Abstract
Male idiopathic infertility accounts for 15–25% of reproductive failure. One of the factors that has been linked to this condition is oxidative stress (OS), defined as the imbalance between antioxidants and reactive oxygen species. Amongst the different factors that protect the cell against OS, the members of the glutathione S-transferase (GST) superfamily play an important role. Interestingly, reduction or lack of some GSTs has been associated to infertility in men. Therefore, and to clarify the relationship between GSTs and male fertility, the aim of this work is to describe the role that GSTs play in the male reproductive tract and in sperm physiology. To that end, the present review provides a novel perspective on the triple role of GSTs (detoxification, regulation of cell signalling and fertilisation), and reports their localisation in sperm, seminal plasma and the male reproductive tract. Furthermore, we also tackle the existing correlation between some GST classes and male fertility. Due to the considerable impact of GSTs in human pathology and their tight relationship with fertility, future research should address the specific role of these proteins in male fertility, which could result in new approaches for the diagnosis and/or treatment of male infertility.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03405-w) contains supplementary material, which is available to authorized users.
Keywords: GSTs, Infertility, Detoxification, Cell signaling, Fertilisation
Background
In humans, about 30–50% of reproductive failure is associated with some form of male factor infertility. Multiple factors can lead to a reduction in fertility, including lifestyle, environmental and genetic factors, that can act alone or in combination. In most cases, these factors lead to infertility related to low sperm count, reduced motility, or abnormal sperm size and morphology [1]. However, in over 50% of patients, the aetiology is unknown and thus, the subject is classified as presenting male idiopathic infertility [2]. Two factors that can potentially play a key role in male idiopathic infertility are oxidative stress (OS) and reactive oxygen species (ROS). Oxidative stress reflects the imbalance between the production of ROS and the antioxidant capacity of sperm [3]. On the other hand, ROS are defined as free radicals that have one or more unpaired electrons (i.e., electrophile). To stabilize their electronic structure, ROS interact with nucleophilic substances such as lipids, proteins, nucleic acids and carbohydrates, which can lead to cell damage [4, 5]. Examples of ROS are: superoxide anion, hydrogen peroxide, hydroxyl radical, lipid hydroperoxides and peroxyl radicals.
It has been well established that ROS play a dual role in the cell, having a beneficial or harmful effect depending on their concentration, localisation or exposure time [1]. For example, in sperm, appropriate levels of ROS are required to undergo capacitation, hyperactivation, acrosome reaction and sperm–oocyte fusion [6]. However, excessive ROS may cause a reduction in motility, lipid peroxidation, DNA damage and apoptosis-like events [7–9]. In the testis, the continuous division of germ cells entails a high rate of mitochondrial activity that is related to high levels of ROS production, which could have detrimental effects on spermatogenesis [10]. Nevertheless, many antioxidant systems have been described to control the physiological levels of ROS, and can thus be involved in the protection of sperm and germ cells from OS. Glutathione S-transferases (GSTs; EC 2.5.1.18) are some of the enzymes most commonly implicated in the regulation of OS.
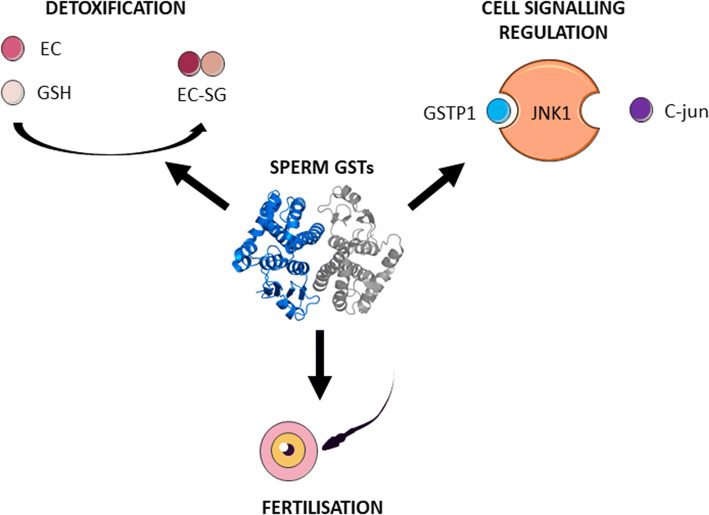
GSTs are a large group of cytosolic and membrane-bound, multi-gene and multi-functional isoenzymes that catalyse a number of reduced glutathione-dependent reactions, which are involved in cellular protection against OS by the removal of electrophilic substances [11]. Different GST isoforms have been previously described in sperm [12], seminal plasma [13] and male reproductive tissues [14]. Although such enzymes operate as antioxidants in most mammalian cell types, sperm GSTs play a triple role (1) in cell detoxification (preventing lipid membrane peroxidation and subsequent OS) [15]; (2) in cell signalling regulation (involved in spermatogenesis and sperm capacitation) [16, 17]; and (3) in fertilisation (since specific GSTs members are involved in sperm–oocyte binding and acceleration of sperm nuclear decondensation) [18–20] (Fig. 1).
Fig. 1.
Triple role of GSTs in the male reproductive tract. The functions of sperm GSTs in detoxification, cell signalling regulation, and allowing fertilisation. Detoxification: electrophilic compounds (EC) react with the reduced nucleophilic thiol group of GSH, in a reaction catalysed by GSTs, forming the reaction product (EC-SG). Cell signalling regulation: GSTs are involved in the regulation of signalling pathways, such as the JNK-C-jun pathway that regulates the apoptosis process. Fertilisation: GSTs mediate sperm–oocyte binding and sperm nucleus decondensation
In this regard, the aim of the present review is to describe the three functions attributed to the GST superfamily as they relate to male fertility. In addition, GST biochemistry and localisation in sperm, seminal plasma and the male reproductive tract are discussed. To this end, a critical review of literature available through PubMed was carried out between November 2018 and June 2019.
Classification, genetics and structure of GSTs
Classification and nomenclature of GSTs
In 1961, GST activity was reported for the first time in rat liver preparations by Combes and Stakelum [21]. Following this discovery, the scientific community uncovered a large range of glutathione-dependent liver enzymes that conjugate with several different substrates which were termed glutathione S-transferases. GSTs are present in most mammalian cell types and are classified into three superfamilies: (1) canonical soluble GSTs; (2) distantly related soluble kappa class GSTs; and (3) hydrophobic GSTs (Membrane-Associated Proteins in Eicosanoid and Glutathione metabolism; MAPEG). Generally, in mammals, canonical soluble GSTs are cytosolic proteins, soluble kappa class GSTs are mitochondrial and/or peroxisomal proteins, and MAPEG members are found in microsomal fractions [22]. Because the predominant GST superfamily in mammalian cells is the canonical soluble GSTs, the present work will focus on this group.
Canonical soluble GST-isoforms were classified in 1992 by Mannervik and colleagues, which introduced the current nomenclature (Table 1). Classification of these isoenzymes was made on the basis of their extremely variable C-terminal α-helical domain, resulting in seven GST classes: alpha (GSTA), mu (GSTM), omega (GSTO), pi (GSTP), sigma (GSTS), theta (GSTT), and zeta (GSTZ) [23, 24]. Moreover, Mannervik et al. [25, 26] evidenced the presence of homo- and hetero-dimerization of GST isoforms within the same class, which allows modular enzymatic activities. Consequently, GST isoforms are currently named according to their class (e.g., GSTM: Mu class) and their dimerization (e.g., GSTM1-1: homodimerization of two GSTM1; and GSTM1–2: heterodimerization of GSTM1 and GSTM2) [23].
Table 1.
Classification of canonical soluble GSTs.
