Abstract
Hearing loss is a common affection mainly resulting from irreversible loss of the sensory hair cells of the cochlea; therefore, developing therapies to replace missing hair cells is essential. Understanding the mechanisms that drive their formation will not only help to unravel the molecular basis of deafness, but also give a roadmap for recapitulating hair cells development from cultured pluripotent stem cells. In this review, we provide an overview of the molecular mechanisms involved in hair cell production from both human and mouse embryonic stem cells. We then provide insights how this knowledge has been applied to differentiate induced pluripotent stem cells into otic progenitors and hair cells. Finally, we discuss the current limitations for properly obtaining functional hair cell in a Petri dish, as well as the difficulties that have to be overcome prior to consider stem cell therapy as a potential treatment for hearing loss.
Keywords: Stem cells, Differentiation, Inner ear, Otic progenitors, Hair cells
Introduction
Hearing loss affects more than 16% of the worldwide population and this percentage may increase as a result of increased life expectancy. For the majority of patients, irreversible deafness results from an alteration of the auditory portion of the inner ear, the so-called sensorineural hearing loss. This type of deafness is mainly due to a loss of hair cells, the sensory cells that transform the mechanical signal of sound waves into electrical signals. The survival of hair cells can be impaired by exposure to loud sounds, environmental toxins or ototoxic drugs, and mutations in numerous genes can also contribute to their death. Unfortunately, the absence of hair cells prevents sound amplification provided by hearing aids. Moreover, the lack of hair cells and the essential neurotrophic factors they secrete lead secondarily to spiral ganglion neuron death, precluding the use of cochlear implants since these otic neurons constitute the first auditory relay to the central nervous system. Therefore, there is a need to develop stem cell therapy to be able to replace missing hair cells. In this review, we first provide a brief overview of the main steps involved in hair cell formation in vivo. We then describe impressive progress made to date in differentiating stem cells into hair cell-like cells, but also the numerous hurdles that still have to be considered and overcome before considering stem cell therapy to treat deafness.
Embryonic development of the cochlea
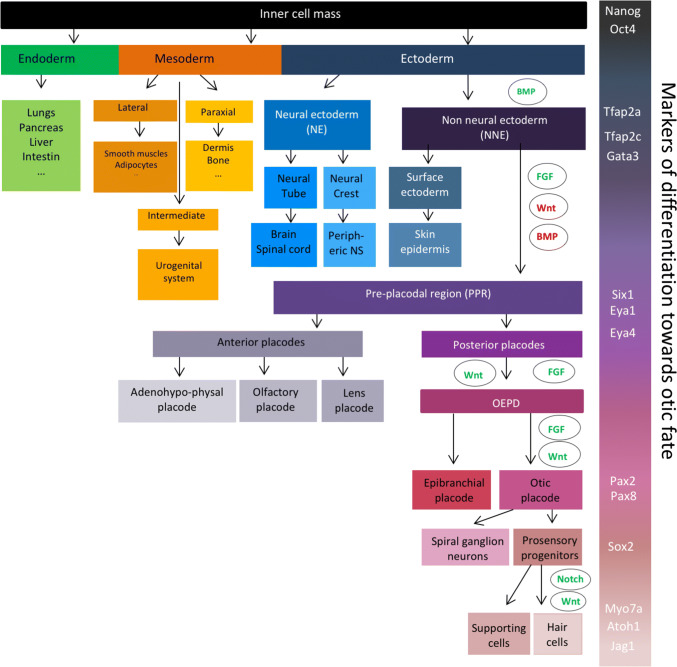
Understanding the molecular mechanisms underlying inner ear development is crucial to enable the successful differentiation of pluripotent stem cells into hair cells in vitro. At the beginning of mammalian embryonic development, the fertilized egg—the zygote—undergoes a series of duplications to form the blastocyst, a structure composed of the trophoblast and the inner cell mass, which will give rise to the extraembryonic tissues and the embryo, respectively. The pluripotent inner cell mass cells will then generate the three germ layers. The first step of the inner ear development consists of the formation of the non-neural ectoderm (NNE) (Fig. 1), a part of the anterior ectoderm. The ectodermal germ layer starts to differentiate into NNE following BMP signalling activation around embryonic day 6.5–7.5 (E6.5–7.5) in mice [1] and approximately around E17–18 in humans [2]. The NNE is characterised by the expression of different transcription factors, including Tfap2a, Tfap2c, Foxi1 and Gata3. The successive inhibition of BMP and Wnt pathways together with the activation of FGF signalling allows the formation of the preplacodal region (PPR) within the NNE [1, 3]. Several genes can be used as markers of the PPR, such as Six1, Six4, Eya1 and Eya4 [4]. The PPR further differentiates into patches that will give rise to the different cranial sensory organs around E8–9 in mice and E22 in humans. The combined expression of Pax8 and Pax2 defines the otic epibranchial placode domain (OEPD) [5, 6]. The specification of the otic placode from the OEPD is mediated by Wnt signalling that induces an increase of Pax2 