Abstract
Fetal and neonatal development represents a critical window for setting a path toward health throughout life. In this review, we focus on intestinal immunity, how it develops, and its implications for subsequent neonatal diseases. We discuss maternal nutritional and environmental exposures that dictate outcomes for the developing fetus. Although still controversial, there is evidence in support of an in utero microbiome. Specific well-intentioned and routine applications of antibiotics, steroids, and surgical interventions implemented before, during, and after birth skew the neonate towards pro-inflammatory dysbiosis. Shortly after birth, a consortium of maternal and environmentally derived bacteria, through cross-talk with the developing host immune system, takes center stage in developing or disrupting immune homeostasis at the intestinal interface. We also examine subsequent immunological cross-talks, which involve neonatal myeloid and lymphoid responses, and their potential impacts on health and disease such as necrotizing enterocolitis and sepsis, especially critical disease entities for the infant born preterm.
Keywords: Microbiota, Breastmilk, Sterile womb, C-section, Prematurity, Gut
Introduction
As years go by, care for premature infants continues to improve. Progress has been made in preventing neural tube defects and treating respiratory distress associated with prematurity. Necrotizing enterocolitis (NEC) and late-onset sepsis (LOS), on the other hand, continue to confuse investigators trying to identify their pathophysiological origins. Although decades of research have been dedicated to elucidating etiologies of these two major causes of morbidity and mortality, specific mechanisms remain enigmatic.
New avenues in research relating to these conditions may provide answers. A few decades ago, we would not consider bacteria normally present in amniotic fluid, the placenta, and the fetal gastrointestinal tract, nor would we consider how their interaction with the infant’s developing immune system would dictate its development. This notion is changing. We also need to understand, using multi-omics technologies, how the susceptible immature preterm’s intestinal tolerance of gut bacteria often goes awry, and how environmental factors like breastmilk can influence the fledgling microbiome and immune system. Only then might we begin to understand the root causes of two of the most devastating diseases of prematurity, NEC and LOS.
This review summarizes important factors of each developmental stage pertaining to neonatal intestinal immunity, namely: maternal nutrition and the in utero environment, the perinatal period, including mode of delivery, and the post-natal period, including breastfeeding. We apply a special focus on preterm infants, born before 37 weeks of gestational age, and how they differ from those born at term. Finally, we summarize the current body of evidence relating these factors to the development of LOS and NEC.
Maternal nutrition and the in utero environment
The interplay between maternal microbiota, stress, and nutrition critically dictates major characteristics of fetal development. Maternal exposures, including diet, modulate the maternal microbiota, which in turn influences the formation of the neonatal microbiota and subsequently its immune system [1]. Overnutrition and undernutrition both directly and strongly link maternal nutrition to unhealthy pregnancy [2, 3]. Specific micronutrient and macronutrient consumption by the pregnant mother is also associated with subsequent outcomes related to neonatal immunology and the developing gut microbiota. This interplay between maternal nutrition and neonatal outcomes is summarized in Table 1.
Table 1.
Maternal nutrition during pregnancy and immunological or developmental impacts on the subsequently born neonate
| Maternal nutrition | Effect on neonate |
|---|---|
| Caloric intake | |
|
Excess caloric intake/obesity (overnutrition) |
Preterm birth risk increased [2] C-section risk increased [182] Lactation dysfunction [183] IUGR risk increased [4] Miscarriage/stillbirth risk increased [184] Neonatal gut dysbiosis risk increased [185, 186] Risk for pro-inflammatory chronic disease later in life increased [4] |
| Inadequate caloric intake/underweight (undernutrition) |
Preterm birth risk increased [2] Perinatal infant mortality risk increased [187] Birth defect risks increased [187] Neonatal gut dysbiosis risk increased [186] Heightened stress response [5, 52] Risk for chronic disease later in life [188] |
| Macronutrients | |
| High fat diet | Maternal and neonatal gut dysbiosis risk increased [189–191] |
| Omega-3 fatty acids |
Reduced preterm birth and low birth weight rate [3] Prevention of intrauterine infection [192] Epigenetic upregulation of immunomodulatory genes [193] |
| Micronutrients | |
| Vitamin D supplementationa |
Reduced preterm birth rate [194, 195] Increased birthweight [194] |
| Vitamin A deficiency | Decreased lymphoid organ, B cell, myeloid cell, and LTi development [196] |
| Selenium deficiency | Preterm birth risk increased [197] |
| Zinc deficiency |
Decreased lymphoid organ and immunocyte development [4, 198, 199] |
IUGR intrauterine growth restriction, LTi lymphoid tissue inducer cell
aSerum vitamin D deficiency not related with birth outcomes
In utero development: questioning the dogmatic “sterile womb” hypothesis
It has been postulated for over a century that the normal fetus is devoid of colonizing commensals. Through indirect mechanisms alone, the maternal microbiota can be quite impactful on fetal development. It is known that the developing immune system of the fetus and neonate is shaped by circulating metabolites originating from the mother’s intestinal microbiome, entering the fetus and neonate simultaneously with maternal antibodies via the placenta and breastmilk, respectively [4]. This indirect early immunological influence critically sets the developing fetus and neonate on a path towards or away from immunological homeostasis later in life [5]. Exposure to large amounts of viral and bacterial pathogen-associated molecular patterns (PAMPS), made worse by maternal stress and malnutrition, exacerbates the inflammatory stress response. Since it is known that maternal microbiota and their metabolites may have the potential to influence neonatal and birthing outcomes, the topic of maternal probiotics to prevent preterm birth and negative health outcomes has been explored. However, this practice fails to conclusively show benefits [6].
We now have reason to question the dogmatic view of a “sterile womb”. A recent commentary in nature succinctly sums up the growing evidence against this notion [7]. Using 16S sequencing, our group demonstrated that meconium from preterm infants contained microbial DNA, suggesting the womb is not sterile as previously postulated [8, 9]. In addition, a growing body of evidence supports the concept of an intrauterine environment that is not sterile, as previously thought, and that the formation of the neonatal microbiome may originate in utero [9–12]. Bacterial DNA has been found in the human placenta as well as amniotic fluid, suggesting a unique placental microbiome [10, 13] that might impact the immune balance of the fetus directly [14]. If cervicovaginal microbiota composition can already be used as an indicator of prospective preterm birth risk [15–18], understanding the in utero microbial composition might perhaps serve to predict birth outcomes to an even larger extent and fill a critical gap in knowledge in this field. This is just one example of what might come from investigating uterine bacteria during pregnancy.
Despite the recent series of findings in favor of the notion, there is still debate concerning the validity of the “in utero colonization” theory. Critics claim that bacterial DNA is not strong enough evidence of bacterial presence in utero due to possible experimental contamination or circulation of cell-free bacterial DNA [19]. They claim that this highlights the need for further studies to elucidate better culturing methods or other ways to definitively show bacterial presence and function in utero [7, 20]. To address this criticism, a small study of 5 samples showed that most bacterial DNA reported in meconium comes from living bacterial cells by eliminating non-viable bacterial DNA with a chemical compound [21]. However, recent studies suggest the lack of a commensal in utero microbiome during healthy pregnancy [22, 23]. Despite the skepticism, it remains true that the majority of recent investigations have found bacterial DNA in components of the placenta and amniotic fluid, and if we have faith in our current methods of detection, it is not a far-cry to conclude that bacteria or at the very least, their components, reside along with the developing fetus in utero. If that is the case after additional rigorously performed studies with appropriate quality control and assay blanks, it will open an entirely new window of developmental immunology at the fetal–maternal interface with potential for great leaps in mechanistic and therapeutic understanding.
Impact of the perinatal and post-natal period on intestinal homeostasis
At the beginnings of extra-uterine life, the preterm newborn is thrust into a sea of antigens to which it must selectively and appropriately respond, despite underdeveloped mechanisms to do so. The neonate’s birthing conditions and exposure and response to imminent environmental antigens will culminate to dictate its subsequent development.
Mode of delivery: surgical vs vaginal
Preterm infants are particularly likely to undergo cesarean section (C-section) delivery. Surgical C-section delivery is a life-saving procedure. In some areas of the world, it is not used enough to deliver infants that need it, but in other parts of the world its use exceeds medical necessity and is often done out of convenience. This has been thought to set the stage for dysbiotic neonatal microbiota entirely different from what has been selected for by millions of years of co-evolution. Differences in microbial composition among infants’ feces born via C-section have been found to be present up to 7 years [24]. Moreover, it can take months to institute a solid gut microbiome in a surgically delivered infant [25]. Thereby, type of delivery appears to be a key factor in the development of the infant gut microbiome.
Surgically delivered infant microbiota is thought to consist of overall decreased bacterial diversity, less protective vaginally derived Bacteroidetes colonization, and more (perhaps skin-derived) Staphylococcus, Propionibacteria, and Streptococcus species until 1–2 years of age [26–28]. It is for this reason that it is postulated that the physical passing of the infant through the birth canal inoculates a symbiotic intestinal flora into the infant. In support of this idea, it has been observed that Lactobacillus, Prevotella, or Sneathia spp. are more prevalent in vaginally delivered babies, and within months there is an appreciable spread of Bifidobacterium and Bacteroides. Bifidobacterium is thought to have an especially beneficial role in the maturation of the developing gut lining and neonatal immune tolerance [29]. Metagenomic microbiome signatures vary accordingly between neonates born to surgical or vaginal delivery and might explain immune response differences between them [30].
Mouse studies have found that surgical delivery of pups leads to an increased inflammatory and dysbiotic state, which can be partly corrected with prebiotics [31]. The study also found that some aspects of surgical delivery complications in neonatal mice were not able to be reversed by prebiotics and are thus microbiota-independent. This neonatal dysbiosis associated with surgical birth is either compounded or perhaps caused by the prophylactic antibiotics given to mothers during cesarean section birth [32].
The decision to perform a C-section is often necessary, but with it comes with risks for many gastrointestinal and immunity-related problems for the newborn, including breastfeeding complications, food allergy, and, later in life, obesity and asthma, and potentially juvenile arthritis, immune deficiencies, and inflammatory bowel disease (IBD) [27, 33, 34].
There continues to be skepticism about the mechanism explaining C-section-mediated intestinal dysbiosis, as well as the “vaginal seeding” practice whereupon the surgically delivered fetus is iatrogenically exposed to microbes from the birth canal [35, 36]. A recent study by the Aagaard group finds no causative relationship (P = 0.057) between delivery mode and infant gut microbiome structure or function [37]. The results were almost statistically significant but contradict what many other investigators have found, and thus should not be looked at in isolation. However, another recent high-profile study echoed the finding that mode of delivery does not leave persistent microbiome alterations when confounders present in other studies are accounted for [38].
Despite the aforementioned two recent studies finding no relationship between mode of delivery and the neonatal microbiome, the fact remains that the majority of investigations with human subjects focusing on this issue point to impaired neonatal gut microbiota and elevated risk for immune-related diseases correlated with mode of delivery. Clinically, eliminating any and all unnecessary and non-medically indicated surgical deliveries, which have been estimated to encompass nearly half of all surgical births in the US, should still be an urgent and top medical priority [39].
