Abstract
Vertebrate cranial mesoderm is a discrete developmental unit compared to the mesoderm below the developing neck. An extraordinary feature of the cranial mesoderm is that it includes a common progenitor pool contributing to the chambered heart and the craniofacial skeletal muscles. This striking developmental potential and the excitement it generated led to advances in our understanding of cranial mesoderm developmental mechanism. Remarkably, recent findings have begun to unravel the origin of its distinct developmental characteristics. Here, we take a detailed view of the ontogenetic trajectory of cranial mesoderm and its regulatory network. Based on the emerging evidence, we propose that cranial and posterior mesoderm diverge at the earliest step of the process that patterns the mesoderm germ layer along the anterior–posterior body axis. Further, we discuss the latest evidence and their impact on our current understanding of the evolutionary origin of cranial mesoderm. Overall, the review highlights the findings from contemporary research, which lays the foundation to probe the molecular basis of unique developmental potential and evolutionary origin of cranial mesoderm.
Keywords: Head mesoderm, Cardiopharyngeal field, Head muscles, Vertebrate head evolution, Mesoderm development
Introduction
Somites are the basis of the segmental body plan of vertebrates. Starting from the developing neck to the tail tip, the paraxial mesoderm, which runs parallel to the embryonic body axis, segments to form somites. In contrast, mesoderm in the developing cranium does not form somites (Fig. 1). Adding to this conspicuous morphological difference, studies over the last 2 decades have revealed several fundamental differences between the cranial and the somite-forming posterior mesoderm. Together, these studies have established that cranial mesoderm is a discrete developmental unit.
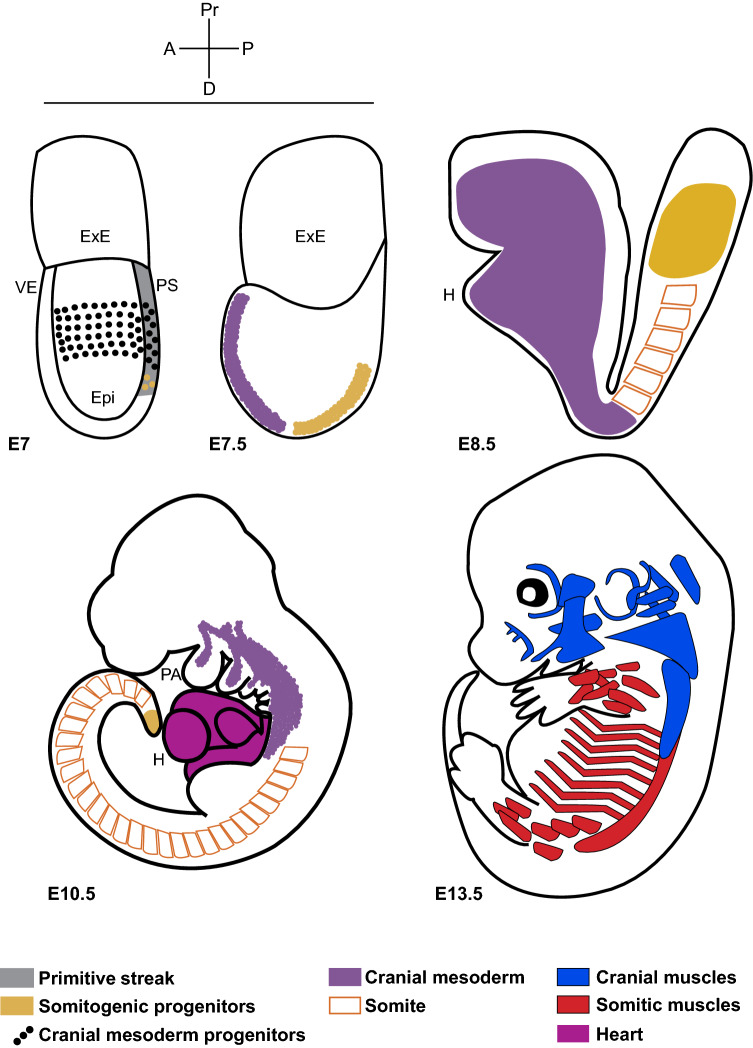
Fig. 1.
Schematic illustrating the origin and derivatives of cranial mesoderm. Cartoons of mouse embryos from mid-gastrula stage showing cranial and somitic mesoderm progenitors and their muscle derivatives. Embryonic day E7 all mesodermal progenitors emerge from the posterior end, where the primitive streak is formed. E7 and E7.5 cranial mesoderm migrates to the anterior pole, while somitogenic progenitors develop on the posterior side. E8.5 and E10.5 lateral plate of cranial mesoderm forms the heart and the paraxial component spreads as a non-segmented mesenchyme and eventually patterns into streams entering the pharyngeal arches. This contrasts with posterior paraxial mesoderm forming somites. E13.5 the pharyngeal mesodermal core contributes to different cranial skeletal muscle groups (in blue). Somite-derived skeletal muscles are shown in red. VE visceral endoderm, ExE extra-embryonic ectoderm, Epi epiblast, PS primitive streak (marked in grey), H heart, PA pharyngeal arches, A anterior, P posterior, Pr proximal, D distal. E7, 7.5 (based on evidence from [40]), E8.5 (based on evidence from Mesp1-cre/R26R; [153]), E10.5 [129] and E13.5 [53]
A further remarkable feature of vertebrate cranial mesoderm is that it contains a common progenitor pool, which gives rise to cardiomyocytes and skeletal muscles of the head. Elaborate head skeletal musculature including jaw muscles, facial and neck muscles are unique to vertebrates and are considered key to the evolution of their predatory lifestyle ([1]; see Box #1). Nevertheless, the cardiogenic/myogenic common progenitor, referred to as the cardiopharyngeal field, is conserved in Ciona [2, 3], which belongs to Urochordata—the likely sister group of craniates, which includes hagfish and vertebrates [4]. Thus, the cardiopharyngeal subset of cranial mesoderm appears to be a discrete developmental unit shared at least by Olfactores (a taxonomic clade within the Chordata that comprises urochordates and craniates). Contemporary studies have begun dissecting the developmental mechanism specifying the common progenitor (see Box #2). This understanding is beginning to shed light on the phylogenetic origin of cardiopharyngeal mesoderm and thus, on the evolution of cranial mesoderm.
Research groups investigating early mesoderm development in the 90s uncovered broad differences in the anterior and posterior mesoderm development. Loss of function of early mesoderm T-box factors and components of FGF and canonical Wnt signaling pathways selectively affect the development of somitogenic mesoderm posterior to forelimb level. A broad anterior compartment including cranial mesoderm is spared in these mutants, highlighting differences in the genetic program between anterior and posterior mesoderm. Recent studies are unraveling the early steps underlying the formation of cranial mesoderm. These studies begin to trace the divergent trajectory of cranial mesoderm development vis-a-vis the program of posterior somite-forming mesoderm. Here, we review the literature on the developmental specificities of cranial mesoderm focusing on its distinct trajectory and discuss the implications of recent findings in addressing the evolutionary origin of cranial mesoderm.
Box 1: Anatomy and derivatives of cranial mesoderm.
Vertebrate cranial mesoderm is bilaterally adjacent to the developing brain from the forebrain to hindbrain level. Cranial mesoderm populates the core of branchial/pharyngeal arches to give rise to the skeletal muscles (Fig. 1)—of the jaw that aid prey capture and mastication, of the face, which allow facial expression, as well as of the pharynx and larynx that help in swallowing, breathing and vocal expression (reviewed in [5]. The cucullaris group of neck muscles, which connects head to the shoulder blade, as well as the striated muscle in the anterior end of esophagus, are also of cranial mesoderm origin [6–8]. Cardiomyocytes of the heart derive from the lateral plate of cranial mesoderm. Endothelium of blood vessels in head, as well as some of the posterior skull bones also have cranial mesoderm origin [5, 9–15]. A subset of the extraocular muscles, which enable eye movements, is thought to derive from cranial mesoderm, while the rest from the prechordal mesoderm [12, 14, 16]. The prechordal mesoderm, although present in the cranial region, does not appear to share a close cell lineage relationship with cranial mesoderm [17].
Box 2: Spotlight on cranial mesoderm: cranial muscles and cardiopharyngeal field.
