Abstract
The downregulation of AMP-activated protein kinase (AMPK) activity contributes to numerous pathologies. Recent reports suggest that the elevation of cellular cAMP promotes AMPK activity. However, the source of the cAMP pool that controls AMPK activity remains unknown. Mammalian cells possess two cAMP sources: membrane-bound adenylyl cyclase (tmAC) and intracellularly localized, type 10 soluble adenylyl cyclase (sAC). Due to the localization of sAC and AMPK in similar intracellular compartments, we hypothesized that sAC may control AMPK activity. In this study, sAC expression and activity were manipulated in H9C2 cells, adult rat cardiomyocytes or endothelial cells. sAC knockdown depleted the cellular cAMP content and decreased AMPK activity in an EPAC-dependent manner. Functionally, sAC knockdown reduced cellular ATP content, increased mitochondrial ROS formation and led to mitochondrial depolarization. Furthermore, sAC downregulation led to EPAC-dependent mitophagy disturbance, indicated by an increased mitochondrial mass and unaffected mitochondrial biogenesis. Consistently, sAC overexpression or stimulation with bicarbonate significantly increased AMPK activity and cellular ATP content. In contrast, tmAC inhibition or stimulation produced no effect on AMPK activity. Therefore, the sAC–EPAC axis may regulate basal and induced AMPK activity and support mitophagy, cellular energy and redox homeostasis. The study argues for sAC as a potential target in treating pathologies associated with AMPK downregulation.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03152-y) contains supplementary material, which is available to authorized users.
Keywords: ADCY10, cAMP, AMPK, ROS, ATP, Mitophagy
Introduction
AMP-activated protein kinase (AMPK) regulates diverse metabolic and physiological processes, such as lipid and glucose metabolism, protein synthesis, autophagy and redox homeostasis [1]. Emerging evidence highlights the role of AMPK in controlling cellular energy and redox homeostasis, particularly by supporting mitochondrial health [2].
Due to the association of dysregulated AMPK activity with aging and major chronic diseases, e.g., obesity, inflammation and diabetes [3–5], efforts have been made to understand the molecular mechanisms that control AMPK activity. AMPK activity regulation is a complex process. Two well-defined mechanisms of AMPK activation include the binding of AMP/ADP to the γ-subunit and the phosphorylation of the -subunit at T172. Several signaling pathways, such as ROS, Akt, PKD1 and S6K [6–9], regulate AMPK activity. An emerging role of cAMP signaling has also been suggested. cAMP signaling promotes AMPK activity in endothelial cells [10], muscle cells [11] and adipocytes [12]. These effects have been partially attributed to the activation of EPAC, an exchange protein directly activated by cAMP [11]. In contrast, the inhibitory effect of the PKA-dependent phosphorylation of the AMPK -subunit at S173, S485/491 or S497 has been observed [13, 14], although a stimulatory effect of PKA on AMPK by the phosphorylation and activation of the upstream kinase LKB1 has also been identified [15]. The discrepancy in the cAMP signaling effects on AMPK activity may be due to the unspecific elevation of cellular cAMP, e.g., PDE inhibition [11]. PDE inhibition or treatment with cAMP analogs leads to the uncontrolled diffusion of cAMP throughout the cytosol, followed by the unselective activation of numerous cAMP targets within diverse compartments. Another widely used approach, the activation of tmAC [14, 16], while specific, leads to cAMP elevation primarily within the sub-plasmalemmal compartment due to PDE activity [17], i.e., far from AMPK compartments within the cytosol or nucleus. Apart from tmAC, a second source of cAMP, type 10 soluble adenylyl cyclase (sAC), has been demonstrated for mammalian cells [18]. sAC has been shown to localize in various intracellular compartments [18, 19] and provides both specificity and selectivity by allowing cAMP generation in close proximity to its intracellular targets. Of note, cytosolic and nuclear sAC localization allows the building of the cAMP pool in proximity to the main AMPK compartments. Whether the sAC-generated cAMP pool may modulate AMPK activity and, thereby, mitochondrial biology remains unknown and was the aim of the present study.
Using cardiac and endothelial cells, we demonstrated that sAC downregulation diminished AMPK activity, whereas sAC upregulation or activation promoted it. Reduced sAC activity was associated with disturbed mitochondrial clearance that culminated in oxidative stress and energy imbalance. Contrary to sAC, elevation of cellular cAMP via tmAC activation did not affect AMPK activity, which suggests an exclusive role of the sAC-dependent cAMP pool in regulating AMPK activity.
Materials and methods
Cell culture and treatments
Cardiac rat embryonic myoblasts (H9C2, ATCC CRL-1446) were purchased from American Type Culture Collection. Cells were expanded and frozen in aliquots within 4 weeks of purchase. For the experiments, cells were thawed and cultured for no more than four further passages and were not allowed to grow to more than 60% confluence. Cells were cultured in phenol red-free Dulbecco’s modified Eagle’s medium (DMEM, Sigma, #D2902), supplemented with 10% fetal bovine serum (FBS), 21 mmol/l sodium bicarbonate and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) in a 5% CO2 incubator at 37 °C.
In the bicarbonate stimulation experiments, cells were incubated in DMEM containing no bicarbonate (0% CO2), 21 mmol/l (5% CO2) or 42 mmol/l bicarbonate (10% CO2) and supplemented with 15 mmol/l HEPES and 3% FBS. NaCl was used to adjust osmolarity and pH was set to 7.4 with sodium hydroxide and hydrochloric acid.
Treatments with 1 and 10 µmol/l forskolin (Sigma, Germany), 200 µmol/l 2′,3′-dideoxyadenosine (Sigma, Germany), 200 µmol/l IBMX (Sigma, Germany), 200 µmol/l 6-Bnz-cAMP (BioLog, Germany), 200 µmol/l 8-CPT-2Me-cAMP (BioLog, Germany), 10 µmol/l antimycin A (Sigma, Germany), 1 mmol/l H2O2 (Sigma, Germany), 1 mmol/l NaCN (Sigma, Germany), 5 mmol/l 2-deoxy-d-glucose (Sigma, Germany), 50 µmol/l resveratrol (Sigma, Germany), 10 µmol/l A769662 (Tocris, Germany) or 10 µmol/l compound C (Sigma, Germany) were performed as indicated.
Isolation of adult rat coronary endothelial cells
Treatment of rats to isolate endothelial cells was performed in consent with the directive of the EU (2010/63/EU).
Coronary endothelial cells were obtained and cultured as described previously [20]. For this purpose, adult male rats (200–300 g) were euthanized using deep isoflurane (5%) anesthesia, hearts were rapidly excised, washed with ice-cold 0.9% NaCl and connected to the perfusion system for perfusion with collagenase type II (300 U/ml). Anesthesia depth was monitored by limb withdrawal using toe pinching. Myocytes-free cell suspension was treated with trypsin for 10 min and seeded in cell culture dishes for 1 h in DMEM supplemented with 15% FBS followed by vigorous washing. This protocol excludes all non-endothelial cells, while leaving endothelial cells in sufficient quantity. The purity of the cell culture (> 98%) was confirmed by immunochemical staining with antibodies against von Willebrand factor (Sigma, Germany). Experiments were performed with monolayers reaching 90% confluence.
sAC overexpression and knockdown
For sAC overexpression experiments, cells were transfected with GFP or sAC (50 kDa) encoding plasmids (pTurbo-CMV) kindly provided by Dr. R. Acin-Perez (Centro Nacional de Investigaciones Cardiovasculares, Madrid). Briefly, 400,000 cells resuspended in 100 μl electroporation buffer (5 mmol/l KCl, 15 mmol/l MgCl2, 50 mmol/l mannitol, 86.4 mmol/l Na2HPO4, 33.6 mmol/l NaH2PO4, pH = 7.2) containing 8 µg plasmids and were electroporated using T-020 program for H9C2 cells and S-005 for coronary endothelial cells in Amaxa Nucleofector II Device (Lonza). The protocol was optimized by a series of experiments with GFP-targeted plasmids and shows 81.5 ± 3.8% (n = 5) GFP-positive cells 24 h after transfection determined by fluorescence microscopy (Online Resource, Supplementary Fig. 1). All downstream analyses were carried out 24 h after transfection.
