Abstract
In contrast to the insidious and poorly immunogenic human papillomavirus (HPV) infections, vaccination with the HPV virus-like particles (vlps) is non-infectious and stimulates a strong neutralizing-antibody response that protects HPV-naïve vaccinees from viral infection and associated cancers. However, controversy about alleged adverse events following immunization (AEFI) with the vlps have led to extensive reductions in vaccine acceptance, with countries like Japan dropping it altogether. The AEFIs are grouped into chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). In this review, we present a hypothesis that the AEFIs might arise from malfunctions within the immune system when confronted with the unusual antigen. In addition, we outline how the pathophysiology of the AEFIs can be cost-effectively investigated with the holistic principles of systems vaccinology in a two-step process. First, comprehensive immunological profiles of HPV vaccinees exhibiting the AEFIs are generated by integrating the data derived from serological profiling for prominent HPV antibodies and serum cytokines, with data from serum metabolomics, peripheral white blood cells transcriptomics and gut microbiome profiling. Next, the immunological profiles are compared with corresponding profiles generated for matched (a) HPV vaccinees without AEFIs; (b) non-HPV-vaccinated individuals with CFS/ME-like symptoms; and (c) non-HPV-vaccinated individuals without CFS/ME. In these comparisons, any causal links between HPV vaccine and the AEFIs, as well as the underlying molecular basis for the links will be revealed. Such a study should provide an objective basis for evaluating HPV vaccine safety and for identifying biomarkers for individuals at risk of developing AEFI with HPV vaccination.
Keywords: ‘Omics technologies, Systems biology, Chronic fatigue syndrome/myalgic encephalomyelitis, Vaccine safety
Introduction
Vaccines are keystones for huge and cost-effective improvements to both human and animal health worldwide. Detailed knowledge of the natural biology of papillomaviruses and strong associations of the chronic infections of certain HPV types to cervical cancer (the fourth most frequent cancer in women worldwide (WHO vaccines, 2016 update [1]) drove efforts to create prophylactic vaccines that are solely based on the major capsid protein of the oncogenic virus. DNA sequences coding for the HPV capsid protein were cloned and expressed in yeast or insect cells. The purified proteins packaged as virus-like particles (vlps, i.e. viral particle devoid of genome) are presented as antigens during immunization [2]. Thus, in HPV vaccination, the major capsid protein is used to trigger an “exquisitely specific adaptive immune response” [3], which ensures that only subclinical infections are experienced when the vaccinated individual later encounters the virus.
However, the controversy surrounding several adverse events following immunization (AEFI) with the HPV vlps have led to extensive reductions in vaccine acceptance rates in different countries, with some like Japan dropping it altogether [4]. The AEFIs include symptoms of non-migraine-like headaches, orthostatic intolerance and chronic fatigue or nausea, collectively termed chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) [5]. With the exception of a small increased risk to Guillain–Barre syndrome in a French cohort, reviews of pre- and post-licensure data provided no clear evidence of an association between HPV vaccines and the AEFIs. In the latest systematic survey of safety concerns about HPV vaccines, the WHO-appointed Global Advisory Committee on Vaccine Safety (GACVS) found that the incidence of the AEFIs was not higher in vaccinees as compared to background levels. The GACVS also concluded that with so few adverse events, the long-lasting cancer prevention benefits provided to the majority of women far outweighed any vaccine safety issues [6]. For example, the Danish 2016 HPV immunization statistics shows that of 590,000 vaccinees, there were 2119 cases of AEFI (823 involving hospitalization) [7]. Nevertheless, the experiences were severe, and with the rapid spread of information via social media, vaccination rates quickly dropped from 49% in 2014 for the 2002 birth cohort to 24% in 2015 for the 2003 birth cohort.
If this scepticism or mistrust for the HPV vaccine persists, the potential it has for protecting women from ovarian cancer will be lost. Therefore, there is the need to investigate the aetiology of the alleged AEFI. If the HPV vaccine does trigger the AEFI, the next challenge will be to find a simple test for predicting the few at risk of developing the AEFIs, so that the overwhelming majority, who are obviously not at risk, can be immunized. In the resultant environment where the bulk of the target population is protected against infection with the oncogenic HPV types, the drop in viral carriage rate will indirectly protect the unimmunized.
Recent technological advances have enabled studies of the human immune system and revealed that though there is high variability between individuals, the composition of immune cells and associated proteins is relatively stable over time within a given person [8, 9]. The gold standard for finding causal links between any vaccine and associated adverse side effects is by comparing the rates of the event occurring in vaccinated and non-vaccinated groups in controlled and randomized clinical trials. Conducting such a trial in the HPV milieu would require an extremely large number of study participants to catch the rare adverse event. That kind of study design would be unethical, as a large group of the placebo subjects would have remain unprotected against HPV for almost a lifetime.
Hence, we propose a case control study, in which the pathophysiology of a patient group, who have allegedly experienced the adverse events after HPV vaccination, is elucidated using the holistic and hypothesis-generating principles of Systems Vaccinology. In this approach, systems biology methods are used to investigate the immune status of the test subjects by identifying and quantifying the active components of the immune systems in circulating blood samples, as represented by immune cells and the signalling molecules (cytokines and chemokines), by which they communicate [8, 10]. Based on the comparison of the immune responses (IR) to natural HPV infections and IR to HPV vlps, we hypothesize that the AEFI with HPV vaccination may be due to one or several dysfunctions in the recipient’s immune processes. Therefore, by comparing the IR (as reflected in their biomolecules) in HPV vaccinees with IR of carefully selected and well-matched control groups, it will be evident if AEFI are or are not triggered by the HPV vaccine.
We believe that this approach will provide a greater understanding of the primary causes of the AEFI with HPV vlps, since it is the interactions of the different biomolecules in the many signalling pathways and molecular sub- and major networks that drive and define the phenotype of the cellular machinery. For example, the systems biology approach was applied for identifying early gene ‘signatures’, comprising complement protein C1qB and eukaryotic translation initiation factor 2 alpha kinase 4 in humans vaccinated with yellow fever vaccine YF-17D in a blinded study, with a 90% accuracy. In 100% of the study subjects, the induction of neutralizing antibody to YF-17D was associated with a distinct gene signature, which included the B cell growth factor TNFRS17 [11]. Similarly, nine genes with significantly increased expression and six genes with significantly reduced expression were identified in young adults who were high responders to influenza vaccination by comparing the transcriptomic profiles of their peripheral blood mononuclear cells to that from poor responders [12].
Life cycle and epidemiology of HPV infections vs HPV vlps
In this section, the physiology of natural HPV infections is described and compared with the HPV vaccine preparations that are administered during HPV immunization.
HPV viral structure
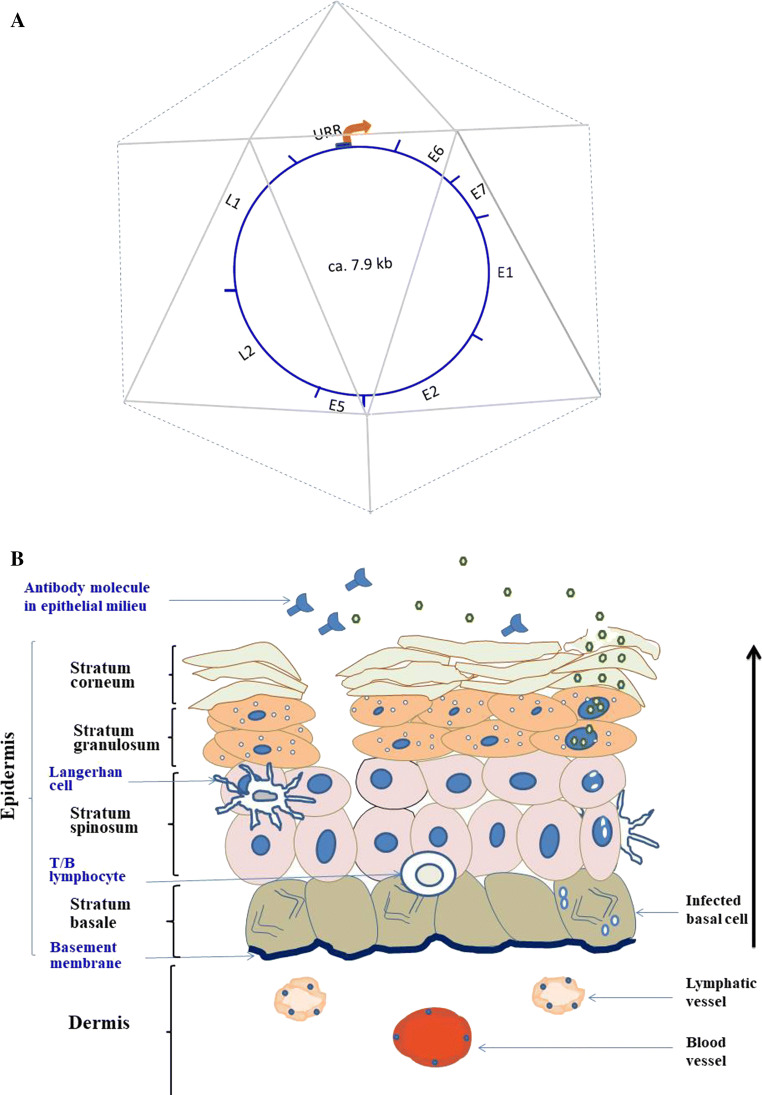
The HPV virus is a naked, icosahedral particle that is 50–60 nm in diameter [13, 14]. The particle encloses an approximately 7.9 kb double-stranded, circular DNA genome that encodes eight open reading frames (ORFs)—E1, E2, E4, E5, E6 and E7, L1 and L2; refer to Fig. 1a [15]. All the ORFs are transcribed from one of the DNA strands, which can be divided into three functional regions, a noncoding upstream regulatory region (URR), an early region and a late gene region. The URR ranges in size between 800 and 1000 bp, varies substantially in nucleotide sequences between different HPV types [15], and contains one keratinocyte-specific promoter that is usually bound by the product of the viral E2 gene, as well as several host transcription factors during episomal viral infection [16]. The early genes are arranged as follows: E6, E7, E1, E2, E4 and E5. The protein E1 is known to have helicase function. As mentioned above, pE2 binds to URR, driving viral DNA transcription and replication. It is absent when HPV DNA integrates into host chromosomal DNA, as recombination with host genome occurs via E2 gene sequences. Protein E4 is only produced during viral replication and seems to control genome amplification, virus maturation and collapse of keratinocyte for release of viral particles [14]. Like its equivalent in bovine papillomavirus infections, E5 promotes oncogenicity by downregulating the expression of surface MHC class I molecules and, hence, the presentation of viral antigens to cytotoxic T lymphocytes and NK cells [17]. The third or late gene region encodes the structural capsid proteins, L1 and L2.
