and 72 h (Fig. 5 and Supplementary Fig. 7). The knockdown experiments with LNA GapmeR degrade RNA in an RNase H-dependent manner [38]. A negative control with a LNA GapmeR (CTR_LNA) was also included to confirm that the phenotypes obtained with this GapmeRs are not simply the result of a general response to LNA oligonucleotides (beyond mock control, with transfection reagent). The real-time RT-qPCR results showed that the FA-SAT ncRNA levels were reduced by 99% (in the 48 h assay) in the cat primary cells, and by 59% (in the 72 h experiment) in the HeLa cell line, in comparison with the respective mock controls (Fig. 5a, b, Supplementary Fig. 7A and Table 6). The cells transfected with FA-SAT_LNA were easily visualized once the LNA GapmeR was FAM labelled (Fig. 5c, d and Supplementary Fig. 7B). Depletion of FA-SAT transcripts caused cell death in both cat and human cells as can be observed in Fig. 5c, d, which was afterwards confirmed by flow cytometry as being apoptosis (Fig. 5e, f and Supplementary Fig. 7C, D). In the FA-SAT_LNA knockdown experiments (48 h after transfection in cat primary cells and 72 h in the HeLa cell line, Fig. 5g and Supplementary Table 6) was also verified that the PKM2 RNA levels were reduced by 99% in cat primary cells and by 96% in the HeLa cell line. We also assessed the PKM2 protein by immunofluorescence (IF) and western blotting (WB), and both assays showed a PKM2 protein reduction (Fig. 5h, i and Supplementary Fig. 8). The IF further demonstrated a high reduction of nuclear PKM2 signals: in cat primary cells by 67% and in HeLa cell line by 71% (Fig. 5h). Thus, FA-SAT depletion caused a consistent reduction in the PKM2 levels, both RNA and protein (specifically in the nuclear PKM2 protein), resulting the cell phenotype in apoptosis.
Fig. 5.
FA-SAT knockdown leads to apoptosis. a, bFA-SAT expression analysis of FA-SAT knockdown (FA-SAT_LNA, with a customized LNA GapmeR) in FCA primary cells (a) and in HeLa cells (b) by real-time RT-qPCR using mock as reference at 48 and 72 h. An LNA GapmeR negative control (CTR_LNA) was used in the experiment. a In FCA cells, at 48 h, a high decrease in FA-SAT ncRNA was observed. At 72 h, the FA-SAT RNA percentage was similar to that of the negative control, likely due to the RNA extraction of non-transfected cells (as the majority was dead). b In HeLa cells, the high decrease of FA-SAT ncRNA was observed at 72 h. c, d Cell imaging of the FCA primary cells (c) and HeLa cells (d) at 24, 48 and 72 h in the mock, negative control and FA-SAT_LNA. The scale bar represents 100 µm. e, f Flow cytometry assay results in FCA primary cells (e) and in HeLa cells (f) with annexin-V and propidium iodide (PI) staining of the mock, negative control and FA-SAT_LNA. Early apoptosis was considered positive for annexin-V staining, and late apoptosis was considered positive for annexin-V and PI staining. Apoptosis in the transfected cells was also analyzed with annexin-V and LNA-FAM labelling. g Percentage of PKM2 expression in the FA-SAT-depleted cells in FCA primary cells and in HeLa cells compared with mock by real-time RT-qPCR, demonstrating the high decrease in the PKM2 RNA in FA-SAT-knockdown cells. h PKM2 content/nucleus of the mock, negative control and FA-SAT_LNA confocal images in FCA primary cells and HeLa cells, showing the PKM2 protein decrease at the nucleus in the depleted cells (a minimum of 30 cells were analyzed). i PKM2 protein (red) visualization by immunofluorescence in FCA primary cells and HeLa cells in the mock, negative control and FA-SAT_LNA (green) resulting from projections of several plans (confocal imaging). The scale bar represents 10 µm. The data are presented as the mean ± SD (of three independent experiments in real-time RT-qPCR). ns non-significant p > 0.05, *p ≤0.05, ***p ≤0.001, ****p ≤0.0001, as determined by Student’s t test (when two samples are compared) or one-way ANOVA (when more than two samples are compared)
