Abstract
CD4 T-helper (Th) cells secret a variety of inflammatory cytokines and play critical roles in host defense against invading foreign pathogens. On the other hand, uncontrolled inflammatory responses mediated by Th cells may result in tissue damage and inflammatory disorders including autoimmune and allergic diseases. Thus, the induction of anti-inflammatory cytokine expression becomes an important “brake” to repress and/or terminate aberrant and/or unnecessary immune responses. Interleukin-10 (IL-10) is one of the most important anti-inflammatory cytokines to limit inflammatory Th cells and immunopathology and to maintain tissue homeostasis. Many studies have indicated that Th cells can be a major source of IL-10 under specific conditions both in mouse and human and that extracellular signals and cell intrinsic molecular switches are required to turn on and off Il10 expression in different Th cells. In this review, we will highlight the recent findings that have enhanced our understanding on the mechanisms of IL-10 induction in distinct Th-cell subsets, including Th1, Th2, and Th17 cells, as well as the importance of these IL-10-producing anti-inflammatory Th cells in immunity and inflammation.
Keywords: T-cell activation, T-helper cell differentiation, Immunopathology, T-cell homeostasis, Transcriptional regulation, Cytokine expression, C-Maf, Bhlhe40
Introduction
In response to foreign- or self-antigens, naïve CD4 T cells differentiate into type 1 T helper (Th1), Th2, Th17, follicular T helper (Tfh), or peripherally derived T regulatory (pTreg) cells [1–3]. Master transcription factors (T-bet for Th1, GATA3 for Th2, and RORγt for Th17) control the fate determination of T-helper (Th) cells and the production of effector pro-inflammation cytokines [4, 5]. An appropriate immune response is critical for pathogen clearance; however, uncontrolled inflammatory responses, sometimes linked to a cytokine storm, can often result in severe immunopathology or chronic inflammation in the host. Regulatory cytokine IL-10, encoded by the Il10 gene, was originally named as cytokine synthesis inhibitory factor and it is mainly expressed by Th2 cells and inhibits Th1 cell differentiation [6]. As a multifunctional anti-inflammatory cytokine, IL-10 targets various innate and adaptive leukocytes to repress their activation and functions, through which it suppresses inflammatory cytokine eruption, prevents host damage, and maintains functional tissue integrity [7–9]. Up to date, many different cell types including CD4 T cells, CD8 T cells, dendritic cells (DCs), macrophages, mast cells, conventional natural killer cells (cNKs), eosinophils, neutrophils, B cells, as well as some innate lymphoid cells (ILCs) are reported to produce IL-10. Among the CD4 T cells, Foxp3+ Treg cells were thought to be the professional IL-10-producing CD4 T-cell subset [10, 11]. However, majority of the CD4+CD25+ Treg cells, except for intestinal Treg cells, do not produce IL-10 when they are immediately re-stimulated ex vivo [12], and retroviral transduction of Foxp3 fails to induce Il10 expression [13], suggesting that IL-10 production is not a unique feature of Treg cells. Nevertheless, IL-10 produced by Treg cells while not essential for controlling early onset of systemic autoimmunity, and it plays an important role in limiting immune responses in colon and lungs [14]. Foxp3-negative IL-10-producing CD4 T cells have been designated as Tr1 cells [15, 16]. However, in contrast to other effector Th-cell subsets, Tr1 cells do not have lineage-specific master transcription factor(s) to define their differentiation. Accumulating evidence shows that all Th-cell subsets, including Th1, Th2, and Th17 cells, have the capability to produce IL-10 both in vitro and in vivo [6, 17–19], and these Th cells may control their own inflammatory and anti-inflammatory functions through switching on and off IL-10 production. Thus, Tr1 cells may be very heterogeneous and they may not represent a separate lineage. Tr1 cells, by definition, include IL-10-producing cells from distinct effector Th-cell subsets. The molecular mechanisms of Il10 induction are still not fully understood partly due to its regulation being cell-type specific or environment dependent. In this review, we will summarize our knowledge on similarities and differences of molecular switches including important transcription factors for regulating Il10 expression in Th1, Th2, and Th17 cells, the presence of IL-10-producing Th-cell subsets in disease settings (both in human and in mouse), and their potential functions in regulating inflammatory and anti-inflammatory immune responses.
IL-10-producing Th cells in diseases
IL-10-producing Th2 cells in diseases
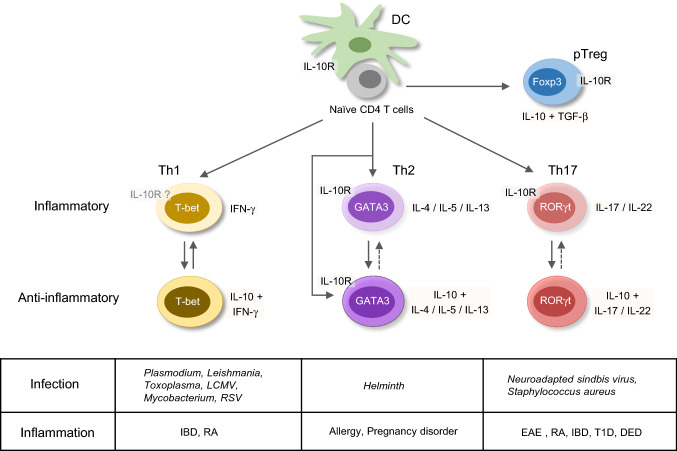
The function of IL-10-producing Th cells is brought into attention in both human and murine physiological and pathologic conditions (Fig. 1). IL-10 was first regarded as a Th2 cytokine. Indeed, peripheral blood mononuclear cells (PBMCs) from house dust mite (HDM)-sensitized asthmatic patients express both IL-5 and IL-10 after re-stimulated with antigen ex vivo [20]. In mouse skin, repeated infection with helminth Schistosoma mansoni larvae that elicits a strong Th2 response induces IL-10+ CD4 T-cell population which do not express Treg makers such as Foxp3, Helios, Nrp1, and CD223 [21]. Moreover, an in vitro proliferation assay shows that those IL-10+ CD4 T cells suppress the proliferation of CD4 T cells from the skin draining lymph nodes (dLNs). Furthermore, IL-4-producing Th2 cells are recognized as one of the major sources of IL-10 production during helminth infection [21] and in HDM-induced allergic airway inflammation [22]. In addition, IL-4- and IL-10-producing Th2 cells are important in tolerance during a successful pregnancy and in allograft, which has been reviewed by Chatterjee et al. [23].
Fig. 1.
IL-10-producing T-helper cells in diseases. Upon T-cell receptor engagement and co-stimulation signaling, naïve CD4 T cells differentiate into type 1 T-helper (Th1), Th2, and Th17 cells, as well as IL-10-producing anti-inflammatory peripherally derived regulatory T (pTreg) cells. Under certain conditions, Th cells also have capability to express IL-10 in addition to their effector cytokines. IL-10+ Th1 cells are identified in Plasmodium induced malaria, and during Leishmania, Toxoplasma, lymphocytic choriomeningitis virus (LCMV), Mycobacterium and respiratory syncytial virus (RSV) infection, as well as in inflammatory bowel disease (IBD) and rheumatoid arthritis (RA) disease. Th1 and IL-10+ Th1 cells may switch between each other, and Th1 cells express low levels of IL-10R. IL-10+ Th2 cells are induced in helminth infection, allergy, and pregnancy disorder conditions. Both Th2 and IL-10+ Th2 cells express IL-10R. IL-10+ Th17 cells are found in neuroadapted sindbis virus and Staphylococcus aureus infection, experimental autoimmune encephalomyelitis (EAE), RA, IBD, type 1 diabetes (T1D), and dry eye disease (DED). The IL-10R-expressing Th17 cells play critical roles in inflammatory diseases
IL-10-producing Th1 cells in diseases
IL-10 production by Th1 cells has also been reported in many disease settings. Malaria-specific IL-10+IFN-γ+ CD4 T cells are induced in children exposed to Plasmodium, although whether these co-producing CD4 T cells are associated with future protection is still controversial [24–26]. Nevertheless, it has been shown that IL-10+IFN-γ+ CD4 T cells are present in a higher frequency in uncomplicated malaria than that in severe malaria [26]. A recent review has summarized the role of IL-10 in malaria [27]. In another important human infectious disease leishmaniasis, IL-10-producing Th cells have been implicated in regulating pathogen clearance versus chronic infection. In cutaneous leishmaniasis patients, the major source of IL-10 is the CD25−CD127−/lowFOXP3− CD4 T cells, and the Il10 mRNA is correlated with IFN-γ, IL-27, and IL-21 [28]. During chronic Leishmania donovani infection, IL-10+IFN-γ+T-bet+ Th1 cells are dramatically expanded [29]. It is consistent with the observation that in a murine model of clinical isolated Leishmania major infection, IFN-γ+IL-10+Foxp3− Th1 cells contribute to repressing the healing process [30]. IL-10 produced by T cell are also clinically relevant to tuberculosis pathogenesis [31–33]. Upon tubercle bacillus (TB) antigen- or anti-CD3 antibody-triggered stimulation, IL-10-producing CD4 T cells including Th1 (IL-10+IFN-γ+T-bet+), Th2 (IL-10+IL-4+GATA3+), Th17 (IL-10+IL-17+), and Treg cells (IL-10+CD4+CD25+Foxp3+) from PBMCs of the pulmonary tuberculosis (PTB) patients are expanded [34]. In addition, the frequency of IL-10+ Th1 cells and Treg cells is related to TB burden and disease severity [34]. Bronchoalveolar lavages (BAL) derived T-cell clones from TB patients produce significant IFN-γ and IL-10 [31]. A significant population of IL-10+T-bet+CD44hi CD4 T cells are found 21 day post Mycobacterium tuberculosis (MTB) infection, and repress inflammatory Th1 cell function [35]. Such IL-10-producing Th1 cells may impair bacterial clearance and contribute to chronic infection [35]. IL-10+IFN-γ+ CD4 T cells are also observed in experimental murine models of acute respiratory syncytial virus (RSV) and Toxoplasma gondii (T. gondii) infection [36, 37]. The latter is a very useful model for investigating the dual functions of IL-10+ Th1 cells in pathogen clearance and in immunopathology, as deleting Il10 in T cells results in an enhanced control of parasite expansion but with severe immunopathology [37, 38].