Fig. 1.
a HPV viral capsid with genome. The regions in the genome coding for the early genes E6, E7, E1, E2 and E5 are indicated. E4 (not indicated) is coded for by DNA sequences from within the E5 gene and translated from spliced polycistronic transcripts, originating from the early core promoter located within the URR (upper regulatory region). DNA coding for the late transcripts L2 and L1 is also indicated. b The life cycle of HPV infections starting from episomal existence in basal epidermal cells (stratum basale) to replication of viral genome and package of viral particles within differentiating suprabasal keratinocytes (stratum granulosum and stratum corneum). The black arrow denotes the direction of epidermal cell migration as they differentiate after cell division in the stratum basale. The epidermis is maintained by the more complex underlying dermis, which is made up of intertwining elastic, reticular and collagen fibres (not shown) embedded in connective tissue. The dermis is crisscrossed by blood vessels, which bring nutrients, circulating lymphocytes and platelets, and also by lymphatic vessels, which drain the interstitial fluids. The presence of HPV-neutralizing antibodies prevents viral attachment to rifts in the epithelium and, as consequence, obstructs viral infection
The mature viral capsid is formed from an aggregation of the two late structural proteins, pL1 and pL2 that are organized into 72 capsomeres. Each capsomere contains five monomeric 55 kDa units of pL1 and a variable number of pL2 (about 12/virion), held together by intra- and inter-pentameric disulphide bonds [18]. Capsid assembly is believed to proceed via the pL1 pentamers linking to pL2 that seals the centre of the assembled pentavalent units. In analogy to other icosahedral viruses, pL2 may therefore be acting as “capsid glue” [19, 20].
Natural HPV infections
Papillomaviruses replicate and assemble exclusively within the nucleus of differentiating keratinocytes or epithelial cells of the skin or mucous membranes as shown in the right side of Fig. 1b [21]. Infections with HPV start with the viral entry through breaks or openings in the suprabasal layers of skin or mucous membranes (left side of Fig. 1b). Cell attachment and entry of HPV genome into the nucleus of the infected cell are mediated by the capsid proteins L1 and L2, respectively, as viral infections can be blocked by the neutralizing antibodies to both proteins [19]. Viral DNA replication takes place alongside normal cell DNA replication within the infected basal cells and the genome is maintained as episomes in the terminally differentiating cells of the suprabasal layers. Synthesis of numerous copies of viral genome, viral proteins and packaging of viral particles occurs in the stratum granulosum and stratum corneum of the epithelia (right side of Fig. 1b). Expression of the early genes from the keratinocyte-specific early promoter on URR provides the oncogenic protein E6 which (a) binds host PDZ protein, uncoupling the polarity control that normally exists in differentiated epithelium [22]; (b) binds and degrades the tumour-suppressor protein P53; and (c) promotes the maintenance of the telomere by activating the expression of the catalytic subunit of telomerase. The protein E7 also promotes chronic HPV infection within the epithelium by binding and degrading several cellular growth-regulatory factors like RB tumour suppressor and CDK inhibitors [23]. Acting in concert, E6 and E7 supress the expression of the Toll-like receptor, TLR9 in keratinocytes [24], thereby blocking the production of cytokines and chemokines which would have been released to alert IRs [25]. The early HPV transcripts also provide E1 and E2 that drive a rolling-circle mode of DNA replication for amplification of the viral genome, a process which in turn triggers transcription of the late genes for the capsid proteins L2 and L1. Upon successful assembly of viral particles, the product of the E4 gene orchestrates the release of the mature viral particles into the environment, during the shedding of the topmost corneal epidermal layer [15].
Epidemiology of HPV infections
Genital HPV infections are often asymptomatic and most common in sexually active young women, aged between 18 and 30 years, with a sharp decrease in prevalence after 30 years of age. Sexual intercourse is not a prerequisite for infection, as mucosal HPV can be transmitted via intimate contacts of the genitalia or other mucosal surfaces [26]. In addition, the viral particle is relatively resistant to desiccation, surviving 56 °C for 1 h. Since the virus is shed from the epidermis in the dehydrated corneal layer, transmission of cutaneous HPV also occurs via fomites from contaminated clothes and towels [27]. Over 90% of HPV infections are self-limiting and regress spontaneously within a few months. However, about 10% of the cases persist and the chronic infection may progress to pre-neoplastic cervical and squamous epithelial cancers [26]. As cervical cancer is more common in women older than 35 years, the consensus is that infections at a younger age remain latent and slowly progress to full-blown cancer in the later years.
Human papillomavirus is highly heterogeneous, presenting with more than 200 genotypes that are classified into types, lineages, and sub-lineages based on variations in their major capsid protein. The L1 capsid protein in the different types differ by at least 10% of their amino acid sequences, while those grouped as lineages differ by only ca. 1% [28]. When considering their association with cervical cancer and precursor lesions, HPVs are grouped as high-risk (HR-HPV—associated with 99% of malignant cervical cancers) and low-risk HPV (LR-HPV) types [29]. The most prevalent HR-HPV types worldwide are types 16, 18, 31, 33, and 45 [28, 30], which explains why their L1 proteins were incorporated into the HPV vaccine (see below). In long-term HR-HPV infection, the viral genome may be integrated into host chromosomes, usually with disruptions in the viral gene E2. In the absence of E2, the control on DNA replication is relieved, while expression and activities of the oncogenic E6 and E7 are increased. Integration of viral genomes occurs more commonly with HR-HPV types 16, 18, and 45, when compared to the types 31 and 33 that only display episomal viral genomes in the cancerous samples [31]. The authors, therefore, concluded that integration of oncogenic HPV genomes in cervical lesions is not required for oncogenicity and may be a consequence rather than the cause of transformation induced by deregulated HR-HPV E6 and E7.
HPV viral-like particle preparations
There are currently two licensees for HPV vaccines: Cervarix®, a bivalent HPV 16/18 vaccine from GlaxoSmithKline Biologicals (GSK); and two versions—quadrivalent HPV6/11/16/18 Gardasil® vaccine and the nine-valent Gardasil® variety (against HPV6/11/16/18/31/33/45/52/58) from MSD Merck. In addition to adjuvant and preservatives, the Cervarix and Gardasil preparations contain bio-manufactured HPV virus-like particles (vlps) produced in insect and yeast host cells, respectively. The vlps are empty icosahedral particles composed of self-aggregates of the L1 capsid proteins of the HR-HPV types indicated in the vaccine name. The vaccine preparations are administered in 0.5 ml doses as follows: the quadrivalent HPV vaccine contains 20 µg of HPV-6, 40 µg of HPV-11, 40 µg of HPV-16 and 20 µg of HPV-18 vlps with 225 µg of adjuvant amorphous aluminium hydroxyphosphate sulphate (AAHS); the nine-valent vaccine contains 30 µg of HPV-6, 40 µg of HPV-11, 60 µg of HPV-16, 40 µg of HPV-18, 20 µg of HPV-31, 20 µg of HPV-33, 20 µg of HPV-45, 20 µg of HPV-52 and 20 µg of HPV-58 vlps, as well as 500 µg of AAHS [32].
The declared “Summary of Products’ Characteristics” of the Gardasil vaccine preparations states that the preparation contains some yeast proteins, without indicating if these are specific or unknown contaminants from the vector expression system. By contrast to natural HPV viral particles that contain pL2, it is therefore not clear if these “vector–host contaminants” play any role in HPV vlps aggregate formation, for example, as serendipitous “glue proteins” in the vaccine preparations or if they play any role in the immunogenicity of the vaccine.
Immune reactions to HPV infections contra HPV immunization
Exogenous antigens arriving at the skin or mucous membranes are confronted not only by the solid barrier of the epidermal layer and microbiota that already colonize the sites, but also by a plethora of specialized cells that are the arsenal of the innate or natural immune response (IR), naive and antigen-primed B and T cells (adaptive immunity), as well as humoral factors, and signal molecules supplied by the surrounding interstitial fluids. Scientific advances have promoted studies of the types and levels of specific cell surface markers (usually receptors) and signal molecules expressed by the different immune cells. The properties and functions of the innate and adaptive immune cells are summarized in Table 1.
Table 1.
Properties and functions of immune cells
| Immune cells | Characteristics | % of WBC | Functions | Life span | References |
|---|---|---|---|---|---|
| Innate immune cells | |||||
| Macrophages |
Plastic physiology which changes in response to environmental signals Found in all tissues Have proliferative capacity Exist as subsets named according to stimuli inducing their polarization and the cytokine profile they deliver: M1, M2, tumor associated, CD169+, T cell receptor positive |
2–8% |
1. Clearance of cell debris in tissue remodeling and wound healing 2. Phagocytosis of MCOs and dead or dying cells during resolution of infection or inflammation 3. Antigen-presenting cells (APCs) 4. In response to IFNγ, produce pro-inflammatory cytokines, superoxide anions, oxygen and nitrogen radicals |
Several months and years | [33–35] |
| Neutrophils |
Granulocytes Rapidly localize to sites of infection Undergo phagocytosis-induced cell death (PICD) Express CD11b, CD16 and CD66b |
40–70% |
1. First line of defence against exogenous MCOs 2. Phagocytosis and resolution of infection by PICD 3. APCs |
5 h–7 days | [36–38] |
| Dendritic cells (DC) |
Numerous membrane processes that protrude from the main cell body Specialized receptors for antigen uptake and efficient antigen-processing and -presenting pathways Express migratory, homing and lymphocyte-binding functions to assist in moving towards lymphoid tissues Distinct subsets of different origins found in blood, lymph and tissues—e.g. myeloid and plasmacytoid in blood; CD14+ or interstitial DCS, Langerhans cells and microglia, two self-renewing DC populations found in stratified squamous epithelium and parenchyma of the brain, respectively Markers include CD103, CD45, MHC-II, CD11c and Flt3 |
1. Immunomodulatory link for innate and adaptive immune systems as major APCs 2. DC are involved with development of immune tolerance in T cells by driving deselection of those responding to self-antigens in thymus 3. A class of DC known as follicular DC forms complexes with antigen and antibody at lymph nodes, serving as ‘reservoir’ for antibody, by providing sustained stimulation of the relevant B cells |
2–3 days (FDC may last for months or years) | [39–43] | |
| Eosinophils |
Granulocytes, rich in cationic proteins, cytokines, chemokines and growth factors Rapidly release a variety of mediators without the need for de novo protein synthesis Cell surface markers include: IL-5Rα, CCR3, Siglec-8, EMR1, CD11b |
1–4% |
1. Involved in allergies and reactions to parasites 2. Phagocytic and APCs, although less efficient 3. Maintains epithelial barrier function 4. Orchestrates tissue remodelling events |
8–12 days | [44–46] |
| Basophils |
Granulocytes rich in IL-4 Express the high-affinity receptor for IgE, FcεRI Constitutively express MHC class II and costimulatory molecules such as CD40, CD80 and CD86 |
Rare, ~ 0.5% |
1. Are primary source of IL-4 in vivo for generation of Th2 cells and boosting antibody response 2. Act as APCs, especially for soluble antigens of parasites 3. Mediate long-term allergic reactions such as asthma or skin allergies |
1–2 days | [47, 48] |
| Mast cells | Similar to basophils; also produce IL-1 family | Found in tissues around blood vessels |
1. Regulate innate and adaptive immune responses 2. Promote inflammatory reactions |
[49, 50] | |
| Natural killer cell |
Granulocytes that constitutively express genes for perforin and granzymes in cytotoxic organelles for killing both viral infected and tumor cells, without prior antigen exposure Like B and T cells, NK cells establish a small pool of long-lived antigen- specific ‘memory’ cells after initial antigen encounter Molecular markers: Neural cell adhesion molecule CD56, CD4±, CD8− TCRαβlow, CD24−, CD44+, NK1.1+ |
5–15% |
1. Directly bind and kill infected, foreign and cancer cells, especially those that would escape adaptive T cell response caused by MHC-I downregulation 2. Engage in immunomodulatory interactions with dendritic cells, macrophages, T cells and endothelial cells |
[51–54] | |
| Adaptive immune cells | |||||
| Plasma or effector B cells |
Express the B cell immunoreceptor Molecular markers: B220+, CD19+, CD79, CD43−, surface IgM+ |
2–5% |
1. Produce antibody against foreign or non-self-antigens 2. Regulate diverse immune responses through their production of cytokines and other immune mediators |
[54, 55] | |
| CD8+ T cells |
Constitutively express the T cell receptor–CD3 complex Cytotoxic T cells Molecular markers: CD4−, CD8+, TCRαβ+, CD24± |
5–30% | Antigen-specific killing of infected, foreign or cancer cells | [54] | |
| CD4+ T helper cells |
Express the T cell receptor–CD3+ complex Proliferative in response to IL-2 Molecular markers: CD4+, CD8−, TCRαβ+, CD24± |
20–40% |
Depending on the subtype, the helper cells secrete cytokines, growth factors and chemokines that 1. Help B cells make antibodies 2. Stimulate cells involved in cell-mediated immunity 3. Induce macrophages to develop enhanced microbicide activity 4. Recruit neutrophils, eosinophils, and basophils to sites of infection and inflammation 5. Dampen the immune response |
[54, 56, 57, 67] | |
| Memory B or T cells |
Can be B or T cell type Express the B cell receptor or T cell receptor–CD3+ complex, respectively |
Months and years | |||
MCOs microorganisms, WBC white blood cells
The list is not exhaustive, as more rigorous analyses of receptors on T cell clones and the cytokines they secrete have revealed CD4+ T helper cell diversity that extends well beyond the characterized Th1, Th2, Th17 and Treg varieties [58]. In addition, a novel family of lymphocytes involved in innate immunity, the so-called innate lymphoid cells (ILCs), have been identified [59]. The ILCs differentiate from the same progenitor cells that give rise to T and B cells, though they differ by not expressing antigen-specific receptors (see below). So far, ILCs are thought to drive rapid and cytokine-dependent responses to infection, chronic inflammation, metabolic disease and cancer. Interestingly, ILCs exhibit transcription factor profiles and cytokine-producing capabilities that are strikingly similar to those produced by CD4+ T cells. Hepworth and Sonnenberg [60] have therefore suggested that ILCs may have evolved to provide a critical early source of cytokines and chemokines that are used to contain a potentially deleterious situation in naïve animals, especially as the initiation of a full adaptive IR can last days.
