Abstract
Throughout the animal kingdom sucrose is one of the most palatable and preferred tastants. From an evolutionary perspective, this is not surprising as it is a primary source of energy. However, its overconsumption can result in obesity and an associated cornucopia of maladies, including type 2 diabetes and cardiovascular disease. Here we describe three physiological levels of processing sucrose that are involved in the decision to ingest it: the tongue, gut, and brain. The first section describes the peripheral cellular and molecular mechanisms of sweet taste identification that project to higher brain centers. We argue that stimulation of the tongue with sucrose triggers the formation of three distinct pathways that convey sensory attributes about its quality, palatability, and intensity that results in a perception of sweet taste. We also discuss the coding of sucrose throughout the gustatory pathway. The second section reviews how sucrose, and other palatable foods, interact with the gut–brain axis either through the hepatoportal system and/or vagal pathways in a manner that encodes both the rewarding and of nutritional value of foods. The third section reviews the homeostatic, hedonic, and aversive brain circuits involved in the control of food intake. Finally, we discuss evidence that overconsumption of sugars (or high fat diets) blunts taste perception, the post-ingestive nutritional reward value, and the circuits that control feeding in a manner that can lead to the development of obesity.
Keywords: Sugars, Sweetness, Hedonic taste value, Nutritional value, Gut-reward, AgRP, LHA GABA neurons, Obesity
Introduction
The gustatory system has evolved to identify and ingest a large array of appetitive substances like sucrose, and to avoid unpleasant tasting compounds, like quinine, that are usually perceived as bitter or disgusting [1]. In this manner, the gustatory system must make a rapid decision as to whether a substance is safe or dangerous to swallow. Of course, the decision is dependent on many factors, including the substances’ familiarity and whether culturally, it is acceptable to be eaten [2, 3]. In parallel, the taste system also initiates important physiological reflexes such as salivary secretions and the cephalic phase insulin secretion that facilitates the absorption of nutrients [4]. In the mammalian taste system, we initially review the peripheral transduction mechanisms for sweet tastants and then show how these responses are projected to the higher cortical areas that result in ingestion or aversion.
Sugars and other carbohydrates
Natural sugars such as the disaccharide, sucrose, and the hexoses, glucose and fructose (Fig. 1), are the primary products of photosynthesis and are produced by plants and fruits during the no-light phase of photosynthesis [5, 6]. These sugars, all belong to a group of substances called carbohydrates (Table 1). The simple carbohydrates are divided into monosaccharides (e.g., glucose, fructose, and galactose) and disaccharides (sucrose, maltose, and lactose). Although most simple carbohydrates taste sweet to humans, not all carbohydrates evoke a sweet taste. For example, complex carbohydrates such as starch, a polysaccharide consisting of a large number of glucose units joined by glycosidic bond, is tasteless to humans (but see [7]). Likewise, non-digestible carbohydrates, such as fiber or cellulose, are also tasteless. As compared to sucrose, other caloric sugars such as the sugar alcohols (e.g., sorbitol and mannitol) are generally less sweet (on a weight basis) and have a lower caloric content (Table 1). In this regard, to obtain the same sweetness as sucrose (hedonic value), one would have to ingest a greater number of calories [5, 6].
Fig. 1.
Chemical structures of selected sweet tastants and lactisole, a human sweet taste inhibitor. Molecular structures of some monosaccharides (fructose and glucose), the disaccharide sucrose, sugar alcohols (xylitol, erythritol), the aminoacid d-tryptophan, non-caloric sweet tastants (acesulfame, aspartame, neotame, cyclamate, saccharin, and sucralose), proteins (monellin and brazzein) and a sweet taste inhibitor (lactisole). Monellin and brazzein
Adapted from [270]
Table 1.
Sweet tasting molecules and sweet inhibitors
| Molecule | Composition | Sweetness (relative to wt of sucrose) | Binding site | Obtained from | References | |
|---|---|---|---|---|---|---|
| Caloric Carbohydrates | ||||||
| Mono-saccharides | Fructose | Monomer | 1.2 | T1R2 (ATD) | Fruits, honey and certain root vegetables | [5, 6] |
| Galactose | Monomer | – | – | Milk | [6] | |
| Glucose | Monomer | 0.7 | T1R2 (ATD) | Fruits and vegetables | [5, 6, 46] | |
| Di-saccharides | Lactose | Glucose–Galactose | 0.4 | – | Milk | [6] |
| Maltose | Glucose–Glucose | 0.45 | – | Fruits and vegetables | [6] | |
| Sucrose | Glucose–Fructose | 1 | T1R2 (ATD) T1R3 (ATD) | Fruits and vegetables | [5, 6, 46] | |
| Polysaccharides | Starch sugars (Dextrins) | Polymers of glucose | – | – | Store energy in plants | [6] |
| Maltodextrin | – | – | Rice, potato, corn | [6] | ||
| Glycogen | Branch glucose polymer | – | – | Energy store in animals | [6] | |
| High fructose corn syrup (via glucose isomerase) | 42–55% | 0.8–1 | – | Processed food, soft drinks | [6] | |
| High-maltose corn syrup | Maltose | – | – | Hard candy | [6] | |
| Sugar alcohols (polyols) | Erythritol | – | 06–0.7 | – | – | [5, 6] |
| Isomalt | – | 0.5 | – | – | [6] | |
| Lactilol | – | 0.4 | – | – | [6] | |
| Maltilol | – | 0.9 | – | – | [6] | |
| Mannitol | – | 0.7 | – | – | [6] | |
| Sorbitol | – | 0.6 | – | – | [6] | |
| Xylitol | – | 1 | – | – | [5, 6] | |
| Aminoacids | d-Alanine | – | – | – | – | [46] |
| d-Asparagine | – | – | – | – | [46] | |
| d-Glutamine | – | – | – | – | [46] | |
| d-Histidine | – | – | – | – | [46] | |
| d-Phenylalanine | – | – | – | – | [46] | |
| d-tryptophan | – | – | T1R2 (ATD) | – | [43, 44, 46] | |
| Glycine | – | – | – | – | [46] | |
| Proteinsa | Brazzeina | – | 1900–2000 | T1R2 (ATD) T1R3 (CRD) | Pentadiplandra brazzeana | [5, 44, 46] |
| Neoculin (Curculin) | – | 550 | – | Curculigo latifolia | [46] | |
| Mabinlin | – | 375 | – | Capparis masakai | [46] | |
| Miraculin | – | – | – | Synsepalum dulcificum | [5] | |
| Monellina | – | 3000 | T1R2 (ATD) | Dioscoreophyllum cumminsii | [5, 43, 46] | |
| Pentadin | – | 500 | – | Pentadiplandra brazzeana | [46] | |
| Thaumatina | – | 1600–3000 | T1R3 (CRD) | Thaumatococcus danielli | [5, 43, 46] | |
| High intensity synthetic sweeteners (Non-sugar sweeteners) | Acesulfame-k | – | 130–200 | T1R2 (ATD) | Sulfamate | [5, 43] |
| Advantame | – | 200 | – | Derivative of aspartame | [5] | |
| Aspartamea | – | 180–250 | T1R2 (ATD) | Peptide | [5, 44] | |
| Cyclamatea | – | 30–50 | T1R3 (TMD) | Derivative of sulfamate | [5, 44, 54] | |
| Neohesperidina dihydrochalcone | – | 1000 | T1R3 (TMD) | Obtained from the alkali hydrogenation of neohesperidin isolated from citrus peel | [5, 46, 54] | |
| Neotamea | – | 11,000 | T1R2 (ATD) | Peptide derivative of aspartame | [5, 44] | |
| Rebaudioside A | – | 242–450 | – | Stevia rebaudiana | [5, 46] | |
| Saccharine | – | 150–300 | T1R2 (ATD) | Sulfamate | [5] | |
| Stevioside | – | 210 | – | Stevia rebaudiana | [46] | |
| Sucralose | – | 755 | T1R2 (ATD) T1R3 (ATD) | Derivative of sugar | [5, 46, 54] | |
| Sweet inhibitors | Fibrates | – | – | T1R3 (TMD) | – | [54] |
| Gymnemic acids | – | – | T1R3 (TMD) | Gymnema sylvestre | [54] | |
| Lactisole | – | – | T1R3 (TMD) | Roasted coffee beans | [44, 46, 54] | |
| Phenoxy herbicides | – | – | T1R3 (TMD) | – | [54] | |
ATD amino terminal domain, CRD cystein-rich domain, TMD transmembrane domain
aSweet for humans not for rodents
The tongue: taste receptor cells and their response to tastants
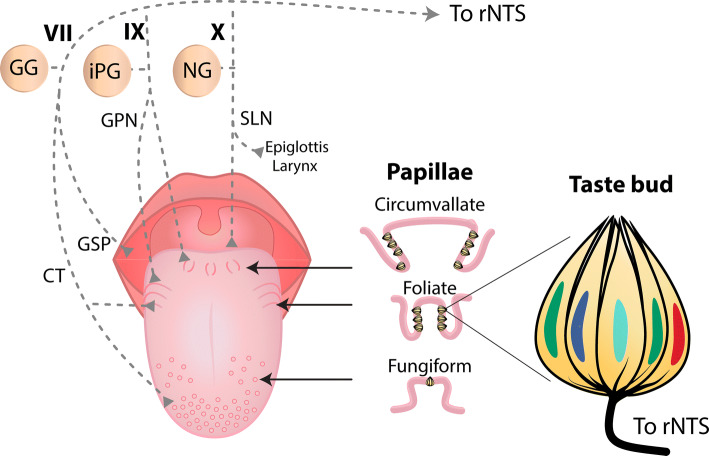
The gustatory system detects a large variety of molecules via activation of receptors located on the apical tips of taste cells located throughout the oral cavity (Fig. 2 and [8]). These neuroepithelial cells are arranged in “garlic-like” shape structures called taste buds that are composed of 50–100 taste cells. On the tongue, taste buds found on the anterior, lateral, and posterior regions are embedded in fungiform, foliate, and circumvallate papillae, respectively (Fig. 3). As described below, when stimulated, taste cells release neurotransmitters and peptides that activate afferent fibers from cranial nerve (CN) branches of the facial (VII, innervating two-third of anterior tongue and soft palate), glossopharyngeal (IX, one-third of posterior tongue), and vagus (X, taste buds in epiglottis [9–11]) that, in turn, transmit information to the central nervous system (CNS) about the attributes of tastant quality, intensity, and hedonic value (see below).
Fig. 2.

A figure of the structure of the human sweet taste receptor hT1R2/T1R3 showing the presence of multiple binding sites. The heterodimer hT1R2 (red) + hT1R3 (green) is primarily constituted by an extracellular Amino Terminal Domain (ATD) which is composed of a Venus Flytrap Domain (VFD) and a Cysteine-Rich Domain (CRD). The VFD consists of two lobes that change their conformation to “open” or “closed,” resembling that of the carnivorous plant. It is linked via the CRD to the seven helical Transmembrane Domain (TMD). Sweet tastants exert their effects by binding to different sites of the receptor (arrows) that result in conformational changes eliciting downstream signaling cascades as shown in Fig. 4.
Figure with permission from [39]
Fig. 3.
Human tongue, taste papillae, and their afferent nerve fibers. Dorsal surface of a human tongue showing the location of the three types of papillae containing taste buds and their associated afferent nerves. In fungiform papillae, taste buds are on the anterior surface, while in foliate and circumvallate papillae they are in trenches on the side and posterior areas of the tongue, respectively (middle panel). Note that the chorda tympani (CT) nerve, a branch of CN VII, innervates both the fungiform papillae and the anterior part of foliate papillae, whereas the posterior part of foliate papillae and the circumvallate papillae are innervated by a sensory branch of CN IX, the glossopharyngeal nerve (GPN). The soft palate is innervated by the greater superficial petrosal (GSP) branch of CN VII, while the root of the tongue, epiglottis, and larynx are innervated by the superior laryngeal (SLN) branch of the vagus nerve (CN X). At right panel shows a longitudinal section of a taste bud showing five different taste receptor cells. GG geniculate ganglion, PG inferior petrosal ganglion, NG nodose ganglion, rNTS rostral portion of the Nucleus Tractus Solitarius
Despite a large array of compounds, only five different primary taste qualities: sweet, salty, bitter, sour, and umami have been described (for humans, there is a debate about the taste of fat [8, 12]). Receptors for each of these qualities have been identified in one of the several types of Taste Receptor Cells (TRCs). Presently, there are four well-characterized types of TRCs. Type I cells, the most prevalent just above 50%, are “glial-like” cells, and one report has them being activated by the hedonically positive amiloride-sensitive responses to NaCl [13]. Type II (19%) TRCs are selective to either sweet (Fig. 2), bitter, and umami (monosodium glutamate) tastants [14, 15], and recently to hedonically negative amiloride-insensitive responses to high concentration of NaCl [16]. The receptors for the first three stimuli are primarily G-protein coupled receptors (GPCRs) [8], although other receptor types are also involved (see below and [17]). For salt (NaCl) taste, in rodents at least, amiloride-sensitive Epithelial Na+ Channels (ENaCs) are involved [18], but for humans, the receptor for low NaCl concentrations appears to be amiloride-insensitive, but remains to be discovered [15]. Likewise, there is little agreement about the identification of the TRCs used to transduce NaCl responses to the brain. Various studies have suggested TRCs Types I [13], II [19, 20], and III [21]. Recently, Roebber et al. [22] implemented a whole mice lingual slice preparation to image amiloride-insensitive NaCl evoked Ca2+ responses directly from the anterior tongue. They revealed that > 80% of NaCl responsive- cells were Type II TRCs, whereas Type III never responded to NaCl [22]. It is well established, however, that Type III cells (15%) are activated by acidic stimuli both by binding to receptors on their apical side and possibly for weak acids by lowering the intracellular pH [19, 23]. Recently, it was discovered that otopetrin-1 (OTOP1), a proton-selective ion channel, functions as an (or the) acid (sour) taste receptor [24–26]. Likewise, lowering intracellular pH has been suggested to block K+ channels and depolarize the TRCs [27]. Activation of these Type III cells evokes serotonin (5-hydroxytryptamine, 5-HT) and ATP release onto neurons synapsed to them [28]. Also, acid-sensing Type III TRCs may mediate taste responses to water [29] and carbonation [30]. Finally, Type IV TRCs, or “basal cells,” are post-mitotic precursor cells that can differentiate into other types of TRCs [31]. Since this review is primarily about sweeteners, we will now focus almost exclusively on Type II cells.
Type II taste cells
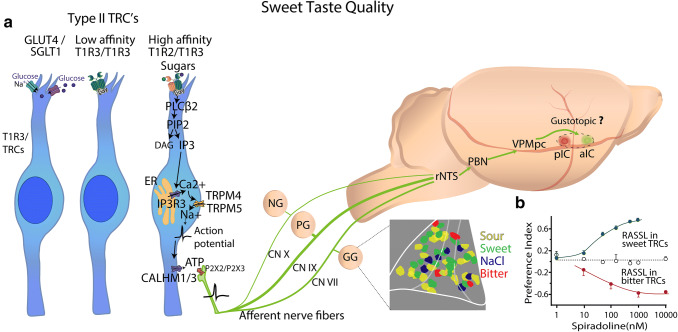
Type II cells lack classical synaptic structures, and thus, they use a non-vesicular ATP-release pathway. On their microvilli at their apical ends, Type II cells contain GPCRs that are generally selective to chemicals associated with sweet, bitter, and umami tastes [8]. Once bound to their receptors, they activate these taste cells via a signal transduction cascade (Fig. 4a) involving the activation -gustducin, PLC-β2 (phospholipase C beta 2), which produces diacylglycerol (DAG), and inositol triphosphate (IP3) from phosphatidylinositol 4,5-bisphosphate (PIP2). In turn, IP3 activates inositol 1,4,5-trisphosphate receptor type 3 (IP3R3) receptors in the endoplasmic reticulum producing an increase in intracellular Ca2+, which then activates TRPM5 (transient receptor potential cation channel subfamily M member 5) channels [32] on their basolateral surface. It has been recently found that TRPM4 also plays a role in sweet taste processing [33]. Thus, the activation of both TRPM4 and TRPM5 by intracellular Ca2+ gates open these channels to permit the entry of Na+ that depolarizes the cell-evoking action potentials that will, via Na channels and the hetero-hexameric voltage-dependent Ca2+ homeostasis modulator 1 (CALHM1) and CALHM3 ion channel [34, 35], release the transmitter ATP which subsequently binds to P2X2/P2X3 receptors on the afferent neurons associated with the particular cell (tastant) and causes them to transmit their information to the CNS [36]. Several receptors for sweet tastants have been suggested including the heterodimer T1R2/T1R3, the homodimer T1R3/T1R3, and glucose transporters (see below and Fig. 4a).
Fig. 4.
Sweet taste transduction and taste pathway into the rodent brain. a Sweet tastants are detected in Type II taste receptor cells (TRCs) by high and low affinity T1R2/T1R3 heterodimers (Fig. 2), a glucose receptor 4 (GLUT4), and a Sodium-Glucose Linked Transporter 1 (SGLT1). The TRC on the right depicts the signaling cascade originated by the binding of sweet tastants [i.e., glucose (purple) and sucrose (green)] which promotes intracellular calcium increases via a PIP2 cascade that binds to IP3R3 receptors in the endoplasmic reticulum (ER) that opens the sodium-selective cation channels TRPM4 and TRPM5, eliciting depolarization and action potentials. Membrane voltage depolarization promotes fast extracellular release of ATP through the hetero-hexameric calcium homeostasis modulator 1/3 (CALHM1/3) ion channel. ATP activates P2X2/P2X3 purinergic receptors that are localized in the afferents of the facial (CN VII) and glossopharyngeal (CN IX) nerves. In addition, vagal (CN X) cranial nerves innervate epiglottis and esophagus. Taste information travels through the Geniculate, Petrosal, and Nodose Ganglia (GG and PG, and NG, respectively) to higher brain centers. The first central relay of gustatory processing is the rostral portion of the Nucleus Tractus Solitarius (rNTS). Then, in rodents (but not humans) information is carried to the Parabrachial Nucleus (PBN) followed by input to the parvocellular portion of the Ventroposteromedial (VPMpc) thalamus until it reaches the primary gustatory or Insular Cortex (IC). In rodents, within the IC (dashed circle) is suggested to display a gustotopic organization with bitter-posterior (pIC red) and sweet-anterior (aIC green) areas [81] relative to the medial cerebral artery, and dorsal to the rhinal vein. See text for additional discussion about this controversial point. The inset in the GG depicts that the topographic organization, or proposed hotspots, for taste qualities, are not seen at the level of the GG. Heterogenic distribution of sour (yellow), sweet (magenta), salty (green), and bitter (blue) taste-preferred cells in the GG. The white lines delineated the GG. b Consumption preference index, defined as spiradoline intake divided by spiradoline + water intake, as a function of spiradoline concentration (a tasteless κ opioid agonist) in mice expressing receptor activated solely by a synthetic ligand (RASSL) in sweet (green) and bitter (red) taste receptor cells (TRCs). When RASSL is expressed in sweet and bitter TRCs, mice are strongly attracted and repelled, respectively, to drink a spiradoline solution, implying a peripheral labeled-line coding scheme. White circles are control mice with no expression of RASSL receptors.
Molecular mechanisms of sucrose sweet taste detection
Sweet detection is largely mediated by a single type of GPCR, namely the heterodimer T1R2/T1R3 [37]. Genetic studies in rodents identified that the Sac locus, in the distal part of chromosome 4, contains the tas1r3 gene (taste 1 receptor member 3) that encodes the T1R3 protein [38]. Using a heterologous expression system in HEK cells, it was further shown that T1R2 and T1R3 dimerize to function as the primary receptor for sweet tastants. These receptors share a common structure that includes a large amino-terminal domain (ATD), a venus flytrap domain (VFD), and a short cysteine-rich domain (CRD), which connects the ATD to the canonical α-helical seven-transmembrane domain (TMD) that are characteristic of GPCRs (Fig. 2 and [39]). T1R2/T1R3s are activated by natural sugars, non-caloric artificial and natural sweeteners, some D-amino acids, and, in primates, “sweet-tasting” proteins ([40] and Fig. 2). In humans, more than 50 substances, exhibiting a myriad of sizes and structures, have been shown to produce the perception of sweet taste (Table 1; Fig. 1). Although all these molecules activate T1R2/T1R3, they may bind to different sites on the protein (Fig. 2). Each T1R2 and T1R3 subunit can directly bind sweet tastants, albeit with distinct affinities, in the VFD cleft (e.g., glucose, sucrose, sucralose, aspartame, neotame), and produce different conformational changes that may increase the receptive range of the heterodimer [41]. Thaumatin (22,206 Da) is a “sweet-tasting” protein that binds in the CRD of hT1R3 [42], whereas monellin (11,086 Da) binds to the ATD of hT1R2 [43]. In contrast, brazzein (6,473 Da) interacts in multiple binding sites on the hT1R2/hT1R3 receptor (see Fig. 2) [41, 44].
