Abstract
Epigenetic modifications play a central role in cell differentiation and development. In the current study, we have recognized lysine demethylase 4A (KDM4A) as a novel epigenetic regulator of osteoblast and adipocyte differentiation. Kdm4a expression was upregulated during osteogenesis and adipogenesis of primary marrow stromal cells and established stromal ST2 line. Overexpression of wild-type Kdm4a promoted adipogenic differentiation and blocked osteogenic differentiation of the progenitor cells. This effect was largely alleviated when the catalytically dead mutation was made. Conversely, depletion or inactivation of Kdm4a in undifferentiated progenitor cells inhibited the formation of adipocytes and promoted the differentiation of osteoblasts. Mechanism explorations showed that overexpression of Kdm4a upregulated the expression of secreted frizzled-related protein 4 (Sfrp4) and CCAAT/enhancer-binding protein α (C/ebpα). Chromatin immunoprecipitation assay demonstrated that KDM4A directly bound the promoters of Sfrp4 and C/ebpα, removed the histone methylation mark H3K9me3, and reduced DNA methylation levels of CpG in promoter regions of C/ebpα and Sfrp4. Furthermore, overexpression of Kdm4a inactivated canonical Wnt signaling. Moreover, activation of canonical Wnt signaling through silencing of Sfrp4 in ST2 attenuated the inhibition of osteogenic differentiation and the enhancement of adipogenic differentiation by KDM4A. These data have identified KDM4A as a novel regulator of osteoblast and adipocyte differentiation and suggest KDM4A inhibition as a potential therapeutic target for treating metabolic disorders such as osteoporosis.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03289-w) contains supplementary material, which is available to authorized users.
Keywords: Lysine demethylase 4A, Adipocyte, Osteoblast, Differentiation, Secreted frizzled-related protein 4, CCAAT/enhancer-binding protein α, Wnt/β-catenin
Introduction
Mesenchymal stem cells (MSCs) are common progenitor cells with self-renewal capacity and potential of differentiation into several cell lineages, including osteoblasts, chondrocytes, and adipocytes [1, 2]. The adipogenesis and osteogenesis of MSCs are competing and reciprocal. The imbalance between adipogenesis and osteogenesis results in bone-related diseases [3]. MSCs fate determination is governed by a complex network of signaling pathways, including canonical Wnt signaling, transforming growth factor-β/bone morphogenic protein signaling [4–7], as well as multiple key transcription factors such as runt-related transcription factor 2 (Runx2), osterix (Osx), CCAAT element binding protein (C/EBP) members and peroxisome proliferator-activated receptor γ (PPARγ) [8, 9].
Epigenetic modification has been found to play a key regulatory role in directing cell lineage commitment [10, 11]. Investigations in recent years have deduced and demonstrated significant roles of histone demethylases in MSCs fate determination by removing epigenetic marks on essential genes to facilitate or block differentiation towards a particular lineage [12]. Histone demethylases KDM4B and KDM6B have recently been identified to play pivotal roles in osteogenic commitment of MSCs. Depletion of KDM4B or KDM6B favored adipogenic differentiation at the expense of osteogenic differentiation. The mechanisms involved are associated with the removal of silencing mark H3K27me3 by KDM6B to increase homeobox (HOX) expression, or the removal of silencing mark H3K9me3 by KDM4B to promote distal-less homeobox (DLX) expression [13]. KDM4B also promotes chondrogenic differentiation of human MSCs due to its recruitment to the chondrogenic gene SOX9 promoter, removing the silencing H3K9me3 marks, and thus activating the transcription of SOX9 [14].
Lysine-specific demethylase 4A (KDM4A), also known as JMJD2A, removes methyl residues from trimethylated or dimethylated histone 3 at lysines 9 and 36 (H3K9me2/me3, H3K36me2/me3) [15]. KDM4A expression has been reported to be deregulated in several cancer types, such as prostate [16], bladder [17], lung [18] and breast cancers [19]. These, combined with a number of functional studies, have suggested KDM4A as an attractive target for cancer therapy [20, 21]. KDM4A may correlate with tumour aggressiveness due to its upregulating hypoxia-inducible factor 1α (HIF-1α) through removing H3K9me3 on HIF-1α promoter [22]. To date, little is known about the role of KDM4A in MSCs differentiation.
In this study, we have demonstrated that KDM4A plays a role in lineage commitment of MSCs. KDM4A is recruited to secreted frizzled-related protein 4 (sfrp4) and C/ebpα promoter regions and leads to the reduced methylation levels of both H3K9 and DNA. The enhanced expression of SFRP4 and C/EBPα promotes adipocyte formation and inhibits osteogenic differentiation.
Materials and methods
Cells
Stromal line ST2 cells were maintained in DMEM containing 10% fetal bovine serum (FBS). To induce adipogenic differentiation, confluent cells were grown for 3 days in the adipogenic medium of a-MEM containing 10% FBS, 5 μg/ml insulin, 0.5 μM dexamethasone, 0.25 mM methylisobutylxanthine and 50 μM indomethacin. Then the cells were cultured for additional 2 d in presence of 5 μg/ml insulin alone. To allow osteogenic differentiation, 80% confluent cells were induced for 14 d in osteogenic medium of a-MEM containing 10% FBS, 50 μg/ml ascorbic acid and 5 mM β-glycerophosphate.
Bone marrow stromal cells (BMSCs) were isolated from the tibias and femurs of 6-week-old C57BL/6J mice, grown and passaged in α-MEM containing 10% FBS. BMSCs at passages 3–5 were induced to differentiate with adipogenic or osteogenic medium at appropriate confluence.
Cell proliferation assay
Cells were plated in 96-well plates at 1.5 × 104 cells per well and were transfected with Kdm4a expression plasmid or vector using Attractene transfection reagent (QIAGEN, Hilden, Germany). 24 h after transfection, cell growth rate was determined using a CCK-8 assay kit (Dojindo Molecular Technology, Kumamoto, Japan). Cell growth rate was also assayed in cells treated for 48 h with ML324, a selective KDM4A inhibitor, or vehicle (DMSO).
Quantitative RT-PCR
At specified time points, total RNA was extracted from cells using an RNA isolation kit (Gmbiolab, Taiwan). Reverse transcription was performed using RevertAid first-strand cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA) with 1 μg of total RNA as the template. qPCR was performed using SYBR Green fluorescence PCR kit (Sangon Biotech, Shanghai, China). The primers are listed in Supplemental Table 1. The following program was used for cDNA amplification: 1 cycle at 95 °C for 5 min followed by 40 cycles of heating at 95 °C for 10 s, annealing at 57 °C for 10 s, and extension at 72 °C for 10 s. The expression levels of the target genes were normalized against β-actin and analysis was performed using the ΔΔCt method.
Constructs, siRNAs and transfections
The expression construct of wild-type (WT) Kdm4a was obtained from Dharmacon (Lafayette, CO, USA). The mutant mouse Kdm4a construct with a point mutation of H188A [22] was made using a mutagenesis kit (Vazyme Biotech, Nanjing, China). For the Kdm4a gain-of-function studies, we transfected ST2 cells of 70% confluence with the WT or mutant Kdm4a expression plasmid, or the empty vector using Attractene transfection reagent (QIAGEN) for 16 h. For the loss of function studies, 30 nM Kdm4a siRNAs or negative control siRNA (Genepharma, Shanghai, China) were transfected for 20 h into ST2 cells of 50% confluence using lipofectamine RNAi-Max (Life technologies, Gaithersburg, MD, USA). When the cells attained full or 80% confluence, adipogenic or osteogenic medium was added to induce the cells to differentiate.
For the mechanism study, the ST2 cells at 50% confluence were co-transfected for 16 h with WT Kdm4a expression plasmid or the vector, and Sfrp4 siRNA or the negative control siRNA using Attractene transfection reagent. Adipogenic or osteogenic induction was performed to allow the cells to differentiate. The siRNA sequences used are listed in Supplemental Table 2.
