Abstract
We evaluated the energy metabolism of human mesenchymal stem cells (MSC) isolated from umbilical cord (UC) of preterm (< 37 weeks of gestational age) and term (≥ 37 weeks of gestational age) newborns, using MSC from adult bone marrow as control. A metabolic switch has been observed around the 34th week of gestational age from a prevalently anaerobic glycolysis to the oxidative phosphorylation. This metabolic change is associated with the organization of mitochondria reticulum: preterm MSCs presented a scarcely organized mitochondrial reticulum and low expression of proteins involved in the mitochondrial fission/fusion, compared to term MSCs. These changes seem governed by the expression of CLUH, a cytosolic messenger RNA-binding protein involved in the mitochondria biogenesis and distribution inside the cell; in fact, CLUH silencing in term MSC determined a metabolic fingerprint similar to that of preterm MSC. Our study discloses novel information on the production of energy and mitochondrial organization and function, during the passage from fetal to adult life, providing useful information for the management of preterm birth.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-017-2665-z) contains supplementary material, which is available to authorized users.
Keywords: CLUH, Endothelial cells, Energy metabolism, Mesenchymal stem cells, OXPHOS, Preterm newborns, Term newborns
Introduction
Human mesenchymal stem cells (MSCs) are somatic cells that display a great proliferation capacity, multi-lineage differentiation and an extensive self-renewal ability [1]. At first, MSCs have been isolated from adult tissues, such as bone marrow (BM) or adipose tissue, but these cells seem to lose their proliferative potential with aging [1]. Recently, also the umbilical cord (UC) has been considered as a great source of MSC, because it contains a considerable number of MSCs with properties similar to those of MSCs from bone marrow and adipose tissue [2], characterized by higher proliferative rates [3].
There is an increasing interest in understanding relationships between energy metabolism, differentiation, and proliferative potential. It is known that murine embryonic stem cells (mESCs) express a high glycolytic flux for their energy production probably to minimize reactive oxygen species (ROS) production [4–6]. Moreover, the anaerobic metabolism shifts to aerobic metabolism only during the differentiation in somatic cells [7, 8], suggesting a mitochondrial role in the bioenergetic functions associated with the differentiation of stem cells [9, 10]. For example, Shum et al. have observed that BM-MSCs displayed a high OXPHOS metabolism during osteogenic differentiation, albeit maintaining the glycolysis rate similar to that of undifferentiated cells [11]. Other authors have observed that adult stem cells remain in a prevalent quiescent state, down-regulating metabolic activity and relying mainly on glycolysis. However, glycolysis is not, per se, associated with low pace of proliferation since induced pluripotent stem cells (iPS) may proliferate indefinitely while maintaining high glycolytic rate and globular immature mitochondria [12].
Although mitochondria are considered the powerhouse of the cells, their activity is not limited to the energy production, but they are also involved in the regulation of cell cycle and proliferation, through ROS production and the apoptosis processes [13].
Recently, we have described that only the MSC-derived exosomes from full-term newborns [≥ 37 weeks of gestational age (GA)] are able to conduct a complete oxidative phosphorylation (OXPHOS) activity, producing ATP, while exosomes from preterm newborns (< 37 weeks of GA) consumed oxygen, but did not produce ATP [14]. These data anticipated that the MSCs undergo a “metabolic shift” from anaerobic to aerobic, during gestational development.
After those initial observations, in this work, we have evaluated the asset of energy metabolism and the function of mitochondria in the development of UC-MSCs isolated from preterm and term newborns.
Materials and methods
Cell cultures
After written informed consent, umbilical cords from full-term (gestational age (GA) ≥ 37 weeks, n = 89, term) and preterm (GA 26, 28, 34 and 36 weeks, n = 88 for each age) babies were collected following elective cesarean section [15]. Study was approved by the Institutional Review Board of the IRCCS G. Gaslini, Genoa, Italy (no. 164, 22nd July 2013).
The MSCs were isolated from umbilical cord (UC) using a non-enzymatic digestion protocol [16]. Briefly, the adherent cells growing from small pieces of cord were collected after 14 days of culture and re-plated at low density (500/cm2), using the same culture conditions until confluence, in a 75-cm2 culture flask. Finally, cells were harvested, counted, and identified by flow cytometry using standard mesenchymal markers, at P2 [17]. Only samples presenting a purity ≥ 95% have been evaluated for the energetic status. MSCs isolated from adult bone marrow [18] were used as positive control.
All cells were cultured in Dulbecco’s modified eagle medium (DMEM), low glucose medium (Gibco Invitrogen, Carlbad, CA, USA) containing penicillin/streptomycin, Glutamine (Gibco Invitrogen, Carlbad, CA, USA) heparin (PharmaTex Italia, Milan, Italy), and 5% platelet lysate at 37 °C in 5% carbon dioxide (CO2) and in 21% O2 (normoxic condition). For hypoxic condition, UC-MSCs were incubated for 48 h in a humidified hypoxic chamber (Binder, Tuttlingen, Germany) at 5% CO2 level and 1% O2 balanced with N2.
Endothelial cells were obtained from cord blood samples of premature and full-term infants as described elsewhere [19]. The cells were cultured in endothelial cell growth medium EGM-2 Bullet Kit (Lonza-Euroclone) at 37 °C in 5% CO2 and used in passage numbers 2–4.
Proliferative potential
Adherent cells growing from small pieces of cords were harvested when they became confluent in a 100-mm Petri dish and were counted (p1MSC).
Adherent cells growing from 3.8 × 104 p1MSC were harvested and counted when they were confluent in a 75-cm2 tissue culture flask (p2MSC).
MSC immunophenotyping
Immunophenotyping of the expanded term or preterm UC-derived MSCs was done using flow cytometry, at passage 2 [14]. After detachment using 0.05% trypsin/EDTA, cells were washed, pelleted, and stained with fluorescein isotiocyanate-, phycoerythrin-, allophycocyanin- or VioBlue-conjugated monoclonal antibodies specific for CD14 (345784, BD Biosciences), CD31 (560984, BDBiosciences), CD34 (130-095-393, MiltenyiBiotec), CD45, (560976, BDBiosciences), CD73 (130-097-947, MiltenyiBiotec), CD90 (130-095-400, MiltenyiBiotec), CD105 (130-094-941, MiltenyiBiotec), CD166 (559263, BDpharmigen) and SSEA-4 (130-098-369, MiltenyiBiotec). Nonspecific staining was determined using isotype-matched nonreactive mouse IgGs and 7-AAD (EBioScience). 7-AAD (EBioScience) was used to evaluate cell viability. After washing, at least 5000 events were acquired on MACSQuant Analyzer 10 (MiltenyiBiotec) and the multicolor analysis was performed using FlowJo 10.0.6 software (Tree Star, Ashland, OR, USA).
Intracellular staining for the detection of pluripotency gene was performed on UC-MSC as previously described [19]. Cells were fixed/permeabilized using Fix/Perm buffer kit (BD Biosciences) according to manufacturer’s indications. The following PE-conjugated antibodies were used: Nestin, (Pharmigen), OCT3/4 (EBioScience), and Nanog (BD Pharmigen). Samples were then acquired on MACSQuant and data were analyzed by FlowJo.
Small interfering RNA and transfection
Predesigned siRNAs against human CLUH (5′-CCACCAGCUGGACCACGUCUUUAAA-3′) and nonspecific siRNA (named scramble) for control were obtained from Life Technologies. Transfection of siRNA was performed as described previously [20]. Briefly, MSCs were grown in either six-well or eight-well Lab-Tek chamber slide at 5 × 104 cells/cm2 in DMEM with 5% platelet lysate. At 70–80% confluence, cells were incubated with siRNA transfection solution at 37 °C 5% CO2. After 24 h of down-regulation, metabolic assays and confocal microscopy analyses were performed.
Evaluation of ATP/AMP ratio
To calculate the ATP/AMP ratio, the intracellular concentration of ATP and AMP was evaluated spectrophotometrically [21]. For each assay, 20 µg of total protein was used. ATP was assayed, following NADP reduction at 340 nm, in a solution containing: 50 mM Tris–HCl, pH 8.0, 1 mM NADP, 10 mM MgCl2, and 5 mM glucose in 1 ml of final volume. Samples were analyzed before and after the addition of 4 µg of purified hexokinase/glucose-6-phosphate dehydrogenase.
