Abstract
Cystic fibrosis (CF) is the most common autosomal-recessive disease in Caucasians caused by mutations in the CF transmembrane regulator (CFTR) gene. Patients are usually diagnosed in infancy and are burdened with extensive medical treatments throughout their lives. One of the first documented biochemical defects in CF, which predates the cloning of CFTR gene for almost three decades, is an imbalance in the levels of polyunsaturated fatty acids (PUFAs). The principal hallmarks of this imbalance are increased levels of arachidonic acid and decreased levels of docosahexaenoic acids (DHA) in CF. This pro-inflammatory profile of PUFAs is an important component of sterile inflammation in CF, which is known to be detrimental, rather than protective for the patients. Despite decades of intensive research, the mechanistic basis of this phenomenon remains unclear. In this review we summarized the current knowledge on the biochemistry of PUFAs, with a focus on the metabolism of AA and DHA in CF. Finally, a synthetic retinoid called fenretinide (N-(4-hydroxy-phenyl) retinamide) was shown to be able to correct the pro-inflammatory imbalance of PUFAs in CF. Therefore, its pharmacological actions and clinical potential are briefly discussed as well.
Keywords: Cystic fibrosis, Lipids, PUFAs, CFTR, Fenretinide
Polyunsaturated fatty acids and their metabolism in CF
Metabolism of PUFAs
Unsaturated fatty acids are carboxylic acids that contain one or more double bonds and more than four carbons in the acyl chain. Monounsaturated fatty acids contain one double bond, whereas polyunsaturated fatty acids (PUFAs) contain two or more double bonds. The most common naturally occurring PUFAs are ω-3 and ω-6 PUFAs. The designation ω refers to the position of the most distal double bond counted from the last carbon of the acyl chain (e.g., ω-3 PUFAs have its first double-bond three carbons away from the most distal methyl group). Alternatively, the n letter instead of the ω can be used with the same meaning and fatty acids can be designated as n-3 and n-6. For example, docosahexaenoic acid is a ω-3 fatty acid that has 22 carbons and 6 alternating double bonds counted from the most distal carbon atom and thus can be categorized as 22:6n-3. Similarly, arachidonic acid is a ω-6 PUFA that has 20 carbons and 4 alternating double bonds counted from the 20th carbon and, thus, can be labeled as 20:4n-6.
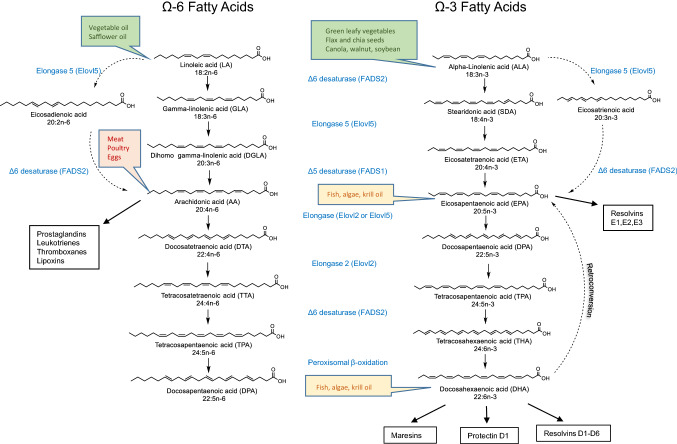
Mammals do not have the ability to introduce double bonds in fatty acids beyond carbon 9 and carbon 10 (counted from the carboxyl carbon), and therefore, they must obtain some PUFAs from their food [1]. The two essential fatty acids for humans are linoleic acid (18:2n-6) and α-linolenic acid (18:3n-3), which are metabolized through the pathways shown in Fig. 1. Therefore, linoleic acid is the precursor for AA, and α-linolenic acid is the precursor for DHA. Importantly, both AA and DHA can also be introduced in the metabolism directly from food. Indeed, AA and its precursors are found in plant-derived oils (corn, peanut, and sunflower) and red meat, while DHA is found in fish oil and algae [2], as illustrated with the coloured boxes in Fig. 1. Some earlier studies expressed concerns that rodents may not be good models to study the metabolism of DHA with relevance to humans, because microsomal desaturase activity seemed much more prominent in rats than in humans and, consequently, conversion of α-linolenic acid would occur at a much faster rate in rats than in humans [3, 4]. However, the methods used to estimate the rate of DHA production from α-linolenic acid in these two studies were not the same. To address this issue, Domenichiello et al. [5] orally administered radioactively labeled 2H5-α-linolenic acid to rats and sampled blood over a 6 h experiment to measure the amount of 2H5-DHA, and they applied calculations used previously in human studies with a single oral bolus of labeled α-linolenic acid [5]. The percentage of α-linolenic acid that was converted to DHA in this study ranged from 0.12 to 0.64%, which is not higher than the previously estimated α-linolenic acid to DHA ratio in humans [6–8]. Based on these results, rodents may represent good models for the study of unsaturated fatty acids modulation. However, the same group conducted a study with 12 week DHA supplementation in humans, and despite a 130% increase in plasma EPA concentrations, there was no change in the plasma δ13 C-EPA levels suggesting that there was no or very little retroconversion of DHA to EPA. Plasma δ13 C-EPA remained similar to plasma δ13 C-ALA, which means that, virtually, all of the increase in plasma EPA with DHA supplementation was derived from ALA, which supports the slower turnover of EPA responsible for this phenomenon [9]. Therefore, even though retroconversion of DHA to EPA appears to be a real phenomenon in both rodents and humans, the increases in plasma and tissue EPA levels following DHA supplementation are not the result of increased flux through the retroconversion pathway, but are largely due to the slowed metabolism and accumulation of EPA.
Fig. 1.
Metabolism of ω-6 and ω-3 fatty acids starting with essential fatty acids obtained from food. The food sources that are rich in specific fatty acids are shown in the boxes placed next to the name of the corresponding fatty acid
Both AA and DHA are transported in the bloodstream either in the esterified form (incorporated into triglycerides or cholesteryl esters) or as “free fatty acids” bound to albumin and/or lipoproteins, but once inside the cell, they then get incorporated into phospholipids, and other complex lipid moieties which are principal lipid constituents of cellular membranes. Upon activation of various signaling pathways initiated either by endogenous (hormones and cytokines) or exogenous factors (bacterial lipopolysaccharide), AA and DHA are released from phospholipids through the action of enzymes called phospholipases. There are three major categories of phospholipases: cytosolic phospholipases, secreted phospholipases (sPLA2), which are Ca2+-dependent and Ca2+-independent phospholipases (iPLA2), and intracellular phospholipases, cPLA2 and iPLA2.
Each of these categories of enzymes is coded by multiple paralogous genes. For example, in the human genome, there are six genes for cytosolic phospholipases (PLA2G4A, PLA2G4B, PLA2G4C, PLA2G4D, PLA2G4E, and PLA2G4F coding for cPLA2-α, cPLA2-β, cPLA2-γ, cPLA2-δ, cPLA2-ε, and cPLA2-ζ proteins respectively), 11 genes for secreted phospholipases (PLA2G2A, PLA2G2D, PLA2G2F, PLA2G3, PLA2G5, PLA2G10, PLA2G1B, PLA2G2C, PLA2G2E, PLA2G12A, and PLA2G12B coding for group IIA sPLA2, group IID sPLA2, group IIF sPLA2, group III sPLA2, group V sPLA2, group X sPLA2, sPLA2-IB, sPLA2-IIC, sPLA2-IIE, sPLA2-XIIA, and sPLA2-XIIB proteins, respectively), and six genes for iPLA2 (PNPLA9, PNPLA8, PNPLA6, PNPLA3, PNPLA2, and PNPLA4 coding for iPLA2β, iPLA2γ, iPLA2δ, iPLA2ε, iPLA2ζ and iPLA2η proteins, respectively) [10, 11].