As illustrated in Fig. 1b, the interstitial fluids that surround the cells of the stratum basale and stratum spinosum in cutaneous and mucosal epithelia abound with the dendritic Langerhans cells and T and B lymphocytes. The underlying dermal layer is well supplied with blood vessels that bring nutrition and all the circulating immune cells, which include macrophages, dendritic cells, natural killer cells and some B, T and IL cells. The immune cells migrate into the intercellular spaces between the fibres and connective tissue that form the dermis, scavenging dying or infected cells. During immune surveillance, the distinction between self- and non-self-antigens starts with the establishment of contact between special cell surface proteins or receptors on the immune cells with other infected or transformed host cells or soluble derivatives therefrom. Distinguishing features of the receptors borne on the different immune cells are summarized in Table 2. Briefly, the receptors of immune cells, involved in rapid and innate IRs, lack antigen specificity, but are well equipped to confront exogenous pathogens (by recognizing metabolites unique to microbial pathogens—lipopolysaccharides, peptidoglycan, flagellin, N-formylmethionine) as well as endogenous waste biological material that have passed their “shelf-life”. By contrast, the progenitor of both the adaptive B and T cells undergoes somatic recombination (SR) to generate the highly diverse repertoire of Ig-specific receptors (BCRs) and TCRs found on B and T cells, respectively. SR involves rearrangement and assembly of DNA sequences that encode the V, D and J regions of the heavy and light chain for BCR or the clonotypic chains of TCR. The myriad of possibilities available with SR ensues that only a few lymphocytes exists with specificity for any antigen or potential pathogen (whether known or unknown) in the naïve or unexposed state [54]. The flip side to this desirable quality is that some of the immunoreceptors generated during the development of B and T lymphocytes may bind to and signal the destruction of self-antigens. Not surprisingly, the immune system has evolved sophisticated systems to prevent self-recognition or autoimmunity by (a) triggering apoptosis of self-reactive B or T cells, (b) receptor editing, or (c) developing non-responsiveness (anergy) in the otherwise auto-recognizing B or T cells, and d) generation of regulatory T cells [63–66].
Table 2.
Immune cell receptors
| Cell type | Immunoreceptors in target recognition | References |
|---|---|---|
| Eukaryotic cells in general, including macrophages, neutrophils, dendritic cells, eosinophils and basophils |
Integral membrane proteins (e.g. Toll-like and NOD-like receptors) specific for Pathogen-associated molecular patterns (PAMPs) Damage-associated molecular patterns (DAMPs) |
[3, 44, 61, 62] |
| NK cells | Several inhibitory and activating receptors act in concert to “sense” the absence or diminished expression of class I major histocompatibility complex (MHC) molecules on cell membranes | [51] |
| B cells |
B cell or immunoglobulin receptor (BCR) Is composed of signal proteins CD79A, CD79B Cell membrane version of the antibody that the B cell will produce on encounter with its cognate antigen Specificity is directed at a great variety of different antigens from bacteria, viruses and other disease-causing organism. Directly bind antigen |
[35, 54] |
| T cells |
T cell antigen receptor (TCR) Composed of two clonotypic chains αβ (95% of humans) or γδ which confer antigenic specificity The invariant CD3 signal transduction complex (composed of at least five distinct polypeptides) Specificity is directed at short peptide fragments of pathogen proteins, bound to MHC molecules on the surfaces of APC |
[36, 54] |
Dysfunction in either the innate or adaptive IR can provoke or exacerbate disease progression in individuals [67]. Such disorders are traditionally classified as (a) hypersensitivity—reactions caused by an overactive IR, (b) immunodeficiency, characterized as ineffective IRs and (c) autoimmunity that is defined as inappropriate and destructive immune reaction to self-antigens. Immunodeficiency can be due to primary or genetic defects in one or more of the many components of the immune system. The underlying causes of immunodeficiency can also be secondary in nature, i.e. the loss of function in IR is caused by another disease, drug treatment or environmental exposure to toxins. Clinically, different types of immune disorders can co-occur in individuals.
Though the different immune cells perform unique roles in the IR by exhibiting lineage-specific gene expression patterns [67], the different immune cells and nearly all eukaryotic cells including keratinocytes are capable of sensing foreign agents of microbial origin and derivatives of dying cells [61]. They generally act in synergy to protect the host, while maintaining immune homeostasis and self-tolerance. Therefore, the status and efficiency of the IR mounted at any time depends on which immune cells are active, the quality and quantity of their effector molecules, all acting in balance with contributions from the established microbiota.
Immunogenicity in natural HPV infection
Attempts at studying the direct involvement of innate immunity in IRs to natural HPV infections have been limited because of their asymptomatic nature and the difficulty of growing papillomaviruses in standard cell culture. HPV viral replication is totally linked to the differentiating cells of the stratified epithelium as illustrated in Fig. 1b. However, individuals with mutations that confer human primary immunodeficiency syndromes such as classical or functional natural killer cell deficiencies display amplified susceptibility to HPV or Herpesviridae members [53]. Serological screenings of human blood samples with recombinant yeast- or insect cells-expressed HPV proteins revealed the presence of IgG or IgM to HPV16 E4, E7, L1 and L2 in children with no clinically apparent HPV infections, normal women, patients with papillomavirus-related benign lesions such as hand warts, and in patients with overt cancer (reviewed in IARC, 1995 [68]). Seroconversion in genital HPV infection was followed by detectable neutralizing antibody to the major capsid protein L1 in infected women [69]. The response was slow (average time to seroconversion after the first detection of HPV16 DNA was 8–9 months) and antibody titters in 50–70% of the screened women (n = 588) were low, but the L1 antibody persisted in many of the subjects for at least 10 years [70]. Nevertheless, the presence and levels of the antibodies generated from natural HPV infections were not useful as indicators of cervical cancer prognosis [71]. For example, amongst the 43% anti-HPV L1-seropositive unvaccinated women (n = 953) attending their regular Pap smear control in Girardot, Colombia, from 2006 to 2007, the antibody level was significantly higher in the few women having cervical abnormalities (2.7% with atypical squamous cells or high-grade squamous intraepithelial lesions), when compared with those having normal cytology [72].
Cell-mediated IRs to HPV E4, E6, E7 and L1 proteins have also been reported in healthy humans and in patients with HPV-associated diseases [73, 74]. As with antibody-mediated IRs, T cell responses to HPV infections are investigated by using synthetic peptides derived from HPV proteins to stimulate the proliferation of polymorphonuclear cells isolated from sera of the test subjects. Amongst HPV-infected women routinely attending a colposcopy clinic, the proportion of patients with T cell determinants for epitopes of the L1, E6 and E7 proteins of HPV 52 and HPV 58 was less than 20% [75, 76]. Although the study population was small (95 with HPV 52 and 91 with HPV 58), T cell responses against L1 peptides were predominantly registered for subjects who had cleared the HPV infection, while positive T cell responses against E6 and E7 peptides were largely confined to patients who had developed cervical intraepithelial neoplasia (CIN) grade I (CIN I), CIN II or invasive cervical cancers. These observations indicate that T cell determinants of the viral surface protein, L1 are highly protective and that in patients where the HPV infection had progressed to CIN II and beyond, the virus had already escaped immune surveillance from L1 epitopes. Indeed, the apparent inability to clear HPV infections, seen in some individuals could be associated with an increase in tolerogenic CD4+ regulatory T cells and a decrease in killer CD8+ T cells [77, 78]. In the largest prospective and longitudinal study of disease progression in women with low-grade squamous intraepithelial lesions, failure to mount an HPV16 E2, and in some cases E7-specific T cell immunity, was closely associated with disease persistence and progression to cancer [74]. This strong correlation between HPV’s capacity to induce an effective cell-mediated immunity and subsequent viral clearance is also evident in the high incidence of cutaneous and genital warts in individuals with inherited immune deficiencies [52], in immune-compromised patients undergoing renal transplantation [79] or with active HIV infection [80].
In line with the heterogeneity and type variation in HPV L1 proteins mentioned earlier, infection with one HPV type is permissive for co-infection with other HPV types, especially as cell attachment during viral infection is dependent on the L1 capsid protein, [81].
Immune reactions in HPV vlp immunization
Intra-muscular (i.m) administration of the Cervarix and Gardasil preparations have been shown in randomized and controlled clinical trials to be highly effective in preventing new HPV infections and hence diseases associated with the HR-HPV types from which the constituent L1 originates [2, 82]. The superiority of the IRs generated to the HPV vaccines when compared to immunity arising from natural HPV viral infections can be attributed to (a) the direct and systemic i.m antigen deliverance, which bypasses the epithelial barriers characteristic of natural HPV infection, and (b) the generation of high titres of neutralizing L1 antibodies with high avidity, as well as the generation of their long-lasting memory B cells [83–85] that are well distributed in serum, cervico-vaginal secretions, as well as in oral mucosal fluids of vaccinees [86]. In a long-term follow-up study, 89–96% of preadolescents and adolescents administered a three-dose regimen (at day 1, month 2 and month 6) of the quadrivalent HPV remained seropositive and were clinically protected from HPV infections [87]. Therefore, HPV vaccines provide type-specific protection, are totally prophylactic and incapable of eliminating an established infection or protecting against the oncogenic concerns surrounding ongoing HPV infections. Hence, the HPV vaccination is highly recommended for protecting preadolescent girls and boys before their sexual debut.
