Abstract
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by a progressive loss of dopamine (DA) neurons through apoptotic, inflammatory and oxidative stress mechanisms. The octadecaneuropeptide (ODN) is a diazepam-binding inhibitor (DBI)-derived peptide, expressed by astrocytes, which protects neurons against oxidative cell damages and apoptosis in an in vitro model of PD. The present study reveals that a single intracerebroventricular injection of 10 ng ODN 1 h after the last administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) prevented the degeneration of DA neurons induced by the toxin in the substantia nigra pars compacta of mice, 7 days after treatment. ODN-mediated neuroprotection was associated with a reduction of the number of glial fibrillary acidic protein-positive reactive astrocytes and a strong inhibition of the expression of pro-inflammatory genes such as interleukins 1β and 6, and tumor necrosis factor-α. Moreover, ODN blocked the inhibition of the anti-apoptotic gene Bcl-2, and the stimulation of the pro-apoptotic genes Bax and caspase-3, induced by MPTP in the substantia nigra pars compacta. ODN also decreased or even in some cases abolished MPTP-induced oxidative damages, overproduction of reactive oxygen species and accumulation of lipid oxidation products in DA neurons. Furthermore, DBI knockout mice appeared to be more vulnerable than wild-type animals to MPTP neurotoxicity. Taken together, these results show that the gliopeptide ODN exerts a potent neuroprotective effect against MPTP-induced degeneration of nigrostriatal DA neurons in mice, through mechanisms involving downregulation of neuroinflammatory, oxidative and apoptotic processes. ODN may, thus, reduce neuronal damages in PD and other cerebral injuries involving oxidative neurodegeneration.
Electronic supplementary material
The online version of this article (10.1007/s00018-017-2727-2) contains supplementary material, which is available to authorized users.
Keywords: Neuropeptide ODN, MPTP, Parkinson’s disease, Neurodegeneration, Neuroprotection, Inflammation and oxidative response
Introduction
Parkinson’s disease (PD), a chronic neurodegenerative disorder that affects around 2% of people aged over 65 years worldwide [1, 2], is characterized by motor deteriorations such as postural instability, rigidity and bradykinesia [3, 4]. The pathological hallmarks of PD include a loss of dopaminergic neurons within the substantia nigra pars compacta (SNpc), responsible for a decrease of striatal dopamine (DA) levels and associated dysfunction of neuronal network integrity [5, 6]. It is generally accepted that PD develops as a result of interplay between genetic and environmental factors [7–9] and involves inflammatory and oxidative stress processes [10–12]. In particular, increased levels of pro-inflammatory mediators and free radicals in the SNpc, as observed in both PD models and PD patients, exacerbate DA neuron degeneration [10, 13]. Various treatments are used to improve motor functions in PD patients, including administration of levodopa, surgical approaches and electrical stimulation [7, 14, 15]. However, all the current therapeutic approaches remain palliative and do not cure the disease [16]. Furthermore, these treatments have drawbacks and, in some cases, can produce disabling side effects [17]. Thus, there is a need to find efficient neuroprotective agents which could lead to the development of new therapies for effective treatments of the disease.
It is well established that astrocytes play an important role in the protection of brain damages induced by inflammation and oxidative stress, and recent studies have highlighted the neuroprotective effect of various factors expressed by astrocytes in PD models [18, 19]. Concurrently, several findings have provided evidence that reactive astrocytes can exert beneficial effects and confer endogenous neuroprotection by secreting neurotrophic factors such as glial cell line-derived neurotrophic factor (GDNF) [20], ciliary neurotrophic factor (CNTF) [21] and mesencephalic astrocyte-derived neurotrophic factor (MANF) [22]. Therefore, investigation of the beneficial effects of reactive astrocytes, particularly the capability of the factors they express to limit inflammatory damages, oxidative stress insults and apoptosis, appears crucial to protect DA neurons. It is notably important to identify new factors expressed by astrocytes, which can prevent both oxidative stress and proinflammatory mediators responsible for apoptosis-induced DA neuron degeneration. In this context, the octadecaneuropeptide (ODN), which is exclusively produced by astroglial cells in the central nervous system (CNS) of mammals [23–27] and exerts a potent neuroprotective activity against 6-hydroxydopamine (6-OHDA)-induced oxidative stress on cultured cerebellar granule cells [28], could be a promising factor. ODN is a gliopeptide generated through proteolytic cleavage of an 86-amino acid precursor called diazepam-binding inhibitor (DBI) [29, 30], also known as acyl-CoA binding protein (ACBP) [31]. The primary structure of DBI has been well preserved during evolution [32, 33], suggesting that DBI-derived peptides play important biological functions. Indeed, DBI and its derived products regulate food intake, behavioral activities and energy metabolism [34–37]. In addition, ACBP/DBI regulates brain lipid metabolism, and fatty acid metabolism-related gene expression is compromised in brain slices and astrocytes cultured from ACBP/DBI-deficient mice [38]. Interestingly, ODN has been shown to stimulate neurogenesis in the adult mouse brain [39], to increase glutathione (GSH) biosynthesis and antioxidant enzyme activity in cultured neurons and astroglial cells [28, 40, 41], and to prevent oxidative stress-induced reactive oxygen species (ROS) accumulation and cellular damages in cultured astrocytes [40, 42]. Experimental and clinical studies have also shown that ODN concentrations are increased in the plasma of rats and humans subjected to systemic inflammation [43]. Consistent with these observations, we have recently shown that ODN release, as well as expression of its precursor DBI, are induced in astrocytes in response to mild oxidative insult, and contribute to prevent cell death [44]. Altogether, these data suggest that ODN acts as a neurotrophic factor regulating proliferation and/or survival of neuronal cells, and may, thus, contribute to prevent pro-inflammatory and oxidative damages under injury conditions.
The prodrug 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which replicates in human and non-human mammals clinical hallmarks of PD syndrome [45–47], is widely used to induce DA neuronal cell death in animal models [48, 49]. MPTP per se is not deleterious, but it is oxidized by monoamine oxydase B expressed by astrocytes into 1-methyl-4-phenylpyridinium (MPP+), which is toxic. MPP+ is then released into the extracellular fluid and conveyed by DA transporters into nerve terminals of DA neuron. MPP+ induces DA neuron apoptosis through the production of ROS, causing inhibition of complex I of the mitochondrial respiratory chain [50–52] associated with the expression of the pro-apoptotic protein Bax and activation of caspase-3 [53].
Although there is clear evidence that ODN exerts a strong protective activity against oxidative stress-induced apoptosis on cultured neurons and glial cells, the potential neuroprotective activity of ODN in vivo has not been tested so far. Therefore, the aim of the present study was to investigate whether intracerebroventricular (i.c.v.) injection of ODN could prevent DA neuron degeneration in a MPTP-induced mouse model of PD, and to explore the vulnerability of ODN precursor knockout (KO) mice to MPTP-induced neurotoxicity.
Materials and Methods
Animals
Animals used in this study were 10-week-old C57BLJ/6 male mice purchased from Charles Rivers Laboratories (L’Obrestle, France), and C57BL/6JBomTac wild-type (ACBP/DBI+/+), DBI heterozygous (ACBP/DBI+/−) and DBI homozygous KO (ACBP/DBI−/−) mice obtained from the intercross of heterozygous animals after characterization by PCR analysis of DNA isolated from tail biopsies [54]. Primers used for the genotyping were: rev: 5′GTATCTGCTCATCTATTCGGCTTGG3′; fwd: 5′CCGCTGAGGAGGTGAAGCGCC3′. One week before the beginning of the treatments, animals were housed four per cage on a 12-h light/dark cycle at 21 °C with free access to food and tap water for acclimatization. Experiments were approved by the regional ethic committee for animal experimentation (CENOMEXA) and conducted by authorized investigators according to the recommendations of the European Union.
I.c.v. injections
I.c.v. injections of saline or ODN solutions (10 µl/mouse) were conducted according to the procedure of Haley and McCormick [55], using a microsyringe (50 µl; Hamilton, Bonaduz, Switzerland) connected to a needle (diameter 0.5 mm), of which the bevel protruded only 3.5 mm from a guard, limiting its penetration into the brain. The free-hand i.c.v. injections in immobilized mice lasted approximately 5 s and were conducted as previously described [35]. I.c.v. injections were performed by an experienced investigator who frequently controlled the regularity and success of the injections, using methylene blue dye, and who observed (after killing and frontal brain sectioning) that the injection was successful in more than 95% of the trials.
In vivo treatment and experimental design
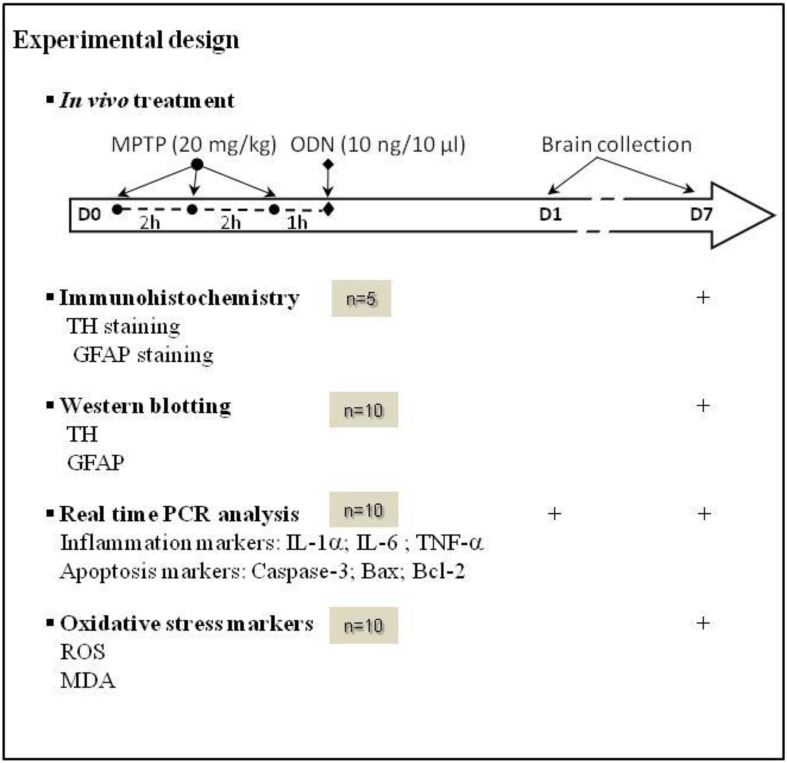
Animals were divided into four groups, i.e, control (n = 15), MPTP (n = 15), ODN (n = 15) and MPTP plus ODN (n = 15). On the first day of treatment (D0), each mice received three intraperitoneal (i.p.) injections of MPTP (20 mg/kg) in 100 µl of 0.9% NaCl at 2-h intervals, and a 10 µl i.c.v injection of ODN (10 ng) 1 h after the last injection of MPTP (Table 1). Control animals received saline solution instead of MPTP with or without ODN. Seven days after the beginning of the treatment (D7), mice were deeply anesthetized and killed to collect brains for real-time PCR, Western blot, immunohistochemistry and biochemical experiments (see Table 1). For experiments using mice with targeted deletion of ACBP/DBI (DBI−/− and DBI+/−), since the objective was to observe a difference of toxicity between wild-type and DBI−/− animals, the experimental design was similar except the doses of MPTP, which were reduced to 10 mg/kg.
Table 1.
