To the Editor,
COVID-19 vaccination in patients with kidney disease has generally been stated not to cause accelerated eGFR decline or an increase in proteinuria [1]. However, from the observational studies that have been performed thus far, no firm conclusions can be drawn, as no comprehensive longitudinal analyses were performed. Here, we report, to our knowledge, the first data on longitudinal kidney function before and after COVID-19 vaccination in patients with end-stage kidney disease and after kidney transplantation. Additionally, we studied proteinuria and the formation of HLA antibodies after COVID-19 vaccination.
For this study, we included patients with chronic kidney disease grade 4/5 (CKD G4/5), on dialysis and kidney transplant recipients (KTR) who received two mRNA-1273 COVID-19 vaccinations in the context of a clinical trial investigating the immune response after COVID-19 vaccination in kidney patients [2, 3]. A subset of KTR who did not seroconvert after two vaccinations continued in a subsequent randomized clinical trial investigating different vaccination strategies where they received a third COVID-19 vaccination [4]. Estimated GFR slopes were compared in 169 CKD G4/5 and 294 KTR six months pre- and post-vaccination (Table S1). Of these KTR, 53 were included for eGFR slope comparison after a third vaccination. In a subset of the eGFR cohort (CKD G4/5 n = 92, KTR n = 167), urine protein creatinine ratios were available (Table S2). Additionally, we selected 21 CKD G4/5, 26 dialysis patients, and 55 KTR to determine HLA antibody status pre- and post-vaccination (Table S3) (see Figs. S1 and S2 for a patient flowchart). A detailed description of the methods can be found in the Supplementary Methods.
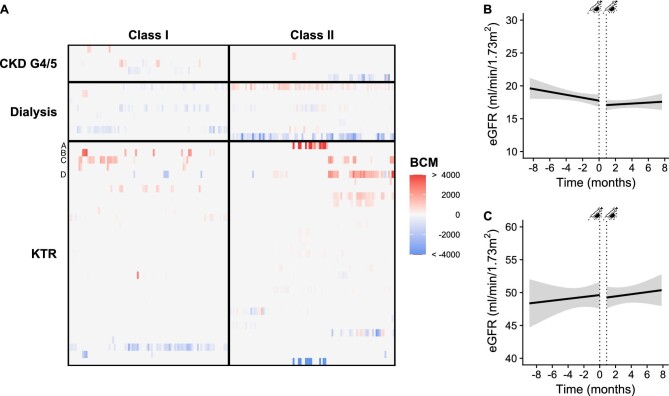
After two vaccinations, no de novo HLA antibodies were detected in each group. In KTR with pre-existing HLA antibodies, some individuals had considerable changes (Fig. 1A; Table S4). One KTR with a substantial rise in donor-specific HLA antibodies had an increase in urine protein creatinine ratio and a decline in eGFR. The kidney biopsy showed chronic active antibody-mediated rejection, and consequently, this patient received a higher dosage of mycophenolate mofetil (Patient A in Fig. 1A). Combining our data with other published studies on HLA antibody formation after COVID-19 vaccinations, a total of 642 KTR and 583 waitlisted patients were investigated.S1–S16 Of these patients, 12 (1.9%) KTR and 6 (1.0%) waitlisted patients developed de novo HLA antibodies. The reported 1%–2% of patients with de novo HLA antibody formation after COVID-19 vaccination seems to be in line with the incidence reported during regular follow-up with an interval of eight months between measurements after kidney transplantation [5].
Figure 1:
Kidney-related outcomes after COVID-19 vaccination. (a) Heatmap with changes in specific HLA antibodies of positive patients per patient group and class. Alphabetic letters are the individuals whose characteristics are presented in Table S6. Estimated GFR slope before and after mRNA-1273 COVID-19 vaccination in (b) CKD G4/5 patients and (c) KTR. Change in eGFR slope was calculated by segmented mixed effects linear regression. Dotted lines represent the first and second vaccination. BCM, background corrected mean fluorescence intensity.
The rate of eGFR loss over time was less steep in CKD G4/5 patients after two vaccinations (βbefore −3.65 (CI: −7.3, −1.83) versus βafter2 −1.46 (CI: −3.29–0.37) ml/min/1.73 m2 per year; P = 0.01; Fig. 1a). Results were not different for various subgroups (Table S5). In KTR, there was no change in the annual rate of eGFR loss after two vaccinations (βbefore 1.10 (CI: −0.37–2.56) vs. βafter2 0.73 (CI: −0.37–2.19) mL/min/1.73 m2 per year; P = 0.83; Fig. 1b) nor after three vaccinations (βafter3 −0.99 (CI: −2.58–0.59) vs. βbefore −0.92 (CI: −4.69, 2.84) ml/min/1.73 m2 per year; P = 0.99). No changes in proteinuria were observed in both groups [CKD G4/5 86.6 (31.7–178.4) vs. 86.1 (36.1–174.2) mg/mmol; P = 0.73 and KTR 14.3 (9.5–36.5) vs. 16.7 (10.0–40.0) mg/mmol; P = 0.35], or according to subgroups (Table S6).
