Abstract
Thoracic aorta perivascular adipose tissue (T-PVAT) has critical roles in regulating vascular homeostasis. However, the developmental characteristics and cellular lineage of adipocyte in the T-PVAT remain unclear. We show that T-PVAT contains three long strip-shaped fat depots, anterior T-PVAT (A-T-PVAT), left lateral T-PVAT (LL-T-PVAT), and right lateral T-PVAT (RL-T-PVAT). A-T-PVAT displays a distinct transcriptional profile and developmental origin compared to the two lateral T-PVATs (L-T-PVAT). Lineage tracing studies indicate that A-T-PVAT adipocytes are primarily derived from SM22α+ progenitors, whereas L-T-PVAT contains both SM22α+ and Myf5+ cells. We also show that L-T-PVAT contains more UCP1+ brown adipocytes than A-T-PVAT, and L-T-PVAT exerts a greater relaxing effect on aorta than A-T-PVAT. Angiotensin II-infused hypertensive mice display greater macrophage infiltration into A-T-PVAT than L-T-PVAT. These combined results indicate that L-T-PVAT has a distinct development from A-T-PVAT with different cellular lineage, and suggest that L-T-PVAT and A-T-PVAT have different physiological and pathological functions.
Electronic supplementary material
The online version of this article (10.1007/s00018-018-2970-1) contains supplementary material, which is available to authorized users.
Keywords: Thoracic aorta perivascular adipocyte, Development, Lineage tracing, SM22α, Myf5
Introduction
Cardiovascular disease is the leading cause of morbidity and mortality worldwide. There is increasing evidence that adipose tissue dysfunction is a major risk factor for cardiovascular diseases such as hypertension and atherosclerosis [1–3]. Essentially, all arteries are surrounded by differing amounts of perivascular adipose tissue (PVAT) [4, 5]. PVAT has been reported to have crucial roles in vascular homeostasis by secreting adipokines, inflammatory factors, and hormones [6–10].
Adipose tissue includes white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is presumed to be the main depot for lipid storage, whereas BAT dissipates energy though uncoupled respiration and thermogenesis and protects against metabolic disorders [11]. Adipose tissue in different anatomical locations has different developmental dynamics [12]. For example, subcutaneous fat development occurs during embryonic days 14–18, whereas gonadal fat starts to develop in postnatal mice [13, 14]. Although essentially all adipocytes differentiate from mesodermal stem cells, brown and white adipocytes arise from different progenitors [12]. Brown adipocytes are derived primarily from Myf5-expressing dermomyotome, whereas most white adipocytes are derived from PDGFRα-positive progenitors [15–17]. A recent study reported that PVAT could be deleted by crossing the adipogenic transcription factor PPARγ-flox mice to SM22α-Cre knock-in mice [18]. Vascular resident progenitors have the potential to differentiate into different cell types including adipocytes [19]. Thus, the underlying origin of PVAT is not completely understood.
PVAT differs between species and anatomical locations [20]. PVAT contains both BAT and WAT, and displays differing BAT/WAT ratios according to the type of vessel it surrounds [11]. Thoracic aortic PVAT (T-PVAT) shares structural, genetic, and proteomic features with BAT [18], including several small, multilocular lipid droplets and abundant mitochondria. Mounting evidence indicates that T-PVAT secretes a wide range of bioactive molecules and affects vascular function in normal and pathological vessels [21, 22]. Structural and functional changes in T-PVAT are involved in regulating vascular function [8, 23]. The Framingham Heart Study considers T-PVAT hyperplasia as an independent risk factor in human cardiovascular disease [24]. However, little is known about the developmental characteristics of T-PVAT.
In this study, we hypothesize that T-PVAT is a heterogeneous adipose tissue derived from multiple progenitor cells, and different anatomical locations of T-PVAT may have different origins and distinct physiological functions. We investigated the structure and adipogenesis of T-PVAT. We found that T-PVAT was unevenly distributed around the aorta and had different developmental dynamics at different anatomical sites. RNA-seq was performed to map the transcriptional profiles in different parts of T-PVAT. A lineage tracing study indicates that T-PVAT is derived from different adipocyte progenitor cells at different locations, which is associated with distinct physiological and pathological functions.
Materials and methods
Animals
All C56/B6 mice were purchased from Shanghai Slac Laboratory Animal Co., Ltd., China. One-month-old and 5-month-old male mice were used in all experiments. Myf5-Cre knock-in mice (Jackson Lab, USA, stock 007893) and Rosa26-RFP reporter mice [25] were bred to generate Myf5-specific RFP-expressing mice (Myf5-Cre;Rosa26RFP/+). SM22α CreERT2 knock-in mice [26] and Rosa26-RFP reporter mice were bred to generate tamoxifen-inducible SM22α-specific RFP-expressing mice (SM22α CreERT2;Rosa26RFP/+). Tamoxifen (ABCONE, Shanghai, China, Cat. T56488) was dissolved in 10 mg/ml corn oil and administered to SM22α-CreERT2;Rosa26RFP/+ mice by intraperitoneal injection (ip) (0.15 mg/g body weight) to induce Cre activity at the indicated time. All mice were backcrossed to C57/B6 mice for more than eight generations. Mice were housed in the Laboratory Animal Facility at Shanghai Jiao Tong University School of Medicine at 25 °C. All animal experiments were approved by the Animal Care Committee of Shanghai Jiao Tong University.
Histological analysis
Mice were anesthetized by isoflurane inhalation and killed by CO2 asphyxiation. T-PVAT was dissected for histological analysis as described previously [27]. Tissues were fixed in 4% paraformaldehyde overnight at 4 °C and then embedded in paraffin for histological analysis or optimal cutting temperature (OCT) compound for immunofluorescent staining. Transverse sections were cut from paraffin blocks into 10-μm sections for hematoxylin and eosin (H&E) staining and from OCT blocks into 8-μm sections for immunofluorescent staining. At least ten sections per sample and four samples per genotype were analyzed.
