Abstract
As an important chemokine receptor, the role of CX3CR1 has been studied extensively on the migration of lymphocytes including T and B cells. Although CX3CR1+ B cells have immune suppressor properties, little is known about its role on the regulation of BCR signaling and B cell differentiation as well as the underlying molecular mechanism. We have used CX3CR1 KO mice to study the effect of CX3CR1 deficiency on BCR signaling and B cell differentiation. Interestingly, we found that proximal BCR signaling, such as the activation of CD19, BTK and SHIP was reduced in CX3CR1 KO B cells upon antigenic stimulation. However, the activation of mTORC signaling was enhanced. Mechanistically, we found that the reduced BCR signaling in CX3CR1 KO B cells was due to reduced BCR clustering, which is caused by the enhanced actin accumulation by the plasma membrane via increased activation of WASP. This caused an increased differentiation of MZ B cells in CX3CR1 KO mice and an enhanced generation of plasma cells (PC) and antibodies. Our study shows that CX3CR1 regulates BCR signaling via actin remodeling and affects B cell differentiation and the humoral immune response.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03416-7) contains supplementary material, which is available to authorized users.
Keywords: CX3CR1, B cell, BCR signaling, Metabolism
Introduction
CX3 chemokine receptor 1 (CX3CR1), also known as the fractalkine receptor or G-protein coupled receptor 13 (GPR13) is encoded by the CX3CR1 gene in humans [1, 2]. CX3CR1 binds the chemokine CX3CL1 that is a transmembrane protein and chemokine involved in the adhesion and migration of leukocytes [3]. CX3CR1 is also expressed by monocytes and plays a critical role in the survival of monocytes [4].
The role of CX3CR1 has been studied recently in T and B cells. Human peripheral CX3CR1+ B cells could efficiently inhibit effector CD4+T cell activation and have immune suppressor functions. Food allergy patients have fewer CX3CR1+ B cells in their peripheral blood [5]. During respiratory syncytial virus (RSV) infection, the B cell receptor of neonatal Breg (nBreg) cells interacts with RSV protein F and induces the upregulation of CX3CR1 expression. CX3CR1 interacts with RSV glycoprotein G, leading to IL-10 production of nBreg that dampens T helper 1 (Th1) cytokine production [6]. The CX3CR1/CX3CL1 axis is involved in the interaction of chronic lymphocytic leukemia (CLL) cells with their microenvironment [7]. A fraction of CD4+ T cells expressing CX3CR1 in patients with asthma and CX3CR1-deficient mice have reduced lung disease upon allergen sensitization by inhibiting survival of T helper cells by reducing Bcl2 expression [8].
CX3CR1 signaling has been associated with cell metabolism in cancer. CX3CR1 is expressed in pancreatic cancer cell lines and overexpression of CX3CL1 increases cell metabolism such as glucose uptake and lactate secretion [9]. Mechanistically, the CX3CL1/CX3CR1 reprograms cell metabolism through the HIF-1 in pancreatic cancer cells and CX3CL1 stimulates HIF-1 expression via PI3K/Akt and MAPK pathways [9]. CX3CL1 induces cell migration by enhancing intercellular adhesion molecule-1 (ICAM-1) expression via CX3CR1/PI3K/Akt/NF-κB pathway in human osteosarcoma cells [10]. CX3CL1 stimulates angiogenesis by activating the RAF-1/MEK/ERK and PI3K/Akt/eNOS/NO signal pathways via CX3CR1 [11]. These data indicate that CX3CR1 regulates multiple cell activities via the PI3K/Akt pathway. mTOR mediates the PI3K/Akt signal transduction pathway and mTORC2 stimulates the activation of Akt. After receiving a proliferative signal mediated by the PI3K/Akt pathway, mTORC1 phosphorylates and activates S6K1 in Jurkat cells. In turn, S6K1 phosphorylates and activates the 40S ribosomal S6 protein [12–14]. The role of CX3CR1 in B cell metabolism remains poorly defined.
BCR signaling is vital for the survival and clonal expansion of naïve B cells as well as the differentiation of peripheral B cells [15–19]. When Ag binds to the BCR, it induces the conformational change of the BCR, which leads to the oligomerization of BCRs and reorganization of the actin cytoskeleton [20–24]. In turn, this leads to the initiation of BCR signaling by the recruitment of Src kinases, such as Lyn, to the ITAM domain of the cytoplasmic region of BCR-Igα and Igβ. The phosphorylation of ITAM recruits Syk that activates downstream signaling events, such as the phosphorylation of BTK and PLCγ as well as the activation of SHIP [25]. The differentiation of FO B cells is favored by strong BCR signaling while differentiation of MZ B cells is favored by weaker BCR signaling [19]. CD19-mediated BTK signaling is critical for the survival and maintenance of MZ B cells [15]. CX3CR1+ B cells express high amount of TGF-β upon BCR stimulation and suppress the proliferation of CD4+ CD25− T cells when co-culturing [5]. However, the role of CX3CR1 in regulation of BCR signaling and differentiation of B cells is unknown.
The induction of BCR signaling can induce the actin reorganization via activating several actin regulators such as WASP, N-WASP, Abp1 and Ezrin [25–31]. The antigenic stimulation of BCRs causes dephosphorylation of Ezrin, which is accompanied by the initial actin depolymerization. Later actin polymerization is triggered by the phosphorylation of WASP [32–34]. Interestingly, the actin reorganization can offer feedback to the BCR signaling by altering the velocity of BCR movement in the actin cortical network [23, 24, 29]. Previous research has shown that CX3CR1 is linked to the actin polymerization in dendritic cells [35]. It remains to be determined if CX3CR1 can regulate BCR signaling via modulating the actin reorganization.
In this study, we found that the deficiency of CX3CR1 reduced the proximal BCR signaling, such as the phosphorylation of CD19, BTK and SHIP as well as total tyrosine proteins. Intriguingly, the activation of distal BCR signaling molecules related with metabolic reprogramming such as PI3K and mTORC was enhanced in CX3CR1 KO B cells. The reduction of the proximal BCR signaling was associated with increased differentiation of MZ B cells, and an autoimmune phenotype in CX3CR1 KO mice. Mechanistically, the loss of CX3CR1 caused overactivation of WASP but weak dephosphorylation of Ezrin, resulting in enhanced accumulation of actin at the plasma membrane. This led to reduced BCR clustering and BCR signalosome recruitment in the contact zone of B cells stimulated with membrane antigens. Although the BCR signaling was reduced, it caused elevated plasma cell (PC) response and antibody production after immunization or Schistosoma japonicum infection. Overall, our study has established a molecular mechanism for how CX3CR1 regulates BCR signaling and B cell differentiation via modulation of actin remodeling.
Material and methods
Mice and cells
CX3CR1 KO Mice on the C57BL/6 background were purchased from the Taconic Biosciences. All mice were raised under the specific pathogen-free condition. Mouse experiments were performed at 6–8 weeks according to the guidelines of the Chinese Council on Animal Care and approved by the Ethics Committee of Animal Experimentation of Tongji Medical College (Wuhan, China). Splenic lymphocytes were isolated by Ficoll density centrifugation at 2000 rpm, 20 min, 25 °C. After that splenic B cells were purified by depleting T cells with anti-mouse CD90.2 mAb (105310, Biolegend) and guinea pig complement (C300-0500, Rockland Immunochemicals) and incubated with 75 cm2 flask in cell culture incubator for 1 h to remove adherent cells.
Flow cytometry
Red blood cells in bone marrow (BM) were lysed with ACK (RT122-01, Takara) and BM cells were stained with specific antibodies including FITC-anti-CD127, APC-anti-CD43, PE- anti-BP-1, Percp/cy5.5-anti-B220, PE/Cy7-anti-CD24 and Brilliant Violet 421 (BV421)-anti-IgM (BioLegend). Splenic cells were stained with other specific antibodies including FITC-anti-CD19, FITC-anti-CD95, FITC-anti-AnnexinV, FITC-anti-B220, Percp-anti-B220, BV510-anti-B220, PE-anti-CD23, Percp-anti-IgD, APC-anti-CD21, AF647-anti-GL7, PB-anti-IgM (BioLegend). For intracellular staining, PE-Cy7-anti-Ki-67 (25-5698-82, eBioscience) was used after cells were fixed and permeabilized with Fixation/Permeabilization Kit (00-5123 00-5223, eBioscience). Cells were detected with a BD LSR II (BD Biosciences) and data were analyzed with the Flow Jo (Tree Star) software.
