Abstract
The reproductive life span in women starts at puberty and ends at menopause, following the exhaustion of the follicle stockpile termed the ovarian reserve. Increasing data from experimental animal models and epidemiological studies indicate that exposure to a number of ubiquitously distributed reproductively toxic environmental chemicals (RTECs) can contribute to earlier menopause and even premature ovarian failure. However, the causative relationship between environmental chemical exposure and earlier menopause in women remains poorly understood. The present work, is an attempt to review the current evidence regarding the effects of RTECs on the main ovarian activities in mammals, focusing on how such compounds can affect the ovarian reserve at any stages of ovarian development. We found that in rodents, strong evidence exists that in utero, neonatal, prepubescent and even adult exposure to RTECs leads to impaired functioning of the ovary and a shortening of the reproductive lifespan. Regarding human, data from cross-sectional surveys suggest that human exposure to certain environmental chemicals can compromise a woman’s reproductive health and in some cases, correlate with earlier menopause. In conclusion, evidences exist that exposure to RTECs can compromise a woman’s reproductive health. However, human exposures may date back to the developmental stage, while the adverse effects are usually diagnosed decades later, thus making it difficult to determine the association between RTECs exposure and human reproductive health. Therefore, epidemiological surveys and more experimental investigation on humans, or alternatively primates, are needed to determine the direct and indirect effects caused by RTECs exposure on the ovary function, and to characterize their action mechanisms.
Keywords: Premature menopause, Earlier menopause, Reproductive lifespan, Endocrine disrupting chemicals
Introduction
The ovaries of women are able to generate fertilizable oocytes for a defined period of time, spanning from puberty to menopause. This period lasts about 35 years starting at the onset of puberty, estimated from the time of the first menstruation (menarche: between 13 and 15 years of age), to when menstruation stops, typically between 49 and 52 years of age. This limited timeframe is thought to be due to the fact that mammalian females harbor a fixed stockpile of oocytes inherently, which progressively decreases with age through atresia and ovulation until nearing exhaustion [1–3]. Even if, as suggested by some scientists, the mammalian ovary maintains the ability to form new oocytes from an ovarian stem cell population (oogonia stem cells, OSCs or female germ stem cells, FGSCs) [4, 5], the ovarian follicle pool and the reproductive capabilities of mammalian females invariably decrease and end with age. A recent study estimated that, on average, women lose 90% of their oocytes by the time they are 30 years old, and have only about 3% remaining by the time they are 40 years old, while at menopause only 100–1000 oocytes remain [6, 7]. In a few mammalian species, including humans, the cessation of reproductive capabilities is mainly contributed by the continuous consumption of ovarian follicles with the ovary. The reason for this is unknown, but an increasing number of conditions that can accelerate the depletion of the ovary reserve, the trend of later-in-life pregnancies, and the current prolonging of the post-reproductive lifespan in women, require enhancing our understanding of the mechanisms controlling the establishment and dynamics of the ovarian reserve.
It is estimated that by 20 weeks of gestation the developing human foetal ovaries contain the maximum number of about 5 million oogonia/oocytes. For reasons that remain unclear, more than two-thirds of these germ cells will degenerate in the following months, leaving a much smaller population of oocytes at birth. Oocyte numbers at birth range anywhere from half to one million [8]. This stockpile of oocytes (the ovarian reserve) is housed in structures called primordial follicles (PFs). Although several follicles start to develop in waves prior to each ovulation, generally only one achieves ovulation to release a fertilizable oocyte with the rest being removed through atresia. During her reproductive life, a woman ovulates about 400 oocytes, approximately 1% of the follicles making up the ovarian reserve [9, 10]. As indicated above, the exhaustion of the ovarian reserve leads to menopause in women, which is associated with the cessation of menstruation [11–19]. The mean age at the onset of menopause varies due to many factors including socio-demographics, health status and others [20, 21]. Despite the fact that an increasing number of women worldwide are suffering from early menopause and premature ovarian failure (POF) (around 0.4% under the age of 35 and 1% under the age of 40 [22]), there is still little information concerning the etiologies of such pathologies [23]. Genetic, autoimmune, infectious, metabolic and iatrogenic or environmental factors all appear to potentially play a role [24–29]. Whatever the factor/s, such conditions are almost invariably associated with exhaustion of the ovarian reserve. Two main reasons can be at the origin of such a depletion: (1) insufficient quantity of germ cells formed during the establishment of the ovarian reserve (mainly in the prenatal period), resulting in a deficient ovarian follicle pool at birth and/or puberty, and (2) accelerated depletion of the established ovarian reserve, including increased activation of dormant PFs and enhanced follicular atresia [25]. The number of ovarian follicles in the ovary (ovarian reserve) and the ovarian follicle depletion rate is clearly a key determinant for the onset of menopause, and factors affecting such balance are crucial in determining the onset time.
In this regard, numerous papers demonstrate that RTECs can affect the reproductive health of female mammals and suggest such exposures can lead to POF in women [25, 30, 31]. One class of the most-studied RTECs is endocrine disrupting chemicals (EDCs) as these compounds have been shown to interfere with hormone-related signaling, however, it is important to note that there are many other environmental chemicals that can induce ovarian toxicology independent of hormone signaling [32]. Evidence is increasing that certain ovary pathologies and infertility conditions in women might be due to the exposure to RTECs during prenatal, pubertal or adult life [33]. Of particular concerns is that RTECs are ubiquitously distributed in daily human life. Generally, they are man-made and found in various materials such as pesticides, metals, personal care products and as additives or as food contaminants [33]. The most common RTECs in our daily life include bisphenol A (a commonly used plasticizer), methoxychlor (an organochlorine pesticide), phthalates (plasticizers), genistein (a soy isoflavone), acetaminophen/paracetamol (drug used for relieving pain and fever), zearalenone (ZEA, a fungal mycotoxin), polycyclic aromatic hydrocarbons (PAHs) and others [34–40]. Considering the high incidence of human exposure to RTECs, it is imperative to figure out if and through which mechanisms an RTEC can induce ovarian dysfunctions and contribute to POF and eventually to infertility. However, the analysis of the causal relationship between exposure to RTECs and POF in women is problematic since such pathology is diagnosed at an advanced age, mainly in their 40s–50s, while the exposure leading to the pathology might occur decades earlier during prepubescence and even during prenatal stages. Moreover, the chemical nature of RTECs can be difficult to define and characterize at the cellular and molecular levels. For these reasons, studies performed in rodents and primates with shorter reproductive lifespans can provide useful insights into the aetiology of women suffering from POF and in identifying environmental chemicals, their cellular targets and molecular modes of action.
In the present review, we focused on recent studies looking at the impact of selected RTECs on the development of the mammalian ovary and folliculogenesis. In particular, we focus on recapitulating how such chemicals affect the establishment and dynamics of the ovarian reserve, central in determining the reproductive lifespan and the onset of menopause in women.
Establishment of the ovarian reserve
The emergence of the germline: the specification and migration of the primordial germ cells
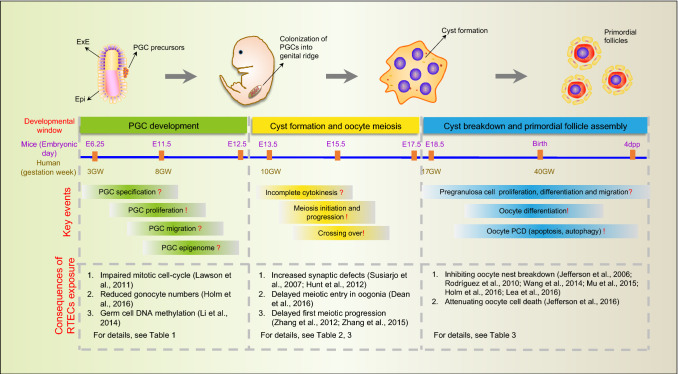
The establishment of ovarian reserve traces back to embryonic day 5.5–6.5 in mice and between the second and third weeks of embryo development in human, during which the precursors of the oogonia, the primordial germ cells (PGCs), are specified in extraembryonic tissues before the beginning of gastrulation [41, 42]. After their formation, PGCs undergo mitosis and begin to move towards the genital ridges developing inside the embryo proper [42–44]. Conceivably, any defects in these processes may result in a reduced germ cell population responsible for establishing the ovarian reserve, resulting in adverse effects on the future fertility and the ovarian-dependent physiology of the female.
It is to be pointed out that during this period, PGC development relies on many epigenetic changes involving both histone modifications, X reactivation, imprinting erasure and global DNA demethylation, likely crucial for reprogramming their genome and assuring the correct establishment of the germline [45]. Evidence is growing that aberrant epigenetic modifications can be detrimental not only for the oogenesis and fertility of the affected individuals but also for the next generations suggesting that they can be transferred through the germline [46–48].
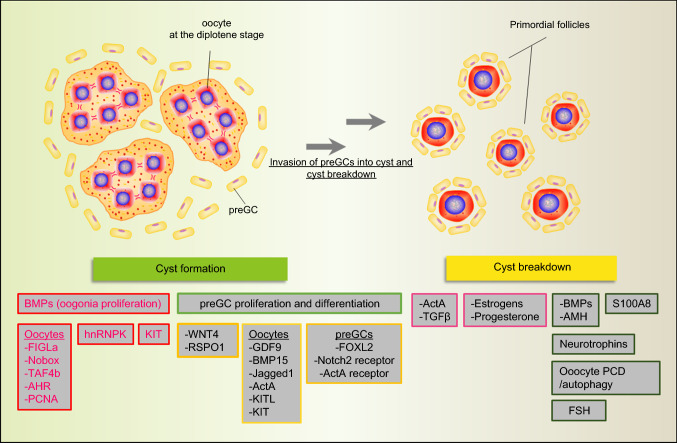
Germ cell cyst formation and the beginning of meiosis
When PGCs migrate into the gonadal ridges, they undergo mitosis with incomplete cytodieresis, which gives rise to clonal germ cell groups, termed germ cell “cysts” or “nests”, featuring up to 30 cells linked by intercellular cytoplasmic bridges [49, 50]. In mice and probably in humans, external stimuli from the surrounding and/or neighboring (mesonephros) tissues including retinoic acid (RA), Activin A (Act A), and perhaps WNT4 and RSPO1, induce mitotic oogonia in cysts to enter meiosis and become primary oocytes [51–58]. One of the key genes controlling meiotic entry during meiotic prophase I (MPI) induced by RA in PGCs/oogonia is Stimulated by retinoic acid 8 (Stra8) [51, 52, 59, 60]. STRA8 promotes the expression of the synaptonemal complex proteins (SCPs) [61, 62] and is involved in the initiation of homologous recombination through elevating the expression of the DNA meiotic recombinase 1 (DMC1), and meiotic recombination protein SPO11 [59, 60]. Ablation of Stra8, or defects in DNA homologous recombination, impair the establishment of the ovarian reserve. Finally, in line with these observations, studies in humans found that mutations of DMC1, SPO11, MSH4 (MutS homolog protein), and MSH5 correlate with women POI [63].