IL-10-producing Th17 cells in diseases
Th17 cells can also express IL-10. The relationship between IL-10-producing Th17 cells and autoimmune diseases, including experimental autoimmune encephalomyelitis (EAE), type 1 diabetes (T1D), rheumatoid arthritis (RA), and inflammatory bowel disease (IBD), has been reviewed recently [39]. Clinically, higher Il10 mRNA in Th17 cells is found in clinically stable patients compared with patients with active multiple sclerosis (MS) [40]; myelin-reactive CD4 T-cell clones from healthy controls produce higher IL-10 compared to those from the MS patients that express higher levels of IFN-γ, IL-17, and GM-CSF [41]. These observations suggest that IL-10-producing Th17 cells may be beneficial in controlling autoimmunity and that these cells are either repressed or switched to the IL-10− Th17 cells in the MS patients. In addition, during the acute dry eye disease (DED), reduced frequency of IL-10+ Th17 cells is associated with an increased production of IL-17 and IL-23 and a higher frequency of IL-17+ CD4 T cells [42], suggesting that the IL-10-producing Th17 cells are important to repress the inflammatory Th17 cells. IL-10-producing Th17 cells are also found during pathogen infection. For example, Staphylococcus aureus-primed human naïve CD4 T cells will differentiate into IL-10-producing Th17 cells [43]. In Neuroadapted sindbis virus (NSV) infection murine model, Il10−/− Th17 cells in the CNS have Th1/Th17 phenotype and seem to be more pathogenic [44, 45].
Genomic organization of the Il10 locus in T cells
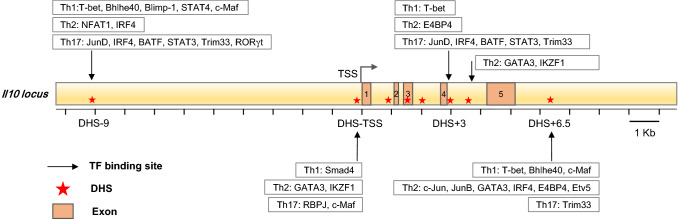
The Il10 locus includes 5 exons, and several DNase I hypersensitivity sites (DHSs) that have been identified in different immune cells [19, 46] (Fig. 2). DHSs are functionally related to transcriptional activity and necessary for the binding of many transcription factors. While naive CD4 T cells lack DHSs at the Il10 locus, Th1 and Th2 cells display different inducible DHSs at the Il10 locus, indicating that Th1 and Th2 cells utilize different mechanisms to induce Il10 expression [46–48].
Fig. 2.
Genomic regulatory elements at the Il10 locus in T-helper cells. The murine Il10 gene comprises five exons and many cis-regulatory elements, which are identified as DNase I hypersensitivity sites (DHSs). In Th cells, there are four major functional DHSs: DHS-9, DHS-transcriptional start site (DHS-TSS), DHS + 3, and DHS + 6.5. Different transcription factors bind to those sites to regulate IL-10 expression in different cell types
Il10 locus in Th2 cells
Four important DHSs are found at the Il10 locus in different Th cells: DHS-9, DHS-transcriptional start site (DHS-TSS), DHS + 3, and DHS + 6.5 (Fig. 2). Distinct from Il10 regulation in Th1 cells, the Il10 locus in Th2 cells seems to be fully prepared to produce IL-10, since no additional DHS is induced upon reactivation when higher levels of Il10 mRNA are induced in differentiated Th2 cells [47]. In Th2 cells, DHS + 6.5 (6.5 kb downstream of the TSS), conserved between murine and human, is a strong DHS and bound by c-Jun and JunB; however, this site is not bound by c-Jun and JunB in Th1 cells [49]. More importantly, DHS + 6.5, but not the Il10 promoter, responds to phorbol myristate acetate (PMA) and ionomycin stimulation [49]. In addition, E4BP4, IRF4, c-Jun, JunB, and Etv5 bind to the DHS + 6.5 region rather than the Il10 promoter to induce IL-10 expression in Th2 cells [50]. The master transcription factor (TF) of Th2 cells, GATA3, binds to DHS-TSS [51, 52] and DHS-3.7 [51] in addition to DHS + 6.5 [50]. These data suggest that DHS + 6.5 is the major Il10 trans-activity site, at least in Th2 cells. Similarly, DHS-9 bound by NFAT1 and IRF4 is critical for Il10 induction in Th2 cells, not Th1 cells [53]. Even for the transcription factor c-Maf, which was initially reported to regulate IL-10 production in macrophages [54, 55] and seems to be a common Il10 inducer in different cell types [56, 57], its binding patterns in different Th-cell subsets are lineage-specific.
Il10 locus in Th1 and Th17 cells
STAT4, Blimp-1, and c-Maf co-bind to DHS-9 in Th1 cells; however, the binding of c-Maf is independent of Blimp-1 [58]. Recent studies also show that Th1 cell master TF T-bet and Bhlhe40 co-bind to DHS-9 and DHS + 6.5 [59, 60], but DHS + 3 is only bound by T-bet [59]. TGF-β downstream molecular Smad4 also binds to the Il10 promoter in Th1 cells [61, 62]. In Th17 cells, JunD, IRF4, BATF, and STAT3 bind to DHS + 3 and DHS-9 [63]. Th17 cell master TF RORγt and Trim33 co-bind to DHS-9 [64]. Canonical Notch signaling molecule RBPJ may bind to the Il10 promoter and repress c-Maf-mediated Il10 expression, although the binding of RBPJ to the Il10 is weak [65].
Shared molecular switches of inflammatory and IL-10 producing anti-inflammatory Th cells
IL-27 is a potent Il10 inducer
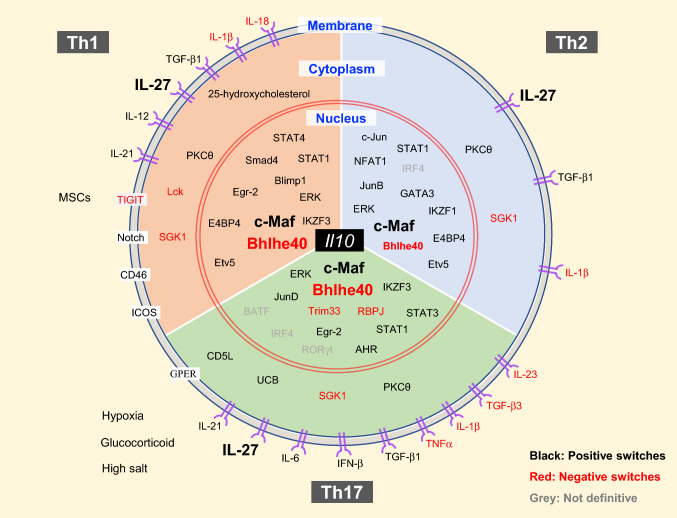
As mentioned above, different Th-cell subsets may utilize distinguished mechanisms to regulate Il10 expression, but they also share several similar signaling pathways (Fig. 3). IL-27 is a powerful cytokine for Il10 induction in all Th cells. IL-27 is initially reported to promote Th1-cell polarization together with IL-12 [66]. Later studies suggest that IL-27 may also exert anti-inflammatory function [67, 68]. Indeed, IL-27 could induce c-Maf, IL-21, IL-21R, and ICOS expression in CD4 T cells [69]. c-Maf is an important transcription factor for inducing IL-10 expression both in mice and in humans [70, 71]; IL-21, which can be produced by Th1, Th2, and Th17 cells, has been reported to promote Th1 and Th17 cells, but not Th2 cells to produce IL-10 in vitro [72]; in steady state, ICOShigh CD4 cell population in the secondary lymphoid organs is highly correlated with IL-10 production [73]. Under the in vitro Th1 cell-polarizing condition, IL-27 promotes IFN-γ+IL-10+ Th1 cell population, but has no effect on IFN-γ production; in Th2 cell-culture condition, IL-27 increases IL-10 production, but reduces IL-13 expression. IL-27 together with TGF-β enhances IL-10 expression and inhibits Foxp3 expression [74]. IL-27 and TGF-β also increase IFN-γ+IL-10+ cells in vitro neutral condition [75]. Furthermore, Il27ra−/− mice infected with T. gondii fail to produce IL-10+ CD4 T cells [74]. In vitro neutral condition, IL-27-induced Il10 expression depends on STAT1 and STAT3, but not STAT4 [74, 76]. From the PBMCs of PTB patients, IL-27 expands the IL-10+ Th1, Th17, and Treg cell population, and TGF-β promotes the expansion of IL-10+ Th2, Th17, and Treg cell population [34].