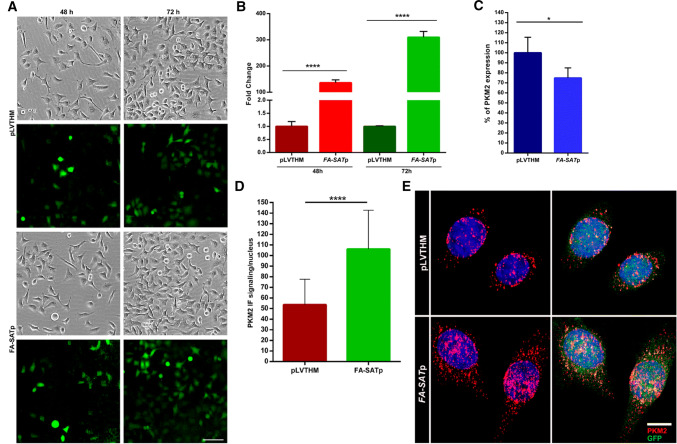
Following, two additional experiments were performed in HeLa cells. First, we used two siRNAs to target the PKM2 mRNA (Fig. 6a, Supplementary Table 6 and Fig. 8 for WB with PKM2 antibody). As can be checked in Fig. 6b, c (and Supplementary Fig. 9), the cell phenotype regarding the depletion of PKM2 is the same as obtained by the FA-SAT silencing, that is apoptosis (confirmed by flow cytometry only for siPKM2_3, as both siRNAs generated similar PKM2 silencing levels/profile). The apoptotic phenotype as a consequence of PKM2 knockdown has already been reported by other authors [39–45], further supporting our findings. It is also important to highlight that the interference with PKM2 RNA caused a decrease in the levels of FA-SAT ncRNAs in more than 40%, a similar percentage of PKM2 RNA decrease in PKM2 knockdown (Fig. 6d and Supplementary Table 6). In a second experiment, we ectopically overexpressed FA-SAT (Fig. 7a, b) using a pLVTHM construct. FA-SAT-overexpressing cells demonstrated a tremendous high increase of FA-SAT RNAs (Fig. 7b and Supplementary Table 7), with no increase in the PKM2 RNA levels (Fig. 7c and Supplementary Table 7) but with an increase in the PKM2 protein nuclear content (Fig. 7d, e). The most parsimonious explanation is that the overexpression of FA-SAT results in the translocation of the PKM2 protein to the nucleus, highlighting the association of these two molecules. Even so, the cells appeared to be healthy, without obvious defects or alterations considering cell-cycle progression. Indeed, cell division seemed to be comparable to the pLVTHM control (Fig. 7a), suggesting that an increased amount of FA-SAT ncRNAs does not negatively interfere with the cell-cycle progression. A similar conclusion was reported in cancer cell lines overexpressing PKM2, described to promote cell proliferation [40, 46].
Fig. 6.
PKM2 knockdown leads to apoptosis and to FA-SAT ncRNA decreased level. a Percentage of PKM2 RNA in PKM2-depleted cells with the siPKM2_1 and siPKM2_3 by real-time RT-qPCR using mock as a reference. The decrease in the PKM2 RNA level was similar in both siRNAs. b Cell imaging of the HeLa cells at 24, 48, and 72 h of the mock and siPKM2. Cell death increase was evident until 72 h. The scale bar represents 100 µm. c Graphical representation of the flow cytometry assay results with annexin-V and PI staining of mock and siPKM2 in HeLa cells. Early apoptosis was considered positive for annexin-V staining and late apoptosis was considered positive for annexin-V and PI staining. This result proves that the cell death previously observed was apoptosis. d Percentage of FA-SAT ncRNA in the PKM2-depleted cells compared with mock by real-time RT-qPCR, demonstrating the decrease in FA-SAT ncRNA in PKM2-knockdown cells (with both siRNAs). The data are presented as the mean ± SD (of three independent experiments by real-time RT-qPCR). *p ≤0.05, **p ≤0.01, ****p ≤0.0001 as determined by Student’s t test
Fig. 7.