Oocyte cyst breakdown and the formation of primordial follicles
The oocyte cyst breakdown (CBD) and PF formation are prerequisites for the establishment of the ovarian reserve. Synchronized development of oocytes and GCs, as well as their mutual communication are required for efficient CBD and the subsequent PF formation. Both in mouse and human, mutations of WNT4 and RSPO1 signaling genes induce failure of preGC differentiation, impaired CBD, and finally result in POF [64]. In addition, failure of oocytes to reach the diplotene stage impairs CBD and PF formation as indicated by an SCP1 knockdown assay [65]. Moreover, oocyte-specific transcription factor Figlα mutations in woman has been reported to be correlated with POF [66–68]. Two other oocyte transcription factors, TAF4b and AHR, also appears to play a role in CBD and PF formation both in mice and humans [69, 70]. Finally, siRNA knockdown of the hnRNPK (nuclear heterogeneous ribonucleoprotein K) in the rat, caused a block in CBD and PF formation [71].
Signal exchange between oocytes and preGCs are also implicated in such processes (Fig. 1), such as BMP signaling, Notch signaling, JNK signaling and others [72, 73]. Other factors and compounds such as the KITL/KIT system [74], TGFβ and its family members ActA [75], Inhibin [76], Follistatin [77], anti-Mullerian hormone [78], estrogen [79], progesterone [80] and finally FSH have been found to play a role in CBD and PF assembly and the references are summarized in Fig. 1 as several reviews focusing on them are readily available [3, 50, 81, 82].
Fig. 1.
Various factors involved in germ cell cyst formation and breakdown. For cyst breakdown, compounds exerting negative and positive action are displayed in red and green rectangles, respectively
Programmed cell death in foetal and early postnatal oocytes
In many mammalian species, large numbers of oocytes are removed through atresia during CBD [83]. It has been reported that germ cell death during the fetal and early postnatal period occurs following various types of programmed cell death (PCD) that can be apoptotic or not apoptotic [84]. Likewise, oocytes appear to degenerate via several mechanisms during CBD, including classical Caspase 2-dependent apoptosis [85, 86], cytoprotective autophagy [87, 88], and direct extrusion from the ovaries [87, 89].
It is thought that CBD may be reliant on oocyte death as these two processes are temporally correlated in the mouse ovary [85]. As evidenced by the fact that a lack of the pro-apoptotic BCL2 family protein BAX led to a decrease in oocyte death and a reduction in CBD [90, 91]. Furthermore, inhibition of autophagy causes germ cell loss during the CBD period, suggesting autophagy may act as a protection or survival mechanism during this process [92]. On the contrary, exacerbated autophagy, due to the deprivation of nutrients at birth, can lead to increased apoptosis of oocytes and preGCs [93, 94]. In this regard, ablation of Ngf or its Ntrk1 and Ntrk1 receptor genes caused a permanence of germ cell cysts and reduced PFs [95, 96].
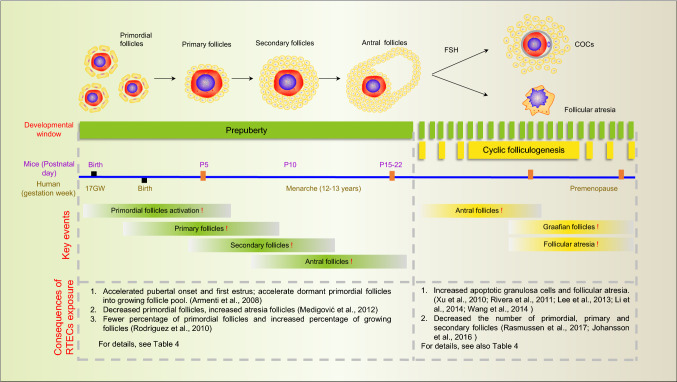
Exhaustion of the ovarian reserve: initial recruitment, cyclic recruitment and follicular atresia
As reported above, mammalian reproductive aging is determined by the exhaustion of the ovarian reserve in women and the exhaustion of ovarian follicle pool generally means the onset of menopause [3]. Three fates are possible for the PFs in the ovarian reserve: (1) keep dormant, which constitutes the ovarian follicle pool; (2) directly undergo apoptosis, which results in female aging; (3) to be activated from the dormant state and undergo follicular activation, which maintains hormonal homeostasis in women and during menstrual cycles. The balance between the quiescence, atresia, and recruited PFs is critical in maintaining a proper reproductive lifespan.
During initial activation, intraovarian and/or other unknown factors stimulate an undetermined number of PFs to initiate growth. The activation of mTORC1 in GCs seems to be an important aspect of PF activation, as enhanced mTORC1 promotes the expression of KITL in dormant oocytes [97, 98]. Besides, many growth factors, such as LIF [99], bFGF [100] and PDGF [101] have also been reported to be efficient in regulating the activation of PFs in vitro. In comparison, reproductive lifespan is more attributable to follicle atresia by PCD with two main factors leading to PF degeneration: shortage of survival factors affecting GCs, oocytes or both and irreparable damage of the oocyte DNA. Studies performed in animal models have suggested that the viability of primordial and primary follicles can be determined by an insufficient availability of growth factors such as KITL, EGF, IGF-1, LIF, or GDF9 [102]. In mice, lack of GDF9 prevents the development of the follicles from the primary to early secondary stages, causing their atresia [103]. Along with this the BCL2 family member MCL1 has been demonstrated to be the essential survival factor required for the maintenance of the PF pool, growing follicle survival and effective oocyte mitochondrial bioenergetics [90, 104].
Effects of RTECs throughout oogenesis and folliculogenesis
The notion that exposure to an RTEC or mixture of RTECs during each of the above-reviewed stages of oogenesis can alter the establishment of the ovarian reserve and the dynamics of its exhaustion is supported by a growing body of evidence [105] (Figs. 2 and 3). In addition, the possibility of transgenerational transmission of epigenetic modifications caused by such exposure that are deleterious for health and reproduction has also been advanced by some studies [106, 107]. However, the identification and the effects on reproduction of RTECs for women remain elusive for several reasons including the difficulty of performing experiments on female germ cells, the scarcity of systematic and reliable epidemiological studies, and a problem in linking the aetiology of certain ovary pathologies such as POI diagnosticated at advanced ages from an exposure that possibly occurred decades earlier [108].
Fig. 2.
Developmental windows sensitive to RTECs during establishment of the ovarian reserve
Fig. 3.
Developmental windows sensitive to RTECs during folliculogenesis
Effects of RTECs on primordial germ cell development
Currently, studies on the effects of RTECs on PGC development are relatively limited (Table 1). By analyzing PGC-related gene expression, researchers demonstrated that in utero exposure to RTECs during the early PGC development stage decreased the expression of mitotic genes and germ cell-specific genes, such as Stra8, Dazl and Nobox [109, 110]. In another study, intrauterine exposure by gavage of pregnant mice to acetaminophen/paracetamol, a widely used drug with EDC effects, negatively affected germ cells within fetal the ovaries as inferred by decreased levels of the transcripts for Mvh [111]. These together demonstrated that environmental chemical exposure during the early PGC differentiation stage may interfere with key PGC related gene expression.
Table 1.
RTEC exposure impairs PGC development
| References | Type of EDC | Exposure stage (dpc) | Exposure | Affected processes |
|---|---|---|---|---|
| La Sala et al. [113] (CD-1 mice) | 15–30 mg/kg; 10–20 nM lindane | 11.5 | Gavage | Increased apoptosis |
| Lawson et al. [110] (C57BL/6J mice) | 20 ng/g BPA | 11.5–12.5 | Oral | Down-regulation of mitotic cell-cycle genes |
| Zhang et al. [116] (CD1 mice) | 40–160 μg/kg BPA | 0.5–12.5 | Oral | Decreased expression of germ cell specific genes; DNA methylation |
| Li et al. [154] (CD-1 mice) | 40 μg/kg DEHP | 0.5–18.5 | Oral | DNA methylation (female and male transgenerational transmitted) |
| Holm et al. [111] (C57BL/6J mice) | 50, 150 mg/kg paracetamol | 7–13.5 | Gavage | Decreased proliferation and/or/increased apoptosis |
Some EDCs, termed xenoestrogens, have been reported to perturb the expression of the two canonical mammalian estrogen receptors ERα and ERβ [112]. Under in vitro conditions, the AKT (protein kinase B) activity, central for signaling of several growth factors, significantly increased in mouse PGCs upon exposure to ZEA while it decreased following exposure to lindane (γ-HCH) [113]. Other studies indicated that EDC exposure may also exert their effects via affecting germ cell adhesion [114]. Finally, Del-Mazo et al. [115] screened differentially expressed genes altered by MEHP exposure in 12.5–13.5 dpc female mouse PGCs in vitro and found that MEHP induced differentially expressed genes mainly enriched in cell survival pathways.
Increasing research has demonstrated that RTECs might interfere with epigenetic modification and disturb germ cell development [107]. In line with such a possibility, Zhang and colleagues [116] reported that exposure of pregnant mice from 0.5 to 12.5 dpc to BPA resulted in decreased DNA methylation of imprinted genes in 12.5 dpc fetal PGCs and the germ cell-specific genes expression and increased ERα and ERβ expression in female and male PGCs. Similar alteration of DNA methylation, although at a subsequent developmental stage, was reported by Chao and colleagues, they investigated the methylation status of two imprinted genes Igf2r and Peg3 using bisulfite sequencing and found that these two genes were hypo-methylated after BPA exposure, besides, elevated ER expression at both mRNA and protein levels was also demonstrated [117]. Even more worrisome is the fact that aberrant methylation status in germ cells may be inherited by subsequent generations through transgenerational mechanisms. Several studies have reported that EDCs induced epigenetic modification can be inherited by the next generation in female rats and, strikingly, showed POF-like phenotypes [118], thus, it is plausible that RTECs can affect epigenetic marks which are exempt from epigenetic erasure [119, 120]. Research from Skinner’s group supports this idea and showed that exposure to the fungicide vinclozolin (VCZ) during PGC development induced abnormal phenotypes that can be inherited even to the F4 offspring in male germ cells [121]. Similar defects in the F1–F4 offspring, were reported following mouse maternal exposure to DEHP (di 2-ethyl hexyl phthalate) from 7 to 14 dpc [122]. Likewise, Li et al. [123], investigated the effects of DEHP exposure during pregnancy in mice on DNA methylation of imprinted genes and found that the methylation status of imprinted genes were altered as early as 12.5 dpc PGCs, besides, these altered DNA methylations can be inherited in the growing oocytes of the F1 and F2 generations. Recently, Pocar and Zhou et al. [124, 125], reported that maternal exposure to DEHP might be associated with reproductive dysfunctions across several generations, indicating that DEHP exposure might interfere with transgenerational genetic information transmission between generations.