expression [7]. Between E10 and E12 in mice, Sox2-positive otic progenitor cells proliferate in a region called the prosensory domain and will progressively become post-mitotic according to an apex-to-base wave [8]. Shortly after cell cycle exit, the Sox2-positive otic progenitors differentiate either in sensory hair cells or in supporting cells in an opposite base-to apex wave [9–11]. The hair cell fate is profoundly linked to the expression of the transcription factor Atoh1. Indeed, Atoh1 is initially expressed in nascent hair cells and activates its own transcription, as well as the expression of hair cell specific genes such as Pou4f3 [12]. Moreover, lateral inhibition of Atoh1 mediated by Notch signalling pathway leads to the differentiation of adjacent cells into supporting cells. Hair cells express Notch ligands—such as DELTA-1 or JAGGED-2—that will activate trans-membrane Notch receptors of adjacent cells and lead to the upregulation of transcriptional inhibitors of Atoh1 such as Hes1 or Hes5 [13, 14]. First hair cells appear around E15.5 in mice and around the gestational weeks 10–11 in humans, and are classically identified with specific markers such as parvalbumin, Myosin 6 (MYO6) or Myosin 7a (MYO7A). Supporting cells can be identified following immunostaining with P27KIP1, HES5 or SOX2 antibodies [15].
Fig. 1.
Different steps of embryonic development from the blastocyst to the inner ear hair cells. Pluripotent inner cell mass of the blastocyst can generate the three germ layers: the endoderm, the mesoderm and the ectoderm. Transient BMP activation induces the differentiation of the ectoderm into the non-neural ectoderm (NNE), characterised by the expression of Tfap2a, Tfap2c, and Gata3. Combination of FGF activation, and Wnt and BMP inhibition leads to the formation of the preplacodal region (PPR) expressing Six 1, Eya1 and Eya4 transcription factors. The difference cranial sensory organs arise from this PPR. Its most posterior part, the otic and epibranchial placode domain (OEPD) further differentiates into the otic placode under the control of FGF and Wnt pathways. Some otic placode-derived progenitors will then differentiate into spiral ganglion neurons as other will proliferate in a region called the prosensory domain to give rise to the Sox2-positive prosensory progenitors. Through lateral inhibition of Notch signalling pathway, those progenitors will differentiate into hair cells and supporting cells
Mouse embryonic stem cells
Mouse embryonic stem cells (mESCs) have been cultured for the first time in 1981 [16] on a feeder layer of mitotically inactivated mouse embryonic fibroblasts (MEFs) in presence of fetal calf serum (FCS). Several years later, feeder-free cultures of mESCs appeared following the identification of leukemia inhibitory factor (LIF) as the main factor secreted by fibroblasts [17, 18]. Later on, the activation of Wnt signalling using a GSK3 inhibitor has been reported to stimulate self-renewal of mESCs [19, 20]. In addition, the combination of CHIR99021 (CHIR), a highly selective GSK3 inhibitor, with a MEK1/2 inhibitor (PD0325901) preserves the pluripotent and proliferative state of mESCs [20]. This so-called 2i medium replaces efficiently FCS to maintain mESCs in culture.
It has been shown that mESCs display important transcriptomic and DNA methylation differences depending on the medium used for their maintenance [21–23]. It seems that when maintained in 2i/LIF medium, mESCs are close to E3.75–E4.5 pre-implantation embryonic cells and are the so-called ground or naïve stem cells. When mESCs are cultured in serum/LIF condition, they present a pluripotency state closer to the one of post-implantation blastocyst cells, called epiblast stem cells or EpiSCs [24]. Interestingly, it is possible to switch from one condition to another in one passage just by changing the culture medium [25, 26]. It has been recently reported that culturing mESCs in 2i medium dramatically impacts their developmental potential and their chromosomal stability due to the long-term inhibition of MEK1/2. However, it seems that this issue can be overcome using a Src inhibitor instead of a MEK1/2 inhibitor [27].