A different population of Type II TRCs, ~ 6% in fungiform papillae, only express T1R3 [38, 45]. In these cells, the T1R3 forms a low-affinity T1R3/T1R3 homomeric dimer that is only sensitive to high concentrations of monosaccharides and disaccharides (see Fig. 4a) [38, 46]. In contrast, T1R1/T1R2 heterodimer appears not to be functional [39].
Perceptual differences to sweeteners are due to variations in T1R2/T1R3 receptor
Although T1Rs are somewhat conserved among species, with a 70% homology between humans and rodents [38], this difference is sufficient to impact sweet taste perception between these species. Ligand affinities for T1R3 subunit can be drastically reduced by changing a single amino acid in the Sac locus [41]. For example, changing the protein sequence I60T increased the dissociation constant (Kd) for sucrose and glucose from 2.9 to 20 mM and 7.3 to 32 mM respectively; thus explaining in mice different behavioral sensitivities in taste preference [41, 47]. In this regard, slight changes in the receptor may explain differences between animals’ ability to detect a sweet tastant. For example, artificial sweeteners, including aspartame, neotame, cyclamate, and neohesperidin dihydrochalcone, as well as sweet-tasting proteins, such as brazzein, monellin, and thaumatin are perceived sweet by humans, but not by rodents (Table 1). Likewise, rodents do not exhibit the same preference for sucralose as humans [48]. Domestic cats, tigers, and cheetahs are “blind” to sweet tasting stimuli (including artificial sweeteners) as a consequence of a microdeletion of 247 bp in the Tas1r2 gene that results in a pseudogene [49]. Likewise, birds, including chickens, turkeys, and zebra finches do not have T1R2 and thus are unresponsive to natural sugars. The main exception are hummingbirds that depend on nectar. Hummingbirds recently evolved a novel sugar receptor by repurposing the ancestral umami receptor (T1R1/T1R3) to function as a carbohydrate receptor [50].
Allosteric modulation of the sweet taste receptor
From a societal viewpoint, it would be beneficial to reduce the amount of sugar in food while retaining its palatability or pleasantness. This is one reason that led to the development of artificial sweeteners. However, although they taste sweet, they do not precisely have the same taste as sucrose. Moreover, at higher concentrations, some have a bitter taste that arises from their binding to selected T2R (bitter) receptors [16, 51]. For these reasons, it is desirable to develop tasteless molecules that can change the conformation of the “sweet receptor” (by partially closing the VFD) so that less sugar will be needed to fully activate the receptor. Three such allosteric molecules have been identified: SE-2, SE-3, and SE-4 [52]. These molecules act on the hT1R2 VFD (Fig. 2) hinge to produce a synergistic positive allosteric modulation. Specifically, application of SE-3 and SE-4 allows a reduction of up to 50% sucrose while maintaining its sweetness intensity. They are currently used in soft drinks to reduce their caloric content [52]. Another example is, SE-2, which itself is not sweet, but significantly enhances (sixfold) the potency of sucralose while keeping its sweetness intensity [52]. In this regard, it is used to reduce the aftertaste of sucralose found at high concentrations.
Presently, two inhibitors of sweet taste that function as negative allosteric modulators have been identified: lactisole, a carboxylic acid salt, binds to the TMD region of hT1R3, and gurmarin, a peptide isolated from the leaves of Gymnema sylvestre, both inhibit sweetness [44, 53–55]. Eating lactisole together with a teaspoon of sugar makes the latter tasteless and perceived as having the mouthfeel of sand [56]. Therefore, the food industry can use more sugar in products (that act as preservatives or to depress the freezing point in ice cream), but without the sweetness side effect. In this regard, people can be unaware that they may be ingesting significant quantities of sugar.
Sweet sensing mechanisms independent of T1Rs
T1R-independent mechanisms such as the sodium-glucose linked transporter 1 (SGLT1) that utilizes the sodium gradient to co-transport glucose into the cell and glucose transporter 4 (GLUT4), a glucose/hexose transporter of glucose, are also present in TRCs [57, 58]. In the tongue, SGLT1 and GLUT4 have been shown to transport glucose into sweet-sensing taste cells (T1R3-expressing TRCs; Fig. 4a), leading to a transient elevation of intracellular ATP, which binds to KATP, closing these channels thereby depolarizing the sweet responsive TRCs [59]. On the tongue, the enzyme sucrase–isomaltase rapidly breaks down disaccharides (i.e., sucrose to glucose and fructose), which can then recruit the glucose transporter pathway [15, 60]. Sucrase–isomaltase is expressed in Type II TRCs (TRPM5 positive), and in nearly all T1R3-expressing taste cells [60].
Although it is currently unknown as to exactly what roles the T1Rs-independent pathways play in sweet taste perception, it has been argued that glucose transporters may function as a secondary detection mechanism to ensure carbohydrate intake [61, 62]. In this regard, since they are not activated by artificial sweeteners, the differences in sweet taste between natural and artificial sweeteners may also arise from this pathway. Also, since this pathway is selective for natural sugars, it raises the possibility that it could act as a lingual calorie sensor for substances that are both sweet tasting and have caloric value [60].
From a physiological perspective, the activation of T1R-independent glucose transporters on the tongue triggers physiological reflexes involved in a cephalic-phase insulin release [4]. Orally applied sugar has been shown within minutes (< 5 min) to produce a small elevation in plasma insulin levels, that occurs long before the sugar is absorbed in the gut. It has been proposed that this cephalic response facilitates nutrient absorption and assimilation [4]. Importantly, in Tas1r3-knockout mice [4] sugar-induced cephalic-phase insulin secretion persists, suggesting that even in the absence of sweet taste perception by these T1R receptors, sugars can still elicit post-ingestive effects. In this regard, it has been shown that “sweet blind” TRPM5−/− mice still develop a preference for sucrose’s caloric content [63]. However, this result may need to be re-interpreted in the light of new evidence indicating that TRPM5−/− mice could still exhibit a residual sweet taste -sensitivity [33]. Specifically, it has been shown that both TRPM4 and TRPM5 are required to transduce sweet taste since only the double knockout TRMP4−/−/TRPM5−/− mice completely lost sensitivity to sweet tastants [33].
Cellular/neural mechanisms of sweet taste quality
In an impressive demonstration of neural hardwiring from types of TRCs to the nervous system, Zhao et al. expressed opiate receptors (receptors activated solely by synthetic ligand, RASSL) that are activated by spiradoline, a tasteless synthetic ligand, in T1R2-expressing TRCs [40]. Since T1R2 subunits are always co-expressed with T1R3s [38], they control the activity of most, if not all, Type II TRC sweet-sensor cells [38]. As shown in Fig. 4b, in a two-bottle preference test, these transgenic mice exhibited concentration-dependent ingestion of spiradoline, showing that activation of a specific population of Type II TRCs and their associated nerve fibers are sufficient to trigger licking responses to a normally innocuous compound [40]. Similarly, when RASSL is expressed in T2R- (bitter) expressing TRCs, aversive behavioral responses, such as gaping, were elicited [64] (see Fig. 4b). Like mammals, invertebrates, like flies, contain on “taste bristles” on their proboscis both “sweet-sensing cells (Gr5a)” and “bitter-sensing cells (Gr66a)” [65]. Taken together, these data suggest a labeled-line coding scheme, although as described below, there is some controversy about this issue [66].
Nevertheless, these data indicate a profound constraint on coding, at least at the periphery. However, some unresolved issues remain. First, the role of the different Type II TRCs expressing the low-affinity homodimer (T1R3/T1R3) needs to be identified and determined to see if they can induce appetitive responses or sweet perception. Since this subunit is also involved in hedonically positive umami taste quality (T1R1/T1R3), then what perception will be triggered, sweet or umami or both? If it is sweet taste, then how come there appears a complete absence of responses in KO T1R3 mice [37]. Second, the brief-access test task and the two-bottle test only measure the palatability reflex (or taste preference), and thus they are not designed to indicate whether an animal experiences the perception of sweet [67]. To address this issue, a key experiment would be to perform optogenetic stimulations to selectively turn on and off Type II sweet sensing TRCs, while mice perform a psychophysical sucrose detection task (where they need to report actively, in 50% of trials, the presence of any of multiple concentrations of sucrose vs. in the other 50% of trials, water). Thus, the optogenetic activation of sweet TRCs, for example, during water trials will induce an erroneous misdetection of water.
Taste coding: specialist, generalist, and gustotopic coding
Having established that T1R2/T1R3 receptors in Type II TRCs are the primary transducers for sweet tastants, several studies have shown that information from these cells is sent to a set of primary afferent neurons called “sweet-best” or “sweet-selective” [15, 68–70]. That is, these neurons, at isointense concentrations, respond best to sweet tastants among a battery of taste stimuli or respond exclusively to sweet tastants, respectively. These types of narrowly tuned responses were found throughout the gustatory pathway (see Fig. 5). However, the question of how TRCs transmit taste quality information to higher brain centers it has been a matter of much debate [8, 66] with the extremes suggesting that taste quality is encoded by a labeled-line coding scheme as described above [25, 71], whereas at the opposing scheme suggests the combinatorial activity profile (across fiber pattern) from broadly tuned cells dictates quality (Fig. 5a) [72, 73]. We note that although the division of specialists and generalists may be real, some of the division may be a result of where the experimenter sets the threshold (e.g., for detecting a significant Ca+ transient). Naturally, the higher the threshold, the more selective will be the response. Likewise, this classification also depends on the tastant concentration used [74].
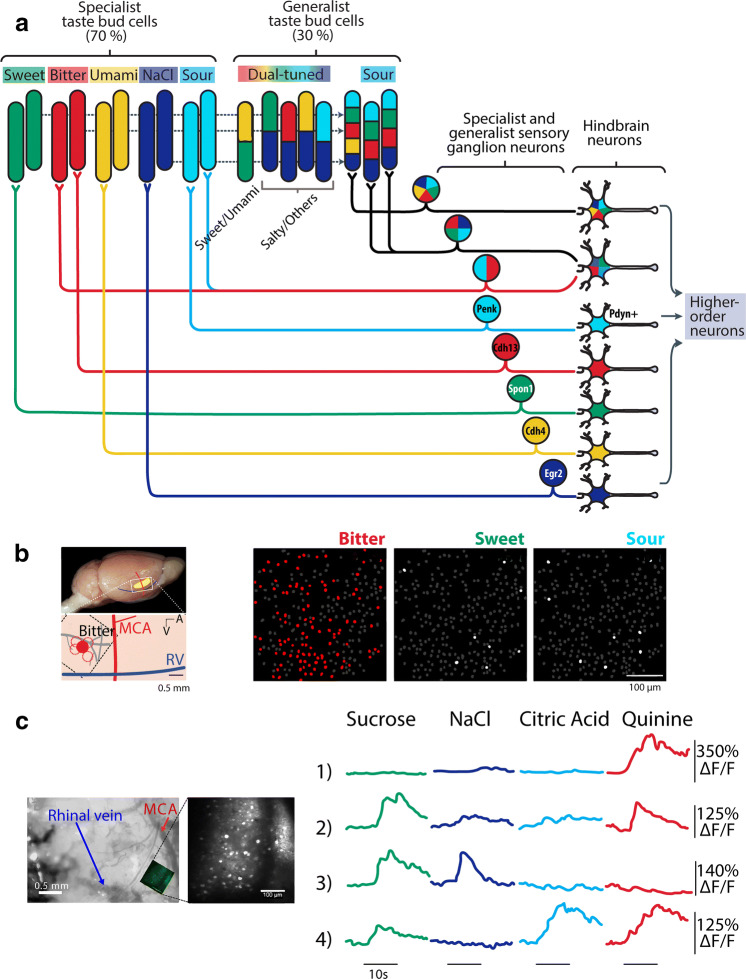
Fig. 5.
Taste coding in specialist and generalist taste cells, associated primary neurons, and in the mouse insular cortex. a Responses of five primary tastants at different levels of the taste system that goes from TRCs to higher-order neurons (sweet—green, NaCl—dark blue, citric acid—cyan, and quinine—red). Shown are mention some ‘specialist’ cells that exhibit selective responses to one taste quality, and ‘generalist’ cells that respond to more than one tastant. For simplicity, not all connections are drawn. The gene marker for geniculate ganglion neurons selective to each taste quality is proenkephalin (Penk), Cadherin 4 (Cdh4) and 13 (Cdh13), Spondin-1 (Spon-1) and Early Growth Response 2 (Egr2). The sour sensitive neurons in the rNTS are marked by the Pdyn (prodynorphin) gen. Modified from [15]. b The dorsal portion of the mouse IC, which is above the rhinal vein (RV), is subdivided into posterior (pIC) and anterior (aIC) relative to the medial cerebral artery (MCA). The extent of IC is depicted in yellow. A hotspot for bitter tastants was reported to be in the pIC (see red dot). Right panels, two-photon calcium imaging of “bitter-hotspot” identified in the pIC. In this report on this area of the mouse cortex most IC neurons preferentially responded to bitter (red), in comparison to other sapid stimuli. Reused from [81]. c In contrast, another calcium imaging study in the mouse pIC (see inset of recording window), revealed that calcium transient responses of pIC neurons were broadly tuned to tastants, sweet (sucrose, green), salty (NaCl, dark blue), sour (citric acid, cyan), and bitter (quinine, red). In this study a bitter hotspot was not found. Each row shows a neuron, note that neurons 2 and 4 responded to both sweet and bitter tastants. Reused with permission from [91]
Taste cells
At the periphery, at least at low concentrations, the sweet, bitter, and umami qualities are detected by some, but not all, narrowly tuned Type II TRCs (see above and [75]). In this regard, they respond as labeled-lines or ‘specialists’ to one taste quality (Figs. 4, 5a). At higher concentrations, one study found that some of these become more broadly tuned [16] and thus do not respond as fixed labeled-lines. In fungiform taste buds, 70% of TRCs were found to be “specialists” and thus convey information about just one taste quality. In contrast, 30% of TRCs are “broadly tuned” most frequently are activated by two tastants that are of the form sweet/umami (10% of TRCs) and salty/the other qualities (20%) with the most frequent combinations being sour/salt and bitter/salt (Fig. 5a and [45]). Notably, TRCs responding to sweet and bitter tastants were not found [45] although, interestingly, such cells were found in geniculate ganglion (GG) and the cortex (Fig. 5). Similarly, acid-sensitive Type III cells are inherently broadly tuned (Fig. 5a) and therefore are more consistent with a combinatorial coding scheme [72, 73]. However, a recent elegant study revealed a population of Type III TRCs expressing acid-sensitive OTOP1 receptors that exhibited selective narrowly tuned responses to a wide range of acid (sour) stimuli, that were abolished in the OTOP1−/− knockout mice, supporting a dedicated labeled-line for acid (sour) taste quality [25].
Primary afferent taste nerves
Taste buds in fungiform papillae and anterior part of foliate papillae are innervated by afferent nerve fibers of the chorda tympani (CT) branch of the facial nerve (CN VII) that has cell bodies in the GG. The posterior part of foliate papillae and the circumvallate papillae are innervated by a sensory branch of the glossopharyngeal nerve (GPN; CN IX) that has cell bodies in the inferior petrosal ganglion (iPG) (Fig. 3). Both these nerves convey taste information via the rostral, gustatory portion of the Nucleus Tractus Solitarius (rNTS) to the Parabrachial Nucleus (PBN) (for rodents but not humans) to the parvocellular portion of the Ventroposteromedial thalamus (VPMpc) (Fig. 4a) and then to the primary taste cortex (the Insular Cortex). The root of the tongue, epiglottis, and larynx are innervated by the superior laryngeal branch (SLN) of vagal nerve (CN X) that has cell bodies in the nodose ganglion (NG) that project to the NTS (Figs. 3, 4a) [76]. The SLN and GPN are also involved in oral reflexes such as swallowing and gagging [76, 77]. All these three cranial nerves (VII, IX, and X) exhibited responses to tastants [9, 78]. Classic recordings in 1941 by Pfaffman of taste nerve fibers in the cat found that some afferent CT fibers were selective to only one tastant, whereas other fibers responded to multiple tastants [79]. In rats and primates, recent recordings of single taste fibers from CT have further confirmed the existence of both specialist and generalist taste responses [80]. Others, using calcium imaging experiments from mice GG neurons, found that most responses were specialists in that they respond to a single tastant while a minority were generalists responding to multiple tastants [71]. In other words, they are like the responses of TRCs. These authors also found using T1R3 KO mice that sweet/umami specialist responses were abolished [81], a result consistent with a labeled-line coding scheme. However, doing similar calcium imaging experiments from the mouse GG, others found that increasing the concentration of tastants (the stimulus strength or intensity) converts seemingly narrowly tuned neurons into broadly tuned ones, and neurons with one best stimulus at low concentration may acquire a different ‘best stimulus’ at higher concentrations [74]. These observations are inconsistent with a fixed first order labeled-line model of taste coding. Thus, at the periphery, there is some evidence supporting a labeled-line coding of taste quality, although, even at this early stage of processing, some broadly tuned responses are already present. What is needed to settle this issue is optogenetic stimulation of a specific type of TRC while simultaneously recording from GG activity [25]. In this regard, an elegant study where single-cell RNA sequencing was performed to find a unique genetic marker for GG cells mediating each of the five basic taste qualities. Briefly, they revealed that GG neurons mediating umami responses were marked by selective expression of the Cdh4 (cadherin) gene, the Cdh13 gene for bitter-sensitive neurons, the Egr2 gene for salt-sensitive neurons, the spon1 (spondin-1) gene for sweet GG neurons and the Penk (proenkephalin) gene for acid (sour) neurons [25]. Finally, they showed that Penk + GG neurons make a synaptic connection with neurons in the rostral portion of the NTS (rNTS) that express the Pdyn (prodynorphin) gene (see Fig. 5a). More importantly, in mice, the optogenetic activation of this rNTS circuit evokes an aversive perception, presumably of sour taste [25]. Taken together, these data support the labeled-line coding scheme at least to the level of the GG.
Higher brain centers
What happens beyond the periphery? At higher brain centers, it is commonly found that there are more broadly tuned responses than in TRCs and GG cell bodies. Electrophysiological recordings throughout the gustatory pathway including the rNTS [82] (but see [25]), PBN [83], VPMpc [84–87], and IC [70, 88–90] consistently found neurons with not only narrowly tuned responses (e.g., specialist, “best-neurons”) but also, and more commonly, generalist (“broadly tuned”) neural responses. As noted, broadly tuned neurons respond to multiple taste stimuli. An example, seen in Fig. 5c shows an area of a mouse primary taste cortex, the posterior Insular Cortex (pIC), showing four neuronal responses. It is seen that neurons labeled 2 and 4 responded to both sucrose and quinine, a bitter tastant, suggesting, but not proving, that taste quality can also be encoded, not only for “specialists,” but also for more broadly tuned neurons. Taken together, there is no doubt that the gustatory system is composed of specialist and generalist responses. What is controversial is the knowledge of which of these responses is necessary for taste quality perception. If specialists transmit quality information, then what functions are there for the generalists? Thus, one of the most important issues in the sweet taste physiology is to demonstrate if narrowly, or broadly tuned neurons, or both, are necessary for sweet taste perception.