Antibiotic use
It has been suggested that inflammation associated with the mode of delivery and administration of intrapartum antibiotics together alter the bacterial composition of the breast milk, and through the milk are mainly responsible for the development of dysbiosis observed in C-section delivered babies [40]. Intrapartum antibiotics are frequently used in C-section deliveries in the US and very often around the world. Some postulate that they are the major reason why infants born surgically have been observed to exhibit different microbiota profiles [35]. Though lifesaving when used correctly, these drugs may set the developing infant microbiota on a path towards dysbiosis and disease.
When 18 mothers received intrapartum antimicrobial prophylaxis (IAP) due to confirmed or suspected vaginal colonization by Group-B-streptococci, a reduction of potentially protective Actinobacteria and Bacteroidetes and an increase of Proteobacteria and Firmicutes abundance was later observed in the fecal microbiomes of babies from IAP-treated mothers compared to babies from mothers not exposed to antibiotics. Additionally, infants born to mothers in the IAP group showed reduced proportions of putatively mutualistic microorganisms, such as the family Bifidobacteriaceae, but increased proportions of potentially pathogenic microorganisms including Campylobacteriaceae and Helicobacteriaceae [41]. Broad-spectrum antibiotics for neonatal LOS prevention during the first months after birth significantly contribute to decreased species richness [42] and delayed colonization of Bifidobacterium in the neonate as well [43].
Antibiotic exposure during the first year after birth is linked with development of immunological issues such as wheezing and eczema by 8 years of age [44]. Exposure to antibiotics at this developmental period was also linked with an increased risk for development of IBD as well as type 2 diabetes mellitus later in life [45]. The increased risk of diseases like chronic asthma and allergies in this demographic can be attributed to the frequency of antibiotic use throughout the first month after birth and beyond [46–48].
Antenatal corticosteroids are commonly given to pregnant women expecting preterm delivery between 24 and 33 weeks of gestation in order to prevent neonatal respiratory distress syndrome, intraventricular hemorrhage, premature rupture of membranes, NEC, and LOS [49, 50]. Use of antenatal steroid medications was found to be an additional risk factor for developing neonatal intestinal dysbiosis [51].
Post-natal microbiota and intestinal development
The human gastrointestinal tract is a complex and specialized organ that arises during embryonic and fetal life from a simple tube. This multifaceted organ will eventually house the largest microbial reservoir of the body and interface with the outside world. The last trimester of gestation represents a critical developmental window for the fetal intestine that is often shortened by preterm birth. At term birth in humans, intestinal motility, mature villus and crypt anatomy, and feeding reflexes are fully developed to help the infant establish proper nutrition and a functioning immune system [52]. If the infant is born preterm, these are not fully developed [52]. Refer to this excellent review about more details of early embryonic and fetal gastrointestinal development [53].
The gut microbiota, often referred to as the “forgotten organ”, is central to neonatal mucosal immunity and aforementioned intestinal anatomical development [54]. Shortly after birth, gut colonization is thought to be influenced by the birth canal in addition to microbes from colostrum. Its development during the first 2 weeks after birth represents a critical window, especially for premature infants who have underdeveloped intestinal barrier function. The microbiota composition helps determine the integrity of the intestinal barrier, formed by tight junction proteins between intestinal epithelial cells (IECs) that prevent escape of inflammatory microorganisms and molecules into circulation [55]. Gut colonization by commensal bacteria is a vital part of development of the mucosal barrier and modulates risk of developing systemic inflammation, NEC, LOS, and other pathologies. Depending on the conditions of the gastrointestinal tract and composition of commensal bacteria, the microbiota can either degrade or enrich the mucus barrier of the gut. Some commensals provide stimulation to goblet cells to coat the intestinal surface with a glycocalyx (mucin), which serves as a protective barrier between commensal microbes and IECs. On the other hand, pathogenic or opportunistic bacteria actively degrade it, making sepsis more likely. Commensal bacteria can also stimulate epithelial transcription and translation of tight junction proteins to close the intercellular route for the uptake of large molecules and microbiota, while dysbiotic microbiotas with opportunistic pathogens weaken intestinal integrity in order to proliferate and translocate. At the same time, the release of defensins and cathelicidins (which act as anti-microbial molecules) by Paneth cells and IECs protects the mucosa from pathogenic bacteria [56].
Underdevelopment of the preterm intestine leads to a highly permeable intestinal surface and pathogenic bacterial colonization because of reduced gastrointestinal motility as well as limited enteric nervous system function, all of which sets the stage for destructive dysbiosis, chronic inflammation, and microbial translocation through the weakened intestinal barrier, leading to potentially lethal diseases of prematurity described later in this review.
Premature infants have a vastly different intestinal milieu than term infants. The majority of them are vulnerable to the pathogenic hospital environment, which stresses their microbiota further. As a consequence of all factors associated with prematurity, their microbiota diversity is reduced, and its composition is very different compared with healthy term infants [57]. The compositional effect of gestational age on microbiota can last up to 4 years [58]. Preterm neonates have diminished microbial species alpha-diversity, reduced protective bacterial genera (Bifidobacterium, Lactobacillus), and increased proportions of potentially pathogenic bacteria such as Clostridium difficile and bacteria of the γ-proteobacterial class, namely Pseudomonas, Klebsiella, and Escherichia coli [59–63]. This dysbiosis is thought to be implicated with a major intestinal disease of prematurity, namely NEC.
Neonatal nutrition and microbiota development
Recent data suggest both the physical and nutritional process of breastfeeding is critical for the development of a symbiotic neonatal microbiome (e.g., Bifidobacterium-dominant) and prevention of NEC [63]. Alternative feeding methods, provided by inserting temporary tubes through the nose or mouth into the stomach or proximal intestine as well as intravenous catheters, are required to supply nutrition in preterm infants until they are developmentally ready to feed by breast or bottle, but they lack many protective factors and overlook the interplay between nutrition and microbiota. For example, inappropriately supplementing iron to deficient neonates may promote dysbiosis in addition to increased risks of death and LOS, as it circumvents maternal breastmilk and neonatal mechanisms to restrict iron from pathogens that readily take it up and become dominant [64, 65]. Preterm babies may be further disadvantaged due to their underdeveloped suckling and swallowing reflexes, which can make actual breastfeeding of colostrum not possible at birth [63].
The glycans of the neonatal gut and prebiotic fiber (or lack thereof) in breastmilk or formula further play a role in selection of commensal bacteria. Specific genes encoding glycosyltransferases determine the composition of the intestinal glycans. Expression patterns of these genes are epigenetically downregulated during the post-natal period; the breastmilk-derived fermentable and bioactive components are expected to make up for the subsequent lack of mucin and innate defenses [66]. Consumed fucosylated oligosaccharides from milk pass undigested into the colon, where they are used as carbon sources to select for beneficial Bifidobacteria, Bacteroidetes, and other anaerobes that are key for symbiosis [67, 68]. This process is disturbed by parenteral nutrition and lack of enteral feeding commonly experienced by preterm infants in neonatal intensive care units.
Breastfeeding dictates neonatal immune trajectories
Human breast milk (HBM) feeding is the optimal nutrition for the human newborn in normal conditions. HBM feeding has been shown to reduce infant mortality by 12% compared to formula feeding and has shown to be greatly beneficial in preventing several neurological, respiratory, and gastrointestinal diseases, including NEC [69].
The ideal carbohydrate source for the infant is lactose, as the infant’s intestine contains lactase enzyme activity at birth, and symbiotic bacteria have evolved to prefer it as a carbon source. Human milk oligosaccharides (HMOs) can additionally expand the metabolism and modulate the composition of the infant microbiota [70]. These carbohydrate sources, in addition to lactose, serve as fermentable carbohydrate prebiotics that positively select for beneficial gut bacteria, though we do not fully understand how HMOs function in the preterm intestine, which may have markedly different effects than what has been observed in a term gut [71]. These carbohydrate sources also prevent pathogens from binding to IECs and instead carve out a niche for beneficial mutualistic bacteria such as Bifidobacteria. In doing so, they serve as carbon sources that facilitate production of beneficial metabolites by those commensals, such as microbial short-chain fatty acids (SCFA) and folate (vitamin B9), both of which act as potent epigenetic regulators. Higher levels of immunomodulatory SCFAs acetate and propionate (but not butyrate) have been observed in the feces of HBM-fed preterm infants than were seen in feces of formula-fed preterm infants [72]. SCFAs acetate, propionate, and butyrate are all known to enhance epithelial integrity, which is critical in maintaining systemic immune homeostasis. These bacterial metabolites, and others, dictate immune development in the preterm infant. In contrast to HBM-fed infants, many infant formula products contain sucrose and glucose, no HMOs, and some do not contain lactose at all, all of which modulates the gut bacteria in a way that does not appear to be physiological for the infant. The formula-fed infant is therefore at a greater risk for post-natal pathologies and death, despite ingesting proper micronutrient and near-adequate macronutrients proportions [69].
Various immunoglobulins and immune cells are a component unique to colostrum, the “first milk”, and are not present in mature breast milk. Consumption of colostrum by the infant is a well-known mechanism of passive immunity transfer from the mother, though consumption of colostrum may not be possible in the preterm neonate due to potentially underdeveloped feeding behaviors [73].
A physical, biochemical and immune barrier forms the intestinal mucosa and “gut closure” which refers to the permeability of the neonatal gut and is mainly characterized by (1) the passage of large molecules from the intestinal lumen to the blood through tight junctions; (2) active transport of immunoglobulin G and immune complexes from breastmilk into intestinal submucosae and (3) maternal microchimerism, which allows the transference and nesting of maternal cells from breastmilk to infant mucosa when the intestinal permeability is high [74]. This transfer of maternal cells may help with immune responses, tissue repair, and regulation of the neonatal immune response and tolerance.
Maternal immunoglobulins play a critical role in shaping neonatal immunity. The human newborn relies solely on maternal IgG for the first 3 months of life due to a lack of functional plasma cells [75]. Preterm infants were at one point thought to be compromised because the reduced developmental time in utero was thought to prevent adequate transfer of maternal IgG to the infant [76]. However, a recent study has shown that this is not the case, and that maternal antibodies from gestation are not responsible for the reduced immunity observed in human preterm infants [77].
Another major way the mother confers passive immunity to the neonate is through secretory IgA (sIgA) in the breast milk, which makes up over 90% of all antibodies present in the milk [78]. sIgA is known to shape the characteristics of the commensal bacteria and foster their symbiotic relationship with the host through selection pressure for symbiotic bacteria [79]. All newborns lack sIgA at birth, during which time they are completely reliant on the mother’s milk for sIgA [78]. Deficiencies in sIgA are associated with a range of immune inadequacies in immunocytes isolated from human preterm infants [80]. By 2 weeks after birth, mice begin producing their own IgA from plasma cells in the Peyer’s patches (PP) and eventually mucosal lamina propria in a process known to be influenced by the gut microbiome [78, 81]. In piglet models, it has been shown that bacterial colonization drives the isotype switch from neonatal IgM to sIgA at the ileal PP, further supporting this view [82]. sIgA is known to prevent bacterial translocation by interacting with intestinal M cells and enhancing agglutination of pathogenic bacteria [83].