AA is released from the phospholipids mostly through the actions of cPLA2 and sPLA2 enzymes [10, 11] while DHA can be released through the action of iPLA2 enzymes [12, 13] and sPLA2 [14, 15] (Fig. 2). Interestingly, once released from the membrane, DHA can inhibit cPLA2 and thus limit the release of AA [16].
Fig. 2.
Subcellular metabolism of ω-6 and ω-3 fatty acids starting with essential fatty acids obtained from food (LA and ALA) and from the complex phospholipid membrane (AA and DHA)
Once released from the membrane, AA and DHA are metabolized through a series of enzymatic steps into molecules with potent pro- and anti-inflammatory properties.
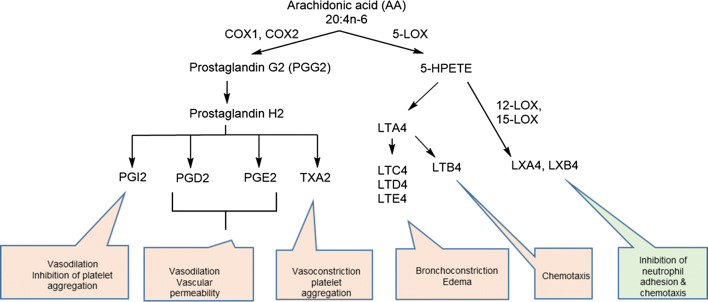
AA is the substrate for cyclooxygenases: constitutively expressed COX-1 and inflammation induced COX-2 are both well-known targets of aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) [17]. These two enzymes initially convert AA into an unstable intermediate called prostaglandin H2, which is converted to diverse biologically active prostanoids depending on the presence of different tissue-specific isomerases. These are prostaglandins (PGE2, PGD2, and PGF2α), prostacyclin (PGI2), and thromboxanes (TXA2 and TXB2) [18]. AA is also the substrate for lipooxygenases (5-LOX, 12-LOX and 15-LOX), which convert it into leukotrienes, lipoxins, and hepoxilins [19]. 5-LOX produces leukotriene A4 (LTA4), which leads to cysteinyl-leukotrienes (LTC4, LTD4, and LTE4), leukotriene B4 (LTB4), and lipoxins (LXA4 and LXB4). 12-LOX converts AA into 12-hydroxyeicosatetraenoic acid (12-HETE), and 15-LOX converts AA into LXA4 and LXB4 [20]. Indeed, LXA4 exerts potent anti-inflammatory activities most notably by counteracting LTB4-induced neutrophil migration [21–23] and mice treated with LXA4 analogs resolved P. aeruginosa lung infection more efficiently than placebo-treated controls [24]. Importantly, the LXA4/LTB4 ratio is strongly decreased in the lower airways of children with CF [25] (Figs. 3 and 4)
Fig. 3.
Lipid mediators derived from arachidonic acid. Most of the lipid mediators derived from arachidonic acid have pro-inflammatory properties (red), with the notable exception of lipoxins which are anti-inflammatory (green). In one branch of the pathway, arachidonic acid is a substrate for cyclooxygenases and resulting mediators are prostacyclin, prostaglandins, and thromboxanes, all of which have pro-inflammatory properties. Alternatively, arachidonic acid serves as the substrate for 5-lipooxygenase, which leads to the synthesis of leukotrienes and lipoxins. Lipoxins are the only known anti-inflammatory lipid mediators derived from arachidonic acid
Fig. 4.
Lipid mediators derived from Ω-3 fatty acids. All known lipid mediators derived from Ω-3 fatty acids have anti-inflammatory properties. EPA-derived mediators are resolvins of E series, whereas resolvins of D series, maresins, and protectins are derived from DPA and DHA through the series of intermediates. All of these intermediary compounds are short-living, unstable species, and their physiological functions, if any, remain unknown
All secondary metabolites produced from AA, with the notable exception of lipoxins, are potent pro-inflammatory molecules. The activation of numerous G-protein coupled receptors (GPCRs) through which these derivatives of AA act leads to the well-known hallmarks of inflammation, such as synthesis of pyrogenic cytokines which raise the body’s temperature, increased chemotaxis of neutrophils, and degranulation of platelets at their site of production [26].
On the other hand, all known metabolites of DHA have anti-inflammatory properties [27]. Once released from the membrane by the action of phospholipases, DHA serves as the substrate for 15-LOX and 12-LOX enzymes. 15-LOX converts DHA into the intermediate 17S-hydroperoxy-DHA which is further converted by neutrophil-derived 5-LOX to the D series of resolvins (RvD1–RvD4) [28, 29]. DHA can also be converted by 15-LOX into 16, 7-epoxy-protectin intermediate and then to protectin D1 [28, 29]. Finally, 12-LOX in macrophages converts DHA into maresins (Macrophage Mediators in Resolving Inflammation) [30, 31]. Resolvins, protectins, and maresins are potent anti-inflammatory molecules that also act through specific GPCRs and activate multiple signaling pathways that lead to the resolution of inflammation [27]. These include reduction of neutrophils chemotaxis, stimulation of phagocytosis of apoptotic neutrophils, clearance of allergens, and many others [27]. A detailed discussion of the physiological actions and biosynthetic pathways of these metabolites can be found in the other recent excellent reviews on this topic [32–36].
PUFAs in CF
The first association of abnormal metabolism of PUFAs with CF was made back in 1962 when Kuo et al. [37] described abnormally low levels of linoleic acid (18:2n-6) in the plasma and tissues of children with CF. The next two important milestones were the discoveries that the proportion of DHA to AA is decreased in different tissues of CF patients examined post mortem [38], whereas the proportion of AA to DHA is increased in leukocytes [39–41]. These historical findings were later confirmed in both CF patients [42] and CFTR-KO mice [43, 44]. Taking into consideration the previously discussed pro- and anti-inflammatory roles of AA and DHA, respectively, these findings collectively defined what is nowadays known as a pro-inflammatory imbalance of fatty acids in CF, which is also frequently illustrated as a high AA/DHA or low DHA/AA ratio.
However, with the cloning of the CFTR gene in 1989, which sparked the hope that the genetic defect itself could be corrected by gene therapy, the initial description of pro-inflammatory imbalance of fatty acids fell into obscurity. New interest in this field was rekindled in the early 2000s with the finding that the dietary supplementation with DHA of cftr −/− mice ameliorated many of the CF-related pathologic phenotypes, including reduction of villi hypertrophy, reversal of pancreatic duct dilation, and most importantly, restrained LPS-stimulated pulmonary inflammation [43, 45]. Although an improvement of liver function in mice was observed [43, 45–47], the supplementation of DHA alone and other n-3 PUFAs alone did not confer a significant clinical benefit to patients with CF [48–50]. Nevertheless, it was shown that the pharmacological correction of the pro-inflammatory AA/DHA ratio by fenretinide that leads to the simultaneous decrease in AA and increase in DHA (which may not be achieved with DHA treatment alone) resulted in the significantly improved ability of cftr −/− mice to resolve pulmonary infection with P. aeruginosa [44].