Experimental design of the study showing treatments and assays
Mice were exposed to MPTP (20 mg/kg, 3 times at 2 h interval), ODN (10 ng/10 µl), MPTP and ODN or saline solution (Nacl 0.9%, Sham). On Day 0 (D0), animals received three intraperitoneal injections of 100 µl MPTP at 2-h intervals and 1 h after the last injection of MPTP, mice received 10 µl of intracerebroventricular injection of ODN (10 ng; ODN and MPTP + ODN treated mice) or saline solution (control and MPTP-treated mice). On D7, mice were killed and striatum as well as substantia nigra tissues were removed and analyzed by immunofluorescence, Western blotting and RT-qPCR approaches to assess tyrosine hydroxylase (TH), glial fibrillary acidic protein (GFAP), apoptotic and proinflammatory gene and protein expression. Tissue samples were also used to measure ROS and MDA abundance. Brain from each group was also collected at D1 for real-time PCR analysis. The number of animals used in each experiment (n) is indicated.
Immunohistochemistry
Animals were anesthetized with isoflurane and intracardially perfused with 0.9% NaCl to rinse blood vessels, and then with 4% paraformaldehyde (PFA; Sigma Aldrich, Saint-Quentin-Fallavier, France) to fix tissues. After decapitation, the brains were removed and post-fixed overnight in a 4% PFA solution at 4 °C. The brains were then immerged for cryopreservation in 15 and 30% sucrose solution for 24 h each. Frozen brains were sectioned in the frontal plane at the levels of the SNpc and striatum into 10 µm thick slices with a cryomicrotome (CM3050; Leica Microsystems, Nanterre, France), and mounted onto chromium potassium sulphate and gelatine-coated glass slides. Three to four tissue sections from each brain were preincubated for 90 min in blocking solution containing 1:50 normal donkey serum, 1% bovine serum albumin, and 0.3% Triton X-100 (VWR International, Strasbourg, France) in phosphate buffered saline (PBS). Three–four tissue sections from each brain were then incubated overnight with an anti-tyrosine hydroxylase (TH) rabbit primary antibody (1:1000 dilution; Millipore, Molsheim, France) and others with glial fibrillary acidic protein (GFAP) rabbit primary antibody (1:1000 dilution; Dako, Les Ulis, France) at 4 °C. After three washes in PBS, the tissues were incubated with secondary antibodies for 90 min at room temperature, i.e., Alexa 488-conjugated donkey anti-rabbit IgG or Alexa 594-conjugated donkey anti-rabbit IgG (1:400 dilution each; Invitrogen Life technologies, Longjumeau, France). Finally, the slices were rinsed thrice in PBS and cover slipped with Mowiol to perform image acquisition using a TCS SP2 upright confocal laser-scanning microscope (Leica Microsystems, Nanterre, France). Fluorescence quantification was conducted with the video imaging software ImageJ.
Preparation of brain tissue extracts for Western blot and biochemical experiments
Animals were deeply anesthetized with pentobarbital (Sigma Aldrich, 40 mg/kg body weight i.p.). After decapitation, SNpc and striatum from each animal were dissected, homogenized in 1 ml lysis buffer containing 1% Triton X-100, 50 mM Tris–HCl, 10 mM EDTA plus Pierce EDTA free protease inhibitor tablets (Thermo Scientific, Paris, France) and centrifuged (14,000g, 4 °C, 15 min).
Western blot
Whole protein content in brain tissue extracts was measured by using the Bradford reagent method and normalized. The homogenate was centrifuged (14,000g, 4 °C, 15 min) and the proteins contained in the supernatant were precipitated at 4 °C by addition of ice-cold 10% trichloroacetic acid (TCA). Precipitated proteins were then recovered by centrifugation (12,000g, 4 °C, 15 min) and washed thrice with an alcohol/ether solution (30:70; v/v). The proteins were finally denatured in 50 mM Tris–HCl (pH 7.5) containing 20% glycerol, 0.7 M 2-mercaptoethanol, 0.004% (w/v) bromophenol blue and 3% (v/v) SDS at 100 °C for 5 min.
Protein samples (~ 20 µg) were subjected to a 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and the gel was transferred onto a nitrocellulose membrane (Amersham, Les Ulis, France). The membrane was first incubated at room temperature for 1 h in a blocking solution containing 5% skim milk in 50 mM Tris-buffered saline solution completed with 0.1% Tween 20 (TBST). The membranes were then incubated with primary antibodies against TH (1:1000 dilution; Millipore, Molsheim, France), GFAP (1:1000 dilution; Dako, Les Ulis, France) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:4000 dilution; Santa Cruz, Nanterre, France) overnight at 4 °C. The next day, membranes were washed with TBST and then incubated in TBS containing 5% skim milk with a goat anti-rabbit secondary antibody (1:5000 dilution; Santa Cruz) for 1 h at room temperature. After washing, proteins were revealed using a chemiluminescence detection kit (ECL System, GE Healthcare, Aulnay-Sous-Bois, France) and measured with an image analysis system (BioRad, Châtillon, France).
Caspase-3 activity
An aliquot of 100 µl of brain tissue extract was incubated with the Apo-ONE™ homogeneous caspase-3/7 assay kit (Promega). Fluorescence intensity was measured over a 3-h period with a FlexStation III microplate reader (Molecular Devices, Sunnyvale, USA) at an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
Measurement of oxidative stress markers
Measurement of intracellular ROS formation
ROS were detected by measuring the fluorescence of 2′,7′-dichlorofluorescein (DCF) which is derived from the deacetylation and oxidation of the non-fluorescent compound DCFH2-DA. An aliquot of 100 µl of brain tissue extract (0.24 ± 0.02 mg/ml from SNpc and 0.6 ± 0.03 mg/ml from striatum) was incubated with 10 µM DCFH2-DA at 37 °C for 30 min in the dark and fluorescence was measured with a fluorescence microplate reader FL800TBI (Bio-Tek Instruments, Winooski, VT, USA) using 485- and 538-nm excitation and emission filters, respectively.
Measurement of malondialdehyde formation
Malondialdehyde (MDA, i.e, a lipid oxidation product) content was determined using an MDA kit (Sigma Aldrich) according to the manufacturer’s instructions. Briefly, 200 µl of brain tissue extract (~ 0.24 mg/ml from SNpc and ~ 0.6 mg/ml from striatum) was mixed with a solution containing 1% butylhydroxytoluene and 20% TCA, and then centrifuged (1000g, 5 min, 4 °C). The supernatant was mixed with a solution containing 0.5 M HCl, 120 mM 2-thio-barbituric acid plus 26 mM Tris–HCl (pH 7.5) and heated at 95 °C for 60 min. After cooling, the absorbance was measured at 532 nm (FlexStation III Molecular Devices, Sunnyvale, USA).
Real-time PCR analysis
Total RNAs from the mouse SNpc or striatum, obtained at 1 and 7 days after MPTP treatment, were extracted using Tri-Reagent (Sigma Aldrich) and purified with the NucleoSpin RNA kit (Macherey–Nagel, Hoerdt, France). The concentration of RNA was measured with a Thermo-Scientific Nanodrop 2000 spectrophotometer (Labtech, Palaiseau, France) at an absorbance of 260 nm. One micro gram of total RNA from each sample was converted into single stranded cDNA using the Improm-II reverse transcriptase kit (Promega, Madison, WI, USA) with random primers (0.5 µg/ml). Three ng cDNA were then amplified in the presence of 1X SYBR Green Mastermix (Applied Biosystems, Courtaboeuf, France) containing preset concentrations of dNTPs and MgCl2 with forward and reverse primers (Table 2), using an ABI Prism 7500 Sequence Detection System (Applied Biosystems). The relative amount of cDNA in each sample was calculated using the comparative quantification cycle (Cq) method and expressed as 2−∆∆Cq using GAPDH, which was constant in all treatment conditions, as an internal standard for variations in amounts of input mRNA.
Table 2.
Sequences of the primers used for real-time PCR experiments
| Gene | GenBank accession number | Sequence | Blast E value |
|---|---|---|---|
| IL-6 | NM_031168.1 | ||
| Forward | GAAACCGCTATGAAGTTCCTCTCTG | 0.00003 | |
| Reverse | TGTTGGGAGTGGTATCCTCTGTGA | 0.0001 | |
| IL-β | NM_008361.3 | ||
| Forward | GAAGAAGAGCCCATCCTCTGT | 0.005 | |
| Reverse | TGTTCATCTCGGAGCCTGTA | 0.013 | |
| TNF-α | NM_013693.2 | ||
| Forward | CACCGTCAGCCGATTTGC | 0.14 | |
| Reverse | TGAGTTGGTCCCCCTTCTCC | 0.013 | |
| Caspase-3 | NM_009810 | ||
| Forward | GAGGCTGACTTCCTGTATGCTT | 0.001 | |
| Reverse | AACCACGACCCGTCCTTT | 0.14 | |
| Bax | NM_007527 | ||
| Forward | GTGAGCGGCTGCTTGTCT | 0.14 | |
| Reverse | GGTCCCGAAGTAGGAGAGGA | 0.013 | |
| Bcl-2 | NM_009741 | ||
| Forward | GTACCTGAACCGGCATCTG | 0.035 | |
| Reverse | GGGGCCATATAGTTCCACAA | 0.013 | |
Statistical analysis
The data are presented as the mean ± SEM of at least three independent experiments. Statistical analysis was performed using the PRISM software (GraphPad Software, San Diego, CA, USA). A two-way ANOVA, followed by a Bonferroni’s post hoc test was used, and differences were considered statistically significant when P ≤ 0.05.