In an important finding of taste responses in the anesthetized mouse IC, Chen et al. found that within the IC they are arranged in a cortical spatial gustotopic map of taste quality (where the pIC contains bitter taste responses, while the aIC contains sweet taste responses). This implies that taste quality information is represented by spatially segregated clusters “hotspots” of IC neurons with specialist responses to only one taste quality [81] (see red and green circles in Figs. 4, 5b). Also consistent with the presence of hotspots is that in KO mice, they disappear without the related TRC receptors, indicating a direct continuation of the proposed labeled-line form sweet or bitter TRC to a “sweet-taste” or “bitter-taste” cortex [81]. A similar result was found in flies [65]. What is the function of areas between the hot spots is not clear. That said, not all studies found the presence of hot spots. That is, their presence has not been replicated in three subsequent calcium imaging studies in the pIC [91–93] and two electrophysiological studies in the IC [88, 94]. For example, as seen in Fig. 5c, Fletcher et al. recorded in the pIC nearly overlapping the “bitter-taste cortex” reported by Chen et al. [81, 91]. However, instead of finding only “bitter-best” or selective responses (Fig. 5b), they found broadly tuned responses. Indeed, as noted, some of these responded to both bitter and sweet tastants (Fig. 5c; see neurons 2 and 4). A recent study using mice found that the pIC neurons responded more to aversive stimuli, such as tail shocks (28%) and quinine (49%) than to sucrose (10%). Nevertheless, most pIC neurons were multisensory, responding to painful stimuli, quinine, and sucrose [95].
In human fMRI experiments, primary taste responses revealed a more complex gustotopic map than in mice, with larger overlap across taste qualities and variability among subjects [96, 97]. In a recent high-resolution 7 T scan and using a multivariate searchlight analysis across the entire brain found that within the mid-insula region taste quality was not represented topographically, but by a combinatorial spatial code, both within primary taste cortex as well as regions involved in processing the hedonic and aversive properties of taste [98]. Intriguingly, they observed that the activation patterns of taste-specific responses were not only highly variable across subjects, but also within subjects. In other words, in the same subject, brain regions within the insula responding to taste quality changed from day to day. Thus, they found a lack of evidence supporting clear gustotopic representation of taste quality in the human brain, their results, however, were more consistent with the idea that taste quality is encoded by a distributed and wide brain network [98]. In another fMRI study, narrowly tuned clusters within the human gustatory cortex selective to one taste quality were identified. However, changing the tastant concentrations (low or high) completely changed the location of these clusters [97, 99]. Moreover, they found only a small subset, if any, of coactive clusters (concentration-invariant) that represent taste quality regardless of concentration [99].
Presently, the gustotopic coding of taste quality lacks reproducible evidence. Clearly, this important point needs to be resolved. Subsequent optogenetic stimulation of the apparent “sweet-hotspot” or “bitter-hotspot” suggests that even in the absence of taste stimulation, they could function as an “appetitive” or “aversive” hotspots since its stimulation can induce place preference [100] or avoidance to a spatial context, respectively [95, 100]. Likewise, it was shown that optogenetic stimulation of the pIC (“bitter hotspot”) induces a broad spectrum of anxiogenic-like behaviors, suggesting that the pIC is not only involved in taste processing, but also in evoking aversive states [95].
Other sweet taste attributes
Chemical stimulation of TRCs elicits signals that are transduced into neural representations of multiple sensory attributes, such as taste quality, palatability (hedonic value), and intensity (the strength or concentration of a stimulus). These attributes form a single percept [1]. Having discussed sweet taste quality, in the following sections, we will address other attributes, including palatability and intensity.
Hedonic value (palatability)
In addition to its sweet taste quality sucrose also evokes a hedonic value or palatability attribute (i.e., whether it is appetitive or aversive). Sucrose’s palatability is the primary reason for why it is over-consumed and one reason underlying its attraction across species. In rodents, palatability is measured by an increase (or decrease) in oromotor responses due to increase in tastant concentration [101]. To measure a tastants’ palatability in rodents a brief-access taste test was developed, which consists of allowing access to a tastant for a brief period (< 5 s), thus limiting the impact of post-ingestional effects [102]. For hedonically positive tastants, like sucrose, the animal’s lick rate will increase sigmoidally with concentration and decrease with increasing concentration for hedonically negative tastants, like quinine [101]. In rodents, palatability is necessarily linked to an oromotor response (licking or gaping) [103, 104] and should not be confused with a conscious hedonic sensation of pleasant taste that is felt by humans [105, 106]. Although sweet taste naturally elicits positive palatability responses, taste and palatability attributes do not always change together, suggesting they are distinct. For example, palatability responses can change with experience, as when animals develop a conditioned taste aversion to sucrose when it is paired with gastric malaise [107]. Under these conditions, animals still perceive the sweet taste of sucrose, but avoid ingesting it because of learning.
Neural correlates of palatability
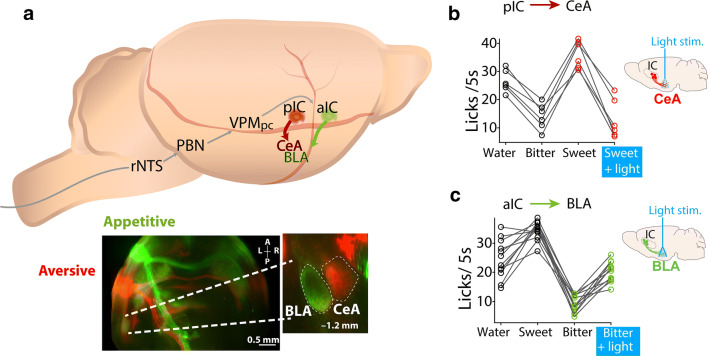
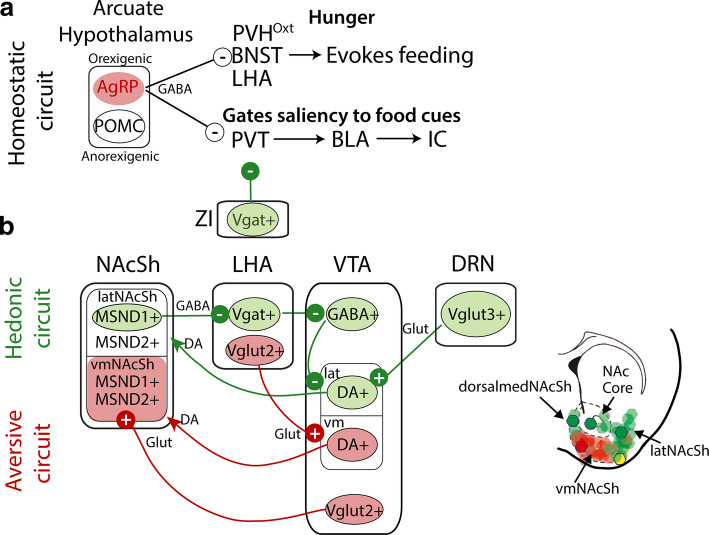
Throughout the gustatory pathway, electrophysiological recordings in freely moving animals have uncovered neuronal responses related to palatability (Fig. 6). These include recordings from the PBN [108], VPMpc [84], IC [109], and Amygdala [110]. Such types of responses were also found in the forebrain and diencephalon, that is regions, that are involved in reward and feeding including the Nucleus Accumbens Shell (NAcSh) [67] and the lateral hypothalamic area (LHA) [111], indicating that palatability is encoded in a widespread brain network (Fig. 6a–e). Nevertheless, which brain areas are necessary to assign palatability awaits further investigation. With regard to the issue of palatability, as noted, it was suggested that the IC contains gustotopic representation of “sweet” and “bitter” hotspots with neurons responding to sweet or bitter tastants [81]. We note that subsequent studies using optogenetic stimulation of these hotspots suggested that rather than act as pure taste sensors, they may be appetitive and aversive hotspots [95, 100]. Similar findings were previously reported using electrical stimulation in the primate IC [112]. In this latter study, it was found that stimulation of one region of the primate IC induced appetitive behaviors (i.e., licking), whereas stimulation of another insula area resulted in aversion and facial expressions associated with unpleasant tastes [112]. In mice, further evaluation of the hotspots projections found they projected to two different nuclei of the amygdala (Fig. 7a). The appetitive hotspot innervates the basolateral amygdala (anterior IC to BLA) [113], while the aversive hotspot projected to the central amygdala (posterior IC to CeA) [95, 113]. Upon manipulating these projections, Wang et al. demonstrated that it is possible to impose a positive or negative valence on a neutral water stimulus, and even to reverse the hedonic value of a sweet or bitter tastant (Fig. 7b, c, see Sweet + light and Bitter + light, respectively) [113]. Importantly, these manipulations can change the palatability without affecting the identification of taste quality, supporting the idea that these two sensory attributes recruit two distinct neural substrates [113]. Moreover, the aIC to BLA and the pIC to CeA represent two parallel pathways that appear to be responsible for imposing and triggering a hardwired stereotypic attractive and aversive taste responses. Further studies should unravel the mechanisms responsible for changes in palatability induced by learning [92, 114].
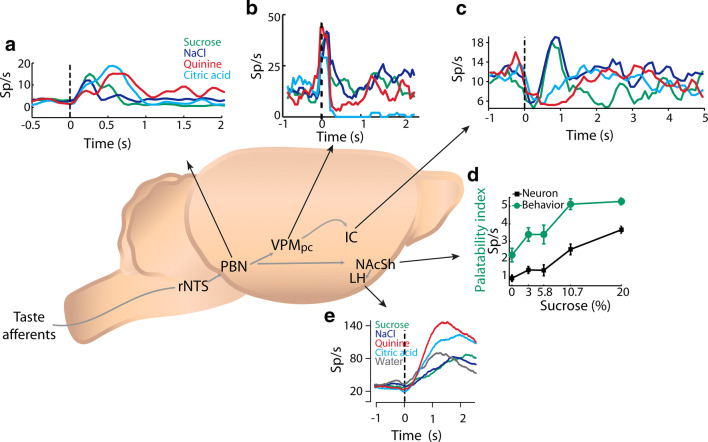
Fig. 6.
Palatability is encoded in a widespread brain network. a Example of the response of a Parabrachial Nucleus (PBN) neuron that exhibited larger firing rates in response to aversive tastants [citric acid (light blue) and quinine (red)] than to appetitive stimuli [sucrose (green) and sodium chloride (dark blue)]. From [108]. b Mean PSTHs of a representative palatability-related thalamic neuron with higher firing rates (from 0.2 to 1 s) in response to hedonically positive stimuli (sucrose and NaCl)), and lower activity in response to aversive stimuli (Quinine and citric acid). Same conventions as in a. From [84]. Notably, as seen in a and b, throughout the gustatory axis there are both aversive- and hedonic-selective cells that display higher responses to unpleasant or to palatable tastants, respectively. c. Example of a hedonic-selective IC neuron with greater activity in response to sucrose and NaCl (from 0.2 to 1 s) in comparison to aversive stimuli. Same conventions as in a. From [109]. d An example of a Nucleus Accumbens Shell (NAcSh) neuron whose mean firing rate increases as a function of sucrose concentration, shown in black, and that covaries with the palatability behavioral index (green). The palatability index is the lick rate during a 5 s reward epoch where sucrose was available to the animal to consume. From [67]. e Example of a Lateral Hypothalamic Area (LHA) aversive-selective cell with increasing activity from positive (water, NaCl, and sucrose) to negative (citric acid and quinine) valence sapid stimuli. Same conventions as in a, water is depicted in gray. With permission from [111]
Fig. 7.
Optogenetic manipulation of IC projections to the amygdala can reverse innate palatability responses. a Schematic of the rat’s brain taste pathway from the rNTS to the posterior pIC and anterior aIC, and then to two different amygdalar nuclei (Central Amygdala (CeA) and Basolateral Amygdala (BLA)). Below, a horizontal brain slice showing distinct projections from the anterior IC/“appetitive” (green) and posterior IC/“aversive” (red) “hotspots” to the BLA and the CeA, respectively. b The consumption of a sweet tastant solution is decreased when paired with optogenetic stimulation of the “aversive” pIC to CeA projections (see sketch of brain on the right side) in comparison to sweet solution alone, which is more preferred than water or a bitter solution. It is plotting the number of licks given in 5 s during a brief access test task. c. Consumption of a bitter-tasting stimulus increases when paired with photostimulation of the “appetitive” aIC to BLA projections (see right-side of the brain) in comparison to the bitter solution alone which is less preferred than water or a sweet solution. With permission from [113]. Thus, activation of these projections can assign a new-opposite valence to tastants
The idea that palatability and taste quality are independent processes raises the possibility that they could run in parallel [113]. In this regard, parallel processing is consistent with behavioral observations in thirsty and well-trained rats showing they could identify a tastant and its palatability, within < 400 ms, suggesting these attributes must either occur in parallel or if serially with marked overlap [115]. This is consistent with Halpern’s classic studies and with more recent studies [116] on humans showing that they can rapidly discriminate between tastes and, in parallel, perform hedonic computations [117]. However, when tastants are delivered passively (in non-water deprived mice), say by intraoral cannulae, palatability responses in the IC occur slowly (> 500–1500 ms) and after tastant identification [88, 118].
The intensity of sucrose
In addition to the taste attributes of quality and palatability, sucrose (and other tastants) also triggers an attribute related to their intensity. Interestingly, unlike NaCl, which becomes aversive at high concentrations [119], sucrose remains hedonically positive at all concentrations [101], a property that allows more caloric intake. Thus, sucrose’s sweet intensity is one attribute contributing to the overconsumption of high-energy palatable foods since it provides a sensory cue predicting the presence of immediate energy sources. Historically, the neural representation of sweet taste intensity has been characterized by firing rates (spike counts) that linearly or sigmoidally increases as the sucrose concentration is increased. Sweet tastant concentration responses have been found along the gustatory pathway including GG neurons [71, 74], the rNTS [82], PBN [120] (Fig. 8a–c), the primary (IC) [89, 121] and the orbitofrontal cortex (OFC- sometimes referred to as the secondary taste cortex) [122, 123]. Importantly, those responses were obtained in either anesthetized animals [71, 74], or during passive intraoral delivery of tastants [121, 124], or in behavioral tasks where animals do not have to make any decision other than to lick [89, 125]. In rodents, sucrose’s palatability is easier to study than intensity since animals increase positive oromotor responses (e.g., licking) as the concentration of sucrose increases [104]. In contrast, the intensity attribute cannot be directly measured by any licking response per se, as to study the neural basis of intensity an animal must actively report the perceived concentration of sucrose in a process necessarily involving decision making. In this regard, Fonseca et al. trained rats in a novel sucrose intensity discrimination task where animals needed to report, by visiting one of two lateral ports, that a drop of 3 wt% sucrose is low, and visit another lateral port if they received 18 wt% sucrose (to indicate it is high) while recording electrophysiological responses in the pIC, aIC, and the OFC [94]. They found that a small population of neurons that were widely distributed in these three taste-activated cortices exhibited linear increases or decreases in firing rate with increasing sucrose concentration (Fig. 8d–f). These responses were not limited to a single hotspot but were widely distributed indicating they are inconsistent with proposed “gustotopic map” of taste quality [81] since, even in the pIC which contains the putative “bitter hotspot,” there were neurons sensitive to sucrose’s intensity (see pIC Fig. 8d; see red circle “bitter-cortex” in Fig. 4a; also see [95]). Importantly, when the psychometric curve (the behavioral judgment made about sucrose’s intensity: black curve in Fig. 8g–h) was contrasted against the neurometric curve, it revealed neuronal responses that were significantly correlated with the perceptual judgments of sucrose’s intensity. These neurons were present in the aIC (Fig. 8g), and OFC, but not in the pIC. In the OFC responses were closely related to the rat’s decisions (see overlapped sigmoids; Fig. 8h) [94].
Fig. 8.
The concentration-dependent and intensity responses to sucrose are found throughout the rat gustatory pathway. a When sucrose is applied to the tongue, calcium imaging measurements from the mouse geniculate ganglion (GG) showed sucrose concentration-dependent increases. From [74]. b Mean response from rat rNTS cells that track sucrose intensity by increasing (upper) or decreasing (lower) firing rates. From [82]. c PBN neurons with increased activity as a function of sucrose intensity (in [moles]). From [120]. d Mean activity of pIC cells that tracks sucrose’s intensity either by monotonically increasing (red) or decreasing (blue) firing rates. e–f Same conventions as in d for aIC, and OFC, respectively. g Mean firing rate of aIC neurons that covaries their activity with the subjective judgment of “how sweet” that a subject perceived a sucrose concentration. h Same conventions as in g but for OFC neurons. Note that activity of figures a–f shows the encoding of the sensory/physical dimension of the stimuli (whether intensity increased), while activity in figures g and h shows the encoding of the perceptual/psychological dimension. That is, it shows how the intensity was perceived and classified in an active self-reporting generalization task. With permission from [94]
Taken together, the first level of the gustatory system deals with detecting and transducing taste stimulation in the mouth into, at least, three distinct taste attributes in higher brain centers. Here we propose that in thirsty and freely licking motivated animals, these attributes are processed in a nearly parallel manner (or if serially with a large overlap [115]). Nevertheless, in all cases, they recruit areas throughout the entire gustatory-limbic system. It is important that the existence of three distinct traces does not imply they are completely independent. It is possible that a single neuron could, simultaneously convey quality and intensity [68] or even palatability [88], given that it may be part of several neural networks [126].
In sum, stimulation of the tongue with sucrose triggers the formation of three attributes: taste quality, palatability, and intensity. This information rapidly evolves and deploys across the gustatory-limbic pathways. One open question is whether these three attributes are later integrated by a “hub brain region” that serves as an ideal observer. Alternatively, an interesting possibility is that taste perception does not require further integration and that it is precisely the nearly parallel processing and the widespread recruitment of brain circuits sufficient to perceived a sweet tastant [73, 94, 127]. Finally, one challenge of the taste field is to determine the cellular identity (the cell-types) and the brain circuits responsible for these taste attributes.
Sweetness is reduced by cooling
Perception of foods is temperature dependent. Psychophysical measurements have uncovered that of the primary taste qualities sweet taste is the most temperature sensitive [128]. Indeed, changes in the tongue’s temperature can lead to changes in taste perception [129]. For example, warming the anterior part of the tongue (innervated by CT nerve) from a cold temperature to a warmer temperature can by itself evoke a perception of sweetness [129]. Furthermore, cold sweetened sodas are more refreshing then when they are warm because when it is warm, it is often perceived as too sweet. Furthermore, cooling a taste solution to 5–12 °C reduces the sweetness intensity of sucrose [130]. The effect of the suppressing effect of cooling varies across stimuli. For glucose and fructose, their perceived sweetness was reduced by cooling whereas saccharin was unaffected [128]. Likewise, neuronal responses evoked by sweeteners are attenuated as temperature decreases [131–134]. In mice, CT responses to caloric and non-caloric sweeteners diminishes when the temperature decreased from 35 to 15 °C [134]. Similarly, in single-unit recordings from the rat GG, sucrose-specialist neuronal activity was reduced at low temperatures [131]. Similar responses are found in the mouse NTS; that is responses of sucrose-“best neurons” diminished when the taste solution is at 18° C, in comparison to when is 37 and 22 °C [135, 136]. Importantly, in sweet-blind TRPM5−/− mice, temperature dependent modulations are abolished [134], suggesting that temperature dependent effects are, in part, mediated by a reduction in the excitability of TRPM5 that leads to a decrease in sweetness [134, 137]. However, it is not clear why temperature affects sweet tastes more than other TRPM5-dependent taste qualities (e.g., umami and bitter).
Leptin selectively modulates sweet taste perception
Leptin is an anorexigenic hormone produced by adipocytes that promotes weight loss through appetite suppression and the activation of metabolic processes [138]. It acts by binding to the “obese receptor,” a type I cytokine receptor, that is highly expressed in hypothalamic nuclei [139]. More recently, it has been found to be expressed in circumvallate and fungiform taste bud cells [140, 141]. Mice with genetically induced leptin receptor deficiency (db/db mice) were shown to selectively display greater CT responses to sweet tasting compounds in comparison to a non-diabetic control group, whereas no differences were found for salty, sour, or bitter sapid stimuli [142]. These findings indicate that leptin may selectively diminish sweet taste sensitivity [143]. Indeed, in lean mice, it was demonstrated that systemic administration of leptin suppresses responses of the CT and glossopharyngeal nerves to sucrose and saccharin while responses to other basic sapid stimuli were unaltered. In contrast, db/db mouse showed no response suppression. Moreover, in lean mice, pharmacological blockade of leptin receptors results in a dose-dependent increase in CT responses for sucrose and saccharin [144]. In addition, in ob/ob mice, that do not produce leptin but exhibit normal leptin receptor activity, the decrease in neural and behavioral responses induced by sucrose and saccharin were diminished after leptin administration as well as in control mice, but not in db/db mice [141]. The leptin-induced sweet taste diminished responses have been suggested to be induced by increasing potassium outward currents via an ATP-sensitive K+ channels that hyperpolarizes sweet-responsive Type II TRCs, thus reducing their excitability [140, 143].