Lentiviral packaging and infection
The sense and antisense sequences (Supplemental Table 3) coding for mouse Kdm4a shRNA were annealed, then cloned into the pLVX-shRNA2 (Clontech, Palo Alto, CA, USA) at the EcoRI/BamHI sites. The lentiviruses were packaged in 293T cells with the lentiviral packaging system (Jiman Biotech, Shanghai, China). Primary BMSCs were infected with the viruses with MOI of 20. Adipogenic or osteogenic induction was performed when the cells attained appropriate confluence. The lentiviruses packaged with the empty vector were used as control.
Oil-red O staining
Lipid accumulation in differentiated adipocytes was visualized by staining with oil-red O. In brief, 5 days after adipogenic induction, the cells were fixed in 4% paraformaldehyde for 10 min, washed with deionized water, then stained in 60% saturated oil-red O solution for 5 min. To quantify retention of oil-red O, isopropanol was added to dissolve the stain and absorbance was measured by spectrophotometry at 520 nm.
ALP staining
14 days after osteogenic treatment, differentiated osteoblasts were fixed in 4% paraformaldehyde for 10 min. The activity of ALP enzyme was determined by staining for 15 min using a NBT/BCIP staining kit (Sangon Biotech, Shanghai, China).
Western blot analysis
Protein extracts from total cells were harvested in RIPA buffer, run on 12% SDS-PAGE, and electrotransferred onto nitrocellulose membranes. The membranes were blocked in 5% nonfat dry milk and immunoblotted with primary antibodies overnight at 4 °C. The primary antibodies used included rabbit pABs or mAbs by Abcam (Cambridge, MA, USA): anti-H3K9me3 (ab176916), anti-KDM4A (ab105953), anti-LRP6 (ab134146), anti-C/EBPα (ab40764), anti-β-catenin (ab32572), anti-osterix (ab94744), and anti-ALP (ab108337); rabbit mAbs by Cell Signaling Technology (Danvers, MA, USA): anti-non-phospho-β-catenin (19807), anti-phopho-GSK-3β (Ser9) (5558) and anti-phospho-LRP6 (2568); rabbit polyclonal antibodies by Proteintech (Wuhan, China): anti-FABP4 (12802-1-AP) and anti-β-actin (66009-1-Ig). After being washed with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 90 min at room temperature. Protein bands were visualized with chemiluminescence reagent (Advansta, Menlo Park, CA, USA).
KDM4A inhibitor treatment
ST2 cells were grown in 24-well plates or 6 cm dishes and maintained in a-MEM containing 10% FBS. To induce adipogenic differentiation, confluent cells were cultured in adipogenic medium supplemented with various concentrations of ML324 (2, 4, 8 μM) or vehicle (DMSO). To induce osteoblast differentiation, 80% confluent cells were cultured in osteogenic medium supplemented with ML324 or DMSO.
Chromatin immunoprecipitation assay
We performed chromatin immunoprecipitation (ChIP) assay using a kit from Cell Signaling Technology (Danvers, MA, USA) following the supplier’s instructions. Immunoprecipitations were performed overnight at 4 °C with 4 μg anti-KDM4A, anti-H3K9me3 antibody or IgG, and the bound complexes were collected using protein G magnetic beads. The de-crosslinked DNA was purified and the enrichment of mouse C/ebpα- or Sfrp4-specific sequences was analyzed using PCR.
Co-immunoprecipitation
Immunoprecipitation was performed using a kit from Beaver (Suzhou, China) following the supplier’s instructions. In brief, cells were lysed with a lysis buffer supplemented with protease inhibitor cocktail. The cell lysate was incubated with either anti-KDM4A or anti-IgG overnight at 4 °C followed by incubation with protein A/G beads for 1 h at 4 °C. The immunocomplexes were eluted with elution buffer and the eluted proteins were subjected to SDS-PAGE and Western blotting.
DNA methylation analysis
Genomic DNA was extracted using the E.Z.N.A. DNA purification Kit (Omega Bio-Tek). Bisulfite treatment of DNA was carried out using an EpiTect Fast DNA Bisulfite Kit (Qiagen), followed by the methylation-specific PCR (MSP). Methylated sequence (Sfrp4: − 91 ~ + 41; CEBPα: − 32 ~ + 114) and unmethylated sequence (Sfrp4: − 94 ~ + 44; CEBPα: − 34 ~ + 115) were amplified separately using specific primers. The amplifications consisted of 35 cycles (95 °C for 30 s, 52 °C for 30 s, and 72 °C for 20 s) after an initial denaturation of 5 min at 95 °C. The primers were designed based on CpG sites on the promoters of Sfrp4 and C/ebpα (Supplementary Table 3). DNA methylation analysis was also performed with a real-time qPCR method as previously reported [23]. In brief, the bisulfite-converted genomic DNA was amplified using a SYBR Green fluorescence PCR kit (Sangon Biotech). β-actin was used as a reference gene to normalize for DNA input in each sample. The running conditions were: 95 °C for 5 min followed by 40 cycles of 95 °C for 30 s, 52 °C for 30 s, and 72 °C for 20 s.
Luciferase reporter assay
A wild-type 1.5-kb mouse Kdm4a promoter fragment was PCR-amplified and the resulting fragment was cloned into the vector pGL4.14[luc2/Hygro] (Promega, Madison, WI, USA) at EcoRV/HindIII sites using the ClonExpress II One Step Cloning Kit (Vazyme, Nangjing, China). In silico analysis of the cis-acting elements within the promoter was done using the online program Jaspar (http://jaspar.binf.ku.dk/cgi-bin/jaspar_db.pl). Deletion mutation to the TCF7L2 binding sequence at − 638 nt (CCTTTAATGA) was made using the primers listed in supplemental Table 3 with a mutagenesis kit (Vazyme Biotech) following the supplier’s instructions. To clarify if β-catenin transcriptionally modulates Kdm4a expression, ST2 cells were co-transfected with the WT or mutant Kdm4a promoter construct, and β-catenin expression plasmid or vector using Attractene transfection reagent. pRL-SV40 was included in the mixture to monitor the transfection efficiency. Cells were harvested 36 h after transfection and luciferase assays were done with a dual-Luciferase reporter assay kit (Promega). The relative luciferase activity was calculated by dividing the firefly luciferase activity with renilla luciferase activity.
Statistical analysis
Data were expressed as mean ± SD. For the quantifications of mRNA expression and luciferase activity, the means of the control groups were set to 1. Statistical analysis was performed with independent t test or one-way ANOVA. If one-way ANOVA indicates significant difference, a post hoc comparison was performed with the Student–Newman–Keuls test. A value of P < 0.05 is considered significantly different.
Results
Kdm4a was abundant in bone and the expression level changed during differentiation of stromal ST2 cells
Expression levels of Kdm4a were investigated in various tissues of 6-week-old C57BL/6J mice. The results showed that Kdm4a mRNA was abundant in bone and skeletal muscle, and moderately expressed in heart, kidney and brain (Fig. 1a). We then studied if Kdm4a expression changes during differentiation of MSCs. During osteogenic differentiation, Kdm4a expression increased during osteogenic differentiation until day 8, and stayed high at least until day 14 of culture (Fig. 1b). It also increased during adipogenic differentiation, stayed at a high level at day 1 through day 5 after adipogenic treatment (Fig. 1c). These results suggest a potential role of KDM4A to regulate adipogenic and osteogenic differentiation of MSCs.
Fig. 1.
Kdm4a expression increased during osteogenic and adipogenic differentiation. Kdm4a expression was examined using RT-PCR in various tissues of mice. The level of Kdm4a in intestine was set to 1 (a). Kdm4a expression in ST2 after osteogenic (b) and adipogenic (c) treatment was examined using qRT-PCR. The level of Kdm4a at d 0 was set to 1. Values are mean ± SD (n = 3). *P < 0.05 vs. vehicle
Enforced expression of Kdm4a in ST2 cells promoted adipogenesis at the expense of osteogenesis
qRT-PCR results indicated that the expression level of Kdm4a was efficiently increased in ST2 cells after transfection with either WT or mutant Kdm4a construct (Fig. 2a). Western blotting showed that, when overexpressed, the wild-type KDM4A significantly reduced the level of H3K9me3 while the mutant KDM4A did not (Fig. 2b). Cell proliferation assay demonstrated that Kdm4a overexpression in ST2 cells slightly promoted cell growth (Fig. 2c).
