Abstract
Amyloid precursor protein (APP) is a transmembrane protein expressed largely within the central nervous system. Upon cleavage, it does not produce the toxic amyloid peptide (Aβ) only, which is involved in neurodegenerative progressions but via a non-amyloidogenic pathway it is metabolized to produce a soluble fragment (sAPPα) through α-secretase. While a lot of studies are focusing on the role played by APP in the pathogenesis of Alzheimer’s disease, sAPPα is reported to have numerous neuroprotective effects and it is being suggested as a candidate with possible therapeutic potential against Alzheimer’s disease. However, the mechanisms through which sAPPα precisely works remain elusive. We have presented a comprehensive review of how sAPPα is regulating the neuroprotective effects in different biological models. Moreover, we have focused on the role of sAPPα during different developmental stages of the brain, neurogenic microenvironment in the brain and how this metabolite of APP is regulating the neurogenesis which is regarded as a compelling approach to ameliorate the impaired learning and memory deficits in dementia and diseases like Alzheimer’s disease. sAPPα exerts beneficial physiological, biochemical and behavioral effects mitigating the detrimental effects of neurotoxic compounds. It has shown to increase the proliferation rate of numerous cell types and promised the synaptogenesis, neurite outgrowth, cell survival and cell adhesion. Taken together, we believe that further studies are warranted to investigate the exact mechanism of action so that sAPPα could be developed as a novel therapeutic target against neuronal deficits.
Keywords: Neurogenesis, Amyloid precursor protein, Neurogenic niche, Neurodegeneration, Dementia, Brain development, sAPPα, Learning and memory, Alzheimer’s disease
Introduction
Amyloid precursor protein (APP) plays a vital role in development and plasticity of the nervous system. The major non-amyloidogenic cleavage at alpha (α) secretase site within the amyloid sequence results in release of a soluble long N-terminal APP fragment (sAPPα). This metabolite of APP has reported to have neurotrophic and neuroprotective properties [1]. Apart from earlier known neuroprotective properties like neurite outgrowth, synaptogenesis, modulation of LTP, attenuation of neurotoxicity, rescue of spatial learning and synaptic plasticity deficits by sAPPα [2–5]. It is now being investigated as a candidate with potential neurogenic potential which may ascertain to benefit against the impaired learning and memory in dementia and disease like Alzheimer’s disease. Neurogenesis is an endogenous complex multistep process that involves synchronized proliferation, differentiation and migration of neural precursor cells [6]. In adult brain, there are two distinct regions where neurogenesis is continuously thought to occur throughout the lifespan in mammals. These regions include the subventricular zone (SVZ), lining the lateral ventricles, and the subgranular layer (SGL) at the border of the dentate gyrus and hilus in the hippocampal formation [7]. Among the neurogenic zones in the adult brain, adult hippocampal neurogenesis attracts the most attention, because it is involved in higher cognitive function, most notably memory processes, and certain affective behaviors [8]. This persistent production of new neurons in the adult hippocampus was first reported by Altman and colleagues [9]. The genesis of these new neurons becomes integrated synaptically into hippocampal circuits over the passage of weeks’ time, and once sufficiently mature, they are thought to contribute toward encoding new memories [10]. The two discrete adult neurogenic brain niches comprise resident neural stem/progenitor cells (NSPCs), which have been found to have a great potential for self-renewal and exhibit multipotent potential. In subventricular zone/olfactory bulb (SVZ/OB) system, the resident stem cells found at the SVZ divide to form neuroblasts, and these cells migrate through the rostral migratory path to reach the olfactory bulb (OB), where these immature cells become fully mature neurons [11]. A similar process occurs in the SGZ of the dentate gyrus in the hippocampus, where the neural stem cells divide to form neuroblasts, which migrate a short distances into the granule cell layer and finally differentiate into hippocampal granular cells [12, 13].Although a recent study demonstrated that adult hippocampal neurogenesis is non-existent beyond adolescence, showing undetectable levels of proliferating cells and immature neurons in both aged non-human primates and young or adult humans [14], but it was immediately followed by the reports demonstrating that adult hippocampal neurogenesis is sustained through aging in the human brain, albeit at lower levels than in the young human brain and even this process was found to exist in Alzheimer’s disease [15–17]. Reports have demonstrated that the Aβ and in particular soluble form of amyloid precursor protein alpha (sAPPα) play a neurodevelopmental role due to its integral membrane interactions [18] and there are several studies that have addressed the effects of APP on different events of the neurogenic process using neural stem/progenitor cells (NSPCs) [19–21]. Studies have further demonstrated that influence of neurogenesis by APP is deliberated differently via its two separate domains, soluble secreted APPs (sAPPα) and APP intracellular domain (AICD). The sAPPα has been shown to be neuroprotective and important to neurogenesis, whereas AICD was found to negatively modulate neurogenesis [22]. Here, we will be discussing the neuromodulatory role of sAPPα and how this metabolite is regulating the neurogenic niches and thus helps in attenuating different neuronal deficits.
Amyloid precursor protein processing
Human amyloid precursor protein (APP) belongs to a highly conserved family of type 1 transmembrane glycoproteins which constitutes APP and the mammalian homologs APLP-1 and APLP-2, both of them lacking the Aβ sequence [23–25]. It comprises a long extracellular N-terminal domain, a transmembrane region and a short intracellular C-terminal domain [24]. The biological function of APP is yet to be fully elucidated. The evolutionary conservation of APP gene family also extends to invertebrate species with its orthologs APPL in Drosophila and APL-1 in Caenorhabditis elegans, respectively [26, 27]. These proteins share numerous conserved motifs within the large extracellular domain and a short cytoplasmic region which displays the highest sequence homology [28]. The human APP gene contains 18 exons spanning more than 170 kbp [29]. The region encoding the Aβ sequence consists part of exons 16 and 17 and is composed of 40–43 amino acid residues that extend from the ectodomain into the transmembrane domain of this protein. Alternative splicing of the APP gene leads to at least 3 different isoforms encoding 695, 751 and 770 amino acid proteins: the latter two containing an N-terminal domain similar to Kunitz-type serine protease inhibitors (KPI) [30, 31]. In CNS, APP695 is highly enriched in neurons while astrocytes and microglia express similar amounts of all three isoforms that appear to be mainly internalized to vesicles and more resistant to cleavage [32]. The presence of multiple distinct domains located within the extracellular portion includes a signal peptide (SP), a copper binding domain (CuBD), a heparin-binding/growth factor-like domain1 (HPBD1), a Kunitz-type protease inhibitor domain (KPI), a second heparin-binding domain 2 (HPBD2), a zinc-binding domain (ZnBD), a random coil region (RC) and the Aβ sequence. The remaining region consists of the cytoplasmic tail of APP, including AICD [33, 34]. Recently, a study has found that the average protein sequence identity between human and Chinese tree shrew was 88.29% higher than that of between human and mouse (85.11%). Moreover, the mouse only had 52 genes with a high sequence identity, while the Chinese tree shrew had 68 such genes indicating how this gene family is conserved across different species [35]. Constitutive APP mutations and copy number variations (duplications) are thought to cause rare forms of familial Alzheimer’s disease and related neuropathology in Down’s syndrome supporting the idea that they have a pathogenic role when present mosaically in sporadic Alzheimer’s disease [36, 37]. Recent reports investigating the APP genomic loci have shown that individuals with sporadic Alzheimer’s disease had a marked shift in the forms and abundance of genomic cDNAs when compared with healthy controls including the three- to fivefold increase in genomic cDNAs in all brains from individuals with sporadic Alzheimer’s disease examined. They have further identified 11 somatic single nucleotide variants that were earlier reported to be pathogenic in familial Alzheimer’s disease and absent here from non-diseased controls [36, 38].
Proteolytic processing of APP
APP undergoes extensive proteolytic cleavages by four enzymatic actions (α-, β-, γ-, and η-secretase) resulting in numerous intra-and extracellular metabolites based on the type of cleavage. APP processing results either homo or hetero-dimerization via N-terminal domain along with its mammalian paralogs, the APLP-1 and 2, promoting intercellular adhesion [39, 40]. The cleavage of APP takes place via amyloidogenic (leading to plaque formation) pathway, where APP is cleaved first by a different enzyme, β-secretase (a transmembrane aspartic protease), yielding a soluble N-terminal fragment (sAPPβ) and a membrane-bound C-terminal fragment (CTFβ). This cut is made closer to the N-terminal end of APP than with α-secretase, making CTFβ longer than CTFα. Upon subsequent cleavage of CTFβ via γ-secretase, there is generation of a membrane-bound C-terminal fragment (AICD), and a soluble N-terminal fragment (amyloid-β, or Aβ) which is longer than p3. Though Aβ is required for neuronal function, it can accumulate in the extracellular space of the brain, where it aggregates to form amyloid plaques. Aβ can exert deleterious effects on neuronal and synaptic function, ultimately causing neuronal cell death. Another cleavage pathway is non-amyloidogenic; here, the APP is cleaved first by α-secretase to yield a soluble N-terminal fragment (sAPPα) and a C-terminal fragment (CTFα). ADAM10 is the main α-secretase that cleaves APP in this pathway. Its increased activity has been found to protect the brain from β-amyloid deposition in AD and this approach has been proved to be effective in treating several neurodegenerative diseases. Nonetheless, ADAM10 is controlled in a very complex manner at transcriptional, translational and post-translational levels [41] and the other members of this family like ADAM17 and ADAM19 as well as BACE2 that share a substantial amino acid sequence homology with β-secretase have shown to cleave APP at or close to the α-cleavage site in APP [42–44]. sAPPα may be involved in the enhancement of synaptogenesis, neurite outgrowth and neuronal survival, and is considered to be neuroprotective. CTFα is retained in the membrane, where it is further cleaved by presenilin-containing γ-secretase to yield a soluble N-terminal fragment (p3) and a membrane-bound C-terminal fragment (AICD, or APP intracellular domain). AICD may be involved in nuclear signaling via transcriptional regulation as well as axonal transport through its ability to associate with a host of different proteins [45]. Moreover, another recently discovered cleavage of APP by η-secretase has added to the complexity of APP processing; it results in the generation of the carboxy-terminal fragment-η (CTF-η or η-CTF) and N-terminal sAPPη [46]. The cleavage is regulated either through inhibition of lysosomal-cathepsin degradation pathway [47] or through inhibition of BACE1 [48]. Cleavage by η-Secretase starts initially at 504–505 amino acids of APP695, releasing a truncated ectodomain. Following this, CTF-η is further processed by ADAM10 and BACE1 to release long and short Aη peptides (termed Aη-α and Aη-β). CTFs produced by η-secretase have been found to be enriched in dystrophic neurites in an AD mouse model and in human AD brains [48].
