Abstract
The determination of sex is an important hallmark in the life cycle of organisms, in which the fate of gonads and then the individual sex are defined. In gonochoristic teleost fish, this process is characterized by a high plasticity, considering that in spite of genotypic sex many environmental factors can cause shifts from one to another molecular pathway, resulting in organisms with mismatching genotypic and phenotypic sexes. Interestingly, in most instances, both female-to-male or male-to-female sex-reversed individuals develop functional gonads with normal gametogenesis and respective progenies with full viability. The study of these mechanisms is being spread to other non-model species or to those inhabiting more extreme environmental conditions. Although water temperature is an important mechanism involved in sex determination, there are other environmental stressors affected by the climate change which are also implicated in stress response-induced masculinization in fish. In this regard, the brain has emerged as the transducer of the environment input that can influence the gonadal fate. Furthermore, the evaluation of other environmental stressors or their synergic effect on sex determination at conditions that simulate the natural environments is growing gradually. Within such scope, the concerns related to climate change impacts rely on the fact that many of biotic and abiotic parameters reported to affect sex ratios are expected to increase concomitantly as a result of increased greenhouse gas emissions and, particularly worrying, many of them are related to male bias in the populations, such as high temperature, hypoxia, and acidity. These environmental changes can also generate epigenetic changes in sex-related genes affecting their expression, with implications on sex differentiation not only of exposed individuals but also in following generations. The co-analysis of multi-stressors with potential inter- and transgenerational effects is essential to allow researchers to perform long-term predictions on climate change impacts in wild populations and for establishing highly accurate monitoring tools and suitable mitigation strategies.
Keywords: Sex determination, Sex reversal, Brain and gonadal connection, Stress, Epigenetic
Introduction
In vertebrates, several reproduction events are under the control of chemical-physical stimuli from the surrounding environment. Those signals are sensed by the organism which in turn triggers a series of physiological responses, such as metabolism of nutrients and shifting in energy allocation, which promotes proper synchronization among conspecifics and to seasonal conditions, thus enabling improved reproductive performance. The alterations on climate conditions associated with the unprecedented high levels of carbon dioxide in the atmosphere [1] are likely to affect those processes in a large number of vertebrate groups. Due to particular characteristics of aquatic environments, especially the higher thermal convection properties of water, subtle changes in hydrological parameters can induce a more direct and drastic modifications in metabolic and physiological processes of aquatic organisms, particularly in ectotherms [2].
In the last years, there has been growing evidence on the fundamental role of stress in response to environmental changes, driving the evolution of sex-determining mechanisms in teleost fish [3]. Although the stress hormone cortisol has generally a negative connotation associated with detrimental impacts in vertebrates, the stress response mechanism plays fundamental roles in the maintenance of many biological process essential for the organism’s homeostasis [4]. The involvement of cortisol in the physiological adaptation in catadromous or anadromous species during migrations between freshwater and marine environments [5] is one of many examples that demonstrate how essential the stress response is in the life cycle. Here we compiled the information on the involvement of stress hormone cortisol in environment-dependent sex determination with a focus on gonochoristic species and correlate them with recent advances on the regulation of stress axis at the brain level. We also present some considerations on sexual plasticity based on information from gonochorists and discuss new prospects in this field such as alternative mechanisms that potentially influence sex ratios, epigenetic-mediated sex reversal mechanisms, and finally concerns/perspectives related to current global climate change scenario.
Sexual plasticity in fish
Among vertebrates, the teleost fish appear as a group with a huge variability on sex determination mechanisms (Fig. 1), which together direct the gonadal fate decision. This large repertory of strategies is seen in closely related species or even in populations from the same species [6]. The reproductive organs in fish are extremely simple structures compared to those of mammals, composed mainly by gonads with somatic supporting-cells and germ cells. In most species, the gonads are typically paired organs, suspended along the roof of the coelom cavity, but in some species, one of the lobules regresses or fuses to another, resulting in a single, unpaired gonad in adults [7]. The gonadal primordia present an exclusive characteristic in terms of organ development because it differentiates antagonistically into two dissimilar organs, the ovary or the testis. In the beginning, the primordial germ cells (PGCs) migrate actively towards the marginal zone, reaching the coelom and establishing the gonadal primordium [8, 9]. As the PGCs colonize the gonadal primordium, they are arrested at G1/G0 phase of cell cycle, now termed embryonic germ stem cells (EGSCs), and become the precursors of spermatogonial stem cells (SSCs) in the testis and oogonial stem cells (OSCs) in the ovary. Then, during early gonadal differentiation, the somatic supporting-cell and steroidogenic cell lineages differentiate into Sertoli and Leydig cells in testes and granulosa and theca cells in the ovary, respectively [10].
Fig. 1.

Schematic representation of sex-determination systems diversity in vertebrates. The analysis was performed using the database of The Tree of Sex consortium [6]. The number of species reported and used in this analysis is represented below by drawings for each group of vertebrates. The size of the circle is proportional to the number of sex determination strategies in each group of vertebrates
An important characteristic of germ cells in fish that differs strikingly in relation to mammals is the presence of germ cells with stemness in adult gonads [11]. It has also been demonstrated that spermatogonial cells transplanted into a female host are able to generate oocytes [12, 13]. Conversely, oogonial cells transplanted into male recipients are able to differentiate and generate viable spermatozoa [14, 15]. The ability of oogonial cells to differentiate into male gametes has also been corroborated by masculinization induction in adult ovaries of Nile tilapia (Oreochromis niloticus) through long-term administration of an inhibitor of aromatase (involved in the synthesis of estrogens) [16]. Remarkably, this study also evidenced that ovarian somatic cells have undergone transdifferentiation to testicular somatic cells, with interstitial and granulosa cells being converted to Leydig and Sertoli cells, respectively. Although the plasticity of adult somatic cells had been already demonstrated for hermaphrodite species [17] and postulated for gonochorists (both categories of gonadal differentiation are explained below) with basis on the recurrent appearance of intersex gonads upon hormonal manipulation in adults [7], the elucidation of the molecular process involved in such differentiation demonstrates that somehow the liability of gonadal sex in gonochorists persists even in adulthood. Thus, this plasticity in both germ and somatic cell lines, and the rudimentary gonadal structure may somehow underlie the sexual plasticity of fish gonads. At environmentally relevant or normal conditions, gonochorists may probably rely on suppressing mechanisms that avoid the formation of sexual ambiguity or complete sex reversal at later stages, as proposed by some authors [18, 19].
Gonochorists and hermaphrodites are differently categorized based on the way the gonad differentiation progresses [7]. In gonochorists, the gonadal sex is generally determined during embryonic or larval stages following genetically programmed or environmentally modulated pathways, strategies described below and known as sex determination mechanisms. In this category, the destiny of the gonad cannot be reverted anymore, at least under normal conditions. On the other hand, the hermaphrodites include species in which gonadal differentiation can shift from one to another gonadal sex depending mainly on social factors or those that can bear simultaneously both testicular and ovarian tissues [20]. However, the fact that hermaphroditism has arisen independently several times during teleost evolution [21] (e.g. carps and seabasses) illustrates that gonochorism–hermaphroditism transitions undergo with a high likelihood in this group of vertebrates. The majority of fish species are characterized as gonochorist of the differentiated type, but there are also few cases of undifferentiated gonochorists, in which a “juvenile hermaphrodite/intersex” stage is preceded by either ovarian or testicular differentiation [9, 22].
At the molecular level, fish also show high plasticity in the differentiation process of the gonad, conferring to this group a great repertoire of possibilities in front of diverse aquatic environments, being able to occupy different ecological niches. For this reason, alteration in environmental conditions, particularly temperature, has a tremendous impact on reproduction in fish species [23]; this effect is especially perdurable during the early steps of organogenesis, such as the gonadal fate decision.
Environmental sex determination and stress response
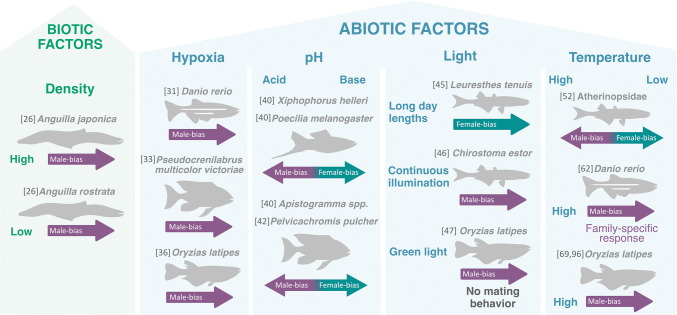
In ectotherm animals, such as fish, amphibians, and reptiles, environmental changes can affect severely whole-body homeostasis. Several environmental cues have been related to sexual fate, particularly in fish [19, 22, 24, 25], whose habitats are susceptible to variations in many physico-chemical factors [2]. This occurs through the action of an array of environmental parameters that can be divided into biotic and abiotic factors (Fig. 2). Social factors are biotic factors that can change the sex ratio and represent a fascinating adaptive sex determination mechanism, mostly in hermaphrodite fish. However, in some gonochoristic fish, the population density can also affect the sex ratio. For instance, in Anguilliformes, the population density seems to be crucial for sex determination (revised by [26]) and dependent on the species, whereby high (Anguilla japonica) or low rearing densities (A. rostrata) induced male-biased sex ratios. Thus, sex reversal in Anguilliformes fish is observed only in the opposite density where these fish commonly live in the wild.
Fig. 2.