RTECs effects the meiosis of foetal oocytes
It is commonly accepted that meiosis is susceptible to RTECs disturbance [126] (Table 2). Susiarjo et al. [127] investigated the effects of BPA exposure on pregnant mice and found that BPA exposure disturbed meiotic synapsis and homologous recombination in germ cells. Similarly, elevated level of homologous recombination was also observed in primate models and in vitro cultured human ovary model after exposure to BPA [128–130]. Two studies in the mouse, showed that daily oral administration of 0.02–0.08 mg/kg BPA [109] or 40 μg/kg DEHP [131] during gestation significantly delayed meiotic progression of the exposed primary oocytes. Moreover, decreased expression of the meiotic genes Stra8, Dmc1, Rec8 and Scp3 were observed. Alarmingly, it has been reported that exposure to the anti-inflammatory drug indomethacin or the analgesic drug acetaminophen (8 or 350 mg/kg, respectively) delayed meiotic entry of oogonia in Wistar rat fetal ovaries [132–134]. Although these results suggest that RTECs can deregulate meiosis-related genes, the exact molecular mechanisms of all these effects remain to be determined.
Table 2.
RTEC exposure impairs meiotic prophase I (MPI) and germ cell cyst formation
| References | Type of EDCs | Exposure stage | Exposure way | Main conclusions |
|---|---|---|---|---|
| Susiarjo et al. [127] (C57BL/6 mice) | 20 μg/kg BPA | 11.5–18.5 dpc | Subcutaneous implanted | Increased oocyte aneuploidy |
| Lawson et al. [110] (C57BL/6J mice) | 20 ng/g BPA | 11.5–14.5 dpc | Oral | Increased expression of Stra8 and other meiotic genes |
| Hunt et al. [128] (Rhesus) |
0.51 + 0.21 ng/mL 0.31–0.13 ng/mL BPA |
50–100 GD GD 100-term |
Oral or subcutaneous implantation | Increased chromosome synaptic defects, recombination and multi-oocyte follicles |
| Zhang et al. [116] (CD1 mice) | 0.02–0.08 mg/kg BPA | 12.5–18.5 dpc | Oral | Increased number of oocytes in nests; decreased primordial follicles; delayed MPI; decreased expression of meiotic genes |
| Li et al. [154] (CD1 mice) | 40 μg/kg DEHP | 0.5–18.5 dpc | Oral | Reduced percentage of methylated CpG sites in Igf2r and Peg3 differentially methylated regions (DMRs), inherited to F2 offspring |
| Zhang et al. [131] (CD1 mice) | 40 μg/kg DEHP | 0.5–18.5 dpc | Oral | Decreased synthesis of estradiol; delayed MPI progression; accelerated activation of primordial follicles in F1 and F2 |
| Dean et al. [134] (Wistar rats) |
8 mg/kg indomethacin 350 mg/kg paracetamol |
15.5–18.5 dpc | Subcutaneous injection and gavage | Delayed meiotic entry; decreased germ cell number and ovarian size in F1 offspring; reduced follicle numbers during puberty/adulthood in F2 |
RTECs impair germ cell cyst breakdown and primordial follicle assembly
Substantial works demonstrated that exposure to RTECs adversely affects CBD and subsequent PF assembly (Table 3), thus warranting public attention that RTECs may induce POF in woman [135]. In rhesus monkeys, researches have investigated the effects of continuous BPA exposure on pregnancy and found that BPA exposure caused a significant increase in the frequency of MOF in the offspring [128]. In rodent, disturbances in CBD and PF formation were observed when pregnant mice were exposed to this compound [136], and attributed to inhibition of MPI progression [109]. Similarly, Holm and colleagues showed significant reduction of the ovarian reserve in pubertal mice exposed in utero to 50–150 mg/kg acetaminophen/paracetamol [111]. Besides, Lea and colleagues demonstrated when pregnant ewes were fed with RTECs derived from sewage sludge, the fetal ovarian reserve was significantly decreased [137].
Table 3.
RTEC exposure impairs germ cell cyst breakdown (CBD) and primordial follicle assembly
| References | Type of EDCs | Exposure stage | Exposure way | Main conclusions |
|---|---|---|---|---|
| Jefferson et al. [140] (CD1 mice) | 50 mg/kg genistein | 1–5 dpp | Subcutaneous injection | Inhibition of oocyte nest breakdown |
| Rodriguez et al. [141] (Wistar rats) | 0.05–20 mg/kg BPA | 1–7 dpp | Subcutaneous injection | Decreased primordial follicle pool |
| Karavan and Pepling [143] (CD1 mice) | 5–50 mg/kg BPA | 1–4 dpp | Subcutaneous injection | Reduced single oocyte; impaired follicle activation |
| Ahn et al. [142] (Wistar rats) | 62.5–250 mg/kg or 1000 mg/kg methyl-propyl- or butyl-parabens | 1–7 dpp | Subcutaneous injection | Inhibition of early phase of folliculogenesis; inhibition of Foxl2 expression |
| Wang et al. [160] (FVB mice) | 0.5, 20, and 50 μg/kg/BPA | 11.5 dpc-birth | Oral | Inhibited nest breakdown; advanced age of first estrus; higher percentage of dead pups in F1 |
| Mu et al. [139] (BALB mice) | 2.5, 5, 10 μg/g DEHP | 0–4 dpp | Intraperitoneal injection | Impaired cyst breakdown |
| Holm et al. [111] (C57BL/6J mice) | 50, 150 mg/kg paracetamol | 7 dpc-birth | Gavage | Shortening of the anogenital distance; diminished follicle reserve |
| Lea et al. [137] (sheep) | 2.25 metric tons of dry matter per ha ECs fertilized with sewage sludge | Early, mid or late gestation | Grazing | Reduced the proportion of healthy early stage fetal follicles; reduced ovarian reserve |
Along with in utero exposure, several studies have demonstrated that neonatal exposure to RTECs impairs CBD and PF assembly. In fact, Zhang et al. [138], demonstrated that BPA exposure in vitro significantly decreased the percentage of CBD and PFs in 0 dpp mouse ovaries. Noteworthy, Mu and colleagues demonstrated that DEHP may exert its effects by deregulating ERβ and progesterone receptor expression and Notch signalling in the cultured ovaries [139]. Moreover, Jefferson and colleagues reported that exposure of mouse pups to 50 mg/kg genistein from 1 to 5 dpp impaired CBD [140]. Similar results were reported in Wistar rats using 0.05–20 mg/kg BPA or 62.5–250 mg/kg or 1000 mg/kg methyl-propyl- or butyl-parabens by subcutaneous injection from 1 to 7 dpp [141, 142]. Furthermore, Karavan et al. [139, 143], demonstrated that neonatal exposure to BPA (5 mg/kg or 50 mg/kg) and DEHP (2.5–10 mg/kg) via subcutaneous or intraperitoneal injection significantly inhibited CBD. While in rodents the inhibitory effects of xenoestrogenic EDCs on CBD can be explained with the analogous action of estrogens, in primates where estrogens do not appear to exert such effects (see a previous section), alternative mechanisms such as epigenetic modifications and oxidative stress, must be envisaged.
RTECs effect prepuberal and adult folliculogenesis
Along with affecting the establishment of the ovarian reserve, RTECs can act in accelerating the exhaustion of the PF pool. In fact, a number of studies report that prepuberty exposure to RTECs accelerated follicle exhaustion via increased PF activation and/or atresia of the growing follicles [37, 141, 144–148] (Table 4).
Table 4.
RTEC exposure alters the folliculogenesis dynamics
| References | Type of EDCs | Exposure stage | Exposure way | Main conclusions |
|---|---|---|---|---|
| Jurisicova et al. [156] (C57BL/6 mice) | 1 mg/kg each BaP and DMBA | 3 dpp–3 weeks | Breast milk | Maternal exposure to PAHs during lactation decreased ovarian reserve by 2/3 in female offspring |
| Armenti et al. [144] (CDF rats) | 20–100 μg/kg, methoxychlor |
19 dpc 7 dpp |
Intraperitoneal injection | Altered steroidogenesis; increased preantral and early antral follicle maturation |
| Pru et al. [155] (C57BL/6 mice) | 50 mg/kg DMBA | Prepubertal | Intraperitoneal | PAH upregulated a series apoptotic related gene in oocyte via Ahr |
| Xu et al. [159] (SD rats) |
5–300 mg/kg B[a]P 10–600 mg/kg DEHP |
6 weeks | Gavage | Increased granulosa cell apoptosis; Impaired steroidogenesis |
| Rodriguez et al. [141] (Wistar rats) | 0.05–20 mg/kg BPA | 1–7 dpp | Subcutaneous injection | Reduction of the primordial follicle pool; accelerated primordial follicle activation |
| Zhuang et al. [147] (SD rats) | 160 mg/kg genistein | 4–15 months | Gavage | Altered folliculogenesis dynamics |
| Rivera et al. [146] (Lambs) |
50 μg/kg BPA 5 μg/kg DES |
1–14 dpp | Subcutaneous injections | Reduced ovarian weight and increased number of multi-oocyte follicles; Increased atresia of antral follicles |
| Medigovic et al. [151] (Wistar rats) | 50 mg/kg genistein | 18–20 dpp | Subcutaneous injections | Decreased number of primordial and primary follicles; Stimulating of preantral to antral stage transition |
| Lee et al. [157] (SD rats) | 0.001–0.1 mg/kg BPA | 8 weeks | Gavage | Decreased E2 secretion; increased follicular atresia and accelerated luteal regression |
| Li et al. [154] (Wistar rats) | 10–160 mg/kg BPA | 2–35 dpp | Intraperitoneal injection | Reduced ovarian weights and follicle numbers; increased number of atretic follicles |
| Hannon et al. [152] (CD1 mice) | 20–750 mg/kg DEHP | 40 dpp | Oral | Disruption of estrous cyclicity; prolonged duration of estrus and accelerated primordial follicle recruitment |
| Wang et al. [160] (Wistar rats) | 50–200 mg/kg soy isoflavones |
21 dpp 3 months |
Oral | Increased granulosa cell apoptosis |
| Rasmussen et al. [148] (CD1 mice) | 5–10 mg/kg acetyl-tributyl-citrate | 88 dpp | Oral | Decreased number of primordial, primary and secondary follicles |
| Johansson et al. [145] (Wistar rats) | Mixed BPA, butylparaben, paracetamol |
7–21 dpc 1–22 dpp |
Gavage | Decreased primordial follicles; irregular estrous cycles in 1-year old rats |
A high dose (100 mg/kg/day) of methoxychlor (MXR), an organochlorine pesticide with estrogenic properties [149, 150], injected intraperitoneally in rats between 19 dpc and 7 dpp increased the percentage of preantral and early antral follicles and reduced the percentage of corpora lutea [144]. This effect was associated with reduced expression of ERβ and LH receptors in large antral follicles. Medigovic and colleagues [151] injected Wistar rats with 50 mg/kg of genistein subcutaneously from 18 to 20 dpp and analysed ovarian dynamics at 21 dpp. Similarly, to a parallel injection of estradiol genistein reduced the number of PFs but increased the number of primary follicles suggesting increased PF activation. A study by Rodriguez and collaborators [141] also provided evidence that BPA exposure during the first wave of ovarian follicle recruitment was associated with accelerated PF recruitment. Daily exposure to DHEP have been demonstrated to perturb oestrous cycle and PFs activation via PI3K pathway [152]. BPA exposure during ovarian development have been demonstrated to impair folliculogenesis [153], and transgenerational inheritance of the accelerated PF recruitment induced by maternal DEHP exposure has been also reported in the mouse [131]. Intraperitoneally injection of BPA to prepubescent female Wistar rats significantly reduced the number of growing follicles, and increased follicle atresia [154]. Further investigations found that following BPA exposure, the expression of FIGLA and the oocyte-specific histone H1 variant (H1FOO), key regulators of oocyte meiotic maturation, were reduced while the expression of AMH was increased. In particular, exposure to PAHs, a common RTECs found in tobacco smoke, have been shown to have reproductively toxic effects in both maternal and offspring. Pru et al. found that prepubertal exposure to dimethylbenz[a]anthracene (a PAH) accelerated oocyte depletion via increasing the expression of apoptosis-related genes in oocytes Pru et al. [155]. Jurisicova et al. [156] exposed lactating mice to PAHs and investigated whether such exposure could induce any adverse effects in the offspring. Noteworthy, the authors found that the ovarian reserve of the female offspring was decreased by about two-thirds. Further investigation demonstrated that the aryl hydrocarbon receptor (Ahr) dependent cell death pathway was activated in the immature ovarian follicles.