We are beginning to understand the role of breast milk exosomes, which are nanovesicles containing immunologically relevant components that direct immune responses. In a study, rat milk was collected to determine the effect of milk-derived exosomes on IECs [84]. Exosomes from the milk were found to enhance proliferation and stimulate intestinal stem cell activity. Furthermore, it has been demonstrated that human milk exosomes have the capacity to survive simulated digestion conditions of infants. Results of these studies indicate that nutritional HBM exosome biology in the neonate is worth investigating further [85].
The physiological state of the mother also impacts the health of the breast-fed infant via the composition of human breast milk. Mothers that bear premature infants have higher proportions of protein content in their colostrum than mothers that bear term infants [86]. Human mothers that themselves have IBD have been shown to produce breast milk that is overall more potentially inflammatory than healthy mothers’ milk, suggesting an inflammatory axis shared between mother and child [87]. HBM derived from healthy mothers is richer in protective sIgA, 2-aminobutyrate, and lactose [87]. Lactose, as discussed previously, is important for providing carbon for neonatal bacteria to produce beneficial short-chain fatty acids (SCFAs), namely, acetate and propionate, which are critically important for maintaining gut integrity, promoting regulatory immune responses, and preventing excessive immune responses to commensal bacteria [88, 89]. The HBM derived from mothers with IBD contained relatively higher levels of pro-inflammatory cytokines and higher putatively pro-inflammatory bacterial energy metabolites lactate and succinate than milk from healthy mothers [90, 91].
Milk-derived cytokines, including TGF-B, IL-1B, Il-6, IL-10, IL-12, TNF-a, IFN-γ, and GM-CSF present in human colostrum, have been shown to survive digestion and mechanistically modulate the piglet intestinal inflammatory environment when consumed via milk from the sow, which is likely also the case in humans [92–94].
Breast milk also contains live microbes, which may play an important role in colonizing the neonate. Bacterial colonization of breast milk is thought to occur via bacterial translocation from the maternal intestine to lymph nodes to mammary glands to milk [95]. Infants fed their own mother’s milk develop a different and potentially more protective microbiota than those fed pasteurized donor milk or formula. Pasteurization of donor or mother’s own breast milk, while preventing the growth of pathogens, results in loss of some biologically active components such as sIgA, lactoferrin, lysozyme, cytokines, lipase, cellular components, and live microbes [96]. UV-C radiation and high-pressure processing have been explored as alternative ways to sterilize donor breast milk and preserve bioactive components, but human studies must be carried out before clinical conclusions and therapeutic decisions are made about using them routinely [97, 98]. Pumping of breastmilk, compared to breastfeeding, reduces its efficacy in beneficially modulating the infant microbiota [99]. The notion of sharing unpasteurized human breast milk has become increasingly popular recently, though doing so without a physician’s prescription is not currently recommended by the Food and Drug Administration (FDA) due to safety concerns [100, 101].
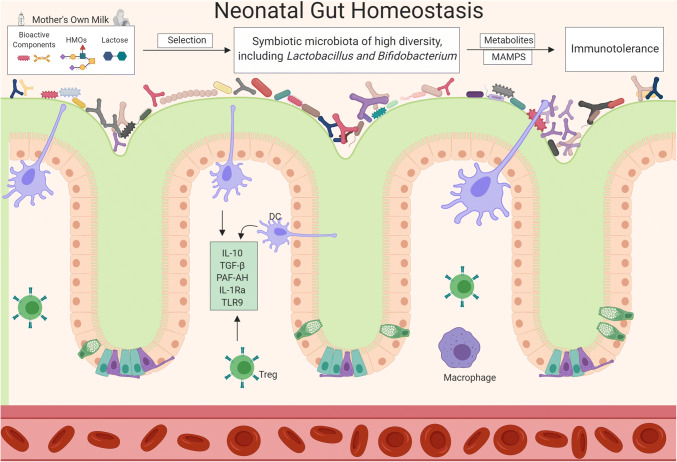
An overall summary of how mother’s milk promotes homeostatic mechanisms in the gastrointestinal tract is illustrated in Fig. 1. HBM significantly influences both the bacterial composition and intestinal immune response of the neonate, both of which are known to dictate disease risk throughout life [102, 103].
Fig. 1.
Compositional factors in mother’s milk including live microbes promote production of bioactive molecules that are thought to protect the mucosal barrier, promote the growth of commensal bacteria, and provide a balance between pro and anti-inflammatory processes. MAMPS microbial-associated molecular patterns
Neonatal immune system
Much like an army defends a nation against outside invaders and a police force defends a nation from autoreactive citizens, the mucosal immune system in the preterm neonate must respond to foreign antigens and self in a targeted and selectively tolerant fashion from birth [104]. Immune development begins in the womb. What is currently known about fetal immune ontogeny has been covered in an excellent review [65]. Our knowledge of this subject might expand further once we understand the likely substantial effects of uterine microorganisms and their components/metabolites on the fetus, both during steady-state and infection. For example, exposure to infection in utero is associated with an increased risk for NEC development in the subsequently born neonate, hypothetically by tuning the developing fetal immune system towards a pro-inflammatory state prior to delivery [105, 106].
Here, we focus on intestinal immune development into the neonatal period. The neonatal immune system is immature but rapidly developing. Animal models and in vitro/ex vivo experiments, despite not providing fully adequate replicas of the human neonate due to differences in rates of immune maturity and cellular milieu, are one of the primary ways causal relationships are determined pertaining to neonatal immune ontogeny. For example, murine studies consistently conclude that neonatal regulatory T cells (Tregs) are unable to suppress autoreactive cytotoxic cells, while Tregs isolated from human fetuses already functionally suppress T cell populations expressing markers of early activation [107, 108]. This reflects the general trend that the intestinal integrity and mucosal immunity of mice born with normal gestation are much more immature than their human counterparts for which they serve as models [52]. Relying too heavily on these models may provide erroneous conclusions, but they still provide useful information that we are not able to obtain from humans, so they will be utilized here.
Blood leukocytes from preterm human infants are of markedly inferior composition and cell count than term counterparts, with results including diminished pattern recognition receptor (PRR) function, reduced bacterial elimination, and hindered endothelial adhesive rolling [109]. The human newborn lacks adult levels of mature B cells and T cells, but its innate immune system, including intestinal dendritic cell and macrophage populations, is intact [52]. The human neonatal Th2 and Th17-type immune responses are equipped to handle extracellular pathogens in the gut, but Th1 type defenses against intracellular pathogens are silent during homeostasis in early stages of neonatal development [110]. In cases of microbial dysbiosis, the neonatal immune system may skew towards a more Th1-dominant response, leading to excessive inflammatory responses to commensals, though it is still debated whether this skew is the case in NEC [27].
At birth, cytokines IL-23, IL-12, IL-1B, Il-6, and TNF-α are the main pro-inflammatory messengers of the innate immune system, but they are largely suppressed in preterm infants due to reduced toll-like receptor 4 (TLR4) function and epigenetic modifications associated with premature birth and diseases of prematurity [109, 111]. Platelet-activating factor (PAF) also plays a role in the inflammatory intestinal milieu associated with prematurity, putatively increasing luminal expression of TLR4 and contributing to NEC based on association studies from humans and causal findings in mouse models [112, 113]. Treg-derived IL-10 is the main anti-inflammatory cytokine at this stage, though it cannot effectively counteract exacerbated inflammatory responses originating from platelet-activating factor (PAF) and TLR4-signaled NFκB in diseases such as NEC [55, 114]. Human studies have found increased serum IL-10 levels to be correlated with NEC, further suggesting that high IL-10 levels represent the body’s futile attempt to tone down the destructive inflammation seen in NEC [115].
Toll-like receptors
The innate Toll-like receptor (TLR) response is compromised in preterm infants [111]. Molecular patterns expressed and concealed on the bacterial cell surface can stimulate IECs, dendritic cells, and lymphocytes to prompt PRRs such as TLRs on their cell surface to induce an acute inflammatory response to block penetration of pathogens into the epithelium.
Murine studies, if serving as accurate models for disease, are best employed to elucidate mechanisms of neonatal immunity, so information derived from them will be used here. TLRs communicate their signal to the newborn’s dendritic cells (DCs) and initiate immunological cascades. Specific TLRs in the neonate epithelium are centrally involved in the microbiota-mediated mucosal development. TLR2, responsible for detecting and clearing coagulase-negative staphylococci that are often involved in neonatal LOS, is expressed at adult levels in preterm infants, just as it is in term infants. TLR4, responding to lipopolysaccharide by signaling production of pro-inflammatory NFκB and other products, is regulated during neonate development, dysfunctional in preterm infants, and is implicated in the pathology of NEC. In brief, lipopolysaccharide (LPS) from gram-negative bacteria interacts with TLR4 receptors through the adapter protein MYD88 to induce a transduction cascade through NFκB, which is a transcription factor that stimulates general pro-inflammation, including the transcription and translation of the chemokine IL-8, which in turn induces migration of neutrophils to the site of infection. TNF-α is subsequently released to activate an inflammatory response. This process, gone awry, is involved in the pathology of NEC [116, 117].
TLR4 overactivation is thought to be a major factor in the destruction of intestinal integrity observed in NEC. Prematurity leads to enhanced expression of PAF and reduced expression of the enzyme that degrades it, PAF-AH, in human neonates [113]. Enhanced PAF has been shown to increase intestinal TLR4 expression in rodent models and is therefore thought to contribute to NEC development in human preterm infants [112]. TLR9, based on observations from human neonates and mechanistic studies on fetal mice, responds to DNA rich in cytosine–phosphate–guanine (CpG) islands and is thought to counteract TLR4 activation and tone down excessive TLR4-driven inflammation [118]. In cases of human NEC, TLR9 and PAF-AH expression is low while PAF and TLR4 expression is abnormally high [118]. While these factors are thought to be important in the pathophysiology of NEC, there are many others at play as we still do not fully understand the disease.
Neonatal innate lymphoid cells
Neonates express markedly higher cell lineage marker negative innate lymphoid cell (ILC) frequencies in the thymus than they do later in life [119]. Murine studies indicate that the neonatal mouse thymus is uniquely dominated by Group 3 ILCs (ILC3s), which are strongly involved in regulating pathogenic and commensal microorganism abundance in the intestine, and in the case of lymphoid tissue inducer (LTi) ILC3 cells, assisting in the developing gut-associated lymphoid tissue (GALT) of the neonate [119]. ILC3 development is driven by maternal microbiota-derived aryl hydrocarbon receptor ligands, which are found in the placenta and breast milk [120]. This phenomenon is neonate specific, as neonate development after birth results in a rapid loss of ILC3s, which are largely replaced with a more dominant ILC2 population that remains throughout the rest of life.