In summary, natural HR-HPV infection is insidious and avoids notice by the Langerhans cells and macrophages of the epithelium, because its E6 protein downregulates the function of the toll-like TLR9 receptors in keratinocytes. Free HPV particles are assembled in the differentiated layers of the epithelium that are devoid of immune cells and are destined to be shed. Therefore, there is neither viremia nor systemic exposure to the virus. However, HPV infections do not completely escape the immune system because T cell-mediated IRs are implicated in the resolution of 90% of natural HPV infections [77, 88]. In addition, a high incidence of HPV-induced cutaneous and genital warts occurs in individuals with inherited primary immune deficiencies [52], in immunocompromised patients undergoing renal transplantation [79] and those infected with HIV [80]. By contrast, follow-up studies reveal that HPV vaccinees remain seropositive and are protected from infection by the HR-HPV types for up to 10 years post-vaccination. Substantively high titres of neutralizing antibodies that neutralized HPV pseudovirions in vitro and memory B cells specific for the capsid L1 protein were recorded [86], indicating that the immune responses and, hence, processes evolved in the vertebrate alongside papillomavirus infections are very different from that elicited in humans confronted with the prophylactic HPV vaccines.
The hypothesis
In comparison with natural HPV infections, HPV vaccines represent a great potential for reducing the incidence of HR-HPV infections and therefore abolishing cervical and anogenital cancers worldwide. However, HPV vaccinees are undoubtedly confronted with the HPV antigen in a novel way, since the HPV vlp preparation is administered in a sizable dose, which is higher than what the human body would naturally encounter systemically. While papillomavirus infections are ubiquitous in a wide variety of animals, they are highly host specific and depend on an exclusively intraepithelial existence in differentiated cells [13]. HPV phylogenetic studies outlined in the International Agency for Research on Cancer monograph of 1995 [68] suggest that the viruses have so successfully co-evolved with their hosts that the infection process is finely tuned and effectively evades the host’s immune system. This fact is reinforced by the experimental observations that antibodies to HPV16 E4, E7, L1 and L2 proteins are detectable in both healthy and sick people, and seroconversion in natural HPV infections is slow (taking an average of 8–9 months) and occurs in only 50–70% of exposed individuals. Interestingly, 100% of these seropositive individuals remain susceptible to reinfection by HPVs that are homologous or non-homologous to the virus encountered in the earlier infection. Therefore, the rapid seroconversion and strong neutralizing antibody response generated during immunization with HPV vlps is different and will involve immune factors that do not normally participate in natural HPV infections.
Therefore, we hypothesize that: in a small subset of HPV vaccinees encountering the “novel” HPV vlps, their immunological status is such that they undergo exaggerated immune reactions which present with the CFS/ME phenotype, referred to as the AEFI with HPV vlps.
Therefore, the key questions here are:
Which immune cells are active in that small subset of HPV vaccinees?
How is their immunological profile unique or different from that in age- and gender-matched HPV vaccinees that did not experience the AEFI, or non-HPV vaccinees with and without symptoms of CFS/ME?
Proposed method
The current practice of investigating adverse events associated with vaccinations is dependent on a passive surveillance system, which is subject to a high variability in the quality of the reports that can be biased or incomplete, as well as lacking enough information for determination of the underlying causes [89, 90]. By contrast, our approach of gathering comprehensive data of the immunological status of the vaccinees with alleged AEFI and comparing these to similar data collected from vaccinees without the AEFI and non-vaccinees presenting with similar disease profiles will help in establishing a connection between the vaccine and the AEFI. Where an association exists, the holistic approach provides enough information for identifying the molecular factors underlying the immune reactions and predicting the distinguishing factor(s) for those at risk of developing the AEFI with HPV vlps.
In this section, we outline the methodologies that should provide robust and sufficient data for defining comprehensive immune profiles of the test and control groups. In addition, we will highlight the strengths and limitations from generating and handling such large amounts of data, as well as discuss current scientific developments that address these challenges.
Study design
The gold standard for finding a causal link between a vaccine and an AEFI is by comparing the rates of the event occurring in vaccinated and non-vaccinated groups in randomized and controlled clinical trials (RCT). As it would take an extremely large number of participants to catch the rare AEFI, conducting an RCT in the HPV milieu would be unethical, because very large numbers of the control subjects would have to remain unvaccinated for years. Hence, we recommend testing our hypothesis by conducting a case–control study, in which the immune profiles of patient groups with different HPV immunization outcomes are compared, so as to identify associations between the vaccine and the AEFI. The study groups should consist of the following: (a) HPV vaccinees presenting with symptoms classified as CFS-ME (HPV–AEFI), (b) healthy HPV vaccinees without CFS-ME, (HPV–non-AEFI), (c) healthy subjects who have not been HPV vaccinated and do not have symptoms of CFS-ME (non-HPV–non-AEFI) and (d) non-HPV-vaccinated individuals with symptoms of CFS-ME (non-HPV–AEFI).
-
b.
Human population and bio-samples to be tested
The alleged AEFI with the HPV vlps include indications that are collectively referred to as CFS/ME [5]. A report of the American Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome describes CFS/ME as a malaise of the nervous system that is characterized by extreme tiredness, often involving cognitive dysfunction, sleep disturbances, non-migraine-like headaches and orthostatic intolerance, all generally made worse by any physical exertion [91]. Dantoft et al. [92] made a distinction between acute fatigue or general tiredness and the chronic or persistent tiredness associated with CFS/ME as the latter being the kind, which worsens with any physical labour. The pathophysiology of this exhaustion which worsens with any exertion has been shown to be linked with muscular metabolic disorders [93] that is, in turn, associated with mitochondrial dysfunction [94, 95].
Other common features in patients with CFS/ME are autoimmunity and additional derangements in immunity [96, 97]. For example, the severity of CFS/ME symptoms could be correlated to increases in a predominantly pro-inflammatory cytokine profile [98], alteration in levels and types of immune cells (reflected in reduced NK cell activity, but increased invariant-NK and naïve CD8 T cell cytotoxicity [99, 100], chronic inflammation [101], as well as in increased levels of auto-antibodies against the autonomic nervous system and the resulting orthostatic intolerance [102]. Indeed, HPV16 L1 protein was found to share 82 heptapeptides and 2 octapeptides with various human proteins important for cell proliferation, immunomodulation, lipid metabolism and myelination [103, 104], implying that HPV vlps have the potential to trigger autoimmune reactions that can disrupt important life processes. In addition, activated brain mast cells have been implicated in experimental allergic encephalomyelitis (EAE) in rat [105]. Under normal conditions, mast cells patrol and ply the cerebral microvasculature without expressing their surface growth factor (c-kit) receptor [106]. When activated by pro-inflammatory cytokines, mast cell-derived products instigate the breakdown of the blood–brain barrier and allow passage of inflammatory factors that enhance the development of EAE. [107].
Although CFS/ME symptoms are good diagnostic pointers, the underlying causes are poorly understood because the symptoms affect so many different organs and tissues. It has therefore been difficult to determine which of the pathological indicators are primary and which of them arise as consequences of the disease [108]. Hence, we maintain that by comparing comprehensive data that define the immune status in CFS/ME patients with and without the HPV vaccination, it should be clear whether there is an association between the two events. If an association exists, our proposed method of applying systems vaccinology (see below) will ensure that the nature of the association is evident.
The immune status in individuals can be described in their characteristic serological reactions, as well as in the global profiling of mRNA transcripts, regulatory microRNA and small metabolite populations present in their venous blood samples. Blood is an ideal tissue to be sampled because it permeates all tissues of the body and so becomes a repository for the currently active immune cells and the mixture of signalling molecules that orchestrate their activities. Besides, blood samples can be easily harvested with minimum discomfort to the study subjects when carried out by competent technicians. Information of the serological events triggered when immune cells encounter and react to antigens can be gathered from using biochemical techniques, genetic manipulations and modern imaging approaches enabled by fluorescent labelling [109]. As a result, a large number of signalling molecules (including antigen-specific receptors, humoral factors, enzymes and second messengers) that are involved in lymphocyte activation and responses have been identified and the information is currently stored in databases [110]. In addition, extensive characterizations of the different immune cell types and assignment of their roles and contributions have been made possible by elucidation of the molecular programmes in the different immune cells isolated after cell sorting with flow cytometry [111]. In total, there are now robust reference libraries that catalogue the functional hierarchy of the biomolecules involved in immune reactions.
-
c.
The systems vaccinology approach
Whereas the genome is constant in all somatic cells of an individual, the proteome or expressed proteins can vary with cell type, and in accordance with time and external influences. Invariably, specific tissues and cells will have different gene expression signatures, some of which can be biomarkers of the particular cells under consideration. While techniques such as western blot, immunohistochemical staining, enzyme-linked immunosorbent assay (ELISA), ELISPOT and flow cytometry have revealed valuable information about single or a small number of biomarker proteins in cells, the high-throughput methods developed in systems biology have proven to be more suited for exploring the complexity and dynamics of the interactions that result in variations between cells, tissues, individuals and populations in either health or disease [112–114]. The application of the principles of systems biology to the study of the molecular mechanisms by which vaccines induce, maintain or lose protective IRs is known as systems vaccinology [115–117].
Specifically, by global profiling of the mRNA transcripts, regulatory microRNA populations and metabolites in blood samples, the active molecular pathways (whether immunologic or metabolic) can be identified. An intrinsic advantage to this holistic approach is that relevant mRNA population in the situation being studied can be corroborated with the regulatory miRNA and metabolite profiles. Any pathways that are exclusively activated by the HPV vaccine should be easily identified as occurring only in the appropriate study group. In the background of the heterogeneity evident in patients with CFS/ME, we propose that a parallel determination of the types and levels of cytokines, chemokines and other immunological components be carried out for the study groups. Supporting information from these proteome-based analyses will help in specifying effects that come from the HPV vaccine and those that are not or simply arise from random events. Blood samples for harvesting the lymphocyte and serum fractions should be collected from study subjects by trained personnel in environments that are relaxed. We also recommend the inclusion of the characterization of the gut microbiome of the study groups, as recent studies have shown that bacterial communities within the host’s gut strongly influence immune reactions and autoimmune diseases. In particular, shifts towards pro-inflammatory microbes such as Methanobrevibacter sp. have recently been associated with exacerbations of the CFS/ME-like symptoms in multiple sclerosis [118].