Increasing data has demonstrated that exposure to RTECs by an adult can inhibit the growth of follicles at various developmental stages and increase follicular atresia. Lee and colleagues investigated the effects of 0.001–0.1 mg/kg BPA exposure on 9-week-old rats for 3 months via gavage and showed that such exposure significantly decreased serum 17β-estradiol levels and increased atresia of the growing follicles [157]. Further investigation demonstrated that BPA exposure significantly reduced aromatase P450arom expression in GCs and reduced StAR expression in theca cells [157]. Therefore, it is plausible that BPA may exert its effects via perturbed steroidogenesis. Besides, multiple studies have shown consistently that MXR and DEHP along with their metabolites and genistein also inhibit ovarian steroidogenesis (for a review, see [34]).
Several studies showed that exposure to RTECs induced GC apoptosis and as a result increased follicular atresia [158]. Xu and colleagues investigated the effects of exposure to benzo[a]pyrene (B[a]P) and/or a DEHP mixture on 6-week-old Sprague–Dawley rats by oral gavage for 60 days, and demonstrated that either a single exposure of B[a]P, DEHP or their combination prolonged oestrous cycles, decreased serum 17β-estradiol levels, and increased GC apoptosis [159]. Further investigation indicated that the expression of aromatase P450arom was significantly decreased, while the expression of PPAR-γ protein was significantly increased following the exposure to B[a]P and DEHP. Wang and colleagues investigated the effects of gavage administration of 50–200 mg/kg phytoestrogen Soy isoflavones (SIFs) and genistein to Wistar rats on ovarian follicle development from 21 dpp to 3 months of age. They found that SIF exposure significantly increased apoptosis-dependent atresia in GCs [160]. In mouse GCs, it was demonstrated that 100 µM BPA exposure increased apoptosis as revealed by the increased level of BAX and decreased level of BCL2 [161]. In adult female rats dosed with 0.001–0.1 mg/kg/day BPA for 90 days, the percentage of atretic follicles was significantly increased, and elevated Caspase3 mediated apoptosis was observed in their follicles [157]. Phthalates have also been shown to perturb folliculogenesis. 600 mg/kg DEHP exposure via gavage for 2 months significantly decreased the percentage of primary and secondary follicles, while increased the percentage of atretic follicles [159]. Besides, it was found that MEHP also perturbs folliculogenesis via increased level of reactive oxygen species [162].
RTECs exposure in a human scenario
Studies on the effects of the exposure to RTECs on the female reproductive system have long been investigated and substantial evidence exists that RTECs exposure, either during foetus or adult stage, perturbs female reproductive health in model animals. However, species differences and the relatively high dosage used in model animals make it difficult to analogize a human scenario. In addition, as previously mentioned, women are usually diagnosed with POF in their 40s–50s while the exposure to RTECs may occur decades earlier during prepubescence and even during prenatal development, making the cause difficult to determine. Further complicating the situation is the fact that human exposure to RTECs is ubiquitous and various kinds of RTECs may exert their effects at the same time, thus making it is elusive whether RTECs exposure may contribute to the aetiology of premature menopause [25, 163, 164].
Assaying the evidence of RTEC exposure affecting human female reproductive health
The available data regarding the association of RTEC exposure and human POF or earlier menopause mainly comes from cross-sectional surveys of public databases, such as the National Health and Nutrition Examination Survey (NHANES, https://www.cdc.gov/nchs/nhanes/index.htm), the Seveso Women’s Health Study (SWHS, https://cerch.berkeley.edu/research-programs/seveso-womens-health-study), the InCHIANTI study (http://inchiantistudy.net/wp/) and others (Table 5). These studies have found links between several common RTEC exposure levels and reproductive pathologies. Evidence exists that exposure to RTECs by women may be associated with sex endocrine homeostasis, and may, in some cases cause earlier menopause [31, 165, 166].
Table 5.
RTECs exposure in a human exposure scenario
| References | Exposure level | Exposure stage | Exposure way | Main conclusions |
|---|---|---|---|---|
| Eskenazi et al. [168] (616 women) | Serum concentration about 20–120 ppt TCDD | Postpubertal to adult | Chemical plant explosion | Correlate with TCDD serum level and earlier menopause (about 100 ppt TCDD, but not above) |
| Galloway et al. [167] (60 premenopausal women) | Urinary BPA concentration was 3.59 ng/mL (Geometric mean) | Between 20 and 74 years old | NA | Associated with (sex hormone-binding globulin) SHBG concentrations in the 60 premenopausal women |
| Mok-Lin et al. [176] (84 women) | Urinary BPA concentrations: 0.4–25.5 μg/L | 35.6 (mean age) years old women undergoing IVF | NA | Decreased the number of oocytes retrieved per cycle and peak serum estradiol levels |
| Bloom et al. [177] (44 women) | Serum BPA: 7.22 ng/L | 35.8 (mean age) years old women undergoing IVF | NA | Reduced E2 response during IVF |
| Knox et al. [166] (25,957 women) | Serum perfluorooctane sulfonate: 15.0–21.5 ng/L | 18–65 years old women | NA | Affect estradiol level |
| Fujimoto et al. [178] (58 women) | Serum BPA 0.31–1.10 ng/L | 58 infertile female patients | NA | Decreased oocyte quality during IVF |
| Grindler et al. [31] (31,575 women) | PCB (3.61–26.3 ng/g); dioxin/furan (7.22 ng/g); MEHP (17.1 ng/g); all median value | About 50–60 years old women | NA | 1.9–3.8 years earlier of menopause entry. 6 times more likely to be menopausal than non-exposed women |
| Taylor et al. [165] (2732 women) | Serum polyfluoroalkyl chemicals (PFC): 0.9–17.5 ng/mL | 20–65 years old women | NA | Earlier menopause age |
The first study regarding the association between BPA and woman reproductive health comes from the research by Galloway and colleagues according to the InCHIANTI study (InCHIANTI 2010) report. By analysing urinary BPA concentration, the authors found that urinary BPA concentration was associated with the level of the sex hormone binding globulin (SHBG) but not estradiol or thyroid-stimulating hormone [167]. More direct evidence of RTEC exposure affecting the age of menopause in women came from the data in the SWHS. Eskenazi and colleagues evaluated the association of menopausal age and serum dioxin concentrations in women who lived in a dioxin polluted area (caused by a chemical plant explosion) for about 20 years. They found that serum dioxin concentrations correlated with an increasing risk of earlier menopause [168].
Another cross-sectional survey using data from NHANES in the USA from 1999 to 2008 also demonstrated that RTECs exposure correlated with earlier menopause [31]. These authors analysed data from a large population of women (n = 31,575) in the USA and evaluated 111 RTECs known to be toxicants to the reproductive system. By analysing these data, the authors concluded that women with high serum levels of polychlorinated biphenyls (PCBs, such as polychlorinated biphenyl congeners—70, 99, 105, etc.), phthalates (MEHP), pesticides (Beta-hexachlorocyclohexane, p,p′-DDE, etc.) and dioxin/furan reached menopause about 3 years earlier than those having lower levels of these chemicals. Taylor and colleagues also analysed the relevance between RTEC exposure and menopause age among women in 20–65 years old using NHANES. These authors evaluated the serum level of a series of polyfluoroalkyl chemicals from five NHANES sample groups (1999–2000, 2003–2004, 2005–2006, 2007–2008, and 2009–2010) and demonstrated that high serum levels of polyfluoroalkyl chemicals were associated with earlier menopause [165]. Although data from these cross-sectional surveys indicate a potential association between RTEC exposure and the early onset of menopause, the developmental window during exposure, RTEC type, exposure level, the mechanism of RTEC induced early onset of menopause and/or POF, are not clarified in these reports.
Potential machinery of RTECs contributing to female POF
Due to the lack of information on the molecular machinery involved regarding the association between the exposure to RTECs and human early menopause and/or POF, studies performed on non-human primates provide a source of valuable information that may lead to a better understanding of the mechanisms involved. In 2011, Aldad and colleagues [169] investigated the effects of BPA exposure on endometrial expression of the progesterone receptor in African green monkeys and demonstrated that BPA exposure decreased estrogen-induced progesterone receptor expression. Although these authors did not investigate ovary physiology, it is plausible that BPA exposure may affect ovarian health via perturbing the function of reproductive hormones in primates.
Contrasting with studies performed using model animals of which the exact exposure window of RTECs is controlled, the association of RTECs exposure and women reproductive health is mainly investigated in post-puberty women. Along with this, it is difficult in human studies, to accurately determine specific RTEC exposure and when exposure occurred (prenatal, postnatal, prepubescent and post-pubescent). Therefore, confounding the determination of the aetiology of POF and/or earlier menopause. This makes it nearly impossible to focus on the two key developmental stages of human oogenesis occurring in utero: namely the initiation of meiosis and primordial follicle formation. Only one study has investigated the effects of EDC exposure on in uterus oogenesis in non-human primates. In 2012, Hunt and colleagues investigated the role of human circulating levels of BPA on the initiation of meiosis and primordial follicle formation during the second trimester [gestational day (GD) 50–100] and the third trimester (GD 100-term) and found that: (1) pachytene oocytes from the BPA exposed group showed a significantly higher (P < 0.001) rate of recombination as evidenced by an SCP3 and MLH1 co-staining assay. (2) BPA exposure decreased the percentage of secondary and antral follicles while increased the percentage of MOFs [128]. These findings here emphasize that BPA exposure during the foetal stage may interfere with MPI, similar to the findings using rodent models. BPA exposure was also found to result in decreasing the percentage of normal secondary and antral follicles, which functionally results in the reduction of the ovarian follicle pool. Taken together, these data suggest that the exposure to RTECs during the foetus stage may also interfere with MPI and follicle formation, two key events pivotal for ovarian follicle formation. Particularly in primates, whether other RTEC may function similarly still warrants further study.
Currently, our understanding on the effects of RTECs on the reproductive health of women remains limited. According to findings from studies involving human and primates, we can infer that RTECs may exert their effects similar to those observed in rodent models: interfere with foetal oogenesis (including MPI and primordial follicle formation) and/or accelerate ovarian follicle exhaustion (earlier onset of menopause and POF). It is plausible that RTECs in a human scenario may also exert their effects via disrupting the balance between the ovarian reserve establishment (fetal oogenesis) and the rate of ovarian follicle depletion (ovarian follicle recruitment, atresia and ovulation). This may as a consequence induce the aetiology of POF and the early onset of menopause. However, our current study regarding the effects of RTEC exposure on the aetiology of POF and/or premature menopause remains limited while the occurrence of POF is increasing. All of this makes studies in this area of great value for both understanding the aetiology of human reproductive disorders and developing potential medical treatments.