Fig. 2.
Kdm4a overexpression in ST2 promoted adipogenic differentiation and inhibited osteogenic differentiation. Overexpression of Kdm4a in ST2 was verified using qRT-PCR (a). The protein levels of KDM4A and H3K9me3 were examined (b). Cell growth rate was examined in ST2 following overexpression of Kdm4a (c). Differentiated adipocytes were stained with oil-red O (d). Oil-red O extracted with isopropanol was measured at OD520 (e). The mRNA (f) and protein (g) levels of adipogenic factors were examined. Differentiated osteoblasts were subjected to ALP staining (h). The mRNA (i) and protein (j) levels of osteogenic factors were examined. Values are mean ± SD. a, b, e, f, g, i, jn = 3; cn = 8. *P < 0.05 vs. vector
When cultured under adipogenic medium, the ST2 cells overexpressing Kdm4a-WT had a greater adipogenic differentiation capacity, with 104% increase of triglyceride content, as compared to the vector-transfected cells (Fig. 2d, e). Accordingly, the transcript and protein expression levels of adipogenic factors including PPARγ, C/EBPα, FABP4 and adipsin were remarkably elevated in the cells overexpressing Kdm4a-WT vs. those in the control, 48 h and 72 h, respectively, after adipogenic treatment. However, the catalytically dead mutant of KDM4A showed largely reduced adipogenic potential in ST2 cells (Fig. 2f, g).
By contrast, in the presence of osteogenic medium, overexpression of Kdm4a-WT in ST2 cells inhibited osteogenic differentiation, as shown by alleviated ALP staining of differentiated osteoblasts vs. vector transfection (Fig. 2h). Consistently, Kdm4a-WT overexpression decreased the transcript and protein levels of osteogenic factors including Runx2, Osterix (OSX), and ALP 72 h after osteogenic induction. In contrast, the mutant construct of Kdm4a failed to affect the osteogenic commitment of ST2 cells in terms of either ALP staining, or the expression levels of osteogenic factors (Fig. 2i, j). These data suggest that the demethylase activity of KDM4A is required for its pro-adipogenic and anti-osteogenic function.
Inactivation of KDM4A blocked adipogenic differentiation and promoted osteogenic differentiation
ML324 was reported to be an effective inhibitor of KDM4A activity [24]. As shown in Fig. 3a, Western blot analysis showed that ML324 dramatically increased H3K9me3 in ST2 (Fig. 3a). Furthermore, ML324 suppressed proliferation of ST2 cells in a dose-dependent manner (Fig. 3b). Functional studies showed that ML324 treatment reduced mature adipocytes formed under adipogenic treatment in a dose-dependent manner (Fig. 3c, d). Accordingly, the mRNA and protein levels of adipogenic factors were decreased by ML324 in a dose-dependent manner after adipogenic treatment as compared with the vehicle-treated cells (Fig. 3e, f). By contrast, ML324 stimulated osteogenic differentiation, as evidenced by the enhanced ALP staining (Fig. 3g) and the induction of the mRNA and protein levels of osteogenic factors (Fig. 3h, i).
Fig. 3.
ML324 treatment blocked adipocyte formation and promoted osteoblast differentiation. Effect of ML324 on H3K9me3 was examined using Western blotting (a). Cell growth rate was examined in ST2 following ML324 treatment at 2, 4 and 8 μM (b). Differentiated adipocytes were stained with oil-red O (c). Oil-red O extracted with isopropanol was measured at OD520 (d). The mRNA (e) and protein (f) levels of adipogenic factors were examined. Differentiated osteoblasts were subjected to ALP staining (g). The mRNA (h) and protein (i) levels of osteogenic factors were examined. Values are mean ± SD. a, d, e, f, h, in = 3; bn = 8. *P < 0.05 vs. vehicle treatment
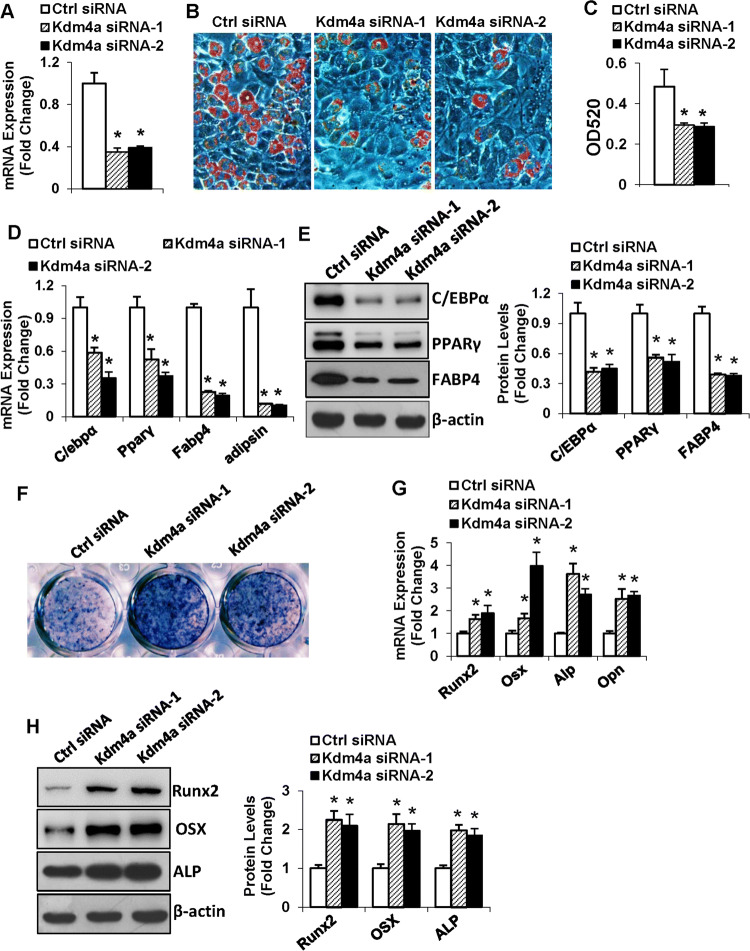
To further confirm the role of KDM4A in modulating differentiation of MSCs, we silenced Kdm4a expression using two independent siRNAs (Fig. 4a). In the presence of adipogenic medium, silencing of Kdm4a significantly reduced the formation of adipocytes, revealed by the decrease in the triglyceride content (Fig. 4b, c). Consistently, the mRNA and protein levels of the adipogenic factors were downregulated in Kdm4a-silenced cells, 48 h and 72 h, respectively, after adipogenic treatment (Fig. 4d, e).
Fig. 4.
Silencing of Kdm4a in ST2 inhibited adipogenic differentiation and promoted osteogenic differentiation. The knockdown of Kdm4a mRNA in ST2 cells was verified using qRT-PCR (a). Differentiated adipocytes were stained with oil-red O (b). Oil-red O extracted with isopropanol was measured at OD520 (c). The mRNA (d) and protein (e) levels of adipogenic factors were examined. Differentiated osteoblasts were subjected to ALP staining (f). The mRNA (g) and protein (h) levels of osteogenic factors were examined. Values are mean ± SD (n = 3). *P < 0.05 vs. control siRNA
By contrast, knockdown of Kdm4a remarkably enhanced the osteogenic differentiation of ST2 (Fig. 4f). Moreover, 72 h after osteogenic induction, the mRNA and protein levels of the osteogenic factors were significantly increased in Kdm4a-silenced cells compared to the control cells (Fig. 4g, h).