APP trafficking
Within the neurons of central nervous system, the transport of APP varies slightly to that of other systems. It is synthesized and translated in the endoplasmic reticulum before traveling to the Golgi complex [49, 50]. During its trafficking from the ER to the plasma membrane, APP undergoes several post-translational modifications. After protein synthesis in membrane-bound polysomes, APP is N-glycosylated in the endoplasmic reticulum (ER) and then transported to the Golgi apparatus where it is further O- and N-glycosylated, phosphorylated and sulphonated at tyrosine motifs [50]. APP is concentrated in the Golgi complex and, from here, travels through the central vacuolar system to the plasma membrane. Reports from in vitro studies have demonstrated that only 10% of APP goes to the plasma membrane, whereas the majority of APP protein remains in the Golgi and trans-Golgi network (TGN) [51]. α-Secretase is principally enriched at the cell surface and competes with BACE1 for APP processing. BACE1 is primarily localized in the TGN and endosomes. Within trans-Golgi network (TGN), α-Secretase competes with β-secretase, whereas protein kinase C stimulates α-secretase activity to moderately decrease β-cleavage [52, 53]. Endosomal acidic compartments provide a low pH environment, which is more favorable for BACE1 activity. Furthermore, BACE1 is rapidly internalized from the cell surface and degraded by the ubiquitin–proteasome pathway [54–56]. Prompt degradation of BACE1 by ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) reduces C99 and Aβ production [57]. Hence, the majority of cell surface APP is processed through the non-amyloidogenic pathway, whereas intracellular APP processing predominantly involves the amyloidogenic pathway [58]. A trivial fraction of γ-secretase complex components is found on the cell surface, and the rest are mainly localized at the ER, Golgi/TGN and endosomes [59]. APP is transported along the axon in post-TGN vesicles or in elongated tubular structures using kinesin 1 as a motor protein. Using kinesin and microtubules, it travels via fast axonal transport to the presynapse [49, 60]. Here, it is assimilated into the presynaptic membrane, precisely into the presynaptic active zone, and to a lesser extent to free synaptic vesicles, signifying a role in the physiology of neurotransmitter release [49].
Neuroactive/neuromodulatory role of sAPPα
Among the metabolites of the APP pathway, sAPPα has been reported with a maximum number of neuroprotective activities. It has shown to enhance the long-term neuronal survival and neuronal extensions [61], protected cultured rat hippocampal, septal neurons and human cortical neurons against hypoglycemic damage, calcium-mediated hypoglycemia and glutamate excitotoxicity [62]. Further, it has demonstrated to attenuate elevated intracellular calcium levels, increased reactive oxygen species, iron-induced oxidative injury, NMDA and Aβ-induced toxicities in cultured rat hippocampal neurons (Fig. 1 ) [63]. Moreover, it has shown to regulate the function of full-length APP enhancing neurite outgrowth in mouse and rat hippocampal neurons [64] and progenitor proliferation in the sub-ventricular zone of lateral ventricle of mouse epidermal growth factor responsive neurospheres [65]. In impact-acceleration model of diffused traumatic brain injury (TBI) in rats, sAPPα has shown to improve motor outcome and attenuate axonal injury and neuronal cell loss [66]; it has further improved the cortical and hippocampal injury and behavioral outcomes in controlled cortical impact (focal) with intra-cerebro ventricular infusion mice model of TBI [67]. sAPPα increases long-term potentiation [2] and has revealed to rescue the spatial learning and synaptic plasticity deficits of APP knockout mice and in Alzheimer’s disease (AD) mouse model. It has helped in recruiting microglia to the site of amyloid deposits, resulting in upregulating the insulin degrading enzymes (IDE) and thus contributing toward clearance of Aβ [4, 5]. A recent study has found that SET (SET/I2PP2A-a nuclear protein) and α3-NKA are required for sAPPα-induced axonal elongation and neuroprotection and axonal elongation are differently regulated by sAPPα [68] sAPPα can inhibit BACE1-mediated β-secretase activity and decrease Aβ generation and amyloid pathology in cells and mouse models of AD [69]; it has further inhibited tau phosphorylation through modulation of GSK3β signaling pathway (Fig. 1) [70]. Numerous studies have reported the activation of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) in response to sAPPα [71, 72]. These kinases become activated upon binding of extracellular ligands to growth factor receptors and are paired with neurite elongation following neural stimulation. Activation of cell proteins involved in cell proliferation such as phosphatidylinositol-3 kinase, Akt kinase, and p42/p44 MAPKs has also been implicated in sAPPα-mediated neurotrophic and neuroprotective mechanisms (Fig. 1) [73]. Binding of sAPPα to the cell surface suggests an interaction with membrane-bound receptors as reported from the studies, where sAPPα and p75NTR have been co-immuno-precipitated in vitro and co-localized in COS-7 cells. This interaction promoted neurite outgrowth in mouse cortical neurons, an effect that was abolished with p75NTR siRNA-mediated knockdown [74]. Increasing sAPPα levels have proven to be a therapeutic treatment that mitigates the effects of Aβ and rescue cognitive functions. Reports have recently shown that lentivirus-mediated expression of a human sAPPα construct in a mouse model of AD (APPswe/PS1dE9), before the onset of plaque pathology, could prevent later behavioral and electrophysiological deficits. Transgenic mice expressing sAPPα have performed significantly better than untreated littermates in all aspects of the spatial water maze task. Moreover, the increased expression of sAPPα has led to partial rescue of long-term potentiation (LTP) as well (Fig. 1) [75].
Fig. 1.
Neuroprotective effects of sAPPα: Schematic diagram representing how sAPPα, the soluble metabolite of APP, was able to protect the neuronal cells against Aβ and NMDA-induced toxicities, increased the synaptogenesis and neurite outgrowth and was able to restore the impaired memory and thus synaptic plasticity in mice. In addition, sAPPα is able to modulate several cell signaling pathways like PI3K, MAPK, and GSK3β and has shown to inhibit tau hyperphosphorylation and afford neuroprotection in different in vitro models
APP and sAPPα in brain development
APP family members have revealed an essential role in brain development and maintenance during development, young/adulthood as well as at aging stages. Evidence from APPL knockout Drosophila has found to be viable but with slight behavioral defects (conditional learning defects) [76] and defects in maintaining the integrity of synaptic boutons at the neuromuscular junctions [77], and was susceptible to brain injury [78]. In another study, APL-1 knockout condition in Caenorhabditis elegans has been found to be lethal due to molting defect [79, 80]. APP has a crucial role to play in neuronal migration during early embryogenesis. The neuronal precursor cells migrate from the ventricular zone into the cortical plate and this process has been found to be impaired when APP levels are reduced in the cortex. Migration defects caused by APP loss were found to be salvaged by increasing the expression of human APP695, APP751 isoforms, mutant APP (Swedish and Indiana) but not by truncated APP constructs [81, 82]; APP expression levels are at their highest during the early stages of synaptogenesis [83] and varying expression levels appear to be important during normal development. APP levels have been found to be highest in the second postnatal week, overlapping with the timing of brain maturation and completion of synaptic connections [84]; the increased levels suggest a role for APP in synapse formation or maintenance. APP is predominantly cleaved by α-secretase throughout the normal physiology of a cell, and sAPPα embarks its presence throughout the lifespan, including early brain development. Reports have demonstrated that the increased levels of sAPPα are important for synaptic pruning and growth [85]. It has been found to be a critical factor during development contributing toward synaptic stabilization and purging via protein synthesis [86]. Studies have found that in postnatal days 21 and 28 Fragile X mental retardation protein (FMR1) knockout mice, there was an increased expression of APP and ADAM10, as compared to their wild-type littermates. This further resulted in an increase in the cleavage of APP through the non-amyloidogenic pathway, which could be found by looking at the lack of increased cell surface APP in the knockout mice and, therefore, there was a surplus of sAPPα. Interestingly, this increase in APP, ADAM10, and sAPPα was also found in fibroblasts derived from FXS patients [86]. The increased expression of sAPPα in the mouse model has led to an increase in the number of thin, immature dendritic spines, which is a key pathological feature of the disorder. The authors have advocated that increased protein synthesis may be the reason which involves the ability of sAPPα to activate the mGluR5 pathway [86, 87]. APP knockout mice had abnormal brain weight, and behavior deficits like decrease in grip strength, locomotor activity, and impaired learning and memory, which were found to be rescued in mice with sAPPα knockin on the APP null background, suggesting the involvement of sAPPα fragment of APP to ameliorate these neurological functionalities [4]. Administration of exogenous recombinant sAPPα or overexpressing the secreted fragment of APP gene by viral gene transfer has led to enhanced spatial memory and stimulated memory consolidation in healthy adult rodents. It has further resulted in regulating hippocampal NMDA receptor function and rescuing long-term potentiation (LTP) in the APP knockout mice [2, 4, 5]. sAPPα has a well-established role in neurite outgrowth during adulthood and this is due to cell surface receptor binding and one such potential receptor for this is p75 neurotrophic receptor (p75NTR) [74]. Further, sAPPα has shown to stimulate membrane-associated guanylate cyclase [74], regulating synaptic scaffolding which led to a decrease in N-methyl-d-aspartate (NMDA) receptor currents [88]. However, during aging reports have shown a dramatic decrease in sAPPα levels and this decreased has resulted in spatial memory deficits [89]. Moreover, these levels were also decreased in patients with mild cognitive impairment and Alzheimer’s diseases [90, 91]. sAPPα overexpression studies in aged APP/PS1ΔE9 mice with plaques have resulted in enhanced synaptic plasticity via LTP induction and partial rescue in dendritic spines, and decrease in Aβ levels and plaque load, which may be associated with the improvements in animal’s spatial reference memory [5]. Although the exact mechanism of sAPPα action remains elusive, the overall effects so far are promising at different developmental stages of the brain.