Confocal and TIRFm
For confocal analysis, purified splenic B cells were first incubated with biotinylated AF594-mB- F(ab′)2–anti-Ig(M + G) (115-586-068, Jackson ImmunoResearch) on ice for 30 min and streptavidin (16000114, Jackson) for 10 min. Then the cells were stimulated for 0, 5, 10 and 30 min at 37 °C, and then fixed with paraformaldehyde (28908, Thermo) and permeabilized with 0.05% saponin prior to staining with anti-CX3CR1 (abs136616, Absin), anti-pY (05-321, mcrck-millipore), anti-pBTK (ab52192, abcam), anti-pCD19 (ab203615, abcam), anti-pSHIP (3941S, Cell Signaling Technology), anti-pWASP (A300-205A, Bethyl) and AF488-phalloidin (R37110, Thermo Fisher). Samples were analyzed by confocal microscope (Zeiss, LSM 780) with 405, 488 and 546 nm lasers. Colocalization was measured in a single confocal plane with the ZEN 2.3 (blue edition) software. For TIRFm analysis, purified B cells were labeled with AF546-mB-Fab′-anti-Ig and activated by mB-Fab′-anti-Ig tethered lipid bilayer prepared as described previously [29]. Then the cells were fixed with 4% paraformaldehyde (Thermo) and permeabilized with 0.05% saponin, and then stained with anti-pBTK, anti-pCD19, anti-pSHIP, anti-pWASP and AF488-phalloidin. The unclustered BCRs were considered as background fluorescence and subtracted for quantification. For each image we chose an area without BCR clustering that has similar background as the area that has BCR clustering in each cell as the background fluorescence. The MFI of this area that has background fluorescence was subtracted automatically from the MFI of the BCR clustering in each cell. TIRFm images were captured with TIRF system on an inverted microscope (Nikon Eclipse Ti-PFS), and data was measured by NIS-Elements AR 3.2 software from more than 50 individual cells per group.
Phos flow
Purified B cells were stained with Percp-anti-B220 at 4 °C for 30 min. Cells were incubated with biotinylated F(ab′)2 Anti-Mouse Ig(M + G) (115-066-068, Jackson ImmunoResearch) for 30 min at 4 °C and with streptavidin at 37 °C for 0, 5, 10 and 30 min. After that, cells were immediately fixed and permeabilized with Phosflow Lyse/Fix buffer and Phosflow Perm buffer III (BD Biosciences) and stained with anti-pWASP and AF488-phalloidin. Cells were detected with a BD LSR II and data were analyzed with the Flow Jo software.
Western blot
Purified B cells were stimulated with biotinylated F(ab′)2 Anti-Mouse Ig(M + G) (10 μg/ml) plus streptavidin (20 μg/ml) for 0, 5, 10 and 30 min or were stimulated with LPS (L2880-10 mg, sigma) (10 μg/ml) for 0, 10, 30 and 60 min at 37 °C. Then cells were lysed by RIPA buffer (P0013B, Beyotime) containing protease inhibitor cocktail (G2006, Servicebio), NaF (1M, G2007-1, Servicebio) and Na3VO3 (100Mm, G2007-1, Servicebio). Lysates were analyzed by SDS-PAGE, and transferred onto a nitrocellulose membrane and probed with anti-pCD19 (3571S, Cell Signaling Technology), anti-pY (05-321, Merck Millipore), anti-pBTK (5082S, Cell Signaling Technology), anti-pSHIP1 (3941S, Cell Signaling Technology), anti-pAkt (4060L, Cell Signaling Technology), anti-pS6 (4856S, Cell Signaling Technology), anti-pFoxO1 (9461S, Cell Signaling Technology), anti-pmTOR (5536S, Cell Signaling Technology), anti-pPI3K p85/p55 (4228S, Cell Signaling Technology), anti-WASP (sc-13139 SantaCruz), anti-pWASP (A300-205A, Bethyl), anti-WIP (sc-271113SantaC), anti-BTK (8547S, Cell Signaling Technology), anti-SHIP1 (2728S, Cell Signaling Technology), anti-PI3K (4292S,Cell Signaling Technology), anti-FoxO1 (2880S,Cell Signaling Technology), anti-Akt (9272S,Cell Signaling Technology), anti-S6 (2217S,Cell Signaling Technology), anti-mTOR (2983S,Cell Signaling Technology), anti-HIF-1α (39665, Active Motif), anti-NF-κB (D14E12, Cell Signaling Technology), anti-pNF-κB (3033S, Cell Signaling Technology), anti-pIKKα/β (2697S, Cell Signaling Technology), anti-IKKβ (D30C6, Cell Signaling Technology) and β-actin (60008-1-IG-10 proteintech). The total proteins were used as the loading control.
Immunization and ELISA
For NP-KLH immunization, 6-week old C57BL/6 WT and CX3CR1 KO mice were immunized i.p with 50 μg of 4-hydroxy-3-nitrophenylacetyl-keyhole Limpet Hemocyanin (NP-KLH) (MPL + TDM, N-5060-25, BIOSEARCH) precipitated in adjuvant. After 14 days, splenic lymphocytes were collected and stained with antibodies including FITC-anti-CD95, PE-NP (Biosearch Technologies), Percp-anti-B220 (BioLegend), AF647-anti-GL7 (BioLegend), PB-anti-IgM (BioLegend), BV510-anti-B220 (BioLegend), FITC-anti-B220 (BioLegend), APC-anti-CD21 (BioLegend). After 28 days, mice were immunized with the same dose of NP-KLH for the second immunization. After 5 days, splenic lymphocytes were collected and stained with antibodies and the serum was analyzed by ELISA using NP-BSA (Biosearch Technologies) coated plates and stained with IgM and IgG1 specific secondary Ab (Bethyl Laboratories) to measure the antibody levels of NP-specific subclasses.
Quantitative RT-PCR analysis
Purified B cells were used for measuring gene expression of WT and CX3CR1 KO mice. Total RNA was extracted by AxyPrep Multisource RNA Kit (AP-MN-MS-RNA-50, Axygen). PrimeScript RT Reagent Kit with gDNA Eraser (RR047A, Takara) was used to gain cDNA. The expression of different genes was determined using SYBR Premix ex taq (RR047A, Takara) on a StepOne Real-Time PCR System (Allied Biosystems). The primer sequences for the test genes are as follows:
cd19 5′ primer: ggacagtgaacgtggaggat and 3′ primer: gggcacatacaggctttgtt
btk 5′ primer: cgccattacgttgtgtgttc and 3′ primer: tagaaggcgcgtttttgttt.
wipf3 5′ primer:gctccctatcaaagccatca and 3′ primer: gatggattcaccttggttgg
pik3r6 5′ primer: acaggagctgagctgtccat and 3′ primer: ttggaggacttgaccacctc
dnm3 5′ primer: cactcttcaacaccgagcaa and 3′ primer: ggttgcgtatggtctccact
cd80 5′ primer:gagtctggaaacccatctgc and 3′ primer: tctgacacgtgagcatctcc
Bone marrow chimeras
For chimera analysis, BM cells (5 × 106) were obtained from WT (CD45.2+) or CX3CR1 KO (CD45.2+) mice and mixed with C57BL/6 (CD45.1+) BM cells at a 1:1 ratio and then were injected intravenously into C57BL/6 (CD45.1+) mice with 7 Gy irradiated. After 8 weeks of reconstitution, recipients were used for flow cytometry analysis as described [36].
Anti-double stranded DNA analysis
The serums of WT and CX3CR1 KO mice were collected to analyze the level of anti-dsDNA antibody by ELISA using the protocol as described [25].
Schistosoma japonicum infection
Mice were percutaneously infected with 25 cercariae of Schistosoma japonicum. About 5 weeks later, the splenic cells of infected mice were collected for flow cytometry.
Seahorse
Purified B cells were plated 2 × 106/well in a 24 well cluster and were incubated with F(ab′)2 Anti-Mouse Ig(M + G) for 2 h. Then cells were collected and plated into the Seahorse XF24 V7 PET 24-well microplates, which had been coated with poly-D-lysin 50 μg/ml (C0132, Beyotime) overnight at 4 °C. After that OCR (oxygen consumption rate) was measured in Seahorse XF 24 Assay Medium containing 25 mM glucose (G8769, Sigma), 2 mM L-glutamine (G6392, Sigma) and 1 mM sodium pyruvate (S8636, Sigma) under basal condition and in response to 1.5 μM oligomycin (abs42024304, Absin), 1 μM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) (C2920, Sigma) and 0.5 μM rotenone (R8875, Sigma) plus 1 μM antimycin A (abs42013402, Absin) with the XF-24.