Overexpression of FA-SAT causes a “normal” cell proliferation. a Cell imaging of the HeLa cells transduced with pLVTHM (control) and FA-SATp (lentivirus with pLVTHM and FA-SAT) at 24, 48, and 72 h with evidence for cell transduction efficiency by the GFP. No difference in cell proliferation was observed. The scale bar represents 100 µm. b Fold change of FA-SAT ncRNA of the FA-SATp transduced cells by real-time RT-qPCR using pLVTHM transduced cells as a reference at 48 and 72 h. The highest increase of FA-SAT RNA is observed at 72 h. c Percentage of PKM2 RNA in the FA-SATp transduced cells by real-time RT-qPCR using pLVTHM transduced cells as a reference. d PKM2 content/nucleus of the pLVTHM and FA-SATp confocal images in HeLa cells, showing the PKM2 protein increase at the nucleus in the FA-SAT overexpressing cells (a minimum of 22 cells were analyzed). e Representative images of the PKM2 protein (red) by IF in HeLa cells in pLVTHM (control) and FA-SATp transduced cells (green), showing the increase of PKM2 nuclear content in FA-SAT overexpressing cells. The scale bar represents 10 µm. The data are presented as the mean ± SD (of three independent experiments for real-time RT-qPCR). ns non-significant. *p ≤ 0.05, ****p ≤ 0.0001 as determined by Student’s t test
Taking all these functional data into consideration, it seems that the FA-SAT transcripts are most likely responsible for recruiting nuclear PKM2 protein to cell-cycle progression, and its depletion results in the cells’ signaling to apoptosis. To further confirm this hypothesis, we analyzed two important molecules involved in these cellular processes: MYC and P53. When FA-SAT is depleted, MYC RNA was reduced by 59% in cat primary cells and by 56% in the HeLa cell line (Fig. 8, Supplementary Table 6 and Fig. 8 for decrease of MYC protein). Simultaneously, the P53 protein amount was increased in both cell lines (Supplementary Fig. 8). In addition, the knockdown of PKM2 in HeLa cells also decreased the amount of MYC RNA by 88% (Fig. 8, with a simultaneously decrease in MYC protein, Supplementary Fig. 8) and increased the P53 protein (Supplementary Fig. 8). Some studies also described that when PKM2 is knocked down in PC3 and HepG2 cells, a decrease in MYC RNA and/or protein is observed, resulting in cell apoptosis [41, 45]. Thus, it seems that both the proliferative (most probably involving the MYC protein) and the apoptosis (via-p53) pathways are associated with the FA-SAT ncRNA/PKM2 protein complex, deserving to be further explored in future work.
Fig. 8.

FA-SAT and PKM2 knockout leads to MYC RNA decrease. Percentage of MYC RNA in FA-SAT and PKM2 depleted cells by real-time RT-qPCR using mock as reference. FA-SAT knockdown or PKM2 silencing in FCA primary cells and HeLa cells results in the decrease of MYC RNA. The data are presented as the mean ± SD of three independent experiments. ****p ≤ 0.0001 as determined by Student’s t test
Discussion
In a previous study, it was demonstrated that the FA-SAT satDNA is conserved, for more than 570 MY, in several Bilateria species, including humans, and that it is transcribed in these genomes [3]. With this premise in mind, we questioned the role of FA-SAT satellite RNAs in proliferative cells.
Our first experiments were restricted to the cat genome, where this sequence was innately amplified [3], and its transcripts could be more easily followed in the cells. We generally characterized the FA-SAT transcripts; these are nucleus specific, polyadenylated, generated mainly from up to two repeating units with a ladder RACE pattern typical of a tandem repeated sequence, as found in other satellite transcripts [28]. In addition, FA-SAT DNA does not exhibit any regular open reading frame, as confirmed by in silico analysis of this sequence (data not shown). These molecular and cellular patterns exhibited by FA-SAT transcripts are characteristic of satellite non-coding RNAs (similar to what was reported with other satDNA sequence [28]).