sAPPα and neurogenesis
Neurogenic regions in the brain
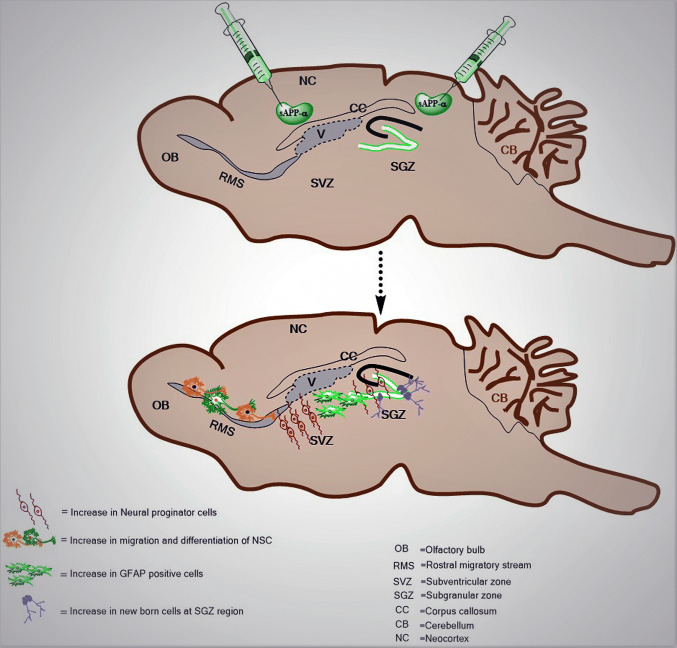
Neurogenesis was thought to take place only during embryonic and perinatal stages in mammals until studies from Altman and Das reported the presence of newly generated dentate granule cells in the postnatal rat hippocampus [9]. However, the functional integration of these new neurons in the adult central nervous system (CNS) was demonstrated later on in songbirds [92]. Adult neurogenesis is highly vibrant and controlled by multiple physiological stimuli and pathological states. It persists in specific regions of the brain [subventricular zone (SVZ) and subgranular layer (SGL)] known as neurogenic niches (Fig. 2). The neurogenic region embodies a dedicated microenvironment which plays major role in maintaining and regulating neural stem cell (NSC) proliferation [93]. Neurogenesis in other adult CNS regions is generally believed to be very limited under normal physiological conditions, but could be induced after injury [94]. Controlled cortical impact (CCI) model of severe traumatic brain injury (TBI) has proven to enhance neural stem cell (NSC) proliferation in the hippocampal dentate gyrus [95]. In developing brain, most of the neurogenesis occurs in the ventricular and sub-ventricular proliferative zones and in adult brain; neurogenesis contracts to the subependymal zone and the dentate gyrus (subgranular zone) of the hippocampus [96]. A huge population of neural stem/precursor cells (NSCs) has been found to keep on in the ventricular–subventricular zone (V–SVZ) located in the walls of the lateral brain ventricles. Ventricular–SVZ neural cells are reportedly producing huge number of neuroblasts that finally migrate a long distance via rostral migratory stream (RMS) into the olfactory bulb (OB), where they differentiate into local circuit interneurons [97]. Neural stem cells in the SGZ give rise to granular neurons that integrate into the functional circuits in hippocampus [93]. These newly born neurons play critical roles in facilitating memory function. The cellular population of these neurogenic niches includes ependymal cells, astrocytes, endothelial cells, mature neurons, microglia and progeny of adult neural precursors [98]. Vascular cells play a prominent role in regulating proliferation of adult neural precursors. Studies have demonstrated increased neuronal differentiation of adult rat SVZ explants when co-cultured with endothelial cells [99]. The vasculature was found to play a significant role by maintaining the substrate for new neuronal migration after injury in the adult striatum [100]. Adult SVZ NSCs (Type B cells) are reported to extend a basal process to terminate on blood vessels and extend an apical process with a primary cilium which pushes through the ependymal cell layer to make connections with the cerebrospinal fluid (CSF) in the ventricle [101]. These Type B NSCs have shown to contribute toward escalating new transient amplifying progenitors (Type C cells) [7], which follow some divisions before turning into neuroblasts (Type A cells). These neuroblasts then grow in a series and migrate into the olfactory bulb where they migrate radially and differentiate into different subtypes of interneurons. Radial glia-like (RGLs)-NSCs (Type 1 cells) in the SGZ, at the edge between the inner granule cell layer and hilus, give rise to intermediate progenitor cells (IPCs) [102]; however, they go through a few repeats of proliferation before generating new neuroblasts [103]. These neuroblasts migrate tangentially along the SGZ and develop into immature neurons, which migrate radially into the granule cell layer to differentiate into dentate granule neurons [104]. In neurogenic regions, the vasculature is highly structured with distinctive architecture throughout both adult SGZ [104] and SVZ [105] and adult NSCs extend processes that are closely associated with blood vessels and are affected by the factors carried through blood. Endothelial cells have shown to secrete vascular endothelial growth factor (VEGF) which promotes NSC self-renewal, and neurotrophin-3 (NT-3), which stimulates quiescence and long-term maintenance [106]. Additionally, the blood–brain barrier near the clusters of proliferating NSCs is found to be somewhat leaky, permitting these cells an easy access toward the factors in the blood [107]. Among the supporting cell types astrocytes in SGZ and SVZ are vital for the adult neurogenic niche, due to their contributions toward promoting proliferation and differentiation of NSCs. Astrocytes have shown to provide a physical support for newborn neurons and facilitate their migration and integration into neuronal circuits [108]. In the neurogenic niche, astrocytes express ciliary neurotrophic factor (CNTF), while the receptor, CNTFRα, is predominantly expressed in neural progenitor cells and hippocampal neurons [109]. The trophic factors have an immense role in maintenance and differentiation of NSC and neurogenic niches and there is a complex interplay of distinct signaling effectors within the neurogenic niche that serve to maintain neurogenesis through to adulthood.
Fig. 2.
Neurogenic role of sAPPα: Sagittal view of rodent brain showing the two distinct regions of the brain with neurogenic potential. Schematic diagram showing how sAPPα plays a vital role in generation, migration and differentiation of neural stem cells at subgranular zone and subventricular zone along with rostral migratory route. sAPPα acts as a vascular niche signal in the subventricular zone (SVZ) and it has an ability to bind to the surface of neural stem cells and regulate their proliferation and differentiation. It increases the number of neural progenitor cells and GFAP-positive cells and helps in migration and differentiation of the neural stem cells which finally migrate via RMS and differentiate into local circuit interneurons
Neurotrophic factors and neurogenesis
Growth factors have shown to play a key role in neurogenesis right from embryonic life to adult neurogenesis. Recently, it has been reported that growth factors and neurotrophins play a significant role in neural stem cell fate determination [110]. Endogenous neurotrophic growth factors, which include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glia-derived nerve factor (GDNF) and insulin-like growth factor 1 (IGF-1), have vibrant roles in stimulating NSC proliferation, differentiation and central nervous system (CNS) development [111–113]. NGF regulates the differentiation of NSC into mature neural phenotypes, a trait inhibited by EGF. It has shown its effects by differentiating NSC into neurons and astrocytes and further into functional glutamatergic and sensory neurons. Its differentiation effects have reportedly regulated via down-regulation of ATF5 and upregulation of TIMP-2 metalloproteinase inhibitor expression [114, 115]. Neurotrophic factors have shown to stimulate the activity of tropomyosin-related kinase (Trk) receptors, which in turn activates intracellular signaling pathways that regulate NSC self-renewal and fate like BDNF–TrkB signaling has been found to be essential in the upregulation of hippocampal neurogenesis and for the survival of new neurons throughout adult neurogenesis [116, 117]. Altered levels of neurotrophic factors have been found in different dementia and degenerative disorders like Alzheimer’s disease and Parkinson’s disease. BDNF is reported as a potential neurotrophin that affects dendritic development of SVZ-derived neurons via its high affinity receptor TrkB. Changes in BDNF levels were reported in dopaminergic neurons of the substantia nigra in PD patients [118] and a reduction has been found in NGF levels in cholinergic neurons of forebrain in AD patients as well as in aged rats [119, 120]. ICV injection of these neurotrophic factors has promoted the adult hippocampal neurogenesis and new neuron survival in young adult rats and enhanced cognitive functions in rats as a result of improved neurogenesis in the hippocampus [121–123]. In addition to these basic growth factors, NT-3, another growth factor similar to these in basic structure, has shown to affect the migration and neurite outgrowth. NSC overexpressing NT-3 exhibited lengthier neurites in vitro and promoted neurite outgrowth in in vivo conditions [124, 125]. It is strongly involved in neuronal specification and NSCs expressing NT-3 had a higher viability compared to controls [124]. Apart from these, insulin-like growth factor 1 (IGF-1), epidermal growth factor (EGF), fibroblast growth factor (FGF), glial cell line-derived neurotrophic factor (GDNF), platelet-derived growth factor (PDGF) have immense role in regulation of NSC migration, proliferation and differentiation. The problem with most of the growth factors is the restricted entry due to blood–brain barrier and second the delivery of clinically beneficial levels of neurotrophic factors has proven challenging, so we need to find a better way so that the beneficial effects of these neurotrophic and growth factor could be utilized and in this context our laboratory is evaluating the sAPPα overexpressing stem cell approach which shows promising results till now; however, further studies are going on to reach a logical conclusion.