Summary of the biotic and abiotic factors characterized in the Environmental sex determination system. The scheme shows the outcomes of single environmental factors and not the interaction between them. Numbers between square bracket are the references for each example: Geffroy and Bardonnet [26], Shang et al. [31], Thomas and Rahman [33], Cheung et al. [36], Rubin [40], Reddon and Hurd [42], Brown et al. [45], Corona-Herrera et al. [46], Hayasaka et al. [47], Strüssmann et al. [52], Ribas et al. [62], Hattori et al. [69], y Castañeda-Cortés [96]
Among abiotic factors, changes in dissolved oxygen (DO) is an important source of environmental stress. Conditions of hypoxia (low levels of DO) occur naturally in estuaries, seasonal coasts, and inland waters due to periodic variations. Nevertheless, those hypoxic conditions tend to increase significantly due to large discharges of pollutants by anthropogenic activities in water bodies [27–30]. The critical scenario is highly influenced by increasing water temperature due to climate change which reduces the levels of DO. Currently, it is considered as one of the most important threats to aquatic environments, with implications on ocean productivity, biodiversity, and biogeochemical cycles [30]. In relation to sex determination, hypoxia has been highly correlated with male-biased sex ratio in zebrafish (Danio rerio), medaka (Oryzias latipes) and African cichlid (Pseudocrenilabrus multicolor victoriae) [31–35].
Another abiotic factor that can induce sex bias is the pH. The acidification of waters by increased CO2 emissions associated with climate change is predicted to cause widespread changes on marine and freshwater ecosystems, with concomitant effects on fish growth, behavior, and reproduction, which in turn affects species interactions and ecological processes [36–38], including transgenerational effects [39]. Moreover, high CO2 influences the sexual differentiation in the offspring of several species of poeciliids (swordtail Xiphophorus helleri and black-bellied limia Poecilia melanogaster) and cichlids (Apistogramma spp. and Pelvicachromis pulcher) [40–42], by inducing male- or female-biased ratios, at acid or basic pH, respectively. Impacts of pH have to be better evaluated in marine species since acidification is also progressing in the oceans.
Fish sex determination is also susceptible to physical factors, such as light and temperature. Light is an important physical factor in the aquatic environment and it has been linked to growth and survival, skin color, behavior, reproduction, and stress response in fish [43, 44]. Although photoperiod has been mostly studied in relation to the modulation of the reproductive season [45], few studies have been focused on the effects of light on the fate of sex. In the California grunion (Leuresthes tenuis), in which female-biased sex ratios are associated with long day lengths at early breeding season, similar photoperiod was consistent with more females and likely adaptive through increased size and fecundity in females [45]. In another silverside, the Chirostoma estor, rearing under continuous illumination induced a male-biased sex ratio [46], reinforcing the high susceptibility of sex to photoperiod conditions. In this sense, phenological changes associated with climate change in spawning could also make offspring to be exposed to longer or shorter photoperiods, with concomitant impact on sex ratios in wild populations. Recently, a study in medaka embryos irradiated with green light during the gonadal sex determination period triggered sex reversal of genotypic female (XX) to phenotypic males, with secondary sexual characteristics and full testis development [47]. Interestingly, when these neo-males (XX) were paired with wild-type females (XX), no sexual behavior and spawning were observed, demonstrating an impact on mating behavior. Furthermore, the artificial insemination of neo-male (XX) sperm with wild-type (XX) female produced all-XX offspring with female phenotype [47], without intergenerational impacts on sex ratio.
Water temperature is probably the physical factor that receives more attention from the climate change perspective and the most studied form of environmental sex determination in fish [24, 48–50]. The relative easy manipulation of temperature and several ways to record thermal data also make this factor to be investigated in many fish species. A shared characteristic of most fish species in which thermal stress (especially high temperatures) can alter sex bias during early development is the masculinization. Exposure to cool temperatures only modified the sex ratio to females in a few species [3, 22, 24, 51]. One of the most studied families is the Atherinopsidae, which presents several species with well-characterized temperature-dependent sex determination (TSD) [52]. In many atherinopsids or silversides, female-biased progenies can be obtained when larvae are maintained during the critical period of sex determination at low temperatures and, conversely, male-biased at high temperatures; this group is considered as the teleosts with highest TSD pattern reported to date. In these species, apart from masculinization, which is expected due to global warming, feminized fish have also been detected in the wild [53, 54]. Indeed, in spite of a gradual increase in average global temperature, the frequency of extremely low or high thermal conditions has increased significantly [55] and the unusually colder temperatures could be implicated in feminization. In other species with reported thermal influences on sex ratios, extreme temperatures, especially the highest ones, are more effective in causing sex ratio distortions [22, 24, 49, 56]. Although the mechanisms of low-temperature-induced feminization are not clarified in fish, the masculinization of genotypic females (XX) exposed to high temperatures is known to involve stress response, whereby cortisol acts as a key mediator between high temperature and maleness [24, 25, 51, 57, 58]; cortisol is considered an important player in other forms of Environmental Sex Determination (ESD; described below).
Another interesting point of thermosensitivity in fish, which could be operational for other forms of environmental stressors, is the high heritability and the existence of different patterns of response, as demonstrated in tilapia [59–61] and zebrafish [62]. In fact, it has been well demonstrated that fish can display different responses when they are subjected to environmental stimuli since stress response is a physiological process that can be influenced by factors such as genetic background, developmental stage, previous exposition to the stressor, exposition of parental fish, and rearing in captivity or in the wild [4]. Those factors could justify the differences in response to the same parameter not only under laboratory conditions [62–64] but also in the way that wild populations may cope with single or multi-stressors, biotic and/or abiotic factors, in natural environments.
The functional plasticity of sex-determining and sex-related genes
The plasticity on the mechanisms of sex determination in fish is also observed in the genes involved in both ovarian and testicular determination/differentiation. From the early theories that both environmental and genotypic sex determination (GSD) systems were mutually exclusive [65], the findings of sex-determining genes in species characterized as typical TSD and, conversely, the description of TSD in species with well-characterized sex-determining genes have provided clear evidence that both systems can co-exist in the same species [53, 66–69]. Also, since the boundary between these two mechanisms is dependent on several conditions of the external environment and intrinsic features of the organism (e.g. genetic background, physiological status), both systems are considered as extremes of a continuum.
Our understanding of the sex determination/differentiation plasticity has been increasing due to the discovery of new sex-determining genes in gonochoristic teleosts. Our current view supports that the evolution of those genes does not follow a strict hierarchy and universal conserved trend. Considering that in many groups, it is common to find species with male or female heterogametic systems such as medaka, tilapia, and also in both gonochoristic and hermaphrodite species, which is the case of seabasses, demonstrate the choice for different reproductive strategies, even in phylogenetically closely related species. The susceptibility of sex determination to environmental factors also represents another source of instability for GSD and may promote either “the fall or the rise” of new sex-determining genes at higher frequencies compared to other homoeothermic vertebrates [70].
The large repertoire of sex-determining genes (and candidates) described in fish [71–75] includes the well-known dmrt1- and sox3-related genes, such as sox3Y and dmy in medakas, and dmrt1 in tongue sole (Cynoglossus semilaevis), which are transcription factors from the same families of eutherian mammals Sry, Dm-w of African clawed frog, and DMRT1 in birds. However, there are some players with functions that are not necessarily linked with sex determination, such as sdY and hsd17b1. Another group that is receiving great attention belong to the TGF-β (transforming growth factor-beta) superfamily (gsdf, amh, amhrII, and gdf6), especially the amh (anti-Müllerian hormone) gene, which has been recruited for determination of sex by many phylogenetically unrelated groups (amhy in silversides, tilapia, and northern pike) [75, 76].
In the case of hermaphrodite species, no sex-determining gene has been described so far. Since these species rely on an environmental sex-determination system that can produce the minority sex or gametes according to the population demand, it would become superfluous and thus negatively selected along evolution. In this regard, groups with species possessing both gonochorists and hermaphrodite populations [9] could be an interesting material to explore the evolutionary transitional process between these reproductive strategies.
Along with the rise of new major testis-determining genes that have been unraveled, a reorganization or subfunctionalization of downstream genes involved in the process of gonad differentiation seems to occur, especially in the male pathway. While the female pathway seems to be more conserved, especially the ovarian aromatase cyp19a1a and its regulator foxl2, the male pathway shows several alternative players, even in closely-related species, such as in South American silversides Odontesthes bonariensis and O. hatcheri [77]. The plasticity of the downstream mechanism seems to be a common trait along with diverse animal groups. In view of the female default hypothesis, it seems plausible that sex-determining genes have to act to block the female pathway [78], as demonstrated through functional analysis of many sex-determining genes. However, it is also intriguing that the suppression of female-related genes such as foxl2 and cyp19a1a [79] may be sufficient to induce testicular differentiation in some cases. It is becoming evident that many of these genes, apart from promoting proper gonadal development, act by suppressing continuously the opposite sex. In fact, this is a very peculiar characteristic of the development of gonads in gonochorists, which are committed to either the female or male outcomes, whereby disruption of a testis or ovary-related gene generally promotes the differentiation of a normal organ of the opposite sex, without compromising its functionality, i.e., either female or male gonads are formed with the production of viable gametes. These findings converge to the notion that mutually controlled antagonism [80] is also a prevailing rule of sex determination, at least in gonochoristic teleosts. The maintenance of suppression over sex-related genes of the opposite sex even at later developmental stages would be critical to avoid ovary-to-testis or testis-to-ovary transdifferentiation in adults, which is a key property of hermaphrodite species.