Hair cell differentiation from mESCs
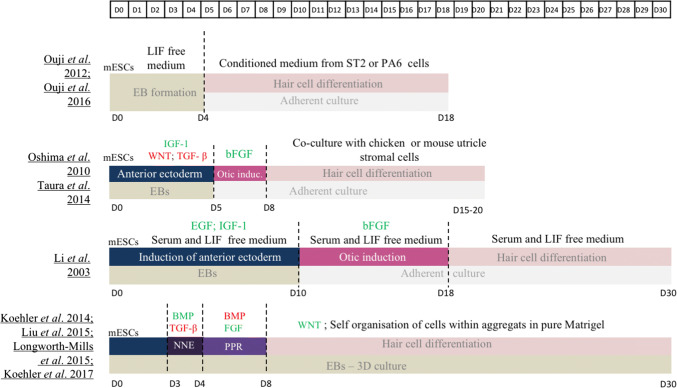
Over the years, numerous protocols have been developed to differentiate mESCs into hair cells (Fig. 2). For all of them, an initial step of embryonic bodies (EBs) formation is performed prior to culturing cells in adherent conditions to trigger the differentiation toward an ectodermal fate, confirming that EBs promote the secretion of differentiation factors. Several factors can be added to support the anterior ectodermal induction, e.g. transforming growth factor beta (TGF-β) and Wnt inhibitors, known to induce ectodermal fate, or insulin-like growth factor 1 (IGF-1), promoting anterior ectoderm formation [28–32]. Activation of the FGF signalling pathway seems to be necessary for otic induction as it increases the expression level of different otic markers such as Pax2, Pax8 or Six1 [28–32]. Withdrawal of growth factors and serum was sufficient to observe specific markers of hair cells such as MYO7A and ESPIN [28]. Co-culture with different stromal cell types, including inactivated chicken utricle or mouse bone marrow ST2 line, helps to further differentiate the otic progenitors into more mature hair cells that harbour electrophysiologically functional hair bundles at their apical surface [30, 32, 33].
Fig. 2.
Comparison of published protocols for hair cell-like cells differentiation of mESCs. BMP bone morphogenetic protein, EB embryoid bodies, EGF epidermal growth factor, FGF fibroblast growth factor, IGF-1 insulin-like growth factor-1, LIF leukemia inhibitory factor, mESCs mouse embryonic stem cells, NNE non neural ectoderm, PPR pre placodal region, TGF-β transforming growth factor-β
To be closer to in vivo embryogenesis, 3D culture models recently emerged with the advantage to favour self-organisation of cells, the only way to obtain mature cell types in vitro. In these protocols, aiming at reproducing all the developmental steps leading to otic placode differentiation, mESCs are cultured on ultra-low adherence plates to form aggregates. EBs are then exposed to TGF-β inhibitor (SB-431542) and BMP4 for a short period to induce a NNE fate. Cells are treated with BMP inhibitor (LDN-193189) to give rise to PPR cells and with FGF2 to direct them towards OEPD stage. This differentiation protocol considerably shortens the process of generating OEPD cells, as they are obtained after only 8 days in culture. At that time, aggregates are cultured in a maturation medium containing a Wnt activator (CHIR99021) and cells continue their self-organisation in complex structures. After 12 days of maturation, aggregates present inner ear organoids containing a high number of mechanosensitive hair cells (around 1500 per aggregate) but also cells expressing supporting cell markers [1, 34–36].
Human models
Mouse models are powerful research tools to study developmental mechanisms as well as pathologies. However, many fundamental disparities exist between mice and human. Considering the inner ear development, the mouse cochlea is still immature at birth, while human cochlea is completely functional at embryonic week 20. Moreover, numerous promising drugs failed to translate into the clinic mainly due to large discrepancies between mouse and human intracellular machinery. Several studies showed that genetic, molecular, immunologic and anatomic differences between humans and rodents hinder the ability to effectively mimic disease and predict toxicity [37–39]. Therefore, both human embryonic stem cells (hESCs) and human-induced pluripotent stem cells (hiPSCs) constitute a unique opportunity to create human-specific models.