In humans, a link between leptin and reduced sweet taste sensitivity showed that in healthy non-obese humans, plasma leptin exhibit diurnal changes that covary with sweet taste recognition thresholds [145]. These changes were sweet tastant-selective and independent of blood glucose levels. Furthermore, if leptin diurnal variations were shifted by shifting meals, sweet taste thresholds shifted in parallel [145]. Similarly, higher levels of salivary leptin were found in young adults with low sweet sensitivity in comparison to the highly sensitive group [146]. In contrast, no link has been found between leptin levels and sweet taste sensitivity in overweight and obese humans, probably due to the high basal leptin levels that result in small daily fluctuations [147]. Overall, these studies clearly showed that leptin selectively modulates sweet taste neuronal responses and taste perception through activation of oral leptin receptors in TRCs. Thus, leptin exerts its anorexigenic properties through both central and peripheral pathways.
Taste receptors in other organs than the tongue
Other than their presence in the mouth, gustatory receptors are expressed in a wide variety of other tissues (see Table 2). The G-protein coupled sweet, bitter, and umami gustatory receptors together with other gustatory signaling transduction proteins are expressed in the digestive system [148–152], respiratory system [153, 154], brain [155, 156], adipocytes [157, 158], retinal endothelium [159], testicles [160–163], heart [164], pancreas [151, 165–167], urinary bladder [168], liver [167], and spermatozoa [169]. However, the functions of taste receptors in extraoral tissues, with the possible exception for those found in the gastrointestinal tract and pancreas, remain largely unexplored. Taste receptors in the gastrointestinal tract are involved in glucose homeostasis and secretion of incretins and insulin [39]. In the stomach, T1R3 and -gustducin are expressed in enteroendocrine cells (EECs) and brush border cells. Moreover, ghrelin, a peptide released in the stomach that signals hunger, is expressed in T1R3 EECs in the stomach’s fundus. It has been suggested that increases in blood glucose suppress ghrelin release, possibly through a T1R3-dependent pathway [39].
Table 2.
Sweet TRCs distribution
| Organs | Function | References |
|---|---|---|
| Adipocytes | Stimulates adipogenesis and suppreses lipolysis | [157, 158] |
| Arcuate nucleus | Glucose homeostasis and food intake regulation | [155] |
| Bladder | Augments bladder contraction | [168] |
| Brain cortex | Membrane-bound sensor | [158] |
| Heart | Nutrient sensor | [164] |
| Hypothalamus | Membrane-bound glucose sensor | [156] |
| Intestine | Promotes secretion of GLP-1 and glucose absortion | [150, 151] |
| Liver | Regulates digestion by sensing bile juice composition? | [167] |
| Pancreas | Stimulates insulin secretion and blood glucose clearance | [151, 165] |
| Retinal endothelium | Attenuates aberrant vascularisation processes | [159] |
| Stomach | Nutrient detection and regulating ghrelin release | [149, 150] |
| Testis | Regulates spermatogenesis | [169] |
| Upper respiratory airways | Suppression of the T2R-induced secretion of antibacterial peptides | [153, 154] |
In the small intestine, T1R2 and T1R3 are expressed throughout EECs that are involved in the release of two satiety-inducing hormones: glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). GLP-1 is released in response to both natural sugars and the noncaloric sweetener, sucralose. Sugar-induced GLP-1 release is inhibited by lactisole or gurmarin [55, 151], supporting the idea that sweet-sensitive TRCs are involved in regulating the secretion of incretins.
In the pancreas, insulin-secreting β-pancreatic cells contain T1R2, T1R3, -gustducin, PLC-β2, and TRPM5, that is, all the components necessary for the detection of glucose and sweet-tasting compounds [166]. Moreover, fructose also activates “sweet receptors” on β-cells and evokes glucose-induced release of insulin [170]. Importantly, genetic ablation or lactisole pretreatment of T1R2 blocks fructose-induced insulin release [170]. In summary, taste receptors for sweeteners are found throughout the body and serve a myriad of functions beyond that of a transducer for tastants.
Gut-nutritional reward value
Having discussed the effect of sugars on the tongue and mouth, we now focus on its effects on the gut. Sugars not only induce a sweet taste, but they have “post-ingestional” rewarding effects that produce a preference learning for energy rich solutions, and especially for those containing d-glucose [171–173]. This is perhaps expected since it is the preferred energy fuel of the brain. In a classic paper using rats, Holman paired an arbitrary oral stimulus with intragastric (IG) infusions of food and concluded that “IG injections of liquid food have the power to enhance the value of oral stimuli [174].” It is now known that an IG infusion of d-glucose is the most potent stimulus to induce preference learning [175, 176]. That is, the preference for sweet tastants has an innate component elicited by activation of sweet- responsive TRC’s and a learned component that is evoked by post-ingestional factors [175]. Indeed, both newborn human babies and decerebrate rats show an innate liking for sucrose [177, 178]. The impact of the post-ingestional effects can be seen upon pairing an orosensory conditioned stimulus (e.g., sucralose) with IG infusions of d-glucose. After learning, animals enhance their intake of sucralose [179]. Using a two-bottle test (water vs. sucrose), it was found that based on its nutritional value, “sweet blind” trpm5−/− mice develop a preference for sucrose, but not for non-nutritious sucralose [63]. This indicates that the nutritional value of d-glucose is taste (sweet) independent. It follows that the gut–brain system uses a separate sensory pathway for post-ingestive factors.
Mechanisms of gut-nutritional responses
Despite extensive research, the gut-sensing mechanism of sugars that underlies their learned preference behavior remains to be elucidated [176, 180]. What is clear, however, is that the nutritional reinforcing effects occur beyond the stomach since d-glucose infusions directly into the duodenum and jejunum support food preference learning, but not when it is infused into the stomach or distal ileum [172, 179, 181]. Furthermore, this effect is not mediated by T1R1/T1R3 sweet taste receptors as T1R3−/− mice exhibit food preference [182]. In contrast, d-glucose-induced learned flavor preference was blocked by phloridzin or phloretin, SGLT1/3 and GLUT2 antagonists, respectively [183]. In addition, SGLT1−/− mice failed to develop flavor conditioning to an IG infusion of d-glucose [62], suggesting a possible role of glucose transporters as sensors of the positive reinforcing post-ingestive effects [62]. One unexpected result was that a non-metabolizable (nonnutritive) glucose analog, α-methyl-D-glucopyranoside (MDG), could serve as rewarding stimuli [62, 183], although it is less potent than d-glucose to induce preference learning [176, 181]. Since MDG and glucose have similar affinities to SGLT1, it suggests that SGLT1-independent mechanisms participate in the stronger rewarding-post ingestive effects of d-glucose, with GLUT2 being a likely candidate [176]. Also, in trpm5−/− sweet-blind mice, d-glucose preference is better correlated with glucose oxidation than with blood glucose levels, suggesting that glucose utilization is a key factor for its rewarding effects [184]. Nevertheless, the signal that acts as a glucose gut sensor remains to be uncovered [176, 180].
Glucose is known to circulate from the proximal gut, which is the duodenum and jejunum, into the mesenteric portal system of the liver [185]. Indeed, low and high intraportal infusions of glucose are sensed by SGLT1 and GLUT2 transporters, respectively [61]. Hepatoportal d-glucose infusions support the acquisition of food preferences [171, 176, 186]. To this point, enteric infusion of d-glucose, at a concentration that is sufficient to elicit conditioned preference, leads to a glycemic profile similar to that observed after a hepatoportal, but not jugular, infusion of a low glucose concentration [171]. These nutrient-rewarding properties of intraportal d-glucose are likely mediated by SGLT1 and GLUT2, pathways that transport glucose from the proximal gut into the portal systems [62, 176, 183]. Overall, these findings support a major role of the portal system for sensing the nutritional value of glucose.
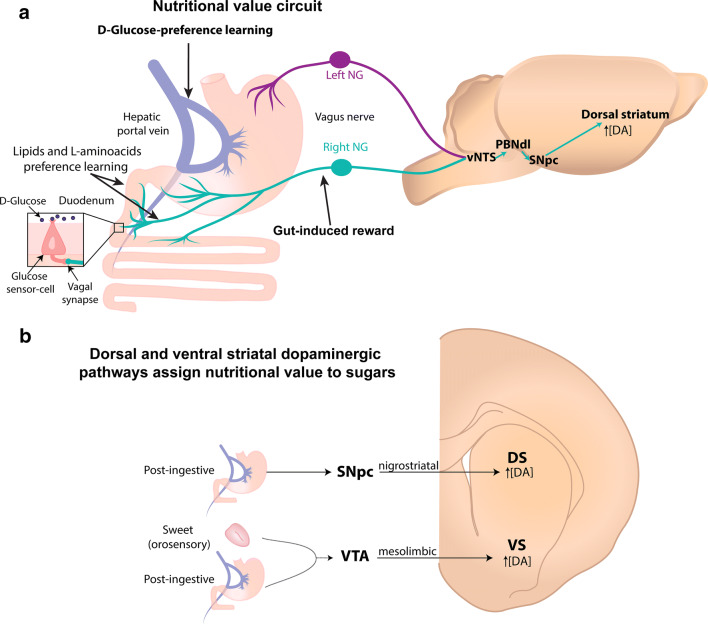
The portal vein is innervated abundantly by the left NG and to a lesser extent, by the right NG of the vagus nerve [187]. In mice, the left NG innervates the stomach’s fundus, and the right NG innervates the pylorus and proximal intestine [188]. Recently, it was demonstrated that the ascending vagal fibers from the right NG are concentrated in the ventromedial portion of the NTS and that photostimulation of these NTS terminals promotes self-stimulation, place preference, and flavor conditioning to non-caloric solutions (Fig. 9a) [188]. These studies demonstrate that interactions in the gut can induce rewarding behaviors. In contrast, the left NG, which only slightly innervates the NTS, did not sustain any of the aforementioned reward-related behaviors [188]. Thus, the activation of the right, but not the left NG produces gut-induced reward behaviors [188]. However, despite the reinforcing effect of the right vagus stimulation and its communication with the portal vein, the action of the right vagal nerve does not support d-glucose conditioned preference since subdiaphragmatic vagotomy does not disrupt the rewarding nutritional effect of IG infusions of d-glucose [172, 189]. That is, in either vagotomized mice or those having a duodenal-jejunal bypass intestinal rerouting, fat and protein preference are selectively impaired, but glucose is not [172, 190]. Thus, for fat and L-aminoacids learned preferences depend on both duodenal–jejunal and vagal systems, whereas d-glucose-learned preference relies on glucose sensors on the portal vein (see Fig. 9a) [172, 176, 179, 189].
Fig. 9.
Gut-induced reward pathway. a Left panel: The right (cyan) and left (purple) Nodose Ganglia (NG) of the vagus nerve differentially innervates the digestive system. The left NG projects from the stomach’s fundus (see upper left side), while the right NG projects from the pyloric antrum and small intestine (lower left side). Both synapse in the ventral NTS (vNTS). The gut-induced reward information is produced exclusively by activation of the right vagal nerve and reaches the vNTS, the dorsolateral portion of the PBN (PBNdl). Subsequently, the SNpc, which activity increases dorsostriatal dopamine (DA) efflux. Preference learning for lipids and l-aminoacids requires vagal terminals and sensing in the duodenum. In contrast, glucose-preference learning is mediated by a vagal-independent and taste-independent pathway via a post-absorptive mechanism in the hepatic portal vein. b Mesolimbic (orosensory) and nigrostriatal (post-ingestive) dopamine pathways assign nutritional value to sugars. Schematics of the two separate dopaminergic pathways involved in the nutritional value of d-glucose. Note that the mesolimbic pathways require sweet taste activation to induced learned preference to d-glucose. In contrast, the nigrostriatal pathway is more dedicated to sense and detect energetic foods in the gut. SNpc substantia nigra pars compacta, VTA ventral tegmental area, DS dorsal striatum, and VS ventral striatum
Neuropods and vagal nerve communication
EECs are a heterogeneous population of isolated cells that serve as “sensory sentinels” that are found throughout the GI system [191]. They release hormones and transmitters, are electrically excitable, and are capable of forming glutamatergic synapses with vagal nerves [192]. A subset of EECs in the small intestine and colon, express cholecystokinin (CCK) and/or peptide YY (PYY) and synapse with vagal nerves. These special EECs are named neuropods (Fig. 9a). Neuropods function as gut-sensor cells since after luminal stimulation with sucrose or d-glucose (but not to fructose) they evoke action potentials [192]. Furthermore, neuropods express SGLT1 and its inhibition by phloridzin blocks the sucrose-induced action potentials on vagal nodose neurons [192]. Neuropods via the nodose ganglion have a monosynaptic projection between the gut and the vNTS demonstrating that the gut has a rapid pathway to sense sugars (in the subsecond range) well before it is metabolized [192]. Nevertheless, neuropod-independent sensing mechanisms of the nutrient value of sugars must also exist since vagotomized mice can still develop learned preferences for IG d-glucose infusions [172].
In sum, considering the large energetic demands of the brain, the post-ingestive effects of sugars are at least as important for the body as its taste. Although it is established that glucose metabolism is a key factor for its reinforcing properties, additional research is needed to clarify the gut-sensing mechanisms responsible for the post-ingestive learned preference of sugars.
Two independent dopaminergic pathways assign the nutritional value to sugars
Recently, it has been found that increases in midbrain dopamine (DA) levels are involved in assigning gut nutritional values to sugars [193]. The mesolimbic dopaminergic system consists, in part, of DA neurons in the Ventral Tegmental Area (VTA) that project to the Ventral Striatum (VS). Similarly, sweet-sensitive taste responses that originate from taste cells also project to the VTA and then to the VS [194, 195]. To better understand the workings of this pathway, researchers expressed channelrhodopsin (ChR2) in VTADA+ neurons in DAT-cre mice (Dopamine Transporter promotor) and then, during a two-bottle test, paired optogenetic stimulation in the VTA to the mice licking out of just one the two bottles [193]. First, they paired optogenetic stimulation of DA neurons to water (noncaloric + light) vs. sucrose alone (sweet and caloric) and found the animals preferred sucrose, suggesting that the value of sugar is higher than the optogenetic-induced “nutritional” value state. In contrast, when they paired lick-induced activation of DA neurons when the animals were drinking water (noncaloric + light) vs. one where they were drinking sucralose (noncaloric but sweet), they found the animals preferred water (noncaloric + light), suggesting that the optogenetic “nutritional value” is preferred over only sweet taste. In well-fed mice, the activation of DA neurons paired to sucralose (noncaloric + light) vs. sucrose (sweet and caloric), showed the animals preferred sucralose (noncaloric +light). These results suggest that the mesolimbic dopaminergic pathway can assign nutritional value to sugars, but it necessarily requires concomitant activation of the lingual sweet-sensing taste receptors (Fig. 9b) [193]. The take-home message is that natural sugars induce DA release in the VS for their hedonic sweet taste as well as for their post-ingestive nutritional effects [63, 194].
The above experiments suggest that the brain has a secondary pathway to assign nutritional value to energy-rich substances that are independent (or weakly dependent) of taste quality. Such a system was uncovered by Tellez et al. when they identified a population of midbrain dopamine neurons located in the Substantia Nigra pars compacta (SNpc) that project to Dorsal Striatum (DS) (see Fig. 9b) [173]. They simultaneously measured DA efflux in both VS and DS, while mice developed a learning preference for licking sucralose paired with infusions of IG sucrose or IG sucralose [173]. During licking for sucralose (i.e., an orosensory cue), regardless of which IG solution was infused, they observed increases in DA release in the VS, showing that, as expected, DA efflux in VS depends on sweet taste input. However, they found that DA release in the DS was only seen when sucralose intake was followed by IG infusions of d-glucose. Thus, regardless of taste, DA efflux in DS could function as a gut sensor of nutritional value. Next, they explored if this pathway would allow animals to develop post-ingestive learning preferences for tastants having a caloric value, but with a bitter taste quality. To this end, the same group altered the sweet taste of sucralose by mixing it with denatonium benzoate (a bitter tastant) and then the sucralose/denatonium solution was accompanied by IG infusions of d-glucose. Despite that the taste quality was bittersweet and aversive, DA efflux was only observed in DS but not in VS, suggesting that regardless of taste quality, DA efflux in DS is dedicated to detecting post-ingestive effects. Their elegant results demonstrate the existence of separate circuits to encode the hedonic (orosensory) and nutritional value (post-ingestive) of sugars [173] (see Fig. 9b).
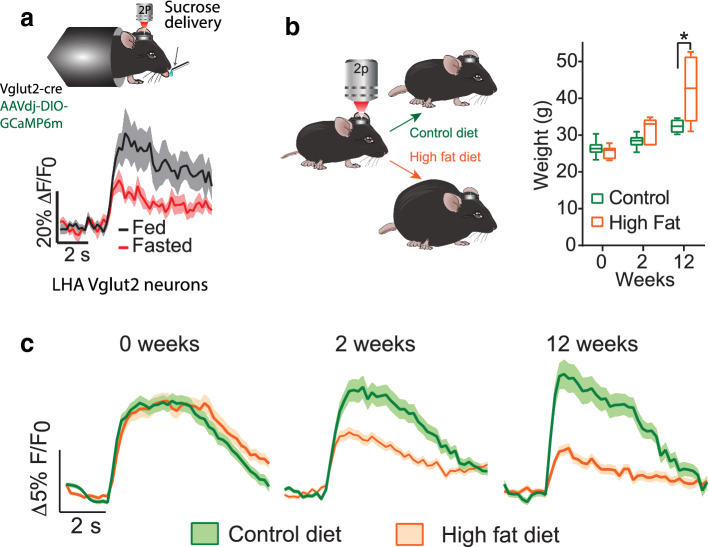
The striatum contains a vast number of neurons, most being of the Medium Spiny Neuron (MSN) type. MSNs project to different brain regions depending on whether they are in DS or VS, and if the type of DA receptor they express is of the D1 or D2 subtype [196]. In the DS, MSNs that express the dopamine D1 receptor (MSND1+) are necessary and sufficient to induce preference to bittersweet tastants (e.g., sucralose/denatonium) when paired with a nutritious IG d-glucose infusion [173]. Recently, a PET and fMRI study with humans uncovered two temporally distinct peaks of DA release after drinking milkshakes: an early orosensory one and a second delayed one related to post-ingestive effects. The early DA release correlated with NAc activity, whereas the delayed peak correlated with the caudate head nucleus (rats have no homologous region) [197]. In sum, the mesolimbic and nigrostriatal DA systems represent two separate pathways, both fundamental to assigning nutritional value to sugars (see Fig. 9b). Since DA is an important factor in the post-ingestive effects to sugars, it is not surprising that for high fat diet (HFD) obese rats, obese humans [198], or rats that binge drink sucrose solutions [199], that they exhibit diminished dopaminergic neurotransmission in the striatum [200]. Concerning the worldwide epidemic of obesity, it has been proposed that obese subjects overeat as a compensatory mechanism to release more DA [198–204]. In this regard, obesity impairs the interoceptive awareness of the gut to sense the caloric content.