Oil Red staining
Oil Red O Stain kits (Abcam, Cambridge, MA, USA, Cat. ab150678) were used for Oil Red staining according to the manufacturer’s protocol. Paraformaldehyde-fixed whole-mount T-PVAT tissue was briefly washed with phosphate-buffered saline (PBS) three times for 5 min, rinsed with 60% isopropanol, and stained with freshly prepared Oil Red working solution for 15 min. The samples were then rinsed with 60% isopropanol and finally rinsed with distilled water. Photographs were taken of the stained tissue by using Leica M205 FA stereo microscopes.
Immunofluorescence staining
Mouse tissues were fixed in 4% paraformaldehyde overnight, washed with PBS, soaked in 30% sucrose overnight at 4 °C, and then embedded in OCT compound. Cryosections of OCT-embedded T-PVAT were cut into 8-μm thickness. The primary antibodies used in this study were goat anti-perilipin (1:100, Abcam, Cambridge, MA, USA, Cat. ab61682) and rabbit anti-UCP1 (1:100, Abcam, Cambridge, MA, USA, Cat. ab10983). Secondary antibodies conjugated to Alexa Fluor 488, 555, and 647 (1:500; ThermoFisher, USA, Cat.A-11055, Cat.A-21127, and Cat.A-21244) were used to detect the corresponding primary antibodies. Goat serum and rabbit serum were used as negative controls for anti-perilipin antibody and anti-UCP1 antibody, respectively. Photographs were taken of the stained tissue by using Zeiss Cell Observer confocal microscope
RNA sequencing
Total RNA was extracted from A-T-PVAT, LL-T-PVAT, and RL-T-PVAT using RNAiso Plus Total RNA Extraction Reagent (TAKARA, Takara Bio Inc., Japan, Cat#9109). The RNA quality was checked using an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). Qualified total RNA was used for amplification of complementary DNA (cDNA) using SuperScriptII (Invitrogen, Carlsbad, CA, USA 18064014) according to the manufacturer’s instructions. The sample was amplified with PCR and sequenced on the Illumina HiSeq2500 platform at Shanghai Biotechnology Corporation. Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) analyses were performed to identify the functions of unigenes and distributions of gene functions, especially focusing on genes related to the development of adipose tissue and blood vessels.
Thoracic aorta ring assay
The thoracic aorta ring assay was performed as described previously [28]. Five-month-old C56/B6 mouse thoracic aorta were dissected out together with whole T-PVAT and cut into 2-mm rings [we use thoracic aortas and T-PVAT around the 7th–9th thoracic vertebra (T-vertebra) for this experiment]. Then, the sample was separated into three groups, with each group only keeping A-T-PVAT, LL-T-PVAT, or RL-T-PVAT on the aorta. All T-PVAT tissue was weighed after the experiment to ensure similar comparisons. Thoracic aortas together with different parts of T-PVAT (A-T-PVAT, LL-T-PVAT, or RL-T-PVAT) were fixed on isometric force transducers (Danish Myo Technology Model 610 M, Denmark) in a 5 ml organ bath and aerated with 95% O2 and 5% CO2 under an initial resting tension of 2.5 mN. Force was recorded in a Power Lab/8sp data acquisition system (A.D. Instruments, Castle Hill, Australia). After 1 h of incubation in oxygenated Krebs medium (containing: KCl 4.7 mmol/L, NaCl 118 mmol/L, CaCl2 2.5 mmol/L, KH2PO4 1.2 mmol/L, MgSO4 1.2 mmol/L, glucose 11 mmol/L and NaHCO3 25 mmol/L) at pH 7.4 and 37 °C, ring contractility was tested three times in high K+ medium (60 mM KCI) to stabilize the contraction. Cumulative response curves of phenylephrine (10−8 to 10−4 mol/L) were performed to assess the vasoconstriction response. Cumulative concentration–response curves of acetylcholine (10−8 to 10−4 mol/L) were constructed with phenylephrine pre-contraction (3 µmol/L).
Angiotensin II (Ang II) infusion
Five-month-old C57/B6 male mice were subjected to PVAT inflammatory response generated by infusion of AngII (Sigma-Aldrich, St. Louis, MO, USA, Cat. A9525) via subcutaneously embedded osmotic minipump (Alzet, Cupertino, CA, USA, model 2002). Mice were randomly assigned into the following two groups (n = 5 per group): group 1, mice infused with vehicle (saline); group 2, mice treated with Ang II (1000 ng/kg per min) for 2 weeks.
Systolic blood pressure was measured by a tail-cuff method using a noninvasive blood pressure instrument (BP-98A, Softron, Beijing, China) 3 days before and 14 days after AngII infusion as described previously [29]. Significantly different blood pressures at the two time points were considered as successful AngII-induced PVAT inflammatory response as described previously [29].
Flow cytometry
Single-cell suspensions of T-PVAT were prepared as described previously [30]. A-T-PVAT and L-T-PVAT of angiotensin II-infused mice were carefully dissected under a Leica M205 FA stereo microscopes, minced with opposing scalpels, and digested with 0.2 mg/ml Collagenase I (Sigma-Aldrich, St. Louis, MO, USA, Cat. C0130) in DMEM medium containing 0.5% FBS at 37 °C. After 1 h of digestion, the digested tissue was filtered through 100-mm cell filters (BD Falcon). Cells were labeled with fluorochrome-conjugated antibody to F4/80 (1:200) (Bio-Rad, Hercules, CA, USA, MCA497A488T). Stained cells were assessed via flow cytometry using a FACSCalibur flow cytometer (BD Biosciences), and data were analyzed with FlowJo software.