Fig. 3.
Regulators of IL-10 expression in T-helper cells. IL-10-producing Th cells can be induced and regulated through different mechanisms which include: extracellular physical and chemical factors; cytokine and membrane proteins that trigger signaling; cytoplasmic signaling regulators; transcription factors in the nucleus. Th cells may share some common mechanism and regulators to induce IL-10 expression, such as PKCθ, c-Maf, Bhlhe40, etc. However, each Th-cell subset also has its own lineage-specific regulators for switching on and off IL-10 production
Other common Il10 regulators
Many signaling molecules have been identified to regulate Il10 expression in all Th cells. For example, ERK1 and ERK2 activation are required for IL-10 production by Th1, Th2, and Th17 cells [77]. Serine/threonine kinase glycogen synthase kinase-3 (GSK3) inhibitor does not affect Il10 expression in recently activated T cells; however, it dramatically up-regulates IL-10 and c-Maf production and regulates histone H3 acetylation and methylation in long-term re-stimulated Th1, Th2, and Th17 cells in vitro [78]. From experiments using Tg4 T-cell receptor transgenic mice, protein kinase C theta (PKCθ) has been shown as an essential regulator in myelin basic protein (MBP) Ac1-9[4Y] antigen-induced IL-10+ CD4 T cells [79]. Since c-Maf is down-regulated in PKCθ-deficient cells and c-Maf is a major inducer of Il10 expression in a variety of cells [19, 80], the effect of PKCθ in Il10 induction may be through c-Maf upregulation.
Although many factors and pathways are common in different Th-cell subsets in regulating IL-10 production, there are also Th-cell subset-specific mechanisms of Il10 induction; such mechanisms involve TFs and extracellular cytokines, as well as cell-surface proteins, intracellular kinases, and metabolism-related molecules (Fig. 3). Below, we will discuss molecular switches for IL-10 regulation in each Th-cell subset.
Switches of inflammatory and IL-10-producing anti-inflammatory Th2 cells
IL-10 production has been considered as a natural capability of Th2 cells [6, 81]. Il10 expression is down-regulated in Gata3-knockdown Th2 clones using an antisense Gata3 construct, but up-regulated in CD4-Gata3 transgenic Th1 cells [81]. GATA3 binds to the Il10 promoter region, and ectopic expression of GATA3 promotes histone acetylation at the Il10 locus; however, reporter assay indicates that GATA3 does not enhance the transcription activity of the Il10 promoter [51]. Therefore, the main function of GATA3 is possibly to make the Il10 locus more accessible in Th2 cells. It has been reported that the binding of GATA3 to the Il10 promoter is higher in IL-10+ Th2 cells than in IL-10− Th2 cells [52]. Th2 cytokines are also shown to regulate Il10 expression. In vitro and ex vivo experiments suggest that repeated IL-4 treatment is essential for Th2 cells to express high levels of IL-10 [52]. IL-4-signaling engagement induces IL-10 production under the Th1 cell-culture conditions without affecting the expression of IFN-γ and T-bet [82]; similarly, IL-13 increases IL-10 production but only in polarized Th17 cells [83]. Since IL-4-mediated STAT6 activation is also responsible for GATA3 upregulation, whether STAT6 directly or indirectly induce Il10 expression needs further investigation. Interestingly, it has been recently reported that inflammatory cytokine IL-1 promotes IL-13 production, but inhibits IL-4 and IL-10 production by Th2 cells [84].
Transcription factors E4BP4, GATA3, IRF4, c-Jun, JunB, and Etv5 can bind to the Il10 locus at the DHS + 6.5 region rather than the promoter to induce Il10 expression in Th2 cells [50]. E4BP4, encoded by Nfil3, is essential for IL-10 production in both Th1 and Th2 cells by regulating histone modifications at the Il10 locus [85]. Etv5 also regulates IL-10 production in Th1 and Th2 cells, but it doesn’t affect IFN-γ and IL-4 production. Etv5 does not regulate the expression of Nfil3, Gata3, Irf4, Jun, and Junb, suggesting that its effect on Il10 expression is independent of regulating the other known IL-10 regulators [50]. NFAT1 directly binds to the Il10 locus at the DHS-9 region in Th2 cells, but not in Th1 cells, and NFAT1 is essential for IL-10 production in Th2 cells upon stimulation of PMA/ionomycin [53]. Furthermore, Ikaros (IKZF1) has been reported to positively regulate IL-10 production in Th2 cells and IKZF1 strongly binds to DHS-TSS region of the Il10 gene [86].
Self-regulation of IL-10-producing Th2 cells
Under in vitro Th2 cell-culture conditions, higher percentage of IL-4, IL-5, and IL-13-producing CD4 T cells can be generated from Il10ra−/− CD4 T cells than from wild-type CD4 T cells, and in vivo, more IL-4, IL-5 and IL-13-producing CD4 T cells are detected in the lung and mediastinal LN of HDM-immunized Il10ra−/− mice compared to wild-type mice [22]. These results suggest that IL-10 may directly act on T cells to inhibit Th2-cell differentiation and function; however, whether all Th2 cells express IL-10R and whether IL-10R expression is related to IL-10 production in Th2 cells are unknown. An early study also shows that IL-10 treatment together with allergen re-challenge through intratracheal inhibits IL-4 and IL-5 secretion in the bronchoalveolar lavage [87]. However, another study indicates that IL-10 produced by DC and T cells is important for CD4 T cells to produce IL-4 and IL-10 inhibits IFN-γ production in tape stripping-mediated allergic dermatitis [88]. IL-10 produced by Th2 cells could either repress IL-10R+ non-Th2 cells to boost a Th2 response or inhibit IL-10R+ Th2 cells to dampen a Th2 response. Considering the “natural” IL-10-producing capability of Th2 cells, regulating IL-10R expression on Th2 cells may be a critical self-regulatory mechanism.
Switches of inflammatory and IL-10-producing anti-inflammatory Th1 cells
Cytokines that regulate IL-10-producing Th1 cells
A strong Th1 response is essential for pathogen clearance; however, uncontrolled production of Th1 effector cytokine IFN-γ may lead to severe tissue damage and sometimes death of the host due to severe immunopathology [19, 89–91]. The space–time-dependent balance between IFN-γ+ and IL-10+IFN-γ+ Th1 cells is essential for an effective immune response with limited tissue damage. An important anti-inflammatory function of IL-10+IFN-γ+ Th1 cell has been highlighted in the T. gondii infection-induced type 1 immune response. Il10−/− mice suffer from cytokine storm-mediated immunopathology and ultimate death, although the parasite is effectively controlled [92]. Several switches in controlling the balance between IFN-γ+ and IL-10+IFN-γ+ Th1 cells have been reported. In the Plasmodium chabaudi infection-induced malaria murine model, Il27ra−/− mice failed to generate the IL-10+IFN-γ+ CD4 T-cell population [93]. During the course of blood-stage malaria infection, IFN-γ+ CD4 T cells are the major source of IL-10 both in the liver and lung, and IL-10 expression is controlled by IL-27R-mediated signaling in CD4 T cells [94]. Thus, IL-27 signaling plays a critical role in IL-10+ Th1-cell induction in vivo. A strong and long-lasting IL-12-STAT4 signaling, which is essential for T-bet expression and Th1-cell differentiation, together with high-dose TCR engagement can induce the development of IL-10-producing Th1 cells both in vitro and in vivo [77]. Results from both in vitro and ex vivo experiments indicate that repeated IL-12 exposure is able to induce Th1 cells to express high levels of IL-10 [52]. Interestingly, in the T. gondii STAg-immunized genetically resistant BALB/c and susceptible C57BL/6 mice, IL-10 expression by IFN-γ+ Th1 cells is independent of IL-12 [95], which suggests that IL-12 signaling is sufficient but not necessary for Th1 producing IL-10. Interestingly, although TGF-β inhibits Th1-cell differentiation through repressing T-bet and IFN-γ, it promotes antigen-specific effector Th1 cells to express IL-10 and up-regulates IFN-γ without affecting T-bet expression [62, 96]. Under in vitro Th1 or Th2 cell-culture conditions, either retrovirus-mediated TGF-β1 over-expression or addition of recombinant TGF-β1 promotes IL-10 production [61]. Under un-skewing cell-culture conditions, TGF-β could further promote IL-27-mediated IL-10 production independent of Blimp-1 [58]. TGF-β1 also causes Smad4 binding to the Il10 promoter and promotes Il10 transcription in Th1 cells [61, 62]. However, TGF-β has also been reported to inhibit Il10 expression in Th1 cells and to suppress Blimp-1 expression both in Th1 and Th17 cells [58, 97]. Altogether, it is possible that TGF-β could stabilize IL-10+IFN-γ+ cell population and that the functions of TGF-β in regulating Il10 expression are cell content dependent. By contrast, IL-1 family members including IL-1β and IL-18 suppress IL-10 production in Th1 cells [98].