Calcium flux
Purified B cells were plated 2 × 106/well in a 96-well plates with Fluo-4 (1 μM, S1060, Beyotime) at 37 °C for 30 min. After washing three times with 1 × PBS, the cells were transferred to flow tubes followed by adding F(ab′)2 Anti-Mouse Ig(M + G) antibodies and then use flow cytometry to measure the relative level of intracellular calcium upon BCR stimulation over time according to the Fluo-4 manufacturer’s instructions.
Plasma cell differentiation in vitro and B cell proliferation assay
Purified B cells (7 × 105) were seeded in 96-well plates and stimulated with 5 µg/ml LPS for 96 h. Then, the cells labeled with FITC-anti-B220, 7-AAD (6084701, BD Pharmingen), BV510-anti-CD138 were detected by flow cytometry. The plasma cells can be examined by analyzing the percentage of B220lowCD138+ cells. B cell proliferation was detected by Cell trace violet (CTV) (C34557, Thermo Fisher) stain kit. B cells were labeled with CTV and stimulated with LPS or F(ab′)2 Anti-Mouse Ig(M + G) plus anti-CD40 (BE0016-2-1MG) for 36 h and 72 h, respectively. Then, the cells labeled with FITC-anti-B220, 7-AAD were detected by flow cytometry.
Co-immunoprecipitation
Purified B cells were lysed by RIPA buffer (P0013B, Beyotime) containing protease inhibitor cocktail (G2006, Servicebio). The cell lysates (644 µg of total protein) were incubated for 1 h 30 min at 4 °C with 2 µg of anti-CX3CR1 (abs136616, Absin) monoclonal antibody and control IgG (sc-2025, SantaCruz), respectively. Antibody complexes were pulled out of solution by the addition of protein G sepharose (17061801, GE life) at 4 °C, 12 h. After washing five times of 1 × TBST, the IP sepharose were denatured by boiling in 30 µl 1 × SDS loading buffer for 10 min at 95 °C. Denatured agarose were examined by Western blotting with anti-CD79b (ab134147, abcam) monoclonal antibody.
Statistical analysis
Student t test and the Mann–Whitney U test were carried out with GraphPad Prism 6 Software (San Diego, CA) to assess the statistical significance. The difference was considered significant when p < 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data were from three independent experiments.
Results
CX3CR1 is important for the development of bone marrow and peripheral B cells
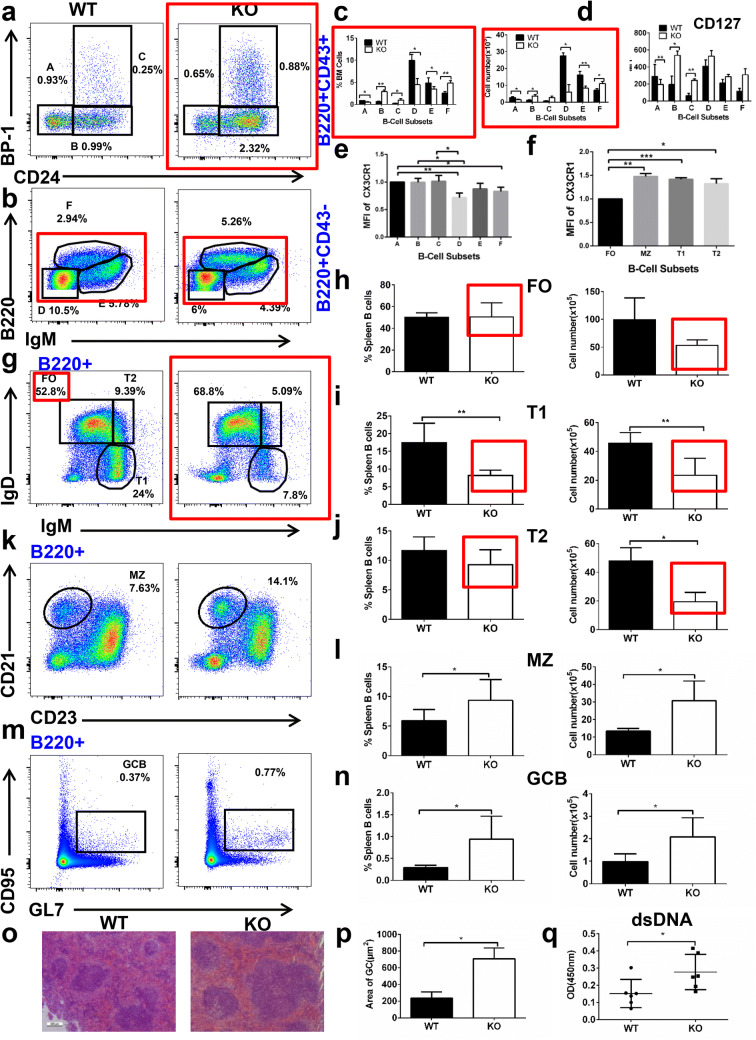
To determine whether CX3CR1 affects the development of bone marrow B cells or not, the subpopulations of bone marrow B cells of CX3CR1 KO mice were stained for BP-1 and CD24 to distinguish pre-pro, pro and early-pre while B220 and IgM were used to distinguish late-pre, immature and recirculating B cells. Although the frequency and number of pre-pro B cells were decreased in CX3CR1 KO mice, that of pro and early-pre B cells was increased except for the number of early-pre B cells (Fig. 1a, c). The percentage and number of late-pre and immature B cells were decreased, but that of recirculating B cells was increased in CX3CR1 KO mice (Fig. 1b, c). These results suggest that the development of bone marrow B cells was partly blocked at early-pre B stage. IL7R (CD127) is essential for the early development of bone marrow B cells, and therefore the CD127 expression in different subpopulations was examined with flow cytometry. In correspondence with the changes of pre-pro, pro and early-pre B cells, CD127 expression was reduced in pre-pro B cells, but enhanced in pro and early-pre B cells (Fig. 1d). We found that the increase in pro B cells and decrease in late-pre B cells is B cell intrinsic using mixed CD45.1 (WT) and CD45.2 (CX3CR1 KO) bone marrow chimera mice (Figure S1A). We examined the expression of CX3CR1 in different subsets of bone marrow B cells and found the expression of CX3CR1 was decreased in late-pre B cells compared to other subsets. The expression of CX3CR1 in recirculating B cells was reduced when compared to pre-pro B cells (Fig. 1e). Next we examined the expression of CX3CR1 in the different subsets of peripheral B cells and found that the expression of CX3CR1 was significantly enhanced in MZ, T1 and T2 compared to FO B cells (Fig. 1f). We further investigated how loss of CX3CR1 affects the peripheral B cells by staining for IgM and IgD to define the T1, T2 and follicular (FO) B cells, CD23 and CD21 to characterize the marginal zone (MZ) B cells, and GL7 and CD95 to define the germinal center (GC) B cells. The frequency and number of FO B cells was similar in WT and CX3CR1 KO mice (Fig. 1g, h), but the T1 B cells were decreased in CX3CR1 KO mice (Fig. 1g, i). Although the frequency of T2 B cells was normal, the number of T2 B cells was decreased in CX3CR1 KO mice (Fig. 1g, j). Interestingly, the percentage and number of MZ B cells were increased in CX3CR1 KO mice (Fig. 1k, l). The percentage and number of GC B cells were increased in CX3CR1 KO mice in the absence of immunization (Fig. 1m, n). This increase in MZ is cell intrinsic by as shown in mixed CD45.1 (WT) and CD45.2 (CX3CR1 KO) bone marrow chimera mice (Figure S1B). The increase in CD45.2+ GC B cells was small, so there might be an effect of CX3CR1 KO in CD4 T cells that might lead to increased GC formation in whole body KO mice. This was associated with enlarged GC areas in the spleen of CX3CR1 KO mice by hematoxylin and eosin staining (Fig. 1o, p). The increase in spontaneous GC B cells may lead to autoimmune disease [37] thus the titer of dsDNA was evaluated by ELISA. We found that serum titers of dsDNA antibodies were increased in CX3CR1 KO mice (Fig. 1q). All these results suggest that CX3CR1 is important for B cell development and differentiation in bone marrow and spleen and for controlling the autoimmunity.
Fig. 1.