Statistical analysis
GraphPad prism 6.01 was used for statistical analysis. Data were presented as mean ± SD. The Shapiro–Wilk normality test was applied to confirm the normality distribution of a variable. After comparing variances via the F test, Student’s t test was used for two-group comparisons. Multiple comparisons were tested by one-way ANOVA followed by the post hoc Dunnett’s test (Levine’s tests were performed for equal variance). Dunnett’s T3 test was used for post hoc test comparison for the analysis of unequal variances (Welch and Brown–Forsythe test). Differences were considered as statistically significant at P < 0.05.
Results
Anatomy and distribution of T-PVAT
We performed a dissection analysis of T-PVAT and subsequent Oil Red staining to distinguish adipose tissue surrounding the thoracic aorta. We detected T-PVAT primarily between the aortic arch at the T4 vertebra and the aortic hiatus of the diaphragm at the T10–T11 vertebrae. T-PVAT was composed of three long strip-shaped adipose depots (Fig. 1a–h, Supplemental Fig. S1A-H), which we named left lateral T-PVAT (LL-T-PVAT), right lateral T-PVAT (RL-T-PVAT), and anterior T-PVAT (A-T-PVAT) (Fig. 1c–e). The two L-T-PVAT clung to the posterior chest wall and were merged with the paravertebral fat, whereas the A-T-PVAT was attached to the anterior wall of the thoracic aorta and filled the triangular prism space between the thoracic aorta and lung. These results suggest that A-T-PVAT and L-T-PVAT have different anatomical characteristics.
Fig. 1.
Anatomy of mouse T-PVAT. a Gross picture of adult mouse T-PVAT. b Whole-mount Oil Red staining of adult mouse T-PVAT. b1 Magnification, upper segment of T-PVAT. b2. Magnification, lower segment of T-PVAT. c–e Whole-mount Oil Red staining of transverse sections of adult mouse T-PVAT at T4 (c), T7 (d), and T9 (e) vertebra levels; n = 8 mice. f–h Hematoxylin and eosin staining of transverse sections of adult mouse T-PVAT at T4 (f), T7 (g), and T9 (h) vertebra levels; n = 8 mice. Scale bar: 400 μm. PAT paravertebral adipose tissue, AP A-T-PVAT, LLP LL-T-PVAT, RLP RL-T-PVAT, TA thoracic aorta, SC spinal cord, V vertebra
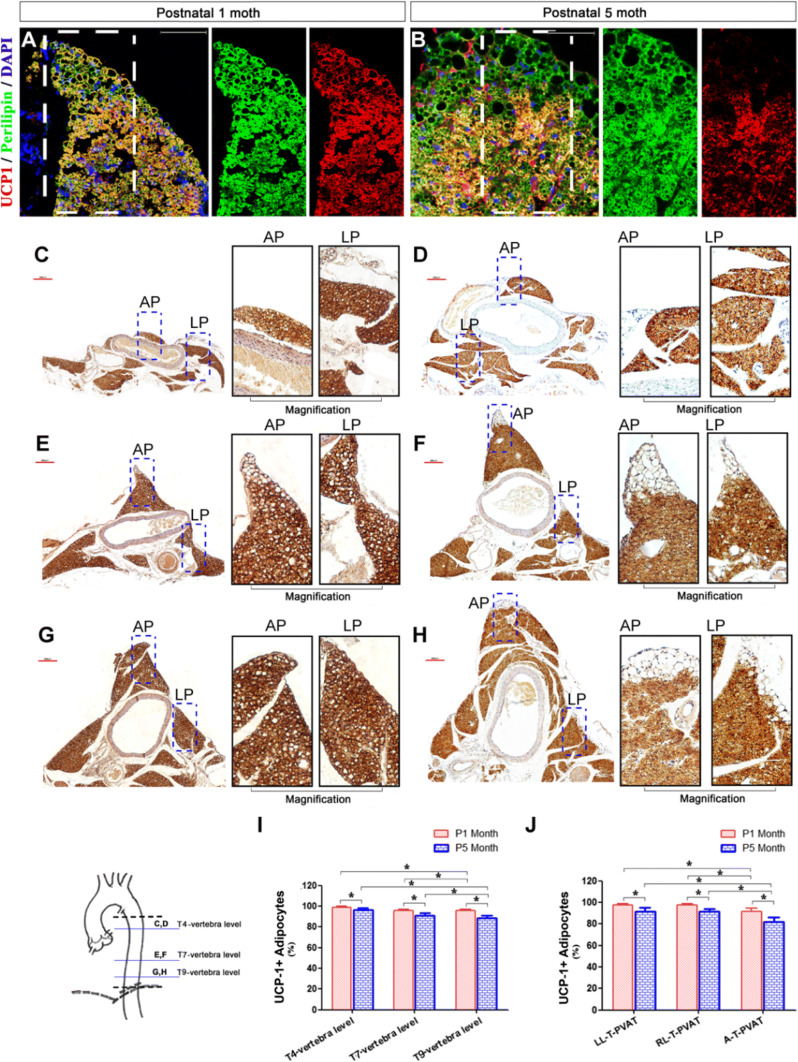
Since PVAT is composed of both BAT and WAT, we next determined the adipocyte type in T-PVAT by performing immunohistochemical staining for perilipin (adipocyte marker) and UCP1 (BAT marker) in L-T-PVAT and A-T-PVAT from 1-month-old and 5-month-old mice (Fig. 2a–h). At these developmental states, T-PVAT formed a complete fat sheath surrounding the aorta (1-month-old) and the mouse body weight was almost the heaviest throughout the lifetime (5-month-old) [31]. T-PVAT contained primarily perilipin+;UCP1+ brown adipocytes, and only a small fraction of perilipin+;UCP1− white adipocytes were found at the edge of T-PVAT (Fig. 2a, b). We calculated the percentage of UCP1+ adipocytes in T-PVAT at the T4, T7, and T9 vertebral levels (Fig. 2c–h); 1-month-old mice contained 98.8 ± 1.0%, 95.7 ± 1.5%, and 95.7 ± 1.3% UCP-1+ adipocytes, respectively, and 5-month-old mice contained 96.3 ± 1.7%, 91.1 ± 2.1%, and 88.4 ± 2.8% UCP1+ adipocytes, respectively (Fig. 2I). The percentages of UCP1+ adipocytes in LL-T-PVAT, RL-T-PVAT, and A-T-PVAT at the 9th T-vertebra level were calculated as follows: 1-month-old mice contained 97.8 ± 1.0%, 97.7 ± 1.4%, and 91.8 ± 3.1% UCP1+ adipocytes, respectively, and 5-month-old mice contained 91.9 ± 3.1%, 91.7 ± 2.6%, and 81.7 ± 4.6% UCP1+ adipocytes, respectively (Fig. 2J). These results indicate that the number of brown adipocytes declines in both L-T-PVAT and A-T-PVAT during aging, L-T-PVAT contains more brown adipocytes than A-T-PVAT, and more BAT surrounds the proximal aorta than the distal aorta with respect to the aortic arch in 5-month-old mice.