The FA-SAT ncRNAs cell-cycle analysis allowed to verify that its transcription is cell-cycle dependent, starting at the G0/early G1 phase with a preferential accumulation in late G1 and in the G1/S transition. Other satellite transcripts also accumulated at certain stages of the cell cycle, including G1/S transition [47–49]. Lu and Gilbert [47] have demonstrated that mouse pericentromeric satDNAs start their transcription in G1 and increase in the G1/S transition or S phase [47]. Regarding telomeric satellite transcripts, there is only one report [50] that discloses pervasively telomeric satellite RNAs, which were found in the asynchronous cells analyzed.
The RNA pull-down assays [51, 52] allowed us to disclose several proteins that potentially interact with FA-SAT ncRNAs. However, one of these, PKM2, stood out as a promising interactor due to the high fold interaction in both analyzed conditions; these data were also validated by a co-localization assay. The FA-SAT ncRNA/PKM2 protein complex was further confirmed by RIP and in silico analyzed by a bioinformatic binding propensity prediction tool. Regarding this last analysis, the FA-SAT ncRNA/PKM2 complex pair showed the highest propensity to bind and most probably occurring at the NLS motif, what is in agreement with the nuclear location of this ribonucleoprotein complex. In 2012, Castello et al. [53] reported that PKM2 protein could bind to a polyadenylated RNA. Later, in 2015, Beckmann et al. [54] classified the PKM2 protein as an enigmRBP (an RNA-binding protein with an unidentified RNA sequence). Here, we report in first-hand, that the RNA molecule, FA-SAT ncRNA, can interact with the PKM2 protein.
To discover in which cellular pathways this ribonucleoprotein complex could be involved, we designed a set of functional assays. The knockdown of FA-SAT in both cat primary and human tumor cells resulted in the same cellular phenotype, apoptosis. In addition, the depletion of FA-SAT caused a reduction in the RNA and protein levels of PKM2 (being also observed a decrease of the PKM2 protein at the nucleus). Reciprocally, the knockout of PKM2 in HeLa cells had a similar outcome (apoptosis) with a reduction in the FA-SAT ncRNA levels. On the contrary, the ectopic overexpression of FA-SAT in HeLa cells did not affect cell-cycle progression, being observed an accumulation of the PKM2 protein at the nucleus. Other studies on PKM2 overexpression reported a similar fate—i.e., cell proliferation [40, 46]. Both functional assays suggest the involvement of FA-SAT ncRNAs in the nuclear distribution of PKM2, being the nuclear PKM2 auto-regulating its own expression [55]. Assembling all these functional experiments, it seems that FA-SAT ncRNA/PKM2 complex is likely to crosstalk between the mitogenic and apoptotic pathways. To get some more clues about this hypothesis, we further analyzed two molecules that could be involved in these pathways, MYC and P53. When the cells went to apoptosis (via FA-SAT or PKM2 knockdown), a reduction in MYC RNA and protein levels and an increase of P53 protein were observed. The depletion of PKM2 inducing apoptosis was also reported in some previous studies, linking this knockdown effect to: a decrease in MYC and CYCLIN D1 expression [41, 45]; a decrease in the anti-apoptotic proteins levels of BCL-XL [40] and BCL-2 [42, 45]; and an increase in the pro-apoptotic proteins BIM [43], BAD [44], and BAX [45]. Moreover, it was already proven that PKM2 is responsible for MYC and Cyclin D1 transcription, hence promoting cell-cycle progression [56, 57]. All of these findings are in agreement with our results, reinforcing the role of PKM2 in cell proliferation and apoptosis. Most importantly, our results reveal a new player in these processes: a non-coding RNA, FA-SAT satellite RNA, which most likely recruits the nuclear PKM2 protein to somehow carry out cell proliferation. Therefore, if FA-SAT RNAs or PKM2 are knocked down, the FA-SAT ncRNA/nuclear PKM2 complex cannot interact and the cell switches to the apoptotic pathway (Fig. 9). It seems that this complex FA-SAT ncRNA/PKM2 has an anti-apoptotic effect, being its presence essential to avoid triggering the apoptotic pathway, and with a conserved function in cat and human cells.
Fig. 9.
