Abstract
ROS (superoxide and oxygen peroxide in this paper) play a dual role as signalling molecules and strong oxidizing agents leading to oxidative stress. Their production mainly occurs in mitochondria although they may have other locations (such as NADPH oxidase in particular cell types). Mitochondrial ROS production depends in an interweaving way upon many factors such as the membrane potential, the cell type and the respiratory substrates. Moreover, it is experimentally difficult to quantitatively assess the contribution of each potential site in the respiratory chain. To overcome these difficulties, mathematical models have been developed with different degrees of complexity in order to analyse different physiological questions ranging from a simple reproduction/simulation of experimental results to a detailed model of the possible mechanisms leading to ROS production. Here, we analyse experimental results concerning ROS production including results still under discussion. We then critically review the three models of ROS production in the whole respiratory chain available in the literature and propose some direction for future modelling work.
Keywords: ROS, Superoxide, Oxygen peroxide, Respiratory chain, Modelling
Introduction
Accumulating evidence has suggested that reactive oxygen species (ROS), such as superoxide, hydrogen peroxide and other reactive forms of oxygen, play an important role in a broad range of cellular signalling processes [1–4]. However, at high concentrations, ROS damage proteins, lipid membranes, DNA and triggers PTP opening [5, 6] generating what is called oxidative stress. Oxidative stress is defined as a perturbation in the balance between the production of reactive oxygen species (free radicals) and antioxidant defences and contributes to pathologies such as cancer, ischemic cardiac injury and stroke, neurodegenerative diseases and other age-related degenerative conditions [7, 8]. Given their deleterious effects, ROS production is usually finely tuned by ROS-scavenging systems.
The mitochondrial electron transport chain is one of the major providers of ROS in most cells. In C2C12 myoblasts, Wong et al. [9] show that 45% of ROS comes from mitochondria and 40% from NADPH oxidase. Work by many investigators (see [10] for a review) has largely established that complexes I and III of the mitochondrial respiratory chain are the major sources of reactive oxygen species (ROS), in the form of superoxide (O•−2) and hydrogen peroxide H2O2. However, despite intensive biochemical and biophysical studies of electron and proton transfer in the respiratory chain (for reviews, see [9, 11–15]) many questions about the mechanisms of O•−2 generation, particularly in physiological conditions, remain unsolved.
The production of superoxide/hydrogen peroxide is difficult to assess, particularly their site of production and their dependence upon the experimental conditions (respiratory substrate, inhibitors). Furthermore, when working with the whole respiratory chain in isolated mitochondria or in whole cells, it is difficult to assess the relative contribution of each separate site and to take into account contribution of the scavenging systems. This is why theoretical models of ROS generation can be useful to facilitate the quantitative analysis of the features controlling mitochondrial O•−2 production and help in the elucidation of experimental results and eventually predict new discriminant experimental protocols. Several theoretical models of ROS production by complex I and III exist [16–21] and also one involving complex II [22]. However, we found only three theoretical models in the literature aiming at describing ROS production by the whole respiratory chain under different conditions. First, we will describe the main experimental results concerning ROS production for which a large consensus exists, and that theoretical models should reproduce, as well as experimental results leading to contradictory hypotheses, between which theoretical models might help to decide. We will emphasize the main points that a theoretical model must explain/simulate and finally we will proceed to the critical description of the three theoretical models.
Experimental data: the different sites of ROS production and the role of inhibitors
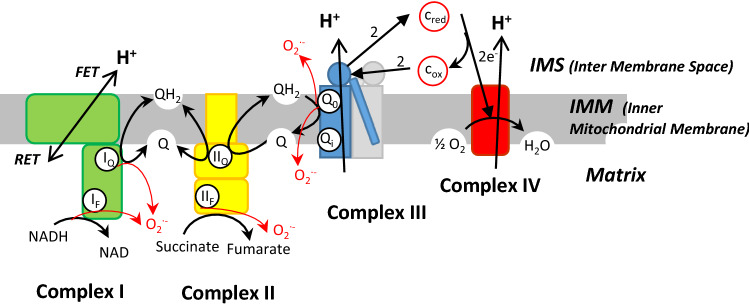
Using specific inhibitors of different sites of ROS production (superoxide and/or hydrogen peroxide), particularly inhibitors that do not prevent electron flow and varying the respiratory substrates, the group of Martin Brand has finely dissected the different sites of mitochondrial ROS production [9, 10]. They identified eleven distinct sites associated with respiratory complexes or enzymes and they gave an estimation of the maximal ROS production flux for each site. If we limit our description to the respiratory chain complexes (Fig. 1), the main producers are: the Flavin site of complex I (IF), the ubiquinone reducing site of complex I (IQ), the Flavin site of complex II (IIF) and the ubiquinone oxidizing site of complex III (IIIQo), (see Fig. 1 in [10] and Fig. 1b in [23]). Site IIIQo has, at least, twice the capacity of any other site (see Fig. 2 in [10]).
Fig. 1.
Possible sites of ROS production (red arrows) in respiratory chain. In the text, the site Qo and Qi of complex III are called IIIQo and IIIQi. All ROS are generated in the matrix except for ROS in IIIQo which are partly extruded in the IMS. FET forward electron transport (with NADH), RET reverse electron transport (with succinate)
ROS production by complex I
Site IF has long been proposed as a site of superoxide production [24, 25]. However, Brand et al. [10, 26] show that much of the ROS production previously ascribed to site IF truly arises from other dehydrogenases, particularly sites OF (2-oxoglutarate dehydrogenase) and PF (pyruvate dehydrogenase).