AMP was assayed following the NADH oxidation at 340 nm. The medium contained 100 mM Tris–HCl, pH 8.0, 75 mM KCl, 5 mM MgCl2, 0.2 mM ATP, 0.5 mM phosphoenolpyruvate, 0.2 mM NADH, 10 IU adenylate kinase, 25 IU pyruvate kinase, and 15 IU of lactate dehydrogenase.
Oxygen consumption measurements
O2 consumption was evaluated using 100,000 preterm or term UC-MSC or endothelial cells, permeabilized with 0.03% digitonin by a thermostatically controlled oxygraph apparatus equipped with an amperometric electrode (Unisense-Microrespiration, Unisense A/S, Denmark). Before each experiment, the electrode was equilibrated with the respiration medium containing: 137 mM NaCl, 5 mM KCl, 0.7 mM KH2PO4, 25 mM Tris–HCl, pH 7.4, and 25 mg/ml ampicillin. The addition of the respiring substrates was performed with a Hamilton syringe, in the following order: 10 mM pyruvate plus 5 mM malate, 40 µM rotenone, 20 mM succinate, and 50 µM antimycin A. Pyruvate and malate were used to stimulate the pathway composed by Complexes I, III and IV, while succinate was used for the pathway formed by Complexes II, III and IV. To observe the ADP-stimulated respiration rates, 0.08 mM ADP was added after the addition of pyruvate and malate or succinate.
The respiratory rates were expressed as nmol O/min/106 cells [22].
Fo–F1 ATP synthase activity assay
To evaluate the Fo–F1 ATP synthase activity, 100,000 preterm or term UC-MSC or endothelial cells were incubated for 10 min at 37 °C in a medium containing: 10 mM Tris–HCl, pH 7.4, 100 mM KCl, 5 mM KH2PO4, 1 mM EGTA, 2.5 mM EDTA, and 5 mM MgCl2, 0.6 mM ouabain and 25 mg/ml ampicillin. Afterward, ATP synthesis was induced by the addition of 10 mM pyruvate plus 5 mM malate or 20 mM succinate, to stimulate the pathways composed by Complexes I, III, and IV pathway or Complexes II, III, and IV, respectively. The reaction was monitored for 2 min, every 30 s, in a luminometer (GloMax® 20/20n Luminometer, Promega Italia, Milano, Italy), by the luciferin/luciferase chemiluminescent method. ATP standard solutions between 10−8 and 10−5 M was employed (luciferin/luciferase ATP bioluminescence assay kit CLSII, Roche, Basel, Switzerland). Data were expressed as nmol ATP produced/min/106 cells [23].
The oxidative phosphorylation efficiency (P/O ratio) was calculated as the ratio between the concentration of the produced ATP and the amount of consumed oxygen in the presence of respiring substrate and ADP. When the oxygen consumption is completely devoted to the energy production, the P/O ratio should be around 2.5 and 1.5 after pyruvate plus malate or succinate addition, respectively [24].
Evaluation of glycolysis flux
To assay the glycolytic flux, the activities of hexokinase, phosphofructokinase, pyruvate kinase, and lactate dehydrogenase were measured at room temperature on 20 µg of preterm or term UC-MSCs or endothelial cells total protein. Enzymatic activity was expressed as mU/mg of total protein (nmol/min/mg of protein). NADH molar extinction coefficient was considered 6.22 mM-1 cm-1, at 340 nm. The reaction mixtures used for the determination of each enzyme activity were the following [25]:
Hexokinase (EC 2.7.1.1): 100 mM Tris–HCl pH 7.4, 5 mM MgCl2, 200 mM glucose, 1 mM ATP, 0.91 mM NADP, and 0.55 IU/ml of glucose 6-phosphate dehydrogenase.
Phosphofructokinase (EC 2.7.1.11): 100 mM Tris–HCl, pH 7.4, 2 mM MgCl2, 5 Mm KCl, 2 mM fructose 6-phosphate, 1 mM ATP, 0.5 mM phosphoenolpyruvate, 0.2 mM NADH, and 2 IU/ml of pyruvate kinase and lactate dehydrogenase.
Pyruvate kinase (EC 2.7.1.40): 100 mM Tris–HCl, pH 7.6, 2.5 mM MgCl2, 10 mM KCl, 0.6 mM phosphoenolpyruvate, 0.2 mM NADH, 5 mM ADP, and 1 IU/ml of lactate dehydrogenase.
Lactate dehydrogenase (EC 1.1.1.27): 100 mM Tris–HCl pH 7.4, 0.2 mM NADH, and 5 mM pyruvate.
The lactate concentration was measured in the growth medium, following the reduction of NAD+. The assay mixture contained: 100 mM Tris–HCl pH 7.4, 5 mM NAD+, and 4 IU of lactate dehydrogenase.
Electrophoretic separation and semiquantitative WB
Denaturing electrophoresis (sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed following a Laemmli protocol with minor modifications in a Mini-PROTEAN III (BioRad, Hercules, CA, USA) apparatus. Separating gel was a gradient gel from 6 to 20% w/v acrylamide. For each sample, 20 µg of total protein was loaded. Run was performed at 4 °C, at 70 mA for each gel, for 120–150 min.
Electrophoretically separated samples were transferred onto nitrocellulose (NC) membranes by electroblotting, at 400 mA for 1 h, at 4 °C. NC was incubated with the specific Abs (overnight at 4 °C) diluted in PBS plus 0.15% tween (PBSt): anti-MTCO2 (subunit II of complex IV, ab110258, Abcam); anti-ATP5B (β subunit of ATP synthase, HPA001528, Sigma Aldrich); anti-OPA1 (ab42364, Abcam); anti-DRP1 (ab54038, Abcam); anti-eIF3X (CLUH, A301-764A, Bethyl Lab); SOD-2 (superoxide dismutase isoform 2, sc-18503, Santa Cruz Biotechnology) and anti-β-actin antibody (sc-47778, Santa Cruz Biotechnology). Secondary HPR-conjugated Abs were diluted in PBS 1:10,000. Bands were visualized by chemiluminescence with Immuno-Star WesternC kit (BioRad Lab, Hercules, CA) and images acquired by means of the molecular imager ChemiDoc XRS + System (Bio-Rad). Quantitative densitometry was performed using the ChemiDoc XRS + System software and data expressed as relative optical density (R.O.D.).
Transmission electron microscopy
For this analysis, UC-MSCs pellets from preterm and term newborns were employed. Cell pellets were fixed with 2.5% glutaraldehyde/0.1 M cacodylate buffer at pH 7.6 for 1 h at room temperature. After post-fixation with 1% OsO4 in cacodylate buffer for 1 h, pellets were dehydrated in an ethanol series and embedded in Epon resin. Ultrathin sections stained with uranyl-acetate and lead citrate were observed with a Jeol Jem-1011 transmission electron microscope. 200 mitochondria were examined for each sample.
Determination of lipid peroxidation: malondialdehyde assay
To assess the lipid peroxidation in preterm and term UC-MSCs or endothelial cells, malondialdehyde (MDA) levels were assayed, using the thiobarbituric acid reactive substances (TBARS) assay, with minor modifications [26]. This method is based on the reaction of MDA, a breakdown product of lipid peroxides, with thiobarbituric acid TBA. To evaluate the basal concentration of MDA, 50 μg of total protein dissolved in 300 μl of milliQ water was added to 600 μl of TBARS solution, containing 15% trichloroacetic acid in 0.25 N HCl and 26 mM TBA.
The mix was incubated for 40 min at 100 °C, centrifuged at 14,000 rpm for 2 min, and the supernatant was analyzed spectrophotometrically, at 532 nm.
Different MDA concentrations (0.75, 1, and 2 μM) were used to obtain a standard curve.
Catalase activity assay
Catalase activity was evaluated following the peroxidation of H2O2, at 240 nm. The assay medium containing: 100 mM Tris–HCl, pH 7.4, and 2 mM H2O2. For each assay, 20 µg of total protein was employed.