FA-SAT recruits PKM2 to promote cell proliferation and apoptosis inhibition. Schematic analysis of the results, proving that the FA-SAT ncRNA/PKM2 complex blocks apoptosis and allows cell proliferation. Meaning that, when PKM2 or FA-SAT ncRNA is absent from the cell (with the complex disruption), the cell cycle is blocked and the apoptotic pathway is triggered
Future work will focus on detailing the relationship between P53 and FA-SAT ncRNA/nuclear PKM2 complex, to understand how the phenotype observed, apoptosis via P53, is modulated. It will be also mandatory to disclose how FA-SAT ncRNA/PKM2 complex promotes cell proliferation (involving the MYC pathway). Nevertheless, the present work demonstrated a new key player, the FA-SAT ncRNA, which seems to be important to considered in cellular processes such as cancer, since they are involved in critical-cancer pathways. This and other non-coding RNAs are increasingly related to these important processes [58], which highlight the importance of these non-coding molecules.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the PhD Grants (SFRH/BD/80446/2011, SFRH/BD/98122/2013, SFRH/BD/81495/2011) all from the Science and Technology Foundation (FCT) from Portugal and for the projects with the reference PTDC/NEU-NMC/0205/2012, PTDC/NEU-SCC/7051/2014, UID/NEU/04539/2013 and POCI-01-0145-FEDER-007440, also from FCT and co-financed by “COMPETE Programa Operacional Factores de Competitividade” QREN, the European Union (FEDER—Fundo Europeu de Desenvolvimento Regional) and UID/MULTI/04046/2019 Research Unit grant from FCT (to BioISI). We also want to acknowledge to Raúl Pérez (from CITAB, Vila Real) for the technical support in Flow Cytometry, Elsa Logarinho (PI of the Aging and Aneuploidy group from I3S, Porto) for the support in the lentivirus production and Paula Lopes (from BioISI, Vila Real) for the English revision.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adega F, Guedes-Pinto H, Chaves R. Satellite DNA in the karyotype evolution of domestic animals—clinical considerations. Cytogenet Genome Res. 2009;126(1–2):12–20. doi: 10.1159/000245903. [DOI] [PubMed] [Google Scholar]
- 2.Tsoumani KT, Drosopoulou E, Mavragani-Tsipidou P, Mathiopoulos KD. Molecular characterization and chromosomal distribution of a species-specific transcribed centromeric satellite repeat from the olive fruit fly, Bactrocera oleae. PLoS One. 2013;8(11):e79393. doi: 10.1371/journal.pone.0079393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaves R, Ferreira D, Mendes-da-Silva A, Meles S, Adega F. FA-SAT is an old satellite DNA frozen in several Bilateria genomes. Genome Biol Evol. 2017;9(11):3073–3087. doi: 10.1093/gbe/evx212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ugarkovic D. Functional elements residing within satellite DNAs. EMBO Rep. 2005;6(11):1035–1039. doi: 10.1038/sj.embor.7400558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plohl M, Luchetti A, Mestrovic N, Mantovani B. Satellite DNAs between selfishness and functionality: structure, genomics and evolution of tandem repeats in centromeric (hetero)chromatin. Gene. 2008;409(1–2):72–82. doi: 10.1016/j.gene.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Enukashvily NI, Ponomartsev NV. Mammalian satellite DNA: a speaking dumb. Adv Protein Chem Struct Biol. 2013;90:31–65. doi: 10.1016/B978-0-12-410523-2.00002-X. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira D, Meles S, Escudeiro A, Mendes-da-Silva A, Adega F, Chaves R. Satellite non-coding RNAs: the emerging players in cells, cellular pathways and cancer. Chromosome Res. 2015;23(3):479–493. doi: 10.1007/s10577-015-9482-8. [DOI] [PubMed] [Google Scholar]
- 8.Rošić S, Erhardt S. No longer a nuisance: long non-coding RNAs join CENP-A in epigenetic centromere regulation. Cell Mol Life Sci. 2016;73(7):1387–1398. doi: 10.1007/s00018-015-2124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biscotti MA, Canapa A, Forconi M, Olmo E, Barucca M. Transcription of tandemly repetitive DNA: functional roles. Chromosome Res. 2015;23(3):463–477. doi: 10.1007/s10577-015-9494-4. [DOI] [PubMed] [Google Scholar]