Modified from Mannervik et al. [23]
| Class | Member | RefSeq [30] | UniProt [31] | Chromosome | Confirmed number of isoforms [30, 31] | Nucleotide length (bp) [30] | Amino acid length (aa) [31] |
|---|---|---|---|---|---|---|---|
| Alpha | GSTA1 | NM_145740 | P08263 | 6p12 | 2 | 1218 | 222 |
| GSTA2 | NM_000846 | P09210 | 6p12 | 1 | 1221 | 222 | |
| GSTA3 | NM_000847 | Q16772 | 6p12 | 2 | 908 | 222 | |
| GSTA4 | NM_001512 | O15217 | 6p12 | 1 | 1240 | 222 | |
| GSTA5 | NM_153699 | Q7RTV2 | 6p12 | 1 | 845 | 222 | |
| Mu | GSTM1 | NM_146421 | P09488 | 1p13 | 2 | 1155 | 218 |
| GSTM2 | NM_000848 | P28161 | 1p13 | 2 | 1166 | 218 | |
| GSTM3 | NM_000849 | P21266 | 1p13 | 1 | 4144 | 225 | |
| GSTM4 | NM_147148 | Q03013 | 1p13 | 2 | 1372 | 218 | |
| GSTM5 | NM_000851 | P46439 | 1p13 | 1 | 1561 | 218 | |
| Omega | GSTO1 | NM_004832 | P78417 | 10q24.3 | 3 | 813 | 241 |
| GSTO2 | NM_183239 | Q9H4Y5 | 10q24.3 | 4 | 6715 | 243 | |
| Pi | GSTP1 | NM_000852 | P09211 | 11q13 | 1 | 986 | 210 |
| Sigma | GSTS1 | NM_014485 | O60760 | 4q22.3 | 1 | 1615 | 199 |
| Theta | GSTT1 | NM_000853 | P30711 | 22q11.2 | 9 | 1109 | 240 |
| GSTT2 | NM_000854 | P0CG29 | 22q11.2 | 1 | 1231 | 244 | |
| Zeta | GSTZ1 | NM_145870 | O43708 | 14q24.3 | 4 | 1186 | 216 |
Nucleotide and amino acid lengths correspond to the canonical isoform. Transcript variants of each isoform are illustrated in Supplementary Figures 1–4
Genetic heterogeneity of GSTs
All members of a single GST class are encoded by individual genes grouped in a cluster of a specific genomic region (Table 1). The common organization of class-clustered GST genes in both plant and animal genomes reveals their importance during evolutionary history [27]. Additionally, the genetic organization of GSTs is related to their expression pattern, since each class of GSTs is expressed in distinct tissue-specific patterns [28].
As it has been noted above, the soluble GSTs superfamily comprises a considerable number of isoforms which give rise to a diversity of protein functions. This vast functional heterogeneity is further increased by alternative splicing of some GST members [29], which can modify the substrate specificity of the new GST isoform. As a result, even more enzymatic and non-enzymatic variability is generated. In mammals, alternative splicing in soluble GSTs has been reported to occur in the Alpha, Mu, Omega, Theta, and Zeta classes (Table 1 and Supplementary Fig. 1).
Protein structure of GSTs
In the cell, almost every member of the canonical soluble GSTs is active as dimers, in both homodimer and heterodimer forms within the same class [25–27]. Each dimer subunit is composed by a 23–30 kDa protein with 199–244 amino acids [30, 31]. While the sequence similarity between classes is low, the folding of the different members is similar [32]. Each subunit consists of two distinct domains: the N-terminal and C-terminal domains, which are linked by an α-helix. Even though the C-terminal α-helical domain of soluble GSTs is extremely variable, the N-terminal domain is conserved among isoforms. The glutathione-binding site (G-site) is extremely conserved, and is constituted by the N-terminal fold. On the other hand, the modular binding site for the electrophilic substrate (H-site) depends highly on the C-terminal domain and is variable between isoforms [22, 32]. As GST isoform classification is carried out based on the C-terminal domain, each class recognises a specific type of electrophilic substrate.
Expression and localisation of GSTs
Sperm GSTs: species-specific and maturation-status-dependent localisation patterns
The first evidence of GSTs in sperm was reported in 1978 by Mukhtar et al., who demonstrated GST activity in rat, mouse and human sperm [33]. Later, during the 1990s, Gopalakrishnan et al. [12, 34] identified a 24-kDa rat testicular protein with GST activity, which shared a similar N-terminus to GSTM. Simultaneously, in 1995, Fulcher et al. [35] identified a member of GSTM in mouse sperm for the first time. This 26 kDa protein was found to be associated with the fibrous sheath [35]. In 1998, Gopalakrishnan et al. [36] provided evidence on the presence of both Mu- and Pi-GST class members on the surface of goat sperm. Furthermore, studies from Hemachand et al. [18] showed that, despite GSTM and GSTP being soluble isoforms of GSTs, they were attached to the plasma membrane of goat sperm via non-covalent interactions. Both GSTs have also been shown to relocalise during sperm epididymal maturation in this species. While GSTPs migrate from the post-equatorial segment to the anterior acrosome and post-equatorial segment [35], GSTMs relocalise from the entire sperm surface to the sperm head [35]. This localisation differs to that of buffalo- and boar-ejaculated sperm, where GSTMs are found in the mid-, principal- and end-piece of the tail [37, 38]. Although, to the best of our knowledge, there is no information regarding GSTPs localisation on ejaculated sperm, both GSTMs and GSTPs have been shown to undergo relocalisation during in vitro capacitation. In goat sperm, after capacitation, GSTPs can be found in the sperm tail, in addition to their pre-capacitation presence in the anterior acrosome and post-equatorial segment. On the other hand, GSTMs relocalise from the entire head to the acrosome and the post-equatorial region [36].
Recently, another GST family member has been identified in sperm and the male reproductive tract. GSTO2 has been characterised within the post-acrosomal sheath of the perinuclear theca of bovine, porcine, and rodent sperm [39, 40]. This enzyme has been shown to actively participate in murine sperm nuclear descondensation, probably facilitated by the reducing power of glutathione (GSH) [20].
In summary, GSTs can exhibit differences in their sperm localisation patterns based on the species or the maturation status of the cell. The shifts in localisation during epididymal maturation or capacitation could be due to the specific physiological requirements of sperm, acting as a mechanism to maintain oxidative homeostasis and physiological status [38]. However, further studies are needed to elucidate this hypothesis.
GST family members are secreted differentially along the male reproductive tract
Although sperm contains several antioxidant enzymes in the plasma membrane, many ROS-scavengers are secreted along the male reproductive tract. The epididymis and seminal vesicles are considered as the main antioxidant-secretion organs [41]. However, while many proteomic studies have been carried out to characterize the human secretions of testis, epididymis, seminal vesicle and prostate, the degree of contribution of the secretion of each of these organs to the ejaculated seminal plasma is unclear [42].
Secretion of GSTs has been described in rat seminiferous tubules [34]. Similarly, Klys et al. [43] demonstrated the presence of GSTP, GSTA and GSTM in human testes through immunostaining. These GSTs were shown to be confined to Sertoli and Leydig cells [43]. However, a recent in-depth proteome study of human testis failed to identify any GST family members [44]. In the rat, Sertoli cells seem to be responsible for germ cell detoxification through secretion of, mainly, GST isoforms and GSH [34, 45]. However, at the time of writing this review, no published studies were available on the role of Leydig cells in detoxification through GSTs. In addition to Sertoli cells, rodent germ cells also produce GSTs. In the rat, germ cells synthesize, but do not secrete, GSTs [45]. While, in the mouse, Gstm2 is highly expressed in both type A and type B spermatogonia, gene expression is dramatically downregulated to near-undetectable levels in mature germ cells [46]. Similarly, Paz and colleagues found Gstm2 to be downregulated during mice sperm development in the testis, with higher levels in Sertoli cells and spermatogonia, and lower levels in spermatocytes and spermatids [47]. Finally, GSTO2 has been described to follow similar developmental localisation as other post-acrosomal sheath proteins, appearing along the different steps of rat spermiogenesis [39].