Kdm4a depletion in primary marrow stromal cells stimulated osteogenic differentiation at the expense of adipogenic differentiation
As shown in Fig. 5a, the Kdm4a shRNA lentivirus substantially silenced the endogenous Kdm4a mRNA in primary marrow stromal cells. Kdm4a depletion inhibited adipogenesis of primary marrow stromal cells (Fig. 5b), as revealed by the decreased triglyceride content (Fig. 5c) and the reduced expression levels of adipogenic factors (Fig. 5d).
Fig. 5.
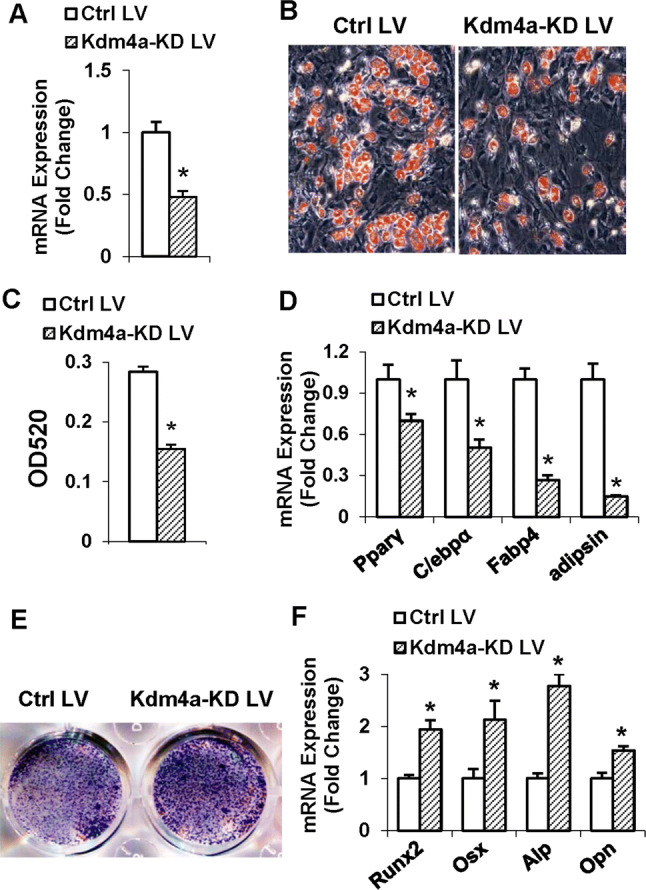
Kdm4a depletion in primary marrow stromal cells stimulated osteogenic differentiation at the expense of adipogenic differentiation. The silencing of Kdm4a mRNA in MSCs was verified using qRT-PCR (a). Differentiated adipocytes were stained with oil-red O (b). Oil-red O extracted with isopropanol was measured at OD520 (c). The mRNA levels of adipogenic factors were examined (d). Differentiated osteoblasts were subjected to ALP staining (e). The mRNA levels of osteogenic factors were examined (f). Values are mean ± SD (n = 3). *Significant vs. control lentivirus, P < 0.05
On the contrary, in presence of osteogenic medium, Kdm4a depletion in primary marrow stromal cells potentiated osteogenic differentiation, as evidenced by the enhanced ALP staining (Fig. 5e), and the increased expression levels of osteogenic factors (Fig. 5f).
KDM4A controlled lineage commitment by demethylating H3K9me3 on Sfrp4 and C/ebpα promoters
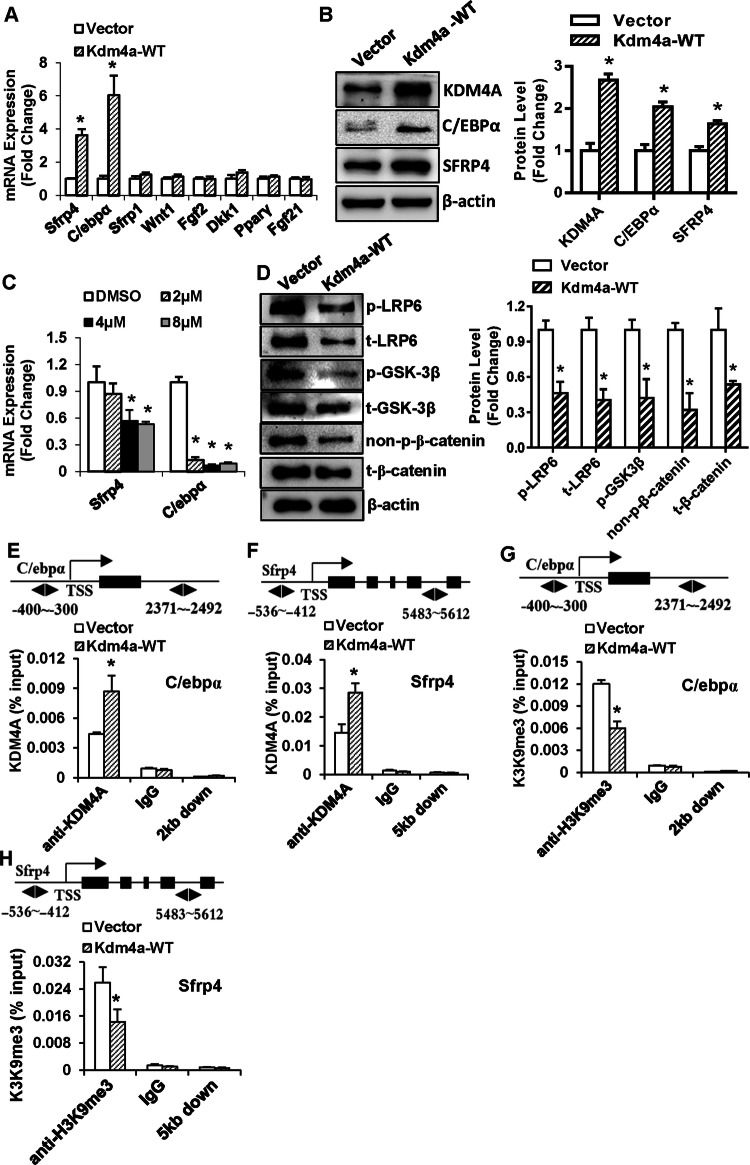
To investigate the genetic network regulated by KDM4A, we detected the endogenous expression of several key regulators of cell fate 24 h after Kdm4a overexpression. The mRNA and protein levels of endogenous C/ebpα and Sfrp4 were remarkably elevated in the Kdm4a-overexpressing ST2 cells (Fig. 6a, b). By contrast, the expression levels of Sfrp1, Wnt1, fibroblast growth factor 2 (Fgf2), dickkopf Wnt signaling pathway inhibitor 1 (Dkk1), Pparγ and Fgf21 were not changed (Fig. 6a). Consistently, ML324 decreased the mRNA levels of C/ebpα and Sfrp4 in ST2 cells, indicating that the regulation of C/ebpα and Sfrp4 is associated with the demethylase activity of KDM4A (Fig. 6c). It is known that SFRP4 binds directly to Wnt ligands to prevent receptor binding and activation of Wnt signaling. To determine whether KDM4A really affects Wnt signaling through regulation of SFRP4, we detected the major components of Wnt signaling. Western blot analysis revealed that Kdm4a overexpression decreased the protein levels of active β-catenin, phosphorylated LRP6 and GSK-3β (Ser9) (Fig. 6d). These data provide evidences that KDM4A inactivates canonical Wnt signaling.
Fig. 6.
KDM4A controlled lineage commitment by demethylating H3K9me3 on Sfrp4 and C/ebpα promoters. The mRNA (a, c) and protein (b) levels of C/EBPα and SFRP4 were examined after Kdm4a overexpression (a, b) or ML324 treatment (c). The protein levels of the major components of canonical Wnt signaling were examined following Kdm4a overexpression (d). KDM4A occupancy on the promoters of C/ebpα and Sfrp4 was examined using ChIP assay (e, f). Binding of H3K9me3 to the promoters of C/ebpα and Sfrp4 was examined using ChIP assay (g, h). Values are mean ± SD (n = 3). *P < 0.05 vs. vector
We then performed ChIP assay to assess the physical occupancy of KDM4A and the histone methylation status on the promoter regions of its target genes. Following overexpression of Kdm4a, the occupancy of KDM4A was observed to be markedly higher on the promoters of C/ebpα and Sfrp4 in ST2 cells (Fig. 6e, f). Meanwhile, H3K9me3 mark in the promoter regions of C/ebpα and Sfrp4 were significantly decreased (Fig. 6g, h). These suggest that KDM4A is recruited to the C/ebpα and Sfrp4 promoters, removing the silencing epigenetic mark H3K9me3, thereby activating the transcription of C/ebpα and Sfrp4.