For sAC knockdown experiments, cells were transfected either with scramble or with sAC shRNA-encoding plasmids. The electroporation procedure was the same as for sAC overexpression. All downstream analyses were carried out 3 days after transfection. sAC shRNA (5′-GGGGTACCAAAAAAGTGGAAAGTGGAACGAAAGCATCTCTTGAATGCTTTCGTTCCACTTTCCACAAACAAGGCTTTTCTCCAAG-3′) was expressed by the U6 promoter and a randomized sequence (scramble) based on sAC shRNA sequence was used as a control. Hairpin loop in the sequence is underlined.
Western blotting
Cells were lysed in Laemmli buffer and proteins were quantified using Pierce 660 nm Assay Kit. Equal amounts of total proteins were separated by SDS–polyacrylamide gels and transferred onto nitrocellulose membrane. The blots were blocked with 5% BSA in TBST for 1 h at room temperature and incubated overnight with the primary antibodies: ACC (Cell Signaling, Germany), AMPK (Cell Signaling, Germany), GAPDH (MD Millipore, Germany), pACC (Cell Signaling, Germany), pAMPK (Cell Signaling, Germany), PGC1α (Abcam, Germany), sAC (R21, CEP Biotech, NY), β-actin (Santa Cruz, Germany), TFAM (Acris, Germany), α-tubulin (Sigma, Germany). After incubation with the HRP-conjugated secondary antibodies, specific bands were visualized by chemiluminescence using an ECL kit (Promega, Germany). To confirm equal protein loading, membranes were stripped and immunostained for actin, tubulin or GAPDH.
FRET-based assay of cytosolic cAMP
For FRET-based live cell imaging, cells were transfected with plasmids encoding EPAC-based cAMP sensor, H187 [21] for cAMP analysis. Transfection was performed by electroporation as mentioned above and cells were seeded on 24 mm glass coverslips and cultured for 48 h, followed by 24 h starvation in DMEM supplemented with 3% FBS.
The FRET-based cytosolic cAMP assay was performed as previously described [22]. cAMP probes were excited at 430 nm and emission light was acquired at 470 nm for CFP and 530 nm for YFP channel. Images were acquired with an inverted microscope (oil immersion objective 40x, Zeiss, Germany) and an imaging system (Visitron, Germany) every 6 s. The analysis of the FRET signal was performed by VisiView software (Visitron, Germany). Emission signals obtained in the cell-free region (background) were subtracted from the corresponding emission signals obtained within the region of interest and presented as ratio of CFP/YFP.
Analysis of mitochondrial membrane potential
H9C2 cells were loaded with TMRM (50 nmol/l; Thermo Scientific, Germany) and MitoGreen (100 nmol/l; Thermo Scientific, Germany) at 37 °C for 20 min. Cells were then washed, trypsinized and resuspended in ice-cold PBS and subjected to the flow cytometry analysis applying MACSQuant Analyzer 10 (Miltenyi Biotec, Germany). Cells were excited at 488 nm and the emission fluorescence was collected at 535 nm for MitoGreen and 590 nm for TMRM. Mitochondrial membrane potential is expressed as the fluorescence ratio of TMRM to MitoGreen fluorescence. At least 10,000 events were recorded per sample.
In some experiments, TMRM and MitoGreen fluorescence was analyzed applying imaging system. Images were acquired with an inverted microscope (oil immersion objective 40×, Zeiss, Germany) and an imaging system (Visitron, Germany) every 10 s. The analysis of the emission signal was performed by VisiView software (Visitron, Germany). Emission signals obtained in the cell-free region (background) were subtracted from corresponding emission signals obtained within the region of interest and presented as ratio of TMRM/MitoGreen.
ROS analyses
To examine total cellular reactive oxygen species (ROS), cells were loaded with DCF (2′,7′-dichlorodihydrofluorescein diacetate, succinimidyl ester, 10 µmol/l, Invitrogen, Germany), a nonfluorescent dye, which is converted into the highly fluorescent DCF in the presence of free radicals. DCF fluorescence was analyzed by excitation at 488 nm and emission at 530 nm applying ELISA reader. Values are presented as a ratio of DCF fluorescence to total protein amount per well.
Mitochondrial ROS was examined by loading cells with MitoSox (10 µmol/l, Invitrogen, Germany), a fluorescent dye that accumulates in mitochondria. It is rapidly oxidized by superoxide anion and produces red fluorescence. MitoSox fluorescence was analyzed by excitation at 510 nm and emission at 580 nm with ELISA reader. Values are presented as a ratio of DCF fluorescence or MitoSox fluorescence to total protein amount per well.
Analysis of mitochondrial mass
Amounts of nuclear DNA and mitochondrial DNA (mtDNA) were determined by quantitative real-time PCR. The ratio of mtDNA to nuclear DNA was used as a measure of the mitochondrial content. Total genomic DNA was isolated using Quick-gDNA Miniprep Kit (Zymo Research, Germany). Quantitative PCR was performed by using the following primers (mtDNA specific cytochrome b primers: forward 5ʹ-CCACATCTGCCGAGACGTAA-3ʹ and reverse 5ʹ-TAGTCCTCGTCCCACATGGA-3ʹ; nuclear DNA specific β-globin primers: forward 5ʹ-AAGTACCACTAAGCCCCCTTTC-3ʹ and reverse 5ʹ-GGGAACACAAAAGACCTCTTCTGG-3ʹ) and SYBR Green PCR Kit in a StepOnePlus real-time PCR system (Applied Biosystem, USA).
MitoGreen dye (Invitrogen, Germany) accumulates in mitochondria irrespectively of mitochondrial membrane potential [23] and, thus, widely used for determination of mitochondrial mass. Briefly, cells were loaded with MitoGreen (100 nmol/l) at 37 °C for 20 min. Cells were then washed, trypsinized and resuspended in ice-cold PBS and subjected to flow cytometry analysis applying MACSQuant Analyzer 10 (Miltenyi Biotec, Germany). Cells were excited at 490 nm and the emission fluorescence was collected at 535 nm. At least 10,000 events were analyzed per sample.
Quantitative real-time PCR
Total RNA was isolated by using the RNA-Bee reagent (AMS Biotechnology, Germany), according to the manufacturer’s instructions. RNA was subsequently reverse transcribed to cDNA by using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Germany). The mRNA levels were measured by real-time PCR using iTaq Universal SYBR Green Supermix and the CFX96 qPCR system (Bio Rad, Germany). Quantitative PCR was performed by using the following primers for mitochondria-encoded genes (COX1: forward primer 5′-ATCGCAATTCCTACAGGCGT-3′; reverse primer 5′-GGCTCATAATATGGCGGGGG-3′; ATP6: forward primer 5′-CGCCTAATCAGCAACCGACT-3′; reverse primer 5′-TTAGGGCTCAGGTTCGTCCT-3′; ND4: forward primer 5′-ACCCCCTACCCTCAACATGA-3′; reverse primer 5′-GCTTCTACGTGGGCTTTTGG-3′; Cytb: forward primer 5′-TGCCGAGACGTAAACTACGG-3′; reverse primer 5′-TAGTCCTCGTCCCACATGGA-3′) and nuclear-encoded mitochondrial genes (Atp5b: forward primer 5′-AGCAGACTGGTTTTGGAGGT-3′; reverse primer 5′-GGCCTCTAACCAAGCCTTCA-3′; COX5B: forward primer 5′-GATAGCAGCACAGAGGGGAC-3′; reverse primer 5′-CGGATGGGACTAGATTGGGG-3′; COX7B: forward primer 5′-TAGTCACCGCAGTTCCATCC-3′; reverse primer 5′-CTTGCCACCACTTGCTGAATG-3′; NDUFS1: forward primer 5′-GGCAGGACTTGCCAAAGGAT-3′; reverse primer 5′-GCAGCCCCTGGGAGAATAAC-3′; NDUFS7: forward primer 5′-CTACTCGGTTGTTCGTGGCT-3′; reverse primer 5′-AGCTGCAAGATGCCGTAGAG-3′).