Gastric bypass surgery changes food preference by exacerbating DA efflux in DS
To date, Roux-en-Y gastric bypass is the most effective long-term treatment for morbid obesity [205]. After surgery patients shift their preference from high-fat to low-fat foods and exhibit smaller cravings for palatable foods, they also reduce their intake and preference for sugars [206]. As fat is ingested various lipid species are generated in the gut that communicate with the brain. One such lipid signal is oleoylethanolamide (OEA), an endogenous agonist of the peroxisome proliferator-associated receptor-α (PPAR-α) receptors, which, in turn, activates vagal afferents to curb fat intake [207]. Interestingly, in HFD obese mice, OEA levels are reduced [208]. In rats, it has been shown that gastric bypass enhances the production of OEA, which is accompanied by increases in vagus-evoked DA release in the DS [207]. Notably, gastric bypass upregulates D1r signaling in DS that correlates with a higher preference for low fat food [207]. Importantly, the beneficial effects of gastric bypass are reversed by interfering with local OEA by blocking PPAR-α receptors, vagal and DS D1r signaling pathways [207]. This suggests that the dramatic changes in food preference observed after surgery are mediated by overactivation of this pathway. These effects are not induced by losing weight after food restriction. Moreover, gastric bypass not only changes fat preference, in rats and humans, it also reduces sugar intake [209]. The mechanism by which gastric bypass curbs the appetite for sweet tastants may also involve DA efflux in the DS because direct infusion of d-glucose in the duodenum induces greater release of DA in DS than similar jejunum infusions in healthy rats. However, after duodenal-jejunal bypass surgery, rats exhibited no increase in IG glucose-induced nigrostriatal DA release in DS [181]. Thus, gastric bypass surgery impinges in the nigrostriatal DA system to curb the appetite for both fat and sweet tastants.
Feeding circuits
Having discussed the effects of sugars in the gut, we now address its impact on the brain. Hunger and satiety are two internal motivational states that directly control feeding in that they correct negative and positive deviations from body energy homeostasis. Although many of the neural correlates of hunger and satiety have remained elusive, the development of optogenetics and the design of genetically encoded calcium indicators, such as GCaMP6, has accelerated the identification of a variety of cell types and their associated circuits involved in the neural aspects of appetite control [210]. These circuits can be coarsely divided into three major feeding circuits: homeostatic, hedonic, and aversive (Figs. 10, 11). Briefly, in the arcuate nucleus of the hypothalamus (ARC), the homeostatic circuit can be thought to be comprised of two groups of neurons: one expressing the gene Agouti-related protein (AgRP) and the other expressing the Proopiomelanocortin (POMC) protein (Fig. 11a). The activation of ARCAgRP neurons elicits a state of hunger that promotes the voracious feeding of foods with nutritional value (e.g., increases sucrose intake but not of non-caloric sweeteners) [211]. In contrast, ARCPOMC neurons are anorexigenic, and their activation leads to the suppression of food intake and subsequent weight loss [212]. The hedonic circuit encompasses multiple brain regions, including the NAcSh, the LHA, and VTA as well as multiple cell types (Fig. 11b, green color). The critical neurons of this circuit are in the LHA and are GABAergic and express the vesicular GABA transporter (LHAVgat+). Optogenetic activation of LHAVgat+ neurons elicits feeding regardless of the food’s nutritional value [213, 214]. As its name indicates, the activation of this circuit produces a robust reward signal via its projection to VTA GABA interneurons that, in turn, disinhibits VTA DA neurons releasing DA in the NAcSh [215]. Finally, the aversive dopaminergic circuit is intermingled within the same regions of the hedonic circuit, but it is recruited by aversive stimuli including air puffs, electric shocks, bitter tastants, and nociceptive stimuli [216]. Its main components are the glutamatergic neurons in the LHAVglut2+ and a subpopulation of DA neurons in the medial VTA [216, 217]. Optogenetic activation of any element along the circuit produces an aversive behavior [216]. The coordinated activity of these three intermingled networks controls food intake, either by reinforcing the approach and consumption of foods with nutritional value or by negative valence signals punishing not attending to the bodies energy demands [218].
Fig. 10.
Model of how ARCAgRP neurons control feeding behavior and track the number of calories ingested. The population activity of ARCAgRP neurons encodes a “hunger drive” that is released by food cues (see text for details). The population AgRP neuronal activity is plotted vs. time from the last meal, when AgRP neuron activity increases (the “hunger drive” also increases; see red lines). The valence of food is assigned according to whether the food can inhibit (positive, see green colors) -or not- AgRP neuron activity (negative, red colors show when food is inaccessible). Presentation of food that can be seen and smelled but not consumed (food inaccessible) inhibited ARCAgRP neuron activity that is reversed within minutes. However, when a food cue is followed by intake of a caloric meal, a sustained inhibition is observed [220, 226]. The magnitude of the transition from high to low ARCAgRP neuron activity drives feeding in the same proportion [221] and encodes the number of calories ingested [219, 220]. Thus, caloric food intake resets the “hunger drive”. Note that the kcal axis on the right increases in a downward direction
Fig. 11.
Schematic diagrams of the neuronal homeostatic, hedonic, and aversive feeding circuits in the brain. a Homeostatic pathway: The Arcuate nucleus of the hypothalamus (Arc) has both orexigenic (Agouti-related peptide, AgRP, in red) and an anorexigenic population (Proopiomelanocortin, POMC). ARCAgRP neurons are GABAergic and promote food intake by inducing a sensation of hunger or by increasing the attractiveness of food-predicting. Hunger is evoked via the inhibition (negative arrow) of the oxytocin-expressing cells in the hypothalamic Paraventricular nucleus (PVHOxt), Bed Nucleus Stria Terminalis (BNST), and Lateral Hypothalamic Area (LHA). ARCAgRP neurons also encode the food’s saliency due to Insular Cortex (IC) activation via the Paraventricular Thalamus (PVT) and Basolateral Amygdala (BLA), respectively. b In the NAcSh, the hedonic circuit (green) induces feeding via GABAergic inhibitory projections (negative arrow) from the Medium Spiny Neurons expressing D1 receptors (MSND1+) in the lateral Nucleus Accumbens Shell (latNAcSh) to the GABA+ cells of the LHAVgat+. LHAVgat+ inhibits VTAGABA+ interneurons, thus disinhibiting lateral Ventral Tegmental Area dopaminergic neurons (latVTADA+) which, in turn, are excited by the reward signal of neurons expressing the Vesicular glutamate transporter type-3 (Vglut3) from the Dorsal Raphe Nucleus (DRN). The aversive circuit (red) involves activation (positive arrow) of the NAcSh from the VTAVglut2+, and positive modulatory projections (arrowheads) to the ventromedial nucleus accumbens shell (vmNAcShMSN+) from the vmVTADA+, which, in turn, are activated by LHAVglut2+. Thus, the NAcSh displays a lateral-ventromedial gradient encoding rewarding (green)-aversive (red) stimuli (see right panel, from [216]). The GABA neurons in the Zona Incerta (ZIVgat+) project and inhibit glutamatergic PVT neurons inducing rapid binge-like eating for high fat and sugar foods
Homeostatic circuit
The ARC area is located adjacent to the third ventricle and the median eminence, which is a site partially devoid of blood–brain barrier and therefore ARCAgRP neurons can “sense” peptides and other compounds present in the peripheral bloodstream. For this reason, ARCAgRP neurons can be activated or inhibited by hormones signaling energy deficit (e.g., ghrelin) or abundance (e.g., leptin), respectively. In addition, ARCAgRP neurons are also sensitive to gut peripheral satiety signals, including CCK, PYY, and amylin [219, 220]. Their activation is associated with the induction of a “hunger brain state,” whereas the transition from an active to an inactive state drives and sustained food intake (see Fig. 10) [212, 221, 222]. In contrast, their long-lasting inhibition suppresses feeding [222], and their ablation results in anorexia [223]. ARCAgRP neurons project to multiple brain areas [224], and the evoked food intake can be triggered by activating the LHA, paraventricular hypothalamus (PVH) terminals in oxytocin expressing neurons (ARCAgRP to PVHoxt), as well as ARCAgRP terminals in the Bed Nucleus of the Stria Terminalis (BNST) (see Fig. 11a) [221].
ARCAgRP neuron activation is also involved in assigning a negative and/or positive valence to foods. For example, Betley et al. found that mice developed a lower preference for foods paired with optogenetic activation of ARCAgRP neurons and suggested that foods that failed to suppress ARCAgRP activity are no longer preferred [225]. In contrast, foods that successfully suppress ARCAgRP activity are positively reinforced [221]. For this reason, ARCAgRP neurons may also assign either positive (or negative) valence to foods depending on whether they can (or cannot) decrease their activity (see Fig. 10) [221, 225]. Importantly, in mice, calcium imaging studies or optrode recordings have both shown that ARCAgRP neurons are more active in food-deprived (hunger) than in well-fed (sated) states [225, 226]. Interestingly, in food-deprived mice, the activity of ARCAgRP neurons could decrease just by the sight of food, or after presenting sensory cues predicting food delivery (Fig. 10) [219, 225]. Also, ARCAgRP neuron activity decreases with increasing concentrations of IG d-glucose infusions, suggesting that even in the absence of taste cues, they can directly sense the caloric content of sugars (and other macronutrients such as lipids and proteins) [220]. In this regard, ARCAgRP neurons can “learn” to signal the intragastric caloric content of foods because even a single exposure to a caloric solution is sufficient to “train” AgRP neurons to decrease their firing to the sight, smell, and taste of food [219]. In this regard, ARCAgRP neurons can both sense and predict the intragastric nutritional value of sugars [219, 220].
In sum, ARCAgRP neurons encode a “hunger drive,” which increases as the day passes from the last meal to when the “hunger drive” gradually develops hours later. The transition from an elevated level of activity to a lower activity unleashes this “hunger drive.” The stronger the magnitude of this transition, the greater the induced food intake (Fig. 10) [221]. Importantly, this inhibition is proportional to the number of calories ingested [220], suggesting that ARCAgRP neurons contain a central representation of the energy consumed (Fig. 10). In food-deprived animals, any exteroceptive cue previously associated with intragastric caloric content can control this inhibition [219, 220]. In sum, under physiological conditions, the inhibition in ARCAgRP neuron activity is what drives feeding (Fig. 10) [221].
In contrast, to AGRP neurons, ARCPOMC neurons are anorexigenic in that their activation decreases feeding [212]. Since ARCAgRP and ARCPOMC neurons are reciprocally connected [227], and since they both are GABAergic, it follows that the activation of one population will result in the inactivation of the other. For this reason, it was expected that ARCAgRP could indirectly elicit feeding by decreasing ARCPOMC activity [227]. However, the simultaneous co-activation of both ARCAgRP and ARCPOMC neurons did not inhibit ARCAgRP evoked feeding, showing that ARCAgRP does not require ARCPOMC suppression to promote food intake [224]. This indicates that in efforts to control feeding, it is more promising to (pharmacologically) target ARCAgRP rather than ARCPOMC neurons [228].
ARCAgRP neurons gate saliency to food cues in the gustatory insular cortex (IC) and recapitulate hunger drive
Hunger is a strong motivational drive that could enhance the saliency of sensory cues predicting food [93, 229]. Dysregulation of this saliency enhancement mechanism is a contributory factor in failed diets, obesity, and eating disorders [230–233]. By performing calcium imaging in the gustatory cortex (IC) while hungry mice performed a visual discrimination task, Livneh et al. found that most IC neurons responded to food cues (a visual cue predicting a caloric food delivery). They also responded to the actual consumption of a caloric liquid. In contrast, in well-fed mice, IC neurons did not respond to food cues [93], suggesting that the state of hunger “gates” IC neuronal sensitivity to food cues. To show that when mice are in a sated state, the activation of ARCAgRP neurons will mimic the hunger state, they activated ARCAgRP neurons and found that IC neurons responded to food cues as if mice were naturally hungry [93]. Since ARCAgRP neurons do not directly project to the IC, upon tracing the circuit, they found that an energy deficiency increases ARCAgRP activity that, in turn, inhibits neurons in the paraventricular thalamus (PVT) that project to BLA and then to the IC (Fig. 11a) [93]. Thus, ARCAgRP neurons not only evoke a sustained state of hunger to drive feeding, but also increase the saliency to food cues (Fig. 11a; homeostatic circuit).
The hedonic circuit
Hedonics reflects the pleasant sensation of highly palatable foods. Although this is a natural response to get energy-rich food, dysregulation of this system can lead to obesity [234]. Hence, it is important to elucidate the brain circuits that permit overeating beyond energetic demands. In this regard, even in the absence of energy deficiency, the hedonic circuit is involved in producing both the seeking and consumption of highly palatable foods. The circuit includes (as detailed below) neurons from multiple brain regions and cell types. These include MSND1s+ in the NAcSh, LHAVgat+ neurons, and GABAergic interneurons in the VTA that disinhibit dopaminergic neurons in the same region and then release DA into the NAcSh, thus closing the rewarding loop circuit (Fig. 11b) [215].
The circuit from the nucleus accumbens shell (NAcSh) to the LHA
The NAcSh is the only region of the striatum that projects to the LHA, thus enabling it to exert direct control overfeeding [235, 236]. Its anatomical connections suggest the NAcSh is an important center that can translate motivation into action [237], and hence, it is a pharmacological target for appetite suppressants [238, 239]. In the NAcSh, 95% of neurons are MSNs. About half are positive for the DA receptor 1 (MSND1+), and the other half for the DA receptor type 2 (MSND2+). The other 5% of striatal neurons comprise several types of interneurons [196]. All MSNs are GABAergic, and they exhibit a low spontaneous activity [196]. Thus, their firing reflects the balance and integration of three main neurochemical inputs: glutamatergic inputs from the cortex (and other brain regions), the mesolimbic DA from the VTA, and 5-HT from dorsal raphe neurons [196].
The NAcSh receives strong glutamatergic inputs from the prefrontal cortex, BLA, ventral hippocampus (vHipp), and thalamus, and its activation is rewarding [240]. Using a GCaMP6-based fiber photometry system to measure the activity of mice glutamatergic afferent fibers into the NAc, Reed et al. found during the consumption of sucrose there was a coordinated reduction of glutamatergic inputs to the NAc from the vHipp, thalamus, and BLA, whereas silencing these fibers promoted sucrose intake [241]. In contrast, in fasted mice, global optogenetic activation of glutamatergic inputs to the NAcSh rapidly stopped mice from licking for sucrose [236]. Thus, the glutamatergic afferents to the NAcSh bidirectionally control feeding behavior. Importantly, O’Connor et al. reported that optogenetic stimulation of MSND1+neurons was sufficient to stop feeding, whereas silencing MSND1+ neurons prolonged feeding [235]. Moreover, MSND1+ activation inhibits LHAVgat+ neurons (also see below and Fig. 11b, the circuit in green), rationalizing why these NAcSh neurons authorize feeding, since silencing (or stimulating) LHAVgat+ neurons decreases (or increases) feeding, respectively [214].
The LHA to the ventral tegmental area (VTA) circuit
The LHA is commonly referred to as the “feeding center” since its electrical stimulation enhances feeding [242, 243], and its ablation leads to starvation and death [244]. The LHA is a heterogeneous region primarily comprised of four neuronal populations. The melanin-concentrating hormone (MCH) and the Orexin expressing neurons (Ox) are two small populations involved in arousal and in stabilizing sleep–wake cycles, respectively [245]. The two larger populations include the glutamatergic neurons expressing the vesicular glutamate transporter type-2 (LHAVglut2+), and a GABAergic (LHAVgat+) population. As outlined below, these latter two populations play opposite roles in feeding [213, 217]. Optogenetic stimulation of LHAVgat+ neurons evokes robust responses to feeding (regardless of caloric content). Nevertheless, they are rewarding since mice will self-stimulate to activate these neurons [213]. In contrast, LHAVglut2+ activation halts sucrose consumption and is aversive [217, 246]. Moreover, LHAVgat+ neurons inhibit local GABAergic interneurons in the VTA (VTAGABA+) that, in turn, disinhibit VTADA+ neurons and lead to DA release in the NAcSh (see Fig. 11b). Thus, optogenetic stimulation of LHAVgat+ neurons (or non-specific electrical stimulation of LHA) results in reward by releasing DA into the NAcSh via the VTA [215, 247]. In addition, the hedonic circuit is complete with a distinct glutamatergic population located in the dorsal raphe nucleus (DRN) expressing Vglut3 (DRNVglut3+), which provides a reward signal to DA+ neurons located in the lateral part of VTA that innervates the lateral NAc (latVTADA+ to latNAcSh neurons, see Fig. 11b green dots in NAc). Thus, mice have been shown to self-stimulate these dopaminergic latVTADA+ to latNAcSh neurons, indicating their rewarding nature [216].
The zona incerta (ZI) to PVT hedonic-homeostatic feeding circuit
Despite the fact that less attention has been paid regarding the involvement of ZI on feeding, recently, a careful observation in mice unveiled that ZIVgat+ neurons projecting to PVT induce a robust preference toward high fat and sugar pellets, over normal Chow food. Activation of ZIVgat+ neurons is rewarding, and it promotes robust binge-like eating, even stronger than that triggered by LHAVgat+ neurons [248]. ZIVgat+ neurons are more active during food deprivation than in a well-fed state, and they are activated by ghrelin a hunger-gut hormone. Its effects in feeding are mediated via an inhibitory projection from ZI to the PVT. Furthermore, soma inhibition of PVT glutamatergic neurons also evokes feeding [248]. Thus, the ZIVgat+ ⟶ PVT circuit could serve as a bridge between the hedonic and homeostatic feeding circuits.
The aversive circuit
Unlike the rewarding ZI/LHAVgat+ neurons, the LHAVglut2+ population is aversive and hence decreases feeding [217]. One unique characteristic of LHAVglut2+ neurons is that they are more active during energy surfeit states (Fig. 12a). That is they are more active in well-fed mice and show suppression in firing during food deprivation, suggesting they may function as a satiety signal that results in the stoppage of feeding [217].
Fig. 12.
High-fat diets progressively attenuate a neural satiety “stop-feeding” signal. a VGlut2-cre mice were injected with the calcium indicator, GCaMP6m, to measure neuronal activity via in vivo two-photon calcium imaging, while the mice consumed a 10% sucrose solution. Figure showing two-photon imaging setup and control (healthy) mouse feeding (licking for sucrose) together with a representative example of a response from an LHAVglut2+ neuron that displayed a diminished activity in response to sucrose consumption when the same mouse had fasted for 24 h (red) in comparison when it had ad libitum access to food (Fed, black). b Preparation of study in Vglut2-cre mice regarding how obesity affects LHAVglut2+ responses. Mice fed for 12 weeks with a high-fat diet (orange) gained significantly more weight in comparison to lean control (green). c LHAVglut2+ population responses to sucrose during the development of obesity. Note that as obese subjects (orange) gained weight, the response of LHAVglut2+ neurons to sucrose consumption was attenuated in comparison to lean control (green) showing that obesity dampens the “brake on satiety” feeding signal.
From [217]
Although mesolimbic DA efflux is usually associated with reward, recently a subpopulation of dopaminergic neurons (vmVTADA+ to vmNAc) has been shown to release DA after stimulation with aversive stimuli, such as airpuffs and electric shocks (see Fig. 11b red dots in a coronal section of rat’s NAc brain slice). Thus, mesolimbic DA is not only associated with reward but also with aversion [216]. In this regard, LHAVglut2+ neurons project to aversive vmVTADA+ to vmNAc neurons. Interestingly, Rossi et al. tracked the activity of the same LHAVglut2+ neurons throughout the time mice were fed with a high-fat diet (HFD, Fig. 12b) [217]. As the mice became obese, the LHAVglut2+ neurons gradually diminished their activity (after licking for sucrose Fig. 12b, c). They found that obese mice upon feeding had a smaller satiety signal, which may explain why they are less prone to stop eating after they are sated (Fig. 12c). Importantly, they showed that the activity of a population of neurons involved in the neural control of food intake is directly affected by obesity [217].
Recently, another aversive glutamatergic population of neurons has been identified in the VTA and projects to the vmNAc (Fig. 11b; the VTAVglut2+ to vmNAc pathway). Additional studies are needed to determine what role they play in feeding and what other elements of the aversive circuit are altered by overconsumption of sucrose or high fat-induced obesity.
The feeding circuits described here are not only related to the control of sucrose intake but are involved in appetitive (approach) and consummatory behaviors regardless of the nature of food, or its nutritional value. Thus, these circuits likely play a general role in the control of food intake and the development of obesity.