Although there are conflicting reports on the presence of GSTs in the testis, mounting evidence supports their presence along the epididymis. Li et al. [48] carried out a systematic mapping of the human epididymal fluid proteome and different GST members were shown to be expressed in a region-specific manner. For example, GSTP1 and GSTM3 were present at higher concentrations in corpus and cauda, whereas GSTM2, GSTM5 and GSTO1 were highly secreted in the caput. In addition, Thimon et al. [49] described the presence of GSTM2–3 and GSTP1 in epididymosomal membranes, and hypothesised that proteins found in these extracellular vesicles can bind to the sperm surface during their transport along the epididymis. This may be the system through which GSTM2–3 and GSTM5 bind to sperm, since, as noted previously, they can be found on the sperm membrane from the caput region onwards [48]. Evidence of a similar event was reported by Suryawanshi et al. [50], who identified GSTM5 incorporation to sperm during epididymal maturation. Thus, epididymal secretions contain GSTs that are able to bind to sperm as they migrate through the lumen and may play a role in sperm maturation. One likely function of sperm-surface GSTs is the protection against OS, which contributes to the sperm survival during epididymal storage [51].
Together with the epididymis, seminal vesicles are the main source of antioxidants in the male reproductive tract. However, no GST family members have been identified in these glands so far, neither through proteomic nor transcriptomic analysis.
Another accessory gland, the prostate, has been reported to secrete GSTP1 through prostasomes [52]. Prostasomes seem to play an important role in sperm motility enhancement [53] and prevention of premature acrosome reaction [54]. Furthermore, GSTP1 has been proposed as a prostate cancer biomarker, due to aberrant methylation of GSTP1 occurring in more than 90% of prostate tumours [55].
The production and secretion of GSTs by so many tissues of the male reproductive tract (Fig. 2) suggest an important role for these enzymes in male fertility. In the testicle, GSTs protect germ cells against OS, ensuring successful spermatogenesis. In addition, epididymal and accessory gland secretions contain GSTs that have the potential to bind to sperm and may play a role in sperm maturation and function. Future studies in this likely ability of secreted GSTs to bind to the sperm surface, and their role in sperm physiology should be conducted.
Fig. 2.
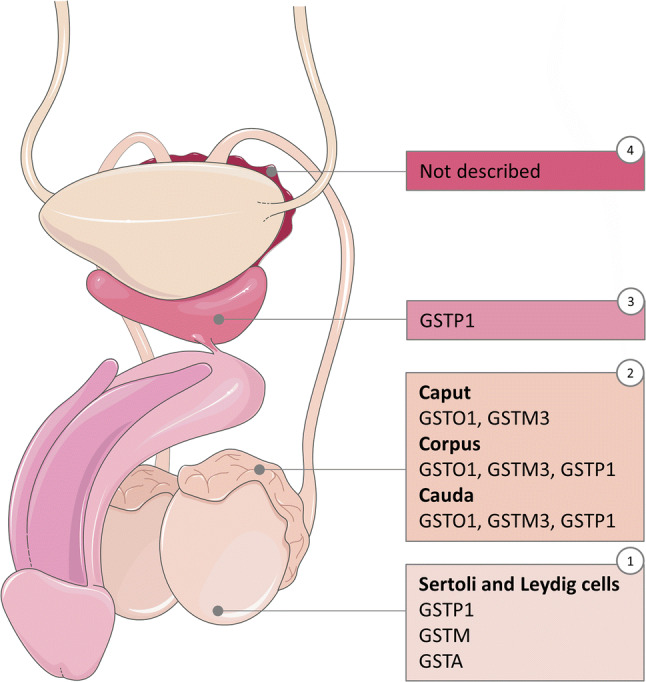
Proteomic annotation of GSTs in male reproductive tract. Mapping of the secretion of GSTs along the male reproductive tract: (1) testis, (2) epididymis, (3) prostate, (4) seminal vesicles
Triple activity of GSTs
GSTs are involved in sperm detoxification
The main role of GSTs in any cell type is cell detoxification, i.e., protecting macromolecules from attack by reactive electrophiles, including environmental carcinogens, ROS and chemotherapeutic agents [56]. This detoxification role is apparent by the fact that GST expression patterns are altered by xenobiotics. For example, while GSTO2 is affected by cisplatin in HeLa cells [57], both GSTO2 and GSTM5 have been reported to be affected by fluoride in the epididymis [58].
This detoxification function of membrane-bound GSTs in goat sperm prevents lipid membrane peroxidation (a process highly damaging to membrane integrity [7]), by eliminating electrophilic compounds via extracellular GSH [15]. Therefore, sperm seem to use membrane-bound GSTs and extracellular GSH to maintain their motility, viability, mitochondrial status, oocyte-binding capacity and fertilising abilities during exposure to H2O2 and/or lipid peroxidation products [15]. Moreover, higher levels of GSTM3 have been related to lower cryotolerance in boar sperm, suggesting it as a freezability marker of sperm [38].
Furthermore, the importance of these membrane-bound GSTs is highlighted by the fact that 85% of GST activity is located in the plasma membrane rather than in other sperm compartments [15]. Moreover, GSH is present in extracellular fluids in both male and female reproductive tracts (seminiferous tubular fluid: 0.99 ± 0.08 mM; epididymal fluid: 0.21 ± 0.03 mM; seminal fluid: 0.76 ± 0.08 mM; oviductal fluid: 0.11 ± 0.03 mM) [15]. Since sperm has little cytoplasm, it is very likely that the main source of GSH comes from these fluids.
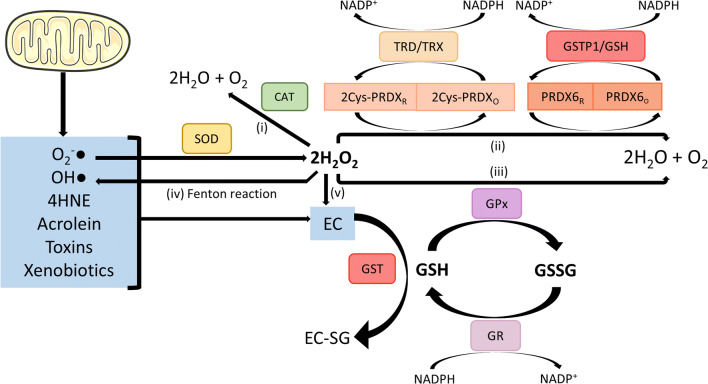
GSH is a natural reservoir of reducing power, which can be quickly used as an antioxidant. This tripeptide exists in the reduced (GSH) and the oxidized (GSSG) forms, both of which can interact with their associated enzymes (glutathione peroxidases [GPX], glutathione reductases [GSR] and glutathione s-transferases [GST]) to provide protection against OS [56, 59] through the thiol group (–SH) of their cysteines. The mechanism through which the sperm cell prevents OS through the equilibrium between the generation of electrophilic compounds and the antioxidant capacity of GSH is illustrated in Fig. 3. Briefly, either external and/or internal electrophilic compounds (EC; Fig. 3 blue box) react with the reduced nucleophilic -SH of extracellular GSH. This reaction, catalysed by GSTs, neutralises the EC, giving rise to the reaction product (EC-SG). Other enzymes such as glutathione peroxidases (GPX), glutathione reductase (GSR), superoxide dismutase (SOD) [60], catalase (CAT) [61], and peroxiredoxins (PRDX) [62] can also participate in sperm and seminal plasma detoxification.
Fig. 3.