Human embryonic stem cells (hESCs) were derived for the first time in 1998 by James Thomson and co-workers from human blastocysts [40]. These hESCs share common characteristics with mESCs, such as their capacity to proliferate indefinitely and their pluripotent status. However, major differences between mouse and human early embryonic development exist, especially regarding the extra-embryonic structures, and suggest that significant differences could also exist between hESCs and mESCs. While both ES cells can be maintained in an undifferentiated state on MEF feeder cells, feeder-free cultures supplemented by LIF are not appropriate to maintain pluripotency of hESCs [40, 41], suggesting that other factors play a role to ensure their stem cell identity. The inability to maintain hESCs in 2i medium reinforces this hypothesis.
Induced pluripotent stem cells
Although working with ESCs was a major step forward in disease modeling and a promising tool to find new therapeutic approaches, the ethical considerations surrounding the use of ESCs but also their non-autologous state limits their potential for clinical applications. Takahashi and Yamanaka have overcome this issue in 2006 and 2007 with the transduction of mouse or human fibroblasts with four key transcription factors, C-MYC, KLF4, SOX2 and OCT3/4, inducing their successful reprogramming. This process resulted in induced pluripotent stem cells (iPSCs) that have self-renewal and pluripotency properties comparable to ESCs [42, 43]. While SOX2 and OCT3/4 directly act to maintain pluripotency [44, 45], the roles of the two tumour-related factors C-MYC and KLF4 are less clear. It seems that C-MYC may allow the binding of OCT3/4 and SOX2 to their target genes through histone acetylation [46], while KLF4 might reduce apoptosis associated to C-MYC activation [42, 43]. However, in addition to the use of these two tumour-related factors, this reprogramming technique involves retroviral vectors that are integrated in the host genome, increasing the risk of mutagenesis and cancer and limiting their potential for clinical applications [47]. Therefore, finding non-integrating safer options for reprogramming adult cells became essential, and several methods quickly emerged to transfect cells with the 4 transcription factors such as non-integrative Sendai viral vectors [48], recombinant proteins [49] or synthetic modified mRNAs [50].
Mouse ESCs are easy to obtain and maintain in culture. Therefore, the advantage of working with mouse induced pluripotent stem cells (miPSCs) instead of mESCs for differentiation into hair cells is not crucial, explaining why so little has been done with these cells.
Hair cell differentiation from hSCs
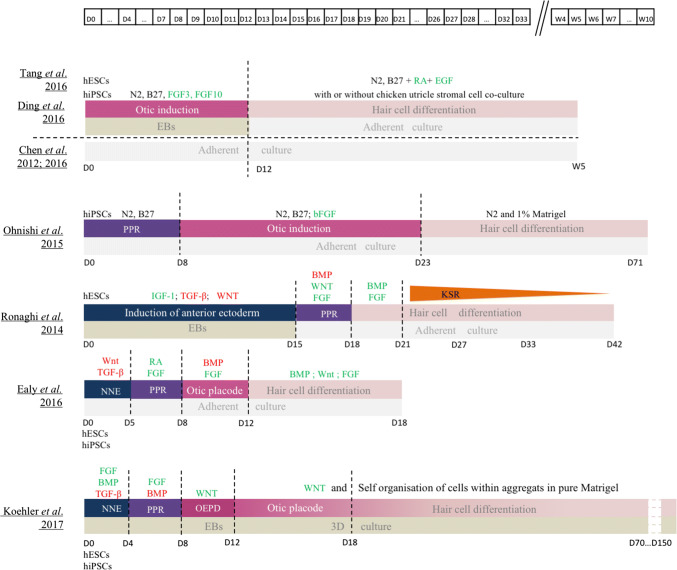
Two different strategies have been developed for generating hair cells from human ES or iPS cells (both called hSCs). While one method relies on the direct induction of otic progenitors from hSCs, the other one involves intermediate stages of NNE and PPR differentiation (Fig. 3).
Fig. 3.