Results
ODN protects dopaminergic neurons against MPTP-induced degeneration
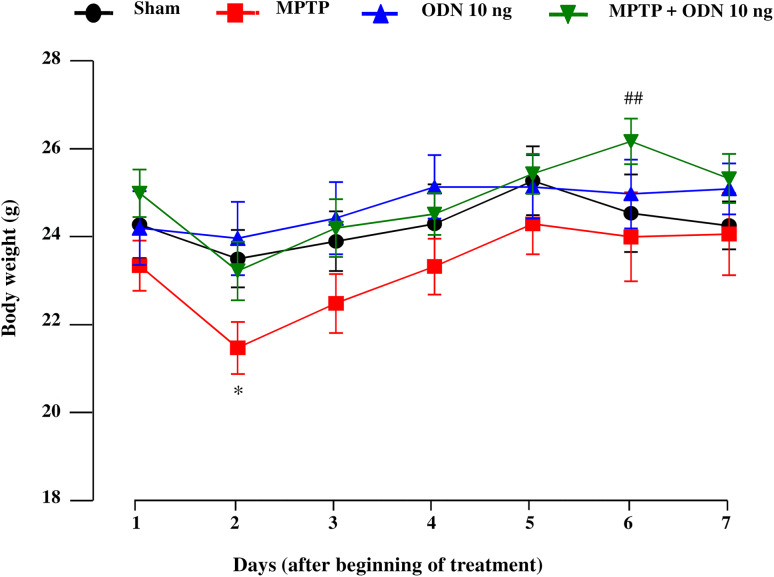
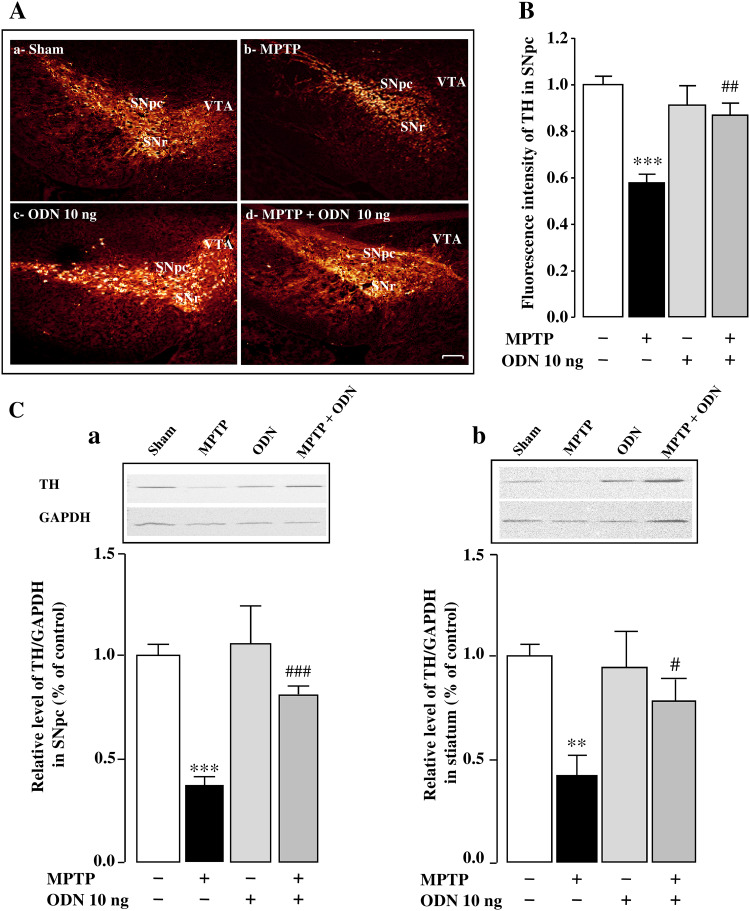
MPTP administration induced a significant decrease in body weight within 2 days after the beginning of the treatment, while ODN administration, which had no effect compared to control, counteracted body weight loss during all the period of treatment in MPTP-treated mice (Fig. 1). Although the body weight of MPTP-treated animals remained slightly lower than in the other groups over the following 6 days, the differences were no longer significant (Fig. 1). To evaluate the efficiency of MPTP administration, immunohistochemical and Western blot experiments were performed (Fig. 2). Consistent with previous reports [56, 57], acute administration of MPTP (20 mg/kg, 3 times at 2 h interval) induced a massive loss of DA neurons in the SNpc within 7 days after intoxication when compared to saline-treated animals (Fig. 2A-a,b). Quantification of the fluorescence mean intensity of TH expression in the SNpc revealed a 43% decrease in MPTP-treated animals compared to sham animals (Fig. 2B). Western blot analysis also confirmed that the TH protein level was decreased in the SNpc (− 62%; Fig. 2C-a) and the striatum (− 58%; Fig. 2C-b) of MPTP-treated mice. We next examined whether ODN could prevent MPTP-induced cell death of DA neurons. While i.c.v. injection of ODN (10 ng/10 µl) alone had no effect on TH expression (Fig. 2A-c, B), administration of ODN to MPTP-treated mice suppressed the loss of DA neurons induced by the toxin in the SNpc and prevented the degeneration of nerve fibers in the striatum (Fig. 2A-d, C). Quantification of the fluorescence mean intensity of TH expression in the SNpc revealed that ODN counteracted by 86% (P < 0.01) the decrease of TH-immunoreactive neurons induced by MPTP (Fig. 2B). Western blot experiments confirmed that ODN administration to MPTP-treated mice significantly prevented the reduction of TH protein expression in the SNpc (116% vs MPTP group) and in the striatum (86% vs MPTP group) within 7 days after treatment (Fig. 2C). ODN alone had no effect on TH expression in the SNpc (Fig. 2C-a) and the striatum (Fig. 2C-b) when compared to sham-treated animals.
Fig. 1.
Time-course of the effect of ODN on body weight of MPTP-treated mice. On day 1, mice received 3 intraperitoneal (i.p.) injections of saline solution (0.9% NaCl; Sham filled circle) or MPTP (20 mg/kg; red square) at 2 h of interval, followed by intracerebroventricular (i.c.v.) injection of saline or ODN (10 ng/10 µl) 1 h after the last i.p. injection of saline (blue triangle) or MPTP (green inverted triangle) solution. Body weight of each mouse was measured daily from day 1 to day 7 after MPTP treatment. Each value represents the mean (±SEM) from 15 animals and statistical analysis was conducted by ANOVA followed by Bonferroni’s test. *P < 0.05 vs saline-treated mice; ## P < 0.01 vs MPTP-treated mice
Fig. 2.
ODN reverses the reduction of tyrosine hydroxylase expression in MPTP-treated mice. A Representative images of tyrosine hydroxylase (TH) immunostaining in the substantia nigra pars compacta (SNpc) 7 days after treatment with NaCl (Sham; A-a), MPTP (3 × 20 mg/kg; A-b), ODN (10 ng/10 µl; A-c) and MPTP + ODN (A-d). Scale bar = 100 µm SNpc (substantia nigra pars compacta), SNr (substantia nigra pars reticulate), VTA (ventral tegmental area). B Relative TH immunofluorescence intensity of surviving dopaminergic neurons in the SNpc 7 days after MPTP treatment alone or with ODN. C Densitometric analysis of TH protein levels in the SNpc (C-a) and striatum (C-b) of sham-, MPTP-, ODN-, and MPTP + ODN-treated mice. Digital photographs illustrate the expression of TH after immunoblotting, and graphs display the relative abundance of TH measured by densitometry of the bands obtained in immunoblots and standardized with GAPDH. All values are expressed as mean ± SEM from 5 animals and statistical analysis was conducted by ANOVA followed by Bonferroni’s test. **P < 0.01, ***P < 0.001 vs saline-treated mice; # P < 0.05, ## P < 0.01, ### P < 0.001 vs MPTP-treated mice
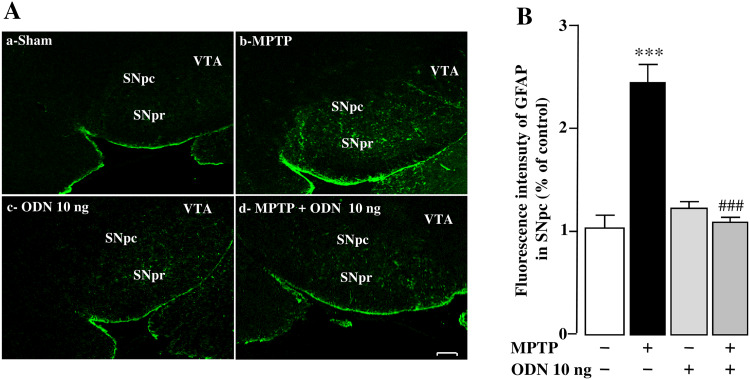
In agreement with previous results [58], a strong increase in GFAP immunostaining, a hallmark of astrogliosis, was detected throughout the SNpc 7 days after MPTP intoxication, paralleling the loss of DA neurons (Fig. 3A-a, b). Administration of ODN alone did not modify the expression of GFAP compared to sham animals (Fig. 3A-a, c), but administration of ODN to MPTP-treated mice completely blocked the astrogliosis induced by the toxin (Fig. 3A-d). Observation of GFAP-positive cell morphology at high magnification in the SNpc revealed that, in MPTP-treated mice, astrocytes display a hypertrophic and activated phenotype (data not shown). Quantification of the fluorescence mean intensity of GFAP expression in the SNpc showed a significant increase (+ 133%) of activated glial cells in MPTP-treated mice compared with saline-treated animals (Fig. 3B). This increase in GFAP expression induced by MPTP was totally blocked by ODN treatment.
Fig. 3.
ODN suppresses astrogliosis in MPTP-treated mice. A Representative images of GFAP immunostaining in the substantia nigra pars compacta (SNpc) 7 days after treatment with NaCl (Sham; A-a), MPTP (3 × 20 mg/kg; A-b), ODN (10 ng/10 µl; A-c) and MPTP + ODN (A-d). Scale bar = 100 µm. SNpc (substantia nigra pars compacta), SNr (substantia nigra pars reticulate), VTA (ventral tegmental area) B Relative GFAP immunofluorescence intensity in the SNpc 7 days after sham, MPTP, ODN, and MPTP + ODN treatment. All values are expressed as mean ± SEM from five animals and statistical analysis was conducted by ANOVA followed by Bonferroni’s test. ***P < 0.001 vs saline-treated mice; ### P < 0.001 vs MPTP-treated mice
ODN inhibits MPTP-induced pro-inflammatory and pro-apoptotic gene expression
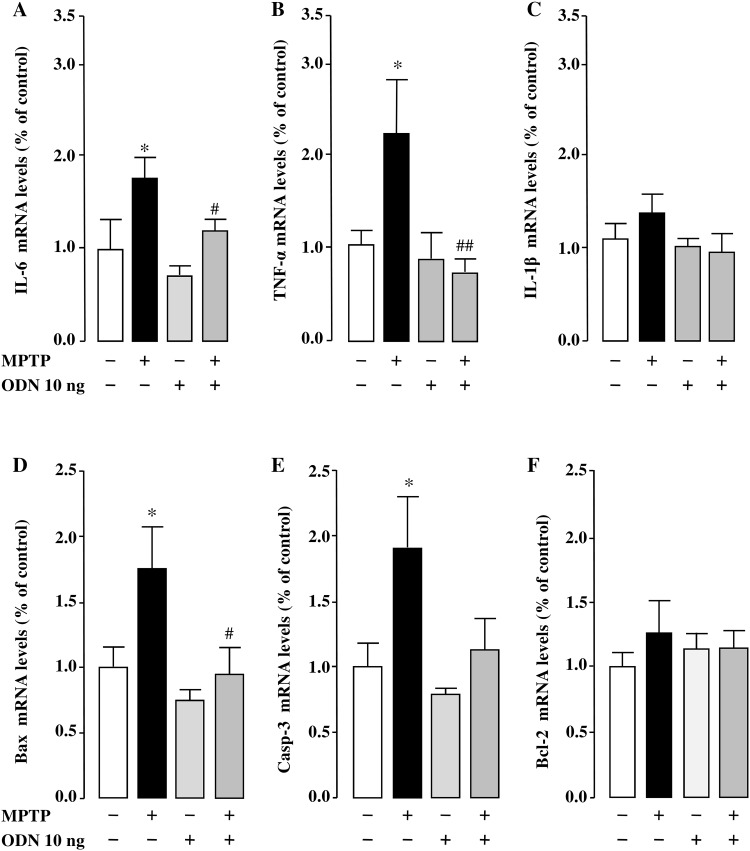
Since MPTP induces an inflammatory response that facilitates neurodegeneration, we examined the effect of ODN on the expression of pro-inflammatory genes in MPTP-treated mice by means of real-time PCR. Consistent with the literature [10, 59], the mRNA levels of interleukin 6 (Il-6; + 76.9% vs sham group) and tumor necrosis factor-α (Tnf-α; +115% vs sham group) were markedly increased in the SNpc of MPTP-treated mice 7 days after the beginning of the treatment (Fig. 4a, b). An i.c.v. injection of ODN to MPTP-treated animals abolished the stimulatory effect of MPTP on Il-6 (-32.8% vs MPTP group) and Tnf-α (-67.4% vs MPTP group) mRNA levels (Fig. 4a, b). While Il-1β gene expression was not increased on day 7 in MPTP-treated animals (Fig. 4c), it was significantly enhanced 1 day after administration of the toxin (supplementary data, Fig. 1A), showing a time-dependent evolution of the pro-inflammatory response. This early increase of Il-1β expression was also blocked by ODN injection to MPTP-treated animals (supplementary data, Fig. 1A).
Fig. 4.