GSH-related antioxidant capacity Electrophilic compounds (EC) in sperm may have both an external and internal source. Internally, the transfer of electrons through the respiratory chain leads to the generation of superoxide anion (O−·2) [60]. Additionally, highly damaging lipid peroxidation products such as 4-hydroxynonenal (4HNE) and acrolein may also be generated in sperm [100]. Either externally and/or internally generated EC react with the reduced nucleophilic thiol group (-SH) of extracellular GSH in a reaction catalysed by GSTs. This process neutralises EC, forming the reaction product (EC-SG). O−·2 and OH· can be directly eliminated by GST/GSH system. On the other hand, superoxide dismutase (SOD) catalyses the dismutation of O−·2 to hydrogen peroxide (H2O2), which is highly reactive and leads to pathophysiological processes in sperm such as lipid peroxidation [60]. This molecule may subsequently be eliminated by five different methods: (1) 2H2O2 can be rapidly transformed to 2H2O and O2 by a reaction catalysed by catalase (CAT) [61]; (2) reduced peroxiredoxins (PRDX) can reduce 2H2O2 to 2H2O and O2, oxidising themselves [62]. Subsequently, the thioredoxin reductase/thioredoxin system (TRD/TRX) can re-reduce 2Cys-PRDX (PRDX1–5) to allow for another cycle of H2O2 scavenging [101], while 1Cys-PRDX (PRDX6) is re-reduced by the GSTP1/GSH system [102]. In both TRD/TRX and GSTP1/GSH, reducing power (NADPH to NADP+) is needed. Finally, (3) sperm glutathione peroxidases (GPX) reduce 2H2O2 to 2H2O and O2 by the oxidation of GSH to GSSG. Following this, oxidised GSSG is re-reduced to GSH by glutathione reductase (GR), which oxides NADPH to NADP+ as reducing power [103]. Additionally, (4) formation of the hydroxyl radical (OH·) from H2O2 may occur by the Fenton reaction [104]. Finally, (5) H2O2 can also be directly neutralised by GST/GSH system
Sperm GSTs regulate cellular signalling pathways
Mitogen-activated protein kinases (MAPKs) are regulatory enzymes which conform signalling transduction cascades implicated in many cell functions, such as cellular differentiation, apoptosis and stress response [63]. Three different MAPK pathways have been characterizsed in many cell types: extracellular signal-regulation kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK. All of these pathways have been shown to play a role in spermatogenesis [64]. In addition, ERK is involved in sperm capacitation [65, 66] and MAPK is essential for sperm motility [67] and oocyte fertilisation [68].
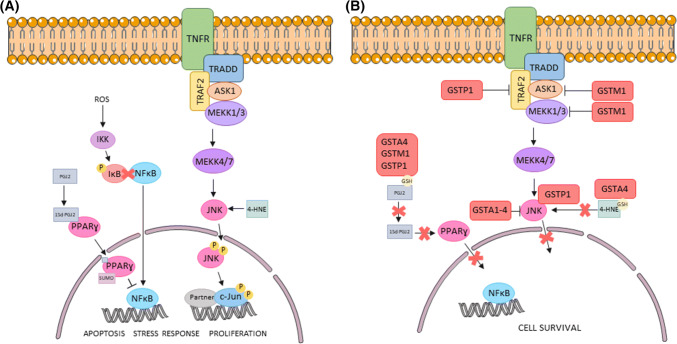
Adler and colleagues first identified an interaction between GSTP1 and JNK in ETE/4A fibroblasts [16]. They observed that GSTP1 could inhibit JNK and kidnap c-Jun in non-stressed cells. However, under OS, GSTP1 oligomerised and, as a result, JNK was released and c-Jun was phosphorylated and activated, leading to apoptosis. This was later confirmed with in vivo studies, in which Gstp1–/– mice exhibited enhanced JNK activity [69]. Similar interactions have been identified in GSTA1, GSTM1 and GSTO1. Desmots et al. [70] described that JNK and GSTA1 co-immunoprecipitated in liver extracts and that GSTA1 mRNA increased during OS via JNK pathway. On the other hand, Cho et al. [71] described an interaction between GSTM1 and ASK1 (a protein belonging to MAPK family) using the yeast two-hybrid technology. Finally, the overexpression of GSTO1 in HeLa cells has also been associated with the inhibition of apoptosis through JNK, among other signalling pathways related to cell survival [72]. All of these findings suggest that GSTA1, GSTM1, GSTO1 and GSTP1 could play a role in the regulation of apoptosis in most cell types (Fig. 4). Recently, Luna et al. [17] reported that MAPK molecular signalling pathways involved in sperm capacitation could be shared with sperm apoptosis. Based on this evidence, it could be hypothesised that sperm GSTs play a role in sperm capacitation and apoptosis-like changes.
Fig. 4.
Regulation of C-Jun and NFκB signalling pathways by GSTs. a Apoptosis, stress response and proliferation signalling pathways. After activation of TNFR, it triggers a downstream cascade which results in JNK phosphorylation. This phosphorylation can be also promoted by 4-HNE. Regardless of the signal that leads to JNK phosphorylation, JNK-P translocates into the nucleus to phosphorylate c-Jun and activate apoptosis, stress response and proliferation related genes. On the other hand, ROS is able to activate IKK, which can phosphorylate and inactivate IκB. Therefore, NFκB can translocate into the nucleus and act as a transcription factor to promote cell survival. However, if PPARɣ binds 15d-PGJ2 and SUMO, a protein that can inhibit NFκB and enhance apoptosis, stress response and proliferation. b GST regulation. The different GST family members can inhibit these pathways directly, or via binding of reduced glutathione (GSH) to 4-HNE or to PGJ2. Both inhibitions may lead to cell survival
Besides these protein–protein interactions, GSTs probably have an indirect role in the regulation of other pathways. Specifically, GSTA4 has been described to participate in the detoxification of 4-HNE, a product of lipid peroxidation which is involved in apoptosis and differentiation, and activates proteins such as adenylyl cyclase or JNK (Fig. 4) [73]. Furthermore, GSTA1, GSTM1 and GSTP1 conjugate prostaglandin J2 (PGJ2) with GSH [74]. PGJ2 can act as an activator ligand of peroxisome proliferator-activated receptor gamma (PPARγ), which induces apoptosis [75]; and as an inhibitor of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway [76], which results in the transcription of anti-apoptotic genes [77]. Thus, by neutralising different factors, GSTs are able to modify cellular fate. This ability of GSTs highlights the importance of further research into the interaction between PPARγ and NF-κB pathways during spermatogenesis.
Two other functions of GSTs, S-glutathionylation and nitric oxide (NO) storage, have been recently described. The first study that pointed out that GSTP1 could promote S-glutathionylation was carried out by Townsend et al. [78]. The authors evidenced that, although S-glutathionylation spontaneously occurs in many cell types to protect from cellular stress, it is significantly enhanced by GSTP. Moreover, self-S-glutathionylation of GSTP1 inhibits GSTP-JNK dimerization, which allows for JNK activation [78], leading to apoptosis. On the other hand, GSTP1 and multidrug resistance protein 1 (MRP1) are thought to be essential in protecting the cell against NO-mediated cytotoxicity. NO is a messenger associated with physiologic and cytotoxic processes which interact with iron–sulphur clusters to form dinitrosyl–dithiol iron-complexes (DNICs), the natural storage form of NO [79]. Dinitrosyl–dithiol iron-complexes can be removed from the cell by MRP1 [80] to prevent the cytotoxic effect of NO, which includes the GSH depletion through formation of dinitrosyl–diglutathionyl–iron complexes (DNDGIC). It has been suggested that GSTP1 could displace GSH from DNDGIC to prevent NO cytotoxicity [81]. Therefore, it seems that GSTP1 have a crucial role in preventing NO cytotoxicity.
Although none of these studies have been carried out on sperm, it is likely that GSTs also play a role in their signalling pathways. Due to the aforementioned importance of MAPK pathways in spermatogenesis and sperm functions, further research into the modulation of these pathways by GSTs could help to deepen or understanding of sperm physiology and, subsequently, male fertility.
Role of sperm GSTs in fertilisation
Sperm GSTM3 act as an early sperm–oocyte binding protein essential for fertilisation
Early in 1990, Shaha et al. [82] reported that the inhibition of a 24-kDa testicular protein reduced fertility in rats, identifying this protein as a member of the GST family. These authors described the localisation of GSTs in the acrosome of rat and goat sperm, and confirmed that antibody inhibition of these proteins interfered in rat and goat sperm–oocyte interaction, while maintaining their detoxification activity [34, 36]. Through more inhibition studies, GSTM was found to interfere in acrosin release and the binding to zona pellucida (ZP) [18], and it was suggested that the binding of sperm GSTM to ZP is a crucial step to achieve successful fertilisation. Since a covalent interaction between GSTM and the plasma membrane was not found, it has been suggested that these proteins are anchored through an integral membrane protein, which could activate a signalling pathway promoting membrane fusion and acrosome reaction [36].