Estimating sample size
Given the complexity of CFS/ME, a major challenge with identifying the molecular and clinically relevant factors that correctly differentiate the study groups is finding the minimum sample size required. High-throughput procedures like metabolomics are costly, so the choice of sample size is a difficult trade-off between limited research resources and the number that will provide statistically meaningful results [119]. Although several methods for calculating ‘study power’ for arrays have been published [120–122], the expected statistical difference for each factor under consideration and its variance must be known for efficient calculation of the number of samples required for detection of the difference. Since we lack a prior study with the necessary information of the expected statistical differences and their variance, we have to rely on practical experience. In consultation with investigators experienced with metabolomics analysis, we learned that a minimum of 35 people in the test and control groups was sufficient for identification of a serum-based metabolic signature for detecting and quantifying liver damage (personal communication, Juan Falcon-Perez [123]. In transcriptomics, the sample size required varies with the strength of the biological signal being investigated (personal communication, Steen Knudsen and James Timmons). For example, we learnt that while n = 20 worked well for determining statistically significant differences in gene expression levels of pre-exercise versus post-exercise muscles [124], a study size, n ≥ 500, was required for detecting statistically significant differences in a muscle insulin resistance study [125]. However, when considering differences in ‘gene sets’ instead of single genes, n = 62 was sufficient in the muscle insulin resistance study. As we propose the consideration of differences in gene networks for this study (see below), we recommend a minimum sample size of 70 (i.e. double the minimum sample size validated for metabolomics) in each of the study groups.
Characterizing the serologically important serum proteins
The kinds and levels of different proteins present in the serum of the test subjects can be studied using specific assays designed for them. For example, evaluations of (HPV)-specific antibodies after HPV vaccination or natural infection can be carried out using any of the ELISA-based techniques that are commercially available [126]. There are also several commercial ELISA-based kits for determination of serum cytokine levels. Classification of the proteins as pro- or anti-inflammatory or as involved in the immunological reactions to HPV vlps can later be used to corroborate the findings after bioinformatics data integration (see below).
Transcriptomics
For transcriptomics, the starting material of interest is the total RNA in the sample. The major target as regards our proposal is the identification and quantification of messenger RNA (mRNA) or transcripts of genes simultaneously expressed in the lymphocytes of the study subjects. The mRNAs are distinguished by their poly-adenylated tails. Another important RNA fraction or subset is the microRNA (miRNAs), short nucleotide sequences that negatively regulate the expression of thousands of genes at the post-transcriptional level by binding to the 3′ untranslated regions of their target mRNAs. Current transcriptomic technologies include microarray analysis (a hybridization approach where fluorescence-tagged probes are used to identify and quantify transcripts of interest) and RNA sequencing (RNA-seq—a high-throughput sequencing approach for the discovery of novel miRNA and mRNA species, noncoding RNA analysis, alternative splicing analysis and changes in gene expression). These transcriptomics analyses should reveal the set of mRNA and miRNA that are consistently changed in the study groups, thus providing information about the active molecular pathways in the lymphocytes, as well as reveal which control factors are operative.
In isolating miRNA, care has to be taken to safeguard the small RNA fraction. Widely used commercially available products such as miRNeasy (Qiagen), mirVana™ (Ambion) and PureLink™ (Invitrogen) miRNA isolation kits are based on chemical extraction using concentrated chaotropic salts such as guanidine thiocyanate (e.g. Trizol and QIAzol® reagents) followed by a solid-phase extraction procedure on silica columns [127]. As earlier mentioned, plasma and serum samples are very rich in RNAses. Hence, RNAse inhibitors should be added to the blood samples at collection to ensure maximum recovery of whole RNAs. The miRNAs are short (ca. 22 nucleotides) and not as diverse as mRNA; therefore, RNA sequencing of the small RNA fraction is a powerful approach for both profiling and discovering new species of miRNA [128]. Laboratory costs, investigator and intra-subject variation can be reduced by employing the help of reliable vendors that receive patient samples, extract RNA, conduct high throughput automated mRNA assays and carry out bioinformatics analyses for qualitative and quantitative evaluations of the patients’ transcriptomes. The results are typically presented as graphical representations of data within a 2-D matrix referred to as heat maps, in which the gene expression levels are represented by colours.
Metabolomics
Biochemical analyses of small metabolites (≤ 1500 Da) in body fluids (e.g. glucose levels in urine for diabetes or blood urea nitrogen levels for kidney function) have always being used as hallmarks for determining health and disease states. In our proposal, we suggest a systematic study of the unique chemical fingerprints left behind by the ongoing immune processes in the study participants by subjecting their sera to both qualitative and quantitative analysis of the metabolites in a metabolomics platform consisting of ultraperformance liquid or gas chromatography coupled to mass spectrometry (UPLC–MS). Due to the wide range of concentrations and extensive chemical diversity of the metabolites in biological samples, there is no single platform or method that can reveal the complete metabolome or comprehensive set of all small molecules in a cell, organ or tissue [129, 130]. Therefore, it is more appropriate to use multiple UPLC–MS platforms, which are optimized to provide coverage of the target metabolome [131]. Individual metabolites are then identified by comparing the ion features in the experimental samples to reference libraries of chemical standards [132].
Just as with genes and proteins expressed in a biological system, changes in the biological status of any tissue are also reflected in the types and concentrations of the endogenous metabolites [133]. Consequently, interests in finding metabolite biomarkers that will facilitate early disease diagnosis, therapy monitoring and assessments of the pathogenesis of complex diseases have driven advances in the field of “metabolomics” and led to the generation of the Human Metabolome Database (HMDB). HMDB is currently the most complete and comprehensive curated collection of human metabolite data and the corresponding physiological information [134]. For example, there are about 2200 records of endogenous metabolites gathered from books, journals, electronic databases and additional experimental metabolite concentration data compiled from hundreds of MS and NMR metabolomics analyses of urine, blood and CS fluid samples of both diseased and healthy states. Individual HMDB metabolite entries contain an average of 90 separate data fields providing a comprehensive compound description, such as chemical name and synonyms, structural information, physio-chemical data, reference NMR and MS spectra, biofluid concentrations and disease associations, information regarding associated enzymes, gene sequences of these proteins, SNP and mutation data, as well as cross-referencing to chemical structures, library references and other public genomic and protein databases.
Major challenges in carrying out metabolite profiling include reproducibility of assay results in different laboratories, as well as the identification of unknown, but interesting metabolites due to NMR spectral complexity and signal overlap. As a result, laboratory costs and investigator and intra-subject variation can be reduced by employing the services of single and experienced vendors, who have optimized metabolite extraction procedures, routinely receive patient samples and conduct high-throughput automated metabolomics assays and bioinformatics analyses to quantitate and quantify the results. Metabolomics profiles are also presented as 2D heat maps.
Microbiome profiling
The discovery that the microbial quality of the human gut affects the immune system and was critical for the development of an autoimmune disease like type 1 diabetes in susceptible NOD mice [135] sparked off a new field of research into the biomolecular mechanisms linking microbial communities or microbiome of the human skin, gut and vagina to diseases. Apart from the obvious associations between the gut microbiome and inflammatory bowel disease [136], colorectal cancer [137] and the metabolic syndromes [138], where disease pathologies can be traced to autoIRs directly affecting the gastrointestinal tract, the composition of the gut microbial flora has now been implicated in anxiety and depression [139], as well as in exacerbations of CFS–ME-like symptoms in multiple sclerosis [118].
The idea that commensal microorganisms promote human health is an old concept dating back to the foundations of microbiology, e.g. Döderlein’s (1892) explanation of the role of lactobacilli as gatekeepers of the vaginal ecosystem as well Metchnikoff’s observation that life could be prolonged with ingestion of fermented milk (cited by Belkaid and Hand [140]). More recent research has confirmed that commensals in “healthy microbial communities” modify colonization by pathogens through several mechanisms which include (a) reduction of local pH by lactobacilli [141]; (b) production of antimicrobials such as bacteriocins by Escherichia coli or antimicrobial peptides by the nosocomial Staphylococcus epidermidis, which directly prevent pathogen growth or survival in gut and the skin, respectively [142]; (c) alteration of nutrient availability to pathogens resulting in the reduced expression of virulence genes and growth rate of entero-haemorragic E. coli, or Clostridia difficile [143, 144]; and d) by producing signalling agents like short chain fatty acids (SCFAs), which cause the downregulation of virulence genes encoding the type 3 secretion system in Salmonella enterica and Salmonella typhimurium [145].
The microbiome also manipulates the host immune system into thwarting the establishment of pathogens at distal niches by promoting and adjusting both innate and adaptive immunity [146]. This microbiome influence on the host immune system is facilitated by the availability of the many microbiome-derived signal molecules to the mucosa-associated lymphoid tissues lining the entire human gastrointestinal tract [147]. Mucosal macrophages and dendritic cells sensitized by PAMPs, via their Toll-like and Nod-like families of receptors, carry the information about the commensals to neighbouring lymph nodes and organs, resulting in the induction of the systemic adaptive immunity. Therefore, the microbiota condition the host’s IR to both local and systemic infectious challenges. Indeed, the dramatic rise in autoimmune and inflammatory disorders currently prevalent in high-income countries has been attributed to an imbalance in the symbiotic relationship established between the microbiota and the host’s immune system [148]. Indeed, the specific immune cells which mediate these microbiota-associated inflammatory and autoimmune disorders have been identified. For example, in a murine model of rheumatoid arthritis, a significant reduction in the invasion of joints by immune complexes that was accompanied by a lack of the proinflammatory Th17 response was observed with germ-free animals. By contrast, arthritic phenotypes were completely restored in germ-free mice colonized with segmented filamentous bacteria, which drive the differentiation and dissemination of both intestinal and splenic Th17 cells [149].
Faecal samples are easily collected by study subjects themselves following one of the well-established self-guided procedures currently available. The carefully packaged samples are then sent to expert laboratories where the gut microbiome profiles are studied with metagenome- and metatranscriptome sequencing of total microbiome DNA and RNA, respectively. In addition to these techniques that bypass the need for microbial cultivation, robust bioinformatics tools are also now available for classifying the bacteria into both taxa and functional groups, while avoiding contamination with human genetic material [150, 151]. An interesting question here would be: are there overlaps in community compositions between any of the study groups?
-
d.
Bioinformatics data analysis
Network medicine
Nowadays, the network-based approach is the first choice for the analysis and modelling of biological systems. This has led to an emerging field, known as network medicine, a combination of systems medicine and network science. The main concept is a simplified analysis that groups single genes, identified as being involved in the physiological state under consideration, into sets of functionally related and interacting proteins. Typically, pathway modelling of a cellular process starts with identifying the extracellular signalling molecule that activates a specific receptor protein on the cell surface, and tracing the chain of protein–protein or protein–small molecule interactions involved in the entire process. In this way, a greater explanatory power is achieved in identifying “differentially expressed” pathways, vis-à-vis simply listing “differentially expressed” genes, when comparing two different biological conditions (e.g. healthy versus sick states). Such approaches improve the understanding of how changes in cellular processes can lead to pathologies and complex diseases, as well as allow the identification of the essential molecules and phenotype-associated modules of biological systems. Also, emerging network-based computational tools offer a platform to systematically explore the molecular complexity of a particular disease through the identification of disease pathways.
Early work in this field ignored the pathway-interacting topology and used over-representation to compare the number of interesting genes that are involved, assuming a chance hit in the situation being studied. Later studies used functional class scoring to identify coordinated changes in the expression of genes in the same pathway. Yet, other approaches focused on the effect of the upstream genes relative to the downstream genes and coupled classical enrichment analysis along with the perturbation of a specific pathway to quantify the impact of upstream genes [125].