Analyses of total cellular ATP and cAMP
Total cellular ATP was measured using ATPLite Luminescence Assay System (PerkinElmer, USA). Briefly, cells were washed with PBS and lysed using the ice-cold lysis buffer provided by the manufacturer. Cell lysates were transferred to a white-walled 96-well plate alongside the series of ATP standards used for calibration. Bioluminescence produced after the addition of ATPLite substrate was measured using the Victor Luminometer (PerkinElmer, USA) and the amount of ATP in the lysates was calculated using the calibration curve. Values were presented as a ratio of ATP to total protein content.
The analysis of the total cellular cAMP content was performed using a Direct cAMP ELISA Kit (Enzo, Germany). Briefly, cells were washed with PBS and lysed using the ice-cold lysis buffer provided by the manufacturer. The measured absorbance at 405 nm was used to calculate the concentration of the cAMP by applying a calibration curve. Values were presented as a ratio of cAMP to total protein content.
Data and statistical analysis
The data are given as mean ± SEM, where mean indicates biological replicates. Comparisons of the mean between two groups were performed using unpaired two-tailed t tests assuming equal variance. For more than two groups, two-way ANOVA followed by Bonferroni post-test was used. Statistical significance was accepted when P < 0.05.
Results
sAC–EPAC axis controls basal AMPK activity
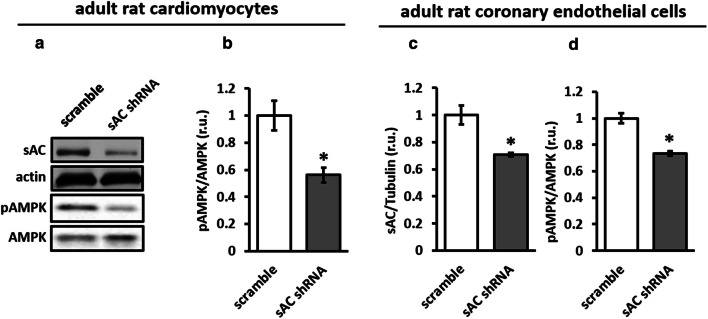
To test the role of sAC in basal AMPK activity in cardiac cells, sAC knockdown was performed in H9C2 cells. The downregulation of sAC expression significantly reduced cellular cAMP and the phosphorylation of AMPK and its direct target ACC, a marker of AMPK activity (Fig. 1). In addition, by applying the protein samples from a study performed with a primary culture of adult rat cardiomyocytes [24], we found that sAC knockdown significantly reduced AMPK phosphorylation (Fig. 2a, b). A similar effect of sAC knockdown was found in rat coronary endothelial cells (Fig. 2c, d). Therefore, sAC supports the basal activity of AMPK independently of cell type.
Fig. 1.
sAC knockdown suppressed AMPK activity. Transfection of H9C2 cells with plasmids encoding either sAC-targeted (sAC shRNA) or scramble shRNA significantly reduced sAC expression (a), cellular cAMP content (b) and the phosphorylation of AMPK (c) and its direct target ACC (d). Data are means ± SEM. n = 10 for a, c, d and n = 6 for b. *P < 0.05 vs. scramble
Fig. 2.
Downregulation of sAC expression reduced AMPK activity in adult rat cardiomyocytes and coronary endothelial cells. Expression analyses of sAC and phosphorylated AMPK performed in adult rat cardiomyocytes (a, b) and rat coronary endothelial cells (c, d) transfected with plasmids encoding either sAC-targeted (sAC shRNA) or scramble shRNA (for methodological details see [24]). Data are means ± SEM. n = 8 for b and n = 4 for c, d. *P < 0.05 vs. scramble
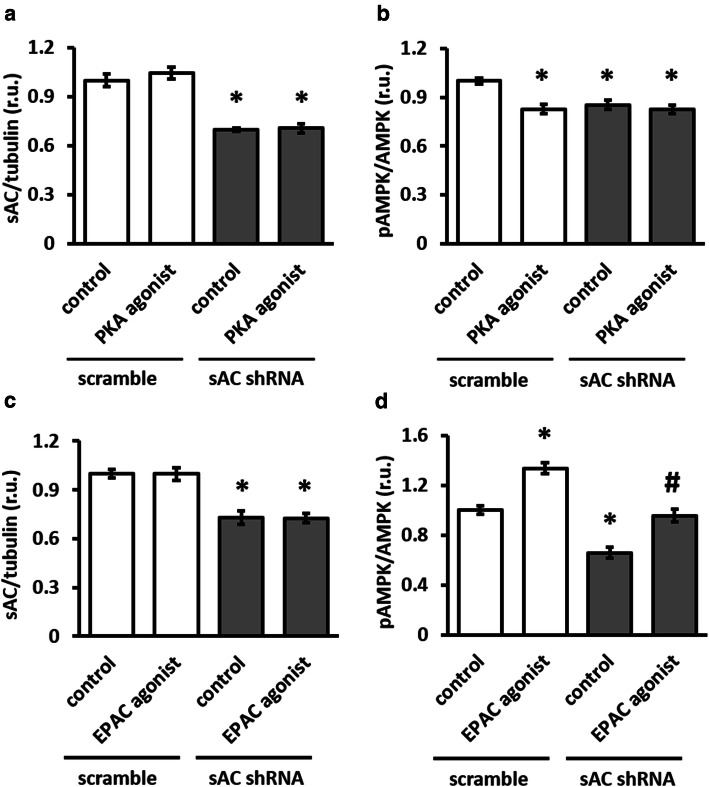
Because sAC knockdown was accompanied by a significant reduction in cellular cAMP (Fig. 1b), we aimed to determine which downstream target of cAMP, PKA and/or EPAC, may be involved in the regulation of AMPK activity by sAC. Treatment with specific PKA agonist had no effect on AMPK phosphorylation in sAC knockdown cells, but reduced AMPK phosphorylation in control cells (Fig. 3a, b). In contrast, EPAC agonist rescued the effect of sAC knockdown on AMPK phosphorylation (Fig. 3c, d). Thus, the sAC–EPAC axis is involved in regulating basal AMPK activity.
Fig. 3.
Activation of EPAC, not PKA, rescued AMPK phosphorylation. Effect of 1 h treatment with 200 µmol/l PKA agonist 6-Bnz-cAMP (a–b) or with 200 µmol/l EPAC agonist 8-CPT-2Me-cAMP (c–d). Treatments were performed in H9C2 cells transfected with plasmids encoding either sAC-targeted (sAC shRNA) or scramble shRNA. Data are means ± SEM. n = 4 for a–c and n = 6 for d. *P < 0.05 vs. scramble control, #P < 0.05 vs. sAC shRNA control
sAC downregulation jeopardizes mitophagy, as well as cellular energy and redox balances
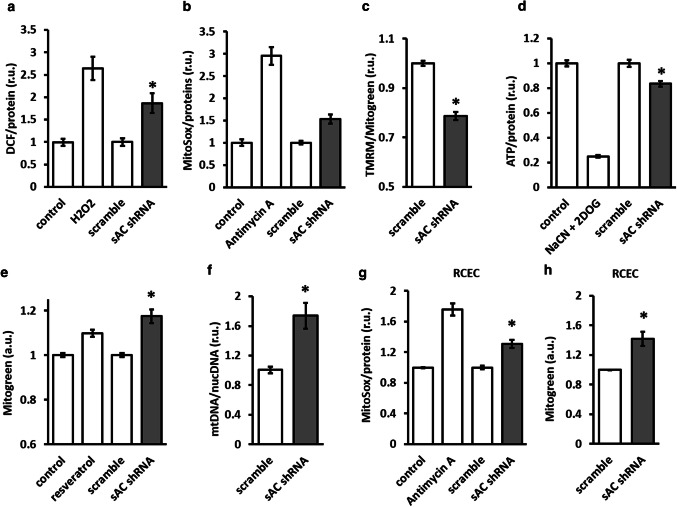
Because AMPK activity is a key regulator of cellular redox and energy homeostasis as well as mitochondrial biology [2], we examined the functional effects of sAC knockdown. The downregulation of sAC expression led to the elevation of total cellular (DCF fluorescence) and mitochondria-specific (MitoSox fluorescence) ROS formation (Fig. 4a, b). These effects were accompanied by mitochondrial depolarization and a significant reduction in the total cellular ATP level (Fig. 4c, d).