For a long time, it was thought that the pro-inflammatory imbalance of fatty acids observed in patients with CF is the consequence of pancreatic insufficiency and the resultant lipid malabsorption [51–53]. However, several independent lines of evidence undoubtedly disproved the malabsorption hypothesis. First, fatty acid imbalance persists in CF patients in whom pancreatic insufficiency has been successfully corrected with enzyme replacement and nutritional support [54, 55]. Second, the pro-inflammatory imbalance of fatty acids is seen in cftr −/− mice [43, 44], mostly in tissues that in littermate, wt (cftr + / +) mice express high levels of CFTR protein such as the lungs, pancreas, and intestine, but not in the brain, kidney, or heart [43, 56]. Finally, the presence of the pro-inflammatory imbalance of fatty acids in cultured cells derived from the lungs of CF patients demonstrates that this phenomenon is not secondary to pancreatic insufficiency or pathogen-induced lung inflammation, but is indeed causally linked to lack of functional CFTR protein and is, therefore, an intrinsic defect of CF [57, 58].
Several non-conflicting hypotheses were proposed to explain the pro-inflammatory imbalance of fatty acids in CF, but none of them provides a mechanistic connection between the absence of functional CFTR protein and high AA/DHA ratio. Historically, the first described abnormality of fatty acids in CF, i.e., the LA deficiency, as well as the increased level of AA could simply be explained by the rapid turnover of LA into AA [59]. Indeed, increased conversion of radioactively labeled LA into AA was demonstrated in CF cells compared to wild-type controls [60]. These changes parallel with the increased expression of Δ5- and Δ6-desaturases (coded by FADS1 and FADS2 genes, respectively) and could be induced by the treatment of cells with a small molecule inhibitor of CFTR (CFTRinh-172). However, these results do not fully explain the low levels of DHA observed in CF.
Interestingly, DHA is known to suppress the expression of Δ5- and Δ6-desaturases in cultured cells [61] and mice [43, 56], but the causal connection between CFTR deficiency and low DHA levels remains unclear. It was demonstrated that the metabolism of EPA to DHA is decreased in cultured CF cells [60], although in that study, the levels of 22:5n-3 (DPA), an immediate downstream product of EPA, were not decreased. On the other hand, retroconversion of DHA to EPA which occurs through the modified β-oxidation in peroxisomes [62, 63] was also described in CF [61]. Abnormal metabolism of phosphatidylcholine (PC) was proposed as a possible explanation for lower levels of DHA in CF. PC formed de novo through the methylation of phosphatidylethanolamine (PE) is known to have higher DHA content than PC formed using choline derived from the diet [64, 65]. CF cells appear to have a defect in the metabolism of methyl groups; therefore, the de novo synthesis of PC is favored, thereby depleting DHA levels [66, 67]. Whatever the mechanism of DHA deficiency in CF is, it is clear that this abnormality may result in a lower level of anti-inflammatory lipid mediators derived from DHA and EPA, as it was indeed reported for Resolvin E1 in CF patients, impairing their ability to resolve inflammatory responses induced by frequent lung infections [68]. Consistent with the anti-inflammatory role not only of DHA, but also of other metabolites from the n-3 pathway, lipidomic analysis of plasma samples revealed that the higher levels of docosapentaenoic acid (DPA) showed a very strong positive correlation with higher FEV1 in patients with CF, whereas lower levels of α-linolenic acid were associated with chronic infection with P. aeruginosa [69].
Finally, numerous studies have convincingly demonstrated that the increased release of AA in CF cells from the cell membrane is mediated by cytosolic phospholipases (cPLA2) [39, 70–73]. In this scenario, the increased conversion of LA to AA could be merely a compensatory response to this change, which inadvertently contributes to even higher levels of AA. The association between these two pathways, if any, remains to be explored.
Nevertheless, it has never been established which one out of the six cytosolic phospholipases contributes the most to the increased levels of AA. We examined the mRNA levels of all six cytosolic phospholipases in CFBE 41o-cells derived from a ΔF508/ΔF508 patient and we could not observe any difference in mRNA between parental cells and those transfected with wt-CFTR protein [58]. However, this leaves an open possibility that the protein levels and/or activities of cPLA2 enzymes are affected by posttranscriptional modifications. Indeed, the treatment of CFBE41o- parental cells with fenretinide, an orphan drug which was previously reported to decrease the level of AA and increase the level of DHA [44], was found to decrease the total cPLA2 activity assayed by colorimetric assay [58]. The mechanism of this change remains to be further explored.
Finally, an important role for anti-inflammatory metabolites of DHA in CF is starting to emerge. Indeed, resolvin D1 (RvD1) has been detected in the plasma and sputum of CF patients, and an increased RvD1/IL-8 ratio is associated with a better pulmonary function and higher FEV1 in CF patients [74]. Interestingly, RvD1 can increase airway surface liquid height in CF bronchial epithelial cells, restoring the nasal trans-epithelial potential difference in CF mice by decreasing the amiloride-sensitive sodium absorption and by stimulating CFTR-independent chloride secretion [75]. RvD1 decreased TNFα-induced IL-8 secretion and enhanced the phagocytic and bacterial killing capacity of alveolar macrophages from CF patients [75].
Taken together, these studies underline the importance of restoring the normal levels of DHA and AA in patients with CF and suggest that CF patients would benefit from such a biochemical correction.
Pro-inflammatory fatty acid imbalance in CF and its normalization by treatment with fenretinide
Understanding the mechanisms of pro-inflammatory fatty acid imbalance in CF is of direct relevance to dietary therapy [76, 77]. Unfortunately, the typical modern Western diet contains a high ratio of ω-6 to ω-3 PUFAs [78, 79], and, therefore, may exacerbate the already present intrinsic lipid imbalance resulting from high AA and low DHA production in CF patients. This, in turn, may result in an increased production of pro-inflammatory lipid mediators either triggered directly by AA-mediated phosphorylation of signaling molecules such as phospho-ERK or via decreased production of anti-inflammatory lipid mediators for which DHA serves as the starting substrate, such as D-class resolvins. Unfortunately, supplementation of DHA alone and other n-3 PUFAs alone did not confer a significant clinical benefit to patients with CF [48–50]. The outcomes of these trials raise many questions regarding the physiological role of not only AA and DHA but also of their precursors and downstream metabolites. For example, dihommo-γ-linolenic acid (DGLA) is a known substrate for cyclooxygenases, giving rise to PGE1 and for 15-LOX, increasing 15-hydroxy-eicosatrienoic acid levels, both of which are anti-inflammatory eicosanoids [80]. Additionally, the supplementation of γ-linolenic acid, the immediate upstream precursor of dihommo-γ-linolenic acid, was shown to decrease the production of pro-inflammatory LTB4 by neutrophils ex vivo [81–83]. Several studies involving an increased intake of GLA reported an increment of DGLA in PMBCs and neutrophils as expected, but surprisingly found no effect on the levels of AA [81, 82, 84–86]. An epidemiological study assessing 1123 subjects in Italy found no correlation between the concentrations of LA in plasma and eight markers of inflammation (IL-6, soluble IL-6R, IL-1β, IL-1 receptor antagonist, TNF-α, IL-10, TGF-β, and CRP) [87]. Although subjects in the lowest quartile of plasma LA concentrations had the highest concentrations of pro-inflammatory markers (IL-6 and CRP) and the lowest concentrations of anti-inflammatory cytokines (IL-10 and TGF-β) [87]. Finally, even though AA is a known downstream metabolite of LA, increased levels of LA are not necessarily associated with high levels of AA [88–91] and it is still unknown why. This clearly implies that our linear view of PUFA metabolism was oversimplified thus far.