ODN prevents pro-inflammatory and pro-apoptotic gene expression in MPTP-treated mice. Quantification of Il-6 (a), Tnf-α (b), Il-1β (c), Bax (d), Caspase-3 (e) and Bcl-2 (f) mRNA levels in the substantia nigra pars compacta (SNpc) of the mouse brain 7 days after treatment with NaCl (Sham), MPTP (3 × 20 mg/kg), ODN (10 ng/10 µl) and MPTP + ODN. Data were normalized using the GAPDH housekeeping gene as an internal control and the results are expressed as a percentage of control. Data are mean ± SEM obtained from 10 animals and statistical analysis was conducted by ANOVA followed by Bonferroni’s test. *P < 0.05 vs saline-treated mice; # P < 0.05, ## P < 0.01 vs MPTP-treated mice
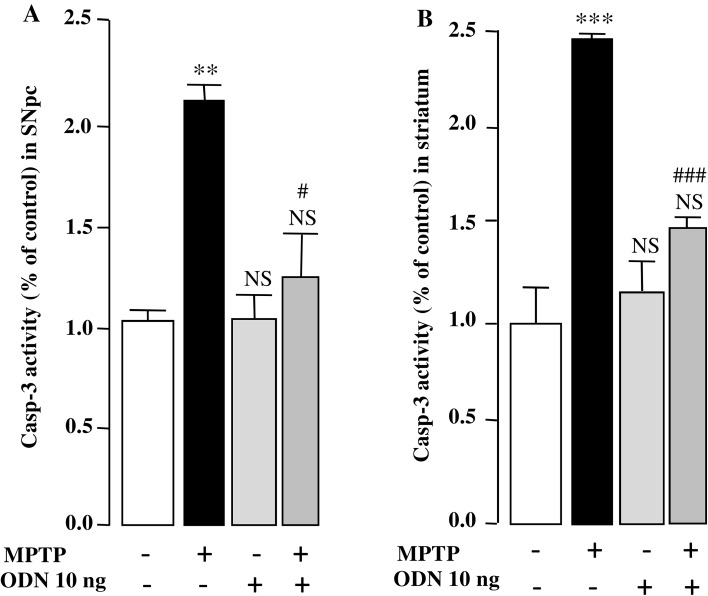
To explore the effect of ODN on MPTP-induced apoptosis, the expression of several anti- and pro-apoptotic genes was investigated. The results showed that Bax and caspase-3 mRNA expression levels were increased by 76 and 91%, respectively, in the SNpc (Fig. 4d, e), and that ODN totally blocked the MPTP-induced increase of Bax and caspase-3 expression (− 81 and − 77%, respectively, vs MPTP group; Fig. 4d, e). No change in Bcl-2 mRNA expression level was detected 7 days after MPTP treatment (Fig. 4f) but a significant decrease of the gene expression (− 48% vs sham group) was observed 1 day after administration of the toxin, and this effect was blocked in animals that received ODN (supplementary data Fig. 1B). Besides its effect on gene expression, ODN also prevented MPTP-induced stimulation of caspase-3 activity within 7 days of treatment, in both the SNpc and striatum (− 89 and − 96% vs MPTP group, respectively; Fig. 5a, b).
Fig. 5.
ODN suppresses stimulation of caspase-3 activity in MPTP-treated mice. Caspase-3 (Casp-3) activity was determined in the substantia nigra pars compacta (SNpc) (a) and striatum (b) 7 days after treatment with NaCl (Sham), MPTP (3 × 20 mg/kg), ODN (10 ng/10 µl) and MPTP + ODN. Each value, expressed as a percentage of control, represents the mean (±SEM) obtained from 10 animals and statistical analysis was conducted by ANOVA followed by Bonferroni’s test. *P < 0.05, ***P < 0.001, NS, Ns, not statistically different vs saline-treated mice; # P < 0.05; ### P < 0.01 vs MPTP-treated mice
ODN prevents the effect of MPTP on ROS accumulation and on oxidative damages
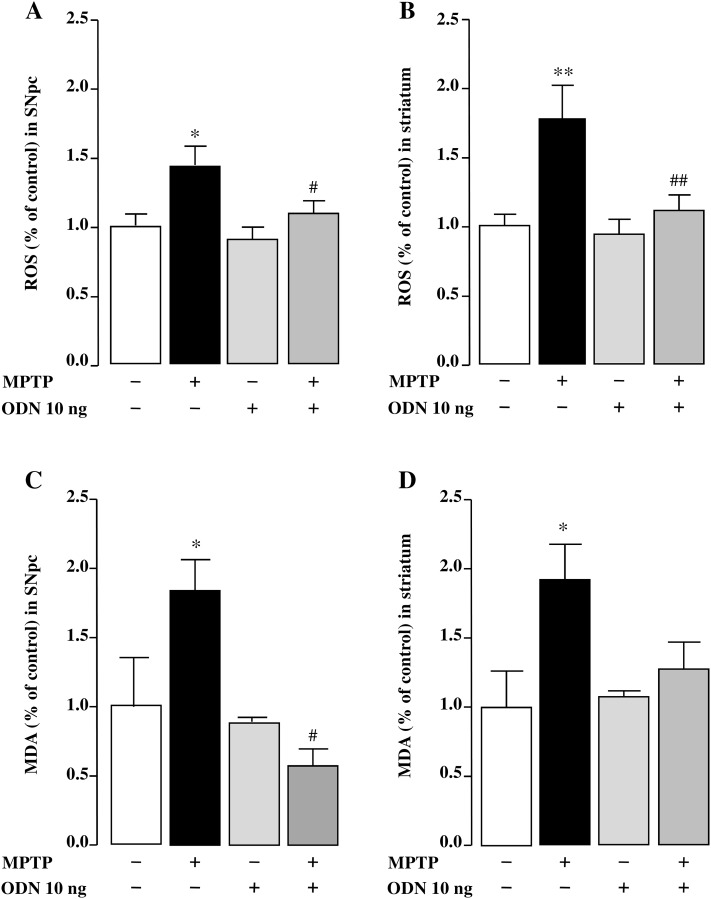
To investigate the ability of ODN to block MPTP-induced oxidative stress, ROS content in SNpc and striatum homogenates was measured with the CM-H2DCFDA probe, which becomes fluorescent upon oxidation by ROS. Seven days after MPTP treatment, the level of ROS was significantly increased in comparison to saline-treated animals both in the SNpc (+ 44%; Fig. 6a) and in the striatum (+ 77%; Fig. 6b). The action of MPTP on ROS accumulation was associated with a significant increase of MDA, a product of the oxidative breakdown of highly unsaturated fatty acids, both in the SNpc (+ 43%; Fig. 6c) and the striatum (+ 66%; Fig. 6d). ODN administration totally prevented the increase of ROS and MDA levels induced by MPTP intoxication in both SNpc and striatum (Fig. 6).
Fig. 6.
ODN inhibits reactive oxygen species and lipid oxidation product malondialdehyde accumulation in MPTP-treated mice. Quantification of the levels of reactive oxygen species (ROS) and malondialdehyde (MDA) in the substantia nigra pars compacta (SNpc) (a, c) and striatum (b, d) 7 days after treatment with NaCl (Sham), MPTP (3 × 20 mg/kg), ODN (10 ng/10 µl) and MPTP + ODN. Each value, expressed as a percentage of control, represents the mean (± SEM) obtained from 10 animals and statistical analysis was conducted by ANOVA followed by Bonferroni’s test. *P < 0.05, **P < 0.01 vs saline-treated mice; # P < 0.05; ## P < 0.01 vs MPTP-treated mice
MPTP induced toxicity is enhanced in DBI-KO mice
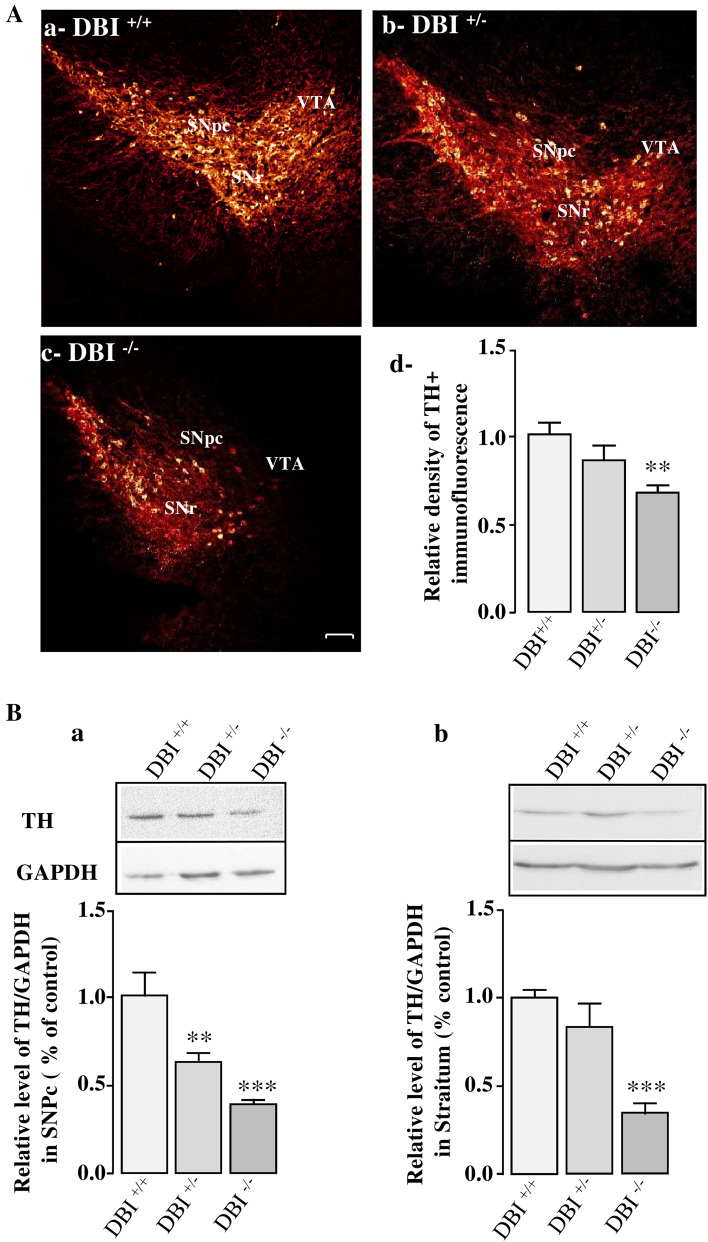
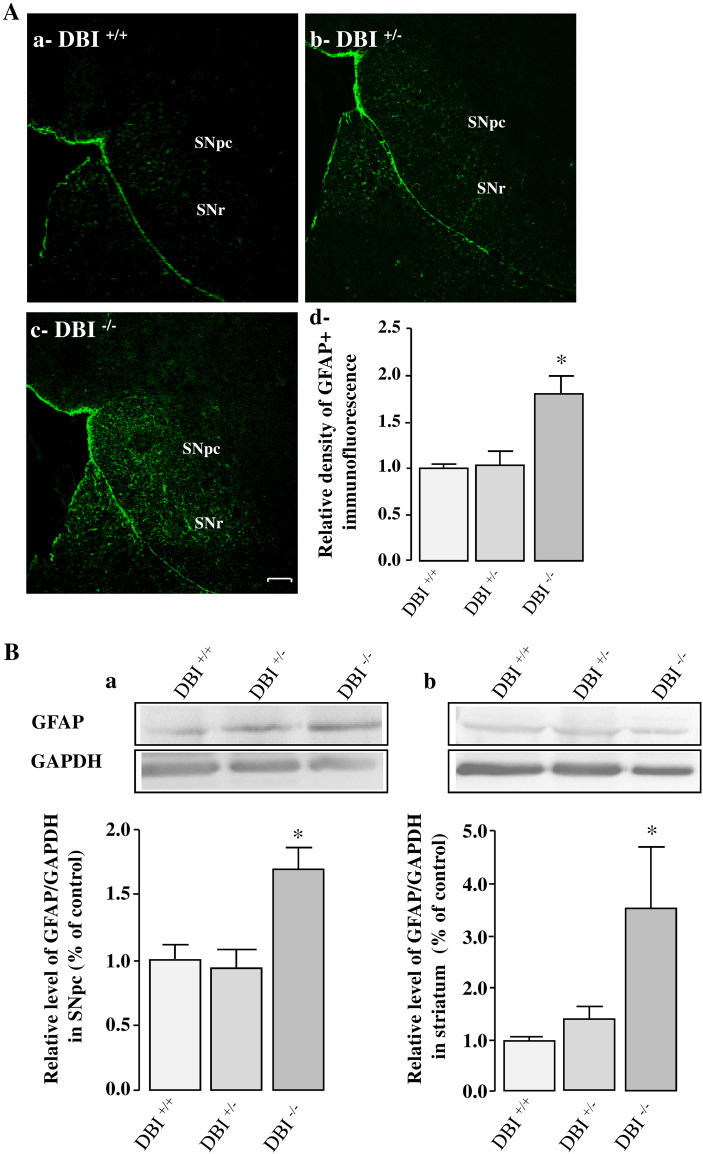
We next investigated the impact of the deficiency of the ODN precursor, i.e, DBI, on DA neuron loss, glial activation, and expression of pro-inflammatory and apoptotic mediators in the SNpc and striatum of MPTP-treated mice. For these experiments, DBI+/+, DBI+/− and DBI−/− mice received 3 injections of MPTP (10 mg/kg) at 2 h interval on day 0, and were killed on day 7. As illustrated on Figs. 7A, DA neurons of DBI−/− mice were more sensitive to MPTP than DBI+/− and DBI+/+ animals. In particular, a deficiency in DBI strongly potentiated MPTP-induced DA neuron apoptosis in the ventral tegmental area (VTA), indicating that the endogenous DBI-derived peptide ODN may be involved in the wounding and protection of DA VTA neurons. Western blot quantification of TH expression levels in the SNpc (Fig. 7B-a) and striatum (Fig. 7B-b) confirmed that MPTP induced a greater loss of DA neurons in DBI−/− mice than in wild-type animals. Furthermore, Q-PCR analysis revealed that i.c.v. injection of ODN (10 ng) prevented MPTP-evoked inhibition of TH gene expression in wild-type mice (supplementary data, Fig. 2A) and that ODN deficiency in DBI−/− mice leads to a decrease of TH mRNA level in DBI−/− mice (supplementary data, Fig. 2B). GFAP immunostaining and Western blot analysis showed that MPTP treatment significantly increased the number of activated GFAP-positive cells (Fig. 8A) and the GFAP expression level (Fig. 8B) in the SNpc and striatum of DBI−/− mice when compared to wild-type animals.