KDM4A decreased DNA methylation in Sfrp4 and C/ebpα promoter regions
A previous study demonstrated that H3K9me and DNA methylation systems could synergize to regulate silenced chromatin domains at major and minor satellite repeats in mammals [25]. We herein tested if KDM4A enhancement of C/ebpα and Sfrp4 expression is linked to DNA methylation. We employed primers that amplify unmethylated and methylated sequences to investigate the methylation state of CpG sites proximal to the transcription start site (TSS) of C/ebpα and Sfrp4 promoters, respectively (Fig. 7a, b). Agarose electrophoresis indicated that the amplicons of the methylated sequences on the C/ebpα and Sfrp4 promoters were reduced after Kdm4a overexpression in ST2 cells (Fig. 7c). Consistently, real-time qPCR also demonstrated the reduction of these methylated sequences in Kdm4a overexpressing cells (Fig. 7d).
Fig. 7.
KDM4A decreased DNA methylation on Sfrp4 and C/ebpα promoters. The methylation state of CpG sites proximal to the transcription start sites (TSS) of C/ebpα and Sfrp4 promoters are shown (a, b). The bisulfite-converted amplicons of C/ebpα and Sfrp4 promoters in ST2 after Kdm4a overexpression were run on gel. “M” indicates methylated sequence-specific PCR; “U” indicates unmethylated sequence-specific PCR (c). The level of DNA methylation was also determined using real-time qPCR (d). The interaction among H3K9me3, DNMT3B and MeCP2 were detected using co-IP (e). *P < 0.05 vs. vector
To date, the mechanisms underlying the crosstalk between histone modifications and DNA methylation is not fully understood. We carried out co-IP to reveal how KDM4A affects DNA methylation in CpG sites. The interaction among H3K9me3, DNMT3B and MeCP2 was detected. Moreover, overexpression of Kdm4a decreased the protein level of H3K9me3 (Fig. 7e).
Knockdown of Sfrp4 attenuated KDM4A promotion of adipocyte differentiation and suppression of osteoblast differentiation
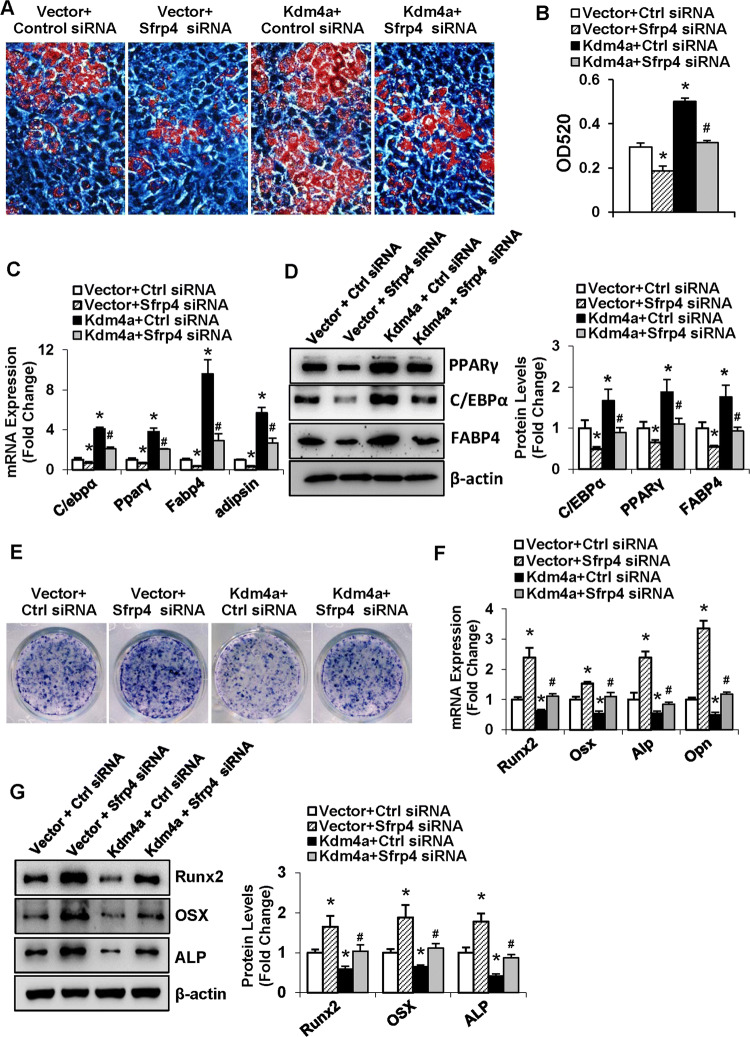
To clarify whether SFRP4/Wnt signaling mediates the function of KDM4A in modulating cell fate of progenitor cells, we undertook KDM4A gain-of-function experiments under the background of Sfrp4 silencing in ST2. The data revealed that the stimulatory effect of KDM4A on adipocyte formation was largely attenuated when co-transfected with Sfrp4 siRNA (Fig. 8a, b). Accordingly, the mRNA and protein levels of the adipogenic factors were lower in the cells co-transfected with Kdm4a expression plasmid and Sfrp4 siRNA than in the cells co-transfected with Kdm4a expression plasmid and control siRNA (Fig. 8c, d).
Fig. 8.
Silencing of Sfrp4 attenuated KDM4A promotion of adipocyte differentiation and suppression of osteoblast differentiation. ST2 cells were co-transfected with Kdm4a-WT or the vector, and Sfrp4 siRNA or the control siRNA. Differentiated adipocytes were stained with oil-red O (a). Oil-red O extracted with isopropanol was measured at OD520 (b). The mRNA (c) and protein (d) levels of adipogenic factors were examined. Differentiated osteoblasts were subjected to ALP staining (e). The mRNA (f) and protein (g) levels of osteogenic factors were examined. Values are mean ± SD (n = 3). *P < 0.05 vs. Vector plus Ctrl siRNA, #P < 0.05 vs. Kdm4a plus Ctrl siRNA
By contrast, the inhibitory effect of KDM4A on osteogenic differentiation was attenuated as well when co-transfected with Sfrp4 siRNA (Fig. 8e). Accordingly, the mRNA and protein levels of the osteogenic factors were higher in the cells co-transfected with Kdm4a expression plasmid and Sfrp4 siRNA than in the cells co-transfected with Kdm4a expression plasmid and control siRNA (Fig. 8f, g).
β-Catenin transcriptionally regulated Kdm4a expression
In silico analysis of Kdm4a promoter revealed that there exists a putative transcription factor 7 like 2 (TCF7L2) binding motif within the promoter of Kdm4a located 638 nt upstream of the transcription start (Fig. 9a). Overexpression of β-catenin in ST2 reduced the transcript level of Kdm4a (Fig. 9b), whereas knockdown of β-catenin increased the protein level of KDM4A (Fig. 9c). The promoter luciferase assay showed that the wild-type promoter construct of Kdm4a had transcriptional activity in ST2 cells as compared to the vector pGL4.14 [Luc2/Hygro]. Of note, the transcriptional activity of the WT promoter was significantly decreased following β-catenin overexpression (Fig. 9d). The removal of the putative TCF7L2 binding site at − 638 nt significantly increased the transcriptional activity of the Kdm4a promoter. Of more interest, β-catenin overexpression failed to show inhibitory effect on the mutant construct (Fig. 9e).
Fig. 9.