Fenretinide [4-hydroxy(phenyl)retinamide; 4-HPR] is a synthetic analog of retinoic acid which has a phenyl moiety instead of carboxylic group linked through an amide bond (Fig. 5).
Fig. 5.
Structures of fenretinide and retinoic acid
We have previously reported that 5–10 mg/kg doses of fenretinide correct pro-inflammatory lipid imbalance in CFTR-KO mice by decreasing the levels of AA while simultaneously upregulating the level of DHA [44]. Fenretinide was used in the long-term chemopreventive trial (5 years daily) at a 100 mg per day dosage in patients with breast cancers [92] and relatively high doses (several times higher) for treatment of patients with neuroblastoma [93]. In both cases, fenretinide was shown to be a relatively safe agent and the most common adverse effects reported were minor and reversible visual disturbances such as diminished dark adaptation. This adverse effect is most likely due to the ability of fenretinide to decrease endogenous retinol concentrations by competing for the same transport protein RBP4 [94]. Fenretinide binds strongly to RBP4, but this complex does not bind to transthyretin [95]. However, fenretinide–RBP4 complex is eliminated through renal filtration, thereby depleting the level of RBP4 necessary to transport retinol, which eventually results in lower retinol levels in fenretinide-treated patients [95]. However, a few days of interruption of treatment after every 4 weeks of treatment to recuperate the RBP4 levels were sufficient to resolve this issue.
Similarly to retinoic acid, which is a physiologically active metabolite of retinol, fenretinide binds to retinoid receptors (RARs), which belong to the family of nuclear receptors. Upon binding of their ligand (which may either be all-trans-retinoic acid or 9-cis retinoic acid), several isoforms of RARs, known as RAR-α, -β, and -γ, bind to the DNA sequence known as retinoic acid receptor elements (RAREs) and regulate transcription of hundreds of genes [96]. On the other hand, retinoid X receptors (RXR-α, -β, and -γ) bind only 9-cis retinoic acid [97]. Fenretinide was found to bind strongly to RARγ, weakly to RARβ [98, 99] and not at all to RXRα [99]. Additionally, fenretinide activates transcription by a nuclear receptor called PPARγ [100, 101], although there is no evidence for the direct interaction with PPARγ. The consequences of these actions are certainly cell-type dependent and result in the down-regulation or up-regulation of the transcription of the genes normally controlled by these nuclear receptors. Importantly, PPARγ receptor has long been known to be reduced in CF [102–104] and pharmacological activation of PPARγ by rosiglitazone was shown to confer some therapeutic benefits in CF mice [105]. However, rosiglitazone has been recalled from the market due to serious side effects such as elevated risk of heart attacks in diabetic patients [106]. Fenretinide was found to prevent the activation of the NF–κB pathway by inhibiting the phosphorylation of IκBα kinase [107] and to inhibit phosphorylation of Erk kinase [108], thereby inhibiting the pathways generated from multiple Tyr-phosphorylated growth factor receptors.
Due to its pleiotropic effects, outlined above, it is challenging to pinpoint the exact mechanism whereby fenretinide affects the activity of cytosolic phospholipases. Additionally, fenretinide was found to increase the level of anti-inflammatory very-long-chain ceramides while decreasing the levels of pro-inflammatory long-chain ceramides and this effect could be attributed to the down-regulation of Cers5 enzyme [109].
Even though the exact mechanism of fenretinide action on different lipid species is still not fully elucidated, fenretinide is a safe agent for the pharmacological correction of aberrant lipid metabolism in patients with CF. Long-term safety of fenretinide was well established in the population of breast cancer patients [110, 111]. The safety profile of fenretinide in CF patients was already evaluated in a Phase Ib clinical trial with a novel orally bioavailable formulation of fenretinide (LAU-7B). The reassuring safety profile and excellent pharmacokinetics of the LAU-7B formulation obtained in a dose escalation (100, 200, and 300 mg per day) Phase Ib trial, led to the Phase II (APPLAUD) trial (NCT03265288). This is a currently ongoing international Phase II, double-blind, randomized, placebo-controlled study to confirm the safety of long-term (6 months) treatment using 300 mg once-daily dosage and to evaluate the efficacy of LAU-7b in CF patients.
Finally, since the signaling through retinoid receptors and NF–κB pathway is known to be essential for constitutive and pathogen-induced expression of mucin genes [112–114], we examined how fenretinide treatment affects the expression of MU5AC and MUC5B genes in a goblet cell line derived from rat lungs. Interestingly, our recent results demonstrate that the low dose of fenretinide (1.25 µM) selectively prevents MUC5AC overexpression, without significantly affecting MUC5B expression [58, 115]. These results are especially important considering significant biological differences between MUC5AC and MUC5B [116, 117]. It was recently demonstrated that MUC5B is required for mucociliary clearance and immunological defense of the lungs, whereas MUC5AC is dispensable and is, in fact, responsible for pathological plugging and airway obstruction [117]. Nevertheless, the mechanistic connection between pro-inflammatory lipid imbalance and the regulation of mucin gene expression remains unexplored. Most likely, such a connection would be indirect, through the metabolites of fatty acids such as eicosanoids [118] or 12(R)-HETE [119].
Conclusion
In conclusion, the pro-inflammatory imbalance of PUFAs reflected in a high AA/DHA ratio is an important component of sterile inflammation which is detrimental for the lung defense of CF patients. This underexplored biochemical aspect of CF may lead to the development of novel therapeutic interventions in the future. To this end, fenretinide holds promise as a safe pharmacological agent for the correction of abnormal lipid metabolism and as a novel mucoregulatory agent.
Acknowledgements
We want to acknowledge financial support from Canadian Institutes of Health Research (POP90155, account 6071), Canadian Cystic Fibrosis Foundation via McGill University (account 3645), PSVT2B Grant 2768 (Ministère de l'Économie et de l’Innovation via ALIGO Innovation), and the Department for Administration and Development project, Ministry of Education, Youth and Sport, Czech Republic, Molecular and Cellular Clinical Approach to Healthy Ageing (ENOCH project) via IMTM (CZ.02.1.01/0.0/0.0/16_019/0000868 and LM2015064).
Author contributions
DG and DR wrote the manuscript. DCD created the figures and edited the text. JS and JBdS edited the text, performed literature search, and provided valuable comments.