Brain and gonadal connection during environmental sex determination
The high plasticity of the gonadal fate in fish, which is related to environment-induced sex reversal, is also evidenced in the brain [81]. The communication between the brain and gonad has been largely studied, with most of the focus on the hormonal connection during reproduction through the hypothalamic–pituitary–gonadal (HPG) axis [82]. This axis regulates the secretion of gonadotropin-releasing hormone (GnRH), which in turn modulates the release of two gonadotropins by the pituitary, namely follicle-stimulating (FSH) and luteinizing (LH) hormones. These two hormones reach the main target reproductive organs (ovary or testis), bind to their specific receptors, and finally regulate the synthesis of sex steroids in the gonad, such as testosterone, 11 keto-testosterone, progesterone, estradiol, among others. These hormones are key modulators of gametogenesis and act by regulating downstream pathways not only in the gonad but also in the brain through reciprocal positive and negative feedbacks [83, 84]. Although the participation of the hypothalamus and pituitary on gonadal development and maturation in teleost fish has been largely studied, their role in sex determination is limited to a few species. For instance, in European sea bass (Dicentrarchus labrax L.) it was observed that gnrh and fsh transcripts were up-regulated at the time of morphological gonadal differentiation [85]. Moreover, FSH bioactivity in plasma showed dimorphic capability intensity, higher in females than in males in this [86] and other teleosts [87–89]. FSH was also reported to induce direct stimulation of gonadal aromatase (cyp19a1a) [90, 91] and a mutation on fsh receptor decreased estradiol synthesis and induced female-to-male sex reversal in medaka [92]. In pejerrey, it was observed that GnRH and both gonadotropins were increased at high masculinizing temperature in the preoptic area (POA) of the brain and in the pituitary, respectively [87, 89]. In this regard, Blázquez and Somoza [93] have also postulated that the brain participates in the late gonadal differentiation through the regulation of GnRH and brain aromatase (a paralogue of gonadal aromatase, named cyp19a1b), which together reinforce the importance of estrogens in the development of ovaries and highlight the participation of the HPG axis on early gonadal development. In a recent study, gnrh3-null zebrafish exhibited a high proliferation of embryonic germ stem cells and a male-biased sex ratio, which also supported the involvement of the gnrh with sexual fate [94]. However, in spite of such progresses on the brain and gonad connection, the direct influence of the brain on gonadal fate, including the role on ESD still needs to be more explored (Fig. 3).
Fig. 3.
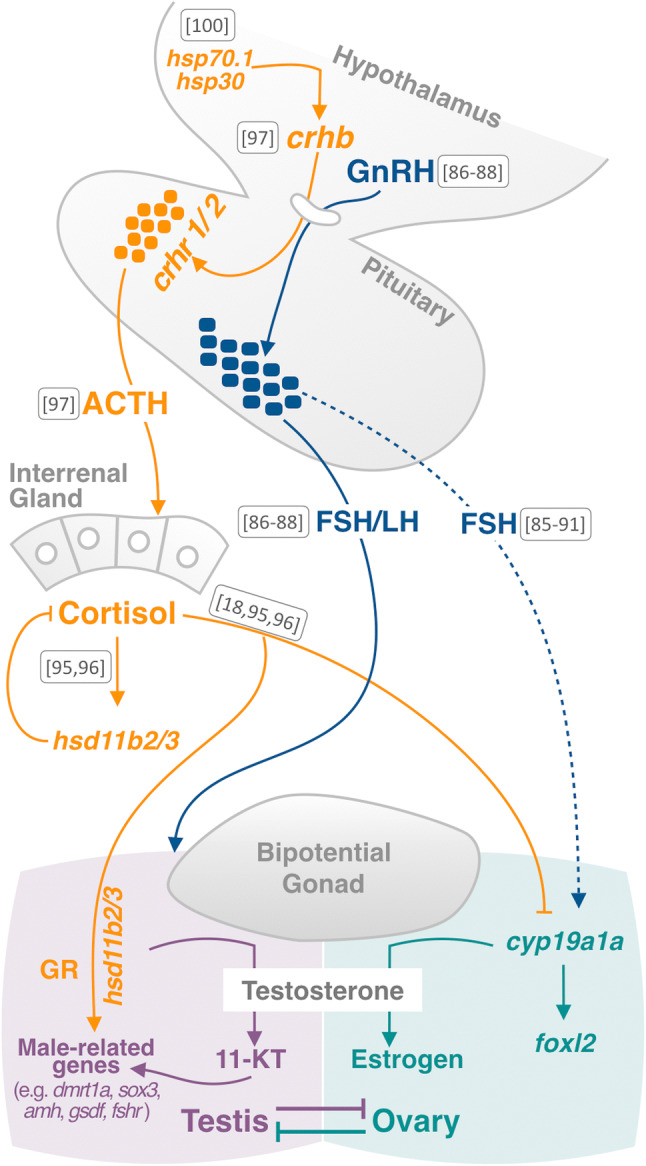
Schematic representation of brain and gonadal connection during Environmental Sex Determination (ESD). The action of hypothalamic–pituitary–gonadal axis in ESD is represented by blue labels and arrows, blue dashed arrows represent the participation of FSH in normal conditions of sex determination. The role of hypothalamic–pituitary–adrenal/interrenal axis is represented by orange labels and arrows. Numbers between square brackets are the references for each step, Adolfi et al. [18], Molés et al. [85], Molés et al. [86], Mianda [87], Chen et al. [88], Miranda et al. [89], Montserrat et al. [90], Yamaguchi et al. [91], Fernandino et al. [95], Castañeda-Cortés [96], Uchimura et al. [100]
As we mentioned above, female-to-male sex reversal in fish is induced by elevation in the levels of the stress hormone cortisol during the thermolabile period of sex determination [57]. This glucocorticoid is synthesized in the adrenal/interrenal in response to environmental stress and released into blood circulation. If we consider that all factors involved in ESD, both biotic and abiotic, can be sensed as stress [24], it is reasonable to establish the cortisol as the key hormone in environmentally induced sex reversal, as corroborated in several fish species [51]. Based on these findings, two major questions related to the role of cortisol have arisen. The first one is how cortisol was able to trigger sex reversal. In this regard, it has been elucidated that the elevation of cortisol by an environmental stressor implies the elevation of enzymes involved in its deactivation. These enzymes, encoded by hsd11b2 and hsd11b3 are shared by the androgen pathway in the synthesis of 11-ketotestosterone (11-KT), the most potent androgen in fish and whose elevation causes the development of testes. Thus, the masculinization induced by environmental stressors can be considered as a consequence of cortisol biogenesis with the concomitant synthesis of 11-KT [95]. This elevation of cortisol and androgen synthesis were reported to regulate the transcription of downstream genes involved in the differentiation of the gonads in different species, such as dmrt1a, gsdf, cyp19a1a, and fshr [58, 96, 97] (Fig. 3).
The second question is how cortisol regulation occurs during early stages of development. It is well known that the hypothalamic–pituitary–adrenal/interrenal (HPA/I) axis is involved as the transducer of environmental signals, such as stress, which is well-characterized in adults [82, 98, 99]. Recently, the brain has been established as the switch of the environmental sex determination mechanism in fish, directing the development of the bipotential gonad into the testes direction when embryos are exposed to a thermal treatment [96]. This is possible by a temporal coincidence between the high expression of corticosteroid-related hormone type b (chrb) in the brain and the high plasticity of the gonadal primordium during the gonadal sex determination period. Thus, the synthesis of Crhb in the hypothalamus upon environmental stress induces the release of the adrenocorticotropic hormone (Acth) from the pituitary through binding to two Crh receptors, to promote the synthesis and release of cortisol from the interrenal gland [96]. To build up connections between crhb regulation and thermal stress, the involvement of chaperones was investigated in medaka and it was demonstrated that the heat shock proteins hsp70.1 and hsp30 were able to up-regulate the transcription of crhb upon heat stress [100]. In vertebrates, all stress-related inputs are conveyed to the hypothalamus nucleus where CRH is synthesized. In this regard, some neurotransmitters, such as norepinephrine (NE) have also been found to regulate CRH in mice [101, 102]. Thus, the connection between the regulatory mechanisms of Crh in relation to other forms of environmental stress may represent an interesting field of study to furthering the upstream regulation of ESD in fish.
Epigenetic contribution to gonadal sex differentiation
It has been well established that environmental conditions can shape chromatin landscapes, as DNA methylation or histones modification, emphasizing the importance of studying epigenetics in the context of the phenotypic plasticity observed in fish gonadal differentiation. Gonadal plasticity influenced by environmental conditions can be considered more as a rule rather than the exception in fish. This could tell us that in fish the epigenetic pathways may represent a conserved adaptive mechanism that integrates both genomic and environmental information to control the destiny of gonads.
DNA methylation
Changes in DNA methylation, typically in the position 5 of cytosine, affect many developmental processes by repressing some regulatory regions of the genome. Sexual differentiation in gonochorists involves a certain antagonism between testis and ovary development pathways, wherein DNA methylation seems to play a pivotal role in the mechanisms of gene suppression [103]. In 2011, Navarro-Martin et al. [104] first described in juvenile males of European sea bass that the promoter of gonadal aromatase (cyp19a1a) had twice more methylation in relation to females. Moreover, exposure to high temperature increased the cyp19a1a promoter methylation levels compared with females, indicating that induced masculinization involves the transcriptional repression mediated by DNA methylation in female-related genes. In agreement with this, for other gonochoristic species, such as Japanese flounder and Nile tilapia, promoter regions of cyp19a1a were also differentially methylated and correlated with expression levels [105, 106]. Methylation in promoters of other candidate genes, such as dmrt1 and foxl2, shows a similar pattern [106]. In this regard, the idea on epigenetic repression of gonadal sex differentiation genes has been postulated by Piferrer et al. [103] and implies that antagonistic DNA methylation of “pro-female” and “pro-male” genes comprises one of the main molecular mechanisms of gonadal differentiation. However, this binary epigenetic regulation is still a debate on whether DNA methylation is a cause or a consequence of the final outcome to initiate and/or to maintain sexual identity.