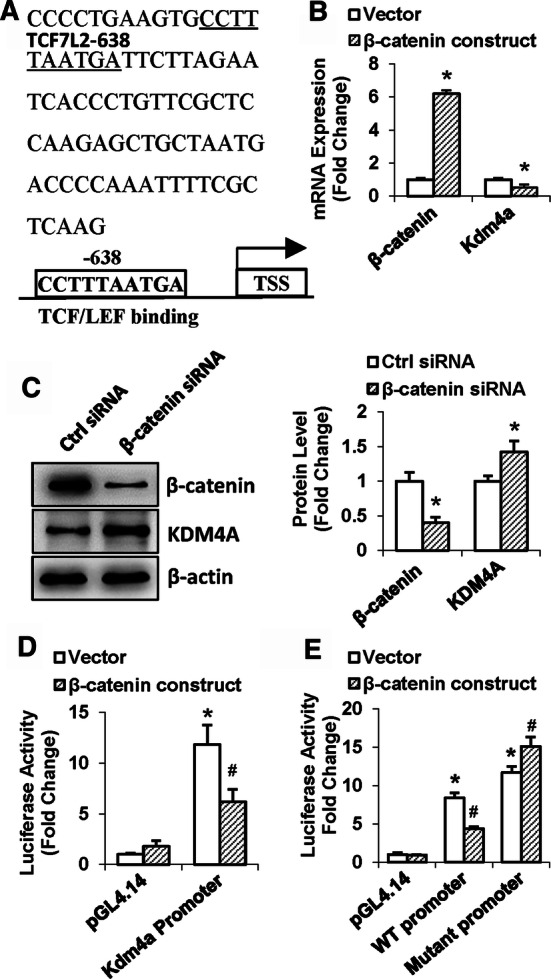
β-catenin transcriptionally regulated Kdm4a expression. Putative β-catenin binding sites on the mouse Kdm4a promoter are shown (a). The effect of β-catenin on the expression level of KDM4A was studied using qRT-PCR (b) and Western blotting (c). Luciferase assay was done to measure the promoter activity of wild-type (d) or mutant (e) Kdm4a promoter. Values are mean ± SD. b, cn = 3; d, en = 4. *P < 0.05 vs. vector (b) or Ctrl siRNA (c). *P < 0.05 vs. pGL4.14 plus vector; #P < 0.05 vs. WT or mutant promoter plus vector
Discussion
Abundant experimental evidences have proved that epigenetic factors are pivotal for marrow stromal cell lineage commitment [26]. A large number of coregulators at critical gene promoters set up specific patterns of DNA methylation, histone acetylation and methylation, and nucleosome rearrangement that act as an epigenetic code to modulate the correct progress of adipocyte and osteoblast differentiation [11, 27]. This study examined the mRNA expression levels of the H3K9 demethylase, KDM4A, in various tissues in mice and revealed high expression levels of Kdm4a in bone and skeletal muscle. Additionally, Kdm4a exhibited an increased expression pattern during either osteogenic or adipogenic differentiation of mouse stromal cells. These data suggest the potential of KDM4A to regulate adipocyte and osteoblast differentiation.
Thereafter, we tested the epigenetic function of KDM4A in adipogenic and osteogenic commitment of marrow stromal cells. Enforced Kdm4a expression induced adipocyte differentiation and blocked osteoblast differentiation in either ST2 line or primary marrow stromal cells. Of interest, the catalytically dead KDM4A with mutation to disrupt the non-heme metal-binding site essential for its demethylase activity lost most of its function in regulating reciprocal commitment of marrow stromal cells to adipocytes and osteoblasts. Additionally, inactivation of KDM4A by its chemical inhibitor or siRNAs exhibited opposite effect on adipogenic and osteogenic differentiation. These suggest that the demethylase activity is responsible and indispensable for the role of KDM4A in adipogenesis and osteogenesis. Of note, the anti-osteogenic function of KDM4A may arouse surprise since its expression increased during osteogenic differentiation. Similar pattern was previously reported for LncRNA H19, which was induced during myoblast differentiation but decelerated muscle differentiation [28]. The induction of KDM4A during osteoblast differentiation might be interpreted as a mechanism of cell self-balance to limit excessive osteogenesis.
To elucidate the mechanism for KDM4A to control the differentiation of marrow stromal cells, we detected the expression profiles of several regulators of cell fate following overexpression of Kdm4a and observed the upregulation of C/ebpα and Sfrp4. While C/EBPα is a adipogenic transcription factor that directly modulates adipocyte formation through interacting with PPARγ, SFRP4 is a secreted antagonist of canonical Wnt signaling which encodes a cysteine-rich domain similar to the Wnt-binding site of Frizzled receptors. Subsequent investigations demonstrated that KDM4A inactivated canonical Wnt signaling. The in-depth KDM4A gain-of-function studies under the background of Sfrp4 silencing proved that the potential of KDM4A to induce adipocyte formation and to inhibit osteoblast differentiation was largely attenuated when Sfrp4 was silenced. Taken together, all the data we presented demonstrated that KDM4A may promote adipogenesis and inhibit osteogenesis from marrow stromal progenitor cells at least partially through blocking canonical Wnt signaling.
Previous studies have shown that the differentiation of progenitor cells is associated with the regulation of key transcription factors such as C/EBPα, PPARγ, Runx2, osterix and β-catenin [8, 29, 30]. Recent experiments have provided evidences that changes in histone methylation marks of key transcription factors are pivotal during adipogenic and osteogenic differentiation [31]. The higher occupancy of H3K4me2 and lower occupancy of H3K9me3/me2 on the C/ebpα promoter facilitated the expression of C/EBPα, leading to increased adipogenesis [32, 33]. In addition to KDM4B and KDM6B, KDM5A and KDM2A have also been recognized as negative osteogenic regulators. The mechanism study uncovered that KDM5A attenuated the expression of Runx2 in BMSCs through decreasing H3K4me3 levels on the promoter of Runx2 [34], while KDM2A reduced secreted frizzled-related protein 2 (Sfrp2) in stem cells of the apical papilla by removing histone H3K4 and H3K36 methylation from the Sfrp2 promoter [35]. We have recently reported that KDM7A is able to reciprocally regulate osteogenic and osteogenic differentiation through removing the histone methylation marks H3K9me2 and H3K27me2 from the promoters of C/ebpα and Sfrp1, thus transactivating C/ebpα and Sfrp1 [36]. In the current study, to evaluate whether the H3K9me3 mark is present in the promoter regions of target genes, we performed ChIP assay. ChIP-qPCR results validated the presence of an H3K9me3 marks on the promoters of C/ebpα and Sfrp4, which were decreased after Kdm4a overexpression.
Epigenetic methylation of CpG islands in DNA often negatively correlates with gene expression and is involved in the modulation of survival, lineage specification, and transformation of progenitor cells [37]. DNA methylation levels were associated with the osteogenic lineage commitment of mesenchymal stem cells [38] and transcriptional activation of the osteocalcin gene in osteoblasts [39]. Demethylation of Runx2 promoter by plant homeodomain finger protein 2 (PHF2) enhanced osteocalcin transcription and osteogenic differentiation of C2C12 cells [31].
As two major mechanisms for epigenetic regulation, DNA methylation and histone modifications are expected to crosstalk with each other [40]. Hypermethylation in H3K9 and CpG islands always means gene expression repressing, and H3K9me3 plays a role to promote DNA methylation in mammals [41]. Suv39h-mediated H3K9 methylation has been reported to direct DNA methylation at pericentric heterochromatin through heterochromatin protein 1 (HP1)-DNA methyltransferase 3B (DNMT3B) interaction. DNMT3A/B are recruited to H3K9-methylated heterochromatin by direct interactions with HP1 [25]. In our research, we validated if histone demethylase is linked to DNA methylation. As expected, KDM4A reduced DNA methylation levels of CpG in promoter regions of C/ebpα and Sfrp4. Furthermore, we detected the association of H3K9me3, DNMT3B and MeCP2. KDM4A might impair their interaction through demethylating H3K9me3. These results suggest that H3K9me3 may be an adaptor to recruit the DNMT3A/B and other molecules to enhance DNA methylation, and KDM4A may alter the methylation status of histone and hence affect DNA chromatin structure to activate the transcription of target genes.