Later studies have supported the notion that GSTM3 plays a role in sperm–oocyte binding. Petit et al. [19] performed Far Western blot and mass spectrometry assays using recombinant human ZP2, ZP3 and ZP4 glycoproteins to screen for sperm-head ZP-binding proteins. These authors identified an interaction between sperm GSTM3 and ZP3 and ZP4, which was confirmed by subsequent co-immunoprecipitation experiments. It is interesting to note that GSTM3 is absent from sperm after the acrosome reaction, suggesting that the binding of GSTM3 to ZP3 and ZP4 occurs within the first steps of gamete recognition [19]. These results do confirm the crucial role of GSTM3 binding to ZP4 during the first steps of gamete recognition to allow fertilisation to occur.
Sperm GSTOs enhance nuclear decondensation of sperm during fertilisation
Recently, Hamilton et al. [20] reported that specific inhibition of sperm GSTO2 delayed nuclear decondensation and head decompartmentalization of mouse sperm in the ooplasm. This highlights the importance of this sperm protein, as oocyte cytosolic GSTOs do not appear to be sufficient to carry out these important steps in fertilisation. The study of Hamilton et al. provides evidence for the hypothesis that GSH plays a role in sperm nucleus decondensation and male pronucleus formation [83] by reducing protamine–disulphide bonds [84]. However, further studies should elucidate the specific mechanism of GSTO2 and GSH in sperm nuclear decondensation, and the implication of other perinuclear theca proteins into this process.
Role of GSTs in male fertility
Many studies of various ethnic populations have shown that the GSTM1, GSTP1, GSTT1–/– genotype is higher in infertile than in fertile men [85–89]. Moreover, three meta-analyses performed between 2012 and 2013 evidenced that GSTM1–/– and GSTT1–/– genotypes are a risk factor for male idiopathic infertility [90–92]. Interestingly, GSH levels, and GPX and GSR activity, have been described to be substantially reduced in GSTT1–/– sperm, supporting the idea that GSTs play a crucial role in sperm physiology [87]. There is also evidence suggesting that GSTM1–/– men with idiopathic infertility have increased ROS in seminal plasma and sperm [93]. Based on these data, it could be suggested that the infertility phenotype of GSTM1–/–, GSTT1–/– and GSTP1–/– men is due to the inability to reduce OS and prevent sperm DNA damage. Supporting this notion, a previous study described that GSTM–/– men have higher susceptibility to sperm DNA damage when exposed to air pollution [94]. In addition, GSTM1–/– genotype has also been associated to higher PAH-DNA adducts in sperm caused by the exposure to polycyclic aromatic hydrocarbons (PAHs), which has been considered a direct sign of DNA damage due to air pollutants [95].
Several proteomic studies have been performed to characterize GSTM3 abundance as a marker of men and boar infertility. Using two-dimensional proteomic analysis in human sperm, Botta et al. [96] found a 3.7-fold increase of GSTM3 content in oligozoospermic samples in comparison with normospermic samples of proven fertility. Moreover, sperm GSTM3 was found to be overexpressed in infertile men with unilateral varicocele, suggesting its detoxification action in these patients [97]. Furthermore, GSTM3 was increased in seminal plasma of men with mitochondrial-altered sperm [98]. In pigs, Kwon et al. [99] found that higher GSTM3 levels in boar sperm were associated with smaller litter sizes. All these data indicate that higher relative levels of GSTM3 are linked to boar and men infertility, and as the study of Itanski et al. suggests, the mechanisms through which GSTM3 is associated to infertility could be mitochondrial-related.
In conclusion, GSTs play an essential role in maintaining sperm physiology and fertilising capabilities. While the molecular mechanisms behind GST-regulation of sperm function and fertility are yet to be determined, these enzymes have the potential to serve as robust infertility markers.
Concluding remarks
To the best of our knowledge, and despite being extensively characterised as antioxidant proteins, this is the first work describing the triple role of sperm GSTs in (1) detoxification, (2) regulation of cellular signalling and (3) fertilising ability. Many GSTs-family members have been described to be differentially expressed along the male reproductive tract, and localisation patterns of sperm GSTs are known to be variable across species and sperm maturation status. In addition, as opposed to other cell types, mounting evidence demonstrates the extracellular localisation of sperm GSTs: (1) GSTM3 and GSTP1 are secreted in the epididymis during sperm maturation; (2) the main detoxification function of GSTs occurs at the sperm plasma membrane level; (3) the principal GSH source is extracellular; and (4) its zona pellucida-binding capacity requires an extracellular orientation. Nevertheless, we cannot discard the intracellular activity of GSTs in post-testicular spermatozoa, which would explain its regulatory role in signalling pathways that still remain to be elucidated. Furthermore, an intracellular GST family member (GSTO2) has recently been localised in the perinuclear theca of sperm, where it has a role in male nuclear decondensation during fertilisation. What is more, it is important to highlight once again that several studies have shown a correlation of several GST isoforms (GSTM1, GSTM3, GSTP1 and GSTT1) with men infertility and reduced sperm quality. Impaired performance of any of the described GSTs functions (detoxification, cell signalling regulation and fertilising ability) may be the main cause of such effects. Therefore, we suggest that new research ventures should look at the relationship of GSTs with male infertility, as this could also address the potential of these proteins as infertility markers and/or therapeutic targets.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Confirmed transcript variants of GSTA1-GSTA5. Boxes represent exons and lines represent introns. In all family members, the first transcript variant corresponds to the canonical variant. Information was retrieved from RefSeq (https://www.ncbi.nlm.nih.gov/refseq/) [30] and Uniprot (https://www.uniprot.org/) [31] databases (TIFF 96 kb)
Confirmed transcript variants of GSTM1-GSTM5. Boxes represent exons and lines represent introns. In all family members, the first transcript variant corresponds to the canonical variant. The red transcript variants do not codify for any isoform. Information was retrieved from RefSeq (https://www.ncbi.nlm.nih.gov/refseq/) [30] and Uniprot (https://www.uniprot.org/) [31] databases (TIFF 128 kb)
Confirmed transcript variants of GSTO1, GSTO2, GSTP1 and GSTS1. Boxes represent exons and lines represent introns. In all family members, the first transcript variant corresponds to the canonical variant. Information was retrieved from RefSeq (https://www.ncbi.nlm.nih.gov/refseq/) [30] and Uniprot (https://www.uniprot.org/) [31] databases. (TIFF 101 kb)
Confirmed transcript variants of GSTT1, GSTT2 and GSTZ1. Boxes represent exons and lines represent introns. In all family members, the first transcript variant corresponds to the canonical variant. Information was retrieved from RefSeq (https://www.ncbi.nlm.nih.gov/refseq/) [30] and Uniprot (https://www.uniprot.org/) [31] databases (TIFF 155 kb)
Acknowledgements
The authors acknowledge the support from the European Commission (H2020-MSCA-IF-79212); the Ministry of Science, Innovation and Universities, Spain (Grants RYC-2014-15581, AGL2016-81890-REDT, AGL2017-88329-R and FJCI-2017-31689); and Regional Government of Catalonia, Spain (2017-SGR-1229). The authors would also like to thank Servier Medical Art for their image bank used to create all figures.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marc Llavanera and Yentel Mateo-Otero have contributed equally to the work.
Beatriz Fernández-Fuertes and Marc Yeste have contributed equally to the direction of this study.
