Abstract
Despite the experimental evidence pointing to a significant role of the Wnt family of proteins in physiological and pathological rodent spinal cord functioning, its potential relevance in the healthy and traumatically injured human spinal cord as well as its therapeutic potential in spinal cord injury (SCI) are still poorly understood. To get further insight into these interesting issues, we first demonstrated by quantitative Real-Time PCR and simple immunohistochemistry that detectable mRNA expression of most Wnt components, as well as protein expression of all known Wnt receptors, can be found in the healthy human spinal cord, supporting its potential involvement in human spinal cord physiology. Moreover, evaluation of Frizzled (Fz) 1 expression by double immunohistochemistry showed that its spatio-temporal and cellular expression pattern in the traumatically injured human spinal cord is equivalent to that observed in a clinically relevant model of rat SCI and suggests its potential involvement in SCI progression/outcome. Accordingly, we found that long-term lentiviral-mediated overexpression of the Fz1 ligand Wnt1 after rat SCI improves motor functional recovery, increases myelin preservation and neuronal survival, and reduces early astroglial reactivity and NG2+ cell accumulation, highlighting the therapeutic potential of Wnt1 in this neuropathological situation.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03427-4) contains supplementary material, which is available to authorized users.
Keywords: Wnt, Frizzled 1, Wnt1, Spinal cord injury, Human, Rat
Introduction
Wnts are a highly complex and conserved family of secreted lipid-modified glycoproteins which exerts essential functions in development, adult homeostasis, and disease in multiple tissues and organs. To date, 19 Wnt ligands have been identified in mammals which are able to bind different receptors that can be classified into conventional Frizzled (Fz) receptors (Fz1-10) and non-conventional receptors such as the receptor tyrosine kinase-like orphan receptor (Ror) 1 and 2, Ryk, and protein tyrosine kinase 7 (PTK7). Moreover, different Wnt co-receptors [low-density lipoprotein receptor protein (LRP) 5 and 6] and soluble modulators [Dickkopf (Dkk) 1–4, Wnt inhibitory factor 1 (Wif1), R-spondin 1–4, and secreted Frizzled-related proteins (sFRP) 1–5] have been also identified. Finally, Wnt signaling pathways can be divided into canonical Wnt/β-catenin and non-canonical Wnt/Ca2+ and Wnt/planar cell polarity signaling pathways [1–3]. Specifically regarding the central nervous system (CNS), different studies performed in rodents have demonstrated that, besides its crucial functions during CNS development [4–7], the Wnt family of proteins is also involved in relevant aspects of CNS functioning during adulthood [8–13]. However, only a few studies have pointed to a potential role of this family of proteins in physiological adult human CNS activity, mostly by assessing the expression of a small selection of its components in the healthy human brain and spinal cord. Specifically in the healthy adult human spinal cord, we have recently shown that specific Wnt ligands, receptors, co-receptors, and soluble modulators are expressed at the mRNA level in the ependymal region [14] and in the anterior horn [15]. However, the broad expression of the different components of the Wnt family of proteins at the mRNA and protein level in the healthy adult human spinal cord is currently unknown. As a consequence and to further ascertain the potential involvement of this family of proteins in physiological adult human spinal cord functioning, we first aimed to evaluate the mRNA expression of the different Wnt ligands, receptors, co-receptors, and soluble modulators, as well as the protein expression of all known conventional and non-conventional Wnt receptors, in the adult human spinal cord under physiological conditions.
Otherwise, during the last years, we and others have consistently demonstrated that the Wnt family of proteins also plays a relevant role in many different neuropathologies, including spinal cord injury (SCI) [16–22]. As it has been extensively reviewed [23, 24], SCI is a major neuropathological condition characterized by a primary injury phase, which involves the initial mechanical damage, and a secondary injury phase, encompassing a wide range of cellular and molecular processes that generally lead to the affectation of initially spared tissue surrounding the injury site and, thus, to a worst histopathological and functional outcome. Unfortunately, despite the SCI-associated lifelong disabilities, the huge social and economic burden, and the significant improvement in our understanding of its pathophysiology, there is no currently clinically accepted treatment for this neuropathology [25–29]. Therefore, a great effort is being performed to better understand the molecular and cellular mechanisms that determine the injury progression and outcome and thus unveil new targets for therapeutic intervention.
In this context, we have previously shown that the expression of most Wnt ligands, receptors, co-receptors, and soluble modulators was clearly dysregulated during the progression of SCI [30–33]. Moreover, different studies have demonstrated that specific components of the Wnt family of proteins as well as glycogen synthase kinase 3β (GSK-3β), which is a main element of the canonical Wnt/β-catenin signaling pathway [34], are involved in the regulation of at least axonal regeneration, astroglial and microglia/macrophage reactivity, glial and fibrous scar formation, inflammatory response, cell death, motor cortex remapping, myelin preservation and/or remyelination, and the differentiation of endogenous neural precursors after SCI [35–48]. Furthermore, the administration or blockade of specific components of the Wnt family of proteins, as well as the modulation of the canonical Wnt/β-catenin signaling pathway, is able to improve functional recovery after SCI [35, 38, 40, 43, 45–48]. Altogether, these observations strongly support that the modulation of the Wnt family of proteins might be a promising therapeutic approach to ameliorate the histopathological and functional sequelae of SCI, although its therapeutic potential is still poorly understood.
Interestingly, previous studies have shown that the expression of Fz1 suffered significant variations in cultured CNS cells subjected to different insults [49, 50], as well as in a variety of neuropathologies in vivo [33, 51–56]. More importantly, several reports have pointed to a neuroprotective role of this receptor both in vitro [49, 57] and in vivo in animal models of brain ischemic stroke [55], Parkinson’s disease (PD) [49], and subarachnoid hemorrhage [56]. Accordingly, it has been shown that Wnt1, which is able to exert its functions through Fz1 [49, 56, 58–60], induces neuroprotection both in vitro in neuronal cultures subjected to different noxious stimuli and in vivo in different neuropathologies such as PD, brain ischemia, and subarachnoid hemorrhage [49, 56, 61, 62], and that its neuroprotective function depends on the presence of Fz1 at least in PD [49] and subarachnoid hemorrhage [56]. However, despite the neuroprotective role of Fz1 and Wnt1 and the necessity of unveil new therapeutic targets for the treatment of SCI, the potential involvement of these molecules in the progression and outcome of this neuropathology is currently unexplored. Therefore, we here aimed to evaluate the spatio-temporal and cellular expression pattern of Fz1 in the traumatically injured human and rat spinal cord, as a first essential step to ascertain the potential relevance of this receptor in this neuropathological condition, as well as to determine the therapeutic potential of Wnt1 overexpression in a clinically relevant rat model of SCI.
Materials and methods
Production and titration of lentiviral vectors
Production of self-inactivating lentivirus was carried out following the method already described with several modifications. Briefly, 293T cells were cultured in 0.1 µm filtered Dulbecco’s Modified Eagle Medium (DMEM) (41966-029, Gibco) supplemented with 100 IU/ml of penicillin and 100 μg/ml of streptomycin (15070-083, Gibco), 10% of fetal bovine serum (10270, Gibco), 0.4% Tylosin (T3397, Sigma-Aldrich), and 7.5 mM of HEPES buffer (15630-056, Gibco). When the culture reached 60% confluence, the transfer plasmid (15 µg) (see below for details), the packaging plasmid (5 µg) (psPAX2) (12260, Addgene), and the envelope plasmid containing the VSV-G gene (5 µg) (pMD2.G) (12259, Addgene) were added in a 2 M CaCl2 HEPES-buffered saline solution. Twenty-four hours later, lentiviral particles in the medium were concentrated by ultracentrifugation at 31,500g for 2 h in a Beckman Coulter Optima L-100 XP ultracentrifuge. The pellet containing the lentiviral particles was resuspended in phosphate-buffered saline (PBS) and stored in working aliquots at − 80 °C until use. The bicistronic pWPI transfer plasmid (12254, Addgene) was used, as it allows for simultaneous expression of the transgene and GFP. Two different lentiviral vectors were generated: (1) one that only induces the expression of the green fluorescence protein (GFP) (LV-GFP) and (2) another that induces the expression of both GFP and Wnt1 (LV-Wnt1). For this purpose, the nucleotide sequence encoding Wnt1 (GenBank acc. no: NM_021279) was cloned into the pWPI plasmid by GeneScript Biotech Corporation. Lentiviral stock titration was carried out following the protocol already described with slight modifications [63, 64]. In summary, 70% confluent B1a rat fibroblasts, cultured in the same medium described above, were transduced by incubation during 24 h with decreasing doses of the lentiviral concentrate in DMEM. Seventy-two hours later, the percentage of GFP positive cells was quantified by flow cytometry (see “Flow cytometry” for details) and the lentiviral titer was calculated as transducing units per ml.
Evaluation of lentiviral vector generated to overexpress Wnt1
Cell culture and transduction
All experiments performed to evaluate the proper functioning of LV-Wnt1 were carried out in B1a cells. Cells were cultured, in DMEM supplemented with 10% of fetal bovine serum, 7.5 mM of HEPES buffer, 100 IU/ml of penicillin, and 100 μg/ml of streptomycin, in non-coated 24- or 6-well culture plates (140675, Nunc) depending on whether they were used for flow cytometry or quantitative Real-Time PCR (qRT-PCR) and western blot, respectively. Cell transduction with the corresponding lentiviral vector at the desired multiplicity of infection (MOI) (infectious particles/cells) was performed as described above.
Flow cytometry
Flow cytometry was used to: (1) quantify the percentage of GFP positive cells during lentiviral stock titration and (2) assess total cell number, transduction efficiency (% of GFP positive transduced cells), and GFP intensity in non-transduced B1a cells and after transduction with different MOIs (MOI 5, 10, 20, and 40) of LV-GFP. In all cases, B1a cells were detached using 0.25% trypsin–EDTA (25200-056, Gibco), resuspended in PBS, and analyzed in an FACS Canto II Flow Cytometer (BD Biosciences). The existence of significant differences between groups and evaluated times post-transduction was assessed by two-way ANOVA followed by Bonferroni post hoc test using the GraphPad Prism 5.01 software. A value of p ≤ 0.05 was considered statistically significant.
qRT-PCR
We used qRT-PCR to assess whether LV-Wnt1 induced the expression of the carried transgene at the mRNA level. Total RNA isolation, sample processing, and amplification of the complementary DNA (cDNA) corresponding to Wnt1 and 18s, as endogenous control, were performed as we have previously described [30–33, 65]. Wnt1 cDNA amplification was carried out using 125 ng of total reverse transcribed RNA per sample and the following specific primers: forward: 5′-CTTCGGCAAGATCGTCAACC-3′, reverse: 5′-GCGAAGATGAACGCTGTTTCT-3′. All gene expression analyses were performed in duplicate for each sample and cycle threshold (Ct) values above 35 were considered as undetectable. The existence of statistically significant differences between groups in ΔCt values was determined by one-way ANOVA followed by Bonferroni post hoc test using the GraphPad Prism 5.01 software. A value of p ≤ 0.05 was considered statistically significant.
Western blot
Western blot-based analysis was carried out to evaluate whether LV-Wnt1 efficiently induced Wnt1 protein overexpression, as well as to analyze whether overexpressed Wnt1 properly functioned at the Wnt signaling level. Quantification of Wnt1 and active β-catenin protein levels in cell lysates was performed following the experimental protocol that we have used in a previous report [50], using 50 µg of total protein per lane and the following primary and secondary antibodies: rabbit anti-Wnt1 (ab15251, Abcam) (1:500), mouse anti-active β-catenin (05-665, Millipore) (1:500), mouse anti-glyceraldehyde 3-phosphate dehydrogenase (ab8245, Abcam) (1:10,000), horseradish peroxidase (HRP)-linked anti-mouse (31430, Pierce) (1:7000), and HRP-linked anti-rabbit (31460, Thermo Scientific) (1:5000). Evaluation of Wnt1 protein levels in conditioned medium was carried out following the same protocol, although in this case, 30 µl of conditioned medium were added in each lane and the bands were not normalized against a housekeeping protein. The existence of statistically significant differences between groups in normalized band densities was determined by one-way ANOVA followed by Bonferroni post hoc test using the GraphPad Prism 5.01 software. A value of p ≤ 0.05 was considered statistically significant.
Preparation of conditioned medium
Conditioned medium was used to assess the correct secretion of the overexpressed Wnt1 and to evaluate its proper functionality at the Wnt signaling level. To generate the different conditioned mediums used, non-transduced B1a cells or transduced with LV-GFP or LV-Wnt1 were cultured as described above. The culture medium was maintained during 72 h to ensure the accumulation of overexpressed Wnt1. The conditioned medium was then recovered, filtered through a 0.2 µm filter to eliminate any potential cross-contaminating cell, and stored in working aliquots at − 20 °C until use.
Human samples
Donation of human spinal cord samples included a written informed consent from donors while alive or from their families after death. Fresh-frozen and paraffin-embedded samples from donor individuals, deceased without clinical or histopathological involvement of the spinal cord, were obtained from the BioB-HVS BioBank and were used as non-lesioned (NL) controls. Paraffin-embedded lesioned spinal cord samples were obtained from Dr. Michael Norenberg (Department of Pathology, Miller School of Medicine, University of Miami, USA) (see Table 1 for details about human samples). Data from donors and handling of samples were carried out after approval by the Clinical Research Ethical Committee in Toledo (Permit number 76/2017, Spain) and in accordance with the Spanish law and International Guidelines (LOPD 15/1999; RD 1720/2007; Helsinki declaration 2008).
Table 1.
Human samples
| Case | Tissue processing | Cause of death/injury | Gender | Age | Coded as | Time post-SCI | IHQ | RT-qPCR |
|---|---|---|---|---|---|---|---|---|
| 15A59 | Fresh frozen/paraffin embedded | Heart failure | Male | 81 | Control | – | X | X |
| 15A9 | Fresh frozen/paraffin embedded | Multiorganic failure/septic shock | Female | 68 | Control | – | X | X |
| 16A8 | Fresh frozen/paraffin embedded | Renal failure | Male | 44 | Control | – | X | X |
| 15A58 | Fresh frozen/paraffin embedded | Hepatic failure | Male | 35 | Control | – | X | X |
| 16A11 | Fresh frozen | Respiratory failure | Female | 63 | Control | – | X | X |
| 15A12 | Fresh frozen | Cardiac surgery complication | Female | 68 | Control | – | X | |
| 16A7 | Fresh frozen | Cardiorespiratory arrest | Male | 41 | Control | – | X | |
| 218 | Paraffin embedded | Gunshot wound | Male | 26 | Lesioned | 1 day | X | |
| 119 | Paraffin embedded | Altercation | Male | 37 | Lesioned | 2 days | X | |
| 162 | Paraffin embedded | Fall | Male | 84 | Lesioned | 3 days | X | |
| 176 | Paraffin embedded | Motor vehicle accident | Male | 73 | Lesioned | 3 days | X | |
| 120 | Paraffin embedded | Motor vehicle accident | Male | 88 | Lesioned | 5 days | X | |
| 206 | Paraffin embedded | Motor vehicle accident | Male | 16 | Lesioned | 5 days | X |
Animals and surgical procedures
A total of 87 adult female Wistar rats were used (3 months; ≃ 300 g). Animal housing and experimental procedures were carried out in accordance with the Spanish (Royal Decree 53/2013) and the European Union (2010/63/EU) laws, and they were approved by the Bioethics Committee at The National Hospital of Paraplegics (Toledo, Spain) (Permit numbers 51/2009 and 45/2008). The spinal cord contusions were performed as we have described in previous reports [30–32, 65]. When required, the viral particles were intraparenchymatically injected immediately after the spinal cord contusion. Two different injection methods were initially tested. In the first one, one injection per rostro-caudal level (stereotaxic coordinates: 0 mm lateral and 1 mm depth) was performed in three rostro-caudal levels corresponding to the lesion epicenter, 1.5 mm rostral and 1.5 mm caudal. In the second one, two injections per rostro-caudal level (stereotaxic coordinates: 0.6/− 0.6 mm lateral and 1 mm depth) were performed in the same rostro-caudal levels detailed above. In both cases, a volume of 1 µl of vehicle alone (PBS) or containing 5 × 105 lentiviral particles were injected in each injection point at a rate of 0.5 μl/min using a 33G needle and a 10 μl Hamilton syringe attached to a microinjector (KDS-311, KD Scientific) and a stereotaxic device (Kopf). The needle was maintained during 4 further minutes in each injection point to minimize reflux of the solution. Based in the results obtained, the second injection method was used to evaluate the effects exerted by Wnt1 overexpression. The postoperative cares were the same that we have used in previous works [30–32, 65]. All efforts were done during the whole experimental process to minimize animal suffering.
qRT-PCR
To analyze the mRNA expression of the different Wnt ligands, receptors, co-receptors, and soluble modulators, total mRNA was isolated from fresh-frozen human spinal cord samples using the RNeasy Lipid Tissue Mini Kit (74804, Quiagen) following the manufacturer’s instructions. Reverse transcription of total mRNA was performed as previously described [30]. cDNA amplification was performed using the same experimental protocol and customized TaqMan Low Density Arrays (Applied Biosystems), prepared with TaqMan Assays (Applied Biosystems) predesigned and validated for humans, that we have described in a previous report [14], although in this case, 20.8 ng per well were used.
Histology
Tissue processing
Under anesthesia, those rats used to perform the different histological techniques were sacrificed by intraaortical perfusion with 1 mg/kg of 4% paraformaldehyde (P6148, Sigma-Aldrich). A 2 cm spinal cord stretch containing the lesion was extracted, postfixed during 4 h in the same fixative, cryoprotected by immersion in 30% sucrose (84100, Sigma-Aldrich) during 72 h, and frozen embedded in Neg-50 medium (6502, Richard-Allan Scientific). Frozen rat and human spinal cord samples were cut in a cryostat (HM560, Microm) to obtain parallel sections of 30 μm or 10 µm thick, respectively, which were mounted on slides (J1800AMNZ, Thermo Scientific) and stored at − 20 °C until use. Paraffin-embedded human spinal cord samples were cut in a microtome to obtain 5 µm-thick sections, which were mounted on slides and stored at room temperature (RT) until use.