Confocal microscopy
Cells were washed with growth medium and incubated with 200 nM MitoTracker-633 probe (Life Technologies Ltd, Monza, MB, Italy), for 20 min at room temperature. After the incubation, sample was washed with PBS and immediately analyzed using a laser scanning spectral confocal microscope TCS SP2 AOBS (Leica, Heidelberg, Germany), equipped with Argon ion, He–Ne 543 nm, and He–Ne 633 nm lasers. Images were acquired through a HCX PL APO CS 40 ×/1.25 oil UV objective and processed with Leica. Images were acquired as single transcellular optical sections.
Gene expression analysis
Total RNA was isolated from 0.5 to 1 × 105 term or preterm UC-MSC, using the RNeasyPlus Mini kit (QIAGEN), according to the manufacturer’s protocol. Amount and purity of RNA were assessed using a NanoDrop spectrophotometer (Nanodrop Technologies) and 1 µg of total RNA was used to synthesize cDNA by reverse transcription, using iScript Reverse Transcriptase (BioRad). SYBR Green q-PCR was performed on an iQ5 (Bio-Rad). Results were normalized into two reference genes, β-actin and TATA-box binding protein, selected by NormFinder for their highest stability among a pool of common housekeeping genes [27]. Primers were designed by NCBI/primer-BLAST primer designing tool. Statistical analysis of the results was performed using the 2ΔCt method [28], and results were expressed relative to term UC-MSC expression values. CLUH primers sequences were TACATCATGGGCGACTACGC (forward primer) and GGCCAGGTGCATGTATTCCT (reverse primer).
Statistical analysis
GraphPad Prism 4.03 for Windows (GraphPad Software, La Jolla, CA, USA) was used to carry out statistical analysis. Two-way repeated-measures ANOVA was used to evaluate the changes among term and preterm samples; the Bonferroni post hoc test was then used to evaluate the difference between treatments at specific time points.
Results
Characterization of preterm and term UC-MSCs
UC-MSCs derived from preterm and term newborns were characterized by flow cytometry at passage 2. Both samples met the standard criteria [17]. In fact, the majority of collected preterm and term UC-MSCs were highly positive for the main MSCs markers CD73, CD90, and CD105, as well as of CD166, but negative for the typical hematopoietic and endothelial markers, such as CD14, CD31, CD34, and CD45 (Figure 1 Supplementary, panel a). Furthermore, the expression of stemness markers, such as Nestin, Oct3/4, Nanog, and SSEA-4, did not differ in UC-MSC from preterm and term samples (Figure 1 Supplementary, panel b).
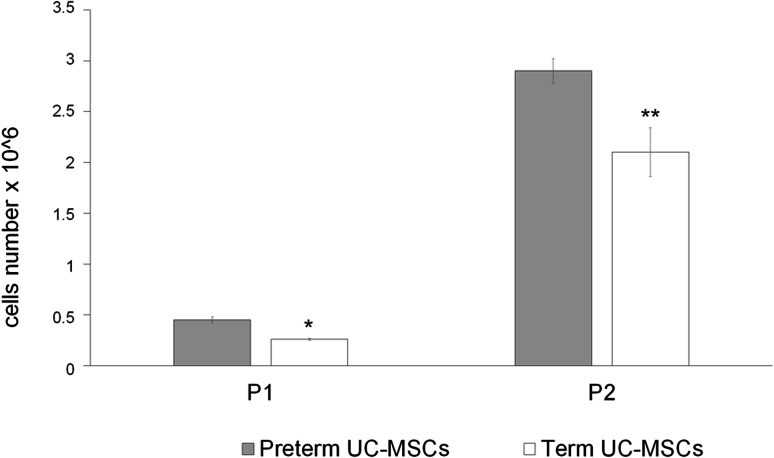
In addition, as reported in Fig. 1, preterm cord samples (n = 89) grew a higher number of MSC both in the first and in the second generation compared to term cords (n = 88) (p = 0.01 and p = 0.003, respectively), although both samples maintained a high proliferation rate.
Fig. 1.
Proliferation rate of preterm and term UC-MSCs. Graph shows the proliferation rate of preterm (gray columns) and term (white columns) UC-MSCs at first or the second passage in culture (P1 and P2, respectively). Preterm cord samples (no. 89) grew a higher number of MSCs both at the first than at the second generation compared to term cords (no. 88) (p = 0.01 and p = 0.003, respectively). Asterisk indicates a p < 0.003 between preterm and term at P1. Double asterisk indicates a p < 0.001 between preterm and term at P2
The identity of endothelial cells from preterm and term newborns was previously reported [19].
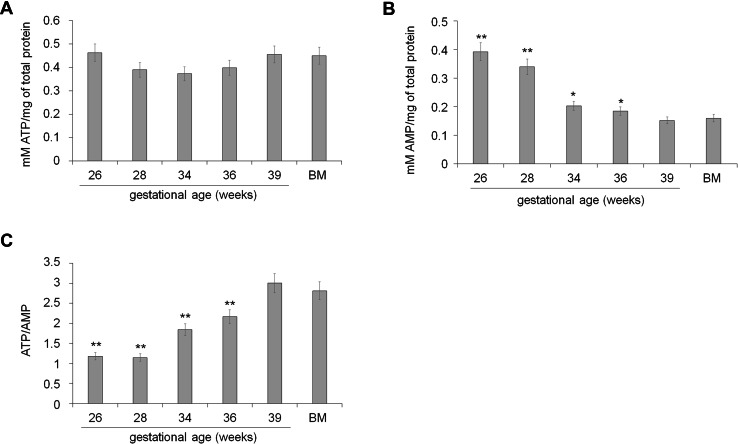
Energy status of preterm and term UC-MSCs
The intracellular level of ATP appeared similar in each sample (Fig. 2, panel a), while the AMP concentration decreased with GA progressing (Fig. 2, panel b). This determined an increment of the ATP/AMP ratio proportionally with the GA of the sample, reaching a maximum in term UC-MSCs and BM-MSCs, used as positive control (Fig. 2, panel c).
Fig. 2.
Energetic status of preterm and term UC-MSCs. a shows the intracellular concentration of ATP in preterm (26–36 weeks of GA), term (39 weeks of GA), and BM-MSCs. The values appear similar in all samples, without significant differences. b Reports the intracellular concentration of AMP in preterm (26–36 weeks of GA), term (39 weeks of GA), and BM-MSCs. The values increase proportional to the age of the samples. The same trend was observed for the ATP/AMP ratio, showed in c. All data are expressed as mean ± SD and are representative of at least four experiments. There is not significance different among term UC-MSCs (39 weeks of GA) and BM-MSCs. Double asterisk indicates a significant difference for p < 0.001 between preterm (26–34 weeks of GA) and term UC-MSCs (39 weeks of GA). Asterisk indicates a significant difference for p < 0.005 between preterm (26–34 weeks of GA) and term UC-MSCs (39 weeks of GA)
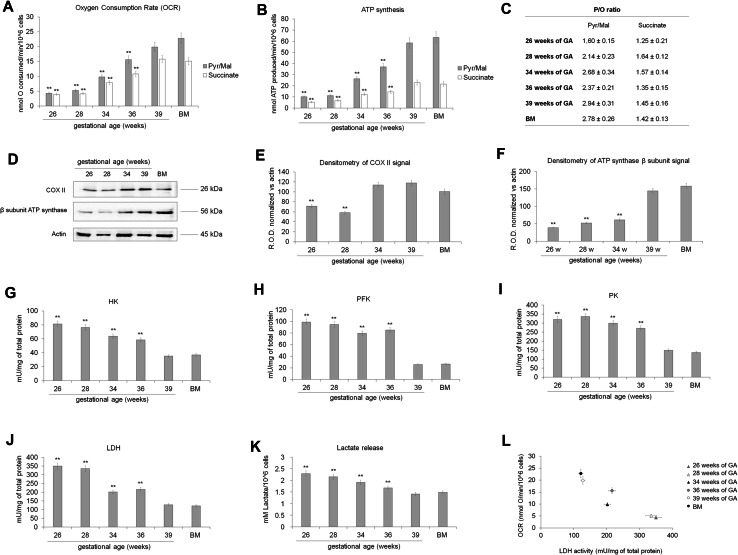
Energy metabolism in preterm and term UC-MSCs
The difference observed in the ATP/AMP ratio may depend by the pathways that UC-MSCs employ for the energy production, thus, the activity of the oxidative phosphorylation (OXPHOS; in term of oxygen consumption and ATP synthesis), as well as the activity of some glycolytic enzymes was investigated. Data show that the oxygen consumption and the ATP synthesis (Fig. 3, panels a, b, respectively) were very low in the early preterm stage (26–28 weeks) and increased concurrently with the GA.