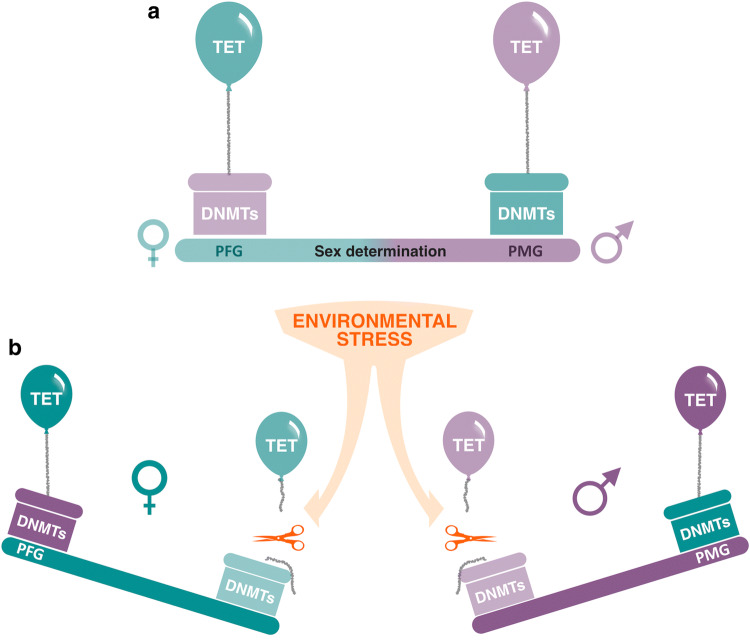
According to this notion, sex-related differences in global DNA methylation have been observed in various chromosomal DNA of Nile tilapia gonads [107]. This difference may indicate the important role of epigenetic modifications in fish gonadal development under normal conditions. However, apart from high temperature-induced males, high temperature-treated females also presented an increase in methylation levels on various chromosomes [107]. A recent study in the tiger pufferfish (Takifugu rubripes) using DNA from gonads of male, female, and pseudo male (by using a low-temperature treatment on females) concluded that changes in DNA methylation observed at higher or lower temperatures may just represent a consequence of the reared temperatures and not a specific epigenetic pathway activated to induce masculinization [108]. Nevertheless, another study conducted in the Chinese pond turtle (Mauremys reevesii), a species with a strong TSD in which female differentiation is induced at higher temperature, it has been demonstrated that some genes involved in the DNA methylation and demethylation processes are significantly different in developing gonads [109]. Specifically, the expression of DNA methyltransferases (dnmt1 and dnmt3b) and demethylases (tdg, tet1, tet2, and tet3) were higher at male- than at female-inducing temperature. This result raised the interesting possibility that the neofunctionalization of epigenetic regulators, via genomic duplication, contributes to the sexual plasticity seen across teleost fishes. Moreover, they observed higher methylation on male-related genes (dmrt1, sox9, and amh) at female-promoting temperatures; oppositely, higher methylation on female-related genes (cyp19a1a and foxl2) were found at male-promoting temperatures, establishing a balance of methylation, to determine the individual sex (Fig. 4). Nonetheless, it should be noted that all previous studies have been made on the entire gonads and DNA methylation patterns may differ depending on the cell type. Thus, DNA methylation levels reported up to now in the gonads represent the combined values of the different cell types and, therefore, further studies focusing on single-cell analysis approaches would provide a more accurate view behind these mechanisms.
Fig. 4.
Model of sex determination in environmental stress mediated by the epigenetic balance between DNMTs and TET enzymes. a Bipotential stage of sex determination, Promoter of female genes (PFG) and promoter of male genes (PMG) are at the poised state. b Environmental stressors affect the methylation/demethylation status of PMG or PFG and the respective sex
Histone modifications
Another epigenetic mechanism of transcriptional regulation is the post-translational modification of histones, such as methylation, acetylation, deacetylation, phosphorylation, ubiquitination, and sumoylation [110]. Histone methylation is associated with both active and repressive transcriptions [111]. For example, the H3K4me3 (Histone 3 Lysine 4 trimethylation) established at Trithorax group (TrxG) proteins is indicative of transcriptionally permissive chromatin states and is mostly found in the promoter regions. H3K36me3 is associated with euchromatic regions involved with active transcription and primarily found in gene bodies. In contrast, H3K27me3 catalyzed by Polycomb group (PcG) proteins and H3K9me3 catalyzed by G9a methyltransferase are associated with transcriptional repression [112]. On the other side, histone acetylation mediated by histone acetyltransferases (HATs) is associated with active transcription while histone de-acetylation exerted by deacetylases (HDACs) silences transcription [113]. Although many aspects of histone modifications and nucleosome architecture have been extensively described in several biological systems, very little is known in the context of gonadal sex differentiation.
In European sea bass, exposure of larvae to high masculinizing temperatures increased the expression of genes encoding PcG proteins (pcgf2, jarid2a, and suz12) and histone-modifying enzymes (ehmt2 and hdac11) in early-differentiating female gonads [114]. In rice field eel, repression of cyp19a1a in developing testis is associated with epigenetic repressive marks, including H3K9 deacetylation and trimethylation [115]. These data support a central role of histone modifications and chromatin remodeling in shaping gonadal differentiation. More studies in gonochoristic fish will be determinant to clarify the participation of these epigenetic modifications, especially on key sex-related genes, under normal and environmentally stressful conditions.
Environment-mediated intergenerational implications of sex reversal
The intra- and trans-generational heritability is receiving special attention in recent years, owing to the characterization of epigenetic inheritance mechanisms, especially to those related to the effects of early exposure to stressors. One of the most surprising examples of epigenetic plasticity is the environmentally sensitive sexual development observed in the flatfish half-smooth tongue sole. In this species, under normal temperature conditions, ∼14% of ZW genetic females are reversed to phenotypic males (pseudomales) and at higher temperatures, the sex reversal rate reaches ∼73%. Interestingly, sex-reversed pseudomales are fertile, and their ZW-F1 offspring exhibit an extremely high sex reversal rate (∼94%) at normal temperatures [61]. Remarkably, methylation patterns in testis from pseudomales (ZWm) resemble those in true parental males (ZZ), but also in ZWm-F1 offspring (less than 0.01% variation among them). In contrast, differentially methylated regions between testis and ovary represent approximately 4% of the genome and are enriched in sex-determining pathways. This male bias was generated by the epigenetic transmission of DNA methylation-altered states in other genes involved in the testis development, resulting in similar expression levels observed in the well-know compensatory mechanism in ZZ males. These results indicate that environmental sex reversal can override the predisposed genotypic sex through epigenetic regulation, and this mechanism can be transmitted across generations; however, the mechanisms implicated in this intergenerational inheritance are largely unknown.
Concerns related to environmental stress impacts on fish sex determination
Throughout this review, we have provided numerous examples of environmental factors that are able to affect the normal development of the gonads and change the biological sexual fate in fish, as well as the respective associated molecular, epigenetic, and endocrine mechanisms. The evolution of this high diversity of reproductive strategies seems to have been supported by the extraordinary plasticity of the gonads, especially during early stages of gonadogenesis. Moreover, there have been growing pieces of evidence that place the mechanisms of the stress response as one of the key players behind the remarkable sexual plasticity in this vertebrate group, with the stress hormone cortisol as a key endocrine activator.
In this regard, there has been an increased concern on the impacts of environmental stress associated with global climate change due to higher frequency of extremely low or high temperatures, outside the range of optimum or tolerable conditions in recent years. These changes represent important implications because natural selection normally directs the evolution and adaptation of the organism to an environment in a process that occurs for many generations; however, anthropogenic changes in the environment are occurring rapidly, which could exceed the adaptive capacity of organisms. It must be pointed out that global climate change poses a threat especially to species with ESD if adaptation does not occur rapidly enough to avoid highly biased sex ratios and their respective population consequences [116]. For example, this rapid adaptation has been studied in turtles with TSD, presenting potential adaptive directions to local climate change in their populations [117]. Although similar adaptations to different environments have been observed in fish populations [118], the effects of human impact are obvious albeit largely unknown in natural conditions, especially in terms of species adaptability.
Since the last decade, from the studies under laboratory conditions, we begin to estimate the impact of environmental stressors on wild populations of pejerrey, predicting that they are at risk of masculinization due to global increase in water temperatures. Recently, a good correlation of high temperature and male bias in laboratory and wild populations has been observed in another fish species, the Southern flounder (Paralichthys lethostigma) [119]. Nevertheless, there may be differences between the estimated effects on the wild environment based on experiments at laboratory conditions and the real effects in the wild, considering that in the first the results are generally for a single environmental factor whereas in the wild there is a wide range of environmental factors in constant change that could impact additively the process of sex determination. In this regard, integrative studies using a combination of environmental stressors are necessary to clarify the synergic, inhibitory or antagonistic effect of several stressors on gonadal sex determination.