Comparision of published protocols of human ES and iPS cell differentiation into hair cell-like cells. BMP bone morphogenetic protein, EB embryoid bodies, EGF epidermal growth factor, FGF fibroblast growth factor, hESCs human embryonic stem cells, hPSCs human induced pluripotent sten cells, IGF-1 insulin-like growth factor 2, LIF leukemia inhibitory factor, NNE non neural ectoderm, OEPD otic epibranchial placode domain, PPR pre placodal region, RA retinoic acid, TCF-β transforming growth factor-β
The induction of otic progenitors directly from hSCs has been achieved with the activation of FGF signalling pathway, reliably giving rise to two kinds of otic progenitors: otic neural progenitors (ONPs) and otic epithelial progenitors (OEPs) [51, 52]. Enrichment of OEPs was performed with sequential dissociation and maintenance in a proliferative medium. OEPs were then derived in cells expressing specific hair cell markers such as ATOH1, BRN3C and/or MYO7A after 2–4 weeks exposure to all-trans retinoic acid (RA) and epidermal growth factor (EGF), two factors previously demonstrated to promote hair cell differentiation in the mammalian cochlea. However, these differentiated cells failed to exhibit stereociliary hair bundles at their apical surface [51, 52], indicating that these factors were not sufficient to drive the full program of hair cell maturation. However, electrophysiologically functional hair cell-like cells were derived from OEPs when co-cultured with chicken utricle stromal cells complemented or not with RA and EGF treatment [52–54], demonstrating that, as for mES cells, chicken utricle stromal cells provide factors required for human hair cell development. Adding a step of PPR induction prior to the otic induction step also gave rise to hair cell-like cells [55]. Unfortunately, the hair cell differentiation efficiency of these protocols remained very low.
Recently, more complex protocols starting with a step of anterior NNE induction have been developed to reproduce/simulate more adequately the normal embryonic developmental stages. As demonstrated for mESC, a medium containing TGF-β and WNT pathway inhibitors allows NNE generation from hESCs [56, 57], but replacing WNT inhibitor by activators of FGF and BMP pathways seems even more efficient [2]. Then, activation of the FGF pathway is necessary to induce PPR fate [2, 56, 57] but high yields of PPR cells necessitate either RA [58] or inhibition of BMP pathway [2]. To progress throughout normal development, transient activation of Wnt signalling is necessary to obtain otic placode precursors. Indeed, starting from competent preplacodal tissue, activated Wnt pathway promotes the generation of otic placode tissue at the expense of the epidermis in embryonic mice [59]. Finally, induction towards hair cells can be actively led by culturing otic placode precursors in 3D [2] while activation of BMP and FGF pathway failed to efficiently trigger full maturation [57] even in combination with Wnt activation [56].
Culturing cells in 3D allowed secretion of undefined factors favouring the differentiation process but also the self-organisation of cells, giving rise to complex vesicles containing hair cell-like cells, which harbour apical kinocilium and stereocilia bundles, as well as neurons expressing synaptic ribbon proteins specific of the mammalian sensory organs. However, derived hair cells exhibited electrophysiological properties typical of vestibular hair cells in accordance with the vestibular morphology of their stereocilia bundles, suggesting that additional signalling modulations are necessary to obtain cochlear hair cells. Taken together, these data prove how delicate the differentiation process is and the fundamental importance of deeply understanding the molecular mechanisms regulating the physiological inner ear development in humans. Indeed, modulating signalling pathways at the wrong time point could dramatically reduce the efficiency of the differentiation into hair cell-like cells [57]. Moreover, single cell analysis throughout the process of hSC monolayer differentiation into posterior placode cells revealed crucial differences between hESCs and hiPSCs cells [56] and even between two different iPS lines, indicating that culture conditions should be adapted to each cell line to promote otic lineage differentiation and finally hair cell-like cells.
Potentials and limitations of stem cells therapies
Replacement therapies relying on stem cells have been successfully led for more than 20 years. Indeed, mesenchymal stem cells (MSC) were first used in 1995 for bone marrow recovery after cancer treatment [60], and they are now routinely used as curative therapy. Moreover, more than 2500 clinical trials involving stem cells have been performed and 644 are still ongoing (https://clinicaltrials.gov/), making them attractive for treating many diseases including deafness. In 2015, a clinical trial was conducted to test the effectiveness of autologous bone marrow-derived MSC transplantation in patients with sensorineural hearing loss [61]. Cells were intravenously injected in two patients with no side effects reported 3 years after the injection. Unfortunately, there was no hearing improvement 1, 6 or 12 months later. More recently, injection of autologous umbilical cord stem cells has started for the treatment of acquired hearing loss in 11 children. Audition, but also language development, is recorded for 1 year after the transplantation (https://clinicaltrials.gov/). These clinical trials represent the starting point towards stem cell therapy for hearing loss.