Fig. 2.
T-PVAT contains BAT and WAT. a, b Perilipin (green) and UCP1 (red) immunofluorescence staining of A-T-PVAT in P1 month (a) and P5 month (b) mice; Scale bar: 50 μm. c–h UCP1 immunohistochemical staining of T-PVAT in P1 month (c, e, g) and P5 month (d, f, h) mice at T4 (c, d), T7 (e, f), and T9 (g, h) vertebra levels; Scale bar: 200 μm. i Statistics of the ratio of UCP1+ adipose cells in T-PVAT at T4, T7, and T9 vertebra levels in P1 month and P5 month mice. j Statistics of the ratio of UCP1+ adipose cells in A-T-PVAT and LL/RL-T-PVAT at the 9th T-vertebra level in P1 month and P5 month mice; n = 10 mice per group. *P < 0.05
Transcriptome analysis of T-PVAT
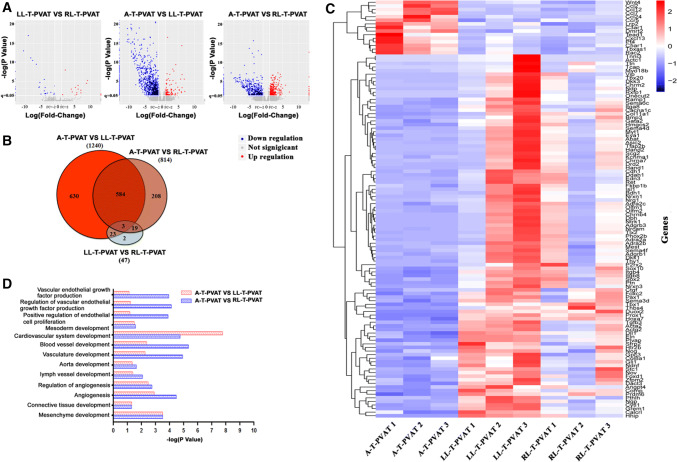
To determine whether anatomical and distributional parameters of L-T-PVAT and A-T-PVAT are associated with different transcriptional profiles, we performed RNA sequencing of LL-T-PVAT, RL-T-PVAT, and A-T-PVAT from 5-month-old C57/B6 male mice. Volcano plots and Venn diagrams showed large overlaps between LL-T-PVAT and RL-T-PVAT, whereas those for A-T-PVAT were distinctly different (Fig. 3a, b). To further investigate the biological specificity between A-T-PVAT and LL/RL-T-PVAT, we performed cluster and GO analyses. The heatmap identified several GO terms related to biological process, cellular component, and molecular function (Fig. 3c, d). We detected an abundance of differently expressed genes in the developmental regulation of A-T-PVAT and LL/RL-T-PVAT, including aorta development, angiogenesis, connective tissue development, and adipogenesis (Fig. 3d). These results suggest that A-T-PVAT and L-T-PVAT may have different developmental programs and origins, which may reflect differences in anatomical parameters between A-T-PVAT and L-T-PVAT.
Fig. 3.