Neonatal B and T lymphocytes
The adaptive immune system in neonates is underdeveloped, which leaves them vulnerable to critical viral and bacterial infections. B lymphocytes are present at birth in the human neonatal intestine, but their functional maturation into sIGA secreting plasma cells does not complete until 1 year after birth, during which time the neonate relies on the sIgA provided from colostrum [121]. In human newborns, PPs have begun to develop during gestation but PP germinal centers, necessary for B cell proliferation, differentiation, isotype switching, and mutation of antibody-related genes, are not fully developed [122, 123]. Thus, functional immaturity of B lymphocytes and associated cells and tissues is observable at birth in humans.
The abundant yet immature naive T lymphocytes in the periphery of the newborn express higher levels of intestine-homing integrins at this stage of development than they will ever express in adult life, indicating the increased priority for T cell activity at the GALT and PP of the newborn. T lymphocytes, only at the neonatal stage of life, are able to respond to TLR4 ligand lipopolysaccharide (LPS) and TLR5 ligand flagellin by differentiating towards activated memory cells [124].
Neonatal immune responses to γ-proteobacteria dysbiosis are characterized by excessive pro-inflammatory responses to commensal intestinal flora. There is confusion about the precise nature of the response to this dysbiosis, especially in conditions such as NEC. Some investigators have concluded that the response is dominated by a pathogenic Type 1 T helper (TH1) and IL-12 response, while others conclude that there is not enough evidence to observe a skew towards any of the T helper subtypes [125]. A more thorough understanding of the T helper response in neonatal intestinal inflammation is needed.
Term and preterm neonatal Tregs both have a diminished ability to prevent dendritic cell (DC) and effector T cell aggregation and activation compared to adult Tregs [126]. Hallmark adult regulatory T cell products, including IL-10 and TGF-B, are markedly lower in neonates compared to adults (but similar between term and preterm neonates), resulting in an enhanced potential for relatively unchecked pro-inflammatory sequelae in neonates [127]. High IL-10 levels do not necessarily indicate homeostasis, as levels of IL-10 in cases of human NEC are elevated, which likely represents a futile attempt to tone down the destructive inflammatory response to commensals common to all classical cases of NEC [115]. A better immunological indicator of the pro-inflammatory state inherent to human NEC appears to be an increased ratio of Th17 to Treg cells as well as the presence of a dysfunctional Treg cell subset that resembles Th17 cells in their IL-17 production [128]. Despite this diminished neonatal Treg function, these cells are still thought to contribute to resisting, though not entirely preventing, potentially disastrous exacerbated neonatal effector T cell responses to commensals at PPs. These findings highlight the unique yet reduced roles of B and T cells in neonatal mucosal immunology.
Diseases of prematurity: necrotizing enterocolitis and late-onset sepsis
Among the diseases associated with prematurity and deaths in preterm infants, NEC and LOS are highly prevalent. Deaths from both conditions are increasing in some regions, despite progress in combatting respiratory problems and other issues associated with prematurity. One of the major problems is not having a clear definition of either one of these diseases [129, 130]. On one hand, the classic form of NEC [131] represents a hyperactive immune system that damages the host in response to commensal bacteria, whereas infection and LOS occur due to the immune system not reacting strongly enough and being overrun by bacterial pathogens. We will, therefore, focus on both NEC and LOS in the preterm neonate and examine the role of the immune system and microbiota in each of these conditions.
Necrotizing enterocolitis
NEC is a devastating disease affecting primarily premature infants, most of which are very-low-birthweight (VLBW). NEC has been the subject of decades of research, but its etiology and definition remain poorly understood. Its incidence in preterm infants varies between 3 and 15% [131, 132], with a mortality rate as high as 30% in infants requiring surgery. Other risk factors besides prematurity are formula feeding and microbial dysbiosis [133, 134]. We have not learned how to prevent or effectively treat this disease, and it continues to cause great mortality and morbidity.
The greater the degree of prematurity of the infant, the longer it takes to develop NEC. Thus, the time from birth to the onset of NEC is inversely proportional with the length of gestation [135]. The key factors associated with the pathogenesis of NEC are intestinal immaturity, enteral feeds, the intestinal microbiome, inflammation, local ischemia, and reperfusion injury. The development of NEC peaks around 29–32 weeks postmenstrual age [132, 136–138].
To evaluate specific mechanisms for the pathophysiology of NEC, reliance on studies in human infants is suboptimal. Cell lines and animal models have been utilized in attempts to more clearly delineate the potential mechanisms implicated in NEC pathogenesis. Commonly used cells to evaluate intestinal epithelium include the immortalized colon cancer cell line, Caco-2. Human fetal non-transformed cell lines such as the H4 line have also been employed [139]. Another model that shows promise is the use of intestinal stem cells that produce enteroids or organoids [140]. These have some advantages over many other cell lines in that they can be derived from humans, can grow relatively long-term in culture, and simulate characteristics of epithelial cells growing in vivo [141].
Animal models have been developed with the intent to be utilized to better understand prevention, pathophysiology, and treatment for NEC. Unfortunately, the gastrointestinal tract and immune system of the animals differ from humans. Most commonly, rodents and piglets are used to model NEC. In rodents, the gut is in a less advanced stage of development at birth. Piglets transfer immunoglobulins across their GI tract after birth, whereas in humans the transfer is mostly via the placenta.
In the rodent models commonly used, formula feeds, cold stress, and hypoxia induce damage to the gastrointestinal tract. The rationale for the use of these models is based on studies in the 1970s when it was thought that hypoxia was a major antecedent of NEC [142]. However, a hypoxic event is not characteristic for the development of NEC. There is no temporal association found clinically between hypoxia and NEC [143], thus use of this model may result in erroneous interpretations if the major initiating event is the pathogenic inflammation. Therefore, the lack of a suitable animal model is a major barrier to clearly study NEC. However, when animals are used carefully as models of intestinal injury rather than direct models of NEC, we may be better able to delineate discrete candidate components of the pathophysiologic cascade implicated in NEC.
Numerous risk factors are implicated in the pathogenesis of NEC. Among these, premature birth is the major risk factor. Other commonly cited risk factors include intestinal dysbiosis, impaired mucosal protective mechanisms, altered immune responses, modified mucosal development with subsequent increases in permeability, formula feeds, antibiotics, H2-blockers, altered gut perfusion, transfusion associated-gut injury, and dysmotility. Here we will discuss a few of these.
Intestinal dysbiosis likely plays a significant role in the development of NEC. A harmonious intestinal microbiota is known to control gut homeostasis through numerous protective pathways. The concept of dysbiosis being a major factor in NEC pathogenesis has been considered for many years [144], but identification of specific causative microbes or metabolites remains elusive [145]. Intriguingly, although C-section babies can have markedly different microbiota, they are not at an increased risk for NEC development [146]. It is very important to mention the role of multi-omics investigations when looking into the pathophysiology of NEC; the interaction between host genetics, the environment, and how the host responds to the metabolism of the microbes will help us understand disease mechanisms in an integrative way, and at same time could give us a better understanding on how to develop treatment strategies in the future [147, 148].
Antibiotics are known to alter the microbial ecology of the preterm neonatal gastrointestinal tract, resulting in complex resistomes that we are only beginning to understand [149]. Use of antibiotics for “rule out sepsis” workups has been standard of care in the treatment of infants delivered preterm. Recent studies suggest an increased risk for NEC in infants receiving antibiotic treatment in the absence of sepsis. The risk of developing NEC increases significantly if the exposure to antibiotics is prolonged to more than 10 days [150]. This finding has been seen in other studies as well [151–153]. Alternatives to prophylactic antibiotics, such as bacteriophage therapy, might prove to be promising solutions [154, 155].
Multiple studies involving a relatively low number of patients failed to identify consistent bacteria as culprits. We found in NEC cases an increase in γ-proteobacteria and decrease in Firmicutes, as well as a low diversity index, with regional variation in the Bacteroidetes load [156, 157]. A systematic review and meta-analysis of sequence data from several different studies support the concept of an increase in Proteobacteria and decreases in Firmicutes and Bacteroidetes prior to the development of NEC in preterm infants [137]. However, there is regional variability of Bacteroidetes levels during the week of diagnosis, a trend inherent to most microbiome studies.
Human milk feeding in preterm infants was shown almost three decades ago to be associated with a lower incidence of NEC [158]. Numerous additional studies support this protective role of mothers’ milk, but none are prospective and/or randomized. The mechanism through which human milk confers protection against NEC is not fully understood. It is, most likely, the result of an additive effect of multiple bioactive components with immune, microbial, or nutritional properties.
There are potential prevention and therapeutic strategies for NEC reflected in recent studies, either involving the TLR4 inflammatory signaling pathway, repair of intestinal barrier function, probiotics, antioxidative stress, breastfeeding and immunomodulators, but large and good-quality trials are needed in order to elucidate if any of the mentioned strategies are valid [134].
Neonatal late-onset sepsis
LOS is a life-threatening organ dysfunction caused by an impairment of host response to infection. In the US, positive blood culture sepsis is present in 2 of every 1000 births and the incidence of LOS is remarkably higher in premature infants, especially VLBW infants of less than 1000 g, compared to term infants and those with birth weight greater than 1000 g [159].
As an important cause of morbidity and mortality in the newborn preterm population, it is vital to mention that neonatal LOS is different from adult sepsis because the immune response is managed largely by the innate immune system [160]. Pathogenic bacteria like Salmonella typhimurium or Campylobacter jejuni modify host responses and disrupt intestinal microbiota in order to colonize and cause more damage [161]. These bacteria interfere with host cell receptors, such as the epidermal growth factor (EGF) receptors in epithelial cells. They also delay recognition by TLR4 and inhibit inflammatory responses like those promoted by NFĸΒ. Such mechanisms favor pathologic bacterial translocation [162] in the premature neonatal immune system.
The gut microbiome has been implicated in the development of neonatal LOS [163]. A cohort study analyzing stool samples from LOS preterm infants used combined -omics to evaluate associations between the microbiome and metabolome, and found that the same strains of dominant bacteria in gut of LOS preterm infants were also isolated from their blood, which supports bacterial translocation from the intestine as a key for development of LOS [62, 163].
In vitro studies [164] show that intestinal bacteria can use either transcellular or paracellular mechanisms for bacterial translocation. Bacteria can use either a zipper or trigger mechanism to enter the IEC. After inflammation is induced, there is an increased expression of zonulin proteins (ZO-1, ZO-2, ZO-3, and occludin) [165, 166] inducing the disruption of tight junctions leading to a predisposition to bacterial translocation. At the level of the enterocyte, TLRs aim for identification of such bacteria but at the same time mobilize phagocytes and translocation of bacteria through the intestinal barrier [167]. Once the tight junctions between enterocytes have been destroyed by exacerbated responses to toxins like flagellin, endotoxin, and exotoxins, opportunistic pathogens can then utilize paracellular mechanisms to enter circulation. After bacteria penetrate mucus and epithelial barriers, a cytokine-induced anticholinergic pathway will occur via the vagus nerve in which intestinal macrophages will attempt protection against bacterial translocation using M cells [168].