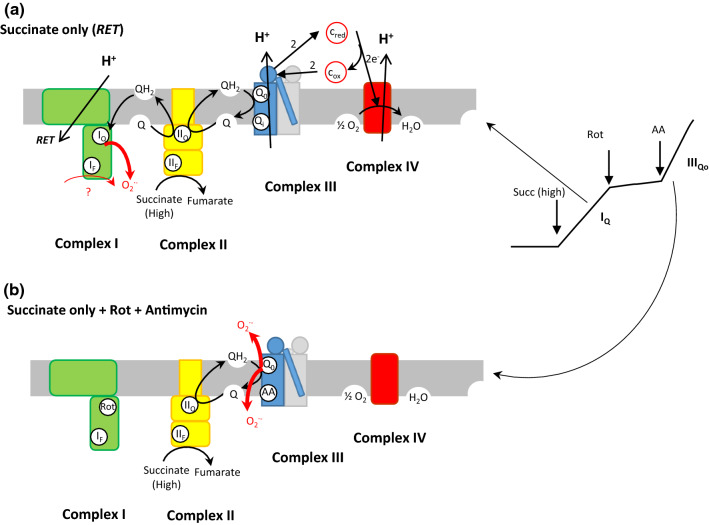
Site IQ. During reverse electron transport (RET), the majority of ROS arises from site IQ. In RET (Fig. 2a), electrons are forced back into complex I by the high QH2/Q ratio and the high proton motive force generated by electron flow through complexes III and IV. In this process, the electron flow is driven backwards by the consumption of proton motive force. Thus, production of ROS at site IQ during reverse electron transport has a strong dependence on proton motive force [27–32]. However, several authors showed that it is much more sensitive to the magnitude of the pH gradient than of the membrane potential, even at constant proton motive force [30, 31, 33]. These results suggest that site IQ is linked to an electroneutral proton-translocating step in the proton-pumping mechanism of complex I [34, 35]. The localization at site IQ is confirmed by the inhibition of ROS production at this site by rotenone. Then addition of antimycin stimulates ROS production at site IIIQo (Figs. 2b, 4).
Fig. 2.
ROS production in the respiratory chain by complex I and III with succinate. a RET results from the addition of succinate alone at high concentration. The thickness of the red arrows corresponds to the first addition of succinate without any inhibitor. Rotenone inhibits this ROS production. b High ROS production by complex III in the presence of antimycin A (AA) [a similar ROS production occurs at complex III with antimycin when electrons are supplied by NADH in complex I in the absence of succinate and rotenone (Rot)]
Fig. 4.
Modified Q-cycle and ROS generation in complex III. The mechanism of modified Q-cycle [41, 42] involves the bifurcation of electrons in Qo. The first electron (green pathway) reduces a FeS centre in the head of the Rieske protein which moves towards c1 (dotted green arrows). An unstable semiquinone (SQ) can be formed before the second electron (blue pathway) is transferred to a Q molecule in Qi to form a stable semiquinone (bold SQ). A second cycle of QH2 oxidation in Qo is necessary to completely reduce the SQ in Qi in a new QH2 molecule. After the two cycles, two Cytox have been reduced in two Cytcred and a net QH2 has been oxidized to Q
ROS production by complex II
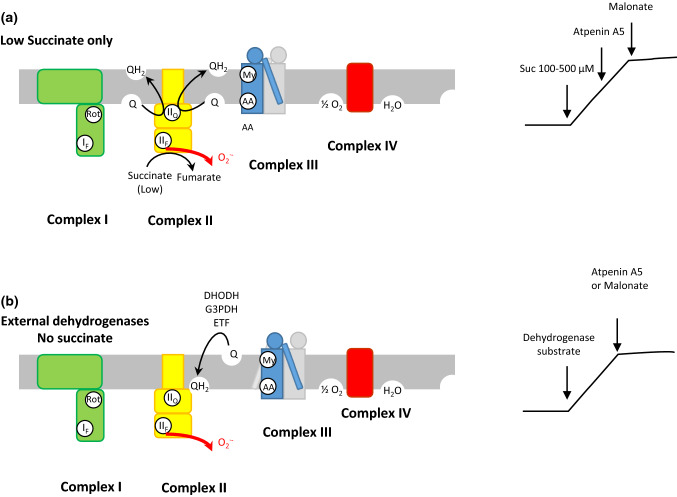
The Flavin site IIF (Fig. 3) is supposed to be the site of ROS production in complex II and displays a similar maximal capacity of ROS production as site IQ [10]. However, Grivennikova et al. [22] claim that their data are indicative of the [3Fe–4S] centre, close to the ubiquinone reduction site, as the site of superoxide generation in this complex. Wild-type complex II makes little contribution to ROS production in isolated mammalian mitochondria under normal conditions [36]. However, mutations in this complex can lead to abundant ROS production and cause pathologies [37]. ROS production by the Flavin site of complex II, site IIF, in isolated mitochondria [23] requires two conditions: there must be a source of electrons to reduce the Flavin (succinate), and the site must be open, probably to allow access of oxygen [10].
Fig. 3.
ROS production at site IIF (a), complex II in the presence of rotenone, myxothiazol and antimycine generates ROS in the forward reaction: succinate around its KM (100–500 µM) supplies electrons to reduce Q to QH2 which accumulates and blocks electron pathway generating ROS in IIF. Malonate or high succinate concentrations cancel ROS formations but not Atpenin A5 binding in IIQ. b ROS production by complex II in the reverse reaction with electrons supplied by other dehydrogenases to accumulate QH2 and provide electrons in IIF to form ROS. In these conditions, ROS formation is inhibited by Atpenin A5 or Malonate or high succinate concentrations (after [10, 23])
This behaviour results in a bell-shaped response of ROS production to succinate concentration, with a maximum in the region of the KM of complex II for succinate (100–500 µM) [22, 23, 38]. The capacity for ROS production at site IIF can be measured in two particular conditions illustrated in Fig. 3 in the presence of inhibitors of complexes I and III [10]. In rat skeletal muscle mitochondria, the maximum capacity for ROS production of site IIF is very high, of the same order as site IQ (Fig. 2 in [10]).