Sucrose intake and its role in obesity
Overconsumption of simple carbohydrates has been proposed as a primary factor in the development of the world-wide epidemic of obesity [249]. Psychophysical studies revealed that obese people, even though they like sweets more than non-obese people, exhibited a reduced sweetness perception [250]. Likewise, obese rodents and fruit flies fed with highly palatable diets have a dulled sense of sweet taste that leads to a compensatory overconsumption mechanism [250–258]. However, it is unclear whether this reduction in sweet perception is a metabolic consequence of obesity or the effect of drinking sucrose per se. In other words, whether overconsumption is developed as a compensatory mechanism to adjust to a diminished sweet taste sensation, or whether overconsumption of sugars blunts sweet taste perception remains to be elucidated.
We begin this discussion with the studies of Drosophila melanogaster, the fruit fly, where the link between drinking sugary liquids and obesity is better understood then in mammals. Fruit flies fed for several weeks a 30 wt% sucrose diet developed obesity, metabolic syndrome, peripheral insulin resistance, and recapitulate the hallmarks of kidney and heart disease in their corresponding organs [252]. In this regard, they exhibit very similar responses to those found in mammals [252]. In Drosophila, taste cells are neurons that sense the environment through taste hairs found on the labellum at the tip of the proboscis, the main taste organ in the fly [259]. Tastant-sensitive neurons that express receptors for only one taste modality send their projections to the subesophageal zone, a taste processing center in the fly brain, where taste modalities remain segregated in a labeled-line like coding scheme [259]. By exploiting the relative simplicity of the Drosophila taste system, where there are “sweet-sensing neurons” that directly project to the brain, the group of Monica Dus addressed and answered three important questions [252]: First, do changes in taste sensation occur with diet-induced obesity? They found that fruit flies fed on a high sugar diet show a diminished sense of sweet taste because sweet-tastant responding neurons exhibited diminished responses to sugar. Second, they asked if these changes are a consequence of the altered physiology of the obese state, or do they result from chronic exposure to a high nutrient diet? Rather than obesity per se, they found the diminished activity of the sweet sensing neurons was mediated by the increased activity of the sugar sensor, O-linked N-Acetylglucosamine Transferase [260, 261]. Interestingly, flies fed with sucralose diet did not exhibit a diminished sweet taste, suggesting that glucose metabolism plays a critical role in altering taste sensation. Third, and finally, they asked if changes in taste function occurred, what role do they play in the etiology of obesity? Optogenetic excitability of the “sweet-taste sensing neurons” showed that a reduced sweet taste response leads to overfeeding and obesity. More importantly, they found that preventing a decrease in sweet taste sensation prevents overfeeding and obesity-development in animals exposed to the high sugar diet. Overall, these findings implicate deficits in sweet taste as a main driver of obesity [252].
Having discussed the electrophysiological responses evoked by sugar in the rat’s brain (Figs. 5, 6, and 8), in this section, we focus on the behavioral impact of sugars. In rodents, results about the role of sucrose intake on weight gain have revealed that the sucrose presentation (liquid vs. solid) plays a key role in promoting obesity. Togo et al. demonstrated that, in comparison to mice consuming solid sucrose (added to standard rodent chow diet), mice fed with aqueous solutions of sucrose became obese, had greater adiposity and hepatic inflammation and also had an impairment in blood glucose levels [262]. Rats consumed more sucrose in liquid form than in solid form [262]. The difference between drinking sugar-sweetened beverages and sugar added in food is that the liquid consumption of sweet tastants increases total caloric intake despite a compensatory reduction in solid chow food intake that rats exhibited (that is sucrose devaluated the reward value of healthy chow food), thereby increasing the probability of becoming obese [262].
Since the classic discovery by Nora Volkov and colleagues that showed that the brains of morbidly obese people exhibited a decrease in striatal D2 receptors similar those found in people with addictions to substances of abuse [198], it has been generally accepted that people could develop addiction-like behaviors towards sugars and other highly palatable foods [199]. The presence of multiple secondary pathways to perceive sugars sweetness and energetic content (Figs. 4a, 9b, 11), only highlights the importance for the body to ensure the intake of high energy foods. Given that glucose is the preferred energy source for the brain, this is perhaps expected. Moreover, the overall consensus of studies suggests that overconsumption of sugars results in a blunted sweet taste perception that leads to compensatory mechanisms and compulsive behaviors toward sugars that resemble, in many respects, addictive-like behaviors [199, 200, 204, 257].
In humans, most access to sugars comes from ingestion of sugar-sweetened beverages (SSBs) which contain caloric sweeteners, including sucrose or high-fructose corn syrup [263, 264]. SSBs generate and maintain a positive energy balance due to their associated weak satiety- inducing state and poor compensatory dietary adjustment on subsequent meals that may lead to weight gain and ultimately to obesity [265, 266]. As noted, the ingestion of a liquid form of sugar has a key role in promoting overconsumption, since a liquid caloric solution should produce a diminished gastric distention and an aberrant endocrine response in comparison to an isocaloric solid food [265]. In addition, the consumption of SSBs are associated with higher risk of diabetes type 2, coronary heart disease, dyslipidemia, and stroke [263, 267]. Indeed, a large longitudinal study uncovered a positive association between the intake of SSBs and artificially sweetened beverages with cardiovascular disease mortality, but only SSBs were also associated with cancer mortality [263]. Moreover, middle-aged chronic consumers of SSBs had 29% more visceral adipose tissue in comparison to non-consumers [268]. Since the obesity pandemic is a matter of public health and the undesirable effects that SSB consumption has on people’s health, it is important to educate consumers on matters of dietary healthier choices so they can select foods based mostly on their nutritional benefits and not merely its taste [269].
Acknowledgements
This project was supported in part by Productos Medix 3247, Fundación Miguel Alemán, Cátedra Marcos Moshinsky, CONACyT Grants Fronteras de la Ciencia 63, and Problemas Nacionales 464 (R.G.). We thank Professor Carlos Cerda for help in Fig. 1. We also thank Professors Stephen Roper, Luis Tellez, Diego Bohorquez, and Monica Hernandez-Luna for their perceptive comments.
Abbreviations
- 5-HT
5-Hydroxytryptamine or serotonin
- AgRP
Agouti-related protein
- aIC
Anterior insular cortex
- ARC
Arcuate nucleus of the hypothalamus
- ATD
Amino-terminal domain
- BLA
Basolateral amygdala
- BNST
Bed nucleus of the stria terminalis
- CALHM1/3
Ca2+-activated and voltage-dependent Ca2+ homeostasis modulator 1/3 hetero-hexameric ion channel
- CCK
Cholecystokinin
- Cdh4
Cadherin 4
- CeA
Central amygdala
- ChR2
Channelrhodopsin-2
- CN
Cranial nerve
- CNS
Central nervous system
- CRD
Cystein-rich domain
- CT
Chorda tympani
- DA
Dopamine
- DAG
Diacylglycerol
- DAT
Dopamine transporter promotor
- DS
Dorsal striatum
- EEC
Enteroendocrine cells
- Egr2
Early growth response 2
- ENaC
Epithelial Na+ channel
- GG
Geniculate ganglion
- GIP
Glucose-dependent insulinotropic peptide
- GLP-1
Glucagon-like peptide-1
- GPN
Sensory branch of the glossopharyngeal nerve
- IC
Insular cortex
- IG
Intragastric
- IP3
Inositol triphosphate
- IP3R3
Inositol 1,4,5-trisphosphate receptor type 3
- iPG
Inferior petrosal ganglion
- GLUT4
Glucose transporter 4
- GPCR
G-protein coupled receptor
- GPN
Sensory branch of the glossopharyngeal nerve
- GSP
Great superficial petrosal nerve
- HFD
High fat diet
- IX
Ninth glossopharyngeal cranial nerve
- latNAc
Lateral nucleus accumbens
- latVTA
Lateral ventral tegmental area
- LHA
Lateral hypothalamic area
- LHAVgat+
Lateral hypothalamic area neuron expressing vesicular GABA transporter
- LHAVglut2+
Lateral hypothalamic area neuron expressing vesicular glutamatergic transporter 2
- MCH
Melanin-concentrating hormone
- MSN
Medium spiny neuron
- MSND1+
Medium spiny neuron expressing dopamine D1 receptor
- MSND2+
Medium spiny neuron expressing dopamine D2 receptor
- NAcSh
Nucleus accumbens shell
- NG
Nodose ganglion
- NTS
Nucleus tractus solitarius
- OEA
Oleoylethanolamide
- OFC
Orbitofrontal cortex
- OTOP1
Otopetrin-1
- Ox
Orexin
- PBN
Parabrachial nucleus
- Pdyn
Prodynorphin gene
- Penk
Proenkephalin gene
- pIC
Posterior insular cortex
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- POMC
Proopiomelanocortin protein
- PPAR-α
Peroxisome proliferator-associated receptor-α
- PVH
Paraventricular hypothalamus
- PVT
Paraventricular thalamus
- PYY
Peptide YY
- RASSL
Receptors activated solely by synthetic ligands
- rNTS
Rostral portion of the nucleus tractus solitarius
- SGLT1
Sodium-glucose linked transporter 1
- SLN
Superior laryngeal branch
- SNpc
Substantia nigra pars compacta
- spon1
Spondin-1 gene
- SSB
Sugar-sweetened beverages
- tas1r3
Taste 1 receptor member 3 gene
- TMD
Transmembrane domain
- TRC
Taste receptor cell
- TRPM4
Transient receptor potential cation channel subfamily M member 4
- TRPM5
Transient receptor potential cation channel subfamily M member 5
- VFD
Venus flytrap domain
- vHipp
Ventral hippocampus
- VII
Seventh facial cranial nerve
- vmNAc
Ventromedial nucleus accumbens
- vmVTA
Ventromedial ventral tegmental area
- VPMpc
Parvocellular portion of the ventroposteromedial thalamus
- VS
Ventral striatum
- VTA
Ventral tegmental area
- VTAGABA+
GABAergic interneurons in the ventral tegmental area
- X
CN10th vagus nerve
- ZI
Zona incerta
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Breslin PAS. An evolutionary perspective on food and human taste. Curr Biol. 2013;23:R409–R418. doi: 10.1016/j.cub.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutierrez R, Simon SA. Why do people living in hot climates like their food spicy? Temp Multidiscip Biomed J. 2015;3:48–49. doi: 10.1080/23328940.2015.1119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon SA, de Araujo IE, Gutierrez R, Nicolelis MAL. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci. 2006;7:890. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- 4.Glendinning JI, Stano S, Holter M, et al. Sugar-induced cephalic-phase insulin release is mediated by a T1r2 + T1r3-independent taste transduction pathway in mice. Am J Physiol Regul Integr Comp Physiol. 2015;309:R552–R560. doi: 10.1152/ajpregu.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belloir C, Neiers F, Briand L. Sweeteners and sweetness enhancers. Curr Opin Clin Nutr Metab Care. 2017;20:279–285. doi: 10.1097/MCO.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 6.McGee H. On food and cooking: the science and lore of the kitchen. New York: Scribner; 2004. [Google Scholar]
- 7.Lapis TJ, Penner MH, Lim J. Evidence that humans can taste glucose polymers. Chem Senses. 2014;39:737–747. doi: 10.1093/chemse/bju031. [DOI] [PubMed] [Google Scholar]
- 8.Yarmolinsky DA, Zuker CS, Ryba NJP. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David Dickman J, Smith DV. Response properties of fibers in the hamster superior laryngeal nerve. Brain Res. 1988;450:25–38. doi: 10.1016/0006-8993(88)91541-7. [DOI] [PubMed] [Google Scholar]
- 10.Ohkuri T, Horio N, Stratford JM, et al. Residual chemoresponsiveness to acids in the superior laryngeal nerve in “taste-blind” (P2X2/P2X3 double-KO) mice. Chem Senses. 2012;37:523–532. doi: 10.1093/chemse/bjs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith DV, Hanamori T. Organization of gustatory sensitivities in hamster superior laryngeal nerve fibers. J Neurophysiol. 1991;65:1098–1114. doi: 10.1152/jn.1991.65.5.1098. [DOI] [PubMed] [Google Scholar]
- 12.Mouillot T, Szleper E, Vagne G, et al. Cerebral gustatory activation in response to free fatty acids using gustatory evoked potentials in humans. J Lipid Res. 2019;60:661–670. doi: 10.1194/jlr.M086587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roper SD. The taste of table salt. Pflugers Arch. 2015;467:457–463. doi: 10.1007/s00424-014-1683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roper SD, Chaudhari N. Taste buds: cells, signals and synapses. Nat Rev Neurosci. 2017;18:485–497. doi: 10.1038/nrn.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oka Y, Butnaru M, von Buchholtz L, et al. High salt recruits aversive taste pathways. Nature. 2013;494:472–475. doi: 10.1038/nature11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasumatsu K, Ogiwara Y, Takai S, et al. Umami taste in mice uses multiple receptors and transduction pathways. J Physiol. 2012;590:1155–1170. doi: 10.1113/jphysiol.2011.211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 19.Chandrashekar J, Kuhn C, Oka Y, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bigiani A, Cuoghi V. Localization of amiloride-sensitive sodium current and voltage-gated calcium currents in rat fungiform taste cells. J Neurophysiol. 2007;98:2483–2487. doi: 10.1152/jn.00716.2007. [DOI] [PubMed] [Google Scholar]
- 21.Lewandowski BC, Sukumaran SK, Margolskee RF, Bachmanov AA. Amiloride-insensitive salt taste is mediated by two populations of type III taste cells with distinct transduction mechanisms. J Neurosci Off J Soc Neurosci. 2016;36:1942–1953. doi: 10.1523/JNEUROSCI.2947-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roebber JK, Roper SD, Chaudhari N. the role of the anion in salt (NaCl) detection by mouse taste buds. J Neurosci. 2019;39:6224–6232. doi: 10.1523/JNEUROSCI.2367-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsey IS, DeSimone JA. Otopetrin-1: a sour-tasting proton channel. J Gen Physiol. 2018;150:379–382. doi: 10.1085/jgp.201812003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu Y-H, Cooper AJ, Teng B, et al. An evolutionarily conserved gene family encodes proton-selective ion channels. Science. 2018;359:1047–1050. doi: 10.1126/science.aao3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Jin H, Zhang W, et al. Sour sensing from the tongue to the brain. Cell. 2019 doi: 10.1016/j.cell.2019.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Teng B, Wilson CE, Tu Y-H, et al. Cellular and neural responses to sour stimuli require the proton channel Otop1. Curr Biol. 2019 doi: 10.1016/j.cub.2019.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye W, Chang RB, Bushman JD, et al. The K + channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction. Proc Natl Acad Sci. 2016;113:E229–E238. doi: 10.1073/pnas.1514282112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson CE, Vandenbeuch A, Kinnamon SC (2019) Physiological and behavioral responses to optogenetic stimulation of PKD2L1 + type III taste cells. eNeuro 6. 10.1523/ENEURO.0107-19.2019 [DOI] [PMC free article] [PubMed]
- 29.Zocchi D, Wennemuth G, Oka Y. The cellular mechanism for water detection in the mammalian taste system. Nat Neurosci. 2017;20:927–933. doi: 10.1038/nn.4575. [DOI] [PubMed] [Google Scholar]
- 30.Chandrashekar J, Yarmolinsky D, von Buchholtz L, et al. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang R, Dzowo YK, Wilson CE, et al (2019) Three-dimensional reconstructions of mouse circumvallate taste buds using serial blockface scanning electron microscopy: I. cell types and the apical region of the taste bud. bioRxiv 610410. 10.1101/610410 [DOI] [PMC free article] [PubMed]
- 32.Pérez CA, Huang L, Rong M, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 33.Dutta Banik D, Martin LE, Freichel M, et al. TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc Natl Acad Sci U S A. 2018;115:E772–E781. doi: 10.1073/pnas.1718802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taruno A, Vingtdeux V, Ohmoto M, et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Z, Taruno A, Ohmoto M et al (2018) CALHM3 is essential for rapid ion channel-mediated purinergic neurotransmission of GPCR-mediated tastes. Neuron 98:547–561.e10. 10.1016/j.neuron.2018.03.043 [DOI] [PMC free article] [PubMed]
- 36.Finger TE, Danilova V, Barrows J, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 37.Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 38.Nelson G, Hoon MA, Chandrashekar J, et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 39.Laffitte A, Neiers F, Briand L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr Opin Clin Nutr Metab Care. 2014;17:379–385. doi: 10.1097/MCO.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao GQ, Zhang Y, Hoon MA, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 41.Nie Y, Vigues S, Hobbs JR, et al. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol CB. 2005;15:1948–1952. doi: 10.1016/j.cub.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 42.Masuda T, Taguchi W, Sano A, et al. Five amino acid residues in cysteine-rich domain of human T1R3 were involved in the response for sweet-tasting protein, thaumatin. Biochimie. 2013;95:1502–1505. doi: 10.1016/j.biochi.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 43.DuBois GE. Molecular mechanism of sweetness sensation. Physiol Behav. 2016;164:453–463. doi: 10.1016/j.physbeh.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Cui M, Jiang P, Maillet E, et al. The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Curr Pharm Des. 2006;12:4591–4600. doi: 10.2174/138161206779010350. [DOI] [PubMed] [Google Scholar]
- 45.Han J, Choi M (2018) Comprehensive functional screening of taste sensation in vivo. bioRxiv 371682. 10.1101/371682
- 46.Behrens M, Meyerhof W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol Behav. 2011;105:4–13. doi: 10.1016/j.physbeh.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Reed DR, Li S, Li X, et al. Polymorphisms in the taste receptor gene (Tas1r3) Region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loney GC, Blonde GD, Eckel LA, Spector AC. Determinants of taste preference and acceptability: quality versus hedonics. J Neurosci. 2012;32:10086–10092. doi: 10.1523/JNEUROSCI.6036-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Li W, Wang H, et al. Cats lack a sweet taste receptor. J Nutr. 2006;136:1932S–1934S. doi: 10.1093/jn/136.7.1932S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldwin MW, Toda Y, Nakagita T, et al. Evolution of sweet taste perception in hummingbirds by transformation of the ancestral umami receptor. Science. 2014;345:929–933. doi: 10.1126/science.1255097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiffman SS, Booth BJ, Losee ML, et al. Bitterness of sweeteners as a function of concentration. Brain Res Bull. 1995;36:505–513. doi: 10.1016/0361-9230(94)00225-P. [DOI] [PubMed] [Google Scholar]
- 52.Servant G, Tachdjian C, Li X, Karanewsky DS. The sweet taste of true synergy: positive allosteric modulation of the human sweet taste receptor. Trends Pharmacol Sci. 2011;32:631–636. doi: 10.1016/j.tips.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Ninomiya Y, Imoto T. Gurmarin inhibition of sweet taste responses in mice. Am J Physiol-Regul Integr Comp Physiol. 1995;268:R1019–R1025. doi: 10.1152/ajpregu.1995.268.4.R1019. [DOI] [PubMed] [Google Scholar]
- 54.Sanematsu K, Shigemura N, Ninomiya Y. Binding properties between human sweet receptor and sweet-inhibitor, gymnemic acids. J Oral Biosci. 2017;59:127–130. doi: 10.1016/j.job.2017.05.004. [DOI] [Google Scholar]
- 55.Sigoillot M, Brockhoff A, Meyerhof W, Briand L. Sweet-taste-suppressing compounds: current knowledge and perspectives of application. Appl Microbiol Biotechnol. 2012;96:619–630. doi: 10.1007/s00253-012-4387-3. [DOI] [PubMed] [Google Scholar]
- 56.O’Connell S. Sugar: the grass that changed the world. London: Virgin; 2012. [Google Scholar]