Compliance with ethical standards
Conflict of interest
Dr. Danuta Radzioch is a scientific advisor and a shareholder in Laurent Pharmaceuticals Inc., which is currently running Phase II (APPLAUD) multicenter clinical trial aimed to assess the efficacy of fenretinide in patients with CF.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Di Pasquale MG. The essentials of essential fatty acids. J Diet Suppl. 2009;6:143–161. doi: 10.1080/19390210902861841. [DOI] [PubMed] [Google Scholar]
- 2.Horrocks LA, Yeo YK. Health benefits of docosahexaenoic acid (DHA) Pharmacol Res. 1999;40:211–225. doi: 10.1006/phrs.1999.0495. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez A, Sarda P, Nessmann C, Boulot P, Leger CL, Descomps B. Delta6- and delta5-desaturase activities in the human fetal liver: kinetic aspects. J Lipid Res. 1998;39:1825–1832. [PubMed] [Google Scholar]
- 4.Biagi PL, Hrelia S, Stefanini GF, Zunarelli P, Bordoni A. Delta-6-desaturase activity of human liver microsomes from patients with different types of liver injury. Prostaglandins Leukot Essent Fatty Acids. 1990;39:39–42. doi: 10.1016/0952-3278(90)90169-l. [DOI] [PubMed] [Google Scholar]
- 5.Domenichiello AF, Chen CT, Trepanier MO, Stavro PM, Bazinet RP. Whole body synthesis rates of DHA from alpha-linolenic acid are greater than brain DHA accretion and uptake rates in adult rats. J Lipid Res. 2014;55:62–74. doi: 10.1194/jlr.M042275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emken EA, Adlof RO, Gulley RM. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim Biophys Acta. 1994;1213:277–288. doi: 10.1016/0005-2760(94)00054-9. [DOI] [PubMed] [Google Scholar]
- 7.Gillingham LG, Harding SV, Rideout TC, Yurkova N, Cunnane SC, Eck PK, Jones PJ. Dietary oils and FADS1-FADS2 genetic variants modulate [13C]alpha-linolenic acid metabolism and plasma fatty acid composition. Am J Clin Nutr. 2013;97:195–207. doi: 10.3945/ajcn.112.043117. [DOI] [PubMed] [Google Scholar]
- 8.McCloy U, Ryan MA, Pencharz PB, Ross RJ, Cunnane SC. A comparison of the metabolism of eighteen-carbon 13C-unsaturated fatty acids in healthy women. J Lipid Res. 2004;45:474–485. doi: 10.1194/jlr.M300304-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Metherel AH, Irfan M, Klingel SL, Mutch DM, Bazinet RP. Compound-specific isotope analysis reveals no retroconversion of DHA to EPA but substantial conversion of EPA to DHA following supplementation: a randomized control trial. Am J Clin Nutr. 2019;110:823–831. doi: 10.1093/ajcn/nqz097. [DOI] [PubMed] [Google Scholar]
- 10.Murakami M, Yamamoto K, Miki Y, Murase R, Sato H, Taketomi Y. The roles of the secreted phospholipase A2 gene family in immunology. Adv Immunol. 2016;132:91–134. doi: 10.1016/bs.ai.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011;111:6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheon Y, Kim HW, Igarashi M, Modi HR, Chang L, Ma K, Greenstein D, Wohltmann M, Turk J, Rapoport SI, et al. Disturbed brain phospholipid and docosahexaenoic acid metabolism in calcium-independent phospholipase A(2)-VIA (iPLA(2)beta)-knockout mice. Biochim Biophys Acta. 2012;1821:1278–1286. doi: 10.1016/j.bbalip.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ Br J Pharmacol. 2003;139:1014–1022. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murase R, Sato H, Yamamoto K, Ushida A, Nishito Y, Ikeda K, Kobayashi T, Yamamoto T, Taketomi Y, Murakami M. Group X secreted phospholipase A2 releases omega3 polyunsaturated fatty acids, suppresses colitis, and promotes sperm fertility. J Biol Chem. 2016;291:6895–6911. doi: 10.1074/jbc.M116.715672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami M, Taketomi Y, Sato H, Yamamoto K. Secreted phospholipase A2 revisited. J Biochem. 2011;150:233–255. doi: 10.1093/jb/mvr088. [DOI] [PubMed] [Google Scholar]
- 16.Shikano M, Masuzawa Y, Yazawa K, Takayama K, Kudo I, Inoue K. Complete discrimination of docosahexaenoate from arachidonate by 85 kDa cytosolic phospholipase A2 during the hydrolysis of diacyl- and alkenylacylglycerophosphoethanolamine. Biochim Biophys Acta. 1994;1212:211–216. doi: 10.1016/0005-2760(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 17.Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2009;50(Suppl):S29–34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGiff JC. Prostaglandins, prostacyclin, and thromboxanes. Annu Rev Pharmacol Toxicol. 1981;21:479–509. doi: 10.1146/annurev.pa.21.040181.002403. [DOI] [PubMed] [Google Scholar]
- 19.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 20.Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50:115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168:1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 25.Ringholz FC, Buchanan PJ, Clarke DT, Millar RG, McDermott M, Linnane B, Harvey BJ, McNally P, Urbach V. Reduced 15-lipoxygenase 2 and lipoxin A4/leukotriene B4 ratio in children with cystic fibrosis. Eur Respir J. 2014;44:394–404. doi: 10.1183/09031936.00106013. [DOI] [PubMed] [Google Scholar]
- 26.Serhan CN, Ward PA. Molecular and cellular basis of inflammation. Totowa: Humana Press; 1999. [Google Scholar]
- 27.Duvall MG, Levy BD. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol. 2016;785:144–155. doi: 10.1016/j.ejphar.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 29.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, Serhan CN. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013;27:2573–2583. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim Biophys Acta. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med. 2017;58:114–129. doi: 10.1016/j.mam.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilroy DW, Bishop-Bailey D. Lipid mediators in immune regulation and resolution. Br J Pharmacol. 2019;176:1009–1023. doi: 10.1111/bph.14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kytikova O, Novgorodtseva T, Denisenko Y, Antonyuk M, Gvozdenko T. Pro-resolving lipid mediators in the pathophysiology of asthma. Medicina (Kaunas) 2019 doi: 10.3390/medicina55060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014;19:21–36. doi: 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuo PT, Huang NN, Bassett DR. The fatty acid composition of the serum chylomicrons and adipose tissue of children with cystic fibrosis of the pancreas. J Pediatr. 1962;60:394–403. doi: 10.1016/S0022-3476(62)80065-1. [DOI] [PubMed] [Google Scholar]
- 38.Underwood BA, Denning CR, Navab M. Polyunsaturated fatty acids and tocopherol levels in patients with cystic fibrosis. Ann N Y Acad Sci. 1972;203:237–247. doi: 10.1111/j.1749-6632.1972.tb27879.x. [DOI] [PubMed] [Google Scholar]
- 39.Carlstedt-Duke J, Bronnegard M, Strandvik B. Pathological regulation of arachidonic acid release in cystic fibrosis: the putative basic defect. Proc Natl Acad Sci USA. 1986;83:9202–9206. doi: 10.1073/pnas.83.23.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilljam H, Strandvik B, Ellin A, Wiman LG. Increased mole fraction of arachidonic acid in bronchial phospholipids in patients with cystic fibrosis. Scand J Clin Lab Invest. 1986;46:511–518. doi: 10.3109/00365518609083706. [DOI] [PubMed] [Google Scholar]
