Abstract
We propose a two-stage clinical trial in patients with Staphylococcus aureus bacteremia (SAB). In Stage 1 we will evaluate metagenomic next generation sequencing from blood as a quantitative biological surrogate for clinical endpoint in patients with SAB, similar to quantitative HIV viral load in HIV-infected patients. In Stage 2, we will conduct a randomized controlled trial to individualize duration of antibiotic therapy for based upon the presence of S. aureus genetic material in patients’ blood. The proposed study addresses two critical aspects of treatment of patients with SAB: the identification of a surrogate biological endpoint for future clinical trials, and a new approach by which to individualize patient management.
Introduction
As part of a series of invited commentaries in Clinical Microbiology and Infection (1), we propose a clinical trial to evaluate whether highly sensitive next generation sequencing platforms that quantify pathogen genetic material in plasma can serve as a surrogate for clinical response to therapy, individualize antibiotic therapy in patients with Staphylococcus aureus bacteremia (SAB), and ultimately improve patient outcomes.
SAB is common and potentially lethal, with 30-day mortality estimates as high as 30% (2). In current practice, the duration of antibiotic therapy is based on distinguishing uncomplicated from complicated SAB (typically treated for either 14 days or 28-42 days, respectively) (3). However, this distinction is often difficult early in the clinical course. Moreover, the conventional approach of 7-day increments to define durations of therapy for SAB is arbitrary and dogmatic. As a result, patients with SAB are likely to be both over- and undertreated.
Registrational trials in SAB have been complicated by the need for a biological endpoint to confirm clinical response. In an attempt to provide such biological confirmation, regulatory authorities have required sponsors of Phase 3 trials in SAB to include post-treatment blood cultures as part of treatment success (4, 5). Despite clinical cure, and without an alternative biomarker to serve as a surrogate for clinical response, patients without post-treatment blood cultures are defined as treatment failures (6, 7). Clinical trials in HIV were transformed by the acceptance of HIV quantitative viral load as a surrogate for clinical response by regulatory authorities. Unfortunately, trials in S. aureus infection do not yet have a comparable biological surrogate for clinical response. The presence of such a biomarker may improve both the execution of SAB clinical trials and their generalizability.
Metagenomic next generation sequencing (mNGS) can be utilized to speciate and quantify pathogen DNA in the blood of patients with a variety of infections, including SAB (8, 9). The high sensitivity and specificity of these platforms for both endovascular and extravascular infections offer the potential to individualize therapy. For example, Eichenberger et al. reported mNGS sensitivity of 86% for detection of SAB. Microbial cell free DNA (mcfDNA) identified the presence of S. aureus in patients for over 7 times longer than conventional blood cultures (15 vs 2 days, p<0.001) (9). Importantly, the presence of metastatic sites of infection was associated with longer durations of mcfDNA detection (median 22 vs 8 days, p=0.0054), with each additional day nearly tripling the odds of metastatic infection (OR 2.89, 95%CI 1.53-5.46). Furthermore, patients with definitive source control or effective antibiotic therapy often exhibited rapid declines in mcfDNA (9, 10). Collectively, these findings suggest that mNGS could complement current treatment of SAB and ultimately serve as a much-needed biomarker for efficacy assessments in registrational clinical trials of antibacterial agents.
Proposed trial
We propose a clinical trial to evaluate whether the quantification of bacterial DNA in plasma can individualize the duration of antibiotic therapy in patients with SAB. Specifically, we hypothesize that antibiotic treatment can be safely discontinued for patients with SAB when S. aureus DNA is no longer detectable by mNGS. To test this hypothesis, we propose a two-stage clinical trial (Figure 1).
Figure 1.