- 10.Fanning TG. Origin and evolution of a major feline satellite DNA. J Mol Biol. 1987;197(4):627–634. doi: 10.1016/0022-2836(87)90469-4. [DOI] [PubMed] [Google Scholar]
- 11.Pontius JU, O’Brien SJ. Artifacts of the 1.9x feline genome assembly derived from the feline-specific satellite sequence. J Hered. 2009;100(Suppl 1):S14–S18. doi: 10.1093/jhered/esp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos S, Chaves R, Guedes-Pinto H. Chromosomal localization of the major satellite DNA family (FA-SAT) in the domestic cat. Cytogenet Genome Res. 2004;107(1–2):119–122. doi: 10.1159/000079581. [DOI] [PubMed] [Google Scholar]
- 13.Santos S, Chaves R, Adega F, Bastos E, Guedes-Pinto H. Amplification of the major satellite DNA family (FA-SAT) in a cat fibrosarcoma might be related to chromosomal instability. J Hered. 2006;97(2):114–118. doi: 10.1093/jhered/esj016. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira D, Escudeiro A, Adega F, Chaves R. DNA methylation patterns of a satellite non-coding sequence—FA-SAT in cancer cells: its expression cannot be explained solely by DNA methylation. Front Genet. 2019;10:101. doi: 10.3389/fgene.2019.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris I, McCracken S, Mak TW. PKM2: a gatekeeper between growth and survival. Cell Res. 2012;22(3):447–449. doi: 10.1038/cr.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamada M, Suematsu M, Saya H. Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells. Clin Cancer Res. 2012;18(20):5554–5561. doi: 10.1158/1078-0432.CCR-12-0859. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal MA, Gupta V, Gopinath P, Mazurek S, Bamezai RN. Pyruvate kinase M2 and cancer: an updated assessment. FEBS Lett. 2014;588(16):2685–2692. doi: 10.1016/j.febslet.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Wong N, Ojo D, Yan J, Tang D. PKM2 contributes to cancer metabolism. Cancer Lett. 2015;356(2 Pt A):184–191. doi: 10.1016/j.canlet.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Wiznerowicz M, Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol. 2003;77(16):8957–8961. doi: 10.1128/JVI.77.16.8957-8951.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNulty SM, Sullivan LL, Sullivan BA. Human centromeres produce chromosome-specific and array-specific alpha satellite transcripts that are complexed with CENP-A and CENP-C. Dev Cell. 2017;42(3):226–240. doi: 10.1016/j.devcel.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellucci M, Agostini F, Masin M, Tartaglia GG. Predicting protein associations with long noncoding RNAs. Nat Methods. 2011;8(6):444–445. doi: 10.1038/nmeth.1611. [DOI] [PubMed] [Google Scholar]
- 22.Anjo SI, Santa C, Manadas B. Short GeLC-SWATH: a fast and reliable quantitative approach for proteomic screenings. Proteomics. 2015;15(4):757–762. doi: 10.1002/pmic.201400221. [DOI] [PubMed] [Google Scholar]
- 23.Vizcaino JA, Csordas A, Del-Toro N, Dianes JA, Griss J, Lavidas I, Mayer G, Perez-Riverol Y, Reisinger F, Ternent T, Xu QW, Wang R, Hermjakob H. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016;44(22):11033. doi: 10.1093/nar/gkw880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2008;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 25.da Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Croft D, Mundo AF, Haw R, Milacic M, Weiser J, Wu G, Caudy M, Garapati P, Gillespie M, Kamdar MR, Jassal B, Jupe S, Matthews L, May B, Palatnik S, Rothfels K, Shamovsky V, Song H, Williams M, Birney E, Hermjakob H, Stein L, D’Eustachio P. The reactome pathway knowledgebase. Nucleic Acids Res. 2014;42:472–477. doi: 10.1093/nar/gkt1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novikova IV, Hennelly SP, Sanbonmatsu KY. Tackling structures of long noncoding