Immunohistochemistry
Chromogen-based simple immunohistochemistry was performed to: (1) analyze the protein expression of conventional (Fz1-10) and non-conventional Wnt receptors (PTK7, Ryk and Ror1 and 2) as well as of the active phosphorylated form of LRP6 (pLRP6) in fresh-frozen human NL spinal cord samples, (2) evaluate the spatio-temporal expression pattern of Fz1 in the NL and lesioned rat spinal cord, and (3) quantify by densitometrical analysis the potential variations induced by Wnt1 overexpression in the presence of astrocytes, microglia/macrophages, and NG2+ cells after rat SCI in sections processed for the visualization of glial fibrillary acidic protein (GFAP), ionized calcium-binding adaptor molecule 1 (Iba1) and NG2, respectively. For this purpose, we used the same experimental protocol that we have detailed in previous publications [30–32, 65], with slight modifications in human samples as, in this case, the spinal cord sections were fixed by immersion during 15 min at RT in 4% paraformaldehyde before starting the immunohistochemical procedure. Fluorescence-based simple immunohistochemistry was performed, following the same experimental protocol that we have used in previous works [53], to evaluate the potential changes induced by Wnt1 overexpression in neuronal and oligodendroglial cell number as well as in the presence of 5-HT+ axons after rat SCI in sections processed for the visualization of neuronal nuclei (NeuN), adenomatous polyposis coli (APC), and 5-HT, respectively. Fluorescence-based double immunohistochemistry was performed to evaluate the cellular protein expression pattern of Fz1 in the rat and human NL and lesioned spinal cord. The experimental protocol used in rat samples has been conveniently detailed in previous works performed by our group [31, 65]. The experimental protocol used in paraffin-embedded human samples included several modifications. Briefly, before starting the immunohistochemical procedure, sections were deparaffinized by immersion in xylene and then in decreasing graded ethanol solutions. Subsequently, the sections were subjected to antigen retrieval as previously described [66]. At the end of the experimental protocol and to reduce tissue autofluorescence, sections were incubated during 5 min at RT in a solution of Sudan Black B (199664, Sigma-Aldrich) prepared as described elsewhere [67]. The different primary and secondary antibodies used for the distinct immunohistochemical procedures are listed in Table 2. To confirm a lack of undesired cross-reactivity, both sections processed without the primary antibodies and/or sections processed without the second primary antibody were used as controls. No non-specific staining was observed in any case. Finally, to ensure the specificity of the primary antibody used to visualize Fz1, we pre-incubated this antibody, following the protocol previously described [15, 68], with its corresponding blocking peptide (ab260468, Abcam) with 20-fold weight/weight excess. As shown in the Online Resource 1, antibody pre-adsorption completely abolished Fz1 immunostaining.
Table 2.
Primary and secondary antibodies used for immunohistochemistry
| Tissue species | Immunohistochemical method | Antibody | Reference | Dilution |
|---|---|---|---|---|
| Rat | Chromogen-based simple immunohistochemistry | Rb anti-Fz1 | ab71342, Abcam | 1:250 |
| Rb anti-Iba1 | 019-19741, Wako | 1:1000 | ||
| Mo anti-GFAP | G3893, Sigma-Aldrich | 1:1000 | ||
| Rb anti-NG2 | AB5320, Millipore | 1:250 | ||
| Biotinilated Go anti-Rb | BA-1000, Vector | 1:500 | ||
| Biotinilated Ho anti-Mo | BA-2001, Vector | 1:500 | ||
| Fluorescence-based simple immunohistochemistry | Mo anti-APC | OP80, Calbiochem | 1:100 | |
| Rb anti-NeuN | MABN140, Millipore | 1:100 | ||
| Rb anti-serotonin | S5545, Sigma-Aldrich | 1:500 | ||
| Dylight 594-linked Go anti-Rb | ab96897, Abcam | 1:500 | ||
| Dylight 594-linked Go anti-Mo | ab96881, Abcam | 1:500 | ||
| Fluorescence-based double immunohistochemistry | Rb anti-Fz1 | ab71342, Abcam | 1:50 | |
| Mo anti-GFAP | G3893, Sigma-Aldrich | 1:1000 | ||
| Mo anti-NeuN | MABN140, Millipore | 1:100 | ||
| Mo anti-APC | OP80, Calbiochem | 1:100 | ||
| Mo anti-NF200 | N0142, Sigma-Aldrich | 1:2000 | ||
| Mo anti-OX42 | ab58457, Abcam | 1:500 | ||
| Mo anti-NG2 | 37-2700, Zymed | 1:100 | ||
| Mo anti-RECA1 | MCA970GA, AbD Serotec | 1:250 | ||
| Dylight 594-linked Go anti-Rb | ab96897, Abcam | 1:500 | ||
| Dylight 594-linked Go anti-Mo | ab96881, Abcam | 1:500 | ||
| Human | Chromogen-based simple immunohistochemistry | Rb anti-Fz1 | ab71342, Abcam | 1:500 |
| Rb anti-Fz2 | ab94913, Abcam | 1:500 | ||
| Rb anti-Fz3 | ab188974, Abcam | 1:50 | ||
| Rb anti-Fz4 | ab83042, Abcam | 1:1000 | ||
| Rb anti-Fz5 | ab75234, Abcam | 1:500 | ||
| Rb anti-Fz6 | ab150545, Abcam | 1:100 | ||
| Rb anti-Fz7 | ab64636, Abcam | 1:1000 | ||
| Rb anti-Fz8 | ab75235, Abcam | 1:50 | ||
| Rb anti-Fz9 | ab61430, Abcam | 1:50 | ||
| Rb anti-Fz10 | ab83044, Abcam | 1:500 | ||
| Rb anti-Ryk | AP7677a, Abgent | 1:500 | ||
| Rb anti-Ror1 | AP7671d, Abgent | 1:50 | ||
| Rb anti-Ror2 | AP7672d, Abgent | 1:50 | ||
| Mo anti-PTK7 | 400005754-M06, Abnova | 1:500 | ||
| Rb anti-pLRP6 (Ser 1490) | 2568L, Cell Signaling | 1:100 | ||
| Biotinilated Go anti-Rb | BA-1000, Vector | 1:500 | ||
| Biotinilated Ho anti-Mo | BA-2001, Vector | 1:500 | ||
| Fluorescence-based double immunohistochemistry | Rabbit anti-Fz1 | ab71342, Abcam | 1:100 | |
| Mo anti-GFAP | G3893, Sigma-Aldrich | 1:1000 | ||
| Mo anti-CNPase | SMI-91R, Covance | 1:100 | ||
| Mo anti-CD31 | #3528, Cell Signaling | 1:100 | ||
| Mo Pan Neuronal Marker | MAB2300, Millipore | 1:50 | ||
| Go anti-Iba1 | ab5076, Abcam | 1:100 | ||
| Dylight 594-linked Go anti-Rb | DI-1594, Vector | 1:500 | ||
| Dylight 488-linked Ho anti-Mo | DI-2488, Vector | 1:500 | ||
| Alexa 488-linked Do anti-Go | A11055, Invitrogen | 1:1000 |
Evaluation of transduction in vivo
The evaluation of the transduction efficiency after lentiviral injection in vivo was performed in: (1) a set of parallel longitudinal sections per animal from animals that were used to determine which of the injection methods initially tested induced a higher transduction in the lesioned areas and (2) a set of parallel transversal sections per animal from animals that were used to analyze the effects exerted by Wnt1 overexpression. Briefly, after several washes in TBS, sections were incubated with DAPI (1:10,000) during 5 min at RT, washed in TB, and coverslipped using Immumount (9990402, Thermo Scientific). As sections from animals sacrificed at 126 days post-injury (dpi) displayed high levels of tissue autofluorescence that hindered the identification of transduced GFP+ cells, these sections were incubated with Sudan Black B as described above.
Eriochrome cyanine
To assess whether Wnt1 overexpression induced variations in myelin preservation after SCI, we performed eriochrome cyanine (ECy) staining, in a set of parallel transversal sections per animal, following the protocol that we have used in previous publications [65].
Densitometrical analysis
Densitometrical analysis was performed in a set of parallel transverse spinal cord sections per animal processed by Ecy staining and GFAP, Iba1, and NG2 chromogen-based simple immunohistochemistries to evaluate the potential existence of variations in myelin preservation, and in the presence of astrocytes, microglia/macrophages, and NG2+ cells after SCI due to Wnt1 overexpression. For this purpose, composite 10 × images of complete sections corresponding to different rostro-caudal levels (see “Image acquisition” section for further details about the image acquisition process), that include the whole lesion, were analyzed using the Fiji software following the method that we have described in previous reports [65]. All images were obtained using the same acquisition settings. Briefly, sections were carefully delineated and a threshold was established according to the histological signal. The selected threshold was maintained in all analyzed sections at each evaluated time post-injury. Total and stained areas were calculated in each section. Ecy, GFAP, Iba1, and NG2-positive areas were normalized against total spinal cord area in each rostro-caudal level. Densitometrical analysis was also performed to evaluate the presence of 5-HT axons in the lesioned rat spinal cord as already described with minor variations [69]. Briefly, 20 × composite images from both ventral horns were obtained at two rostral levels (8.58 and 7.92 mm from the lesion epicenter) and at different caudal levels per animal (2.64, 3.30, 3.96, 4.62, 5.28, 5.94, 6.60, 7.26, 7.92, 8.58, and 9.24 mm from the lesion epicenter) (see “Image acquisition” section for further details about the image acquisition process). Subsequently, a square ROI (150 × 150 µm) was selected in the lower limit of the ventral horns and the area occupied by 5-HT axons in this region was quantified using the same threshold in all images. In each animal, the 5-HT+ area observed in the different caudal levels was normalized to the 5-HT+ area observed in the rostral levels. In all cases, the existence of significant differences between groups at each rostro-caudal level was assessed by two-way ANOVA followed by Bonferroni post hoc test using the GraphPad Prism 5.01 software. A value of p ≤ 0.05 was considered statistically significant.
Cell count
Automatized cell count was performed, using the Fiji software, in a set of parallel transverse spinal cord sections per animal processed by APC and NeuN fluorescence-based simple immunohistochemistry to evaluate whether Wnt1 overexpression induced changes in oligodendroglial and neuronal cell number after SCI. Composite 10 × images of complete sections corresponding to the evaluated rostro-caudal levels were used (see “Image acquisition” section for further details about the image acquisition process). All images were obtained using the same acquisition settings. Briefly, spinal cord sections were carefully delineated and total section area was quantified. Subsequently, a restrictive threshold was established according to the histological signal in images showing APC, NeuN, or DAPI staining. The selected thresholds were maintained in all analyzed sections. After binarization, APC and NeuN images were merged with images showing the corresponding DAPI nuclear counterstaining. The number of APC- or NeuN-positive particles containing a nucleus in their cytoplasm was calculated using the Analyze Particle tool of the Fiji software. Only those APC- or NeuN-positive particles that displayed a minimum size of 20 µm2 were counted to minimize the potential influence of the few oligodendroglial or neuronal projections that were stained. To determine the validity of this automatic cell count method, randomly blind selected images were manually counted, and the data obtained using both methods showed an extremely high correlation degree. Cell number data were presented as APC- or NeuN-positive cells per mm2. The existence of significant differences between groups at each rostro-caudal level was assessed by two-tailed t test using the GraphPad Prism 5.01 software. A value of p ≤ 0.05 was considered statistically significant.
Image acquisition
An DP71 camera (Olympus) attached to a BX61 Motorized Research Microscope (Olympus), a UPlanSApo 10 ×/0.40 objective (Olympus), and the Visiopharm Integrator System acquisition software were used to obtain composite images of human spinal cord sections processed for the visualization of the different Wnt receptors and co-receptors, and of rat spinal cord sections processed for the visualization of Fz1 by simple immunohistochemistry, myelin, APC, NeuN, NG2, GFAP, and Iba1. The same acquisition equipment and software was used to obtain high magnification images from human sections processed for the visualization of the different Wnt receptors and co-receptors, high magnification images from rat spinal cord sections processed for the visualization of NG2, GFAP, and Iba1, as well as images from rat spinal cord sections processed for the visualization of serotonin, although, in these cases, a UPlanSApo 20 ×/0.75 objective (Olympus) was used. Composite images from transversal rat spinal cord sections used to visualize the presence and distribution of GFP+ transduced cells were obtained in a TCS SP5 Resonant Scanner confocal microscope (Leica Microsystems) using a PL APO 20 ×/0.40 (Leica Microsystems) and the Leica Application Suite X acquisition software. The same confocal microscope, software, and a PL APO 40 ×/1.25–0.75 objective (Leica Microsystems) were used to obtain composite images from rat and human spinal cord sections used to determine the specificity of the antibody anti-Fz1. High magnification images from transversal rat spinal cord sections used to visualize the presence and distribution of GFP+ transduced cells, as well as images from human and rat spinal cord sections used to evaluate the spatio-temporal and cellular expression pattern of Fz1 by fluorescence-based double immunohistochemistry, were obtained using a TCS SP5 confocal microscope (Leica Microsystems), the Leica Application Suite X acquisition software, and an HCX PL APO 40 ×/1.25–0.75 or an HCX PL APO 63 ×/1.4–0.6 objective. Finally, a DFC 350 FX camera (Leica Microsystems) attached to a DMI6000B microscope (Leica Microsystems), an HP PL FLUOTAR 10 ×/0.30 objective (Leica Microsystems), and the Leica Application Suite X acquisition software were used to obtain images from longitudinal rat spinal cord sections used to evaluate the presence and distribution of GFP+ transduced cells. In all cases, images were obtained at RT (≃ 24 °C).
Functional evaluation
To evaluate whether Wnt1 overexpression induced variations in motor functional recovery, the 21-point Basso, Beattie, and Bresnahan (BBB) open-field test was carried out at 1, 3, 7, 14, 21, 28, 35, 49, 63, 77, 91, 105, and 119 dpi as previously described [65, 70]. The BBB analysis was performed by two assessors who were blinded to the experimental groups and the consensus score taken. The existence of differences in motor functional recovery was further determined through the evaluation of different gait parameters using the CatWalk® gait analysis system (version 7.1, Noldus) [71, 72] either before (to obtain pre-injury values) or at the end of the study (126 dpi). Only those animals that displayed a minimum BBB score of 10 (consistent stepping) after SCI were evaluated. A minimum of four runs per animal, performed at a consistent pace and containing at least three complete step cycles, were used in the analysis. Moreover, as the velocity is a critical factor influencing the different gait parameters [73], we first calculated the crossing velocity of the different runs as previously described [74]. To reduce the velocity-dependent variability, we determined the crossing velocity average of all acquired runs and only those included in a range between a 20% higher and lower to this average were used. The following gait parameters were evaluated: regularity index, frequency of Ab step patterns, hind paws base of support, print positions, hind paws stride length, hind paws duty cycle, hind paws swing duration, hind paws swing speed, and hind paws stand duration (see [71] for a full description of the different gait parameters evaluated). No left–right differences were observed in any of the gait parameters analyzed and, thus, the corresponding left and right values were averaged. Two-way ANOVA or one-way ANOVA following by Bonferroni post hoc test was used to determine the existence of significant differences between groups in data obtained from the BBB and Catwalk-based analysis, respectively. In both cases, the GraphPad Prism 5.01 software was used and a value of p < 0.05 was considered statistically significant.
Results
Expression of the Wnt family of proteins in the healthy adult human spinal cord
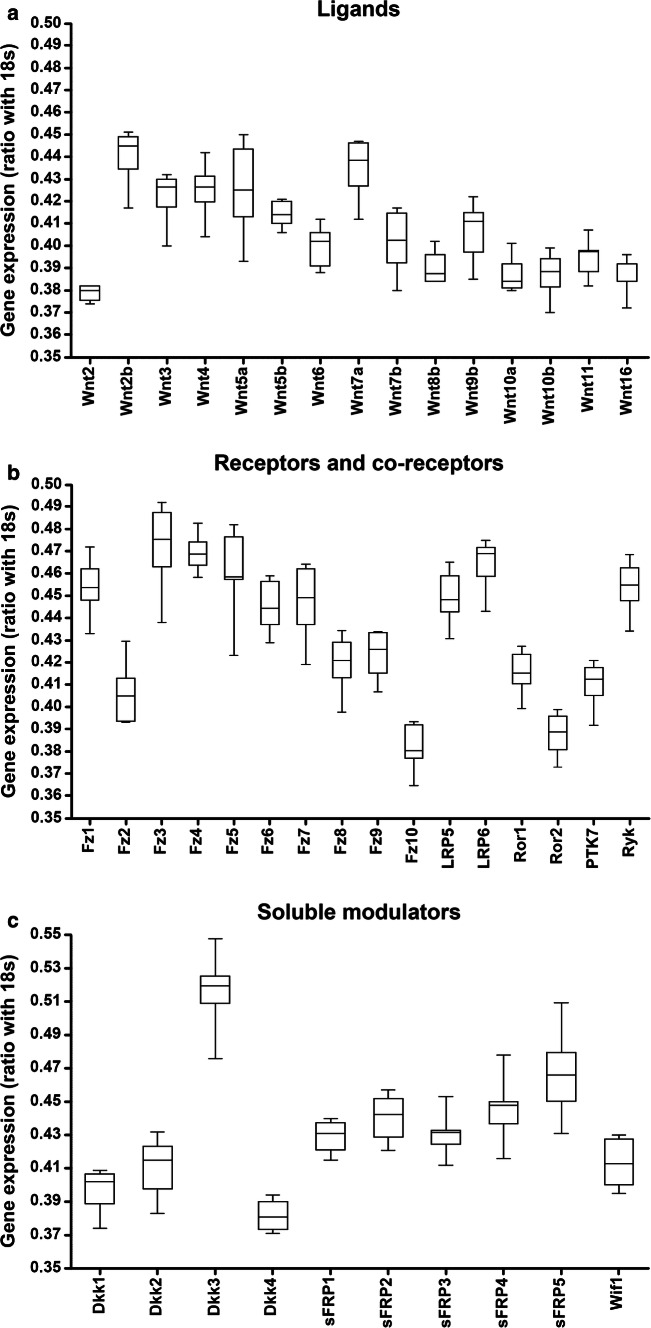
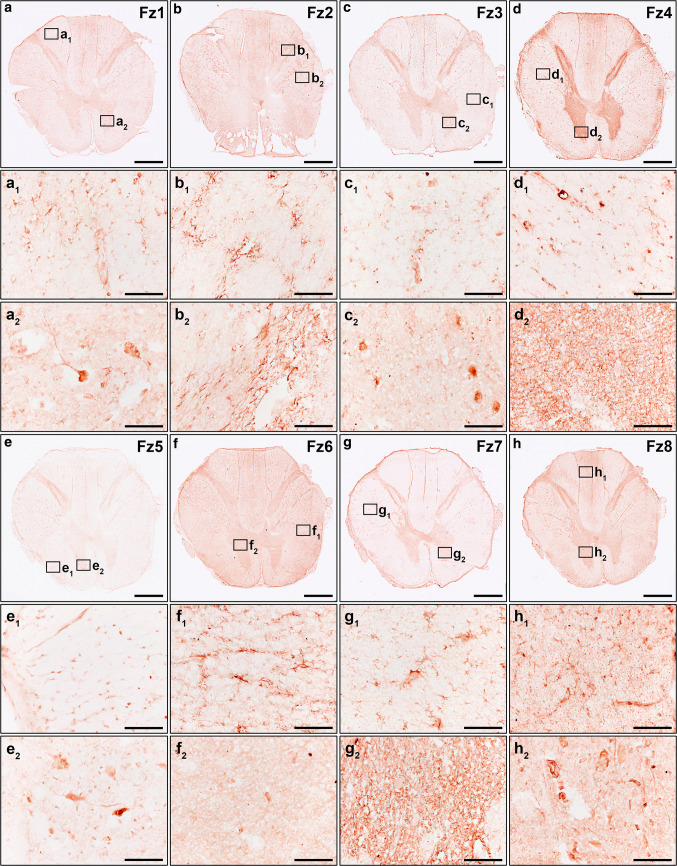
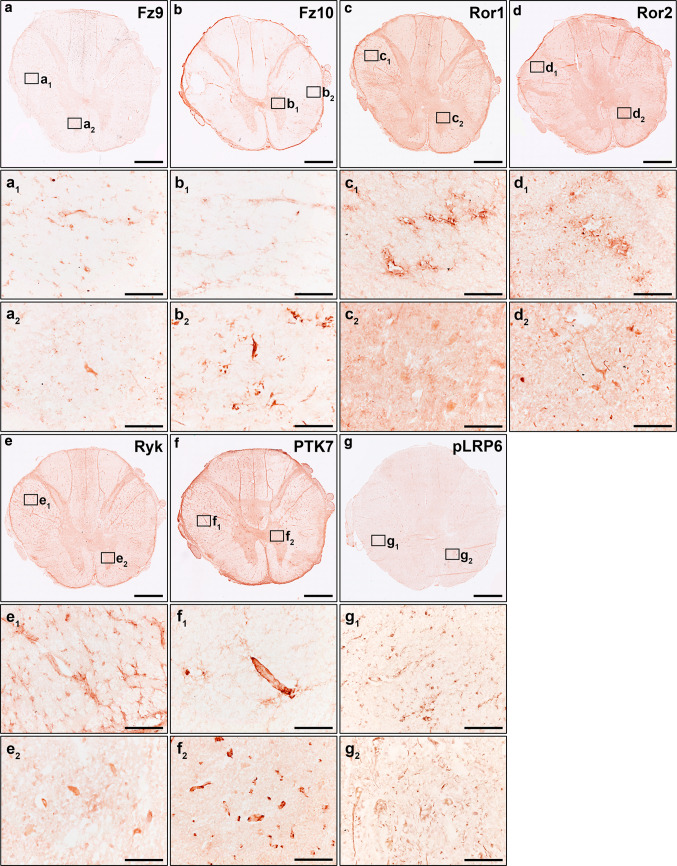
We recently demonstrated that, in the healthy adult human spinal cord, specific components of the Wnt family of proteins were expressed at the mRNA level in the ependymal region [14] and in the anterior horn [15]. However, the broad expression of the different components of the Wnt family of proteins at the mRNA and protein levels in the adult human spinal cord under physiological conditions is currently unknown. Hence, to further ascertain the potential relevance of the Wnt family of proteins in physiological adult human spinal cord functioning, we first analyzed the mRNA expression of the different Wnt ligands, receptors, co-receptors, and soluble modulators, as well as the protein expression of all known conventional and non-conventional Wnt receptors, in NL human spinal cord samples. Interestingly, we found detectable mRNA expression levels of most Wnt ligands (Wnt2, 2b, 3, 4, 5a, 5b, 6, 7a, 7b, 8b, 9b, 10a, 10b, 11, and 16) (Fig. 1a), receptors (Fz1-10, Ryk, PTK7, and Ror1 and 2) (Fig. 1b), co-receptors (LRP5 and 6) (Fig. 1b), and soluble modulators (Dkk1-4, sFRP1-5, and Wif1) (n = 7, Table 1) (Fig. 1c), but not of Wnt1, 3a, 8a and 9a. Moreover, immunohistochemical analysis of the different Wnt receptors showed that Fz1 (Fig. 2a–a2), 2 (Fig. 2b–b2), 3 (Fig. 2c–c2), 4 (Fig. 2d–d2), 5 (Fig. 2e–e2), 6 (Fig. 2f–f2), 7 (Fig. 2g–g2), 8 (Fig. 2h–h2), 9 (Fig. 3a–a2) and 10 (Fig. 3b–b2), Ror1 (Fig. 3c–c2) and 2 (Fig. 3d–d2), Ryk (Fig. 3e–e2) and PTK7 (Fig. 3f–f2) were expressed at the protein level with specific expression patterns in the adult human spinal cord under physiological conditions (n = 5, Table 1). Furthermore, we also found that pLRP6, which is an essential co-receptor for the activation of canonical Wnt/β-catenin signaling pathway [75], was also widely expressed at the protein level in the NL adult human spinal cord (Fig. 3g–g2).