For the last 15 years, impressive work has been performed in the field of otic differentiation from both mouse and human SCs. Numerous studies succeeded to obtain functional hair cell-like cells, even if the differentiation efficiency still remains low. However, these derived-hair cell-like cells exhibit a vestibular phenotype, characterised by a distinctive hair bundle at their surface and a typical electrophysiological response [1, 2, 34, 35, 62, 63], suggesting that more investigations have to be pursued to unravel the factors implicated in cochlear hair cell differentiation. Nevertheless, the ability to derive hair cell-like cells from SCs combined with the latest gene editing tool—CRISPR/Cas9 technology—is a new promising step for treating human hereditary deafness [53, 54]. Indeed, CRISPR/CAS9 system was successfully used to correct deafness-associated mutations in hiPSCs. The stem cell-derived hair cell-like cells that present mutation in MYO7 or MYO15 gene harboured defects in stereocilia bundles. The correction of gene mutation using CRISPR/CAS9 technology restored the morphology of hair bundles and the functionality of the derived hair cells [53, 54].
Numerous issues need to be addressed before testing the efficacy of human hair cell-like cell transplantation. First, we need a homogeneous hair-cell like population to avoid transplantation of non-differentiated cells and potential tumour formation [64]. Second, the grafted cells have to be able to integrate into the organ of Corti following their injection. This is not a trivial problem since the compartment of cell injection, the scala media, is filled with endolymph a unique compartment containing a particularly high potassium concentration that may be toxic for injected cells [65–67]. Moreover, if cells survive, they have to reach the damaged portion of the sensory epithelium and correctly integrate into it. Finally, the grafted cells should also establish functional synaptic connections with spiral ganglion neurons.
Many pitfalls have to be surpassed before using hiPSCs as a treatment for hearing loss. The emergence of hiPSCs offered a priceless answer to the ethical considerations of using ES cells and to the immuno-incompatibility of non-autologous transplantations [68]. Unfortunately, significant variability exists between hiPSC lines [69] and consequently developing iPSC lines for each patient is currently too expensive and time-consuming to be conceivable [68]. Nevertheless, in vitro human models are an essential tool for new drug discovery. Indeed, many clinical trials failed because of discrepancies between human and mouse metabolisms. Therefore, human stem cell-derived cultures represent an interesting alternative to animal models.
In summary, stem cell therapy has still many challenges to address prior applying it for hearing restoration. However, this field has quickly progressed over the last decade, and promises to make further major strides in the next few years, remaining an ambitious but relevant line of research for hearing loss treatment.
References
- 1.Koehler KR, Mikosz AM, Molosh AI, et al. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature. 2013;500:217–221. doi: 10.1038/nature12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koehler KR, Nie J, Longworth-Mills E, et al. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat Biotechnol. 2017;35:583–589. doi: 10.1038/nbt.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pieper M, Ahrens K, Rink E, et al. Differential distribution of competence for panplacodal and neural crest induction to non-neural and neural ectoderm. Development. 2012;139:1175–1187. doi: 10.1242/dev.074468. [DOI] [PubMed] [Google Scholar]
- 4.Saint-Jeannet JP, Moody SA. Establishing the pre-placodal region and breaking it into placodes with distinct identities. Dev Biol. 2014;389:13–27. doi: 10.1016/j.ydbio.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basch ML, Brown RM, Jen HI, Groves AK. Where hearing starts: the development of the mammalian cochlea. J Anat. 2016;228:233–254. doi: 10.1111/joa.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freter S, Muta Y, Mak S-S, et al. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–3424. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- 7.Hidalgo-Sánchez M, Alvarado-Mallart R, Alvarez IS. Pax2, Otx2, Gbx2 and Fgf8 expression in early otic vesicle development. Mech Dev. 2000;95(1–2):225–9. doi: 10.1016/S0925-4773(00)00332-4. [DOI] [PubMed] [Google Scholar]