RNAs. Int J Mol Sci. 2013;14(12):23672–23684. doi: 10.3390/ijms141223672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosic S, Kohler F, Erhardt S. Repetitive centromeric satellite RNA is essential for kinetochore formation and cell division. J Cell Biol. 2014;207(3):335–349. doi: 10.1083/jcb.201404097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jolly C, Metz A, Govin J, Vigneron M, Turner BM, Khochbin S, Vourc’h C. Stress-induced transcription of satellite III repeats. J Cell Biol. 2004;164(1):25–33. doi: 10.1083/jcb.200306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouzinba-Segard H, Guais A, Francastel C. Accumulation of small murine minor satellite transcripts leads to impaired centromeric architecture and function. Proc Natl Acad Sci USA. 2006;103(23):8709–8714. doi: 10.1073/pnas.0508006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valgardsdottir R, Chiodi I, Giordano M, Rossi A, Bazzini S, Ghigna C, Riva S, Biamonti G. Transcription of satellite III non-coding RNAs is a general stress response in human cells. Nucleic Acids Res. 2008;36(2):423–434. doi: 10.1093/nar/gkm1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sengupta S, Parihar R, Ganesh S. Satellite III non-coding RNAs show distinct and stress-specific patterns of induction. Biochem Biophys Res Commun. 2009;382(1):102–107. doi: 10.1016/j.bbrc.2009.02.137. [DOI] [PubMed] [Google Scholar]
- 33.Pezer Z, Ugarkovic D. Satellite DNA-associated siRNAs as mediators of heat shock response in insects. RNA Biol. 2012;9(5):587–595. doi: 10.4161/rna.20019. [DOI] [PubMed] [Google Scholar]
- 34.Tilman G, Arnoult N, Lenglez S, Van Beneden A, Loriot A, De Smet C, Decottignies A. Cancer-linked satellite 2 DNA hypomethylation does not regulate Sat2 non-coding RNA expression and is initiated by heat shock pathway activation. Epigenetics. 2012;7(8):903–913. doi: 10.4161/epi.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Barros FR, Goissis MD, Caetano HV, Paula-Lopes FF, Peres MA, Assumpcao ME, Visintin JA. Serum starvation and full confluency for cell cycle synchronization of domestic cat (Felis catus) foetal fibroblasts. Reprod Domest Anim. 2010;45(1):38–41. doi: 10.1111/j.1439-0531.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- 36.Bertero T, Gastaldi C, Bourget-Ponzio I, Mari B, Meneguzzi G, Barbry P, Ponzio G, Rezzonico R. CDC25A targeting by miR-483-3p decreases CCND-CDK4/6 assembly and contributes to cell cycle arrest. Cell Death Differ. 2013;20(6):800–811. doi: 10.1038/cdd.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cirillo D, Blanco M, Armaos A, Buness A, Avner P, Guttman M, Cerase A, Tartaglia GG. Quantitative predictions of protein interactions with long noncoding RNAs. Nat Methods. 2016;14(1):5–6. doi: 10.1038/nmeth.4100. [DOI] [PubMed] [Google Scholar]
- 38.Kauppinen S, Vester B, Wengel J. Locked nucleic acid (LNA): high affinity targeting of RNA for diagnostics and therapeutics. Drug Discov Today Technol. 2005;2(3):287–290. doi: 10.1016/j.ddtec.2005.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg MS, Sharp PA. Pyruvate kinase M2-specific siRNA induces apoptosis and tumor regression. J Exp Med. 2012;209(2):217–224. doi: 10.1084/jem.20111487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon OH, Kang TW, Kim JH, Kim M, Noh SM, Song KS, Yoo HS, Kim WH, Xie Z, Pocalyko D, Kim SY, Kim YS. Pyruvate kinase M2 promotes the growth of gastric cancer cells via regulation of Bcl-xL expression at transcriptional level. Biochem Biophys Res Commun. 2012;423(1):38–44. doi: 10.1016/j.bbrc.2012.05.063. [DOI] [PubMed] [Google Scholar]
- 41.Lu L, Wang L, Jiang GS, Zhang CH, Zeng FQ. Silencing pyruvate kinase M2 sensitizes human prostate cancer PC3 cells to gambogic acid-induced apoptosis. Natl J Androl. 2013;19(2):102–106. [PubMed] [Google Scholar]
- 42.Chu B, Wang J, Wang Y, Yang G. Knockdown of PKM2 induces apoptosis and autophagy in human A549 alveolar adenocarcinoma cells. Mol Med Rep. 2015;12(3):4358–4363. doi: 10.3892/mmr.2015.3943. [DOI] [PubMed] [Google Scholar]