- 41.Strandvik B, Gronowitz E, Enlund F, Martinsson T, Wahlstrom J. Essential fatty acid deficiency in relation to genotype in patients with cystic fibrosis. J Pediatr. 2001;139:650–655. doi: 10.1067/mpd.2001.118890. [DOI] [PubMed] [Google Scholar]
- 42.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, Weed DA, Gelrud A, Regan MM, Laposata M, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 43.Freedman SD, Katz MH, Parker EM, Laposata M, Urman MY, Alvarez JG. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr (−/−) mice. Proc Natl Acad Sci USA. 1999;96:13995–14000. doi: 10.1073/pnas.96.24.13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guilbault C, Wojewodka G, Saeed Z, Hajduch M, Matouk E, De Sanctis JB, Radzioch D. Cystic fibrosis fatty acid imbalance is linked to ceramide deficiency and corrected by fenretinide. Am J Respir Cell Mol Biol. 2009;41:100–106. doi: 10.1165/rcmb.2008-0279OC. [DOI] [PubMed] [Google Scholar]
- 45.Freedman SD, Weinstein D, Blanco PG, Martinez-Clark P, Urman S, Zaman M, Morrow JD, Alvarez JG. Characterization of LPS-induced lung inflammation in cftr −/− mice and the effect of docosahexaenoic acid. J Appl Physiol. 2002;92:2169–2176. doi: 10.1152/japplphysiol.00927.2001. [DOI] [PubMed] [Google Scholar]
- 46.Beharry S, Ackerley C, Corey M, Kent G, Heng YM, Christensen H, Luk C, Yantiss RK, Nasser IA, Zaman M, et al. Long-term docosahexaenoic acid therapy in a congenic murine model of cystic fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G839–848. doi: 10.1152/ajpgi.00582.2005. [DOI] [PubMed] [Google Scholar]
- 47.Portal C, Gouyer V, Leonard R, Husson MO, Gottrand F, Desseyn JL. Long-term dietary (n-3) polyunsaturated fatty acids show benefits to the lungs of Cftr F508del mice. PLoS ONE. 2018;13:e0197808. doi: 10.1371/journal.pone.0197808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lloyd-Still JD, Powers CA, Hoffman DR, Boyd-Trull K, Lester LA, Benisek DC, Arterburn LM. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients: a randomized, controlled study. Nutrition. 2006;22:36–46. doi: 10.1016/j.nut.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Coste TC, Armand M, Lebacq J, Lebecque P, Wallemacq P, Leal T. An overview of monitoring and supplementation of omega 3 fatty acids in cystic fibrosis. Clin Biochem. 2007;40:511–520. doi: 10.1016/j.clinbiochem.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Van Biervliet S, Devos M, Delhaye T, Van Biervliet JP, Robberecht E, Christophe A. Oral DHA supplementation in DeltaF508 homozygous cystic fibrosis patients. Prostaglandins Leukot Essent Fatty Acids. 2008;78:109–115. doi: 10.1016/j.plefa.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Hubbard VS, Dunn GD, di Sant'Agnese PA. Abnormal fatty-acid composition of plasma-lipids in cystic fibrosis. A primary or a secondary defect? Lancet. 1977;2:1302–1304. doi: 10.1016/S0140-6736(77)90359-2. [DOI] [PubMed] [Google Scholar]
- 52.Rogiers V, Vercruysse A, Dab I, Baran D. Abnormal fatty acid pattern of the plasma cholesterol ester fraction in cystic fibrosis patients with and without pancreatic insufficiency. Eur J Pediatr. 1983;141:39–42. doi: 10.1007/BF00445666. [DOI] [PubMed] [Google Scholar]
- 53.Farrell PM, Mischler EH, Engle MJ, Brown DJ, Lau SM. Fatty acid abnormalities in cystic fibrosis. Pediatr Res. 1985;19:104–109. doi: 10.1203/00006450-198501000-00028. [DOI] [PubMed] [Google Scholar]
- 54.Roulet M, Frascarolo P, Rappaz I, Pilet M. Essential fatty acid deficiency in well nourished young cystic fibrosis patients. Eur J Pediatr. 1997;156:952–956. doi: 10.1007/s004310050750. [DOI] [PubMed] [Google Scholar]
- 55.Aldamiz-Echevarria L, Prieto JA, Andrade F, Elorz J, Sojo A, Lage S, Sanjurjo P, Vazquez C, Rodriguez-Soriano J. Persistence of essential fatty acid deficiency in cystic fibrosis despite nutritional therapy. Pediatr Res. 2009;66:585–589. doi: 10.1203/PDR.0b013e3181b4e8d3. [DOI] [PubMed] [Google Scholar]
- 56.Mimoun M, Coste TC, Lebacq J, Lebecque P, Wallemacq P, Leal T, Armand M. Increased tissue arachidonic acid and reduced linoleic acid in a mouse model of cystic fibrosis are reversed by supplemental glycerophospholipids enriched in docosahexaenoic acid. J Nutr. 2009;139:2358–2364. doi: 10.3945/jn.109.110999. [DOI] [PubMed] [Google Scholar]
- 57.Andersson C, Al-Turkmani MR, Savaille JE, Alturkmani R, Katrangi W, Cluette-Brown JE, Zaman MM, Laposata M, Freedman SD. Cell culture models demonstrate that CFTR dysfunction leads to defective fatty acid composition and metabolism. J Lipid Res. 2008;49:1692–1700. doi: 10.1194/jlr.M700388-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garić D, De Sanctis JB, Dumut DC, Shah J, Peña MJ, Youssef M, Petrof BJ, Kopriva F, Hanrahan JW, Hajduch M, et al. Fenretinide favorably affects mucins (MUC5AC/MUC5B) and fatty acid imbalance in a manner mimicking CFTR-induced correction. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1865:158538. doi: 10.1016/j.bbalip.2019.158538. [DOI] [PubMed] [Google Scholar]
- 59.Al-Turkmani MR, Andersson C, Alturkmani R, Katrangi W, Cluette-Brown JE, Freedman SD, Laposata M. A mechanism accounting for the low cellular level of linoleic acid in cystic fibrosis and its reversal by DHA. J Lipid Res. 2008;49:1946–1954. doi: 10.1194/jlr.M800035-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Njoroge SW, Seegmiller AC, Katrangi W, Laposata M. Increased Delta5- and Delta6-desaturase, cyclooxygenase-2, and lipoxygenase-5 expression and activity are associated with fatty acid and eicosanoid changes in cystic fibrosis. Biochim Biophys Acta. 2011;1811:431–440. doi: 10.1016/j.bbalip.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Njoroge SW, Laposata M, Katrangi W, Seegmiller AC. DHA and EPA reverse cystic fibrosis-related FA abnormalities by suppressing FA desaturase expression and activity. J Lipid Res. 2012;53:257–265. doi: 10.1194/jlr.M018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hiltunen JK, Karki T, Hassinen IE, Osmundsen H. beta-Oxidation of polyunsaturated fatty acids by rat liver peroxisomes. A role for 2,4-dienoyl-coenzyme A reductase in peroxisomal beta-oxidation. J Biol Chem. 1986;261:16484–16493. [PubMed] [Google Scholar]
- 63.Gronn M, Christensen E, Hagve TA, Christophersen BO. Peroxisomal retroconversion of docosahexaenoic acid (22:6(n-3)) to eicosapentaenoic acid (20:5(n-3)) studied in isolated rat liver cells. Biochim Biophys Acta. 1991;1081:85–91. doi: 10.1016/0005-2760(91)90254-F. [DOI] [PubMed] [Google Scholar]