RNA-seq profiling of A-T-PVAT and L/RL-T-PVAT. a RNA-seq volcano plots showing differential gene expression between A-T-PVAT and LL/RL-T-PVAT. b Venn diagrams showing the unsupervised clustering analysis of genome-wide expression indicated that LL-T-PVAT and RL-T-PVAT have very similar gene expression, but A-T-PVAT has unigene expression compared to LL/RL-T-PVAT. c Heatmap showing differential gene expression profiles in A-T-PVAT, LL-T-PVAT, and RL-T-PVAT. d Histograms from GO analysis depict some developmental pathways that are differentially expressed in A-T-PVAT and LL/RL-T-PVAT; n = 3 mice
Developmental programs of L-T-PVAT and A-T-PVAT
To determine whether L-T-PVAT and A-T-PVAT have different developmental programs, we performed Oil Red staining of T-PVAT from postnatal day 1 to 1-month-old (Fig. 1a–j, Supplemental Fig. S2A–G, Supplemental Fig. S3A–H). At P1, paravertebral fat was visible at the posterior chest wall along with vertebration, whereas adipocyte-positive staining was not detected surrounding the thoracic aorta (Fig. 4a, f, Supplemental Fig. S2A). Both L-T-PVAT were visible at P2 (Fig. 4g, Supplemental Fig. S2B), grew very quickly (Fig. 4c, d, h, i, k–m, Supplemental Fig. S2C–F), and fused with paravertebral fat to finally form L-T-PVAT (Fig. 4e, j, Supplemental Fig. S2G). By contrast, A-T-PVAT was first detected at P3 (Fig. 4b, k, green arrow, Supplemental Fig. S2C) and reached the T5 vertebral level at P7 (Fig. 4d, i, Supplemental Fig. 3a–h). About 1 month after birth, mouse T-PVAT was fully developed (Fig. 4e, j, Supplemental Fig. S2G). These combined results suggested that L-T-PVAT has at least has two different origins. Some adipocytes were derived from paravertebral fat, which extended toward the vertebra and touched the thoracic aorta, whereas other adipocytes were derived from resident progenitor cells. By contrast, essentially all A-T-PVAT cells were derived from vascular resident progenitor cells (Fig. 4n–r). Cultures of ex vivo adventitia together with the thoracic aorta anterior wall of P1 newborn mice showed positive Oil Red staining, which further indicated that A-T-PVAT was derived from vascular resident progenitor cells (Supplemental Fig. S4).
Fig. 4.
Development of mouse T-PVAT. a–j Direct front view (a–e) and left side view (f–j) of whole-mount Oil Red staining of mouse T-PVAT at different developmental stages: postnatal day 1 (a, f), 2 (g), 3 (b), 4 (h), 5 (c), 7 (d, i), and postnatal 1 month (e, j); n = 8 mice per stage. k–m Hematoxylin and eosin staining of T-PVAT at the T7 vertebra level at different developmental stages: postnatal day 3 (k), day 5 (l), and day 7 (m); n = 8 mice per stage. n Diagram of transverse surface of T-PVAT. o–r Diagram of T-PVAT developmental progression. Scale bar: a–g 1 mm; k–m 100 μm
SM22α-CreERT2 lineage tracing of L-T-PVAT and A-T-PVAT
To determine whether the origin of T-PVAT is related to vascular cells, we generated SM22α-CreERT2;Rosa26RFP/+ mice, which were used for lineage tracing of vascular smooth muscle cells (VSMCs) and myofibroblasts. Two pulses of tamoxifen were administered from E8.5 in SM22α-CreERT2;Rosa26RFP/+ pregnant mice to label SM22α+ cells. The T-PVAT was collected at postnatal 1 month when the T-PVAT was well developed (Fig. 5a). Immunohistochemical staining with RFP and the adipocyte marker perilipin showed that 88.6 ± 5.5% of adipocytes in A-T-PVAT were RFP positive (Fig. 5b, d), whereas 61.6 ± 9.9% of adipocytes in L-T-PVAT were RFP positive. (Figure 5b, c). Essentially all T-PVAT adipocytes were RFP negative in E16.5 tamoxifen-treated mice, although T-PVAT development began on postnatal day 2 (Fig. 5b, e, f). Administration of three pulses of tamoxifen in adult mice blocked T-PVAT adipocyte labeling with RFP (Fig. 5b, g, h). These results suggest that adipocyte progenitor cell fate decision occurs earlier than adipocyte differentiation during T-PVAT development, and T-PVAT progenitor cells transiently express SM22α during early development.
Fig. 5.
Most T-PVAT adipose progenitors transiently express SM22α. a Schematic diagram showing the strategy for genetic labeling of SM22α+ cells by tamoxifen treatment. b Ratios of SM22α+ adipocytes in T-PVAT at different tamoxifen treatment time points. c–h Immunostaining for RFP, perilipin (green), and DAPI (blue) in sections of T-PVAT from SM22α-CreER;Rosa26RFP/+ mice with tamoxifen induction at E8.5 (c, d), E16.5 (e, f), and P8 week (g, h); n = 8 mice per stage, *P < 0.05. Scale bar 50 μm
Myf5-Cre lineage tracing of L-T-PVAT and A-T-PVAT
To determine whether T-PVAT is derived from BAT progenitor cells, we generated Myf5-Cre; Rosa26RFP/+ mice, which are used for tracing brown adipocytes and skeletal muscle progenitor cells. Consistent with the previous anatomical experiments, perilipin+ adipocytes developed earlier in L-T-PVAT than in A-T-PVAT from P3 to P7. L-T-PVAT contained many more RFP+ adipocytes than A-T-PVAT (Fig. 6a, b, f, Supplemental Fig. S5), which supported the previous evidence that T-PVAT is partly derived from paravertebral Myf5+ BAT precursors (Fig. 4o–r). Next, we observed the distribution of Myf5+ adipocytes in adult mice. L-T-PVAT contained 27.0 ± 6.0% of brown adipocytes (UCP1+), whereas A-T-PVAT contained 9.4 ± 3.2% of brown adipocytes (UCP1+) labeled with RFP (Fig. 6a, b, e). Most subscapular brown adipocytes (99.2 ± 1.0%) were labeled with RFP (Fig. 6c), whereas only few gonadal adipocytes (2.4 ± 0.8%) were labeled with RFP (Fig. 6d). These results indicate that some adipocytes in L-T-PVAT are derived from Myf5+ precursor cells, whereas none displays this lineage in A-T-PVAT.
Fig. 6.