- 43.Hu W, Lu SX, Li M, Zhang C, Liu LL, Fu J, Jin JT, Luo RZ, Zhang CZ, Yun JP. Pyruvate kinase M2 prevents apoptosis via modulating Bim stability and associates with poor outcome in hepatocellular carcinoma. Oncotarget. 2015;6(9):6570–6583. doi: 10.18632/oncotarget.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miao Y, Lu M, Yan Q, Li S, Feng Y. Inhibition of proliferation, migration, and invasion by knockdown of pyruvate kinase-M2 (PKM2) in ovarian cancer SKOV3 and OVCAR3 cells. Oncol Res Featur Preclin Clin Cancer Ther. 2016;24(6):463–475. doi: 10.3727/096504016X14685034103671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J, Yu Z, Li J, Zhang A, Zhang X, Kan Q. Impact of PKM2 gene silencing on biological behavior of HepG2 cells. Int J Clin Exp Med. 2016;7(9):13475–13483. [Google Scholar]
- 46.Zhou CF, Li XB, Sun H, Zhang B, Han YS, Jiang Y, Zhuang QL, Fang J, Wu GH. Pyruvate kinase type M2 is upregulated in colorectal cancer and promotes proliferation and migration of colon cancer cells. IUBMB Life. 2012;64(9):775–782. doi: 10.1002/iub.1066. [DOI] [PubMed] [Google Scholar]
- 47.Lu J, Gilbert DM. Proliferation-dependent and cell cycle regulated transcription of mouse pericentric heterochromatin. J Cell Biol. 2007;179(3):411–421. doi: 10.1083/jcb.200706176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen ES, Zhang K, Nicolas E, Cam HP, Zofall M, Grewal SI. Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature. 2008;451(7179):734–737. doi: 10.1038/nature06561. [DOI] [PubMed] [Google Scholar]
- 49.Ferri F, Bouzinba-Segard H, Velasco G, Hube F, Francastel C. Non-coding murine centromeric transcripts associate with and potentiate Aurora B kinase. Nucleic Acids Res. 2009;37(15):5071–5080. doi: 10.1093/nar/gkp529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trofimova I, Popova D, Vasilevskaya E, Krasikova A. Non-coding RNA derived from a conservative subtelomeric tandem repeat in chicken and Japanese quail somatic cells. Mol Cytogenet. 2014;7(1):102. doi: 10.1186/s13039-014-0102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S, Kopp F, Chang TC, Sataluri A, Chen B, Sivakumar S, Yu H, Xie Y, Mendell JT. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164(1–2):69–80. doi: 10.1016/j.cell.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang Y, Chen X, Wu Y, Li J, Zhang S, Wang K, Guan X, Yang K, Bai Y. LncRNA CASC9 promotes esophageal squamous cell carcinoma metastasis through upregulating LAMC2 expression by interacting with the CREB-binding protein. Cell Death Differ. 2018 doi: 10.1038/s41418-018-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, Krijgsveld J, Hentze MW. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149(6):1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 54.Beckmann BM, Horos R, Fischer B, Castello A, Eichelbaum K, Alleaume AM, Schwarzl T, Curk T, Foehr S, Huber W, Krijgsveld J, Hentze MW. The RNA-binding proteomes from yeast to man harbour conserved enigmRBPs. Nat Commun. 2015;6:10127. doi: 10.1038/ncomms10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, Lyssiotis CA, Aldape K, Cantley LC, Lu Z. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol. 2012;14(12):1295–1304. doi: 10.1038/ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, Gao X, Aldape K, Lu Z. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature. 2011;480(7375):118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, Aldape K, Hunter T, Alfred Yung WK, Lu Z. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012;150(4):685–696. doi: 10.1016/j.cell.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitagawa M, Kitagawa K, Kotake Y, Niida H, Ohhata T. Cell cycle regulation by long non-coding RNAs. Cell Mol Life Sci. 2013;70(24):4785–4794. doi: 10.1007/s00018-013-1423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.