Fig. 7.
Effect of DBI gene knockout on MPTP toxicity in mice. A Illustration of TH immunoreactivity in the substantia nigra pars compacta (SNpc) of wild-type (DBI+/+; A-a), DBI heterozygote (DBI−/+, A-b) and DBI KO homozygote (DBI−/−, A-c) mice 7 days after MPTP injection (3 × 10 mg/kg). Scale bar = 100 µm. SNpc (substantia nigra pars compacta), SNr (substantia nigra pars reticulate), VTA (ventral tegmental area). A-d. Relative TH immunofluorescence intensity of surviving DA neurons in the SNpc of DBI+/+, DBI−/+ and DBI−/− MPTP-treated mice. B Densitometric analysis of TH protein levels in the SNpc (a) and striatum (b) of DBI+/+, DBI−/+ and DBI−/− MPTP-treated mice. Digital photographs illustrate the expression of TH after immunoblotting, and graphs display the relative expression of TH protein measured by densitometry and standardized to GAPDH levels. Each value, expressed as percentage of controls, represents the mean ± SEM obtained from nine animals and statistical analysis was conducted by ANOVA followed by Bonferroni’s test. **P < 0.01, ***P < 0.001 vs DBI+/+ mice
Fig. 8.
Effect of DBI gene knockout on astrocytes reactivity evoked by MPTP intoxication. A Representative photomicrographs illustrating GFAP immunoreactivity in the substantia nigra pars compacta (SNpc) of DBI wild-type (DBI+/+; A-a), DBI heterozygote (DBI−/+, A-b) and DBI KO homozygote (DBI−/−, A-c) mice 7 days after MPTP injection (3 x 10 mg/kg). Scale bar = 100 µm. SNpc (substantia nigra pars compacta), SNr (substantia nigra pars reticulate). A-d Relative GFAP immunofluorescence quantification in SNpc of DBI+/+, DBI−/+ and DBI−/− MPTP-treated mice. B Densitometric analysis of the expression of GFAP in SNpc (a) and striatum (b) of DBI+/+, DBI−/+ and DBI−/− MPTP-treated mice. Digital photographs illustrate the expression of GFAP protein after immunoblotting and graphs display the relative expression of GFAP protein measured by densitometry and standardized to GAPDH levels. Each value, expressed as percentage of controls, represents the mean ± SEM obtained from nine animals and statistical analysis was conducted by ANOVA followed by Bonferroni’s test. *P < 0.05 vs DBI+/+ mice
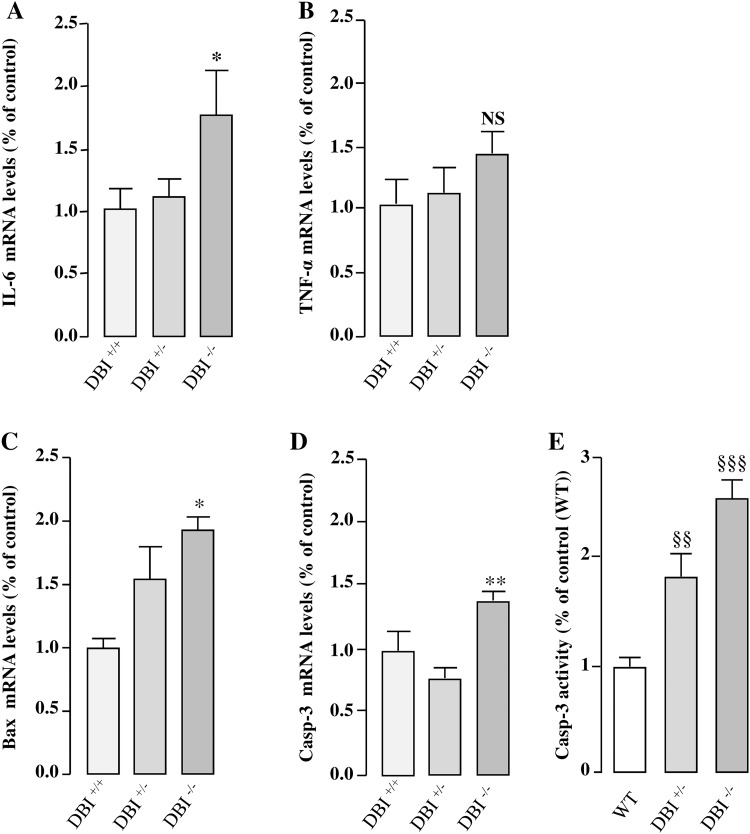
In addition to astroglial activation, 7 days after injection, MPTP treatment induced a significant increase of Il-6 mRNA level in DBI−/− animals (Fig. 9a) while Tnf-α mRNA level was not significantly modified. Concurrently, a significant increase of Bax (+ 92% vs DBI+/+ group) and caspase-3 (+ 40% vs DBI+/+ group) gene expression occurred (Fig. 9c, d). Finally, the level of activated caspase-3 is higher in DBI−/− mice than in wild-type mice (Fig. 9e).
Fig. 9.
DBI gene knockout promotes the expression of pro-inflammatory and pro-apoptotic genes in mice. Brain tissues were collected from wild-type (DBI+/+), DBI heterozygote (DBI−/+) and DBI KO homozygote. (DBI−/−) mice 7 days after MPTP treatment, and Il-6 (a), Tnf-α (b), Bax (c) and caspase-3 (d) mRNA levels were measured by quantitative RT-PCR. Data were normalized using the GAPDH housekeeping gene as an internal control and the results are expressed as a percentage of DBI +/+ mice. Caspase-3 activity (e) was assessed by measuring clivage of the profluorescent caspase-3/7 substrate, Z-DEVD-R110. Data are mean ± SEM obtained from nine animals and statistical analysis was conducted by ANOVA followed by the Bonferroni’s test. *P < 0.05, ***P < 0.001 vs DBI+/+ mice. §§ P < 0.01, §§§ P < 0.001 vs wild-type mice
Discussion
Previous studies performed on cultured neurons and astrocytes have established the antiapoptotic activity of ODN against oxidative insults and cell death [28, 40, 42], but the neuroprotective effect of ODN had not been investigated in vivo yet. The present report provides the first evidence that i.c.v. administration of ODN is able to counteract MPTP-induced degeneration of nigrostriatal DA neurons, oxidative damages and neuroinflammation in an in vivo model of PD. It also appears, using ACBP/DBI KO animals, that ODN precursor deficiency increases brain sensitivity to MPTP toxicity, highlighting the neuroprotective role of endogenous ODN against neuronal degeneration.
ODN exerts a neuroprotective effect in the MPTP-treated mice model of PD
A single i.c.v. injection of ODN, 1 h after the last administration of MPTP, protected TH-positive cells from death in the SNpc and prevented the degeneration of dopaminergic nerve fibers in the striatum. It is noteworthy that ODN was effective to protect DA neurons from the cytotoxic action of MPTP at low doses. Consistent with this observation, in vitro studies have shown the capacity of ODN to prevent 6-OHDA-induced cerebellar granule cell death at very low concentrations, i.e., within the picomolar range, with no remaining protective effect at higher concentrations. As for ODN, other astroglial-derived neuroprotective compounds such as activity-dependent neurotrophic factor (ADNF), activity-dependent neuroprotective protein (ADNP) and ADNP-derived peptide (NAP) have been reported to prevent, at femtomolar concentrations, neuronal cell death induced by various neurotoxins or brain injuries, including ischemia and Alzheimer’s diseases [60–63]. Protection of DA neurons from apoptosis has also been observed with other femtomolar-acting neuroprotective compounds including dynorphin and its glycine–glycine–phenylalanine (GGF) peptide fragment [64]. Thus, the present data identify ODN as a new player of this growing family of femtomolar-acting gliopeptides that exhibit potent neuroprotective activity. Indeed, considering the antiapoptotic effect of ODN on granule neurons and on glial cells in vitro [28, 40], its protective effect on nigrostriatal neurons in vivo may result from both a direct effect on DA neurons and/or an indirect effect, through the release of neuroprotective factors from ODN-activated astrocytes.
ODN acts by preventing astrogliosis, inflammation, oxidative stress and apoptosis induced by MPTP
Astroglial cells exhibit a reactive phenotype in response to neurodegenerative diseases, ischemia and various neurotoxic insults [65, 66]. Furthermore, reactive astrogliosis is involved in the initial and acute stages of the development of PD [67–69]. In particular, astrocytes become persistently activated in the substantia nigra from 5 h up to 21 days after MPTP treatment in a mouse model of PD [67, 69], as observed in the present study showing a significant increase of GFAP-immunopositive cells in the SNpc and striatum after MPTP exposure. In parallel to its antiapoptotic activity, ODN suppressed the effects of MPTP on GFAP, suggesting that the gliopeptide exerts its neuroprotective activity through a modulation of astrocytic activation. This hypothesis is supported by growing evidence showing the pivotal role of reactive astrocytes in the progression of PD, by either protecting or exacerbating DA neuron loss [65, 66, 70]. Although reactive astrocytes in the striatum of PD animal models express a panel of protective antioxidant and neurotrophic factors, they also produce proinflammatory cytokines which can impede neuronal survival [12, 67]. For instance, reactive astrocytes in the SNpc have been reported to overexpress several factors known to promote neuronal damage such as S-100β, iNOS and NF-κB within the first 3 days after MPTP treatment when degenerescence of DA neurons is maximum [67, 71, 72]. Consistent with the hypothesis of a harmful-astrocytic status, the present study shows that the genic expression of the neuroinflammatory markers Il-1β, Il-6 and Tnf-α is enhanced in the SNpc of MPTP-treated animals. One difficulty to get a complete view of the mechanisms involved in DA neuron death comes from the fact that some genes such as IL-1β are induced within 1 day after MPTP injection while others such as IL-6 and TNF-α are activated a week after administration of the toxin. Interestingly, ODN prevented MPTP-induced increase in expression of these pro-inflammatory mediators, 1 day but also a week after treatment, indicating that the peptide exerts both rapid and sustained effects.