More recently, pathway analysis has evolved into subpathway analysis, where searches for sub-areas on the overall network topology are made, so as to interpret the related biological phenomena. Subpathways are local subnetworks within the pathway topology which can be associated with small-scale biological functions, within the boundaries of the major pathway, and whose deregulation can give rise to a disease. Thus, the subpathway provides more targeted and context-specific molecular candidate signatures for pathophysiology or disease aetiology. Subpathway-based bioinformatics tools, with their capacity to scan the entire pathway network and zoom into the specific subareas that are deregulated, can deeper explore the biological significance of disease-associated variations identified by genome-wide association studies. Hence, we describe algorithms of such bioinformatics tools designed with this perspective in mind. They offer new horizons in the growing field known as network medicine [152–154].
Algorithm for the proposed data analysis
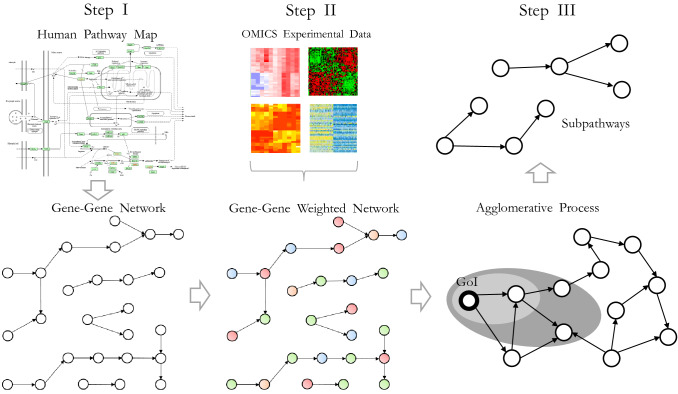
Based on the above, we present the design for a network-based approach for the identification of pathology/disease-related network biomarkers, using multi-omics experimental expression data (transcriptomics, metabolomics etc.). The three main steps are illustrated in Fig. 2 and consist of:
Step I Construction and conversion of human pathway maps to a gene–gene network based on association rules.
Step II OMICS data are incorporated onto the gene–gene network in the form of weights in their edges.
Step III Design and implementation of subnetwork identification method based on an agglomerative technique starting from seed genes of interest (GoI).
Fig. 2.
Identifying unique subpathways in the study groups. Construction and conversion of human pathway maps to a gene–gene network based on association rules (Step I); the incorporation of omics data onto gene–gene networks in the form of weights in their edges (Step II); and design and implementation of the subnetwork identification method based on an agglomerative technique starting from seed genes of interest (GoI) (Step III)
In more detail, the first step involves bio-inspired association rules for converting a pathway map to a gene–gene network. Publicly available databases, such as the Kyoto Encyclopedia of Genes and Genomes (KEGG), provide extensive pathway maps with several entry elements (proteins, chemical compounds, genes, protein complexes, enzymes, etc.), in addition to several types of relationships among the elements, such as activation, inhibition, dissociation, phosphorylation and ubiquitination [155]. To incorporate omics information onto the pathway maps, an efficient conversion step is required. Reliable conversion steps have been researched and are available in the literature [153, 156, 157].
The incorporation of the omics data onto the gene–gene network in step II is a crucial step, since data arising from various omics techniques are mapped in by creating a network with highly condensed information. To accomplish this, the appropriate way is to imprint the omics information onto the network in the form of weights on their nodes and/or edges. A weighting scheme for nodes or edges indicates the statistically significant difference between the two states (e.g. control and pathological/disease state) or the expression correlation between two different biological processes.
Afterwards, subnetworks are extracted from the weighted gene–gene network indicating “active subpathways” in the form of local topologies of the entire network. Local methods are more relevant for the entire framework as described above. Such methods use agglomerative processes to grow modules from seed nodes; and ensemble methods merge alternative clustering sampled either from stochastic runs of a given method or from a set of different methods.
Outputs of the above workflow contain “active subnetworks”, which can be considered as the potential “network biomarkers” for the case under study. Indeed, these biomarkers define the mechanisms of the case being examined in a more realistic manner.
Integrative data analysis
Initially, metabolomics and transcriptomics profiles from each patient category can be mapped onto the gene–gene network as described above. After this, four different weighted human genetic networks are constructed for the categories (a) HPV vaccinees exhibiting the AEFIs, (b) HPV vaccinees without AEFIs, (c) non-HPV-vaccinated individuals with CFS/ME-like symptoms and (d) non-HPV-vaccinated individuals without CFS/ME. Each network imprints the information from ‘omics data onto its edges. Next, starting from a GoI, subpathways are extracted for each network. GoI are genes with statistically significant high differential expression. As a result, we obtain a pool of “active subpathways”, four for each GoI and one for each study group.
The next step is to examine the overlap among the subpathways with shared starting gene node. Here, we can detect shared processes and differences between categories (a)–(b) and (c)–(d). In the first comparison, we have samples from HPV vaccinees with (a) and without (b) the AEFI-like symptoms. In the second comparison, we have samples from non-HPV vaccinated with (c) and without (d) AEFI-like symptoms. Thus, any overlaps from these two comparisons will clearly indicate which subpathways are influenced by AEFI-like symptoms, as well as point out whether their underlying physiology is related to HPV vaccination. Similarly, if there are “active subpathways” in category (a) that are absent in the three other categories, then HPV vaccination must have triggered the creation of an active subpathway in the entire pathway topology.
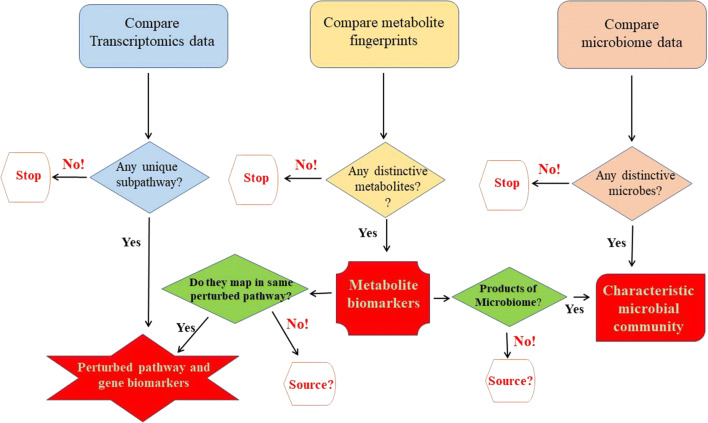
If the AEFIs truly arise from disturbances caused by HPV vaccination, data analyses should give rise to models describing the perturbed subpathways with genetic and metabolic biomarkers, as well as the dominant gut microbial community for HPV vaccinees with AEFIs, as depicted in Fig. 3. As the metabolites result from the biological reactions triggered and catalysed by the proteins expressed, a majority of the metabolite biomarkers should corroborate and map onto the same subpathways as identified with the transcribed genes. A major advantage with this proposed study approach is that the sources of the metabolites are easily traceable either to the perturbed subpathways or as contributed by the gut microbiota. For all the data types, the subpathways, metabolites or microbial groups that are uniquely absent in the HPV vaccinees with AEFI, during the comparison with the other test groups, are just as important as those that are exclusively present.
Fig. 3.
Expectations from the integrative data analysis. Transcriptomics, metabolomics and microbiomics data sets from (a) HPV–AEFIs are compared with the corresponding data sets from (b) HPV–non-AEFIs, (c) non-HPV–AEFIs; and the fourth group, (d) non-HPV–AEFIs to yield genetic and metabolic biomarkers, as well as distinctive microbiota
Validation studies and conclusions
At the end of the integrated bioinformatics data analyses outlined above, it should be possible to establish if there is a genuine connection between HPV vaccination and the AEFIs. An extension of these studies might be in the analysis of the roles of the identified miRNAs in controlling gene expression for the different immune status by exploring miRNA:mRNA or miRNA:protein–protein interactions. Any potential biomarkers identified in the study can be validated by testing for their presence or absence in pre-vaccination blood samples (where available), in the proteome assays of reference blood samples taken and stored from the test groups described above before the beginning of the assays (internal validation), as well as in matching cohorts that were not part of the original study (external validation).
Abbreviations
- HPV
Human papillomavirus
- AEFI
Adverse events following immunization
- CFS/ME
Chronic fatigue syndrome/myalgic encephalomyelitis
- DC
Dendritic cells
- APC
Antigen-presenting cell
- BCR
B cell receptor
- TCR
T cell receptor
References
- 1.World Health Organization (WHO) (2018) Human papillomavirus (HPV) and cervical cancer at http://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accessed 8 Oct 2018
- 2.Mariani L, Venuti A. HPV vaccine: an overview of immune response, clinical protection, and new approaches for the future. J Transl Med. 2010;8:105. doi: 10.1186/1479-5876-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul WE. Bridging innate and adaptive immunity. Cell. 2011;147(6):1212–1215. doi: 10.1016/j.cell.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Larson HJ, Wilson R, Hanley S, et al. Tracking the global spread of vaccine sentiments: the global response to Japan’s suspension of its HPV vaccine recommendation. Hum Vaccines Immunother. 2014;10(9):2543–2550. doi: 10.4161/21645515.2014.969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinth L, Pors K, Hoppe AAG, et al. Is chronic fatigue syndrome/myalgic encephalomyelitis a relevant diagnosis in patients with suspected side effects to human papilloma virus vaccine? Int J Vaccines Vaccin. 2015;1(1):00003. doi: 10.15406/ijvv.2015.01.00003. [DOI] [Google Scholar]
- 6.Global Advisory Committee on Vaccine Safety (2017) Safety update of HPV vaccines at http://www.who.int/vaccine_safety/committee/reports/June_2017/en/. Accessed 8 Oct 2018
- 7.Danish Health Authority, news (2016) Sharp fall in HPV vaccination rate at https://www.sst.dk/en/news/2016/sharp-fall-in-hpv-vaccination-rate. Accessed 8 Oct 2018
- 8.Brodin P, Jojic V, Gao T, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160:37–47. doi: 10.1016/j.cell.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodin P, Davis MM. Human immune system variation. Nat Rev Immunol. 2017;17(1):21–29. doi: 10.1038/nri.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagana T, Nakaya HI, Subramaniam S, et al. Systems vaccinology: enabling rational vaccine design with systems biological approaches. Vaccine. 2015;33(40):5294–5301. doi: 10.1016/j.vaccine.2015.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Querec TD, Akondy RS, Lee EK, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avey S, Cheung F, Fermin D, et al. Multicohort analysis reveals baseline transcriptional predictors of influenza vaccination responses (HIPC-CHI Signatures Project Team and HIPC-I Consortium) Sci Immunol. 2017;2(14):eaal4656. doi: 10.1126/sciimmunol.aal4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16(1):1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004;68(2):362–372. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehrmann F, Laimins LA. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003;22:5201–5207. doi: 10.1038/sj.onc.1206554. [DOI] [PubMed] [Google Scholar]
- 16.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121(3):621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 17.Ashrafi GH, Haghshenas MR, Marchetti B, et al. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA. Int J Cancer. 2005;113:276–283. doi: 10.1002/ijc.20558. [DOI] [PubMed] [Google Scholar]
- 18.Sapp M, Volpers C, Muller M, Streck RE. Organization of the major and minor capsid proteins in human papillomavirus type 33 virus-like particles. J Gen Virol. 1995;76:2407–2412. doi: 10.1099/0022-1317-76-9-2407. [DOI] [PubMed] [Google Scholar]
- 19.Buck CB, Cheng N, Thompson CD, et al. Arrangement of L2 within the papillomavirus capsid. J Virol. 2008;82:5190–5197. doi: 10.1128/JVI.02726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conway MJ, Meyers C. Replication and assembly of human papillomaviruses. J Dent Res. 2009;88(4):307–317. doi: 10.1177/0022034509333446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Z-M, Baker CC. Papillomavirus genome structure, expression and post-transcriptional regulation. Front Biosci. 2006;11:2286–2302. doi: 10.2741/1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterlinko GH, Weber M, Elston R, et al. Inhibition of E6-induced degradation of its cellular substrates by novel blocking peptides. J Mol Biol. 2004;335:971–985. doi: 10.1016/j.jmb.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 23.Yim EK, Park JS. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 2005;37(6):319–324. doi: 10.4143/crt.2005.37.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasan UA, Bates E, Takeshita F, et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol. 2007;178:3186–3197. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 25.Kanodia S, Fahey LM, Kast WM. Mechanisms used by human papillomaviruses to escape the host immune response. Curr Cancer Drug Targets. 2007;7:79–89. doi: 10.2174/156800907780006869. [DOI] [PubMed] [Google Scholar]