A great concern related to environmental parameters that affect sex determination is that most of the climate change-associated disturbances such as high/extreme temperatures, acidification or hypoxia are associated with male-skewed sex ratios and even with all-male progenies in many fish species. Other factors that are expected to be affected include phenological alterations in spawning due to changes in marine currents or increased stochastic events (typhoons or storms) which have been associated to global warming, making fish to be exposed to unusual photoperiod or temperature conditions that could induce sex reversal or sex ratios distortions. In rivers and lakes, the intensification of droughts during either El Niño or La Nina events can bring about alterations in water quality parameters (higher concentration of ions, pollutants or organic matter) and also in biotic factors (density, species interactions or harmful algal blooms) that could act synergistically and thus enhance significantly the induction of stress responses in these environments. Therefore, the assessment of climate change impacts does require the analysis of multiple biotic/abiotic parameters at many conditions (not necessarily extreme) in association with in situ evaluations and wild population surveys for sex genotype–phenotype mismatches, since the process of population collapse driven by reduced reproductive potential and population growth due to male-skewed sex ratios [67] could be already in course for many fish species.
Another factor that should be considered in futures studies of environmental influence on sex ratio is the combinatory effect with endocrine-disrupting chemicals (EDCs) exposure. Due to the high influence of sex steroids on gonadal fate, the imbalance of sex steroid action due to the presence of anthropogenic EDCs [120] presents a growing concern in the study of Environmental EDCs by their high presence in aquatic habitats. EDCs can act as (anti)estrogenic and (anti)androgenic compounds, affecting the normal gonadal differentiation [120]. Moreover, their synergic effect with abiotic factors has been demonstrated [121], which makes it necessary to extend these evaluations at environmentally relevant concentrations biotic and abiotic factors.
Besides assessing those environmental impacts, integrative approaches including the evaluation of the capacity for fish populations to cope with those adverse conditions are critically important. Will fish be able to adjust or buffer the impacts of sex ratio distortions in response to the fast pace of climate changes? At what conditions the transgenerational effects can bring advantages to overcome the negative effects of sex ratio bias? Can maternal influences affect transgenerationally offspring ESD? More accurate and long-term evaluation of individual responses to climate change impacts will be crucial to answer those queries and to increase our understanding of the remarkable diversity and plasticity of fish sex determination.
Acknowledgements
We thank Agencia Nacional de Promoción Científica y Tecnológica (Grants PICT 2015-2501 to J.I.F. and PICT 2016-0747 to P.H.S.-M.) and to Sao Paulo Research Foundation (FAPESP2014/50790-0 and 2018/20200-8 to R.S.H.). D.C.C.C. and L.F.A.P. were supported by a PhD fellowship from the National Research Council (CONICET). J.I.F. and P.H.S.-M. are members of the career of scientific researcher at the CONICET.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Willeit M, Ganopolski A, Calov R, Brovkin V. Mid-Pleistocene transition in glacial cycles explained by declining CO2 and regolith removal. Sci Adv. 2019;5:eaav7337. doi: 10.1126/sciadv.aav7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinsky ML, Eikeset AM, McCauley DJ, Payne JL, Sunday JM. Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature. 2019;569:108–111. doi: 10.1038/s41586-019-1132-4. [DOI] [PubMed] [Google Scholar]
- 3.Geffroy B, Douhard M. The adaptive sex in stressful environments. Trends Ecol Evol. 2019;34:628–640. doi: 10.1016/j.tree.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Barton BA. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol. 2002;42:517–525. doi: 10.1093/icb/42.3.517. [DOI] [PubMed] [Google Scholar]
- 5.Björnsson BT, Stefansson SO, McCormick SD. Environmental endocrinology of salmon smoltification. Gen Comp Endocrinol. 2011;170:290–298. doi: 10.1016/j.ygcen.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Ashman TL, Bachtrog D, Blackmon H, Goldberg EE, Hahn MW, Kirkpatrick M, Kitano J, Mank JE, Mayrose I, Ming R, Otto SP, Peichel CL, Pennell MW, Perrin N, Ross L, Valenzuela N, Vamosi JC. Tree of sex: a database of sexual systems. Sci Data. 2014;1:140015. doi: 10.1038/sdata.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364. doi: 10.1016/S0044-8486(02)00057-1. [DOI] [Google Scholar]
- 8.Nishimura T, Tanaka M. The mechanism of germline sex determination in vertebrates. Biol Reprod. 2016;95:30–30. doi: 10.1095/biolreprod.115.138271. [DOI] [PubMed] [Google Scholar]
- 9.Capel B. Vertebrate sex determination: evolutionary plasticity of a fundamental switch. Nat Rev Genet. 2017;18:675–689. doi: 10.1038/nrg.2017.60. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura T, Tanaka M. Gonadal development in fish. Sex Dev. 2014;8:252–261. doi: 10.1159/000364924. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura S, Kobayashi K, Nishimura T, Higashijima S-i, Tanaka M. Identification of germline stem cells in the ovary of the teleost medaka. Science. 2010;80(328):1561–1563. doi: 10.1126/science.1185473. [DOI] [PubMed] [Google Scholar]
- 12.Hattori RS, Yoshinaga TT, Katayama N, Hattori-Ihara S, Tsukamoto RY, Takahashi NS, Tabata YA. Surrogate production of Salmo salar oocytes and sperm in triploid Oncorhynchus mykiss by germ cell transplantation technology. Aquaculture. 2019;506:238–245. doi: 10.1016/j.aquaculture.2019.03.037. [DOI] [Google Scholar]
- 13.Okutsu T, Suzuki K, Takeuchi Y, Takeuchi T, Yoshizaki G. Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc Natl Acad Sci USA. 2006;103:2725–2729. doi: 10.1073/pnas.0509218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshizaki G, Yazawa R. Application of surrogate broodstock technology in aquaculture. Fish Sci. 2019;85:429–437. doi: 10.1007/s12562-019-01299-y. [DOI] [Google Scholar]
- 15.Majhi SK, Hattori RS, Rahman SM, Strussmann CA. Surrogate production of eggs and sperm by intrapapillary transplantation of germ cells in cytoablated adult fish. PLoS ONE. 2014;9:e95294. doi: 10.1371/journal.pone.0095294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L-N, Jiang X-L, Xie Q-P, Yuan J, Huang B-F, Tao W-J, Zhou L-Y, Nagahama Y, Wang D-S. Transdifferentiation of differentiated ovary into functional testis by long-term treatment of aromatase inhibitor in Nile Tilapia. Endocrinology. 2014;155:1476–1488. doi: 10.1210/en.2013-1959. [DOI] [PubMed] [Google Scholar]
- 17.Wu G-C, Chang C-F. Oocytes survive in the testis by altering the soma fate from male to female in the protandrous black porgy, Acanthopagrus schlegeli. Biol Reprod. 2013;88:19. doi: 10.1095/biolreprod.112.104398. [DOI] [PubMed] [Google Scholar]
- 18.Adolfi MC, Nakajima RT, Nobrega RH, Schartl M. Intersex, hermaphroditism, and gonadal plasticity in vertebrates: evolution of the Mullerian duct and amh/amhr2 signaling. Annu Rev Anim Biosci. 2019;7:149–172. doi: 10.1146/annurev-animal-020518-114955. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura T, Sato T, Yamamoto Y, Watakabe I, Ohkawa Y, Suyama M, Kobayashi S, Tanaka M. foxl3 is a germ cell-intrinsic factor involved in sperm-egg fate decision in medaka. Science. 2015;80(349):328–331. doi: 10.1126/science.aaa2657. [DOI] [PubMed] [Google Scholar]
- 20.Gemmell NJ, Todd EV, Goikoetxea A, Ortega-Recalde O, Hore TA. Current topics in developmental biology. 1. Amsterdam: Elsevier Inc.; 2019. Natural sex change in fish; pp. 71–117. [DOI] [PubMed] [Google Scholar]
- 21.Avise JC, Mank JE. Evolutionary perspectives on hermaphroditism in fishes. Sex Dev. 2009;3:152–163. doi: 10.1159/000223079. [DOI] [PubMed] [Google Scholar]