It is well established that increased levels of pro-inflammatory cytokines in PD cause neuronal degeneration by apoptosis through the induction of apoptosis-related factor [67, 73]. It has been previously demonstrated that ODN can regulate the expression of Bcl-2 family members to prevent apoptotic cell death provoked by oxidative insult in cultured neurons and astrocytes [28, 40, 74]. In agreement with these data, the present study reveals that ODN decreased MPTP-induced expression of the pro-apoptotic gene Bax and suppressed the inhibitory effect of MPTP on the anti-apoptotic gene Bcl-2. As a consequence, ODN prevented the stimulatory effect of MPTP on caspase-3 expression and activation. These findings are in accordance with in vitro studies showing that ODN inhibits the mitochondrial intrinsic apoptotic pathway by preventing collapse of the mitochondrial potential and stimulation of caspase-3 activity induced by 6-OHDA in cultured cerebellar granule neurons [28].
ROS are another key player involved in the neurotoxic activity of MPTP [73, 75]. Accordingly, the present study showed that the level of ROS was increased in both the SNpc and striatum of MPTP-treated mice, leading to the formation and accumulation of lipid oxidation products. Interestingly, ODN, which had no significant effect by itself on ROS content and MDA generation, totally suppressed the deleterious effects of MPTP, i.e., excessive production of ROS and lipid oxidative damages. The fact that ODN is able (1) to increase the level of the major free radical scavenger in the brain, i.e. glutathione (GSH), as well as the expression and activity of the antioxidant enzymes superoxide dismutase (SOD) and catalase, and (2) to prevent inhibition of endogenous ROS defense system under oxidative stress in cultured neuronal cells [28, 41, 74], suggests that the peptide could block ROS overproduction and dampen the oxidative processes through an upregulation of ROS-detoxifying enzymes in vivo. Overexpression of the transcription factor NF-E2-related factor (Nrf2), which binds to antioxidant response element [76] to induce antioxidant enzyme expression, has been found to be protective against oxidative damage and to provide neuroprotection in the MPTP mouse model, while reduction of Nrf2/ARE activity is associated with an exacerbation of oxidative assaults and sensitivity to the neurodegenerative effects of MPTP [76]. Furthermore, in vitro studies have shown that addition of GSH to the culture medium prevents degeneration of a human DA cell line induced by MPTP/MPP+ [77]. All these data support the hypothesis that the endogenous antioxidant system is involved in the neuroprotective effect of ODN against MPTP-induced DA cell death. Consistent with this notion, it has been reported that inhibition of the endogenous antioxidant system in astrocytes suppresses the cell survival-promoting effect of ODN under oxidative conditions [74]. A possible effect of ODN on the expression of genes involved in the control of oxidative mechanisms in MPTP-treated mice may, thus, be a key player which deserves further investigations.
The intracellular signaling pathways triggering the neuroprotective effect of ODN on MPTP intoxication in vivo remains to be fully elucidated. Nevertheless, previous in vitro studies indicate that ODN protects both glial cells and cerebellar granule neurons against oxidative stress through activation of the metabotropic receptor of ODN and via a PKC/MAK- or PKA/MAK-dependent mechanism, respectively [28, 40]. In both cell types, ODN increases SOD and catalase expression and activity, and preserves mitochondrial integrity through inhibition of oxidative stress-induced increase of Bax gene expression [22, 41, 42]. It is, thus, conceivable that DA neurons also express the ODN metabotropic receptor and that similar signaling pathways are involved in the protective effect of ODN in vivo.
DBI deficiency increases MPTP sensitivity in mice
Experiments conducted in ACBP/DBI KO mice highlight the ability of DBI and/or its derived peptides to mobilize endogenous defense mechanisms against inflammatory and oxidative insults produced by MPTP, leading to an increase of DA cell death in DBI−/− mice treated with MPTP. Strikingly, the number of nigral TH-positive cells was lower in DBI−/− animals but was not modified in heterozygote mice (DBI+/−). This effect on cell survival correlates well with the observed upregulation of the expression of pro-inflammatory genes, i.e. Il-6 and Tnf-α, and pro-apoptotic genes, i.e. Bax and caspase-3, in DBI−/− mice treated with MPTP. These findings are in agreement with data showing that, while ODN injection stimulates neurogenesis in the adult mouse brain, DBI silencing with siRNA causes growth arrest and cell death of neural progenitors [39]. In fact, transfection with ACBP/DBI siRNA also leads to growth arrest and apoptosis in various mammalian cell lines [78, 79]. Despite ACBP/DBI deficiency is associated with an increased sensitivity of cell lines to oxidative stress [79], a neuroprotective effect of the DBI precursor polypeptide by itself has never been reported to our knowledge. Since the protective action of ODN is clearly established in vitro on both astrocytes and neurons [28, 40, 41] and since the production and secretion of ODN from astroglial cells is directly correlated with the level of expression of its precursor DBI [44], it appears that the greater sensitivity of ACBP/DBI knockout mice can be specifically ascribed to the absence of the ODN fragment. Such observations, together with studies showing the presence of ODN in several brain structures, including the nigrostriatal region [26, 27], strongly suggest that endogenous ODN may act as an autocrine and/or paracrine trophic factor promoting survival of neuronal cells, and could thus contribute to decrease tissue damages in injury conditions.
In conclusion, the results of the present study reveal that i.c.v. injection of low doses of ODN causes a very strong neuroprotective action against degeneration of DA neurons in MPTP-treated mice. Moreover, DBI−/−mice are more sensitive to MPTP-induced inflammatory and oxidative brain damages, suggesting that endogenous DBI and ODN may also be neuroprotective. Further studies are required to characterize the receptor and the signaling pathways implicated in this neuroprotective effect of ODN, as well as to develop new methods of peptide administration. The current findings indicate that, based on its anti-oxidative, anti-inflammatory and anti-apoptotic effects, the gliopeptide ODN could lead to the development of effective therapeutic agents for the treatment of cerebral injuries involving oxidative neurodegeneration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1 Protective effect of intracerebroventricular administration of ODN against MPTP-induced pro-inflammatory and pro-apoptotic genes expression in the substantia nigra pars compacta. Quantification of interleukin-1β (Il-1β;A) and Bcl-2 (B) mRNA levels in the substantia nigra pars compacta (SNpc) 1 day after sham-, MPTP-, ODN-, and MPTP + ODN-treatment. Data were normalized using the GAPDH housekeeping gene as an internal control and the results are expressed as a percentage of control. Data are mean ± SEM obtained from at least 9 animals and statistical analysis was conducted by ANOVA followed by Bonferroni’s test. * P < 0.05, ** P < 0.01 vs. saline-treated mice; # P < 0.05 vs MPTP-treated mice. (TIFF 770 kb)
Supplementary Fig. 2 Protective effect of intracerebroventricular administration of ODN against MPTP-induced reduction of tyrosine hydroxylase gene expression in the substantia nigra pars compacta in wild-type and DBI knockout mice. A. Quantification of tyrosine hydroxylase (TH) mRNA levels in the substantia nigra pars compacta (SNpc) of wild-type DBI mice 8 days after sham (0.9% Nacl)-, MPTP (20 mg/kg)-, ODN (10 ng)-, and MPTP + ODN-treatment. B. Brain tissues were collected from DBI wild-type (DBI+/+), DBI heterozygote (DBI−/+) and DBI knockout (DBI−/−) mice 8 days after MPTP (10 mg/kg) treatment. TH mRNA levels were measured by quantitative RT-PCR and data were normalized using the GAPDH housekeeping gene as an internal control. Data are mean ± SEM of at least 8 animals and statistical analysis was conducted by ANOVA followed by Bonferroni’s test. * P < 0.05vs. saline-treated mice; #P < 0.05 vs. MPTP-treated mice; §§ P < 0.05 vs DBI+/+ mice. (TIFF 791 kb)
Acknowledgements
This work was supported by a France-Tunisia CMCU-Campus France/PHC Utique 16G0820/34940PK exchange program (to Olfa Masmoudi-Kouki and David Vaudry), an Erasmus Mundus Battuta grant from Rouen Normandy University (Erasmus), INSERM (U1239), Normandy Region, an alternance scholarship of Tunisian Higher Education Ministry, the Laboratory of Functional Neurophysiology and Pathology UR/11ES09 and European Union. Europe gets involved in Normandy with European Regional Development Fund (ERDF) supporting the PACT-CBS project. Funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Seyma Bahdoudi, Ikram Ghouili and Mansour Hmiden were recipients of fellowships from the University of Tunis El Manar and a France-Tunisia exchange program.
Abbreviations
- ACBP
Acyl-CoA binding protein
- CNS
Central nervous system
- DA
Dopamine
- DBI
Diazepam-binding inhibitor
- GSH
Glutathione
- GFAP
Glial fibrillary acidic protein
- i.c.v.
Intracerebroventricular
- IL
Interleukin
- i.p.
Intraperitoneal
- KO
Knockout
- MAO
Monoamine oxidase
- MDA
Malondialdehyde
- MPP+
1-Methyl-4-phenylpyridinium
- MPTP
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- ODN
Octadecaneuropeptide
- PD
Parkinson’s disease
- SNpc
Substantia nigra pars compacta
- TH
Tyrosine hydroxylase
- TNF-α
Tumor necrosis factor-α
Contributor Information
Olfa Masmoudi-Kouki, Email: olfa.masmoudi@fst.utm.tn.
David Vaudry, Email: david.vaudry@univ-rouen.fr.