CX3CR1 is essential for the development of bone marrow and peripheral B cells. Flow cytometry analysis of BM cells (a, b) obtained from WT and CX3CR1 KO mice (n = 7) stained with Abs specific for surface markers of the subsets pre-pro (a), pro (b), early-pre (c), late-pre (d), immature (e) and recirculating mature (f) B cells. Shown are the average percentages (± SEM) and total cell number of the subpopulations of BM cells (c) and the mean fluorescence intensity (MFI) of CD127 in B cell subsets (d). The expression of CX3CR1 in the different subsets of BM (e) and peripheral B cells (f). Splenic B cells were harvested and surface-stained for transitional 1 (T1), transitional 2 (T2), follicular (FO), marginal zone (MZ) and germinal center (GC) B cells. Samples were analyzed using Flow Jo (Tree Star) software and the summary statistics of percentage (± SEM) and absolute numbers of splenic B cells subsets were shown (g–n). Hematoxylin and eosin staining of spleen from WT and CX3CR1 KO were analyzed (o). The GC areas in the spleen of CX3CR1 KO mice were analyzed by HE staining (p). The levels of dsDNA in the serum of WT and CX3CR1 KO mice were analyzed by ELISA (q) (n = 6). *p < 0.05, **p < 0.01
CX3CR1 is involved in BCR activation and positively regulates the proximal BCR signaling
Differentiation of peripheral B cells is dependent on antigenic BCR signaling strength [19]. We examined whether CX3CR1 is involved in BCR activation by confocal microscopy. WT splenic B cells were stimulated with soluble antigen for varying lengths of time and then stained with an antibody specific for CX3CR1. The colocalization between BCR and CX3CR1 was examined by the Pearson’s correlation coefficient and it was found that the colocalization increased gradually up to 30 min (Fig. 2a, b). We found that BCR (CD79b) associated with CX3CR1 upon antigenic stimulation by co-immunoprecipitation (Fig. 2c). We next examined the effect of CX3CR1 deficiency on the spatiotemporal organization of pCD19, important to stabilize the BCR signaling complex. The colocalization between pCD19 and the BCR was significantly reduced in CX3CR1 KO B cells upon 10 min of stimulation (Fig. 2d, e). The pCD19 and total CD19 levels were also examined with immunoblotting or flow cytometry, whereby splenic B cells stimulated with soluble anti-Ig for varying lengths of time were lysed and probed with antibodies specific for pCD19 and CD19. The level of pCD19 was significantly reduced in CX3CR1 KO B cells, suggesting that CX3CR1 not only affects the spatiotemporal organization of pCD19 but also its expression (Fig. 2f–h). To examine the total levels of BCR signaling, we examined the overall tyrosine phosphorylation by staining for phosphotyrosine proteins (pY) and phosphorylated Bruton tyrosine kinase (pBTK), BTK being the immediate downstream signaling molecule of CD19. Using confocal microscopy, the colocalization between pY/pBTK and BCR was significantly decreased in CX3CR1 KO B cells at 5, 10 and 30 min (Fig. 2i, j). By Western blotting, we found that the levels of pY and pBTK were significantly reduced as well in CX3CR1 KO B cells (Fig. 2k–m). Altogether, these results indicate that CX3CR1 regulates the activation of BTK mediated by CD19.
Fig. 2.
CX3CR1 is involved in BCR activation and positively regulates the proximal BCR signaling. Purified B cells were incubated with biotinylated AF594-mB-F(ab′)2-anti-Ig(M + G) for 30 min at 4 °C to label the BCR before cells were either incubated with streptavidin or with medium alone (0 min) as a control at 37 °C for indicated times. After fixation and permeabilization, B cells were stained for CX3CR1 and analyzed using confocal microscopy (a). The Pearson’s correlation coefficients between the labeled BCR and CX3CR1 were quantified using the ZEN 2.3 (blue edition) software (b). Purified B cells were stimulated with biotinylated F(ab′)2 Anti-Mouse Ig(M + G) (10 µg/ml) plus streptavidin (20 µg/ml) for 0 and 5 min at 37 °C. Stimulated B cells were lysed by RIPA buffer containing protease inhibitor cocktail. The cell lysates (644 µg of total protein) were incubated for 1 h 30 min at 4 °C with 2 µg of anti-CX3CR1 monoclonal antibody or isotype-matched control IgG, respectively. Antibody complexes were pulled out of solution by addition of protein G sepharose at 4 °C, 12 h. After washing five times with 1 × TBST, the IP sepharose were denatured by boiling in 30 µl 1 × SDS loading buffer for 10 min at 95 °C. Denatured agarose were examined by Western blotting with anti-CD79b monoclonal antibody (c). Splenic B cells from WT and CX3CR1 KO mice were stained for pCD19 (d), pY and pBTK (i). Correlation coefficients between the labeled BCR and pCD19 (e), pY and pBTK (j) were quantified using the ZEN 2.3 (blue edition) software. Western blot analysis of the levels of pCD19 (g), pBTK, BTK and pY (k) in splenic B cells with β-actin or total proteins as the loading control. Shown are the representative blots and the ratio of phosphorylated/total proteins from three independent experiments (h, m, l). Shown are representative images from one of three independent experiments. *p < 0.05, **p < 0.01 ****p < 0.0001
We examined the effect of CX3CR1 deficiency on the colocalization between pSHIP and BCR, SHIP being a proximal negative regulator of BCR signaling. Splenic B cells were stimulated with soluble anti-Ig for varying lengths of time and then stained with antibodies specific for pSHIP by confocal microscopy and Western blotting. The correlation coefficient between BCR and pSHIP was significantly reduced in CX3CR1 KO B cells at 5, 10 and 30 min (Fig. 3a, b), and the activation levels of SHIP were only reduced in CX3CR1 KO B cells at 5 min compared to that of WT B cells, suggesting a delayed early activation of SHIP (Fig. 3c, d). Altogether these results suggest that CX3CR1 positively regulates proximal BCR signaling.
Fig. 3.
CX3CR1 deficiency promotes the PI3K-Akt-mTORC1 mediated metabolic signaling pathway. Splenic B cells from WT and CX3CR1 KO mice were labeled with biotinylated AF594-mB-F (ab′)2-anti-Ig(M + G) without (−) or with streptavidin (sAg) at 4 °C, then washed and activated for varying times. After fixation and permeabilization, cells were stained for pSHIP and analyzed using confocal microscopy (a). The Pearson’s correlation coefficients between BCR and pSHIP were determined using the ZEN 2.3 (blue edition) software (b). Western blot analysis the expression of pSHIP and SHIP (c), pPI3K, PI3K, pFoxO1, FoxO1, pAkt, Akt, pS6, S6, pmTOR, mTOR, pNF-κB, NF-κB, pIKKα/β, IKKα/β and HIF-1α in splenic B cells with total proteins as the loading control (e). Calcium assay of CX3CR1 KO splenic B cells was analysed by flow cytometry (g). OCR of CX3CR1 KO mice was measured by Seahorse XF 24 (l). Shown are representative blots and the ratio of phosphorylated/total proteins from three independent experiments (d, f, h–o). *p < 0.05 **p < 0.01 ****p < 0.0001
CX3CR1 deficiency promotes the PI3K-Akt-mTORC1 mediated metabolic signaling pathway
To further investigate the underlying molecular mechanism of how CX3CR1 regulates BCR signaling, we found that the expression of Pik3r6 (regulatory subunit 6 of PI3K) was decreased by mRNA sequencing (data not shown) and this was confirmed by RT-PCR (Figure S2A). However, the levels of PI3K were not altered in CX3CR1 KO B cells. We next examined the PI3K-activated metabolic signaling pathways by mTORC1 and mTORC2. CX3CR1 KO splenic B cells stimulated with soluble anti-Ig for varying lengths of time were lysed and probed with antibodies specific for pPI3K and surprisingly the activation levels of pPI3K were enhanced (Fig. 3e, f). To examine the discrepancy of CX3CR1 KO B cells having reduced proximal BCR signaling while distal signaling by PI3K and mTOR was increased, we compared the ability of CX3CR1 KO B cells to transmit the BCR-driven signal using calcium mobilization and found that the calcium flux was reduced in CX3CR1 KO B cells upon antigenic stimulation (Fig. 3g). Next, we examined the activation of mTORC2 signaling molecules, pAkt and pFoxO1. The levels of pAkt and pFoxO1 were increased in CX3CR1 KO B cells upon stimulation (Fig. 3e, h–i). We next examined the activation of the Akt-mTORC1 downstream molecule S6 by determining the pS6 levels that were elevated as well (Fig. 3e, j). Finally, we examined the activation levels of the common subunit of mTORC1 and found that mTORC2-pmTOR was enhanced in CX3CR1 KO B cells upon stimulation (Fig. 3e, k). The change of mTORC activity is directly associated to cell metabolism. Seahorse XF technology was used to analyze oxidative phosphorylation in real time. The oxygen-consumption rate (OCR) was used as a measurement of oxidative phosphorylation. Cells were first treated with oligomycin to block mitochondrial ATP production and then with carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) to induce maximal amounts of oxygen consumption and rotenone plus antimycin to inhibit the electron transport chain. After stimulation of the cells with anti-Ig(M + G), both basal and maximal rates of respiration were found to be higher in CX3CR1 KO B cells when compared to WT B cells, which is consistent with the increased mTORC activity in CX3CR1 KO B cells (Fig. 3l). To further examine the biological consequence of the increased metabolic activity, the proliferation of CX3CR1 KO B cells was examined upon anti-Ig or LPS stimulation. We found that KO B cells proliferated more extensively in response to anti-Ig or LPS than the control B cells (Figure S3A), but had the same cell size (Figure S3B, C). Additionally the expression of pPI3K was higher at 30 min in CX3CR1 KO B cells upon LPS stimulation (Figure S3D). These results imply that CX3CR1 inhibits the B cell metabolism via downregulating the mTORC1 and mTORC2 signaling pathways.