- 64.Pynn CJ, Henderson NG, Clark H, Koster G, Bernhard W, Postle AD. Specificity and rate of human and mouse liver and plasma phosphatidylcholine synthesis analyzed in vivo. J Lipid Res. 2011;52:399–407. doi: 10.1194/jlr.D011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeLong CJ, Shen YJ, Thomas MJ, Cui Z. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J Biol Chem. 1999;274:29683–29688. doi: 10.1074/jbc.274.42.29683. [DOI] [PubMed] [Google Scholar]
- 66.Innis SM, Davidson AG. Cystic fibrosis and nutrition: linking phospholipids and essential fatty acids with thiol metabolism. Annu Rev Nutr. 2008;28:55–72. doi: 10.1146/annurev.nutr.27.061406.093625. [DOI] [PubMed] [Google Scholar]
- 67.Watkins SM, Zhu X, Zeisel SH. Phosphatidylethanolamine-N-methyltransferase activity and dietary choline regulate liver-plasma lipid flux and essential fatty acid metabolism in mice. J Nutr. 2003;133:3386–3391. doi: 10.1093/jn/133.11.3386. [DOI] [PubMed] [Google Scholar]
- 68.Yang J, Eiserich JP, Cross CE, Morrissey BM, Hammock BD. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic Biol Med. 2012;53:160–171. doi: 10.1016/j.freeradbiomed.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ollero M, Astarita G, Guerrera IC, Sermet-Gaudelus I, Trudel S, Piomelli D, Edelman A. Plasma lipidomics reveals potential prognostic signatures within a cohort of cystic fibrosis patients. J Lipid Res. 2011;52:1011–1022. doi: 10.1194/jlr.P013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berguerand M, Klapisz E, Thomas G, Humbert L, Jouniaux AM, Olivier JL, Bereziat G, Masliah J. Differential stimulation of cytosolic phospholipase A2 by bradykinin in human cystic fibrosis cell lines. Am J Respir Cell Mol Biol. 1997;17:481–490. doi: 10.1165/ajrcmb.17.4.2734. [DOI] [PubMed] [Google Scholar]
- 71.Dif F, Wu YZ, Burgel PR, Ollero M, Leduc D, Aarbiou J, Borot F, Garcia-Verdugo I, Martin C, Chignard M, et al. Critical role of cytosolic phospholipase A2{alpha} in bronchial mucus hypersecretion in CFTR-deficient mice. Eur Respir J. 2010;36:1120–1130. doi: 10.1183/09031936.00183409. [DOI] [PubMed] [Google Scholar]
- 72.Levistre R, Lemnaouar M, Rybkine T, Bereziat G, Masliah J. Increase of bradykinin-stimulated arachidonic acid release in a delta F508 cystic fibrosis epithelial cell line. Biochim Biophys Acta. 1993;1181:233–239. doi: 10.1016/0925-4439(93)90026-W. [DOI] [PubMed] [Google Scholar]
- 73.Miele L, Cordella-Miele E, Xing M, Frizzell R, Mukherjee AB. Cystic fibrosis gene mutation (deltaF508) is associated with an intrinsic abnormality in Ca2+-induced arachidonic acid release by epithelial cells. DNA Cell Biol. 1997;16:749–759. doi: 10.1089/dna.1997.16.749. [DOI] [PubMed] [Google Scholar]
- 74.Eickmeier O, Fussbroich D, Mueller K, Serve F, Smaczny C, Zielen S, Schubert R. Pro-resolving lipid mediator resolvin D1 serves as a marker of lung disease in cystic fibrosis. PLoS ONE. 2017;12:e0171249. doi: 10.1371/journal.pone.0171249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ringholz FC, Higgins G, Hatton A, Sassi A, Moukachar A, Fustero-Torre C, Hollenhorst M, Sermet-Gaudelus I, Harvey BJ, McNally P, et al. Resolvin D1 regulates epithelial ion transport and inflammation in cystic fibrosis airways. J Cyst Fibros. 2018;17:607–615. doi: 10.1016/j.jcf.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 76.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35:246–259. doi: 10.1097/00005176-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Smith C, Winn A, Seddon P, Ranganathan S. A fat lot of good: balance and trends in fat intake in children with cystic fibrosis. J Cyst Fibros. 2012;11:154–157. doi: 10.1016/j.jcf.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 78.Simopoulos AP. Overview of evolutionary aspects of omega 3 fatty acids in the diet. World Rev Nutr Diet. 1998;83:1–11. doi: 10.1159/000059674. [DOI] [PubMed] [Google Scholar]
- 79.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sergeant S, Rahbar E, Chilton FH. Gamma-linolenic acid, dihommo-gamma linolenic, eicosanoids and inflammatory processes. Eur J Pharmacol. 2016;785:77–86. doi: 10.1016/j.ejphar.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ziboh VA, Fletcher MP. Dose-response effects of dietary gamma-linolenic acid-enriched oils on human polymorphonuclear-neutrophil biosynthesis of leukotriene B4. Am J Clin Nutr. 1992;55:39–45. doi: 10.1093/ajcn/55.1.39. [DOI] [PubMed] [Google Scholar]
- 82.Johnson MM, Swan DD, Surette ME, Stegner J, Chilton T, Fonteh AN, Chilton FH. Dietary supplementation with gamma-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J Nutr. 1997;127:1435–1444. doi: 10.1093/jn/127.8.1435. [DOI] [PubMed] [Google Scholar]
- 83.Ziboh VA, Naguwa S, Vang K, Wineinger J, Morrissey BM, Watnik M, Gershwin ME. Suppression of leukotriene B4 generation by ex-vivo neutrophils isolated from asthma patients on dietary supplementation with gammalinolenic acid-containing borage oil: possible implication in asthma. Clin Dev Immunol. 2004;11:13–21. doi: 10.1080/10446670410001670445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yaqoob P, Pala HS, Cortina-Borja M, Newsholme EA, Calder PC. Encapsulated fish oil enriched in alpha-tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions. Eur J Clin Invest. 2000;30:260–274. doi: 10.1046/j.1365-2362.2000.00623.x. [DOI] [PubMed] [Google Scholar]
- 85.Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with gamma-linolenic acid or fish oil decreases T lymphocyte proliferation in healthy older humans. J Nutr. 2001;131:1918–1927. doi: 10.1093/jn/131.7.1918. [DOI] [PubMed] [Google Scholar]
- 86.Chilton L, Surette ME, Swan DD, Fonteh AN, Johnson MM, Chilton FH. Metabolism of gammalinolenic acid in human neutrophils. J Immunol. 1996;156:2941–2947. [PubMed] [Google Scholar]
- 87.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 88.Adam O, Wolfram G, Zollner N. Influence of dietary linoleic acid intake with different fat intakes on arachidonic acid concentrations in plasma and platelet lipids and eicosanoid biosynthesis in female volunteers. Ann Nutr Metab. 2003;47:31–36. doi: 10.1159/000068906. [DOI] [PubMed] [Google Scholar]
- 89.Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012;112(1029–1041):1041.e1021–1015. doi: 10.1016/j.jand.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 90.Liou YA, King DJ, Zibrik D, Innis SM. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr. 2007;137:945–952. doi: 10.1093/jn/137.4.945. [DOI] [PubMed] [Google Scholar]
- 91.Rett BS, Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab (Lond) 2011;8:36. doi: 10.1186/1743-7075-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Veronesi U, De Palo G, Marubini E, Costa A, Formelli F, Mariani L, Decensi A, Camerini T, Del Turco MR, Di Mauro MG, et al. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst. 1999;91:1847–1856. doi: 10.1093/jnci/91.21.1847. [DOI] [PubMed] [Google Scholar]