References
- 1.Moore O, Kreitler S, Ehrenfeld M, Giladi N. Quality of life and gender identity in Parkinson’s disease. J Neural Transm. 2005;112:1511–1522. doi: 10.1007/s00702-005-0285-5. [DOI] [PubMed] [Google Scholar]
- 2.Schapira AH. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol Sci. 2009;30:41–47. doi: 10.1016/j.tips.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Chan-Palay V. Alterations in the locus coeruleus in dementias of Alzheimer’s and Parkinson’s disease. Prog Brain Res. 1991;88:625–630. doi: 10.1016/S0079-6123(08)63839-X. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher DA, Schapira AH. Etiopathogenesis and treatment of Parkinson’s disease. Curr Top Med Chem. 2009;9:860–868. [PubMed] [Google Scholar]
- 5.Lindholm D, Mäkelä J, Di Liberto V, Mudò G, Belluardo N, Eriksson O, Saarma M. Current disease modifying approaches to treat Parkinson’s disease. Cell Mol Life Sci. 2016;73:1365–1379. doi: 10.1007/s00018-015-2101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toulorge D, Schapira AH, Hajj R. Molecular changes in the postmortem parkinsonian brain. J Neurochem. 2016;1:27–58. doi: 10.1111/jnc.13696. [DOI] [PubMed] [Google Scholar]
- 7.Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target alpha-synuclein pathology. Exp Neurol. 2017;S0014–4886(17):30245. doi: 10.1016/j.expneurol.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 9.Elbaz A, Moisan F. Update in the epidemiology of Parkinson’s disease. Curr Opin Neurol. 2008;21:454–460. doi: 10.1097/WCO.0b013e3283050461. [DOI] [PubMed] [Google Scholar]
- 10.Gordon R, Singh N, Lawana V, et al. Protein kinase Cdelta upregulation in microglia drives neuroinflammatory responses and dopaminergic neurodegeneration in experimental models of Parkinson’s disease. Neurobiol Dis. 2016;93:96–114. doi: 10.1016/j.nbd.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deleidi M, Gasser T. The role of inflammation in sporadic and familial Parkinson’s disease. Cell Mol Life Sci. 2013;70:4259–4273. doi: 10.1007/s00018-013-1352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov Disord. 2011;26:1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- 13.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 14.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 15.Oueslati A, Sgambato-Faure V, Melon C, Kachidian P, Gubellini P, Amri M, Kerkerian-Le Goff L, Salin P. High-frequency stimulation of the subthalamic nucleus potentiates l-DOPA-induced neurochemical changes in the striatum in a rat model of Parkinson’s disease. J Neurosci. 2007;27:2377–2386. doi: 10.1523/JNEUROSCI.2949-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Athauda D, Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol. 2015;11:25–40. doi: 10.1038/nrneurol.2014.226. [DOI] [PubMed] [Google Scholar]
- 17.Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM. The pharmacology of l-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev. 2013;65:171–222. doi: 10.1124/pr.111.005678. [DOI] [PubMed] [Google Scholar]
- 18.Carta AR, Mulas G, Bortolanza M, Duarte T, Pillai E, Fisone G, Vozari RR, Del-Bel E. l-Dopa-induced dyskinesia and neuroinflammation: do microglia and astrocytes play a role? Eur J Neurosci. 2017;45:73–91. doi: 10.1111/ejn.13482. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki I, Murakami S, Torigoe N, Kitamura Y, Asanuma M. Neuroprotective effects of levetiracetam target xCT in astrocytes in parkinsonian mice. J Neurochem. 2016;136:194–204. doi: 10.1111/jnc.13405. [DOI] [PubMed] [Google Scholar]
- 20.Sidorova YA, Saarma M. Glial cell line-derived neurotrophic factor family ligands and their therapeutic potential. Mol Biol. 2016;50:589–598. doi: 10.1134/S0026893316040105. [DOI] [PubMed] [Google Scholar]
- 21.Nam JH, Park ES, Won SY, Lee YA, Kim KI, Jeong JY, Baek JY, Cho EJ, Jin M, Chung YC, Lee BD, Kim SH, Kim EG, Byun K, Lee B, Woo DH, Lee CJ, Kim SR, Bok E, Kim YS, Ahn TB, Ko HW, Brahmachari S, Pletinkova O, Troconso JC, Dawson VL, Dawson TM, Jin BK. TRPV1 on astrocytes rescues nigral dopamine neurons in Parkinson’s disease via CNTF. Brain. 2015;138:3610–3622. doi: 10.1093/brain/awv297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Chen C, Gu H, Li C, Fu X, Jiang M, Sun H, Xu J, Fang J, Jin L. Mesencephalic astrocyte-derived neurotrophic factor reduces cell apoptosis via upregulating GRP78 in SH-SY5Y cells. Cell Biol Int. 2016;40:803–811. doi: 10.1002/cbin.10621. [DOI] [PubMed] [Google Scholar]
- 23.Butterworth RF, Tonon MC, Desy L, Giguere JF, Vaudry H, Pelletier G. Increased brain content of the endogenous benzodiazepine receptor ligand, octadecaneuropeptide (ODN), following portacaval anastomosis in the rat. Peptides. 1991;12:119–125. doi: 10.1016/0196-9781(91)90177-Q. [DOI] [PubMed] [Google Scholar]
- 24.Malagon M, Vaudry H, Van Strien F, Pelletier G, Gracia-Navarro F, Tonon MC. Ontogeny of diazepam-binding inhibitor-related peptides (endozepines) in the rat brain. Neuroscience. 1993;57:777–786. doi: 10.1016/0306-4522(93)90023-9. [DOI] [PubMed] [Google Scholar]
- 25.Rouet-Smih F, Tonon MC, Pelletier G, Vaudry H. Characterization of endozepine-related peptides in the central nervous system and in peripheral tissues of the rat. Peptides. 1992;13:1219–1225. doi: 10.1016/0196-9781(92)90032-X. [DOI] [PubMed] [Google Scholar]
- 26.Tonon MC, Desy L, Nicolas P, Vaudry H, Pelletier G. Immunocytochemical localization of the endogenous benzodiazepine ligand octadecaneuropeptide (ODN) in the rat brain. Neuropeptides. 1990;15:17–24. doi: 10.1016/0143-4179(90)90155-R. [DOI] [PubMed] [Google Scholar]
- 27.Yanase H, Shimizu H, Yamada K, Iwanaga T. Cellular localization of the diazepam binding inhibitor in glial cells with special reference to its coexistence with brain-type fatty acid binding protein. Arch Histol Cytol. 2002;65:27–36. doi: 10.1679/aohc.65.27. [DOI] [PubMed] [Google Scholar]
- 28.Kaddour H, Hamdi Y, Vaudry D, Basille M, Desrues L, Leprince J, Castel H, Vaudry H, Tonon MC, Amri M, Masmoudi-Kouki O. The octadecaneuropeptide ODN prevents 6-hydroxydopamine-induced apoptosis of cerebellar granule neurons through a PKC-MAPK-dependent pathway. J Neurochem. 2013;125:620–633. doi: 10.1111/jnc.12140. [DOI] [PubMed] [Google Scholar]
- 29.Alho H, Bovolin P, Jenkins D, Guidotti A, Costa E. Cellular and subcellular localization of an octadecaneuropeptide derived from diazepam binding inhibitor: immunohistochemical studies in the rat brain. J Chem Neuroanat. 1989;2:301–318. [PubMed] [Google Scholar]
- 30.Ferrero P, Santi MR, Conti-Tronconi B, Costa E, Guidotti A. Study of an octadecaneuropeptide derived from diazepam binding inhibitor (DBI): biological activity and presence in rat brain. Proc Natl Acad Sci USA. 1986;83:827–831. doi: 10.1073/pnas.83.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neess D, Bek S, Bloksgaard M, Marcher AB, Faergeman NJ, Mandrup S. Delayed hepatic adaptation to weaning in ACBP−/− mice is caused by disruption of the epidermal barrier. Cell Rep. 2013;5:1403–1412. doi: 10.1016/j.celrep.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Rose TM, Schultz ER, Todaro GJ. Molecular cloning of the gene for the yeast homolog (ACB) of diazepam binding inhibitor/endozepine/acyl-CoA-binding protein. Proc Natl Acad Sci USA. 1992;89:11287–11291. doi: 10.1073/pnas.89.23.11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tonon MC, Leprince J, Morin F, Gandolfo P, Compère V, Pelletier G, Malagon M, Vaudry H. Endozepines. In: Kastin AJ, editor. Handbook of biological active peptides. 2. Oxford, UK: Elsevier; 2013. pp. 760–765. [Google Scholar]
- 34.de Mateos-Verchere JG, Leprince J, Tonon MC, Vaudry H, Costentin J. The octadecaneuropeptide [diazepam-binding inhibitor (33–50)] exerts potent anorexigenic effects in rodents. Eur J Pharmacol. 2001;414:225–231. doi: 10.1016/S0014-2999(01)00771-3. [DOI] [PubMed] [Google Scholar]
- 35.do Rego JC, Orta MH, Leprince J, Tonon MC, Vaudry H, Costentin J. Pharmacological characterization of the receptor mediating the anorexigenic action of the octadecaneuropeptide: evidence for an endozepinergic tone regulating food intake. Neuropsychopharmacology. 2007;32:1641–1648. doi: 10.1038/sj.npp.1301280. [DOI] [PubMed] [Google Scholar]
- 36.Gach K, Belkacemi O, Lefranc B, Perlikowski P, Masson J, Walet-Balieu ML, do Rego JC, Galas L, Schapman D, Lamtahri R, Tonon MC, Vaudry D, Chuquet J, Leprince J. Detection, characterization and biological activities of [bisphospho-thr3,9]ODN, an endogenous molecular form of ODN released by astrocytes. Neuroscience. 2015;290:472–484. doi: 10.1016/j.neuroscience.2015.01.045. [DOI] [PubMed] [Google Scholar]
- 37.Lanfray D, Arthaud S, Ouellet J, Compere V, do Rego JL, Leprince J, Lefranc B, Castel H, Bouchard C, Monge-Roffarello B, Richard D, Pelletier G, Vaudry H, Tonon MC, Morin F. Gliotransmission and brain glucose sensing: critical role of endozepines. Diabetes. 2013;62:801–810. doi: 10.2337/db11-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouyakdan K, Taib B, Budry L, Zhao S, Rodaros D, Neess D, Mandrup S, Faergeman NJ, Alquier T. A novel role for central ACBP/DBI as a regulator of long-chain fatty acid metabolism in astrocytes. J Neurochem. 2015;133:253–265. doi: 10.1111/jnc.13035. [DOI] [PubMed] [Google Scholar]
- 39.Alfonso J, Le Magueresse C, Zuccotti A, Khodosevich K, Monyer H. Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell. 2012;10:76–87. doi: 10.1016/j.stem.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Hamdi Y, Kaddour H, Vaudry D, Bahdoudi S, Douiri S, Leprince J, Castel H, Vaudry H, Tonon MC, Amri M, Masmoudi-Kouki O. The octadecaneuropeptide ODN protects astrocytes against hydrogen peroxide-induced apoptosis via a PKA/MAPK-dependent mechanism. PLoS One. 2012;7:e42498. doi: 10.1371/journal.pone.0042498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamdi Y, Kaddour H, Vaudry D, Douiri S, Bahdoudi S, Leprince J, Castel H, Vaudry H, Amri M, Tonon MC, Masmoudi-Kouki O. The stimulatory effect of the octadecaneuropeptide ODN on astroglial antioxidant enzyme systems is mediated through a GPCR. Front Endocrinol. 2012;3:138. doi: 10.3389/fendo.2012.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamdi Y, Kaddour H, Vaudry D, Leprince J, Zarrouk A, Hammami M, Vaudry H, Tonon MC, Amri M, Masmoudi-Kouki O. Octadecaneuropeptide ODN prevents hydrogen peroxide-induced oxidative damage of biomolecules in cultured rat astrocytes. Peptides. 2015;71:56–65. doi: 10.1016/j.peptides.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Clavier T, Tonon MC, Foutel A, Besnier E, Lefevre-Scelles A, Morin F, Gandolfo P, Tuech JJ, Quillard M, Veber B, Dureuil B, Castel H, Compere V. Increased plasma levels of endozepines, endogenous ligands of benzodiazepine receptors, during systemic inflammation: a prospective observational study. Crit Care. 2014;18:633. doi: 10.1186/s13054-014-0633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghouili I, Bahdoudi S, Morin F, Amri F, Hamdi Y, Coly PM, Walet-Balieu M-L, Leprince J, Zekri S, Vaudry H, Vaudry D, Castel H, Amri M, Tonon M-C, Masmoudi-Kouki O. Endogenous Expression of ODN-related peptides in astrocytes contributes to cell protection against oxidative stress: astrocyte-neuron crosstalk relevance for neuronal survival. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0630-3. [DOI] [PubMed] [Google Scholar]