Proteobacteria, the same phylum of bacteria associated with intestinal dysbiosis and NEC development in humans, appear to enhance resistance to polymicrobial sepsis via increased serum IgA when part of the microbiota of mice [169]. Perhaps the enhanced immune response to proteobacteria to prevent sepsis in the body comes at the expense of an increased risk for NEC. This highlights the delicate balance the immune system plays in working to prevent LOS and NEC.
State of the science: preventing NEC and LOS
Our understanding of how to prevent and effectively treat both of these conditions is currently inadequate. While feeding of mother’s own milk appears to be protective toward the development of NEC, LOS, and overall mortality in preterm infants, the prophylactic potential of any specific milk component in isolation, such as lactoferrin, antibodies, HMOs, and putatively probiotic milk bacteria, has been either negative or contradictory in humans [170–176].
Lactoferrin, a major human milk protein found to have antimicrobial, antioxidant, antifungal, and immunomodulatory properties, has been debated for its use in the prevention of LOS and NEC in preterm infants. A 2018 study concluded that prophylactic use of lactoferrin does indeed reduce the incidence of NEC and LOS as well as hospital-acquired infection in preterm infants, but with low-quality evidence [174]. A Cochrane review [177] that considered the aforementioned study in its search criteria did not find any eligible randomized trials properly evaluating lactoferrin for treatment of sepsis or NEC, implying that the study did not meet high quality standards, and concluded that there is no high-quality evidence to either support or refute the use of enteral lactoferrin by late 2018. Subsequently, a more sufficiently powered, well-executed, randomized placebo controlled trial published in Lancet in early 2019 demonstrated that administering enteral bovine lactoferrin to very preterm infants (less than 32 weeks of gestation) at or before 72 h after birth does not reduce the risk of late-onset sepsis, any other morbidities, or mortality, relative to a sucrose control [176]. Therefore, the best evidence suggests that isolated lactoferrin has no prophylactic role in preterm infants.
Other administered milk components in isolation have yielded similarly negative results. Despite many small or poorly designed studies claiming that probiotics may have prophylactic potential for preterm infants, when put to the test in high-quality trials, putatively probiotic milk-derived bacteria such as Bifidobacterium breve in isolation fail to consistently prevent sepsis, NEC, and death in preterm infants [178, 179]. Maternal sIGA analysis in human preterm infant stools provides interesting associations, but the most recent Cochrane review on the subject fails to show any prophylactic efficacy of administered antibodies in preterm neonates, though no studies have thoroughly investigated specific isotypes and subclasses of prophylactic antibodies, including sIGA specifically, in preterm infants [175, 180]. While there is some evidence to suggest that infant formula supplemented with specific HMOs is both safe and beneficial for infant health, no adequately powered RCTs to date have provided results about the prophylactic role of HMOs toward NEC, LOS, and mortality in formula-fed preterm infants [181].
While it is probable that most or all of these breast milk components play important physiological roles in protecting the health of the breastfed neonate, current human studies have been unable to convincingly recapitulate their protective prophylactic roles in isolation.
Future directions and conclusion
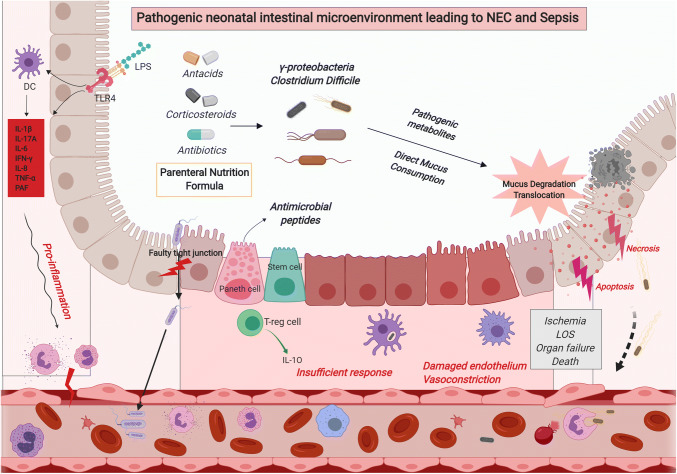
This review summarizes the recent findings related to the intertwined preterm neonatal intestinal immunity and commensal microbes of the intestinal tract. The inflammatory and microbial signatures of the fetal and neonatal periods have lasting consequences throughout life. It is during these periods that the microbiome and immune systems co-develop and are most susceptible to intervention. There is potential during these stages to set the course for immune homeostasis, just as there is vulnerability to be set astray towards pathogenic inflammation, dysbiosis, and chronic disease. What is currently thought about the former is summarized in Fig. 1, while our best concept of the latter of these conditions is summarized in Fig. 2. With better understanding and implementation of principles outlined in this review, it is our hope that preterm neonates will receive improved therapies and subsequent outcomes in the future.
Fig. 2.
Pathogenic neonatal intestinal microenvironment leading to NEC and sepsis, especially in the vulnerable preterm infant
Public health initiatives involving proper pregnancy practices and breastfeeding should continue to be a top priority for the improvement of neonatal intestinal mucosal immunity and health outcomes in the US. Beyond public health approaches, an increased multi-omics-based understanding of cross-talk between the neonatal preterm intestinal microbiota and the associated mucosal immunity will serve to educate future interventional strategies to treat and prevent diseases such as necrotizing enterocolitis and neonatal late-onset sepsis, which currently have progress stymied for lack of effective new approaches. Novel strategies might include practices that currently lack enough understanding for implementation, such as improved infant formula, donor breast milk supplementation, engineered bacteriophage treatment, altered antibiotic and steroid use, birthing practices, and more. Learning to engineer microbial community compositions of preterm infants and specific individual gut microbes and phages within them for therapeutic use might prove to be a promising approach toward remedying the imbalance of incoming basic science to the relative dearth of applied translational therapies involving the neonatal microbiome.
Improvements in culture methods might further our understanding of the “in utero” microbiome more than the current bacterial DNA harvested from healthy placenta samples. As evidence mounts, the questions investigators ask may extend past “Is the womb sterile?” Finally, improved models for necrotizing enterocolitis and neonatal late-onset sepsis, such as innovations of more realistic organoid and murine models, will further our understanding of the root causes of pathophysiological mechanisms along with novel therapeutics that target them. Only time will tell what outcomes may come of these avenues.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Valentine G, Chu DM, Stewart CJ, Aagaard KM. Relationships between perinatal interventions, maternal-infant microbiomes, and neonatal outcomes. Clin Perinatol. 2018;45(2):339–355. doi: 10.1016/j.clp.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergstrom A, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American, and Australian cohorts. BJOG. 2019 doi: 10.1111/1471-0528.15661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst Rev. 2018;11:CD003402. doi: 10.1002/14651858.cd003402.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macpherson AJ, de Aguero MG, Ganal-Vonarburg SC. How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol. 2017;17(8):508–517. doi: 10.1038/nri.2017.58. [DOI] [PubMed] [Google Scholar]
- 5.Torow N, Hornef MW. The neonatal window of opportunity: setting the stage for life-long host-microbial interaction and immune homeostasis. J Immunol (Baltimore, Md: 1950) 2017;198(2):557–563. doi: 10.4049/jimmunol.1601253. [DOI] [PubMed] [Google Scholar]
- 6.Grev J, Berg M, Soll R. Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database of Syst Rev. 2018 doi: 10.1002/14651858.cd012519.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willyard C. Could baby’s first bacteria take root before birth? Nature. 2018;553(7688):264–266. doi: 10.1038/d41586-018-00664-8. [DOI] [PubMed] [Google Scholar]
- 8.Mshvildadze M, Neu J, Schuster J, Theriaque D, Li N, Mai V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr. 2010;156(1):20–25. doi: 10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ardissone AN, Cruz DM, Davis-Richardson AG, Rechcigl KT, Li N, Drew JC, et al. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS One. 2014 doi: 10.1371/journal.pone.0090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med. 2012;17(1):2–11. doi: 10.1016/j.siny.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Funkhouser LJ, Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11(8):e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Nitert MD. Contributions of the maternal oral and gut microbiome to placental microbial colonization in overweight and obese pregnant women. Sci Rep. 2017;7(1):2860. doi: 10.1038/s41598-017-03066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elovitz MA, Gajer P, Riis V, Brown AG, Humphrys MS, Holm JB, et al. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat Commun. 2019;10(1):1305. doi: 10.1038/s41467-019-09285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown RG, Chan D, Terzidou V, Lee YS, Smith A, Marchesi JR, et al. Prospective observational study of vaginal microbiota pre- and post-rescue cervical cerclage. BJOG. 2019;126(7):916–925. doi: 10.1111/1471-0528.15600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, et al. The vaginal microbiome and preterm birth. Nat Med. 2019 doi: 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serrano MG, Parikh HI, Brooks JP, Edwards DJ, Arodz TJ, Edupuganti L, et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat Med. 2019 doi: 10.1038/s41591-019-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim ES, Rodriguez C, Holtz LR. Amniotic fluid from healthy term pregnancies does not harbor a detectable microbial community. Microbiome. 2018;6(1):87. doi: 10.1186/s40168-018-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5(1):48. doi: 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stinson LF, Keelan JA, Payne MS. Characterization of the bacterial microbiome in first-pass meconium using propidium monoazide (PMA) to exclude nonviable bacterial DNA. Lett Appl Microbiol. 2019;68(5):378–385. doi: 10.1111/lam.13119. [DOI] [PubMed] [Google Scholar]
- 22.Theis KR, Romero R, Winters AD, Greenberg JM, Gomez-Lopez N, Alhousseini A, et al. Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. Am J Obstet Gynecol. 2019;220(3):267. doi: 10.1016/j.ajog.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Goffau MC, Lager S, Sovio U, Gaccioli F, Cook E, Peacock SJ, et al. Human placenta has no microbiome but can contain potential pathogens. Nature. 2019 doi: 10.1038/s41586-019-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang I, Corwin EJ, Brennan PA, Jordan S, Murphy JR, Dunlop A. The infant microbiome: implications for infant health and neurocognitive development. Nurs Res. 2016;65(1):76–88. doi: 10.1097/nnr.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gronlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28(1):19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Miller S, Abalos E, Chamillard M, Ciapponi A, Colaci D, Comandé D, et al. Beyond too little, too late and too much, too soon: a pathway towards evidence-based, respectful maternity care worldwide. Lancet. 2016;388(10056):2176–2192. doi: 10.1016/S0140-6736(16)31472-6. [DOI] [PubMed] [Google Scholar]