Conclusions
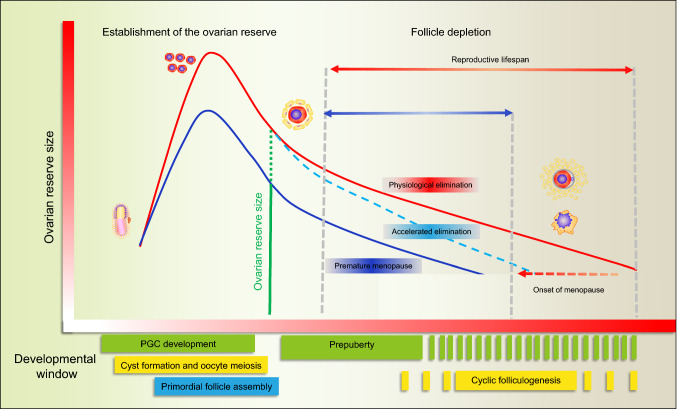
It is estimated that 1% of women under the age of 40 and 0.1% of women under the age of 30 are suffering from POI, while only about 25% of the patients have a definite etiology for this pathology [22, 23, 170, 171]. Growing evidence is accumulating that environmental chemicals, including RTECs, may interfere with oogenesis at any stage and disturb the two main activities of this organ: the production of fertilizable oocytes and the secretion of reproductive hormones [37, 172]. Noteworthy, it is reported that occupational exposure to RTECs by women are associated with POI [173], suggesting that the industrialization of modern society significantly increased the risk of RTEC exposure [174, 175]. Strong evidence, gained using animal models, exists that in utero, neonatal, prepubescent and even adult exposure to RTECs leads to impaired functioning of the ovary and a shortening of the reproductive lifespan. Specifically, in such models, in utero and neonatal exposure to RTECs either alone or combination are proven to decrease the size of the ovarian reserve. On the other hand, prepubescent and adult exposure to RTECs are reported to accelerate the exhaustion of the ovarian follicle pool and interfere with the cyclic recruitment of antral follicles for ovulation [25]. Regarding humans, reasons for the lack of knowledge of RTECs effects on ovarian function can be summarized as follows: (1) the complexity of the processes regulating oogenesis and the still scant information about the molecular mechanisms underlying, (2) the fact that RTECs may exert their effects collectively and continuously throughout the lifetime, while the current studies usually use a single point of RTEC exposure, and (3) the diagnosis of early menopause mainly happens in women when they are 40–50 years old while the deleterious RTEC exposure may happen decades before making it hard to find out the etiology of this pathology (Fig. 4). Therefore, epidemiological surveys and more experimental investigation on humans are needed to identify RTECs, determine their direct and indirect effects on the ovary function, and to characterize their action mechanisms. The effects of RTECs on folliculogenesis, follicle/oocyte health, and steroidogenesis can have lasting effects both on reproductive and non-reproductive health because ovary endocrine functions are essential not only for fertility but also for the regulation of skeletal, cardiovascular, and brain health. Furthermore, more evidence that RTECs may modify the germ cell epigenome are particularly devious as they are potentially transmitted to future generations resulting in deleterious consequences for reproduction, as well as cancer, cardiovascular and neurological diseases.
Fig. 4.
Hypothetical diagram of RTEC induced earlier menopause. The red line represents normal ovarian dynamics during aging while the blue line represents ovarian dynamics in a premature menopause scenario
Acknowledgements
This work was supported by National Key Research and Development Program of China (2016YFD0501207) and National Nature Science Foundation (31471346, 31572225 and 31671554).
Compliance with ethical standards
Conflict of interest
The author declares no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Massimo De Felici, Email: defelici@uniroma2.it.
Wei Shen, Email: wshen@qau.edu.cn.
References
- 1.De Felici M, Klinger FG, Farini D, Scaldaferri ML, Iona S, Lobascio M. Establishment of oocyte population in the fetal ovary: primordial germ cell proliferation and oocyte programmed cell death. Reprod Biomed Online. 2005;10(2):182–191. doi: 10.1016/S1472-6483(10)60939-X. [DOI] [PubMed] [Google Scholar]
- 2.Monget P, Bobe J, Gougeon A, Fabre S, Monniaux D, Dalbies-Tran R. The ovarian reserve in mammals: a functional and evolutionary perspective. Mol Cell Endocrinol. 2012;356(1–2):2–12. doi: 10.1016/j.mce.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 3.Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142(15):2554–2563. doi: 10.1242/dev.125211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Felici M. Germ stem cells in the mammalian adult ovary: considerations by a fan of the primordial germ cells. Mol Hum Reprod. 2010;16(9):632–636. doi: 10.1093/molehr/gaq006. [DOI] [PubMed] [Google Scholar]
- 5.De Felici M, Barrios F. Seeking the origin of female germline stem cells in the mammalian ovary. Reproduction. 2013;146(4):R125–R130. doi: 10.1530/REP-13-0069. [DOI] [PubMed] [Google Scholar]
- 6.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 7.Forabosco A, Sforza C, De Pol A, Vizzotto L, Marzona L, Ferrario VF. Morphometric study of the human neonatal ovary. Anat Rec. 1991;231(2):201–208. doi: 10.1002/ar.1092310208. [DOI] [PubMed] [Google Scholar]
- 8.Tilly JL, Kolesnick RN. Sphingolipids, apoptosis, cancer treatments and the ovary: investigating a crime against female fertility. Biochim Biophys Acta. 2002;1585(2–3):135–138. doi: 10.1016/S1388-1981(02)00333-5. [DOI] [PubMed] [Google Scholar]
- 9.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 10.Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab. 2010;21(2):96–103. doi: 10.1016/j.tem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin N Am. 2011;38(3):425–440. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, LaVasseur BI, Barton DL, Novotny PJ, Dakhil SR, Rodger K, Rummans TA, Christensen BJ. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356(9247):2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 13.Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobbee DE, van der Schouw YT. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16(4):556–562. doi: 10.1097/01.ede.0000165392.35273.d4. [DOI] [PubMed] [Google Scholar]
- 14.van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347(9003):714–718. doi: 10.1016/S0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- 15.Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Kondo T, Watanabe Y, Koizumi A, Inaba Y, Tamakoshi A. Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: the JACC study. J Epidemiol. 2006;16(5):177–184. doi: 10.2188/jea.16.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J Clin Epidemiol. 1999;52(4):303–307. doi: 10.1016/S0895-4356(98)00170-X. [DOI] [PubMed] [Google Scholar]
- 17.Penoni DC, Fidalgo TK, Torres SR, Varela VM, Masterson D, Leao AT, Maia LC. Bone density and clinical periodontal attachment in postmenopausal women: a systematic review and meta-analysis. J Dent Res. 2017;96(3):261–269. doi: 10.1177/0022034516682017. [DOI] [PubMed] [Google Scholar]
- 18.Monninkhof EM, van der Schouw YT, Peeters PH. Early age at menopause and breast cancer: are leaner women more protected? A prospective analysis of the Dutch DOM cohort. Breast Cancer Res Treat. 1999;55(3):285–291. doi: 10.1023/A:1006277207963. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs I, Davies AP, Bridges J, Stabile I, Fay T, Lower A, Grudzinskas JG, Oram D. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ. 1993;306(6884):1030–1034. doi: 10.1136/bmj.306.6884.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14(2):103–115. doi: 10.1016/0378-5122(92)90003-M. [DOI] [PubMed] [Google Scholar]
- 21.Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, Lee JS, Thurston R, Vuga M, Harlow SD. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178(1):70–83. doi: 10.1093/aje/kws421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67(4):604–606. [PubMed] [Google Scholar]
- 23.Beck-Peccoz P, Persani L. Premature ovarian failure. Orphanet J Rare Dis. 2006;1:9. doi: 10.1186/1750-1172-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin Y, Jiao X, Simpson JL, Chen ZJ. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21(6):787–808. doi: 10.1093/humupd/dmv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vabre P, Gatimel N, Moreau J, Gayrard V, Picard-Hagen N, Parinaud J, Leandri RD. Environmental pollutants, a possible etiology for premature ovarian insufficiency: a narrative review of animal and human data. Environ Health. 2017;16(1):37. doi: 10.1186/s12940-017-0242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iorio R, Castellucci A, Ventriglia G, Teoli F, Cellini V, Macchiarelli G, Cecconi S. Ovarian toxicity: from environmental exposure to chemotherapy. Curr Pharm Des. 2014;20(34):5388–5397. doi: 10.2174/1381612820666140205145319. [DOI] [PubMed] [Google Scholar]
- 27.Dragojevic-Dikic S, Vasiljevic M, Nikolic B, Pazin V, Tasic L, Jurisic A, Dikic S, Perisic Z. Premature ovarian failure: immunological aspects and therapeutic strategies. Vojnosanit Pregl. 2013;70(11):1051–1055. doi: 10.2298/VSP1311051D. [DOI] [PubMed] [Google Scholar]
- 28.Hewlett M, Mahalingaiah S. Update on primary ovarian insufficiency. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):483–489. doi: 10.1097/MED.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker EJ, Grover SR, Bachelot A, Touraine P, Sinclair AH. Premature ovarian insufficiency: new perspectives on genetic cause and phenotypic spectrum. Endocr Rev. 2016;37(6):609–635. doi: 10.1210/er.2016-1047. [DOI] [PubMed] [Google Scholar]
- 30.Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, Maisog J, Kim S, Chen Z, Barr DB. Persistent environmental pollutants and couple fecundity: the LIFE study. Environ Health Perspect. 2013;121(2):231–236. doi: 10.1289/ehp.1205301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grindler NM, Allsworth JE, Macones GA, Kannan K, Roehl KA, Cooper AR. Persistent organic pollutants and early menopause in U.S. women. PLoS One. 2015;10(1):e0116057. doi: 10.1371/journal.pone.0116057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craig ZR, Wang W, Flaws JA. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. 2011;142(5):633–646. doi: 10.1530/REP-11-0136. [DOI] [PubMed] [Google Scholar]
- 33.Richardson MC, Guo M, Fauser BC, Macklon NS. Environmental and developmental origins of ovarian reserve. Hum Reprod Update. 2014;20(3):353–369. doi: 10.1093/humupd/dmt057. [DOI] [PubMed] [Google Scholar]
- 34.Patel S, Zhou C, Rattan S, Flaws JA. Effects of endocrine-disrupting chemicals on the ovary. Biol Reprod. 2015;93(1):20. doi: 10.1095/biolreprod.115.130336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Badach H, Nazimek T, Kaminska IA. Pesticide content in drinking water samples collected from orchard areas in central Poland. Ann Agric Environ Med. 2007;14(1):109–114. [PubMed] [Google Scholar]