sAPPα: an important neurotrophic factor regulating neurogenesis
Accumulative evidence has shown that neurogenesis in the adult hippocampus plays an essential role in learning and memory [10], and impairments in this neurogenic process are concomitant with cognitive impairments in AD [126]. A recent report has demonstrated that reduction in sAPPα levels could serve as a significant compounding factor in disease progression, as sAPPα exerts beneficial physiological, biochemical and behavioral effects that may mitigate the detrimental effects of Aβ accumulation [75] Further, studies have provided evidence that expression of human sAPPα in the mouse hippocampus not only prevented the development of an AD-like phenotype, but it also rescued the synaptic plasticity once the phenotype has developed and synaptic plasticity plays a major role in learning and memory which is anomalous in AD [75]. Aberrant neurogenesis has been found in transgenic mouse models of AD in which Aβ and its precursor APP levels were increased [127–129]. Studies have demonstrated neurotrophic properties of sAPPα in numerous cell types. It has shown to aid as a proliferation factor for NPCs of the adult brain (Fig. 2). There has been an abundant expression of sAPP and α- secretase in SVZ region. Using a broad-spectrum matrix-metalloproteinase inhibitor that inhibits α-secretase enzymes, it was shown that α-secretase inhibition reduced NPC proliferation and this reduction was further on reversed by treating the cells with sAPPα. Not only in NPCs, sAPPα treatment in mesenchymal stem cells and human placental stem cells increased the proliferation rate implying that sAPPα could act as a potent proliferation factor for stem cells of various lineages [130]. Apart from the physiological functions of sAPPα that have been implicated in the enhancement of synaptogenesis, neurite outgrowth, cell survival and cell adhesion [71, 131] in vivo reports have found the neurogenic potential of sAPPα [64]. Crystal structure of sAPP has revealed that it contains a growth factor like domain similar to cysteine-rich growth factors [132], which possibly makes it a potential metabolite of APP to stimulate the proliferation of a number of different cell types. It was further reported that these growth-promoting properties of sAPPα are possibly mediated by the ability of sAPPα to downregulate CDK5 and inhibit tau hyperphosphorylation [133]. sAPPα has initially shown to protect cultured neurons against glutamate neurotoxicity and hypoglycemia damage through the activation of potassium channels, which in turn mediates the ability of sAPPα to inhibit calcium influx and, therefore, modulate the neuronal excitability [62, 134]. Studies have recently found in adult brain of conditional double knockout mice (cDKO) lacking APP and the related APLP2 that sAPPα is able to efficiently rescue the deficits in spine density, synaptic plasticity (LTP and PPF), and spatial reference memory [135]. sAPPα has an ability to bind to the surface of neural stem cells and regulate their proliferation and differentiation.(Figure 2) [136, 137]. Moreover, it has been found that sAPPα signaling is required for depolarization-induced neurite outgrowth in NPC-derived neurons [71]. It has been able to increase the population of GFAP-positive cells from an average of 45% in controls (no sAPP) to an average of 83% with sAPP treatment under serum-free conditions in human neuronal stem cells (hNSCs). This increase has been hypothesized due to JAK/STAT and Notch signaling pathways by studying the expression of the different proteins (LIF, CNTF, JAK1, and STAT3) and (Delta, Jagged1, and Hes1) in NT-2 cells. Additionally, studies have found that hNSCs migration and differentiation were reduced in APP knockout mice [138]. Looking at the synergistic effects of growth factors, sAPPα has been found to function independently of the prominent proliferation factors; epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), but in association with ERK signaling and MAP-kinase signaling pathways. It has been found that it works as a proliferation factor of adult NPCs, mesenchymal stem cells (MSCs), and human decidua parietalis placenta stem cells (hdpPSCs). Inhibition of alpha secretase activity has reduced the proliferation of these stem cell populations in a dose-dependent manner. Stem cell proliferation was found to be rescued by the addition of sAPPα in a dose-dependent manner, but not in cells where media were depleted of sAPPα [139]. Though epidermal growth factor (EGF)-responsive neural progenitor cells (NPCs) in the sub-ventricular zone (SVZ) have binding sites for sAPP for regulating the proliferation of NPCs [64], reports have shown that deficiency of the sortilin-related receptor with type-A repeats (SORLA) results in enhancement of sAPP production, extracellular signal-regulated kinase (ERK) stimulation, increased proliferation and survival of NPCs in both the SVZ and subgranular layer of the dentate gyrus (SGL) [140]. However, at the same time sAPPα has an ability to work independently regulating as an essential proliferation factor for neural and non-neural adult stem cells [139]. Studies have suggested that sAPPα as well as sAPPβ increases the proliferation of NPC derived from the SGL and the SVZ of adult rats in vitro, and enhances their glial fate [141]. Not only in neuronal stem cells, sAPPα has been found to regulate the proliferation of non-NSCs suggesting a potential justification for the ubiquitous expression of APP and ADAMs. Remarkably, following cerebral ischemia, an increase in ADAM17 proteolytic activity and APP expression led to an increased proliferation in the SVZ [142]. In developing brain, overexpression of APP had shown migration of progenitors past the cortical plate boundary [81]. The neurogenic microenvironment plays an important role for the genesis and processing of new cells. Vascular niche has demonstrated to regulate the neural stem cell (NSC) quiescence and growth signals. Recent reports have shown that soluble amyloid precursor protein (sAPP) acts as a vascular niche signal in the subventricular zone (SVZ) of the lateral ventricle of the adult mouse brain [143]. These reports are consistent with the previous studies where mice with a human mutant form of APP (APPIndiana) initially had normal number of new born cells in the dentate gyrus and eventually after a year, proliferation was reduced compared to wild-type mice [144]. On the other hand, mice carrying an alternative human mutant form of APP (APPSwedish) had fewer proliferating (BrdU+) cells in the dentate gyrus [145] and sub-ventricular zone [146], as compared to wild-type mice; however, most of the new cells survived and as such APPSwedish mice ultimately accumulated more new dentate granule neurons than wild-type mice [147]. AAV-mediated intracranial expression of sAPPα has shown to mitigate the Aβ-related synaptic deficits of APP/PS1 mice in vivo [5]. Moreover, it has lowered the Aβ levels by directly binding to and inhibiting BACE [69]. Recent studies have demonstrated that the CTa16 domain of sAPPα (that is lacking in sAPPB) is able to facilitate LTP to the same extent as sAPPα, in a mechanism that involves functional nicotinic a7 acetylcholine receptors (a7-nAChRs). Further, they have shown that nanomolar concentrations of sAPPα can directly potentiate a7-nAChRs-mediated currents upon heterologous expression in Xenopus oocytes and increase in the apparent agonist affinity as a positive allosteric modulator [135]. sAPPα-mediated trophic effects are activity dependent and stimulated by the activation of 5-HT4 and NMDAR [71, 148], signifying them to be a realistic adaptive strategy in LTP, plasticity and excitotoxicity. Interestingly, sAPPα levels were shown to decrease in the cerebrospinal fluid (CSF) of AD individuals, while infusion of sAPPα into the brain increased synaptic density and improved memory retention [89, 149]. Taken together, these findings raised the possibility that sAPPα may contribute to neurogenesis in adult brain and sAPPα might be used for AD patients clinically, while decrease of sAPPα levels in brain may be a crucial precondition for pathogenesis of Alzheimer’s disease (Table 1).
Table 1.