- 27.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22:713. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- 28.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63(4):559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 29.Underwood MA, German JB, Lebrilla CB, Mills DA. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatr Res. 2015;77(1–2):229–235. doi: 10.1038/pr.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wampach L, Heintz-Buschart A, Fritz JV, Ramiro-Garcia J, Habier J, Herold M, et al. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat Commun. 2018;9(1):5091. doi: 10.1038/s41467-018-07631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zachariassen LF, Krych L, Rasmussen SH, Nielsen DS, Kot W, Holm TL, et al. Cesarean section induces microbiota-regulated immune disturbances in C57BL/6 mice. J Immunol (Baltimore, Md: 1950) 2018 doi: 10.4049/jimmunol.1800666. [DOI] [PubMed] [Google Scholar]
- 32.Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014;10:CD007482. doi: 10.1002/14651858.cd007482.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adeyeye TE, Yeung EH, McLain AC, Lin S, Lawrence DA, Bell EM. Wheeze and food allergies in children born via cesarean section—the upstate KIDS study. Am J Epidemiol. 2018 doi: 10.1093/aje/kwy257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keag OE, Norman JE, Stock SJ. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: systematic review and meta-analysis. PLoS Med. 2018;15(1):e1002494. doi: 10.1371/journal.pmed.1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stinson LF, Payne MS, Keelan JA. A critical review of the bacterial Baptism hypothesis and the impact of cesarean delivery on the infant microbiome. Front Med. 2018;5:135. doi: 10.3389/fmed.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22(3):250–253. doi: 10.1038/nm.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017;23(3):314–326. doi: 10.1038/nm.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumann-Dudenhoeffer AM, D’Souza AW, Tarr PI, Warner BB, Dantas G. Infant diet and maternal gestational weight gain predict early metabolic maturation of gut microbiomes. Nat Med. 2018;24(12):1822–1829. doi: 10.1038/s41591-018-0216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witt WP, Wisk LE, Cheng ER, Mandell K, Chatterjee D, Wakeel F, et al. Determinants of cesarean delivery in the US: a lifecourse approach. Matern Child Health J. 2015;19(1):84–93. doi: 10.1007/s10995-014-1498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hermansson H, Kumar H, Collado MC, Salminen S, Isolauri E, Rautava S. Breast milk microbiota is shaped by mode of delivery and intrapartum antibiotic exposure. Front Nutr. 2019;6:4. doi: 10.3389/fnut.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nogacka A, Salazar N, Suárez M, Milani C, Arboleya S, Solís G, et al. Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full-term neonates. Microbiome. 2017;5(1):93. doi: 10.1186/s40168-017-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson MK, Crofts TS, Dantas G. Antibiotics and the developing infant gut microbiota and resistome. Curr Opin Microbiol. 2015;27:51–56. doi: 10.1016/j.mib.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henderickx JGE, Zwittink RD, van Lingen RA, Knol J, Belzer C. The preterm gut microbiota: an inconspicuous challenge in nutritional neonatal care. Front Cell Infect Microbiol. 2019 doi: 10.3389/fcimb.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Risnes KR, Belanger K, Murk W, Bracken MB. Antibiotic exposure by 6 months and asthma and allergy at 6 years: findings in a cohort of 1,401 US children. Am J Epidemiol. 2011;173(3):310–318. doi: 10.1093/aje/kwq400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012;130(4):e794–e803. doi: 10.1542/peds.2011-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penders J, Kummeling I, Thijs C. Infant antibiotic use and wheeze and asthma risk: a systematic review and meta-analysis. Eur Respir J. 2011;38(2):295–302. doi: 10.1183/09031936.00105010. [DOI] [PubMed] [Google Scholar]
- 47.Metsala J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clin Exp Allergy. 2015;45(1):137–145. doi: 10.1111/cea.12356. [DOI] [PubMed] [Google Scholar]
- 48.Zwittink RD, Renes IB, van Lingen RA, van Zoeren-Grobben D, Konstanti P, Norbruis OF, et al. Association between duration of intravenous antibiotic administration and early-life microbiota development in late-preterm infants. Eur J Clin Microbiol Infect Dis. 2018;37(3):475–483. doi: 10.1007/s10096-018-3193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ment LR, Oh W, Ehrenkranz RA, Philip AGS, Duncan CC, Makuch RW. Antenatal steroids, delivery mode, and intraventricular hemorrhage in preterm infants. Am J Obstet Gynecol. 1995;172(3):795–800. doi: 10.1016/0002-9378(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 50.Abbasi S, Oxford C, Gerdes J, Sehdev H, Ludmir J. Antenatal Corticosteroids prior to 24 weeks’ gestation and neonatal outcome of extremely low birth weight infants. Am J Perinatol. 2010;27(01):061–066. doi: 10.1055/s-0029-1223269. [DOI] [PubMed] [Google Scholar]
- 51.Ho TTB, Groer MW, Kane B, Yee AL, Torres BA, Gilbert JA, et al. Dichotomous development of the gut microbiome in preterm infants. Microbiome. 2018;6(1):157. doi: 10.1186/s40168-018-0547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torow N, Marsland BJ, Hornef MW, Gollwitzer ES. Neonatal mucosal immunology. Mucosal Immunol. 2017;10(1):5–17. doi: 10.1038/mi.2016.81. [DOI] [PubMed] [Google Scholar]
- 53.Drozdowski LA, Clandinin T, Thomson AB. Ontogeny, growth and development of the small intestine: understanding pediatric gastroenterology. World J Gastroenterol. 2010;16(7):787–799. doi: 10.3748/wjg.v16.i7.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. 2016;13(10):590–600. doi: 10.1038/nrgastro.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker WA. Intestinal colonization and programming of the intestinal immune response. J Clin Gastroenterol. 2014;48:S8–S11. doi: 10.1097/mcg.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel AL, Mutlu EA, Sun Y, Koenig L, Green S, Jakubowicz A, et al. Longitudinal survey of microbiota in hospitalized preterm very-low-birth-weight infants. J Pediatr Gastroenterol Nutr. 2016;62(2):292–303. doi: 10.1097/mpg.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fouhy F, Watkins C, Hill CJ, O’Shea CA, Nagle B, Dempsey EM, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi: 10.1038/s41467-019-09252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carlisle EM, Morowitz MJ. The intestinal microbiome and necrotizing enterocolitis. Curr Opin Pediatr. 2013;25(3):382–387. doi: 10.1097/MOP.0b013e3283600e91. [DOI] [PubMed] [Google Scholar]
- 60.Cilieborg MS, Boye M, Sangild PT. Bacterial colonization and gut development in preterm neonates. Early Hum Dev. 2012;88:S41–S49. doi: 10.1016/j.earlhumdev.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3(8):944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chernikova DA, Madan JC, Housman ML, Zain-ul-abideen M, Lundgren SN, Morrison HG, et al. The premature infant gut microbiome during the first 6 weeks of life differs based on gestational maturity at birth. Pediatr Res. 2018;84(1):71–79. doi: 10.1038/s41390-018-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biagi E, Aceti A, Quercia S, Beghetti I, Rampelli S, Turroni S, et al. Microbial community dynamics in mother’s milk and infant’s mouth and gut in moderately preterm infants. Front Microbiol. 2018;9:2512. doi: 10.3389/fmicb.2018.02512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alexeev EE, He X, Slupsky CM, Lönnerdal B. Effects of iron supplementation on growth, gut microbiota, metabolomics and cognitive development of rat pups. PLoS One. 2017;12(6):e0179713. doi: 10.1371/journal.pone.0179713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity. 2017;46(3):350–363. doi: 10.1016/j.immuni.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Meyer-Hoffert U, Hornef MW, Henriques-Normark B, Axelsson LG, Midtvedt T, Putsep K, et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57(6):764–771. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]
- 67.Newburg DS, He Y. Neonatal gut microbiota and human milk glycans cooperate to attenuate infection and inflammation. Clin Obstet Gynecol. 2015;58(4):814–826. doi: 10.1097/grf.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 68.Bozzi Cionci N, Baffoni L, Gaggia F, Di Gioia D. Therapeutic microbiology: the role of bifidobacterium breve as food supplement for the prevention/treatment of paediatric diseases. Nutrients. 2018 doi: 10.3390/nu10111723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boquien C-Y. Human milk: an ideal food for nutrition of preterm newborn. Front Pediatr. 2018;6:295. doi: 10.3389/fped.2018.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oozeer R, van Limpt K, Ludwig T, Ben Amor K, Martin R, Wind RD, et al. Intestinal microbiology in early life: specific prebiotics can have similar functionalities as human-milk oligosaccharides. Am J Clin Nutr. 2013;98(2):561s–571s. doi: 10.3945/ajcn.112.038893. [DOI] [PubMed] [Google Scholar]
- 71.Bering SB. Human milk oligosaccharides to prevent gut dysfunction and necrotizing enterocolitis in preterm neonates. Nutrients. 2018 doi: 10.3390/nu10101461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pourcyrous M, Nolan VG, Goodwin A, Davis SL, Buddington RK. Fecal short-chain fatty acids of very-low-birth-weight preterm infants fed expressed breast milk or formula. J Pediatr Gastroenterol Nutr. 2014;59(6):725–731. doi: 10.1097/mpg.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 73.Hassiotou F, Hepworth AR, Metzger P, Tat Lai C, Trengove N, Hartmann PE, et al. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin Transl Immunol. 2013;2(4):e3. doi: 10.1038/cti.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moles JP, Tuaillon E, Kankasa C, Bedin AS, Nagot N, Marchant A, et al. Breastmilk cell trafficking induces microchimerism-mediated immune system maturation in the infant. Pediatr Allergy Immunol. 2018;29(2):133–143. doi: 10.1111/pai.12841. [DOI] [PubMed] [Google Scholar]
- 75.Conway SP, Dear PR, Smith I. Immunoglobulin profile of the preterm baby. Arch Dis Child. 1985;60(3):208–212. doi: 10.1136/adc.60.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van den Berg JP, Westerbeek EA, van der Klis FR, Berbers GA, van Elburg RM. Transplacental transport of IgG antibodies to preterm infants: a review of the literature. Early Hum Dev. 2011;87(2):67–72. doi: 10.1016/j.earlhumdev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Pou C, Nkulikiyimfura D, Henckel E, Olin A, Lakshmikanth T, Mikes J, et al. The repertoire of maternal anti-viral antibodies in human newborns. Nat Med. 2019 doi: 10.1038/s41591-019-0392-8. [DOI] [PubMed] [Google Scholar]
- 78.McElroy SJ, Weitkamp JH. Innate immunity in the small intestine of the preterm infant. NeoReviews. 2011;12(9):e517–e526. doi: 10.1542/neo.12-9-e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28(6):740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kallman J, Schollin J, Schalen C, Erlandsson A, Kihlstrom E. Impaired phagocytosis and opsonisation towards group B streptococci in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 1998;78(1):F46–F50. doi: 10.1136/fn.78.1.F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shroff KE, Cebra JJ. Development of mucosal humoral immune responses in germ-free (GF) mice. Adv Exp Med Biol. 1995;371:441–446. doi: 10.1007/978-1-4615-1941-6_92. [DOI] [PubMed] [Google Scholar]