Fig. 4.
sAC knockdown disturbed cellular redox and energy homeostasis and increased mitochondrial mass. Analyses of total cellular ROS formation (DCF fluorescence, a), mitochondrial ROS formation (MitoSox fluorescence, b), mitochondrial membrane potential (c), total cellular ATP (d), and mitochondrial mass (e–f) performed with H9C2 cells. Analyses of mitochondrial ROS formation (g) and mitochondrial mass (h) performed with adult rat coronary endothelial cells (RCEC). Cells were transfected with plasmids encoding either sAC-targeted (sAC shRNA) or scramble shRNA. Data are means ± SEM. n = 6–8. *P < 0.05 vs. scramble. Treatments with H2O2 (1 mmol/l, 1 h), antimycin A (10 µmol/l, 1 h), 1 mmol/l NaCN + 5 mmol/l 2DOG (40 min) and resveratrol (50 µmol/l, 24 h) were used as positive controls
AMPK plays a substantial role in regulating mitochondrial quality control, or mitophagy [2]. In line with these data, downregulation of sAC expression in H9C2 cells significantly increased mitochondrial mass, as demonstrated by an elevation in MitoGreen staining, a mitochondrial membrane potential-independent mitochondrial dye [23], and by the mtDNA/nDNA ratio (Fig. 4e, f). Similarly, the knockdown of sAC in rat coronary endothelial cells significantly increased mitochondrial ROS formation and mitochondrial mass (Fig. 4g, h).
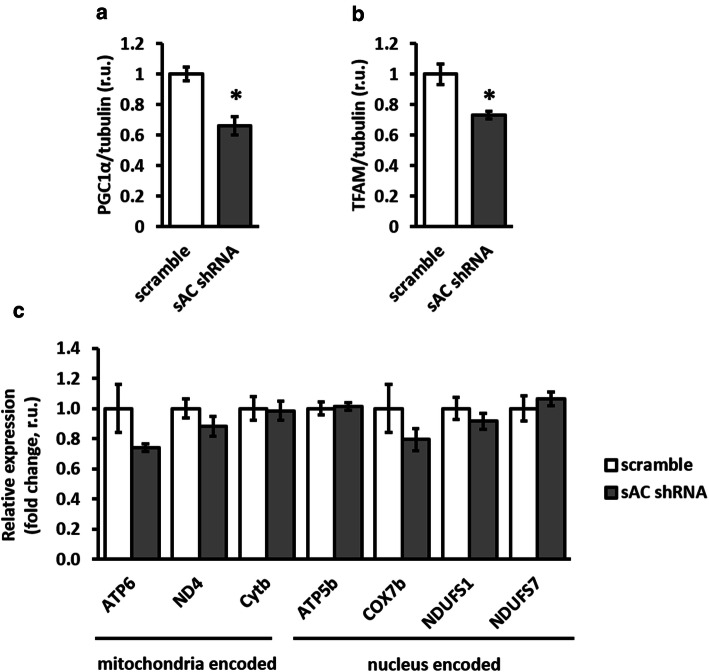
To exclude the potential effect of mitochondrial biogenesis on mitochondrial mass, expression analyses of nuclear (PGC-1alpha) and mitochondrial (TFAM) transcription factors (western blot) as well as nuclear- and mitochondria-encoded mitochondrial proteins (qPCR) were performed. We found significant downregulation of both transcription factors and no effect on mitochondrial gene expression (Fig. 5). Thus, sAC knockdown likely does not affect mitochondrial mass via upregulating mitochondrial biogenesis, but rather due to disturbed mitochondrial clearance.
Fig. 5.
sAC knockdown reduced the expression of mitochondria-related transcription factors, but did not affect the expression of mitochondrial genes. Expression analysis of transcription factors PGC1α and TFAM (western blot, a–b), and mitochondria- and nucleus-encoded mitochondrial genes (c) performed in H9C2 cells transfected with plasmids encoding either sAC-targeted (sAC shRNA) or scramble shRNA. Data are means ± SEM. n = 5. *P < 0.05 vs. scramble
To strengthen these findings, a direct pharmacological suppression of AMPK activity, i.e., treatment with AMPK inhibitor compound C [25], was applied. Compound C markedly reduced AMPK phosphorylation, which was accompanied by significant mitochondrial depolarization and the elevation of mitochondrial mass (Online Resource, Supplementary Fig. 2).
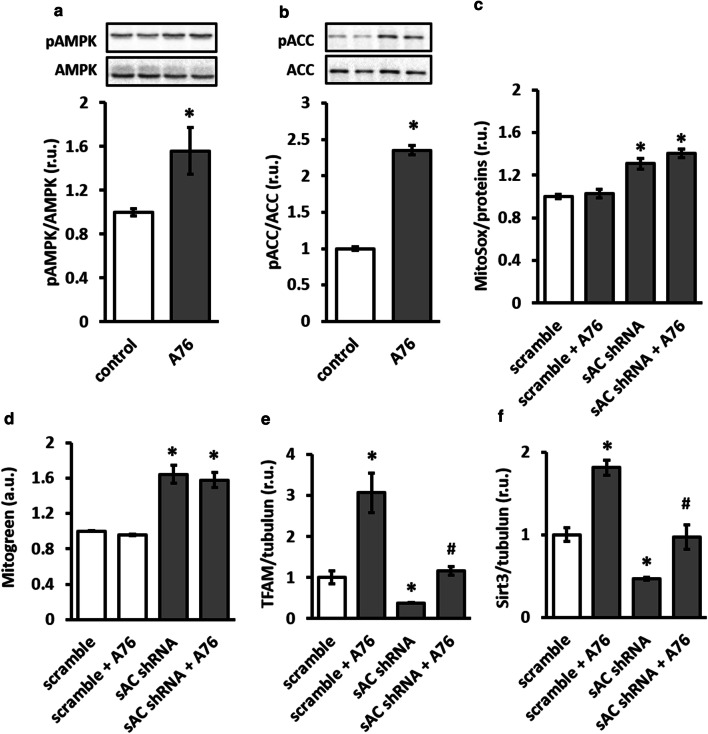
To prove the causal role of AMPK downregulation in disturbed mitochondrial clearance and redox balance, treatment with AMPK agonist A769662 was performed in control and sAC-depleted cells. Though AMPK agonist significantly elevated AMPK and ACC phosphorylation (Fig. 6a, b), it did not prevent elevation of mitochondrial mass and ROS formation induced by sAC knockdown (Fig. 6c, d). In contrast, treatment with AMPK agonist rescued the sAC knockdown-induced downregulation of TFAM and Sirt3 (Fig. 6e, f), two key mitochondrial proteins involved in the expression of mitochondria-encoded genes and activation of numerous mitochondrial enzymes, respectively.
Fig. 6.