Fig. 3.
Energy metabolism in preterm and term UC-MSCs. The mitochondrial oxygen consumption (a) and the ATP synthesis from Fo–F1 ATP synthase (b) were assayed to evaluate the mitochondrial oxidative metabolism in preterm (26, 28, 34 and 36 weeks of GA), term (39 weeks of GA) and BM-MSCs. To stimulate the pathway composed by Complexes I, III, and IV, pyruvate plus malate (Pyr/Mal; gray columns) was employed; while succinate (white columns) was used to stimulate the pathway formed by complexes II, III, and IV. In both cases, the mitochondrial function is very low in the early preterm samples (26–28 weeks of GA), increasing proportionally to the age with a maximum in term (39 weeks of GA) and BM MSC. All data are expressed as mean ± SD and are representative of at least five experiments. There is not significance different between term (39 weeks of GA) and BM MSC. Double asterisk indicates a significant difference for p < 0.001 with respect the value of term (39 weeks of GA) and BM MSC. c Reports the P/O value after the addition of pyruvate plus malate or succinate. This parameter indicates the efficiency of the OXPHOS machinery and must be around 2.5 for pyruvate plus malate induction and 1.5 for the succinate addition. All data are expressed as mean ± SD and are representative of at least five experiments. d shows the WB analysis against COXII, a subunit of complex IV, and the β subunit of ATP synthase. The loaded samples were: preterm UC-MSCs (28, 32 and 34 weeks of GA), term UC-MSCs (39 weeks of GA), and BM-MSCs used as positive control. The densitometric analysis of the WB signals is shown in e and f, respectively. d–f are representative of at least four experiments and double asterisk indicates a significant difference for p < 0.001 with respect to the value of term (39 weeks of GA) and BM MSC. To evaluate the glycolytic flux and the anaerobic metabolism in preterm (26–36 weeks of GA), term (39 weeks of GA), and BM-MSCs, the activities of hexokinase, (HK, g), phosphofructokinase (PFK, h), pyruvate kinase (PK, i) lactate dehydrogenase (LDH, j) and the lactate release (k) were assayed. In all cases, the activity of these enzymes and the lactate concentration decrease proportionally with the age of the sample, reaching a minimum value in term (39 weeks of GA) and BM-MSCs. The correlation between the oxygen consumption rate (OCR) and the LDH activity is shown in l. Data are expressed as mean ± SD and are representative of at least five experiments. There is not significance different between term (39 weeks of GA) and BM MSC. Double asterisk indicates a significant difference for p < 0.001 among preterm UC-MSCs with respect to the value of term UC-MSCs (39 weeks of GA) and BM-MSCs
In addition, when early preterm UC-MSCs were stimulated with pyruvate plus malate in comparison with succinate stimulation, they displayed a lower P/O ratio compared to samples with higher GA, suggesting that the OXPHOS machinery displays low efficiency (Fig. 3, panel c).
The increment of the OXPHOS activity during fetal life was confirmed by the expression of COXII, a complex IV subunit codified by mitochondrial DNA and by the expression of β subunit of Fo–F1 ATP synthase, codified by nuclear DNA (Fig. 3, panels d–f). By contrast, the activity of hexokinase (HK), phosphofructokinase (PFK), pyruvate kinase (PK) and lactate dehydrogenase (LDH) as well as the lactate release were very high in preterm UC-MSCs and decrease proportionally with their maturation (Fig. 3, panels g–k). Moreover, as reported in Fig. 3, panel l, the LDH activity is inversely proportional to the oxygen consumption rate.
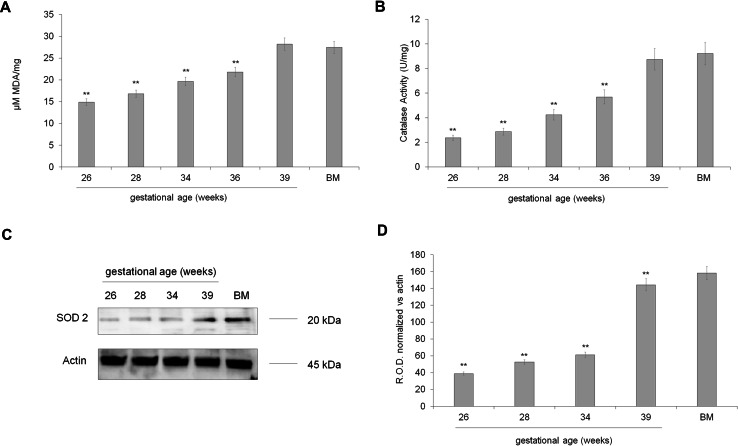
Oxidative stress production
The mitochondrial activity is always associated with the oxidative stress production. Therefore, we evaluated the MDA level, a marker of lipid peroxidation, the catalase activity, and SOD2 expression. As reported in Fig. 4, panel a, MDA concentration was low in preterm UC-MSCs until the 28th week of GA, increased around the 34th week of GA, reaching a maximum around the term stage. On the other side, both catalase activity (Fig. 4, panel b) and SOD2 expression (Fig. 4, panels c, d) increased concurrently with the UC-MSC maturation, suggesting an improvement of the endogenous antioxidant defenses.
Fig. 4.
Oxidative stress production in preterm and term UC-MSCs. a shows the concentration of MDA, a lipid peroxidation marker, in preterm (26–36 weeks of GA), term (39 weeks of GA), and BM-MSCs. b shows the catalase activity in preterm (26–36 weeks of GA), term (39 weeks of GA), and BM-MSCs. In these panels, data are expressed as mean ± SD and are representative of at least five experiments. There is not significance different among term (39 weeks of GA) and BM MSC. Double asterisk indicates a significant difference for p < 0.001 among preterm UC-MSCs with respect to the value of term UC-MSCs (39 weeks of GA) and BM-MSCs. c Reports the expression of SOD2, an antioxidant mitochondrial enzyme. The loaded samples are: preterm UC-MSCs (28, 32 and 34 weeks of GA), term UC-MSCs (39 weeks of GA), and BM-MSCs used as positive control. The densitometric analysis of the WB signals is shown in d. c, d are representative of at least four experiments. Double asterisk indicates a significant difference for p < 0.001 among preterm UC-MSCs with respect to the value of term UC-MSCs (39 weeks of GA) and BM-MSCs
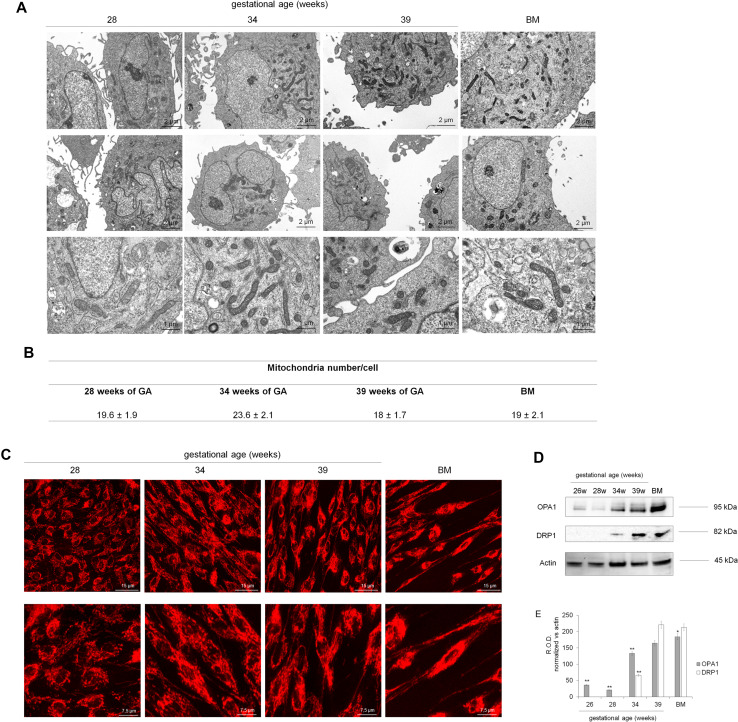
Mitochondria morphology, organization, and biogenesis
To understand whether the limited aerobic metabolism in preterm UC-MSCs depends on either a lower mitochondria density or alterations of mitochondria structures, TEM and confocal microscopy analysis were performed. (Fig. 5, panels a, b). Preterm as well as term and bone marrow MSC showed comparable number of well-developed mitochondria, with rounded or elongated shape, characterized by normally structured cristae and dense matrix. However, the mitotracker staining showed that while in term and BM-MSCs mitochondria were organized, as expected, in a reticulum (Fig. 5, panel c), in preterm samples, they were scattered within the cytoplasm.