To a great extent, the developmental mechanisms of cranial mesoderm were brought to focus by studies on cranial skeletal muscle development. Early studies in the field indicated that the craniofacial muscle development is divergent from the somite-derived muscles of the trunk and limbs. They showed that the signaling environment driving the lineage progression of muscle progenitors is different in cranial mesoderm and somites [18, 19]. Subsequently, cranial mesoderm was shown to be a discrete domain delineated from the rest of posterior mesoderm by largely confined marker gene expression [20]. Furthermore, the cell lineage contributing to and the genetic program regulating the development of cranial muscles were demonstrated to be distinct from that governing somitic muscles [5, 21–27]. Remarkably, a recent single-cell transcriptome study has also identified the myogenic lineage from cranial mesoderm as a distinct developmental trajectory compared to somitic myogenic trajectories [28]. These findings underscored a deeper divide in the mesodermal subsets from which they derive. In parallel, a growing body of evidence had begun unraveling the deep ontogenetic link between the branchiomeric (pharyngeal arch-derived) muscles and heart [8, 29–36]. These studies showed that a discrete developmental unit, known as the cardiopharyngeal field (reviewed in [37]), harbors the common progenitors of heart and cranial muscles. Subsequent interest in the cardiopharyngeal mesoderm, a major subset, began shedding light on the development of cranial mesoderm as a whole.
Signals in primitive streak trigger commitment to cranial mesoderm fate
At the onset of gastrulation in mouse embryos, pluripotent epiblast cells converge towards the midline at the posterior pole of the embryo and undergo epithelial to mesenchymal transition to form the primitive streak (PS; [38, 39]. Both the cranial and posterior mesoderm subtypes emerge from PS (Fig. 1). The reporter tracing and grafting studies in mouse had established that anterior primitive streak cells of early to mid-gastrula stage embryos give rise to cranial mesoderm [40]. In contrast, anterior PS at mid-late streak stage contributes to posterior mesoderm derivatives [40]. While epiblast domains map to distinct fates [41, 42], there is no evidence for fate priming in epiblast. Instead, as shown by prospective labelling and tracing of PS cells as well as by orthotopic grafting of labelled donor PS cells, the order of ingression, i.e., the spatiotemporal domain of PS during ingression influences the fate choice (Fig. 1; [40, 43]).
The signaling environment of the early anterior PS, from which cranial mesoderm emerges, has been reported to prime the mesoderm progenitors for cranial fate (Fig. 2). The anterior PS in the early mouse gastrula experiences BMP and Nodal (TGFβ-Smad2/3) signaling along with Wnt3 and FGF. In contrast, anterior PS at late gastrula, which gives rise to posterior mesoderm is marked primarily by Wnt3A and FGF signaling [38, 39]. In fact, exposure to early anterior PS cues, BMP and Activin A (a substitute for Nodal), induces cardiac mesoderm from human pluripotent cells in vitro [44–46]. Similarly, induction with canonical Wnt (using GSK3β inhibitor) and FGF2, which represents the late PS environment, drives somitic mesoderm differentiation [46]. Recent demonstration of distinct gene expression patterns along the anterior–posterior axis of PS in chick as well as mouse gastrulae supports the commencement of fate restriction in PS [47, 48]. Together, these studies demonstrate that the discrete signaling environment of distinct spatiotemporal PS domains initiate fate commitment allowing segregation of cranial mesoderm and posterior somitogenic mesoderm. Moreover, the spatiotemporal domains could be narrowed down further for discrete subsets within cranial mesoderm. Temporally controlled induction of cranial mesoderm selective lineage tracer, Mesp1Cre, demonstrated that early PS contributes to the first heart field, which contributes primarily to the left ventricle and the atria; mid-anterior PS gives rise to the second heart field, which is the progenitor of right ventricle and the outflow tract, as well as the myogenic pharyngeal mesoderm [31]. Furthermore, single-cell analysis in mouse showed that fate restriction into first and second heart field subsets occurs early during gastrulation [31]. Moreover, the latest single-cell transcriptome data from a time series covering key stages in mouse gastrulation revealed dynamic nature of the PS cell regulatory state during the course of development [49]. All this evidence point to the initiation of fate restriction as mesoderm progenitors ingress through the streak. However, the role of this early step in driving cranial mesoderm identity is unclear. In fact, the fate commitment in PS is yet to be characterized at the molecular level. Nevertheless, the differentiation potential of in vitro human PS-like progenitors into cardiomyocytes is blunted upon exposure to late PS signaling cues, which favors posterior fate [46]. This observation emphasizes the significance of PS cues in cranial mesoderm trajectory.
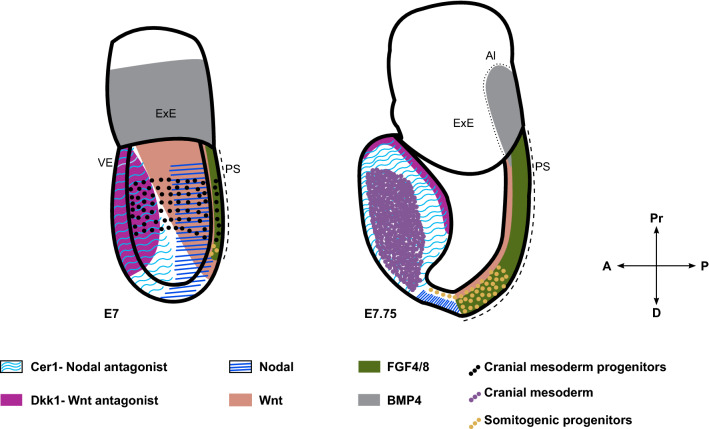
Fig. 2.
Signaling cues driving progressive specification of cranial and posterior somitogenic mesoderm. Schematic highlighting the expression domains of key signaling cues influencing mesoderm patterning. Anatomical locations of cranial and somitic mesoderm and their progenitors at these temporal windows are indicated. A anterior, P posterior, Pr proximal, D distal, ExE extra-embryonic ectoderm, Al Allantois (marked by dotted line), VE visceral endoderm, PS primitive streak (marked by dashed line)
Once generated at PS, cranial mesoderm progenitors migrate (laterally in cylindrical mouse gastrulae; anteriorly in disc-shaped chick gastrulae) to the anterior end of the embryo. Live imaging of labelled mesoderm progenitors in mouse embryos using light sheet microscopy reveals that the lateral wings of mesoderm cells display filopodia projections [50]. Based on transcriptome analysis the report suggests a role for guidance molecules such as netrin and ephrins in the mechanism for cranial mesoderm migration to anterior destination [50]. In contrast, posterior mesoderm continues to develop at the posterior pole. It is possible that the fate commitment occurring in PS may underlie this crucial difference in behavior. The mechanism by which cranial mesoderm progenitors respond selectively to the guidance cues for anterior migration is an open question.
In summary, the diversification of cranial mesoderm from posterior mesoderm commences at PS. Subsequently, cranial and posterior mesoderm develop further at anterior or posterior pole of the embryo, respectively. Latest studies have elucidated the role of distinct signaling environment at the destination in the divergent trajectories of cranial and posterior mesoderm.
Anterior cues instruct cranial mesoderm identity
The fate commitment of progenitors during ingression through PS is reversible, as evidenced by their plasticity [51]. Moreover, epiblast cells, prior to ingression through PS, when heterotopically grafted in the anterior region adopt cardiac fate [52]. This indicates that although the fate commitment occurs at PS, this step is not necessary for acquiring cardiac mesoderm fate. The grafting experiments also indicate that the signal(s) in the anterior destination is sufficient to drive cardiac mesoderm specification. This idea is supported by the fact that the genes that drive cranial mesoderm regulatory network and used widely to mark the subtype, such as Tbx1, Nkx2.5 and Isl1, are turned on only at the anterior destination [20, 53].