AMPK activator rescued the expression of mitochondrial proteins under sAC knockdown in H9C2 cells. Treatment with A769662 (A76, 10 µmol/l) for 24 h significantly increased phosphorylation of AMPK (a) and its direct target ACC (b). Analyses of mitochondrial ROS formation (MitoSox fluorescence, c), mitochondrial mass (MitoGreen, d) and expression of mitochondrial proteins TFAM and Sirt3 (e, f) under sAC knockdown with or without treatment with A76 10 µM for 24 h. Data are means ± SEM. n = 3–4 for a, b, e, f and n = 6–8 for c and d. *P < 0.05 vs. control or scramble and #P < 0.05 vs. sAC shRNA
Because EPAC in our study is involved in regulating AMPK activity, we were also interested in its role in mitochondrial homeostasis regulation. Consistently, the sAC knockdown-induced elevation of mitochondrial mass was significantly attenuated by treatment with EPAC but not PKA agonist (Online Resource, Supplementary Fig. 3). Similarly, EPAC agonist partly reversed the elevation of mitochondrial ROS formation and prevented mitochondrial depolarization, though it did not affect reduction of cellular ATP content in sAC-depleted cells (Online Resource, Supplementary Fig. 4).
These results suggest that sAC knockdown leads to the impaired mitophagy that is accompanied by disturbed cellular energy and redox balances.
Stimulation of sAC, but not tmAC, promotes AMPK activity
Several studies have demonstrated that the upregulation of cAMP signaling affects AMPK activity, although the results are controversial, and AMPK activation and inhibition have been reported [11, 26]. Thus, to prove whether the upregulation of sAC signaling may affect AMPK activity, two models were applied.
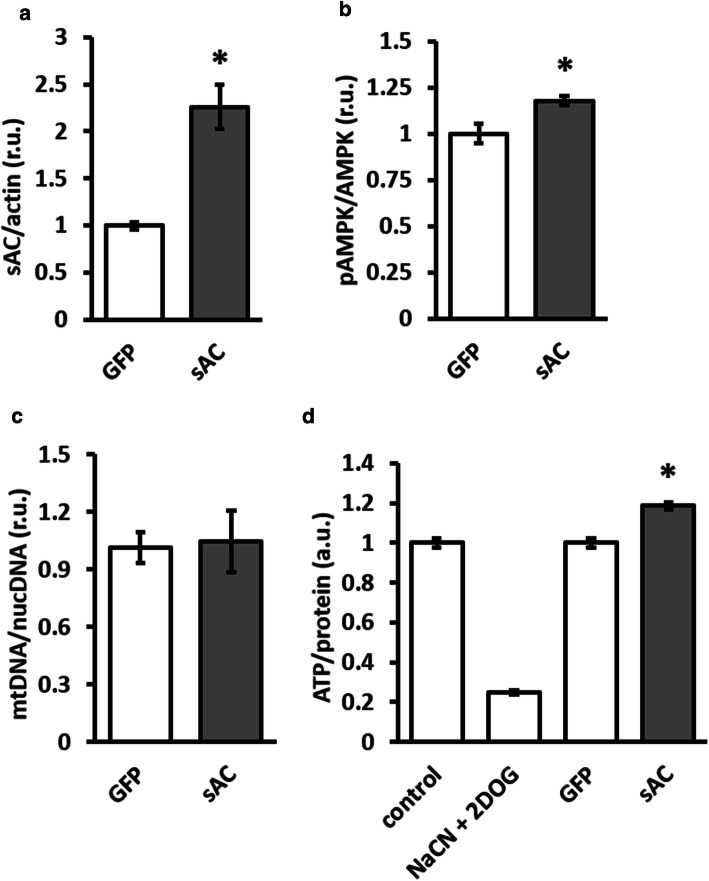
First, the overexpression of active 50 kDa sAC isoform in H9C2 cells was performed. sAC overexpression significantly elevated cellular cAMP concentration and increased AMPK phosphorylation (Fig. 7). sAC overexpression was accompanied by a significant increase in cellular ATP content and had no effect on mitochondrial mass (Fig. 7).
Fig. 7.
sAC overexpression increased AMPK phosphorylation and the cellular ATP content. Analyses of sAC expression (a), AMPK phosphorylation (b), mitochondrial mass (d) and total cellular ATP content (d) performed in H9C2 cells transfected with plasmids encoding either 50-kDa sAC isoform (sAC) or GFP. Data are means ± SEM. n = 4. *P < 0.05 vs. GFP. Treatment with 1 mmol/l NaCN + 5 mmol/l 2DOG (40 min) was used as a positive control
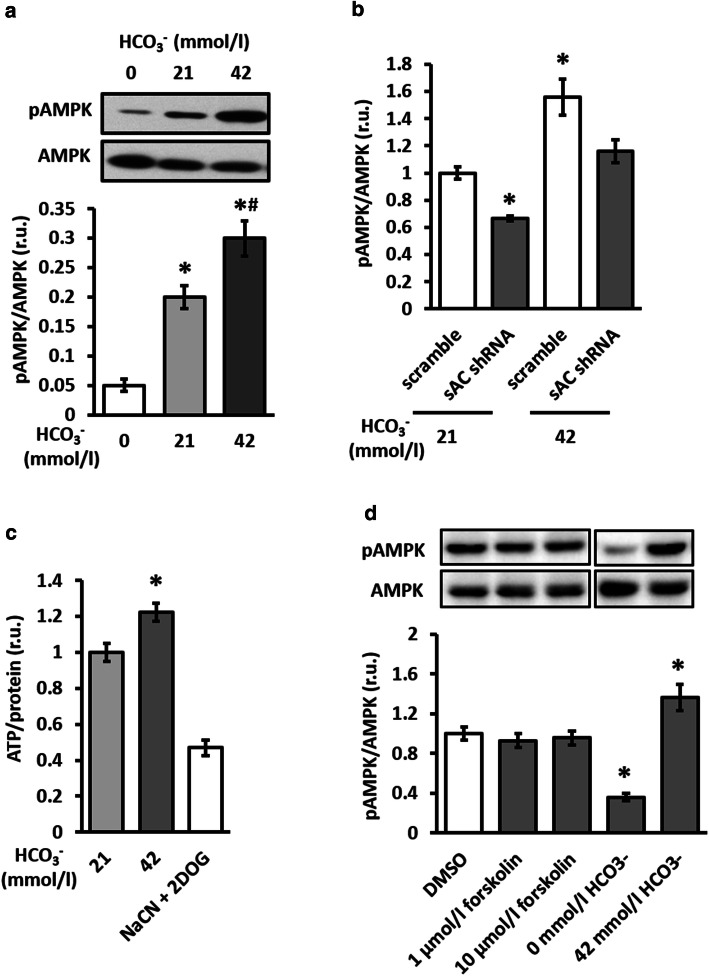
Second, stimulation of sAC with bicarbonate, a natural sAC activator [27, 28], was applied. Bicarbonate led to the stable (until 24 h) and dose-dependent elevation of AMPK activity (Fig. 8a and Online Resource, Supplementary Fig. 5). Because this effect could be prevented by sAC knockdown (Fig. 8b), we suggest a causal role of sAC in bicarbonate-induced AMPK activation. Similarly to sAC overexpression, treatment with bicarbonate increased the cellular ATP content (Fig. 8c).
Fig. 8.
Stimulation of sAC, but not tmAC increased AMPK phosphorylation and cellular ATP content. Analyses of AMPK phosphorylation (a, b, d) and total cellular ATP content (c) performed in control, untransfected H9C2 cells, or in cells transfected with plasmids encoding either sAC-targeted (sAC shRNA) or scramble shRNA. Cells were incubated in medium containing 0, 21 or 42 mmol/l bicarbonate for 1 h in (a, b, d) and for 4 h in (c). In d, cells were treated with 1 or 10 µmol/l forskolin, or vehicles (DMSO) in medium containing 21 mmol/l bicarbonate for 1 h. Data are means ± SEM. n = 16 for (a, d), n = 6 for (b) and n = 4 for (c). *P < 0.05 vs. 0 mmol/l HCO3− in (a). *P < 0.05 vs. scramble at 21 mmol/l HCO3− in (b). *P < 0.05 vs. 21 mmol/l HCO3− in (c). *P < 0.05 vs. DMSO in (d). #P < 0.05 vs. 21 mmol/l HCO3− in (a)
To determine whether tmAC stimulation may also promote AMPK activity, treatment with forskolin was performed. A western blot assay demonstrated that tmAC stimulation had no effect on cellular AMPK phosphorylation (Fig. 8d), although in the present study (Online Resource, Supplementary Fig. 6a) and in a previous study applying a similar model [22] it significantly elevated cytosolic cAMP content. Consistently, tmAC suppression with 2′,5′-dideoxyadenosine had no effect on AMPK phosphorylation, although it significantly inhibited tmAC activity (Online Resource, Supplementary Fig. 6). Altogether, these findings demonstrate that AMPK activity is supported by sAC, but not tmAC.