Transcription factors that regulate IL-10-producing Th1 cells
Recently, several studies have demonstrated that the transcription factor Bhlhe40 represses IL-10 production in Th1 cells during Mycobacterium tuberculosis (MTB) or T. gondii infection, or in Th17 cells during EAE disease induction [59, 60, 99]. Yu et al. show that Bhlhe40 inhibits IL-10 expression, but promotes IFN-γ production without affecting T-bet expression [99]. Bhlhe40−/− mice with increased IL-10 production fail to control parasites; on the other hand, Bhlhe40 deficiency results in loss of the pathogenicity of CD4 T cell in inducing colitis [99]. Huynh et al. show that CD4 T cells and other immune cells including dendritic cells require Bhlhe40 to repress Il10 expression during MTB infection [60]. Lin et al. also reported that Bhlhe40 deficiency results in increased IL-10 production in Th1 and Th17 cells [59]. Although Bhlhe40−/− Th2 cells have normal IL-10 production in this study [59], Yu et al. have reported that Bhlhe40 also regulates IL-10 production in Th2 cells [99]. Bhlhe40 directly binds to several sites at the Il10 locus [59]. Interestingly, Bhlhe40 binds to the DHS + 6.5 site, where c-Maf binds in Th1 cells [60, 100]. Furthermore, in Bhlhe40−/− Th1 cells, the expression of c-Maf and an Ikaros family member IKZF3 is up-regulated [99]. IKZF3 has also been shown to induce IL-10 production in Th17 cells [101]. Thus, Bhlhe40 may repress Il10 induction directly through its binding to the Il10 gene or indirectly through inhibiting the expression of two IL-10 inducers, c-Maf and IKZF3.
Another important TF involved in inducing IL-10+ Th1 cells is Blimp-1 [58]. However, Blimp-1 is not induced in Th2 or Th17 cells and thus is dispensable for IL-10 expression in these cells. Together with the IL-12-STAT4 signaling, Blimp-1 promotes IL-10 production in Th1 cells both in vitro and in vivo with T. gondii infection [58]. Interestingly, Blimp-1, STAT4, and c-Maf co-bind to the DHS-9 region of the Il10 locus, although STAT4 but not Blimp-1 regulates histone modifications at the DHS-9 region [58]. During chronic LCMV infection, Blimp-1 is required for viral-specific Th1 cells to produce IL-10 accompanied by reduced inflammatory function of these cells, and such function of Blimp-1 seems to be independent of the expression of c-Maf, AhR, NFIL3, or RBPJκ [102]. Single-cell RNA-Seq analysis performed on the antigen-specific IL-10-producing cells shows that Prdm1 (encoding Blimp-1) and Maf are co-expressed in the IL-10-expressing CD4 T cells [103]. Low levels of c-Maf expression are detected in Th1 cells, and c-Maf binding to the DHS-9 site is independent of Blimp-1 in Th1 cells [58]. In addition, IL-27-mediated induction of IL-10 and Blimp-1 depends on another transcription factor early growth response gene 2 (Egr2), whose induction depends on IL-27-mediated STAT3 activation. Luciferase reporter assay indicates that direct binding of Egr2 to the Prdm1 promoter trans-activates the expression of Prdm1 [104]. E4BP4 and Etv5, which regulate IL-10 production in Th2 cells as mentioned above, may also regulate Il10 expression in Th1 cells [50, 85].
Surface and intracellular molecules that regulate IL-10-producing Th1 cells
Besides cytokine-mediated signaling, other cell-surface protein-triggered signaling such as Notch pathway may enhance IL-10 production in Th1 cells [58, 105]. Dll4 stimulation enhances the binding of c-Maf to the DHS-9 region, and constitutively active form of Notch can no longer induce IL-10 production in Th1 cells in the absence of Blimp-1 [58]. Therefore, Notch-mediated induction of Il10 expression in Th1 cells requires both Blimp-1 and c-Maf. Moreover, when high levels of IL-2 are present, complement regulator CD46 may convert IFN-γ+ Th1 cells into IL-10+IFN-γ+ Th1 cells [106]. ERK and/or JNK signaling may be involved in CD46-mediated induction of IL-10+ Th1 cells [89]. In human CD4 T cells, knocking-down the expression of the cell-surface protein T-cell Ig and ITIM domain (TIGIT), which binds to DC surface protein CD155, results in increased T-bet and IFN-γ expression, but reduced Il10 expression, indicating that TIGIT plays a positive role in IL-10 induction [107].
Some intracellular molecules that are involved in the induction of IL-10+ Th1 cells have been summarized by Cope et al. [89]. For example, Src family kinase Lck is known to regulate Th2-cell differentiation. However, Lck−/− Th1 cells express higher levels of IL-10 and c-Maf, but normal levels of IFN-γ and T-bet compared to wild-type Th1 cells [108]. Recently, the cholesterol biosynthesis pathway but not the cellular cholesterol content has been found to regulate IL-10 production in IFN-γ+ and TNF-α+ human Th1 cells, but not in Th17 cells [109]. Physiological level of 25-hydroxycholesterol is sufficient to repress c-Maf expression [109]. It has also been reported that cord-blood-derived mesenchymal stromal cells (MSCs) display immune regulatory properties in autoimmune disease, and induce IFN-γ+ IL-10+ Th1-cell population [110, 111].
No self-regulation of IL-10-producing Th1 cells
Since only a small population of Th1 cells express IL-10R, and IL-10+IFN-γ+ Th1 cells do not repress T-bet and IFN-γ expression by other Th1 cells [62], it is unlikely that IL-10 produced by anti-inflammatory IL-10+IFN-γ+ Th1 cells directly acts on inflammatory IFN-γ+ Th1 cells. It has also been noticed that IFN-γ-producing capability is similar between IL-10− Th1 and IL-10+ Th1 cells isolated from T. gondii-infected mice. When co-cultured with T. gondii-infected macrophages, IL-10+ Th1 cells significantly suppress IL-12 production by these macrophages and this suppressive effect is reversed by the addition of the IL-10R-neutralizing antibody [37]. Therefore, it is possible that IL-10+IFN-γ+ Th1 cells attenuate pro-inflammatory cytokine production and thus pro-inflammatory Th1 response through repressing antigen presenting cells (APCs). Another interesting observation is that ex vivo IL-10− Th1 cells can express IL-10 when they are re-activated with anti-CD3 antibodies, and IL-10+ Th1 cells will lose IL-10 production when resting in culture [37]. Thus, different from the IL-10-producing Th2 cells, IL-10+IFN-γ+ Th1 cells may represent a transient stage of Th1 cells.
Switches of inflammatory and IL-10-producing anti-inflammatory Th17 cells
Cytokines that regulate IL-10-producing Th17 cells
Th17 cells are best known as pro-inflammatory effector cells in many autoimmune diseases and during certain pathogen infection [112]. Recently, IL-10-producing anti-inflammatory Th17 cells have been reported, especially in the protection barrier of the intestine. Although IL-10 is difficult to be detected in human Th17 cells ex vivo after PMA/ionomycin treatment, TCR-stimulated human Th17 cell clones can produce IL-10, and both IL-10+IL-17A+ and IL-10+IFN-γ+ CD4 T cells are found in mouse small intestine [40, 113]. TGF-β1 and IL-6 can induce non-pathogenic Th17 cells that do not induce EAE in a transfer model, and these Th17 cells express high levels of anti-inflammatory cytokine IL-10 and c-Maf, but lower levels of chemokine gene Ccl5, Cxcl10, Ccl2, Cxcl2, and Ccl22 expression. By contrast, IL-23 induces inflammatory Th17 cells, which express high levels of pathogenic signature genes Il23r, Sgk1, Ccl4, Gzmb, Lrmp, Grp65, Plzp, Toso, and Lag3 [114, 115]. IL-6 cooperates with TGF-β to promote IL-10 and c-Maf expression in vitro and c-Maf induces IL-10 production during Th17 cell differentiation [116]. However, it has also been reported that IL-6 induces naïve CD4 T cells to produce IL-10 independent of IL-27 and TGF-β [117]. In the absence of TGF-β1, IL-23 may induce pathogenic Th17 cells that do not express IL-10 [118]. Interestingly, IL-23 induces the expression of TGF-β3 which together with IL-6 preferentially induces pathogenic Th17 cells by down-regulating the expression of IL-10 and its inducer c-Maf and AhR; all these molecules are up-regulated by TGF-β1 in the presence of IL-6 [11, 115, 119, 120]. Interestingly, IL-10+ Th17 cells express high levels of IL-27R in the peritoneal fluid of endometriosis patients compared to healthy controls. WSX-1 (an IL-27R subunit)-expressing Th17 cells in peritoneal fluid from endometriosis mice express higher levels of IL-10, Blimp1, and c-Maf, but low levels of RORγt compared to the WSX-1− Th17 cells [121]. These results indicate a critical role of IL-27 signaling in the induction of Il10 expression in both humans and mice. Thus, TGF-β1, together with IL-27, may educate inflammatory Th17 cells to become anti-inflammatory Th17 cells. Other cytokines are also involved in regulating Il10 induction in Th17 cells. For example, Type I IFNs including IFN-β repress IL-17 and RORγt, but induce IL-10 expression under Th17 cell-polarizing culture conditions; however, whether IFN-β regulates IL-10+IL-17+ co-producing Th17 cells or inhibits Th17 cell differentiation to Th2 cell phenotype still needs further investigation [122–124]. On the other hand, IL-1β inhibits IL-10 production in S. aureus-primed Th17 cells [43] similar to the function of IL-1β in regulating IL-10 production in Th2 cells as mentioned previously [84]. Interestingly, IL-1β has been reported to induce the expression of Bhlhe40 in Th17 cells, which is known to suppress IL-10 [59, 125]. Furthermore, blocking another inflammatory cytokine TNFα induces IL-10 production in Th17 cells through up-regulating IKZF3 [101].