Some T-PVAT adipocytes are derived from Myf5+ precursors. a–d Immunostaining for RFP, perilipin (green), and UCP1 (gray) in L-T-PVAT (a), A-T-PVAT (b), subscapular BAT (c), and perigonadal WAT (d) from Myf5-Cre;Rosa26RFP/+adult mice. e Statistics indicate that 27.0 ± 6.0% of L-T-PVAT adipocytes and 9.4 ± 3.2% of A-T-PVAT adipocytes are derived from Myf5+ precursors; conversely, 99.2 ± 1.0% of subscapular adipocytes and 2.4 ± 0.8% of gonadal adipocytes are derived from Myf5+ precursors; n = 8. f Statistics indicate that L-T-PVAT and A-T-PVAT adipocytes are derived from Myf5+ precursors at different developmental stages; n = 8 mice per stage. *P < 0.05. Scale bar 50 μm
Biological functions of L-T-PVAT and A-T-PVAT
To determine whether L-T-PVAT and A-T-PVAT heterogeneity has different effects on aorta functional regulation, we measured phenylephrine-induced contraction and acetylcholine-induced relaxation in aorta together with different parts of T-PVAT (Fig. 7a, Supplemental Fig. S6). There is no significant difference in regulating phenylephrine-induced aorta contraction among different parts of T-PVAT (Supplemental Fig. S6), but A-T-PVAT displayed less relaxation on aortas than LL/RL-T-PVAT in 5-month-old mice (Fig. 7b).
Fig. 7.
A-T-PVAT has distinct functions compared to L-T-PVAT. a Representative hematoxylin and eosin staining of thoracic aortas including LL-T-PVAT, A-T-PVAT, or RL-A-T-PVAT. b Dose-dependent vasorelaxation induced by acetylcholine in 5-month-old mouse aorta with A-T-PVAT, RL-T-PVAT, or LL-T-PVAT. Endothelium-denuded aorta with whole T-PVAT was used as negative control; n = 10 mice per group. c Single-cell suspensions of A-T-PVAT, RL-T-PVAT, or LL-T-PVAT were subjected to flow cytometric analysis with antibodies against F4/80 from control (Con) or angiotensin II-infused (Ang II) mice. d Quantitative analysis of F4/80 in A-T-PVAT, RL-T-PVAT, and LL-T-PVAT; n = 5 mice per group
Angiotensin II (AngII) induced aorta adventitial and PVAT inflammation [32]. We performed flow cytometry analysis of T-PVAT from Ang II-infused hypertensive mice and observed many more F4/80-positive macrophages in A-T-PVAT than L-T-PVAT (Fig. 7c, d), indicating that A-T-PVAT may have a more important role in AngII-induced aorta adventitial and PVAT inflammation than L-T-PVAT. These combined results suggest that L-T-PVAT and A-T-PVAT have different physiological and pathological functions in regulating vascular health and disease.
Discussion
In this study, we showed that T-PVAT had distinct characteristics and developmental dynamics at different anatomical sites. A-T-PVAT was derived primarily from local SM22α+ progenitor cells, whereas L-T-PVAT was derived from SM22α+ and Myf5+ progenitor cells. L-T-PVAT contained more UCP1+ brown adipocytes than A-T-PVAT. Differences in PVAT adipocyte developmental and distributional parameters result in different vasorelaxation effects of L-T-PVAT and A-T-PVAT on aortas.
Mature adipocytes are post-mitotic and are generated through the proliferation and differentiation of precursor cells [13]. T-PVAT shows similar morphological characteristics and transcriptional profiles as BAT [20]. Typical brown adipocytes such as interscapular adipose tissue are derived primarily from Myf5-expressing dermomyotome [17]. Here, we showed that Myf5-Cre labeled some adipocytes in T-PVAT, suggesting that T-PVAT has a different origin than typical BAT. Although adipocytes also can originate from endothelial and neural crest cells [33, 34], we found that very few T-PVAT adipocytes were labeled with Tie2-Cre or Wnt1-Cre (data not shown). Chang et al. reported that T-PVAT in SM22α-Cre;Rosa 26 LacZ mice could be marked by LacZ staining [18]. In this study, we performed genetic lineage tracing studies on T-PVAT using inducible SM22α-CreERT2 mice. We found that only tamoxifen administration induced SM22α-Cre expression in the early embryonic stage (E8.5), and T-PVAT adipocytes were labeled with RFP. If SM22α-Cre expression was induced at E16.5 or in adulthood, the T-PVAT adipocytes would not be labeled. The previous study showed that PPARγ deletion using SM22α-Cre knock-in mice caused complete PVAT deletion in aortic regions [18]. We further showed that VSMCs in the thoracic aorta express Myf5-Cre. Therefore, we speculate that some adipocyte progenitors first express Myf5 and SM22α, and then, respectively, differentiate into VSMCs or adipocytes. By contrast, major adipocytes are derived from Myf5−SM22α+ progenitors. Although lineage tracing can have some limitations, our unique observations suggest at least three important characteristics of T-PVAT development: (1) the vascular resident T-PVAT progenitors transiently express SM22α during early development; (2) adipocyte progenitor cell fate decision occurs earlier than adipocyte differentiation during T-PVAT development; and (3) adult VSMC could not differentiate into adipocytes in PVAT. Our studies also indicate that the origin and development of PVAT are much more complex than previously anticipated. Additional studies are required to clarify the origin of PVAT, which is crucial to determine the functional role of PVAT in vascular regulation.