Fig. 1.
mRNA expression of the Wnt family of proteins in the healthy human spinal cord. This figure shows data obtained from the evaluation, by quantitative Real-Time PCR, of the mRNA expression of the different components of the Wnt family of proteins in the healthy human spinal cord (n = 7). As shown, we found detectable mRNA expression of most Wnt ligands (Wnt2, 2b, 3, 4, 5a, 5b, 6, 7a, 7b, 8b, 9b, 10a, 10b, 11, and 16) (a), receptors [Frizzled (Fz)1–10, receptor tyrosine kinase-like orphan receptor (Ror) 1 and 2, protein tyrosine kinase 7 (PTK7) and Ryk] (b), co-receptors [low-density lipoprotein receptor protein (LRP) 5 and 6] (b), and soluble modulators [Dickkopf (Dkk) 1–4, secreted Frizzled-related proteins (sFRP) 1–5 and the Wnt inhibitory factor 1 (Wif1)] (c) in the human spinal cord under physiological conditions. Data are presented as the ratio between each gene of interest and 18s
Fig. 2.
Protein expression of Wnt receptors in the healthy human spinal cord I. This figure shows representative images from the immunohistochemical evaluation of the protein expression of Frizzled (Fz) 1 (a, a1, a2), 2 (b, b1, b2), 3 (c, c1, c2), 4 (d, d1, d2), 5 (e, e1, e2), 6 (f, f1, f2), 7 (g, g1, g2), and 8 (h, h1, h2) in the healthy human spinal cord (n = 5). Squares in a–h indicate the areas shown in the corresponding higher magnification images. Scale bars in a–h = 2 mm; scale bars in a1–h2 = 100 µm
Fig. 3.
Protein expression of Wnt receptors in the healthy human spinal cord II. This figure shows representative images from the immunohistochemical evaluation of the protein expression of Frizzled (Fz) 9 (a, a1, a2) and 10 (b, b1, b2), receptor tyrosine kinase-like orphan receptor (Ror) 1 (c, c1, c2) and 2 (d, d1, d2), Ryk (e, e1, e2), protein tyrosine kinase 7 (PTK7) (f, f1, f2), and the phosphorylated active form of the low-density lipoprotein receptor protein 6 (pLRP6) (g, g1, g2) in the healthy human spinal cord (n = 5). Squares in a–g indicate the areas shown in the corresponding higher magnification images. Scale bars in a–g = 2 mm; scale bars in a1–g2 = 100 µm
Spatial and cellular expression pattern of Fz1 in the NL and lesioned human spinal cord
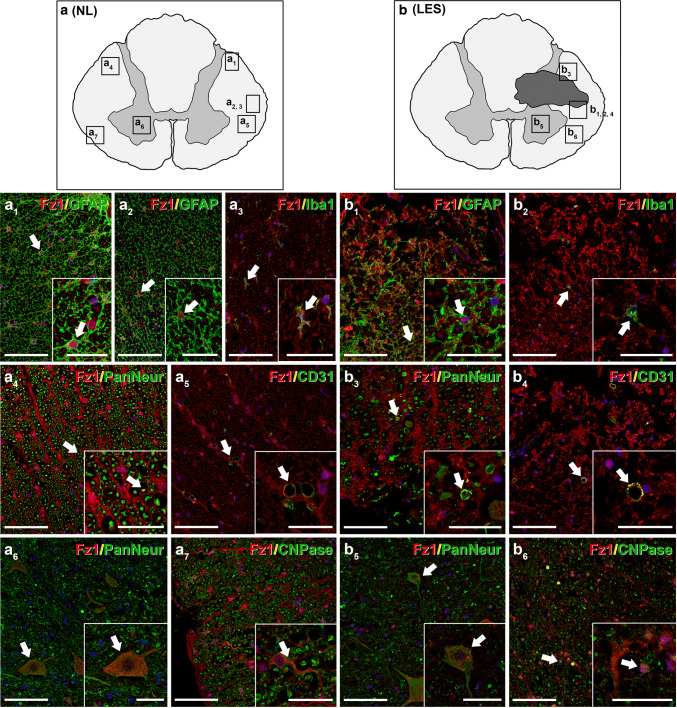
Among the different Wnt receptors, it has been shown that Fz1 was expressed in the adult rodent CNS under physiological conditions [14, 33, 49, 51–56, 76, 77] and that its expression suffered evident changes in different neuropathologies, including SCI [33, 36, 51–56]. Moreover, several reports pointed to a neuroprotective role of this receptor both in vitro [49, 57] and in vivo [49, 55, 56]. However, there is a complete lack of information about the potential involvement of this receptor in the traumatically injured human spinal cord. Therefore, and as a first essential step to shed light on the potential functions of this receptor in this neuropathological situation, we subsequently evaluated the cellular expression pattern of Fz1 in NL (n = 4; Table 1) and lesioned human spinal cord samples (n = 6; Table 1). We found that, in the NL human spinal cord, Fz1 was expressed in white matter astrocytes that were mainly located in areas near the pial surface (Fig. 4a, a1, a2), quiescent microglial cells (Fig. 4a, a3), axons (Fig. 4a, a4), blood vessels (Fig. 4a, a5), neurons (Fig. 4a, a6), and oligodendrocytes (Fig. 4a, a7). After injury, an increase in the presence of Fz1-expressing astroglial cells was observed surrounding the injured tissue (Fig. 4b, b1), which was more evident at 5 dpi than at earlier times after SCI. Moreover, Fz1 expression was also detected in the injured areas in activated amoeboid microglia/macrophages (Fig. 4b, b2), axons (Fig. 4b, b3), and blood vessels (Fig. 4b, b4). Finally, Fz1-expressing neurons (Fig. 4b, b5) and oligodendrocytes (Fig. 4b, b6) were still present in non-injured areas near the affected regions. Overall, these observations strongly suggest that, besides its potential neuroprotective role, Fz1 may play a relevant role in different biological processes involved in the progression and outcome of SCI.
Fig. 4.
Expression pattern of Fz1 in the non-lesioned (NL) and injured human spinal cord. This figure shows representative images from the immunohistochemical evaluation of Frizzled (Fz) 1 expression in astrocytes [glial fibrillary acidic protein (GFAP)] (a, a1, a2, b, b1), microglia/macrophages [ionized calcium-binding adaptor molecule 1 (Iba1)] (a, a3, b, b2), axons (a, a4, b, b3), and neuronal somas (a, a6, b, b5) [Pan Neuronal Marker (PanNeur)], endothelial cells (CD31) (a, a5, b, b4) and oligodendrocytes [2′,3′-cyclic nucleotide-3′-phosphodiesterase (CNPase)] (a, a7, b, b6) in the NL (n = 4) (a, a1–7) and traumatically injured human spinal cord (LES) (n = 6) (b, b1–6). a, b correspond to schematic drawings of prototypical NL and lesioned human spinal cord sections, respectively, indicating the approximate areas shown in the corresponding images. Scale bars in a1–7 and b1–6 = 100 µm; scale bars in image insets = 40 µm
Spatio-temporal and cellular expression pattern of Fz1 in the NL and lesioned rat spinal cord
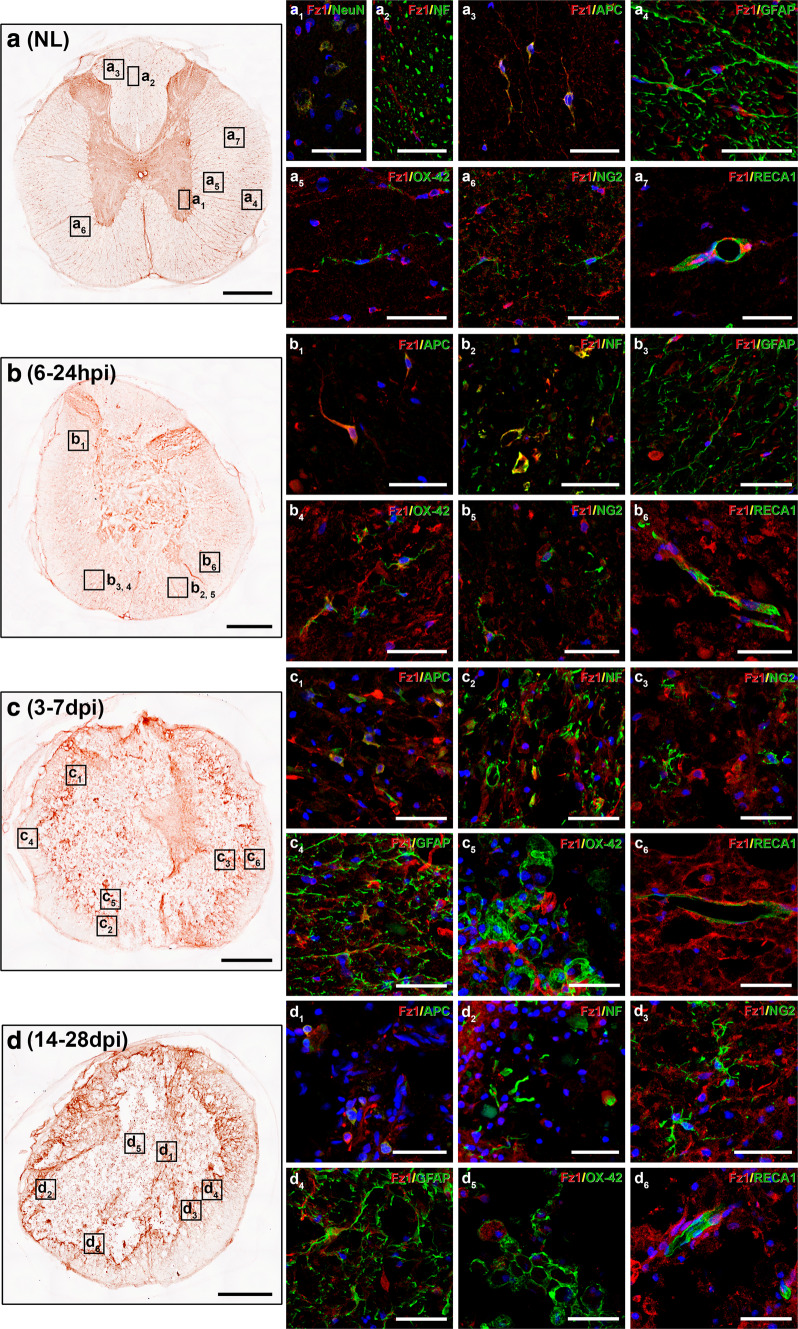
We next analyzed the spatio-temporal and cellular expression pattern of Fz1 in the NL rat spinal cord and after contusive SCI, to assess whether this animal model of SCI can be used to evaluate the potential beneficial effects derived from the modulation of this receptor. As observed in human spinal cord samples, in the NL rat spinal cord (n = 5), we found Fz1 expression in neurons (Fig. 5, a1), axons (Fig. 5a, a2), oligodendrocytes (Fig. 5a, a3), white matter astrocytes located near the pial surface (Fig. 5a, a4), quiescent microglial cells (Fig. 5a, a5), NG2+ cells (Fig. 5a, a6), and blood vessels (Fig. 5a, a7). After SCI, at 6 (n = 5) and 24 h post-injury (n = 5), Fz1 expression was observed in oligodendrocytes (Fig. 5b, b1), axons (Fig. 5b, b2), astrocytes (Fig. 5b, b3), ramified microglia (Fig. 5b, b4), NG2+ cells (Fig. 5b, b5), and blood vessels (Fig. 5b, b6) in the affected areas. At 3 (n = 5) and 7 dpi (n = 5), Fz1-expressing oligodendrocytes (Fig. 5, c1) and axons (Fig. 5c, c2) were still present in the injured tissue. We also found an evident increase in the presence of Fz1-expressing astrocytes (Fig. 5c, c4) and, to a lesser extent, NG2+ cells (Fig. 5c, c3) in areas surrounding the injury site. Moreover, the expression of Fz1 was nearly disappeared in activated amoeboid microglia/macrophages (Fig. 5c, c5) and lost in endothelial cells (Fig. 5c, c6) at these times post-injury. At 14 (n = 5) and 28 dpi (n = 5), Fz1 expression in the injured tissue was still observed in oligodendrocytes (Fig. 5, d1), axons (Fig. 5d, d2) and glial scar forming NG2+ cells (Fig. 5d, d3) and astrocytes (Fig. 5d, d4), but not in endothelial cells (Fig. 5d, d6). Again, weak or absent Fz1 immunostaining was observed in activated microglia/macrophages (Fig. 5d, d5), although in some cases, intense Fz1 immunolabeling was detected in intracellular vesicular profiles in these cell types, probably due to the phagocytosis of Fz1-expressing cells. Finally, at all evaluated times post-injury neuronal Fz1 expression was observed in those regions where surviving neurons were still present. In summary, these observations demonstrate that the expression pattern of Fz1 the NL rat spinal cord and after SCI is extremely similar to that observed in the human.
Fig. 5.
Expression pattern of Fz1 in the non-lesioned (NL) and injured rat spinal cord. This figure shows representative images obtained from the evaluation by simple (a–d) and double immunohistochemistry (a1–d6) of the spatial and cellular expression of Frizzled (Fz) 1 in the NL rat spinal cord and after SCI at 6 and 24 h post-injury (hpi) and 3, 7, 14, and 28 days post-injury (dpi) (n = 5 per group). Double immunohistochemistry was used to evaluate Fz1 expression in neurons [neuronal nuclei (NeuN)] (a1) axons [neurofilament 200 (NF)] (a2, b2, c2, d2), oligodendrocytes [adenomatous polyposis coli (APC)] (a3, b1, c1, d1), astrocytes [glial fibrillary acidic protein (GFAP)] (a4, b3, c4, d4), microglia/macrophages (OX-42) (a5, b4, c5, d5), NG2+ cells (NG2) (a6, b5, c3, d3), and endothelial cells (RECA1) (a7, b6, c6, d6). Squares in a–d indicate the approximate areas shown in the corresponding images. Scale bars in a–d = 500 µm; scale bars in a1–d6 = 40 µm
Potential beneficial role of lentiviral-mediated overexpression of Wnt1 after SCI
Different studies have demonstrated that Wnt1 is able to induce neuroprotection both in vitro [49, 51, 61, 78–80] and in vivo in animal models of PD, cerebral ischemia, and subarachnoid hemorrhage [49, 56, 61], and that the neuroprotective role of Wnt1 depended on the presence of Fz1 at least in PD and subarachnoid hemorrhage [49, 56], in agreement with previous reports, demonstrating that Wnt1 is able to exert its functions through this Wnt receptor [49, 56, 58–60]. Therefore, we next aimed to evaluate the potential beneficial effects exerted by the lentiviral-mediated overexpression of Wnt1 in the traumatically injured rat spinal cord.
For this purpose and once we determined the proper functioning of the lentiviral vector generated to induce the expression of Wnt1 (see Online Resource 2 for details about the different experiments carried out) and the best injection method of the lentiviral particles in the lesioned spinal cord in vivo (see Online Resource 3 for details about the different experiments carried out), we performed two different groups to evaluate the potential beneficial role of Wnt1 overexpression in the traumatically injured spinal cord: (1) a control group composed by animals subjected to spinal cord contusion and injected with LV-GFP and (2) a group composed by animals subjected to spinal cord contusion and injected with LV-Wnt1, which were sacrificed for histological evaluation at 7 (LV-GFP group, n = 5; LV-Wnt1 group, n = 5), 14 (LV-GFP group, n = 5; LV-Wnt1 group, n = 5), and 126 dpi (LV-GFP group, n = 10; LV-Wnt1 group, n = 7).
In agreement with the observations performed during the determination of the lentiviral injection method (Online Resource 3), cell transduction in these animals at 7 dpi was mostly observed in close relationship with the lesioned areas (Online Resource 4a–k). Briefly, transduced GFP+ cells were mainly observed in rostral and caudal levels adjacent to the lesion epicenter (Online Resource 4a, c, c1, h, h1, e, e1, j, and j1) and, to a lesser extent, in the lesioned dorsal columns at rostral and caudal levels distant from the lesion epicenter (Online Resource 4a, b, b1, g, g1, f, f1, k, and k1). Moreover, transduced GFP+ cells were also observed within the injured tissue and in the dorsal meninges in the lesion epicenter (Online Resource 4a, d, d1, i and i1). At 14 (Online Resource 5) and 126 dpi (Online Resource 6), the distribution of transduced GFP+ cells was almost exactly the same, although the presence of these cells in the lesion epicenter was lower at 14 dpi and nearly absent at 126 dpi.