Transcription factors that regulate IL-10-producing Th17 cells
Transcription factor RORγt and tripartite motif-containing 33 (Trim33), which regulates TGF-β/Smad-signaling pathway [126], are required for Th17-cell differentiation both in vivo and in vitro, and both repress IL-10 production [64]. Trim33 and RORγt co-bind to several sites at the Il17a and Il10 locus, including the DHS-9 region at the Il10 locus, and regulate histone modifications at these regions [64, 127]. It has been reported that in Th17 cells, c-Maf directly binds to and trans-activates the Il10 promoter [116]. However, c-Maf expression is not altered in Trim33−/− Th17 cells [64]. This suggests that Trim33 may directly repress Il10 expression. Canonical Notch signaling downstream molecule RBPJ binds to the Il23r and Il10 promoter [65]. While RBPJ promotes Il23r expression, it represses c-Maf-mediated Il10 induction. Therefore, RBPJ may facilitate the differentiation of inflammatory Th17 cells through both inhibiting Il10 induction and promoting IL-23 signaling.
Cellular homeostasis and IL-10-producing Th17 cells
Intracellular protein CD5L binds to cytosolic fatty acid synthase and regulates lipid metabolism [128]. CD5L deficiency does not affect Th17 cell differentiation; normal expression of Th17 signature genes Il17f, Il21, Il23r, Rorc, and Rora, but reduced expression of Il10 and Maf is found in Cd5l−/− Th17 cells [129]. RORγt binding to the Il10 DHS-9 is reduced in Cd5l−/− Th17 cells, and the ratio of polyunsaturated fatty acid (PUFA) and saturated (SFA) correlates with the RORγt binding to the Il10 DHS-9 [129], although the importance of such binding in regulating Il10 expression is still unknown. Anyway, this study indicates that intracellular fatty acid composition is involved in regulating the differentiation of IL-10-producing anti-inflammatory Th17 cells. One of the heme oxidation products, unconjugated bilirubin (UCB), is also reported to favor the differentiation of IL-10+IL-17+ anti-inflammatory Th17 cells in the dextran sulfate sodium (DSS)-induced colitis model. In the presence of UCB, CD4 T cells from PBMCs of healthy individuals are more likely to differentiate into IL-10-producing Th17 cells when polarized in vitro in presence of IL-6, IL-1β, and TGF-β [130]. Taken together, metabolism pathways may play important roles in the differentiation of IL-10-producing Th17 cells.
A critical factor for a successful pregnancy is the immune tolerance to the fetus. Interestingly, patients with multiple sclerosis suffer a reduced clinical symptom during their pregnancy [131], suggesting that pregnancy may induce anti-inflammatory cells. It has been reported that estrogen may reduce EAE symptoms through G-protein-coupled estrogen receptor (GPER) [132]. Under Th17 cell-culture conditions in vitro, GPER small molecule agonist G-1 up-regulates IL-10 production in Th17 cells [133]. Furthermore, glucocorticoid dexamethasone induces human memory CD4 T cells to co-express IL-17 and IL-10 or IFN-γ and IL-10 [134]. Hypoxia condition (1% O2) is reported to significantly induce Il10 expression in Th17 cells compared with normal 21% O2 [135]. High salt (NaCl) diet potentiates inflammatory Th17 cell differentiation and enhance EAE induction through serine/threonine kinase serum glucocorticoid kinase 1 (SGK1) and by up-regulating IL-23R expression [136]. All above findings suggest that the balance between inflammatory and anti-inflammatory Th17 cells is quite sensitive to environment changes and subsequent alterations in regulatory host factors.
Self-regulation of IL-10-producing Th17 cells
Th17 cells express high levels of IL-10Rα, and IL-10 inhibits IL-17A+IFN-γ+ and IL-17A+IFN-γ−, but not IL-17A−IFN-γ+ cell population in the T-cell transfer colitis model [113]. This indicates that IL-10 can directly repress IL-10R+ Th17 cells, although IL-10R expression by Treg cells is also essential for inhibiting Th17-mediated inflammation in the colitis model [137]. Th17 cells may also lose IL-10 production during long-term activation [40, 41]. Interestingly, strong TCR/CD28 stimulation not only suppresses human Th17-cell differentiation, but also represses c-Maf expression in Th17 cells [138].
IL-10-producing innate lymphoid cells (ILCs)
ILCs are considered as the innate counterparts of CD4 T-helper cells [4, 139]. Interestingly, just as T-helper cells, ILCs can be classified into at least three different groups: group 1 ILC (ILC1), group 2 ILC (ILC2), and group 3 ILC (ILC3) that resemble Th1, Th2, and Th17 cells, respectively. Compared to numerous studies on IL-10 regulation in Th cells, there are relatively fewer studies on IL-10 production by ILCs. In 2009, Prf1−/− NK cells were found to express IL-10 [140]. In 2010, a Lin−IL-13+T1/ST2+IL-17BR+ cell population (now known as ILC2s) were identified and they have capability to produce IL-10 upon IL-25/IL-33 treatment or helminth infection [141]. However, in 2016, single-cell transcriptomic analysis on small intestinal ILCs did not classify the IL-10-producing cell subset [142]. In 2017, Wang et al. identified a Lin−CD45+CD127+IL-10+ ILCs population in small intestine, and TGF-β is required for their expansion [143]. No IL-10-producing ILCs are present in the lung of naïve mice, but upon IL-33 and papain treatment, Lin−CD45+Thy-1.2+ST2+IL-10+ ILC2s are induced [144]. Because of limited reports on IL-10 induction in ILCs, it is not clear whether the regulation and production of IL-10 in ILCs shares similar mechanisms that are found in Th cells. Further studies on the identification of IL-10-producing ILC subsets both in humans and mice under physiological and pathological conditions, and on the molecules and DNA elements that regulate IL-10 production in ILCs are required to expand our knowledge on the regulation of inflammatory and anti-inflammatory ILCs.
Conclusions
A well-balanced immune response, involving both inflammatory and anti-inflammatory lymphocytes, is critical for providing effective host protection against pathogen invasion and for preventing chronic inflammatory diseases. IL-10 is a well-studied member of an important anti-inflammatory cytokine family [91]. Although IL-10 can be expressed by many cell types, lymphocytes are a major source of IL-10 production. Besides regulatory T cells, all the effector CD4 T cells are capable of expressing IL-10. IL-10-producing cells are very heterogeneous and contain subsets that are derived from distinct effector CD4 T cells. Even within the IL-10-producing Th1 cells, it has been recently shown that only the IL-10-producing cells co-expressing co-inhibitory receptors are anti-inflammatory, whereas the IL-10-producing cells that do not express co-inhibitory receptors are actually inflammatory, suggesting that there is another level of functional heterogeneity within the IL-10-producing cells [145].
Regulation of IL-10 production in distinct lymphocytes is cell-type specific. While IL-10 production by Th2 cells may be a stable phenotype, Th1 and Th17 cells often transiently express IL-10, suggesting that there are on-and-off switch-like controls. Cell-type-specific IL-10 regulation may depend on the differential chromatin accessibility at different regulatory regions of the Il10 locus in different cell types. Master transcription factors may influence such epigenetic differences. Furthermore, unique expression pattern of a given transcription factor that is involved in IL-10 regulation in different cell subsets may also contribute to cell-type-specific IL-10 regulation. Finally, the levels of some IL-10 regulators may be dynamically expressed. For example, the expression of Bhlhe40 is highly sensitive to TCR stimulation through which IL-10 production may be differently regulated by this molecule at distinct activation stages. In addition, because of the dynamic nature of Bhlhe40 expression, the wild-type cells at certain stages when Bhlhe40 is under-expressed may behave like Bhlhe40 knockout cells; this may offer an explanation for different effects of Bhlhe40 deletion on IL-10 production in Th2 cells.
Transcription factor c-Maf is one of the most important regulators for IL-10 production in Th1, Th2, and Th17 cell subsets as well as in regulatory T cells, macrophages, and B cells [55–57, 146]. Similarly, Bhlhe40 can regulate IL-10 expression in distinct Th-cell subsets. Nevertheless, c-Maf and Bhlhe40 seems to cross-regulate each other’s expression [99, 100, 147]. The physiologic importance of the balance between c-Maf and Bhlhe40 may be well beyond IL-10 regulation. Indeed, while c-Maf suppresses IL-2 production, Bhlhe40 induces IL-2 production [100, 148]. Therefore, further investigation on the regulation of Bhlhe40 and c-Maf expression as well as their relationship in controlling the balance between anti-inflammatory and inflammatory Th-cell subsets will allow us to better understand immune regulation.