The anterior pole is established by inhibiting Wnt and Nodal signaling pathways [54–59], which would otherwise induce posterior fate by triggering PS formation [60–64]. In the early mouse gastrula, symmetry breaking occurs with the polarized expression of Nodal and its antagonists. Nodal expression is progressively restricted from throughout the epiblast to the presumptive posterior of embryo [38, 65]; Fig. 2). Similarly, expression of Wnt ligands are restricted to posterior pole [58, 66–72] (Fig. 2). Simultaneously, the anterior signaling center expressing antagonists of Nodal and Wnt such as Lefty1, Cer1 and Dkk1 [54–58, 73]) establish the anterior pole of the embryo [60–64] (Fig. 2). While these cues act to set up anterior–posterior axis coincident with the initiation of gastrulation, they persist till later, when the cranial mesoderm progenitors arrive at the anterior pole [53]. In fact, Wnt inhibition is known to be an instructive cue for cardiac differentiation [45, 74–78]. Furthermore, attempts to generate cardiomyocytes in vitro showed that inhibition of canonical Wnt signal in the mesoderm derived from pluripotent stem cells promoted cardiac fate [44–46, 79]. In the same vein, Wnt inhibition is key for skeletal muscle fate induction in the pharyngeal arches [19]. This is in contrast with myogenesis in somites, which is promoted by Wnt signal [80–84]. While these reports underscored the role of anterior cues in the differentiation of cranial mesoderm derivatives, the role of inhibitory anterior cues, including Nodal antagonism, in cranial mesoderm specification remained unaddressed. Recently, we showed that Wnt and Nodal inhibition commit PS-like early mesoderm cells derived from mouse as well as human embryonic stem cells into cranial mesoderm-like cells ([53]; Fig. 2). Moreover, countering Wnt inhibition by forced activation of canonical Wnt signal in the entire embryo ex vivo appears to blunt cranial mesoderm fate acquisition [53]. Taking this evidence from the literature together, we could conclude that the earliest cues for fate commitment in PS prime the cranial mesoderm fate, while the ‘instructive’ anterior inhibitory cues seal the fate.
Distinct specification network governs divergent cranial mesoderm trajectory?
Wnt/β-catenin signal regulates a genetic network, involving FGF pathway and mesoderm T-box factors T (brachyury) and Tbx6, which controls the development of posterior somitogenic mesoderm. Loss of Wnt3a function results in suppression of T induction and causes complete loss of somitogenic mesoderm below the forelimb level [85–87]. T is a key mesoderm specification factor and T null mouse embryos phenocopy Wnt3a mutation [88]. Similarly, null mutants of Tbx6, which acts downstream of T [89, 90], as well as those of FGFR1 and double null for FGF4/8 also manifest loss of posterior somitogenic mesoderm [91, 92]. Moreover, T and FGF pathway positively feedback on Wnt/β-catenin signaling [87, 91] indicating that sustained Wnt signal and its downstream network are required for posterior somite-forming mesoderm [87, 91, 93]. In fact, recent studies show that sustained Wnt/β-catenin and FGF signals are crucial for the induction and maintenance of the stem cells/progenitors of the posterior mesoderm [46, 93–96]. These progenitors are referred to as neuromesoderm progenitors as they contribute to somites as well as to the spinal cord below neck [93, 97–101]. This dual potential of these Wnt/FGF-dependent progenitors is underscored by the dramatic phenotype of ectopic spinal cords forming at the expense of somites in the mutants of Wnt3a, FGFR1, T and Tbx6 [87, 92, 102]. Based on this evidence, the emerging idea is that the necessity of Wnt3a/FGF/T/Tbx6 axis is specific to the neuromesoderm-derived posterior mesoderm. Remarkably, a broad anterior mesodermal compartment including cranial mesoderm, occipital and cervical somites are spared in these mutants. We had shown that while the mutant somites display patterning defects, cranial mesoderm development is unperturbed in T and Tbx6 mutants [53]. While the finding that Wnt inhibition specifies cranial mesoderm fits with and complements our understanding of posterior network from these studies, the genetic network governing cranial mesoderm remains poorly defined (Fig. 3).
Fig. 3.
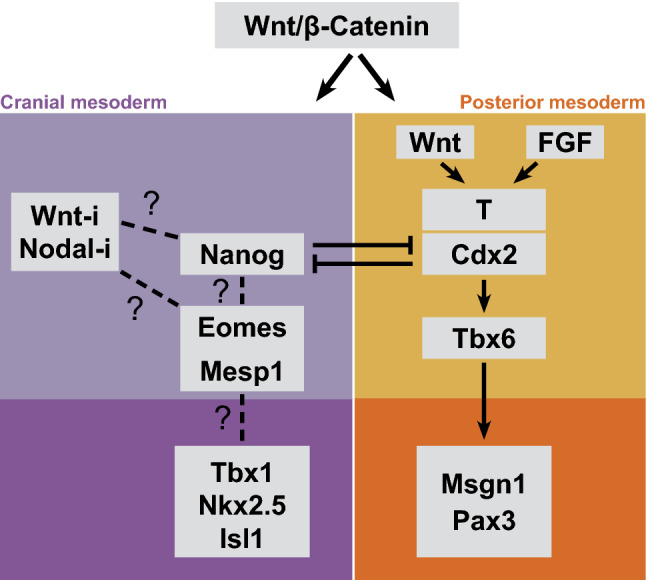
Divergent regulatory network governing cranial and posterior somitogenic mesoderm. Wnt/β-catenin signal is central for the generation of all mesoderm. The regulatory network of somite-forming posterior mesoderm is well studied. Recent studies reveal key components of the cranial mesoderm program; however, the network is unclear. Wnt-i Wnt inhibition, Nodal-i Nodal inhibition
Although, heart morphogenesis is affected in T and Tbx6 mouse mutants, resulting from left–right patterning defect [103–105], the cardiac lineage specification progresses and heart develops in these mutants. Moreover, our work demonstrated that the development and patterning of cranial skeletal musculature in the pharyngeal arches is unaffected in T and Tbx6 mutants ([53]; Fig. 3). Therefore, T and Tbx6 are dispensable for cranial mesoderm development. Thus, cranial mesoderm development appears to be governed by a regulatory program distinct from Wnt3a/FGF/T/Tbx6 network. Though dispensable for the program conferring cranial mesoderm identity, a redundant role for T/Tbx6 in specifying mesodermal fate of PS cells destined to the cranial domain cannot be ruled out.
While inhibition of Wnt is central to cranial mesoderm specification, initially, Wnt signal is required at the streak for the emergence of progenitors of cranial mesoderm. Whereas mutation in Wnt3a specifically ablates posterior somitogenic mesoderm [85], loss of Wnt3 in mice abrogates PS formation and hence, no mesoderm is generated [106]. Do Wnt3 and Wnt3A have different downstream effects? The difference in mutant phenotypes could simply be explained by the timing of their expression; Wnt3 is induced first concomitant to PS induction, while Wnt3a is induced at a later stage, when posterior mesoderm progenitors emerge from PS [39]. However, cascade downstream of Wnt3 appears dissimilar to that of Wnt3a since Lef1:Tcf1 double knockout phenocopies Wnt3a but not Wnt3 mutation [106, 107]. Whether differences between Wnt3 and Wnt3a pathways already prime the divergent trajectories of cranial and posterior mesoderm remains to be addressed. This further highlights the mechanism driving specifically the cranial mesoderm progenitors towards the anterior pole. The mechanism is not yet understood and probing the function of genes selectively expressed in the lineage will help uncover it.
Early studies revealed that expression of a number of regulatory genes uniquely mark the cranial mesoderm domain [20]. A recent single-cell study has also discovered a number of genes preferentially expressed in pharyngeal mesoderm [49]. However, the function of these genes in cranial mesoderm network remains to be investigated. In this context, there has been a significant breakthrough recently. Homeobox transcription factors, Cdx2 and Nanog, repress each other and have opposing functions in the choice between posterior versus cranial mesoderm [46]. Notably, Cdx2–Nanog module provides a mechanistic link between Wnt pathway and the network, which governs the differential specification of mesoderm along anterior–posterior axis. Activating posterior mesoderm network is only one aspect of the function of canonical Wnt pathway, which has an evolutionarily conserved function in establishing the posterior identity [108–110]. Wnt induces caudal homeobox factors, Cdx [111–115], which, in turn, promote the expression of posterior Hox genes [116–118]. Similar to Wnt, Cdx is central to establish posterior identity [101, 118, 119]. Notably, in addition to promoting the posterior fate, Cdx appears to actively repress the cranial fate. This is revealed by the fact that the combined loss-of-function of paralogous Cdx factors reduces the strength of Wnt inhibitory cues required to induce cardiac differentiation in vitro [45]. This set of evidence indicates that the suppression of Wnt–Cdx module could be crucial for cranial mesoderm formation (Fig. 3). In this context, the demonstration that Nanog transcriptionally represses Cdx2 and functions to promote cardiac differentiation [46] reveals a key role in cranial mesoderm. Thus, it appears that Nanog may work in concert with Wnt inhibitory cues to specify cranial mesoderm.