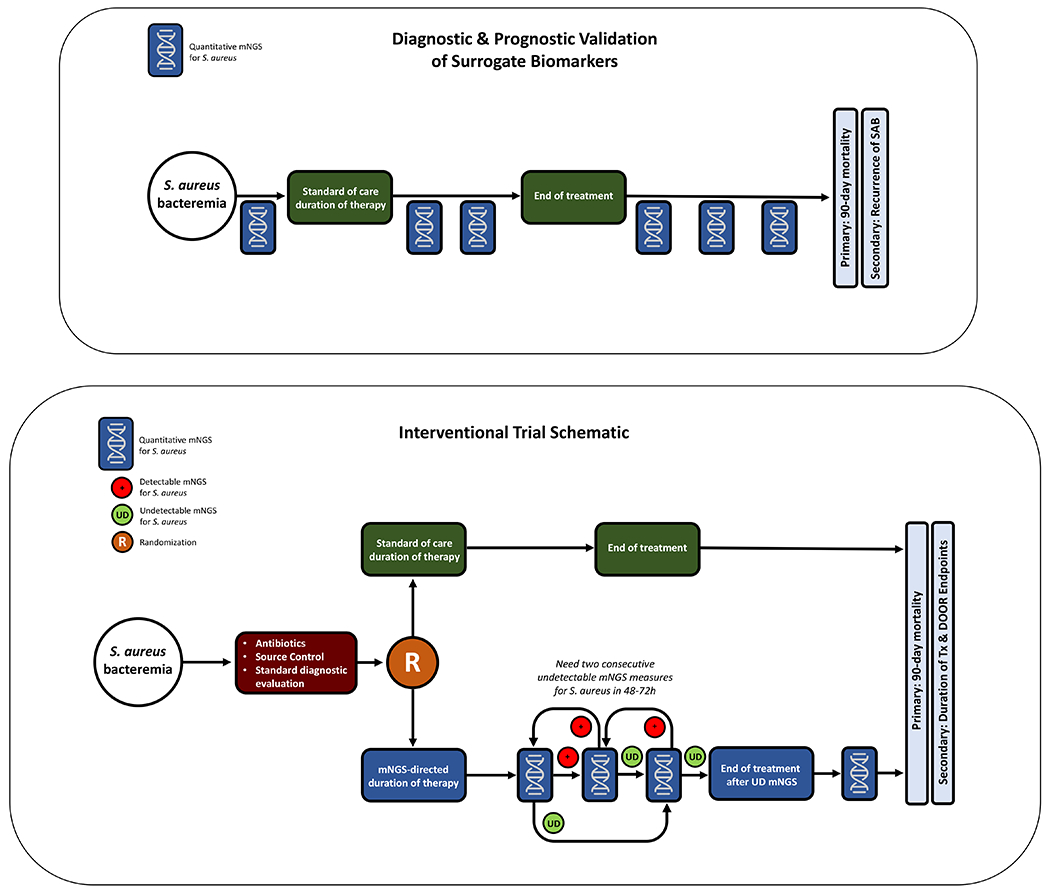
a. Study schematic for a diagnostic and prognostic validation study of mNGS for S. aureus tests.
b. Study schematic for a randomized controlled trial of mNGS-guided management of S. aureus bacteremia.
Stage 1: Performance of mNGS platforms as biological surrogates of clinical response in patients with SAB
The goal of this first stage is to evaluate the utility of mNGS as a biological surrogate for clinical response in patients with SAB. Using a design similar to the Master Protocol for Evaluating Multiple Infection Diagnostics (MASTERMIND) (11), patients with SAB, with or without adequate source control, will be enrolled. Quantitative mNGS for S. aureus will be measured every 2-3 days until a definitive course of therapy is assigned by the treating clinician. At that time, mNGS for S. aureus will be measured twice weekly for two weeks while on therapy, then twice monthly through 90 days of post-treatment follow-up (Figure 1a). Multiple quantitative mNGS platforms can be evaluated simultaneously, with the most promising platforms advancing to the interventional portion of the study. The primary outcome will be a collapsed endpoint of either mortality or recurrent S. aureus infection, defined as isolation of S. aureus by culture from any sterile body site, within 90 days of initial S. aureus blood culture. Quantitative mNGS levels will be compared to patient clinical response. This will establish the sensitivity, specificity, positive and negative predictive value of quantitative mNGS for SAB.
Stage 2: RCT of mNGS-guided duration of therapy vs standard of care for patients with SAB
In Stage 2, patients with SAB will be randomized to mNGS-guided management vs standard of care (SoC) (Figure 1b). Patients with low probability of curable infection who will require long term suppressive antibiotic therapy, such as those with retained infected hardware, will be excluded. Routine follow-up blood cultures will be obtained as directed by the clinical team in both arms. In the mNGS arm, quantitative mNGS for S. aureus DNA will be obtained every 2-3 days. Once planned source control procedures are complete and definitive antibiotic therapy assigned (e.g., a glycopeptide, daptomycin, or anti-staphylococcal β-lactam), mNGS for S. aureus will be measured every 2-3 days until undetectable on two consecutive measures. Patients randomized to mNGS-guided therapy who achieve consecutive undetectable mNGS will discontinue antibiotics. If a patient does not achieve undetectable mNGS for S. aureus after 28-42 days from initiation of therapy, additional diagnostic evaluation will be at the discretion of the treating clinician. No minimum treatment duration will be specified; however, on a practical basis, patients will receive at least 7 days of therapy given the requirement for serial negative mNGS results.