Il10-deficient mice spontaneously develop inflammatory bowel disease in the steady state with the presence of normal microbiota [149] and display more severe immunopathology after parasite infection when compared to wild-type mice [9, 19, 90]. In humans, mutations in IL-10 receptors have been associated with patients of inflammatory bowel disease [150]. Thus, targeting molecules and/or pathways that regulate IL-10 should be considered as effective strategies to treat many immunological diseases. For example, modulating IL-10 production and/or IL-10-producing Th cells, negatively or positively, may help tumor eradication or resolution of chronic inflammation, respectively. In general, understanding the molecular switches that control the balance and/or transition between inflammatory and anti-inflammatory states of distinct CD4 T-cell subsets is essential for designing new strategies to modulate immune responses in a cell-type- or disease-specific manner for the benefit of a wide range of patients in immunological disease conditions.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases (No. 1-ZIA-AI001169).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Difeng Fang, Email: difeng.fang@nih.gov.
Jinfang Zhu, Email: jfzhu@niaid.nih.gov.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 3.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang D, Zhu J. Dynamic balance between master transcription factors determines the fates and functions of CD4 T cell and innate lymphoid cell subsets. J Exp Med. 2017;214(7):1861–1876. doi: 10.1084/jem.20170494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorentino DF. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol. 2013;4:129. doi: 10.3389/fimmu.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5(4):271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 9.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 10.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2(9):816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 11.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8(9):931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 12.Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172(10):5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 13.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 14.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 16.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 17.Gerosa F. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J Exp Med. 1996;183(6):2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150(2):353–360. [PubMed] [Google Scholar]
- 19.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 20.Adachi M, Oda N, Kokubu F, Minoguchi K. IL-10 induces a Th2 cell tolerance in allergic asthma. Int Arch Allergy Immunol. 1999;118(2–4):391–394. doi: 10.1159/000024145. [DOI] [PubMed] [Google Scholar]
- 21.Sanin DE, Prendergast CT, Bourke CD, Mountford AP. Helminth infection and commensal microbiota drive early IL-10 production in the skin by CD4+ T cells that are functionally suppressive. PLoS Pathog. 2015;11(5):e1004841. doi: 10.1371/journal.ppat.1004841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coomes SM, Kannan Y, Pelly VS, Entwistle LJ, Guidi R, Perez-Lloret J, et al. CD4(+) Th2 cells are directly regulated by IL-10 during allergic airway inflammation. Mucosal Immunol. 2017;10(1):150–161. doi: 10.1038/mi.2016.47. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol. 2014;5:253. doi: 10.3389/fimmu.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagannathan P, Eccles-James I, Bowen K, Nankya F, Auma A, Wamala S, et al. IFNgamma/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children. PLoS Pathog. 2014;10(1):e1003864. doi: 10.1371/journal.ppat.1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portugal S, Moebius J, Skinner J, Doumbo S, Doumtabe D, Kone Y, et al. Exposure-dependent control of malaria-induced inflammation in children. PLoS Pathog. 2014;10(4):e1004079. doi: 10.1371/journal.ppat.1004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, Deininger S, et al. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 2009;5(4):e1000364. doi: 10.1371/journal.ppat.1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar R, Ng S, Engwerda C. The role of IL-10 in malaria: a double edged sword. Front Immunol. 2019;10:229. doi: 10.3389/fimmu.2019.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa DL, Cardoso TM, Queiroz A, Milanezi CM, Bacellar O, Carvalho EM, et al. Tr-1-like CD4+CD25−CD127−/lowFOXP3− cells are the main source of interleukin 10 in patients with cutaneous leishmaniasis due to Leishmania braziliensis. J Infect Dis. 2015;211(5):708–718. doi: 10.1093/infdis/jiu406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owens BM, Beattie L, Moore JW, Brown N, Mann JL, Dalton JE, et al. IL-10-producing Th1 cells and disease progression are regulated by distinct CD11c(+) cell populations during visceral leishmaniasis. PLoS Pathog. 2012;8(7):e1002827. doi: 10.1371/journal.ppat.1002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204(2):285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerosa F, Nisii C, Righetti S, Micciolo R, Marchesini M, Cazzadori A, et al. CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin Immunol. 1999;92(3):224–234. doi: 10.1006/clim.1999.4752. [DOI] [PubMed] [Google Scholar]
- 32.Chowdhury IH, Ahmed AM, Choudhuri S, Sen A, Hazra A, Pal NK, et al. Alteration of serum inflammatory cytokines in active pulmonary tuberculosis following anti-tuberculosis drug therapy. Mol Immunol. 2014;62(1):159–168. doi: 10.1016/j.molimm.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Harling K, Adankwah E, Guler A, Afum-Adjei Awuah A, Adu-Amoah L, Mayatepek E, et al. Constitutive STAT3 phosphorylation and IL-6/IL-10 co-expression are associated with impaired T-cell function in tuberculosis patients. Cell Mol Immunol. 2019;16(3):275–287. doi: 10.1038/cmi.2018.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar NP, Moideen K, Banurekha VV, Nair D, Sridhar R, Nutman TB, et al. IL-27 and TGFbeta mediated expansion of Th1 and adaptive regulatory T cells expressing IL-10 correlates with bacterial burden and disease severity in pulmonary tuberculosis. Immun Inflamm Dis. 2015;3(3):289–299. doi: 10.1002/iid3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreira-Teixeira L, Redford PS, Stavropoulos E, Ghilardi N, Maynard CL, Weaver CT, et al. T cell-derived IL-10 impairs host resistance to Mycobacterium tuberculosis infection. J Immunol. 2017;199(2):613–623. doi: 10.4049/jimmunol.1601340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss KA, Christiaansen AF, Fulton RB, Meyerholz DK, Varga SM. Multiple CD4+ T cell subsets produce immunomodulatory IL-10 during respiratory syncytial virus infection. J Immunol. 2011;187(6):3145–3154. doi: 10.4049/jimmunol.1100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, et al. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204(2):273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, Stenzel W, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200(10):1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X, Tian J, Wang S. Insight into non-pathogenic Th17 cells in autoimmune diseases. Front Immunol. 2018;9:1112. doi: 10.3389/fimmu.2018.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu D, Notarbartolo S, Croonenborghs T, Patel B, Cialic R, Yang TH, et al. Transcriptional signature of human pro-inflammatory TH17 cells identifies reduced IL10 gene expression in multiple sclerosis. Nat Commun. 2017;8(1):1600. doi: 10.1038/s41467-017-01571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao Y, Goods BA, Raddassi K, Nepom GT, Kwok WW, Love JC, et al. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci Transl Med. 2015;7(287):287ra74. doi: 10.1126/scitranslmed.aaa8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong Qi YC, Inomata T, Amouzegar A, Dana R (2017) IL-10-producing Th17 cells: a potentially regulatory cell population in dry eye disease. J Immunol Immunother 2(1:5)
- 43.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484(7395):514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 44.Kulcsar KA, Baxter VK, Greene IP, Griffin DE. Interleukin 10 modulation of pathogenic Th17 cells during fatal alphavirus encephalomyelitis. Proc Natl Acad Sci USA. 2014;111(45):16053–16058. doi: 10.1073/pnas.1418966111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulcsar KA, Griffin DE. T cell-derived interleukin-10 is an important regulator of the Th17 response during lethal alphavirus encephalomyelitis. J Neuroimmunol. 2016;295–296:60–67. doi: 10.1016/j.jneuroim.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Im SH, Hueber A, Monticelli S, Kang KH, Rao A. Chromatin-level regulation of the IL10 gene in T cells. J Biol Chem. 2004;279(45):46818–46825. doi: 10.1074/jbc.M401722200. [DOI] [PubMed] [Google Scholar]
- 47.Jones EA, Flavell RA. Distal enhancer elements transcribe intergenic RNA in the IL-10 family gene cluster. J Immunol. 2005;175(11):7437–7446. doi: 10.4049/jimmunol.175.11.7437. [DOI] [PubMed] [Google Scholar]
- 48.Kang KH, Im SH. Differential regulation of the IL-10 gene in Th1 and Th2 T cells. Ann N Y Acad Sci. 2005;1050:97–107. doi: 10.1196/annals.1313.011. [DOI] [PubMed] [Google Scholar]
- 49.Wang ZY, Sato H, Kusam S, Sehra S, Toney LM, Dent AL. Regulation of IL-10 gene expression in Th2 cells by Jun proteins. J Immunol. 2005;174(4):2098–2105. doi: 10.4049/jimmunol.174.4.2098. [DOI] [PubMed] [Google Scholar]
- 50.Koh B, Hufford MM, Sun X, Kaplan MH. Etv5 regulates IL-10 production in Th cells. J Immunol. 2017;198(5):2165–2171. doi: 10.4049/jimmunol.1600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shoemaker J, Saraiva M, O’Garra A. GATA-3 directly remodels the IL-10 locus independently of IL-4 in CD4+ T cells. J Immunol. 2006;176(6):3470–3479. doi: 10.4049/jimmunol.176.6.3470. [DOI] [PubMed] [Google Scholar]