- 22.Maitre D, Selmoni OM, Uppal A, Marques da Cunha L, Wilkins LGE, Roux J, Mobley KB, Castro I, Knörr S, Robinson-Rechavi M, Wedekind C. Sex differentiation in grayling (Salmonidae) goes through an all-male stage and is delayed in genetic males who instead grow faster. Sci Rep. 2017;7:15024. doi: 10.1038/s41598-017-14905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miranda LA, Chalde T, Elisio M, Strussmann CA. Effects of global warming on fish reproductive endocrine axis, with special emphasis in pejerrey Odontesthes bonariensis. Gen Comp Endocrinol. 2013;192:45–54. doi: 10.1016/j.ygcen.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Fernandino JI, Hattori RS, Moreno Acosta OD, Strüssmann CA, Somoza GM. Environmental stress-induced testis differentiation: androgen as a by-product of cortisol inactivation. Gen Comp Endocrinol. 2013;192:36–44. doi: 10.1016/j.ygcen.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Cotta JBHD. The reversible sex of gonochoristic fish: insights and consequences. Sex Dev. 2016 doi: 10.1159/000452362. [DOI] [PubMed] [Google Scholar]
- 26.Geffroy B, Bardonnet A. Sex differentiation and sex determination in eels: consequences for management. Fish Fish. 2016;17:375–398. doi: 10.1111/faf.12113. [DOI] [Google Scholar]
- 27.Wu RSS. Hypoxia: from molecular responses to ecosystem responses. Mar Pollut Bull. 2002;45:35–45. doi: 10.1016/S0025-326X(02)00061-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Gilbert D, Gooday A, Levin L, Naqvi SWA, Middelburg JJ, Scranton M, Ekau W, Pena A, Dewitte B. Natural and human-induced hypoxia and consequences for coastal areas: synthesis and future development. Biogeosciences. 2010;7:1443–1467. doi: 10.5194/bg-7-1443-2010. [DOI] [Google Scholar]
- 29.Gray JS, Wu RSS, Ying YO. Effects of hypoxia and organic enrichment on the coastal marine environment. Mar Ecol Prog Ser. 2002;238:249–279. doi: 10.3354/meps238249. [DOI] [Google Scholar]
- 30.Breitburg D, Levin LA, Oschlies A, Grégoire M, Chavez FP, Conley DJ, Garçon V, Gilbert D, Gutiérrez D, Isensee K, Jacinto GS, Limburg KE, Montes I, Naqvi SWA, Pitcher GC, Rabalais NN, Roman MR, Rose KA, Seibel BA, Telszewski M, Yasuhara M, Zhang J. Declining oxygen in the global ocean and coastal waters. Science (80-) 2018;359:eaam7240. doi: 10.1126/science.aam7240. [DOI] [PubMed] [Google Scholar]
- 31.Shang EHH, Yu RMK, Wu RSS. Hypoxia affects sex differentiation and development, leading to a male-dominated population in zebrafish (Danio rerio) Environ Sci Technol. 2006;40:3118–3122. doi: 10.1021/es0522579. [DOI] [PubMed] [Google Scholar]
- 32.Breitburg DL, Hondorp DW, Davias La, Diaz RJ. Hypoxia, nitrogen, and fisheries: integrating effects across local and global landscapes. Ann Rev Mar Sci. 2009;1:329–349. doi: 10.1146/annurev.marine.010908.163754. [DOI] [PubMed] [Google Scholar]
- 33.Thomas P, Rahman MS. Extensive reproductive disruption, ovarian masculinization and aromatase suppression in Atlantic croaker in the northern Gulf of Mexico hypoxic zone. Proc R Soc B Biol Sci. 2012;279:28–38. doi: 10.1098/rspb.2011.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollock MS, Dubé MG, Schryer R. Investigating the link between pulp mill effluent and endocrine disruption: attempts to explain the presence of intersex fish in the Wabigoon River, Ontario, Canada. Environ Toxicol Chem. 2010;29:952–965. doi: 10.1002/etc.118. [DOI] [PubMed] [Google Scholar]
- 35.Cheung CHY, Chiu JMY, Wu RSS. Hypoxia turns genotypic female medaka fish into phenotypic males. Ecotoxicology. 2014;23:1260–1269. doi: 10.1007/s10646-014-1269-8. [DOI] [PubMed] [Google Scholar]
- 36.Ou M, Hamilton T, Eom J, Lyall E, Gallup J, Jiang A, Lee J, Close D, Yun S-S, Brauner C. Responses of pink salmon to CO2-induced aquatic acidification. Nat Clim Chang. 2015;5:950–955. doi: 10.1038/nclimate2694. [DOI] [Google Scholar]
- 37.Nagelkerken I, Munday PL. Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob Chang Biol. 2016;22:974–989. doi: 10.1111/gcb.13167. [DOI] [PubMed] [Google Scholar]
- 38.Lefevre S. Are global warming and ocean acidification conspiring against marine ectotherms? A meta-analysis of the respiratory effects of elevated temperature, high CO2 and their interaction. Conserv Physiol. 2016;4:1–31. doi: 10.1093/conphys/cow009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schunter C, Welch MJ, Ryu T, Zhang H, Berumen ML, Nilsson GE, Munday PL, Ravasi T. Molecular signatures of transgenerational response to ocean acidification in a species of reef fish. Nat Clim Chang. 2016;6:1014–1018. doi: 10.1038/nclimate3087. [DOI] [Google Scholar]
- 40.Rubin DA. Effect of ph on sex ratio in Cichlids and a Poecilliid (Teleostei) Copeia. 1985;1985:233–235. doi: 10.2307/1444818. [DOI] [Google Scholar]
- 41.Römer U, Beisenherz W. Environmental determination of sex in Apistogrammai (Cichlidae) and two other freshwater fishes (Teleostei) J Fish Biol. 1996;48:714–725. doi: 10.1111/j.1095-8649.1996.tb01467.x. [DOI] [Google Scholar]
- 42.Reddon AR, Hurd PL. Water pH during early development influences sex ratio and male morph in a West African cichlid fish, Pelvicachromis pulcher. Zoology. 2013;116:139–143. doi: 10.1016/j.zool.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Karakatsouli N, Papoutsoglou SE, Pizzonia G, Tsatsos G, Tsopelakos A, Chadio S, Kalogiannis D, Dalla C, Polissidis A, Papadopoulou-Daifoti Z. Effects of light spectrum on growth and physiological status of gilthead seabream Sparus aurata and rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquac Eng. 2007;36:302–309. doi: 10.1016/j.aquaeng.2007.01.005. [DOI] [Google Scholar]
- 44.Villamizar N, Garcia-Alcazar A, Sanchez Vazquez FJ. Effect of light spectrum and photoperiod on the growth, development and survival of European sea bass (Dicentrarchus labrax) larvae. Aquaculture. 2009;292:80–86. doi: 10.1016/j.aquaculture.2009.03.045. [DOI] [Google Scholar]
- 45.Brown EE, Baumann H, Conover DO. Temperature and photoperiod effects on sex determination in a fish. J Exp Mar Bio Ecol. 2014;461:39–43. doi: 10.1016/j.jembe.2014.07.009. [DOI] [Google Scholar]
- 46.Corona-Herrera GA, Arranz SE, Martínez-Palacios CA, Navarrete-Ramírez P, Toledo-Cuevas EM, Valdez-Alarcón JJ, Martínez-Chávez CC. Experimental evidence of masculinization by continuous illumination in a temperature sex determination teleost (Atherinopsidae) model: is oxidative stress involved? J Fish Biol. 2018;93:229–237. doi: 10.1111/jfb.13651. [DOI] [PubMed] [Google Scholar]
- 47.Hayasaka O, Takeuchi Y, Shiozaki K, Anraku K, Kotani T. Green light irradiation during sex differentiation induces female-to-male sex reversal in the medaka Oryzias latipes. Sci Rep. 2019;9:2383. doi: 10.1038/s41598-019-38908-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baroiller JF, D’Cotta H, Saillant E. Environmental effects on fish sex determination and differentiation. Sex Dev. 2009;3:118–135. doi: 10.1159/000223077. [DOI] [PubMed] [Google Scholar]
- 49.Ospina-Alvarez N, Piferrer F. Temperature-dependent sex determination in fish revisited: prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS ONE. 2008;3:e2837. doi: 10.1371/journal.pone.0002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobayashi Y, Nagahama Y, Nakamura M. Diversity and plasticity of sex determination and differentiation in fishes. Sex Dev. 2013;7:115–125. doi: 10.1159/000342009. [DOI] [PubMed] [Google Scholar]
- 51.Goikoetxea A, Todd EV, Gemmell NJ. Stress and sex: does cortisol mediate sex change in fish? Reproduction. 2017;154:R149–R160. doi: 10.1530/REP-17-0408. [DOI] [PubMed] [Google Scholar]
- 52.Strüssmann CA, Conover DO, Somoza GM, Miranda LA. Implications of climate change for the reproductive capacity and survival of New World silversides (family Atherinopsidae) J Fish Biol. 2010;77:1818–1834. doi: 10.1111/j.1095-8649.2010.02780.x. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto Y, Zhang Y, Sarida M, Hattori RS, Strüssmann CA. Coexistence of genotypic and temperature-dependent sex determination in pejerrey Odontesthes bonariensis. PLoS ONE. 2014;9:1–8. doi: 10.1371/journal.pone.0102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hattori RS, Somoza GM, Fernandino JI, Colautti DC, Miyoshi K, Gong Z, Yamamoto Y, Strüssmann CA. The duplicated y-specific amhy gene is conserved and linked to maleness in silversides of the genus Odontesthes. Genes (Basel) 2019;10:E679. doi: 10.3390/genes10090679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brierley AS, Kingsford MJ. Impacts of climate change on marine organisms and ecosystems. Curr Biol. 2009;19:R602–R614. doi: 10.1016/j.cub.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 56.Penman DJ, Piferrer F. Fish Gonadogenesis. Part I: genetic and environmental mechanisms of sex determination. Rev Fish Sci. 2008;16:16–34. doi: 10.1080/10641260802324610. [DOI] [Google Scholar]