After completion of antibiotic therapy, mNGS for S. aureus will be obtained weekly for the first two weeks, then monthly through the end of the follow-up period. Clinicians will be informed if mNGS for S. aureus reverts to detectable in any of these follow-up measurements, and evaluation for relapsed S. aureus infection will be at their discretion. In the SoC arm, patients will be assigned a treatment course ranging from 14-42 days of antibiotic therapy depending on their clinical designation as uncomplicated or complicated SAB, in accordance with published guidelines (12).
Outcomes and sample size
The non-inferiority primary endpoint will be a composite of mortality or recurrent S. aureus infection within 90 days. Outcomes will also be evaluated utilizing validated desirability of outcomes ranking (DOOR) endpoints (Table 1) that incorporate treatment failure and incidence of adverse events (13, 14). DOOR endpoints provide the advantages of a superiority analysis to compare interventions, evaluate competing risks, assess the risks and benefits of the intervention simultaneously, and can incorporate the patient experience (including quality of life measures). Assuming a 90-day mortality or recurrent S. aureus infection rate of 30%, two-sided alpha level of 0.05, power of 0.8, and non-inferiority margin of 10% difference in the primary outcome, the sample size for this study would be approximately 260 in each arm.
Table 1.
Desirability of Outcomes Ranking (DOOR) for Staphylococcus aureus bacteremia trials
|
|
|||||
|---|---|---|---|---|---|
| Possible Unfavorable Outcomes |
|||||
| Rank | Alive | Treatment failure | Infectious Complications | Ongoing Symptoms | Grade 4 Adverse Events |
| 1 (most desirable) | Yes | 0 of 4 | |||
|
| |||||
| 2 | Yes | Any 1 of 4 | |||
|
| |||||
| 3 | Yes | Any 2 of 4 | |||
|
| |||||
| 4 | Yes | Any 3 of 4 | |||
|
| |||||
| 5 | Yes | 4 of 4 | |||
|
| |||||
| 6 (least desirable) | No | Any | |||
Adapted from ( 4 )
Limitations
Limitations of this study design include mNGS costs, availability, and turn-around time. For example, at our institution, mcfDNA testing is offered by a single vendor, currently costs approximately $3,000 per test, and requires 48-72 hours to obtain results. As such, the cost-effectiveness of a mNGS-guided treatment strategy will depend on the balance between test cost and potential savings that result from shorter durations of therapy. Given that the costs of mNGS are likely to diminish over time as more commercial providers become available, the cost-effectiveness of a mNGS strategy may increase in the future. A second potential limitation is that patients randomized to mNGS-guided therapy may undergo more diagnostic testing and source control procedures, as clinicians respond to reports of detectable S. aureus DNA. However, such interventions would be prompted by the results of the mNGS itself, and we would not know a priori if they were necessary or beneficial. Therefore, these differences between arms would inform the benefit-risk tradeoffs with the mNGS strategy.
Conclusion
In summary, our proposed clinical trial evaluating mNGS-guided duration of SAB therapy has three principal advantages: 1) it evaluates the feasibility of mNGS as a biological surrogate for clinical efficacy assessment in antibacterial trials, potentially providing a much-needed biomarker that could pave the way for new registrational trials; 2) using mNGS, it assesses a strategy to individualize SAB treatment so that patients receive a necessary but not excessive duration of therapy; and 3) includes patients across the full spectrum of disease, enhancing generalizability. As we seek to improve stubbornly poor outcomes from this deadly infection, these advantages may transform care for the next generation of patients with SAB.
Acknowledgements
VGF was supported by 1R01-AI165671-01.
Footnotes
Disclosures
VGF reports personal fees from Novartis, Debiopharm, Genentech, Achaogen, Affinium, Medicines Co., MedImmune, Bayer, Basilea, Affinergy, Janssen, Contrafect, Regeneron, Destiny, Amphliphi Biosciences, Integrated Biotherapeutics; C3J, Armata, Valanbio; Akagera, Aridis, Roche, grants from NIH, MedImmune, Allergan, Pfizer, Advanced Liquid Logics, Theravance, Novartis, Merck; Medical Biosurfaces; Locus; Affinergy; Contrafect; Karius; Genentech, Regeneron, Deep Blue, Basilea, Janssen; Royalties from UpToDate, stock options from Valanbio and ArcBio, Honoraria from Infectious Diseases Society of America for his service as Associate Editor of Clinical Infectious Diseases, and a patent sepsis diagnostics pending.