Summarizing the role of different growth factors in neuroprotection and neurogenesis
| sAPPα | Other neurotrophic factors | |||
|---|---|---|---|---|
| Function | References | Growth factor | Function | References |
| Enhanced memory retention by regulating synaptic structure in adult rats | [150] | BDNF | Stimulated cell proliferation, neural differentiation and cell survival in vitro | [151] |
| sAPPα exerts beneficial physiological, biochemical effects; preventing the development of memory and plasticity deficits in a mouse model of Alzheimer’s disease | [75] | Intra-hippocampal infusion of BDNF in adult rats has increased the number of newborn neurons and resulted in migration of newborn neurons | [152] | |
| sAPPα significantly increased the proliferation rate of stem cells of various lineages | [130] | Higher levels of BDNF expression have been implicated in long-term potentiation and synaptic plasticity | [153, 154] | |
| sAPPα has shown to regulate the proliferation of neural precursor cells in SVZ of mice | [64] | Decreased levels of BDNF in older adults may lead to compromised memory and cognitive deficits and neurodegeneration | [155] | |
| Crystal structure of sAPP has revealed that it contains a growth factor like domain similar to cysteine-rich growth factors | [156] | BDNF has significantly promoted the in vivo proliferation, initiated differentiation, axonal path migration, and resulted in maturation of the neural stem cells in the dentate gyrus area of hippocampus | [153, 157–159] | |
| sAPPα has shown to protect cultured neurons against glutamate-induced excitotoxicity and hypoglycemia damage | [62] | BDNF | BDNF expression in the cerebral cortex and hippocampus was markedly reduced in an AD mouse model (APP/PS1 transgenic mice) at 3 and 9 months old | [160] |
| sAPPα has significantly rescued the deficits in spine density, synaptic plasticity (LTP and PPF), and spatial reference memory in conditional double knock mice lacking APP and APLP2 | [135] | Lentiviral BDNF gene delivery into the entorhinal cortex of transgenic mice expressing the APP with mutations at age 2 months improved hippocampal-dependent contextual fear conditioning after 3 months | [161] | |
| sAPPα enhanced neurite outgrowth and survival in embryonic rat neocortical cultures | [162] | During traumatic brain injury, BDNF can stimulate and regulate the growth of new neurons from neuronal stem cells | [163] | |
| ICV injections of sAPPα in a while after traumatic brain injury improved motor functions and reduced the number of apoptotic neurons in CA3 of hippocampus and cortex after 3 days of TBI | [66] | BDNF, through TrkB signaling, was shown to have an essential role in the regulation of dendritic complexity as well as synaptic formation, maturation and plasticity of newborn neurons | [164–166] | |
| sAPPα promoted glial differentiation via Notch signaling | [167] | NGF | NGF regulates the differentiation of NSC into mature neural phenotypes, a trait inhibited by EGF. Its differentiation effects have reportedly regulated via downregulation of ATF5 and upregulation of TIMP-2 metalloproteinase inhibitor expression | [114, 115] |
| sAPPα has shown to regulate the presynaptic bouton density by maintaining the cortical cholinergic, glutamatergic and GABAergic transmission | [168] | Reduced levels of NGF have been found in cholinergic neurons of forebrain in AD patients as well as in aged rats | [119, 120] | |
| sAPPα has shown to enhance neurite outgrowth in differentiating NPCs and regulate the synaptic structure | [71, 150] | NGF | Trophic failure of NGF is responsible for the cholinergic atrophy in AD | [169] |
| sAPPα has an ability to bind to the surface of neural stem cells and regulate their proliferation and differentiation | [136, 137] | NGF | NGF levels have been found dysregulated in the pathophysiology of depression in some models of peripheral nerve damage and stress | [170] |
| sAPPα signaling is required for depolarization-induced neurite outgrowth in NPC-derived neurons | [75] | IGF-1 | IGF-I treatment has shown to increase the proliferation and differentiation of NPCs in vitro | [171–173] |
| sAPPα works as a proliferation factor of adult NPCs, mesenchymal stem cells (MSCs), and human decidua parietalis placenta stem cells (hdpPSCs) | [139] | Treatment of IGF-I in vitro has rescued impairments in differentiation/maturation of newborn neurons exposed to corticosterone (a stress-related hormone) | [174] | |
| Following cerebral ischemia, an increase in ADAM17 proteolytic activity and APP expression leads to increased proliferation in the SVZ | [142] | Insulin-like growth factor I and IGF-II are widely expressed in nervous tissue from early embryonic life and higher levels of IGF-I expression are associated with proliferating neural precursors | [175] | |
| In developing brain overexpression of APP had shown migration of progenitors past the cortical plate boundary | [81] | IGF-1 overexpressing mice have increased postnatal brain growth and the mice with IGF-1 deficiency have impaired neuronal somatic and dendritic growth | [176, 177] | |
| soluble amyloid precursor protein (sAPP) acts as a vascular niche signal in the subventricular zone (SVZ) of the lateral ventricle of the adult mouse brain | [143] | GDNF is involved in the development and maintenance of mesencephalic and adult dopaminergic neurons | [178–180] | |
| AAV-mediated intracranial injection of sAPPα has shown to mitigate the Aβ-related synaptic deficits of APP/PS1 mice in vivo | [5] | GDNF has shown to regulate the normal functioning of the dopamine transporter in the striatum | [181] | |
| sAPPα has been found to directly potentiate a7-nAChRs-mediated currents upon heterologous expression in Xenopus oocytes and increase in the apparent agonist affinity as a positive allosteric modulator | [135] | GDNF | GDNF is a well-known neuroprotective therapeutic candidate for Parkinson’s disease (PD) | [182] |
| sAPPα-mediated trophic effects are activity-dependent and stimulated by activation of 5-HT4 and NMDAR | [71, 148] | It has shown to facilitate the differentiation of adult dentate gyrus-derived neural precursor cells into astrocytes via STAT3 | [183] | |
| sAPPα, but not sAPPβ, protects neurons against Aβ oligomer-induced dendritic spine loss and increased tau phosphorylation | [184] | |||
| sAPP has recently shown to regulate GABABR1a function, sAPP–GABABR1a binding suppressed synaptic transmission and enhanced short-term facilitation in mouse hippocampal synapses via inhibition of synaptic vesicle release | [185] | |||
Conclusion
The reports from diverse studies about the soluble form of amyloid precursor protein alpha (sAPPα) having beneficial effects against myriad of neuronal ailments and in particular its role at different developmental stages and as a potent proliferating factor are encouraging. Here, we increased our knowledge about various aspects beginning from embryonic stage through adulthood to aging in connection with physiological and pathological conditions of the brain that are regulated by sAPPα, although the neuroactive properties like synaptogenesis, neurite outgrowth, attenuation of neurotoxic effects, neurogenic role of sAPPα are promising. However, no dedicated receptor has been reported yet for this metabolite. Over a decade, the focus on sAPPα is increasing and taking new directions. We believe that activating α-secretase-dependent endoproteolytic APP processing to surge sAPPα shedding from the neuronal plasma membrane would protect dendritic spines and phospho-tau-dependent toxicity in Alzheimer’s disease. The neurogenic role of sAPPα we have presented here seems to be overwhelmingly leading toward approachable targets. In conclusion, this review opens up new research directions to elucidate the sAPPα in more details by investigating the α-secretases and recently found new secretases, using gene therapy approaches to understand the relation of this metabolite with diverse receptors/transcription factors, so that it could be used as a better therapeutic target against neuronal deficits like impairments in dementia and Alzheimer’s disease.
Acknowledgements
The research is currently supported by grants to GWG from St. Boniface Hospital Research Foundation (SBHF-7069), Winnipeg, Canada. We would like to thank Dr. JA Bhat (University of Rochester, NY) for his constant advice throughout manuscript preparation.
Abbreviations
- APP
Amyloid precursor protein
- sAPPα
Secreted amyloid precursor protein alpha
- ADAM 10
A disintegrin and metalloproteinase domain-containing protein 10
- BACE1
Beta-secretase enzyme 1
- CCI
Controlled cortical impact
- IDE
Insulin degrading enzyme
- NSC
Neural stem cell
- NMDA
N-Methyl-d-aspartate
- SGZ
Subgranular zone
- SVZ
Sub-ventricular zone
- TBI
Traumatic brain injury
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dorard E, et al. Soluble amyloid precursor protein alpha interacts with alpha3-Na, K-ATPAse to induce axonal outgrowth but not neuroprotection: evidence for distinct mechanisms underlying these properties. Mol Neurobiol. 2018;55:5594–5610. doi: 10.1007/s12035-017-0783-0. [DOI] [PubMed] [Google Scholar]
- 2.Taylor CJ, et al. Endogenous secreted amyloid precursor protein-α regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol Dis. 2008;31(2):250–260. doi: 10.1016/j.nbd.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Moreno L, et al. sAβPPα improves hippocampal NMDA-dependent functional alterations linked to healthy aging. J Alzheimers Dis. 2015;48(4):927–935. doi: 10.3233/JAD-150297. [DOI] [PubMed] [Google Scholar]
- 4.Ring S, et al. The secreted β-amyloid precursor protein ectodomain APPsα is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci. 2007;27(29):7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fol R, et al. Viral gene transfer of APPsα rescues synaptic failure in an Alzheimer’s disease mouse model. Acta Neuropathol. 2016;131(2):247–266. doi: 10.1007/s00401-015-1498-9. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 7.Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/S0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 8.Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015;7(9):a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 10.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Buylla A, Garcıa-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14(2):186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Babu H, et al. Synaptic network activity induces neuronal differentiation of adult hippocampal precursor cells through BDNF signaling. Front Neurosci. 2009;3:1. doi: 10.3389/neuro.22.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorrells SF, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555(7696):377. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boldrini M, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22(4):589–599. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartt AN, et al. Considerations for assessing the extent of hippocampal neurogenesis in the adult and aging human brain. Cell Stem Cell. 2018;23(6):782–783. doi: 10.1016/j.stem.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno-Jiménez EP, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25(4):554. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 18.Plant LD, et al. The production of amyloid β peptide is a critical requirement for the viability of central neurons. J Neurosci. 2003;23(13):5531–5535. doi: 10.1523/JNEUROSCI.23-13-05531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Toledano MA, Shelanski ML. Neurogenic effect of β-amyloid peptide in the development of neural stem cells. J Neurosci. 2004;24(23):5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heo C, et al. Effects of the monomeric, oligomeric, and fibrillar Aβ42 peptides on the proliferation and differentiation of adult neural stem cells from subventricular zone. J Neurochem. 2007;102(2):493–500. doi: 10.1111/j.1471-4159.2007.04499.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Dong C. Aβ40 promotes neuronal cell fate in neural progenitor cells. Cell Death Differ. 2009;16(3):386. doi: 10.1038/cdd.2008.94. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Z-D, et al. The roles of amyloid precursor protein (APP) in neurogenesis: implications to pathogenesis and therapy of Alzheimer disease. Cell Adhes Migr. 2011;5(4):280–292. doi: 10.4161/cam.5.4.16986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldgaber D, et al. Isolation, characterization, and chromosomal localization of human brain cDNA clones coding for the precursor of the amyloid of brain in Alzheimer’s disease, Down’s syndrome and aging. J Neural Transm Suppl. 1987;24:23–28. [PubMed] [Google Scholar]