References
- 1.Aitken RJ, Smith TB, Jobling MS, et al. Oxidative stress and male reproductive health. Asian J Androl. 2014;16:31–38. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghuman N, Ramalingam M. Male infertility. Obstet Gynaecol Reprod Med. 2017;28:7–14. doi: 10.1016/j.ogrm.2017.10.007. [DOI] [Google Scholar]
- 3.Betteridge DJ. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/S0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 4.Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2004;2:1–7. doi: 10.1186/1477-7827-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griveau JF, Le Lannou D. Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl. 1997;20:61–69. doi: 10.1046/j.1365-2605.1997.00044.x. [DOI] [PubMed] [Google Scholar]
- 6.García Rodríguez A, de la Casa M, Johnston S, et al. Association of polymorphisms in genes coding for antioxidant enzymes and human male infertility. Ann Hum Genet. 2019;83:63–72. doi: 10.1111/ahg.12286. [DOI] [PubMed] [Google Scholar]
- 7.Kodama H, Kuribayashi Y, Gagnon C. Effect of sperm lipid peroxidation on fertilization. J Androl. 1996;17:151–157. doi: 10.1002/j.1939-4640.1996.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith MA, Perry G, Pryor WA. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. 2016;28:1–10. doi: 10.1016/S0891-5849(02)00793-1. [DOI] [PubMed] [Google Scholar]
- 10.Aitken RJ, Curry BJ. Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxidants Redox Signal. 2011 doi: 10.1089/ars.2010.3186. [DOI] [PubMed] [Google Scholar]
- 11.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 12.Shaha C, Suri A, Talwar GP. Identification of sperm antigens that regulate fertility. Int J Androl. 1988;11:479–491. doi: 10.1111/j.1365-2605.1988.tb01022.x. [DOI] [PubMed] [Google Scholar]
- 13.Raijmakers MTM, Roelofs HMJ, Steegers EAP, et al. Glutathione and glutathione S-transferases A1-1 and P1-1 in seminal plasma may play a role in protecting against oxidative damage to spermatozoa. Fertil Steril. 2003;79:169–172. doi: 10.1016/S0015-0282(02)04404-7. [DOI] [PubMed] [Google Scholar]
- 14.Dierickx P. Soluble glutathione S-transferases from rat testes: isoenzyme pattern and lack of inducibility by drug metabolizing enzyme inducers. Toxicol Res Appl. 1982;4:47–51. [PubMed] [Google Scholar]
- 15.Hemachand T, Shaha C. Functional role of sperm surface glutathione S-transferases and extracellular glutathione in the haploid spermatozoa under oxidative stress. FEBS Lett. 2003;538:14–18. doi: 10.1016/S0014-5793(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 16.Adler V, Yin Z, Fuchs SY, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luna C, Mendoza N, Casao A, et al. c-Jun N-terminal kinase and p38 mitogen-activated protein kinase pathways link capacitation with apoptosis and seminal plasma proteins protect sperm by interfering with both routes. Biol Reprod. 2017;96:800–815. doi: 10.1093/biolre/iox017. [DOI] [PubMed] [Google Scholar]
- 18.Hemachand T, Gopalakrishnan B, Salunke DM, et al. Sperm plasma-membrane-associated glutathione S-transferases as gamete recognition molecules. J Cell Sci. 2002;115:2053–2065. doi: 10.1242/jcs.115.10.2053. [DOI] [PubMed] [Google Scholar]
- 19.Petit FM, Serres C, Bourgeon F, et al. Identification of sperm head proteins involved in zona pellucida binding. Hum Reprod. 2013;28:852–865. doi: 10.1093/humrep/des452. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton LE, Suzuki J, Aguila L, et al. Sperm-borne glutathione-S-transferase omega 2 accelerates the nuclear decondensation of spermatozoa during fertilization in mice. Biol Reprod. 2019;101:368–376. doi: 10.1093/biolre/ioz082. [DOI] [PubMed] [Google Scholar]
- 21.Combes B, Stakelum GS. A liver enzyme that conjugates sulfobromophthalein sodium with glutathione. J Clin Invest. 1961;40:981–988. doi: 10.1172/JCI104337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta. 2013;1830:3217–3266. doi: 10.1016/j.bbagen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Mannervik B, Board PG, Hayes JD, et al. Nomenclature for mammalian soluble glutathione transferases. Methods Enzymol. 2005;401:1–8. doi: 10.1016/S0076-6879(05)01001-3. [DOI] [PubMed] [Google Scholar]
- 24.Morel F, Rauch C, Petit E, et al. Gene and protein characterization of the human glutathione S-transferase kappa and evidence for a peroxisomal localization. J Biol Chem. 2004;279:16246–16253. doi: 10.1074/jbc.M313357200. [DOI] [PubMed] [Google Scholar]
- 25.Mannervik B, Jensson H. Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J Biol Chem. 1982;257:9909–9912. [PubMed] [Google Scholar]
- 26.Mannervik B, Danielson UH. Glutathione transferases—structure and catalytic activity. Crit Rev Biochem. 1988;23:283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- 27.Di Pietro G, LA Magno V, Rios-Santos F. Glutathione S-transferases: an overview in cancer research. Expert Opin Drug Metab Toxicol. 2010;6:153–170. doi: 10.1517/17425250903427980. [DOI] [PubMed] [Google Scholar]
- 28.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:521–600. doi: 10.3109/10409239509083492. [DOI] [PubMed] [Google Scholar]
- 29.Wongsantichon J, Ketterman AJ. Alternative splicing of glutathione S-transferases. Methods Enzymol. 2005;401:100–116. doi: 10.1016/S0076-6879(05)01006-2. [DOI] [PubMed] [Google Scholar]
- 30.O’Leary NA, Wright MW, Brister JR, et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44:733–745. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The UniProt Consortium UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:506–515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheehan D, Meade G, Foley V, Dowd C. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukhtar H, Lee IP, Bend JR. Glutathione S-transferase activities in rat and mouse sperm and human semen. Biochem Biophys Res Commun. 1978;83:1093–1098. doi: 10.1016/0006-291X(78)91507-3. [DOI] [PubMed] [Google Scholar]
- 34.Aravinda S, Gopalakrishnan B, Dey CS, et al. A testicular protein important for fertility has glutathione S-transferase activity and is localized extracellularly in the seminiferous tubules. J Biol Chem. 1995;270:15675–15685. doi: 10.1074/jbc.270.26.15675. [DOI] [PubMed] [Google Scholar]
- 35.Fulcher KD, Welch JE, Klapper DG, et al. Identification of a unique μ-class glutathione S-transferase in mouse spermatogenic cells. Mol Reprod Dev. 1995;42:415–424. doi: 10.1002/mrd.1080420407. [DOI] [PubMed] [Google Scholar]
- 36.Gopalakrishnan B, Aravinda S, Pawshe CH, et al. Studies on glutathione S-transferase important for sperm function: evidence of catalytic activity-independent functions. Biochem J. 1998;329:231–241. doi: 10.1042/bj3290231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar R, Singh VK, Atreja SK. Glutathione-S-transferase: role in buffalo (Bubalus bubalis) sperm capacitation and cryopreservation. Theriogenology. 2014;81:587–598. doi: 10.1016/j.theriogenology.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Llavanera M, Delgado-Bermúdez A, Fernandez-Fuertes B, et al. GSTM3, but not IZUMO1, is a cryotolerance marker of boar sperm. J Anim Sci Biotechnol. 2019 doi: 10.1186/s40104-019-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamilton LE, Acteau G, Xu W, et al. The developmental origin and compartmentalization of glutathione-s-transferase omega 2 isoforms in the perinuclear theca of eutherian spermatozoa. Biol Reprod. 2017;97:612–621. doi: 10.1093/biolre/iox122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Protopapas N, Hamilton LE, Warkentin R, et al. The perforatorium and postacrosomal sheath of rat spermatozoa share common developmental origins and protein constituents. Biol Reprod. 2019;100:1461–1472. doi: 10.1093/biolre/ioz052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batruch I, Lecker I, Kagedan D, et al. Proteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital system. J Proteome Res. 2011;10:941–953. doi: 10.1021/pr100745u. [DOI] [PubMed] [Google Scholar]