- 57.Damak S, Rong M, Yasumatsu K, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 58.Merigo F, Benati D, Cristofoletti M, et al. Glucose transporters are expressed in taste receptor cells. J Anat. 2011;219:243–252. doi: 10.1111/j.1469-7580.2011.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yee KK, Sukumaran SK, Kotha R, et al. Glucose transporters and ATP-gated K + (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci USA. 2011;108:5431–5436. doi: 10.1073/pnas.1100495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sukumaran SK, Yee KK, Iwata S, et al. Taste cell-expressed α-glucosidase enzymes contribute to gustatory responses to disaccharides. Proc Natl Acad Sci. 2016;113:6035–6040. doi: 10.1073/pnas.1520843113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delaere F, Duchampt A, Mounien L, et al. The role of sodium-coupled glucose co-transporter 3 in the satiety effect of portal glucose sensing. Mol Metab. 2012;2:47–53. doi: 10.1016/j.molmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sclafani A, Koepsell H, Ackroff K. SGLT1 sugar transporter/sensor is required for post-oral glucose appetition. Am J Physiol-Regul Integr Comp Physiol. 2016;310:R631–R639. doi: 10.1152/ajpregu.00432.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 64.Mueller KL, Hoon MA, Erlenbach I, et al. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- 65.Marella S, Fischler W, Kong P, et al. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 66.Ohla K, Yoshida R, Roper SD, et al. Recognizing taste: coding patterns along the neural axis in mammals. Chem Senses. 2019;44:237–247. doi: 10.1093/chemse/bjz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villavicencio M, Moreno MG, Simon SA, Gutierrez R. Encoding of sucrose’s palatability in the nucleus accumbens shell and its modulation by exteroceptive auditory cues. Front Neurosci. 2018;12:265. doi: 10.3389/fnins.2018.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frank ME, Contreras RJ, Hettinger TP. Nerve fibers sensitive to ionic taste stimuli in chorda tympani of the rat. J Neurophysiol. 1983;50:941–960. doi: 10.1152/jn.1983.50.4.941. [DOI] [PubMed] [Google Scholar]
- 69.Tokita K, Boughter JD. Topographic organizations of taste-responsive neurons in the parabrachial nucleus of C57BL/6J mice: an electrophysiological mapping study. Neuroscience. 2016;316:151–166. doi: 10.1016/j.neuroscience.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste responses of cortical neurons in freely ingesting rats. J Neurophysiol. 1989;61:1244–1258. doi: 10.1152/jn.1989.61.6.1244. [DOI] [PubMed] [Google Scholar]
- 71.Barretto RPJ, Gillis-Smith S, Chandrashekar J, et al. The neural representation of taste quality at the periphery. Nature. 2015;517:373–376. doi: 10.1038/nature13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erickson RP. The evolution and implications of population and modular neural coding ideas. Prog Brain Res. 2001;130:9–29. doi: 10.1016/S0079-6123(01)30003-1. [DOI] [PubMed] [Google Scholar]
- 73.Erickson RP (2008) A study of the science of taste: on the origins and influence of the core ideas. Behav Brain Sci 31:59–75. 10.1017/S0140525X08003348(discussion 75–105) [DOI] [PubMed]
- 74.Wu A, Dvoryanchikov G, Pereira E, et al. Breadth of tuning in taste afferent neurons varies with stimulus strength. Nat Commun. 2015;6:8171. doi: 10.1038/ncomms9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomchik SM, Berg S, Kim JW, et al. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ootani S, Umezaki T, Shin T, Murata Y. Convergence of afferents from the SLN and GPN in cat medullary swallowing neurons. Brain Res Bull. 1995;37:397–404. doi: 10.1016/0361-9230(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 77.Spector AC. Linking gustatory neurobiology to behavior in vertebrates. Neurosci Biobehav Rev. 2000;24:391–416. doi: 10.1016/S0149-7634(00)00013-0. [DOI] [PubMed] [Google Scholar]
- 78.Danilova V, Danilov Y, Roberts T, et al. Sense of taste in a new world monkey, the common marmoset: recordings from the chorda tympani and glossopharyngeal nerves. J Neurophysiol. 2002;88:579–594. doi: 10.1152/jn.2002.88.2.579. [DOI] [PubMed] [Google Scholar]
- 79.Pfaffmann C. Gustatory afferent impulses. J Cell Comp Physiol. 1941;17:243–258. doi: 10.1002/jcp.1030170209. [DOI] [Google Scholar]
- 80.Breza JM, Nikonov AA, Contreras RJ. response latency to lingual taste stimulation distinguishes neuron types within the geniculate ganglion. J Neurophysiol. 2010;103:1771–1784. doi: 10.1152/jn.00785.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen X, Gabitto M, Peng Y, et al. A gustotopic map of taste qualities in the mammalian brain. Science. 2011;333:1262–1266. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roussin AT, D’Agostino AE, Fooden AM, et al. Taste coding in the nucleus of the solitary tract of the awake, freely licking rat. J Neurosci Off J Soc Neurosci. 2012;32:10494–10506. doi: 10.1523/JNEUROSCI.1856-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sammons JD, Weiss MS, Victor JD, Di Lorenzo PM. Taste coding of complex naturalistic taste stimuli and traditional taste stimuli in the parabrachial pons of the awake, freely licking rat. J Neurophysiol. 2016;116:171–182. doi: 10.1152/jn.01119.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu H, Fontanini A. State dependency of chemosensory coding in the gustatory thalamus (VPMpc) of alert rats. J Neurosci. 2015;35:15479–15491. doi: 10.1523/JNEUROSCI.0839-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nomura T, Ogawa H. The taste and mechanical response properties of neurons in the parvicellular part of the thalamic posteromedial ventral nucleus of the rat. Neurosci Res. 1985;3:91–105. doi: 10.1016/0168-0102(85)90024-0. [DOI] [PubMed] [Google Scholar]
- 86.Ogawa H, Nomura T. Receptive field properties of thalamo-cortical taste relay neurons in the parvicellular part of the posteromedial ventral nucleus in rats. Exp Brain Res. 1988;73:364–370. doi: 10.1007/bf00248229. [DOI] [PubMed] [Google Scholar]
- 87.Verhagen JV, Giza BK, Scott TR. Responses to taste stimulation in the ventroposteromedial nucleus of the thalamus in rats. J Neurophysiol. 2003;89:265–275. doi: 10.1152/jn.00870.2001. [DOI] [PubMed] [Google Scholar]
- 88.Levitan D, Lin J-Y, Wachutka J, et al. Single and population coding of taste in the gustatory cortex of awake mice. J Neurophysiol. 2019 doi: 10.1152/jn.00357.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stapleton JR, Lavine ML, Wolpert RL, et al. Rapid taste responses in the gustatory cortex during licking. J Neurosci Off J Soc Neurosci. 2006;26:4126–4138. doi: 10.1523/JNEUROSCI.0092-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yaxley S, Rolls ET, Sienkiewicz ZJ. Gustatory responses of single neurons in the insula of the macaque monkey. J Neurophysiol. 1990;63:689–700. doi: 10.1152/jn.1990.63.4.689. [DOI] [PubMed] [Google Scholar]
- 91.Fletcher ML, Ogg MC, Lu L, et al. Overlapping representation of primary tastes in a defined region of the gustatory cortex. J Neurosci Off J Soc Neurosci. 2017;37:7595–7605. doi: 10.1523/JNEUROSCI.0649-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lavi K, Jacobson GA, Rosenblum K, Lüthi A. Encoding of conditioned taste aversion in cortico-amygdala circuits. Cell Rep. 2018;24:278–283. doi: 10.1016/j.celrep.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 93.Livneh Y, Ramesh RN, Burgess CR, et al. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature. 2017;546:611–616. doi: 10.1038/nature22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fonseca E, de Lafuente V, Simon SA, Gutierrez R (2018) Sucrose intensity coding and decision-making in rat gustatory cortices. eLife 7:e41152. 10.7554/eLife.41152 [DOI] [PMC free article] [PubMed]
- 95.Gehrlach DA, Dolensek N, Klein AS, et al. Aversive state processing in the posterior insular cortex. Nat Neurosci. 2019;22:1424–1437. doi: 10.1038/s41593-019-0469-1. [DOI] [PubMed] [Google Scholar]
- 96.Chikazoe J, Lee DH, Kriegeskorte N, Anderson AK. Distinct representations of basic taste qualities in human gustatory cortex. Nat Commun. 2019;10:1048. doi: 10.1038/s41467-019-08857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Canna A, Prinster A, Cantone E, et al. Intensity-related distribution of sweet and bitter taste fMRI responses in the insular cortex. Hum Brain Mapp. 2019;40:3631–3646. doi: 10.1002/hbm.24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Avery JA, Liu AG, Ingeholm JE, et al (2019) Taste quality representation in the human brain. bioRxiv 726711. 10.1101/726711
- 99.Porcu E, Benz K, Ball F, et al (2019) Information-based taste maps in insular cortex are shaped by stimulus concentration. Neuroscience [DOI] [PMC free article] [PubMed]
- 100.Peng Y, Gillis-Smith S, Jin H, et al. Sweet and bitter taste in the brain of awake behaving animals. Nature. 2015;527:512–515. doi: 10.1038/nature15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci. 1998;112:678–694. doi: 10.1037/0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- 102.Young PT, Burright RG, Tromater LJ. Preferences of the white rat for solutions of sucrose and quinine hydrochloride. Am J Psychol. 1963;76:205–217. doi: 10.2307/1419157. [DOI] [PubMed] [Google Scholar]
- 103.Mukherjee N, Wachutka J, Katz DB (2019) Impact of precisely-timed inhibition of gustatory cortex on taste behavior depends on single-trial ensemble dynamics. eLife 8:e45968. 10.7554/eLife.45968 [DOI] [PMC free article] [PubMed]
- 104.Spector AC, Smith JC. A detailed analysis of sucrose drinking in the rat. Physiol Behav. 1984;33:127–136. doi: 10.1016/0031-9384(84)90023-4. [DOI] [PubMed] [Google Scholar]
- 105.Berridge KC, Grill HJ. Alternating ingestive and aversive consummatory responses suggest a two-dimensional analysis of palatability in rats. Behav Neurosci. 1983;97:563–573. doi: 10.1037/0735-7044.97.4.563. [DOI] [PubMed] [Google Scholar]
- 106.Sclafani A. Conditioned food preferences and appetite. Appetite. 1991;17:71–72. doi: 10.1016/0195-6663(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 107.Garcia J, Lasiter PS, Bermudez-Rattoni F, Deems DA. A general theory of aversion learning. Ann N Y Acad Sci. 1985;443:8–21. doi: 10.1111/j.1749-6632.1985.tb27060.x. [DOI] [PubMed] [Google Scholar]
- 108.Baez-Santiago MA, Reid EE, Moran A, et al. Dynamic taste responses of parabrachial pontine neurons in awake rats. J Neurophysiol. 2016;115:1314–1323. doi: 10.1152/jn.00311.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jezzini A, Mazzucato L, Camera GL, Fontanini A. Processing of hedonic and chemosensory features of taste in medial prefrontal and insular networks. J Neurosci. 2013;33:18966–18978. doi: 10.1523/JNEUROSCI.2974-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sadacca BF, Rothwax JT, Katz DB. Sodium concentration coding gives way to evaluative coding in cortex and amygdala. J Neurosci. 2012;32:9999–10011. doi: 10.1523/JNEUROSCI.6059-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li JX, Yoshida T, Monk KJ, Katz DB. Lateral hypothalamus contains two types of palatability-related taste responses with distinct dynamics. J Neurosci. 2013;33:9462–9473. doi: 10.1523/JNEUROSCI.3935-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jezzini A, Caruana F, Stoianov I, et al. Functional organization of the insula and inner perisylvian regions. Proc Natl Acad Sci USA. 2012;109:10077–10082. doi: 10.1073/pnas.1200143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang L, Gillis-Smith S, Peng Y, et al. The coding of valence and identity in the mammalian taste system. Nature. 2018;558:127–131. doi: 10.1038/s41586-018-0165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Devineni AV, Sun B, Zhukovskaya A, Axel R (2019) Acetic acid activates distinct taste pathways in Drosophila to elicit opposing, state-dependent feeding responses. eLife 8:e47677. 10.7554/eLife.47677 [DOI] [PMC free article] [PubMed]
- 115.Perez IO, Villavicencio M, Simon SA, Gutierrez R. Speed and accuracy of taste identification and palatability: impact of learning, reward expectancy, and consummatory licking. Am J Physiol-Regul Integr Comp Physiol. 2013;305:R252–R270. doi: 10.1152/ajpregu.00492.2012. [DOI] [PubMed] [Google Scholar]
- 116.Halpern BP. Constraints imposed on taste physiology by human taste reaction time data. Neurosci Biobehav Rev. 1986;10:135–151. doi: 10.1016/0149-7634(86)90024-2. [DOI] [PubMed] [Google Scholar]
- 117.Wallroth R, Ohla K (2018) As soon as you taste it: evidence for sequential and parallel processing of gustatory information. eNeuro 5:e0269. 10.1523/ENEURO.0269-18.2018 [DOI] [PMC free article] [PubMed]
- 118.Katz DB, Simon SA, Nicolelis MAL. Taste-specific neuronal ensembles in the gustatory cortex of awake rats. J Neurosci Off J Soc Neurosci. 2002;22:1850–1857. doi: 10.1523/JNEUROSCI.22-05-01850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frank M, Pfaffmann C. Taste nerve fibers: a random distribution of sensitivities to four tastes. Science. 1969;164:1183–1185. doi: 10.1126/science.164.3884.1183. [DOI] [PubMed] [Google Scholar]
- 120.Kovacs P, Hajnal A. Altered pontine taste processing in a rat model of obesity. J Neurophysiol. 2008;100:2145–2157. doi: 10.1152/jn.01359.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scott TR, Plata-Salaman CR, Smith VL, Giza BK. Gustatory neural coding in the monkey cortex: stimulus intensity. J Neurophysiol. 1991;65:76–86. doi: 10.1152/jn.1991.65.1.76. [DOI] [PubMed] [Google Scholar]
- 122.Rolls ET, Yaxley S, Sienkiewicz ZJ. Gustatory responses of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. J Neurophysiol. 1990;64:1055–1066. doi: 10.1152/jn.1990.64.4.1055. [DOI] [PubMed] [Google Scholar]
- 123.Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: neuronal activity in the behaving monkey. Exp Brain Res. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- 124.Maier JX, Katz DB. Neural dynamics in response to binary taste mixtures. J Neurophysiol. 2013;109:2108–2117. doi: 10.1152/jn.00917.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rosen AM, Di Lorenzo PM. Neural coding of taste by simultaneously recorded cells in the nucleus of the solitary tract of the rat. J Neurophysiol. 2012;108:3301–3312. doi: 10.1152/jn.00566.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tellez LA, Perez IO, Simon SA, Gutierrez R. Transitions between sleep and feeding states in rat ventral striatum neurons. J Neurophysiol. 2012;108:1739–1751. doi: 10.1152/jn.00394.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Allen WE, Chen MZ, Pichamoorthy N, et al (2019) Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 364:eaav3932. 10.1126/science.aav3932 [DOI] [PMC free article] [PubMed]
- 128.Green BG, Frankmann SP. The effect of cooling on the perception of carbohydrate and intensive sweeteners. Physiol Behav. 1988;43:515–519. doi: 10.1016/0031-9384(88)90127-8. [DOI] [PubMed] [Google Scholar]
- 129.Cruz A, Green BG. Thermal stimulation of taste. Nature. 2000;403:889–892. doi: 10.1038/35002581. [DOI] [PubMed] [Google Scholar]
- 130.Green BG, Nachtigal D. Temperature affects human sweet taste via at least two mechanisms. Chem Senses. 2015;40:391–399. doi: 10.1093/chemse/bjv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol. 2006;95:674–685. doi: 10.1152/jn.00793.2005. [DOI] [PubMed] [Google Scholar]
- 132.Lemon CH. Modulation of taste processing by temperature. Am J Physiol-Regul Integr Comp Physiol. 2017;313:R305–R321. doi: 10.1152/ajpregu.00089.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lemon CH. Perceptual and neural responses to sweet taste in humans and rodents. Chemosens Percept. 2015;8:46–52. doi: 10.1007/s12078-015-9177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Talavera K, Yasumatsu K, Voets T, et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 135.Wilson DM, Lemon CH. Temperature systematically modifies neural activity for sweet taste. J Neurophysiol. 2014;112:1667–1677. doi: 10.1152/jn.00368.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li J, Lemon CH. Influence of stimulus and oral adaptation temperature on gustatory responses in central taste-sensitive neurons. J Neurophysiol. 2015;113:2700–2712. doi: 10.1152/jn.00736.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liman ER. TRPM5 and taste transduction. In: Flockerzi V, Nilius B, editors. Transient receptor potential (TRP) channels. Berlin Heidelberg, Berlin, Heidelberg: Springer; 2007. pp. 287–298. [Google Scholar]
- 138.Kwon O, Kim KW, Kim M-S. Leptin signalling pathways in hypothalamic neurons. Cell Mol Life Sci CMLS. 2016;73:1457–1477. doi: 10.1007/s00018-016-2133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meister B. Control of food intake via leptin receptors in the hypothalamus. Vitam Horm. 2000;59:265–304. doi: 10.1016/S0083-6729(00)59010-4. [DOI] [PubMed] [Google Scholar]
- 140.Kawai K, Sugimoto K, Nakashima K, et al. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci. 2000;97:11044–11049. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shigemura N, Ohta R, Kusakabe Y, et al. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology. 2004;145:839–847. doi: 10.1210/en.2003-0602. [DOI] [PubMed] [Google Scholar]
- 142.Ninomiya Y, Sako N, Imai Y. Enhanced gustatory neural responses to sugars in the diabetic db/db mouse. Am J Physiol-Regul Integr Comp Physiol. 1995;269:R930–R937. doi: 10.1152/ajpregu.1995.269.4.R930. [DOI] [PubMed] [Google Scholar]
- 143.Yoshida R, Niki M, Jyotaki M, et al. Modulation of sweet responses of taste receptor cells. Semin Cell Dev Biol. 2013;24:226–231. doi: 10.1016/j.semcdb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 144.Niki M, Jyotaki M, Yoshida R, et al. Modulation of sweet taste sensitivities by endogenous leptin and endocannabinoids in mice. J Physiol. 2015;593:2527–2545. doi: 10.1113/JP270295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nakamura Y, Sanematsu K, Ohta R, et al. Diurnal variation of human sweet taste recognition thresholds is correlated with plasma leptin levels. Diabetes. 2008;57:2661–2665. doi: 10.2337/db07-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Han P, Keast RSJ, Roura E. Salivary leptin and TAS1R2/TAS1R3 polymorphisms are related to sweet taste sensitivity and carbohydrate intake from a buffet meal in healthy young adults. Br J Nutr. 2017;118:763–770. doi: 10.1017/S0007114517002872. [DOI] [PubMed] [Google Scholar]
- 147.Sanematsu K, Nakamura Y, Nomura M, et al. Diurnal variation of sweet taste recognition thresholds is absent in overweight and obese humans. Nutrients. 2018;10:297. doi: 10.3390/nu10030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fujita T. Taste cells in the gut and on the tongue. Their common, paraneuronal features. Physiol Behav. 1991;49:883–885. doi: 10.1016/0031-9384(91)90198-W. [DOI] [PubMed] [Google Scholar]
- 149.Hass N, Schwarzenbacher K, Breer H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res. 2010;339:493–504. doi: 10.1007/s00441-009-0907-6. [DOI] [PubMed] [Google Scholar]
- 150.Höfer D, Drenckhahn D. Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol. 1998;110:303–309. doi: 10.1007/s004180050292. [DOI] [PubMed] [Google Scholar]
- 151.Jang H-J, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee RJ, Kofonow JM, Rosen PL, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124:1393–1405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in Solitary Chemosensory Cells of rodent airways. BMC Pulm Med. 2011;11:3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kohno D, Koike M, Ninomiya Y, et al. Sweet taste receptor serves to activate glucose- and leptin-responsive neurons in the hypothalamic arcuate nucleus and participates in glucose responsiveness. Front Neurosci. 2016;10:502. doi: 10.3389/fnins.2016.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ren X, Zhou L, Terwilliger R, et al. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci. 2009;3:12. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Masubuchi Y, Nakagawa Y, Ma J, et al. A novel regulatory function of sweet taste-sensing receptor in adipogenic differentiation of 3T3-L1 cells. PLoS One. 2013;8:e54500. doi: 10.1371/journal.pone.0054500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Simon BR, Parlee SD, Learman BS, et al. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J Biol Chem. 2013;288:32475–32489. doi: 10.1074/jbc.M113.514034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lizunkova P, Enuwosa E, Chichger H. Activation of the sweet taste receptor T1R3 by sucralose attenuates VEGF-induced vasculogenesis in a cell model of the retinal microvascular endothelium. Graefes Arch Clin Exp Ophthalmol. 2019;257:71–81. doi: 10.1007/s00417-018-4157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gong T, Wei Q, Mao D, Shi F. Expression patterns of taste receptor type 1 subunit 3 and α-gustducin in the mouse testis during development. Acta Histochem. 2016;118:20–30. doi: 10.1016/j.acthis.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 161.Kiuchi S, Yamada T, Kiyokawa N, et al. Genomic structure of swine taste receptor family 1 member 3, TAS1R3, and its expression in tissues. Cytogenet Genome Res. 2006;115:51–61. doi: 10.1159/000094801. [DOI] [PubMed] [Google Scholar]