- 24.Kang J, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325(6106):733. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 25.Wasco W, et al. Identification of a mouse brain cDNA that encodes a protein related to the Alzheimer disease-associated amyloid beta protein precursor. Proc Natl Acad Sci. 1992;89(22):10758–10762. doi: 10.1073/pnas.89.22.10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daigle I, Li CA. apl-1, a Caenorhabditis elegans gene encoding a protein related to the human beta-amyloid protein precursor. Proc Natl Acad Sci. 1993;90(24):12045–12049. doi: 10.1073/pnas.90.24.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen DR, et al. A Drosophila gene encoding a protein resembling the human beta-amyloid protein precursor. Proc Natl Acad Sci. 1989;86(7):2478–2482. doi: 10.1073/pnas.86.7.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gralle M, Ferreira ST. Structure and functions of the human amyloid precursor protein: the whole is more than the sum of its parts. Prog Neurobiol. 2007;82(1):11–32. doi: 10.1016/j.pneurobio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Yoshikai S-I, et al. Genomic organization of the human amyloid beta-protein precursor gene. Gene. 1990;87(2):257–263. doi: 10.1016/0378-1119(90)90310-N. [DOI] [PubMed] [Google Scholar]
- 30.Ponte P, et al. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988;331(6156):525. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka S, et al. Three types of amyloid protein precursor mRNA in human brain: their differential expression in Alzheimer’s disease. Biochem Biophys Res Commun. 1988;157(2):472–479. doi: 10.1016/S0006-291X(88)80273-0. [DOI] [PubMed] [Google Scholar]
- 32.Haass C, Hung AY, Selkoe DJ. Processing of beta-amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion. J Neurosci. 1991;11(12):3783–3793. doi: 10.1523/JNEUROSCI.11-12-03783.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitaguchi N, et al. Novel precursor of Alzheimer’s disease amyloid protein shows protease inhibitory activity. Nature. 1988;331(6156):530. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- 34.König G, et al. Identification and differential expression of a novel alternative splice isoform of the beta A4 amyloid precursor protein (APP) mRNA in leukocytes and brain microglial cells. J Biol Chem. 1992;267(15):10804–10809. [PubMed] [Google Scholar]
- 35.Fan Y, et al. Does the genetic feature of the Chinese tree shrew (Tupaia belangeri chinensis) support its potential as a viable model for Alzheimer’s disease research? J Alzheimers Dis. 2018;61(3):1015–1028. doi: 10.3233/JAD-170594. [DOI] [PubMed] [Google Scholar]
- 36.Murrell J, et al. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science. 1991;254(5028):97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- 37.Wiseman FK, et al. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci. 2015;16(9):564. doi: 10.1038/nrn3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee M-H, et al. Somatic APP gene recombination in Alzheimer’s disease and normal neurons. Nature. 2018;563(7733):639. doi: 10.1038/s41586-018-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheuermann S, et al. Homodimerization of amyloid precursor protein and its implication in the amyloidogenic pathway of Alzheimer’s disease. J Biol Chem. 2001;276(36):33923–33929. doi: 10.1074/jbc.M105410200. [DOI] [PubMed] [Google Scholar]
- 40.Soba P, et al. Homo-and heterodimerization of APP family members promotes intercellular adhesion. EMBO J. 2005;24(20):3624–3634. doi: 10.1038/sj.emboj.7600824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peron R, et al. Alpha-secretase ADAM10 regulation: insights into Alzheimer’s disease treatment. Pharmaceuticals. 2018;11(1):12. doi: 10.3390/ph11010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farzan M, et al. BACE2, a β-secretase homolog, cleaves at the β site and within the amyloid-β region of the amyloid-β precursor protein. Proc Natl Acad Sci. 2000;97(17):9712–9717. doi: 10.1073/pnas.160115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanabe C, et al. ADAM19 is tightly associated with constitutive Alzheimer’s disease APP α-secretase in A172 cells. Biochem Biophys Res Commun. 2007;352(1):111–117. doi: 10.1016/j.bbrc.2006.10.181. [DOI] [PubMed] [Google Scholar]
- 44.Asai M, et al. Putative function of ADAM9, ADAM10, and ADAM17 as APP α-secretase. Biochem Biophys Res Commun. 2003;301(1):231–235. doi: 10.1016/S0006-291X(02)02999-6. [DOI] [PubMed] [Google Scholar]
- 45.Lopez Sanchez MIG, van Wijngaarden P, Trounce IA. Amyloid precursor protein-mediated mitochondrial regulation and Alzheimer’s disease. Br J Pharmacol. 2019;176(18):3464–3474. doi: 10.1111/bph.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrew RJ, et al. A Greek tragedy: the growing complexity of Alzheimer amyloid precursor protein proteolysis. J Biol Chem. 2016;291(37):19235–19244. doi: 10.1074/jbc.R116.746032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, et al. Cathepsin L mediates the degradation of novel APP C-terminal fragments. Biochemistry. 2015;54(18):2806–2816. doi: 10.1021/acs.biochem.5b00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willem M, et al. η-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature. 2015;526(7573):443. doi: 10.1038/nature14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laßek M, et al. Amyloid precursor proteins are constituents of the presynaptic active zone. J Neurochem. 2013;127(1):48–56. doi: 10.1111/jnc.12358. [DOI] [PubMed] [Google Scholar]
- 50.Tomita S, Kirino Y, Suzuki T. Cleavage of Alzheimer’s amyloid precursor protein (APP) by secretases occurs after O-glycosylation of APP in the protein secretory pathway Identification of intracellular compartments in which APP cleavage occurs without using toxic agents that interfere with protein metabolism. J Biol Chem. 1998;273(11):6277–6284. doi: 10.1074/jbc.273.11.6277. [DOI] [PubMed] [Google Scholar]
- 51.Placido A, et al. The role of endoplasmic reticulum in amyloid precursor protein processing and trafficking: implications for Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis. 2014;1842(9):1444–1453. doi: 10.1016/j.bbadis.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Skovronsky DM, et al. Protein kinase C-dependent α-secretase competes with β-secretase for cleavage of amyloid-β precursor protein in the trans-Golgi network. J Biol Chem. 2000;275(4):2568–2575. doi: 10.1074/jbc.275.4.2568. [DOI] [PubMed] [Google Scholar]
- 53.Vassar R, et al. β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 54.Huse JT, et al. Maturation and endosomal targeting of β-site amyloid precursor protein-cleaving enzyme the Alzheimer’s disease β-secretase. J Biol Chem. 2000;275(43):33729–33737. doi: 10.1074/jbc.M004175200. [DOI] [PubMed] [Google Scholar]
- 55.Pastorino L, et al. The carboxyl-terminus of BACE contains a sorting signal that regulates BACE trafficking but not the formation of total Aβ. Mol Cell Neurosci. 2002;19(2):175–185. doi: 10.1006/mcne.2001.1065. [DOI] [PubMed] [Google Scholar]
- 56.Qing H, et al. Degradation of BACE by the ubiquitin-proteasome pathway. FASEB J. 2004;18(13):1571–1573. doi: 10.1096/fj.04-1994fje. [DOI] [PubMed] [Google Scholar]
- 57.Zhang M, et al. Control of BACE1 degradation and APP processing by ubiquitin carboxyl-terminal hydrolase L1. J Neurochem. 2012;120(6):1129–1138. doi: 10.1111/j.1471-4159.2011.07644.x. [DOI] [PubMed] [Google Scholar]
- 58.Haass C, et al. Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992;357(6378):500. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- 59.Chyung JH, Raper DM, Selkoe DJ. γ-Secretase exists on the plasma membrane as an intact complex that accepts substrates and effects intramembrane cleavage. J Biol Chem. 2005;280(6):4383–4392. doi: 10.1074/jbc.M409272200. [DOI] [PubMed] [Google Scholar]
- 60.Koo EH, et al. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci. 1990;87(4):1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Araki W, et al. Trophic effect of β-amyloid precursor protein on cerebral cortical neurons in culture. Biochem Biophys Res Commun. 1991;181(1):265–271. doi: 10.1016/S0006-291X(05)81412-3. [DOI] [PubMed] [Google Scholar]
- 62.Mattson MP, et al. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the β-amyloid precursor protein. Neuron. 1993;10(2):243–254. doi: 10.1016/0896-6273(93)90315-I. [DOI] [PubMed] [Google Scholar]
- 63.Goodman Y, Mattson MP. Secreted forms of β-amyloid precursor protein protect hippocampal neurons against amyloid β-peptide-induced oxidative injury. Exp Neurol. 1994;128(1):1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- 64.Caillé I, et al. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131(9):2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- 65.Young-Pearse TL, et al. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural Dev. 2008;3(1):15. doi: 10.1186/1749-8104-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thornton E, et al. Soluble amyloid precursor protein α reduces neuronal injury and improves functional outcome following diffuse traumatic brain injury in rats. Brain Res. 2006;1094(1):38–46. doi: 10.1016/j.brainres.2006.03.107. [DOI] [PubMed] [Google Scholar]
- 67.Corrigan F, et al. Evaluation of the effects of treatment with sAPPα on functional and histological outcome following controlled cortical impact injury in mice. Neurosci Lett. 2012;515(1):50–54. doi: 10.1016/j.neulet.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 68.Dorard E, et al. Soluble amyloid precursor protein alpha interacts with alpha3-Na, K-ATPAse to induce axonal outgrowth but not neuroprotection: evidence for distinct mechanisms underlying these properties. Mol Neurobiol. 2018;55(7):5594–5610. doi: 10.1007/s12035-017-0783-0. [DOI] [PubMed] [Google Scholar]