Loss of CX3CR1 enhances the accumulation of F-actin mediated by WASP and WIP
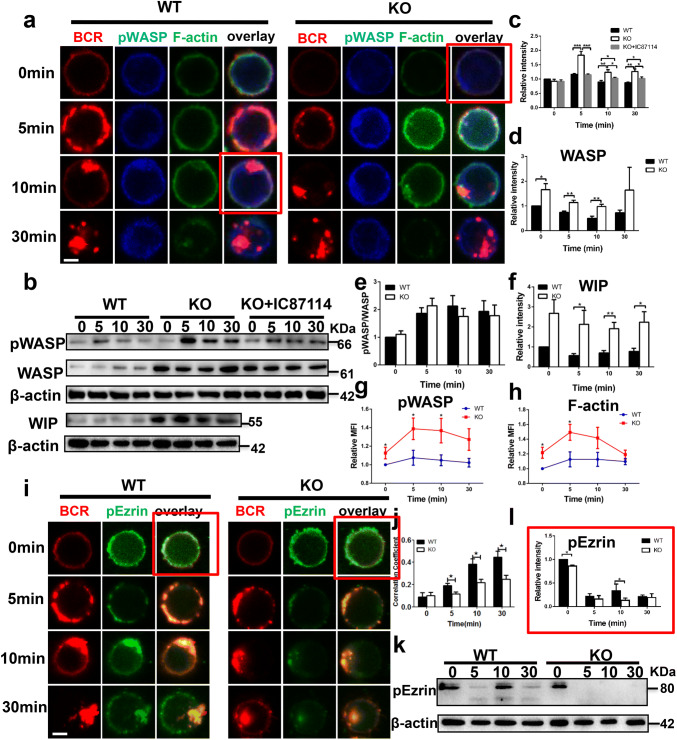
Previous research has shown that Fractalkine, the ligand of CX3CR1 induces actin polymerization and chemotaxis in a dose-dependent manner in DCs [35]. To investigate the correlation between CX3CR1 and actin in B cells, WT and CX3CR1 KO B cells were stimulated with soluble anti-Ig for varying lengths of time. Cells were fixed, permeabilized and stained for pWASP and F-actin, and then analyzed by confocal microscopy, immunoblot and phosflow. We found that the total and phosphorylated WASP were enhanced by both immunoblot and phosflow (Fig. 4a–e, g), which correlated with increased F-actin accumulation (Fig. 4h). However, the ratio of pWASP to WASP was similar when comparing WT and CX3CR1 KO B cells (Fig. 4e). To understand how CX3CR1 upregulated the expression of WASP, we examined expression of WAS/WASL-interacting protein family member 3 (Wipf3) and found less Wipf3 mRNA by RT-PCR (Figure S2B). Expression of WIP stabilizes pWASP and protects it from degradation. We examined WIP expression by immunoblot and found that the expression of WIP was elevated in CX3CR1 KO B cells (Fig. 4b, f). Previous research has shown that the activation of PI3K promotes the activation of WASP [28]. We used a PI3K inhibitor, IC87114, to treat CX3CR1 KO B cells and then examine the expression of pWASP. We found that the activation of pWASP in CX3CR1 KO B cells treated with IC87114 restored to the degree of that of WT B cells (Fig. 4b, c). These results imply that the increased expression of WASP and pWASP could be due to the enhanced expression of WIP in CX3CR1 KO B cells. We also examined the expression of another important actin regulator, Ezrin, the phosphorylation of which maintains the connection between the submembrane actin cytoskeleton and membrane proteins in CX3CR1 KO B cells by confocal microscopy and immunoblot and we found that the colocalization between BCR and pEzrin was significantly reduced at 5, 10 and 30 min, and the rephosphorylation of Ezrin was not observed at 10 min upon stimulation with soluble anti-Ig in CX3CR1 KO B cells (Fig. 4i–l). These results suggested that the increased activation of WASP was in accordance with decreased activation of Ezrin to positively regulate the actin accumulation in CX3CR1 KO B cells upon stimulation.
Fig. 4.
Loss of CX3CR1 enhances the accumulation of F-actin mediated by WASP and WIP. Confocal analysis of pWASP and F-actin staining (a) and pEzrin (i) from WT and CX3CR1 KO splenic B cells upon antigenic stimulation. The Pearson’s correlation coefficients between BCR and pEzrin (j) were determined using the ZEN 2.3 (blue edition) software. Western blot analysis of the expression levels of pWASP, WASP and WIP (b) and pEzrin (k) in splenic B cells upon antigenic stimulation. Shown are representative blots, blots’ relative band intensities and the ratio of pWASP, WASP, pWASP to WASP, WIP and pEzrin from three independent experiments (c–f, l). Splenic B cells were incubated with Percp-anti-B220 for 30 min at 4 °C. Then the cells were either incubated with streptavidin or with medium alone (0 min) as a control at 37 °C for indicated time. After fixation and permeabilization the cells were stained for pWASP and F-actin for phosflow cytometry. Relative MFI of pWASP and F-actin in splenic B cells was quantified using Flow Jo software (g, h). Shown are representative images and mean values (± SEM) from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
The enhanced actin in CX3CR1 KO B cells leads to reduced BCR clustering and positive signalosome recruitment
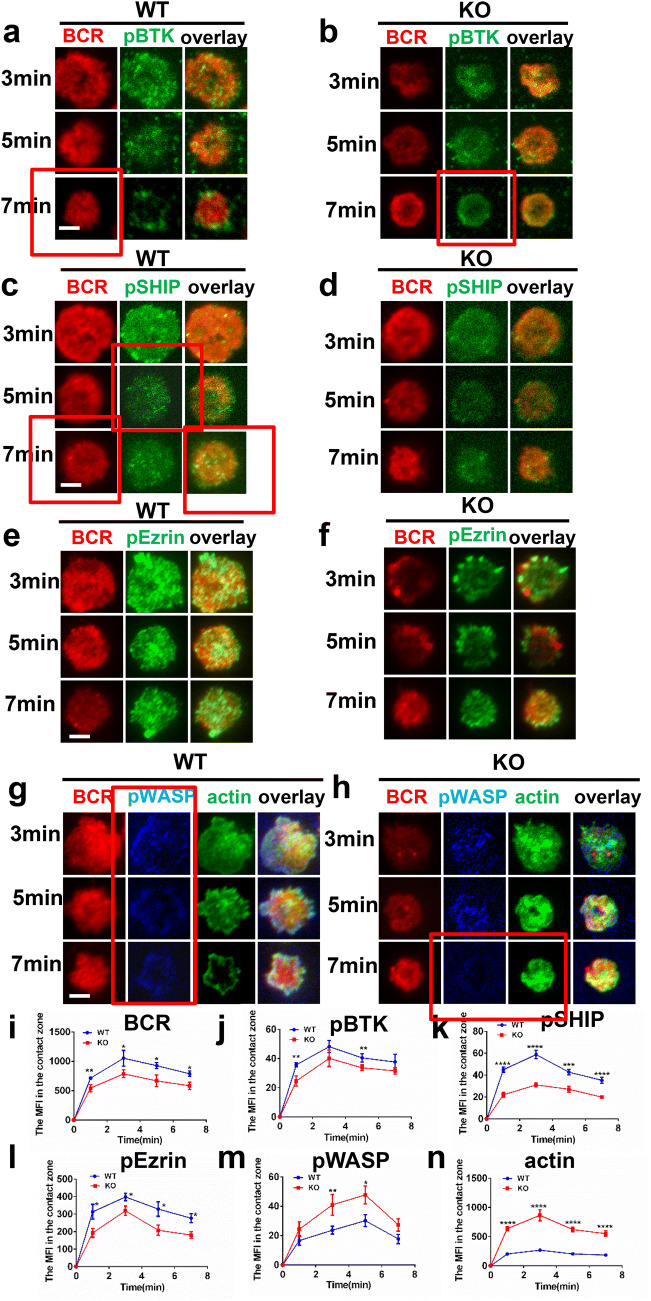
Our previous research has shown that the enhanced intensity of F-actin by the plasma membrane reduces the velocity of BCRs and BCR clustering as well as BCR signaling [29]. To determine the effect of the deficiency of CX3CR1 on the early activation of B cells including BCR clustering and signalsome accumulation, we used total internal reflection fluorescence microscopy (TIRF) to observe B cells stimulated with membrane tethered antigens (mAg). Splenic B cells activated with mAg for varying lengths of time were stained for pBTK, pSHIP, pWASP, pEzrin and F-actin. We found that BCR clustering measured by mean fluorescence intensity (MFI) in the contact zone was significantly reduced in CX3CR1 KO B cells (Fig. 5i). Meanwhile, the recruitment of pBTK and pSHIP signalosomes in the B cell contact zone was significantly decreased in CX3CR1 KO B cells (Fig. 5a–d, j, k). Consistent with the established model [38], the recruitment of pWASP and F-actin was also significantly increased while pEzrin was significantly decreased in the contact zone of CX3CR1 KO B cells (Fig. 5e–h, l–n). Altogether, these results indicate that the deficiency of CX3CR1 increases the recruitment of F-actin mediated by WASP at the plasma membrane, which leads to the reduced BCR clustering and BCR signaling.