- 162.Li F. Taste perception: from the tongue to the testis. MHR Basic Sci Reprod Med. 2013;19:349–360. doi: 10.1093/molehr/gat009. [DOI] [PubMed] [Google Scholar]
- 163.Max M, Shanker YG, Huang L, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. doi: 10.1038/88270. [DOI] [PubMed] [Google Scholar]
- 164.Foster SR, Porrello ER, Purdue B, et al. Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS One. 2013;8:e64579. doi: 10.1371/journal.pone.0064579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kochem M. Type 1 taste receptors in taste and metabolism. Ann Nutr Metab. 2017;70(Suppl 3):27–36. doi: 10.1159/000478760. [DOI] [PubMed] [Google Scholar]
- 166.Nakagawa Y, Nagasawa M, Yamada S, et al. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One. 2009;4:e5106. doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Taniguchi K. Expression of the sweet receptor protein, T1R3, in the human liver and pancreas. J Vet Med Sci. 2004;66:1311–1314. doi: 10.1292/jvms.66.1311. [DOI] [PubMed] [Google Scholar]
- 168.Elliott RA, Kapoor S, Tincello DG. Expression and distribution of the sweet taste receptor isoforms T1R2 and T1R3 in human and rat bladders. J Urol. 2011;186:2455–2462. doi: 10.1016/j.juro.2011.07.083. [DOI] [PubMed] [Google Scholar]
- 169.Meyer D, Voigt A, Widmayer P, et al. Expression of Tas1 taste receptors in mammalian spermatozoa: functional role of Tas1r1 in regulating basal Ca2+ and cAMP concentrations in spermatozoa. PLoS One. 2012;7:e32354. doi: 10.1371/journal.pone.0032354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci USA. 2012;109:E524–E532. doi: 10.1073/pnas.1115183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oliveira-Maia AJ, Roberts CD, Walker QD, et al. Intravascular food reward. PLOS One. 2011;6:e24992. doi: 10.1371/journal.pone.0024992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Qu T, Han W, Niu J, et al. On the roles of the Duodenum and the Vagus nerve in learned nutrient preferences. Appetite. 2019;139:145–151. doi: 10.1016/j.appet.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tellez LA, Han W, Zhang X, et al. Separate circuitries encode the hedonic and nutritional values of sugar. Nat Neurosci. 2016;19:465–470. doi: 10.1038/nn.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Holman GL. Intragastric reinforcement effect. J Comp Physiol Psychol. 1969;69:432–441. doi: 10.1037/h0028233. [DOI] [PubMed] [Google Scholar]
- 175.Sclafani A, Cardieri C, Tucker K, et al. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol-Regul Integr Comp Physiol. 1993;265:R320–R325. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- 176.Zhang L, Han W, Lin C, et al. Sugar metabolism regulates flavor preferences and portal glucose sensing. Front Integr Neurosci. 2018 doi: 10.3389/fnint.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978;201:267–269. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- 178.Berridge KC. Wanting and liking: observations from the neuroscience and psychology laboratory. Inq Oslo Nor. 2009;52:378. doi: 10.1080/00201740903087359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ackroff K, Yiin Y-M, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav. 2010;99:402–411. doi: 10.1016/j.physbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sclafani A, Ackroff K. Commentary: sugar metabolism regulates flavor preferences and portal glucose sensing. Front Integr Neurosci. 2019 doi: 10.3389/fnint.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Han W, Tellez LA, Niu J, et al. Striatal dopamine links gastrointestinal rerouting to altered sweet appetite. Cell Metab. 2016;23:103–112. doi: 10.1016/j.cmet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and nonmetabolizable sugar analogs. Am J Physiol-Regul Integr Comp Physiol. 2013;305:R840–R853. doi: 10.1152/ajpregu.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ren X, Ferreira JG, Zhou L, et al. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Strubbe JH, Steffens AB. Blood glucose levels in portal and peripheral circulation and their relation to food intake in the rat. Physiol Behav. 1977;19:303–307. doi: 10.1016/0031-9384(77)90342-0. [DOI] [PubMed] [Google Scholar]
- 186.Tordoff MG, Friedman MI. Hepatic portal glucose infusions decrease food intake and increase food preference. Am J Physiol. 1986;251:R192–R196. doi: 10.1152/ajpregu.1986.251.1.R192. [DOI] [PubMed] [Google Scholar]
- 187.Berthoud H-R. Anatomy and function of sensory hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280A:827–835. doi: 10.1002/ar.a.20088. [DOI] [PubMed] [Google Scholar]
- 188.Han W, Tellez LA, Perkins MH, et al. A neural circuit for gut-induced reward. Cell. 2018;175:887–888. doi: 10.1016/j.cell.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 189.Sclafani A, Lucas F. Abdominal vagotomy does not block carbohydrate-conditioned flavor preferences in rats. Physiol Behav. 1996;60:447–453. doi: 10.1016/S0031-9384(96)80018-7. [DOI] [PubMed] [Google Scholar]
- 190.Uematsu A, Tsurugizawa T, Uneyama H, Torii K. Brain–gut communication via vagus nerve modulates conditioned flavor preference. Eur J Neurosci. 2010;31:1136–1143. doi: 10.1111/j.1460-9568.2010.07136.x. [DOI] [PubMed] [Google Scholar]
- 191.Worthington JJ, Reimann F, Gribble FM. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 2018;11:3–20. doi: 10.1038/mi.2017.73. [DOI] [PubMed] [Google Scholar]
- 192.Kaelberer MM, Buchanan KL, Klein ME et al (2018) A gut-brain neural circuit for nutrient sensory transduction. Science 361:eaat5236. 10.1126/science.aat5236 [DOI] [PMC free article] [PubMed]
- 193.Domingos AI, Vaynshteyn J, Voss HU, et al. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14:1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 195.Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav. 2006;89:531–535. doi: 10.1016/j.physbeh.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Klawonn AM, Malenka RC. Nucleus accumbens modulation in reward and aversion. Cold Spring Harb Symp Quant Biol. 2019 doi: 10.1101/sqb.2018.83.037457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Thanarajah SE, Backes H, DiFeliceantonio AG, et al. Food intake recruits orosensory and post-ingestive dopaminergic circuits to affect eating desire in humans. Cell Metab. 2019;29:695.e4–706.e4. doi: 10.1016/j.cmet.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 198.Wang G-J, Volkow ND, Logan J, et al. Brain dopamine and obesity. The Lancet. 2001;357:354–357. doi: 10.1016/S0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 199.Wiss DA, Avena N, Rada P. Sugar addiction: from evolution to revolution. Front Psychiatry. 2018 doi: 10.3389/fpsyt.2018.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Blum K, Thanos PK, Gold MS. Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol. 2014 doi: 10.3389/fpsyg.2014.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30:13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pritchett CE, Hajnal A. Obesogenic diets may differentially alter dopamine control of sucrose and fructose intake in rats. Physiol Behav. 2011;104:111–116. doi: 10.1016/j.physbeh.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.DiFeliceantonio AG, Small DM. Dopamine and diet-induced obesity. Nat Neurosci. 2019;22:1–2. doi: 10.1038/s41593-018-0304-0. [DOI] [PubMed] [Google Scholar]
- 205.Shoar S, Saber AA. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: a systematic review and meta-analysis of comparative studies. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2017;13:170–180. doi: 10.1016/j.soard.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 206.Mathes CM. Taste- and flavor-guided behaviors following Roux-en-Y gastric bypass in rodent models. Appetite. 2019 doi: 10.1016/j.appet.2019.104422. [DOI] [PubMed] [Google Scholar]
- 207.Hankir MK, Seyfried F, Hintschich CA, et al. Gastric bypass surgery recruits a gut PPAR-α-striatal D1R pathway to reduce fat appetite in obese rats. Cell Metab. 2017;25:335–344. doi: 10.1016/j.cmet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 208.Tellez LA, Medina S, Han W, et al. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science. 2013;341:800–802. doi: 10.1126/science.1239275. [DOI] [PubMed] [Google Scholar]
- 209.Tsouristakis AI, Febres G, McMahon DJ, et al. Long-term modulation of appetitive hormones and sweet cravings after adjustable gastric banding and Roux-en-Y gastric bypass. Obes Surg. 2019 doi: 10.1007/s11695-019-04111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sternson SM, Eiselt A-K. Three pillars for the neural control of appetite. Annu Rev Physiol. 2017;79:401–423. doi: 10.1146/annurev-physiol-021115-104948. [DOI] [PubMed] [Google Scholar]
- 211.Burnett CJ, Li C, Webber E, et al. Hunger-driven motivational state competition. Neuron. 2016;92:187–201. doi: 10.1016/j.neuron.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jennings JH, Ung RL, Resendez SL, et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160:516–527. doi: 10.1016/j.cell.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Navarro M, Olney JJ, Burnham NW, et al. Lateral hypothalamus gabaergic neurons modulate consummatory behaviors regardless of the caloric content or biological relevance of the consumed stimuli. Neuropsychopharmacology. 2016;41:1505–1512. doi: 10.1038/npp.2015.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nieh EH, Vander Weele CM, Matthews GA, et al. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron. 2016;90:1286–1298. doi: 10.1016/j.neuron.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.de Jong JW, Afjei SA, Pollak Dorocic I, et al. A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron. 2019;101:133.e7–151.e7. doi: 10.1016/j.neuron.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rossi MA, Basiri ML, McHenry JA, et al. Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science. 2019;364:1271–1274. doi: 10.1126/science.aax1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sternson SM. Hunger: the carrot and the stick. Mol Metab. 2016;5:1–2. doi: 10.1016/j.molmet.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Su Z, Alhadeff AL, Betley JN. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep. 2017;21:2724–2736. doi: 10.1016/j.celrep.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Beutler LR, Chen Y, Ahn JS, et al. Dynamics of gut-brain communication underlying hunger. Neuron. 2017;96:461.e5–475.e5. doi: 10.1016/j.neuron.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen Y, Lin Y-C, Zimmerman CA et al (2016) Hunger neurons drive feeding through a sustained, positive reinforcement signal. eLife 5. 10.7554/eLife.18640 [DOI] [PMC free article] [PubMed]
- 222.Krashes MJ, Koda S, Ye C, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 224.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Betley JN, Xu S, Cao ZFH, et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mandelblat-Cerf Y, Ramesh RN, Burgess CR, et al (2015) Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife 4:e07122. 10.7554/eLife.07122 [DOI] [PMC free article] [PubMed]
- 227.Rau AR, Hentges ST. The relevance of AgRP neuron-derived GABA inputs to POMC neurons differs for spontaneous and evoked release. J Neurosci. 2017;37:7362–7372. doi: 10.1523/JNEUROSCI.0647-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wei Q, Krolewski DM, Moore S, et al. Uneven balance of power between hypothalamic peptidergic neurons in the control of feeding. Proc Natl Acad Sci. 2018;115:E9489–E9498. doi: 10.1073/pnas.1802237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Weingarten HP. Meal initiation controlled by learned cues: basic behavioral properties. Appetite. 1984;5:147–158. doi: 10.1016/S0195-6663(84)80035-5. [DOI] [PubMed] [Google Scholar]
- 230.Robinson MJ, Burghardt PR, Patterson CM, et al. Individual differences in cue-induced motivation and striatal systems in rats susceptible to diet-induced obesity. Neuropsychopharmacology. 2015;40:2113–2123. doi: 10.1038/npp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Burger KS, Stice E. Greater striatopallidal adaptive coding during cue–reward learning and food reward habituation predict future weight gain. NeuroImage. 2014;99:122–128. doi: 10.1016/j.neuroimage.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Reppucci CJ, Petrovich GD. Learned food-cue stimulates persistent feeding in sated rats. Appetite. 2012;59:437–447. doi: 10.1016/j.appet.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Derman RC, Ferrario CR. Junk-food enhances conditioned food cup approach to a previously established food cue, but does not alter cue potentiated feeding; implications for the effects of palatable diets on incentive motivation. Physiol Behav. 2018;192:145–157. doi: 10.1016/j.physbeh.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Denis RGP, Joly-Amado A, Webber E, et al. Palatability can drive feeding independent of AgRP neurons. Cell Metab. 2015;22:646–657. doi: 10.1016/j.cmet.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.O’Connor EC, Kremer Y, Lefort S, et al. Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron. 2015;88:553–564. doi: 10.1016/j.neuron.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 236.Prado L, Luis-Islas J, Sandoval OI, et al. Activation of glutamatergic fibers in the anterior NAc shell modulates reward activity in the aNAcSh, the lateral hypothalamus, and medial prefrontal cortex and transiently stops feeding. J Neurosci. 2016;36:12511–12529. doi: 10.1523/JNEUROSCI.1605-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 238.Kalyanasundar B, Perez CI, Luna A, et al. D1 and D2 antagonists reverse the effects of appetite suppressants on weight loss, food intake, locomotion, and rebalance spiking inhibition in the rat NAc shell. J Neurophysiol. 2015;114:585–607. doi: 10.1152/jn.00012.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rothman RB, Baumann MH. Balance between dopamine and serotonin release modulates behavioral effects of amphetamine-type drugs. Ann N Y Acad Sci. 2006;1074:245–260. doi: 10.1196/annals.1369.064. [DOI] [PubMed] [Google Scholar]
- 240.Britt JP, Benaliouad F, McDevitt RA, et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Reed SJ, Lafferty CK, Mendoza JA, et al. Coordinated reductions in excitatory input to the nucleus accumbens underlie food consumption. Neuron. 2018;99:1260.e4–1273.e4. doi: 10.1016/j.neuron.2018.07.051. [DOI] [PubMed] [Google Scholar]
- 242.Valenstein ES, Cox VC, Kakolewski JW. Modification of motivated behavior elicited by electrical stimulation of the hypothalamus. Science. 1968;159:1119–1121. doi: 10.1126/science.159.3819.1119. [DOI] [PubMed] [Google Scholar]
- 243.Frank RA, Preshaw RL, Stutz RM, Valenstein ES. Lateral hypothalamic stimulation: stimulus-bound eating and self-deprivation. Physiol Behav. 1982;29:17–21. doi: 10.1016/0031-9384(82)90359-6. [DOI] [PubMed] [Google Scholar]
- 244.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24:123–140. [PMC free article] [PubMed] [Google Scholar]
- 245.de Lecea L. Optogenetic control of hypocretin (orexin) neurons and arousal circuits. Curr Top Behav Neurosci. 2015;25:367–378. doi: 10.1007/7854_2014_364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Stamatakis AM, Swieten MV, Basiri ML, et al. Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J Neurosci. 2016;36:302–311. doi: 10.1523/JNEUROSCI.1202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.You ZB, Chen YQ, Wise RA. Dopamine and glutamate release in the nucleus accumbens and ventral tegmental area of rat following lateral hypothalamic self-stimulation. Neuroscience. 2001;107:629–639. doi: 10.1016/s0306-4522(01)00379-7. [DOI] [PubMed] [Google Scholar]
- 248.Zhang X, van den Pol AN. Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science. 2017;356:853–859. doi: 10.1126/science.aam7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.WHO. Sugars intake for adults and children. In: WHO. http://www.who.int/nutrition/publications/guidelines/sugars_intake/en/. Accessed 16 Aug 2019
- 250.Bartoshuk LM, Duffy VB, Hayes JE, et al. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci. 2006;361:1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Berthoud H-R, Zheng H. Modulation of taste responsiveness and food preference by obesity and weight loss. Physiol Behav. 2012;107:527–532. doi: 10.1016/j.physbeh.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.May CE, Vaziri A, Lin YQ, et al. High dietary sugar reshapes sweet taste to promote feeding behavior in Drosophila melanogaster. Cell Rep. 2019;27:1675.e7–1685.e7. doi: 10.1016/j.celrep.2019.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Overberg J, Hummel T, Krude H, Wiegand S. Differences in taste sensitivity between obese and non-obese children and adolescents. Arch Dis Child. 2012;97:1048–1052. doi: 10.1136/archdischild-2011-301189. [DOI] [PubMed] [Google Scholar]
- 254.Pasquet P, Frelut ML, Simmen B, et al. Taste perception in massively obese and in non-obese adolescents. Int J Pediatr Obes IJPO Off J Int Assoc Study Obes. 2007;2:242–248. doi: 10.1080/17477160701440521. [DOI] [PubMed] [Google Scholar]
- 255.Proserpio C, Laureati M, Bertoli S, et al. Determinants of obesity in Italian adults: the role of taste sensitivity, food liking, and food neophobia. Chem Senses. 2016;41:169–176. doi: 10.1093/chemse/bjv072. [DOI] [PubMed] [Google Scholar]
- 256.Sartor F, Donaldson LF, Markland DA, et al. Taste perception and implicit attitude toward sweet related to body mass index and soft drink supplementation. Appetite. 2011;57:237–246. doi: 10.1016/j.appet.2011.05.107. [DOI] [PubMed] [Google Scholar]
- 257.Weiss MS, Hajnal A, Czaja K, Di Lorenzo PM. Taste responses in the nucleus of the solitary tract of awake obese rats are blunted compared with those in lean rats. Front Integr Neurosci. 2019 doi: 10.3389/fnint.2019.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kaufman A, Choo E, Koh A, Dando R. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 2018;16:e2001959. doi: 10.1371/journal.pbio.2001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Scott K. Gustatory processing in Drosophila melanogaster. Annu Rev Entomol. 2018;63:15–30. doi: 10.1146/annurev-ento-020117-043331. [DOI] [PubMed] [Google Scholar]
- 260.Hanover JA. Epigenetics gets sweeter: O-GlcNAc joins the “Histone Code”. Chem Biol. 2010;17:1272–1274. doi: 10.1016/j.chembiol.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hardivillé S, Hart GW. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab. 2014;20:208–213. doi: 10.1016/j.cmet.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Togo J, Hu S, Li M, et al. Impact of dietary sucrose on adiposity and glucose homeostasis in C57BL/6J mice depends on mode of ingestion: liquid or solid. Mol Metab. 2019 doi: 10.1016/j.molmet.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 264.Barquera S, Hernandez-Barrera L, Tolentino ML, et al. Energy intake from beverages is increasing among Mexican adolescents and adults. J Nutr. 2008;138:2454–2461. doi: 10.3945/jn.108.092163. [DOI] [PubMed] [Google Scholar]
- 265.Cassady BA, Considine RV, Mattes RD. Beverage consumption, appetite, and energy intake: what did you expect? Am J Clin Nutr. 2012;95:587–593. doi: 10.3945/ajcn.111.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Shearrer GE, O’Reilly GA, Belcher BR, et al. The impact of sugar sweetened beverage intake on hunger and satiety in minority adolescents. Appetite. 2016;97:43–48. doi: 10.1016/j.appet.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Collin LJ, Judd S, Safford M, et al. Association of sugary beverage consumption with mortality risk in US adults: a secondary analysis of data from the regards study. JAMA Netw Open. 2019;2:e193121–e193121. doi: 10.1001/jamanetworkopen.2019.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jiantao Ma, McKeown Nicola M, Shih-Jen Hwang, et al. Sugar-Sweetened beverage consumption is associated with change of visceral adipose tissue over 6 years of follow-up. Circulation. 2016;133:370–377. doi: 10.1161/CIRCULATIONAHA.115.018704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Reed DR, Mainland JD, Arayata CJ. Sensory nutrition: the role of taste in the reviews of commercial food products. Physiol Behav. 2019;209:112579. doi: 10.1016/j.physbeh.2019.112579. [DOI] [PubMed] [Google Scholar]
- 270.Temussi PA. Why are sweet proteins sweet? Interaction of brazzein, monellin and thaumatin with the T1R2-T1R3 receptor. FEBS Lett. 2002;526:1–4. doi: 10.1016/s0014-5793(02)03155-1. [DOI] [PubMed] [Google Scholar]