- 26.Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(Suppl 3):S3-42–S3-51. doi: 10.1016/j.vaccine.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Roden RBS, Lowy DR, Schiller JT. Papillomavirus is resistant to desiccation. J Infect Dis. 1997;176:1076–1079. doi: 10.1086/516515. [DOI] [PubMed] [Google Scholar]
- 28.zur Hausen H H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]
- 29.Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Laimins LA. The biology of human papillomaviruses: from warts to cancer. Infect Agents Dis. 1993;2(2):74–86. [PubMed] [Google Scholar]
- 31.Vinokurova S, Wentzensen N, Kraus I, et al. Type-dependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res. 2008;68(1):307–313. doi: 10.1158/0008-5472.CAN-07-2754. [DOI] [PubMed] [Google Scholar]
- 32.Joura EA, Giuliano AR, Ole-Erik Iversen O, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 33.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epelman S, Kory J, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chávez-Galán L, Olleros ML, Vesin D, Garcia I. Much more than M1 and M2 macrophages, there are also CD169+ and TCR+ macrophages. Front Immunol. 2015;6:263. doi: 10.3389/fimmu.2015.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi SD, DeLeo FR. Towards a comprehensive understanding of the role of neutrophils in innate immunity: a systems biology-level approach. Wiley Interdiscip Rev Syst Biol Med. 2009;1(3):309–333. doi: 10.1002/wsbm.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tak T, Tesselaar K, Pillay J, et al. What’s your age again? Determination of human neutrophil half-lives revisited. J Leukoc Biol. 2013;94:595–601. doi: 10.1189/jlb.1112571. [DOI] [PubMed] [Google Scholar]
- 38.Lakschevitz FS, Hassanpour S, Rubin A, et al. Identification of neutrophil surface marker changes in health and inflammation using high-throughput screening flow cytometry. Exp Cell Res. 2016;342(2):200–209. doi: 10.1016/j.yexcr.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Steinman RM (2006) Introduction to some of the issues and mysteries considered in this book on dendritic cells. In: Lutz MB, Romani N, Steinkasserer A (eds) Handbook of Dendritic Cells. Biology, Diseases, and Therapies, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 3–11
- 40.Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages and dendritic cells. Science. 2010;327(5966):656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology. 2013;140(1):22–30. doi: 10.1111/imm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helft J, Ginhoux F, Bogunovic M, Merad M. Review Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234(1):55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 43.Merad M, Sathe P, Helft J, et al. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013 doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 2011;343(1):57–83. doi: 10.1007/s00441-010-1049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi T, Kouzaki H, Kita H. Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J Immunol. 2010;184(11):6350–6358. doi: 10.4049/jimmunol.0902673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JJ, Jacobsen EA, Ochkur SI, et al. Human vs. mouse eosinophils: “That which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol. 2012;130(3):572–584. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokol CL, Medzhitov R. Role of basophils in the initiation of Th2 responses. Curr Opin Immunol. 2010;22:73–77. doi: 10.1016/j.coi.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Min B, Brown MA, LeGros G. Understanding the roles of basophils: breaking dawn. Immunology. 2012;135(3):192–197. doi: 10.1111/j.1365-2567.2011.03530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 50.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327(5963):296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 51.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–910. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132(3):515–526. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ham H, Billadeau DD. Human immunodeficiency syndromes affecting human natural killer cell cytolytic activity. Front Immunol. 2014;5:2. doi: 10.3389/fimmu.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothenberg EV. Transcriptional control of early T and B cell developmental choices. Annu Rev Immunol. 2014;32:283–321. doi: 10.1146/annurev-immunol-032712-100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wan YY. GATA3: a master of many trades in immune regulation. Trends Immunol. 2014;35(6):233–242. doi: 10.1016/j.it.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125(2):S33–S40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 59.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 60.Hepworth MR, Sonnenberg GF. Regulation of the adaptive immune system by innate lymphoid cells. Curr Opin Immunol. 2014;27:75–82. doi: 10.1016/j.coi.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Franchi L, Warner N, Viani K, Nuñez G. Function of Nod-like receptors in microbial recognition and host defence. Immunol Rev. 2009;227(1):106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halverson R, Torres R, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5(6):645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 64.Schwarz BA, Bhandoola A. Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis. Immunol Rev. 2006;209:47–57. doi: 10.1111/j.0105-2896.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- 65.Takaba H, Morishita Y, Tomofuji Y, et al. Fezf2 orchestrates a thymic program of self-antigen expression for immune tolerance. Cell. 2015;163:975–987. doi: 10.1016/j.cell.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J Exp Med. 1982;156:1565–1576. doi: 10.1084/jem.156.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warrington R, Watson W, Kim HL, Antonetti FR. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 2011;7(Suppl 1):S1. doi: 10.1186/1710-1492-7-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.International Agency for Research on Cancer (IARC) (1995) Monographs on the evaluation of carcinogenic risks to humans, no. 64.1, human papillomavirus (HPV) infection. https://www.ncbi.nlm.nih.gov/books/NBK424405/. Accessed 8 Oct 2018
- 69.Carter JJ, Koutsky LA, Hughes JP, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181(6):1911–1919. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 70.Af Geijersstam V, Kibur M, Wang Z, et al. Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis. 1998;177:1710–1714. doi: 10.1086/517428. [DOI] [PubMed] [Google Scholar]
- 71.Lehtinen M, Pawlita M, Zumbach K, et al. Evaluation of antibody response to human papillomavirus early proteins in women in whom cervical cancer developed 1 to 20 years later. Am J Obstet Gynecol. 2003;188(1):49–55. doi: 10.1067/mob.2003.98. [DOI] [PubMed] [Google Scholar]
- 72.Leon S, Sánchez R, Patarroyo MA, et al. Prevalence of HPV-DNA and anti-HPV antibodies in women from Girardot, Colombia. Sex Transm Dis. 2009;36(5):290–296. doi: 10.1097/OLQ.0b013e318195762c. [DOI] [PubMed] [Google Scholar]
- 73.de Jong A, van Poelgeest MI, van der Hulst JM, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004;64:5449–5455. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 74.Woo YL, van den Hende M, Sterling JC, et al. A prospective study on the natural course of low-grade squamous intraepithelial lesions and the presence of HPV16 E2-, E6- and E7-specific T-cell responses. Int J Cancer. 2010;126:133–141. doi: 10.1002/ijc.24804. [DOI] [PubMed] [Google Scholar]
- 75.Chan PKS, Liu SJ, Cheung TH, et al. T-cell response to human papillomavirus type 58 L1, E6, And E7 peptides in women with cleared infection, cervical intraepithelial neoplasia, or invasive cancer. Clin Vaccine Immunol. 2010;17(9):1315–1321. doi: 10.1128/CVI.00105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chan PKS, Liu SJ, Cheung JL, et al. T-cell response to human papillomavirus type 52 L1, E6, and E7 peptides in women with transient infection, cervical intraepithelial neoplasia, and invasive cancer. J Med Virol. 2011;83(6):1023–1030. doi: 10.1002/jmv.21889. [DOI] [PubMed] [Google Scholar]
- 77.van der Burg SH, Piersma SJ, de Jong A, et al. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci USA. 2007;104(29):12087–12092. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Molling JW, de Grujil TD, Glim J, et al. CD4(+) CD25hi regulatory T-cell frequency correlates with persistence of human papillomavirus type 16 and T helper cell responses in patients with cervical intraepithelial neoplasia. Int J Cancer. 2007;121(8):1749–1755. doi: 10.1002/ijc.22894. [DOI] [PubMed] [Google Scholar]
- 79.Benton C, Shahidulhah H, Hunter JAA (1992) Human papillomaviruses in the immuno-suppressed; pp 23–26 in IARC Working Group on the evaluation of carcinogenic risk to humans. Human papillomaviruses. Lyon (FR): International Agency for Research on Cancer; 1995 (IARC monographs on the evaluation of carcinogenic risks to humans, no. 64.). https://www.ncbi.nlm.nih.gov/books/NBK424408/. Accessed 8 Oct 2018
- 80.Braun L. Role of human immunodeficiency virus infection in the pathogenesis of human papillomavirus-associated cervical neoplasia. Am J Pathol. 1994;144:209–214. [PMC free article] [PubMed] [Google Scholar]
- 81.Wheeler CM, Hunt WC, Schiffman M, Castle PE. Human papillomavirus genotypes and the cumulative 2-year risk of cervical pre-cancer. J Infect Dis. 2006;194:1291–1299. doi: 10.1086/507909. [DOI] [PubMed] [Google Scholar]
- 82.Stanley M. HPV—immune response to infection and vaccination. Infect Agents Cancer. 2010;5:19. doi: 10.1186/1750-9378-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giannini SL, Hanon E, Moris P, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24(33–34):5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 84.De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine. 2010;28:6247–6255. doi: 10.1016/j.vaccine.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Scherpenisse M, Schepp RM, Mollers M, et al. Characteristics of HPV-specific antibody responses induced by infection and vaccination: cross-reactivity, neutralizing activity, avidity and IgG subclasses. PLoS One. 2013;8(9):e74797. doi: 10.1371/journal.pone.0074797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Handisurya A, Schellenbacher C, Haitel A, et al. Human papillomavirus vaccination induces neutralising antibodies in oral mucosal fluids. Br J Cancer. 2016;114:409–416. doi: 10.1038/bjc.2015.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferris DG, Samakoses R, Block SL, et al. 4-Valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140(6):e20163947. doi: 10.1542/peds.2016-3947. [DOI] [PubMed] [Google Scholar]
- 88.Narayan S, Choyce A, Linedale R, et al. Epithelial expression of human papillomavirus type 16 E7 protein results in peripheral CD8 T-cell suppression mediated by CD4+ CD25+ T cells. Eur J Immunol. 2009;39:481–490. doi: 10.1002/eji.200838527. [DOI] [PubMed] [Google Scholar]
- 89.Varricchio F, Iskander J, Destefano F, et al. Understanding vaccine safety information from the vaccine adverse event reporting system. Pediatr Infect Dis J. 2004;23(4):287–294. doi: 10.1097/00006454-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 90.Xie J, Zhao L, Zhou S, He Y. Statistical and ontological analysis of adverse events associated with monovalent and combination vaccines against hepatitis A and B diseases. Sci Rep. 2016;6:34318. doi: 10.1038/srep34318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine (2015) Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. National Academies Press (US), Washington (DC). https://www.ncbi.nlm.nih.gov/books/NBK274235/. Accessed 8 Oct 2018