ROS production by complex III (bc1 complex) at site IIIQo
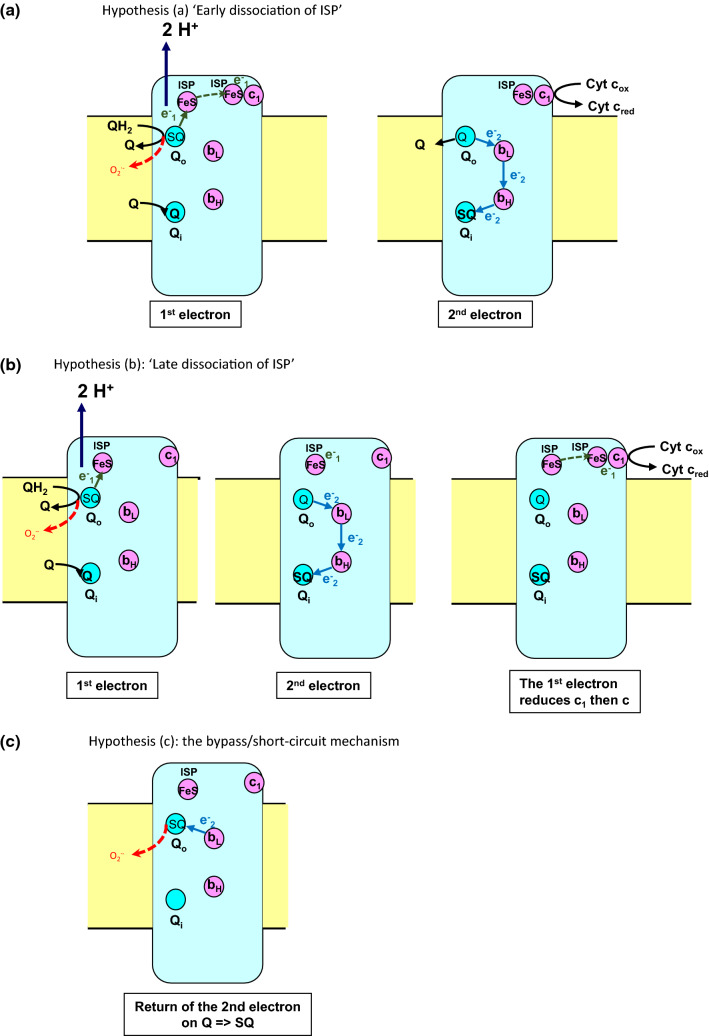
Complex III (Fig. 4) at site Qo (IIIQO site) has the highest capacity of ROS production (Fig. 2 in [10]). It is commonly accepted that the complex III ROS production is due to the formation during the catalytic process of an instable semiquinone SQ in Qo. However, the mechanism of a semiquinone formation in Qo is still the matter of controversy, which has to be taken into account for modelling. It is widely accepted that the modified Q-cycle mechanism proposed by Mitchell [39, 40] and subsequently refined by Crofts [41, 42] correctly describes the bc1 complex operation. It is based on a bifurcation of the two electrons coming from the QH2 molecule bound at the Qo site. The first electron is transferred to the iron sulphur protein (ISP) and the second to the lower potential heme bL. The electron on heme bL moves within the cytochrome b to reduce the higher potential heme bH, which in turn reduces ubiquinone (Q) at a second ubiquinone binding site Qi (Fig. 4). The transfer of an electron from quinol to cytochrome c is a complex process involving: (i) a first electron transfer from quinol bound at the catalytic Qo site to a [2Fe–2S] cluster situated in the head of the Rieske iron sulphur protein (ISP) anchored in the inner mitochondrial membrane (ii) a large-scale movement of the head of reduced ISP towards cytochrome c1, (iii) the reduction of cytochrome c1 and eventually, (iv) the reduction of cytochrome c by cytochrome c1 and the return of ISP head to site Qo. How the steps following the transfer of the first electron on ISP interweave with the transfer of the second electron on bL then bH, is still debated. Three main scenarios (Fig. 5) have been proposed which may have implications for the semiquinone formation/lifetime and its reaction with oxygen:
Fig. 5.
ISP movement, transfer of the second electron and ROS formation. The dotted red arrows indicate the possible formation of O•−2 from the semiquinone SQ in Qo a ‘early dissociation hypothesis’: O•−2 formation can occur in the same time as reduced ISP leaves Qo site, i.e. before the second electron is transferred to heme bL.; b ‘late dissociation hypothesis’: reduced ISP leaves Qo site after the second electron is transferred from bL to bH and c return of the electron of reduced bL on a Q molecule in Qo forming the semiquinone which can react with oxygen
a. The ISP leaves the Qo site to reduce c1 before the second electron jumps on bL and then bH [43, 44] (Fig. 5a).
b. The ISP leaves the Qo site to reduce c1 after the second electron transfers from bL to bH [45, 46] (Fig. 5b).
c. A bypass/short-circuit mechanism can occur when reduced bL transfers its electron not on bH but in the reverse direction on a quinone in Qo, which can be either the product of the reaction or of a newly bound quinone [47–49] (Fig. 5c).