- 37.Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front Endocrinol (Lausanne) 2015;6:8. doi: 10.3389/fendo.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinli K, Block G. Phytoestrogen content of foods—a compendium of literature values. Nutr Cancer. 1996;26(2):123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- 39.Kristensen DM, Mazaud-Guittot S, Gaudriault P, Lesne L, Serrano T, Main KM, Jegou B. Analgesic use—prevalence, biomonitoring and endocrine and reproductive effects. Nat Rev Endocrinol. 2016;12(7):381–393. doi: 10.1038/nrendo.2016.55. [DOI] [PubMed] [Google Scholar]
- 40.Jegou B. Paracetamol-induced endocrine disruption in human fetal testes. Nat Rev Endocrinol. 2015;11(8):453–454. doi: 10.1038/nrendo.2015.106. [DOI] [PubMed] [Google Scholar]
- 41.Ge W, Chen C, De Felici M, Shen W. In vitro differentiation of germ cells from stem cells: a comparison between primordial germ cells and in vitro derived primordial germ cell-like cells. Cell Death Dis. 2015;6:e1906. doi: 10.1038/cddis.2015.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Felici M. Origin, migration, and proliferation of human primordial germ cells. In: Coticchio G, editor. Oogenesis. London: Springer; 2013. pp. 19–37. [Google Scholar]
- 43.De Felici M. The formation and migration of primordial germ cells in mouse and man. Results Probl Cell Differ. 2016;58:23–46. doi: 10.1007/978-3-319-31973-5_2. [DOI] [PubMed] [Google Scholar]
- 44.Kojima Y, Sasaki K, Yokobayashi S, Sakai Y, Nakamura T, Yabuta Y, Nakaki F, Nagaoka S, Woltjen K, Hotta A, Yamamoto T, Saitou M. Evolutionarily distinctive transcriptional and signaling programs drive human germ cell lineage specification from pluripotent stem cells. Cell Stem Cell. 2017;21(4):517–532 e5. doi: 10.1016/j.stem.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 45.De Felici M. Nuclear reprogramming in mouse primordial germ cells: epigenetic contribution. Stem Cells Int. 2011;2011:425863. doi: 10.4061/2011/425863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilsson EE, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of disease susceptibility. Transl Res. 2015;165(1):12–17. doi: 10.1016/j.trsl.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei Y, Schatten H, Sun QY. Environmental epigenetic inheritance through gametes and implications for human reproduction. Hum Reprod Update. 2015;21(2):194–208. doi: 10.1093/humupd/dmu061. [DOI] [PubMed] [Google Scholar]
- 48.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157(1):95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Felici M. Primordial germ cell biology at the beginning of the XXI century. Int J Dev Biol. 2009;53(7):891–894. doi: 10.1387/ijdb.082815mf. [DOI] [PubMed] [Google Scholar]
- 50.Wang C, Zhou B, Xia G. Erratum to: Mechanisms controlling germline cyst breakdown and primordial follicle formation. Cell Mol Life Sci. 2017;74(14):2567. doi: 10.1007/s00018-017-2499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006;312(5773):596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 52.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103(8):2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Bouffant R, Guerquin MJ, Duquenne C, Frydman N, Coffigny H, Rouiller-Fabre V, Frydman R, Habert R, Livera G. Meiosis initiation in the human ovary requires intrinsic retinoic acid synthesis. Hum Reprod. 2010;25(10):2579–2590. doi: 10.1093/humrep/deq195. [DOI] [PubMed] [Google Scholar]
- 54.Childs AJ, Cowan G, Kinnell HL, Anderson RA, Saunders PT. Retinoic acid signalling and the control of meiotic entry in the human fetal gonad. PLoS ONE. 2011;6(6):e20249. doi: 10.1371/journal.pone.0020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mu X, Wen J, Guo M, Wang J, Li G, Wang Z, Wang Y, Teng Z, Cui Y, Xia G. Retinoic acid derived from the fetal ovary initiates meiosis in mouse germ cells. J Cell Physiol. 2013;228(3):627–639. doi: 10.1002/jcp.24172. [DOI] [PubMed] [Google Scholar]
- 56.Liang GJ, Zhang XF, Wang JJ, Sun YC, Sun XF, Cheng SF, Li L, De Felici M, Shen W. Activin A accelerates the progression of fetal oocytes throughout meiosis and early oogenesis in the mouse. Stem Cells Dev. 2015;24(20):2455–2465. doi: 10.1089/scd.2015.0068. [DOI] [PubMed] [Google Scholar]
- 57.Chassot AA, Gregoire EP, Lavery R, Taketo MM, de Rooij DG, Adams IR, Chaboissier MC. RSPO1/beta-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS ONE. 2011;6(10):e25641. doi: 10.1371/journal.pone.0025641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu CF, Parker K, Yao HH. WNT4/beta-catenin pathway maintains female germ cell survival by inhibiting activin betaB in the mouse fetal ovary. PLoS ONE. 2010;5(4):e10382. doi: 10.1371/journal.pone.0010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell. 2005;8(6):949–961. doi: 10.1016/j.devcel.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 60.Griswold MD, Hogarth CA, Bowles J, Koopman P. Initiating meiosis: the case for retinoic acid. Biol Reprod. 2012;86(2):35. doi: 10.1095/biolreprod.111.096610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tedesco M, Desimio MG, Klinger FG, De Felici M, Farini D. Minimal concentrations of retinoic acid induce stimulation by retinoic acid 8 and promote entry into meiosis in isolated pregonadal and gonadal mouse primordial germ cells. Biol Reprod. 2013;88(6):145. doi: 10.1095/biolreprod.112.106526. [DOI] [PubMed] [Google Scholar]
- 62.Tedesco M, La Sala G, Barbagallo F, De Felici M, Farini D. STRA8 shuttles between nucleus and cytoplasm and displays transcriptional activity. J Biol Chem. 2009;284(51):35781–35793. doi: 10.1074/jbc.M109.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandon-Pepin B, Touraine P, Kuttenn F, Derbois C, Rouxel A, Matsuda F, Nicolas A, Cotinot C, Fellous M. Genetic investigation of four meiotic genes in women with premature ovarian failure. Eur J Endocrinol. 2008;158(1):107–115. doi: 10.1530/EJE-07-0400. [DOI] [PubMed] [Google Scholar]
- 64.Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13(11):1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- 65.Paredes A, Garcia-Rudaz C, Kerr B, Tapia V, Dissen GA, Costa ME, Cornea A, Ojeda SR. Loss of synaptonemal complex protein-1, a synaptonemal complex protein, contributes to the initiation of follicular assembly in the developing rat ovary. Endocrinology. 2005;146(12):5267–5277. doi: 10.1210/en.2005-0965. [DOI] [PubMed] [Google Scholar]
- 66.Tosh D, Rani HS, Murty US, Deenadayal A, Grover P. Mutational analysis of the FIGLA gene in women with idiopathic premature ovarian failure. Menopause. 2015;22(5):520–526. doi: 10.1097/GME.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 67.Zhao H, Chen ZJ, Qin Y, Shi Y, Wang S, Choi Y, Simpson JL, Rajkovic A. Transcription factor FIGLA is mutated in patients with premature ovarian failure. Am J Hum Genet. 2008;82(6):1342–1348. doi: 10.1016/j.ajhg.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jagarlamudi K, Rajkovic A. Oogenesis: transcriptional regulators and mouse models. Mol Cell Endocrinol. 2012;356(1–2):31–39. doi: 10.1016/j.mce.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 69.Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305(5687):1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- 70.Grive KJ, Seymour KA, Mehta R, Freiman RN. TAF4b promotes mouse primordial follicle assembly and oocyte survival. Dev Biol. 2014;392(1):42–51. doi: 10.1016/j.ydbio.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang N, Zhang P, Guo X, Zhou Z, Sha J. Hnrnpk, a protein differentially expressed in immature rat ovarian development, is required for normal primordial follicle assembly and development. Endocrinology. 2011;152(3):1024–1035. doi: 10.1210/en.2010-0797. [DOI] [PubMed] [Google Scholar]
- 72.Niu W, Wang Y, Wang Z, Xin Q, Feng L, Zhao L, Wen J, Zhang H, Wang C, Xia G. JNK signaling regulates E-cadherin junctions in germline cysts and determines primordial follicle formation in mice. Development. 2016;143(10):1778–1787. doi: 10.1242/dev.132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao L, Du X, Huang K, Zhang T, Teng Z, Niu W, Wang C, Xia G. Rac1 modulates the formation of primordial follicles by facilitating STAT3-directed Jagged1, GDF9 and BMP15 transcription in mice. Sci Rep. 2016;6:23972. doi: 10.1038/srep23972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones RL, Pepling ME. KIT signaling regulates primordial follicle formation in the neonatal mouse ovary. Dev Biol. 2013;382(1):186–197. doi: 10.1016/j.ydbio.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 75.Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006;298(1):132–148. doi: 10.1016/j.ydbio.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 76.Knight PG, Satchell L, Glister C. Intra-ovarian roles of activins and inhibins. Mol Cell Endocrinol. 2012;359(1–2):53–65. doi: 10.1016/j.mce.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 77.Yao HH, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230(2):210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of Anti-Mullerian Hormone (AMH) on ovarian primordial follicle assembly. PLoS ONE. 2011;6(5):e20087. doi: 10.1371/journal.pone.0020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y, Breen K, Pepling ME. Estrogen can signal through multiple pathways to regulate oocyte cyst breakdown and primordial follicle assembly in the neonatal mouse ovary. J Endocrinol. 2009;202(3):407–417. doi: 10.1677/JOE-09-0109. [DOI] [PubMed] [Google Scholar]
- 80.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148(8):3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- 81.Pepling ME. Follicular assembly: mechanisms of action. Reproduction. 2012;143(2):139–149. doi: 10.1530/REP-11-0299. [DOI] [PubMed] [Google Scholar]
- 82.Pelosi E, Forabosco A, Schlessinger D. Genetics of the ovarian reserve. Front Genet. 2015;6:308. doi: 10.3389/fgene.2015.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klinger FG, Rossi V, De Felici M. Multifaceted programmed cell death in the mammalian fetal ovary. Int J Dev Biol. 2015;59(1–3):51–54. doi: 10.1387/ijdb.150063fk. [DOI] [PubMed] [Google Scholar]
- 84.De Felici M, Lobascio AM, Klinger FG. Cell death in fetal oocytes: many players for multiple pathways. Autophagy. 2008;4(2):240–242. doi: 10.4161/auto.5410. [DOI] [PubMed] [Google Scholar]
- 85.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234(2):339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 86.Xu B, Hua J, Zhang Y, Jiang X, Zhang H, Ma T, Zheng W, Sun R, Shen W, Sha J, Cooke HJ, Shi Q. Proliferating cell nuclear antigen (PCNA) regulates primordial follicle assembly by promoting apoptosis of oocytes in fetal and neonatal mouse ovaries. PLoS ONE. 2011;6(1):e16046. doi: 10.1371/journal.pone.0016046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodrigues P, Limback D, McGinnis LK, Plancha CE, Albertini DF. Multiple mechanisms of germ cell loss in the perinatal mouse ovary. Reproduction. 2009;137(4):709–720. doi: 10.1530/REP-08-0203. [DOI] [PubMed] [Google Scholar]