We showed that T-PVAT contained three long strip-shaped fat depots, LL-T-PVAT, RL-T-PVAT, and A-T-PVAT. L-T-PVAT development began earlier than A-T-PVAT development. Both L-T-PVAT contained SM22α+ and Myf5+ cells, whereas most adipocytes in A-T-PVAT were derived from SM22α+ cells. L-T-PVAT contained more UCP1+ brown adipocytes than A-T-PVAT. Brown adipocytes stimulate energy expenditure, and BAT activation has become a trending topic for preventing metabolic and related cardiovascular disease [8, 35, 36]. Our RNA-seq analyses showed distinct transcriptional profiles in A-T-PVAT and L-T-PVAT. Both WAT and BAT are active endocrine organs that release abundant adipokines that function in endocrine and paracrine regulation of vascular functions. However, the adipokines in brown adipocytes are different from those in white adipocytes [37, 38]. These combined data suggest that L-T-PVAT and A-T-PVAT may have different paracrine roles. In agreement with this hypothesis, our vascular ring experiment showed that L-T-PVAT exerts a significantly enhanced vasorelaxation effect on the aorta compared to A-T-PVAT. A previous study also showed that mesenteric and omental depots, two visceral fat tissues located in close vicinity, exhibit distinct gene expression profiles and are not functionally equivalent [39]. Macrophage infiltration-induced inflammation is a key regulator of adipose tissue dysfunction, which also contributes to hypertensive vascular remodeling [30, 40]. Here, we showed that A-T-PVAT has much more macrophage infiltration than L-T-PVAT. These combined results suggest that differences in the development and origin of A-T-PVAT and L-T-PVAT may lead to distinct functional properties.
In conclusion, our study mapped the development of T-PVAT and identified the adipocyte precursor cells. Our unique observations indicate that T-PVAT adipocytes were derived from multiple cell lineages, which may have different roles in regulating aorta function. Our results revealed the heterogeneous characteristics of A-T-PVAT and L-T-PVAT, which suggest that future experimental results on T-PVAT should be obtained from the same anatomical location in mice. Given the significant distinctions between L-T-PVAT and A-T-PVAT, their potentially different pathological roles in regulating vascular disease deserve to be further investigated in future studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 14476 kb)
Acknowledgements
This work was funded by grants from the National Natural Science Foundation of China (91539202, 81570221, 81770495, 91739303, 81200067), Natural Science Foundation of Shanghai (17ZR1423800), Science and Technology Commission of Shanghai Municipality (18140903402), and Shanghai Municipal Commission of Health and Family Planning (2017YQ076, 201540222).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 2.Almabrouk TA, Ewart MA, Salt IP, Kennedy S. Perivascular fat, AMP-activated protein kinase and vascular diseases. Br J Pharmacol. 2014;171:595–617. doi: 10.1111/bph.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karastergiou K, Fried SK. Multiple adipose depots increase cardiovascular risk via local and systemic effects. Curr Atherosclerosis Rep. 2013;15:361. doi: 10.1007/s11883-013-0361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–1539. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Withers SB, Bussey CE, Saxton SN, Melrose HM, Watkins AE, Heagerty AM. Mechanisms of adiponectin-associated perivascular function in vascular disease. Arterioscler Thromb Vasc Biol. 2014;34:1637–1642. doi: 10.1161/ATVBAHA.114.303031. [DOI] [PubMed] [Google Scholar]
- 6.Aghamohammadzadeh R, Heagerty AM. Obesity-related hypertension: epidemiology, pathophysiology, treatments, and the contribution of perivascular adipose tissue. Ann Med. 2012;44(Suppl 1):S74–S84. doi: 10.3109/07853890.2012.663928. [DOI] [PubMed] [Google Scholar]
- 7.Bussey CE, Withers SB, Aldous RG, Edwards G, Heagerty AM. Obesity-related perivascular adipose tissue damage is reversed by sustained weight loss in the rat. Arterioscler Thromb Vasc Biol. 2016;36:1377–1385. doi: 10.1161/ATVBAHA.116.307210. [DOI] [PubMed] [Google Scholar]
- 8.Friederich-Persson M, Nguyen Dinh Cat A, Persson P, Montezano AC, Touyz RM (2017) Brown adipose tissue regulates small artery function through NADPH oxidase 4-derived hydrogen peroxide and redox-sensitive protein kinase G-1alpha. Arterioscler Thromb Vasc Biol 37:455–465 [DOI] [PubMed]
- 9.Gollasch M. Adipose-vascular coupling and potential therapeutics. Annu Rev Pharmacol Toxicol. 2017;57:417–436. doi: 10.1146/annurev-pharmtox-010716-104542. [DOI] [PubMed] [Google Scholar]
- 10.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 11.Cinti S. Between brown and white: novel aspects of adipocyte differentiation. Ann Med. 2011;43:104–115. doi: 10.3109/07853890.2010.535557. [DOI] [PubMed] [Google Scholar]
- 12.Hepler C, Vishvanath L, Gupta RK. Sorting out adipocyte precursors and their role in physiology and disease. Genes Dev. 2017;31:127–140. doi: 10.1101/gad.293704.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science (New York, N.Y.) 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nature Commun. 2014;5:4099. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokkinopoulos I, Wong MM, Potter CMF, Xie Y, Yu B, Warren DT, Nowak WN, Le Bras A, Ni Z, Zhou C, Ruan X, Karamariti E, Hu Y, Zhang L, Xu Q. Adventitial SCA-1(+) progenitor cell gene sequencing reveals the mechanisms of cell migration in response to hyperlipidemia. Stem cell Rep. 2017;9:681–696. doi: 10.1016/j.stemcr.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34:1621–1630. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang L, Xiong W, Zhao X, Fan Y, Guo Y, Garcia-Barrio M, Zhang J, Jiang Z, Lin JD, Chen YE (2018) Bmal1 in perivascular adipose tissue regulates resting phase blood pressure through transcriptional regulation of angiotensinogen. Circulation [DOI] [PMC free article] [PubMed]