Summary of the main points to consider in a mathematical model of mitochondrial ROS generation
The main site of ROS production by the respiratory chain are IIIQO, IQ and IF with a decreased maximum capacity in this order.
In IIIQO and IQ, ROS are produced by the semiquinone Q•−. The ROS production in IF is through the fully reduced FMNH− species.
All ROS species by respiratory chain are produced in the matrix except for IIIQO site, which produces ROS both in the matrix and in the intermembrane space.
ROS production can also occur in the matrix at the FMN site of complex II (IIF) at low succinate concentration around 100–500 µM, with a maximal capacity analogous to the one of IQ.
The relative contributions of distinct mitochondrial sites depend on many factors: the substrates being oxidized, the energetic demands of the cell, the transmembrane potential, the amount of QH2 pool and ultimately the cell type.
Mathematical models of ROS production through the whole respiratory chain
Models of the group of Aon and Cortassa
In [50], Kembro et al. extended previous mathematical models of the mitochondrial respiratory chain [51, 52] to account for ROS production. Their ROS production model is purely phenomenological, with a function called ‘shunt’ which is a small percentage of the rate of respiration (VO2) and depends on the state 3 or 4 of the respiration rate (Table 2 in [50]). In addition, the model involves the important contribution of ROS scavenging systems to study the balance between ROS production and scavenging in different redox environments. Model simulations were compared with experiments from isolated heart mitochondria reported in the same paper. However, in their conclusions the authors note that their model “is unable to simulate the increase in ROS levels when mitochondria evolve into state 4 respiration” [50].
Shortly after, the same authors published a new model [53] which is, above all, a detailed respiratory chain model including variables describing the concentrations of ubiquinone, ubiquinol, and ubisemiquinone, along with the oxidation states of cytochrome c and the redox centres in complex III, i.e., the high- and low-potential b-hemes (bH and bL) and cytochrome c1. They use the forward and reverse rate constants for electron transfer of complexes II–IV from the model of Demin et al. [54, 55]. A model of complex I was based on the non‐equilibrium thermodynamic description from Magnus and Keizer’s mitochondrial model [56]. First, the authors fit the experimental results in which the variables such as bL and bH reduction states [57, 58] are measured because these variables play an important role in semiquinone concentration at the Qo site of complex III. The production of ROS in complex I is supposed to occur from FMNH− according to Pryde and Hirst [59], although the authors test the possibility of ROS production in site IQ (but in the absence of ROS production by FMNH−); they claim that they obtain similar results (not shown) and conclude that “Given that the modelling results for the two different hypotheses were identical, this modelling experiment was unable to distinguish between the two mechanisms” [53]. They also reproduce the pH dependence of ROS production by complex I observed in [33] (Fig. 5 in [53]). The ROS production from complex III is modelled from Demin and involves the semiquinone in Qo, a highly reduced quinone pool and a high proton motive force [54, 55]. The authors were aware of other hypotheses [47, 60] but did not take them into account in their model.
In addition, they added to their model of ROS production a previous minimal model of ROS scavenging [61], which allowed them to draw a U-shaped dependence of the ROS balance between production and scavenging as a function of mitochondrial redox environment (sum of redox potentials for NADH, NADPH and GSH weighted by their respective concentrations [62]): the measured ROS production is high in reducing environment which favours ROS production which cannot be destroyed at limited scavenging mechanisms, but are also high in oxidizing environment, as, despite a lower ROS production, the scavenging mechanisms are also less efficient (less regenerated in oxidizing environment). The intermediate environment corresponds to a minimum in the ROS production.
More recently, the same authors proposed an experimental and theoretical approach to assess the effects of β-oxidation in the heart on redox and energy metabolism [63]. They described the antagonist effects of fatty acids as respiratory substrates but also as uncouplers on the respiration rate (VO2) and on the ROS production rate in relationship with the level of the antioxidant system.
Markevich’s model [64]
In order to study the mitochondrial production of ROS in different conditions of respiratory substrates and membrane potential (Δψ), Markevich and Hoek developed an elaborate computational model of the whole respiratory chain. Complex II, modelled by only one simple rate equation, is not taken into account in their work for ROS production. Electron transfers inside complex I and III are detailed with several sites of ROS production. In accordance with Kussmaul and Hirst [24], they proposed that O•−2 is formed by the transfer of one electron from the fully reduced Flavin FMNH− to O2. In addition, they assumed that the semiquinone Q•− in site IQ is a second site of O•−2 formation in Complex I. Regarding complex III, they considered, as generally accepted, that the unstable semiquinone Q•− in site Qo is the site of O•−2 formation. According to the various mechanisms of electron transfer that have been proposed in Qo (see above), Markevich and Hoek compare three variations of their model. The first one considers that O•−2 formation occurs at the same time as the reduced Rieske protein (Iron Sulphur Protein or ISP) leaves the site Qo to transfer its electron to c1 (‘early dissociation of ISP’, Fig. 5a) i.e. before the second electron is transferred to the heme bL [43, 65]. The second variation suggests that the Rieske protein leaves the Qo site after the transfer of the second electron to bL and then to bH (‘late dissociation of ISP’, Fig. 5b) [46]. In a third variation proposed by Dröse and Brandt [47] they considered that the oxidized quinone can leave the Qo site before ISP and before bL transfers its electron to bH. This may allow the return of the reduced bL electron on an oxidized Q molecule (bypass/short-circuit mechanism also proposed in [48, 49]) by the reversion of the reactions to form a semiquinone which can react with O2 and explain the activating role of the oxidized quinone in O•−2 formation by complex III (Fig. 5c).