- 42.Zubkova EV, Robaire B. Effect of glutathione depletion on antioxidant enzymes in the epididymis, seminal vesicles, and liver and on spermatozoa motility in the aging brown Norway rat. Biol Reprod. 2004;71:1002–1008. doi: 10.1095/biolreprod.104.028373. [DOI] [PubMed] [Google Scholar]
- 43.Klys HS, Whillis D, Howard G, Harrison DJ. Glutathione S-transferase expression in the human testis and testicular germ cell neoplasia. Br J Cancer. 1992;66:589–593. doi: 10.1038/bjc.1992.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu M, Hu Z, Qi L, et al. Scanning of novel cancer/testis proteins by human testis proteomic analysis. Proteomics. 2013;13:1200–1210. doi: 10.1002/pmic.201200489. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee SB, Aravinda S, Gopalakrishnan B, et al. Secretion of glutathione S-transferase isoforms in the seminiferous tubular fluid, tissue distribution and sex steroid binding by rat GSTM1. Biochem J. 1999;340:309. doi: 10.1042/0264-6021:3400309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu Z, Guo R, Ge Y, et al. Gene expression profiles in different stages of mouse spermatogenic cells during spermatogenesis1. Biol Reprod. 2003;69:37–47. doi: 10.1095/biolreprod.102.012609. [DOI] [PubMed] [Google Scholar]
- 47.Paz M, Morín M, del Mazo J. Proteome profile changes during mouse testis development. Comp Biochem Physiol. 2006;1:404–415. doi: 10.1016/j.cbd.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Liu F, Wang H, et al. Systematic mapping and functional analysis of a family of human epididymal secretory sperm-located proteins. Mol Cell Proteomics. 2010;9:2517–2528. doi: 10.1074/mcp.m110.001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thimon V, Frenette G, Saez F, et al. Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum Reprod. 2008;23:1698–1707. doi: 10.1093/humrep/den181. [DOI] [PubMed] [Google Scholar]
- 50.Suryawanshi AR, Khan SA, Gajbhiye RK, et al. Differential proteomics leads to identification of domain-specific epididymal sperm proteins. J Androl. 2011;32:240–259. doi: 10.2164/jandrol.110.010967. [DOI] [PubMed] [Google Scholar]
- 51.Dacheux JL, Belleannée C, Jones R, et al. Mammalian epididymal proteome. Mol Cell Endocrinol. 2009;306:45–50. doi: 10.1016/j.mce.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Utleg AG, Yi EC, Xie T, et al. Proteomic analysis of human prostasomes. Prostate. 2003;56:150–161. doi: 10.1002/pros.10255. [DOI] [PubMed] [Google Scholar]
- 53.Carlsson L, Ronquist G, Stridsberg M, Johansson L. Motility stimulant effects of prostasome inclusion in swim-up medium on cryopreserved human spermatozoa. Arch Androl. 1997;38:215–221. doi: 10.3109/01485019708994880. [DOI] [PubMed] [Google Scholar]
- 54.Cross N, Mahasreshti P. Prostasome fraction of human seminal plasma prevents sperm from becoming acrosomally responsive to the agonist progesterone. Arch Androl. 1997;39:30–44. doi: 10.3109/01485019708987900. [DOI] [PubMed] [Google Scholar]
- 55.Crocitto LE, Korns D, Kretzner L, et al. Prostate cancer molecular markers GSTP1 and hTERT in expressed prostatic secretions as predictors of biopsy results. Urology. 2004;64:821–825. doi: 10.1016/j.urology.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Armstrong RN, Morgenstern R, Board PG. Glutathione transferases. In: McQueen C, editor. Comprehensive toxicology. 3. Amsterdam: Elsevier; 2017. pp. 326–362. [Google Scholar]
- 57.Maselli J, Hales BF, Chan P, Robaire B. Exposure to bleomycin, etoposide, and cis-platinum alters rat sperm chromatin integrity and sperm head protein profile1. Biol Reprod. 2012;86:1–10. doi: 10.1095/biolreprod.111.098616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Z, Li S, Yu Y, et al. Alterations in epididymal proteomics and antioxidant activity of mice exposed to fluoride. Arch Toxicol. 2018;92:169–180. doi: 10.1007/s00204-017-2054-2. [DOI] [PubMed] [Google Scholar]
- 59.Knapen MFCM, Zusterzeel PLM, Peters WHM, Steegers EAP. Glutathione and glutathione-related enzymes in reproduction: a review. Eur J Obstet Gynecol Reprod Biol. 1999;82:171–184. doi: 10.1016/S0301-2115(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 60.Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeulin C, Soufir JC, Weber P, et al. Catalase activity in human spermatozoa and seminal plasma. Gamete Res. 1989;24:185–196. doi: 10.1002/mrd.1120240206. [DOI] [PubMed] [Google Scholar]
- 62.O’Flaherty C, Rico de Souza A. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol Reprod. 2010;84:238–247. doi: 10.1095/biolreprod.110.085712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 64.Chen W, Ni Y, Pan Y, et al. GABA, progesterone and zona pellucida activation of PLA 2 and regulation by MEK-ERK1/2 during acrosomal exocytosis in guinea pig spermatozoa. FEBS Lett. 2005;579:4692–4700. doi: 10.1016/j.febslet.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 65.De Lamirande E, Gagnon C. The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol Hum Reprod. 2002;8:124–135. doi: 10.1093/molehr/8.2.124. [DOI] [PubMed] [Google Scholar]
- 66.Nixon B, Bielanowicz A, Anderson AL, et al. Elucidation of the signaling pathways that underpin capacitation-associated surface phosphotyrosine expression in mouse spermatozoa. J Cell Physiol. 2010;224:71–83. doi: 10.1002/jcp.22090. [DOI] [PubMed] [Google Scholar]
- 67.Almog T, Lazar S, Reiss N, et al. Identification of extracellular signal-regulated kinase 1/2 and p38 MAPK as regulators of human sperm motility and acrosome reaction and as predictors of poor spermatozoan quality. J Biol Chem. 2008;283:14479–14489. doi: 10.1074/jbc.M710492200. [DOI] [PubMed] [Google Scholar]
- 68.Plessis SS, Page C, Franken DR. Extracellular signal-regulated kinase activation involved in human sperm-zona pellucida binding. Andrologia. 2002;34:55–59. doi: 10.1046/j.0303-4569.2001.00475.x. [DOI] [PubMed] [Google Scholar]
- 69.Elsby R, Kitteringham NR, Goldring CE, et al. Increased constitutive c-Jun N-terminal kinase signaling in mice lacking glutathione S-transferase Pi. J Biol Chem. 2003;278:22243–22249. doi: 10.1074/jbc.M301211200. [DOI] [PubMed] [Google Scholar]
- 70.Desmots F, Rissel M, Gilot D, et al. Pro-inflammatory cytokines tumor necrosis factor α and interleukin-6 and survival factor epidermal growth factor positively regulate the murine GSTA4 enzyme in hepatocytes. J Biol Chem. 2002;277:17892–17900. doi: 10.1074/jbc.M112351200. [DOI] [PubMed] [Google Scholar]
- 71.Cho S, Lee YH, Park H, et al. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem. 2001;276:12749–12755. doi: 10.1074/jbc.M005561200. [DOI] [PubMed] [Google Scholar]
- 72.Piaggi S, Raggi C, Corti A, et al. Glutathione transferase omega 1-1 (GSTO1-1) plays an anti-apoptotic role in cell resistance to cisplatin toxicity. Carcinogenesis. 2010;31:804–811. doi: 10.1093/carcin/bgq031. [DOI] [PubMed] [Google Scholar]
- 73.Awasthi YC, Sharma R, Cheng JZ, et al. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Mol Asp Med. 2003;24:219–230. doi: 10.1016/S0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- 74.Bogaards JJP, Venekamp JC, Van Bladeren PJ. Stereoselective conjugation of prostaglandin A2 and prostaglandin J2 with glutathione, catalyzed by the and P1-1. Chem Res Toxicol. 1997;10:310–317. doi: 10.1021/tx9601770. [DOI] [PubMed] [Google Scholar]