In addition to Nanog, two other developmental genes, Eomes and Mesp1, are implicated in the commitment of PS progenitors to cranial mesoderm fate. Both show preferential expression in the spatiotemporal domain of PS that generates cranial mesoderm [120, 121]. Mesp1 (paradoxically named Mesoderm posterior1), a bHLH factor, is also expressed selectively in anterior mesoderm [122, 123]. Mesp1 mutation affects heart morphogenesis [122]; however, cranial mesoderm appears unaffected. The possible redundancy with the paralog Mesp2 in cranial mesoderm has not been addressed since double knockout mouse embryos fail to generate any mesoderm [124]. Nonetheless, forced expression of Mesp1 programs cranial mesoderm differentiation in pluripotent stem cells [125]. Remarkably, MespB, the ortholog in Ciona intestinalis marks the progenitor cells, which give rise to the entire cardiopharyngeal lineage [3, 126]. Eomes, a T-box factor, is induced at the start of gastrulation and its expression is downregulated in PS around the time of posterior somite formation [120]. Lineage tracing experiments support its preferential expression in the anterior mesodermal compartment [127]. Compound mutants heterozygous for Nodal and Eomes, wherein Eomes is specifically targeted in epiblast, cause severe anterior truncations with no effect on posterior structures [128]. Cranial mesoderm development has not been assessed in these mutants. However, analysis of Eomes null embryonic stem cells reveals that they lack cardiomyogenic potential [127]. Moreover, Mesp1 expression is diminished during mesoderm differentiation in Eomes mutant cells [127]. This evidence implies a central role for Eomes–Mesp1 in cranial mesoderm ([122, 127, 129]; Fig. 3). At this juncture, addressing the connections among Wnt inhibition, Nanog–Cdx module, Eomes and Mesp1 will provide mechanistic insight into the regulatory network governing cranial mesoderm.
As the progenitors of cranial mesoderm emigrate away from the streak and come under the influence of the anterior signaling center, Eomes and Mesp1 are downregulated [120, 121]. The acquisition of cranial mesoderm identity is marked by the expression of a different set of regulatory genes: Tbx1, a T-box factor, homeobox factors Nkx2.5 and Pitx2, as well as Isl1, a LIM-homeodomain factor are induced after the arrival of the population at the anterior end (reviewed in [20, 37]). These factors form the core network driving lineage progression in cranial mesoderm and their roles are reviewed in detail elsewhere [27]. The mechanistic link between Wnt inhibition–Nanog–Eomes–Mesp1 and this core cranial mesoderm network is a major gap and addressing it is critical for a comprehensive understanding of the cranial mesoderm regulatory program.
Interaction with cranial neural crest sustains divergent trajectory of cranial mesoderm
Skeletal muscle is a single-cell type in functional terms, irrespective of somite or cranial mesoderm origin. In both cases, Myod family of bHLH factors including Myf5 and Myod form the gateway into myogenesis. However, cranial mesoderm never induces Pax3 during myogenesis [130], a paired box factor critical for myogenesis from somites [23]. Thus, cranial mesoderm appears to follow a distinct trajectory till the induction of muscle differentiation. Moreover, when placed in the somitic environment, cranial mesoderm fails to follow its distinct trajectory [18, 130]. This underscores differences in the signaling environment governing cranial mesoderm and somitic lineage progression.
Neural crest plays a significant role in posterior paraxial mesoderm; a fleeting kiss and run by migratory neural crest activates Myf5 via notch signaling to bootstrap myogenesis in the dorsomedial compartment of somites [131]. The interaction of head neural crest with cranial mesoderm is not only critical, it is also extensive and lasting [17]. The connective tissues associated with the head musculature derived from cranial mesoderm are of cranial neural crest origin [7, 14, 15, 17, 132, 133]. In contrast, the connective tissue associated with posterior somite-derived muscles is of mesodermal origin [132]. Thus, the neighboring tissue types that make up the signaling environment of cranial mesoderm during downstream differentiation are distinct.
Wnt inhibition is a leitmotif in cranial mesoderm development. Akin to early requirement in specification of cranial mesoderm, the initiation of myogenesis in mesodermal core of arches also requires Wnt inhibition. Neural crest cells that surround the core secrete antagonists of Wnt pathway [19]. In fact, cranial neural crest is required for the development of cranial mesoderm occupying the core of pharyngeal arches [134]. Thus, since its birth at the primitive streak till its differentiation, at least into skeletal muscle, the trajectory of cranial mesoderm development is divergent from that of posterior somitogenic mesoderm. The divergent trajectory underlies the distinct regulatory cascade driving commitment and differentiation of head muscles (reviewed in [5, 135]).
Cranial mesoderm: a phylogenetically distinct ontogenetic unit
Gans and Northcutt proposed an influential hypothesis that the vertebrate head is a de novo addition to the ancestral chordate body plan since it is made primarily of evolutionarily new cell types, the neural crest and neurogenic placodes. They also proposed that the cranial mesoderm enabled a key innovation during vertebrate evolution—pharyngeal musculature [1]. Initially involved in efficient respiratory gas exchange, the pharyngeal muscles eventually gave rise to the jaw musculature. This enabled the transition from passive filter feeding, characteristic of invertebrate chordates, to an active predatory lifestyle seen among vertebrates [1, 37]. Furthermore, the chambered heart, another cranial mesoderm derivative, allowed increased growth and metabolism and thus, contributed to the success of vertebrates [1, 37]. Therefore, cranial mesoderm is central in vertebrate evolution and hence, the origin of cranial mesoderm is a significant question.
Whether the non-segmented cranial mesoderm is a novel embryonic tissue similar to neural crest1* and placodes* [5, 136, 137] or emerged from anterior somites of chordate ancestor that secondarily lost their segmentation [138, 139] is hotly disputed. Settling this dispute has far reaching implications to resolve a long-standing controversy; whether the vertebrate head is a new non-segmental addition to the basic segmental chordate body plan or it is only a modification that arose by selective loss of anterior segmentation [136–138].
The segmentalist view of vertebrate head comes from the body plan of amphioxus, in which mesoderm forms somites along the entire length of the anterior–posterior axis. As a basal chordate, amphioxus serves as a proxy for the chordate ancestor. There is growing evidence, based on the developmental program, that the anterior somites of amphioxus are homologous to vertebrate somites [139, 140]. However, the ventral/visceral part of the somites expresses cardiac progenitor marker Csx, an ortholog of vertebrate Nkx2.5 as well as Hand and Tbx4/5 [141, 142]. Significantly, progenitors from the ventral somites generate the myocardial progenitors of the pulsatile vessels in amphioxus, which are considered a ‘decentralized heart’ [141, 142]. Since somites extend till the anterior end and ventral aspect of somites are cardiogenic-like, it has been proposed recently that vertebrate cranial mesoderm emerged from the visceral component of anterior somites of the chordate ancestor [139]. The hypothesis is that the dorsal parts of the anterior somites were lost and the visceral component lost segmentation and expanded dorsally evolving into cranial mesoderm; the expansion into non-visceral territory created the condition for acquiring novel developmental potential characteristic of cranial mesoderm. In other words, the non-segmented vertebrate cranial mesoderm with novel potential arose from a segmented ancestral state [139]. This proposal is a significant advance; however, further efforts are needed to investigate the presence of tissue homologous to vertebrate cranial mesoderm in amphioxus. At this juncture, addressing the mechanisms diversifying cranial mesoderm from somitogenic mesoderm will provide the framework to systematically address cranial mesoderm origin. This, in turn, will aid inquiries into vertebrate head evolution.