- 82.Butler JE, Santiago-Mateo K, Wertz N, Sun X, Sinkora M, Francis DL. Antibody repertoire development in fetal and neonatal piglets. XXIV. Hypothesis: the ileal Peyer patches (IPP) are the major source of primary, undiversified IgA antibodies in newborn piglets. Dev Comp Immunol. 2016;65:340–351. doi: 10.1016/j.dci.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 83.Bollinger RR, Everett ML, Palestrant D, Love SD, Lin SS, Parker W. Human secretory immunoglobulin A may contribute to biofilm formation in the gut. Immunology. 2003;109(4):580–587. doi: 10.1046/j.1365-2567.2003.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hock A, Miyake H, Li B, Lee C, Ermini L, Koike Y, et al. Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg. 2017;52(5):755–759. doi: 10.1016/j.jpedsurg.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 85.Kahn S, Liao Y, Du X, Xu W, Li J, Lonnerdal B. Exosomal microRNAs in milk from mothers delivering preterm infants survive in vitro digestion and are taken up by human intestinal cells. Mol Nutr Food Res. 2018;62(11):e1701050. doi: 10.1002/mnfr.201701050. [DOI] [PubMed] [Google Scholar]
- 86.Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14:216. doi: 10.1186/1471-2431-14-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meng X, Dunsmore G, Koleva P, Elloumi Y, Wu RY, Sutton RT, et al. The profile of human milk metabolome, cytokines, and antibodies in inflammatory bowel diseases versus healthy mothers, and potential impact on the newborn. J Crohns Colitis. 2019;13(4):431–441. doi: 10.1093/ecco-jcc/jjy186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kien CL. Digestion, absorption, and fermentation of carbohydrates in the newborn. Clin Perinatol. 1996;23(2):211–228. doi: 10.1016/S0095-5108(18)30239-2. [DOI] [PubMed] [Google Scholar]
- 89.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;6145:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Macias-Ceja DC, Ortiz-Masiá D, Salvador P, Gisbert-Ferrándiz L, Hernández C, Hausmann M, et al. Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunol. 2018 doi: 10.1038/s41385-018-0087-3. [DOI] [PubMed] [Google Scholar]
- 91.Lei W, Ren W, Ohmoto M, Urban JF, Jr, Matsumoto I, Margolskee RF, et al. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc Natl Acad Sci USA. 2018;115(21):5552–5557. doi: 10.1073/pnas.1720758115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elahi S, Thompson DR, Van Kessel J, Babiuk LA, Gerdts V. protective role of passively transferred maternal cytokines against bordetella pertussis infection in newborn piglets. Infect Immun. 2017;85(4):e01063-16. doi: 10.1128/IAI.01063-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dawod B, Marshall JS. Cytokines and soluble receptors in breast milk as enhancers of oral tolerance development. Front Immunol. 2019;10:16. doi: 10.3389/fimmu.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin N Am. 2013;60(1):49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perez PF, Dore J, Leclerc M, Levenez F, Benyacoub J, Serrant P, et al. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007;119(3):e724–e732. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- 96.Arslanoglu S, Corpeleijn W, Moro G, Braegger C, Campoy C, Colomb V, et al. Donor human milk for preterm infants: current evidence and research directions. J Pediatr Gastroenterol Nutr. 2013;57(4):535–542. doi: 10.1097/MPG.0b013e3182a3af0a. [DOI] [PubMed] [Google Scholar]
- 97.Wesolowska A, Sinkiewicz-Darol E, Barbarska O, Strom K, Rutkowska M, Karzel K, et al. New achievements in high-pressure processing to preserve human milk bioactivity. Front Pediatr. 2018;6:323. doi: 10.3389/fped.2018.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y, Nguyen DN, de Waard M, Christensen L, Zhou P, Jiang P, et al. Pasteurization procedures for donor human milk affect body growth, intestinal structure, and resistance against bacterial infections in preterm pigs. J Nutr. 2017;147(6):1121–1130. doi: 10.3945/jn.116.244822. [DOI] [PubMed] [Google Scholar]
- 99.Moossavi S, Sepehri S, Robertson B, Bode L, Goruk S, Field CJ, et al. Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host Microbe. 2019;25(2):324–335. doi: 10.1016/j.chom.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 100.Perrin MT, Goodell LS, Allen JC, Fogleman A. A mixed-methods observational study of human milk sharing communities on facebook. Breastfeed Med. 2014;9(3):128–134. doi: 10.1089/bfm.2013.0114. [DOI] [PubMed] [Google Scholar]
- 101.Palmquist AEL, Doehler K. Contextualizing online human milk sharing: structural factors and lactation disparity among middle income women in the US. Soc Sci Med. 2014;122:140–147. doi: 10.1016/j.socscimed.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 102.Urbaniak C, McMillan A, Angelini M, Gloor GB, Sumarah M, Burton JP, et al. Effect of chemotherapy on the microbiota and metabolome of human milk, a case report. Microbiome. 2014;2:24. doi: 10.1186/2049-2618-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morelli L. Postnatal development of intestinal microflora as influenced by infant nutrition. J Nutr. 2008;138(9):1791S–1795S. doi: 10.1093/jn/138.9.1791S. [DOI] [PubMed] [Google Scholar]
- 104.Yu JC, Khodadadi H, Malik A, Davidson B, Salles E, Bhatia J, et al. Innate immunity of neonates and infants. Front Immunol. 2018;9:1759. doi: 10.3389/fimmu.2018.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64(1):38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Investig. 2015 doi: 10.1172/JCI83356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Michaëlsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176(10):5741. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- 108.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202(7):901. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharma AA, Jen R, Butler A, Lavoie PM. The developing human preterm neonatal immune system: a case for more research in this area. Clin Immunol (Orlando, Fla) 2012;145(1):61–68. doi: 10.1016/j.clim.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol (Baltimore, Md: 1950) 2009;183(11):7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med. 2011;24(1):25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- 112.Soliman A, Michelsen KS, Karahashi H, Lu J, Meng FJ, Qu X, et al. Platelet-activating factor induces TLR4 expression in intestinal epithelial cells: implication for the pathogenesis of necrotizing enterocolitis. PLoS One. 2010;5(10):e15044. doi: 10.1371/journal.pone.0015044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frost BL, Caplan MS. Necrotizing enterocolitis: pathophysiology, platelet-activating factor, and probiotics. Semin Pediatr Surg. 2013;22(2):88–93. doi: 10.1053/j.sempedsurg.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 114.Kennedy D, Zarandi ER, Aminzadeh F, Hassanshahi G, Khorramdelazad H, Arababadi MK, et al. Cytokines in preterm delivery. Lab Med. 2012;43(4):27–30. doi: 10.1309/LMY9ILPGSETU2CO0. [DOI] [Google Scholar]
- 115.Benkoe T, Baumann S, Weninger M, Pones M, Reck C, Rebhandl W, et al. Comprehensive evaluation of 11 cytokines in premature infants with surgical necrotizing enterocolitis. PLoS One. 2013;8(3):e58720. doi: 10.1371/journal.pone.0058720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One. 2011;6(3):e17776. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hackam DJ, Sodhi CP. Toll-like receptor-mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell Mol Gastroenterol Hepatol. 2018;6(2):229–238. doi: 10.1016/j.jcmgh.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol (Baltimore, Md: 1950) 2009;182(1):636–646. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jones R, Cosway EJ, Willis C, White AJ, Jenkinson WE, Fehling HJ, et al. Dynamic changes in intrathymic ILC populations during murine neonatal development. Eur J Immunol. 2018;48(9):1481–1491. doi: 10.1002/eji.201847511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gomez Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, et al. The maternal microbiota drives early postnatal innate immune development. Science (New York, NY) 2016;351(6279):1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 121.Gleeson M, Cripps AW. Development of mucosal immunity in the first year of life and relationship to sudden infant death syndrome. FEMS Immunol Med Microbiol. 2004;42(1):21–33. doi: 10.1016/j.femsim.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 122.Heel KA, McCauley RD, Papadimitriou JM, Hall JC. Review: peyer’s patches. J Gastroenterol Hepatol. 1997;12(2):122–136. doi: 10.1111/j.1440-1746.1997.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 123.Maclennan KA. Reaction patterns of the lymph node. Part 1 cell types and functions. J Clin Pathol. 1991;44(12):1039. doi: 10.1136/jcp.44.12.1039-a. [DOI] [Google Scholar]
- 124.Crespo M, Martinez DG, Cerissi A, Rivera-Reyes B, Bernstein HB, Lederman MM, et al. Neonatal T-cell maturation and homing receptor responses to Toll-like receptor ligands differ from those of adult naive T cells: relationship to prematurity. Pediatr Res. 2012;71(2):136–143. doi: 10.1038/pr.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cho SX, Berger PJ, Nold-Petry CA, Nold MF. The immunological landscape in necrotising enterocolitis. Expert Rev Mol Med. 2016;18:e12. doi: 10.1017/erm.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rueda CM, Moreno-Fernandez ME, Jackson CM, Kallapur SG, Jobe AH, Chougnet CA. Neonatal regulatory T cells have reduced capacity to suppress dendritic cell function. Eur J Immunol. 2015;45(9):2582–2592. doi: 10.1002/eji.201445371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Neu J. Necrotizing enterocolitis: the mystery goes on. Neonatology. 2014;106(4):289–295. doi: 10.1159/000365130. [DOI] [PubMed] [Google Scholar]
- 128.Weitkamp J-H, Koyama T, Rock MT, Correa H, Goettel JA, Matta P, et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut. 2013;62(1):73–82. doi: 10.1136/gutjnl-2011-301551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wynn JL, Polin RA. Progress in the management of neonatal sepsis: the importance of a consensus definition. Pediatr Res. 2018;83(1–1):13–15. doi: 10.1038/pr.2017.224. [DOI] [PubMed] [Google Scholar]
- 130.Neu J, Modi N, Caplan M. Necrotizing enterocolitis comes in different forms: historical perspectives and defining the disease. Semin Fetal Neonatal Med. 2018;23(6):370–373. doi: 10.1016/j.siny.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 131.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129(2):e298–e304. doi: 10.1542/peds.2011-2022. [DOI] [PubMed] [Google Scholar]
- 133.Jin YT, Duan Y, Deng XK, Lin J. Prevention of necrotizing enterocolitis in premature infants—an updated review. World J Clin Pediatr. 2019;8(2):23–32. doi: 10.5409/wjcp.v8.i2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang K, Tao G, Sylvester KG. Recent advances in prevention and therapies for clinical or experimental necrotizing enterocolitis. Dig Dis Sci. 2019 doi: 10.1007/s10620-019-05618-2. [DOI] [PubMed] [Google Scholar]
- 135.Gonzalez-Rivera R, Culverhouse RC, Hamvas A, Tarr PI, Warner BB. The age of necrotizing enterocolitis onset: an application of Sartwell’s incubation period model. J Perinatol. 2011;31(8):519–523. doi: 10.1038/jp.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gordon PV, Clark R, Swanson JR, Spitzer A. Can a national dataset generate a nomogram for necrotizing enterocolitis onset? J Perinatol. 2014;34(10):732–735. doi: 10.1038/jp.2014.137. [DOI] [PubMed] [Google Scholar]
- 137.Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5(1):31. doi: 10.1186/s40168-017-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eaton S, Rees CM, Hall NJ. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology. 2017;111(4):423–430. doi: 10.1159/000458462. [DOI] [PubMed] [Google Scholar]
- 139.Sanderson IR, Ezzell RM, Kedinger M, Erlanger M, Xu ZX, Pringault E, et al. Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci USA. 1996;93(15):7717–7722. doi: 10.1073/pnas.93.15.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Serra D, Mayr U, Boni A, Lukonin I, Rempfler M, Challet Meylan L, et al. Self-organization and symmetry breaking in intestinal organoid development. Nature. 2019;569(7754):66–72. doi: 10.1038/s41586-019-1146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18(3):246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 142.Pitt J, Barlow B, Heird WC. Protection against experimental necrotizing enterocolitis by maternal milk. I. Role of milk leukocytes. Pediatr Res. 1977;11(8):906–909. doi: 10.1203/00006450-197708000-00011. [DOI] [PubMed] [Google Scholar]