Fig. 5.
The enhanced actin in CX3CR1 KO B cells leads to reduced BCR clustering and positive signalosome recruitment. Purified splenic B cells from WT and CX3CR1 KO mice were stained with AF546-mB-Fab-anti-Ig tethered to lipid bilayers and activated for indicated time and then fixed, permeabilized and stained for pBTK (a, b), pSHIP (c, d), pEzrin (e, f), pWASP and F-actin (g, h). Cells were analyzed using TIRFm. Shown are representative images the MFI of BCR (i), pBTK (j), pSHIP (k), pEzrin (l), pWASP (m) and F-actin (n) in the B cell contact zone from three independent experiments. The TIRFm analysis was measured by NIS-Elements AR 3.2 software. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
CX3CR1 deficiency leads to increased PC and antibody production in the T-dependent immune response
To examine the effect of CX3CR1 deficiency on the humoral immune response, mice were immunized with NP-KLH twice to examine the T-cell-dependent immune response. At day 14 after primary immunization, the mice were euthanized and splenocytes and serum were collected for flow cytometry and serological analysis. After immunization, the frequency and number of FO B cells did not change in CX3CR1 KO mice either before or after immunization compared to that of WT mice (Figure S4A, B). Interestingly, the percentage and number of T1 B cells were both decreased in CX3CR1 KO mice, as compared to WT mice before or after immunization. Additionally, the number of T1 B cells was decreased in CX3CR1 KO mice before immunization and did not change after immunization, but that of T1 B cells in CX3CR1 KO mice was increased after immunization compared to non-immunized mice (Figure S4A, C). Although the frequency of T2 B cells did not change between WT and CX3CR1 KO mice before or after immunization, the number of T2 B cells was decreased in CX3CR1 KO mice before immunization and did not change after immunization (Figure S4A, D). Although the frequency and number of MZ B cells was increased in CX3CR1 KO mice before immunization, MZ B cells were similar in CX3CR1 KO and WT mice after immunization (Figure S4E, F). The frequency and number of GCB cells was increased in CX3CR1 KO mice when compared to WT mice before immunization, but not after immunization. The frequency of GCB cells was increased after immunization in both WT and CX3CR1 KO mice (Figure S4G, H). Lastly, the frequency and number of MBC and PC was both increased in CX3CR1 KO mice after immunization (Figure S4I–L), suggesting an overall increased primary response to NP-KLH. Because the CX3CR1 KO B cells have a tendency of increased plasma cell (PC) formation, we performed a plasma cell differentiation assay in vitro to test that by stimulating naïve B cells with LPS, and we found increased percentage of PC and decreased percentage of PBC for CX3CR1 naïve KO B cells (Figure S3E–G). When compared to WT mice, the titers of NP-specific IgG1 and IgM were increased in CX3CR1 KO mice after immunization probably due to the increase of PC (Figure S4M, N and S5N, O). Additionally, the size of GC in CX3CR1 KO mice was larger and darker when compared to WT mice after immunization by HE and immunofluorescence staining (Figure S4O–Q). After second immunization, the different B cell subsets were similar in WT and CX3CR1 KO mice (Figure S5A–H). The percentage and number of PBC was increased and the number of MBC was decreased in CX3CR1 KO mice (Figure S5I–M), which was different from the results of first immunization. Lastly, the percentage of NP+ GCB cells was higher in CX3CR1 KO mice when compared to WT mice after second immunization (Figure S3H). All these results suggest that CX3CR1 inhibits the humoral immune response elicited by T cell dependent antigens.
CX3CR1 is critical for the humoral immune response elicited by Schistosoma japonicum infection
To further explore the role of CX3CR1 in the humoral immune response in a real physiological infection, mice were infected with Schistosoma japonicum and euthanized five weeks later. Splenocytes were collected and B cell subsets were examined. In contrast to the NP-KLH immunization, the percentage and number of FO B cells in CX3CR1 KO mice were decreased compared to that of WT mice after infection. The frequency or number of FO B cells was increased in both WT and CX3CR1 KO mice after infection, as compared to mock infection (Fig. 6a, b). Although the frequency of T1 B cells was increased after infection, the number of T1 B cells was decreased in CX3CR1 KO mice when compared to WT mice. The percentage of T1 cells was decreased in WT mice, but increased in CX3CR1 KO mice after infection compared to mock infection. However, the number of T1 cells was increased in CX3CR1 KO mice compared to mock infection (Fig. 6a, c). The frequency and number of T2 cells were reduced in CX3CR1 KO mice when compared to WT mice after infection. The percentage or number of T2 cells was increased in WT mice after infection compared to mock infection (Fig. 6a, d). Although the percentage of MZ B cells was increased in CX3CR1 KO mice, the number of MZ B cells was decreased when compared to WT mice after infection. The number of MZ B cells was increased in WT mice after infection compared to mock infection (Fig. 6e, f). The frequency and number of GC B cells were increased after infection in WT or CX3CR1 KO mice compared to mock infection, but the frequency and number of GC B cells were decreased in CX3CR1 KO mice when compared to of WT mice (Fig. 6g, h). Although the frequency and number of PBC were decreased, PC was increased in CX3CR1 KO mice when compared to WT mice after infection (Fig. 6i–k). The frequency of unswitched and switched B cells was increased and decreased, respectively, in CX3CR1 KO mice when compared to WT mice after infection (Fig. 6l–n). However, we did observe reduced size of GCs in CX3CR1 KO mice when compared to WT mice after infection, which is different from the result of NP-KLH immunization (Fig. 6o, p).
Fig. 6.
CX3CR1 is critical for the humoral immune response elicited by Schistosoma japonicum Infection. 6–8 weeks old C57BL/6 WT and CX3CR1 KO mice were percutaneously infected with 25 cercariae of Schistosoma japonicum and splenic B cells were collected after 5 weeks. Isolated splenic B cells were stained for T1, T2 and FO (a), MZ (e), GC (g), PC and PBC (i), B cells switched and unswitched (l) and assessed using flow cytometry. Shown are representative flow cytometry plots from three independent experiments and the summary statistics of percentage (± SEM) and absolute numbers of extracted splenic B cells subsets were shown (b–d, f, h, j, k, m and n). Hematoxylin and eosin staining of spleen from WT and CX3CR1 KO mice (o). The GC areas in the spleen of CX3CR1 KO mice by HE staining (p) *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Discussion
This research unveils the underlying molecular mechanism of how CX3CR1 regulates BCR signaling and peripheral B cell differentiation. The loss of CX3CR1 led to enhanced activation of WASP as well as increased actin accumulation at the plasma membrane, although there is reduced BCR clustering and reduced activation of proximal BCR signaling molecules CD19, BTK and SHIP. Surprisingly, the activation of metabolic relevant BCR signaling molecules, mTORC1 and mTORC2 as well as OCR, was enhanced in CX3CR1 KO B cells upon antigenic stimulation. This alteration in BCR signaling was associated with increased differentiation of MZ B cells and an autoimmune phenotype in CX3CR1 KO mice. However, the reduced BCR signaling caused increased generation of PC and antibody titers after immunization and Schistosoma japonicum infection. To resolve this contradiction, we need to further examine whether the affinity of the antibodies generated are of high affinity by sequencing the somatic hypermutations. Additionally, the possibility that loss of CX3CR1 in other immune cells could influence B cell development and activation, and that a B cell-specific KO is required to definitively show that the defects are cell-intrinsic.