The values of the kinetic parameters of superoxide production were chosen such that the computer-simulated rates of ROS generation were close to those observed in liver mitochondria [66, 67].
Their model is detailed enough to dissect ROS production at each site in different experimental conditions, particularly as a function of the transmembrane potential Δψ with different respiratory substrates (NADH alone, succinate alone, NADH+ succinate and NADH+ Rotenone (ROT), Fig. 3 in [64]). The difference in ROS production when pH or Δψ were changed is noteworthy (Fig. 4 in [64]). Furthermore, comparing the different variations of electron transfer in site Qo of complex III, they proposed that the scenario with ‘late dissociation of ISP’ is more likely. Finally, their third variation of the model (with ‘late dissociation of ISP’ and with binding of Q when cytbL is reduced) qualitatively reproduced the results of Dröse and Brand [47] and of Quinlan et al. [19] (see also Fig. 12 in [64]). They dissected this behaviour by analysing the amount of individual species, oxidized Q, reduced bL and the b−L.Q.ISP complex (Fig. 12B in [64]). Their results stressed the necessity of a detailed modelling in the multifaceted field of ROS production by the respiratory chain. Furthermore Markevich and Hoek showed another advantage of modelling, i.e. generating testable hypotheses, some of them were confirmed later on, such as the ROS production by RET in IQ [10]. Incidentally, they offer a careful model of the calculation of the different volumes and concentrations of the different mitochondrial compartments (membrane, matrix, etc.). In fact, this very detailed model is not far from a complete model of the respiratory chain, i.e. the expression of VO2 as a function of different substrates (Fig. 3E in their paper) and can certainly be used to test other hypotheses.
Bazil and Vinnakota’s model [68]
Bazil et al. [68] integrated their previous models of superoxide and hydrogen peroxide production by complexes I [20] and III [69] into an updated Beard’s model of oxidative phosphorylation [70] that can simulate both the respiratory dynamics associated with ATP production and the kinetics of ROS production in a single integrated system. The authors distinguished hydrogen peroxide and superoxide generation.
The authors showed that the kinetic control is distributed and depends upon the experimental conditions as experimentally reported by many authors [71–73] and they fitted experimental results obtained using isolated rat heart mitochondria at low (1 mM) and high (5 mM) Pi concentrations.
They showed that ROS production depends indirectly on Pi concentration through changes in pH and Δψ.
Model simulations predicted that complex III is responsible for more ROS production during physiological working conditions relative to complex I in the condition of forward electron transport, where electrons are transferred along the Electron Transfer Chain (ETC) from NADH and succinate to O2. However, simulating ischemia/reperfusion mechanism, they showed that an accumulation of succinate leading to a highly reduced quinone pool can explain a burst of ROS generated in complex I corroborating the experimental results of Chouchani et al. [8] (who also used a mathematical model of ROS production in complex I [74]). Of note the authors explored the bistability behaviour reported in [16–18] characterized by different rates of ROS production in the same conditions. This is mainly due to their model of ROS production by complex III [69] as emphasized in several complex III models aimed to simulate this phenomenon [16–18, 21].
Conclusion and future prospective
We have analysed in this review the three models currently available for ROS production by the whole respiratory chain. Two of them focused on ROS production in heart [50, 53, 68] in which ROS production during ischemia–reperfusion is a medical concern. The third one by Markevich and Hoek [64] estimated parameters from liver mitochondria but is in fact rather general. It is difficult to compare these models because their aims were different. The models of Aon and Cortassa and of Bazil and Vinnakota tried to simulate in the simplest way possible the experimental results in the heart. Furthermore, Aon and Cortassa studied the physiological interplay of the scavenging mechanisms with ROS production. On the contrary, the Markevich and Hoek model [64] aimed at understanding the intimate mechanisms of ROS production in different conditions. Their model is more detailed than the two others and analysing the consequences of different scenarios for the complex reaction of complex III, proposed a mechanism of the controversial mode of bifurcation of electrons in the Qo site of bc1 complex. This illustrates two purposes of modelling, either to derive a phenomenological simple model which can be used to study physiological question such as the balance between ROS production and scavenging in different redox situations or to test different mechanistic hypotheses of ROS production as done by Markevich and Hoek [64]. However, even in this latter case some simplifications were made. For instance, they bring together almost all FeS centres redox reactions of complex I in one reaction and concerning complex III, they assume the release of reduced ISP from Qo in the same time as the second electron transfer on bL, which is far from acknowledged.
Another hypothesis, made in all models, is the localization of ROS production. A general consensus is emerging that IF, IQ, IIF and IIIQo are the main sites of ROS production in the respiratory chain and there are good evaluations of their maximal capacities [10]. All models take these sites as ROS production sites, excluding site IIF. In addition, some indications exist of the possibility of ROS production at other sites. In principle, any reduced redox centre with midpoint potential close to that of the O2/O•−2 couple (− 160 mV), should be able to produce O•−2 when O2 is (spatially) close enough to accept an electron. The respiratory chain complexes contain iron–sulphur centres and hemes which are, in principle, able to transfer their electrons to O2. The limiting factor will be the distance, the O2 molecule being at the closest at the surface of the protein. This is perhaps the reason why the binding sites, opened on the external medium and allowing O2 to diffuse near the redox active site, are favoured for ROS generation. Although left aside by a number of experimenters, there have been published experiments proposing such redox centres as producing superoxide. For complex I, the iron–sulphur centre N2 which is close to the quinone binding site has been proposed in [75], as well as the iron–sulphur centre N1a which is not very far from the FMN in [76]. The Fe3S4 iron–sulphur centre of complex II which is close to the quinone binding site was proposed as a superoxide producing site [22]. Similarly, it has been proposed that reduced heme bL is able to generate ROS in complex III [77]. One way of approaching this problem without any a priori would be to calculate the probability of reacting with the oxygen of all the redox centres of the respiratory complexes by using the equations developed by Moser and Dutton [78], that considers the distance and the difference in redox potential between the redox centres. Such a stochastic treatment takes into account all possible reactions (i.e. the reactions with a reasonable probability) and all the different oxidized/reduced species, but only when they are produced contrary to what occurs using differential equations (see for instance [79, 80]).