- 143.Young CM, Kingma SD, Neu J. Ischemia-reperfusion and neonatal intestinal injury. J Pediatr. 2011;158(2):e25–e38. doi: 10.1016/j.jpeds.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 144.Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J. 2001;15(8):1398–1403. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]
- 145.Wandro S, Osborne S, Enriquez C, Bixby C, Arrieta A, Whiteson K. The microbiome and metabolome of preterm infant stool are personalized and not driven by health outcomes, including necrotizing enterocolitis and late-onset sepsis. mSphere. 2018 doi: 10.1128/msphere.00104-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Son M, Grobman WA, Miller ES. Is mode of delivery associated with the risk of necrotizing enterocolitis? Am J Obstet Gynecol. 2016;215(3):389.e1–389.e4. doi: 10.1016/j.ajog.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 147.Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18(1):83. doi: 10.1186/s13059-017-1215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Neu J. Multiomics-based strategies for taming intestinal inflammation in the neonate. Curr Opin Clin Nutr Metab Care. 2019;22(3):217–222. doi: 10.1097/mco.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 149.Gibson MK, Wang B, Ahmadi S, Burnham CA, Tarr PI, Warner BB, et al. Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome. Nat Microbiol. 2016;1:16024. doi: 10.1038/nmicrobiol.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159(3):392–397. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abdel Ghany EA, Ali AA. Empirical antibiotic treatment and the risk of necrotizing enterocolitis and death in very low birth weight neonates. Ann Saudi Med. 2012;32(5):521–526. doi: 10.5144/0256-4947.2012.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of enterobacter. J Pediatr. 2014 doi: 10.1016/j.jpeds.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Furfaro LL, Chang BJ, Payne MS. Applications for bacteriophage therapy during pregnancy and the perinatal period. Front Microbiol. 2018;8:2660. doi: 10.3389/fmicb.2017.02660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dedrick RM, Guerrero-Bustamante CA, Garlena RA, Russell DA, Ford K, Harris K, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med. 2019;25(5):730–733. doi: 10.1038/s41591-019-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Torrazza RM, Ukhanova M, Wang X, Sharma R, Hudak ML, Neu J, et al. Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. PLoS One. 2013;8(12):e83304. doi: 10.1371/journal.pone.0083304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011;6(6):e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730):1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 159.Shermadou ES, Mavrogeorgos G. Neonatal sepsis. Treasure Island: StatPearls Publishing LLC; 2018. [Google Scholar]
- 160.Raymond SL, Stortz JA, Mira JC, Larson SD, Wynn JL, Moldawer LL. Immunological defects in neonatal sepsis and potential therapeutic approaches. Front Pediatr. 2017;5:14. doi: 10.3389/fped.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tourneur E, Chassin C. Neonatal immune adaptation of the gut and its role during infections. Clin Dev Immunol. 2013;2013:270301. doi: 10.1155/2013/270301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gebbers JO, Laissue JA. Bacterial translocation in the normal human appendix parallels the development of the local immune system. Ann N Y Acad Sci. 2004;1029:337–343. doi: 10.1196/annals.1309.015. [DOI] [PubMed] [Google Scholar]
- 163.Madan JC, Salari RC, Saxena D, Davidson L, O’Toole GA, Moore JH, et al. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):F456–F462. doi: 10.1136/fetalneonatal-2011-301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Burns JL, Griffith A, Barry JJ, Jonas M, Chi EY. Transcytosis of gastrointestinal epithelial cells by Escherichia coli K1. Pediatr Res. 2001;49(1):30–37. doi: 10.1203/00006450-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 165.Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci. 2012;1258(1):25–33. doi: 10.1111/j.1749-6632.2012.06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Anand RJ, Leaphart CL, Mollen KP, Hackam DJ. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock. 2007;27(2):124–133. doi: 10.1097/01.shk.0000239774.02904.65. [DOI] [PubMed] [Google Scholar]
- 167.Sherman MP. New concepts of microbial translocation in the neonatal intestine: mechanisms and prevention. Clin Perinatol. 2010;37(3):565–579. doi: 10.1016/j.clp.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sansonetti PJ, Phalipon A. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin Immunol. 1999;11(3):193–203. doi: 10.1006/smim.1999.0175. [DOI] [PubMed] [Google Scholar]
- 169.Wilmore JR, Gaudette BT, Gomez Atria D, Hashemi T, Jones DD, Gardner CA, et al. Commensal microbes induce serum IgA responses that protect against polymicrobial sepsis. Cell Host Microbe. 2018;23(3):302–311. doi: 10.1016/j.chom.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302(13):1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 171.Sherman MP. Lactoferrin and necrotizing enterocolitis. Clin Perinatol. 2013;40(1):79–91. doi: 10.1016/j.clp.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ashraf RN, Jalil F, Zaman S, Karlberg J, Khan SR, Lindblad BS, et al. Breast feeding and protection against neonatal sepsis in a high risk population. Arch Dis Child. 1991;66(4):488–490. doi: 10.1136/adc.66.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Herrmann K, Carroll K. An exclusively human milk diet reduces necrotizing enterocolitis. Breastfeed Med. 2014;9(4):184–190. doi: 10.1089/bfm.2013.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.He Y, Cao L, Yu J. Prophylactic lactoferrin for preventing late-onset sepsis and necrotizing enterocolitis in preterm infants: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2018;97(35):e11976. doi: 10.1097/md.0000000000011976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gopalakrishna KP, Macadangdang BR, Rogers MB, Tometich JT, Firek BA, Baker R, et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat Med. 2019;25(7):1110–1115. doi: 10.1038/s41591-019-0480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Group Eti Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial. Lancet. 2019;393(10170):423–433. doi: 10.1016/s0140-6736(18)32221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pammi M, Abrams SA. Enteral lactoferrin for the treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database Syst Rev. 2019;5:CD007138. doi: 10.1002/14651858.cd007138.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Costeloe K, Bowler U, Brocklehurst P, Hardy P, Heal P, Juszczak E, et al. A randomised controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: the Probiotics in Preterm infantS (PiPS) trial. Health Technol Assess. 2016;20(66):1–194. doi: 10.3310/hta20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lenfestey MW, Neu J. Probiotics in newborns and children. Pediatr Clin N Am. 2017;64(6):1271–1289. doi: 10.1016/j.pcl.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 180.Foster JP, Seth R, Cole MJ. Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth weight neonates. Cochrane Database Syst Rev. 2016;4:CD001816. doi: 10.1002/14651858.cd001816.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Reverri EJ, Devitt AA, Kajzer JA, Baggs GE, Borschel MW. Review of the clinical experiences of feeding infants formula containing the human milk oligosaccharide 2′-fucosyllactose. Nutrients. 2018;10(10):1346. doi: 10.3390/nu10101346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Al-Kubaisy W, Al-Rubaey M, Al-Naggar RA, Karim B, Mohd Noor NA. Maternal obesity and its relation with the cesarean section: a hospital based cross sectional study in Iraq. BMC Pregnancy Childbirth. 2014;14:235. doi: 10.1186/1471-2393-14-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Marshall NE, Lau B, Purnell JQ, Thornburg KL. Impact of maternal obesity and breastfeeding intention on lactation intensity and duration. Matern Child Nutr. 2019;15(2):e12732. doi: 10.1111/mcn.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Salihu HM. Maternal obesity and stillbirth. Semin Perinatol. 2011;35(6):340–344. doi: 10.1053/j.semperi.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 185.Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun. 2018;9(1):4462. doi: 10.1038/s41467-018-06929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li Y. Epigenetic mechanisms link maternal diets and gut microbiome to obesity in the offspring. Front Genet. 2018;9:342. doi: 10.3389/fgene.2018.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nnam NM. Improving maternal nutrition for better pregnancy outcomes. Proc Nutr Soc. 2015;74(4):454–459. doi: 10.1017/s0029665115002396. [DOI] [PubMed] [Google Scholar]
- 188.Buklijas T. Food, growth and time: Elsie Widdowson’s and Robert McCance’s research into prenatal and early postnatal growth. Stud Hist Philos Biol Biomed Sci. 2014;47:267–277. doi: 10.1016/j.shpsc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 189.Mandal S, Godfrey KM, McDonald D, Treuren WV, Bjornholt JV, Midtvedt T, et al. Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome. 2016;4(1):55. doi: 10.1186/s40168-016-0200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M, et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016;8(1):77. doi: 10.1186/s13073-016-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. 2014;5:3889. doi: 10.1038/ncomms4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Garcia-So J, Zhang X, Yang X, Rubinstein MR, Mao Y, Kitajewski J, et al. Omega-3 fatty acids suppress Fusobacterium nucleatum-induced placental inflammation originating from maternal endothelial cells. JCI Insight. 2019 doi: 10.1172/jci.insight.125436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Acevedo N, Frumento P, Harb H, Alashkar Alhamwe B, Johansson C, Eick L, et al. Histone acetylation of immune regulatory genes in human placenta in association with maternal intake of olive oil and fish consumption. Int J Mol Sci. 2019 doi: 10.3390/ijms20051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.De-Regil LM, Palacios C, Lombardo LK, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2016;1:CD008873. doi: 10.1002/14651858.cd008873.pub3. [DOI] [PubMed] [Google Scholar]
- 195.Wang B, Cruz Ithier M, Parobchak N, Yadava SM, Schulkin J, Rosen T. Vitamin D stimulates multiple microRNAs to inhibit CRH and other pro-labor genes in human placenta. Endocr Connect. 2018 doi: 10.1530/ec-18-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Palmer AC, Schulze KJ, Khatry SK, De Luca LM, West KP., Jr Maternal vitamin A supplementation increases natural antibody concentrations of preadolescent offspring in rural Nepal. Nutrition (Burbank, Los Angeles County, Calif) 2015;31(6):813–819. doi: 10.1016/j.nut.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 197.Hallman M, Haapalainen A, Huusko JM, Karjalainen MK, Zhang G, Muglia LJ, et al. Spontaneous premature birth as a target of genomic research. Pediatr Res. 2018 doi: 10.1038/s41390-018-0180-z. [DOI] [PubMed] [Google Scholar]
- 198.Gernand AD, Schulze KJ, Stewart CP, West KP, Jr, Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol. 2016;12(5):274–289. doi: 10.1038/nrendo.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2015;2:000230. doi: 10.1002/14651858.cd000230.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]