Fig. 5.
Mitochondrial morphology and organization of mitochondrial reticulum in preterm and term UC-MSCs. a Reports the TEM analysis to evaluate the morphology of mitochondria in preterm (28 and 35 weeks of GA), in term (39 weeks of GA), and BM-MSCs, while table in b shows the number of mitochondria/cell, expressed as mean ± SD. No relevant difference in mitochondrial morphology or number is observable in the samples. c shows the structure of mitochondrial reticulum, stained with mitotracker deep red. In early preterm sample (28 weeks of GA), mitochondria appear punctuated and not organized in a network. In preterm sample (34 w of GA), the mitochondrial reticulum is more evident and becomes well organized in term (39 weeks of GA) and BM-MSCs. d Reports the expression of OPA1 and DRP1, involved, respectively, in the mitochondria fusion and fission. The loaded samples are: preterm UC-MSCs (26–34 weeks of GA), term UC-MSCs (39 weeks of GA), and BM-MSCs, used as positive control. The densitometric analysis of the WB signals is shown in e. Each panel is representative of at least four experiments. Double asterisk indicates a significant difference for p < 0.001 among preterm UC-MSCs with respect to the value of term UC-MSCs (39 weeks of GA), while asterisk indicates a significant difference for p < 0.005 between term UC-MSCs and BM-MSCs
We have further evaluated, by western blot analyses, the expression of OPA1, a protein belonging to the inner membrane involved in the fusion, and DRP1, a protein expressed in the outer mitochondria membrane involved in the fission. Both proteins were virtually absent in preterm UC-MSCs, while they were expressed in term and BM-MSCs (Fig. 5, panel d). Data are confirmed by the densitometric analysis reported in Fig. 5, panel e.
Role of CLUH in mitochondrial physiology
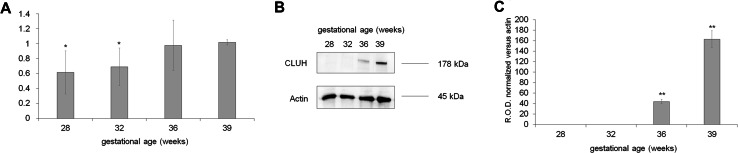
To understand whether the changes in mitochondria endoplasmic reticulum organization correlated with the expression of CLUH, a ribosomal factor involved in the mitochondria biogenesis and distribution [20, 29], RT-PCR, and western blot for CLUH was performed. In preterm samples, CLUH mRNA expression and protein signal were low but increased concurrently with the GA (Fig. 6, panel a–c, respectively).
Fig. 6.
Expression of CLUH in preterm and term UC-MSCs. The figure shows the q-PCR (a) and WB analysis (b signals and c densitometric analysis) to evaluate the expression of CLUH in preterm (26, 28 and 34 weeks of GA) and term (39 weeks of GA) UC-MSCs. The q-PCR data are representative of at least five experiments and asterisk indicates a significant difference for a p < 0.005. The WB data are representative of at least four experiments. Double asterisk indicates a significant difference for p < 0.001 among preterm UC-MSCs with respect to the value of term UC-MSCs (39 weeks of GA)
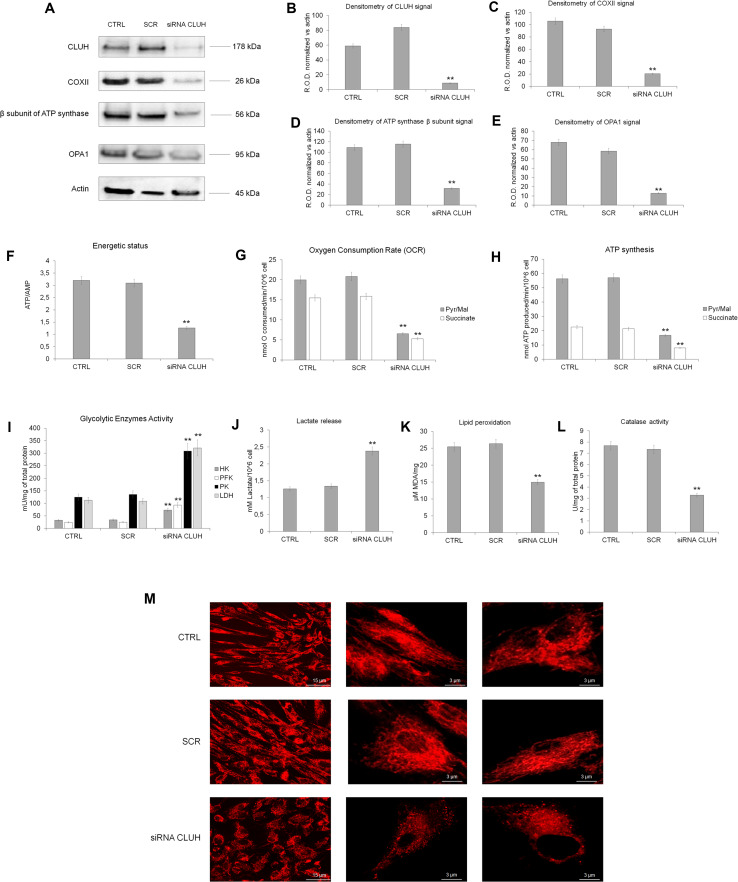
In addition, by silencing CLUH in term UC-MSCs, a shift from aerobic to anaerobic metabolism was documented with a significant decrement of OXPHOS proteins and OPA1 expression compared to the control and the scramble sample (Fig. 7, panels a–e). The decrease of OXPHOS proteins expression accomplished an impairment of ATP/AMP ratio, oxygen consumption, ATP synthesis, MDA production, and catalase activity (Fig. 7, panel f–l). Finally, the mitochondrial reticulum of term UC-MSCs appeared less organized with respect to the controls (Fig. 7, panel m). By contrast, the activities of glycolytic enzymes (HK, PFK, PK, and LDH; Fig. 7, panel i) as well as the lactate production (Fig. 7, panel j) were increased in the CLUH silencing sample in comparison with the controls.
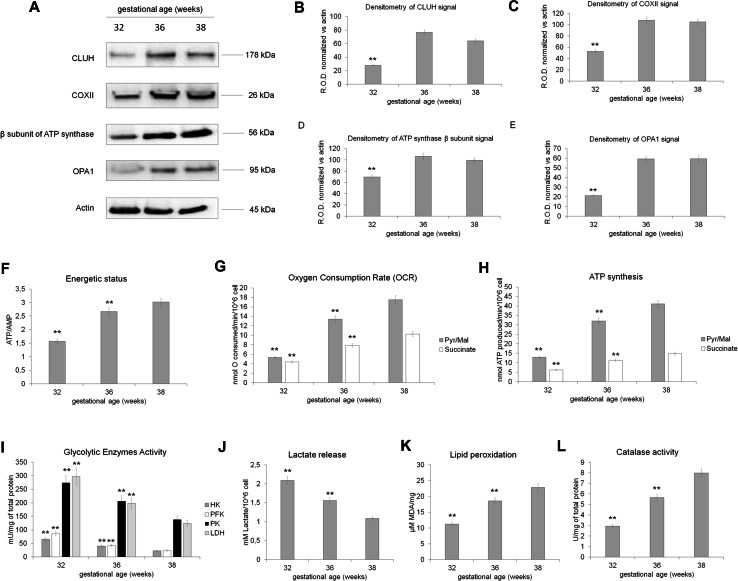
Fig. 7.