- 92.Dantoft TM, Ebstrup JF, Linneberg A, et al. Cohort description: the Danish study of functional disorders. Clin Epidemiol. 2017;9:127–139. doi: 10.2147/CLEP.S129335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong R, Lopaschuk G, Zhu G, et al. Skeletal muscle metabolism in the chronic fatigue syndrome. In vivo assessment by 31P nuclear magnetic resonance spectroscopy. Chest. 1992;102(6):1716–1722. doi: 10.1378/chest.102.6.1716. [DOI] [PubMed] [Google Scholar]
- 94.Filler K, Lyon D, Bennett J, et al. Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clin. 2014;1:12–23. doi: 10.1016/j.bbacli.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rutherford G, Manning P, Newton JL. Understanding muscle dysfunction in chronic fatigue syndrome. J Aging Res. 2016;2016:2497348. doi: 10.1155/2016/2497348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lacourt TE, Vichaya EG, Chiu GS, et al. The high costs of low-grade inflammation: persistent fatigue as a consequence of reduced cellular-energy availability and non-adaptive energy expenditure. Front Behav Neurosci. 2018;12:78. doi: 10.3389/fnbeh.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blomberg J, Gottfries CG, Elfaitouri A, et al. Infection elicited autoimmunity and myalgic encephalomyelitis/chronic fatigue syndrome: an explanatory model. Front Immunol (Hypothesis Theory) 2018;9:229. doi: 10.3389/fimmu.2018.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Montoya JG, Holmes TH, Anderson JN, et al. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc Natl Acad Sci USA. 2017;114:E7150–E7158. doi: 10.1073/pnas.1710519114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brenu EW, van Driel ML, Staines DR, et al. Immunological abnormalities as potential biomarkers in chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med. 2011;9:81. doi: 10.1186/1479-5876-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hardcastle SL, Brenu EW, Johnston S, et al. Longitudinal analysis of immune abnormalities in varying severities of chronic fatigue syndrome/myalgic encephalomyelitis patients. J Transl Med. 2015;13:299. doi: 10.1186/s12967-015-0653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nacul L, O’Donovan DG, Eliana M, et al. Considerations in establishing a post-mortem brain and tissue bank for the study of myalgic encephalomyelitis/chronic fatigue syndrome: a proposed protocol. BMC Res Notes. 2014;7:370. doi: 10.1186/1756-0500-7-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schondorf R, Benoit J, Wein T, Phaneuf D. Orthostatic intolerance in the chronic fatigue syndrome. J Auton Nerv Syst. 1999;75(2–3):192–201. doi: 10.1016/S0165-1838(98)00177-5. [DOI] [PubMed] [Google Scholar]
- 103.Kanduc D. Quantifying the possible cross-reactivity risk of an HPV16 vaccine. J Exp Ther Oncol. 2009;8(1):65–76. [PubMed] [Google Scholar]
- 104.Kanduc D, Shoenfeld Y. From HBV to HPV: designing vaccines for extensive and intensive vaccination campaigns worldwide. Autoimmun Rev. 2016;15(11):1054–1061. doi: 10.1016/j.autrev.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 105.Lu C, Diehl SA, Noubade R, et al. Endothelial histamine H1 receptor signaling reduces blood-brain barrier permeability and susceptibility to autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107(44):18967–18972. doi: 10.1073/pnas.1008816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shanas U, Bhasin R, Sutherland AK, et al. Brain mast cells lack the c-kit receptor: immunocytochemical evidence. J Neuroimmunol. 1998;90(2):207–211. doi: 10.1016/S0165-5728(98)00137-4. [DOI] [PubMed] [Google Scholar]
- 107.Letourneau R, Rozniecki JJ, Dimitriadou V, Theoharides TC. Ultrastructural evidence of brain mast cell activation without degranulation in monkey experimental allergic encephalomyelitis. J Neuroimmunol. 2003;145:18–26. doi: 10.1016/j.jneuroim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 108.Twisk FNM. The status of and future research into myalgic encephalomyelitis and chronic fatigue syndrome: the need of accurate diagnosis, objective assessment, and acknowledging biological and clinical subgroups. Front Physiol. 2014;5:109. doi: 10.3389/fphys.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Balagopalan L, Sherman E, Barr VA, Samelson LE. Imaging techniques for assaying lymphocyte activation in action. Nat Rev Immunol. 2011;11(1):21–33. doi: 10.1038/nri2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schietinger A, Delrow JJ, Basom RS, et al. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335:723–727. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35(2):51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aderem A. Systems biology: its practice and challenges. Cell. 2005;20:511–513. doi: 10.1016/j.cell.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 113.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 114.Daniel E, Zak DE, Aderem A. Systems biology of innate immunity. Immunol Rev. 2009;227(1):264–282. doi: 10.1111/j.1600-065X.2008.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Querec TD, Akondy RS, Lee EK, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010;33(4):516–529. doi: 10.1016/j.immuni.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakaya HI, Li S, Pulendran B. Systems vaccinology: learning to compute the behavior of vaccine induced immunity. Wiley Interdiscip Rev Syst Biol Med. 2012;4:193–205. doi: 10.1002/wsbm.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jangi S, Gandhi R, Cox LM, Li N, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nyamundanda G, Gormley IC, Fan Y, et al. MetSizeR: selecting the optimal sample size for metabolomic studies using an analysis based approach. BMC Bioinform. 2013;14:338. doi: 10.1186/1471-2105-14-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muller P, Parmigiani G, Robert C, Rousseau J. Optimal sample size for multiple testing. J Am Stat Assoc. 2004;99(468):990–1000. doi: 10.1198/016214504000001646. [DOI] [Google Scholar]
- 121.Tibshirani R. A simple method for assessing sample sizes in microarray experiments. BMC Bioinform. 2006;7:106. doi: 10.1186/1471-2105-7-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin WJ, Hsueh HM, Chen JJ. Power and sample size estimation in microarray studies. BMC Bioinform. 2010;11:48. doi: 10.1186/1471-2105-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gonzalez E, van Liempd S, Conde-Vancells J, Gutierrez-de Juan V, Perez-Cormenzana M, Mayo R, Berisa A, Alonso C, Marquez CA, Barr J, Lu SC, Mato JM, Falcon-Perez JM. Serum UPLC-MS/MS metabolic profiling in an experimental model for acute-liver injury reveals potential biomarkers for hepatotoxicity. Metabolomics. 2012;8(6):997–1011. doi: 10.1007/s11306-011-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Timmons JA, Knudsen S, Rankinen T, et al. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J Appl Physiol. 2010;108:1487–1496. doi: 10.1152/japplphysiol.01295.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gallagher IJ, Scheele C, Keller P, et al. Integration of microRNA changes in vivo identifies novel molecular features of muscle insulin resistance in type 2 diabetes. Genome Med. 2010;2:9. doi: 10.1186/gm130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Scherpenisse M, Schepp RM, Mollers M, et al. Comparison of different assays to assess human papillomavirus (HPV) type 16- and 18-specific antibodies after HPV infection and vaccination. Clin Vaccine Immunol. 2013;20(8):1329–1332. doi: 10.1128/CVI.00153-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2015;13(5):358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bizuayehu TT, Lanes CF, Furmanek T, et al. Differential expression patterns of conserved miRNAs and isomiRs during Atlantic halibut development. BMC Genom. 2012;13:11. doi: 10.1186/1471-2164-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baker M. Metabolomics:from small molecules to big ideas. Nat Methods. 2011;8(2):117–121. doi: 10.1038/nmeth0211-117. [DOI] [Google Scholar]
- 130.Duportet X, Aggio RB, Carneiro S, Villas-Bôas S. The biological interpretation of metabolomics data can be misled by the extraction method used. Metabolomics. 2012;8(3):410–421. doi: 10.1007/s11306-011-0324-1. [DOI] [Google Scholar]
- 131.Martinaz-Arranz I, Mayo R, Perez-Cormenzana M, et al. Data in support of enhancing metabolomics research through data mining. Data Br. 2015;3:155–164. doi: 10.1016/j.dib.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo L, Milburn MV, Ryals JA, et al. Plasma metabolomics profiles enhance precision medicine for volunteers of normal health. Proc Natl Acad Sci. 2015 doi: 10.1073/pnas.1508425112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nagana Gowda GA, Zhang S, Gu H, et al. Metabolomics-based methods for early disease diagnostics: a review. Exp Rev Mol Diagn. 2008;8(5):617–633. doi: 10.1586/14737159.8.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wishart DS, Tzur D, Knox C, et al. HMDB: the human metabolome database. Nucleic Acids Res. 2007;35:D521–D526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wen L, Ley RE, Volchkov PV, et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455(7216):1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;5:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 137.Arthur JC, Perez-Chanona E, Mühlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;5:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Delzenne NM, Neyrinck AM, Backhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;5:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- 139.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 140.Belkaid Y, Hand T. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Turovskiy Y, Sutyak NK, Chikindas ML. The aetiology of bacterial vaginosis. J Appl Microbiol. 2011;110:1105–1128. doi: 10.1111/j.1365-2672.2011.04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Iwase T, Uehara Y, Shinji H, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465:346–349. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- 143.Kamada N, Chen GY, Inohara N, Nunez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pacheco AR, Curtis MM, Ritchie JM, et al. Fucose sensing regulates bacterial intestinal colonization. Nature. 2012;492:113–117. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gantois I, Ducatelle R, Pasmans F, et al. Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Appl Environ Microbiol. 2006;72:946–949. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Molloy MJ, Grainger JR, Bouladoux N, et al. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe. 2013;14:318–328. doi: 10.1016/j.chom.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sansonetti PJ, Di Santo JP. Debugging how bacteria manipulate the immune response. Immunity. 2007;26:149–161. doi: 10.1016/j.immuni.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 148.Manzel A, Muller DN, Hafler DA, et al. Role of “Western Diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2014;14(1):404. doi: 10.1007/s11882-013-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu HJ, Ivanov II, Darce J, et al. Gut residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kearse M, Moir R, Wilson A, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Khatri P, Sirota M, Butte AJ. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput Biol. 2012;8:e1002375. doi: 10.1371/journal.pcbi.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vrahatis AG, Dimitrakopoulou K, Balomenos P, et al. CHRONOS: a time-varying method for microRNA-mediated sub-pathway enrichment analysis. Bioinformatics. 2015;32:884–892. doi: 10.1093/bioinformatics/btv673. [DOI] [PubMed] [Google Scholar]
- 154.Vrahatis AG, Balomenos P, Tsakalidis AK, Bezerianos A. DEsubs: an R package for flexible identification of differentially expressed subpathways using RNA-seq experiments. Bioinformatics. 2016;32:3844–3846. doi: 10.1093/bioinformatics/btw544. [DOI] [PubMed] [Google Scholar]
- 155.Kanehisa FM, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Judeh T, Johnson C, Kumar A, Zhu D. TEAK: topology enrichment analysis framework for detecting activated biological subpathways. Nucleic Acids Res. 2013;41:1425–1437. doi: 10.1093/nar/gks1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li C, Han J, Yao Q, et al. Subpathway-GM: identification of metabolic subpathways via joint power of interesting genes and metabolites and their topologies within pathways. Nucleic Acids Res. 2013;41:e101. doi: 10.1093/nar/gkt161. [DOI] [PMC free article] [PubMed] [Google Scholar]