The extraordinary potential of cranial mesoderm pertains to the bipotential nature of the cardiopharyngeal field to make both cardiomyocytes and skeletal muscles. The close cell lineage kinship in the ontogeny between these two cell types is remarkable. The split of smooth muscle and striated skeletal muscle cell types appear to have occurred at the base of bilateria [143, 144]. Cardiomyocytes are hypothesized to have evolved from smooth muscle since the core transcriptional factors of cardiogenic network such as Nkx2.5 and GATA4 are paralogs of those that specify smooth muscle (Nkx3.2 and GATA6), while the core myogenic network of skeletal muscle consisting of Myod family is distinct. The striation-related gene set is thought to have been activated later during the course of cardiomyocyte evolution [143, 144]. Thus, cardiomyocytes and skeletal myocytes are fundamentally divergent cell types. In this context, the close lineage relationship between these cell types in cranial mesoderm is intriguing, as it indicates that the bipotent mesoderm may have emerged either from an ancestral cardiogenic mesoderm or myogenic mesoderm. The former would imply that cranial skeletal muscles have an origin independent of somite-derived muscles, from an ancestral cardiogenic progenitor pool. Analysis of cardiogenic fields deeper in phylogeny will shed light on these fundamental questions.
The role of Wnt signal and Wnt inhibitory cues in the developmental divergence between cranial and posterior mesoderm raises the possibility of a deep phylogenetic divergence. As outlined above, Wnt signaling and its effectors T and Cdx drive posterior mesoderm formation. Remarkably, Wnt/T and Wnt/Cdx modules [145–149] are deeply conserved. In contrast, emerging evidence show that cranial mesoderm is T independent and is induced by Wnt inhibition, and suppression of Cdx factors. This dichotomy is significant in the light of the emerging theory that Wnt/β-catenin signal and its inhibition are deeply conserved cues for symmetry breaking and establishing embryonic posterior and anterior pole identities, respectively [108–110, 150–152]. This prompts the speculation that divergent mesodermal programs based on opposing Wnt cues could also have deeper origin in phylogeny. In other words, the divergent nature of anterior mesoderm is possibly ancient and fundamental.
Perspectives
Efforts in the last decade by a number of groups have yielded insight into the biology of cranial mesoderm and the evidence generated has established that it represents a discrete mesodermal subtype. However, several important questions remain to be addressed: (1) the mechanism guiding cranial mesoderm progenitors from primitive streak to the anterior destination, (2) the global genetic program linking the anterior signaling cues and the key regulatory transcription factors in a detailed network and (3) the identity of embryonic tissue homologous to vertebrate cranial mesoderm in the chordate ancestor. Renewed efforts through classical developmental biology studies to tease apart the regulatory network of cranial mesoderm, single-cell transcriptome studies to elucidate developmental trajectories of mesoderm progenitors in early gastrula and evolutionary developmental biology approaches tracing the origin of cranial mesoderm will help address these open questions and further illuminate the development and evolution of this important embryonic cell type.
Footnotes
*Studies in Ciona suggest that evolutionary precursors of neural crest and placode were already present in the ancestors of olfactores [3, 154, 155]. These reports provide reason to refine the idea of novelty of the embryonic cell types.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bhakti Vyas and Nitya Nandkishore contributed equally.
References
- 1.Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science (80-) 1983;220:268–273. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- 2.Stolfi A, et al. Early chordate origins of the vertebrate second heart field. Science (80-) 2010 doi: 10.1126/science.1190181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan N, Razy-Krajka F, Christiaen L. Regulation and evolution of cardiopharyngeal cell identity and behavior: insights from simple chordates. Curr Opin Genet Dev. 2015;32:119–128. doi: 10.1016/j.gde.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delsuc F, et al. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- 5.Sambasivan R, Kuratani S, Tajbakhsh S. An eye on the head: the development and evolution of craniofacial muscles. Development. 2011;138:2401–2415. doi: 10.1242/dev.040972. [DOI] [PubMed] [Google Scholar]
- 6.Gopalakrishnan S, Comai G, Sambasivan R, et al. A cranial mesoderm origin for esophagus striated muscles. Dev Cell. 2015;34:694–704. doi: 10.1016/j.devcel.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Heude E, Tesarova M, Sefton EM, et al. Unique morphogenetic signatures define mammalian neck muscles and associated connective tissues. Elife. 2018;7:1–26. doi: 10.7554/eLife.40179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lescroart F, Hamou W, Francou A, et al. Clonal analysis reveals a common origin between nonsomite-derived neck muscles and heart myocardium. Proc Natl Acad Sci. 2015;112:1446–1451. doi: 10.1073/pnas.1424538112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noden DM. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat. 1983;168:257–276. doi: 10.1002/aja.1001680302. [DOI] [PubMed] [Google Scholar]
- 10.Evans DJR, Noden DM. Spatial relations between avian craniofacial neural crest and paraxial mesoderm cells. Dev Dyn. 2006;235:1310–1325. doi: 10.1002/dvdy.20663. [DOI] [PubMed] [Google Scholar]
- 11.Jacob M, et al. Ontogeny of avian extrinsic muscles. Cell Tissue Res. 1984 doi: 10.1007/bf00228439. [DOI] [PubMed] [Google Scholar]
- 12.Couly GF, Coltey PM, Le Douarin NM. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Kuratani S. Craniofacial development and the evolution of the vertebrates: the old problems on a new background. Zoolog Sci. 2005;19:19. doi: 10.2108/zsj.22.1. [DOI] [PubMed] [Google Scholar]
- 14.Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- 15.Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- 16.Bothe I, Ahmed MU, Winterbottom FL, et al. Extrinsic versus intrinsic cues in avian paraxial mesoderm patterning and differentiation. Dev Dyn. 2007;236:2397–2409. doi: 10.1002/dvdy.21241. [DOI] [PubMed] [Google Scholar]
- 17.Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. J Anat. 2005;207:575–601. doi: 10.1111/j.1469-7580.2005.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mootoosamy RC, Dietrich S. Distinct regulatory cascades for head and trunk myogenesis. Development. 2002;129:573–583. doi: 10.1242/dev.129.3.573. [DOI] [PubMed] [Google Scholar]
- 19.Tzahor E, Kempf H, Mootoosamy RC, et al. Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev. 2003;17:3087–3099. doi: 10.1101/gad.1154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bothe I, Dietrich S. The molecular setup of the avian head mesoderm and its implication for craniofacial myogenesis. Dev Dyn. 2006;235:2845–2860. doi: 10.1002/dvdy.20903. [DOI] [PubMed] [Google Scholar]
- 21.Harel I, Maezawa Y, Avraham R, et al. Pharyngeal mesoderm regulatory network controls cardiac and head muscle morphogenesis. Proc Natl Acad Sci. 2012;109:18839–18844. doi: 10.1073/pnas.1208690109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, Chang P, Valdez R, et al. Control of facial muscle development by MyoR and capsulin. Science. 2010;298:2378–2381. doi: 10.1126/science.1078273. [DOI] [PubMed] [Google Scholar]
- 23.Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/S0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- 24.Kelly RG, Jerome-Majewska LA, Papaioannou VE. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum Mol Genet. 2004;13:2829–2840. doi: 10.1093/hmg/ddh304. [DOI] [PubMed] [Google Scholar]
- 25.Shih HP, Gross MK, Kioussi C. Cranial muscle defects of Pitx2 mutants result from specification defects in the first branchial arch. Proc Natl Acad Sci. 2007;104:5907–5912. doi: 10.1073/pnas.0701122104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong F, Sun X, Liu W, et al. Pitx2 promotes development of splanchnic mesoderm-derived branchiomeric muscle. Development. 2006;133:4891–4899. doi: 10.1242/dev.02693. [DOI] [PubMed] [Google Scholar]