Metabolic changes in term MSCs silenced for CLUH. a Reports the WB analysis against CLUH, COX II, β subunit of ATP synthase and OPA1 in term MSCs (CTRL), scramble MSCs (SCR) and term silenced for CLUH (siRNA CLUH). b–e show the relative densitometric analyses normalized on the actin signal showed in a. The WB data are representative of at least three experiments. Double asterisk indicates a significant difference for p < 0.001 among CLUH silencing term UC-MSCs with respect to the value of term UC-MSCs (CTRL). All the metabolic analyses were performed on term MSCs (CTRL), scramble MSCs cells (SCR), and term MSCs silenced for CLUH (siRNA CLUH). The energy status in terms of ATP/AMP ratio is reported in f. The OXPHOS activity, as oxygen consumption and ATP synthesis are shown in g and h, respectively. i Reports the activity of hexokinase (HK), phosphofructokinase (PFK), pyruvate kinase (PK), and lactate dehydrogenase (LDH), while j shows the lactate released. The lipid peroxidation and the catalase activity are reported in k and l, respectively. The organization of mitochondrial reticulum was observed to confocal microscopy, using mitotracker as fluorescence probe (m). Each panel is representative of at least three experiment and double asterisk indicates a significant difference for p < 0.001 among CLUH silencing term UC-MSCs with respect to the value of term UC-MSCs (CTRL)
Effect of hypoxia on the metabolism of preterm and term UC-MSCs
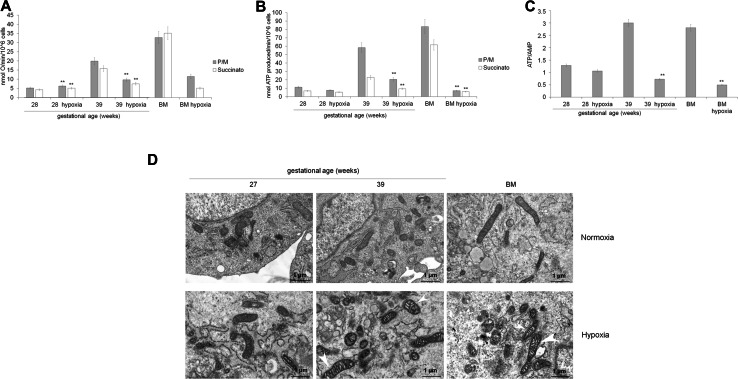
To confirm that preterm UC-MSCs are characterized by an anaerobic metabolism, the preterm and term UC-MSCs, as well as BM-MSCs have been grown in hypoxic condition (1% oxygen tension for 48 h) (Fig. 8). Preterm UC-MSCs grown in hypoxic condition showed comparable values of ATP/AMP ratio and OXPHOS activity with respect the same cells cultured in normoxia (panels a–c). Moreover, also the TEM analysis did not show significant morphologic changes (panel d). By contrast, term UC-MSCs (39 w) and BM-MSCs grown in hypoxia show a great impairment of ATP/AMP ratio (panel a) and aerobic metabolism (panels b, c). The low oxygen tension also caused evident swelling of mitochondrial cristae, which appear less-defined (panel d).
Fig. 8.
Energy metabolism, oxidative status, and mitochondrial morphology in preterm and term UC-MSCs grown in hypoxic condition. All data show a comparison of preterm (28 weeks of GA) and term (39 weeks of GA) UC-MSCs and BM-MSCs grown in normoxic or hypoxic condition (1% O2, for 48 h). a, b report the mitochondrial oxygen consumption and the ATP synthesis trough Fo–F1 ATP synthase. To stimulate the pathway composed by complexes I, III, and IV, pyruvate plus malate (Pyr/Mal; gray columns) was employed; while succinate (white columns) was used to stimulate the pathway formed by complexes II, III, and IV. c shows the ATP/AMP ratio. d Reports the TEM analysis. Mitochondria from UC-MSC (preterm) patients appeared morphologically normal before and after hypoxia treatments. On the contrary, both term than BM-MSC showed mitochondria with swelling cristae (white arrowheads) after hypoxia treatments. (Scale bars, 1 μm). Each Panel is representative of at least five experiments. Double asterisk indicates a p < 0.001 between the sample grown in hypoxia and grown in normoxia
Metabolic changes in endothelial cells form umbilical cord blood of preterm and term newborns
To evaluate whether other tissues display a metabolic shift from anaerobic to aerobic metabolism, during the fetal development, the energy metabolism was evaluated also in endothelial cells.
WB analysis shows that preterm endothelial cells (32 weeks of GA) displayed a lower expression of CLUH, COXII, β subunit of ATP synthase, and OPA1, as reported for preterm UC-MSC, with respect to the endothelial cells of 36 and 38 weeks of GA (Fig. 9, panels a–e). The difference in the expression of OXPHOS proteins suggested that preterm endothelial cells are characterized by an anaerobic metabolism, which shifts to an aerobic respiration proportionally to the GA. In particular, preterm endothelial cells are characterized by a lower ATP/AMP ratio (Fig. 9, panel f), oxygen consumption (Fig. 9, panel g), and ATP synthesis (Fig. 9, panel h) with respect to the term cells. Moreover, also the lipid peroxidation (Fig. 9, panel k) and catalase activity appeared diminished than in term endothelial cells. This low OXPHOS activity is compensated by a high glycolytic rate (Fig. 9, panel i) and lactate production (Fig. 9, panel j).
Fig. 9.
Metabolic characterization of endothelial cells isolated from umbilical cord of preterm and term newborns. a Reports the WB analysis against CLUH, COX II, β subunit of ATP synthase, and OPA1 in early preterm endothelial cells (32 weeks of GA), preterm endothelial cells (36 weeks of GA) and in term endothelial cells (38 weeks of GA). b–e show the relative densitometric analysis normalized on the actin signal showed in a. The WB data are representative of at least three experiments. Double asterisk indicates a significant difference for p < 0.001 among preterm endothelial cells (32 weeks of GA) with respect to the value of term endothelial cells (38 weeks of GA). All the metabolic analyses were performed on early preterm endothelial cells (32 weeks of GA), preterm endothelial cells (36 weeks of GA), and term endothelial cells (38 weeks of GA). The energy status in terms of ATP/AMP ratio is reported in f. The OXPHOS activity, as oxygen consumption and ATP synthesis are shown in g, h, respectively. i Reports the activity of hexokinase (HK), phosphofructokinase (PFK), pyruvate kinase (PK), and lactate dehydrogenase (LDH), while j shows the lactate released. The lipid peroxidation and the catalase activity are reported in k and l, respectively
Discussion
Specific metabolic pathways involved in the energy production influence cell functions. Two main pathways are active in mammalian cells during development: the oxidative phosphorylation (OXPHOS) and the anaerobic glycolysis, which are finely regulated according to cell requirements and specification.
OXPHOS that occurs in mitochondria represents the principal site of aerobic energy production, synthesizing ATP by oxygen consumption [30]. Anaerobic glycolysis is considered as a source of energy that could be advantageous either when a low O2 consumption is required either in limited oxygen availability conditions [31]. Even if anaerobic glycolysis is less efficient than OXPHOS in terms of energy produced, it does not determine an oxidative stress production, limiting the structural damages [32] and the relative aging of the cell [6]. The balance between anaerobic and aerobic metabolism influences the chromatin structure, gene transcription, and maintenance of pluripotency [33].
In this work, we have analyzed the energy metabolism of preterm and term UC-MSCs, observing a metabolic switch from anaerobic to aerobic metabolism, during fetal development. In fact, oxygen consumption and ATP synthesis were very low in early preterm samples and increased around the 34th week of GA at the expense of glycolytic flux (Fig. 3). These data were particularly evident when the mitochondrial function was stimulated with pyruvate plus malate, which induces the OXPHOS activity through the complexes I, III, and IV, the principal pathway of the electron transport chain.