One remaining issue is the detailed underlying mechanism of how CX3CR1 signaling regulates cell metabolism. In our study, the deficiency of CX3CR1 enhanced the activation of the mTORC1 and mTORC2 signaling molecules. However, CX3CR1, as a surface molecule, may regulate the expression of these metabolic genes through transcriptional factors. HIF-1 and NF-κB have been reported to be critical for hypoxia-induced CX3CR1 expression, which is correlated with increased migratory and invasive potential of prostate cancer cells [42]. NF-κB also regulates the basal level of HIF-1, which is essential to regulate the human metabolism [29, 39]. We speculated that the absence of CX3CR1 may also affect the activity of HIF-1 and NF-κB. HIF-1 is a transcription factor that can respond to hypoxia [39, 40]. Not surprisingly we found the increased expression of HIF-1α in CX3CR1 KO B cells (Fig. 3e, m). However, the activation of NF-κB signaling is reduced upon BCR stimulation (Fig. 3e, n), which is line with the reduction of calcium signaling that is the stimulator of NF-κB signaling. mTORC1 inhibition also prevents the TGF-β-stimulated increase in both hypoxia-responsive element (HRE) activity and HIF-1α protein expression [41]. Overall, the regulation axis CX3CR1-HIF-1/NF-κB-mTORC1 may exist to control the B cell metabolism.
Another remaining issue is how CX3CR1 regulates the PI3K signaling pathway. Previous researches have suggested that the fractalkine/CX3CR1 axis promotes tumor progression by activating PI3K/Akt signaling [42, 43]. However, in our case, the absence of CX3CR1 promotes the activation of PI3K/Akt signaling, but this difference could be due to the specific cell type examined. The PI3K/Akt/NF-κB signaling cascade is responsible for fractalkine-induced ICAM-1 expression and cell migration [10]. It is possible that the CX3CR1-mediated activation of NF-κB merges with the regulation axis CX3CR1-HIF-1/ NF-κB-mTORC1 on cell metabolism. It is possible that NF-κB also regulates the gene expression of PI3K via a feedback loop of CX3CR1. A chromatin immunoprecipitation would reveal a possible direct interaction between NF-κB and PI3K if NF-κB expression is enhanced in CX3CR1 KO B cells. Interestingly, the regulation of the PI3K/Akt axis by CX3CR1 has been reported as well [44]. Therefore, the regulation of CX3CR1 on PI3K signaling is reciprocal.
Finally, it is still puzzling how CX3CR1 regulates the actin reorganization via WASP. Although fractalkine/CX3CR1 axis has been shown to induce actin polymerization in human dendritic cells and stimulate cytoskeleton changes and F-actin reorganization in NK cells, the detailed process is still unknown [35, 45]. Previous research has shown that the activation of PI3K promotes the activation of WASP. In our results, the deficiency of CX3CR1 promoted the activation of PI3K, so it is reasonable that the activation of WASP is enhanced. Interestingly we found the treatment of CX3CR1 KO B cells with PI3K inhibitor reduced the overactived pWASP to the degree of WT B cells. It is interesting that the activation of Ezrin is reduced in CX3CR1 KO B cells because our previous studies have shown that the activation of Ezrin promotes the actin polymerization [29]. These results imply that WASP and Ezrin counteract each other during the stimulation of actin polymerization. It might be insightful to use a WASP inhibitor such as wiskostatin on the CX3CR1 KO B cells and then to examine the activation of Ezrin. If the activation of Ezrin is increased, this would indicate that the wiskotatin treatment skews the activation from WASP to Ezrin.
In summary, our data have shown that CX3CR1 can regulate metabolic BCR signaling via inhibiting mTORC1 and mTORC2 activity. Moreover, CX3CR1 can affect the actin reorganization through affecting the levels of WIP that is critical for the stability of WASP, which offers a feedback to proximal BCR signaling. This is associated with a role of CX3CR1 for the differentiation of peripheral B cells and control of B cell autoimmunity as well as the humoral immune response. Our research may offer therapeutic design for the treatment of autoimmune disease caused by the CX3CR1 deficiency.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the Natural Science Foundation of China (81861138002, 81722002 and 31970839) and the start-up funding from Huazhong University of Science and Technology.
Author contributions
NL and PJ drafted the initial manuscript. CL designed the research, reviewed and revised the manuscript. AC performed the TRIFm experiments, XL and NL performed Seahorse experiments. NL and YJ performed the confocal experiments. NL, YJ, LY, PJ, DK, JL, JC, QC carried out the flow cytometry assay, ELISA, and Western blotting. NL analyzed the data and generated figures. JJ, HM, LW, CW, QG assisted with the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Na Li and Panpan Jiang contributed equally to this work.
Change history
5/13/2020
In the original published version of the article, the red squares in the figures which indicated the corrections.
Contributor Information
Quan Gong, Email: gongquan1998@163.com.
Chaohong Liu, Email: chaohongliu80@126.com.
References
- 1.Combadiere C, Ahuja SK, Murphy PM. Cloning, chromosomal localization, and RNA expression of a human beta chemokine receptor-like gene. DNA Cell Biol. 1995;14:673–680. doi: 10.1089/dna.1995.14.673. [DOI] [PubMed] [Google Scholar]
- 2.Combadiere C, Salzwedel K, Smith ED, Tiffany HL, Berger EA, Murphy PM. Identification of CX3CR1. A chemotactic receptor for the human CX3C chemokine fractalkine and a fusion coreceptor for HIV-1. J Biol Chem. 1998;273:23799–23804. doi: 10.1074/jbc.273.37.23799. [DOI] [PubMed] [Google Scholar]
- 3.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 4.Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z. CX3CR1(+) B cells show immune suppressor properties. J Biol Chem. 2014;289:22630–22635. doi: 10.1074/jbc.M114.569459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhivaki D, Lemoine S, Lim A, Morva A, Vidalain PO, Schandene L, Casartelli N, Rameix-Welti MA, Herve PL, Deriaud E, Beitz B, Ripaux-Lefevre M, Miatello J, Lemercier B, Lorin V, Descamps D, Fix J, Eleouet JF, Riffault S, Schwartz O, Porcheray F, Mascart F, Mouquet H, Zhang X, Tissieres P, Lo-Man R. Respiratory syncytial virus infects regulatory B cells in human neonates via chemokine receptor CX3CR1 and promotes lung disease severity. Immunity. 2017;46:301–314. doi: 10.1016/j.immuni.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferretti E, Bertolotto M, Deaglio S, Tripodo C, Ribatti D, Audrito V, Blengio F, Matis S, Zupo S, Rossi D, Ottonello L, Gaidano G, Malavasi F, Pistoia V, Corcione A. A novel role of the CX3CR1/CX3CL1 system in the cross-talk between chronic lymphocytic leukemia cells and tumor microenvironment. Leukemia. 2011;25:1268–1277. doi: 10.1038/leu.2011.88. [DOI] [PubMed] [Google Scholar]
- 8.Mionnet C, Buatois V, Kanda A, Milcent V, Fleury S, Lair D, Langelot M, Lacoeuille Y, Hessel E, Coffman R, Magnan A, Dombrowicz D, Glaichenhaus N, Julia V. CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nat Med. 2010;16:1305–1312. doi: 10.1038/nm.2253. [DOI] [PubMed] [Google Scholar]
- 9.Ren H, Zhao T, Sun J, Wang X, Liu J, Gao S, Yu M, Hao J. The CX3CL1/CX3CR1 reprograms glucose metabolism through HIF-1 pathway in pancreatic adenocarcinoma. J Cell Biochem. 2013;114:2603–2611. doi: 10.1002/jcb.24608. [DOI] [PubMed] [Google Scholar]
- 10.Liu JF, Tsao YT, Hou CH. Fractalkine/CX3CL1 induced intercellular adhesion molecule-1-dependent tumor metastasis through the CX3CR1/PI3K/Akt/NF-kappaB pathway in human osteosarcoma. Oncotarget. 2017;8:54136–54148. doi: 10.18632/oncotarget.11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SJ, Namkoong S, Kim YM, Kim CK, Lee H, Ha KS, Chung HT, Kwon YG, Kim YM. Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways. Am J Physiol Heart Circ Physiol. 2006;291:H2836–2846. doi: 10.1152/ajpheart.00113.2006. [DOI] [PubMed] [Google Scholar]
- 12.Fumarola C, La Monica S, Alfieri RR, Borra E, Guidotti GG. Cell size reduction induced by inhibition of the mTOR/S6K-signaling pathway protects Jurkat cells from apoptosis. Cell Death Differ. 2005;12:1344–1357. doi: 10.1038/sj.cdd.4401660. [DOI] [PubMed] [Google Scholar]