- 69.Obregon D, et al. Soluble amyloid precursor protein-alpha modulates beta-secretase activity and amyloid-beta generation. Nat Commun. 2012;3:777. doi: 10.1038/ncomms1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng J, et al. Soluble amyloid precursor protein alpha inhibits tau phosphorylation through modulation of GSK3beta signaling pathway. J Neurochem. 2015;135(3):630–637. doi: 10.1111/jnc.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gakhar-Koppole N, et al. Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur J Neurosci. 2008;28(5):871–882. doi: 10.1111/j.1460-9568.2008.06398.x. [DOI] [PubMed] [Google Scholar]
- 72.Greenberg SM, Kosik KS. Secreted beta-APP stimulates MAP kinase and phosphorylation of tau in neurons. Neurobiol Aging. 1995;16(3):403–407. doi: 10.1016/0197-4580(94)00172-W. [DOI] [PubMed] [Google Scholar]
- 73.Cheng G, et al. Phosphatidylinositol-3-kinase-Akt kinase and p42/p44 mitogen-activated protein kinases mediate neurotrophic and excitoprotective actions of a secreted form of amyloid precursor protein. Exp Neurol. 2002;175(2):407–414. doi: 10.1006/exnr.2002.7920. [DOI] [PubMed] [Google Scholar]
- 74.Hasebe N, et al. Soluble beta-amyloid precursor protein alpha binds to p75 neurotrophin receptor to promote neurite outgrowth. PLoS ONE. 2013;8(12):e82321. doi: 10.1371/journal.pone.0082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan VTY, et al. Lentivirus-mediated expression of human secreted amyloid precursor protein-alpha prevents development of memory and plasticity deficits in a mouse model of Alzheimer’s disease. Mol Brain. 2018;11(1):7. doi: 10.1186/s13041-018-0348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo L, Tully T, White K. Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron. 1992;9(4):595–605. doi: 10.1016/0896-6273(92)90024-8. [DOI] [PubMed] [Google Scholar]
- 77.Torroja L, et al. The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J Neurosci. 1999;19(18):7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leyssen M, et al. Amyloid precursor protein promotes post-developmental neurite arborization in the Drosophila brain. EMBO J. 2005;24(16):2944–2955. doi: 10.1038/sj.emboj.7600757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hornsten A, et al. APL-1, a Caenorhabditis elegans protein related to the human beta-amyloid precursor protein, is essential for viability. Proc Natl Acad Sci U S A. 2007;104(6):1971–1976. doi: 10.1073/pnas.0603997104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wiese M, Antebi A, Zheng H. Intracellular trafficking and synaptic function of APL-1 in Caenorhabditis elegans. PLoS ONE. 2010;5(9):e12790. doi: 10.1371/journal.pone.0012790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Young-Pearse TL, et al. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci. 2007;27(52):14459–14469. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Young-Pearse TL, et al. Biochemical and functional interaction of disrupted-in-schizophrenia 1 and amyloid precursor protein regulates neuronal migration during mammalian cortical development. J Neurosci. 2010;30(31):10431–10440. doi: 10.1523/JNEUROSCI.1445-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Priller C, et al. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26(27):7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loffler J, Huber G. Beta-amyloid precursor protein isoforms in various rat brain regions and during brain development. J Neurochem. 1992;59(4):1316–1324. doi: 10.1111/j.1471-4159.1992.tb08443.x. [DOI] [PubMed] [Google Scholar]
- 85.Corbett NJ, Hooper NM. Soluble amyloid precursor protein alpha: friend or foe? Adv Exp Med Biol. 2018;1112:177–183. doi: 10.1007/978-981-13-3065-0_13. [DOI] [PubMed] [Google Scholar]
- 86.Pasciuto E, et al. Dysregulated ADAM10-mediated processing of APP during a critical time window leads to synaptic deficits in fragile X syndrome. Neuron. 2015;87(2):382–398. doi: 10.1016/j.neuron.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 87.Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5(3):e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Furukawa K, Mattson MP. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem. 1998;70(5):1876–1886. doi: 10.1046/j.1471-4159.1998.70051876.x. [DOI] [PubMed] [Google Scholar]
- 89.Anderson JJ, et al. Reduced cerebrospinal fluid levels of alpha-secretase-cleaved amyloid precursor protein in aged rats: correlation with spatial memory deficits. Neuroscience. 1999;93(4):1409–1420. doi: 10.1016/S0306-4522(99)00244-4. [DOI] [PubMed] [Google Scholar]
- 90.Lannfelt L, et al. Decreased alpha-secretase-cleaved amyloid precursor protein as a diagnostic marker for Alzheimer’s disease. Nat Med. 1995;1(8):829–832. doi: 10.1038/nm0895-829. [DOI] [PubMed] [Google Scholar]
- 91.Dobrowolska JA, et al. Diurnal patterns of soluble amyloid precursor protein metabolites in the human central nervous system. PLoS ONE. 2014;9(3):e89998. doi: 10.1371/journal.pone.0089998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paton JA, Nottebohm FN. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225(4666):1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- 93.Mu Y, Lee SW, Gage FH. Signaling in adult neurogenesis. Curr Opin Neurobiol. 2010;20(4):416–423. doi: 10.1016/j.conb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8(6):481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- 95.Wang X, et al. Traumatic brain injury severity affects neurogenesis in adult mouse hippocampus. J Neurotrauma. 2016;33(8):721–733. doi: 10.1089/neu.2015.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stolp HB, Molnar Z. Neurogenic niches in the brain: help and hindrance of the barrier systems. Front Neurosci. 2015;9:20. doi: 10.3389/fnins.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim DA, Alvarez-Buylla A. The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb Perspect Biol. 2016;8(5):a018820. doi: 10.1101/cshperspect.a018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leventhal C, et al. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13(6):450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 100.Kojima T, et al. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28(3):545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- 101.Mirzadeh Z, et al. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seri B, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bond AM, Ming GL, Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015;17(4):385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun GJ, et al. Tangential migration of neuronal precursors of glutamatergic neurons in the adult mammalian brain. Proc Natl Acad Sci U S A. 2015;112(30):9484–9489. doi: 10.1073/pnas.1508545112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3(3):289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Delgado AC, et al. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron. 2014;83(3):572–585. doi: 10.1016/j.neuron.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 107.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee SW, Clemenson GD, Gage FH. New neurons in an aged brain. Behav Brain Res. 2012;227(2):497–507. doi: 10.1016/j.bbr.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Richardson PM. Ciliary neurotrophic factor: a review. Pharmacol Ther. 1994;63(2):187–198. doi: 10.1016/0163-7258(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 110.Oliveira SL, et al. Functions of neurotrophins and growth factors in neurogenesis and brain repair. Cytometry A. 2013;83(1):76–89. doi: 10.1002/cyto.a.22161. [DOI] [PubMed] [Google Scholar]
- 111.Auld DS, Mennicken F, Quirion R. Nerve growth factor rapidly induces prolonged acetylcholine release from cultured basal forebrain neurons: differentiation between neuromodulatory and neurotrophic influences. J Neurosci. 2001;21(10):3375–3382. doi: 10.1523/JNEUROSCI.21-10-03375.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weissmiller AM, Wu C. Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl Neurodegener. 2012;1(1):14. doi: 10.1186/2047-9158-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aberg MA, et al. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20(8):2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Angelastro JM, et al. Regulated expression of ATF5 is required for the progression of neural progenitor cells to neurons. J Neurosci. 2003;23(11):4590–4600. doi: 10.1523/JNEUROSCI.23-11-04590.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jaworski DM, Perez-Martinez L. Tissue inhibitor of metalloproteinase-2 (TIMP-2) expression is regulated by multiple neural differentiation signals. J Neurochem. 2006;98(1):234–247. doi: 10.1111/j.1471-4159.2006.03855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 118.Mogi M, et al. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett. 1999;270(1):45–48. doi: 10.1016/S0304-3940(99)00463-2. [DOI] [PubMed] [Google Scholar]
- 119.Calissano P, Matrone C, Amadoro G. Nerve growth factor as a paradigm of neurotrophins related to Alzheimer’s disease. Dev Neurobiol. 2010;70(5):372–383. doi: 10.1002/dneu.20759. [DOI] [PubMed] [Google Scholar]
- 120.Cooper JD, Lindholm D, Sofroniew MV. Reduced transport of [125I]nerve growth factor by cholinergic neurons and down-regulated TrkA expression in the medial septum of aged rats. Neuroscience. 1994;62(3):625–629. doi: 10.1016/0306-4522(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 121.Frielingsdorf H, et al. Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol Dis. 2007;26(1):47–55. doi: 10.1016/j.nbd.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 122.Pinnock SB, Herbert J. Brain-derived neurotropic factor and neurogenesis in the adult rat dentate gyrus: interactions with corticosterone. Eur J Neurosci. 2008;27(10):2493–2500. doi: 10.1111/j.1460-9568.2008.06250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Birch AM, Kelly AM. Chronic intracerebroventricular infusion of nerve growth factor improves recognition memory in the rat. Neuropharmacology. 2013;75:255–261. doi: 10.1016/j.neuropharm.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 124.Lu H, et al. Retrovirus delivered neurotrophin-3 promotes survival, proliferation and neuronal differentiation of human fetal neural stem cells in vitro. Brain Res Bull. 2008;77(4):158–164. doi: 10.1016/j.brainresbull.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 125.Kamei N, et al. BDNF, NT-3, and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine (Phila Pa 1976) 2007;32(12):1272–1278. doi: 10.1097/BRS.0b013e318059afab. [DOI] [PubMed] [Google Scholar]
- 126.Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener. 2011;6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chuang TT. Neurogenesis in mouse models of Alzheimer’s disease. Biochim Biophys Acta. 2010;1802(10):872–880. doi: 10.1016/j.bbadis.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 128.Lazarov O, Marr RA. Neurogenesis and Alzheimer’s disease: at the crossroads. Exp Neurol. 2010;223(2):267–281. doi: 10.1016/j.expneurol.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 2011;33(6):1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- 130.Demars MP, et al. Soluble amyloid precursor protein-alpha rescues age-linked decline in neural progenitor cell proliferation. Neurobiol Aging. 2013;34(10):2431–2440. doi: 10.1016/j.neurobiolaging.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mattson MP. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev. 1997;77(4):1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- 132.Rossjohn J, et al. Crystal structure of the N-terminal, growth factor-like domain of Alzheimer amyloid precursor protein. Nat Struct Biol. 1999;6(4):327–331. doi: 10.1038/7562. [DOI] [PubMed] [Google Scholar]