In addition, the evaluation of OXPHOS efficiency showed that in preterm UC-MSCs, oxygen consumption is not completely linked to ATP synthesis, indicating a partially uncoupling (Fig. 3). In fact, in a complete coupling condition, the P/O value after the induction with pyruvate plus malate should be around 2.5 [24], while in early preterm UC-MSCs, the P/O value was around 1.6–2.1 (Fig. 3, panel c).This less efficient mitochondria metabolism determines a high intracellular AMP concentration that decreases during the GA progression (Fig. 2). However, both preterm and term UC-MSCs contained a similar ATP intracellular concentration, indicating that, at any age, cells are able to produce the necessary amount of energy to sustain their functions. In fact, preterm UC-MSCs displayed a higher proliferative potential in comparison with term UC-MSCs (Fig. 1), giving rise to a large progeny when cultured in vitro. Our results suggest that preterm MSC display a metabolic asset very similar to what has been shown in ES or iPS that mainly use a glycolytic metabolism without impairment of their proliferation capacity [34].This pathway seems associated with higher protection to oxidative stress and DNA damage [6]. The same results were observed by evaluating the energy metabolism in endothelial cells isolated from umbilical cord of preterm and term newborn (Fig. 9).
Interestingly, we observed that low aerobic metabolism in preterm UC-MSCs is not due to an alteration of mitochondrial morphology or to a low number of mitochondria within the cell. Rather, it seems linked to the lack of an organized mitochondria reticulum (Fig. 5), a complex structure that renders the oxidative metabolism highly efficient, providing the necessary connection between biochemical parameters and qualitative features fundamental for the functionality of living cells [35]. In fact, only term UC-MSCs and BM-MSCs show such an organized mitochondria reticulum that is not observed in preterm cells. The immaturity of the mitochondria apparatus was confirmed by the expression of OPA1 and DRP1, two proteins involved in fusion/fission processes, which increased proportionally with the GA (Fig. 5). Modification of bioenergetic profile was observed also in MSCs from mice, during the commitment, due to the changes in mitochondrial morphology and fission/fusion process, suggesting a central role for mitochondrial dynamics in the maintenance/commitment of MSCs [36]. In particular, mitochondrial elongation and an increased expression of fusion proteins were observed in the early steps of adipo and osteogenesis differentiation, while chondrogenesis has been characterized by a fragmented mitochondrial phenotype, associated with an increment of fission proteins expression [36].
These metabolic changes seem under the control of CLUH, a cytosolic messenger ribonucleic acid (RNA; mRNA)-binding protein involved in the mitochondria biogenesis and distribution inside the cell [20]. As shown in Fig. 6, both the mRNA and the protein expression of CLUH appeared less represented in preterm sample and increased proportionally with the GA.
To verify whether the metabolic switch during the fetal development is led by CLUH, a silencing of this protein was performed in term MSCs (Fig. 7). Data show that CLUH siRNA-treated term MSCs displayed an energy metabolism and a production of oxidative stress similar to that observed in the preterm MSCs, confirming the hypothesis that the hypo-function of mitochondria in preterm MSCs is linked to the low CLUH expression. These results further extend the observation by other authors [20, 29], who described an association between CLUH expression and mitochondrial function and corroborate the concept that CLUH favors the organization of mitochondrial reticulum promoting the oxidative metabolism.
The aerobic metabolism is always associated with an increment of oxidative stress production [37, 38]. However, the proportional increment of malondialdehyde (MDA) level, during the metabolic switch, was balanced by the catalase activity and the expression of SOD2 (Fig. 4), suggesting that UC-MSCs have sufficient endogenous antioxidant defenses. On the other hand, it is important to note that early preterm UC-MSCs produce MDA, despite the high glycolytic metabolism. This lipid peroxidation may be due to the uncoupled OXPHOS metabolism, as demonstrated by the low P/O value (Fig. 3). In fact, one of the causes of the oxidative stress is the uncoupling between the electron transport chain and the ATP synthase activity [39], because, in this condition, the oxygen consumption rate considerably increases, favoring the formation of reactive oxygen species (ROS).
It is important to consider that the results described herein have been obtained on UC-MSCs grown in normoxic condition, while in vivo they reside in hypoxic environments [40]. In fact, a comparison of arterial blood pO2 has shown that fetal oxygenation is close to the values that determined a hypoxic condition in adult [41, 42]. This condition seems to play a pivotal role in the fetal development [43, 44], considering that hypoxia plays a critical role in the formation and development of the heart [43], through the HIF modulation which regulates numerous functions, such as energy metabolism, erythropoiesis, cell survival and death, vascularization, angiogenesis, and differentiation [44–46]. However, it was observed that UC-MSCs expanded under normoxia seem to display a high O2 consumption rate, suggesting that glycolysis may be an environmental adaptation for UC-MSCs [47]. To evaluate whether metabolic switch observed in UC-MSCs depends by the growth conditions, experiments were performed also in hypoxic condition (1% of O2, for 48 h). As reported in Fig. 8, the ATP/AMP ratio (panel c), the OXPHOS activity (panels a, b), and the mitochondrial morphology (panel d) of preterm sample did not show significant difference in comparison with those maintained in normoxia. By contrast, in term and BM-MSCs grown in hypoxic condition, the same parameters resulted as severely impaired, suggesting that the metabolism of these cells is “irreversibly oxygen-dependent”.
Therefore, if we consider the UC-MSCs as a model of fetal cells [48, 49], data reported herein suggest that, in preterm newborn tissues, the immature OXPHOS machinery, not able to use correctly the oxygen, determines an enhancement of the oxidative stress and increased inflammation response and tissue damage. In fact, it is known that the exposure of preterm newborns to oxygen leads to the accumulation of reactive oxygen species (ROS), which results in the generation of hydrogen and lipid peroxides [50], causing tissue damages [51].
In conclusion, our study provides new information on the production of energy and mitochondrial metabolism during the passage from fetal to adult life, and discloses the changes necessary to optimize the mitochondria function; moreover, it extends the notion that CLUH plays a role in such organization. These data shed new light on the pathophysiology of damages occurring in preterm newborns [51, 52] and may provide useful information for the clinical management of these patients to individuate new protective therapeutic strategies aiming to reduce entity and number of complications of prematurity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 Suppl Figure 1 Phenotypical characterization of UC-MSC from preterm and term neonates. Phenotypical characterization of UC-MSC from preterm and term neonates. Panel a shows histograms of flow cytometry analyses demonstrating the expression of surface molecules of preterm MSC (preterm) compared with term MSC (term) and normal bone marrow-derived MSC (NBM). b Reports a representative flow cytometric analysis of Nestin, OCT3/4, NANOG, and SSEA-4 expression by preterm and term MSC. The gray histograms show the region of fluorescent intensity of the specific antibody and bold empty histograms represent staining of respective isotype-matched control immunoglobulins. Values are represented as percentage (%) of positive cells (TIFF 10330 kb)
Acknowledgements
This work was supported by funds from Cinque per mille e Ricerca Corrente, Ministero della Salute, to Istituto Giannina Gaslini; a Compagnia di San Paolo Grant (2014AAI637.U/812/AR pv 2013.0958 to F.F) and a Grant FIRB (2012# RBFR1299K0_002 to C.F.). The authors are indebted to Dr. Federica Raggi for providing the hypoxia incubator.