- 57.Hattori RS, Fernandino JI, Kishil A, Kimura H, Kinno T, Oura M, Somoza GM, Yokota M, Strüssmann CA, Watanabe S. Cortisol-induced masculinization: does thermal stress affect gonadal fate in pejerrey, a teleost fish with temperature-dependent sex determination? PLoS ONE. 2009;4(8):e6548. doi: 10.1371/journal.pone.0006548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adolfi MC, Fischer P, Herpin A, Regensburger M, Kikuchi M, Tanaka M, Schartl M. Increase of cortisol levels after temperature stress activates dmrt1a causing female-to-male sex reversal and reduced germ cell number in medaka. Mol Reprod Dev. 2019;86:1405–1417. doi: 10.1002/mrd.23177. [DOI] [PubMed] [Google Scholar]
- 59.Wessels S, Hörstgen-Schwark G. Selection experiments to increase the proportion of males in Nile tilapia (Oreochromis niloticus) by means of temperature treatment. Aquaculture. 2007;272:80–87. doi: 10.1016/j.aquaculture.2007.08.009. [DOI] [Google Scholar]
- 60.Wessels S, Sharifi RA, Luehmann LM, Rueangsri S, Krause I, Pach S, Hoerstgen-Schwark G, Knorr C. Allelic variant in the anti-müllerian hormone gene leads to autosomal and temperature-dependent sex reversal in a selected nile tilapia line. PLoS ONE. 2014;9(8):e104795. doi: 10.1371/journal.pone.0104795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen S, Zhang G, Shao C, Huang Q, Liu G, Zhang P, Song W, An N, Chalopin D, Volff JN, Hong Y, Li Q, Sha Z, Zhou H, Xie M, Yu Q, Liu Y, Xiang H, Wang N, Wu K, Yang C, Zhou Q, Liao X, Yang L, Hu Q, Zhang J, Meng L, Jin L, Tian Y, Lian J, Yang J, Miao G, Liu S, Liang Z, Yan F, Li Y, Sun B, Zhang H, Zhang J, Zhu Y, Du M, Zhao Y, Schartl M, Tang Q, Wang J. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat Genet. 2014;46:253–260. doi: 10.1038/ng.2890. [DOI] [PubMed] [Google Scholar]
- 62.Ribas L, Liew WC, Díaz N, Sreenivasan R, Orbán L, Piferrer F. Heat-induced masculinization in domesticated zebrafish is family-specific and yields a set of different gonadal transcriptomes. Proc Natl Acad Sci USA. 2017;114:E941–E950. doi: 10.1073/pnas.1609411114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liew WC, Bartfai R, Lim Z, Sreenivasan R, Siegfried KR, Orban L. Polygenic sex determination system in zebrafish. PLoS ONE. 2012;7:e34397. doi: 10.1371/journal.pone.0034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Hattori RS, Sarida M, Garcia EL, Strussmann CA, Yamamoto Y. Expression profiles of amhy and major sex-related genes during gonadal sex differentiation and their relation with genotypic and temperature-dependent sex determination in pejerrey Odontesthes bonariensis. Gen Comp Endocrinol. 2018;265:196–201. doi: 10.1016/j.ygcen.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 65.Bull JJ. Sex determination in reptiles. Q Rev Biol. 1980;55:3–21. doi: 10.1086/411613. [DOI] [Google Scholar]
- 66.Holleley CE, Sarre SD, O’Meally D, Georges A. Sex reversal in reptiles: reproductive oddity or powerful driver of evolutionary change? Sex Dev. 2016;10:279–287. doi: 10.1159/000450972. [DOI] [PubMed] [Google Scholar]
- 67.Xiong Y, Wang S, Gui J-F, Mei J. Artificially induced sex-reversal leads to transition from genetic to temperature-dependent sex determination in fish species. Sci China Life Sci. 2020;63:157–159. doi: 10.1007/s11427-019-1568-7. [DOI] [PubMed] [Google Scholar]
- 68.Li X-Y, Gui J-F. Diverse and variable sex determination mechanisms in vertebrates. Sci China Life Sci. 2018;61:1503–1514. doi: 10.1007/s11427-018-9415-7. [DOI] [PubMed] [Google Scholar]
- 69.Hattori RS, Gould RJ, Fujioka T, Saito T, Kurita J, Strüssmann CA, Yokota M, Watanabe S. Temperature-dependent sex determination in Hd-rR medaka Oryzias latipes: gender sensitivity, thermal threshold, critical period, and dmrt1 expression profile. Sex Dev. 2007;1:138–146. doi: 10.1159/000100035. [DOI] [PubMed] [Google Scholar]
- 70.Cotton S, Wedekind C. Population consequences of environmental sex reversal. Conserv Biol. 2009;23:196–206. doi: 10.1111/j.1523-1739.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- 71.Herpin A, Schartl M. Plasticity of gene-regulatory networks controlling sex determination: of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Rep. 2015;16:1260–1274. doi: 10.15252/embr.201540667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernandino JI, Hattori RS. Sex determination in neotropical fish: implications ranging from aquaculture technology to ecological assessment. Gen Comp Endocrinol. 2019;273:172–183. doi: 10.1016/j.ygcen.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Reichwald K, Petzold A, Koch P, Downie BR, Hartmann N, Pietsch S, Baumgart M, Chalopin D, Felder M, Bens M, Sahm A, Szafranski K, Taudien S, Groth M, Arisi I, Weise A, Bhatt SS, Sharma V, Kraus JM, Schmid F, Priebe S, Liehr T, Gorlach M, Than ME, Hiller M, Kestler HA, Volff J-N, Schartl M, Cellerino A, Englert C, Platzer M. Insights into sex chromosome evolution and aging from the genome of a short-lived fish. Cell. 2015;163:1527–1538. doi: 10.1016/j.cell.2015.10.071. [DOI] [PubMed] [Google Scholar]
- 74.Koyama T, Nakamoto M, Morishima K, Yamashita R, Yamashita T, Sasaki K, Kuruma Y, Mizuno N, Suzuki M, Okada Y, Ieda R, Uchino T, Tasumi S, Hosoya S, Uno S, Koyama J, Toyoda A, Kikuchi K, Sakamoto T. A SNP in a steroidogenic enzyme is associated with phenotypic sex in seriola fishes. Curr Biol. 2019;29:1901–1909.e8. doi: 10.1016/j.cub.2019.04.069. [DOI] [PubMed] [Google Scholar]
- 75.Pan Q, Feron R, Yano A, Guyomard R, Jouanno E, Vigouroux E, Wen M, Busnel J-M, Bobe J, Concordet J-P, Parrinello H, Journot L, Klopp C, Lluch J, Roques C, Postlethwait J, Schartl M, Herpin A, Guiguen Y. Identification of the master sex determining gene in Northern pike (Esox lucius) reveals restricted sex chromosome differentiation. PLOS Genet. 2019;15:e1008013. doi: 10.1371/journal.pgen.1008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hattori RS, Murai Y, Oura M, Masuda S, Majhi SK, Sakamoto T, Fernandino JI, Somoza GM, Yokota M, Strüssmann CA. A Y-linked anti-Müllerian hormone duplication takes over a critical role in sex determination. Proc Natl Acad Sci U S A. 2012;109:2955–2959. doi: 10.1073/pnas.1018392109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamamoto Y, Hattori RS, Patiño R, Strüssmann CA (2019) Chapter Two: Environmental regulation of sex determination in fishes: insights from atheriniformes. In: Capel BBT-CT in DB (ed) Sex determination in vertebrates. Academic Press, pp 49–69 [DOI] [PubMed]
- 78.Bertho S, Herpin A, Branthonne A, Jouanno E, Yano A, Nicol B, Muller T, Pannetier M, Pailhoux E, Miwa M, Yoshizaki G, Schartl M, Guiguen Y. The unusual rainbow trout sex determination gene hijacked the canonical vertebrate gonadal differentiation pathway. Proc Natl Acad Sci. 2018;115:12781–12786. doi: 10.1073/pnas.1803826115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang X, Li M, Ma H, Liu X, Shi H, Li M, Wang D. Mutation of foxl2 or cyp19a1a results in female to male sex reversal in XX Nile Tilapia. Endocrinology. 2017;158:2634–2647. doi: 10.1210/en.2017-00127. [DOI] [PubMed] [Google Scholar]
- 80.Warr N, Greenfield A. The molecular and cellular basis of gonadal sex reversal in mice and humans. Wiley Interdiscip Rev Dev Biol. 2012;1:559–577. doi: 10.1002/wdev.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chi W, Gao Y, Hu Q, Guo W, Li D. Genome-wide analysis of brain and gonad transcripts reveals changes of key sex reversal-related genes expression and signaling pathways in three stages of Monopterus albus. PLoS ONE. 2017;12:e0173974. doi: 10.1371/journal.pone.0173974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Acevedo-Rodriguez A, Kauffman AS, Cherrington BD, Borges CS, Roepke TA, Laconi M. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J Neuroendocrinol. 2018;30:e12590. doi: 10.1111/jne.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young G, Kusakabe M, Nakamura I, Lokman PM, Goetz FW. Hormones and their receptors in fish reproduction. Singapore: World Scientific; 2005. Gonadal steroidogenesis in teleost fish; pp. 155–223. [Google Scholar]
- 84.Levavi-Sivan B, Bogerd J, Mananos EL, Gomez A, Lareyre JJ. Perspectives on fish gonadotropins and their receptors. Gen Comp Endocrinol. 2010;165:412–437. doi: 10.1016/j.ygcen.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 85.Molés G, Carrillo M, Mañanós E, Mylonas CC, Zanuy S. Temporal profile of brain and pituitary GnRHs, GnRH-R and gonadotropin mRNA expression and content during early development in European sea bass (Dicentrarchus labrax L.) Gen Comp Endocrinol. 2007;150:75–86. doi: 10.1016/j.ygcen.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 86.Molés G, Gómez A, Carrillo M, Rocha A, Mylonas CC, Zanuy S. Determination of fsh quantity and bioactivity during sex differentiation and oogenesis in European Sea Bass. Biol Reprod. 2011;85:848–857. doi: 10.1095/biolreprod.111.091868. [DOI] [PubMed] [Google Scholar]