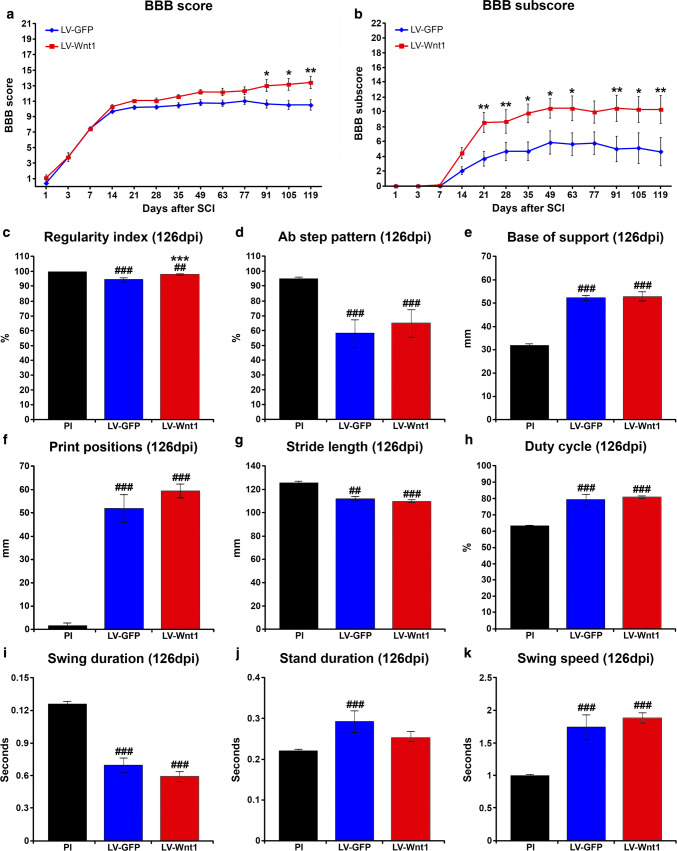
Evaluation of motor functional recovery using the BBB open-field test showed that, when compared to the LV-GFP control group, animals injected with LV-Wnt1 displayed a non-significant increasing trend in the BBB score from 21 dpi, which reached statistical significance at 91, 105, and 119 dpi (Fig. 6a). Accordingly, analysis of the BBB subscore showed that Wnt1 overexpression induced a significant increase in this parameter at almost all evaluated times post-injury from 21 to 119 dpi (Fig. 6b). Moreover, when the different individual aspects of locomotion analyzed were evaluated separately (Online Resource 7a–e), we observed that those animals injected with LV-Wnt1 displayed significantly higher values in critical aspects of locomotion such as coordination (Online Resource 7a), paw positions (Online Resource 7b), and tail position (Online Resource 7c), as well as an evident non-significant increasing trend in stepping values (Online Resource 7d). Subsequently, we further analyzed the motor function by evaluating different gait parameters at 126 dpi in those animals which displayed consistent stepping (LV-GFP group, n = 3; LV-Wnt1 group, n = 5) using the CatWalk® gait analysis system. As shown and in agreement with the data obtained from the analysis of coordination (Online Resource 7a), Wnt1 overexpression induced a significant increment in the regularity index (Fig. 6c), while no differences were observed in the rest of gait parameters evaluated (Fig. 6d–k).
Fig. 6.
Wnt1 overexpression in motor functional recovery after SCI. This figure shows the BBB score (a) and subscore (b) data obtained from the evaluation of motor functional recovery using the 21-point BBB open-field test, as well as data obtained from the analysis of relevant gait parameters using the CatWalk® gait analysis system (c–k), in lesioned animals injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1). The 21-point BBB open-field test was carried out at 1, 3, 7, 14, 21, 35, 49, 63, 77, 91, 105, and 119 days post-injury (dpi), and data obtained from all animals used to evaluate the effects exerted by Wnt1 overexpression, which were sacrificed at 7 (n = 5 per group), 14 (n = 5 per group), and 126 dpi (LV-GFP, n = 10; LV-Wnt1, n = 7), were included in the analysis. The analysis of the different gait parameters was performed either before injury [pre-injury (PI)] or at 126 dpi in animals displaying consistent stepping (LV-GFP, n = 3; LV-Wnt1, n = 5) (c–k). The existence of statistically significant differences between groups in a and b was assessed by two-way ANOVA, while, in c–k, one-way ANOVA was used. In both cases, Bonferroni post hoc test was used. Data are presented as the mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. LV-GFP; ##p < 0.01 and ###p < 0.001 vs. PI
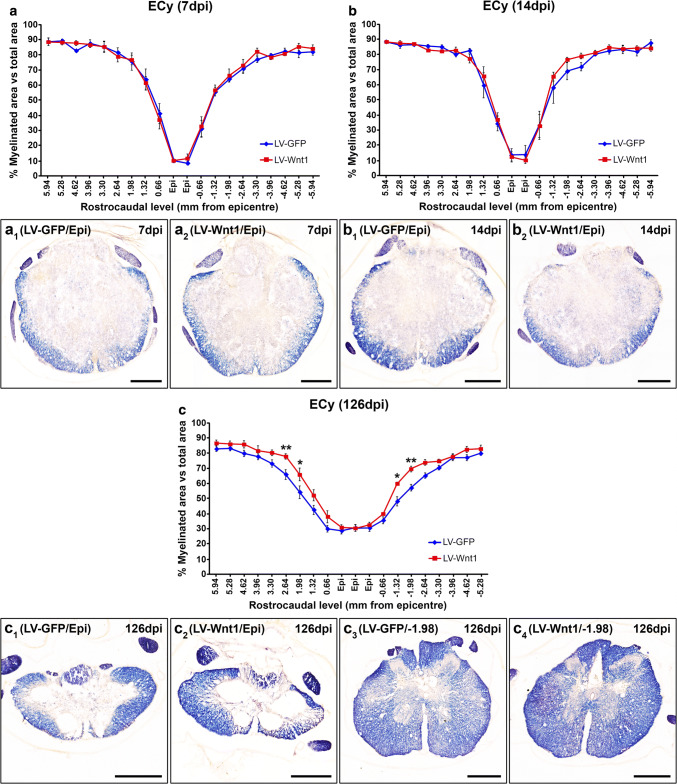
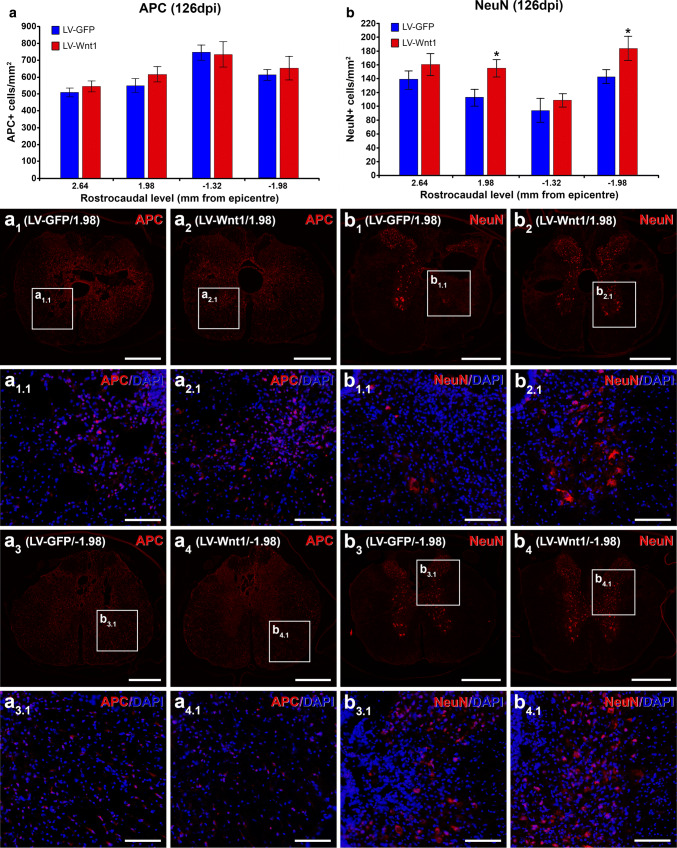
As a first step to assess the mechanisms that might be underlying the beneficial effects of Wnt1 overexpression in motor functional recovery after SCI, we subsequently analyzed the potential existence of differences in myelin preservation (Fig. 7). Although no differences between groups in this parameter were observed at 7 (Fig. 7a, a1, a2) and 14 dpi (Fig. 7b, b1, b2), at 126 dpi, those animals injected with LV-Wnt1 displayed a significantly higher myelinated area in both rostral and caudal levels adjacent to the injury epicenter (Fig. 7c, c1–4) where, interestingly, we found the higher presence of transduced GFP+ cells. We next aimed to assess whether the increase in myelin preservation induced by Wnt1 overexpression at 126 dpi correlated with variations in the presence of oligodendroglial cells, since are the myelinating cells in the CNS [81]. For this purpose, we counted the number of oligodendrocytes in the same rostro-caudal levels where the significant differences in myelin preservation were found. As shown (Fig. 8a, a1–4, a1.1–4.1), we did not find significant changes in oligodendroglial cell density in these rostro-caudal levels, indicating that the previously detailed variations in myelin preservation induced by Wnt1 overexpression were not due to changes in the presence of oligodendroglial cells. Moreover and as previously stated, different reports have consistently pointed to a role of Wnt1 in neuron survival in different experimental conditions [49, 51, 61, 78–80], although its potential neuroprotective role after SCI is still unknown. To evaluate whether Wnt1 overexpression induced neuroprotection in this neuropathological condition, we quantified the neuronal cell number at 126 dpi in the same rostro-caudal levels where Wnt1 overexpression led to higher myelin preservation which, as previously detailed, correspond to those levels where the maximum cell transduction was found. As shown (Fig. 8b, b1–4, b1.1–4.1), those animals injected with LV-Wnt1 displayed a significantly higher neuronal density, clearly pointing to a neuroprotective role of this Wnt ligand after SCI.
Fig. 7.
Wnt1 overexpression in myelin preservation after SCI. This figure shows the data (a–c) and representative images (a1–c4) obtained from the quantification of myelin preservation in eriochrome cyanine staining (ECy) processed spinal cord sections from lesioned animals injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP) (n = 10) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1) (n = 7). Evaluation was performed at 7 (a, a1, a2), 14 (b, b1, b2), and 126 (c, c1–4) days post-injury (dpi). Scale bars in a1–c4 = 500 µm. The existence of statistically significant differences between groups was assessed by two-way ANOVA followed by Bonferroni post hoc test. Data are presented as the mean ± SEM. *p < 0.05 and **p < 0.01 vs. LV-GFP
Fig. 8.
Wnt1 overexpression in oligodendroglial and neuronal cell number after SCI. This figure shows the data and representative images obtained from the quantification of oligodendroglial (a–a4.1) and neuronal (b–b4.1) cell number in spinal cord sections, processed for the immunohistochemical visualization of adenomatous polyposis coli (APC) or neuronal nuclei (NeuN), from lesioned animals injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP) (n = 10) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1) (n = 7). The analysis was performed at 126 days post-injury (dpi) in spinal cord levels where the significant differences in myelin preservation were observed (2.64, 1.98, − 1.32, and − 1.98 mm from epicenter). Squares in a1–4 and b1–4 indicate the areas shown in the corresponding images. Scale bars in a1–4 and b1–4 = 500 µm; scale bars in a1.1–4.1 and b1.1–4.1 = 100 µm. The existence of statistically significant differences between groups in each analyzed rostro-caudal level was assessed by two-tailed t test. Data are presented as the mean ± SEM. *p < 0.05 vs. LV-GFP
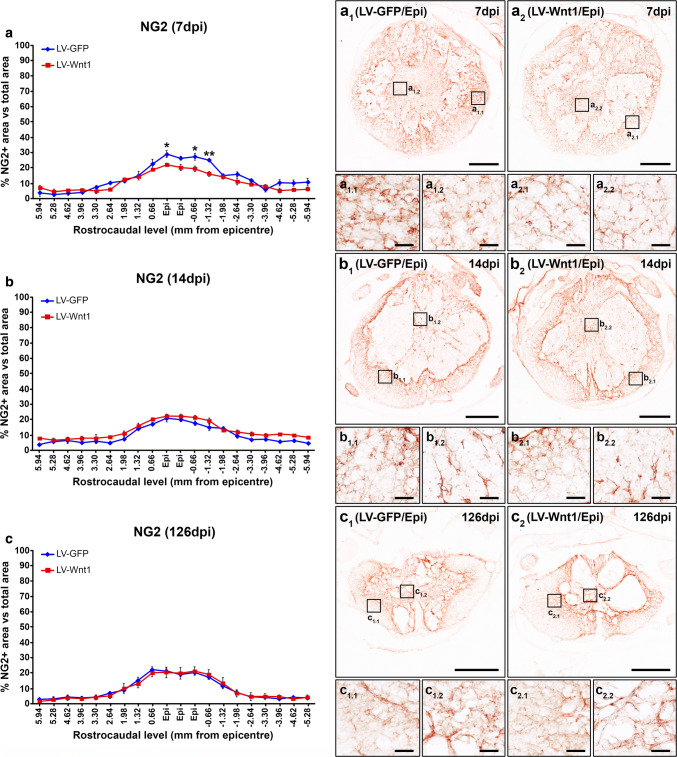
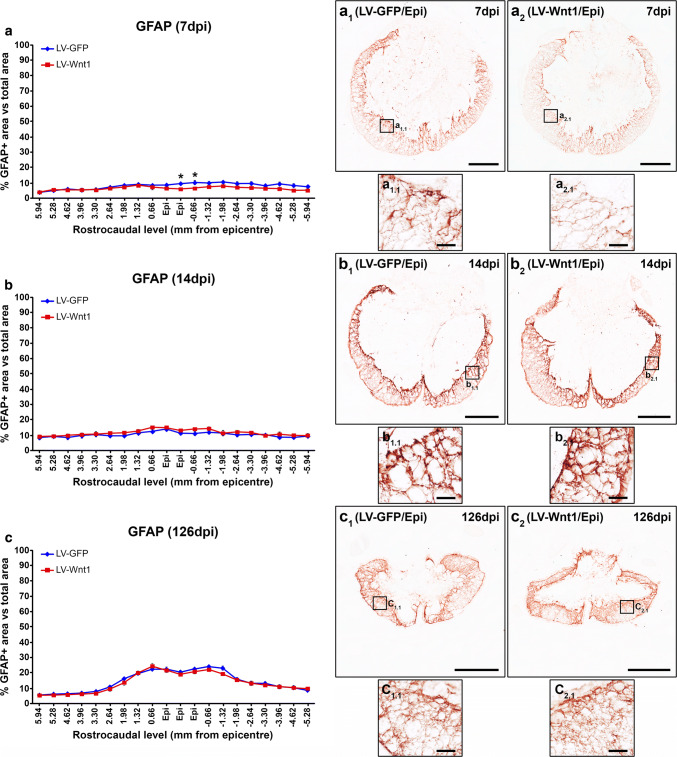
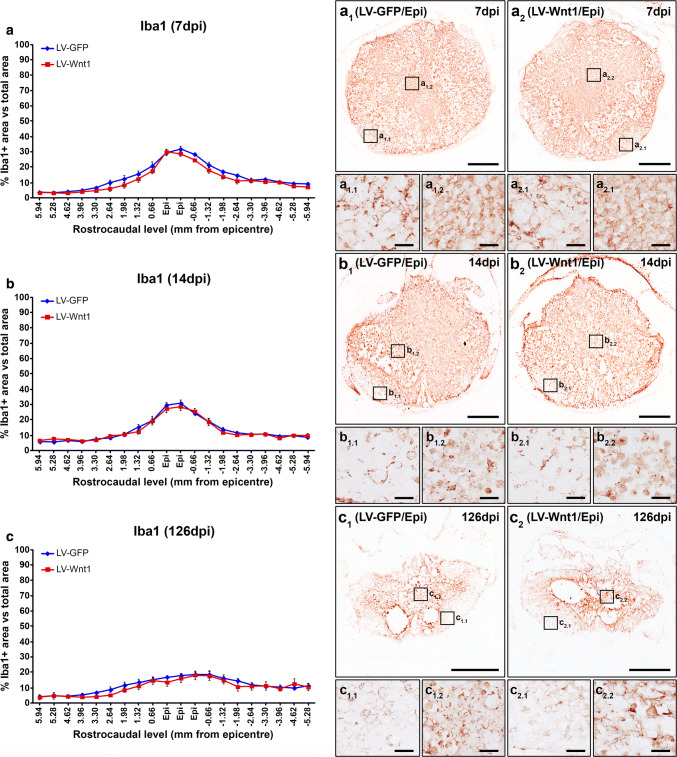
Beside oligodendroglial and neuronal cells, NG2+ cells, astrocytes, and microglia/macrophages exert critical functions that greatly determine the progression and outcome in this neuropathological situation [82–87]. Therefore, we subsequently evaluated whether Wnt1 overexpression led to changes in the injury-induced accumulation of NG2+ cells as well as in the astroglial and microglia/macrophage reactivity that take place in the injured areas after SCI. We found that, at 7 dpi, Wnt1 overexpression induced a significant reduction in the accumulation of NG2+ cells in the lesion epicenter and the adjacent caudal levels (Fig. 9a, a1–2, a1.1–2.2), which was mainly observed in the external ring of spared tissue. No differences in this parameter were detected at 14 (Fig. 9b, b1–2, b1.1–2.2) and 126 dpi (Fig. 9c, c1–2, c1.1–2.2). Similarly, those animals injected with LV-Wnt1 displayed a significant reduction in the presence of astroglial cells at 7 dpi in the lesion epicenter and the adjacent caudal levels (Fig. 10a, a1–2, a1.1–2.1), which also showed evident qualitative morphological alterations such as a lower hypertrophy of their cell body and processes (Fig. 10a1–2, a1.1–2.1), clearly indicating that Wnt1 overexpression reduced the astroglial reactivity at this time post-injury. Again, no differences in these parameters were observed at 14 (Fig. 10b, b1–2, b1.1–2.1) and 126 dpi (Fig. 10c, c1–2, c1.1–2.1). Regarding the SCI-associated microglia/macrophage reactivity, we did not find differences between groups neither in the presence of these cell types in the lesioned areas nor in their morphology at any of the evaluated times post-injury (Fig. 11).
Fig. 9.
Wnt1 overexpression in NG2 immunoreactivity after SCI. This figure shows the data (a–c) and representative images (a1–c2) obtained from the quantification of the area occupied by NG2 immunoreactivity in spinal cord sections from lesioned animals injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP) (n = 10) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1) (n = 7). Evaluation was performed at 7 (a, a1–2 and a1.1–2.2), 14 (b, b1–2 and b1.1–2.2), and 126 (c, c1–2 and c1.1–2.2) days post-injury (dpi). Squares in a1–c2 indicate the areas shown in the corresponding images. Scale bars in a1–c2 = 500 µm; scale bars in a1.1–c2.2 = 40 µm. The existence of statistically significant differences between groups was assessed by two-way ANOVA followed by Bonferroni post hoc test. Data are presented as the mean ± SEM. *p < 0.05 and **p < 0.01 vs. LV-GFP
Fig. 10.