- 75.Kim EH, Surh YJ. The role of 15-deoxy-Δ12,14-prostaglandin J2, an endogenous ligand of peroxisome proliferator-activated receptor γ, in tumor angiogenesis. Biochem Pharmacol. 2008;76:1544–1553. doi: 10.1016/j.bcp.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 76.Straus DS, Pascual G, Li M, et al. 15-Deoxy-Delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci. 2002;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-κB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 78.Townsend DM, Manevich Y, He L, et al. Novel role for glutathione S-transferase π. J Biol Chem. 2008;284:436–445. doi: 10.1074/jbc.m805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pajaud J, Kumar S, Rauch C, et al. Regulation of signal transduction by glutathione transferases. Int J Hepatol. 2012 doi: 10.1155/2012/137676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richardson DR, Lok HC. The nitric oxide-iron interplay in mammalian cells: transport and storage of dinitrosyl iron complexes. Biochim Biophys Acta. 2008;1780:638–651. doi: 10.1016/j.bbagen.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 81.Cesareo E, Parker LJ, Pedersen JZ, et al. Nitrosylation of human glutathione transferase P1-1 with dinitrosyl diglutathionyl iron complex in vitro and in vivo. J Biol Chem. 2005;280:42172–42180. doi: 10.1074/jbc.M507916200. [DOI] [PubMed] [Google Scholar]
- 82.Shaha C, Suri A, Talwar GP. Induction of infertility in female rats after active immunization with 24 kD antigens from rat testes. Int J Androl. 1990;13:17–25. doi: 10.1111/j.1365-2605.1990.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 83.Sutovsky P, Schatten G. Depletion of glutathione during bovine oocyte maturation reversibly blocks the decondensation of the male pronucleus and pronuclear apposition during fertilization1. Biol Reprod. 1997;56:1503–1512. doi: 10.1095/biolreprod56.6.1503. [DOI] [PubMed] [Google Scholar]
- 84.Yeste M. Boar spermatozoa within the oviductal environment (III): Fertilisation. In: Bonet S, Casas I, Holt WV, Yeste M, editors. Boar reproduction. 1. Heidelberg: Springer; 2013. pp. 407–467. [Google Scholar]
- 85.Aydos SE, Ph D, Taspinar M, et al. Association of CYP1A1 and glutathione S-transferase polymorphisms with male factor infertility. Fertil Steril. 2009;92:541–547. doi: 10.1016/j.fertnstert.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 86.Vani GT, Mukesh N, Siva Prasad B, et al. Role of glutathione S-transferase Mu-1 (GSTM1) polymorphism in oligospermic infertile males. Andrologia. 2010;42:213–217. doi: 10.1111/j.1439-0272.2009.00971.x. [DOI] [PubMed] [Google Scholar]
- 87.Kolesnikova LI, Kurashova NA, Bairova TA, et al. Role of glutathione-S-transferase family genes in male infertility. Bull Exp Biol Med. 2017;163:643–645. doi: 10.1007/s10517-017-3869-9. [DOI] [PubMed] [Google Scholar]
- 88.Safarinejad MR, Shafiei N, Safarinejad S. The association of glutathione-S-transferase gene polymorphisms (GSTM1, GSTT1, GSTP1) with idiopathic male infertility. J Hum Genet. 2010;55:565–570. doi: 10.1038/jhg.2010.59. [DOI] [PubMed] [Google Scholar]
- 89.Lakpour N, Mirfeizollahi A, Farivar S, et al. The association of seminal plasma antioxidant levels and sperm chromatin status with genetic variants of GSTM1 and GSTP1 (Ile105Val and Ala114Val) in infertile men with oligoasthenoteratozoospermia. Dis Markers. 2013;34:205–210. doi: 10.3233/DMA-120954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang M, Wang S, Wang W, et al. The glutathione-S-transferase gene polymorphisms (GSTM1 and GSTT1) and idiopathic male infertility risk: a meta-analysis. Gene. 2012;511:218–223. doi: 10.1016/j.gene.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 91.Kan HP, Wu FL, Bin Guo W, et al. Null genotypes of GSTM1 and GSTT1 contribute to male factor infertility risk: a meta-analysis. Fertil Steril. 2013;99:690–696. doi: 10.1016/j.fertnstert.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 92.Song X, Zhao Y, Cai Q, et al. Association of the glutathione S-transferases M1 and T1 polymorphism with male infertility: a meta-analysis. J Assist Reprod Genet. 2013;30:131–141. doi: 10.1007/s10815-012-9907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aydemir B, Onaran I, Kiziler AR, et al. Increased oxidative damage of sperm and seminal plasma in men with idiopathic infertility is higher in patients with glutathione S-transferase Mu-1 null genotype. Asian J Androl. 2007;9:108–115. doi: 10.1111/j.1745-7262.2007.00237.x. [DOI] [PubMed] [Google Scholar]
- 94.Rubes J, Selevan SG, Sram RJ, et al. GSTM1 genotype influences the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutat Res. 2007;625:20–28. doi: 10.1016/j.mrfmmm.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 95.Paracchini V, Chang SS, Santella RM, et al. GSMT1 deletion modifies the levels of polycyclic aromatic hydrocarbon-DNA adducts in human sperm. Mutat Res Genet Toxicol Environ Mutagen. 2005;586:97–101. doi: 10.1016/j.mrgentox.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 96.Botta T, Blescia S, Martínez-Heredia J, et al. Identificación de diferencias proteómicas en muestras oligozoospérmicas. Rev Int Androl. 2009;7:14–19. doi: 10.1016/S1698-031X(09)70257-2. [DOI] [Google Scholar]
- 97.Agarwal A, Sharma R, Durairajanayagam D, et al. Major protein alterations in spermatozoa from infertile men with unilateral varicocele. Reprod Biol Endocrinol. 2015;13:1–22. doi: 10.1186/s12958-015-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Intasqui P, Camargo M, Antoniassi MP, et al. Association between the seminal plasma proteome and sperm functional traits. Fertil Steril. 2016;105:617–628. doi: 10.1016/j.fertnstert.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 99.Kwon WS, Oh SA, Kim YJ, et al. Proteomic approaches for profiling negative fertility markers in inferior boar spermatozoa. Sci Rep. 2015;5:1–10. doi: 10.1038/srep13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Allocati N, Masulli M, Di Ilio C, Federici L. Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis. 2018 doi: 10.1038/s41389-017-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pannala VR, Dash RK. Mechanistic characterization of the thioredoxin system in the removal of hydrogen peroxide. Free Radic Biol Med. 2015;78:42–55. doi: 10.1016/j.freeradbiomed.2014.10.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manevich Y, Feinstein SI, Fisher AB. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with GST. Proc Natl Acad Sci. 2004;101:3780–3785. doi: 10.1073/pnas.0400181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 104.Lloyd RV, Hanna PM, Mason RP. The origin of the hydroxyl radical oxygenin the Fenton reaction. Free Radic Biol Med. 1997;22:885–888. doi: 10.1016/S0891-5849(96)00432-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Confirmed transcript variants of GSTA1-GSTA5. Boxes represent exons and lines represent introns. In all family members, the first transcript variant corresponds to the canonical variant. Information was retrieved from RefSeq (https://www.ncbi.nlm.nih.gov/refseq/) [30] and Uniprot (https://www.uniprot.org/) [31] databases (TIFF 96 kb)
Confirmed transcript variants of GSTM1-GSTM5. Boxes represent exons and lines represent introns. In all family members, the first transcript variant corresponds to the canonical variant. The red transcript variants do not codify for any isoform. Information was retrieved from RefSeq (https://www.ncbi.nlm.nih.gov/refseq/) [30] and Uniprot (https://www.uniprot.org/) [31] databases (TIFF 128 kb)
Confirmed transcript variants of GSTO1, GSTO2, GSTP1 and GSTS1. Boxes represent exons and lines represent introns. In all family members, the first transcript variant corresponds to the canonical variant. Information was retrieved from RefSeq (https://www.ncbi.nlm.nih.gov/refseq/) [30] and Uniprot (https://www.uniprot.org/) [31] databases. (TIFF 101 kb)
Confirmed transcript variants of GSTT1, GSTT2 and GSTZ1. Boxes represent exons and lines represent introns. In all family members, the first transcript variant corresponds to the canonical variant. Information was retrieved from RefSeq (https://www.ncbi.nlm.nih.gov/refseq/) [30] and Uniprot (https://www.uniprot.org/) [31] databases (TIFF 155 kb)