- 87.Miranda L. Gonadotropin-releasing hormone neuronal development during the sensitive period of temperature sex determination in the pejerrey fish, Odontesthes bonariensis. Gen Comp Endocrinol. 2003;132:444–453. doi: 10.1016/S0016-6480(03)00117-5. [DOI] [PubMed] [Google Scholar]
- 88.Chen W, Ge W. Ontogenic expression profiles of Gonadotropins (fshb and lhb) and growth hormone (gh) during sexual differentiation and puberty onset in female Zebrafish. Biol Reprod. 2012;86:1–11. doi: 10.1095/biolreprod.111.094730. [DOI] [PubMed] [Google Scholar]
- 89.Miranda LA, Strüssmann CA, Somoza GM. Immunocytochemical identification of gth1 and gth2 cells during the temperature-sensitive period for sex determination in pejerrey, Odontesthes bonariensis. Gen Comp Endocrinol. 2001;124:45–52. doi: 10.1006/gcen.2001.7687. [DOI] [PubMed] [Google Scholar]
- 90.Montserrat N, González A, Méndez E, Piferrer F, Planas JV. Effects of follicle stimulating hormone on estradiol-17β production and P-450 aromatase (CYP19) activity and mRNA expression in brown trout vitellogenic ovarian follicles in vitro. Gen Comp Endocrinol. 2004;137:123–131. doi: 10.1016/j.ygcen.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 91.Yamaguchi T, Yamaguchi S, Hirai T, Kitano T. Follicle-stimulating hormone signaling and Foxl2 are involved in transcriptional regulation of aromatase gene during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochem Biophys Res Commun. 2007;359:935–940. doi: 10.1016/j.bbrc.2007.05.208. [DOI] [PubMed] [Google Scholar]
- 92.Murozumi N, Nakashima R, Hirai T, Kamei Y, Ishikawa-Fujiwara T, Todo T, Kitano T. Loss of follicle-stimulating hormone receptor function causes masculinization and suppression of ovarian development in genetically female medaka. Endocrinology. 2014;155:3136–3145. doi: 10.1210/en.2013-2060. [DOI] [PubMed] [Google Scholar]
- 93.Blázquez M, Somoza GM. Fish with thermolabile sex determination (TSD) as models to study brain sex differentiation. Gen Comp Endocrinol. 2010;166:470–477. doi: 10.1016/j.ygcen.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 94.Feng K, Cui X, Song Y, Tao B, Chen J, Wang J, Liu S, Sun Y, Zhu Z, Trudeau VL, Hu W (2020) Gnrh3 regulates pgc proliferation and sex differentiation in developing zebrafish. Endocrinology 161. 10.1210/endocr/bqz024 [DOI] [PubMed]
- 95.Fernandino JI, Hattori RS, Kishii A, Strüssmann CA, Somoza GM. The cortisol and androgen pathways cross talk in high temperature-induced masculinization: the 11β-hydroxysteroid dehydrogenase as a key enzyme. Endocrinology. 2012;153:6003–6011. doi: 10.1210/en.2012-1517. [DOI] [PubMed] [Google Scholar]
- 96.Castañeda Cortés DC, Arias Padilla LF, Langlois VS, Somoza GM, Fernandino JI. The central nervous system acts as a transducer of stress-induced masculinization through corticotropin-releasing hormone B. Development. 2019;146:172866. doi: 10.1242/dev.172866. [DOI] [PubMed] [Google Scholar]
- 97.Hayashi Y, Kobira H, Yamaguchi T, Shiraishi E, Yazawa T, Hirai T, Kamei Y, Kitano T. High temperature causes masculinization of genetically female medaka by elevation of cortisol. Mol Reprod Dev. 2010;77:679–686. doi: 10.1002/mrd.21203. [DOI] [PubMed] [Google Scholar]
- 98.Kovács KJ. CRH: the link between hormonal-, metabolic- and behavioral responses to stress. J Chem Neuroanat. 2013;54:25–33. doi: 10.1016/j.jchemneu.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 99.Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol. 2012;33:67–84. doi: 10.1016/j.yfrne.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Uchimura T, Hara S, Yazawa T, Kamei Y, Kitano T. Involvement of heat shock proteins on the transcriptional regulation of corticotropin-releasing hormone in medaka. Front Endocrinol (Lausanne) 2019;10:529. doi: 10.3389/fendo.2019.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma X-M, Aguilera G. Differential regulation of corticotropin-releasing hormone and vasopressin transcription by glucocorticoids. Endocrinology. 1999;140:5642–5650. doi: 10.1210/endo.140.12.7214. [DOI] [PubMed] [Google Scholar]
- 102.McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87:605–620. doi: 10.1016/j.neuron.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piferrer F, Anastasiadi D, Valdivieso A, Sánchez-Baizán N, Moraleda-Prados J, Ribas L. The model of the conserved epigenetic regulation of sex. Front Genet. 2019;10:1–13. doi: 10.3389/fgene.2019.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Navarro-Martín L, Viñas J, Ribas L, Díaz N, Gutiérrez A, Di Croce L, Piferrer F. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 2011;7:e1002447. doi: 10.1371/journal.pgen.1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang YY, Sun LX, Zhu JJ, Zhao Y, Wang H, Liu HJ, Ji XS. Epigenetic control of cyp19a1a expression is critical for high temperature induced Nile tilapia masculinization. J Therm Biol. 2017;69:76–84. doi: 10.1016/j.jtherbio.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 106.Wen AY, You F, Sun P, Li J, Xu DD, Wu ZH, Ma DY, Zhang PJ. CpG methylation of dmrt1 and cyp19a promoters in relation to their sexual dimorphic expression in the Japanese flounder Paralichthys olivaceus. J Fish Biol. 2014;84:193–205. doi: 10.1111/jfb.12277. [DOI] [PubMed] [Google Scholar]
- 107.Sun LX, Wang YY, Zhao Y, Wang H, Li N, Ji XS. Global DNA methylation changes in Nile tilapia gonads during high temperature-induced masculinization. PLoS ONE. 2016;11:1–16. doi: 10.1371/journal.pone.0158483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou H, Zhuang Z-X, Sun Y-Q, Chen Q, Zheng X-Y, Liang Y-T, Mahboob S, Wang Q, Zhang R, Al-Ghanim KA, Shao C-W, Li Y-J. Changes in DNA methylation during epigenetic-associated sex reversal under low temperature in Takifugu rubripes. PLoS ONE. 2019;14:e0221641. doi: 10.1371/journal.pone.0221641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dong J, Xiong L, Ding H, Jiang H, Zan J, Nie L. Characterization of deoxyribonucleic methylation and transcript abundance of sex-related genes during temperature-dependent sex determination in Mauremys reevesii. Biol Reprod. 2019;1:ioz147. doi: 10.1093/biolre/ioz147. [DOI] [PubMed] [Google Scholar]
- 110.Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N, Lu Z, Ye Z, Zhu Q, Wysocka J, Ye Y, Khochbin S, Ren B, Zhao Y. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146:1016–1028. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 112.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 114.Díaz N, Piferrer F. Lasting effects of early exposure to temperature on the gonadal transcriptome at the time of sex differentiation in the European sea bass, a fish with mixed genetic and environmental sex determination. BMC Genomics. 2015 doi: 10.1186/s12864-015-1862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Y, Zhang S, Liu Z, Zhang L, Zhang W. Epigenetic modifications during sex change repress Gonadotropin stimulation of Cyp19a1a in a Teleost Ricefield Eel (Monopterus albus) Endocrinology. 2013;154:2881–2890. doi: 10.1210/en.2012-2220. [DOI] [PubMed] [Google Scholar]
- 116.Mitchell NJ, Janzen FJ. Temperature-dependent sex determination and contemporary climate change. Sex Dev. 2010;4:129–140. doi: 10.1159/000282494. [DOI] [PubMed] [Google Scholar]
- 117.Refsnider JM, Janzen FJ. Temperature-dependent sex determination under rapid anthropogenic environmental change: evolution at a turtle’s pace? J Hered. 2016;107:61–70. doi: 10.1093/jhered/esv053. [DOI] [PubMed] [Google Scholar]
- 118.Conover DO. Adaptive significance of temperature-dependent sex determination in a fish. Am Nat. 1984;123:297–313. doi: 10.1086/284205. [DOI] [Google Scholar]
- 119.Honeycutt JL, Deck CA, Miller SC, Severance ME, Atkins EB, Luckenbach JA, Buckel JA, Daniels HV, Rice JA, Borski RJ, Godwin J. Warmer waters masculinize wild populations of a fish with temperature-dependent sex determination. Sci Rep. 2019;9:1–13. doi: 10.1038/s41598-019-42944-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kloas W, Urbatzka R, Opitz R, Sven W, Behrends T, Hofmann F, Jagnytsch O, Kroupova H, Lorenz C, Neumann N, Pietsch C, Trubiroha A, Van BC, Wiedemann C, Lutz I. Endocrine disruption in aquatic vertebrates. Ann N Y Acad Sci. 2009;1163:187–200. doi: 10.1111/j.1749-6632.2009.04453.x. [DOI] [PubMed] [Google Scholar]
- 121.Hooper MJ, Ankley GT, Cristol DA, Maryoung LA, Noyes PD, Pinkerton KE. Interactions between chemical and climate stressors: A role for mechanistic toxicology in assessing climate change risks. Environ Toxicol Chem. 2013;32:32–48. doi: 10.1002/etc.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]