- 27.Sambasivan R, et al. Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev Cell. 2009;16:810–821. doi: 10.1016/j.devcel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Cao J, Spielmann M, Qiu X, et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature. 2019;566:496–502. doi: 10.1038/s41586-019-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly RG, Brown NA, Buckingham ME, Kingdom U. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/S1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 30.Lescroart F, Kelly RG, Le Garrec J-F, et al. Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development. 2010;137:3269–3279. doi: 10.1242/dev.050674. [DOI] [PubMed] [Google Scholar]
- 31.Lescroart F, Chabab S, Lin X, et al. Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat Cell Biol. 2014;16:829–840. doi: 10.1038/ncb3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaffran S, Odelin G, Stefanovic S, et al. Ectopic expression of Hoxb1 induces cardiac and craniofacial malformations. Genesis. 2018;56:1–13. doi: 10.1002/dvg.23221. [DOI] [PubMed] [Google Scholar]
- 33.Kelly RG, Buckingham ME. The anterior heart-forming field: voyage to the arterial pole of the heart. Trends Genet. 2002;18:210–216. doi: 10.1016/S0168-9525(02)02642-2. [DOI] [PubMed] [Google Scholar]
- 34.Tirosh-Finkel L. Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development. 2006;133:1943–1953. doi: 10.1242/dev.02365. [DOI] [PubMed] [Google Scholar]
- 35.Nathan E, Monovich A, Tirosh-Finkel L, et al. The contribution of Islet1-expressing splanchnic mesoderm cells to distinct branchiomeric muscles reveals significant heterogeneity in head muscle development. Development. 2008;135:647–657. doi: 10.1242/dev.007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grifone R, Kelly RG. Heartening news for head muscle development. Trends Genet. 2007;23:365–369. doi: 10.1016/j.tig.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Diogo R, Kelly RG, Christiaen L, et al. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature. 2015;520:466–473. doi: 10.1038/nature14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10:91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- 39.Ramkumar and Anderson SnapShot: mouse primitive streak. Cell. 2011;146:488. doi: 10.1016/j.cell.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 40.Kinder SJ, Tsang TE, Quinlan GA, et al. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development. 1999;126:4691–4701. doi: 10.1242/dev.126.21.4691. [DOI] [PubMed] [Google Scholar]
- 41.Tam PP, Beddington RS. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development. 1987;99:109–126. doi: 10.1242/dev.99.1.109. [DOI] [PubMed] [Google Scholar]
- 42.Lawson KA, Pedersen RA (1992) Clonal analysis of cell fate during gastrulation and early neurulation in the mouse. In: Ciba foundation symposium, pp 3–26 [DOI] [PubMed]
- 43.Tam PP, Parameswaran M, Kinder SJ, Weinberger RP. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development. 1997;124:1631–1642. doi: 10.1242/dev.124.9.1631. [DOI] [PubMed] [Google Scholar]
- 44.Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR + embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 45.Rao J, Pfeiffer MJ, Frank S, et al. Stepwise clearance of repressive roadblocks drives cardiac induction in human ESCs. Cell Stem Cell. 2016;18:341–353. doi: 10.1016/j.stem.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 46.Mendjan S, Mascetti VL, Ortmann D, et al. NANOG and CDX2 pattern distinct subtypes of human mesoderm during exit from pluripotency. Cell Stem Cell. 2014;15:310–325. doi: 10.1016/j.stem.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Peng G, Suo S, Chen J, et al. Spatial transcriptome for the molecular annotation of lineage fates and cell identity in mid-gastrula mouse embryo. Dev Cell. 2016;36:681–697. doi: 10.1016/j.devcel.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Vermillion KL, Bacher R, Tannenbaum AP, et al. Spatial patterns of gene expression are unveiled in the chick primitive streak by ordering single-cell transcriptomes. Dev Biol. 2018;439:30–41. doi: 10.1016/j.ydbio.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Pijuan-Sala B, Griffiths JA, Guibentif C, et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature. 2019;566:490–495. doi: 10.1038/s41586-019-0933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saykali B, Mathiah N, Nahaboo W, et al. Distinct mesoderm migration phenotypes in extra-embryonic and embryonic regions of the early mouse embryo. Elife. 2019;8:1–27. doi: 10.7554/eLife.42434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trainor PA, Tan SS, Tam PP. Cranial paraxial mesoderm: regionalisation of cell fate and impact on craniofacial development in mouse embryos. Development. 1994;120:2397–2408. doi: 10.1242/dev.120.9.2397. [DOI] [PubMed] [Google Scholar]
- 52.Parameswaran M, Tam PPL. Regionalisation of cell fate and morphogenetic movement of the mesoderm during mouse gastrulation. Dev Genet. 1995;17:16–28. doi: 10.1002/dvg.1020170104. [DOI] [PubMed] [Google Scholar]
- 53.Nandkishore N, Vyas B, Javali A, et al. Correction: divergent early mesoderm specification underlies distinct head and trunk muscle programmes in vertebrates. Development. 2018;4529:4522–4529. doi: 10.1242/dev.173187. [DOI] [PubMed] [Google Scholar]
- 54.Takaoka K, Yamamoto M, Hamada H. Origin and role of distal visceral endoderm, a group of cells that determines anterior-posterior polarity of the mouse embryo. Nat Cell Biol. 2011;13:743–752. doi: 10.1038/ncb2251. [DOI] [PubMed] [Google Scholar]
- 55.Meno C, Gritsman K, Ohishi S, et al. Mouse lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell. 1999;4:287–298. doi: 10.1016/S1097-2765(00)80331-7. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto M, Saijoh Y, Perea-Gomez A, et al. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature. 2004;428:387–392. doi: 10.1038/nature02418. [DOI] [PubMed] [Google Scholar]
- 57.Finley KR, Tennessen J, Shawlot W. The mouse Secreted frizzled-related protein 5 gene is expressed in the anterior visceral endoderm and foregut endoderm during early post-implantation development. Gene Expr Patterns. 2003;3:681–684. doi: 10.1016/S1567-133X(03)00091-7. [DOI] [PubMed] [Google Scholar]
- 58.Kemp C, Willems E, Abdo S, et al. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005;233:1064–1075. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- 59.Perea-Gomez A, Camus A, Moreau A, et al. Initiation of gastrulation in the mouse embryo is preceded by an apparent shift in the orientation of the anterior-posterior axis. Curr Biol. 2004;14:197–207. doi: 10.1016/S0960-9822(04)00044-2. [DOI] [PubMed] [Google Scholar]
- 60.Kimura C, Yoshinaga K, Tian E, et al. Visceral endoderm mediates forebrain development by suppressing posteriorizing signals. Dev Biol. 2000;225:304–321. doi: 10.1006/dbio.2000.9835. [DOI] [PubMed] [Google Scholar]
- 61.Perea-Gomez A, Vella FDJ, Shawlot W, et al. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell. 2002;3:745–756. doi: 10.1016/S1534-5807(02)00321-0. [DOI] [PubMed] [Google Scholar]
- 62.Thomas P, Beddington R. Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Curr Biol. 1996;6:1487–1496. doi: 10.1016/S0960-9822(96)00753-1. [DOI] [PubMed] [Google Scholar]
- 63.Perea-Gomez Rhinn M, Ang SL. Role of the anterior visceral endoderm in restricting posterior signals in the mouse embryo. Int J Dev Biol. 2001;45:311–320. [PubMed] [Google Scholar]
- 64.Perea-Gomez Lawson KA, Rhinn M, et al. Otx2 is required for visceral endoderm movement and for the restriction of posterior signals in the epiblast of the mouse embryo. Development. 2001;128:753–765. doi: 10.1242/dev.128.5.753. [DOI] [PubMed] [Google Scholar]
- 65.Brennan J, Lu CC, Norris DP, et al. Nodal signalling in the epiblast patterns the early mouse embryo Nature. 2001;8716:965–969. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- 66.Tortelote GG, Hernández-Hernández JM, Quaresma AJC, et al. Wnt3 function in the epiblast is required for the maintenance but not the initiation of gastrulation in mice. Dev Biol. 2013;374:164–173. doi: 10.1016/j.ydbio.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barrow JR, Howell WD, Rule M, et al. Wnt3 signaling in the epiblast is required for proper orientation of the anteroposterior axis. Dev Biol. 2007;312:312–320. doi: 10.1016/j.ydbio.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 68.Tam PP, Loebel DA, Tanaka SS. Building the mouse gastrula: signals, asymmetry and lineages. Curr Opin Genet Dev. 2006;16:419–425. doi: 10.1016/j.gde.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Rivera-Pérez JA, Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Mohamed OA, Clarke HJ, Dufort D. β-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn. 2004;231:416–424. doi: 10.1002/dvdy.20135. [DOI] [PubMed] [Google Scholar]