Wnt1 overexpression in astroglial reactivity after SCI. This figure shows the data (a–c) and representative images (a1–c2) obtained from the quantification of the area occupied by glial fibrillary acidic protein (GFAP) immunoreactivity in spinal cord sections from lesioned animals injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP) (n = 10) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1) (n = 7). Evaluation was performed at 7 (a, a1–2 and a1.1–2.1), 14 (b, b1–2 and b1.1–2.1), and 126 (c, c1–2 and c1.1–2.1) days post-injury (dpi). Squares in a1–c2 indicate the areas shown in the corresponding images. Scale bars in a1–c2 = 500 µm; scale bars in a1.1–c2.1 = 40 µm. The existence of statistically significant differences between groups was assessed by two-way ANOVA followed by Bonferroni post hoc test. Data are presented as the mean ± SEM. *p < 0.05 vs. LV-GFP
Fig. 11.
Wnt1 overexpression in microglia/macrophage reactivity after SCI. This figure shows the data (a–c) and representative images (a1–c2) obtained from the quantification of the area occupied by ionized calcium-binding adaptor molecule 1 (Iba1) immunoreactivity in spinal cord sections from lesioned animals injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP) (n = 10) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1) (n = 7). Evaluation was performed at 7 (a, a1–2 and a1.1–2.2), 14 (b, b1–2 and b1.1–2.2), and 126 (c, c1–2 and c1.1–2.2) days post-injury (dpi). Scale bars in a1–c2 = 500 µm; scale bars in a1.1–c2.2 = 40 µm. The existence of statistically significant differences between groups was assessed by two-way ANOVA followed by Bonferroni post hoc test. Data are presented as the mean ± SEM
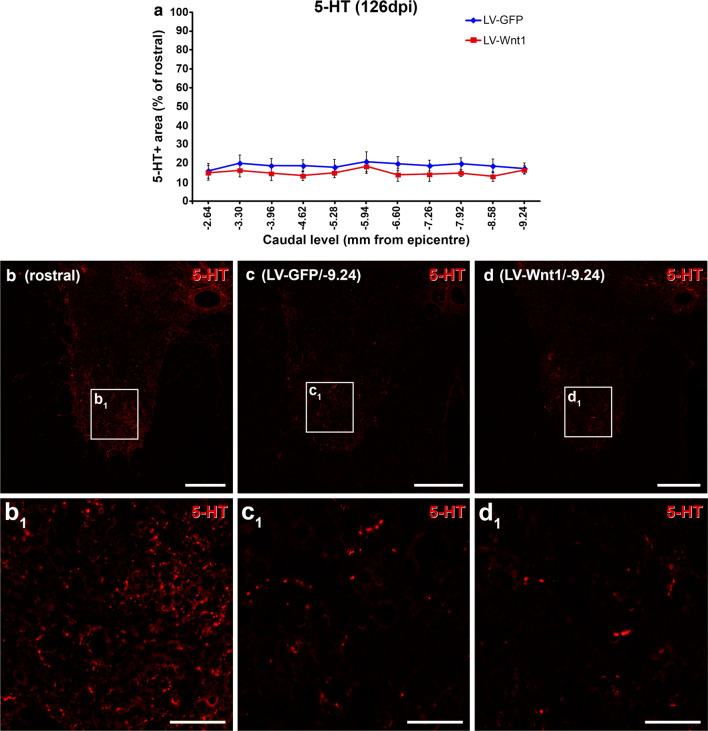
Finally, since the Wnt family of proteins is able to play a prominent role in axonal regeneration after SCI [88–90] and the great relevance of this biological process in the progression and outcome of this neuropathology [91, 92], we next aimed to evaluate whether Wnt1 overexpression led to changes in axonal preservation and/or regeneration in the traumatically injured rat spinal cord. For this purpose, we quantified the descending serotonergic innervation of the ventral horn motor regions caudally to the injury site at the end of the study (126 dpi), since the serotonergic system plays a major role in the recovery of motor function and is particularly affected after SCI [93]. As shown, (Fig. 12), no significant differences between groups were observed in the presence of serotonin (5-HT)+ axons in the ventral horns at any of the different levels analyzed.
Fig. 12.
Wnt1 overexpression in motor serotonergic innervation after SCI. This figure shows the data (a) and representative images (b–d1) obtained from the densitometrical quantification of the area occupied by serotonin (5-HT) immunoreactivity in spinal cord sections from lesioned animals injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP) (n = 10) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1) (n = 7). The analysis was carried out at 126 days post-injury (dpi) in the ventral horns of spinal cord sections corresponding to different spinal cord levels caudal to the injury site. Squares in b–d indicate the areas shown in the corresponding images. Scale bars in b–d = 150 µm; scale bars in b1–d1 = 40 µm. The existence of statistically significant differences between groups was assessed by two-way ANOVA followed by Bonferroni post hoc test. Data are presented as the mean ± SEM
Discussion
Over the last decade, a growing amount of studies have challenged the initial prevailing dogma that supported a lack of Wnt-related protein expression in the adult mammal spinal cord under physiological conditions. From the first experimental studies describing the absence of detection of all evaluated Wnt ligands and receptors in the healthy adult mice spinal cord [36], many different experimental works have found detectable physiological expression of almost all known Wnt ligands, conventional and non-conventional receptors, co-receptors, and soluble modulators in the healthy mammal adult spinal cord from different animal species, at both mRNA and protein levels and using different detection methods [14, 15, 30, 31, 33, 52, 53, 94–96]. Accordingly, different studies performed in rodents have shown that the Wnt family of proteins exerts major functions in the adult CNS, such as blood–brain barrier maintenance, synaptic plasticity and activity, neurogenesis, energy balance, memory regulation, and ependymal cell functioning [8–13]. However, up to date, only a few studies have suggested its potential involvement in the physiological adult human CNS activity, mostly through the assessment of the expression of selected components of this family of proteins. More specifically, detectable mRNA expression of most components of the Wnt family of proteins [97, 98], as well as protein expression of at least Wnt1, 2, 5a and 7b, Fz9, LRP6, Ryk, Ror2, Dkk3, and Wif1 [98–104] have been found in the healthy adult human brain. Regarding the healthy adult human spinal cord, we have recently shown that specific Wnt ligands, conventional and non-conventional receptors, co-receptors, and soluble modulators are expressed at the mRNA level in the ependymal region [14] and in the anterior horn [15]. To further complete this picture, we here found physiological mRNA expression of most Wnt ligands (Wnt2, 2b, 3, 4, 5a, 5b, 6, 7a, 7b, 8b, 9b, 10a, 10b, 11, and 16), receptors (Fz1-10, Ryk, PTK7, and Ror1 and 2), co-receptors (LRP5 and 6), and soluble modulators (Dkk1-4, sFRP1-5, and Wif1) in adult NL human samples of the complete spinal cord. Moreover, we also demonstrate that all known Fz receptors (Fz1-10) and non-conventional Wnt receptors (Ryk, PTK7, Ror1 and 2), as well as pLRP6, which is an essential component for the induction of the canonical Wnt/β-catenin signaling pathway [75], are expressed at the protein level in the healthy adult human spinal cord with specific expression patterns. Overall, these observations strongly point to the involvement of the Wnt family of proteins and its canonical signaling pathway in physiological adult human spinal cord functioning, although the specific functions of each component and elicited intracellular signaling pathways are currently unknown and should be evaluated in the future.
Among the different Fz receptors, it has been reported that Fz1 is expressed in the physiological adult rodent CNS [33, 49, 51–56, 76], while we and others have shown that the expression of this receptor suffered evident changes in cultured CNS cells subjected to different noxious stimuli [49, 50] as well as in different neuropathologies such as PD [51], amyotrophic lateral sclerosis [52–54], brain ischemic stroke [55], subarachnoid hemorrhage [56], and SCI [33, 36], suggesting a potential role for this receptor in the pathological CNS response. Accordingly, several reports have pointed to a neuroprotective role of Fz1 both in vitro [49, 57] and in vivo in experimental models of PD [49], subarachnoid hemorrhage [56], and brain ischemic stroke [55]. Despite these interesting observations, the potential involvement of this receptor in the traumatically injured human spinal cord is currently unknown. As we show here, in the traumatically injured human spinal cord, Fz1 is expressed in reactive astroglial cells surrounding the injury site, in activated microglia/macrophages, axons, and blood vessels located in the affected tissue, and in neurons and oligodendrocytes located in areas adjacent to the injury site, which are thought to be affected by the secondary cell death after SCI. To our knowledge, these observations constitute the first experimental evidence pointing to a potential role of this receptor in the traumatically injured human spinal cord and suggest that, besides its putative neuroprotective function, this receptor may be involved in the pathological response of different cell types to SCI. Intriguingly, we have recently found that, in the traumatically injured mice spinal cord, Fz1 expression was observed in neurons and oligodendrocytes, but not in axons and reactive astrocytes and microglia/macrophages [33], pointing to the potential existence of between-species differences in the expression of different Wnt components in the damaged spinal cord, as we have suggested in previous works [30, 33]. Remarkably, we here also demonstrate that, after rat spinal cord contusion, the spatio-temporal and cellular expression pattern of Fz1 is extremely similar to that observed in the human and, therefore, that this experimental model is suitable to evaluate the potential functions of this receptor in the progression and outcome of SCI.
In this line, different reports have demonstrated that Wnt1 is able to exert its functions through Fz1 [49, 56, 58–60]. More importantly, Wnt1 is able to induce neuroprotection both in vitro [49, 51, 61, 78–80] and in vivo under neuropathological conditions such as PD, subarachnoid hemorrhage and brain ischemia [49, 56, 61, 62], and its neuroprotective role depends on the presence of Fz1 at least in PD [49] and subarachnoid hemorrhage [56]. Moreover, the administration or blockade of specific components of the Wnt family of proteins as well as the interaction with its associated signaling pathways is able to improve functional recovery after SCI [35, 38, 40, 43, 45–48, 105]. However, despite the neuroprotective role of Wnt1 and the promising therapeutic potential of the modulation of the Wnt family of proteins in SCI, the putative beneficial effects of Wnt1 after SCI have not been evaluated so far. Interestingly, we and others have previously shown that, after SCI, the mRNA and protein expression of Wnt1 is acutely increased from 6 h post-injury to 3dpi [30, 33, 106]. Accordingly, it has been recently demonstrated that, in the injured spinal cord, the amount of Wnt1-expressing cells is greatly increased in the affected areas at 2 dpi [107]. However, at later times post-injury, its expression levels returned to that observed in NL controls, suggesting that the loss of increased levels of Wnt1 may favor the secondary progression of the injury. In agreement, we here show that, after rat SCI, long-term overexpression of Wnt1 led to a better motor functional recovery by improving different crucial aspects of locomotion, clearly supporting that inducing a sustained increase in Wnt1 levels during the progression of SCI might be used as a therapeutic approach to ameliorate the functional deficits associated to this neuropathological condition and, by extrapolation, the therapeutic potential of modulating the Wnt family of proteins in SCI.
Otherwise, since myelin loss and/or subsequent endogenous remyelination are major features of SCI that greatly determines the impact on functional recovery [108, 109], we next aimed to evaluate whether the previously detailed improvement in motor functional recovery due to Wnt1 overexpression was associated with changes in myelin preservation. In this regard, different reports have evaluated the potential involvement of specific components of the Wnt family of proteins and the Wnt/β-catenin signaling pathway in CNS myelination and remyelination, showing apparently disparate results and highlighting its complex role in physiological and pathological myelin generation in the CNS, which seems to depend on different aspects including the pathological condition [6, 17, 110]. Specifically in SCI, it has been shown that Wnt3a administration as well as GSK-3β inhibition significantly reduced myelin loss [45, 48]. Moreover, we found that, at 126 dpi, Wnt1 overexpression led to a prominent increase in the myelinated area in rostro-caudal spinal cord levels adjacent to the injury site, where we observed the major accumulation of transduced cells. However, we did not observe differences neither in the density of oligodendroglial cells nor in the presence of NG2+ cells in the same spinal cord levels and time post-injury, suggesting that Wnt1 overexpression might promote the myelination capacity of oligodendroglial cells rather than influence oligodendroglial cell survival or the recruitment and differentiation of oligodendrocyte precursor cells (OPCs). Accordingly, the only study that has previously assessed the potential role of Wnt1 in myelin formation showed that, in oligodendrocyte-enriched cultures, this Wnt ligand induced a robust increase in the expression of myelin proteolipid protein [111], which is the most abundant protein in CNS myelin and plays a major role in physiological and pathological myelin functions [112, 113].
On the other hand and as previously stated, different studies have pointed to a role of Wnt1 in neuronal survival. More specifically, Wnt1 administration in neuronal cell cultures reduced cell death after serum deprivation, oxygen–glucose deprivation, or administration of 6-OHDA, MPP, or β-amyloid [49, 51, 61, 78–80]. In addition, the neuroprotective role of Wnt1 has been also addressed in vivo in rodent experimental models of PD [49, 62], subarachnoid hemorrhage [56], and brain ischemia [61]. In this regard, we found that those animals injected with LV-Wnt1 exhibited a higher neuronal density in rostral and caudal spinal cord levels adjacent to the injury site, which are susceptible to be affected by the extension of damage along the secondary stage after SCI and co-localize with those areas where we found a major accumulation of transduced cells and increase in myelin presence induced by Wnt1 overexpression. These results demonstrate that Wnt1 is able to promote neuron survival also in this neuropathological condition, further highlighting the neuroprotective potential of this Wnt ligand.
Another major hallmark of SCI is the proliferation and accumulation of NG2+ cells in and surrounding the injury site, where they exert a wide range of functions that greatly influence the progress and result of the injury [82, 83]. Remarkably, different reports have assessed the involvement of specific components of the Wnt family of proteins as well as the Wnt/β-catenin signaling pathway in physiological and pathological OPC recruitment, generation, and differentiation obtaining contradictory results [6]. Again, these likely conflicting results clearly indicate the complexity of the relationship between the Wnt family of proteins and OPC biology, since the effects observed seem to depend on the specific experimental context. Specifically after SCI, we have shown that the presence of NG2+ cells expressing different Wnt receptors is increased in the affected tissue [31, 32], suggesting the involvement of the Wnt family of proteins in the NG2+ cell response to this neuropathological situation. Accordingly, the deletion of β-catenin in OPCs greatly reduced its proliferation and accumulation in the affected areas as well as the injury-induced morphological changes in these cells after SCI [39]. Moreover, we here show that Wnt1 is able to modulate the injury response of OPCs in the traumatically injured spinal cord, since Wnt1 overexpression led to a reduced accumulation of NG2+ cells at 7 dpi in the lesion site. This is somewhat surprising, since Wnt1 has been classically considered as a prototypical “canonical” Wnt ligand owing to its widely described capacity to activate the Wnt/β-catenin signaling pathway [12, 114], although an increasing number of studies have questioned this probably oversimplified point of view [78, 79, 115–117]. Finally, although the lack of effects in the presence of NG2+ cells at 14 and 126 dpi may indicate the existence of compensatory mechanisms, another plausible explanation is that the presence of transduced cells in spinal cord levels corresponding to the lesion epicenter was greatly reduced at 14 dpi and nearly disappeared at 126 dpi.
Otherwise, after SCI, astroglial and microglial cells are rapidly activated in response to different injury-related stimuli and thus suffered dramatic morphological and functional changes, which allow them to exert both beneficial and detrimental functions by influencing a wide range of cellular and molecular processes that characterize and/or determine the progression and outcome of the injury [84–87, 118, 119]. Regarding the potential involvement of the Wnt family of proteins in the injury response of these cell types to SCI, previous in vitro experiments have shown that both astroglial and microglial cells express a wide range of Wnt receptors [50, 120], that its expression levels suffered evident variations after activation at least in astroglial cells [50], and that both cell types are able to respond to different Wnt ligands [50, 120–123]. In addition, specific components of this family of proteins and/or its associated signaling pathways are able to modulate cell proliferation, glutamate uptake, and the expression of glutamate transporters, pro-inflammatory cytokines, trophic factors, and potassium and water channels in astroglial cells [50, 124–128], as well as to regulate cell proliferation and migration and the expression of a battery of inflammatory mediators in microglial cells [120–123], which are critical features of the astroglial and microglial response to SCI [86, 87, 129–134]. Moreover, Wnt3a or Wnt5a overexpression in the healthy spinal cord led to an injury-like reaction of astroglial and microglial cells [39], while, as we have previously shown, the expression of different Wnt receptors in these cell types suffered evident variations after SCI [31, 32]. Finally, β-catenin inhibition in NG2+ cells reduced astroglial scarring and microglia/macrophage reactivity, while the inhibition of GSK-3β led to increased astroglial migration, earlier glial scar formation, and increased compaction of microglia/macrophage cells after SCI [39, 45]. Within this context, we here found that Wnt1 overexpression induced a significant reduction of astroglial reactivity in the lesion epicenter at 7 dpi by diminishing the accumulation of activated astroglial cells surrounding the damaged tissue, which also showed a reduced hypertrophy of their cell soma and processes. Similarly to that observed in NG2+ cells, the reduction of the astroglial response due to Wnt1 overexpression was only observed at 7 dpi but not at 14 and 126 dpi, which again may be due to the disappearance of transduced cells in the lesion epicenter at these times post-injury. On the contrary, we found that the overexpression of this Wnt ligand did not induce variations neither in microglia/macrophage accumulation in the damaged areas nor in the injury-related morphological changes of these cell types at any of the evaluated times post-injury.
Besides, the injury-associated axonal degeneration and regeneration are among the most relevant processes determining the loss of motor and sensory function after SCI [91, 92, 135, 136]. Noticeably, mounting evidence has pointed to a crucial role of the Wnt family of proteins in the regulation of axon preservation and/or regeneration after SCI [89, 90, 137], since it has been demonstrated that at least Ryk, Wnt3a and 4, sFRP2, Wif1, Dkk1, and the Wnt/β-catenin signaling pathway are involved in the modulation of these biological processes in the traumatically injured spinal cord [35–38, 41–43, 47, 48, 105]. To our knowledge, only one study has assessed the potential involvement of Wnt1 in axon growth [138]. In this work, Liu and collaborators showed that, during development, Wnt1 and Wnt5a are expressed in an anterior-high/posterior-low gradient in the mouse spinal cord, in those areas where descending corticospinal (CST) axon growth and at the proper time period when this developmental process occurs. Moreover, the authors demonstrated that Wnt1- or Wnt5a-transfected cells inhibit neurite outgrowth from neonatal motor cortex explants, an effect that is abolished by blocking antibodies against the Ryk receptor, which is expressed in developing CST axons. Finally, the administration of anti-Ryk antibodies in vivo reduced the developmental growth of CST axons posterior to the site of injection. Altogether, these observations indicated that Wnt1 could act as a potent repellent factor for CST axon growth during development. Moreover, a subsequent study from the same group showed that, after SCI, increased Wnt1 and Wnt5a expression was found surrounding the injury site, while Ryk expression was reinduced in the damaged CST axons [36]. Furthermore, this work also showed that the in vivo administration of a blocking antibody against Ryk increased CST axon regeneration after SCI, suggesting that the previously detailed Wnt axon guidance system during development also governs axonal regeneration in the injured spinal cord. In this regard, we here show that, after SCI, Wnt1 overexpression did not induce any variation in the preservation and/or regeneration of the descending serotonergic axons, which play a major role in the recovery of motor function locomotion and are particularly affected in SCI [93]. This interesting observation suggests that Wnt1 might play different roles depending on the specific axonal tract, as it has been demonstrated for other Wnt ligands such as Wnt5a, which repels CST and dopaminergic but attracts serotonergic axons [139]. Noticeably, serotonergic axons display and enhanced ability to regenerate or sprout after injury, probably due to the differential expression of at least distinct receptors, proteases, and integrins, which allow them to overcome the inhibitory environment for axonal regeneration in the damaged tissue [140]. Interestingly, the functions elicited by Wnt ligands, even at the axonal level, greatly depend on the receptor which is activated. For instance, it has been shown that Ryk, which mediated the previously detailed inhibitory effect of Wnt1 on CST development in vitro, is able to induce either axon repulsion or growth in response to Wnt5a depending on whether it acts in combination or not with Fz receptors [141]. Therefore, the differential expression of Wnt receptors in each specific axonal tract might underlie the discrepancies between our observations in serotonergic axons and those performed in the CST, although this hypothesis should be evaluated in future studies. Finally, although the previously detailed potential role of Wnt1 as a repellent of CST regeneration after SCI could be in apparent contrast with the improvement in the motor functional recovery that we found in the present study, it should be first noted that different studies have shown that CST injury and/or regeneration have little or no influence on unskilled locomotor function in rodents [142]. In this way, since the motor functional tests that we have used evaluate unskilled overground locomotion, it is plausible that the potential deleterious effect of Wnt1 in CST regeneration would not or minimally influence the improvement of motor functional recovery observed. Moreover, it also should be noted that Wnt1 overexpression induced a significant increase in myelin preservation and neuroprotection after SCI, and that these beneficial effects may compensate the potential deleterious role of Wnt1 in CST regeneration.