- 8.Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- 9.Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18:7811–7821. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laine H, Sulg M, Kirjavainen A, Pirvola U. Cell cycle regulation in the inner ear sensory epithelia: role of cyclin D1 and cyclin-dependent kinase inhibitors. Dev Biol. 2010;337:134–146. doi: 10.1016/j.ydbio.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Oesterle EC, Campbell S, Taylor RR, et al. Sox2 and Jagged1 expression in normal and drug-damaged adult mouse inner ear. JARO. 2008;9:65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu DK, Kelley MW. Molecular mechanisms of inner ear development. Cold Spring Harb Perspect Biol. 2012;4:a008409. doi: 10.1101/cshperspect.a008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanford PJ, Lan Y, Jiang R, et al. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Jeong H-S, Cho H-H. Atoh1 as a coordinator of sensory hair cell development and regeneration in the cochlea. Chonnam Med J Chonnam Med J. 2017;53:37–46. doi: 10.4068/cmj.2017.53.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White PM, Doetzlhofer A, Lee YS, et al. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 16.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 17.Smith AG, Heath JK, Donaldson DD, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 18.Williams RL, Hilton DJ, Pease S, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 19.Sato N, Meijer L, Skaltsounis L, et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 20.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habibi E, Brinkman AB, Arand J, et al. Whole-genome bisulfite sequencing of two distinct interconvertible DNA methylomes of mouse embryonic stem cells. Cell Stem Cell. 2013;13:360–369. doi: 10.1016/j.stem.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Leitch HG, McEwen KR, Turp A, et al. Naive pluripotency is associated with global DNA hypomethylation. Nat Struct Mol Biol. 2013;20:311–316. doi: 10.1038/nsmb.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks H, Kalkan T, Menafra R, et al. The transcriptional and epigenomic foundations of ground state pluripotency. Cell. 2012;149:590–604. doi: 10.1016/j.cell.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boroviak T, Loos R, Lombard P, et al. Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis. Dev Cell. 2015;35:366–382. doi: 10.1016/j.devcel.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi O, Wang SY, Kuznetsova T, et al. Dynamic reorganization of extremely long-range promoter-promoter interactions between two states of pluripotency. Cell Stem Cell. 2015;17:748–757. doi: 10.1016/j.stem.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Martin Gonzalez J, Morgani SM, Bone RA, et al. Embryonic stem cell culture conditions support distinct states associated with different developmental stages and potency. Stem Cell Reports. 2016;7:177–191. doi: 10.1016/j.stemcr.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi J, Huebner J, Clement K, et al. Prolonged Mek1/2 suppression impairs the developmental potential of embryonic stem cells. Nature. 2017;548(7666):219–223. doi: 10.1038/nature23274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Sci USA. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouji Y, Sakagami M, Omori H, et al. Efficient induction of inner ear hair cell-like cells from mouse ES cells using combination of Math1 transfection and conditioned medium from ST2 stromal cells. Stem Cell Res. 2017;23:50–56. doi: 10.1016/j.scr.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Ouji Y, Ishizaka S, Nakamura-Uchiyama F, Yoshikawa M. In vitro differentiation of mouse embryonic stem cells into inner ear hair cell-like cells using stromal cell conditioned medium. Cell Death Dis. 2012;3:e314. doi: 10.1038/cddis.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taura A, Ohnishi H, Ochi S, et al. Effects of mouse utricle stromal tissues on hair cell induction from induced pluripotent stem cells. BMC Neurosciences. 2014;15:121. doi: 10.1186/s12868-014-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oshima K, Shin K, Diensthuber M, et al. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–716. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshikawa M, Ouji Y. Induction of inner ear hair cells from mouse embryonic stem cells in vitro. Methods Mol Biol. 2016;1516:257–267. doi: 10.1007/7651_2016_328. [DOI] [PubMed] [Google Scholar]
- 34.Longworth-Mills E, Koehler KR, Hashino E. Generating inner ear organoids from mouse embryonic stem cells. Methods Mol Biol. 2016;1341:391–406. doi: 10.1007/7651_2015_215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koehler KR, Hashino E. 3D mouse embryonic stem cell culture for generating inner ear organoids. Nat Protoc. 2014;9:1229–1244. doi: 10.1038/nprot.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abboud N, Fontbonne A, Watabe I, et al. Culture conditions have an impact on the maturation of traceable, transplantable mouse embryonic stem cell-derived otic progenitor cells. Med: J Tissue Eng Regen; 2016. [DOI] [PubMed] [Google Scholar]
- 37.Martignoni M, Groothuis GMM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- 38.Uhl EW, Warner NJ. Mouse models as predictors of human responses: evolutionary medicine. Curr Pathobiol Rep. 2015;3:219–223. doi: 10.1007/s40139-015-0086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 40.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 41.Thomson JA, Itskovitz-eldor J, Shapiro SS et al (2007) Embryonic stem cell lines derived from human blastocysts. Science (80-) 1145:. 10.1126/science.282.5391.1145 [DOI] [PubMed]