We then testified how KDM4A expression in the progenitor cells is regulated. Bioinformatic analysis revealed potential response element of TCF7L2, which is the coactivator of β-catenin in canonical Wnt signaling. Wnt signaling represses adipogenesis by blocking the induction of PPARγ and C/EBPα [42, 43], and regulates osteoblast differentiation through directly regulating osterix transcription [44]. In the current study, the WT promoter construct showed transcriptional activity, which was further reduced when co-transfected with the β-catenin construct. In contrast, mutation of the TCF7L2 binding sequence resulted in enhanced transcriptional activity and rendered the promoter not to lose activity in response to β-catenin. These data thus demonstrate the negative transcriptional regulation of Kdm4a by canonical Wnt signaling.
In summary, the current study has provided evidences that a network exists among KDM4A, SFRP4 and Wnt/β-catenin signaling which functions to balance osteogenic and adipogenic differentiation. Kdm4a expression changes during adipogenesis and osteogenesis of marrow stromal progenitor cells. Wnt/β-catenin signaling transcriptionally controls the expression of Kdm4a. KDM4A promotes adipogenic differentiation and inhibits osteogenic differentiation from marrow stromal progenitor cells. This is based on the activation of C/EBPα and SFRP4 expression via epigenetic regulations (Fig. 10). These findings suggest that KDM4A may be an attractive potential target for new therapies that are aimed at controlling metabolic disorders such as osteoporosis.
Fig. 10.
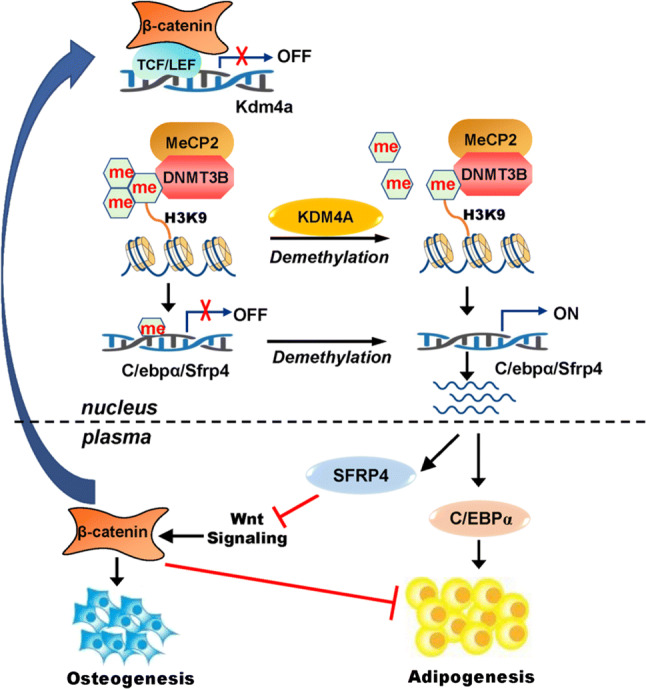
Schematic diagram depicting the mechanism for KDM4A in regulating MSCs differentiation
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The work was funded by National Natural Science Foundation of China (Grants nos. 81871741, 81672116 and 81772297), Natural Science Foundation of Tianjin City Municipal Science and Technology Commission (Grants nos. 18JCZDJC32200 and 18JCQNJC12900) and by Graduate student innovation fund of Tianjin Medical University (Grant no. YJSCX201803).
ABBREVIATIONS
- ALP
Alkaline phosphatase
- FABP4
Fatty acid binding protein 4
- C/EBP
CCAAT/enhancer-binding protein
- DLX
Distal-less homeobox
- DNMT3B
DNA methyltransferase 3
- HOX
Homeobox
- HP1
Heterochromatin protein 1 (HP1)
- KDM4A
Lysine demethylase 4A
- Lrp6
Low-density lipoprotein receptor-related protein 6
- MSC
Mesenchymal stem cell
- Osx
Osterix
- PPARγ
Peroxisome proliferator-activated receptor γ
- Runx2
Runt-related transcription factor 2
- SFRP4
Secreted frizzled-related protein 4
- TCF7L2
Transcription factor 7 like 2
Author contributions
QQ, YW, XW, JY, YX and JZ: collection and assembly of data, data analysis and interpretation, and final approval of manuscript; XL: conception and design and final approval of manuscript; BW: conception and design, manuscript writing, and final approval of manuscript.
Compliance with ethical standards
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qi Qi and Yi Wang contributed equally to the work.
References
- 1.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arpornmaeklong P, Brown SE, Wang Z, Krebsbach PH. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem Cells Dev. 2009;18(7):955–968. doi: 10.1089/scd.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Ayala I, Shannon C, Fourcaudot M, Acharya NK, Jenkinson CP, Heikkinen S, Norton L. The diabetes gene and Wnt pathway effector TCF7L2 regulates adipocyte development and function. Diabetes. 2018;67(4):554–568. doi: 10.2337/db17-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sottile V, Seuwen K. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone) FEBS Lett. 2000;475(3):201–204. doi: 10.1016/s0014-5793(00)01655-0. [DOI] [PubMed] [Google Scholar]
- 6.Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, Chen J, Bi Y, He BC, Park JK, Jiang W, Tang Y, Huang J, Su Y, Zhu GH, He Y, Yin H, Hu Z, Wang Y, Chen L, Zuo GW, Pan X, Shen J, Vokes T, Reid RR, Haydon RC, Luu HH, He TC. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18(4):545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grafe I, Alexander S, Peterson JR, Snider TN, Levi B, Lee B, Mishina Y. TGF-beta family signaling in mesenchymal differentiation. Cold Spring Harb Perspect Biol. 2018;10(5):a022202. doi: 10.1101/cshperspect.a022202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siersbaek R, Nielsen R, Mandrup S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol Metab. 2012;23(2):56–64. doi: 10.1016/j.tem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B. PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem. 2009;106(2):232–246. doi: 10.1002/jcb.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JC, Chua M, Bellon RB, Jacobs CR. Epigenetic changes during mechanically induced osteogenic lineage commitment. J Biomech Eng. 2015;137(2):020902. doi: 10.1115/1.4029551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnsdorf EJ, Tummala P, Castillo AB, Zhang F, Jacobs CR. The epigenetic mechanism of mechanically induced osteogenic differentiation. J Biomech. 2010;43(15):2881–2886. doi: 10.1016/j.jbiomech.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet. 2007;8(11):829–833. doi: 10.1038/nrg2218. [DOI] [PubMed] [Google Scholar]
- 13.Ye L, Fan Z, Yu B, Chang J, Al Hezaimi K, Zhou X, Park NH, Wang CY. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell. 2012;11(1):50–61. doi: 10.1016/j.stem.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HL, Yu B, Deng P, Wang CY, Hong C. Transforming growth factor-beta-induced KDM4B promotes chondrogenic differentiation of human mesenchymal stem cells. Stem Cells. 2016;34(3):711–719. doi: 10.1002/stem.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, Spooner E, Li E, Zhang G, Colaiacovo M, Shi Y. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125(3):467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Kim TD, Jin F, Shin S, Oh S, Lightfoot SA, Grande JP, Johnson AJ, van Deursen JM, Wren JD, Janknecht R. Histone demethylase JMJD2A drives prostate tumorigenesis through transcription factor ETV1. J Clin Investig. 2016;126(2):706–720. doi: 10.1172/JCI78132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kogure M, Takawa M, Cho HS, Toyokawa G, Hayashi K, Tsunoda T, Kobayashi T, Daigo Y, Sugiyama M, Atomi Y, Nakamura Y, Hamamoto R. Deregulation of the histone demethylase JMJD2A is involved in human carcinogenesis through regulation of the G(1)/S transition. Cancer Lett. 2013;336(1):76–84. doi: 10.1016/j.canlet.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Mallette FA, Richard S. JMJD2A promotes cellular transformation by blocking cellular senescence through transcriptional repression of the tumor suppressor CHD5. Cell Rep. 2012;2(5):1233–1243. doi: 10.1016/j.celrep.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 19.Li LL, Xue AM, Li BX, Shen YW, Li YH, Luo CL, Zhang MC, Jiang JQ, Xu ZD, Xie JH, Zhao ZQ. JMJD2A contributes to breast cancer progression through transcriptional repression of the tumor suppressor ARHI. Breast Cancer Res. 2014;16(3):R56. doi: 10.1186/bcr3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metzger E, Stepputtis SS, Strietz J, Preca BT, Urban S, Willmann D, Allen A, Zenk F, Iovino N, Bronsert P, Proske A, Follo M, Boerries M, Stickeler E, Xu J, Wallace MB, Stafford JA, Kanouni T, Maurer J, Schule R. KDM4 inhibition targets breast cancer stem-like cells. Cancer Res. 2017;77(21):5900–5912. doi: 10.1158/0008-5472.CAN-17-1754. [DOI] [PubMed] [Google Scholar]