- 93.Children's Oncology G, Villablanca JG, Krailo MD, Ames MM, Reid JM, Reaman GH, Reynolds CP. Phase I trial of oral fenretinide in children with high-risk solid tumors: a report from the Children's Oncology Group (CCG 09709) J Clin Oncol. 2006;24:3423–3430. doi: 10.1200/JCO.2005.03.9271. [DOI] [PubMed] [Google Scholar]
- 94.Berni R, Formelli F. In vitro interaction of fenretinide with plasma retinol-binding protein and its functional consequences. FEBS Lett. 1992;308:43–45. doi: 10.1016/0014-5793(92)81046-O. [DOI] [PubMed] [Google Scholar]
- 95.Berni R, Clerici M, Malpeli G, Cleris L, Formelli F. Retinoids: in vitro interaction with retinol-binding protein and influence on plasma retinol. FASEB J. 1993;7:1179–1184. doi: 10.1096/fasebj.7.12.8375617. [DOI] [PubMed] [Google Scholar]
- 96.Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res. 2013;54:1761–1775. doi: 10.1194/jlr.R030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leblanc BP, Stunnenberg HG. 9-cis retinoic acid signaling: changing partners causes some excitement. Genes Dev. 1995;9:1811–1816. doi: 10.1101/gad.9.15.1811. [DOI] [PubMed] [Google Scholar]
- 98.Bu P, Wan YJ. Fenretinide-induced apoptosis of Huh-7 hepatocellular carcinoma is retinoic acid receptor beta dependent. BMC Cancer. 2007;7:236. doi: 10.1186/1471-2407-7-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fanjul AN, Delia D, Pierotti MA, Rideout D, Yu JQ, Pfahl M. 4-Hydroxyphenyl retinamide is a highly selective activator of retinoid receptors. J Biol Chem. 1996;271:22441–22446. doi: 10.1074/jbc.271.37.22441. [DOI] [PubMed] [Google Scholar]
- 100.Lin CH, Lee SY, Zhang CC, Du YF, Hung HC, Wu HT, Ou HY. Fenretinide inhibits macrophage inflammatory mediators and controls hypertension in spontaneously hypertensive rats via the peroxisome proliferator-activated receptor gamma pathway. Drug Des Dev Ther. 2016;10:3591–3597. doi: 10.2147/DDDT.S114879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harris G, Ghazallah RA, Nascene D, Wuertz B, Ondrey FG. PPAR activation and decreased proliferation in oral carcinoma cells with 4-HPR. Otolaryngol Head Neck Surg. 2005;133:695–701. doi: 10.1016/j.otohns.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 102.Dekkers JF, van der Ent CK, Kalkhoven E, Beekman JM. PPARgamma as a therapeutic target in cystic fibrosis. Trends Mol Med. 2012;18:283–291. doi: 10.1016/j.molmed.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 103.Ollero M, Junaidi O, Zaman MM, Tzameli I, Ferrando AA, Andersson C, Blanco PG, Bialecki E, Freedman SD. Decreased expression of peroxisome proliferator activated receptor gamma in cftr−/− mice. J Cell Physiol. 2004;200:235–244. doi: 10.1002/jcp.20020. [DOI] [PubMed] [Google Scholar]
- 104.Perez A, van Heeckeren AM, Nichols D, Gupta S, Eastman JF, Davis PB. Peroxisome proliferator-activated receptor-gamma in cystic fibrosis lung epithelium. Am J Physiol Lung Cell Mol Physiol. 2008;295:L303–313. doi: 10.1152/ajplung.90276.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harmon GS, Dumlao DS, Ng DT, Barrett KE, Dennis EA, Dong H, Glass CK. Pharmacological correction of a defect in PPAR-gamma signaling ameliorates disease severity in Cftr-deficient mice. Nat Med. 2010;16:313–318. doi: 10.1038/nm.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 107.Shishodia S, Gutierrez AM, Lotan R, Aggarwal BB. N-(4-hydroxyphenyl)retinamide inhibits invasion, suppresses osteoclastogenesis, and potentiates apoptosis through down-regulation of I(kappa)B(alpha) kinase and nuclear factor-kappaB-regulated gene products. Cancer Res. 2005;65:9555–9565. doi: 10.1158/0008-5472.CAN-05-1585. [DOI] [PubMed] [Google Scholar]
- 108.Lachance C, Wojewodka G, Skinner TA, Guilbault C, De Sanctis JB, Radzioch D. Fenretinide corrects the imbalance between omega-6 to omega-3 polyunsaturated fatty acids and inhibits macrophage inflammatory mediators via the ERK pathway. PLoS ONE. 2013;8:e74875. doi: 10.1371/journal.pone.0074875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garic D, De Sanctis JB, Wojewodka G, Houle D, Cupri S, Abu-Arish A, Hanrahan JW, Hajduch M, Matouk E, Radzioch D. Fenretinide differentially modulates the levels of long- and very long-chain ceramides by downregulating Cers5 enzyme: evidence from bench to bedside. J Mol Med (Berl) 2017;95:1053–1064. doi: 10.1007/s00109-017-1564-y. [DOI] [PubMed] [Google Scholar]
- 110.Camerini T, Mariani L, De Palo G, Marubini E, Di Mauro MG, Decensi A, Costa A, Veronesi U. Safety of the synthetic retinoid fenretinide: long-term results from a controlled clinical trial for the prevention of contralateral breast cancer. J Clin Oncol. 2001;19:1664–1670. doi: 10.1200/JCO.2001.19.6.1664. [DOI] [PubMed] [Google Scholar]
- 111.Veronesi U, Mariani L, Decensi A, Formelli F, Camerini T, Miceli R, Di Mauro MG, Costa A, Marubini E, Sporn MB, et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol. 2006;17:1065–1071. doi: 10.1093/annonc/mdl047. [DOI] [PubMed] [Google Scholar]
- 112.Morin C, Cantin AM, Rousseau E, Sirois M, Sirois C, Rizcallah E, Fortin S. Proresolving action of docosahexaenoic acid monoglyceride in lung inflammatory models related to cystic fibrosis. Am J Respir Cell Mol Biol. 2015;53:574–583. doi: 10.1165/rcmb.2014-0223OC. [DOI] [PubMed] [Google Scholar]
- 113.Binker MG, Binker-Cosen MJ, Richards D, Binker-Cosen AA, Freedman SD, Cosen-Binker LI. Omega-3 PUFA docosahexaenoic acid decreases LPS-stimulated MUC5AC production by altering EGFR-related signaling in NCI-H292 cells. Biochem Biophys Res Commun. 2015;463:1047–1052. doi: 10.1016/j.bbrc.2015.06.056. [DOI] [PubMed] [Google Scholar]
- 114.Blanco R, Iwakawa R, Tang M, Kohno T, Angulo B, Pio R, Montuenga LM, Minna JD, Yokota J, Sanchez-Cespedes M. A gene-alteration profile of human lung cancer cell lines. Hum Mutat. 2009;30:1199–1206. doi: 10.1002/humu.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Medjane S, Raymond B, Wu Y, Touqui L. Impact of CFTR DeltaF508 mutation on prostaglandin E2 production and type IIA phospholipase A2 expression by pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L816–824. doi: 10.1152/ajplung.00466.2004. [DOI] [PubMed] [Google Scholar]
- 116.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, McGing MA, McElwee MM, Williams OW, Sanchez E, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun. 2015;6:6281. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marom Z, Shelhamer JH, Kaliner M. Effects of arachidonic acid, monohydroxyeicosatetraenoic acid and prostaglandins on the release of mucous glycoproteins from human airways in vitro. J Clin Invest. 1981;67:1695–1702. doi: 10.1172/JCI110207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garcia-Verdugo I, BenMohamed F, Tattermusch S, Leduc D, Charpigny G, Chignard M, Ollero M, Touqui L. A role for 12R-lipoxygenase in MUC5AC expression by respiratory epithelial cells. Eur Respir J. 2012;40:714–723. doi: 10.1183/09031936.00023111. [DOI] [PubMed] [Google Scholar]