Overall, our body of results provides for a solid proof of concept supporting that intraparenchyma expression of Wnt1 from the acute stage after SCI might be a clinically promising therapy. Besides, based on the spatio-temporal and cellular pattern of Fz1 in this neuropathological condition, the second major finding of our study is the identification of this receptor as a potential direct therapeutic target. In this regard and accordingly to the different reports demonstrating that Wnt1 is able to act through Fz1 [49, 58–60], we strongly consider that this receptor may be mediating the effects exerted by Wnt1 overexpression after SCI. However, since several works have shown that Wnt1 is able to act through other Wnt receptors [138, 143], we cannot conclude it and, thus, this interesting hypothesis should be evaluated in the following studies. Finally, it should be noted that Wnt therapies are largely limited by their short half-life [144] and that the use of lentiviral vectors, although constitutes a consistent experimental approach to evaluate the effects elicited by a sustained expression of a Wnt ligand, also has major limitations such as the impossibility to determine neither the optimum dose nor the therapeutic window. Therefore, future research should focus on the development of novel therapeutic strategies addressed to overcome these limitations and optimize the different parameters necessary to pave for a clinically feasible Wnt1 and, by extension, other Wnt-related therapeutic design.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1 Determination of the primary antibody specificity used to visualize Fz1. This figure shows representative images obtained from non-lesioned (NL) rat spinal cord sections (a1-6), lesioned rat spinal cord sections (7 days post-injury) (b1-6) and NL human spinal cord sections (c1-6) used to determine the specificity of the anti-Fz1 primary antibody. (a1-2), (b1-2) and (c1-2) correspond to images obtained from spinal cord sections processed for the immunohistochemical visualization of Fz1 and used as positive controls (C+); (a3-4), (b3-4) and (c3-4) correspond to images obtained from spinal cord sections processed for the immunohistochemical visualization of Fz1 without the primary antibody and used as negative controls (C-); (a5-6), (b5-6) and (c5-6) correspond to images obtained from spinal cord sections processed for the immunohistochemical visualization of Fz1 with the primary antibody pre-adsorbed with its corresponding blocking peptide (1:20 antibody/blocking peptide weight excess) (1/20). (a), (b) and (c) correspond to schematic drawings of prototypical NL rat, lesioned rat and and NL human spinal cord sections respectively, indicating the approximate areas shown in the corresponding images. Scale bars = 100 µm. (TIF 64125 kb)
Online Resource 2 Determination of the proper functioning of the lentiviral vector generated to overexpress Wnt1. This figure shows the data and representative images obtained from the different studies performed in cultured B1a cells to evaluate the proper functioning of the lentiviral vector generated to overexpress Wnt1. Briefly, to determine the multiplicity of infection (MOI) that should be used to conduct this set of experiments we tested the effects of various MOI (MOI 5, 10, 20 and 40) on different aspects such as cell number (a), percentage of transduced cells (b) and GFP intensity (c) at 3 and 7 days post-transduction (dpt) (n = 3 per group) by flow cytometry. Based in the results obtained we selected MOI 10 to perform the subsequent experiments, since it induced a similar transduction efficiency to that observed with higher MOIs (MOI 20 and 40) (b), led to a high transgene expression (c) and did not affect cell viability and/or proliferation (a). The existence of significant differences between groups in a-c was assessed by Two-way ANOVA followed by Bonferroni post-hoc test (#, ## and ### = p < 0.05, p < 0.01 and p < 0.001 vs. 3 dpt; *, ** and *** = p < 0.05, p < 0.01 and p < 0.001 vs. non-transduced cells (NT); &, && and &&& = p < 0.05, p < 0.01 and p < 0.001 vs. the immediately lower MOI). We next determined by Real Time PCR and western blot that, in comparison with NT cells and those transduced with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP), transduction with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1) induced a robust increase in Wnt1 expression at both the mRNA (d, n = 3 per group) and protein levels (e, n = 3 per group) at 7 dpt, when we have previously observed the maximum transduction efficiency (b) and transgene expression (c). The existence of statistically significant differences between groups in e was assessed by One-way ANOVA followed by Bonferroni post-hoc test (*** represent p < 0.001 vs. NT; $$$ represent p < 0.001 vs. LV-GFP). It should be noted that Wnt1 is a soluble protein that acts in both autocrine and paracrine fashions and, thus, its correct secretion to the extracellular environment is a critical step to exert its multiple biological activities. In this regard, we found that the protein levels of Wnt1 were also strongly upregulated in the culture medium of cells transduced with LV-Wnt1 in comparison with those of NT and cells transduced with LV-GFP (f, n = 3 per group), demonstrating that Wnt1 was conveniently secreted to the extracellular space. We next aimed to evaluate whether overexpressed Wnt1 was able to activate the canonical Wnt/β-catenin signaling pathway which is the main Wnt signaling pathway that is activated by Wnt1. For this purpose, we determined that incubation of cell cultures with recombinant Wnt1 (100 and 200 ng/ml) (120-17, Peprotech) during 1 h led to the activation of the canonical Wnt/β-catenin signaling pathway (g) (n = 3 per group and time of incubation). The existence of significant differences between groups in g was assessed by Two-way ANOVA followed by Bonferroni post-hoc test (* = p < 0.05 vs. control). Finally, we found that, in comparison with cell cultures incubated with conditioned medium from cells transduced with LV-GFP (1:5) (CM LV-GFP), cell cultures incubated during 1 h with conditioned medium from cells cultures transduced with LV-Wnt1 (1:5) (CM LV-Wnt1) induced a similar activation of this signaling pathway than that observed in cell cultures incubated during 1 h with conditioned medium from cell cultures transduced with LV-GFP (1:5) and supplemented with 200 ng/ml of recombinant Wnt1 (CM LV-GFP+ Wnt1) (h, n = 3 per group), demonstrating that overexpressed Wnt1 is biologically active. The existence of significant differences between groups in h was assessed by One-way ANOVA followed by Bonferroni post-hoc test (* = p < 0.05 vs. CM LV-GFP). In all cases, data are presented as the mean + SEM. (TIF 4260 kb)
Online Resource 3 Determination of the injection method of lentiviral vectors in vivo. This figure shows the results obtained from the experiments performed to establish the in vivo intraparenchymal injection method of the lentiviral vectors in the lesioned rat spinal cord. (a) and (b) shows schematic drawings from the two methods tested. In the first one (3ip), one injection per rostro-caudal level (stereotaxic coordinates: 0 mm lateral and 1 mm depth) was performed in three rostro-caudal levels corresponding to the lesion epicenter, 1.5 mm rostral and 1.5 mm caudal (a). In the second one (6ip), two injections per rostro-caudal level (stereotaxic coordinates: 0.6/-0.6 mm lateral and 1 mm depth) were performed in the same rostro-caudal levels detailed above (b). Evaluation of transduction at 7 days post-injury showed that both injection methods induced a similar transduction in longitudinal sections corresponding to the median line, where transduced green fluorescence protein (GFP)+ cells were observed surrounding the lesioned area, mainly rostral and caudal to the lesion epicenter, and to a lesser extent in the injury site (c-e) (non-injected (NT) group, n = 2; 3ip group, n = 3; 6ip group, n = 3) (arrows in d and e point the areas where GFP+ cells were observed). However, those animals that received 6 injections of lentiviral particles displayed a wider lateral distribution of transduced GFP+ cells than those that received 3 injections (f). As a consequence, we selected the second injection method for the subsequent experiments performed to evaluate the effects exerted by Wnt1 overexpression in the traumatically injured spinal cord. Scale bars in c-e represent 1 mm. (TIF 7420 kb)
Online Resource 4 Evaluation of transduction at 7 days after SCI. This figure shows representative images from the evaluation of transduction at 7 days post-injury in those animals used to evaluate the effects of Wnt1 overexpression after SCI, which were injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP control group) (n = 5) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1 group) (n = 5). (a), shows a schematic drawing of a prototypical lesioned longitudinal spinal cord section and indicates the approximate rostro-caudal levels where the representative images were obtained. b, b1, c, c1, d, d1, e, e1, f and f1 shows representative images from the LV-GFP control group, while g, g1, h, h1, i, i1, j, j1, k and k1 show representative images from the LV-Wnt1 group. Squares in a-k indicate the areas shown in the corresponding higher magnification images. Arrows in a1-k1 point to the cells shown in the corresponding insets. Scale bars in a-k represent 500 µm, while those in a1-k1 and in their corresponding insets represent 100 and 20 µm, respectively. (TIF 17121 kb)
Online Resource 5 Evaluation of transduction at 14 days after SCI. This figure shows representative images from the evaluation of transduction at 14 days post-injury in those animals used to evaluate the effects of Wnt1 overexpression after SCI, which were injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP control group) (n = 5) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1 group) (n = 5). (a), shows a schematic drawing of a prototypical lesioned longitudinal spinal cord section and indicates the approximate rostro-caudal levels where the representative images were obtained. b, b1, c, c1, d, d1, e, e1, f and f1 shows representative images from the LV-GFP control group, while g, g1, h, h1, i, i1, j, j1, k and k1 show representative images from the LV-Wnt1 group. Squares in a-k indicate the areas shown in the corresponding higher magnification images. Arrows in a1-k1 point to the cells shown in the corresponding insets. Scale bars in a-k represent 500 µm, while those in a1-k1 and in their corresponding insets represent 100 and 20 µm, respectively. (TIF 12872 kb)
Online Resource 6 Evaluation of transduction at 126 days after SCI. This figure shows representative images from the evaluation of transduction at 126 days post-injury in those animals used to evaluate the effects of Wnt1 overexpression after SCI, which were injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP control group) (n = 10) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1 group) (n = 7). (a), shows a schematic drawing of a prototypical lesioned longitudinal spinal cord section and indicates the approximate rostro-caudal levels where the representative images were obtained. b, b1, c, c1, d, d1, e, e1, f and f1 shows representative images from the LV-GFP control group, while g, g1, h, h1, i, i1, j, j1, k and k1 show representative images from the LV-Wnt1 group. Squares in a-k indicate the areas shown in the corresponding higher magnification images. Arrows in a1-k1 point to the cells shown in the corresponding insets. Scale bars in a-k represent 500 µm, while those in a1-k1 and in their corresponding insets represent 100 and 20 µm, respectively. (TIF 17167 kb)
Online Resource 7 Separate analysis of different individual aspects of locomotion analyzed in the 21-point BBB open-field test. This figure shows data obtained from the separate analysis of different individual aspects of locomotion such as coordination (a), paw positions (b), tail position (c), stepping (d), and toe clearance (e), which were evaluated using the 21-point BBB open-field test. The analysis was performed in lesioned animals injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP control group) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1 group) at 1, 3, 7, 14, 21, 35, 49, 63, 77, 91, 105 and 119 days post-injury (dpi). Data obtained from all animals used to evaluate the effects exerted by Wnt1 overexpression, which were sacrificed at 7 (LV-GFP group, n = 5; LV-Wnt1 group, n = 5), 14 (LV-GFP group, n = 5; LV-Wnt1 group, n = 5) and 126 dpi (LV-GFP group, n = 10; LV-Wnt1 group, n = 7), were included in the analysis. The existence of statistically significant differences between groups was assessed by Two-way ANOVA followed by Bonferroni post-hoc test. Data are presented as the mean + SEM. *, ** and *** represent p < 0.05, p < 0.01 and p < 0.001 vs. LV-GFP. (TIF 4261 kb)
Acknowledgements
We would like to thank Virginia Pérez, Sandra Vázquez, and the technical staff from the Service of Microscopy and Image Analysis and the Service of Flow Cytometry at the National Hospital for Paraplegics for their outstanding technical help, especially to Javier Mazarío for his essential participation in the design of the automatized cell count method. We also like to thank Sam David for his help to obtain the lesioned human spinal cord samples. This work has been supported by the Fondo de Investigación Sanitaria (FIS) of Instituto de Salud Carlos III (Grant number PI12/2895; FEDER co-funded) and the Ministerio de Ciencia, Innovación y Universidades (Grant number RTI2018-097775-B-I00; FEDER co-funded).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pau González and Carlos González-Fernández have contributed equally to this work.
Contributor Information
Pau González, Email: paug@sescam.jccm.es.
Francisco Javier Rodríguez, Email: fjrodriguez@sescam.jccm.es.