Discussion
The aim of this study was to prove the role of sAC in AMPK activity regulation and its functional significance in mitochondrial biology. The main findings are as follows: the downregulation of sAC expression (i) significantly reduced basal AMPK activity in an EPAC-dependent manner and (ii) disturbed mitochondrial clearance, cellular energy and redox balances. (iii) The upregulation of sAC expression or activity promoted AMPK activity and improved cellular energy balance. (iv) In contrast to sAC, the stimulation or suppression of tmAC had no effect on AMPK activity.
Although several studies suggest the regulation of AMPK activity by cAMP, the results are controversial [11, 14, 26], and only a tmAC-dependent source of cAMP was suggested. Of note, cAMP synthesized by tmAC is mainly restricted within the sub-plasmalemmal compartment as long as PDEs are active. In contrast, numerous functional compartments distributed throughout the cell can be built by sAC [29]. Particularly, sAC is localized within the nucleus and in the cytosol near the main AMPK compartments [30–32]. Therefore, sAC is an ideal candidate in regulating AMPK activity by cAMP, particularly under basal conditions. Indeed, applying sAC knockdown in H9C2 cells a significant downregulation of AMPK activity was observed in the present study. This effect appears to not be restricted to a particular cell type and was also observed in primary cardiomyocytes and coronary endothelial cells. Altogether, sAC plays an essential role in supporting basal AMPK activity independently of cell type.
In contrast to our findings, Valsecci et al. [33] have shown an increased AMPK activity in mouse embryonic fibroblast derived from sAC knockout mice compared to cells from wild-type mice. The elevated AMPK activity might be due to the metabolic stress, a primary stimulus for AMPK activation, as mitochondrial sAC plays a key role in ATP synthesis [34]. Indeed, ATP synthesis rate was significantly reduced in the study. The discrepancy in our results, i.e., reduction of AMPK activity, may be due to the partial suppression of sAC expression in our study, which is not sufficient to reach a threshold for AMPK activation due to metabolic stress. Thus, the present study uncovers a unique mechanism of the AMPK activity regulation, which was masked in the study of Valsecci et al. [33] under sAC knockout due to metabolic stress.
By analyzing the potential downstream target of sAC involved in basal AMPK activity regulation, we examined the contribution of PKA and EPAC. Previous studies suggested their controversial roles in regulating AMPK activity, i.e., a stimulating effect of EPAC [11] and an inhibitory effect of PKA [14, 26]. In agreement with these reports, we determined that the activation of EPAC, not PKA, rescued AMPK activity in sAC knockdown cells. Additionally, control cells treated with EPAC agonist promoted AMPK activity, whereas PKA agonist suppressed it. Therefore, the sAC–EPAC axis supports AMPK activity under basal conditions. Though we did not further investigate the signaling link between EPAC and AMPK, previous studies clearly demonstrated the contribution of Rap1/2—phospholipase C—calcium/calmodulin-dependent kinase kinase β in various cell types, including cardiomyocytes [11, 35].
Due to the significance of AMPK in regulating cell metabolism and the association of its downregulation with aging and metabolic diseases [2], intensive efforts have been made to develop tools that promote AMPK activity, such as AMPK agonists and resveratrol [11, 36–38]. Therefore, we were interested in whether sAC upregulation or stimulation promotes AMPK activity. Since bicarbonate is a natural sAC stimulator [27], cells were treated with bicarbonate and a stable, at least within 24 h, and, dose-dependent upregulation of AMPK activity was observed. The effect of bicarbonate is likely due to the activation of sAC because (i) in a similar model, bicarbonate leads to cAMP elevation in an sAC-dependent manner [22] and (ii) sAC knockdown prevented a bicarbonate effect on AMPK (Fig. 8b). Similarly to the sAC stimulation using bicarbonate, the overexpression of sAC significantly upregulated AMPK activity. In agreement with these findings, a previous study by Jaitovich et al. [39] demonstrated that hypercapnia, a state of increased intracellular bicarbonate concentration due to the activity of carbonic anhydrases [40], leads to AMPK activation in skeletal muscle, although no mechanistic analyses have been performed.
In contrast to sAC, the activation of tmAC with forskolin had no significant effect on AMPK phosphorylation or activity. In line with our finding, negative effects of tmAC stimulation on AMPK activity have also been identified in several cell types by other studies and were attributed to the PKA-dependent phosphorylation of the AMPK -subunit at S173, S485/491 or S497 [13, 14]. Thus, the two cAMP sources in the cell, tmAC and sAC, play controversial roles in AMPK regulation.
AMPK is a well-known key regulator of mitochondrial biogenesis, dynamics and clearance, and hence the cellular energy and redox homeostasis [2]. Therefore, we were interested in whether the sAC-dependent regulation of AMPK activity may be translated into mitochondrial biology and cellular ATP content. In agreement with previous reports [33, 34, 41], we discovered that sAC knockdown led to mitochondrial depolarization, increased mitochondrial ROS formation and reduced cellular ATP content, which are typical fingerprints of mitochondrial dysfunction. Surprisingly, we also observed a significant increase in mitochondrial mass, whereas no effect on mitochondrial biogenesis was observed. These observations argue for a disturbed mitochondrial clearance, or mitophagy, in sAC-depleted cells. Similar mitochondrial depolarization and mitochondrial mass elevation have been found with pharmacological approaches, such as the suppression of AMPK activity with compound C.
The role of AMPK in regulating mitophagy has only recently been appreciated. A recent study by Toyama et al. [42] demonstrated that the AMPK-dependent phosphorylation of MFF resulted in the mitochondrial recruitment of Drp1 and consequent mitochondrial fission, a key step in generating mitochondrial particles that are sufficiently sized for autophagosome engulfment. Another recent study demonstrated that in ULK1-knockout murine embryonic fibroblasts, neither metabolic stress nor starvation was able to induce autophagy [43]. The study also reported that the increased accumulation of defective mitochondria was due to a lack of mitophagy in cells expressing a mutated form of ULK1 that cannot be phosphorylated by AMPK.
Applying AMPK activator A769662 to prove the causal role of AMPK in mitophagy disturbance induced by sAC knockdown, we found no effect of the AMPK agonist. This finding suggests that sAC knockdown may affect mitophagy independently of AMPK. Interestingly, a recent report of Rahman et al. [19] demonstrated an essential role of sAC in lysosomal function via supporting lysosomal acidification. Since lysosomes play a key role at the mitophagy process, one may suppose that sAC knockdown-induced failure in lysosomal acidification may cause mitophagy disturbance.
Nevertheless, AMPK activity seems to play a causal role in mitochondrial homeostasis disturbed by sAC knockdown. Particularly, sAC knockdown-induced downregulation of two mitochondrial proteins playing key roles in expression of mitochondria-encoding genes and regulation of enzyme activity in mitochondria, i.e., TFAM and Sirt3, was rescued by treatment with AMPK agonist (Fig. 6e, f). Altogether, the study suggests that sAC knockdown affects mitochondrial biology in AMPK-dependent as well as AMPK-independent manner.
Although no data are available regarding the role of sAC in mitochondrial dynamics, the PKA localized on the outer mitochondrial membrane suppresses mitochondrial fission by the phosphorylation of Drp1 at Ser637 [44, 45] or Ser656 [46] and by the destabilization of PINK1 [47]. Because mitochondrial fission is a key step in mitophagy [48], PKA may suppress mitophagy. Thus, PKA is unlikely to be involved in disturbed mitophagy under sAC knockdown. Indeed, treatment with PKA agonist in our study failed to rescue mitochondrial mass elevation in sAC-depleted cells (Online Resource, Supplementary Fig. 3).