- 42.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Boyer LA, Lee TI, Cole MF, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loh Y-H, Wu Q, Chew J-L, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez PC, Frank SR, Wang L, et al. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17:1115–1129. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng T, Dong Y, Zhu G, Xie D. Induced pluripotent stem cells: landscape for studying and treating hereditary hearing loss. J Otol. 2014;9:151–155. doi: 10.1016/j.joto.2015.02.001. [DOI] [Google Scholar]
- 48.Fusaki N, Ban H, Nishiyama A, et al. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim D, Kim C-H, Moon J-I, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen W, Jongkamonwiwat N, Abbas L, et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490:278–282. doi: 10.1038/nature11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding J, Tang Z, Chen J, et al. Induction of differentiation of human embryonic stem cells into functional hair-cell-like cells in the absence of stromal cells. Int J Biochem Cell Biol. 2016;81:208–222. doi: 10.1016/j.biocel.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Tang Z-H, Chen J-R, Zheng J, et al. Genetic correction of induced pluripotent stem sells from a deaf patient with MYO7A mutation results in morphologic and functional recovery of the derived hair cell-like cells. Stem Cells Transl Med. 2016;5:561–571. doi: 10.5966/sctm.2015-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J-R, Tang Z-H, Zheng J, et al. Effects of genetic correction on the differentiation of hair cell-like cells from iPSCs with MYO15A mutation. Cell Death Differ. 2016;23:1347–1357. doi: 10.1038/cdd.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohnishi H, Skerleva D, Kitajiri S, et al. Limited hair cell induction from human induced pluripotent stem cells using a simple stepwise method. Neurosci Lett. 2015;599:49–54. doi: 10.1016/j.neulet.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 56.Ealy M, Ellwanger DC, Kosaric N, et al. Single-cell analysis delineates a trajectory toward the human early otic lineage. Proc Natl Acad Sci. 2016;113:8508–8513. doi: 10.1073/pnas.1605537113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ronaghi M, Nasr M, Ealy M, et al. Inner ear hair cell-like cells from human embryonic stem cells. Stem Cells Dev. 2014;23:1275–1284. doi: 10.1089/scd.2014.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ealy M, Ellwanger DC, Kosaric N, et al. Single-cell analysis delineates a trajectory toward the human early otic lineage. Proc Natl Acad Sci. 2016;113:201605537. doi: 10.1073/pnas.1605537113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohyama T, Mohamed OA, Taketo MM, et al. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–875. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- 60.Lazarus HM, Haynesworth SE, Gerson SL, et al. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transpl. 1995;16:557–564. [PubMed] [Google Scholar]
- 61.Lee HS, Kim WJ, Gong JS, Park KH. Clinical safety and efficacy of autologous bone marrow-derived mesenchymal stem cell transplantation in sensorineural hearing loss patients. J Audiol Otol. 2018;22:105–109. doi: 10.7874/jao.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nie J, Koehler KR, Hashino E. Directed differentiation of mouse embryonic stem cells into inner ear sensory epithelia in 3D culture. Methods Mol Biol. 2017;1597:67–83. doi: 10.1007/978-1-4939-6949-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X-P, Koehler KR, Mikosz AM, et al. Functional development of mechanosensitive hair cells in stem cell-derived organoids parallels native vestibular hair cells. Nat Commun. 2016;7:11508. doi: 10.1038/ncomms11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee AS, Tang C, Rao MS et al (2013) Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 10.1038/nm.3267 [DOI] [PMC free article] [PubMed]
- 65.Okano T, Kelley MW. Stem cell therapy for the inner ear. Trends Amplif. 2012;16:4–18. doi: 10.1177/1084713812440336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu Z, Ulfendahl M. The potential of stem cells for the restoration of auditory function in humans. Regen Med. 2013;8:309–318. doi: 10.2217/rme.13.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kürşat Gökcan M, Mülazimoğlu S, Ocak E, et al. Turkish journal of medical sciences study of mouse induced pluripotent stem cell transplantation into Wistar albino rat cochleae after hair cell damage. Turk J Med Sci. 2016;46:1603–1610. doi: 10.3906/sag-1510-136. [DOI] [PubMed] [Google Scholar]
- 68.Pauley S, Kopecky B, Beisel K, et al. Stem cells and molecular strategies to restore hearing. Panminerva Med. 2008;50:41–53. [PMC free article] [PubMed] [Google Scholar]
- 69.Ortmann D, Vallier L, Wang J, Esteban M. Variability of human pluripotent stem cell lines This review comes from a themed issue on Cell reprogramming. Curr Opin Genet Dev. 2017;46:179–185. doi: 10.1016/j.gde.2017.07.004. [DOI] [PubMed] [Google Scholar]