Contributor Information
Ahmad Mourad, Department of Medicine, Division of Infectious Diseases, Duke University Medical Center, Durham, North Carolina, USA.
Vance G. Fowler, Jr, Department of Medicine, Division of Infectious Diseases, Duke University Medical Center, Durham, North Carolina, USA; Duke Clinical Research Institute, Durham, North Carolina, USA.
Thomas L. Holland, Department of Medicine, Division of Infectious Diseases, Duke University Medical Center, Durham, North Carolina, USA; Duke Clinical Research Institute, Durham, North Carolina, USA; Duke Box 102359, Durham, NC 27710; Room 163 Hanes House, Trent Drive, Durham, NC 27710.
References:
- 1.Leibovici L, Paul M, Doernberg SB. Which randomized controlled trial do we need? Clin Microbiol Infect. 2022;28(12):1525. [DOI] [PubMed] [Google Scholar]
- 2.Bai AD, Lo CKL, Komorowski AS, Suresh M, Guo K, Garg A, et al. Staphylococcus aureus bacteraemia mortality: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28(8):1076–84. [DOI] [PubMed] [Google Scholar]
- 3.Eichenberger EM, Fowler VG Jr., Holland TL. Duration of antibiotic therapy for Staphylococcus aureus bacteraemia: the long and the short of it. Clin Microbiol Infect. 2020;26(5):536–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holland TL, Chambers HF, Boucher HW, Corey GR, Coleman R, Castaneda-Ruiz B, et al. Considerations for Clinical Trials of Staphylococcus aureus Bloodstream Infection in Adults. Clin Infect Dis. 2019;68(5):865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolby HW, Clifford SA, Laurenson IF, Fowler VG, Russell CD. Heterogeneity in Staphylococcus aureus Bacteraemia Clinical Trials Complicates Interpretation of Findings. J Infect Dis. 2022;226(4):723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler VG Jr., Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653–65. [DOI] [PubMed] [Google Scholar]
- 7.Hamed K, Engelhardt M, Jones ME, Saulay M, Holland TL, Seifert H, et al. Ceftobiprole versus daptomycin in Staphylococcus aureus bacteremia: a novel protocol for a double-blind, Phase III trial. Future Microbiol. 2020;15(1):35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blauwkamp TA, Thair S, Rosen MJ, Blair L, Lindner MS, Vilfan ID, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol. 2019;4(4):663–74. [DOI] [PubMed] [Google Scholar]
- 9.Eichenberger EM, de Vries CR, Ruffin F, Sharma-Kuinkel B, Park L, Hong D, et al. Microbial Cell-Free DNA Identifies Etiology of Bloodstream Infections, Persists Longer Than Conventional Blood Cultures, and Its Duration of Detection Is Associated With Metastatic Infection in Patients With Staphylococcus aureus and Gram-Negative Bacteremia. Clin Infect Dis. 2022;74(11):2020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichenberger EM, Degner N, Scott ER, Ruffin F, Franzone J, Sharma-Kuinkel B, et al. Microbial Cell-Free DNA Identifies the Causative Pathogen in Infective Endocarditis and Remains Detectable Longer Than Conventional Blood Culture in Patients with Prior Antibiotic Therapy. Clin Infect Dis. 2023;76(3):e1492–e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel R, Tsalik EL, Petzold E, Fowler VG Jr., Klausner JD, Evans S, et al. MASTERMIND: Bringing Microbial Diagnostics to the Clinic. Clin Infect Dis. 2017;64(3):355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55. [DOI] [PubMed] [Google Scholar]
- 13.Doernberg SB, Tran TTT, Tong SYC, Paul M, Yahav D, Davis JS, et al. Good Studies Evaluate the Disease While Great Studies Evaluate the Patient: Development and Application of a Desirability of Outcome Ranking Endpoint for Staphylococcus aureus Bloodstream Infection. Clin Infect Dis. 2019;68(10):1691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans SR, Rubin D, Follmann D, Pennello G, Huskins WC, Powers JH, et al. Desirability of Outcome Ranking (DOOR) and Response Adjusted for Duration of Antibiotic Risk (RADAR). Clin Infect Dis. 2015;61(5):800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