References
- 1.Ding D-C, Chang Y-H, Shyu W-C, Lin S-Z. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24:339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 2.Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao S, Wehner R, Bornhäuser M, et al. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2010;19:607–614. doi: 10.1089/scd.2009.0345. [DOI] [PubMed] [Google Scholar]
- 4.Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahlqvist KJ, Suomalainen A, Hämäläinen RH. Stem cells, mitochondria and aging. Biochim Biophys Acta. 2015;1847:1380–1386. doi: 10.1016/j.bbabio.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Ocampo A, Izpisua Belmonte JC. Stem cells. Holding your breath for longevity. Science. 2015;347:1319–1320. doi: 10.1126/science.aaa9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C-T, Hsu S-H, Wei Y-H. Upregulation of mitochondrial function and antioxidant defense in the differentiation of stem cells. Biochim Biophys Acta. 2010;1800:257–263. doi: 10.1016/j.bbagen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Lonergan T, Bavister B, Brenner C. Mitochondria in stem cells. Mitochondrion. 2007;7:289–296. doi: 10.1016/j.mito.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker GC, Acsadi G, Brenner CA. Mitochondria: determinants of stem cell fate? Stem Cells Dev. 2009;18:803–806. doi: 10.1089/scd.2009.1806.edi. [DOI] [PubMed] [Google Scholar]
- 10.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 11.Shum LC, White NS, Mills BN, et al. Energy metabolism in mesenchymal stem cells during osteogenic differentiation. Stem Cells Dev. 2016;25:114–122. doi: 10.1089/scd.2015.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folmes CDL, Nelson TJ, Martinez-Fernandez A, et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antico Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC. Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal. 2012;16:1150–1180. doi: 10.1089/ars.2011.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panfoli I, Ravera S, Podestà M, et al. Exosomes from human mesenchymal stem cells conduct aerobic metabolism in term and preterm newborn infants. FASEB J. 2016;30:1416–1424. doi: 10.1096/fj.15-279679. [DOI] [PubMed] [Google Scholar]
- 15.Almgren M, Schlinzig T, Gomez-Cabrero D, et al. Cesarean delivery and hematopoietic stem cell epigenetics in the newborn infant: implications for future health? Am J Obstet Gynecol. 2014;211:502.e1–502.e8. doi: 10.1016/j.ajog.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Capelli C, Gotti E, Morigi M, et al. Minimally manipulated whole human umbilical cord is a rich source of clinical-grade human mesenchymal stromal cells expanded in human platelet lysate. Cytotherapy. 2011;13:786–801. doi: 10.3109/14653249.2011.563294. [DOI] [PubMed] [Google Scholar]
- 17.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 18.Sessarego N, Parodi A, Podestà M, et al. Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica. 2008;93:339–346. doi: 10.3324/haematol.11869. [DOI] [PubMed] [Google Scholar]
- 19.Podestà M, Bruschettini M, Cossu C, et al. Preterm cord blood contains a higher proportion of immature hematopoietic progenitors compared to term samples. PLoS ONE. 2015;10:e0138680. doi: 10.1371/journal.pone.0138680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Schatton D, Martinelli P, et al. CLUH regulates mitochondrial biogenesis by binding mRNAs of nuclear-encoded mitochondrial proteins. J Cell Biol. 2014;207:213–223. doi: 10.1083/jcb.201403129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bianchi G, Martella R, Ravera S, et al. Fasting induces anti-Warburg effect that increases respiration but reduces ATP-synthesis to promote apoptosis in colon cancer models. Oncotarget. 2015;6:11806–11819. doi: 10.18632/oncotarget.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravera S, Vaccaro D, Cuccarolo P, et al. Mitochondrial respiratory chain complex I defects in Fanconi anemia complementation group A. Biochimie. 2013;95:1828–1837. doi: 10.1016/j.biochi.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Ravera S, Aluigi MG, Calzia D, et al. Evidence for ectopic aerobic ATP production on C6 glioma cell plasma membrane. Cell Mol Neurobiol. 2011;31:313–321. doi: 10.1007/s10571-010-9624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinkle PC. P/O ratios of mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 2005;1706:1–11. doi: 10.1016/j.bbabio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Ravera S, Bartolucci M, Calzia D, et al. Tricarboxylic acid cycle-sustained oxidative phosphorylation in isolated myelin vesicles. Biochimie. 2013;95:1991–1998. doi: 10.1016/j.biochi.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Ravera S, Bartolucci M, Cuccarolo P, et al. Oxidative stress in myelin sheath: the other face of the extramitochondrial oxidative phosphorylation ability. Free Radic Res. 2015 doi: 10.3109/10715762.2015.1050962. [DOI] [PubMed] [Google Scholar]
- 27.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 28.Schefe JH, Lehmann KE, Buschmann IR, et al. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s CT difference” formula. J Mol Med (Berl) 2006;84:901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 29.Schatton D, Pla-Martin D, Marx M-C, et al. CLUH regulates mitochondrial metabolism by controlling translation and decay of target mRNAs. J Cell Biol. 2017;216:675–693. doi: 10.1083/jcb.201607019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 31.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondoh H, Lleonart ME, Bernard D, Gil J. Protection from oxidative stress by enhanced glycolysis; a possible mechanism of cellular immortalization. Histol Histopathol. 2007;22:85–90. doi: 10.14670/HH-22.85. [DOI] [PubMed] [Google Scholar]
- 33.Moussaieff A, Kogan NM, Aberdam D. Concise review: Energy metabolites: key mediators of the epigenetic state of pluripotency. Stem Cells. 2015;33:2374–2380. doi: 10.1002/stem.2041. [DOI] [PubMed] [Google Scholar]
- 34.Daley GQ. Stem cells and the evolving notion of cellular identity. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140376. doi: 10.1098/rstb.2014.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westermann B. Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys Acta. 2012;1817:1833–1838. doi: 10.1016/j.bbabio.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 36.Forni MF, Peloggia J, Trudeau K, et al. Murine mesenchymal stem cell commitment to differentiation is regulated by mitochondrial dynamics. Stem Cells. 2016;34:743–755. doi: 10.1002/stem.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai D-F, Chiao YA, Marcinek DJ, et al. Mitochondrial oxidative stress in aging and healthspan. Longev Heal. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging 11. This article is dedicated to the memory of our dear friend, colleague, and mentor Lars Ernster (1920–1998), in gratitude for all he gave to us. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 39.Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta Bioenerg. 2003;1604:77–94. doi: 10.1016/S0005-2728(03)00027-6. [DOI] [PubMed] [Google Scholar]
- 40.Chen C-T, Shih Y-RV, Kuo TK, et al. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence J, Xiao D, Xue Q, et al. Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J Pharmacol Exp Ther. 2008;324:331–341. doi: 10.1124/jpet.107.132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds JD, Penning DH, Dexter F, et al. Ethanol increases uterine blood flow and fetal arterial blood oxygen tension in the near-term pregnant ewe. Alcohol. 1996;13:251–256. doi: 10.1016/0741-8329(95)02051-9. [DOI] [PubMed] [Google Scholar]
- 43.Patterson AJ, Zhang L. Hypoxia and fetal heart development. Curr Mol Med. 2010;10:653–666. doi: 10.2174/156652410792630643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Covello KL, Kehler J, Yu H, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Semenza GL, Agani F, Iyer N, et al. Regulation of cardiovascular development and physiology by hypoxia-inducible factor 1. Ann N Y Acad Sci. 1999;874:262–268. doi: 10.1111/j.1749-6632.1999.tb09241.x. [DOI] [PubMed] [Google Scholar]
- 46.Adelman DM, Gertsenstein M, Nagy A, et al. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pattappa G, Heywood HK, de Bruijn JD, Lee DA. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J Cell Physiol. 2011;226:2562–2570. doi: 10.1002/jcp.22605. [DOI] [PubMed] [Google Scholar]
- 48.Wyburn GM. The formation of the umbilical cord and the umbilical region of the anterior abdominal wall. J Anat. 1939;73:289–310. [PMC free article] [PubMed] [Google Scholar]
- 49.Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248–269. [PMC free article] [PubMed] [Google Scholar]
- 50.Lavoie PM, Lavoie J-C, Watson C, et al. Inflammatory response in preterm infants is induced early in life by oxygen and modulated by total parenteral nutrition. Pediatr Res. 2010;68:248–251. doi: 10.1203/PDR.0b013e3181eb2f18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Donovan DJ, Fernandes CJ. Free radicals and diseases in premature infants. Antioxid Redox Signal. 2004;6:169–176. doi: 10.1089/152308604771978471. [DOI] [PubMed] [Google Scholar]
- 52.Perrone S, Tataranno LM, Stazzoni G, et al. Brain susceptibility to oxidative stress in the perinatal period. J Matern Fetal Neonatal Med. 2015;28(Suppl 1):2291–2295. doi: 10.3109/14767058.2013.796170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 Suppl Figure 1 Phenotypical characterization of UC-MSC from preterm and term neonates. Phenotypical characterization of UC-MSC from preterm and term neonates. Panel a shows histograms of flow cytometry analyses demonstrating the expression of surface molecules of preterm MSC (preterm) compared with term MSC (term) and normal bone marrow-derived MSC (NBM). b Reports a representative flow cytometric analysis of Nestin, OCT3/4, NANOG, and SSEA-4 expression by preterm and term MSC. The gray histograms show the region of fluorescent intensity of the specific antibody and bold empty histograms represent staining of respective isotype-matched control immunoglobulins. Values are represented as percentage (%) of positive cells (TIFF 10330 kb)