- 22.Ruan CC, Zhu DL, Chen QZ, Chen J, Guo SJ, Li XD, Gao PJ. Perivascular adipose tissue-derived complement 3 is required for adventitial fibroblast functions and adventitial remodeling in deoxycorticosterone acetate-salt hypertensive rats. Arterioscler Thromb Vasc Biol. 2010;30:2568–2574. doi: 10.1161/ATVBAHA.110.215525. [DOI] [PubMed] [Google Scholar]
- 23.Saxton SN, Ryding KE, Aldous RG, Withers SB, Ohanian J, Heagerty AM (2018) Role of sympathetic nerves and adipocyte catecholamine uptake in the vasorelaxant function of perivascular adipose tissue. Arterioscler Thromb Vasc Biol [DOI] [PubMed]
- 24.Britton KA, Pedley A, Massaro JM, Corsini EM, Murabito JM, Hoffmann U, Fox CS. Prevalence, distribution, and risk factor correlates of high thoracic periaortic fat in the Framingham Heart Study. J Am Heart Assoc. 2012;1:e004200. doi: 10.1161/JAHA.112.004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye M, Zhang Q, Xu X, Zhang Q, Ge Y, Geng P, Yan J, Luo L, Sun Y, Liang X. Loss of JAM-C leads to impaired esophageal innervations and megaesophagus in mice. Dis Esophagus. 2016;29:864–871. doi: 10.1111/dote.12383. [DOI] [PubMed] [Google Scholar]
- 26.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 27.Ye M, Coldren C, Liang X, Mattina T, Goldmuntz E, Benson DW, Ivy D, Perryman MB, Garrett-Sinha LA, Grossfeld P. Deletion of ETS-1, a gene in the Jacobsen syndrome critical region, causes ventricular septal defects and abnormal ventricular morphology in mice. Hum Mol Genet. 2010;19:648–656. doi: 10.1093/hmg/ddp532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, An X, Chen D, Ye M, Shen W, Han W, Zhang Y, Gao P. Chronic exercise training improved aortic endothelial and mitochondrial function via an AMPKalpha2-dependent manner. Front Physiol. 2016;7:631. doi: 10.3389/fphys.2016.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheng LJ, Ruan CC, Ma Y, Chen DR, Kong LR, Zhu DL, Gao PJ. Beta3 adrenergic receptor is involved in vascular injury in deoxycorticosterone acetate-salt hypertensive mice. FEBS Lett. 2016;590:769–778. doi: 10.1002/1873-3468.12107. [DOI] [PubMed] [Google Scholar]
- 30.Ruan CC, Ge Q, Li Y, Li XD, Chen DR, Ji KD, Wu YJ, Sheng LJ, Yan C, Zhu DL, Gao PJ. Complement-mediated macrophage polarization in perivascular adipose tissue contributes to vascular injury in deoxycorticosterone acetate-salt mice. Arterioscler Thromb Vasc Biol. 2015;35:598–606. doi: 10.1161/ATVBAHA.114.304927. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Turer E, Li X, Zhan X, Choi M, Tang M, Press A, Smith SR, Divoux A, Moresco EM, Beutler B. Insulin resistance and diabetes caused by genetic or diet-induced KBTBD2 deficiency in mice. Proc Natl Acad Sci USA. 2016;113:E6418–E6426. doi: 10.1073/pnas.1614467113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang ZB, Ruan CC, Lin JR, Xu L, Chen XH, Du YN, Fu MX, Kong LR, Zhu DL, Gao PJ. Perivascular adipose tissue-derived PDGF-D contributes to aortic aneurysm formation during obesity. Diabetes. 2018;67:1549–1560. doi: 10.2337/db18-0098. [DOI] [PubMed] [Google Scholar]
- 33.Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, Corvera S, Cinti S. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowa Y, Imura T, Numajiri T, Takeda K, Mabuchi Y, Matsuzaki Y, Nishino K. Adipose stromal cells contain phenotypically distinct adipogenic progenitors derived from neural crest. PLoS One. 2013;8:e84206. doi: 10.1371/journal.pone.0084206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang L, Garcia-Barrio MT, Chen YE. Brown adipose tissue, not just a heater. Arterioscler Thromb Vasc Biol. 2017;37:389–391. doi: 10.1161/ATVBAHA.116.308909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aldiss P, Davies G, Woods R, Budge H, Sacks HS, Symonds ME. ‘Browning’ the cardiac and peri-vascular adipose tissues to modulate cardiovascular risk. Int J Cardiol. 2017;228:265–274. doi: 10.1016/j.ijcard.2016.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheideler M, Herzig S, Georgiadi A (2017) Endocrine and autocrine/paracrine modulators of brown adipose tissue mass and activity as novel therapeutic strategies against obesity and type 2 diabetes. Hormon Mol Biol Clin Investig 31 [DOI] [PubMed]
- 38.Giralt M, Cereijo R, Villarroya F. Adipokines and the endocrine role of adipose tissues. Handb Exp Pharmacol. 2016;233:265–282. doi: 10.1007/164_2015_6. [DOI] [PubMed] [Google Scholar]
- 39.Billon N, Dani C. Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev. 2012;8:55–66. doi: 10.1007/s12015-011-9242-x. [DOI] [PubMed] [Google Scholar]
- 40.Zieger K, Weiner J, Kunath A, Gericke M, Krause K, Kern M, Stumvoll M, Kloting N, Bluher M, Heiker JT. Ablation of kallikrein 7 (KLK7) in adipose tissue ameliorates metabolic consequences of high fat diet-induced obesity by counteracting adipose tissue inflammation in vivo. CMLS. 2018;75:727–742. doi: 10.1007/s00018-017-2658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 14476 kb)