- 88.Gawriluk TR, Hale AN, Flaws JA, Dillon CP, Green DR, Rucker EB., 3rd Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction. 2011;141(6):759–765. doi: 10.1530/REP-10-0489. [DOI] [PubMed] [Google Scholar]
- 89.Motta PM, Makabe S. Elimination of germ cells during differentiation of the human ovary: an electron microscopic study. Eur J Obstet Gynecol Reprod Biol. 1986;22(5–6):271–286. doi: 10.1016/0028-2243(86)90115-2. [DOI] [PubMed] [Google Scholar]
- 90.Jones RL, Pepling ME. Role of the antiapoptotic proteins BCL2 and MCL1 in the neonatal mouse ovary. Biol Reprod. 2013;88(2):46. doi: 10.1095/biolreprod.112.103028. [DOI] [PubMed] [Google Scholar]
- 91.Greenfeld CR, Babus JK, Furth PA, Marion S, Hoyer PB, Flaws JA. BAX is involved in regulating follicular growth, but is dispensable for follicle atresia in adult mouse ovaries. Reproduction. 2007;133(1):107–116. doi: 10.1530/REP-06-0144. [DOI] [PubMed] [Google Scholar]
- 92.Sun YC, Wang YY, Sun XF, Cheng SF, Li L, Zhao Y, Shen W, Chen H. The role of autophagy during murine primordial follicle assembly. Aging (Albany NY) 2018;10(2):197–211. doi: 10.18632/aging.101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang YY, Sun YC, Sun XF, Cheng SF, Li B, Zhang XF, De Felici M, Shen W. Starvation at birth impairs germ cell cyst breakdown and increases autophagy and apoptosis in mouse oocytes. Cell Death Dis. 2017;8(2):e2613. doi: 10.1038/cddis.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Watanabe R, Kimura N. Non-suckling starvation of neonatal mice promotes primordial follicle formation with activation of ovarian autophagy. J Reprod Dev. 2018;64(1):89–94. doi: 10.1262/jrd.2017-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dissen GA, Romero C, Hirshfield AN, Ojeda SR. Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology. 2001;142(5):2078–2086. doi: 10.1210/endo.142.5.8126. [DOI] [PubMed] [Google Scholar]
- 96.Kerr B, Garcia-Rudaz C, Dorfman M, Paredes A, Ojeda SR. NTRK1 and NTRK2 receptors facilitate follicle assembly and early follicular development in the mouse ovary. Reproduction. 2009;138(1):131–140. doi: 10.1530/REP-08-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update. 2015;21(6):779–786. doi: 10.1093/humupd/dmv037. [DOI] [PubMed] [Google Scholar]
- 98.Klinger FG, De Felici M. In vitro development of growing oocytes from fetal mouse oocytes: stage-specific regulation by stem cell factor and granulosa cells. Dev Biol. 2002;244(1):85–95. doi: 10.1006/dbio.2002.0592. [DOI] [PubMed] [Google Scholar]
- 99.Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol. 2002;188(1–2):65–73. doi: 10.1016/S0303-7207(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 100.Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol. 2001;175(1–2):123–130. doi: 10.1016/S0303-7207(01)00391-4. [DOI] [PubMed] [Google Scholar]
- 101.Nilsson EE, Detzel C, Skinner MK. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction. 2006;131(6):1007–1015. doi: 10.1530/rep.1.00978. [DOI] [PubMed] [Google Scholar]
- 102.Driancourt MA, Reynaud K, Cortvrindt R, Smitz J. Roles of KIT and KIT LIGAND in ovarian function. Rev Reprod. 2000;5(3):143–152. doi: 10.1530/ror.0.0050143. [DOI] [PubMed] [Google Scholar]
- 103.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383(6600):531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 104.Omari S, Waters M, Naranian T, Kim K, Perumalsamy AL, Chi M, Greenblatt E, Moley KH, Opferman JT, Jurisicova A. Mcl-1 is a key regulator of the ovarian reserve. Cell Death Dis. 2015;6:e1755. doi: 10.1038/cddis.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee DH, Jacobs DR., Jr Methodological issues in human studies of endocrine disrupting chemicals. Rev Endocr Metab Disord. 2015;16(4):289–297. doi: 10.1007/s11154-016-9340-9. [DOI] [PubMed] [Google Scholar]
- 106.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21(4):214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arendrup FS, Mazaud-Guittot S, Jegou B, Kristensen DM. EDC IMPACT: is exposure during pregnancy to acetaminophen/paracetamol disrupting female reproductive development? Endocr Connect. 2018;7(1):149–158. doi: 10.1530/EC-17-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang HQ, Zhang XF, Zhang LJ, Chao HH, Pan B, Feng YM, Li L, Sun XF, Shen W. Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Mol Biol Rep. 2012;39(5):5651–5657. doi: 10.1007/s11033-011-1372-3. [DOI] [PubMed] [Google Scholar]
- 110.Lawson C, Gieske M, Murdoch B, Ye P, Li Y, Hassold T, Hunt PA. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol Reprod. 2011;84(1):79–86. doi: 10.1095/biolreprod.110.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Holm JB, Mazaud-Guittot S, Danneskiold-Samsoe NB, Chalmey C, Jensen B, Norregard MM, Hansen CH, Styrishave B, Svingen T, Vinggaard AM, Koch HM, Bowles J, Koopman P, Jegou B, Kristiansen K, Kristensen DM. Intrauterine exposure to paracetamol and aniline impairs female reproductive development by reducing follicle reserves and fertility. Toxicol Sci. 2016;150(1):178–189. doi: 10.1093/toxsci/kfv332. [DOI] [PubMed] [Google Scholar]
- 112.Massimo De Felici GLS. Epigenetic reprogramming in the mammalian germ line: possible effects by endocrine disruptors on primordial germ cells. Open Biotechnol J. 2016;10(Suppl-1, M4):36–41. doi: 10.2174/1874070701610010036. [DOI] [Google Scholar]
- 113.La Sala G, Farini D, De Felici M. Proapoptotic effects of lindane on mouse primordial germ cells. Toxicol Sci. 2009;108(2):445–451. doi: 10.1093/toxsci/kfp027. [DOI] [PubMed] [Google Scholar]
- 114.Iona S, Klinger FG, Sisti R, Ciccalese R, Nunziata A, De Felici M. A comparative study of cytotoxic effects of N-ethyl-N-nitrosourea, adriamycin, and mono-(2-ethylhexyl)phthalate on mouse primordial germ cells. Cell Biol Toxicol. 2002;18(2):131–145. doi: 10.1023/A:1015336318623. [DOI] [PubMed] [Google Scholar]
- 115.Del-Mazo J, Brieno-Enriquez MA, Garcia-Lopez J, Lopez-Fernandez LA, De-Felici M. Endocrine disruptors, gene deregulation and male germ cell tumors. Int J Dev Biol. 2013;57(2–4):225–239. doi: 10.1387/ijdb.130042jd. [DOI] [PubMed] [Google Scholar]
- 116.Zhang XF, Zhang LJ, Feng YN, Chen B, Feng YM, Liang GJ, Li L, Shen W. Bisphenol A exposure modifies DNA methylation of imprint genes in mouse fetal germ cells. Mol Biol Rep. 2012;39(9):8621–8628. doi: 10.1007/s11033-012-1716-7. [DOI] [PubMed] [Google Scholar]
- 117.Chao HH, Zhang XF, Chen B, Pan B, Zhang LJ, Li L, Sun XF, Shi QH, Shen W. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochem Cell Biol. 2012;137(2):249–259. doi: 10.1007/s00418-011-0894-z. [DOI] [PubMed] [Google Scholar]
- 118.Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova MI, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS ONE. 2012;7(5):e36129. doi: 10.1371/journal.pone.0036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seisenberger S, Andrews S, Krueger F, Arand J, Walter J, Santos F, Popp C, Thienpont B, Dean W, Reik W. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell. 2012;48(6):849–862. doi: 10.1016/j.molcel.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, Surani MA. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science. 2013;339(6118):448–452. doi: 10.1126/science.1229277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Skinner MK, Guerrero-Bosagna C, Haque M, Nilsson E, Bhandari R, McCarrey JR. Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS ONE. 2013;8(7):e66318. doi: 10.1371/journal.pone.0066318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod. 2013;88(5):112. doi: 10.1095/biolreprod.112.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li L, Zhang T, Qin XS, Ge W, Ma HG, Sun LL, Hou ZM, Chen H, Chen P, Qin GQ, Shen W, Zhang XF. Exposure to diethylhexyl phthalate (DEHP) results in a heritable modification of imprint genes DNA methylation in mouse oocytes. Mol Biol Rep. 2014;41(3):1227–1235. doi: 10.1007/s11033-013-2967-7. [DOI] [PubMed] [Google Scholar]
- 124.Zhou C, Gao L, Flaws JA. Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology. 2017;158(6):1739–1754. doi: 10.1210/en.2017-00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pocar P, Fiandanese N, Berrini A, Secchi C, Borromeo V. Maternal exposure to di(2-ethylhexyl)phthalate (DEHP) promotes the transgenerational inheritance of adult-onset reproductive dysfunctions through the female germline in mice. Toxicol Appl Pharmacol. 2017;322:113–121. doi: 10.1016/j.taap.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 126.Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ., Jr Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90(4):911–940. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3(1):e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, Hassold T, VandeVoort CA. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci USA. 2012;109(43):17525–17530. doi: 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brieno-Enriquez MA, Robles P, Camats-Tarruella N, Garcia-Cruz R, Roig I, Cabero L, Martinez F, Caldes MG. Human meiotic progression and recombination are affected by Bisphenol A exposure during in vitro human oocyte development. Hum Reprod. 2011;26(10):2807–2818. doi: 10.1093/humrep/der249. [DOI] [PubMed] [Google Scholar]
- 130.Brieno-Enriquez MA, Reig-Viader R, Cabero L, Toran N, Martinez F, Roig I, Garcia Caldes M. Gene expression is altered after bisphenol A exposure in human fetal oocytes in vitro. Mol Hum Reprod. 2012;18(4):171–183. doi: 10.1093/molehr/gar074. [DOI] [PubMed] [Google Scholar]
- 131.Zhang XF, Zhang T, Han Z, Liu JC, Liu YP, Ma JY, Li L, Shen W. Transgenerational inheritance of ovarian development deficiency induced by maternal diethylhexyl phthalate exposure. Reprod Fertil Dev. 2015;27(8):1213–1221. doi: 10.1071/RD14113. [DOI] [PubMed] [Google Scholar]
- 132.Liew Z, Ritz B, Rebordosa C, Lee PC, Olsen J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 2014;168(4):313–320. doi: 10.1001/jamapediatrics.2013.4914. [DOI] [PubMed] [Google Scholar]
- 133.Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol. 2005;193(3 Pt 1):771–777. doi: 10.1016/j.ajog.2005.02.100. [DOI] [PubMed] [Google Scholar]