- 71.Kelly OG. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 72.Andre P, Song H, Kim W, et al. Wnt5a and Wnt11 regulate mammalian anterior-posterior axis elongation. Development. 2015;142:1516–1527. doi: 10.1242/dev.119065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoshino H, Shioi G, Aizawa S. AVE protein expression and visceral endoderm cell behavior during anterior-posterior axis formation in mouse embryos: asymmetry in OTX2 and DKK1 expression. Dev Biol. 2015;402:175–191. doi: 10.1016/j.ydbio.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 74.Schneider VA, Mercola M. Spatially distinct head and heart inducers within the Xenopus organizer region. Curr Biol. 1999;9:800–809. doi: 10.1016/S0960-9822(99)80363-7. [DOI] [PubMed] [Google Scholar]
- 75.Schneider and Mercola Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mazzotta S, Neves C, Bonner RJ, et al. Distinctive roles of canonical and noncanonical Wnt signaling in human embryonic cardiomyocyte development. Stem Cell Rep. 2016;7:764–776. doi: 10.1016/j.stemcr.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marvin MJ, Di Rocco G, Gardiner A, et al. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palpant NJ, Pabon L, Roberts M, et al. Inhibition of β-catenin signaling respecifies anterior-like endothelium into beating human cardiomyocytes. Development. 2015;128:e1.2. doi: 10.1242/jcs.180588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minami I, Yamada K, Otsuji TG, et al. A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep. 2012;2:1448–1460. doi: 10.1016/j.celrep.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 80.Münsterberg AE, Kitajewski J, Bumcrot DA, et al. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- 81.Capdevila J, Tabin C, Johnson RL. Control of dorsoventral somite patterning by Wnt-1 and β-catenin. Dev Biol. 1998;193:182–194. doi: 10.1006/dbio.1997.8806. [DOI] [PubMed] [Google Scholar]
- 82.Ikeya M, Takada S. Wnt signaling from the dorsal neural tube is required for the formation of the medial dermomyotome. Development. 1998;125:4969–4976. doi: 10.1242/dev.125.24.4969. [DOI] [PubMed] [Google Scholar]
- 83.Tajbakhsh S, Borello U, Vivarelli E, et al. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998;125:4155–4162. doi: 10.1242/dev.125.21.4155. [DOI] [PubMed] [Google Scholar]
- 84.Cossu G, Borello U. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999;18:6867–6872. doi: 10.1093/emboj/18.24.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takada S, Stark KL, Shea MJ, et al. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- 86.Dunty WC, Biris KK, Chalamalasetty RB, et al. Wnt3a/β-catenin signaling controls posterior body development by coordinating mesoderm formation and segmentation. Development. 2007;135:85–94. doi: 10.1242/dev.009266. [DOI] [PubMed] [Google Scholar]
- 87.Yamaguchi TP, Takada S, Yoshikawa Y, et al. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herrmann BG. Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development. 1991;113:913–917. doi: 10.1242/dev.113.3.913. [DOI] [PubMed] [Google Scholar]
- 89.Chapman DL, Agulnik I, Hancock S, et al. Tbx6, a mouse T-box gene implicated in paraxial mesoderm formation at gastrulation. Dev Biol. 1996;180:534–542. doi: 10.1006/dbio.1996.0326. [DOI] [PubMed] [Google Scholar]
- 90.Javali A, Misra A, Leonavicius K, et al. Co-expression of Tbx6 and Sox2 identifies a novel transient neuromesoderm progenitor cell state. Development. 2017;144:4522–4529. doi: 10.1242/dev.153262. [DOI] [PubMed] [Google Scholar]
- 91.Boulet AM, Capecchi MR. Signaling by FGF4 and FGF8 is required for axial elongation of the mouse embryo. Dev Biol. 2012;371:235–245. doi: 10.1016/j.ydbio.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001;1:37–49. doi: 10.1016/S1534-5807(01)00017-X. [DOI] [PubMed] [Google Scholar]
- 93.Martin BL, Kimelman D. Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev Cell. 2012;22:223–232. doi: 10.1016/j.devcel.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Turner DA, Hayward PC, Baillie-Johnson P, et al. Wnt/β-catenin and FGF signalling direct the specification and maintenance of a neuromesodermal axial progenitor in ensembles of mouse embryonic stem cells. Development. 2014;141:4243–4253. doi: 10.1242/dev.112979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garriock RJ, Chalamalasetty RB, Kennedy MW, et al. Lineage tracing of neuromesodermal progenitors reveals novel Wnt-dependent roles in trunk progenitor cell maintenance and differentiation. Development. 2015;142:1628–1638. doi: 10.1242/dev.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gouti M, Tsakiridis A, Wymeersch FJ, et al. In vitro generation of neuromesodermal progenitors reveals distinct roles for Wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol. 2014 doi: 10.1371/journal.pbio.1001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cambray N, Wilson V. Two distinct sources for a population of maturing axial progenitors. Development. 2007;134:2829–2840. doi: 10.1242/dev.02877. [DOI] [PubMed] [Google Scholar]
- 98.Tzouanacou E, Wegener A, Wymeersch FJ, et al. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell. 2009;17:365–376. doi: 10.1016/j.devcel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 99.McGrew MJ, Sherman A, Lillico SG, et al. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development. 2008;135:2289–2299. doi: 10.1242/dev.022020. [DOI] [PubMed] [Google Scholar]
- 100.Henrique D, Abranches E, Verrier L, Storey KG. Neuromesodermal progenitors and the making of the spinal cord. Development. 2015;142:2864–2875. doi: 10.1242/dev.119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Steventon B, Martinez Arias A. Evo-engineering and the cellular and molecular origins of the vertebrate spinal cord. Dev Biol. 2017;432:3–13. doi: 10.1016/j.ydbio.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 102.Chapman DL, et al. Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature. 1998;391:695–697. doi: 10.1038/35624. [DOI] [PubMed] [Google Scholar]
- 103.King T, Beddington RSP, Brown NA. The role of the brachyury gene in heart development and left-right specification in the mouse. Mech Dev. 1998;79:29–37. doi: 10.1016/S0925-4773(98)00166-X. [DOI] [PubMed] [Google Scholar]
- 104.Kitaguchi T, Mizugishi K, Hatayama M, et al. Xenopus Brachyury regulates mesodermal expression of Zic3, a gene controlling left-right asymmetry. Dev Growth Differ. 2002;44:55–61. doi: 10.1046/j.1440-169x.2002.00624.x. [DOI] [PubMed] [Google Scholar]
- 105.Hadjantonakis AK, Pisano E, Papaioannou VE. Tbx6 regulates left/right patterning in mouse embryos through effects on nodal cilia and perinodal signaling. PLoS One. 2008 doi: 10.1371/journal.pone.0002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu P, Wakamiya M, Shea MJ, et al. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 107.Galceran J, Fariñas I, Depew MJ, et al. Wnt3a−/−-like phenotype and limb deficiency in Lef−/−Tcf1−/− mice. Genes Dev. 1999;13:709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Robertis EM. Evo-Devo: variations on ancestral themes. Cell. 2008;132:185–195. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niehrs C. On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137:845–857. doi: 10.1242/dev.039651. [DOI] [PubMed] [Google Scholar]
- 110.Petersen CP, Reddien PW. wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 111.Ikeya M, Takada S. Wnt-3a is required for somite specification along the anteroposterior axis of mouse embryo and for regulation of Cdx-1 expression. Mech Dev. 2001;103:27–33. doi: 10.1016/S0925-4773(01)00338-0. [DOI] [PubMed] [Google Scholar]
- 112.Nordström U, Maier E, Jessell TM, Edlund T. An early role for Wnt signaling in specifying neural patterns of Cdx and Hox gene expression and motor neuron subtype identity. PLoS Biol. 2006;4:1438–1452. doi: 10.1371/journal.pbio.0040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pilon N, Oh K, Sylvestre JR, et al. Cdx4 is a direct target of the canonical Wnt pathway. Dev Biol. 2006;289:55–63. doi: 10.1016/j.ydbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 114.Shimizu T, Bae YK, Muraoka O, Hibi M. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev Biol. 2005;279:125–141. doi: 10.1016/j.ydbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 115.van de Ven C, Bialecka M, Neijts R, et al. Concerted involvement of Cdx/Hox genes and Wnt signaling in morphogenesis of the caudal neural tube and cloacal derivatives from the posterior growth zone. Development. 2011;138:3859. doi: 10.1242/dev.072462. [DOI] [PubMed] [Google Scholar]