- 21.Berry WL, Janknecht R. KDM4/JMJD2 histone demethylases: epigenetic regulators in cancer cells. Cancer Res. 2013;73(10):2936–2942. doi: 10.1158/0008-5472.CAN-12-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobrynin G, McAllister TE, Leszczynska KB, Ramachandran S, Krieg AJ, Kawamura A, Hammond EM. KDM4A regulates HIF-1 levels through H3K9me3. Sci Rep. 2017;7(1):11094. doi: 10.1038/s41598-017-11658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eads CA, Lord RV, Wickramasinghe K, Long TI, Kurumboor SK, Bernstein L, Peters JH, DeMeester SR, DeMeester TR, Skinner KA, Laird PW. Epigenetic patterns in the progression of esophageal adenocarcinoma. Can Res. 2001;61(8):3410–3418. [PubMed] [Google Scholar]
- 24.Ishiguro K, Watanabe O, Nakamura M, Yamamura T, Matsushita M, Goto H, Hirooka Y. Inhibition of KDM4A activity as a strategy to suppress interleukin-6 production and attenuate colitis induction. Clin Immunol. 2017;180:120–127. doi: 10.1016/j.clim.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Lehnertz B, Ueda Y, Derijck AA, Braunschweig U, Perez-Burgos L, Kubicek S, Chen T, Li E, Jenuwein T, Peters AH. Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr Biol. 2003;13(14):1192–1200. doi: 10.1016/s0960-9822(03)00432-9. [DOI] [PubMed] [Google Scholar]
- 26.Vincent A, Van Seuningen I. Epigenetics, stem cells and epithelial cell fate. Differentiation. 2009;78(2–3):99–107. doi: 10.1016/j.diff.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Lee JE, Ge K. Transcriptional and epigenetic regulation of PPARgamma expression during adipogenesis. Cell Biosci. 2014;4:29. doi: 10.1186/2045-3701-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kallen AN, Zhou XB, Xu J, Qiao C, Ma J, Yan L, Lu L, Liu C, Yi JS, Zhang H, Min W, Bennett AM, Gregory RI, Ding Y, Huang Y. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell. 2013;52(1):101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan Z, Li Q, Luo S, Liu Z, Luo D, Zhang B, Zhang D, Rao P, Xiao J. PPARgamma and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther. 2016;11(3):216–225. doi: 10.2174/1574888x10666150519093429. [DOI] [PubMed] [Google Scholar]
- 30.Abdallah BM, Jafari A, Zaher W, Qiu W, Kassem M. Skeletal (stromal) stem cells: an update on intracellular signaling pathways controlling osteoblast differentiation. Bone. 2015;70:28–36. doi: 10.1016/j.bone.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 31.Kim HJ, Park JW, Lee KH, Yoon H, Shin DH, Ju UI, Seok SH, Lim SH, Lee ZH, Kim HH, Chun YS. Plant homeodomain finger protein 2 promotes bone formation by demethylating and activating Runx2 for osteoblast differentiation. Cell Res. 2014;24(10):1231–1249. doi: 10.1038/cr.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musri MM, Corominola H, Casamitjana R, Gomis R, Parrizas M. Histone H3 lysine 4 dimethylation signals the transcriptional competence of the adiponectin promoter in preadipocytes. J Biol Chem. 2006;281(25):17180–17188. doi: 10.1074/jbc.M601295200. [DOI] [PubMed] [Google Scholar]
- 33.Jang MK, Kim JH, Jung MH. Histone H3K9 demethylase JMJD2B activates adipogenesis by regulating H3K9 methylation on PPARgamma and C/EBPalpha during adipogenesis. PLoS One. 2017;12(1):e0168185. doi: 10.1371/journal.pone.0168185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Wang J, Li J, Hu G, Shan S, Li Q, Zhang X. KDM5A controls bone morphogenic protein 2-induced osteogenic differentiation of bone mesenchymal stem cells during osteoporosis. Cell Death Dis. 2016;7(8):e2335. doi: 10.1038/cddis.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu G, Wang J, Lin X, Diao S, Cao Y, Dong R, Wang L, Wang S, Fan Z. Demethylation of SFRP2 by histone demethylase KDM2A regulated osteo-/dentinogenic differentiation of stem cells of the apical papilla. Cell Prolif. 2016;49(3):330–340. doi: 10.1111/cpr.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Wang G, Wang Y, Zhou J, Yuan H, Li X, Liu Y, Wang B. Histone demethylase KDM7A reciprocally regulates adipogenic and osteogenic differentiation via regulation of C/EBPalpha and canonical Wnt signalling. J Cell Mol Med. 2019;23(3):2149–2162. doi: 10.1111/jcmm.14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumura Y, Nakaki R, Inagaki T, Yoshida A, Kano Y, Kimura H, Tanaka T, Tsutsumi S, Nakao M, Doi T, Fukami K, Osborne TF, Kodama T, Aburatani H, Sakai J. H3K4/H3K9me3 bivalent chromatin domains targeted by lineage-specific DNA methylation pauses adipocyte differentiation. Mol Cell. 2015;60(4):584–596. doi: 10.1016/j.molcel.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 38.Berdasco M, Melguizo C, Prados J, Gomez A, Alaminos M, Pujana MA, Lopez M, Setien F, Ortiz R, Zafra I, Aranega A, Esteller M. DNA methylation plasticity of human adipose-derived stem cells in lineage commitment. Am J Pathol. 2012;181(6):2079–2093. doi: 10.1016/j.ajpath.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Villagra A, Gutierrez J, Paredes R, Sierra J, Puchi M, Imschenetzky M, Wijnen Av A, Lian J, Stein G, Stein J, Montecino M. Reduced CpG methylation is associated with transcriptional activation of the bone-specific rat osteocalcin gene in osteoblasts. J Cell Biochem. 2002;85(1):112–122. [PubMed] [Google Scholar]
- 40.Du J, Johnson LM, Jacobsen SE, Patel DJ. DNA methylation pathways and their crosstalk with histone methylation. Nat Rev Mol Cell Biol. 2015;16(9):519–532. doi: 10.1038/nrm4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Q, Zhang J, Chen R, Wang L, Li B, Cheng H, Duan X, Zhu H, Wei W, Li J, Wu Q, Han JD, Yu W, Gao S, Li G, Wong J. Dissecting the precise role of H3K9 methylation in crosstalk with DNA maintenance methylation in mammals. Nat Commun. 2016;7:12464. doi: 10.1038/ncomms12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282(19):14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 43.Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277(34):30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 44.Nemoto E, Sakisaka Y, Tsuchiya M, Tamura M, Nakamura T, Kanaya S, Shimonishi M, Shimauchi H. Wnt3a signaling induces murine dental follicle cells to differentiate into cementoblastic/osteoblastic cells via an osterix-dependent pathway. J Periodontal Res. 2016;51(2):164–174. doi: 10.1111/jre.12294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.