- 45.Grondin R, Bedard PJ, Hadj Tahar A, Gregoire L, Mori A, Kase H. Antiparkinsonian effect of a new selective adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology. 1999;52:1673–1677. doi: 10.1212/WNL.52.8.1673. [DOI] [PubMed] [Google Scholar]
- 46.Gubellini P, Kachidian P. Animal models of Parkinson’s disease: an updated overview. Rev Neurol. 2015;171:750–761. doi: 10.1016/j.neurol.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Segura-Aguilar J, Paris I, Muñoz P. The need of a new and more physiological preclinical model for Parkinson’s disease. Cell Mol Life Sci. 2016;73:1381–1382. doi: 10.1007/s00018-016-2140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.German DC, Dubach M, Askari S, Speciale SG, Bowden DM. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonian syndrome in Macaca fascicularis: which midbrain dopaminergic neurons are lost? Neuroscience. 1988;24:161–174. doi: 10.1016/0306-4522(88)90320-X. [DOI] [PubMed] [Google Scholar]
- 49.Cho KL, Searle K, Webb M, Yi H, Ferreira PA. Ranbp2 haploinsufficiency mediates distinct cellular and biochemical phenotypes in brain and retinal dopaminergic and glia cells elicited by the Parkinson neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Cell Mol. 2012;69:3511–3527. doi: 10.1007/s00018-012-1071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bajpai P, Sangar MC, Singh S, Tang W, Bansal S, Chowdhury G, Cheng Q, Fang JK, Martin MV, Guengerich FP, Avadhani NG. Metabolism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by mitochondrion-targeted cytochrome P450 2D6: implications in Parkinson disease. J Biol Chem. 2013;288:4436–4451. doi: 10.1074/jbc.M112.402123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 52.Shang T, Kotamraju S, Kalivendi SV, Hillard CJ, Kalyanaraman B. 1-Methyl-4-phenylpyridinium-induced apoptosis in cerebellar granule neurons is mediated by transferrin receptor iron-dependent depletion of tetrahydrobiopterin and neuronal nitric-oxide synthase-derived superoxide. J Biol Chem. 2004;279:19099–19112. doi: 10.1074/jbc.M400101200. [DOI] [PubMed] [Google Scholar]
- 53.Yamada M, Kida K, Amutuhaire W, Ichinose F, Kaneki M. Gene disruption of caspase-3 prevents MPTP-induced Parkinson’s disease in mice. Biochem Biophys Res Commun. 2010;402:312–318. doi: 10.1016/j.bbrc.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neess D, Bloksgaard M, Bek S, Marcher AB, Elle IC, Helledie T, Due M, Pagmantidis V, Finsen B, Wilbertz J, Kruhoffer M, Faergeman N, Mandrup S. Disruption of the acyl-CoA-binding protein gene delays hepatic adaptation to metabolic changes at weaning. J Biol Chem. 2011;286:3460–3472. doi: 10.1074/jbc.M110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 57.Park J, Lim CS, Seo H, Park CA, Zhuo M, Kaang BK, Lee K. Pain perception in acute model mice of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Mol Pain. 2015;11:28. doi: 10.1186/s12990-015-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El Massri N, Johnstone DM, Peoples CL, Moro C, Reinhart F, Torres N, Stone J, Benabid AL, Mitrofanis J. The effect of different doses of near infrared light on dopaminergic cell survival and gliosis in MPTP-treated mice. Int J Neurosci. 2016;126:76–87. doi: 10.3109/00207454.2014.994063. [DOI] [PubMed] [Google Scholar]
- 59.Episcopo FL, Tirolo C, Testa N, Caniglia S, Morale MC, Marchetti B. Reactive astrocytes are key players in nigrostriatal dopaminergic neurorepair in the MPTP mouse model of Parkinson’s disease: focus on endogenous neurorestoration. Curr Aging Sci. 2013;6:45–55. doi: 10.2174/1874609811306010007. [DOI] [PubMed] [Google Scholar]
- 60.Arya A, Meena R, Sethy NK, Das M, Sharma M, Bhargava K. NAP (davunetide) protects primary hippocampus culture by modulating expression profile of antioxidant genes during limiting oxygen conditions. Free Radic Res. 2015;49:440–452. doi: 10.3109/10715762.2015.1011153. [DOI] [PubMed] [Google Scholar]
- 61.Busciglio J, Pelsman A, Helguera P, Ashur-Fabian O, Pinhasov A, Brenneman DE, Gozes I. NAP and ADNF-9 protect normal and Down’s syndrome cortical neurons from oxidative damage and apoptosis. Curr Pharm Des. 2007;13:1091–1098. doi: 10.2174/138161207780618957. [DOI] [PubMed] [Google Scholar]
- 62.Gozes I, Divinski I. The femtomolar-acting NAP interacts with microtubules: novel aspects of astrocyte protection. J Alzheimers Dis. 2004;6:S37–S41. doi: 10.3233/JAD-2004-6S605. [DOI] [PubMed] [Google Scholar]
- 63.Zamostiano R, Pinhasov A, Bassan M, Perl O, Steingart RA, Atlas R, Brenneman DE, Gozes I. A femtomolar-acting neuroprotective peptide induces increased levels of heat shock protein 60 in rat cortical neurons: a potential neuroprotective mechanism. Neurosci Lett. 1999;264:9–12. doi: 10.1016/S0304-3940(99)00168-8. [DOI] [PubMed] [Google Scholar]
- 64.Qin L, Block ML, Liu Y, Bienstock RJ, Pei Z, Zhang W, Wu X, Wilson B, Burka T, Hong JS. Microglial NADPH oxidase is a novel target for femtomolar neuroprotection against oxidative stress. FASEB J. 2005;19:550–557. doi: 10.1096/fj.04-2857com. [DOI] [PubMed] [Google Scholar]
- 65.Chen LW, Yung KL, Chan YS. Reactive astrocytes as potential manipulation targets in novel cell replacement therapy of Parkinson’s disease. Curr Drug Targets. 2005;6:821–833. doi: 10.2174/138945005774574506. [DOI] [PubMed] [Google Scholar]
- 66.Sun XL, Chen BY, Duan L, Xia Y, Luo ZJ, Wang JJ, Rao ZR, Chen LW. The proform of glia cell line-derived neurotrophic factor: a potentially biologically active protein. Mol Neurobiol. 2014;49:234–250. doi: 10.1007/s12035-013-8515-6. [DOI] [PubMed] [Google Scholar]
- 67.Aoki E, Yano R, Yokoyama H, Kato H, Araki T. Role of nuclear transcription factor kappa B (NF-kappaB) for MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced apoptosis in nigral neurons of mice. Exp Mol Pathol. 2009;86:57–64. doi: 10.1016/j.yexmp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 68.Chen LW, Wei LC, Qiu Y, Liu HL, Rao ZR, Ju G, Chan YS. Significant up-regulation of nestin protein in the neostriatum of MPTP-treated mice. Are the striatal astrocytes regionally activated after systemic MPTP administration? Brain Res. 2002;925:9–17. doi: 10.1016/S0006-8993(01)03253-X. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe Y, Kato H, Araki T. Protective action of neuronal nitric oxide synthase inhibitor in the MPTP mouse model of Parkinson’s disease. Metab Brain Dis. 2008;23:51–69. doi: 10.1007/s11011-007-9080-3. [DOI] [PubMed] [Google Scholar]
- 70.McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 71.Kato H, Kurosaki R, Oki C, Araki T. Arundic acid, an astrocyte-modulating agent, protects dopaminergic neurons against MPTP neurotoxicity in mice. Brain Res. 2004;1030:66–73. doi: 10.1016/j.brainres.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 72.Muramatsu Y, Kurosaki R, Watanabe H, Michimata M, Matsubara M, Imai Y, Araki T. Expression of S-100 protein is related to neuronal damage in MPTP-treated mice. Glia. 2003;42:307–313. doi: 10.1002/glia.10225. [DOI] [PubMed] [Google Scholar]
- 73.Sun XL, Chen BY, Zhao HK, Cheng YY, Zheng MH, Duan L, Jiang W, Chen LW. Gas1 up-regulation is inducible and contributes to cell apoptosis in reactive astrocytes in the substantia nigra of LPS and MPTP models. J Neuroinflamm. 2016;13:180. doi: 10.1186/s12974-016-0643-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamdi Y, Masmoudi-KParkouki O, Kaddour H, Belhadj F, Gandolfo P, Vaudry D, Mokni M, Leprince J, Hachem R, Vaudry H, Tonon MC, Amri M. Protective effect of the octadecaneuropeptide on hydrogen peroxide-induced oxidative stress and cell death in cultured rat astrocytes. J Neurochem. 2011;118:416–428. doi: 10.1111/j.1471-4159.2011.07315.x. [DOI] [PubMed] [Google Scholar]
- 75.Mudò G, Mäkelä J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, Eriksson O, Mälkiä A, Bonomo A, Kairisalo M, Aguirre JA, Korhonen L, Belluardo N, Lindholm D. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci. 2012;69:1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: critical role for the astrocyte. Proc Natl Acad Sci USA. 2009;106(8):2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Efremova L, Schildknecht S, Adam M, Pape R, Gutbier S, Hanf B, Burkle A, Leist M. Prevention of the degeneration of human dopaminergic neurons in an astrocyte co-culture system allowing endogenous drug metabolism. Br J Pharmacol. 2015;172:4119–4132. doi: 10.1111/bph.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faergeman NJ, Knudsen J. Acyl-CoA binding protein is an essential protein in mammalian cell lines. Biochem J. 2002;368:679–682. doi: 10.1042/bj20021413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shin SW, Yun SH, Park ES, Jeong JS, Kwak JY, Park JI. Overexpression of PGC1alpha enhances cell proliferation and tumorigenesis of HEK293 cells through the upregulation of Sp1 and Acyl-CoA binding protein. Int J Oncol. 2015;46:1328–1342. doi: 10.3892/ijo.2015.2834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Protective effect of intracerebroventricular administration of ODN against MPTP-induced pro-inflammatory and pro-apoptotic genes expression in the substantia nigra pars compacta. Quantification of interleukin-1β (Il-1β;A) and Bcl-2 (B) mRNA levels in the substantia nigra pars compacta (SNpc) 1 day after sham-, MPTP-, ODN-, and MPTP + ODN-treatment. Data were normalized using the GAPDH housekeeping gene as an internal control and the results are expressed as a percentage of control. Data are mean ± SEM obtained from at least 9 animals and statistical analysis was conducted by ANOVA followed by Bonferroni’s test. * P < 0.05, ** P < 0.01 vs. saline-treated mice; # P < 0.05 vs MPTP-treated mice. (TIFF 770 kb)
Supplementary Fig. 2 Protective effect of intracerebroventricular administration of ODN against MPTP-induced reduction of tyrosine hydroxylase gene expression in the substantia nigra pars compacta in wild-type and DBI knockout mice. A. Quantification of tyrosine hydroxylase (TH) mRNA levels in the substantia nigra pars compacta (SNpc) of wild-type DBI mice 8 days after sham (0.9% Nacl)-, MPTP (20 mg/kg)-, ODN (10 ng)-, and MPTP + ODN-treatment. B. Brain tissues were collected from DBI wild-type (DBI+/+), DBI heterozygote (DBI−/+) and DBI knockout (DBI−/−) mice 8 days after MPTP (10 mg/kg) treatment. TH mRNA levels were measured by quantitative RT-PCR and data were normalized using the GAPDH housekeeping gene as an internal control. Data are mean ± SEM of at least 8 animals and statistical analysis was conducted by ANOVA followed by Bonferroni’s test. * P < 0.05vs. saline-treated mice; #P < 0.05 vs. MPTP-treated mice; §§ P < 0.05 vs DBI+/+ mice. (TIFF 791 kb)