- 52.Chang HD, Helbig C, Tykocinski L, Kreher S, Koeck J, Niesner U, et al. Expression of IL-10 in Th memory lymphocytes is conditional on IL-12 or IL-4, unless the IL-10 gene is imprinted by GATA-3. Eur J Immunol. 2007;37(3):807–817. doi: 10.1002/eji.200636385. [DOI] [PubMed] [Google Scholar]
- 53.Lee CG, Kang KH, So JS, Kwon HK, Son JS, Song MK, et al. A distal cis-regulatory element, CNS-9, controls NFAT1 and IRF4-mediated IL-10 gene activation in T helper cells. Mol Immunol. 2009;46(4):613–621. doi: 10.1016/j.molimm.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 54.Cao S, Liu J, Chesi M, Bergsagel PL, Ho IC, Donnelly RP, et al. Differential regulation of IL-12 and IL-10 gene expression in macrophages by the basic leucine zipper transcription factor c-Maf fibrosarcoma. J Immunol. 2002;169(10):5715–5725. doi: 10.4049/jimmunol.169.10.5715. [DOI] [PubMed] [Google Scholar]
- 55.Cao S, Liu J, Song L, Ma X. The protooncogene c-Maf is an essential transcription factor for IL-10 gene expression in macrophages. J Immunol. 2005;174(6):3484–3492. doi: 10.4049/jimmunol.174.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ, et al. c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature. 2018;554(7692):373–377. doi: 10.1038/nature25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neumann C, Blume J, Roy U, Teh PP, Vasanthakumar A, Beller A, et al. c-Maf-dependent Treg cell control of intestinal TH17 cells and IgA establishes host-microbiota homeostasis. Nat Immunol. 2019;20(4):471–481. doi: 10.1038/s41590-019-0316-2. [DOI] [PubMed] [Google Scholar]
- 58.Neumann C, Heinrich F, Neumann K, Junghans V, Mashreghi MF, Ahlers J, et al. Role of Blimp-1 in programing Th effector cells into IL-10 producers. J Exp Med. 2014;211(9):1807–1819. doi: 10.1084/jem.20131548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin CC, Bradstreet TR, Schwarzkopf EA, Sim J, Carrero JA, Chou C, et al. Bhlhe40 controls cytokine production by T cells and is essential for pathogenicity in autoimmune neuroinflammation. Nat Commun. 2014;5:3551. doi: 10.1038/ncomms4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huynh JP, Lin CC, Kimmey JM, Jarjour NN, Schwarzkopf EA, Bradstreet TR, et al. Bhlhe40 is an essential repressor of IL-10 during Mycobacterium tuberculosis infection. J Exp Med. 2018;215(7):1823–1838. doi: 10.1084/jem.20171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-beta1-mediated fibrosis. J Exp Med. 2003;198(8):1179–1188. doi: 10.1084/jem.20030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huss DJ, Winger RC, Cox GM, Guerau-de-Arellano M, Yang Y, Racke MK, et al. TGF-beta signaling via Smad4 drives IL-10 production in effector Th1 cells and reduces T-cell trafficking in EAE. Eur J Immunol. 2011;41(10):2987–2996. doi: 10.1002/eji.201141666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li P, Spolski R, Liao W, Wang L, Murphy TL, Murphy KM, et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature. 2012;490(7421):543–546. doi: 10.1038/nature11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanaka S, Jiang Y, Martinez GJ, Tanaka K, Yan X, Kurosaki T, et al. Trim33 mediates the proinflammatory function of Th17 cells. J Exp Med. 2018;215(7):1853–1868. doi: 10.1084/jem.20170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meyer Zu Horste G, Wu C, Wang C, Cong L, Pawlak M, Lee Y, et al. RBPJ controls development of pathogenic Th17 cells by regulating IL-23 receptor expression. Cell Rep. 2016;16(2):392–404. doi: 10.1016/j.celrep.2016.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16(6):779–790. doi: 10.1016/S1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 67.Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, et al. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19(5):657–667. doi: 10.1016/S1074-7613(03)00298-X. [DOI] [PubMed] [Google Scholar]
- 68.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19(5):645–655. doi: 10.1016/S1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 69.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183(2):797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aschenbrenner D, Foglierini M, Jarrossay D, Hu D, Weiner HL, Kuchroo VK, et al. An immunoregulatory and tissue-residency program modulated by c-MAF in human TH17 cells. Nat Immunol. 2018;19(10):1126–1136. doi: 10.1038/s41590-018-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11(9):854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182(5):2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Löhning M, Hutloff A, Kallinich T, Mages HW, Bonhagen K, Radbruch A, et al. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197(2):181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8(12):1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 75.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8(12):1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 76.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8(12):1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 77.Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O’Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31(2):209–219. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hill EV, Ng TH, Burton BR, Oakley CM, Malik K, Wraith DC. Glycogen synthase kinase-3 controls IL-10 expression in CD4(+) effector T-cell subsets through epigenetic modification of the IL-10 promoter. Eur J Immunol. 2015;45(4):1103–1115. doi: 10.1002/eji.201444661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Britton GJ, Mitchell RE, Burton BR, Wraith DC. Protein kinase C theta is required for efficient induction of IL-10-secreting T cells. PLoS One. 2017;12(2):e0171547. doi: 10.1371/journal.pone.0171547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gabrysova L, Howes A, Saraiva M, O’Garra A. The regulation of IL-10 expression. Curr Top Microbiol Immunol. 2014;380:157–190. doi: 10.1007/978-3-662-43492-5_8. [DOI] [PubMed] [Google Scholar]
- 81.Zheng WP, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89(4):587–596. doi: 10.1016/S0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 82.Mitchell RE, Hassan M, Burton BR, Britton G, Hill EV, Verhagen J, et al. IL-4 enhances IL-10 production in Th1 cells: implications for Th1 and Th2 regulation. Sci Rep. 2017;7(1):11315. doi: 10.1038/s41598-017-11803-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newcomb DC, Boswell MG, Huckabee MM, Goleniewska K, Dulek DE, Reiss S, et al. IL-13 regulates Th17 secretion of IL-17A in an IL-10-dependent manner. J Immunol. 2012;188(3):1027–1035. doi: 10.4049/jimmunol.1102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caucheteux SM, Hu-Li J, Guo L, Bhattacharyya N, Crank M, Collins MT, et al. IL-1beta enhances inflammatory TH2 differentiation. J Allergy Clin Immunol. 2016;138(3):898–901.e4. doi: 10.1016/j.jaci.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Motomura Y, Kitamura H, Hijikata A, Matsunaga Y, Matsumoto K, Inoue H, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol. 2011;12(5):450–459. doi: 10.1038/ni.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Umetsu SE, Winandy S. Ikaros is a regulator of Il10 expression in CD4+ T cells. J Immunol. 2009;183(9):5518–5525. doi: 10.4049/jimmunol.0901284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Scott MR, Justice JP, Bradfield JF, Enright E, Sigounas A, Sur S. IL-10 reduces Th2 cytokine production and eosinophilia but augments airway reactivity in allergic mice. Am J Physiol Lung Cell Mol Physiol. 2000;278(4):L667–L674. doi: 10.1152/ajplung.2000.278.4.L667. [DOI] [PubMed] [Google Scholar]
- 88.Laouini D, Alenius H, Bryce P, Oettgen H, Tsitsikov E, Geha RS. IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J Clin Investig. 2003;112(7):1058–1066. doi: 10.1172/JCI18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cope A, Le Friec G, Cardone J, Kemper C. The Th1 life cycle: molecular control of IFN-gamma to IL-10 switching. Trends Immunol. 2011;32(6):278–286. doi: 10.1016/j.it.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 90.O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7(6):425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 91.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 92.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157(2):798–805. [PubMed] [Google Scholar]
- 93.Freitas do Rosario AP, Lamb T, Spence P, Stephens R, Lang A, Roers A, et al. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol. 2012;188(3):1178–1190. doi: 10.4049/jimmunol.1102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Villegas-Mendez A, Shaw TN, Inkson CA, Strangward P, de Souza JB, Couper KN. Parasite-specific CD4+ IFN-gamma+ IL-10+ T cells distribute within both lymphoid and nonlymphoid compartments and are controlled systemically by interleukin-27 and ICOS during blood-stage malaria infection. Infect Immun. 2016;84(1):34–46. doi: 10.1128/IAI.01100-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jankovic D, Kullberg MC, Hieny S, Caspar P, Collazo CM, Sher A. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 2002;16(3):429–439. doi: 10.1016/S1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 96.Huss DJ, Winger RC, Peng H, Yang Y, Racke MK, Lovett-Racke AE. TGF-beta enhances effector Th1 cell activation but promotes self-regulation via IL-10. J Immunol. 2010;184(10):5628–5636. doi: 10.4049/jimmunol.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salehi S, Bankoti R, Benevides L, Willen J, Couse M, Silva JS, et al. B lymphocyte-induced maturation protein-1 contributes to intestinal mucosa homeostasis by limiting the number of IL-17-producing CD4+ T cells. J Immunol. 2012;189(12):5682–5693. doi: 10.4049/jimmunol.1201966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blom L, Poulsen LK. IL-1 family members IL-18 and IL-33 upregulate the inflammatory potential of differentiated human Th1 and Th2 cultures. J Immunol. 2012;189(9):4331–4337. doi: 10.4049/jimmunol.1103685. [DOI] [PubMed] [Google Scholar]