- 134.Dean A, van den Driesche S, Wang Y, McKinnell C, Macpherson S, Eddie SL, Kinnell H, Hurtado-Gonzalez P, Chambers TJ, Stevenson K, Wolfinger E, Hrabalkova L, Calarrao A, Bayne RA, Hagen CP, Mitchell RT, Anderson RA, Sharpe RM. Analgesic exposure in pregnant rats affects fetal germ cell development with inter-generational reproductive consequences. Sci Rep. 2016;6:19789. doi: 10.1038/srep19789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang W, Hafner KS, Flaws JA. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol Appl Pharmacol. 2014;276(2):157–164. doi: 10.1016/j.taap.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lea RG, Amezaga MR, Loup B, Mandon-Pepin B, Stefansdottir A, Filis P, Kyle C, Zhang Z, Allen C, Purdie L, Jouneau L, Cotinot C, Rhind SM, Sinclair KD, Fowler PA. The fetal ovary exhibits temporal sensitivity to a ‘real-life’ mixture of environmental chemicals. Sci Rep. 2016;6:22279. doi: 10.1038/srep22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang T, Li L, Qin XS, Zhou Y, Zhang XF, Wang LQ, De Felici M, Chen H, Qin GQ, Shen W. Di-(2-ethylhexyl) phthalate and bisphenol A exposure impairs mouse primordial follicle assembly in vitro. Environ Mol Mutagen. 2014;55(4):343–353. doi: 10.1002/em.21847. [DOI] [PubMed] [Google Scholar]
- 139.Mu X, Liao X, Chen X, Li Y, Wang M, Shen C, Zhang X, Wang Y, Liu X, He J. DEHP exposure impairs mouse oocyte cyst breakdown and primordial follicle assembly through estrogen receptor-dependent and independent mechanisms. J Hazard Mater. 2015;298:232–240. doi: 10.1016/j.jhazmat.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 140.Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod. 2006;74(1):161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- 141.Rodriguez HA, Santambrosio N, Santamaria CG, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod Toxicol. 2010;30(4):550–557. doi: 10.1016/j.reprotox.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 142.Ahn HJ, An BS, Jung EM, Yang H, Choi KC, Jeung EB. Parabens inhibit the early phase of folliculogenesis and steroidogenesis in the ovaries of neonatal rats. Mol Reprod Dev. 2012;79(9):626–636. doi: 10.1002/mrd.22070. [DOI] [PubMed] [Google Scholar]
- 143.Karavan JR, Pepling ME. Effects of estrogenic compounds on neonatal oocyte development. Reprod Toxicol. 2012;34(1):51–56. doi: 10.1016/j.reprotox.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 144.Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233(2):286–296. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Johansson HK, Jacobsen PR, Hass U, Svingen T, Vinggaard AM, Isling LK, Axelstad M, Christiansen S, Boberg J. Perinatal exposure to mixtures of endocrine disrupting chemicals reduces female rat follicle reserves and accelerates reproductive aging. Reprod Toxicol. 2016;61:186–194. doi: 10.1016/j.reprotox.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 146.Rivera OE, Varayoud J, Rodriguez HA, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol. 2011;32(3):304–312. doi: 10.1016/j.reprotox.2011.06.118. [DOI] [PubMed] [Google Scholar]
- 147.Zhuang XL, Fu YC, Xu JJ, Kong XX, Chen ZG, Luo LL. Effects of genistein on ovarian follicular development and ovarian life span in rats. Fitoterapia. 2010;81(8):998–1002. doi: 10.1016/j.fitote.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 148.Rasmussen LM, Sen N, Liu X, Craig ZR. Effects of oral exposure to the phthalate substitute acetyl tributyl citrate on female reproduction in mice. J Appl Toxicol. 2017;37(6):668–675. doi: 10.1002/jat.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cummings AM. Methoxychlor as a model for environmental estrogens. Crit Rev Toxicol. 1997;27(4):367–379. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- 150.Hall DL, Payne LA, Putnam JM, Huet-Hudson YM. Effect of methoxychlor on implantation and embryo development in the mouse. Reprod Toxicol. 1997;11(5):703–708. doi: 10.1016/S0890-6238(97)00026-9. [DOI] [PubMed] [Google Scholar]
- 151.Medigovic I, Ristic N, Trifunovic S, Manojlovic-Stojanoski M, Milosevic V, Zikic D, Nestorovic N. Genistein affects ovarian folliculogenesis: a stereological study. Microsc Res Tech. 2012;75(12):1691–1699. doi: 10.1002/jemt.22117. [DOI] [PubMed] [Google Scholar]
- 152.Hannon PR, Peretz J, Flaws JA. Daily exposure to di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod. 2014;90(6):136. doi: 10.1095/biolreprod.114.119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hannon PR, Brannick KE, Wang W, Flaws JA. Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biol Reprod. 2015;92(5):120. doi: 10.1095/biolreprod.115.129148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li Y, Zhang W, Liu J, Wang W, Li H, Zhu J, Weng S, Xiao S, Wu T. Prepubertal bisphenol A exposure interferes with ovarian follicle development and its relevant gene expression. Reprod Toxicol. 2014;44:33–40. doi: 10.1016/j.reprotox.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 155.Pru JK, Kaneko-Tarui T, Jurisicova A, et al. Induction of proapoptotic gene expression and recruitment of p53 herald ovarian follicle loss caused by polycyclic aromatic hydrocarbons. Reprod Sci. 2009;16(4):347–356. doi: 10.1177/1933719108327596. [DOI] [PubMed] [Google Scholar]
- 156.Jurisicova A, Taniuchi A, Li H, Shang Y, Antenos M, Detmar J, Xu J, Matikainen T, Benito Hernandez A, Nunez G, Casper RF. Maternal exposure to polycyclic aromatic hydrocarbons diminishes murine ovarian reserve via induction of Harakiri. J Clin Investig. 2007;117(12):3971–3978. doi: 10.1172/JCI28493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee SG, Kim JY, Chung JY, Kim YJ, Park JE, Oh S, Yoon YD, Yoo KS, Yoo YH, Kim JM. Bisphenol A exposure during adulthood causes augmentation of follicular atresia and luteal regression by decreasing 17beta-estradiol synthesis via downregulation of aromatase in rat ovary. Environ Health Perspect. 2013;121(6):663–669. doi: 10.1289/ehp.1205823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58(1):44–50. doi: 10.1262/jrd.2011-012. [DOI] [PubMed] [Google Scholar]
- 159.Xu C, Chen JA, Qiu Z, Zhao Q, Luo J, Yang L, Zeng H, Huang Y, Zhang L, Cao J, Shu W. Ovotoxicity and PPAR-mediated aromatase downregulation in female Sprague–Dawley rats following combined oral exposure to benzo[a]pyrene and di-(2-ethylhexyl) phthalate. Toxicol Lett. 2010;199(3):323–332. doi: 10.1016/j.toxlet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 160.Wang W, Sun Y, Liu J, Li Y, Li H, Xiao S, Weng S, Zhang W. Soy isoflavones administered to rats from weaning until sexual maturity affect ovarian follicle development by inducing apoptosis. Food Chem Toxicol. 2014;72:51–60. doi: 10.1016/j.fct.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 161.Xu J, Osuga Y, Yano T, Morita Y, Tang X, Fujiwara T, Takai Y, Matsumi H, Koga K, Taketani Y, Tsutsumi O. Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochem Biophys Res Commun. 2002;292(2):456–462. doi: 10.1006/bbrc.2002.6644. [DOI] [PubMed] [Google Scholar]
- 162.Wang W, Craig ZR, Basavarajappa MS, Hafner KS, Flaws JA. Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol Reprod. 2012;87(6):152. doi: 10.1095/biolreprod.112.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Johansson HKL, Svingen T, Fowler PA, Vinggaard AM, Boberg J. Environmental influences on ovarian dysgenesis—developmental windows sensitive to chemical exposures. Nat Rev Endocrinol. 2017;13(7):400–414. doi: 10.1038/nrendo.2017.36. [DOI] [PubMed] [Google Scholar]
- 164.Schmidt CW. Age at menopause: do chemical exposures play a role? Environ Health Perspect. 2017;125(6):062001. doi: 10.1289/EHP2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Taylor KW, Hoffman K, Thayer KA, Daniels JL. Polyfluoroalkyl chemicals and menopause among women 20–65 years of age (NHANES) Environ Health Perspect. 2014;122(2):145–150. doi: 10.1289/ehp.1306707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Knox SS, Jackson T, Javins B, Frisbee SJ, Shankar A, Ducatman AM. Implications of early menopause in women exposed to perfluorocarbons. J Clin Endocrinol Metab. 2011;96(6):1747–1753. doi: 10.1210/jc.2010-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Galloway T, Cipelli R, Guralnik J, Ferrucci L, Bandinelli S, Corsi AM, Money C, McCormack P, Melzer D. Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environ Health Perspect. 2010;118(11):1603–1608. doi: 10.1289/ehp.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Eskenazi B, Warner M, Marks AR, Samuels S, Gerthoux PM, Vercellini P, Olive DL, Needham L, Patterson D, Jr, Mocarelli P. Serum dioxin concentrations and age at menopause. Environ Health Perspect. 2005;113(7):858–862. doi: 10.1289/ehp.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Aldad TS, Rahmani N, Leranth C, Taylor HS. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertil Steril. 2011;96(1):175–179. doi: 10.1016/j.fertnstert.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck Keizer-Schrama S, Hogervorst E, Janse F, Liao L, Vlaisavljevic V, Zillikens C, Vermeulen N. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–937. doi: 10.1093/humrep/dew027. [DOI] [PubMed] [Google Scholar]
- 171.Goswami D, Conway GS. Premature ovarian failure. Horm Res. 2007;68(4):196–202. doi: 10.1159/000102537. [DOI] [PubMed] [Google Scholar]
- 172.Brown S. Endocrine disrupting chemicals associated with earlier menopause. Post Reprod Health. 2015;21(1):5–6. doi: 10.1177/2053369115574688. [DOI] [PubMed] [Google Scholar]
- 173.Beranger R, Hoffmann P, Christin-Maitre S, Bonneterre V. Occupational exposures to chemicals as a possible etiology in premature ovarian failure: a critical analysis of the literature. Reprod Toxicol. 2012;33(3):269–279. doi: 10.1016/j.reprotox.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 174.Gascon M, Vrijheid M, Nieuwenhuijsen MJ. The built environment and child health: an overview of current evidence. Curr Environ Health Rep. 2016;3(3):250–257. doi: 10.1007/s40572-016-0094-z. [DOI] [PubMed] [Google Scholar]
- 175.Haruty B, Friedman J, Hopp S, Daniels R, Pregler J. Reproductive health and the environment: counseling patients about risks. Cleve Clin J Med. 2016;83(5):367–372. doi: 10.3949/ccjm.83a.14070. [DOI] [PubMed] [Google Scholar]
- 176.Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2010;33:385–393. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bloom MS, Kim D, Vom Saal FS, Taylor JA, Cheng G, Lamb JD, Fujimoto VY. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil Steril. 2011;96:672–677e2. doi: 10.1016/j.fertnstert.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fujimoto VY, Kim D, vom Saal FS, Lamb JD, Taylor JA, Bloom MS. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil Steril. 2011;95:1816–1819. doi: 10.1016/j.fertnstert.2010.11.008. [DOI] [PubMed] [Google Scholar]