- 133.Han P, et al. Suppression of cyclin-dependent kinase 5 activation by amyloid precursor protein: a novel excitoprotective mechanism involving modulation of tau phosphorylation. J Neurosci. 2005;25(50):11542–11552. doi: 10.1523/JNEUROSCI.3831-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Furukawa K, et al. Activation of K+ channels and suppression of neuronal activity by secreted beta-amyloid-precursor protein. Nature. 1996;379(6560):74–78. doi: 10.1038/379074a0. [DOI] [PubMed] [Google Scholar]
- 135.Richter MC, et al. Distinct in vivo roles of secreted APP ectodomain variants APPsalpha and APPsbeta in regulation of spine density, synaptic plasticity, and cognition. EMBO J. 2018;37(11):e98335. doi: 10.15252/embj.201798335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hayashi Y, et al. Alzheimer amyloid protein precursor enhances proliferation of neural stem cells from fetal rat brain. Biochem Biophys Res Commun. 1994;205(1):936–943. doi: 10.1006/bbrc.1994.2755. [DOI] [PubMed] [Google Scholar]
- 137.Ohsawa I, et al. Amino-terminal region of secreted form of amyloid precursor protein stimulates proliferation of neural stem cells. Eur J Neurosci. 1999;11(6):1907–1913. doi: 10.1046/j.1460-9568.1999.00601.x. [DOI] [PubMed] [Google Scholar]
- 138.Kwak YD, et al. Amyloid precursor protein regulates differentiation of human neural stem cells. Stem Cells Dev. 2006;15(3):381–389. doi: 10.1089/scd.2006.15.381. [DOI] [PubMed] [Google Scholar]
- 139.Demars MP, et al. Soluble amyloid precursor protein: a novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. Stem Cell Res Ther. 2011;2(4):36. doi: 10.1186/scrt77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rohe M, et al. Sortilin-related receptor with A-type repeats (SORLA) affects the amyloid precursor protein-dependent stimulation of ERK signaling and adult neurogenesis. J Biol Chem. 2008;283(21):14826–14834. doi: 10.1074/jbc.M710574200. [DOI] [PubMed] [Google Scholar]
- 141.Baratchi S, et al. Secreted amyloid precursor proteins promote proliferation and glial differentiation of adult hippocampal neural progenitor cells. Hippocampus. 2012;22(7):1517–1527. doi: 10.1002/hipo.20988. [DOI] [PubMed] [Google Scholar]
- 142.Katakowski M, et al. Stroke-induced subventricular zone proliferation is promoted by tumor necrosis factor-alpha-converting enzyme protease activity. J Cereb Blood Flow Metab. 2007;27(4):669–678. doi: 10.1038/sj.jcbfm.9600390. [DOI] [PubMed] [Google Scholar]
- 143.Sato Y, et al. Soluble APP functions as a vascular niche signal that controls adult neural stem cell number. Development. 2017;144(15):2730–2736. doi: 10.1242/dev.143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Donovan MH, et al. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495(1):70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- 145.Dong H, et al. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127(3):601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 146.Haughey NJ, et al. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem. 2002;83(6):1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 147.Ermini FV, et al. Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. Am J Pathol. 2008;172(6):1520–1528. doi: 10.2353/ajpath.2008.060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cochet M, et al. 5-HT4 receptors constitutively promote the non-amyloidogenic pathway of APP cleavage and interact with ADAM10. ACS Chem Neurosci. 2013;4(1):130–140. doi: 10.1021/cn300095t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Almkvist O, et al. Cerebrospinal fluid levels of α-secretase—cleaved soluble amyloid precursor protein mirror cognition in a Swedish family with Alzheimer disease and a gene mutation. Arch Neurol. 1997;54(5):641–644. doi: 10.1001/archneur.1997.00550170111022. [DOI] [PubMed] [Google Scholar]
- 150.Roch J-M, et al. Increase of synaptic density and memory retention by a peptide representing the trophic domain of the amyloid beta/A4 protein precursor. Proc Natl Acad Sci. 1994;91(16):7450–7454. doi: 10.1073/pnas.91.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li T, et al. In-vitro effects of brain-derived neurotrophic factor on neural progenitor/stem cells from rat hippocampus. NeuroReport. 2009;20(3):295–300. doi: 10.1097/WNR.0b013e32832000c8. [DOI] [PubMed] [Google Scholar]
- 152.Scharfman H, et al. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192(2):348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 153.Korte M, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci. 1995;92(19):8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lu B, et al. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14(6):401. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 155.Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5(6):311. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- 156.Rossjohn J, et al. Crystal structure of the N-terminal, growth factor-like domain of Alzheimer amyloid precursor protein. Nat Struct Mol Biol. 1999;6(4):327. doi: 10.1038/7562. [DOI] [PubMed] [Google Scholar]
- 157.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc B Biol Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nakata H, Nakamura S. Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett. 2007;581(10):2047–2054. doi: 10.1016/j.febslet.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 159.Waterhouse EG, et al. BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J Neurosci. 2012;32(41):14318–14330. doi: 10.1523/JNEUROSCI.0709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xia D-Y, et al. PGC-1α or FNDC5 is involved in modulating the effects of Aβ1−42 oligomers on suppressing the expression of BDNF, a beneficial factor for inhibiting neuronal apoptosis, Aβ deposition and cognitive decline of APP/PS1 Tg mice. Front Aging Neurosci. 2017;9:65. doi: 10.3389/fnagi.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nagahara AH, et al. Early BDNF treatment ameliorates cell loss in the entorhinal cortex of APP transgenic mice. J Neurosci. 2013;33(39):15596–15602. doi: 10.1523/JNEUROSCI.5195-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ohsawa I, Takamura C, Kohsaka S. The amino-terminal region of amyloid precursor protein is responsible for neurite outgrowth in rat neocortical explant culture. Biochem Biophys Res Commun. 1997;236(1):59–65. doi: 10.1006/bbrc.1997.6903. [DOI] [PubMed] [Google Scholar]
- 163.Habtemariam S. The brain-derived neurotrophic factor in neuronal plasticity and neuroregeneration: new pharmacological concepts for old and new drugs. Neural Regen Res. 2018;13(6):983. doi: 10.4103/1673-5374.233438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chan JP, et al. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci. 2008;39(3):372. doi: 10.1016/j.mcn.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gao X, Smith GM, Chen J. Impaired dendritic development and synaptic formation of postnatal-born dentate gyrus granular neurons in the absence of brain-derived neurotrophic factor signaling. Exp Neurol. 2009;215(1):178–190. doi: 10.1016/j.expneurol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 166.Wang L, et al. Autocrine action of BDNF on dendrite development of adult-born hippocampal neurons. J Neurosci. 2015;35(22):8384–8393. doi: 10.1523/JNEUROSCI.4682-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kwak Y-D, et al. Involvement of notch signaling pathway in amyloid precursor protein induced glial differentiation. Eur J Pharmacol. 2011;650(1):18–27. doi: 10.1016/j.ejphar.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bell KF, et al. ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol Aging. 2008;29(4):554–565. doi: 10.1016/j.neurobiolaging.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 169.Bruno MA, et al. Increased matrix metalloproteinase 9 activity in mild cognitive impairment. J Neuropathol Exp Neurol. 2009;68(12):1309–1318. doi: 10.1097/NEN.0b013e3181c22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mondal AC, Fatima M. Direct and indirect evidences of BDNF and NGF as key modulators in depression: role of antidepressants treatment. Int J Neurosci. 2019;129(3):283–296. doi: 10.1080/00207454.2018.1527328. [DOI] [PubMed] [Google Scholar]
- 171.Zhang X, et al. IGF-1 promotes Brn-4 expression and neuronal differentiation of neural stem cells via the PI3K/Akt pathway. PLoS ONE. 2014;9(12):e113801. doi: 10.1371/journal.pone.0113801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Åberg MA, et al. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24(1):23–40. doi: 10.1016/S1044-7431(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 173.Mir S, et al. IGF-1 mediated neurogenesis involves a novel RIT1/Akt/Sox2 cascade. Sci Rep. 2017;7(1):3283. doi: 10.1038/s41598-017-03641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Odaka H, et al. Chronic glucocorticoid exposure suppressed the differentiation and survival of embryonic neural stem/progenitor cells: possible involvement of ERK and PI3K/Akt signaling in the neuronal differentiation. Neurosci Res. 2016;113:28–36. doi: 10.1016/j.neures.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 175.O’Kusky J, Ye P. Neurodevelopmental effects of insulin-like growth factor signaling. Front Neuroendocrinol. 2012;33(3):230–251. doi: 10.1016/j.yfrne.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mathews LS, et al. Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology. 1988;123(6):2827–2833. doi: 10.1210/endo-123-6-2827. [DOI] [PubMed] [Google Scholar]
- 177.Cheng CM, et al. Insulin-like growth factor 1 is essential for normal dendritic growth. J Neurosci Res. 2003;73(1):1–9. doi: 10.1002/jnr.10634. [DOI] [PubMed] [Google Scholar]
- 178.Lin LF, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 179.Pascual A, et al. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11(7):755. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- 180.Pascual A, et al. GDNF and protection of adult central catecholaminergic neurons. J Mol Endocrinol. 2011;46(3):R83–R92. doi: 10.1530/JME-10-0125. [DOI] [PubMed] [Google Scholar]
- 181.Kopra JJ, et al. Dampened amphetamine-stimulated behavior and altered dopamine transporter function in the absence of brain GDNF. J Neurosci. 2017;37(6):1581–1590. doi: 10.1523/JNEUROSCI.1673-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Grondin R, et al. GDNF revisited: a novel mammalian cell-derived variant form of GDNF increases dopamine turnover and improves brain biodistribution. Neuropharmacology. 2019;147:28–36. doi: 10.1016/j.neuropharm.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 183.Boku S, et al. GDNF facilitates differentiation of the adult dentate gyrus-derived neural precursor cells into astrocytes via STAT3. Biochem Biophys Res Commun. 2013;434(4):779–784. doi: 10.1016/j.bbrc.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 184.Tackenberg C, Nitsch RM. The secreted APP ectodomain sAPPα, but not sAPPβ, protects neurons against Aβ oligomer-induced dendritic spine loss and increased tau phosphorylation. Mol Brain. 2019;12(1):27. doi: 10.1186/s13041-019-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rice HC, et al. Secreted amyloid-β precursor protein functions as a GABABR1a ligand to modulate synaptic transmission. Science. 2019;363(6423):eaao4827. doi: 10.1126/science.aao4827. [DOI] [PMC free article] [PubMed] [Google Scholar]