- 13.Lang CH, Frost RA. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E-BP1, and S6K1 in skeletal muscle. J Cell Physiol. 2005;203:144–155. doi: 10.1002/jcp.20207. [DOI] [PubMed] [Google Scholar]
- 14.Fiano V, Ghimenti C, Imarisio S, Silengo L, Schiffer D. PAkt, cyclin D1 and p27/Kip.1 in glioblastomas with and without EGFR amplification and PTEN mutation. Anticancer Res. 2004;24:2643–2647. [PubMed] [Google Scholar]
- 15.Otero DC, Anzelon AN, Rickert RC. CD19 function in early and late B cell development: I. Maintenance of follicular and marginal zone B cells requires CD19-dependent survival signals. J Immunol (Baltimore, Md. : 1950) 2003;170:73–83. doi: 10.4049/jimmunol.170.1.73. [DOI] [PubMed] [Google Scholar]
- 16.Thomas MD, Srivastava B, Allman D. Regulation of peripheral B cell maturation. Cell Immunol. 2006;239:92–102. doi: 10.1016/j.cellimm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 18.De S, Barnes BJ. B cell transcription factors: potential new therapeutic targets for SLE. Clin Immunol (Orlando, Fla.) 2014;152:140–151. doi: 10.1016/j.clim.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14:603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Meckel T, Tolar P, Sohn HW, Pierce SK. Intrinsic properties of immunoglobulin IgG1 isotype-switched B cell receptors promote microclustering and the initiation of signaling. Immunity. 2010;32:778–789. doi: 10.1016/j.immuni.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tolar P, Hanna J, Krueger PD, Pierce SK. The constant region of the membrane immunoglobulin mediates B cell-receptor clustering and signaling in response to membrane antigens. Immunity. 2009;30:44–55. doi: 10.1016/j.immuni.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolar P, Sohn HW, Pierce SK. The initiation of antigen-induced B cell antigen receptor signaling viewed in living cells by fluorescence resonance energy transfer. Nat Immunol. 2005;6:1168–1176. doi: 10.1038/ni1262. [DOI] [PubMed] [Google Scholar]
- 23.Mattila PK, Feest C, Depoil D, Treanor B, Montaner B, Otipoby KL, Carter R, Justement LB, Bruckbauer A, Batista FD. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity. 2013;38:461–474. doi: 10.1016/j.immuni.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Treanor B, Depoil D, Gonzalez-Granja A, Barral P, Weber M, Dushek O, Bruckbauer A, Batista FD. The membrane skeleton controls diffusion dynamics and signaling through the B cell receptor. Immunity. 2010;32:187–199. doi: 10.1016/j.immuni.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Bai X, Wu J, Sharma S, Upadhyaya A, Dahlberg CI, Westerberg LS, Snapper SB, Zhao X, Song W. N-wasp is essential for the negative regulation of B cell receptor signaling. PLoS Biol. 2013;11:e1001704. doi: 10.1371/journal.pbio.1001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao S, August A. Actin depolymerization transduces the strength of B-cell receptor stimulation. Mol Biol Cell. 2005;16:2275–2284. doi: 10.1091/mbc.E04-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seeley-Fallen MK, Liu LJ, Shapiro MR, Onabajo OO, Palaniyandi S, Zhu X, Tan TH, Upadhyaya A, Song W. Actin-binding protein 1 links B-cell antigen receptors to negative signaling pathways. Proc Natl Acad Sci USA. 2014;111:9881–9886. doi: 10.1073/pnas.1321971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma S, Orlowski G, Song W. Btk regulates B cell receptor-mediated antigen processing and presentation by controlling actin cytoskeleton dynamics in B cells. J Immunol (Baltimore Md. : 1950) 2009;182:329–339. doi: 10.4049/jimmunol.182.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang L, Zhang Y, Xu C, Gu X, Niu L, Wang J, Sun X, Bai X, Xuan X, Li Q, Shi C, Yu B, Miller H, Yang G, Westerberg LS, Liu W, Song W, Zhao X, Liu C. Rictor positively regulates B cell receptor signaling by modulating actin reorganization via ezrin. PLoS Biol. 2017;15:e2001750. doi: 10.1371/journal.pbio.2001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pore D, Huang E, Dejanovic D, Parameswaran N, Cheung MB, Gupta N. Cutting edge: deletion of Ezrin in B cells of lyn-deficient mice downregulates lupus pathology. J Immunol. 2018;201:1353–1358. doi: 10.4049/jimmunol.1800168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pore D, Matsui K, Parameswaran N, Gupta N. Cutting edge: Ezrin regulates inflammation by limiting B cell IL-10 production. J Immunol. 2016;196:558–562. doi: 10.4049/jimmunol.1502098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treanor B, Depoil D, Bruckbauer A, Batista FD. Dynamic cortical actin remodeling by ERM proteins controls BCR microcluster organization and integrity. J Exp Med. 2011;208:1055–1068. doi: 10.1084/jem.20101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parameswaran N, Enyindah-Asonye G, Bagheri N, Shah NB, Gupta N. Spatial coupling of JNK activation to the B cell antigen receptor by tyrosine-phosphorylated ezrin. J Immunol. 2013;190:2017–2026. doi: 10.4049/jimmunol.1201292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pore D, Gupta N. The ezrin-radixin-moesin family of proteins in the regulation of B-cell immune response. Crit Rev Immunol. 2015;35:15–31. doi: 10.1615/critrevimmunol.2015012327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dichmann S, Herouy Y, Purlis D, Rheinen H, Gebicke-Harter P, Norgauer J. Fractalkine induces chemotaxis and actin polymerization in human dendritic cells. Inflamm Res. 2001;50:529–533. doi: 10.1007/PL00000230. [DOI] [PubMed] [Google Scholar]
- 36.Ha N, Pham DH, Shahsafaei A, Naruse C, Asano M, Thai TH. HP-1gamma controls high-affinity antibody response to T-dependent antigens. Front Immunol. 2014;5:271. doi: 10.3389/fimmu.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domeier PP, Schell SL, Rahman ZS. Spontaneous germinal centers and autoimmunity. Autoimmunity. 2017;50:4–18. doi: 10.1080/08916934.2017.1280671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C, Miller H, Hui KL, Grooman B, Bolland S, Upadhyaya A, Song W. A balance of Bruton’s tyrosine kinase and SHIP activation regulates B cell receptor cluster formation by controlling actin remodelling. J Immunol (Baltimore Md.: 1950) 2011;187:230–239. doi: 10.4049/jimmunol.1100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith TG, Robbins PA, Ratcliffe PJ. The human side of hypoxia-inducible factor. Br J Haematol. 2008;141:325–334. doi: 10.1111/j.1365-2141.2008.07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkins SE, Abboud MI, Hancock RL, Schofield CJ. Targeting Protein-Protein Interactions in the HIF System. ChemMedChem. 2016;11:773–786. doi: 10.1002/cmdc.201600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rozen-Zvi B, Hayashida T, Hubchak SC, Hanna C, Platanias LC, Schnaper HW. TGF-beta/Smad3 activates mammalian target of rapamycin complex-1 to promote collagen production by increasing HIF-1alpha expression. Am J Physiol Renal Physiol. 2013;305:F485–494. doi: 10.1152/ajprenal.00215.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shulby SA, Dolloff NG, Stearns ME, Meucci O, Fatatis A. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Can Res. 2004;64:4693–4698. doi: 10.1158/0008-5472.CAN-03-3437. [DOI] [PubMed] [Google Scholar]
- 43.Gaudin F, Nasreddine S, Donnadieu AC, Emilie D, Combadiere C, Prevot S, Machelon V, Balabanian K. Identification of the chemokine CX3CL1 as a new regulator of malignant cell proliferation in epithelial ovarian cancer. PLoS ONE. 2011;6:e21546. doi: 10.1371/journal.pone.0021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saitoh Y, Koizumi K, Sakurai H, Minami T, Saiki I. RANKL-induced down-regulation of CX3CR1 via PI3K/Akt signaling pathway suppresses Fractalkine/CX3CL1-induced cellular responses in RAW264.7 cells. Biochem Biophys Res Commun. 2007;364:417–422. doi: 10.1016/j.bbrc.2007.09.137. [DOI] [PubMed] [Google Scholar]
- 45.El-Shazly AE, Doloriert HC, Bisig B, Lefebvre PP, Delvenne P, Jacobs N. Novel cooperation between CX3CL1 and CCL26 inducing NK cell chemotaxis via CX3CR1: a possible mechanism for NK cell infiltration of the allergic nasal tissue. Clin Exp Allergy. 2013;43:322–331. doi: 10.1111/cea.12022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.