References
- 1.Grainger S, Willert K (2018) Mechanisms of Wnt signaling and control. Wiley Interdiscip Rev Syst Biol Med. 10.1002/wsbm.1422 [DOI] [PMC free article] [PubMed]
- 2.van Amerongen R. Alternative Wnt pathways and receptors. Cold Spring Harb Perspect Biol. 2012;4(10):a007914. doi: 10.1101/cshperspect.a007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13(12):767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 4.Engelhardt B, Liebner S. Novel insights into the development and maintenance of the blood–brain barrier. Cell Tissue Res. 2014;355(3):687–699. doi: 10.1007/s00441-014-1811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brafman D, Willert K. Wnt/beta-catenin signaling during early vertebrate neural development. Dev Neurobiol. 2017;77(11):1239–1259. doi: 10.1002/dneu.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo F, Lang J, Sohn J, Hammond E, Chang M, Pleasure D. Canonical Wnt signaling in the oligodendroglial lineage—puzzles remain. Glia. 2015;63(10):1671–1693. doi: 10.1002/glia.22813. [DOI] [PubMed] [Google Scholar]
- 7.Salinas PC. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb Perspect Biol. 2012;4(2):a008003. doi: 10.1101/cshperspect.a008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helfer G, Tups A. Hypothalamic Wnt signalling and its role in energy balance regulation. J Neuroendocrinol. 2016;28(3):12368. doi: 10.1111/jne.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortress AM, Frick KM. Hippocampal Wnt signaling: memory regulation and hormone interactions. Neuroscientist. 2016;22(3):278–294. doi: 10.1177/1073858415574728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohata S, Nakatani J, Herranz-Perez V, Cheng J, Belinson H, Inubushi T, Snider WD, Garcia-Verdugo JM, Wynshaw-Boris A, Alvarez-Buylla A. Loss of Dishevelleds disrupts planar polarity in ependymal motile cilia and results in hydrocephalus. Neuron. 2014;83(3):558–571. doi: 10.1016/j.neuron.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood–brain barrier. Nat Med. 2013;19(12):1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliva CA, Montecinos-Oliva C, Inestrosa NC. Wnt signaling in the central nervous system: new insights in health and disease. Prog Mol Biol Transl Sci. 2018;153:81–130. doi: 10.1016/bs.pmbts.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Xing L, Anbarchian T, Tsai JM, Plant GW, Nusse R. Wnt/beta-catenin signaling regulates ependymal cell development and adult homeostasis. Proc Natl Acad Sci USA. 2018;115(26):E5954–E5962. doi: 10.1073/pnas.1803297115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Fernandez C, Arevalo-Martin A, Paniagua-Torija B, Ferrer I, Rodriguez FJ, Garcia-Ovejero D. Wnts are expressed in the ependymal region of the adult spinal cord. Mol Neurobiol. 2016;54(8):6342–6355. doi: 10.1007/s12035-016-0132-8. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Fernandez C, Gonzalez P, Andres-Benito P, Ferrer I, Rodriguez FJ. Wnt signaling alterations in the human spinal cord of amyotrophic lateral sclerosis cases: spotlight on Fz2 and Wnt5a. Mol Neurobiol. 2019;56(10):6777–6791. doi: 10.1007/s12035-019-1547-9. [DOI] [PubMed] [Google Scholar]
- 16.L'Episcopo F, Tirolo C, Caniglia S, Testa N, Morale MC, Serapide MF, Pluchino S, Marchetti B. Targeting Wnt signaling at the neuroimmune interface for dopaminergic neuroprotection/repair in Parkinson's disease. J Mol Cell Biol. 2014;6(1):13–26. doi: 10.1093/jmcb/mjt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie C, Li Z, Zhang GX, Guan Y. Wnt signaling in remyelination in multiple sclerosis: friend or foe? Mol Neurobiol. 2014;49(3):1117–1125. doi: 10.1007/s12035-013-8584-6. [DOI] [PubMed] [Google Scholar]
- 18.Lambert C, Cisternas P, Inestrosa NC. Role of Wnt signaling in central nervous system injury. Mol Neurobiol. 2015;53(4):2297–2311. doi: 10.1007/s12035-015-9138-x. [DOI] [PubMed] [Google Scholar]
- 19.Libro R, Bramanti P, Mazzon E. The role of the Wnt canonical signaling in neurodegenerative diseases. Life Sci. 2016;158:78–88. doi: 10.1016/j.lfs.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Suwala AK, Hanaford A, Kahlert UD, Maciaczyk J. Clipping the wings of glioblastoma: modulation of WNT as a novel therapeutic strategy. J Neuropathol Exp Neurol. 2016;75(5):388–396. doi: 10.1093/jnen/nlw013. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y, Lee JK, Ahn SH, Lee J, Nam DH. WNT signaling in glioblastoma and therapeutic opportunities. Lab Investig. 2016;96(2):137–150. doi: 10.1038/labinvest.2015.140. [DOI] [PubMed] [Google Scholar]
- 22.Tapia-Rojas C, Inestrosa NC. Loss of canonical Wnt signaling is involved in the pathogenesis of Alzheimer's disease. Neural Regen Res. 2018;13(10):1705–1710. doi: 10.4103/1673-5374.238606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15(3):415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 2011;71(2):281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 25.Reilly P. The impact of neurotrauma on society: an international perspective. Prog Brain Res. 2007;161:3–9. doi: 10.1016/S0079-6123(06)61001-7. [DOI] [PubMed] [Google Scholar]
- 26.Priebe MM, Chiodo AE, Scelza WM, Kirshblum SC, Wuermser LA, Ho CH (2007) Spinal cord injury medicine. 6. Economic and societal issues in spinal cord injury. Arch Phys Med Rehabil 88 (3 Suppl 1):S84–88. 10.1016/j.apmr.2006.12.005 [DOI] [PubMed]
- 27.Witiw CD, Fehlings MG. Acute spinal cord injury. J Spinal Disord Tech. 2015;28(6):202–210. doi: 10.1097/BSD.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 28.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25(5):E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 29.Ahuja CS, Fehlings M. Concise review: bridging the gap: novel neuroregenerative and neuroprotective strategies in spinal cord injury. Stem Cells Transl Med. 2016;5(7):914–924. doi: 10.5966/sctm.2015-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, Maqueda A, Arenas E, Rodriguez FJ. Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS ONE. 2011;6(11):e27000. doi: 10.1371/journal.pone.0027000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez P, Fernandez-Martos CM, Gonzalez-Fernandez C, Arenas E, Rodriguez FJ. Spatio-temporal expression pattern of frizzled receptors after contusive spinal cord injury in adult rats. PLoS ONE. 2012;7(12):e50793. doi: 10.1371/journal.pone.0050793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez P, Fernandez-Martos CM, Arenas E, Rodriguez FJ. The Ryk receptor is expressed in glial and fibronectin-expressing cells after spinal cord injury. J Neurotrauma. 2013;30(10):806–817. doi: 10.1089/neu.2012.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Fernandez C, Fernandez-Martos CM, Shields SD, Arenas E, Javier Rodriguez F. Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma. 2014;31(6):565–581. doi: 10.1089/neu.2013.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35(3):161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyashita T, Koda M, Kitajo K, Yamazaki M, Takahashi K, Kikuchi A, Yamashita T. Wnt-ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J Neurotrauma. 2009;26(7):955–964. doi: 10.1089/neu.2008.0776. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, Zou Y. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008;28(33):8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollis ER, 2nd, Zou Y. Reinduced Wnt signaling limits regenerative potential of sensory axons in the spinal cord following conditioning lesion. Proc Natl Acad Sci USA. 2012;109(36):14663–14668. doi: 10.1073/pnas.1206218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suh HI, Min J, Choi KH, Kim SW, Kim KS, Jeon SR. Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta Neurochir (Wien) 2011;153(5):1003–1010. doi: 10.1007/s00701-011-0945-1. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez JP, Coulter M, Miotke J, Meyer RL, Takemaru K, Levine JM. Abrogation of beta-catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS injury. J Neurosci. 2014;34(31):10285–10297. doi: 10.1523/JNEUROSCI.4915-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollis ER, 2nd, Ishiko N, Pessian M, Tolentino K, Lee-Kubli CA, Calcutt NA, Zou Y. Remodelling of spared proprioceptive circuit involving a small number of neurons supports functional recovery. Nat Commun. 2015;6:6079. doi: 10.1038/ncomms7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wehner D, Tsarouchas TM, Michael A, Haase C, Weidinger G, Reimer MM, Becker T, Becker CG. Wnt signaling controls pro-regenerative collagen XII in functional spinal cord regeneration in zebrafish. Nat Commun. 2017;8(1):126. doi: 10.1038/s41467-017-00143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strand NS, Hoi KK, Phan TMT, Ray CA, Berndt JD, Moon RT. Wnt/beta-catenin signaling promotes regeneration after adult zebrafish spinal cord injury. Biochem Biophys Res Commun. 2016;477(4):952–956. doi: 10.1016/j.bbrc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Park JH, Min J, Baek SR, Kim SW, Kwon IK, Jeon SR. Enhanced neuroregenerative effects by scaffold for the treatment of a rat spinal cord injury with Wnt3a-secreting fibroblasts. Acta Neurochir (Wien) 2013;155(5):809–816. doi: 10.1007/s00701-013-1663-7. [DOI] [PubMed] [Google Scholar]
- 44.Zhang G, Lei F, Zhou Q, Feng D, Bai Y. Combined application of Rho-ROCKII and GSK-3beta inhibitors exerts an improved protective effect on axonal regeneration in rats with spinal cord injury. Mol Med Rep. 2016;14(6):5180–5188. doi: 10.3892/mmr.2016.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renault-Mihara F, Katoh H, Ikegami T, Iwanami A, Mukaino M, Yasuda A, Nori S, Mabuchi Y, Tada H, Shibata S, Saito K, Matsushita M, Kaibuchi K, Okada S, Toyama Y, Nakamura M, Okano H. Beneficial compaction of spinal cord lesion by migrating astrocytes through glycogen synthase kinase-3 inhibition. EMBO Mol Med. 2011;3(11):682–696. doi: 10.1002/emmm.201100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cuzzocrea S, Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Collin M, Esposito E, Bramanti P, Thiemermann C. Glycogen synthase kinase-3 beta inhibition reduces secondary damage in experimental spinal cord trauma. J Pharmacol Exp Ther. 2006;318(1):79–89. doi: 10.1124/jpet.106.102863. [DOI] [PubMed] [Google Scholar]
- 47.Hollis ER, 2nd, Ishiko N, Yu T, Lu CC, Haimovich A, Tolentino K, Richman A, Tury A, Wang SH, Pessian M, Jo E, Kolodkin A, Zou Y. Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat Neurosci. 2016;19(5):697–705. doi: 10.1038/nn.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin ZS, Zu B, Chang J, Zhang H. Repair effect of Wnt3a protein on the contused adult rat spinal cord. Neurol Res. 2008;30(5):480–486. doi: 10.1179/174313208X284133. [DOI] [PubMed] [Google Scholar]
- 49.L'Episcopo F, Serapide MF, Tirolo C, Testa N, Caniglia S, Morale MC, Pluchino S, Marchetti B. A Wnt1 regulated frizzled-1/beta-catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: therapeutical relevance for neuron survival and neuroprotection. Mol Neurodegener. 2011;6:49. doi: 10.1186/1750-1326-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez P, Rodriguez FJ. Analysis of the expression of the Wnt family of proteins and its modulatory role on cytokine expression in non activated and activated astroglial cells. Neurosci Res. 2016;114:16–29. doi: 10.1016/j.neures.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 51.L'Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Cossetti C, D'Adamo P, Zardini E, Andreoni L, Ihekwaba AE, Serra PA, Franciotta D, Martino G, Pluchino S, Marchetti B. Reactive astrocytes and Wnt/beta-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Neurobiol Dis. 2011;41(2):508–527. doi: 10.1016/j.nbd.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu L, Guan Y, Wu X, Chen Y, Liu Z, Du H, Wang X. Wnt Signaling is altered by spinal cord neuronal dysfunction in amyotrophic lateral sclerosis transgenic mice. Neurochem Res. 2013;38(9):1904–1913. doi: 10.1007/s11064-013-1096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Fernandez C, Mancuso R, Del Valle J, Navarro X, Rodriguez FJ. Wnt signaling alteration in the spinal cord of amyotrophic lateral sclerosis transgenic mice: special focus on frizzled-5 cellular expression pattern. PLoS ONE. 2016;11(5):e0155867. doi: 10.1371/journal.pone.0155867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S, Guan Y, Chen Y, Li X, Zhang C, Yu L, Zhou F, Wang X. Role of Wnt1 and Fzd1 in the spinal cord pathogenesis of amyotrophic lateral sclerosis-transgenic mice. Biotechnol Lett. 2013;35(8):1199–1207. doi: 10.1007/s10529-013-1199-1. [DOI] [PubMed] [Google Scholar]
- 55.Matei N, Camara J, McBride D, Camara R, Xu N, Tang J, Zhang JH. Intranasal Wnt3a attenuates neuronal apoptosis through Frz1/PIWIL1a/FOXM1 pathway in MCAO rats. J Neurosci. 2018;38(30):6787–6801. doi: 10.1523/JNEUROSCI.2352-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Bao DJ, Xu B, Cheng CD, Dong YF, Wei XP, Niu CS. Neuroprotection mediated by the Wnt/Frizzled signaling pathway in early brain injury induced by subarachnoid hemorrhage. Neural Regen Res. 2019;14(6):1013–1024. doi: 10.4103/1673-5374.250620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chacon MA, Varela-Nallar L, Inestrosa NC. Frizzled-1 is involved in the neuroprotective effect of Wnt3a against Abeta oligomers. J Cell Physiol. 2008;217(1):215–227. doi: 10.1002/jcp.21497. [DOI] [PubMed] [Google Scholar]
- 58.Dijksterhuis JP, Petersen J, Schulte G. WNT/Frizzled signalling: receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3. Br J Pharmacol. 2014;171(5):1195–1209. doi: 10.1111/bph.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gazit A, Yaniv A, Bafico A, Pramila T, Igarashi M, Kitajewski J, Aaronson SA. Human frizzled 1 interacts with transforming Wnts to transduce a TCF dependent transcriptional response. Oncogene. 1999;18(44):5959–5966. doi: 10.1038/sj.onc.1202985. [DOI] [PubMed] [Google Scholar]
- 60.Kajiwara K, Kamamoto M, Ogata S, Tanihara M. A synthetic peptide corresponding to residues 301–320 of human Wnt-1 promotes PC12 cell adhesion and hippocampal neural stem cell differentiation. Peptides. 2008;29(9):1479–1485. doi: 10.1016/j.peptides.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 61.Chong ZZ, Shang YC, Hou J, Maiese K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010;3(2):153–165. doi: 10.4161/oxim.3.2.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J, Gotz S, Vogt Weisenhorn DM, Simeone A, Wurst W, Prakash N. A WNT1-regulated developmental gene cascade prevents dopaminergic neurodegeneration in adult En1(+/−) mice. Neurobiol Dis. 2015;82:32–45. doi: 10.1016/j.nbd.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 63.Giry-Laterriere M, Verhoeyen E, Salmon P. Lentiviral vectors. Methods Mol Biol. 2011;737:183–209. doi: 10.1007/978-1-61779-095-9_8. [DOI] [PubMed] [Google Scholar]
- 64.Barde I, Salmon P, Trono D (2010) Production and titration of lentiviral vectors. Curr Protoc Neurosci Chapter 4:Unit 4 21. 10.1002/0471142301.ns0421s53 [DOI] [PubMed]
- 65.Fernandez-Martos CM, Gonzalez P, Rodriguez FJ. Acute leptin treatment enhances functional recovery after spinal cord injury. PLoS ONE. 2012;7(4):e35594. doi: 10.1371/journal.pone.0035594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Ovejero D, Arevalo-Martin A, Paniagua-Torija B, Florensa-Vila J, Ferrer I, Grassner L, Molina-Holgado E. The ependymal region of the adult human spinal cord differs from other species and shows ependymoma-like features. Brain. 2015;138(Pt 6):1583–1597. doi: 10.1093/brain/awv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyronet D, Dorey A, Massoma P, Rey C, Alix E, Silva K, Perrin C, Quadrio I, Perret-Liaudet A, Streichenberger N, Thomasset N, Honnorat J, Arzberger T, Kretzschmar H. The workflow from post-mortem human brain sampling to cell microdissection: a Brain Net Europe study. J Neural Transm (Vienna) 2015;122(7):975–991. doi: 10.1007/s00702-015-1378-4. [DOI] [PubMed] [Google Scholar]
- 68.Paniagua-Torija B, Arevalo-Martin A, Molina-Holgado E, Molina-Holgado F, Garcia-Ovejero D. Spinal cord injury induces a long-lasting upregulation of interleukin-1beta in astrocytes around the central canal. Neuroscience. 2015;284:283–289. doi: 10.1016/j.neuroscience.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 69.Hong LTA, Kim YM, Park HH, Hwang DH, Cui Y, Lee EM, Yahn S, Lee JK, Song SC, Kim BG. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat Commun. 2017;8(1):533. doi: 10.1038/s41467-017-00583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139(2):244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 71.Hamers FP, Koopmans GC, Joosten EA. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J Neurotrauma. 2006;23(3–4):537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- 72.Hamers FP, Lankhorst AJ, van Laar TJ, Veldhuis WB, Gispen WH. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J Neurotrauma. 2001;18(2):187–201. doi: 10.1089/08977150150502613. [DOI] [PubMed] [Google Scholar]
- 73.Batka RJ, Brown TJ, McMillan KP, Meadows RM, Jones KJ, Haulcomb MM. The need for speed in rodent locomotion analyses. Anat Rec (Hoboken) 2014;297(10):1839–1864. doi: 10.1002/ar.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leszczynska AN, Majczynski H, Wilczynski GM, Slawinska U, Cabaj AM. Thoracic hemisection in rats results in initial recovery followed by a late decrement in locomotor movements, with changes in coordination correlated with serotonergic innervation of the ventral horn. PLoS ONE. 2015;10(11):e0143602. doi: 10.1371/journal.pone.0143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4(12):a007880. doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mardones MD, Andaur GA, Varas-Godoy M, Henriquez JF, Salech F, Behrens MI, Couve A, Inestrosa NC, Varela-Nallar L. Frizzled-1 receptor regulates adult hippocampal neurogenesis. Mol Brain. 2016;9:29. doi: 10.1186/s13041-016-0209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Y, Zhang Q, Xi J, Xiao B, Li Y, Ma C. Neuroprotective effect of fasudil on inflammation through PI3K/Akt and Wnt/beta-catenin dependent pathways in a mice model of Parkinson's disease. Int J Clin Exp Pathol. 2015;8(3):2354–2364. [PMC free article] [PubMed] [Google Scholar]
- 78.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19(6):1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bournat JC, Brown AM, Soler AP. Wnt-1 dependent activation of the survival factor NF-kappaB in PC12 cells. J Neurosci Res. 2000;61(1):21–32. doi: 10.1002/1097-4547(20000701)61:1<21::AID-JNR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 80.Wei L, Sun C, Lei M, Li G, Yi L, Luo F, Li Y, Ding L, Liu Z, Li S, Xu P. Activation of Wnt/beta-catenin pathway by exogenous Wnt1 protects SH-SY5Y cells against 6-hydroxydopamine toxicity. J Mol Neurosci. 2013;49(1):105–115. doi: 10.1007/s12031-012-9900-8. [DOI] [PubMed] [Google Scholar]
- 81.Simons M, Nave KA. Oligodendrocytes: myelination and axonal support. Cold Spring Harb Perspect Biol. 2015;8(1):a020479. doi: 10.1101/cshperspect.a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levine J. The reactions and role of NG2 glia in spinal cord injury. Brain Res. 2016;1638(Pt B):199–208. doi: 10.1016/j.brainres.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hackett AR, Lee JK. Understanding the NG2 glial scar after spinal cord injury. Front Neurol. 2016;7:199. doi: 10.3389/fneur.2016.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karimi-Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol. 2012;46(2):251–264. doi: 10.1007/s12035-012-8287-4. [DOI] [PubMed] [Google Scholar]
- 86.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12(7):388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 87.Zhou X, He X, Ren Y. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen Res. 2014;9(20):1787–1795. doi: 10.4103/1673-5374.143423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Onishi K, Hollis E, Zou Y. Axon guidance and injury-lessons from Wnts and Wnt signaling. Curr Opin Neurobiol. 2014;27:232–240. doi: 10.1016/j.conb.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hollis ER., 2nd Axon guidance molecules and neural circuit remodeling after spinal cord injury. Neurotherapeutics. 2016;13(2):360–369. doi: 10.1007/s13311-015-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garcia AL, Udeh A, Kalahasty K, Hackam AS. A growing field: the regulation of axonal regeneration by Wnt signaling. Neural Regen Res. 2018;13(1):43–52. doi: 10.4103/1673-5374.224359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Filous AR, Schwab JM. Determinants of axon growth, plasticity, and regeneration in the context of spinal cord injury. Am J Pathol. 2018;188(1):53–62. doi: 10.1016/j.ajpath.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O'Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J Clin Investig. 2017;127(9):3259–3270. doi: 10.1172/JCI90608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghosh M, Pearse DD. The role of the serotonergic system in locomotor recovery after spinal cord injury. Front Neural Circuits. 2014;8:151. doi: 10.3389/fncir.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 95.Zhang YK, Huang ZJ, Liu S, Liu YP, Song AA, Song XJ. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Investig. 2013;123(5):2268–2286. doi: 10.1172/JCI65364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi Y, Shu J, Gelman BB, Lisinicchia JG, Tang SJ. Wnt signaling in the pathogenesis of human HIV-associated pain syndromes. J Neuroimmune Pharmacol. 2013;8(4):956–964. doi: 10.1007/s11481-013-9474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ftouh S, Akbar MT, Hirsch SR, de Belleroche JS. Down-regulation of Dickkopf 3, a regulator of the Wnt signalling pathway, in elderly schizophrenic subjects. J Neurochem. 2005;94(2):520–530. doi: 10.1111/j.1471-4159.2005.03239.x. [DOI] [PubMed] [Google Scholar]