- 99.Yu F, Sharma S, Jankovic D, Gurram RK, Su P, Hu G, et al. The transcription factor Bhlhe40 is a switch of inflammatory versus antiinflammatory Th1 cell fate determination. J Exp Med. 2018;215(7):1813–1821. doi: 10.1084/jem.20170155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gabrysova L, Alvarez-Martinez M, Luisier R, Cox LS, Sodenkamp J, Hosking C, et al. c-Maf controls immune responses by regulating disease-specific gene networks and repressing IL-2 in CD4(+) T cells. Nat Immunol. 2018;19(5):497–507. doi: 10.1038/s41590-018-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Evans HG, Roostalu U, Walter GJ, Gullick NJ, Frederiksen KS, Roberts CA, et al. TNF-alpha blockade induces IL-10 expression in human CD4+ T cells. Nat Commun. 2014;5:3199. doi: 10.1038/ncomms4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parish IA, Marshall HD, Staron MM, Lang PA, Brustle A, Chen JH, et al. Chronic viral infection promotes sustained Th1-derived immunoregulatory IL-10 via BLIMP-1. J Clin Investig. 2014;124(8):3455–3468. doi: 10.1172/JCI66108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xin G, Zander R, Schauder DM, Chen Y, Weinstein JS, Drobyski WR, et al. Single-cell RNA sequencing unveils an IL-10-producing helper subset that sustains humoral immunity during persistent infection. Nat Commun. 2018;9(1):5037. doi: 10.1038/s41467-018-07492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iwasaki Y, Fujio K, Okamura T, Yanai A, Sumitomo S, Shoda H, et al. Egr-2 transcription factor is required for Blimp-1-mediated IL-10 production in IL-27-stimulated CD4+ T cells. Eur J Immunol. 2013;43(4):1063–1073. doi: 10.1002/eji.201242942. [DOI] [PubMed] [Google Scholar]
- 105.Rutz S, Janke M, Kassner N, Hohnstein T, Krueger M, Scheffold A. Notch regulates IL-10 production by T helper 1 cells. Proc Natl Acad Sci USA. 2008;105(9):3497–3502. doi: 10.1073/pnas.0712102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11(9):862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188(8):3869–3875. doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kemp KL, Levin SD, Stein PL. Lck regulates IL-10 expression in memory-like Th1 cells. Eur J Immunol. 2010;40(11):3210–3219. doi: 10.1002/eji.201040699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perucha E, Melchiotti R, Bibby JA, Wu W, Frederiksen KS, Roberts CA, et al. The cholesterol biosynthesis pathway regulates IL-10 expression in human Th1 cells. Nat Commun. 2019;10(1):498. doi: 10.1038/s41467-019-08332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dryden GW. Overview of stem cell therapy for Crohn’s disease. Expert Opin Biol Ther. 2009;9(7):841–847. doi: 10.1517/14712590902956615. [DOI] [PubMed] [Google Scholar]
- 111.Selleri S, Dieng MM, Nicoletti S, Louis I, Beausejour C, Le Deist F, et al. Cord-blood-derived mesenchymal stromal cells downmodulate CD4+ T-cell activation by inducing IL-10-producing Th1 cells. Stem Cells Dev. 2013;22(7):1063–1075. doi: 10.1089/scd.2012.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 113.Huber S, Gagliani N, Esplugues E, O’Connor W, Jr, Huber FJ, Chaudhry A, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(−) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34(4):554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 115.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13(10):991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, et al. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009;182(10):6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jin JO, Han X, Yu Q. Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation. J Autoimmun. 2013;40:28–44. doi: 10.1016/j.jaut.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467(7318):967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183(11):7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang KK, Liu LB, Jin LP, Zhang B, Mei J, Li H, et al. IL-27 triggers IL-10 production in Th17 cells via a c-Maf/RORgammat/Blimp-1 signal to promote the progression of endometriosis. Cell Death Dis. 2017;8(3):e2666. doi: 10.1038/cddis.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang L, Yuan S, Cheng G, Guo B. Type I IFN promotes IL-10 production from T cells to suppress Th17 cells and Th17-associated autoimmune inflammation. PLoS One. 2011;6(12):e28432. doi: 10.1371/journal.pone.0028432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martin-Saavedra FM, Gonzalez-Garcia C, Bravo B, Ballester S. Beta interferon restricts the inflammatory potential of CD4+ cells through the boost of the Th2 phenotype, the inhibition of Th17 response and the prevalence of naturally occurring T regulatory cells. Mol Immunol. 2008;45(15):4008–4019. doi: 10.1016/j.molimm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 124.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16(4):406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin CC, Bradstreet TR, Schwarzkopf EA, Jarjour NN, Chou C, Archambault AS, et al. IL-1-induced Bhlhe40 identifies pathogenic T helper cells in a model of autoimmune neuroinflammation. J Exp Med. 2016;213(2):251–271. doi: 10.1084/jem.20150568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MA, Massague J. Hematopoiesis controlled by distinct TIF1gamma and Smad4 branches of the TGFbeta pathway. Cell. 2006;125(5):929–941. doi: 10.1016/j.cell.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 127.Xiao S, Yosef N, Yang J, Wang Y, Zhou L, Zhu C, et al. Small-molecule RORgammat antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity. 2014;40(4):477–489. doi: 10.1016/j.immuni.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kurokawa J, Arai S, Nakashima K, Nagano H, Nishijima A, Miyata K, et al. Macrophage-derived AIM is endocytosed into adipocytes and decreases lipid droplets via inhibition of fatty acid synthase activity. Cell Metab. 2010;11(6):479–492. doi: 10.1016/j.cmet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 129.Wang C, Yosef N, Gaublomme J, Wu C, Lee Y, Clish CB, et al. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell. 2015;163(6):1413–1427. doi: 10.1016/j.cell.2015.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Longhi MS, Vuerich M, Kalbasi A, Kenison JE, Yeste A, Csizmadia E, et al. Bilirubin suppresses Th17 immunity in colitis by upregulating CD39. JCI Insight. 2017;2(9):92791. doi: 10.1172/jci.insight.92791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339(5):285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 132.Yates MA, Li Y, Chlebeck PJ, Offner H. GPR30, but not estrogen receptor-alpha, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunol. 2010;11:20. doi: 10.1186/1471-2172-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brunsing RL, Prossnitz ER. Induction of interleukin-10 in the T helper type 17 effector population by the G protein coupled estrogen receptor (GPER) agonist G-1. Immunology. 2011;134(1):93–106. doi: 10.1111/j.1365-2567.2011.03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mann EH, Gabrysova L, Pfeffer PE, O’Garra A, Hawrylowicz CM. High-dose IL-2 skews a glucocorticoid-driven IL-17(+)IL-10(+) memory CD4(+) T cell response towards a single IL-10-producing phenotype. J Immunol. 2019;202(3):684–693. doi: 10.4049/jimmunol.1800697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Volchenkov R, Nygaard V, Sener Z, Skalhegg BS. Th17 polarization under hypoxia results in increased IL-10 production in a pathogen-independent manner. Front Immunol. 2017;8:698. doi: 10.3389/fimmu.2017.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34(4):566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Revu S, Wu J, Henkel M, Rittenhouse N, Menk A, Delgoffe GM, et al. IL-23 and IL-1beta drive human Th17 cell differentiation and metabolic reprogramming in absence of CD28 costimulation. Cell Rep. 2018;22(10):2642–2653. doi: 10.1016/j.celrep.2018.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 140.Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med. 2009;206(10):2235–2251. doi: 10.1084/jem.20082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell. 2016;166(5):1231–1246.e13. doi: 10.1016/j.cell.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 143.Wang S, Xia P, Chen Y, Qu Y, Xiong Z, Ye B, et al. Regulatory innate lymphoid cells control innate intestinal inflammation. Cell. 2017;171(1):201–216.e18. doi: 10.1016/j.cell.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 144.Seehus CR, Kadavallore A, Torre B, Yeckes AR, Wang Y, Tang J, et al. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat Commun. 2017;8(1):1900. doi: 10.1038/s41467-017-02023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brockmann L, Soukou S, Steglich B, Czarnewski P, Zhao L, Wende S, et al. Molecular and functional heterogeneity of IL-10-producing CD4(+) T cells. Nat Commun. 2018;9(1):5457. doi: 10.1038/s41467-018-07581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu M, Zhao X, Ma Y, Zhou Y, Deng M, Ma Y. Transcription factor c-Maf is essential for IL-10 gene expression in B cells. Scand J Immunol. 2018;88(3):e12701. doi: 10.1111/sji.12701. [DOI] [PubMed] [Google Scholar]
- 147.Gabrysova L, O’Garra A. Regulating the regulator: Bhlhe40 directly keeps IL-10 in check. J Exp Med. 2018;215(7):1767–1769. doi: 10.1084/jem.20180824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sun H, Lu B, Li RQ, Flavell RA, Taneja R. Defective T cell activation and autoimmune disorder in Stra13-deficient mice. Nat Immunol. 2001;2(11):1040–1047. doi: 10.1038/ni721. [DOI] [PubMed] [Google Scholar]
- 149.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–274. doi: 10.1016/0092-8674(93)80068-P. [DOI] [PubMed] [Google Scholar]
- 150.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361(21):2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]