Of note, all models listed above are studied at steady-state. Let us also emphasize that experimental studies of transient phases of ROS production might be more informative on the intimate mechanisms than the simple consideration of steady-states.
To sum up, it appears that a complete model of ROS production by the respiratory chain still remains to be developed by incorporating at least the generation of ROS by the dehydrogenases of the Krebs cycle (including the IIF site of the succinate dehydrogenase) in a deterministic (differential equations) or/and a stochastic approach.
Acknowledgements
This work was supported by the Agence Nationale de la Recherche and the Conseil National de la Recherche Scientifique (CNRS). The authors wish to thank Prof. Michel Rigoulet for careful proofreading of the manuscript and constructive discussions.
Author contributions
JPM conceived and coordinated the study and wrote the paper. SR and AD contributed to the writing of the paper, the design of the figures and the analysis of the literature. All authors approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
The corresponding author declares no conflict of interests on behalf of all authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 2.Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 3.Sies H. On the history of oxidative stress: concept and some aspects of current development. Curr Opin Toxicol. 2018;7:122–126. doi: 10.1016/j.cotox.2018.01.002. [DOI] [Google Scholar]
- 4.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petronilli V, Costantini P, Scorrano L, et al. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J Biol Chem. 1994;269:16638–16642. [PubMed] [Google Scholar]
- 6.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 8.Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong H-S, Benoit B, Brand MD. Mitochondrial and cytosolic sources of hydrogen peroxide in resting C2C12 myoblasts. Free Radic Biol Med. 2019;130:140–150. doi: 10.1016/j.freeradbiomed.2018.10.448. [DOI] [PubMed] [Google Scholar]
- 10.Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Detaille D, Pasdois P, Sémont A, et al. An old medicine as a new drug to prevent mitochondrial complex I from producing oxygen radicals. PLoS ONE. 2019;14:e0216385. doi: 10.1371/journal.pone.0216385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreyev AY, Kushnareva YE, Murphy AN, Starkov AA. Mitochondrial ROS metabolism: 10 years later. Biochem Mosc. 2015;80:517–531. doi: 10.1134/S0006297915050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouchez C, Devin A. Mitochondrial biogenesis and mitochondrial reactive oxygen species (ROS): a complex relationship regulated by the cAMP/PKA signaling pathway. Cells. 2019;8:287. doi: 10.3390/cells8040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubouchaud H, Walter L, Rigoulet M, Batandier C. Mitochondrial NADH redox potential impacts the reactive oxygen species production of reverse electron transfer through complex I. J Bioenerg Biomembr. 2018;50:367–377. doi: 10.1007/s10863-018-9767-7. [DOI] [PubMed] [Google Scholar]
- 16.Selivanov VA, Votyakova TV, Pivtoraiko VN, et al. Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. PLoS Comput Biol. 2011;7:e1001115. doi: 10.1371/journal.pcbi.1001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selivanov VA, Votyakova TV, Zeak JA, et al. Bistability of mitochondrial respiration underlies paradoxical reactive oxygen species generation induced by anoxia. PLoS Comput Biol. 2009;5:e1000619. doi: 10.1371/journal.pcbi.1000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selivanov VA, Cascante M, Friedman M, et al. Multistationary and oscillatory modes of free radicals generation by the mitochondrial respiratory chain revealed by a bifurcation analysis. PLoS Comput Biol. 2012;8:e1002700. doi: 10.1371/journal.pcbi.1002700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinlan CL, Gerencser AA, Treberg JR, Brand MD. The mechanism of superoxide production by the antimycin-inhibited mitochondrial Q-cycle. J Biol Chem. 2011;286:31361–31372. doi: 10.1074/jbc.M111.267898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazil JN, Pannala VR, Dash RK, Beard DA. Determining the origins of superoxide and hydrogen peroxide in the mammalian NADH: ubiquinone oxidoreductase. Free Radic Biol Med. 2014 doi: 10.1016/j.freeradbiomed.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guillaud F, Dröse S, Kowald A, et al. Superoxide production by cytochrome bc1 complex: a mathematical model. Biochim Biophys Acta (BBA) Bioenerg. 2014;1837:1643–1652. doi: 10.1016/j.bbabio.2014.05.358. [DOI] [PubMed] [Google Scholar]
- 22.Grivennikova VG, Kozlovsky VS, Vinogradov AD. Respiratory complex II: ROS production and the kinetics of ubiquinone reduction. Biochim Biophys Acta (BBA) Bioenerg. 2017;1858:109–117. doi: 10.1016/j.bbabio.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Quinlan CL, Orr AL, Perevoshchikova IV, et al. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J Biol Chem. 2012;287:27255–27264. doi: 10.1074/jbc.M112.374629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. PNAS. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirst J, King MS, Pryde KR. The production of reactive oxygen species by complex I. Biochem Soc Trans. 2008;36:976–980. doi: 10.1042/BST0360976. [DOI] [PubMed] [Google Scholar]
- 26.Quinlan CL, Goncalves RLS, Hey-Mogensen M, et al. The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. J Biol Chem. 2014;289:8312–8325. doi: 10.1074/jbc.M113.545301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997;29:89–95. doi: 10.1023/A:1022420007908. [DOI] [PubMed] [Google Scholar]