The role of cAMP target EPAC in regulating the mitochondrial clearance process remains unknown. A recent report argues for the supporting role of EPAC1 in mitochondrial fission [49]. The findings of the present study that treatment with EPAC agonist, not PKA agonist, rescued both (i) the sAC knockdown-induced mitophagy disturbance and (ii) AMPK inactivation provide the first clue for the role of the sAC–EPAC axis in regulating mitochondrial clearance.
Accumulating data suggest the involvement of sAC-dependent cAMP pool in numerous pathologies accompanied by excessive cell death [24, 50, 51] or growth [52, 53]. In a recent study, Wang et al. [54] showed that advanced heart failure in rats is accompanied by a significant downregulation of sAC that leads to a reduced mitochondrial resistance to Ca2+, which demonstrates a potential role of sAC in cardiac pathology. Whether this sAC downregulation may contribute to AMPK activity and mitochondrial biology is yet to be investigated.
In conclusion, this study suggests that the sAC–EPAC axis controls basal AMPK activity and supports mitochondrial clearance, cellular energy and redox homeostasis. The overexpression or activation of sAC promotes AMPK activity and improves the cellular energy balance. In contrast to sAC, tmAC does not affect AMPK activity. This study suggests sAC as a potential target in diseases accompanied by mitochondrial dysregulation such as heart failure [54].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our gratitude to N. Haritonow, A. Kuehne, V. Riese, S. Pozdniakova and E. Dworatzek for their assistance with the theoretical and technical components of this research. This study was supported by the European Union (Radox Grant FP7-PEOPLE-2012-ITN) to VRZ, by DZHK (German Centre for Cardiovascular Research) partner site Berlin (Grant 81Z2100201) to VRZ and by Margarete-Ammon foundation to VRZ. Parts of the study were part of V. Jayarajan’s thesis project submitted in fulfillment of the requirements for the degree of Doctor of Philosophy at the Freie Universität Berlin (Germany).
Abbreviations
- ACC
Acetyl-CoA carboxylase
- AMP
Adenosine monophosphate
- ADP
Adenosine diphosphate
- AMPK
AMP-activated protein kinase
- ATP
Adenosine triphosphate
- cAMP
3′-5′-Cyclic adenosine monophosphate
- CFP
Cyan fluorescence protein
- Drp1
Dynamin-like protein 1
- EPAC
Exchange protein activated by cAMP
- FRET
Föster resonance energy transfer
- GFP
Green fluorescence protein
- LKB1
Liver kinase B1
- MFF
Mitochondrial fission factor
- PDE
Phosphodiesterase
- PGC1α
Peroxisome proliferator-activated receptor‑γ co-activator 1α
- PKA
Protein kinase A
- ROS
Reactive oxygen species
- sAC
Soluble adenylyl cyclase
- TFAM
Mitochondrial transcription factor A
- tmAC
Transmembrane adenylyl cyclase
- ULK1
unc-51 like autophagy activating kinase 1
- YFP
Yellow fluorescence protein
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jeon S-M. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48(7):e245–e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277(28):25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 4.Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51(8):2420. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S., Yamashita S., Noda M., Kita S., Ueki K., Eto K., Akanuma Y., Froguel P., Foufelle F., Ferre P., Carling D., Kimura S., Nagai R., Kahn B.B., Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Medicine. 2002;8(11):1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 6.Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB. p70S6 kinase phosphorylates AMPK on serine 491 to mediate Leptin’s effect on food intake. Cell Metab. 2012;16(1):104–112. doi: 10.1016/j.cmet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawley SA, Ross FA, Gowans GJ, Tibarewal P, Leslie NR, Hardie DG. Phosphorylation by Akt within the ST loop of AMPK-α1 down-regulates its activation in tumour cells. Biochem J. 2014;459(2):275–287. doi: 10.1042/BJ20131344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha RA, Singh BK, Zhou J, Wu Y, Farah BL, Ohba K, Lesmana R, Gooding J, Bay B-H, Yen PM. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy. 2015;11(8):1341–1357. doi: 10.1080/15548627.2015.1061849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coughlan KA, Valentine RJ, Sudit BS, Allen K, Dagon Y, Kahn BB, Ruderman NB, Saha AK. PKD1 inhibits AMPKα2 through phosphorylation of serine 491 and impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2016;291(11):5664–5675. doi: 10.1074/jbc.M115.696849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.M-l Chen, Yi L, Jin X, X-y Liang, Zhou Y, Zhang T, Xie Q, Zhou X, Chang H, Y-j Fu, J-d Zhu, Q-y Zhang, M-t Mi. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy. 2013;9(12):2033–2045. doi: 10.4161/auto.26336. [DOI] [PubMed] [Google Scholar]
- 11.Park S-J, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148(3):421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omar B, Zmuda-Trzebiatowska E, Manganiello V, Göransson O, Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal. 2009;21(5):760–766. doi: 10.1016/j.cellsig.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferretti AC, Tonucci FM, Hidalgo F, Almada E, Larocca MC, Favre C. AMPK and PKA interaction in the regulation of survival of liver cancer cells subjected to glucose starvation. Oncotarget. 2016;7(14):17815–17828. doi: 10.18632/oncotarget.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem. 2006;281(48):36662–36672. doi: 10.1074/jbc.M606676200. [DOI] [PubMed] [Google Scholar]
- 15.Kimball SR, Siegfried BA, Jefferson LS. Glucagon represses signaling through the mammalian target of rapamycin in rat liver by activating AMP-activated protein kinase. J Biol Chem. 2004;279(52):54103–54109. doi: 10.1074/jbc.M410755200. [DOI] [PubMed] [Google Scholar]
- 16.Damm E, Buech TRH, Gudermann T, Breit A. Melanocortin-induced PKA activation inhibits AMPK activity via ERK-1/2 and LKB-1 in hypothalamic GT1-7 cells. Mol Endocrinol (Baltimore, Md) 2012;26(4):643–654. doi: 10.1210/me.2011-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal SR, Clancy CE, Harvey RD. Mechanisms restricting diffusion of intracellular cAMP. Sci Rep. 2016;6:19577. doi: 10.1038/srep19577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2002;17(1):82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 19.Rahman N, Ramos-Espiritu L, Milner TA, Buck J, Levin LR. Soluble adenylyl cyclase is essential for proper lysosomal acidification. J Gen Physiol. 2016;148(4):325–339. doi: 10.1085/jgp.201611606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Kostin S, Flacke J-P, Reusch HP, Ladilov Y. Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells. J Biol Chem. 2009;284(22):14760–14768. doi: 10.1074/jbc.M900925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klarenbeek J, Goedhart J, van Batenburg A, Groenewald D, Jalink K. Fourth-generation epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity. PLoS One. 2015;10(4):e0122513–e0122513. doi: 10.1371/journal.pone.0122513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozdniakova S, Guitart-Mampel M, Garrabou G, Di Benedetto G, Ladilov Y, Regitz-Zagrosek V. 17beta-Estradiol reduces mitochondrial cAMP content and cytochrome oxidase activity in a phosphodiesterase 2-dependent manner. Br J Pharmacol. 2018;175(20):3876–3890. doi: 10.1111/bph.14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pendergrass W, Wolf N, Poot M. Efficacy of MitoTracker Green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytom A. 2004;61(2):162–169. doi: 10.1002/cyto.a.20033. [DOI] [PubMed] [Google Scholar]