In conclusion, we did not observe changes in kidney-related outcomes longitudinally assessed in kidney patients before and after mRNA-1273 COVID-19 vaccination. Neither did we observe a change in the development of de novo HLA antibodies. These findings imply that the COVID-19 vaccination is safe and does not worsen kidney damage or function in patients with end-stage kidney disease and kidney transplantation recipients.
Supplementary Material
ACKNOWLEDGEMENTS
We would especially like to thank Joost de Jong for his assistance with the statistical analyses of this manuscript. Furthermore, we would like to thank the RECOVAC collaborators: Debbie van Baarle, Carla C. Baan, Renate G. van der Molen, Ester B.M. Remmerswaal, Rory D. de Vries, Dimitri A. Diavatopoulos, Vera Koomen, Yvette den Hartog, Rob van Binnendijk, Corine H. Geurts van Kessel, Gerco den Hartog, Marion P.G. Koopmans, Wouter B. Mattheussens, Alferso C. Abrahams, Pim Bouwmans, Marc A.G.J. ten Dam, Marc H. Hemmelder, Nynke Rots, Priya Vart, and Aiko P.J. de Vries. We would like to thank the technicians of the laboratory of Transplantation Immunology and specifically Abeer Hamad for performing the HLA antibody assays.
Contributor Information
Céline Imhof, Department of Internal Medicine, Division of Nephrology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands.
A Lianne Messchendorp, Department of Internal Medicine, Division of Nephrology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands.
Laura B Bungener, Transplantation Immunology, Department of Laboratory Medicine, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Bouke G Hepkema, Transplantation Immunology, Department of Laboratory Medicine, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Marcia M L Kho, Department of Internal Medicine, Nephrology and Transplantation, Erasmus MC Transplant Institute, Erasmus Medical Center, Rotterdam, the Netherlands.
Marlies E J Reinders, Department of Internal Medicine, Nephrology and Transplantation, Erasmus MC Transplant Institute, Erasmus Medical Center, Rotterdam, the Netherlands.
Frederike J Bemelman, Renal Transplant Unit, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands.
Luuk B Hilbrands, Department of Nephrology, Radboud University Medical Center, Nijmegen, the Netherlands.
Ron T Gansevoort, Department of Internal Medicine, Division of Nephrology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands.
Jan Stephan F Sanders, Department of Internal Medicine, Division of Nephrology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands.
RECOVAC Consortium:
Debbie van Baarle, Carla C Baan, Renate G van der Molen, Ester B M Remmerswaal, Rory D de Vries, Dimitri A Diavatopoulos, Vera Koomen, Yvette den Hartog, Rob van Binnendijk, Corine H Geurts van Kessel, Gerco den Hartog, Marion P G Koopmans, Wouter B Mattheussens, Alferso C Abrahams, Pim Bouwmans, Marc A G J ten Dam, Marc H Hemmelder, Nynke Rots, Priya Vart, and Aiko P J de Vries
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
R.G. and J.S. designed the study protocol. M.R., D.B., F.B., R.M., R.v.d.M., D.D., E.R., R.V., and L.H. contributed to the protocol design. C.I. participated in the performance of the research. C.I., A.M., and L.B. participated in data analysis. C.I., A.M., R.G., and J.S. participated in the writing of the paper. F.B., M.R., and L.H. contributed to the intellectual content.
FUNDING
Funding was supplied by The Netherlands Organization for Health Research and Development (ZonMW, project number: 10430072010002).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request. Research proposals can be submitted to the Consortium members via the corresponding author.
CLINICAL TRIAL NOTATION
REFERENCES
- 1. Al Jurdi A, Gassen RB, Borges TJet al. . Non-invasive monitoring for rejection in kidney transplant recipients after SARS-CoV-2 mRNA vaccination. Front Immunol 2022;13:1–14. 10.3389/fimmu.2022.838985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kho MML, Reinders MEJ, Baan CCet al. . The RECOVAC IR study: the immune response and safety of the mRNA-1273 COVID-19 vaccine in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Nephrol Dial Transplant 2021;36:1761–4. Published online 2021. 10.1093/ndt/gfab186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders JSF, Bemelman FJ, Messchendorp ALet al. . The RECOVAC immune-response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation 2022;106:821–34. 10.1097/TP.0000000000003983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kho MML, Messchendorp AL, Frölke SCet al. . Alternative strategies to increase the immunogenicity of COVID-19 vaccines in kidney transplant recipients not responding to two or three doses of an mRNA vaccine (RECOVAC): a randomised clinical trial. Lancet Infect Dis 2023;23:307–19. 10.1016/S1473-3099(22)00650-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stringer D, Gardner L, Shaw Oet al. . Optimized immunosuppression to prevent graft failure in renal transplant recipients with HLA antibodies (OuTSMART): a randomised controlled trial. EClin Med 2023;56:1–17. 10.1016/j.eclinm.2022.101819 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request. Research proposals can be submitted to the Consortium members via the corresponding author.