- 28.Miwa S, St-Pierre J, Partridge L, Brand MD. Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free Radic Biol Med. 2003;35:938–948. doi: 10.1016/S0891-5849(03)00464-7. [DOI] [PubMed] [Google Scholar]
- 29.Votyakova TV, Reynolds IJ. ΔΨm-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 30.Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH: ubiquinone oxidoreductase (complex I) J Biol Chem. 2004;279:39414–39420. doi: 10.1074/jbc.M406576200. [DOI] [PubMed] [Google Scholar]
- 31.Liu S. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci Rep. 1997;17:259–272. doi: 10.1023/A:1027328510931. [DOI] [PubMed] [Google Scholar]
- 32.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/S0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 33.Lambert AJ, Brand MD. Superoxide production by NADH: ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J. 2004;382:511–517. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treberg JR, Quinlan CL, Brand MD. Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I) J Biol Chem. 2011;286:27103–27110. doi: 10.1074/jbc.M111.252502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Treberg JR, Brand MD. A model of the proton translocation mechanism of complex I. J Biol Chem. 2011;286:17579–17584. doi: 10.1074/jbc.M111.227751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 37.Ackrell BAC. Cytopathies involving mitochondrial complex II. Mol Aspects Med. 2002;23:369–384. doi: 10.1016/S0098-2997(02)00012-2. [DOI] [PubMed] [Google Scholar]
- 38.Siebels I, Dröse S. Q-site inhibitor induced ROS production of mitochondrial complex II is attenuated by TCA cycle dicarboxylates. Biochim Biophys Acta (BBA) Bioenerg. 2013;1827:1156–1164. doi: 10.1016/j.bbabio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell P. Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol. 1976;62:327–367. doi: 10.1016/0022-5193(76)90124-7. [DOI] [PubMed] [Google Scholar]
- 40.Trumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem. 1990;265:11409–11412. [PubMed] [Google Scholar]
- 41.Crofts AR, Meinhardt SW, Jones KR, Snozzi M. The role of the quinone pool in the cyclic electron-transfer chain of Rhodopseudomonas sphaeroides a modified Q-cycle mechanism. Biochim Biophys Acta (BBA) Bioenerg. 1983;723:202–218. doi: 10.1016/0005-2728(83)90120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crofts AR. The Q-cycle—a personal perspective. Photosynth Res. 2004;80:223–243. doi: 10.1023/B:PRES.0000030444.52579.10. [DOI] [PubMed] [Google Scholar]
- 43.Crofts AR, Hong S, Wilson C, et al. The mechanism of ubihydroquinone oxidation at the Qo-site of the cytochrome bc1 complex. Biochim Biophys Acta (BBA) Bioenerg. 2013;1827:1362–1377. doi: 10.1016/j.bbabio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Victoria D, Burton R, Crofts AR. Role of the -PEWY-glutamate in catalysis at the Qo-site of the Cyt bc1 complex. Biochim Biophys Acta (BBA) Bioenerg. 2013;1827:365–386. doi: 10.1016/j.bbabio.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esser L, Gong X, Yang S, et al. Surface-modulated motion switch: capture and release of iron–sulfur protein in the cytochrome bc1 complex. PNAS. 2006;103:13045–13050. doi: 10.1073/pnas.0601149103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu C-A, Cen X, Ma H-W, et al. Domain conformational switch of the iron–sulfur protein in cytochrome bc1 complex is induced by the electron transfer from cytochrome bL to bH. Biochim Biophys Acta (BBA) Bioenerg. 2008;1777:1038–1043. doi: 10.1016/j.bbabio.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 47.Dröse S, Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J Biol Chem. 2008;283:21649–21654. doi: 10.1074/jbc.M803236200. [DOI] [PubMed] [Google Scholar]
- 48.Osyczka A, Moser CC, Daldal F, Dutton PL. Reversible redox energy coupling in electron transfer chains. Nature. 2004;427:607. doi: 10.1038/nature02242. [DOI] [PubMed] [Google Scholar]
- 49.Osyczka A, Moser CC, Dutton PL. Fixing the Q cycle. Trends Biochem Sci. 2005;30:176–182. doi: 10.1016/j.tibs.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Kembro JM, Aon MA, Winslow RL, et al. Integrating mitochondrial energetics, redox and ROS metabolic networks: a two-compartment model. Biophys J. 2013;104:332–343. doi: 10.1016/j.bpj.2012.11.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cortassa S, Aon MA, Winslow RL, O’Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J. 2004;87:2060–2073. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei A-C, Aon MA, O’Rourke B, et al. Mitochondrial energetics, pH regulation, and ion dynamics: a computational–experimental approach. Biophys J. 2011;100:2894–2903. doi: 10.1016/j.bpj.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gauthier LD, Greenstein JL, Cortassa S, et al. A computational model of reactive oxygen species and redox balance in cardiac mitochondria. Biophys J. 2013;105:1045–1056. doi: 10.1016/j.bpj.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demin OV, Kholodenko BN, Skulachev VP. A model of O2.-generation in the complex III of the electron transport chain. Mol Cell Biochem. 1998;184:21–33. doi: 10.1023/A:1006849920918. [DOI] [PubMed] [Google Scholar]
- 55.Demin OV, Gorianin II, Kholodenko BN, Westerhoff HV. Kinetic modeling of energy metabolism and generation of active forms of oxygen in hepatocyte mitochondria. Mol Biol (Mosk) 2001;35:1095–1104. doi: 10.1023/A:1013211007465. [DOI] [PubMed] [Google Scholar]