- 24.Appukuttan A, Kasseckert SA, Micoogullari M, Flacke J-P, Kumar S, Woste A, Abdallah Y, Pott L, Reusch HP, Ladilov Y. Type 10 adenylyl cyclase mediates mitochondrial Bax translocation and apoptosis of adult rat cardiomyocytes under simulated ischaemia/reperfusion. Cardiovasc Res. 2012;93(2):340–349. doi: 10.1093/cvr/cvr306. [DOI] [PubMed] [Google Scholar]
- 25.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Investig. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, Vaahtomeri K, Auchli Y, Rechsteiner H, Brunisholz RA, Viollet B, Mäkelä TP, Wallimann T, Neumann D, Krek W. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 2010;29(2):469–481. doi: 10.1038/emboj.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289(5479):625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 28.Steegborn C. Structure, mechanism, and regulation of soluble adenylyl cyclases—similarities and differences to transmembrane adenylyl cyclases. Biochim Biophys Acta Mol Basis Dis. 2014;1842(12, Part B):2535–2547. doi: 10.1016/j.bbadis.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Pozdniakova S, Ladilov Y. Functional significance of the Adcy10-dependent intracellular cAMP compartments. J Cardiovasc Dev Dis. 2018;5(2):29. doi: 10.3390/jcdd5020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase: synergism between calcium and bicarbonate. J Biol Chem. 2003;278(18):15922–15926. doi: 10.1074/jbc.m212475200. [DOI] [PubMed] [Google Scholar]
- 31.Zippin JH, Chen Y, Straub SG, Hess KC, Diaz A, Lee D, Tso P, Holz GG, Sharp GWG, Levin LR, Buck J. CO2/HCO3(-)- and calcium-regulated soluble adenylyl cyclase as a physiological ATP sensor. J Biol Chem. 2013;288(46):33283–33291. doi: 10.1074/jbc.M113.510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zippin JH, Farrell J, Huron D, Kamenetsky M, Hess KC, Fischman DA, Levin LR, Buck J. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J Cell Biol. 2004;164(4):527–534. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valsecchi F, Konrad C, D’Aurelio M, Ramos-Espiritu LS, Stepanova A, Burstein SR, Galkin A, Magranè J, Starkov A, Buck J, Levin LR, Manfredi G. Distinct intracellular sAC-cAMP domains regulate ER Ca(2+) signaling and OXPHOS function. J Cell Sci. 2017;130(21):3713–3727. doi: 10.1242/jcs.206318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9(3):265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurent A-C, Bisserier M, Lucas A, Tortosa F, Roumieux M, De Régibus A, Swiader A, Sainte-Marie Y, Heymes C, Vindis C, Lezoualc’h F. Exchange protein directly activated by cAMP 1 promotes autophagy during cardiomyocyte hypertrophy. Cardiovasc Res. 2015;105(1):55–64. doi: 10.1093/cvr/cvu242. [DOI] [PubMed] [Google Scholar]
- 36.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3(6):403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Myers RW, Guan H-P, Ehrhart J, Petrov A, Prahalada S, Tozzo E, Yang X, Kurtz MM, Trujillo M, Gonzalez Trotter D, Feng D, Xu S, Eiermann G, Holahan MA, Rubins D, Conarello S, Niu X, Souza SC, Miller C, Liu J, Lu K, Feng W, Li Y, Painter RE, Milligan JA, He H, Liu F, Ogawa A, Wisniewski D, Rohm RJ, Wang L, Bunzel M, Qian Y, Zhu W, Wang H, Bennet B, LaFranco Scheuch L, Fernandez GE, Li C, Klimas M, Zhou G, van Heek M, Biftu T, Weber A, Kelley DE, Thornberry N, Erion MD, Kemp DM, Sebhat IK. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science. 2017;357(6350):507–511. doi: 10.1126/science.aah5582. [DOI] [PubMed] [Google Scholar]
- 38.Xiao B, Sanders MJ, Carmena D, Bright NJ, Haire LF, Underwood E, Patel BR, Heath RB, Walker PA, Hallen S, Giordanetto F, Martin SR, Carling D, Gamblin SJ. Structural basis of AMPK regulation by small molecule activators. Nat Commun. 2013;4:3017. doi: 10.1038/ncomms4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaitovich A, Angulo M, Lecuona E, Dada LA, Welch LC, Cheng Y, Gusarova G, Ceco E, Liu C, Shigemura M, Barreiro E, Patterson C, Nader GA, Sznajder JI. High CO2 levels cause skeletal muscle atrophy via AMP-activated kinase (AMPK), FoxO3a protein, and muscle-specific Ring finger protein 1 (MuRF1) J Biol Chem. 2015;290(14):9183–9194. doi: 10.1074/jbc.M114.625715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J, Lecuona E, Briva A, Welch LC, Sznajder JI. Carbonic anhydrase II and alveolar fluid reabsorption during hypercapnia. Am J Respir Cell Mol Biol. 2008;38(1):32–37. doi: 10.1165/rcmb.2007-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Rasmo D, Signorile A, Santeramo A, Larizza M, Lattanzio P, Capitanio G, Papa S. Intramitochondrial adenylyl cyclase controls the turnover of nuclear-encoded subunits and activity of mammalian complex I of the respiratory chain. Biochim Biophys Acta Mol Cell Res. 2015;1853(1):183–191. doi: 10.1016/j.bbamcr.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Toyama EQ, Herzig S, Courchet J, Lewis TL, Jr, Losón OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science (New York, NY) 2016;351(6270):275–281. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science (New York, NY) 2011;331(6016):456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282(30):21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 45.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8(10):939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dagda RK, Gusdon AM, Pien I, Strack S, Green S, Li C, Van Houten B, Cherra SJ, 3rd, Chu CT. Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson’s disease. Cell Death Differ. 2011;18(12):1914–1923. doi: 10.1038/cdd.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akabane S, Uno M, Tani N, Shimazaki S, Ebara N, Kato H, Kosako H, Oka T. PKA regulates PINK1 stability and parkin recruitment to damaged mitochondria through phosphorylation of MIC60. Mol Cell. 2016;62(3):371–384. doi: 10.1016/j.molcel.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 48.Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27(2):433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H, Robichaux WG, Wang Z, Mei FC, Cai M, Du G, Chen J, Cheng X. Inhibition of Epac1 suppresses mitochondrial fission and reduces neointima formation induced by vascular injury. Sci Rep. 2016;6:36552. doi: 10.1038/srep36552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chagtoo M, George N, Pathak N, Tiwari S, Godbole MM, Ladilov Y. Inhibition of intracellular type 10 adenylyl cyclase protects cortical neurons against reperfusion-induced mitochondrial injury and apoptosis. Mol Neurobiol. 2018;55(3):2471–2482. doi: 10.1007/s12035-017-0473-y. [DOI] [PubMed] [Google Scholar]
- 51.Rinaldi L, Pozdniakova S, Jayarajan V, Troidl C, Abdallah Y, Aslam M, Ladilov Y. Protective role of soluble adenylyl cyclase against reperfusion-induced injury of cardiac cells. Biochim Biophys Acta Mol Basis Dis. 2019;1865(1):252–260. doi: 10.1016/j.bbadis.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 52.Flacke J-P, Flacke H, Appukuttan A, Palisaar R-J, Noldus J, Robinson BD, Reusch HP, Zippin JH, Ladilov Y. Type 10 soluble adenylyl cyclase is overexpressed in prostate carcinoma and controls proliferation of prostate cancer cells. J Biol Chem. 2013;288(5):3126–3135. doi: 10.1074/jbc.M112.403279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schirmer I, Bualeong T, Budde H, Cimiotti D, Appukuttan A, Klein N, Steinwascher P, Reusch P, Mügge A, Meyer R, Ladilov Y, Jaquet K. Soluble adenylyl cyclase: a novel player in cardiac hypertrophy induced by isoprenaline or pressure overload. PLoS One. 2018;13(2):e0192322–e0192322. doi: 10.1371/journal.pone.0192322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, Liu D, Varin A, Nicolas V, Courilleau D, Mateo P, Caubere C, Rouet P, Gomez AM, Vandecasteele G, Fischmeister R, Brenner C. A cardiac mitochondrial cAMP signaling pathway regulates calcium accumulation, permeability transition and cell death. Cell Death Dis. 2016;7:e2198. doi: 10.1038/cddis.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.