- 98.Riise J, Plath N, Pakkenberg B, Parachikova A. Aberrant Wnt signaling pathway in medial temporal lobe structures of Alzheimer's disease. J Neural Transm (Vienna) 2015;122(9):1303–1318. doi: 10.1007/s00702-015-1375-7. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Z, Schittenhelm J, Guo K, Buhring HJ, Trautmann K, Meyermann R, Schluesener HJ. Upregulation of frizzled 9 in astrocytomas. Neuropathol Appl Neurobiol. 2006;32(6):615–624. doi: 10.1111/j.1365-2990.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 100.Miyaoka T, Seno H, Ishino H. Increased expression of Wnt-1 in schizophrenic brains. Schizophr Res. 1999;38(1):1–6. doi: 10.1016/S0920-9964(98)00179-0. [DOI] [PubMed] [Google Scholar]
- 101.Bruggink KA, Kuiperij HB, Gloerich J, Otte-Holler I, Rozemuller AJ, Claassen JA, Kusters B, Verbeek MM. Dickkopf-related protein 3 is a potential Abeta-associated protein in Alzheimer's disease. J Neurochem. 2015;134(6):1152–1162. doi: 10.1111/jnc.13216. [DOI] [PubMed] [Google Scholar]
- 102.Kim Y, Hong M, Do IG, Ha SY, Lee D, Suh YL. Wnt5a, Ryk and Ror2 expression in glioblastoma subgroups. Pathol Res Pract. 2015;211(12):963–972. doi: 10.1016/j.prp.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 103.Wu W, Tian Y, Wan H, Song Y, Li J, Zhang L. The expressions of Wnt/beta-catenin pathway-related components in brainstem gliomas. Can J Neurol Sci. 2013;40(3):355–360. doi: 10.1017/S031716710001430X. [DOI] [PubMed] [Google Scholar]
- 104.Yang Z, Wang Y, Fang J, Chen F, Liu J, Wu J. Expression and aberrant promoter methylation of Wnt inhibitory factor-1 in human astrocytomas. J Exp Clin Cancer Res. 2010;29:26. doi: 10.1186/1756-9966-29-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seo DK, Kim JH, Min J, Yoon HH, Shin ES, Kim SW, Jeon SR. Enhanced axonal regeneration by transplanted Wnt3a-secreting human mesenchymal stem cells in a rat model of spinal cord injury. Acta Neurochir (Wien) 2017;159(5):947–957. doi: 10.1007/s00701-017-3097-0. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J, Li S, Wu Y. Recovery of spinal cord injury following electroacupuncture in rats through enhancement of Wnt/beta-catenin signaling. Mol Med Rep. 2017;16(2):2185–2190. doi: 10.3892/mmr.2017.6801. [DOI] [PubMed] [Google Scholar]
- 107.Shinozuka T, Takada R, Yoshida S, Yonemura S, Takada S (2019) Wnt produced by stretched roof-plate cells is required for the promotion of cell proliferation around the central canal of the spinal cord. Development 146(2):dev159343. 10.1242/dev.159343 [DOI] [PubMed]
- 108.Alizadeh A, Karimi-Abdolrezaee S. Microenvironmental regulation of oligodendrocyte replacement and remyelination in spinal cord injury. J Physiol. 2016;594(13):3539–3552. doi: 10.1113/JP270895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Papastefanaki F, Matsas R. From demyelination to remyelination: the road toward therapies for spinal cord injury. Glia. 2015;63(7):1101–1125. doi: 10.1002/glia.22809. [DOI] [PubMed] [Google Scholar]
- 110.Gaesser JM, Fyffe-Maricich SL. Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp Neurol. 2016;283(Pt B):501–511. doi: 10.1016/j.expneurol.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tawk M, Makoukji J, Belle M, Fonte C, Trousson A, Hawkins T, Li H, Ghandour S, Schumacher M, Massaad C. Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J Neurosci. 2011;31(10):3729–3742. doi: 10.1523/JNEUROSCI.4270-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jahn O, Tenzer S, Werner HB. Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol. 2009;40(1):55–72. doi: 10.1007/s12035-009-8071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Greer JM, Lees MB. Myelin proteolipid protein—the first 50 years. Int J Biochem Cell Biol. 2002;34(3):211–215. doi: 10.1016/S1357-2725(01)00136-4. [DOI] [PubMed] [Google Scholar]
- 114.Kikuchi A, Yamamoto H, Sato A, Matsumoto S. New insights into the mechanism of Wnt signaling pathway activation. Int Rev Cell Mol Biol. 2011;291:21–71. doi: 10.1016/B978-0-12-386035-4.00002-1. [DOI] [PubMed] [Google Scholar]
- 115.Spinsanti P, De Vita T, Caruso A, Melchiorri D, Misasi R, Caricasole A, Nicoletti F. Differential activation of the calcium/protein kinase C and the canonical beta-catenin pathway by Wnt1 and Wnt7a produces opposite effects on cell proliferation in PC12 cells. J Neurochem. 2008;104(6):1588–1598. doi: 10.1111/j.1471-4159.2007.05111.x. [DOI] [PubMed] [Google Scholar]
- 116.Schiavone D, Dewilde S, Vallania F, Turkson J, Di Cunto F, Poli V. The RhoU/Wrch1 Rho GTPase gene is a common transcriptional target of both the gp130/STAT3 and Wnt-1 pathways. Biochem J. 2009;421(2):283–292. doi: 10.1042/BJ20090061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Curr Neurovasc Res. 2011;8(4):270–285. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kilander MB, Halleskog C, Schulte G. Recombinant WNTs differentially activate beta-catenin-dependent and -independent signalling in mouse microglia-like cells. Acta Physiol (Oxf) 2011;203(3):363–372. doi: 10.1111/j.1748-1716.2011.02324.x. [DOI] [PubMed] [Google Scholar]
- 121.Halleskog C, Mulder J, Dahlstrom J, Mackie K, Hortobagyi T, Tanila H, Kumar Puli L, Farber K, Harkany T, Schulte G. WNT signaling in activated microglia is proinflammatory. Glia. 2011;59(1):119–131. doi: 10.1002/glia.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Halleskog C, Dijksterhuis JP, Kilander MB, Becerril-Ortega J, Villaescusa JC, Lindgren E, Arenas E, Schulte G. Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation. J Neuroinflammation. 2012;9:111. doi: 10.1186/1742-2094-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Halleskog C, Schulte G. WNT-3A and WNT-5A counteract lipopolysaccharide-induced pro-inflammatory changes in mouse primary microglia. J Neurochem. 2013;125(6):803–808. doi: 10.1111/jnc.12250. [DOI] [PubMed] [Google Scholar]
- 124.Chen X, Hao J, Fu T, Liu J, Yu M, He S, Qian R, Zhang F. Temporal and spatial expression of LGR5 after acute spinal cord injury in adult rats. Neurochem Res. 2016;41(10):2645–2654. doi: 10.1007/s11064-016-1977-y. [DOI] [PubMed] [Google Scholar]
- 125.Endo M, Ubulkasim G, Kobayashi C, Onishi R, Aiba A, Minami Y. Critical role of Ror2 receptor tyrosine kinase in regulating cell cycle progression of reactive astrocytes following brain injury. Glia. 2017;65(1):182–197. doi: 10.1002/glia.23086. [DOI] [PubMed] [Google Scholar]
- 126.Lutgen V, Narasipura SD, Sharma A, Min S, Al-Harthi L. beta-Catenin signaling positively regulates glutamate uptake and metabolism in astrocytes. J Neuroinflammation. 2016;13(1):242. doi: 10.1186/s12974-016-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ding S, Xu Z, Yang J, Liu L, Huang X, Wang X, Zhuge Q. The involvement of the decrease of astrocytic Wnt5a in the cognitive decline in minimal hepatic encephalopathy. Mol Neurobiol. 2017;54(10):7949–7963. doi: 10.1007/s12035-016-0216-5. [DOI] [PubMed] [Google Scholar]
- 128.Yang J, Zhang X, Wu Y, Zhao B, Liu X, Pan Y, Liu Y, Ding Y, Qiu M, Wang YZ, Zhao G. Wnt/beta-catenin signaling mediates the seizure-facilitating effect of postischemic reactive astrocytes after pentylenetetrazole-kindling. Glia. 2016;64(6):1083–1091. doi: 10.1002/glia.22984. [DOI] [PubMed] [Google Scholar]
- 129.Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33(31):12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oklinski MK, Skowronski MT, Skowronska A, Rutzler M, Norgaard K, Nieland JD, Kwon TH, Nielsen S (2016) Aquaporins in the spinal cord. Int J Mol Sci 17(12). 10.3390/ijms17122050 [DOI] [PMC free article] [PubMed]
- 131.Olsen ML, Campbell SC, McFerrin MB, Floyd CL, Sontheimer H (2010) Spinal cord injury causes a wide-spread, persistent loss of Kir4.1 and glutamate transporter 1: benefit of 17 beta-oestradiol treatment. Brain 133 (Pt 4):1013–1025. 10.1093/brain/awq049 [DOI] [PMC free article] [PubMed]
- 132.Lepore AC, O'Donnell J, Bonner JF, Paul C, Miller ME, Rauck B, Kushner RA, Rothstein JD, Fischer I, Maragakis NJ. Spatial and temporal changes in promoter activity of the astrocyte glutamate transporter GLT1 following traumatic spinal cord injury. J Neurosci Res. 2011;89(7):1001–1017. doi: 10.1002/jnr.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lepore AC, O'Donnell J, Kim AS, Yang EJ, Tuteja A, Haidet-Phillips A, O'Banion CP, Maragakis NJ. Reduction in expression of the astrocyte glutamate transporter, GLT1, worsens functional and histological outcomes following traumatic spinal cord injury. Glia. 2011;59(12):1996–2005. doi: 10.1002/glia.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boyce VS, Mendell LM. Neurotrophic factors in spinal cord injury. Handb Exp Pharmacol. 2014;220:443–460. doi: 10.1007/978-3-642-45106-5_16. [DOI] [PubMed] [Google Scholar]
- 135.Darian-Smith C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist. 2009;15(2):149–165. doi: 10.1177/1073858408331372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu J, Yang X, Jiang L, Wang C, Yang M. Neural plasticity after spinal cord injury. Neural Regen Res. 2012;7(5):386–391. doi: 10.3969/j.issn.1673-5374.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Onishi K, Hollis E, Zou Y. Axon guidance and injury-lessons from Wnts and Wnt signaling. Curr Opin Neurobiol. 2014;27C:232–240. doi: 10.1016/j.conb.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song XJ, Zou Y. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8(9):1151–1159. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- 139.Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, Goffinet A, Zou Y, Pasterkamp RJ. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci. 2010;30(47):16053–16064. doi: 10.1523/JNEUROSCI.4508-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Perrin FE, Noristani HN. Serotonergic mechanisms in spinal cord injury. Exp Neurol. 2019;318:174–191. doi: 10.1016/j.expneurol.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 141.Li L, Hutchins BI, Kalil K (2010) Wnt5a induces simultaneous cortical axon outgrowth and repulsive turning through distinct signaling mechanisms. Sci Signal 3 (147):pt2 [DOI] [PubMed]
- 142.Serradj N, Agger SF, Hollis ER., 2nd Corticospinal circuit plasticity in motor rehabilitation from spinal cord injury. Neurosci Lett. 2017;652:94–104. doi: 10.1016/j.neulet.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 143.Voloshanenko O, Gmach P, Winter J, Kranz D, Boutros M. Mapping of Wnt-Frizzled interactions by multiplex CRISPR targeting of receptor gene families. FASEB J. 2017;31(11):4832–4844. doi: 10.1096/fj.201700144R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dhamdhere GR, Fang MY, Jiang J, Lee K, Cheng D, Olveda RC, Liu B, Mulligan KA, Carlson JC, Ransom RC, Weis WI, Helms JA. Drugging a stem cell compartment using Wnt3a protein as a therapeutic. PLoS ONE. 2014;9(1):e83650. doi: 10.1371/journal.pone.0083650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1 Determination of the primary antibody specificity used to visualize Fz1. This figure shows representative images obtained from non-lesioned (NL) rat spinal cord sections (a1-6), lesioned rat spinal cord sections (7 days post-injury) (b1-6) and NL human spinal cord sections (c1-6) used to determine the specificity of the anti-Fz1 primary antibody. (a1-2), (b1-2) and (c1-2) correspond to images obtained from spinal cord sections processed for the immunohistochemical visualization of Fz1 and used as positive controls (C+); (a3-4), (b3-4) and (c3-4) correspond to images obtained from spinal cord sections processed for the immunohistochemical visualization of Fz1 without the primary antibody and used as negative controls (C-); (a5-6), (b5-6) and (c5-6) correspond to images obtained from spinal cord sections processed for the immunohistochemical visualization of Fz1 with the primary antibody pre-adsorbed with its corresponding blocking peptide (1:20 antibody/blocking peptide weight excess) (1/20). (a), (b) and (c) correspond to schematic drawings of prototypical NL rat, lesioned rat and and NL human spinal cord sections respectively, indicating the approximate areas shown in the corresponding images. Scale bars = 100 µm. (TIF 64125 kb)
Online Resource 2 Determination of the proper functioning of the lentiviral vector generated to overexpress Wnt1. This figure shows the data and representative images obtained from the different studies performed in cultured B1a cells to evaluate the proper functioning of the lentiviral vector generated to overexpress Wnt1. Briefly, to determine the multiplicity of infection (MOI) that should be used to conduct this set of experiments we tested the effects of various MOI (MOI 5, 10, 20 and 40) on different aspects such as cell number (a), percentage of transduced cells (b) and GFP intensity (c) at 3 and 7 days post-transduction (dpt) (n = 3 per group) by flow cytometry. Based in the results obtained we selected MOI 10 to perform the subsequent experiments, since it induced a similar transduction efficiency to that observed with higher MOIs (MOI 20 and 40) (b), led to a high transgene expression (c) and did not affect cell viability and/or proliferation (a). The existence of significant differences between groups in a-c was assessed by Two-way ANOVA followed by Bonferroni post-hoc test (#, ## and ### = p < 0.05, p < 0.01 and p < 0.001 vs. 3 dpt; *, ** and *** = p < 0.05, p < 0.01 and p < 0.001 vs. non-transduced cells (NT); &, && and &&& = p < 0.05, p < 0.01 and p < 0.001 vs. the immediately lower MOI). We next determined by Real Time PCR and western blot that, in comparison with NT cells and those transduced with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP), transduction with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1) induced a robust increase in Wnt1 expression at both the mRNA (d, n = 3 per group) and protein levels (e, n = 3 per group) at 7 dpt, when we have previously observed the maximum transduction efficiency (b) and transgene expression (c). The existence of statistically significant differences between groups in e was assessed by One-way ANOVA followed by Bonferroni post-hoc test (*** represent p < 0.001 vs. NT; $$$ represent p < 0.001 vs. LV-GFP). It should be noted that Wnt1 is a soluble protein that acts in both autocrine and paracrine fashions and, thus, its correct secretion to the extracellular environment is a critical step to exert its multiple biological activities. In this regard, we found that the protein levels of Wnt1 were also strongly upregulated in the culture medium of cells transduced with LV-Wnt1 in comparison with those of NT and cells transduced with LV-GFP (f, n = 3 per group), demonstrating that Wnt1 was conveniently secreted to the extracellular space. We next aimed to evaluate whether overexpressed Wnt1 was able to activate the canonical Wnt/β-catenin signaling pathway which is the main Wnt signaling pathway that is activated by Wnt1. For this purpose, we determined that incubation of cell cultures with recombinant Wnt1 (100 and 200 ng/ml) (120-17, Peprotech) during 1 h led to the activation of the canonical Wnt/β-catenin signaling pathway (g) (n = 3 per group and time of incubation). The existence of significant differences between groups in g was assessed by Two-way ANOVA followed by Bonferroni post-hoc test (* = p < 0.05 vs. control). Finally, we found that, in comparison with cell cultures incubated with conditioned medium from cells transduced with LV-GFP (1:5) (CM LV-GFP), cell cultures incubated during 1 h with conditioned medium from cells cultures transduced with LV-Wnt1 (1:5) (CM LV-Wnt1) induced a similar activation of this signaling pathway than that observed in cell cultures incubated during 1 h with conditioned medium from cell cultures transduced with LV-GFP (1:5) and supplemented with 200 ng/ml of recombinant Wnt1 (CM LV-GFP+ Wnt1) (h, n = 3 per group), demonstrating that overexpressed Wnt1 is biologically active. The existence of significant differences between groups in h was assessed by One-way ANOVA followed by Bonferroni post-hoc test (* = p < 0.05 vs. CM LV-GFP). In all cases, data are presented as the mean + SEM. (TIF 4260 kb)
Online Resource 3 Determination of the injection method of lentiviral vectors in vivo. This figure shows the results obtained from the experiments performed to establish the in vivo intraparenchymal injection method of the lentiviral vectors in the lesioned rat spinal cord. (a) and (b) shows schematic drawings from the two methods tested. In the first one (3ip), one injection per rostro-caudal level (stereotaxic coordinates: 0 mm lateral and 1 mm depth) was performed in three rostro-caudal levels corresponding to the lesion epicenter, 1.5 mm rostral and 1.5 mm caudal (a). In the second one (6ip), two injections per rostro-caudal level (stereotaxic coordinates: 0.6/-0.6 mm lateral and 1 mm depth) were performed in the same rostro-caudal levels detailed above (b). Evaluation of transduction at 7 days post-injury showed that both injection methods induced a similar transduction in longitudinal sections corresponding to the median line, where transduced green fluorescence protein (GFP)+ cells were observed surrounding the lesioned area, mainly rostral and caudal to the lesion epicenter, and to a lesser extent in the injury site (c-e) (non-injected (NT) group, n = 2; 3ip group, n = 3; 6ip group, n = 3) (arrows in d and e point the areas where GFP+ cells were observed). However, those animals that received 6 injections of lentiviral particles displayed a wider lateral distribution of transduced GFP+ cells than those that received 3 injections (f). As a consequence, we selected the second injection method for the subsequent experiments performed to evaluate the effects exerted by Wnt1 overexpression in the traumatically injured spinal cord. Scale bars in c-e represent 1 mm. (TIF 7420 kb)
Online Resource 4 Evaluation of transduction at 7 days after SCI. This figure shows representative images from the evaluation of transduction at 7 days post-injury in those animals used to evaluate the effects of Wnt1 overexpression after SCI, which were injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP control group) (n = 5) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1 group) (n = 5). (a), shows a schematic drawing of a prototypical lesioned longitudinal spinal cord section and indicates the approximate rostro-caudal levels where the representative images were obtained. b, b1, c, c1, d, d1, e, e1, f and f1 shows representative images from the LV-GFP control group, while g, g1, h, h1, i, i1, j, j1, k and k1 show representative images from the LV-Wnt1 group. Squares in a-k indicate the areas shown in the corresponding higher magnification images. Arrows in a1-k1 point to the cells shown in the corresponding insets. Scale bars in a-k represent 500 µm, while those in a1-k1 and in their corresponding insets represent 100 and 20 µm, respectively. (TIF 17121 kb)
Online Resource 5 Evaluation of transduction at 14 days after SCI. This figure shows representative images from the evaluation of transduction at 14 days post-injury in those animals used to evaluate the effects of Wnt1 overexpression after SCI, which were injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP control group) (n = 5) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1 group) (n = 5). (a), shows a schematic drawing of a prototypical lesioned longitudinal spinal cord section and indicates the approximate rostro-caudal levels where the representative images were obtained. b, b1, c, c1, d, d1, e, e1, f and f1 shows representative images from the LV-GFP control group, while g, g1, h, h1, i, i1, j, j1, k and k1 show representative images from the LV-Wnt1 group. Squares in a-k indicate the areas shown in the corresponding higher magnification images. Arrows in a1-k1 point to the cells shown in the corresponding insets. Scale bars in a-k represent 500 µm, while those in a1-k1 and in their corresponding insets represent 100 and 20 µm, respectively. (TIF 12872 kb)
Online Resource 6 Evaluation of transduction at 126 days after SCI. This figure shows representative images from the evaluation of transduction at 126 days post-injury in those animals used to evaluate the effects of Wnt1 overexpression after SCI, which were injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP control group) (n = 10) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1 group) (n = 7). (a), shows a schematic drawing of a prototypical lesioned longitudinal spinal cord section and indicates the approximate rostro-caudal levels where the representative images were obtained. b, b1, c, c1, d, d1, e, e1, f and f1 shows representative images from the LV-GFP control group, while g, g1, h, h1, i, i1, j, j1, k and k1 show representative images from the LV-Wnt1 group. Squares in a-k indicate the areas shown in the corresponding higher magnification images. Arrows in a1-k1 point to the cells shown in the corresponding insets. Scale bars in a-k represent 500 µm, while those in a1-k1 and in their corresponding insets represent 100 and 20 µm, respectively. (TIF 17167 kb)
Online Resource 7 Separate analysis of different individual aspects of locomotion analyzed in the 21-point BBB open-field test. This figure shows data obtained from the separate analysis of different individual aspects of locomotion such as coordination (a), paw positions (b), tail position (c), stepping (d), and toe clearance (e), which were evaluated using the 21-point BBB open-field test. The analysis was performed in lesioned animals injected with a lentiviral vector that only induce the expression of the green fluorescence protein (GFP) (LV-GFP control group) or with a lentiviral vector that induce the expression of both GFP and Wnt1 (LV-Wnt1 group) at 1, 3, 7, 14, 21, 35, 49, 63, 77, 91, 105 and 119 days post-injury (dpi). Data obtained from all animals used to evaluate the effects exerted by Wnt1 overexpression, which were sacrificed at 7 (LV-GFP group, n = 5; LV-Wnt1 group, n = 5), 14 (LV-GFP group, n = 5; LV-Wnt1 group, n = 5) and 126 dpi (LV-GFP group, n = 10; LV-Wnt1 group, n = 7), were included in the analysis. The existence of statistically significant differences between groups was assessed by Two-way ANOVA followed by Bonferroni post-hoc test. Data are presented as the mean + SEM. *, ** and *** represent p < 0.05, p < 0.01 and p < 0.001 vs. LV-GFP. (TIF 4261 kb)