- 56.Magnus G, Keizer J. Minimal model of beta-cell mitochondrial Ca2+ handling. Am J Physiol Cell Physiol. 1997;273:C717–C733. doi: 10.1152/ajpcell.1997.273.2.C717. [DOI] [PubMed] [Google Scholar]
- 57.Kim N, Ripple MO, Springett R. Measurement of the mitochondrial membrane potential and pH gradient from the redox poise of the hemes of the bc1 complex. Biophys J. 2012;102:1194–1203. doi: 10.1016/j.bpj.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown GC, Brand MD. Thermodynamic control of electron flux through mitochondrial cytochrome bc1 complex. Biochem J. 1985;225:399–405. doi: 10.1042/bj2250399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pryde KR, Hirst J. Superoxide is produced by the reduced flavin in mitochondrial complex I a single, unified mechanism that applies during both forward and reverse electron transfer. J Biol Chem. 2011;286:18056–18065. doi: 10.1074/jbc.M110.186841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borek A, Sarewicz M, Osyczka A. Movement of the iron–sulfur head domain of cytochrome bc1 transiently opens the catalytic Qo site for reaction with oxygen. Biochemistry. 2008;47:12365–12370. doi: 10.1021/bi801207f. [DOI] [PubMed] [Google Scholar]
- 61.Aon MA, Stanley BA, Sivakumaran V, et al. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: an experimental-computational study. J Gen Physiol. 2012;139:479–491. doi: 10.1085/jgp.201210772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/S0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 63.Cortassa S, Sollott SJ, Aon MA. Mitochondrial respiration and ROS emission during β-oxidation in the heart: an experimental–computational study. PLoS Comput Biol. 2017;13:e1005588. doi: 10.1371/journal.pcbi.1005588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markevich NI, Hoek JB. Computational modeling analysis of mitochondrial superoxide production under varying substrate conditions and upon inhibition of different segments of the electron transport chain. Biochim Biophys Acta. 2015;1847:656–679. doi: 10.1016/j.bbabio.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crofts AR, Lhee S, Crofts SB, et al. Proton pumping in the bc1 complex: a new gating mechanism that prevents short circuits. Biochim Biophys Acta (BBA) Bioenerg. 2006;1757:1019–1034. doi: 10.1016/j.bbabio.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Batandier C, Guigas B, Detaille D, et al. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr. 2006;38:33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- 67.Hoffman DL, Brookes PS. Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions. J Biol Chem. 2009;284:16236–16245. doi: 10.1074/jbc.M809512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bazil JN, Beard DA, Vinnakota KC. Catalytic coupling of oxidative phosphorylation, ATP demand, and reactive oxygen species generation. Biophys J. 2016;110:962–971. doi: 10.1016/j.bpj.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bazil JN, Vinnakota KC, Wu F, Beard DA. Analysis of the kinetics and bistability of ubiquinol: cytochrome c oxidoreductase. Biophys J. 2013;105:343–355. doi: 10.1016/j.bpj.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beard DA. A biophysical model of the mitochondrial respiratory system and oxidative phosphorylation. PLoS Comput Biol. 2005;1:e36. doi: 10.1371/journal.pcbi.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Groen AK, Wanders RJ, Westerhoff HV, et al. Quantification of the contribution of various steps to the control of mitochondrial respiration. J Biol Chem. 1982;257:2754–2757. [PubMed] [Google Scholar]
- 72.Gellerich FN, Bohnensack R, Kunz W. Control of mitochondrial respiration. The contribution of the adenine nucleotide translocator depends on the ATP- and ADP-consuming enzymes. Biochim Biophys Acta. 1983;722:381–391. doi: 10.1016/0005-2728(83)90086-5. [DOI] [PubMed] [Google Scholar]
- 73.Letellier T, Malgat M, Mazat JP. Control of oxidative phosphorylation in rat muscle mitochondria: implications for mitochondrial myopathies. Biochim Biophys Acta. 1993;1141:58–64. doi: 10.1016/0005-2728(93)90189-M. [DOI] [PubMed] [Google Scholar]
- 74.Smith AC, Robinson AJ. A metabolic model of the mitochondrion and its use in modelling diseases of the tricarboxylic acid cycle. BMC Syst Biol. 2011;5:102. doi: 10.1186/1752-0509-5-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Genova ML, Ventura B, Giuliano G, et al. The site of production of superoxide radical in mitochondrial complex I is not a bound ubisemiquinone but presumably iron–sulfur cluster N2. FEBS Lett. 2001;505:364–368. doi: 10.1016/S0014-5793(01)02850-2. [DOI] [PubMed] [Google Scholar]
- 76.Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation–reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/bj20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nohl H, Jordan W. The mitochondrial site of superoxide formation. Biochem Biophys Res Commun. 1986;138:533–539. doi: 10.1016/S0006-291X(86)80529-0. [DOI] [PubMed] [Google Scholar]
- 78.Moser CC, Keske JM, Warncke K, et al. Nature of biological electron transfer. Nature. 1992;355:796. doi: 10.1038/355796a0. [DOI] [PubMed] [Google Scholar]
- 79.Ransac S, Parisey N, Mazat J-P. The loneliness of the electrons in the bc1 complex. Biochim Biophys Acta (BBA) Bioenerg. 2008;1777:1053–1059. doi: 10.1016/j.bbabio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Ransac S, Mazat J-P. How does antimycin inhibit the bc1 complex? A part-time twin. Biochim Biophys Acta (BBA) Bioenerg. 2010;1797:1849–1857. doi: 10.1016/j.bbabio.2010.05.014. [DOI] [PubMed] [Google Scholar]