- 116.van den Akker E, Forlani S, Chawengsaksophak K, et al. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development. 2002;129:2181–2193. doi: 10.1242/dev.129.9.2181. [DOI] [PubMed] [Google Scholar]
- 117.Neijts R, Amin S, van Rooijen C, Deschamps J. Cdx is crucial for the timing mechanism driving colinear Hox activation and defines a trunk segment in the Hox cluster topology. Dev Biol. 2017;422:146–154. doi: 10.1016/j.ydbio.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 118.Young T, Rowland JE, van de Ven C, et al. Cdx and hox genes differentially regulate posterior axial growth in mammalian embryos. Dev Cell. 2009;17:516–526. doi: 10.1016/j.devcel.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 119.van Rooijen C, Simmini S, Bialecka M, et al. Evolutionarily conserved requirement of Cdx for post-occipital tissue emergence. Development. 2012;139:2576–2583. doi: 10.1242/dev.079848. [DOI] [PubMed] [Google Scholar]
- 120.Ciruna BG, Rossant J. Expression of the T-box gene eomesodermin during early mouse development. Mech Dev. 1999;81:199–203. doi: 10.1016/S0925-4773(98)00243-3. [DOI] [PubMed] [Google Scholar]
- 121.Saga Y, Hata N, Taketo MM, et al. MesP1: a novel basic helix-loop-helix protein expressed in the nascent mesodermal cells during mouse gastrulation. Development. 1996;122:2769–2778. doi: 10.1242/dev.122.9.2769. [DOI] [PubMed] [Google Scholar]
- 122.Saga Y, Miyagawa-Tomita S, Takagi A, et al. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- 123.Harel I, Nathan E, Tirosh-Finkel L, et al. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell. 2009;16:822–832. doi: 10.1016/j.devcel.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kitajima S, Takagi A, Inoue T, Saga Y. MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development. 2000;127:3215–3226. doi: 10.1242/dev.127.15.3215. [DOI] [PubMed] [Google Scholar]
- 125.Chan SSK, Shi X, Toyama A, et al. Mesp1 patterns mesoderm into cardiac, hematopoietic, or skeletal myogenic progenitors in a context-dependent manner. Cell Stem Cell. 2013;12:587–601. doi: 10.1016/j.stem.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Satou Y, et al. The ascidian Mesp gene specifies heart precursor cells. Development. 2004;131:2533–2541. doi: 10.1242/dev.01145. [DOI] [PubMed] [Google Scholar]
- 127.Costello I, Pimeisl IM, Dräger S, et al. The T-box transcription factor eomesodermin acts upstream of Mesp1 to specify cardiac mesoderm during mouse gastrulation. Nat Cell Biol. 2011;13:1084–1092. doi: 10.1038/ncb2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arnold SJ, Hofmann UK, Bikoff EK, Robertson EJ. Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Development. 2008;135:501–511. doi: 10.1242/dev.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bondue A, Blanpain C. Mesp1: a key regulator of cardiovascular lineage commitment. Circ Res. 2010;107:1414–1427. doi: 10.1161/CIRCRESAHA.110.227058. [DOI] [PubMed] [Google Scholar]
- 130.Hacker A, Guthrie S. A distinct developmental programme for the cranial paraxial mesoderm in the chick embryo. Development. 1998;125:3461–3472. doi: 10.1242/dev.125.17.3461. [DOI] [PubMed] [Google Scholar]
- 131.Rios AC, Serralbo O, Salgado D, Marcelle C. Neural crest regulates myogenesis through the transient activation of NOTCH. Nature. 2011;473:532–535. doi: 10.1038/nature09970. [DOI] [PubMed] [Google Scholar]
- 132.Köntges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- 133.Grenier J, Teillet MA, Grifone R, et al. Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS One. 2009 doi: 10.1371/journal.pone.0004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rinon A, Lazar S, Marshall H, et al. Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development. 2007;134:3065–3075. doi: 10.1242/dev.002501. [DOI] [PubMed] [Google Scholar]
- 135.Rios AC, Marcelle C. Head muscles: aliens who came in from the cold? Dev Cell. 2009;16:779–780. doi: 10.1016/j.devcel.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 136.Kuratani S, Schilling T. Head segmentation in vertebrates. Integr Comp Biol. 2008;48:604–610. doi: 10.1093/icb/icn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Onai T, Adachi N, Kuratani S. Metamerism in cephalochordates and the problem of the vertebrate head. Int J Dev Biol. 2017;61:621–632. doi: 10.1387/ijdb.170121to. [DOI] [PubMed] [Google Scholar]
- 138.Holland LZ, Holland ND, Gilland E. Amphioxus and the evolution of head segmentation. Integr Comp Biol. 2008;48:630–646. doi: 10.1093/icb/icn060. [DOI] [PubMed] [Google Scholar]
- 139.Aldea D, Subirana L, Keime C, et al. Genetic regulation of amphioxus somitogenesis informs the evolution of the vertebrate head mesoderm. Nat Ecol Evol. 2019 doi: 10.1038/s41559-019-0933-z. [DOI] [PubMed] [Google Scholar]
- 140.Bertrand S, Camasses A, Somorjai I, et al. Amphioxus FGF signaling predicts the acquisition of vertebrate morphological traits. Proc Natl Acad Sci. 2011;108:9160–9165. doi: 10.1073/pnas.1014235108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Holland ND, Venkatesh TV, Holland LZ, et al. AmphiNk2-tin, an amphioxus homeobox gene expressed in myocardial progenitors: insights into evolution of the vertebrate heart. Dev Biol. 2003;255:128–137. doi: 10.1016/S0012-1606(02)00050-7. [DOI] [PubMed] [Google Scholar]
- 142.Pascual-Anaya J, Albuixech-Crespo B, Somorjai IML, et al. The evolutionary origins of chordate hematopoiesis and vertebrate endothelia. Dev Biol. 2013;375:182–192. doi: 10.1016/j.ydbio.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 143.Achim K, Arendt D. Structural evolution of cell types by step-wise assembly of cellular modules. Curr Opin Genet Dev. 2014;27:102–108. doi: 10.1016/j.gde.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 144.Brunet T, Fischer AHL, Steinmetz PRH, et al. The evolutionary origin of bilaterian smooth and striated myocytes. Elife. 2016;5:1–24. doi: 10.7554/eLife.19607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Amacher SL, Draper BW, Summers BR, Kimmel CB. The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development. 2002;3323:3311–3323. doi: 10.1242/dev.129.14.3311. [DOI] [PubMed] [Google Scholar]
- 146.Baillie-johnson P, Hayward P. The chick caudolateral epiblast acts as a permissive niche for generating neuromesodermal progenitor behaviours. Cell Tissue Organs. 2018 doi: 10.1159/000494769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Attardi A, Fulton T, Florescu M, et al. Correction: neuromesodermal progenitors are a conserved source of spinal cord with divergent growth dynamics. Development. 2019;146:dev175620. doi: 10.1242/dev.175620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ansari S, Troelenberg N, Dao VA, et al. Double abdomen in a short-germ insect: zygotic control of axis formation revealed in the beetle Tribolium castaneum. Proc Natl Acad Sci. 2018 doi: 10.1073/pnas.1716512115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fritzenwanker JH, Uhlinger KR, Gerhart J, et al. Untangling posterior growth and segmentation by analyzing mechanisms of axis elongation in hemichordates. Proc Natl Acad Sci. 2019 doi: 10.1073/pnas.1817496116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet. 2004;5:425–434. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- 151.De Robertis E. Wnt signaling in axial patterning and regeneration: lessons from planaria. Sci Signal. 2010;3:2008–2011. doi: 10.1126/scisignal.3127pe21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Loh KM, van Amerongen R, Nusse R. Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Dev Cell. 2016;38:643–655. doi: 10.1016/j.devcel.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 153.Yoshida T, Vivatbutsiri P, Morriss-Kay G, et al. Cell lineage in mammalian craniofacial mesenchyme. Mech Dev. 2008;125:797–808. doi: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 154.Graham A, Shimeld SM. The origin and evolution of the ectodermal placodes. J Anat. 2013;222:32–40. doi: 10.1111/j.1469-7580.2012.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Abitua PB, Wagner E, Navarrete IA, Levine M. Identification of a rudimentary neural crest in a non-vertebrate chordate. Nature. 2012;492:104–107. doi: 10.1038/nature11589. [DOI] [PMC free article] [PubMed] [Google Scholar]